Abstract
The Ca2+-activated Cl− channel, anoctamin 1 (Ano1, also known as transmembrane protein 16A) contributes to intestinal pacemaking, fluid secretion, cellular excitability, and tissue development. The human ANO1 promoter contains binding sites for the glioma-associated oncogene (Gli) proteins. We investigated regulation of ANO1 transcription by Gli. ANO1 promoter activity was determined using a luciferase reporter system. Binding and functional effects of Glis on ANO1 transcription and expression were demonstrated by chromatin immunoprecipitation, small interfering RNA knockdown, PCR, immunolabeling, and recordings of Ca2+-activated Cl− currents in human embryonic kidney 293 (HEK293) cells. Results from previous genome-wide association studies were used to test ANO1 promoter polymorphisms for association with disease. Gli1 and Gli2 bound to the promoter and repressed ANO1 transcription. Repression depended on Gli binding to a site close to the ANO1 transcriptional start site. Mutation of this site prevented Gli binding and transcriptional repression. Knockdown of Gli expression and inhibition of Gli activity increased expression of ANO1 RNA and Ca2+-activated Cl− currents in HEK293 cells. A single-nucleotide polymorphism prevented Gli binding and showed association with irritable bowel syndrome. We conclude that Gli1 and Gli2 repress ANO1 by a novel mechanism that is independent of Gli cleavage and that has a role in gastrointestinal function.—Mazzone, A., Gibbons, S. J., Eisenman, S. T., Strege, P. R., Zheng, T., D’Amato, M., Ordog, T., Fernandez-Zapico, M. E., Farrugia, G. Direct repression of anoctamin 1 (ANO1) gene transcription by Gli proteins.
Keywords: ion channels, gastrointestinal tract, calcium-activated chloride currents, TMEM16A, SNP
Expression of the widely expressed Ca2+-activated Cl− channel, anoctamin 1 (Ano1, also known as transmembrane protein 16A, or TMEM16A) needs to be tightly regulated because both decreased and increased expression are associated with altered physiologic function and disease. Ca2+-dependent bulk Cl− transport by Ano1 is coupled to fluid secretion in various epithelia (1–5). In the nervous system, more dynamic regulation of Ano1, following rises in intracellular Ca2+, couples both photoreceptors and vomeronasal sensory neurons to downstream sensory pathways (6, 7) and regulates cellular excitability on a fast time scale by modifying the rise times and durations of action potentials (8). Ano1 also contributes to membrane depolarization and maintenance of tone in vascular smooth muscle (9, 10) and phasic contractility in visceral smooth muscles (11–15). In the gastrointestinal tract, intestinal pacemaking and neuromuscular signaling are both dependent on Ano1 (16), which is selectively expressed in interstitial cells of Cajal but not gastrointestinal smooth muscle (17). Decreased expression and differences in the representation of transcriptional variants of Ano1 are associated with diabetic gastroparesis, a condition identified as slowed emptying of stomach contents in the absence of a physical obstruction (18). In development, the role of Ano1 is indicated by the lethal phenotype of Ano1 knockout mice, which are runted and have multiple defects (19), possibly because of the contribution of Ano1 to cell-cycle progression at the G1-S transition (20, 21), and a role in the cell cycle may contribute to the association of high expression of Ano1 with several cancers, including squamous cell carcinomas (22, 23) and gastrointestinal stromal tumors, in which Ano1 is a defining marker (24, 25).
The abundance and variety of Ano1 proteins derive from inclusion or skipping of exons because of alternative splicing or the use of alternative promoters in the ANO1 gene (1, 18, 26–28). Alternative isoforms not only have altered kinetics and changes in their sensitivity to Ca2+ (29, 30) but their expression is tissue dependent and has pathophysiological implications in disorders such as cancer, pain, and gastroparesis (18, 27, 31–33).
The human ANO1 promoter has been recently identified by Hui et al. as an active, dynamically regulated promoter containing multiple response elements including binding sites for Gli proteins (glioma-associated oncogenes), which are zinc-finger transcription factors with evolutionarily conserved roles in animal development (34) and causal links to human cancers (35). Glis are well known as downstream targets of the Hedgehog signaling pathway (34) but are also regulated by mechanisms that are independent of Hedgehog signaling, including p53 (36–38). Complex cellular responses to changes in Gli activity result from well-characterized transcriptional regulation of many genes, but the consequences of Gli binding to the ANO1 gene have not been reported. In vertebrates, 3 Gli genes have been identified, and varied patterns of gene expression result from activation of 1 or more of these proteins because of transcriptional activation or repression of target genes (34). In mice, Gli2 is the predominant activator of transcription, and Gli3 is predominantly inhibitory (39–43), although both proteins have the capacity to act in the opposite fashion (44, 45). Gli1 does not contain the amino acid sequences in the N-terminal region commonly associated with transcriptional repression, and up to now it has been considered to be only a transcriptional activator (44, 46).
The identification of consensus sequences for binding of Glis to the ANO1 gene indicated that Gli can regulate Ano1 expression. Therefore, we tested the hypothesis that Gli does regulate ANO1 expression. We found that Gli repressed Ano1 transcription in human embryonic kidney 293 (HEK293) cells by a previously unreported mechanism. This mechanism is prevented by a human single-nucleotide polymorphism (SNP) preliminarily linked to irritable bowel syndrome (IBS), a common gastrointestinal disorder. We propose that this is a mechanism by which Gli proteins can alter Ano1 expression and tissue function that can be exploited as a therapeutic tool for regulating Ano1 expression and function in multiple tissues and diseases.
MATERIALS AND METHODS
Cell cultures
HEK293 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured and passaged according to specifications. For luciferase assays, the cells were transiently transfected with plasmids of interest using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After 4 h, the medium was changed to serum-containing medium, and the medium was collected after 48 h and used for the luciferase assay as described below.
Luciferase assays
The activity of the ANO1 promoter was analyzed using the Ready-to-Glow secreted luciferase reporter system (Clontech Laboratories, Mountain View, CA, USA) according to the manufacturer’s instructions, as previously described by Ferrera et al. (28). This system uses secreted Metridia luciferase as a reporter molecule by sampling medium supernatant, without the need for cell lysis. To normalize for transfection efficiencies, the cells were cotransfected with the phosphorylated secreted alkaline phosphatase 2 control (Clontech Laboratories) vector that expresses as a reporter molecule a secreted form of human placental alkaline phosphatase. Luciferase assays were carried out by transfecting the promoter-luciferase chimeric constructs in HEK293 cells.
Site-directed mutagenesis
Gli binding sites on the ANO1 promoter region were modified to AAAAAAA using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions. The integrity of the constructs and the presence of the desired mutations were verified by DNA sequencing. The primers used are listed in Table 1.
TABLE 1.
Sequences of primers used for mutagenesis
| Primer, 5′–3′ |
||
|---|---|---|
| Primer | Forward | Reverse |
| Purple | GGGCTGGTCCTCAGCACCAAACCAGAAAAAAACTTGGGGTGCAGAAGTTGTCAGATG | CATCTGACAACTTCTGCACCCCAAGTTTTTTTCTGGTTTGGTGCTGAGGACCAGCCC |
| Yellow | CCGGCACTGCAGTTCCCCACGCAAAAAAACCGTAGGGCCCACATTGCTGCT | AGCAGCAATGTGGGCCCTACGGTTTTTTTGCGTGGGGAACTGCAGTGCCGG |
| Red | CTCGGGGGCTGGCTCAAGGCCCAAAAAAATAGTCAACACCCCTTCCCTGGG | CCCAGGGAAGGGGTGTTGACTATTTTTTTGGGCCTTGAGCCAGCCCCCGAG |
Chromatin immunoprecipitation
DNA and proteins from HEK293 cells were cross-linked with 1% formaldehyde, followed by cell lysis. DNA was sheared by 30 repeated cycles of 30 s of sonication followed by 30 s of rest using a Bioruptor 300 (Diagenode, Liège, Belgium). The chromatin immunoprecipitation (ChIP) protocol was as previously described by Mazzone et al. (28). The antibodies used for the immunoprecipitation are listed in Table 2. Quantitative PCR was performed for each sample as described below. All the primers used for quantitative PCR are listed in Table 3.
