Abstract
Exposure to spaceflight and microgravity causes physiologic and psychologic changes including bone loss, cardiovascular dysfunction, and immune dysfunction. Anemia and hematopoietic disorders are observed in astronauts after spaceflight. Hematopoietic stem and progenitor cells (HSPCs), which can self-renew and give rise to all blood cells, play vital roles in hematopoiesis and homeostasis; however, the molecular mechanisms responsible for the impacts of microgravity on the proliferation of HSPCs remain unclear. We maintained mouse bone marrow HSPCs in the presence of stem cell factor for 12 d under spaceflight and simulated microgravity conditions, respectively, and analyzed cell proliferation and gene expression. Both spaceflight and simulated microgravity significantly decreased the number of HSPCs, mainly by blocking cell cycle at G1/S transition, but did not affect their differentiation abilities. RNA-sequencing data indicated that genes related to cell proliferation were down-regulated, whereas the genes related to cell death were up-regulated under microgravity. Among the gene signatures, we identified that the Kit-Ras/cAMP–cAMP response element-binding protein pathway might be one of the major microgravity-regulated pathways during HSPC proliferation. Furthermore, the quantification of notable genes was validated at the mRNA levels under simulated microgravity condition. Overall, these results would help us to understand the intracellular molecular mechanisms regulating microgravity-inhibited proliferation of HSPCs.—Wang, P., Tian, H., Zhang, J., Qian, J., Li, L., Shi, L., Zhao, Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays.
Keywords: HSPCs, signaling pathway, SJ-10 satellite, Tianzhou-1 cargo ship
Spaceflight provides an environment that is significantly different from the normal earth environment. During spaceflight, astronauts and laboratory animals are exposed to outer space containing various harmful factors, such as microgravity, ionizing radiation, and altered circadian rhythms (1). Microgravity during spaceflight may produce a wide range of physiologic and psychologic changes affecting multiple systems and causing severe spaceflight-related health consequences, including cognitive impairment, cephalad fluid shift–induced optic nerve and ocular changes, irreversible bone resorption, cardiovascular dysfunction, and immunosuppression (2–6). All astronauts show anemia and hematopoietic disorders, such as thrombocytopenia, after spaceflights (6–9). Numerous studies have indicated that short- and long-duration spaceflights can affect a wide variety of hematopoietic and immunologic responses, including decreased plasma and blood cell mass, altered blood flow, lymphocyte, and eosinophil numbers, and increased IgA and M levels (10, 11). Most space biologic studies have been conducted on an in-orbit space shuttle, which has supported short-term experiments on the effects of spaceflight (12). Colony-forming assays showed that bone marrow (BM) cells from animals subjected to spaceflight on Spacelab Life Sciences-1 and Cosmos formed fewer colony-forming unit-erythroid in vitro (13, 14). Experiments on hematopoietic cell proliferation and differentiation during the space shuttle missions STS-63 and STS-69 showed that microgravity during spaceflight accelerated the maturation and differentiation of BM CD34+ progenitor cells toward the macrophage lineage (15). However, because of the high cost and rare chances of spaceflight experiments, researchers have used a rotating wall vessel (RWV) bioreactor to simulate the effects of microgravity. Simulated microgravity inhibited cell migration, cell cycle progression, and differentiation patterns in primitive CD34+ BM hematopoietic progenitor cells (16, 17). Hematopoietic stem and progenitor cells (HSPCs) are widely accepted as the origin of all blood cells in adults and have the potential to self-renew and differentiate into myeloid and lymphoid cells. However, despite advances in space biology over recent decades, studies of the effects of microgravity on the proliferation and maintenance of HSPCs are rare and the regulatory mechanisms remain unknown.
It is a great honor that we have participated in 2 flight projects to explore the effects of microgravity on the proliferation and maintenance of HSPCs. The Tianzhou-1 cargo ship program (18), which uses an active vibration isolation system to provide a high-level microgravity environment, clearly showed that the number of HSPCs was reduced under a space microgravity environment. Furthermore, HSPCs remained alive for further analysis after being deployed on China’s first scientific microgravity satellite, the SJ-10 recoverable satellite (19, 20). In the meantime, a series of ground tests of the flight microgravity have been conducted using the RWV bioreactor to supplement and validate flight experiments. By detecting cell proliferation and high-depth transcriptomic profiling, we provided exact validation of the finding that HSPCs display reduced proliferation in a microgravity environment. Cell cycle analysis demonstrated that simulated microgravity inhibited the G1/S transition. Importantly, RNA-sequencing (RNA-Seq) and bioinformatics analysis clarified clearer gene signatures and deeper molecular mechanisms for microgravity inhibiting HSPC proliferation.
MATERIALS AND METHODS
Flight hardware
This research was conducted as a subset of the Tianzhou-1 cargo ship program (18) and the SJ-10 space program (19, 20). Sorted mouse HSPCs were cultured in 2 special culture vessels (Supplemental Fig. S1) that allow automatic replacement of the culture medium aboard the Tianzhou-1 cargo ship or the SJ-10 recoverable satellite for 12 d. All cell culture devices for spaceflight (21) and the RWV bioreactor in the laboratory (22) were designed and supplied by the National Center of Space Science, Chinese Academy of Sciences. Throughout the duration of the Tianzhou-1 spaceflight experiment, real-time photomicrographs of the HSPCs were taken every 24 h using space teleoperation technology and transmitted back to the ground when the satellite passed though Chinese airspace. The HSPCs on the SJ-10 satellite were brought back safely for subsequent experimental analysis.
Preparation of murine HSPCs
C57BL/6 mice (6–8 wk old) were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). BM cells were obtained from the femur, tibia, and lilac bone and prepared into single cell suspensions (23). After lysis of red blood cells, the cells were labeled with various lineage-specific markers by BD Mouse Biotin Lineage Panel (BD Biosciences, San Jose, CA, USA), which includes 5 prediluted biotinylated antibodies (Biotin Hamster anti-Mouse CD3e, Biotin Rat anti-Mouse CD45R, Biotin Rat anti-Mouse Ly-6G and Ly-6C, Biotin Rat anti-Mouse TER-119/Erythroid cells, and Biotin Rat anti-Mouse CD11b) in order to remove lymphoid, myeloid, and erythroid cells. The labeled cells were then separated over IMag Streptavidin Particles Plus-DM (BD Biosciences) designed for depletion (24). High-purity mouse HSPCs were finally prepared for cell culture under spaceflight and simulated microgravity conditions.
