Abstract
Healthy repair of cutaneous injury is a coordinated response of inflammatory cells, secreted factors, and biologically active extracellular vesicles (EVs). Although constitutive release of EVs into biologic fluids is a hallmark of cultured cells and tumors, their payload and biologic activity appears to be tightly regulated. We show that Tre-2/Bub2/Cdc16 (TBC1) domain family member 3 (TBC1D3) drives the release of an EV population that causes a decrease in phosphorylation of the transcription factor signal transducer and activator of transcription 3 in naive recipient cells. To explore the biologic activity of EVs in vivo, we used a mouse model of sterile subcutaneous inflammation to determine the payload and biologic activity of EVs released into the microenvironment by committed myeloid lineages and stroma. Expression of TBC1D3 in macrophages altered the payload of their released EVs, including RNA-binding proteins, molecular motors, and proteins regulating secretory pathways. A wound-healing model demonstrated that closure was delayed by EVs released under the control of TBC1D3. We show that modulating the secretory repertoire of a cell regulates EV payload and biologic activity that affects outcomes in tissue repair and establishes a strategy for modifying EVs mediating specific biologic responses.—Qin, S., Dorschner, R. A., Masini, I., Lavoie-Gagne, O., Stahl, P. D., Costantini, T. W., Baird, A., Eliceiri, B. P. TBC1D3 regulates the payload and biological activity of extracellular vesicles that mediate tissue repair.
Keywords: macrophage activation, wound healing, exosome, RabGAP, foreign body response
Extracellular vesicles (EVs) deliver proteins, lipids, and nucleic acids to recipient cells that support functional crosstalk between stroma and immune cells (1–7). As a class, these EVs represent a ubiquitous mechanism of cell-cell communication that is critically important for inflammation (8–11), cancer (12–17), infection (18), immune tolerance and privilege (19, 20), arthritis (21, 22), and wound healing. For example, EV transfer from macrophages to lymphocytes controls expansion and differentiation through the immune synapse (23–25), amplifies immune responses to infection (26–28), controls the immune response to tumors (9, 29, 30), and even controls tolerance and immunogenicity (31–35). In the case of wound healing, EVs accelerate wound closure (36), but the mechanisms regulating their temporal spatial release, payload, and biologic activity during the injury response are unknown (37). The release of EVs is mediated by the fusion of intracellular multivesicular endosomes with the plasma membrane (38–40) or by evagination of the plasma membrane and is regulated by the endosomal sorting complexes required for transport family of sorting proteins, Rabs and Rab GTPase activating proteins (RabGAPs) (41, 42). Here, we explored whether one such Rab-binding protein, TBC1 domain family member 3 (TBC1D3), could alter the payload of EVs and, if so, whether their adoptive transfer to the wound bed would alter the natural course of repair. TBC1D3 expression enhances growth factor signaling by modulating phosphorylation and ubiquitination (43–45) and accelerates micropinocytosis, a process known to enhance antigen uptake and presentation (46–49). We show here that expression of TBC1D3 regulates EV payload and biologic activity that modulates leukocyte recruitment and signaling.
MATERIALS AND METHODS
Mice, sponge implants, EV recovery, adoptive transfer, and wound-healing assay
All mouse procedures were approved by the University of California–San Diego Institutional Animal Care and Use Committee. C57Bl/6 male mice 8–10 wk of age were used to implant sterile polyvinyl alcohol (PVA) sponges (PVA Unlimited, Warsaw, IN, USA) prepared as previously described by Baird et al. (50). Briefly, 3 individual sponges, each 4 mm in diameter, were placed in the dorsum of animals that had been prepared aseptically. PVA implants were then used to analyze leukocyte content, the EVs released into the conditioned sponge, and for lentiviral gene delivery. PVA sponges were harvested at indicated time points, washed in 1 ml PBS, and subjected to centrifugation at 300 g for 5 min; infiltrating leukocytes were either analyzed by flow cytometry or lysed in Trizol (Thermo Fisher Scientific, Waltham, MA, USA) for gene expression analysis. The supernatant was further centrifuged at 3000 g and 10,000 g at 4°C, and the supernatants were collected as previously described by Thery et al. (51). The EVs were then concentrated from the supernatant with a 100,000 g centrifugation for 70 min at 4°C in a Beckman Optima Max-XP Ultracentrifuge with a TLA120.2 rotor (Beckman Coulter, Brea, CA, USA) (clearance factor = 7), and particles were quantified by nanoparticle tracking analysis (NTA) (NS300; Spectris, Egham, United Kingdom) using a standardized operating procedure (Acquisition software, NTA 3.1). A scientific complementary metal-oxide–semiconductor camera acquired 1500 frames, 25 frames per second, and a detection threshold of 5 and blur size control set to automatic. EV analysis using NTA was used to establish the absolute number for the adoptive transfers described below and estimate the mean size of EVs. The adoptive transfer of EVs from these donor PVA sponges was accomplished by instilling 2e10 EVs in 25 µl of PBS into a fresh, full-thickness punch wound (4 mm diameter) splinted with a neoprene ring (6-mm outer diameter and 4-mm inner diameter), fastened with nylon sutures, and covered with Tegaderm dressing (3M, Maplewood, MN, USA). Wound closure was monitored by regular imaging with a digital camera and scale bar, and the wound area was quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA) by a blinded observer. EVs collected from PVA implants for transfer into the wound bed were labeled with PKH26 per the manufacturer’s recommended conditions (PKH26GL; MilliporeSigma, Burlington, MA, USA). Transfer of EVs validated by NTA and collected from THP-1–transduced cells was accomplished by the incubation of THP-1 transduced cells (i.e., donor cells), as described below, in RPMI1640 supplemented with exosome-depleted fetal bovine serum (EXO-FBS-50A-1; System Biosciences, Palo Alto, CA, USA) for 18 h followed by collection of conditioned medium, enrichment of EVs by serial centrifugation (as described above), and addition to naive recipient THP-1 cells resuspended in EV-containing medium (2 × 1010 EVs in 2 ml serum-free medium) for 18 h. The same amount of EV-depleted fetal bovine serum was used for each condition; therefore, the differences observed are unlikely to be due to exogenous serum. Recipient THP-1 cells were then fixed, permeabilized, and stained as described below.
Cell culture and lentiviral gene transduction
Human premonocytic THP-1 cells [TIB-202; American Type Culture Collection (ATCC), Manassas, VA, USA] and mouse RAW264.7 macrophage cells (TIB-71; ATCC) were cultured in Roswell Park Memorial Insitutute (RPMI) 1640 medium (Thermo Fisher Scientific) with 10% fetal calf serum at low passage. Cells were transduced with a fourth-generation lentivirus using a high titer 1-step packaging system based on vesicular stomatitis virus G (631276; Takara Bio, Kusatsu, Japan) using Lenti-293T cells (632180; Takara Bio) and concentrated with Lenti-X concentrator (631231; Takara Bio) yielding titers > 107 infectious U/ml. TBC1D3 (NM_001123391, RC225941; Origene, Rockville, MD, USA) was subcloned into the lentiviral (lv) vector pLenti-EF1a-C-Myc-DDK-IRES-Puro (PS100085, lv-TBC1D3; Origene) with empty vector used as a negative control (lv-vector). THP-1 cells and RAW264.7 cells were transduced with lv-TBC1D3 or lv-empty and used for studies within 7 d of transduction. Expression was verified by reverse transcription (iScript, 1708890; Bio-Rad, Hercules, CA, USA), amplification by PCR with primers to human TBC1D3 (up, 5′-AACAGGAGCATGTGGTAGCC-3′; down, 5′-GGTTTGGCTGGGGGTGG-3′), human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (QT01658692, Quantitect; Qiagen, Venlo, The Netherlands), and agarose gel electrophoresis. Quantification of gene expression was performed by quantitative RT-PCR as described above with primers optimized for an amplicon for quantitative RT-PCR using primers to human TBC1D3 (up, 5′-TACGAAAAGGGACACCGAGC-3′; down, 5′-TCTCCCAGCATATCCACCCA-3′) and the same human GAPDH primers from Qiagen. TBC1D3 primer sets were validated by analysis of HEK293 cells transfected to overexpress TBC1D3. In vivo gene delivery used the same lentiviral vectors, packaging plasmids and cells, and concentration strategies, with the virus being concentrated to a final titer of 107 infectious units in 100 µl for in vivo injections into PVA sponges that had been implanted subcutaneously and preincubated for 3 d to increase the transduction of infiltrating leukocytes in the PVA sponge model. Cells and EVs from the conditioned wound fluid of the PVA model were isolated as described above.
