Abstract
Blockade of immune-checkpoint programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 can enhance effector T-cell responses. However, the lack of response in many patients to checkpoint-inhibitor therapies emphasizes the need for combination immunotherapies to pursue maximal antitumor efficacy. We have previously demonstrated that antagonism of C-X-C chemokine receptor type 4 (CXCR4) by plerixafor (AMD3100) can decrease regulatory T (Treg)-cell intratumoral infiltration. Therefore, a combination of these 2 therapies might increase antitumor effects. Here, we evaluated the antitumor efficacy of AMD3100 and anti–PD-1 (αPD-1) antibody alone or in combination in an immunocompetent syngeneic mouse model of ovarian cancer. We found that AMD3100, a highly specific CXCR4 antagonist, directly down-regulated the expression of both C-X-C motif chemokine 12 (CXCL12) and CXCR4 in vitro and in vivo in tumor cells. AMD3100 and αPD-1 significantly inhibited tumor growth and prolonged the survival of tumor-bearing mice when given as monotherapy. Combination of these 2 agents significantly enhanced antitumor effects compared with single-agent administration. Benefits of tumor control and animal survival were associated with immunomodulation mediated by these 2 agents, which were characterized by increased effector T-cell infiltration, increased effector T-cell function, and increased memory T cells in tumor microenvironment. Intratumoral Treg cells were decreased, and conversion of Treg cells into T helper cells was increased by AMD3100 treatment. Intratumoral myeloid-derived suppressor cells were decreased by the combined treatment, which was associated with decreased IL-10 and IL-6 in the ascites. Also, the combination therapy decreased suppressive leukocytes and facilitated M2-to-M1 macrophage polarization in the tumor. These results suggest that AMD3100 could be used to target the CXCR4-CXCL12 axis to inhibit tumor growth and prevent multifaceted immunosuppression alone or in combination with αPD-1 in ovarian cancer, which could be clinically relevant to patients with this disease.—Zeng, Y., Li, B., Liang, Y., Reeves, P. M., Qu, X., Ran, C., Liu, Q., Callahan, M. V., Sluder, A. E., Gelfand, J. A., Chen, H., Poznansky, M. C. Dual blockade of CXCL12-CXCR4 and PD-1–PD-L1 pathways prolongs survival of ovarian tumor–bearing mice by prevention of immunosuppression in the tumor microenvironment.
Keywords: CXCR4 antagonist, immune checkpoint inhibitor, combination immunotherapy
Ovarian cancer is the most lethal malignancy in women (1). Surgical debulking followed by platinum-based chemotherapy remains the current standard-of-care treatment to which ∼80% of patients will respond favorably. However, >60% of patients who initially achieve remission eventually experience relapse of their cancer and <40% will survive beyond 5 yr. (2). Thus, novel treatments for ovarian cancer are urgently needed. Clinical trials of various immunotherapeutic modalities have not yet yielded significant benefit in ovarian cancer (3). The failures might be caused by the complexities of tumor-induced dysregulation of the antitumor immune response. These previous therapies have been limited but have provided insight into how to design future therapies.
The intratumoral microenvironment mediates induction of the programmed cell death protein 1 (PD-1) pathway and accumulation of regulatory T (Treg) cells, which are thought to contribute to the dampening of antitumor immunity (4, 5). PD-1 is an inhibitory surface receptor expressed on T cells and can bind the immunosuppressive programmed cell death ligand 1 (PD-L1) up-regulated on tumor cells. The receptor-ligand interaction can inhibit antitumor cytokine production and cytolytic activity of PD-1+ tumor–infiltrating T cells and thus silence the immune system (6). Checkpoint blockade of the interaction between PD-1 and PD-L1 potentiates T-cell immune responses and mediates antitumor activity (4, 7–9). However, the lack of response in many patients to the checkpoint-inhibitor therapies emphasizes the potential synergy of other treatments (10, 11).
C-X-C motif chemokine 12 (CXCL12) and its receptor C-X-C chemokine receptor type 4 (CXCR4) constitute a chemokine-receptor axis that is known to be overexpressed in a broad range of tumors and mediates trafficking and retention of various immune cells to and within the intratumoral microenvironment, respectively (12). Treg cells are thought to modulate antitumor immune responses through selective migration and accumulative retention at tumor sites, thereby playing a central role in the immunopathogenesis of tumors (13). It was reported that basal-like breast cancers behaved more aggressively despite the presence of dense lymphoid infiltrate because of Treg cell recruitment driven by hypoxia-induced up-regulation of CXCR4 in Treg cells (14). In our previous study, blockage of the CXCL12-CXCR4 axis with plerixafor (AMD3100), a CXCR4 antagonist, elicited multimodal effects on tumor pathogenesis associated with selective reduction of intratumoral Treg cells by conversion of Treg cells into T-helper–like cells and conferred a significant survival advantage to the ovarian tumor– (15) and mesothelioma (16)–bearing mice.
Although unimodal immunotherapies have produced promising clinical responses, pursuit of the maximal antitumor immune response is likely to require combinatorial immunotherapies (17), which create the possibility of synergistic and additive effects between the different immune regulatory pathways (18). Therefore, we postulated that targeted interventions against the immunosuppressive PD-1 pathway and removing Treg cells while enhancing effector T-cell entry into the intratumoral microenvironment could help augment antitumor immunity. In the current study, we developed a novel combination immunotherapy for ovarian cancer which involves blockade of both PD-1–PD-L1 and CXCL12-CXCR4 pathways. We found that combinatorial blockade of CXCR4 and PD-1 greatly reduces specific cellular and functional elements within the immunosuppressive tumor microenvironment and augments tumor-specific cell-mediated immune responses to yield more powerful control of ovarian cancer in an immunocompetent syngeneic mouse model.
MATERIALS AND METHODS
AMD3100 and anti–PD-1/anti–PD-L1
AMD3100 octahydrochloride was purchased from Abcam (Cambridge, United Kingdom) (ab120718). AMD3100 was reconstituted in PBS to desired concentration for in vitro and in vivo application. Anti–PD-1 (αPD-1) mAb was purchased from Bio X Cell (West Lebanon, NH, USA) (BE0273 and BE0101).
Tumor cell and proliferation assay
Epithelial ovarian cancer cell line ID8 was a kind gift from Dr. Kathy Roby (University of Kansas Medical Center, Kansas City, KS, USA) (19). ID8 cells were transfected with lentivirus encoding luciferase, then termed luciferized ID8 (ID8-luc) cells. A total of 500 cells were seeded in 96-well plates and cultured overnight at 37°C in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 100 U/ml penicillin and streptomycin (Corning, Corning, NY, USA), and 2 mM l-glutamine (Thermo Fisher Scientific). Each group was set up in sextuplicate. AMD3100 or vehicle was added to the wells the next day, and the medium was changed. CyQuant assay (C7026; Thermo Fisher Scientific) based on measuring the amount of DNA was utilized to quantitate cell proliferation following the manufacturer’s instructions.
Wound healing assay
Cells were cultured in 12-well plates overnight to reach 90% confluence. Wound gaps were then made using a 200-μl pipette tip. After washing twice to get rid of detached cells, fresh medium and varying concentrations of AMD3100 were added to the wells. A total of 3 wounds were made for each concentration. Marks were made for imaging at the identical location. Migration distance was determined by photography and measured at 6, 12, and 24 h post-initiation of the wound gap.
Transwell invasion assay
1 × 106 ID8-luc cells/ml were prepared in DMEM supplemented with 10% fetal bovine serum. A total of 100 μl cell suspension was added into the upper chamber of the transwell insert with 5-µm pore size and incubated at 37°C and 5% CO2 for 10 min. A total of 600 μl medium was added to the bottom of the lower chamber of 24-well plates to make contact with the membrane in the upper well. AMD3100 at certain concentrations was added both on top of the well and the lower chamber and incubated for 24 h. The transwell insert was then removed and fixed in 70% ethanol. Crystal violet (0.2%) was applied for 10 min to stain cells. After washing and removal of cells from the top of the membrane, the membrane was observed underneath using an inverted microscope, and cells were counted in different fields of view to get a mean sum of cells that had migrated through the membrane. All experiments were performed in quadruplicate for each concentration of AMD3100.
