Abstract
Background
Venous leg ulcers (VLUs) are open skin wounds on the lower leg that occur because of poor blood flow in the veins of the leg; leg ulcers can last from weeks to years, and are both painful and costly. Prevalence in the UK is about 2.9 cases per 10,000 people. First‐line treatment for VLUs is compression therapy, but around 60% of people have unhealed ulcers after 12 weeks' treatment and about 40% after 24 weeks; therefore, there is scope for further improvement. Limited evidence suggests non‐healing leg ulcers may have persisting elevated levels of proteases, which is thought to deter the later stages of healing; thus, timely protease‐modulating matrix (PMM) treatments may improve healing by physically removing proteases from the wound fluid.
Objectives
To determine the effects of protease‐modulating matrix (PMM) treatments on the healing of venous leg ulcers, in people managed in any care setting.
Search methods
In September 2016 we searched: the Cochrane Wounds Specialised Register; CENTRAL; Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We searched for published or unpublished randomised controlled trials (RCTs) that evaluated PMM treatments for VLUs. We defined PMM treatments as those with a purposeful intent of reducing proteases. Wound healing was the primary endpoint.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We included 12 studies (784 participants) in this review; sample sizes ranged from 10 to 187 participants (median 56.5). One study had three arms that were all relevant to this review and all the other studies had two arms. One study was a within‐participant comparison. All studies were industry funded. Two studies provided unpublished data for healing.
Nine of the included studies compared PMM treatments with other treatments and reported results for the primary outcomes. All treatments were dressings. All studies also gave the participants compression bandaging. Seven of these studies were in participants described as having 'non‐responsive' or 'hard‐to‐heal' ulcers. Results, reported at short, medium and long durations and as time‐to‐event data, are summarised for the comparison of any dressing regimen incorporating PMM versus any other dressing regimen. The majority of the evidence was of low or very low certainty, and was mainly downgraded for risk of bias and imprecision.
It is uncertain whether PMM dressing regimens heal VLUs quicker than non‐PMM dressing regimens (low‐certainty evidence from 1 trial with 100 participants) (HR 1.21, 95% CI 0.74 to 1.97).
In the short term (four to eight weeks) it is unclear whether there is a difference between PMM dressing regimens and non‐PMM dressing regimens in the probability of healing (very low‐certainty evidence, 2 trials involving 207 participants).
In the medium term (12 weeks), it is unclear whether PMM dressing regimens increase the probability of healing compared with non‐PMM dressing regimens (low‐certainty evidence from 4 trials with 192 participants) (RR 1.28, 95% CI 0.95 to 1.71). Over the longer term (6 months), it is also unclear whether there is a difference between PMM dressing regimens and non‐PMM dressing regimens in the probability of healing (low certainty evidence, 1 trial, 100 participants) (RR 1.06, 95% CI 0.80 to 1.41).
It is uncertain whether there is a difference in adverse events between PMM dressing regimens and non‐PMM dressing regimens (low‐certainty evidence from 5 trials, 363 participants) (RR 1.03, 95% CI 0.75 to 1.42). It is also unclear whether resource use is lower for PMM dressing regimens (low‐certainty evidence, 1 trial involving 73 participants), or whether mean total costs in a German healthcare setting are different (low‐certainty evidence, 1 trial in 187 participants). One cost‐effectiveness analysis was not included because effectiveness was not based on complete healing.
Authors' conclusions
The evidence is generally of low certainty, particularly because of risk of bias and imprecision of effects. Within these limitations, we are unclear whether PMM dressing regimens influence venous ulcer healing relative to dressing regimens without PMM activity. It is also unclear whether there is a difference in rates of adverse events between PMM and non‐PMM treatments. It is uncertain whether either resource use (products and staff time) or total costs associated with PMM dressing regimens are different from those for non‐PMM dressing regimens. More research is needed to clarify the impact of PMM treatments on venous ulcer healing.
Plain language summary
Protease‐modulating matrix treatments for healing venous leg ulcers
Review question
We reviewed the evidence about the effects of treatments designed to lower the levels of protease in venous leg ulcers. Protease is an enzyme, a chemical produced by the body. High levels of protease in a wound are thought to slow down wound healing. We wanted to find out if treatments that remove protease from wounds could help venous leg ulcers to heal more quickly, and if these treatments were harmful in any way.
Background
Venous leg ulcers are open skin wounds on the lower leg that can last weeks, months or even years. Leg ulcers can be painful, may become infected, and may affect mobility and quality of life. In 2012 in the UK, it cost about GBP 1700 per year to treat each person with an open venous leg ulcer.
The usual treatment for venous leg ulcers is compression therapy (for example, compression bandages), but even this does not work for everyone (about a third of people still have wounds that have not healed after six months). Therefore, we need to try additional treatments, and various dressings have been used alongside compression therapy. One of these is a 'protease‐modulating matrix' (PMM) type of dressing. Research suggests that wounds are slow to heal when there are high levels of a substance called 'protease'. The PMM dressing is designed to remove these proteases from wound fluid, and this is expected to help the wound heal.
In this study, we investigated whether there is any evidence that PMM dressings heal leg ulcers more quickly than other types of dressings.
Study characteristics
In September 2016 we searched for as many relevant studies as we could find that had a reliable design (randomised controlled trials) and had compared PMM treatments with other treatments for venous leg ulcers. We found 12 studies involving a total of 784 people. Ten studies gave results we could use and all treatments were dressings. All these studies gave all the participants compression therapy as well as the dressings. Most of the people in the trials had wounds that were not getting better or had been there a long time.
Key results
Findings from four trials are unclear as to whether there is a benefit of PMM dressings on venous ulcer healing compared with other dressings. Five trials reported on wound side effects and their results are unclear as to whether there is a difference in rates of side effects between PMM dressings and other dressings. It is also unclear whether PMM dressings result in decreases in the amount of saline used and the time taken during dressing changes, and whether there is an effect on total costs.
Certainty of the evidence Overall, the certainty of the evidence was judged to be low: most studies we found were small and could have been better conducted, so it was difficult to be sure how meaningful the results were. The next step would be to do more research of better quality to see whether PMM dressings do heal venous ulcers more quickly than other dressings.
This plain language summary is up to date as of September 2016.
Summary of findings
Summary of findings for the main comparison. Protease‐modulating matrix dressing regimen compared to any other dressing regimen for healing venous leg ulcers.
| Protease‐modulating matrix dressing compared to advanced dressings/no dressing for venous leg ulcers | |||||
| Patient or population: people with venous leg ulcers Intervention: PMM dressing regimen Comparison: other dressing regimen; different comparators across studies | |||||
| Outcomes | Absolute effect* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with other dressing regimen | Risk with PMM dressing regimen | ||||
| TIme to complete healing | Estimated median time to complete healing: 3 months | Estimated median time to complete healing: 4.5 months | HR 1.21 (0.74 to 1.97) | 100
(1 RCTǂ) 66 events |
⊕⊕⊝⊝ LOW 1 |
| Difference in estimated median time to complete healing: approximately 1.5 months shorter | |||||
| Proportion of participants healed ‐ short term (4‐8 weeks) | 287 per 1000 | 210 per 1000 | RR 0.73 (0.34 to 1.58) | 207
(2 RCTs) 21 events |
⊕⊝⊝⊝ VERY LOW 2 |
|
Difference: 77 fewer wounds healed per 1000 (95% CI 167 more to 190 fewer) | |||||
| Proportion of participants healed ‐ medium term (12 weeks) | 400 per 1000 | 512 per 1000 | RR 1.28 (0.95 to 1.71) | 192
(4 RCTs) 89 events |
⊕⊕⊝⊝ LOW 3 |
|
Difference: 112 more wounds healed per 1000 (95% CI 20 fewer to 284 more) | |||||
| Proportion of participants healed ‐ long term (over 24 weeks) | 640 per 1000 | 678 per 1000 (512 to 902) | RR 1.06 (0.80 to 1.41) | 100
(1 RCTǂ) 66 events |
⊕⊕⊝⊝ LOW 4 |
|
Difference: 38 more wounds healed per 1000 (95% CI 128 fewer to 262 more) | |||||
| Proportion of participants with 1 or more adverse events at 2‐12 weeks | 172 per 1000 | 178 per 1000 | RR 1.03 (0.75 to 1.42) | 363
(5 RCTs) 99 events |
⊕⊕⊝⊝ LOW 5 |
|
Difference: 6 more adverse events per 1000 (95% CI 43 fewer to 72 more) | |||||
| *The risk without the intervention is based on the median control group risk across studies. The corresponding risk with the intervention (and the 95% confidence interval for the difference) is based on the overall relative effect (and its 95% confidence interval). ǂ Same study (Petkov 1997) CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||||
|
GRADE Working Group grades of evidence High: It is very likely that the effect will be close to what was found in the research. Moderate: It is likely that the effect will be close to what was found in the research, but there is a possibility that it will be substantially different. Low: It is likely that the effect will be substantially different from what was found in the research, but the research provides an indication of what might be expected. Very low: The anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected | |||||
1 Imprecision (downgraded twice): 66 events, wide CI; assumptions in calculation of HR ‐ no censoring (not downgraded); data extracted from graph (not downgraded)
2 Risk of bias (downgraded once): majority of information at high risk of bias. Imprecision (downgraded twice): 21 events and wide CI
3 Risk of bias (downgraded once): majority of information at high risk of bias. Imprecision (downgraded once): 89 events, CI consistent with no effect and benefit
4 Imprecision (downgraded twice): 66 events, wide CI around absolute effect
5 Risk of bias (not downgraded): majority of information at low risk of bias. Imprecision (downgraded twice): 99 events, CI wide around relative effect
Background
Description of the condition
Venous leg ulcers are open skin ulcers (wounds) on the lower leg (from below the ankle up to mid‐calf), that can last weeks, months or even years, and are a consequence of problems in either the superficial or deep veins or both. Damage to the valves or vein blockages result in malfunctioning of the venous system, reducing the efficient return of blood to the heart and increasing the pressure in the leg veins (Ghauri 2010; Vlajinac 2014), which, if prolonged may result in venous leg ulcers (VLUs). The precise chain of events that links the high venous pressures with skin breakdown and a chronic wound is not fully understood (Coleridge 1988; Ghauri 2010; Valencia 2001). Leg ulcers are frequently associated with venous disease in combination with vascular disease, which impairs arterial blood supply, and such ulcers are said to have a 'mixed aetiology'.
Accurate current estimates of leg ulcer prevalence are hard to identify because most surveys do not differentiate between causes of leg ulceration, or do so per limb but not per participant (Moffatt 2004; Srinivasaiah 2007; Vowden 2009). Estimates of the prevalence of open leg ulceration (any cause) range from 0.4 to 4.8 cases per 1000 (Graham 2003; Johnson 1995;Walker 2002). A recent estimate suggests that venous ulceration has a point prevalence of 0.29 cases per 1000 in the UK, whilst mixed arterial/venous leg ulceration has a point prevalence of 0.11 per 1000 (Hall 2014).
Venous disease is a chronic condition which is characterised by periods of ulceration (i.e. an open wound) followed by healing and then recurrence, though published contemporary data are lacking (Callam 1987). An early cross‐sectional study reported that half of current or recent ulcers had been open for up to nine months and that 35% of people with leg ulcers had experienced four or more episodes (Callam 1987). This picture was supported by a subsequent cross‐sectional study (Nelzén 1994). Cohort data from 20,000 people have shown that initial wound area and duration accurately predict healing (Margolis 2004). An ulcer that is smaller than 10 cm² and has a duration shorter than 12 months at first visit has a 29% chance of not healing by the 24th week of care, whilst one larger than 10 cm² and duration longer than 12 months has a 78% chance of not healing by 24 weeks (Margolis 2004).
The first line treatment for VLUs is compression therapy in the form of bandages, stockings or mechanical devices (O'Meara 2012). This application of external pressure around the lower leg assists venous return and reduces venous reflux (Fletcher 2013; O'Meara 2012). Alongside compression, dressings are almost always applied to open ulcers. The primary rationale for using a dressing is to protect the surface of the ulcer, however other considerations such as absorption of exudate or antimicrobial properties also play a role in treatment selection (O'Meara 2014). Other treatments for VLUs include venous surgery (removal of incompetent superficial veins) (SIGN 2010); and drugs such as pentoxifylline (Jull 2012).
Leg ulcers are associated with considerable cost to patients and to healthcare providers. Two systematic reviews summarised the literature on health‐related quality of life in people with leg ulcers (Herber 2007; Persoon 2004). Both included qualitative and quantitative evaluations, and reported that presence of leg ulceration was associated with pain, restriction of work and leisure activities, impaired mobility, sleep disturbance, reduced psychological well‐being and social isolation. Ulcers can be painful, malodorous, prone to infection, and may severely affect people's mobility and quality of life (Dumville 2009; Herber 2007). In severe cases, ulceration can lead to limb amputation, though this is more likely in people who also have arterial insufficiency (Dumville 2009; Nelzén 2008; Valencia 2001). Recent research suggests that people with complex wounds, including those with VLUs, commonly see complete ulcer healing as the most important outcome to them (Madden 2014).
The financial cost of treating a person with an open venous leg ulcer in the UK has been estimated at around GBP 1700 per year at 2012 prices. A large part of ulcer treatment cost comprises nursing time (Ashby 2014). Another evaluation estimated the average cost of treating a person with a venous leg ulcer in the UK (based on costs for material for dressing changes) as between EUR 814 and EUR 1994 and, in Sweden as between EUR 1332 and EUR 2585 (price year 2002), with higher costs associated with larger and more chronic wounds (Ragnarson Tennvall 2005). Data from a German study, which estimated total costs, including those classified as indirect or intangible costs, estimated mean annual costs of treating leg ulcers as EUR 9060 per patient (2006 prices). This figure is higher than other estimates because it includes non‐health service costs to the patient and to society (Augustin 2012). A recent Australian cost‐effectiveness study estimated the mean cost per person per week for treating 905 people with a chronic leg or foot ulcer below the knee for 24 weeks as AUD 53.31 (which corresponds to AUD 2772 per year); costs included consultations with healthcare professionals, compression bandaging, other dressings and treatments, and community care services, such as Meals‐on‐Wheels and home help (Graves 2014).
Description of the intervention
It has been suggested that one cause of non‐healing in chronic ulcers generally is a prolonged high concentration of proteases in the wound in the later stages of wound healing (Harding 2011; Hart 2002; Palolahti 1993). 'Protease‐modulating' matrix (protease‐inhibiting) treatments are designed to reduce these levels of proteases.
Proteases are enzymes that break down proteins into peptides and amino acids. The principal proteases involved in wound healing are the matrix metalloproteinases and the serine proteases that break down extracellular matrix and connective tissue proteins such as collagen and elastin (Ladwig 2002; Nwomeh 1999; Velnar 2009).
Proteases are active in all of the phases of wound healing (haemostasis, inflammation, proliferation and remodelling) and are therefore thought to have a number of roles in the normal wound healing process (Trengove 1999; Velnar 2009). It is thought that there is a burst of protease activity at the start of acute wound healing, and that in normally‐healing wounds, the activity peaks in the first few days and then declines to very low levels by one week, as healing progresses (Harding 2011; Nwomeh 1998).
In non‐healing wounds, however, it is thought that complex inflammatory mechanisms may result in proteases reaching higher levels and persisting for longer than in normally‐healing wounds (Trengove 1999). This persistent proteolytic activity is thought to damage newly formed tissue and to degrade growth factors, leading to non‐healing wounds (Cullen 2002; Harding 2011; Wlaschek 1997; Yager 1997). Limited evidence suggests correlations between elevated levels of matrix metalloproteinases and delayed healing in people with pressure ulcers (Ladwig 2002), or in foot ulcers in people with diabetes (Liu 2009), as well as in people with VLUs (Mwaura 2006; Serra 2013).
For VLUs in particular, studies of protease levels in wound fluid suggest that there are significantly higher levels of proteases in ulcer tissue compared with healthy tissue, and that these levels decrease following compression treatment in wounds that heal (Beidler 2008). Furthermore, it has been suggested that bacteria present in infected wounds may also produce proteases and these may work synergistically with host proteases to direct tissue degradation (McCarty 2012).
It is logical therefore to postulate that interventions that reduce protease levels may promote wound healing where there are high levels of protease activity.
There is a lack of clarity in the literature as to what constitutes a protease‐modulating matrix (PMM) treatment. For example, some authors have categorised super absorbant dressings as "protease‐modulating" (Wound Care Handbook 2016)), whilst other texts do not describe them as such. For the purposes of this review we defined a PMM treatment as a product that had a purposeful intent of reducing proteases. With important clinical input, we produced a taxonomy for defining PMM treatments to be those specifically marketed as having protease‐modulating activity, with this being a key feature of the product; and where no commercial product was named, the study reported a specific intent of modulating proteases. Common PMM treatments are described below. Products are listed by their generic names and, when possible, with examples of corresponding trade names and manufacturers. Both dressings and ointments are available; some dressings have silver ions incorporated, which are intended to reduce wound pathogens.
Types of PMM treatment include the following (BNF 2016; Wound Care Handbook 2016Young 2012):
starch based ointment: Cadesorb® (Smith & Nephew)
collagen matrix: Suprasorb® C (Activa); Catrix® (Cranage)
collagen and oxidised regenerated cellulose matrix dressing: Promogran® (Systagenix)
collagen, silver and oxidised regenerated cellulose matrix dressing: Promogran® Prisma® Matrix (Systagenix)
cellulose acetate matrix, impregnated with polyhydrated ionogens ointment in polyethylene glycol basis dressing: Tegaderm® Matrix (3M)
adherent polymer matrix dressing containing nano‐oligosaccharide factor (NOSF), with polyurethane foam film backing: UrgoStart® (Urgo)
non‐adherent wound contact dressing containing NOSF: UrgoStart® Contact (Urgo)
cellulose and polymer in a polypropylene sachet: DryMax® Extra (Aspen Medical).
Costs range from GBP 2.96 to GBP 9.18 (median GBP 4.75) (BNF 2016). These costs are higher than for the advanced wound dressings typically used for leg ulcers including alginate dressings (median GBP 0.82) and hydrogels (median GBP 1.92) (BNF 2016). Annual prescribing volumes (as categorised by the BNF) in England for the period October 2008 to September 2009 are available, and state that 6.3% (about 0.2 million items) of advanced wound dressings were protease modulating (MeReC 2010).
How the intervention might work
PMM treatments are used with the aim of increasing wound healing via a reduction in the levels of proteases. The principle of PMM treatments is both to absorb and bind excess proteases from wound fluids, thereby reducing levels of protease at the wound bed (Cullen 2002).These treatments do not, however, appear to affect the expression of proteases on a cellular level (Lobmann 2006). Treatments can target specific proteases or can be more broad spectrum, designed to inhibit the activity of more than one protease. It is likely that in trials, PMM treatments have been given to people who have already had other treatments (particularly compression). However, this review is also interested in protease‐modulating treatment as first line therapy. Point‐of‐care tests are currently being marketed that are intended to identify wounds with persistently high protease levels in order to target treatment appropriately (Norman 2016).
Why it is important to do this review
VLUs are a relatively common, complex type of wound that have a negative impact on people's lives and incur high costs for health services. Leg ulcers are painful, malodorous, prone to infection, and may severely affect patients' mobility and quality of life. In severe cases VLUs may lead to limb amputation. There are a number of treatments for VLUs available and in use, especially compression treatment. However many people experience ulcers that have been open for several months or that recur, or both. Evidence from one large randomised controlled trial (RCT) (Iglesias 2004) in two types of compression treatment showed that healing occurred in 55% and 68% after 24 weeks' treatment, so there is still scope for further improvement from other treatments. PMM treatments are designed to improve the healing of these hard‐to‐heal venous ulcers.
We have been unable to identify an existing systematic review of RCTs investigating the effectiveness of these treatments for VLUs, although PMM treatments are included as comparators in one Cochrane review (O'Meara 2013); we concluded that an up‐to‐date and transparent evidence summary was required on the use of PMM treatments for VLUs.
Objectives
To determine the effects of protease‐modulating matrix treatments on the healing of venous leg ulcers, in people managed in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished RCTs, including cluster RCTs (for which the participant with multiple ulcers was the 'cluster'), irrespective of language of report. Crossover trials would only be included if they reported outcome data at end of the first treatment period and prior to crossover. We excluded studies using quasi‐random methods of allocation (such as alternation).
Types of participants
We included studies recruiting people with a VLU, managed in any care setting. Studies recruiting people described as having VLU were eligible for inclusion. The method of diagnosis of venous ulceration was expected to vary, so we accepted definitions as used in the studies.
We included trials recruiting people with VLUs, alongside people with other types of wounds (e.g. arterial ulcers, pressure ulcers, diabetic foot ulcers), provided the results for people with venous ulcers were presented separately, or if the majority of participants (at least 75% in each arm at randomisation) had leg ulcers of venous aetiology.
We included participants at any stage in their treatment pathway, for example, participants with or without hard‐to‐heal ulcers; and participants selected on the basis of high protease levels, or unselected participants, or people without protease measurements. We also included participants irrespective of infection status at baseline: any available data on these were recorded.
Types of interventions
The primary intervention of interest was a PMM treatment of any type, including dressings and topical treatments. We defined PMM treatments as those specifically marketed as having protease‐modulating activity, with this being a key feature of the product and where no commercial product was named, the study reported a specific intent of modulating proteases. Dressings could be collagen alone, collagen plus oxidised regenerated cellulose (ORC), collagen plus ORC plus other treatments (such as silver, i.e. a combined role), etc. We included any RCTs in which the PMM treatment was the only systematic difference between intervention groups.
We anticipated that likely initial comparisons would include (i) any PMM treatment versus any conventional dressing, (ii) any PMM treatment versus treatment as usual, and (iii) comparisons of different PMM treatments. We planned to treat PMM interventions as a class, combining all types in the meta‐analysis.
For first line PMM treatment, the timing of the intervention was expected to be an important feature; we also planned to include studies that compared different application timings or durations, as long as the difference in timing was the only systematic difference between groups.
Studies in which both groups of participants received compression (adjunct) would be initially meta‐analysed with those that did not give compression to either group, and later examined in subgroup analyses if there was heterogeneity: compression is known to be an effective treatment in terms of reducing time to ulcer healing (O'Meara 2012). We would not include studies in which the provision of compression varied between study groups because the PMM treatment would not be the only systematic difference between groups.
We described the polymeric material and reported generic names where possible, and also provided trade names and manufacturers where these were available.
We excluded from this review evaluations of 'test‐and‐treat' approaches that initiate PMM treatments on the basis of formal measurement of protease levels. These trials are reported in the concurrent Cochrane review, "A test and treat policy for elevated wound protease activity for healing in venous leg ulcers" (Norman 2016).
