Abstract
Salivary glands are a major component of the mucosal immune system that confer adaptive immunity to mucosal pathogens. As previously demonstrated, immunization of the submandibular gland with tissue culture–derived murine cytomegalovirus (tcMCMV) or replication-deficient adenoviruses expressing individual murine cytomegalovirus (MCMV) genes protected mice against a lethal MCMV challenge. Here, we report that salivary gland inoculation of BALB/cByJ mice with tcMCMV or recombinant adenoviruses differentially activates T helper (Th)1, -2, and -17 cells in the salivary glands vs. the associated lymph nodes. After inoculation with tcMCMV, lymphocytes from the submandibular gland preferentially express the transcription factor T-cell–specific T-box transcription factor (T-bet), which controls the expression of the hallmark Th1 cytokine, IFN-γ. Lymphocytes from the periglandular lymph nodes (PGLNs) express both T-bet and GATA-binding protein 3 (GATA3), which promotes the secretion of IL-4, -5, and -10 from Th2 cells. In contrast, after inoculation with replication-deficient adenoviruses, lymphocytes from the submandibular gland express T-bet, GATA3, and RAR-related orphan receptor γ, thymus-specific isoform (RORγt) (required for differentiation of Th17 cells) and forkhead box P3 (Foxp3) (required for the differentiation of regulatory T cells). Lymphocytes from the PGLNs were not activated. The differential induction of Th responses in the salivary gland vs. the PGLNs after inoculation with attenuated virus vs. a nominal protein antigen supports the use of the salivary as an alternative mucosal route for administering vaccines.—Liu, G., Zhang, F., Wang, R., London, S. D., London, L. Salivary gland immunization via Wharton’s duct activates differential T-cell responses within the salivary gland immune system.
Keywords: mucosal immunity, vaccine development, T helper cells
Because mucosal surfaces of the body are a significant portal of entry for many pathogens (especially viruses), understanding the mechanisms of defense in these tissues is an important endeavor in vaccine development (1–4). The salivary glands (parotid, submandibular, and sublingual glands) are major components of the mucosal immune system of the oral cavity. We previously demonstrated that immunization of the salivary gland with tissue culture–derived murine cytomegalovirus (tcMCMV) or replication-deficient adenovirus expressing individual murine cytomegalovirus (MCMV) genes induced salivary gland ectopic follicles that displayed both functional and phenotypic characteristics of mucosal inductive site germinal centers (GCs), including cognate interactions of B and T cells with follicular dendritic cells, proliferating cells, class switching, plasma cell differentiation, and protection against a lethal systemic challenge with MCMV (5–7). In addition, both systemic and mucosal MCMV-specific antibodies and MCMV-specific T-cell responses were induced after immunization with either tcMCMV or replication-deficient adenovirus expressing individual MCMV genes (5–7). T helper (Th)1, -2, and -17 cells are the prototypical CD4 T-cell subsets that originally were described based on their cytokine profiles, with Th1 cells predominantly secreting IFN-γ, Th2 cells secreting IL-4 and -5, and Th17 cells secreting IL-17 (8–11). Their differentiation is driven by specific transcription factors, including T-cell–specific T-box transcription factor (T-bet), a Th1-specific T-box transcription factor that controls the development of Th1 cells and expression of the hallmark Th1 cytokine, IFN-γ (12); GATA binding protein 3 (GATA3), which controls the development of Th2 cells and expression of IL-4, -5, and -10; and RAR-related orphan receptor γ, thymus-specific isoform (ROR-γt), which controls the development of Th17 cells and expression of IL-17 (11, 12). In addition to its role in Th1 development, T-bet plays important roles in other Th cell populations, including T follicular helper (Tfh) cells, which are critical for providing help to B cells during GC responses (13, 14). Regulatory T (Treg) cells are an additional subset of CD4 T cells with potent immunosuppressive properties that play a central role in the maintenance of immune homeostasis (15–17). These cells are defined by stable expression of the lineage-specific transcription factor forkhead box P3 (Foxp3) and high amounts of the IL-2 receptor α-chain (CD25) (15–18).
Studies exploring the salivary gland as an immunization site have involved immunization with pathogens, protein antigens, and subunit DNA vaccines (4, 19). Although many of these studies demonstrated the production of both systemic IgG and mucosal IgA (salivary glands and gastrointestinal tract) after immunization near and within the salivary glands, these studies did not investigate the development of Th cell function, including the production of signature cytokines. In this study, we evaluated the expression of transcription factors responsible for Th1, -2, -17, and Treg differentiation after salivary gland immunization and profiled cytokine expression related to Th cell immune responses.
MATERIALS AND METHODS
Virus
Female CD1 mice (Charles River Laboratories, Wilmington, MA, USA) were used to propagate MCMV in vivo. Smith strain MCMV [105 plaque-forming units (PFU)/mouse; American Type Culture Collection (ATCC), Manassas, VA, USA] was injected intraperitoneally into CD1 mice. After 14 d, salivary glands were homogenized using a BeadBeater (BioSpec, Bartlesville, OK, USA) and tittered for MCMV PFU via a standard plaque assay utilizing 3T12 cells. The homogenate was used to infect naive CD1 mice (105 PFU/mouse). This process was repeated twice, and third-passage MCMV was used for inoculation (referred to as MCMV). Attenuated tcMCMV was generated by infecting 3T12 mouse embryo fibroblasts (ATCC) with third-passage MCMV (multiplicity of infection 0.1). After 6 d of culture, tcMCMV was isolated from the supernatant and infected fibroblasts and purified using sucrose density gradient centrifugation (6, 20). Recombinant adenoviruses expressing glycoprotein (gp)-B, gpH, or immediate early (IE)-1 of MCMV were constructed by inserting those genes into a replicate-deficient adenoviral vector (Ad) and transfection into AD-293 cells to generate infectious virus (Ad-gpB, -gpH, and -IE1) (21, 22). The replication-deficient recombinant adenoviruses and FG140, the replication-deficient adenovirus lacking an insert, were amplified by 3 passages in AD-293 cells, tittered via a standard plaque assay utilizing AD-293 cells, and stored at −80°C until use. All recombinant adenoviruses, FG140, and the AD-193 cell line were kindly provided by Dr. John Shanley (University of Connecticut, Farmington, CT, USA).
Animals and immunization protocol
Salivary gland immunization via the Wharton’s duct was performed as previously described (7). Briefly, 5–6-wk-old female BALB/cByJ mice (The Jackson Laboratory, Bar Harbor, ME, USA) were subcutaneously injected with atropine sulfate monohydrate (0.5 mg/kg) to prevent salivary secretions and anesthetized intraperitoneally with ketamine (90 mg/kg) and xylazine (10 mg/kg) in 0.9% saline. The mice were placed on a custom-made plastic platform in the ventral position. The maxillary incisors were locked on a metal wire, and the mandibular incisors were hooked on an elastic string to hold the mouth open. A 0.58-mm diameter polyethylene tube (PE-10; Braintree Scientific, Braintree, MA, USA) was inserted 3–5 mm inside Wharton’s duct, and an insulin syringe with a 29-gauge needle was inserted into the other end of the polyethylene tube. A total of 100 µl (50 μl/duct) of immunogen was slowly injected into the submandibular salivary gland. BALB/cByJ mice were inoculated with 105 PFU/mouse tcMCMV in 60 mM sodium bicarbonate or the equivalent volume of saline via retrograde perfusion of the salivary gland. For prime-boost experiments, 21 d after primary inoculation, mice were boosted with either 105 PFU/mouse tcMCMV in 60 mM sodium bicarbonate or the equivalent volume of saline via retrograde perfusion. Prime-boost experiments were analyzed 10 d after boost. In protection studies, saline-inoculated BALB/cByJ mice were challenged systemically (intraperitoneally) with 2 × 104 PFU/mouse MCMV (100 μl), and tcMCMV-inoculated mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV (100 μl). A total of 2 × 104 PFU/mouse MCMV was used in saline-inoculated mice because challenge with a lethal inoculum of MCMV (5 × 104 PFU/mouse) resulted in the death of all mice by d 8 postchallenge (7). In experiments utilizing replication-deficient recombinant adenoviruses, BALB/cByJ mice were inoculated and boosted on d 30, with 106 PFU/mouse replication-deficient recombinant adenovirus expressing a combination of MCMV genes gpB, gpH, and IE1 (Ad-Combo) (106 PFU/mouse of each recombinant virus). Mice inoculated with 106 PFU/mouse FG140 or saline (mock) served as negative controls. All animal protocols were approved by the Stony Brook University Institutional Animal Care and Use Committee Review Boards.
