Abstract
Background
Infectious mononucleosis (IM) is a clinical syndrome, usually caused by the Epstein Barr virus (EPV), characterised by lymphadenopathy, fever and sore throat. Most cases of symptomatic IM occur in older teenagers or young adults. Usually IM is a benign self‐limiting illness and requires only symptomatic treatment. However, occasionally the disease course can be complicated or prolonged and lead to decreased productivity in terms of school or work. Antiviral medications have been used to treat IM, but the use of antivirals for IM is controversial. They may be effective by preventing viral replication which helps to keep the virus inactive. However, there are no guidelines for antivirals in IM.
Objectives
To assess the effects of antiviral therapy for infectious mononucleosis (IM).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, March 2016), which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (1946 to 15 April 2016), Embase (1974 to 15 April 2016), CINAHL (1981 to 15 April 2016), LILACS (1982 to 15 April 2016) and Web of Science (1955 to 15 April 2016). We searched the World Health Organization (WHO) International Clinical Trials Registry Platform and ClinicalTrials.gov for completed and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) comparing antivirals versus placebo or no treatment in IM. We included trials of immunocompetent participants of any age or sex with clinical and laboratory‐confirmed diagnosis of IM, who had symptoms for up to 14 days. Our primary outcomes were time to clinical recovery and adverse events and side effects of medication. Secondary outcomes included duration of abnormal clinical examination, complications, viral shedding, health‐related quality of life, days missing from school or work and economic outcomes.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed the included studies' risk of bias and extracted data using a customised data extraction sheet. We used the GRADE criteria to rate the quality of the evidence. We pooled heterogeneous data where possible, and presented the results narratively where we could not statistically combine data.
Main results
We included seven RCTs with a total of 333 participants in our review. Three trials studied hospitalised patients, two trials were conducted in an outpatient setting, while the trial setting was unclear in two studies. Participants' ages ranged from two years to young adults. The type of antiviral, administration route, and treatment duration varied between the trials. The antivirals in the included studies were acyclovir, valomaciclovir and valacyclovir. Follow‐up varied from 20 days to six months. The diagnosis of IM was based on clinical symptoms and laboratory parameters.
The risk of bias for all included studies was either unclear or high risk of bias. The quality of evidence was graded as very low for all outcomes and so the results should be interpreted with caution. There were statistically significant improvements in the treatment group for two of the 12 outcomes. These improvements may be of limited clinical significance.
There was a mean reduction in 'time to clinical recovery as assessed by physician' of five days in the treatment group but with wide confidence intervals (CIs) (95% CI ‐8.04 to ‐1.08; two studies, 87 participants). Prospective studies indicate that clinical signs and symptoms may take one month or more to resolve and that fatigue may be persistent in approximately 10% of patients at six‐month follow‐up, so this may not be a clinically meaningful result.
Trial results for the outcome 'adverse events and side effects of medication' were reported narratively in only five studies. In some reports authors were unsure whether an adverse event was related to medication or complication of disease. These results could not be pooled due to the potential for double counting results but overall, the majority of trials reporting this outcome did not find any significant difference between treatment and control groups.
There was a mean reduction in 'duration of lymphadenopathy' of nine days (95% CI ‐11.75 to ‐6.14, two studies, 61 participants) in favour of the treatment group.
In terms of viral shedding, the overall effect from six studies was that viral shedding was suppressed while on antiviral treatment, but this effect was not sustained when treatment stopped.
For all other outcomes there was no statistically significant difference between antiviral treatment and control groups.
Authors' conclusions
The effectiveness of antiviral agents (acyclovir, valomaciclovir and valacyclovir) in acute IM is uncertain. The quality of the evidence is very low. The majority of included studies were at unclear or high risk of bias and so questions remain about the effectiveness of this intervention. Although two of the 12 outcomes have results that favour treatment over control, the quality of the evidence of these results is very low and may not be clinically meaningful. Alongside the lack of evidence of effectiveness, decision makers need to consider the potential adverse events and possible associated costs, and antiviral resistance. Further research in this area is warranted.
Keywords: Adolescent; Adult; Child; Child, Preschool; Female; Humans; Male; Young Adult; Acute Disease; Acyclovir; Acyclovir/adverse effects; Acyclovir/analogs & derivatives; Acyclovir/therapeutic use; Antiviral Agents; Antiviral Agents/adverse effects; Antiviral Agents/therapeutic use; Guanine; Guanine/adverse effects; Guanine/analogs & derivatives; Guanine/therapeutic use; Infectious Mononucleosis; Infectious Mononucleosis/drug therapy; Randomized Controlled Trials as Topic; Valacyclovir; Valine; Valine/adverse effects; Valine/analogs & derivatives; Valine/therapeutic use
Plain language summary
Antiviral medication for the treatment of infectious mononucleosis (glandular fever)
Review question
We investigated the benefits and side effects of antiviral treatment for people with glandular fever compared with fake treatment or standard care.
Background
Glandular fever is usually caused by the Epstein Barr virus. Although not generally serious, it can lead to significant time off school or work due to intense tiredness. Rarely, it can lead to potentially life‐threatening complications. Treating people with complications is costly both in terms of healthcare costs and lost productivity. Reducing complications would benefit patient care, so it is important to identify effective treatments for people with glandular fever.
Antiviral medications are expensive, may cause side effects and can lead to antiviral resistance. Good justification is needed to ensure best outcomes when antivirals are used. There is no agreement about whether antivirals are effective for treating people with glandular fever.
Search date
April 2016.
Study characteristics
We included seven studies that involved a total of 333 people; two were conducted in Europe and five in the USA. Three studies took place in hospitals, one each in a student health centre and a children's clinic, but the setting was unclear in two studies. Three different antiviral drugs were studied: acyclovir, valomaciclovir and valacyclovir, as well as dosage, comparison treatment (fake or no treatment), and how long people were treated and followed up.
Study funding
One study did not report study funding, but the other six studies appeared to have some industry support. None declared conflicts of interest, but one included two authors from a drug company.
Key results
We wanted to investigate several outcomes: time to recovery; medication side effects; duration of: fever, sore throat, swollen lymph nodes, enlarged spleen and liver; development of glandular fever complications; how long it took to eliminate the virus from the throat; health‐related quality of life; days off school or work; and economic outcomes.
We found improvements in participants who received antiviral for two outcomes.
There was an improvement of five days in time taken to recover among people who received antiviral treatment, but this result was not very precise, and the way it was measured was not clearly defined. Other studies show that glandular fever symptoms can take a month or more to get better, and tiredness may occur in about one in every 10 of patients six months later. This improvement may be of limited clinical significance.
Most studies that examined adverse effects did not find any differences between people who received antivirals and those who did not.
Time taken to resolve lymph node swelling improved to nine days when antivirals were used. However, studies that reported on this, measured lymph node swelling in different ways so we cannot be sure about the accuracy of the result.
Quality of the evidence
Evidence quality was rated as very low for all results, which means that we cannot know the exact effect of using antivirals for glandular fever. Better studies are needed so we can draw firm conclusions.
Summary of findings
Summary of findings for the main comparison. Antivirals compared with placebo/no treatment for infectious mononucleosis (glandular fever).
| Antivirals compared with placebo/no treatment for infectious mononucleosis (glandular fever) | ||||||
| Patient or population: patients diagnosed with clinical and laboratory‐confirmed diagnosis of infectious mononucleosis (glandular fever) Setting: hospitalised patients or outpatient setting Intervention: antivirals Comparison: placebo / no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with antivirals | |||||
| Time to clinical recovery doctor judgement | The mean time to clinical recovery doctor judgement was 20 days | The mean time to clinical recovery doctor judgement in the intervention group was 5 days fewer (8.04 fewer to 1.08 fewer) | ‐ | 87 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Statistically significant reduction in favour of treatment group. Andersson 1987 had 3 patients in the treatment group who had a co‐administered steroid whereas none of the placebo group had this |
| Time to clinical recovery patient judgement | The mean time to clinical recovery patient judgement was 42 days | The mean time to clinical recovery patient judgement in the intervention group was 6 days fewer (26.23 fewer to 15.05 more) | ‐ | 87 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | No statistically significant difference between groups. Andersson 1987 had 3 patients in the treatment group who had a co‐administered steroid whereas none of the placebo group had this |
| Adverse events and side effects | See comments | ‐ | 248 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | Reported narratively only in five studies. In some reports authors were unsure whether adverse event was related to medication or complication of disease | |
| Duration of lymphadenopathy | The mean duration of lymphadenopathy was 41 days | The mean duration of lymphadenopathy in the intervention group was 9 days fewer (11.75 fewer to 6.14 fewer) | ‐ | 61 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | Statistically significant difference in favour of treatment group. One study weighted very heavily due to high variance in other study |
| Development of complications of Infectious mononucleosis | see comments | ‐ | 108 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Three studies reported complications narratively. There did not seem to be any difference in the incidence of complications between treatment and control groups | |
| Viral shedding | see comments | ‐ | 268 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | Overall effect from all six studies was that viral shedding was suppressed while on antiviral treatment but this was not sustained when treatment stopped | |
| Days missing from school / work | The mean days missing from school / work was 20 days | Mean days missing from school/work in the intervention group was 1 day fewer (6.53 fewer to 4.74 more) | ‐ | 87 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | No statistically significant difference between groups. Andersson 1987 had 3 patients in the treatment group who had a co‐administered steroid whereas none of the placebo group had this |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels for imprecision where sample size was very small (< 200 participants)
2 Downgraded one level for risk of bias due to the majority of studies included in this outcome having an unclear or high risk of bias
3 Downgraded one level for indirectness as no study reported adverse events as a measurable outcome
4 Downgraded one level for imprecision due to small sample sizes or wide confidence intervals for this outcome
5 Downgraded one level for inconsistency due to wide variance of point estimates across studies and differences in setting, type of antiviral, or route of medication administration
Background
For definitions of terminology see the Glossary of terms (Appendix 1).
Description of the condition
Infectious mononucleosis (IM) is a clinical syndrome, characterised by lymphadenopathy, fever and sore throat (Hurt 2007). Ninety per cent of cases are caused by the Epstein‐Barr virus (EBV), about 5% to 7% are caused by cytomegalovirus (CMV), and less than 1% are caused by Toxoplasma gondii (T gondii) (Evans 1978). EBV is a widespread virus, transmitted primarily through infected saliva.
EBV infection may be subclinical during childhood years, without causing the overt syndrome of IM. However, the incidence of symptomatic infection rises in adolescents and adults, and studies have shown that EBV eventually infects over 95% of adults (Luzuriaga 2010). The overall incidence of IM in the USA is about 500 cases per 100,000 people per year (Luzuriaga 2010). In high‐income countries, the incidence of IM peaks in the late teens and falls after the age of 35 (Auwaerter 1999). In contrast, in low‐income countries most children are infected with EBV before they reach adolescence and symptomatic IM is uncommon (Straus 1993).
Following infection with EBV, the incubation period is four to eight weeks (Ebell 2004). Symptoms of IM usually peak one week from onset, and generally start to resolve over the next one to four weeks (Macsween 2010; Rea 2001). Occasionally, fatigue after acute IM can be severe and persistent. Persistent fatigue was present in 12% of cases at six months after illness onset in one prospective study (Buchwald 2000), and another cohort study found that 9% to 22% of patients with IM were classified as having Chronic Fatigue Syndrome six months after the acute illness (White 2001). The virus can continue to shed in saliva for a median duration of six months (Balfour 2013).
IM is regarded as a benign disease in the majority of cases and is associated with typical features of fever, pharyngitis, lymphadenopathy, fatigue and atypical lymphocytosis (Luzuriaga 2010).
IM is usually diagnosed clinically, based on characteristic signs and symptoms. Typical features of IM include lymphadenopathy, fever, sore throat and fatigue. However, there are no definitive diagnostic criteria. Blood testing during acute IM also usually reveals (atypical) lymphocytosis and abnormal liver function tests (LFTs) (Charles 2003). Laboratory tests are usually not required for diagnosis. However, specific antibody tests may be required to confirm diagnosis or to identify the cause of illness in atypical cases. A definitive diagnosis can be made by testing for IgG and IgM antibodies against viral capsid antigens, early antigens and EBV nuclear antigen proteins (Luzuriaga 2010). Recent studies have proposed a number of biomarkers for monitoring disease severity in IM caused by EBV (Kawano 2013; van de Veerdonk 2012).
