Abstract
Skin resident T cells provide immediate immunologic responses at their specific location and play a role in the pathogenesis of skin diseases such as psoriasis. Recently, IL-9–producing T cells were described as a major T-cell subtype present in the skin, but knowledge on the biology and in situ regulation of this T-cell subtype is scarce. Here, we investigated the cytokine influence on skin T cells with focus on IL-9–producing T cells because a better understanding of their biology may identify novel therapeutic approaches. Healthy human skin biopsies were cultured either in the presence of IL-2, IL-4, and TGF-β [T helper (Th)9–promoting condition (Th9-PC)] or IL-2 and IL-15 [standard condition (SC)]. Paired analysis of enzymatically isolated skin T cells and emigrated T cells after 4 wk of skin culture showed significant alterations of T-cell phenotypes, cytokine production, and IL-9–producing T-cell frequency. RNA sequencing analysis revealed differentially regulated pathways and identified CXCL8 and CXCL13 as top up-regulated genes in Th9-PC compared with SC. Functionally supernatant of stimulated skin-derived T cells, CXCL8 and CXCL13 increased neutrophil survival. We report that the cytokine environment alters skin-derived T-cell phenotype and functional properties.—Kienzl, P., Polacek, R., Reithofer, M., Reitermaier, R., Hagenbach, P., Tajpara, P., Vierhapper, M., Gschwandtner, M., Mildner, M. Jahn-Schmid, B., Elbe-Bürger, A. The cytokine environment influence on human skin–derived T cells.
Keywords: T-cell plasticity, IL-9, neutrophil, IL-8, CXCL13
Human skin not only forms a physical barrier to the outside environment but also harbors a multitude of cells of the innate and adaptive immune system, thereby constituting the immunologic barrier (1–4). Clark et al. (5) estimated that ∼20 billion resident T cells are present in the healthy skin of an average individual, which react locally and rapidly to invading pathogens. A major role for their retainment in the skin is attributed to the early activation marker CD69 that is able to negatively regulate the migration molecule sphingosine-1-phosphate receptor 1, and thereby prevents T cells from leaving the skin (6–8). CD103 was described as another skin homing marker (9, 10). Cutaneous lymphocyte-associated antigen (CLA) allows T cells to tether to blood vessel walls, enabling them to exit the bloodstream and migrate into skin (11, 12), and C-C motif chemokine receptor 4 senses chemokine signals that guide the cell from the bloodstream into skin tissue (13).
T helper (Th)9 cells, characterized by the expression of IL-9, have recently been described as the Th cell subset present in the skin apart from regulatory T (Treg) cells, Th1, Th2, and Th17 cells and are also found under steady-state conditions (14–16). They are involved in the immune reaction against extracellular pathogens, especially against Candida albicans, and mediate antitumor immunity against melanoma in a mouse model (14, 17). In addition, IL-9+ T cells are found in psoriatic and atopic dermatitis lesions, suggesting a possible role in inflammatory skin diseases (14) as well as in mycosis fungoides (18).
The combined effects of IL-2, IL-4, and TGF-β on naive T cells promote their differentiation into Th9 cells (19, 20). Skin can potentially provide a milieu favoring Th9 differentiation because keratinocytes are well-known producers of TGF-β (21–23), and IL-4 is released by activated antigen-presenting cells (3, 21, 24) and type 2 innate lymphoid cells (25). IL-2 is primarily produced by activated CD4+ T cells (26) as demonstrated for inflammatory skin diseases (27). IL-2 promotes IL-9 gene expression via the signal transducer and activator of transcription (STAT) 5 and regulates the expression of IL-4Rα (19, 28). IL-4 does not impact IL-9 expression directly but rather through the sSTAT6-mediated induction of the IL-9 transcription factor IFN regulatory factor 4 (IRF4), among others. PU.1 (SPI1) was described as an important transcription factor for IL-9 production and is regulated by TGF-β (29, 30). Nonetheless, no master transcription factor as found in other Th cell subsets has been identified yet (20). In addition, to our knowledge, it is currently not possible to isolate viable IL-9–producing T cells because of a lack of specific surface marker proteins.
Here, we studied the influence of the cytokine milieu on emigrating skin-derived T cells and found significant phenotypical and functional alterations. Because most studies on Th9 cells were conducted in mice, we aimed to characterize IL-9–producing T cells in healthy human skin in more detail. Skin IL-9–producing T cells exhibited a plastic phenotype dependent on the cytokine milieu but did not coproduce signature cytokines from Th1, Th2, or Th17 cells.
MATERIALS AND METHODS
Details on experimental procedures are provided in the Supplemental Data.
Study approval
This study was approved by the Ethics Committee of the Medical University of Vienna. Healthy human skin was obtained from patients undergoing cosmetic surgeries after informed consent was obtained and according to the Declaration of Helsinki. Experiments were performed within the first 24 h after surgical removal.
Skin T-cell isolation
For freshly isolated (FI) T-cell stimulation experiments, Collagenase P (Roche, Basel, Switzerland) digestion of fresh human skin was used essentially as previously described (31). In brief, dermatome-cut skin specimens were digested in complete Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (complete RPMI) containing 1 mg/ml Collagenase P (Roche) at 37°C on a rotator for 3 h. Next, 200 U/ml DNase I (Roche) were added and incubated for 15 min at 37°C. The cell suspension was diluted with 10 mM EDTA/PBS and filtered through 100- and 40-µm cell strainers. Dead cells were eliminated using a Dead Cell Removal Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), and T cells were enriched (purity range: 91–98%) with CD2 microbeads (Miltenyi Biotec) according to manufacturer’s instructions. Next, either IL-9 staining was performed or cells were further stimulated. For flow-cytometric analysis of surface proteins on FI skin T cells, the Whole Skin Dissociation Kit (Miltenyi Biotec) was used according to manufacturer’s instructions to digest human skin.
Skin explant cultures
Skin explant cultures were prepared as previously described in Clark et al. (5) with modifications. Briefly, skin punch biopsies (5 mm) were minced, subsequently loaded onto collagen G (Biochrom, Berlin, Germany)–coated tantalum cell foam matrix grids (Ultramet, Pacoima, CA, USA), and placed in 24-well plates submerged in complete RPMI 1640, either with 100 U/ml IL-2, 2 ng/ml IL-4, and 10 ng/ml TGF-β or 100 U/ml IL-2 and 10 ng/ml IL-15 (Peprotech, Rocky Hill, NJ, USA). The IL-4 and TGF-β concentrations for maximal differentiation and expansion of Th9 cells in our skin explant model had to be evaluated in a set of experiments (unpublished results) because, to our knowledge, there are no publications utilizing this specific cytokine combination in this setting in humans. Cells were harvested after 4 wk of culture and either analyzed by flow cytometry or further stimulated.
T-cell stimulation
Cells were seeded in round-bottom 96-well plates at a density of 5 × 105/ml in complete RPMI 1640 medium containing 50 µM 2-mercaptoethanol (Thermo Fisher Scientific) and Dynabeads Human T-Activator CD3/CD28 (bead-to-cell ratio of 1:1; Thermo Fisher Scientific). After 3 d of culture, beads were magnetically removed and the cells incubated in complete RPMI 1640 medium supplemented with 1× cell-stimulation cocktail (CSC; Thermo Fisher Scientific) plus transport inhibitors for 4 h. After harvesting, an aliquot was stained for flow cytometry, and the other cells were layered on Ficoll Paque Plus (GE Healthcare, Waukesha, WI, USA) for density gradient centrifugation to remove dead cells and debris. Finally, adhesion slides, RNA, and protein lysates were prepared.