Collection of lymphocyte populations
Single-cell suspensions of salivary gland cells were obtained as previously described, with modifications (5–7). Briefly, salivary glands were separated from the periglandular lymph nodes (PGLNs)—also called superficial cervical lymph nodes—and adipose and connective tissues using a dissecting microscope. Salivary glands were minced into small fragments and incubated 3 times in digestion medium containing CaCl2 (100 mM), collagenase type IV (0.5 mg/ml; MilliporeSigma, Burlington, MA, USA), and DNase type I (0.1 mg/ml; Roche, Indianapolis, IN, USA) in Rosewell Park Memorial Institute (RPMI) 1640 + 5% newborn calf serum (Bio-Techne, Minneapolis, MN, USA) for 20 min at 37°C with stirring. Salivary gland lymphocytes were isolated using a discontinuous Percoll gradient (MP Biomedicals, Santa Ana, CA, USA) spun at 1800 rpm for 20 min at 20°C in a Heraeus Multifuge ×3R (Thermo Fisher Scientific, Waltham, MA, USA). Lymphocytes were collected at the interface between 44 and 67.5% Percoll. Spleens and PGLNs were homogenized by grinding between 2 frosted glass slides, and debris was removed by passing the homogenate through a nylon mesh. Cells were pelleted by spinning at 1200 rpm for 10 min at 4°C in a Heraeus Multifuge ×3R. Red blood cells were lysed in ammonium chloride/potassium bicarbonate/EDTA buffer for 5 min at room temperature. Cells were then washed twice with cold PBS. Live cells were counted via trypan blue dye exclusion.
Collection of saliva and serum
Saliva was stimulated via a subcutaneous injection of pilocarpine nitrate (5 mg/ml, 0.15 mg/30 µl; MilliporeSigma) and collected using a pipette into microcentrifuge tubes on ice. Saliva was collected for a total of 20 min. We have previously demonstrated that in mice inoculated intraperitoneally with MCMV salivary output (saliva) was significantly diminished and normal salivary output was maintained after intraglandular inoculation with tcMCMV (5–7). In addition, mice were protected from diminished salivary output when inoculated intraglandularly with tcMCMV prior to challenge intraperitoneally with MCMV (5, 7). Similarly, in this study, mice inoculated intraglandularly with Ad-Combo had similar saliva output compared with control mice, and these mice were also protected from diminished salivary production in response to intraperitoneal MCMV (unpublished results). Whole blood was collected from the vascular bundle located at the rear of the jawbone using Goldenrod Lancets (Medipoint, Mineola, NY, USA) weekly or by cardiac puncture at the time of euthanasia. Blood was allowed to clot at room temperature. Serum was separated by centrifugation at 10,000 rpm for 5 min in an Eppendorf 5415R (Eppendorf North America, Hauppauge, NY, USA), removed to new microcentrifuge tubes, and stored at −20°C until assay.
Flow cytometric analysis of lymphocyte populations
Cells (106) were washed twice with 1 ml staining buffer (PBS, 1% fetal calf serum, 0.02% sodium azide, pH 7.0) and then resuspended in 100 μl staining buffer containing antibodies for cell surface marker expression. Cells were incubated with directly labeled primary antibodies for 30 min on ice in the dark and then washed twice with staining buffer. The following directly labeled antibodies were used in this study: CD8-α [clone 53-6.7, allophycocyanin (APC)–labeled; BD Biosciences, San Jose, CA, USA], CD4 (clone H129.19, FITC-labeled; Thermo Fisher Scientific), CD103 (clone 2E7, FITC-labeled; Thermo Fisher Scientific), and CD62L (clone MEL-14, FITC-labeled; BioLegend, San Diego, CA, USA). Intracellular staining for Foxp3 was performed with Foxp3 Staining Buffer Set (Thermo Fisher Scientific) following the manufacturer’s instructions, washed twice with staining buffer, and resuspended in 300 μl staining buffer. The absolute number of positive cells for each subpopulation was obtained by multiplying cell yields by the percentage positive for each marker. Flow cytometry data were acquired on an Accuri C6 flow cytometer (BD Biosciences) and analyzed using FlowJo software (BD Biosciences).
Th1/Th2/Th17 cytokine bead array
A commercial BD Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences) was used to determine the concentration (pg/ml) of IL-2, -4, -6, -10, TNF-α, IFN-γ, and IL-17A in serum and saliva samples, according to the manufacturer’s instructions. Fluorescence was analyzed using a flow cytometer (FacsCalibur; Thermo Fisher Scientific), and cytokine levels were determined using BD Cytometric Bead Array Software (FCAP Array v.1; BD Biosciences).
RNA preparation and quantitative RT-PCR
Salivary glands, PGLNs, and spleens were homogenized in the presence of Tri Reagent solution, and total RNA was isolated according to the manufacturer’s instructions (Thermo Fisher Scientific). Specific primers for mouse T-bet, GATA3, Foxp3, ROR-γt, IL-2, -6, -10, -12p40, IFN-γ, TGF-β, TNF-α, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are presented in Table 1. All primers were selected from PrimerBank (http://pga.mgh.harvard.edu/primerbank/index.html) and were obtained from MilliporeSigma. Quantitative RT-PCR (qRT-PCR) was carried out in 20 µl total volume containing 100 ng of RNA and 200 nM of each primer using a Power Sybr Green RNA-to-CT 1-Step Kit (Applied Biosystems). After reverse transcription for 30 min at 48°C and a starting denaturation for 10 min at 95°C, 40 PCR cycles (15 s at 95°C and 1 min at 60°C) were performed using the ABI StepOne Plus Real-Time PCR System (Thermo Fisher Scientific). Each sample was evaluated in triplicate. Specificity of PCR products was evaluated at the end of each run by a melt curve analysis. A nontemplate control and an endogenous control (GAPDH) were used for relative quantification. All quantitations (Ct values) were normalized to that of GAPDH to generate ΔCt, and the difference between the ΔCt value of the sample and that of the reference [saline (control)] was calculated as ΔΔCt. The relative level of gene expression was expressed as 2−ΔΔCt (23).
TABLE 1.
Th1/Th2/Th17 related primers used for qRT-PCR
| Primer sequence, 5−3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| T-bet | AGCAAGGACGGCGAATGTT | GGGTGGACATATAAGCGGTTC |
| GATA3 | CTCGGCCATTCGTACATGGAA | GGATACCTCTGCACCGTAGC |
| Foxp3 | CCCATCCCCAGGAGTCTTG | ACCATGACTAGGGGCACTGTA |
| ROR-γt | ACCTCCACTGCCAGCTGTGTGCTGTC | TCATTTCTGCACTTCTGCATGTAGACTGTCCC |
| IL-2 | TGAGCAGGATGGAGAATTACAGG | GTCCAAGTTCATCTTCTAGGCAC |
| IL-6 | GAGGATACCACTCCCAACAGACC | AGTGCATCATCGTTGTTCATACA |
| IL-10 | CCCTTTGCTATGGTGTCCTT | TGGTTTCTCTTCCCAAGACC |
| IL-12b | TGGTTTGCCATCGTTTTGCTG | ACAGGTGAGGTTCACTGTTTCT |
| IFN-γ | GCGGCTGACTGAACTCAGATTGTAG | TCACAGTTTTCAGCTGTATAGGG |
| TGF-β1 | CTCCCGTGGCTTCTAGTGC | GCCTTAGTTTGGACAGGATCTG |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| GAPDH | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA |
Statistical analysis
A Student’s t test with 2-tailed distribution and 2-sample, unequal variance was performed to determine statistical significance in pair-wise comparisons. Values of P < 0.05 were considered significant. Data points were excluded from analysis by using Grubb’s test for detecting outliers.
RESULTS
Retrograde perfusion of the submandibular salivary gland with tcMCMV activated Th1/Th2/Th17 transcription factors associated with adaptive immunity
Because we demonstrated that both intraglandular inoculation (5, 6) and retrograde perfusion of the submandibular salivary gland (7) with tcMCMV generated MCMV-specific T-cell immunity, we evaluated the Th1/Th2/Th17 immune responses after both tcMCMV inoculation and a lethal MCMV challenge. BALB/cByJ mice were inoculated with saline or 105 PFU/mouse tcMCMV via retrograde perfusion of the submandibular gland. On d 28 after inoculation, saline-inoculated mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV, and tcMCMV-inoculated mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. RNA was prepared from enriched salivary gland lymphocytes after removal of the associated lymph nodes prior to inoculation (−/−) or challenge (+/−) and postchallenge (−/+ or +/+) on d 14 and analyzed for key transcription factors that control the differentiation of Th1/Th2/Th17 cells and expression of key cytokines by qRT-PCR.
