Abstract
Background
Choice of antibiotic, and the use of single or combined therapy are controversial areas in the treatment of respiratory infection due to Pseudomonas aeruginosa in cystic fibrosis (CF). Advantages of combination therapy include wider range of modes of action, possible synergy and reduction of resistant organisms; advantages of monotherapy include lower cost, ease of administration and reduction of drug‐related toxicity. Current evidence does not provide a clear answer and the use of intravenous antibiotic therapy in cystic fibrosis requires further evaluation. This is an update of a previously published review.
Objectives
To assess the effectiveness of single compared to combination intravenous anti‐pseudomonal antibiotic therapy for treating people with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register, comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.
Most recent search of the Group's Trials Register: 14 October 2016.
Selection criteria
Randomised controlled trials (RCTs) comparing a single intravenous anti‐pseudomonal antibiotic with a combination of that antibiotic plus a second anti‐pseudomonal antibiotic in people with CF.
Data collection and analysis
Two authors independently assessed trial quality and extracted data.
Main results
We identified 45 trials, of which eight trials (356 participants) comparing a single anti‐pseudomonal agent to a combination of the same antibiotic and one other, were included.
There was a wide variation in the individual antibiotics used in each trial. In total, the trials included seven comparisons of a beta‐lactam antibiotic (penicillin‐related or third generation cephalosporin) with a beta‐lactam‐aminoglycoside combination and three comparisons of an aminoglycoside with a beta‐lactam‐aminoglycoside combination. These two groups of trials were analysed as separate subgroups.
There was considerable heterogeneity amongst these trials, leading to difficulties in performing the review and interpreting the results. The meta‐analysis did not demonstrate any significant differences between monotherapy and combination therapy, in terms of lung function; symptom scores; adverse effects; and bacteriological outcome measures.
These results should be interpreted cautiously. Six of the included trials were published between 1977 and 1988; these were single‐centre trials with flaws in the randomisation process and small sample size. Overall, the methodological quality was poor.
Authors' conclusions
The results of this review are inconclusive. The review raises important methodological issues. There is a need for an RCT which needs to be well‐designed in terms of adequate randomisation allocation, blinding, power and long‐term follow up. Results need to be standardised to a consistent method of reporting, in order to validate the pooling of results from multiple trials.
Plain language summary
A comparison of single and combined intravenous drug therapy for people with cystic fibrosis infected with Pseudomonas aeruginosa
Review question
We reviewed the evidence about the different effects of using a single intravenous (given directly into a vein) antibiotic compared to using a combination of intravenous antibiotics in people with cystic fibrosis infected with Pseudomonas aeruginosa.
Background
Cystic fibrosis is a serious genetic disease that affects cells in the exocrine glands (sweat glands and others). People with cystic fibrosis have a greater risk of chronic lung infections, often due to bacteria called Pseudomonas aeruginosa. They receive antibiotics, either a single drug or a combination of different drugs, by injection to treat these infections. Both the choice of antibiotic and the use of single or combined therapy vary. We looked for randomised controlled trials which compared a single intravenous antibiotic with a combination of that antibiotic plus another one in people with cystic fibrosis. This is an updated version of the review.
Search date
The evidence is current to: 14 October 2016.
Study characteristics
We included eight trials with a total of 356 people. Six of these were published before 1988, were each based in a single centre and used a range of different drugs. These factors made it difficult to combine and analyse the results.
Key results
We did not find any differences between the two therapies for lung function, symptom scores, side effects or bacteriological outcome measures. We conclude that there is not enough evidence to compare the different therapies. More research is needed, particularly looking at side effects of treatment.
Quality of the evidence
Six of the included trials were quite old (published between 1977 and 1988). They did not include many people and had flaws in the way the people taking part were put into the different treatment groups. Overall, the quality of the trials' design was poor.
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting inherited disorder affecting white populations, with chronic, progressive lung disease being the major cause of morbidity and shortened survival. The continuous cycle of infection and inflammation is responsible for the severe airway damage and loss of respiratory function (Cantin 1995; Konstan 1997).
Recurrent infection, in particular with Pseudomonas aeruginosa (P aeruginosa) is the main feature of the lung involvement in CF. Administration of intravenous (IV) antibiotic therapy for a period of around two weeks is standard practice for treatment of pulmonary infections in most CF centres. Some studies have shown this approach to be effective in improving sputum colony counts (Regelmann 1990). Regular, elective IV therapy, as well as other interventions such as early treatment and cohorting have been shown to have a beneficial effect on the prevalence of P aeruginosa (Frederiksen 1999). However, this relationship was not demonstrated in a small study by Wolter (Wolter 1999). The inconsistency of these results suggests that the use of this therapy requires further evaluation.
Description of the intervention
Currently, treatment of chronic P aeruginosa infection in people with CF usually involves one of the following strategies. One approach is to use IV antibiotics to treat people with CF only when they become acutely unwell, on the grounds of clinical, radiological or pulmonary function parameters (subsequently referred to as symptomatic regimen). Alternatively, chronic infection may be treated with elective IV antibiotics at regular intervals (e.g. three‐monthly) (Hoiby 1993), to try to prevent long‐term deterioration (subsequently referred to as elective regimen). Trials using either of these strategies will be considered for this review, if the same strategy was used for treatment and comparison groups.
Choice of antibiotic, single or combined therapy and the duration of treatment are controversial areas in the treatment of infection with IV antibiotics in CF. Current practice in the UK is variable, with one study from 1993 showed 80% choosing a combination and 20% using monotherapy (Taylor 1993). However, more recently it was reported that monotherapy is now used in only 1 out of 23 centres in the UK who replied to a postal survey (Tan 2002).
How the intervention might work
Most centres perceive dual or combination IV antibiotic therapy in CF to be more effective than single therapy. It has been suggested that a clinic policy of using monotherapy with a beta‐lactam antibiotic may be responsible for the emergence of resistant strains of P aeruginosa (Cheng 1996). Use of a beta‐lactam alone offers advantages for the individual because of ease of administration and avoidance of the need to measure aminoglycoside levels.
Why it is important to do this review
Intravenous antibiotic therapy may have contributed to improved survival among people with CF; however, the multiple use of potent and highly selective antibiotics may increase the likelihood of adverse effects and lead to the development of resistant strains of organisms (Levy 1998). This version of the review is an update of previous review versions (Elphick 2002; Elphick 2005; Elphick 2014).
Objectives
1. To assess the effectiveness of single compared to combination IV anti‐pseudomonal antibiotic therapy in the treatment of people with CF. 2. To assess whether the use of combination IV anti‐pseudomonal antibiotic therapy leads to an increase in adverse effects or the development of resistant strains of organisms in CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Trials in which individuals are treated according to a symptomatic or an elective regimen (see above) were included if the only difference between the treatment and comparison group is whether the participants receive single or combination antibiotic therapy. Trials where quasi‐randomisation methods such as alternation were used were included, when there was sufficient evidence that the treatment and comparison groups were comparable in terms of clinical status.
Types of participants
Children and adults with defined CF diagnosed clinically and by sweat or genetic testing including all ages and all degrees of severity.
Types of interventions
Any single IV anti‐pseudomonal antibiotic compared to a combination of two or more IV anti‐pseudomonal antibiotics. Trials comparing a single anti‐pseudomonal agent to a combination of the same antibiotic and one other anti‐pseudomonal agent (drug A versus drug A plus drug B) were included. Trials which compared a single anti‐pseudomonal antibiotic agent with a combination of two further anti‐pseudomonal antibiotics (drug A versus drug B plus drug C) were not included.
Types of outcome measures
We aimed to assess whether a combination of IV anti‐pseudomonal antibiotics is more effective than a single IV anti‐pseudomonal antibiotic for the following outcomes:
subjective improvement;
clinical improvement;
bacteriological improvement;
adverse effects.
Short‐term results
(i.e. at end of course of antibiotics)
Primary outcomes
-
Clinical improvement
improvement in spirometric lung function (e.g. forced expiratory volume in one second (FEV1) and forced vital capacity (FVC))
-
Bacteriological improvement
improvement in quantitative bacteriology of sputum
Adverse effects to antibiotics, e.g. renal and auditory impairment, serum sickness and sensitivity reactions
Secondary outcomes
-
Subjective improvement
quality of life assessment, in terms of measures of the individual's well‐being
-
Clinical improvement
nutritional status as noted by weight gain, body mass index, z score or other indices of nutritional state
additional treatment required
duration of hospitalisation
time to next course of IV antibiotics
chest X‐ray (CXR) scores
improvement in symptoms
-
Bacteriological improvement
Changes in inflammatory markers (in sputum or blood)
Long‐term results
(measured at 6 to 12 months after course of antibiotics; if long‐term outcomes are measured at other time intervals, consideration will be given to these also)
Primary outcomes
-
Clinical improvement
prevention of deterioration of lung function
-
Bacteriological change
development of antibiotic‐resistant strains ofP aeruginosa and other organisms
Adverse effects to antibiotics, e.g. renal and auditory impairment, serum sickness and sensitivity reactions
Secondary outcomes
-
Subjective improvement
quality of life assessment
-
Clinical improvement
number of courses of IV antibiotics in the following year
Search methods for identification of studies
Relevant trials were identified from the Group's Cystic Fibrosis Trials Register using the terms: antibiotics AND intravenous.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's CF Trials Register: 14 October 2016.
Data collection and analysis
Selection of studies
Two authors from different centres independently reviewed all trials to select which were to be included in the review. If disagreement arose on the suitability of a trial for inclusion in the review, the authors reached a consensus by discussion.
Data extraction and management
Each author independently extracted data using standard data acquisition forms. If disagreement arose on the quality of a trial, the authors reached a consensus by discussion.
If there had been sufficient numbers of trials using quasi‐randomisation methods, then the authors would have analysed this group separately.
The authors grouped data into short‐term results and long‐term results. They defined short‐term results as those at the end of the course of antibiotics; they considered long‐term results to be 6 to 12 months after the course of antibiotics. The authors will also consider other time intervals for long‐term outcomes if these are reported.
For binary outcome measures the authors recorded the number of events and the number of participants for each group. For continuous outcomes, the authors recorded either the mean change from baseline for each group or mean post‐treatment or intervention values and the standard deviation or standard error for each group.
Assessment of risk of bias in included studies
In order to establish a risk of bias for each trial, the authors independently assessed the methodological quality of each trial. In particular, authors examined details of the randomisation method (generation and concealment of allocation), the degree of blinding in the trial, whether intention‐to‐treat analyses were possible from the available data and if the investigators recorded the number of participants lost to follow up or subsequently excluded from the trial.
