Abstract
Background
Root canal therapy is a sequence of treatments involving root canal cleaning, shaping, decontamination and obturation. It is conventionally performed through a hole drilled into the crown of the affected tooth, namely orthograde root canal therapy. For teeth that cannot be treated with orthograde root canal therapy, or for which it has failed, retrograde root filling, which seals the root canal from the root apex, is a good alternative. Many materials, such as amalgam, zinc oxide eugenol and mineral trioxide aggregate (MTA), are generally used. Since none meets all the criteria an ideal material should possess, selecting the most efficacious material is of utmost importance.
Objectives
To determine the effects of different materials used for retrograde filling in children and adults for whom retrograde filling is necessary in order to save the tooth.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 13 September 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 13 September 2016); MEDLINE Ovid (1946 to 13 September 2016); Embase Ovid (1980 to 13 September 2016); LILACS BIREME Virtual Health Library (1982 to 13 September 2016); and OpenSIGLE (1980 to 2005). ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. We also searched Chinese BioMedical Literature Database (in Chinese, 1978 to 20 September 2016); VIP (in Chinese, 1989 to 20 September 2016); China National Knowledge Infrastructure (in Chinese, 1994 to 20 September 2016); and Sciencepaper Online (in Chinese, to 20 September 2016). No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We selected randomised controlled trials (RCTs) only that compared different retrograde filling materials, with reported success rate that was assessed by clinical or radiological methods for which the follow‐up period was at least 12 months.
Data collection and analysis
Two review authors extracted data independently and in duplicate. Original trial authors were contacted for any missing information. Two review authors independently carried out risk of bias assessments for each eligible study following Cochrane methodological guidelines.
Main results
We included six studies (916 participants with 988 teeth) reported in English. All the studies had high risk of bias. The six studies examined five different comparisons, including MTA versus intermediate restorative material (IRM), MTA versus super ethoxybenzoic acid cement (Super‐EBA), Super‐EBA versus IRM, dentine‐bonded resin composite versus glass ionomer cement and glass ionomer cement versus amalgam. There was therefore little pooling of data and very little evidence for each comparison.
There is weak evidence of little or no difference between MTA and IRM at the first year of follow‐up (risk ratio (RR) 1.09; 95% confidence interval (CI): 0.97 to 1.22; 222 teeth; quality of evidence: low). Insufficient evidence of a difference between MTA and IRM on success rate at the second year of follow‐up (RR 1.06; 95% CI: 0.89 to 1.25; 86 teeth, 86 participants; quality of evidence: very low). All the other outcomes were based on a single study. There is insufficient evidence of any difference between MTA and Super‐EBA at the one‐year follow‐up (RR 1.03; 95% CI: 0.96 to 1.10; 192 teeth, 192 participants; quality of evidence: very low), and only weak evidence indicating there might be a small increase in success rate at the one‐year follow‐up in favour of IRM compared to Super‐EBA (RR 0.90; 95% CI: 0.80 to 1.01; 194 teeth; quality of evidence: very low). There was also insufficient and weak evidence to show that dentine‐bonded resin composite might be a better choice for increasing retrograde filling success rate compared to glass ionomer cement at the one‐year follow‐up (RR 2.39; 95% CI: 1.60 to 3.59; 122 teeth, 122 participants; quality of evidence: very low). And there was insufficient evidence of a difference between glass ionomer cement and amalgam at both the one‐year (RR 0.98; 95% CI: 0.86 to 1.12; 105 teeth; quality of evidence: very low) and five‐year follow‐ups (RR 1.00; 95% CI: 0.84 to 1.20; 82 teeth; quality of evidence: very low).
None of these studies reported an adverse event.
Authors' conclusions
Based on the present limited evidence, there is insufficient evidence to draw any conclusion as to the benefits of any one material over another. We conclude that more high‐quality RCTs are required.
Plain language summary
Materials for retrograde filling in root canal therapy
Review question
This review examined the effects of different materials used for retrograde filling in children and adults for whom this treatment is necessary in order to save the tooth.
Background
The living part of the tooth, also known as the tooth pulp, can become irreversibly inflamed as a result of damage or bacterial infection due to tooth decay. To deal with this problem, the dentist has to drill a hole to access the inner space of the tooth or root canal system, and remove the infected tissue and toxic irritants by a combination of mechanical cleaning and irrigation. After this is done, the dentist fills the space with an inert packing material and seals the opening. This procedure is known as root canal therapy. Although results are generally good, a small number of failures do occur. This may be attributed to the complexity of the root canal system, which has many small additional pathways communicating with each other, making it difficult to completely eliminate all of the toxins and irritants. These can spread, causing the infection around the root to last indefinitely. When root canal therapy fails, a retreatment called retrograde filling is a good alternative to save the tooth. During retrograde filling the dentist cuts a flap in the gum and creates a hole in the bone to get access to the bottom tip of the root. After cutting off the tip, then thorough preparation, the apex is sealed (the apical seal) and the hole made by the dentist filled with a dental material. This sealing process is thought to be the single most important factor in achieving success in a retrograde root filling. Many materials have been developed to seal the root tip, mineral trioxide aggregate is the material of interest at present, but there is no consensus about which material is best.
Study characteristics
The evidence in this review, which was carried out together with Cochrane Oral Health, is up‐to‐date as of 13th September 2016. We included six studies that evaluated 916 participants and 988 teeth, who were undergoing retrograde filling using different types of filling material: mineral trioxide aggregate (MTA), intermediate restorative material (IRM), super ethoxybenzoid acid (Super‐EBA), dentine‐bonded resin composite, glass ionomer cement, and amalgam. Five studies were conducted in Europe and one in Asia. Studies measured the success rate with clinical or radiological methods. None of the studies reported possible side effects.
Key results
The limited evidence is insufficient to draw any conclusion as to the benefits of any one material over another, so we are not able to recommend which material is best to use in retrograde filling at present.
Quality of the evidence
The evidence presented is of very low quality due to the small amount of available studies, all at high risk of bias, results were imprecise and may not be applicable to other settings/countries.
Summary of findings
Background
Description of the condition
Root canal therapy is a sequence of treatments for the infected pulp of a tooth which results in the elimination of that infection and the subsequent protection of the decontaminated tooth from future microbial invasion (Cohen 2006). It involves the removal of the infected pulp, the subsequent shaping, cleaning, decontamination of the hollow tooth core and obturation. Traditionally, treatment is carried out through a hole drilled on the top of the crown of the tooth, and it is known as orthograde root canal therapy (Figure 1 (A and B)). With the development of new materials and techniques, orthograde root canal therapy has been demonstrated to provide satisfactory results for patients in most cases. However, because of the well‐known complexity of the root canal system and the acknowledged difficulty of completely eliminating all bacteria, their by‐products and toxins, from the canal system, failures occur at a reported rate of 4% to 15% (Sjogren 1990; Swartz 1983; Wong 2004). A higher failure rate was ascertained by Eriksen's review of multiple clinical studies of success and failure (Eriksen 1991). There are many causes for such failures, such as untreated canals, ledges formation, perforations, and overextensions of root‐filling materials. To plan treatment effectively, the clinician may place the aetiological factors into four groups (Sundqvist 1998): 1. persistent or reintroduced intraradicular micro‐organisms; 2. extraradicular infection; 3. foreign body reaction; 4. true cysts.
1.
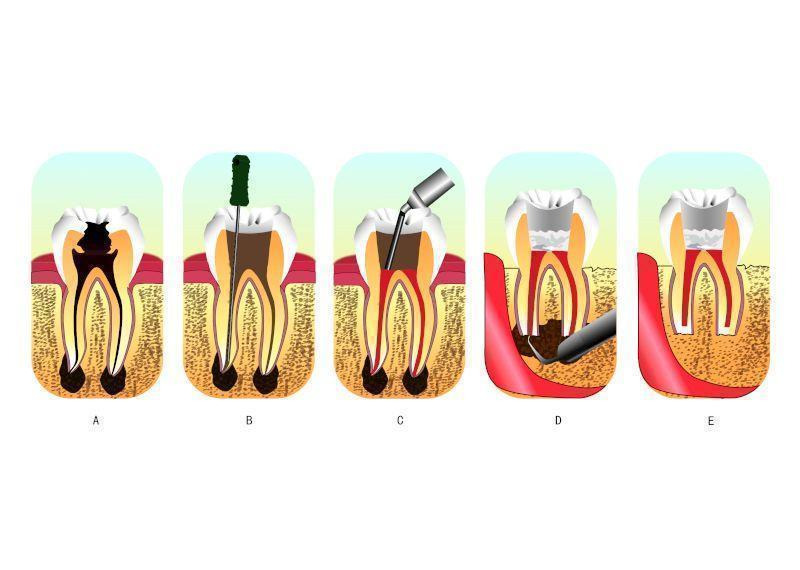
A: an infected tooth. The inner space of the tooth is the root canal system, where the pulp is located. The pulp of this tooth is irreversibly inflamed from bacterial infection due to decay.
B and C: the process of root canal therapy. B: a hole has been drilled from the top of the crown of the tooth. The dentist could then remove the infected tissues and toxic irritants by a combination of mechanical cleaning and irrigation in the root canal system through the hole. C: after cleaning and irrigation, the dentist fills the space with an inert packing material and seals the opening.
D and E: the process of retrograde filling. D: when retrograde filling is indicated, the dentist needs to cut a flap in the gum and creates a hole in the bone to get access to the bottom tip of the root. E: after cutting off the tip, then thorough preparation, the apex is sealed (the apical seal) and the hole made by the dentist filled with a dental material.
For these treatment failures, conventional orthograde endodontic retreatment is always the first choice. Although it is a highly predictable option in most cases, periradicular surgery may be indicated for teeth with persistent periradicular pathosis that have not responded to non‐surgical approaches (Lee 2004). Sometimes, apical surgery is preferred to orthograde treatment for expediency or if a tight‐fitting post, especially a fibre post, is present. At this circumstance, conventional retreatment needs to remove more dentine to acquire a pathway into the original root canal, which may cause root perforation or root fracture.
Periradicular surgery should be considered as an extension to non‐surgical treatment, because the underlying aetiology of the disease process and the treatment objectives are the same: the prevention or elimination of apical periodontitis (Hargreaves 2015). Periradicular surgery, also called retrograde filling, always requires root‐end preparation and obturation (Figure 1 (D and E)). The former aims to expose the apical root via flap elevation and bone removal prior to cavity preparation on the apical root where the apical foramina is located. Following that, materials are placed in the cavity for apical sealing. Harty reported that the apical seal was the single most important factor in achieving success in such surgery (Harty 1970). This apical seal is established by retrograde filling materials obturated between the root canal system and the surrounding tissues (Gutmann 1991). Thus, subsequent studies have evaluated many retrograde materials so as to determine which is most efficacious.
Description of the intervention
Amalgam has been used as a retrograde filling material for many years. Its earliest use as a root‐end filling following resection has been reported in 1884 (Vasudev 2003). It has the advantages of being easily available, inexpensive and easy to handle. Therefore, for many years, amalgam was accepted as the material of choice for root‐end filling, and the clinical application of amalgam was well documented in several clinical studies with a reported success rate of 50% to 80% (Dalal 1983; Finne 1977; Grung 1990; Hirsch 1979; Persson 1974; Rud 1972). However, in recent years, the efficacy of amalgam has been questioned due to initial marginal leakage, corrosion, moisture sensitivity, mercury contamination of periapical tissue and the potential hazards associated with mercury‐containing materials (Eley 1993; Gartner 1992). The disadvantages associated with amalgam and the potential long‐term damage to the environment has led to the research and development of alternative materials.
In the past decades, amalgam has slowly given way to zinc oxide eugenol (ZOE) containing materials, such as intermediate restorative material (IRM), which has 20%, by weight, polymethacrylate added to the base zinc oxide powder and super ethoxybenzoic acid (Super‐EBA), which contains ethoxybenzoic acid. In vitro leakage studies, animal studies, and retrospective in vivo studies indicate that these ZOE‐containing materials are superior to amalgam in terms of sealability and biocompatibility (Dorn 1990; Kim 2006; King 1990). Shortcomings of the currently available ZOE‐containing cements are their mild to moderate toxicity, when freshly mixed, and their radiopacity, which is similar to that of gutta‐percha (Johnson 1999).