Flow cytometry and vesicle flow cytometry
For the in vivo studies, cells harvested from the PVA sponge were subjected to flow cytometry to detect infiltrating monocytes, macrophages, and granulocytes using the following cell surface markers: CD45-VioGreen (130-110-803; Miltenyi Biotec, Auburn, CA, USA), CD11b-APC-Vio770 (130-109-288; Miltenyi Biotec), F4/80-PE (130-102-433; Miltenyi Biotec), Ly6G-FITC (130-107-422; Miltenyi Biotec), and propidium iodide (130-093-233; Miltenyi Biotec) to exclude dead cells. The EVs harvested from the conditioned wound fluid of the PVA sponge model were characterized by NTA as described above and subjected to vesicle flow cytometry (VFC) (52, 53) by first incubating EVs isolated from ultracentrifugation with the fluorescent membrane dye di8-ANEPPS (MilliporeSigma)—a dye that undergoes an increase in fluorescence upon binding in the lipid bilayer as previously described in refs. 52 and 53. Configuration of the flow cytometer (MacsQuant10 Analyzer; Miltenyi Biotec) to trigger events on the basis of di8-ANEPPS fluorescence enabled the detection of high stain index (i.e., allophycocyanin)–conjugated antibodies to the tetraspanins CD9, CD63, and CD81. All VFC data shown are based on triggered di8-ANEPPS events followed by antibody staining and overlaying with the same samples subjected to 0.1% Triton X-100 and reanalyzed on the flow cytometer (Miltenyi Biotec) to verify the membrane specificity of the VFC analysis. For the in vitro studies, recipient THP-1 cells that had been fixed and permeabilized as described above were subjected to analysis of intracellular phosphorylation of the transcription factor signal transducer and activator of transcription 3 (STAT3; 9131; Cell Signaling Technology, Danvers, MA, USA).
Mass spectrometry
To determine the protein payload of EVs, either conditioned medium of cultured RAW264.7 macrophages or wound fluid from PVA sponge implants was subjected to serial centrifugation (including ultracentrifugation), size validation by NTA as previously described, normalization of input protein by bicinchoninic acid assay followed by protease digestion, and analysis by liquid chromatography coupled with tandem mass spectrometry at the University of California–San Diego Biomolecular and Proteomics Mass Spectrometry Facility. Briefly, samples were diluted in TNE buffer (50 mM Tris pH 8.0, 100 mM NaCl, 1 mM EDTA) with 0.1% RapiGest SF surfactant (Waters, Milford, MA, USA). Tris (2-carboxyethyl) phosphine was added to a 1-mM final concentration, carboxymethylated with 0.5 mg/ml of iodoacetamide, and then neutralized with 2 mM Tris (2-carboxyethyl) phosphine. Samples were digested with trypsin (trypsin:protein ratio, 1:50) with RapiGest, inactivated, and the soluble peptides extracted and desalted. Peptides were quantified using bicinchoninic acid assay, and a total of 1 µg of peptides were injected for liquid chromatography coupled with tandem mass spectrometry analysis using nanospray ionization using an Orbitrap Fusion Lumos Tribrid Mass Spectrometer (Thermo Fisher Scientific) interfaced with nanoscale reversed-phase ultra–high-pressure liquid chromatography (Dionex UltiMate 3000 RSLCnano System; Thermo Fisher Scientific). Mass spectrometer (MS) parameters were as follows: MS1 survey scan using the Orbitrap detector [mass range (m/z): 400–1500 (using quadrupole isolation), 120,000 resolution setting, spray voltage of 2200 V, ion transfer tube temperature of 275°C, automatic gain control target of 400,000, and maximum injection time of 50 ms]. Peaks Studio 8.5 (Bioinformatics Solutions, Waterloo, ON, Canada) was used for protein identification and label-free quantification and searched against the mouse database with a modified target decoy to determine the minimum peptide spectrum matching score threshold to meet the false discovery rate requirement. Highly differentially expressed proteins between d 1 and 7 in the PVA sponge proteomics or in TBC1D3-transduced vs. vector control cells were established by the Peaks en-suite statistical analysis tool. All uncharacterized proteins were excluded from analysis of both the RAW264.7 cells and the cells from the PVA sponge time course. In addition, the RAW264.7 proteomic data were subjected to further crossanalysis on a Bos taurus database, in which any peptide hit that was indistinguishable from mouse was eliminated from the analyses, thereby addressing the potential for bovine peptides present in the exosome-depleted fetal bovine serum from contaminating the results of the RAW264.7 proteomic analyses (54). Proteomic data are reported here using published reporting guidelines detailed in the Supplemental Data (55), and more detail on the Peaks algorithms is available from Zhang et al. (56).
Statistical analysis
All statistical analyses were performed with Prism 6.0h (Graphpad Software, La Jolla, CA, USA). A Student’s t test was performed for the analysis of flow cytometry, whereas, for the analysis of wound closure kinetics, differences between multiple groups at each time point were evaluated using two-way ANOVA, with a value of P < 0.05 used to determine statistical significance. Data are presented as the means ± sd and are representative of at least 3 independent experiments.
RESULTS
TBC1D3 decreases proteins relevant to inflammation in the EV payload
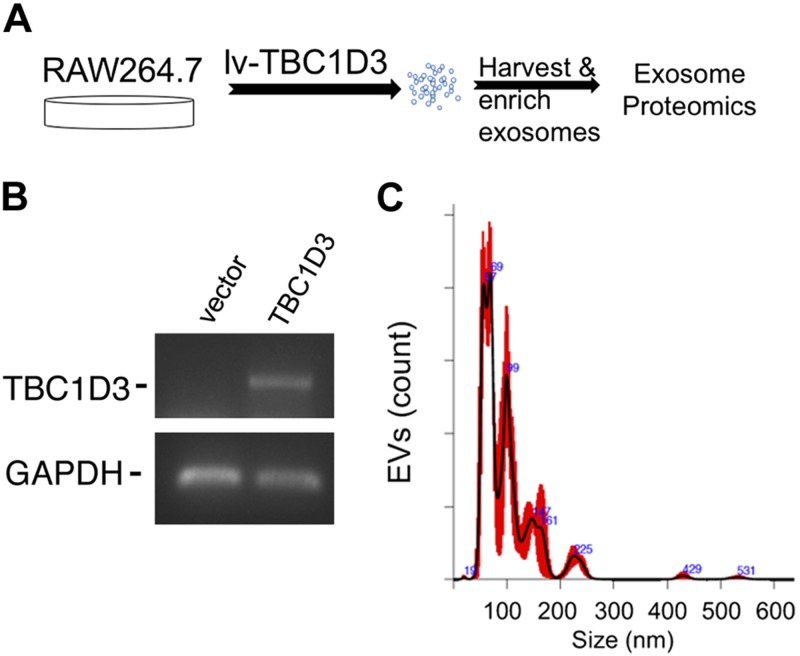
To determine whether TBC1D3 regulates the payload of EVs released into culture medium, RAW264.7 macrophages were transduced with a lentiviral vector expressing TBC1D3 or an empty vector control (Fig. 1A) and the expression of TBC1D3 validated by RT-PCR (Fig. 1B). Conditioned medium containing EVs was enriched from the RAW264.7 cells as described in Materials and Methods by serial ultracentrifugation and analyzed by NTA to determine size distribution and concentration with a representative plot shown in Fig. 1C. Duplicate samples were subjected to analysis by mass spectrometry using a Mus musculus database and absent from a B. taurus database to identify at least 2 unique peptides for each protein (Tables 1 and 2 and Supplemental Data). NTA histograms of EVs released from vector and TBC1D3-transduced RAW264.7 cells had vesicle diameters with mode values of 59.5 ± 4 nm and 81 ± 15 nm (n = 3) that were not statistically different. Mass spectrometry analysis of normalized spectral counts was used to identify proteins on or in EVs released into the conditioned medium and analyzed on a mouse protein database. The Materials and Methods section and Supplemental Table S1 provide additional details on the data acquisition and analysis of the mass spectrometry studies. Table 1 shows that the top 10 EV proteins that were increased in the EVs from TBC1D3-transduced cells were compared with control vector–transduced cells, and Table 2 shows the top 10 proteins that were decreased in TBC1D3-transduced EVs compared with vector control. A list of the proteins increased and decreased by TBC1D3 is provided in Supplemental Table S2. As shown in Table 1, we identified TBC1D3-mediated changes in the EV payload, with increases in proteins regulating RNA binding and nucleotide binding (e.g., Ddx17 and Ruvbl1), vesicle trafficking (e.g., Rab-11a), signaling (e.g., glycine-tRNA ligase and Rack1), and components of proteasomes and ribosomes. In Table 2, we identified TBC1D3-mediated decreases in EV payload that included peroxidases (e.g., Prdx4), membrane trafficking (e.g., syntenin-1), biosynthesis enzymes (e.g., Mat2a and Aldoa), growth factors (e.g., lactadherin), and proteins relevant in remodeling of the microenvironment (e.g., lysozyme, hypoxia up-regulated protein 1, murinoglobulin, and protocadherin Fat4). Along with the annotated TBC1D3 changes shown in Tables 1 and 2, a list of these proteins, along with additional proteins that were unchanged, is detailed in Supplemental Table S2. We observed decreases in factors relevant to the inflammation phase of tissue repair (e.g., peroxiredoxin, lysozyme, and syntenin), whereas TBC1D3-mediated increases in factors related to nucleic acid binding (Ddx17 and Ruvbl1) and EV trafficking (e.g., Rab-11A) were identified, suggesting that TBC1D3 expression in macrophages may decrease the level of inflammatory mediators in EVs while increasing factors normally associated with EV biogenesis and membrane dynamics. These findings demonstrate that TBC1D3 may reprogram subsets of EVs produced by macrophages.