Measures of treatment effect
For binary outcome measures, the authors calculated a pooled estimate of the treatment effect for each outcome across trials using the Peto odds ratio with 95% confidence intervals (CIs) where appropriate. For continuous outcomes, the authors calculated a pooled estimate of treatment effect by calculating the mean difference with 95% CIs.
Unit of analysis issues
One trial included in the review was of cross‐over design (Pedersen 1986). Ideally when conducting a meta‐analysis combining results from cross‐over trials the authors would have liked to use the inverse variance methods that are recommended by Elbourne (Elbourne 2002). However, due to restrictions on the data that were available from the included trial, the only method that they have been able to use was to treat the cross‐over trial as if it was a parallel trial (assuming a correlation of zero as the most conservative estimate). Elbourne says that this approach produces conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant appears in both the treatment and control group, so the two groups are not independent.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, the authors collected data on the number of participants with each outcome event by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up.
Assessment of heterogeneity
The authors tested for heterogeneity between trial results using a standard Chi2 test.
Data synthesis
The authors analysed the data using a fixed‐effect model. In future updates, if they identify a moderate to high degree of heterogeneity, they plan to analyse the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If there were sufficient trials, the authors planned to carry out subgroup analyses of adults separately from children; of participants on a symptomatic regimen separately from those on an elective regimen; and also of those who were colonised with P aeruginosa (i.e. those people with CF who are sputum positive on three consecutive occasions) separately from those who were not colonised.
Sensitivity analysis
The authors planned to perform a sensitivity analysis based on the methodological quality of the trials.
Results
Description of studies
Results of the search
A total of 45 trials were identified by the searches. No trials were found through contact with pharmaceutical companies. We included eight trials in the review and excluded 37 trials.
Included studies
Eight trials (including 356 participants) were included in this review; of these three were published only as abstracts from conference proceedings (Costantini 1982; Huang 1982; Master 1997).
Seven trials were stated to be randomised controlled trials (Costantini 1982; Huang 1982; Master 1997; McCarty 1988; McLauglin 1983; Pedersen 1986; Smith 1999). The method of randomisation was stated in only one of these, in which it was described as "computer‐generated" (Smith 1999). In the remaining trial, treatment was assigned as an alternate allocation with good evidence of similar groups at baseline (Parry 1977). This trial was included as a quasi‐randomised trial.
In seven of the eight trials, participants were included during an exacerbation of symptoms (symptomatic regimen) in a parallel group design (Costantini 1982; Huang 1982; Master 1997; McCarty 1988; McLauglin 1983; Parry 1977; Smith 1999). In the remaining trial, participants were treated using a three‐monthly elective regimen and were re‐entered into the trial during consecutive courses of antibiotics in a cross‐over design (Pedersen 1986). In five of the trials, evidence of P aeruginosa in the sputum was an inclusion requirement (Costantini 1982; Master 1997; Parry 1977; Pedersen 1986; Smith 1999); of the remaining three trials, one stated that 98% had P aeruginosa (McLauglin 1983).
Criteria for diagnosis of CF were stated in only one of the eight trials (Smith 1999). Sample sizes varied from 14 to 83 participants, with a total of 356 participants recruited. All trials either stated that they included both adults and children, or did not state the age range. No trial looked at the effects of single versus combination antibiotic therapy in children alone. One trial included 17 children, but included three children twice, giving a total of 20 treatment courses (McCarty 1988).
There was a wide variation in the individual antibiotics used in each trial (seePublished notes: Description of pharmacological properties of antibiotics used). Two trials made two comparisons, therefore, in total, the eight trials included seven comparisons of a beta‐lactam antibiotic (penicillin‐related or third generation cephalosporin) to a beta‐lactam‐aminoglycoside combination and three comparisons of an aminoglycoside to a beta‐lactam‐aminoglycoside combination. These two groups of trials were analysed as separate subgroups.
Two trials compared two single agents with the combination of the same two antibiotics: carbenicillin versus sisomycin versus carbenicillin and sisomycin (Costantini 1982); ticarcillin versus gentamicin versus ticarcillin and gentamicin (Parry 1977). One other trial looked at an aminoglycoside as the single agent: tobramycin versus tobramycin and ceftazidime (Master 1997). Of the remaining five trials, one studied ceftazidime as the single agent (ceftazidime and tobramycin (Pedersen 1986)) and the remaining four compared an agent from the penicillin group of antibiotics: piperacillin (McCarty 1988); ticarcillin (Huang 1982); azlocillin (McLauglin 1983; Smith 1999) with a combination of that agent with tobramycin.
Outcomes were studied at the end of the treatment course in all trials. Treatment duration varied from 10 to 14 days. Three trials included a follow‐up period, which varied from two to eight weeks (McLauglin 1983; Smith 1999) to six months for the cross‐over trial (Pedersen 1986).
Excluded studies
A total of 37 trials were excluded. Eleven trials compared a single antibiotic agent with the existing combination of two other antibiotics (drug A versus drug B plus drug C) (Balsamo 1986; Beaudry 1980; Bosso 1988; Church 1997; De Boeck 1989; De Boeck 1999; Gold 1985; Jewett 1985; Permin 1983; Stack 1985; Wesley 1988). Fourteen trials compared different dosage regimens of the same antibiotic (Adeboyeku 2011; Al‐Ansari 2006; Aminimanizani 2002; Beringer 2012; Conway 1997; Hubert 2009; Keel 2011; McCabe 2013; Prayle 2013; Noah 2010; Riethmueller 2009; Semykin 2010; Turner 2013; Whitehead 2002). One trial was excluded as it compared two single drugs (Levy 1982) and another trial as it compared two different combinations of antibiotics (Blumer 2005). One trial compared intravenous antibiotics administered in hospital compared to at home (Donati 1987) and another trial looked at an eradication regimen (Kenny 2009). Five further trials were excluded as allocation was not by randomisation and because there were marked differences in baseline characteristics between the treatment and comparison groups (Hoogkamp 1983; Hyatt 1981; Krause 1979; Nelson 1985; Roberts 1993). Two trials were excluded as they evaluated tools to assess treatment response (Hatziagorou 2013; Kuni 1992).
One trial included 30 participants. However, 17 of these received more than one course of treatment (Padoan 1987). In total, 40 courses of treatment took place (20 in each intervention group). The trial was cross‐over in design; but re‐randomisation took place between courses of treatment, resulting in some participants possibly receiving two or more courses of the same treatment, or a mixture of different treatments. Since the number of participants receiving each treatment was unclear, results could not be included in the analysis of this review and therefore the trial was excluded. Individual patient data are being requested from the authors of this trial so that data from this trial may be included in future updates (Padoan 1987).
Risk of bias in included studies
In order to assess the risk of bias in the trials, the methodological quality of each trial was assessed using criteria described by Schulz (Schulz 1995). For each trial the following were assessed: concealment of allocation schedule; generation of allocation sequences; inclusion in the analysis of all randomised participants; and double‐blinding.
Allocation
One trial stated that treatment was allocated to participants using a computer‐generated randomisation (Smith 1999); and one trial was stratified for age and disease severity during randomisation (Master 1997). We assessed these trials as having a low risk of bias. Five trials stated that allocation was randomised, but did not specify the method of generation, so we rated the risk of bias in each of these as unclear (Costantini 1982; Huang 1982; McCarty 1988; McLauglin 1983; Pedersen 1986). One trial used alternation but does not discuss how the first participant was randomised to their treatment group and we assessed this as inadequate, thus having a high risk of bias (Parry 1977).
Four trials were assessed as adequate, Master stated that the code was only broken on completion of the study (Master 1997); another trial stated they used sequentially numbered envelopes (McCarty 1988); a further trial employed sealed envelopes prepared by pharmacy (McLauglin 1983); and the fourth trial stated central randomisation (Smith 1999). We judged these trials to have a low risk of bias. We assessed one trial as inadequate, since the investigators used alternate allocation and so we judged this to have a high risk of bias (Parry 1977). We included this trial as a quasi‐randomised trial. In the remaining three trials the method of allocation concealment was unclear and so we judged these to have an unclear risk of bias (Costantini 1982; Huang 1982; Pedersen 1986).
Blinding
Five of the trials were described as double‐blinded (Huang 1982; Master 1997; McLauglin 1983; Smith 1999). In each trial, saline was used for the placebo injection. We judged these trials to have a low risk of bias. One trial did not explicitly state that blinding had taken place, but did state that both interventions were given with the same volume and in the same way, so it can be assumed that there was some degree of blinding leading to a low risk of bias (Pedersen 1986). Two further trials stated that no blinding had taken place, thus we deemed these to have a potential risk of bias (McCarty 1988; Parry 1977); and in the remaining trial it was not clear whether blinding had taken place and we judge this to have an unclear risk of bias (Costantini 1982).
Incomplete outcome data
An intention‐to‐treat analysis was not stated in any of the included trials. In four, however, there appeared to be no withdrawals (Costantini 1982; Huang 1982; McCarty 1988; Parry 1977). In one trial, seven of the 41 participants did not complete the trial, six from the single therapy group and one from the combination group (reasons given) (McLauglin 1983) and another described reasons why three participants out of a cohort of 20 withdrew (Pedersen 1986). One trial published a flow chart showing numbers randomized and included or excluded (with reasons) at each stage in paper (Master 1997). A further trial gave the numbers and reasons for withdrawals in a table (Smith 1999).
Effects of interventions
Only those primary and secondary outcomes of this review, which were reported within the included studies, are listed below. No trial included follow up for longer than six months.
Single compared to combination therapy
Pooling of results was difficult because of missing data, differences in method of expression of the results and missing standard deviations.
Primary outcomes
1. Clinical improvement
a. Effect on lung function
Seven of the eight trials included lung function as an outcome measure. However, there was great variety between the trials in the tests used, the time at measurement and the method of expression of the results.
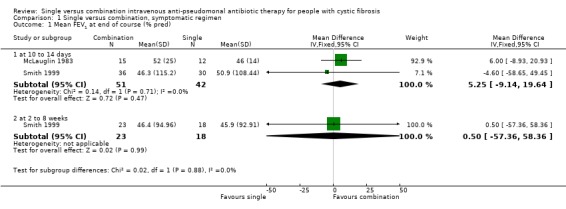
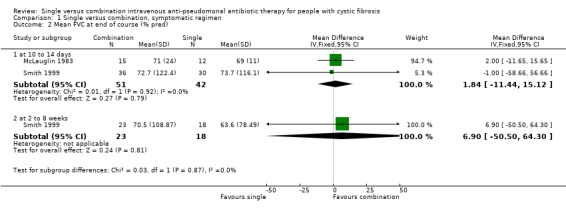
Although the outcome measure given in our protocol was improvement in spirometric lung function, no trial included these data. Two trials measured mean FEV1 and FVC at the end of the treatment course, expressed as per cent predicted (McLauglin 1983; Smith 1999). These trials were randomised and gave baseline data, clearly stating that there was no significant difference between the single and combination treatment groups at baseline. We therefore analysed these outcomes.
