Abstract
The response to highly active antiretroviral treatment (HAART) and predictors of mortality among patients with advanced HIV infection (CD4+ cell count <50 cells/mm3) in Botswana are described. Clinical and laboratory data for 349 patients with CD4 < 50 cells/mm3 initiating HAART from January 23 to November 18, 2002 at Princess Marina Hospital in Gaborone, Botswana were extracted from clinical charts and electronic patient management systems. The Kaplan–Meier method was used to estimate survival and log-rank tests used for group comparisons. Cox regression was used to identify independent predictors of survival. A total of 349 adults initiated HAART. In all, 78.2% (95% CI: 73.7%, 82.9%) of patients survived 1 year. Among survivors, the mean CD4+ cell count increase was 239.8 cells/mm3 (95% CI: 217.0, 262.8) at 12 months; 92.1% (95% CI: 87.8%, 94.9%) of patients (as treated) had plasma HIV-1 RNA ≤400 copies/ml at 9 months declining to 59.9% (95% CI: 54.7%, 64.9%) (ITT). There was a 2-fold higher mortality rate among patients with CD4+ ≤10 cells/mm3 compared to 11–49 cells/mm3, hazard ratio (HR) = 1.91 (95% CI: 1.16, 3.14). A 10 cell/mm3 higher CD4+ cell count corresponded to a 22% decrease in hazard of death (HR = 0.78; 95% CI: 0.64, 0.94). Lower baseline CD4+ cell count (p < 0.001) and WHO clinical stage 4 HR = 2.41 (95% CI: 1.32, 4.38) were independent predictors of poorer survival. HAART confers significant benefit even among persons with advanced immunosuppression. Adults with CD4+ cell counts ≤10 cells/mm3 and/or WHO clinical stage 4 disease at the time of HAART initiation have a higher risk of death.
Introduction
Highly active antiretroviral therapy (HAART) has been the standard of care in the developed world since 1996.1 The introduction of HAART has had an enormous impact on the health of persons infected with HIV in Western Europe and the United States.2–4 Sub-Saharan Africa has the highest prevalence of HIV/AIDS worldwide,5 and HIV-1C is the predominant HIV-1 subtype in the region.6 In 2002, Botswana became the first country in Africa to offer HAART through its public health system at the outpatient Infectious Disease Care Clinic (Princess Marina Hospital) in the capital city of Gaborone. Due to huge patient demands and limited physical infrastructure when the program first began, the majority of initially screened and prioritized patients were the “sickest of the sick,” with many having CD4+ cell counts below 50 cells/mm3.7–9 These patients consumed considerable resources as many of them required multiple clinic visits prior to HAART initiation for stabilization and treatment of active opportunistic infections, and many also required multiple hospitalizations. Such “late presenters” are at heightened risk of clinical events while their CD4+ cell count values are low, and those who initiate HAART with very low CD4+ cell counts are reported to be less likely to have a sustained virologic response compared to patients starting at higher baseline CD4+ cell counts.10–12 Although other studies have documented the response to HAART among patients with low baseline CD4+ cell counts,13–15 these studies were conducted primarily among cohorts of HAART-treated HIV-1B infected patients in Western Europe and the United States. As countries in Africa are rolling out antiretroviral treatment programs, more information is needed about the overall response to HAART among this group of severely immuno-compromised adults.
We report our experience with the first group of severely immunosuppressed antiretroviral (ARV)-naive adults initiated on HAART as part of Botswana's national ARV treatment program. The overall impact of HAART in this setting, including clinical outcomes and laboratory evaluation, is presented.