Animal model and treatment
C57BL/6J female mice (4–6 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the Massachusetts General Hospital animal facility. After 1-wk accommodation in the animal facility, 3 × 106 ID8-luc cells were administered intraperitoneally per mouse. The establishment of the tumor model was identified by positive signal (total photons >1 × 105) using an In Vivo Imaging System (IVIS), bioluminescent imaging 3-wk posttumor cell inoculation. The mice with low or no signal (total photons <1 × 105) were excluded from experiments. All tumor-bearing mice were randomly divided into 6 groups (n = 15–20 in each group) and received saline, AMD3100, αPD-1, or AMD3100 + αPD-1 treatments. AMD3100 has been used in the clinic for mobilization of hematopoietic stem cells at the concentration of 0.24 mg/kg human body weight and no more than 40 mg/d. Based on Food and Drug Administration guidelines for conversion doses between animals and humans (20), the equivalent concentration for mice is 3 mg/kg mouse body weight. AMD3100 was administered intraperitoneally 10 times every other day from d 20 post–tumor implantation dosing at 1 mg/kg or 3 mg/mg in 100 μl. αPD-1 was applied at 200 μg/100 μl per mouse every other day starting from d 23 for 9 doses. For anesthesia, when needed, the mice were administrated intraperitoneally with 9 mg/ml ketamine and 9 mg/ml xylazine (K2753, X1126; MilliporeSigma, Burlington, MA, USA) at 0.1 ml/10 g of body weight. All animal studies were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Tumor growth and mouse survival
Tumor growth was evaluated by IVIS (PerkinElmer, Waltham, MA, USA) at d 20, 30, and 40 post–tumor cell inoculation. Luciferin (1-360222; Regis Technologies, Morton Grove, IL, USA) was dissolved in Dulbecco's PBS to the concentration of 30 mg/ml. The mice received 100 μl, i.p. and were kept for 5 min before imaging. Total photons under the region of interest captured by IVIS were used for statistical analysis. Survival time was calculated as life span from the day of tumor inoculation. In some cases, the Massachusetts General Hospital Center for Comparative Medicine faculties evaluated severe midsection swelling of the mice and poor body condition, and the life spans of the mice were recorded at the day of euthanasia.
Handling of cells from ascites
At 2 wk after the last dose of treatment at wk 7 post–tumor inoculation, the mice were euthanized by CO2 and processed to obtain all tissue samples. At this time point, the majority of control and experimental mice were observed to develop ascites. Ascites enriched with immune cells is another important sample in assessing the phenotypic and functional profiles of immune responses in order to understand the tumor microenvironment. Ascites was aspirated using a syringe and 34G needle to puncture the peritoneal cavity. Ascites supernatant was collected after centrifuge and stored in a −80°C freezer for ELISA. All harvested cells were lysed by red blood cells lysis buffer (420301; BioLegend, San Diego, CA, USA) twice until the majority of red blood cells were eliminated. The bulk ascites cells were then immunostained for cytometric analysis.
Processing tumor cells
ID8-luc cell–established tumors disperse as small friable intraperitoneal micronodules attached to the mesentery 2–7 mm in diameter. The tumor micronodules present in each mouse were gently dissociated from the mesentery and collected. The tumor tissues were validated by histology with hematoxylin and eosin staining. The tumor tissues were then gently sliced into 1–2-mm pieces using a scalpel and dissociated in DMEM supplemented with 2 mg/ml collagenase type IV (17104-019; Thermo Fisher Scientific), 2 mg/ml hyaluronidase (H3506; MilliporeSigma), and 2 mg/ml bovine serum albumin (05470; MilliporeSigma) for 1.5 h at 37°C, shaking at a very slow speed for mixing. The resultant cell suspension was then filtered through 70-μm cell strainers.
Flow cytometry
Cell suspensions collected as described above were washed with PBS and stained with Zombie UV viability dye (423108; BioLegend) and then washed with cell-staining buffer (420201; BioLegend). The cells were then stained with multiple fluorophore–conjugated cell-surface antibodies (Supplemental Table S1). Intracellular staining was initiated by fixation and permeabilization reagents (424401; BioLegend). Cells were then stained with multiple fluorophore–conjugated intracellular antibodies. These antibodies were arranged into different panels, and compensation settings were established before running the stained cells on a Fortessa X-20 (BD Biosciences, San Jose, CA, USA). Storage events were gated on populations of interest (Supplemental Fig. S1). Flow data were analyzed using FlowJo v.10 (FlowJo, Ashland, OR, USA).
ELISA
Cytokines CXCL12, IL-2, IL-10, and IL-6 were evaluated in the ascites supernatant by Duoset ELISA Kit purchased from R&D Systems (Minneapolis, MN, USA). Following the manufacturer’s instructions, 2- or 3 times–diluted supernatant was reacted with capture antibody and detection antibody. A microplate reader (PerkinElmer, Waltham, MA, USA) was used to read the 96-well plates at 450 nm.
Statistical analyses
One-way ANOVA, 2-way ANOVA, and Student’s t test were applied for most statistical analysis. Prism 7 (Graphpad Software, La Jolla, CA, USA) was used to make graphs and calculate P values. Survival curves were analyzed by the Kaplan-Meier method and log-rank test. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
AMD3100 inhibits ovarian cancer cell growth and migration in vitro
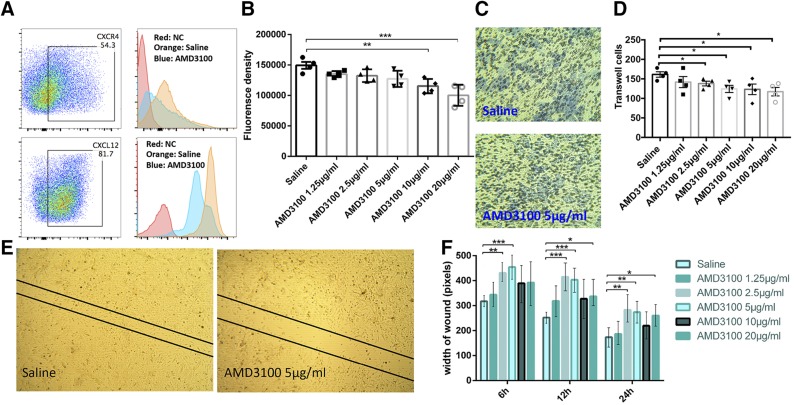
ID8-luc cells were shown to express high levels of CXCR4 and CXCL12 by flow cytometry (Fig. 1A). AMD3100 down-regulated the expression of both CXCR4 and CXCL12 in ID8-luc cells after 24 h stimulation (Fig. 1A). In CyQuant assays, AMD3100 significantly inhibited proliferation of ID8-luc cells at concentrations of 10 and 20 μg/ml (Fig. 1B). In transwell assays, AMD3100 significantly inhibited cell invasion and migration at concentrations of 2.5 μg/ml and above (Fig. 1C, D). Wound healing assays also revealed that AMD3100 inhibited ID8 cell migration at concentrations of ≥2.5 μg/ml (Fig. 1E, F). Taken together, these data suggest that AMD3100 can inhibit ovarian cancer cell growth and migration in vitro by antagonizing the CXCR4-CXCL12 axis.
Figure 1.
