Abstract
Human autosomal dominant polycystic kidney disease (ADPKD) is characterized by bilateral renal cysts that lead to a decline in kidney function. Previous studies reported aquaporin (AQP)-3 expression in cysts derived from collecting ducts in ADPKD. To study the role of AQP3 in cyst development, we generated 2 polycystic kidney disease (PKD) mouse models: kidney-specific Pkd1 knockout mice and inducible Pkd1 knockout mice, each without and with AQP3 deletion. In both models, kidney sizes and cyst indexes were significantly reduced in AQP3-null PKD mice compared with AQP3-expressing PKD mice, with the difference seen mainly in collecting duct cysts. AQP3-deficient kidneys showed significantly reduced ATP content, increased phosphorylated (p)-AMPK, and decreased p-ERK and p-mammalian target of rapamycin (mTOR). In a matrix-grown Madin-Darby canine kidney cyst model, AQP3 expression promoted cyst enlargement and was associated with increased expression of hypoxia-inducible factor 1-α and glucose transporter 1 and increased glucose uptake. Our data suggest that the slowed renal cyst enlargement in AQP3 deficiency involves impaired energy metabolism in the kidney through AMPK and mTOR signaling and impaired cellular glucose uptake. These findings implicate AQP3 as a novel determinant of renal cyst enlargement and hence a potential drug target in ADPKD.—Wang, W., Geng, X., Lei, L., Jia, Y., Li, Y., Zhou, H., Verkman, A. S., Yang, B. Aquaporin-3 deficiency slows cyst enlargement in experimental mouse models of autosomal dominant polycystic kidney disease.
Keywords: water channel, ADPKD, glucose metabolism, AMPK/mTOR signaling, HIF1-α
Autosomal dominant polycystic kidney disease (ADPKD) is a chronic progressive disease that is caused by mutations in 1 of 2 genes, polycystin (Pkd)1 and Pkd2, which encode the proteins polycystin-1 and polycystin-2, respectively (1). ADPKD affects between 1 in 400–1000 individuals and is characterized by massive enlargement of fluid-filled cysts of renal tubular origin (2, 3). Cysts originate from all segments of the renal tubule and are seen in up to 5% of nephrons (4, 5). Gradual expansion of cysts compresses and eventually replaces the normal tissue, causing end-stage renal disease (4, 5). There remains a need for effective treatment for ADPKD (1, 6, 7).
In a previous study, we demonstrated that deficiency in water channel aquaporin (AQP)-1, which is expressed in the kidney proximal tubule and thin descending limb of Henle, slowed cyst enlargement in polycystic kidney disease (PKD) mice, embryonic kidney cultures, and a Madin-Darby canine kidney (MDCK) cyst model (8). Several mechanisms appeared to be involved, including stabilization of the destruction complex in the Wnt signaling pathway, and pathways involved in cyst epithelial cell differentiation and migration.
Several lines of evidence suggest the possible involvement of AQP3 in cyst epithelial proliferation in ADPKD. AQP3 is a water-, glycerol-, and H2O2-transporting protein that is expressed at the basolateral plasma membrane in the kidney collecting duct. Approximately 30% of cysts in ADPKD express AQP3 (9). The glycerol- and H2O2-transporting functions of AQP3 have been implicated in several cell signaling processes involved in cellular energy metabolism, proliferation, and the inflammatory response (10, 11). Altered metabolism has been proposed as an important determinant in ADPKD (12, 13); for example, Pkd1-null cells consume 2-fold more glucose than normal cells for energy production by aerobic glycolysis. AQP3 may thus be involved in renal cyst enlargement, perhaps in a manner related to its non–water-transporting functions.
To test the role of AQP3 in renal cyst development in PKD, here we generated Pkd1flox/flox; kidney-specific protein (Ksp)-Cre; AQP3−/− PKD mice and inducible Pkd1flox/flox; MxCre; AQP3−/− PKD mice. We found that AQP3 deficiency slowed cyst growth in the collecting ducts of PKD mice. In vitro studies using a MDCK cell cyst model identified potential mechanisms of AQP3-dependent cyst development.
MATERIALS AND METHODS
Pkd1flox/flox; Ksp-Cre; AQP3−/− mice
AQP3 knockout mice in a C57BL/6 genetic background were generated by targeted gene disruption as reported by Ma et al. (14). Pkd1flox/flox mice (Center for PKD Research, Yale University, New Haven, CT, USA) and Ksp-Cre transgenic mice (George M. O’Brien Kidney Research Core Center, University of Texas Southwestern Medical Center, Dallas, TX, USA), in which Cre recombinase is driven by the Ksp promoter, in a C57BL/6 genetic background were generated as previously described by Shibazaki et al. (15). Male mice were crossed with AQP3−/− female mice, resulting in Pkd1flox/+; Ksp-Cre; AQP3+/− mice. Pkd1flox/+; Ksp-Cre; AQP3+/− mice were then interbred to generate Pkd1flox/flox; Ksp-Cre; AQP3−/− mice. Neonatal mice (age 1 d) were genotyped by genomic PCR. Body weight was measured at age 1, 3, 5, and 7 d. Kidneys from Pkd1flox/+; Ksp-Cre; AQP3+/−, Pkd1flox/+; Ksp-Cre; AQP3−/−, Pkd1flox/flox; Ksp-Cre; AQP3+/−, and Pkd1flox/flox; Ksp-Cre; AQP3−/− mice at postnatal d 1, 3, 5, and 7 were removed, weighed, and processed for immunofluorescence and Western blot analysis immediately. The kidney index (kidney weight/body weight) was calculated.
Pkd1flox/flox; Mx-Cre; AQP3−/− mice
Male Pkd1flox/+; Mx-Cre mice were crossed with AQP3−/− female mice, resulting in Pkd1flox/+; Mx-Cre; AQP3+/− mice. Pkd1flox/+; Mx-Cre; AQP3+/− mice were then interbred to generate Pkd1flox/flox; Mx-Cre; AQP3−/− mice. Pkd1flox/+; Mx-Cre; AQP3+/− mice and Pkd1flox/flox; Mx-Cre; AQP3−/− mice were injected intraperitoneally with 250 μl of saline or 1 mg/ml of Poly(I)-Poly(C) (MilliporeSigma, Burlington, MA, USA) on postnatal d 1, 3, and 5 as control, AQP3-expressing, and AQP3-null PKD mice, respectively. Mice were euthanized 30 d later, and kidneys were removed, weighed, and fixed for histologic examination.
Cyst index
The extent of tubular cyst formation was quantified in sagittal sections of whole kidneys. For each of 6 mice (including 3 mice of the inducible PKD model), 5 representative renal hematoxylin and eosin (H&E) images were captured. Images were analyzed with ImageJ software (National Institutes of health, Bethesda, MD, USA) [cyst index (%) = (total cystic area/total kidney area) × 100].
Cell culture
MDCK cells (CCL-34; American Type Culture Collection, Manassas, VA, USA) and AQP3-MDCK cells were cultured in DMEM and incubated at 37°C in 5% CO2 and 95% air. Cells were seeded at 2 × 104 cells per 60-mm plate (Corning, Corning, NY, USA). In some experiments, cells were incubated with low-glucose DMEM (Thermo Fisher Scientific, Waltham, MA, USA). For some experiments, cells were incubated with 10 μM AMPK inhibitor compound C (P5499; MilliporeSigma) for 24 h or 25 μM MAPK inhibitor PD98059 (21300; MilliporeSigma) for 2 h.
Cell proliferation
A Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Kumamoto, Japan) was used to assay cell proliferation in MDCK and AQP3-MDCK cells. Cells were plated in 96-well plates at a density of 103 cells per well. At each time point, 100 μl of CCK-8 solution at a dilution of 1:10 with 10% fetal bovine serum DMEM was added to each well for 2 h at 37°C. Absorbance at 450 nm was measured with a microplate reader (MQX200; BioTek Instruments, Winooski, VT, USA).
MDCK cyst model
In vitro cyst assays were performed as previously described. In brief, 400 MDCK cells were suspended in 0.4 ml of ice-cold modified Eagle’s medium containing 2.9 mg/ml collagen (PureCol; Inamed Biomaterials, Fremont, CA, USA), 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (pH 7.4). The cell suspension was plated onto 24-well plates. After incubation for 90 min at 37°C, 1.5 ml of the MDCK cell medium containing 10 μM forskolin was then added to each well, and plates were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Cells were incubated in medium containing 10 μM forskolin for 12 d with twice-daily changes.
Micrographs of the same cysts in collagen gels were taken on d 4, 6, 8, 10, and 12 after seeding. A cyst was defined as a spherical structure with a distinct cell wall and central cavity >50 μm in diameter. For analysis of cyst enlargement, 5 random visual fields per well were photographed with an Olympus microscope (original magnification, ×10). Diameters of cysts that were measured using ImageJ software.
Immunofluorescence
Kidneys were fixed in 4% paraformaldehyde and equilibrated in 20% sucrose overnight before embedding in optimal cutting temperature compound. Kidney sections were cut on a cryostat. The sections were blocked overnight at 4°C and incubated with primary mAbs to AQP3 (1:500; MilliporeSigma) at 4°C, followed by secondary antibody (tetramethylrhodamine goat anti-rabbit, 1:200) and Lotus tetragonolobus lectin (LTL) (1:400; Vector Laboratories, Burlingame, CA, USA) or Dolichos biflorus agglutinin (DBA) (1:400; Vector Laboratories) for 1 h. Hoechst 33342 (1:1000; Leagene, Thermo Fisher Scientific) was used to label nuclei. Images were captured by a Leica fluorescence microscope (Leica Biosystems, Wetzlar, Germany). Dilated tubules with diameter >50 μm were considered cysts. The number of cysts in 5 sections per mouse from 6 mice were averaged (3 mice of inducible PKD model).
