Abstract
Gulf War illness (GWI) is a chronic multisymptom disorder that is prominent in Gulf War veterans. Major unexplained symptoms of GWI include functional gastrointestinal disorders and undiagnosed illnesses, including neurologic disorders. Exposure to the antinerve gas drug pyridostigmine bromide (PB) is linked to the development of GWI, but the exact mechanisms remain unclear. Here, we tested the hypothesis that PB alters gut function by disrupting the neural and immune systems of the intestine. We exposed male and female mice to physiologically comparable amounts of PB that match the dose, route, and time frame of exposure experienced by Gulf War veterans and assessed the acute and chronic impacts on gastrointestinal functions, the functional architecture of the enteric nervous system, and immune responses in the gut and brain. Exposure to PB drove acute alterations to colonic motility and structure in both male and female mice that transitioned to chronic changes in gut functions. PB drove acute alterations to enteric neural and glial activity, glial reactivity, and neuron survival with glial reactivity persisting into the chronic phase in male mice. Despite having no effect on colonic permeability, exposure to PB caused major shifts in the expression of proinflammatory cytokines and chemokines in the colon and brain that suggest immunosuppressive effects. Interestingly, immune disruption was still evident in the colon and brain in female animals at 1 mo following exposure to PB. Together, our results show that the paradigm of PB exposure experienced by veterans of the Persian Gulf War contributes to long-lasting pathophysiology by driving enteric neuroinflammation, promoting immunosuppression, and altering functional anatomy of the colon in a sex-dependent manner.—Hernandez, S., Fried, D. E., Grubišić, V., McClain, J. L., Gulbransen, B. D. Gastrointestinal neuroimmune disruption in a mouse model of Gulf War illness.
Keywords: pyridostigmine bromide, cholinergic signaling, enteric nervous system
Gulf War illness (GWI) is a prominent condition affecting Gulf War (GW) veterans that is characterized by a spectrum of chronic medically unexplained symptoms including fatigue, insomnia, dizziness, respiratory disorders, headaches, neurologic disorders, and functional gastrointestinal (GI) disorders (1, 2). GI disorders are one of the most debilitating symptoms and are 3 times more common in GW veterans than in non-GW veterans (3). GI complications include diarrhea, constipation, bloating, and abdominal pain (4–6). To date, the cause of GI dysfunction in GWI is not understood, and there are no therapies available.
Although the precise etiology of GWI is unknown, exposure to anti-acetylcholinesterase (AChE) drugs such as permethrin, N,N-diethyl-meta-toluamide, and pyridostigmine bromide (PB) is considered an important factor (7). PB, in particular, has emerged as a potential causative factor for the development of GWI, and in 2008, the U.S. Congress concluded that there is a clear link between PB and the development of GWI (1, 8). In support, the amount of PB consumed directly predicts declines in health following the war (9), and individuals with genetic variants that impair the clearance of PB have a 40-fold risk of developing chronic health problems (8, 10). Yet the mechanisms that link this potential causal relationship remain unresolved.
PB is a reversible AChE inhibitor that was administered to 250,000–300,000 American troops as prophylactic protection against Iraqi nerve gas attacks in a daily dose of 90 mg for a maximum of 7 d (1). Blocking AChE permits an increase in availability and prolongation of time for acetylcholine (Ach) to remain in contact with muscarinic and nicotinic receptors (11, 12). ACh is particularly important in the autonomic nervous system where parasympathetic and enteric branches use ACh as their main excitatory neurotransmitter. Cholinergic neurotransmission in the enteric nervous system (ENS) contributes to the regulation of key GI functions, such as motility, fluid exchange across the mucosa, and the regulation of local blood flow (13). The acute disruption of cholinergic transmission in the intestine with AChE inhibitors such as PB causes massive and spontaneous contractions (14) while longer-term inhibition of AChE causes cholinergic signaling to desensitize and leads to a catastrophic failure of gut motility (15). These acute effects of PB may lead to long-lasting GI issues by altering the neural circuitry of the ENS (16). Likewise, the regulation of intestinal immune homeostasis involves parasympathetic pathways that utilize ACh as their main transmitter (12, 17). Vagal stimulation, in particular, is thought to act through a cholinergic anti-inflammatory pathway to suppress proinflammatory cytokine release from gut immune cells, such as macrophages (18–21). Despite the potential effects of PB on gut neural and immune systems and the clear link between PB and GWI, the mechanisms that connect the acute effects of PB with the multiple dysfunctions remain unclear.
We addressed this issue by testing the hypothesis that PB alters gut functions by disrupting the neural and immune systems of the intestine. We tested our hypothesis using a mouse model in which PB exposure matches the dose, time frame, and route of exposure experienced by soldiers in the GW, and the acute and chronic effects on in vivo and ex vivo gut functions, the structure and function of the ENS, and immune responses in the gut and brain are tested. Our results show that exposure to PB causes both acute and chronic disturbances in gut motility, altered excitability and survival of neurons and glia in the ENS, and a dysregulation of cytokines and chemokines in the colon and brain. Based on these data, we propose that the acute effects of PB on the intestinal nervous and immune systems contribute to the chronic GI pathophysiology of GWI.
MATERIALS AND METHODS
Animals
All experimental protocols were approved by the Michigan State University Institutional Animal Care and Use Committee. C57BL/6 male and female mice were purchased from Charles River Laboratories (Wilmington, MA, USA) at 5 wk of age. Mice were maintained in a temperature-controlled environment (Innocage system with ALPHA-dri bedding; Innovive, Thermo Fisher Scientific, Waltham, MA, USA) on a 12-h light/dark cycle with access to acidified water and a minimal phytoestrogen diet (Diet 2919; Envigo, Huntington, United Kingdom) ad libitum.
Vaginal smears were performed in female mice 5 d before and after treatment to verify if they were cycling properly. Vaginal smears were performed as previously described by Caligoni (22). Cells present in vaginal fluid were evaluated by light microscopy to classify the 4 stages of estrous cycle (22).
PB treatment
PB was purchased from MilliporeSigma (Burlington, MA, USA). Mice (n = at least 8/group) were arbitrarily assigned to each group and administered PB dissolved in drinking water for 7 d. The 2 doses of PB (9 and 90 μg/ml) were chosen based on calculated conversions from known human dosing in the GW (1). Conversions from human to mouse dosages based on body weight yielded a mouse dose of 9 μg/ml and conversions based on body surface area yielded a mouse dose of 90 μg/ml. Controls were given water only.
Animals were euthanized at 7 or 30 d. Samples of blood, colon, and brain were collected at the time of euthanization. Macroscopic damage was assessed and scored as the colon was removed using a well-characterized scale to quantify colonic length, fecal blood, and diarrhea (23).
Ca2+ imaging
Live whole-mounts of the colonic myenteric plexus were prepared for Ca2+ imaging as previously described (24). Briefly, colonic segments were collected in ice-cold DMEM and transferred to Sylgard-coated open diamond-shaped bath recording chambers. Tissue segments were opened along the mesenteric border, and the mucosa, submucosa, and longitudinal muscle were removed by microdissection to expose the myenteric plexus. The resulting longitudinal muscle myenteric plexus (LMMP) whole mount preparations were incubated for 15 min at room temperature in enzyme mixture consisting of 150 U/ml collagenase type II and 1 U/ml Dispase (Thermo Fisher Scientific) dissolved in DMEM. Whole-mounts were washed 2 times with DMEM and then loaded with 4 μM Fluo-4 AM, 0.02% pluronic F-127, and 200 μM water-soluble probenecid (Thermo Fisher Scientific) in DMEM for 45 min at 37°C (5% CO2, 95% air). Myenteric plexus preparations were washed and incubated with 200 μM probenecid in DMEM for 15 min to de-esterify before imaging. Images were acquired every 1 s through ×40 water-immersion objective (LUMPlanFI, 0.8 numerical aperture) of an upright Olympus BX51wI fixed stage microscope (Olympus, Tokyo, Japan) using NIS-Elements software (v.4.5; Nikon, Tokyo, Japan) and an Andor Zyla sCMOS camera (Oxford Instruments, Abingdon, United Kingdom). Whole-mounts were superfused with Krebs buffer, (37°C) at 2–3 ml/min. We dissolved ADP (100 μM) and PB (250 μM) in Krebs solution and bath applied for 30 s or 7 min, respectively.
In situ neuroinflammation
Enteric neuron death was assessed as previously described (25) by incubating live colonic LMMP whole-mount preparations from 8 to 12 wk old C57BL/6 mice with 250 µM PB for 4 h in 95% air: 5% CO2 at 37°C. LMMP preparations were rinsed with fresh Krebs and fixed in Zamboni’s fixative overnight and processed for immunohistochemistry.
NO imaging
Intracellular NO was assessed in live colonic LMMP whole-mount preparations from 8 to 12 wk old C57BL/6 mice following application of 250 µM PB for 4 h in 95% air: 5% CO2 at 37°C. LMMP preparations were rinsed with fresh Krebs and loaded with 4 µM 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM; Thermo Fisher Scientific) for 45 min at 37°C as described in Brown et al. (23). Images were acquired with a Neo sCMOS digital camera (Oxford Instruments) through the ×40 water-immersion objective (LUMPlan N, 0.8 numerical aperture) of an upright Olympus BX51W1 fixed-stage microscope controlled by Andor IQ3 (Oxford Instruments) software.
In vivo colonic motility
Pellet production
Fecal pellet output was measured as previously described (26, 27). Briefly, mice were individually housed without water or food for 1 h starting at 8:00 am (zeitgeber +2). Fecal pellets were collected, wet weight was measured immediately, and the dry weight was measured after an overnight dehydration at 60°C.
Colon bead assay
Distal colonic transit time was measured using glass beads (2 mm in diameter) as described in Nasser et al. (28). Briefly, mice were lightly anesthetized with isofluorane, and a glass bead was inserted through the anus and gently pushed 2 cm aborally by a customized syringe. The syringe was carefully withdrawn, and the bead expulsion latency was measured.
Ex vivo colonic function
Contractility studies
Isometric muscle tension recordings were performed in circular muscular segments of the distal colon under 2 g passive tension. Circular muscle strips were mounted in a tissue bath with Krebs solution at 37°C oxygenated (28). Each muscle strip was attached to an isometric force transducer, and data were charted with LabChart 8 software (ADInstruments, Sydney, NSW, Australia) as described in Fried et al. (29). Tissue segments were equilibrated for 20 min under 0.5 g initial tension. Electrical field stimulation (EFS; 20 V, 1–30 Hz) was applied through 2 platinum concentric electrodes and a GRASS stimulator (S88; GRASS Telefactor, West Warwick, RI, USA) to evoke neurogenic contractions and relaxation. EFS was applied to obtain neurogenic contraction. After EFS, maximal muscle contractions were obtained by adding carbachol (10 μM, a cholinergic agonist; MilliporeSigma) to the bath. After being rinsed and stabilized, prostaglandin F2α (1 μM) was added to the bath, and tissues were subjected to EFS to obtain neurogenic relaxation recordings (24).