IM can be associated with a variety of complications affecting multiple organ systems. As previously mentioned, fatigue after IM can be prolonged. Haematological complications are observed in 25% to 50% of cases and are generally mild (Luzuriaga 2010). Rare complications, such as airway occlusion secondary to oedema of the soft palate and tonsils and peritonsillar abscess, can occur (Monem 1999). Upper airway obstruction is seen in approximately 1% of cases (Luzuriaga 2010). Splenomegaly is seen in approximately 50% of patients with IM, but usually begins to resolve by the third week of the illness (Carter 1969). Splenic rupture is rare ‐ occurring in 0.1% to 0.2% of cases (Aldrete 1992) ‐ but potentially fatal (Brichkov 2006). EBV has also been associated with other complications including pneumonia, pleural effusions (Chen 2003), hepatitis (Devereaux 1999) and cholestasis, myocarditis and cardiac conduction abnormalities, acute renal failure (Lei 2000), and in 1% to 5% of cases, neurological complications (Luzuriaga 2010).
IM is generally self‐limiting, and there is no specific treatment. The mainstay of treatment for IM is supportive care. Patients should be encouraged to maintain adequate fluid and nutrition intake. Over‐the‐counter medications are recommended to relieve symptoms of sore throat, fever and malaise (Luzuriaga 2010). Corticosteroids may be used in the treatment of complications. However, a Cochrane review evaluating the effectiveness of corticosteroids for the control of symptoms concluded that there was insufficient evidence of clinical benefit (Rezk 2015). Metronidazole, an anaerobic antibacterial agent, has recently been studied in severe cases of patients with IM who were hospitalised and found to reduce length of hospital stay Lennon 2014. Antiviral medications have been used to treat IM, but the use of antivirals for IM is controversial.
Description of the intervention
Antivirals for IM have been studied previously in a 1999 meta‐analysis of five randomised controlled trials (RCTs) of acyclovir for the treatment of IM (Torre 1999). This systematic review showed less oropharyngeal EBV shedding at the end of therapy but failed to show a clinical benefit in terms of pharyngitis, weight loss and absence from school or work compared to placebo. A randomised pilot study comparing valacyclovir with no treatment in young adults with IM showed a transient reduction of oropharyngeal EBV shedding during therapy and a decrease in the number and severity of reported symptoms in the valacyclovir group, but with no difference between the two groups in the peripheral blood EBV load (Balfour 2007).
How the intervention might work
There are several antiviral agents, but the two that have been studied most with respect to IM are acyclovir and valacyclovir. Acyclovir is a nucleoside analogue that selectively inhibits the replication of certain viruses. It is active against herpes simplex virus types 1 and 2 (HSV‐1, HSV‐2), varicella zoster virus (VZV) and EBV. Valacyclovir acts as an oral prodrug and is converted in vivo to acyclovir. Other antiviral agents that have been shown to have in vitro activity against herpes viruses are penciclovir, famciclovir, ganciclovir, valganciclovir, cidofovir and foscarnet (Kimberlin 2007). All these agents act by preventing viral replication by inhibiting viral DNA synthesis. This helps to keep the virus inactive.
Antiviral medications are generally well tolerated. However, the most commonly reported side effects of acyclovir (observed in between 1/10 and 1/100 of cases) are nausea, vomiting, diarrhoea and abdominal pain, headache, dizziness, fatigue and fever, as well as skin rashes (including photosensitivity and itching) (HPRA 2016).
Why it is important to do this review
IM is a commonly encountered illness in primary care settings. A general practice with 10,000 patients can expect to see approximately seven new cases of IM a year (Candy 2002). Although generally not considered a serious illness, IM can lead to significant loss of time from school or work due to profound fatigue (Macsween 2010), or the development of chronic illness (Candy 2002). Also, in rare cases, it can lead to potentially life‐threatening complications such as splenic rupture, encephalitis and severe upper airway obstruction (Jenson 2000). If the incidence of complications could be reduced, by implementing evidence‐based treatment, it would impact positively on patient care. These complications also have economic implications ‐ both in terms of healthcare costs and loss of productivity. As such, there is great interest in developing regimens for treating IM with antiviral agents.
Antiviral medications are known to be expensive. Another consideration is the emergence of resistance to antiviral agents. There needs to be an evidence base for using these medications so that the available resources are used efficiently and effectively. To our knowledge, there are no professional society guidelines for the management of IM. This indicates a lack of clarity regarding the current evidence in relation to antiviral treatment for IM. It is hoped that the findings of this review will inform the preparation of a clinical guideline or policy document.
The previous meta‐analysis examining acyclovir for the treatment of IM showed some benefit in reducing oropharyngeal EBV shedding but no evidence to support its clinical effectiveness (Torre 1999). These data are now 15 years old and only included one antiviral agent, acyclovir. We feel it is necessary to search, appraise and summarise RCTs of antiviral agents for IM.
Objectives
To assess the effects of antiviral therapy for infectious mononucleosis (IM).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that examine the benefits and side effects of antiviral medication in infectious mononucleosis (IM).
Types of participants
Immunocompetent participants of any age or sex with both clinical and laboratory‐confirmed diagnosis of IM, who had symptoms for up to 14 days. Laboratory diagnosis is by monospot test or atypical lymphocytosis or EBV‐specific serology.
Types of interventions
Antiviral medication (acyclovir, valacyclovir, penciclovir, famciclovir, ganciclovir, valganciclovir, cidofovir and foscarnet) used for any duration or at any dose or by any route of administration. We included RCTs comparing antivirals with placebo or no treatment.
Types of outcome measures
Primary outcomes
Time to clinical recovery.
Adverse events and side effects of medication: as reported in the original studies by patients and clinicians.
Secondary outcomes
-
Duration of abnormal clinical examination (as assessed clinically by physician). We included:
fever (> 37.5° C);
pharyngitis;
lymphadenopathy;
splenomegaly;
hepatomegaly.
Development of complications of IM.
Viral shedding (as reported in the original studies).
-
Patient‐reported outcome measures:
health‐related quality of life (as reported in the original studies);
days missing from school or work.
Economic outcomes: based on collecting cost data from studies, where available.
Search methods for identification of studies
See Figure 1 for study flow diagram.
1.
Study flow diagram.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, March 2016), which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (1946 to 15 April 2016), Embase (1974 to 15 April 2016), CINAHL (1981 to 15 April 2016), LILACS (1982 to 15 April 2016) and Web of Science (1955 to 15 April 2016). We used the search strategy in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). We adapted the search strategy to search the other databases. We imposed no language, publication date or publication status restrictions on the electronic database searches.
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp) and ClinicalTrials.gov for completed and ongoing trials. We checked the reference lists of included trials to ensure that the main search has not missed any trials. We contacted the authors of included trials that were published in the last 10 years for the purpose of identifying missing trials. We contacted the European Medicines Agency (EMA) to ask for clinical study reports for any relevant trials.
Data collection and analysis
Selection of studies
We retrieved all titles and abstracts to assess eligibility against the inclusion criteria, as well as to identify multiple reports from single studies. We obtained full text copies of all papers considered to be potentially eligible and two review authors (MDP, SS) independently assessed these for suitability for inclusion. We resolved any disagreement by discussion between pairs of review authors, and where necessary, a third review author. We contacted the authors of a number of the primary studies for clarification. We excluded any papers that did not meet the inclusion criteria.
Data extraction and management
Two review authors (MDP, KOB) independently completed data extraction using a standard data extraction form. The pair of review authors and, where necessary, a third review author, resolved any disagreement by discussion.
Assessment of risk of bias in included studies
A combination of two authors (MDP, KOB) assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias according to the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias (other sources of bias related to particular trial design (cross‐over and cluster‐randomised) or specific circumstances).
We classified the risk of bias as: low risk, high risk or unclear risk of bias (Higgins 2011). We completed an overall 'Risk of bias' assessment graph displaying the review authors' judgements about each risk of bias item presented as percentages across all included studies.
Where necessary, we contacted study authors for clarification. We resolved any disagreement by discussion between the two review authors and, where necessary, a third review author.
Measures of treatment effect
We measured treatment effect by using either dichotomous data or an ordinal rating scale. For continuous data, we calculated the mean difference (MD) (using the method described in Hozo 2005). We planned to use the standardised mean difference (SMD) if different measures had been used. Where results were reported as mean and standard error, standard deviations were calculated as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) by multiplying the standard error of a mean by the square root of the sample size.
Unit of analysis issues
We considered the individual the unit of analysis. If we had identified any non‐standard design RCTs, we planned to follow the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted lead study investigators or corresponding authors for missing trial data, along with data missing from published reports and for additional clarification. Three authors responded with additional information but only one was in a position to provide selected original data.
For data reported as median and range, we used the method described in Hozo 2005 to convert it to mean and standard deviation. Where there were data missing from a study, we have explicitly stated this and reported it in the 'Risk of Bias' table. We have commented on the potential impact of missing data on the review findings in the Discussion.
Assessment of heterogeneity
We assessed included studies for clinical heterogeneity. We pooled minimal data for analysis across trials as we found diversity in the intervention (which drug was administered, route of administration, co‐administered medication, use of placebo), outcomes (which outcomes were reported at which time points and whether they were continuous or dichotomous outcomes), and length of follow‐up.
Assessment of reporting biases
We tried to minimise reporting bias by conducting a comprehensive search for studies that met the eligibility criteria, including grey literature and unpublished trials, and by contacting trial authors for missing information. We planned to assess the potential for publication bias in funnel plot analysis if we had sufficient and appropriate trial data to combine.
Data synthesis
We had intended to perform a meta‐analysis to calculate a weighted intervention effect for our primary outcome across trials. However, we found that the trial outcomes were too heterogeneous. We pooled the results of some of the studies where appropriate. We performed the statistical analyses using Review Manager software (RevMan 2014).
We prepared a 'Summary of findings' table to present the results for five of the outcomes, including adverse effects, as outlined in the Types of outcome measures section (with results synthesised mainly narratively). We used the five GRADE (Atkins 2004) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. The GRADE approach specifies four levels of quality. The highest quality rating is for randomised trial evidence. However, randomised trial evidence may be downgraded to moderate, low or even very low quality evidence, depending on the presence of the above five considerations (Higgins 2011). We used the GRADEproGDT software to prepare the table (GRADEpro GDT 2014). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We had planned to pursue subgroup analyses based on patient age, setting and placebo versus no treatment controls with sufficient data but unfortunately this was not possible.
Sensitivity analysis
We had planned to carry out a sensitivity analysis to explore the impact of risk of bias on study findings but all included studies were at a moderate or high risk of bias, so this was not possible.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We obtained a total of 1691 abstracts from electronic searches. We found an additional 22 studies from searching other sources. From the screening of titles and abstracts, we found 22 studies to be potentially relevant. On full‐text retrieval, we excluded 13 studies leaving nine to be analysed. Three (Andersson 1985; Andersson 1986; Ernberg 1986) appear to be different reports of the same study. We tried to contact the trial authors for confirmation of this but did not receive any response. We used Andersson 1986 as the main paper for this trial, thus leaving us with seven included studies.
Included studies
We included seven trials (Andersson 1986; Andersson 1987; Balfour 2007a; Balfour 2007b; Pagano 1983; Simon 2003; van der Horst 1991) and summarised them in the Characteristics of included studies table.
Intervention
Six of the seven studies explored the effects of antivirals versus placebo and one trial compared antiviral treatment with no drug (Balfour 2007b). The antivirals examined were acyclovir, valomaciclovir and valacyclovir. One of the trials had a third study arm which compared antiviral and steroid versus placebo (Simon 2003).
The dose of antiviral, route of administration, and duration of treatment varied between the trials.
