Abstract
G0/G1 switch gene 2 (G0S2) is a specific inhibitor of adipose triglyceride lipase (ATGL), the rate-limiting enzyme for intracellular lipolysis. Recent studies show that G0S2 plays a critical role in promoting triacylglycerol (TG) accumulation in the liver, and its encoding gene is a direct target of a major lipogenic transcription factor liver X receptor (LXR)α. Here we sought to investigate a lipolysis-independent role of G0S2 in hepatic triglyceride synthesis. Knockdown of G0S2 decreased hepatic TG content in mice with ATGL ablation. Conversely, overexpression of G0S2 promoted fatty acid incorporation into TGs and diacylglycerols in both wild-type and ATGL-deficient hepatocytes. Biochemical characterization showed that G0S2 mediates phosphatidic acid synthesis from lysophosphatidic acid (LPA) and acyl-coenzyme A. In response to a high-sucrose lipogenic diet, G0S2 is up-regulated via LXRα and required for the increased TG accumulation in liver. Furthermore, deletion of a distinct 4-aa motif necessary for the LPA-specific acyltransferase (LPAAT) activity impaired G0S2’s ability to mediate TG synthesis both in vitro and in vivo. These studies identify G0S2 as a dual-function regulator of lipid metabolism as well as a novel mechanism whereby hepatic TG storage is promoted in response to lipogenic stimulation. In addition to its role as a lipolytic inhibitor, G0S2 is capable of directly promoting TG synthesis by acting as a lipid-synthesizing enzyme.—Zhang, X., Xie, X., Heckmann, B. L., Saarinen, A. M., Gu, H., Zechner, R., Liu, J. Identification of an intrinsic lysophosphatidic acid acyltransferase activity in the lipolytic inhibitor G0/G1 switch gene 2 (G0S2).
Keywords: LPA acyltransferase, fatty liver disease, hepatic steatosis, lipolysis, lipogenesis
Nonalcoholic fatty liver disease (NAFLD) is currently the most common form of chronic liver disease not related to alcohol consumption. NAFLD is developed when hepatic steatosis becomes sustained due to disruptions of the equilibrium between metabolic pathways for triacylglycerol (TG) synthesis, hydrolysis, and secretion (1–4). The major sources of fatty acids (FAs) contributing to hepatic TG synthesis are the nonesterified FAs from adipose tissue, dietary FAs, and FAs derived from lipoprotein remnant, and newly synthesized FAs through de novo lipogenesis (DNL) (5, 6). Although TG synthesis is generally recognized to follow the classic sn-glycerol-3-phosphate or Kennedy pathway (7), deletion of key TG-synthesizing enzymes such as 1-acylglycerol-3-phosphate o-acyltransferase (AGPAT)2 and lysophosphatidic acid acyl transferase (LPAAT)-β or lipin-1 failed to prevent hepatic TG accumulation and steatosis (8, 9). Thus, the molecular mechanisms and identity of proteins that regulate the incorporation of FAs from different origins to the liver TG pool are still incompletely defined.
Aside from enhanced FA and TG synthesis, emerging data have pointed to impaired intrahepatic TG clearance through lipolysis and FA oxidation as another possible contributor to liver steatosis. Earlier work by several groups has established adipose triglyceride lipase (ATGL)/desnutrin/patatin-like phospholipase domain containing 2 as the rate-limiting TG hydrolase in adipose tissue and many nonadipose tissues including liver (10–15). Genetic disruption of ATGL in mice resulted in blocked adipose lipolysis as well as accumulation of TGs in liver and striated muscles. Liver-specific ablation of either ATGL or the ATGL coactivator comparative gene identification-58 both led to the impairment in PPARα activation and FA oxidation along with the development of hepatic steatosis (16–18). Conversely, adenovirus-mediated hepatic overexpression of ATGL promoted FA oxidation, reduced hepatic lipotoxicity, and ameliorated steatosis in mice with diet-induced obesity (19, 20). In humans, liver ATGL expression is decreased in NAFLD patients (21). Collectively, these studies have established ATGL as a key regulator of hepatic TG and energy metabolism. Despite recent evidence showing a communication between ATGL action and lipid synthesis in adipose tissue (22), whether TG synthetic and lipolytic pathways crosstalk and converge in liver remains an open question.
Previous studies by our lab have established a small basic protein encoded by G0/G1 switch gene 2 (G0S2) as a selective endogenous inhibitor of ATGL (23). G0S2 functions to prevent intracellular TG hydrolysis and lipid droplet degradation catalyzed by ATGL (23–25). Mechanistically, G0S2 binds directly to the patatin-like domain of ATGL and inhibits ATGL in a noncompetitive manner (26). In contrast to ATGL deletion, both global- and liver-specific G0S2 ablation in mice led to impaired hepatic unfed response in terms of TG accumulation and glycogen depletion (27–29). The G0S2-deficient mice exhibited increased hepatic lipolysis, FA oxidation, and ketogenesis. In response to high-fat diet (HFD) treatment, these mice showed improved insulin sensitivity and were completely resistant to the development of hepatic steatosis (27).
Recently, we have obtained evidence that hepatic G0S2 is a direct target of liver X receptor α (LXRα) (30), a major transcription factor mediating DNL. In the present study, we demonstrate that G0S2 plays a lipogenic role downstream of LXRα that is in addition to its established function as an ATGL inhibitor. Specifically, G0S2 is capable of acting as a TG-synthesizing enzyme, namely LPAAT. Our data indicate that in response to lipogenic stimulation, G0S2 plays a dual role as both an LPAAT and a lipolytic inhibitor in promoting hepatic TG accumulation.
MATERIALS AND METHODS
Reagents
[9, 10 (n)-3H]triolein; [9, 10 (n)-[3H]] oleic acid; (1-14C)oleoyl-coenzyme A (CoA); and [1-oleoyl-9, 10-3H] lysophosphatidic acid (LPA) were purchased from PerkinElmer (Waltham, MA, USA). 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate; 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC); 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (LPE); 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1'-rac-glycerol) (LPG); 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1'-myo-inositol) (LPI); 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-l-serine (LPS); 1,2-dielaidoyl-sn-glycero-3-phosphocholine; 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; 1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol); 1,2-dioleoyl-sn-glycero-3-phospho-(1'-myo-inositol); 1,2-dioleoyl-sn-glycero-3-phospho-l-serine; and docosahexaenoyl CoA were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Sodium oleate; 1,2-dioleoyl-sn-glycerol (DAG); 1,2-diacyl-sn-glycero-3-phosphate; glycerol-3-phosphate; butyryl CoA; Octanoyl CoA; Lauroyl CoA, palmitoyl CoA; oleoyl CoA; Linoleoyl CoA; Arachidonoyl CoA; and bovine serum albumin (BSA) (essentially FA free) were purchased from MilliporeSigma (Burlington, MA, USA). T0901317 (T09) was obtained from Tocris Bioscience (Bristol, United Kingdom). The anti-ATGL antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-G0S2 antibody was produced as previously described by Yang et al. (23). Horseradish peroxidase–linked secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The protease inhibitor mini tablet cocktail was obtained from Roche Diagnostics (Basel, Switzerland). The PureLink RNA Mini Kit and High-Capacity cDNA Reverse Transcription Kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Thin-layer chromatography (TLC) Glass Silica 60 F254 plates were purchased from MilliporeSigma. pET His6 MBP cloning vector was a gift from Scott Gradia (Plasmid 29708; Addgene, Watertown, MA, USA).