Western blot analysis
Tissues or cells were homogenized in RIPA lysis buffer (89901; Thermo Fisher Scientific) containing protease inhibitor cocktail (11873580001; Roche, Basel, Switzerland) and phosphatase inhibitor mixture (P1260; Applygen, Beijing, China). Blots were incubated with pAb against AQP3 (MilliporeSigma), proliferating cell nuclear antigen (PCNA) (Cell Signaling Technology, Danvers, MA, USA), AMPK (Cell Signaling Technology), phosphorylated (p)-AMPK (Cell Signaling Technology), S6 (Cell Signaling Technology), p-S6 (Cell Signaling Technology), acetyl–coenzyme A carboxylase (ACC) (MilliporeSigma), p-ACC (MilliporeSigma), hypoxia-inducible factor (HIF) 1α (Santa Cruz Biotechnology, Dallas, TX, USA), and glucose transporter 1 (GLUT1) (Cell Signaling Technology). Goat anti-rabbit IgG (Abcam, Cambridge, MA, USA) and goat anti-mouse IgG (Santa Cruz Biotechnology) were added, and the blots were developed with an ECL Plus Kit (GE Healthcare, Chicago, IL, USA). Protein expression was quantified by optical density. The relative amount of protein was calculated in relation to β-actin expression.
Measurement of ATP, glycerol, glucose, and l-lactate
MDCK cells and AQP3-MDCK cells were cultured for 48 h in DMEM. The culture supernatant was collected and glycerol content was measured using a Glycerol Assay Kit (MilliporeSigma). Cell monolayers were washed twice with PBS. ATP, glucose, and l-lactate concentrations were measured using an ATP Assay Kit (Vigorous Biotechnology, Beijing, China), Glucose Assay Kit (EnzyChrom, Kampenhout, Belgium), and Lactate Assay Kit (EnzyChrom) per the manufacturer’s instructions (MQX200; BioTek Instruments). Data were normalized to protein concentration.
Fluorescent glucose uptake
Glucose uptake in MDCK and AQP3-MDCK cells was measured using 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) as previously described by Maekawa et al. (16). Cells were serum starved for 12 h, washed 3 times with Krebs-Henseleit buffer (0.5% bovine serum albumin, 2 mM sodium pyruvate, and 6 mM mannitol), and then incubated in Krebs-Henseleit buffer containing 50 μM 2-NBDG at 37°C for 1 h. Cells were washed 3 times with PBS, and 2-NBDG content was determined using a microplate fluorimeter (Infinite M200; Tecan, Mannedorf, Switzerland) and normalized by cell number.
Statistical analyses
All results are expressed as means ± sem. For multiple comparisons, statistical analysis was performed by using a Student’s t test or 2-way ANOVA. Values of P < 0.05 were considered statistically significant.
RESULTS
AQP3 deficiency reduces renal cyst enlargement in neonatal kidney-specific Pkd1 knockout mice
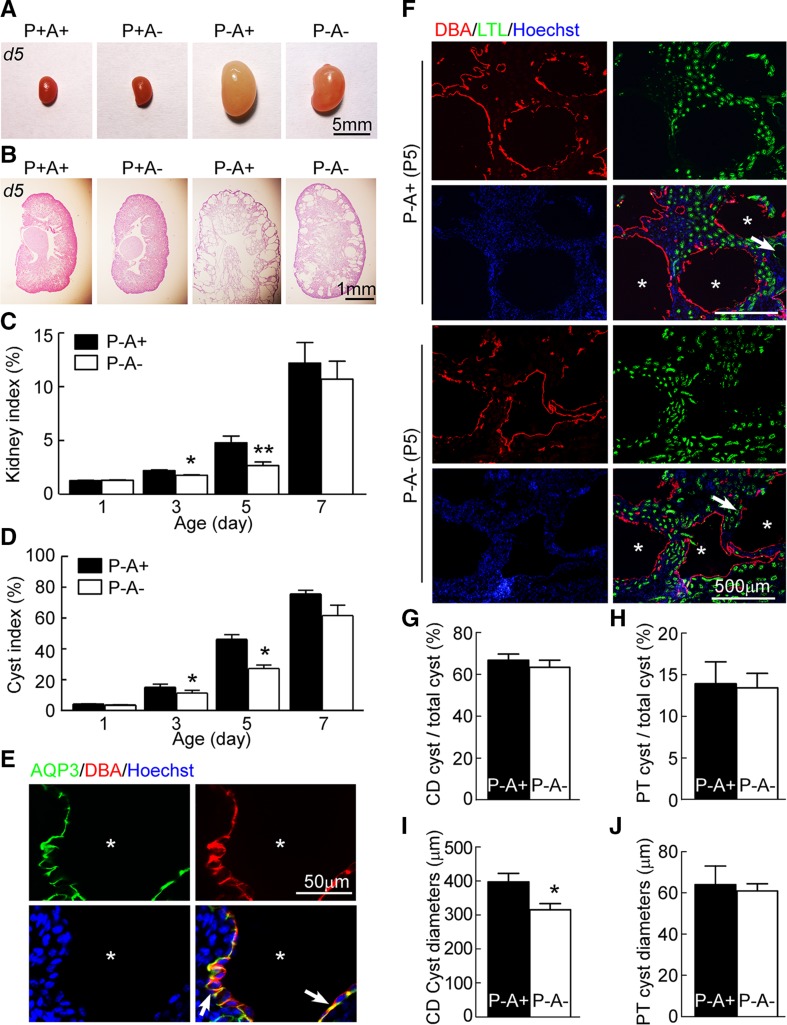
AQP3-null PKD mice were generated by the intercrossing of Pkd1flox/+; Ksp-Cre mice and AQP3+/− mice. Pkd1flox/flox; Ksp-Cre; AQP3+/− mice were used as AQP3-expressing PKD mice to provide an adequate supply of litter-matched AQP3-expressing and AQP3-null PKD mice. Kidney size in AQP3-null PKD mice was significantly smaller than that in AQP3-expressing PKD mice on postnatal day 3 and 5, as seen from the ratio of kidney weight to body weight (kidney index, Fig. 1A, C). Image analysis of H&E-stained sections showed a significantly lower cyst index (percentage area occupied by cysts) in kidneys from AQP3-null PKD mice than from AQP3-expressing PKD mice on postnatal d 3 and 5 (Fig. 1B, D).
Figure 1.
AQP3 deficiency reduces kidney index and cyst index in collecting ducts (CDs) in PKD mice. A) Representative images of kidneys at postnatal d 5 from mice with wild-type phenotype (P+A+, Pkd1flox/+; Ksp-Cre; AQP3+/− genotype), AQP3 knockout mice (P+A−, Pkd1flox/+; Ksp-Cre; AQP3−/− genotype), AQP3-expressing PKD mice (P-A+, Pkd1flox/flox; Ksp-Cre; AQP3+/− genotype), and AQP3-null PKD mice (P−A−, Pkd1flox/flox; Ksp-Cre; AQP3−/− genotype). B) H&E-stained sections. C) Kidney index (ratio of kidney to body weight). D) Cyst index. Means ± sem; P−A+, n = 6; P−A−, n = 6. *P < 0.05, **P < 0.01 compared with AQP3-expressing PKD mice [Student’s t test (C, D)]. E) AQP3 immunofluorescence in AQP3-expressing PKD (P−A+) kidneys at postnatal d 5. Sections were stained with AQP3 (green), DBA (red), and Hoechst 33342 (blue). Arrows indicate AQP3 localized at the basolateral membrane of cyst epithelial cells. Asterisks indicate cysts. F) DBA and LTL immunofluorescence of AQP3-expressing PKD (P−A+) and AQP3-null PKD (P−A−) kidneys at postnatal d 5. Asterisks indicate cysts derived from CD. The arrows indicate cysts derived from proximal tubule. G) Percentage of cysts derived from collecting duct. H) Percentage of cysts derived from proximal tubule (PT). I) Diameters of cysts (≥50 μm) derived from collecting duct. J) Diameters of cysts (≥50 μm) derived from PT. Means ± sem; n = 6. *P < 0.05 compared with AQP3-expressing PKD mice [Student’s t test (G–J)].
Consistent with a previous report from Gattone et al. (17), AQP3 immunofluorescence showed AQP3 localization at the basolateral membrane of cyst epithelial cells in ADPKD mice (Fig. 1E). To determine which part of the nephron was affected by AQP3 deletion, sections were stained with LTL (a selective marker of proximal tubules) and DBA (a selective marker of collecting ducts) (Fig. 1F). The proportions of collecting duct cysts were similar in AQP3-null and AQP3-expressing PKD mice (Fig. 1F, G). The mean diameter of DBA-stained AQP3-null cysts (315 ± 13 μm) was significantly smaller than that in DBA-stained AQP3-expressing cysts (391 ± 25 μm) (Fig. 1I). However, no significant difference was found in the number or size of LTL-stained cysts derived from proximal tubules (Fig. 1F, H, J). These results indicate more reduced kidney sizes and cyst indexes in AQP3-null PKD mice than in AQP3-expressing PKD mice, with the difference due mainly to smaller cysts derived from collecting ducts.
