Abstract
Background
Stroke is a major cause of long‐term disability in adults. Several systematic reviews have shown that a higher intensity of training can lead to better functional outcomes after stroke. Currently, the resources in inpatient settings are not always sufficient and innovative methods are necessary to meet these recommendations without increasing healthcare costs. A resource efficient method to augment intensity of training could be to involve caregivers in exercise training. A caregiver‐mediated exercise programme has the potential to improve outcomes in terms of body function, activities, and participation in people with stroke. In addition, caregivers are more actively involved in the rehabilitation process, which may increase feelings of empowerment with reduced levels of caregiver burden and could facilitate the transition from rehabilitation facility (in hospital, rehabilitation centre, or nursing home) to home setting. As a consequence, length of stay might be reduced and early supported discharge could be enhanced.
Objectives
To determine if caregiver‐mediated exercises (CME) improve functional ability and health‐related quality of life in people with stroke, and to determine the effect on caregiver burden.
Search methods
We searched the Cochrane Stroke Group Trials Register (October 2015), CENTRAL (the Cochrane Library, 2015, Issue 10), MEDLINE (1946 to October 2015), Embase (1980 to December 2015), CINAHL (1982 to December 2015), SPORTDiscus (1985 to December 2015), three additional databases (two in October 2015, one in December 2015), and six additional trial registers (October 2015). We also screened reference lists of relevant publications and contacted authors in the field.
Selection criteria
Randomised controlled trials comparing CME to usual care, no intervention, or another intervention as long as it was not caregiver‐mediated, aimed at improving motor function in people who have had a stroke.
Data collection and analysis
Two review authors independently selected trials. One review author extracted data, and assessed quality and risk of bias, and a second review author cross‐checked these data and assessed quality. We determined the quality of the evidence using GRADE. The small number of included studies limited the pre‐planned analyses.
Main results
We included nine trials about CME, of which six trials with 333 patient‐caregiver couples were included in the meta‐analysis. The small number of studies, participants, and a variety of outcome measures rendered summarising and combining of data in meta‐analysis difficult. In addition, in some studies, CME was the only intervention (CME‐core), whereas in other studies, caregivers provided another, existing intervention, such as constraint‐induced movement therapy. For trials in the latter category, it was difficult to separate the effects of CME from the effects of the other intervention.
We found no significant effect of CME on basic ADL when pooling all trial data post intervention (4 studies; standardised mean difference (SMD) 0.21, 95% confidence interval (CI) ‐0.02 to 0.44; P = 0.07; moderate‐quality evidence) or at follow‐up (2 studies; mean difference (MD) 2.69, 95% CI ‐8.18 to 13.55; P = 0.63; low‐quality evidence). In addition, we found no significant effects of CME on extended ADL at post intervention (two studies; SMD 0.07, 95% CI ‐0.21 to 0.35; P = 0.64; low‐quality evidence) or at follow‐up (2 studies; SMD 0.11, 95% CI ‐0.17 to 0.39; P = 0.45; low‐quality evidence).
Caregiver burden did not increase at the end of the intervention (2 studies; SMD ‐0.04, 95% CI ‐0.45 to 0.37; P = 0.86; moderate‐quality evidence) or at follow‐up (1 study; MD 0.60, 95% CI ‐0.71 to 1.91; P = 0.37; very low‐quality evidence).
At the end of intervention, CME significantly improved the secondary outcomes of standing balance (3 studies; SMD 0.53, 95% CI 0.19 to 0.87; P = 0.002; low‐quality evidence) and quality of life (1 study; physical functioning: MD 12.40, 95% CI 1.67 to 23.13; P = 0.02; mobility: MD 18.20, 95% CI 7.54 to 28.86; P = 0.0008; general recovery: MD 15.10, 95% CI 8.44 to 21.76; P < 0.00001; very low‐quality evidence). At follow‐up, we found a significant effect in favour of CME for Six‐Minute Walking Test distance (1 study; MD 109.50 m, 95% CI 17.12 to 201.88; P = 0.02; very low‐quality evidence). We also found a significant effect in favour of the control group at the end of intervention, regarding performance time on the Wolf Motor Function test (2 studies; MD ‐1.72, 95% CI ‐2.23 to ‐1.21; P < 0.00001; low‐quality evidence). We found no significant effects for the other secondary outcomes (i.e. patient: motor impairment, upper limb function, mood, fatigue, length of stay and adverse events; caregiver: mood and quality of life).
In contrast to the primary analysis, sensitivity analysis of CME‐core showed a significant effect of CME on basic ADL post intervention (2 studies; MD 9.45, 95% CI 2.11 to 16.78; P = 0.01; moderate‐quality evidence).
The methodological quality of the included trials and variability in interventions (e.g. content, timing, and duration), affected the validity and generalisability of these observed results.
Authors' conclusions
There is very low‐ to moderate‐quality evidence that CME may be a valuable intervention to augment the pallet of therapeutic options for stroke rehabilitation. Included studies were small, heterogeneous, and some trials had an unclear or high risk of bias. Future high‐quality research should determine whether CME interventions are (cost‐)effective.
Plain language summary
Caregiver‐mediated exercises for improving outcomes after stroke
Review question
What is the effect of performing exercises with a caregiver after stroke on outcome for people with stroke and burden for caregivers?
Background
Stroke is a major cause of acquired adult disability. Research has shown that more time spent on exercise therapy in the first weeks to months after stroke leads to better functioning. Due to lack of personnel and resources, in practice it is difficult to spend more time on exercise therapy in this period. One method to increase this exercise time, is to involve caregivers in performing exercise training together with a person with stroke. During this exercise training a therapist coaches patient and caregiver and an evaluation is planned on a regular basis.
Study characteristics
We identified nine clinical trials to October 2015, which all investigated some form of caregiver‐mediated exercises compared with usual care, no treatment (intervention), or another intervention that was not caregiver‐mediated.
Key results
We included 333 patient‐caregiver couples in the review. We found trials in which caregiver‐mediated exercises themselves were the studied subject (called CME‐core). In addition, we found trials in which the caregiver was the provider of another, already existing intervention. In the latter category, it was difficult to separate the effect of caregiver‐mediated exercises from the effect of the other intervention.
We found evidence that caregiver‐mediated exercises could have a positive effect on patients' standing balance (low‐quality evidence) and quality of life (very low‐quality evidence) directly after the intervention. In the long term, we found very low‐quality evidence for a positive effect on walking distance. For speed of use of the arm and hand, we found low‐quality evidence in favour of the control group.
We found no significant side effects or beneficial effects on caregiver strain; we judged the quality of this evidence as moderate (after intervention) to very low (long term). Furthermore, we found no significant effects for basic activities of daily living, such as dressing and bathing, after intervention (moderate‐quality evidence) or follow‐up (low‐quality evidence). In addition, we found no significant effects for extended activities of daily living, such as cooking and gardening, after intervention or at follow‐up (both low‐quality evidence).
In the CME‐core analysis, we found moderate‐quality evidence for a positive effect of caregiver‐mediated exercises for basic activities of daily living.
It can be concluded that caregiver‐mediated exercises may be a promising form of therapy to add to usual care.
Quality of the evidence
The number of included trials was small and the level of evidence was of very low to moderate quality. Therefore, results should be interpreted with caution.
Summary of findings
Summary of findings for the main comparison. Caregiver‐mediated exercises compared with control intervention for people with stroke.
| Caregiver‐mediated exercises compared with control intervention for people with stroke | ||||||
|
Patient or population: people with stroke Settings: inpatient and outpatient settings Intervention: caregiver‐mediated exercises Comparison: control, i.e. usual care, other intervention, no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control intervention | Caregiver‐mediated intervention | |||||
|
Patient: ADL measures Barthel Index. Scale 0 to 100 (follow‐up: 2 studies; 3/6 months) FIM. Scale 7 to 126 (no follow‐up) |
The mean Barthel Index score ranged across control groups from 78 to 84 1 study: The mean FIM score in the control group was 65 |
The mean Barthel Index score in the intervention groups was
5.09 higher (‐2.88 to 13.07 higher) 1 study: The mean FIM score in the intervention group was 11 higher (‐1.59 to 23.67 higher) |
‐ | Barthel Index: 247
(3) FIM: 48 (1) Total: 295 |
⊕⊕⊕⊝ Moderate | Higher scores are better More than half of the studies at low risk of bias (3 low risk of bias, 1 at unclear risk of bias) There was clinical heterogeneity SMD 0.21 (‐0.02 to 0.44) |
|
Caregiver: measures of mood, burden and QoL: burden Caregiver Strain Index Scale. 0 to 13 (follow‐up 3 months) Caregiver Burden Scale. 22 to 88 (no follow‐up) |
The mean Caregiver Strain Index score in the control group was
3.4 The mean Caregiver Burden Scale score in the control group was 46.6 |
The mean Caregiver Strain Index score in the intervention group was 0.50 higher (‐0.81 to 1.81 higher) The mean Caregiver Burden Scale score in the intervention group was 1.30 lower (‐4.88 to 7.48 lower) |
‐ | Caregiver Strain Index: 40 (1) Caregiver Burden Scale: 51 (1) Total: 91 |
⊕⊕⊕⊝ Moderate | Lower scores are better Both studies at low risk of bias Small total number of participants SMD ‐0.04 (‐0.45 to 0.37) |
|
Gait and gait‐related measures: walking speed in m/s (follow‐up: 1 study, 9 months) |
The mean walking speed ranged across control groups from 0.26 m/s to 0.46 m/s | The mean walking speed in the intervention group was 0.08 m/s higher (‐0.03 to 0.18) | ‐ | 71 (2) |
⊕⊝⊝⊝ Very low | |
|
Gait and gait‐related measures: walking distance measured with the Six‐Minute Walk Test in metres walked in 6 minutes (follow‐up: 1 study, 3 months) |
The mean distance walked ranged across control groups from 157 m to 166 m | The mean distance walked in the intervention groups was 30.98 m higher (‐20.22 to 82.19 higher) | ‐ | 91 (2) | ⊕⊕⊕⊝ moderate | Lower scores are better 1 study at unclear risk of bias Small total number of participants MD 0.04 (‐0.10 to 0.18) |
|
Measures of mood and QoL of the patient: Stroke Impact Scale Stroke Impact Scale mobility scale. Scale 9 to 45. (no follow‐up) |
The mean Stroke Impact Scale mobility score in the control group was 66.8 | The mean Stroke Impact Scale mobility score in the intervention group was 18.2 higher (7.54 to 28.86 higher) | ‐ | 51 (1) |
⊕⊝⊝⊝ Very low | Higher scores are better 1 study at low risk of bias Small total number of participants MD 18.2 (7.54 to 28.86) |
|
Length of stay: length of stay in rehabilitation unit in days |
The mean length of stay in a rehabilitation unit in the control group was 52.3 days | The mean length of stay in a rehabilitation unit in the intervention group was 12 days lower (‐10.88 to 34.88) | ‐ | 20 (1) |
⊕⊝⊝⊝ very low | Higher scores are better 1 study at low risk of bias and 1 at unclear or high risk of bias Small total number of participants There was clinical heterogeneity MD 0.08 m/s (‐0.03 to 0.18) |
|
Adverse outcomes: falls number of falls/patient (no follow‐up) |
1 study: the mean number of falls/patient in the control group was 0.08 | 1 study: the mean number of falls/ patient in the intervention group was 0.04 lower (‐0.10 to 0.18 lower) | ‐ | 48 (1) | ⊕⊝⊝⊝ Very low | Higher scores are better Both studies at low risk of bias Small total number of participants MD 30.98 m (‐20.22 to 82.19) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; FIM: Functional Independence Measure; MD: mean difference; QoL: quality of life; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Stroke is a major cause of long‐term disability in adults with effects on activities of daily living (ADL) and quality of life (QoL). Although most people leave the rehabilitation setting with some level of independent walking, many have residual walking disabilities and it has been reported that following rehabilitation, only 7% of stroke survivors can walk at a level commensurate with community participation (Ada 2009). Twelve months after stroke about 28% of people with stroke remain dependent in their basic ADLs, such as dressing, toileting, and indoor mobility (Ullberg 2015). Pettersen and colleagues reported that 32% of people with stroke living at home after three years were inactive in extended ADL (Pettersen 2002). Any treatment that improves functional outcome can potentially reduce the burden of this illness for the person, their caregivers, and society.
Description of the intervention
Several systematic reviews have shown that a higher intensity of training in terms of time spent on exercise therapy can lead to better functional outcome in people with stroke in terms of ADL and functional performance (French 2010; Galvin 2008a; Kwakkel 2004; Kwakkel 2006; Langhorne 2011; Lohse 2014; Veerbeek 2011; Veerbeek 2014). One resource‐efficient method to increase intensity of training could be to involve caregivers in exercise training (De Weerdt 2002). We define caregiver‐mediated exercises (CME) as the person with stroke performing exercises together with a caregiver under the auspices of a physical or occupational therapist. "Under the auspices" means that the therapist is involved as a coach by instructing both patient and caregiver on how to perform the exercises, and evaluating them on a regular basis. Hereby, the exercises are aimed at improving ADL including mobility, such as making transfers, standing, and walking.
How the intervention might work
Performing exercises together with a caregiver has the potential to augment the intensity of practice without increasing healthcare costs. This could improve outcomes in terms of body functions, activities, and participation as well as cost effectiveness in people with stroke.
In addition, caregivers are more actively involved in CME than in the usually applied rehabilitation services, which may increase feelings of empowerment with reduced levels of caregiver burden (Brereton 2002; Smith 2004a). CME could lead to a reduced length of inpatient stay or outpatient treatment in hospitals, rehabilitation, and nursing settings, and may improve outcomes in self‐management, empowerment, and QoL of patients and caregivers.
Why it is important to do this review
Several systematic reviews have indicated that additional exercise therapy and repetitive task training have a significant, favourable effect on functional outcome after stroke, and concluded that the more time spent on exercise therapy (Galvin 2008a; Kwakkel 2004; Kwakkel 2006; Lohse 2014; Veerbeek 2011), and the higher the number of repetitions, the better the outcome (French 2010; Langhorne 2011; Veerbeek 2014). Therefore, clinical guidelines recommend that people who are in a rehabilitation setting should have the opportunity to train intensively (ESO 2008; NICE 2013; SIGN 2010; Veerbeek 2014). For example, the stroke guideline in the UK recommends a daily dose of 45 minutes of exercise therapy (NICE 2013).
Currently, the resources in inpatient settings are not sufficient to meet these recommendations. Most people admitted to stroke units, rehabilitation wards, and nursing homes spend most of their waking time during the working week inactive (Bernhardt 2004; Smith 2008; West 2012), and on weekends, rehabilitation services (including exercise therapy) in most hospital and rehabilitation settings are not available (Otterman 2012). Therefore, it is important to find innovative methods, such as CME, to enhance intensity of training after stroke, without increasing costs.
However, the caregiver taking the role of a therapist (instead of a family role) may burden the caregiver with yet another task (Gordon 2004). Therefore, it is important to study the mood, burden, and QoL of caregivers when involving them in CME systematically. No systematic review has yet been conducted to evaluate the effect of caregiver participation in exercise training on functional outcome after stroke, or to evaluate the effect on mood and burden of the caregiver when involved in CME.
Objectives
To determine if caregiver‐mediated exercises (CME) improve functional ability and health‐related quality of life in people with stroke, and to determine the effect on caregiver burden.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs. One group of the trial must have received CME and we considered this group as the experimental group for this review. The other (control) group could have received usual treatment, no treatment, or any other type of rehabilitation intervention or attention‐control as long as it was not caregiver‐mediated. We accepted usual treatment when it was described as usual care in the setting of the participant.
Types of participants
People, at least 18 years old, who had had a stroke. Stroke is defined by the World Health Organization as "a clinical syndrome typified by rapidly developing signs of focal or global disturbance of cerebral functions, lasting more than 24 hours or leading to death, with no apparent causes other than of vascular origin" (WHO 1989). We included RCTs regardless of timing after stroke and setting.
Types of interventions
One group of the RCT must have included CME, whereas the caregiver involvement was not explicitly asked for in the other group of the RCT. We included trials in which the patient and their caregiver were trained or instructed together, as well as trials in which the caregiver was trained or instructed alone. There was no limit to the number of sessions or to the frequency of delivery. We included all types of exercises as long as they were aimed at improving patients' abilities to perform daily activities. Therefore, we excluded RCTs of speech, swallowing, or cognitive interventions done together with a caregiver. We defined a caregiver or carer as an unpaid or partially paid person who voluntarily helped an impaired person with his or her ADL. In other words, the mediated services were not applied by a professional in health care but in most cases, someone who was close to the patient and voluntarily offered his or her services. This may have been a partner, family member, or friend, but it can also have been a volunteer. We argued that this person was 'not a professional' such as a 'therapy assistant'. When a professional in health services applied the mediated exercises, we excluded the RCT. We included interventions delivered at any location, for example at home, in hospital, or in a rehabilitation setting. Because a caregiver can be the provider of an intervention, we did not exclude trials that combined CME with an existing intervention. However, we did differentiate between trials in which CME was the only intervention (CME‐core) and trials in which a caregiver was used to deliver another, existing intervention. We contacted trial authors when it was unclear whether a trial met our definition.
Types of outcome measures
Primary outcomes
Patient: basic ADL measures, such as the Barthel index (BI) (Collin 1988; Mahoney 1965), Functional Independence Measure (FIM) (Dodds 1993), modified Rankin Scale (mRS) (De Haan 1995; Dromerick 2003); extended ADL measures, such as the Nottingham Extended Activities of Daily Living (NEADL) Index (Nouri 1987), or Frenchay Activities Index (FAI) (Wade 1985). When found, we combined scales with the same construct.
Caregiver: measures of burden, for example Caregiver Strain Index (CSI) (Robinson 1983). When found, we combined scales with the same construct.
When possible we distinguished between caregivers who were family or friends and other types of caregivers, such as volunteers, for the above‐mentioned measures of outcome.
Secondary outcomes
Measures of motor impairment: Motricity Index (MI) (Collin 1990), Fugl‐Meyer Assessment (FMA) (Duncan 1983; Sanford 1993; Shelton 2001).
Gait and gait‐related measures: walking speed, walking distance, Timed‐Up‐and‐Go test (TUG) (Collen 1990; Flansbjer 2005), Rivermead Mobility Index (RMI) (Collen 1991; Hsieh 2000; Hsueh 2003), Berg Balance Scale (BBS) (Berg 1992; Berg 1995; Mao 2002; Stevenson 2001).
Measures of upper limb activities or function, for example, Action Research Arm Test (ARAT) (Chen 2012; Hsieh 1998; Platz 2005).
Measures of mood and QoL of the patient, for example, measured by the Stroke Impact Scale (SIS) (Duncan 1999; Duncan 2002; Duncan 2003), and Hospital Anxiety and Depression Scale (HADS) (Aben 2002; Bjelland 2002; Herrmann 1997; Zigmond 1983).
Measures of fatigue of the participant, for example, measured by the Fatigue Severity Scale (FSS) (Valko 2008).
Length of stay in hospital, rehabilitation centre, or nursing home, or treatment in an outpatient clinic.
Adverse outcomes, for example, pain, injury, or falls. When possible, we compared the total number of falls between groups, and the number of patients experiencing at least one fall between groups.
Caregiver: measures of mood and QoL, for example, HADS (Aben 2002; Bjelland 2002; Herrmann 1997; Zigmond 1983), or CarerQoL (Brouwer 2006; Hoefman 2011).
When we found scales measuring the same construct, we combined them. If studies reported outcome measures other than the ones mentioned above, we verified if they measured the same construct. If this was the case, we pooled them; if they did not measure the same construct, we reported these outcomes separately.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of papers where necessary. Due to time limitations, we were unable to perform the review within one year after the first search (April 2014). Therefore, it was necessary to update our search in October 2015. We used the same search strategy but due to different availability of Information Specialists and providers of databases, we adjusted the search strategies accordingly: Embase.com instead of Ovid/Embase, and EBSCO/AMED instead of Ovid/AMED. We limited the update searches between 2014 and 2016.
Electronic searches
We searched the following databases and trials registers.
Cochrane Stroke Group Trials Register (last searched October 2015).
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2015, Issue 10) (Appendix 1).
Cochrane Database of Systematic Reviews (CDSR) (the Cochrane Library, last searched October 2015) (Appendix 1).
Cochrane Methodology Register (CMR) (the Cochrane Library, last searched October 2015) (Appendix 1).
Database of Abstracts of Reviews of Effects (DARE) (the Cochrane Library, last searched October 2015) (Appendix 1).
Health Technology Assessment Database (HTA) (the Cochrane Library, last searched October 2015) (Appendix 1).
NHS Economic Evaluation Database (NHS EED) (the Cochrane Library, last searched October 2015) (Appendix 1).
MEDLINE (Ovid) (from 1946 to October 2015) (Appendix 2).
Embase (Ovid from 1980 to April 2014 and Embase.com from 2014 to December 2015) (Appendix 3; Appendix 4).
CINAHL (Cumulative Index of Nursing and Allied Health Literature) (EBSCO) (from 1982 to December 2015) (Appendix 5).
SPORTDiscus (EBSCO) (from 1985 to December 2015) (Appendix 6).
AMED (Alternative and Complementary Medicine) (Ovid from 1985 to April 2014 and EBSCO from 1985 to December 2015) (Appendix 7; Appendix 8).
Physiotherapy Evidence Database (PEDro) (from 1929 to October 2015) (www.pedro.org.au/).
REHABDATA (from 1956 to October 2015) (www.naric.com/?q=en/REHABDATA).
ClinicalTrials.gov (www.clinicaltrials.gov/).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Stroke Trials Registry (www.strokecenter.org/trials/).
Current Controlled Trials (www.controlled‐trials.com).
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/).
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Trials Search Co‐ordinator (Brenda Thomas) and adapted this for the other databases. Search strategies for the main databases are included. For a complete overview of the search, see Appendix 9.
Searching other resources
To identify further published, unpublished, and ongoing studies we:
searched the reference lists of all included articles;
contacted experts and authors in the field;
used Science Citation Index Cited Reference Search for forward tracking of important articles.
Data collection and analysis
Selection of studies
Two review authors (JV, MM) independently screened the titles of records obtained from the electronic searches and excluded obviously irrelevant studies. Subsequently, we screened the remaining abstracts and excluded those that were irrelevant. Finally, we obtained the full‐text articles for the remaining studies and the same two review authors selected studies for inclusion in the review based on the inclusion criteria described previously. We resolved any disagreement by discussion and, where necessary, in consultation with a third review author (EvW).
Data extraction and management
Two review authors (JV, MM) conducted data extraction and reviewed risk of bias of the eligible trials. The review authors were not blinded to study authors, journals, or outcomes. We resolved any disagreement about risk of bias by discussion. If we could not reach consensus, a third review author (EvW) made the final decision. One review author (JV) extracted data and a second review author (MM) cross‐checked the extracted data using a standard checklist, including randomisation method, study population, intervention methods and delivery, outcome measures, and follow‐up.
Assessment of risk of bias in included studies
We used the tool for assessing risk of bias in included RCTs as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed allocation (selection bias), blinding (performance and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias, such as management of dropouts (no intention‐to‐treat analysis). We presented the results in 'Risk of bias' tables. We provided our judgement ('low risk', 'high risk' or 'unclear risk') for each entry, followed by a description of the judgement. We made our judgements transparent, and used comments or quotes when necessary.
Measures of treatment effect
We extracted means and standard deviations (SDs) of postintervention scores and follow‐up scores. Where available, we also extracted means and SDs of change from baseline.
For continuous outcomes using similar measurement scales, we used the mean difference (MD) with 95% confidence intervals (CIs). If similar outcomes were measured on different scales, we used Hedges' g, calculated the 95% CI and standard mean difference (SMD).
We reported the direction of the effect for every scale to align the treatment effects between outcome scales. For scales in which a low score reflected a favourable outcome and a high score an unfavourable outcome, we multiplied scores by ‐1.
We used Review Manager 5 for all quantitative analyses (RevMan 2014).
Unit of analysis issues
We took into account that studies can apply different randomisation methods, for example, at the level of a participant or at the level of a group of participants (cluster randomisation).
In selected studies with multiple intervention groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups. We made sure that participants were not double‐counted in the analysis.
Dealing with missing data
If data were missing or were not in a form suitable for quantitative pooling, we contacted the trial authors to request additional information.
Assessment of heterogeneity
We assessed the impact of heterogeneity in the meta‐analysis for each outcome with the I2 statistic (Higgins 2011). When there was substantial statistical heterogeneity (I2 greater than 50%) we used a random‐effects model, otherwise we used a fixed‐effect model for meta‐analysis.
Assessment of reporting biases
Because we identified fewer than 10 studies, we did not assess reporting bias by a funnel plot in which effect estimates and precision (standard error) of individual RCTs are plotted, as we had planned.
Data synthesis
We performed a meta‐analysis of the comparison CME versus control group (usual care, no intervention, or any other intervention) where there were two or more RCTs with a low risk of bias in which study population, intervention, and outcome measures were the same. We determined the quality of evidence using GRADE levels of evidence.
We included a 'Summary of findings' table using the Cochrane template, and included the following seven outcomes: ADL measures, burden of the caregiver, walking speed, walking distance, mood of the patient, length of stay, and adverse events (falls) (see Table 1). For each outcome, we included the number of participants, the overall quality of the evidence using GRADE levels of evidence, the magnitude of the effect, a measure of burden of the outcome, and comments (Guyatt 2008a; Guyatt 2008b).
In the text and tables, we have systematically described those studies that could not be included in the meta‐analysis. In the same way, we systematically reported other outcome measures that we could not include in a meta‐analysis because they did not measure the same construct as our predefined outcome measures.
We used Review Manager 5 for the analyses (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Where two or more studies per subgroup were available, we performed subgroup analysis for:
interventions with a higher dose of training in the intervention group than the control group versus interventions with a same dose of training in intervention and control groups;
interventions within six months after stroke and interventions beyond six months after stroke;
interventions aimed at the upper extremity and interventions aimed at the lower extremity.
Sensitivity analysis
A caregiver could be a provider of an existing intervention, for example constraint‐induced movement therapy (CIMT). We included trials investigating this form of CME. However, in these trials, it was difficult to separate the effects of CME from the effects of the intervention. In the other trials, CME itself was considered as the only intervention under study. Therefore, we performed a sensitivity analysis in which only these trials were included (CME‐core). A priori, we did not plan this sensitivity analysis, but decided afterwards to include this analysis in light of the type of studies that we identified. In this sensitivity analysis, we also repeated the subgroup analyses.