Materials and Methods
Patient population and procedures
The patients were seen at the Adult Infectious Disease Care Clinic (IDCC) of Princess Marina Hospital established for outpatient care of HIV-1-infected adults. Patients with AIDS-defining illnesses and/or CD4+ cell count <200 cells/mm3 were offered HAART in accordance with existing Botswana National Antiretroviral Treatment Guidelines.16 Of the 1983 patients registered in the IDCC between January 23, 2002 and November 18, 2002, 349 were antiretroviral naive with CD4+ cell counts less than 50 cells/mm3 prior to HAART initiation. We enrolled the first 349 ARV-naive adult patients with CD4+ cell counts less than 50 cells/mm3 at baseline who initiated HAART at the IDCC. These patients initiated HAART between February 6 and March 31, 2002.
The recommended first line regimens for adult patients in Botswana in 2002 were combivir (zidovudine plus lamivudine) (CBV) and either efavirenz (EFV) for all men and for women without reproductive potential or nevirapine (NVP) for women with reproductive potential. A few patients were initiated on didanosine, lamivudine, or stavudine in combination with either EFV or NVP, if they had preexisting anemia, defined as baseline hemoglobin of less than 7.0 g/dl.
At the initial visit, patients’ basic demographic information and the date of the first positive HIV test were recorded. All patients underwent a comprehensive medical history and physical examination, including body weight measurement. The baseline tests included complete blood count, CD4+ cell count, plasma HIV-1 RNA, liver function tests, albumin, blood urea nitrogen (BUN), and serum creatinine. CD4+ cell counts obtained at CD4+ cell count screening clinics within 3 months prior to registering at the IDCC were used to determine the need for HAART initiation, unless patients had an AIDS-defining illness, which immediately qualified them for HAART initiation. Chest radiography was performed on all patients and three sputum specimens for acid-fast bacilli were obtained when clinically indicated. Patients found to have syphilis, tuberculosis, or HIV-associated medical conditions were treated in accordance with national guidelines. After their initial visit, all patients were scheduled to return within 2 weeks for HAART initiation.
All patients initiated on nevirapine-containing HAART were scheduled to return to the IDCC 2 weeks following HAART initiation for blood draw (AST and ALT) and dose escalation from 200 mg once daily to 200 mg twice daily. One month following HAART initiation, all patients returned for adherence education and review and blood was drawn for chemistry and hematology. Subsequently, blood was drawn for hematology and chemistry at months 2 and 3 and then every 3 months thereafter. CD4+ cell count was repeated at 3 months and then every 3 months thereafter where possible. Blood was obtained for HIV-1 RNA at 3 months and if, when compared to baseline, there was “adequate” viral suppression (defined as a 1.0 log or greater reduction in plasma HIV-1 RNA), then plasma HIV-1 RNA determinations were obtained every 6 months thereafter. Patient weight was measured at baseline and quarterly thereafter. Abnormal laboratory results were flagged and attended to by the treating physician. All patients who missed three consecutive scheduled clinic visits were contacted by phone or at home and were requested to come to the IDCC. If the patient could not be reached, their designated contact person was called. If unsuccessful after two such attempts, they were considered lost to follow-up. The hospital (for in-patient deaths) or the patient's family for those who died outside the facility notified the clinic of patients’ deaths. Patients were asked to designate a family member or friend as an “adherence partner” to aid with antiretroviral medication adherence and potential toxicity recognition. This “partner” was usually a spouse, family member, or close friend. The partner helped remind the patient to take ARV medications and to attend scheduled appointments. Patients were given sufficient ARV medication supplies to last 30 days with an additional 3 day supply to cover emergencies.
Laboratory methods
All patients had a confirmed positive HIV-1 enzyme-linked immunosorbent assay or rapid HIV test at enrollment that was performed at public voluntary counseling and testing centers, government clinics/hospitals, or in private practitioner clinics. Plasma HIV-1 RNA was quantified using the Amplicor HIV-1 Monitor test version 1.5 (Roche Diagnostics Systems, Branchburg, NJ). The lower limit of quantification is 400 copies/ml and the upper limit is 750,000 copies/ml for this assay. CD4+ cell counts were determined within 4 h of obtaining the blood sample using the FACSCalibur™ flow cytometer (Becton Dickinson, San Jose, CA) with CD3/4/8/45 Multiset reagents.