Paracellular permeability of the gut wall
Intestinal barrier function was assessed using Ussing chambers as previously described (30). Briefly, segments of distal colon were mounted in the Ussing chambers (aperture 0.3 cm2, EasyMount Ussing Chamber system; Physiologic Instruments, San Diego, CA, USA), equilibrated for 20 min, and a cell-impermeant fluorescein-5-(and-6)-sulfonate (478.32 Da; Thermo Fisher Scientific) was added to the mucosal chamber (0.05 mg/ml). Samples from the serosal chamber were taken before the dye was added and then every 20 min until 2 h (100 μl duplicates and buffer was replenished). Fluorescent intensity was measured on the Infinite M1000 PRO microplate reader (wavelength 495/520 nm; Tecan, Männedorf, Switzerland) using i-control microplate reader software (v.1.6.19.2; Tecan). The permeability of the gut wall was assessed from the slope of fluorescence values of the last 4 time points.
Whole-mount immunohistochemistry
Whole-mount preparations of mouse colonic myenteric plexus were prepared from segments of intestine preserved in Zamboni’s fixative as previously described (25). LMMP whole-mounts were rinsed 3 times for 10 min each with 0.1% Triton X-100 in PBS (PBS-Triton) followed by a 45-min incubation in blocking solution (4% normal goat or normal donkey serum, 0.4% Triton X-100, and 1% bovine serum albumin). Preparations were incubated with primary antibodies overnight at room temperature and secondary antibodies (Table 1) for 2 h at room temperature before mounting. Fluorescent labeling was evaluated using the ×20 or 40 objective (0.75 numerical aperture, Plan Fluor; Nikon) of an upright epifluorescence microscope (Nikon Eclipse Ni) with a Retiga 2000R camera (QImaging, Surrey, BC, Canada) controlled by QCapture Pro 7.0 (QImaging) software (23, 24). Representative images were acquired through the ×60 oil immersion objective (Plan-Apochromat, 1.42 numerical aperture) of an inverted Fluoview FV1000 confocal microscope (Olympus). Alexa Fluor 405, 488, or 568 secondary antibodies were excited with 405-, 488-, or 543-nm wavelengths and detected using SDM560 dichroic mirror and BA505-525 bandpass or BA560IF longpass filter sets (30).
TABLE 1.
Details of primary and secondary antibodies used in this study
| Antibody | Source | Dilution | Catalog no. |
|---|---|---|---|
| Primary antibodies | |||
| Goat anti-calretinin | Swant, Marly, Switzerland | 1:1000 | CG1 |
| Chicken anti-GFAP | Abcam, Cambridge, MA, USA | 1:1000 | AB4674 |
| Biotinylated anti-mouse HuC/D | Thermo Fisher Scientific | 1:200 | A21272 |
| Rabbit anti–IL-1β | Abcam | 1:500 | AB9722 |
| Sheep anti-nNOS | MilliporeSigma | 1:500 | AB1529 |
| Secondary antibodies | |||
| Alexa Fluor 350–conjugated streptavidin | Thermo Fisher Scientific | 1:200 | S-11249 |
| Alexa Fluor 488 goat anti-chicken | Thermo Fisher Scientific | 1:200 | A-11039 |
| Alexa Fluor 488 donkey anti-goat | Jackson ImmunoResearch Laboratories, West Grove, PA, USA | 1:200 | 705-545-003 |
| Alexa Fluor 488 donkey anti-sheep | Jackson ImmunoResearch Laboratories | 1:200 | 713-545-003 |
| Alexa Fluor 568 donkey anti-goat | Thermo Fisher Scientific | 1:200 | A-11057 |
| Alexa Fluor 594 donkey anti-rabbit | Jackson ImmunoResearch Laboratories | 1:200 | 711-585-152 |
| Alexa Fluor 594–conjugated streptavidin | Jackson ImmunoResearch Laboratories | 1:200 | 016-580-084 |
Multiplex cytokine assay
Brain and colonic tissues collected at the time of euthanization were homogenized in a Tris-buffered saline Tween buffer to extract protein. Cytokine/chemokine levels were evaluated using bead-based multiplex immunoassays (Eve Technologies, Calgary, AB, Canada).
Solutions
Calcium imaging experiments were performed in modified Krebs buffer consisting of (in millimolars): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, 8 glucose (pH adjusted to 7.4 with NaOH) with 3 mM nicardipine, and 1 mM scopolamine to inhibit muscle contractions. Muscle contractility and Ussing chambers studies were performed in normal Krebs buffer consisting of (in millimolar): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. Live tissue was maintained in DMEM/F-12 nutrient mixture (Thermo Fisher Scientific) containing 3 mM nicardipine and 1 mM scopolamine during microdissection and incubations.
Chemical and reagents
All chemicals and reagents were purchased from MilliporeSigma. PB was dissolved in water at 9 or 90 μg/ml and made available to mice over the 7 d ad libitum in drinking water. For in vitro experiments, PB was dissolved in Krebs at 250 μM and applied via a gravity-fed perfusion system in Ca2+ imaging experiments or added to the bath for 4 h in cell survival assays. Carbachol (10 μM) was directly added to organ baths for isometric muscle tensions recordings.
Data analysis
Cell counts, ganglionic expression, and NO imaging data were analyzed offline using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Cell counts were performed using the cell counter plugin of ImageJ software. Enteric neuron and glial cell numbers are presented as ganglionic packing density, which was calculated by tracing the ganglionic area and counting the number of HuC/D-immunoreactive neurons or S100β-immunoreactive glia within the defined ganglionic area. The relative ganglionic expression of glial fibrillary acidic protein (GFAP) and IL-1β was measured by recording the mean gray values of GFAP and IL-1β fluorescence within a defined ganglionic area. The relative NO fluorescence of neurons and glia was measured by recording the mean gray values of neurons and glia, identified by morphology. Cell counts, ganglionic expression data, and NO imaging were performed on a minimum of 10 ganglia per animal and averaged to obtain a value for that animal. The number of animals in each experiment is represented by n values, and data are expressed as percent buffer control.
Statistical analysis
Data were analyzed with Prism 7 (GraphPad Software, La Jolla, CA, USA) and are shown as means  sem. Ca2+ imaging, NO imaging, and in situ data were analyzed by Student’s t test. Remaining data were analyzed by 1- and 2-way, where appropriate, ANOVA with a Tukey posttest. A value of P < 0.05 was considered statistically significant.
sem. Ca2+ imaging, NO imaging, and in situ data were analyzed by Student’s t test. Remaining data were analyzed by 1- and 2-way, where appropriate, ANOVA with a Tukey posttest. A value of P < 0.05 was considered statistically significant.
RESULTS
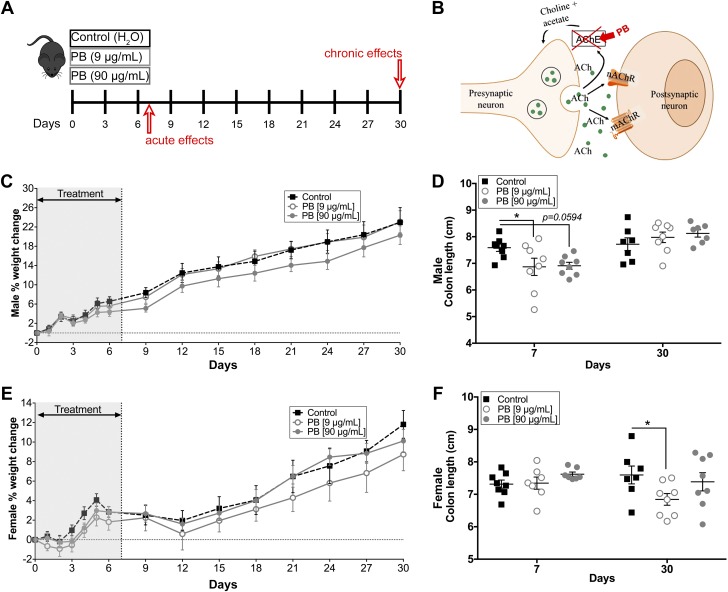
American ground troops in the GW received daily oral doses of 90 mg PB for a maximum of 7 d. Given that the average weight and body surface area of Americans in 1990 was 161 lbs (73 kg) and 1.85 m2, respectively (31), this equals a daily dose of ∼1.23 mg/kg based on body weight and 48.6 mg/m2 based on body surface area. We extrapolated these doses to mice to create a mouse model that mirrors the route, time frame, and dosing of PB experienced by GW veterans. C57Bl/6 mice in our facility drink an average of 3.5 ml/d, weigh ∼25 g at 3 mo of age, and have a body surface area of ∼0.007 m2. Therefore, the correct daily oral dose in mice via drinking water is 9 µg/ml (31.5 µg/d) based on body weight and 90 µg/ml (340 µg/d) based on body surface area, which is a more accurate extrapolation of dosing between mice and humans. We administered these 2 relevant doses of PB to male and female mice over 7 d and studied the acute effects immediately following the exposure to PB (d 7) and the chronic effects at 30 d (Fig. 1A).
Figure 1.
A) Model paradigm and effects of PB on body weight and colonic length. Model of experimental timeline to assess the acute (7 d) and chronic (30 d) effects of a 7-d exposure of PB in mice. B) Model showing the mechanism of action of PB in the ACh pathway. C, E) Body weight of acute and chronic male (C) or female (E) mice of either drinking water or water containing PB. There was no difference in weight between treated and untreated animals. D) Summary data showing decreased colonic length measurements in PB-treated male mice at d 7, but no difference at d 30. F) Females show no difference at d 7 but have decreased colonic length at d 30 (F) (n = 7–8 animals/group). *P < 0.05 compared with control.
Mice did not avoid water containing PB and drank comparable amounts to mice with normal water (unpublished results). Likewise, there was no difference in body weight between mice drinking water and mice drinking water containing PB during the course of the study (Males Fig. 1C; Females Fig. 1E) and mice exposed to PB did not exhibit overt macroscopic changes indicative of colitis such as bloody diarrhea or mucosal ulceration (unpublished results). Vaginal smears performed in female mice 5 d before and after treatment showed no significant effect on cycling (unpublished results). However, colon length, one of the macroscopic measure of fibrosis in mouse colitis models, was significantly decreased in male mice at the 7 d time point and in female mice at the 30 d time point (Fig. 1D, F). These results are in line with human GWI where functional GI issues are observed in the absence of organic intestinal disease.