Immunofluorescence staining
Antibodies are specified in Supplemental Table S1. Species and isotype-specific secondary antibodies αrabbit Alexa Fluor 488 and αmouse (H+L) Alexa Fluor 546 (Thermo Fisher Scientific) were used. Images were acquired using an AX70 Microscope (Olympus, Tokyo, Japan) with the imaging software MetaMorph (Molecular Devices, Sunnyvale, CA, USA) or an LSM 500 Microscope (Carl Zeiss, Oberkochen, Germany) equipped with Zen software (Carl Zeiss).
Flow cytometry
Fluorochrome-labeled antibodies are listed in Supplemental Table S1. Samples were acquired on a BD FacsVerse Flow Cytometer (BD Biosciences, San Jose, CA, USA) with FACSuite v.1.0.5.3841 software (BD Biosciences) and analyzed using FlowJo v.10.0.7 (BD Biosciences).
Analysis of cytokine concentrations
Supernatants from skin explant cultures after 4 wk and after αCD3 and αCD28 bead stimulation of emigrated skin T cells after 4 wk of explant culture were collected for bead array analysis with LegendPlex Human Th Cytokine Panel (13-plex; BioLegend, San Diego, CA, USA). The assay was performed according to the manufacturer’s instructions. Cytokine concentrations were calculated using the LegendPlex v.7.0 software tool (BioLegend).
Western blot
FI, bead-stimulated, and CSC-restimulated skin T cells were lysed in SDS-PAGE loading buffer, sonicated, and denatured with 0.1 M DL-DTT (MilliporeSigma).
Transcriptome analyses
E.Z.N.A. MicroElute Total RNA Kit (Omega Bio-tek, Norcross, GA, USA) with DNase I On-Column Digestion was used for the isolation of total RNA according to the manufacturer’s instructions. Sequencing libraries were prepared (Core Facility Genomics, Medical University of Vienna) using the NebNext Ultra Directional RNA Library Prep Kit for Illumina according to the manufacturer’s protocols (New England Biolabs, Ipswich, MA, USA).
Isolation of peripheral blood mononuclear cells and culture
Living peripheral blood mononuclear cells (PBMCs) and protein lysates thereof (Fig. 1D) were prepared essentially as previously described in Gschwandtner et al. (32). Cells were cultured in serum-free TexMacs (Miltenyi Biotec) medium alone or with chemokine (C-X-C motif) ligand 8 (100 ng/ml), CXCL13 (100 ng/ml), or both cytokines combined in round-bottom 96-well plates at 37°C, 5% CO2, and 95% humidity for 48 h. Dead cells were excluded with fixable viability dye eFluor 450 (Thermo Fisher Scientific). The following antibodies were used for flow cytometry staining: CD3-PE, CD4-Brilliant Violet 510, CD8-allophycocyanin (APC)-Vio770, CLA-FITC, CCR4-APC (clone 205410), CD69-PE-Cy7, and CD103-PerCP-eFluor710 (Supplemental Table S1). Analysis was carried using BD FACSVerse.
Figure 1.
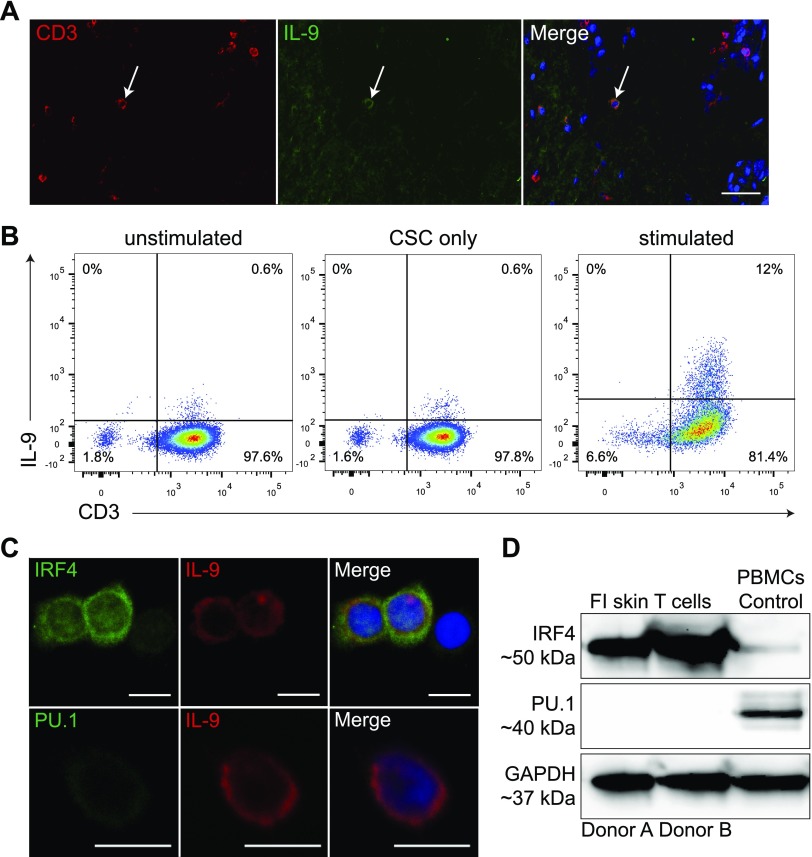
IL-9–producing T cells in healthy human skin express IRF4. A) Representative 2-color immunofluorescence staining of CD3 and IL-9 in cryosections of human skin (n = 3). White arrow marks an IL-9+ T cell within the lower dermal compartment. Scale bar, 50 µm. B) Flow cytometric analysis of IL-9+ T cells in FI unstimulated (left), FI CSC-stimulated (middle), and 3-d αCD3- and αCD28-stimulated and CSC-restimulated skin-derived T cells (right). Quadrants were set according to isotype-matched control staining. C) Two-color immunofluorescence staining for IRF4 and IL-9 (upper panel) and PU.1 and IL-9 (lower panel). FI skin T cells were αCD3 and αCD28 bead–stimulated and CSC-activated. Shown is one representative experiment of 3 independent donors. Scale bar, 10 µm. D) Protein expression of indicated markers from 3-d αCD3 and αCD28 bead–stimulated and CSC-restimulated FI skin T cells from 2 representative donors was analyzed by Western blot, and 2 representative donors of 5 are shown. PBMC lysate was included as control.
Migration assay
PBMCs and neutrophils (purity >95%) were isolated as described in Gschwandtner et al. and Polak et al. (32, 33), and 2 × 105 cells in 30 μl RPMI 1640 medium were added onto the membrane of the migration system (ChemoTx; Neuro Probe, Gaithersburg, MD, USA). The lower compartment was filled with 300 μl of 100 ng/ml CXCL8 (neutrophils) or CXCL13 (PBMCs). After 2 h, cells were harvested and counted using BD FACSVerse.