Types of outcome measures
We list primary and secondary outcomes below. If a study was otherwise eligible (i.e. correct study design, population and intervention/comparator) but did not report a listed outcome then we contacted the study authors where possible to establish whether an outcome of interest here was measured but not reported.
It is important to take time into account in the reporting of outcome measures. Where possible, we used or calculated time‐to‐event data. Otherwise, we categorised outcomes data as follows:
one week or less to 8 weeks = short‐term outcome;
more than 8 weeks to 24 weeks = medium‐term outcome; and
more than 24 weeks = long‐term outcome.
If results were given at more than one time point in a study, we reported outcome measures at the latest time point available (assumed to be length of follow‐up if not specified) or the time point specified in the study report's Methods section as being of primary interest (if this was different from latest time point available). This avoided statistical issues inherent in the use of multiple time points. 'Follow‐up' is defined as the time from randomisation to outcome measurement. The review authors' judgement was used as to whether statistical pooling within the above time categories was appropriate.
We analysed the data separately for the three durations for the complete healing outcome, but combined durations for adverse events (reasoning that local adverse events would probably occur fairly quickly).
Primary outcomes
1. Complete ulcer healing
The primary outcome for the review was complete ulcer healing. We regarded the following as the most relevant and rigorous measures of this outcome:
time to complete ulcer healing (correctly analysed using survival, time‐to‐event approaches, ideally with adjustment for relevant covariates such as baseline size);
the proportion of people with ulcers completely healed.
Where both of these outcomes were reported we planned to present all data in a summary outcome table for reference, but to regard time to healing as having primacy. When time was analysed as a continuous measure, but it was not clear whether all ulcers healed, we planned to document the use of the outcome in the study, but not to extract, summarise or use the data in any meta‐analysis.
2. Adverse events
Events defined, and grouped together, as 'adverse events' by studies were reported where a clear methodology for the collection of adverse event data was provided. This methodology should have made it clear whether (i) events were reported at the participant level; or (ii) if multiple events per person were reported, that an appropriate adjustment was made for data clustering. Where available, we planned to extract data on all serious adverse events and all non‐serious adverse events. We anticipated that adverse events for PMM treatments would be likely to be similar to those for conventional treatments (e.g. deterioration, infection, maceration, pruritis).
Secondary outcomes
3. Health‐related quality of life
We included health‐related quality of life where it was reported using a validated scale such as the SF‐36 or EQ‐5D or a validated disease‐specific questionnaire such as the Cardiff Wound Impact Schedule. Ideally, reported data would be adjusted for the baseline score. We did not include ad hoc measures of quality of life that were unlikely to be validated and would not be common to multiple trials.
4. Pain (including pain at dressing change)
Mean pain scores were included only where they were reported either as presence or absence of pain, or as a continuous outcome using a validated scale such as a visual analogue scale (VAS).
5. Infection
We noted whether wounds were infected at baseline and investigated, where possible, any reduction in infection (efficacy) or incidence of infection (adverse events), or both. We did not consider measurement of bacterial counts.
6. Change in ulcer size
If there were no ulcer healing data for a particular comparison, we planned to consider using data on the change (and percentage change) in ulcer size, with adjustment for baseline size (contacting study authors to request adjusted means when not presented). Where change in ulcer size was reported without adjustment for baseline size, use of the outcome in the study would have been documented, but data would not have been extracted, summarised or used in any meta‐analysis.
7. Resource use
Mean or median summaries of resource use were reported (including measurements of resource use such as number of dressing changes, nurse visits, length of hospital stay and re‐operation/intervention).
8. Costs
Mean costs associated with resource use (as described above) and estimates of cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify relevant RCTs:
Cochrane Wounds Specialised Register (searched 19 September 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 8);
Ovid MEDLINE (1946 to 19 September 2016);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 19 September 2016);
Ovid Embase (1974 to 16 September 2016);
EBSCO CINAHL Plus (1937 to 16 September 2016).
The search strategies for CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase randomised trials filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the randomised trials filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov)
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/Default.aspx)
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included studies as well as relevant systematic reviews, meta‐analyses, and health‐technology assessment reports.
We contacted corresponding study authors for further information (where necessary) and three responded (Cullen 2012; Hanft 2006; Meaume 2012).
We also checked the results of the search conducted for a related review (Norman 2016), and the PRISMA diagram is given for the combined records (Liberati 2009; Figure 1).
1.

Study flow diagram
Data collection and analysis
Selection of studies
Two review authors independently assessed the search results (titles and abstracts) against the eligibility criteria. After this initial assessment, we obtained full‐text copies of all studies considered to be potentially eligible. Two review authors independently checked the full papers for eligibility; we resolved disagreements by discussion and, where required, with the input of a third review author. Where required and possible, we contacted study authors where the eligibility of a study was unclear. We recorded all reasons for exclusion of studies for which we had obtained full copies. We completed a PRISMA flowchart to summarise this process (Liberati 2009; Figure 1).
Where studies were reported in multiple publications/reports we obtained all of them. Whilst we only included the study once in the review, we extracted data from all reports to ensure that we obtained maximal relevant data.
Data extraction and management
Two review authors extracted data independently and resolved disagreements by discussion, drawing on a third review author where required. Where data were missing from reports, we attempted to contact the study authors to obtain this information.
We planned that, where a study with more than two randomised interventions was included, we would only extract data from groups that met the eligibility criteria and would simply note any additional arms. However, there were no studies in which this occurred.
We extracted the following data where possible by treatment group for the pre‐specified interventions and outcomes in this review. We collected outcome data for relevant time points as described in Types of outcome measures:
Country in which study conducted
Unit of randomisation: cluster, participant; wounds (for split‐site or split‐body study); foot/leg
Trial design e.g. parallel; cluster; ulcer randomisation; crossover trials with first period results
Publication status of study
Source of funding
Care setting
Number of participants randomised to each trial arm and a note taken of additional excluded intervention arms, with numbers randomised
Inclusion and exclusion criteria (including selection on basis of protease levels)
-
Population baseline characteristics:
age
sex
duration of venous leg ulcer
ulcer area at baseline
proportion of participants with infected ulcers at baseline
protease levels at baseline
-
Treatment received by each group:
details of treatment regimen, including polymer type/structure
mode of delivery of treatment (e.g. dressing or topical treatment)
number of applications of treatment
timing of treatment (initiation relative to time of randomisation)
duration of treatment and duration of follow‐up
details of any co‐interventions, especially compression interventions
details of any background treatment and any subsequent treatment post randomisation
Prior treatment (type, if any, or treatment naive)
Primary and secondary outcome(s) (with definitions)
Unit of analysis
-
Details of analysis
e.g. time‐to‐event analysis method such as Cox proportional hazards; regression adjusted for which list of covariates
where mean or median time to healing without survival analysis has been conducted (i.e. time to healing treated as a continuous measure without censoring and whether this was done as all ulcers healed)
Outcome data for primary and secondary outcomes (by group)
Withdrawals per treatment group with numbers and reasons.
Assessment of risk of bias in included studies
Two review authors independently assessed included studies using the Cochrane tool for assessing risk of bias (Higgins 2011a). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues (Appendix 2). In this review, we also recorded issues with the unit of analysis, for example where a cluster trial had been undertaken but analysed at the individual level in the study report (Appendix 3). Additionally, we recorded in the notes the comparability of participant characteristics at baseline across the two groups, especially the values of continuous outcomes at baseline, and whether an adjusted analysis was conducted. We used these data to help inform decisions on the risk of selection bias.
We assessed blinding and completeness of outcome data for each of the review outcomes separately. We note that, since judgement is exercised in determining when ulcer healing has actually occurred, the outcome of healing can be at high risk of detection bias when outcome assessment is not masked to treatment allocation.
We presented our assessment of risk of bias using two 'Risk of bias' summary figures; one of which shows a cross‐tabulation of each trial by all of the risk of bias items (Figure 2), and a second which is a summary of bias for each item across all studies (Figure 3). We classed studies with an assessment of high risk of bias for the randomisation sequence or the allocation concealment domain or the blinded outcome assessment domain or incomplete outcome data (or combinations thereof) as being at overall high risk of bias (for the specified outcome for that study), and noted if there were two or more domains contributing to the overall risk of bias.
2.
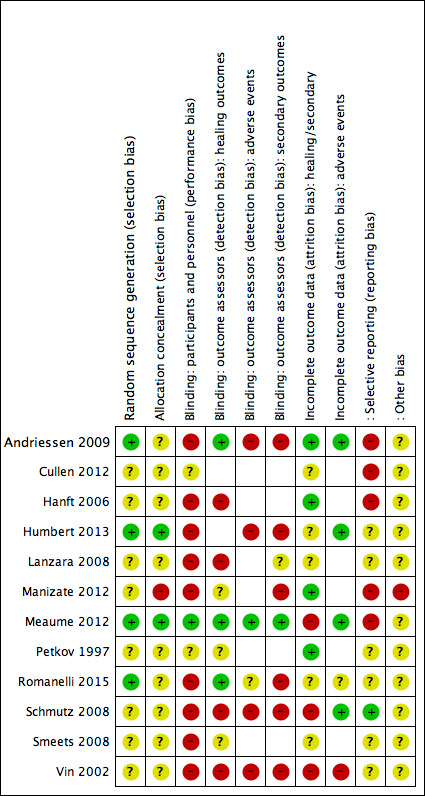
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
If there were any trials using cluster randomisation, we planned to consider the risk of bias in terms of recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials (Higgins 2011b) (Appendix 3). However, no studies had cluster randomisation.
Measures of treatment effect
For dichotomous outcomes the risk ratio (RR) was calculated with its 95% confidence interval (CI). Where there were no events in either arm, the study was included in the analysis (but did not contribute to the summary estimate). Where the event risk was less than 1% in any one arm, we calculated a Peto Odds Ratio (OR) with its 95% CI.
For continuous outcome data we used the mean difference (MD) with its 95% CI, if all trials used the same or similar (magnitude) assessment scale. If trials used different magnitude assessment scales, we would have used the standardised mean difference (SMD) with 95% CIs.
For time‐to‐complete ulcer healing, we reported data as hazard ratios (HR) (with their 95% CI), in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio, we estimated this using other reported data, such as the numbers of events, with application of available statistical methods (Parmar 1998; Tierney 2007; Wang 2013). In the absence of these measures, if there had been any studies in which all ulcers healed, we would have considered the mean or median time to healing without survival analysis as a valid outcome (i.e. if the trial authors regarded time to healing as a continuous measure because there was no censoring). However, no studies met these criteria.
Unit of analysis issues
We planned to treat the participant as the unit of analysis if studies randomised at the participant level, measured outcomes at the ulcer level (e.g. ulcer healing), and the number of ulcers assessed appeared to be equal to the number of participants (e.g. one wound per person). However, this issue did not arise for any studies.
We anticipated a possible unit‐of‐analysis issue if individual participants with multiple ulcers were randomised, the allocated treatment was used on multiple ulcers per participant (or perhaps only for some participants) and then data were presented and analysed by ulcer not person. This is a type of clustered data, such that the participant is the 'cluster', and presents a unit of analysis error which inflates precision. If there had been studies that contained some or all clustered data we would have reported this alongside information on whether data had been (incorrectly) treated as independent. We would have recorded this as part of the 'Risk of bias' assessment. We would not have undertaken further calculation to adjust for clustering. However, no studies of this type were included.
We also noted when randomisation used a split‐site or split‐body design, and assessed whether the correct paired analysis had been undertaken in the study. Again, we recorded issues in the 'Risk of bias' section. If an incorrect analysis had been undertaken and the required data had been available from the study report or the study authors, we would have approximated a correct analysis (Altman 2000; Elbourne 2002). However, this was not available for the included study with a split‐site design. If the majority of the evidence had had incorrect analyses, we would have considered conducting separate meta‐analyses for incorrectly analysed data and adjusting the 'Risk of bias' assessment accordingly. However, only one study had a split‐site design. We included this study in the meta‐analysis, accepting that its contribution would be reduced.
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants post‐randomisation, or ignoring those participants who withdraw from the trial or are lost to follow‐up, compromises the randomisation and potentially introduces bias into the trial. Where there were missing data that the review authors thought should be included in the analyses, the relevant study authors were contacted to request whether these data were available and to determine reasons for 'missingness'; however, we noted it was likely that data would often be missing due to loss to follow‐up.
Where data remained missing for the primary outcome of proportion healed, we assumed participants did not have the outcome (i.e. they were considered in the denominator but not the numerator). We conducted a sensitivity analysis using an alternative imputation approach (available case analysis) to examine this assumption.
For continuous variables, for example, quality of life, we presented available data from the study reports/study authors and did not impute missing data.
For adverse events and all secondary dichotomous outcomes we used an available case analysis, where possible, for all studies; and failing that, used whatever the study authors reported. Where measures of variance were missing, we calculated these wherever possible (Higgins 2011a). If these data were not available and calculation was not possible, we would have contacted the study authors, and if this was unsuccessful, we would have excluded the study from any relevant meta‐analyses that were conducted. However, this issue did not arise.
Assessment of heterogeneity
Assessment of heterogeneity comprised initial assessment of clinical and methodological heterogeneity and the appropriateness of combining study results: that is the degree to which the included studies varied in terms of participant, intervention, outcome and characteristics such as length of follow‐up. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity of the results — assessed using the Chi² test (we considered a significance level of P < 0.10 to indicate statistically significant heterogeneity) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). In general I² values of 25%, or less, may mean a low level of heterogeneity (Higgins 2003), and values of 75%, or more, indicate very high heterogeneity (Deeks 2011). We also examined the variability of the point estimates and the overlap of the confidence intervals, when I² values were less than 50%. Where there was possible heterogeneity we explored this further: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias, an across‐studies reporting bias, is one of a number of possible causes of 'small study effects', that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the effect estimates from individual RCTs against some measure of trial size or precision (Sterne 2011). If we had had meta‐analyses with 10 RCTs or more, we would have presented funnel plots using Cochrane's Review Manager (RevMan) 5 software (RevMan 2014). However, we did not have sufficient studies for this.
We also considered the publication status of the studies and their funding.
Data synthesis
We reviewed details of included studies at the level of comparison between intervention and comparator, stratified by outcomes by time point.
We considered clinical and methodological heterogeneity (based on the items in the section on Data extraction and management) and pooling was undertaken when studies appeared appropriately similar in terms of wound type, intervention type, outcome measurement time and outcome type, such that synthesis was considered viable.
In terms of meta‐analytical approach, when we considered meta‐analysis viable in the presence of clinical heterogeneity (review author judgement) or evidence of statistical heterogeneity, or both, we used a random‐effects model. We considered a fixed‐effect approach only when clinical heterogeneity was thought to be minimal and statistical heterogeneity was estimated as non‐statistically significant for the Chi² value and 0% for the I² assessment (Kontopantelis 2012). This approach was adopted because it is recognised that statistical assessments can miss potentially important between‐study heterogeneity in small samples hence the preference for the more conservative random‐effects model (Kontopantelis 2012). Where clinical heterogeneity was thought to be acceptable or of interest we conducted meta‐analyses even when statistical heterogeneity was high, and attempted to interpret the causes behind this heterogeneity, using pre‐defined sensitivity analyses and pre‐specified subgroup analyses (see below); if we had had sufficient studies, we would have considered using meta‐regression for that purpose, but there were too few studies (Thompson 1999; Thompson 2002).
We have presented data using forest plots where possible. For dichotomous outcomes, we have presented the summary estimate as a risk ratio (RR) with 95% CI. If we had had more than one study reporting continuous outcomes measured in the same way across studies, we would have presented a pooled mean difference (MD) with 95% CI; we would have pooled standardised mean difference (SMD) estimates if studies measured the same outcome using different magnitude scales. However, we did not identify more than one study reporting particular continuous outcomes. For time‐to‐event data, we reported estimates of hazard ratios and 95% CIs, either as presented in the study reports, or as calculated by us using alternative data (Tierney 2007). If we had identified more than one study reporting time‐to‐event data, we would have pooled the hazard ratios and their standard errors using the generic inverse variance method in RevMan 2014. If there had been any studies in which time to healing was analysed as a continuous measure, but not all ulcers were healed or it was not clear if all ulcers were healed, we would have documented use of the outcome in the study, but data would not have been summarised or used in any meta‐analysis. However, no studies were found with outcomes of this type.
We obtained pooled estimates of treatment effect using RevMan 2014.
Subgroup analysis and investigation of heterogeneity
Where possible, we included all studies in the analysis, and for the primary outcomes carried out a sensitivity analysis, excluding from the analysis studies at overall high risk of bias, provided this did not reduce the analysis to one study.
If there was heterogeneity in the primary outcome of complete healing, we investigated it using the following pre‐specified subgroup analyses, provided there were at least two studies per subgroup:
presence versus absence of compression treatment
comparator treatments as basic contact dressings versus advanced wound dressings
silver‐containing treatments versus non silver‐containing treatments
infected ulcers versus non‐infected ulcers at baseline; preferably this subgroup analysis was at the study level (all participants with, or all participants without infection at baseline) or the subgroup analysis was based on the authors' pre‐specified within‐trial analyses
duration of leg ulcer (12 months or more versus less than 12 months); because it is suggested that hard‐to‐heal ulcers (such as those of a longer duration) have higher protease activity and thus will respond better to PMM treatments (relative to control), compared with wounds of shorter duration. A duration of 12 months or more is an independent risk factor for harder‐to‐heal wounds (Margolis 2004). Preferably this subgroup analysis was based on pre‐specified within‐trial analyses performed by study authors. In the absence of this, we intended to consider between‐trial subgroup analyses on the basis of the median duration of leg ulcer in the study, provided there were at least two studies per subgroup.
We conducted the standard test for homogeneity across subgroup results (rather than across individual study results) as part of the assessment of the credibility of the subgroup analyses: an I² statistic was computed for subgroup differences; this describes the percentage of the variability in effect estimates from the different subgroups that is due to genuine subgroup differences rather than sampling error (chance) (Deeks 2011).
Sensitivity analysis
If there was heterogeneity, we carried out a sensitivity analysis for the outcome of complete healing in which we excluded RCTs classified as being at overall high risk of bias, provided this did not reduce the analysis to one study.
For the outcome 'proportion of participants completely healed', we conducted a sensitivity analysis based on available cases.
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the certainty (formerly, quality) of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We have presented the following outcomes in the 'Summary of findings' tables, with a separate table for each key comparison:
time‐to‐complete ulcer healing where analysed using appropriate survival analysis methods
proportion of ulcers completely healed during the trial period
adverse events.
Where it was not possible to pool the data or if the evidence consisted of single studies, we conducted the GRADE assessment for each comparison and presented this narratively within the results section without the presentation of separate 'Summary of findings' tables.
For assessing imprecision, we took into consideration the number of events and the width of the 95% CI with respect to GRADE 'default' values of RR = 1.25 and 0.75.
For calculating absolute risk differences for dichotomous and time‐to‐event outcomes, we used the median of the risks in the control groups at particular time points.
Elements of this methods section are based on the standard Cochrane Wounds Protocol Template.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies
Results of the search
The search generated 377 records of which we obtained 79 in full text (Figure 1). We excluded 58 studies (see Characteristics of excluded studies). Nineteen records containing 12 RCTs were eligible for inclusion. One further study is awaiting classification pending further communication from the study authors (see Characteristics of studies awaiting classification). We are also aware of one ongoing study (Characteristics of ongoing studies).
We located no new studies by searching reference lists, as any relevant studies had been identified in the electronic searching.
Included studies
This review includes 12 studies (Andriessen 2009; Cullen 2012; Hanft 2006; Humbert 2013; Lanzara 2008; Manizate 2012; Meaume 2012; Petkov 1997; Romanelli 2015; Schmutz 2008; Smeets 2008; Vin 2002), which together contained 784 participants. For these studies, the median and range sample sizes were 56.5 (10 to 187). Four studies were reported only as conference abstracts or posters (Cullen 2012; Hanft 2006; Lanzara 2008; Petkov 1997). We contacted the study authors to seek further information on all studies, obtaining information from four (Cullen 2012; Hanft 2006; Meaume 2012; Petkov 1997): the Cullen 2012 authors gave some further information, but could not supply results data. One study (Andriessen 2009) had three arms, and all the other studies had two. Two studies were conducted in the USA (Hanft 2006; Manizate 2012), nine in Europe; in one case it was unclear (Cullen 2012). The majority of studies were conducted in an outpatient setting. Eleven studies randomised individual participants whilst one (Manizate 2012) randomised legs (i.e. a within‐participant study); this latter study did not take into account pairing either in their analysis or the reporting of their results. No studies randomised clusters of participants.
All studies were funded by manufacturers of the PMM treatments, although two studies (Meaume 2012; Schmutz 2008) stated that their analyses were conducted by independent companies.
Participant characteristics
All studies included participants with VLUs. The Schmutz 2008 study also included people with VLUs with aetiologies described as venous (55%), postphlebitic (17%) and with arterial participation (28%); we regarded this as an 'indirect' population.
Nine of the 12 studies were in participants described as having 'non‐responsive' or 'hard‐to‐heal' ulcers; explanations are given in the Characteristics of included studies, where information was available; Manizate 2012 and Petkov 1997 did not give sufficient information and in Cullen 2012 the only indicator of 'hard‐to‐heal' was 23% with elevated protease levels. Five studies reported prior treatment of the wounds (Andriessen 2009; Humbert 2013; Meaume 2012; Romanelli 2015; Schmutz 2008), one study reported no prior treatment (Petkov 1997) and the other studies did not mention prior treatment.
Two studies reported that over 50% of the ulcers were recurrent (Meaume 2012; Schmutz 2008).
Interventions assessed
A range of PMM treatments was evaluated; all were dressings (see below). Four studies (Cullen 2012; Hanft 2006; Lanzara 2008; Manizate 2012) randomised PMM dressings that incorporated silver; we treated these silver‐containing products as a different type of PMM dressing, noting that there may be additional benefits from the combination with silver.