Figure 1.
Expression of TBC1D3 in macrophages regulates the payload of released EVs. A) Mouse RAW264.7 macrophages were transduced with lentivirus-expressing TBC1D3 or vector control. B) The cells validated for expression of TBC1D3 by RT-PCR. C) EVs isolated and subjected to NTA with a representative plot shown from vector-transduced cells. No significant differences were observed in the NTA between vector and TBC1D3-transduced RAW264.7 cell EVs.
TABLE 1.
Identification of EV proteins increased by TBC1D3-expressing cells
| Protein | NCBI ref seq ID | Fold change | Gene | Molecular function |
|---|---|---|---|---|
| ATP-dependent RNA helicase DDX17 isoform 4 | NP_001035277.1 | 8.1 | Ddx17 | Nucleotide binding |
| RuvB-like 1 | NP_062659.1 | 7.4 | Ruvbl1 | Nucleotide binding |
| Glycine-tRNA ligase | NP_851009.2 | 3.1 | Gars | Signaling ligase |
| Receptor of activated protein C kinase 1 | NP_032169.1 | 3.1 | Rack1 | Signaling scaffold |
| Ras-related Rab-11A | NP_059078.2 | 2.9 | Rab11a | Membrane trafficking |
| Multifunctional protein ADE2 | NP_080215.1 | 2.1 | Paics | Purine metabolism |
| 26S proteasome non-ATPase regulatory subunit 3 | NP_033465.1 | 1.7 | Psmd3 | Proteasome regulation |
| 40S ribosomal protein SA | NP_035159.3 | 1.6 | Rpsa | Ribosome component |
| Vimentin | NP_035831.2 | 1.6 | Vim | Intermediate filament |
| 60S acidic ribosomal protein P0 | NP_031501.1 | 1.5 | Rplp0 | Ribosome component |
NCBI, National Center for Biotechnology Information.
TABLE 2.
Identification of EV proteins decreased by TBC1D3-expressing cells
| Protein | NCBI ref seq ID | Fold change | Gene | Molecular function |
|---|---|---|---|---|
| Peroxiredoxin-4 isoform 1 | NP_001300640.1 | −8.2 | Prdx4 | Thiol-specific peroxidase |
| Syntenin-1 isoform 1 | NP_001091697.1 | −7.1 | Sdcbp | Transmembrane trafficking |
| Lactadherin isoform 2 | NP_001038954.1 | −6.1 | Mfge8 | Putative growth factor |
| Lysozyme C-2 precursor | NP_059068.1 | −5.6 | Lyz2 | Bacteriolytic agent |
| Hypoxia up-regulated protein 1 | NP_067370.3 | −5.6 | Hyou1 | Regulated by oxygen deprivation |
| Collagen α-1(I) chain | NP_031768.2 | −5.4 | Col1a1 | Extracellular matrix structure |
| Murinoglobulin-1 | NP_032671.2 | −5.1 | Mug1 | Endopeptidase inhibitor |
| S-adenosylmethionine synthase isoform type 2 | NP_663544.1 | −5.1 | Mat2a | Biochemical synthesis |
| Fructose-bisphosphate aldolase A isoform 1 | NP_001170778.1 | −4.4 | Aldoa | Biochemical synthesis |
| Protocadherin Fat 4 | NP_899044.3 | −4.3 | Fat4 | Cell-cell interaction |
NCBI, National Center for Biotechnology Information.
EV biogenesis in sterile subcutaneous PVA sponge implants
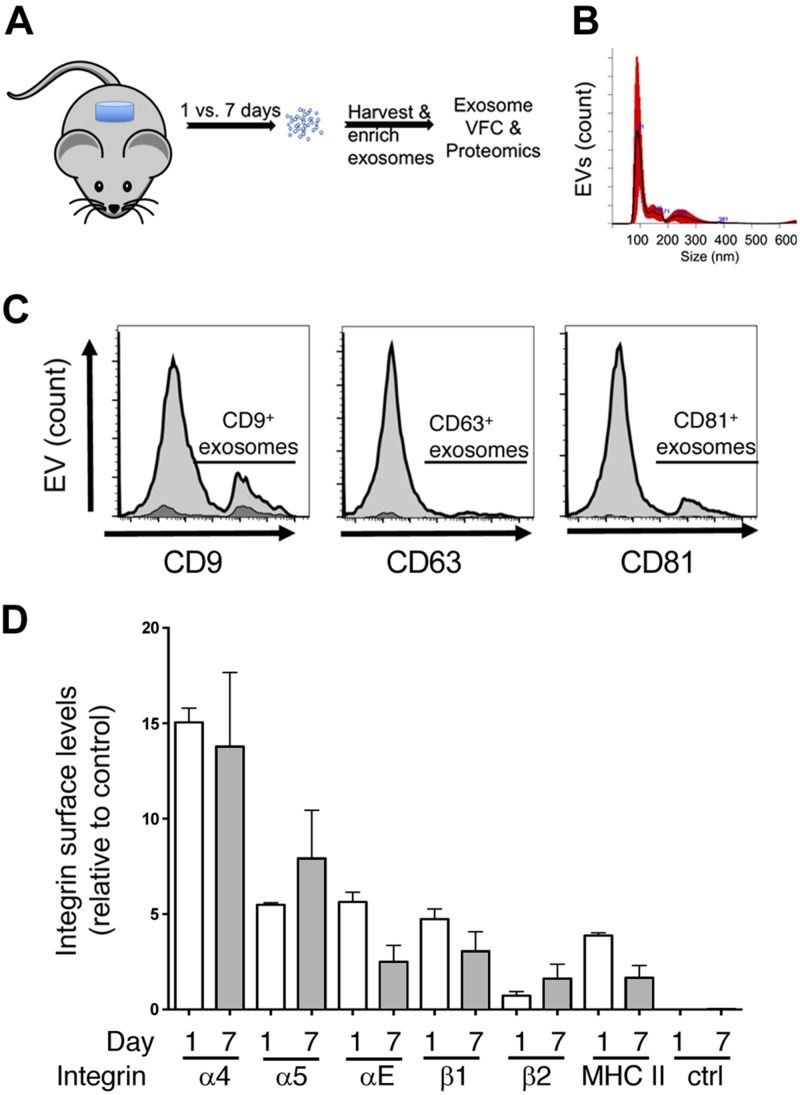
We and others have used subcutaneous implants of PVA sponge as an in vivo model for the study of leukocyte recruitment in the sterile inflammation of a cutaneous injury (50, 57). This model has many parallels with the inflammation response observed in cutaneous wounds and is characterized by the infiltration of macrophages and inflammatory monocytes within the first week of implantation. Therefore, we used this model to collect EVs released in the wound fluid of PVA sponges collected 1 or 7 d after placement into mice as described in Materials and Methods (Fig. 2A). We used both NTA (Fig. 2B) and VFC to characterize EVs on the basis of protein epitopes measured on detergent-soluble EVs using antibodies directed to tetraspanins (Fig. 2C) and integrins (Fig. 2D). NTA was used to measure size and distribution with no significant difference in the diameter of d 1 (mode 125 ± 23.8 nm, n = 3) vs. d 7 EVs (mode 144.9 ± 51.5 nm), and as shown by a representative NTA from a d 7 EV sample (Fig. 2B), and quantification of EV concentration over a 7-d timecourse (Supplemental Fig. S1). Although we have observed that there are low numbers of vesicles of larger diameter in the PVA sponge model, these were of low abundance in terms of relative concentration. Using VFC to assess the levels of the tetraspanins CD9, CD63, and CD81 on EVs from d 7 PVA sponge implants, we observed higher levels of both CD9 and CD81 relative to CD63. To establish that the particles being analyzed were lipid vesicles, we subjected samples to the detergent Triton X-100 and re-analyzed samples where histogram overlays defined the amount of detergent-soluble vesicles (Fig. 2C, light gray) vs. detergent-insoluble particles (Fig. 2C, dark gray). Based on the importance of integrins in mediating binding and affecting tropism in binding the wound microenvironment, we performed VFC with antibodies directed to specific integrin subunits. In each case, only detergent-soluble EVs were quantified with no significant changes in the integrin profile observed between day 1 and 7 EVs (Fig. 2D). Together, these findings define a population of EVs that is generally similar in terms of tetraspanins, integrins, and size.