Where we applied a fixed‐effect model, we subsequently applied a random‐effects model to assess the robustness of the results to the method used.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
Through electronic searches we found 8107 citations. In addition, one potentially relevant trial was already known to us, but not found through electronic searches (Wall 1987). After removing duplicates, we screened 5640 citations. Based on screening of titles, we excluded 5201 obviously irrelevant studies and screened the remaining 439 abstracts. Subsequently, we excluded 307 studies based on the abstract. Finally, we assessed 132 full‐text articles or trial registry entries for eligibility. After an extensive search, we still could not obtain full‐text articles for four studies ("THE DAYS AFTER"; "Family boosts results of poststroke therapy"; Liu 2012; Wang 2014). We identified 11 relevant systematic reviews, which we screened for trials (Bakas 2014; Brereton 2007; Glasdam 2010; Klinke 2015; Lawler 2013; Legg 2011; Morris 2014; Parke 2015; Pollock 2014a; Pollock 2014b; Warner 2015). In total, we identified 46 potentially relevant trials. The results of the search are summarised in Figure 1. We were able to include nine trials for final analysis (see Characteristics of included studies table), and we included six trials in the meta‐analysis (Abu Tariah 2010; Barzel 2015; Dai 2013; Galvin 2011; Wall 1987; Wang 2015).
1.

Study flow diagram.
We excluded three trials from the meta‐analysis because of poor methodological quality (Agrawal 2013; Gómez 2014) or no reporting of required data (i.e. means or SDs, or both, of outcome measures) (Agrawal 2013; Gómez 2014; Souza 2015), or both. We had no success contacting the corresponding authors to request the necessary data.
We excluded 37 trials, 35 with reasons given in the Characteristics of excluded studies table. Two trials are ongoing (see Characteristics of ongoing studies table).
Included studies
Participants
Characteristics
In the nine included studies, 456 stroke survivors and their caregivers were randomised to CME or control interventions. A total of 342 people with stroke‐caregiver couples were included in the six trials included in the meta‐analysis. In these six trials, nine patient‐caregiver couples were not analysed according to intention‐to‐treat principles and no information about these withdrawals was published. Therefore, we have presented information about 333 stroke survivors and their caregivers (ranging from 18 to 156 patient‐caregiver couples per trial) in the meta‐analysis.
The mean age in all studies was around 60 years. The mean time since onset of symptoms ranged from 15 days to 10 years. One trial did not report mean time since onset of symptoms (Gómez 2014).
Three studies defined inclusion or exclusion criteria for the caregiver, for example "willing to participate", "medically stable and physically able" (Galvin 2011), "being defined as primary caregivers" (Dai 2013), and "caregivers were excluded if they were in poor physical health, had mental or behavioural disorders" (Wang 2015).
Four studies described an inclusion criterion for the patient about the caregiver: "live with family caregiver at home" (Abu Tariah 2010), "patients with family support" (Gómez 2014), "had a caregiver who was prepared to be a non‐professional coach (e.g., family member)" (Barzel 2015), and "availability of a family member to supervise home exercises" (Souza 2015). Two studies gave information about the caregiver: "about 50% of the caregivers were nursing attendants" (Dai 2013), and "majority were patients' spouse" (Wang 2015).
Sample size
Five trials included fewer than 50 participants: 20 participants (Abu Tariah 2010; Wall 1987), 24 participants (Souza 2015), 30 participants (Agrawal 2013), and 40 participants (Galvin 2011). Four trials included more than 50 participants: 51 participants (Wang 2015), 55 participants (Dai 2013), 60 participants (Gómez 2014), and 156 participants (Barzel 2015).
Interventions
The content of the training and the timing was different between trials. Details of each intervention are summarised in Table 2.
1. Outline of included studies.
| Study ID | Form of training | Upper or lower body | Timing since stroke | Task caregiver | Routine care continued | Control group | Programme (length ‐frequency‐ duration) | Contact with therapist | Place |
| Abu Tariah 2010 | CIMT | Upper | > 2 months | Carried out the intervention with support of therapists | No | Neurodevelopmental training, same intensity | 2 months ‐ daily ‐ 2 hours | 3 or 4 sessions | Home |
| Agrawal 2013 | Exercise therapy | Upper | "Sub‐acute stroke" | Encouragement, participating, and help | Yes | Usual care | 4 weeks ‐5 days/week ‐ 60 to 90 minutes | Weekly | Inpatient? |
| Barzel 2015 | CIMT | Upper | > 6 months | Supervision, help, and maintaining training diary | No | Usual care, frequency of seeing a therapist was the same | 4 weeks ‐ Every weekday (not weekend) ‐ 2 hours |
5 x 60 minutes | Home |
| Dai 2013 | Vestibular rehabilitation | Both | < 6 months | Guidance and supervision (in third and fourth week) |
Yes | Usual care | 4 weeks ‐ 10 sessions per 2 weeks ‐ 30 minutes | 2 to 4 sessions in first 2 weeks | Inpatient? |
| Galvin 2011 | Exercise therapy | Lower | Assessment 2 weeks after stroke onset | Encouragement and help | Yes | Usual care | 8 weeks ‐every day ‐ 35 minutes | Weekly | Inpatient or at home |
| Gómez 2014 | CIMT | Upper | < 6 months | Monitoring and supervising | Yes | Usual care | 14 days ‐ every day* ‐ 5.5 hours* | 1.5 hours per day* | Inpatient |
| Souza 2015 | CIMT: 1.5 hours with therapist and 1.5 hours with caregiver | Upper | < 24 months** | Supervision and making notes | No | CIMT: 3 hours with therapist | 22 days ‐ 10 sessions ‐ 3 hours | 10 x 90 minutes | Outpatient and home |
| Wall 1987 | Exercise therapy | Lower | After discharge of rehabilitation | Supervision | No | No intervention | 6 months ‐ twice a week ‐ 1 hour | 1 group: twice a week 1 group: once a week 1 group: 'monitoring' |
Outpatient or at home |
| Wang 2015 | Exercise programme aimed at body functions, activities, and participation | Both | > 6 months | Encouragement and help | No | Usual care | 12 weeks ‐ minimal twice a week, if possible every day ‐ minimal 50 to 60 minutes | Weekly 90 minutes | Home |
CIMT: constraint‐induced movement therapy. * Details of the intervention are not completely clear, contact with the authors was not successful. ** But mean time since stroke was 27 and 35 months since stroke, unclear why.
Two trials were aimed at the lower body (Galvin 2011; Wall 1987), five at the upper body (Abu Tariah 2010; Agrawal 2013; Barzel 2015; Gómez 2014; Souza 2015), and two at both upper and lower body (Dai 2013; Wang 2015). Four studies included patients within six months after stroke (Agrawal 2013; Dai 2013; Galvin 2011; Gómez 2014), three studies included patients beyond six months after stroke (Barzel 2015; Wall 1987; Wang 2015), one study included patients from two months after stroke or later (Abu Tariah 2010), one study included patients if they had a stroke in the last 24 months (Souza 2015). The task of the caregiver ranged across trials from supervision, guidance, encouragement, to physical help. In four trials, usual care continued, so CME were applied in addition to usual care (Agrawal 2013; Dai 2013; Galvin 2011; Gómez 2014). The frequency, duration, and programme length differed between studies, with training frequencies ranging from twice a week (Wall 1987; Wang 2015), to every day (Abu Tariah 2010; Galvin 2011), with a duration per session ranging from 30 minutes (Dai 2013), to three hours (Souza 2015), and a programme length ranging from 14 days (Gómez 2014), to six months (Wall 1987). In four trials, patients had weekly contact with the supervising therapist (Agrawal 2013; Barzel 2015; Galvin 2011; Wang 2015). Two trials planned two to four sessions with a therapist (Abu Tariah 2010; Dai 2013). One trial had 10 sessions with a therapist in 22 days (Souza 2015). One trial consisted of four groups, the amount of contact with the therapist differed between trial groups (Wall 1987). The frequency and duration of one trial was not clearly reported (Gómez 2014). Three trials were carried out at home (Abu Tariah 2010; Barzel 2015; Wang 2015), one trial was carried out in an inpatient setting (Gómez 2014), three trials were carried out when patients were inpatient, outpatient, or at home (Galvin 2011; Souza 2015; Wall 1987), and two trials were unclear about the location of the intervention (Agrawal 2013; Dai 2013).
Two trials had more than one trial group. The study by Agrawal 2013, which was not included in meta‐analysis, had two experimental trial groups with different duration of intervention (60 and 90 minutes, five days a week) and one control group. Wall 1987 had two intervention groups (CME, CME plus physiotherapy) and two control groups (physiotherapy, no intervention). We decided to combine the intervention groups and the control groups into one comparison because of the small total number of participants (20).
Compliance
Five studies recorded compliance: "frequency of training and tasks completed was recorded" (Wang 2015), "the amount of training was noted in a diary by patients' families" (Abu Tariah 2010), "compliance with therapy time was documented through the use of an exercise diary, in which the number of exercises completed and time taken to complete the exercises were recorded daily" (Galvin 2011), "a log sheet per participant to record the total number of minutes completed per day" (Agrawal 2013), and "compliance was assessed in all participants via a form (standard therapy group) or a training diary (home CIMT group)" (Barzel 2015). Two trials reported these outcomes in the results. Galvin 2011 reported that 245 minutes of additional exercise therapy was planned for each participant and that a mean of 227 minutes was actually delivered. Barzel 2015 reported a mean exercise time of 27.7 hours within the four‐week intervention. They also noted 12 cases of participants not adhering to the protocol. In Souza 2015, compliance about wearing of the sling was reported in the results, but no information about compliance to the CME was provided. Agrawal 2013 mentioned "inability to monitor patient's compliance with the home exercise programme which might have influenced the study".
Comparisons
Interventions consisted of CME in addition to usual care (Agrawal 2013; Dai 2013; Galvin 2011; Gómez 2014), or instead of usual care (Abu Tariah 2010; Barzel 2015; Souza 2015; Wall 1987; Wang 2015). Two studies included a control intervention (Abu Tariah 2010; Souza 2015), seven included usual care as control (Agrawal 2013; Barzel 2015; Dai 2013; Galvin 2011; Gómez 2014; Wall 1987; Wang 2015), one had no control intervention (Wall 1987). Furthermore, there were different forms of interventions in terms of type of exercise therapy, duration of the intervention, and timing of the intervention.
Outcome measures
All trials reported outcome measures at the end of intervention. Five trials reported outcome measures after three to six months' follow‐up (Abu Tariah 2010; Barzel 2015; Galvin 2011; Souza 2015; Wall 1987). Two trials reported outcome measures during the intervention period (Dai 2013; Wall 1987). Some outcome measures were not reported at baseline, but only at post intervention and at follow‐up. In some instances there were no SDs of outcome measures given, for which we imputed other SDs from the same study when possible (i.e. Galvin 2011: no SD at post intervention for NEADL Index, CSI and Reintegration to Normal Living Index was available and follow‐up SD was used; Abu Tariah 2010: no SD at post intervention or follow‐up for Wolf Motor Function test ‐ performance time was given and SD from baseline was used). Walking speed was reported in different units and were converted to metres/second. Where available, we also extracted mean changes from baseline (Abu Tariah 2010; Barzel 2015; Galvin 2011; Wang 2015), and in those cases where postintervention scores were not available, we used the mean change from baseline. Abu Tariah 2010 and Wang 2015 gave no SDs, but provided CIs. We calculated the SDs for these outcomes using the Z‐score.
One trial reported two outcome measures for extended ADL (Galvin 2011). Based on that, the NEADL Index is developed for people with stroke and widely used in stroke research, we restricted to NEADL Index in the main analysis.
Insufficient information was available regarding the type of caregiver, rendering it impossible to distinguish between caregivers who were family or friends and other (voluntary) caregivers for the different outcome measures. One study mentioned that "about 50% of the caregivers were nursing attendants" (Dai 2013), and one study included four paid workers (Wang 2015). We did not take this professional background into account during the analyses.
The trials used a variety of outcome measures. Some outcome measures were identical, but most differed between trials. We combined outcome measures when they appeared to measure the same construct.
Excluded studies
We excluded 35 articles based on the full texts because they did not meet the inclusion criteria (Adie 2014; Araujo 2015; Barzel 2009; Baskett 1999; Bertilsson 2014; Cameron 2015; Chang 2015; Chinchai 2010; El‐Senousey 2012; Evans 1984; Forster 2013; Goldberg 1997; Grasel 2005; Harrington 2010; Harris 2009; Hebel 2014; Hirano 2012; Jones 2015; Kalra 2004; Koh 2015; Larson 2005; Lin 2004; Maeshima 2003; Marsden 2010; McClellan 2004; Mudzi 2012; NCT00908479; Osawa 2010; Parker 2012; Redzuan 2012; Schure 2006; Shyu 2010; Smith 2004b; Van de Port 2012; Walker 1996). See Characteristics of excluded studies table.
The most common reasons for exclusion were: interventions were educational for patient and caregiver but they performed no, or minimal, exercises together (Chinchai 2010; El‐Senousey 2012; Evans 1984; Forster 2013; Harrington 2010; Larson 2005; Marsden 2010; Mudzi 2012; Parker 2012; Schure 2006; Shyu 2010; Smith 2004a); caregivers were involved and encouraged to participate but caregiver participation was not mandatory (Adie 2014; Baskett 1999; Bertilsson 2014; Harris 2009; Jones 2015; Lin 2004; McClellan 2004; NCT00908479; Van de Port 2012; Walker 1996); and the intervention concerned 'skill training' (Araujo 2015; Chang 2015; El‐Senousey 2012; Forster 2013; Grasel 2005; Hebel 2014; Kalra 2004; Mudzi 2012). Skill training is primarily aimed at training of the caregiver in performing ADL and mobility together with the patient to improve functioning together in the home situation. Skill training is given to the caregiver in a limited number of sessions by a professional, like a therapist or a nurse, but it is not considered progressive training to improve functioning of the patient.
Furthermore, there are some non‐randomised studies about CME (Barzel 2009; Hirano 2012; Maeshima 2003; Osawa 2010). Because of their relevance for the topic of this review they are listed in Characteristics of excluded studies table. However, it is important to note that our search was not aimed at identifying non‐randomised studies and, therefore, we may not be complete in reporting these studies.
Risk of bias in included studies
Assessments for 'Risk of bias' in individual studies are shown in the Characteristics of included studies table. See also Figure 2 and Figure 3 for a summary of the results.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
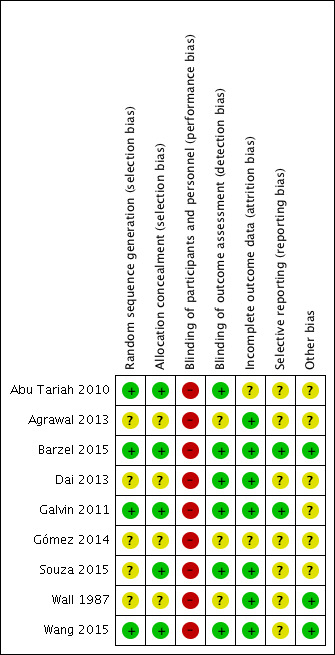
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All trials used random allocation to an intervention or control group, of which four adequately described how the randomisation procedure took place and provided sufficient information to determine that the allocation procedure was concealed (Abu Tariah 2010; Barzel 2015; Galvin 2011; Wang 2015). One study was unclear about the randomisation procedure, but did provide sufficient information about allocation procedure (Souza 2015). The other four studies did not describe the randomisation procedure sufficiently (Agrawal 2013; Dai 2013; Gómez 2014; Wall 1987).
Blinding
Participant blinding
Due to the nature of the intervention, participants included in the trials could not be blinded for treatment allocation.
Investigator blinding
Six studies blinded the outcome assessors to treatment allocation (Abu Tariah 2010; Barzel 2015; Dai 2013; Galvin 2011; Souza 2015; Wang 2015). Three studies did not report anything about an outcome assessor (Agrawal 2013; Gómez 2014; Wall 1987). Five studies used participant‐reported outcomes (questionnaires, report of number of falls) (Barzel 2015; Dai 2013; Galvin 2011; Souza 2015; Wang 2015). For these outcomes, the assessor (patient or caregiver) was aware of the treatment allocation. This may have biased the results.
Incomplete outcome data
Three studies had no withdrawals and, therefore, reported complete outcome data (Agrawal 2013; Wall 1987; Wang 2015). Four studies had withdrawals, but reasons were well described and comparable in the intervention and control group (Barzel 2015; Dai 2013; Galvin 2011; Souza 2015). One study reported only withdrawals in the control group (Abu Tariah 2010). Reasons for withdrawal were not documented by the participants, making the risk of bias unclear. One trial did not describe withdrawals, making the risk of bias unclear (Gómez 2014).
Selective reporting
For two included trials (Barzel 2015; Galvin 2011), we identified a trial registry (NCT00666744) and published protocol (Barzel 2013; Galvin 2008b). Galvin 2011 reported no exclusion criteria in the trial paper in contrast to the protocol paper (Galvin 2008b) and trial registration (NCT00666744). Not all outcome measures that were reported in the protocol paper of Barzel 2013 were reported in the trial paper (Barzel 2015), such as the EQ‐5D and healthcare costs. There were an insufficient number of studies (fewer than 10) to reliably examine the effects of risk of bias on estimates of effect and thus we generated no funnel plots.
Other potential sources of bias
Three trials did not perform an intention‐to‐treat analysis. This could be a potential source of bias (Abu Tariah 2010; Dai 2013; Souza 2015). Three trials did not report means or SDs for (a part of) the study outcomes (Agrawal 2013; Galvin 2011; Souza 2015). In one trial, means and SDs for outcome measures were not given, the included outcomes were insufficiently described, and intervention and timing of measurements needed clarification (Gómez 2014). We identified no other potential sources of bias for the remaining trials (Barzel 2015; Wall 1987; Wang 2015).
Grading the quality of the evidence
We determined the quality of the evidence using GRADE levels of evidence. We downgraded effects based on one trial by two levels of evidence and effects based on a small total number of participants (fewer than 200 participants) (BMJ Clinical Evidence 2012) by one level. When half, or more, of the included trials for an outcome measure were of unclear or high risk of bias, we downgraded the level of evidence by one level. When we found substantial unexplained statistical heterogeneity or clinical heterogeneity, we also downgraded the level of evidence by one level. In addition, when we found publication bias, we downgraded the level of evidence by one level.
Effects of interventions
See: Table 1
Caregiver‐mediated exercises versus control (Comparison 1 and 2): primary outcomes
Patient: activities of daily living measures
End of intervention
Three trials assessed the BI (100‐point version) (Barzel 2015; Galvin 2011; Wang 2015). We found no significant summary effect (mean difference (MD) 5.09, 95% CI ‐2.88 to 13.07; P = 0.21; Table 3). One trial used the FIM (Dai 2013). The effect of CME on the FIM was not significant (MD 11.04, 95% CI ‐1.59 to 23.67; P = 0.09; Table 3). Overall, we found no significant summary effect on basic ADL (standardised mean difference (SMD) 0.21, 95% CI ‐0.02 to 0.44; P =0.07; Analysis 1.1). The quality of evidence for effects on basic ADL was moderate; it was downgraded one level due to clinical heterogeneity between studies.
2. (Standard) Mean differences which are not reported in section 'data and analysis'.
| Outcome | Outcome measure | Fixed‐effect or random‐effects model | Mean difference | Confidence interval | Heterogeneity | P value |
| 1.1 Patient: ADL measures ‐ Combined |
1.1.1 Barthel Index | Random‐effects | 5.09 | ‐2.88 to 13.07 | 58% | 0.21 |
| 1.1.2 Functional Independence Measure |
Fixed‐effect | 11.04 | ‐1.59 to 23.67 | ‐ | 0.09 | |
| 1.2 Patient: ADL measures ‐ extended ADL | 1.2.1 Nottingham Extended Activities of Daily Living Index |
Fixed‐effect | 5.50 | ‐5.83 to 16.83 | ‐ | 0.34 |
| 1.2.2 IADL | Fixed‐effect | 0.02 | ‐0.72 to 0.76 | ‐ | 0.96 | |
| 1.3 Caregiver: burden | 1.3.1 Caregiver Strain Index | Fixed‐effect | ‐0.50 | ‐1.81 to 0.81 | ‐ | 0.46 |
| 1.3.2 Caregiver Burden Scale | Fixed‐effect | 1.30 | ‐4.88 to 7.48 | ‐ | 0.68 | |
| 1.6 Gait and gait‐related measures: balance |
1.6.1 Berg Balance Scale | Fixed‐effect | 6.35 | 1.64 to 11.06 | 0% | 0.008 |
| 1.6.2 Postural Assessment for Stroke patients |
Fixed‐effect | 3.50 | ‐0.52 to 7.52 | ‐ | 0.09 | |
| 2.2 Patient: ADL measures ‐ extended ADL |
2.2.1 Nottingham Extended Activities of Daily Living Index |
Fixed‐effect | 9.50 | ‐1.83 to 20.83 | ‐ | 0.10 |
| 2.2.2 IADL | Fixed‐effect | 0.02 | ‐0.77 to 0.81 | ‐ | 0.96 | |
| 3.1 Patient: ADL measures ‐ combined | 3.1.1 < 6 months | Fixed‐effect | 0.44* | 0.01 to 0.86 | 0% | 0.04 |
| 3.1.2 > 6 months | Random‐effects | 4.90 | ‐7.56 to 17.36 | 77% | 0.44 | |
| 8.1 Patient ADL measures ‐ extended ADL ‐ end of intervention | 8.1.1 Reintegration to normal living Index | Fixed‐effect | 0.20 | ‐3.76 to 4.16 | ‐ | 0.92 |
| 8.1.2 IADL | Fixed‐effect | 0.02 | ‐0.72 to 0.76 | ‐ | 0.96 | |
| 8.2 Patient ADL measures ‐ extended ADL ‐ end of follow‐up | 8.2.1 Reintegration to normal living Index | Fixed‐effect | 4.50 | 0.54 to 8.46 | ‐ | 0.03 |
| 8.2.2 IADL | Fixed‐effect | 0.02 | ‐0.77 to 0.81 | ‐ | 0.96 |
ADL: activities of daily living; IADL: instrumental activities of daily living.
*Standardised mean difference.
1.1. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 1 Patient: activities of daily living (ADL) measures: combined.
Two trials assessed extended ADL (Barzel 2015; Galvin 2011). We found no significant effects of CME on the NEADL Index (MD 5.50, 95% CI ‐5.83 to 16.83; P = 0.34; Table 3) or on the Instrumental Activities of Daily Living (IADL) (MD 0.02, 95% CI ‐0.72 to 0.76; P = 0.96; Table 3). Overall, we found no significant summary effect on extended ADL (SMD 0.07, 95% CI ‐0.21 to 0.35; P = 0.64; Analysis 1.2). This effect was based on two trials with low risk of bias, but with clinical heterogeneity between studies and a small total number of participants for this outcome measure, resulting in a low quality of evidence.
1.2. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 2 Patient: ADL measures: extended ADL: combined.
Follow‐up
Two trials assessed basic ADL and extended ADL at three months' follow‐up (Galvin 2011) and six months' follow‐up (Barzel 2015). We found no significant summary effect of CME on basic ADL (MD 2.69, 95% CI ‐8.18 to 13.55; P = 0.63; Analysis 2.1). This effect was based on two trials with low risk of bias, but with clinical heterogeneity between studies and a small total number of participants for this outcome measure, resulting in a low quality of evidence. The substantial statistical heterogeneity between trials (I2 =69%), can be explained by different timing post stroke (within six months versus beyond six months) and thus there was no reason to downgrade the level of evidence further.
2.1. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 1 Patient: activities of daily living (ADL) measures: ADL.
The effect of CME on extended ADL measured with the NEADL Index (MD 9.50, 95% CI ‐1.83 to 20.83; P = 0.10; Table 3), or measured with the IADL (MD 0.02, 95% CI ‐0.77 to 0.81; P = 0.96; Table 3) was not significant. Overall, there was no significant summary effect of CME on extended ADL (SMD 0.11, 95% CI ‐0.17 to 0.39; P = 0.45; Analysis 2.2). The quality of evidence was low, based on two trials with low risk of bias, but with clinical heterogeneity between studies and a small total number of participants for this outcome measure.
2.2. Analysis.
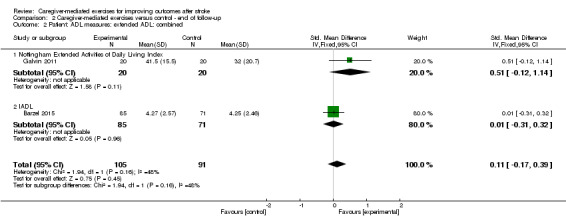
Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 2 Patient: ADL measures: extended ADL: combined.
Caregiver: measures of burden
End of intervention
One trial used the CSI to assess caregiver burden (Galvin 2011); we found no significant effect (MD –0.50, 95% CI ‐1.81 to 0.81; P = 0.46; Table 3). Another trial used the Caregiver Burden Scale (Wang 2015), and again we found no significant effect (MD 1.30, 95% CI ‐4.88 to 7.48; P = 0.68; Table 3). Overall, we found no significant summary effect of CME on caregiver strain (SMD ‐0.04, 95% CI ‐0.45 to 0.37; P = 0.86; Analysis 1.3). These findings were based on two trials with low risk of bias, but with a small total number of participants for this outcome measure, resulting in moderate quality of evidence.
1.3. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 3 Caregiver: burden: combined.
Follow‐up
One study reported follow‐up of caregiver burden by using the CSI, three months after termination of the intervention (Galvin 2011). We found no significant effect of CME on caregiver strain compared with the control group (MD 0.60, 95% CI ‐0.71 to 1.91; P = 0.37; Analysis 2.3). The quality of the evidence for this finding was very low, since it is based on only one trial with a small number of participants.
2.3. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 3 Caregiver: burden.
Caregiver‐mediated exercises versus control (Comparison 1 and 2): secondary outcomes
Measures of motor impairment
One study assessed the FMA lower extremity score (Galvin 2011). We found no significant effect after the intervention (MD 3.10, 95% CI ‐2.02 to 8.22; P = 0.24; Analysis 1.4) or at follow‐up (MD 3.40, 95% CI ‐1.74 to 8.54; P = 0.19; Analysis 2.4). These findings were based on one trial with a small number of participants, resulting in a very low quality of evidence.
1.4. Analysis.
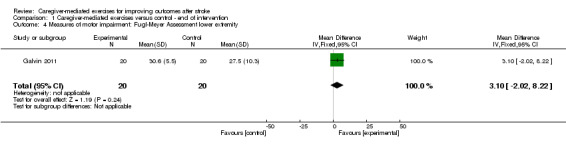
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 4 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity.
2.4. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 4 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity.