Animal experiments
Wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the animal facility at Mayo Clinic Arizona. ATGL+/− mice were generated by targeted homologous recombination as previously described by Haemmerle et al. (31). Heterozygous ATGL+/− mice on C57/BL6J background were used to generate the whole-body ATGL−/− mice. LXRα−/− mice were purchased from The Jackson Laboratory along with aged matched WT mice on C57BL6/J background. G0S2−/− mice were produced on C57/BL6N as previously reported by Zhang et al. (27) and backcrossed to C57/BL6J for 10 generations. Unless otherwise noted, all mice had free access to water and were fed a chow diet (5001, 10% calories as fat; Test Diet, Richmond, IN, USA). All animal experiments were done in accordance with protocols approved by the Mayo Clinic Institutional Animal Care and Use Committee.
To induce hepatic lipid accumulation, the LXR agonist T09 suspended in PBS containing 0.5% Tween-80 was intraperitoneally injected into mice. For liver-specific overexpression of G0S2, mice were injected with 2.5 × 109 plaque-forming units (pfu) of adenovirus-encoding murine G0S2 or a control adenovirus (Vector Biolabs, Malvern, PA, USA) via the retro-orbital sinus. For liver-specific knockdown of G0S2 or ATGL, stealth control, G0S2, or ATGL-specific small interfering RNA (siRNA) or invovofectamin mixture was made as previously described by Zhang et al. (27) and injected into mice via the retro-orbital sinus. The sequences of siRNA oligonucleotides (Thermo Fisher Scientific) are as follows: For mouse G0S2, sense 5′-CAUGCUGUUUCAAGGUGCCACCGAA-3′ and antisense 5′-UUCGGUGGCACCUUGAAACAGCAUG-3′; for mouse ATGL, sense 5′-UCAGACGGAGAGAACGUCAUCAUAU-3′ and antisense 5′-AUAUGAUGACGUUCUCUCCGUCUGA-3′. Control oligonucleotides with comparable guanine-cytosine (GC) content were also obtained from Thermo Fisher Scientific.
Liver lipid metabolite analysis
Liver lipids were extracted by choloroform/methonal (2:1), dried under nitrogen gas, and resuspended in 1% Triton X-100. Then total triglyceride content was qualified by using the TG assay kit from Thermo Fisher Scientific. Analysis of hepatic lipid profile was custom performed by the West Coast Metabolomics Center, University of California-Davis (Davis, CA, USA).
In vitro transcription-translation expression
In vitro transcription-translation was carried out by using TNT SP6 High-Yield Protein Expression System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Specifically, reactions consisting of 30 μl TNT SP6 High-Yield Wheat Germ Master Mix and 5 μg vector DNA, made up to 50 μl with molecular biology–grade water were incubated for 120 min at 25°C. Then the mixture was diluted to 1 ml in the assay buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM ETDA), and immunoprecipitation was performed with anti-DYKDDDDK peptide (FLAG) agarose beads to purify FLAG fusion proteins. After washing with the assay buffer 3 times, the agarose beads in 50 μl of the assay buffer were directly used for the assay of LPAAT or glycerol-3-phosphate acyltransferase (GPAT) activity.
Production and purification of bacterially expressed G0S2
The full-length mouse G0S2 cDNA was subcloned by standard PCR into pET His6 MBP vector (Plasmid 29708; Addgene) producing a G0S2 fusion protein with a His6-MBP tag at the N-terminal end. G0S2 fusion protein was produced in BL21(DE3) Escherichia coli (Agilent Technologies, Santa Clara, CA, USA) with induction by addition of 0.5 mM IPTG (MilliporeSigma) at an A600 of 0.4–0.6 at 15°C and harvested after culturing for an additional 16 h. The cells were lysed by sonication-collagenase; the expressed G0S2 fusion protein was purified using nickel nitrilotriacetic acid (NTA) agrose beads according to the commercial protocol. The purified protein was then injected onto an HPLC-driven amylose affinity column equilibrated with a buffer consisting of 20 mM Tris, pH 7.5; and 300 mM NaCl (buffer A). After flow through, and absorption at 280 nm returned to ∼0 mAU, 100% buffer B, consisting of 20 mM Tris, pH 7.5; 300 mM NaCl; and 50 mM maltose, replaced buffer A and the eluted protein was collected. For further purification, a Superdex 200 Column (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) was run with the eluted protein, and the resulting chromatogram showed a dominant peak at the 7.5-ml void volume where G0S2 fusion protein was confirmed by SDS-PAGE analysis followed by Coomassie Brilliant Blue staining. The sample was then concentrated to 5 mg/ml and dialyzed into the assay buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1 mM EDTA.
PCR cloning of cDNA and site-directed mutagenesis
The complete open reading frame of mouse ATGL, GPAT4, AGPAT2, and G0S2 were cloned into pRK vector without tags or pKF vector with a FLAG epitope tag at the C-terminal end as previously reported by Liu et al. (32). Deletion mutations were generated by using the QuickChange Site-Directed Mutagenesis Kit (Agilent Technologies) according to the manufacturer’s guidelines. pRF-G0S2 constructs were used as templates for mutagenesis reactions.
Assay of acyltransferase activities
Enzyme activities of GPAT and LPAAT were determined by measuring the production of LPA and phosphatidic acid (PA), respectively. Unless otherwise stated, the enzymatic reaction was assembled in 50 μl of the assay buffer above containing 10 μM of [14C]oleoyl-CoA and 100 μM of either glycerol 3-phosphate for GPAT or LPA for LPAAT. The reaction was initiated by adding 50 μl of protein purified from BL21(DE3) E. coli lysate or anti-FLAG immunoprecipitates from the in vitro transcription-translation yeast lysate, followed by incubation for 10 min at 37°C. At the end of the incubation, the reaction was terminated by the addition of 600 μl of chloroform:methanol (2:1) and 400 μl of acidified water (2% orthophosphoric acid). The labeled lipid in the lower phase was resolved by TLC using Silica Gel 60 TLC plates in a solvent system consisting of chloroform:methanol:acetone:glacial acetic acid:water (50:10:20:15:5). Radioactive spots comigrating with unlabeled LPA or PA standard were scraped and quantified by scintillation counting.
Assay for TG hydrolase activity
HeLa cells expressing ATGL, G0S2, or its mutants were lysed on ice by sonication in a buffer containing 0.25 M sucrose, 1 mM EDTA, 1 mM DTT, and protease inhibitors (1 minitablet/10 ml volume). The cell extracts were clarified by centrifugation at 15,000 g for 10 min. The TG hydrolase activity against 3H-labeled triolein was measured as previously described in refs 14 and 33 by mixing 0.1 ml of extracts with 0.1 ml of substrate solution.