Cytokine stimulation of neutrophils and human myeloperoxidase ELISA
Isolated neutrophils were cultured in 200 μl RPMI 1640 medium at a cell density of 103/μl containing 11% supernatant of stimulated T cells derived from 4-wk skin explant cultures or complete RPMI 1640 medium as control (Supplemental Fig. S3D, E) or in RPMI 1640 medium containing 100 ng/ml CXCL8 or CXCL13 for 24 h. Before and after culture, viability of neutrophils was assessed using Annexin V and 7-aminoactinomycin D (7-AAD) staining. For this, cells were first incubated with Annexin V Alexa Fluor 488 antibody (Thermo Fisher Scientific) in Annexin V Binding Buffer (BioLegend) for 15 min, and then 7-AAD (BD Biosciences) was added before analysis by flow cytometry.
Human myeloperoxidase (MPO) concentrations in cell and skin culture supernatants were determined by the Human MPO DuoSet ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Absorbance was measured using a Multiskan photometer (Thermo Fisher Scientific) at 450 nm, and background was subtracted before fitting the values to a 4-parametric logistic model of the standards.
Statistical analysis
Graphical illustrations and statistical computations were performed using Prism 6 (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were regarded as significant. The designation n in figure legends generally refers to biologic replicates, except when stated otherwise.
RESULTS
The transcription factor IRF4, but not PU.1, is expressed in dermal IL-9–producing T cells
Two-color immunofluorescence staining on healthy human skin cryosections revealed that most of the CD3+IL-9+ T cells were located in the dermis but never in the epidermis (Fig. 1A and Supplemental Fig. S1A), confirming previously published data in Schlapbach et al. (14). In addition, CD3−IL-9+ cells were found within the dermis and most likely represent mast cells or innate lymphoid cells, which are known to secrete IL-9 (34–38). Seventeen percent of αCD3 and αCD28 bead–stimulated and CSC-restimulated FI dermal T cells were positive for IL-9, whereas only very few unstimulated or CSC-stimulated dermal T cells were IL-9+ (Fig. 1B). IRF4, but not PU.1, was identified in the cytoplasm and nucleus in IL-9+ T cells (Fig. 1C). The staining patterns of IRF4 and IL-9 are similar to previously published results in thymic cells and skin T cells, respectively (14, 39). Immunofluorescence data were confirmed by qualitative Western blot analysis with identical results (Fig. 1D).
Cytokine environment influences the phenotype of human skin–derived T cells
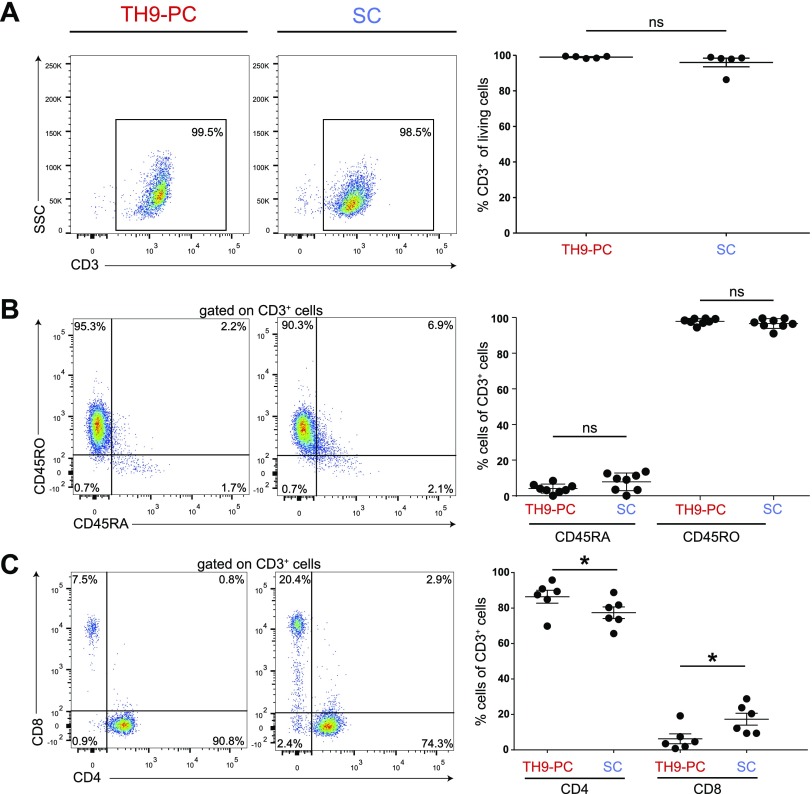
Next, we investigated the cytokine influence on human skin–derived T cells and the potential to propagate IL-9–producing T cells in an adapted ex vivo skin culture model to learn more about their biology (40). In the presence of either Th9-promoting conditions (Th9-PC; IL-2, IL-4, and TGF-β) or standard conditions (SCs; IL-2 and IL-15), the number of cells emigrated per well steadily increased. Approximately 15% of the cells, irrespective of the cytokines provided, were Ki-67+ after 4 wk, suggesting that proliferation contributes to overall cell numbers (Supplemental Fig. S1B, C). Emigrated cells from Th9-PC were exclusively CD45RO+CD45RA−CD3+ memory T cells, whereas from SC (besides the overwhelming majority of CD45RO+CD45RA−CD3+ T cells) (Fig. 2A, B), a small CD3− population (range: 1.7–16.0%) was consistently observed and consisted mainly of CD56+ natural killer (NK) cells (Supplemental Fig. S1D). Under both conditions, we repeatedly found more CD4+ T cells than CD8+ T cells (Fig. 2C). Equal numbers of cells expressed the Treg cell master transcription factor forkhead box P3 under both culture conditions (Supplemental Fig. S1E). However, Treg-differentiating cytokines (IL-2 and TGF-β) increased FoxP3+ T-cell densities significantly (Supplemental Fig. S1E). As hypothesized, significantly more IL-9+ T cells were found in Th9-PC as compared with SC (Fig. 2D). Frequencies of CLA+ and CD103+ T cells were unchanged under both culture conditions. In contrast, CCR4+ and CD69+ T-cell frequencies were extremely low under SC and Th9-PC, respectively (Fig. 2E, F). In accordance with our flow cytometry data (Fig. 2D), a significantly higher IL-9 concentration was observed in Th9-PC compared with SC when supernatants were analyzed after 4 wk of culture. The Th2-associated cytokine IL-13 was significantly higher under SC, and IFN-γ levels were below detection limit under Th9-PC (Supplemental Fig. S1F).
Figure 2.
Phenotypic profile of T cells harvested from skin explant cultures after 4 wk. Representative flow cytometry dot plots (left) and quantification statistics (right; n = 5–8) of the indicated surface markers on cells cultured under Th9-PC or SC (SSC/CD3, A; CD45RO/CD45RA, B; CD8/DC4, C, IL-9/CD3, D; CCR4/CLA, E; CD69/CD103, F). Cells (D) were αCD3 and αCD28 bead–stimulated and CSC-restimulated 3 d. Quadrants were set according to isotype-matched control staining. Ns, not significant. Means ± sem are shown, and data were analyzed using Wilcoxon’s rank-sum test. *P ≤ 0.05.