One study assessed the FMA upper extremity score (Abu Tariah 2010). We found no significant effect of CME at the end of intervention (MD 4.43, 95% CI ‐2.09 to 10.95; P = 0.18; Analysis 1.5) or at follow‐up (MD 2.75, 95% CI ‐8.24 to 13.74; P = 0.62; Analysis 2.5). These findings were based on only one trial with a small number of participants. Therefore, the quality of evidence was very low.
1.5. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 5 Measures of motor impairment: Fugl‐Meyer Assessment upper extremity.
2.5. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 5 Measures of motor impairment: Fugl‐Meyer Assessment upper extremity.
Gait and gait‐related measures
Balance
Two trials reported the BBS (Galvin 2011; Wang 2015). We found a significant summary effect (MD 6.35, 95% CI 1.64 to 11.06; P = 0.008; Table 3). One study assessed the Postural Assessment Scale for Stroke Patients (Dai 2013), and found no significant effect of CME (MD 3.50, 95% CI ‐0.52 to 7.52; P = 0.09; Table 3). Overall, we found a significant summary effect of CME on standing balance performance at the end of the intervention (SMD 0.53, 95% CI 0.19 to 0.87; P = 0.002; Analysis 1.6). These findings were based on a small total number of participants and there was clinical heterogeneity between studies resulting in a low quality of evidence. One trial was of unclear risk of bias (Dai 2013), but more than half of the trials were of low risk of bias (Galvin 2011; Wang 2015), and thus there was no reason to downgrade the level of evidence further.
1.6. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 6 Gait and gait‐related measures: balance: combined.
Only one trial assessed standing balance performance at three months' follow‐up (Galvin 2011). There was no significant effect (MD 8.40, 95% CI ‐1.04 to 17.84; P = 0.08; Analysis 2.6). This effect was based on one trial with a small number of participants resulting in a very low quality of evidence.
2.6. Analysis.
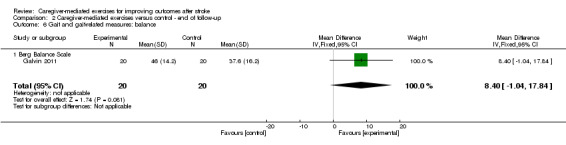
Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 6 Gait and gait‐related measures: balance.
Walking distance
Two trials used the Six‐Minute Walk Test to assess walking distance (Galvin 2011; Wang 2015). We found no significant summary effect of CME at the end of the intervention period (MD 30.98 m, 95% CI ‐20.22 to 82.19; P = 0.24; Analysis 1.7). These findings were based on two trials with a low risk of bias, but with a small total number of participants for this outcome measure, resulting in a moderate quality of evidence.
1.7. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 7 Gait and gait‐related measures: Six‐Minute Walk Test.
Only one trial assessed the Six‐Minute Walk Test at three months' follow‐up (Galvin 2011). There was a significant effect in favour of CME (MD 109.50 m, 95% CI 17.12 to 201.88; P = 0.02; Analysis 2.7). This finding was based on one trial with a small number of participants, resulting in a very low quality of evidence.
2.7. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 7 Gait and gait‐related measures: Six‐Minute Walking Test.
Walking speed
Two trials reported comfortable walking speed (Wall 1987; Wang 2015). We found no significant summary effect of CME on walking speed (MD 0.08 m/s, 95% CI ‐0.03 to 0.18; P = 0.17; Analysis 1.8). This effect was based on one trial with low risk of bias (Wang 2015) and one trial with an unclear risk of bias (Wall 1987). In addition, there was a small total number of participants. Therefore, the quality of evidence was low.
1.8. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 8 Gait and gait‐related measures: walking speed.
Only Wall 1987 reported follow‐up data three months after termination of the intervention. We found no significant effect of CME on walking speed (MD 0.10 m/s, 95% CI ‐0.02 to 0.22; P = 0.10; Analysis 2.8). The quality of evidence was very low, because the effect was based on only one trial of unclear risk of bias with a small total number of participants.
2.8. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 8 Gait and gait‐related measures: walking speed.
Measures of upper limb activities or function
Two trials with low risk of bias used the Wolf Motor Function test and the Motor Activity Log (Abu Tariah 2010; Barzel 2015). However, there may be publication bias, because all studies excluded for meta‐analysis were about upper limb training (Agrawal 2013; Gómez 2014; Souza 2015). In addition, there was a small total number of participants for these outcome measures and we detected substantial unexplained statistical heterogeneity between trials. We graded the quality of the evidence as very low, except the Wolf Motor Function test ‐ performance time and the Motor Activity Log ‐ amount of use at the end of intervention, and the Motor Activity Log ‐ quality of movement at both end of intervention and follow‐up. We did not detect any substantial statistical heterogeneity in these cases and, therefore, we graded the quality of evidence as low.
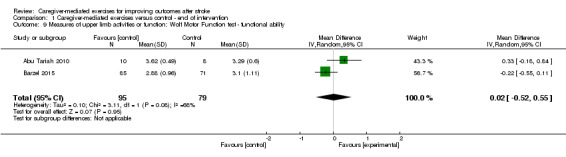
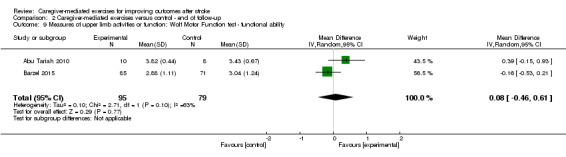
We found no significant summary effect of CME on the Wolf Motor Function test ‐ functional ability (end of intervention: MD 0.02, 95% CI ‐0.52 to 0.55; P = 0.95; Analysis 1.9; follow‐up four to six months after termination: MD 0.08, 95% CI ‐0.46 to 0.61; P = 0.77; Analysis 2.9), the Motor Activity Log ‐ amount of use (end of intervention: MD 0.01, 95% CI ‐0.36 to 0.38; P = 0.96; Analysis 1.11; follow‐up four to six months after termination: MD 0.21, 95% CI ‐0.65 to 1.08; P = 0.63; Analysis 2.11), and Motor Activity Log ‐ quality of movement (end of intervention: MD 0.08, 95% CI ‐0.26 to 0.42; P = 0.64; Analysis 1.12; follow‐up four to six months after termination: MD ‐0.03, 95% CI ‐0.43 to 0.37; P = 0.89; Analysis 2.12).
1.9. Analysis.
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 9 Measures of upper limb activities or function: Wolf Motor Function test ‐ functional ability.
2.9. Analysis.
Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 9 Measures of upper limb activities or function: Wolf Motor Function test ‐ functional ability.
1.11. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 11 Measures of upper limb activities or function: Motor Activity Log (MAL) ‐ amount of use.
2.11. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 11 Measures of upper limb activities or function: Motor Activity Log ‐ amount of use.
1.12. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 12 Measures of upper limb activities or function: MAL ‐ quality of movement.
2.12. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 12 Measures of upper limb activities or function: Motor Activity Log ‐ quality of movement.
For the Wolf Motor Function test ‐ performance time, we found a significant summary effect in favour of the control group post intervention (MD ‐1.72, 95% CI ‐2.23 to ‐1.21; P < 0.00001; Analysis 1.10), but not at follow‐up (MD 1.85, 95% CI ‐8.78 to 12.48; P = 0.73; Analysis 2.10).
1.10. Analysis.
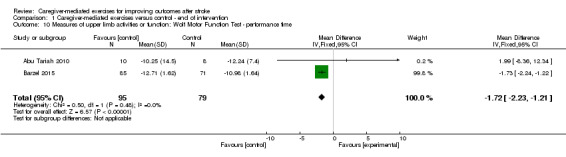
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 10 Measures of upper limb activities or function: Wolf Motor Function Test ‐ performance time.
2.10. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 10 Measures of upper limb activities or function: Wolf Motor Function test ‐ performance time.
One trial used the Nine Hole Peg test (Barzel 2015). We found no significant effect post intervention (MD ‐0.04, 95% CI ‐0.11 to 0.03; P = 0.26; Analysis 1.13) or at follow‐up (MD ‐0.05, 95% CI ‐0.12 to 0.02; P = 0.17; Analysis 2.13). This evidence was based on one trial with a small number of participants, resulting in a very low quality of evidence.
1.13. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 13 Measures of upper limb activities or function: Nine Hole Peg test.
2.13. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 13 Measures of upper limb activities or function: Nine Hole Peg test.
Measures of mood and quality of life of the patient
One trial assessed QoL of the patients with the SIS 3.0 at the end of the intervention (Wang 2015), and one trial assessed only SIS hand function (Barzel 2015).
The effect of CME was significant for the composite physical scale (MD 12.40, 95% CI 1.67 to 23.13; P = 0.02; Analysis 1.14), mobility scale (MD 18.20, 95% CI 7.54 to 28.86; P = 0.0008; Analysis 1.17), and general recovery scale (MD 15.10, 95% CI 8.44 to 21.76; P < 0.00001; Analysis 1.23).
1.14. Analysis.
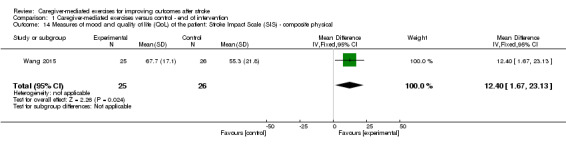
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 14 Measures of mood and quality of life (QoL) of the patient: Stroke Impact Scale (SIS) ‐ composite physical.
1.17. Analysis.
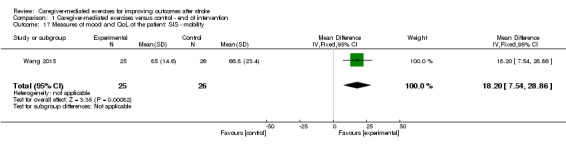
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 17 Measures of mood and QoL of the patient: SIS ‐ mobility.
1.23. Analysis.
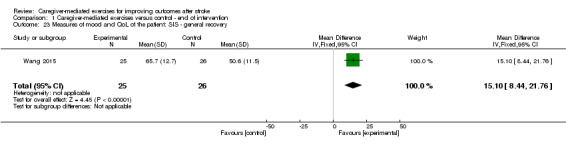
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 23 Measures of mood and QoL of the patient: SIS ‐ general recovery.
For SIS hand function at follow‐up (Barzel 2015), we found no significant effect (MD ‐2.20, 95% CI ‐12.46 to 8.06; P = 0.67; Analysis 2.14). These findings were based on one trial with a small number of participants resulting in a very low quality of evidence. The reported effects on SIS hand function were based on two trials with low risk of bias, but with clinical heterogeneity between studies, resulting in a moderate quality of evidence.
2.14. Analysis.

Comparison 2 Caregiver‐mediated exercises versus control ‐ end of follow‐up, Outcome 14 Measures of mood and quality of life of the patient: Stroke Impact Scale (SIS) ‐ hand function.
Measures of fatigue of the patient
None of the trials reported on effects of CME on fatigue of the patient after intervention or at follow‐up.
Length of stay
None of the included trials reported length of stay as an outcome measure. However, Galvin 2011 did state that mean length of hospital stay for the intervention group was 35.7 days (SD 10.5) and for the control group was 40.1 days (SD 15). Mean length of stay in a rehabilitation unit was 40.3 days (SD 9.6) for the intervention group and 52.3 days (SD 40) for the control group. Patients were recruited in a hospital and a rehabilitation unit. We found no significant differences for length of stay in a hospital (MD 4.40 days, 95% CI ‐3.91 to 12.71; P = 0.30; Analysis 1.24) or length of stay in a rehabilitation unit (MD 12.0 days, 95% CI ‐10.88 to 34.88; P = 0.30; Analysis 1.25). These effects were based on one trial, and length of stay was reported for a small number of participants (n = 20). Therefore, we graded the quality of the evidence as very low.
1.24. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 24 Length of stay ‐ hospital.
1.25. Analysis.
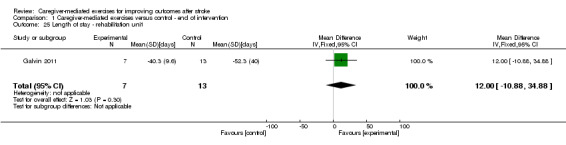
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 25 Length of stay ‐ rehabilitation unit.
Adverse outcomes
One trial reported falls among participants (Dai 2013). We found no significant effect of CME on the number of falls reported (MD 0.04, 95% CI ‐0.10 to 0.18; P = 0.57; Analysis 1.26). There was no follow‐up in this trial. This effect was based on one trial with unclear risk of bias and a small number of participants, resulting in a very low quality of evidence.
1.26. Analysis.
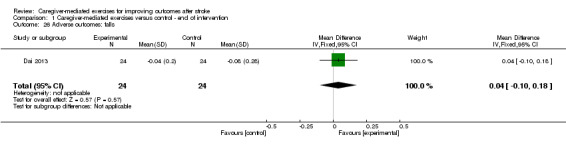
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 26 Adverse outcomes: falls.
Caregiver: measures of mood and quality of life
None of the included trials reported measures of mood or QoL of the caregiver.
Other outcomes
See Table 4.
3. Results 'other outcomes' (not included in meta‐analysis).
| Outcome |
Control group (mean (SD)) |
Intervention group (mean (SD)) |
||||
| Baseline | Post intervention | Follow‐up | Baseline | Post intervention | Follow‐up | |
| Behavioural Inattention Test Conventional (Dai 2013) | 48.79 (44.64) | 68.83 (44.72) | ‐ | 49.71 (39.63) | 88.71 (44.56) | ‐ |
| Motor Assessment Scale (Galvin 2011) | 29.7 (12.9) | 34.5 (11.6) | 35.2 (10.8) | 24.3 (11.1) | 36.1 (10.2) | 37.9 (9.7) |
SD: standard deviation.
Wall 1987 reported on gait parameters such as duration of single support phase and asymmetry ratio. We did not summarise these findings because they were beyond the scope of this review.
Dose of training
In three trials, the dose of training was comparable between the intervention and control groups (Abu Tariah 2010; Souza 2015; Wall 1987).
In six trials, the dose of training in the intervention group was higher than the dose of training in the control group (Agrawal 2013; Barzel 2015; Dai 2013; Galvin 2011; Gómez 2014; Wang 2015). In four of these trials, there was as higher dose of training in the intervention group because the intervention was additional to usual care and the control group received only usual care (Agrawal 2013; Dai 2013; Galvin 2011; Gómez 2014). In one trial, the intensity of training in the intervention group was higher due to the differences between interventions in the intervention and control groups (Wang 2015). The study compared a 90‐minute visit of a therapist and performing activities at least twice weekly, and if possible, every day in the intervention group, with a weekly visit or telephone call of the therapist and maintaining daily routines in the control group. In one trial, daily CIMT, which is a high‐intensity training intervention, was compared with usual care (Barzel 2015). With that, the intensity of training in the intervention group was higher than the dose of training in the control group.
We could not perform subgroup analysis for dose of training (higher dose of training versus same dose of training). For most outcome measures, all included trials had a higher dose of training in the intervention group, so no comparison could be made. For walking speed and upper arm function (Wolf Motor Function test and Motor Activity Log), one included trial was in the higher dose of training group and one included trial was in the same dose of training group. Because there was only one study per subgroup for these outcome measures, we did not perform a subgroup analysis.
Timing post stroke (Comparison 3)
We performed subgroup analyses for trials that included patients within six months after stroke (Agrawal 2013; Dai 2013; Galvin 2011; Gómez 2014) versus trials that included patients beyond six months after stroke (Barzel 2015; Wall 1987; Wang 2015). One trial included patients from beyond two months after stroke (Abu Tariah 2010), and another included patients directly after stroke (Souza 2015); however, the reported mean time since stroke was about nine months after stroke in the Abu Tariah 2010 study and 30 months after stroke in the Souza 2015 study. Therefore, we included both trials in the chronic phase group.
Because of the low number of included trials, we could only perform a subgroup analysis for the outcome measure basic ADL at the end of intervention. We found no difference between trials that included participants within six months after stroke when compared with trials that included patients beyond six months after stroke (P = 0.21; Analysis 3.1). The quality of evidence for this comparison was low, due to clinical heterogeneity between studies and a small total number of participants per subgroup.
3.1. Analysis.

Comparison 3 Timing post stroke ‐ end of intervention, Outcome 1 Patient: activities of daily living measures: combined.
For all other outcome measures, the number of included trials per subgroup was too low to test for subgroup differences.
Upper and lower extremity
Five trials were aimed at the upper extremity (Abu Tariah 2010; Agrawal 2013; Barzel 2015; Gómez 2014; Souza 2015), and four of these trials were about CIMT (Abu Tariah 2010; Barzel 2015; Gómez 2014; Souza 2015). However, Agrawal 2013, Gómez 2014, and Souza 2015 were not included in meta‐analysis.
Two trials were specifically aimed at the lower extremity (Galvin 2011; Wall 1987).
Basic and extended ADL were the only outcome measures in common when comparing upper and lower extremity trials. Due to the low number of trials per subgroup, we could not perform a subgroup analysis.
Reported mean changes (Comparison 4)
Mean change from post intervention to follow‐up
Galvin 2011 reported mean change at follow‐up (three months after termination of the intervention) from post intervention, using the outcome measures BI, CSI, NEADL Index, Reintegration to Normal Living Index, FMA lower extremity score, BBS, Six‐Minute Walk Test, and the Motor Assessment Scale.
This study found a significant effect in favour of CME for the Reintegration to Normal Living Index, CSI and the Six‐Minute Walk Test. The other mean changes were not significantly different. This result was based on one trial with a small number of participants, resulting in a very low quality of evidence.
Sensitivity analysis (Comparisons 5 and 6)
CME‐core
In five trials, CME was the only intervention (CME‐core) (Agrawal 2013; Galvin 2011; Souza 2015; Wall 1987; Wang 2015). Four trials studied the effect of another, existing intervention provided by the caregiver (Abu Tariah 2010; Barzel 2015; Dai 2013; Gómez 2014). In these four trials, it was difficult to separate the effects of CME from the effects of the other intervention (e.g. CIMT). Therefore, we performed a sensitivity analysis that included only CME‐core trials
Three CME‐core trials were suitable for meta‐analyses (Galvin 2011; Wall 1987; Wang 2015). We found a significant summary effect for basic ADL post intervention in favour of CME (2 studies; MD 9.45, 95% CI 2.11 to 16.78; P = 0.01; Analysis 5.1). This effect was based on two studies with low risk of bias, but with a small total number of participants for this outcome measure, resulting in a moderate quality of evidence.
5.1. Analysis.

Comparison 5 Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of intervention, Outcome 1 Patient: activities of daily living (ADL) measures: Barthel Index.
We found no significant effect at follow‐up (1 study; MD 9.00, 95% CI ‐1.29 to 19.29; P = 0.09; Analysis 6.1). This effect was based on one study with a small number of participants, resulting in a very low quality of evidence.
6.1. Analysis.

Comparison 6 Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of follow‐up, Outcome 1 Patient: activities of daily living (ADL) measures: Barthel Index.
For extended ADL, we found no significant summary effect post intervention (1 study; MD 5.50, 95% CI ‐5.83 to 16.83; P = 0.34; Analysis 5.2) or at follow‐up (1 study; MD 9.50, 95% CI ‐1.83 to 20.83; P = 0.10; Analysis 6.2). These effects were based on one study with a small number of participants resulting in a very low quality of evidence. For outcome measures relating to caregiver burden, we found no significant differences between the CME and control groups (see Analysis 1.3; moderate quality of evidence; and Analysis 2.3: very low quality of evidence).
5.2. Analysis.

Comparison 5 Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of intervention, Outcome 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index.
6.2. Analysis.

Comparison 6 Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of follow‐up, Outcome 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index.
For the secondary outcome measures, we found significant effects in favour of CME post intervention for standing balance (2 studies; MD 6.35, 95% CI 1.64 to 11.06; P = 0.008; Analysis 5.3; moderate quality of evidence) and QoL, concerning the composite physical subscale (1 study; MD 12.40, 95% CI 1.67 to 23.13; P = 0.02; Analysis 1.14; very low quality of evidence), mobility subscale (1 study; MD 18.20, 95% CI 7.54 to 28.86; P = 0.0008; Analysis 1.17; very low quality of evidence), and general recovery subscale of the SIS (1 study; MD 15.10, 95% CI 8.44 to 21.76; P < 0.00001; Analysis 1.23; very low quality of evidence). We found a significant effect in favour of CME for walking distance at follow‐up (1 study; MD 109.50 m, 95% CI 17.12 to 201.88; P = 0.02; Analysis 2.7; very low quality of evidence).
5.3. Analysis.

Comparison 5 Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of intervention, Outcome 3 Gait and gait‐related measures: balance: Berg Balance Scale.
The included trials did not report the outcome measures FMA upper extremity, upper limb activities or function, length of stay, and adverse outcome for this sensitivity analysis.
The total number of included trials per subgroup within this sensitivity analysis was too small to test for subgroup differences.
Robustness of the results
In all analyses where we applied a fixed‐effect model, we subsequently applied a random‐effects model. This did not affect the overall results.
For one study, we combined two intervention groups (CME; CME plus physiotherapy) and two control groups (physiotherapy; no intervention) (Wall 1987). When we made separate comparisons of each intervention group versus each control group, we found no differences in the results (Comparison 7).
One study reported two outcome measures for extended ADL: the NEADL Index and the Reintegration to Normal Living Index (Galvin 2011). To prevent double counting this trial in our meta‐analysis, we included the NEADL Index in our primary analysis. We performed a sensitivity analysis in which we replaced the NEADL Index with the Reintegration to Normal Living Index (Comparison 8). Changing the outcome measure did not affect the direction or magnitude of the effect, neither did it affect the significance level of the meta‐analysis.
Qualitative synthesis
We could not include three trials in meta‐analyses: the various reasons are described in the Results of the search section (Agrawal 2013; Gómez 2014; Souza 2015).
All three trials were aimed at the upper extremity, with two trials applying CIMT (Gómez 2014; Souza 2015). For details of these trials, see the Characteristics of included studies table.
Agrawal 2013 comprised exercise training for the upper extremity in addition to usual care for two months. The three groups included a total of 30 participants. The results of each group are separately summarised in Table 5.
4. Results Agrawal 2013 (study not included in meta‐analysis).
| Outcome |
Control group (mean scores) |
GRASP 60 group (mean scores) |
GRASP 90 group (mean scores) |
|||
| Baseline | Post intervention | Baseline | Post intervention | Baseline | Post intervention | |
| Fugl‐Meyer Assessment upper extremity | 31.3 | 37.0 | 32.9 | 44.0 | 34.7 | 48.2 |
| Chedoke Arm and Hand Activities Inventory | 20.3 | 26.8 | 21.0 | 30.0 | 24.4 | 37.0 |
Gómez 2014 studied CIMT with a caregiver in addition to usual care compared with usual care alone. The trial included a total of 60 participants and the intervention lasted 14 days. The goal of this trial was to determine if family support could increase eligibility for CIMT and to study the influence of social and family support. Reported outcomes were a description of the included participants and their level of social and family support. Furthermore, correlations were calculated between ADL, cognitive functioning, and level of social and family support, and were all found to be significant. Means and SDs were not reported. Gómez 2014 concluded that family can play a crucial role in delivering a CIMT protocol and that social and family support has a positive influence on functional outcome of the patient.
Souza 2015 studied CIMT (partly) performed together with a caregiver versus CIMT performed with a therapist. The trial included a total of 24 participants and had a follow‐up of six months. The study authors published effectiveness indexes for the outcome measures Motor Activity Log ‐ quality of movement, FMA upper extremity scale, and Stroke Specific Quality of Life Scale (SSQoL). There were no differences between experimental and control groups and the authors concluded that CIMT therapy (partly) together with a caregiver is equally effective as CIMT therapy with a therapist, but less expensive.
Discussion
Summary of main results
For an overview of the results, see the Table 1.
Effects on outcome measures
This review aimed to determine the effectiveness of CME versus control in people with stroke. We included nine out of 46 potentially relevant trials. The meta‐analyses included 333 patient‐caregiver couples. Four trials assessed the primary outcome measure of ADL. We found no significant summary effect on basic ADL at the end of intervention (Analysis 1.1; moderate quality of evidence) or at follow‐up (Analysis 2.1; low quality of evidence). For extended ADL, there were two trials, in which we found no significant summary effect at the end of intervention (Analysis 1.2; low quality of evidence) or follow‐up (Analysis 2.2; low quality of evidence). Two trials assessed the primary outcome measure of caregiver burden at the end of intervention and one trial at follow‐up. For both time points, we found no significant summary effects of CME (at the end of intervention: Analysis 1.3; moderate quality of evidence; at follow‐up: Analysis 2.3; very low quality of evidence).
With regard to secondary outcome measures, we found a significant effect in favour of CME at the end of intervention for standing balance (three studies; Analysis 1.6; low quality of evidence) and QoL (one study: composite physical (Analysis 1.14), mobility (Analysis 1.17), and general recovery (Analysis 1.23) subscales; very low quality of evidence). The composite physical scale is a sum score of the scales strength, hand function, mobility, and ADL/IADL. We found a significant effect on walking distance at follow‐up (one study; Analysis 2.7; very low quality of evidence). On the Wolf Motor Function test ‐ performance time at the end of intervention there was a significant effect in favour of the control group (two studies; Analysis 1.10; low quality of evidence). We found no significant effects for walking distance post intervention or for standing balance at follow‐up, and QoL was not reported at follow‐up. We found no significant effects for FMA upper and lower extremity scores, walking speed, measures of upper limb activities or function, length of hospital stay, and adverse events (falls) at both post intervention and at follow‐up (where assessed). None of the included trials reported on measures of fatigue of the patient or mood and QoL of the caregiver.
Unfortunately, due to the small number of included trials, we could not apply subgroup analyses with respect to the dose of training and focus of CME training aimed at the upper or lower extremity. In the subgroup analysis regarding timing since stroke onset (within six months after stroke versus beyond six months after stroke), we could only make a comparison for basic ADL at the end of intervention. For the other outcome measures, the number of included studies per subgroup was too small. Timing since stroke did not have an effect on basic ADL at the end of intervention (Analysis 3.1; low quality of evidence).
One trial reported mean changes from post intervention to follow‐up. Most reported mean changes were in favour of CME. The mean change of caregiver burden from post intervention to follow‐up was significantly in favour of the CME group (Analysis 4.4; very low quality of evidence).
4.4. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 4 Caregiver: Caregiver Strain Index.
CME‐core
We included all trials of CME in the primary analysis. However, several trials used CME as the only intervention (CME‐core), where in others a caregiver provided an existing intervention, for example CIMT. In the latter trials, it is difficult to separate the effects of CME from the effects of the other intervention.