Exposure to PB alters gut motility
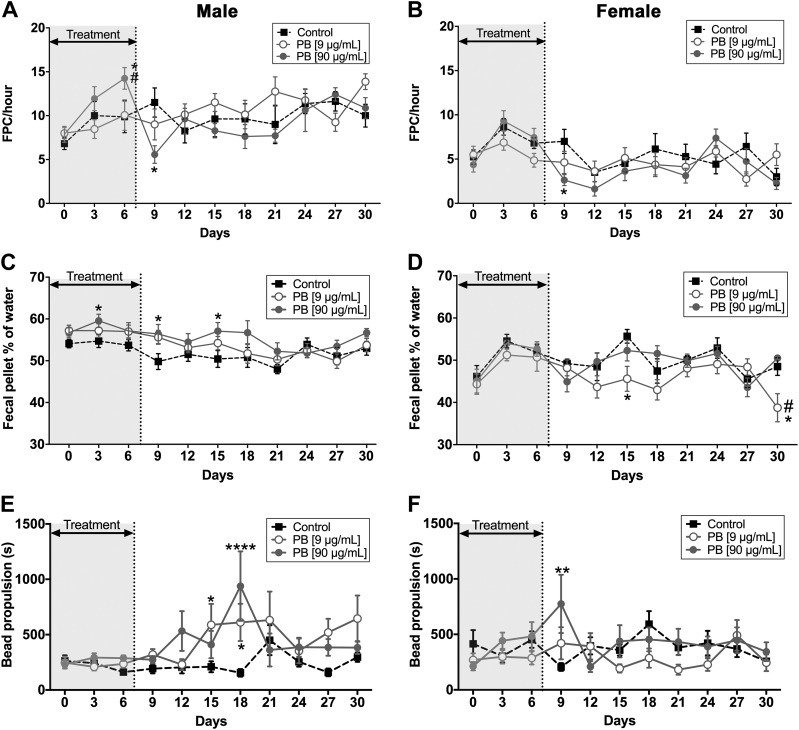
We began testing how exposure to PB affects gut functions by recording in vivo measures of colonic motility, such as fecal pellet production per hour, fecal fluid content, and colonic bead expulsion time (Fig. 2). PB (90 µg/ml) caused an acute increase in pellet production in male mice during the treatment (d 6) (Fig. 2A) but eventually reduced pellet production in both male and female mice following treatment with PB (both d 9) (Fig. 2A, B). Fecal fluid content was acutely increased in male mice exposed to 90 µg/ml PB (d 3, 9, and 15) (Fig. 2C). Conversely, fecal fluid content was acutely decreased in female mice exposed to 9 µg/ml (d 15 and 30) (Fig. 2D). We measured distal colonic transit time using glass beads and found that colonic transit was delayed in both male (9 and 90 µg/ml doses; d 15 and 18, respectively) and female (90 µg/ml dose; d 9) mice following the treatment with PB (Fig. 2E, F). These data show that exposure to PB over a time course relevant to GW veterans is sufficient to cause significant changes to colonic motility in mice.
Figure 2.
Effects of PB on gut motility in vivo. Data showing the effects of PB (9 and 90 μg/ml) on gut motility in vivo in male and female mice. A, B) PB (90 µg/ml) caused an acute increase in pellet production in male mice during the treatment (A), but eventually reduced pellet production in both male and female mice following treatment with PB (B). C, D) Fecal fluid content was acutely increased in male mice exposed to 90 µg/ml PB (C) but was acutely decreased in female mice exposed to 9 µg/ml (D). E, F) Distal colonic transit time was measured using glass beads and found that colonic transit was delayed in both male (9 and 90 µg/ml doses) and female (90 µg/ml dose) mice following the treatment with PB. FPC, fecal pellet count (n = 7–8 animals/group). *P < 0.05, **P < 0.01, ***P < 0.001compared with controls, #P < 0.05 compared with 9 μg/ml PB.
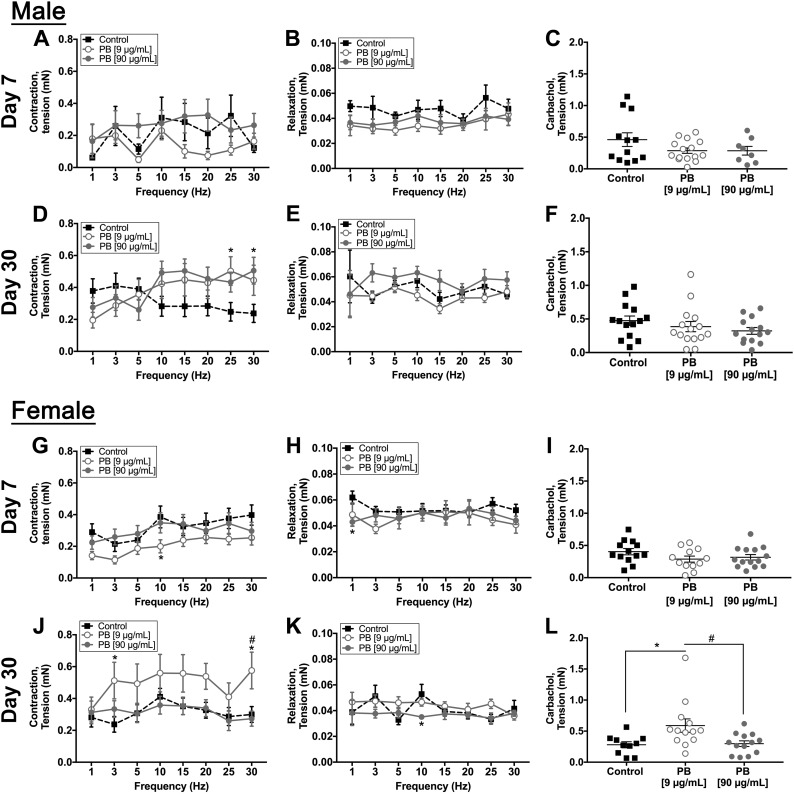
To study specific changes in neuromuscular transmission that might underlie the effects of PB on colonic motility, we conducted isometric muscle tension recordings in segments of colon. PB did not alter neurogenic contractions or relaxation in male mice immediately following exposure to PB (Fig. 3A, B). However, PB significantly increased neurogenic contractions (103% in PB 9 µg/ml and 112.5% in 90 µg/ml) in male mice at d 30 with no significant effect on neurogenic relaxations (Fig. 3D, E). PB caused divergent effects in female mice and decreased neurogenic contractions acutely while increasing neurogenic contractions at d 30 (Fig. 3G, H). Exposure to PB also decreased neurogenic relaxations by up to 30.5% at d 7 and 44.6% at d 30 in female mice (Fig. 3J, K). To assess whether changes in neuromuscular transmission reflect neuronal impairments or a reduced ability of smooth muscle cells to respond to cholinergic transmission, we directly stimulated intestinal smooth muscle with the cholinergic agonist carbachol (10 µM). Exposure to PB had no significant effect on contractions driven by carbachol in male mice (both time points) or in female mice (acute), indicating that changes in neuromuscular transmission mainly reflect alterations to enteric neurons (Fig. 3C, F, I). In contrast, female mice exposed to 9 µg/ml PB exhibited a heightened response to carbachol at d 30 (Fig. 3L), suggesting that different mechanisms underlie the effects of PB on gut motility in male and female animals. Together, our ex vivo (Fig. 3) and in vivo (Fig. 2) motility data show that exposure to PB has lasting effects on colonic contractility and motility that correlate with some of the GI issues observed in GW veterans. Additionally, our data show that the effects of PB on gut function involve differing mechanisms in male and female mice.
Figure 3.
Effects of PB on gut motor functions ex vivo. Data from isometric muscle tension recordings showing the effects of PB on ex vivo gut motor functions at d 7 and 30 in male (A–F) and female (G–L) mice. Frequency (Hz) response curves for neurogenic contractions are shown in A, D, G, J, and relaxations in B, E, H, K. Responses to the cholinergic agonist carbachol are shown in D, F, I, L. PB significantly enhanced neurogenic contractions in male mice at 30 d (D) decreased contractions in female mice at 7 d (G) (9 μg/ml PB compared with control), and increased contractions in female mice at 30 d (J).*P < 0.05 compared with control, #P < 0.05 compared with 90 μg/ml PB. Female animals exhibited a decrease in neurogenic relaxations at d 7 and 30 (H, K). *P < 0.05 compared with control. Samples from female mice at d 30 also show elevated contractions in the presence of carbachol (L) (n = 7–8 animals/group). *P < 0.05 compared with control, #P < 0.05 compared with 90 μg/ml PB.
PB alters the activity of enteric glia and neurons in the myenteric plexus
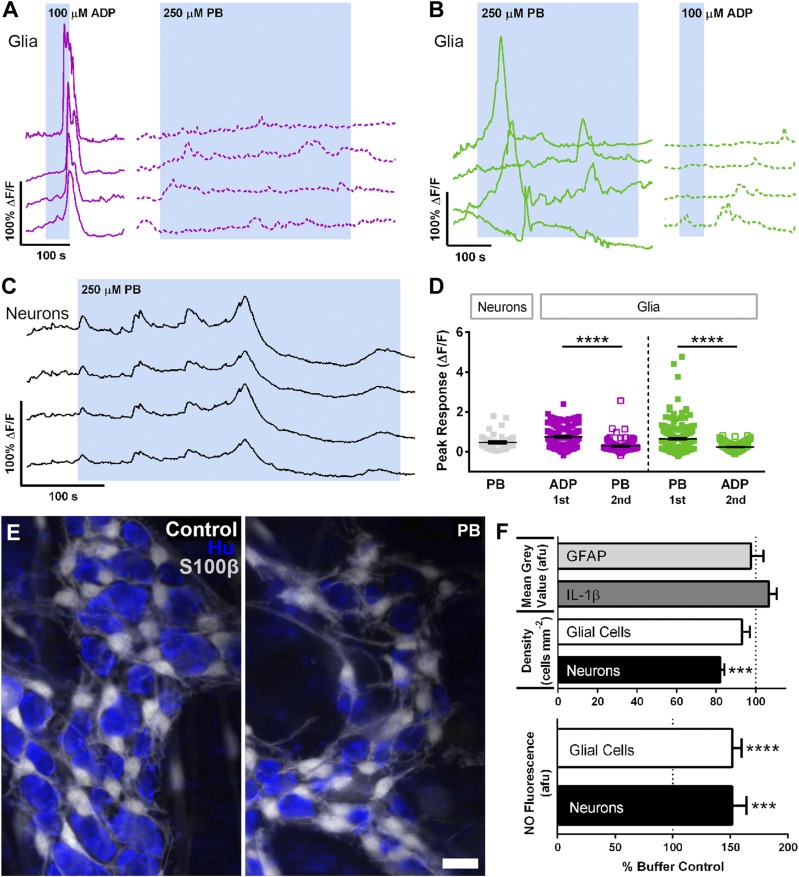
Our muscle tension recordings suggest that PB primarily affects colonic motility through effects on the ENS. ACh is the primary excitatory neurotransmitter in the ENS (14, 32, 33) and activates nicotinic and muscarinic receptors on enteric neurons and glia before being degraded by AChE and recycled. Therefore, we used Ca2+ imaging to understand how PB affects the activity of enteric neurons and glia. We used ADP (100 µM) as a positive control for glial responses (Fig. 4). Directly exposing isolated whole-mount preparations of myenteric plexus to PB (250 µM) drove Ca2+ responses in enteric glia (Fig. 4A, B, D) and neurons (Fig. 4C, D). PB initially drove an increase in neuronal activity, but eventually caused a decrease in activity during the continued presence of PB (Fig. 4C). The magnitude of glial Ca2+ responses driven by PB were comparable to responses driven by the P2Y1 agonist ADP and glial responses to PB and ADP showed cross-desensitization. For example, the application of PB followed by the application of ADP produced glial responses to PB that were 164.7% larger than peak responses driven by ADP. Reversing the drug application order (ADP then PB) produced ADP responses that were 180.9% larger than responses to PB (Fig. 4D). These results show that PB initially increases the activity of enteric neurons and glia but that the desensitization of endogenous transmitter pathways during the continued presence of PB leads to an overall decrease in cellular signaling within the ENS.