Interventions
The following PMM dressings were reported in the included studies:
PMM: collagen/oxidised regenerated cellulose matrix dressing (Promogran ®) ‐ four studies (Cullen 2012; Schmutz 2008; Smeets 2008; Vin 2002)
PMM: collagen dressing ‐ two studies: Suprasorb C ® (Andriessen 2009) and Proheal ® (Romanelli 2015)
PMM: polyacrylate‐based hydrogel (Hydroclean ®) ‐ one study (Humbert 2013)
PMM‐FOAM combination dressing: non‐adherent wound contact dressing (foam) containing nano‐oligosaccharide factor (NOSF; UrgoStart ®) ‐ two studies (Meaume 2012; Schmutz 2008)
PMM‐ALGINATE combination dressing: collagen alginate dressing (Fibracol ®)* ‐ one study (Petkov 1997)
PMM‐SILVER: collagen/oxidised regenerated cellulose matrix with silver dressing (Promogran Prisma ®) ‐ three studies (Cullen 2012; Hanft 2006; Lanzara 2008)
PMM‐SILVER: collagen plus silver dressing ‐ one study (Manizate 2012).
*The product Fibracol is described as protease‐modulating on the Portuguese version of Systagenix’s web site (Systagenix Portugal 2016), which shows a column chart reporting levels of proteases, so it is reasonable to be considered as a PMM dressing. It is not widely available.
Comparisons
There were a number of different comparisons, which we have grouped under the following broad headings. Further details are given in Table 2:
1. Comparisons table.
| Comparison type* | Study | Intervention group | Control group | ||
| Primary dressing | Secondary dressing | Primary dressing | Secondary dressing | ||
| 1 | Vin 2002 | PMM | Basic wound contact (BWC) |
BWC | ‐ |
| 2a | Andriessen 2009 | PMM | Foam | Foam | ‐ |
| Hanft 2006 | PMM‐silver | Hydrocolloid | Hydrocolloid | ‐ | |
| Lanzara 2008 | PMM‐silverR | Foam | Foam | ‐ | |
| Romanelli 2015 | PMM + BWC (interfacial dressing) |
Alginate | Alginate | ‐ | |
| Smeets 2008 | PMM | Hydrocolloid | Hydrocolloid | ‐ | |
| 2b | Andriessen 2009 | PMM | Foam | BWC | ‐ |
| 2c | Manizate 2012 | PMM‐silver | Foam | Hydrocolloid‐silver | Foam |
| Humbert 2013 | PMM | ‐ | Hydrogel | BWC | |
| 2d | Meaume 2012 | PMM‐foam | ‐ | Foam | ‐ |
| Petkov 1997 | PMM‐alginateE | ‐ | Alginate | ‐ | |
| 3 | Schmutz 2008 | PMM‐foam | ‐ | PMM | ‐ |
| Cullen 2012 | PMM‐silver | ‐ | PMM | ‐ | |
* Comparison types:
1. PMM dressing regimen versus basic wound contact dressing regimen
2a. PMM dressing regimen versus advanced dressing regimen with the secondary dressing in the experimental group the same as the primary dressing in the control group
2b. PMM dressing regimen versus advanced dressing regimen with the secondary dressing in the experimental group being similar but different from the primary dressing in the control group
2c. PMM dressing regimen versus advanced dressing regimen with the same secondary dressings in both groups or no secondary dressings or secondary dressings only in the control group
2d. PMM dressing regimen versus advanced dressing regimen: PMM/advanced combination dressing versus advanced dressing
3. PMM dressing 1 versus PMM dressing 2
PMM dressing regimen versus basic wound contact dressing regimen (Vin 2002)
-
PMM dressing regimen versus advanced dressing regimen
with the secondary dressing in the experimental group the same as the primary dressing in the control group (Andriessen 2009; Hanft 2006; Lanzara 2008; Romanelli 2015; Smeets 2008). The Romanelli 2015 study reported additional use of a non‐adherent petrolatum‐impregnated dressing as an interface between the PMM dressing and the secondary dressing, and this was not used in the control group
with the secondary dressing in the experimental group being similar but different from the primary dressing in the control group (Andriessen 2009)
with the same secondary dressings in both groups or no secondary dressings or secondary dressings only in the control group (Humbert 2013; Manizate 2012)
PMM/advanced dressing combination dressing versus advanced dressing (Meaume 2012; Petkov 1997)
PMM dressing 1 versus PMM dressing 2 (Cullen 2012; Schmutz 2008).
In accordance with the protocol, and on clinical advice, we combined comparisons 1, 2a, 2b, 2c and 2d in a single analysis, thereby comparing any PMM dressing regimen with any other (non‐PMM) dressing regimen. This approach was expected to answer the clinically important question regarding whether PMM dressings per se (and as a class) are associated with positive or negative effects relative to other dressings. This assumes that the PMM 'class' not only includes different types of PMM dressing, but also includes combinations of PMM dressing with any other dressing type. It also assumes that secondary dressings are unimportant.
Eleven of the 12 studies reported concurrent compression therapy; the Smeets 2008 full paper did not mention compression. We have given further details of compression therapy used in the Characteristics of included studies table.
Trial duration
Four studies had a duration of follow‐up of eight weeks or less: Andriessen 2009 (four weeks); Humbert 2013 (two weeks); Manizate 2012 and Meaume 2012 (eight weeks) and five studies had 12 weeks' follow‐up (Lanzara 2008; Romanelli 2015; Schmutz 2008; Smeets 2008; Vin 2002). One study reported results at both four and 12 weeks (Hanft 2006). One study reported results at six months and graphically at one, two, three, four and five months (Petkov 1997). The Humbert 2013 study was stopped early for benefit in a planned interim analysis; this was at two weeks, assessed on their primary outcome of reduction in the proportion of fibrin and necrosis.
Outcomes
Not all 12 studies reported all the outcomes.
Only one study reported sufficient data to allow calculation of time‐to‐complete healing (Petkov 1997)
Eight reported the proportion with complete healing (Hanft 2006; Lanzara 2008; Manizate 2012; Meaume 2012; Petkov 1997; Romanelli 2015; Schmutz 2008; Vin 2002)
Six studies reported on adverse events (Andriessen 2009, Humbert 2013; Meaume 2012; Romanelli 2015; Schmutz 2008; Vin 2002)
Five studies reported pain (Andriessen 2009, Humbert 2013; Meaume 2012; Romanelli 2015; Vin 2002 ); five reported infection (Humbert 2013; Lanzara 2008; Manizate 2012; Meaume 2012; Vin 2002); one study reported quality of life (Meaume 2012); one study reported resource use (Vin 2002) and one study reported cost data in a German healthcare setting (Meaume 2012)
One study (Smeets 2008) only reported the secondary outcome of change in ulcer size. We dId not report the results for this outcome because the review's primary healing outcomes were reported for this comparison by other studies (as per our protocol)
For one study, we are awaiting results from the study authors (Cullen 2012).
Excluded studies
We excluded 57 studies from the review for the following reasons (see Characteristics of excluded studies):
nine studies were not RCTs (Bolton 2003; Gardner 2013; Hodde 2006; Karim 2006; Metzner 1997; Mian 1992; Ronfard 2012; Serra 2013; Wollina 2005);
eight studies were in an ineligible or mixed wound population (Anichini 2013; Palmieri 1992; Ramirez 1994; Shanahan 2013; Sheehan 2003; Veves 2001; Veves 2002; Wethers 1994);
38 had an ineligible intervention (Brown 2014; Brown‐Etris 2000a; Caprio 1995a; Curran 2002; Demling 2004; Ebell 1998; Falabella 1998; Falanga 1998a; Falanga 2006; Falanga 1998b; Falanga 2000; Gilligan 2014; Goedkoop 2010; Gravante 2013; Lantis 2013; Marston 2012; Moffatt 2014; Morimoto 2012; Morimoto 2013; Mostow 2005; Planinsek 2007; Robson 1995; Romanelli 2006b; Romanelli 2007; Romanelli 2006a; Romanelli 2008b; Romanelli 2008a; Romanelli 2010; Romanelli 2011; Serra 2014; Smith 1994; Stojadinovic 2014; Thomas 1997; Trial 2010; Varelias 2002; Vowden 2006; Vowden 2007a; Vowden 2007b). The majority of these studies investigated skin substitutes/bioengineered matrix treatments;
two studies did not report a relevant outcome and healing was not the objective of the trial (Chaloner 1992; Varelias 2006).
Risk of bias in included studies
Figure 2 shows risk of bias judgements for each study (and by outcome for attrition and outcome assessor blinding). Judgements for each domain across studies are shown in Figure 3. We itemised blinding of outcome assessors separately for the healing outcomes, adverse events and secondary outcomes (pain and infection); risk of attrition bias was reported separately for adverse events and other outcomes. We have displayed risk of bias assessments for the studies in each analysis at the side of each forest plot, but only the domains contributing to overall risk of bias are shown. We have given further information on each risk of bias item in the Characteristics of included studies.
Overall risk of bias
We assessed overall risk of bias in terms of high risk of bias in one or more domains from selection bias, detection bias (outcome assessor not blinded), and attrition bias.
For the healing outcomes, we considered six studies (of nine) to have high overall risk of bias (Hanft 2006; Lanzara 2008; Manizate 2012; Meaume 2012; Schmutz 2008; Vin 2002), two of which had two domains of high risk of bias (Schmutz 2008;Vin 2002).
For adverse events, four studies (of six) had high overall risk of bias (Andriessen 2009; Humbert 2013; Schmutz 2008; Vin 2002); one of which had two domains (Vin 2002).
For the secondary outcomes, six studies (of eight) had high risk of bias (Andriessen 2009; Humbert 2013; Manizate 2012; Romanelli 2015; Schmutz 2008; Vin 2002); three of which had two domains (Manizate 2012; Schmutz 2008; Vin 2002).
Effects of interventions
See: Table 1
See: Table 1 and Table 3 for extracted outcome data. In this section, we report the effects of any PMM dressing regimen versus any comparison dressing regimen.
2. Outcomes table.
| Study | Comparison type* | Interventions (dressings) | Length of follow‐up | Proportion healed | Time to healing | Adverse events | Proportion with pain | Proportion with infection |
Quality of life mean (SD) |
Resource use |
| Andriessen 2009 | 2a and 2b | Group 1: PMM + foam (n = 4); Group 2: foam (n = 4) Group 3: basic wound contact (n = 4) |
4 weeks | Not reported | Not reported | Group 1: 0/4 Group 2: 0/4 Group 3: 0/4 | Group 1: 0/4 Group 2: 0/4 Group 3: 4/4 Peto OR for 1 vs 3: 0.03 (95% CI 0.00 to 0.40) |
Not reported | Not reported | |
| Cullen 2012 | 3 | Group 1: PMM1 (n = 32); Group 2: PMM2 (n = 32) |
4 weeks | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Hanft 2006 | 2a | Group 1: PMM‐silver + hydrocolloid (n = 22); Group 2: hydrocolloid (n = 27) |
4 weeks and 12 weeks |
4 weeks Group 1: 5/22 Group 2: 3/27 RR 2.05 (95% CI 0.55 ‐ 7.63) 12 weeks Group 1: 14/22 Group 2: 16/27 |
Not reported | Not reported | No results reported (though in protocol) | No results reported (though in protocol) | No results reported (though in protocol) | |
| Humbert 2013 | 2c | Group 1: PMM (n = 34); Group 2: hydrogel (n = 41) |
2 weeks | Not reported | Not reported | Group 1: 15/34 Group 2: 18/41 |
Group 1: 7/32 Group 2: 0/39 |
Group 1: 0/32 Group 2: 2/39 |
Not reported | |
| Lanzara 2008 | 2a | Group 1: PMM‐silver + foam (n = 15); Group 2: foam (n = 15) |
12 weeks | Group 1: 11/15 Group 2: 7/15 |
Not reported | Not reported | Not reported | Group 1: 4/15 Group 2: 5/15 |
Not reported | |
| Manizate 2012 | 2c | Group 1: PMM‐silver (n = 10); Group 2: hydrocolloid + silver (n = 10) |
8 weeks | Group 1: 3/10 Group 2: 5/10 |
Not reported | Not reported | Not reported | Group 1: 0/10 Group 2: 0/10 |
Not reported | |
| Meaume 2012 | 2d | Group 1: PMM‐foam (n = 93); Group 2: foam (n = 94) |
8 weeks | Group 1: 6/93 Group 2: 7/94 |
Not reported | Group 1: 29/89 Group 2: 27/88 |
Group 1: 1/89 Group 2: 1/88 | Group 1: 7/89 Group 2: 6/88 |
EQ‐5D: Pain/discomfort: Group 1: mean 1.53 (SD 0.53) n = 89 Group 2: 1.74 (0.65) n = 88 MD: ‐0.21 (95% CI ‐0.38 to ‐0.04) Anxiety/depression: 1.35 (0.53) and 1.54 (0.60) MD: ‐0.19 (95% CI ‐0.36 to ‐0.02) numbers analysed assumed |
Mean total treatment costs over 8 weeks (Germany): Group 1: EUR 557.51 Group 2: EUR 526.19 Cost effectiveness analysis was based on number with at least 40% wound area reduction and so are not reported here |
| Petkov 1997 | 2d | Group 1: PMM‐alginate Group 2: alginate |
6 months |
6 months: Group 1: 34/50 Group 2: 32/50 RR: 1.06 (95% CI 0.80 to 1.41) |
Graph of cumulative number of healed ulcers. HR calculated (Tierney 2007): 1.21 (95% CI 0.75 to 1.97), assuming no censoring | Not reported | Not reported | Not reported | QoL/Resources not reported. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Number healed at other times (from graph); 50 randomised per group): 1 month: 2 and 0 2 months: 12 and 8 3 months: 26 and 20 4 months: 29 and 22 5 months: 34 and 31 |
|
| Romanelli 2015 | 2a | Group 1: PMM + basic wound contact dressing + alginate (n = 20); Group 2: alginate (n = 20) |
12 weeks | Group 1: 6/20 Group 2: 5/20 | Not reported | Group 1: 0/20 Group 2: 0/20 |
Group 1: 0/20 Group 2: 0/20 | Not reported | Not reported | |
| Schmutz 2008 | 3 | Group 1: PMM1 (n = 57); Group 2: PMM2 (n = 60) |
12 weeks | Group 1: 10/57 Group 2: 8/60 RR: 1.32 (95% CI 0.56 to 3.10) |
Not reported | Group 1: 14/46 Group 2: 23/49 RR: 0.65 (95% CI 0.38 to 1.10) |
Group 1: 4/40 Group 2: 12/36 |
Group 1: 1/40 Group 2: 6/36 |
Not reported | |
| Smeets 2008 | 2a | Group 1: PMM + hydrocolloid (n = 17); Group 2: hydrocolloid (n = 10) |
12 weeks | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Vin 2002 | 1 | Group 1: PMM + basic wound contact dressing (n = 37); Group 2: basic wound contact dressing (n = 36) |
12 weeks | Group 1: 18/37 Group 2: 12/36 |
Not reported | Group 1: 5/30 Group 2: 5/29 |
Severe pain: Group 1: 6/27 Group 2: 3/24 Constant pain: Group 1: 7/27 Group 2: 4/24 RR: 1.56 (95% CI 0.52 to 4.67) |
Group 1: 0/27 Group 2: 5/24 |
Saline vials per treatment:
Group 1: mean 1.06 (SD 0.78)
Group 2: mean 1.27 (SD 0.78) MD: ‐0.21 (95% CI ‐0.31 to ‐0.11) Number of gauzes: Group 1: 3.8 (2.5) Group 2: 3.7 (2.2) MD: 0.10 (95% CI ‐0.17 to 0.37) (SD 201) seconds MD: ‐40.0 (95% CI ‐128.9 to 48.9) |
* Comparison types:
1. PMM dressing regimen versus basic wound contact dressing regimen
2a. PMM dressing regimen versus advanced dressing regimen with the secondary dressing in the experimental group the same as the primary dressing in the control group
2b. PMM dressing regimen versus advanced dressing regimen with the secondary dressing in the experimental group being similar but different from the primary dressing in the control group
2c. PMM dressing regimen versus advanced dressing regimen with the same secondary dressings in both groups or no secondary dressings or secondary dressings only in the control group
2d. PMM dressing regimen versus advanced dressing regimen: PMM/advanced combination dressing versus advanced dressing
3. PMM dressing 1 versus PMM dressing 2
1. Comparison of PMM dressing regimens versus other dressing regimens (nine trials, 503 participants)
We investigated whether any dressing regimen that incorporated a PMM was more effective than any other (non‐PMM) dressing regimen; the term 'regimen' includes primary and secondary dressings, as appropriate. Therefore, we combined the results from nine studies (Andriessen 2009; Hanft 2006; Humbert 2013; Lanzara 2008; Manizate 2012; Meaume 2012; Petkov 1997; Romanelli 2015; Vin 2002). A tenth study (Smeets 2008) reported none of the primary outcomes. One study (Petkov 1997) reported the proportion with complete healing at six time points, which we used to calculate a hazard ratio, assuming there was no censoring.
1.1. Results
Primary outcomes: complete wound healing and adverse events
Time‐to‐complete healing
Low‐certainty evidence from one study (Petkov 1997) (100 participants, of whom 66 healed at 6 months' follow‐up) is unclear whether PMM dressings heal wounds quicker than alginate dressings (HR 1.21, 95% CI 0.74 to 1.97; Table 3). The 95% CI is consistent with both clinically important benefit and clinically important harm, and the evidence certainty was downgraded for imprecision (twice).
Proportion healed
Short‐term follow‐up ‐ four to eight weeks: it is unclear whether there is a difference in the probability of healing between PMM dressing regimens and other dressing regimens, because the evidence is of very low certainty (downgraded for risk of bias and imprecision (twice)); RR 0.73 (95% CI 0.34 to 1.58) (random effects) (2 studies (Manizate 2012; Meaume 2012); 207 participants, of whom 21 healed; Analysis 1.1).
1.1. Analysis.
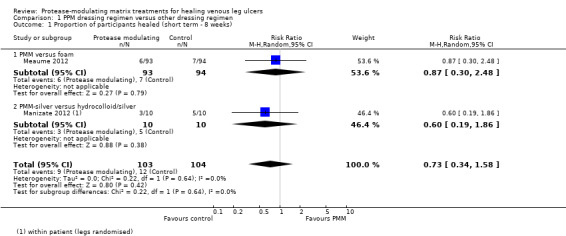
Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 1 Proportion of participants healed (short term ‐ 8 weeks).
Medium‐term follow‐up ‐ 12 weeks: low‐certainty evidence from four studies (Hanft 2006; Lanzara 2008; Romanelli 2015; Vin 2002) (192 participants, of whom 89 healed) is unclear whether PMM dressing regimens increase the probability of healing compared with other dressing regimens at 12 weeks' follow‐up (RR 1.28, 95% CI 0.95 to 1.71; Analysis 1.2); the 95% CI is consistent with both clinically important benefit and no difference. Evidence certainty was downgraded for risk of bias and imprecision.
1.2. Analysis.

Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 2 Proportion of participants healed (medium term ‐ 12 weeks).
Long‐term follow‐up ‐ six months: low‐certainty evidence from one study (Petkov 1997) (100 participants, of whom 66 healed) is unclear whether there is a difference in healing at six months between PMM dressings and other dressings (RR 1.06, 95% CI 0.80 to 1.41; Table 3); the 95% CI is consistent with both no difference and clinically important benefit, and the evidence certainty was downgraded twice for imprecision.
Adverse events
Low‐certainty evidence from five studies (Andriessen 2009; Humbert 2013; Meaume 2012; Romanelli 2015; Vin 2002) (363 participants, of whom 99 had at least one adverse event) is unclear whether there is a difference between PMM dressing regimens and other dressing regimens in adverse events (RR 1.03, 95% CI 0.75 to 1.42; Analysis 1.3); the 95% CI is consistent with both a clinically important benefit and a clinically important harm. Data were pooled across all durations. Evidence certainty was downgraded for imprecision (twice).
1.3. Analysis.
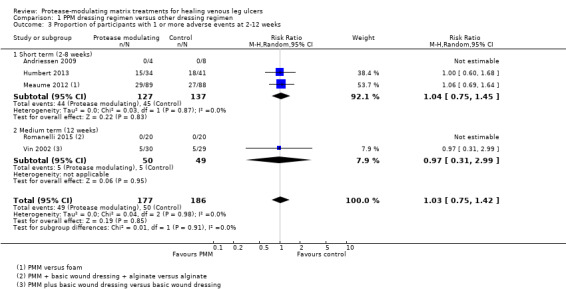
Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 3 Proportion of participants with 1 or more adverse events at 2‐12 weeks.
Secondary outcomes: pain, infection, quality of life, resource use
Pain
It is unclear whether there is a difference in the risk of pain between PMM dressing regimens and other dressing regimens, because the evidence is of very low certainty (downgraded for risk of bias, inconsistency (twice) and imprecision (twice)); (5 studies (Andriessen 2009; Humbert 2013; Meaume 2012; Romanelli 2015; Vin 2002); 356 participants, of whom 22 had pain; Analysis 1.4). Results were not pooled because of the different types of pain measured and durations of follow‐up. There is substantial heterogeneity, which may be due to differences in the comparator dressings.
1.4. Analysis.
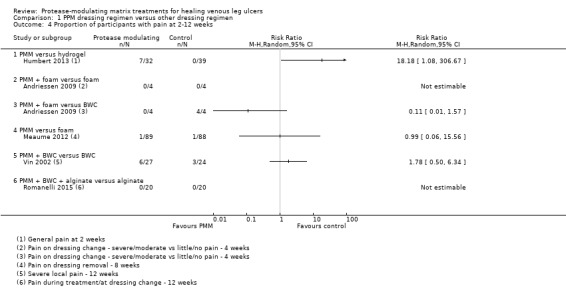
Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 4 Proportion of participants with pain at 2‐12 weeks.
Infection
It is unclear whether there is a difference in the risk of infection between PMM dressing regimens and other dressing regimens, because the evidence is of very low certainty (downgraded for risk of bias, inconsistency and imprecision (twice)); RR 0.69 (95% CI 0.29 to1.68) (5 studies (Humbert 2013; Lanzara 2008; Manizate 2012; Meaume 2012; Vin 2002); 349 participants, of whom 29 had infection; Analysis 1.5).
1.5. Analysis.

Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 5 Proportion of participants with infection at 2‐12 weeks.
Quality of life
It is unclear whether there is a difference in quality of life between PMM dressing regimens and other dressing regimens, because the evidence is of very low certainty (downgraded for risk of bias, indirectness and imprecision). Two sub‐scales of EQ 5D were selectively reported on the basis of statistical significance: pain‐discomfort sub‐scale: MD ‐0.21 (95% CI ‐0.38 to ‐0.04); anxiety‐depression sub‐scale, MD ‐0.19 (95% CI ‐0.36 to ‐0.02) (scale 1 to 5, high as poor outcome) (1 study (Meaume 2012), 177 participants). However, these results are not meaningful because the 5 points on the scale are not intended to be used arithmetically (Euroqol 2015).