Sensitivity analysis with the three trials investigating CME‐core showed one important difference compared with the primary analysis. We found a significant effect in favour of CME‐core on basic ADL post intervention (Analysis 5.1; moderate quality of evidence). On secondary outcome measures, we found the same significant effects in favour of CME as in the primary analysis at the end of intervention for standing balance (Analysis 5.3; moderate quality of evidence) and QoL (composite physical: Analysis 1.14; mobility: Analysis 1.17; general recovery scale: Analysis 1.23; all very low quality of evidence), and at follow‐up for walking distance (Analysis 2.7; very low quality of evidence). We could not perform subgroup analysis.
It is important to note that in the CME‐core analysis only lower extremity trials could be included. An ADL outcome, such as the BI, is more sensitive to lower extremity improvement than to upper extremity improvement (Kwakkel 2004).
These positive effects of CME‐core on basic ADL and standing balance may suggest improved and earlier independence, similar to early supported discharge interventions.
Importance of the CME‐core analysis
There are a limited number of trials and outcome measures included in this meta‐analysis. Due to the number of participants in the trial of Barzel (n = 156), this trial has a large effect on the results (Barzel 2015). The main affected outcome measures are basic ADL, extended ADL, and measures of upper limb activities or function (Wolf Motor Function test and Motor Activity Log).
In this trial, CIMT provided by a caregiver was compared with standard therapy; therefore, this trial is one of the trials in which the effects of CME are difficult to separate from the effects of the other intervention (CIMT). Therefore, we believe that the sensitivity analysis, in which only CME‐core trials are included, is especially important. The effects found in the analysis of CME‐core are probably the most robust to answer the objective of this review.
Activities of daily living
We found no significant effects on basic or extended ADL in the primary analyses. These results were not robust because CME had a significant positive effect on basic ADL at the end of the intervention in the sensitivity analysis of CME‐core. There was no positive effect on basic ADL at follow‐up. This may be attributed to the ceiling effect of outcome measures of basic ADL (Quinn 2011). Therefore, it is important that measures of extended ADL are included in studies investigating CME.
CME has the potential to increase intensity of training. In most included trials in this review, CME did increase intensity of training (Agrawal 2013; Barzel 2015; Dai 2013; Galvin 2011; Gómez 2014; Wang 2015). Several systematic reviews have shown that a higher intensity of training can lead to better outcome in people with stroke in terms of ADL (French 2010; Galvin 2008a; Kwakkel 2004; Kwakkel 2006; Langhorne 2011; Lohse 2014; Veerbeek 2011; Veerbeek 2014), and, therefore, one may expect favourable outcomes in terms of ADL. However, based on the low number of proof‐of‐concept trials and moderate‐quality evidence, our results are not conclusive yet and more trials assessing ADL are needed.
Caregiver burden
CME are yet another task for the caregiver and, therefore, one could hypothesise that CME will lead to an increase in caregiver burden. However, several authors have argued that caregiver burden could actually decrease during CME, due to concurrent education of both patient and caregiver and increased caregiver support, by providing caregivers with more knowledge about the capabilities of the person with stroke and themselves (Galvin 2011; Kalra 2004; Wang 2015). This may potentially increase feelings of self‐efficacy and control of the caregiver (van den Heuvel 2001). When combining data in this review from two trials that assessed caregiver burden, we found no significant effects, that is, there was no increase or decrease in caregiver burden (Galvin 2011; Wang 2015). Quality of the evidence was moderate. Reported mean change on the CSI from post intervention to follow‐up was in favour of CME (Galvin 2011).
Veerbeek 2014 did show a significant homogeneous positive significant effect size on caregiver strain in its meta‐analysis of trials about CME. The difference with our analysis is that they included Kalra 2004, which we excluded because we implemented a different definition of CME. Kalra 2004 applied skill training of the caregiver, which strictly speaking is not the same as CME as it is not a progressive training intervention. However, as skill training and CME may be closely related, the current results on the effect of CME on caregiver burden are not robust. So, results on caregiver burden are inconclusive and more trials assessing caregiver burden in CME are needed.
Adherence to safety
Adherence to safety is essential in CME. Only one included trial assessed adverse events in terms of number of falls, and there were no differences between the intervention and control groups. These findings suggest that, at the least, CME are equally safe as usual care. However, the quality of the evidence was very low. Since a caregiver is not a professional therapist, specific screening, training, and instruction are needed to address safety risks (e.g. falling). Therefore, an important part of each CME protocol should be addressing safety during CME.
Dose of training
Veerbeek 2014 found strong evidence in favour of physiotherapy interventions with intensive, high repetitive, task‐oriented, and task‐specific training in all phases post stroke. This is in line with several other meta‐analyses that showed that intensity of training and repetitive task training are crucial aspects of stroke rehabilitation, suggesting that more exercise therapy is better (French 2010; Galvin 2008a; Kwakkel 2004; Kwakkel 2006; Langhorne 2011; Lohse 2014; Pollock 2014a; Veerbeek 2011; Veerbeek 2014). Pollock and colleagues suggested that a dose of 30 to 60 minutes per day, delivered five to seven days per week, has a surplus value in terms of activities. However, no conclusions could be drawn regarding to the total duration of the intervention due to substantial heterogeneity in the analyses (Pollock 2014a).
All trials included in the high‐intensity training group had a dose of at least 30 to 60 minutes per day delivered five to seven days per week. In all trials, except for Dai 2013, the intervention group received at least 16 hours of exercise treatment compared with the control group (Kwakkel 2004; Veerbeek 2011). In one trial, the intervention group received an extra 10 hours of treatment compared with the control group (Dai 2013).
Unfortunately, in the present review, we could not perform a subgroup analysis of the augmented dose of training compared to dose‐matched trials.
Timing post stroke
The first two months after stroke are considered the optimal time for recovery of function (Cramer 2008; Hankey 2007; Jørgensen 1995; Jørgensen 1999; Kwakkel 2003; van Kordelaar 2014). Pollock 2014a found evidence of greater benefit of an intervention associated with a shorter time since stroke onset. Therefore, increasing intensity of training with CME seems especially meaningful in the first months after stroke. We could only perform one subgroup analysis (basic ADL post intervention), and we found no difference between participants who started the intervention in the first six months after stroke and participants who started the intervention beyond six months after stroke. However, it is not possible to conclude if there are any differences in effect of CME at different time points after stroke due to the low number of included trials in subgroup analyses.
Upper versus lower extremity
Five of the nine included trials were aimed at the upper extremity (Abu Tariah 2010; Agrawal 2013; Barzel 2015; Gómez 2014; Souza 2015), and four of these trials were about CIMT (Abu Tariah 2010; Barzel 2015; Gómez 2014; Souza 2015). CIMT has proven to be an effective therapy (Nijland 2011). However, CIMT can be a time‐consuming therapy and asking for the help of a caregiver can decrease the time spent by a therapist, so the intervention is still enforceable. Souza and colleagues performed an important trial by comparing CIMT therapy (partly) together with a caregiver to CIMT therapy done with a therapist. They found no differences between experimental and control groups and concluded that these forms of therapy provision are equally effective, but that training with a caregiver is less expensive when compared to training with a therapist (Souza 2015).
In our primary analysis, we found a significant effect in favour of the control intervention on the performance time of the Wolf Motor Function test at post intervention, but not at follow‐up. This result is largely determined by a single study with a large number of participants (Barzel 2015), and should, therefore, be considered with caution.
Only two included trials were specifically aimed at the lower extremity (Galvin 2011; Wall 1987). A disadvantage of interventions aimed at the lower extremity is the safety aspect. The risk of adverse events (e.g. tripping or falling) is much higher when standing or walking is practiced compared with practicing the use of the upper extremity. However, evidence for intensity trials focused on the lower limb showed them to be more effective than those aimed at the upper paretic limb after stroke. This latter finding makes focusing CME on gait and gait‐related activities meaningful.
Overall completeness and applicability of evidence
We found a limited number of trials (nine) with substantial variation in type of CME, duration, timing of training (i.e. within six months or beyond six months after stroke), and outcome measures, which hampered summarising and combining data in a meta‐analysis. However, for both primary outcome measures we found two or more trials of relative good quality.
Due to the limited number of included studies, not enough good quality trials were available to perform subgroup analyses, with the exception of timing post stroke (i.e. within six months or beyond six months) for the outcome basic ADL.
Two studies included paid as well as unpaid caregivers, which could not be separated in the results (Dai 2013; Wang 2015). Therefore, this review could not compare the effect of paid and unpaid caregivers. The effects of exercising with a paid caregiver may be different compared with exercising with an unpaid caregiver, especially when there is a difference in the relationship between patient and caregiver.
There may be cultural, ethnic, and societal differences between regions and countries that can influence the applicability and effectiveness of CME interventions. Where ethnicity in itself may not be a limitation for individualised CME programmes after stroke, potential facilitators and barriers may be present that relate to the capacity of the professional to navigate cultural and ethnic differences effectively (Norris 2014).
In addition, involving caregivers during the rehabilitation process can be more or less easy to implement and may be more or less accepted as self‐evident in certain cultures for several reasons. In some countries, rehabilitation services are not readily available and communities are required to help, so‐called 'community‐based rehabilitation' (WHO CBR). One of the excluded trials performed CME in both groups (Redzuan 2012). When contacted, the study author explained that caregivers (or paid workers) are often asked to help in Malaysia. Conversely, caregivers in Western cultures, with advanced healthcare systems and different social practices may be more inclined to leave healthcare services to professionals. However, due to constant changes and budget cuts in Western health care, more pressure is put on the family to provide care. Therefore, CME could have very different implications in different cultures.
Quality of the evidence
The risk of bias of the nine included trials was generally low or unclear (Characteristics of included studies table). Unfortunately, there was insufficient data (fewer than 10 trials) to examine the effects of risk of bias on the calculated estimates of effect reliably by funnel plots.
The overall quality of evidence was very low to moderate. Details of GRADE levels of evidence are presented in the Effects of interventions section. The meta‐analysis could include only six trials and these included trials were small, considering the number of included participants per trial. Therefore, we downgraded most of the evidence one level due to a small total number of participants (fewer than 200 participants). For some outcome measures (mainly aimed at upper extremity functioning), there was substantial unexplained statistical heterogeneity and we downgraded the level of evidence one level. For other outcome measures, there was substantial clinical heterogeneity. There is substantial variation between type of exercises performed with a caregiver between trials. We differentiated between CIMT trials (Abu Tariah 2010; Barzel 2015), trials with mobility exercises (Galvin 2011; Wall 1987; Wang 2015), and other trials (Dai 2013). When these trials were combined, we downgraded the level of evidence because there was clinical heterogeneity. In addition, there may be publication bias in the comparisons about upper extremity functioning, because all trials not included in meta‐analyses were aimed at upper extremity functioning, and, therefore, we downgraded the level of evidence for these outcome measures.
For an overview of the quality of evidence per outcome measure see Table 1.
Potential biases in the review process
In some countries, CME appears to be more necessary or is more accepted, or both, in daily practice due to lack of formal rehabilitation services or because of cultural attitudes. Although speculative, the implementation of CME could, therefore, be different across countries, suggesting that compliance should be systematically measured in CME trials. As we did not identify any completed trials from, for example, Africa, Asia, and South America, information on such cultural differences remains elusive.
In the current review, we made a distinction between CME and skill training of the caregiver, whereby we excluded trials about skill training as skill training does not pertain specifically to a couple performing exercises together. There could potentially be some overlap between these two forms of training. By excluding trials about skill training, potentially useful information from these trials may have been missed. However, we are confident that our current results do adequately reflect the effects of CME.
Regarding the data‐analysis, we employed imputation or extrapolation procedures where SDs were not reported or could not be obtained from the study authors. In four analyses, the SDs from the same trial were used, for example from baseline. For mean changes, we used 95% CI and the Z‐score to calculate SDs. Although this could be a potential source of bias, it is unlikely that results were impacted in a major way.
Authors' conclusions
Implications for practice.
Currently, there is evidence of very low to moderate quality that caregiver‐mediated exercises (CME) can improve patients' functional performance in terms of standing balance and quality of life (QoL) at the end of intervention and walking distance at the end of follow‐up, with no significant increase or decrease effect on caregiver burden and no significant effects on (extended) activities of daily living (ADL). Separate analyses of only CME‐core trials suggest favourable effects in terms of basic ADL at the end of intervention. However, the results should be interpreted with caution since the included phase II trials were small, had potential bias, and had methodological shortcomings including multiple testing. In addition, one outcome measure was in favour of the control group (Wolf Motor function test ‐ performance time), although this result was mainly influenced by one study with a relatively large number of participants.
The findings in this review suggest that CME may be a valuable and resource‐efficient intervention to augment intensity of rehabilitation services after stroke. The effect of CME may be explained by, at least in part, an increase in intensity of training. However, due to the small number of included trials, we could not confirm or reject this hypothesis. In addition, CME can be a treatment option when an increase in intensity of training is useful, for example in constraint‐induced movement therapy (CIMT). To implement CME, it is essential that study protocols are published explaining in detail the type, intensity, and content of exercises as well as safety instructions. Finally, CME can be used in inpatient settings as well as in outpatient settings and may be used in acute, subacute, and chronic phase after stroke.
Implications for research.
Further studies are needed to get a more complete overview of the different aspects of CME such as timing, duration, and frequency, to assess the most suitable target audience, and to assess (long‐term) effects. In addition, it is important to study caregiver burden in relation to CME further, and to assess self‐efficacy and study empowerment of people with stroke and their caregivers, which may allow stroke patients to return earlier to the community and stay independently at home (van Vliet 2015). At the moment, only nine trials have been published that use different outcome measures and measurement tools, making it difficult to summarise and combine outcome measures.
In addition, studies about cost‐effectiveness are needed. CME have the potential to achieve a higher intensity of training, resulting in better functional outcome, without increasing healthcare costs. One included trial recorded length of stay and showed a positive trend (Galvin 2011). However, more studies are needed to determine if CME can be cost‐effective by reducing length of stay, supporting early supported discharge, improving outcomes, and therewith reducing direct and indirect healthcare costs.
To visualise exercises, measure compliance, or keep contact with a supporting therapist, the use of e‐health appears promising. This could also be a cost‐effective method. E‐health in combination with CME has not been studied to date, but two similar clinical trials conducted in different countries (i.e. Adelaide, Australia and Amsterdam, the Netherlands) are currently ongoing (Care4Stroke trial 2014). In particular, because of the impact of availability of community‐based stroke services as well as cultural differences with respect to the role of the caregiver as a co‐therapist, CME cannot be implemented around the world in the same way. Due to these cross‐cultural differences, exercising with a caregiver will be interpreted and implemented differently and so it will be necessary to identify these differences before implementation.
In conclusion, future trials should obey the current CONSORT statements for reporting randomised controlled trials (CONSORT 2010). In addition, they should be powered in a more robust way by including more participants and provide larger treatment contrasts of additional (caregiver‐mediated) exercises when compared with the control group as suggested in several meta‐analyses with respect to intensity of exercise therapy, include a long‐term follow‐up, use a consensus‐based set of clinical outcome measures (particularly with respect to primary outcomes such as basic ADL and extended ADL), as well as perceived burden of the caregiver. Preferably, these trials should include an economical evaluation alongside to investigate the cost‐effectiveness of these services.
Acknowledgements
We acknowledge the support of Brenda Thomas and Hazel Fraser from the Cochrane Stroke Group, Remke Albers from Amsterdam Rehabilitation Research Centre Reade and the Dutch Cochrane Centre for their help in preparing the protocol and the assistance in developing the search strategies. In addition, we wish to acknowledge all external peer reviewers, with special thanks to the consumer reviewer Marion Foreman.
Appendices
Appendix 1. Cochrane search strategy
The Cochrane Library search strategy
‐ Cochrane Central Register of Controlled Trials (CENTRAL)
‐ Cochrane Database of Systematic Reviews (CDSR)
‐ Cochrane Methodology Register (CMR)
‐ Database of Abstracts of Reviews of Effects (DARE)
‐ Health Technology Assessment Database (HTA)
‐ NHS Economic Evaluation Database (NHS EED)
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"]
#2 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 [mh ^hemiplegia] or [mh paresis]
#6 (hempar* or hemipleg* or brain injur*):ti,ab
#7 [mh ^"Gait Disorders, Neurologic"]
#8 {or #1‐#7}
#9 [mh ^caregivers] or [mh ^friends] or [mh parents] or [mh ^spouses] or [mh ^"visitors to patients"]
#10 [mh ^"voluntary workers"] or [mh ^"hospital volunteers"] or [mh ^"home health aides"] or [mh "parent‐child relations"] or [mh "interpersonal relations"]
#11 [mh ^family] or [mh "family characteristics"] or [mh ^"family relations"] or [mh ^"intergenerational relations"]
#12 [mh ^"family therapy"] or [mh ^"family health"]
#13 (carer* or caregiver* or care next giver* or care‐giver*):ti,ab
#14 (family* or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners or neighbour or neighbours):ti,ab
#15 "next of kin":ti,ab
#16 (("non‐professional" or "non professional" or informal or volunteer* or relative or relatives) near/5 (exercise* or rehabilitat* or therap* or train*)):ti,ab
#17 {or #9‐#16}
#18 [mh ^rehabilitation] or [mh ^"activities of daily living"] or [mh "exercise therapy"] or [mh ^"occupational therapy"] or [mh ^"physical therapy modalities"] or [mh "exercise movement techniques"] or [mh Exercise] or [mh ^"Physical Fitness"] or [mh ^"physical endurance"] or [mh ^"early ambulation"] or [mh ^walking] or [mh "Physical and Rehabilitation Medicine"]
#19 (rehabilitat* or "activities of daily living" or ADL or exercis* or physiotherap* or occupational next therap* or physical next therap* or "physical fitness" or "physical endurance" or ambulat* or walk* or progressive next resist*):ti,ab
#20 (muscle near/5 strengthen*):ti,ab
#21 #18 or #19 or #20
#22 #8 and #17 and #21
#23 [mh ^"cerebrovascular disorders"/RH] or [mh "basal ganglia cerebrovascular disease"/RH] or [mh "brain ischemia"/RH] or [mh "carotid artery diseases"/RH] or [mh "intracranial arterial diseases"/RH] or [mh "intracranial arteriovenous malformations"/RH] or [mh "intracranial embolism and thrombosis"/RH] or [mh "intracranial hemorrhages"/RH] or [mh ^stroke/RH] or [mh "brain infarction"/RH] or [mh ^"vasospasm, intracranial"/RH] or [mh ^"vertebral artery dissection"/RH] or [mh ^"brain injuries"/RH] or [mh ^"brain injury, chronic"/RH] or [mh ^hemiplegia/RH] or [mh paresis/RH] or [mh ^"Gait Disorders, Neurologic"/RH]
#24 #17 and #23
#25 #22 or #24
Appendix 2. MEDLINE search strategy (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ or brain injuries/ or brain injury, chronic/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hempar$ or hemipleg$ or brain injur$).tw. 7. Gait Disorders, Neurologic/ 8. or/1‐7 9. caregivers/ or friends/ or exp parents/ or spouses/ or visitors to patients/ 10. voluntary workers/ or hospital volunteers/ or home health aides/ or exp parent‐child relations/ or exp interpersonal relations/ 11. family/ or exp family characteristics/ or family relations/ or intergenerational relations/ 12. family therapy/ or family health/ 13. (carer$ or caregiver$ or care giver$ or care‐giver$).tw. 14. (family$ or families or spous$ or parent or parents or father$ or mother$ or friend or friends or husband$ or wife or wives or partner or partners or neighbour or neighbours).tw. 15. next of kin.tw. 16. ((non‐professional or non professional or informal or volunteer$ or relative or relatives) adj5 (exercise$ or rehabilitat$ or therap$ or train$)).tw. 17. or/9‐16 18. rehabilitation/ or "activities of daily living"/ or exp exercise therapy/ or occupational therapy/ or physical therapy modalities/ or exp exercise movement techniques/ or exp Exercise/ or Physical Fitness/ or physical endurance/ or early ambulation/ or walking/ or exp "Physical and Rehabilitation Medicine"/ 19. (rehabilitat$ or activities of daily living or ADL or exercis$ or physiotherap$ or occupational therap$ or physical therap$ or physical fitness or physical endurance or ambulat$ or walk$ or progressive resist$).tw. 20. (muscle adj5 strengthen$).tw. 21. 18 or 19 or 20 22. 8 and 17 and 21 23. cerebrovascular disorders/rh or exp basal ganglia cerebrovascular disease/rh or exp brain ischemia/rh or exp carotid artery diseases/rh or exp intracranial arterial diseases/rh or exp intracranial arteriovenous malformations/rh or exp "intracranial embolism and thrombosis"/rh or exp intracranial hemorrhages/rh or stroke/rh or exp brain infarction/rh or vasospasm, intracranial/rh or vertebral artery dissection/rh or brain injuries/rh or brain injury, chronic/rh or hemiplegia/rh or exp paresis/rh or Gait Disorders, Neurologic/rh 24. 17 and 23 25. 22 or 24 26. Randomized Controlled Trials as Topic/ 27. random allocation/ 28. Controlled Clinical Trials as Topic/ 29. control groups/ 30. clinical trials as topic/ 31. double‐blind method/ 32. single‐blind method/ 33. cross‐over studies/ 34. Therapies, Investigational/ 35. Research Design/ 36. randomized controlled trial.pt. 37. controlled clinical trial.pt. 38. clinical trial.pt. 39. (random$ or RCT or RCTs).tw. 40. (controlled adj5 (trial$ or stud$)).tw. 41. (clinical$ adj5 trial$).tw. 42. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 46. (cross‐over or cross over or crossover).tw. 47. trial.ti. 48. (assign$ or allocate$).tw. 49. or/26‐48 50. 25 and 49
51. exp animals/ not humans.sh
52. 50 not 51.
Appendix 3. Embase search strategy (Ovid)
1. stroke/ or cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke patient/ or stroke unit/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/ or paresis/ or brain injury/ or acquired brain injury/ or exp neurologic gait disorder/
6. (hempar$ or hemipleg$ or brain injur$).tw.
7. or/1‐6
8. caregiver/ or friend/ or relative/ or exp parent/ or family/ or extended family/ or exp family relation/ or exp nuclear family/ or spouse/ or volunteer/ or voluntary worker/ or family centered care/ or family health/ or family interaction/ or family therapy/ or family life/
9. (carer$ or caregiver$ or care giver$ or care‐giver$).tw.
10. (family$ or families or spous$ or parent or parents or father$ or mother$ or friend or friends or husband$ or wife or wives or partner or partners or neighbour or neighbours).tw.
11. next of kin.tw.
12. ((non‐professional or non professional or informal or volunteer$ or relative or relatives) adj5 (exercise$ or rehabilitat$ or therap$ or train$)).tw.
13. 8 or 9 or 10 or 11 or 12
14. rehabilitation/ or occupational therapy/ or daily life activity/ or exp exercise/ or exp kinesiotherapy/ or physiotherapy/ or fitness/ or endurance/ or mobilization/ or exp walking/ or rehabilitation medicine/
15. (rehabilitat$ or activities of daily living or ADL or exercis$ or physiotherap$ or occupational therap$ or physical therap$ or physical fitness or physical endurance or ambulat$ or walk$ or progressive resist$).tw.
16. (muscle adj5 strengthen$).tw.
17. 14 or 15 or 16
18. 7 and 13 and 17
19. stroke/rh or cerebrovascular disease/rh or exp basal ganglion hemorrhage/rh or exp brain hematoma/rh or exp brain hemorrhage/rh or exp brain infarction/rh or exp brain ischemia/rh or exp carotid artery disease/rh or cerebral artery disease/rh or exp cerebrovascular accident/rh or exp cerebrovascular malformation/rh or exp intracranial aneurysm/rh or exp occlusive cerebrovascular disease/rh or hemiparesis/rh or hemiplegia/rh or paresis/rh or brain injury/rh or acquired brain injury/rh or exp neurologic gait disorder/rh
20. 13 and 19
21. 18 or 20
22. Randomized Controlled Trial/
23. Randomization/
24. Controlled Study/
25. control group/
26. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
27. Crossover Procedure/
28. Double Blind Procedure/
29. Single Blind Procedure/ or triple blind procedure/
30. placebo/
31. (random$ or RCT or RCTs).tw.
32. (controlled adj5 (trial$ or stud$)).tw.
33. (clinical$ adj5 trial$).tw.
34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
35. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
36. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
37. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
38. (cross‐over or cross over or crossover).tw.
39. (placebo$ or sham).tw.
40. trial.ti.
41. (assign$ or allocat$).tw.
42. controls.tw.