Measurement of lipid synthesis in primary hepatocytes
Primary hepatocytes were isolated by the collagenase perfusion method from 8-wk old WT or ATGL−/− mice as previously described by Chen et al. (34). Recombinant adenovirus under the control of a cytomegalovirus promoter were custom generated by Vector Biolabs. A cytomegalovirus-null virus [null adenovirus (Ad-Null)] was also obtained for use in control experiments. Briefly, primary hepatocytes were seeded into a 6-well plate in the medium with low glucose DMEM/1% fetal bovine serum at 37°C under 5% CO2. Three hours later, cells were infected with 2 × 107 pfu per well of adenovirus for 40 h in the presence of 2.5 μg/ml of polybrene. Then cells were washed with PBS and treated with 2 ml of oleate/BSA complex containing 0.3 mM sodium oleate, 0.3% BSA, and 1 μCi/ml [9,10-3H]-oleate in serum-free DMEM medium. After 6 h of incubation, cellular lipid was extracted and separated by TLC. Radioactivity comigrating with the TG or DAG band was excised and quantified by scintillation counting. The rate of incorporation of [3H] oleate into TG or DAG normalized by protein contents was calculated to assess lipid synthesis.
Immunoblotting
Liver samples or primary hepatocytes were lysed at 4°C in a buffer containing 50 mM Tris-HCl (pH 7.4), 135 mM NaCl, 10 mM NaF, 1% Nonidet P-40 (MilliporeSigma), 0.1% SDS, 0.5% sodium deoxycholate, 1.0 mM EDTA, 10% glycerol, and protease inhibitors (1 tablet/10 ml buffer). The lysates were clarified by centrifugation at 20,000 g, 4°C for 10 min, and then mixed with equal volume of 2× SDS sample buffer. Equivalent amounts of protein were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Individual proteins were blotted with primary antibodies at appropriate dilutions. Peroxide-conjugated secondary antibodies were incubated with the membrane at a dilution of 1:5000. The signals were then visualized using ECL substrate (Pierce, Rockford, IL, USA).
Statistical analysis
Values are expressed as means ± sd. Statistical significance was evaluated by a 2-tailed, unpaired Student’s t test. Differences were considered significant at P < 0.05.
RESULTS
Effect of G0S2 on hepatic TG accumulation independent of ATGL inhibition
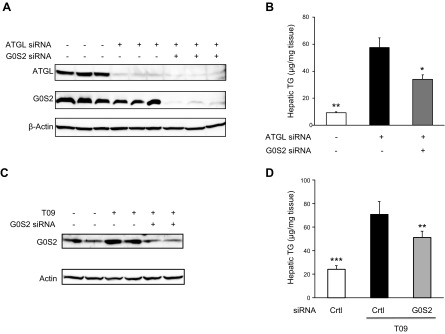
Previous studies show that hepatic ablation of ATGL results in steatosis. To determine whether G0S2 promotes hepatic TG accumulation solely through its inhibitory effect on ATGL, we silenced G0S2 expression in mouse liver where ATGL was also depleted. Immunoblotting analysis demonstrated that the hepatic ATGL expression was effectively knocked down upon injection of mice with an siRNA oligo specifically targeting ATGL when compared with a GC-matched control oligo (Fig. 1A). Subsequent coknockdown of G0S2 did not affect the knockdown efficiency of ATGL. As shown in Fig. 1B, knockdown of ATGL led to a near 6-fold increase in the unfed liver TG content. Interestingly, coknockdown of G0S2 with ATGL resulted in a 43% reduction in the hepatic TG levels compared to the knockdown of ATGL alone (Fig. 1B). The result implies the possibility that G0S2 is able to substantially enhance TG accumulation in the absence of ATGL.
Figure 1.
The ATGL-independent effect of G0S2 on hepatic lipid metabolism. A, B) Eight-week-old C57BL/6j female mice fed a chow diet were injected with ATGL-specific or control siRNA. Ten hours later, mice were injected with G0S2-specific or control siRNA. After 3 d of siRNA treatment, mice were unfed for 6 h. Immunoblotting of liver extract (A) and measurement of hepatic TG content (B) (n = 6/group) were performed. Results are expressed as means ± sem. *P < 0.05, **P < 0.01 vs. single ATGL knockdown. C, D) Eight-week-old ATGL−/− mice fed a chow diet were intraperitoneally injected with 20 mg/kg per days of T09. G0S2-specific or control siRNA was injected into mice at d 4 after T09 injection. After 7 d of T09 treatment, plasma and tissues were collected. Immunoblotting of liver extract (C) and measurement of hepatic TG content (D) (n = 6/group) were performed. **P < 0.01, ***P < 0.001 vs. T09 + control siRNA.
Since our previous study identified G0S2 as a direct target of LXRα (30), we next determined whether G0S2 contributes to hepatic TG accumulation during LXR-mediated lipogenic stimulation. To exclude the influence of ATGL, we performed hepatic G0S2 knockdown in the whole-body ATGL−/− mice that were pretreated with vehicle alone or an LXR agonist T0901317 (T09). In mice receiving the control siRNA, T09 treatment resulted in a marked increase in the hepatic G0S2 expression (Fig. 1C) as well as a near 2.9-fold increase in the liver TG content (Fig. 1D). Injection of siRNA targeting G0S2 was effective in inducing a >60% reduction in the G0S2 levels in T09-treated mice (Fig. 1C). Importantly, silencing of G0S2 decreased the liver TG content significantly in the T09-treated ATGL−/− animals by 28% (Fig. 1D). Thus, in addition to food withdrawal, hepatic G0S2 is also critically involved in LXR-activated TG accumulation when ATGL is absent.
G0S2 promotes lipid synthesis in ATGL−/− hepatocytes
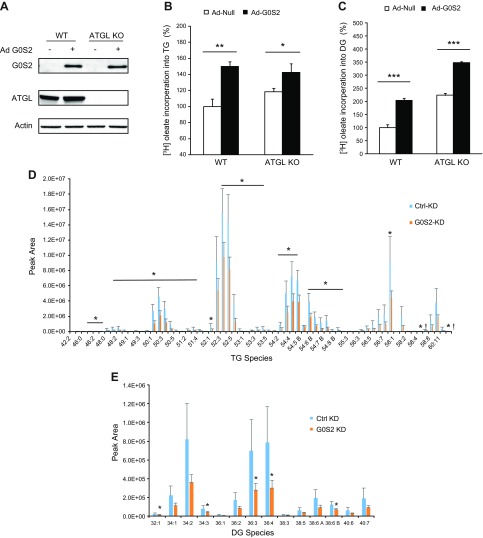
The findings that G0S2 promoted TG accumulation independently of ATGL and in response to LXR agonism hinted a role of G0S2 in lipid synthesis. To test this, mouse primary hepatocytes were isolated and infected with either a recombinant adenovirus-encoding G0S2 (Ad-G0S2) or a control null virus (Ad-Null). The rates of incorporation of radiolabeled oleate into TG and diacylglycerol (DG) were measured as an index of lipid synthesis. As shown in Fig. 2A, infection with Ad-G0S2 resulted in comparable expression of G0S2 in WT and ATGL−/− hepatocytes. Interestingly, overexpression of G0S2 induced a significant increase in the rates of DG and TG synthesis in both cell types (Fig. 2B, C), indicating a role of G0S2 in promoting glycerolipid synthesis in an ATGL-independent manner. In accordance, we also found that knockdown of G0S2 in vivo led to significant decreases in not only TG species but also various DG species in the mouse liver (Fig. 2D, E).