Data collection and statistical analysis
Data were obtained from clinical charts and the electronic integrated patient management system (IPMS) that was maintained in Microsoft Access. Charts of all consecutively seen adults who initiated HAART between January 23 and November 18, 2002 at the IDCC were reviewed until 350 patients initiating HAART with CD4 < 50 cells/mm3 were identified for study inclusion. One patient was excluded because he did not actually initiate HAART prior to death. Clinical and laboratory information was abstracted into Microsoft Excel and exported to SAS 8.2 (SAS Institute Inc. Cary, NC) for analysis.
We used all available follow-up data in the analysis starting from time of HAART initiation. If the patient stopped or changed treatment his data were included according to his initial regimen. However, changes in CD4+ cell count and body weight and percentage with detectable HIV-1 RNA included only patients in active follow-up. For each patient, time was counted from HAART initiation. Kaplan–Meier methods were used to estimate survival and loss to follow-up distributions, and group comparisons of survival were made using the log-rank test. Cox regression was used to calculate hazard ratios and to identify independent predictors of survival, loss to follow-up, and death.. The following factors were included in a multiple Cox regression model: CD4 count (continuous scale), log-transformed HIV-1 RNA (continuous scale), sex, WHO clinical stage 4, and HAART regimen (see Table 1). All statistical tests were two-sided at the 0.05 significance level and no adjustments were made for multiple comparisons.
Table 1.
Cox Regression Models
| Univariate models | Adjusted model | |||
|---|---|---|---|---|
| Characteristic | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value |
| CD4 count (HR is for 10 cell/mm3 higher count) | 0.75 (0.62, 0.91) | 0.003b | 0.78 (0.64, 0.94) | <0.001b |
| Log HIV-1 RNA (HR is for 1 log copy/ml higher value) | 0.58 (0.33, 1.01) | 0.062b | 0.69 (0.39, 1.22) | 0.21b |
| Sex | ||||
| Female | 0.83 (0.52, 1.33) | 0.43a | 0.68 (0.33, 1.38) | 0.28b |
| Male | 1.00 (referent) | 1.00 (referent) | ||
| WHO clinical stage | ||||
| 1–3 | 1.00 (referent) | <0.001a | 1.00 (referent) | 0.002b |
| 4 | 2.49 (1.42, 4.36) | 2.41 (1.32, 4.38) | ||
| HAART regimenc | ||||
| CBV + NVP | 0.84 (0.51, 1.39) | 0.49b | 1.36 (0.64, 2.90) | 0.58b |
| CBV + EFV | 1.00 (referent) | 1.00 (referent) | ||
p-value from log-rank test.
p-value from likelihood ratio test.
An indicator for other HAART regimens was included in the model but results are not displayed because of the small number of patients on these regimens.
Ethical approval was obtained from the Princess Marina Hospital Institutional Research and Ethics Committee, the Health Research Development Unit (Ministry of Health, Botswana), and the Harvard School of Public Health's Human Subjects Committee (HSC).
Results
Patient characteristics
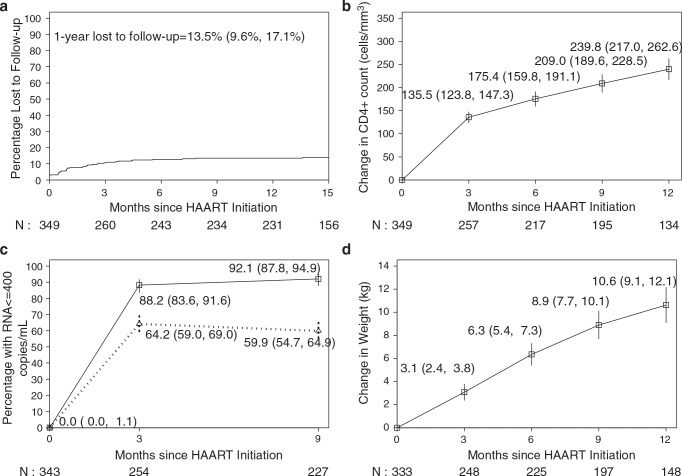
The median age was 35 years (range 17–68) and 59% were women (Table 2). The median body weight was 53 kg for men and 48 kg for women and the median body mass index (BMI) was 17.0 kg/m2. Ninety percent had WHO clinical stage 3 or 4 disease. The median CD4+ cell count was 22 cells/mm3 (range 0–49) and plasma HIV-1 RNA was 534,000 copies/ml (range 12,200–750,000). Eighty-five percent of the HAART-treated men were treated with CBV/EFV and 70% of the HAART-treated women were treated with CBV/NVP. The median follow-up was 1.2 years (range 0–692 days). Four patients transferred to other public ARV treatment sites and 44 were lost to follow-up. At 1 year, 13.5% (95% CI: 9.6%, 17.1%) (Fig. 1a) were lost to follow-up.