43. or/22‐42
44. 21 and 43
45. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
46. 44 not 45
Appendix 4. Embase.com search strategy
1. 'cerebrovascular accident'/de OR 'basal ganglion hemorrhage'/exp OR 'brain ischemia'/exp OR 'carotid artery disease'/exp OR 'cerebral artery disease'/exp OR 'brain arteriovenous malformation'/exp OR 'thromboembolism'/exp OR 'brain hemorrhage'/exp OR 'brain infarction'/exp OR 'brain vasospasm'/de OR 'artery dissection'/de OR 'brain injury'/de OR stroke:ab,ti OR poststroke:ab,ti OR cerebrovasc*:ab,ti OR (brain NEXT/1 vasc*):ab,ti OR (cerebral NEXT/1 vasc*):ab,ti OR cva*:ab,ti OR apoplex*:ab,ti OR sah:ab,ti OR ((brain* OR cerebr* OR cerebell* OR intracran* ORintracerebral) NEAR/5 (ischemi* OR ischaemi* OR infarct* OR thrombo* OR emboli* OR occlus*)):ab,ti OR ((brain* OR cerebr* OR cerebell* OR intracerebral OR intracranial OR subarachnoid) NEAR/5 (haemorrhage* OR hemorrhage* OR haematoma* OR hematoma* OR bleed*)):ab,ti OR'hemiplegia'/de OR 'paresis'/exp OR hemipar*:ab,ti OR hemipleg*:ab,ti OR (brain NEXT/1 injur*):ab,ti OR 'neurologic gait disorder'/de
2. 'caregiver'/exp OR 'friend'/de OR 'parent'/exp OR 'spouse'/de OR 'voluntary worker'/de OR 'child parent relation'/exp OR 'human relation'/exp OR 'family'/de OR 'family size'/exp OR 'family relation'/exp OR 'human relation'/de OR 'family therapy'/de OR 'family health'/de OR carer*:ab,ti OR caregiver*:ab,ti OR (care NEXT/1 giver*):ab,ti OR family*:ab,ti OR families:ab,ti OR spous*:ab,ti OR parent:ab,ti OR parents:ab,ti OR father*:ab,ti OR mother*:ab,ti OR friend:ab,ti OR friends:ab,ti OR husband*:ab,ti OR wife:ab,ti OR wives:ab,ti OR partner:ab,ti OR partners:ab,ti OR neighbour:ab,ti OR neighbours:ab,ti OR 'next of kin':ab,ti OR (('non professional' OR informal OR volunteer* OR relative OR relatives) NEAR/5 (exercise* OR rehabilitat* OR therap* OR train*)):ab,ti
3. 'rehabilitation'/de OR 'daily life activity'/de OR 'kinesiotherapy'/exp OR 'occupational therapy'/de OR 'physiotherapy'/de OR 'exercise'/exp OR 'fitness'/exp OR 'endurance'/exp OR 'mobilization'/de OR 'walking'/de OR 'rehabilitation medicine'/exp OR rehabilitat*:ab,ti OR 'activities of daily living':ab,ti OR adl:ab,ti OR exercis*:ab,ti OR physiotherap*:ab,ti OR (occupational NEXT/1 therap*):ab,ti OR (physical NEXT/1 therap*):ab,ti OR (physical NEXT/1 fitness):ab,ti OR (physical NEXT/1 endurance):ab,ti OR ambulat*:ab,ti OR walk*:ab,ti OR (progressive NEXT/1 resist*):ab,ti OR (muscle NEAR/5 strengthen*):ab,ti
4. #1 AND #2 AND #3
5. 'cerebrovascular accident'/dm_rh OR 'basal ganglion hemorrhage'/exp/dm_rh OR 'brain ischemia'/exp/dm_rh OR 'carotid artery disease'/exp/dm_rh OR 'cerebral artery disease'/exp/dm_rh OR 'brain arteriovenous malformation'/exp/dm_rh OR 'thromboembolism'/exp/dm_rh OR 'brain hemorrhage'/exp/dm_rh OR 'brain infarction'/exp/dm_rh OR 'brain vasospasm'/dm_rh OR 'artery dissection'/dm_rh OR 'brain injury'/dm_rh OR 'hemiplegia'/dm_rh OR 'paresis'/exp/dm_rh OR 'neurologic gait disorder'/dm_rh
6. #2 AND #5
7. #4 OR #6
8. 'randomized controlled trial (topic)'/de OR 'randomization'/de OR 'controlled clinical trial (topic)'/de OR 'control group'/de OR 'clinical trial (topic)'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR 'crossover procedure'/de OR 'experimental therapy'/de OR 'methodology'/de OR 'randomized controlled trial'/exp OR 'controlled clinical trial'/de OR 'clinical trial'/de OR random*:ab,ti OR rct:ab,ti OR rcts:ab,ti OR (controlled NEAR/5 (trial* OR stud*)):ab,ti OR (clinical* NEAR/5 trial*):ab,ti OR ((control OR treatment OR experiment* OR intervention) NEAR/5 (group* OR subject* OR patient*)):ab,ti OR (quasi NEXT/1 random*):ab,ti OR (pseudo NEXT/1 random*):ab,ti OR ((control OR experiment* OR conservative) NEAR/5 (treatment OR therapy OR procedure OR manage*)):ab,ti OR ((singl* OR doubl* OR tripl* OR trebl*) NEAR/5 (blind* OR mask*)):ab,ti OR 'cross over':ab,ti OR crossover:ab,ti OR trial:ti OR assign*:ab,ti OR allocate*:ab,ti
9. #7 AND #8
10. #9 NOT ([animals]/lim NOT [humans]/lim)
11. #10 AND [2014‐2016]/py
Appendix 5. CINAHL search strategy (EBSCO)
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") or (MH "Hypoxia, Brain")
S2 .(MH "Stroke Patients") OR (MH "Stroke Units")
S3 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S4 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 .(MH "Hemiplegia")
S11 .TI ( hemipleg* or hemipar* or brain injur*) or AB ( hemipleg* or hemipar* or brain injur*)
S12 .(MH "Brain Damage, Chronic") OR (MH "Brain Injuries")
S13 .(MH "Gait Disorders, Neurologic")
S14 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 OR S12 OR S13
S15 .(MH "Caregivers") OR (MH "Family") OR (MH "Extended Family+") OR (MH "Family Characteristics+") OR (MH "Family Functioning+") OR (MH "Family Relations+") OR (MH "Nuclear Family+") OR (MH "Spouses") OR (MH "Parents+") OR (MH "Visitors to Patients") OR (MH "Volunteer Workers") OR (MH "Home Health Aides") OR (MH "Family Health") OR (MH "Family Therapy")
S16 .TI ( carer* or caregiver* or care giver* or care‐giver* or family* or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners or neighbour or neighbours or next of kin ) OR AB ( carer* or caregiver* or care giver* or care‐giver* or family* or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners or neighbour or neighbours or next of kin )
S17 .TI ( non‐professional or non professional or informal or volunteer* or relative or relatives ) OR AB ( non‐professional or non professional or informal or volunteer* or relative or relatives )
S18 .TI ( exercise* or rehabilitat* or therap* or train* ) OR AB ( exercise* or rehabilitat* or therap* or train* )
S19 .S17 AND S18
S20 .S15 OR S16 OR S19
S21 .(MH "Rehabilitation") OR (MH "Activities of Daily Living+") OR (MH "Early Ambulation") OR (MH "Home Rehabilitation+") OR (MH "Occupational Therapy+") OR (MH "Physical Therapy+") OR (MH "Rehabilitation, Community‐Based") OR (MH "Exercise+") OR (MH "Therapeutic Exercise+") OR (MH "Physical Fitness") OR (MH "Physical Endurance")
S22 .TI ( rehabilitat* or activities of daily living or ADL or exercis* or physiotherap* or occupational therap* or physical therap* or physical fitness or physical endurance or ambulat* or walk* or progressive resist* ) OR AB ( rehabilitat* or activities of daily living or ADL or exercis* or physiotherap* or occupational therap* or physical therap* or physical fitness or physical endurance or ambulat* or walk* or progressive resist* )
S23 .TI ( muscle and strengthen* ) OR AB ( muscle and strengthen* )
S24 .S21 OR S22 OR S23
S25 .S14 AND S20 AND S24
S26 .PT randomized controlled trial or clinical trial
S27 .(MH "Random Assignment") or (MH "Random Sample+")
S28 .(MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies")
S29 .(MH "Control (Research)") or (MH "Control Group")
S30 .(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials")
S31 .(MH "Placebo Effect") or (MH "Placebos")
S32 .(MH "Clinical Research") or (MH "Clinical Nursing Research")
S33 .(MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design")
S34 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S35 .TI ( controlled N5 (trial* or stud*) ) OR AB ( controlled N5 (trial* or stud*) )
S36 .TI (clinical N5 trial*) OR AB (clinical N5 trial*)
S37 .TI ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) ) OR AB ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) )
S38 .TI ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* ) OR AB ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* )
S39 .TI ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) ) OR AB ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) )
S40 .TI ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) )
S41 .TI ( cross‐over or cross over or crossover ) OR AB ( cross‐over or cross over or crossover )
S42 .TI trial
S43 .TI ( assign* or allocate* ) OR AB ( assign* or allocate* )
S44 .S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43
S45 .S25 AND S44
Appendix 6. SPORTDiscus search strategy (EBSCO)
S1 .DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" OR DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "BRAIN damage"
S2 .DE "CEREBROVASCULAR disease ‐‐ Patients"
S3 .DE "HEMIPLEGIA" OR DE "HEMIPLEGICS"
S4 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH )
S5 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S6 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S7 .S5 and S6
S8 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S9 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S10 .S8 and S9
S11 .TI ( hemipleg* or hemipar* or brain injur*) or AB ( hemipleg* or hemipar* or brain injur* )
S12 .DE "GAIT disorders"
S13 .S1 or S2 or S3 or S4 or S7 or S10 or S11 or S12
S14 .(DE "CAREGIVERS") OR (DE "VOLUNTEER workers in recreation")
S15 .TI ( carer* or caregiver* or care giver* or care‐giver* or family* or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners or neighbour or neighbours or next of kin ) OR AB ( carer* or caregiver* or care giver* or care‐giver* or family* or families or spous* or parent or parents or father* or mother* or friend or friends or husband* or wife or wives or partner or partners or neighbour or neighbours or next of kin )
S16 .TI ( non‐professional or non professional or informal or volunteer* or relative or relatives ) OR AB ( non‐professional or non professional or informal or volunteer* or relative or relatives )
S17 .TI ( exercise* or rehabilitat* or therap* or train* ) OR AB ( exercise* or rehabilitat* or therap* or train* )
S18 .S16 AND S17
S19 .S14 OR S15 OR S18
S20 . DE "REHABILITATION" OR DE "MEDICAL rehabilitation" OR DE "OCCUPATIONAL therapy" OR DE "ACTIVITIES of daily living training" OR DE "EXERCISE" OR DE "RESISTANCE bands (Exercise equipment)" OR DE "EXERCISE physiology" OR DE "ISOMETRIC exercise" OR DE "EXERCISE therapy" OR DE "TREADMILLS (Exercise equipment)" OR DE "EXERCISE intensity" OR DE "ISOTONIC exercise" OR DE "ISOKINETIC exercise" OR DE "EXERCISE ‐‐ Equipment & supplies" OR DE "PHYSICAL fitness" OR DE "PHYSICAL training & conditioning" OR DE "SPORTS physical therapy" OR DE "PHYSICAL fitness for people with disabilities" OR DE "PHYSIOLOGICAL therapeutics" OR DE "PHYSICAL therapy" OR DE "WALKING" OR DE "FITNESS walking"
S21 .TI ( rehabilitat* or activities of daily living or ADL or exercis* or physiotherap* or occupational therap* or physical therap* or physical fitness or physical endurance or ambulat* or walk* or progressive resist* ) OR AB ( rehabilitat* or activities of daily living or ADL or exercis* or physiotherap* or occupational therap* or physical therap* or physical fitness or physical endurance or ambulat* or walk* or progressive resist* )
S22 .TI ( muscle and strengthen* ) OR AB ( muscle and strengthen* )
S23 .S20 OR S21 OR S22
S24 .S13 AND S19 AND S23
Appendix 7. AMED search strategy (Ovid)
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ or hemiplegia/ or brain injuries/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. (hempar$ or hemipleg$ or brain injur$).tw.
6. 1 or 2 or 3 or 4 or 5
7. caregivers/ or exp parents/ or spouses/ or exp voluntary workers/ or family/ or exp family relations/ or family therapy/ or professional family relations/
8. (carer$ or caregiver$ or care giver$ or care‐giver$).tw.
9. (family$ or families or spous$ or parent or parents or father$ or mother$ or friend or friends or husband$ or wife or wives or partner or partners or neighbour or neighbours).tw.
10. next of kin.tw.
11. ((non‐professional or non professional or informal or volunteer$ or relative or relatives) adj5 (exercise$ or rehabilitat$ or therap$ or train$)).tw.
12. 7 or 8 or 9 or 10 or 11
13. rehabilitation/ or rehabilitation modalities/ or rehabilitation techniques/
14. exp physical therapy modalities/ or physical therapy specialty/ or physiotherapists/
15. occupational therapy modalities/ or occupational therapy techniques/ or occupational therapy specialty/ or occupational therapists/
16. "activities of daily living"/ or exp exercise/ or exp exercise therapy/ or physical fitness/ or physical endurance/ or exp walking/ or early ambulation/
17. (rehabilitat$ or activities of daily living or ADL or exercis$ or physiotherap$ or occupational therap$ or physical therap$ or physical fitness or physical endurance or ambulat$ or walk$ or progressive resist$).tw.
18. (muscle adj5 strengthen$).tw.
19. 13 or 14 or 15 or 16 or 17 or 18
20. 6 and 12 and 19
21. research design/ or clinical trials/ or randomized controlled trials/ or comparative study/ or double blind method/ or random allocation/ or single blind method/ or placebos/
22. (random$ or RCT or RCTs).tw.
23. (controlled adj5 (trial$ or stud$)).tw.
24. (clinical$ adj5 trial$).tw.
25. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
27. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
28. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
29. (cross‐over or cross over or crossover).tw.
30. (placebo$ or sham).tw.
31. trial.ti.
32. (assign$ or allocat$).tw.
33. controls.tw.
34. or/21‐33
35. 20 and 34
Appendix 8. AMED search strategy (EBSCO)
(DE "CEREBROVASCULAR DISORDERS" OR DE CEREBROVASCULARACCIDENT" OR DE "CEREBRAL HEMORRHAGE" OR DE "CEREBRAL INFARCTION" OR DE "CEREBRAL ISCHEMIA" OR DE "STROKE" OR DE "HEMIPLEGIA" OR DE "BRAIN INJURIES" OR TX (stroke OR poststroke OR post‐stroke OR cerebrovasc* OR brain vasc* OR cerebral vasc* OR cva* OR apoplex* OR SAH) OR TX ((brain* OR cerebr* OR cerebell* OR intracran* OR intracerebral) N5 (ischemi* OR ischaemi* OR infarct* OR thrombo* OR emboli* OR occlus*)) OR TX ((brain* OR cerebr* OR cerebell* OR intracerebral OR intracranial OR subarachnoid) N5 (haemorrhage* OR hemorrhage* OR haematoma* OR hematoma* OR bleed*)) OR TX (hempar* OR hemipleg* OR brain injur*)) AND
(DE "CAREGIVERS" OR DE "PARENTS" OR DE "SPOUSES" OR DE "VOLUNTARY WORKERS" OR DE "FAMILY" OR DE "FAMILY RELATIONS" OR DE "FAMILY THERAPY" OR DE "PROFESSIONAL FAMILY RELATIONS" OR TX (carer* OR caregiver* OR care giver* OR care‐giver*) OR TX (family* OR families OR spous* OR parent OR parents OR father* OR mother* OR friend OR friends OR husband* OR wife OR wives OR partner OR partners OR neighbour OR neighbours) OR TX (next of kin) OR TX ((non‐professional OR non professional OR informal OR volunteer* OR relative OR relatives) N5 (exercise* OR rehabilitat* OR therap* OR train*))) AND (DE "REHABILITATION" OR DE "REHABILITATION MODALITIES" OR DE "REHABILITATION TECHNIQUES" OR DE "PHYSICAL THERAPY MODALITIES" OR DE "PHYSICAL THERAPY SPECIALITY" OR DE "PHYSIOTHERAPISTS" OR DE "OCCUPATIONAL THERAPY MODALITIES" OR DE "OCCUPATIONAL THERAPY SPECIALITY" OR DE "OCCUPATIONAL THERAPY TECHNIQUES" OR DE "OCCUPATIONAL THERAPISTS" OR DE "ACTIVITIES OF DAILY LIVING" OR DE "EXERCISE" OR DE "EXERCISE THERAPY" OR DE "PHYSICAL ENDURANCE" OR DE "PHYSICAL FITNESS" OR DE "WALKING" OR DE "EARLY AMBULATION" OR TX (rehabilitat* OR activities of daily living OR ADL OR exercis* OR physiotherap* OR occupational therap* OR physical therap* OR physical fitness OR physical endurance OR ambulat* OR walk* OR progressive resist*) OR TX (muscle N5 strengthen*)) AND (DE "RESEARCH DESIGN" OR DE "CLINICAL TRIALS" OR DE "RANDOMIZED CONTROLLED TRIALS" OR DE "COMPARATIVE STUDY" OR DE "DOUBLE BLIND METHOD" OR DE "RANDOM ALLOCATION" OR DE "SINGLE BLIND METHOD" OR DE "PLACEBOS" OR TX (random* OR RCT OR RCTs) OR TX (controlled N5 (trial* OR stud*)) OR TX (clinical* N5 trial*) OR TX ((control OR treatment OR experiment* OR intervention) N5 (group* OR subject* OR patient*)) OR TX (quasi‐random* OR quasi random* OR pseudo‐random* OR pseudo random*) OR TX ((control OR experiment* OR conservative) N5 (treatment OR therapy OR procedure OR manage*)) OR TX ((singl* OR doubl* OR tripl* OR trebl*) N5 (blind* OR mask*)) OR TX (cross‐over OR cross over OR crossover) OR TX (placebo* OR sham) OR TI trial OR TX (assign* OR allocat*) OR TX controls)
Appendix 9. Search overview
| Database | Provider | Date original search 2014 |
Date update search 2015 (limited to 2014 to 2016) |
Appendix number |
| Cochrane Central Register of Controlled Trials (CENTRAL) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 | 1 |
| Cochrane Database of Systematic Reviews (CDSR) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 | 1 |
| Cochrane Methodology Register (CMR) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 | 1 |
| Database of Abstracts of Reviews of Effects (DARE) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 # | 1 |
| Health Technology Assessment Database (HTA) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 | 1 |
| NHS Economic Evaluation Database (NHS EED) | The Cochrane Library, latest issue | 3 April 2014 | 19 October 2015 # | 1 |
| MEDLINE | Ovid | 14 May 2014 | October 2015 | 2 |
| Embase | Ovid | 14 May 2014 | X | 3 |
| Embase | Embase.com | X | 2 December 2015 | 4 |
| CINAHL (Cumulative Index of Nursing and Allied Health Literature) | EBSCO | 14 May 2014 | 9 December 2015 | 5 |
| SPORTDiscus | ‐ | 14 May 2014 | 9 December 2015 | 6 |
| AMED (Alternative and Complementary Medicine) | Ovid | 14 May 2014 | X | 7 |
| AMED | EBSCO | X | 14 December 2015* | 8 |
| Physiotherapy Evidence Database (PEDro) | Website | 7 August 2014 | 1 November 2015 | ‐ |
| REHABDATA | Website | 7 August 2014 | 1 November 2015 | ‐ |
| ClinicalTrials.gov | Website | 3 April 2014 | 19 October 2015 | ‐ |
| EU Clinical Trials Register | Website | 3 April 2014 | 19 October 2015 | ‐ |
| Stroke Trials Registry | Website | 3 April 2014 | 19 October 2015 | ‐ |
| Current Controlled Trials | Website | 3 April 2014 | 19 October 2015 | ‐ |
| World Health Organization International Clinical Trials Registry Platform | Website | 3 April 2014 | 19 October 2015 | ‐ |
| Australian New Zealand Clinical Trials Registry | Website | 3 April 2014 | 19 October 2015 | ‐ |
# From January 2015 no new records/commentaries have been added to DARE and NHS EED. Searches of DARE were continued until the end of the 2014. Bibliographic records were published on DARE and NHS EED until 31 March 2015 and included in Issue 4, 2015 on the Cochrane Library.
* Search not limited to 2014 to 2016.
Data and analyses
Comparison 1. Caregiver‐mediated exercises versus control ‐ end of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living (ADL) measures: combined | 4 | 295 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.02, 0.44] |
| 1.1 Barthel Index | 3 | 247 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.09, 0.41] |
| 1.2 Functional Independence Measure | 1 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.09, 1.06] |
| 2 Patient: ADL measures: extended ADL: combined | 2 | 196 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.21, 0.35] |
| 2.1 Nottingham Extended Activities of Daily Living Index | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.33, 0.92] |
| 2.2 Instrumental Activities of Daily Living (IADL) | 1 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.31, 0.32] |
| 3 Caregiver: burden: combined | 2 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.45, 0.37] |
| 3.1 Caregiver Strain Index | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.85, 0.39] |
| 3.2 Caregiver Burden Scale | 1 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.44, 0.66] |
| 4 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐2.02, 8.22] |
| 5 Measures of motor impairment: Fugl‐Meyer Assessment upper extremity | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.43 [‐2.09, 10.95] |
| 6 Gait and gait‐related measures: balance: combined | 3 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.19, 0.87] |
| 6.1 Berg Balance Scale | 2 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.14, 0.98] |
| 6.2 Postural Assessment Scale for Stroke Patients | 1 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.09, 1.06] |
| 7 Gait and gait‐related measures: Six‐Minute Walk Test | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 30.98 [‐20.22, 82.19] |
| 8 Gait and gait‐related measures: walking speed | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.18] |
| 9 Measures of upper limb activities or function: Wolf Motor Function test ‐ functional ability | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.52, 0.55] |
| 10 Measures of upper limb activities or function: Wolf Motor Function Test ‐ performance time | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.72 [‐2.23, ‐1.21] |
| 11 Measures of upper limb activities or function: Motor Activity Log (MAL) ‐ amount of use | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.36, 0.38] |
| 12 Measures of upper limb activities or function: MAL ‐ quality of movement | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.26, 0.42] |
| 13 Measures of upper limb activities or function: Nine Hole Peg test | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 14 Measures of mood and quality of life (QoL) of the patient: Stroke Impact Scale (SIS) ‐ composite physical | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [1.67, 23.13] |
| 15 Measures of mood and QoL of the patient: SIS ‐ strength | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 12.20 [‐0.08, 24.48] |
| 16 Measures of mood and QoL of the patient: SIS ‐ ADL/IADL | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 11.4 [‐1.11, 23.91] |
| 17 Measures of mood and QoL of the patient: SIS ‐ mobility | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 18.20 [7.54, 28.86] |
| 18 Measures of mood and QoL of the patient: SIS ‐ hand function | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | 2.64 [‐5.87, 11.15] |
| 19 Measures of mood and QoL of the patient: SIS ‐ memory | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 6.30 [‐1.65, 14.25] |
| 20 Measures of mood and QoL of the patient: SIS ‐ communication | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.34, 8.34] |
| 21 Measures of mood and QoL of the patient: SIS ‐ emotion | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐4.35, 8.55] |
| 22 Measures of mood and QoL of the patient: SIS ‐ social participation | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐1.69, 15.09] |
| 23 Measures of mood and QoL of the patient: SIS ‐ general recovery | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 15.10 [8.44, 21.76] |
| 24 Length of stay ‐ hospital | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 4.40 [‐3.91, 12.71] |
| 25 Length of stay ‐ rehabilitation unit | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [‐10.88, 34.88] |
| 26 Adverse outcomes: falls | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.10, 0.18] |
1.15. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 15 Measures of mood and QoL of the patient: SIS ‐ strength.
1.16. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 16 Measures of mood and QoL of the patient: SIS ‐ ADL/IADL.
1.18. Analysis.
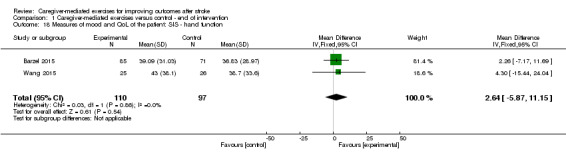
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 18 Measures of mood and QoL of the patient: SIS ‐ hand function.
1.19. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 19 Measures of mood and QoL of the patient: SIS ‐ memory.
1.20. Analysis.
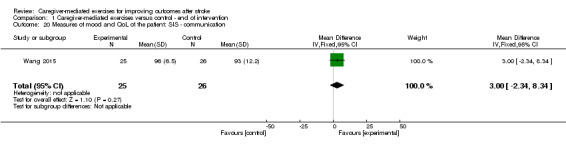
Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 20 Measures of mood and QoL of the patient: SIS ‐ communication.
1.21. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 21 Measures of mood and QoL of the patient: SIS ‐ emotion.
1.22. Analysis.

Comparison 1 Caregiver‐mediated exercises versus control ‐ end of intervention, Outcome 22 Measures of mood and QoL of the patient: SIS ‐ social participation.
Comparison 2. Caregiver‐mediated exercises versus control ‐ end of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living (ADL) measures: ADL | 2 | 196 | Mean Difference (IV, Random, 95% CI) | 2.69 [‐8.18, 13.55] |
| 1.1 Barthel Index | 2 | 196 | Mean Difference (IV, Random, 95% CI) | 2.69 [‐8.18, 13.55] |
| 2 Patient: ADL measures: extended ADL: combined | 2 | 196 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.17, 0.39] |
| 2.1 Nottingham Extended Activities of Daily Living Index | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.12, 1.14] |
| 2.2 IADL | 1 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.31, 0.32] |
| 3 Caregiver: burden | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.71, 1.91] |
| 3.1 Caregiver Strain Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.71, 1.91] |
| 4 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐1.74, 8.54] |
| 5 Measures of motor impairment: Fugl‐Meyer Assessment upper extremity | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 2.75 [‐8.24, 13.74] |
| 6 Gait and gait‐related measures: balance | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 8.40 [‐1.04, 17.84] |
| 6.1 Berg Balance Scale | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 8.40 [‐1.04, 17.84] |
| 7 Gait and gait‐related measures: Six‐Minute Walking Test | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 109.50 [17.12, 201.88] |
| 8 Gait and gait‐related measures: walking speed | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.02, 0.22] |
| 9 Measures of upper limb activities or function: Wolf Motor Function test ‐ functional ability | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.46, 0.61] |
| 10 Measures of upper limb activities or function: Wolf Motor Function test ‐ performance time | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 1.85 [‐8.78, 12.48] |
| 11 Measures of upper limb activities or function: Motor Activity Log ‐ amount of use | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.65, 1.08] |
| 12 Measures of upper limb activities or function: Motor Activity Log ‐ quality of movement | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.43, 0.37] |
| 13 Measures of upper limb activities or function: Nine Hole Peg test | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 14 Measures of mood and quality of life of the patient: Stroke Impact Scale (SIS) ‐ hand function | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐12.46, 8.06] |
Comparison 3. Timing post stroke ‐ end of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living measures: combined | 4 | 295 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.02, 0.44] |
| 1.1 < 6 months | 2 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.01, 0.86] |
| 1.2 > 6 months | 2 | 207 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.16, 0.39] |
Comparison 4. Mean change from post intervention ‐ end of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living (ADL) measures: Barthel Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [‐3.95, 8.55] |
| 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 4.00 [‐0.99, 8.99] |
| 3 Patient: ADL measures: extended ADL ‐ reintegration to normal living index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 4.3 [2.03, 6.57] |
| 4 Caregiver: Caregiver Strain Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.45, 1.75] |
| 5 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.21, 2.81] |
| 6 Gait and gait‐related measures: balance: Berg Balance Scale | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐4.78, 2.98] |
| 7 Gait and gait‐related measures: Six‐Minute Walking Test | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 43.3 [15.11, 71.49] |
| 8 Other outcomes: Motor Assessment Scale | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐0.92, 3.12] |
4.1. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 1 Patient: activities of daily living (ADL) measures: Barthel Index.
4.2. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index.
4.3. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 3 Patient: ADL measures: extended ADL ‐ reintegration to normal living index.
4.5. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 5 Measures of motor impairment: Fugl‐Meyer Assessment lower extremity.
4.6. Analysis.

Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 6 Gait and gait‐related measures: balance: Berg Balance Scale.
4.7. Analysis.
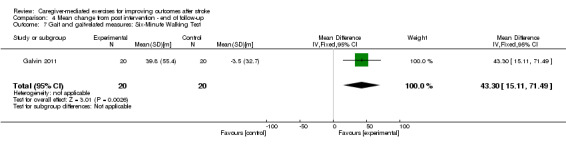
Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 7 Gait and gait‐related measures: Six‐Minute Walking Test.