One trial did compare mean change in FEV1 and FVC, expressed as per cent predicted, but did not include standard deviations (SD) in the results, so we could not include the data (Master 1997). No data were given in a further three trials (Huang 1982; McCarty 1988; Parry 1977) and one trial expressed the results in terms of a median and range (Pedersen 1986).
There were no significant differences between the single and combination treatment groups in the lung function parameters, which we were able to include.
b. Effect on clinical scores
Measures of change in clinical status were used in most trials. In all but two, either no data or measure of variance were given. One trial measured mean Schwachman score at the end of treatment (McLauglin 1983). This was a randomised trial with no significant difference between the groups at baseline. There was no significant difference between single and combination groups for this parameter.
2. Bacteriological improvement
a. Effect on sputum bacteriology
One trial reported change in sputum P aeruginosa density in colony forming units per gram (cfu/g) (Smith 1999). This trial showed a significant decrease in P aeruginosa density in both treatment groups, at the end of treatment, with a greater decrease in the combination group. The mean difference in the mean change in density was ‐1.60 (95% confidence interval (CI) ‐9.51 to 6.31), i.e. the mean decrease in P aeruginosa density was 1.6 cfu/g greater in the combination treatment group than for the single treatment group. However, on follow up, the density of P aeruginosa in the sputum was similar in both groups.
3. Effect on adverse events
Three trials reported adverse events (Master 1997; McCarty 1988; Smith 1999). The most commonly reported were: local erythema at the injection site; generalised rash; fever; renal impairment and proteinuria; auditory impairment; and hypersensitivity reaction, with no differences found between treatment groups.
Secondary outcomes
2. Clinical improvement
c. Hospitalisation
Two trials measured the number of participants readmitted to hospital within given time periods of one month (Huang 1982) and 80 days (Smith 1999). Huang found no significant difference between the two groups (Huang 1982). The results from the Smith trial favoured combination therapy with a Peto odds ratio of 0.30 (95% CI 0.12 to 0.73) (Smith 1999).
d. Effect on time to next course of antibiotics
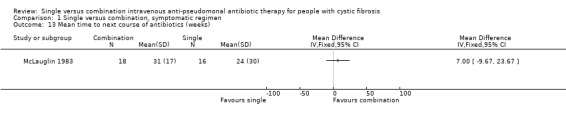
Only one trial reported the effect on the time to next course of antibiotics (McLauglin 1983). There was no significant difference between the two groups.
3. Bacteriological improvement
a. Effect on inflammatory markers
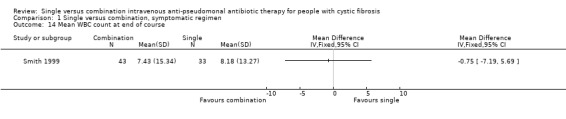
One trial reported blood or sputum markers of inflammation (Smith 1999). This trial reported mean white blood cell (WBC) count at the end of the antibiotic course and involved treatment groups with similar baseline characteristics. There was no significant difference between the groups.
b. Effect on antibiotic resistant strains of P aeruginosa
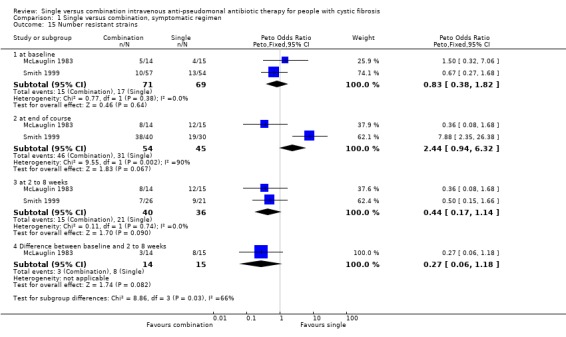
Six of the eight trials examined sputum for drug sensitivity at the beginning of the trial; five of these defined sensitivity in terms of minimum inhibitory concentration (MIC). One trial found that the disc diffusion method did not identify resistant strains (Smith 1999). The trials varied in their definitions of resistance, e.g. resistance to tobramycin was defined as MIC greater than 8 μg/ml in one trial (McLauglin 1983) and as MIC greater than 32 μg/ml in another (Master 1997). No trial used antibiotic sensitivity in their entry criteria. In one trial, the bacteria were clearly sensitive to the antibiotics used (Pedersen 1986). This trial gave mean MIC values at baseline and the end of the treatment course and found no significant change. Parry stated MIC values for all the isolates for ticarcillin and carbenicillin, but did not define antibiotic resistance (Parry 1977). This trial found that the median MIC value for ticarcillin at the end of treatment was same as the pre‐treatment value (3.1 μg/ml). One further trial stated that there was no significant difference between the single and combination groups at the beginning of the treatment period, and that emergence of resistance was not seen with any isolate (McCarty 1988). The two remaining trials gave the number or per cent of bacterial sensitivity, but did not comment on whether the two groups were significantly different at baseline (Costantini 1982; Huang 1982).
In the two trials included in the analysis, the number of participants developing resistant strains of P aeruginosa at baseline, end of treatment and follow up at between two and eight weeks was reported (McLauglin 1983; Smith 1999). For completeness, we have shown each of these analyses on the forest plot, as well as the difference between baseline and follow up in two of the trials. The second trial could not be represented in the latter analysis, as the total numbers of participants changed from baseline to follow up (Smith 1999). McLaughlin classified bacteria as resistant to tobramycin if the MIC was greater than 8 μg/ml and to azlocillin if the MIC was greater than 125 μg/ml (McLauglin 1983). Smith defined resistance to tobramycin if the MIC was greater than 8 μg/ml and for azlocillin, if the MIC was greater than 100 μg/ml (Smith 1999).
The result of the analysis showed that the difference between the single and combination therapy groups was not significant at baseline or at the end of the treatment course. At two to eight weeks follow up, both trials individually showed an increase in the number of participants with resistant strains of P aeruginosa with single therapy, but the aggregated results showed that the difference was not significant. However, the aggregation of the studies at follow up included a relatively small number of participants, with a total of 40 participants in each group. Calculation of the difference between the numbers of participants with resistant strains from baseline to two to eight weeks post‐treatment also favoured combination treatment, although the difference was not significant.
Symptomatic compared to elective regimen
One trial studied outcomes at the end of an elective course of antibiotics and again after a second course three months later, using a cross‐over design (Pedersen 1986). Results were expressed as median and range in all outcomes and therefore meta‐analysis using RevMan within this review was not possible.
Beta‐lactam versus beta‐lactam‐aminoglycoside combination compared to aminoglycoside versus beta‐lactam‐aminoglycoside combination
Insufficient data on outcome measures were given in all of the three comparisons of aminoglycoside with aminoglycoside‐beta‐lactam combination therapy and therefore meta‐analysis using RevMan within this review was not possible (Costantini 1982; Master 1997; Parry 1977).
Discussion
Choice of anti‐pseudomonal antibiotic and the use of single or combined therapy are controversial areas in the treatment of respiratory infection in cystic fibrosis (CF). Current practice is variable. Advantages of combination therapy include a wider range of modes of action, possible synergy and reduction of resistant organisms; whereas advantages of monotherapy include lower cost and reduction of drug‐related toxicity. From the perspective of the individual with CF, the use of a beta‐lactam alone offers such advantages as ease of administration and no requirement for blood sampling to measure aminoglycoside levels. Current evidence does not provide a clear answer and, therefore, the use of intravenous (IV) antibiotic therapy in CF requires further evaluation.
This review has found eight trials that examined the effect of single compared to combination IV anti‐pseudomonal antibiotic therapy for acute exacerbations in CF. There was considerable heterogeneity amongst these trials, which led to difficulties in performing the review and interpreting the results. We were unable to perform adequate meta‐analysis for most outcome measures. The overall results showed that there was no significant difference between monotherapy and combination therapy in terms of clinical outcome measures, such as lung function and symptom scores, or in terms of bacteriological outcomes.
These results should be interpreted with caution. All but two of the included trials were published between 1977 and 1988; these were single‐centre trials with flaws in the randomisation process. Furthermore, the sample sizes were too small to have the power to detect a difference between the two groups. Three of the eight trials were not published as full papers. Overall, the methodological quality was poor: only one trial was considered to have adequate randomisation allocation and concealment (Smith 1999); only four were double‐blinded; and none stated any intention‐to‐treat analysis. The review raises some interesting methodological issues, including the difficulties of pooling results from a number of small trials that are of poor quality.
The trials were very heterogeneous in terms of design, drugs used, duration of treatment and follow up and outcome measures. The combinations of antibiotics in each trial were different and therefore we aggregated the two groups according to the class of antibiotics: beta‐lactams and aminoglycosides. Inconsistencies in expression of results and statistical reporting made meta‐analysis impossible in most cases and individual patient data would need to be collected from authors to clarify these issues. It was disappointing that only four trials included data that were possible to analyse. Most of the outcome measures analysed included data from only one or two trials. Due to the small number of trials, it was not possible to examine for effects of trial quality, type of antibiotic or treatment regimen using sensitivity and subgroup analyses.
In our protocol we stated that we would like to compare the differences between adults and children. This was not possible, as no trial looked at children alone. No trial looked specifically at quality of life scores. There were no long‐term outcome measures such as the development of resistant bacterial strains or side effects, such as ototoxicity to aminoglycosides. This may be relevant particularly to children, in whom there is emerging evidence of ototoxicity due to chronic use of aminoglycosides (Katbamna 1998; Mulherin 1991). Only four trials stated that they had looked for adverse effects; therefore there may have been side effects that have not been identified. The longest follow up in this systematic review was six months, but the majority of trials did not have any follow up after the acute course of antibiotics. Potential problems with development of drug‐resistant bacteria, which may shorten long‐term survival, may not be detected in trials of such short duration covered by this review.
Authors' conclusions
Implications for practice.