Cell culture in spaceflight incubator
The sorted mouse HSPCs were cultured in a culture device that provided automatic replacement of 5-ml culture medium in space every day (1 ml/h, 5 h/d) at 37°C and 0% CO2 for ∼12 d. For ground controls, HPCs were cultured in the same cell culture device (0% CO2) and rested in a 37°C incubator. Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Corning, Manassas, USA) containing 10 ng/ml IL-3, 20 ng/ml IL-6, 20 ng/ml stem cell factor (SCF), 10% fetal bovine serum (FBS), 100 U/ml streptomycin, and 100 U/ml penicillin. After being cultured for 12 d, the cells were collected for a series of analyses, including counting, proliferation analysis, RNA extraction, and RNA-Seq analysis.
Cell culture under simulated microgravity
For the simulated microgravity condition, the sorted mouse HSPCs were cultured for 12 d under simulated microgravity in the RWV bioreactor with a 12-rpm rotation speed (generate a microgravity from 10−2 g to infinitesimal) at 37°C and 5% CO2 as previously described (22). Meanwhile, we also cultured sorted mouse HSPCs under normal gravity condition in a humidified 37°C incubator with 5% CO2 for 12 d. The culture medium for cell proliferation included RPMI-1640 medium, 10 ng/ml IL-3, 20 ng/ml IL-6, 20 ng/ml SCF, 10% FBS, 100 U/ml streptomycin, and 100 U/ml penicillin. The medium was changed at the same frequency as in the cultures on the of SJ-10 recoverable satellite in space. In addition, to induce macrophage differentiation of these cultured HSPCs, these collected cells were further cultured in RPMI-1640 medium containing 100 ng/ml M-CSF, 10% FBS, 100 U/ml streptomycin, and 100 U/ml penicillin for 7 d. The percentages of differentiated macrophages were detected by flow cytometry.
Flow cytometry
The cell cycle was analyzed in the collected cells using flow cytometry (25). A total of 1 × 106 cells were collected, washed twice using cold PBS, and then fixed in 70% ethanol overnight at 4°C. The ethanol was discarded, and the cells were washed twice with PBS, followed by the addition of 50 μg/ml propidium iodide and 2% Triton X-100 for 30 min at 4°C. The DNA content was then tested using FACSCalibur flow cytometer (BD Biosciences). The results were analyzed by ModFit LT 4.1 software (Verity Software House, Topsham, ME, USA).
Intranuclear staining for Ki67 expression was performed using the Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific, Waltham, MA, USA). In brief, cells were washed with PBS and fixed and permeabilized with Fix/Perm buffer for 20 min prior to the addition of the conjugated antibodies. Antibodies used were PE anti–mouse/human Ki-67 Antibody (BioLegend, San Diego, CA, USA) (26). For extracellular staining, anti–mCD11b-FITC, anti–mF4/80-PE-Cy5, anti–mCD3e-FITC, anti–mTCRβ-PE, anti–mCD45R/B220-PE, and anti–mCD11c-PE were purchased from BD Biosciences. Cells (1 × 106) were collected and resuspended in 100 μl staining buffer, then fluorochrome-conjugated antibodies were added and kept at 4°C for 30 min (27). Data acquisition followed on a BD FACSCalibur flow cytometer (BD Biosciences) and data analysis was performed using the Flowing Software v.2.5 (http://flowingsoftware.btk.fi/) (28).
RNA-Seq and analysis
For RNA-Seq, total RNA was extracted using Trizol (Thermo Fisher Scientific) according to the manufacturer’s instruction. The purity and quality of the total RNA were checked using Nanodrop 2000 (Thermo Fisher Scientific). The total RNAs were sent to Beijing Novogene Bioinformatics Technology (Beijing, China) for RNA-Seq analysis (29). cDNA libraries were prepared, and the samples were then sequenced by Illumina HiSeq 3000 sequencer (Illumina, San Diego, CA). To allow the comparison of gene expression profiles, values for each transcript were normalized and calculated as fragments per kilobase per million mapped reads. Differentially expressed genes (DEGs) were calculated using the DEGseq package (30) of R software (R Foundation for Statistical Computing, Vienna, Austria).
Pathway and network analysis
All significant DEGs between the transcriptomes of HSPCs subjected to spaceflight or simulated microgravity and normal gravity were subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. We applied the Database for Annotation, Visualization, and Integrated Discovery to performed GO analysis, and signaling pathway analysis was performed using the Kobas 3.0 online tool (http://kobas.cbi.pku.edu.cn) (31) and Java gene set enrichment analysis (GSEA). Visualization was performed using the R packages ggplot2 (v.2.2.1) and pheatmap (v.1.0.8). For GSEA analysis, gene sets of apoptosis, cell cycle, and hematopoietic cell lineage pathway derived from the “canonical” mSigDB geneset (KEGG pathway database).
The metabolic network was analyzed using the network tool Shiny GAM: integrated analysis of genes and metabolites (https://artyomovlab.wustl.edu/shiny/gam/) (32) to identify interactions between metabolites and DEGs generated by spaceflight and simulated microgravity. The criteria were limited by a fold change >2 and a value of P < 0.05. The results were visualized using Cytoscape software (https://cytoscape.org/) (33).
Real-time PCR analysis
For real-time PCR, total RNA was extracted from cultured cells using a total RNA Kit (Promega, Madison, WI, USA), and cDNA was synthesized with a cDNA Reverse Transcription Kit (Takara Bio, Kusatsu, Japan). Real-time quantitative PCR was performed on the CFX96 real-time system (Bio-Rad, Hercules, CA, USA) with TB Green Premix Ex Taq (Takara Bio) (34, 35). All real-time PCR reactions were carried out in optical 96-well reaction plate and run in triplicate. Each plate included 2 no-template controls. The primers used in the present study are summarized in Supplemental Table S1. The real-time PCR data were analyzed by the comparative Ct method and were normalized to the hypoxanthine phosphoribosyltransferase housekeeping gene.
Statistical analysis
All data were presented as means ± sd. Data were compared between 2 groups using 2-tailed Student’s t tests. Comparisons were considered significant at a value of P < 0.05.