Figure 2.
Kinetics of EV release defined by changes in protein profile and payload in the foreign-body response in vivo. A) Subcutaneous implants of PVA sponge were used as a model for sterile inflammation where EVs are isolated from the conditioned supernatant of infiltrating leukocytes. B) Representative NTA analysis of d 7 EVs showing a mode value of 144.9 ± 51, with no significant differences with d 1 EVs. C) VFC analysis was performed on vesicles released in the PVA sponge implant after 7 d using antibodies directed to the tetraspanins CD9, CD63, and CD81 and are indicated under the line segment (light gray). Treatment of samples with the detergent Triton X-100 was used to establish background values (dark gray shading). D) VFC analysis of integrin subunits and major histocompatibility complex (MHC) II detected on EVs collected at d 7 postimplantation to assess the profile of integrins present on EVs. No significant differences in integrins were observed between d 1 and d 7 EVs. Ctrl, control.
Based on previous experience defining the cutaneous inflammation response on the basis of leukocyte recruitment (50), we next explored the possibility of defining the timecourse of the inflammation response on the basis of EV protein payload. A proteomic approach was used to compare protein changes in d 1 vs. d 7 EVs (Tables 3 and 4). EVs were subjected to protease digestion, fractionated by high-performance liquid chromatography, and analyzed by mass spectrometry. After normalizing changes observed in d 7 EVs to the protein profile from d 1 EVs, we found increases in factors relevant to vascular remodeling (e.g., kallikrein), signaling (e.g., TGF-β-induced protein, Niban/Fam129a, and epidermal growth factor receptor), inflammation (e.g., leukotriene hydrolase), actin filament polymerization (Actr3 and Wdr1), chaperonins (e.g., Hsp5a), and metabolism (e.g., Aldh2 and Fth1). We observed decreases in proteins related to energy transduction (e.g., creatine kinase and glycogen phosphorylase), DNA binding (e.g., Hist1h1d), leukocytes (e.g., Ly6C1), and 40S and 60S ribosome components (e.g., Rpl7, Rpl18, Rps26, Rpl14, and Rpl15). Changes in additional proteins relevant to plasma membrane dynamics and EV-related signaling and trafficking (e.g., actin-related protein 2/3, VAT1, Rab8b, dynein, and RhoA) beyond the top 10 are shown here, and others that were observed in the TBC1D3-regulated EVs (e.g., ruvbl1), are included in Supplemental Table S3.
TABLE 3.
Identification of EV proteins increased in d 7 vs. d 1 PVA implants
| Protein | NCBI ref seq ID | Fold change | Gene | Molecular function |
|---|---|---|---|---|
| Plasma kallikrein | NP_032481.2 | 72.7 | Klkb1 | Endopeptidase |
| Protein Niban | NP_071301.2 | 71.4 | Fam129a | Regulate protein phosphorylation |
| 78 kDa glucose-regulated protein | NP_071705.3 | 57.3 | Hspa5 | Chaperonin |
| Leukotriene A-4 hydrolase isoform 1 | NP_032543.2 | 46.2 | Lta4h | Epoxide hydrolase |
| Aldehyde dehydrogenase, mitochondrial isoform 1 | NP_033786.1 | 44.9 | Aldh2 | Alcohol metabolism |
| Epidermal growth factor receptor isoform 1 | NP_997538.1 | 33.4 | Egfr | Growth factor receptor |
| WD repeat–containing protein 1 | NP_035845.1 | 24.1 | Wdr1 | Actin filament polymerization |
| Actin-related protein 3 | NP_076224.1 | 20.8 | Actr3 | Actin filament polymerization |
| Ferritin heavy chain | NP_034369.1 | 19.5 | Fth1 | Iron homeostasis |
| TGF-β–induced protein ig-h3 | NP_033395.1 | 18.8 | Tgfbi | Cell adhesion |
NCBI, National Center for Biotechnology Information; ref seq ID, reference sequence identification.
TABLE 4.
Identification of EV proteins decreased in d 7 vs. d 1 PVA implants
| Protein | NCBI ref seq ID | Fold change | Gene | Molecular function |
|---|---|---|---|---|
| Creatine kinase M-type | NP_031736.1 | −12.2 | Ckm | Energy transduction |
| Histone H1.3 | NP_663759.3 | −8.8 | Hist1h1d | DNA binding |
| 60S ribosomal protein L7 | NP_035421.2 | −7.3 | Rpl7 | Ribosome component |
| Glycogen phosphorylase, muscle form | NP_035354.1 | −7.2 | Pygm | Carbohydrate metabolism |
| 60S ribosomal protein L18 | NP_033103.2 | −6.5 | Rpl18 | Ribosome component |
| 40S ribosomal protein S26 | NP_038793.2 | −6.2 | Rps26 | Ribosome component |
| Lymphocyte antigen 6C1 isoform 2 precursor | NP_001238985.1 | −6.1 | Ly6c1 | Lymphocyte antigen |
| 60S ribosomal protein L14 | NP_080250.1 | −6.0 | Rpl14 | Ribosome component |
| Heterogeneous nuclear RNP C1/C2 | XP_017171348.1 | −5.8 | Hnrnpc | Ribosome component |
| 60S ribosomal protein L15 | NP_079862.1 | −5.2 | Rpl15 | Ribosome component |
NCBI, National Center for Biotechnology Information.