Figure 2.
G0S2 promotes TG synthesis in an ATGL-independent way. A, B) Primary hepatocytes infected with 2 × 107 of Ad-Null or G0S2-expressing adenovirus (Ad-G0S2) were treated with BSA-complexed oleate (0.3 mM unlabeled plus 1 µCi/ml [9,10-3H]oleate and 0.3% BSA) for 6 h. C) Immunoblotting (IB) of G0S2 and ATGL in cells was performed. D, E) Lipid synthesis was assessed by the rate of incorporation of [3H] oleate into TG (D) or DG (E). The data are representative of 3 independent experiments. Eight-week-old C57BL/6j female mice were injected with control (Ctrl KD) or G0S2-specific siRNA (G0S2 KD). Three days later, mice were unfed for 6 h. Then TG and DG species were profiled as described in Materials and Methods (n = 6). KD, knockdown; KO, knockout. *P < 0.05, **P < 0.01, ***P < 0.001.
Identification of an intrinsic LPAAT activity in G0S2
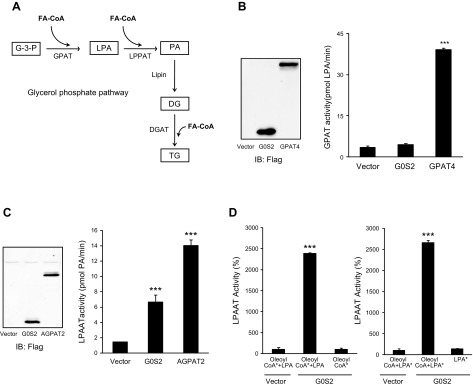
The main pathway for TG synthesis is the glycerol phosphate pathway, where 3 FAs are sequentially added to a glycerol backbone by glycerol kinase, GPATs, LPAATs/AGPATs, and DG acyltransferase, respectively (Fig. 3A). Given the G0S2’s ability to promote the synthesis of both DG and TG, we asked whether G0S2 would be involved in acyl-CoA–dependent lipid synthesis. An in vitro transcription-translation system was used to produce FLAG epitope–tagged G0S2, GPAT4, and AGPAT2. Immunoprecipitated proteins were then incubated with radiolabeled oleoyl-CoA as an acyl donor and various acylglycerols as acceptor molecules. As revealed by the TLC analysis, G0S2 displayed no notable activity as a GPAT while GPAT4 robustly catalyzed the production of LPA from glycerol-3-phosphate and oleoyl-CoA (Fig. 3B). Instead, G0S2 was capable of efficiently catalyzing the acyl-CoA-dependent acylation of LPA to generate PA (Fig. 3C). When used in comparable amounts, G0S2 exhibited a level of LPAAT activity that was near 50% of that displayed by AGPAT2. Figure 3D further illustrates that the LPAAT activity of G0S2 is specific because G0S2 was unable to produce PA in the absence of either LPA or oleoyl-CoA. In addition, no significant acyltransferase activity was detected when other lipids (e.g., monoacylglycerols, DGs) were used as acyl acceptors (unpublished results).
Figure 3.
G0S2 possesses a LPAAT activity. A) Outline of the glycerol phosphate pathway for TG synthesis in liver. B, C) Evaluation of the GPAT or LPAAT activity of G0S2. C-terminal Flag-tagged G0S2, GPAT, or LPAAT was expressed using a yeast cell-free lysate and then immunoprecipitated by Flag agarose beads followed by the assay of GPAT (B) or LPAAT activity under standard conditions (C). D) The LPAAT activity was measured by using 10 µM [14C]oleoyl-CoA (oleoyl CoA*) in the presence or absence of 100 µM LPA (left panel) or by using 20 µM ([3H]) LPA (LPA*) in the presence or absence of 100 µM oleoyl-CoA (right panel). The data are representative of 3 independent experiments. ***P < 0.001 vs. vector alone.
Kinetic behavior of G0S2 as an LPA-specific acyltransferase
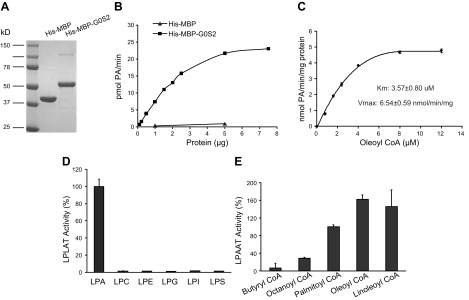
To further validate the LPAAT activity of G0S2, we cloned mouse G0S2 into a His-MBP vector and expressed the doubly tagged fusion protein in E. coli cells. The MBP tag was used to enhance the solubility of G0S2, and the double tag allowed for sequential purification through NI-NTA agarose resin and amylose affinity column prior to the final size-exclusion chromatography (Fig. 4A). As shown in Fig. 4B, the His-MBP-G0S2 fusion protein dose dependently catalyzed the formation of PA in the presence of oleoyl-CoA and LPA. In comparison, the LPAAT activity of His-MBP alone was negligible, indicating that our method of purification effectively eliminated a previously reported by McMahon et al. (35) contaminating activity present in E. coli. The steady-state kinetic analysis of G0S2 LPAAT activity using varying concentrations of oleoyl-CoA revealed a Km value at 3.57 µM, a Vmax of 6.54 nmol/min/mg, and a Vmax/Km ratio of 1.83 (Fig. 4C). These results confirm that G0S2 possesses significant and intrinsic LPAAT activity.
Figure 4.
Characterization of the LPAAT activity of purified recombinant G0S2. His-MBP-G0S2 was purified from Ecoli. and used to characterize the LPAAT activity of G0S2. A) Coomassie blue staining of recombinant G0S2 was shown on the SDS-PAGE gel. B) LPAAT activity was monitored across various protein concentrations in the presence of LPA and [14C]oleoyl-CoA under standard procedures. C) The kinetic study of LPAAT activity was performed using 100 μM LPA and varying [14C]oleoyl-CoA concentrations at 1 µg of recombinant G0S2. D) Substrate specificity of recombinant G0S2 for acyl acceptors was determined at 1 µg of purified protein using 10 µM [14C]oleoyl-CoA as acyl donor and 100 µM of various acyl acceptors (LPA, LPC, LPE, LPG, LPI, LPS). E) Substrate specificity of recombinant G0S2 for acyl-CoA donors was determined at 1 µg of purified protein using 20 µM ([3H]) LPA as an acceptor and 100 µM various short, medium, and long chain fatty acyl-CoA as donors. The data are representative of 3 independent experiments. LPLAT, 1-acylglycerol-3-phosphate o-acyltransferase.