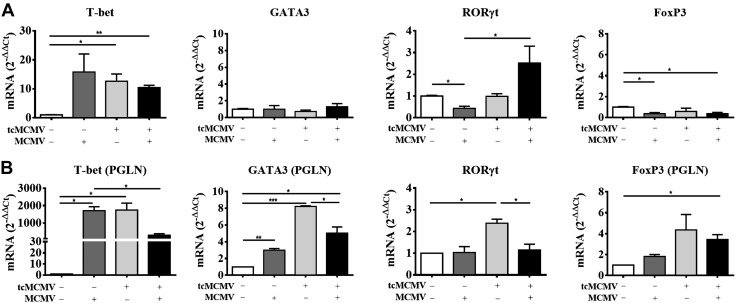
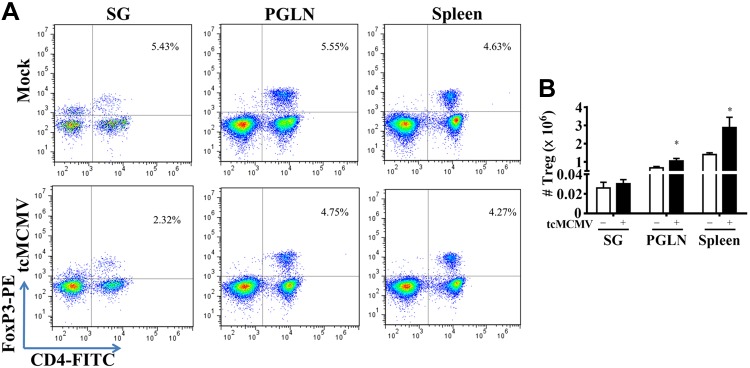
Compared with control mice, T-bet, a Th1-specific transcription factor that controls the expression of the hallmark Th1 cytokine, IFN-γ (12), was increased in both the salivary gland (Fig. 1A) and PGLNs (Fig. 1B) in challenged (−/+), primed (+/−), and primed/challenged (+/+) mice. Although no significant expression of GATA3, which promotes the secretion of IL-4, -5, and -10 from Th2 cells (8, 12), was observed in the salivary glands (Fig. 1A), in the PGLNs, GATA3 was increased in challenged (−/+), primed (+/−), and primed/challenged (+/+) mice (Fig. 1B) compared with controls. In addition, in the PGLNs, expression of both T-bet and GATA3 was reduced in primed/challenged (+/+) compared with primed (+/−) mice (Fig. 1B). In the salivary gland, expression of ROR-γt, required for differentiation of Th17 cells (9, 10, 12), was increased in primed/challenged (+/+) mice only (Fig. 1A), whereas expression of Foxp3, required for the development and function of Treg cells (12, 15–17), was not significantly expressed (Fig. 1A). Alternatively, in the PGLNs, ROR-γt was increased in primed (+/−) compared with control mice and was reduced in primed/challenged (+/+) compared with primed mice (+/−) (Fig. 1B). In the PGLNs, Foxp3 was increased in primed (+/−) and primed/challenged (+/+) mice compared with control mice (Fig. 1B). In addition, we have stained selected samples via immunohistochemistry, including mock (control; open bars, Fig. 1A) and primed/challenged mice, on d 14 postchallenge (black bars, Fig. 1A) with antibodies against T-bet and GATA3. Our results demonstrated no staining for either T-bet or GATA3 from control mice (unpublished data). However, we observed positive staining for T-bet and no staining for GATA3 in lymphoid follicles within the salivary glands from primed/challenged mice, which mirrors the results we obtained with mRNA expression (unpublished data). We also verified the mRNA expression pattern of Foxp3 in isolated lymphocytes from the salivary glands, PGLNs, and spleen by intracellular staining for Foxp3 via flow cytometry. We observed that control salivary glands contained a population of CD4+Foxp3+ Treg cells that was similar in percentage to that observed in the PGLNs and the spleen (Fig. 2A, top row). However, although the percentage of CD4+Foxp3+ Treg cells decreased ∼2-fold in the salivary glands of tcMCMV-inoculated mice compared with control mice, the absolute number of Treg cells in the salivary gland remained the same (Fig. 2B). In contrast, whereas the percentage of CD4+Foxp3+ Treg cells was similar in the PGLN and spleen in control and tcMCMV-inoculated mice (Fig. 2A, bottom row), the absolute number of CD4+Foxp3+ Treg cells increased in the PGLNs and the spleen in tcMCMV-inoculated mice (Fig. 2B). These data are consistent with mRNA expression of Foxp3 observed in Fig. 1.
Figure 1.
Retrograde perfusion of the submandibular salivary gland with tcMCMV resulted in the expression of transcription factors associated with Th1/Th2/Th17 cytokines after a lethal challenge with MCMV. BALB/cByJ mice were inoculated with saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion of the salivary gland. On d 28 after inoculation, saline mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV, and tcMCMV mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. RNA was prepared from the PGLNs and the lymphocytes obtained from the whole salivary glands after removal of the associated lymph nodes in saline control (mock) (−/−), primed (+/−), or postchallenge (−/+ or +/+) mice on d 14 and analyzed for expression of the indicated transcription factors by qRT-PCR. A) Salivary glands. B) PGLNs. Data are the means ± sd of 5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
Retrograde perfusion of the submandibular gland with tcMCMV modulated CD4+Foxp3+ Treg cells. BALB/cByJ mice were inoculated with either saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion of the salivary gland. Single-cell suspensions were prepared from lymphocytes obtained from the whole salivary glands, PGLNs, and spleens on d 14 after inoculation and analyzed for the coexpression of the T-cell surface marker CD4 and the intracellular marker Foxp3. A) Dot plots demonstrate lymphocytes stained with CD4 (x axis) and Foxp3 (y axis). The percentage of dual-staining cells is shown in the upper right quadrant. B) The histogram demonstrates the absolute numbers of CD4+Foxp3+ Treg cells from saline- (open bars) and tcMCMV- (solid bar) inoculated mice. Data are the means ± sd from 5 individual mice/group. *P < 0.05 vs. saline
Retrograde perfusion of the submandibular salivary gland with tcMCMV activated specific Th1/Th2/Th17 cytokine expression associated with adaptive immunity
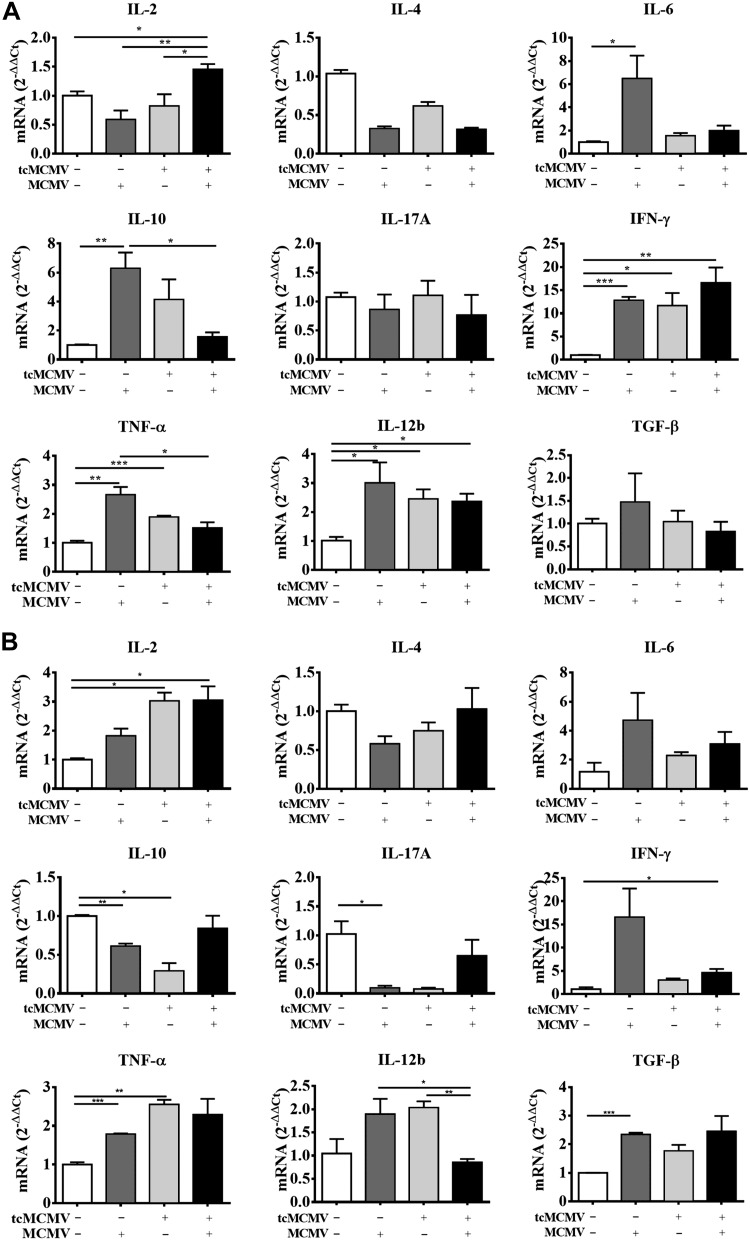
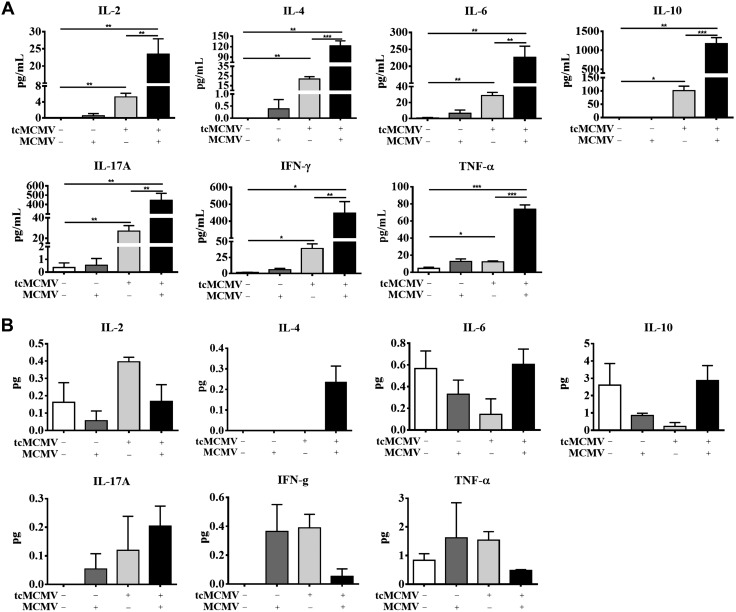
