Abstract
Background
Pneumonia is a leading cause of morbidity and mortality in children younger than five years of age. Most deaths occur during infancy and in low‐income countries. Daily zinc supplements have been reported to prevent acute lower respiratory tract infection (LRTI) and reduce child mortality. This is an update of a review first published in 2010.
Objectives
To evaluate the effectiveness of zinc supplementation in the prevention of pneumonia in children aged two to 59 months.
Search methods
We searched CENTRAL (Issue 21 October 2016), MEDLINE (1966 to October 2016), Embase (1974 to October 2016), LILACS (1982 to October 2016), CINAHL (1981 to October 2016), Web of Science (1985 to October 2016) and IMSEAR (1980 to October 2016).
Selection criteria
Randomised controlled trials (RCTs) evaluating zinc supplementation for the prevention of pneumonia in children aged from 2 months to 59 months.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
We did not identify any new studies for inclusion in this update. We included six studies that involved 5193 participants.
Analysis showed that zinc supplementation reduced the incidence of pneumonia by 13% (fixed‐effect risk ratio (RR) 0.87; 95% confidence interval (CI) 0.81 to 0.94, six studies, low‐quality evidence) and prevalence of pneumonia by 41% (random‐effects RR 0.59; 95% CI 0.35 to 0.99, one study, n = 609, low‐quality evidence). On subgroup analysis, we found that zinc reduced the incidence of pneumonia defined by specific clinical criteria by 21% (i.e. confirmation by chest examination or chest radiograph) (fixed‐effect RR 0.79; 95% CI 0.71 to 0.88, four studies, n = 3261), but had no effect on lower specificity pneumonia case definition (i.e. age‐specific fast breathing with or without lower chest indrawing) (fixed‐effect RR 0.95; 95% CI 0.86 to 1.06, four studies, n = 1932).
Authors' conclusions
Zinc supplementation in children is associated with a reduction in the incidence and prevalence of pneumonia.
Plain language summary
Zinc supplementation for the prevention of pneumonia in children aged two to 59 months
Review question
We evaluated the effectiveness of zinc supplementation to prevent pneumonia in children aged from two to 59 months.
Background
Zinc is an essential element for children's growth and development. Too little zinc is associated with increased risk of infection, particularly diarrhoea and pneumonia. Children are more prone to zinc deficiency because they are less able to absorb dietary zinc and some children, especially in low‐income countries, may not have received enough zinc from their mothers before birth. Zinc supplements for children has been reported to prevent pneumonia.
Search date
We searched the literature up to October 2016. This is an update of a review published in 2010. We did not find any new studies for this update.
Study characteristics
We included six studies that investigated zinc supplements to prevent pneumonia. The studies were conducted in Bangladesh, India, Peru and South Africa and involved 5193 children aged from two to 59 months. Children received either zinc or a similar‐looking treatment that did not contain zinc. In two studies, children were also given vitamin A.
Study funding sources
All included studies were funded. Of these, three explicitly mentioned that funding agencies had no role in the design and results of the study.
Key results
Zinc supplementation was significantly associated with reducing the incidence and prevalence of pneumonia among children aged from two to 59 months. On subgroup analysis, we found that a more stringent diagnosis (radiological examination) increased the reduction in pneumonia incidence.
Quality of the evidence
Overall, evidence quality was assessed as low on GRADE assessment.
Summary of findings
Summary of findings for the main comparison. Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months.
| Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months | ||||||
| Patient or population: children aged 2 months to 59 months Settings: Bangladesh, India, Peru, South Africa Intervention: zinc supplementation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplementation | |||||
| Pneumonia incidence | 343 per 1000 | 299 per 1000 (278 to 323) | RR 0.87 (0.81 to 0.94) | 5193 (6 studies) | ⊕⊕⊝⊝ low1,2 | |
| Pneumonia prevalence | 22 per 1000 | 13 per 1000 (8 to 22) | RR 0.59 (0.35 to 0.99) | 609 (1 study) | ⊕⊕⊝⊝ low3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Studies have unclear information on allocation concealment, blinding and reporting biases. 2 Pneumonia diagnosis criteria was used differently. 3 Studies have limited information on blinding of outcomes assessors and reporting bias. 4 Total number of events were less than 300.
Background
Description of the condition
Pneumonia is the largest cause of childhood mortality, accounting for 15% of all childhood deaths under five years (WHO 2015), and 19% of all childhood deaths in low‐income countries (Bryce 2005; Rudan 2008). Pneumonia is diagnosed by the presence of either fast breathing or lower chest wall indrawing (WHO 2015) in children aged under five years. Presenting features in viral or bacterial pneumonia are similar, however wheezing is more common in children with viral pneumonia.
Pneumonia can be prevented by immunising against Haemophilus influenzae type B (Hib), pneumococcus, measles and pertussis (whooping cough) (WHO 2015). Nutrition including breastfeeding for the first six months of life plays a major role by boosting immunity against causative organisms of pneumonia (WHO 2015). Good hygiene and clean indoor air also help in preventing pneumonia (WHO 2015).
Rudan 2013 identified five risk factors for pneumonia: malnutrition, low birth weight, nonexclusive breast feeding, solid fuel use and overcrowding. By 2010, pneumonia episodes for children aged under four years in low‐ and middle‐income countries (LMICs) decreased by 25% over the past decade to about 0.22 episodes per child per year when exposure to the five risk factors decreased by 20% to 30% (Rudan 2013). Therefore, any interventions that can improve child survival from pneumonia are important.
According to the World Health Organization (WHO), zinc deficiency accounts for 13% of all lower respiratory tract infection (LRTIs) (WHO 2009). Acute LRTI is a leading cause of mortality among children aged under five years (Bryce 2005; Rudan 2008), and which is responsible for nearly two million deaths annually, mainly in low‐income countries.
Description of the intervention
In 2005, zinc intake was inadequate among 17.3% of the world's population; rates of zinc adequacy are lower in sub‐Saharan Africa and South Asia (Wessells 2012). The World Bank described multiple factors such as low animal food intake, less bioavailability of dietary zinc, and zinc loss during events of diarrhoea (Bhutta 1999; Black 1998) that could potentially lead to zinc deficiency in low‐income settings (World Bank 2016). The WHO estimated that zinc deficiency may be related to 800,000 deaths a year, with more than 50% occurring in children under five years of age. Children are more susceptible to zinc deficiency because of lower rates of intestinal absorption and from starting life with low body stores due to intrapartum under‐nutrition (Krebs 2014).
Mild‐to‐moderate zinc deficiency is associated with impaired physical growth, delayed sexual maturity, behavioural disturbances, anorexia, affected permeability of the intestinal tract and decreased immunocompetence as well as subclinical inflammation; moderate‐to‐severe deficiency can lead to diarrhoea; and severe deficiency produces dermatitis and alopecia (Krebs 2014).
There is evidence that preventative zinc supplementation reduces pneumonia morbidity (Bhutta 1999; Yakoob 2011) and is beneficial in reducing diarrhoeal episodes in children with acute and persistent diarrhoea (Bhandari 1994; Zinc Group 2000). However, there is insufficient evidence to support preventive zinc supplementation or adjunctive zinc supplementation with antibiotics to reduce pneumonia‐specific mortality (Haider 2011; Yakoob 2011). Zinc supplements (zinc sulphate, zinc gluconate, zinc acetate) can be administered as tablets, syrup or powder. However, zinc supplements need to be administered with caution because excess levels may cause toxicity.
How the intervention might work
Zinc plays an important role in cell regeneration, immunity and growth (Krebs 2014; Shankar 1998). Zinc deficiency decreases T‐lymphocytes and T‐helper, impairs macrophage function and reduced killer cells (Ibs 2003; Ravaglia 2000), and adversely impacts innate immunity affecting interferon (IFN) gamma production, interleukin‐2 (IL‐2) and tumour necrosis factor‐α (TNF‐α) (Krebs 2014). Zinc supplementation in children increases levels of complement in the blood that modulate the function of T‐lymphocytes, T‐helper, macrophages and neutrophils and hence improves the ability to fight infection. Zinc supplementation improves circulating levels of T‐lymphocytes and other macrophages that enhance ability to fight infection (Fraker 1993). Zinc is not stored in the body, but repletion is straightforward. Children with diarrhoea can become zinc deficient quickly. Furthermore, if zinc deficiency occurs during periods of growth, it can be conducive to growth faltering as is shown by a review that found correlation between dietary zinc inadequacy and stunting among children aged under four years (Krebs 2014; Wessells 2012).
Why it is important to do this review
Bhutta 1999 showed that zinc was associated with a reduced incidence of pneumonia. Aggarwal 2007 included more recent studies, but included children aged between birth and 59 months who were given zinc supplements for at least a few months. Roth 2010 calculated the effect size by case definition of pneumonia, and included studies that administered zinc supplements to children aged between birth and 59 months. Yakoob 2011 focused on zinc supplementation for more than three months in children aged under five years and found evidence to support the positive impact of zinc supplementation on diarrhoeal and pneumonia morbidity and mortality to include it in the LiST tool. However Yakoob 2011 did not include low birth weight or small‐for‐gestational age infants, nor provide a specific case definition for pneumonia. Similarly, a review by Bhutta 2013 of diarrhoea and pneumonia interventions reported a non‐significant impact of preventive zinc supplementation on acute lower respiratory infection (ALRI)‐related mortality.
We investigated zinc supplementation for at least three months in children aged from two months to 59 months and conducted a meta‐analysis of data published up to 2011. However, with new and upcoming trials, this review required updating.
Objectives
To evaluate the effectiveness of zinc supplementation in the prevention of pneumonia in children aged two to 59 months.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) evaluating supplementation of zinc for the prevention of pneumonia in children aged from two to 59 months. We included studies that defined an episode of pneumonia in the following ways.