Figure 4.
Effects of PB on the activity and survival of enteric neurons and glia. A–C) Representative traces from Ca2+ imaging recordings of enteric glial (A, B) and neuronal (C) activity. Each trace represents an individual glial cell (A, B) and neuron (C). ADP drives large Ca2+ responses in glia and the subsequent application of PB elicits minimal activity (A). However, PB application prior to ADP drives large glial Ca2+ responses and desensitizes subsequent responses to ADP (B). Neurons initially show heightened activity in the presence of PB but then decrease activity as receptor pathways desensitize (C). D) Summary data showing peak glial and neuronal responses to either PB or ADP (n = 160–211 glia, n = 39–46 neurons). ****P < 0.001. E) Representative images of myenteric ganglia showing glia (S100β, grayscale) and neurons (Hu, blue). Scale bar, 20 μm. F) Summary data showing the quantification of ganglionic GFAP, IL-1β, glial and neuronal density, and glial and neuronal NO levels quantified by DAF-FM fluorescence (n = 4 animals). ***P < 0.001, ****P < 0.001 compared with buffer control.
Exposure to PB causes cellular remodeling within the ENS
Altering enteric neurotransmission could drive persistent network dysfunction by altering the survival and/or phenotype of enteric neurons and glia. We tested this possibility by measuring glial reactivity with GFAP expression, analyzing neuron survival with HuC/D labeling, and assessing the enteric neurochemical coding with markers of major excitatory and inhibitory neuron subtypes. Exposing isolated whole-mount preparations of myenteric plexus to PB (250 µM, 4 h) in vitro drove a decrease in enteric neuron survival without affecting glial survival (Fig. 4F). In prior work, we showed that purines drive enteric neuron death through mechanisms that involve an increase in NO production by enteric glia (23). We did not observe significant changes in glial GFAP and IL-1β expression at 4 h (Fig. 4F). However, PB significantly increased the fluorescence of the NO indicator, DAF-FM in both neurons and glial cells (Fig. 4F), suggesting that NO production by enteric glia may contribute to the early neurotoxic effects of PB.
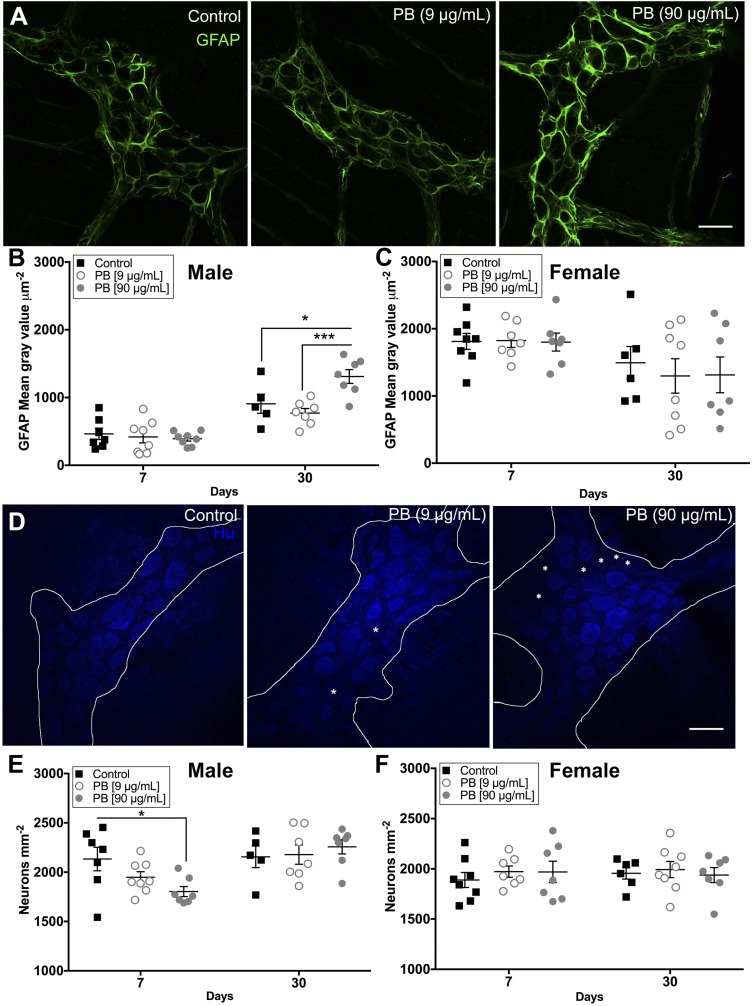
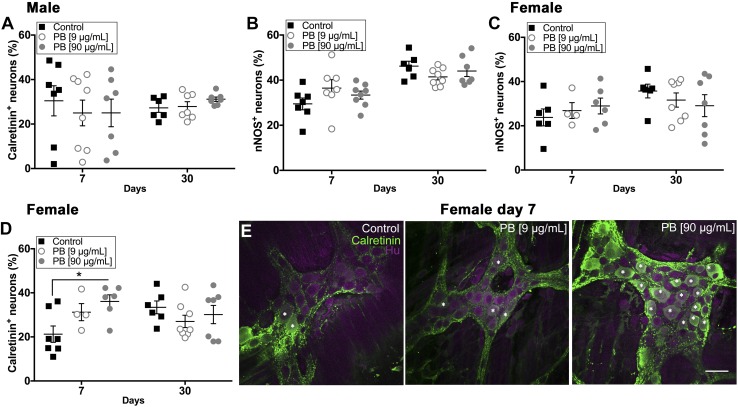
Our in vitro results suggested that PB has the potential to drive neurodegeneration and, possibly, the transition of enteric glia to a reactive state. In agreement, we observed an increase in GFAP expression in the myenteric plexus of the colon of male mice treated with 90 µg/ml PB at 30 d (Fig. 5A, B). These animals also exhibited significant enteric neurodegeneration as early as d 7 immediately following PB treatment (18.3% decrease compared to control, Fig. 5E). Surprisingly, female mice did not exhibit changes in GFAP expression or neuronal density following exposure to PB (Fig. 5C, F). However, female mice exhibited a shift in the proportion of excitatory neurons labeled with calretinin at d 7 (69.8% increase, Fig. 6D), whereas males did not (Fig. 6A). We did not observe differences in the proportion of inhibitory neurons expressing neuronal NO synthase (nNOS) in any group (Fig. 6B, C).
Figure 5.
Exposure to PB drives reactive gliosis and enteric neurodegeneration. A) Representative confocal images of myenteric ganglia showing GFAP expression (GFAP, green) in control and PB-treated male mice at d 30. Scale bar, 30 μm. B, C) Summary data show the quantification of GFAP immunoreactivity in male (B) and female (C) mice. GFAP expression is elevated in males at d 30 with PB (90 μg/ml).*P < 0.05 compared with control (B), ***P < 0.001 compared with PB (9 μg/ml) (but it is not altered in females) (C). D) Representative confocal images of myenteric ganglia labeled with anti-Hu antibodies to identify enteric neurons (Hu, blue) in control and PB-treated male mice at d 7. Scale bar, 30 μm. E, F) Summary data show the quantification of myenteric neuron packing density in male (E) and female (F) mice. Exposure to PB (90 μg/ml) causes neurodegeneration in the ENS at d 7 in male animals (E) (10.1% loss). *P < 0.05 compared to control (but does not affect the survival of enteric neurons in female animals) (F). Asterisk highlights areas lacking Hu immunoreactive neurons (n = 7–8 animals/group).
Figure 6.
PB affects the expression of excitatory enteric neurons in the myenteric plexus. A–D) Data showing the proportion of calretinin (A, D) or nNOS (B, C) immunoreactive enteric neurons in male and female animals following exposure to PB. Exposure to PB did not alter the proportion of calretinin and nNOS neurons in males, but did significantly increase the proportion of calretinin neurons in female animals exposed to PB (90 μg/ml) at d 7 PB (D); 67.7% increased. *P < 0.05 compared with control. E) Representative confocal overlay images show myenteric ganglia labeled with antibodies against the excitatory neuron marker calretinin (calretinin, green) and the pan-neuronal marker Hu (Hu, magenta) in control and PB-treated female mice at d 7. Asterisk indicates cells positive for calretinin and Hu (n = 7–8 animals/group). Scale bar, 30 μm.