Resource use
Low‐certainty evidence from one study (Vin 2002) (73 participants; Table 3) is unclear whether resource use is lower with the PMM dressing than the other dressing. For the participants who used the dressings allocated at randomisation throughout the study, there were 619 and 529 dressing changes for the PMM dressing compared with the other dressing. Number of vials of saline: MD per treatment ‐0.21 (95% CI ‐0.31 to ‐0.11). Number of gauzes used: MD 0.10 (95% CI ‐0.17 to 0.37). Time to complete a dressing change: MD = ‐40 seconds (95% CI ‐62.4 to ‐16.8). The evidence was downgraded for risk of bias (twice).
Costs
Low‐certainty evidence from a cost‐effectiveness analysis based on data from Meaume 2012 (187 participants; Table 3) in a German healthcare setting is uncertain whether there is a difference in mean total treatment costs. Results are EUR 557.51 for the PMM dressing and EUR 526.19 for the other dressing (mean difference 31.32 EUR higher) after eight weeks of treatment; no standard deviations were reported. The cost effectiveness analysis was based on the number of participants with at least 40% wound area reduction (rather than the number with complete healing) and so results are not reported here. The evidence was downgraded for imprecision (twice).
1.2. Summary
Overall, low‐certainty evidence (downgraded for risk of bias and imprecision) is unclear whether PMM dressing regimens are either quicker or more likely to heal VLUs at four to eight weeks, 12 weeks or longer‐term follow‐up than other dressing regimens.
The evidence for any difference in the rate of adverse events is similarly uncertain. It is unclear whether there is a difference in pain, infection and quality of life. Finally, there is uncertainty around resource use (vials of saline, dressing changes, and the number of gauzes used) and around mean total costs in a German healthcare system (Table 1 and Table 3).
2. Comparison of two different PMM dressing regimens (one trial; 117 participants)
One study (n = 117) directly compared two different PMM dressings, non‐adherent wound contact dressing containing NOSF (UrgoStart) and collagen/oxidised regenerated cellulose matrix (Promogran), over 12 weeks (medium term) (Schmutz 2008) and a second study (Cullen 2012) compared two PMM dressings but did not report the results per group. We downgraded the certainty of the evidence from the Schmutz 2008 study for indirectness because some of the participants had leg ulcers that the authors classified as other than venous (17% were postphlebitic and 28% had arterial participation (see Included studies).
2.1. Results
Primary outcomes: complete wound healing and adverse events
Time‐to‐complete healing
No studies reported the time‐to‐complete healing outcome.
Proportion healed
Medium‐term follow‐up ‐ 12 weeks: it is unclear whether there is a difference between UrgoStart and Promogran in healing rates at 12 weeks' follow‐up because the evidence is of very low certainty (downgraded for risk of bias (twice), indirectness and imprecision (twice)); RR 1.32 (95% CI 0.56 to 3.10) (1 study (Schmutz 2008); 117 participants, of whom 18 healed; Table 3).
Adverse events
Medium‐term follow‐up ‐ 12 weeks: it is unclear whether there is a difference in adverse events because the evidence is of very low certainty (as above); RR 0.65 (95% CI 0.38 to 1.10) (1 study (Schmutz 2008); 95 participants for this outcome, in whom 37 had adverse events, which included pain and infection).
Secondary outcomes
Schmutz 2008 also reported on pain between dressing changes and infection (Table 3). The evidence certainty is very low (downgraded for risk of bias (twice), indirectness and imprecision (twice)). RR for pain: 0.30 (95% CI 0.11 to 0.85); RR for infection is 0.15 (95% CI 0.02 to 1.19).
2.2. Summary
There is too much uncertainty to determine whether there is a difference between two different types of PMM dressing (UrgoStart and Promogran) in the rates of healing, adverse events, pain and infection.
3. Subgroup and Sensitivity analyses
We conducted prespecified sensitivity and subgroup analyses (see section: Subgroup analysis and investigation of heterogeneity) for the medium‐term healing outcome because there was a small amount of variability in the point estimates, and a sensitivity analysis for the short‐term healing outcome for the comparison, PMM dressing regimens versus other dressing regimens.
Sensitivity analyses
For the medium‐term outcome, three of the four studies were at overall high risk of bias (Hanft 2006; Lanzara 2008; Vin 2002) so risk of bias could not be examined, either for this outcome or for the short‐term outcome (which only had two studies).
Sensitivity analysis, assuming an available case analysis, was conducted (Analysis 1.6) and gave a risk ratio of 0.72 (95% CI 0.33 to 1.56) for the short‐term outcome and 1.26 (95% CI 0.96 to 1.64) for the medium‐term outcome. The results of these analyses were very similar to the imputed intention‐to‐treat analyses.
1.6. Analysis.

Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 6 Sensitivity analysis ‐ available case ‐ proportion of participants healed.
Subgroup analyses
Subgroup analyses could only be conducted for the medium‐term outcome. Of the pre‐specified subgroup analyses, only the comparison of silver‐containing PMM treatments versus non silver‐containing treatments gave subgroups with more than one study in each subgroup. The results of this analysis are shown in Analysis 1.7. the test for subgroup differences gives I² = 0% and P = 0.68, and the RR for each subgroup was: silver‐containing PMM subgroup, RR = 1.22 (95% CI 0.85 to 1.75) and no silver subgroup, RR = 1.39 (95% CI 0.85 to 2.29). There was no heterogeneity in either subgroup.
1.7. Analysis.

Comparison 1 PPM dressing regimen versus other dressing regimen, Outcome 7 Subgroup analysis: (+/‐) silver ‐ proportion of participants healed medium term.
As a result of these sensitivity and subgroup analyses, we continued with the original analyses.
Discussion
Summary of main results
The review includes 12 RCTs involving a total of 784 participants, however two of these studies (Cullen 2012; Smeets 2008) did not report results for any of the outcomes prespecified for this review. Of the studies reporting outcomes relevant to this review, nine compared PMM dressing regimens with other dressing regimens, whilst one directly compared two PMM dressing regimens. All studies for which we had relevant outcome data also gave the participants compression bandaging. All but two of these studies (Manizate 2012; Petkov 1997) were in participants described as having 'non‐responsive' or 'hard‐to‐heal' ulcers.
For the overall estimates of effectiveness and safety, we summarised results from nine studies; seven of these reported healing outcomes. The main findings reported here are for the comparison of a regimen that includes a PMM dressing versus a regimen with other (non‐PMM) dressings. This is a broad comparison that has a number of assumptions: firstly, that all PMM dressings can be treated as a single class and, secondly, that the nature of the comparator dressings is unimportant. We discuss these assumptions below (Overall completeness and applicability of evidence).
The evidence for the effects of PMM on healing is of low or very low certainty (see Quality of the evidence). The main results are that in the short term (4 to 8 weeks), the evidence is of very low certainty. In the medium term (12 weeks), it is unclear whether there is a benefit in using a PMM dressing regimen, compared with other dressing regimens: meta‐analysis of four studies in 192 participants gave a RR of 1.28 (95% CI 0.95 to 1.71), which corresponds to an absolute risk difference of 112 more people healed per 1000 (from 20 fewer to 284 more) over 12 weeks (for a median control group risk of 400 per 1000). Low‐certainty evidence from a single study (100 participants) at longer term (6 months) is unclear whether there is a difference between PMM dressing regimens and other dressing regimens (RR 1.06, 95% CI 0.80 to 1.41). Low‐certainty evidence from the same study is uncertain whether wounds treated with PMM‐containing dressing regimens heal quicker than other dressing regimens. The HR is 1.21(95% CI 0.74 to 1.97) and the median time to healing is estimated to be 1.5 months quicker for PMM dressing regimens. However, the 95% CI for the HR is consistent with both clinically important benefit and clinically important harm, and the findings are uncertain.
Low‐certainty evidence (5 studies, 363 participants) on adverse events across all durations of follow‐up is unclear whether there is a difference in adverse events between dressing regimens that do, and do not incorporate PMM (RR 1.03, 95% CI 0.75 to 1.42). It is unclear whether there is a difference for the specific adverse events of pain and infection, as well as quality of life because the evidence is of very low certainty.
For resource use, low certainty evidence from one study (73 participants) of 12 weeks duration is unclear whether there is a difference between PMM dressing regimens and other dressing regimens in the use of saline vials and the time taken to complete dressing changes; mean differences were fairly small (0.21 vials per treatment and 40 seconds). For costs, low‐certainty evidence from one study (187 participants) of eight weeks' duration is unclear whether there is a difference in total costs per person for the PMM dressing regimen (mean difference versus control = 31 EUR) in a German healthcare system.
Overall completeness and applicability of evidence
The evidence on PMM treatments is fairly limited: the ten relevant studies were generally small, with numerically few events. The population covered was also limited: participants in all studies but two (Manizate 2012; Petkov 1997), which gave no details, were described as having non‐responsive or 'hard‐to‐heal' ulcers. Therefore, the findings of the review may only be directly relevant to people with ulcers which are in some way hard to heal and who are also receiving compression treatment.
We have investigated healing at three pre‐specified time durations and also as a time‐to‐event analysis. Different studies with different characteristics (see below) inform the different durations, and both the time‐to‐event data and any inference regarding time dependence is reliant on a single study.
We treated all PMM dressing regimens as a single 'class', but there were several variations, including the type of PMM dressing and the presence or absence of secondary dressings and their type: the evidence for the medium‐term (12‐week) healing outcome involved a meta‐analysis of four studies and in each of these the experimental group dressing regimen consisted of a primary PMM dressing plus a non‐PMM secondary dressing, and the control group had the same non‐PMM dressing as its primary dressing. Conversely, two studies for the short‐term (4 to 8‐week) healing outcome compared a PMM‐containing dressing with an advanced dressing. The long‐term outcome (and the time‐to‐event data) involved a study in which the experimental group received a PMM‐alginate combination dressing and the control group received an alginate dressing. One study in 117 participants potentially allowed us to investigate the appropriateness of the 'class' assumption, randomising head‐to‐head two different types of PMM dressing. However, the evidence was of very low certainty and we could not draw conclusions. We cannot be confident from the low‐certainty evidence that there are differences between healing outcomes at different durations, but even if the differences are real, we cannot determine if they are a consequence of the different types of comparison, the duration of follow‐up, the certainty of the evidence, or some other factor.
All the identified studies were industry funded, but there were insufficient studies to examine publication bias statistically. We obtained results from the authors of two conference abstracts, one of which had not been published for 10 years (Hanft 2006) and the other for nearly 20 years (Petkov 1997). The addition of one of these studies to the meta‐analysis for medium‐term healing reduced the effect estimate from RR 1.46 (95% CI 0.99 to 2.15) to 1.28 (95% CI 0.95 to 1.71) and smaller effects at the same interim time point were also found for the other study. These additional data do not affect the qualitative finding of a potential benefit in the medium term of using PMM‐containing dressing regimens, but they do imply a lack of robustness in the current evidence and the need for a large trial with outcomes measured at appropriate intervals in order to examine the overall effect of PMM treatments.
Overall, we cannot be confident that the application of any dressing that claims to be protease‐modulating will lead to benefits in healing, neither can we be sure that our consideration of all PMM dressings as a 'class' has not diluted the effectiveness of a particular dressing. The 'class' assumption may not be reasonable and generalisability of the results to all PMM dressings may not be appropriate.
Quality of the evidence
The evidence in this review is marked by uncertainty, not only because of its sparsity, but also because of risk of bias.
The evidence is of low or very low certainty for all outcomes. This is because of high risk of bias for outcomes in most studies, particularly regarding a lack of blinding of outcome assessors and also attrition bias. In addition, studies were small, with numerically few events, which gives rise to imprecision in the pooled effect estimates for healing.
As discussed above, the meta‐analysis for medium‐term healing is sensitive to the addition of new studies. There is insufficient evidence to carry out sensitivity analyses on the basis of risk of bias or to investigate heterogeneity using subgroups, and publication bias could not be investigated.
Potential biases in the review process
We carefully defined PMM treatments to be those specifically marketed as having protease‐modulating activity, with this being a key feature of the product, or if no commercial product was named, we examined whether the study reported a specific intent of modulating proteases. This meant that a number of studies were excluded because of the interventions, even though matrix dressings were described (but without an indication of protease‐reducing mechanisms). It was important to have a clear and transparent definition of the class, and this definition was derived with important clinical input. This is not so much a potential bias, but a position with which others may disagree, however, inclusion of other studies may have affected the results.
Agreements and disagreements with other studies or reviews
Evidence on PMM treatments for healing VLUs is sparse: one systematic review investigated one type of PMM dressing (collagen oxidised regenerated cellulose) in people with venous, arterial and pressure ulcers (Galea 2015). Two studies were identified for VLUs, one of which was excluded from our review (Wollina 2005). Overall, the review concluded that, "although there is some evidence to support the use of collagen ORC, there is a clear need for further evidence". We have been unable to identify any further systematic reviews on PMM treatments.
An overview of reviews of treatments for VLU has recently been published (Nelson 2016), but does not report on PMM treatments.
Authors' conclusions
Implications for practice.
Low‐certainty evidence in participants with hard‐to‐heal VLUs is unclear whether the use of PMM dressing regimens (in comparison with other dressings), over and above the effect of compression bandaging, increases either the speed of healing or the rate of healing at short‐, medium‐ and long‐term follow‐up. Low‐certainty evidence is unclear whether there is a difference in adverse events compared with other (non‐PMM) dressings, and there is also uncertainty around whether there is a difference in resource use or costs for PMM dressings.
The GRADE meaning of 'low‐certainty evidence' is that "our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect". Additionally, "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate" (Balshem 2011). Further research is proposed (see Implications for research), but clinicians wishing to use these products should be aware of the uncertainty around the findings of this review.
Implications for research.
The findings of this review are that it is uncertain whether PMM treatments result in quicker and more healing for people with hard‐to‐heal VLUs, who are receiving compression treatment, but the limited evidence suggests a possibility that they might. If this is true, such that PMM dressings can give healing times that are shorter by a few weeks compared to other dressings or there is a moderate improvement in the probability of medium term healing, this could be important to patients. The existing evidence is uncertain and so there is a need for further investigation in a large RCT. Such a trial could usefully compare two different PMM dressings and an advanced dressing: two PMM dressings are proposed because we cannot be sure there is a class effect. We suggest the PMM dressings should be those specifically designed and marketed as protease‐modulating (e.g. Promogran and UrgoStart). The population would be people with hard‐to‐heal VLUs or it might be useful to include both venous and mixed venous‐arterial ulcers, as stratified groups. Healing should be investigated as a time‐to‐event outcome, with regular monitoring times and at least six months' follow‐up. It might be useful to additionally monitor protease levels.
What's new
| Date | Event | Description |
|---|---|---|
| 20 April 2017 | Amended | Additional source of support added. |
Acknowledgements
The review authors would like to acknowledge the contribution of Cochrane Wounds editor Liz McInnes and peer referees Abraham Janis and Durhane Wong Rieger for their comments on the protocol; and peer referees Andrea Nelson, Anne‐Marie Glenny, Sarah Rhodes, Una Adderley, Ross Atkinson and Linda Lehman for their comments on the review. They are grateful to copy editor Denise Mitchell for her contribution.
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Leg Ulcer] explode all trees #2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris or foot ulcer*):ti,ab,kw (Word variations have been searched) #3 {or #1‐#2} #4 MeSH descriptor: [Protease Inhibitors] explode all trees #5 MeSH descriptor: [Collagen] explode all trees #6 MeSH descriptor: [Doxycycline] explode all trees #7 MeSH descriptor: [Amelogenin] explode all trees #8 (proteas* or proteinas*):ti,ab,kw (Word variations have been searched) #9 metalloproteas*:ti,ab,kw (Word variations have been searched) #10 Cadesorb* or Catrix* or Xelma* or Promogran* or Tegaderm* or UrgoStart*:ti,ab,kw (Word variations have been searched) #11 matrix:ti,ab,kw (Word variations have been searched) #12 ((Starch* or Collagen* or Doxycycline* or Amelogenin* or NOSF or TLC‐NOSF or "nano‐oligosaccharide factor") near/3 (dressing* or ointment*)):ti,ab,kw (Word variations have been searched) #13 {or #4‐#12} #14 {and #3, #13} in Trials
Ovid MEDLINE
1 exp Leg Ulcer/ 2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris or foot ulcer*).tw. 3 or/1‐2 4 exp Protease Inhibitors/ 5 exp Collagen/ or Doxycycline/ or Amelogenin/ or exp Starch/ 6 (proteas* or proteinas*).ti,ab. 7 metalloproteas*.ti,ab. 8 (Cadesorb* or Catrix* or Xelma* or Promogran* or Tegaderm* or UrgoStart*).af. 9 matrix.ti,ab. 10 ((Starch* or Collagen* or Doxycycline* or Amelogenin* or NOSF or TLC‐NOSF or "nano‐oligosaccharide factor") adj3 (dressing* or ointment*)).ti,ab. 11 or/4‐10 12 and/3,11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomi?ed.ab. 16 placebo.ab. 17 clinical trials as topic.sh. 18 randomly.ab. 19 trial.ti. 20 or/13‐19 21 exp animals/ not humans.sh. 22 20 not 21 23 and/12,22
Ovid Embase
1 leg ulcer/ or foot ulcer/ or leg varicosis/ 2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris or foot ulcer*).tw. 3 or/1‐2 4 exp Proteinase Inhibitor/ 5 exp collagen/ or doxycycline/ or amelogenin/ or starch/ 6 (proteas* or proteinas*).ti,ab. 7 metalloproteas*.ti,ab. 8 (Cadesorb* or Catrix* or Xelma* or Promogran* or Tegaderm* or UrgoStart*).af. 9 matrix.ti,ab. 10 ((Starch* or Collagen* or Doxycycline* or Amelogenin* or NOSF or TLC‐NOSF or "nano‐oligosaccharide factor") adj3 (dressing* or ointment*)).ti,ab. 11 or/4‐10 12 and/3,11 13 Randomized controlled trials/ 14 Single‐Blind Method/ 15 Double‐Blind Method/ 16 Crossover Procedure/ 17 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 18 (doubl* adj blind*).ti,ab. 19 (singl* adj blind*).ti,ab. 20 or/13‐19 21 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 22 human/ or human cell/ 23 and/21‐22 24 21 not 23 25 20 not 24 26 and/12,25
EBSCO CINAHL Plus
S27 S13 AND S26 S26 S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 TI allocat* random* or AB allocat* random* S24 MH "Quantitative Studies" S23 TI placebo* or AB placebo* S22 MH "Placebos" S21 TI random* allocat* or AB random* allocat* S20 MH "Random Assignment" S19 TI randomi?ed control* trial* or AB randomi?ed control* trial* S18 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S17 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S16 TI clinic* N1 trial* or AB clinic* N1 trial* S15 PT Clinical trial S14 MH "Clinical Trials+" S13 S3 AND S12 S12 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S11 TI ( ((Starch* or Collagen* or Doxycycline* or Amelogenin* or NOSF or TLC‐NOSF or "nano‐oligosaccharide factor") N3 (dressing* or ointment*)) ) OR AB ( ((Starch* or Collagen* or Doxycycline* or Amelogenin* or NOSF or TLC‐NOSF or "nano‐oligosaccharide factor") N3 (dressing* or ointment*)) ) S10 TI matrix OR AB matrix S9 TI ( (Cadesorb* or Catrix* or Xelma* or Promogran* or Tegaderm* or UrgoStart*) ) OR AB ( (Cadesorb* or Catrix* or Xelma* or Promogran* or Tegaderm* or UrgoStart*) ) S8 TI metalloproteas* OR AB metalloproteas* S7 TI ( (proteas* or proteinas*) ) OR AB ( (proteas* or proteinas*) ) S6 (MH "Doxycycline") S5 (MH "Collagen") S4 (MH "Protease Inhibitors+") S3 S1 OR S2 S2 TI ( (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or or ulcus cruris or ulcer cruris or foot ulcer*) ) OR AB ( (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or or ulcus cruris or ulcer cruris or foot ulcer*) ) S1 (MH "Leg Ulcer+")
Appendix 2. Assessment of risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially‐numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Either participants or some key study personnel were not blinded, and the non‐blinding was likely to introduce bias.
Unclear
Either of the following:
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified.
One or more reported primary outcomes of the study were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important additional risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias in cluster randomised trials
In cluster‐randomised trials, particular biases to consider include: (i) recruitment bias; (ii) baseline imbalance; (iii) loss of clusters; (iv) incorrect analysis; and (v) comparability with individually randomised trials (Higgins 2011b).
(i) Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited.
(ii) Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
(iii) Occasionally complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
(iv) Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
(v) In a meta‐analysis including both cluster and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by discussion of a Cochrane review of hip protectors. The cluster trials showed large positive effect whereas individually randomised trials did not show any clear benefit. One possibility is that there was a ‘herd effect’ in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such ‘contamination’ would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and ‘herd effects’ may be different for different types of cluster.