Most known LPAATs exhibit acyltransferase activity toward other lysophospholipids. However, G0S2 appears to be a more LPA-specific acyltransferase because the purified G0S2 showed less activity to esterify LPC, LPE, LPI, or LPS substrates and nearly no activity toward LPG (Fig. 4D). In parallel, we also determined the FA specificity for G0S2-mediated LPAAT reaction. Oleoyl-CoA and linoleoyl CoA were found to be the preferred acyl donors, followed by palmitoyl CoA (Fig. 4E). G0S2 was incapable of utilizing any of the short-chain fatty acyl CoAs such as butyryl CoA and octanoyl CoA (Fig. 4E). Thus, G0S2 protein is a substrate-specific acyltransferase.
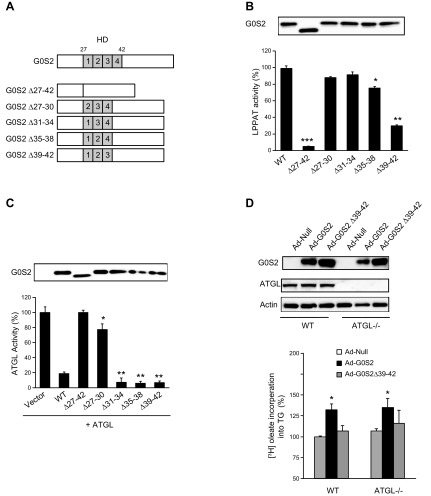
Residues 39–42 of G0S2 are required for LPAAT activity but dispensable for ATGL inhibition
We have previously shown that the hydrophobic domain (HD) of G0S2 (aa 27–42) is necessary for mediating ATGL inhibition. To determine whether separate sequence motifs are responsible for G0S2’s activities as ATGL inhibitor and LPAAT, we generated a series of internal deletion mutants, with each lacking 4 residues in the HD domain (Fig. 5A). When compared to the full-length protein, an internal deletion mutant lacking the entire HD domain (G0S2∆27-42) showed a 95% decrease in LPAAT activity (Fig. 5B) as well as being disabled in inhibiting ATGL (Fig. 5C). Interestingly, deletion of the central 8 residues (∆31–34 and ∆35–38) elicited no considerable effects on either LPAAT activity or ATGL inhibition. However, the G0S2∆39-42 mutant that lacks a Val-Leu-Gly-Leu motif lost ∼70% of the LPAAT activity (Fig. 5B) while retaining the same, if not greater, capacity to inhibit ATGL as the full-length protein (Fig. 5C). Conversely, deletion of residues 27–30 resulted in a 77% loss of ATGL inhibitory activity (Fig. 5C) without impacting the LPAAT activity (Fig. 5B). Taken together, these data demonstrate that distinct motifs within the HD domain of G0S2 are responsible for mediating ATGL inhibition and catalyzing LPAAT reaction. Whereas the former requires the N-terminal aa 27–30, the C-terminal aa 39–42 of the HD domain are critically needed for the latter.
Figure 5.
Identifying the domain for the LPAAT activity of G0S2. A) Schematic showing the structures of engineered mutants of G0S2. The location of the HD is indicated by gray bars starting from aa 27 to 42. The HD domain was divided to 4 parts, 27–30 (1), 31–34 (2), 35–38 (3), and 39–42 (4), respectively, and the corresponding mutants were constructed with a C-terminal Flag-tag. B) Flag-tagged mutants were purified by anti-FLAG immunoprecipitation following the in vitro transcription-translation reactions. The LPAAT activity of each mutant was detected under standard conditions. The data are representative of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT. C) Extracts of HeLa cells singly expressing ATGL or G0S2 mutants were mixed and TAG hydrolase activity was measured using [3H] triolein as a substrate. The data are representative of 3 independent experiments. *P < 0.05, **P < 0.01 vs. vector. D) Primary hepatocytes infected with 2 × 106 pfu/ml of Ad-Null or adenovirus-expressing G0S2 were treated with BSA-complexed oleate [0.3 mM unlabeled plus 1 µCi/ml (9,10-[3H]) oleate and 0.3% BSA] for 6 h. Then immunoblotting was used to detect G0S2 and ATGL, and the rate of incorporation of [3H] oleate into TG was measured. The data are representative of 3 independent experiments. *P < 0.05 vs. Ad-Null.
Because G0S2 was able to promote TG synthesis in an ATGL-independent fashion, we examined whether the LPAAT activity of G0S2 would be involved in this process. In this regard, we generated a recombinant adenovirus encoding G0S2∆39-42 (Ad-G0S2∆39-42) the mutant as demonstrated above to be fully competent in inhibiting ATGL but largely deficient as an LPAAT. In agreement with the results shown in Fig. 2B, adenoviral expression of the full-length G0S2 induced similar increases in the rates of FA incorporation into TGs in both WT and ATGL−/− hepatocytes (Fig. 5D). By contrast, expression of the LPAAT-deficient G0S2∆39-42 mutant conferred no significant effect on TG synthesis in either cell type (Fig. 5D). These results suggest that G0S2’s function in promoting TG synthesis is dependent on its LPAAT activity.
G0S2 is required for hepatic TG accumulation induced by high-sucrose diet
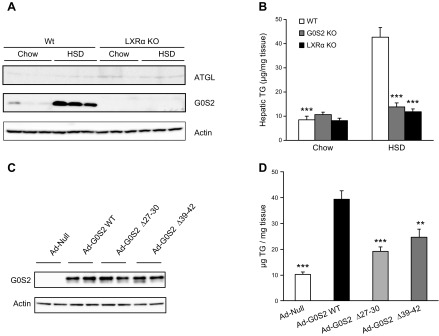
To further explore the role of G0S2 in TG synthesis in vivo, we fed mice with either normal chow diet or a high-sucrose diet (HSD) to stimulate hepatic DNL. Compared with chow diet under ad libitum feeding condition, HSD caused an ∼10-fold increase in the hepatic levels of G0S2 protein (Fig. 6A). However, this effect of HSD was completely abolished in LXRα−/− mice, confirming G0S2 as a downstream target of LXRα. On the other hand, the low-level expression of ATGL in liver under ad libitum feeding condition was unaffected by either HSD feeding or LXRα deletion. Interestingly, although HSD feeding markedly increased hepatic content of TG in the WT mice, knockout of G0S2 or LXRα both completely prevented such an effect (Fig. 6B). These data suggest that differential up-regulation of hepatic G0S2 via LXRα activation may be prerequisite for the development of liver steatosis in response to lipogenic stimulation.
Figure 6.