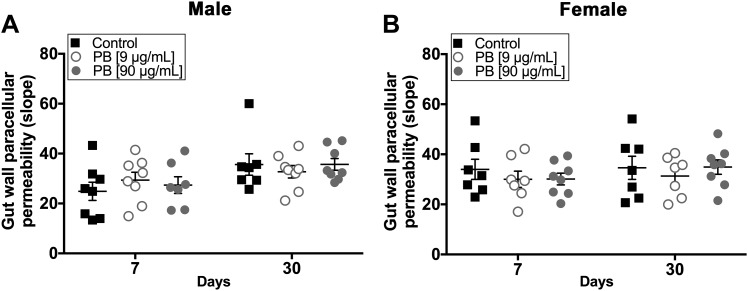
PB does not affect gut permeability
One potential mechanism for enteric and/or central neurologic dysfunction in GWI is that changes in the microbiome cause gut permeability and altered immune functions (34). To address this possibility, we tested paracellular permeability of the colon in Ussing chambers using a cell-impermeant fluorescein-5-(and-6)-sulfonate (478.32 Da) dye. Our data show that exposure to PB did not significantly affect gut permeability in either male or female animals at d 7 and 30 (Fig. 7A, B). These data suggest that altered gut permeability is not a major factor in symptom development following exposure to PB.
Figure 7.
Exposure to PB alone is not sufficient to alter gut permeability. Summary data showing gut paracellular permeability in tissue samples from male (A) and female (B) animals assessed by dye flux in Ussing chambers. Neither dose of PB caused a significant change in gut wall permeability in male (A) or female (B) mice at d 7 or 30 (n = 7–8 animals/group).
Exposure to PB alters immune profiles in the gut and brain
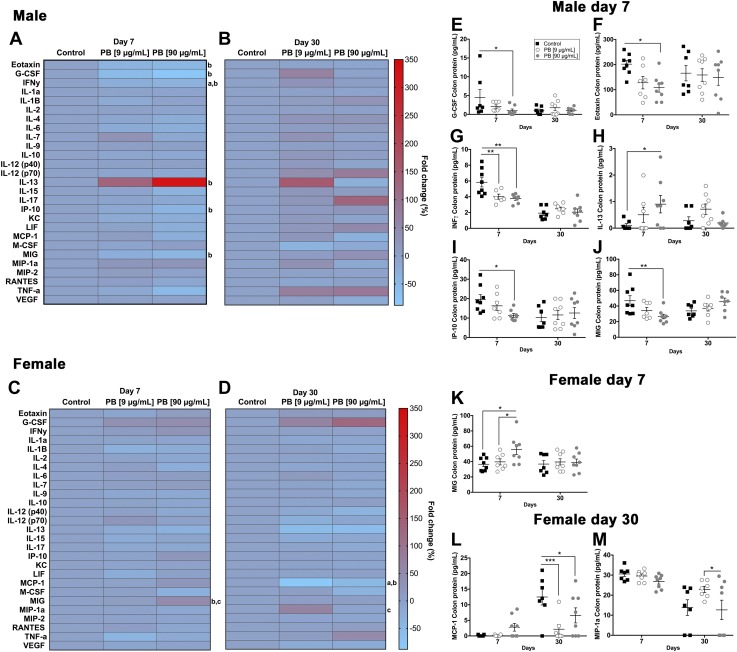
Bidirectional signaling between the immune and nervous systems is mediated, in part, by cholinergic transmission. To understand how alterations to cholinergic signaling during exposure to PB impacts peripheral and central immune responses, we assessed the expression of pro- and anti-inflammatory cytokines and chemokines in the colon and brain of mice exposed to PB. Immediately following the 7-d treatment with PB in the colon, male mice (90 µg/ml group) exhibited a decrease in eotaxin, granulocyte colony stimulation factor (G-CSF), IFN-γ, INF-γ–induced protein 10 (IP-10; also known as CXCL10), and monokine induced by IFN-γ (MIG; also known as CXCL9) and an increase in the expression of IL-13 (Fig. 8A, Table 2). These alterations were not observed at d 30 (Fig. 8B). In contrast, female mice exhibited an increase in the expression of MIG at the acute time point (Fig. 8C), an increase in the expression of macrophage inflammatory protein (MIP)-1a,d and a decrease in monocyte chemoattractant protein (MCP)-1 at 30 d (Fig. 8D). These results show that the main effect of PB on immune responses in the colon is a decrease in the expression of proinflammatory mediators and that this effect is most pronounced in male mice.
Figure 8.
Exposure to PB alters colonic cytokines/chemokines. Data from multiplex cytokine/chemokine arrays showing the effects of exposure to PB on colonic immune responses in male (A, B, E–J) and female (C, D, K–M) mice. Heat maps show the fold change in cytokines and chemokines in males and females at d 7 (A, C) and d 30 (B, D). Significant differences are indicated for control vs. 9 μg/ml PB (a), control vs. 90 μg/ml PB (b), and 9 μg/ml PB vs. 90 μg/ml PB (c). Summary data (right) show significant differentially regulated cytokines and chemokines in males (E–J) and females (K–M). At d 7, male mice (90 µg/ml group) exhibited a decrease in G-CSF. *P < 0.05 compared with control (E), *P < 0.05 compared with control (eotaxin, F), **P < 0.01 compared with control (IFN-γ, G), *P < 0.05 compared with control (IP-10; also known as CXCL10, I), **P < 0.01 compared with control (MIG; also known as CXCL9, J), *P < 0.05 compared with control (increase in the expression of IL-13, H). These alterations were not observed in male mice at d 30 (B). In contrast, female mice exhibited an increase in the expression of MIG at d 7 (C, K), *P < 0.05 compared with PB (9 μg/ml), *P < 0.05 compared with control; a decrease in MCP-1 at d 30 (D, L), ***P < 0.001 (9 μg/ml) vs. control and *P < 0.05 (90 μg/ml) vs. control; and an increase in the expression of MIP-1a at d 30 (M), *P < 0.05 compared with PB (9 μg/ml) (n = 7–8 animals/group).
TABLE 2.
Summary of cytokines/chemokines in the colon affected by PB treatment
| Chemokine/cytokine | Expression | P | Treatment |
|---|---|---|---|
| Male (7 d) | |||
| Eotaxin | Decreased | 0.05 | PB vs. controla |
| G-CSF | Decreased | 0.05 | PB vs. controla |
| IFN-γ | Decreased | 0.01 | PB and PB vs. controlb |
| IL-13 | Increased | 0.05 | PB vs. controla |
| IP-10 | Decreased | 0.05 | PB vs. controla |
| MIG | Decreased | 0.05 | PB vs. controla |
| Female (7 d) | |||
| MIG | Increased | 0.05 | PB vs. control and PBc |
| Female (30 d) | |||
| MCP-1 | Decreased | 0.05; 0.001 | PB and PB vs. controlb |
| MIP-1a | Increased | 0.05 | PB vs. PBd |
PB (90 μg/ml) vs. control; bPB (90 μg/ml) and PB (9 μg/ml) vs. control; cPB (90 μg/ml) vs. control and PB (9 μg/ml); dPB (90 μg/ml) vs. PB (9 μg/ml).
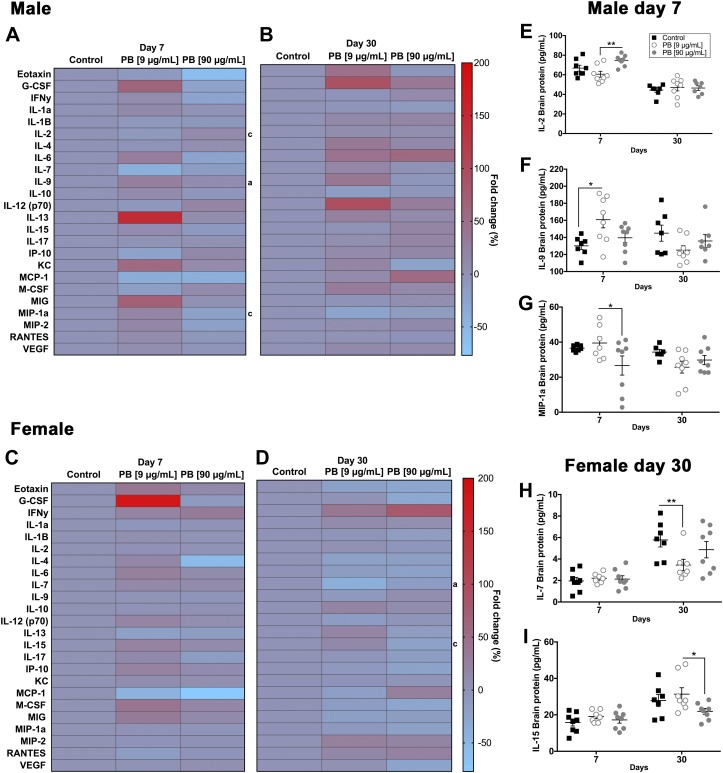
In the brain, exposure to PB (90 µg/ml dose) caused a decrease in the expression of MIP-1a and increased the expression of IL-2 and IL-9 in male mice at d 7 (Fig. 9A, Table 3). In contrast, female mice exhibited a decrease in IL-7 and IL-15 at 30 d (Fig. 9D). No significant differences were observed in the brain immune profiles of male mice at d 30 (Fig. 9B) and female mice at d 7 (Fig. 9C). Together, these data show that the disruption of proinflammatory cytokines and chemokines in the brains of male mice occurs acutely and that changes to immune responses in the female brain evolve more slowly and involve different mediators.
Figure 9.
Exposure to PB alters brain cytokines/chemokines. Data from multiplex cytokine/chemokine arrays showing the effects of exposure to PB on brain immune responses in male (A, B, E–G) and female (C, D, H, I) mice. Heat maps show the fold change in cytokines and chemokines in males and females at d 7 (A, C) and d 30 (B, D). Significant differences are indicated for control vs. 9 μg/ml PB (a), control vs. 90 μg/ml PB (b), and 9 μg/ml PB vs. 90 μg/ml PB (c). Summary data (right) show significant differentially regulated cytokines and chemokines in males (E–G) and females (H, I). Exposure to 90 µg/ml PB causes an increase in the expression of: IL-2, **P < 0.01 compared with PB (9 μg/ml) (E); and IL-9, *P < 0.05 compared with control (F); and a decrease in the expression of MIP-1a, *P < 0.05 compared with PB (9 μg/ml) in male mice at d 7 (G). In contrast, female mice exhibited a decrease in: IL-7, **P < 0.01 compared with control (H); and IL-15, **P < 0.05 compared with PB (9 μg/ml) at 30 d (I). No significant differences were observed in the brain immune profiles of male mice at d 30 (B) and female mice at d 7 (C) (n = 7–8 animals/group).
TABLE 3.
Summary of cytokines/chemokines in the brain affected by PB treatment
| Chemokine/cytokine | Expression | P | Treatment |
|---|---|---|---|
| Male (7 d) | |||
| IL-2 | Increased | 0.01 | PB vs. PBa |
| IL-9 | Increased | 0.05 | PB vs. controlb |
| MIP-1a | Decreased | 0.05 | PB vs. PBa |
| Female (30 d) | |||
| IL-7 | Decreased | 0.01 | PB vs. controlb |
| IL-15 | Decreased | 0.05 | PB vs. PBa |
PB (90 μg/ml) vs. PB (9 μg/ml); bPB (9 μg/ml) vs. control.