In recent years a promising new root‐end filling material, mineral trioxide aggregate (MTA), developed at Loma Linda University, California, USA (Torabinejad 1993) has received widespread attention (Lee 1993). Its major components are similar to Portland cement, a mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, and tetracalcium aluminoferrite (Camilleri 2005). Although it is an expensive material and requires additional skill and equipment to use satisfactorily, the clinician can handle it satisfactorily after suitable training (Wang 2010). MTA has major advantages, including excellent biocompatibility (Camilleri 2006), ideal adherence to cavity walls, low solubility (Poggio 2007), and the ability of inducing cementogenesis at the root surface, with deposition of new cementum onto the exposed dentine and MTA surfaces (Baek 2005). MTA is an excellent bioactive material. When it is placed in direct contact with human tissues, it will form calcium hydroxide that releases calcium ions for cell attachment and proliferation (Takita 2006); modulates cytokine production (Koh 1998), and encourages proliferation and migration of progenitors followed by their differentiation into odontoblast‐like cells (Kuratate 2008). However, the mean setting time of MTA is 165 ± 5 minutes, which is longer than amalgam, Super‐EBA, and IRM (Torabinejad 1995), which is potentially problematic in endodontic surgery.
In addition to polymers, glass ionomer cements, polycarboxylate cements, zinc phosphate cements, calcium phosphate cements and composite resins have all been employed and several cases reported (Hauman 2003). A new material, Biodentine, which is reported to have reparative dentine synthesis properties (Laurent 2012), is awaiting clinical evaluation as a possible retrograde filling material. All these materials have different characteristics and are potential alternatives to traditional materials, although potential harm should be carefully considered before widespread use is considered.
How the intervention might work
Studies have proved that the main contributory factor in endodontic failure is persistent microbial infection in the root canal system and periapical region (Siqueira Jr 2003). Chemical and mechanical preparation may not reach every corner of the complex root canal system. Bacteria in isthmuses, ramifications, irregularities and dentinal tubules may persist and some necrotic tissue debris may also remain. Bacteria may gain access to the periapical region if complete sealing is not achieved, leading to pathological lesions (Lin 1991; Siqueira 2001).
To control microbial infection is always a high priority in periradicular surgery. The surgery removes the pathogenic agents and establishes an environment facilitating the regeneration of damaged tissue first. Then the procedure usually involves root‐end exposure and resection, in addition to preparing a Class I cavity and retrofilling with packing materials (Torabinejad 1995). Hence, these materials can form a proper seal of the internal root canal contents from the external periradicular tissues and therefore repair root defects (Chong 2004).
Why it is important to do this review
Periapical surgery is the last resort to save a tooth in endodontics. If it is not successful, the tooth might be lost. The use of proven retrograde filling materials is critical for apical sealing, which is the single most important factor in achieving success in periradicular surgery. To maintain a perfect apical seal, an ideal endodontic retrograde filling material is required to adhere to the tooth structure, be insoluble in tissue fluids, be dimensionally stable, non‐resorbable, radiopaque, and exhibit biocompatibility, if not bioactivity (Johnson 1999; Kratchman 2004). In order to find the best, if not ideal, retrograde filling material, many clinical trials have been conducted in an attempt to evaluate the efficacy and safety of different materials. Secondly, the sample size of most clinical trials is small and some of the results conflict with one another. Therefore, the purpose of our systematic review, through the use of strict criteria to integrate small sample size trials, was to clarify the clinical effect and safety of different materials for retrograde filling in root canal therapy.
Objectives
To determine the effects of different materials used for retrograde filling in children and adults for whom retrograde filling is necessary in order to save the tooth.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomised controlled trials (RCTs), including cluster, split‐mouth and cross‐over RCTs.
Types of participants
We included participants for whom orthograde root canal filling or retreatment was not possible and where periapical surgery was used to save the tooth. There were no age or gender limitations.
Types of interventions
Intervention group: retrograde obturation with any material.
Control group: retrograde obturation with any materials other than those used in the intervention group.
Types of outcome measures
Primary outcomes
Success rate: this was assessed by either clinical or radiological methods, or a combination of the two. Minimum follow‐up was 12 months.
Clinical methods included the assessment of clinical symptoms including pain, pain only on percussion or palpation, tenderness, increased tooth mobility, sinus tract formation or any other subjective discomfort. Any one of these was counted as treatment failure.
Radiological methods were used to detect periapical bone regeneration on medical images. Lack of apical bone regeneration compared to the baseline was counted as treatment failure.
The combination of clinical and radiological methods was used to assess the success of retrograde fillings in this review, unless specifically stated otherwise.
All cases not assessed as treatment failure were considered as successes.
Secondary outcomes
Adverse events.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health's Trials Register (to 13th September 2016) (Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 13th September 2016) (Appendix 2);
MEDLINE Ovid (1946 to 13th September 2016) (Appendix 3);
Embase Ovid (1980 to 13th September 2016) (Appendix 4);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; from 1982 to 13th September 2016) (Appendix 5);
OpenSIGLE (1980 to 2005) (Appendix 6).
We also searched the following databases:
Chinese BioMedical Literature Database (in Chinese, 1978 to 20th September 2016);
VIP (in Chinese, 1989 to 20th September 2016);
China National Knowledge Infrastructure (in Chinese, 1994 to 20th September 2016).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
Cochrane Oral Health's Information Specialist searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 13th September 2016) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 13th September 2016) (Appendix 7).
We also searched Sciencepaper Online (in Chinese, to 20th September 2016).
The following journals were handsearched:
Chinese Journal of Stomatology (January 2001 to December 2010)
Stomatology (January 2001 to December 2010)
West China Journal of Stomatology (January 2001 to December 2010)
Journal of Practical Stomatology (January 2001 to December 2010)
Journal of Clinical Stomatology (January 2001 to December 2010)
Journal of Comprehensive Stomatology (January 2001 to December 2010)
Journal of Modern Stomatology (January 2001 to December 2010)
Chinese Journal of Conservative Dentistry (January 2001 to December 2010)
Journal of Maxillofacial Surgery (January 2001 to December 2010)
Shanghai Journal of Stomatology (January 2001 to December 2010)
Chinese Journal of Dental Material and Devices (January 2001 to December 2010)
Beijing Journal of Stomatology (January 2001 to December 2010)
Chinese Journal of Dental Prevention and Treatment (January 2001 to December 2010)
Chinese Journal of Orthodontics (January 2001 to December 2010)
Chinese Journal of Implantology (January 2001 to December 2010)
Journal of International Stomatology (January 2001 to December 2010)
Chinese Journal of Prosthodontics (January 2001 to December 2010)
China Journal of Oral and Maxillofacial Surgery (2003 to December 2010)
Chinese Journal of Geriatric Dentistry (2002 to December 2010).
The reference lists from the included studies were also searched. We contacted the authors of eligible studies to see if there was any additional published or unpublished studies. Related manufacturers of different materials were contacted to identify if there were any unpublished trials on the material.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Three review authors (Xiangyu Ma (XM), Yan Wang (YW) and Trevor M Johnson (TMJ)) reviewed independently and in duplicate the titles and abstracts (if available) of the articles identified by the search to locate articles that met the inclusion criteria. If eligibility could not be assessed from the title and abstract, the full‐text article was obtained and further reviewed. Any disagreement was resolved by discussion among the three review authors. Non‐English and non‐Chinese papers were examined with the help of Cochrane Oral Health (COH).
Data extraction and management
Two review authors (Chunjie Li (CL) and XM) independently extracted the data from relevant articles with the help of a data extraction form designed specifically for this review. These forms had been piloted on several papers and modified as required before use.
For each study, the following data were recorded.
Basic information: date of the study, year of publication, country of origin.
Study type: details of sequence generation, allocation concealment and blinding.
Participants: inclusion and exclusion criteria, characteristics of the participants, sample size calculation.
Intervention: characteristics of the interventions (instruments used, cavity form prepared, and material used).
Outcome: outcome measures and detailed follow‐up information.
Any disagreement was resolved by discussion. The study authors were contacted for clarification if necessary.
Assessment of risk of bias in included studies
The risk of bias assessment was delivered by two review authors (CL and XM), also independently and in duplicate, according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion. Seven domains were considered for the risk of bias assessment.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias which is not covered by the first six (including confounding bias, baseline imbalance, co‐intervention and contamination).
For each study included in the review, the risk of bias in every domain was judged as either low, high or unclear, according to the included studies or from correspondence with the author.
The summary assessment was also prepared according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and presented graphically.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
The success rate and adverse events were classified as dichotomous data. For dichotomous outcomes, we expressed the estimate of effect of an intervention as risk ratios (RRs) together with 95% confidence intervals (CIs).
Unit of analysis issues
We treated individual participants as units of analysis where possible, otherwise each tooth or root was considered the unit of analysis (but this had to be clearly stated).
Dealing with missing data
For papers with missing data, we contacted the first and corresponding authors in an attempt to retrieve such data. Failing that, some special methods were used following guidance included in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We used the Chi2 test to calculate statistical heterogeneity. Statistical heterogeneity was classified into four categories according to I2 which was used to describe the percentage of the statistical variability in effect estimates. P < 0.1 indicated statistical heterogeneity:
0% to 40% implied slight heterogeneity
30% to 60% moderate heterogeneity
50% to 90% substantial heterogeneity
75% to 100% considerable heterogeneity.
Assessment of reporting biases
Publication and other reporting biases were planned to be assessed with the help of a funnel plot. If the funnel plot appeared asymmetric, bias was further planned to be investigated via Begg's test (Begg 1994).
Data synthesis
A meta‐analysis was only performed if there were studies of similar comparisons reporting the same outcomes and there was not considerable heterogeneity (I2 > 75%). A fixed‐effect model was considered if the number of studies was smaller than four. Otherwise, a random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
If clinical or methodological heterogeneity existed, subgroup analysis was performed. Such analysis would have been based on the different materials used, and short‐term and long‐term observations.
If unexplained heterogeneity existed, metaregression would have been adopted to investigate it.
Sensitivity analysis
Sensitivity analyses were planned to be performed on the basis of risk of bias by excluding studies from the analysis which exhibited high and unclear risk of bias.
Presentation of main results
For each comparison, we created a 'Summary of findings' table, which reflected the quality assessment of the body of evidence for each outcome under each comparison. The quality of evidence included assessment of risk of bias at study level, directness of the evidence, heterogeneity, precision of effect estimates and risk of publication bias.
We adopted the GRADE system for evaluating the quality of evidence with the help of GRADEpro GDT software (GRADEpro GDT). The quality of a body of evidence was classified into four categories: high, moderate, low and very low (Atkins 2004).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Initial searches from all sources identified 759 articles (including 625 in English, 106 in Chinese, 19 in Portuguese, 8 in Spanish and 1 in German). After scanning the titles and abstracts, 20 articles were considered to be possibly eligible. The full‐texts of these reports were obtained, and six studies (seven articles) were finally considered for inclusion in this systematic review. The remaining thirteen studies were excluded.
The process of study selection is presented in Figure 2.
2.
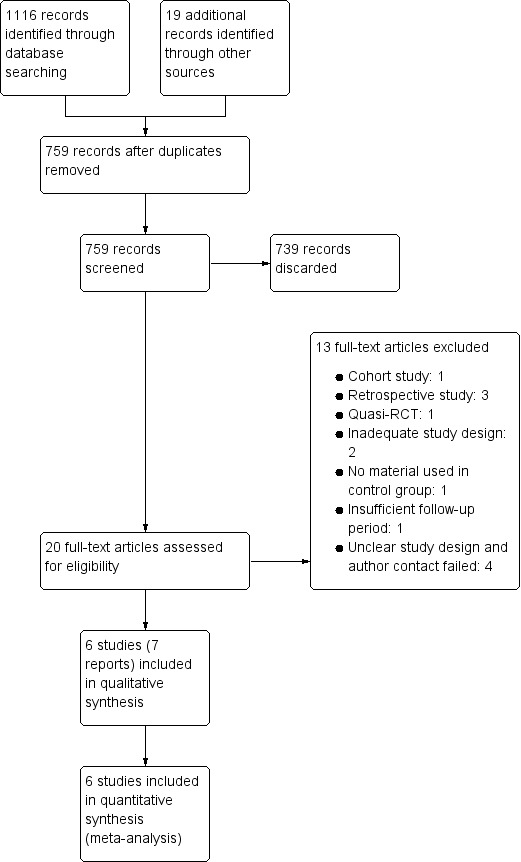
Study flow diagram.
Included studies
This review included six randomised controlled trials (RCTs) (Chong 2003; Jensen 2002; Jesslen 1995; Lindeboom 2005; Song 2012; Wälivaara 2011), which were published between 1995 and 2012. The details of the included studies are listed in the Characteristics of included studies tables.
Characteristics of the trial designs and settings
All included studies used a parallel design (Chong 2003; Jensen 2002; Jesslen 1995; Lindeboom 2005; Song 2012; Wälivaara 2011). Five studies were conducted in Europe and one in Asia. They are listed as follows:
Europe
United Kingdom (Chong 2003)
Denmark (Jensen 2002)
Sweden (Jesslen 1995; Wälivaara 2011)
the Netherlands (Lindeboom 2005)
Asia
South Korea (Song 2012).
