Abstract
Novel psychoactive substances have maintained a prominent role in the global drug culture, despite increased regulation by governing bodies. Novel compounds continue to become available on the market, often in “Ecstasy” or “Molly” formulations in lieu of MDMA, at a much faster rate than they can be properly characterized. The current study aimed to investigate the discriminative stimulus and locomotor effects of three putatively entactogenic compounds that have become increasingly prevalent on the drug market: 5-(2-aminopropyl)-benzofuran (5-APB), 6-(2-aminopropryl)-2,3-dihydrobenzofuran (6-APDB), and 4-fluoroamphetamine (4-FA). Locomotor stimulant effects were assessed in an open-field assay for locomotor activity using Swiss-Webster mice. Discriminative stimulus effects were assessed in Sprague-Dawley rats trained to discriminate either cocaine, methamphetamine, DOM, or MDMA from vehicle. The benzofuran compounds produced locomotor stimulation whereas 4-FA depressed locomotor activity. The benzofurans substituted for the discriminative stimulus effects of MDMA, but only partially or not at all for methamphetamine, cocaine, and DOM, whereas 4-FA fully substituted for MDMA, methamphetamine and cocaine, but not DOM. These results indicate an MDMA-like pattern of abuse might be expected for the benzofurans, whereas 4-FA may be substituted for psychostimulants and MDMA.
Keywords: benzofuran, amphetamine, drug discrimination, rat
1. Introduction
Despite increased regulation, use of novel psychoactive substances (NPS) remains a prominent component of global drug culture and is especially popular among club- and rave-goers (Palamar et al., 2015; 2016a; 2016b). In addition to voluntary use, many individuals have inadvertently taken NPS as adulterants in “Ecstasy” or “Molly” formulations (UNODC, 2014; Palamar, 2016c). Many chemical classes comprise the “Ecstasy”-like NPS, but para-substituted and benzofuran analogs of amphetamine are especially popular alternatives to 3,4-methylenedioxymethamphetamine (MDMA) (Palamar et al., 2016c).
Benzofuran analogs of amphetamine (benzofurans) were initially synthesized as part of investigation into structure-activity relations of ring-substituted amphetamines (Monte et al., 1993). The derivatives 5- and 6-(2-aminpropyl)-benzofuran (5- and 6-APB) and 5- and 6-(2-aminopropyl)-2,3-dihydrobenzofuran (5- and 6-APDB) were commonly used in Benzofury formulations, which could readily be purchased online prior to scheduling (Jebadurai et al., 2013). Chemical structures are shown in figure 1. User reports of the subjective experiences associated with benzofuran compounds indicate both mild hallucinogenic and entactogenic effects, described as a “more intense” version of MDMA (Erowid.org, 2015). Similarly, para-substituted amphetamines have been associated with entactogen-like effects and used as adulterants in “Ecstasy” formulations, with para-methylamphetamine, para-methoxyamphetamine, and 4-fluoroamphetamine (4-FA) being amongst the most prevalent compounds in this class (Elliot & Evans, 2014; Palamar et al., 2016c). 4-FA is the most commonly reported para-substituted analog and is sought out specifically for its effects, in addition to inadvertent, adulterated “Ecstasy” use (Linsen et al., 2015). Its chemical structure is shown in figure 1.
Figure 1.
Structures of amphetamine and the analogs tested in the current study.
Like MDMA, benzofuran analogs of amphetamine act as substrates at the monoamine transporters with a greater affinity for the serotonin transporter (SERT) than the dopamine transporter (Rickli et al., 2015b; Baumann et al., 2011). The para-substituted amphetamine analogs have a greater serotonergic efficacy in terms of their capacity for promoting transmitter release than their unsubstituted amphetamine counterparts, but are still more selective for dopamine and norepinephrine transporters relative to the serotonin transporter (Nugteren-van Lonkhuyzen et al., 2015; Eshleman et al., 2016; Marona-Lewicka et al., 1995; Rickli et al., 2015a; Bauman et al., 2011). The reverse transport of dopamine by 5-APB demonstrated in vitro has been replicated in vivo using cyclic voltammetry (Dawson et al., 2014). This pharmacodynamic profile, relative to traditional, predominantly dopaminergic stimulants, is associated with unique effects and patterns of use, in that they tend to promote feelings of openness and empathy with fairly-limited or episodic consumption (Nichols, 1986; Degenhardt et al., 2010). As has been the case with many novel psychoactive substances, numerous adverse effects have been associated with benzofuran and para-substituted amphetamine use including hyperthermia, cardiovascular complications, psychotic episodes, and death (Chan et al., 2013; Seetohul & Pounder, 2013; Kamour et al., 2014; Hondebrink et al., 2015).