Reported cough or difficulty in breathing with a respiratory rate above the WHO‐defined age‐specific values (respiratory rate of 50 breaths per minute or more for children aged two to 11 months, or respiratory rate of 40 breaths per minute or more for children aged 12 to 59 months), and either documented fever over 38°C or chest in‐drawing (WHO 1990).
A diagnosis of pneumonia based on chest examination by a physician.
A diagnosis of pneumonia based on a chest radiograph.
We included trials published in languages other than English. Quasi‐RCTs were not eligible for inclusion.
Types of participants
We included children aged from two months to 59 months.
Types of interventions
Oral supplement containing at least the USA's recommended daily allowance of zinc versus either an oral supplement without zinc or placebo. We excluded trials in which children were given additional supplements unless the co‐interventions other than zinc were the same in both groups. The recommended daily allowance for infants is 5 mg of elemental zinc per day and 10 mg per day for children aged from one to five years (RDA 1989). We included trials in which supplements were administered for at least three months and outcome surveillance was carried out for at least four weeks.
Types of outcome measures
Primary outcomes
Numbers of new episodes of pneumonia in children aged from two months to 59 months.
Secondary outcomes
Prevalence (number of cases of pneumonia at a given time per total days of observation) of pneumonia in children aged from two months to 59 months.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library (www.thecochranelibrary.com, accessed 21 October 2016), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (November 2009 to 21 October 2016) and Embase (January 2010 to 21 October 2016). We searched LILACS (1982 to 21 October 2016) using a new search strategy. We also searched CINAHL (1981 to 21 October 2016) and Web of Science (1985 to 21 October 2016). Details of previous searches are presented in Appendix 1.
We used the search strategy described in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011) . We adapted the search strategy to search Embase (Appendix 3), LILACS (Virtual Health Library) (Appendix 4), CINAHL (Appendix 5) and Web of Science (Appendix 6). We searched IMSEAR using the search terms "pneumonia" and "zinc". We imposed no language or publication restrictions.
Figure 1 illustrates the search and study selection processes.
1.
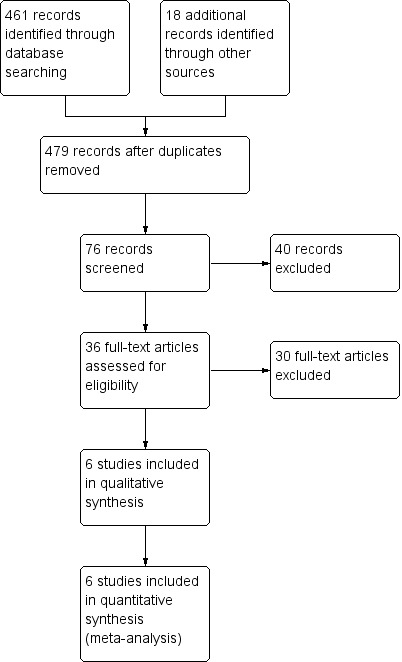
Study flow diagram
Searching other resources
We searched the WHO ICTRP (www.who.int/ictrp) and ClinicalTrials.gov trials registries (latest search 8 July 2016). We also searched related conference proceedings for relevant abstracts. We also checked the reference lists of all trials identified.
Data collection and analysis
Selection of studies
Two review authors independently assessed all potential studies identified for inclusion. We resolved any disagreement through discussion, and if required, consulted the third review author.
Data extraction and management
We designed a data extraction form. Two review authors extracted data. We resolved discrepancies through discussion, and if required, we consulted the third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. There were no new studies identified for inclusion in this update.
Assessment of risk of bias in included studies
There were no new studies identified for inclusion for this update. For the 2010 version of this review (Lassi 2010), two review authors (ZSL, BAH) independently assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion with the third review author (ZAB).
Measures of treatment effect
For dichotomous outcomes, we extracted the total number of participants for each group and numbers of participants experiencing an event. We used risk ratio (RR) and 95% confidence intervals (CIs) to describe effect sizes.
Unit of analysis issues
We carried out statistical analysis using RevMan 2014). Initially, we used fixed‐effect inverse variance meta‐analysis to combine data where trials examined the same intervention, and study populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we conducted random‐effects meta‐analysis. When we identified substantial heterogeneity in a fixed‐effect meta‐analysis, we noted this and repeated the analysis using the random‐effects method.
Dealing with missing data
We noted levels of attrition among included studies. As far as possible, we performed analyses on an intention‐to‐treat (ITT) basis. We attempted to include all participants randomised to each group in the analyses.
Assessment of heterogeneity
We measured heterogeneity among the trials by calculating the I² statistic, and Chi² P value. If the I² statistic exceeded 50%, and the Chi² P value was less than 0.1, we would have considered heterogeneity to be substantial. We did not find any heterogeneity; therefore, we did not attempt subgroup analyses based on differences in zinc dosage, participants' age, supplementation in healthy children compared with children recovering from an episode of illness, and pre‐intervention zinc levels. However, we attempted to look for the differential effect of zinc supplementation on the case definition of ALRI.
Assessment of reporting biases
We aimed to assess reporting bias by comparing published study reports with study protocols. We planned to assess for the presence of publication bias by looking for funnel plot asymmetry; however, the low number of included studies meant this was not feasible.
Data synthesis
We carried out statistical analysis using RevMan 2014 software. In the absence of significant heterogeneity, we used the fixed‐effect meta‐analysis model for combining data.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on diagnosis of pneumonia.
Sensitivity analysis
We planned to carry out a sensitivity analysis to explore the effect of trial quality (assessed by concealment of allocation), by excluding studies with clearly inadequate allocation concealment. However, only two studies had adequate allocation concealment, therefore we did not run the sensitivity analysis.
Results
Description of studies
See Characteristics of included studies, Table 2 and Characteristics of excluded studies tables.
1. Zinc supplement schedule and duration.
| Study | Supplement | Schedule | Duration | Surveillance | |
| Zinc | Control | ||||
| Bhandari 2002 |
Zinc gluconate 10 mg |
Both groups vitamin A | Daily | 4 months | Once weekly |
| Bobat 2005 |
Zinc sulphate 10 mg |
Placebo | Daily | 6 months | Every 2 weeks |
| Brooks 2005 |
Zinc acetate 35 mg to infants 70 mg to children aged > 12 months |
Placebo | Weekly | 12 months | Once weekly |
| Luabeya 2007 |
Zinc gluconate 10 mg |
Both groups vitamin A | Daily | (Continued until 24 months of age) | Once weekly |
| Penny 2004 |
Zinc gluconate 10 mg |
Placebo | Daily | 6 months | Once weekly |
| Sazawal 1998 |
Zinc gluconate 10 mg |
Placebo | Daily | 4 months | Every 5th day |
Results of the search
This is an update of a review previously published in 2010 (Lassi 2010). The most recent search (to October 2016) did not identify any new studies for inclusion.
Included studies
We included six trials. These studies were from Bangladesh (Brooks 2005), India (Bhandari 2002; Sazawal 1998), Peru (Penny 2004), and South Africa (Bobat 2005; Luabeya 2007).
Penny 2004 conducted a randomised, double‐blind, placebo‐controlled community‐based trial in Lima, Peru that involved 238 children with diarrhoea for more than 14 days who were aged from six to 36 months. Children were randomised to receive zinc alone, zinc plus vitamins or placebo for two weeks. Childrens' characteristics at baseline were similar except for sex and length‐for‐age Z scores.
Brooks 2005 conducted a study in a low‐income population in an urban setting of Dhaka, Bangladesh. The study included 1665 children aged from 60 days to 12 months who were randomly allocated to receive oral zinc (70 mg) or placebo once weekly. Children were assessed weekly in the children's homes. Children with suggestive respiratory disease or diarrhoea were referred to clinics where they were assessed and diagnoses made based on the WHO criteria.
Bhandari 2002 included 2482 children aged six to 30 months who were from urban slums in New Delhi, India. Children received daily elemental zinc (10 mg for infants; 20 mg for older children) or placebo for four months. Both groups received single dose vitamin A (100,000 IU for infants and 200,000 IU for older children) at enrolment. Both groups were comparable on socio‐demographic profile, anthropometry, child feeding practices, morbidity in the previous 24 hours, and plasma zinc concentration.
Luabeya 2007 included children aged from four to six months from the KwaZulu‐Natal Province of South Africa. Children were randomised to two intervention arms and one control arm. Children in the control arm received vitamin A only; in the first intervention arm they were given vitamin A plus zinc; and in the second intervention arm children received vitamin A, zinc and multiple micronutrients. Children received supplements until 24 months of age, thus each child received zinc supplements for 18 to 20 months. Among the participants, 32 children were born with HIV positive status, and 154 were born without HIV infection to HIV positive mothers. Groups differed on weight‐for‐length scores.
The double‐blind controlled trial by Sazawal 1998 was conducted in a low socioeconomic population of 609 children aged between six and 35 months in urban India. Children were assigned to zinc supplementation (n = 298) and placebo (n = 311) groups. Children in the treatment group received 10 mg elemental zinc and placebo group children received a substance similar in colour and taste. The daily fixed dose of 5 mL per child was given to all enrolled children for six months, but this was increased to 10 mL for children with diarrhoeal illnesses. The baseline characteristics of included children were similar for age, sex, nutritional status and baseline plasma zinc.
Bobat 2005 conducted a randomised, double‐blind, placebo‐controlled equivalence trial of zinc supplementation at Grey's Hospital in Pietermaritzburg, South Africa. The study included 96 children aged from six to 60 months with HIV 1 infection who were randomly assigned to receive 10 mg of elemental zinc sulphate or placebo daily for six months. Baseline characteristics of included children were similar.