Two studies performed sample size calculation (Chong 2003; Song 2012). Chong 2003 did not mention the method of sample size calculation, Song 2012 calculated the sample size according to the method described by Walters (Walters 2004). The remaining four studies did not mention sample size calculation at all (Jensen 2002; Jesslen 1995; Lindeboom 2005; Wälivaara 2011).
Four studies did not state their funding sources (Jensen 2002; Jesslen 1995; Lindeboom 2005; Wälivaara 2011) and the remaining two studies stated that they received non‐industry funding (Chong 2003; Song 2012).
Characteristics of the participants
This review involved 916 participants and 988 teeth. A total of 166 participants were lost during the follow‐up.
| Studies | Targeted disease | Periodontal condition | Previous treatment | Age | Sex ratio (male/female) | Follow‐up period | Lost to follow‐up ratio |
| Chong 2003 | Teeth had clear periapical lesions and required retrograde filling | No serious periodontitis or apicomarginal communication | Root canal treatment before | Unclear | Unclear | 1 year and 2 years | < 10% |
| Jensen 2002 | Unclear | Mean age of 49 years | 48/86 | 1 year | < 10% | ||
| Jesslen 1995 | Unclear | Unclear | Unclear | Unclear | 1 year and 5 years | 10% to 20% | |
| Lindeboom 2005 | No serious periodontitis or apicomarginal communication | Root canal treatment before | 17 to 64 years | 33/57 | 1 year | 0% | |
| Song 2012 | Unclear | Unclear | Unclear | 1 year | 20% | ||
| Wälivaara 2011 | Unclear | Unclear | 65/99 | 1 year | < 10% |
Characteristics of the interventions
The interventions used in the included studies were:
mineral trioxide aggregate (MTA);
intermediate restorative material (IRM);
super ethoxybenzoid acid (Super‐EBA);
dentine‐bonded resin composite;
glass ionomer cement;
amalgam.
These interventions were all used singly. We evaluated the following comparisons:
MTA versus IRM (Chong 2003; Lindeboom 2005);
MTA versus Super‐EBA (Song 2012);
Super‐EBA versus IRM (Wälivaara 2011);
dentine‐bonded resin composite versus glass ionomer cement (Jensen 2002);
glass ionomer cement versus amalgam (Jesslen 1995).
The preparation of the root tip differed between studies. The two earliest studies (Jesslen 1995; Jensen 2002) prepared the root tip with cone or diamond burs. The other studies (Chong 2003; Lindeboom 2005; Song 2012; Wälivaara 2011) used ultrasonic apical preparation. Two studies (Song 2012; Wälivaara 2011) used magnification equipment. The lengths of apical root resection were 2 to 3 mm (Jensen 2002; Song 2012), 3 mm (Lindeboom 2005), and 3 to 4 mm (Wälivaara 2011).
Characteristics of the outcomes
The primary outcome in our review was success rate which was assessed via clinical or radiological methods or a combination of the two. It was defined as no pain, pain on percussion or palpation, tenderness, increased tooth mobility, sinus tract formation or any other subjective discomfort; and no presence of apical absorption or no bony change compared to the baseline indicated treatment failure.
All included studies followed the definition of success rate using the specified criteria provided by Molven 1987 and Rud 1972 (Chong 2003; Jensen 2002; Lindeboom 2005; Song 2012; Wälivaara 2011) or Zetterqvist 1991 (Jesslen 1995). Via these criteria, treatment results were classified into four types:
complete healing;
incomplete or scar healing (improvement);
uncertain healing (no improvement);
unsatisfactory healing.
As described in these specified criteria, the rate of complete healing, incomplete or scar healing (improvement) were all considered as meeting the definition of success rate used in our review; the other two (uncertain healing (no improvement), unsatisfactory healing) were considered to be failures.
All included studies reported the success rate assessed via the combination of clinical and radiological methods. And only Jensen 2002 reported the success rate solely assessed by radiological methods.
None of the included studies reported any information on adverse events, this review's secondary outcome.
Excluded studies
We have listed all the studies that were excluded and the reasons for their exclusion in the Characteristics of excluded studies tables.
We excluded three studies (Pantchev 2009; Rud 1991; Schwartz‐Arad 2003) because they were retrospective. We excluded von Arx 2012 because it was a cohort study; Wälivaara 2009 was excluded because it was a quasi‐RCT. For Christiansen 2009, the control group did not have retrograde obturation with any material. For Hou 2008, no radiological outcome was recorded. And for Platt 2004, the methods used to prepare the cavity differed in both randomised groups. One study (Silva 2016) was excluded because the following‐up period was too short (only 6 months). We excluded four studies (Burstein 2001; Nordenram 1970; Pantschev 1994; Rud 1996) because the authors did not mention randomisation. We have tried to contact the study authors, but no response was obtained.
Risk of bias in included studies
All six studies were assessed as being at high overall risk of bias (Figure 3; Figure 4). A detailed explanation is presented in the Characteristics of included studies table.
3.
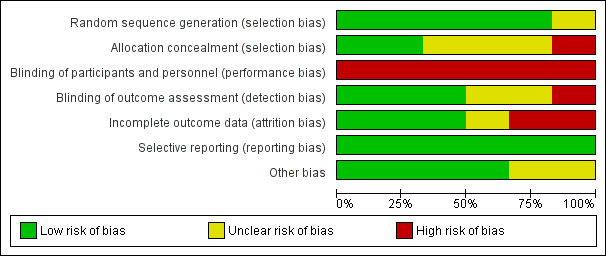
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
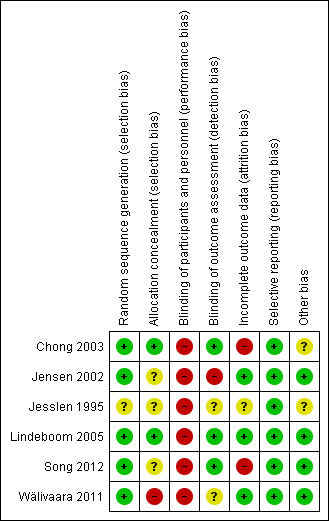
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Five out of the six studies reported adequate methods of randomisation and were graded as at low risk of bias in this domain, the other was graded as at unclear risk of bias; both Chong 2003 and Lindeboom 2005 used sealed envelopes randomly picked from a pack; Song 2012 used a "minimization method" described by Pocock (Pocock 1983); Wälivaara 2011 allocated according to a randomisation table; and Jensen 2002 used a computer‐generated random table; only Jesslen 1995 did not report a clear method.
Allocation concealment
Two studies (Chong 2003; Lindeboom 2005) used sealed envelopes, so that the allocation was adequately concealed, and were judged to be at low risk of bias for this domain. Three studies had unclear allocation concealment and were therefore graded as at unclear risk (Jensen 2002; Jesslen 1995; Song 2012). One study was judged at high risk of bias (Wälivaara 2011), the study author confirming that the allocation process was not concealed via correspondence.
Blinding
Blinding of participants and personnel (performance bias)
It was not possible to blind the surgeons, so all the included studies were ranked as at high risk in terms of performance bias. In Jensen 2002; Lindeboom 2005; Song 2012; Wälivaara 2011 surgeons were not blinded according to authors' correspondence. The other studies (Chong 2003; Jesslen 1995) did not mention the blinding of participants and personnel so they were ranked as at high risk of performance bias.
Blinding of outcome assessment (detection bias)
Three studies had low risk of detection bias (Chong 2003; Lindeboom 2005; Song 2012) as they clearly reported the blinding of the outcome assessors. Two studies had unclear risk (Jesslen 1995; Wälivaara 2011) and one had high risk (Jensen 2002) as the author replied that the assessors were not blinded to the treatment.
Although some studies reported the blinding of outcome assessment, different materials may be identifiable via radiological assessment and this might influence the risk of bias.
Incomplete outcome data
In Chong 2003; Jesslen 1995 and Song 2012, too many participants were lost during follow‐up and the study authors did not do an intention‐to‐treat (ITT) analysis.
Selective reporting
Selective data reporting was not detected in the studies included in the review as they all fully reported the outcomes they stated in the methods section.
Other potential sources of bias
In Chong 2003 and Jesslen 1995, the issue of whether baseline demographic characteristics were comparable was not clearly reported and therefore we classified them as at unclear risk of other bias. For the other four studies, there was no other potential source of bias detected.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. MTA versus IRM for retrograde filling in root canal therapy.
| MTA versus IRM for retrograde filling in root canal therapy | ||||||
| Patient or population: patients needing retrograde filling in root canal therapy Settings: UK and the Netherlands Intervention: MTA versus IRM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IRM | MTA | |||||
| Success rate ‐ 1‐year outcome (teeth as unit of analysis) Assessed by combination of clinical and radiological methods Follow‐up: mean 1 year | 806 per 1000 | 879 per 1000 (782 to 983) | RR 1.09 (0.97 to 1.22) | 222 (2 studies) | ⊕⊕⊝⊝ low1 | Weak evidence of little or no difference between MTA and IRM at the first year of follow‐up Insufficient evidence of a difference between MTA and IRM after 2 years of follow‐up (RR 1.06; 95% CI 0.89 to 1.25; participants = 86; studies = 1; quality of evidence: very low2) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; IRM: intermediate restorative material; MTA: mineral trioxide aggregate; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Downgraded by 2 due to (1) high risk of bias: both studies had the personnel unblinded due to the nature of the study design, and Chong 2003 had incomplete outcome data reported; and (2) imprecision: only 2 studies were included. 2 Downgraded by 4 due to (1) risk of bias (downgraded by 1): the only study included had high risk of bias on incomplete data reporting and personnel blinding; (2) imprecision (downgraded by 2): only 1 study included; and (3) indirectness (downgraded by 1): participants were from the UK and therefore the results may not be applicable elsewhere.
Summary of findings 2. MTA versus Super‐EBA for retrograde filling in root canal therapy.
| MTA versus Super‐EBA for retrograde filling in root canal therapy | ||||||
| Patient or population: patients needing retrograde filling in root canal therapy Settings: South Korea Intervention: MTA versus Super‐EBA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Super‐EBA | MTA | |||||
| Success rate ‐ 1‐year outcome (participants as unit of analysis) Assessed by combination of clinical and radiological methods Follow‐up: mean 1 year | 931 per 1000 | 959 per 1000 (894 to 1000) | RR 1.03 (0.96 to 1.10) | 192 (1 study) | ⊕⊝⊝⊝ very low1 | Insufficient evidence of a difference between MTA and Super‐EBA on retrograde filling after 1‐year follow‐up |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; MTA: mineral trioxide aggregate; RR: risk ratio; Super‐EBA: super ethoxybenzoid acid | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Downgraded by 4 due to (1) high risk of bias (downgraded by 1): the only included study (Song 2012) had high risk of bias regarding incomplete data reporting and personnel blinding; (2) indirectness (downgraded by 1): participants were from South Korea and therefore the results may not be applicable elsewhere; and (3) imprecision (downgraded by 2): only 1 study included and the small population might cause serious imprecision.
Summary of findings 3. Super‐EBA versus IRM for retrograde filling in root canal therapy.
| Super‐EBA versus IRM for retrograde filling in root canal therapy | ||||||
| Patient or population: patients needing retrograde filling in root canal therapy Settings: Sweden Intervention: Super‐EBA versus IRM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IRM | Super‐EBA | |||||
| Success rate ‐ 1‐year outcome (teeth as unit of analysis) Assessed by combination of clinical and radiological methods Follow‐up: mean 1 year | 906 per 1000 | 815 per 1000 (725 to 915) | RR 0.90 (0.80 to 1.01) | 194 (1 study) | ⊕⊝⊝⊝ very low1 | A very low grade of evidence showed that there might be some possibility that the usage of IRM might slightly increase success rate for retrograde filling compared to Super‐EBA |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; IRM: intermediate restorative material; RR: risk ratio; Super‐EBA: super ethoxybenzoid acid | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Downgraded by 4 due to (1) high risk of bias (downgraded by 1): high risk of bias existed in the only included study (Wälivaara 2011) on allocation concealment and personnel blinding; (2) indirectness (downgraded by 1): participants were from Sweden and therefore the results may not be applicable elsewhere; and (3) imprecision (downgraded by 2): only 1 study included and the small population might cause serious imprecision.
