Abstract
Background
Pterygium, a growth of the conjunctiva over the cornea, is a progressive disease leading in advanced stages to visual impairment, restriction of ocular motility, chronic inflammation and cosmetic concerns. Surgical removal is the treatment of choice, but recurrence can be a problem. Currently the best surgical option in terms of recurrence is conjunctival autograft. To date the most common surgical methods of attaching conjunctival autografts to the sclera are through suturing or fibrin glue. Each method presents its own advantages and disadvantages. Sutures require considerable skill from the surgeon and can be associated with a prolonged operation time, postoperative discomfort and suture‐related complications, whereas fibrin glue may give a decreased operation time, improve postoperative comfort and avoid suture‐related problems.
Objectives
To assess the effectiveness of fibrin glue compared to sutures in conjunctival autografting for the surgical treatment of pterygium.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2016), Embase (January 1980 to October 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 October 2016.
Selection criteria
We included randomised controlled trials (RCTs) in any setting where fibrin glue was compared with sutures to treat people with pterygium.
Data collection and analysis
Two review authors independently screened the search results, assessed trial quality, and extracted data using standard methodological procedures expected by Cochrane. Our primary outcome was recurrence of pterygium defined as any re‐growth of tissue from the area of excision across the limbus onto the cornea. The secondary outcomes were surgical time and complication rate. We graded the certainty of the evidence using GRADE.
Main results
We included 14 RCTs conducted in Brazil, China, Egypt, India, Malaysia, New Zealand, Philippines, Saudi Arabia, Sweden and Turkey. The trials were published between 2004 and 2016, and were assessed as a mixture of unclear and low risk of bias with three studies at high risk of attrition bias. Only adults were enrolled in these studies.
Using fibrin glue for the conjunctival autograft may result in less recurrence of pterygium compared with using sutures (risk ratio (RR) 0.47, 95% CI 0.27 to 0.82, 762 eyes, 12 RCTs; low‐certainty evidence). If pterygium recurs after approximately 10 in every 100 surgeries with sutures, then using fibrin glue may result in approximately 5 fewer cases of recurrence in every 100 surgeries (95% CI 2 fewer to 7 fewer cases). Using fibrin glue may lead to more complications compared with sutures (RR 1.92; 95% CI 1.22 to 3.02, 11 RCTs, 673 eyes, low‐certainty evidence). The most common complications reported were: graft dehiscence, graft retraction and granuloma. On average using fibrin glue may mean that surgery is quicker compared with suturing (mean difference (MD) ‐17.01 minutes 95% CI ‐20.56 to ‐13.46), 9 RCTs, 614 eyes, low‐certainty evidence).
Authors' conclusions
The meta‐analyses, conducted on people with pterygium in a hospital or outpatient setting, show fibrin glue may result in less recurrence and may take less time than sutures for fixing the conjunctival graft in place during pterygium surgery. There was low‐certainty evidence to suggest a higher proportion of complications in the fibrin glue group.
Plain language summary
Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery
What is the aim of this review? The aim of this Cochrane Review was to find out if it is better to use fibrin glue or sutures (stitches) when operating on a pterygium (unwanted growth of tissue on the front of the eye). The operation involves replacing the pterygium with a piece of tissue from another part of the eye (autograft). Cochrane researchers collected and analysed all relevant studies to answer this question and found 14 studies.
Key messages Using fibrin glue when doing the graft may result in a lower chance of recurrence of the pterygium. The operation may take less time as well. Fibrin glue may be associated with more complications such as a rupture of the graft, the graft shrinking and the development of an area of inflammation (granuloma).
What was studied in the review? Sometimes a piece of tissue can grow on the front of the eye, and if it grows large enough it can affect vision. This tissue is known as a pterygium. People who live in hot, dusty places with high sunlight are more likely to get a pterygium. A pterygium can be uncomfortable and itchy, and may affect the appearance of the eye.
Doctors can remove this tissue and replace it with tissue from another part of the body, usually from another part of the conjunctiva (which covers the white part of the eye). This is known as a graft or autograft.
Cochrane researchers looked at two different methods of attaching this graft during pterygium surgery, either with fibrin glue or with stitches.
What are the main results of the review? The review authors found 14 relevant studies. The studies were from Brazil, China, Egypt, India, Malaysia, New Zealand, Philippines, Saudi Arabia, Sweden and Turkey. These studies compared fibrin glue to stitches in people having their pterygium removed and a graft of tissue from the conjunctiva.
Using fibrin glue during pterygium surgery may result in fewer cases of recurrence of pterygium compared with using stitches (low‐certainty evidence). It may take less time to do a pterygium operation and graft with fibrin glue (low‐certainty evidence). There may be a higher chance of some complications with fibrin glue, such as a rupture of the graft, the graft shrinking or development of an area of inflammation (granuloma).
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to 14 October 2016.
Summary of findings
for the main comparison.
| Fibrin glue compared with sutures for primary pterygium | ||||||
|
Patient or population: individuals with pterygium Settings: hospital or outpatients Intervention: fibrin glue Comparison: sutures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sutures | Fibrin glue | |||||
|
Recurrence of pterygium (follow‐up 2 to 24 months) |
100 per 1000 | 47 per 1000 (27 to 82) | RR 0.47 (0.27 to 0.82) | 762 (12 RCTs) |
⊕⊕⊝⊝ low1 2 | Large variability in duration of follow‐up |
| Occurrence of complication | 70 per 1000 | 134 per 1000 (85 to 211) | RR 1.92 (1.22 to 3.02) | 673 (11 RCTs) |
⊕⊕⊝⊝ low1 2 | |
| Operating time | The mean operating time ranged across control groups from 27 to 67 minutes | The mean operating time in the intervention groups was17.0 minutes less (from 20.6 minutes less to 13.5 minutes less) | 614 (9 RCTs) |
⊕⊕⊝⊝ low1 3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; Std: standardised | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low‐certainty: we are very uncertain about the estimate | ||||||
1Downgraded 1 level for risk of bias due to study limitations, in particular generation of allocation sequence, allocation concealment, and incomplete outcome data. 2.Downgraded 1 level for imprecision as there were relatively few events. 3 Downgraded 1 level for inconsistency (I2 = 96%) although all studies favoured fibrin glue.
Background
Primary pterygium is one of the most common eye diseases, affecting a large percentage of the population, especially those living between the Tropics of Cancer and Capricorn. Effectively the incidence is high in geographic areas with high ultraviolet radiation (Moran 1984) or hot, dry, windy, dusty and smoky environments (Nakaishi 1997; Norn 1991), however hereditary factors also play a role (Anguria 2014; Romano 2016). The estimated prevalence of pterygium is variable, ranging from 0.7% to 31%.
Surgical removal is the treatment of choice. For many years, the surgical management of pterygium involved a simple excision of the excessive tissue mass overlying the cornea and the adjacent sclera, leaving a wide area of bare sclera. However, recurrence of the pterygium was unacceptably high, in as many as 89% of cases (Cameron 1965; Youngson 1972). To improve surgical results, two strategies have been adopted: the destructive approach, which enhances the effect of excision by radiation and chemotherapy (mitomycin C (MMC), thiotepa, 5‐fluorouracil, beta‐irradiation) and the reconstructive approach, namely transplantation of various tissue grafts (conjunctival autograft, amniotic membrane transplantation, mucous membrane graft, conjunctival limbal transplantation). Recurrences after conjunctival autograft vary from 0% to 39% (Cano‐Parra 1995; Chen 1995; Hirst 2009; Jaros 1988; Kenyon 1985; Lewallen 1989; Sebban 1991a; Sebban 1991b) and from 0% to 70% after adjunctive chemotherapy. Most studies have demonstrated that conjunctival autograft and MMC application are highly successful and equally effective (0% to 38% recurrence rate) (Cano‐Parra 1995; Chen 1995; Hayasaka 1988; Mahar 1993; Singh 1988). However, severe complications may occur following MMC therapy, such as melting of the conjunctiva and sclera, and even perforation (Dunn 1991; Mackenzie 1991; Rubinfeld 1992). Currently several studies have demonstrated that conjunctival autograft is the best method to avoid recurrence (Al Fayez 2002; Chen 1995; Kenyon 1985; Prabhasawat 1997). To date the most common surgical methods of attaching conjunctival autografts to the sclera are through suturing or fibrin glue. Each one presents its own advantages and disadvantages. Sutures require major ability of the surgeon and can be associated with a prolonged operation time, postoperative discomfort and suture‐related complications, whereas fibrin glue gives a decreased operation time, improves postoperative comfort and avoids suture‐related problems. However currently the results of efficacy of fibrin glue and sutures in pterygium surgery are not completely consistent.
The aim of this review is to summarise and compare outcomes of tissue glue and sutures that are currently used to attach conjunctival autografts in pterygium surgery.
Description of the condition
Primary pterygium is a fibrovascular wing of tissue extending onto the cornea, generally situated on the nasal side. Before it enters the optical zone, an advancing pterygium can cause localised flattening of the horizontal meridian of the cornea to the leading apex (Bedrossian 1960; Fong 1998; Gasser 2016; Hansen 1980; Holladay 1985; Lin 1998; Oldenburg 1990; Starck 1991; Stern 1998; Tomidokoro 1999; Walland 1994), often resulting in with‐the‐rule astigmatism (Lin 1997). The pterygium is conventionally divided into three parts of a triangle: a 'head', a 'neck' and a 'body'. The former is the apex of the triangle that points towards the cornea and it is connected to the 'body' by the 'neck'. The pathogenesis of pterygium is still not fully understood. Recent studies have shown alterations of limbal stem cells along the affected pterygial area. UV‐B causes mutations at the limbal edge, resulting in apoptosis and altering the production of various growth factors. Indications for surgery include visual impairment (Bedrossian 1960; Oldenburg 1990; Starck 1991), restriction of ocular motility, chronic inflammation and cosmetic concerns. Many procedures have been suggested, with or without adjunctive techniques that aim to lower the recurrence rate (Akarsu 2003; Avisar 2003; Chapman‐Smith 1992; Dadeya 2002; Mackenzie 1991).