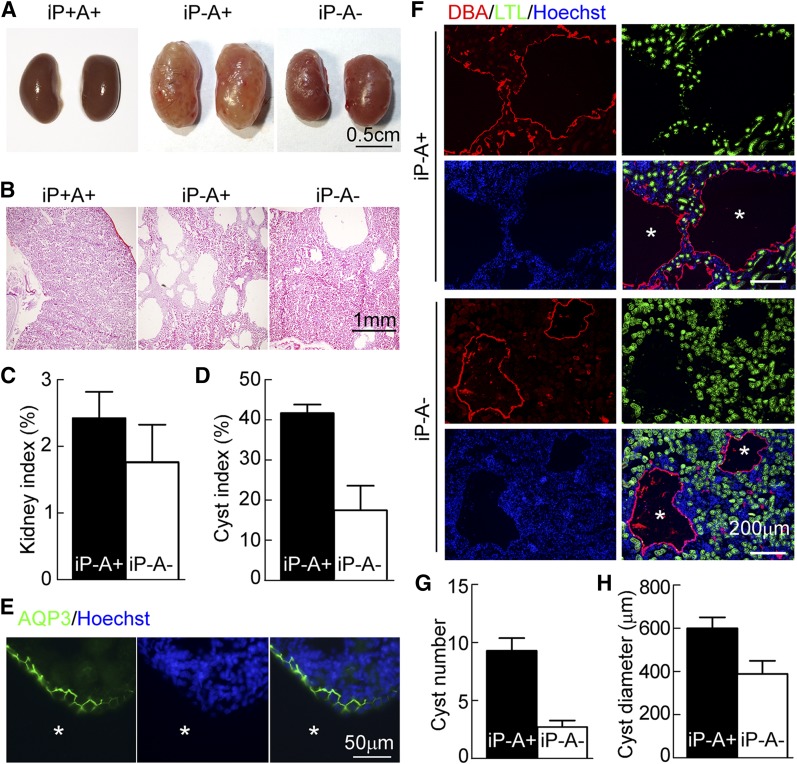
AQP3 deficiency slows cyst enlargement in inducible PKD mice
Compared with kidney-specific Pkd1 knockout mice, the progression of ADPKD is substantially slower in adult mice following conditional inactivation of polycystins or cilia (18, 19). To determine whether AQP3 deletion slows cyst enlargement in this more indolent adult model of PKD, Pkd1flox/flox; MxCre–inducible PKD mice were studied (20). Kidney sizes and kidney indexes in AQP3-null PKD mice were smaller than those in AQP3-expressing PKD mice (Fig. 2A, C). Image analysis of H&E-stained sections showed a ∼55% lower cyst index in AQP3-null PKD mice than in AQP3-expressing PKD mice (Fig. 2B, D). Immunofluorescence showed AQP3 localization at the basolateral membrane of cysts (Fig. 2E). As shown in Fig. 2F, cysts from the proximal tubule were absent, as expected, because MxCre is not expressed in the proximal tubule (21). Notably, AQP3 deficiency greatly reduced the number of cysts from the collecting duct (9.3 ± 1.1 vs. 2.7 ± 0.6) and their diameter (598 ± 51 vs. 388 ± 60 μm) (Fig. 2F–H).
Figure 2.
AQP3 deficiency reduces cyst growth in inducible PKD mice. A) Representative images of kidneys from P35 mice with noncystic phenotype (iP+A+, Pkd1flox/flox; Mx-Cre; AQP3+/− genotype with vehicle administration), AQP3-expressing PKD (iP−A+, Pkd1flox/flox; Mx-Cre; AQP3+/− genotype with pI:pC administration), and AQP3-null PKD mice (iP−A−, Pkd1flox/flox; Mx-Cre; AQP3−/− genotype with polyinosinic-polycytidylic acid administration). B) H&E-stained kidney sections. C) Kidney index (ratio of kidney to body weight). D) Cyst index. Means ± sem; iP−A+, n = 8; iP−A−, n = 3. E) AQP3 immunofluorescence in AQP3-expressing PKD (iP−A+) kidneys at postnatal d 35. The sections were stained with AQP3 (green) and Hoechst 33342 (blue). Asterisks indicate cysts. F) DBA and LTL immunofluorescence of AQP3-expressing PKD (iP−A+) and AQP3-null PKD (iP−A−) kidneys at postnatal d 35. Asterisks indicate cysts derived from the collecting duct (CD). G) Number of cysts derived from CD. H) Diameters of cysts derived from CD. Means ± sem; n = 3 (G, H).
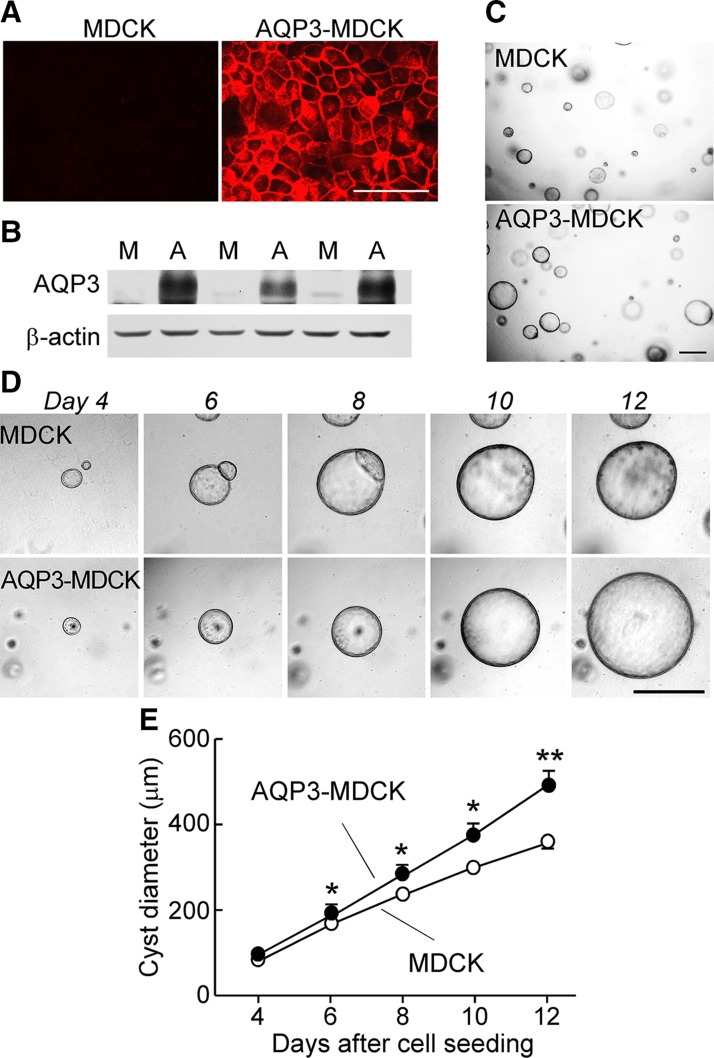
AQP3 promotes cyst enlargement and cell proliferation in an MDCK cell cyst model
To study the effect of AQP3 expression on cyst formation and enlargement in vitro, we used an MDCK cell line stably expressing human AQP3 (Fig. 3A, B). The cyst diameter of AQP3-MDCK cells was significantly greater than that of MDCK cells (Fig. 3C–E).
Figure 3.
AQP3 promotes cyst formation in matrix-grown MDCK cells. A) AQP3 immunostaining in MDCK and AQP3-MDCK cells. Scale bar, 50 μm. B) Western blot of AQP3 in MDCK and AQP3-MDCK cells. M, MDCK cells; A, AQP3-MDCK cells. C) Light micrographs of cysts on d 12 after culture with forskolin. Scale bar, 500 μm. D) Light micrographs of cysts on d 4–12. Each series of photographs shows the same cyst on successive days in culture. Scale bar, 500 μm. E) Cyst diameters on d 4–12. Means ± sem; >30 cysts analyzed; *P < 0.05, **P < 0.01 vs. MDCK cells (Student’s t test).
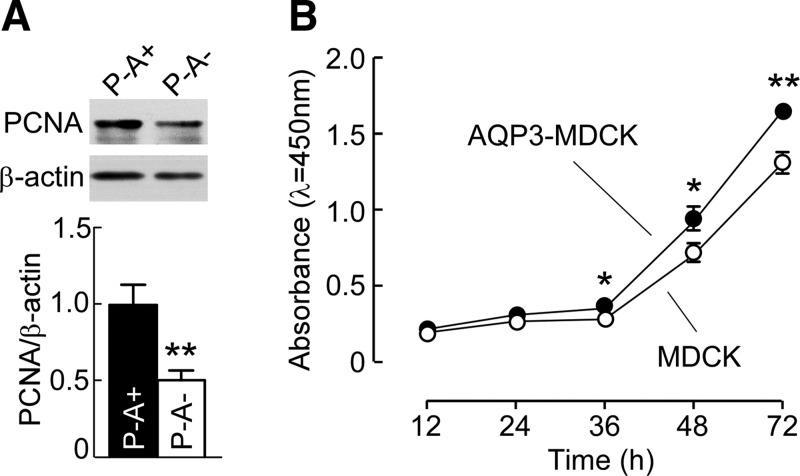
Proliferation is increased early in cyst formation in polycystic kidneys (22). We studied the role of AQP3 on cyst epithelial cell proliferation in vivo and in vitro. Western blot revealed that expression of PCNA in AQP3-null PKD kidneys was reduced as compared with control PKD kidneys (Fig. 4A). In vitro, the proliferation of MDCK and AQP3-MDCK cells was quantified using a CCK-8 assay. Figure 4B shows significantly greater proliferation in AQP3-MDCK cells than in MDCK cells.