TABLE 2.
Antibodies used for the study
| Antibody | Host | Source | Catalog no. | Application |
|---|---|---|---|---|
| Gli1 | Rabbit | Novus Biologicals (Centennial, CO, USA) | NB600-600 | ChIP, transient ChIP |
| Gli2 | Rabbit | Novus Biologicals | NB600-874 | ChIP, transient ChIP |
| Gli3 | Goat | R&D Systems (Minneapolis, MN, USA) | AF3690 | ChIP |
| Goat IgG | Goat | R&D Systems | AB-108-C | ChIP |
| Rabbit IgG | Rabbit | MilliporeSigma (Burlington, MA, USA) | 12-370 | ChIP, transient ChIP |
| DOG1 | Mouse | Leica Microsystems (Buffalo Grove, IL, USA) | NCL-L-DOG-1 | IHC |
DOG1, discovered on gastrointestinal stromal tumor 1; IHC, immunohistochemistry.
TABLE 3.
Sequences of primers used for ChIP in this study
| Sequence, 5′–3′ |
|||
|---|---|---|---|
| Primer | Forward | Reverse | Application (ChIP) |
| hChIP-9 | CTCAGGTACCAATCCCAGCAG | CTTCACCTAGTGCCCAGCTTT | Human |
| hChIP-1 | AAAGCTGGGCACTAGGTGAAG | AGAATGCTAAGTGGCTCCGTG | Human |
| hChIP-8 | GAGGTTTGTGGGTACACAAGGA | GTCCCCGACCCTTCCTCTTC | Human |
| hChIP-5 | CTGTGGCTTGCGGGAGG | GACCCCGACGAATCACTGTC | Human |
| hChIP-6 | GACAGTGATTCGTCGGGGTC | TGCACCCCCAGCCAGG | Human |
| hChIP-7 | GCTCCTCTGCAGCGTCC | GGTCCCGGGCAGACCT | Human |
| TChIP | GGAAACAGCTATGACCATGATTACG | ATTTGTCACACGAGGAGGCTG | Transient |
Transient ChIP
For this approach, we engineered 2 plasmids containing the control promoter sequence and a mutant version containing the SNP sequence by cloning the 2 regions of DNA into pCR2.1 using TA cloning technology. The plasmids were constructed by cloning the “red” region of the ANO1 promoter containing either the wild-type allele (with sequence 5′-CCTCCTA-3′) or reference SNP 7940681 (rs7940681) mutation (with sequence 5′-CCTCCTG-3′) into the pCR2.1 vector. hChIP-6 primers were used, and are listed in Table 3. Each of these plasmids was then cotransfected in HEK293 cells with expression vectors for Gli1 or Gli2. Forty-eight hours after transfection, DNA and proteins were cross-linked with 1% formaldehyde, followed by cell lysis. DNA was sheared by 30 repeated cycles of 30 s of sonication followed by 30 s of rest using a Bioruptor 300 (Diagenode), and immunoprecipitation was carried out using anti-Gli1 or anti-Gli2 antibodies (Table 2). For quantitative PCR, a forward primer designed within the cloned DNA region and a reverse primer aligning with the pCR2.1 plasmid DNA were used to ensure amplification of the band immunoprecipitated from the transfected plasmids rather than that from HEK293 genomic DNA. The sequences of the primers used are listed in Table 3.
Small interfering RNA knockdown
On-Target Plus SmartPools small interfering RNAs (siRNAs) against human Gli1 and Gli2 and nontargeting control pools were transiently transfected into HEK293 cells at a final concentration of 25 nM using the Lipofectamine 2000 Reagent (all from Thermo Fisher Scientific). The cells were incubated for 48 h after siRNA transfection, and RNA was extracted and used for quantitative RT-PCR experiments.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the RNABee reagent (Tel-Test, Friendswood, TX, USA) according to standard procedures, followed by an in-column clean-up with an RNeasy Kit (Qiagen, Germantown, MD, USA). cDNA for quantitative RT-PCR expression analyses was synthesized from total RNA using the SuperScript Vilo cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative PCR was performed with Sybr Green I Master Mix (Roche, Basel, Switzerland) on the LightCycler 480 (Roche) according to standard procedures, using primers specific for all genes of interest purchased from Qiagen. RNA expression levels were normalized to hypoxanthine guanine phosphoribosyl transferase.
Chemical compounds
Gli antagonist 61 (GANT61; Tocris Bioscience, Bristol, United Kingdom) was used at 20 µM final concentration. Transmembrane protein 16A inhibitor A01 (T16Ainh-A01; Tocris Bioscience) was diluted in DMSO on the day of the experiment to a final concentration of 10 µM from a 5 mM stock solution. For both compounds, the vehicle control condition was 0.2% DMSO.
Ano1 staining
After treatment with GANT61 or vehicle for 48 h, HEK293 cells were fixed for 10 min in ice cold acidic ethanol (25% acidic acid, 75% absolute ethanol) and blocked using a buffer composed+ of 1% bovine serum albumin and 0.3% Triton X-100 diluted in PBS for 2 h. Primary antibody against ANO1 (also known as DOG1, discovered on gastrointestinal stromal tumor 1) was applied overnight at 4°C to a final concentration of 47 µg/ml. The secondary antibody was a cyanine 3–conjugated anti-mouse IgG diluted to a final concentration of 15 µg/ml. The coverslips were mounted using SlowFade Gold Antifade Mounting Medium (Thermo Fisher Scientific) containing DAPI for nuclear counterstaining and observed using a FV1000 confocal microscope (Olympus, Tokyo, Japan). The antibodies used are listed in Table 2.
Patch-clamp
Whole cell electrophysiology
Pipettes were pulled from KG12 glass (DWK Life Sciences, Rockwood, TN, USA) on a Sutter P-97 puller (Sutter Instrument, Novato, CA, USA) to a resistance of 1–5 MΩ and coated with heat-cured R6101 polymer (DowDuPont, Midland, MI, USA). Whole cell currents were recorded in standard configuration on an Axopatch 200B, Digidata 1400, with CyberAmp 220 hardware and Clampex v.10.4 software (Molecular Devices, Sunnyvale, CA, USA). Currents were analyzed with Clampfit v.10.6 (Molecular Devices), Excel 2010 (Microsoft, Redmond, WA, USA), and SigmaPlot v.12.5 (Systat Software, San Jose, CA, USA). Vehicle- (0.2 µl/ml DMSO) or GANT61- (10 µM) treated HEK293 cells (ATCC) were held at −100 mV and, every 2 s, were stepped for 1 s to voltages 20 mV apart, ranging from −100 to +120 mV.
Solutions
The intracellular solution was (in millimolars) 150 Cl− (0 CH3SO3−) or 35 Cl− (120 CH3SO3−), 150 Cs+, 300 nM free Ca2+ buffered with 2 EGTA, and 10 HEPES; pH 7.0 (CsOH), 290 mmol/kg. The extracellular solution was (mM) 160 Cl−, 150 N-methyl d-glucamine, 5 K+, 2.5 Ca2+, 10 HEPES, and 5.5 glucose; pH 7.35 (HCl), 305 mmol/kg. Where noted, T16Ainh-A01 (30 µM) was added to the extracellular solution as an inhibitor of Ano1 current.