Table 2.
Baseline Characteristics of Study Population (n = 349)a
| Characteristic | Total n = 349 | Men n = 144 | Women n = 205 |
|---|---|---|---|
| Age (years): median (min, max) | 35 (17, 68) | 36 (17, 64) | 34 (19, 68) |
| Weight (kg): median (min, max) | 50 (25, 92) | 53 (29, 76) | 48 (25, 92) |
| BMI (kg/m2): median (min, max) | 17 (10, 35) | 17 (11, 25) | 18 (10, 35) |
| WHO clinical stage: N = 343 (%) | |||
| 1 | 9 (3%) | 2 (1%) | 7 (4%) |
| 2 | 25 (7%) | 10 (7%) | 15 (7%) |
| 3 | 100 (29%) | 39 (28%) | 61 (30%) |
| 4 | 209 (61%) | 90 (64%) | 119 (59%) |
| CD4+ cell count (cells/mm3): median (min, max) | 22 (0, 49) | 22 (0, 48) | 22 (1, 49) |
| 534,000 | 451,000 | 624,000 | |
| HIV-1 RNA (copies/ml): median (min, max) | (12,200, ≥750,000) | (12,200, ≥750,000) | (20,100, ≥750,000) |
Age was available for N = 348, weight for N = 333, BMI for N = 126, WHO clinical stage for N = 343, CD4+ cell count for N = 349, and HIV-1 RNA for N = 343 patients studied.
FIG. 1.
(a) Kaplan–Meier lost to follow-up estimate. (b) Change in absolute CD4+ cell count following HAART Initiation (95% CI). (c) Percentage with undetectable plasma HIV-1 RNA following HAART initiation. Solid line is among those alive and on active follow-up and dotted line is among all participants with those dying or lost to follow-up treated as not achieving undetectable levels. (d) Change in body weight following HAART initiation.
Response to treatment
The mean increase in CD4+ cell count 3 months after initiation of HAART was 135.5 cells/mm3 (95% CI: 123.8, 147.3) and after 12 months was 239.8 cells/mm3 (95% CI: 217.0, 262.6, p < 0.001). The mean absolute CD4+ cell count 12 months after treatment initiation was 262.6 cells/mm3 (95% CI: 239.4, 285.9) (Fig. 1b)
While all patients had detectable HIV-1 RNA at initiation, 88.2% (95% CI: 83.6%, 91.6%) were virologically suppressed (defined as HIV RNA ≤400 copies/ml) at month 3 and 92.1% (95% CI: 87.8%, 94.9%) at month 9 (as treated). This declined to 64.2% (95% CI: 59.0%, 69.0%) at month 3 and 59.9% (95% CI: 54.7%, 64.9%) at month 9 when those patients who died or were lost to follow-up was classified as not achieving virologic suppression (Fig. 1c). The mean weight gain for those followed for 12 months after initiating treatment was 10.6 kg (95% CI: 9.1, 12.1, p < 0.001) (Fig. 1d). Men had a mean weight gain of 8.1 kg (95% CI: 6.1, 10.2) and women had a mean weight gain of 12.1 kg (95% CI: 10.1, 14.2) at 12 months.