Data and analyses
Comparison 1. PPM dressing regimen versus other dressing regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants healed (short term ‐ 8 weeks) | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.58] |
| 1.1 PMM versus foam | 1 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.30, 2.48] |
| 1.2 PMM‐silver versus hydrocolloid/silver | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.19, 1.86] |
| 2 Proportion of participants healed (medium term ‐ 12 weeks) | 4 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.95, 1.71] |
| 2.1 PMM + BWC versus BWC | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.83, 2.58] |
| 2.2 PMM + BWC + alginate versus alginate | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.44, 3.30] |
| 2.3 PMM‐silver + foam versus foam | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.84, 2.92] |
| 2.4 PMM‐silver + hydrocolloid versus hydrocolloid | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.67] |
| 3 Proportion of participants with 1 or more adverse events at 2‐12 weeks | 5 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.42] |
| 3.1 Short term (2‐8 weeks) | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.75, 1.45] |
| 3.2 Medium term (12 weeks) | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.31, 2.99] |
| 4 Proportion of participants with pain at 2‐12 weeks | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 PMM versus hydrogel | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 PMM + foam versus foam | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 PMM + foam versus BWC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 PMM versus foam | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 PMM + BWC versus BWC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 PMM + BWC + alginate versus alginate | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Proportion of participants with infection at 2‐12 weeks | 5 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.68] |
| 5.1 PMM versus hydrogel (2 weeks) | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.87] |
| 5.2 PMM‐silver versus hydrocolloid + silver (8 weeks) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 PMM versus foam (8 weeks) | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.40, 3.30] |
| 5.4 PMM + BWC versus BWC (12 weeks) | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.40] |
| 5.5 PMM‐silver + foam versus foam (12 weeks) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.27, 2.41] |
| 6 Sensitivity analysis ‐ available case ‐ proportion of participants healed | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Short term (4‐8 weeks) | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.56] |
| 6.2 Medium term (12 weeks all studies) | 4 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.96, 1.64] |
| 6.3 Long term (over 24 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.80, 1.41] |
| 7 Subgroup analysis: (+/‐) silver ‐ proportion of participants healed medium term | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Silver in PMM arm | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.85, 1.75] |
| 7.2 No silver in PMM arm | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.85, 2.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andriessen 2009.
| Methods | RCT, parallel; single‐centre trial, participants randomised Setting: phlebology clinic. Country: Italy Duration of follow‐up (intervention period): 4 weeks Funding: industry‐funded trial ‐ limited grant from Lohmann & Rauscher (manufacturer of both interventions) Unit of analysis: participant | |
| Participants | 12 participants with VLUs (hard‐to‐heal: wounds that had not reduced in size after 4 weeks' standard care). Number of wounds: not reported; if participants had more than one ulcer, the largest was selected (no indication of how many per participant)
Age: mean (range): Group 1: 79 (70‐91); Group 2: 78.25 (70‐81); Group 3: 76.5 (74‐79) years. Sex (M/F): overall: 4/8. Duration of ulcer: mean (median, range): Group 1: 7.75 (9, 4–14); Group 2: 11.25 (14, 4–22); Group 3: 26.25 (11, 4–84) months. Ulcer size: mean (range, assumed): Group 1: 23.97 (12.4–56); Group 2: 27.55 (16–62); Group 3: 17.37 (8.48–29) cm². No infected wounds at baseline Inclusion criteria: transcutaneous oxygen partial pressure measurement of < 40 mmHg; VLU not reduced in size despite 4 weeks of standard care, aged > 18 years Exclusion criteria: clinical signs of infection, necrotic tissue or predominance of slough, significant arterial disease (ABPI < 0.8), ulcers less than 4 cm² or circumferential; other causes of ulceration, oral or topical corticosteroids, participation in a leg ulcer trial in previous year, dementia or disorientation, known allergy for latex/trial products |
|
| Interventions | Group 1: PMM dressing + foam dressing: collagen dressing plus secondary foam dressing (Suprasorb® C (Activa) plus Suprasorb® P (secondary)); (n = 4; duration 4 weeks) Group 2: foam dressing (Suprasorb® P (Lohmann & Rauscher)); i.e. same dressing as secondary dressing for intervention 1 (n = 4; duration 4 weeks) Group 3: basic wound contact dressing ‐ paraffin gauze (manufacturer not stated); (n = 4; duration 4 weeks) Co‐interventions: all participants wore short‐stretch high compression bandages Dressing procedure: the clinician cleansed the VLU with saline and then applied the assigned treatment. Dressing change frequency was at the clinician’s discretion, but on average this took place twice weekly and was based on exudate levels only. Prior treatments: a variety of other modern wound dressings and compression bandaging systems had been used before entry into the study. No participants had previously used the foam or collagen dressings. | |
| Outcomes | Primary outcomes of the review: complete healing not reported; adverse events Secondary outcomes: pain on dressing change (moderate/severe versus little/no pain), change in ulcer size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by a computer‐generated allocation scheme." Comment: adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised by a computer‐generated allocation scheme, using sealed envelopes." Comment: partial allocation concealment ‐ envelopes not said to be sequentially numbered or opaque. In addition, there were some baseline differences: in ulcer area (mean 24 versus 28 versus 17 cm²) and duration of ulcer (median 9 versus 14 versus 11 months) |
| Blinding participants and personnel (performance bias) | High risk | Comment: the "clinician" changed the dressings and performed the assessments. Dressings were sufficiently different to be unblinded (2 dressings versus 1 dressing) |
| Blinding outcome assessors (detection bias): healing outcomes | Low risk | Quote: "Assessors were blinded to the treatment given for all of these tests. Ulcer area and wound‐bed characteristics..." Comment: outcome assessors blinded for ulcer area outcome |
| Blinding outcome assessors (detection bias): adverse events | High risk | Comment: the "clinician" changed the dressings and performed the assessments. Dressings were sufficiently different to be unblinded (2 dressings versus 1 dressing for the two comparisons included in this review) |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quote: "Patients reported pain at each dressing removal on a 10cm visual analogue scale (VAS)." Comment: the patient was the outcome assessor. Dressings were sufficiently different to be unblinded (2 dressings versus 1 dressing) |
| Incomplete outcome data (attrition bias) healing/secondary | Low risk | No missing data |
| Incomplete outcome data (attrition bias) adverse events | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Comment: paper stated that the "patients who healed before 4 weeks returned to the clinic for a final evaluation", but did not report the number of participants healed. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Cullen 2012.
| Methods | RCT, parallel; number of centres not stated, participants randomised Setting: not reported. Country: unclear Duration of follow‐up (intervention period): 4 weeks Funding: industry‐funded trial ‐ authors employed by Systagenix (manufacturers of both interventions) Unit of analysis: not stated | |
| Participants | *64 participants with VLUs (hard‐to‐heal: 23% reported to have elevated protease levels, but no other indicators reported). Number of wounds: not reported Age: not reported. Sex (M/F): not reported. Duration of ulcer: not reported. Ulcer size: not reported. Infected wounds at baseline: not reported. Total number of participants with elevated protease activity (EPA) 13/64 (56 analysed); not reported per group and *participants not stratified by EPA level before randomisation Inclusion criteria: not reported Exclusion criteria: not reported | |
| Interventions | Group 1: PMM + silver dressing: collagen, silver & oxidised regenerated cellulose matrix dressing (Promogran® Prisma® (Systagenix)); (*n = 32; duration 4 weeks) Group 2: PMM dressing: collagen & oxidised regenerated cellulose matrix dressing (Promogran® (Systagenix)); (*n = 32; duration 4 weeks) Co‐interventions: compression was required to be at least 40 mmHg Dressing procedure: not reported Prior treatment: not reported | |
| Outcomes | Primary outcomes of the review: complete healing not reported (4 weeks); adverse events not reported Secondary outcomes: complete healing defined as > 30% reduction in wound area, but results not given per group | |
| Notes | Abstract and some communication with author (marked with an asterisk) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding participants and personnel (performance bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) healing/secondary | Unclear risk | *Overall 8/64 (12.5%) excluded because of protocol violations. Overall event rate was 63% for "healing", but results per group not reported, so assigned unclear risk of bias |
| Selective reporting (reporting bias) | High risk | Quote from author communication: "I am still trying to access this data – however I have attached the poster which provides more info than the abstract that was published" Comment: results only given overall |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Hanft 2006.
| Methods | RCT, parallel; multicentre, participants randomised (3 centres) Setting: wound clinic. Country: USA Duration of follow‐up (intervention period): 12 weeks Funding: industry‐funded trial ‐ Ethicon (manufacturers of intervention 1) Unit of analysis: not stated | |
| Participants | 49 participants with VLUs (hard‐to‐heal: mean duration 4‐5 months). Number of wounds: not reported Age: not reported. Sex (M/F): not reported Duration of ulcer*: Group 1: 4.3 month; Group 2: 5.1 months. Ulcer size*: Group 1: 6.9 cm²; Group: 5.6 cm². No infected wounds at baseline Inclusion criteria: ≥ 18 years; ulcer area > 3 cm² but < 25 cm²; ulcer open for > 1 month but < 18 months; ABI > 0.8, HbA1c < 10; free of clinical signs of infection Exclusion criteria: prior treatment with becaplermin or other topical recombinant therapy within 30 days; prior treatment with skin substitute or growth factor; significant acute or chronic disease; enzymatic debridement in previous 7 days | |
| Interventions | Group 1*: protease‐modulating matrix + silver dressing + hydrocolloid dressing: collagen, silver & oxidised regenerated cellulose matrix dressing + hydrocolloid (Collagen/ORC+silver + Adaptic®); (n = 22; duration 12 weeks) Group 2*: hydrocolloid dressing: non‐adherent petrolatum impregnated dressing (Adaptic® (Johnson & Johnson)); (n = 27; duration 12 weeks) Co‐interventions: standardised compression therapy Dressing procedure: not stated Prior treatment: 1 week run in with standardised leg compression; debridement | |
| Outcomes | Primary outcomes of the review*: proportion completely healed (12 weeks) (also 4 weeks); adverse events not reported Secondary outcomes: protocol included pain, infection and quality of life, but no results reported | |
| Notes | Published as two protocol abstracts. Communication with study authors gave additional information on results (indicated with asterisk). NCT00235209 on ClinicalTrials.gov* |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding participants and personnel (performance bias) | High risk | Quote: "Randomized, prospective, open‐label, multicenter, comparative trial" |
| Blinding outcome assessors (detection bias): healing outcomes | High risk | Quote: "Randomized, prospective, open‐label, multicenter, comparative trial" Comment: outcome assessors likely to be unblinded |
| Incomplete outcome data (attrition bias) healing/secondary | Low risk | Quote from author communication: "intervention group 9% (2/22) did not complete the study, 1 was lost to follow up and 1 chose to withdraw. In the control group 11% (3/27) did not complete, 2 subjects died from severe AEs (unrelated to the study interventions) and one chose to withdraw". Healing risks were 64% and 59% |
| Selective reporting (reporting bias) | High risk | Limited reporting of results ‐ some obtained from the author, but some protocol outcomes not reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Humbert 2013.
| Methods | RCT, parallel; multi‐centre trial, participants randomised (21 French hospitals, clinics and private practices) Setting: both inpatients and outpatients implied. Country: France Duration of follow‐up (intervention period): 2 weeks Funding: industry‐funded trial ‐ Paul Hartmann AG (manufacturer of protease‐modulating dressing) Unit of analysis: participant | |
| Participants | 75 participants with VLUs randomised (hard‐to‐heal: defined below). Number of wounds: 75. One wound per participant, but unclear if selected. 'Hard‐to‐heal' wounds defined as those with "a duration of >6 months (69%), an ulcer surface of >10 cm² (85%) and a wound bed that was covered by >70% with slough and necrotic tissue (73%)"; 22/34 (65%) and 30/41 (73%) > 6 months' duration
Age: Group 1: mean 74.8 (SD 11.7); Group 2: 73.7 (SD 9.6). Sex (M/F): Group 1: 13/21; Group 2: 9/32. Duration of ulcer: Group 1: mean 2.32 (SD 3.22) years, median (range) 1.7 (0.1‐16.5); Group 2: 3.32 (SD 4.37), median (range) 1.5 (0.1‐22.6). Ulcer size: Group 1: mean 31.0 (SD 28.9), median 21.0; Group 2: 26.1 (SD 20.1), median 18.0 cm². Infected wounds at baseline: not reported Inclusion criteria: aged > 18 years; ECOG score < 2; leg ulcer duration > 4 weeks; ABI > 0.8; wound coverage > 70% fibrin and/or necrotic tissue; wound size 8‐100 cm2; concomitant compression therapy with stockings or bandages Exclusion criteria: prior treatment with study or control dressing in previous 4 weeks; mechanical and enzymatic debridement or use of gels in previous 2 weeks; surgical debridement in previous 8 weeks; severe concomitant disease; blood haemoglobin ≤ 8g/L; serum albumin ≤ 25g/L; HbA1c ≥ 8.5% |
|
| Interventions |
Group 1: PMM dressing ‐ polyacrylate‐based hydrogel (Hydroclean®/Tenderwet® (Paul Hartmann AG)); (n = 34; duration 2 weeks) Group 2: hydrogel (Intrasite® (Smith & Nephew)): secondary dressings such as gauze were used for group 2 only; (n = 41; duration 2 weeks) Co‐interventions: compression bandages: 29 and 35 (90% for each); stockings 3 (9%) and 4 (10%); 35 participants (46.7%) received a concomitant medication at baseline (analgesics 25.3%, systemic antibiotics 8.3%) Dressing procedure: sodium chloride solution (for rinsing) and compresses and tapes for fixation were permitted; dressings were changed on average every 1.1 days (0.9‐2.0) for Group 1 and every 1.4 days (0.8‐2.3) for Group 2 Prior treatment: in 69 participants (92.0%), the wound had received a dressing prior to inclusion (contact layers 52%, hydrofibre/alginate 42.7%, silver‐releasing dressings 22.7% and foam 20.0%. |
|
| Outcomes | Primary outcomes of the review: complete healing not reported (2 weeks); adverse events Secondary outcomes: pain (general), infection | |
| Notes | Trial stopped at 2 weeks because of benefit to intervention group in a planned interim analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization list using the PLAN procedure of the SAS software (SAS Institute, Cary, NC, USA) was centrally generated" Comment: adequate sequence generation using a computer |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomization list ... was centrally generated, controlled and accessed through a secured website" Comment: adequate allocation concealment because randomisation plan was centrally generated by computer via a secured website. |
| Blinding participants and personnel (performance bias) | High risk | Quote: "Study blinding was impossible because of the different aspect of the two dressing types" |
| Blinding outcome assessors (detection bias): adverse events | High risk | Quote "AE and patient complaints about discomfort were recorded at each visit." Comment: adverse events assessed by participants and investigators who were not blinded |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quote "AE and patient complaints about discomfort were recorded at each visit." Comment: secondary outcomes assessed by participants and investigators who were not blinded |
| Incomplete outcome data (attrition bias) healing/secondary | Unclear risk | Quote: "Two patients in each group [6 and 5%] discontinued the study before day 14 because of an AE. 10 participants had a major deviation from the protocol, with 2 participants in each group failing to perform adequate compression therapy." Comment: this level of missing data could affect outcomes with a low event rate (i.e. infection and pain). It is unclear which AEs led to discontinuation (i.e. they could be infection or pain), so assessed as unclear risk of bias for these outcomes |
| Incomplete outcome data (attrition bias) adverse events | Low risk | Quote: "Two patients in each group [6 and 5%] discontinued the study before day 14 because of an AE. 10 participants had a major deviation from the protocol, with 2 participants in each group failing to perform adequate compression therapy." Comment: the missing data were due to adverse events, so this does not constitute attrition bias for the adverse events outcome |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported according to methods section, but change in size was reported as a dichotomised outcome |
| Other bias | Unclear risk | Study stopped early on the basis of a positive response in a planned interim analysis. Significantly more fibrin and necrotic tissue at baseline for group 1 |
Lanzara 2008.
| Methods | RCT, parallel; single‐centre trial, participants randomised (implied one centre (only 30 participants)) Setting: not reported. Country: Italy Duration of follow‐up (intervention period): 12 weeks Funding: industry‐funded trial ‐ Systagenix Unit of analysis: not stated | |
| Participants | 30 participants with "non‐responsive" VLUs (hard‐to‐heal: description of non‐responsive) Number of wounds: not reported
Age: overall: mean 73 (SD 20) years. Sex (M/F): overall: 14/16. Duration of ulcer: disease duration 30 days‐20 years. Ulcer size: Group 1: 6 cm²; Group 2: 9 cm². Infected wounds at baseline: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Group 1: PMM + silver dressing + foam dressing ‐ collagen, silver & oxidised regenerated cellulose matrix dressing + hydropolymer foam (Collagen/ORC + silver (Systagenix) + Tielle Family® (Systagenix) + Tielle Family® (Systagenix)): dressing changes every week; (n = 15; duration 12 weeks)
Group 2: foam dressing (Tielle Family® (Systagenix)); (n = 15; duration 12 weeks) Co‐interventions: short stretch multi‐layer compression Dressing procedure: not reported. Prior treatment: not reported |
|
| Outcomes | Primary outcomes of the review: proportion completely healed (12 weeks); adverse events not reported Secondary outcomes: infection | |
| Notes | Conference abstract plus additional information from Systagenix website poster | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients... were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients... were randomized" Comment: Baseline differences in ulcer size: 6 cm² versus 9 cm², so some indication of selection bias |
| Blinding participants and personnel (performance bias) | High risk | Comment: dressings were sufficiently different for participants and personnel to be unblinded ‐ two dressings versus one dressing |
| Blinding outcome assessors (detection bias): healing outcomes | High risk | Quote: "Study duration was 12 weeks, with dressing changes every week as well as measurements on wound size and assessment of wound appearance." Comment: implication that outcome assessors were also responsible for dressing changes, who were not blinded as above |
| Blinding outcome assessors (detection bias): secondary outcomes | Unclear risk | Comment: unclear who were the outcome assessors |
| Incomplete outcome data (attrition bias) healing/secondary | Unclear risk | Apparently no missing data, but no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting. Some results (healing) only reported on the Systagenix website |
| Other bias | Unclear risk | Abstract. Insufficient information to assess whether an important risk of bias exists |
Manizate 2012.
| Methods | RCT, parallel; single‐centre trial, legs randomised (within‐participant) Setting: tertiary‐care referral wound practice. Country: USA Duration of intervention: 8 weeks Funding: industry‐funded trial ‐ Medline Industries; Mundelein, Illinois Unit of analysis: ulcer | |
| Participants | 10 participants with VLUs (hard‐to‐heal: ulcer size). Number of wounds: 20. 2 per participant randomised to different groups; unclear if ulcers selected. (9/10 participants had VLU; 1 had DFU (apparently all data reported))
Age: not reported. Sex (M/F): not reported. Duration of ulcer: not reported. Ulcer size: Group 1: 14.9 (SD 13.3) cm² versus Group 2: 9.8 (SD 9.7) cm². No infected wounds at baseline (but bacterial loads reported for both groups; no conversion to infection). Bilateral comparable wounds Inclusion criteria: aged > 18 years, full thickness venous stasis or diabetic or neuropathic lower‐extremity wounds, greater or lesser saphenous insufficiency; venous perforator incompetency and deep venous system incompetency or diabetes and HbA1c 6%‐14% and ABI 0.7‐1.2 Exclusion criteria: known history of poor compliance or allergy to products evaluated; NPWT in previous 14 days; skin substitutes or skin grafts in previous 60 days; participants requiring corticosteroids or with immune disorders |
|
| Interventions |
Group 1: PMM + silver dressing ‐ bovine native collagen plus silver (manufacturer not stated); secondary foam dressing (Optifoam); (n = 10; duration 8 weeks)
Group 2: hydrocolloid + silver dressing ‐ carboxymethylcellulose plus silver (manufacturer not stated); secondary foam dressing (Optifoam); (n = 10; duration 8 weeks) Co‐interventions: 4‐layer multilayer wrap for compression (4‐Layer Compression Bandaging System) Dressing procedure: sharp debridement; cleansing with normal saline; secondary foam dressing (Optifoam). Dressings were changed weekly. Prior treatment: not reported |
|
| Outcomes | Primary outcomes of the review: proportion completely healed (8 weeks ‐ assumed); adverse events not reported Secondary outcomes: infection, change in ulcer size. Pain (general) measured on a pain scale, but no results given | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "1 limb was randomized to treatment with either CMC or BDC, whereas the contralateral wound was treated with the other dressing" |
| Allocation concealment (selection bias) | High risk | Quote: "1 limb was randomized to treatment with either CMC or BDC, whereas the contralateral wound was treated with the other dressing". Comment: Baseline differences in wound size ‐ mean (SD): BDC 14.9 (SD13.3) and CMC 9.8 (SD 9.7) cm². Additionally, the absolute rate of wound closure was bigger in BDC, but the percentage (of wound volume) rate of closure was smaller in BDC. The difference was not statistically significant, but this was a small study and the differences in an important prognostic factor for healing, together with the lack of information on allocation concealment in a within‐participant trial, suggests high risk of selection bias |
| Blinding participants and personnel (performance bias) | High risk | Quote: "This is a prospective, randomized, nonblinded trial." |
| Blinding outcome assessors (detection bias): healing outcomes | Unclear risk | Quote: "At the weekly study, site dressing changes, subjective assessments of .. any signs of erythema (no reddening, pink, red, beet red), the level of pain (linear analog scale 1 through 10) were recorded. Digital images also were taken and used to assess wound healing over time. Moreover, the total surface area (in centimeters squared) of the participant’s reference ulcers was measured." Comment: unclear who the outcome assessors were for healing |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quote: "This is a prospective, randomized, nonblinded trial." Comment: outcome assessors were the participants for pain |
| Incomplete outcome data (attrition bias) healing/secondary | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | High risk | Quote from the methods section: "...and the level of pain (linear analog scale 1 through10) were recorded. Digital images also were taken and used to assess wound healing over time." Comment: the study authors do not report pain data, although measured. Additionally, the methods section mentions wound healing over time, but no results are reported. |
| Other bias | High risk | No account taken of paired data. Healing results calculated from percentages and number randomised |
Meaume 2012.