Analysis
Mean Cl− currents (ICl) were normalized to whole cell capacitance (Cm), ICl/Cm. The Cl− current remaining following T16Ainh-A01 blockade was expressed as a percentage of the GANT61-induced current (IGANT61) at any given voltage step. The predicted reversal potential for Cl− (ECl) was −39 mV with asymmetric Cl− (160 mM [Cl−]o ÷ 35 mM [Cl−]i) or −2 mV with symmetric Cl− (160 mM [Cl−]o ÷ 150 mM [Cl−]i), where o = extracellular, and i = intracellular. The recorded reversal potential (EREV) was calculated by subtracting the predicted liquid junction potential (VL) from the x-intercept of the current (I)-voltage (V) curve: {[V(−20 mV) × I(0 mV) − V(0 mV) × I(−20 mV)] ÷ [I(0 mV) − I(−20 mV)]} − VL. Data are expressed as means ± se, and significance was assigned when P < 0.05 by a nonparametric 2-tailed Student’s t test.
SNP and linkage analysis
IBS association data for the rs7940681 SNP were extracted from 2 genome-wide association studies reported in previous publications by Bonfiglio et al. (47, 48), where quality controls and the analytical pipeline are also described in detail. Meta-analysis was implemented using the METAL bioinformatic tool (https://genome.sph.umich.edu/wiki/METAL) by applying an inverse variance-weighted fixed-effect model.
RESULTS
Gli1 and Gli2, but not Gli3, repress the activity of the human ANO1 promoter
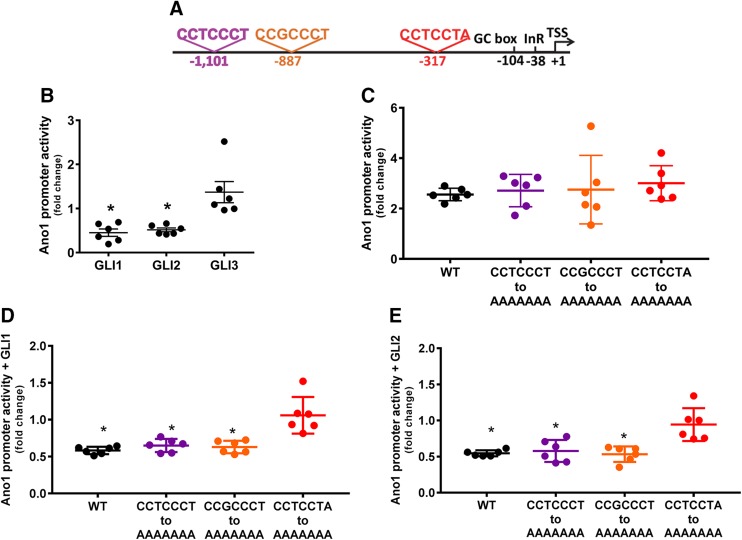
Bioinformatics analyses showed that sequences that are close matches to the consensus Gli binding sites are present in the human ANO1 promoter (Fig. 1A). Therefore, we tested whether Gli proteins regulated the activity of the human ANO1 promoter. We cotransfected, into HEK293 cells, expression plasmids for Gli1, Gli2, or Gli3 together with a luciferase reporter plasmid constructed by fusing the full-length human ANO1 promoter to a Metridia gene (28). In luciferase reporter assays, ANO1 promoter activity was significantly repressed by cotransfection with Gli2 (0.52 ± 0.1%, Fig. 1B). No effect was observed in cells cotransfected with Gli3 expression vector (1.37 ± 0.59%, Fig. 1B). Surprisingly, cotransfection with Gli1, known for its role as a transcriptional activator, also repressed the activity of the ANO1 promoter (0.45 ± 0.2%, Fig. 1B). Next, we determined whether the 3 identified putative Gli binding sites were necessary or sufficient for Gli modulation of ANO1 transcription. For this purpose, we mutated each Gli binding site to inactivate the site and examined the effects on promoter activity. Introduction of the mutated binding sites did not change the basal activity of the ANO1 promoter (Fig. 1C). Inactivation of the Gli binding site closest to the transcriptional start site (TSS; Fig. 1A, red) significantly and reproducibly prevented Gli1 (Fig. 1D) and Gli2 (Fig. 1E) repression of ANO1 promoter activity. No significant effect was recorded after inactivation of the more upstream (Fig. 1A, purple) and the intermediate (Fig. 1A, orange) Gli binding sites (Fig. 1D, E).
Figure 1.
Gli1 and Gli2 repress the activity of the human ANO1 promoter. A) Schematic of the human ANO1 promoter. Previously characterized core promoter elements are indicated in black. In colors are marked the 3 putative Gli binding sites with their sequences. B) Expression of the human ANO1 promoter-luciferase chimeric constructs in the HEK293 cell line. Promoter activity was calculated by fold increase over the luciferase activity of the ANO1 promoter-luciferase chimeric construct coexpressed with the empty expression vector used for Glis. The activity of the human ANO1 promoter was significantly repressed in cells cotransfected with Gli1 and Gli2 but not Gli3 expression vectors. C) Activity of the wild-type (WT) and mutated human ANO1 promoter-luciferase chimeric constructs in the HEK293 cell line in basal conditions. Promoter activity was calculated by fold increase over the luciferase activity of the luciferase empty vector. D, E) Effect of inactivation of Gli binding sites by site-directed mutagenesis on Gli1 (D) and Gli2 (E) repression of the human ANO1 promoter. The colors indicated the mutagenesis done on the more downstream (red), the intermediate (orange) or the more upstream (red) binding sites, as indicated in the promoter schematic in A. Promoter activity was calculated by fold increase over the luciferase activity of the ANO1 promoter-luciferase chimeric construct coexpressed with the empty expression vector used for Glis (n = 6). *P < 0.05, Kruskal-Wallis with Dunn’s multiple comparisons test.
Inhibition of endogenous Gli1 and Gli2 activity in HEK293 cells results in an increase in ANO1 protein expression and channel activity
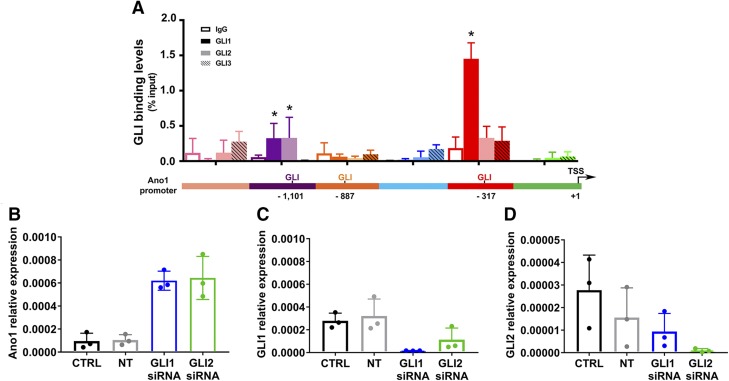
Direct binding of endogenous Gli proteins to the ANO1 promoter was examined by ChIP in HEK293 cells. Using primer sets covering the entire ANO1 promoter region (Table 3), ChIP assays demonstrated pull-down by Gli1 of DNA sequences from the human ANO1 promoter. Genomic DNA was pulled down with anti-Gli1 antibodies from the binding site closest to the TSS in the human ANO1 promoter under basal conditions (Fig. 2A, red, at −317 bp). The middle sequence was not found to bind any Gli (Fig. 2A, orange, at −887 bp). There was also low but detectable binding of both Gli1 and Gli2 to the binding site farthest from the TSS (Fig. 2A, purple, at −1101 bp). However, site-directed mutagenesis experiments indicated that this site was not important for the Gli repression of ANO1 promoter activity. No binding of Gli3 was detected on any of the sites examined in the ANO1 promoter.
Figure 2.