Adoptive transfer of EVs to naive PVA implant enhances macrophage recruitment
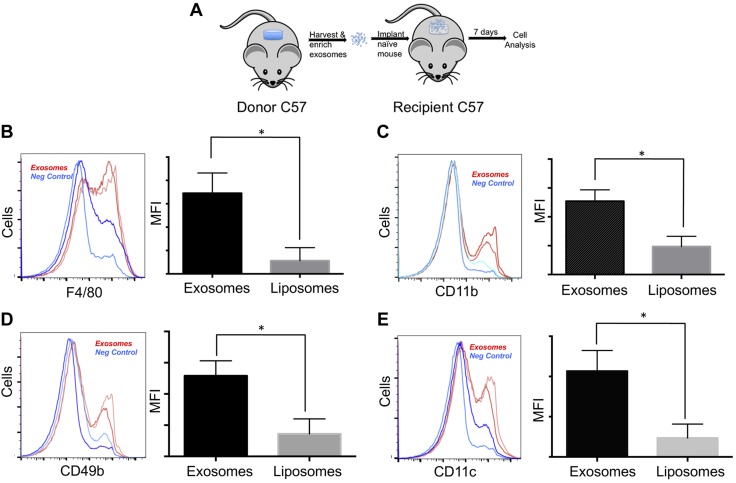
To determine the biologic relevance of changes in the EV payloads, EVs isolated from d 7 PVA implants (i.e., donor EVs) were transferred into fresh PVA implants in naive recipient mice (Fig. 3A) using liposomes as a control for the effects of lipids on leukocyte recruitment in the PVA model (58). As shown in Fig. 3B, transfer of d 7 donor sponge EVs (red curve) resulted in a significant increase in the number of wound-bed monocytes and macrophages expressing the macrophage marker F4/80 and the integrins CD11b, CD11c, and CD49b (Fig. 3C). These observations establish that injury-derived EVs can accelerate the recruitment of monocytes and macrophages.
Figure 3.
Adoptive transfer of EVs from 7 d donor site promotes macrophage recruitment in vivo. A) Adoptive transfer of EVs from donor site PVA sponge implants that are then purified and adoptively transferred to naive PVA sponge implants to monitor leukocyte recruitment. B–E) Flow cytometry analysis of leukocytes infiltrating recipient PVA sponges that had received EVs isolated from d 7 donor PVA implants, incubated for an additional 7 d post-transfer. EVs (red lines) are compared to liposome controls (blue lines) from duplicate samples. For each group, an analysis of F4/80 levels by histogram and quantitation of mean fluorescence intensity (MFI) is provided (B), along with an analysis of CD11b+ cells by histogram and MFI (C), CD49b+ cells by histogram and MFI (D), and CD11c+ cells by histogram and MFI (E). For each marker, n = 2. *P < 0.05.
Adoptive transfer of TBC1D3-EVs delays wound closure
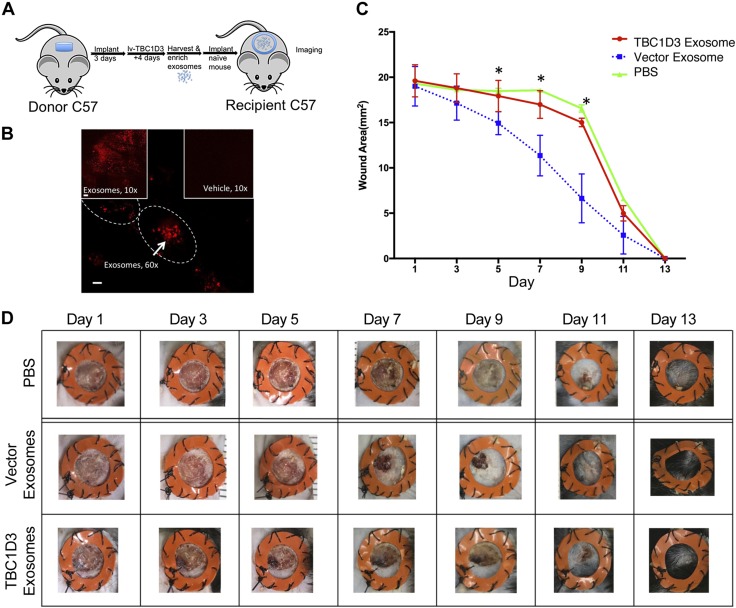
Because the adoptive transfer of d 7, PVA-normal EVs into a naive PVA recipient alters recruitment of inflammatory cells (Fig. 3), we evaluated the effect of d 7 PVA EVs on an excisional wound. We transferred wild-type normal EVs into a 4-mm wound bed (Fig. 4A) and used the membrane dye PKH26 to establish EV uptake (Fig. 4B). The wound bed was imaged 4 h postimplantation by laser scanning confocal microscopy, and PKH26-labeled EVs were identified by the clustering of PKH26-positive intracellular staining (Fig. 4B, outlined) compared with vehicle control. These findings show that EVs generated in PVA sponge implants are internalized in the wound bed of recipient mice. We next focused on the effect of TBC1D3 and examined the effect of TBC1D3-regulated EVs on wound closure by first transducing the donor PVA site with TBC1D3 followed by transfer of EVs into a recipient wound site (Fig. 4C). Lentivirus-expressing TBC1D3 or empty vector was injected into the PVA donor site to transduce infiltrating leukocytes and expression validated by RT-PCR (unpublished results) followed by the collection of EVs from the conditioned medium of the sponge implants 4 d later. The donor EVs released under the regulation of TBC1D3 in the PVA donor site were then transferred to a splinted, 4-mm, full-thickness punch wound. Wound closure was determined by quantification of the wound area (Fig. 4C) and supported by representative images at each time point (Fig. 4D). EVs isolated from vector controls accelerated wound closure (Fig. 4C, blue squares), consistent with reports showing that EVs accelerate wound closure (36, 59–61). In contrast, we observed that TBC1D3-regulated EVs failed to accelerate healing (Fig. 4C, red circles). These results suggest that the expression of TBC1D3 abrogates the proreparative properties of EVs.
Figure 4.
Expression of TBC1D3 regulates the biologic activity of a class of EVs that uncouples the proreparative effects of EVs on wound closure. A) Overview of the lentiviral transduction of leukocytes infiltrating the PVA donor site from which EVs are isolated for adoptive transfer of EVs into recipient 4-mm full-thickness cutaneous punches. B) Donor EVs labeled with fluorescent PKH26 were transferred to fresh wound sites, incubated for 18 h, imaged by confocal microscopy, and compared with vehicle controls. Cell membrane is indicated with a tracing to show intracellular clustering of EV uptake. Scale bar, 60 µm. C) Quantification of the kinetics of wound closure was determined in splinted wound sites following the addition of EVs harvested from the PVA donor site cells expressing TBC1D3 (red) vs. EVs isolated from vector control cells (blue). PBS was used as a control for the normal kinetics of wound closure in the absence of EVs (green). *P < 0.05 between TBC1D3 and vector groups; n = 6 for each group. D) Representative images of the time course of wound closure following addition of TBC1D3-regulated EVs, vector control EVs, and buffer control.
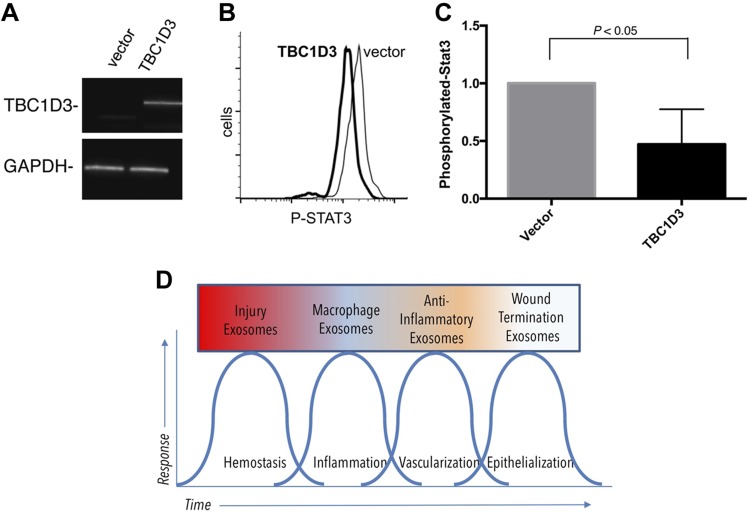
TBC1D3 EVs reduce STAT3 phosphorylation in recipient cells in vitro
Because TBC1D3-mediated EVs lost their ability to accelerate wound closure, we used an in vitro system to better understand the mechanism. We examined the ability of TBC1D3 to influence STAT3 phosphorylation, a transcription factor relevant in monocyte and macrophage differentiation and previously shown to be responsive to treatment with EVs (62–64). EVs were enriched from the conditioned medium of THP-1 cells expressing TBC1D3 (Fig. 5A) and added to naive recipient THP-1 cells to assess changes in the phosphorylation state of STAT3. As shown in Fig. 5B, C, we observed a decrease in STAT3 phosphorylation in recipient cells mediated by the addition of EVs released from donor cells expressing TBC1D3 vs. EVs from donor cells transduced with a vector control (data normalized to vector control). Based on our findings that the payload and biochemical activity of EVs can be regulated by genes such as TBC1D3, we propose a model in which the phases of the wound-repair response can be defined on the basis of distinct EV populations released in each of the canonical phases of tissue repair (Fig. 5D).