In Andersson 1987, three patients in the treatment group required intravenous (IV) antiviral and steroid for 10 days, whereas none of the patients in the placebo group had this treatment. In Andersson 1986, 11 of 15 in the treatment group and nine of 16 in the placebo group had antibiotics pre admission. In Balfour 2007b, some participants had oral steroids co‐administered, but it is not reported how many from each group had this. In Pagano 1983, two patients in the placebo group and one in the treatment group had steroids co‐administered.
Setting
Two of the studies were conducted in Europe (Andersson 1986; Andersson 1987), while the other five took place in the USA. The European trials along with Pagano 1983 took place in an inpatient setting. Balfour 2007b and Simon 2003 took place in outpatient settings, with Balfour 2007b set in a student health centre and Simon 2003 in a paediatric clinic. The trial setting was unclear in two of the studies (Balfour 2007a; van der Horst 1991). All trials were undertaken by researchers either located in hospitals or at academic institutions.
Participants
Participants ranged in age from young children (aged from two) to young adults (although no maximum age was specified in the exclusion criteria of most of the trials). Four trials mentioned a maximum age: 25 years in Andersson 1986; 30 years in Andersson 1987; 24 years in Balfour 2007a; and 18 years in Simon 2003. Balfour 2007b reported median ages of 19.3 years in the treatment group and 21.4 in the control group. Pagano 1983 reported a mean age of 19.5 years in the treatment group and a mean age of 21 years in the placebo group. van der Horst 1991 did not report the age of participants. In the six trials that reported gender, there were consistently more males than females. One trial did not report the participants' ages (van der Horst 1991). One trial did not report the gender of participants (Pagano 1983), One of the inclusion criteria for this review were that diagnosis of infectious mononucleosis (IM) was based on clinical symptoms and laboratory parameters. The laboratory tests used in the studies included positive heterophile test, monospot test, atypical lymphocytosis, and antibody testing. Our inclusion criteria also specified that symptoms should be present for 14 days or less and that participants should be immunocompetent. In Pagano 1983, the duration of symptoms and immunocompetence of the participants was unclear. The average time from symptom onset to trial inclusion was variably reported; Andersson 1987 reported the time from clinical onset to treatment but it was unclear whether this was trial treatment or otherwise, Andersson 1986 reported number of days with symptoms before admission but again it was unclear as to whether admission referred to trial inclusion or hospitalisation; Balfour 2007a and Balfour 2007b reported number of days ill at baseline.
Outcomes and follow‐up assessment
Outcomes examined to evaluate the effectiveness of antivirals were quite heterogenous between studies.
One of our primary outcomes ‐ time to clinical recovery ‐ was reported as the number of days in two studies (Andersson 1986; Andersson 1987). van der Horst 1991 also reported the outcome in a dichotomous way; as recovery by day five or 10.
Adverse events and side effects of medication (which was the other primary outcome) were reported narratively in five trials (Andersson 1986; Andersson 1987; Balfour 2007a; Balfour 2007b; van der Horst 1991).
Viral shedding was the most evaluated outcome, reported by six trials. Other outcomes reported by more than one study included: duration of abnormal clinical examination (Andersson 1986; Andersson 1987; Simon 2003), development of complications (Andersson 1986; Andersson 1987; Balfour 2007a), days missing from school or work (Andersson 1986; Andersson 1987).
Outcomes were assessed at different times in the different studies. The length of follow‐up was not clear from some of the studies and it was inferred from information in the results tables, etc. Follow‐up varied from 20 days (Simon 2003), 35 days (van der Horst 1991), 120 days (Pagano 1983), 170 days (Balfour 2007b) to six months (Andersson 1986; Andersson 1987; Balfour 2007a).
Excluded studies
Studies were excluded for a variety of reasons based on study design and intervention criteria. Full descriptions of such exclusions are detailed in the Characteristics of excluded studies table.
Risk of bias in included studies
All seven included studies were at either unclear or high risk of bias (see Characteristics of included studies table). The 'Risk of bias' summary and 'Risk of bias' graph are presented in Figure 2 and Figure 3, respectively.
2.
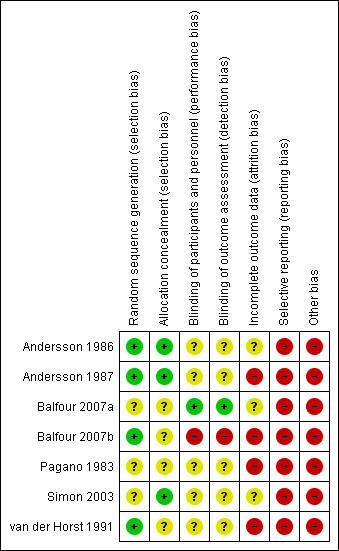
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
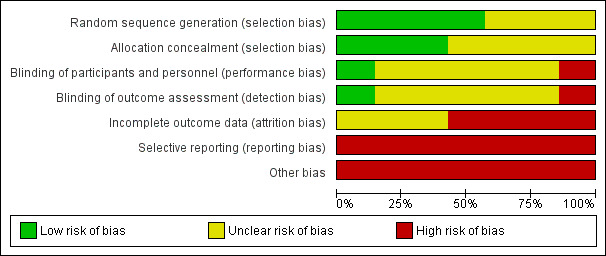
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three trials provided adequate details on allocation concealment (Andersson 1986; Andersson 1987; Simon 2003); in the remaining four trials this was unclear (Balfour 2007a; Balfour 2007b; Pagano 1983; van der Horst 1991).
Blinding
Five of the trials were 'double‐blinded' but did not specify who was blinded (Andersson 1986; Andersson 1987; Pagano 1983; Simon 2003; van der Horst 1991). In Balfour 2007a details were found on clinicaltrials.gov specifying that participant, caregiver, investigator and outcomes assessor were blinded. In Balfour 2007b clinical observers and participants were not blinded.
Incomplete outcome data
In four trials there was a high risk of attrition bias (Andersson 1987; Balfour 2007b; Pagano 1983; van der Horst 1991), and in the remaining three trials there was an unclear risk of attrition bias (Andersson 1986; Balfour 2007a; Simon 2003).
Selective reporting
The risk of selective reporting in all included trials was high. For Andersson 1986, the outcome difficulty with swallowing was stated in the methods section but not reported in the results section. Similarly in Andersson 1987, lymphadenopathy was not reported although it states in the methods section that it was measured at visits. In Balfour 2007a, clinical examination or symptom scores were not individually reported, but were reported as a composite score of 10 parameters. This was also the case in Simon 2003; individual symptom scores were not all reported, but only reported as composite scores. In Balfour 2007b, clinic visits took place on days 5, 10, 15, 18, 21, 42, 84, and 168, but clinical data were only reported up to day 15. Clinical outcomes were not fully reported for Pagano 1983; only reported narratively with no actual results given. In van der Horst 1991, it was stated that some of the outcomes were measured on days 3, 5, 10, and 30, but these were only reported in baseline data.
Other potential sources of bias
In the trial Andersson 1986, some patients received IV fluids, some also had antibiotics before the trial. In the trial Andersson 1987, there were differences in the severity of illness between patients and three patients who had IV rather than oral antiviral and steroids were included in the analysis. In Balfour 2007b, some participants had oral steroids co‐administered, but it is not reported how many from each group had this. Balfour 2007a is an unpublished trial, and therefore not peer reviewed. In Pagano 1983, it was unclear whether patients had symptoms for 14 days or less. Two patients in the placebo group and one in the treatment group had steroids. The groups in the trial van der Horst 1991 had significant baseline imbalances in that more acyclovir recipients had a temperature > 37.5°C, and the mean pharyngitis score was slightly greater for acyclovir recipients.
Most of the studies appeared to have industry funding. Andersson 1986 and Andersson 1987 were both supported by the Wellcome foundation. Balfour 2007a was supported by an investigator‐initiated grant from Epiphany Biosciences. Balfour 2007b was supported by grants including an investigator‐initiated grant from Roche Laboratorie. Pagano 1983 and van der Horst 1991 were both supported by a grant from Burroughs‐Wellcome company. In Simon 2003, the study funding source and conflicts of interest were not stated. However, two of the authors were from a drug company.
Effects of interventions
See: Table 1
We included seven randomised controlled trials (RCTs) with a total of 333 participants in the review. The trials dated from 1983 to 2007 and were heterogeneous in terms of outcome assessment and how they were reported, therefore few trial results were pooled. There were statistically significant improvements in the treatment group for two of 12 outcomes. The quality of evidence was graded as very low for all outcomes See Table 1.
Primary outcomes
1. Time to clinical recovery
Six of the seven included studies reported time to clinical recovery but reported this in different formats. When the two trials (Andersson 1986; Andersson 1987) that reported this homogenously were pooled, there was an overall statistically significant reduction in favour of treatment group for this outcome as measured by physician but not by patient assessment.
Andersson 1986 and Andersson 1987 reported the time to clinical recovery as a continuous outcome and subdivided this into 'patient assessment' and 'physician assessment'. It should be noted that in Andersson 1987 three patients in the treatment group had a co‐administered steroid, whereas none of the placebo group had this.
Andersson 1986 reported a statistically significant reduction in time to clinical recovery as assessed by physician of five days (95% CI ‐8.21 to ‐0.79) in favour of the treatment group. This study also reported a non statistically significant reduction in time to clinical recovery as assessed by patient of 18 days (95% CI ‐44.23 to 7.23) in favour of the treatment group.
Andersson 1987 reported a non statistically significant reduction in time to clinical recovery as assessed by physician of five days (95% CI ‐14.97 to 4.97) in favour of the treatment group. This study also reported a non statistically significant reduction in time to clinical recovery as assessed by patient of three days (95% CI ‐14.48 to 20.48) in favour of the control group.
When the results of Andersson 1986 and Andersson 1987 were pooled for 'time to clinical recovery as assessed by physician' there was a statistically significant mean reduction of five days in the treatment group but with wide confidence intervals (95% CI ‐8.04 to ‐1.08, 87 participants) (Analysis 1.1). Pooling the results of Andersson 1986 and Andersson 1987 for the outcome 'time to clinical recovery as assessed by patient' did not yield any statistically significant result (Analysis 1.2). The result for this analysis was a reduction of six days (95% CI ‐26.23 to 15.05, 87 participants) in favour of the treatment group.
1.1. Analysis.
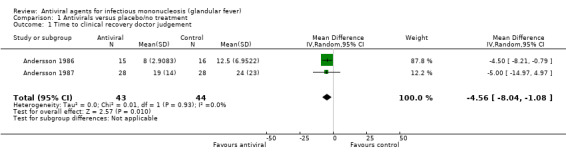
Comparison 1 Antivirals versus placebo/no treatment, Outcome 1 Time to clinical recovery doctor judgement.
1.2. Analysis.
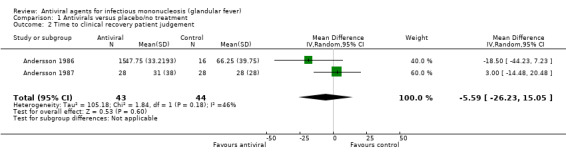
Comparison 1 Antivirals versus placebo/no treatment, Outcome 2 Time to clinical recovery patient judgement.
van der Horst 1991 reported this as a dichotomous outcome at two time points; the number of participants with recovery or not by day five, or day 10. There was a non statistically significant risk ratio of 1.74 (95% CI 0.69 to 4.41) in favour of a higher proportion of participants recovered in the treatment group at day five. There was a non statistically significant risk ratio of 1.10 (95% CI 0.74 to 1.64) in favour of a higher proportion of participants recovered in the treatment group at day 10.
Balfour 2007a reported the SF12 (Ware 1996) composite score at days one and 28 and determined the amount of change from the baseline for each group. It was not possible to access the original data to assess these outcomes individually. In the SF12; Physical and Mental Health Composite Scores (PCS & MCS) are calculated using the scores of 12 questions and range from zero to 100, where a zero score indicates the lowest level of health and 100 indicates the highest level of health. The median change from baseline at day 28 in the treatment group in this study was +17.1, whereas the median change from baseline in the control group was +11.2 indicating that there was a difference of 5.9 points in favour of the treatment group. Given that this score ranges from zero to 100, this is likely to be a modest clinical improvement in the antiviral group. The authors did not report ranges or standard deviation for these results, but did report that the differences were not statistically significant.