Inhibition of endogenous Gli1 and Gli2 activity results in an increase in human ANO1 expression. A) ChIP–quantitative PCR showing Gli1 (solid, dark shade), Gli2 (solid, light shade), Gli3 (striped), or IgG (empty) relative enrichment over input on the human ANO1 promoter of HEK293 cells under basal conditions (n = 4). *P < 0.05, 2-way ANOVA with Dunnett’s multiple comparison test. B) Effect on ANO1 expression of siRNA knockdown of Gli1 or Gli2 in HEK293 cells. C) Expression of Gli1 after siRNA knockdown of Gli1 or Gli2. D) Expression of Gli2 after siRNA knockdown of Gli1 or Gli2. Ctrl, mock transfected control; NT, nontarget siRNA. Data (B–D) are plotted as expressions relative to housekeeping (hypoxanthine guanine phosphoribosyl transferase) and are represented as means ± sd (n = 3). *P < 0.05, 1-way ANOVA with Dunnet’s multiple comparison test.
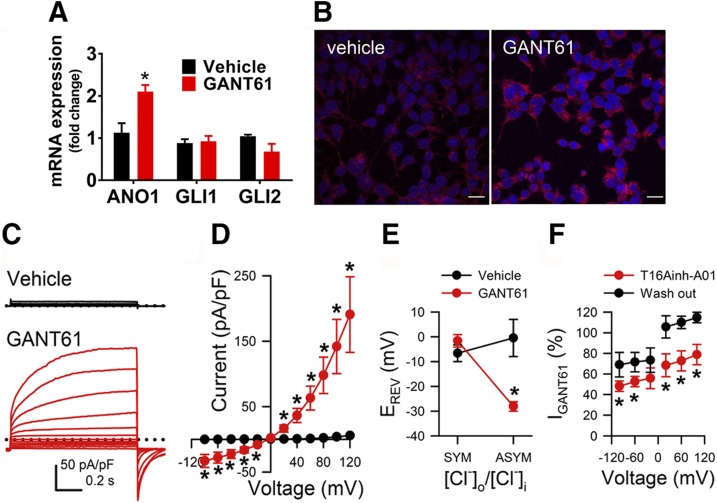
To test whether altering the endogenous expression of Gli1 and Gli2 in HEK293 functionally regulates ANO1 expression, we used siRNA to silence expression of Glis and then measured ANO1 mRNA expression. Cells transfected with siRNA against either Gli1 or Gli2 had ∼6-fold higher ANO1 expression compared with mock transfected controls, whereas no effect was observed with nontarget siRNA (Fig. 2B–D). To confirm those data, we pharmacologically inhibited Gli signaling in HEK293 cells using GANT61, a small molecule that directly inhibits binding of Gli1 and Gli2 to DNA (49). Treatment with 20 µM GANT61 for 48 h produced a 2.1 ± 0.26–fold increase in ANO1 mRNA expression with no change in Gli1 or Gli2 mRNA as measured by quantitative RT-PCR (Fig. 3A). Brighter fluorescent immunolabeling against ANO1 indicated increased ANO1 protein expression in HEK293 cells treated with GANT61 compared with vehicle-treated cells (Fig. 3B). Whole cell voltage clamp recordings from GANT61-treated HEK293 cells identified large outwardly rectifying currents that were not present in cells treated with vehicle (Fig. 3C, D). These currents were characteristic of Ca2+-activated Cl− currents, which was confirmed when we reduced the intracellular Cl− concentration from 150 to 35 mM and observed that the zero current potential for the whole cell currents shifted toward the equilibrium potential for Cl− of −35 mV (1.6 ± 2.5 mV, 160/150 [Cl−]o ÷ [Cl−]i; 28.1 ± 1.9 mV, 160/35 [Cl−]o ÷ [Cl−]i; n = 6–13, P < 0.01 by a 2-tailed Student’s t test; Fig. 3E, red). Changing the Cl− distribution had no effect on cells treated with vehicle (−6.6 ± 3.4 mV, 160/150 [Cl−]o ÷ [Cl−]i; 0.5 ± 7.5 mV, 160/35 [Cl−]o ÷ [Cl−]i; P > 0.05 by a 2-tailed Student’s t test; Fig. 3E, black). The currents were also significantly and reversibly inhibited by the selective inhibitor T16Ainh-A01 (plateau IGANT61 at +100 mV: 310 ± 72 pA/pF, control; 258 ± 71 pA/pF, 30 µM T16Ainh-A01; P < 0.05 by a 2-tailed Student’s t test, n = 4, Fig. 3F). The recorded currents were biophysically similar to heterologously expressed ANO1(0) currents (29, 30) with comparable time constants of activation (τACT at +100 mV: 664 ± 223 ms, IANO1(0); 330 ± 50 ms, IGANT61; n = 6–7, P > 0.05, by a 2-tailed Student’s t test). We conclude from these studies that blocking the binding of Gli1 and Gli2 to the ANO1 promoter DNA resulted in an increase in ANO1 protein expression and channel function.
Figure 3.
Inhibition of endogenous Gli1 and Gli2 activity results in an increase ANO1 channel activity. A) Effect of inhibition of endogenous Gli function on ANO1 RNA expression in HEK293 cells. Quantitative RT-PCR data are shown as fold changes over untreated cells. Data are expressed as the mean ± se of 3 independent experiments (n = 3). *P < 0.05, 2-way ANOVA with Bonferroni’s multiple comparison test. B) Representative confocal images of HEK293 cells immunostaining using an anti-ANO1 antibody (red) after treatment with either vehicle or GANT61 compound for 48 h. In blue is the nuclear counterstain with DAPI. Scale bar, 20 µm. C) Representative traces of functional Ca2+-activated Cl− channels recorded from HEK293 cells treated with vehicle (black) or GANT61 (20 µM, red). Cells were assayed by whole cell voltage clamp recordings optimized for ANO1. D) Quantification of voltage clamp data recorded from HEK293 cells treated with vehicle (black) or GANT61 (red) represented as current (I)-voltage (V) plots (n = 6–13 cells). *P < 0.05, 2-tailed Student’s t test. E) Effect of asymmetric chloride ratio on measured chloride currents after treatment of HEK293 cells with GANT61 (red) or vehicle (black). On the y axis is indicated the measured reversal potential for chloride in mV (EREV). The chloride ratio between extracellular ([Cl]o) and intracellular ([Cl]i) solutions is plotted on the x axis (n = 6–13). *P < 0.05, 2-tailed Student’s t test. ASYM, asymmetrical chloride distribution; SYM, symmetrical chloride distribution. F) Effect of the inhibitor T16Ainh-A01 (30 µM) on measured chloride currents from HEK293 cells treated with GANT61. The currents measured after GANT61 treatment are plotted on the y axis; the voltage (mV) are plotted on the x axis (n = 4). *P < 0.05, 2-tailed Student’s t test.