Culture conditions change skin homing–marker expression and are crucial for maintaining the IL-9–expressing phenotype
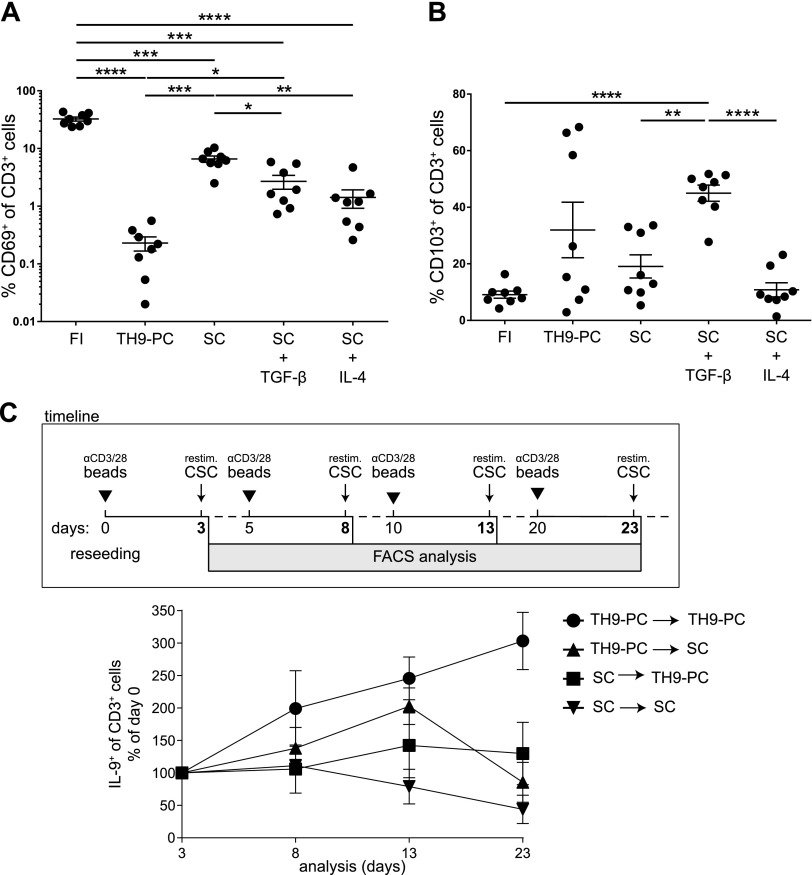
To compare the skin homing–marker profile on T cells before and after culture from the same donors (paired data), T cells were isolated from whole-skin specimens directly (FI) and harvested after 4 wk of skin explant culture. FI T cells expressed very high levels of CD69, whereas a significantly lower frequency of CD69+ T cells was observed under both culture conditions, especially under Th9-PC (Fig. 3A). To identify the responsible cytokine, cells from FI, Th9-PC, SC, SC plus TGF-β, or SC plus IL-4 were analyzed. Both cytokines significantly diminished the frequency of CD69+ T cells, but the stronger effect was exerted by IL-4 (Fig. 3A). Analysis of FI T cells revealed a low frequency of CD103+ T cells. Surprisingly, their density increased upon skin explant culture and was higher under Th9-PC as compared with SC when using the same culture setup as described for CD69. Here, the effect was mediated by TGF-β but not by IL-4 (Fig. 3B). Applying the same experimental strategy, we studied CCR4 expression on skin T cells and changes upon the addition of either TGF-β or IL-4. Neither TGF-β nor IL-4 restored the CCR4+ T-cell frequency when compared with the Th9-PC group, suggesting an additive effect of TGF-β and IL-4 on CCR4 expression (Supplemental Fig. S3A).
Figure 3.
Culture conditions alter CD69 and CD103 expression on skin T cells. A, B) Percentage of CD69 and CD103 cells among skin T cells (FI) or harvested after 4 wk from skin explant cultures under indicated conditions. Paired data are means ± sem and were analyzed using repeated measures with 1-way ANOVA (Holm-Sidak post hoc). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Only significant differences are indicated. C) T cells from 4-wk cultures were reseeded (d 0; 105 T cells/well) and stimulated as well as restimulated according to the scheme. Percentage of IL-9+ T cells upon reseeding T cells under indicated conditions. Before each analysis, cells were αCD3 and αCD28 bead–stimulated and CSC-restimulated 3 d at the indicated time points (n = 3). Data are means ± sem.
To test the stability of the IL-9–producing T-cell phenotype, T cells from both culture conditions were reseeded after 4 wk either in their previous cytokine milieu or in the respective other cytokine condition (Fig. 3C, scheme). As expected, the IL-9+ T-cell frequency steadily increased under continuous Th9-PC, whereas it initially slightly increased and then remained constant when cells were reseeded from SC to Th9-PC. Intriguingly, the IL-9+ T-cell frequency among T cells that were previously cultured under Th9-PC and further cultured under SC increased up to d 10 and later decreased (Fig. 3C). Taken together, these data suggest a cytokine influence on skin homing–marker expression and a dependence of IL-9 expression of T cells on the cytokine environment.
The vast majority of IL-9–producing T cells do not coexpress Th1, Th2, or Th17 signature cytokines
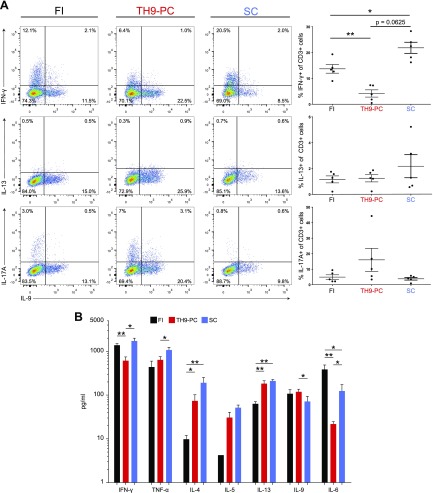
IL-9 was originally described as Th2 cytokine because it can be coproduced together with the classic Th2 cytokines. Furthermore, Th17 cells can express IL-9. However, bona fide Th9 cells are considered to solely produce IL-9. The characterization of the cytokine profile of FI as well as emigrated T cells from skin explants after 4 wk of culture and after αCD3 and αCD28 and CSC stimulation, irrespective of the culture conditions, revealed that IL-9–producing T cells did not generally coexpress cytokines of other Th cell subsets but represented a distinct T-cell subset (Fig. 4A). Among multiple Th-type cytokines tested in supernatants of αCD3 and αCD28 bead–stimulated FI and emigrated skin T cells, IFN-γ and TNF-α concentrations were highest. IL-4, IL-5, and IL-13 were extremely low in FI T-cell supernatants and low in supernatants of emigrated T cells, irrespective of the culture conditions. In contrast, IL-9 levels were significantly higher under Th9-PC compared with SC. IL-6 was significantly lower in supernatants of cultured T cells and lowest under Th9-PC compared with FI T cells (Fig. 4B). Taken together, IL-9–producing T cells do not coexpress other important Th-type cytokines. Furthermore, FI and emigrated skin T cells have a differential cytokine expression profile.
Figure 4.
IL-9+ skin-derived T cells do not coexpress Th1, Th2, or Th17 signature cytokines. A) Flow cytometric dot plots with indicated cytokine analyses and gating on CD3 (left panel) after cells were stimulated with αCD3 and αCD28 beads for 3 d and restimulated with CSC for 4 h. Quadrants were set according to isotype-matched control staining. Percentage of cytokine-expressing T cells among total CD3+ T cells was determined from 5 different donors (right panel). B) Cytokine concentrations in supernatants from αCD3 and αCD28 bead–stimulated cultures 3 d were determined by cytokine bead array (n = 7/group). In the IL-5 group with FI cells, cytokine concentrations of only 2 donors were above the detection limit of the bead array. Data represent means ± sem. FI vs. Th9-PC and SC were compared using a Mann-Whitney U test and Th9-PC vs. SC using Wilcoxon’s rank-sum test. *P ≤ 0.05, **P ≤ 0.01.