| Methods | RCT, parallel; multi‐centre, participants randomised (number of centres not stated) Setting: hospital inpatient or outpatient (81%). Country: France Duration of follow‐up (intervention period): 8 weeks Funding: industry‐funded trial ‐ Laboratoires URGO (Chenôve, France), manufacturer of both interventions However, data were managed, interpreted and analysed by an independent organisation. Unit of analysis: participant | |
| Participants | 187 participants with VLUs (hard‐to‐heal: ulcer description). Number of wounds: 203. Where more than 1 ulcer was present the one that best met the inclusion criteria was selected; this had to be at least 3 cm distant from any other wound. 55% and 52% of selected ulcers were recurrent. *Following prior treatment, 42% and 44% of wounds were stagnating and 13% and 9% were worsening)
Age: Group 1: mean 72.6 years (SD 13.0); Group 2: 74.4 (SD 12.1). Sex (M/F): 31/62 and 34/60. Duration of ulcer: Group 1: mean 15.6 months (SD 9.1); Group 2: 15.1 (SD 8.7); median (range): 12 (3‐35) and 12 (6‐36). Ulcer size: Group 1: mean 17.0 cm² (SD 15.6); Group 2: 16.6 (SD 15.8); median (range) 12.9 (2.3‐86.9) and 10.5 (2.7‐85.3). 58.1% and 51.1% had ulcers > 10 cm². No infected wounds at baseline Inclusion criteria: VLU, aged > 18 years, ulcer 5‐50 cm², 6‐36 month duration; ABPI 0.8‐1.3; ≥ 50% wound bed covered with granulation tissue with no black necrotic tissue Exclusion criteria: suspected clinical infection that could require systemic antibiotic, known contact dermatitis to carboxymethylcellulose, venous surgery within previous 2 months, DVT in previous 3 months, severe morbid disease/poor health threatening 8‐week follow‐up; malignant wound degeneration, immunosuppressive agents or high dose oral corticosteroids |
|
| Interventions |
Group 1: PMM dressing ‐ adherent polymer matrix dressing containing nano‐oligosaccharide factor (NOSF), with polyurethane foam film backing (UrgoStart® (Urgo)): lipido colloid technology dressing; (n = 93; duration 8 weeks)
Group 2: foam dressing (Urgotul® Absorb (Urgo)): lipido colloid technology dressing; (n = 94; duration 8 weeks) Co‐interventions: compression therapy appropriate to participant and ulcer status (physician judgement) ‐ 2 groups equally distributed to monolayer and multilayer. Use of topical antibiotics, antimicrobial paste/cream, or antiseptics was not allowed; all other general and local treatments were allowed. Dressing procedure: all ulcers were appropriately debrided at baseline; only sterile saline used for wound cleaning during dressing change. Dressing change recommended at least every 2–4 days or more frequently, depending on the level of exudate and the clinical aspect of the wound. *Prior treatment: Foam 30% and 33%, alginate 16% and 17%, greasy gauze 14% and 13%, silver dressing 12% and 9%, interface 4% and 10%, hydrocolloid 1% and 3%, others 23% and 16% |
|
| Outcomes | Primary outcomes of the review: proportion completely healed (8 weeks); adverse events Secondary outcomes: health‐related quality of life, pain (on dressing removal), infection | |
| Notes | Additional information from study author communication marked with an asterisk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization code was generated in blocks of two using a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual sterile dressings were packed in boxes of 35 dressings per participant. Each box and dressing was identified by a center identification number and participant number corresponding to the chronological participant inclusion number... the procedure to break the randomization code was not provided to the participating centers." Comment: probably sufficient for low risk of bias |
| Blinding participants and personnel (performance bias) | Low risk | Quote: "Prior to the start of the trial, an assessment team examined the two dressings and found no distinguishing features, indicating that they could be used in a double‐blind trial." |
| Blinding outcome assessors (detection bias): healing outcomes | Low risk | Quote: "The VLU was evaluated by the investigating physician... At each visit, the wound evaluations were repeated (clinical assessment, acetate tracing, and wound photo)." Comment: double blind trial and outcome assessors were the investigators |
| Blinding outcome assessors (detection bias): adverse events | Low risk | Quote: "The VLU was evaluated by the investigating physician... Investigators were required to notify any unexpected local adverse events" Comment: double‐blind trial and outcome assessors were the investigators |
| Blinding outcome assessors (detection bias): secondary outcomes | Low risk | Quote: "all local procedures were recorded by health‐care professionals... The parameters
(..pain at removal, and between dressing changes and periwound maceration) were subjectively assessed" Comment: double‐blind trial and outcome assessors were the health care professionals |
| Incomplete outcome data (attrition bias) healing/secondary | High risk | 4/93 (4%) and 6/94 (6%) withdrew and were lost to follow‐up. An additional 11/93 (12%) and 11/94 (12%) switched to "another" dressing, but were followed up in the groups to which they were randomised. Number missing comparable with number of events for healing (6 and 7) and infection (4 and 5), and more than for pain (1 and 1) |
| Incomplete outcome data (attrition bias) adverse events | Low risk | 4/93 (4%) and 6/94 (6%) withdrew and were lost to follow‐up. An additional 11/93 (12%) and 11/94 (12%) switched to another dressing, but were followed up in the groups to which they were randomised. Number of missing participants lower than the number of adverse events (29 and 27), so low risk of bias assigned. Similarly the risk of bias for the continuous quality of life outcome was considered at low risk of bias. |
| Selective reporting (reporting bias) | High risk | EQ‐5D only reported for the two sub‐scales that gave significant differences in favour of the test dressing |
| Other bias | Unclear risk | There were some differences at baseline, for example, 58% and 51% had an ulcer size > 10 cm². |
Petkov 1997.
| Methods | RCT, parallel; single‐centre trial, participants randomised Setting: unclear. Country: Poland Duration of follow‐up (intervention period): 6 months (but graph showing 1, 2, 3, 4 , 5 months too) Funding: industry‐funded trial ‐ Johnson & Johnson Unit of analysis: participant | |
| Participants | 100 participants with vVLUs. Number of wounds: not reported. Implied 1 per person Age: not reported. Sex (M/F): not reported. Duration of ulcer: not reported. Ulcer size: not reported, but < 100 cm². No infected wounds at baseline Inclusion criteria: VLU, aged > 18 years, ulcer < 100 cm²; ABPI > 0.7; moderate to severe exudate Exclusion criteria: clinically infected ulcers; treatment with topical medications; dry necrotic tissue layer; any therapy that may retard wound healing; pregnant/lactating women; silver sulphadiazine in last 7 days; participation in any research study for ulcer treatment in past 3 months | |
| Interventions | Group 1: PMM dressing ‐ collagen alginate dressing (Fibracol® (Johnson & Johnson)); (n = 50; duration 6 months) Group 2: alginate dressing ‐ alginate (Kaltostat® (Convatec)]; (n = 50; duration 6 months) Co‐interventions: standardised compression therapy (Secure Forte Johnson & Johnson elastic cohesive bandage) Dressing procedure: redressed as required Prior treatment: study author correspondence, "None of the patients was treated for more but infection, mainly antibiotics and never used compression before" | |
| Outcomes | Primary outcomes of the review: proportion completely healed (6 months) (also 1, 2, 3, 4, 5 months); adverse events not reported Secondary outcomes: not reported | |
| Notes | Only published as an abstract on a follow‐up study from an unpublished trial. However, author communication gave many more details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "100 patients ... were randomised" Comment: no details on method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "100 patients ... were randomised" Comment: no details on allocation concealment |
| Blinding participants and personnel (performance bias) | Unclear risk | Comment: no information |
| Blinding outcome assessors (detection bias): healing outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) healing/secondary | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine if reporting bias |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Romanelli 2015.
| Methods | RCT, parallel; single‐centre trial, participants randomised Setting: outpatients attending wound healing unit. Country: Italy Duration of follow‐up (intervention period): 12 weeks Funding: industry‐funded trial ‐ unrestricted grant from Medskin Solutions (manufacturer of intervention 1) Unit of analysis: participant | |
| Participants | 40 participants with 'hard‐to‐heal' VLUs (non‐responsive ulcers (no measurable improvement after 6 weeks' standard treatment)); participants with venous insufficiency. Number of wounds: not reported. Age: Group 1: mean 68 (SD 5); Group 2: 65 (SD 2). Sex (M/F): Group 1: 7/13; Group 2: 5/15. Duration of ulcer: wound age: Group 1 ‐ mean 24 (SD 6) weeks; Group 2: 20 (SD 4) weeks. Ulcer size: Group 1: mean 26 (SD 4); Group 2: 24 (SD 5) cm². No infected wounds at baseline Inclusion criteria: participants with venous insufficiency and a VLU, which did not respond to 6 weeks' treatment with short‐stretch compression and moist wound healing Exclusion criteria: participants who had diabetes, autoimmune disease or peripheral arterial disease; ABPI < 0.8; participants who smoked or who had VLU(s) with clinical signs of infection | |
| Interventions | Group 1: PMM dressing + basic wound contact dressing + alginate dressing ‐ collagen membrane + non‐adherent petrolatum impregnated dressing + alginate (Proheal® (MedSkin Solutions) + Adaptic® (Systagenix) + Curasorb® (Kendall)): non‐adherent dressing was used as an interface with the secondary dressing; (n = 20; duration 12 weeks) Group 2: alginate dressing ‐ alginate (Curasorb® (Kendall)); (n = 20; duration 12 weeks) Co‐interventions: short‐stretch compression bandaging system Dressing procedure: dressing changes performed twice a week with a saline solution used to cleanse the wound. The collagen dressing was then applied over the wound bed. The short‐stretch compression bandaging system was applied by an experienced nurse and maintained over the lower leg. Prior treatments: all participants had received prior treatment of 6 weeks of compression therapy and moist wound healing. | |
| Outcomes | Primary outcomes of the review: proportion completely healed (12 weeks); adverse events Secondary outcomes: pain (during treatment or at dressing change) | |
| Notes | Group 1 had a non‐adherent dressing as an interface between the collagen membrane and the secondary alginate dressing. Group 2 only had the alginate dressing. We do not consider this to be a substantial difference between interventions, rather a variation on the protease‐modulating dressing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was established by a random permuted block of five patients, prepared in advance." Comment: Probably computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation was established by a random permuted block of five patients, prepared in advance." Comment: allocation concealment method unclear |
| Blinding participants and personnel (performance bias) | High risk | Comment: dressings were sufficiently different for participants and personnel to be unblinded ‐ three dressings versus one dressing |
| Blinding outcome assessors (detection bias): healing outcomes | Low risk | Quote: "To improve the quality of this trial and exclude bias during wound assessment, patients were evaluated in a standard room ambient, laying in the same position at each visit. The VLU area was measured with a non‐invasive laser scanning system (Silhouette, Aranz, New Zealand) and by the same two nurses especially trained for this study." Comment: this measurement will have led to the assessment of complete healing. Therefore low risk of bias |
| Blinding outcome assessors (detection bias): adverse events | Unclear risk | Quote: "No significant side effects were detected in either group." Comment: no further information on who assessed the adverse events |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quote: "Patients of both groups were satisfied with their treatment and healing progress, and did not report any problems with pain during treatment or at dressing changes" Comment: participants were the outcome assessors and they would have known whether they had three dressings or one |
| Incomplete outcome data (attrition bias) healing/secondary | Unclear risk | Comment: number of participants with missing data not reported |
| Incomplete outcome data (attrition bias) adverse events | Unclear risk | Comment: number of participants with missing data not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain and adverse events vaguely reported and the number of participants analysed was uncertain. The paper only reported the number randomised per group in the study characteristics table. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Schmutz 2008.
| Methods | RCT, parallel; multicentre trial, participants randomised (22 French hospital units and 5 UK specialist wound centres) Setting: hospitalised and outpatients (82%). Country: France and UK Duration of follow up (intervention period): 12 weeks Funding: unclear ‐ mentions that statistical analysis was conducted by a company independent of the "sponsor"; one author was employed by Urgo (manufacturer of intervention 1) Unit of analysis: participant | |
| Participants | 117 participants with VLUs (hard‐to‐heal: description of ulcers, duration and size). Number of wounds: not reported. In the case of multiple ulcers, a single ulcer was selected as target wound. Wound details: VLU 32 (56.1% Group 1) and 32 (53.3% Group 2); post‐phlebitic 8 (14%) and 12 (20%); arterial participation 17 (29.8%) and 16 (26.7%); 54% and 67% were recurrent and 65% and 72% were stagnating.
Age: Group 1: 71.5 (SD 13.1); Group 2: 71.0 (SD 13.9) years. Sex (M/F): 24/33 and 24/36. Duration of ulcer: mean 10.4 (SD 7.1) months and 12.1 (SD 7.7); median 8.0 and 12.0 months. Ulcer size: mean 11.4 (SD 10.1) cm² and 10.4 (SD 8.4); median 9.0 and 7.9 cm²; 54% and 58% had an ulcer duration > 6 months. No infected wounds at baseline Inclusion criteria: adult inpatient or outpatients with VLU with ABPI ≥ 0.8 and concordant with compression therapy. Ulcer area between 5‐25cm² and duration 3‐24 months Exclusion criteria: black or necrotic tissue; venous surgery within previous 2 months, DVT in previous 3 months; suspicion of clinical infection or malignant wound degeneration, poor health status, current treatment with immunosuppressive agents, radiotherapy or high dose of oral corticosteroids |
|
| Interventions |
Group 1: PMM dressing ‐ non‐adherent wound contact dressing containing nano‐oligosaccharide factor (UrgoStart® (Urgo)): 10 x 10 cm; (n = 57; duration 12 weeks)
Group 2: PMM dressing ‐ collagen & oxidised regenerated cellulose matrix dressing (Promogran® (Systagenix)): 28 cm²; (n = 60; duration 12 weeks) Co‐interventions: 88% and 95% were concordant with compression bandages Dressing procedure: before each dressing change (every 3 days or more frequently as required), wounds were cleansed exclusively with normal saline. If necessary, mechanical debridement was performed. Dressings were applied to completely cover the wound surface and covered with a non woven absorbent pad. Prior treatments: participants had previously received "appropriate care". |
|
| Outcomes | Primary outcomes of the review: proportion completely healed (12 weeks); adverse events Secondary outcomes: pain (between dressing changes), infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients...were randomly allocated to be treated" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients...were randomly allocated to be treated" Comment: insufficient information to determine if the allocation was concealed. In addition, there were some differences at baseline which could have affected the outcome: recurrent ulcer, diabetes, ulcer duration, stagnating ulcer (all of which were less for group 1) and size (less for group 2) |
| Blinding participants and personnel (performance bias) | High risk | Quote: "open, randomised trial" |
| Blinding outcome assessors (detection bias): healing outcomes | High risk | Quote: "open, randomised trial" and "Efficacy, which was the primary endpoint of the
study, was assessed by the investigating physician at each weekly clinical evaluation" Comment: investigating physician was the outcome assessor and they were not blinded |
| Blinding outcome assessors (detection bias): adverse events | High risk | Quote: "open, randomised trial" and "the tolerance (occurrence of local adverse events documented by the investigating physician)" Comment: investigating physician was the outcome assessor and they were not blinded |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quotes: "open, randomised trial" and "During the study, acceptability of the dressings was monitored by using open questions" and "the tolerance (occurrence of local adverse events documented by the investigating physician) and the acceptability of the tested dressings were assessed (by the nursing staff) during the 12‐week follow‐up." Comment: outcome assessors were the nursing staff and the participants and neither were blinded |
| Incomplete outcome data (attrition bias) healing/secondary | High risk | 17/57 (30%) and 24/60 (40%) participants were withdrawn from the study (though authors report an ITT analysis with imputation of non‐event). Main reasons: local adverse events 6 and 13; ulcer aggravation 7 and 5. This is high compared with the number of events for the healing (10 and 8), pain (4 and 12) and infection (1 and 6) outcomes, and there is differential missing data too. |
| Incomplete outcome data (attrition bias) adverse events | Low risk | 17/57 (30%) and 24/60 (40%) participants were withdrawn from the study (though authors report an ITT analysis with imputation of non‐event). Main reasons: local adverse events 6 and 13; ulcer aggravation 7 and 5. So level of missing data not due to AE was 11/57 and 11/60; this level may not affect the adverse events outcome (14 and 23). |
| Selective reporting (reporting bias) | Low risk | Outcomes in the methods section reported in the results |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Smeets 2008.
| Methods | RCT, parallel; single‐centre trial, participants randomised (implied 1 centre (only 27 participants)) Setting: not reported. Country: Germany Duration of follow‐up (intervention period): 12 weeks Funding: industry‐funded trial ‐ research grant from Ethicon GmbH Unit of analysis: participant | |
| Participants | 27 participants with VLUs (hard‐to‐heal: reported baseline and follow‐up values of proteases, which suggested non‐healing wounds). Number of wounds: not reported
Age: Group 1: 68 (SD 9); Group 2: 66 (SD 10) years. Sex (M/F): not reported, "majority were female". Duration of ulcer: between 30 days and 3 months. Ulcer size: not reported. Infected wounds at baseline: not reported. No systemic inflammatory diseases or malignant tumours Inclusion criteria: people with a VLU Exclusion criteria: not reported |
|
| Interventions |
Group 1: PMM dressing + hydrocolloid dressing ‐ collagen & oxidised regenerated cellulose matrix dressing + hydrocolloid secondary dressing (manufacturer not stated); (n = 17; duration 12 weeks)
Group 2: hydrocolloid dressing (manufacturer not stated); (n = 10; duration 12 weeks) Co‐interventions: not reported Dressing procedure: not reported Prior treatment: not reported |
|
| Outcomes | Primary outcomes of the review: complete healing not reported; adverse events not reported Secondary outcomes: change in ulcer size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients randomly divided into two groups" |
| Blinding participants and personnel (performance bias) | High risk | Comment: Dressings were sufficiently different to be unblinded (two dressings versus one dressing). |
| Blinding outcome assessors (detection bias): healing outcomes | Unclear risk | Quote: "All ulcers were photographed on admission and at each wound exudates collection time point to provide a visual record of any changes in appearance of the ulcer and to determine healing rate. The surface area of all ulcers was measured by planimetry" Comment: unclear who were the outcome assessors |
| Incomplete outcome data (attrition bias) healing/secondary | Unclear risk | Not stated if data were missing |
| Selective reporting (reporting bias) | Unclear risk | Few outcomes reported |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Vin 2002.
| Methods | RCT, parallel; multicentre trial, participants randomised (14 centres ‐ 9 hospitals, 5 private practice centres) Setting: hospital or private practice, most participants treated as outpatients. Country: France Duration of follow‐up (intervention period): 12 weeks Funding: industry‐funded trial ‐ Johnson & Johnson; Wound Management, France Unit of analysis: participant | |
| Participants | 73 participants with stagnating VLUs (hard‐to‐heal: description of ulcers). Number of wounds: Group 1: 30 participants with 1 ulcer, 6 with 2 ulcers, 4 with 3‐5 ulcers; median 1 (81%). Group 2: 22 participants with 1 ulcer, 7 with 2 ulcers, 7 with 3‐5 ulcers; median 1 (61%). In people with multiple ulcers, the largest ulcer was selected as the trial ulcer and was ≥ 3 cm from any other ulcer.