Excluded studies
We excluded 30 studies that did not satisfy inclusion criteria. Seven studies included children outside the age limits of our review criteria (Lira 1998; McDonald 2015; Osendarp 2002; Sur 2003; Taneja 2009; Tielsch 2007 (also reported a different response to intervention definition than we used); Vakili 2009). We excluded seven studies that provided supplements for less than three months (Baqui 2002; Castillo‐Duran 1987; Chandyo 2010; Feiken 2014; Rahman 2001; Roy 1999; Sempértegui 1996). We excluded 14 studies that applied different case definitions for ALRI/pneumonia, which were not based on WHO criteria, radiograph or chest examination by physician (Baqui 2003; Larson 2010; Long 2006; Lind 2004; Malik 2014; Mazoomar 2010; Ninh 1996; Reul 1997; Richard 2006; Sampaio 2013; Sanchez 2014; Soofi 2013; Rosado 1997; Umeta 2000). We excluded one study because children received zinc supplement in a fortified drink (Bates 1993). We also excluded one study (Adhikari 2016) because it had included children with recurrent infections.
Please refer to Characteristics of excluded studies for further details.
Risk of bias in included studies
Figure 2 and Figure 3 provide graphical summaries of the 'Risk of bias' assessment for the six included studies. With one exception (Penny 2004), studies were assessed as providing low or unclear risk of bias in relation to methods. Luabeya 2007 had the lowest risk of bias; Penny 2004 was assessed at unclear risk of bias.
2.
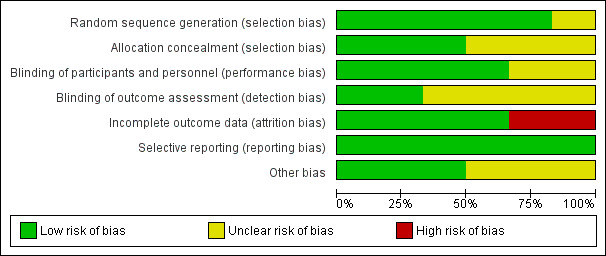
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
3.
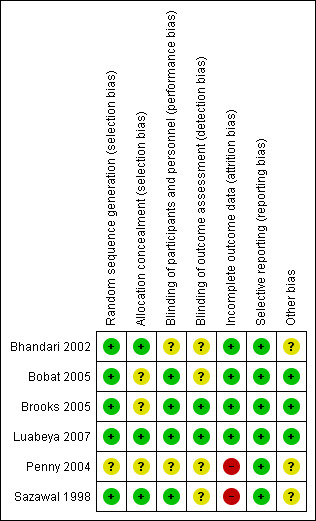
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Most included studies were assessed as demonstrating adequate sequence generation and used computer‐generated sequencing techniques (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007; Sazawal 1998). Allocation concealment was adequate three studies (Bhandari 2002; Luabeya 2007; Sazawal 1998); the remainder did not report in sufficient detail to enable assessment (Bobat 2005; Brooks 2005; Penny 2004).
Blinding
Blinding of participants and study personnel was achieved in four studies (Bobat 2005; Brooks 2005; Luabeya 2007; Sazawal 1998). The remaining studies reported insufficient information to permit judgement (Bhandari 2002; Penny 2004).
Blinding of outcome assessors was achieved in Brooks 2005 and Luabeya 2007. Brooks 2005 reported that blinding was not affected because a proportion of children in both the zinc and placebo groups reacted to the taste such that treatment could not be distinguished; Luabeya 2007 reported that outcomes assessors were blinded to assignment.
Incomplete outcome data
Attrition and exclusions were described in all except studies Penny 2004 and Sazawal 1998. The other four studies (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007) reported reasons for exclusions; these included refusal to participate, moved outside the study site, taste of the syrup, and death.
Selective reporting
Only Luabeya 2007 was registered with a trials register. None of the other studies had published protocols (Bhandari 2002; Bobat 2005; Brooks 2005; Penny 2004; Sazawal 1998). However, all proposed outcomes were reported.
Other potential sources of bias
The funding agencies were reported to have had no input to design or study results in three studies (Bobat 2005; Brooks 2005; Luabeya 2007). Bhandari 2002; Penny 2004 and Sazawal 1998 did not mention the role of funding agencies explicitly.
Effects of interventions
See: Table 1
All six studies reported the incidence of pneumonia (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007; Penny 2004; Sazawal 1998), while one study (Sazawal 1998) also reported prevalence rates.
Incidence of pneumonia
Administration of zinc supplementation showed a statistically significant impact on reducing pneumonia incidence by 13% (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.81 to 0.94, fixed‐effect model, six studies, n = 5193, low‐quality evidence). There was no heterogeneity (I² statistic = 28%, Chi² test P = 0.20; Analysis 1.1).
1.1. Analysis.
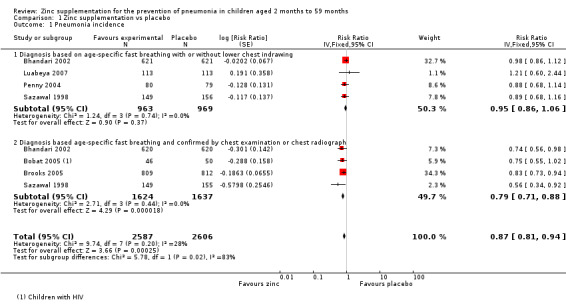
Comparison 1 Zinc supplementation vs placebo, Outcome 1 Pneumonia incidence.
We pooled studies that used similar case definitions. Studies that used clinical definitions of age‐specific fast‐breathing, with or without lower chest indrawing did not exhibit impact from zinc supplementation on reducing pneumonia (RR 0.95, 95% CI 0.86 to 1.06, fixed‐effect model, four studies, n = 1932). There was no heterogeneity (I² statistic = 0%, Chi² test P = 0.74).
Studies that applied case definition for pneumonia based on chest examination or chest radiograph exhibited significant impact from zinc supplementation on reducing incidence of pneumonia by 21% (RR 0.79, 95% CI 0.71 to 0.88, fixed‐effect model, four studies, n = 3261). There was no heterogeneity (I² statistic = 0%, Chi² test P = 0.44). Bobat 2005 included children with HIV. When this study was removed from the analysis there was no difference in results (RR 0.88, 95% CI 0.81 to 0.95, fixed‐effect model, three studies, n = 3165). Evidence was low quality (Table 1).
Prevalence of pneumonia
Administration of zinc supplementation showed a statistically significant impact on reducing the pneumonia prevalence by 41% among children aged from two to 59 months (RR 0.59, 95% CI 0.35 to 0.99, fixed‐effect model, one study, n = 609, low‐quality evidence; Analysis 1.2). (Table 1).
1.2. Analysis.
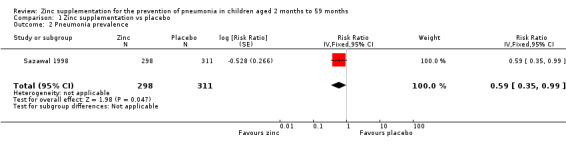
Comparison 1 Zinc supplementation vs placebo, Outcome 2 Pneumonia prevalence.
Discussion
Summary of main results
This is an update of a review first published in 2010 and included no new studies. Previous conclusions are unchanged.
We included six randomised controlled trials (RCTs) evaluating the impact of zinc supplementation in children aged from two to 59 months. Our meta‐analysis indicated that zinc supplementation led to reductions in the incidence of pneumonia by 13% and pneumonia prevalence by 41%. The greater overall reduction of pneumonia prevalence result is due to prevalence data originating from one study.
We found that zinc reduced pneumonia incidence (defined by specific clinical criteria and confirmed by chest examination or chest radiograph) by 21%. We found an expected association of acute lower respiratory infection (ALRI) case definition with an effect size (P = 0.02). We found benefits of zinc supplementation when ALRI was diagnosed following clinical examination or chest radiography suggesting infection.
Reduction in pneumonia incidence supports the use of zinc supplements among children two to 59 months of age. Because zinc is not stored in the body, adequate zinc needs to be available in daily diet (Sanstead 1995). Children, particularly those from low‐income countries who have inadequate intake of food that contains zinc (mainly foods of animal origin) should receive supplements to address deficiency.
Overall completeness and applicability of evidence
Our findings from can be extrapolated for children living in low‐ and middle‐income countries because most included studies were from disadvantaged urban areas of low‐income countries (Bangladesh, India, Peru, and South Africa). In two studies, either children or their mothers had HIV positive status (Bobat 2005; Luabeya 2007). In most studies, children were excluded if they had co‐morbidities such as tuberculosis, congenital heart disease, less than 60% median weight‐for‐age Z score, and nutritional oedema. Penny 2004 recruited children on their breastfeeding status. The use of consistent and accurate definitions of pneumonia, with emphasis on clinical documentation of key signs or radiographic diagnosis, would avoid misclassification and lead to greater confidence in study findings.
Quality of the evidence
We assessed that review outcomes were low according to GRADE criteria. We downgraded evidence quality because outcomes were ascertained using different criteria and 'Risk of bias' assessment. Of note, half of the included studies reported pneumonia according to the WHO definition (WHO 1990); the remainder relied on clinical examination and chest radiographs. We therefore looked for differential effects on outcome estimates with respect to case definition and reported their impact separately.
Allocation concealment was adequately described overall. All included studies were deemed to be adequately blinded for treatment assignment. Completion rates were greater than 90% in four included studies; Penny 2004 reported completion by 16% of participants and Sazawal 1998 did not report numbers of participants who dropped out. The low levels of missing data meant that we chose not to assess the impact on overall estimates because it was not anticipated to cause significant bias in the study results.
Potential biases in the review process
We undertook a systematic search of the literature to identify all studies that met our inclusion criteria. Study selection and data extraction decisions were cross‐checked independently by the review authors. Included studies were not free from bias; only one study was assessed at low risk of bias for all domains. We could not assess reporting bias because of the limited numbers of included studies.