The cytokine environment influences the transcriptional profile of emigrated T cells
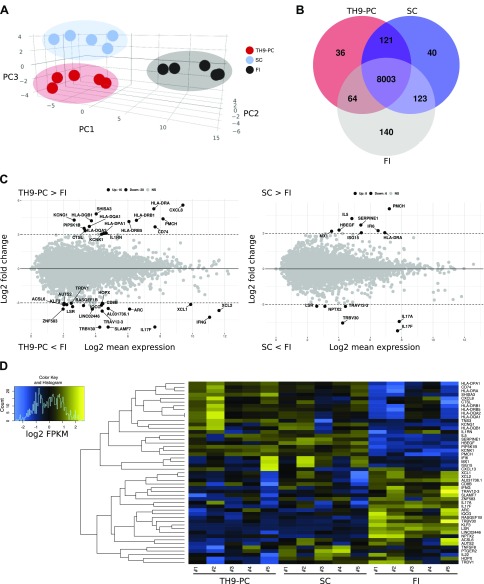
RNA-sequencing (RNA-seq) analysis was performed, and principal component analysis revealed distinct populations of FI, Th9-PC, and SC (Fig. 5A). Ninety-three percent of detected genes were present in T cells in all analyzed conditions (Fig. 5B). The CXCL8 (IL-8) gene as well as genes associated with antigen presentation, such as human leukocyte antigen (HLA)-DP, HLA-DQ, and HLA-DR, were up-regulated, whereas IFN-γ, IL-17F, CD8, and variants of T-cell receptor α, β, and δ chains were down-regulated genes in Th9-PC compared with FI T cells (Fig. 5C, D, and Supplemental Table S2). Genes involved in lymphocyte-mediated immunity, leukocyte activation, and antigen binding were shared between Th9-PC and FI T cells. In addition, functional annotation analysis showed enrichment in gene sets associated with cell-cell adhesion (Supplemental Fig. S2D). Conversely, genes associated with antiviral response (MX1, ISG15, and IFI6) were higher in SC than in FI (Fig. 5D and Supplemental Fig. S2D). CXCL8 and CXCL13 ranged among the most up-regulated genes, whereas IL-22 and IFN-γ were down-regulated genes in Th9-PC compared with SC T cells (Supplemental Fig. S2A). IFN-γ was similarly regulated on the protein level (Fig. 4A). IL-17A and IL-17F were significantly down-regulated in SC compared with FI (Fig. 5C), a trend that was also seen on the protein level (Fig. 4A). Determination of HLA-DRB1 expression levels on T cells by flow cytometry confirmed a higher HLA-DR–positive T-cell frequency in Th9-PC than in SC (Supplemental Fig. S2B). Although IRF4 transcripts were identified by RNA-seq, PU.1 transcripts were undetectable (Supplemental Table S2), which is in line with our data on the protein level (Fig. 1D, E). Importantly, IL-9 mRNA was significantly higher in Th9-PC than in SC (P = 0.03) (Supplemental Fig. S2C). Our data indicate that the cytokine milieu influences the transcriptional profile of skin T cells.
Figure 5.
Culture conditions affect the transcriptional profile of skin T cells. RNA-seq analysis of Th9-PC, SC, and FI T cells of 5 healthy individuals. A) Principal component analysis of the 200 most significantly differentially regulated genes. Color-coded dots represent individual donors from indicated conditions. Principal components 1 and 2 accounted for 83.1% of the total variation. Color-coded ellipses mark clusters. B) Venn diagram of expressed genes [mean fragments/kb of transcript/106 (FPKM) >1] among each group. C) M (log ratio)/A (mean average)-plot (log2 fold change against log2 mean expression) showing differential gene expression between indicated conditions. Significantly regulated genes with more than 8-fold change were labeled with their respective gene symbol. D) Heatmap illustrating gene expression of differentially regulated genes selected from MA-plot analyses in 5 individuals per condition. Rows represent clustered genes and columns represent donors of each condition.
To investigate the potential involvement of our top hits CXCL8 and CXCL13 in skin homing of T cells, we treated PBMCs isolated from human peripheral blood with either cytokine alone or a combination of both. After 48 h of culture, we determined the percentage of CLA+, CCR4+, CD69+, and CD103+ T cells using flow cytometry. No significant differences were observed between the tested groups (Supplemental Fig. S3B). As a positive control experiment, we performed a migration assay, which showed that significantly more purified neutrophils and PBMCs migrated through the filter membrane when the respective cytokines were present compared with the medium control (Supplemental Fig. S3C).
Previous work showed that CXCL8 is an important mediator of neutrophil survival via the effects of the serine and threonine protein kinase B (41). To test if the supernatant produced by stimulated T cells derived from 4-wk skin explant cultures affect neutrophil survival, purified neutrophils from human blood were cultured in medium containing supernatant for 24 h. Thereafter, cell survival was assessed by Annexin V and 7-AAD staining. Indeed, we found that the supernatant of stimulated T cells increased the survival of neutrophils significantly when compared with medium control. No significant difference was observed with supernatants from stimulated T cells derived from either Th9-PC or SC cultures (Supplemental Fig. S3D). MPO is an important enzyme for the generation of reactive oxygen species (42) because it can be produced by neutrophils and kill microbiota (43). When the MPO concentration was determined at the end of the neutrophil culture, we found that compared with the medium control, significantly less MPO was measured in the presence of supernatants derived from stimulated T cells of Th9-PC or SC cultures (Supplemental Fig. S3E). Unexpectedly, both recombinant CXCL8 and CXCL13 significantly increased neutrophil survival (Supplemental Fig. S3F). These data highlight a role of the factors produced by stimulated skin-derived T cells and, in particular, CXCL8 as well as CXCL13 in neutrophil immunobiology.
DISCUSSION
Over the years, several T-cell subsets have been identified and characterized. Adding more to the complexity is the fact that T cells can be very flexible and, for example, are able to express not just one but many signature cytokines, which is especially evident in vivo (44). In this study, we aimed to shed more light on the local regulation of T cells within the skin in terms of the cytokine profile and surface-marker expression in response to cytokine stimulation.
Apart from IFN-γ–producing T cells, here we report that IL-9–producing T cells are a frequent T-cell subtype (mean = 17% of skin T cells) in healthy human skin, thus confirming and extending a previous report (14). Candida species are important pathogens that are common in the environment and colonize the skin of up to 70% of healthy humans (45). Unambiguously, it has been shown that Th9 cells are specific for Candida antigen (14). Because we observed that a substantial number among skin-derived T cells is able to produce IL-9, it is tempting to speculate that IL-9–producing T cells might indeed contribute to controlling Candida skin infections. It is conceivable that after infection, IL-9–producing T cells continue to reside locally in the skin. Along this line, RNA-seq identified CXCL13 and CXCL8 (IL-8), which recruits neutrophils, cells that are able to mediate antifungal defense, to be among the most up-regulated genes in Th9-PC compared with SC (46).