HSD feeding-induced liver steatosis is mediated by dual function of G0S2. A, B) Eleven- to 12-wk-old female WT, LXRα KO, or G0S2 KO mice were fed with an HSD (D12450B, 10% calories as fat and 70% calories as carbohydrate) for 5 wk. Expression of G0S2, ATGL and β-actin in liver was analyzed by immunoblotting (A), and hepatic triglyceride content was measured biochemically (n = 6–8 mice/group) (B). ***P < 0.001 vs. WT HSD. C, D) Eleven- to 12-wk-old female LXRα KO mice were fed an HSD for 4 wk. Then mice were injected once with adenoviruses encoding different G0S2 variants (2 × 107/mice) and continued to be fed an HSD for another 5 d. G0S2 and β-actin expression in liver was analyzed by immunoblotting (C), and hepatic triglyceride content was measured biochemically (n = 5) (D). **P < 0.01, ***P < 0.001 vs. Ad WT. KO, knockout.
To gain insight into the dual role of G0S2 in vivo, we administered LXRα−/− mice on HSD with adenoviruses encoding different G0S2 variants, namely full-length G0S2, G0S2Δ27-30, and G0S2Δ39-42. Although G0S2Δ27-30 is deficient in inhibiting ATGL, G0S2Δ39-42 is impaired as an LPAAT. Figure 6C shows that adenoviral infection resulted in comparable expression of all 3 proteins in the mouse liver tissues. Under the condition of continuous HSD feeding, overexpression of the full-length G0S2 in the LXRα−/− background caused a near 4-fold increase in the hepatic TG levels (Fig. 6D). Although mice infected with viruses encoding G0S2Δ27-30 or G0S2Δ39-42 both exhibited significant increases in their liver TG content, the ability of these 2 deletion mutants to drive TG accumulation was substantially compromised when compared to the full-length G0S2 (Fig. 6D). These results suggest that in response to lipogenic stimulation, hepatic TG accumulation is mediated by the dual role of G0S2 as both an ATGL inhibitor and an LPAAT.
DISCUSSION
Increased availability of free FAs is generally believed to be the driving force for the induction of hepatic TG accumulation and steatosis. Yet, the mechanisms that regulate the TG synthesis in hepatocytes from different sources of FAs are still elusive. In the present study, we have obtained several lines of evidence that are supportive of a novel function for G0S2 in the TG synthetic pathway, which is separate from and in addition to its well-recognized role as a lipolytic inhibitor. Firstly, knockdown of G0S2 significantly reduced hepatic TG levels in the ATGL-ablated mice during food withdrawal or in response to LXR agonism, suggesting that G0S2 promotes lipolysis-independent TG storage in vivo. Secondly, overexpression of G0S2 was able to promote FA incorporation into DG and TG similarly in WT and ATGL−/− mouse primary hepatocytes. Thirdly, purified recombinant G0S2 proteins produced from both an in vitro translation system and E. coli exhibited an acyl-CoA–dependent LPAAT activity. For E. coli–expressed proteins, the absence of measurable LPAAT activity with His-MBP alone demonstrates that our method combining the sequential double affinity purification with size-exclusion chromatography was effective in eliminating potential contaminants (Fig. 4B). In addition, the LPAAT activity is dependent on a distinct Val39-Leu-Gly-Leu42 motif in the HD domain of G0S2 that is separate from the one responsible for ATGL inhibition, indicating that it is intrinsic to G0S2. Fourthly, expression of the LPAAT-deficient mutant of G0S2 failed to fully promote TG synthesis in both primary hepatocytes and mouse liver, demonstrating the functional relevance of G0S2 as a LPAAT in vivo. Lastly, the fact that G0S2 is required for the HSD-induced liver steatosis indicates a critical role of G0S2 in TG synthesis downstream of lipogenic stimulation.
The finding that G0S2 can function as an LPAAT is surprising, considering that it bears no structural resemblance to any known acyltransferases, including members of the AGPAT family (36, 37). Thus far, AGPAT2 has been the best-characterized AGPAT that shows the most robust LPAAT activity in the liver (8). A direct comparison between in vitro–translated proteins reveals that G0S2 possesses an LPA-specific activity that is close to 50% of that displayed by AGPAT2 with a comparable Vmax/Km value (38). Like AGPAT2 (39, 40), G0S2 exhibited strict acyl acceptor specificity for LPA. Whereas AGPAT2 and G0S2 both show broad acyl donor specificity for acyl-CoA, G0S2 prefers unsaturated FAs such as C18:2 and C18:1 over the saturated C16:0-CoA. This is consistent with the general metabolic phenomenon that TG synthesis is more responsive to increased cellular levels of unsaturated FAs. In particular, endogenously generated unsaturated FAs play an important role in hepatic TG synthesis, as demonstrated by lack of hepatic TG accumulation in stearoyl-CoA desaturase 1-deficient mice fed a lipogenic diet (41).
We recognize that depletion of G0S2 did not affect the LPAAT activity or LPA:PA ratio as measured in the whole liver homogenates (unpublished results). This is in accordance with the previous study finding that a majority of the total measured LPAAT activity in the liver is accounted for by AGPAT2 (8). It is known that AGPAT2 deficiency causes congenital generalized lipodystrophy in humans (42) as well as severe lipodystrophy in mice (8). However, in contrast to its conceived role as the key hepatic LPAAT, AGPAT2 ablation failed to reduce TG content in the liver. Instead, the absence of AGPAT2 caused a profound hepatic steatosis along with increased levels of PA and DG even under normal chow diet conditions (8). More intriguingly, adenoviral expression of human AGPAT2 in AGPAT2−/− mice elicited no effect on the liver TG levels, despite increasing the total hepatic LPAAT activity by 10-fold (38). Thus, there appears to be a dissociation of the total measured LPAAT activity and the TG content in hepatocytes. It was speculated that the role of AGPAT2 in the liver might be to provide PA for synthesis of cellular membranes or the outer monolayer of the lipid droplets (38). In addition, increased activity of the monoacylglycerol pathway was previously suggested to be causal for the enhanced liver TG storage in AGPAT2−/− mice (8). However, a recent study showed that deletion of monoacylglycerol o-acyltransferase 1 failed to ameliorate hepatic steatosis in these animals. Thus, data in support of AGPAT2 as a key enzyme mediating hepatic TG synthesis are still lacking. In light of these observations, the present study has provided evidence pointing to G0S2 as an unconventional LPAAT that catalyzes the LPA-PA step in the liver TG synthesis. Further investigation is needed to determine whether G0S2 and AGPAT2 work coordinately in regulating different aspects of lipid synthesis. For example, due to reduced storage capacity of their lipodystrohpic adipose tissue, mice with AGPAT2 deficiency likely develop the hepatic steatosis similarly as animals on HFD (i.e., through increased uptake and incorporation of dietary FAs to liver TGs). Given that G0S2 knockout mice are completely protected against HFD-induced steatosis (27), it would be tempting to examine the effect of liver-specific deletion of G0S2 on the steatotic development in an AGPAT2−/− background.