Agreements and disagreements with other studies or reviews
We found RCT evidence from both high‐ and low‐income countries showing an effect of zinc in decreasing morbidity and mortality in children due to respiratory and gastro‐intestinal infections (Hambidge 1999; Sazawal 1998). The effect of zinc against infectious diseases is therapeutic as well as preventive.
Findings reported by the Zinc Investigators’ Collaborative Group in (Bhutta 1999) and Aggarwal 2007 were consistent with our review. Bhutta 1999 reported that children who received zinc supplements had positive effects for pneumonia (odds ratio (OR) 0.59, 95% CI 0.41 to 0.83). Moreover, Bhutta 1999 included trials that administered zinc supplements with therapeutic intent, which might have led to an overestimation of the potential preventive effects of zinc. Aggarwal 2007 included trials that recruited children aged from birth to 59 months who were provided zinc supplementation for at least three months; analysis showed 20% decreased incidence of respiratory illness among children supplemented with zinc compared with placebo. Similar findings were reported by Roth 2010 who also assessed specific case definitions for impact evaluation of children aged from birth to five years. Roth 2010 reported that zinc reduced ALRI incidence defined by specific clinical criteria (incidence rate ratio (IRR) 0.65, 95% CI 0.52 to 0.82), compared with no effect on lower‐specificity ALRI case definitions based on caregiver reports (IRR 1.01, 95% CI 0.91 to 1.12) or WHO non‐severe pneumonia (IRR 0.96, 95% CI 0.86 to 1.08).
Authors' conclusions
Implications for practice.
We found sufficient evidence to support the use of zinc supplementation for children aged from two to 59 months to prevent pneumonia. Our analysis supports use of preventive zinc supplementation to improve child health.
Implications for research.
More well‐designed, large‐scale randomised controlled trials (RCTs) are needed to establish the benefit of zinc supplementation for preventing pneumonia among children aged from two to 59 months. Development of effective and feasible interventions to improve zinc status in children is essential. Enhancement of bioavailable zinc in foods by genetic engineering, plant breeding and periodical supplementation are other possible intervention strategies that should be evaluated.
Given the rising burden of child mortality due to respiratory infections, particularly pneumonia, and considering its decreasing impact with zinc supplementation, further reviews should be considered in which the effectiveness of zinc supplementation should be assessed for acute pneumonia provided that cases are well‐defined by strict clinical criteria.
Feedback
Feedback comment by Joseph L. Mathew, 10 February 2011
Summary
Please note the following feedback.
1. The forest plot in Analysis 1.1 shows that participants in the trial by Bhandari et al, have been split to present two different outcomes. Half the participants in each arm are included for the outcome "Diagnosis based on age‐specific fast breathing with or without lower chest indrawing" and half for the outcome "Diagnosis based age‐specific fast breathing AND confirmed by chest examination or chest radiograph". Both outcomes have been presented as subgroup analysis in the same forest plot; although the trial report mentions that both outcomes were evaluated in all participants. Please confirm whether the procedure adopted in this review is standard practice; and the purpose of doing this.
Answer: We presented the findings after dividing the participants equally in the four cells so that participants do not repeat across analysis. The number of participants were 1241 in each group and we divided them as 620/621 for each of the four cells).
2. A similar adjustment seems to have been made with the participants in the trial by Sazawal et al.
Answer: Adjusted
3. The reference section mentions three citations under Sazawal 1998a; and one under Sazawal 1998b. The third citation in Sazawal 1998a is the same as Sazawal 1998b. Further, none of the citations reports 3300 participants in the trial; whereas this is the number included in the meta‐analysis. Sazawal's 1995 publication in the New England Journal of Medicine mentions 937 participants; while the 1998 publication in Pediatrics reported 609 participants. Please confirm the basis for using the numbers in the meta‐analysis.
Answer: Those were the two parts of the same study. We have now merged it and presented it as one study.
4. The meta‐analysis has pooled data from children with HIV infection, along with otherwise healthy children. While it seems reasonable to pool children with unknown HIV status with otherwise healthy children, it would be better to analyse HIV infected children separately since pneumonia in immuno‐compromised children could be quite different from otherwise healthy children.
Answer: In the text only, we presented the results on removing Bobat trial as well.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
We thank Dr Mathew for these comments.
Contributors
Joseph L. Mathew
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2017 | Amended | Minor data transcription error corrected |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 26 October 2015 | New search has been performed | A new review author joined us to update this review. We excluded 11 new trials (Adhikari 2016; Chandyo 2010; Feiken 2014; Larson 2010; Malik 2014; Mazoomar 2010; McDonald 2015; Sampaio 2013; Sanchez 2014; Soofi 2013; Vakili 2009). |
| 26 October 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 26 October 2015 | New search has been performed | Searches updated, |
| 8 May 2013 | New search has been performed | Searches conducted |
| 10 February 2012 | Amended | Authors replied to Feedback comment |
| 8 August 2011 | Feedback has been incorporated | Feedback added to review. |
Acknowledgements
The authors wish to thank Ammad Saeed, who assisted in the development of protocol and Ms Sarah Thorning for her assistance with the literature search. The review authors would like to thank the following people for commenting on the draft review: Abdullah Brooks, Robert Black, Teenah Handiside and Jiaan‐Der Wang, Mark Griffin, and John Holden.The draft protocol was written by Dr Batool A Haider (BAH) who also took part in the initial stages of the 2010 review and assisted in data extraction.
Appendices
Appendix 1. Previous search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, issue 2), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to January Week 2, 2010), Embase (1974 to January 2010) and LILACS (1985 to January 2010).
We used the following search strategy to search MEDLINE and CENTRAL. The search strategy incorporates the search strategy devised by Boluyt 2008 to identify child studies. Due to the small number of search results we chose not to use a search filter to identify randomised trials. We adapted the search strategy to search Embase (see Appendix 2) and LILACS (see Appendix 3).
MEDLINE (Ovid)
1 exp Pneumonia/ 2 pneumon*.tw. 3 lower respiratory tract infection*.tw. 4 lower respiratory infection*.tw. 5 lrti.tw. 6 or/1‐5 7 Zinc/ 8 (zinc or zn).tw,nm. 9 or/7‐8 10 exp Infant/ 11 (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*).tw. 12 exp Child/ 13 (child* or schoolchild* or school age* or preschool* or kid or kids or toddler*).tw. 14 Adolescent/ 15 (adoles* or teen* or boy* or girl*).tw. 16 Minors/ 17 Puberty/ 18 (minor* or pubert* or pubescen*).tw. 19 exp Pediatrics/ 20 (pediatric* or pediatric*).tw. 21 exp Schools/ 22 (nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw. 23 or/10‐22 24 6 and 9 and 23
Embase search strategy
14. #5 AND #9 AND #13 13. #10 OR #11 OR #12 12. child*:ab,ti OR schoolchild*:ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti OR kindergar*:ab,ti OR (school* NEAR/2 (nursery OR primary OR elementary OR age*)):ab,ti 11. infant*:ab,ti OR infancy:ab,ti OR newborn*:ab,ti OR baby*:ab,ti OR babies:ab,ti OR neonat*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti 10. 'infant'/exp OR 'child'/exp OR 'pediatrics'/exp OR 'school'/exp 9. #6 OR #7 OR #8 8. zinc:ab,ti OR zn:ab,ti 7. 'gluconate zinc'/exp 6. 'zinc'/exp 5. #1 OR #2 OR #3 OR #4 4. 'lower respiratory tract infection':ab,ti OR 'lower respiratory tract infections':ab,ti OR 'lower respiratory infection':ab,ti OR 'lower respiratory infections':ab,ti OR lrti:ab,ti 3. 'lower respiratory tract infection'/de 2. pneumon*:ab,ti 1. 'pneumonia'/exp
LILACS search strategy
pneumon$ or namonia or pulmonia or neumonia [Words] and zinc$ or cinc [Words]
"PNEUMONIA" [Subject descriptor] and "ZINC" [Subject descriptor]
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Pneumonia/ 2 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 3 ((respiratory tract infect* or respiratory infect*) adj3 lower).tw. 4 (lower respiratory adj3 infect*).tw. 5 lrti.tw. 6 or/1‐5 7 Zinc/ 8 (zinc* or zn).tw,nm. 9 Dietary Supplements/ 10 trace elements/ or zinc/ 11 Micronutrients/ 12 exp Zinc Compounds/ 13 ((essential or trace) adj1 (mineral* or nutrient*)).tw. 14 micronutrient*.tw. 15 or/7‐14 16 6 and 15
Appendix 3. Embase.com search strategy
#24 #15 AND #23 #23 #18 NOT #22 #22 #19 NOT #21 #21 #19 AND #20 #20 'human'/de AND [embase]/lim #19 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #18 #16 OR #17 #17 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #16 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #15 #6 AND #14 #14 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #13 micronutrient*:ab,ti #12 ((essential OR trace) NEAR/1 (mineral* OR nutrient*)):ab,ti #11 'zinc derivative'/de #10 'trace element'/exp #9 'diet supplementation'/de #8 zinc*:ab,ti OR zn:ab,ti #7 'zinc'/de #6 #1 OR #2 OR #3 OR #4 OR #5 #5 lrti:ab,ti #4 'lower respiratory tract infection':ab,ti OR 'lower respiratory tract infections':ab,ti OR 'lower respiratory infection':ab,ti OR 'lower respiratory infections':ab,ti #3 'lower respiratory tract infection'/de #2 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti #1 'pneumonia'/exp
Appendix 4. LILACS (Virtual Health Library) search strategy
> Search > (MH:pneumonia OR pneumon$ OR Neumonía OR MH:C08.381.677$ OR MH:C08.730.610$ OR Pulmonía OR Bronchopneumonia OR Bronconeumonía OR Broncopneumonia OR Pleuropneumonia OR Pleuroneumonía OR MH:"Respiratory Tract Infections" OR "Infecciones del Sistema Respiratorio" OR "Infecções Respiratórias" OR "lower respiratory infection" OR "lower respiratory infections" OR "lower respiratory tract infection" OR "lower respiratory tract infections" OR lrti) AND (MH:zinc OR zinc OR zinco OR zn OR MH:"Zinc Compounds" OR MH:D01.975$ OR MH:"Dietary Supplements" OR "Suplementos Dietéticos" OR "Suplementos Dietéticos" OR MH:"Trace Elements" OR Oligoelementos OR MH:Micronutrients OR Micronutrientes)
Appendix 5. CINAHL (EBSCO) search strategy
S23 S13 and S22 S22 S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 (MH "Quantitative Studies") S20 TI placebo* OR AB placebo* S19 (MH "Placebos") S18 TI random* OR AB random* S17 TI ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) S16 TI clinic* trial* OR AB clinic* trial* S15 PT clinical trial Search modes ‐ S14 (MH "Clinical Trials+") S13 S4 and S12 S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TI micronutrient* OR AB micronutrient* S10 TI ((essential or trace) N1 (mineral* or nutrient*)) OR AB ((essential or trace) N1 (mineral* or nutrient*)) S9 (MH "Micronutrients") S8 (MH "Trace Elements") S7 (MH "Dietary Supplements") S6 TI (zinc* or zn) OR AB (zinc* or zn) S5 (MH "Zinc") OR (MH "Zinc Compounds+") S4 S1 or S2 or S3 S3 TI (lower respiratory tract infect* or lower respiratory infect* or lrti) OR AB (lower respiratory tract infect* or lower respiratory infect* or lrti) S2 TI (pneumon* or bronchopneumon* or pleuropneumon*) OR AB (pneumon* or bronchopneumon* or pleuropneumon*) S1 (MH "Pneumonia+")
Appendix 6. Web of Science (Thomson Reuters)
Topic=(pneumonia or bronchopneumonia or pleuropneumonia) AND Topic=(zinc) Refined by: Topic=(random* or placebo* or clinical trial* or double blind*) Timespan=All Years. Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC. Lemmatization=Off
Data and analyses
Comparison 1. Zinc supplementation vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pneumonia incidence | 6 | 5193 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 1.1 Diagnosis based on age‐specific fast breathing with or without lower chest indrawing | 4 | 1932 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.2 Diagnosis based age‐specific fast breathing and confirmed by chest examination or chest radiograph | 4 | 3261 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 2 Pneumonia prevalence | 1 | 609 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.35, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhandari 2002.