Figure 4.
AQP3 increases cyst epithelial cell proliferation. A) Western blot (top) of kidney lysates analyzed with PCNA and β-actin antibodies. Bar graph (bottom) shows the ratios of PCNA:β-actin. Means ± sem, n = 6. **P < 0.01 vs. P−A+ kidneys (Student’s t test). B) Cell proliferation measured by CCK-8 assay. Means ± sem, n = 6. *P < 0.05, **P < 0.01 vs. MDCK cells (Student’s t test).
AQP3 increases ATP amount and suppresses AMPK phosphorylation
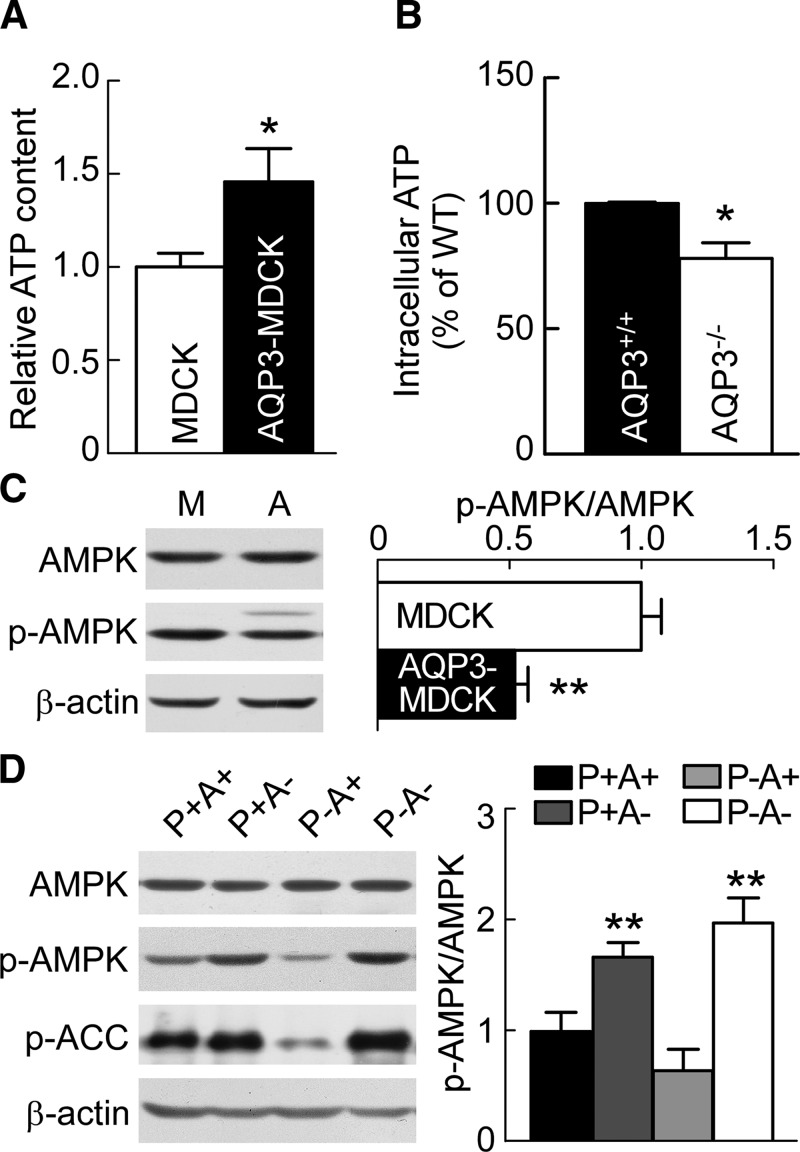
AQP3 has been reported as a key determinant of cellular ATP in epidermal cells (23) and gastric epithelias cells (24). To determine whether the greater proliferation rate in AQP3-MDCK cells could be the consequence of greater cytoplasmic ATP concentration, intracellular ATP in MDCK and AQP3-MDCK cells was measured. AQP3-MDCK cells had a significantly higher amount of ATP than MDCK cells (Fig. 5A). Notwithstanding the heterogeneous cellular makeup of the whole kidney, the amount of ATP in AQP3-null kidneys was significantly lower than that in AQP3-expressing kidneys (Fig. 5B), which is consistent with the results in vitro.
Figure 5.
AQP3 increases cellular ATP and reduces AMPK phosphorylation. A) Intracellular ATP in MDCK and AQP3-MDCK cells. Means ± sem, n = 6. *P < 0.05 vs. MDCK cells (Student’s t test). B) Intracellular ATP in AQP3+/+ and AQP3−/− mouse kidney. WT, wild type. Means ± sem, n = 6. *P < 0.05 vs. AQP3+/+ mice (Student’s t test). C) Western blot (left) of lysates of MDCK and AQP3-MDCK cells analyzed with AMPK, p-AMPK, and β-actin antibodies. Bar graph (right) shows the ratios of p-AMPK:AMPK. M, MDCK cells; A, AQP3-MDCK cells. Means ± sem, n = 6. **P < 0.01 vs. MDCK cells (Student’s t test). D) Western blots (left) of lysates of P+A+, P+A−, P−A+, and P−A− kidneys analyzed with AMPK, p-AMPK, p-ACC, and β-actin antibodies. Bar graph (right) shows the ratios of p-AMPK:AMPK. Means ± sem, n = 6. p-AMPK/AMPK: Fpkd1(1,20) = 0.24, P > 0.05; FAQP3(1,20) = 60.6, P < 0.01; FInteraction (1,20) = 4.03, P > 0.05 (2-way ANOVA). Bonferroni: AQP3 null kidneys (A−) vs. AQP3 expression kidneys (A+). **P < 0.01 [pkd1-expression kidneys (P+)], **P < 0.01 [pkd1-null kidneys (P−)].
AMPK is a major cellular energy sensor (25, 26). We found a lower level of AMPK phosphorylation in AQP3-MDCK cells than in MDCK cells (Fig. 5C), which is consistent with the higher amount of ATP. In vivo, p-AMPK in AQP3-null kidneys was significantly greater than that in wild-type kidneys. The amount of p-AMPK in PKD mouse kidneys was 1.7-fold lower than in wild-type control kidneys, in agreement with previous data from Rowe et al. (12). AQP3 gene deletion in PKD mice restored the reduced p-AMPK level (Fig. 5D). To determine whether this effect was correlated with increased phosphorylation of an AMPK target, we determined the effect of AQP3 deletion on AMPK-mediated inhibition of ACC phosphorylation. p-ACC was significantly increased in AQP3-null kidneys (Fig. 5D). These results suggest that AQP3 inhibits AMPK phosphorylation, which is in accord with the greater ATP in AQP3-expressing cells.
AQP3 increases ERK and mTOR signaling
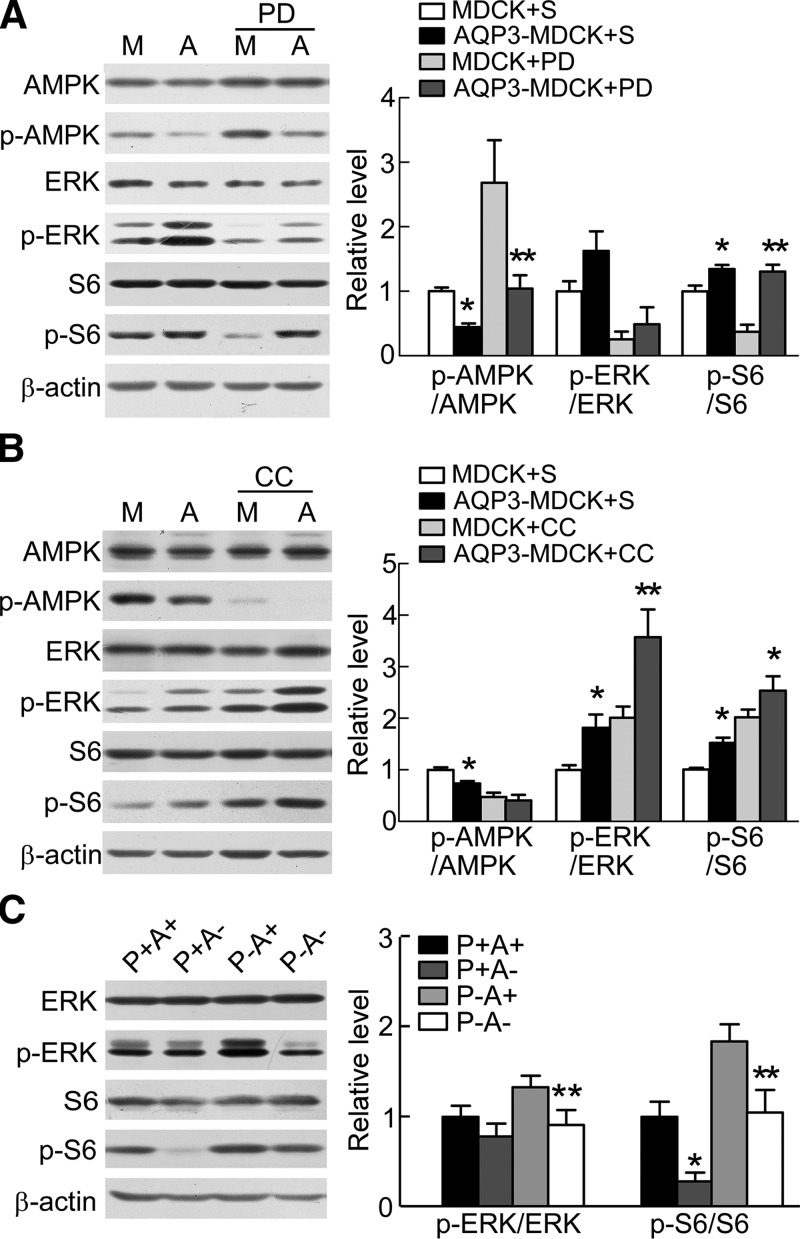
AMPK also inhibits the activity of the mammalian target of rapamycin (mTOR), an essential kinase for cell growth and proliferation (27). To determine whether AQP3 induces AMPK-mediated stimulation of mTOR, we measured the phosphorylated form of the mTOR downstream target S6. S6 phosphorylation in AQP3-MDCK cells was 1.3-fold greater than that in MDCK cells (Fig. 6A, B). Western blot revealed that p-S6 in AQP3-null kidneys was 28% of that in wild-type kidneys and that p-S6 in PKD kidneys was 1.8-fold greater than that in wild-type kidneys. AQP3 gene deletion in PKD mice reversed the increase in p-S6 (Fig. 6C).