Balfour 2007b reported a composite score; 'Severity of Illness Score', which was developed by the authors and described in a previous study (Balfour 2005). It was not possible to access the original data to assess these outcomes individually. The severity of illness score is the sum of the evaluations of physical activity limitation and symptom or pain intensity. Scores range from zero (completely asymptomatic) to six (severely affected). The median 'severity of illness score' for each group was reported graphically at study enrolment, day five, day 10, day 15 and day 20, but a range was only reported for the day‐15 result. At day 15, the median score was 1 in the treatment group, and 2 in the control group. This meant that there was a statistically significant mean difference (MD) of ‐1.00 (95% CI ‐1.82 to ‐0.18) in favour of the treatment group at this time point.
Simon 2003 reported composite scores (which were developed by the authors) at certain time points but it was not possible to access the original data to assess these outcomes individually. Four scoring systems were developed by the authors: 'total score' (composite score incorporating sore throat, stomach ache, fatigue, swollen glands, headache, vomiting, rash, nausea, sweats, chills, swollen eyes, runny nose, cough); 'selected score' (composite score incorporating sore throat, fatigue, swollen glands, nausea and chills); 'feeling bad' and 'fatigue'. Results were reported for these four outcomes in terms of 'analysis of change from baseline scores at day 20'. We felt that 'total score' was the outcome likely to equate most closely with recovery. Unfortunately, it was not clear what the maximum value for 'total score' was, but a higher score indicated that a participant was more unwell. The highest average (unclear whether this was mean or median) 'total score' at baseline in the treatment group was 10.7, and 8.7 in the control group. There was a non statistically significant mean difference in change from baseline of 'total score' at day 20 of ‐4.46 (95% CI ‐9.02 to 0.10) in favour of the treatment group.
Pagano 1983 did not report this outcome; there was a summary given of the clinical outcome results but no clinical data reported in the paper.
2. Adverse events and side effects of medication
Five of the seven studies reported adverse events of medication narratively and the majority reported no significant difference between groups for this outcome. It was not possible to pool the results for this outcome because of the potential for double counting results. Trial participants may have had more than one adverse outcome and this was not clear in the reporting of the original studies.
Balfour 2007b reported that no adverse events were observed.
van der Horst 1991 reported that there was no difference between the two treatment groups in terms of side effects despite the large dose of acyclovir given. It was also reported in this study that “Ten acyclovir recipients had a rising SGOT or SGPT level on day 10 or 30 compared with 15 placebo recipients. All abnormalities resolved.”
Balfour 2007a listed the adverse events in a summary format: abdominal pain, headache, nausea, vomiting, rash. Of these; nausea was the only symptom where a statistically significant difference was observed between the two treatment groups with seven participants in the treatment group and one patient in the placebo group affected (P value 0.03). One serious adverse event was reported ‐ a case of pancreatitis but the authors stated that this was more likely to be a complication of IM rather than a side effect of medication.
Andersson 1986 reported that an asymptomatic, transient elevation of serum creatinine and urea was noted in two patients from the treatment group and in none in the placebo group. Thrombophlebitis was found in four patients from both treatment groups. Skin rashes were found in three of the patients treated with antiviral, but these patients were also treated with ampicillin pre‐admission) and six patients from the placebo group (of whom four were given ampicillin pre‐admission). During the six‐month follow‐up, a total of 12 cases of upper respiratory tract infection or tonsillitis were noted. Ten of the 12 were diagnosed as bacterial complications, and were evenly distributed in the two groups.
Andersson 1987 reported that three patients had an unexplained late occurrence of exanthema, one week after admission, which could have been due to acyclovir or to penicillin treatment preceding enrolment in the study. One patient in the acyclovir group suffered from diarrhoea and abdominal pain that resolved once acyclovir was withdrawn. No side effects were noticed in the placebo‐treated patients. Serum creatinine elevation of > 10% above the normal level was found in three placebo and five acyclovir‐treated patients, all of which normalised within one month.
Secondary outcomes
1. Duration of abnormal clinical examination (assessed by physician)
1a. Duration of fever (> 37.5° C)
Four of the seven included studies reported this outcome and one of these found a statistically significant difference between groups.
Simon 2003 reported this as shift in change from baseline score at day 20. van der Horst 1991 also reported a dichotomous outcome: number of patients in each treatment group with fever at day 10. There was no statistically significant difference between the two groups in either of these studies.
Andersson 1986 and Andersson 1987 reported this as a continuous outcome: number of days. Andersson 1986 found that there was a small statistically significant reduction in this in favour of the antiviral group (median number of days four versus six in the placebo group), whereas Andersson 1987 found that there was no statistically significant difference between the two groups.
1b. Duration of pharyngitis
Five of the seven included studies reported this outcome with one of these studies finding a significant effect in favour of antiviral treatment.
Original data obtained from the lead author of the Simon 2003 study showed a statistically significant difference between the two groups for this outcome. Duration of pharyngitis in the treatment group was a mean of six days versus 12 in the placebo group.
Balfour 2007b reported the presence of sore throat for the two groups at different time points: day 1, 6, 10, and 15 (there was missing data on day 15) with no statistically significant difference between the groups at any of these time points.
Andersson 1986 and Andersson 1987 reported this as a continuous outcome: number of days. Both reported no statistically significant difference between the two groups.
van der Horst 1991 reported a dichotomous outcome: number of patients in each treatment group with severe pharyngitis at day 10 with no statistically significant difference between the two groups.
Pooling the results from Simon 2003, Andersson 1986 and Andersson 1987 indicated no statistically significant difference between groups (mean difference (MD) of one day fewer in the treatment group (95% CI ‐5.68 to 2.92, 117 participants)) (Analysis 1.5)
1.5. Analysis.
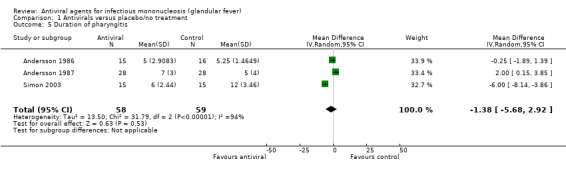
Comparison 1 Antivirals versus placebo/no treatment, Outcome 5 Duration of pharyngitis.
1c. Duration of lymphadenopathy
Three of the seven included studies reported this outcome and one reported a statistically significant improvement in the anti‐viral treatment group.
Original data obtained from the lead author of the Simon 2003 study showed that there was a statistically significant difference between the two groups for this outcome. Duration of lymphadenopathy in the treatment group was a mean of 11 days versus 20 in the placebo group.
Andersson 1986 and Andersson 1987 reported this as a continuous outcome: number of days. Andersson 1986 found no statistically significant difference between the two groups. Andersson 1987 reported this outcome for three patients in the treatment group who were also administered steroids. It was not reported for the rest of the treatment group, or the placebo group.
When the results of Andersson 1986 and Simon 2003 were pooled, there was a mean reduction in number of days of nine (95% CI ‐11.75 to ‐6.14, 61 participants) (Analysis 1.6) in favour of the treatment group. The study Simon 2003 was weighted more than 99% in this analysis as the results for mean and SD in Andersson 1986 were so large (the original median and range from Andersson 1986 were converted to mean and SD using the method by Hozo 2005, already mentioned above).
1.6. Analysis.
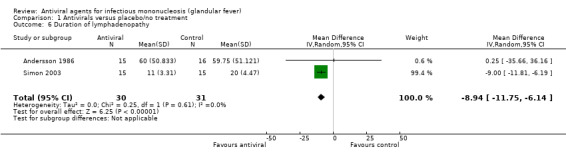
Comparison 1 Antivirals versus placebo/no treatment, Outcome 6 Duration of lymphadenopathy.
1d. Duration of splenomegaly
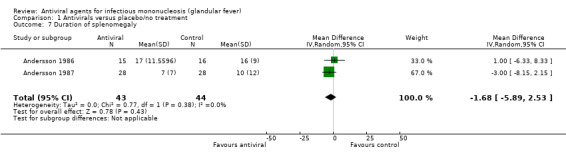
Two of the seven included studies reported this as a continuous outcome: number of days with no statistically significant difference between the two groups (Andersson 1986; Andersson 1987). See Analysis 1.7.
1.7. Analysis.
Comparison 1 Antivirals versus placebo/no treatment, Outcome 7 Duration of splenomegaly.
1e. Duration of hepatomegaly
Two of the seven included studies reported this as a continuous outcome: number of days with no statistically significant difference between the two groups (Andersson 1986; Andersson 1987). See Analysis 1.8.
1.8. Analysis.
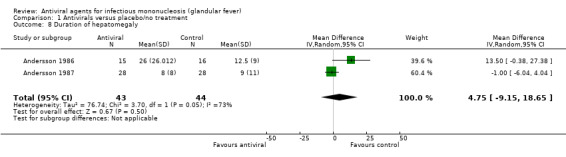
Comparison 1 Antivirals versus placebo/no treatment, Outcome 8 Duration of hepatomegaly.
2. Development of complications of infectious mononucleosis (IM)
Three of the seven included studies reported this outcome. We had planned to examine chronic fatigue (fatigue present for ≥ six months) as one of the specific complications of IM. Hoowever, this was not reported in the included studies.
In Balfour 2007a, a 24‐year‐old participant who was in the treatment arm developed acute pancreatitis on the 10th study day. The authors considered that this was more likely to be a complication of IM rather than an adverse drug event.
Andersson 1986 reported that one patient in the treatment group had to be tracheotomised, and acquired a transient, bilateral hypoglossal nerve palsy. One patient from the placebo group was operated on 12 days after admission because of abdominal pain with a surgical diagnosis of pseudo appendicitis, and another patient in the placebo group suffered from hepatitis with mild icterus persisting for seven months, where no other aetiologic agent could be demonstrated. During the six‐month follow‐up, a total of 12 cases of upper respiratory tract infection or tonsillitis were noted. Ten of the 12 were diagnosed as bacterial complications, and were evenly distributed in the two groups.
Andersson 1987 reported that there were three patients in the antiviral treatment group with over‐whelming clinical symptoms;two of the patients had severe upper respiratory obstruction which responded to combined steroid‐acyclovir therapy, the third patient developed disseminated intravascular coagulopathy, and had a fever of three weeks’ duration along with hepatitis and pancytopenia, despite effective inhibition of oropharyngeal EBV production during treatment.
Simon 2003 stated 'no patients developed thrombocytopenia or anaemia during the study period'.
3. Viral shedding
All of the studies except for Simon 2003 reported this outcome in some way. All reported similar techniques for detection of the Epstein‐Barr virus (EBV) oropharyngeal shedding.
van der Horst 1991 reported inadequate sampling handling, but reported the percentage of patients who were culture positive at days zero and 10.The study reported that the differences between the groups were not statistically significant. It was unclear how many patients in each group these percentages were based on.
Balfour 2007b reported the quantity of EBV DNA in oral wash cells and supernatant at certain time points but this was only represented graphically and we were unable to extrapolate from this graph or obtain original data.
Balfour 2007a reported the number of patients with ≥2log₁₀ decrease in EBV copies/mL in oral cells, supernatant and whole blood at the end of treatment. Of these, only the data for supernatant showed a statistically significant result in favour of the antiviral, with a risk of 7.27 (95% CI 1.09 to 48.35). It also reported the median log₁₀ copies EBV/mL of the oral supernatant and oral cells at certain time points but this was only represented graphically and we were unable to extrapolate from this graph or obtain original data.
Pagano 1983 reported this as a dichotomous outcome: oropharyngeal excretion of EBV at certain time points but there are a lot of missing data here making it difficult to interpret the findings.
Andersson 1986 reported the proportion of oropharyngeal EBV shedders in treatment versus placebo groups at certain time points and found a statistically significant result on days four and seven, which was not sustained at days 28 and 180 after treatment was stopped.