We next evaluated whether the expression of transcription factors evaluated in Fig. 1 correlated with cytokine mRNA expression from enriched salivary gland lymphocytes and PGLNs (Fig. 3) and cytokine protein expression in the saliva and serum (Fig. 4). The expression of mRNA for the Th1 cytokines IFN-γ and IL-12β was significantly increased in salivary gland lymphocytes from challenged (−/+), primed (+/−), and primed/challenged (+/+) mice (Fig. 3A). The expression of mRNA for IL-2 was increased only in the primed/challenged (+/+) mice, whereas the expression of TNF-α was increased in the challenged (−/+) and primed (+/−) mice only (Fig. 3A). The expression of the Th2 cytokine IL-10 was increased in primed (+/−) and challenged (−/+) mice, whereas expression of IL-6 was only increased in challenged (−/+) mice. Th2 cytokine IL-4, the Th17 cytokine IL-17, and TGF-β were not significantly expressed under any inoculation protocol (Fig. 3A). Lymphocytes obtained from the PGLNs significantly expressed mRNA for IL-2 in the primed (+/−) and primed/challenged (+/+) mice, for IFN-γ in the challenged (−/+) mice, and for IL-12β and TNF-α in the challenged (−/+) and primed (+/−) mice (Fig. 3B). Significant expression of TGF-β was only observed in the challenged (−/+) mice (Fig. 3B). There was no significant mRNA expression of IL-10, -17A, -4, or -6 in any group examined (Fig. 3B). These results were consistent with significant cytokine protein expression for the Th1 cytokines (IL-2, IFN-γ, TNF-α) and the Th2 cytokines (IL-10 and -6) in the serum (Fig. 4A) and for expression of Th1 cytokines (IL-2, IFN-γ, TNF-α) in the saliva (Fig. 4B). Taken together, these data provide direct evidence that lymphocytes obtained from the salivary gland preferentially express the necessary transcription factors to differentiate along the Th1 cell pathway, whereas lymphocytes obtained from the PGLNs have the potential to differentiate along either the Th1 or Th2 cell pathways, which is reflected in the production of cytokines either locally (saliva) or systemically (serum).
Figure 3.
Retrograde perfusion of the submandibular gland with tcMCMV resulted in Th1/Th2/Th17 cytokine mRNA expression after a lethal challenge with MCMV. BALB/cByJ mice were inoculated with saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion of the salivary gland. On d 28 after inoculation, saline mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV and tcMCMV mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. RNA was prepared from PGLNs and lymphocytes obtained from whole salivary glands after removal of the associated lymph nodes in saline control (mock) (−/−), primed (+/−), or postchallenge (−/+ or +/+) mice on d 14 and analyzed for expression of the indicated cytokines/chemokines by qRT-PCR. A) Salivary glands. B) PGLNs. Data are the means ± sd of 5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
Retrograde perfusion of the submandibular gland with tcMCMV resulted in Th1/Th2/Th17 cytokine protein expression after a lethal challenge with MCMV. BALB/cByJ mice were inoculated with saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion of the salivary gland. On d 28 after inoculation, saline mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV and tcMCMV mice were challenged with 5 × 104 PFU/mouse MCMV. Serum and saliva samples were collected from saline control (mock) (−/−), primed (+/−), or postchallenge (−/+ or +/+) mice on d 14 and subjected to a Th1/Th2/Th17 cytokine bead array assay. A) Serum (pg/ml). B) Saliva (total pg) recovered to normalize for differences in the volume of saliva collected from individual animals. Data are the means ± sd of 5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001.
The percentage of lymphocytes obtained from the submandibular salivary gland expressing the integrin αE is increased after retrograde perfusion with tcMCMV
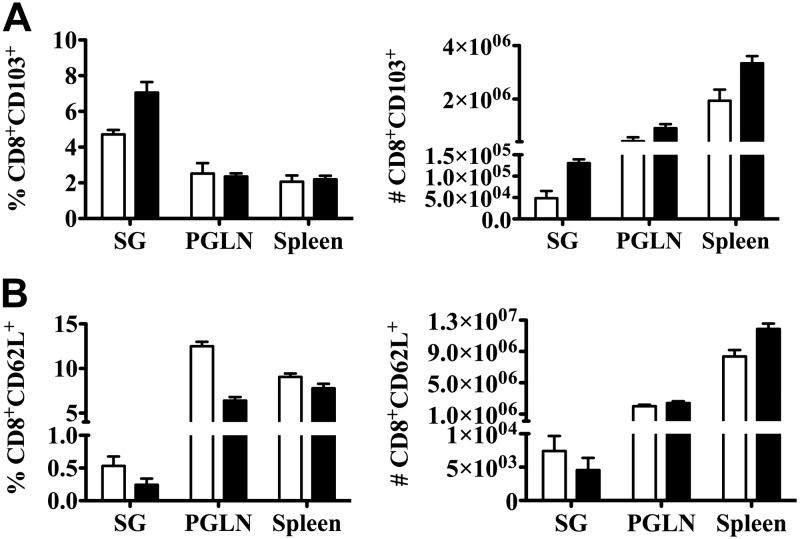
To evaluate homing receptor expression on lymphocytes obtained from the submandibular gland as a marker of mucosal induction, BALB/cByJ mice were inoculated with saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion. On d 28 after inoculation, saline-inoculated mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV, and tcMCMV-inoculated mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. Single-cell suspensions were prepared from lymphocytes obtained from the salivary glands, PGLNs, and spleens on d 14 after challenge and analyzed for the coexpression of the T-cell surface marker CD8 and the adhesion molecules CD62L or -103. Both the percentage and absolute number of CD8+ T cells expressing CD103, a component of the heterodimeric integrin molecule αEβ7 associated with mucosal homing, was increased in lymphocytes obtained from the salivary gland after prime/challenge (black bars) compared with mock (saline/saline) inoculated mice (Fig. 5A). Concomitantly, both the percentage and absolute number of CD8+ T-cells expressing CD62L (l-selectin), the dominant adhesion molecule involved in homing of naive T cells to lymph nodes, was decreased in lymphocytes obtained from the salivary gland after prime/challenge (black bars) compared with mock (saline/saline) inoculated mice (Fig. 5B). These data suggest that lymphocytes activated in the salivary gland up-regulate the expression of the homing receptor CD103 and therefore have the potential to home to mucosal tissues.
Figure 5.