RESULTS
Spaceflight microgravity decreased proliferation of HSPCs
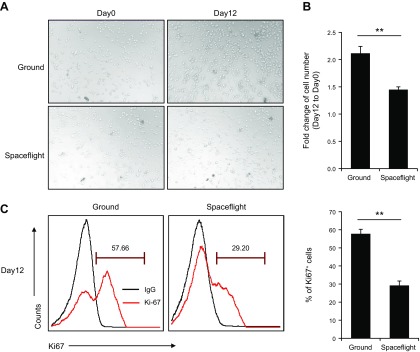
HSPCs from C57BL/6 mice were isolated by magnetic cell sorting, stained with lineage-specific antibodies to CD3ε, TCR-β, B220, CD11b, F4/80, and CD11c, and detected by flow cytometry (Supplemental Fig. S2). The sorted HSPCs did not express mature immune cell–specific markers. We examined the effects of spaceflight microgravity on the proliferative capacity of the mouse HSPCs by comparing microscope images of the cultured HSPCs between the spaceflight and ground (control) groups on d 0 and 12. Based on microscopic appearance, there were obviously more cells in the ground control cultures (Fig. 1A) and accurate counting confirmed that the number of HSPCs was significantly decreased in the spaceflight group (Fig. 1B). We further examined the proliferative ability of the HSPCs in both groups by detecting expression levels of the cell proliferation marker Ki67 (36). Ki67 expression was significantly lower in mouse HSPCs after spaceflight than that in cells under normal gravity (Fig. 1C). These data collectively indicated that the spaceflight microgravity significantly inhibited the proliferation of mouse HSPCs.
Figure 1.
Spaceflight microgravity inhibits proliferation of murine HSPCs. A) Microscopic appearance of cultured HSPCs under normal ground control and spaceflight conditions. Original magnification, ×20. B) Change in cell number of HSPCs after culture under normal ground control and spaceflight conditions for 12 d. C) Ki67 staining analysis of HSPC proliferation after culture under normal ground control and spaceflight conditions for 12 d. **P < 0.01.
Simulated microgravity decreased proliferation of HSPCs
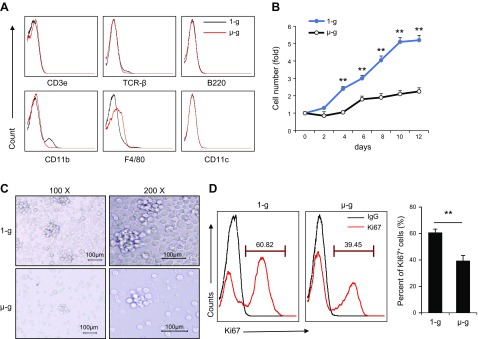
We determined if simulated microgravity had a similar effect on the proliferation of murine HSPCs to spaceflight microgravity by culturing mouse HSPCs in an RWV system. According to the data from previous studies (37), about 50% HSPCs complete a cell cycle after 6 d and cell proliferation is exponentially increased within 12 d. Therefore, we also analyzed HSPCs after 12 d in simulated microgravity experiments to match the experimental duration which performed in the cargo ship. We detected expressions of the lineage-specific markers including CD3ε, TCR-β, B220, CD11b, F4/80 and CD11c in HSPCs cultured under simulated and normal gravity conditions for 12 d by flow cytometry, and compared their abilities to differentiate into macrophages. HSPCs cultured under simulated microgravity and normal gravity nearly did not express T cell, B cell, macrophage, and dendritic cell lineage–specific markers (Fig. 2A). We also counted cell numbers and compared microscope images of proliferated HSPCs under simulated microgravity and normal gravity on d 12, and we showed that cell numbers were significantly reduced under simulated microgravity (Fig. 2B, C). We further examined cell proliferation ability by detecting expression levels of Ki67 on d 12 and showed that Ki67 expression was significantly reduced in HSPCs under simulated microgravity (Fig. 2D). In addition, the expanded cells cultured for 12 d under either simulated microgravity or normal gravity were then cultured with M-CSF for an additional 7 d to see their potential ability to differentiate into macrophages under normal gravity. The data shows that HSPCs pre-experienced under either simulated microgravity or normal gravity differentiated into macrophages similarly (Supplemental Fig. S3), indicating that these cells might have identical potential ability to differentiate into macrophages.
Figure 2.
Simulated microgravity inhibits proliferation of murine HSPCs. A) Expression of lineage-specific markers CD3ε, TCR-β, B220, CD11b, F4/80, and CD11c in murine HSPCs after 12 d of culture under normal and simulated microgravity conditions. B) Growth curve of HSPCs by living-cell counting method during 12 d of culture under normal gravity and simulated microgravity conditions. C) HSPCs after 12 d of culture under normal and simulated microgravity conditions. D) Percentages of Ki67+ HSPCs after culture under normal ground control and simulated microgravity conditions for 12 d. µ-g, simulated microgravity; 1-g, normal gravity. **P < 0.01.
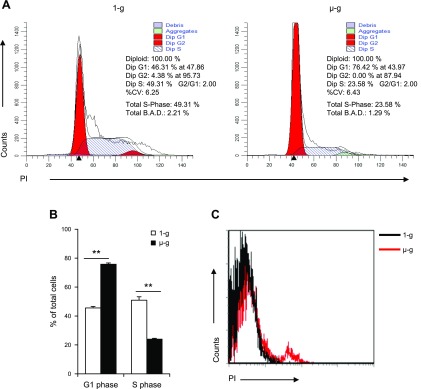
We also determined if the simulated microgravity decreased HSPC proliferation by blocking cell cycle progression or by promoting apoptosis of cultured HSPCs. We detected cell cycle progression and apoptosis in HSPCs after being cultured for 12 d under normal and simulated microgravity conditions. More HSPCs cultured under simulated microgravity stayed in G0/G1 phase and fewer were in S phase compared with HSPCs cultured under normal gravity (Fig. 3A, B). Meanwhile, HSPCs cultured under simulated microgravity also produced more apoptotic cells than HSPCs cultured under normal gravity (Fig. 3C). Simulated microgravity thus decreased the proliferation of HSPCs by both blocking cell cycle and increasing cell death.
Figure 3.
Effects of simulated microgravity on cell cycle and apoptosis of HSPCs. A) Distribution of each phase of the cell cycle was evaluated in HSPCs after 12 d of culture under normal and simulated microgravity conditions. %CV (coefficient of variation) indicates the numerical dispersion degree. Total B.A.D (Background, Aggregates and Debris) indicates the amount of debris and aggregates found within the region between the first G1 and the last G2 peak. B) Ratio of G1 and S-phase murine HSPCs after 12 d of culture under normal and simulated microgravity conditions. C) Expression of propidium iodide (PI) in cultured HSPCs under simulated microgravity for 12 d. µ-g, simulated microgravity; 1-g, normal gravity. **P < 0.01.