Age: Group 1: 74.1 (SD 12.1); Group 2: 71.7 (SD 11.4) years. Sex (M:F): Group 1: 59.5% female; Group 2: 69.4%. Duration of ulcer: Group 1: 8.5 (SD 11); Group 2: 9.9 (SD 20.2) months. Ulcer size: Group 1: mean 7.0 (SD 6.8; range 1.6‐35.5 ); Group 2: 9.5 (SD 9.5; range 1.2‐34.5) cm². No infected wounds at baseline Inclusion criteria: VLU > 30 days' duration with measurement 2‐10 cm in any one dimension; aged > 18 years; ABPI ≥ 0.8 Exclusion criteria: infected ulcers; unwilling to wear compression bandages continuously; immobile or unable to care for themselves; comorbidity such as carcinoma, vasculitis, connective tissue disease, immune system disorder, systemic or topical corticosteroids, immunosuppressive agents, radiation or chemotherapy in prior 30 days |
|
| Interventions |
Group 1: PMM dressing + basic wound contact dressing ‐ Collagen & oxidised regenerated cellulose matrix dressing + non‐adherent wound contact dressing (Promogran® (Systagenix) + Adaptic® (Johnson & Johnson)): dressing 10.2 x 10.2 cm cut to fit; then covered with non‐adherent petrolatum‐impregnated dressing (Adaptic); (n = 37; duration not stated.) Group 2: basic wound contact dressing ‐ non‐adherent petrolatum impregnated dressing (Adaptic® (Johnson & Johnson)); (n = 36; duration 12 weeks) Co‐interventions: Biflex compression bandaging worn continuously Dressing procedure: wound was cleaned with warm sterile normal saline before dressings were re‐applied. The surrounding tissue was dried. Gauze pads were applied as secondary dressings. Dressings were changed at least twice weekly. Prior treatments: participants with diabetes could be included provided the target ulcer was venous. Prior wound treatment not reported |
|
| Outcomes | Primary outcomes of the review: proportion completely healed (12 weeks); adverse events Secondary outcomes: infection, resource use and pain (reported as the number of dressing changes with pain associated; also reported as 'constant pain" and "severe pain" (local)) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly allocated to the Promogran or the control group." Comment: no details on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "subjects were randomly allocated to the Promogran or the control group." Comment: no details on allocation concealment. In addition, there were differences at baseline that could have affected the pain outcome: number of participants with severe pain (10.8 and 5.6%) c.f. pain outcome, "number with constant pain" ‐ 18.9 and 11.1% |
| Blinding participants and personnel (performance bias) | High risk | Quote: "this study was a randomised controlled prospective open‐label ..study." |
| Blinding outcome assessors (detection bias): healing outcomes | High risk | Quotes: "Investigators assessed the overall ulcer progress and local care acceptability/tolerability." and "Dressings were changed at least twice weekly at the investigators’ facility, either by the investigator and/or the same nurse team." Comment: outcome assessors were not blinded |
| Blinding outcome assessors (detection bias): adverse events | High risk | Quote: "At the final visit, investigators assessed the ... local care acceptability/tolerability. Subjects were asked to assess their satisfaction with the treatment received." Comment: investigators and participants were the outcome assessors and they were not blinded |
| Blinding outcome assessors (detection bias): secondary outcomes | High risk | Quote: "At the final visit, investigators assessed the ... local care acceptability/tolerability. Subjects were asked to assess their satisfaction with the treatment received." Comment: investigators and participants were the outcome assessors and they were not blinded |
| Incomplete outcome data (attrition bias) healing/secondary | High risk | 10/36 (28%) and 12/37 (32%) said to have an "early end to follow up", for the following reasons: Group 1 ‐ general adverse event (unrelated) 2, local adverse event 3, poor acceptability 2, stagnating ulcer 1, other (holiday) 2; Group 2: consent withdrawal 3, local adverse event 5, poor acceptability 2, stagnating ulcer 1, other (holiday) 1. This is high in relation to the number of events for infection (0 and 5), pain (7 and 4) and local adverse events outcomes (3 and 5, as described), and comparable for healing (18 and 12), so high risk of bias assigned |
| Incomplete outcome data (attrition bias) adverse events | High risk | 10/36 (28%) and 12/37 (32%) said to have an "early end to follow up", for the following reasons: group 1 ‐ general adverse event (unrelated) 2, local adverse event 3, poor acceptability 2, stagnating ulcer 1, other (holiday) 2; group 2: consent withdrawal 3, local adverse event 5, poor acceptability 2, stagnating ulcer 1, other (holiday) 1. The number of adverse events are 3 and 5 (as described as having early end to follow up), so the remaining missing data could have affected the effect estimate for the adverse events outcome |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes were specified vaguely in the methods section, and the number of participants with any adverse effect was not reported. The pain outcome unclear ‐ some participants had pain at baseline |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
ABPI: Ankle Brachial Pressure Index EPA: elevated protease activity ECOG: Eastern Co‐operative Oncology Group (a performance score) ITT: intention‐to‐treat NPWT: negative pressure wound therapy ORC: oxidised regenerated cellulose PMM: protease‐modulating matrix RCT: randomised controlled trial VLU: venous leg ulcer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anichini 2013 | Ineligible patient population |
| Bolton 2003 | Invited editor piece, "Evidence corner" ‐ discussion of two trials; also incorrect population ‐ diabetic foot ulcer |
| Brown 2014 | Ineligible intervention ‐ silica gel fibre matrix. Not marketed or reported to have a specific intent of modulating proteases |
| Brown‐Etris 2000a | Ineligible intervention ‐ composite cultured skin containing keratinocytes and fibroblasts. Not marketed or reported to have a specific intent of modulating proteases |
| Caprio 1995a | Ineligible intervention ‐ lyophilised collagen tablets covered by gauze. Not marketed or reported to have a specific intent of modulating proteases |
| Chaloner 1992 | Ineligible population/outcomes. Authors stated that as healing rate was not one of the objectives of the study, it was decided to include both arterial and venous ulcers in the study. |
| Curran 2002 | Ineligible intervention ‐ skin substitute (Apligraf). Not marketed or reported to have a specific intent of modulating proteases |
| Demling 2004 | Ineligible intervention ‐ biomaterial derived from porcine small intestinal submucosa. Not marketed or reported to have a specific intent of modulating proteases |
| Ebell 1998 | Ineligible intervention ‐ human skin equivalent. Not marketed or reported to have a specific intent of modulating proteases |
| Falabella 1998 | Ineligible intervention ‐ ointment consisting of a combination of 2 proteolytic enzymes, fibrinolysin and desoxyribonuclease (DNAse). Not marketed or reported to have a specific intent of modulating proteases |
| Falanga 1998a | Ineligible intervention ‐ skin substitute (Apligraf). Not marketed or reported to have a specific intent of modulating proteases |
| Falanga 1998b | Ineligible intervention ‐ skin substitute (Apligraf). Not marketed or reported to have a specific intent of modulating proteases |
| Falanga 2000 | Ineligible intervention ‐ skin substitute (Apligraf). Not marketed or reported to have a specific intent of modulating proteases |
| Falanga 2006 | Ineligible intervention ‐ skin substitute (Apligraf). Not marketed or reported to have a specific intent of modulating proteases |
| Gardner 2013 | Ineligible study design |
| Gilligan 2014 | Ineligible intervention ‐ extracellular matrix. Not marketed or reported to have a specific intent of modulating proteases |
| Goedkoop 2010 | Ineligible intervention ‐ HP802‐247, a new‐generation, allogeneic tissue engineering product consisting of growth‐arrested, human keratinocytes and fibroblasts. Not marketed or reported to have a specific intent of modulating proteases |
| Gravante 2013 | Ineligible intervention ‐ Bionect Start, a topical ointment based on hyaluronic acid sodium salt by fermentation (0.2% w/w), and bacterial collagenase obtained from non‐pathogenic Vibrio alginolitycus (> 2.0 nkat/g). Not marketed or reported to have a specific intent of modulating proteases |
| Hodde 2006 | Ineligible study design |
| Karim 2006 | Ineligible study design |
| Lantis 2013 | Ineligible intervention ‐ HP802‐247, an investigational allogeneic living cell bioformulation consisting of neonatal keratinocytes and fibroblasts in a fixed ratio of 1:9, maintained through growth arrest using gamma irradiation. Not marketed or reported to have a specific intent of modulating proteases |
| Marston 2012 | Ineligible intervention ‐ fibrin matrix with growth‐arrested neonatal fibroblasts and keratinocytes. Not marketed or reported to have a specific intent of modulating proteases |
| Metzner 1997 | Ineligible study design |
| Mian 1992 | Ineligible study design |
| Moffatt 2014 | Ineligible intervention and non‐specific comparator ‐ Oxyzyme/Iodozyme versus standard care (continuation with current treatment regimen) |
| Morimoto 2012 | Ineligible intervention ‐ skin substitute, collagen/gelatin sponge impregnated with basic fibroblast growth factor. Not marketed or reported to have a specific intent of modulating proteases |
| Morimoto 2013 | Ineligible intervention ‐ skin substitute, collagen/gelatin sponge impregnated with basic fibroblast growth factor. Not marketed or reported to have a specific intent of modulating proteases |
| Mostow 2005 | Ineligible intervention ‐ extracellular matrix graft (OASIS Wound Matrix). Not marketed or reported to have a specific intent of modulating proteases |
| Palmieri 1992 | Ineligible patient population |
| Planinsek 2007 | Ineligible intervention ‐ autologous platelet releasate. Not marketed or reported to have a specific intent of modulating proteases |
| Ramirez 1994 | Ineligible patient population |
| Robson 1995 | Ineligible interventions ‐ bovine transforming growth factor‐R2 plus collagen matrix versus collagen matrix placebo vehicle versus a standard dressing. Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2006a | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2006b | Ineligible intervention ‐ Oasis versus hyaluronic acid wound dressing. Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2007 | Ineligible intervention ‐ extracellular matrix, Oasis versus Hyaloskin. Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2008a | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2008b | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2010 | Ineligible intervention ‐ OASIS (biomaterial derived from the porcine small‐intestine submucosa). Not marketed or reported to have a specific intent of modulating proteases |
| Romanelli 2011 | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Ronfard 2012 | Ineligible study design |
| Serra 2013 | Ineligible study design |
| Serra 2014 | Ineligible intervention ‐ mixed glycosaminoglycan formulations (Sulodexide). Not marketed or reported to have a specific intent of modulating proteases |
| Shanahan 2013 | Ineligible patient population |
| Sheehan 2003 | Ineligible patient population |
| Smith 1994 | Ineligible interventions ‐ alginate versus hydrocolloid |
| Stojadinovic 2014 | Ineligible intervention ‐ bilayered living cellular construct. Not marketed or reported to have a specific intent of modulating proteases |
| Thomas 1997 | Ineligible intervention ‐ alginate versus hydrogel |
| Trial 2010 | Ineligible intervention ‐ ionic silver alginate matrix (Askina Calgitrol Ag). Not marketed or reported to have a specific intent of modulating proteases |
| Varelias 2002 | Ineligible intervention ‐ mitogenic bovine whey extract containing growth factors. Not marketed or reported to have a specific intent of modulating proteases |
| Varelias 2006 | Ineligible population/outcomes: only 50% of the participants had VLUs. Also, purpose of the study was solely to investigate changes in protease levels |
| Veves 2001 | Ineligible patient population |
| Veves 2002 | Ineligible patient population |
| Vowden 2006 | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Vowden 2007a | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Vowden 2007b | Ineligible intervention ‐ extracellular matrix biocompatible protein, amelogenin (Xelma). Not marketed or reported to have a specific intent of modulating proteases |
| Wethers 1994 | Ineligible patient population |
| Wolcott 2015 | Ineligible intervention: wound gel/biofilm. Not marketed or reported to have a specific intent of modulating proteases |
| Wollina 2005 | Ineligible study design |
Characteristics of studies awaiting assessment [ordered by study ID]
Braumann 2008.
| Methods | Study design unclear (participants were allocated to treatments "depending on the initial bacterial status of wounds"). We had no response to requests for further information; multicentre trial (3 university centres) Setting: not reported. Country: Germany Duration of follow‐up (intervention period): 6 weeks Funding: unclear Unit of analysis: ulcer |
| Participants | 60 participants with VLUs and other wounds (chronic leg ulcers, pressure sores, abdominal wounds, skin defects after cancer resection, and diabetic foot ulcers ‐ proportions not stated). Number of wounds: not reported Age: not reported. Sex (M/F): not reported. Duration of ulcer: not reported. Ulcer size: not reported. No infected wounds at baseline (none of the wounds required systemic antibiotics or were associated with lymphangitis or fever) Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Group 1: PMM + silver dressing + foam dressing ‐ collagen, silver & oxidised regenerated cellulose matrix dressing + foam: secondary foam dressing; (n = not stated; duration 6 weeks) Group 2: alginate + silver dressing ‐ silver‐releasing hydro‐alginate: secondary foam dressing; (n = not stated; duration 6 weeks) Co‐interventions: not reported (including compression) Prior treatment: not reported |
| Outcomes | Primary outcomes of the review: proportion completely healed (6 weeks, not reported per group); adverse events not reported Secondary outcomes: none reported |
| Notes | No response to request for further information |
PMM: protease‐modulating matrix VLU: venous leg ulcer
Characteristics of ongoing studies [ordered by study ID]
NCT 01537003.
| Trial name or title | WOUNDCHEK™ Protease Status Point of Care (POC) Diagnostic Test |
| Methods | Randomised controlled trial Open label efficacy study Primary purpose diagnostic |
| Participants |
Inclusion criteria: adults (aged at least 18 years) with VLU (ABPI ≥ 0.8) willing/able to use appropriate compression therapy. Ulcers with a duration between 6 weeks and 3 years and area between 1 cm² and 100 cm² (maximum length 10 cm). Ulcers needed to show no signs of local or systemic infection; C‐reactive protein needed to be normal and leukocyte levels below 10,000. Wounds could not be treated with PROMOGRAN dressing in the 4 weeks prior to study entry. Exclusion criteria: known hypersensitivity to wound dressings used; local or systemic antibiotics in week prior to inclusion; cancer treated by radiotherapy or chemotherapy; prolonged treatment with immunosuppressive agents/high dose corticosteroids; current illness or condition which may interfere with wound healing in the last 30 days (carcinoma, connective tissue disease, autoimmune disease or alcohol or drug abuse); life expectancy of < 6 months; uncontrolled diabetes; participation in a clinical trial on wound healing within the past month; unable to understand aims and objectives of the trial; known history of non‐adherence with medical treatment; pregnancy; HIV/AIDS; viral hepatitis |
| Interventions | Participants with low EPA: Collagen/ORC dressing (PROMOGRAN®) plus 2 layer compression bandage Participants with low EPA: 2 layer compression bandage only Participants with high EPA: Collagen/ORC dressing (PROMOGRAN®) plus 2 layer compression bandage Participants with high EPA: 2 layer compression bandage only |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | October 2012 |
| Contact information | Breda Cullen, PhD, Systagenix Wound Management |
| Notes | Contacted June 2015 |
ABPI: Ankle Brachial Pressure Index EPA: elevated protease activity ORC: oxidised regenerated cellulose
Differences between protocol and review
We did not directly contact the manufacturers and distributors of PMM treatments or topical treatments regarding relevant unpublished research.
We stated in the protocol that we would use an imputed intention‐to‐treat analysis for adverse events, but in the review we used an available case analysis because it was more appropriate.
We included one study that randomised legs in the same analysis as studies that randomised participants, even though a paired analysis could not be performed. We considered this to give a conservative estimate of the standard error. We did not exclude the data from the meta‐analysis and record it separately.
We conducted subgroup analyses if there was a possibility of heterogeneity (including variability in the point estimates), rather than if there was high heterogeneity. We conducted a sensitivity analysis on the assumption around imputation of missing data, regardless of whether there were different rates of missing data between treatment arms.
Contributions of authors
Maggie Westby: designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; wrote to study author/ experts/companies; and is a guarantor of the review.
Gill Norman: extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; checked the quality of the statistical analysis; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; and wrote to study author/experts /companies.
Jo Dumville: conceived and designed the review; contributed to writing or editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; secured funding; and performed previous work that was the foundation of the current review.
Nikki Stubbs: contributed to writing or editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; and performed previous work that was the foundation of the current review.
Nicky Cullum: conceived, designed and coordinated the review; analysed or interpreted data; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; secured funding; and performed previous work that was the foundation of the current review.
Contributions of the editorial base
Joan Webster (Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to submission.
Andrea Nelson (Editor): edited the protocol; advised on methodology, interpretation and content; approved the final protocol prior to submission.
Gill Rizzello/Sally Bell‐Syer (Managing Editors): co‐ordinated the editorial process; advised on content; edited the protocol and review.
Reetu Child (Information Specialist): designed the search strategy, ran the search and edited the search methods section.
Ursula Gonthier (Editorial Assistant): contacted study authors to request unpublished data.
Sources of support
Internal sources
School of Nursing, Midwifery and Social Work, University of Manchester, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 ‐ High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester, UK.
Nicky Cullum and Jo Dumville, authors of this review, were partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Maggie Westby: my employment at the University of Manchester is funded by the NIHR and focuses on high priority Cochrane reviews in the prevention and treatment of wounds.
Gill Norman: my employment at the University of Manchester is funded by the NIHR and focuses on high priority Cochrane reviews in the prevention and treatment of wounds.
Jo Dumville: I receive research funding from the NIHR for the production of systematic reviews focusing on high priority Cochrane reviews in the prevention and treatment of wounds.
Nikki Stubbs: funding from pharmaceutical companies supports training and education events in the service, and payments have been received by the author for non product related educational sessions. These have been unrelated to the subject matter of the systematic review and have never been in support or in pursuit of the promotion of products.
Nicky Cullum: I receive research funding for wounds related research and systematic reviews from the NIHR.
Edited (no change to conclusions)
References
References to studies included in this review
Andriessen 2009 {published data only (unpublished sought but not used)}
- Andriessen A, Polignano R, Abel M. Monitoring the microcirculation to evaluate dressing performance in patients with venous leg ulcers. Journal of Wound Care 2009;18(4):145‐6, 148. [DOI] [PubMed] [Google Scholar]
Cullen 2012 {published and unpublished data}
- Cullen B, Gibson M, Nisbet L. Targeted use of collagen/ORC improves clinical outcome. woundchek.com/uploads/downloads/posters_articles/Targeted‐Use‐of‐CollagenORC‐Improves‐Clinical‐Outcome.pdf (accessed 5 January 2016).
- Cullen B, Gibson M, Nisbet L. Targeted use of collagen/ORC improves clinical outcomes. Wound Repair and Regeneration 2012;20(5):A93. [Google Scholar]
Hanft 2006 {published and unpublished data}
- Hanft J, Serena T, Snyder R. Evaluation of the clinical effectiveness of a collagen‐ORC antimicrobial matrix in venous leg ulcers. 16th Conference of the European Wound Management Association. 2006 May 18‐20; Prague, Czech Republic. 2006:151.
- Hanft JR, Serena T, Snyder R. A study to evaluate the clinical effectiveness of a Collagen‐ORC Antimicrobial Matrix in venous leg ulcers. Wound Repair and Regeneration 2006;14(2):A64. [Google Scholar]
Humbert 2013 {published data only (unpublished sought but not used)}
- Humbert P, Faivre B, Veran Y, Debure C, Truchetet F, Becherel PA, et al. Protease‐modulating polyacrylate‐based hydrogel stimulates wound bed preparation in venous leg ulcers: a randomized controlled trial. Journal of the European Academy of Dermatology and Venereology 2013;28(12):1742‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanzara 2008 {published data only (unpublished sought but not used)}
- Lanzara S, Tacconi G, Gianesini S, Menegatti E, Federici F, Liboni A, et al. A pilot randomized trial to determine the effects of a new active dressing on wound healing of venous leg ulcers. EWMA Journal 2008;8(2 Suppl):76. [Google Scholar]
Manizate 2012 {published data only (unpublished sought but not used)}
- Manizate F, Fuller A, Gendics C, Lantis JC. A prospective, single‐center, nonblinded, comparative, postmarket clinical evaluation of a bovine‐derived collagen with ionic silver dressing versus a carboxymethylcellulose and ionic silver dressing for the reduction of bioburden in variable‐etiology, bilateral lower‐extremity wounds. Advances in Skin and Wound Care 2012;25(5):220‐5. [DOI] [PubMed] [Google Scholar]
Meaume 2012 {published and unpublished data}
- Augustin M, Herberger K, Kroeger K, Muenter KC, Goepel L, Rychlik R. Cost‐effectiveness of treating vascular leg ulcers with UrgoStart® and UrgoCell® Contact. International Wound Journal 2016;13:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaume S, Truchetet F, Cambazard F, Lok C, Debure C, Dalac S, et al. A randomized, controlled, double‐blind prospective trial with a Lipido‐Colloid Technology‐Nano‐Oligo Saccharide Factor wound dressing in the local management of venous leg ulcers. 3rd Congress of the World Union of Wound Healing Societies meeting; 2008 June 4‐8;Toronto, Canada 2012;20(4):500‐11. [DOI] [PubMed] [Google Scholar]
Petkov 1997 {published and unpublished data}
- Petkov L. A comparative study of the influence of two topical wound dressings used during healing on subsequent venous ulcer recurrence. 6th European Conference on Advances in Wound Management: Wound Healing Therapy: a critique of current practice and opportunities for improvement; 1996 October 1‐4 October; Amsterdam, Netherlands. 1997:267.
Romanelli 2015 {published data only}
- Romanelli M, Mulder G, Paggi B, Macchia M, Panduri S, Dini V. The use of a collagen matrix in hard‐to‐heal venous leg ulcers. Journal of Wound Care 2015;24(11):543‐7. [DOI] [PubMed] [Google Scholar]
Schmutz 2008 {published data only}
- Dompmartin A, Schmutz JL, Collier M, Smith J, Bohbot S. Management of venous leg ulcers with two active wound dressings. Protocol of a randomized clinical trial. 17th Conference of the European Wound Management Association; 2007 May 2‐4; Glasgow, Scotland. 2007:55.
- Meaume S, Dompmartin A, Schmutz J‐L, Ourabah Z, Thirion V. Management of venous leg ulcers with two active wound dressings. Results of a randomized clinical trial. 18th Conference of the European Wound Management Association; 2008 May 14‐16; Lisbon, Portugal. 2008; Vol. 8, issue 2 Suppl:Abstract 38.
- Meaume S, Schmutz J‐L, Dompmartin A, Fays S, Ourabah Z, Thirion V, et al. Management of venous leg ulcers with two active wound dressings. Results of a randomized clinical trial. 3rd Congress of the World Union of Wound Healing Societies meeting; 2008 June 4‐8; Toronto, Canada. 2008:Abstract OR047.
- Schmutz J‐L, Meaume S, Fays S, Ourabah Z, Guillot B, Thirion V, et al. Evaluation of the nano‐oligosaccharide factor lipido‐colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. International Wound Journal 2008;5(2):172‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeets 2008 {published data only (unpublished sought but not used)}
- Smeets R, Ulrich D, Unglaub F, Wöltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. International Wound Journal 2008;5(2):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vin 2002 {published data only}
- Delchambre J. Venous leg ulcers trials. Journal of Wound Care 2002;12(3 Suppl):6‐7. [Google Scholar]
- Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. Journal of Wound Care 2002;11(9):335‐41. [DOI] [PubMed] [Google Scholar]
- Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. Phlebologie 2003;56(1):77‐86. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anichini 2013 {published data only}
- Anichini R, Tedeschi A, Bernini A, Barbanera L, Bellis A. Detecting and treating Elevated Protease Activity (EPA) in chronic diabetic wounds. EWMA Journal 2013;13(1 Suppl):275. [Google Scholar]
Bolton 2003 {published data only}
- Bolton LL. Evidence corner: randomized controlled trial of collagen/oxidized regenerated cellulose dressing. Wounds 2003;15(6 Suppl):19‐20. [Google Scholar]
Brown 2014 {published data only}
- Brown A, Augustin M, Junger M, Zutt M, Dissemond J, Rabe E, et al. Randomized standard‐of‐care‐controlled trial of a silica gel fibre matrix in the treatment of chronic venous leg ulcers. European Journal of Dermatology 2014;24(2):210‐16. [DOI: 10.1684/ejd.2014.2344] [DOI] [PubMed] [Google Scholar]
Brown‐Etris 2000a {published data only}
- Brown‐Etris M, Shields DK, Galluzzi KE, Steinberg JS, Shalen AB, Barnes HR, et al. Preliminary clinical outcomes on the use of composite cultured skin for the healing of venous ulcers. 13th Annual Symposium on Advanced Wound Care; 2000 April 1‐4; Dallas (TX). 2000:D4.
Caprio 1995a {published data only}
- Caprio B, Murgiano GA, Ricci E, Siciliano G, Zurleni G. The cost effectiveness of modern dressings in leg ulcer care. 4th European Conference on Advances in Wound Management; 1994 Sept 6‐9; Copenhagen, Denmark. 1995:199.
Chaloner 1992 {published data only}
- Chaloner D, Fletcher M, Milward P. Clinical trials: comparing dressings. Nursing Standard 1992;7(7):9‐11. [DOI] [PubMed] [Google Scholar]
Curran 2002 {published data only}
- Curran MP, Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs 2002;16(6):439‐55. [DOI: 10.2165/00063030-200216060-00005] [DOI] [PubMed] [Google Scholar]
Demling 2004 {published data only}
- Demling RH, Niezgoda JA, Haraway GD, Mostow EN. Small intestinal submucosa wound matrix and full‐thickness venous ulcers: preliminary results. Wounds 2004;16(1):18‐22. [Google Scholar]
Ebell 1998 {published data only}
- Ebell M. Is human skin equivalent more effective than Unna paste boots in the treatment of venous ulcers?. Evidence‐Based Practice 1998;1(6):1. [Google Scholar]
Falabella 1998 {published data only}
- Falabella AF, Carson P, Eaglstein WH, Falanga V. The safety and efficacy of a proteolytic ointment in the treatment of chronic ulcers of the lower extremity. Journal of the American Academy of Dermatology 1998;39(5):737‐40. [DOI] [PubMed] [Google Scholar]
Falanga 1998a {published data only}
- Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Archives of Dermatology 1998;134(3):293‐300. [DOI] [PubMed] [Google Scholar]
Falanga 1998b {published data only}
- Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF(TM)) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair and Regeneration 1999;7(4):201‐7. [DOI] [PubMed] [Google Scholar]
Falanga 2000 {published data only}
- Falanga VJ. Tissue engineering in wound repair. Advances in Skin and Wound Care 2000;13(2 Suppl):15‐19. [PubMed] [Google Scholar]
Falanga 2006 {published data only}
- Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatologic Therapy 2006;19(6):383‐90. [DOI: 10.1111/j.1529-8019.2006.00096.x] [DOI] [PubMed] [Google Scholar]
Gardner 2013 {published data only}
- Gardner S. Using treatment pathways to improve healing of venous leg ulceration. Wounds UK 2013;9(1):67‐75. [Google Scholar]
Gilligan 2014 {published data only}
- Gilligan AM, Waycaster C. Cost‐effectiveness of small intestinal submucosa extracellular matrix on wound closure in patients with difficult‐to‐heal wound of mixed arterial/venous and venous etiology. Value in Health 2014;17(3):A272‐3. [DOI: 10.1016/j.jval.2014.03.1587] [DOI] [Google Scholar]
Goedkoop 2010 {published data only}
- Goedkoop R, Juliet R, You PHK, Daroczy J, Roos K‐P, Lijnen R, et al. Wound stimulation by growth‐arrested human keratinocytes and fibroblasts: HP802‐247, a new‐generation allogeneic tissue engineering product. Dermatology 2010;220(2):114‐20. [DOI: 10.1159/000277380] [DOI] [PubMed] [Google Scholar]
Gravante 2013 {published data only}
- Gravante G, Sorge R, Giordan N, Georgescu SR, Morariu SH, Stoicescu I, et al. Multicenter clinical trial on the performance and tolerability of the Hyaluronic acid‐collagenase ointment for the treatment of chronic venous ulcers: a preliminary pilot study. European Review for Medical and Pharmacological Sciences 2013;17(20):2721‐7. [PubMed] [Google Scholar]
Hodde 2006 {published data only}
- Hodde JP, Hiles MC, Sillings N, Metzger DW. Characterization of the local wound environment following treatment of chronic leg ulcers with SIS wound matrix: small intestinal submucosa. 19th Annual Symposium on Advanced Wound Care; 2006 April 30‐May 3; San Antonio (TX). 2006; Vol. 4:105‐6.