Retrograde perfusion of the submandibular gland with tcMCMV resulted in increased expression of integrin αE. BALB/cByJ mice were inoculated with saline (mock) or 105 PFU/mouse tcMCMV via retrograde perfusion of the salivary gland. On d 28 after inoculation, saline mice were challenged intraperitoneally with 2 × 104 PFU/mouse MCMV and tcMCMV mice were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. Single-cell suspensions of lymphocytes were prepared from the whole salivary glands, PGLNs, and spleens on d 14 postchallenge and analyzed for the coexpression of the T-cell surface marker CD8 and the adhesion molecules CD62L or -103. A) The histogram demonstrates the percentage and absolute numbers of CD8+CD103+ in mock (−/−) (open bars) or tcMCMV postchallenge with MCMV (solid bar). B) The histogram demonstrates the absolute numbers of CD8+CD62L+ in mock (−/−) (open bars) or tcMCMV postchallenge with MCMV (solid bar). Data are the mean ± sd from 5 individual mice/group.
Retrograde perfusion of the submandibular salivary gland with replication-deficient recombinant adenovirus expressing MCMV genes activated Th1/Th2/Th17 adaptive immunity
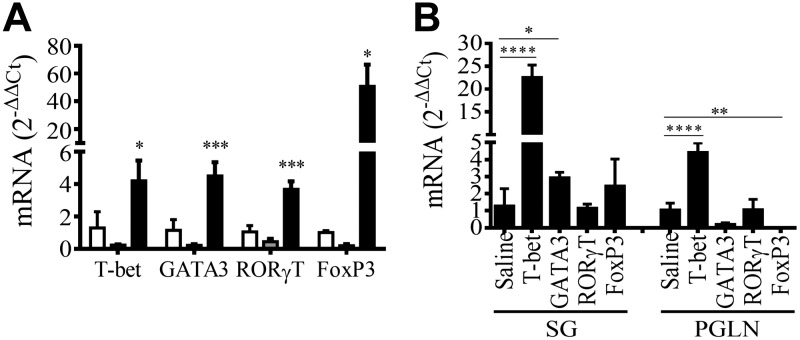
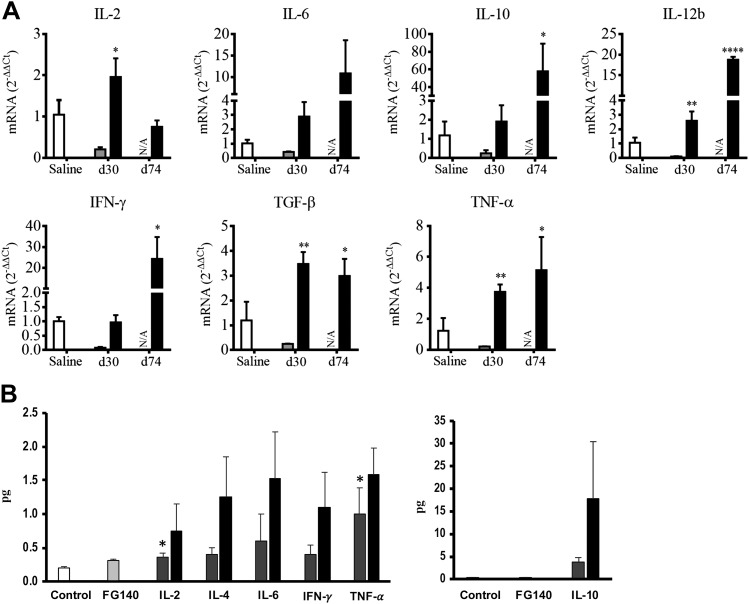
BALB/cByJ mice were inoculated (d 0) and boosted (d 30) with saline, 106 PFU/mouse of FG140 (replication-deficient adenovirus lacking MCMV inserts), or 106 PFU/mouse of replication-deficient recombinant adenoviruses expressing gpB, gpH, and Immediate Early (IE)-1 genes of MCMV (Ad-Combo) via retrograde perfusion of the submandibular salivary gland. On d 30 after boost, mice inoculated with saline were challenged intraperitoneally with saline, and mice inoculated with FG140 or Ad-Combo were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. RNA was prepared from the PGLNs and lymphocytes obtained from the whole salivary glands after removal of the associated lymph nodes on d 30 after primary inoculation and 2 wk postchallenge (d 72) for evaluation of Th1/Th2/Th17 immune response via qRT-PCR. After inoculation with Ad-Combo (solid bar), mRNA expression for the transcription factors T-bet (Th1), GATA3 (Th2), ROR-γt (Th17), and Foxp3 (Treg cells) were all activated within the salivary gland (Fig. 6A). No significant expression of any transcription factors was observed in the salivary glands after immunization with either saline (open bar) or FG140 (gray bar) (Fig. 6A). No significant expression of any transcription factors was observed in the PGLNs after prime/boost with saline, FG140, or Ad-Combo via retrograde perfusion of the submandibular gland (unpublished data). After prime/boost with Ad-Combo via retrograde perfusion of the submandibular salivary gland and challenge intraperitoneally with MCMV, significant expression of T-bet and GATA3 in the salivary gland and T-bet in the PGLNs was observed (Fig. 6B). As a result of activation of Th1/Th2/Th17 transcription factors, the mRNA for Th1-related cytokines (IL-2, -12β, IFN-γ, TNF-α), Th2-related cytokines (IL-6, -10), and a Th17-related cytokine (TGF-β) were expressed in the salivary glands after prime with Ad-Combo (d 30) or prime/boost with Ad-Combo and challenge with MCMV (d 74) (Fig. 7A). Similarly, protein expression for the Th1-related cytokines (IL-2, IFN-γ, TNF-α) and Th2-related cytokines (IL-6, -4, -10) was observed in the saliva (Fig. 7B). Taken together, these data suggest that inoculation of the salivary glands with nominal antigen (Ad-Combo) activates the transcription factors necessary for the induction of Th1/Th2/Th17 cells and the cytokines associated with these cell types.
Figure 6.
Retrograde perfusion of the submandibular salivary gland with replication-deficient recombinant adenovirus expressing MCMV genes resulted in the expression of transcription factors associated with Th1/Th2/Th17 responses. BALB/cByJ mice were inoculated (d 0) and boosted on d 30 with either saline, 106 PFU/mouse of FG140, or a combination of Ad-gpB, -gpH, and -IE1 (106 PFU/mouse of each recombinant virus, Ad-Combo) via retrograde perfusion of the submandibular salivary gland. On d 30 after boost, mice inoculated with saline were challenged intraperitoneally with saline and mice inoculated with FG140 or Ad-Combo were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. RNA was prepared from the whole salivary glands after removal of the associated lymph nodes and from the PGLNs and were analyzed for expression of the indicated transcription factors by qRT-PCR. A) Salivary gland RNA was analyzed from mice inoculated (d 0) and boosted on d 30 (prechallenge) with saline (mock, open bars), 106 PFU/mouse of FG140 (negative control, gray bars), or with Ad-Combo (solid bars) for expression of Th-related transcription factors. B) RNA from the salivary glands and PGLNs at 2 wk postchallenge was analyzed from mice inoculated and boosted (d 0, d 30) with Ad-Combo and challenged with 5 × 104 PFU/mouse MCMV (d 72) (solid bars) for expression of Th-related transcription factors. Data are the means ± sd of ≤5 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 7.
Retrograde perfusion of the submandibular salivary gland with replication-deficient recombinant adenovirus expressing MCMV genes resulted in the expression of cytokines associated with Th1/Th2/Th17 responses. BALB/cByJ mice were inoculated (d 0) and boosted on d 30 with either saline, 106 PFU/mouse of FG140, or a combination of Ad-gpB, -gpH, and -IE1 (106 PFU/mouse of each recombinant virus, Ad-Combo) via retrograde perfusion of the submandibular salivary gland. On d 30 after boost, mice inoculated with saline were challenged intraperitoneally with saline, and mice inoculated with FG140 or Ad-Combo were challenged intraperitoneally with 5 × 104 PFU/mouse MCMV. A) RNA was prepared from the whole salivary glands after removal of the associated lymph nodes and was analyzed for Th-related cytokine expression at d 30 after primary inoculation and boost (prechallenge) and at 2 wk post-boost/challenge (d 74). Saline (open bars), FG140 (negative control, gray bars), and Ad-Combo (solid bars). Data were not available for FG140-inoculated mice after lethal challenge (d 74) due to death. B) Saliva samples were collected from mice inoculated with saline (control, open bars), from FG140 (negative control, gray bars), from Ad-Combo at d 30 after primary inoculation and boost (prechallenge, hashed bars), and from Ad-Combo mice challenged intraperitoneally with MCMV (d 74 postchallenge, solid bars) and were analyzed via a Th1/Th2/Th17 cytokine bead array assay. Saliva was expressed as total picograms recovered to normalize for differences in the volume of saliva collected from individual animals. Data are the mean ± sd of ≤5 mice/group. *P < 0.05, **P < 0.01, ****P < 0.0001.