Summary of findings 4. Dentine‐bonded resin composite versus glass ionomer cement for retrograde filling in root canal therapy.
| Dentine‐bonded resin composite versus glass ionomer cement for retrograde filling in root canal therapy | ||||||
| Patient or population: patients needing retrograde filling in root canal therapy Settings: Denmark Intervention: dentine‐bonded resin composite versus glass ionomer cement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Glass ionomer cement | Dentine‐bonded resin composite | |||||
| Success rate ‐ 1‐year outcome PP analysis (participants as unit of analysis) Assessed by combination of clinical and radiological methods Follow‐up: mean 1 year | 306 per 1000 | 731 per 1000 (490 to 1000) | RR 2.39 (1.60 to 3.59) | 122 (1 study) | ⊕⊝⊝⊝ very low1 | There was insufficient and weak evidence showing that the usage of dentine‐bonded composite might result in higher success rate assessed by clinical and radiological methods compared to glass ionomer cement |
| Success rate ‐ 1‐year outcome PP analysis (root as unit of analysis) Assessed by combination of clinical and radiological methods Follow‐up: mean 1 year | 519 per 1000 | 825 per 1000 (623 to 1000) | RR 1.59 (1.20 to 2.09) | 127 (1 study) | ⊕⊝⊝⊝ very low1 | There was insufficient and weak evidence showing that the usage of dentine‐bonded composite might result in higher success rate assessed by radiological methods compared to glass ionomer cement |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; PP: per‐protocol; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Downgraded by 4 because of (1) high risk of bias (downgraded by 1): the included study (Jensen 2002) did not have participants, personnel or outcome assessors blinded; (2) indirectness (downgraded by 1): participants were from Denmark and therefore the results may not be applicable elsewhere; and (3) imprecision (downgraded by 2): only 1 study included and the small population might cause serious imprecision.
Summary of findings 5. Glass ionomer cement versus amalgam for retrograde filling in root canal therapy.
| Glass ionomer cement versus amalgam for retrograde filling in root canal therapy | ||||||
| Patient or population: patients needing retrograde filling in root canal therapy Settings: Sweden Intervention: glass ionomer cement versus amalgam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Amalgam | Glass ionomer cement | |||||
| Success rate ‐ 5‐year outcome Clinical and radiological methods Follow‐up: mean 5 years | 854 per 1000 | 854 per 1000 (717 to 1000) | RR 1.00 (0.84 to 1.20) | 82 (1 study) | ⊕⊝⊝⊝ very low1 | There was insufficient evidence of a difference between glass ionomer cement and amalgam on success rate of retrograde filling during 5 years of follow‐up There was also insufficient evidence of a difference between glass ionomer cement and amalgam on success rate of retrograde filling during 1 year of follow‐up (RR 0.98; 95% CI 0.86 to 1.12; tooth = 105; studies = 1; quality of evidence: very low) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Downgraded by 4 due to (1) high risk of bias (downgraded by 1): the included study (Jesslen 1995) was at high risk of bias as the personnel were impossible to be blinded considering the nature of the study; (2) indirectness (downgraded by 1): participants were from Sweden and therefore the results may not be applicable elsewhere; and (3) imprecision (downgraded by 2): only 1 study included and the small population might cause serious imprecision.
Generally, each participant was considered as the unit of analysis in this review, or if that was not possible, each tooth. But for success rate evaluated solely by radiological methods, the root could be considered as the unit of analysis if clearly stated.
Comparison 1: MTA versus IRM
Both Chong 2003 and Lindeboom 2005 compared mineral trioxide aggregate (MTA) versus intermediate restorative material (IRM). There was no significant clinical heterogeneity, and we pooled their data.
Success rate
Both studies reported this outcome. Chong 2003 described this in one‐ and two‐year follow‐ups and Lindeboom 2005 reported only the one‐year follow‐up outcome.
One‐year outcome
Data from the two studies at high risk of bias with 222 participants were pooled, with 114 teeth in the MTA group and 108 teeth in the IRM group. There is weak evidence of little or no difference between MTA and IRM at the first year of follow‐up (risk ratio (RR) 1.09; 95% confidence interval (CI) 0.97 to 1.22) (Analysis 1.1).
1.1. Analysis.
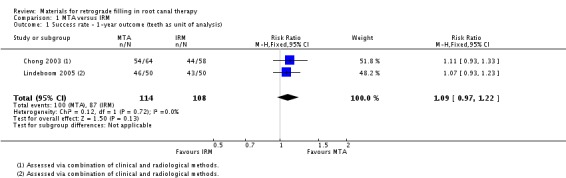
Comparison 1 MTA versus IRM, Outcome 1 Success rate ‐ 1‐year outcome (teeth as unit of analysis).
Two‐year outcome
At the second‐year follow‐up appointment, there was insufficient evidence of a difference between MTA and IRM on the success rate of retrograde filling (RR 1.06; 95% CI 0.89 to 1.25), with 47 patients (with 47 teeth) in the MTA group and 39 patients (39 teeth) in the IRM group (Analysis 1.2).
1.2. Analysis.
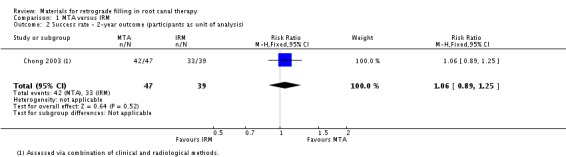
Comparison 1 MTA versus IRM, Outcome 2 Success rate ‐ 2‐year outcome (participants as unit of analysis).
Adverse events
Adverse events were not reported.
Comparison 2: MTA versus Super‐EBA
Only Song 2012 compared MTA with super ethoxybenzoid acid (Super‐EBA).
Success rate
One‐year outcome
After one year, 192 participants (192 teeth) were followed up (90 in the MTA group and 102 in the Super‐EBA group). In the MTA group, 86 participants (86 teeth) were successfully treated while the Super‐EBA group had 95 participants (95 teeth). There was insufficient evidence of a difference in success rate between MTA usage and that of super‐EBA in retrograde filling at the one‐year follow‐up (RR 1.03; 95% CI 0.96 to 1.10) (Analysis 2.1).
2.1. Analysis.
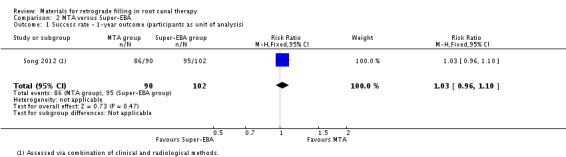
Comparison 2 MTA versus Super‐EBA, Outcome 1 Success rate ‐ 1‐year outcome (participants as unit of analysis).
Adverse events
Adverse events were not reported.
Comparison 3: Super‐EBA versus IRM
Only Wälivaara 2011 compared Super‐EBA with IRM.
Success rate
One‐year outcome
At the one‐year follow‐up, 194 teeth were considered (98 in the Super‐EBA group and 96 in the IRM group). Following analysis, there was weak evidence of a small increase in success rate due to the use of IRM for retrograde filling compared to Super‐EBA (RR 0.90; 95% CI 0.80 to 1.01) (Analysis 3.1).
3.1. Analysis.
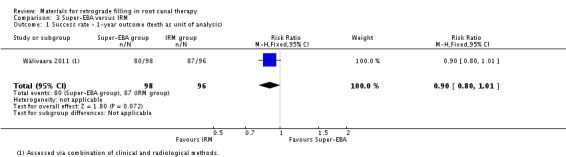
Comparison 3 Super‐EBA versus IRM, Outcome 1 Success rate ‐ 1‐year outcome (teeth as unit of analysis).
Adverse events
Adverse events were not reported.
Comparison 4: dentine‐bonded resin composite versus glass ionomer cement
Only Jensen 2002 compared dentine‐bonded resin composite with glass ionomer cement.
Success rate
One‐year outcome
In this comparison, participants were considered as the analysis unit. Both per‐protocol (PP) and intention‐to‐treat (ITT) analyses ('worst‐case scenario' analysis: all lost to follow‐up participants in the intervention group were considered as failed cases) were adopted.
Within the dentine‐bonded resin composite group (60 participants (60 teeth)), 44 participants (44 teeth) were successfully healed. In the glass ionomer cement group (62 participants (62 teeth)) only 19 participants (19 teeth) were considered as a clinical and radiological success. The result provided only insufficient and weak evidence that dentine‐bonded resin composite is superior to glass ionomer cement (RR 2.39; 95% CI 1.60 to 3.59) (Analysis 4.1). The quality of the body of evidence was very low.
4.1. Analysis.
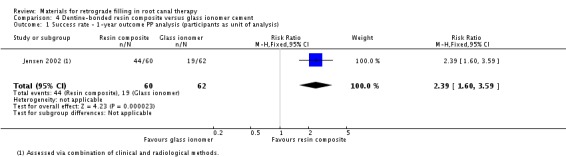
Comparison 4 Dentine‐bonded resin composite versus glass ionomer cement, Outcome 1 Success rate ‐ 1‐year outcome PP analysis (participants as unit of analysis).
The result of the ITT analysis also supported the results of the PP analysis (RR 1.83; 95% CI 1.27 to 2.64) (Analysis 4.2) with 67 participants (67 teeth) in both groups.
4.2. Analysis.
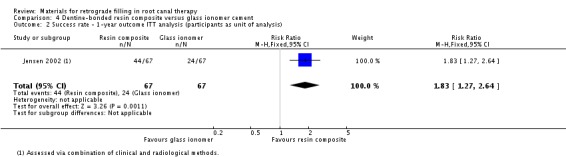
Comparison 4 Dentine‐bonded resin composite versus glass ionomer cement, Outcome 2 Success rate ‐ 1‐year outcome ITT analysis (participants as unit of analysis).
We also collected data using the root of the teeth as the unit of analysis. However, instead of using combined methods for evaluation, only the radiological method was adopted in this case. As there was a clear description of the participants who were lost to follow‐up, both PP and ITT analyses ('worst‐case scenario' analysis) were performed. Insufficient and weak evidence indicated that dentine‐bonded resin composite had a greater effect than glass ionomer cement on success rate (RR 1.59; 95% CI 1.20 to 2.09) with 73 roots in the dentine‐bonded resin composite group and 54 roots in the glass ionomer cement group (Analysis 4.3). ITT analysis ('worst‐case scenario' analysis) was adopted in this section, which supported the results of the PP analysis (RR 2.31; 95% CI 1.62 to 3.29) with 89 roots in each group (Analysis 4.4).
4.3. Analysis.
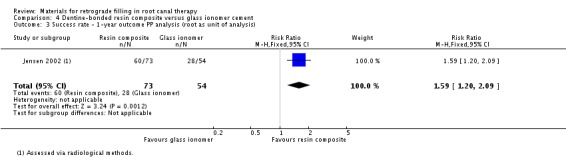
Comparison 4 Dentine‐bonded resin composite versus glass ionomer cement, Outcome 3 Success rate ‐ 1‐year outcome PP analysis (root as unit of analysis).
4.4. Analysis.
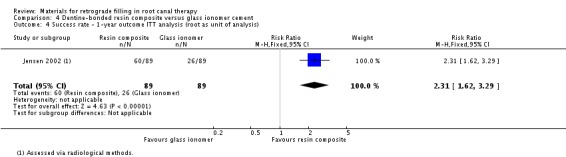
Comparison 4 Dentine‐bonded resin composite versus glass ionomer cement, Outcome 4 Success rate ‐ 1‐year outcome ITT analysis (root as unit of analysis).
Adverse events
Adverse events were not reported.
Comparison 5: glass ionomer cement versus amalgam
Only Jesslen 1995 compared glass ionomer cement with amalgam.
Success rate
One‐year outcome
After the first year, 105 teeth were followed up (53 in the glass ionomer cement group and 52 in the amalgam group). Each group had 47 teeth which had complete or partial healing and were therefore considered as a treatment success. There was insufficient evidence of a difference between the treatments on success rate at one‐year follow‐up (RR 0.98; 95% CI 0.86 to 1.12) (Analysis 5.1).
5.1. Analysis.
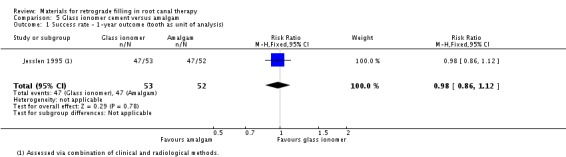
Comparison 5 Glass ionomer cement versus amalgam, Outcome 1 Success rate ‐ 1‐year outcome (tooth as unit of analysis).
Five‐year outcome
In the fifth follow‐up year, 82 teeth were included (41 in the glass ionomer group and 41 in the amalgam group). There were 35 teeth in each group with complete or partial healing. There was insufficient evidence of a difference between glass ionomer cement and amalgam on success rate (RR 1.00; 95% CI 0.84 to 1.20) (Analysis 5.2).
5.2. Analysis.
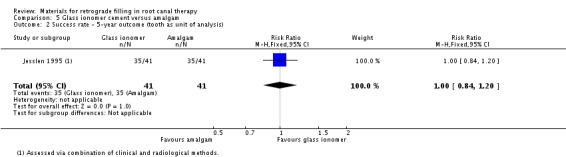
Comparison 5 Glass ionomer cement versus amalgam, Outcome 2 Success rate ‐ 5‐year outcome (tooth as unit of analysis).