AMD3100 inhibits ID8-luc cell growth and migration in vitro. A) Representative data showing that CXCR4 and CXCL12 are highly expressed on ID8-luc cells (left). Comparative flow results showing that AMD3100 at 10 μg/ml down-regulated the expression of CXCR4 and CXCL12 (right). NC, negative controls (unstained cells). B) CyQuant proliferation assays. C) Representative images showing the inhibition of cancer cell invasion by AMD3100 proved by transwell assay. The blue color represents transmembrane cells. D) Results of transwell assay stimulated by different concentrations of AMD3100. E) Representative images showing the impact of AMD3100 on ID8-luc cell migration in the wound healing assay. F) Statistical results of wound healing assay. Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Combination therapy augments tumor control and mouse survival
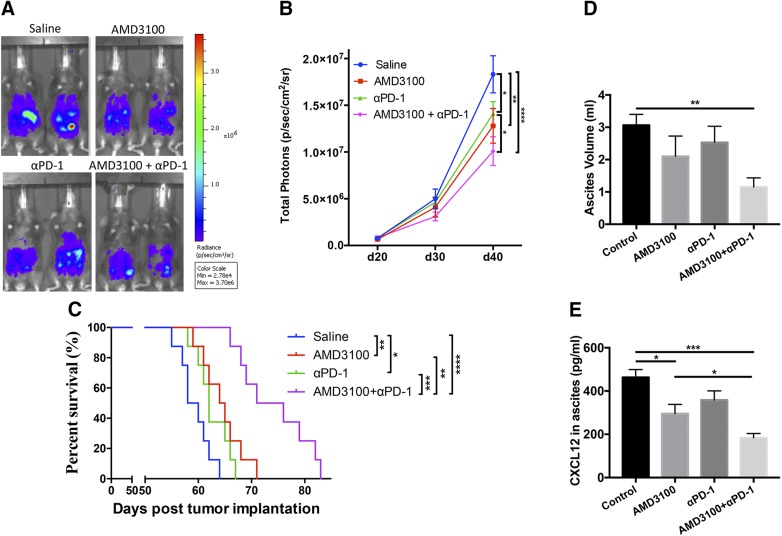
We established an intraperitoneal ovarian cancer model in immunocompetent C57BL/6J mice using luciferase-expressing syngeneic ID8-luc cells (21). Here, we tested the effect of AMD3100 and αPD-1 used alone or in combination on tumor growth and animal survival in ovarian tumor–bearing mice. In animals treated with AMD3100 (3 mg/kg of mouse body weight, an allometrically-scaled equivalent dose to the approved human dose of 0.24 mg/kg human body weight) or αPD-1 (200 μg per mouse) alone, tumor growth as recorded by tumor bioluminescence signals was significantly inhibited (Fig. 2A, B) (P < 0.01, P < 0.05, respectively), and animal survival was significantly prolonged (Fig. 2C) (P < 0.01, P < 0.05, respectively) compared with that in saline-treated mice. The combination treatment with AMD3100 and αPD-1 further significantly enhanced tumor control (P < 0.0001) and prolonged animal survival (P < 0.0001) compared with the saline control. Moreover, the combination treatment showed significantly improved antitumor efficacy on inhibition of tumor growth (P < 0.05) compared with αPD-1 monotherapy and prolongation of mouse survival (P < 0.01, P < 0.001) compared with AMD3100 and αPD-1 monotherapy, respectively. Ascites volumes were found to be significantly lower in the combination therapy group compared with saline control (P < 0.01) (Fig. 2D). ELISA results showed that treatment with AMD3100 alone or in combination with αPD-1 reduced the concentration of CXCL12 in ascites (Fig. 2E). These data indicate that the combination immunotherapy of anti-PD1 and AMD3100 significantly enhances the antitumor effect compared with monotherapy with either agent.
Figure 2.
Combination therapy augments tumor control and mouse survival. A) Representative IVIS images showing tumor growth at d 40 post–tumor inoculation among different treated groups. B) Tumor growth of ID8-luc cells in C57BL/6 mice treated with αPD-1, AMD3100, or in combination (n = 11–13). C) Survival curve showing most prolonged survival in AMD3100 in combination with αPD-1 therapy in comparison with mice receiving monotherapy or N-saline. D) Ascites volume in control and experimental groups at the time of euthanization 2 wk after last treatment (n = 6). E) ELISA results of CXCL12 in the ascites supernatant (n = 6). Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
Combination therapy increases intratumoral T-cell infiltration
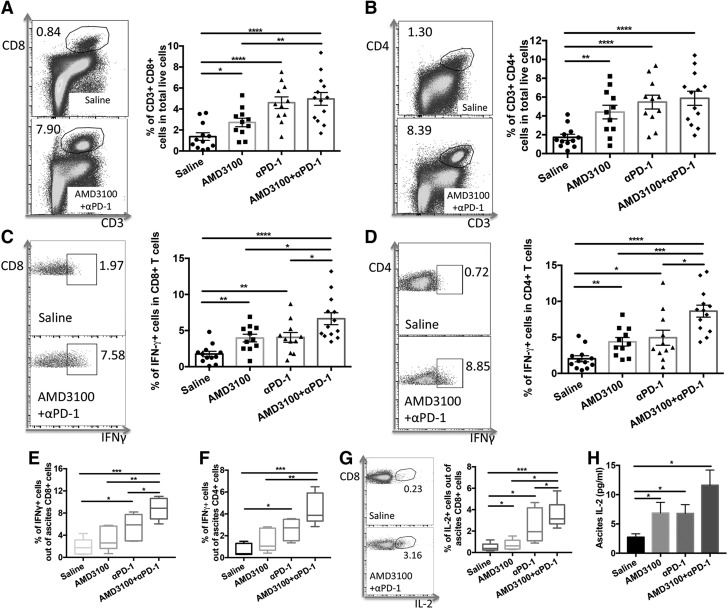
Intraperitoneal tumors were collected from tumor-bearing mice 1 wk after the final treatment dose was given. Single cells were prepared from tumors and analyzed by flow cytometry. The proportion of CD8+ and CD4+ T cells among total live cells in tumors was significantly increased in the AMD3100 (P < 0.05, P < 0.01, respectively), αPD-1 (P < 0.0001, P < 0.0001, respectively), or combination (P < 0.0001, P < 0.0001, respectively) treatment groups compared with that in the saline control group (Fig. 3A, B). The combination therapy further increased intratumoral CD8+ T-cell infiltration compared with AMD3100 monotherapy (P < 0.01).
Figure 3.
Combination therapy enhanced intratumoral T-cell infiltration and effector T-cell function. A) Representative cytometric dot plots of CD3+CD8+ T cells in tumor (left) and the proportions of CD3+CD8+ T cells in tumor in different treatment groups (right). B) Representative cytometric dot plots of CD3+CD4+ T cells in tumor (left) and the proportions of CD3+CD4+ T cells in tumor in different treatment groups (right). C) Representative cytometric dot plots showing intratumoral CD8+ IFN-γ+ cells in saline and AMD3100 and αPD-1 combination groups (left), and percentage of intratumoral CD8+ IFN-γ+ cells showing that AMD3100 and αPD-1 combination could great enhance IFN-γ expressing on CD8+ cells (right). D) Representative dot plots showing intratumoral CD4+ IFN-γ+ cells (left), and percentage of intratumoral CD4+ IFN-γ+ cells (right). E, F) Percentage of ascites CD8+ IFN-γ+ (E) and CD4+ IFN-γ+ (F) cells showing that combination therapy could significantly enhance IFN-γ expressing on CD8+ and CD4+ T cells. G) Representative dot plots showing ascites CD8+ IL-2+ cells in saline and AMD3100 and αPD-1 combination groups (left), and percentage of ascites CD8+ IL-2+ cells (right). H) ELISA results showing IL-2 amount in the ascites (n = 6 for ascites). Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
Combination therapy enhances effector T-cell function
We next analyzed intracellular IFN-γ in CD8+ and CD4+ T cells. IFN-γ expression in both CD8+ and CD4+ T cells was significantly greater in AMD3100 (P < 0.01, P < 0.01, respectively), αPD-1 (P < 0.01, P < 0.05, respectively), or combination (P < 0.0001, P < 0.0001, respectively) treatment groups compared with that in the saline control group (Fig. 3C, D). The combination therapy further significantly enhanced IFN-γ expression in both CD8+ and CD4+ T cells compared with AMD3100 or αPD-1 monotherapy. We also found that IFN-γ was highly expressed in ascites CD8+ and CD4+ T cells in all the treated groups but was at its highest in the combination therapy group (Fig. 3E, F). The combination therapy also significantly enhanced IL-2 expression in ascites CD8+ T cells compared with AMD3100, αPD-1, or saline control treatment (Fig. 3G). ELISA results showed that IL-2 was significantly increased in ascites in AMD3100 or αPD-1 treatment group, and the combination of these 2 agents further increased IL-2 in ascites (Fig. 3H). Together, these data indicate that combination of AMD3100 and αPD-1 increases intratumoral T-cell infiltration and enhances effector T-cell function.