DISCUSSION
Cytokines are critical to the host defense against MCMV infection. Both TNF-α and IFN-γ have been associated with protective effects during acute infection, and IL-4, -6, and -10 have also been reported to play a role in MCMV infection (24, 25). Many of these studies utilized a systemic immunization protocol with MCMV (intraperitoneal) and assessed serum levels or systemic cytokine responses. After intraperitoneal immunization with MCMV, the induction pattern of cytokines is organ specific, and cytokine production correlates with organ-specific viral load and disease severity (26). In regard to the salivary gland, cytokine levels (IL-10 and IFN-γ) stayed low early when viral replication was limited and rose later at d 15 in concert with the later increases in viral load (26). Additional studies demonstrated a striking difference in the immune response to MCMV infection between the submandibular gland and draining lymph nodes after intraperitoneal inoculation with MCMV (27, 28). Cytokine and chemokine profiles demonstrated that the expression of IFN-γ, IL-10, and CC chemokines was significantly greater in immune cells of the submandibular gland than in spleen and lymph node cells (27, 28). Our studies support and extend these findings. Using retrograde perfusion of the submandibular gland via Wharton’s duct, we evaluated the expression of cytokines in the serum (systemic), saliva (mucosal), and in the lymphocytes obtained from the submandibular gland and draining lymph nodes. In the submandibular gland or saliva, IFN-γ, TNF-α, and IL-2 (prototypical Th1 cytokines) were expressed, whereas little to no expression of Th2 cytokines (IL-4, -6, -10) was observed. In contrast, in the PGLNs, the expression of both Th1 and Th2 cytokines was observed at the mRNA level. These results were verified via the expression of key transcription factors associated with the Th1 (T-bet) and Th2 (GATA3) T-cell lineages. We also demonstrated that the Th1 (T-bet) but not the Th2 (GATA3) pathway was induced in the salivary gland after cannulation of the submandibular salivary gland with tcMCMV. In contrast, transcription factors necessary for induction of both Th1 (T-bet) and Th2 (GATA3) T-cell pathways were up-regulated in the PGLNs. Thus, we demonstrated a differential expression of Th1 vs. -2 responses in the primary site of inoculation (salivary gland) vs. the draining PGLNs. These data support the concept that immunization of the salivary gland results in the expression of a number of key cytokines, providing a milieu for the differentiation of Th cell populations necessary for the induction of both humoral- and cell-mediated immune responses. Thus, protection against pathogens that invade the host through mucosal surfaces may be induced through direct inoculation of the salivary glands, supporting the use of the salivary as an alternative mucosal route for administering vaccines.
To our knowledge, our studies are some of the only studies that have investigated cytokine expression in the salivary gland in either a viral infection model or after inoculation with a nominal (Ad-Combo) antigen. Much of the data concerning expression of specific cytokines in the salivary glands is in reference to cytokine expression in Sjögren’s syndrome (SS). SS is a chronic autoimmune disease of the exocrine glands, including the salivary glands, with the predominant infiltration of CD4+ T cells leading to destruction of and dysregulated function of the gland (29, 30). An increase in expression of all Th subset–related molecules and a strong association of Th2- and Tfh-related molecules was associated closely with lymphocytic accumulation in whole labial salivary glands from SS patients (31). In addition, expression of Th1- and Th17-related molecules in infiltrating lymphocytes without ectopic GC was higher than those with ectopic GC, whereas expression of Th2- and Tfh-related molecules in infiltrating lymphocytes with ectopic GC was higher than in those without ectopic GC (30–32). In other reports, minor salivary glands from primary SS (pSS) patients contained IL-7–expressing cells and IL-17–expressing cells as a dominant population within inflammatory lesions and correlated with disease severity (33–35). In addition, TGF-β, IL-6, -12, and -23, which are requisite promoters of Th17 differentiation, were abundantly expressed (35). However, although TGF-β is a differentiation factor for Foxp3+ Treg cells, only a mild increase in Foxp3+ Treg cells was observed in SS patients (36, 37). Further studies demonstrated that IL-18 expressed in salivary gland cells together with IL-17 activates the secretion of inflammatory cytokines IL-6 and -8 (38). In SS, both IL-7 and -23 are capable of promoting IL-17 polarization of mucosal-associated invariant T cells, a novel subpopulation of nonconventional/innate-like T cells, through distinct molecular pathways involving RAR-related orphan receptor C or signal transducer and activator of transcription 3 and hypoxia-inducible factor 1α expression (39, 40). Unlike what is observed in SS, our data demonstrate that lymphocytes obtained from the submandibular gland after retrograde perfusion with tcMCMV preferentially express the transcription factor T-bet, and significant levels of the cytokine IFN-γ were induced. Little to no expression of Th17cytokines was observed. Thus, in concert with our previous data, these data evaluate the cytokines present in the salivary gland after inoculation with a viral or nominal antigen and further demonstrate that the salivary gland provides a necessary milieu to act as a mucosal inductive site for the induction of both humoral- and cell-mediated immunity (5–7).
In these studies, we also demonstrated that inoculation with either tcMCMV or MCMV suppressed the Foxp3+ Treg cells in the salivary glands. Conversely, immunization with replication-deficient adenoviruses expressing individual genes of MCMV increased the Foxp3 expression in the salivary glands. Treg cells are involved with maintaining immune homeostasis, self-tolerance, and limiting tissue damage, and they have dual and opposite manifestations during viral infections (16, 41). For example, Treg cell frequencies are decreased in the peripheral blood of HIV and simian immunodeficiency virus infections (42, 43) but highly increased in chronic hepatitis B virus infection (44). Tessmer et al. (45) observed a decrease of Treg in salivary gland of MCMV-infected mice. However, an increase of Foxp3+ Treg cells in the salivary glands of primary (p) SS pSS patients, which is correlated with gland infiltration, suggests that natural Treg cells play an important role in the pathogenesis of pSS (46). pSS patients had an increased frequency of ROR-γ+CD161+CD4+ T-cell (Th17) population that correlated with auto-antibody positivity and hypergammaglobulinemia, which supports the hypothesis that Th17 cells might play a role in pSS pathogenesis (47). Further studies have provided evidence for a role of ROR-γt overexpression in CD4+ T cells in the development of spontaneous sialadenitis-like SS through down-regulation of CD4+CD25+Foxp3+ Treg cells, which occurs via suppression of IL-2–induced phosphorylation of signal transducer and activator of transcription 5, suggesting that regulation of ROR-γt expression is a potentially useful therapeutic strategy against SS (48). In support of a role for ROR-γt in the pathogenesis of SS, a recent study demonstrated that a ROR-γt antagonist suppressed IL-17 and IFN-γ production by M3 muscarinic acetylcholine receptor–specific T cells and may be a potential treatment strategy for SS-like sialadenitis (49). Via the analysis of critical transcription factors in the Treg (Foxp3) and Th17 (ROR-γt) pathways and protein expression of IL-17, we found that neither the Treg nor Th17 pathways were induced in the salivary gland after cannulation of the submandibular gland with tcMCMV, but these same T-cell pathways were up-regulated in the PGLNs. However, salivary gland immunization with a nominal antigen (Ad-Combo) did up-regulate the Treg pathway in the salivary gland but not in the PGLN. The lack of expression of Th transcription factors in the PGLNs after salivary gland immunization with Ad-Combo was not surprising because we previously demonstrated that no RNA specific for gpB, gpH, or IE1 was detected from the PGLNs, spleen, or mesenteric lymph nodes of inoculated mice (7), further demonstrating that the salivary gland acts as a mucosal inductive site. Thus, in addition to the differential expression of Th1 vs. Th2 responses in the primary site of inoculation (salivary gland) vs. the draining PGLNs, a similar differential expression of Treg and Th17 cells was also observed. To our knowledge, this is the first description of either Foxp3+ Treg cells or Th17 cells in the salivary gland after an immunization protocol.