Existing evidence indicates that glucose- and insulin-signaling pathways are coordinated in the carbohydrate feeding to synergistically activate LXRs in the liver (43–45). The LXR activation leads to elevated SREBP1c, ACC, FAS, and SCD1, which together cause increased de novo synthesis of unsaturated FAs, providing a source of acyl residues to produce TGs through the glycerol phosphate pathway. The present study adds to this model by demonstrating that by mediating G0S2 expression, LXRα also functions to promote the net deposition of newly synthesized FAs in TGs, thereby promoting hepatic steatosis during high sucrose feeding. A separate study has previously demonstrated adiponutrin, a protein encoded by patatin-like phospholipase domain containing 3, as a nutritionally regulated LPAAT (46). In particular, an increased LPAAT activity was observed in an adiponutrin mutant (e.g., I148M) that is highly linked to various forms of fatty liver disease and liver cirrhosis in humans (47). However, the functional relationship between G0S2 and adiponutrin remains to be determined because ablation of adiponutrin, unlike that of G0S2, did not significantly affect TG content in mouse liver (46, 48, 49).
In summary, we have identified an ATGL-independent function for the G0S2 protein. Specifically, our data demonstrate that G0S2 acts as both a lipid-synthesizing enzyme and an ATGL inhibitor downstream of LXRα in response to high sucrose-induced lipogenic stimulation. The TG-accumulating effects by LXRα through G0S2 may lead to new therapeutic opportunities for controlling TG accumulation in NAFLD and its associated pathologies.
ACKNOWLEDGMENTS
The authors thank the West Coast Metabolomics Center (University of California–Davis, Davis, CA, USA) for expertise and assistance in lipid profiling. This work was supported by research grants from the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK089178 and DK109096 to J.L.), as well as predoctoral funding to B.L.H. from the Mayo Foundation for Medical Education and Research. The authors declare no conflicts of interest.
Glossary
- Ad-G0S2
adenovirus-encoding G0S2
- Ad-Null
null adenovirus
- AGPAT
1-acylglycerol-3-phosphate o-acyltransferase
- ATGL
adipose triglyceride lipase
- BSA
bovine serum albumin
- CoA
coenzyme A
- DAG
1,2-dioleoyl-sn-glycerol
- DG
diacylglycerol
- DNL
de novo lipogenesis
- FA
fatty acid
- FLAG
DYKDDDDK peptide
- G0S2
G0/G1 switch gene 2
- GPAT
glycerol-3-phosphate acyltransferase
- HD
hydrophobic domain
- HFD
high-fat diet
- HSD
high-sucrose diet
- LPA
lysophosphatidic acid
- LPAAT
LPA acyltransferase
- LPC
1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine
- LPE
1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine
- LPG
1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1'-rac-glycerol)
- LPI
1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1'-myo-inositol)
- LPS
1-oleoyl-2-hydroxy-sn-glycero-3-phospho-l-serine
- LXR
liver X receptor
- NAFLD
nonalcoholic fatty liver disease
- PA
phosphatidic acid
- pfu
plaque-forming unit
- siRNA
small interfering RNA
- TG
triacylglycerol
- TLC
thin-layer chromatography
- WT
wild type
AUTHOR CONTRIBUTIONS
X. Zhang, X. Xie, and J. Liu conceptualized the study; X. Zhang, X. Xie, H. Gu, and J. Liu assessed the methodology of the study; X. Zhang, X. Xie, B. L. Heckmann, A. M. Saarinen and H. Gu performed the investigations; H. Gu, R. Zechner, and J. Liu provided resources; X. Zhang, X. Xie, R. Zechner, and J. Liu wrote and edited the manuscript; J. Liu supervised the study; and J. Liu acquired the funding.
REFERENCES
- 1.Malhi H., Gores G. J. (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 28, 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petta S., Gastaldelli A., Rebelos E., Bugianesi E., Messa P., Miele L., Svegliati-Baroni G., Valenti L., Bonino F. (2016) Pathophysiology of non alcoholic fatty liver disease. Int. J. Mol. Sci. 17, e2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashek D. G., Khan S. A., Sathyanarayan A., Ploeger J. M., Franklin M. P. (2015) Hepatic lipid droplet biology: getting to the root of fatty liver. Hepatology 62, 964–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J. C., Horton J. D., Hobbs H. H. (2011) Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou-Bonafonte J. M., Arnal C., Osada J. (2011) New genes involved in hepatic steatosis. Curr. Opin. Lipidol. 22, 159–164 [DOI] [PubMed] [Google Scholar]
- 6.Barrows B. R., Timlin M. T., Parks E. J. (2005) Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes 54, 2668–2673 [DOI] [PubMed] [Google Scholar]
- 7.Green C. J., Pramfalk C., Morten K. J., Hodson L. (2015) From whole body to cellular models of hepatic triglyceride metabolism: man has got to know his limitations. Am. J. Physiol. Endocrinol. Metab. 308, E1–E20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortés V. A., Curtis D. E., Sukumaran S., Shao X., Parameswara V., Rashid S., Smith A. R., Ren J., Esser V., Hammer R. E., Agarwal A. K., Horton J. D., Garg A. (2009) Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 9, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweitzer G. G., Chen Z., Gan C., McCommis K. S., Soufi N., Chrast R., Mitra M. S., Yang K., Gross R. W., Finck B. N. (2015) Liver-specific loss of lipin-1-mediated phosphatidic acid phosphatase activity does not mitigate intrahepatic TG accumulation in mice. J. Lipid Res. 56, 848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girousse A., Langin D. (2012) Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int. J. Obes. 36, 581–594 [DOI] [PubMed] [Google Scholar]
- 11.Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., Madeo F. (2012) FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 13.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 15.Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50, 3–21 [DOI] [PubMed] [Google Scholar]
- 16.Wu J. W., Wang S. P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K. G., Yang G., Mitchell G. A. (2011) Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54, 122–132 [DOI] [PubMed] [Google Scholar]
- 17.Ong K. T., Mashek M. T., Bu S. Y., Greenberg A. S., Mashek D. G. (2011) Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53, 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F., Ma Y., Kadegowda A. K., Betters J. L., Xie P., Liu G., Liu X., Miao H., Ou J., Su X., Zheng Z., Xue B., Shi H., Yu L. (2013) Deficiency of liver comparative gene identification-58 causes steatohepatitis and fibrosis in mice. J. Lipid Res. 54, 2109–2120; erratum: 2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid B. N., Ables G. P., Otlivanchik O. A., Schoiswohl G., Zechner R., Blaner W. S., Goldberg I. J., Schwabe R. F., Chua S. C., Jr., Huang L. S. (2008) Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 283, 13087–13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turpin S. M., Hoy A. J., Brown R. D., Rudaz C. G., Honeyman J., Matzaris M., Watt M. J. (2011) Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 54, 146–156 [DOI] [PubMed] [Google Scholar]