Figure 6.
AQP3 increases mTOR and ERK signaling. A) Representative Western blots (left) of lysates of MDCK and AQP3-MDCK cells treated with saline (S) or 25 μM PD98059 (PD, ERK inhibitor) for 2 h and then analyzed with AMPK, p-AMPK, ERK, p-ERK, S6, p-S6, and β-actin antibodies. Bar graph (right) shows the ratios of p-AMPK:AMPK, p-ERK:ERK, and p-S6:S6. Means ± sem, n = 8. M, MDCK cells; A, AQP3-MDCK cells. p-AMPK/AMPK: FPD(1,26) = 45.4, P < 0.01; FAQP3(1,26) = 38.5, P < 0.01; FInteraction (1,26) = 5.9, P < 0.05 (2-way ANOVA). Bonferroni: MDCK cells vs. AQP3-MDCK cells. *P < 0.05 (S), **P < 0.01 (PD). p-ERK/ERK: FPD(1,26) = 18.1, P < 0.01; FAQP3(1,26) = 2.27, P > 0.05; FInteraction (1,26) = 0.35, P > 0.05. Bonferroni: MDCK cells vs. AQP3-MDCK cells. P > 0.05 (S), P > 0.05 (PD). p-S6/S6: FPD(1,26) = 11.6, P < 0.01; FAQP3(1,26) = 36.8, P < 0.01; FInteraction (1,26) = 8.847, P < 0.01. Bonferroni: MDCK cells vs. AQP3-MDCK cells. *P < 0.05 (S), **P < 0.01 (PD). B) Representative Western blots (left) of lysates of MDCK and AQP3-MDCK cells treated with or without 10 μM compound C (CC, AMPK inhibitor) for 24 h. Bar graph (right) shows the ratios of p-AMPK:AMPK, p-ERK:ERK, and p-S6:S6. Means ± sem, n = 8. p-AMPK/AMPK: FCC(1,27) = 31.9, P < 0.01; FAQP3(1,27) = 4.7, P < 0.05; FInteraction (1,27) = 1.74, P > 0.05 (2-way ANOVA). Bonferroni: MDCK cells vs. AQP3-MDCK cells. *P < 0.05 (S), P > 0.05 (CC). p-ERK/ERK: FCC(1,27) = 18.8, P < 0.01; FAQP3(1,27) = 13.9, P < 0.01; FInteraction (1,27) = 1.38, P > 0.05. Bonferroni: MDCK cells vs. AQP3-MDCK cells. *P < 0.05 (S), **P < 0.01 (CC). p-S6/S6: FCC(1,27) = 37.01, P < 0.01; FAQP3(1,27) = 9.648. **P < 0.01. FInteraction (1,27) = 0.004, P > 0.05. Bonferroni: MDCK cells vs. AQP3-MDCK cells. *P < 0.05 (S), *P < 0.05 (CC). C) Representative Western blots (left) of lysates of P+A+, P+A−, P−A+, and P−A− kidneys analyzed with ERK, p-ERK, S6, and p-S6 antibodies. Bar graph (right) shows the ratios of p-ERK:ERK and p-S6:S6. Means ± sem, n = 6. p-ERK/ERK: Fpkd1(1,20) = 6.3, P < 0.05; FAQP3(1,20) = 9.3, P < 0.01; FInteraction (1,20) = 2.19, P > 0.05 (2-way ANOVA). Bonferroni: AQP3-null kidneys (A−) vs. AQP3-expressing kidneys (A+); pkd1-expressing kidneys (P+): P > 0.05; pkd1-null kidneys (P−). **P < 0.01. p-S6/S6: Fpkd1(1,20) = 18.62, P < 0.01; FAQP3(1,20) = 16.4, P < 0.01; FInteraction (1,20) = 0.04, P > 0.05. Bonferroni: AQP3-null kidneys (A−) vs. AQP3-expressing kidneys (A+). *P < 0.01 [pkd1-expressing kidneys (P+)], **P < 0.01 [pkd1-null kidneys (P−)].
Rowe et al. (12) previously showed that increased mTOR complex 1 (mTORC1) activity is driven largely by ERK up-regulation. ERK phosphorylation in AQP3-MDCK cells was 1.5-fold greater than that in MDCK cells (Fig. 6A, B), and PKD kidneys had a higher level of p-ERK than wild-type kidneys. AQP3 deletion reduced ERK signaling in both wild-type and PKD mouse kidneys (Fig. 6C).
Because AQP3 appears to be involved in activation of ERK signaling, an ERK inhibitor (PD98059) was used to investigate whether AQP3 inhibited AMPK phosphorylation through the ERK signaling pathway. PD98059 inhibited ERK phosphorylation in both MDCK and AQP3-MDCK cells. p-AMPK was 2.3-fold increased and p-S6 was 2.4-fold decreased in MDCK cells after PD98059 treatment (Fig. 6A). p-AMPK was 2.3-fold increased in AQP3-MDCK cells after PD98059 treatment. Interestingly, p-S6 was affected little in AQP3-MDCK cells after PD98059 treatment. An AMPK inhibitor (compound C) inhibited AMPK phosphorylation in both MDCK and AQP3-MDCK cells. Both p-ERK and p-S6 were about 2-fold increased in both MDCK and AQP3-MDCK cells after compound C treatment (Fig. 6B). The increases in p-ERK and p-S6 were similar in MDCK and AQP3-MDCK cells, suggesting that compound C did not reverse the effect of AQP3 on p-ERK and p-S6, and hence, the AQP3 effect may be upstream of AMPK. Both ERK and AMPK can activate mTOR signaling directly or indirectly in the MDCK cell model.
AQP3 promotes glycolysis
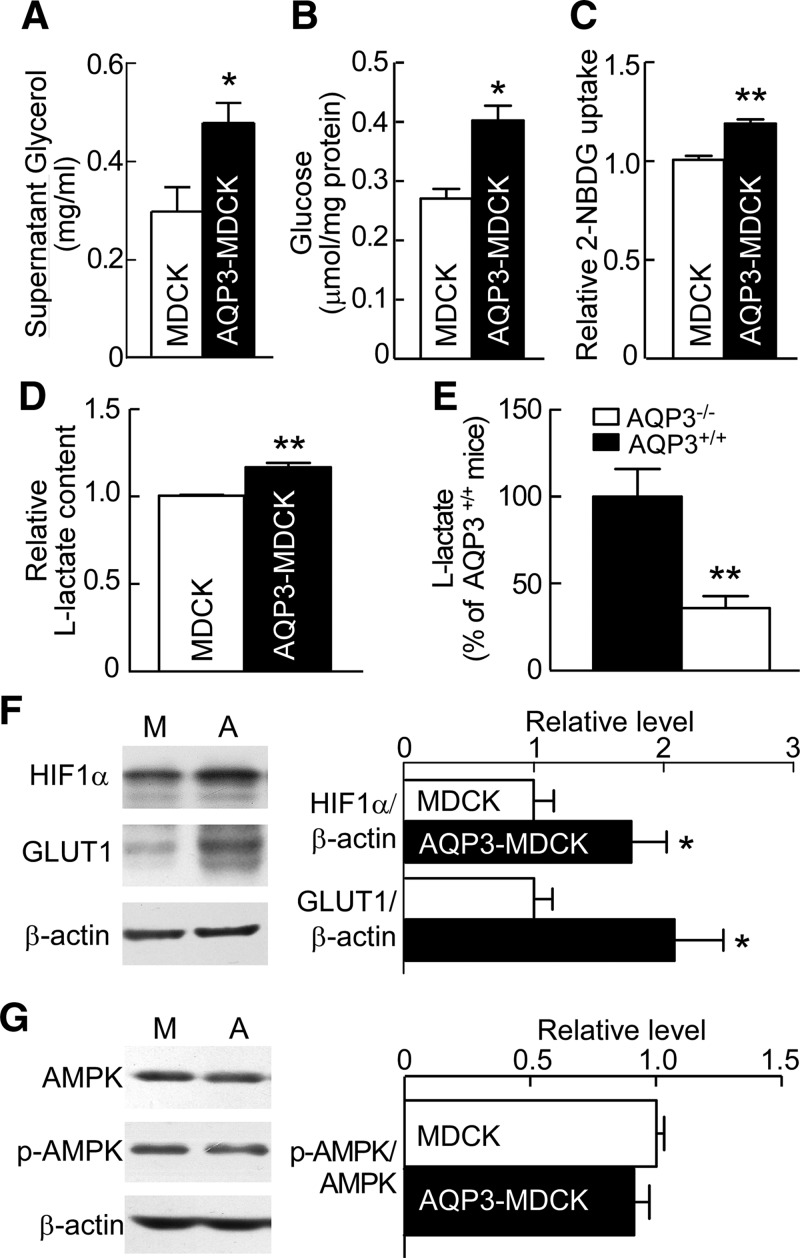
To investigate the source of ATP, glycerol content was measured in the culture supernatant. Glycerol (which is transported by AQP3) in the supernatant of AQP3-MDCK cells was 1.6-fold greater than in MDCK cells (Fig. 7A). Because there was no detectable glycerol in the culture medium without cells, the measured glycerol was produced by cells and secreted into the supernatant.