For migration into local mucosal areas, lymphocytes express mucosal homing receptors, with the caveat that the micro-environment where B and T cells undergo differentiation determines their expression of mucosal homing receptors (50–52). With the aim of further establishing whether the salivary glands act as novel sites for mucosal immunization, we evaluated the expression of 2 homing receptors, CD62L and -103, on CD8+ lymphocytes obtained from the salivary gland vs. the PGLNs and spleen after retrograde perfusion of the submandibular gland and lethal challenge with virulent MCMV. We demonstrated an increase in both the number and percentage of CD8+ cells expressing CD103, with a concomitant decrease in the expression of CD62L from lymphocytes obtained from the submandibular salivary glands. The integrin αE (CD103) is frequently used to define tissue-resident memory T cells (53, 54). Our results are in agreement with 2 recent studies that demonstrated that either polyinosinic:polycytidylic acid or lymphocytic choriomeningitis virus increased the frequency of CD8+ cells in salivary glands by an α4β1 integrin–dependent mechanism and is important for accumulation of virus-specific CD8+ tissue memory cells in the salivary glands after viral infections (55, 56). An additional report extended these studies to suggest that the salivary gland is able to constitutively recruit CD8+ T cells to naive and infected salivary glands in an α4 integrin– and C-X-C motif chemokine receptor 3–dependent manner and subsequently induce and sustain tissue-resident memory populations in the absence of infection, antigen, or inflammation (57). However, in contrast to our studies, which evaluated receptor expression on salivary gland–derived lymphocytes after direct salivary gland immunization, these studies evaluated lymphocytes obtained from the salivary gland after parenteral immunization. Although the data we present are limited to expression of CD103 and -62L expression on salivary gland–derived lymphocytes after a focused MCMV inoculation, the data nonetheless support a role for α4 integrin in this process and, in agreement with this study, suggest that enhanced expression of α4 integrin could potentially be used to amplify mucosal immune responses in the salivary gland.
Using a novel method of focused salivary gland immunization (retrograde perfusion of the submandibular gland via Wharton’s duct), we demonstrated that salivary gland vaccination with tcMCMV and recombinant adenoviruses expressing MCMV proteins (nominal antigen) modulated the expression of CD4+Foxp3+ Treg cells and differentially activated Th1, -2, and -17 cell subsets. These new data demonstrate that the salivary gland can provide the necessary milieu, including the expression of key transcription factors necessary for the differentiation of various Th types leading to the expression of cytokines, required for the induction of both humoral- and cell-mediated immunity. Although it is known that macrophages and dendritic cells can contribute to the expression of cytokines necessary for induction of Th1 and -2 responses, including both IL-12 and -10 (58–60), in these studies, we demonstrated cytokine expression from semipurified lymphocytes obtained from the salivary gland, which are greatly depleted of macrophages and dendritic cells. However, we also evaluated cytokine expression from whole salivary gland tissue and demonstrated that, although the pattern of expression of cytokines, including IL-10 and -12, was similar to that observed from semipurified lymphocyte preparations, the magnitude of expression was enhanced, especially in regard to IL-12 expression (unpublished data). These data suggest that other cells, including dendritic cells or macrophages in the environment of the salivary gland, are also contributing to cytokine expression and thus participate in creating an environment that supports the differentiation of both Th2 and -1 cells. Therefore, together with our previously published data (5–7), this route of inoculation was able to induce an adaptive immune response via the submandibular salivary gland, and thus, immunization via the salivary glands has the potential to be further developed into a strategy for the induction of protective immune responses to a number of additional pathogens that invade the host via mucosal surfaces.
ACKNOWLEDGMENTS
The authors thank Dr. John D. Shanley and Dr. Carol A. Wu (University of Connecticut, Farmington, CT, USA) for providing recombinant adenovirus vectors and cell lines. This project was supported by U.S. National Institutes of Health/National Institute of Dental and Craniofacial Research Grant 1R01 DE016652 (to S.D.L.) and 1R15DE024313 (to S.D.L.). The authors declare no conflicts of interest.
Glossary
- Ad
adenoviral vector
- Ad-Combo
recombinant adenovirus expressing a combination of MCMV genes gB, gH, and IE1
- Foxp3
forkhead box P3
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- gp
glycoprotein
- GATA3
GATA-binding protein 3
- GC
germinal center
- IE
immediate early
- MCMV
murine cytomegalovirus
- PFU
plaque-forming unit
- PGLN
periglandular lymph node
- pSS
primary Sjögren’s syndrome
- qRT-PCR
quantitative RT-PCR
- ROR-γt
RAR-related orphan receptor γ, thymus-specific isoform
- SS
Sjögren’s syndrome
- T-bet
T-cell–specific T-box transcription factor
- tcMCMV
tissue culture–derived MCMV
- Tfh
T follicular helper
- Th
T helper
- Treg
regulatory T
AUTHOR CONTRIBUTIONS
G. Liu, S. D. London, and L. London designed the research and experimental plan; G. Liu, S. D. London, and L. London analyzed the data; G. Liu, F. Zhang, and R. Wang performed the research; G. Liu, S. D. London, and L. London wrote the manuscript; and all authors approved the final manuscript.
REFERENCES
- 1.Takahashi I., Nochi T., Yuki Y., Kiyono H. (2009) New horizon of mucosal immunity and vaccines. Curr. Opin. Immunol. 21, 352–358 [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. (2007) Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 [DOI] [PubMed] [Google Scholar]
- 3.Neutra M. R., Kozlowski P. A. (2006) Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6, 148–158 [DOI] [PubMed] [Google Scholar]
- 4.Tucker S. N., Lin K., Stevens S., Scollay R., Bennett M. J., Olson D. C. (2004) Salivary gland genetic vaccination: a scalable technology for promoting distal mucosal immunity and heightened systemic immune responses. Vaccine 22, 2500–2504 [DOI] [PubMed] [Google Scholar]
- 5.Grewal J. S., Pilgrim M. J., Grewal S., Kasman L., Werner P., Bruorton M. E., London S. D., London L. (2011) Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site-restricted MCMV infection. FASEB J. 25, 1680–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilgrim M. J., Kasman L., Grewal J., Bruorton M. E., Werner P., London L., London S. D. (2007) A focused salivary gland infection with attenuated MCMV: an animal model with prevention of pathology associated with systemic MCMV infection. Exp. Mol. Pathol. 82, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G., Zhang F., Wang R., London L., London S. D. (2014) Protective MCMV immunity by vaccination of the salivary gland via Wharton’s duct: replication-deficient recombinant adenovirus expressing individual MCMV genes elicits protection similar to that of MCMV. FASEB J. 28, 1698–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J. (2015) T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 75, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockinger B., Omenetti S. (2017) The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 17, 535–544 [DOI] [PubMed] [Google Scholar]
- 10.Nalbant A., Eskier D. (2016) Genes associated with T helper 17 cell differentiation and function. Front. Biosci. (Elite Ed.) 8, 427–435 [DOI] [PubMed] [Google Scholar]
- 11.Gagliani N., Huber S. (2017) Basic aspects of T helper cell differentiation. Methods Mol. Biol. 1514, 19–30 [DOI] [PubMed] [Google Scholar]
- 12.Zhu J., Yamane H., Paul W. E. (2010) Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 28, 445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallies A., Good-Jacobson K. L. (2017) Transcription factor T-bet orchestrates lineage development and function in the immune system. Trends Immunol. 38, 287–297 [DOI] [PubMed] [Google Scholar]
- 14.Wali S., Sahoo A., Puri S., Alekseev A., Nurieva R. (2016) Insights into the development and regulation of T follicular helper cells. Cytokine 87, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plitas G., Rudensky A. Y. (2016) Regulatory T cells: differentiation and function. Cancer Immunol. Res. 4, 721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khor B. (2017) Regulatory T cells: central concepts from ontogeny to therapy. Transfus. Med. Rev. 31, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min B. (2017) Heterogeneity and stability in Foxp3+ regulatory T cells. J. Interferon Cytokine Res. 37, 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao Y. C., Pan Y. H., Ling W., Tian F., Chen Y. L., Zhang X. X., Zhao H. L. (2017) The Yin and Yang of regulatory T cell and therapy progress in autoimmune disease. Autoimmun. Rev. 16, 1058–1070 [DOI] [PubMed] [Google Scholar]