Survival
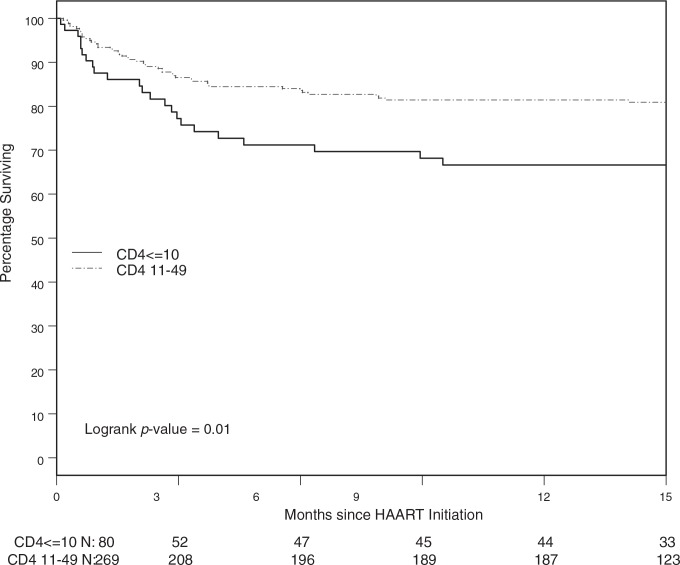
Seventy-eight percent (95% CI: 73.7%, 82.9%) of patients survived 1 year. Most deaths occurred early following HAART initiation. Thus of 70 persons that died during the first year, 34% died in the first month, 50% died in the first 2 months, and 71% died in the first 3 months of initiation of HAART. Univariate analysis showed survival varied by CD4+ cell count. Among patients having baseline CD4+ cell counts of less than or equal to 10 cells/mm3, 66.7% (56.4%, 78.8%) were alive at 1 year compared to 81.4% (76.1%, 86.0%) of patients with baseline CD+ cell counts between 11 and 49 cells/mm3 (p = 0.010) (Fig. 2). The hazard ratio for death comparing those with CD4+ count less than or equal to 10 cells/mm3 vs. those with 11–49 cells/mm3 was 1.91 (95% CI: 1.16, 3.14). CD4+ cell count at baseline measured on a continuous scale was even more predictive of death (p = 0.003) (see Table 1). Baseline CD4+ cell count and WHO clinical stage 4 were independent risk factors for death in adjusted models. A 10 cell/mm3 higher CD4+ cell count corresponded to a 22% decrease in hazard of death [HR = 0.78 (0.64, 0.94)] (p < 0.001) and those who were WHO clinical stage 4 had a relative hazard of death of 2.41 (1.32, 4.38) compared to those in stages 1–3 (p = 0.002). Baseline plasma HIV-1 RNA was not an independent predictor of death in adjusted models (p = 0.21). No significant differences in survival by initial HAART regimen was observed in unadjusted or adjusted models HR = 0.84 (95% CI: 0.51, 1.39) and 1.36 (95% CI: 0.64, 2.90), respectively.
FIG. 2.
Kaplan–Meier survival estimates stratified by CD4+ cell count ≤10 vs. 11–49.
Loss to follow-up
Patients with WHO stage 4 were approximately twice as likely [HR = 2.03 95% CI: (1.01, 4.03), p = 0.040] to be lost to follow-up, and those taking CBV + EFV were marginally more likely to be lost to follow-up than those on CBV + NVP [HR = 1.91 95% CI: (0.98, 3.74), p = 0.054]. An multiple Cox regression model that included WHO stage 4 and HAART regimen showed that WHO stage 4 was a marginally significant predictor (p = 0.074) but HAART regimen was not (p = 0.11). However, the adjusted hazard ratios were similar to unadjusted hazard ratios. We also investigated death/long-term follow-up (LTFU) as a composite endpoint. Results were similar to the death endpoint; WHO stage 4 and CD4 cell count were significant predictors of death/LTFU.