RNA-Seq analysis of HSPCs under spaceflight microgravity and simulated microgravity
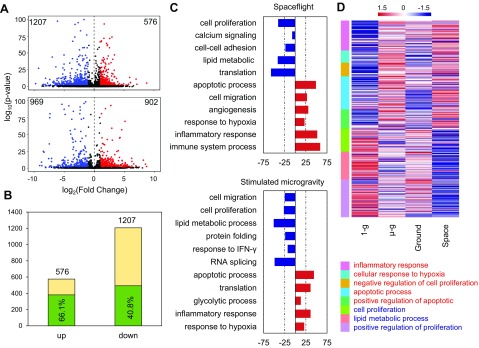
We investigated how spaceflight microgravity and simulated microgravity impacted the proliferation ability of HSPCs. We detected the gene expression profiles of HSPCs from the spaceflight and time-matched ground control groups and from the simulated microgravity and normal gravity control groups after culture for 12 d. We then analyzed the DEGs between the spaceflight and simulated microgravity groups and their respective controls. A total of 1783 DEGs were identified in the spaceflight HSPCs compared with the ground control group, including 576 up-regulated and 1207 down-regulated genes. A total of 1872 DEGs were identified in HSPCs cultured under simulated microgravity compared with the normal gravity controls, including 903 up-regulated and 969 down-regulated genes (Fig. 4A). Among the 576 up-regulated and 1207 down-regulated DEGs under spaceflight, 66.1 and 40.8% had similar trends under simulated microgravity, respectively (Fig. 4B), indicating similarity between the spaceflight microgravity–regulated and simulated microgravity–regulated transcriptomes of HSPCs. To identify the biologic functions affected by spaceflight and simulated microgravity, respectively, we performed GO–biological process (BP) enrichment analysis for the identified DEGs using the Database for Annotation, Visualization, and Integrated Discovery. DEGs up-regulated under spaceflight and simulated microgravity were enriched in the processes of apoptosis, hypoxic stress, hematopoietic capacity, and inflammatory response (Fig. 4C), whereas down-regulated DEGs were enriched in the process of cell proliferation (Fig. 4C). There was a slight difference in other BPs between the spaceflight–down-regulated and simulated microgravity–down-regulated DEGs, in that spaceflight–down-regulated BPs were mainly related to calcium signaling, cell adhesion, lipid metabolism, and translation process (Fig. 4C), whereas simulated microgravity–down-regulated BPs were related to lipid metabolism, protein folding, and response to IFN-γ (Fig. 4C). DEGs enriched in the BPs commonly regulated under both spaceflight and simulated microgravity included genes involved in the inflammatory response, cellular response to hypoxia, apoptotic processes, positive regulation of apoptosis, cell proliferation, positive regulation of cell proliferation, and negative regulation of cell proliferation (Fig. 4D). We also verified the expression of some DEGs enriched in the biologic processes regulated by both spaceflight and simulated microgravity by real-time PCR, including Hif1α, Vegfa, and Hyou1 in the process of cellular response to hypoxia; Lif, Osm, Apoe, Rac2, Kit, and Tiam1 in the process of positive regulation of cell proliferation; Trib3, Sox4, and Pim2 in process of negative regulation of cell proliferation; and Tnfrsf6 and Bcl2l11 in apoptotic processes. These results were all consistent with the RNA-Seq results (Supplemental Fig. S4).
Figure 4.
Quantitative analysis and GO-BP enrichment analysis of microgravity-responsive genes. A) Differential expression of spaceflight (above) and simulated (below) microgravity-responsive genes relative to normal gravity conditions presented by volcano plot. Colors are restricted by P < 0.05 and fold change >1.5. B) Histogram showing the degree of similarity between differential microgravity and ground microgravity. Percentages indicate the ratio of overlapping genes to spaceflight DEGs. C) Horizontal histograms representing the top 10 categories of enriched DEGs in GO-BP generated by spaceflight and simulated microgravity. Red indicates up-regulated genes caused by microgravity, and blue indicates down-regulated genes. D) The classified heatmap is used to present log2 fold change of specific genes in BP consistent with changes in spaceflight and simulated microgravity. Data normalized using z score in column. µ-g, simulated microgravity; 1-g, normal gravity; Ground, ground control for spaceflight; Space, spaceflight.
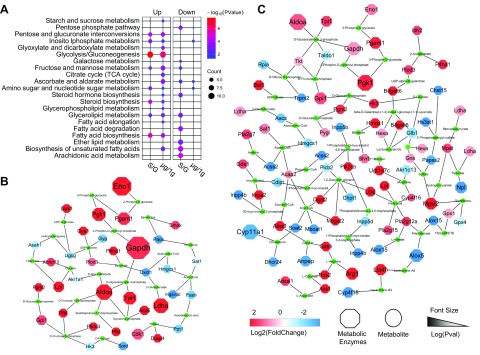
Growth-related metabolic dysfunction caused by microgravity
Similar metabolic pathways were identified as enriched by KEGG analysis after exposure to microgravity by either spaceflight or simulated microgravity. The primary classification of the metabolic pathway grading system is carbohydrate metabolism, lipid metabolism, and amino acid metabolism. Spaceflight and simulated microgravity elicited different effects on carbohydrate and lipid metabolism mainly by up-regulating the metabolic pathways including pentose and glucuronate interconversions, inositol phosphate metabolism, glycolysis, fructose and mannose metabolism, amino sugar and nucleotide sugar metabolism, steroid biosynthesis, glycerolipid metabolism, and fatty acid biosynthesis. However, the metabolic pathways including ether lipid metabolism, biosynthesis of unsaturated fatty acids, arachidonic acid metabolism, fatty acid degradation, and glycerolipid metabolism were all strongly inhibited under spaceflight but not in simulated microgravity (Fig. 5A). We visualized significant DEGs under simulated microgravity using Shiny GAM (genome-scale model metabolic network analysis) and Cytoscape software. Important kinases in the glycolytic pathway, including glyceraldehyde phosphate dehydrogenase, phosphoglycerate kinase, phosphoglycerate mutase-1, enolase, triosephosphate isomerase, aldolase A, phosphofructokinase-1, phosphoglycerate kinase-1, and phosphoglucomutase-2, were all up-regulated under the influences of spaceflight and simulated microgravity (Fig. 5B). However, simulated microgravity had a broader impact on intracellular metabolic activity compared with spaceflight (Fig. 5C), and some lipid metabolism related kinases, including arachidonate 5-lipoxygenase, arachidonate 15-lipoxygenase, inositol polyphosphate phosphatase (Inpp4b, Inpp5b, and Inpp5d), acyl-CoA synthetase-2, sterol acyltransferase-2, and alanyl aminopeptidase, were specifically up-regulated by simulated microgravity.
Figure 5.