DISCUSSION
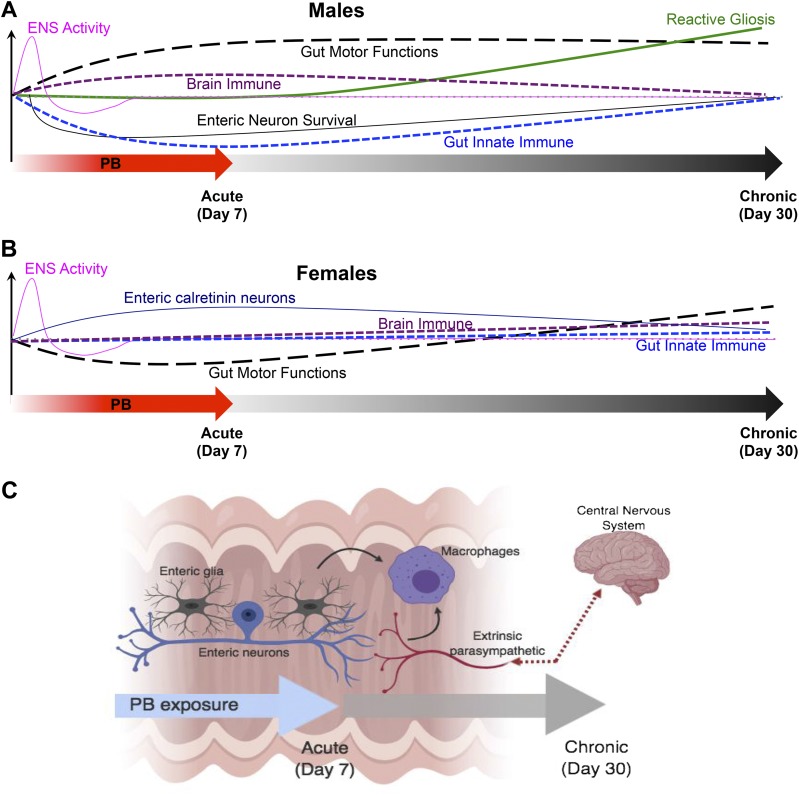
The spectrum of chronic medically unexplained symptoms involved in GWI suggests that multiple mechanisms contribute to the pathogenesis and presentation of the condition (1, 2). Although no unifying hypothesis has emerged to explain the various symptoms, at least some of these are thought to arise because of the inadvertent effects of exposure to the antinerve gas drug PB (7). Strong links between exposure to this drug and the subsequent development of GWI led the U.S. Congress to conclude that there is a clear link between PB and GWI (1, 8), but mechanistic knowledge to explain this link is limited. Here, we used a mouse model that replicates the PB doses to which humans were exposed to study mechanisms that link PB exposure with the development of GWI. We specifically focused on the GI tract because functional GI issues are common and debilitating symptoms of GWI, and the intestine is a key site of systemic immune regulation and health. Our results show that exposure to PB has interesting effects on the neural and immune systems of the GI tract. Namely, our results suggest that exposure to PB disrupts the neural control of gut functions by altering the activity and survival of enteric neurons, driving glial reactivity, and that PB-driven alterations to the immune system involve the suppression of innate immunity (Fig. 10). Additionally, the effects of PB are sex-dependent, and the mechanisms affected differ in male and female animals.
Figure 10.
Model showing the temporal effects of PB on gut functions, the ENS, and gut and brain immune profiles in male and female animals. A, B) Graphic summaries of the main effects of exposure to PB are shown for males (A) and for females (B). C) Model showing the main cellular mechanisms affected by the 7-d exposure to PB. PB alters the coordination of gut reflexes by altering the functional activity of enteric neurons and glia and by driving enteric neuroinflammation. PB also disrupts the cholinergic regulation of innate immunity in the intestine, likely through effects on macrophages that involve enteric and vagal neural pathways.
Functional GI disorders are among the most common symptoms of GWI. Our results show that exposure to PB alone, in the absence of other toxic agents, is sufficient to drive similar effects in mice. The coordination of gut reflexes relies heavily on cholinergic neurotransmission in the ENS. ACh is the main excitatory neurotransmitter in the ENS (14, 32, 33), and, under normal conditions, ACh briefly activates nicotinic and muscarinic Ach receptors on postsynaptic neurons before being degraded by AChE and recycled. This tight control of excitatory transmission is imperative for the maintenance of synaptic transmission and the coordination of polarized reflexes in the intestine. Indeed, acutely blocking ACh degradation with PB drives large, spontaneous contractions in the isolated guinea pig ileum (14). Our motility data show that blocking AChE activity in vivo for 7 d disrupts colonic motility immediately following the treatment (d 7) and has a lasting effect on colonic motility that persists at least until d 30 in both male and female mice. In male mice, PB treatment increased colonic motility and the percentage of fecal fluid at specific peak points, which is likely a consequence of rapid transit in the colon. In contrast, exposure to PB had a biphasic effect on colon motor functions in female mice, and these animals exhibited a decrease in colonic motility at d 7 and an increase in colonic contractility at d 30. Female mice also exhibited a lower percentage of fecal fluid content after PB treatment (d 15 and 30), suggesting that the reduction in colonic contractility allows more time to absorb the fluids. In GW veterans, the most common GI symptoms are diarrhea and increased peristalsis (35). These symptoms align well with our findings of colonic changes and suggest that mechanisms affected could be similar in mice and humans.
The cellular mechanisms underlying the effects of PB on colon motility include the acute activation of cellular signaling, a subsequent dampening as these pathways desensitize, and the development of neuroinflammation. Effects on the nervous system appear to be the main contributing factor to dysmotility in mice exposed to PB. However, alterations to cholinergic signaling in interstitial cells of Cajal and/or smooth muscle cells could contribute because the responsiveness to carbachol was altered in female mice at 30 d. In support, isolated smooth muscle strips of guinea pig ileum exhibit nonspecific desensitization in the continued presence of ACh (15). Similar mechanisms likely underlie the effects of PB on activity within the ENS in which an increased in availability of ACh can cause receptor desensitization. Our Ca2+ imaging data show that PB causes an acute increase in the activity of neurons and glia in the myenteric plexus followed by an overall decrease in activity. The acute increase in activation is likely driven by the activation of neuronal nicotinic receptors (36, 37) and glial muscarinic receptors (38), and desensitization of these receptor pathways leads to a later decrease in responsiveness. Our prior data show that glia express muscarinic ACh receptors that couple to Gq signal transduction pathways that are shared by purinergic receptor pathways (25, 38). Interestingly, glial responses to PB and ADP exhibited cross-desensitization, suggesting that the desensitization of cholinergic receptors could lead to an overall dampening of cellular responsiveness to other neuroligands that signal through shared intracellular signal transduction pathways. Given that glial activity encoded by Ca2+ responses contributes to the regulation of gut motility (24, 38, 39), it is possible that the disruption of glial activity by PB contributes to dysmotility in GWI.
Our data show that PB promotes the development of neuroinflammation in the ENS. Enteric neuroinflammation involves the development of reactive gliosis and neurodegeneration (40), and our data show that both of these outcomes are present in tissues treated with PB and in animals exposed to PB. Bathing isolated tissue preparations of myenteric plexus with PB for 4 h was sufficient to drive neurodegeneration to a similar extent as we have observed with neurotoxic purines acting through P2X7 receptor pathways (23, 25). Likewise, significantly less neuronal density was apparent at the end of the PB treatment period in vivo. Interestingly, enteric neuron density normalized by 30 d, which could suggest that these conditions promote the neuronal differentiation of Sox2 positive precursor cells that contribute to neurogenesis following colitis (41).
At least some of the mechanisms involved in PB-driven enteric neurodegeneration are shared with P2X7 receptor–driven neurodegeneration because both involve an increase in glial NO production (23). The increase in glial NO production is an early danger response that modifies the release of gliotransmitters, such as ATP through connexin-43–dependent mechanisms. These effects take place before significant gliosis is apparent as reflected by changes in glial GFAP expression. In agreement, we did not observe significant changes in GFAP during the acute application of PB in vitro, but changes in GFAP expression did emerge during the longer in vivo paradigm in male animals. GFAP is a classic characteristic of reactive gliosis (42–45) that likely reflects glial remodeling in an effort to protect neuronal networks from damage (46). However, increased glial GFAP is also linked with the production of danger cues that are responsible for driving enteric neuron death (23). The fact that glial GFAP was significantly elevated at 30 d but not immediately following the PB treatment at 7 d, strongly suggests that neuroinflammatory processes driving reactive gliosis continue to evolve despite the removal of the drug.
Some of the slowly evolving effects driven by PB involve alterations to the innate immune system of the intestine. Immediately following the PB treatment, male mice exhibited a decrease in proinflammatory cytokines and chemokines, including eotaxin, G-CSF, IFN-γ, IP-10, and MIG, and an increase in the anti-inflammatory cytokine IL-13. This profile suggests a significant suppression of innate immunity acutely following exposure to PB, but the suppression of innate immunity was transient and did not persist up to d 30. In contrast, female animals only exhibited a significant elevation of MIG immediately following PB treatment and significant alterations in MCP-1 and MIP-1α appeared at 30 d. These data suggest that the effects on the female immune system driven by PB may evolve more slowly than those in males and involve differing signals, although both seem to involve innate immune pathways.
The most likely explanation for the immunosuppressive actions of PB in the intestine is that PB enhances the cholinergic regulation of intestinal immunity by the vagus. Bidirectional neuroimmune communication is now widely recognized as an important mechanism for the regulation of intestinal immunity, and the vagus is considered the primary neural regulator of anti-inflammatory tone (47). In support, activation of the vagus using nicotinic receptor agonists or AChE inhibitors reduces inflammation by suppressing cytokine synthesis (12, 17, 21, 48). ACh released from vagal fibers or other cells in response to vagal stimulation (49) suppresses innate immune mechanisms by activating alpha7 nicotinic receptors expressed by macrophages and other innate immune cells to inhibit cytokine production (17). In the gut, vagal neuroimmune modulation is likely indirectly mediated through the ENS because vagal nerves synapse within the ENS, but do not directly contact gut immune cells (49). The effect of ACh is rather specific for anti-inflammatory pathways in macrophages because the activation of alpha7 nicotinic receptors expressed by human and mouse macrophages in vitro and in vivo suppresses the production of proinflammatory cytokines, such as TNF-α and IL-1β, without affecting the production of anti-inflammatory cytokines (12, 20, 50–52). Despite the expression of components necessary for a functional cholinergic system in other immune cells, such as T cells, B cells, dendritic cells, mast cells, neutrophils, and NK cells, the effect of cholinergic innervation on macrophages seems most pronounced. In support, our data show that the main differentially regulated cytokines and chemokines are proinflammatory mediators secreted by macrophages (G-CSF, IFN-γ, IP-10, MIG, MIP-1a) and anti-inflammatory proteins involved in the inhibition of macrophage and monocyte cytokine production (IL-13) (53–60).