Combination therapy increases intratumoral memory T cells
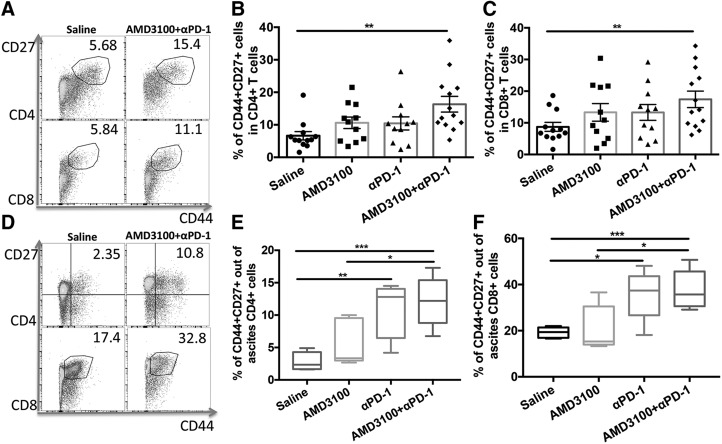
Intratumoral memory T cells were quantitated in tumor-bearing mice treated with AMD3100, αPD-1, and the combination of the 2 agents (Fig. 4A–C). CD4+ or CD8+ memory T cells were indicated by the presence of CD27 and CD44. Although AMD3100 and αPD-1 monotherapy did not significantly increase the memory T cells in the tumor, the combination therapy significantly increased both CD4+ and CD8+ memory T cells in the tumor compared with the saline control group (P < 0.01, P < 0.01, respectively). In ascites, αPD-1–applied groups showed higher proportion of memory T cells in both CD4+ and CD8+ T cells (Fig. 4D–F). The combination therapy further increased the proportion of memory T cells in both ascites CD4+ and CD8+ T cells compared with the control group (P < 0.001, P < 0.001, respectively) or AMD3100-treated group (P < 0.05, P < 0.05, respectively).
Figure 4.
Combination therapy enhanced intratumoral and ascites memory T cells. A) Representative cytometric dot plots of CD44+CD27+ memory T cells in intratumoral CD4 and CD8 T cells. B) Percentages of intratumoral CD4+CD44+CD27+ memory T cells in control and treatment groups. C) Percentages of intratumoral CD8+CD44+CD27+ memory T cells in different groups. D) Representative cytometric dot plots of CD44+CD27+ memory T cells in ascites CD4 and CD8 T cells. E) Percentages of ascites CD4+CD44+CD27+ memory T cells in different groups. F) Percentages of ascites CD8+CD44+CD27+ memory T cells in different groups. Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
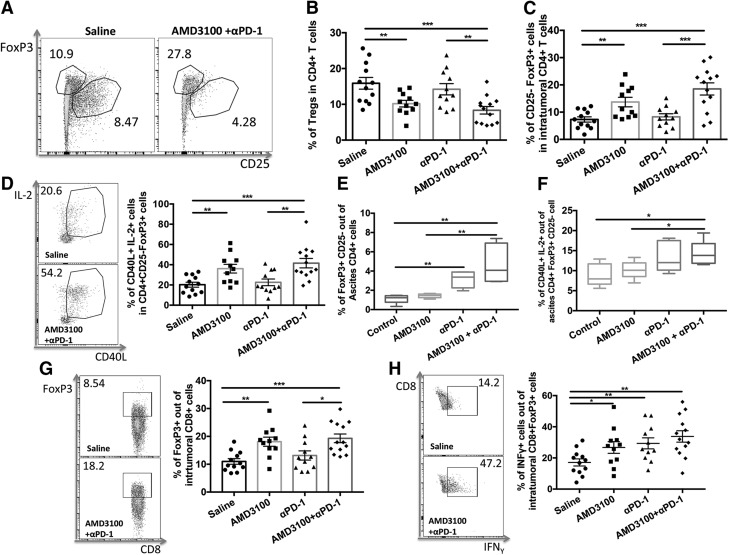
AMD3100 inhibits intratumoral Treg cells and increases forkhead box P3+CD25− CD4+ T-helper cells
Given that we have previously demonstrated that AMD3100 reduced intratumoral Treg cells and immunophenotypically reprogrammed them into T-helper–like cells in a mouse model of mesothelioma (16), we next evaluated the impact of AMD3100 on Treg cells. AMD3100 administered alone or in combination with αPD-1 significantly decreased the proportion of intratumoral Treg cells (P < 0.01, P < 0.001, respectively) compared with saline treatment (Fig. 5A, B). The combination therapy further decreased Treg cells in tumor in comparison with αPD-1 monotherapy (P < 0.01). We also observed that AMD3100 alone or in combination with αPD-1 significantly increased the proportion of forkhead box P3 (FoxP3)+CD25−CD4+ T cells in the tumor (Fig. 5C; P < 0.01, P < 0.001, respectively) compared with the control group. Among the FoxP3+CD25− Treg cell population, significantly more cells were phenotypically CD40L+IL-2+ (Fig. 5D) after AMD3100 monotherapy (P < 0.01) and combination therapy with αPD-1 (P < 0.001) compared with the control group. No differences in the proportion of Treg cells, FoxP3+CD25−, or IL-2+CD40L+FoxP3+CD25− cells between AMD3100 monotherapy and combination therapy groups, as well as between αPD-1 and saline treatment groups, were observed. The combination therapy also significantly enhanced accumulation of FoxP3+CD25− population (Fig. 5E) and increased IL-2+CD40L+FoxP3+CD25− cells in ascites (Fig. 5F) compared with saline treatment.
Figure 5.
AMD3100 inhibited Treg cells and enhanced CD4+FoxP3+CD25− T-helper–cell proportion and function. A) Representative cytometric dot plots of CD4+FoxP3+CD25+ Treg cells in tumor. B) Percentages of intratumoral Treg cells in treatment and control groups. B) Percentages of Treg cells in ascites cells of different groups. C) Percentages of intratumoral CD4+FoxP3+CD25− T-helper–like cells. D) Representative cytometric dot plots and percentages of CD40L+IL-2+ cells in intratumoral CD4+FoxP3+CD25− T-helper–like cells. E) Percentages of ascites CD4+FoxP3+CD25− T-helper–like cells. F) Percentages of CD40L+IL-2+ cells in ascites CD4+FoxP3+CD25− T-helper–like cells (n = 6 for ascites). G) Representative cytometric dot plots and percentages of intratumoral CD8+FoxP3+CD25− T cells in different groups. H) Representative cytometric dot plots and percentages of IFN-γ expression in CD8+FoxP3+CD25− T cells in different groups. Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
In addition, nearly 20% of intratumoral FoxP3+CD8+ T cells were found in AMD3100 monotherapy and combination therapy with αPD-1 (Fig. 5G) compared with about 10% in the saline control group. IFN-γ expression was also significantly increased in FoxP3+CD8+ T cells in monotherapies and further increased in the combination therapy compared with the saline treatment (Fig. 5H). These data are consistent with our previous observations that AMD3100 can drive the reduction of Treg cells and reprogramming of Treg cells into T-helper–like cells (16). This may be associated with increased expression of FoxP3 in both CD4+ and CD8+ T cells without coexpression of CD25.