Karim 2006 {published data only}
- Karim RB, Brito BL, Dutrieux RP, Lassance FP, Hage JJ. MMP‐2 assessment as an indicator of wound healing: a feasibility study. Advances in Skin and Wound Care 2006;19(6):324‐7. [DOI] [PubMed] [Google Scholar]
Lantis 2013 {published data only}
- Lantis JC, Marston WA, Farber A, Kirsner RS, Zhang Y, Lee TD, et al. The influence of patient and wound variables on healing of venous leg ulcers in a randomized controlled trial of growth‐arrested allogeneic keratinocytes and fibroblasts. Journal of Vascular Surgery 2013;58(2):433‐9. [DOI: 10.1016/j.jvs.2012.12.055] [DOI] [PubMed] [Google Scholar]
Marston 2012 {published data only}
- Marston W, Kirsner R, Snyder R, Lee T, Cargill I, Slade H. Variables affecting healing of venous leg ulcers in a randomized, vehicle‐controlled trial of topical cellular therapy. Journal of Vascular Surgery 2012;55(1):303. [DOI: 10.1016/j.jvs.2011.11.027] [DOI] [Google Scholar]
Metzner 1997 {published data only}
- Metzner B, Vanscheidt W, Norgauer J. Influence of platelet‐derived products on mRNA expression of growth factors, inducible growth related genes, metalloproteinases and their inhibitors in ulcers of patients with chronic venous insufficiency. Australasian Journal of Dermatology 1997;38(2 Suppl):175. [Google Scholar]
Mian 1992 {published data only}
- Mian E, Martini P, Beconcini D, Mian M. Healing of open skin surfaces with collagen foils. International Journal of Tissue Reactions 1992;14(Suppl):27‐34. [PubMed] [Google Scholar]
Moffatt 2014 {published data only}
- Moffatt CJ, Stanton J, Murray S, Doody V, Davis PJ, Franks PJ. A randomised trial to compare the performance of Oxyzyme and Iodozyme with standard care in the treatment of patients with venous and mixed venous/arterial ulceration. Wound Medicine 2014;6:1‐10. [DOI: 10.1016/j.wndm.2014.08.002] [DOI] [Google Scholar]
Morimoto 2012 {published data only}
- Morimoto N, Yoshimura K, Niimi M, Ito T, Tada H, Teramukai S, et al. An exploratory clinical trial for combination wound therapy with a novel medical matrix and fibroblast growth factor in patients with chronic skin ulcers: a study protocol. American Journal of Translational Research 2012;4(1):52‐9. [PMC free article] [PubMed] [Google Scholar]
Morimoto 2013 {published data only}
- Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue engineering Part A 2013;19(17‐18):1931‐40. [DOI: 10.1089/ten.tea.2012.0634] [DOI] [PubMed] [Google Scholar]
Mostow 2005 {published data only}
- Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. Journal of Vascular Surgery 2005;41(5):837‐43. [DOI: 10.1016/j.jvs.2005.01.042] [DOI] [PubMed] [Google Scholar]
Palmieri 1992 {published data only}
- Palmieri B. Heterologous collagen in wound healing: a clinical study. International Journal of Tissue Reactions 1992;14(Suppl):21‐5. [PubMed] [Google Scholar]
Planinsek 2007 {published data only}
- Planinsek RT, Lunder T. Stimulation of venous leg ulcers with thrombocytic growth factors: a randomised study. 17th Conference of the European Wound Management Association; 2007 May 2‐4; Glasgow, Scotland. 2007:62.
Ramirez 1994 {published data only}
- Ramirez GM, Wethers DL, Koshy M, Steinberg MH, Phillips G, Siegel RS, et al. Accelerated healing of chronic sickle cell leg ulcers treated with a RGD matrix. 19th Annual Meeting of the National Sickle Cell Disease Program; 1994 March; New York. 1994:Abstract 1.
Robson 1995 {published data only}
- Robson MC, Phillip LG, Cooper DM, Lyle WG, Robson LE, Odom L, et al. Safety and effect of transforming growth factor‐beta(2) for treatment of venous stasis ulcers. Wound Repair and Regeneration 1995;3(2):157‐67. [DOI] [PubMed] [Google Scholar]
Romanelli 2006a {published data only}
- Romanelli M, Ellervee T, Jarve H, Kaha E. Amelogenins (Xelma) in hard‐to‐heal venous leg ulcers, an open regime investigation. 16th Conference of the European Wound Management Association; 2006 May 18‐20; Prague, Czech Republic. 2006:143.
Romanelli 2006b {published data only}
- Romanelli M, Dini V, Brilli C, Bertone M. Oasis wound matrix versus hyaluronic acid in the treatment of difficult‐to‐heal wounds of mixed arterial/venous aetiology. 19th Annual Symposium on Advanced Wound Care & 16th Medical Research Forum on Wound Repair; 2006 April 30‐May 3; San Antonio, Texas, USA; Ostomy Wound Manage. 2006; Vol. 52.
Romanelli 2007 {published data only}
- Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult‐to‐heal wounds of mixed arterial/venous aetiology. International Wound Journal 2007;4(1):3‐7, 39‐40. [DOI: 10.1111/j.1742-481X.2007.00300.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanelli 2008a {published data only}
- Romanelli M, Kaha E, Stege H, Wnorowski JW, Vowden P, Majamaa H, et al. Effect of amelogenin extracellular matrix protein and compression on hard‐to‐heal venous leg ulcers: follow‐up data. Journal of Wound Care 2008;17(1):17‐8, 20‐3. [DOI] [PubMed] [Google Scholar]
Romanelli 2008b {published data only}
- Romanelli M, Vowden P, Price P. An open, randomised, comparative, parallel group, multi‐centre clinical trial of amelogenin extracellular matrix therapy in the treatment of hard‐to‐heal venous leg ulcers: follow‐up data. 3rd Congress of the World Union of Wound Healing Societies; 2008 June 4‐8; Toronto, Canada 2008:Abstract OR100. [Google Scholar]
Romanelli 2010 {published data only}
- Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult‐to‐heal wounds of mixed arterial/venous etiology. Advances in Skin and Wound Care 2010;23(1):34‐8. [DOI: 10.1097/01.ASW.0000363485.17224.26] [DOI] [PubMed] [Google Scholar]
Romanelli 2011 {published data only}
- Romanelli M, Dini V, Bertone M. Histological evaluation of venous leg ulcers treated with amelogenin. EWMA Journal 2011;11(2 Suppl):224. [Google Scholar]
Ronfard 2012 {published data only}
- Ronfard RV, Grover GK. Allogeneic living cell spray: a new concept in the treatment of wounds. Journal of Tissue Engineering and Regenerative Medicine 2012;6(Suppl.1):91. [DOI: 10.1002/term.1586] [DOI] [Google Scholar]
Serra 2013 {published data only}
- Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair and Regeneration 2013;21(3):395‐402. [DOI: 10.1111/wrr.12035] [DOI] [PubMed] [Google Scholar]
Serra 2014 {published data only}
- Serra R, Gallelli L, Conti A, Caridi G, Massara M, Spinelli F, et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Design, Development and Therapy 2014;8:519‐27. [DOI: 10.2147/DDDT.S61770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shanahan 2013 {published data only}
- Shanahan DR. The Explorer study: the first double‐blind RCT to assess the efficacy of TLC‐NOSF on DFUs. Journal of Wound Care 2013;22(2):78‐82. [DOI] [PubMed] [Google Scholar]
Sheehan 2003 {published data only}
- Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26(6):1879‐82. [DOI: 10.2337/diacare.26.6.1879] [DOI] [PubMed] [Google Scholar]
Smith 1994 {published data only}
- Smith BA. The dressing makes the difference. Trial of two modern dressings on venous ulcers. Professional Nurse 1994;9(5):348, 350‐2. [PubMed] [Google Scholar]
Stojadinovic 2014 {published data only}
- Stojadinovic O, Ramirez H, Patel S, Yin N, Bollenbach T, Golden P, et al. Genomic insight into molecular mechanisms of action of bilayered living cellular construct nonhealing venous leg ulcers. Wound Repair and Regeneration 2014;22(2):A60. [DOI: 10.1111/wrr.12155] [DOI] [Google Scholar]
Thomas 1997 {published data only}
- Thomas S, Banks V, Fear M, Hagelstein S, Bale S, Harding K. A study to compare two film dressings used as secondary dressings. Journal of Wound Care 1997;6(7):333‐6. [DOI] [PubMed] [Google Scholar]
Trial 2010 {published data only}
- Trial C, Darbas H, Lavigne JP, Sotto A, Simoneau G, Tillet Y, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
Varelias 2002 {published data only}
- Varelias A, Hughes H, Cowin A, Cowled P, Cooter R, Harries R, et al. Detection and modulation of matrix metalloproteinases in patients with chronic leg ulcers treated with whey‐derived growth factors. Fourth Australian Wound Management Association Conference; 2002 March 7‐10; Adelaide, South Australia. 2002:34.
Varelias 2006 {published data only}
- Varelias A, Cowin AJ, Adams D, Harries RHC, Cooter RD, Belford DA, et al. Mitogenic bovine whey extract modulates matrix metalloproteinase‐2, ‐9, and tissue inhibitor of matrix metalloproteinase‐2 levels in chronic leg ulcers. Wound Repair and Regeneration 2006;14(1):28‐37. [DOI: 10.1111/j.1743-6109.2005.00085.x] [DOI] [PubMed] [Google Scholar]
Veves 2001 {published data only}
- Veves A. Promogran, a cost‐effective new device for treating chronic ulcers. Diabetic Foot 2001;4:S4‐5. [Google Scholar]
Veves 2002 {published data only}
- Veves A, Sheehan P, Pham HT. A randomized controlled trial of Promogran (a collagen/oxidised regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Archives of surgery 2002;137(7):822‐7. [DOI] [PubMed] [Google Scholar]
Vowden 2006 {published data only}
- Vowden P, Romanelli M, Peter R, Boström A, Josefsson A, Stege H. The effect of amelogenins (XELMA) on hard‐to‐heal venous leg ulcers. Wound Repair and Regeneration 2006;14(3):240‐6. [DOI: 10.1111/j.1743-6109.2006.00117.x] [DOI] [PubMed] [Google Scholar]
Vowden 2007a {published data only}
- Vowden P, Romanelli M. Effect of amelogenin matrix protein solution as add‐on treatment to compression in hard‐to‐heal venous leg ulcers: a randomised controlled trial. 17th Conference of the European Wound Management Association; 2007 May 2‐4; Glasgow, Scotland. 2007:54.
Vowden 2007b {published data only}
- Vowden P, Romanelli M, Price P. Effect of amelogenin extracellular matrix protein and compression on hard‐to‐heal venous leg ulcers. Journal of Wound Care 2007;16(5):189‐95. [DOI] [PubMed] [Google Scholar]
Wethers 1994 {published data only}
- Wethers DL, Ramirez GM, Koshy M, Steinberg MH, Phillips G, Siegel RS, et al. Accelerated healing of chronic sickle‐cell leg ulcers treated with RGD peptide matrix. RGD Study Group. Blood 1994;84(6):1775‐9. [PubMed] [Google Scholar]
Wolcott 2015 {published data only}
- Wolcott R. Disrupting the biofilm matrix improves wound healing outcomes. Journal of Wound Care 2015;24(8):366‐71. [DOI] [PubMed] [Google Scholar]
Wollina 2005 {published data only}
- Wollina U, Schmidt W, Kronert C, Nelskamp C, Scheibe A, Fassler D. Some effects of a topical collagen‐based matrix on the microcirculation and wound healing in patients with chronic venous leg ulcers: preliminary observations. International Journal of Lower Extremity Wounds 2005;4(4):214‐24. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Braumann 2008 {published data only}
- Braumann C, Pirlich M, Menenakos C, Lochs H, Mueller JM. Implementation of the clean and close concept for treatment of surgical and chronic wounds in three university centres in Berlin, Germany. EWMA Journal 2008;8(2 Suppl):41. [Google Scholar]
References to ongoing studies
NCT 01537003 {published data only (unpublished sought but not used)}
- NCT01537003. WOUNDCHEK™ protease status point of care (POC) diagnostic test. clinicaltrials.gov/ct2/show/NCT01537003 (accessed 08 July 2016). [Google Scholar]
Additional references
Altman 2000
- Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. 2nd Edition. London: BMJ, 2000. [Google Scholar]
Ashby 2014
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, et al. Clinical and cost‐effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014;383(9920):871‐9. [DOI] [PubMed] [Google Scholar]
Augustin 2012
- Augustin M, Brocatti LK, Rustenbach SJ, Schäfer I, Herberger K. Cost‐of‐illness of leg ulcers in the community. International Wound Journal 2012;11(3):283‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Beidler 2008
- Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair and Regeneration 2008;16(5):642‐8. [DOI] [PubMed] [Google Scholar]
BNF 2016
- Joint Formulary Committee. British National Formulary (BNF). www.evidence.nhs.uk/formulary/bnf/current/a5‐wound‐management‐products‐and‐elasticated‐garments (accessed 14 December 2016).
Callam 1987
- Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. British Medical Journal 1987;294(6584):1389‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleridge 1988
- Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. British Medical Journal 1988;296(6638):1726‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullen 2002
- Cullen B, Smith R, McCulloch E, Silcock D, Morrisson L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair and Regeneration 2002;10(1):16‐25. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dumville 2009
- Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technology Assessment 2009;13(55):1‐182. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Euroqol 2015
- Reenen M, Janssen B. EQ‐5D‐5L User Guide. www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ‐5D‐5L_UserGuide_2015.pdf (accessed 30 March 2015).
Fletcher 2013
- Fletcher J, Moffatt C, Partsch H, Vowden K, Vowden P. Principles of compression in venous disease: a practitioner’s guide to treatment and prevention of venous leg ulcers. Wounds International 2013; available from www.woundsinternational.com (accessed 15 July 2016).
Galea 2015
- Galea E. Managing chronic/stalled arterial, venous and pressure ulcers with collagen and oxidised regenerated cellulose dressings. 2015. www.worldwidewounds.com/2015/August/Galea/wounds‐galea.html (accessed 05 May 2016). [Google Scholar]
Ghauri 2010
- Ghauri AS, Nyamekye IK. Leg ulceration: the importance of treating the underlying pathophysiology. Phlebology/Venous Forum of the Royal Society of Medicine 2010;25(Suppl 1):42‐51. [DOI] [PubMed] [Google Scholar]
Graham 2003
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower‐limb ulceration: a systematic review of prevalence studies. Advances in Skin and Wound Care 2003;16(6):305‐16. [DOI] [PubMed] [Google Scholar]
Graves 2014
- Graves N, Finlayson K, Gibb M, O’Reilly M, Edwards H. Modelling the economic benefits of gold standard care for chronic wounds in a community setting. Wound Practice and Research 2014;22(3):163‐8. [Google Scholar]
Hall 2014
- Hall J, Buckley HL, Lamb KA, Stubbs N, Saramago P, Dumville JC, et al. Point prevalence of complex wounds in a defined United Kingdom population. Wound Repair and Regeneration 2014;22(6):694–700. [DOI] [PubMed] [Google Scholar]
Harding 2011
- Harding K, Armstrong DG, Barrett S, Kaufman H, Lázaro‐Martínez J L, Mayer D, et al. International consensus. The role of proteases in wound diagnostics. An expert working group review. 2011. woundchek.com/uploads/downloads/consensus_documents/Role‐of‐proteases‐in‐wound‐diagnostics‐International.pdf. London, (accessed 15 July 2016).
Hart 2002
- Hart J, Silcock D, Gunnigle S, Cullen B, Light ND, Watt PW. The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. The International Journal of Biochemistry & Cell Biology 2002;34(12):1557–70. [DOI] [PubMed] [Google Scholar]
Herber 2007
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;5:44‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iglesias 2004
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS 1: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technology Assessment 2004;8(29). [DOI] [PubMed] [Google Scholar]
Johnson 1995
- Johnson M. The prevalence of leg ulcers in older people: implications for community nursing. Public Health Nursing 1995;12(4):269‐75. [DOI] [PubMed] [Google Scholar]
Jull 2012
- Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD001733.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontopantelis 2012
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PLoS One 2013;26:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladwig 2002
- Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair and Regeneration 2002;10(1):26‐37. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009
- Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, et al. Increased matrix metalloproteinase‐9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32(1):117‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobmann 2006
- Lobmann R, Zemlin C, Motzkau M, Rescke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing.. Journal of Diabetes and its Complications 2006;20(5):329‐35. [DOI] [PubMed] [Google Scholar]
Madden 2014
- Madden M. Is research in chronic wound care focusing on the outcomes that matter most to patients?. BSA Annual Conference 2014: Changing Society; 23‐25 April 2014, University of Leeds, UK. 2014:232.
Margolis 2004
- Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair and Regeneration 2004;12(2):163‐8. [DOI] [PubMed] [Google Scholar]
McCarty 2012
- McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair and Regeneration 2012;20(2):125–36. [DOI] [PubMed] [Google Scholar]
MeReC 2010
- MeReC publications. Evidence‐based prescribing of advanced wound dressings for chronic wounds in primary care. MeReC Bulletin 2010;21(1). [Google Scholar]
Moffatt 2004
- Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM: Monthly journal of the Association of Physicians 2004;97(7):431‐7. [DOI] [PubMed] [Google Scholar]
Mwaura 2006
- Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase‐2, tissue inhibitor of matrix metalloproteinase‐2 and PDGF‐AA on the chronicity of venous leg ulcers. European Journal of Vascular and Endovascular Surgery 2006;31(3):306‐10. [DOI] [PubMed] [Google Scholar]
Nelson 2016
- Nelson EA, Adderley U. Venous leg ulcers. Systematic review 1902. January 2016. clinicalevidence.bmj.com/x/systematic‐review/1902/overview.html (accessed 8 July 2016).
Nelzén 1994
- Nelzén O, Berqvist D, Lindhagen A. Venous and non‐venous leg ulcers: clinical history and appearance in a population study. British Journal of Surgery 1994;81(2):182‐7. [DOI] [PubMed] [Google Scholar]
Nelzén 2008
- Nelzén O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology 2008;15(4):143‐50. [Google Scholar]
Norman 2016
- Norman G, Westby MJ, Stubbs N, Dumville JC, Cullum N. A 'test and treat' strategy for elevated wound protease activity for healing in venous leg ulcers. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD011753] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nwomeh 1998
- Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP‐1 and MMP‐8 in acute open human dermal wounds. Wound Repair and Regeneration 1998;6(2):127‐34. [DOI] [PubMed] [Google Scholar]
Nwomeh 1999
- Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. Journal of Surgical Research 1999;81(2):189‐95. [DOI] [PubMed] [Google Scholar]
O'Meara 2012
- O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000265.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2013
- O'Meara S, Martyn‐St James M. Foam dressings for venous leg ulcers. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2014
- O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn St‐James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003557.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palolahti 1993
- Palolahti M, Lauharanta J, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Experimental Dermatology 1993;2(1):29‐37. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(30):2815‐34. [DOI] [PubMed] [Google Scholar]
Persoon 2004
- Persoon A, Heinen MM, Vleuten CJM, Rooji MJ, et al. Leg ulcers: a review of their impact on daily life. Journal of Clinical Nursing 2004;13(3):341‐54. [DOI] [PubMed] [Google Scholar]
Ragnarson Tennvall 2005
- Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair and Regeneration 2005;13(1):13‐18. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic venous leg ulcers. National clinical guideline 120. August 2010. www.sign.ac.uk/pdf/sign120.pdf (accessed 05 September 2016).
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html (accessed 8 July 2016).
Srinivasaiah 2007
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north‐east England. Journal of Wound Care 2007;16(10):413‐9. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Systagenix Portugal 2016
- Systagenix Portugal. How does it work? [Como funciona?]. www.systagenix.com.br/our‐products/lets‐promote/fibracol‐plus‐307/how‐does‐it‐work (accessed 19 April 2016).
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(16). [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trengove 1999
- Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair and Regeneration 1999;7(6):442‐52. [DOI] [PubMed] [Google Scholar]
Valencia 2001
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. Journal of the American Academy of Dermatology 2001;44(3):401‐21. [DOI] [PubMed] [Google Scholar]
Velnar 2009
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. Journal of International Medical Research 2009;37(5):1528‐42. [DOI] [PubMed] [Google Scholar]
Vlajinac 2014
- Vlajinac H, Marinkovic J, Maksimovic M, Radak D. Factors related to venous ulceration: a cross‐sectional study. Angiology 2014;65(9):824‐30. [DOI] [PubMed] [Google Scholar]
Vowden 2009
- Vowden KR, Vowden P. The prevalence, management and outcome for acute wounds identified in a wound care survey within one English health care district. Journal of Tissue Viability 2009;18(1):7‐12. [DOI] [PubMed] [Google Scholar]
Walker 2002
- Walker N, Rodgers A, Birchall N, Norton R, MacMahon S. The occurrence of leg ulcers in Auckland: results of a population based study. New Zealand Medical Journal 2002;115(1151):159‐62. [PubMed] [Google Scholar]
Wang 2013
- Wang Y, Zeng T. Response to: Practical methods for incorporating summary time‐to‐event data into meta‐analysis [addendum to Tierney 2007]. Trials 2013;14(391). [DOI: 10.1186/1745-6215-14-391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wlaschek 1997
- Wlaschek M, Peus D, Achterberg V, Meyer‐Ingold W, Scharffetter‐Kochanek K. Protease inhibitors protect growth factor activity in chronic wounds. British Journal of Dermatology 1997;137(4):646‐66. [DOI] [PubMed] [Google Scholar]
Wound Care Handbook 2016
- MA Healthcare. Wound Care Handbook. www.woundcarehandbook.com/ (accessed 10 October 2016).
Yager 1997
- Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair and Regeneration 1997;5(1):23‐32. [DOI] [PubMed] [Google Scholar]
Young 2012
- Young T. Using a protease test to inform wound care treatment decisions. Wounds UK 2012;8(4):74‐80. [Google Scholar]