The results of this review, regarding the benefits and risks of single versus combination anti‐pseudomonal antibiotic therapy in terms of lung function and clinical outcome in people with CF, are inconclusive. In particular, side effects of treatment have not been investigated to a sufficient level, and therefore it is not possible to conclude from this trial that either treatment choice is safe compared to the other. All the trials included in the review looked at different antibiotics, both as a single anti‐pseudomonal agent and in combination therapy and therefore the drug(s) of choice remains uncertain.
Implications for research.
This systematic review raises important questions regarding the use of antibiotic combinations for acute exacerbations in CF, which need to be answered by further randomised controlled trials. These trials need to be designed to overcome the methodological issues highlighted by this review, such as randomisation allocation, blinding, adequate power and long‐term follow up. There is a particular need to compare the effects of single anti‐pseudomonal therapy versus a combination of anti‐pseudomonal antibiotics in terms of long‐term toxicity and the development of drug‐resistant organisms. An observational cohort study, co‐ordinated through national databases, of centres whose practice is either monotherapy or combination therapy may give useful information on a large number of participants for these outcomes. Results need to be standardised to a consistent method of reporting, for example, mean and SD change in FEV1 and FVC expressed as % predicted in order to validate the pooling of results from multiple trials.
This could be particularly pertinent to children, in view of the emergence of long‐term side effects such as ototoxicity with cumulative use of aminoglycoside antibiotics. It would be important to address this issue within a trial.
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2016 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified three new references that were potentially eligible for inclusion in the review. One reference was an additional reference to an already excluded trial (Blumer 2005); the remaining two references to two trials were also excluded (Prayle 2013; Turner 2013). |
| 14 October 2016 | New citation required but conclusions have not changed | Dr Alison Scott has replaced Nikki Jahnke on the review team. The review title has been amended to reflect that the therapies included focus on anti‐pseudomonal antibiotics. No new data were added to the review at this update, therefore our conclusions remain the same. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 29 April 2014 | New citation required but conclusions have not changed | The previous co‐author, Dr Anton Tan, has stepped down and a new co‐author, Nikki Jahnke, has joined the review team. No new references have been added to this review, hence the conclusions remain the same. |
| 29 April 2014 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Cystic Fibrosis Trials Register identified seven references to six potentially eligible studies all of which were excluded from the review (Beringer 2012; Blumer 2005; Hatziagorou 2013; Keel 2011; McCabe 2013; Semykin 2010). |
| 17 October 2012 | Amended | Contact details updated. |
| 3 November 2011 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 15 new references potentially eligible for inclusion in this review. However, none of the references were suitable for inclusion in the review. |
| 15 September 2009 | New search has been performed | A search of the Group's Cystic Fibrosis Register identified no new references which were potentially eligible for inclusion in this review. |
| 11 November 2008 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no new references eligible for inclusion in this review. |
| 11 November 2008 | Amended | Converted to new review format. |
| 20 February 2008 | Amended | The 'Plain Language Summary' has been updated in line with latest guidance from The Cochrane Collaboration. |
| 20 February 2008 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no new references eligible for inclusion in this review. |
| 21 February 2007 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no new references eligible for inclusion in this review. |
| 15 February 2006 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no new references. |
| 9 February 2005 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no new references. One previously included trial has now been excluded from the review (Padoan 1987). In this trial a total of 40 courses of treatment took place (20 in each intervention group). The trial was cross‐over in design, however, re‐randomisation took place between courses of treatment, resulting in some participants possibly receiving two or more courses of the same treatment, or a mixture of different treatments. Since the number of participants receiving each treatment was unclear, results could not be included in the analysis of this review and therefore the trial was excluded. |
| 9 February 2005 | New citation required and conclusions have changed | Substantive amendment |
| 13 November 2002 | New search has been performed | Excluded Studies: One additional study has been added ‐ Krause 1979 . An additional reference to the Nelson 1985 study has also been included. |
Notes
Description of the pharmacological properties of the antibiotics used in the studies included in the review (Kucers 1997).
1. Beta‐Lactams
a. Carbenicillin
Carbenicillin is a semisynthetic penicillin derived from the penicillin nucleus 6 APA and can only be administered parenterally. Its most important feature is its activity against Pseudomonas aeruginosa due to its ability to penetrate the outer cell membrane of the bacteria and is less susceptible than other beta‐lactam antibiotics to at least one beta‐lactamase produced by Pseudomonas aeruginosa. It is also active against other gram positive and negative aerobic organisms including Staphylococcus aureus and Haemophilus influenzae. Principal side effects include hypersensitivity, drug fever and rarely convulsions and effects on platelet function.
b. Ticarcillin
Ticarcillin is very similar to carbenicillin but is at least twice as active against Pseudomonas aeruginosa. It has now replaced carbenicillin for clinical use. As ticarcillin is used in a lower dosage than carbenicillin, it causes fewer side effects, but can be associated with eosinophilia and urticaria.
c. Piperacillin
Piperacillin and azlocillin are semisynthetic penicillins, referred to as 'newer anti‐pseudomonal penicillins' and are considerably more active in vitro than carbenicillin and ticarcillin against Pseudomonas aeruginosa due to their ability to pass through the layers of the cell envelope to reach the enzyme penicillin‐binding protein PBP3, which is responsible for septum formation during bacterial growth and cell division. Piperacillin is not however clinically superior and development of resistant strains have been observed. Piperacillin also has activity against Burkholderia cepacia, Haemophilus influenzae, Staphylococcus aureus and other gram negative organisms. Main side effects are similar to those of carbenicillin.
d. Azlocillin
Azlocillin is a ureido‐penicillin and is similar to piperacillin in its activity against Pseudomonas aeruginosa and acts synergistically with aminoglycosides. Azlocillin‐resistant Pseudomonas aeruginosa strains are uncommon. It has some activity against Haemophilus influenzae.
e. Ceftazidime
Ceftazidime is a third generation cephalosporin, resistant to the usual beta‐lactamases of most gram negative bacteria and its activity against Pseudomonas aeruginosa is one of its most important properties. Ceftazidime‐resistant strains have been described. Ceftazidime can only be administered parenterally and acts in a similar way to penicillin G on the bacterial cell wall and shows an affinity for PBP3. Ceftazidime has low toxicity with a low incidence of hypersensitivity, eosinophilia and reversible elevations in liver enzymes.
2. Aminoglycosides
a. Gentamicin
Gentamicin is an aminoglycoside antibiotic which has particular activity against gram‐negative organisms. Its usefulness has decreased since the mid‐1970s because of the emergence of bacterial resistance. Gentamicin inhibits bacterial growth by inhibiting protein synthesis in a manner similar to streptomycin. It probably also interacts with the cell envelope of some gram‐negative bacilli such as Pseudomonas aeruginosa, resulting in lysis of the cell. Gentamicin has also been shown to inhibit the activity of the extracellular proteases secreted by Pseudomonas aeruginosa, enzymes which contribute to pathogenicity. The major side effects seen with gentamicin are ototoxicity in the form of both cochlear and vestibular toxicity with high prolonged serum levels of the drug and nephrotoxicity due to damage to the proximal tubules, characterised by excretion of casts, oliguria, proteinuria and elevated urea and creatinine. Other side effects include neuromuscular blockade, hypersensitivity reactions and haematological effects.
b. Tobramycin
Tobramycin is an aminoglycoside antibiotic with a similar mode of action to gentamicin but its advantages include greater intrinsic activity against Pseudomonas aeruginosa, activity against some gentamicin‐resistant strains and lesser nephrotoxicity and therefore is often used in preference to gentamicin. The efficacy and safety of tobramycin given as a once‐daily infusion in cystic fibrosis are currently under evaluation.
c. Sisomycin
Sisomycin is another aminoglycoside with similar antimicrobial spectrum to gentamicin. Sisomycin is more active than gentamicin, but less active than tobramycin against Pseudomonas aeruginosa. It has had limited clinical trials and has been available commercially in Europe but not in the UK, USA or Australia. The toxicity is about the same as that of gentamicin.
Acknowledgements
We would like to thank Dr A. Tan for his previous contributions to this review.
Data and analyses
Comparison 1. Single versus combination, symptomatic regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean FEV1 at end of course (% pred) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [‐9.14, 19.64] |
| 1.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐57.36, 58.36] |
| 2 Mean FVC at end of course (% pred) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.84 [‐11.44, 15.12] |
| 2.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 6.90 [‐50.50, 64.30] |
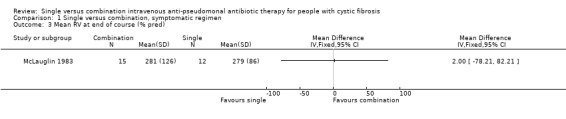
| 3 Mean RV at end of course (% pred) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
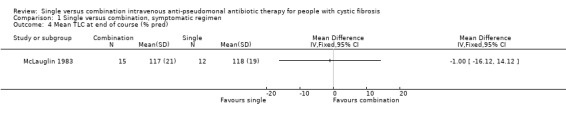
| 4 Mean TLC at end of course (% pred) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean RV/TLC at end of course (% pred) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 at 2 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐40.68, 37.88] |
| 5.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐54.27, 43.87] |
| 6 Mean PFR at end of course (% pred) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 3.21 [‐11.49, 17.91] |
| 6.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐60.90, 65.50] |
| 7 Mean MMEF at end of course (% pred) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 7.17 [‐8.22, 22.55] |
| 7.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐73.27, 69.47] |
| 8 Mean Schwachman score at end of course | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Number of Pseudomonas isolates eradicated at end of course | 3 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.63 [2.12, 14.94] |
| 10 Mean change Pseudomonas density in cfu/g at end of course | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Number adverse events | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 local erythema / irritation | 2 | 131 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.09, 2.36] |
| 11.2 generalised rash | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.16 [0.12, 316.67] |
| 11.3 fever | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.05, 14.14] |
| 11.4 renal impairment (increased creatinine by 50%) | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.15, 15.56] |
| 11.5 auditory impairment | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.86 [0.11, 305.44] |
| 11.6 proteinuria | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.62 [0.68, 19.30] |
| 12 Number readmitted | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 in 1 month | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.30] |
| 12.2 in 80 days | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.12, 0.73] |
| 13 Mean time to next course of antibiotics (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Mean WBC count at end of course | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15 Number resistant strains | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 15.1 at baseline | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.38, 1.82] |
| 15.2 at end of course | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.94, 6.32] |
| 15.3 at 2 to 8 weeks | 2 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 15.4 Difference between baseline and 2 to 8 weeks | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.06, 1.18] |
1.1. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 1 Mean FEV1 at end of course (% pred).
1.2. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 2 Mean FVC at end of course (% pred).
1.3. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 3 Mean RV at end of course (% pred).