Discussion
We believe this to be the first study to be able to stratify treatment outcomes based on CD4+ cell counts less than or equal to 10 compared to 11–49 cells/mm3 in sub-Saharan Africa. Despite the availability of a large-scale national antiretroviral treatment program in Gaborone and well-established referral networks from inpatient medical wards and CD4+ screening clinics, large proportions of patients still had significantly advanced immunosuppression at their time of initial presentation when this study was conducted. Such patients (with baseline CD4+ cell counts less than 50 cells/mm3) were referred to as “late presenters.” Despite late presentation, the 1-year immunologic (mean CD4+ cell count increase of 239.8 cell/smm3) and virologic response (92.1% with undetectable HIV-1 RNA levels) to HAART among these “late presenters” is impressive and is comparable to that reported elsewhere.17–23 However, their overall survival rates are somewhat reduced when compared to the initial group of Botswana adults who initiated HAART in accordance with national guidelines, namely those with an AIDS-defining illness and/or CD4+ cell count <200 cells/mm3.24 Among patients in the national program with baseline CD4 counts <50 cells/mm3, 89% were reported alive at 1 year, which is substantially higher than the 67% of those with baseline CD4 ≤ 10 cells/mm3 described here but similar to survival rates reported in West African cohorts.25,26 Among 153 “late presenters” treated with different protease inhibitor (PI)-sparing HAART regimens as part of the adult IDCC pilot program in Botswana,24 the 1-year survival rate of 84.7% (95% CI: 79.0%, 90.8%) is slightly higher than what we report. When stratified by baseline CD4+ cell counts less than 50 cells/mm3 versus those with baseline CD4+ cell counts between 50 and 200 cells/mm3, those with a CD4+ cell count less than 50 cells/mm3 had a 3.2-fold higher risk of mortality. Our data also show significantly higher mortality rates when we stratify by baseline CD4+ cell count, namely ≤10 cells/mm3 versus 11–49 cells/mm3.
The majority of deaths occurred within the first 3 months of HAART initiation as has been reported elsewhere, and risk of death was elevated among those having advanced clinical disease, namely WHO clinical stage 4 disease and low baseline CD4+ cell counts.27–31 Other studies have identified tuberculosis, wasting syndrome, invasive bacterial and fungal infections, and immune reconstitution disease as common causes of early mortality in patients who initiate HAART with low CD4 counts.30,32,33 Severe immunosuppression is associated with disseminated and subclinical infections that become apparent or deteriorate as pathogen-specific immune responses are restored and may contribute to the high mortality seen after HAART initiation.34 In about 50% of cases, the disease leading to death is present even before the initiation of HAART.35 Overall, initiating HAART with CD4+ counts less than 50 cells/mm3 has been associated with a poorer prognosis, with mortality reported to be six times higher than among individuals with CD4+ counts of at least 200 cells/mm3.36
The comparisons we report should be interpreted with caution because they are not randomized comparisons (including the fact that most men received EFV and women NVP), data were collected retrospectively, and there was considerable missing data. There is potential bias in survival due to those lost to follow-up being more likely WHO stage 4 or CBV + EFV count patients. It is possible that those patients with poorer prognosis are more likely to be lost to follow-up so we may be overstating improvements in CD4+ cell counts, HIV-1 RNA, weight gain, and rates of survival. These analyses also rely on the patient tracking database to be up to date in terms of death, loss to follow-up, and transfer designations and dates. In addition, adherence data were not routinely documented in this care setting.
Only four outcomes were analyzed: CD4 count, plasma HIV-1 RNA, body weight changes, and mortality rates. Although anemia and low BMI are associated with mortality,37 we did not include them in our analysis. We did not collect data on HAART toxicities and immune reconstitution disease (IRD), both of which are important confounding factors. Data were collected on opportunistic infections (OIs) but were excluded from analysis because we could not distinguish incident from prevalent OIs in this retrospective analysis. This may limit the generalizability of our findings. We have not reported on causes of death because autopsies were not routinely conducted to ascertain the definitive causes of death.