PB also drove significant changes in the profile of cytokines and chemokines expressed in the brain. Similar to the effects in the colon, the most significant effects in the male brain were observed immediately following PB treatment and those in the female brain only emerged at 30 d. However, the profiles of cytokines altered by PB in the brain differ from those in the gut and suggest the contribution of differing immune mechanisms. For example, the most prominent effect of PB during the acute phase in male mice in the intestine was a suppression of innate immune mediators, whereas in the brain the most prominent effect was an increase in proinflammatory proteins, such as IL-2 and IL-9, that are mainly produced by T lymphocytes (61, 62). Likewise, innate immune mediators were altered in the colon of female mice at 30 d while IL-7 and IL-15 were the main proteins altered in the brain. IL-7 is expressed by neurons and is important for lymphocyte development and survival (63, 64), whereas IL-15 is expressed by diverse cell types including mast cells, monocytes, and macrophages (65). These data suggest that exposure to PB drives an acute proinflammatory environment within the male brain that involves T lymphocytes and a slower developing proinflammatory environment within the female brain that potentially also involves T lymphocytes and mast cells or monocytes.
One possible explanation for the increase in proinflammatory cytokines in the brain is that PB disrupted intestinal barrier function and the subsequent invasion of pathogens drove systemic immune responses. This possibility is unlikely because we did not detect any change in colonic permeability in our Ussing chamber experiments. In contrast, prior work using a mouse model of GWI in mice driven by a combination of PB, the insecticide permethrin, and the stress hormone corticosterone observed alterations in the gut microbiome and compromised intestinal barrier function (34). Stress alone has been associated with an increase in gut wall permeability (66), which could explain the difference in barrier function between these 2 models. Our results indicate that PB treatment alone is not sufficient to disrupt the gut barrier. Therefore, more plausible explanations for the alterations to brain immune responses include the activation of vagal afferent fibers and the effects of circulating cytokine and chemokines originating in the gut.
An important outcome of our study is the identification of strong sex differences in the effects of PB on gut functions, gut immunity, and brain immunity. Female animals are resistant to many of the most prominent effects of PB in male animals and did not exhibit signs of neuroinflammation in the myenteric plexus, such as changes in GFAP expression or neuron survival. Some of the observed effects in female animals, such as the increase in the percentage of calretinin-positive neurons, might underlie the resistance of females to neurodegeneration. Calretinin is involved in mechanisms that protect against cell death, so it is possible that the up-regulation of calretinin in the female ENS is neuroprotective (67). However, female animals are not entirely resistant to the effects of PB and do exhibit distinct alterations in motility and altered immune responses, albeit to a lesser extent than in male animals. Differences in intestinal motility between men and women are well known, and women tend to defecate less often (68, 69). Our motility data agree with similar effects in mice. One possible explanation for the difference in motility could relate to hormonal changes between sexes, but several studies have confirmed that GI transit is not systematically different during the phases of the menstrual/estrous cycle in humans or rodents (69, 70). An alternate explanation could lie in sex differences between the expression, function, and modulation of cholinergic receptors. For example, the intrathecal injection of selective nicotinic receptor agonists in rats produces significantly greater antinociception in females than in males (71). In addition, the female sex hormones progesterone and E2 act as functional blockers of several nicotinic receptor subtypes (72, 73). These data support the concept that PB treatment disrupts colonic motility by exacerbating these differences in nicotinic receptor function between males and females. Similar effects might also account for the more limited and slower evolving immune responses in female animals.
Together, our results show that exposing mice to a treatment of PB that replicates what was experienced by soldiers in the GW is sufficient to drive major changes to the neural control of GI functions, gut immunity, and immune responses in the brain. Acute and chronic changes in gut motility involve the activation and desensitization of endogenous signaling mechanisms and the development of neuroinflammation. PB also alters the immune system and dampens innate immunity in the intestine. The GI effects likely cause dysfunction in the gut-brain axis, and inflammation in the brain could contribute to lasting neurologic problems. Importantly, the effects of PB are sex-dependent, and PB has a more pronounced acute effect in males but a more pronounced chronic effect in females. Considering that women comprise almost 7% of the nearly 700,000 military personnel who served in the GW and that GWI is more common in female GW veterans than their male counterparts (74–76), understanding the sex-specific mechanisms that underlie the disorder may yield important insights that lead to novel therapies.
ACKNOWLEDGMENTS
This project was supported by grants from the Department of Defense–Congressionally Directed Medical Research Programs (DOD - CDMRP; W81XWH1610631, to B.D.G.) and the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK103723, to B.D.G.). The authors declare no conflicts of interest.
Glossary
- ACh
acetylcholine
- AChE
acetylcholinesterase
- DAF-FM
4-amino-5-methylamino-2′,7′-difluorofluorescein
- EFS
electrical field stimulation
- ENS
enteric nervous system
- G-CSF
granulocyte colony stimulation factor
- GFAP
glial fibrillary acidic protein
- GI
gastrointestinal
- GW
Gulf War
- GWI
Gulf War illness
- IP-10
INF-γ–induced protein 10
- LMMP
longitudinal muscle myenteric plexus
- MCP
monocyte chemoattractant protein
- MIG
monokine induced by IFN-γ
- MIP
macrophage inflammatory protein
- nNOS
neuronal NO synthase
- PB
pyridostigmine bromide
AUTHOR CONTRIBUTIONS
S. Hernandez designed and performed the experiments; D. E. Fried conducted contractility experiments; V. Grubišić performed Ussing experiments; J. L. McClain conducted neurodegeneration and NO experiments; B. D. Gulbransen was responsible for the overall project conception and supervision; and the manuscript was written by S. Hernandez and B. D. Gulbransen, with all authors contributing to the final edited version.
REFERENCES
- 1.Binns J., Barlow C., Bloom F., Clauw D., Golomb B., Graves J., Hardie A., Knox M., Meggs W., Nettleman M., OCallaghan J., Smithson S., Steele L., White R.; Research Advisory Committee on Gulf War Veterans’ Illnesses (2008) Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations, U.S. Government Printing Office, Washington, DC [Google Scholar]
- 2.Zhou Q., Verne M. L., Zhang B., Verne G. N. (2018) Evidence for somatic hypersensitivity in veterans with Gulf war illness and gastrointestinal symptoms. Clin. J. Pain 34, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele L. (2000) Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 152, 992–1002 [DOI] [PubMed] [Google Scholar]
- 4.Dunphy R. C., Bridgewater L., Price D. D., Robinson M. E., Zeilman C. J., III, Verne G. N. (2003) Visceral and cutaneous hypersensitivity in Persian Gulf war veterans with chronic gastrointestinal symptoms. Pain 102, 79–85 [DOI] [PubMed] [Google Scholar]
- 5.Murphy F. M., Kang H., Dalager N. A., Lee K. Y., Allen R. E., Mather S. H., Kizer K. W. (1999) The health status of Gulf war veterans: lessons learned from the department of veterans affairs health registry. Mil. Med. 164, 327–331 [PubMed] [Google Scholar]
- 6.Sostek M. B., Jackson S., Linevsky J. K., Schimmel E. M., Fincke B. G. (1996) High prevalence of chronic gastrointestinal symptoms in a National Guard Unit of Persian Gulf veterans. Am. J. Gastroenterol. 91, 2494–2497 [PubMed] [Google Scholar]
- 7.White R. F., Steele L., O’Callaghan J. P., Sullivan K., Binns J. H., Golomb B. A., Bloom F. E., Bunker J. A., Crawford F., Graves J. C., Hardie A., Klimas N., Knox M., Meggs W. J., Melling J., Philbert M. A., Grashow R. (2016) Recent research on Gulf war illness and other health problems in veterans of the 1991 Gulf war: effects of toxicant exposures during deployment. Cortex 74, 449–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golomb B. A. (2008) Acetylcholinesterase inhibitors and Gulf war illnesses. Proc. Natl. Acad. Sci. USA 105, 4295–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumm W. R., Reppert E. J., Jurich A. P., Bollman S. R., Webb F. J., Castelo C. S., Stever J. C., Kaufman M., Deng L.-Y., Krehbiel M., Owens B. L., Hall C. A., Brown B. F. C., Lash J. F., Fink C. J., Crow J. R., Bonjour G. N. (2002) Pyridostigmine bromide and the long-term subjective health status of a sample of over 700 male reserve component Gulf war era veterans. Psychol. Rep. 90, 707–721 [DOI] [PubMed] [Google Scholar]
- 10.Loewenstein-Lichtenstein Y., Schwarz M., Glick D., Nørgaard-Pedersen B., Zakut H., Soreq H. (1995) Genetic predisposition to adverse consequences of anti-cholinesterases in ‘atypical’ BCHE carriers. Nat. Med. 1, 1082–1085 [DOI] [PubMed] [Google Scholar]
- 11.Fujii T., Mashimo M., Moriwaki Y., Misawa H., Ono S., Horiguchi K., Kawashima K. (2017) Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 134, 1–21 [DOI] [PubMed] [Google Scholar]
- 12.Pavlov V. A., Tracey K. J. (2005) The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 19, 493–499 [DOI] [PubMed] [Google Scholar]
- 13.Furness J. B. (2008) The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol. Motil. 20 (s1, Suppl 1)32–38 [DOI] [PubMed] [Google Scholar]
- 14.Gioia A., Morpurgo C. (1958) Effect of inhibitors of choline acetylation on acetylcholine output and motility in response to anticholinesterases and to distension of the lumen of isolated guinea-pig ileum. Br. J. Pharmacol. Chemother. 13, 467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joiner P. D. (1973) Studies on the loss of acetylcholine sensitivity in ileal muscle. J. Pharmacol. Exp. Ther. 186, 552–561 [PubMed] [Google Scholar]
- 16.De Giorgio R., Bianco F., Latorre R., Caio G., Clavenzani P., Bonora E. (2016) Enteric neuropathies: yesterday, today and tomorrow. Adv. Exp. Med. Biol. 891, 123–133 [DOI] [PubMed] [Google Scholar]
- 17.Wang D.-W., Zhou R.-B., Yao Y.-M. (2009) Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin. J. Traumatol. 12, 355–364 [PubMed] [Google Scholar]
- 18.Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., Tracey K. J. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E. J., Tracey K. J., Ulloa L. (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 [DOI] [PubMed] [Google Scholar]