Adverse events
Adverse events were not reported.
Discussion
Summary of main results
This review aimed at comparing the outcomes of different retrograde filling materials. Only six studies (Chong 2003; Jensen 2002; Jesslen 1995; Lindeboom 2005; Song 2012; Wälivaara 2011) with five comparisons of retrograde filling materials were finally considered to be eligible for this systematic review. The quality of the body of evidence was assessed using GRADE (Atkins 2004). We present five 'Summary of findings' tables where we were able to perform quantitative analysis. They are mineral trioxide aggregate (MTA) versus intermediate restorative material (IRM) (Table 1), MTA versus super ethoxybenzoid acid (Super‐EBA) (Table 2), Super‐EBA versus IRM (Table 3), dentine‐bonded resin composite versus glass ionomer cement (Table 4) and glass ionomer cement versus amalgam (Table 5).
There was little pooled data and very little evidence for each comparison. There is weak evidence of little or no difference between MTA and IRM at the first year follow‐up (risk ratio (RR) 1.09; 95% confidence interval (CI) 0.97 to 1.22; 222 teeth; two studies at high risk of bias; quality of the body of evidence: low). The long setting time (165 ± 5 minutes) is potentially a problem in this type of surgery. Many investigations have been performed to overcome it, such as using a chelating agent or sodium phosphate dibasic (Na2HPO4) setting accelerator (Ber 2007; Huang 2008). Some new and potentially promising materials have been available for root‐end filling, such as Bioceramics (Damas 2011) and Biodentine (Soundappan 2014), but more clinical studies are needed to testify their properties.
All the other outcomes were based on single studies. There was insufficient evidence, from these single studies (mostly with small sample sizes), to determine differences between the following groups.
MTA versus Super‐EBA after one‐year follow‐up (quality of the body of evidence: very low).
Super‐EBA versus IRM after one‐year follow‐up (quality of the body of evidence: very low).
Dentine‐bonded resin composite versus glass ionomer cement after one‐year follow‐up (quality of the body of evidence: very low).
Glass ionomer cement versus amalgam after one‐year follow‐up (quality of the body of evidence: very low).
Glass ionomer cement versus amalgam after five‐year follow‐up (quality of the body of evidence: very low).
None of the included studies reported adverse events.
Overall completeness and applicability of evidence
One of the studies included in the review was conducted in the UK (Chong 2003), one in Denmark (Jensen 2002), two in Sweden (Jesslen 1995; Wälivaara 2011), one in South Korea (Song 2012) and one in the Netherlands (Lindeboom 2005). 916 participants with 988 teeth were randomised. The participants were of different gender and age (the youngest reported was 17 (Lindeboom 2005) and the oldest was 64 (Lindeboom 2005)). The studies included different types of teeth. The applicability of the results could be adopted to different demographics of patients and different types of teeth.
All the studies followed a rigid surgical procedure. The procedures were of little difference except for some detail in the root resection and form of cavity. The lengths of apical root resection were 2 to 3 mm (Jensen 2002; Song 2012), 3 mm (Lindeboom 2005), and 3 to 4 mm (Wälivaara 2011). There was no complete agreement on how much of the root should be resected to satisfy biological principles. Gilheany 1994 suggested that at least 2 mm be removed to minimize bacterial leakage from the canals. An anatomical study of the root apex showed that at least 3 mm of the root‐end must be removed to reduce 98% of apical ramifications and 93% of lateral canals (Kim 2001). Besides the length of resection, the choice of root‐end bevel angle was still in question. Traditionally, the bevel angle which provides best access and visibility was used. But the bevel may open up many channels of communication between the infected canal system and surrounding tissue, allowing the intradental infection to create persistent inflammation. Recent studies indicate that a right angle to the long axis of the root is preferable. In the studies included in this review, only one adopted such a right angle (Chong 2003). In the other five studies, a slightly oblique resection of the root was performed. Jensen 2002 resected at a mean angle of 35 degrees, Lindeboom 2005 10 to 25 degrees and Song 2012 had a bevel angle of 0 to 10 degrees.
Recently the use of a microscope has become popular (Christiansen 2009). In the studies within this review, Song 2012 used a microscope and in two studies (Lindeboom 2005; Wälivaara 2011) the dental surgeon used magnification loupes to acquire a better visual field. Following root resection, cavities were prepared in two ways: four studies used ultrasonic equipment while two used a traditional bur. Jensen 2002 prepared slightly concave cavities, and Jesslen 1995 prepared box‐type cavities. The depth of the cavity was 2 to 3 mm in Lindeboom 2005, 3 mm in Song 2012 and Wälivaara 2011. The condition of the root‐end varied. The modern concept of an ideal root‐end preparation is defined as a Class I cavity at least 3 mm into the root dentine, with walls parallel to and coincident with the anatomic outline of the root canal space, which would achieve the aim of removing the intracanal filling material and associated irritants so as to create a cavity that could be properly filled. From this description, we could consider that the results of the systematic review may not be seriously influenced by heterogeneity of performance of the surgery.
Quality of the evidence
The body of evidence that we identified does not allow for any robust conclusions about which retrograde filling material is best. Six studies, which analysed a total of 916 participants (988 teeth), were included. All the studies had a high risk of bias. All of the eight pieces of evidence produced from the studies were of low quality according to GRADE (Petrisor 2006). When such risk of bias issues were considered alongside the fact that the studies in each comparison/outcome were either single small studies (leading to serious imprecision) or had 95% confidence intervals that prevented the intervention being favoured over the control, the evidence was rated low to very low quality. These GRADE ratings can be interpreted as indicating that there is a lack of confidence in the effect estimates and further research is highly likely to change the estimates, and our confidence in them.
Potential biases in the review process
To discover as many relevant studies as possible, articles were identified irrespective of publication language, status and date through electronic searches and handsearching. The reference lists from each identified article, reviews and related textbooks were also searched. However, we still failed to acquire the data from a potentially relevant study, entitled '18‐month clinical trial of endodontic surgical retrofilling materials' which was presented at the American Association of Endodontists' 58th Annual Session. We have tried all means to contact the author and the American Association of Endodontists, but no reply was received. Another four potentially eligible studies (Burstein 2001; Nordenram 1970; Pantschev 1994; Rud 1996) were excluded because their study type could not be determined from the reports and the authors failed to reply to our contact.
Agreements and disagreements with other studies or reviews
A newly published systematic review (Tang 2010) compared the clinical outcomes of MTA used as root‐end filling with other materials in endodontic surgery. They searched studies conducted on human teeth in vivo, regardless of whether they were prospective studies. Five studies, two that compared MTA with IRM, one that compared MTA with gutta‐percha and two that compared MTA with amalgam, were selected. The analysis suggested that MTA as a root‐end filling is better than amalgam but similar to IRM, which were similar to the results indicated in our review. However, the authors of Tang 2010 had included some studies which seemed non‐existent (we have tried to retrieve the studies they included and discovered at least two studies to which they referred did not actually exist in the corresponding issues of those journals, and no studies with similar titles could be found).
Richard Niederman and his colleagues have carried out two systematic reviews focused on retrograde filling materials. The first one (Niederman 2003) was conducted to identify randomised controlled trials (RCTs) and controlled clinical trials (CCTs), cohort studies and case‐control studies, which were conducted on humans, in vivo. The languages were limited to English, German and French. Only two RCTs (Jesslen 1995; Zetterqvist 1991), which were also analysed in our review, were included in this study. They concluded that glass ionomer cement is almost as effective as amalgam. Furthermore, the other six CCTs and six case‐control studies selected in Niederman's review indicated that EBA cement, composite with Gluma and gold leaf, as well as orthograde gutta‐percha, may be more effective than retrograde amalgam filling. The second review (Theodosopoulou 2005) tested the characteristics of retrograde filling materials in vitro. Thirty‐four studies met all their inclusion and validity criteria. The results indicate that, beyond 10 days in vitro, the most effective retrofilling materials, when measured by dye/ink penetration are: composites, followed by glass ionomer cement, amalgam, orthograde gutta‐percha, and EBA. The results of these in vitro studies are not congruent with in vivo study results, suggesting a need to re‐evaluate the clinical validity and importance of in vitro studies.
Authors' conclusions
Implications for practice.
Based on the present limited evidence, we do not have sufficient evidence to determine the benefits of any one material over another.
Implications for research.
The present results call for further research. We hope future studies could address and answer the following issues.
Participants: studies with a large number of participants from different races.
Intervention and comparison: trials focusing on the materials considered in this systematic review are still needed, and trials using new materials are also required. Many investigations have tried to use a chelating agent or accelerator to overcome the main drawback of mineral trioxide aggregate (MTA), its long setting time, which is also worth more research.
Follow‐up: increasing the follow‐up period to observe the long‐term effects and safety.
To improve the quality of future evidence, we recommend blinding the outcome assessors. Most studies only have the participants and the statistical assessor blinded. But Christiansen 2009 figured out an easy method to blind the radiograph observers. They exported radiographs from the Digora system in TIFF format to Adobe Photoshop format, masking the apical root filling with grey patches. Future studies could make use of this methodology.
History
Protocol first published: Issue 4, 2005 Review first published: Issue 12, 2016
| Date | Event | Description |
|---|---|---|
| 5 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Our thanks go to the Cochrane Oral Health editorial team and external referees for their help in conducting this systematic review. We would like to thank in particular Anne Littlewood, Information Specialist at Cochrane Oral Health, and Sylvia Bickley, for developing the electronic search strategy and running the electronic searches in the English language databases. We would also like to thank Luisa Fernandez Mauleffinch, Managing Editor, and Philip Riley, Editor, at Cochrane Oral Health, for their kind help and guidance in preparing and revising both protocol and review. We thank Dr Qi Wang for the writing of the protocol.
The review authors also send their thanks to Dr Raphael Freitas de Souza and Dr Anette Bluemle for their help in screening the titles and abstracts which were not in English or Chinese. Great thanks should also be sent to Dr Jerome AH Lindeboom, Dr Simon Storgård Jensen, Dr Minju Song and Dr Dan‐Åke Wälivaara, who were the initial investigators of four randomised trials included in this systematic review, for their assistance in providing valuable information about the studies. And thank you to Dr Chenyang Xiang, Wenhang Dong, Qiushi Wang, Zhaoyang Ban, Feng Li from West China College of Stomatology, Sichuan University, for their assistance with handsearching.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
From March 2015, searches of Cochrane Oral Health's Trials Register for this review were undertaken using the Cochrane Register of Studies and the search strategy below:
1 ((apicoectom* or apicectom* or "root canal" or periapical* or periradicular* or endodont* or apical*):ti,ab) AND (INREGISTER) 2 ((retrograd* or retrofill* or retro‐fill* or retroseal* or retro‐seal* or "apical* seal*" or "apical* prepar*" or retroprepar*):ti,ab) AND (INREGISTER) 3 (#1 and #2) AND (INREGISTER)
Previous searches of the Register were undertaken using the Procite software and the search strategy below:
((apicoectom* or apicectom* or "root canal" or periapical* or periradicular* or endodont* or apical*) AND (retrograd* or retrofill* or retro‐fill* or retroseal* or retro‐seal* or "apical* seal*" or "apical* prepar*" or retroprepar*))
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Apicoectomy this term only #2 MeSH descriptor Root Canal Therapy explode all trees #3 (apicect* in All Text or apicoect* in All Text) #4 ("root canal therapy" in All Text or ("root canal*" in All Text near/6 treatment in All Text) or ("root canal*" in All Text near/6 filling* in All Text) or ("root canal*" in All Text near/6 restor* in All Text) ) #5 MeSH descriptor Tooth apex this term only #6 MeSH descriptor Periapical diseases explode all trees #7 endodontic* in All Text #8 ((apex in All Text or apical* in All Text) and (surgery in All Text or surgical in All Text)) #9 (periapical* in All Text or periradicular in All Text) #10 MeSH descriptor Retrograde obturation explode all trees #11 ((retrograde in All Text near/6 fill* in All Text) or retrofill* in All Text or retro‐fill* in All Text or retroseal* in All Text or retro‐seal* in All Text or (retro* in All Text near/6 seal* in All Text) or retro‐seal* in All Text or (root next end in All Text near/6 fill* in All Text) or (root‐end in All Text near/6 fill* in All Text) or (root next end in All Text near/6 seal* in All Text) or (root‐end in All Text near/6 seal* in All Text) or apical* next seal* in All Text or (apical* in All Text near/6 prepar* in All Text) or retroprepar* in All Text or retro‐prepar* in All Text or retrograd* in All Text or (reverse in All Text near/6 obturat* in All Text) or (reverse in All Text near/6 fill* in All Text)) #12 (#10 or #11) #13 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #14 (#12 and #13)
Appendix 3. MEDLINE Ovid search strategy
Apicoectomy/
exp Root Canal Therapy/
(apicect$ or apicoect$).mp.