PU.1 was described as one of the major developmental transcription factors for Th9 cells and was also present in T cells residing in colon mucosa of ulcerative colitis patients (29, 47, 48). However, we and others (18, 49) could not detect PU.1 in IL-9–producing T cells of healthy human skin. IRF4+ T cells were identified in skin from mycosis fungoides lesions and mycosis fungoides cell lines. In allergic contact hypersensitivity, an inflammatory skin disease, PU.1+CD3+ T cells were found in the epidermis as well as in the dermis. Other transcription factors such as IRF4 and IRF8 might regulate the expression of IL-9 in the skin through a different cytokine environment (50). This would argue in favor of skin-resident IL-9–producing T cells being different from blood IL-9–producing T cells, and hence experiments using differentiated naive-blood T cells might not completely reflect the situation in the human skin.
To propagate and to determine the local regulation and biology of T cells with a focus on IL-9–producing T cells, we cultured ex vivo skin in a medium containing Th9-differentiating cytokines IL-2, IL-4, and TGF-β (Th9-PC). As previously reported in Clark et al. (5), emigrated cells were almost exclusively memory T cells with a predominance of CD4 T cells (40). We found a significantly lower expression of CCR4 on cells emigrated from IL-2 and IL-15 conditions. Our data are in contrast with results of a previous report demonstrating higher CCR4 expression after IL-15 stimulation of blood T cells (51). These conflicting results might be explained because of different stimulation periods and differing cell origin. On the contrary, a CD56+ cell population was found in SC, possibly explained by the effects of IL-15, a well-known factor involved in NK cell development (52). In Th9-PC, we observed a significantly lower frequency of CD69-expressing T cells and found that this effect was mediated via IL-4 and TGF-β. In line with our observation are previous results showing inhibition of IL-1β–induced CD69 expression on human naive T cells by TGF-β (53). In vivo, CD69 knockout mice showed a marked reduction of skin-resident T cells, highlighting the importance of CD69 in skin retention (6). Furthermore, CD69 suppressive activity of IL-4 was demonstrated in NK cells and CD3+CD4−CD8− T cells (54). On the contrary, T cells in Th9-PC showed increased CD103 expression, even after loss of skin tissue contact, which could be ascribed to TGF-β function as previously demonstrated in CD8+ T cells (6, 55).
When addressing the stability of skin T cells, we found in reseeding experiments that IL-9 production by skin T cells seems to be dependent on the cytokine milieu. Deprivation of Th9-PC upon 4 wk of culture and reseeding of T cells under SC conditions leads to a significant decrease of IL-9–producing T cells, suggesting a plastic phenotype. In contrast, when skin T cells cultured initially under Th9-PC were reseeded under the same conditions, the IL-9–producing T-cell frequency steadily increased. Analysis of the cytokine profile of single cells revealed that the majority of IL-9–producing T cells did not coexpress any of the other Th cytokines and hence constitute a distinct T-cell subset.
IL-6 is an important survival factor for T cells, a growth factor for B cells (56), boosts T cell activation besides IL-2, promotes Th2 differentiation, and is a critical cytokine produced by antigen presenting cells (56–59). Additionally, IL-6 improves Th9 cell differentiation when added to Th9 polarizing conditions (60). In Fig. 4A, we showed that more T cells secreting IFN-γ (Th1) (61) and IL-13 (Th2) exist in cultures with SC compared with Th9-PC. Because Th1 cells are the main producers of TNF-α, and Th2 cells are an important source of IL-6 among other Th2-related cytokines, it is conceivable that they are responsible for the higher cytokine levels in the SC group compared with the Th9-PC group. In contrast, we found more IL-17+ T cells in the Th9-PC group than in the SC group, which might be due to the presence of the Th17-promoting cytokines TGF-β (added experimentally) and IL-6 (produced by skin cells).
When comparing supernatants derived from T-cell and skin explant cultures, differences with regard to IL-4, IL-6, and TNF-α cytokine levels were observed. Of note, supernatants as shown in Supplemental Fig. S1F were harvested after 4 wk of skin culture, in which, other than T cells, fibroblasts present in the cultures may also release cytokines (62), whereas supernatants in Fig. 4B were obtained from purified and stimulated T cells derived from 4 wk skin explant cultures only. Indeed, we consistently observed more fibroblasts in wells of the Th9-PC group compared with the SC group (unpublished results). Because the numbers of T cells were not determined in skin-cell explant cultures but rather in stimulated T-cell cultures only, different T-cell densities may also explain the different cytokine levels.
RNA-seq and subsequent functional annotation analysis found mainly gene clusters associated with major histocompatibility complex class II–antigen presentation and cell-cell adhesion in Th9-PC as well as endoplasmic reticulum-to-Golgi vesicle–mediated transport and type I IFN signaling pathway in SC compared with FI skin T cells. HLA-DR has been described as a mid-to-late T-cell activation protein, and its enhanced mRNA expression in Th9-PC indicates a higher activation status than in FI T cells. IL-4 is important for HLA-DR up-regulation (63, 64). Contamination with other cell types can be ruled out because typically ∼99% of Th9-PC T cells are CD3+. Interestingly, genes of the type I IFN pathway were up-regulated in SC, which might imply presence of α/β IFN.
We found that the top hits CXCL8 and CXCL13 identified by RNA-seq analysis, which are higher expressed by Th9-PC compared with SC T cells, positively affected neutrophil survival. Skin T cells including IL-9-producing T cells might maintain neutrophils at a site of inflammation via this mechanism. Interestingly, CXCL13 also increased neutrophil survival, although this chemokine was mainly described as a B-cell attraction factor (65).
In summary, we present a comprehensive report about the alteration of the skin-derived T-cell phenotype and functional properties in response to the cytokine environment. This might open new avenues for future studies to elucidate a possible therapeutic influence on T-cell–associated skin diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Wolfgang Bauer, Bärbel Reininger, Johanna Strobl, Christopher Schuster (Medical University of Vienna), and Robert Sabat (University Hospital Charité, Berlin, Germany) for excellent help and valuable discussions and for providing reagents. The authors thank Rachel Clark, Jessica E. Teague (Harvard University, Boston, MA, USA), and Emmanuella Guenova (University of Zürich, Zürich, Switzerland) for the skin explant protocols. The authors thank Christian Schneeberger and Iveta Yotova (Medical Univerity of Vienna) for access to the BD FacsVerse instrument. RNA sequencing was carried out by Markus Jeitler and Martin Bilban (Medical University of Vienna). The authors also thank Barbara Bohle and Dominika Polak (Medical University of Vienna) for the human blood neutrophil isolation protocol. This work was supported by the Austrian Science Fund (FWF; DK W1248-B30). The authors declare no conflicts of interest.
Glossary
- 7-AAD
7-aminoactinomycin D
- APC
allophycocyanin
- CLA
cutaneous lymphocyte-associated antigen
- CSC
cell-stimulation cocktail
- FI
freshly isolated
- HLA
human leukocyte antigen
- IRF4
IFN regulatory factor 4
- MPO
myeloperoxidase
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- RNA-seq
RNA sequencing
- RPMI
Roswell Park Memorial Institute
- SC
standard condition
- STAT
signal transducer and activator of transcription
- Th
T helper
- Th9-PC
Th9-promoting condition
- Treg
regulatory T
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Kienzl designed, conducted, and analyzed the experiments; R. Polacek, M. Reithofer, R. Reitermaier, and P. Hagenbach performed experiments; M. Reithofer, B. Jahn-Schmid, and A. Elbe-Bürger helped to design the experiments; P. Tajpara and M. Vierhapper provided resources; P. Kienzl and A. Elbe-Bürger wrote the manuscript; M. Gschwandtner and M. Mildner edited the manuscript; A. Elbe-Bürger supervised the project and secured funding; and all authors approved the submitted version.