4.8. Analysis.
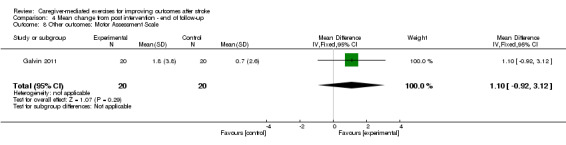
Comparison 4 Mean change from post intervention ‐ end of follow‐up, Outcome 8 Other outcomes: Motor Assessment Scale.
Comparison 5. Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living (ADL) measures: Barthel Index | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 9.45 [2.11, 16.78] |
| 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 5.5 [‐5.83, 16.83] |
| 3 Gait and gait‐related measures: balance: Berg Balance Scale | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 6.35 [1.64, 11.06] |
Comparison 6. Sensitivity analysis ‐ caregiver‐mediated exercise (CME)‐core ‐ end of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: activities of daily living (ADL) measures: Barthel Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐1.29, 19.29] |
| 2 Patient: ADL measures: extended ADL ‐ Nottingham Extended Activities of Daily Living Index | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 9.5 [‐1.83, 20.83] |
Comparison 7. Walking speed, different possibilities study of Wall.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed ‐ caregiver‐mediated exercises (CME) vs physiotherapy ‐ end of intervention | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.07, 0.20] |
| 2 Walking speed ‐ CME vs physiotherapy ‐ end of follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.04, 0.26] |
| 3 Walking speed ‐ CME vs no intervention ‐ end of intervention | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.08, 0.19] |
| 4 Walking speed ‐ CME vs no intervention ‐ end of follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.04, 0.24] |
| 5 Walking speed ‐ CME and physiotherapy vs physiotherapy ‐ end of intervention | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.06, 0.21] |
| 6 Walking speed ‐ CME and physiotherapy vs physiotherapy ‐ end of follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.11, 0.31] |
| 7 Walking speed ‐ CME and physiotherapy vs no intervention ‐ end of intervention | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.20] |
| 8 Walking speed ‐ CME and physiotherapy vs no intervention ‐ end of follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.11, 0.29] |
7.1. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 1 Walking speed ‐ caregiver‐mediated exercises (CME) vs physiotherapy ‐ end of intervention.
7.2. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 2 Walking speed ‐ CME vs physiotherapy ‐ end of follow‐up.
7.3. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 3 Walking speed ‐ CME vs no intervention ‐ end of intervention.
7.4. Analysis.
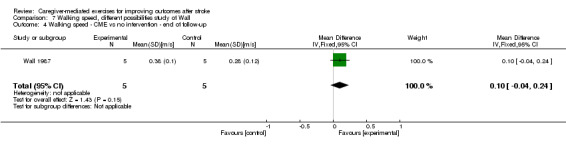
Comparison 7 Walking speed, different possibilities study of Wall, Outcome 4 Walking speed ‐ CME vs no intervention ‐ end of follow‐up.
7.5. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 5 Walking speed ‐ CME and physiotherapy vs physiotherapy ‐ end of intervention.
7.6. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 6 Walking speed ‐ CME and physiotherapy vs physiotherapy ‐ end of follow‐up.
7.7. Analysis.

Comparison 7 Walking speed, different possibilities study of Wall, Outcome 7 Walking speed ‐ CME and physiotherapy vs no intervention ‐ end of intervention.
7.8. Analysis.
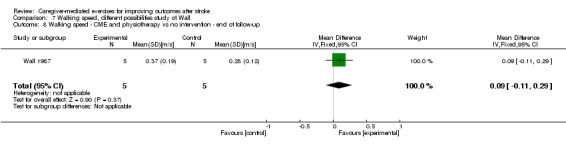
Comparison 7 Walking speed, different possibilities study of Wall, Outcome 8 Walking speed ‐ CME and physiotherapy vs no intervention ‐ end of follow‐up.
Comparison 8. Extended activities of daily living (ADL) ‐ analyses with Reintegration to Normal Living Index (RNLI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient: ADL measures: extended ADL ‐ combined ‐ end of intervention | 2 | 196 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.27, 0.29] |
| 1.1 RNLI | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.59, 0.65] |
| 1.2 Instrumental Activities of Daily Living (IADL) | 1 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.31, 0.32] |
| 2 Patient: ADL measures: extended ADL ‐ combined ‐ end of follow‐up | 2 | 196 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.37, 0.95] |
| 2.1 RNLI | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.05, 1.33] |
| 2.2 IADL | 1 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.31, 0.32] |
8.1. Analysis.
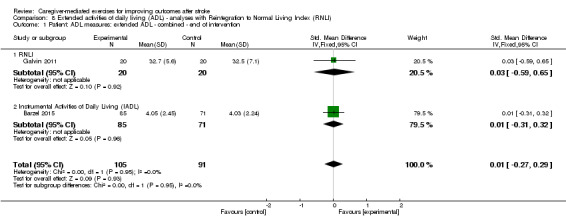
Comparison 8 Extended activities of daily living (ADL) ‐ analyses with Reintegration to Normal Living Index (RNLI), Outcome 1 Patient: ADL measures: extended ADL ‐ combined ‐ end of intervention.
8.2. Analysis.

Comparison 8 Extended activities of daily living (ADL) ‐ analyses with Reintegration to Normal Living Index (RNLI), Outcome 2 Patient: ADL measures: extended ADL ‐ combined ‐ end of follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abu Tariah 2010.
| Methods |
Design: randomised trial of CIMT training vs NDT training Study duration: 6 months (2 months' intervention and 4 months' follow‐up) Randomisation: 20 participants were randomly numbered from 1 to 20; odd numbers participated in the CIMT group, even numbers in the NDT group. Allocation concealment: not applicable: all participants were randomised at the same time. Blinding: assessors blind for group allocation ITT: no |
|
| Participants |
Randomised: 20 participants Withdrawals: 2 participants dropped out of the NDT group at an early stage. There were no reasons given by the participants. Intervention: 10 participants; 8 men and 2 women; mean age 54.8 years (SD 10.9); mean time since stroke 9.2 months (SD 5.79) Control: 8 participants; 4 men and 4 women; mean age 60.6 years (SD 4.9); mean time since stroke 9.6 months (SD 4) Inclusion criteria: stroke > 2 months ago; aged 40 to 75 years; live with family caregivers at their homes; no balance problem that might risk safety Exclusion criteria: recurrent, bilateral or brain stem stroke; inability to actively extend 10° at metacarpophalangeal and interphalangeal joints, and 20° at wrist; substantial use of the involved upper extremity in their life situation: Motor Activity Log ‐ amount of use scale > 2.5; major cognitive deficits (score < 24 points on the Folstein Mini‐Mental State Examination); excessive spasticity and pain, as determined by clinical judgement |
|
| Interventions |
Intervention: CIMT: intensive training of the affected arm 2 hours/day, while restraining the unaffected hand with a resting splint, 7 days/week, for 2 months; 2 trained occupational therapists educated and trained stroke survivors and their caregivers at home in 3 or 4 sessions; detailed information about the training activities to be carried out were given; importance of caregiver commitment was discussed; training activities focuses on patient's ADL/IADL/leisure activities; amount of training was noted in a diary by patients' family. Control: NDT: training consisted of weight bearing and facilitation of arm movement based on conventional NDT procedures; 2 hours/day during weekdays in outpatient clinic and a home programme of 2 hours during the weekend for 2 months; once a week a home visit and follow‐up telephone calls. Setting: outpatient clinic of a large hospital; intervention done at home |
|
| Outcomes |
Included outcomes: Wolf Motor Function test, Motor Activity Log, Fugl‐Meyer Assessment upper extremity Measurements: baseline assessment, post intervention after 2 months, follow‐up 4 months after end of the treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 20 participants were randomly numbered from 1 to 20; odd numbers in CIMT group, even numbers in NDT group. |
| Allocation concealment (selection bias) | Low risk | All participants were randomised at the same time. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blind to the allocation of the group, they provided the evaluation. The investigators were not the therapists who treated the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 withdrawals in the control NDT group, there were no reasons given by the participants. No withdrawals in intervention group. The effect of withdrawal from the control group was unclear. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated. Outcomes were described in results. |
| Other bias | Unclear risk | Small sample size; no ITT analysis |
Agrawal 2013.
| Methods |
Design: randomised trial of exercise training of upper extremity in addition to usual care vs usual care; 3 groups: 90 minutes' exercise training, 60 minutes' exercise training, control Study duration: 4 weeks Randomisation: 'randomly assigned', not described how Allocation concealment: not described Blinding: not described ITT: yes |
|
| Participants |
Randomised: 30 participants Withdrawals: 0 Intervention: Group A (+ 90 minutes): 10 participants; 7 men and 3 women; mean age 55.80 years (SD 4.10); mean time since stroke 3.50 months (SD 1.08) Group B (+ 60 minutes): 10 participants; 5 men and 5 women; mean age 55.70 years (SD 6.24); mean time since stroke 3.70 months (SD 1.34) Group C (control): 10 participants; 7 men and 3 women; mean age 55.20 years (SD 6.12); mean time since stroke 3.50 months (SD 1.08) Inclusion criteria: subacute median carotid artery stroke diagnosed by neuro‐physician on CT or MRI scan; Fugl‐Meyer Assessment upper extremity scale score between 10 and 57; aged 45 to 65 years Exclusion criteria: Mini‐Mental Status Examination score < 20; visual/auditory impairments; presence of any other neurological diagnosis other than stroke or any other major comorbidity; unstable cardiovascular status; non‐co‐operative patients |
|
| Interventions |
Intervention: GRASP (Graded Repetitive Arm Supplementary Program) protocol: self‐administered upper‐limb exercise programme aimed at improving upper‐limb recovery; exercise book and kit tailored according to the motor impairment level; exercise book contained written and pictorial instructions; kit contained inexpensive equipment to complete the exercises; each exercise was graded by varying repetitions to meet each participant's need; exercises included strengthening of the arm, range of motion, and gross and fine motor skills. Repetitive goal and tasks‐oriented activities were designed to simulate partial or whole skill sets required for ADL; 5 days/week, 90 minutes/day (group A) or 60 minutes/day (group B); help of 1 caregiver; weekly meeting with the therapist; plus the education programme: information on stroke recovery and general health. Control: education programme (information on stroke recovery and general health) and conventional physiotherapy (not described) Setting: rehabilitation unit of a hospital |
|
| Outcomes |
Included outcomes: Fugl‐Meyer Assessment upper extremity scale, Chedoke Arm and Hand Activity Inventory Measurements: baseline assessment, post intervention assessment after 4 weeks |
|
| Notes | In results section, SDs were not noted. Contact with authors was unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned"; but not described how. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated |
| Other bias | Unclear risk | None of the SDs in the result section were noted. |
Barzel 2015.
| Methods |
Design: cluster‐randomised trial of home CIMT vs standard therapy Study duration: 4 weeks Randomisation: practices were stratified by region and randomly allocated by an external biometrician (1:1, block size of 4) using a computer‐generated sequence. Allocation concealment: yes, by the computer‐generated sequence. Randomisation was per practice and further allocation concealment was not necessary. Furthermore, patients were included in the study before randomisation of practices to minimise differential self‐selection. Blinding: assessors blind for group allocation; statistician was also masked. ITT: yes |
|
| Participants |
Randomised: 156 participants Withdrawals: 5 withdrawals in the intervention group because of death, poor health, and not wanting to continue; 4 withdrawals in the control group because of moving, death, and poor health Intervention: Home CIMT: 85 participants; 51 men and 34 women; mean age 62.55 years (SD 13.73); mean time since stroke 56.57 months (SD 47.36) Standard therapy: 71 participants; 43 men and 28 women; mean age 65.30 years (SD 12.63); mean time since stroke 45.65 months (SD 57.69) Inclusion criteria:physical and occupational therapy practices: treating adults with upper limb dysfunction after stroke unless they already offered CIMT, with 1 therapist with a professional qualification or at least 2 years of experience in treatment of chronic impairment caused by stroke; patients: > 6 months after stroke, mild‐to‐moderate impairment of arm function and minimal residual hand function (minimum 10° active wrist extension, 10° active thumb abduction or extension, and 10° extension of 2 additional fingers), had a referral for physical or occupational therapy, > 18 years, had a caregiver who was prepared to be a non‐professional coach (e.g. family member). Exclusion criteria: severely impaired verbal communication, inability to give consent, severe neurocognitive deficits (score < 23 in the Mini‐Mental State Examination), terminal illness, or life‐threatening comorbidities, or previously received CIMT. |
|
| Interventions |
Intervention: home CIMT: patients were instructed to train in their home environment for 2 hours each day, accompanied by a coach. Additionally, patients were asked to wear a resting glove during exercises and ADL to immobilise their non‐affected hand. The therapists guided the coach on how to document the time or repetitions per time for each exercise and to assist the patient in keeping a training diary. Therapists used the first of 5 home visits to instruct the patient and the coach in the principles of home CIMT, set individually tailored goals, and work through the first 2 to 3 exercises, focusing on everyday practice. During subsequent weekly home visits, therapists supervised the training, set up new exercises, and applied behavioural techniques. Professional therapy time was not used to practise exercises. Control: conventional physical or occupational therapy, but additional home training was not obligatory. Standard therapy could consist of various therapeutic techniques typical of stroke therapy. The standard therapy group therapists reported details of professional treatment delivery and any agreements (e.g. homework) made with patients via a standardised documentation sheet. Setting: intervention group ‐ home; control group ‐ therapy practice |
|
| Outcomes |
Included outcomes: Motor Activity Log ‐ quality of movement, Wolf Motor Function test ‐ performance time, Motor Activity Log ‐ amount of use, Wolf Motor Function test ‐ functional ability, Nine Hole Peg Test, SIS hand function, Barthel index, IADL Measurements: baseline assessment, post intervention assessment after 4 weeks, follow‐up assessment at 6 months. Interim interview (Motor Activity Log) at 3‐month follow‐up |
|
| Notes | For mean changes of outcomes means and 95% confidence intervals were given. To calculate SDs, we used the Z‐score (1.96). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were stratified and randomly allocated by an external biometrician using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | By computer‐generated sequence. Furthermore, patients were included in the study before randomisation of practices to minimise differential self‐selection. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors and the statistician were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals in intervention group and 4 in the control group; well described and for similar same reasons. Missing data were imputed using correct methods; analyses were by ITT and in case of missing values, a last observation carried forward imputation was performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and preselected outcomes are in the review. There are some minor differences: EQ‐5D, costs and SIS are not described in this paper. |
| Other bias | Low risk | |
Dai 2013.
| Methods |
Design: randomised trial of VR plus conventional rehabilitation vs conventional rehabilitation Study duration: 4 weeks Randomisation: the wards of the same hospital were randomly assigned to the intervention or control group Allocation concealment: not described Blinding: assessors blind for group allocation ITT: no |
|
| Participants |
Randomised: 55 participants Withdrawals: 3 withdrawals in the intervention group because of depression, upper gastrointestinal bleeding, and transfer to another hospital, 4 withdrawals in the control group because of declination (2), asthma attack (1), and transfer to another hospital (1). Intervention: 24 participants; 16 men and 8 women; mean age 57.21 years (SD 12.23); time since stroke 56.88 days (SD 38.93) Control: 24 participants; 12 men and 12 women; mean age 65.54 years (SD 14,67); time since stroke 73.88 days (SD 37.86) Inclusion criteria:for the stroke patients: being diagnosed by physicians by CT or MRI scan of the brain as having experienced a right hemispheric stroke, including haemorrhagic or ischaemic strokes, and first‐time stroke with a duration < 6 months from the stroke onset; meeting the conditions for neglect on any of the 2 scales within the Behavioral Inattention Test Conventional subtest; capable of communicating in Mandarin Chinese or Taiwanese, and understanding instructions; for the primary caregivers: being defined as primary caregivers by patients during inpatient rehabilitation, including family members, friends, employed nursing aides, and foreign caregivers; willing to participate in supervising and guiding the patients' VR training; capable of communicating in Mandarin Chinese or Taiwanese. Exclusion criteria: recurrent stroke with duration > 6 months from stroke onset; < 2 subtests of diagnosed neglect; incapability to communicate; lack of primary caregivers. |
|
| Interventions |
Intervention: VR plus conventional rehabilitation (see control); VR: 1. with their eyes open, the patients moved their head up and down for 20 times or for 1 minute. They also moved their head from side to side for 20 times or for 1 minute, 2. with their eyes closed, the patients moved their head up and down for 20 times or for 1 minute. They also moved their head from side to side for 20 times or for 1 minute, 3. the polypropylene corrugated board was placed on the trainers' thighs. The target was at the same height as the patients' eyes. The patients gazed at the target while moving their head up and down and from side to side for 20 times, 4. the patients rested as necessary. The patients performed steps 1 to 3 repeatedly, and the entire process took approximately 30 minutes; patients were seated in their wheelchairs and their heads and bodies were in the middle position. The instructors verbally reminded the patients to maintain their heads and bodies in the middle position; first and second week a registered trained nurse trained the patients in VR; third and fourth week: patients were guided and supervised by their primary caregivers (the nurse taught the caregivers how to do this in sessions of 5 to 10 minutes, 2 to 4 in total); training once a day for 30 minutes; total of 10 sessions in 2 weeks. Control: conventional rehabilitation: the exercise training for the physiotherapy included passive exercises, active exercises, resistive exercises, ambulation training, and so on. The occupational therapy included maintaining or improving physiological functions such as endurance, balance, and training; to improve ADL, such as dressing, using the toilet, sanitation, home care, and others; 5 days/week for 2 hours. Setting: rehabilitation wards of 2 medical centres |
|
| Outcomes |
Included outcomes: Rivermead Behavioral Inattention Test, Functional Independence Measure, Postural Assessment Scale for people with stroke, falls/person Measurements: baseline assessment, assessment at day 14, and assessment at day 28 |
|
| Notes | "In the Taiwanese health care system, informal caregivers typically assist patients in their activities of daily living" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The wards were randomly assigned, but method not described. |
| Allocation concealment (selection bias) | Unclear risk | The wards were randomly assigned, but method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 withdrawals: 4 in control group and 3 in intervention group. Reasons were well described and about the same. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated |
| Other bias | Unclear risk | No ITT analysis |
Galvin 2011.
| Methods |
Design: randomised trial of exercise therapy (FAME (Fitness And Mobility Exercise) programme) plus 'routine' physiotherapy vs 'routine' physiotherapy Study duration: 5 months (8 weeks' intervention and 3 months' follow‐up) Randomisation: computer‐generated random numbers placed in sealed envelopes. The envelopes were opened by an independent person by enrolment of a participant. Allocation concealment: yes, by the sealed envelopes Blinding: assessor blind for group allocation ITT: yes |
|
| Participants |
Randomised: 40 participants Withdrawals: 2 participants in the intervention group withdrew because of second stroke and myocardial infarction. 1 participant in the control group withdrew because of medically unwell Intervention: 20 participants; 13 men and 7 women; mean age 63.15 years (SD 13.3); time since stroke 18.9 days (SD 2.9) Control: 20 participants; 7 men and 13 women; mean age 69.95 years (SD 11.69); time since stroke 19.7 days (SD 3) Inclusion criteria:for the people with stroke: first unilateral stroke (MRI or CT); no impairment of cognition (> 23 of 30 on the Mini‐Mental State Examination); aged ≥ 18 years; participating in a physiotherapy programme; a family member willing to participate in the programme; 3.2 to 5.2 on the Orpington Prognostic Scale (to control for heterogeneity); for the caregivers: willing to participate in the programme; nominated by the person with stroke as the person that he or she would most like to assist him or her in the performance of the exercises; medically stable and physically able to assist in the delivery of exercises Exclusion criteria (only described in protocol): hemiplegia of a non‐vascular origin; discharge < 2 weeks following stroke; pre‐existing neurological disorder resulting in a motor deficit in addition to that resulting from the stroke; present with any lower extremity orthopaedic condition such as recent fractured femur or amputation; have receptive/expressive dysphasia Suitability was determined after consultation with the individual, their family, and the physiotherapist in charge of the patient's routine care. |
|
| Interventions |
Intervention: FAME programme plus 'routine' physiotherapy (see control); FAME programme: doing exercises together with a nominated family member; daily, 35 minutes, inpatient or at home; weekly were treatment goals set and instructions given by a treating therapist; individual treatment protocol except for the time component; emphasis of the programme was on achieving stability and improving gait velocity and lower limb strength based on patterns derived from findings reported in a systematic review of 151 intervention studies on stroke rehabilitation; a second family member could be involved; compliance was documented with an exercise diary. Control: 'routine' physiotherapy: inpatient or outpatient in either hospital or rehabilitation unit; duration was not recorded; given by staff not linked to the project. Setting: 6 (acute) hospital stroke units or rehabilitation units, or both, in the same hospital or linked to the hospital; inpatient and (if possible) outpatient |
|
| Outcomes |
Included outcomes: lower limb section of the Fugl‐Meyer Assessment, Motor Assessment scale, Berg Balance Scale, Six‐Minute Walk Test, 100‐point original Barthel Index, Reintegration to Normal Living Index, Nottingham Extended Activities of Daily Living Index, Caregiver Strain Index Measurements: baseline, post intervention (8 weeks) and follow‐up 3 months after postintervention assessment |
|
| Notes | No SDs were available for Caregiver Strain Index, Nottingham Extended Activities of Daily Living Index and Reintegration to Normal Living Index at post intervention. Contact with authors was unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in sealed envelopes, which were opened by an independent person. |
| Allocation concealment (selection bias) | Low risk | Random numbers in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was not involved in care and unaware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals in intervention group and 1 in the control group; well described and for similar reasons. Missing data were imputed using correct methods; analyses were by ITT and a last measurement carried forward method was used to account for attrition. |
| Selective reporting (reporting bias) | Low risk | Study protocol was available and all the preselected outcomes were in the review. |
| Other bias | Unclear risk | No SDs were available for Caregiver Strain Index, Nottingham Extended Activities of Daily Living Index and Reintegration to Normal Living Index at post intervention. SDs from follow‐up were imputed. |
Gómez 2014.
| Methods |
Design: randomised trial of CIMT in addition to usual care with help of a caregiver vs usual care Study duration: 14 days Randomisation: simple alternating randomisation Allocation concealment: no Blinding: no information about the assessor of the measurements ITT: not clear: withdrawals were not described. Different numbers were given in the outcome tables. |
|
| Participants |
Randomised: 60 participants Withdrawals: not described Intervention: 30 participants; 20 men and 10 women; mean age 68.03 years (SD 12.43); time since stroke not described Control: 30 participants; 20 men and 10 women; mean age 68.33 years (SD 12.78); time since stroke not described Inclusion criteria: 20 grades extension in the wrist and 10 grades extension in metacarpal joints, subacute phase after stroke, people in wheelchairs or with severe balance problems, people with mild cognitive impairment, people with family support Exclusion criteria: excessive spasticity, behavioural problems |
|
| Interventions |
Intervention: CIMT therapy with a restriction of 75% of the non‐affected arm with a mitt (4 hours free), forced use of the affected arm: daily 1.5 hours with an occupational therapist, 2 hours and ADL monitored by personnel and family and 2 hours of manual activities proposed by the occupational therapist and supervised by family (for 14 days every day?) Patients wore the sling for 14 days. Before the start there was a meeting with the family in which the exercises were explained and a log sheet with activities to be completed every day during the 14 days of the therapy was given. Control: usual care: traditional occupational therapy Setting: a chronic care and long‐stay facility in Spain, inpatient rehabilitation setting |
|
| Outcomes |
Included outcomes: Barthel Index, Index of Lawton and Brody (version 8), Purdue Pegboard, Dynamometer Test, Cognitive Mini Mental Examination of Lobo, modified scale of Socio‐family Gijon; outcomes that needed clarification: cancellation, Nlesulam Measurements: baseline and post intervention (14 days?) |
|
| Notes | Article in Spanish. No means and SDs for outcome measures were given, included outcomes were not all clear, intervention and timing of measurements needed some clarification. However, contact with the authors was unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by simply alternating. However, it was not described which method was used. |
| Allocation concealment (selection bias) | Unclear risk | It was not described which randomisation method was used. Therefore, allocation concealment was unknown. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were not described. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry, nothing stated |
| Other bias | Unclear risk | No means and SDs were described. |
Souza 2015.
| Methods |
Design: randomised trial of CIMT partly supervised by a caregiver vs CIMT supervised by a therapist Study duration: 6 months (22 days' intervention and 6 months' follow‐up) Randomisation: patients were randomised by a staff member not involved in the study. Randomisation information was stored in sealed envelopes that were kept in a cabinet accessible solely to the principal investigator. Allocation concealment: yes, by the sealed envelopes Blinding: assessor blinded for group allocation ITT: no |
|
| Participants |
Randomised: 24 participants Withdrawals: 3 participants in the intervention group withdrew because of fatal recurrent stroke, moving away, and financial limitations; 2 participants in the control group withdrew because of returning to work and finding the exercises too difficult. Intervention: 9 participants; 6 men and 3 women; mean age 61.7 years (SD 12.7); time since stroke 27.6 months (20.9) Control: 10 participants; 9 men and 1 women; mean age 59.5 years (SD 9.1); time since stroke 35.3 months (SD 33.8) Inclusion criteria: aged > 18 years; history of ischaemic or haemorrhagic stroke leading to upper limb paresis in the previous 24 months; minimal active range of motion of 10° for wrist extension, 10° for abduction/extension of the thumb and at least 2 additional digits, 90° for shoulder flexion and abduction, 45° for shoulder external rotation, 30° for elbow extension, 45° for forearm supination and pronation (from neutral position), wrist extension (from neutral), and finger extension of all digits; amount‐of‐use score on the Motor Activity Log > 2.5; balance and stability to move using a glove in the unaffected hand; safe and independent transfer to toilet; ability to stand for 2 minutes with and without the glove (with support of upper limbs, if necessary); availability of a family member to supervise home exercises Exclusion criteria: medical problems or cognitive deficit (Mini‐Mental State Examination score < 24) that could interfere with study completion; aphasia or hemi‐neglect; intended or actual participation in any other study; significant pain (≥ 4 on a visual analogue scale) in any joint; upper limb treatment with antispasticity drugs in the previous 6 months; and severe upper limb spasticity (≥ 3 in the Modified Ashworth Scale) |
|
| Interventions |
Intervention: in the CIMT1.5h_direct group, patients performed exercises with the paretic upper limb for 1.5 hours at an outpatient facility and home exercises, supervised by a caregiver or family member, for additional 1.5 hours. 2 days before treatment started, the caregiver was trained for 1 hour by the researcher providing CIMT on how to supervise the prescribed exercises performed by the patient at home. Each caregiver was instructed to make notes in a log book about the exercises performed, the number of repetitions, and difficulties experienced by the patient. At the beginning of each session, the homework was discussed and when necessary, the level of difficulty was increased or new tasks were prescribed. The CIMT1.5h_direct group received written assignment of practice at home. Control: in the CIMT3h_direct group, patients performed exercises under direct supervision of a therapist, at the outpatient facility, In both groups , training was provided in an individual basis and consisted of shaping principles and task‐specific practice. Shaping exercises comprised a battery of tasks including grasping and releasing objects of different shapes, playing cards and board games, clay activities, drawing, and painting. Tasks were tailored to needs of each patient. Task‐specific practice for both groups involved preparing a snack (sandwiches and juice), including arranging dishes and cutlery on a table, washing and drying them, and putting them in a cupboard. Treatment regimens were designed to ensure that both groups received the same amount of task practice and shaping. Furthermore, in both groups, patients were required to use a padded mitt in the unaffected hand at home, as much as possible during waking hours. The mitt prevented use of the unaffected hand to perform fine motor activities and was used during ADL and household activities. All patients were instructed to record the use time of the mitt and any difficulties perceived at home, in log books. At the beginning of each outpatient session the notes were discussed and, if necessary, problem‐solving strategies were applied. Setting: at home and an outpatient clinic |
|
| Outcomes |
Included outcomes: Motor Activity Log ‐ quality of movement, Fugl‐Meyer Assessment upper extremity scale, Stroke Specific Quality of Life Scale Measurements: baseline, post intervention 2 days after stop of the intervention, and follow‐up 6 months after post intervention assessment |
|
| Notes | No means and SDs were published of post intervention or follow‐up scores, but effectiveness indexes were. However, contact with the authors was unsuccessful. Stroke leading to upper limb paresis in the previous 24 months was named as an inclusion criterion. However, mean time after stroke was 27 months in the intervention group and 35 months in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised by a staff member not involved in the study". But not described how |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded for group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals: 2 in control group and 3 in intervention group. Reasons were well described and similar |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated |
| Other bias | Unclear risk | No ITT No means and SDs were described |
Wall 1987.