Description of the intervention
The goal of surgical treatment is the excision of the lesion, the prevention of its recurrence and the restoration of the integrity of the ocular surface. Several different techniques have been described (Haik 1962; Kenyon 1985; King 1950; Prabhasawat 1997; Singh 1988; Vastine 1982). Briefly, the operation is performed under peribulbar anaesthesia. The eye is prepped and draped in the standard fashion and a lid speculum is inserted. When removing the pterygium, the first step involves dissection and excision of the head off the cornea. This allows for exposure of the underlying fibrovascular tissue that can then be accurately removed by dissection up to the insertion of the medial rectus muscle (if the pterygium is situated on nasal side, as usually happens). It is important in this phase to preserve as much surrounding conjunctival tissue as possible. Abnormal scar tissue on the corneal and scleral surface is also removed. The scleral bed is then measured and a very thin piece of autologous conjunctiva, 1 mm larger than this area, is excised from the superior bulbar conjunctiva. Care is taken not to include Tenon's tissue when dissecting the graft, in order to avoid postoperative oedema and graft retraction. The conjunctival graft is secured to the sclera, taking care to maintain the polarity of the tissue, with interrupted 7.0 to 8.0 Vicryl or 10.0 nylon sutures, or episclerally with fibrin glue added either sequentially or simultaneously with a double‐barrel syringe. This review will consider all types of sutures and glues currently used for conjunctival autograft.
How the intervention might work
The use of natural substances such as fibrin may have significant advantages over traditional suturing techniques (Calson 1987). Fibrin glue is a blood‐derived product that consists of two biologic components: fibrinogen and thrombin. When the components are mixed and fibrinogen is activated by thrombin, an adhesive fibrin network is formed in 30 seconds. Within days, the two components of the fibrin glue are digested, but in the meantime a strong network has been formed between the transplant and the underlying sclera. In the last 10 years, fibrin adhesives have been used to close cataract incisions (Henrick 1987), to attach soft tissue in oculoplastic surgery (Gosain 2002), to attach conjunctiva in strabismus (Dadeya 2001; Mohan 2003; Spierer 1997), to treat leaking blebs (Wright 1998) in glaucoma surgery (O'Sullivan 1996), and to close macular holes in retinal surgeries (Tilanus 1995). Recent studies have demonstrated that the use of fibrin glue in pterygium surgery might be an ideal alternative to suturing because it shortens the time of the surgical procedure, is easy to use and is associated with less postoperative inflammation, discomfort and recurrence.
Why it is important to do this review
The use of sutures in pterygium surgery is associated with postoperative inflammation, discomfort and complications related to the sutures themselves, such as granuloma formation, suture abscesses, buttonholes, tissue necrosis, and giant papillary conjunctivitis (Sridhar 2002; Tan 1997; Ti 2000). This systematic review will compare the results obtained with sutures to those with fibrin glue in conjunctival autografting procedures for the treatment of pterygium, which present advantages related to surgical skills, postoperative complications and patient's discomfort. This information is of interest not only to ophthalmic surgeons and ophthalmologists, but also to other healthcare professionals and patients.
Objectives
To assess the effectiveness of fibrin glue compared to sutures in conjunctival autografting for the surgical treatment of pterygium.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in any setting (hospital or outpatient basis), as planned in the protocol (Romano 2014).
Types of participants
Participants in the trials were people with advancing pterygium, especially people with visual impairment, restriction of ocular motility, chronic inflammation and cosmetic concerns. We excluded trials which recruited participants with connective tissue disease, previous ocular surgery and with other ocular disease.
Types of interventions
We included trials that compared fibrin glue (Tisseel or Full Link or Beriplast P or Quixil) to sutures (Vicryl 8/0 or Vicryl 7/0 or nylon 10‐0).
Types of outcome measures
Primary outcomes
Recurrence of pterygium: any re‐growth of tissue from the area of excision across the limbus onto the cornea, calculated by the frequency of recurrences at six months after surgery. If the study's follow‐up was longer, we considered the last follow‐up.
Secondary outcomes
Surgical time: the time spent to complete the surgery. We considered the mean and standard deviation.
Complication rate: the occurrence of at least one of the following major complications such as dehiscence, displacement or loss of the autograft, infection, haemorrhage, oedema, fibrosis, retraction and other indications that required special treatment.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2016), Embase (January 1980 to October 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 October 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We also searched the reference lists of all potentially relevant trials to identify further reports of studies.
Data collection and analysis
Selection of studies
Two review authors (VR and LC) independently assessed the search results to see if they met the inclusion criteria. We specified reasons for exclusion of studies.
The process for selecting studies for inclusion in a review was in accordance with the following procedures, as described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Merge search results using reference management software (ProCite) and remove duplicate records of the same report.
Examine titles and abstracts to remove obviously irrelevant reports.
Retrieve the full text of potentially relevant reports.
Link together multiple reports of the same study.
Examine full‐text reports for compliance of the studies with the eligibility criteria.
Correspond with investigators, where appropriate, to clarify study eligibility.
Make final decisions on study inclusion and proceed to data collection.
We planned to resolve any disagreements that arose through discussion.
Data extraction and management
Two review authors (VR and LC) independently extracted data from the selected trials using a standardised data extraction form, as suggested in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We considered items related to source, eligibility, methods, participants, interventions, outcomes and results. Further information can be found in Appendix 7.
Assessment of risk of bias in included studies
Each review author independently assessed the risk of bias of each trial using a simple form and followed the domain‐based evaluation as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We compared the assessment results and discussed any discrepancies between ourselves. We aimed to achieve agreement on the final assessment for each criteria by discussion.
We assessed each domain as 'low risk of bias', 'unclear risk of bias' or 'high risk of bias'. We evaluated the following domains.
Randomisation sequence generation
Allocation concealment
Blinding (masking) of outcome assessors
Incomplete outcome data
Selective outcome reporting
Free of other bias (e.g. baseline imbalance, early stopping)
A detailed list of items considered and measures of assessment is provided in Appendix 8.
Measures of treatment effect
We used Review Manager 5.3 (RevMan) (RevMan 2014) to analyse the data.
We have treated the recurrence of pterygium and complication rate as dichotomous variables and have calculated them using the risk ratio (RR) with 95% confidence intervals (CI).
We have treated the surgical time as a continuous variable and calculated it using the mean difference (MD) with 95% CI.
In accordance with Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) we checked for skewed data by calculating the observed mean minus the lowest possible value and dividing this by the standard deviation. A ratio of less than 2 suggested skewness; if the ratio was less than 1 there was strong evidence of a skewed distribution. When the data were skewed, we presented results on a log scale as geometric means and ratios.
Unit of analysis issues
Trials may be parallel group (people randomised to treatment) or within‐person (eyes randomly allocated to treatment). The following table summarises the possible designs and issues in the analysis.
| Type of study | Number of eyes enrolled | Number of eyes reported | Analysis | |
| 1 | Parallel group | One | One | No unit of analysis issue. Criteria for selection of study eye should be specified |
| 2 | Parallel group | Two | One | No unit of analysis issue. Criteria for choice of eye should be specified in the trial protocol and could include worst, best, average of two eyes, right eye, left eye |
| 3 | Parallel group | Two | Two | Ideally effect estimates should be adjusted for within‐person correlation |
| 4 | Within‐person | Two | Two | Ideally study should do a pair‐matched analysis |
We documented the type of study design and approach to the analysis of people and eyes. In the case of categories 3 and 4, which may represent unit of analysis issues, we contacted study investigators for further clarification or data, as needed.
Dealing with missing data
We assessed the percentages of dropouts overall for each included trial and per each randomisation arm and we evaluated whether an intention‐to‐treat (ITT) analysis was performed or could be performed, from the available published information.
In order to allow us to undertake an ITT analysis, we sought data from the trial authors on the number of participants by treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
When additional data were needed, we contacted the corresponding author of each study by email in order to access further information. When missing data were not available, we limited our analysis to the available data.
Assessment of heterogeneity
We used the I2 statistic to measure statistical heterogeneity among the trials in each analysis. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error (Higgins 2003). We considered there to be substantial statistical heterogeneity if the I2 statistic was greater than 50% (Deeks 2011; Higgins 2003; Higgins 2011b). However, we considered the importance of the I2 value by taking into consideration the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P values from the Chi2 test, or confidence intervals for I2). Where possible, we explored clinical heterogeneity according to study setting and characteristics of participants).
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' in Appendix 8), we attempted to contact study authors and asked them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. The assessment focused on the primary outcome and, among secondary outcomes, on complication rate.
We also assessed publication bias (the publication or non‐publication of research findings, depending on the nature and direction of the results) by using a funnel plot to graphically illustrate variability between trials. If asymmetry was detected, we explored causes other than publication bias. We produced a funnel plot if 10 or more RCTs were included.
Data synthesis
We used the random‐effects model and used the fixed‐effect model as a sensitivity analysis for evaluating the possible bias effects of smaller studies, or when there were fewer than three studies.
Subgroup analysis and investigation of heterogeneity
We did not plan to conduct subgroup analysis.
Sensitivity analysis
If a sufficient number of trials was identified, we planned to conduct a sensitivity analysis excluding low‐quality studies (Higgins 2011b). Because the possibility of masking in these trials was unlikely, we used only items in the domain of randomisation (selection bias), incomplete outcome data (attrition bias), selective outcome reporting, and other bias.
'Summary of findings' table
We have included a 'Summary of findings' table (GRADEpro 2014) to give a concise overview and synthesis of the volume and certainty of the evidence for these comparisons.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 174 references (Figure 1). The Cochrane Information Specialist removed 87 duplicate records and we screened the remaining 87 reports. We rejected 70 records after reading the abstracts and obtained the full‐text reports of 17 references for further assessment. We identified 14 studies which met the inclusion criteria, see Characteristics of included studies and excluded one study (CTRI/2013/06/00376). There are two ongoing studies for which results are not currently available, see Characteristics of ongoing studies, If we are able to access the results for these studies, we will include them in further updates of this review.
1.
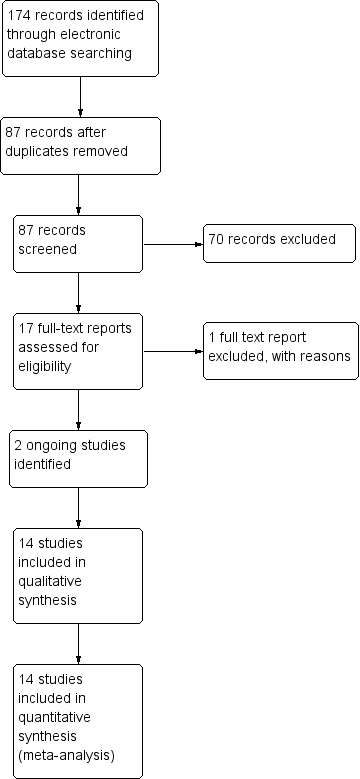
Study flow diagram
Included studies
We included clinical trials which used randomisation. The sample size achieved and analysed was 811 eyes for the recurrence rate, 722 eyes for complication rate and 614 eyes for operating time. The RCTs were conducted in Brazil, China, Egypt, India, Malaysia, New Zealand, Philippines, Saudi Arabia, Sweden and Turkey. The clinical trials were conducted in academic or hospital settings. All the trials included adult participants where there was evidence of visual impairment, restriction of ocular motility, chronic inflammation and cosmetic concerns. All the RCTs compared fibrin glue to suture using the same technique for pterygium excision. The follow‐up range was between 6 months and 24 months. In particular, two studies reported results at 24 months, nine at 12 months, five at 6 months, and one between 9 and 13 months. Not all included studies reported all outcomes evaluated in this meta‐analysis; Al‐Fayez 2008 and Ozdamar 2008 did not report operating time (see Characteristics of included studies for further details).