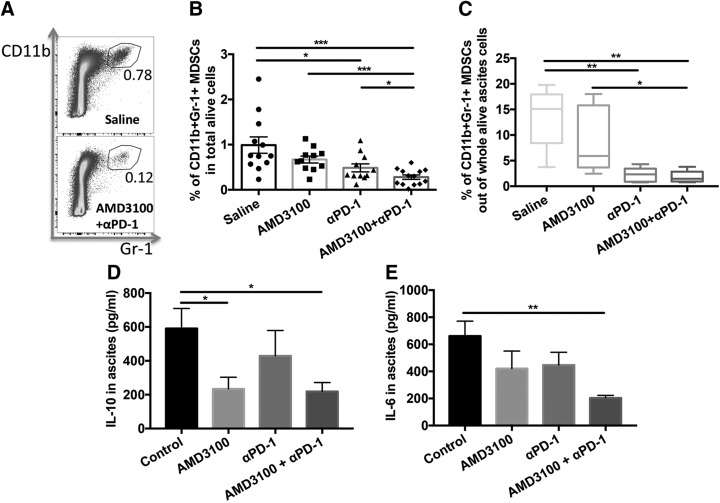
Combination therapy decreases intratumoral myeloid-derived suppressor cells
We next evaluated the impact of the single or combination treatments on intratumoral infiltration of myeloid-derived suppressor cells (MDSCs) (Fig. 6A). CD11b+granulocyte antigen-1+ cells in CD45+ cells were gated to analyze MDSCs (22). αPD-1 treatment significantly decreased the proportion of MDSCs in tumor (P < 0.05) compared with saline treatment (Fig. 6B). Although AMD3100 did not significantly reduce the proportion of MDSCs, when combined with αPD-1, the combination significantly decreased the proportion of intratumoral MDSCs (P < 0.001) compared with saline treatment and further reduced the proportion of MDSCs in tumor compared with αPD-1 monotherapy (P < 0.05). There were much fewer MDSCs in ascites in αPD-1–applied groups (Fig. 6C). IL-10 (23) and IL-6 (24, 25) were reported to be secreted by MDSCs and played a role in immune suppression. The combined treatment significantly decreased the levels of IL-10 (Fig. 6D; P < 0.05) and IL-6 (Fig. 6E; P < 0.01) in ascites compared with the saline control treatment. These data suggest that tumor control and animal survival benefits from the combination therapy may be associated with the decrease of intratumoral MDSCs and their secretion of immunosuppressive cytokines.
Figure 6.
Combination immunotherapy resulted in a marked decrease in immunosuppressive intratumoral MDSCs. A) Representative cytometric dot plots of intratumoral CD11b+granulocyte antigen-1+ MDSCs in saline (top) and combination (bottom) treatment. B) Percentages of intratumoral MDSCs in total alive cells in tumor. C) Percentages of ascites MDSCs in whole alive cell population. D) ELISA results of IL-10, n = 6. E) ELISA results of IL-6, n = 6. Gr-1, granulocyte antigen 1. Error bars represent means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
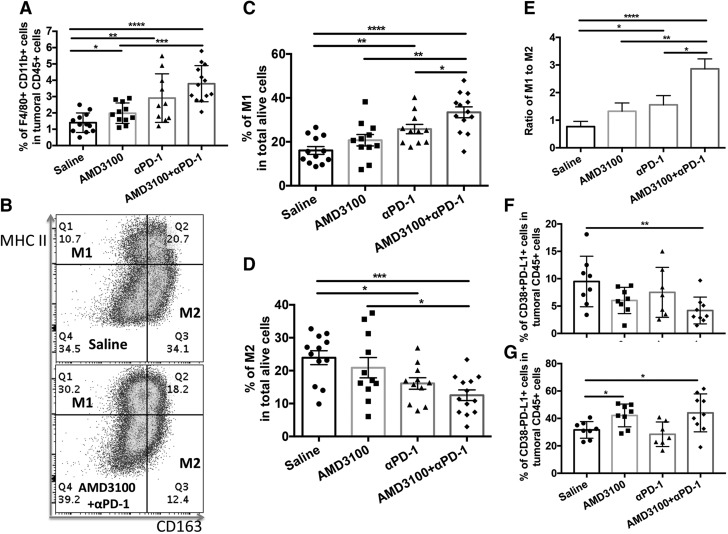
Combination therapy facilitates M1 polarization in tumor
Tumor-associated macrophages were phenotypically defined as EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80)+CD11b+ in the CD45+ cell population in tumor (26, 27). In tumor, the proportion of F4/80+CD11b+ cells was significantly increased in αPD-1 monotherapy and further increased in the combination therapy group (Fig. 7A). Among this population, the proportion of M1 macrophages [CD163−major histocompatibility complex class II (MHC II)+] was significantly increased in αPD-1 and combination treatment groups (P < 0.01, P < 0.0001, respectively) (Fig. 7B, C). In contrast, the proportion of M2 macrophages (CD163+MHC II−) was significantly decreased in αPD-1 and combination treatment groups (P < 0.05, P < 0.001, respectively) (Fig. 7C, D) compared with saline treatment. αPD-1 applied as monotherapy and in combination with AMD3100 significantly increased the ratio of M1:M2 (P < 0.05, P < 0.0001, respectively) compared with saline treatment (Fig. 7E). The combination treatment further increased the ratio of M1:M2 (P < 0.05) compared with αPD-1 treatment. We also found that PD-L1+CD38+ cells in the CD45+ population, which represent suppressive leukocytes (28, 29), were significantly decreased in the tumor in the combination treatment compared with the saline treatment group (Fig. 7F), whereas PD-L1+ CD38− cells, which might be immune-activating leukocytes, were elevated in AMD3100-applied groups (Fig. 7G). Taken together, these data support the view that combination therapy may facilitate M2-to-M1 polarization in the tumor microenvironment and thus enhance antitumor efficacy.
Figure 7.
Combination therapy showed beneficial antitumoral M1 macrophage polarization. A) Percentages of F4/80+CD11b+ macrophages in intratumoral CD45+ cell population. B) Representative dot plots of intratumoral M1 and M2 in saline (top) and combination (bottom) treatment. C) Percentages of M1 (CD163−MHC II+) in intratumoral F4/80+ CD11b+ cell population. D) Percentages of M2 (CD163+MHC II−) in intratumoral F4/80+CD11b+ cell population. E) Ratio of intratumoral M1:M2. F) Percentages of CD38+PD-L1+ cells in intramural CD45+ cell population. G) Percentages of CD38−PD-L1+ cells in intramural CD45+ cell population. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
DISCUSSION
Immune-checkpoint inhibitors have been widely investigated in preclinical studies and tested in clinical trials for various cancers. However, the effectiveness of monotherapy is limited to a minority of patients with cancer (30–32). It is generally accepted that combination immunotherapy is required to achieve maximal antitumor efficacy. Immunomodulators have been used in combination with tumor vaccines or immunotherapies to improve antitumor immune responses, which include removing or inhibiting suppressive cells such as Treg cells, type II NK Treg cells, or MDSCs from the intratumoral microenvironment (33–38). Consistent with previous findings that CXCR4 inhibition or blockade of CXCL12 in the tumor microenvironment facilitates PD-1–PD-L1 blockade immunotherapy in hepatocellular carcinoma (39), pancreatic cancer (40), and colorectal cancer (41), in the current study we demonstrated that a combinational blockade of the CXCL12-CXCR4 pathway by AMD3100 and the PD-1–PD-L1 pathway by αPD-1 enhances tumor control and prolongs animal survival in an immunocompetent mouse ovarian cancer model. Tumor control and animal survival benefits were associated with prevention of an immunosuppressive phenotype in the tumor microenvironment. Specifically, we found that αPD-1 alone or in combination with AMD3100 enhanced intratumoral infiltration of CD8+ T cells. Combination of αPD-1 and AMD3100 greatly enhanced IFN-γ and IL-2 production in intratumoral and ascites CD8+ T cells. Combination therapy increased intratumoral memory T cells. AMD3100 reduced intratumoral infiltration of Treg cells and facilitated conversion of Treg cells into T-helper cells in tumor. αPD-1 alone decreased intratumoral MDSCs and further enhanced this effect in combination with AMD3100. Combination therapy decreased suppressive leukocytes as well as facilitated M1 polarization in tumor. These results indicate that AMD3100 has broad potential in immunomodulation to improve antitumor efficacy when combined with tumor vaccines or other immunotherapeutic strategies.