Andersson 1987 reported the proportion of oropharyngeal EBV shedders in treatment versus placebo groups at certain time points and found a statistically significant during antiviral treatment, which was not sustained after treatment was stopped.
Overall, each study that reported on viral shedding concluded that the antiviral drug suppressed viral shedding during treatment but the effect was not sustained when treatment was stopped.
4. Patient‐reported outcome measures
Five of the seven studies reported patient‐reported outcome measures (PROMs)
4a. Health‐related quality of life (HRQoL)
Three of the included studies reported on HRQoL. van der Horst 1991 reported a 'sense of well being' in the abstract but did not report data for this outcome.
Balfour 2007a reported results for the SF12 composite score which was examined at day and one and day 28. There was no statistically significant difference between treatment and placebo groups.
Simon 2003 reported a composite score ('feeling bad score', scored as absent (0), mild (1), moderate (2), or severe (3) from a scoring system developed by the authors) which found a non significant shift in change from baseline at day 20 between treatment and placebo groups.
4b. Days missing from school of work
Four of the seven included studies reported on days missing from school or work with data available only for two of the studies, which found no difference between groups.
Simon 2003 referred to a 'pattern of activities' and Balfour 2007a reported composite scores, but we were unable to obtain original data for these outcomes.
Andersson 1986 and Andersson 1987 reported this as a continuous outcome: number of days with no statistically significant difference between the two groups. Pooling these two trial results did not give any statistically significant result either (MD ‐0.90, 95% CI ‐6.53 to 4.74, 87 participants) (Analysis 1.11).
1.11. Analysis.
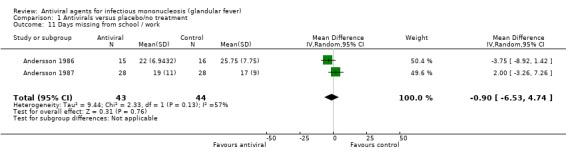
Comparison 1 Antivirals versus placebo/no treatment, Outcome 11 Days missing from school / work.
5. Economic outcomes
Economic outcomes were not mentioned in any of the studies
Subgroups
We had planned to undertake subgroup analyses based on patient age, setting and placebo versus no treatment controls with sufficient data but this was not possible due to lack of appropriate data.
Discussion
Summary of main results
Results of this review need to be interpreted with caution as the quality of the evidence was graded as very low for all outcomes. There was a statistically significant improvement in the treatment group for only two of the 12 outcomes reported in included studies. Both of these outcomes were physician‐assessed outcomes, and may have limited clinical importance.
Pooled results for 'time to clinical recovery as assessed by physician' indicated a mean reduction in the number of days of five days in the treatment group with wide confidence intervals (95% CI ‐8.04 to ‐1.08). However, it was unclear how this outcome was assessed in the studies reporting it. In one of these trials, three of the treatment group had steroids as a co‐intervention while none of the placebo group did, which may have affected the results also (Andersson 1987). Prospective studies report that clinical signs and symptoms start to resolve by one month (Macsween 2010; Rea 2001) and that fatigue may be persistent in approximately 10% of patients at six‐month follow‐up (Buchwald 2000; White 2001), so that a reduction in 'time to clinical recovery as assessed by physician' of five days in the treatment group may not be a clinically meaningful result.
Results for the remaining four studies that examined our primary outcome in some way, all reported modest improvements in the treatment group, but these were mainly non statistically significant findings.
Trial results for the outcome 'adverse events and side effects of medication' were reported narratively only in five studies. In some reports, authors were unsure whether an adverse event was related to medication or complication of disease. These results could not be pooled due to the potential for double counting results but overall, the majority of trials reporting this outcome did not find any significant difference between treatment and control groups.
Results from Andersson 1986 and Simon 2003 were pooled for the outcome 'duration of lymphadenopathy', with a mean reduction of nine days (95% CI ‐11.75 to ‐6.14) in favour of the treatment group. Within this meta‐analysis, Simon 2003 was weighted more than 99% as the standard deviations in Andersson 1986 were so large. These two trials also reported very heterogenous results for this outcome with Andersson 1986 reporting lymphadenopathy of more than 1 cm diameter, whereas Simon 2003 reported the presence of lymphadenopathy as determined by physician assessment, but it is not clear what the criteria for reporting presence or absence was.
The overall effect on viral shedding from the six studies that reported this outcome was that viral shedding was suppressed while on antiviral treatment, but this effect was not sustained when treatment was stopped.
For the eight other outcomes reported in included studies there was no statistically significant effect of anti‐viral therapy in infectious mononucleosis (IM).
Overall completeness and applicability of evidence
Seven trials with a total of 333 participants met the inclusion criteria for this review. The trials were published between 1983 and 2007. In general, the number of participants in each trial was small; only one trial had more than 60 participants (van der Horst 1991). Also, many of the effect sizes had wide confidence intervals (CIs) relating to the small sample sizes, reducing the precision of estimates. It is also possible that these studies were underpowered to detect potential differences in many of the secondary outcomes due to the small sample sizes. The trials were heterogenous in terms of setting (outpatient versus inpatient), severity of illness, antiviral treatment regimens (differences in antiviral used, dose and method of administration), age of participants (children versus young adults) outcomes assessed and reporting of data. Pooling of results was limited as a result of this heterogeneity, with results from three trials or less being pooled for any one outcome. We were unable to perform subgroup or sensitivity analyses for any of the outcomes. All included trials had inadequate outcome reporting. All included trials had either an unclear or high risk of bias. None of the trials reported on economic outcomes. These factors all limit the conclusions that can be drawn.
Quality of the evidence
The quality of the evidence was graded as very low for all outcomes which means that 'the true effect is likely to be substantially different from the estimate of effect' as per the GRADE Working Group grades of evidence. The evidence was downgraded in the domains of risk of bias due to the majority of studies having an unclear or high risk of bias; inconsistency due to wide variance of point estimates across studies, which may reflect differences in setting, type of antiviral, or route of medication administration; imprecision due to small sample sizes or wide CIs.
Overall, the very low quality of the evidence means that we can place very little confidence in the results found.
Potential biases in the review process
We have attempted to limit bias in the review process. Though we used a thorough search strategy, we may not have identified all trials eligible for inclusion, especially unpublished trials.
Agreements and disagreements with other studies or reviews
Although our review includes two additional trials, the results are in agreement with the previous 1999 systematic review Torre 1999, concluding that there is not enough evidence to support the use of antiviral agents for IM. A recent narrative review article also concluded that treatment with acyclovir significantly decreased the rate of oropharyngeal viral shedding, but that there was no evidence to support its use in an acute setting (Lennon 2015).
Studies examining the effect of antivirals for other acute illnesses have generally found small improvements in outcomes in favour of antivirals but with the potential negative effects of medication costs, side effects and potential for antiviral resistance. A 2015 meta‐analysis published in the Lancet found that in adults with a laboratory diagnosis of influenza, oseltamivir accelerated time to clinical symptom alleviation, reduced the risk of antibiotic prescribing for lower respiratory tract infection and hospital admission for any cause (Dobson 2015). There was no benefit conferred to patients who had symptoms of influenza‐like illness but not confirmed infection. A Cochrane review of acyclovir for varicella found that acyclovir was effective in reducing the number of days with fever and the maximum number of lesions but did not have an effect on complications or relief of itch among otherwise healthy children with chickenpox (Klassen 2005).
Authors' conclusions
Implications for practice.
The effectiveness of antiviral agents (acyclovir, valomaciclovir and valacyclovir) in acute infectious mononucleosis (IM) is uncertain. The quality of the evidence is very low; included studies were small, heterogeneous and at unclear or high risk of bias. Outcomes were selectively reported, often reported as composite scores and generally found only modest improvements which may not be clinically meaningful. Alongside the lack of evidence of effectiveness, decision makers need to consider the potential adverse events and possible associated costs, and antiviral resistance.
Implications for research.
More robust clinical trials are required to further assess this research question as the quality of the current evidence is poor and based on small heterogenous studies. Most cases of symptomatic IM are encountered in young adults in a primary care environment, often in student health centres. Trials of commonly used orally‐administered antivirals versus usual care should be established in this setting. Outcomes examined should include effectiveness on acute symptoms, adverse effects, time off work or school, prevention of complications, effect of antivirals on longer‐term outcomes such as fatigue, and evaluation of economic outcomes.
Acknowledgements
We would like to acknowledge the Health Research Board, who funded a Cochrane Fellowship for Muireann de Paor. We would also like to thank the Cochrane ARI Group, who provided ongoing advice and support in writing this review and developing the search strategy. We thank the following people for commenting on the draft protocol and review: Marie Kakhu, Cheryl Jones, Mosarrat Qureshi, Mark Jones, Lubna Al‐Ansary, Ekaterina Yudina and Liliya Ziganshina. We also thank Sushil Agwan and Mohamed Hassan, medical students from Bond University, Gold Coast, Australia, for their assistance in checking the review.
Appendices
Appendix 1. Glossary of terms
Subclinical: not detectable or producing effects that are not detectable by the usual clinical tests. Lymphadenopathy: abnormal enlargement of the lymph nodes.
Lymphocytosis: an increase in the number of lymphocytes (a type of white blood cell) in the blood usually associated with chronic infections or inflammations.
Adenopathy: any disease or enlargement involving glandular tissue; especially involving lymph nodes.
Splenomegaly: abnormal enlargement of the spleen.
Posterior cervical adenopathy: enlargement of the lymph nodes in the posterior cervical area.
Palatal petechiae: minute red or purple spots containing blood that appear in the mucous membrane of the palate as a result of localised haemorrhage.
Airway occlusion: an obstruction of the passageway for air into or out of the lungs.
Cholestasis: total or partial suppression of the flow of bile.
Myocarditis: inflammation of the myocardium (the middle muscular layer of the heart wall).
Encephalomyelitis: concurrent inflammation of the brain and spinal cord.
Cranial nerve palsies: complete or partial paralysis of any of the 12 paired nerves that arise from the lower surface of the brain with one of each pair on each side and passing through openings in the skull to the periphery of the body.
Oropharyngeal shedding: to discharge the virus from the part of the pharynx (the part of the alimentary canal between the mouth and the oesophagus) between the soft palate and the epiglottis.
Appendix 2. MEDLINE search strategy
Infectious Mononucleosis/ OR mononucleosis.tw. OR glandular fever.tw. OR Epstein‐Barr Virus Infections/ OR Herpesvirus 4, Human/ OR ((epstein‐Barr or epstein Barr) adj2 (virus* or viral*)).tw. OR ebv.tw.
AND
exp Antiviral Agents/ OR antiviral*.tw. OR antivirus*.tw. OR exp Acyclovir/ OR (acyclovir or aciclovir).tw,nm. OR (valacyclovir or valaciclovir).tw,nm. OR (gancyclovir or ganciclovir).tw,nm. OR (valganciclovir).tw,nm. OR (cidofovir).tw,nm. OR (foscarnet).tw,nm. OR (penciclovir).tw,nm. OR (famciclovir).tw,nm.
AND
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or randomised.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.)