- 21.Tracey K. J. (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caligioni C. S. (2009) Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown I. A M., McClain J. L., Watson R. E., Patel B. A., Gulbransen B. D. (2016) Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2, 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClain J. L., Fried D. E., Gulbransen B. D. (2015) Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol. 1, 631–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulbransen B. D., Bashashati M., Hirota S. A., Gui X., Roberts J. A., MacDonald J. A., Muruve D. A., McKay D. M., Beck P. L., Mawe G. M., Thompson R. J., Sharkey K. A. (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 18, 600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.France M., Bhattarai Y., Galligan J. J., Xu H. (2012) Impaired propulsive motility in the distal but not proximal colon of BK channel β1-subunit knockout mice. Neurogastroenterol. Motil. 24, e450–e459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Martínez V., Kimura H., Taché Y. (2007) 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G419–G428 [DOI] [PubMed] [Google Scholar]
- 28.Nasser Y., Fernandez E., Keenan C. M., Ho W., Oland L. D., Tibbles L. A., Schemann M., MacNaughton W. K., Rühl A., Sharkey K. A. (2006) Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G912–G927 [DOI] [PubMed] [Google Scholar]
- 29.Fried D. E., Watson R. E., Robson S. C., Gulbransen B. D. (2017) Ammonia modifies enteric neuromuscular transmission through glial γ-aminobutyric acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G570–G580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubišić V., Gulbransen B. D. (2017) Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J. Physiol. 595, 3409–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogden C. L., Fryar C. D., Carroll M. D., Flegal K. M. (2004) Mean body weight, height, and body mass index, United States 1960-2002. Adv. Data 347, 1–17 [PubMed] [Google Scholar]
- 32.McConalogue K., Furness J. B. (1994) Gastrointestinal neurotransmitters. Baillieres Clin. Endocrinol. Metab. 8, 51–76 [DOI] [PubMed] [Google Scholar]
- 33.Furness J. B. (2012) The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 [DOI] [PubMed] [Google Scholar]
- 34.Alhasson F., Das S., Seth R., Dattaroy D., Chandrashekaran V., Ryan C. N., Chan L. S., Testerman T., Burch J., Hofseth L. J., Horner R., Nagarkatti M., Nagarkatti P., Lasley S. M., Chatterjee S. (2017) Altered gut microbiome in a mouse model of Gulf war illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One 12, e0172914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine (US) Committee on Health Effects Associated with Exposures During the Gulf War (2000) Gulf War and Health: Volume 1. Depleted Uranium, Sarin, Pyridostigmine Bromide, Vaccines (Fulco C. E., Liverman C. T., and Sox H. C., eds.), National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 36.Costantini T. W., Krzyzaniak M., Cheadle G. A., Putnam J. G., Hageny A.-M., Lopez N., Eliceiri B. P., Bansal V., Coimbra R. (2012) Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am. J. Pathol. 181, 478–486 [DOI] [PubMed] [Google Scholar]
- 37.Lalo U., Pankratov Y., Krishtal O., North R. A. (2004) Methyllycaconitine, alpha-bungarotoxin and (+)-tubocurarine block fast ATP-gated currents in rat dorsal root ganglion cells. Br. J. Pharmacol. 142, 1227–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delvalle N. M., Fried D. E., Rivera-Lopez G., Gaudette L., Gulbransen B. D. (2018) Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G473–G483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClain J., Grubišić V., Fried D., Gomez-Suarez R. A., Leinninger G. M., Sévigny J., Parpura V., Gulbransen B. D. (2014) Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brierley S. M., Linden D. R. (2014) Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611–627 [DOI] [PubMed] [Google Scholar]
- 41.Belkind-Gerson J., Graham H. K., Reynolds J., Hotta R., Nagy N., Cheng L., Kamionek M., Shi H. N., Aherne C. M., Goldstein A. M. (2017) Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 7, 2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradley J. S., Jr., Parr E. J., Sharkey K. A. (1997) Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 289, 455–461 [DOI] [PubMed] [Google Scholar]
- 43.Gabella G. (1984) Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J. Neurocytol. 13, 73–84 [DOI] [PubMed] [Google Scholar]
- 44.Thacker M., Rivera L. R., Cho H.-J., Furness J. B. (2011) The relationship between glial distortion and neuronal changes following intestinal ischemia and reperfusion. Neurogastroenterol. Motil. 23, e500–e509 [DOI] [PubMed] [Google Scholar]
- 45.Von Boyen G. B. T., Steinkamp M., Reinshagen M., Schäfer K.-H., Adler G., Kirsch J. (2004) Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burda J. E., Sofroniew M. V. (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razani-Boroujerdi S., Behl M., Hahn F. F., Pena-Philippides J. C., Hutt J., Sopori M. L. (2008) Role of muscarinic receptors in the regulation of immune and inflammatory responses. J. Neuroimmunol. 194, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goverse G., Stakenborg M., Matteoli G. (2016) The intestinal cholinergic anti-inflammatory pathway. J. Physiol. 594, 5771–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Browning K. N., Verheijden S., Boeckxstaens G. E. (2017) The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 152, 730–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zdanowski R., Krzyżowska M., Ujazdowska D., Lewicka A., Lewicki S. (2015) Role of α7 nicotinic receptor in the immune system and intracellular signaling pathways. Cent. Eur. J. Immunol. 40, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nouri-Shirazi M., Tinajero R., Guinet E. (2007) Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs). Immunol. Lett. 109, 155–164 [DOI] [PubMed] [Google Scholar]
- 52.Báez-Pagán C. A., Delgado-Vélez M., Lasalde-Dominicci J. A. (2015) Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J. Neuroimmune Pharmacol. 10, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arango Duque G., Descoteaux A. (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris K. T., Khan H., Ahmad A., Weston L. L., Nofchissey R. A., Pinchuk I. V., Beswick E. J. (2014) G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br. J. Cancer 110, 1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosmidis C., Sapalidis K., Koletsa T., Kosmidou M., Efthimiadis C., Anthimidis G., Varsamis N., Michalopoulos N., Koulouris C., Atmatzidis S., Liavas L., Strati T.-M., Koimtzis G., Tsakalidis A., Mantalovas S., Zarampouka K., Florou M., Giannakidis D. E., Georgakoudi E., Baka S., Zarogoulidis P., Man Y.-G., Kesisoglou I. (2018) Interferon-γ and colorectal cancer: an up-to date. J. Cancer 9, 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu M., Guo S., Hibbert J. M., Jain V., Singh N., Wilson N. O., Stiles J. K. (2011) CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 22, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenblum J. M., Shimoda N., Schenk A. D., Zhang H., Kish D. D., Keslar K., Farber J. M., Fairchild R. L. (2010) CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors. J. Immunol. 184, 3450–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berthoud T. K., Dunachie S. J., Todryk S., Hill A. V., Fletcher H. A. (2009) MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J. Immunol. Methods 340, 33–41; erratum: 368, 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hancock A., Armstrong L., Gama R., Millar A. (1998) Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am. J. Respir. Cell Mol. Biol. 18, 60–65 [DOI] [PubMed] [Google Scholar]
- 60.O’Grady N. P., Tropea M., Preas H. L., II, Reda D., Vandivier R. W., Banks S. M., Suffredini A. F. (1999) Detection of macrophage inflammatory protein (MIP)-1alpha and MIP-1beta during experimental endotoxemia and human sepsis. J. Infect. Dis. 179, 136–141 [DOI] [PubMed] [Google Scholar]
- 61.Goswami R., Kaplan M. H. (2011) A brief history of IL-9. J. Immunol. 186, 3283–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Cancer Institute - NIH (2018) Interleukin 2, NCI Dict. Cancer Terms, Bethesda, MD, USA [Google Scholar]
- 63.Hara T., Shitara S., Imai K., Miyachi H., Kitano S., Yao H., Tani-ichi S., Ikuta K. (2012) Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J. Immunol. 189, 1577–1584 [DOI] [PubMed] [Google Scholar]
- 64.Michaelson M. D., Mehler M. F., Xu H., Gross R. E., Kessler J. A. (1996) Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev. Biol. 179, 251–263 [DOI] [PubMed] [Google Scholar]
- 65.Fehniger T. A., Caligiuri M. A. (2001) Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 [DOI] [PubMed] [Google Scholar]
- 66.Söderholm J. D., Yates D. A., Gareau M. G., Yang P.-C., MacQueen G., Perdue M. H. (2002) Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G1257–G1263 [DOI] [PubMed] [Google Scholar]
- 67.Camp A. J., Wijesinghe R. (2009) Calretinin: modulator of neuronal excitability. Int. J. Biochem. Cell Biol. 41, 2118–2121 [DOI] [PubMed] [Google Scholar]
- 68.Heaton K. W., Radvan J., Cripps H., Mountford R. A., Braddon F. E., Hughes A. O. (1992) Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 33, 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Degen L. P., Phillips S. F. (1996) Variability of gastrointestinal transit in healthy women and men. Gut 39, 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matos J. F., Americo M. F., Sinzato Y. K., Volpato G. T., Corá L. A., Calabresi M. F. F., Oliveira R. B., Damasceno D. C., Miranda J. R. A. (2016) Role of sex hormones in gastrointestinal motility in pregnant and non-pregnant rats. World J. Gastroenterol. 22, 5761–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiari A., Tobin J. R., Pan H. L., Hood D. D., Eisenach J. C. (1999) Sex differences in cholinergic analgesia I: a supplemental nicotinic mechanism in normal females. Anesthesiology 91, 1447–1454 [DOI] [PubMed] [Google Scholar]
- 72.Shen J. X., Yakel J. L. (2009) Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 30, 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freitas K., Carroll F. I., Damaj M. I. (2013) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J. Pharmacol. Exp. Ther. 344, 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolfe J., Proctor S. P., Erickson D. J., Hu H. (2002) Risk factors for multisymptom illness in US Army veterans of the Gulf war. J. Occup. Environ. Med. 44, 271–281 [DOI] [PubMed] [Google Scholar]
- 75.Coughlin S. S., McNeil R. B., Provenzale D. T., Dursa E. K., Thomas C. M. (2013) Method issues in epidemiological studies of medically unexplained symptom-based conditions in veterans. J. Mil. Veterans Health 21, 4–10 [PMC free article] [PubMed] [Google Scholar]
- 76.Coughlin S. S., Krengel M., Sullivan K., Pierce P. F., Heboyan V., Wilson L. C. C. (2017) A review of epidemiologic studies of the health of Gulf war women veterans. J. Environ. Heal. Sci. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]