The poor clinical outcome of ovarian cancer has been correlated with overexpression of CXCL12 and CXCR4 in the tumor microenvironment (42). We previously demonstrated that treatment with AMD3100, a CXCR4 antagonist, not only increased tumor apoptosis and necrosis by blockade of CXCL12 pathway but also modulated intratumoral Treg cells and enhanced T-cell–mediated antitumor immune responses in immunocompetent murine tumor models of ovarian cancer (15) and mesothelioma (16). Studies from other groups have also explored antitumor effects of AMD3100 on various cancers that express CXCR4 (43, 44), including colon cancer (45), brain cancer (46), skin cancer (47), breast cancer (48), prostate cancer (49), and bone cancer (50–52). However, most of these studies focused on the impact of AMD3100 on the CXCL12-CXCR4 axis on apoptotic and antimetastatic efficacy instead of exploring its impact on immunomodulation in the tumor microenvironment alone or in combination with immune-checkpoint blockade. In the current study, we investigated antitumor effects of AMD3100 alone or in combination with αPD-1 in an intraperitoneal disseminated orthotopic syngeneic and immunocompetent ovarian cancer mouse model. Both in vitro and in vivo data suggest that AMD3100 not only directly targets CXCR4-CXCL12 axis to inhibit tumor cell proliferation and migration but also prevents multifaceted immunosuppression in the tumor microenvironment by which tumor growth is suppressed and the life span of tumor-bearing mice is extended.
The results of improved antitumor efficacy of the combination of AMD3100 with immune-checkpoint inhibitors in this ovarian cancer mouse model are consistent with a previous report that AMD3100 facilitated αPD-L1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice by preventing the polarization toward an immunosuppressive microenvironment (39). Similarly, in a pancreatic cancer model, AMD3100 induced rapid T-cell accumulation in the tumor microenvironment and acted synergistically with αPD-L1 to diminish cancer cells (40). AMD3100 was also found to interact with effector neutrophils, thus enhancing the therapeutic efficacy of mAb alemtuzumab (anti-CD52) and rituximab (anti-20) in disseminated lymphoma models (53). Most notably, we found that AMD3100 promoted conversion of Treg cells into FoxP3+CD25− T helper–like cells that express IL-2 and CD40L, which is consistent with our previous findings in 2 mouse models of malignant mesothelioma (16). Reprogramming of Treg cells into T-helper–like cells with loss of CD25 and without loss of the transcription factor FoxP3 have also been reported in other studies (52–54). Increased IL-2 levels in the tumor microenvironment seen in this study may be a direct result of increased FoxP3+CD25− T-helper–like cells. In addition, IL-2 may also stimulate effector T-cell proliferation and cytotoxicity against tumor cells. It was previously reported that FoxP3+CD25+CD8+ T cells were a tightly controlled population sharing some developmental and phenotypic properties with FoxP3+CD25+CD4+ Treg cells but lacking potent suppressive activity (55). It was also reported that increased intratumoral FoxP3+CD25+CD8+ cells correlated with tumor progression in human gastric cancer (56). However, in this study, we found that the proportions of intratumoral FoxP3+CD8+ cells, which highly expressed FoxP3 and IFN-γ, were increased in tumor-bearing mice treated with AMD3100 alone or in combination with αPD-1. These data suggest that AMD3100-mediated increase of intratumoral FoxP3+CD8+ cells may be associated with tumor control (57, 58).
AMD3100 alone did not significantly increase intratumoral memory T cells. However, when combined with αPD-1, the combination significantly increased CD44+CD27+ T cells that represent a central memory T-cell phenotype (59). These cells can maintain long-term antitumor effects. AMD3100 did not decrease MDSCs in the tumor microenvironment. However, both αPD-1 monotherapy and the combination therapy significantly decreased MDSCs, which might be a direct result of immune-checkpoint blockade. IL-6 and IL-10 were reported to be secreted by both MDSCs and Treg cells that suppress effector immune cells (23–25). Although AMD3100 did not decrease MDSCs, it significantly inhibited Treg cells, which was associated with lower levels of IL-6 and IL-10 in ascites in AMD3100 monotherapy group. The combination therapy significantly down-regulated IL-6 and IL-10 in ascites.
We also found that the combination therapy induced significant M1 polarization of macrophages in tumor. CXCL12 was previously reported to drive the accumulation of tumor-associated macrophages in the hypoxic tumor environment (60) and support differentiation of monocytes toward an immunosuppressive phenotype (61). AMD3100, through inhibition of CXCL12-CXCR4 axis, facilitated M2-to-M1 polarization when combined with αPD-1 therapy, which would otherwise be reversed by elevated CXCL12 in ovarian tumor environment (62–64). This novel finding stands in contrast to a previous study in a glioblastoma tumor model in which AMD3100 increased the accumulation of both M1 and M2 macrophages in the tumor (65).
These results support the view that AMD3100 initiates immunomodulation of the intratumoral microenvironment through its impact on multiple immune-cell subpopulations which synergize with αPD-1 to more powerfully control tumor growth and prolong animal survival. In summary, we provide robust preclinical evidence for the improved antitumor efficacy of AMD3100 in combination with immune-checkpoint inhibitor αPD-1 for ovarian cancer. We believe this combinatorial strategy can be applied to other cancer types, including those expressing high or low levels of CXCR4 or CXCL12, because AMD3100 not only directly targets CXCR4-CXCL12 axis to inhibit tumor growth and metastasis but also acts as a potent immunomodulator to prevent the development of a multifaceted immunosuppressive intratumoral microenvironment. Our study supports the clinical testing of this combination immunotherapy with these 2 clinically approved agents in the context of ovarian cancer.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The ID8 cell line was a kind gift from Dr. Kathy Roby (University of Kansas Medical Center, Kansas City, KS, USA). This work was supported by the Vaccine and Immunotherapy Center (VIC) Innovation Fund and the Massachusetts General Hospital (MGH) Research Scholar Award (to H.C., Y.Z., and M.C.P.). Cytometric findings reported here were performed in the MGH Department of Pathology Flow and Image Cytometry Research Core, which is supported by the U.S. National Institutes of Health (NIH) Shared Instrumentation Grants 1S10OD012027-01A1, 1S10OD016372-01, 1S10RR020936-01, and 1S10RR023440-01A1. M.C.P. is the Scientific Founder of AperiSys. The remaining authors declare no conflicts of interest.