Excluded studies
We excluded CTRI/2013/06/00376 as this study was a randomised trial that compared autologous blood versus fibrin glue but there was no comparison with sutures.
Risk of bias in included studies
2.
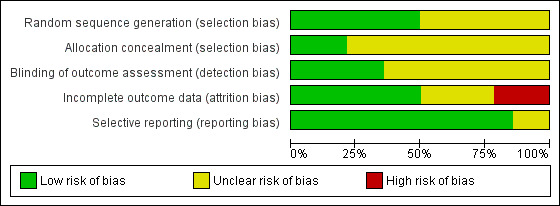
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
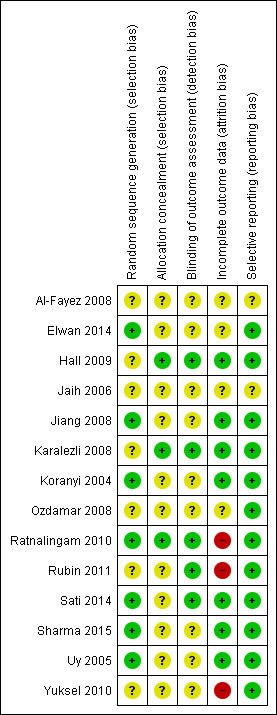
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Generation of allocation sequence
In seven studies (Al‐Fayez 2008; Hall 2009; Jaih 2006; Karalezli 2008; Ozdamar 2008; Rubin 2011; Yuksel 2010) investigators stated that the participants were randomly assigned to interventions and control, but the method used to achieve randomisation was not explicit; thus we judged this domain as 'unclear risk of bias'. The others studies reported how they generated the allocation sequence (e.g. computer‐generated, centrally‐randomised, tossing a coin) and we have judged this domain as 'low risk of bias'.
Allocation concealment
We made a judgement of 'low risk of bias' for this domain in three studies with central randomisation or with measures aimed to reduce intraobserver bias and minimise the influence of the known surgical technique (Hall 2009; Karalezli 2008; Ratnalingam 2010). In the remaining studies it was unclear if adequate measures were taken to ensure that investigators were unaware of the upcoming assignment. Therefore we judged this domain as 'unclear risk of bias' for these studies.
Blinding
We did not assess masking of participants or personnel to the intervention, as it is difficult to mask participants or personnel (clinicians for example) to the intervention. We evaluated the masking of outcome assessor to treatment allocation. However, as trials used two different types of intervention (sutures or glue), masking of outcome assessors could have been problematic, unless assessment was performed by someone not involved in the study.
All the included trials were reported as open‐label studies; however, masking probably had limited importance for more objective outcomes (such as some of the primary outcomes), because the risk of ascertainment bias was limited, but not for all outcomes. We made a judgement of 'unclear risk of bias' for nine studies (Al‐Fayez 2008; Elwan 2014; Jaih 2006; Jiang 2008; Koranyi 2004; Ozdamar 2008; Sharma 2015; Uy 2005; Yuksel 2010), and of 'low risk of bias' for the remaining studies that stated that particular attention was paid to limiting potential judgement biases.
Incomplete outcome data
Seven trials clarified the number of and reasons for dropouts (Hall 2009; Jiang 2008; Karalezli 2008; Koranyi 2004; Sati 2014; Sharma 2015; Uy 2005), and we made a judgement of 'low risk of bias' for this domain. Four trials did not provide sufficient information to permit a judgement (Al‐Fayez 2008; Elwan 2014; Jaih 2006; Ozdamar 2008). Three trials (Ratnalingam 2010; Rubin 2011; Yuksel 2010), judged at 'high risk of bias', were not on ITT basis and did not provide information on reasons and distribution of withdrawal.
Selective reporting
There was no evidence of selective outcome reporting in the large majority of included trials and it appeared that the outcomes reported were comparable to those specified in the methods section of the reports. Two studies, however, did not provide sufficient information to provide a judgement.
Publication bias was assessed by visual inspection of funnel plots (Figure 4; Figure 5. Funnel plots had a symmetric appearance for all the outcomes analysed
4.
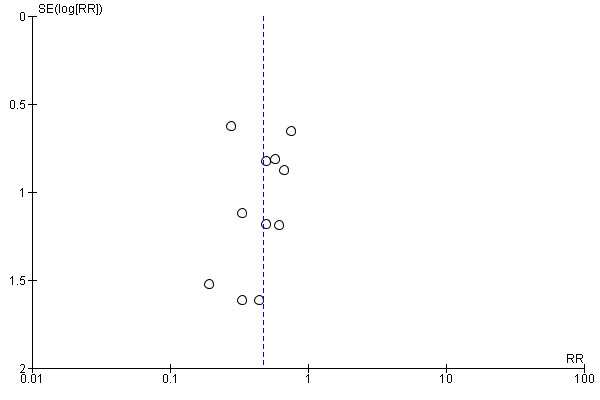
Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.1 Recurrence of pterygium.
5.
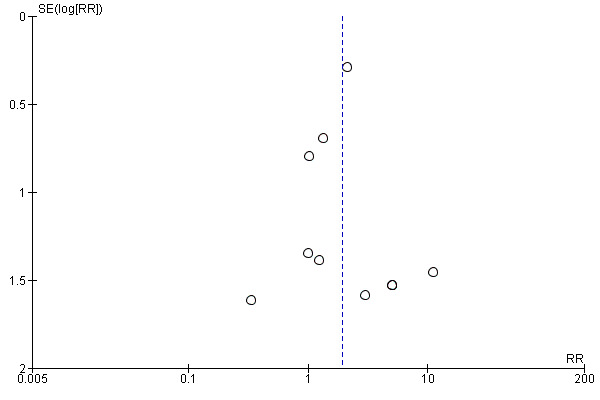
Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.2 Occurrence of 1 or more complications.
Effects of interventions
See: Table 1
See Table 1.
We conducted three meta‐analyses for recurrence of pterygium (primary outcome), surgical time and complication rate (secondary outcomes). The meta‐analyses for recurrence of pterygium shows that the recurrence of pterygium is less in the fibrin glue group (Analysis 1.1) (low‐certainty evidence). Analysing 762 procedures, there were 15 recurrences in the fibrin glue group and 41 in the suture group with a risk ratio of 0.47. Instead, taking into account 673 procedures and looking at the complication rate there was evidence of a higher rate of complications in the fibrin glue group (Analysis 1.2) (low‐certainty evidence), while our analysis reports a significant reduction in surgical time using a fibrin glue (Analysis 1.3) (low‐certainty evidence). The main results of the meta‐analyses (effect estimate and 95% confidence intervals (CIs)) are respectively: risk ratio (RR) 0.47; 95% CI 0.27 to 0.82 for recurrence of pterygium; RR 1.92; 95% CI 1.22 to 3.02 for complication rate, and mean difference ‐17.01; 95% CI ‐20.56 to ‐13.46 for operating time.
1.1. Analysis.
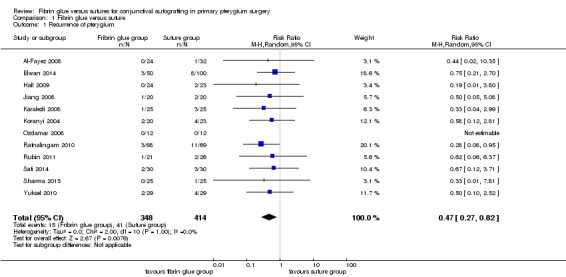
Comparison 1 Fibrin glue versus suture, Outcome 1 Recurrence of pterygium.
1.2. Analysis.
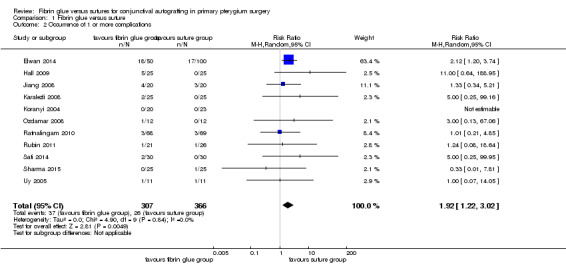
Comparison 1 Fibrin glue versus suture, Outcome 2 Occurrence of 1 or more complications.
1.3. Analysis.
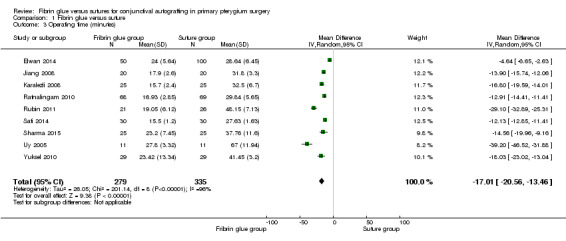
Comparison 1 Fibrin glue versus suture, Outcome 3 Operating time (minutes).
In sensitivity analysis, the exclusion of three studies at high risk of bias (Ratnalingam 2010; Rubin 2011; Yuksel 2010) had marginal impact on effect estimates: RR 0.54; 95% CI 0.27 to 1.08 for recurrence of pterygium (10 studies, 542 eyes; Analysis 2.1); RR 2.16; 95% CI 1.32 to 3.51 for complication rate (9 studies, 489 eyes; Analysis 2.2); and mean difference ‐15.59; 95% CI ‐19.90 to ‐11.28 for operating time (6 studies, 372 eyes; Analysis 2.3). For all the comparisons, we used a random‐effects model.