In conclusion, “late presenters,” whom we define within the context of sub-Saharan Africa as patients who first present for HAART initiation with baseline CD4+ cell counts of less than 50 cells/mm3, still have favorable responses to HAART as defined by immunologic and virologic responses, and survival rates compared to other HAART-treated cohorts. These successful outcomes are a significant finding in a setting in which high proportions of patients present late for HIV diagnosis and treatment.
Acknowledgments
The authors would like to thank the patients who participated in the Botswana public sector HAART program and the management and staff of Princess Marina Hospital and the Infectious Disease Care Clinic. We thank the staff of the Botswana–Harvard School of Public Health AIDS Initiative Partnership who provided clinical, laboratory, and statistical support for this study and the Government of Botswana through the Ministries of Health and Local Government for funding and managing the national treatment program. We also thank Professor Anna Wald (University of Washington) for her editorial comments and support for this manuscript. The Botswana National ARV Program is funded by the Government of Botswana. Part of this work was presented at the XV International AIDS Conference, July 11–16, 2004, Bangkok, Thailand.
Disclosure Statement
No competing financial interests exist.
References
- 1. Panel of Clinical Practices for Treatment of HIV infection: Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services and Henry J. Kaiser Family Foundation. MMWR Recomm Rep 1998;47(RR-5):43–82. Erratum in MMWR Morb Mortal Wkly Rep 1998;47: 619 [PubMed] [Google Scholar]
- 2. Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. : Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 3. Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. : Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998;352:1725–1730 [DOI] [PubMed] [Google Scholar]
- 4. Moore RD. and Chaisson RE: Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS 1999;13:1933–1942 [DOI] [PubMed] [Google Scholar]
- 5. UNAIDS: AIDS epidemic update: December 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf Accessed December5, 2007
- 6. Essex M: Human immunodeficiency viruses in the developing world. Adv Virus Res 1999;53:71–88 [DOI] [PubMed] [Google Scholar]
- 7. Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. : Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA 2006;296:782–793 [DOI] [PubMed] [Google Scholar]
- 8. Stangl AL, Wamai N, Mermin J, Awor AC, and Bunnell RE: Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care 2007;19:626–636 [DOI] [PubMed] [Google Scholar]
- 9. DART Virology Group and Trial Team: Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS 2006; 20:1391–1399 [DOI] [PubMed] [Google Scholar]
- 10. Sabin CA, Smith CJ, Gumley H, Murphy G, Lampe FC, Phillips AN, et al. : Late presenters in the era of highly active antiretroviral therapy: Uptake of and responses to antiretroviral therapy. AIDS 2004;18:2145–2151 [DOI] [PubMed] [Google Scholar]
- 11. Nunez M, Asencio R, Valencia ME, Leal M, Gonzalez-Lahoz J, and Soriano V: Rate, causes, and clinical implications of presenting with low CD4 + cell counts in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses 2003;19:363–368 [DOI] [PubMed] [Google Scholar]
- 12. Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. : Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet 2002;360:119–129 [DOI] [PubMed] [Google Scholar]
- 13. Curtis H, Sabin CA, and Johnson MA: Findings from the first national clinical audit of treatment for people with HIV. HIV Med 2003;4:11–17 [DOI] [PubMed] [Google Scholar]
- 14. Phillips A: Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS 2004;18:51–58 [DOI] [PubMed] [Google Scholar]
- 15. Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. : Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: Analysis of prospective studies. Lancet 2003;362: 679–686 [DOI] [PubMed] [Google Scholar]
- 16. Anabwani G: Botswana Guidelines on Antiretroviral Treatment. Botswana Ministry of Health, 2002 [Google Scholar]
