Abstract
Despite surgical and chemotherapeutic advances over the past few decades, the prognosis for ovarian cancer remains very poor. Although cyclin-dependent kinase (CDK) 9 has an established pathogenic role in various cancers, its function in ovarian cancer remains poorly defined. The purpose of this study was to evaluate the expression of CDK9 and its therapeutic potential in ovarian cancer. CDK9 expression was determined by immunohistochemistry in a unique ovarian cancer tissue microarray constructed with paired primary, metastatic, and recurrent tumor tissues from 26 ovarian cancer patients. CDK9 was highly expressed in human ovarian cancer cell lines and was also elevated in metastatic and recurrent ovarian tumor tissue compared with patient-matched primary ovarian tumor tissue. In addition, increased CDK9 significantly correlated with poor patient prognosis. Inhibition of CDK9 by small interfering RNA or CDK9 inhibitor functionally suppressed RNA transcription elongation, induced apoptosis, and reduced proliferation of ovarian cancer cells. Inhibition of CDK9 also suppressed ovarian cancer cell spheroid growth, clonogenicity formation, and migration activity. Our results reveal CDK9 as a novel prognostic biomarker and a promising therapeutic target for preventing metastasis and recurrence while also improving the overall clinical outcome for ovarian cancer patients.—Wang, J., Dean, D. C., Hornicek, F. J., Shi, H., Duan, Z. Cyclin-dependent kinase 9 (CDK9) is a novel prognostic marker and therapeutic target in ovarian cancer.
Keywords: LDC000067, transcription, apoptosis
Ovarian cancer is currently the most common cause of death among gynecologic malignancies and is the fifth-leading cause of cancer-related death for women in the United States. The American Cancer Society estimated that ∼22,240 new ovarian cancer cases and 14,070 deaths would occur in the United States in 2018 alone (1). The current standard of ovarian cancer treatment entails primary maximal debulking surgery followed by adjuvant combination platinum- and paclitaxel-based chemotherapy (2). This protocol, however, results in more than half of advanced ovarian cancer patients relapsing within the first 5 yr, acquired resistance to standard chemotherapy, and cross-resistance to other functionally and structurally unrelated chemotherapeutic drugs (3). Despite diagnostic and therapeutic advances, the overall 5-yr survival rate for ovarian cancer patients has not significantly improved over the past 3 decades (1, 4). Therefore, there is an urgent need to develop novel therapeutic strategies in order to improve the treatment of ovarian cancer.
Cyclin-dependent kinases (CDKs) are a family of serine/threonine protein kinases whose activity is based on heterodimeric complexes composed of a catalytic kinase subunit and a regulatory cyclin subunit (5, 6). CDKs are classified into 2 groups according to their role in cell cycle progression or transcriptional regulation (7–9). CDK1, -2, -4, and -6 belong to the cell cycle group, whereas CDK8, -9, -12, -13, and -19 are involved in gene transcription (7, 10). CDK7 and CDK20 are linked to both cell cycle and transcription processes (8). Recent studies have demonstrated that CDKs are frequently overexpressed in many cancer types and ultimately result in uncontrolled cell proliferation and drug resistance (11, 12). Therefore, CDKs represent particularly attractive drug targets for human cancer treatment. The dual CDK4/6 inhibitor palbociclib (Ibrance) was approved by the Food and Drug Administration as a first-line treatment of estrogen receptor–positive and human epidermal growth factor receptor 2–negative advanced breast cancer (13). There are currently more than 100 phase I and II clinical trials investigating the effects of CDK inhibitors on various cancer types (http://clinicaltrials.gov/).
Among the several CDKs that have been recognized as crucial regulators of transcription, CDK9 appears to be one of the most important (14). CDK9 is a serine/threonine kinase that stabilizes RNA transcription elongation. Together, CDK9 and cyclin T constitute the positive transcription elongation factor b complex, which promotes transcription elongation via phosphorylation of RNA polymerase II (RNAPII) (15). The carboxyl-terminal domain (CTD) is the largest subunit of RNAPII and consists of 52 tandem heptapeptide repeats with the consensus sequence Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7 (16). CTD phosphorylation occurs at many stages of transcription, including preinitiation, initiation, elongation, and termination (17). More specifically, the CTD is phosphorylated by CDK7 on Ser5 (s5) during transcription initiation and then on Ser2 (s2) by CDK9 to promote transcriptional elongation (18). Recently, CDK9 has been shown to play crucial roles in many types of human cancer, including leukemia, cervical cancer, prostate cancer, glioblastoma, breast cancer, melanoma, and lung cancer (19–25). However, the relationship between CDK9 expression and clinical prognosis and the therapeutic potential of targeting CDK9 in ovarian cancer remains unclear. Here, we report the expression and role of CDK9 in ovarian cancer.
MATERIALS AND METHODS
Human ovarian cancer tissues
Ovarian cancer tissue samples for this study were obtained from the Massachusetts General Hospital Tissue Bank (Boston, MA, USA). Acquisition of tissue samples and clinical information was approved by the Institutional Review Board at Massachusetts General Hospital (Protocol 2007P-002464). All material was collected with written informed consent from patients and in accordance with common rules by the U.S. Department of Health and Human Services. The research was carried out according to the Declaration of Helsinki.
Ovarian cancer tissue microarray
The archived, formalin-fixed, paraffin-embedded tissue microarray (TMA) utilized in the present study was generated from tissue samples obtained from 26 ovarian cancer patients as described in Liu et al. (26) and Wang et al. (27). This TMA was unique in that each of these 26 patients’ tumor tissue blocks were composed of the following: 1) a primary tumor, 2) a synchronous metastasis obtained at the time of the primary surgery, and 3) a metachronous recurrence from the same patient collected at the time of tumor recurrence after combination chemotherapy with paclitaxel and platinum. Clinical information was also collected, including disease-free survival (DFS), defined as the interval between the date of diagnosis and the date of recurrence; overall survival (OS), defined as the interval between the date of surgery to last follow-up or death; the presence or absence of ascites at the time of surgery; and the patient’s status at last follow-up (Table 1).
TABLE 1.