1.4. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 4 Mean TLC at end of course (% pred).
1.5. Analysis.
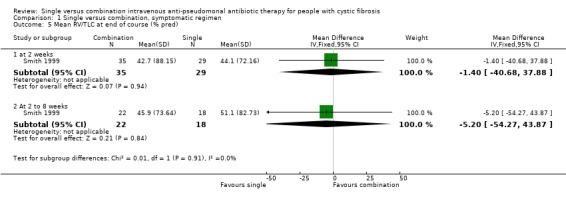
Comparison 1 Single versus combination, symptomatic regimen, Outcome 5 Mean RV/TLC at end of course (% pred).
1.6. Analysis.
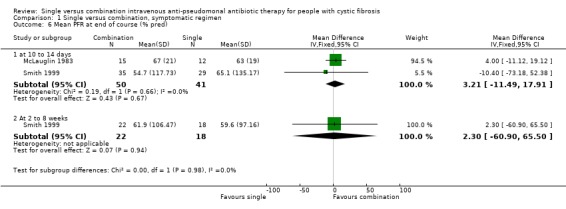
Comparison 1 Single versus combination, symptomatic regimen, Outcome 6 Mean PFR at end of course (% pred).
1.7. Analysis.
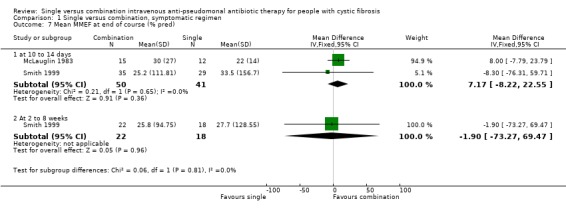
Comparison 1 Single versus combination, symptomatic regimen, Outcome 7 Mean MMEF at end of course (% pred).
1.8. Analysis.
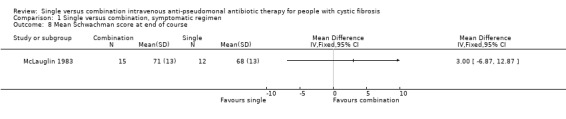
Comparison 1 Single versus combination, symptomatic regimen, Outcome 8 Mean Schwachman score at end of course.
1.9. Analysis.
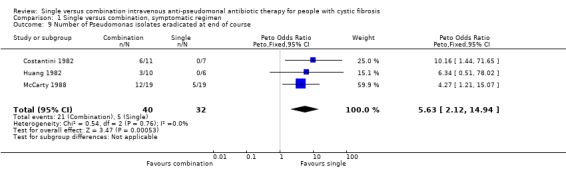
Comparison 1 Single versus combination, symptomatic regimen, Outcome 9 Number of Pseudomonas isolates eradicated at end of course.
1.10. Analysis.
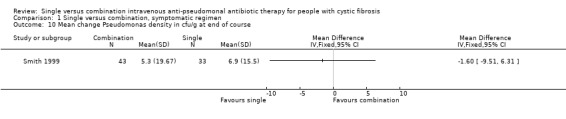
Comparison 1 Single versus combination, symptomatic regimen, Outcome 10 Mean change Pseudomonas density in cfu/g at end of course.
1.11. Analysis.
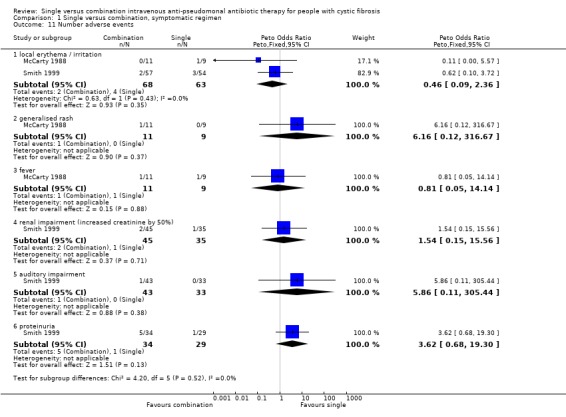
Comparison 1 Single versus combination, symptomatic regimen, Outcome 11 Number adverse events.
1.12. Analysis.
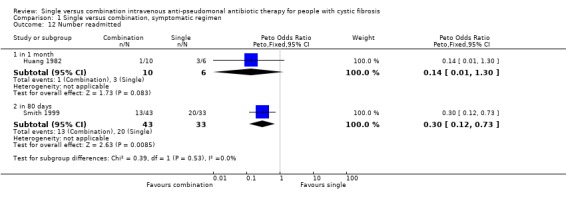
Comparison 1 Single versus combination, symptomatic regimen, Outcome 12 Number readmitted.
1.13. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 13 Mean time to next course of antibiotics (weeks).
1.14. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 14 Mean WBC count at end of course.
1.15. Analysis.
Comparison 1 Single versus combination, symptomatic regimen, Outcome 15 Number resistant strains.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Costantini 1982.
| Methods | No withdrawals, symptomatic regimen. | |
| Participants | 28 participants randomised. PsA colonised, age not stated. | |
| Interventions | Carbenicillin 675 mg/kg/day vs sisomycin 10.5 mg/kg/day vs carbenicillin 590 mg/kg/day plus sisomycin. Variable duration of course. | |
| Outcomes | CXR and symptom scores, bacteriology, development of resistant strains. | |
| Notes | Abstract: no data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | Not directly discussed, but referred to as a controlled clinical trial. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed, but appears to be no withdrawals. |
Huang 1982.
| Methods | Double‐blind trial, no withdrawals, symptomatic regimen. Parallel trial. | |
| Participants | 16 participants randomised. Mixed PsA and non‐PsA, age not stated. | |
| Interventions | Ticarcillin 300 mg/kg/day vs ticarcillin plus tobramycin 6 mg/kg/day. 10‐day course. | |
| Outcomes | Lung function, number readmitted within one month, CXR and symptom scores, bacteriology. | |
| Notes | Abstract: Lung function, CXR and symptom score data not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions randomisation code, but no details given of how it was generated. |
| Allocation concealment (selection bias) | Unclear risk | Mentions randomisation code, but no details of how this may have been concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated as double‐blind, but no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed, but appears to be no drop outs or withdrawals. |
Master 1997.
| Methods | Double‐blind trial, symptomatic regimen. Parallel trial. | |
| Participants | 83 participants randomised. PsA colonised, age not stated. 51 participants randomized, of these, 21 in the tobramycin and ceftazidime group (51 admissions assessed) and 23 in the tobramycin group (47 admissions assessed). 12 participants in the tobramycin and ceftazidime group and 9 participants in the tobramycin group were eligible for long‐term assessment. Participants in both groups experienced an average of 3.1 and 3.0 admissions, respectively, for IV antibiotic treatment during the study period. Tobramycin and ceftazidime group: mean (SD) age 16 (7) years Tobramycin group: mean (SD) age 14 (5) years |
|
| Interventions | Tobramycin 24‐hourly vs tobramycin and ceftazidime 8‐hourly. 10‐day course. | |
| Outcomes | Lung function, adverse events. | |
| Notes | Full paper. Exclusion criteria stated. The study was halted for a period of 3 months when one of the study patients committed suicide by utilizing a study syringe to administer a lethal substance. The study was recommenced after the coroner's finding that this was an unrelated death. During this time of study suspension, there were 14 admissions of patients previously enrolled. Data from these admissions were not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified for age and disease severity. |
| Allocation concealment (selection bias) | Low risk | The treatment code was broken only at the completion of the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medical staff, nursing staff and participants were blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart showing numbers randomized and included/excluded (with reasons) at each stage in paper. |
McCarty 1988.
| Methods | Not double blind, no withdrawals, symptomatic regimen. | |
| Participants | 17 participants treated (8 in piperacillin group, 9 in piperacillin plus tobramycin group); 3 participants treated on more than one occasion (2 initially in piperacillin group and several months later randomised to other group; 1 participant enrolled 2x in piperacillin group). 20 data sets. Mixed PsA and non‐PsA, aged 2 to 12 years. | |
| Interventions | Piperacillin 600 mg/kg/day vs piperacillin plus tobramycin 8 to 10 mg/kg/day, minimum duration 10 days. | |
| Outcomes | Lung function, weight, symptom scores, adverse events, bacteriology. | |
| Notes | No data for lung function, weight and symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment randomly assigned, no further details. |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear explanation of participants in groups, no drop outs occurred. |
McLauglin 1983.
| Methods | Double‐blind trial, symptomatic regimen. | |
| Participants | 41 participants randomised (11 years and older), 34 completed. No ITT analysis. Mean age 21 years. PsA in 98%. | |
| Interventions | Azlocillin 300 mg/kg/day, 4‐hourly plus placebo vs azlocillin plus tobramycin 6 mg/kg/day, 8‐hourly. 10‐day course. Group 1: azlocillin 300 mg/kg/day in 6 divided doses plus tobramycin (6 mg/kg per day) in 3 divided doses. Group 2:azlocillin 300 mg/kg/day in 6 divided doses plus placebo (0.85% NaCl) in 3 divided doses. |
|
| Outcomes | Lung function, symptom scores, development of resistant strains, time to next course. | |
| Notes | Participants were white, of various socioeconomic backgrounds and lived in New England. Exclusion criteria stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly selected, but no further details given. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist used consecutively numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither participants or clinicians knew which regimen they were receiving. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in azlocillin plus placebo group withdrawn due to suspected drug‐related complications; 2 participants discharged improved before completion of antibiotic course; 3 withdrawn due to incomplete outcome data. |
Parry 1977.
| Methods | Not double blind, alternate allocation, no withdrawals, symptomatic regimen. Parallel trial. | |
| Participants | 21 male, 21 female, mean age 15.1 years. PsA colonised. 14 participants in each of 3 treatment groups. Group 1 (ticarcillin): 8 male, 6 female; mean (range) age 16.1 (2 ‐ 30) years Group 2 (ticarcillin & gentamicin): 7 males, 7 females: mean (range) age 16.4 (4 ‐ 30) years Group 3 (gentamicin):6 males, 8 females; mean (range) 12.9 (5 ‐ 31) years |
|
| Interventions | Ticarcillin 300 mg/kg/day, 4‐hourly vs gentamicin 3 ‐ 4 mg/kg/day (adults), 4 ‐ 7 mg/kg/day (children) vs combination. Variable length of course. | |
| Outcomes | Lung function, bacteriology, adverse events, CBC, sedimentation rate, urinalysis, serum electrolytes, blood urea nitrogen, creatinine, liver function tests, chest radiographs, blood gas determinations, sputum cultures, change in cough, weight. | |
| Notes | No data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No discussion of how first participant was assigned to which treatment group. |
| Allocation concealment (selection bias) | High risk | Alternation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Drugs administered in different ways so clinicians and participants couldn't be blinded, no discussion of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop outs or withdrawals. |
Pedersen 1986.
| Methods | Not double blind, elective regimen, crossover ‐ 3 months in between treatment arms. | |
| Participants | 20 participants (10 male, 10 female), mean age 12.6 years. PsA colonised. 3 drop outs, 17 completed trial. | |
| Interventions | Ceftazidime 150 mg/kg/day, 8‐hourly vs ceftazidime plus tobramycin 10 mg/kg/day, 8‐hourly, 14‐day course. | |
| Outcomes | Lung function, inflammatory markers, development of resistant strains. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of method given. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both interventions given with same volume and in same way. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded ‐ reasons given (bacteriological resistance developed between treatment arms in 2 participants and a 3rd withdrew on first day of 2nd treatment arm due to nausea). |
Smith 1999.
| Methods | Double‐blind, computer‐generated randomisation, symptomatic regimen. Parallel trial. | |
| Participants | 37 male, 39 female, mean age 16.3 years.