2.1. Analysis.
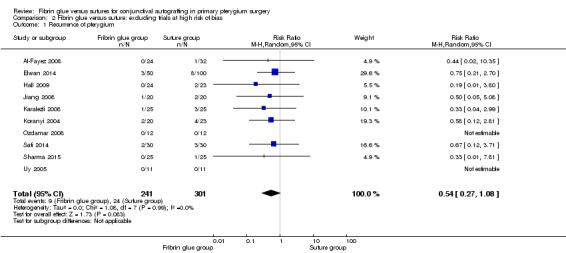
Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 1 Recurrence of pterygium.
2.2. Analysis.
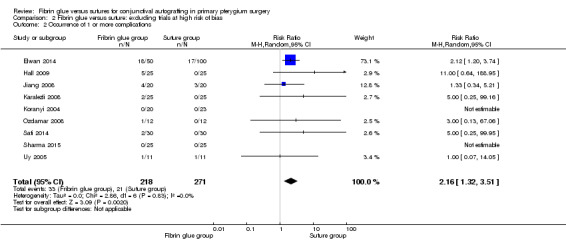
Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 2 Occurrence of 1 or more complications.
2.3. Analysis.
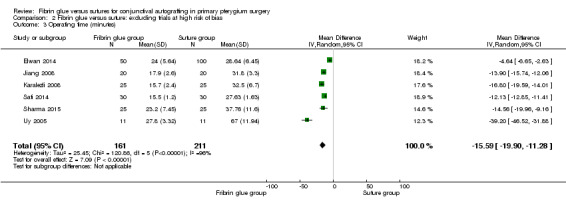
Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 3 Operating time (minutes).
Sensitivity analysis using a fixed‐effect model showed similar estimates.
There were no reports on any adverse event due to fibrin glue or suture use in the studies analysed.
Discussion
Conjunctival autograft transplantation is the most effective surgical procedure to prevent recurrence of pterygium and ensure a white cosmetic conjunctiva, with no persistent symptoms. However there is still no clear evidence to suggest whether the preferred option to fix the conjunctival autograft is fibrin glue or sutures. We conducted three meta‐analyses to answer this question in terms of recurrence of pterygium, complication rate and surgical time.
Summary of main results
Pterygium recurrence is the most common complication after its removal; its etiopathogenesis is multifactorial. Our meta‐analysis points out the benefits of using fibrin glues in terms of recurrence rate (RR 0.47; 95% CI 0.27 to 0.82). Exclusion of studies at high risk of bias led to a reduction of the effect size for the outcome recurrence of pterygium, though a trend towards a reduction of recurrences was still evident (RR 0.54; 95% CI 0.27 to 1.08). The reasons could be attributed to reduced postoperative inflammation (Kheirkhah 2008) and an immediate adherence of the graft, which plays a crucial role inhibiting fibroblast ingrowth, encouraging earlier vascularisation of the graft and reducing the recurrence (Zauberman 1988). Instead this adherence is not achieved by sutures, because they can only fix the edges of the graft, having no direct apposition of the graft in proximity to the underlying episclera. Also the different tensile strength between sutures and fibrin glue could have a determinant role.
The success rate also depends on surgical time, and its increment is directly associated with an increase in postoperative inflammation. The operating time is also correlated to the surgeon's skills. In fact Ti 2000 attributed the variability of success rate of sutured conjunctival autograft to learning curves and different skill levels. The results highlight the benefits of fibrin glue in terms of operating time. Using fibrin glue with conjunctival autograft transplantation shortens the surgical time, and makes the procedure easier with minimal manipulation of the graft and therefore less inflammation. Consequently, better results may be more consistently achieved despite differences in surgical expertise.
Our results report a higher complication rate in the fibrin glue group compared to the suture group (Table 2). It must be noted that the complication rates especially depend on graft preparation, graft manipulation, surgical experience and participant selection. In the fibrin group graft dehiscence is the most common complication (Srinivasan 2007), however it is usually associated with eye trauma or a person rubbing their eyes. A meticulous graft preparation (thin donor conjunctival autograft and free of Tenon's capsule) improves the success rate of graft uptake. Foroutan 2011 reported 13.33% cases of graft dehiscence and attributed this to a low concentration of thrombin and fibrinogen in autologous glue compared to a commercial preparation. Also the graft loss in the case of fibrin glue is a common complication or consequence of dehiscence. The precautions suggested by experts to avoid it are to ensure that the conjunctival autograft and conjunctiva are properly adhered; that fibrin glue is cleared from the ocular surface; and that no Tenon’s capsule remains between the graft and the conjunctiva. Graft retraction is the most common complication in the suture group. Tan 1999 attributed it to sub‐conjunctival fibrosis and suggested a meticulous dissection of sub‐epithelial graft tissue. Different authors (Malik 2012; de Wit 2010) postulated that no direct tension on the free edges, which occurs using fibrin glue, results in reduced stimulus for sub‐conjunctival scar formation; apposition of the eyelids to the bulbar conjunctiva provides a natural biological dressing, and confers a unique wound‐healing environment and a smooth, frictionless surface. The eyelids are able to provide compression and a vascular bed with immune capability in close proximity to the injury site. It must also be noted that intraoperative complications are not very common. However, there are some intraoperative advantages using fibrin glue such as the possibility of still using grafts with buttonholes, and the ability to be more efficient with unco‐operative patients who persistently move their eyes. In these people, the suturing process can be very difficult. We believe that complications, such as graft dehiscence, should be expected but they can be minimised: maintaining a dry scleral bed prior to applying the tissue glue and graft; educating the patient; avoiding eye rubbing postoperatively; and patient selection may reduce this risk.
1. Complications of interventions.
| Complications | Fibrin glue | Suture |
| Conjunctival cyst | 2/372 | 0/439 |
| Dellen | 1/372 | 3/439 |
| Graft dehiscence | 7/372 | 2/439 |
| Graft loss | 3/372 | 0/439 |
| Graft overlying the limbus | 0/372 | 1/439 |
| Graft retraction | 7/372 | 6/439 |
| Granuloma | 4/372 | 11/439 |
| Subconjunctival haemorrhage | 3/372 | 0/439 |
Overall completeness and applicability of evidence
There was variability across studies related to different race of participants, the use of different kinds of sutures and fibrin glue, and different length of follow‐up and in particular some follow‐up time was short.
Certainty of the evidence
We judged the evidence for all three outcomes to be low‐certainty. We downgraded for risk of bias as we judged many studies at unclear risk of selection and detection bias and some at high risk of attrition bias. We downgraded recurrence and complications outcomes for imprecision as there were few events. We downgraded the operating time outcome for inconsistency as there was high statistical heterogeneity.
Potential biases in the review process
As far as we are aware we have minimised potential biases in the review process. We have followed the methods set out in the published protocol (Romano 2014). The only amendment was to add in a summary of findings table and GRADE assessment as required by new Cochrane standards.
Agreements and disagreements with other studies or reviews
Other retrospective studies, such as the large cohort reported by Koranyi 2005 where they studied 461 procedures with follow‐up ranging from 6 to 12 months reported a recurrence rate of 5.3% for the fibrin glue group and 13.5% in the suture group. They also found all the recurrences occurred within six months after surgery. Marticorena 2006 and Uy 2002 suggested that the use of fibrin glue reduced postoperative discomfort. A previous meta‐analysis of pterygium recurrence after surgery concluded that simple bare sclera resection alone is associated with six times higher odds of pterygium recurrence if a conjunctival autograft was not used and 25 times higher odds of recurrence if mitomycin C was not used. The authors recommended avoiding simple bare sclera excision (Sanchez‐Thorin 1998). However, the use of MMC can be associated with sight‐threatening complications such as corneoscleral melt, cataract, uveitis, secondary glaucoma, and symblepharon (Amano 2000; Hayasaka 1988; Rubinfeld 1992).
There are some drawbacks to using the fibrin glue technique, mainly the cost. Most surgeons recommend dividing one vial of fibrin glue between six to 10 patients, reducing the costs, but that means that all the patients must be operated on the same day. However, as the use of fibrin glue reduces the risk of recurrence, the cost of a second surgery will probably be avoided. In addition, reducing the surgical time provides, according to previous published data from the USA, a saving of USD 67.50/min (Montgomery 2007) in terms of surgical operating room expense. Therefore, just a reduction in surgical time would make it cost‐effective compared with sutures and without the need to split the use of the glue over a number of cases. Another theoretical drawback is the risk of transmission of infectious agents, such as parvovirus B19 (Morita 2000) and prions (Hino 2000), as fibrin glue is made from blood products. Other concerns include the risk of transmission of blood‐borne diseases and anaphylaxis. However, careful selection of donors and improved viral inactivation techniques has largely minimised this problem. Autologous fibrin could be the solution, however it is yet to be used widely because of the time taken to procure the fibrin and lack of laboratory facilities at all centres.
Authors' conclusions
Implications for practice.
The meta‐analyses, conducted on people with pterygium in a hospital or outpatient setting, show fibrin glue may result in less recurrence and may take less time than sutures for fixing the conjunctival graft in place during pterygium surgery. There was low‐certainty evidence to suggest a higher proportion of complications in the fibrin glue group. Surgery performed with fibrin glue is technically less demanding than surgery with sutures. However, there were higher rates of complications in the fibrin glue groups, even if some precautions, as suggested by experts, could reduce the number of complications.
Implications for research.
The results of this review suggest that grafts attached with fibrin glue may be better than grafts attached with suture, although it must be taken into consideration limitations to the recurrence outcome. However this should encourage the research on using autologous, in‐situ blood coagulum that shows different advantages: ease of use, almost zero cost, shorter surgical times, and less postoperative discomfort.