("root canal$ therap$" or ("root canal$" adj6 treatment$) or ("root canal$" adj6 filling$) or ("root canal$" adj6 restor$)).mp.
Tooth apex/
exp Periapical diseases/
(periapical$ or periradicular).mp.
endodont$.mp.
((apex or apical$) and (surgery or surgical$)).mp.
or/1‐9
((retrograde adj6 fill$) or retrofill$ or retro‐fill$ or retroseal$ or retro‐seal$ or (retro adj6 seal$) or ("root end" adj6 fill$) or (root‐end adj6 fill$) or ("root end" adj6 seal$) or (root‐end adj6 seal$) or "apical$ seal$" or "apical$ prepar$" or retroprepar$ or retrograd$ or (reverse adj6 obturat$) or (reverse adj6 fill$)).mp.
Retrograde obturation/
11 or 12
10 and 13
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) (Lefebvre 2011).
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/1‐8
exp animals/ not humans.sh.
9 not 10
Appendix 4. Embase Ovid search strategy
Endodontics/
exp Root Canal Filling Material/
(apicect$ or apicoect$).mp.
((root adj canal adj therapy) or (root adj canal$ adj6 treatment) or (root adj canal$ adj6 filling$) or (root canal$ adj6 restor$)).mp.
Tooth Root Canal/ or Tooth root/
Tooth Periapical Disease/
(periapical$ or periradicular or peri‐radicular).mp.
endodontic$.mp.
(apex or apical$).mp.
((retrograde adj6 fill$) or (retrograde adj obturat$) or retrofill$ or retro‐fill$ or retroseal$ or retro‐seal$ or (retro$ adj6 seal$) or retro‐seal$ or (root adj end adj6 fill$) or (root‐end adj6 fill$) or (root adj end adj6 seal$) or (root‐end adj6 seal$) or apical$ next seal$ or apical$ near prepar$ or retroprepar$ or retro‐prepar$ or retrograd$ or (reverse adj6 obturat$) or (reverse adj6 fill$)).mp.
or/1‐9
10 and 11
The above subject search was linked to Cochrane Oral Health's RCT filter for Embase Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. LILACS BIREME Virtual Health Library search strategy
Mh apicoectomy or Mh root canal therapy or apicoect$ or apicect$ or "root canal$ therap$" or "root canal$ treat$" or "root canal fill$" or "root canal restror$" or Mh Tooth apex or Mh Periapical diseases or periapical$ or periradicular or endodont$)) [Words] and (Mh Retrograde obturation or (retrograde and fill$) or retrofill$ or retro‐fill$ or "retro seal$" or "retro‐seal$" or retroseal$ or "root end fill$" or "root‐end fill$" or "root end seal$" or "root‐end seal$" or "apical seal$" or "apical prepar$" or retroprepar$ or retrograd$ or (reverse and obturat$ or (reverse and fill$))
The above subject search was linked to the Brazilian Cochrane Center filter for LILACs via BIREME:
Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)))
Appendix 6. OpenSIGLE search strategy
(("root canal") AND (fill* or restor*? or therap* or treatment*))
((endodont*) AND (fill* or restor*? or therap* or treatment*))
((retrograd*) AND (fill* or restor* or seal* or prepar*))
apicectom* or apicoectom*
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform search strategies
retrograde AND filling
retrograde AND seal
retrograde AND sealant
Data and analyses
Comparison 1. MTA versus IRM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ 1‐year outcome (teeth as unit of analysis) | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 2 Success rate ‐ 2‐year outcome (participants as unit of analysis) | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.25] |
Comparison 2. MTA versus Super‐EBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ 1‐year outcome (participants as unit of analysis) | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
Comparison 3. Super‐EBA versus IRM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ 1‐year outcome (teeth as unit of analysis) | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.01] |
Comparison 4. Dentine‐bonded resin composite versus glass ionomer cement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ 1‐year outcome PP analysis (participants as unit of analysis) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.60, 3.59] |
| 2 Success rate ‐ 1‐year outcome ITT analysis (participants as unit of analysis) | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.27, 2.64] |
| 3 Success rate ‐ 1‐year outcome PP analysis (root as unit of analysis) | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.20, 2.09] |
| 4 Success rate ‐ 1‐year outcome ITT analysis (root as unit of analysis) | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.62, 3.29] |
Comparison 5. Glass ionomer cement versus amalgam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ 1‐year outcome (tooth as unit of analysis) | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 2 Success rate ‐ 5‐year outcome (tooth as unit of analysis) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.84, 1.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chong 2003.
| Methods | Study type: RCT Sample size calculation: yes Follow‐up period: 12 and 24 months Loss to follow‐up: 61 patients with 61 teeth were lost at 12 months, and 75 patients with 75 teeth were lost at 24 months Intention‐to‐treat analysis: not reported Funding: DHSC London. Research & Development, Responsive Funding Programme |
|
| Participants | Country: UK Centres: 1 Inclusion criteria: tooth with apical periodontitis, diagnosed radiologically; the tooth could not be adequately and better managed by root canal retreatment; the tooth had an adequate root canal filling; the crown of the tooth was adequately restored; and periodontal probing depths were < 4 mm except for an unilocular sinus tract Exclusion criteria: participants who failed to satisfy the entry requirements Total recruited: number of participants: 183; number of teeth: 183; age range: unclear; mean age: unclear; gender (male/female): unclear
(Note: the number of participants in each group at baseline was not reported in the article. And the numbers of participants in each group at 12‐month and 24‐month follow‐ups were recorded as following
|
|
| Interventions | Materials
Preparation of the cavity: ultrasonically using CT tips |
|
| Outcomes | Success rate: assessed by Guidelines of the European Society of Endodontology (1994) and Molven 1987 which required an assessment with the combination of clinical and radiological methods Adverse events: not reported |
|
| Notes | Study author contact failed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was carried out on the day of the surgery; one of the two research team members performing the surgery picked a sealed envelope from a pack to reveal which material to use" Comment: low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation process was carried out on the day of the surgery; one of the two research team members performing the surgery picked a sealed envelope from a pack to reveal which material to use" Comment: low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: high risk. It is not possible to blind the personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The observers were unaware of the group from which the radiographs were taken" Comment: low risk. None of the outcomes were patient‐reported outcomes, so once the observers were blinded to the treatment, there would be no assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high risk. Follow‐up was not reported clearly. The study authors did not report the number of participants in each group at baseline. Also, the number of participants lost to follow‐up in the 1st year and 2nd year follow‐ups is too high (61 (33%) participants lost to follow‐up in the 1st year and another 36 participants with 97 in total (53%) lost to follow‐up in the 2nd year, with the total number of participants at baseline reported as 183) |
| Selective reporting (reporting bias) | Low risk | Comment: low risk. The outcomes were reported as planned |
| Other bias | Unclear risk | Comment: unclear risk. The baseline numbers of each group were not clearly reported, and the numbers of lost to follow‐up were unclear |
Jensen 2002.
| Methods | Study type: RCT Sample size calculation: unclear Follow‐up period: 12 months Loss to follow‐up: 12 teeth of 12 participants Intention‐to‐treat analysis: not reported Funding: unclear |
|
| Participants | Country: Denmark Centres: 1 Inclusion criteria: unclear Exclusion criteria: teeth previously subjected to periapical surgery and teeth with apicomarginal communication Total recruited: number of participants: 134; number of teeth: 134; number of roots: 178; age range: unclear; mean age: 49; gender (male/female): 48/86
|
|
| Interventions | Materials
Preparation of the cavity: with a ball‐shaped diamond bur |
|
| Outcomes | Success rate: assessed with criteria from Zuolo 2000 which required an assessment with the combination of clinical and radiological methods. And the study authors also assessed the success rate of each root with the Rud 1972 criteria which only required radiological assessment Adverse events: not reported |
|
| Notes | The study author was contacted and details about randomisation, blinding and allocation concealment were confirmed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (author's reply): "A randomisation scheme was created using the SAS system" Comment: low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote (author's reply): "The surgeon knew at the beginning of the surgery, which materials that would be used for that specific operation" Comment: unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author's reply): "It was not possible to blind the operator and the clinician since the materials looked differently clinically and radiographically. However, the patients and the statistical assessor were blinded" Comment: high risk |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author's reply): "It was not possible to blind the operator and the clinician since the materials looked differently clinically and radiographically. However, the patients and the statistical assessor were blinded" Comment: high risk. None of the outcomes were patient‐reported outcomes, so once the observers were not blinded to the treatment, there would be a risk of assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low risk. 12 participants lost to follow‐up. The lost to follow‐up rate < 10% |
| Selective reporting (reporting bias) | Low risk | Comment: low risk. The outcomes were reported as planned |
| Other bias | Low risk | Comment: low risk |
Jesslen 1995.
| Methods | Study type: RCT Sample size calculation: unclear Follow‐up period: 12 and 60 months Loss to follow‐up: 18 participants with 23 teeth were lost at 60‐month follow‐up Intention‐to‐treat analysis: not reported Funding: unclear |
|
| Participants | Country: Sweden Centres: 1 Inclusion criteria: teeth indicated for periapical surgery (i.e. teeth with periapical lesions not accessible to conventional endodontic treatment) Exclusion criteria: participants who failed to satisfy the entry requirements Total recruited: number of participants: 85; number of teeth: 105; age range: unclear; mean age: unclear; gender (male/female): unclear. (The numbers in this item only indicate the numbers in 12‐month follow‐up, the exact number of participants/teeth in each group was unclear)
|
|
| Interventions | Materials
Preparation of the cavity: with a number 33.5 inverted cone bur |
|
| Outcomes | Success rate: with criteria from Zetterqvist 1991 which required an assessment with the combination of clinical and radiological methods Adverse events: not reported |
|
| Notes | Study author contact failed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each tooth was then filled with either AM (Amalcap non‐gama‐2; Vivadent, Schaan, Liechtenstein) or GC (Chem‐Fil, De Trey, Zurich, Switzerland) in a randomised fashion" Comment: unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: high risk. It was not possible to blind the personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear risk. 18 of 85 participants lost to follow‐up at 60 months; the lost to follow‐up ratio was between 10% and 20% and we decided to rate this domain as at unclear risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: low risk. The outcomes were reported as planned |
| Other bias | Unclear risk | Comment: unclear risk. The study authors did not clearly report the demographic characteristics of the participants in each group and they did not mention whether the two groups were comparable |
Lindeboom 2005.
| Methods | Study type: RCT Sample size calculation: unclear Follow‐up period: 12 months Loss to follow‐up: none Intention‐to‐treat analysis: yes Funding: unclear |
|
| Participants | Country: the Netherlands Centres: 1 Inclusion criteria: teeth with a dental history of a root canal treatment and demonstrated a periradicular lesion of strictly endodontic origin with or without clinical signs or symptoms. Only single‐rooted teeth were included in this study Exclusion criteria: teeth with perforations of the lateral canal walls, periodontal attachment loss (pocket depth < 5 mm), teeth with vertical fractures, and teeth exhibiting radiographic lesions exceeding 1 cm Total recruited: number of participants: 90; number of teeth: 100; age range: 17‐64; mean age: 43.4; gender (male/female): 33/57
|
|
| Interventions | Materials
Preparation of the cavity: ultrasonic apical preparation |
|
| Outcomes | Success rate: using combined criteria (Molven 1987; Rud 1972) which required an assessment with the combination of clinical and radiological methods Adverse events: not reported |
|
| Notes | The study author was contacted and details about blinding, follow‐up and baseline status were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by a nurse who picked a sealed envelope and opened it at the time of placement of the retrograde filling. On a label the filling material was written" Comment: low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out by a nurse who picked a sealed envelope and opened it at the time of placement of the retrograde filling. On a label the filling material was written" Comment: low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author's reply): "The patients, assessors and statisticians were blinded for the materials, surgeons were not blinded for the filling material (since obviously there is a clinical difference between the materials)" Comment: high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (author's reply): "The patients, assessors and statisticians were blinded for the materials, surgeons were not blinded for the filling material (since obviously there is a clinical difference between the materials)" Comment: low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (author's reply): "None of the patients were lost for follow‐up, although this required an extra effort from the researchers since phone‐call or home visits had to be made in order to get the patient info/x‐ray" Comment: low risk |
| Selective reporting (reporting bias) | Low risk | Comment: adequate. The outcomes were reported as planned |
| Other bias | Low risk | Quote (author's reply): "The gender, age and severity of disease were comparable in both groups" Comment: low risk |
Song 2012.