In vivo pharmacological data regarding these compounds are sparse, but some insight into their in vivo mechanistic and reinforcing effects has begun to emerge. 5- and 6-APDB fully substituted for the discriminative stimulus effects of the serotonin-releasing agents MBDB and MMAI (Monte et al., 1993). Assessments of 5-APB’s rewarding and reinforcing effects have demonstrated robust conditioned place preference following 5-APB administration, but limited self-administration in rats (Cha et al., 2016). Conversely, 4-FA substituted fully for the discriminative stimulus effects of d-amphetamine, but not MBDB or MMAI (Marona-Lewicka, et al., 1995). In line with its amphetamine-like discriminative stimulus, 4-FA, under the name PAL-303, was robustly self-administered by rhesus monkeys to a similar degree as cocaine (Wee et al., 2005).
These early data indicate potential mechanistic differences among these compounds that may influence differences in their subjective and reinforcing effects, with the benzofurans potentially maintaining an entactogen-like pharmacodynamic profile, whereas 4-FA may produce more stimulant-like effects. Previous studies from our laboratory have demonstrated abuse potential for the predominately serotonergic “Ecstasy” adulterant MDAI (Gatch et al., 2016), and the current study utilizes a similar approach to investigate the potential abuse liability of 5-APB, 6-APDB, and 4-FA. Assessments of locomotor activity were conducted to determine the active dose range and duration of action of these compounds. Drug discrimination experiments were performed utilizing the predominately dopaminergic training drugs methamphetamine and cocaine, as well as MDMA, which possesses a complex discriminative stimulus mediated by dopamine and 5-HT (Schechter, 1988; Goodwin et al., 2003), and DOM, a hallucinogenic phenethylamine with a 5-HT2A/C-dependent discriminative stimulus. Analyses of the time course of locomotor effects and relative dopaminergic and serotonergic contributions to the discriminative stimuli of these drugs will provide insight into their in vivo pharmacology and allow for predictions regarding their patterns of use in the human population.
2. Methods
2.1. Subjects
Male Swiss–Webster mice (n=136) were obtained from Harlan (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed in cages on a 12:12-h light/dark cycle and were allowed free access to food and water. Male Sprague-Dawley rats (n=61) were obtained from Envigo (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.2. Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) housed within sound-attenuating chambers in sets of two. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination and fans provided an 80-dB ambient noise level within the chamber.
Separate groups of 8 mice were injected with either vehicle (0.9% saline), 5-APB (1, 2.5, 5, 10 or 25 mg/kg), 6-APDB (1, 2.5, 5, 10 or 25 mg/kg), or 4-FA (0.5, 1, 2.5 or 5 mg/kg) immediately prior to locomotor activity testing. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, beginning at 0800 hrs (1 hr after lights on).
2.3. Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, pools of rats previously trained to discriminate either methamphetamine (1 mg/kg) n=12, cocaine (10 mg/kg) n=18, DOM (0.5 mg/kg) n=17, or MDMA (1.5 mg/kg) n=14 from saline as previously described (Gatch et al., 2016) were tested. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever (FR10). The pretreatment times were 10 min for both cocaine and methamphetamine, 15 min for MDMA, and 30 min for DOM. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 4 consecutive sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Each compound was tested in groups of six rats. The dose effect of each compound was tested from no effect to full effect or rate suppression (<20% of vehicle control) or adverse effects using a 1/3 log scale. 6-APDB was tested at 0.25, 0.5 and 1 mg/kg in methamphetamine-, cocaine-, and DOM-trained rats, and at 0.05, 0.1, 0.25, 0.5 and 1 mg/kg in MDMA-trained rats. 5-APB was tested at 0.25, 0.5 and 1 mg/kg in methamphetamine-, cocaine-, and DOM-trained rats, and at 0.1, 0.25, 0.5 and 1 mg/kg in MDMA-trained rats. 4-FA was tested at 0.5, 1 and 2.5 mg/kg in MDMA, cocaine-, and DOM-trained rats, and at 0.25, 0.5, 1 and 2.5 mg/kg in methamphetamine-trained rats. Starting doses and pretreatment times were inferred from the locomotor activity testing. Doses were tested in no particular order. For dose-effect experiments, intraperitoneal (i.p.) injections (1 ml/kg) of vehicle or test compound were administered with a pretreatment of 15 min for 4-FA, 60 min for 5-APB, and 40 min for 6-APDB. A repeated-measures design was used, such that each rat was tested at all doses or all time points of a given drug.