Appendix 3. Embase search strategy
‘Infectious Mononucleosis’/exp OR mononucleosis:ti,ab OR “glandular fever”:ti,ab OR ‘Epstein‐Barr Virus Infections’/exp OR ‘Herpesvirus 4, Human’/exp OR ebv:ti,ab OR (epstein‐Barr NEAR/2 (virus* OR viral*)):ti,ab
AND
‘Antiviral Agents'/exp OR antiviral*:ti,ab OR antivirus*:ti,ab OR 'Acyclovir'/exp OR (acyclovir OR aciclovir OR valacyclovir OR valaciclovir OR gancyclovir OR ganciclovir OR valganciclovir OR cidofovir OR foscarnet OR penciclovir OR famciclovir):ti,ab
AND
random* OR factorial OR crossover OR placebo OR blind OR blinded OR assign OR assigned OR allocate OR allocated OR 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp NOT ('animal'/exp NOT ('animal'/exp AND 'human'/exp))
Appendix 4. CINAHL search strategy
((MH "Infectious Mononucleosis") OR mononucleosis OR glandular fever OR (MH "Epstein‐Barr Virus Infections+") OR ((epstein‐Barr or epstein Barr) N2 (virus* or viral*)) OR ebv)
AND
(MH "Antiviral Agents+") OR antiviral* OR antivirus* OR (MH "Acyclovir+") OR (acyclovir or aciclovir) OR (valacyclovir or valaciclovir) OR (gancyclovir or ganciclovir) OR (valganciclovir) OR (cidofovir) OR (foscarnet) OR (penciclovir) OR (famciclovir)
AND
(MH "Clinical Trials+") OR (MH "Quantitative Studies") OR TI placebo* OR AB placebo* OR (MH "Placebos") OR (MH "Random Assignment") OR TI random* OR AB random* OR TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR TI clinic* trial* OR AB clinic* trial* OR PT clinical trial
Appendix 5. LILACS search strategy
(mononucleosis OR “glandular fever” OR Epstein‐Barr OR “Herpesvirus 4” OR “epstein Barr” OR ebv)
AND
(Antiviral* OR antivirus* OR Acyclovir OR aciclovir OR valacyclovir or valaciclovir OR gancyclovir or ganciclovir OR valganciclovir OR cidofovir OR foscarnet OR penciclovir OR famciclovir)
Appendix 6. Web of Science search strategy
mononucleosis OR “glandular fever” OR Epstein‐Barr OR “Herpesvirus 4” OR “epstein Barr” OR ebv
AND
Antiviral* OR antivirus* OR Acyclovir OR aciclovir OR valacyclovir or valaciclovir OR gancyclovir or ganciclovir OR valganciclovir OR cidofovir OR foscarnet OR penciclovir OR famciclovir
AND
random* or placebo* or allocat* or crossover* or "cross over" or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial)
Appendix 7. Cochrane CENTRAL search strategy
[mh “Infectious Mononucleosis”] OR mononucleosis:ti,ab OR “glandular fever”:ti,ab OR [mh “Epstein‐Barr Virus Infections”] OR [mh “Herpesvirus 4, Human”] OR ((epstein‐Barr or epstein Barr) NEAR2 (virus* or viral*)):ti,ab OR ebv:ti,ab
AND
[mh "Antiviral Agents"] OR antiviral*:ti,ab OR antivirus*:ti,ab OR [mh "Acyclovir"] OR (acyclovir or aciclovir):ti,ab OR (valacyclovir or valaciclovir):ti,ab OR (gancyclovir or ganciclovir):ti,ab OR (valganciclovir):ti,ab OR (cidofovir):ti,ab OR (foscarnet):ti,ab OR (penciclovir):ti,ab OR (famciclovir):ti,ab
Data and analyses
Comparison 1. Antivirals versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to clinical recovery doctor judgement | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐8.04, ‐1.08] |
| 2 Time to clinical recovery patient judgement | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐5.59 [‐26.23, 15.05] |
| 3 Adverse events and side effects | Other data | No numeric data | ||
| 4 Duration of fever > 37.5 degree C | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐6.66, 3.62] |
| 5 Duration of pharyngitis | 3 | 117 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐5.68, 2.92] |
| 6 Duration of lymphadenopathy | 2 | 61 | Mean Difference (IV, Random, 95% CI) | ‐8.94 [‐11.75, ‐6.14] |
| 7 Duration of splenomegaly | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐5.89, 2.53] |
| 8 Duration of hepatomegaly | 2 | 87 | Mean Difference (IV, Random, 95% CI) | 4.75 [‐9.15, 18.65] |
| 9 Development of complications of infectious mononucleosis | Other data | No numeric data | ||
| 10 Viral shedding | Other data | No numeric data | ||
| 11 Days missing from school / work | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐6.53, 4.74] |
1.3. Analysis.
Comparison 1 Antivirals versus placebo/no treatment, Outcome 3 Adverse events and side effects.
| Adverse events and side effects | |
|---|---|
| Study | |
| Andersson 1986 | "Drug reactions. Symptomless, transient elevation (15%) of serum creatinine and serum urea was noted in 2/15 ACV‐treated patients and none of the PLO treated patients." ''Thrombophlebitis occurred in four patients from each group. Skin rash was found in three ACV‐treated patients, all of whom had been given ampicillin before admission, compared with six patients in the placebo group , four of whom had been treated with the antibiotic before admission. No evidence of haematopoietic suppression was observed in the ACV‐treated patients." |
| Andersson 1987 | "Three patients had an unexplained late occurrence of exanthema, one week after admission which could have been due to acyclovir or to penicillin treatment preceding enrolment in the study. One patient in the acyclovir group suffered from diarrhoea and abdominal pain that disappeared once acyclovir was withdrawn. No side effects were noticed in the placebo‐treated patients. Serum creatinine elevation of >10% above the normal level was found in three placebo and five acyclovir‐treated patients, who were all normalized within one month." |
| Balfour 2007a | Adverse events were listed in a summary: Abdominal pain, headache, nausea, vomiting, rash, serious adverse event/complication. Of these; nausea was the only symptom where a statistically significant difference was observed between the two treatment groups with seven participants in the treatment group and one patient in the placebo group affected (P = 0.03). One serious adverse event was reported‐a case of pancreatitis ‐ "the case of pancreatitis was possibly drug‐related although it has been reported to be a complication of infectious mono." |
| Balfour 2007b | "No adverse events were observed. The only abnormal clinical chemistry results were elevations in the ALT and AST levels. The ALT and AST levels were elevated on enrollment in 7 of 10 valacyclovir subjects and in 8 of 10 control subjects. All ALT and AST levels returned to the normal range during the study period. The only other abnormal laboratory findings were mild leukocytosis (five subjects) and thrombocytopenia (four subjects) on enrollment. These abnormalities had all resolved when the subjects were tested again on the third study visit." |
| Pagano 1983 | None mentioned |
| Simon 2003 | None mentioned |
| van der Horst 1991 | Reported that there was no difference between the two treatment groups in terms of side effects despite the large dose of acyclovir given |
1.4. Analysis.
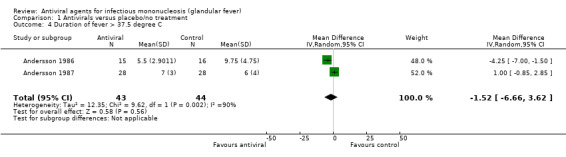
Comparison 1 Antivirals versus placebo/no treatment, Outcome 4 Duration of fever > 37.5 degree C.
1.9. Analysis.
Comparison 1 Antivirals versus placebo/no treatment, Outcome 9 Development of complications of infectious mononucleosis.
| Development of complications of infectious mononucleosis | |
|---|---|
| Study | |
| Andersson 1986 | ACV group: 'the most severely ill patient also had a mild infection with herpes zoster virus in the L2 region at admission. He had a tracheotomy because of an airway obstruction; this was followed by a transient, bilateral hypoglossal nerve palsy." Placebo group: "One patient was operated on on the 12th day after admission because of abdominal pain. Surgical diagnosis was pseudoappendicitis. One patient suffered from hepatitis that persisted for seven months with mild icterus. No other etiologic agent could be demonstrated." |
| Andersson 1987 | There were ‘three patients with over‐whelming clinical symptoms causing airway obstruction and/or disseminated intravascular coagulopathy". These 3 patients were treated with a combination of IV antiviral and steroids |
| Balfour 2007a | "A 24‐year‐old subject developed acute pancreatitis on the 10th study day and was hospitalized for 2 days for medical management. She was taken off clinical trial material and not re‐challenged.The blind was not broken. She is entirely well. When the code was broken, we learned that she was in the valomaciclovir arm. Thus, this SAE is possibly drug‐related, although I considered it more likely caused by EBV infection because pancreatitis has been reported to be a complication of infectious mononucleosis." |
| Balfour 2007b | Not mentioned |
| Pagano 1983 | Not mentioned |
| Simon 2003 | Not mentioned |
| van der Horst 1991 | Not mentioned |
1.10. Analysis.
Comparison 1 Antivirals versus placebo/no treatment, Outcome 10 Viral shedding.
| Viral shedding | |
|---|---|
| Study | |
| Andersson 1986 | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
| Andersson 1987 | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
| Balfour 2007a | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
| Balfour 2007b | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
| Pagano 1983 | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
| Simon 2003 | Not reported |
| van der Horst 1991 | Overall effect was that viral shedding was suppressed while on treatment but this was not sustained when treatment stopped |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersson 1986.
| Methods | Double‐blinded RCT. States 8‐month study period but start and end dates not reported | |
| Participants | 33 hospitalised participants (aged 15 to 25) with symptoms of infectious mononucleosis for 1 week or less. 2 participants excluded because of a change in diagnosis to tonsillitis due to primary herpes simplex or group A strep. Of included patients at enrolment, 21 were male and 10 female | |
| Interventions | Acyclovir (ACV) 10 mg/kg IV 8 hourly for 7 days versus placebo | |
| Outcomes | Assessment of general health by patient and physician, duration of recovery as assessed by patient and physician, duration of weight loss, duration of fever > 37.5°C, duration of sore throat, duration of tonsillar swelling, duration of rash, duration of lymphadenopathy, duration of enlargement of liver and spleen, time away from school/work. Time to normalise WBC count, atypical lymphocytes, liver enzymes. Serological and virological measurements taken also | |
| Notes | Three reports seem to be from the same trial: Andersson 1985, Andersson 1986 and Ernberg 1986 11/15 of the ACV group and 9/16 of the placebo group had antibiotics pre admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation code |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. Each patient was treated in sequential order according to the randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States 'double‐blind' trial but does not report who was actually blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States 'double‐blind' trial but does not report who was actually blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Recording of difficulty with swallowing was stated in the methods section but not reported in the results section. When no statistically significant difference was found between treatment and control groups for any single sign of symptom of infectious mononucleosis, various parameters were combined to find a significant difference. It was unclear which actual parameters were used for this analysis. |
| Other bias | High risk | Some patients in both intervention and placebo groups received IV fluids. Some patients also had antibiotics before the trial. Supported by the Wellcome foundation |
Andersson 1987.
| Methods | Double‐blinded RCT. States that follow‐up was for 6 months after onset of disease but study start and end dates not reported | |
| Participants | 60 adults (aged 15 to 30) hospitalised with symptoms of infectious mononucleosis for 1 week or less. 2 participants excluded because of a change in diagnosis and two excluded for not complying with medication. Of included patients at enrolment, 34 were male and 23 female | |
| Interventions | Acyclovir 800 mg 5 times daily PO for 7 days versus placebo | |
| Outcomes | Duration of: recovery as assessed by patient and physician, weight loss, fever > 37.5°C, difficulty swallowing, sore throat, tonsillar swelling, tiredness, abdominal pain, liver enlargement, elevated liver enzymes, spleen enlargement, absence from school/work, atypical monocytes > 5%, absolute lymphocytosis > 50%. Number of patients with increased serum creatinine during treatment, number of patients with positive monosticon test after six months. Virological outcomes also measured | |
| Notes | Three patients in the acyclovir group required IV acyclovir and prednisolone for 10 days, whereas none of the placebo group had this treatment Virological findings were only provided for 36/56 patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation code |
| Allocation concealment (selection bias) | Low risk | Drawn from sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "double‐blind trial" but does not elaborate further. 3 of the participants had IV acyclovir also had steroids so presume blinding was broken here |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "double‐blind trial" but does not elaborate further |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 of the 60 patients were excluded after randomisation because of a change in the diagnosis, and 2 for not following the medication. Results from these patients were not included in analysis |
| Selective reporting (reporting bias) | High risk | Lymphadenopathy not reported although says in methods that it was measured at visits Virological data only reported for 36 out of 56 patients |
| Other bias | High risk | Differences in severity of illness 3 patients who had IV acyclovir and steroids included in analysis. Supported by the Wellcome foundation |
Balfour 2007a.