| Methods | Study type: RCT Sample size calculation: yes Follow‐up period: 12 months Loss to follow‐up: 68 participants with 68 teeth lost at 12 months Intention‐to‐treat analysis: no Funding: the National Research Foundation of Korea |
|
| Participants | Country: South Korea Centres: 1 Inclusion criteria: all root‐filled cases with symptomatic or asymptomatic apical periodontitis Exclusion criteria: teeth with Class II mobility or greater, horizontal and vertical fractures, and perforations. Through endodontic microsurgery, teeth with a through‐and‐through lesion and/or a lesion of combined periodontal endodontic origin were also excluded Total recruited: number of participants: 260; number of teeth: 260; age range: unclear; mean age: unclear; gender (male/female): unclear
|
|
| Interventions | Materials
Preparation of the cavity: ultrasonic apical preparation, with microscope |
|
| Outcomes | Success rate: using criteria from Molven 1987 and Molven 1996 which required an assessment with the combination of clinical and radiological methods Adverse events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They [teeth] were randomly assigned to either the Super‐EBA group or the MTA group (130 teeth per group) using the 'minimization method' as described by Pocock" Comment: low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The random allocation sequence was generated by an assistant." Author's reply: "When the patient registered, we gave the patient information regarding sex, age, tooth type and got the assignment group" Comment: low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author's reply): "Patients and statistician do not know but the operators know the allocated intervention because the MTA and Super‐EBA is different by just looking" Comment: high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The radiographic findings were evaluated blindly and independently by 2 examiners using the same criteria" Comment: low risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Among the 260 teeth included in this randomized controlled trial, 192 teeth were examined at the 12‐month follow‐up" Comment: Teeh/participants lost to follow‐up reached to 26.1%, high risk |
| Selective reporting (reporting bias) | Low risk | Comment: low risk. The outcomes were reported as planned |
| Other bias | Low risk | Quote: "The following 3 randomization factors were considered: sex, age, and tooth type" Comment: low risk |
Wälivaara 2011.
| Methods | Study type: RCT Sample size calculation: no Follow‐up period: 12 months Loss to follow‐up: 7 participants with 8 teeth were lost at 12 months Intention‐to‐treat analysis: no Funding: unclear |
|
| Participants | Country: Sweden Centres: 1 Inclusion criteria: all teeth were included except those with obvious root fractures or advanced periodontal disease Exclusion criteria: teeth with obvious root fractures or advanced periodontal disease Total recruited: number of participants: 164; number of teeth: 206; age range: unclear; mean age: unclear; gender (male/female): 65/99
|
|
| Interventions | Materials
Preparation of the cavity: ultrasonic apical preparation using × 2.3 magnification operation loupes |
|
| Outcomes | Success rate: using combined criteria (Molven 1987; Rud 1972) which required an assessment with the combination of clinical and radiological methods Adverse events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization procedure was performed using a standard randomization table" Comment: low risk |
| Allocation concealment (selection bias) | High risk | Quote (author's reply): "The allocation to either material group was performed according to a randomization table and thus not concealed" Comment: high risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author's reply): "The patients were informed/consented about the study at the surgery appointment and the operator got the information of which material to use at the start of the surgery. The statistician just received all numbers/figures after the study was completed" Comment: high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear risk. No information on blinding of outcome assessment was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low risk. 7 participants lost to follow‐up. The lost to follow‐up rate < 10% |
| Selective reporting (reporting bias) | Low risk | Comment: low risk. The outcomes were reported as planned |
| Other bias | Low risk | Comment: low risk |
RCT = randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burstein 2001 | Study design: unclear The study author did not mention randomisation. We have tried to contact the authors, but no response was obtained |
| Christiansen 2009 | Study design: the control group does not have retrograde obturation with any material Quote: "The aim of the present study was to compare periapical healing after root‐end resection followed by a root‐end filling with MTA or smoothing of the orthograde gutta‐percha (GP) root filling only" |
| Hou 2008 | Inadequate study design: no radiological outcome was recorded |
| Nordenram 1970 | Study design: unclear The study author did not mention randomisation. We have tried to contact the authors, but no response was obtained |
| Pantchev 2009 | Study design: retrospective Quote: "The study is retrospective and the materials consisted of 186 teeth from 131 consecutive patients who had undergone endodontic surgery during 1993–2003 at a specialist endodontic clinic in Västerås, Sweden" |
| Pantschev 1994 | Study design: unclear The study author did not mention randomisation. We have tried to contact the authors, but no response was obtained |
| Platt 2004 | Inadequate study design: the methods used to prepare the cavity differed in both randomised groups Quote: "A shallow concave apical preparation was filled with a light‐cured compomer with a light‐cured dental adhesive. As a control, a chemically cured glass ionomer was used with a conventional root‐end preparation" |
| Rud 1991 | Study design: retrospective study Quote: "388 cases with retrograde amalgam fillings were selected randomly among patients previously treated by one of the authors (JR) and all controlled 1 year after the operation" |
| Rud 1996 | Study design: unclear The study author did not mention randomisation. We have tried to contact the authors, but no response was obtained |
| Schwartz‐Arad 2003 | Study design: retrospective Quote: "Retrospective. The study collected 228 patients with 262 endodontically treated teeth between 1994 and 1999, operated by 2 oral surgeons" |
| Silva 2016 | Study design: insufficient follow‐up period Quote: "The teeth and surrounding tissues were assessed clinically and by CT scan at the 6‐month follow‐up" |
| von Arx 2012 | Study design: cohort study Quote: "To further elucidate the prognosis of apical microsurgery and the outcome predictors, the purpose of this prospective longitudinal study was to provide evidence for the 5‐year outcome of apical microsurgery in a cohort of patients for whom we previously reported the 1‐year outcome" |
| Wälivaara 2009 | Study design: quasi‐RCT Quote: "160 teeth in 139 consecutive patients (58 men and 81 women) were randomly allocated into 2 groups according to the date of birth" |
CT = computed tomography; MTA = mineral trioxide aggregate; RCT = randomised controlled trial.
Differences between protocol and review
As the protocol was published more than ten years ago and many things changed during these years, a little modification was made on the protocol.
The primary outcome was renamed as success rate; radiological outcome and clinical outcome were all considered criteria or subsets of success rate. The meaning of the outcome was not changed. Adverse events were added as a secondary outcome.
More electronic databases (e.g. LILACS, VIP, China National Knowledge Infrastructure) were added to try to identify more non‐English studies. Ongoing/unpublished studies searching (via US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform, Sciencepaper Online) was also added.
For the risk of bias assessment, seven domains were adopted instead of the previous four domains as it is suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); and the GRADE system was introduced.
The random‐effects model was used when the number of studies for each outcome exceeded four instead of using it throughout.
Intention‐to‐treat analysis was added as one of the sensitivity analysis.
Following the introduction of RevMan 5 (RevMan 2014) and the conversion to a new review format, more subsections have been added to the methods section including: unit of analysis issues, dealing with missing data, assessment of reporting biases, subgroup analysis and investigation of heterogeneity, and presentation of main results.
Contributions of authors
Xiangyu Ma and Chunjie Li were co‐first authors of this review.
Xiangyu Ma included the studies, obtained copies of trials, assessed the risk of bias, extracted data and did the whole writing and revision of the systematic review.
Chunjie Li assessed the risk of bias, extracted data, carried out the analysis and revised the systematic review.
Yan Wang included the studies, obtained copies of trials and revised the whole writing.
Wenwen Liu helped to obtain copies of trials and revised the whole writing.
Liuhe Jia registered the review title, prepared the protocol, provided clinical support and revised the whole writing of the systematic review.
Dingming Huang and Xuedong Zhou provided content expertise on the systematic review and revised the whole writing.
Trevor M Johnson included the studies and provided content expertise on the systematic review and revised the whole writing.
Sources of support
Internal sources
West China College of Stomatology, Sichuan University, China.
External sources
Cochrane Oral Health, UK.
Chinese Cochrane Center, China.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships‐alliances). Contributors over the last year have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
Xiangyu Ma: none known. Chunjie Li: none known. Liuhe Jia: none known. Yan Wang: none known. Wenwen Liu: none known. Xuedong Zhou: none known. Trevor M Johnson: none known. Dingming Huang: none known.
New
References
References to studies included in this review
Chong 2003 {published data only}
- Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of Mineral Trioxide Aggregate and IRM when used as root‐end filling materials in endodontic surgery. International Endodontic Journal 2003;36(8):520‐6. [DOI] [PubMed] [Google Scholar]
Jensen 2002 {published data only}
- Jensen SS, Nattestad A, Egdo P, Sewerin I. A prospective, randomized, comparative clinical study of resin composite and glass ionomer cement for retrograde root filling. Clinical Oral Investigations 2002;6(4):236‐43. [DOI] [PubMed] [Google Scholar]
Jesslen 1995 {published data only}
- Jesslen P, Zetterqvist L, Heimdahl A. Long‐term results of amalgam versus glass ionomer cement as apical sealant after apicectomy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1995;79(1):101‐3. [DOI] [PubMed] [Google Scholar]
- Zetterqvist L, Hall G, Holmlund A. Apicectomy: a comparative clinical study of amalgam and glass ionomer cement as apical sealants. Oral Surgery, Oral Medicine, and Oral Pathology 1991;71(4):489‐91. [DOI] [PubMed] [Google Scholar]
Lindeboom 2005 {published data only}
- Lindeboom JA, Frenken JW, Kroon FH, Akker HP. A comparative prospective randomized clinical study of MTA and IRM as root‐end filling materials in single‐rooted teeth in endodontic surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2005;100(4):495‐500. [DOI] [PubMed] [Google Scholar]
Song 2012 {published data only}
- Song M, Kim E. A prospective randomized controlled study of mineral trioxide aggregate and super ethoxy‐benzoic acid as root‐end filling materials in endodontic microsurgery. Journal of Endodontics 2012;38(7):875‐9. [DOI] [PubMed] [Google Scholar]
Wälivaara 2011 {published data only}
- Wälivaara DÅ, Abrahamsson P, Fogelin M, Isaksson S. Super‐EBA and IRM as root‐end fillings in periapical surgery with ultrasonic preparation: a prospective randomized clinical study of 206 consecutive teeth. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2011;112(2):258‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Burstein 2001 {published data only}
- Burstein J, Ko B, Glick D, White SN. 18 month clinical trial of endodontic surgical retrofilling materials. Journal of Endodontics 2001;27(3):219. [Google Scholar]
Christiansen 2009 {published data only}
- Christiansen R, Kirkevang LL, Horested‐Bindslev P, Wenzel A. Randomized clinical trial of root‐end resection followed by root‐end filling with mineral trioxide aggregate or smoothing of the orthograde gutta‐percha root filling ‐ 1‐year follow‐up. International Endodontic Journal 2009;42(2):105‐14. [DOI] [PubMed] [Google Scholar]
Hou 2008 {published data only}
- Hou WX. Effect of mineral trioxide aggregate in retrograde filling. Journal of Medical Forum 2008;29(21):19‐20. [Google Scholar]
Nordenram 1970 {published data only}
- Nordenram A. Biobond for retrograde root filling in apicectomy. Scandinavian Journal of Dental Research 1970;78(3):251‐5. [DOI] [PubMed] [Google Scholar]