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic searches. We thank Catey Bunce and Gianni Virgili for their comments on the protocol, Cordelia Chan and Jennifer Evans for their comments on the protocol and review and Gabriela Czanner for her comments on the review. We thank Anupa Shah, Managing Editor of CEV for her assistance throughout the editorial process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Pterygium #2 pterygium* #3 (#1 OR #2) #4 MeSH descriptor Tissue Adhesives #5 MeSH descriptor Biological Dressings #6 (fibrin) near/2 (glue or adhesive or seal* or tissue*) #7 tissucol* or tisseel* or beriplast* or crosseal* or Quixil* or full link #8 (#4 OR #5 OR #6 OR #7) #9 MeSH descriptor Sutures #10 MeSH descriptor Suture Techniques #11 MeSH descriptor Polyglactin 910 #12 sutur* #13 vicryl or nylon or mononylon #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#3 AND #8 AND #14)
Appendix 2. MEDLINE (Ovid) search strategy
1. randomised controlled trial.pt. 2. (randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. pterygium/ 14. pterygium$.tw. 15. 13 or 14 16. exp tissue adhesives/ 17. biological dressings/ 18. (fibrin adj2 (glue or adhesive or seal$ or tissue$)).tw. 19. (tissucol$ or tisseel$ or beriplast$ or crosseal$ or Quixil$ or full link).tw. 20. or/16‐19 21. exp sutures/ 22. Suture Techniques/ 23. sutur$.tw. 24. (vicryl or nylon or mononylon).tw. 25. Polyglactin 910/ 26. or/21‐25 27. 15 and 20 and 26 28. 12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomised controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. pterygium/ 34. pterygium$.tw. 35. 33 or 34 36. exp tissue adhesive/ 37. exp bandages/ 38. (fibrin adj2 (glue or adhesive or seal$ or tissue$)).tw. 39. (tissucol$ or tisseel$ or beriplast$ or crosseal$ or Quixil$ or full link).tw. 40. or/36‐39 41. exp sutures/ 42. suturing method/ 43. sutur$.tw. 44. (vicryl or nylon or mononylon).tw. 45. Polyglactin/ 46. or/41‐45 47. 35 and 40 and 46 48. 32 and 47
Appendix 4. ISRCTN search strategy
pterygium and fibrin
Appendix 5. ClinicalTrials.gov search strategy
Pterygium AND Fibrin
Appendix 6. ICTRP search strategy
pterygium AND fibrin
Appendix 7. Data on study characteristics
| Heading in table in RevMan | Proposed subheadings | |
| Methods | Study design | •Parallel group RCT i.e. people randomised to treatment •Paired eye or intra‐individual RCT i.e. eyes randomised to treatment •Cluster RCT i.e. communities randomised to treatment •Cross‐over RCT •Other |
| Eyes | •One eye included in study
•Two eyes included in study, both eyes received same treatment
•Two eyes included in study, eyes received different treatments (pair matched)
|
|
| Participants | Country | Brasil, China, Egypt, India, Malaysia, New Zealand, Philippines, Saudi Arabia, Sweden, and Turkey |
| Setting | ||
| Number of participants | ||
| Number of men | ||
| Number of women | ||
| Average age | ||
| Age range | ||
| Ethnic group | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | Intervention Comparator |
|
| Outcomes | List | |
| Notes | Date conducted | Indicating specific dates of recruitment of participants mm/yr to mm/yr |
| Sources of funding | ||
| Declaration of interest | ||
| Other |
Appendix 8. Assessment of risk of bias
Randomisation sequence generation
We will assess this domain as:
'low risk of bias', if the allocation sequence was generated by a computer or random number table. (Drawing of lots, tossing of a coin, shuffling of cards or throwing dice will be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure);
'unclear risk of bias', if the trial was described as randomised, but the method used for the allocation sequence generation was not described;
'high risk of bias', if a system involving dates, names or admittance numbers was used for the allocation of participants.
Allocation concealment
We will assess this domain as:
'low risk of bias', if the allocation of participants involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes;
'unclear risk of bias', if the trial was described as randomised, but the method used to conceal the allocation was not described;
'high risk of bias', if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding (masking) of participants and personnel
This outcome will not be assessed as it is difficult to mask participants or personnel (clinicians for example) to the intervention.
Blinding (masking) of outcome assessors
masking of outcome assessor to treatment allocation. However, as trials use two different types of intervention (sutures or glue), masking of outcome assessors will be problematic, unless assessment is performed by someone not involved in the study.
Incomplete outcome data
We will assess this domain as:
'low risk of bias', if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals;
'unclear risk of bias', if the report gives the impression that there were no dropouts or withdrawals, but this was not specifically stated;
'high risk of bias', if the number or reasons for dropouts and withdrawals were not described.
We will further examine the percentages of dropouts overall in each trial and per randomisation arm and we will evaluate whether intention‐to‐treat (ITT) analysis has been performed or could be performed, from the published information.
Were all randomised participants analysed in the group to which they were allocated? (ITT analysis).
'Low risk of bias': if it is specifically reported by the authors that ITT was undertaken and this is confirmed on study assessment, or not stated but evident from study assessment that all randomised participants are reported/analysed in the group they were allocated to for the most important time point of outcome measurement (minus missing values) irrespective of non‐compliance and co‐interventions.
Unclear risk of bias': if it is described as ITT analysis, but we are unable to confirm on study assessment, or it is not reported and we are unable to confirm by study assessment.
'High risk of bias': if lack of ITT analysis is confirmed on study assessment (participants who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) regardless of whether ITT is reported or not; 'as treated' analysis was done with substantial departure from allocation; 'complete case' (or 'available case') analysis was done.
Selective outcome reporting
We will assess this domain as:
'low risk of bias': if adequate, pre‐defined or clinically relevant and reasonably expected outcomes are reported on;
'unclear risk of bias': if not all pre‐defined or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not;
'high risk of bias': if one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded (inadequate).
Free of other bias (e.g., baseline imbalance, early stopping)
We will assess this domain as:
'low risk of bias': if the trial appears to be free of other components that could put it at risk of bias;
'unclear risk of bias': if the trial may or may not be free of other components that could put it at risk of bias;
'high risk of bias': if there are other factors in the trial that could put it at risk of bias, e.g., no sample size calculation made, early stopping, industry involvement or an extreme baseline imbalance; moreover, since the Unit of analysis is the person, not the eye, when it was impossible to extract data according to the unit of analysis, the study could receive a High risk of bias score in the Other Bias item.
Data and analyses
Comparison 1. Fibrin glue versus suture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of pterygium | 12 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.82] |
| 2 Occurrence of 1 or more complications | 11 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.22, 3.02] |
| 3 Operating time (minutes) | 9 | 614 | Mean Difference (IV, Random, 95% CI) | ‐17.01 [‐20.56, ‐13.46] |
Comparison 2. Fibrin glue versus suture: excluding trials at high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of pterygium | 10 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.08] |
| 2 Occurrence of 1 or more complications | 9 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.32, 3.51] |
| 3 Operating time (minutes) | 6 | 372 | Mean Difference (IV, Random, 95% CI) | ‐15.59 [‐19.90, ‐11.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Fayez 2008.
| Methods | RCT investigating safety and efficacy of fibrin glue versus suture fixation of conjunctival autograft in treating pterygia. 1 eye enrolled | |
| Participants | Country: Saudi Arabia Number of participants randomised: 56 Number of eyes randomised: 56 Number (%) of participants followed up: 100% Average age: not reported % female: not reported Inclusion criteria: participants with advanced pterygia treated by limbal‐conjunctival autograft Exclusion criteria: not reported |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 24) Comparator: suture fixation of conjunctival autograft (n = 32) |
|
| Outcomes | List outcomes: recurrence of pterygium, complication of interventions Follow‐up: 24 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: participants were prospectively randomised. Comment: methods for the generation of sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: participants were prospectively randomised. Comment: methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Report gives the impression that there were no dropouts or withdrawals, but this was not specifically stated |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained; all outcomes stated in methods reported |
Elwan 2014.
| Methods | RCT. 1 eye enrolled | |
| Participants | Country: Egypt Number of participants randomised: 150 Number of eyes randomised: 150 Number (%) of participants followed up: 100% Average age: 50 % female: 60% Inclusion criteria: participants with pterygium Exclusion criteria: inability to complete the 2‐year follow‐up period, atrophic pterygium, pseudopterygium, ocular surface pathology, infection, previous limbal surgery or double head pterygium |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 50) Comparator: suture fixation of conjunctival autograft (n = 100) |
|
| Outcomes | List outcomes: recurrence of pterygium, complication of interventions, gain in uncorrected visual acuity, duration of surgery, postoperative pain, foreign body sensation, photophobia, hyperemia, chemosis, overall satisfaction Follow‐up: 24 months |
|
| Notes | Funding source: not reported Conflict of interest: no Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by coin toss |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for more objective outcomes (as some of the primary outcomes), because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Hall 2009.
| Methods | RCT. Only 1 eye in each participant had surgery and was studied | |
| Participants | Country: New Zealand Number of participants randomised: 50 Number of eyes randomised: 50 Number (%) of participants followed up: 94% Average age: 49.8 years (range 34–76 years) % female: 46% female Inclusion criteria: participants with pterygium > 4 mm in size Exclusion criteria: other ocular disease, or participant aged younger than 25 years |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 25) Comparator: suture fixation of conjunctival autograft (n = 25) |
|
| Outcomes | List outcomes: recurrence of pterygium, complication of interventions, surgical time, participant discomfort Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: May 2006‐November 2006 Trial registration number: NCT00326560 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eyes randomly allocated to treatment using pre‐allocated codes |
| Allocation concealment (selection bias) | Low risk | Eyes were independently randomised by independent nursing staff, and the surgeon was unaware which technique (sutures or glue) was to be performed until after the conjunctival graft was excised and the circulating nurse entered the room with either glue or sutures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For some of the outcomes, study authors tried to avoid observer influence (e.g. participants filled in the VAS questionnaire prior to follow‐up visits to avoid observer influence). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 3‐month follow‐up, 22/25 participants for the fibrin glue attended their appointment and 22/25 participants for the suture group. At 6‐month follow‐up, 48/50 participants could be contacted, with 2 from the suture group lost to emigration. At 12 months, 47/50 participants could be contacted, with 2 from the suture group and one from the glue group lost due to emigration |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Jaih 2006.
| Methods | RCT | |
| Participants | Country: India Number of participants randomised: 27 Number of eyes randomised: 27 Number (%) of participants followed up: not reported Average age: not reported % female: not reported Inclusion criteria: participants with pterygium Exclusion criteria: not reported |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 12) Comparator: suture fixation of conjunctival autograft (n = 15) |
|
| Outcomes | List outcomes: surgery time and postoperative pain Follow‐up: not reported |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: prospective randomised trial. Comment: methods for the generation of sequence not described |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained; insufficient information to permit judgement |
Jiang 2008.
| Methods | RCT, only 1 eye was enrolled | |
| Participants | Country: China Number of participants randomised: 40 Number of eyes randomised: 40 Number (%) of participants followed up: 100% Average age: 57 % female: 40% Inclusion criteria: participants with nasal primary pterygium Exclusion criteria: ocular pathology, history of previous ocular surgery or trauma, suspect glaucoma, or known hypersensitivity to the glue |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 20) Comparator: suture fixation of conjunctival autograft (n = 20) Tobramycin‐dexamethasone mixed solution was subconjunctivally injected and a pressure patch was applied for 24 h to restrict the eyeball from movement or blinking. Surgery time was recorded from the placement of the lid speculum to its removal. |
|
| Outcomes | List outcomes: operating time, postoperative symptoms, graft success, recurrence rate and complications. Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: June 1‐June 15, 2005 Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By tossing a coin |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for more objective outcomes (e.g. visual acuity, intraocular pressure), because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the 1‐year follow‐up period |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Karalezli 2008.