| Methods | RCT in which the children were individually randomised by a computer‐generated simple randomisation scheme in blocks of 8. Zinc or placebo bottles were labelled with a unique child identification number according to the randomisation scheme. Six bottles, one for each month and two extra, for each child were produced and labelled before enrolment commenced. The supplies for each child were kept separately in labelled plastic bags. The zinc and placebo syrups were similar in appearance, taste, and packaging. Blinding was maintained during analyses by coding the groups as A or B. The study took place in the urban slum of Dakshinpuri in New Delhi, India. For episodes to be counted as individual, there had to be at least 14 intervening days. The children in the two groups were comparable for age, anthropometry, child feeding practices, morbidity in the previous 24 hours, socioeconomic characteristics and plasma zinc concentration. |
|
| Participants | The study included children aged from 6 to 30 months. There were 1241 children in each group, and after dropouts, the number reduced to 1093 in the zinc and 1133 in the placebo groups. Children were excluded if consent was refused, were likely to move out of the study area within the next four months, needed urgent admission to hospital on the enrolment day or had received a massive dose of vitamin A (100,000 IU for infants and 200,000 IU for older children) within the two months before enrolment. | |
| Interventions | Doses of elemental zinc were 10 mg for infants and 20 mg for older children (twice the recommended daily dosage) as zinc gluconate. Zinc or placebo was taken daily for four months. Both groups received single massive doses of vitamin A (100,000 IU for infants and 200,000 IU for older children) at enrolment. Immunisations and treatment for acute illnesses were provided as per WHO guidelines. Children with acute lower respiratory tract infections received cotrimoxazole. Amoxicillin was substituted if the child did not respond within three days. Children were sent to hospital if they had signs and symptoms that warranted referral according to WHO guidelines. | |
| Outcomes | Incidence of ALRI ALRIs were defined by cough and fast breathing or lower chest indrawing as assessed by the physician; other clinical signs were not taken into account. Fast breathing was defined as 2 counts of > 50 breaths/minute for infants and > 40 breaths/minute for older children. Pneumonia was diagnosed either by a combination of cough with crepitations or bronchial breathing by auscultation or as an episode of ALRI associated with at least one of lower chest indrawing, convulsions, not able to drink or feed, extreme lethargy, restlessness or irritability, nasal flaring, or abnormal sleepiness. |
|
| Notes | Funding: European Union (Contract No IC18CT960045), Norwegian Council of Universities' Committee for Development Research and Education (PRO 53/96), Department of Child and Adolescent Health and Development (CAH), WHO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "children were individually randomised by a simple randomisation scheme in blocks of eight. The randomisation scheme was generated by a statistician at Statens Serum Institut, not otherwise involved with this study, using the SAS software" |
| Allocation concealment (selection bias) | Low risk | Quote: "zinc or placebo syrups were prepared and packaged in unbreakable bottles by GK Pharma ApS Koge, Denmark, who also labelled bottles with a unique child identification number according to the randomisation scheme. The supplies of each child were kept separately in labelled plastic bags. The zinc and placebo were similar in appearances, taste and packaging. Masking was maintained during the analysis by coding the groups as A and B" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the supplies for each child were kept separately in labelled plastic bags". "Masking was maintained during analyses by coding the groups as A or B" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (35%) with their reasons documented. Attrition was 12% in the zinc group and 8.7% in the control group. Loss to follow‐ups were mainly because they refused further participation, moved and died (3 died in the placebo group only) |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sources of funding: Not mentioned if they had any role in design or results of study |
Bobat 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial conducted in Grey's Hospital in Pietermaritburg, South Africa. Baseline measurements of plasma HIV‐1 viral load and the percentage of CD4T lymphocytes were established at two study visits before randomisation, and measurements were repeated 3, 6, and 9 months after the start of supplementation. | |
| Participants | 96 children with HIV‐1 infection between the ages of 6 months and 60 months, being cared for as outpatients at Grey’s Hospital, and not receiving anti‐retroviral therapy were recruited. Pneumonia was diagnosed by history and physical examination, including chest auscultation, and confirmed by chest radiograph. | |
| Interventions | Children either received 10 mg of elemental zinc as sulphate or placebo every day for 6 months. The child’s parent or guardian was given one packet at the first two visits and two packets at each monthly follow‐up visit thereafter, and was instructed on how to give the tablet. | |
| Outcomes | The primary outcome measure was plasma HIV‐1 viral load and incidence of pneumonia. | |
| Notes | Outpatient management of children with HIV‐1 infection is provided by a team of paediatricians, medical officers, and nurses who care for about 20 to 30 children per week. After starting zinc or placebo, children were assessed at Grey’s Hospital every 2 weeks for the first month, monthly for 5 months, and a final visit 9 months after zinc or placebo supplementation started. Pneumonia was diagnosed by history and physical examination, including chest auscultation, and confirmed by chest radiograph. Funding: This study was funded by the Johns Hopkins Family Health and Child Survival Cooperative Agreement with the Office of Health, Infectious Diseases, and Nutrition, Global Health Bureau, US Agency for International Development. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were block–randomised in three age strata (6 to 23, 24 to 41, and 42 to 60 months)"; "Randomisation lists were computer generated at the WHO in a fixed block size of eight" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Tablets of zinc sulphate or placebo were produced by the same manufacturer (Nutriset, Bierne, France) and supplied in blister packets of 14 dispersible tablets"; "An investigator at Grey’s Hospital assigned children to the treatment groups. The investigators were unaware of the treatment allocation until follow up was completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An investigator at Grey’s Hospital assigned children to the treatment groups. The investigators were unaware of the treatment allocation until follow up was completed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/105 participants (8.6%) were excluded. 2/46 (4.4%) and 9/50 (18%) participants did not complete the trial in zinc and placebo groups, respectively. The reasons for lost to follow‐up were mainly deaths and refused to participate |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Sources of funding: funding agencies had no role in views and opinions mentioned in the study |
Brooks 2005.