Pantchev 2009 {published data only}
- Pantchev A, Nohlert E, Tegelberg A. Endodontic surgery with and without inserts of bioactive glass PerioGlas ‐ a clinical and radiographic follow‐up. Oral and Maxillofacial Surgery 2009;13(1):21‐6. [DOI] [PubMed] [Google Scholar]
Pantschev 1994 {published data only}
- Pantschev A, Carlsson AP, Andersson L. Retrograde root filling with EBA cement or amalgam. A comparative clinical study. Oral Surgery, Oral Medicine, and Oral Pathology 1994;78(1):101‐4. [DOI] [PubMed] [Google Scholar]
Platt 2004 {published data only}
- Platt AS, Wannfors K. The effectiveness of compomer as a root‐end filling: a clinical investigation. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;97(4):508‐12. [DOI] [PubMed] [Google Scholar]
Rud 1991 {published data only}
- Rud J, Munksgaard EC, Andreasen JO, Rud V. Retrograde root filling with composite and a dentin‐bonding agent. 2. Endodontics & Dental Traumatology 1991;7(3):126‐31. [DOI] [PubMed] [Google Scholar]
Rud 1996 {published data only}
- Rud J, Rud V, Munksgaard EC. Retrograde root filling with dentin‐bonded modified resin composite. Journal of Endodontics 1996;22(9):477‐80. [DOI] [PubMed] [Google Scholar]
Schwartz‐Arad 2003 {published data only}
- Schwartz‐Arad D, Yarom N, Lustig JP, Kaffe I. A retrospective radiographic study of root‐end surgery with amalgam and intermediate restorative material. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2003;96(4):472‐7. [DOI] [PubMed] [Google Scholar]
Silva 2016 {published data only}
- Silva SR, Silva JD Neto, Schnaider TB, Veiga DF, Novo NF, Mesquita M Filho, et al. The use of a biocompatible cement in endodontic surgery. A randomized clinical trial 1. Acta Cirurgica Brasileira 2016;31(6):422‐7. [DOI] [PubMed] [Google Scholar]
von Arx 2012 {published data only}
- Arx T, Jensen SS, Hänni S, Friedman S. Five‐year longitudinal assessment of the prognosis of apical microsurgery. Journal of Endodontics 2012;38(5):570‐9. [DOI] [PubMed] [Google Scholar]
Wälivaara 2009 {published data only}
- Wälivaara DA, Abrahamsson P, Sämfors KA, Isaksson S. Periapical surgery using ultrasonic preparation and thermoplasticized gutta‐percha with AH Plus sealer or IRM as retrograde root‐end fillings in 160 consecutive teeth: a prospective randomized clinical study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2009;108(5):784‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baek 2005
- Baek SH, Plenk H Jr, Kim S. Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root‐end filling materials. Journal of Endodontics 2005;31(6):444‐9. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Ber 2007
- Ber BS, Hatton JF, Stewart GP. Chemical modification of proroot mta to improve handling characteristics and decrease setting time. Journal of Endodontics 2007;33(10):1231‐4. [DOI] [PubMed] [Google Scholar]
Camilleri 2005
- Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dental Materials 2005;21(4):297‐303. [DOI] [PubMed] [Google Scholar]
Camilleri 2006
- Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. International Endodontic Journal 2006;39(10):747‐54. [DOI] [PubMed] [Google Scholar]
Chong 2004
- Chong BS. Chapter 8. A surgical alternative. In: Chong BS editor(s). Managing Endodontic Failure in Practice. Chicago: Quintessence Publishing Co Ltd, 2004:123‐47. [Google Scholar]
Cohen 2006
- Cohen S, Hargreaves KM. Pathways of the Pulp. 9th Edition. Mosby, 2006. [Google Scholar]
Dalal 1983
- Dalal MB, Gohil KS. Comparison of silver amalgam, glass ionomer cement & gutta percha as retrofilling materials, an in vivo & in vitro study. Journal of the Indian Dental Association 1983;55(4):153‐8. [PubMed] [Google Scholar]
Damas 2011
- Damas BA, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. Journal of Endodontics 2011;37(3):372‐5. [DOI] [PubMed] [Google Scholar]
Dorn 1990
- Dorn SO, Gartner AH. Retrograde filling materials: a retrospective success‐failure study of amalgam, EBA, and IRM. Journal of Endodontics 1990;16(8):391‐3. [DOI] [PubMed] [Google Scholar]
Eley 1993
- Eley BM, Cox SW. The release, absorption and possible health effects of mercury from dental amalgam: a review of recent findings. British Dental Journal 1993;175(10):355‐62. [DOI] [PubMed] [Google Scholar]
Eriksen 1991
- Eriksen HM. Endodontology: epidemiologic considerations. Endodontics and Dental Traumatology 1991;7(5):189‐95. [DOI] [PubMed] [Google Scholar]
Finne 1977
- Finne K, Nord PG, Persson G, Lennartsson B. Retro‐grade root filling with amalgam and Cavit. Oral Surgery, Oral Medicine, and Oral Pathology 1977;43(4):621‐6. [DOI] [PubMed] [Google Scholar]
Gartner 1992
- Gartner AH, Dorn SO. Advances in endodontic surgery. Dental Clinics of North America 1992;36(2):357‐78. [PubMed] [Google Scholar]
Gilheany 1994
- Gilheany PA, Figdor D, Tyas MJ. Apical dentin permeability and microleakage associated with root end resection and retrograde filling. Journal of Endodontics 1994;20(1):22‐6. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 25 October 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grung 1990
- Grung B, Molven O, Halse A. Periapical surgery in a Norwegian county hospital: follow‐up findings of 477 teeth. Journal of Endodontics 1990;16(9):411‐7. [DOI] [PubMed] [Google Scholar]
Gutmann 1991
- Gutmann JL, Harrison JW. Surgical Endodontics. Boston: Blackwell Scientific Publications, 1991. [Google Scholar]
Hargreaves 2015
- Hargreaves KM, Berman LH. Cohen's Pathways of the Pulp. 11th Edition. Mosby, 2015. [Google Scholar]
Harty 1970
- Harty FJ, Parkins BJ, Wengraf AM. The success rate of apicectomy. A retrospective study of 1016 cases. British Dental Journal 1970;129(9):407‐13. [DOI] [PubMed] [Google Scholar]
Hauman 2003
- Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 2: Root‐canal filling materials. International Endodontic Journal 2003;36(3):147‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsch 1979
- Hirsch JM, Ahlstrom U, Henrikson PA, Heyden G, Peterson LE. Periapical surgery. International Journal of Oral Surgery 1979;8(3):173‐85. [DOI] [PubMed] [Google Scholar]
Huang 2008
- Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of mineral trioxide aggregate. Journal of Endodontics 2008;34:590‐3. [DOI] [PubMed] [Google Scholar]
Johnson 1999
- Johnson BR. Considerations in the selection of a root‐end filling material. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1999;87(4):398‐404. [DOI] [PubMed] [Google Scholar]
Kim 2001
- Kim S, Pecora G, Rubinstein R. Comparison of traditional and microsurgery in endodontics. Color Atlas of Microsurgery in Endodontics. Philadelphia: WB Saunders, 2001:5‐11. [Google Scholar]
Kim 2006
- Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. Journal of Endodontics 2006;32(7):601‐23. [DOI] [PubMed] [Google Scholar]
King 1990
- King KT, Anderson RW, Pashley DH, Pantera EA Jr. Longitudinal evaluation of the seal of endodontic retrofillings. Journal of Endodontics 1990;16(7):307‐10. [DOI] [PubMed] [Google Scholar]
Koh 1998
- Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. Journal of Endodontics 1998;24(8):543‐7. [DOI] [PubMed] [Google Scholar]
Kratchman 2004
- Kratchman SI. Perforation repair and one‐step apexification procedures. Dental Clinics of North America 2004;48:291‐307. [DOI] [PubMed] [Google Scholar]
Kuratate 2008
- Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. Journal of Endodontics 2008;34:970‐4. [DOI] [PubMed] [Google Scholar]
Laurent 2012
- Laurent P, Camps J, About I. Biodentine(TM) induces TGF‐beta1 release from human pulp cells and early dental pulp mineralization. International Endodontic Journal 2012;45(5):439‐48. [PUBMED: 22188368] [DOI] [PubMed] [Google Scholar]
Lee 1993
- Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. Journal of Endodontics 1993;19(11):541‐4. [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials 2004;25(5):787‐93. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lin 1991
- Lin LM, Pascon EA, Skribner J, Gangler P, Langeland K. Clinical, radiographic and histologic study of endodontic treatment failures. Oral Surgery, Oral Medicine, and Oral Pathology 1991;71(5):603‐11. [DOI] [PubMed] [Google Scholar]
Molven 1987
- Molven O, Halse A, Grung B. Observer strategy and the radiographic classification of healing after endodontic surgery. International Journal of Oral and Maxillofacial Surgery 1987;16(4):432‐9. [DOI] [PubMed] [Google Scholar]
Molven 1996
- Molven O, Halse A, Grung B. Incomplete healing (scar tissue) after periapical surgery ‐ radiographic findings 8 to 12 years after treatment. Journal of Endodontics 1996;22(5):264‐8. [DOI] [PubMed] [Google Scholar]
Niederman 2003
- Niederman R, Theodosopoulou JN. A systematic review of in vivo retrograde obturation materials. International Endodontic Journal 2003;36(9):577‐85. [DOI] [PubMed] [Google Scholar]
Persson 1974
- Persson G, Lennartson B, Lundström I. Results of retro‐grade root‐filling with special reference to amalgam and Cavitas root‐filling materials. Swedish Dental Journal 1974;67(3):123‐34. [PubMed] [Google Scholar]
Petrisor 2006
- Petrisor BA, Keating J, Schemitsch E. Grading the evidence: Levels of evidence and grades of recommendation. Injury 2006;37(4):321‐7. [DOI] [PubMed] [Google Scholar]
Pocock 1983
- Pocock SJ. Clinical Trials: A Practical Approach. Chichester, UK: Wiley, 1983. [Google Scholar]
Poggio 2007
- Poggio C, Lombardini M, Conti A, Rindi S. Solubility of root‐end filling materials: a comparative study. Journal of Endodontics 2007;33(9):1094‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rud 1972
- Rud J, Andreasen JO, Jensen JE. Radiographic criteria for the assessment of healing after endodontic surgery. International Journal of Oral Surgery 1972;1(4):195‐214. [DOI] [PubMed] [Google Scholar]
Siqueira 2001
- Siqueira J. Aetiology of root canal treatment failure: why well‐treated teeth can fail. International Endodontic Journal 2001;34(1):1‐10. [DOI] [PubMed] [Google Scholar]
Siqueira Jr 2003
- Siqueira JF Jr. Microbial causes of endodontic fare‐ups. International Endodontic Journal 2003;36(7):453‐63. [DOI] [PubMed] [Google Scholar]
Sjogren 1990
- Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long‐term results of endodontic treatment. Journal of Endodontics 1990;16(10):498‐504. [DOI] [PubMed] [Google Scholar]
Soundappan 2014
- Soundappan S, Sundaramurthy JL, Raghu S, Natanasabapathy V. Biodentine versus Mineral Trioxide Aggregate versus Intermediate Restorative Material for Retrograde Root End Filling: An invitro study. Journal of Dentistry (Tehran, Iran) 2014;11(2):143‐9. [PMC free article] [PubMed] [Google Scholar]
Sundqvist 1998
- Sundqvist G, Figdor D. Endodontic treatment of apical periodontitis. In: Orstavik D, Pitt Ford TR editor(s). Essential Endodontology: Prevention and Treatment of Apical Periodontitis. London: Wiley‐Blackwell, 1998:242. [Google Scholar]
Swartz 1983
- Swartz DB, Skidmore AE, Griffin JA Jr. Twenty years of endodontic success and failure. Journal of Endodontics 1983;9(5):198‐202. [DOI] [PubMed] [Google Scholar]
Takita 2006
- Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. International Endodontic Journal 2006;39(5):415‐22. [DOI] [PubMed] [Google Scholar]
Tang 2010
- Tang Y, Li X, Yin S. Outcomes of MTA as root‐end filling in endodontic surgery: a systematic review. Quintessence International 2010;41(7):557‐66. [PubMed] [Google Scholar]
Theodosopoulou 2005
- Theodosopoulou JN, Niederman R. A systematic review of in vitro retrograde obturation materials. Journal of Endodontics 2005;31(5):341‐9. [DOI] [PubMed] [Google Scholar]
Torabinejad 1993
- Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. Journal of Endodontics 1993;19(12):591‐5. [PUBMED: 8151252] [DOI] [PubMed] [Google Scholar]
Torabinejad 1995
- Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root‐end filling material. Journal of Endodontics 1995;21(7):349‐53. [DOI] [PubMed] [Google Scholar]
Vasudev 2003
- Vasudev SK, Goel BR, Tyagi S. Root end filling materials ‐ A review. Endodontology 2003;15:12‐8. [Google Scholar]
Walters 2004
- Walters SJ. Sample size and power estimation for studies with health related quality of life outcomes: a comparison of four methods using the SF‐36. Health and Quality of Life Outcomes 2004;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang WH, Wang CY, Shyu YC, Liu CM, Lin CM, Lin CP. Compositional characteristics and hydration behavior of mineral trioxide aggregates. Journal of Dental Science 2010;5(2):53‐9. [Google Scholar]
Wong 2004
- Wong R. Conventional endodontic failure and retreatment. Dental Clinics of North America 2004;48(1):265‐89. [DOI] [PubMed] [Google Scholar]
Zetterqvist 1991
- Zetterqvist L, Hall G, Holmlund A. Apicectomy: a comparative clinical study of amalgam and glass ionomer cement as apical sealants. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology 1991;71(4):489‐91. [DOI] [PubMed] [Google Scholar]
Zuolo 2000
- Zuolo ML, Ferreira MOF, Gutmann JL. Prognosis in periradicular surgery: a clinical prospective study. International Endodontic Journal 2000;33(2):91‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jia 2005
- Jia L, Qi W, Ming HD, Dong ZX. Materials for retrograde filling in root canal therapy. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005517] [DOI] [PMC free article] [PubMed] [Google Scholar]