REFERENCES
- 1.Streilein J. W. (1983) Skin-associated lymphoid tissues (SALT): origins and functions. J. Invest. Dermatol. 80 (Suppl), 12s–16s [DOI] [PubMed] [Google Scholar]
- 2.Bos J. D., Kapsenberg M. L. (1986) The skin immune system its cellular constituents and their interactions. Immunol. Today 7, 235–240 [DOI] [PubMed] [Google Scholar]
- 3.Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. R. (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78 [DOI] [PubMed] [Google Scholar]
- 4.Nestle F. O., Di Meglio P., Qin J. Z., Nickoloff B. J. (2009) Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R. A., Chong B., Mirchandani N., Brinster N. K., Yamanaka K., Dowgiert R. K., Kupper T. S. (2006) The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 [DOI] [PubMed] [Google Scholar]
- 6.Mackay L. K., Rahimpour A., Ma J. Z., Collins N., Stock A. T., Hafon M.-L., Vega-Ramos J., Lauzurica P., Mueller S. N., Stefanovic T., Tscharke D. C., Heath W. R., Inouye M., Carbone F. R., Gebhardt T. (2013) The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 [DOI] [PubMed] [Google Scholar]
- 7.Mackay L. K., Braun A., Macleod B. L., Collins N., Tebartz C., Bedoui S., Carbone F. R., Gebhardt T. (2015) Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194, 2059–2063 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe R., Gehad A., Yang C., Scott L. L., Teague J. E., Schlapbach C., Elco C. P., Huang V., Matos T. R., Kupper T. S., Clark R. A. (2015) Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279ra39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. (1994) Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the α E β 7 integrin. Nature 372, 190–193 [DOI] [PubMed] [Google Scholar]
- 10.Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- 11.Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. (1991) The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174, 1461–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieffer J. D., Fuhlbrigge R. C., Armerding D., Robert C., Ferenczi K., Camphausen R. T., Kupper T. S. (2001) Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: cutaneous lymphocyte antigen. Biochem. Biophys. Res. Commun. 285, 577–587 [DOI] [PubMed] [Google Scholar]
- 13.Yoshie O., Matsushima K. (2015) CCR4 and its ligands: from bench to bedside. Int. Immunol. 27, 11–20 [DOI] [PubMed] [Google Scholar]
- 14.Schlapbach C., Gehad A., Yang C., Watanabe R., Guenova E., Teague J. E., Campbell L., Yawalkar N., Kupper T. S., Clark R. A. (2014) Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 6, 219ra8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez Rodriguez R., Pauli M. L., Neuhaus I. M., Yu S. S., Arron S. T., Harris H. W., Yang S. H., Anthony B. A., Sverdrup F. M., Krow-Lucal E., MacKenzie T. C., Johnson D. S., Meyer E. H., Löhr A., Hsu A., Koo J., Liao W., Gupta R., Debbaneh M. G., Butler D., Huynh M., Levin E. C., Leon A., Hoffman W. Y., McGrath M. H., Alvarado M. D., Ludwig C. H., Truong H. A., Maurano M. M., Gratz I. K., Abbas A. K., Rosenblum M. D. (2014) Memory regulatory T cells reside in human skin. J. Clin. Invest. 124, 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark R. A., Kupper T. S. (2007) IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood 109, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purwar R., Schlapbach C., Xiao S., Kang H. S., Elyaman W., Jiang X., Jetten A. M., Khoury S. J., Fuhlbrigge R. C., Kuchroo V. K., Clark R. A., Kupper T. S. (2012) Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 18, 1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieyra-Garcia P. A., Wei T., Naym D. G., Fredholm S., Fink-Puches R., Cerroni L., Odum N., O’Malley J. T., Gniadecki R., Wolf P. (2016) STAT3/5-dependent IL9 overexpression contributes to neoplastic cell survival in mycosis fungoides. Clin. Cancer Res. 22, 3328–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt E., Germann T., Goedert S., Hoehn P., Huels C., Koelsch S., Kühn R., Müller W., Palm N., Rüde E. (1994) IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J. Immunol. 153, 3989–3996 [PubMed] [Google Scholar]
- 20.Kaplan M. H. (2017) The transcription factor network in Th9 cells. Semin. Immunopathol. 39, 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark R. A., Schlapbach C. (2017) TH9 cells in skin disorders. Semin. Immunopathol. 39, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge M., Green M. R., Langdon J. D., Feldmann M. (1989) Production of TGF-α and TGF-β by cultured keratinocytes, skin and oral squamous cell carcinomas--potential autocrine regulation of normal and malignant epithelial cell proliferation. Br. J. Cancer 60, 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster C., Vaculik C., Fiala C., Meindl S., Brandt O., Imhof M., Stingl G., Eppel W., Elbe-Bürger A. (2009) HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J. Exp. Med. 206, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Graaf C. A. A., Netea M. G., Franke B., Girardin S. E., van der Meer J. W. M., Kullberg B. J. (2006) Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin. Vaccine Immunol. 13, 423–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J. (2015) T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 75, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson B. H. (2004) IL-2, regulatory T cells, and tolerance. J. Immunol. 172, 3983–3988 [DOI] [PubMed] [Google Scholar]
- 27.Michalak-Stoma A., Pietrzak A., Szepietowski J. C., Zalewska-Janowska A., Paszkowski T., Chodorowska G. (2011) Cytokine network in psoriasis revisited. Eur. Cytokine Netw. 22, 160–168 [DOI] [PubMed] [Google Scholar]
- 28.Liao W., Schones D. E., Oh J., Cui Y., Cui K., Roh T. Y., Zhao K., Leonard W. J. (2008) Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 9, 1288–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H.-C., Sehra S., Goswami R., Yao W., Yu Q., Stritesky G. L., Jabeen R., McKinley C., Ahyi A.-N., Han L., Nguyen E. T., Robertson M. J., Perumal N. B., Tepper R. S., Nutt S. L., Kaplan M. H. (2010) The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 11, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goswami R., Jabeen R., Yagi R., Pham D., Zhu J., Goenka S., Kaplan M. H. (2012) STAT6-dependent regulation of Th9 development. J. Immunol. 188, 968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salimi M., Subramaniam S., Selvakumar T., Wang X., Zemenides S., Johnson D., Ogg G. (2016) Enhanced isolation of lymphoid cells from human skin. Clin. Exp. Dermatol. 41, 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gschwandtner M., Kienzl P., Tajpara P., Schuster C., Stipek G., Buchberger M., Mildner M., Mairhofer M., Eppel W., Vierhapper M., Pammer J., Koller R., Elbe-Bürger A., Tschachler E. (2018) The reticulum-associated protein RTN1A specifically identifies human dendritic cells. J. Invest. Dermatol. 138, 1318–1327 [DOI] [PubMed] [Google Scholar]