| Methods | RCT. 1 eye of each participant was included in the study. If the participant had bilateral pterygium, 1 eye was selected randomly and included in the study | |
| Participants | Country: Turkey Number of participants randomised: 50 Number of eyes randomised: 50 Number (%) of participants followed up: 100% Average age: 53‐58 years % female: 56%‐52% Inclusion criteria: participants with primary pterygium Exclusion criteria: participants with immune system, eyelid or ocular surface diseases, with a history of previous ocular surgery or trauma or known hypersensitivity to any component of fibrin glue |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 25) Comparator: suture fixation of conjunctival autograft (n = 25) |
|
| Outcomes | List outcomes: operating time, postoperative symptoms, recurrence rate and complications Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised to 2 groups |
| Allocation concealment (selection bias) | Low risk | To reduce intraobserver bias and minimise the influence of the known surgical technique on the extent of removal and size of the graft, the randomisation was done after the surgeon had harvested the graft. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited. Recurrence was defined as any fibrovascular growth that passed the corneal limbus by more than 1 mm. Reoperation was done if further growth of the recurrent pterygium was observed on any follow‐up examination. To avoid observer influence, participants were asked to fill out a questionnaire on postoperative day 1 and during every follow‐up examination until the 1st month, grading their symptoms using a 5‐point scale |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the 12‐month follow‐up. None was excluded from the study |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Koranyi 2004.
| Methods | RCT; 1 eye of each participant was included in the study | |
| Participants | Country: Sweden Number of participants randomised: 43 Number of eyes randomised: 43 Number (%) of participants followed up: 100% Average age: 44‐48 years % female: 35% Inclusion criteria: participants with primary pterygium Exclusion criteria: not reported |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 20) Comparator: suture fixation of conjunctival autograft (n = 23) |
|
| Outcomes | List outcomes: operating time, postoperative pain, recurrence rate and complications Follow‐up: 6 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: February‐April 2000 Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a computer random function into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the suture group was withdrawn because of inability to fill out the VAS questionnaire |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Ozdamar 2008.
| Methods | RCT; 1 eye of each participant was included in the study | |
| Participants | Country: Turkey Number of participants randomised: 24 Number of eyes randomised: 24 Number (%) of participants followed up: 100% Average age: 42 years % female: 58.3% Inclusion criteria: participants with primary pterygium Exclusion criteria: no reported |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 12) Comparator: suture fixation of conjunctival autograft (n = 12) |
|
| Outcomes | List outcomes: recurrence rate, complications, participant comfort, and histopathologic evaluation Follow‐up: 6 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: a randomised study. Comment: methods used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Ratnalingam 2010.
| Methods | RCT; 1 eye of each participant was included in the study. In case of bilateral pterygium, each eye was randomised separately. | |
| Participants | Country: Malaysia Number of participants randomised: 113 Number of eyes randomised: 137 Number (%) of participants followed up: 100% Average age: 60 years % female: 32% Inclusion criteria: participants with primary pterygium Exclusion criteria: not reported |
|
| Interventions | Intervention: Fibrin glue fixation of conjunctival autograft (n = 68) Comparator: suture fixation of conjunctival autograft (n = 69) |
|
| Outcomes | List outcomes: recurrence rate, surgical time, complications, participant comfort Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: 1 June 2006‐30 January 2007 Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants in each subgroup were randomised into the fibrin adhesive or suture group via block permuted randomised sampling with varying block sizes of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by an independent party who drew a sealed envelope at a different site from the study location. The results of this envelope were then informed to the surgeon via telephone |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The results were not informed to the independent observer, who was grading recurrence. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 137 out of 175 randomised eyes evaluated. No information on reasons and distribution of withdrawal provided |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Rubin 2011.
| Methods | RCT; 1 eye of each participant was included in the study. | |
| Participants | Country: Brazil Number of participants randomised: 47 Number of eyes randomised: 47 Number (%) of participants followed up: 100% Average age: 18‐70 years % female: not reported Inclusion criteria: participants with primary pterygium Exclusion criteria: not reported |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 21) Comparator: suture fixation of conjunctival autograft (n = 26) |
|
| Outcomes | List outcomes: recurrence rate, surgical time, and postoperative pain Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: a randomised study. Comment: methods used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation by an independent observer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial number of withdrawal at 6 months, and reasons not reported |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Sati 2014.
| Methods | RCT; 1 eye of each participant was included in the study. | |
| Participants | Country: India Number of participants randomised: 60 Number of eyes randomised: 60 Number (%) of participants followed up: 100% Average age: 40 years % female: 40% Inclusion criteria: participants with primary pterygium Exclusion criteria: participants with ocular surface disorders, pseudopterygium, hypersensitivity to any blood components and positive serology for human immunodeficiency virus and hepatitis B |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 30) Comparator: suture fixation of conjunctival autograft (n = 30) |
|
| Outcomes | List outcomes: recurrence rate, surgical time, complications rate and postoperative participant discomfort Follow‐up: 12 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using linear systematic sampling |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop out because of excellent communications with participants |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Sharma 2015.
| Methods | RCT; 1 eye of each participant was included in the study | |
| Participants | Country: India Number of participants randomised: 50 Number of eyes randomised: 50 Number (%) of participants followed up: 100% Average age: 43 years % female: 50% Inclusion criteria: participants of all ages and of either sex presenting with primary nasal pterygium Exclusion criteria: recurrent pterygium, glaucoma, retinal pathology requiring surgical intervention, history of previous ocular surgery or trauma |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 25) Comparator: suture fixation of conjunctival autograft (n = 25) |
|
| Outcomes | List outcomes: recurrence rate, surgical time, complications rate and postoperative participant discomfort Follow‐up: 6 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised into 2 groups using a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None noted |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Uy 2005.
| Methods | RCT. Only 1 eye per participant was entered in the study. | |
| Participants | Country: the Philippines Number of participants randomised: 22 Number of eyes randomised: 22 Number (%) of participants followed up: 100% Average age: 45 years % female: 41% Inclusion criteria: participants with primary pterygium Exclusion criteria: participants with ocular pathology other than error of refraction, with a history of previous ocular surgery or trauma, narrow occludable angles, ocular hypertension, physiologic or glaucomatous optic disc cupping, a family history of glaucoma, or known hypersensitivity to any component of fibrin glue |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 11) Comparator: suture fixation of conjunctival autograft (n = 11) |
|
| Outcomes | Graft success, recurrence rate, operating time, participant comfort List outcomes: recurrence rate, surgical time, complications rate and postoperative participant discomfort Follow‐up: 2 months. Due to the short follow‐up we did not take the recurrence outcome into account, we considered just operating time and complication rate. |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: June‐August 2001 Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participant was then randomly assigned by coin toss |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the 2‐month follow‐up period |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
Yuksel 2010.
| Methods | RCT | |
| Participants | Country: Turkey Number of participants randomised: 58 Number of eyes randomised: 58 Number (%) of participants followed‐up: 100% Average age: 48‐52 years % female: 50% Inclusion criteria: participants with primary pterygium Exclusion criteria: participants with ocular surface disorders, pseudopterygium, hypersensitivity to any blood components and positive serology for human immunodeficiency virus and hepatitis B |
|
| Interventions | Intervention: fibrin glue fixation of conjunctival autograft (n = 29) Comparator: suture fixation of conjunctival autograft (n = 29) |
|
| Outcomes | List outcomes: recurrence rate, surgical time, complications rate and cost Follow‐up: 6 months |
|
| Notes | Funding source: not reported Conflict of interest: not reported Date study conducted: January and April 2009 Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: a randomised study. Comment: methods used for random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but methods used for allocation sequence generation not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking probably of limited importance for the specified outcomes, because the risk of ascertainment bias was limited, but could have affected other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 61 eyes of 61 participants were operated. Three participants from group 1 were excluded from the study since their conjunctival grafts were not in place at the third postoperative day. Total 58 eyes of 58 participants were included in study. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained; all outcomes stated in methods reported |
RCT: randomised controlled trial VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CTRI/2013/06/00376 | Randomised trial comparing autologous blood versus fibrin glue, no comparison with sutures |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000986774.
| Trial name or title | Prospective evaluation of pterygium excision and conjunctival autograft with autologous blood, fibrin glue, or Vicryl sutures |
| Methods | Inclusion criteria: age of participant should be more than 18 years. Primary nasal pterygium. Participant willing to be enrolled in the study and available for all subsequent follow‐ups. Exclusion criteria: age of participant less than 18 years. Recurrent pterygium (history of previous pterygium excision). Temporal pterygium. Pterygium > 4 mm. Double‐headed primary pterygium (arising from both medial and lateral side of cornea). Any previous ocular surface surgery. Participant unwilling to participate in the study and all required follow‐ups Age minimum: 18 Years Age maximum: 90 Years Gender: both male and female |
| Participants | 90 |
| Interventions | Excision with conjunctival autograft involves removal of the pterygium leaving an area of bare sclera, which is closed by the movement of a free conjunctival transplant from another part of the ocular surface. The conjunctival autograft will be attached with autologous blood acting as a bioadhesive. The surgical intervention takes approximately 30 minutes and participants will be followed at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year. |
| Outcomes | Graft stability will be assessed on a slit lamp exam, grading stability based on the number of adherent edges Postoperative pain will be assessed based on a 0 (no pain) to 10 (worst pain in life) scale Pterygium recurrence will be assessed on a slit lamp exam |
| Starting date | 1/11/2013 |
| Contact information | Dr Ross MacIntyre, Royal Victorian Eye and Ear Hospital 32 Gisborne Street East Melbourne, VIC 3002, Australia, ross.macintyre@eyeandear.org.au |
| Notes | Not yet recruiting. Trial number: ACTRN12613000986774 |
NCT00326560.
| Trial name or title | Comparison of cut and paste with sutured autograft pterygium excision |
| Methods | Allocation: randomised
Endpoint classification: efficacy study
Intervention model: parallel assignment
Masking: single masked
Primary purpose: treatment Ages eligible for study: 20 years and older (adult, senior) Genders eligible for study: both Accepts healthy volunteers: yes Inclusion criteria: primary pterygium Exclusion criteria: ocular surface disease. Previous pterygium surgery |
| Participants | 40 |
| Interventions | Comparison of cut and paste with sutured autograft pterygium excision |
| Outcomes | Not applicable |
| Starting date | May 2006 |
| Contact information | Reece C Hall, reece.hall@ccdhb.org.nz |
| Notes | Trial number: NCT00326560 |
Differences between protocol and review
We amended the protocol to include a Summary of findings table and GRADE assessment as required by updated Cochrane standards.