Glossary
- αPD-1
anti–PD-1
- AMD3100
plerixafor
- CXCL12
C-X-C motif chemokine 12
- CXCR4
C-X-C chemokine receptor type 4
- F4/80
EGF-like module-containing mucin-like hormone receptor-like 1
- FoxP3
forkhead box P3
- ID8-luc
luciferized ID8
- IVIS
In Vivo Imaging System
- MDSC
myeloid-derived suppressor cell
- MHC II
major histocompatibility complex class II
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- Treg
regulatory T
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Zeng, M. V. Callahan, J. A. Gelfand, H. Chen, and M. C. Poznansky conceived and designed the study; Y. Zeng, B. Li, P. M. Reeves, J. A. Gelfand, H. Chen, and M. C. Poznansky developed methodology; Y. Zeng, B. Li, Y. Liang, X. Qu, C. Ran, and H. Chen acquired data; Y. Zeng, B. Li, Y. Liang, X. Qu, H. Chen, and M. C. Poznansky analyzed and interpreted data; Y. Zeng, C. Ran, Q. Liu, M. V. Callahan, A. E. Sluder, J. A. Gelfand, H. Chen, and M. C. Poznansky participated in the writing, review, and revision of the manuscript; Y. Zeng, A. E. Sluder, H. Chen, and M. C. Poznansky provided administrative, technical, or material support; and H. Chen and M. C. Poznansky supervised the study.
REFERENCES
- 1.Siegel R., DeSantis C., Virgo K., Stein K., Mariotto A., Smith T., Cooper D., Gansler T., Lerro C., Fedewa S., Lin C., Leach C., Cannady R. S., Cho H., Scoppa S., Hachey M., Kirch R., Jemal A., Ward E. (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 62, 220–241 [DOI] [PubMed] [Google Scholar]
- 2.Bast R. C., Jr., Hennessy B., Mills G. B. (2009) The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer 9, 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantia-Smaldone G. M., Corr B., Chu C. S. (2012) Immunotherapy in ovarian cancer. Hum. Vaccin. Immunother. 8, 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duraiswamy J., Freeman G. J., Coukos G. (2013) Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 73, 6900–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad C., Gregorio J., Wang Y. H., Ito T., Meller S., Hanabuchi S., Anderson S., Atkinson N., Ramirez P. T., Liu Y. J., Freedman R., Gilliet M. (2012) Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 72, 5240–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H. (2008) PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H., Strome S. E., Salomao D. R., Tamura H., Hirano F., Flies D. B., Roche P. C., Lu J., Zhu G., Tamada K., Lennon V. A., Celis E., Chen L. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800; erratum: 1039 [DOI] [PubMed] [Google Scholar]
- 8.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 99, 12293–12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Z., Wang X., Cheng D., Xia Z., Luan M., Zhang S. (2014) PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 9, e89350; erratum: 12, e0186965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury P. S., Chamoto K., Honjo T. (2018) Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J. Intern. Med. 283, 110–120 [DOI] [PubMed] [Google Scholar]
- 11.Chodon T., Lugade A. A., Battaglia S., Odunsi K. (2018) Emerging role and future directions of immunotherapy in advanced ovarian cancer. Hematol. Oncol. Clin. North Am. 32, 1025–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luster A. D. (2002) The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14, 129–135 [DOI] [PubMed] [Google Scholar]
- 13.Zou W. (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274 [DOI] [PubMed] [Google Scholar]
- 14.Yan M., Jene N., Byrne D., Millar E. K., O’Toole S. A., McNeil C. M., Bates G. J., Harris A. L., Banham A. H., Sutherland R. L., Fox S. B. (2011) Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 13, R47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Righi E., Kashiwagi S., Yuan J., Santosuosso M., Leblanc P., Ingraham R., Forbes B., Edelblute B., Collette B., Xing D., Kowalski M., Mingari M. C., Vianello F., Birrer M., Orsulic S., Dranoff G., Poznansky M. C. (2011) CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res. 71, 5522–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B., Zeng Y., Reeves P. M., Ran C., Liu Q., Qu X., Liang Y., Liu Z., Yuan J., Leblanc P. R., Ye Z., Sluder A. E., Gelfand J. A., Brauns T. A., Chen H., Poznansky M. C. (2018) AMD3100 augments the efficacy of mesothelin-targeted, immune-activating VIC-008 in mesothelioma by modulating intratumoral immunosuppression. Cancer Immunol. Res. 6, 539–551 [DOI] [PubMed] [Google Scholar]
- 17.Drake C. G. (2012) Combination immunotherapy approaches. Ann. Oncol. 23 (Suppl 8), viii41–viii46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spranger S., Gajewski T. (2013) Rational combinations of immunotherapeutics that target discrete pathways. J. Immunother. Cancer 1, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roby K. F., Taylor C. C., Sweetwood J. P., Cheng Y., Pace J. L., Tawfik O., Persons D. L., Smith P. G., Terranova P. F. (2000) Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 21, 585–591 [DOI] [PubMed] [Google Scholar]
- 20.Sharma V., McNeill J. H. (2009) To scale or not to scale: the principles of dose extrapolation. Br. J. Pharmacol. 157, 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y., Li B., Reeves P., Ran C., Liu Z., Agha-Abbaslou M., Yuan J., Leblanc P., Sluder A., Gelfand J., Brauns T., Poznansky M., Chen H. (2016) Improved antitumoral efficacy of mesothelin targeted immune activating fusion protein in murine model of ovarian cancer. Int. J. Cancer Clin. Res. 3, 051 [Google Scholar]
- 22.Gabrilovich D. I., Bronte V., Chen S. H., Colombo M. P., Ochoa A., Ostrand-Rosenberg S., Schreiber H. (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res. 67, 425; author reply 426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart K. M., Byrne K. T., Molloy M. J., Usherwood E. M., Berwin B. (2011) IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front. Immunol. 2, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Che F., Heng X., Zhang H., Su Q., Zhang B., Chen Y., Zhang Z., Du Y., Wang L. (2017) Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol. Immunother. 66, 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flint T. R., Janowitz T., Connell C. M., Roberts E. W., Denton A. E., Coll A. P., Jodrell D. I., Fearon D. T. (2016) Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24, 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Goncalves R., Mosser D. M. (2008) The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. , Unit 14.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgoudaki A. M., Prokopec K. E., Boura V. F., Hellqvist E., Sohn S., Östling J., Dahan R., Harris R. A., Rantalainen M., Klevebring D., Sund M., Brage S. E., Fuxe J., Rolny C., Li F., Ravetch J. V., Karlsson M. C. (2016) Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 15, 2000–2011 [DOI] [PubMed] [Google Scholar]
- 28.Bu X., Kato J., Hong J. A., Merino M. J., Schrump D. S., Lund F. E., Moss J. (2018) CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis 39, 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karakasheva T. A., Waldron T. J., Eruslanov E., Kim S. B., Lee J. S., O’Brien S., Hicks P. D., Basu D., Singhal S., Malavasi F., Rustgi A. K. (2015) CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res. 75, 4074–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittica G., Genta S., Aglietta M., Valabrega G. (2016) Immune checkpoint inhibitors: a new opportunity in the treatment of ovarian cancer? Int. J. Mol. Sci. 17, 1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X., Lang J. (2016) The significance and therapeutic potential of PD-1 and its ligands in ovarian cancer: a systematic review. Gynecol. Oncol. 142, 184–189 [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Li F., Jiang F., Lv X., Zhang R., Lu A., Zhang G. (2016) A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int. J. Mol. Sci. 17, 1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berzofsky J. A., Terabe M., Wood L. V. (2012) Strategies to use immune modulators in therapeutic vaccines against cancer. Semin. Oncol. 39, 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berzofsky J. A., Ahlers J. D., Janik J., Morris J., Oh S., Terabe M., Belyakov I. M. (2004) Progress on new vaccine strategies against chronic viral infections. J. Clin. Invest. 114, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berzofsky J. A., Terabe M., Oh S., Belyakov I. M., Ahlers J. D., Janik J. E., Morris J. C. (2004) Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J. Clin. Invest. 113, 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi S. (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 [DOI] [PubMed] [Google Scholar]