Figure 7.
AQP3 promotes glucose metabolism. A–D) Glycerol content in MDCK and AQP3-MDCK cell culture supernatants (n = 6) (A), and glucose content (n = 6) (B), glucose uptake (n = 6) (C), and l-lactate content (n = 6) (D) in MDCK and AQP3-MDCK cells. Means ± sem. Student’s t test. *P < 0.05, **P < 0.01 vs. MDCK cells (A–D). E) l-lactate content in AQP3+/+ and AQP3−/− mouse kidneys. Means ± sem, n = 6; Student’s t test. **P < 0.01 vs. AQP3+/+ mice; F) Western blot (left) of lysates of MDCK and AQP3-MDCK cells analyzed with HIF1-α, GLUT1, and β-actin antibodies. Bar graph (right) shows the ratios of HIF1-α:β-actin and GLUT1:β-actin. M, MDCK cells; A, AQP3-MDCK cells. G) Western blot of AMPK and p-AMPK (left) after glucose starvation for 48 h. Bar graph (right) shows the ratios of p-AMPK:AMPK. Means ± sem, n = 6; Student’s t test. *P < 0.05 vs. MDCK cells (F, G).
We also assayed glucose, another source of ATP. Cellular glucose was greater in AQP3-MDCK cells (0.40 ± 0.04 μmol/mg protein) than in MDCK cells (0.27 ± 0.03 μmol/mg protein) (Fig. 7B). Glucose uptake was assayed using 2-NBDG, a fluorescent glucose analog (16). The fluorescence in AQP3-MDCK cells was 1.18-fold greater than in MDCK cells (Fig. 7C), suggesting higher glucose uptake in AQP3-MDCK cells. Because aerobic glycolysis is the principal metabolic pathway for glycerol production from glucose, we also assayed lactate. Intracellular lactate was increased in AQP3-MDCK cells (Fig. 7D). Kidneys from AQP3-null mice had lower amounts of lactate as compared with kidneys from wild-type mice (Fig. 7E), which is consistent with the data in vitro.
Finally, we found that the expression of GLUT1 was 1.7-fold greater than that in AQP3-MDCK cells, as shown by Western blot (Fig. 7F), as was the expression of HIF1-α. AQP3 may thus promote glucose uptake by increasing GLUT1 expression. Indeed, glucose deprivation prevented the decrease of p-AMPK in AQP3-MDCK cells (Fig. 7G).
DISCUSSION
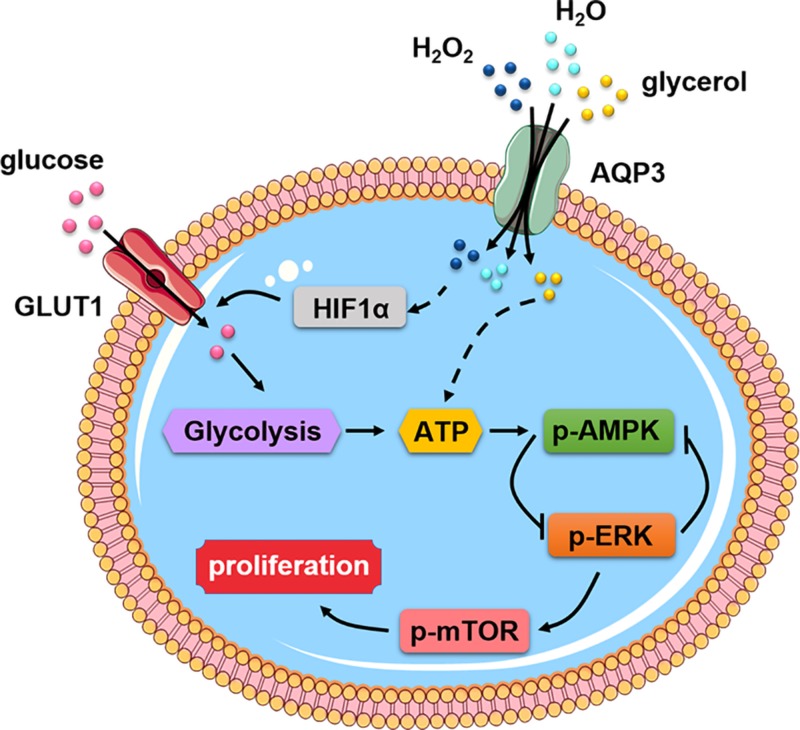
We found here that AQP3 promoted cyst enlargement in both in vivo and in vitro experimental models of ADPKD. Cell proliferation was greater in AQP3-expressing than in AQP3-null MDCK cells, and PCNA expression was decreased in AQP3-null kidneys. Further studies revealed that AQP3 promoted glucose metabolism, which involved increased HIF1-α expression, and AQP3-dependent activation of the AMPK and mTOR signaling pathway. Figure 8 diagrams the pathways that could account for these observations.
Figure 8.
Proposed AQP3-dependent epithelial cell energy metabolism in renal cystogenesis.
AQP3 is reported to influence in several signaling pathways associated with cell proliferation that are relevant to its involvement in renal cytogenesis as found here. We found that p-ERK was increased in AQP3-expressing cells compared with control cells, in agreement with refs. 28 and 29. p-AMPK and AMPK were decreased in AQP3-expressing cells, and p-S6 and S6, which are downstream in the mTORC1 signaling pathway, were increased. In vivo experiments showed increased p-AMPK and AMPK and decreased p-S6 and S6 in AQP3-null kidney tissue, suggesting involvement of AQP3 in activation of AMPK and mTOR signaling.
Several signaling pathways are associated with cyst development, including signal transducer and activator of transcription 3 and signal transducer and activator of transcription 6, AMPK, mTOR, canonical wingless/integrated (Wnt), hedgehog, heat shock protein 90, macrophage migration inhibitory factor, and NF-κB. The AMPK and mTOR pathway is thought to have a relatively proximal role in cyst formation (30). When polycystin-1 is mutated, a polycystin-1–tuberous sclerosis complex 2 complex led to aberrant activation of mTORC1 in tubular epithelial cells in polycystic mice and in patients with PKD (30, 31). Drugs targeting AMPK and mTOR signaling, such as 2-deoxyglucose, saikosaponin-d (32), and food restriction (33), retard cyst development. The observation here of AQP3 regulating AMPK and mTOR signaling suggests AQP3 as a novel target for ADPKD therapy.
AMPK is reported as a negative regulator of ERK signaling (34, 35), with regulation of AMPK by B-raf through ERK (36, 37). ERK was found to inhibit AMPK in Pkd1−/− cells, and ERK inhibitors reversed this effect (12). However, in other studies, inhibition of MEK by PD98059 did not inhibit AMPK activation (38). Thus, there appears to be a complex relationship between AMPK and ERK that requires further clarification. The in vitro experiments here showed ERK inhibition of AMPK and ERK phosphorylation, suggesting a negative feedback relationship.
We found that AQP3 increased GLUT1 expression, glucose uptake, ATP concentration, and lactate production, suggesting a mechanistic link between AQP3 expression and glycolysis. AQP3 might thus increase glycolysis by increasing cellular H2O2 and glycerol permeability.
AQP3 has been shown to function as an H2O2 transporter (10, 39, 40). In a murine ADPKD model, there was an increase in basal reactive oxygen species, including H2O2 (41, 42). Though we did not measure H2O2 here, we speculate that H2O2 is increased in Pkd1−/− mouse kidneys, with decreased intercellular H2O2 following AQP3 deletion. Cells treated with H2O2 showed higher glycolysis (43) and increased expression of glycolytic enzymes (44, 45) and also demonstrated that increased cellular H2O2 activates HIF-1 (43, 46). Intracellular H2O2 triggers redox-dependent inhibition of prolyl hydroxylases and increases HIF1α stability (47). Here, we found greater HIF1-α expression in AQP3-MDCK cells than in MDCK cells. HIF-1α activation appears to play a key role in the glycolysis (48). Cells lacking HIF-1α showed decreased glycolytic capacity (49). H2O2 can also promote glycolysis through activating PKB (50) and the oncogenes Ras, src, and myc (51). We speculate, therefore, that the increased glycolysis in AQP3-expressing cells is the consequence of increased H2O2, though further work is needed to investigate the role of H2O2 in cyst enlargement in ADPKD.