Contributions of authors
Conception and design of study, analysis and interpretation (VR, MC, LC, LF). Drafting the review or commenting on it critically for intellectual content (VR, MC, LC, LF).
Sources of support
Internal sources
No sources of support, Other.
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
VR has no financial or commercial interest in the topic area discussed in this review. LC has no financial or commercial interest in the topic area discussed in this review. LF has no financial or commercial interest in the topic area discussed in this review.
MC has been an advisor/consultant for Bayer, Cephalon and ViiV Healthcare, and has received honoraria for educational lectures from Abbott, ViiV Healthcare and Novartis. These activities were not related to his work with this review.
New
References
References to studies included in this review
Al‐Fayez 2008 {published data only}
- Al‐Fayez M. Fibrin glue vs. suture fixation of limbal conjunctival autograft for pterygium. American Academy of Ophthalmology 2008:198.
Elwan 2014 {published data only}
- Elwan SA. Comparison between sutureless and glue free versus sutured limbal conjunctival autograft in primary pterygium surgery. Saudi Journal of Ophthalmology 2014;28(4):292‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2009 {published data only}
- Hall RC, Logan AJ, Wells AP. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clinical and Experimental Ophthalmology 2009;37(6):584‐9. [DOI] [PubMed] [Google Scholar]
Jaih 2006 {published data only}
- Jaih AK, Singh Sukhija J. Comparison of cut‐and‐paste with cut‐and‐suture technique of pterygium surgery. American Academy of Ophthalmology 2006:215.
Jiang 2008 {published data only}
- Jiang J, Yang Y, Zhang M, Fu X, Bao X, Yao K. Comparison of fibrin sealant and sutures for conjunctival autograft fixation in pterygium surgery: one‐year follow‐up. Ophthalmologica 2008;222(2):105‐11. [DOI] [PubMed] [Google Scholar]
Karalezli 2008 {published data only}
- Karalezli A, Kucukerdonmez C, Akova YA, Altan‐Yaycioglu R, Borazan M. Fibrin glue versus sutures for conjunctival autografting in pterygium surgery: a prospective comparative study. British Journal of Ophthalmology 2008;92(9):1206‐10. [DOI] [PubMed] [Google Scholar]
Koranyi 2004 {published data only}
- Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. British Journal of Ophthalmology 2004;88(7):911‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozdamar 2008 {published data only}
- Ozdamar Y, Mutevelli S, Han U, Ileri D, Onal B, Ilhan O, et al. A comparative study of tissue glue and vicryl suture for closing limbal‐conjunctival autografts and histologic evaluation after pterygium excision. Cornea 2008;27(5):552‐8. [DOI] [PubMed] [Google Scholar]
Ratnalingam 2010 {published data only}
- Ratnalingam V, Eu AL, Ng GL, Taharin R, John E. Fibrin adhesive is better than sutures in pterygium surgery. Cornea 2010;29(5):485‐9. [DOI] [PubMed] [Google Scholar]
Rubin 2011 {published data only}
- Rubin MR, Dantas PE, Nishiwaki‐Dantas MC, Felberg S. Efficacy of fibrin tissue adhesive in the attachment of autogenous conjunctival graft on primary pterygium surgery [Eficacia do adesivo tecidual de fibrina na fixacao de enxerto conjuntival autogeno em cirurgias de pterigio primario]. Arquivos Brasileiros de Oftalmologia 2011;74(2):123‐6. [DOI] [PubMed] [Google Scholar]
Sati 2014 {published data only}
- Sati A, Shankar S, Jha A, Kalra D, Mishra S, Gurunadh VS. Comparison of efficacy of three surgical methods of conjunctival autograft fixation in the treatment of pterygium. International Ophthalmology 2014;34(6):1233‐9. [DOI] [PubMed] [Google Scholar]
Sharma 2015 {published data only}
- Sharma A, Raj H, Gupta A, Raina AV. Sutureless and glue‐free versus sutures for limbal conjunctival autografting in primary pterygium surgery: a prospective comparative study. Journal of Clinical and Diagnostic Research 2015;9(11):NC06‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uy 2005 {published data only}
- Uy HS, Reyes JM, Flores JD, Lim‐Bon‐Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology 2005;112(4):667‐71. [DOI] [PubMed] [Google Scholar]
Yuksel 2010 {published data only}
- Yuksel B, Unsal SK, Onat S. Comparison of fibrin glue and suture technique in pterygium surgery performed with limbal autograft. International Journal of Ophthalmology 2010;3(4):316‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
CTRI/2013/06/00376 {unpublished data only}
- CTRI/2013/06/00376. A prospective randomised controlled trial to compare the efficacy and outcome of autologous blood and fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=6413&EncHid=&modid=&compid=','6413det' (accessed 27 October 2016).
References to ongoing studies
ACTRN12613000986774 {unpublished data only}
- ACTRN12613000986774. Comparison of autologous blood versus fibrin glue versus vicryl sutures for conjunctival autografts in pterygium surgery, evaluating pain scores, graft stability, and recurrence in healthy adult patients. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364878 (accessed 27 October 2016).
NCT00326560 {unpublished data only}
- NCT00326560. Comparison of cut and paste with sutured autograft pterygium excision. clinicaltrials.gov/ct2/show/NCT00326560 (accessed 27 October 2016).
Additional references
Akarsu 2003
- Akarsu C, Taner P, Ergin A. 5‐Fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary report. Cornea 2003;22(6):522‐6. [DOI] [PubMed] [Google Scholar]
Al Fayez 2002
- Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 2002;109(9):1752‐5. [DOI] [PubMed] [Google Scholar]
Amano 2000
- Amano S, Motoyama Y, Oshika T, Eguchi S, Eguchi K. Comparative study of intraoperative mitomycin C and beta irradiation in pterygium surgery. British Journal of Ophthalmology 2000;84(6):618‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anguria 2014
- Anguria P, Kitinya J, Ntuli S, Carmichael T. The role of heredity in pterygium development. International Journal of Ophthalmology 2014;7(3):563‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avisar 2003
- Avisar R, Gaton DD, Loya N, Appel I, Weinberger D. Intraoperative mitomycin C 0.02% for pterygium: effect of duration of application on recurrence rate. Cornea 2003;22(2):102‐4. [DOI] [PubMed] [Google Scholar]
Bedrossian 1960
- Bedrossian RH. The effects of pterygium surgery on refraction and corneal curvature. Archives of Ophthalmology 1960;64:553‐7. [DOI] [PubMed] [Google Scholar]
Calson 1987
- Calson AN, Wilhelmus KR. Giant papillary conjunctivitis associated with cyanoacrylate glue. American Journal of Ophthalmology 1987;104(4):437‐8. [DOI] [PubMed] [Google Scholar]
Cameron 1965
- Cameron ME. Pterygium Throughout the World. Springfield: Thomas, 1965. [Google Scholar]
Cano‐Parra 1995
- Cano‐Parra J, Diaz‐Llopis M, Maldonado MJ, Vila E, Menezo JL. Prospective trial of intraoperative mitomycin C in the treatment of primary pterygium. British Journal of Ophthalmology 1995;79(5):339‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman‐Smith 1992
- Chapman‐Smith JS. Pterygium treatment with triethylene thiophosphoramide. Australian and New Zealand Journal of Ophthalmology 1992;20(2):129‐31. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. American Journal of Ophthalmology 1995;120(2):151‐60. [DOI] [PubMed] [Google Scholar]
Dadeya 2001
- Dadeya S, Ms K. Strabismus surgery: fibrin glue versus vicryl for conjunctival closure. Acta Ophthalmologica Scandinavica 2001;79(5):515‐7. [DOI] [PubMed] [Google Scholar]
Dadeya 2002
- Dadeya S, Kamlesh, Khurana C, Fatima S. Intraoperative daunorubicin versus conjunctival autograft in primary pterygium surgery. Cornea 2002;21(8):766‐9. [DOI] [PubMed] [Google Scholar]
de Wit 2010
- Wit D, Athanasiadis I, Sharma A, Moore J. Sutureless and glue‐free conjunctival autograft in pterygium surgery: a case series. Eye 2010;24(9):1474‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dunn 1991
- Dunn JP, Seamone CD, Ostler HB, Nickel BL, Beallo A. Development of scleral ulceration and calcification after pterygium excision and mitomycin therapy. American Journal of Ophthalmology 1991;112(3):343‐4. [DOI] [PubMed] [Google Scholar]
Fong 1998
- Fong KS, Balakrishnan V, Chee SP, Tan DT. Refractive change following pterygium surgery. CLAO Journal 1998;24(2):115‐7. [PubMed] [Google Scholar]
Foroutan 2011
- Foroutan A, Beigzadeh F, Ghaempanah MJ, Eshghi P, Amirizadeh N, Sianati H, et al. Efficacy of autologous fibrin glue for primary pterygium surgery with conjunctival autograft. Iranian Journal of Ophthalmology 2011;23(1):39–47. [Google Scholar]
Gasser 2016
- Gasser T, Romano V, Seifarth C, Kaye SB, Bechrakis NE, Steger B. Morphometric characterisation of pterygium associated with corneal stromal scarring using high resolution anterior segment optical coherence tomography.. British Journal of Ophthalmology 2016 Aug 3 [Epub ahead of print]. [DOI: 10.1136/bjophthalmol-2016-308685] [DOI] [PubMed]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gosain 2002
- Gosain AK, Lyon VB, Plastic Surgery Educational Foundation DATA Committee. The current status of tissue glues: part II. For adhesion of soft tissues. Plastic and Reconstructive Surgery 2002;110(6):1581‐4. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University, 2014. GRADEpro. Version accessed 14 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014, 2014.