2.4. Drugs
(+)-methamphetamine hydrochloride, (−)-cocaine hydrochloride, (−)-DOM hydrochloride, (±)-MDMA hydrochloride, (±)-4-FA hydrochloride, (±)-5-APB hydrochloride, and (±)-6-APDB hydrochloride were all supplied by the National Institute on Drug Abuse Drug Supply Program. All compounds were dissolved in 0.9% saline except 4-FA, which was dissolved in deionized water. All compounds were administered i.p. in a volume of 1 ml/kg in rats and 10 ml/kg in mice.
2.5. Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data. A two-way repeated measures analysis of variance was conducted on horizontal activity counts/10 min interval. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests.
Drug discrimination data were expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for percent drug-appropriate responding and response rate were plotted as a function of test compound dose (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as >80% drug-appropriate responding and not statistically different from the training drug.
Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data was analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The potencies (ED50 and 95% confidence intervals) of the test compounds in both assays were calculated by fitting straight lines to the linear portion of the dose-response data for each compound by means of Origin Graph (OriginLab Corporation, Northampton, MA).
3. Results
3.1. Locomotor Activity
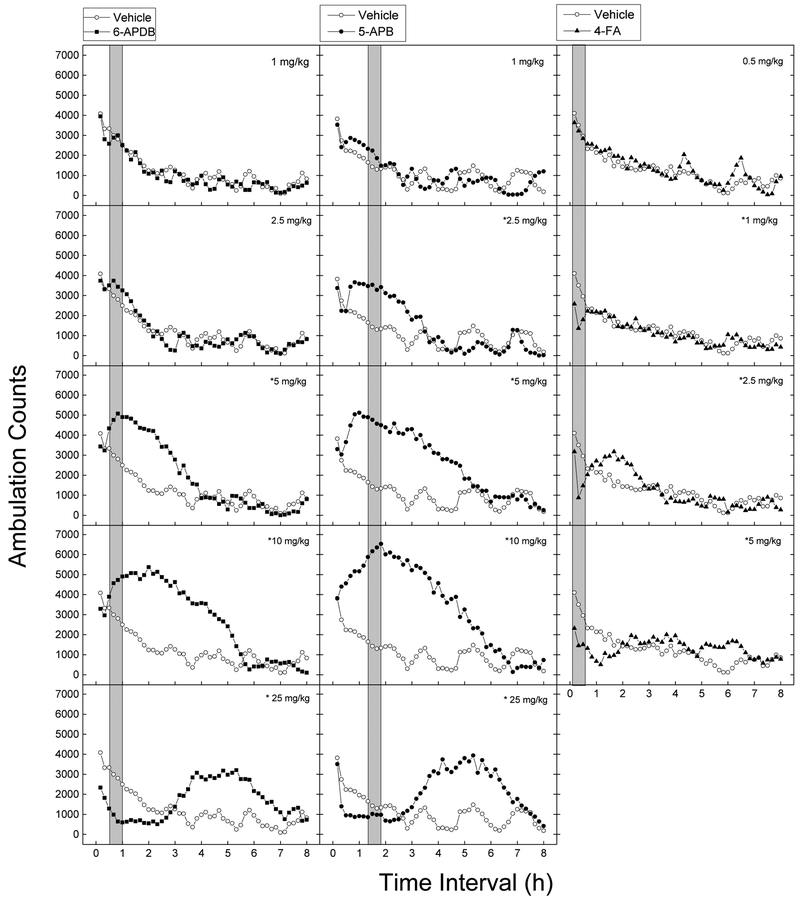
Treatment with 6-APDB resulted in time- and dose-dependent stimulation of locomotor activity following 5, 10 and 25 mg/kg, and depression of activity following 25 mg/kg (Fig. 2). A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effect of Treatment F(5,42)=16.157, p<.001, of 10-Minute Periods F(47,1974)=50.803, p<.001, and the interaction of Periods and Treatment F(235,1974)=10.901, p<.001. Stimulant effects of 5 mg/kg occurred within 30 min following injection. The duration of the stimulant effects increased as dose increased from 5 to 10 mg/kg, and the peak effect (5400 counts) occurred at 2 h following 10 mg/kg. The 25 mg/kg dose produced immediate onset depression of activity that lasted more than 2 h, followed by a delayed stimulant effect lasting from 3 to 7 h that reached a peak effect of 3100 counts at 4 h and 50 min following administration.