Figure 5.
TBC1D3 expression in human hematopoietic and myeloid cells. A) RT-PCR validation of TBC1D3 gene delivery in THP-1 cells. B) Conditioned medium of donor TBC1D3-transduced cells was enriched by ultracentrifugation and added to naive recipient THP-1 cells for 18 h, fixed, permeabilized, and stained for intracellular levels of phosphorylated STAT3 compared with vector control. C) Quantification of TBC1D3-mediated changes on the biologic activity of EVs released under the regulation of TBC1D3 on the phosphorylation of STAT3 (P < 0.05, n = 3). D) Model for the role of EVs in defining phases of wound healing based on the hypothesis that EV classes are dependent on the kinetics of tissue repair.
DISCUSSION
In this study, we have defined a biologic activity for the regulated release of EVs mediated by a Rab-binding protein, TBC1D3. Using adoptive transfer of EVs, we demonstrate that TBC1D3 reprograms the EV payload and decreases the ability of EVs to enhance wound closure in association with decreased STAT3 phosphorylation.
TBC1D3 is a naturally occurring, uniquely hominid variant of the RabGAP superfamily that mediates membrane trafficking (65), likely because of a mutation of a catalytic arginine. Among the family of TBC-domain RabGAPs, TBC1D3 is unique in that it is the only gene in the family that is only expressed in hominids (44, 66). In fact, in the fields of evolutionary biology, neuroscience, and inflammation, there is an emerging interest in unique human genes that encode functional proteins. Such human-specific genes include not only TBC1D3 but other regulators of GTPases such as the GTPase-activating protein, ARHGAP11B (67); the dominant negative regulator of acetylcholine receptor signaling, CHRFAM7A (68); and the intercellular mediator of calcium-signaling differentiation, NOTCH2NL (69). The TBC1D3 gene itself emerged late in primate speciation through the partial duplication of the RNTRE locus and the acquisition of a palmitoylation motif that results in its localization to plasma membrane rafts (41, 42). This may account for the effects of TBC1D3 on signaling, membrane dynamics, and macropinocytosis (43, 45, 65) and the results we show here regarding EV payload and biologic activity. It is also possible that there are intrinsic effects of TBC1D3 on the regulation of macrophage plasticity that may also be important in the wound-repair response. Our recently published studies using a chimeric mouse transplanted with human CD34+ hematopoietic stem cells demonstrated that TBC1D3 is expressed in cells of myeloid lineage, including wound macrophages (50). The effects of TBC1D3 expression on EV-mediated signaling to STAT3 are defined in the human THP-1 cell line to establish a standardized assay for the biochemical activity of EVs released under the regulation of various EV biogenesis pathways. We have used the mouse RAW264.7 cell line as an in vitro tool to validate the capacity for TBC1D3 to reprogram the payload of EVs for our proteomics studies to provide a foundation in a mouse background for the in vivo biologic readouts in mouse wound-healing models.
The scope of this study has been focused primarily on the capacity for TBC1D3 expression to regulate the EV payload and biologic activity, and although the methodologies and standards for this are widely discussed, we selected mass spectrometry as a tool to identify changes at the protein level. Because our work and work by others have shown that EVs can be immunologically active (70–72), we anticipated that the uptake of EVs in the wound microenvironment by inflammatory cells and stroma would affect local remodeling. We note that proteins present in or on the surface of EVs have the potential to mediate rapid signaling at the surface of a recipient cell independent of internalization, as well as the potential to influence classic endosomal uptake and clearance pathways. For example, the EVs released from alveolar macrophages and adipose-derived stem cells can deliver biochemically relevant regulators of STAT3 phosphorylation (64, 73). Although we have focused here on activation of STAT3 to establish a standardized in vitro assay, we anticipate that a systematic analysis of the biochemical effects of EV biogenesis regulators will reveal that different populations of EVs can regulate additional biochemical pathways. We suggest that TBC1D3 affects signaling by modulating phosphorylation on a number of targets that could affect loading of cargo into EVs or, after delivery to recipient cells, manipulation of signaling in recipient cells by selective dephosphorylation events. Based on our experimental approach, we suggest that targeted gene delivery can be used to regulate the biologic activity of EV classes to affect biologic outcomes.
The release of EVs from cells cultured in 2 dimensions has been a standard in the field of cell biology, but recent reports describe the importance of 3-dimensional matrices for the efficient culture of substantially larger quantities of EVs (74). In this context, we suggest that our model of subcutaneous PVA sponge implants is particularly ideal for the study of sterile infiltrating inflammatory leukocytes and the EVs released from these cells. Although the source cells here are relatively heterogeneous compared with single-cell types used in bioreactors, the PVA sponge is well-described as both a model of foreign-body response that recapitulates many aspects of cutaneous leukocyte recruitment (50, 75, 76) and, therefore, an ideal model for use as a local factory for the biogenesis of sterile wound-bed EVs. In conjunction with a primary cell model (i.e., the PVA implants), we have used cultured cell lines as an in vitro model to define the biochemical activity of EVs that can be used to establish standard assays to assess the effect of other mediators of EV biogenesis on the profile and biologic activity of the EVs released. Although our EV activity assays span the range from in vivo wound healing and PVA implant assays to phosphorylation flow cytometry, the scope of additional biologic and biochemical readouts that would be compatible with our overall approach is substantial.
The importance of analytical tools to define populations of EVs is becoming essential to distinguish constitutive EV releases from regulated releases, as we have shown here as a consequence of specific molecular mediators (e.g., TBC1D3) or injury (i.e., cutaneous inflammation). Although batch protein and nucleic analysis of EVs and transmission electron microscopy have led to the identification of molecular mediators and size and localization, respectively, the technologies that address the heterogeneity of EVs will be increasingly critical in the study of EVs. VFC has emerged as one such technique that enables single-EV analysis, which, similar to the advances of flow cytometry for immune-cell analysis, can be used to define subpopulations of detergent-soluble EVs (52). Here, we show that analyses of integrins present on the surface of EVs define at least 1 important class of molecules that may mediate EV adhesion and tropism in the wound microenvironment.
Based on the importance of developing well-defined biologic models for the functional characterization of genes that regulate EV biogenesis, our studies of the biochemical and biologic activity of TBC1D3 demonstrate that the payload of EVs can be regulated by the secretory machinery, with biologically relevant consequences on the outcomes of inflammation and tissue repair. The potential clinical importance of these studies is based on our observations that the biologic activity of EVs can be modified by molecular mediators of EV biogenesis. In the case of TBC1D3, we have identified a mediator that uncouples the protective effects that are generally associated with EVs in the acceleration of wound healing. The clinical importance of defining the specific EV payloads, tropism, and the biologic payload on the basis of the secretory machinery is further underscored by the potential for the adoption of EVs for diagnostic and therapeutic end points. Therefore, it will be of interest to systematically define EV payload and biologic activity on the basis of known mediators of EV biogenesis in laboratory models and in humans.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Ann-Marie Hageny [University of California–San Diego (UCSD)] for expert technical support, and Dr. Antonio DeMaio (UCSD) for use of the Nanoparticle Tracking Analysis Instrument; Dr. Majid Ghassemian (UCSD Biomolecular and Proteomics Mass Spectrometry Facility) provided expert support and analysis for the proteomics studies; Dr. John Nolan (Scintillon Institute, San Diego, CA, USA) provided expert advice on the design of vesicle flow cytometry assays; Dr. Chen Kong (Washington University) helped with consultation on the molecular biology of TBC1D3; and Dr. Lisa A. Hannan provided expert editorial review of the study. Research was supported by operating grants from the U.S. National Institutes of Health, National Institute of General Medical Sciences (1R01GM121530), the UCSD Academic Senate, the UCSD Department of Surgery Reinvestment Fund, the Shock Society, and the Surgical Infection Society. The authors declare no conflicts of interest.