111 participants enrolled, 35 withdrawn. 76 participants in total aged 6 ‐ 18 years. PsA colonised. Group 1 (azlocillin): 33 participants (19 male) mean (SD) age 16.07 (7.4) years Group 2 (azlocillin & tobramycin): 43 participants (18 male) mean (SD) age 16.53 (6.9) years |
|
| Interventions | Azlocillin 450 mg/kg/day, 4‐hourly plus placebo vs azlocillin plus tobramycin 240 mg/m2/day, 6‐hourly, 14 days course. | |
| Outcomes | Lung function, time to next admission, symptom scores, adverse events, bacteriology, inflammatory markers, resistant strains. | |
| Notes | No data for symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation balance by FVC and center. |
| Allocation concealment (selection bias) | Low risk | Code generated by research pharmacist at the core center. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and clinicians blinded, serum concentrations monitored by unblinded 3rd party (research pharmacist). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 35 participants withdrawn (21 from azlocillin group), reasons given in a table. |
CBC: complete blood count CXR: chest x‐ray ITT: intention‐to‐treat PsA: Pseudomonas aeruginosa
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeboyeku 2011 | Comparison of twice daily vs three times daily antibiotics, not single vs combination. |
| Al‐Ansari 2006 | Comparison of once vs multiple daily dosing, not single vs combination. |
| Aminimanizani 2002 | Comparison of single vs multiple daily dosing, not single vs combination. |
| Balsamo 1986 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Beaudry 1980 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Beringer 2012 | Not a comparison of single vs combination antibiotics; a comparison of a single intravenous dose of an antibiotic and multiple oral doses of the same antibiotic. |
| Blumer 2005 | Comparison of two combination regimens. |
| Bosso 1988 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Church 1997 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Conway 1997 | Comparison of colistin with multiple antibiotic combinations. |
| De Boeck 1989 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| De Boeck 1999 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Donati 1987 | Home vs hospital therapy, not single vs combination. |
| Gold 1985 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Hatziagorou 2013 | Not a comparison of a single vs combination antibiotics; evaluation of tool to assess treatment response in children. |
| Hoogkamp 1983 | Non‐randomised study: first 7 participants allocated to single treatment; next 7 to combination treatment with marked differences in baseline characteristics. |
| Hubert 2009 | Comparison of intermittent vs continuous infusions, not single vs combination. |
| Hyatt 1981 | Comparison of anti‐staphylococcal drug (oxacillin) vs oxacillin plus 2 anti‐pseudomonal drugs. |
| Jewett 1985 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Keel 2011 | Not a comparison of a single vs combination antibiotic; comparison of intravenous and oral versions of the same agent. |
| Kenny 2009 | Study of eradication of Pseudomonas aeruginosa, not a comparison of single vs combination. |
| Krause 1979 | Pseudo‐randomised study. Treatment and comparison groups were not sufficiently similar at baseline. |
| Kuni 1992 | Not a comparison of single vs combination antibiotics. |
| Levy 1982 | Comparison of 2 single agents. |
| McCabe 2013 | Not a comparison of single vs combination antibiotics; evaluation of a twice‐daily tobramycin regimen. |
| Nelson 1985 | Review article on single vs combination antibiotic treatment, i.e. not an RCT. |
| Noah 2010 | Inhaled vs systemic antibiotics. |
| Padoan 1987 | Reported number of courses of treatment instead of number of people included. Some participants may have been counted twice or included in both treatment group therefore analysis unclear. |
| Permin 1983 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Prayle 2013 | Not a comparison of single vs combination antibiotics; comparison of morning vs evening intravenous tobramycin. |
| Riethmueller 2009 | Continuous vs intermittent infusions. |
| Roberts 1993 | Randomisation method unclear ‐ participants appeared to have been randomised to single or combination therapy each morning using a cross‐over method. |
| Semykin 2010 | Not a comparison of single vs combination antibiotics; trial of inhaled tobramycin therapy. |
| Stack 1985 | Comparison of single agent compared with two other antibiotics (i.e. drug A vs drug B plus drug C). |
| Turner 2013 | Not a comparison of single vs combination antibiotics; study of continuous vs intermittent infusion piperacillin‐tazobactam. |
| Wesley 1988 | Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). |
| Whitehead 2002 | Efficacy of once daily tobramycin, not a comparison of single vs combination agents. |
CXR: chest X‐ray vs: versus
Contributions of authors
Dr. Anton Tan assisted in the assessment of trial quality and extraction of data up until 2013.
Dr. Heather Elphick performed the updates and acts as guarantor of the review. Dr Alison Scott joined the author team as a co‐author from the update in 2016.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Heather Elphick has no interests to declare.
Dr Alison Scott has no interests to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Costantini 1982 {published data only}
- Costantini D, Padoan R, Brienza A, Lodi G, Assael BM, Giunta A. Clinical evaluation of carbenicillin and sisomycin alone or in combination in CF patients with pulmonary exacerbations [abstract]. Proceedings of the 11th European Cystic Fibrosis Conference. 1982:227. [CFGD Register: PI122]
Huang 1982 {published data only}
- Huang NN, Palmer J, Braverman S, Keith HH, Schidlow D. Therapeutic efficacy of ticarcillin and carbenicillin in patients with cystic fibrosis: a double blind study [abstract]. 23rd Cystic Fibrosis Club Abstracts; 1982 May 14; Washington DC. 1982:124. [CFGD Register: PI114]
Master 1997 {published data only}
- Master V, Martin AJ, Holmes M, Roberts G, Coulthard K. Once daily tobramycin monotherapy versus conventional antibiotic therapy for the treatment of pseudomonal pulmonary exacerbations in cystic fibrosis patients [abstract]. European Respiratory Journal 1997;10(Suppl 25):162s. [CFGD Register: PI148a] [Google Scholar]
- Master V, Roberts GW, Coulhard KP, Baghurst PA, Martin A, Roberts ME, et al. Efficacy of once‐daily tobramycin monotherapy for acute pulmonary exacerbations of cystic fibrosis: a preliminary study. Pediatric Pulmonology 2001;31(5):367‐76. [CFGD Register: PI148b] [DOI] [PubMed] [Google Scholar]
McCarty 1988 {published data only}
- McCarty JM, Tilden SJ, Black P, Craft JC, Blumer J, Waring W, et al. Comparison of piperacillin alone versus piperacillin plus tobramycin for treatment of respiratory infections in children with cystic fibrosis. Pediatric Pulmonology 1988;4(4):201‐4. [CFGD Register: PI58] [DOI] [PubMed] [Google Scholar]
McLauglin 1983 {published data only}
- McLaughlin FJ, Matthews WJ Jr, Strieder DJ, Sullivan B, Goldmann DA. Randomized, double‐blind evaluation of azlocillin for the treatment of pulmonary exacerbations of cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;11 Suppl B:195‐203. [CFGD Register: PI26a] [DOI] [PubMed] [Google Scholar]
- McLaughlin FJ, Matthews WJ, Strieder DJ, Sullivan B, Taneja A, Murphy P, et al. Clinical and bacteriological responses to three antibiotic regimens for acute exacerbations of cystic fibrosis: ticarcillin‐tobramycin, azlocillin‐tobramycin and azlocillin‐placebo. The Journal of Infectious Diseases 1983;147(3):559‐66. [CFGD Register: PI26b] [DOI] [PubMed] [Google Scholar]
Parry 1977 {published data only}
- Parry MF, Neu HC, Merlino M, Gaerlan PF, Ores CN, Denning CR. Treatment of pulmonary infections in patients with cystic fibrosis: a comparative study of ticarcillin and gentamicin. Journal of Pediatrics 1977;90(1):144‐8. [CFGD Register: PI17] [DOI] [PubMed] [Google Scholar]
Pedersen 1986 {published data only}
- Pedersen SS, Pressler T, Pedersen M, Hoiby N, Friis‐Moller A, Kock C. Immediate and prolonged clinical efficacy of Ceftazidime versus Ceftazidime plus Tobramycin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. Scandinavian Journal of Infectious Diseases 1986;18:133‐7. [CFGD Registere: PI42] [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, et al. Comparison of a B‐lactam alone versus B‐lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 1999;134(4):413‐21. [CFGD Register: PI152] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adeboyeku 2011 {published data only}
- Adeboyeku D, Jones AL, Hodson ME. Twice vs three‐times daily antibiotics in the treatment of pulmonary exacerbations of cystic fibrosis. Journal of Cystic Fibrosis 2011;10(1):25‐30. [CFGD Register: PI243] [DOI] [PubMed] [Google Scholar]
Al‐Ansari 2006 {published data only}
- Al Ansari NA, Foweraker J, Mackeown D, Bilton D. Evaluation of once daily tobramycin versus the traditional three times daily for the treatment of acute pulmonary exacerbations in adult cystic fibrosis patients [abstract]. Qatar Medical Journal 2006;15(1):34‐8. [CFGD Register: PI173b] [Google Scholar]
- Al‐Ansari N, McKeon D, Parmer J, Gunn E, Foweraker J, Bilton D. Efficacy of once daily tobramycin for acute pulmonary exacerbations of cystic fibrosis (CF) ‐ a microbiological perspective [abstract]. Thorax 2001;56(Suppl 3):iii85. [CFGD Register: PI173a] [Google Scholar]
Aminimanizani 2002 {published data only}
- Aminimanizani A, Beringer PM, Kang J, Tsang L, Jelliffe RW, Shapiro BJ. Distribution and elimination of tobramycin administered in single or multiple daily doses in adult patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy 2002;50(4):553‐9. [CFGD Register: PI158b] [DOI] [PubMed] [Google Scholar]