Figure 2.
Time course of locomotor stimulant effects. Average horizontal activity counts/10min (ambulation counts) as a function of time and dose for 6-APDB, 5-APB, and 4-FA. Each panel shows the effects of one dose of compound versus the vehicle; n=8 for each dose. *indicates stimulant effects (p<0.05) against vehicle control at any time period.
5-APB produced time- and dose-dependent stimulation of locomotor activity following 2.5 to 25 mg/kg [Treatment F(5,42)=15.352, p<.001, 10-Minute Periods F(47,1974)=46.146, p<.001, and interaction of Periods and Treatment F(235,1974)=11.761, p<.001]. The stimulant effects of 2.5 to 10 mg/kg occurred within 20 to 40 minutes following injection and peak effects of 6500 counts were reached at 110 min following 10 mg/kg. The duration of the stimulant effects increased as dose increased from 3 h following 2.5 mg/kg, to nearly 6 h following 10 mg/kg. Depression of activity occurred within 20 min following 25 mg/kg and lasted about 2 h, followed by a delayed stimulant effect lasting from 3 to 7 h that reached a peak effect of 3900 counts following 5 h and 20 min following administration.
Treatment with 4-FA resulted in time- and dose-dependent depression of locomotor activity following 1, 2.5 and 5 mg/kg [Treatment F(4,35)=0.257, p=.903, but significant effects were observed for the 10-Minute Periods F(47,1645)=25.139, p<.001, and the interaction of Periods and Treatment F(188,1645)=3.441, p<.001]. Depressant effects of 1 to 5 mg/kg occurred within 10 min following injection and lasted 30 min following 1 and 2.5 mg/kg and 90 min following 5 mg/kg. Delayed stimulant effects were observed from 90 to 250 min following 2.5 mg/kg and from 5 h to 6 h 20 min following 5 mg/kg.
The potencies of the locomotor stimulant effects of 5-APB and 6-APDB were calculated from the ascending portion of the dose effect curves taken at the time of peak effect. ED50 values for 5-APB (3.27 mg/kg 95% CI 0.66–16.12) and 6-APDB (2.80 mg/kg 95% CI 0.37–21.14) were not different. There was no difference in the peak effects between the two compounds [F(1,46)=3.008, p=0.09]. To assess the total efficacy of the compounds using both size of peak effect and duration of effect, the area between the vehicle and test compound curves were calculated for the two stimulant compounds. There was a significant effect of dose [F(4,70) = 21.51, p>0.001] and test compound [F(1,70) = 11.40, p=0.001], but no interaction effect [F(4,70)=0.597, p=0.666], such that the locomotor stimulant effects of 5-APB were larger than those of 6-APDB.
3.2. Drug Discrimination
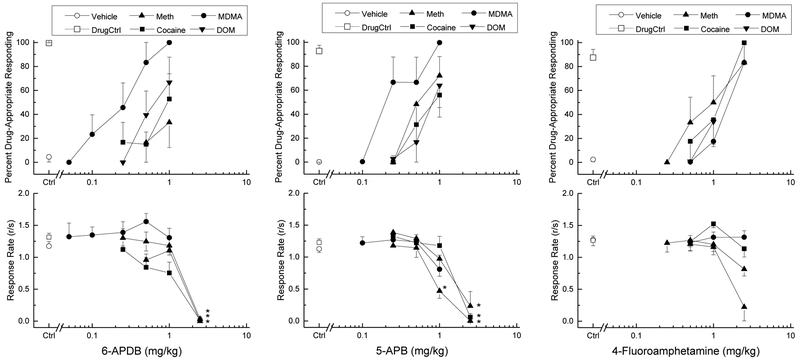
6-APDB fully substituted for the discriminative stimulus effects of MDMA (100% drug-appropriate responding at 1 mg/kg) with an ED50 of 0.21 mg/kg (95% CI 0.02 – 2.33 mg/kg), but produced peaks of only 67±21% in DOM-trained rats, 53±21% in cocaine-trained rats, and 33±21% in methamphetamine-trained rats, all at 1 mg/kg. A higher dose (2.5 mg/kg) produced near complete suppression of responding in DOM-trained rats [F(5,25)=16.88, p<.001], in cocaine-trained rats [F(4,20)=15.26, p<.001], and in methamphetamine-trained rats [F(3,15)=63.34, p<.001].