Glossary
- EV
extracellular vesicle
- NTA
nanoparticle tracking analysis
- PVA
polyvinyl alcohol
- RabGAP
Rab GTPase activating protein
- STAT3
signal transducer and activator of transcription 3
- TBC1D3
TBC1 domain family member 3
- VFC
vesicle flow cytometry
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Qin, R. Dorschner, P. D. Stahl, T. W. Costantini, A. Baird, and B. P. Eliceiri designed the research; S. Qin, I. Masini, and O. Lavoie-Gagne performed the research and analyzed the data; A. Baird and B. P. Eliceiri wrote the manuscript with contributions from all authors; and all authors reviewed and approved the final manuscript.
REFERENCES
- 1.Kojima M., Gimenes-Junior J. A., Langness S., Morishita K., Lavoie-Gagne O., Eliceiri B., Costantini T. W., Coimbra R. (2017) Exosomes, not protein or lipids, in mesenteric lymph activate inflammation: unlocking the mystery of post-shock multiple organ failure. J. Trauma Acute Care Surg. 82, 42–50 [DOI] [PubMed] [Google Scholar]
- 2.Lee H., Zhang D., Zhu Z., Dela Cruz C. S., Jin Y. (2016) Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 6, 35250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D., Liu J., Guo B., Liang C., Dang L., Lu C., He X., Cheung H. Y., Xu L., Lu C., He B., Liu B., Shaikh A. B., Li F., Wang L., Yang Z., Au D. W., Peng S., Zhang Z., Zhang B. T., Pan X., Qian A., Shang P., Xiao L., Jiang B., Wong C. K., Xu J., Bian Z., Liang Z., Guo D. A., Zhu H., Tan W., Lu A., Zhang G. (2016) Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 7, 10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenoda B. B., Ajit S. K. (2016) Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin. Med. Insights Pathol. 9 (Suppl 1), 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garzetti L., Menon R., Finardi A., Bergami A., Sica A., Martino G., Comi G., Verderio C., Farina C., Furlan R. (2014) Activated macrophages release microvesicles containing polarized M1 or M2 mRNAs. J. Leukoc. Biol. 95, 817–825 [DOI] [PubMed] [Google Scholar]
- 6.Truman J. P., Al Gadban M. M., Smith K. J., Jenkins R. W., Mayroo N., Virella G., Lopes-Virella M. F., Bielawska A., Hannun Y. A., Hammad S. M. (2012) Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology 136, 30–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Liu D., Chen X., Li J., Li L., Bian Z., Sun F., Lu J., Yin Y., Cai X., Sun Q., Wang K., Ba Y., Wang Q., Wang D., Yang J., Liu P., Xu T., Yan Q., Zhang J., Zen K., Zhang C. Y. (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 39, 133–144 [DOI] [PubMed] [Google Scholar]
- 8.Bianco N. R., Kim S. H., Morelli A. E., Robbins P. D. (2007) Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol. Biol. 380, 443–455 [DOI] [PubMed] [Google Scholar]
- 9.Hao S., Bai O, Yuan J., Qureshi M., Xiang J. (2006) Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell. Mol. Immunol. 3, 205–211 [PubMed] [Google Scholar]
- 10.Pitt J. M., Charrier M., Viaud S., André F., Besse B., Chaput N., Zitvogel L. (2014) Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J. Immunol. 193, 1006–1011 [DOI] [PubMed] [Google Scholar]
- 11.Quah B. J., O’Neill H. C. (2005) The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol. Dis. 35, 94–110 [DOI] [PubMed] [Google Scholar]
- 12.Viaud S., Thery C., Ploix S., Tursz T., Lapierre V., Lantz O., Zitvogel L., Chaput N. (2010) Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 70, 1281–1285 [DOI] [PubMed] [Google Scholar]
- 13.Rak J. (2013) Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Front. Pharmacol. 4, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logozzi M., De Milito A., Lugini L., Borghi M., Calabrò L., Spada M., Perdicchio M., Marino M. L., Federici C., Iessi E., Brambilla D., Venturi G., Lozupone F., Santinami M., Huber V., Maio M., Rivoltini L., Fais S. (2009) High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One 4, e5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., Nitadori-Hoshino A., Hoffman C., Badal K., Garcia B. A., Callahan M. K., Yuan J., Martins V. R., Skog J., Kaplan R. N., Brady M. S., Wolchok J. D., Chapman P. B., Kang Y., Bromberg J., Lyden D. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 erratum: 22, 1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharaziha P., Ceder S., Li Q., Panaretakis T. (2012) Tumor cell-derived exosomes: a message in a bottle. Biochim. Biophys. Acta 1826, 103–111 [DOI] [PubMed] [Google Scholar]
- 17.Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A. E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J. M., Dumont-Cole V. D., Kramer K., Wexler L. H., Narendran A., Schwartz G. K., Healey J. H., Sandstrom P., Labori K. J., Kure E. H., Grandgenett P. M., Hollingsworth M. A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S. K., Jarnagin W. R., Brady M. S., Fodstad O., Muller V., Pantel K., Minn A. J., Bissell M. J., Garcia B. A., Kang Y., Rajasekhar V. K., Ghajar C. M., Matei I., Peinado H., Bromberg J., Lyden D. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aline F., Bout D., Amigorena S., Roingeard P., Dimier-Poisson I. (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 72, 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson M., Lundin S., Dahlgren U., Kahu H., Pettersson I., Telemo E. (2001) “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 31, 2892–2900 [DOI] [PubMed] [Google Scholar]
- 20.Frängsmyr L., Baranov V., Nagaeva O., Stendahl U., Kjellberg L., Mincheva-Nilsson L. (2005) Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol. Hum. Reprod. 11, 35–41 [DOI] [PubMed] [Google Scholar]
- 21.Kim S. H., Bianco N., Menon R., Lechman E. R., Shufesky W. J., Morelli A. E., Robbins P. D. (2006) Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 13, 289–300 [DOI] [PubMed] [Google Scholar]
- 22.Kim S. H., Lechman E. R., Bianco N., Menon R., Keravala A., Nash J., Mi Z., Watkins S. C., Gambotto A., Robbins P. D. (2005) Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 174, 6440–6448 [DOI] [PubMed] [Google Scholar]
- 23.Gutiérrez-Vázquez C., Villarroya-Beltri C., Mittelbrunn M., Sánchez-Madrid F. (2013) Transfer of extracellular vesicles during immune cell-cell interactions. Immunol. Rev. 251, 125–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittelbrunn M., Vicente Manzanares M., Sánchez-Madrid F. (2015) Organizing polarized delivery of exosomes at synapses. Traffic 16, 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M. A., Bernad A., Sánchez-Madrid F. (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 72, 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colino J., Snapper C. M. (2006) Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 177, 3757–3762 [DOI] [PubMed] [Google Scholar]
- 28.Colino J., Snapper C. M. (2007) Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect. Immun. 75, 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viaud S., Terme M., Flament C., Taieb J., Andre F., Novault S., Escudier B., Robert C., Caillat-Zucman S., Tursz T., Zitvogel L., Chaput N. (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Rα. PLoS One. 4, e4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao S., Bai O., Li F., Yuan J., Laferte S., Xiang J. (2007) Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology 120, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S. H., Bianco N., Menon R., Lechman E. R., Shufesky W. J., Morelli A. E., Robbins P. D. (2006) Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 13, 289–300 [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Meng S., Jiang H., Zhu C., Wu W. (2011) Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J. Surg. Res. 171, 826–832 [DOI] [PubMed] [Google Scholar]
- 33.Peche H., Renaudin K., Beriou G., Merieau E., Amigorena S., Cuturi M. C. (2006) Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am. J. Transplant. 6, 1541–1550 [DOI] [PubMed] [Google Scholar]
- 34.Peche H., Heslan M., Usal C., Amigorena S., Cuturi M. C. (2003) Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 76, 1503–1510 [DOI] [PubMed] [Google Scholar]
- 35.Bianco N. R., Kim S. H., Ruffner M. A., Robbins P. D. (2009) Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 60, 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger A., Walker A., Nissen E. (2015) Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 467, 303–309 [DOI] [PubMed] [Google Scholar]
- 37.Silva A. M., Teixeira J. H., Almeida M. I., Gonçalves R. M., Barbosa M. A., Santos S. G. (2017) Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. Eur. J. Pharm. Sci. 98, 86–95 [DOI] [PubMed] [Google Scholar]