Clinical data for ovarian cancer TMA
| Patient | Histologic subtype | Stage | Grade | DFS (mo) | OS (mo) | Patient status | Ascites |
|---|---|---|---|---|---|---|---|
| 1 | Serous | IV | 3 | 17.5 | 20.7 | Deceased | No |
| 2 | Serous | III | 2 | 13.7 | 32.3 | Deceased | No |
| 3 | Squamous | III | 3 | 9.5 | - | - | Yes |
| 4 | Transitional cell | III | 3 | 26.6 | 162.3 | Alive | Yes |
| 5 | Serous | III | 3 | 8.3 | 12 | Deceased | No |
| 6 | High grade serous | III | 3 | 53.3 | 63.5 | Deceased | No |
| 7 | Serous and endometrioid | III | 2 | 39.8 | 43.1 | Deceased | No |
| 8 | Serous | III | 2 | 14.3 | - | - | Yes |
| 9 | Serous | IV | 3 | 16.5 | 18.8 | Deceased | Yes |
| 10 | Serous | IV | 3 | 12.7 | 43.7 | Deceased | Yes |
| 11 | Serous | III | 3 | 10.5 | 14.5 | Deceased | No |
| 12 | Serous | III | 3 | 14 | 31.5 | Deceased | Yes |
| 13 | Serous | III | 3 | 34 | - | - | Yes |
| 14 | Serous | IV | 3 | 12.2 | 31.6 | Deceased | Yes |
| 15 | Serous | III | 3 | 34.8 | 64.4 | Deceased | Yes |
| 16 | Serous | III | 3 | 24.3 | 146.7 | Deceased | Yes |
| 17 | Serous | IV | 3 | 12.8 | 15.8 | Deceased | Yes |
| 18 | Serous | IV | 3 | 9.5 | 12.5 | Deceased | Yes |
| 19 | Serous | III | 3 | 16.5 | 56.7 | Deceased | No |
| 20 | Serous | IV | 3 | 12 | 13 | Deceased | Yes |
| 21 | Serous | IV | 2 | 38.1 | 50 | Deceased | Yes |
| 22 | Endometroid | III | 3 | 45.1 | 79.6 | Deceased | Yes |
| 23 | Serous | IV | 3 | 11.4 | - | - | Yes |
| 24 | Serous | IV | 1 | 21.9 | 100.7 | Deceased | Yes |
| 25 | Serous | III | 3 | 23.1 | 53.8 | Deceased | No |
| 26 | Endometrioid and clear cell | IV | 3 | 5.3 | 16.9 | Deceased | No |
DFS, interval between the date of diagnosis and the date of recurrence; OS, interval between the date of surgery to last follow-up or death.
Immunohistochemistry
The expression level of CDK9 was determined based on the Immunohistochemistry Protocol (Paraffin; Cell Signaling Technology, Danvers, MA, USA). Briefly, TMA tissue section slides (5 μm thick) were baked at 60°C for 1 h, deparaffinized in xylene (3 times for 5 min each), and then rehydrated through a graded ethanol series (100, 95, 75, and 50%). Following antigen retrieval with Target Retrieval Solution (Agilent Technologies, Santa Clara, CA, USA), the endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide for 10 min. Nonspecific protein content was blocked with blocking solution (Cell Signaling Technology) for 1 h at room temperature before a human CDK9 primary antibody (2316; 1:50 dilution in 1% bovine serum albumin; Cell Signaling Technology) was applied for overnight incubation at 4°C in a humidified chamber. After rinsing with Tris-buffered saline with Tween 20 3 times, the bound antibody on the array was detected by using SignalStain Boost Detection Reagent (Cell Signaling Technology) and SignalStain DAB (Cell Signaling Technology). Finally, the slides were counterstained with Hematoxylin QS (Vector Laboratories, Burlingame, CA, USA) and mounted with VectaMount AQ (Vector Laboratories) for long-term preservation. Evaluations of the percentage of cells with positive nuclear staining were performed separately by 2 independent investigators who were blinded to the clinical data and the other viewer’s score. The nuclear staining patterns of CDK9 were graded into 5 groups: 1+, <10% of cells stained positives; 2+, 10–25% positive cells; 3+, 26–50% positive cells; 4+, 51–75% positive cells; and 5+, >75% positive cells. CDK9-stained images were captured with an Eclipse Ti-U fluorescence microscope (Nikon, Tokyo, Japan) and a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Cell lines and reagents
The ovarian cancer cell lines OVCAR8, SKOV3, and IGROV-1 were used in this study. They were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (GE Healthcare, Waukesha, WI, USA) and supplemented with 10% fetal bovine serum (MilliporeSigma, Burlington, MA, USA) with 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a 5% CO2–humidified atmosphere. The highly selective CDK9 inhibitor LDC000067 (abbreviated to LDC067) was purchased from Selleck Chemicals (S7461; Houston, TX, USA). Paclitaxel was obtained as described in Wang et al. (27). Human nonspecific small interfering RNA (siRNA) and CDK9-targeting siRNA (5′-GCUGCUAAUGUGCUUAUCA-3′) were purchased from MilliporeSigma. Lipofectamine RNAiMax was purchased from Thermo Fisher Scientific. The monoclonal rabbit anti-human CDK9 antibody was purchased from Cell Signaling Technology. The RNAPII-associated antibodies, including RNAPII and phosphorylated RNAPII (s2 and s5), were purchased from Abcam (Cambridge, MA, USA). Apoptosis-related antibodies were obtained from Cell Signaling Technology.
Work flow of lipofectamine-mediated transfection of CDK9 siRNA
Knockdown of CDK9 in ovarian cells was performed by transfection of synthetic CDK9 siRNA. In brief, ovarian cancer cells were seeded into 96-well plates at a density of 2 × 103 cells per well or into 12-well plates at a density of 6 × 104 cells per well and transfected with 10, 30, and 60 nM of synthesized CDK9 siRNA using the Lipofectamine RNAiMax reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. Nonspecific siRNA (60 nM) was used as a negative control.
Methyl thiazolyl tetrazolium assay
Five days after CDK9 siRNA transfection or CDK9 inhibitor LDC067 treatment, the cell viability of ovarian cancer cells was assessed by the methyl thiazolyl tetrazolium [3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide] assay. Briefly, at the end of cell treatment, 20 μl of methyl thiazolyl tetrazolium (5 mg/ml; MilliporeSigma) was added to each well, and the 96-well plates were incubated for an additional 4 h at 37°C. After the removal of supernatants, the resulting intracellular formazan crystals were dissolved in 200 µl per well acid-isopropanol. The absorbance of the samples was assessed using a SpectraMax 340PC Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 490 nm. The cell viability was presented as percent cell viability normalized to the untreated cells. Each experiment was conducted in triplicate to assess the consistency of the results.