- 19.Ponzio T. A., Sanders J. W. (2017) The salivary gland as a target for enhancing immunization response. Trop. Dis. Travel Med. Vaccines 3, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brune W., Hengel H., Koszinowski U. H. (2001) A mouse model for cytomegalovirus infection. Curr. Protoc. Immunol. , Unit 19.7. [DOI] [PubMed] [Google Scholar]
- 21.Shanley J. D., Wu C. A. (2005) Intranasal immunization with a replication-deficient adenovirus vector expressing glycoprotein H of murine cytomegalovirus induces mucosal and systemic immunity. Vaccine 23, 996–1003 [DOI] [PubMed] [Google Scholar]
- 22.Shanley J. D., Wu C. A. (2003) Mucosal immunization with a replication-deficient adenovirus vector expressing murine cytomegalovirus glycoprotein B induces mucosal and systemic immunity. Vaccine 21, 2632–2642 [DOI] [PubMed] [Google Scholar]
- 23.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 24.Geist L. J., Hinde S. L. (2001) Susceptibility to cytomegalovirus infection may be dependent on the cytokine response to the virus. J. Investig. Med. 49, 434–441 [DOI] [PubMed] [Google Scholar]
- 25.Picarda G., Benedict C. A. (2018) Cytomegalovirus: shape-shifting the immune system. J. Immunol. 200, 3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang-Feldman Y. J., Wojtowicz A., Lochhead G. R., Hale M. A., Li Y., Pomeroy C. (2006) Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection. J. Virol. Methods 131, 122–129 [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh V. J., Deng Y., Birkenbach M. P., Slater J. S., Campbell A. E. (2003) Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J. Virol. 77, 1703–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell A. E., Cavanaugh V. J., Slater J. S. (2008) The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med. Microbiol. Immunol. (Berl.) 197, 205–213 [DOI] [PubMed] [Google Scholar]
- 29.Fox R. I. (2005) Sjögren’s syndrome. Lancet 366, 321–331 [DOI] [PubMed] [Google Scholar]
- 30.Hansen A., Lipsky P. E., Dörner T. (2007) B cells in Sjögren’s syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 9, 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maehara T., Moriyama M., Hayashida J. N., Tanaka A., Shinozaki S., Kubo Y., Matsumura K., Nakamura S. (2012) Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin. Exp. Immunol. 169, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szodoray P., Alex P., Jonsson M. V., Knowlton N., Dozmorov I., Nakken B., Delaleu N., Jonsson R., Centola M. (2005) Distinct profiles of Sjögren’s syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin. Immunol. 117, 168–176 [DOI] [PubMed] [Google Scholar]
- 33.Bikker A., van Woerkom J. M., Kruize A. A., Wenting-van Wijk M., de Jager W., Bijlsma J. W., Lafeber F. P., van Roon J. A. (2010) Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjögren’s syndrome correlates with increased inflammation. Arthritis Rheum. 62, 969–977 [DOI] [PubMed] [Google Scholar]
- 34.Hillen M. R., Blokland S. L., Risselada A. P., Bikker A., Lauwerys B. R., Kruize A. A., Radstake T. R., van Roon J. A. (2016) High soluble IL-7 receptor expression in Sjögren’s syndrome identifies patients with increased immunopathology and dryness. Ann. Rheum. Dis. 75, 1735–1736 [DOI] [PubMed] [Google Scholar]
- 35.Mieliauskaite D., Dumalakiene I., Rugiene R., Mackiewicz Z. (2012) Expression of IL-17, IL-23 and their receptors in minor salivary glands of patients with primary Sjögren’s syndrome. Clin. Dev. Immunol. 2012, 187258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsifis G. E., Rekka S., Moutsopoulos N. M., Pillemer S., Wahl S. M. (2009) Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am. J. Pathol. 175, 1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L. W., Zhou P. R., Wei P., Cong X., Wu L. L., Hua H. (2018) Expression of interleukin-17 in primary Sjögren’s syndrome and the correlation with disease severity: a systematic review and meta-analysis. Scand. J. Immunol. 87, e12649 [DOI] [PubMed] [Google Scholar]
- 38.Sakai A., Sugawara Y., Kuroishi T., Sasano T., Sugawara S. (2008) Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J. Immunol. 181, 2898–2906 [DOI] [PubMed] [Google Scholar]
- 39.Wang J. J., Macardle C., Weedon H., Beroukas D., Banovic T. (2016) Mucosal-associated invariant T cells are reduced and functionally immature in the peripheral blood of primary Sjögren’s syndrome patients. Eur. J. Immunol. 46, 2444–2453 [DOI] [PubMed] [Google Scholar]
- 40.Guggino G., Di Liberto D., Lo Pizzo M., Saieva L., Alessandro R., Dieli F., Triolo G., Ciccia F. (2017) IL-17 polarization of MAIT cells is derived from the activation of two different pathways. Eur. J. Immunol. 47, 2002–2003 [DOI] [PubMed] [Google Scholar]
- 41.Van Eden W., van Herwijnen M., Wagenaar J., van Kooten P., Broere F., van der Zee R. (2013) Stress proteins are used by the immune system for cognate interactions with anti-inflammatory regulatory T cells. FEBS Lett. 587, 1951–1958 [DOI] [PubMed] [Google Scholar]
- 42.Hryniewicz A., Boasso A., Edghill-Smith Y., Vaccari M., Fuchs D., Venzon D., Nacsa J., Betts M. R., Tsai W.-P., Heraud J.-M., Beer B., Blanset D., Chougnet C., Lowy I., Shearer G. M., Franchini G. (2006) CTLA-4 blockade decreases TGF-β, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 108, 3834–3842; erratum: 114, 3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oswald-Richter K., Grill S. M., Shariat N., Leelawong M., Sundrud M. S., Haas D. W., Unutmaz D. (2004) HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2, E198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu D., Fu J., Jin L., Zhang H., Zhou C., Zou Z., Zhao J.-M., Zhang B., Shi M., Ding X., Tang Z., Fu Y.-X., Wang F.-S. (2006) Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J. Immunol. 177, 739–747 [DOI] [PubMed] [Google Scholar]
- 45.Tessmer M. S., Reilly E. C., Brossay L. (2011) Salivary gland NK cells are phenotypically and functionally unique. PLoS Pathog. 7, e1001254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarigul M., Yazisiz V., Bassorgun C. I., Ulker M., Avci A. B., Erbasan F., Gelen T., Gorczynski R. M., Terzioglu E. (2010) The numbers of Foxp3 + Treg cells are positively correlated with higher grade of infiltration at the salivary glands in primary Sjogren’s syndrome. Lupus 19, 138–145 [DOI] [PubMed] [Google Scholar]
- 47.Zhao L., Nocturne G., Haskett S., Boudaoud S., Lazure T., Le Pajolec C., Mariette X., Mingueneau M., Banerjee D. (2017) Clinical relevance of RORγ positive and negative subsets of CD161+CD4+ T cells in primary Sjögren’s syndrome. Rheumatology (Oxford) 56, 303–312 [DOI] [PubMed] [Google Scholar]
- 48.Iizuka M., Tsuboi H., Matsuo N., Asashima H., Hirota T., Kondo Y., Iwakura Y., Takahashi S., Matsumoto I., Sumida T. (2015) A crucial role of RORγt in the development of spontaneous Sialadenitis-like Sjögren’s syndrome. J. Immunol. 194, 56–67 [DOI] [PubMed] [Google Scholar]
- 49.Tahara M., Tsuboi H., Segawa S., Asashima H., Iizuka-Koga M., Hirota T., Takahashi H., Kondo Y., Matsui M., Matsumoto I., Sumida T. (2017) RORγt antagonist suppresses M3 muscarinic acetylcholine receptor-induced Sjögren’s syndrome-like sialadenitis. Clin. Exp. Immunol. 187, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivetic A. (2013) Signals regulating L-selectin-dependent leucocyte adhesion and transmigration. Int. J. Biochem. Cell Biol. 45, 550–555 [DOI] [PubMed] [Google Scholar]
- 51.Verma N. K., Kelleher D. (2017) Not just an adhesion molecule: LFA-1 contact tunes the T lymphocyte program. J. Immunol. 199, 1213–1221 [DOI] [PubMed] [Google Scholar]
- 52.Fu H., Wang A., Mauro C., Marelli-Berg F. (2013) T lymphocyte trafficking: molecules and mechanisms. Front. Biosci. 18, 422–440 [DOI] [PubMed] [Google Scholar]
- 53.Bergsbaken T., Bevan M. J. (2015) Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat. Immunol. 16, 406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casey K. A., Fraser K. A., Schenkel J. M., Moran A., Abt M. C., Beura L. K., Lucas P. J., Artis D., Wherry E. J., Hogquist K., Vezys V., Masopust D. (2012) Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woyciechowski S., Hofmann M., Pircher H. (2017) α4 β1 integrin promotes accumulation of tissue-resident memory CD8+ T cells in salivary glands. Eur. J. Immunol. 47, 244–250 [DOI] [PubMed] [Google Scholar]
- 56.Hofmann M., Pircher H. (2011) E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 108, 16741–16746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caldeira-Dantas S., Furmanak T., Smith C., Quinn M., Teos L. Y., Ertel A., Kurup D., Tandon M., Alevizos I., Snyder C. M. (2018) The chemokine receptor CXCR3 promotes CD8+ T cell accumulation in uninfected salivary glands but is not necessary after murine cytomegalovirus infection. J. Immunol. 200, 1133–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng T. H., Britton G. J., Hill E. V., Verhagen J., Burton B. R., Wraith D. C. (2013) Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 4, 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Na H., Cho M., Chung Y. (2016) Regulation of Th2 cell immunity by dendritic cells. Immune Netw. 16, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma X., Yan W., Zheng H., Du Q., Zhang L., Ban Y., Li N., Wei F. (2015) Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000 Res. 4, 1465 [DOI] [PMC free article] [PubMed] [Google Scholar]