| Methods | RCT in which random assignment to zinc or placebo was done with permuted blocks of variable length between 2 and 8. Placebo was designed to be identical to the zinc syrup in colour, odour, and taste. The study was conducted at Kamalapur, southeastern Dhaka, Bangladesh. The medical officer diagnosed pneumonia if crepitations were heard on inspiration with a respiratory rate greater than 50 breaths per minute; severe pneumonia was diagnosed if there was also chest indrawing, or at least one other danger sign. | |
| Participants | Children aged 60 days to 12 months at the time of enrolment and excluded those with known or suspected tuberculosis, chronic respiratory or congenital heart disease, or severe malnutrition requiring hospital admission. Pneumonia was diagnosed if crepitations were heard on inspiration with a respiratory rate greater than 50 breaths per minute; severe pneumonia was diagnosed if there was also chest indrawing, or at least one other danger sign. Children with wheezing or rhonchi with crepitations were also diagnosed with pneumonia. 809 children were randomly assigned to zinc and 812 to placebo. There were no significant differences between groups at baseline, except for a slightly higher proportion of boys in the zinc group. There was no difference between the groups in serum zinc values at baseline. | |
| Interventions | Zinc was given orally as a syrup (35 mg zinc acetate per 5 mL). The placebo was non‐nutritious and vitamin‐free. Compliance required intake of two teaspoons of syrup (10 mL). Children with pneumonia were treated with co‐trimoxazole (10 mg/kg trimethoprim, twice daily for 5 days) for pneumonia. Children on antibiotics were assessed within 72 hours of starting treatment; those who did not improve (i.e. the respiratory rate did not change by more than 5 breaths/minute from baseline) were switched to treatment with amoxicillin (40 mg/kg, three times daily for 5 days). If oral treatment failed, or if they had severe pneumonia, children were referred to hospital for parenteral treatment (ceftriaxone 75 mg/kg intramuscularly per day). Children with only expiratory wheezes or rhonchi were managed with salbutamol syrup (0.3 mg/kg, 3 times daily), or referred to hospital for danger signs. | |
| Outcomes | Pneumonia incidence. Other outcomes included frequency of other illnesses and mortality. | |
| Notes | Sources of funding: The research was funded by Johns Hopkins Family Health and Child Survival Cooperative Agreement with the US Agency for International Development, the Swiss Development Corporation, and a cooperative agreement between the US Agency for International Development (HRN‐A‐00‐96‐90005‐00) and core donors to the Centre for Health and Population Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment to zinc or placebo was done with permuted blocks of variable length between two and eight" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "ACME Laboratories (Dhaka) prepared, labelled and masked the identity of both preparations. Both placebo and treatment were designed to be identical in colour, odour, and taste"; "identity of both the preparations were masked" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding of FRAs was not affected because a proportion of children in both zinc and placebo groups reacted to the taste such that the treatment could not be distinguished" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 9.1%. Withdrawal from both groups was most commonly attributed to the child’s reaction to the taste of the syrup, which sometimes resulted in regurgitation. Most of those who withdrew were young, primarily breast fed infants. The highest proportion (37·1%) of withdrawals for both groups occurred at age 2 months, with 77·1% younger than 6 months |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Study seems to be free from other biases; the funding sources had no role in the study design, data collection, data analysis, interpretation of results, or decision to publish this research Funding: The research was funded by Johns Hopkins Family Health and Child Survival Cooperative Agreement with the US Agency for International Development, the Swiss Development Corporation, and a cooperative agreement between the US Agency for International Development (HRN‐A‐00‐96‐90005‐00) and core donors to the Centre for Health and Population Research |
Luabeya 2007.
| Methods | What was the study design? The study was conducted in northern KwaZulu‐Natal Province, South Africa. Children were enrolled into the study by nurses at five government primary health care clinics. Pneumonia by maternal report was considered to have occurred if there was a history of either fast breathing or chest in‐drawing. Confirmed pneumonia was defined as an elevated respiratory rate at rest measured by the fieldworker using WHO/UNICEF Integrated Management of Childhood Illness guidelines. |
|
| Participants | Add number of participants. Children eligible for study were 4 to 6 months old. Children were excluded from the study if they were: less than 60% of median weight‐for‐age using United States National Center for Health Statistics standards; had nutritional oedema; had received vitamin or micronutrient supplements in the previous month; had diarrhoea for more than seven days at the time of study enrolment; or were enrolled in another study of a clinical intervention. Confirmed pneumonia was defined as an elevated respiratory rate at rest measured by the fieldworker using WHO/UNICEF Integrated Management of Childhood Illness guidelines. | |
| Interventions | The 3 treatment arms were: vitamin A alone; vitamin A plus zinc; and vitamin A, zinc and multiple micronutrients. All supplements were given daily at home from entry into the study until 24 months of age. | |
| Outcomes | Diarrhoea, pneumonia Incidence? prevalence? | |
| Notes | Funding: Supported by grants from the US National Institute of Health (1 UO1 AI45508‐01, 1 K24 AI/HDO1671‐01, D43TW05572‐01 to Dr Bennish) and the Wellcome Trust (Wellcome 62925 to Dr Bennish and Wellcome 063009 to Dr Van den Broeck. The sponsor for the study was the host institution, the Africa Centre for Health and Population Studies, which gave discretion in the investigative team in study design, data analysis, manuscript preparation, and decisions on manuscript submission and publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An allocation list was prepared using computer‐generated random numbers and a block size of six"; assignment to the three treatment arms was done separately for three cohorts of children stratified by HIV status of child and mother: HIV‐infected children and mothers; HIV‐uninfected children of HIV‐infected mothers; and HIV‐uninfected children of HIV‐uninfected mothers |
| Allocation concealment (selection bias) | Low risk | Quote: "The manufacturer prepared numbered packs of tablets corresponding to the allocation list. Children enrolled in the study were assigned by a study physician to one of the three study cohorts after results of the HIV tests became available. The physician then allocated the next pack of tablets from the blocks assigned to that cohort to the participant" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators, study staff and participants were blind to the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators, study staff and participants were blind to the treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (12.7%) and attrition (8.1% in vitamin A + zinc and 8.9% in vitamin A group) data were reported along with their reasons. Thirty‐seven children withdrew and one died before any home visits took place |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of selective reporting. Trial Registration ClinicalTrials.gov NCT00156832. The outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Sources of funding: The sponsor for the study was the host institution, the Africa Centre for Health and Population Studies, which gave discretion in the investigative team in study design, data analysis, manuscript preparation, and decisions on manuscript submission and publication |
Penny 2004.
| Methods | This randomised, double‐blind, placebo‐controlled, community‐based trial was carried out in Canto Grande, a shanty town on the outskirts of Lima, Peru. The study was carried out in two phases. During the first phase researchers evaluated the effect of zinc or multiple micronutrient supplementation on the recovery from persistent diarrhoea. During the second phase researchers assessed the effect of continued supplementation on morbidity from new infections during the following 6 months. | |
| Participants | 412 children aged 6 to 36 months with diarrhoea for 14 days were randomly assigned, after being stratified for breastfeeding status, to receive two weeks of daily supplementation with one of three indistinguishable supplements: placebo; 20 mg zinc daily as zinc gluconate (zinc group); or 20 mg zinc daily as zinc gluconate plus a mixture of other micronutrients, i.e. vitamins and minerals (zinc VM group). A subset of children consisting of the first 246 children enrolled who intended to remain in the study area subsequently received the same assigned supplement at one‐half the initial daily dose (10 mg zinc daily) and continued under observation for a total of 6 months. | |
| Interventions | Supplements were supplied as individual doses of a dry micronutrient mixture with added sugar, colouring and flavouring agents, which were dissolved in clean water in participants’ homes and provided as a liquid beverage under the supervision of study personnel on Monday through Friday and by parents or other caregivers during the weekends. There were two intervention arms, zinc plus vitamins and minerals who were given 10 mg of zinc supplementation along with different combinations of mineral and vitamins. Another interventional arm was given zinc 10 mg and the control group was not given any supplementation. | |
| Outcomes | Changes in plasma zinc, haematocrit, haemoglobin, plasma ferritin, pneumonia incidence. | |
| Notes | In this review, groups with zinc and placebo are included for analysis. Examination included assessment of hydration status, measurement of rectal temperature and monitoring of respiratory rate, which was counted for 1 minute and repeated if the rate was greater than age‐specific upper limits (50/minute for children aged 6 to 11 months and 40/minute for children aged 11 months). Children were referred to the study physician for diagnosis and treatment when the fieldworker or caregiver was concerned about the child’s health status or if the child had any one of several predefined signs of illness, including fever, presentation or worsening of cough with elevated respiratory rate (i.e. fieldworker‐defined acute lower respiratory infection), persistent diarrhoea, diarrhoea with signs of dehydration, or vomiting or skin conditions requiring diagnosis. Sources of funding: Supported primarily by the Thrasher Research Fund and the World Health Organization; additional funds were provided by the University of California Pacific Rim Program. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐masked" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐masked" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/81 (17.3%) in zinc and 13/83 (15.7%) placebo groups lost to follow‐up but no reasons were reported |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sources of funding: funding agencies had no role in views and opinions mentioned in the study |
Sazawal 1998.