Western blot analysis
Protein lysates of ovarian cancer cell lines were extracted using 1 × RIPA lysis buffer (MilliporeSigma), supplemented with complete protease inhibitor cocktail tablets (Roche, Basel, Switzerland). Protein concentrations were calculated by the Protein Assay Reagents (Bio-Rad, Hercules, CA, USA) and a SpectraMax 340PC Microplate Reader from Molecular Devices. Equal amounts of proteins were separated by NuPage 4–12% Bis-Tris Gel (Thermo Fisher Scientific) and subsequently transferred to a nitrocellulose membrane (Bio-Rad), which was then incubated with the following specific primary antibodies at 4°C overnight: CDK9 (2316; 1:1000 dilution; Cell Signaling Technology), RNAPII (ab817; 1:1000 dilution; Abcam), s2 RNAPII (ab5095; 1:1000 dilution; Abcam), s5 RNAPII (ab5131; 1:1000 dilution; Abcam), Mcl-1 (39224; 1:1000 dilution; Cell Signaling Technology), PARP (9532; 1:1000 dilution; Cell Signaling Technology), Bax (2772; 1:1000 dilution; Cell Signaling Technology), and α-tubulin (3873; 1:1000 dilution; Cell Signaling Technology). After being washed 3 times for 5 min with Tris-buffered saline with Tween 20, the membranes were further incubated with goat anti-rabbit IRDye 800CW or goat anti-mouse IRDye 680LT secondary antibody (Li-Cor Biosciences, Lincoln, NE, USA), and scanned by Odyssey CLx equipment (Li-Cor Biosciences) to detect the bands. Finally, protein bands were quantified by densitometry using the Odyssey v.3.0 software (Li-Cor Biosciences).
Three-dimensional cell culture assay
In order to mimic the in vivo environment, a 3-dimensional (3D) cell culture assay was applied to assess the effect of CDK9 on cell growth. Hydrogel of ovarian cancer cell lines was established in 24-well VitroGel 3D cell culture plates (TheWell Bioscience, Newark, NJ, USA) with a density of 2 × 104 cells per well, according to the manufacturer’s protocol. Immediately following this, different cell culture medium (with or without 5 µM of LDC067) was added to cover the hydrogel. The well plate was then placed in an incubator, and its cover media was changed every 48 h to provide sufficient nutrients for the cells. The spheroid formations were photographed with a Nikon microscope (Diagnostic Instruments) every 5 d. At the end of the 15-d period, cell culture medium containing 0.25 μM Calcein AM (Thermo Fisher Scientific) was added to cover the hydrogel. After 15 min of incubation, the spheroids were imaged on the Eclipse Ti-U fluorescence microscope (Nikon) equipped with a Spot RT digital camera (Diagnostic Instruments).
Clonogenic assay
Clonogenic assay was conducted to evaluate the effect of CDK9 on cell viability and proliferation. The ovarian cancer cell lines were seeded into 12-well plates at 100 cells per well and exposed to different concentrations of the CDK9 inhibitor LDC067 (0, 5, 10 µM). After a 14-d incubation period, the cells were fixed with methanol for 10 min, washed 3 times with PBS, and then stained with 10% Giemsa stain (MilliporeSigma) for 20 min. Finally, the colonies were washed with flowing water and allowed to dry. Pictures of the stained colonies were captured using a digital camera (Olympus, Tokyo, Japan).
Wound-healing assay
Cell migration activity was determined with a wound-healing assay. In brief, ovarian cancer cells were seeded into 6-well plates at a density of 4 × 105 cells per well and incubated overnight. The adherent cell layer was then scraped into 2 parallel lines with a sterile 30-μl tip. Immediately afterward, 5 µM of the CDK9 inhibitor LDC067 was added into the cell medium for an additional 72 h of starved incubation with a low-serum medium containing 3% fetal bovine serum. After 0, 24, 48, and 72 h of applied LDC067 treatment, the wounds were photographed with a Nikon microscope (Diagnostic Instruments) equipped with Zen Imaging software (Carl Zeiss, Oberkochen, Germany). The wound width was evaluated by measuring the distance between the 2 edges of the scratch at 5 sites in each image. Cell migration distance was determined with the following formula: wound width at the 0 h time point minus wound width at the observed time point, divided by 2.
Statistical analysis
Statistical analysis was performed using Prism v.7.0 software (GraphPad Software, La Jolla, CA, USA). The correlation between CDK9 expression level and disease survival was analyzed using Kaplan-Meier survival curves with log-rank tests for significance. A paired Student’s t test was used to compare the CDK9 scores among primary tumors, recurrent tumors, and tumors with metastasis. The χ2 test was used to evaluate the relationship between CDK9 expression and clinical pathologic parameters of ovarian cancer patients. Treatment effects of CDK9 siRNA and CDK9 inhibitor in ovarian cancer cells were analyzed by 1-way ANOVA tests. In all cases, results were presented as means ± sem, and values of P < 0.05 were considered statistically significant. All blots represent triple-independent experiments.
RESULTS
CDK9 is highly expressed in metastatic and recurrent ovarian cancer tissues and correlates with poor prognosis
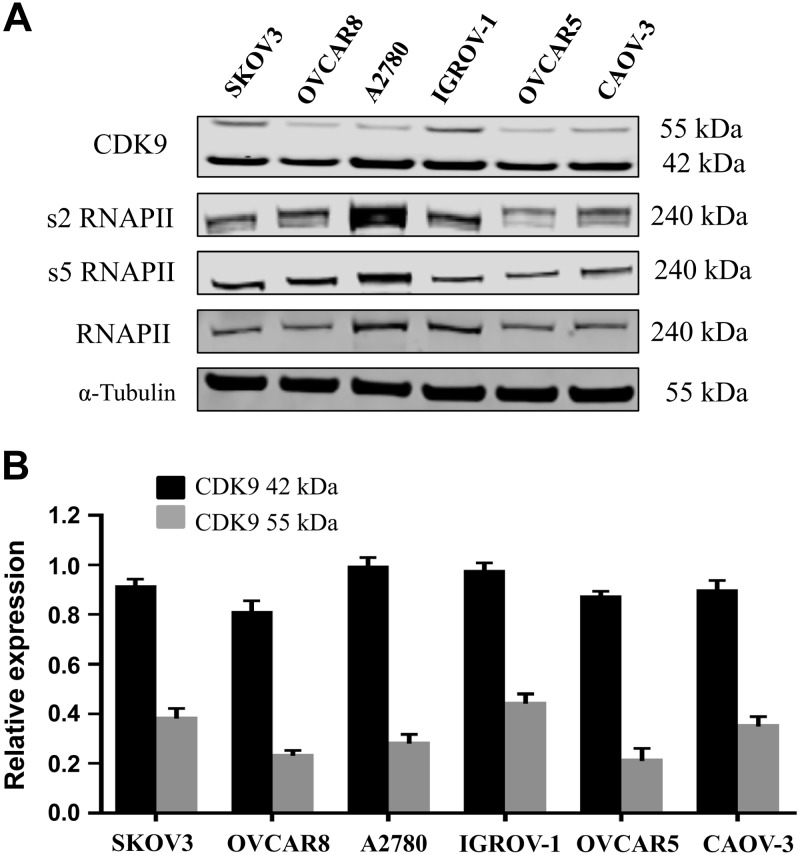