| Methods | Double‐blinded RCT. Study start and end dates (November 2007 to February 2010) obtained from Clinicaltrials.gov | |
| Participants | 23 students (aged over 15 years) with symptoms of infectious mononucleosis for two weeks or less. Setting unclear. Of patients included in data analysis 12 were male and 9 female. Maximum age of participants was 24 years | |
| Interventions | Valomaciclovir 2 g orally twice daily for 21 days, versus placebo | |
| Outcomes | Number of participants with improvement in clinical symptoms and reductions in viral burden from baseline, number of participants who experienced adverse events | |
| Notes | Unpublished study: data obtained from conference slides supplied by author and information on Clinicaltrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 participants were enrolled, however 21 were included in data analysis. 2 participants were excluded; 1 due to not having a primary EBV infection and 1 as they had an adverse event on day 11. In reporting of SF12 questionnaires there are data missing from one of the placebo patients ‐ no details are given on why this might be the case |
| Selective reporting (reporting bias) | High risk | Clinical exam/symptom scores not individually reported; only reported as composite score of 10 parameters |
| Other bias | High risk | Unpublished trial, not peer reviewed Supported by an investigator‐initiated grant from Epiphany Biosciences |
Balfour 2007b.
| Methods | Pilot study. Start date February 2004, end date September 2005 obtained from Clinicaltrials.gov | |
| Participants | 20 students (aged over 18) with symptoms of infectious mononucleosis for 1 week or less. Setting: student health centre. 10 male and 10 female participants. Median age in treatment group was 19.3 years, median age in control group was 21.4 years | |
| Interventions | Valacyclovir 1 g PO every 8 hours for 14 days or no antiviral drug (no placebo given) | |
| Outcomes | The primary outcome was the proportion of participants with laboratory‐confirmed primary EBV infection who had ≥ 2 log10 decrease in EBV copies/mL in oral washes during the treatment period. Secondary outcomes included clinical effects | |
| Notes | This is the same study as 'Controlled Trial of Valacyclovir in Infectious Mononucleosis' found on Clinicaltrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Personnel performing laboratory tests, data entry, and data analysis were blinded to the study group assignment until the trial was completed" "the clinical observers were not blinded" Participants were not blinded as did not get placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Personnel performing laboratory tests, data entry, and data analysis were blinded to the study group assignment until the trial was completed" "the clinical observers were not blinded" Participants were not blinded as did not get placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Data from eight subjects who enrolled were not included in the final analysis. Seven of them were excluded because they did not have primary EBV infections. Five of these seven had past infections and two had no confirmation of EBV infection. One subject who had a primary EBV infection also was not included because we were unable to collect data during the days scheduled for study visits 2–5." Does not mention which group they dropped out from |
| Selective reporting (reporting bias) | High risk | Clinic visits took place on days 5, 10, 15, 18, 21, 42, 84, 168 but clinical data were only reported up to day 15 |
| Other bias | High risk | “Nine subjects (five in the valacyclovir group and four in the control group) received oral corticosteroids. Seven of the nine subjects were given the prednisone taper.... One subject received a 6‐day methylprednisolone taper beginning at 24 mg/day and one student was given 11 days of cortisone (10 mg/day)” The research was supported by grants including an investigator‐initiated grant from Roche Laboratorie. |
Pagano 1983.
| Methods | Double‐blinded RCT. Start and end dates not stated | |
| Participants | 20 adults with relatively severe symptoms of infectious mononucleosis requiring hospitalisation. Gender of participants not reported. No maximum participant age reported but mean age in acyclovir group was 19.5 years and mean age in the placebo group was 21 years | |
| Interventions | Acyclovir (ACV) 500 mg/m² IV 8 hourly for 5 days versus placebo | |
| Outcomes | Unclear whether clinical findings were reported by patient or physician. No table of results for clinical findings. Clinical findings: sore throat, splenomegaly, lethargy, lymphadenopathy, temp, return to baseline body weight. Oropharyngeal EBV shedding measured from 4 participants in each group. Spontaneous outgrowth of EBV‐infected B lymphocytes. Complications and drug reactions not mentioned | |
| Notes | Unclear whether patients had symptoms for 14 days or less. 2 patients in the placebo group and one in the ACV group had steroids | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Randomised code was available only to the pharmacist (pharmacist's role unclear) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States "double blinded" but unclear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States "double blinded" but unclear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete virological outcome table‐no explanation of why results were missing |
| Selective reporting (reporting bias) | High risk | Clinical outcomes not fully reported: only reported narratively with no actual results given |
| Other bias | High risk | Oropharyngeal EBV shedding measured from only 4 participants in each group. Complications and drug reactions not mentioned. Two patients in the placebo group and one in the ACV group had steroids Clinical studies were supported by a grant from Burroughs‐Wellcome company |
Simon 2003.
| Methods | Double‐blinded 3‐arm pilot RCT. Start and end dates not stated | |
| Participants | 45 children (aged 2 to 18) with symptoms of infectious mononucleosis for 1 week or less. Clinic setting. 25 male participants and 20 female participants included | |
| Interventions | 1. Valacyclovir 20 mg/kg tds for 14 days and placebo A OD for 5 days 2. Valacyclovir 20 mg/kg tds for 14 days plus prednisolone 1 mg/kg/day for 5 days 3. Placebo B tds for 14 days plus placebo A OD for 5 days |
|
| Outcomes | Sore throat, stomach ache, fatigue, swollen glands, headache, vomiting, rash, loss of appetite, nausea, sweats, chills, swollen eyes, runny nose, cough, and feeling bad were scored as either absent (0), mild (1), moderate (2), or severe (3) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "An independent pharmacy was in charge of random group assignment, medicine dispersement, and dosing". Unclear how randomisation was performed |
| Allocation concealment (selection bias) | Low risk | "An independent pharmacy was in charge of random group assignment, medicine dispersement, and dosing" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States "double blind" but does not elaborate further |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States "double blind" but does not elaborate further |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Patients that did not complete the study or had a missing result on day 20 had their last observation carried forward to day 20 prior to any analysis". Incomplete data in 1 of the results tables |
| Selective reporting (reporting bias) | High risk | Individual symptom scores were not all reported, but reported as composite scores: "selected score", "feeling bad score", "fatigue score" and "total score" |
| Other bias | High risk | Study funding source and conflicts of interest not stated. However, 2 authors were from a drug company. |
van der Horst 1991.
| Methods | Double‐blinded RCT. Start and end dates not stated | |
| Participants | 132 participants with a clinical and laboratory diagnosis of infectious mononucleosis. Age of participants was not reported. Setting also was unclear. Symptoms present for 1 week or less. 72 male and 48 female participants included in data analysis | |
| Interventions | Acyclovir 600 mg PO 5 times daily for 10 days versus placebo | |
| Outcomes | Clinical outcomes were measured on days 3, 5, 10, 30. Temperature, weight loss, pharyngitis, lymphadenopathy, hepatic and splenic enlargement were weighted and scored according to severity. Hours of bed rest, time to return to normal activities, appetite were also recorded. Virological outcomes were measured on days 0, 10, 30 | |
| Notes | "inadequate handling" of samples reported in study. Also 12 patients dropped out | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation code stratified by participant institution" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States "double blind" but does not elaborate further |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States "double blind" but does not elaborate further |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1. Did not use data from 12 participants who dropped out 2. Data missing from table of results with no explanation of what happened to these data |
| Selective reporting (reporting bias) | High risk | Many outcomes measured on days 3, 5, 10, 30 but only reported baseline data |
| Other bias | High risk | 1. Problem with handling of virological samples likely leading to inaccurate results 2. Baseline imbalances: "The only significant differences between the two groups were that more acyclovir recipients had a temperature > 37.5°C, and the mean pharyngitis score was slightly greater for acyclovir recipients." 3. Acyclovir and financial support provided by the Burroughs Wellcome Company |
EBV: Epstein Barr virus IV: intravenous OD: once daily PO: per oral (medication to be taken by mouth) RCT: randomised controlled trial TDS: ter die sumendum (medication to be taken three times daily) WBC: white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baranova 2013 | Not RCT; seemed to use interferon rather than an antiviral as the intervention |
| Bolden 1972 | Not our intervention of interest; examined corticosteroids |
| Candy 2002 | Not RCT |
| Duan 2009 | Not our intervention of interest; studied Chinese medicine |
| Ebell 2004 | Not RCT |
| Gordeets 2011 | Not RCT |
| Lin 1985 | Only examines in‐vitro studies |
| Sichko 1987 | Not our intervention of interest; studied ribonuclease |
| Sinha 1971 | This study examined methisazone which is an antiviral drug. However, it was never licensed in Ireland, and the UK licence was stopped in 1983 so we felt it was justified not to include this study. |
| Torre 1999 | Not RCT |
| Tynell 1996 | Intervention is acyclovir and steroid versus placebo. No arm with acyclovir on its own |
| Usami 2013 | Not RCT |
| Wood 1997 | Not RCT |
RCT: randomised controlled trial
Differences between protocol and review
For the outcomes ‘time to resolution of fever', 'time to resolution of lymphadenopathy', 'time to resolution of pharyngitis', 'time to resolution of splenomegaly' and 'time to resolution of hepatomegaly’ the review authors felt that these would be more appropriately named ‘duration of fever', 'duration of lymphadenopathy' 'duration of pharyngitis', 'duration of splenomegaly', and 'duration of hepatomegaly’ as they are not actually 'time to event' outcomes.
The title of one of the secondary outcomes was changed from 'Psychosocial outcomes' to 'Patient‐reported outcome measures'. We had planned to examine chronic fatigue (fatigue present for ≥ six months) as one of the specific complications of infectious mononucleosis. However, this was not reported in the included studies.
It was not possible to conduct subgroup analyses, as proposed, due to insufficient data.
Contributions of authors
All review authors have contributed to the production of the review.
Muireann De Paor: contributed to the selection of studies, contributed to the data extraction, 'Risk of bias' assessment, performed the data analyses and wrote the first draft of the review. Kirsty O'Brien: contributed to the selection of studies, data extraction and 'Risk of bias' assessment. Tom Fahey: supervised the data analysis, commented on the draft and contributed to the final version. Susan M Smith: contributed to the selection of studies, supervised the data analysis, commented on the draft and contributed to the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland.
The lead author was awarded a Cochrane Fellowship 2013 by the Health Research Board (HRB) for the purpose of completing this review.
Declarations of interest
Muireann De Paor: none known Kirsty O'Brien: none known Tom Fahey: none known Susan M Smith: none known.
New
References
References to studies included in this review
Andersson 1986 {published data only (unpublished sought but not used)}
- Andersson J, Britton S, Ernberg I, Andersson U, Henle W, Skoldenberg B, et al. Effect of acyclovir on infectious mononucleosis: a double‐blind, placebo‐controlled study. Journal of Infectious Diseases 1986;153:283‐90. [DOI] [PubMed] [Google Scholar]
- Andersson J, Skoldenberg B, Ernberg I, Britton S, Henle W, Andersson U. Acyclovir treatment in primary Epstein‐Barr virus infection. A double‐blind placebo‐controlled study. Scandinavian Journal of Infectious Diseases Supplement 1985;47:107‐15. [PubMed] [Google Scholar]
- Ernberg I, Andersson J. Acyclovir efficiently inhibits oropharyngeal excretion of Epstein‐Barr virus in patients with acute infectious mononucleosis. Journal of General Virology 1986;67:2267‐72. [DOI] [PubMed] [Google Scholar]
Andersson 1987 {published data only (unpublished sought but not used)}
- Andersson J, Skoldenberg B, Henle W, Giesecke J, Ortqvist A, Julander I, et al. Acyclovir treatment in infectious mononucleosis: a clinical and virological study. Infection 1987;15(Suppl 1):14‐20. [DOI] [PubMed] [Google Scholar]
Balfour 2007a {unpublished data only}
- Balfour HH Jr. Activity of valomaciclovir in infectious mononucleosis due to primary Epstein‐Barr virus infection (Mono6). Conference slides received by correspondence with the author. Further details obtained from Clinicaltrials.gov. 2007.
Balfour 2007b {published data only (unpublished sought but not used)}
- Balfour HH Jr. Controlled trial of valacyclovir in infectious mononucleosis. Clinicaltrials.gov Last Updated: October 26, 2011.