5-APB fully substituted for the discriminative stimulus effects of MDMA (99.8±0.2% at 1 mg/kg) with an ED50 of 0.27 mg/kg (95% CI 0.04 – 1.92 mg/kg), but also produced peaks of only 64±18% in DOM-trained rats, 56±18% in cocaine-trained rats, and 77±15% in methamphetamine-trained rats, again all at 1 mg/kg. A higher dose (2.5 mg/kg) significantly decreased responding in DOM-trained rats [F(4,20)=7.08, p=.001], in cocaine-trained rats [F(4,20)=45.49, p<.001], and in methamphetamine-trained rats [F(4,20)=41.98, p<.001]. Response rate was also decreased following 1 mg/kg 5-APB in the methamphetamine trained rats (p<0.05), although all 6 rats finished the session.
In contrast, 4-FA fully substituted for the discriminative stimulus effects of MDMA (83±17%), methamphetamine (83±17%), and cocaine (99.8±0.2%). 4-FA was equipotent across the three training compounds with ED50 values of 1.39 mg/kg (95% CI 0.17 – 11.31 mg/kg) in MDMA-trained rats, 0.95 mg/kg (95% CI 0.08 – 11.29 mg/kg) in methamphetamine-trained rats, and 1.06 mg/kg (95% CI 0.21 – 5.43 mg/kg in cocaine-trained rats. Peak effect was observed at 2.5 mg/kg in each case. A peak effect of only 34±21% drug-appropriate responding was observed in DOM-trained rats at 1 mg/kg. Higher doses were not tested because response rate was decreased following 2.5 mg/kg in the DOM-trained rats such that 5 of 6 rats failed to complete the first FR [F(3,15)=19.04, p<.001]. Response rate was also modestly decreased to 68% of vehicle control following 2.5 mg/kg in methamphetamine-trained rats. [F(4,20)=3.66, p=.021]. Response rate was not changed following any dose of 4-FA in MDMA- or DOM-trained rats.
4. Discussion
The results of the current study indicate differences in the behavioral profiles of 5-APB, 6-APDB, and 4-FA. The benzofuran compounds, 5-APB and 6-APDB, produced comparable patterns of locomotor stimulation and substituted fully for the discriminative stimulus effects of MDMA, but only partially for cocaine and DOM; however, there were slight differences in the methamphetamine-like discriminative stimulus effects of these compounds. 6-APDB failed to substitute for methamphetamine whereas 5-APB produced partial substitution. The para-fluorinated amphetamine analog 4-FA, on the other hand, produced locomotor depression and substituted fully for the discriminative stimulus effects of methamphetamine, cocaine, and MDMA, but produced minimal DOM-appropriate responding. These data provide further evidence of differential behavioral effects between these two classes of drugs and suggest alternative patterns of abuse wherein benzofuran use may mirror the typically episodic drug-taking behavior seen with MDMA (Degenhardt et al., 2010), whereas 4-FA would likely mimic the compulsive use seen with cocaine or methamphetamine.
These studies are the first to evaluate the discriminative stimulus and locomotor effects of 5-APB and expand on previous investigations into the discriminative stimulus effects of 6-APDB (Monte et al., 1993). Both 6-APDB and 5-APB produce robust and long-lasting locomotor stimulation with roughly equal potency in a manner similar to cocaine, methamphetamine, and several synthetic cathinones (Gatch et al., 2013); however, these benzofurans demonstrate a delayed onset of effects relative to other psychostimulants. The onset of locomotor stimulation induced by 5-APB corresponds to the time-course of monoamine release, as previous reports have indicated robust increases in dopamine and 5-HT 20–40 minutes post-administration in mice, which remain elevated up to 3 hours post-administration (Fuwa et al., 2016). The dose-response pattern and time-course of locomotor stimulation is very similar between these compounds, but 5-APB produces a larger amount of locomotor stimulation at 10 mg/kg than 6-APDB.