Analysis and demonstration of microgravity-affected metabolism. A) Metabolic pathways obtained by KEGG enrichment are represented by bubble map. Color in the bubble chart indicates the significance of the enrichment category, and the size indicates the number of enriched genes. Up, up-regulated signal pathway; Down, down-regulated signal pathway; S/G, in S compared with G; μg/1g, in μg compared with 1g; Count, Number of genes enriched in this metabolic pathway. B) The relationship of metabolic enzymes and metabolites is visualized in the network based on spaceflight-affected DEGs. C) The relationship of simulated microgravity–affected metabolic enzymes and corresponding metabolites are displayed. µg, simulated microgravity; 1g, normal gravity; G, ground control for spaceflight; S, spaceflight; TCA, tricarboxylic acid cycle; Pval, P value of differential expression.
Molecular pathways in HSPCs impaired by microgravity
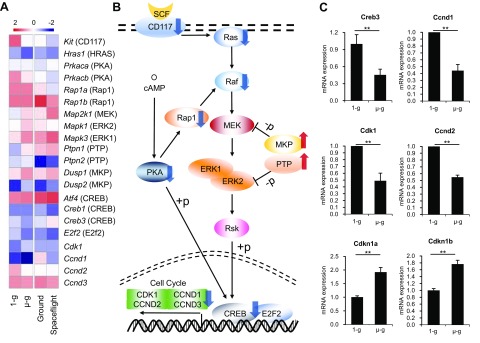
As noted above, the cell cycle of HSPCs was blocked at G0/G1, and HSPCs did not differentiate into other lineages after being cultured under simulated microgravity. We explored the responsible intracellular mechanisms and signaling pathways by KEGG analysis. GSEA showed that up-regulation of the gene expressions in apoptotic pathway was not very obvious, but the gene expressions in cell cycle pathway were significantly down-regulated under spaceflight (Supplemental Fig. S5), which may partially explain the decrease in HSPCs and their cell cycle arrest under spaceflight or simulated microgravity. GSEA also showed genes specific for other lineages were not significantly increased under spaceflight or simulated microgravity (Supplemental Figs. S6 and S7), which again validates the conclusion that these HSPCs do not differentiate into other lineages. We also investigated the potential mechanisms by which simulated microgravity regulated the cell cycle in HSPCs. SCF is a key cytokine known to maintain stem cell characteristics of HSPCs and to promote HSPC expansion in vitro (38). SCF led to rapid HSPC activation and proliferation via the PI3K/AKT and MEK/ERK pathways (39). At the transcriptome level, we showed that the SCF receptor (also called c-Kit or Kit) was significantly down-regulated under both conditions (Fig. 6A and Supplemental Fig. S4), whereas expression levels of the genes encoding MAPK phosphatases and protein tyrosine phosphatases, which negatively regulate SCF pathway, were increased (Fig. 6A), including Dusp1, Dusp2, Ptpn1, and Ptpn2. Activity of SCF pathway was thus decreased under spaceflight. The SCF signaling pathway has been reported to positively regulate the cAMP response element-binding protein (CREB), which can regulate the transcriptional activity of cell cycle genes (40). Decreased SCF signaling may thus cause cell cycle arrest. We also showed that genes involved in the calcium-related cAMP signaling pathway were down-regulated under the influence of microgravity (Fig. 6A), which could also positively regulate CREB (41). Furthermore, the cAMP signaling pathway could regulate the SCF pathway through Rap1. Microgravity may therefore regulate the cell cycle in HSPCs via the SCF pathway and cAMP pathway–mediated CREB (Fig. 6B). Meanwhile, we verified the expression of key genes involved in these 2 pathways and cell cycle–associated genes, such as Cdkn1a encoding P21 and Cdkn1b encoding P27, both of which negatively regulate the cell cycle, and showed that the expression levels of these genes, including Creb3, Ccnd1, Ccnd2, Cdk1, Cdkn1a, and Cdkn1b, were all consistent with the RNA-Seq results (Fig. 6C). Microgravity therefore may mainly inhibit CD117-Ras/cAMP-CREB signaling pathway networks to block the proliferation of HSPCs under microgravity.
Figure 6.
Microgravity regulates cell proliferation and cell cycle via the CD117-Ras/cAMP-CREB pathway. A) The fragments per kilobase per million value of the changed genes downstream of the SCF-cKit pathway was normalized by column in the heatmap. B) Intracellular localization and interaction of SCF-cKit pathway genes. SCF, stem cell factor; RSK, ribosomal S6 kinase; +p, phosphorylation; −p, dephosphorylation. C) CREB-regulated cell cycle genes in the SCF-cKit pathway were validated by real-time PCR in vitro. µ-g, simulated microgravity; 1-g, normal gravity; Ground, ground control for spaceflight. **P < 0.01.
DISCUSSION
The aim of this study was to determine the impact of microgravity on the proliferation and maintenance of HSPCs, to provide a more comprehensive molecular regulatory network affected by microgravity in HSPCs, and to clarify the molecular regulatory networks involved in the effects of microgravity. Our results demonstrated that both spaceflight and simulated microgravity significantly decreased the number and proliferative capacity of HSPCs in vitro, whereas simulated microgravity did not significantly alter the differentiation abilities of HSPCs as evidenced by their efficient differentiation into macrophages in the in vitro induction system. We also carried out next-generation sequencing (NGS) to provide a systematic and in-depth analysis of the transcriptional effects triggered by microgravity. The signature included numerous genes associated with cell proliferation that were down-regulated by microgravity, whereas other genes in BPs associated with cell death were significantly up-regulated. In addition, transcriptional data also revealed that most genes related to energy metabolism were also altered under microgravity compared with normal gravity. Furthermore, the key molecular pathway affecting the cell cycle was significantly down-regulated in HSPCs under microgravity.
Mature immune cells in the blood system have a very short life span and thus need to be replenished by HSPCs throughout adult life, according to the body’s physiologic needs. HSPCs also play a critical role in regulating and maintaining the physiologic balance of various blood cells under stress conditions such as injury and inflammation. HSPC proliferation and maintenance have been widely studied in recent decades and have been shown to be controlled by various factors, molecules, and transcription factors; however, little is known about the effects of microgravity on HSPCs. The Chinese Tianzhou-1 cargo ship provided us with a rare opportunity to observe the effects of spaceflight on cultured HSPCs over 12 d, and the images during spaceflight revealed that HSPC self-renewal was weakened. We were also able to examine the same cells after their return to Earth via the SJ-10 Recoverable Scientific Satellite, and we counted and stained these cell samples and sequenced their transcripts to investigate the effects of spaceflight on cell changes and molecular mechanisms. We also conducted corresponding simulated microgravity experiments. Results of all these experiments suggested that microgravity attenuated cell proliferation. This was consistent with previous clonal assays studies, which found that microgravity decreased the hematopoietic generating capacity by decreasing the number of burst-forming unit-erythroid and colony-forming unit-erythroid in mouse BM stimulated with erythropoietin in vitro (42). HSPCs are pluripotent cells that can give rise to all other blood cells and maintain hematopoiesis and intestinal homeostasis. The direct inhibitory effects of microgravity on HSPC proliferation could thus partially explain the observed anemia and decreased red-cell mass in astronauts after spaceflight.