To determine the relationship between CDK9 expression and clinical prognosis, we compared the expression levels of CDK9 in matched primary, metastatic, and recurrent specimens from 26 ovarian cancer patients. CDK9 was primarily localized in the nucleus. The CDK9 expression levels were found to be considerably lower in primary ovarian cancer tissues (2.58 ± 0.18) compared with patient-paired metastatic (3.23 ± 0.21) and recurrent (3.19 ± 0.17) tissues (Fig. 1A). These values were significantly different from each other (metastatic vs. primary P = 0.003; recurrent vs. primary P = 0.001), whereas there was no significant statistical difference in CDK9 expression between metastatic and recurrent ovarian cancer specimens (metastatic vs. recurrent P = 0.98). Compared with primary ovarian cancer tissues, 54% of patient-paired metastatic tissues had increased CDK9 expression, 35% had unchanged expression, and only 11% had decreased expression (Fig. 1B). Similarly, compared with primary ovarian cancer tissues, 50% of patient-paired recurrent tissues had increased CDK9 expression, 46% were unchanged, and only 4% had decreased expression (Fig. 1C). Patient follow-up data revealed that DFS ranged from 5.3 to 53.3 mo, with the shortest OS being 12 mo, and the most extended follow-up of a living patient being 162.3 mo (Table 1). Kaplan-Meier survival analysis was used to explore the relationship between CDK9 expression and ovarian cancer progression and prognosis (Fig. 1D, E). Patients were subdivided into the following 2 groups based on their CDK9 expression score in the primary ovarian cancer specimens: weak group (CDK9 staining score ≤2; n = 13) and strong group (CDK9 staining score ≥3; n = 13) (Fig. 1F). The patients with low CDK9 expression had significantly better prognostic measures in both DFS (Fig. 1D) and OS (Fig. 1E), with values of P < 0.001 compared with those with high expression of CDK9. In addition, Cox proportional hazards regression analysis revealed that elevated CDK9 expression in ovarian cancer was a significant risk factor for decreased DFS (hazard ratio, 4.1; 95% confidence interval, 1.56–10.73) and OS (hazard ratio, 5.39; 95% confidence interval, 1.6–18.19). However, no statistical differences were observed between CDK9 expression and the stage, grade, ascites, or histologic subtype of ovarian cancer patients (Table 2). Finally, we examined the expression of CDK9 in various human ovarian cancer cell lines through Western blot and confirmed that CDK9 is expressed in all of these cell lines (Fig. 2A). There are 2 isoforms of the CDK9 protein: the 42 kDa isoform and the 55 kDa isoform. The smaller 42 kDa isoform was the first identified isoform. The larger 55 kDa isoform has a characteristic 117 residue terminal expansion. Both isoforms are expressed in human cancer cell lines and tissues at variable quantities (28, 29). These 2 isoforms were expressed in ovarian cancer cell lines (Fig. 2).
Figure 1.
Higher expression of CDK9 was present in metastatic and recurrent ovarian cancer tissues compared with patient-matched primary tumors and correlated with poor patient prognosis. A) Distribution of CDK9 immunohistochemical staining scores among primary, metastatic, and recurrent ovarian cancer tissues. B) Expression of CDK9 between primary and paired metastatic tissues. Red signifies increased, black signifies equal, and green signifies decreased. C) Expression of CDK9 between primary and paired recurrent tissues. D, E) Correlation between expression of CDK9 in the primary ovarian cancer tissues (Weak: CDK9 staining ≤2+; Strong: CDK9 staining ≥3+) and DFS (D) or OS (E) in ovarian cancer patients by Kaplan-Meier survival curve analysis. F) Representative images of hematoxylin and eosin (HE) and nuclear staining intensity for CDK9 in matched primary, metastatic, and recurrent ovarian cancer tissues. CI, confidence interval; HR, hazard ratio.
TABLE 2.
The relationship between CDK9 expression and clinicopathological features of ovarian cancer
| Clinicopathological feature | Cases, n (%) | CDK9 expression |
P | |
|---|---|---|---|---|
| Weak, n (%) | Strong, n (%) | |||
| All patients | 26 (100) | 13 (50) | 13 (50) | |
| Stage | ||||
| IV | 11 (42.3) | 4 (15.4) | 7 (26.9) | 0.234 |
| III | 15 (57.7) | 9 (34.6) | 6 (23.1) | |
| Grade | ||||
| 3 | 21 (80.8) | 10 (38.5) | 11 (42.3) | 0.619 |
| ≤2 | 5 (19.2) | 3 (11.5) | 2 (7.7) | |
| Ascites | ||||
| Yes | 17 (65.4) | 9 (34.6) | 8 (30.8) | 0.68 |
| No | 9 (34.6) | 4 (15.4) | 5 (19.2) | |
| Histologic subtype | ||||
| Serous | 20 | 9 (34.6) | 11 (42.3) | 0.575 |
| Squamous | 1 | 1 (3.8) | ||
| Transitional cell | 1 | 1 (3.8) | ||
| High grade serous | 1 | 1 (3.8) | ||
| Serous and endometrioid | 1 | 1 (3.8) | ||
| Endometroid | 1 | 1 (3.8) | ||
| Endometrioid and clear cell | 1 | 1 (3.8) | ||
Figure 2.
CDK9 was expressed in all human ovarian cancer cell lines. A) Expression levels of CDK9 and related signaling pathway proteins involved in transcription in different human ovarian cancer cell lines by Western blot. There are 2 isoforms of the CDK9 protein: the 42 kDa CDK9 isoform and the 55 kDa isoform. The smaller 42 kDa isoform was the first identified isoform. The larger 55 kDa isoform has a characteristic 117 residue terminal expansion. B) Relative expression of both CDK9 isoforms and α-tubulin in the ovarian cancer cell lines.
CDK9 knockdown by siRNA transfection inhibits ovarian cancer cell proliferation
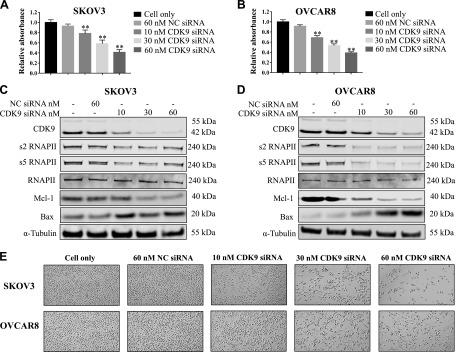
To discern the role of CDK9 expression in ovarian cancer cell proliferation and growth, we transfected both cell lines with CDK9 siRNA to knock down CDK9 expression. Over the 5-d observation period, there was a dose-dependent inhibition of cell viability in both cell lines with increasing concentrations of CDK9 siRNA. This effect was not detected in cells transfected with nonspecific siRNA (Fig. 3A, B). Western blot showed that nonspecific siRNA had no effect on CDK9 expression, whereas CDK9 siRNA significantly reduced CDK9 expression over a 2-d observation period. Knockdown of CDK9 resulted in decreased levels of phosphorylated RNAPII (s2 and s5) and antiapoptotic protein Mcl-1 as well as an increased level of the proapoptotic protein Bax (Fig. 3C, D). CDK9 siRNA transfection resulted in clear morphologic changes and diminished cell proliferation over a 2-d observation period (Fig. 3E). In addition, the effects of CDK9 siRNA on the ovarian endometrioid adenocarcinoma cell line IGROV-1 were also assessed (Supplemental Fig. S1A, B). These data illustrate the critical role of CDK9 in ovarian cancer cell growth and proliferation.