- 33.Polak D., Hafner C., Briza P., Kitzmüller C., Elbe-Bürger A., Samadi N., Gschwandtner M., Pfützner W., Zlabinger G. J., Jahn-Schmid B., Bohle B. (2018) A novel role for neutrophils in IgE-mediated allergy: evidence for antigen presentation in late-phase reactions. [E-pub ahead of print] J. Allergy Clin. Immunol. 10.1016/j.jaci.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stassen M., Arnold M., Hültner L., Müller C., Neudörfl C., Reineke T., Schmitt E. (2000) Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J. Immunol. 164, 5549–5555 [DOI] [PubMed] [Google Scholar]
- 35.Stassen M., Müller C., Arnold M., Hültner L., Klein-Hessling S., Neudörfl C., Reineke T., Serfling E., Schmitt E. (2001) IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J. Immunol. 166, 4391–4398 [DOI] [PubMed] [Google Scholar]
- 36.Stassen M., Klein M., Becker M., Bopp T., Neudörfl C., Richter C., Heib V., Klein-Hessling S., Serfling E., Schild H., Schmitt E. (2007) p38 MAP kinase drives the expression of mast cell-derived IL-9 via activation of the transcription factor GATA-1. Mol. Immunol. 44, 926–933 [DOI] [PubMed] [Google Scholar]
- 37.Licona-Limón P., Henao-Mejia J., Temann A. U., Gagliani N., Licona-Limón I., Ishigame H., Hao L., Herbert D. R., Flavell R. A. (2013) Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39, 744–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm C., Hirota K., Stieglitz B., Van Snick J., Tolaini M., Lahl K., Sparwasser T., Helmby H., Stockinger B. (2011) An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo S., Li Z.-Z., Jiang D.-S., Lu Y. Y., Liu Y., Gao L., Zhang S.-M., Lei H., Zhu L.-H., Zhang X.-D., Liu D.-P., Li H. (2014) IRF4 is a novel mediator for neuronal survival in ischaemic stroke. Cell Death Differ. 21, 888–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark R. A., Chong B. F., Mirchandani N., Yamanaka K., Murphy G. F., Dowgiert R. K., Kupper T. S. (2006) A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Invest. Dermatol. 126, 1059–1070 [DOI] [PubMed] [Google Scholar]
- 41.Cheng G. Z., Park S., Shu S., He L., Kong W., Zhang W., Yuan Z., Wang L.-H., Cheng J. Q. (2008) Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr. Cancer Drug Targets 8, 2–6 [PubMed] [Google Scholar]
- 42.Aratani Y. (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 640, 47–52 [DOI] [PubMed] [Google Scholar]
- 43.El Kebir D., Filep J. G. (2013) Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front. Immunol. 4, 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirahara K., Poholek A., Vahedi G., Laurence A., Kanno Y., Milner J. D., O’Shea J. J. (2013) Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. J. Allergy Clin. Immunol. 131, 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlroth J., Choi B., Spellberg B. (2007) Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45, 321–346 [DOI] [PubMed] [Google Scholar]
- 46.Erwig L. P., Gow N. A. R. (2016) Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 14, 163–176 [DOI] [PubMed] [Google Scholar]
- 47.Gerlach K., Hwang Y., Nikolaev A., Atreya R., Dornhoff H., Steiner S., Lehr H. A., Wirtz S., Vieth M., Waisman A., Rosenbauer F., McKenzie A. N. J., Weigmann B., Neurath M. F. (2014) TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 [DOI] [PubMed] [Google Scholar]
- 48.Jabeen R., Kaplan M. H. (2012) The symphony of the ninth: the development and function of Th9 cells. Curr. Opin. Immunol. 24, 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Harberts E., Tammaro A., Girardi N., Filler R. B., Fishelevich R., Temann A., Licona-Limón P., Girardi M., Flavell R. A., Gaspari A. A. (2014) IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J. Invest. Dermatol. 134, 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humblin E., Thibaudin M., Chalmin F., Derangère V., Limagne E., Richard C., Flavell R. A., Chevrier S., Ladoire S., Berger H., Boidot R., Apetoh L., Végran F., Ghiringhelli F. (2017) IRF8-dependent molecular complexes control the Th9 transcriptional program. Nat. Commun. 8, 2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norii M., Yamamura M., Iwahashi M., Ueno A., Yamana J., Makino H. (2006) Selective recruitment of CXCR3+ and CCR5+ CCR4+ T cells into synovial tissue in patients with rheumatoid arthritis. Acta Med. Okayama 60, 149–157 [DOI] [PubMed] [Google Scholar]
- 52.Cooper M. A., Fehniger T. A., Turner S. C., Chen K. S., Ghaheri B. A., Ghayur T., Carson W. E., Caligiuri M. A. (2001) Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97, 3146–3151 [DOI] [PubMed] [Google Scholar]
- 53.Hebel K., Rudolph M., Kosak B., Chang H.-D., Butzmann J., Brunner-Weinzierl M. C. (2011) IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J. Immunol. 187, 5627–5635 [DOI] [PubMed] [Google Scholar]
- 54.Gerosa F., Tommasi M., Carra G., Gandini G., Tridente G., Benati C. (1992) Different sensitivity to interleukin 4 of interleukin 2- and interferon α-induced CD69 antigen expression in human resting NK cells and CD3+, CD4-, CD8- lymphocytes. Cell. Immunol. 141, 342–351 [DOI] [PubMed] [Google Scholar]
- 55.Wang D., Yuan R., Feng Y., El-Asady R., Farber D. L., Gress R. E., Lucas P. J., Hadley G. A. (2004) Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J. Immunol. 172, 214–221 [DOI] [PubMed] [Google Scholar]
- 56.Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A., Tsunasawa S., Sakiyama F., Matsui H., Takahara Y., Taniguchi T., Kishimoto T. (1986) Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76 [DOI] [PubMed] [Google Scholar]
- 57.Teague T. K., Marrack P., Kappler J. W., Vella A. T. (1997) IL-6 rescues resting mouse T cells from apoptosis. J. Immunol. 158, 5791–5796 [PubMed] [Google Scholar]
- 58.Rincón M., Anguita J., Nakamura T., Fikrig E., Flavell R. A. (1997) Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dienz O., Rincon M. (2009) The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 130, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong M. T., Ye J. J., Alonso M. N., Landrigan A., Cheung R. K., Engleman E., Utz P. J. (2010) Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol. Cell Biol. 88, 624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romagnani S. (1999) Th1/Th2 cells. Inflamm. Bowel Dis. 5, 285–294 [DOI] [PubMed] [Google Scholar]
- 62.Kamimura D., Ishihara K., Hirano T. (2003) IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149, 1–38 [DOI] [PubMed] [Google Scholar]
- 63.Salgado F. J., Lojo J., Fernández-Alonso C. M., Viñuela J., Cordero O. J., Nogueira M. (2002) Interleukin-dependent modulation of HLA-DR expression on CD4 and CD8 activated T cells. Immunol. Cell Biol. 80, 138–147 [DOI] [PubMed] [Google Scholar]
- 64.Bang K., Lund M., Mogensen S. C., Thestrup-Pedersen K. (2005) In vitro culture of skin-homing T lymphocytes from inflammatory skin diseases. Exp. Dermatol. 14, 391–397 [DOI] [PubMed] [Google Scholar]
- 65.Takagi R., Higashi T., Hashimoto K., Nakano K., Mizuno Y., Okazaki Y., Matsushita S. (2008) B cell chemoattractant CXCL13 is preferentially expressed by human Th17 cell clones. J. Immunol. 181, 186–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.