- Tsang L, Aminimanizani A, Beringer PM, Jelliffe R, Shapiro B. Pharmacokinetics of once‐daily tobramycin in adult cystic fibrosis patients [abstract]. Pediatric Pulmonology 2000;30(Suppl 20):284. [CFGD Register: PI158a] [Google Scholar]
Balsamo 1986 {published data only}
- Balsamo V, Bragion E, Iapichino L, Natozi D, Pardo F. Clinical efficacy and "in vitro" activity of some antibiotics, ceftazidime aztreonam or carbenicillin with aminoglycosides against Pseudomonas in Cystic fibrosis patients [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis. 1986:63. [CFGD Register: PI84]
Beaudry 1980 {published data only}
- Beaudry PH, Marks MI, McDougall D, Desmond K, Rangel R. Is anti‐Pseudomonas therapy warranted in acute respiratory exacerbations in children with cystic fibrosis?. Journal of Pediatrics 1980;97(1):144‐7. [CFGD Register: PI21a] [DOI] [PubMed] [Google Scholar]
- Beaudry PH, Marks MI, Rangel R, McDougall D, Desmond K. Is anti‐pseudomonas therapy warranted in acute respiratory exacerbations in children with cystic fibrosis? [abstract]. 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:1. [CFGD Register: PI21b] [DOI] [PubMed]
Beringer 2012 {published data only}
- Beringer P, Owens H, Nguyen A, Benitez D, Boyd‐King A, Rao AP. Safety, pharmacokinetics and preliminary evaluation of the antiinflammatory effect of doxycycline in CF [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:370, Abstract no: 422. [CFGD Register: PI256a; ] [Google Scholar]
- Beringer PM, Owens H, Nguyen A, Benitez D, Rao A, D'Argenio DZ. Pharmacokinetics of doxycycline in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2012;56(1):70‐4. [CFGD Register: PI256b; ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumer 2005 {published data only}
- Blumer JL, Minkwitz M, Saiman L, San Gabriel P, Iaconis J, Melnick D. Meropenem (MEM) compared with ceftazidime (CAZ) in combination with tobramycin (TOB) for treatment of acute pulmonary exacerbations (APE) in patients with cystic fibrosis (CF) infected with Pseudomonas aeruginosa (PA) or Burkholderia cepacia (BC) [abstract]. Pediatric Pulmonology 2003;Suppl 25:294. [CFGD Register: PI179a; ] [Google Scholar]
- Blumer JL, Saiman L, Konstan MW, Melnick D. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest 2005;128(4):2336‐46. [CENTRAL: 531312; CFGD Register: PI179b; CRS: 5500100000006414; PUBMED: 16236892] [DOI] [PubMed] [Google Scholar]
Bosso 1988 {published data only}
- Bosso JA, Black PG. A comparative trial of aztreonam and tobramycin plus azlocillin [abstract]. Excerpta medica, Asia Pacific Congress Series 1988;74:R(c)17. [CFGD Register: PI59a] [Google Scholar]
- Bosso JA, Black PG. Controlled trial of aztreonam vs tobramycin and azlocillin for acute pulmonary exacerbations of cystic fibrosis. Pediatric Infectious Diseases Journal 1988;7(3):171‐6. [CFGD Register: PI59b] [DOI] [PubMed] [Google Scholar]
Church 1997 {published data only}
- Church DA, Kanga JF, Kuhn RJ, Rubio TT, Spohn WA, Stevens JC, et al. Sequential ciprofloxacin therapy in pediatric cystic fibrosis: comparative study vs. ceftazidime/tobramycin in the treatment of acute pulmonary exacerbations. Pediatric Infectious Diseases Journal 1997;16(1):97‐105. [CFGD Register: PI115] [DOI] [PubMed] [Google Scholar]
Conway 1997 {published data only}
- Conway SP, Pond M, Watson A, Etherington C, Robey HL, Goldman MH. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 1997;52(11):987‐93. [CFGD Register: PI103c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SP, Pond M, Watson A, Robey H, Goldman M. A safety profile of intravenous colomycin in adult care [abstract]. 20th European Cystic Fibrosis; 1995 June 18‐21; Brussels, Belgium. 1995:P3. [CFGD Register: PI103a]
- Conway SP, Pond MN, Watson AJ, Robey H, Goldman MH. Colistin alone or in combination with a second antibiotic is effective in the treatment of respiratory exacerbations in cystic fibrosis [abstract]. Pediatric Pulmonology 1996;Suppl 13:296. [CFGD Register: PI103b] [Google Scholar]
De Boeck 1989 {published data only}
- Boeck K, Smet M, Eggermont E. Treatment of Pseudomonas lung infection in cystic fibrosis with piperacillin plus tobramycin versus ceftazidime monotherapy. Pediatric Pulmonology 1989;7(3):171‐3. [CFGD Register: PI61] [DOI] [PubMed] [Google Scholar]
De Boeck 1999 {published data only}
- Boeck K, Sauer K, Vandeputte S. Meropenem versus ceftazidime plus tobramycin for pulmonary disease in CF patients [abstract]. The Netherlands Journal of Medicine 1999;54:S39. [CFGD Register: PI150] [Google Scholar]
Donati 1987 {published data only}
- Donati MA, Guenette G, Auerbach H. Prospective controlled study of home and hospital therapy of cystic fibrosis pulmonary disease. Journal of Pediatrics 1987;111(1):28‐33. [CFGD Register: PI143; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 1985 {published data only}
- Gold R, Overmeyer A, Knie B, Fleming PC, Levison H. Controlled trial of ceftazidime vs ticarcillin and tobramycin in the treatment of acute respiratory exacerbations in patients with cystic fibrosis. Pediatric Infectious Disease 1985;4(2):172‐7. [CFGD Register: PI35] [DOI] [PubMed] [Google Scholar]
Hatziagorou 2013 {published data only}
- Hatziagorou E, Avramidou V, Kirvassilis F, Tsanakas J. Lung clearance index: a tool to assess the response to intravenous treatment among children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2013;12 Suppl 1:S26, Abstract no: WS13.3. [CFGD Register: PI265; ] [Google Scholar]
Hoogkamp 1983 {published data only}
- Hoogkamp‐Korstanje JA, Laag J. Piperacillin and tobramycin in the treatment of Pseudomonas lung infections in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;12(2):175‐83. [CFGD Register: PI140] [DOI] [PubMed] [Google Scholar]
Hubert 2009 {published data only}
- Hubert D, Roux E, Lavrut T, Wallaert B, Scheid P, Manach D, et al. Continuous versus intermittent infusions of ceftazidime for treating exacerbation of cystic fibrosis. Antimicrobial Agents and Chemotherapy 2009;53(9):3650‐6. [CFGD Register: PI180b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert D, Wallaert B, Scheid P, Ramel S, Grenet D, Sermet‐Gaudelus I, et al. Continuous infusion versus intermittent administration of ceftazidime in cystic fibrosis patients [abstract]. Pediatric Pulmonology 2003;Suppl 25:294. [CFGD Register: PI180a] [Google Scholar]
Hyatt 1981 {published data only}
- Hyatt AC, Chipps BE, Kumor KM, Mellits ED, Lietman PS, Rosenstein BJ. A double‐blind controlled trial of anti‐Pseudomonas chemotherapy of acute respiratory exacerbations in patients with cystic fibrosis. Journal of Pediatrics 1981;99(2):307‐11. [CFGD Register: PI23] [DOI] [PubMed] [Google Scholar]
Jewett 1985 {published data only}
- Jewett CV, Ledbetter J, Lyrene RK, Brasfield DM, Tiller RE. Comparison of cefoperazone sodium vs methicillin, ticarcillin and tobramycin in treatment of pulmonary exacerbations in patients with cystic fibrosis. Journal of Pediatrics 1985;106(4):669‐72. [CFGD Register: PI36] [DOI] [PubMed] [Google Scholar]
Keel 2011 {published data only}
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2011;55(7):3393‐8. [CFGD Register: PI251; ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenny 2009 {published data only}
- Kenny S, Hall V, Goldsmith C, Moore J, Rendall JC, Elborn JS. Eradication of Pseudomonas aeruginosa in adults with CF [abstract]. Journal of Cystic Fibrosis 2009;8(Suppl 2):S39, Abstract no: 158. [CFGD Register: PI229] [Google Scholar]
Krause 1979 {published data only}
- Krause PJ, Young LS, Cherry JD, Osher AB, Spencer MJ, Bryson YJ. The treatment of exacerbations of pulmonary disease in cystic fibrosis: netilmicin compared with netilmicin and carbenicillin. Current Therapeutic Research Clinical and Experimental 1979;25:609. [CFGD Register: PI166] [Google Scholar]
Kuni 1992 {published data only}
- Kuni CC, Regelmann WE, duCret RP, Boudreau RJ, Budd JR. Aerosol scintigraphy in the assessment of therapy for cystic fibrosis. Clinical Nuclear Medicine 1992;17(2):90‐3. [CFGD Register: PI236] [DOI] [PubMed] [Google Scholar]
Levy 1982 {published data only}
- Levy J, Baran D, Klastersky J. Comparative study of the antibacterial activity of amikacin and tobramycin during Pseudomonas pulmonary infection in patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy 1982;10(3):227‐34. [CFGD Register: PI134] [DOI] [PubMed] [Google Scholar]
McCabe 2013 {published data only}
- McCabe D, Rodgers HC. Evaluation of a twice daily tobramycin regimen in adult cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2013;12 Suppl 1:S71, Abstract no: 89. [CFGD Register: PI266; ] [Google Scholar]
Nelson 1985 {published data only}
- Jackson MA, Kusmiesz H, Shelton S, Prestidge C, Kramer RI, Nelson JD. Comparison of piperacillin vs. ticarcillin plus tobramycin in the treatment of acute pulmonary exacerbations of cystic fibrosis. Pediatric Infectious Disease 1986;5(4):440‐3. [CFGD Register: PI38b] [DOI] [PubMed] [Google Scholar]
- Nelson JD. Management of acute pulmonary exacerbations in cystic fibrosis: a critical appraisal. Journal of Pediatrics 1985;106:1030‐4. [CFGD Register: PI38a] [DOI] [PubMed] [Google Scholar]
Noah 2010 {published data only}
- Noah T, Ivins S, Abode K, Harris W, Henry M, Leigh M. Comparison of antibiotics for early pseudomonas infection in CF: interim data analysis [abstract]. Pediatric Pulmonology 2007;42 Suppl 30:332. [CFGD Register: PI205a] [Google Scholar]
- Noah TL, Ivins SS, Abode KA, Stewart PW, Michelson PH, Harris WT, et al. Inhaled versus systemic antibiotics and airway inflammation in children with cystic fibrosis and Pseudomonas. Pediatric Pulmonology 2010;45(3):281‐90. [CFGD Register: PI205b] [DOI] [PubMed] [Google Scholar]