Figure 3.
CDK9 knockdown by siRNA transfection suppressed ovarian cancer cell proliferation. A, B) Methyl thiazolyl tetrazolium assay revealed dose-dependent inhibition of cell proliferation after CDK9 siRNA treatment. **P < 0.01. C, D) Expression levels of CDK9 and related signaling pathway proteins involved in transcription and apoptosis after transfection of CDK9 siRNA and nonspecific siRNA in SKOV3 and OVCAR8 cell lines by Western blot. E) Representative images of ovarian cancer cell morphologic changes after transfection of CDK9 siRNA. Original magnification value, ×200.
CDK9 inhibitor suppresses the phosphorylation of RNAPII and induces apoptosis in ovarian cancer cells
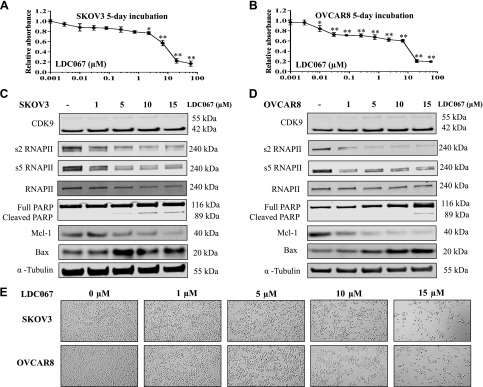
After validating the role of CDK9 on cell viability, we assessed the effects of the CDK9 inhibitor on ovarian cancer cells. Similar to CDK9 siRNA transfection, the CDK9 selective inhibitor LDC067 led to a dose-dependent reduction in both SKOV3 and OVCAR8 cell viability over the allotted 5 d of observation (Fig. 4A, B). To further characterize the effect of CDK9 inhibition on transcription regulation in ovarian cancer cells, we examined the expression of RNAPII and apoptosis-associated proteins. Incubation of SKOV3 and OVACAR8 cells with 1, 5, 10, and 15 µM of LDC067 for 48 h showed a gradual reduction of phosphorylated RNAPII (s2 and s5) without significant change in overall RNAPII expression. Similar to siRNA exposure, the antiapoptotic protein Mcl-1 decreased, and the proapoptotic protein Bax increased in a dose-dependent manner. Moreover, the expression level of cleaved PARP was progressively up-regulated in both cell lines. As expected, there was no apparent influence on CDK9 expression, as the CDK9 inhibitor LDC067 functionally inhibits CDK9 activity without affecting its overall expression (Fig. 4C, D). Morphologic changes and diminished cell proliferation were also observed over 2 d of LDC067 treatment (Fig. 4E). In addition, effects of the CDK9 inhibitor on the ovarian endometrioid adenocarcinoma cell line IGROV-1 were also evaluated (Supplemental Fig. S1C, D). Overall, these data suggest that the inhibitory effect on cell proliferation was likely derived from CDK9-induced apoptosis and dysregulated transcription.
Figure 4.
CDK9 inhibitor reduced ovarian cancer cell proliferation by suppressing transcription elongation and inducing apoptosis in ovarian cancer cells. A, B) Relative cell viability of SKOV3 and OVCAR8 cells after exposure to different concentrations of the CDK9 inhibitor LDC067 for 5 d. *P < 0.05, **P < 0.01 compared with the cell only control group. C, D) Expression levels of CDK9 and related signaling pathway proteins involved in transcription and apoptosis after treatment with LDC067 in SKOV3 and OVCAR8 cells by Western blot. E) Representative images of ovarian cancer cell morphologic changes after treatment with LDC067. Original magnification value, ×200.
Inhibition of CDK9 suppresses ovarian cancer cell spheroid growth and clonogenicity formation
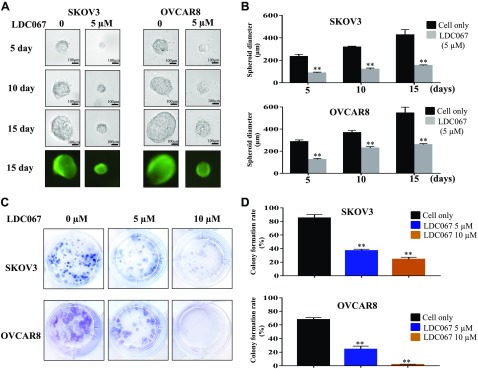
A 3D culture simulates the in vivo environment for cancer cells, namely by permitting the cells to form their natural 3D spheroid shape. The SKOV3 and OVACAR8 cells were exposed to 5 µM LDC067 for a period of 15 d in 3D culture, and their formed spheroids were photographed across multiple time points. Although the diameter of cancer spheroids continuously grew, the spheroids formed from LDC067-treated cells were significantly smaller than the untreated cells (Fig. 5A, B). To further determine the effect of CDK9 inhibition on clonogenicity, we treated both ovarian cancer cell lines with 5 and 10 µM of LDC067 for 14 d. The colony-formation assay demonstrated that LDC067 exposure caused a dose-dependent reduction in the number and size of colonies formed compared with the untreated cells (Fig. 5C, D). In addition, the effects of the CDK9 inhibitor on cell spheroid growth and clonogenicity formation in the ovarian endometrioid adenocarcinoma cell line IGROV-1 were also assessed (Supplemental Fig. S2). These results support the role of CDK9 in enhancing the growth and progression of ovarian cancer cells.
Figure 5.
Inhibition of CDK9 suppressed ovarian cancer cell spheroid growth, and clonogenicity formation. A) Representative images of ovarian cancer cells after treatment with the CDK9 inhibitor LDC067 at different time points (5, 10, and 15 d). B) The relative diameters of spheroids in ovarian cancer cell lines. **P < 0.01 compared with the cell only group. C) Representative images of ovarian cancer cell colony formation after incubation with different concentrations of LDC067 (0, 5, and 10 µM) for 14 d. Original magnification value, ×1. D) Quantification of clonogenicity formation of SKOV3 and OVCAR8 cells after LDC067 treatment. **P < 0.01 compared with the cell only group.