Haik 1962
- Haik GM, Ellis GS, Nowell JF. The management of pterygia, with special reference to surgery combined with beta irradiation. Transactions ‐ American Academy of Ophthalmology and Otolaryngology 1962;66:776‐84. [PubMed] [Google Scholar]
Hansen 1980
- Hansen A, Norn M. Astigmatism and surface phenomena in pterygium. Acta Ophthalmologica 1980;58(2):174‐81. [DOI] [PubMed] [Google Scholar]
Hayasaka 1988
- Hayasaka S, Noda S, Yamamoto Y, Setogawa T. Postoperative instillation of low‐dose mitomycin C in the treatment of primary pterygium. American Journal of Ophthalmology 1988;106(6):715‐8. [DOI] [PubMed] [Google Scholar]
Henrick 1987
- Henrick A, Gaster RN, Silverstone PJ. Organic tissue glue in the closure of cataract incisions. Journal of Cataract and Refractive Surgery 1987;13(5):551‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hino 2000
- Hino M, Ishiko O, Honda KI, Yamane T, Ohta K, Takubo T, et al. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. British Journal of Haematology 2000;108(1):194‐5. [DOI] [PubMed] [Google Scholar]
Hirst 2009
- Hirst LW. Recurrent pterygium surgery using pterygium extended removal followed by extended conjunctival transplant: recurrence rate and cosmesis. Ophthalmology 2009;116(7):1278‐86. [DOI] [PubMed] [Google Scholar]
Holladay 1985
- Holladay JT, Lewis JW, Allison ME, Ruiz RS. Pterygia as cause of post cataract with‐the‐rule astigmatism. Journal ‐ American Intra‐Ocular Implant Society 1985;11(2):176‐9. [DOI] [PubMed] [Google Scholar]
Jaros 1988
- Jaros PA, DeLuise VP. Pingueculae and pterygia. Survey of Ophthalmology 1988;33(1):41‐9. [DOI] [PubMed] [Google Scholar]
Kenyon 1985
- Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 1985;92(11):1461–70. [DOI] [PubMed] [Google Scholar]
Kheirkhah 2008
- Kheirkhah A, Casas V, Sheha H, Raju VK, Tseng SC. Role of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygium. Cornea 2008;27(1):56‐63. [DOI] [PubMed] [Google Scholar]
King 1950
- King JH Jr. The pterygium: brief review and evaluation of certain methods of treatment. A.M.A. Archives of Ophthalmology 1950;44(6):854‐69. [PubMed] [Google Scholar]
Koranyi 2005
- Koranyi G, Seregard S, Kopp ED. The cut‐and‐paste method for primary pterygium surgery: long‐term follow‐up. Acta Ophthalmologica Scandinavica 2005;83:298‐301. [DOI] [PubMed] [Google Scholar]
Lewallen 1989
- Lewallen S. A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology 1989;96(11):1612‐4. [DOI] [PubMed] [Google Scholar]
Lin 1997
- Lin A, Stern GA. Correlation between pterygium size and induced corneal astigmatism. Cornea 1997;17:22‐7. [DOI] [PubMed] [Google Scholar]
Lin 1998
- Lin A, Stern G. Correlation between pterygium size and induced corneal astigmatism. Cornea 1998;17(1):18‐30. [DOI] [PubMed] [Google Scholar]
Mackenzie 1991
- MacKenzie FD, Hirst LW, Kynaston B, Bain C. Recurrence rate and complications after beta irradiation for pterygia. Ophthalmology 1991;98(12):1776‐81. [DOI] [PubMed] [Google Scholar]
Mahar 1993
- Mahar PS, Nwokora GE. Role of mitomycin C in pterygium surgery. British Journal of Ophthalmology 1993;77(7):433‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2012
- Malik KP, Goel R, Gutpa A, Gupta SK, Kamal S, Mallik VK, et al. Efficacy of sutureless and glue free limbal conjunctival autograft for primary pterygium surgery. Nepalese journal of ophthalmology: a biannual peer‐reviewed academic journal of the Nepal Ophthalmic Society: NEPJOPH 2012;4(2):230‐5. [DOI] [PubMed] [Google Scholar]
Marticorena 2006
- Marticorena J, Rodriguez‐Ares MT, Tourino R, Mera P, Valladares MJ, Martinez‐de‐la‐Casa JM, et al. Pterygium surgery: conjunctival autograft using a fibrin adhesive. Cornea 2006;25(1):34‐6. [DOI] [PubMed] [Google Scholar]
Mohan 2003
- Mohan K, Malhi RK, Sharma A, Kumar S. Fibrin glue for conjunctival closure in strabismus surgery. Journal of Pediatric Ophthalmology and Strabismus 2003;40(3):158‐60. [DOI] [PubMed] [Google Scholar]
Montgomery 2007
- Montgomery GH, Bovbjerg DH, Schnur JB, David D, Goldfarb A, Weltz CR, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. Journal of the National Cancer Institute 2007;99(17):1304‐12. [DOI] [PubMed] [Google Scholar]
Moran 1984
- Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. British Journal of Ophthalmology 1984;68(5):343‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morita 2000
- Morita Y, Nishii O, Kido M, Tsutsumi O. Parvovirus infection after laparoscopic hysterectomy using fibrin glue hemostasis. Obstetrics and Gynecology 2000;95(6 Pt 2):1026. [DOI] [PubMed] [Google Scholar]
Nakaishi 1997
- Nakaishi H, Yamamoto M, Ishida M, Someya I, Yamada Y. Pingueculae and pterygia in motorcycle policemen. Industrial Health 1997;35(3):325–9. [DOI] [PubMed] [Google Scholar]
Norn 1991
- Norn M, Franck C. Longterm changes in the outer part of the eye in welders. Prevalence of spheroid degeneration, pinguecula, pterygium and corneal cicatrices. Acta Ophthalmologica 1991;69(3):382‐6. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1996
- O'Sullivan F, Dalton R, Rostron CK. Fibrin glue: an alternative method of wound closure in glaucoma surgery. Journal of Glaucoma 1996;5(6):367‐70. [PubMed] [Google Scholar]
Oldenburg 1990
- Oldenburg JB, Garbus J, McDonnell JM, McDonnell PJ. Conjunctival pterygia. Mechanism of corneal topographic changes. Cornea 1990;9(3):200‐4. [PubMed] [Google Scholar]
Prabhasawat 1997
- Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 1997;104(6):974‐85. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romano 2016
- Romano V, Steger B, Kovacova A, Kaye SB, Willoughby CE. Further evidence for heredity of pterygium. Ophthalmic Genetics 2016;37(4):434‐6. [DOI] [PubMed] [Google Scholar]
Rubinfeld 1992
- Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, Stoleru S, et al. Serious complications of topical mitomycin C after pterygium surgery. Ophthalmology 1992;99(11):1647‐54. [DOI] [PubMed] [Google Scholar]
Sanchez‐Thorin 1998
- Sanchez‐Thorin JC, Rocha G, Yelin JB. Meta‐analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. British Journal of Ophthalmology 1998;82(6):661‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sebban 1991a
- Sebban A, Hirst LW. Pterygium recurrence at the Princess Alexandra Hospital. Australian and New Zealand Journal of Ophthalmology 1991;19(3):203‐6. [DOI] [PubMed] [Google Scholar]
Sebban 1991b
- Sebban A, Hirst LW. Treatment of pterygia in Queensland. Australian and New Zealand Journal of Ophthalmology 1991;19(2):123‐7. [DOI] [PubMed] [Google Scholar]
Singh 1988
- Singh G, Wilson CS, Foster CS. Mitomycin eye drops as treatment for pterygium. Ophthalmology 1988;95(6):813‐21. [DOI] [PubMed] [Google Scholar]
Spierer 1997
- Spierer A, Barequet I, Rosner M, Solomon AS, Martinowitz U. Reattachment of extraocular muscles using fibrin glue in a rabbit model. Investigative Ophthalmology and Visual Science 1997;38(2):543‐46. [PubMed] [Google Scholar]
Sridhar 2002
- Sridhar MS, Bansal AK, Rao GN. Surgically induced necrotizing scleritis after pterygium excision and conjunctival autograft. Cornea 2002;21(3):305–7. [DOI] [PubMed] [Google Scholar]
Srinivasan 2007
Starck 1991
- Starck T, Kenyon KR, Serrano F. Conjunctival autograft for primary and recurrent pterygia: surgical technique and problem management. Cornea 1991;10(3):196‐202. [DOI] [PubMed] [Google Scholar]
Stern 1998
- Stern GA, Lin A. Effect of pterygium excision on induced corneal topographic abnormalities. Cornea 1998;17(1):23‐7. [DOI] [PubMed] [Google Scholar]
Tan 1997
- Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Archives of Ophthalmology 1997;115(10):1235–40. [DOI] [PubMed] [Google Scholar]
Tan 1999
- Tan D. Conjunctival grafting for ocular surface disease. Current Opinion in Ophthalmology 1999;10(4):277‐81. [DOI] [PubMed] [Google Scholar]
Ti 2000
- Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. British Journal of Ophthalmology 2000;84(4):385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilanus 1995
- Tilanus MA, Deutman AF. Full‐thickness macular holes treated with vitrectomy and tissue glue. International Ophthalmology 1994‐1995;18(6):335‐8. [DOI] [PubMed] [Google Scholar]
Tomidokoro 1999
- Tomidokoro A, Oshika T, Amano S, Eguchi K, Eguchi S. Quantitative analysis of regular and irregular astigmatism induced by pterygium. Cornea 1999;18(4):412‐5. [DOI] [PubMed] [Google Scholar]
Uy 2002
- Uy HS, Reyes JMG, Flores JP, Limbonsiong R. Human plasma derived fibrin glue (Beriplast P) in conjunctival autografts for pterygia. In: ARVO. 2002.
Vastine 1982
- Vastine DW, Stewart WB, Schwab IR. Reconstruction of the periocular mucous membrane by autologous conjunctival transplantation. Ophthalmology 1982;89(9):1072‐81. [DOI] [PubMed] [Google Scholar]
Walland 1994
- Walland MJ, Stevens JD, Steele AD. The effect of recurrent pterygium on corneal topography. Cornea 1994;13(5):463‐4. [DOI] [PubMed] [Google Scholar]
Wright 1998
- Wright MM, Brown EA, Maxwell K, Cameron JD, Walsh AW. Laser‐cured fibrinogen glue to repair bleb leaks in rabbits. Archives of Ophthalmology 1998;116(2):199‐202. [DOI] [PubMed] [Google Scholar]
Youngson 1972
- Youngson RM. Recurrence of pterygium after excision. British Journal of Ophthalmology 1972;56(2):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zauberman 1988
- Zauberman H, Hemo I. Use of fibrin glue in ocular surgery. Ophthalmic Surgery 1988;19(2):132‐3. [PubMed] [Google Scholar]
References to other published versions of this review
Romano 2014
- Romano V, Cruciani M, Conti L, Fontana L. Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011308] [DOI] [PMC free article] [PubMed] [Google Scholar]