Padoan 1987 {published data only}
- Padoan R, Cambisano W, Constantini D, Crossignani R, Giunta A. Pseudomonas pulmonary infections in cystic fibrosis: Ceftazidime vs ceftazidime plus sisomicin vs piperacillin plus sisomicin [abstract]. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:Poster 4.21. [CFGD Register: PI52a]
- Padoan R, Cambisano W, Costantini D, Crossignani RM, Danza ML, Trezzi G, et al. Ceftazidime monotherapy vs combined therapy in Pseudomonas pulmonary infections in cystic fibrosis. Pediatric Infectious Diseases Journal 1987;6(7):648‐53. [CFGD Register: PI52b] [DOI] [PubMed] [Google Scholar]
Permin 1983 {published data only}
- Permin H, Koch C, Hoiby N, Christensen HO, Moller AF, Moller S. Ceftazidime treatment of chronic Pseudomonas aeruginosa respiratory tract infection in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):313‐23. [CFGD Register: PI29] [DOI] [PubMed] [Google Scholar]
Prayle 2013 {published data only}
- Prayle A, Jain K, Watson A, Smyth AR. Are morning doses of intravenous tobramycin less nephrotoxic than evening? Evidence from urinary biomarkers in the critic study [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:299, Abstract no: 261. [CENTRAL: 980338; CFGD Register: CO55; CRS: 5500125000000420] [Google Scholar]
Riethmueller 2009 {published data only}
- Riethmueller J, Ballmann M, Schroeter TW, Franke P, Butler R, Claass A, et al. Tobramycin once‐ vs thrice‐daily for elective intravenous antipseudomonal therapy in pediatric cystic fibrosis patients. Infection 2009;37(5):424‐31. [CFGD Register: PI154f] [DOI] [PubMed] [Google Scholar]
- Riethmueller J, Busch A, Franke P, Ziebach R, Butler R, Stern M. Pharmacodynamic variation of intravenous antibiotic treatment with ceftazidime and tobramycin in CF‐patients is equally effective [abstract]. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:166. [CFGD Register: PI154a]
- Riethmueller J, Franke P, Schroeter TW, Claass A, Busch A, Ziebach R, et al. Optimised intravenous antibiotic treatment with ceftazidime (thrice daily vs continuous) and tobramycin (thrice vs once daily) in CF patients [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P192. [CFGD Register: PI154b]
- Riethmueller J, Junge S, Schroeter TW, Kuemmerer K, Franke P, Ballmann M, et al. Continuous vs thrice‐daily ceftazidime for elective intravenous antipseudomonal therapy in cystic fibrosis. Infection 2009;37(5):418‐23. [CFGD Register: PI154e] [DOI] [PubMed] [Google Scholar]
- Schroeter T, Eggert P, Riethmueller J, Stern M, Claass A. Acute‐phase nephrotoxicity of tobramycin in CF patients: A prospective randomized trial of once‐versus thrice‐daily dosing [abstract]. Pediatric Pulmonology 2002;Suppl 24:289. [CFGD Register: PI154d] [Google Scholar]
- Schroeter TW, Eggert P, Riethmueller J, Stern M, Claass A. A prospective randomized trial on the nephrotoxicity of thrice‐daily versus once‐daily tobramycin in cystic fibrosis patients [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P190. [CFGD Register: PI154c]
Roberts 1993 {published data only}
- Roberts GW, Nation RL, Jarvinen AO. Measurement of serum tobramycin in the presence of ticarcillin or piperacillin. Australian Journal of Hospital Pharmacy 1992;22(2):152‐4. [CFGD Register: PI132b] [Google Scholar]
- Roberts GW, Nation RL, Jarvinen AO, Martin AJ. An in vivo assessment of the tobramycin/ticarcillin interaction in cystic fibrosis patients. British Journal of Clinical Pharmacology 1993;36(4):372‐5. [CFGD Register: PI132a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Semykin 2010 {published data only}
- Semykin SY, Polikarpova SV, Dubovik LG, Kashirskaya NY. Efficiency of the inhalational tobramycin therapy in complex antibacterial therapy of lung exacerbation in cystic fibrosis children with chronic pseudomonas aeruginosa infection [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S55, Abstract no: 214. [CFGD Register: PI246; ] [Google Scholar]
Stack 1985 {published data only}
- British Thoracic Society Research Committee. Ceftazidime compared with gentamicin and carbenicillin in patients with cystic fibrosis, pulmonary pseudomonas infection, and an exacerbation of respiratory symptoms. Thorax 1985;40(5):358‐63. [CFGD Register: PI37a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack BHR, Geddes DM, Williams KJ, Dinwiddie R, Selkon JB, Godfrey RC. Ceftazidime compared with a combination of gentamicin and carbenicillin in cystic fibrosis patients with persistent pulmonary pseudomonas infection and an acute exacerbation of respiratory symptoms [abstract]. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:4.16. [CFGD Register: PI37b]
Turner 2013 {published data only}
- Turner R, Biondo LR, Slain D, Phillips U, Cardenas SC, Moffett K. A randomized pilot study of continuous versus intermittent infusion piperacillin‐tazobactam for the treatment of pulmonary exacerbations in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:326, Abstract no: 332. [CENTRAL: 980337; CFGD Register: PI272; CRS: 5500125000000418] [Google Scholar]
Wesley 1988 {unpublished data only}
- Wesley AW, Quested C, Edgar BW, Lennon DR. A double‐blind comparison of ceftazidime with tobramycin and ticarcillin in the treatment of exacerbations of pseudomonas chest infection in children with cystic fibrosis [abstract]. Proceedings of the 10th International Cystic Fibrosis Congress; 1988 March 5‐10; Sydney, Australia. (Excerpta Medica, Asia Pacific Congress Series, No. 74). 1988:13.
Whitehead 2002 {published data only}
- Watson A, Whitehead A, Conway SP, Etherington C. Efficacy and safety of once daily tobramycin in treating acute respiratory exacerbations in adult patients [abstract]. Pediatric Pulmonology 1999;28(19):262‐3. [CFGD Register: PI149b] [Google Scholar]
- Whitehead A, Conway SP, Etherington C, Caldwell NA, Setchfield N, Bogle S. Once‐daily tobramycin in the treatment of adult patients with cystic fibrosis. European Respiratory Journal 2002;19(2):303‐9. [CFGD Register: PI149c] [DOI] [PubMed] [Google Scholar]
- Whitehead A, Conway SP, Etherington C, Dave J. Efficacy and safety of once daily tobramycin in treating acute respiratory exacerbations in adult patients [abstract]. The Netherlands Journal of Medicine 1999;54(Suppl):S36‐7. [CFGD Register: PI149a] [Google Scholar]
Additional references
Cantin 1995
- Cantin A. Cystic fibrosis lung inflammation: early, sustained and severe. American Journal of Respiratory and Critical Care Medicine 1995;151(4):939‐41. [DOI] [PubMed] [Google Scholar]
Cheng 1996
- Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, et al. Spread of beta‐lactam‐resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 1996;348(9028):639‐42. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Frederiksen 1999
- Frederiksen B, Koch C, Hoiby N. Changing epidemiology of Pseudomonas aeruginosa infection in Danish cystic fibrosis patients. Pediatric Pulmonology 1999;28(3):159‐66. [DOI] [PubMed] [Google Scholar]
Hoiby 1993
- Hoiby N. Antibiotic therapy for chronic infection of pseudomonas in the lung. Annual Review of Medicine 1993;44:1‐10. [DOI] [PubMed] [Google Scholar]
Katbamna 1998
- Katbamna B, Homnick DN, Marks JH. Contralateral suppression of distortion product otoacoustic emissions in children with cystic fibrosis: effects of tobramycin. Journal of the American Academy of Audiology 1998;9(3):172‐8. [PubMed] [Google Scholar]
Konstan 1997
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatric Pulmonology 1997;24(2):137‐42. [DOI] [PubMed] [Google Scholar]
Kucers 1997
- Kucers A, Crowe S, Grayson M, Hoy J (editors). The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs. 5th Edition. Oxford: Hodder Arnold, 1997. [ISBN‐10: 0750601558 ] [Google Scholar]
Levy 1998
- Levy SB. Antimicrobial resistance: bacteria on the defence. Resistance stems from misguided efforts to try to sterilise our environment. BMJ 1998;317(7159):612‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulherin 1991
- Mulherin D, Fahy J, Grant W, Keogan M, Kavanagh B, FitzGerald M. Aminoglycoside induced ototoxicity in patients with cystic fibrosis. Irish Journal of Medical Science 1991;160(6):173‐5. [DOI] [PubMed] [Google Scholar]
Regelmann 1990
- Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. American Review of Respiratory Disease 1990;141(4 Pt 1):914‐21. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical Evidence of Bias. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Tan 2002
- Tan KH, Hyman‐Taylor P, Mulheran M, Knox A, Smyth A. Lack of concordance in the use and monitoring of intravenous aminoglycosides in UK cystic fibrosis centers. Paediatric Pulmonology 2002;33(2):165. [DOI] [PubMed] [Google Scholar]
Taylor 1993
- Taylor RF, Hodson ME. Cystic Fibrosis: antibiotic prescribing practices in the United Kingdom and Eire. Respiratory Medicine 1993;87(7):535‐9. [DOI] [PubMed] [Google Scholar]
Wolter 1999
- Wolter JM, Bowler SD, McCormack JG. Are antipseudomonal antibiotics really beneficial in acute respiratory exacerbations of cystic fibrosis?. Australian and New Zealand Journal of Medicine 1999;29(1):15‐21. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elphick 2002
- Elphick HE, Tan A, Ashby D, Smyth RL. Systematic reviews and lifelong diseases. BMJ 2002;325(7360):381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elphick 2005
- Elphick HE, Tan AA. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002007.pub2] [DOI] [PubMed] [Google Scholar]
Elphick 2014
- Elphick HE, Jahnke N. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002007.pub3] [DOI] [PubMed] [Google Scholar]