Inhibition of CDK9 reduces ovarian cancer cell migration in vitro
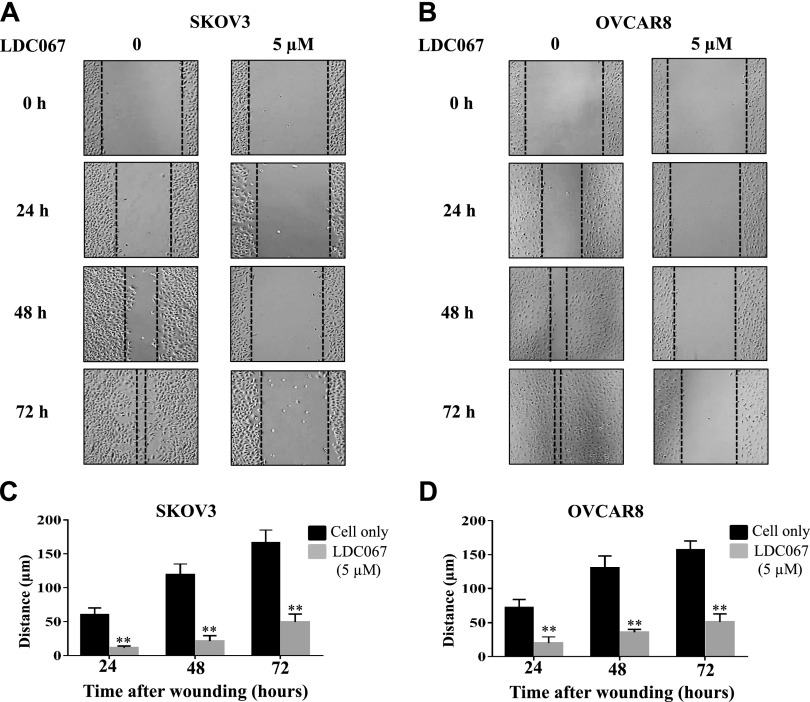
Because cell migration is crucial in cancer metastasis and recurrence, and the TMA revealed a strong correlation between CDK9 expression and metastasis and recurrence in ovarian cancer, we performed a wound-healing assay to assess the effect of CDK9 on cell mobility in vitro. As shown in Fig. 6A, B, the addition of 5 µM of LDC067 for 24, 48, and 72 h led to a time-dependent decrease in cell migration in both cell lines compared with the untreated cells (P < 0.05). After 72 h of LDC067 incubation, SKOV3 and OVCAR8 migrated a total of 49 and 51 µm, respectively, whereas the untreated cells migrated ∼160 µm (Fig. 6C, D). Taken together, these results demonstrate that CDK9 inhibition reduces the migratory distance of ovarian cancer cells.
Figure 6.
Inhibition of CDK9 reduces ovarian cancer cell migration. A, B) Relative migration distance of SKOV3 and OVCAR8 cells at different time points (0, 24, 48, and 72 h) when treated with the CDK9 inhibitor LDC067. Original magnification value, ×200. C, D) Quantification of cell migration distance of SKOV3 and OVCAR8 cells after LDC067 treatment. **P < 0.01 compared with the cell only group.
DISCUSSION
Many studies have found that CDK9 is overexpressed in various types of cancer and plays a critical role in the control of cell growth and proliferation (30–33). A very recent study demonstrated that CDK9 inhibition can reactivate epigenetically silenced genes in cancer, which lead to restored tumor suppressor gene expression and cell differentiation (34). In our present study, we observed that both human ovarian cancer tissues and cell lines expressed high levels of CDK9. Metastatic and recurrent ovarian cancer tumor tissues expressed higher levels of CDK9 compared with patient-matched primary tumor samples. The ovarian cancer patients with lower expression of CDK9 had a longer survival and better outcomes compared with those with high CDK9 expression levels. Cox proportional hazards regression analysis also validated CDK9 expression to be a significant risk factor for the survival of ovarian cancer patients. Our results provide substantial evidence that CDK9 correlates with a poor prognosis in ovarian cancer. These data are consistent with a recent study that reported that CDK9 was highly expressed in pancreatic cancer tissues compared with normal pancreatic tissues, and high CDK9 expression significantly correlated with shortened survival, especially in well-differentiated tumors (35). Another study found CDK9 expression to be associated with the differentiation grade of neuroblastoma and primary neuroectodermal tumor (36). Our study provides evidence that expression of CDK9 can serve as a predictive and prognostic biomarker in ovarian cancer patients.
Dysregulation of cellular transcription and protein synthesis are fundamental features of cancer. Positive transcription elongation factor b is a heterodimeric complex consisting of CDK9 and cyclin T that stimulates the elongation phase of RNA transcription by RNAPII, a crucial enzyme for the transcription of most protein coding genes and an effector of mRNA synthesis (37). Owing to the central role of CDK9 in the regulation of gene transcription, targeting CDK9 has emerged as a highly promising chemotherapeutic strategy. In our study, inhibition of CDK9 with specific siRNA significantly suppressed the growth and proliferation of ovarian cancer cells. Furthermore, knockdown of CDK9 expression led to decreased levels of antiapoptotic protein Mcl-1 and increased levels of proapoptotic protein Bax as a result of reduced expression of phosphorylated RNAPII. Inhibition of cell proliferation and induction of apoptosis by siRNA-mediated CDK9 knockdown have been previously reported in other cancers, including leukemia, breast cancer, prostate cancer, and liver cancer (38–41). Collectively, these studies suggest CDK9 to be a promising target for cancer therapy.
To date, 5 CDK9 inhibitors have been investigated in clinical trials for the treatment of various types of malignancies (42). However, the lack of inhibitor specificity currently limits clinical development. In this study, we evaluated the novel selective CDK9 inhibitor LDC067, which has been used in different kinds of cancers (43–45). Mechanistically, LDC067 inhibited cell viability in a dose-dependent manner by decreasing phosphorylation of RNAPII and inducing apoptosis in ovarian cancer cells. In addition to regulating the expression of Mcl-1 and Bax, cleaved PARP also gradually increased because of the activation of multiple apoptosis signaling pathways. Previous gene expression profiling work with LDC067-treated cells has demonstrated a selective reduction of short-lived mRNAs, including key regulators of proliferation and apoptosis such as Mcl-1 and Myc (44).
We further verified the effect of CDK9 on cell growth and survival by clonogenic assay and 3D cancer models that mimic aspects of in vivo cell biology (46). The average number and size of colonies formed were distinctly suppressed following LDC067 treatment in both ovarian cancer cell lines. There was also a notable decrease in the diameter of the formed cell spheroids. In a recent in vivo experiment, administration of the CDK9 inhibitor dinaciclib in mice elicited potent antitumor responses and significantly prolonged survival in acute myeloid leukemia (21). Migration of ovarian cancer cells has a critical role in tumor metastasis and recurrence. In this study, LDC067 also restrained cell migration, which is consistent with our TMA findings in which CDK9 expression correlated with metastasis and recurrence. These results support CDK9 as a potential contributor to ovarian cancer progression.