We further speculate that the AQP3-activated mTOR pathway may involve AQP3 permeability to H2O2. Previous studies implicated a role for H2O2 on the mTOR signal. H2O2 caused mTOR activation after incubation for 2 h (52), H2O2 induced Akt, and mTOR phosphorylation was prevented by wortmannin, a specific inhibitor of PI3K, indicating that mTOR is downstream of Akt PI3K (53). However, some studies showed that H2O2 induces the dephosphorylation of mTOR at Ser2481 and the p-70 ribosomal protein S6 kinase at Thr389, leading to suppression of the mTOR pathway (54). H2O2 also elevated intracellular [Ca2+], thereby stimulating Ca2+/calmodulin-dependent protein kinase II and inhibiting the mTOR pathway (55). The mechanism for the effect of AQP3 on the mTOR pathway may thus be complex and requires further study.
In addition to H2O2, AQP3 might promote glycolysis through glycerol transport. Glycerol is an energy substrate for glycolysis, and its metabolism is catalyzed by glycerol kinase, which phosphorylates glycerol to glycerol-3-phosphate, a key metabolic intermediate for ATP production (56). Our results support the involvement of AQP3 glycerol transport in glycolytic metabolism.
A previous study by Rowe et al. (12) reported defective glucose metabolism as a hallmark of ADPKD. In Pkd1flox/–; Ksp-Cre mouse kidneys compared with kidneys from control littermates, there was greater uptake of glucose, more conversion to lactate, higher ATP concentration, and transcriptional changes in glycolytic enzymes (12). A subsequent study addressed the role of glycolysis in a nonorthologous model of PKD, the Han:Sprd rat (57). Up-regulation of genes involved in glycolysis (hexokinase 1, hexokinase 2, and lactate dehydrogenase A) and down-regulation of genes involved in gluconeogenesis (glucose-6-phosphatase catalytic subunit and fructose-1,6-biphosphatase 1) were found in the cystic kidneys of Cy/+ rats compared with wild-type rats. Reduced cystic disease in Pkd1 mice treated with the nonmetabolizable glucose analog 2-deoxyglucose (58) and in a nonorthologous rat model treated with the sodium glucose cotransporter inhibitor phlorizin (59) suggested aerobic glycolysis as a potential treatment target in PKD. Here, we found increased GLUT1 expression and intracellular glucose content in AQP3-MDCK cells, suggesting the involvement of AQP3 in cellular glucose uptake. Because aerobic glycolysis has been described as a prominent feature of ADPKD kidneys, we found that lactate, an intermediate product of aerobic glycolysis, was increased in AQP3-expressing cells. Glucose starvation prevented the decrease of p-AMPK in AQP3-MDCK cells, suggesting that glucose metabolism may be involved in the reduced p-AMPK. We thus propose that inhibition of AQP3 might inhibit glucose metabolism and ADPKD progression.
Several limitations of this study are noted. Cellular H2O2 transport and steady-state H2O2 levels were not measured. The MDCK cell studies were done using a single cell clone, so it is difficult to rule out clonal variations as responsible for some of the biochemical differences. No evidence was found for specific up-regulation of HIF1-α and glycolysis in AQP3-expressing cells. The biochemical studies in kidneys were done on whole-kidney homogenates, in which the cyst epithelial cells constitute only a small fraction of total cellular content. Finally, because the mouse phenotype studies were done with complete AQP3 deletion, it is difficult to rule out secondary effects of AQP3 deletion outside of the kidney, though we think they are unlikely.
In summary, notwithstanding these limitations, we found that AQP3 deficiency retarded cyst enlargement in 2 experimental mouse models of ADPKD and in an MDCK cell cyst model. The results support a mechanism involving reduced energy metabolism and AMPK activation in AQP3 deficiency, resulting in inhibition of mTOR and ERK signaling and consequent inhibition of cyst epithelial cell proliferation. These findings reveal a previously unrecognized role for AQP3 in ADPKD and suggest AQP3 as a novel therapeutic target in ADPKD.
ACKNOWLEDGMENTS
The authors thank Peter Igarashi (Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA) and Stefan Somlo (Section of Nephrology, Yale School of Medicine, New Haven, CT, USA) for the Ksp-Cre and Pkd1flox/flox mice. This work was supported by National Natural Science Foundation of China Grants 81620108029, 81261160507, 81330074, 81170632, and 81370783; Beijing Natural Science Foundation Grant 7172113; the 111 Project; U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK101373; and the Fundamental Research Funds for the Central Universities (2018-JYBZZ-XJSJJ012). The authors declare no conflicts of interest.
Glossary
- 2-NBDG
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose
- ACC
acetyl–coenzyme A carboxylase
- ADPKD
autosomal dominant polycystic kidney disease
- AQP
aquaporin
- CCK-8
Cell Counting Kit-8
- DBA
Dolichos biflorus agglutinin
- GLUT1
glucose transporter 1
- H&E
hematoxylin and eosin
- HIF
hypoxia-inducible factor
- Ksp
kidney-specific protein
- LTL
Lotus tetragonolobus lectin
- MDCK
Madin-Darby canine kidney
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- PCNA
proliferating cell nuclear antigen
- PKD
polycystic kidney disease
AUTHOR CONTRIBUTIONS
W. Wang, X. Geng, L. Lei, Y. Jia, and Y. Li performed the experiments and analyzed the data; W. Wang, A. S. Verkman, and B. Yang designed the study; W. Wang and B. Yang wrote the manuscript; and A. S. Verkman revised the manuscript.
REFERENCES
- 1.Harris P. C., Torres V. E. (2009) Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres V. E., Harris P. C. (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornec-Le Gall E., Audrézet M. P., Chen J. M., Hourmant M., Morin M. P., Perrichot R., Charasse C., Whebe B., Renaudineau E., Jousset P., Guillodo M. P., Grall-Jezequel A., Saliou P., Férec C., Le Meur Y. (2013) Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 24, 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres V. E., Harris P. C., Pirson Y. (2007) Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- 5.Grantham J. J., Geiser J. L., Evan A. P. (1987) Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 31, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 6.Takiar V., Caplan M. J. (2011) Polycystic kidney disease: pathogenesis and potential therapies. Biochim. Biophys. Acta 1812, 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres V. E., Boletta A., Chapman A., Gattone V., Pei Y., Qian Q., Wallace D. P., Weimbs T., Wüthrich R. P. (2010) Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin. J. Am. Soc. Nephrol. 5, 1312–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Li F., Sun Y., Lei L., Zhou H., Lei T., Xia Y., Verkman A. S., Yang B. (2015) Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. FASEB J. 29, 1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi M., Yamaji Y., Monkawa T., Yoshida T., Tsuganezawa H., Sasamura H., Kitajima W., Sasaki S., Ishibashi K., Maurmo F., Saruta T. (1997) Expression and localization of the water channels in human autosomal dominant polycystic kidney disease. Nephron 75, 321–326 [DOI] [PubMed] [Google Scholar]
- 10.Miller E. W., Dickinson B. C., Chang C. J. (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 107, 15681–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara-Chikuma M., Satooka H., Watanabe S., Honda T., Miyachi Y., Watanabe T., Verkman A. S. (2015) Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 6, 7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe I., Chiaravalli M., Mannella V., Ulisse V., Quilici G., Pema M., Song X. W., Xu H., Mari S., Qian F., Pei Y., Musco G., Boletta A. (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menezes L. F., Zhou F., Patterson A. D., Piontek K. B., Krausz K. W., Gonzalez F. J., Germino G. G. (2012) Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4α as a disease modifier. PLoS Genet. 8, e1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma T., Song Y., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. (2000) Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 97, 4386–4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibazaki S., Yu Z., Nishio S., Tian X., Thomson R. B., Mitobe M., Louvi A., Velazquez H., Ishibe S., Cantley L. G., Igarashi P., Somlo S. (2008) Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa Y., Ishifune C., Tsukumo S., Hozumi K., Yagita H., Yasutomo K. (2015) Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat. Med. 21, 55–61 [DOI] [PubMed] [Google Scholar]
- 17.Gattone V. H., II, Maser R. L., Tian C., Rosenberg J. M., Branden M. G. (1999) Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev. Genet. 24, 309–318 [DOI] [PubMed] [Google Scholar]
- 18.Piontek K., Menezes L. F., Garcia-Gonzalez M. A., Huso D. L., Germino G. G. (2007) A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma M., Tian X., Igarashi P., Pazour G. J., Somlo S. (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takakura A., Contrino L., Beck A. W., Zhou J. (2008) Pkd1 inactivation induced in adulthood produces focal cystic disease. J. Am. Soc. Nephrol. 19, 2351–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider A., Zhang Y., Guan Y., Davis L. S., Breyer M. D. (2003) Differential, inducible gene targeting in renal epithelia, vascular endothelium, and viscera of Mx1Cre mice. Am. J. Physiol. Renal Physiol. 284, F411–F417 [DOI] [PubMed] [Google Scholar]