- 37.Terabe M., Berzofsky J. A. (2004) Immunoregulatory T cells in tumor immunity. Curr. Opin. Immunol. 16, 157–162 [DOI] [PubMed] [Google Scholar]
- 38.Kusmartsev S., Gabrilovich D. I. (2006) Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol. Immunother. 55, 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Ramjiawan R. R., Reiberger T., Ng M. R., Hato T., Huang Y., Ochiai H., Kitahara S., Unan E. C., Reddy T. P., Fan C., Huang P., Bardeesy N., Zhu A. X., Jain R. K., Duda D. G. (2015) CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61, 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feig C., Jones J. O., Kraman M., Wells R. J., Deonarine A., Chan D. S., Connell C. M., Roberts E. W., Zhao Q., Caballero O. L., Teichmann S. A., Janowitz T., Jodrell D. I., Tuveson D. A., Fearon D. T. (2013) Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 110, 20212–20217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zboralski D., Hoehlig K., Eulberg D., Frömming A., Vater A. (2017) Increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol. Res. 5, 950–956 [DOI] [PubMed] [Google Scholar]
- 42.Scotton C. J., Wilson J. L., Scott K., Stamp G., Wilbanks G. D., Fricker S., Bridger G., Balkwill F. R. (2002) Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 62, 5930–5938 [PubMed] [Google Scholar]
- 43.Xu D., Li R., Wu J., Jiang L., Zhong H. A. (2016) Drug design targeting the CXCR4/CXCR7/CXCL12 pathway. Curr. Top. Med. Chem. 16, 1441–1451 [DOI] [PubMed] [Google Scholar]
- 44.Scala S. (2015) Molecular pathways: targeting the CXCR4-CXCL12 axis--untapped potential in the tumor microenvironment. Clin. Cancer Res. 21, 4278–4285 [DOI] [PubMed] [Google Scholar]
- 45.Heckmann D., Maier P., Laufs S., Wenz F., Zeller W. J., Fruehauf S., Allgayer H. (2013) CXCR4 expression and treatment with SDF-1α or plerixafor modulate proliferation and chemosensitivity of colon cancer cells. Transl. Oncol. 6, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabouret E., Tchoghandjian A., Denicolai E., Delfino C., Metellus P., Graillon T., Boucard C., Nanni I., Padovani L., Ouafik L., Figarella-Branger D., Chinot O. (2015) Recurrence of glioblastoma after radio-chemotherapy is associated with an angiogenic switch to the CXCL12-CXCR4 pathway. Oncotarget 6, 11664–11675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarchio S. N. E., Scolyer R. A., Beaugie C., McDonald D., Marsh-Wakefield F., Halliday G. M., Byrne S. N. (2014) Pharmacologically antagonizing the CXCR4-CXCL12 chemokine pathway with AMD3100 inhibits sunlight-induced skin cancer. J. Invest. Dermatol. 134, 1091–1100 [DOI] [PubMed] [Google Scholar]
- 48.Li H., Wang K., Yang X., Zhou Y., Ping Q., Oupicky D., Sun M. (2017) Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 53, 399–413 [DOI] [PubMed] [Google Scholar]
- 49.Domanska U. M., Timmer-Bosscha H., Nagengast W. B., Oude Munnink T. H., Kruizinga R. C., Ananias H. J., Kliphuis N. M., Huls G., De Vries E. G., de Jong I. J., Walenkamp A. M. (2012) CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia 14, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benslimane-Ahmim Z., Pereira J., Lokajczyk A., Dizier B., Galy-Fauroux I., Fischer A. M., Heymann D., Boisson-Vidal C. (2017) Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR4 axis and promotes tumour development by increasing neovascularization. Cancer Lett. 395, 11–19 [DOI] [PubMed] [Google Scholar]
- 51.Jiang C., Fang X., Zhang H., Wang X., Li M., Jiang W., Tian F., Zhu L., Bian Z. (2017) AMD3100 combined with triptolide inhibit proliferation, invasion and metastasis and induce apoptosis of human U2OS osteosarcoma cells. Biomed. Pharmacother. 86, 677–685 [DOI] [PubMed] [Google Scholar]
- 52.Liao Y. X., Fu Z. Z., Zhou C. H., Shan L. C., Wang Z. Y., Yin F., Zheng L. P., Hua Y. Q., Cai Z. D. (2015) AMD3100 reduces CXCR4-mediated survival and metastasis of osteosarcoma by inhibiting JNK and Akt, but not p38 or Erk1/2, pathways in in vitro and mouse experiments. Oncol. Rep. 34, 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu Y., Gale M., Shields J., Garron C., Swistak M., Nguyen T. H., Jacques G., Fogle R., Siders W., Kaplan J. (2012) Enhancement of the anti-tumor activity of therapeutic monoclonal antibodies by CXCR4 antagonists. Leuk. Lymphoma 53, 130–138 [DOI] [PubMed] [Google Scholar]
- 54.Sharma M. D., Hou D. Y., Baban B., Koni P. A., He Y., Chandler P. R., Blazar B. R., Mellor A. L., Munn D. H. (2010) Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity 33, 942–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma M. D., Huang L., Choi J. H., Lee E. J., Wilson J. M., Lemos H., Pan F., Blazar B. R., Pardoll D. M., Mellor A. L., Shi H., Munn D. H. (2013) An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity 38, 998–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komatsu N., Mariotti-Ferrandiz M. E., Wang Y., Malissen B., Waldmann H., Hori S. (2009) Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA 106, 1903–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer C. T., Floess S., Baru A. M., Lahl K., Huehn J., Sparwasser T. (2011) CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur. J. Immunol. 41, 716–725 [DOI] [PubMed] [Google Scholar]
- 58.Peng L. S., Zhuang Y., Shi Y., Zhao Y. L., Wang T. T., Chen N., Cheng P., Liu T., Liu X. F., Zhang J. Y., Zuo Q. F., Mao X. H., Guo G., Lu D. S., Yu P. W., Zou Q. M. (2012) Increased tumor-infiltrating CD8(+)Foxp3(+) T lymphocytes are associated with tumor progression in human gastric cancer. Cancer Immunol. Immunother. 61, 2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaka A. S., Shaffer D. R., Hartmaier R., Leen A. M., Lu A., Bear A., Rooney C. M., Foster A. E. (2009) Genetic modification of T cells with IL-21 enhances antigen presentation and generation of central memory tumor-specific cytotoxic T-lymphocytes. J. Immunother. 32, 726–736; erratum: 919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S. C., Hong J. H., Hsueh C., Chiang C. S. (2012) Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab. Invest. 92, 151–162 [DOI] [PubMed] [Google Scholar]
- 61.Sánchez-Martín L., Estecha A., Samaniego R., Sánchez-Ramón S., Vega M. A., Sánchez-Mateos P. (2011) The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117, 88–97 [DOI] [PubMed] [Google Scholar]
- 62.Ceradini D. J., Kulkarni A. R., Callaghan M. J., Tepper O. M., Bastidas N., Kleinman M. E., Capla J. M., Galiano R. D., Levine J. P., Gurtner G. C. (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 10, 858–864 [DOI] [PubMed] [Google Scholar]
- 63.Szebeni G. J., Vizler C., Kitajka K., Puskas L. G. (2017) Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm. 2017, 9294018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 [DOI] [PubMed] [Google Scholar]
- 65.Shaaban S., Alsulami M., Arbab S. A., Ara R., Shankar A., Iskander A., Angara K., Jain M., Bagher-Ebadian H., Achyut B. R., Arbab A. S. (2016) Targeting bone marrow to potentiate the anti-tumor effect of tyrosine kinase inhibitor in preclinical rat model of human glioblastoma. Int. J. Cancer Res. 12, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.