- 17. Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. : Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 2004;18:887–895 [DOI] [PubMed] [Google Scholar]
- 18. Hawkins C, Achenbach C, Fryda W, Ngare D, and Murphy R: Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr 2007;45:304–310 [DOI] [PubMed] [Google Scholar]
- 19. Sow PS, Otieno LF, Bissagnene E, Kityo C, Bennink R, Clevenbergh P, et al. : Implementation of an antiretroviral access program for HIV-1-infected individuals in resource-limited settings: Clinical results from 4 African countries. J Acquir Immune Defic Syndr 2007;44:262–267 [DOI] [PubMed] [Google Scholar]
- 20. Spacek LA, Shihab HM, Kamya MR, Mwesigire D, Ronald A, Mayanja H, et al. : Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clin Infect Dis 2006;42:252–259 [DOI] [PubMed] [Google Scholar]
- 21. Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. : Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: Experience from western Kenya. AIDS 2006;20:41–48 [DOI] [PubMed] [Google Scholar]
- 22. Kilaru KR, Kumar A, Sippy N, Carter AO, and Roach TC: Immunological and virological responses to highly active antiretroviral therapy in a non-clinical trial setting in a developing Caribbean country. HIV Med 2006;7:99–104 [DOI] [PubMed] [Google Scholar]
- 23. Sungkanuparph S, Kiertiburanakul S, Manosuthi W, Kiatatchasai W, and Vibhagool A: Initiation of highly active antiretroviral therapy in advanced AIDS with CD4 <50 cells/mm3 in a resource-limited setting: Efficacy and tolerability. Int J STD AIDS 2005;16:243–246 [DOI] [PubMed] [Google Scholar]
- 24. Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter TF, et al. : Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr 2005;40:336–343 [DOI] [PubMed] [Google Scholar]
- 25. Djomand G, Roels T, Ellerbrock T, Hanson D, Diomande F, Monga B, et al. : Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS 2003;17(Suppl. 3): S5–15 [DOI] [PubMed] [Google Scholar]
- 26. Laurent C, Ngom Gueye NF, Ndour CT, Gueye PM, Diouf M, Diakhate N, et al. : Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr 2005;38:14–17 [DOI] [PubMed] [Google Scholar]
- 27. Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, and Harries AD: Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS 2006;20:2355–2360 [DOI] [PubMed] [Google Scholar]
- 28. Jerene D, Endale A, Hailu Y, and Lindtjorn B: Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infect Dis 2006;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. : Scaling up of highly active antiretroviral therapy in a rural district of Malawi: An effectiveness assessment. Lancet 2006;367:1335–1342 [DOI] [PubMed] [Google Scholar]
- 30. Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. : Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: A 7-year cohort study. AIDS 2006;20:1181–1189 [DOI] [PubMed] [Google Scholar]
- 31. Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. : Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. 2007;21:2483–2491. 32. Lawn SD, Bekker LG, Myer L, Orrell C, and Wood R: Cryptococcocal immune reconstitution disease: A major cause of early mortality in a South African antiretroviral programme. AIDS 2005;19:2050–2052 [DOI] [PubMed] [Google Scholar]
- 33. Lawn SD, Myer L, Orrell C, Bekker LG, and Wood R: Early mortality among adults accessing a community-based antiretroviral service in South Africa: Implications for programme design. AIDS 2005;19:2141–2148 [DOI] [PubMed] [Google Scholar]
- 34. Lawn SD, Bekker LG, and Miller RF: Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005;5:361–373 [DOI] [PubMed] [Google Scholar]
- 35. Seyler C, Anglaret X, Dakoury-Dogbo N, Messou E, Toure S, Danel C, et al. : Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d'Ivoire. Antiviral Ther 2003;8:385–393 [PubMed] [Google Scholar]
- 36. Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, and Montaner JS: Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001;286:2568–2577 [DOI] [PubMed] [Google Scholar]
- 37. Marazzi MC, Liotta G, Germano P, Guidotti G, Altan AD, Ceffa S, et al. : Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses 2008;24:555–560 [DOI] [PubMed] [Google Scholar]