| Methods |
Design: randomised trial about exercise therapy; 4 groups: home exercise programme alone, outpatient physiotherapy alone, home exercises + physiotherapy, no intervention Study duration: 9 months (6 months' intervention, 3 months' follow‐up) Randomisation: 'randomly assigned' is stated in article. No further information Allocation concealment: not described Blinding: not described ITT: yes, no withdrawals |
|
| Participants |
Randomised: 20 participants Withdrawals: 0 Intervention and control: 4 interventions; 5 participants per intervention; no information about participants per intervention; in general: aged 45 to 70 years; men and women; time since stroke between 18 months and 10 years Inclusion criteria: not clearly stated (capable of walking with or without a walking stick) Exclusion criteria: negative prognosticators such as serious or unstable medical conditions, major central sensory disorders, homonymous hemianopia, marked cognitive disturbances, intractable pain, motivation defects, incontinence of bowel or bladder |
|
| Interventions |
Intervention: Group B: home exercise programme: 10 exercises over 1 hour. They were designed hierarchically in terms of complexion. Each exercise lasted 5 minutes with the same distribution of exercise and rest. After the fifth and the eighth exercise there was a 5‐minute rest. After 1 month, the most basic exercise was dropped and an additional, more demanding, exercise was added. The exercises were done twice a week. A booklet describing the exercises, duration, and sequence was provided. The programme was undertaken in the person's home with supervision of their spouse or companion. Twice a week for 1 hour. The physiotherapist monitored the programme. Instructional videotapes were available to demonstrate the correct way to do the exercise. These were shown to patients and caregivers when they came for assessment. Group C: outpatient physiotherapy + home exercise programme: exercise programme (as Group B); once a week for 1 hour outpatient physiotherapy and once a week for 1 hour home exercise programme. Control: Group A: outpatient physiotherapy alone; the exercises were taught by a physiotherapist. Feedback and correction was given by this therapist. Twice a week for 1 hour. Group D: control group: no therapy Setting: outpatient |
|
| Outcomes |
Included outcomes: walking speed Other outcomes: measurements of duration of the single support phase of the affected side, measures of the degree of temporal symmetry; asymmetry ratio Measurements: baseline assessment, 1 month interval during treatment, after treatment, and follow‐up after 3 months |
|
| Notes | Inclusion criteria were not clearly stated. There was information about the participants: all participants had residual hemiplegia due to stroke experienced between 18 months and 10 years previously. They all had undergone rehabilitation and were discharged from this. All participants were capable of walking and showed (subjectively) a reduced support phased time of the affected limb. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned", but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated |
| Other bias | Low risk | |
Wang 2015.
| Methods |
Design: randomised trial of a caregiver‐mediated home‐based intervention vs usual care Study duration: 12 weeks Randomisation: with computer‐generated numbers, each approved patient drew a folded piece of paper with 1 of these numbers from a bag. Allocation concealment: yes, folded pieces of paper in a bag Blinding: assessor blind for group allocation ITT: yes, no withdrawals |
|
| Participants |
Randomised: 51 participants Withdrawals: 0 Intervention: 25 participants; 13 men and 12 women; mean age 62.0 years (SD 9.5); time since stroke 18.0 months (SD 15.2) Control: 26 participants; 17 men and 9 women; mean age 65.4 years (SD 10.6); time since stroke 18.5 months (SD 17.1) Inclusion criteria: single ischaemic or haemorrhagic stroke in the cerebral hemisphere, as determined through CT or MRI; > 6 months post onset; mild‐to‐moderate disability (Brunstrom 3 to 5); undergoing rehabilitation activities ≤ 2 times a week; home dwelling; had family members, friends, or paid workers as caregivers; still required assistance to accomplish everyday activities Definition caregiver: a person who was most responsible for person's daily care and who lived with the person. Exclusion criteria: patient: required use of nasogastric feeding, urine tube, tracheal tube; exhibit 1 of the following conditions: recurring stroke, dementia, global or receptive aphasia, severe orthopaedic disability, unstable medical condition; caregiver: poor physical health; mental or behavioural disorders; unable to provide the person at least 2 x 60‐ to 90‐minute sessions of rehabilitation training per week |
|
| Interventions |
Intervention: caregiver‐mediated home‐based intervention (CHI): CHI programme consisted of 3 phases: phase 1 (weeks 1 to 4) to improve person's body functions and structural components; phase 2 (weeks 5 to 8) to improve person's ability to undertake everyday activities within their living environments using task‐specific restorative and compensatory training methods; and phase 3 (weeks 9 to 12) to help the person reintegrate into the society by participating in restorative outdoor leisure activities; a physiotherapist outlined a personalised weekly training schedule according to the CHI programme; weekly visit of the physiotherapist of about 90 minutes: tasks were explained, demonstrated, practiced, and evaluated; individualised training guidelines or illustrations were written; frequency of training and tasks completed was recorded; caregiver was asked to encourage and help if necessary the patient to perform the planned activities twice weekly and if possible every day. Control: usual care: patients maintained their everyday routines; received weekly visits or telephone calls by the therapist to talk about rehabilitation progress, daily activities, and general health; no specific instructions or guidance related to rehabilitation skills. Setting: rehabilitation and neurology departments of teaching hospitals |
|
| Outcomes |
Included outcomes: Barthel Index, Caregiver Burden Scale, Berg Balance Scale, Six‐Minute Walk Test, walking speed, SIS Measurements: baseline assessment, post intervention assessment (12 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated numbers on folded pieces of paper in a bag: each approved patient draw a folded paper". |
| Allocation concealment (selection bias) | Low risk | "Folded pieces of paper in a bag" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blind for the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All outcome measurements were evaluated by an independent physical therapist". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no missing outcomes |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; nothing stated |
| Other bias | Low risk | |
ADL: activities of daily living; CIMT: constraint‐induced movement therapy; CT: computerised tomography; IADL: instrumental activities of daily living; ITT: intention‐to‐treat; MRI: magnetic resonance imaging; NDT: neurodevelopmental treatment; SD: standard deviation; SIS: Stroke Impact Score; VR: vestibular rehabilitation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adie 2014 | No CME intervention. Caregivers were involved in the intervention, but were not obliged. |
| Araujo 2015 | No CME intervention, but skill training and a bit educational intervention. |
| Barzel 2009 | CME, not RCT |
| Baskett 1999 | No CME intervention. Family and caregivers were encouraged to participate during therapy. |
| Bertilsson 2014 | No CME intervention. Caregivers were asked to be involved in the intervention, but were not obliged. |
| Cameron 2015 | No CME intervention. Intervention about how and at which moment to support caregivers. |
| Chang 2015 | No CME intervention. Part of the intervention was skill training. Another part was an intervention for the patient. The 2 interventions could not be separated. |
| Chinchai 2010 | No CME intervention. Educational intervention for the caregiver, with a small part consisting of skill training. |
| El‐Senousey 2012 | No CME intervention but skill training and educational intervention for the caregivers. |
| Evans 1984 | No CME intervention. Educational intervention for patient and caregiver. |
| Forster 2013 | No CME intervention but skill training and educational intervention for the caregivers. |
| Goldberg 1997 | No CME intervention. Intervention consists of a support programme for patient and caregivers at home. |
| Grasel 2005 | Not an RCT, no CME but skill training for the caregivers. |
| Harrington 2010 | No CME intervention. Group community education programme where caregivers were invited to also participate. |
| Harris 2009 | No CME intervention. Family and caregivers were encouraged to participate during therapy. 1 article reported specifically about the role of caregiver involvement in this treatment. |
| Hebel 2014 | No CME intervention, but skill training intervention |
| Hirano 2012 | CME, not RCT |
| Jones 2015 | No CME intervention. "Optional caregiver inclusion" |
| Kalra 2004 | No CME intervention but skill training and educational intervention for the caregivers |
| Koh 2015 | No CME intervention. Caregivers were included to provide safety, but the exercises were done by the patient him‐ or herself. |
| Larson 2005 | No CME intervention. Educational intervention by nurse for caregiver |
| Lin 2004 | No CME intervention. Family and caregivers were encouraged to participate during therapy. |
| Maeshima 2003 | CME, not RCT |
| Marsden 2010 | No CME intervention. Exercises for patient, educational intervention for both patient and caregiver |
| McClellan 2004 | No CME intervention. Family and caregivers were encouraged to participate during therapy. |
| Mudzi 2012 | No CME intervention but skill training and educational intervention for the caregivers |
| NCT00908479 | No CME intervention. Family and caregivers were encouraged to participate during therapy. Only trial information was found. |
| Osawa 2010 | CME, not RCT |
| Parker 2012 | No CME intervention. Educational intervention for the caregiver, with a small part consisting of skill training |
| Redzuan 2012 | Comparison of 2 caregiver‐mediated interventions. Studied intervention was video therapy, not CME. |
| Schure 2006 | No CME intervention. Educational intervention for the caregiver, with a small part consisting of skill training |
| Shyu 2010 | No CME intervention. Educational intervention for the caregiver, with a small part consisting of skill training |
| Smith 2004b | No CME intervention. Educational intervention for patient and caregiver |
| Van de Port 2012 | No CME intervention. Family and caregivers were encouraged to participate during therapy. |
| Walker 1996 | No CME intervention. Intervention aimed at dressing. Family and caregivers were encouraged to participate during therapy. |
CME: caregiver‐mediated exercise; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ATTEND trial 2013.
| Trial name or title | ATTEND trial |
| Methods | RCT |
| Participants | People with stroke, recent ischaemia (< 1 month), residual disability, aged 18 to 99 years, able to identify a nominated caregiver |
| Interventions |
Intervention: trained family‐led caregiver‐delivered, home‐based rehabilitation programme: patient is advised to undergo therapy twice a day for 6 months. Caregiver training is given for approximately 60 minutes/day for up to 3 days. Components: information, joint goal setting, task‐orientated training, discharge planning, exercises. Detailed instructions for exercises will be used from www.physiotherapyexercises.com/ Control: usual care |
| Outcomes | Primary outcome: modified Rankin Scale Secondary outcomes: Barthel Index, Caregiver Burden Scale, health‐related quality of life: WHO Quality of Life ‐ BREF and EuroQoL, patient and caregiver mood: Hospital Anxiety and Depression Scale, Nottingham Extended Activities of Daily Living Index, costs. On 3 and 6 months |
| Starting date | 1 August 2013 Study duration: 4.5 years Sample size: "1200" Information authors: first results are expected in 2016 |
| Contact information | r.lindley@sydney.edu.au jeyarajpandian@hotmail.com |
| Notes | CTRI/2013/04/003557 |
Care4Stroke trial 2014.
| Trial name or title | Care4Stroke program: caregiver mediated exercises with e‐health support for early supported discharge after stroke |
| Methods | RCT |
| Participants | People with stroke, aged > 18 years, in the early rehabilitation phase (24 hours to 3 months), knowing and able to appoint a caregiver who he/she wants to participate in the programme, living independently before the stroke, planned to be discharged home, being able to follow instructions (a Mini‐Mental State Examination score > 23 points), Functional Ambulation Score < 5, score of < 11 on Hospital Anxiety and Depression Scale, motivated for CME, no serious comorbidity Caregivers: aged > 18 years, sufficiently motivated for CME, score of < 11 on the Hospital Anxiety and Depression Scale, medically stable and physically able to perform the exercises with the patient, no significant caregiver strain (< 4 points on Caregiver Strain Index), no serious comorbidity To determine suitability of both patient and partner, an intake exercise session together with a trained therapist will be scheduled prior to inclusion. The therapist will check the inclusion/exclusion criteria and judge if the exercises can be done adequately and safely. |
| Interventions |
Intervention: the Care4Stroke programme consists of 8 weeks of complementary exercise therapy done with a caregiver, alongside usual therapy. 31 standardised exercises are available that can be customised per individual situation. The exercises are presented in a smart phone/ tablet app with videos and voiceover. The patient and their caregiver are asked to do the exercises minimally 5 times/week for 30 minutes on at least both weekend days or the equivalent dosage with an adopted schedule. Patients and their caregiver will have a weekly session with a trained therapist. In this session, the participating couple will be instructed as to which exercises should be performed safely during the next week and evaluate the exercises done last week. All patients and caregivers will be supported by a handbook with instructions. The programme starts when the patient is admitted. When the discharge date of the patient is earlier than the finishing of the programme, the programme continues at home with monitoring from the treating therapist. Control: patients will receive usual care according to the Dutch guidelines for people with stroke and the Royal Dutch Guidelines of Physical Therapy. |
| Outcomes | Primary outcomes: length of stay and the mobility part of the Stroke Impact Scale 3.0 Secondary outcomes: other domains of Stroke Impact Scale 3.0, Fugl‐Meyer Assessment lower extremity Scale, Motricity Index Lower Extremity, Six‐Minute Walk Test, walking speed, Timed‐Up‐and‐Go Test, Berg Balance Scale, Rivermead Mobility Index, Barthel Index, Nottingham Extended Activities of Daily Living Index, modified Rankin Scale, personal opinion questionnaire for empowerment, EuroQol, amount of daily activity For the caregiver: Expanded Caregiver Strain Index, Carer Quality of Life Scale For both patient and caregiver: Hospital Anxiety and Depression Scale, General Self‐efficacy Scale, Fatigue Severity Scale, and (cost) diaries Measurements at baseline, post intervention (8 weeks after randomisation), and follow‐up (12 weeks after randomisation) |
| Starting date | 1 April 2014 Study duration: 2 years Sample size: "66" |
| Contact information | g.kwakkel@vumc.nl r.nijland@reade.nl e.vanwegen@vumc.nl |
| Notes |
www.trialregister.nl/trialreg/admin/rctview.asp?TC=4300 In Australia, a study with the same objective, inclusion and exclusion criteria has been done. Analyses are currently been done. This study is part of the Care4Stroke trial. |
CME: caregiver‐mediated exercises; RCT: randomised controlled trial.
Differences between protocol and review
The second review author who also did the search and cross‐checked data extraction changed from Janne Veerbeek to Marijn Mulder.
Added Johannes Ket to the review team.
Updated 'Description of the intervention' section. Whereby we made clearer that we included interventions which were aimed at improving activities of daily living including mobility (review: 'Hereby, the exercises are aimed at improving activities of daily living including mobility, such as making transfers, standing and walking'), instead of only interventions to improve function (protocol: 'main aim to improve motor function').
Described the definition of 'caregiver' in more detail in the 'Types of interventions' section: a caregiver or carer as an unpaid or partially paid person who voluntarily helps an impaired individual with his or her activities of daily living. In other words, the mediated services were not applied by a professional in health care but in most cases, someone who was close to the patient and voluntarily offered his or her services. This may have been a partner, family member, or friend, but it could have also have been a volunteer. We argued that this person is 'not a professional' such as a 'therapy assistant'.
Included trials that combined caregiver‐mediated exercises (CME) with another intervention in contrary to our description in the protocol ('Types of interventions'). We included these trials because during search and data analysis two forms of CME came forward: trials in which CME was the only intervention (CME‐core) and trials in which caregivers provided an existing intervention. We differentiated between those trials in a sensitivity analysis ('CME‐core').
Changed primary outcome measure 'Caregiver: measures of mood, burden and quality of life' to 'Caregiver: measures of burden. Our primary objective was to learn the effect of CME on caregiver burden. Especially because one can argue that CME gives a caregiver influence and knowledge and, therefore, can lessen caregiver burden, but one can also argue that CME are yet another task for the caregiver in these stressful times and will increase caregiver burden. Moved mood and quality of life to secondary outcomes.
Changed the order of the secondary outcome measures to a more logical order (from impairment to participation).
Renamed the secondary outcome measure 'measures of upper limb activities of function' to 'measures of upper limb activities or function'
Added a sentence under 'Selection of studies' about the screening of abstracts, after the screening of titles.
In the protocol, we proposed under 'Subgroup analysis and investigation of heterogeneity' to do a subgroup analysis of interventions in addition to usual care versus control and interventions instead of usual care versus control. We did not do this subgroup analysis, but a subgroup analysis of interventions with a higher dose of training in the intervention group than the control group versus interventions with a same dose of training in intervention and control group. We changed this because we experienced the importance of difference of dose of training. We noticed that in the group 'interventions instead of usual care' dose of training could still be higher.
Contributions of authors
Judith Vloothuis wrote the protocol with the support of the other authors who directed protocol focus and quality, and commented on the protocol.
Johannes Ket helped to develop search strategies.
Marijn Mulder and Judith Vloothuis screened the references, with the help of Janne Veerbeek.
Erwin van Wegen helped resolving questions and disagreements.
Judith Vloothuis extracted the data and Marijn Mulder cross‐checked these data.
Judith Vloothuis entered data in RevMan and performed analyses.
All authors interpreted the analysis.
Judith Vloothuis drafted the review.
All authors gave input, read, revised, and approved the final version.
Declarations of interest
Judith DM Vloothuis: none known. Marijn Mulder: none known. Janne M Veerbeek: none known. Manin Konijnenbelt: none known. Johanna MA Visser‐Meily: none known. Johannes CF Ket: none known. Gert Kwakkel: none known. Erwin EH van Wegen: none known.
New
References
References to studies included in this review
Abu Tariah 2010 {published data only}
- Abu Tariah H, Almalty AM, Sbeih Z, Al‐Oraibi S. Constraint induced movement therapy for stroke survivors in Jordon: a home‐based model. International Journal of Therapy and Rehabilitation 2010;17(12):638‐46. [Google Scholar]
Agrawal 2013 {published data only}
- Agrawal K, Suchetha PS, Mallikarjunaiah HS. A comparative study on quantity of caregiver support for upper limb functional recovery in post stroke. International Journal of Physiotherapy and Research 2013;3:77‐82. [Google Scholar]
Barzel 2015 {published data only}
- Barzel A, Ketels G, Stark A, Tetzlaff B, Daubmann A, Wegscheider K, et al. Home‐based constraint‐induced movement therapy for patients with upper limb dysfunction after stroke (HOMECIMT): a cluster‐randomised, controlled trial. Lancet Neurology 2015;14(9):893‐902. [DOI] [PubMed] [Google Scholar]
Dai 2013 {published data only}
- Dai CY, Huang YH, Chou LW, Wu SC, Wang RY, Lin LC. Effects of primary caregiver participation in vestibular rehabilitation for unilateral neglect patients with right hemispheric stroke: a randomized controlled trial. Neuropsychiatric Disease and Treatment 2013;9:477‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galvin 2011 {published data only}
- Galvin R, Cusack T, O'Grady E, Murphy TB, Stokes E. Family‐mediated exercise intervention (FAME): evaluation of a novel form of exercise delivery after stroke. Stroke 2011;42(3):681‐86. [DOI] [PubMed] [Google Scholar]
Gómez 2014 {published data only}
- Gómez Martínez M, Tomas Aguirre F, Torregrosa Castellanos C, Labrador Toribio C. The family as a therapeutic collaborator in modified constraint‐induced movement therapy. WFOT Bulletin 2014;70:54‐61. [Google Scholar]
Souza 2015 {published data only}
- Souza WC, Conforto AB, Orsini M, Stern A, André C. Similar effects of two modified constraint‐induced therapy protocols on motor impairment, motor function and quality of life in patients with chronic stroke. Neurology International 2015;7(1):5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 1987 {published data only}
- Wall JC, Turnbull GI. Evaluation of out‐patient physiotherapy and a home exercise program in the management of gait asymmetry in residual stroke. Journal of Neurorehabilitation and Neural Repair 1987;1:115‐23. [Google Scholar]
Wang 2015 {published data only}
- Wang TC, Tsai AC, Wang JY, Lin YT, Lin KL, Chen JJ, et al. Caregiver‐mediated intervention can improve physical functional recovery of patients with chronic stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(1):3‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adie 2014 {published data only}
- Adie K, Schofield C, Berrow M, Wingham J, Freeman J, Humfryes J, et al. Does the use of Nintendo Wii Sports (tm) improve arm function and it is acceptable to patients after stroke? Trial of Wii in stroke ‐ TWIST. International Journal of Stroke 2014;9 Suppl 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araujo 2015 {published data only}
- Araujo O, Lage I, Cabrita J, Teixeira L. Intervention in informal caregivers who take care of older people after a stroke (InCARE): study protocol for a randomised trial. Journal of Advanced Nursing 2015;71(10):2435‐43. [DOI] [PubMed] [Google Scholar]
Barzel 2009 {published data only}
- Barzel A, Liepert J, Haevernick K, Eisele M, Ketels G, Rijntjes M, et al. Comparison of two types of constraint‐induced movement therapy in chronic stroke patients: a pilot study. Restorative Neurology and Neuroscience 2009;27:673‐80. [DOI] [PubMed] [Google Scholar]
Baskett 1999 {published data only}
- Baskett JJ, Broad JB, Reekie G, Hocking C, Green G. Shared responsibility for ongoing rehabilitation: a new approach to home‐based therapy after stroke. Clinical Rehabilitation 1999;13:23‐33. [DOI] [PubMed] [Google Scholar]
Bertilsson 2014 {published data only}
- Bertilsson A‐S, Ranner M, Koch L, Eriksson G, Johansson U, Ytterberg C, et al. A client‐centred ADL intervention: three‐month follow‐up of a randomized controlled trial. Scandinavian Journal of Occupational Therapy 2014;21(5):377‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2015 {published data only}
- Cameron JI, Naglie G, Gignac MA, Bayley M, Warner G, Green T, et al. Randomized clinical trial of the Timing it Right Stroke Family Support Program: research protocol. BMC Health Services Research 2014;17:14‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JI, Naglie G, Green TL, Gignac MA, Bayley M, Huijbregts M, et al. A feasibility and pilot randomized controlled trial of the "Timing it Right Stroke Family Support Program". Clinical Rehabilitation 2015; Vol. 29, issue 11:1129‐40. [DOI] [PubMed]
Chang 2015 {published data only}
- Chang AK, Park YH, Fritschi C, Kim MJ. A family involvement and patient‐tailored health management program in elderly Korean stroke patients' day care centers. Rehabilitation Nursing 2015;40(3):179‐87. [DOI] [PubMed] [Google Scholar]
Chinchai 2010 {published data only}
- Chinchai P, Bunyamark T, Sirisatayawong P. Effects of caregiver education in stroke rehabilitation on the quality of life of stroke survivors. World Federation of Occupational Therapists Bulletin 2010;61:56‐63. [Google Scholar]
El‐Senousey 2012 {published data only}
- El‐Senousey MYA, Aboelsafa AA, El‐Heneedy YA, Khalil MK, Kamel LI, bd‐El‐Aziz AL. Effect of comprehensive training on ischemic stroke patients. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2012;49(3):259‐69. [Google Scholar]
Evans 1984 {published data only}
- Evans R, Held S. Evaluation of family stroke education. International Journal of Rehabilitation Research 1984;7(1):47‐51. [DOI] [PubMed] [Google Scholar]
Forster 2013 {published data only}
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technology Assessment 2013;17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost‐effectiveness analysis. Lancet 2013;382(9910):2069‐76. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J, Nixon J, Kalra L, Smithard D, Patel A, et al. A cluster randomized controlled trial of a structured training programme for caregivers of inpatients after stroke (TRACS). International Journal of Stroke 2012;7(1):94‐9. [DOI] [PubMed] [Google Scholar]
Goldberg 1997 {published data only}
- Goldberg G, Segal ME, Berk SN, Schall RR, Gershkoff AM. Stroke transition after inpatient rehabilitation. Top Stroke Rehabilitation 1997;4(1):64‐79. [DOI] [PubMed] [Google Scholar]
Grasel 2005 {published data only}
- Grasel E, Biehler J, Schmidt R, Schupp W. Intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Controlled clinical trial with follow‐up assessment six months after discharge. Clinical Rehabilitation 2005;19:725‐36. [DOI] [PubMed] [Google Scholar]
- Grasel E, Schmidt R, Biehler J, Schupp W. Long‐term effects of the intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Clinical Rehabilitation 2006;20:577‐83. [DOI] [PubMed] [Google Scholar]
Harrington 2010 {published data only}
- Harrington R. The evaluation of a community based stroke scheme. UK Stroke Forum Conference. Harrogate: Stroke Association, 2006.
- Harrington R, Taylor G, Duggan A, Reed M, Wood V. The evaluation of a community‐based stroke scheme. Disability and Rehabilitation 2007;29(20‐21):1636. [Google Scholar]
- Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood VA. A community‐based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clinical Rehabilitation 2010;24(1):3‐15. [DOI] [PubMed] [Google Scholar]
- Harrington R, Taylor GJ, Duggan A, Reed M, Wood VA. The evaluation of a community‐based stroke scheme. Clinical Rehabilitation 2006;20:1111. [Google Scholar]
- Reed M, Harrington R, Duggan A, Wood VA. Meeting stroke survivors' perceived needs: a qualitative study of a community‐based exercise and education scheme. Clinical Rehabilitation 2010;24:16‐25. [DOI] [PubMed] [Google Scholar]
Harris 2009 {published data only}
- Harris JE, Eng JJ, Miller WC, Dawson AS. A self‐administered graded repetitive arm supplementary program (GRASP) improves arm function during inpatient stroke rehabilitation. A multi‐site randomized controlled trial. Stroke 2009;40:2123‐8. [DOI] [PubMed] [Google Scholar]
- Harris JE, Eng JJ, Miller WC, Dawson AS. The role of caregiver involvement in upper‐limb treatment in individuals with subacute stroke. Physical Therapy 2010;90(9):1302‐10. [DOI] [PubMed] [Google Scholar]
- NCT00359255. The effect of additional arm therapy on arm function after stroke. clinicaltrials.gov/ct2/show/NCT00359255 Date first received: 28 July 2006.