The discriminative stimulus profile of 5-APB and 6-APDB are also similar, with both compounds fully substituting for only MDMA and partially substituting for DOM and cocaine. The discriminative stimulus of 5-APB differs slightly from 6-APDB in that 5-APB produces a greater level methamphetamine-appropriate responding (77%) than 6-APDB (33%), hinting at a potentially greater dopaminergic component of the discriminative stimulus of 5-APB relative to 6-APDB. In vitro characterizations of the benzofurans’ mechanisms have indicated an MDMA-like mechanism, in that both compounds are potent monoamine-releasing agents with a greater relative selectivity for SERT over DAT (Rickli et al., 2015b; Monte et al., 1993). Furthermore, both benzofurans are agonists at the 5-HT2 receptor subtypes, which likely underlies their partial substitution for DOM (Rickli et al., 2015b). Full substitution for DOM was likely hindered by the the benzofurans’ nonselective properties, as evidenced by the partial substitution for cocaine, and, in the case of 5-APB, methamphetamine. Altogether, the discriminative stimulus effects of the benzofurans indicate a probable similarity to the subjective effects of MDMA with potentially hallucinogenic effects, mirroring the subjective effects of 5-APB and 6-APDB reported by users (Erowid.org, 2015). The full substitution for MDMA and partial substitution for DOM, cocaine, and methamphetamine by the benzofurans is indicative of a complex discriminative stimulus mediated by both dopamine and serotonin. The robust, psychostimulant-like locomotor stimulation is curious, given the the benzofurans’ lack of full substitution for cocaine or methamphetamine. Despite full substitution of these compounds for the discriminative stimulus effects of MDMA, the benzofurans produce a different profile of locomotor activity with robust increases in motor activity as opposed to the initial locomotor depression and modest, delayed stimulation typically seen with MDMA (Gatch et al., 2016; Gold et al., 1988).
The discriminative stimulus and locomotor effects of 4-FA are nearly opposite those of the benzofurans in that 4-FA produces dose-dependent, modest locomotor depression and fully substitutes for MDMA, cocaine, and methamphetamine, but not at all for DOM. The discriminative stimulus profile of 4-FA is suggestive of a psychostimulant-like compound, but the locomotor effects indicate otherwise. In fact, the locomotor effects of 4-FA are similar to those of MDMA, flephedrone, the methcathinone analog of 4-FA, and MDAI, a synthetic compound frequently substituted for MDMA as a club drug, or of dimethylamylamine (DMAA), an adrenergic dietary supplement used to enhance the effects of exercise (Dolan et al., 2015; Gatch et al., 2013; 2016). All four compounds, MDMA, flephedrone, MDAI, and DMAA produced dose-dependent depressant effects followed by weak rebound stimulant effects (Gatch et al., 2013; 2016). However, MDMA and MDAI have mixed serotonergic and dopaminergic pharmacology, and DMAA produces its effects primarily through adrenergic receptors (Monte et al., 1993; Iversen et al., 2014). Given the structural and behavioral similarities between flephedrone and 4-FA and their departure from the locomotor and discriminative stimulus of traditional stimulant-like drugs, para-fluorination appears to confer unique effects to amphetamine-like compounds.
Previous assessments of the discriminative stimulus effects of 4-FA found that 4-FA fully substituted for amphetamine, partially for MBDB, and failed to substitute for MMAI (Marona-Lewicka et al., 1995). Our findings parallel those previously reported, in that we demonstrate full substitution of 4-FA for both cocaine and methamphetamine. Furthermore, there is no substitution for the 5-HT2A/C partial agonist DOM in our studies, much like the lack of substitution for MMAI, a selective-serotonin releasing agent, in the previous study (Marona-Lewicka et al., 1995). Our results diverge, however, from those with training drugs with complex, dopaminergic-serotonergic discriminative stimulus effects, in that we found full substitution of 4-FA for MDMA, whereas the previous study found only partial substitution for MBDB, which produces a similar discriminative stimulus as MDMA (Marona-Lewicka et al., 1995; Oberlander & Nichols, 1988; Nichols, 1986). The reasons for the differences in substitution profiles between studies, as well as the psychostimulant-like discrimination, but locomotor depression in the current study are not immediately clear. Given the MDMA-like discriminative stimulus effects of 4-FA, it is possible that 4-FA’s locomotor depressant effects are mediated by serotonin release; however, the lack of substitution in DOM-trained rats limits the likelihood of this mechanism. Methodological differences, including rat strain, reinforcement schedule, etc. may also account in part for the discrepant results. Further assessments of the in vivo pharmacological mechanisms using selective antagonists are necessary to confirm this hypothesis and to dissociate the noradrenergic mechanisms of 4-FA from its dopaminergic and serotonergic effects.