Gli1 and Gli2 repress the activity of the mouse Ano1 promoter
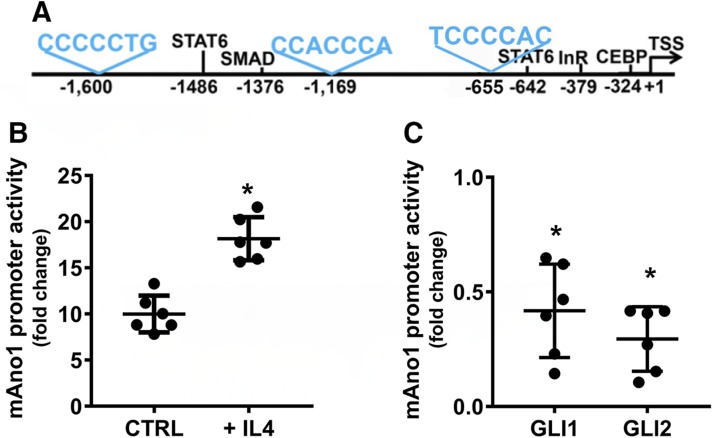
The mouse Ano1 promoter has not been identified and characterized. Using bioinformatics tools, we recognized the region between the nucleotides 144,738,428 and 144,741,026 on the reverse strand of the mouse chromosome 7 as a promoter. Synteny analysis using the Evolutionary Conserved Regions Browser tool indicated this region had an 87.3% homology with the region of the human chromosome 11 between nucleotides 69,829,308 and 69,832,308, which includes the human ANO1 promoter (28). Bioinformatics analyses highlighted the presence in this region of core promoter elements, an array of binding sites for response elements similar to the ones identified on the human ANO1 promoter such as signal transducer and activator of transcription 6, Mothers Against Decapentaplegic Homolog (SMAD) and CCAAT-enhancer-binding protein (28) (Fig. 4A). Luciferase assays in HEK293 cells showed that this is an active promoter and, like the human ANO1 promoter, it is activated following treatment with IL-4 (Fig. 4B). Three putative Gli binding sites were identified in the sequence (Fig. 4A), and cotransfection of the mouse Ano1 promoter-luciferase vector with expression plasmids for mouse Gli1 or Gli2 showed that the promoter activity was significantly repressed by about 60 and 70%, respectively (Fig. 4C). Therefore, the repression of transcription by Glis is conserved in the mouse and human ANO1 genes.
Figure 4.
Gli1 and Gli2 repress the activity of the mouse Ano1 promoter. A) Schematic of the mouse Ano1 promoter. Core promoter elements and putative binding sites for transcription factors identified in the human ANO1 promoter are indicated in black. In blue are the putative Gli binding sites. InR, initiator region. B) Expression of the 1.8 Kb region upstream of the exon 1 of mouse Ano1 in promoter-luciferase chimeric constructs in the HEK293 cell line. The promoter activity is significantly higher than empty vector (8.5 ± 0.6–fold increase), and it is significantly up-regulated after treatment with 10 µM concentration of IL4 for 48 h (17.9 ± 2.1–fold increase over untreated cells). Ctrl, control. Data are expressed as mean ± sd of 6 independent experiments. *P < 0.05, 2-way ANOVA with Sidak’s multiple comparison test. C) Expression of the mouse Ano1 promoter-luciferase chimeric constructs in the HEK293 cell line. Promoter activity was calculated as fold increase over the luciferase activity of the empty vector. The activity of the mouse Ano1 promoter was significantly repressed in cells cotransfected with Gli1 (0.41 ± 0.2) and Gli2 (0.29 ± 0.14) expression vectors (n = 6). *P < 0.05, 1-way ANOVA with Dunn’s multiple comparisons test.
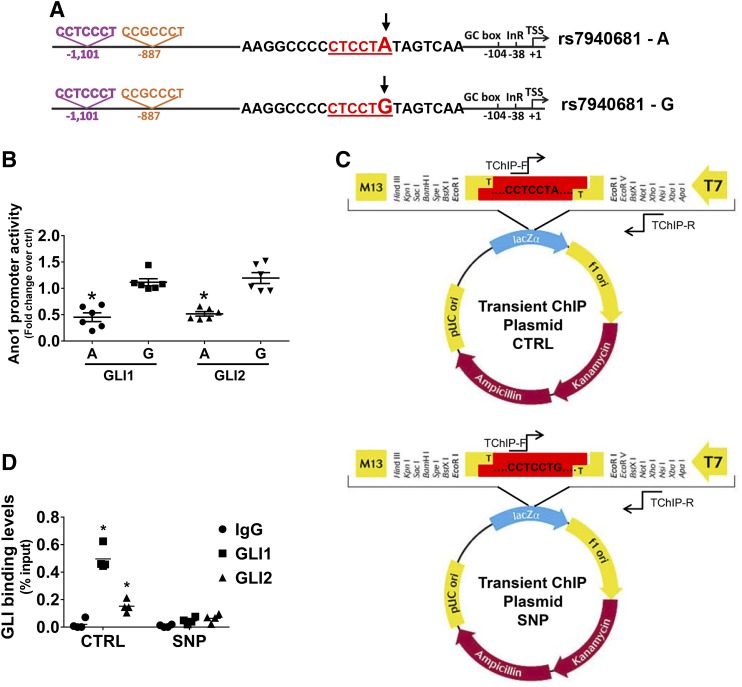
The rs7940681 SNP in the human ANO1 promoter region abolishes Gli-mediated repression of ANO1 promoter and is associated with IBS risk
We observed that there is an SNP mapped to the Gli binding site closest to the TSS of the human ANO1 gene, namely, rs7940681 (Fig. 5A). Both alleles at this SNP are very common in Europeans, with either A or G variant corresponding to the minor or major allele depending on the specific subpopulation [overall Northern Europeans from Utah (CEU) frequencies G = 0.495 and A = 0.505; www.ensembl.org]. We therefore re-examined the activity of the ANO1 promoter by comparing A and G variants (that is, by changing the CCTCCTA sequence to CCTCCTG in the luciferase vector via site-directed mutagenesis; Fig. 5B). Introduction of the G allele significantly and reproducibly prevented the repression by Gli1 and Gli2 of ANO1 promoter activity (Fig. 5B). To investigate whether the presence of a G in the consensus sequence directly inhibits binding of Glis to the human promoter, we carried out a transient ChIP experiment. For this approach we used promoter constructs carrying either rs7940681 variant (A or G) (Fig. 5C) cotransfected in HEK293 cells with expression vectors for Gli1 or Gli2. Our data indicate that Gli1 and, to a lesser extent, Gli2 bind to the A variant of the plasmid containing the control promoter sequence as expected, whereas the presence of the G variant prevented binding of both transcription factors to the ANO1 promoter (Fig. 5D). This observation (allelic differences in Gli-mediated ANO1 repression) may be relevant to the reported role of ANO1 in gastrointestinal motility (11–13) and epithelial function (1, 4, 5), possibly contributing to interindividual variation in these traits. In order to explore this hypothesis, we tested the rs7940681 SNP for association with IBS in a meta-analysis of association data extracted from 2 large genome-wide association studies recently published by Bonfiglio et al. (47, 48): as shown in Table 4, the A allele allowing Gli binding and repression at the ANO1 promoter was also weakly but significantly associated with small effects on IBS risk in this analysis.
Figure 5.
The SNP rs7940681 in the human ANO1 promoter region disrupts repression of ANO1 transcription by Glis. A) Schematic of the ANO1 promoter showing the localization and the sequence changes in the presence of the rs7940681 SNP. B) Expression of the human ANO1 promoter-luciferase chimeric construct modified with the rs7940681 A-to-G SNP transfected in the HEK293 cell line. Promoter activity was calculated by fold increase over the luciferase activity of the empty vector (n = 6). *P < 0.05, 1-way ANOVA with Dunn’s multiple comparisons test. C) Schematic of the plasmid containing the control promoter sequence (CTRL) and the plasmid containing the SNP sequence (SNP) used for transient ChIP (TChIP) experiment. D) Transient ChIP–quantitative PCR experiment to test whether rs7940681 directly inhibits Gli binding to the ANO1 promoter. The data are indicated as relative enrichments over input (n = 4). *P < 0.05, 2-way ANOVA with Sidak’s multiple comparison test.
TABLE 4.