Hebel 2014 {published data only}
- Hebel K, Bieniaszewski L, Kowalewski W. Health education for stroke patient carers: does it affect functional status improvement in patients after ischemic stroke?. Applied Nursing Research 2014;27(3):e7‐e12. [DOI] [PubMed] [Google Scholar]
Hirano 2012 {published data only}
- Physiotherapy with family support is effective for stroke patients in a convalescence rehabilitation ward. Journal of Rehabilitation Medicine (Stiftelsen Rehabiliteringsinformation) 2012:64‐5.
- Hirano Y, Maeshima S, Osawa A, Nishio D, Takeda K, Baba M, et al. The effect of voluntary training with family participation on early home discharge in patients with severe stroke at a convalescent rehabilitation ward. European Neurology 2012;68(4):221‐8. [DOI] [PubMed] [Google Scholar]
Jones 2015 {published data only}
- Jones KM, Bhattacharjee R, Krishnamurthi R, Blanton S, Theadom A, Barker‐Collo S, et al. Methodology of the Stroke Self‐Management Rehabilitation Trial: an international, multisite pilot trial. Journal of Stroke & Cerebrovascular Diseases 2015;24(2):297‐303. [DOI] [PubMed] [Google Scholar]
Kalra 2004 {published data only}
- Training carers of stroke patients improves psychosocial measures in both carer and patient. Evidence‐Based Healthcare and Public Health 2004; Vol. 8:342‐4.
- Training informal carers of stroke patients reduces health and social care costs in the year following a stroke. Evidence‐Based Healthcare and Public Health 2004; Vol. 8:345‐7.
- Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training carers of stroke patients: randomised controlled trial. BMJ 2004;328(7448):1099‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328(7448):1102‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson D. Training informal caregivers of patients with stroke improved patient and caregiver quality of life and reduced costs. Evidence Based Nursing 2004;7(4):118. [DOI] [PubMed] [Google Scholar]
Koh 2015 {published data only}
- Koh GC, Yen SC, Tay A, Cheong A, Ng YS, Silva DA, et al. Singapore Tele‐technology Aided Rehabilitation in Stroke (STARS) trial: protocol of a randomized clinical trial on tele‐rehabilitation for stroke patients. BMC Neurology 2015;15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2005 {published data only}
- Larson J. The impact of a nurse‐led support and education programme for spouses of stroke patients: a randomised controlled trial. Journal of Clinical Nursing 2005;14(8):995‐1003. [DOI] [PubMed] [Google Scholar]
Lin 2004 {published data only}
- Lin JH, Hsieh CL, Lo SK, Chai HM, Liao LR. Preliminary study of the effect of low‐intensity home‐based physical therapy in chronic stroke patients. Kaohsiung Journal of Medical Science 2004;20(1):18‐22. [DOI] [PubMed] [Google Scholar]
Maeshima 2003 {published data only}
- Maeshima S, Matsumoto T, Boh‐oka S, Yoshida M, Ueyoshi A, Ozaki F, et al. Early rehabilitation program for hemiplegic stroke patients: useful training conducted by patient families. 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM); 2001 Jul 6‐13; Amsterdam. Geneva: ISPRM, 2001.
- Maeshima S, Ueyoshi A, Osawa A, Ishida K, Kunimoto K, Shimamoto Y, et al. Mobility and muscle strength contralateral to hemiplegia from stroke: benefit from self‐training with family support. American Journal of Physical Medicine and Rehabilitation 2003;82(6):456‐62. [PubMed] [Google Scholar]
Marsden 2010 {published data only}
- Marsden D, Quinn R, Pond N, Goledge R, Neilson C, White J, et al. A multidisciplinary group programme in rural settings for community‐dwelling chronic stroke survivors and their carers: a pilot randomized controlled trial. Clinical Rehabilitation 2010;24:328‐41. [DOI] [PubMed] [Google Scholar]
McClellan 2004 {published data only}
- McClellan R, Ada L. A six‐week, resource‐efficient mobility program after discharge from rehabilitation improves standing in people affected by stroke: placebo‐controlled, randomised trial. Australian Journal of Physiotherapy 2004;50(3):163‐7. [DOI] [PubMed] [Google Scholar]
- McClellan R, Ada L. Efficacy of a resource‐efficient exercise program in reducing disability and handicap in stroke survivors: a randomised controlled clinical trial. Australian Journal of Physiotherapy 2003;49(4, Suppl):s14. [Google Scholar]
- McClellan R, Ada L. Efficacy of a resource‐efficient exercise program in reducing disability and handicap in stroke survivors: a randomised controlled clinical trial. Internal Medicine Journal 2004;34(1‐2):A11. [Google Scholar]
Mudzi 2012 {published data only}
- Mudzi W, Stewart A, Musenge E. Effect of carer education on functional abilities of patients with stroke. International Journal of Therapy & Rehabilitation 2012;19(7):380‐5. [Google Scholar]
NCT00908479 {published data only}
- NCT00908479. The effect of an inpatient home‐work exercise program on leg function after stroke. clinicaltrials.gov/ct2/show/NCT00908479 Date first received: 26 May 2009.
Osawa 2010 {published data only}
- Osawa A, Maeshima S. Family participation can improve unilateral spatial neglect in patients with acute right hemispheric stroke. European Neurology 2010;63(3):170‐5. [DOI] [PubMed] [Google Scholar]
Parker 2012 {published data only}
- Parker EK, Swint JM, Godwin KM, Ostwald SK. Examining the cost per caregiver of an intervention designed to improve the quality of life of spousal caregivers of stroke survivors. Rehabilitation Nursing 2012;37(5):244‐51. [DOI] [PubMed] [Google Scholar]
Redzuan 2012 {published data only}
- Julia PE, Shahizan MR, Mazlina M, Saini J. Delivering therapy at home: a preliminary result. 7th World Congress for NeuroRehabilitation; 2012 May 16‐19; Melbourne. New York: SAGE Publications Inc, 2012.
- Redzuan NS, Engkasan JP, Mazlan M, Freddy Abdullah SJ. Effectiveness of a video‐based therapy program at home after acute stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93(12):2177‐83. [DOI] [PubMed] [Google Scholar]
Schure 2006 {published data only}
- Schure LM, Heuvel ET, Stewart RE, Sanderman R, Witte LP, Meyboom‐de Jong B. Beyond stroke: description and evaluation of an effective intervention to support family caregivers of stroke patients. Patient Education and Counseling 2006;62(1):46‐55. [DOI] [PubMed] [Google Scholar]
Shyu 2010 {published data only}
- Shyu YL, Kuo L, Chen M, Chen S. A clinical trial of an individualised intervention programme for family caregivers of older stroke victims in Taiwan. Journal of Clinical Nursing 2010;19(11‐12):1675‐85. [DOI] [PubMed] [Google Scholar]
Smith 2004b {published data only}
- Smith J, Forster A, Young J. A randomized trial to evaluate an education programme for patients and carers after stroke. Clinical Rehabilitation 2004;18(7):726‐36. [DOI] [PubMed] [Google Scholar]
Van de Port 2012 {published data only}
- Port I, Valkenet K, Schuurmans M, Visser‐Meily JMA. How to increase activity level in the acute phase after stroke. Journal of Clinical Nursing 2012;21:3574‐8. [DOI] [PubMed] [Google Scholar]
Walker 1996 {published data only}
- Walker MF, Drummond AER, Lincoln NB. Evaluation of dressing practice for stroke patients after discharge from hospital: a crossover design study. Clinical Rehabilitation 1996;10(1):23‐31. [Google Scholar]
References to ongoing studies
ATTEND trial 2013 {published data only}
- Alim M, Lindley R, Felix C, Gandhi DB, Verma SJ, Tugnawat DK, et al. Family‐led rehabilitation after stroke in India: the ATTEND trial, study protocol for a RCT. Trials 2016;17(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix C, Pandian JD. Family‐led rehabilitation after stroke in India: ATTEND trial. International Stroke Conference, 2015 Feb 11‐13; Nashville (TN).
- Gandhi D, Pandian J, Lindley R, Alim M, Maulik P, Murthy GVS, et al. Family‐led rehabilitation after stroke in India: the ATTEND trial. International Journal of Stroke 2015;10:174. [DOI] [PubMed] [Google Scholar]
- Lindley R. The ATTEND Trial: family‐led rehabilitation after stroke in India, 2013. www.georgeinstitute.org/projects/family‐led‐rehabilitation‐after‐stroke‐in‐india‐the‐attend‐trial (last accessed 27 November 2016).
- Lindley R, Pandian JD, Felix C, Alim M, Gandhi DBC, Verma SJ. The ATTEND trial ‐ family‐led rehabilitation after stroke in India: a modified version of early supported discharge with a caregiver delivered home based post‐stroke rehabilitation. European Stroke Conference, 2014 May 6‐9; Nice, France.
- Pandian JD. A multicentre, randomized, blinded outcome assessor, controlled trial, whether a family‐led caregiver‐delivered home‐based rehabilitation intervention versus usual care is an effective, affordable Early Support Discharge strategy for those with disabling stroke in India. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=6195 (date first received 15 April 2013).
- Pandian JD. The ATTEND trial ‐ family‐led rehabilitation after stroke in India: a modified version of early supported discharge with a caregiver delivered home based poststroke rehabilitation. International Journal of Stroke 2014;9:252‐3. [Google Scholar]
- Pandian JD, Felix C, Kaur P, Sharma D, Julia L, Toor G, et al. FAmily‐Led RehabiliTaTion aftEr Stroke in INDia: The ATTEND pilot study. International Journal of Stroke 2015;10:609‐14. [DOI] [PubMed] [Google Scholar]
Care4Stroke trial 2014 {published data only}
- NTR4300. Care 4 Stroke program: caregiver mediated exercises with e‐health support for early supported discharge after stroke. www.trialregister.nl/trialreg/admin/rctview.asp?TC=4300 (date first received 2 December 2013).
- Vloothuis J, Mulder M, Nijland RH, Konijnenbelt M, Mulder H, Hertogh CM, et al. Caregiver‐mediated exercises with e‐health support for early supported discharge after stroke (CARE4STROKE): study protocol for a randomized controlled trial. BMC Neurology 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M, Crotty M, Liu E, Killington M, Kwakkel G, Wegen E. Impact of early supported discharge by caregiver‐mediated exercises and e‐health support after stroke ‐ a proof of concept trial. Stroke 2016; Vol. 47, issue 7:1885‐92. [DOI] [PubMed]
Additional references
"Family boosts results of poststroke therapy."
- "Family boosts results of poststroke therapy". Research Review (International Council on Active Aging) 2011;11(9):3‐3. [Google Scholar]
"THE DAYS AFTER."
- "THE DAYS AFTER". Wellness Options 2005;21:26‐7. [Google Scholar]
Aben 2002
- Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, Hospital Anxiety and Depression Scale, SCL‐90, and Hamilton Depression Rating Scale as screening instruments for depression in stroke patients. Psychosomatics 2002;43(5):386‐93. [DOI] [PubMed] [Google Scholar]
Ada 2009
- Ada L, Dean CM, Lindley R, Lloyd G. Improving community ambulation after stroke: the AMBULATE Trial. BMC Neurology 2009;11(9):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakas 2014
- Bakas T, Clark PC, Kelly‐Hayes M, King RB, Lutz BJ, Miller EL, American Heart Association Council on Cardiovascular and Stroke Nursing and the Stroke Council. Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association and American Stroke Association. Stroke 2014;45(9):2836‐52. [DOI] [PubMed] [Google Scholar]
Barzel 2013
- Barzel A, Ketels G, Tetzlaff B, Krüger H, Haevernick K, Daubmann A, et al. Enhancing activities of daily living of chronic stroke patients in primary health care by modified constraint‐induced movement therapy (HOMECIMT): study protocol for a cluster randomized controlled trial. Trials 2013;14(14):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 1992
- Berg KO, Wood‐Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian Journal of Public Health 1992;83(Suppl 2:S):7‐11. [PubMed] [Google Scholar]
Berg 1995
- Berg K, Wood‐Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scandinavian Journal of Rehabilitation Medicine 1995;27(1):27‐36. [PubMed] [Google Scholar]
Bernhardt 2004
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35(4):1005‐9. [DOI] [PubMed] [Google Scholar]
Bjelland 2002
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research 2002;52(2):69‐77. [DOI] [PubMed] [Google Scholar]
BMJ Clinical Evidence 2012
- What is GRADE?. BMJ Clinical Evidence, 2012:http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665072.html.
Brereton 2002
- Brereton L, Nolan M. 'Seeking': a key activity for new family carers of stroke survivors. Journal of Clinical Nursing 2002;11(1):22‐31. [DOI] [PubMed] [Google Scholar]
Brereton 2007
- Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clinical Rehabilitation 2007;21:867‐84. [DOI] [PubMed] [Google Scholar]
Brouwer 2006
- Brouwer WB, Exel NJ, Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care‐related quality of life of informal caregivers for use in economic evaluations. Quality of Life Research 2006;15(6):1005‐21. [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen HF, Lin KC, Wu CY, Chen CL. Rasch validation and predictive validity of the action research arm test in patients receiving stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 2012;93(6):1039‐45. [DOI] [PubMed] [Google Scholar]
Collen 1990
- Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. International Disability Studies 1990;12(1):6‐9. [DOI] [PubMed] [Google Scholar]
Collen 1991
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13(2):50‐4. [DOI] [PubMed] [Google Scholar]
Collin 1988
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. International Disability Studies 1988;10(2):61‐3. [DOI] [PubMed] [Google Scholar]
Collin 1990
- Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. Journal of Neurology, Neurosurgery and Psychiatry 1990;53(7):576‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cramer 2008
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Annals of Neurology 2008;63(3):272‐87. [DOI] [PubMed] [Google Scholar]
De Haan 1995
- Haan R, Limburg M, Bossuyt P, Meulen J, Aaronson N. The clinical meaning of Rankin 'handicap' grades after stroke. Stroke 1995;26(11):2027‐30. [DOI] [PubMed] [Google Scholar]
De Weerdt 2002
- Weerdt W, Feys H. Assessment of physiotherapy for patients with stroke. Lancet 2002;359(9302):182‐3. [DOI] [PubMed] [Google Scholar]
Dodds 1993
- Dodds TA, Martin DP, Stolov WC, Devo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Archives of Physical Medicine and Rehabilitation 1993;74(5):531‐6. [DOI] [PubMed] [Google Scholar]
Dromerick 2003
- Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. Journal of Rehabilitation Research and Development 2003;40(1):1‐8. [DOI] [PubMed] [Google Scholar]
Duncan 1983
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl‐Meyer assessment of sensorimotor recovery following cerebrovascular accident. Physical Therapy 1983;63(10):1606‐10. [DOI] [PubMed] [Google Scholar]
Duncan 1999
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30(10):2131‐40. [DOI] [PubMed] [Google Scholar]
Duncan 2002
- Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke 2002;33(11):2593‐9. [DOI] [PubMed] [Google Scholar]
Duncan 2003
- Duncan PW, Bode RK, Min LS, Perera S. Rasch analysis of a new stroke‐specific outcome scale: the Stroke Impact Scale. Archives of Physical Medicine and Rehabilitation 2003;84(7):950‐63. [DOI] [PubMed] [Google Scholar]
ESO 2008
- The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. www.karger.com/Article/FullText/131083 (accessed 27 November 2016).
Flansbjer 2005
- Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. Journal of Rehabilitation Medicine 2005;37(2):75‐82. [DOI] [PubMed] [Google Scholar]
French 2010
- French B, Thomas L, Leathley M, Sutton C, McAdam J, Forster A, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta‐analysis. Journal of Rehabilitation Medicine 2010;42(1):9‐14. [DOI] [PubMed] [Google Scholar]
Galvin 2008a
- Galvin R, Murphy B, Cusack T, Stokes E. The impact of increased duration of exercise therapy on functional recovery following stroke ‐ what is the evidence?. Topics in Stroke Rehabilitation 2008;15(4):365‐77. [DOI] [PubMed] [Google Scholar]
Galvin 2008b
- Galvin R, Cusack T, Stokes E. A randomised controlled trial evaluating family mediated exercise (FAME) therapy following stroke. BMC Neurology 2008;20(8):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasdam 2010
- Glasdam S, Timm H, Vittrup R. Support efforts for caregivers of chronically ill persons. Clinical Nursing Research 2010;19(3):233‐65. [DOI] [PubMed] [Google Scholar]
Gordon 2004
- Gordon PA, Perronne KM. When spouses become caregivers: counselling implications for younger couples. Journal of Rehabilitation 2004;80:27‐32. [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, GRADE Working Group. Going from evidence to recommendations. BMJ 2008;336(7652):1049‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hankey 2007
- Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007;68(19):1583‐87. [DOI] [PubMed] [Google Scholar]
Herrmann 1997
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale ‐ a review of validation data and clinical results. Journal of Psychosomatic Research 1997;42(1):17‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoefman 2011
- Hoefman RJ, Exel NJA, Looren de Jong S, Orlewska E, Brouwer W. A new test of the construct validity of the CarerQol instrument: measuring the impact of informal care giving. Quality of Life Research 2011;20(6):875‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 1998
- Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter‐rater reliability and validity of the action research arm test in stroke patients. Age and Ageing 1998;27(2):107‐13. [DOI] [PubMed] [Google Scholar]
Hsieh 2000
- Hsieh CL, Hsueh IP, Mao HF. Validity and responsiveness of the Rivermead Mobility Index in stroke patients. Scandinavian Journal of Rehabilitation Medicine 2000;32(3):140‐2. [DOI] [PubMed] [Google Scholar]
Hsueh 2003
- Hsueh IP, Wang CH, Sheu CF, Hsieh CL. Comparison of psychometric properties of three mobility measures for patients with stroke. Stroke 2003;34(7):1741‐5. [DOI] [PubMed] [Google Scholar]
Jørgensen 1995
- Jørgensen HS, Nakayama H, Raaschou HO, Vive‐Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1995;76(5):406‐12. [DOI] [PubMed] [Google Scholar]
Jørgensen 1999
- Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Physical Medicine and Rehabilitation Clinics of North America 1999;10(4):887. [PubMed] [Google Scholar]
Klinke 2015
- Klinke ME, Hafsteinsdottir TB, Hjaltason H, Jonsdottir H. Ward‐based interventions for patients with hemispatial neglect in stroke rehabilitation: a systematic literature review. International Journal of Nursing Studies 2015;52(8):1375‐403. [DOI] [PubMed] [Google Scholar]
Kwakkel 2003
- Kwakkel G, Kollen BJ, Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181‐6. [DOI] [PubMed] [Google Scholar]
Kwakkel 2004
- Kwakkel G, Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke 2004;35(11):2529‐39. [DOI] [PubMed] [Google Scholar]
Kwakkel 2006
- Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disability and Rehabilitation 2006;28(13‐14):823‐30. [DOI] [PubMed] [Google Scholar]
Langhorne 2011
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377(9778):1693‐702. [DOI] [PubMed] [Google Scholar]
Lawler 2013
- Lawler K, Taylor NF, Shields N. Outcomes after caregiver‐provided speech and language or other allied health therapy: a systematic review. Archives of Physical Medicine and Rehabilitation 2013;94:1139‐60. [DOI] [PubMed] [Google Scholar]
Legg 2011
- Legg LA, Quinn TJ, Mahmood F, Weir CJ, Tierney J, Stott DJ, et al. Non‐pharmacological interventions for caregivers of stroke survivors. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008179.pub2] [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu X, Guan Y, Pu C, Ding X, Zhang S, Xu G, et al. General guidance and training to the caregivers of stroke improves long‐term functional outcome of stroke patients. Cerebrovascular Diseases.
Lohse 2014
- Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose‐response relationships in stroke rehabilitation. Stroke 2014;45(7):2053‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Mao 2002
- Mao H, Hsueh I. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke 2002;33(4):1022. [DOI] [PubMed] [Google Scholar]
Morris 2014
- Morris JH, Macgillivray S, McFarlane S. Interventions to promote long‐term participation in physical activity after stroke: a systematic review of the literature. Archives of Physical Medicine and Rehabilitation 2014;95(5):956‐67. [DOI] [PubMed] [Google Scholar]
NCT00666744
- NCT00666744. A randomised controlled trial of family mediated exercises (FAME) following stoke, 2008. clinicaltrials.gov/ct2/show/NCT00666744 Date first received: 23 April 2008.
NICE 2013
- National Institute for Health and Care Excellence. Stroke rehabilitation in adults. www.nice.org.uk/guidance/cg162 (accessed 27 November 2016).
Nijland 2011
- Nijland R, Kwakkel G, Bakers J, Wegen E. Constraint‐induced movement therapy for the upper paretic limb in acute or sub‐acute stroke: a systematic review. International Journal of Stroke 2011;6:425‐33. [DOI] [PubMed] [Google Scholar]
Norris 2014
- Norris M, Jones F, Kilbride C, Victor C. Exploring the experience of facilitating self‐management with minority ethnic stroke survivors: a qualitative study of therapists' perceptions. Disability and Rehabilitation 2014;36(26):2252‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nouri 1987
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1:301‐5. [Google Scholar]
Otterman 2012
- Otterman NM, Wees PJ, Bernhardt J, Kwakkel G. Physical therapists' guideline adherence on early mobilization and intensity of practice at Dutch acute stroke units: a country‐wide survey. Stroke 2012;43(9):2395‐401. [DOI] [PubMed] [Google Scholar]
Parke 2015
- Parke HL, Epiphaniou E, Pearce G, Taylor SJ, Sheikh A, Griffiths CJ, et al. Self‐management support interventions for stroke survivors: a systematic meta‐review. PLoS One 2015;10(7):e0131448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pettersen 2002
- Pettersen R, Dahl T, Wyller TB. Prediction of long‐term functional outcome after stroke rehabilitation. Clinical Rehabilitation 2002;16(2):149‐59. [DOI] [PubMed] [Google Scholar]
Platz 2005
- Platz T, Pinkowski C, Wijck F, Kim IH, Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl‐Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clinical Rehabilitation 2005;19(4):404‐11. [DOI] [PubMed] [Google Scholar]
Pollock 2014a
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD001920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollock 2014b
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010820.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2011
- Quinn TJ, Langhorne P, Stott DJ. Barthel Index for stroke trials: development, properties, and application. Stroke 2011;42(4):1146‐51. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1983
- Robinson B. Validation of a Caregiver Strain Index. Journal of Gerontology 1983;38(3):344‐8. [DOI] [PubMed] [Google Scholar]
Sanford 1993
- Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl‐Meyer assessment for testing motor performance in patients following stroke. Physical Therapy 1993;73(7):447‐54. [DOI] [PubMed] [Google Scholar]
Shelton 2001
- Shelton FD, Volpe BT, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabilitation and Neural Repair 2001;15(3):229‐37. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network. SIGN Guideline 118. Management of patients with stroke: rehabilitation, prevention and management of complications, and discharge planning. www.sign.ac.uk/pdf/sign118.pdf (accessed 1 September 2013).
Smith 2004a
- Smith LN, Lawrence IM, Kerr SM, Langhorne P, Lees KR. Informal carers' experience of caring for stroke survivors. Journal of Advanced Nursing 2004;46(3):235‐44. [DOI] [PubMed] [Google Scholar]
Smith 2008
- Smith P, Galea M, Woodward M, Said C, Dorevitch M. Physical activity by elderly patients undergoing inpatient rehabilitation is low: an observational study. Australian Journal of Physiotherapy 2008;54(3):209‐13. [DOI] [PubMed] [Google Scholar]
Stevenson 2001
- Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Australian Journal of Physiotherapy 2001;47(1):29‐38. [DOI] [PubMed] [Google Scholar]
Ullberg 2015
- Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke 2015;46(2):389‐94. [DOI] [PubMed] [Google Scholar]
Valko 2008
- Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the Fatigue Severity Scale in a Swiss cohort. Sleep 2008;31(11):1601‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Heuvel 2001
- Heuvel ET, Witte LP, Schure LM, Sanderman R, Meyboom‐de Jong B. Risk factors for burn‐out in caregivers of stroke patients, and possibilities for intervention. Clinical Rehabilitation 2001;15(6):669‐77. [DOI] [PubMed] [Google Scholar]
van Kordelaar 2014
- Kordelaar J, Wegen E, Kwakkel G. Impact of time on quality of motor control of the paretic upper limb after stroke. Archives of Physical Medicine and Rehabilitation 2014;95(2):338‐44. [DOI] [PubMed] [Google Scholar]
van Vliet 2015
- Vliet P, Pomeroy VM, Wolf SL, Kwakkel G. Time to empower people with stroke. Journal of Neurology and Physical Therapy 2015;39(3):139‐41. [DOI] [PubMed] [Google Scholar]
Veerbeek 2011
- Veerbeek JM, Koolstra M, Ket JC, Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke 2011;42(11):3311‐5. [DOI] [PubMed] [Google Scholar]
Veerbeek 2014
- Veerbeek JM, Wegen E, Peppen R, Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta‐analysis. PLoS One 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 1985
- Wade DT, Legh‐Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Disability and Rehabilitation 1985;7(4):176‐81. [DOI] [PubMed] [Google Scholar]
Wang 2014
- Wang TC, Tsai AC, Wang JY, Lin BY, Chen JJ, Lin KL. Enrolling the care‐givers to improve chronic stroke patients' social activity and nutrition ‐ a randomized controlled trial. FASEB Journal 2014; Vol. 28.
Warner 2015
- Warner G, Packer T, Villeneuve M, Audulv A, Versnel J. A systematic review of the effectiveness of stroke self‐management programs for improving function and participation outcomes: self‐management programs for stroke survivors. Disability and Rehabilitation 2015;37(23):2141‐63. [DOI] [PubMed] [Google Scholar]
West 2012
- West T, Bernhardt J. Physical activity in hospitalised stroke patients. Stroke Research and Treatment 2012; Vol. 2012:813765. [DOI] [PMC free article] [PubMed]
WHO 1989
- World Health Organization. Stroke ‐ 1989 recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407‐31. [DOI] [PubMed] [Google Scholar]
WHO CBR
- World Health Organization. Community‐based rehabilitation. www.who.int/disabilities/cbr/en/ (accessed 27 November 2016).
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