Cell proliferation includes 2 successive processes: the preparation of material through various metabolic processes and cell division by cell cycle regulation (43). In this study, we observed that cell metabolism was obviously changed under microgravity. ATP is the original source of power for cell proliferation, and changes in its supply thus seriously affect cell proliferation (44, 45). Under normal gravity conditions, HSPCs are unaffected by external stress and primarily use anaerobic glycolysis for energy production, which represents the best energy state for cells to maintain growth (46). However, glycolysis and hypoxia-inducible factor 1α pathway were activated in HSPCs under microgravity conditions, resulting in cell cycle block at the G1/S phase (47). In addition, an increase or decrease in macromolecular biosynthesis also significantly affects material preparation during cell proliferation, and metabolic changes induced by microgravity will inevitably lead to dysfunction of macromolecular biosynthesis with subsequent effects on the proliferation of HSPCs. In terms of cell cycle regulation, the cell cycle interface is a key factor affecting cell proliferation, and decreased proliferative ability is always concomitant with cell cycle arrest (48, 49). More HSPCs were blocked in G0/G1 phase and fewer were blocked in S phase under microgravity, suggesting that simulated microgravity significantly inhibited cell progression at the G1-to-S phase transition in HSPCs. Similarly, Plett et al. (16) found that modeled microgravity caused cell cycle arrest in primitive hematopoietic progenitor cells after a short period in the RWV system. Previous studies have also shown that growth factors act early in G1 via a growth factor signaling/cell cycle interface to operate the cell cycle signaling system and drive the cell toward the restriction point (R-point), where it becomes irreversibly committed to completing the rest of the cell cycle (43). In the current study, the hematopoietic growth factor receptor c-Kit was a pivotal molecule that accepted the signal from the growth factor SCF (50), leading to a cascade of signals that ultimately regulated the transcription of cyclin D (CCND). CCND is known to control G1 progression and depends on growth factor signaling pathways (51). Expression of the SCF receptor c-Kit, key SCF pathway genes such as Ras, Raf, and Rap1, and the cAMP-PKA pathway were all down-regulated under microgravity. Both these pathways could regulate CREB, which subsequently regulates genes involved in the cell cycle and cell proliferation (41, 52), such as CDK1 and CCND. Of the 3 CCND isoforms, CCND1 was an obvious target regulated by microgravity (either spaceflight or simulated), which may be a key molecule in the microgravity-responsive G1/S blockade.
The mechanisms by which microgravity affects the number of HSPCs have not been well investigated, but the decrease in cell number under microgravity is likely due to both decreased cell proliferation and increased cell death. We investigated the potentially relevant mechanisms by RNA-Seq and bioinformatics analysis. RNA-Seq and GO analysis demonstrated that both spaceflight and simulated microgravity mainly regulated the expressions of genes associated with cell proliferation but also those associated with cell death by regulating cell apoptosis and hypoxic stress. We aim to verify these experimental conclusions in future space programs if possible. The current study had some limitations and weaknesses. Too few cells obtained in spaceflight experience greatly limited our following studies, so many hypotheses can only be validated under simulated microgravity. In fact, spaceflight is still different from simulated microgravity. Gravity (including microgravity, normogravity, and hypergravity) is complex and changeable during spaceflight experiment, although gravitational conditions (microgravity) remained constant in the simulated microgravity experiments. Similarly, the simulated microgravity environment involved not only simple low gravity but also shear force, friction, and other complex forces. It is therefore necessary to combine analyses from spaceflight and simulated microgravity, as in the current study, though this approach is rarely reported. The rarity of the opportunity and the cost of conducting experiments in orbit meant that our study was unable to verify all results obtained during spaceflight. However, we attempted to compensate for this loophole by analyzing the transcriptional profile of the real microgravity samples, detecting similar changes in the transcriptome of samples under simulated microgravity, and finally verifying these changes in simulated microgravity experiments. However, additional studies are needed to confirm these results including studies with appropriate controls (normal gravity in-flight group) and experimental replicates in spaceflight, which should be taken into account in the future.
In summary, the results of the present study provide mechanistic insights into the molecular network responsible for microgravity-inhibited HSPC expansion. Microgravity directly inhibited HSPC proliferation via G1/S transition in the cell cycle. Furthermore, bioinformatics analysis revealed that microgravity may inhibit the proliferation of HSPCs by downregulating the Kit-Ras/cAMP-CREB signaling pathways, accompanied by changes in energy metabolism. These results partly explained the hematopoietic disorders caused by spaceflight microgravity and lay the foundation for further in-depth research into the effects of microgravity on HSPCs.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Huiting Su (State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences) for critical reading of the manuscript. This work was supported by grants from the Program of National Natural Science Foundation of China (U1738111 to Y.Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030301 to Y.Z.), the Mega-Projects of National Science Research for the 13th Five-Year Plan (to L.L.), the Program of National Natural Science Foundation of China (81530049 and 31470860 to Y.Z.), and the China Manned Space Flight Technology Project (TZ-1). The raw RNA-Seq data from this study have been deposited at the Big Data (BIGD) Genome Sequence Archive under accession number CRA001198. The authors declare no conflicts of interest.
Glossary
- BM
bone marrow
- BP
biological process
- CCND
cyclin D
- CREB
cAMP response element-binding protein
- DEG
differentially expressed gene
- FBS
fetal bovine serum
- GO
gene ontology
- GSEA
gene set enrichment analysis
- HSPC
hematopoietic stem and progenitor cell
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- NGS
next-generation sequencing
- RPMI
Roswell Park Memorial Institute
- RWV
rotating wall vessel
- SCF
stem cell factor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Wang and Y. Zhao conceived the study and designed the experiments; P. Wang and L. Shi organized the data and drafted the manuscript; P. Wang, H. Tian, J. Qian, and L. Shi, carried out the experiments; J. Zhang, L. Li, and L. Shi analyzed the raw NGS data; J. Zhang visualized the NGS data as diagrams; L. Shi provided ideas and pipelines for all biological information analysis; and Y. Zhao supervised the project and provided critical feedback at all stages.