The rs7940681 SNP associates with an increased risk of IBS
| SNP | A1 | A2 | A1 frequency | Regression coefficient | P | IBS definition | Case (n) | Ctrl (n) |
|---|---|---|---|---|---|---|---|---|
| rs7940681 | A | G | 0.506 | 1.09 | 0.039 | Questionnaire based (Rome IIIa) | 1335 | 9768 |
| rs7940681 | A | G | 0.54 | 1.03 | 0.097 | Self-reported doctor’s diagnosis | 9576 | 336,499 |
| rs7940681 | A | G | 0.5362 | 1.03 | 0.025 | Meta-analysis | 10,911 | 34,626 |
A1, allele 1; A2, allele 2.
Rome III criteria for diagnosis of IBS (64).
DISCUSSION
Here, we showed that Gli2 and, surprisingly, Gli1 inhibited the activity of the human and mouse ANO1 promoters. This Gli-mediated transcriptional repression was affected by the presence of a common variant in the Gli binding site of the human ANO1 promoter (rs7940681 SNP), possibly resulting in modest effects on IBS risk. These findings identify a physiologic regulator of Ano1 expression, a molecule involved in multiple cellular functions, including but not limited to fluid secretion, cellular excitability, and cancer.
The mechanisms and consequences for transcriptional regulation of ANO1 by any Gli transcription factor have not been previously reported. What we found was unexpected: both Gli1 and Gli2, but not Gli3, repressed the transcriptional activity of the mouse and human ANO1 promoters, an effect that must be mediated by a different mechanism from that previously described by Bai et al. for direct Gli2 and Gli3 repression of promoter activity at other genes (46). Gli1 lacks the N-terminal repressor domain, previously identified by Sasaki et al. and Bai et al., that mediates the repressor functions of Gli2 and Gli3 following proteolytic cleavage of full-length Gli2 and Gli3 (44, 46), but we were able to pull down the ANO1 promoter sequence from a human cell line by ChIP using Gli1. These data indicate that Gli1 was binding directly to the putative Gli1 binding sequence without a canonical repressor domain and directly repressing the activity of the ANO1 promoter recently identified by Mazzone et al. (28). These direct repressor effects due to DNA binding are distinct from a previous report by Chen et al. of Gli1 repression of transcription driven by androgen receptor response elements (50), which was ascribed to Gli1 association with the androgen receptor protein. Based on mutagenesis studies and the effect of introducing the SNP into the promoter sequence, the repressor function depends on only 1 of the 3 putative Gli binding sites in the human ANO1 promoter. We did not detect any activation of human or mouse ANO1 promoter activity by Glis in our studies. It should be noted that although it was an effective repressor of both mouse and human ANO1 promoter activity using the luciferase assay, Gli2 did not bind strongly to putative Gli binding sites in the human ANO1 promoter based on ChIP and transient ChIP experiments in HEK293 cells. This further indicates that Gli1 has a direct repressor effect on the ANO1 promoter sequence.
The functional relevance and physiologic significance of these observations are indicated by the demonstration of regulation of Ca2+-activated Cl− currents by altering Gli function in HEK293 cells. In addition, the preliminary evidence of association with IBS and the demonstration by transient ChIP that the rs7940681 SNP disrupts the repression of ANO1 transcription by Gli indicates that Gli-mediated repression of ANO1 might have consequences for normal gastrointestinal function. There are no clear underlying structural abnormalities in the gastrointestinal tract of people with IBS, despite well-defined characteristics of chronic or recurrent abdominal pain with altered bowel habits, and multiple genetic factors being linked to the syndrome (see ref. 51 for a review). For example, it has been shown that the function of specific genes, and ion channels in particular, can contribute to IBS pathophysiology in subsets of patients (30, 47, 48, 52–55). On the other hand, various and often overlapping other causes such as infections, diet, susceptible microbiome, and other environmental factors are also likely important. Indeed, individualized therapies targeting specific ion channels could be beneficial in multifactorial diseases such as IBS, as indicated by our work on the sodium channel NaV1.5 (53). Ano1 has not been previously linked with IBS, but several characteristics of IBS such as abdominal pain, abnormal secretion, and altered motility are known functions of Ano1 in the gastrointestinal tract, and Ano1 has been linked with the functional gastrointestinal disorder, gastroparesis, in studies showing differential expression of specific variants of the protein in diseased gastric smooth muscle from patients (18). Previous studies in mice support the possibility that impaired function of interstitial cells of Cajal and disrupted motility may be a factor leading to symptoms of IBS in at least 1 subset of patients who carry a rs7940681 risk allele (A) in which ANO1 expression is repressed by Gli. On the contrary, patients with the G allele, which prevents binding of Gli to the ANO1 promoter, appear to be protected and have a lower risk for IBS. The weak but significant evidence of an ANO1-IBS association reported here, which was based on the analysis of population-based cohorts and questionnaire data, certainly warrants further investigation in more relevant, informative case-control cohorts from expert neurogastroenterology clinics.
Ano1 expression is quite broad, and we predict that this novel mechanism could be highly significant given that Ano1 is important for physiologic processes including fluid secretion in many epithelia, nociception by sensory neurons, and excitability of vascular smooth muscle (1–7, 9, 10), as well as tumor growth and metastasis (22–25). The transcriptional specificity conferred by this distinct mechanism for transcriptional repression by Glis indicates that Ano1 expression could be specifically modulated in different cell types by targeting Gli repression of ANO1 transcription. Others have proposed that pharmacological inhibitors or activators of Ano1 have therapeutic potential in diseases including cystic fibrosis, diarrhea, bronchospasm, hypertension, chronic pain, and cancer (56–62). Transcriptional regulation of Ano1 by Gli represents an alternative, potentially cell specific target. Equally significant is that the rs7940681 SNP represents a noncoding polymorphism that disrupts but does not completely inhibit the regulation of gene expression and as such can be a risk allele for these various disorders in association with other SNPs or environmental factors.
In summary, we have provided evidence of Gli-mediated transcriptional repression of ANO1 that adds an additional component to the established “Gli code” (63) wherein Gli1 and Gli2 are transcriptional activators and cleaved Gli2 and Gli3 are transcriptional repressors. Our studies identified a novel repressor function for Gli1 and Gli2 that is not a property of Gli3 and showed that Gli1 and Gli2 repressed the transcriptional activity of the Ano1 promoter. We also provide preliminary evidence that genetic alterations of Gli regulation of ANO1 expression may be relevant to a subset of human diseases. These data, in addition to their relevance for gastrointestinal physiology and disease, are of much broader interest given the expression of Ano1 in a wide range of tissues and its role in numerous physiologic processes.
ACKNOWLEDGMENTS
The authors thank Kristy Zodrow (Enteric NeuroSciences, Mayo Clinic) for assistance in manuscript preparation. This work is supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK57061 (to G.F. and S.J.G.) and DK84567 (to G.F., S.J.G.), the Swedish Research Council (VR) Project 2017-02403 (to M.D.A.), the Health Department of the Basque Government (Grant 2015111133 to M.D.A.), and the Spanish Ministry of Economy and Competitiveness [Instituto de Salud Carlos III (ISCIII) Grant Fondo de Investigación en Salud (FIS) PI17/00308 to M.D.A.]. The authors declare no conflicts of interest.
Glossary
- Ano1
anoctamin 1
- ChIP
chromatin immunoprecipitation
- GANT61
Gli antagonist 61
- Gli
glioma-associated oncogene protein
- HEK293
human embryonic kidney 293
- IBS
irritable bowel syndrome
- rs7940681
reference SNP 7940681
- siRNA
small interfering RNA
- SNP
single-nucleotide polymorphism
- T16Ainh-A01
transmembrane protein 16A–ANO1 inhibitor
- TSS
transcriptional start site
AUTHOR CONTRIBUTIONS
A. Mazzone designed the research, designed and performed experiments, analyzed data, and wrote the manuscript; S. J. Gibbons designed the research, analyzed data, and wrote the manuscript; S. T. Eisenman performed experiments; P. R. Strege performed experiments and analyzed data; T. Zheng analyzed data; M. D’Amato designed research and analyzed data; T. Ordog critically reviewed the manuscript; M. E. Fernandez-Zapico designed research, supplied reagents, and critically reviewed the manuscript; and G. Farrugia designed research and critically reviewed the manuscript.