- Balfour HH Jr, Hokanson KM, Schacherer RM, Fietzer CM, Schmeling DO, Holman CJ, et al. A virologic pilot study of valacyclovir in infectious mononucleosis. Journal of Clinical Virology 2007;39:16‐21. [DOI] [PubMed] [Google Scholar]
Pagano 1983 {published data only (unpublished sought but not used)}
- Pagano JS, Sixbey JW, Lin JC. Acyclovir and Epstein‐Barr virus infection. Journal of Antimicrobial Chemotherapy 1983;12(Suppl B):113‐21. [DOI] [PubMed] [Google Scholar]
Simon 2003 {published and unpublished data}
- Simon MW, Deeter RG, Shahan B. The effect of valacyclovir and prednisolone in reducing symptoms of EBV illness in children: a double‐blind, placebo‐controlled study. International Pediatrics 2003;18:164‐9. [Google Scholar]
van der Horst 1991 {published data only (unpublished sought but not used)}
- Horst C, Joncas J, Ahronheim G, Gustafson N, Stein G, Gurwith M, et al. Lack of effect of peroral acyclovir for the treatment of acute infectious mononucleosis. Journal of Infectious Diseases 1991;164:788‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baranova 2013 {published data only}
- Baranova IP, Kurmaeva DIu. Comparative analysis of the effectiveness of antiviral therapy of children with infectious mononucleosis. Eksperimentalnaia i Klinicheskaia Farmakologiia 2013;76:47‐9. [PubMed] [Google Scholar]
Bolden 1972 {published data only}
- Bolden KJ. Corticosteroids in the treatment of infectious mononucleosis. An assessment using a double blind trial. Journal of the Royal College of General Practitioners 1972;22:87‐95. [PMC free article] [PubMed] [Google Scholar]
Candy 2002 {published data only}
- Candy B, Chalder T, Cleare AJ, Wessely S, White PD, Hotopf M. Recovery from infectious mononucleosis: a case for more than symptomatic therapy? A systematic review. British Journal of General Practice 2002;52:844‐51. [PMC free article] [PubMed] [Google Scholar]
Duan 2009 {published data only}
- Duan HM, Yan HM, Zhen XF, Pan YC, Yao Y, Chen L, et al. Clinically controlled study on children's infectious mononucleosis treated by Chinese medicine. Chinese Journal of Integrative Medicine 2009;15:347‐52. [DOI] [PubMed] [Google Scholar]
Ebell 2004 {published data only}
- Ebell MH. Eipstein‐Barr virus infectious mononucleosis. American Family Physician 2004;70:1279‐87. [PubMed] [Google Scholar]
Gordeets 2011 {published data only}
- Gordeets AV, Savina OG, Beniova SN, Chernikova AA. Infectious mononucleosis: etiology, immunological variants, methods of correction. Eksperimentalnaia i Klinicheskaia Farmakologiia 2011;74:29‐32. [PubMed] [Google Scholar]
Lin 1985 {published data only}
- Lin JC, Smith MC, Pagano JS. Comparative efficacy and selectivity of some nucleoside analogs against Epstein‐Barr virus. Antimicrobial Agents and Chemotherapy 1985;27:971‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sichko 1987 {published data only}
- Sichko ZhV, Kozlova OL, Karas'kova NG. Ribonuclease treatment of infectious mononucleosis in children. Pediatriia 1987;6:87‐8. [PubMed] [Google Scholar]
Sinha 1971 {published data only}
- Sinha AK. A pilot trial of methisazone in infectious mononucleosis and chickenpox. British Journal of Clinical Practice 1971;25:177‐8. [PubMed] [Google Scholar]
Torre 1999 {published data only}
- Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta‐analysis. Scandinavian Journal of Infectious Diseases 1999;31:543‐7. [DOI] [PubMed] [Google Scholar]
Tynell 1996 {published data only}
- Tynell E, Aurelius E, Brandell A, Julander I, Wood M, Yao QY, et al. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double‐blind, placebo‐controlled study. Journal of Infectious Diseases 1996;174:324‐31. [DOI] [PubMed] [Google Scholar]
Usami 2013 {published data only}
Wood 1997 {published data only}
- Wood MJ, Grundy JE, Kinghorn GR. The clinical evaluation of agents for the prophylaxis and treatment of herpesvirus infections. International Journal of Pharmaceutical Medicine 1997; Vol. 11, issue 6:310‐23.
Additional references
Aldrete 1992
- Aldrete JS. Spontaneous rupture of the spleen in patients with infectious mononucleosis. Mayo Clinic Proceedings 1992;67:910‐2. [DOI] [PubMed] [Google Scholar]
Andersson 1985
- Andersson J, Skoldenberg B, Ernberg I, Britton S, Henle W, Andersson U. Acyclovir treatment in primary Epstein‐Barr virus infection. A double‐blind placebo‐controlled study. Scandinavian Journal of Infectious Diseases Supplement 1985;47:107‐15. [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Auwaerter 1999
- Auwaerter PG. Infectious mononucleosis in middle age. Journal of the American Medical Association 1999;281:454‐9. [DOI] [PubMed] [Google Scholar]
Balfour 2005
- Balfour HH Jr, Holman CJ , Hokanson KM , Lelonek MM , Giesbrecht JE , White DR , et al. A prospective clinical study of Epstein‐Barr virus and host interactions during acute infectious mononucleosis. Journal of Infectious Diseases 2005;192:1505‐12. [DOI] [PubMed] [Google Scholar]
Balfour 2007
- Balfour HH Jr, Hokanson KM, Schacherer RM, Fietzer CM, Schmeling DO, Holman CJ, et al. A virologic pilot study of valacyclovir in infectious mononucleosis. Journal of Clinical Virology 2007;39(1):16‐21. [DOI] [PubMed] [Google Scholar]
Balfour 2013
- Balfour HH Jr, Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein‐Barr virus infection in university students. Journal of Infectious Diseases 2013;207(1):80‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brichkov 2006
- Brichkov I, Cummings L, Fazylov R, Horovitz JH. Nonoperative management of spontaneous splenic rupture in infectious mononucleosis: the role for emerging diagnostic and treatment modalities. American Journal of Surgery 2006;72(5):401‐4. [PubMed] [Google Scholar]
Buchwald 2000
- Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. American Journal of Medicine 2000;109:531‐7. [DOI] [PubMed] [Google Scholar]
Carter 1969
- Carter RL, Penman HG. Infectious Mononucleosis. Oxford and Edinburgh: Blackwell Scientific Publication, 1969. [Google Scholar]
Charles 2003
- Charles PGP. Infectious mononucleosis. Australian Family Physician 2003;32:785‐8. [PubMed] [Google Scholar]
Chen 2003
- Chen J, Konstantinopoulos PA, Satyal S, Telonis J, Blair DC. Just another simple case of infectious mononucleosis?. Lancet 2003;361(9364):1182. [DOI] [PubMed] [Google Scholar]
Devereaux 1999
- Devereaux CE, Bemiller T, Brann O. Ascites and severe hepatitis complicating Epstein‐Barr infection. American Journal of Gastroenterology 1999;94(1):236‐40. [DOI] [PubMed] [Google Scholar]
Dobson 2015
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. Lancet 2015;385:1729‐37. [DOI] [PubMed] [Google Scholar]
Ernberg 1986
- Ernberg I, Andersson J. Acyclovir efficiently inhibits oropharyngeal excretion of Epstein‐Barr virus in patients with acute infectious mononucleosis. Journal of General Virology 1986;67:2267‐72. [DOI] [PubMed] [Google Scholar]
Evans 1978
- Evans AS. Infectious mononucleosis and related syndromes. American Journal of the Medical Sciences 1978;276(3):325‐39. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.handbook.cochrane.org 2011.
Hozo 2005
- Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 20 April 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
HPRA 2016
- Health Products Regulatory Authority (HPRA) Ireland. NA [NA]. NA Accessed 19 May 2015; Vol. NA, issue NA:NA.
Hurt 2007
- Hurt C, Tammaro D. Diagnostic evaluation of mononucleosis‐like illnesses. American Journal of Medicine 2007;120(10):911.e1‐8. [DOI] [PubMed] [Google Scholar]
Jenson 2000
- Jenson HB. Acute complications of Epstein‐Barr virus infectious mononucleosis. Current Opinion in Pediatrics 2000;12(3):263‐8. [DOI] [PubMed] [Google Scholar]
Kawano 2013
- Kawano Y, Iwata S, Kawada J, Gotoh K, Suzuki M, Torii Y, et al. Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein‐Barr virus infection. Journal of Infectious Diseases 2013;208:771‐9. [DOI] [PubMed] [Google Scholar]
Kimberlin 2007
- Kimberlin DW, Whitley RJ. Chapter 64: Antiviral therapy of HSV‐1 and ‐2. In: Arvin A, Campadelli‐Fiume G, Mocarski E, et al. editor(s). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PubMed] [Google Scholar]
Klassen 2005
- Klassen TP, Hartling L, Wiebe N, Belseck EM. Acyclovir for treating varicella in otherwise healthy children and adolescents. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002980.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Lei 2000
- Lei PS, Lowichik A, Allen W, Mauch TJ. Acute renal failure: unusual complication of Epstein‐Barr virus‐induced infectious mononucleosis. Clinical Infectious Diseases 2000;31(6):1519‐24. [DOI] [PubMed] [Google Scholar]
Lennon 2014
- Lennon P, O'Neill JP, Fenton JE. Effect of metronidazole versus standard care on length of stay of patients admitted with severe infectious mononucleosis: a randomized controlled trial. Clinical Microbiology and Infection 2014;20(7):45‐2. [DOI] [PubMed] [Google Scholar]
Lennon 2015
- Lennon P, Crotty M, Fenton JE. Infectious mononucleosis. BMJ 2015;350:NA. [DOI] [PubMed] [Google Scholar]
Luzuriaga 2010
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. New England Journal of Medicine 2010;362(21):1993‐2000. [DOI] [PubMed] [Google Scholar]
Macsween 2010
- Macsween KF, Higgins CD, McAulay KA, Williams H, Harrison N, Swerdlow AJ, et al. Infectious mononucleosis in university students in the United Kingdom: evaluation of the clinical features and consequences of the disease. Clinical Infectious Diseases 2010;50(5):699‐706. [DOI] [PubMed] [Google Scholar]
Monem 1999
- Monem SA, O'Connor PF, O'Leary TG. Peritonsillar abscess and infectious mononucleosis: an association or a different presentation of the same condition. Irish Medical Journal 1999;92(2):278‐80. [PubMed] [Google Scholar]
Rea 2001
- Rea TD, Russo JE, Katon W, Ashley RL, Buchwald DS. Prospective study of the natural history of infectious mononucleosis caused by Epstein‐Barr virus. Journal of the American Board of Family Practice / American Board of Family Practice 2001;14:234‐42. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rezk 2015
- Rezk E, Nofal YH, Hamzeh A, Aboujaib MF, AlKheder MA, Al Hammad MF. Steroids for symptom control in infectious mononucleosis. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD004402.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straus 1993
- Straus SE, Cohen JI, Tosato G, Meier J. Epstein‐Barr virus infections: biology, pathogenesis, and management. Annals of Internal Medicine 1993;118:45‐58. [DOI] [PubMed] [Google Scholar]
van de Veerdonk 2012
- Veerdonk FL, Wever PC, Hermans MH, Fijnheer R, Joosten LA, Meer JW, et al. IL‐18 serum concentration is markedly elevated in acute EBV infection and can serve as a marker for disease severity. Journal of Infectious Diseases 2012;206:197‐201. [DOI] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220‐33. [DOI] [PubMed] [Google Scholar]
White 2001
- White PD, Thomas JM, Kangro HO, Bruce‐Jones WD, Amess J, Crawford DH, et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet 2001;358(9297):1946‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
De Paor 2015
- Paor M, O'Brien K, Fahey T, Smith SM. Antiviral agents for infectious mononucleosis (glandular fever). Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011487] [DOI] [PMC free article] [PubMed] [Google Scholar]