Our study demonstrates that high expression of CDK9 significantly correlates with the progression, recurrence, and metastasis of human ovarian cancer and therefore may serve as a novel prognostic and predictive biomarker. Mechanistically, inhibition of CDK9 significantly decreases cell growth and viability by suppressing the phosphorylation of RNAPII and inducing apoptosis in ovarian cancer cells. Our study suggests that CDK9 plays a vital role in the development and progression of ovarian cancer and may therefore be a novel and promising drug target for anticancer therapies. However, further study is needed to fully characterize the molecular mechanisms of CDK9 in ovarian cancer.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported, in part, by a Joint Research Fund (228282) devoted to clinical pharmacy and precision medicine (to Z.D.). Support has also been provided by the National Natural Science Foundation of China (Grants 81402266 and 81372875), and Gattegno and Wechsler funds. Z.D. was supported, in part, by the U.S. National Institutes of Health, National Cancer Institute (Grant UO1, CA151452-01). J.W. was supported by a scholarship from the China Scholarship Council (201607040013). The authors declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- CDK
cyclin-dependent kinase
- CTD
carboxyl-terminal domain
- DFS
disease-free survival
- LDC067
LDC000067
- OS
overall survival
- RNAPII
RNA polymerase II
- s2
RNAPII phosphorylation serine 2
- s5
RNAPII phosphorylation serine 5
- siRNA
small interfering RNA
- TMA
tissue microarray
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Wang curated the data and provided methodology; J. Wang and D. C. Dean provided formal analysis; J. Wang, D. C. Dean, F. J. Hornicek, and Z. Duan wrote and edited the manuscript; H. Shi and Z. Duan supervised and provided project administration; and Z. Duan acquired funds.
REFERENCES
- 1.Siegel R. L., Miller K. D., Jemal A. (2018) Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 [DOI] [PubMed] [Google Scholar]
- 2.Du Bois A., Pfisterer J. (2005) Future options for first-line therapy of advanced ovarian cancer. Int. J. Gynecol. Cancer 15 (Suppl 1), 42–50 [DOI] [PubMed] [Google Scholar]
- 3.Sato S., Itamochi H. (2014) Neoadjuvant chemotherapy in advanced ovarian cancer: latest results and place in therapy. Ther. Adv. Med. Oncol. 6, 293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller K. D., Siegel R. L., Lin C. C., Mariotto A. B., Kramer J. L., Rowland J. H., Stein K. D., Alteri R., Jemal A. (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66, 271–289 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro G. I. (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 24, 1770–1783 [DOI] [PubMed] [Google Scholar]
- 6.Malumbres M. (2014) Cyclin-dependent kinases. Genome Biol. 15, 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loyer P., Trembley J. H., Katona R., Kidd V. J., Lahti J. M. (2005) Role of CDK/cyclin complexes in transcription and RNA splicing. Cell. Signal. 17, 1033–1051 [DOI] [PubMed] [Google Scholar]
- 8.Fisher R. P. (2005) Secrets of a double agent: CDK7 in cell-cycle control and transcription. J. Cell Sci. 118, 5171–5180 [DOI] [PubMed] [Google Scholar]
- 9.Malumbres M., Barbacid M. (2005) Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641 [DOI] [PubMed] [Google Scholar]
- 10.Greifenberg A. K., Hönig D., Pilarova K., Düster R., Bartholomeeusen K., Bösken C. A., Anand K., Blazek D., Geyer M. (2016) Structural and functional analysis of the Cdk13/cyclin K complex. Cell Rep. 14, 320–331 [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Shen J., Choy E., Mankin H., Hornicek F., Duan Z. (2017) Inhibition of CDK4 sensitizes multidrug resistant ovarian cancer cells to paclitaxel by increasing apoptosiss. Cell Oncol. (Dordr.) 40, 209–218 [DOI] [PubMed] [Google Scholar]
- 12.Malumbres M., Barbacid M. (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 [DOI] [PubMed] [Google Scholar]
- 13.Finn R. S., Crown J. P., Lang I., Boer K., Bondarenko I. M., Kulyk S. O., Ettl J., Patel R., Pinter T., Schmidt M., Shparyk Y., Thummala A. R., Voytko N. L., Fowst C., Huang X., Kim S. T., Randolph S., Slamon D. J. (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Fischer P. M. (2008) Cyclin-dependent kinase 9: a key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 29, 302–313 [DOI] [PubMed] [Google Scholar]
- 15.Kohoutek J. (2009) P-TEFb- the final frontier. Cell Div. 4, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterlin B. M., Price D. H. (2006) Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23, 297–305 [DOI] [PubMed] [Google Scholar]
- 17.Hirose Y., Ohkuma Y. (2007) Phosphorylation of the C-terminal domain of RNA polymerase II plays central roles in the integrated events of eucaryotic gene expression. J. Biochem. 141, 601–608 [DOI] [PubMed] [Google Scholar]
- 18.Fuda N. J., Ardehali M. B., Lis J. T. (2009) Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajiro M., Sakai H., Onogi H., Yamamoto M., Sumi E., Sawada T., Nomura T., Kabashima K., Hosoya T., Hagiwara M. (2018) CDK9 inhibitor FIT-039 suppresses viral oncogenes E6 and E7 and has a therapeutic effect on HPV-induced neoplasia. Clin. Cancer Res. 24, 4518–4528 [DOI] [PubMed] [Google Scholar]
- 20.Su Y. T., Chen R., Wang H., Song H., Zhang Q., Chen L. Y., Lappin H., Vasconcelos G., Lita A., Maric D., Li A., Celiku O., Zhang W., Meetze K., Estok T., Larion M., Abu-Asab M., Zhuang Z., Yang C., Gilbert M. R., Wu J. (2018) Novel targeting of transcription and metabolism in glioblastoma. Clin. Cancer Res. 24, 1124–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker A., Gregory G. P., Verbrugge I., Kats L., Hilton J. J., Vidacs E., Lee E. M., Lock R. B., Zuber J., Shortt J., Johnstone R. W. (2016) The CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor effects in preclinical models of MLL-rearranged acute myeloid leukemia. Cancer Res. 76, 1158–1169 [DOI] [PubMed] [Google Scholar]
- 22.Mitra P., Yang R. M., Sutton J., Ramsay R. G., Gonda T. J. (2016) CDK9 inhibitors selectively target estrogen receptor-positive breast cancer cells through combined inhibition of MYB and MCL-1 expression. Oncotarget 7, 9069–9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahaman M. H., Kumarasiri M., Mekonnen L. B., Yu M., Diab S., Albrecht H., Milne R. W., Wang S. (2016) Targeting CDK9: a promising therapeutic opportunity in prostate cancer. Endocr. Relat. Cancer 23, T211–T226 [DOI] [PubMed] [Google Scholar]