- 22.Yuajit C., Muanprasat C., Gallagher A. R., Fedeles S. V., Kittayaruksakul S., Homvisasevongsa S., Somlo S., Chatsudthipong V. (2014) Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem. Pharmacol. 88, 412–421 [DOI] [PubMed] [Google Scholar]
- 23.Hara-Chikuma M., Verkman A. S. (2008) Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 28, 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Li B., Zhang L., Chen L., Sun G., Zhang Q., Wang J., Zhi X., Wang L., Xu Z., Xu H. (2016) The proliferation impairment induced by AQP3 deficiency is the result of glycerol uptake and metabolism inhibition in gastric cancer cells. Tumour Biol. 37, 9169–9179 [DOI] [PubMed] [Google Scholar]
- 25.Hardie D. G. (2011) Signal transduction: how cells sense energy. Nature 472, 176–177 [DOI] [PubMed] [Google Scholar]
- 26.Bland M. L., Birnbaum M. J. (2011) Cell biology. ADaPting to energetic stress. Science 332, 1387–1388 [DOI] [PubMed] [Google Scholar]
- 27.Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 28.Lei L., Wang W., Jia Y., Su L., Zhou H., Verkman A. S., Yang B. (2017) Aquaporin-3 deletion in mice results in renal collecting duct abnormalities and worsens ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1231–1241 [DOI] [PubMed] [Google Scholar]
- 29.Nagaraju G. P., Basha R., Rajitha B., Alese O. B., Alam A., Pattnaik S., El-Rayes B. (2016) Aquaporins: their role in gastrointestinal malignancies. Cancer Lett. 373, 12–18 [DOI] [PubMed] [Google Scholar]
- 30.Pema M., Drusian L., Chiaravalli M., Castelli M., Yao Q., Ricciardi S., Somlo S., Qian F., Biffo S., Boletta A. (2016) mTORC1-mediated inhibition of polycystin-1 expression drives renal cyst formation in tuberous sclerosis complex. Nat. Commun. 7, 10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shillingford J. M., Murcia N. S., Larson C. H., Low S. H., Hedgepeth R., Brown N., Flask C. A., Novick A. C., Goldfarb D. A., Kramer-Zucker A., Walz G., Piontek K. B., Germino G. G., Weimbs T. (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 103, 5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi W., Xu D., Gu J., Xue C., Yang B., Fu L., Song S., Liu D., Zhou W., Lv J., Sun K., Chen M., Mei C. (2018) Saikosaponin-d inhibits proliferation by up-regulating autophagy via the CaMKKβ-AMPK-mTOR pathway in ADPKD cells. Mol. Cell. Biochem. 449, 219–226 [DOI] [PubMed] [Google Scholar]
- 33.Warner G., Hein K. Z., Nin V., Edwards M., Chini C. C., Hopp K., Harris P. C., Torres V. E., Chini E. N. (2016) Food restriction ameliorates the development of polycystic kidney disease. J. Am. Soc. Nephrol. 27, 1437–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J., Yoon M. Y., Choi S. L., Kang I., Kim S. S., Kim Y. S., Choi Y. K., Ha J. (2001) Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. J. Biol. Chem. 276, 19102–19110 [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Whiteman M. W., Lian H., Wang G., Singh A., Huang D., Denmark T. (2009) A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J. Biol. Chem. 284, 21412–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng B., Jeong J. H., Asara J. M., Yuan Y. Y., Granter S. R., Chin L., Cantley L. C. (2009) Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 33, 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damm E., Buech T. R., Gudermann T., Breit A. (2012) Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol. Endocrinol. 26, 643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi A., Lord J. M. (2013) Adiponectin inhibits neutrophil apoptosis via activation of AMP kinase, PKB and ERK 1/2 MAP kinase. Apoptosis 18, 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara-Chikuma M., Watanabe S., Satooka H. (2016) Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem. Biophys. Res. Commun. 471, 603–609 [DOI] [PubMed] [Google Scholar]
- 40.Vieceli Dalla Sega F., Zambonin L., Fiorentini D., Rizzo B., Caliceti C., Landi L., Hrelia S., Prata C. (2014) Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta 1843, 806–814 [DOI] [PubMed] [Google Scholar]
- 41.Brookes Z. L., Ruff L., Upadhyay V. S., Huang L., Prasad S., Solanky T., Nauli S. M., Ong A. C. (2013) Pkd2 mesenteric vessels exhibit a primary defect in endothelium-dependent vasodilatation restored by rosiglitazone. Am. J. Physiol. Heart Circ. Physiol. 304, H33–H41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maser R. L., Vassmer D., Magenheimer B. S., Calvet J. P. (2002) Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J. Am. Soc. Nephrol. 13, 991–999 [DOI] [PubMed] [Google Scholar]
- 43.Metheni M., Echebli N., Chaussepied M., Ransy C., Chéreau C., Jensen K., Glass E., Batteux F., Bouillaud F., Langsley G. (2014) The level of H2O2 type oxidative stress regulates virulence of Theileria-transformed leukocytes. Cell. Microbiol. 16, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W., Liu Z., Zhao L., Sun J., He Q., Yan W., Lu Z., Wang A. (2017) Hexokinase 2 enhances the metastatic potential of tongue squamous cell carcinoma via the SOD2-H2O2 pathway. Oncotarget 8, 3344–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deubzer B., Mayer F., Kuçi Z., Niewisch M., Merkel G., Handgretinger R., Bruchelt G. (2010) H(2)O(2)-mediated cytotoxicity of pharmacologic ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell. Physiol. Biochem. 25, 767–774 [DOI] [PubMed] [Google Scholar]
- 46.Brunelle J. K., Bell E. L., Quesada N. M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R. C., Chandel N. S. (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1, 409–414 [DOI] [PubMed] [Google Scholar]
- 47.Sabharwal S. S., Schumacker P. T. (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla S. K., Purohit V., Mehla K., Gunda V., Chaika N. V., Vernucci E., King R. J., Abrego J., Goode G. D., Dasgupta A., Illies A. L., Gebregiworgis T., Dai B., Augustine J. J., Murthy D., Attri K. S., Mashadova O., Grandgenett P. M., Powers R., Ly Q. P., Lazenby A. J., Grem J. L., Yu F., Mates J. M., Asara J. M., Kim J. W., Hankins J. H., Weekes C., Hollingsworth M. A., Serkova N. J., Sasson A. R., Fleming J. B., Oliveto J. M., Lyssiotis C. A., Cantley L. C., Berim L., Singh P. K. (2017) MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell 32, 71–87.e7; erratum: 392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandjean G., de Jong P. R., James B., Koh M. Y., Lemos R., Kingston J., Aleshin A., Bankston L. A., Miller C. P., Cho E. J., Edupuganti R., Devkota A., Stancu G., Liddington R. C., Dalby K., Powis G. (2016) Definition of a novel feed-forward mechanism for glycolysis-HIF1α signaling in hypoxic tumors highlights aldolase a as a therapeutic target. Cancer Res. 76, 4259–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao S., Li L., Wang S., Yu C., Xiao B., Lin L., Cong W., Cheng J., Yang W., Sun W., Cui S. (2016) H2O2 treatment or serum deprivation induces autophagy and apoptosis in naked mole-rat skin fibroblasts by inhibiting the PI3K/Akt signaling pathway. Oncotarget 7, 84839–84850; erratum: 8, 43593–43594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinq-Frais C., Coatrieux C., Grazide M. H., Hannun Y. A., Nègre-Salvayre A., Salvayre R., Augé N. (2013) A signaling cascade mediated by ceramide, src and PDGFRβ coordinates the activation of the redox-sensitive neutral sphingomyelinase-2 and sphingosine kinase-1. Biochim. Biophys. Acta 1831, 1344–1356 [DOI] [PubMed] [Google Scholar]
- 52.Zhang J., Zhou W., Lin J., Wei P., Zhang Y., Jin P., Chen M., Man N., Wen L. (2016) Autophagic lysosomal reformation depends on mTOR reactivation in H2O2-induced autophagy. Int. J. Biochem. Cell Biol. 70, 76–81 [DOI] [PubMed] [Google Scholar]
- 53.Radisavljevic Z. M., González-Flecha B. (2004) TOR kinase and Ran are downstream from PI3K/Akt in H2O2-induced mitosis. J. Cell. Biochem. 91, 1293–1300 [DOI] [PubMed] [Google Scholar]
- 54.Byun Y. J., Kim S. K., Kim Y. M., Chae G. T., Jeong S. W., Lee S. B. (2009) Hydrogen peroxide induces autophagic cell death in C6 glioma cells via BNIP3-mediated suppression of the mTOR pathway. Neurosci. Lett. 461, 131–135 [DOI] [PubMed] [Google Scholar]
- 55.Liu C., Ye Y., Zhou Q., Zhang R., Zhang H., Liu W., Xu C., Liu L., Huang S., Chen L. (2016) Crosstalk between Ca2+ signaling and mitochondrial H2O2 is required for rotenone inhibition of mTOR signaling pathway leading to neuronal apoptosis. Oncotarget 7, 7534–7549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laforenza U., Bottino C., Gastaldi G. (2016) Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta 1858, 1–11 [DOI] [PubMed] [Google Scholar]
- 57.Riwanto M., Kapoor S., Rodriguez D., Edenhofer I., Segerer S., Wüthrich R. P. (2016) Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 11, e0146654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiaravalli M., Rowe I., Mannella V., Quilici G., Canu T., Bianchi V., Gurgone A., Antunes S., D’Adamo P., Esposito A., Musco G., Boletta A. (2016) 2-Deoxy-d-glucose ameliorates PKD progression. J. Am. Soc. Nephrol. 27, 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X., Zhang S., Liu Y., Spichtig D., Kapoor S., Koepsell H., Mohebbi N., Segerer S., Serra A. L., Rodriguez D., Devuyst O., Mei C., Wüthrich R. P. (2013) Targeting of sodium-glucose cotransporters with phlorizin inhibits polycystic kidney disease progression in Han:SPRD rats. Kidney Int. 84, 962–968 [DOI] [PubMed] [Google Scholar]