REFERENCES
- 1.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 [DOI] [PubMed] [Google Scholar]
- 2.Romanenko V. G., Catalán M. A., Brown D. A., Putzier I., Hartzell H. C., Marmorstein A. D., Gonzalez-Begne M., Rock J. R., Harfe B. D., Melvin J. E. (2010) Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J. Biol. Chem. 285, 12990–13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz B., Faria D., Schley G., Schreiber R., Eckardt K. U., Kunzelmann K. (2014) Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int. 85, 1058–1067 [DOI] [PubMed] [Google Scholar]
- 4.Dutta A. K., Khimji A. K., Kresge C., Bugde A., Dougherty M., Esser V., Ueno Y., Glaser S. S., Alpini G., Rockey D. C., Feranchak A. P. (2011) Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J. Biol. Chem. 286, 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ousingsawat J., Mirza M., Tian Y., Roussa E., Schreiber R., Cook D. I., Kunzelmann K. (2011) Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflugers Arch. 461, 579–589 [DOI] [PubMed] [Google Scholar]
- 6.Mercer A. J., Rabl K., Riccardi G. E., Brecha N. C., Stella S. L., Jr., Thoreson W. B. (2011) Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. J. Neurophysiol. 105, 321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amjad A., Hernandez-Clavijo A., Pifferi S., Maurya D. K., Boccaccio A., Franzot J., Rock J., Menini A. (2015) Conditional knockout of TMEM16A/anoctamin1 abolishes the calcium-activated chloride current in mouse vomeronasal sensory neurons. J. Gen. Physiol. 145, 285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H., Yang Y. D., Lee J., Lee B., Kim T., Jang Y., Back S. K., Na H. S., Harfe B. D., Wang F., Raouf R., Wood J. N., Oh U. (2012) The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 9.Manoury B., Tamuleviciute A., Tammaro P. (2010) TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 588, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinze C., Seniuk A., Sokolov M. V., Huebner A. K., Klementowicz A. E., Szijártó I. A., Schleifenbaum J., Vitzthum H., Gollasch M., Ehmke H., Schroeder B. C., Hübner C. A. (2014) Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J. Clin. Invest. 124, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang S. J., Blair P. J., Britton F. C., O’Driscoll K. E., Hennig G., Bayguinov Y. R., Rock J. R., Harfe B. D., Sanders K. M., Ward S. M. (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 587, 4887–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh R. D., Gibbons S. J., Saravanaperumal S. A., Du P., Hennig G. W., Eisenman S. T., Mazzone A., Hayashi Y., Cao C., Stoltz G. J., Ordog T., Rock J. R., Harfe B. D., Szurszewski J. H., Farrugia G. (2014) Ano1, a Ca2+-activated Cl- channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J. Physiol. 592, 4051–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malysz J., Gibbons S. J., Saravanaperumal S. A., Du P., Eisenman S. T., Cao C., Oh U., Saur D., Klein S., Ordog T., Farrugia G. (2017) Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G228–G245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bijos D. A., Drake M. J., Vahabi B. (2014) Anoctamin-1 in the juvenile rat urinary bladder. PLoS One 9, e106190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sancho M., García-Pascual A., Triguero D. (2012) Presence of the Ca2+-activated chloride channel anoctamin 1 in the urethra and its role in excitatory neurotransmission. Am. J. Physiol. Renal Physiol. 302, F390–F400 [DOI] [PubMed] [Google Scholar]
- 16.Sung T. S., Hwang S. J., Koh S. D., Bayguinov Y., Peri L. E., Blair P. J., Webb T. I., Pardo D. M., Rock J. R., Sanders K. M., Ward S. M. (2018) The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J. Physiol. 596, 1549–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Pinilla P. J., Gibbons S. J., Bardsley M. R., Lorincz A., Pozo M. J., Pasricha P. J., Van de Rijn M., West R. B., Sarr M. G., Kendrick M. L., Cima R. R., Dozois E. J., Larson D. W., Ordog T., Farrugia G. (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzone A., Bernard C. E., Strege P. R., Beyder A., Galietta L. J., Pasricha P. J., Rae J. L., Parkman H. P., Linden D. R., Szurszewski J. H., Ördög T., Gibbons S. J., Farrugia G. (2011) Altered expression of Ano1 variants in human diabetic gastroparesis. J. Biol. Chem. 286, 13393–13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock J. R., Futtner C. R., Harfe B. D. (2008) The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev. Biol. 321, 141–149 [DOI] [PubMed] [Google Scholar]
- 20.Stanich J. E., Gibbons S. J., Eisenman S. T., Bardsley M. R., Rock J. R., Harfe B. D., Ordog T., Farrugia G. (2011) Ano1 as a regulator of proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G1044–G1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzone A., Eisenman S. T., Strege P. R., Yao Z., Ordog T., Gibbons S. J., Farrugia G. (2012) Inhibition of cell proliferation by a selective inhibitor of the Ca(2+)-activated Cl(-) channel, Ano1. Biochem. Biophys. Res. Commun. 427, 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashyap M. K., Marimuthu A., Kishore C. J., Peri S., Keerthikumar S., Prasad T. S., Mahmood R., Rao S., Ranganathan P., Sanjeeviah R. C., Vijayakumar M., Kumar K. V., Montgomery E. A., Kumar R. V., Pandey A. (2009) Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol. Ther. 8, 36–46 [DOI] [PubMed] [Google Scholar]
- 23.Duvvuri U., Shiwarski D. J., Xiao D., Bertrand C., Huang X., Edinger R. S., Rock J. R., Harfe B. D., Henson B. J., Kunzelmann K., Schreiber R., Seethala R. S., Egloff A. M., Chen X., Lui V. W., Grandis J. R., Gollin S. M. (2012) TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 72, 3270–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West R. B., Corless C. L., Chen X., Rubin B. P., Subramanian S., Montgomery K., Zhu S., Ball C. A., Nielsen T. O., Patel R., Goldblum J. R., Brown P. O., Heinrich M. C., van de Rijn M. (2004) The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 165, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinosa I., Lee C. H., Kim M. K., Rouse B. T., Subramanian S., Montgomery K., Varma S., Corless C. L., Heinrich M. C., Smith K. S., Wang Z., Rubin B., Nielsen T. O., Seitz R. S., Ross D. T., West R. B., Cleary M. L., van de Rijn M. (2008) A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 32, 210–218 [DOI] [PubMed] [Google Scholar]
- 26.Ferrera L., Caputo A., Ubby I., Bussani E., Zegarra-Moran O., Ravazzolo R., Pagani F., Galietta L. J. (2009) Regulation of TMEM16A chloride channel properties by alternative splicing. J. Biol. Chem. 284, 33360–33368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondo E., Scudieri P., Tomati V., Caci E., Mazzone A., Farrugia G., Ravazzolo R., Galietta L. J. (2014) Non-canonical translation start sites in the TMEM16A chloride channel. Biochim. Biophys. Acta 1838 (1 Pt B), 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzone A., Gibbons S. J., Bernard C. E., Nowsheen S., Middha S., Almada L. L., Ordog T., Kendrick M. L., Reid Lombardo K. M., Shen K. R., Galietta L. J., Fernandez-Zapico M. E., Farrugia G. (2015) Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6). FASEB J. 29, 152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strege P. R., Bernard C. E., Mazzone A., Linden D. R., Beyder A., Gibbons S. J., Farrugia G. (2015) A novel exon in the human Ca2+-activated Cl- channel Ano1 imparts greater sensitivity to intracellular Ca2. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G743–G749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strege P. R., Gibbons S. J., Mazzone A., Bernard C. E., Beyder A., Farrugia G. (2017) EAVK segment “c” sequence confers Ca2+-dependent changes to the kinetics of full-length human Ano1. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G572–G579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B., Linley J. E., Du X., Zhang X., Ooi L., Zhang H., Gamper N. (2010) The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J. Clin. Invest. 120, 1240–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Driscoll K. E., Pipe R. A., Britton F. C. (2011) Increased complexity of Tmem16a/Anoctamin 1 transcript alternative splicing. BMC Mol. Biol. 12, 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ubby I., Bussani E., Colonna A., Stacul G., Locatelli M., Scudieri P., Galietta L., Pagani F. (2013) TMEM16A alternative splicing coordination in breast cancer. Mol. Cancer 12, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui C. C., Angers S. (2011) Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 27, 513–537 [DOI] [PubMed] [Google Scholar]