- 24.Brägelmann J., Dammert M. A., Dietlein F., Heuckmann J. M., Choidas A., Böhm S., Richters A., Basu D., Tischler V., Lorenz C., Habenberger P., Fang Z., Ortiz-Cuaran S., Leenders F., Eickhoff J., Koch U., Getlik M., Termathe M., Sallouh M., Greff Z., Varga Z., Balke-Want H., French C. A., Peifer M., Reinhardt H. C., Örfi L., Kéri G., Ansén S., Heukamp L. C., Büttner R., Rauh D., Klebl B. M., Thomas R. K., Sos M. L. (2017) Systematic kinase inhibitor profiling identifies CDK9 as a synthetic lethal target in NUT midline carcinoma. Cell Rep. 20, 2833–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittaker S. R., Barlow C., Martin M. P., Mancusi C., Wagner S., Self A., Barrie E., Te Poele R., Sharp S., Brown N., Wilson S., Jackson W., Fischer P. M., Clarke P. A., Walton M. I., McDonald E., Blagg J., Noble M., Garrett M. D., Workman P. (2018) Molecular profiling and combinatorial activity of CCT068127: a potent CDK2 and CDK9 inhibitor. Mol. Oncol. 12, 287–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Gao Y., Shen J., Yang W., Choy E., Mankin H., Hornicek F. J., Duan Z. (2016) Cyclin-Dependent Kinase 11 (CDK11) is required for ovarian cancer cell growth in vitro and in vivo, and its inhibition causes apoptosis and sensitizes cells to paclitaxel. Mol. Cancer Ther. 15, 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Garbutt C., Ma H., Gao P., Hornicek F. J., Kan Q., Shi H., Duan Z. (2018) Expression and role of autophagy-associated p62 (SQSTM1) in multidrug resistant ovarian cancer. Gynecol. Oncol. 150, 143–150 [DOI] [PubMed] [Google Scholar]
- 28.Shore S. M., Byers S. A., Dent P., Price D. H. (2005) Characterization of Cdk9(55) and differential regulation of two Cdk9 isoforms. Gene 350, 51–58 [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Herrmann C. H. (2005) Differential localization and expression of the Cdk9 42k and 55k isoforms. J. Cell. Physiol. 203, 251–260 [DOI] [PubMed] [Google Scholar]
- 30.Chen X. X., Xie F. F., Zhu X. J., Lin F., Pan S. S., Gong L. H., Qiu J. G., Zhang W. J., Jiang Q. W., Mei X. L., Xue Y. Q., Qin W. M., Shi Z., Yan X. J. (2015) Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget 6, 14926–14939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam F., Abbas A. Y., Shao H., Teo T., Adams J., Li P., Bradshaw T. D., Fischer P. M., Walsby E., Pepper C., Chen Y., Ding J., Wang S. (2014) Targeting RNA transcription and translation in ovarian cancer cells with pharmacological inhibitor CDKI-73. Oncotarget 5, 7691–7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R., Keating M. J., Gandhi V., Plunkett W. (2005) Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood 106, 2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano G., Giordano A. (2008) Role of the cyclin-dependent kinase 9-related pathway in mammalian gene expression and human diseases. Cell Cycle 7, 3664–3668 [DOI] [PubMed] [Google Scholar]
- 34.Zhang H., Pandey S., Travers M., Sun H., Morton G., Madzo J., Chung W., Khowsathit J., Perez-Leal O., Barrero C. A., Merali C., Okamoto Y., Sato T., Pan J., Garriga J., Bhanu N. V., Simithy J., Patel B., Huang J., Raynal N. J., Garcia B. A., Jacobson M. A., Kadoch C., Merali S., Zhang Y., Childers W., Abou-Gharbia M., Karanicolas J., Baylin S. B., Zahnow C. A., Jelinek J., Grana X., Issa J. J. (2018) Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell 175, 1244–1258.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kretz A. L., Schaum M., Richter J., Kitzig E. F., Engler C. C., Leithäuser F., Henne-Bruns D., Knippschild U., Lemke J. (2017) CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol. 39, 1010428317694304 [DOI] [PubMed] [Google Scholar]
- 36.De Falco G., Bellan C., D’Amuri A., Angeloni G., Leucci E., Giordano A., Leoncini L. (2005) Cdk9 regulates neural differentiation and its expression correlates with the differentiation grade of neuroblastoma and PNET tumors. Cancer Biol. Ther. 4, 277–281 [DOI] [PubMed] [Google Scholar]
- 37.Hsin J. P., Manley J. L. (2012) The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C. H., Lujambio A., Zuber J., Tschaharganeh D. F., Doran M. G., Evans M. J., Kitzing T., Zhu N., de Stanchina E., Sawyers C. L., Armstrong S. A., Lewis J. S., Sherr C. J., Lowe S. W. (2014) CDK9-mediated transcription elongation is required for MYC addiction in hepatocellular carcinoma. Genes Dev. 28, 1800–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajput S., Khera N., Guo Z., Hoog J., Li S., Ma C. X. (2016) Inhibition of cyclin dependent kinase 9 by dinaciclib suppresses cyclin B1 expression and tumor growth in triple negative breast cancer. Oncotarget 7, 56864–56875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caracciolo V., Laurenti G., Romano G., Carnevale V., Cimini A. M., Crozier-Fitzgerald C., Gentile Warschauer E., Russo G., Giordano A. (2012) Flavopiridol induces phosphorylation of AKT in a human glioblastoma cell line, in contrast to siRNA-mediated silencing of Cdk9: implications for drug design and development. Cell Cycle 11, 1202–1216 [DOI] [PubMed] [Google Scholar]
- 41.Walsby E., Pratt G., Shao H., Abbas A. Y., Fischer P. M., Bradshaw T. D., Brennan P., Fegan C., Wang S., Pepper C. (2014) A novel Cdk9 inhibitor preferentially targets tumor cells and synergizes with fludarabine. Oncotarget 5, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales F., Giordano A. (2016) Overview of CDK9 as a target in cancer research. Cell Cycle 15, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prudencio M., Gonzales P. K., Cook C. N., Gendron T. F., Daughrity L. M., Song Y., Ebbert M. T. W., van Blitterswijk M., Zhang Y. J., Jansen-West K., Baker M. C., DeTure M., Rademakers R., Boylan K. B., Dickson D. W., Petrucelli L., Link C. D. (2017) Repetitive element transcripts are elevated in the brain of C9orf72 ALS/FTLD patients. Hum. Mol. Genet. 26, 3421–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albert T. K., Rigault C., Eickhoff J., Baumgart K., Antrecht C., Klebl B., Mittler G., Meisterernst M. (2014) Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. Br. J. Pharmacol. 171, 55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Löschmann N., Michaelis M., Rothweiler F., Voges Y., Balónová B., Blight B. A., Cinatl J., Jr (2016) ABCB1 as predominant resistance mechanism in cells with acquired SNS-032 resistance. Oncotarget 7, 58051–58064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann D., Conway J. R., Vennin C., Magenau A., Hughes W. E., Morton J. P., Timpson P. (2014) Three-dimensional cancer models mimic cell-matrix interactions in the tumour microenvironment. Carcinogenesis 35, 1671–1679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.