- 21.Kato M., Higuchi N., Enjoji M. (2008) Reduced hepatic expression of adipose tissue triglyceride lipase and CGI-58 may contribute to the development of non-alcoholic fatty liver disease in patients with insulin resistance. Scand. J. Gastroenterol. 43, 1018–1019 [DOI] [PubMed] [Google Scholar]
- 22.Schreiber R., Hofer P., Taschler U., Voshol P. J., Rechberger G. N., Kotzbeck P., Jaeger D., Preiss-Landl K., Lord C. C., Brown J. M., Haemmerle G., Zimmermann R., Vidal-Puig A., Zechner R. (2015) Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc. Natl. Acad. Sci. USA 112, 13850–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Lu X., Lombès M., Rha G. B., Chi Y. I., Guerin T. M., Smart E. J., Liu J. (2010) The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 11, 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweiger M., Paar M., Eder C., Brandis J., Moser E., Gorkiewicz G., Grond S., Radner F. P., Cerk I., Cornaciu I., Oberer M., Kersten S., Zechner R., Zimmermann R., Lass A. (2012) G0/G1 switch gene-2 regulates human adipocyte lipolysis by affecting activity and localization of adipose triglyceride lipase. J. Lipid Res. 53, 2307–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornaciu I., Boeszoermenyi A., Lindermuth H., Nagy H. M., Cerk I. K., Ebner C., Salzburger B., Gruber A., Schweiger M., Zechner R., Lass A., Zimmermann R., Oberer M. (2011) The minimal domain of adipose triglyceride lipase (ATGL) ranges until leucine 254 and can be activated and inhibited by CGI-58 and G0S2, respectively. PLoS One 6, e26349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerk I. K., Salzburger B., Boeszoermenyi A., Heier C., Pillip C., Romauch M., Schweiger M., Cornaciu I., Lass A., Zimmermann R., Zechner R., Oberer M. (2014) A peptide derived from G0/G1 switch gene 2 acts as non-competitive inhibitor of adipose triglyceride lipase. J. Biol. Chem. 289, 32559–32570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Xie X., Heckmann B. L., Saarinen A. M., Czyzyk T. A., Liu J. (2014) Targeted disruption of G0/G1 switch gene 2 enhances adipose lipolysis, alters hepatic energy balance, and alleviates high-fat diet-induced liver steatosis. Diabetes 63, 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Assaad W., El-Kouhen K., Mohammad A. H., Yang J., Morita M., Gamache I., Mamer O., Avizonis D., Hermance N., Kersten S., Tremblay M. L., Kelliher M. A., Teodoro J. G. (2015) Deletion of the gene encoding G0/G 1 switch protein 2 (G0s2) alleviates high-fat-diet-induced weight gain and insulin resistance, and promotes browning of white adipose tissue in mice. Diabetologia 58, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma T., Lopez-Aguiar A. G., Li A., Lu Y., Sekula D., Nattie E. E., Freemantle S., Dmitrovsky E. (2014) Mice lacking G0S2 are lean and cold-tolerant. Cancer Biol. Ther. 15, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heckmann B. L., Zhang X., Saarinen A. M., Schoiswohl G., Kershaw E. E., Zechner R., Liu J. (2017) Liver X receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 expression. JCI Insight 2, e88735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 32.Liu J., Deyoung S. M., Zhang M., Dold L. H., Saltiel A. R. (2005) The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 280, 16125–16134 [DOI] [PubMed] [Google Scholar]
- 33.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. (2006) Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 3, 309–319 [DOI] [PubMed] [Google Scholar]
- 34.Chen Z., Fitzgerald R. L., Averna M. R., Schonfeld G. (2000) A targeted apolipoprotein B-38.9-producing mutation causes fatty livers in mice due to the reduced ability of apolipoprotein B-38.9 to transport triglycerides. J. Biol. Chem. 275, 32807–32815 [DOI] [PubMed] [Google Scholar]
- 35.McMahon D., Dinh A., Kurz D., Shah D., Han G. S., Carman G. M., Brasaemle D. L. (2014) Comparative gene identification 58/α/β hydrolase domain 5 lacks lysophosphatidic acid acyltransferase activity. J. Lipid Res. 55, 1750–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi K., Reue K. (2009) Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296, E1195–E1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita A., Hayashi Y., Matsumoto N., Nemoto-Sasaki Y., Oka S., Tanikawa T., Sugiura T. (2014) Glycerophosphate/Acylglycerophosphate acyltransferases. Biology (Basel) 3, 801–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal A. K., Sukumaran S., Cortés V. A., Tunison K., Mizrachi D., Sankella S., Gerard R. D., Horton J. D., Garg A. (2011) Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J. Biol. Chem. 286, 37676–37691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollenback D., Bonham L., Law L., Rossnagle E., Romero L., Carew H., Tompkins C. K., Leung D. W., Singer J. W., White T. (2006) Substrate specificity of lysophosphatidic acid acyltransferase beta -- evidence from membrane and whole cell assays. J. Lipid Res. 47, 593–604 [DOI] [PubMed] [Google Scholar]
- 40.Eberhardt C., Gray P. W., Tjoelker L. W. (1997) Human lysophosphatidic acid acyltransferase. cDNA cloning, expression, and localization to chromosome 9q34.3. J. Biol. Chem. 272, 20299–20305 [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki M., Kim Y. C., Ntambi J. M. (2001) A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 42, 1018–1024 [PubMed] [Google Scholar]
- 42.Agarwal A. K., Arioglu E., De Almeida S., Akkoc N., Taylor S. I., Bowcock A. M., Barnes R. I., Garg A. (2002) AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 31, 21–23 [DOI] [PubMed] [Google Scholar]
- 43.Tobin K. A., Ulven S. M., Schuster G. U., Steineger H. H., Andresen S. M., Gustafsson J. A., Nebb H. I. (2002) Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J. Biol. Chem. 277, 10691–10697 [DOI] [PubMed] [Google Scholar]
- 44.Chen G., Liang G., Ou J., Goldstein J. L., Brown M. S. (2004) Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA 101, 11245–11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthonisen E. H., Berven L., Holm S., Nygård M., Nebb H. I., Grønning-Wang L. M. (2010) Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 285, 1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumari M., Schoiswohl G., Chitraju C., Paar M., Cornaciu I., Rangrez A. Y., Wongsiriroj N., Nagy H. M., Ivanova P. T., Scott S. A., Knittelfelder O., Rechberger G. N., Birner-Gruenberger R., Eder S., Brown H. A., Haemmerle G., Oberer M., Lass A., Kershaw E. E., Zimmermann R., Zechner R. (2012) Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 15, 691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L. A., Boerwinkle E., Cohen J. C., Hobbs H. H. (2008) Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basantani M. K., Sitnick M. T., Cai L., Brenner D. S., Gardner N. P., Li J. Z., Schoiswohl G., Yang K., Kumari M., Gross R. W., Zechner R., Kershaw E. E. (2011) Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 52, 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W., Chang B., Li L., Chan L. (2010) Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 52, 1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]