- 38.Harding C. V., Heuser J. E., Stahl P. D. (2013) Exosomes: looking back three decades and into the future. J. Cell Biol. 200, 367–371; erratum: 201, 485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding C., Heuser J., Stahl P. (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan B. T., Johnstone R. M. (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 [DOI] [PubMed] [Google Scholar]
- 41.Frasa M. A., Koessmeier K. T., Ahmadian M. R., Braga V. M. (2012) Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat. Rev. Mol. Cell Biol. 13, 67–73; erratum: 476 [DOI] [PubMed] [Google Scholar]
- 42.Fukuda M. (2011) TBC proteins: GAPs for mammalian small GTPase Rab? Biosci. Rep. 31, 159–168 [DOI] [PubMed] [Google Scholar]
- 43.Wainszelbaum M. J., Charron A. J., Kong C., Kirkpatrick D. S., Srikanth P., Barbieri M. A., Gygi S. P., Stahl P. D. (2008) The hominoid-specific oncogene TBC1D3 activates Ras and modulates epidermal growth factor receptor signaling and trafficking. J. Biol. Chem. 283, 13233–13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahl P. D., Wainszelbaum M. J. (2009) Human-specific genes may offer a unique window into human cell signaling. Sci. Signal. 2, pe59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wainszelbaum M. J., Liu J., Kong C., Srikanth P., Samovski D., Su X., Stahl P. D. (2012) TBC1D3, a hominoid-specific gene, delays IRS-1 degradation and promotes insulin signaling by modulating p70 S6 kinase activity. PLoS One 7, e31225; erratum: 2012; 7, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norbury C. C. (2006) Drinking a lot is good for dendritic cells. Immunology 117, 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallusto F., Cella M., Danieli C., Lanzavecchia A. (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt C. D., Ma J. K., Chalouni C., Ebersold M., Bou-Reslan H., Carano R. A., Mellman I., Delamarre L. (2010) Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. USA 107, 4287–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santegoets S. J., van den Eertwegh A. J., van de Loosdrecht A. A., Scheper R. J., de Gruijl T. D. (2008) Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J. Leukoc. Biol. 84, 1364–1373 [DOI] [PubMed] [Google Scholar]
- 50.Baird A., Deng C., Eliceiri M. H., Haghi F., Dang X., Coimbra R., Costantini T. W., Torbett B. E., Eliceiri B. P. (2016) Mice engrafted with human hematopoietic stem cells support a human myeloid cell inflammatory response in vivo. Wound Repair Regen. 24, 1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thery C., Amigorena S., Raposo G., Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3 22 [DOI] [PubMed] [Google Scholar]
- 52.Nolan J. P., Duggan E. (2018) Analysis of individual extracellular vesicles by flow cytometry. Methods Mol. Biol. 1678, 79–92 [DOI] [PubMed] [Google Scholar]
- 53.Van der Vlist E. J., Nolte-’t Hoen E. N., Stoorvogel W., Arkesteijn G. J., Wauben M. H. (2012) Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 7, 1311–1326 [DOI] [PubMed] [Google Scholar]
- 54.Abramowicz A., Marczak L., Wojakowska A., Zapotoczny S., Whiteside T. L., Widlak P., Pietrowska M. (2018) Harmonization of exosome isolation from culture supernatants for optimized proteomics analysis. PLoS One 13, e0205496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latterich M. (2006) Publishing proteomic data. Proteome Sci. 4, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Xin L., Shan B., Chen W., Xie M., Yuen D., Zhang W., Zhang Z., Lajoie G. A., Ma B. (2012) PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell Proteomics. 11, M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crane M. J., Daley J. M., van Houtte O., Brancato S. K., Henry W. L., Jr., Albina J. E. (2014) The monocyte to macrophage transition in the murine sterile wound. PLoS One 9, e86660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J. B., Ofrecio J. M., Wollam J., Hernandez-Carretero A., Fu W., Li P., Olefsky J. M. (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171, 372–84.e12 [DOI] [PubMed] [Google Scholar]
- 59.Bakhtyar N., Jeschke M. G., Herer E., Sheikholeslam M., Amini-Nik S. (2018) Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res. Ther. 9, 193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi E. W., Seo M. K., Woo E. Y., Kim S. H., Park E. J., Kim S. (2018) Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 27, 1170–1172 [DOI] [PubMed] [Google Scholar]
- 61.Kim Y. J., Yoo S. M., Park H. H., Lim H. J., Kim Y. L., Lee S., Seo K. W., Kang K. S. (2017) Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 493, 1102–1108 [DOI] [PubMed] [Google Scholar]
- 62.Cheng L., Liu J., Liu Q., Liu Y., Fan L., Wang F., Yu H., Li Y., Bu L., Li X., Wei W., Wang H., Sun G. (2017) Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through STAT3 pathway in macrophages. Int. J. Biol. Sci. 13, 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorayappan K. D. P., Wanner R., Wallbillich J. J., Saini U., Zingarelli R., Suarez A. A., Cohn D. E., Selvendiran K. (2018) Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene 37, 3806–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Speth J. M., Bourdonnay E., Penke L. R., Mancuso P., Moore B. B., Weinberg J. B., Peters-Golden M. (2016) Alveolar epithelial cell-derived prostaglandin E2 serves as a request signal for macrophage secretion of suppressor of cytokine signaling 3 during innate inflammation. J. Immunol. 196, 5112–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frittoli E., Palamidessi A., Pizzigoni A., Lanzetti L., Garrè M., Troglio F., Troilo A., Fukuda M., Di Fiore P. P., Scita G., Confalonieri S. (2008) The primate-specific protein TBC1D3 is required for optimal macropinocytosis in a novel ARF6-dependent pathway. Mol. Biol. Cell 19, 1304–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodzic D., Kong C., Wainszelbaum M. J., Charron A. J., Su X., Stahl P. D. (2006) TBC1D3, a hominoid oncoprotein, is encoded by a cluster of paralogues located on chromosome 17q12. Genomics 88, 731–736 [DOI] [PubMed] [Google Scholar]
- 67.Florio M., Albert M., Taverna E., Namba T., Brandl H., Lewitus E., Haffner C., Sykes A., Wong F. K., Peters J., Guhr E., Klemroth S., Prüfer K., Kelso J., Naumann R., Nüsslein I., Dahl A., Lachmann R., Pääbo S., Huttner W. B. (2015) Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 347, 1465–1470 [DOI] [PubMed] [Google Scholar]
- 68.Costantini T. W., Dang X., Coimbra R., Eliceiri B. P., Baird A. (2015) CHRFAM7A, a human-specific and partially duplicated α7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J. Leukoc. Biol. 97, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiddes I. T., Lodewijk G. A., Mooring M., Bosworth C. M., Ewing A. D., Mantalas G. L., Novak A. M., van den Bout A., Bishara A., Rosenkrantz J. L., Lorig-Roach R., Field A. R., Haeussler M., Russo L., Bhaduri A., Nowakowski T. J., Pollen A. A., Dougherty M. L., Nuttle X., Addor M. C., Zwolinski S., Katzman S., Kriegstein A., Eichler E. E., Salama S. R., Jacobs F. M. J., Haussler D. (2018) Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 173, 1356–1369.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kojima M., Gimenes-Junior J. A., Chan T. W., Eliceiri B. P., Baird A., Costantini T. W., Coimbra R. (2018) Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 32, 97–110 [DOI] [PubMed] [Google Scholar]
- 71.Robbins P. D., Dorronsoro A., Booker C. N. (2016) Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest. 126, 1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robbins P. D., Morelli A. E. (2014) Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H., Shang Q., Pan Z., Bai Y., Li Z., Zhang H., Zhang Q., Guo C., Zhang L., Wang Q. (2018) Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 67, 235–247 [DOI] [PubMed] [Google Scholar]
- 74.Patel D. B., Santoro M., Born L. J., Fisher J. P., Jay S. M. (2018) Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol. Adv. 36, 2051–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daley J. M., Brancato S. K., Thomay A. A., Reichner J. S., Albina J. E. (2010) The phenotype of murine wound macrophages. J. Leukoc. Biol. 87, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deskins D. L., Ardestani S., Young P. P. (2012) The polyvinyl alcohol sponge model implantation. J. Vis. Exp. 62, 3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.