REFERENCES
- 1.Domaratskaya E. I., Michurina T. V., Bueverova E. I., Bragina E. V., Nikonova T. A., Starostin V. I., Khrushchov N. G. (2002) Studies on clonogenic hemopoietic cells of vertebrate in space: problems and perspectives. Adv. Space Res. 30, 771–776 [DOI] [PubMed] [Google Scholar]
- 2.Wu X., Li D., Liu J., Diao L., Ling S., Li Y., Gao J., Fan Q., Sun W., Li Q., Zhao D., Zhong G., Cao D., Liu M., Wang J., Zhao S., Liu Y., Bai G., Shi H., Xu Z., Wang J., Xue C., Jin X., Yuan X., Li H., Liu C., Sun H., Li J., Li Y., Li Y. (2017) Dammarane sapogenins ameliorates neurocognitive functional impairment induced by simulated long-duration spaceflight. Front. Pharmacol. 8, 315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mader T. H., Gibson C. R., Pass A. F., Kramer L. A., Lee A. G., Fogarty J., Tarver W. J., Dervay J. P., Hamilton D. R., Sargsyan A., Phillips J. L., Tran D., Lipsky W., Choi J., Stern C., Kuyumjian R., Polk J. D. (2011) Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058–2069 [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi K., Soga K., Hoson T., Kotake T., Kojima M., Sakakibara H., Yamazaki T., Higashibata A., Ishioka N., Shimazu T., Kamada M. (2017) Persistence of plant hormone levels in rice shoots grown under microgravity conditions in space: its relationship to maintenance of shoot growth. Physiol. Plant. 161, 285–293 [DOI] [PubMed] [Google Scholar]
- 5.Delp M. D., Charvat J. M., Limoli C. L., Globus R. K., Ghosh P. (2016) Apollo lunar astronauts show higher cardiovascular disease mortality: possible deep space radiation effects on the vascular endothelium. Sci. Rep. 6, 29901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guéguinou N., Huin-Schohn C., Bascove M., Bueb J. L., Tschirhart E., Legrand-Frossi C., Frippiat J. P. (2009) Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 86, 1027–1038 [DOI] [PubMed] [Google Scholar]
- 7.De Santo N. G., Cirillo M., Kirsch K. A., Correale G., Drummer C., Frassl W., Perna A. F., Di Stazio E., Bellini L., Gunga H. C. (2005) Anemia and erythropoietin in space flights. Semin. Nephrol. 25, 379–387 [DOI] [PubMed] [Google Scholar]
- 8.Tavassoli M. (1982) Anemia of spaceflight. Blood 60, 1059–1067 [PubMed] [Google Scholar]
- 9.Borchers A. T., Keen C. L., Gershwin M. E. (2002) Microgravity and immune responsiveness: implications for space travel. Nutrition 18, 889–898 [DOI] [PubMed] [Google Scholar]
- 10.Graebe A., Schuck E. L., Lensing P., Putcha L., Derendorf H. (2004) Physiological, pharmacokinetic, and pharmacodynamic changes in space. J. Clin. Pharmacol. 44, 837–853 [DOI] [PubMed] [Google Scholar]
- 11.Vernikos J. (1996) Human physiology in space. BioEssays 18, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 12.Blaber E., Sato K., Almeida E. A. (2014) Stem cell health and tissue regeneration in microgravity. Stem Cells Dev. 23(Suppl 1), 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange R. D., Gibson L. A., Driscoll T. B., Allebban Z., Ichiki A. T. (1994) Effects of microgravity and increased gravity on bone marrow of rats. Aviat. Space Environ. Med. 65, 730–735 [PubMed] [Google Scholar]
- 14.Udden M. M., Driscoll T. B., Gibson L. A., Patton C. S., Pickett M. H., Jones J. B., Nachtman R., Allebban Z., Ichiki A. T., Lange R. D., Alfrey C. P. (1995) Blood volume and erythropoiesis in the rat during spaceflight. Aviat. Space Environ. Med. 66, 557–561 [PubMed] [Google Scholar]
- 15.Davis T. A., Wiesmann W., Kidwell W., Cannon T., Kerns L., Serke C., Delaplaine T., Pranger A., Lee K. P. (1996) Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 60, 69–76 [DOI] [PubMed] [Google Scholar]
- 16.Plett P. A., Abonour R., Frankovitz S. M., Orschell C. M. (2004) Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp. Hematol. 32, 773–781 [DOI] [PubMed] [Google Scholar]
- 17.Plett P. A., Frankovitz S. M., Abonour R., Orschell-Traycoff C. M. (2001) Proliferation of human hematopoietic bone marrow cells in simulated microgravity. In Vitro Cell. Dev. Biol. Anim. 37, 73–78 [DOI] [PubMed] [Google Scholar]
- 18.Meng D., Jianjun X. (2017) Tianzhou 1 cargo spacecraft leaves orbit. Aerospace China, 18, 62 [Google Scholar]
- 19.Hu W. R., Zhao J. F., Long M., Zhang X. W., Liu Q. S., Hou M. Y., Kang Q., Wang Y. R., Xu S. H., Kong W. J., Zhang H., Wang S. F., Sun Y. Q., Hang H. Y., Huang Y. P., Cai W. M., Zhao Y., Dai J. W., Zheng H. Q., Duan E. K., Wang J. F. (2014) Space program SJ-10 of microgravity research. Microgravity Sci. Technol. 26, 159–169 [Google Scholar]
- 20.Zhao H. G., Qiu J. W., Tang B. C., Kang Q., Hu W. R. (2016) The SJ-10 recoverable microgravity satellite of China. J. Space Explo. 5, 101 [Google Scholar]
- 21.Zhang C., Li L., Jiang Y., Wang C., Geng B., Wang Y., Chen J., Liu F., Qiu P., Zhai G., Chen P., Quan R., Wang J. (2018) Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 32, 4444–4458 [DOI] [PubMed] [Google Scholar]
- 22.Wang C., Chen H., Luo H., Zhu L., Zhao Y., Tian H., Wang R., Shang P., Zhao Y. (2015) Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. 64, 303–311 [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Duan K., Ma H., Niu Z., Peng J., Zhao Y. (2011) An instructive role of donor macrophages in mixed chimeras in the induction of recipient CD4(+)Foxp3(+) Treg cells. Immunol. Cell Biol. 89, 827–835 [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y., Shen X., Na N., Chu Z., Su H., Chao S., Shi L., Xu Y., Zhang L., Shi B., Zhao Y. (2018) mTOR masters monocyte development in bone marrow by decreasing the inhibition of STAT5 on IRF8. Blood 131, 1587–1599 [DOI] [PubMed] [Google Scholar]