| Methods | Double‐blind RCT in which the loss of follow‐up was less than 2% The study was conducted in a low socioeconomic population of urban India. | |
| Participants | Children, 6 to 35 months of age, presenting to a community‐based clinic for acute diarrhoea and before enrolment, a parent of the child was given an explanation of the study and written informed consent was obtained. The baseline characteristics for the child‐periods included in the analysis were similar between the two groups. The zinc group had 298 participants and the placebo one had 311. | |
| Interventions | Children were randomised to receive either zinc or placebo in a liquid preparation containing vitamins A (800 units), B1 (0.6 mg), B2 (0.5 mg), B6 (0.5 mg), D3 (100 IU), and E (3 mg) and niacinamide (10 mg); the zinc preparation contained zinc gluconate (10 mg elemental zinc). The liquid preparation 5 mL was given daily for 6 months to all enrolled children; during diarrhoeal illness this was increased to 10 mL to provide for excess zinc losses. | |
| Outcomes | Incidence and prevalence of ALRI. ALRI was diagnosed as using WHO criteria for respiratory disease episodes based on fast breathing alone. ALRI was also defined as child having cough and at least one assessment documenting: a) an elevated respiratory rate more than the age‐specific value on both 1‐minute estimations; and b) a recorded temperature of more than 101°F or lower chest indrawing. |
|
| Notes | Funding: This work was supported by grants from the WHO Diarrheal Disease Control Program, the Thrasher Research Fund, the Johns Hopkins Family Health and Child Survival Cooperative Agreement with funding from the US Agency for International Development and the US National Institutes of Health (R29 HD34724). The assistance of Ms Usha Dhingra and Mr Dharminder Kashyap in data management and of Sandoz India Ltd for providing the supplements is appreciated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation schedules with permuted blocks of 10 were used for children" |
| Allocation concealment (selection bias) | Low risk | Quote: "Supplements were prepared and coded by Sandoz India Ltd (Mumbai). Both formulation were liquid preparations, similar in colour and taste" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "The Code, which was kept by WHO personnel, was not available to the investigator until the end of the study; "both formulation were liquid preparations, similar in colour and taste" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion and attrition rates with their reasons were not described in the study |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sandoz India provided the supplements. Not clear of their role and other funding agencies |
ALRI: acute lower respiratory infection IU: international unit RCT: randomised controlled trial WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adhikari 2016 | Wrong participants: included children with recurrent respiratory infections |
| Baqui 2002 | Wrong duration: zinc supplementation was given for 2 weeks |
| Baqui 2003 | Wrong diagnosis: ALRI was diagnosed if the child had reported symptoms of cough or difficulty in breathing with rapid breathing with or without chest indrawing |
| Bates 1993 | Wrong mode of supplementation: zinc supplement was delivered in a fortified drink |
| Castillo‐Duran 1987 | Wrong duration; wrong outcomes: zinc was supplemented for 60 days; did not study effects on diarrhoea or respiratory illnesses |
| Chandyo 2010 | Wrong duration: duration of intervention was 2 weeks |
| Feiken 2014 | Wrong duration: duration of intervention was 10 days |
| Larson 2010 | Wrong diagnosis: did not use this review's specific ARI definition |
| Lind 2004 | Wrong diagnosis: considered ‘cough and fever’ as ALRI outcome |
| Lira 1998 | Wrong intervention and outcomes: infants were recruited and supplemented from birth; short course supplementation was provided; only cough was reported |
| Long 2006 | Wrong outcome: respiratory tract infection outcomes were defined as the occurrence of cough alone, cough and fever, or cough and rapid respiratory rate as reported by the mother |
| Malik 2014 | Wrong diagnosis: considered "cough or cold with or without fever. ALRI was diagnosed if the child had symptoms of cough with difficult and/or rapid breathing or chest indrawing as informed by the caregiver" as ALRI |
| Mazoomar 2010 | Wrong diagnosis: considered caregiver’s report of cough or difficulty in breathing along with rapid breathing |
| McDonald 2015 | Wrong population: infants aged less than 2 months at start of intervention (5 to 7 weeks) |
| Ninh 1996 | Wrong diagnosis: respiratory outcome was cough and fever |
| Osendarp 2002 | Wrong population: Infants were recruited and supplemented from 4 weeks of age |
| Rahman 2001 | Wrong duration: supplementation was given for 2 weeks only |
| Reul 1997 | Wrong diagnosis: respiratory infections were defined as the presence of at least two of the following symptoms: runny nose, cough, wheezing, difficulty breathing, or fever |
| Richard 2006 | Wrong diagnosis: ALRI was reported by parent as presence of cough and rapid respiration |
| Rosado 1997 | Wrong diagnosis: respiratory illness was presence of runny nose, common cold, sore throat or cough |
| Roy 1999 | Wrong duration: zinc supplementation period was 2 weeks |
| Sampaio 2013 | Wrong diagnosis: study used Brazilian Ministry of Health Criteria as ARI definition |
| Sanchez 2014 | Wrong diagnosis: presence of two or more of the following symptoms as ARI: "Cough, runny nose, shortness of breath and sore throat two or more days duration" |
| Sempértegui 1996 | Wrong duration: zinc supplementation period was 60 days |
| Soofi 2013 | Wrong diagnosis: definition of ARI: " Signs (fast breathing, chest indrawing) of acute respiratory illness were recorded as reported by the mother. An acute respiratory illness episode was defined as a minimum of 2 days with signs followed by a significant interval of at least 7 days" |
| Sur 2003 | Wrong population: Infants were recruited and supplemented from within 7 days of birth |
| Taneja 2009 | Wrong population: zinc supplementation was given to infants between 2 to 4 weeks and 12 months of age |
| Tielsch 2007 | Wrong diagnosis and population: trial was on children aged 1 to 35 months with data not stratified by age, ARTI: episodes of acute respiratory illness were defined as one or more consecutive days of fever, cough, or difficulty breathing, with all three symptoms on at least 1 day during the episode and at least 7 days between episodes |
| Umeta 2000 | Wrong outcomes: cough was only reported respiratory outcome |
| Vakili 2009 | Wrong population: children included older than review's specified cut off (78 to 120 months) |
ALRI: acute lower respiratory infection ARI: acute respiratory infection ARTI: acute respiratory tract infection
Differences between protocol and review
We have added mandatory sections such as subgroup analysis and sensitivity analysis for this update.
Contributions of authors
Zohra S Lassi (ZSL) entered data, created the comparisons, carried out the analysis and wrote the text of the review under the guidance of Dr Zulfiqar A Bhutta (ZAB). Anoosh Moin (AM) took part in screening results and updating the review.
Sources of support
Internal sources
The Aga Khan University, Pakistan.
External sources
No sources of support supplied
Declarations of interest
Zohra S Lassi: none known.
Anoosh Moin: none known.
Zulfiqar A Bhutta: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bhandari 2002 {published data only}
- Bhandari N, Bahl R, Taneja S, Strand T, Mølbak K, Ulvik RJ, et al. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. BMJ 2002;324(7350):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bobat 2005 {published data only}
- Bobat R, Coovadia H, Stephen C, Naidoo KL, McKerrow N, Black RE, et al. Safety and efficacy of zinc supplementation for children with HIV‐1 infection in South Africa: a randomised double‐blind placebo‐controlled trial. Lancet 2005;366(9500):1862‐7. [DOI] [PubMed] [Google Scholar]
Brooks 2005 {published data only}
- Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener‐West M, et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low‐income population in Bangladesh: randomised controlled trial. Lancet 2005;366(9490):999‐1004. [DOI] [PubMed] [Google Scholar]
Luabeya 2007 {published data only}
- Luabeya KK, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, et al. Zinc or multiple micronutrient supplementation to reduce diarrhoea and respiratory disease in South African children: a randomised controlled trial. PLoS ONE 2007;2(6):e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penny 2004 {published data only}
- Duggan C, Penny ME, Hibberd P, Gil A, Huapaya A, Cooper A, et al. Oligofructose‐supplemented infant cereal: two randomised, blinded, community‐based trials in Peruvian infants. American Journal of Clinical Nutrition 2003;77(4):937‐42. [DOI] [PubMed] [Google Scholar]
- Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lönnerdal B, et al. Randomised community based trial of the effect of zinc supplementation, with and without other micronutrients, on the duration of persistent childhood diarrhoea in Lima, Peru. Journal of Pediatrics 1999;135(2):208‐17. [DOI] [PubMed] [Google Scholar]
- Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lönnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. American Journal of Clinical Nutrition 2004;79(3):457‐65. [DOI] [PubMed] [Google Scholar]
Sazawal 1998 {published data only}
- Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhoea in India. New England Journal of Medicine 1995;333(13):839‐44. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Bhan MK, Jalla S, Bhandari N, Sinha A, et al. Zinc supplementation reduces the incidence of persistent diarrhoea and dysentery among low socioeconomic children in India. Journal of Nutrition 1996;126(‐):443‐50. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infant and preschool children: a double‐blind controlled trial. Pediatrics 1998;102(1 Pt 1):1‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adhikari 2016 {published data only}
- Adhikari DD, Das S. Role of zinc supplementation in the outcome of repeated acute respiratory infections in Indian children: a randomized double blind placebo‐controlled clinical trial. Research Journal of Pharmacy and Technology 2016;9(4):457‐58. [Google Scholar]
Baqui 2002 {published data only}
- Baqui AH, Black RE, Arifeen S, Yunus M, Chakraborty J, Ahmed S, et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. British Medical Journal 2002;325(7372):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baqui 2003 {published data only}
- Baqui AH, Zaman K, Persson LA, Arifeen S, Yunus M, Begum N, et al. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhoea and acute lower respiratory infection in Bangladeshi infants. Journal of Nutrition 2003;133(12):4150–7. [DOI] [PubMed] [Google Scholar]
Bates 1993 {published data only}
- Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop‐Clewes CA, et al. A trial of zinc supplementation in young rural Gambian children. British Journal of Nutrition 1993;69(1):243–55. [DOI] [PubMed] [Google Scholar]
Castillo‐Duran 1987 {published data only}
- Castillo‐Duran C, Heresi G, Fisberg M, Uauy R. Controlled trial of zinc supplementation during recovery from malnutrition:effects on growth and immune function. American Journal of Clinical Nutrition 1987;45(3):602–8. [DOI] [PubMed] [Google Scholar]
Chandyo 2010 {published data only}
- Chandyo RK, Shrestha PS, Valentineer‐Branth P, Mathisen M, Basnet S, Ulak M. Two weeks of zinc administration to Nepalese children with pneumonia does not reduce the incidence of pneumonia or diarrhea during the next six months. Journal of Nutrition 2010;140(9):1677‐82. [DOI] [PubMed] [Google Scholar]
Feiken 2014 {published data only}