- 35.Teglund S., Toftgård R. (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta 1805, 181–208 [DOI] [PubMed] [Google Scholar]
- 36.Kasai K., Inaguma S., Yoneyama A., Yoshikawa K., Ikeda H. (2008) SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res. 68, 7723–7729 [DOI] [PubMed] [Google Scholar]
- 37.Beauchamp E., Bulut G., Abaan O., Chen K., Merchant A., Matsui W., Endo Y., Rubin J. S., Toretsky J., Uren A. (2009) GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J. Biol. Chem. 284, 9074–9082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stecca B., Ruiz I Altaba A. (2010) Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J. Mol. Cell Biol. 2, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz i Altaba A. (1999) Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development 126, 3205–3216 [DOI] [PubMed] [Google Scholar]
- 40.Wang B., Fallon J. F., Beachy P. A. (2000) Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434 [DOI] [PubMed] [Google Scholar]
- 41.Bai C. B., Joyner A. L. (2001) Gli1 can rescue the in vivo function of Gli2. Development 128, 5161–5172 [DOI] [PubMed] [Google Scholar]
- 42.Mao J., Kim B. M., Rajurkar M., Shivdasani R. A., McMahon A. P. (2010) Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137, 1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimeault M., Batra S. K. (2010) Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol. Rev. 62, 497–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki H., Nishizaki Y., Hui C., Nakafuku M., Kondoh H. (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126, 3915–3924 [DOI] [PubMed] [Google Scholar]
- 45.McDermott A., Gustafsson M., Elsam T., Hui C. C., Emerson C. P., Jr., Borycki A. G. (2005) Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development 132, 345–357 [DOI] [PubMed] [Google Scholar]
- 46.Bai C. B., Stephen D., Joyner A. L. (2004) All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 6, 103–115 [DOI] [PubMed] [Google Scholar]
- 47.Bonfiglio F., Henström M., Nag A., Hadizadeh F., Zheng T., Cenit M. C., Tigchelaar E., Williams F., Reznichenko A., Ek W. E., Rivera N. V., Homuth G., Aghdassi A. A., Kacprowski T., Männikkö M., Karhunen V., Bujanda L., Rafter J., Wijmenga C., Ronkainen J., Hysi P., Zhernakova A., D’Amato M. (2018) A GWAS meta-analysis from 5 population-based cohorts implicates ion channel genes in the pathogenesis of irritable bowel syndrome. Neurogastroenterol. Motil. 30, e13358 [DOI] [PubMed] [Google Scholar]
- 48.Bonfiglio F., Zheng T., Garcia-Etxebarria K., Hadizadeh F., Bujanda L., Bresso F., Agreus L., Andreasson A., Dlugosz A., Lindberg G., Schmidt P. T., Karling P., Ohlsson B., Simren M., Walter S., Nardone G., Cuomo R., Usai-Satta P., Galeazzi F., Neri M., Portincasa P., Bellini M., Barbara G., Latiano A., Hübenthal M., Thijs V., Netea M. G., Jonkers D., Chang L., Mayer E. A., Wouters M. M., Boeckxstaens G., Camilleri M., Franke A., Zhernakova A., D’Amato M. (2018) Female-specific association between variants on chromosome 9 and self-reported diagnosis of irritable bowel syndrome. Gastroenterology 155, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agyeman A., Jha B. K., Mazumdar T., Houghton J. A. (2014) Mode and specificity of binding of the small molecule GANT61 to GLI determines inhibition of GLI-DNA binding. Oncotarget 5, 4492–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G., Goto Y., Sakamoto R., Tanaka K., Matsubara E., Nakamura M., Zheng H., Lu J., Takayanagi R., Nomura M. (2011) GLI1, a crucial mediator of sonic hedgehog signaling in prostate cancer, functions as a negative modulator for androgen receptor. Biochem. Biophys. Res. Commun. 404, 809–815 [DOI] [PubMed] [Google Scholar]
- 51.Holtmann G. J., Ford A. C., Talley N. J. (2016) Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 1, 133–146 [DOI] [PubMed] [Google Scholar]
- 52.Beyder A., Farrugia G. (2016) Ion channelopathies in functional GI disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G581–G586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beyder A., Mazzone A., Strege P. R., Tester D. J., Saito Y. A., Bernard C. E., Enders F. T., Ek W. E., Schmidt P. T., Dlugosz A., Lindberg G., Karling P., Ohlsson B., Gazouli M., Nardone G., Cuomo R., Usai-Satta P., Galeazzi F., Neri M., Portincasa P., Bellini M., Barbara G., Camilleri M., Locke G. R., Talley N. J., D’Amato M., Ackerman M. J., Farrugia G. (2014) Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 146, 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henström M., Hadizadeh F., Beyder A., Bonfiglio F., Zheng T., Assadi G., Rafter J., Bujanda L., Agreus L., Andreasson A., Dlugosz A., Lindberg G., Schmidt P. T., Karling P., Ohlsson B., Talley N. J., Simren M., Walter S., Wouters M., Farrugia G., D’Amato M. (2017) TRPM8 polymorphisms associated with increased risk of IBS-C and IBS-M. Gut 66, 1725–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jankipersadsing S. A., Hadizadeh F., Bonder M. J., Tigchelaar E. F., Deelen P., Fu J., Andreasson A., Agreus L., Walter S., Wijmenga C., Hysi P., D’Amato M., Zhernakova A. (2017) A GWAS meta-analysis suggests roles for xenobiotic metabolism and ion channel activity in the biology of stool frequency. Gut 66, 756–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verkman A. S., Galietta L. J. (2009) Chloride channels as drug targets. Nat. Rev. Drug Discov. 8, 153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Namkung W., Thiagarajah J. R., Phuan P. W., Verkman A. S. (2010) Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 24, 4178–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veit G., Bossard F., Goepp J., Verkman A. S., Galietta L. J., Hanrahan J. W., Lukacs G. L. (2012) Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol. Biol. Cell 23, 4188–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danielsson J., Perez-Zoghbi J., Bernstein K., Barajas M. B., Zhang Y., Kumar S., Sharma P. K., Gallos G., Emala C. W. (2015) Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology 123, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B., Li C., Huai R., Qu Z. (2015) Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl- channel, contributes to spontaneous hypertension. J. Mol. Cell. Cardiol. 82, 22–32 [DOI] [PubMed] [Google Scholar]
- 61.Qu Z., Yao W., Yao R., Liu X., Yu K., Hartzell C. (2014) The Ca(2+) -activated Cl(-) channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis. Cancer Med. 3, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takayama Y., Uta D., Furue H., Tominaga M. (2015) Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. USA 112, 5213–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz i Altaba A., Mas C., Stecca B. (2007) The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 17, 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drossman D. A. (2006) The functional gastrointestinal disorders and the Rome III process. Gastroenterology 130, 1377–1390 [DOI] [PubMed] [Google Scholar]