These data provide evidence for a differential behavioral profile of the benzofurans and 4-FA, suggesting that the benzofurans are likely to produce MDMA- or entactogen-like effects, whereas 4-FA is a more psychostimulant-like drug. These conclusions are further supported by previous assessments using intravenous self-administration methods demonstrating limited self-administration of 5-APB (Cha et al., 2016) and robust reinforcing effects of 4-FA (Wee et al., 2005). Given the entactogen-like effects and of the benzofurans, they are less likely to engender compulsive use than 4-FA, with its psychostimulant-like discriminative stimulus. Although the discriminative stimulus effects of these compounds match the self-administration data previously reported, the locomotor data suggest the distinction is not as simple as entactogen versus psychostimulant. Future studies regarding the mechanisms underlying the discrepancies between the locomotor activity and discriminative stimulus effects of these drug classes will be beneficial for understanding their abuse liability or toxicology profiles.
Figure 3.
Substitution for the discriminative stimulus effects of methamphetamine, cocaine, MDMA, or DOM. The top graph in each panel shows the percentage of total responses made on the drug-appropriate lever. The bottom graph shows the rate of responding in responses per second (r/s). Panel A shows the effects of the 6-APDB in methamphetamine-, cocaine-, MDMA-, and DOM-trained rats. Panel B shows the effects of 5-APB, and Panel C shows the effects of 4-FA. n=6 for each compound. Ctrl indicates vehicle and training drug controls averaged across training drugs. * indicates rate effects (p<0.05) against vehicle control.
Acknowledgements
Funding Sources: N01-DA78872, T32 AG020494
References
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB, 2011. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J. Pharmacol. Exp. Ther 337, 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HJ, Lee KW, Eom JH, Kim YH, Shin J, Yun J, Han K, & Kim HS, 2016. 5-(2-aminopropyl)benzofuran and phenazepam demonstrate the possibility of dependence by increasing dopamine levels in the brain. Pharmacol. Biochem. Behav 149, 17–22. [DOI] [PubMed] [Google Scholar]
- Chan WL, Wood DM, Hudson S, & Dargan PI, 2013. Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J. Med. Toxicol 9(3), 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P, Opacka-Juffry J, Moffatt JD, Daniju Y, Dutta N, Ramsey J, & Davidson C, 2014. The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog. Neurophsychopharmacol. Biol. Psychiatry 48, 57–63. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bruno R, & Topp L, 2010. Is ecstasy a drug of dependence? Drug Alcohol Depend. 107(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Dolan SB, Gatch MB (2015). Abuse liability of the dietary supplement dimethylamylamine. Drug and Alcohol Dependence 146: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, & Evans J, 2014. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int 243, 55–60. [DOI] [PubMed] [Google Scholar]
- Erowid. (2015). 6-APB Experience Vault. Retrieved from <https://erowid.org/chemicals/6_apb/6_apb.shtml> Accessed April 24, 2017.