- Feiken DR, Bigogo G, Allan A, Pals SL, Aol G, Mbakaya C. Village‐randomized clinical trial of home distribution of zinc for treatment of childhood diarrhea in rural western Kenya. PLOS ONE 2014;9(5):e94436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2010 {published data only}
- Larson CP, Nasrin D, Saha A, Chowdhury M, Qadri F. The added benefit of zinc supplementation after zinc treatment of acute childhood diarrhoea: a randomized, double‐blind field trial. Tropical Medicine and International Health 2010;15(6):754‐61. [DOI] [PubMed] [Google Scholar]
Lind 2004 {published data only}
- Lind T, Lonnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, et al. A community‐based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. American Journal of Clinical Nutrition 2004;80(3):729–36. [DOI] [PubMed] [Google Scholar]
Lira 1998 {published data only}
- Lira PIC, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function, and growth of low‐birth‐weight, full‐term infants in northeast Brazil. American Journal of Clinical Nutrition 1998;68(2 Suppl):418‐24. [DOI] [PubMed] [Google Scholar]
Long 2006 {published data only}
- Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double‐blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693–700. [DOI] [PubMed] [Google Scholar]
Malik 2014 {published data only}
- Malik A, Taneja DK, Devasenpathy N, Rajeshwari K. Zinc supplementation for prevention of acute respiratory infections in infants: A randomized controlled Ttrial. Indian Pediatrics 2014;51(10):780‐4. [DOI] [PubMed] [Google Scholar]
Mazoomar 2010 {published data only}
- Mazumder S, Taneja S, Bhandari N, Dube B, Agarwal RC, Mahalanabis D. Effectiveness of zinc supplementation plus oral rehydration salts for diarrhoea in infants aged less than 6 months in Haryana state, India. Bulletin of World Health Organization 2010;88(10):754‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2015 {published data only}
- McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW. Daily zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in Tanzanian infants: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Nutrition 2015;145(9):2153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ninh 1996 {published data only}
- Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin‐like growth factor I (IGF‐I) in growth‐retarded Vietnamese children. American Journal of Clinical Nutrition 1996;63(4):514–9. [DOI] [PubMed] [Google Scholar]
Osendarp 2002 {published data only}
- Osendarp SJM, Santosham M, Black RE, Wahed MA, Raaij MA, Fuchs GJ. Effect of zinc supplementation between 1 and 6 months of life on growth and morbidity of Bangladeshi infants in urban slums. American Journal of Clinical Nutrition 2002;76(6):1401‐8. [DOI] [PubMed] [Google Scholar]
Rahman 2001 {published data only}
- Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ 2001;323(7308):314‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reul 1997 {published data only}
- Ruel MT, Rivera JA, Santizo MC, Lonnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhoea and respiratory infections among rural Guatemalan children. Pediatrics 1997;99(6):808–13. [DOI] [PubMed] [Google Scholar]
Richard 2006 {published data only}
- Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhoea, and respiratory infections in children in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 2006;75(1):126–32. [DOI] [PubMed] [Google Scholar]
Rosado 1997 {published data only}
- Rosado JL, Lopez P, Munoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. American Journal of Clinical Nutrition 1997;65(1):13–9. [DOI] [PubMed] [Google Scholar]
Roy 1999 {published data only}
- Roy SK, Tomkins AM, Haider R, Behren RH, Akramuzzaman SM, Mahalanabis D, et al. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. European Journal of Clinical Nutrition 1999;53(7):529–34. [DOI] [PubMed] [Google Scholar]
Sampaio 2013 {published data only}
- Sampaio DLB, Mattosb APD, Ribeiroa TCM, Leitea MEDQ, Colec CR, Costa‐Ribeiro H Jr. Zinc and other micronutrients supplementation through the use of sprinkles: impact on the occurrence of diarrhea and respiratory infections in institutionalized children. Journal of Pediatrics 2013;89(3):286‐93. [DOI] [PubMed] [Google Scholar]
Sanchez 2014 {published data only}
- Sanchez J, Villada OA, Rojas ML, Montoya L, Diaz A, Vargas C. Effect of zinc amino acid chelate and zinc sulfate in the incidence of respiratory infection and diarrhea among preschool children in child care centers [Efecto del zinc aminoquelado y el sulfato de zinc en la incidencia de la infección respiratoria y la diarrea en niños preescolares de centros infantiles]. Biomedica 2014;34(1):79‐91. [DOI] [PubMed] [Google Scholar]
Sempértegui 1996 {published data only}
- Sempértegui F, Estrella B, Correa E, Aguirre L, Saa B, Torres M, et al. Effects of short‐term zinc supplementation on cellular immunity, respiratory symptoms,and growth of malnourished Equadorian children. European Journal of Clinical Nutrition 1996;50:42–6. [PubMed] [Google Scholar]
Soofi 2013 {published data only}
- Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster‐randomised trial. Lancet 2013;382(9886):29‐40. [DOI] [PubMed] [Google Scholar]
Sur 2003 {published data only}
- Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B, Rajendran K, et al. Impact of zinc supplementation on diarrhoeal morbidity and growth pattern of low birth weight infants in Kolkata, India: a randomised, double‐blind, placebo‐controlled, community‐based study. Pediatrics 2003;112(6 Pt 1):1327‐32. [DOI] [PubMed] [Google Scholar]
Taneja 2009 {published data only}
- Taneja S, Bhandari N, Rongsen‐Chandola T, Mahalanabis D, Fontaine O, Bhan MK. Effect of zinc supplementation on morbidity and growth in hospital‐born, low‐birth‐weight infants. American Journal of Clinical Nutrition 2009;90(2):385‐91. [DOI] [PubMed] [Google Scholar]
Tielsch 2007 {published data only}
- Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of daily zinc supplementation on child mortality in southernNepal: a community‐based, cluster randomised, placebo controlled trial. Lancet 2007;370(9594):1230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Umeta 2000 {published data only}
- Umeta M, West CE, Haidar J, Deurenberg P, Hautvast JG. Zinc supplementation and stunted infants in Ethiopia: a randomised controlled trial. Lancet 2000;355(9220):2021–6. [DOI] [PubMed] [Google Scholar]
Vakili 2009 {published data only}
- Vakili R, Vahedian M, Khodaei GH, Mahmoudi M. Effects of zinc supplementation in occurrence and duration of common cold in school aged children during cold season: a double blind placebo controlled trial. Iranian Journal of Pediatrics 2009;19(4):376‐80. [Google Scholar]
Additional references
Aggarwal 2007
- Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhoea and respiratory illnesses: a meta‐analysis. Pediatrics 2007;119(6):1120‐30. [DOI] [PubMed] [Google Scholar]
Bhandari 1994
- Bhandari N, Bhan MK, Sazawal S. Impact of massive dose of vitamin A given to preschool children with acute diarrhoea on subsequent respiratory and diarrhoeal morbidity. BMJ 1994;309(6966):1404‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 1999
- Bhutta ZA, Black RE, Brown KN, Gardner JM, Gore S, Hidayat A, et al. Zinc Investigators Collaborative Group. Prevention of diarrhoea & pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomised controlled trials. Journal of Paediatrics 1999;135(6):689‐97. [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost?. Lancet 2013;381(9875):1417‐29. [DOI] [PubMed] [Google Scholar]
Black 1998
- Black RE. Therapeutic & preventive effects of zinc on serious childhood infectious diseases in developing countries. American Journal of Clinical Nutrition 1998;68(Suppl 2):476‐9. [DOI] [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatric and Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
Bryce 2005
- Bryce J, Boschi‐Pinto C, Shibuya K, Black R. WHO estimates of the causes of death in children. Lancet 2005;365(9465):1147‐52. [DOI] [PubMed] [Google Scholar]
Fraker 1993
- Fraker PG, King LE, Gravy BA. The immunopathology of zinc deficiency in humans and rodents: a possible role for programmed cell death. In: Klurfeld DM editor(s). Nutrition and Immunology. Vol. 267‐83, New York, NY: Springer, 1993. [Google Scholar]
Haider 2011
- Haider BA, Lassi ZS, Ahmed A, Bhutta ZA. Zinc supplementation as an adjunct to antibiotics in the treatment of pneumonia in children 2 to 59 months of age. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007368.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hambidge 1999
- Hambidge M, Krebs N. Zinc, diarrhoea, and pneumonia. Journal of Pediatrics 1999;135(6):661‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org . Chichester, UK: Wiley‐Blackwell.
Ibs 2003
- Ibs KH, Rink L. Zinc‐altered immune function. Journal of Nutrition 2003;133(5):1452S‐56S. [DOI] [PubMed] [Google Scholar]
Krebs 2014
- Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Pediatrics and International Child Health 2014;34(4):279‐88. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ravaglia 2000
- Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, et al. Effect of micronutrient status on natural killer cell immune function in healthy free‐living subjects aged ≥90 y. American Journal of Clinical Nutrition 2000;71(2):590‐8. [DOI] [PubMed] [Google Scholar]
RDA 1989
- Subcommittee on the 10th edition of the RDAs of the Food and Nutrition Board. Recommended Dietary Allowances. 10th Edition. Washington DC: National Academy Press, 1989. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Roth 2010
- Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta‐analysis and meta‐regression of randomized trials. International Journal of Epidemiology 2010;39(3):795‐808. [DOI] [PubMed] [Google Scholar]
Rudan 2008
- Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbelle H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008;86(‐):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudan 2013
- Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S and on behalf of Child Health Epidemiology Reference Group (CHERG). Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of Global Health 2013;3(1):010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanstead 1995
- Sanstead HH. Is zinc deficiency a public health problem?. Nutrition 1995;11(8):87‐92. [PubMed] [Google Scholar]
Shankar 1998
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. American Journal of Clinical Nutrition 1998;68(Suppl 2):44763. [DOI] [PubMed] [Google Scholar]
Wessells 2012
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 2012;7(11):e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1990
- World Health Organization. Acute respiratory infections in children: Case. Programme for the Control of Acute Respiratory Infections. World Health Organization. Geneva, 1990. [WHO/ARI/90.5]
WHO 2009
- World Health Organization. Global Health Risks: WHO Mortality and burden of disease attributable to selected major risks. World Health Organization 2009.
WHO 2015
- WHO. WHO fact sheet: Pneumonia. http://www.who.int/mediacentre/factsheets/fs331/en/ 2015; Vol. Fact sheet N°331.
World Bank 2016
- World Bank. Geographic classifications and data reported for geographic regions are for low‐income and middle‐income economies as defined by the World Bank. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (Accessed 14 July 2016) 2016.
Yakoob 2011
- Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(Suppl 3):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zinc Group 2000
- Zinc Investigators Collaborative Group. Therapeutic effects of oral zinc in acute and persistent diarrhoea in children in developing countries: pooled analysis of randomised controlled trials. American Journal of Clinical Nutrition 2000;72(6):1516‐22. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haider 2006
- Haider BA, Saeed MA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005978] [DOI] [PubMed] [Google Scholar]
Lassi 2010
- Lassi ZS, Haider BA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD005978.pub2] [DOI] [PubMed] [Google Scholar]