- 25.Wu X. L., Yang Z. W., He L., Dong P. D., Hou M. X., Meng X. K., Zhao H. P., Wang Z. Y., Wang F., Baoluri, Wurenqimuge, Agudamu, Jia Y. F., Shi L. (2017) RRS1 silencing suppresses colorectal cancer cell proliferation and tumorigenesis by inhibiting G2/M progression and angiogenesis. Oncotarget 8, 82968–82980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang Z., Zhang L., Su H., Luan R., Na N., Sun L., Zhao Y., Zhang X., Zhang Q., Li J., Zhang L., Zhao Y. (2018) MTOR signaling is essential for the development of thymic epithelial cells and the induction of central immune tolerance. Autophagy 14, 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou Y., Zhu L., Tian H., Sun H. X., Wang R., Zhang L., Zhao Y. (2018) IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell 9, 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu L., Yang T., Li L., Sun L., Hou Y., Hu X., Zhang L., Tian H., Zhao Q., Peng J., Zhang H., Wang R., Yang Z., Zhang L., Zhao Y. (2014) TSC1 controls macrophage polarization to prevent inflammatory disease. Nat. Commun. 5, 4696 [DOI] [PubMed] [Google Scholar]
- 29.Sun B., Zhu L., Tao Y., Sun H. X., Li Y., Wang P., Hou Y., Zhao Y., Zhang X., Zhang L., Na N., Zhao Y. (2018) Characterization and allergic role of IL-33-induced neutrophil polarization. Cell. Mol. Immunol. 15, 782–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Feng Z., Wang X., Wang X., Zhang X. (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 [DOI] [PubMed] [Google Scholar]
- 31.Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., Kong L., Gao G., Li C. Y., Wei L. (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39, W316–W322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sergushichev A. A., Loboda A. A., Jha A. K., Vincent E. E., Driggers E. M., Jones R. G., Pearce E. J., Artyomov M. N. (2016) GAM: a web-service for integrated transcriptional and metabolic network analysis. Nucleic Acids Res. 44(W1), W194–W200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q., Chu Z., Zhu L., Yang T., Wang P., Liu F., Huang Y., Zhang F., Zhang X., Ding W., Zhao Y. (2017) 2-Deoxy-d-glucose treatment decreases anti-inflammatory M2 macrophage polarization in mice with tumor and allergic airway inflammation. Front. Immunol. 8, 637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Zhu L., Chu Z., Yang T., Sun H. X., Yang F., Wang W., Hou Y., Wang P., Zhao Q., Tao Y., Zhang L., Zhang X., Zhao Y. (2018) Characterization and biological significance of IL-23-induced neutrophil polarization. Cell. Mol. Immunol. 15, 518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholzen T., Gerdes J. (2000) The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322 [DOI] [PubMed] [Google Scholar]
- 37.Cheshier S. H., Morrison S. J., Liao X., Weissman I. L. (1999) In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 96, 3120–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopher M. J., Liu F., Hilton M. J., Long F., Link D. C. (2009) Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 114, 1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vajravelu B. N., Hong K. U., Al-Maqtari T., Cao P., Keith M. C., Wysoczynski M., Zhao J., Moore J. B., IV, Bolli R. (2015) C-Kit promotes growth and migration of human cardiac progenitor cells via the PI3K-AKT and MEK-ERK pathways. PLoS One 10, e0140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laresgoiti U., Apraiz A., Olea M., Mitxelena J., Osinalde N., Rodriguez J. A., Fullaondo A., Zubiaga A. M. (2013) E2F2 and CREB cooperatively regulate transcriptional activity of cell cycle genes. Nucleic Acids Res. 41, 10185–10198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y., Lv Q., Zou X. Q., Yan Z. X., Yan Y. X. (2016) Mechanical strain regulates osteoblast proliferation through Ca2+-CaMK-CREB signal pathway. Chin. Med. Sci. J. 31, 100–106 [DOI] [PubMed] [Google Scholar]
- 42.Vacek A., Tkadlecek L., Shvets V. N., Bartonícková A., Viklická S., Rotkovská D., Serova L. V., Michurina T. V. (1982) Space flight effects on haemopoietic stem cells of the bone marrow of rats. Cell Tissue Kinet. 15, 643–649 [DOI] [PubMed] [Google Scholar]
- 43.Berridge M. J. (2014) Module 9: cell cycle and proliferation. Cell Signal Biol. 6, 1–45 [Google Scholar]
- 44.Morettini S., Podhraski V., Lusser A. (2008) ATP-dependent chromatin remodeling enzymes and their various roles in cell cycle control. Front. Biosci. 13, 5522–5532 [DOI] [PubMed] [Google Scholar]
- 45.Sholl-Franco A., Fragel-Madeira L., Macama A. C., Linden R., Ventura A. L. (2010) ATP controls cell cycle and induces proliferation in the mouse developing retina. Int. J. Dev. Neurosci. 28, 63–73 [DOI] [PubMed] [Google Scholar]
- 46.Hsu P., Qu C. K. (2013) Metabolic plasticity and hematopoietic stem cell biology. Curr. Opin. Hematol. 20, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedessem B., Stéphanou A. (2014) A mathematical model of HiF-1α-mediated response to hypoxia on the G1/S transition. Math. Biosci. 248, 31–39 [DOI] [PubMed] [Google Scholar]
- 48.Golias C. H., Charalabopoulos A., Charalabopoulos K. (2004) Cell proliferation and cell cycle control: a mini review. Int. J. Clin. Pract. 58, 1134–1141 [DOI] [PubMed] [Google Scholar]
- 49.Schafer K. A. (1998) The cell cycle: a review. Vet. Pathol. 35, 461–478 [DOI] [PubMed] [Google Scholar]
- 50.Broudy V. C. (1997) Stem cell factor and hematopoiesis. Blood 90, 1345–1364 [PubMed] [Google Scholar]
- 51.Connell-Crowley L., Elledge S. J., Harper J. W. (1998) G1 cyclin-dependent kinases are sufficient to initiate DNA synthesis in quiescent human fibroblasts. Curr. Biol. 8, 65–68 [DOI] [PubMed] [Google Scholar]
- 52.Desdouets C., Matesic G., Molina C. A., Foulkes N. S., Sassone-Corsi P., Brechot C., Sobczak-Thepot J. (1995) Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol. Cell. Biol. 15, 3301–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.