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, & Janowsky AJ, 2016. Structure-activity relationships of substituted cathinones, with transporter binding, uptake and release. J. Pharmacol. Exp. Ther doi: 10.1124/jpet.116.236349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T, Suzuki J, Tanaka T, Inomata A, Honda Y, & Kodama T, 2016. Novel psychoactive benzofurans strongly increase extracellular serotonin level in mouse corpus striatum. J. Toxicol. Sci 41(3), 329–337. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013). Locomotor stimulant and discriminative stimulus effects of “Bath Salt” cathinones. Behavioural Pharmacology 24:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, & Forster MJ, 2016. Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav. Pharmacol 27(6), 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Koob GF, & Geyer MA, 1988. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxymethamphetamine in rats. J. Pharmacol. Exp. Ther 247(2), 547–555. [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, & Baker LE, 2003. Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a three-choice discrimination. Pharmacol. Biochem. Behav 74(4), 987–995. [DOI] [PubMed] [Google Scholar]
- Hondebrink L, Nugteren-van Lonkhuyzen JJ, Van Der Gouwe D, & Brunt TM, 2015. Monitoring new psychoactive substances (NPS) in The Netherlands: data from the drug market and the Poisons Information Centre. Drug Alcohol Depend. 147, 109–115. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, & Roth BL, 2013. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol 700(1–3), 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebadurai J, Schifano F, & Deluca P, 2013. Recreational use of 1-(2-naphthyl)-2-(1-pyrrolidinyl)-1-pentanone hydrochloride (NRG-1), 6-(2-aminopropyl) benzofuran (benzofury/ 6-APB) and NRG-2 with review of available evidence-based literature. Hum. Psychopharmacol 28(4), 356–364. [DOI] [PubMed] [Google Scholar]
- Kamour A, James D, Lupton DJ, Cooper G, Eddleston M, Vale A, Thompson JP, Thanacoody R, Hill SL, & Thomas SH, 2014. Patterns of presentation and clinical features of toxicity after reported use of ([2-aminopropyl]-2,3-dihydrobenzofurans), the ‘benzofuran’ compounds. A report from the United Kingdom National Poisons Information Service. Clin. Toxicol. (Phila) 52(10), 1025–1031. [DOI] [PubMed] [Google Scholar]
- Linsen F, Koning RP, van Laar M, Niesink RJ, Koeter MW, & Brunt TM, 2015. 4-Fluoroamphetamine in the Netherlands: more than a one-night stand. Addiction. 110(7), 1138–1143. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Gyu-Seek R, Sprague JE, & Nichols DE, 1995. Psychostimulant-like effects of p-fluoroamphetamine in the rat. Eur. J. Pharmacol 287, 105–113. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Cozzi NV, & Nichols DE, 1993. Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine. J. Med. Chem 36(23), 3700–3706. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academies Press, Washington, D.C. [Google Scholar]
- Nichols DE (1986). Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: Entactogens. J Psychoactive Drugs, 18(4), 305–313. [DOI] [PubMed] [Google Scholar]
- Nugteren-van Lonkhuyzen JJ, van Riel AJ, Brunt TM, & Hondebrink L, 2015. Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans. Drug Alcohol Depend. 157, 18–27. [DOI] [PubMed] [Google Scholar]
- Oberlander R & Nichols DE, 1988. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology (Berl). 95, 71–76. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Acosta P, Sherman S, Ompad DC, & Cleland CM, 2016a. Self-reported use of novel psychoactive substances among attendees of electronic dance music venues. Am. J. Drug Alcohol Abuse 42(6), 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Barratt MJ, Ferris JA, & Winstock AR, 2016b. Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am. J. Addict 25(5), 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Martins SS, Su MK, & Ompad DC, 2015. Self-reported use of novel psychoactive substances in a US nationally representative survey: Prevalence, correlates, and a call for new survey methods to prevent underreporting. Drug Alcohol Depend. 156, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Vincenti M, & Cleland CM, 2016c. Detection of “bath salts” and other novel psychoactive substances in hair samples of Ecstasy/MDMA/”Molly” users. Drug Alcohol Depend. 161, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, & Liechti ME, 2015a. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol 25(3), 365–376. [DOI] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, & Liechti ME, 2015b. Pharmacological profile of novel psychoactive benzofurans. Br. J. Pharmacol 172(13), 3412–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, 1988. Serotonergic-dopaminergic mediation of 3,4-methlenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol. Biochem. Behav 31(4), 817–824. [DOI] [PubMed] [Google Scholar]
- Seetohul LN, & Pounder DJ, 2013. Four fatalities involving 5-IT. J. Anal. Toxicol 37(7), 447–451. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC)., 2014. Global Synthetic drugs assessment: Amphetamine-type stimulants and new psychoactive substances. New York, New York. [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, & Woolverton WL, 2005. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J. Pharmacol. Exp. Ther 313(2), 848–854. [DOI] [PubMed] [Google Scholar]