Abstract
Cystic fibrosis (CF) is a common genetic disease characterized by defects in the expression of the CF transmembrane conductance regulator (CFTR) gene. Gene therapy offers better hope for the treatment of CF. Adeno-associated viral (AAV) vectors are capable of stable expression with low immunogenicity. Despite their potential in CF gene therapy, gene transfer efficiency by AAV is limited because of pathophysiological barriers in these patients. Although a few AAV serotypes have shown better transduction compared with the AAV2-based vectors, gene transfer efficiency in human airway epithelium has still not reached therapeutic levels. To engineer better AAV vectors for enhanced gene delivery in human airway epithelium, we developed and characterized mutant AAV vectors by genetic capsid modification, modeling the well-characterized AAV2 serotype. We genetically incorporated putative high-affinity peptide ligands to human airway epithelium on the GH loop region of AAV2 capsid protein. Six independent mutant AAV were constructed, containing peptide ligands previously reported to bind with high affinity for known and unknown receptors on human airway epithelial cells. The vectors were tested on nonairway cells and nonpolarized and polarized human airway epithelial cells for enhanced infectivity. One of the mutant vectors, with the peptide sequence THALWHT, not only showed the highest transduction in undifferentiated human airway epithelial cells but also indicated significant transduction in polarized cells. Interestingly, this modified vector was also able to infect cells independently of the heparan sulfate proteoglycan receptor. Incorporation of this ligand on other AAV serotypes, which have shown improved gene transfer efficiency in the human airway epithelium, may enhance the application of AAV vectors in CF gene therapy.
Introduction
Adeno-associated viral (AAV) vectors are emerging as attractive therapeutic gene delivery vehicles for clinical gene therapy because of their nonpathogenicity, durable transgene expression, and safety profiles. Although AAV vectors possess a broad host range, significant variations exist in their transduction efficiency in cell types and tissues. Some of the newer AAV serotypes have been identified by screening human and nonhuman primate tissues for the presence of rescuable AAV genomes. These efforts have resulted in more than 40 genomic variants (Gao et al., 2002, 2003). On the basis of heterogeneity in the amino acid composition of the capsid protein, some of the serotypes use different cellular receptors for internalization (Büning et al., 2003, 2004). Studies have also shown the superior gene transfer efficiency of some of the serotypes (Zabner et al., 2000; Halbert et al., 2001; Auricchio et al., 2002; Limberis and Wilson, 2006).
One of the human genetic diseases molecularly well characterized and suited for gene therapy is cystic fibrosis (CF). CF is the most common autosomal recessive disease in the white population and affects approximately 70,000 individuals worldwide (Rosenecker et al., 2006). Cloning of the CF transmembrane conductance regulator (CFTR) gene and a better understanding of the disease pathophysiology, and promising proof-of-principle preclinical studies on CFTR gene transfer, led to the initiation of human clinical trials. Among the viral vectors for CF gene therapy, the potential of AAV led to the initiation of several clinical trials, all with AAV2-based vectors (Carter, 2005). Despite encouraging safety profiles in phase I and phase II trials, none of the primary end points changed significantly (Wagner et al., 1998; Aitken et al., 2001). A major limitation for this is the lack of efficient vector transduction to airway epithelial cells through the apical surface. Thus, it was apparent that molecular alterations to enhance AAV transduction to airway epithelium would advance their clinical utility.
Several alternative approaches are being attempted with AAV vectors to achieve high-efficiency transduction of target tissues, such as airway epithelium; these approaches include the use of a shortened AAV cassette (Ostedgaard et al., 2005) and intramolecular joining of DNA or RNA from independent vectors (Nakai et al., 2000; Yan et al., 2000; Halbert et al., 2002; Pergolizzi et al., 2003). Additional strategies to increase the permeability of the apical surface of the airway epithelium to AAV, using chemicals and a combination of transduction-enhancing compounds after vector transduction, have resulted in significant augmentation of gene expression in airway epithelium (Duan et al., 2000; Ding et al., 2003; Yan et al., 2004). Thus, it is apparent that further advancements in targeted transduction of AAV vectors to airway epithelium will positively impact on their clinical utility.
Genetic capsid modification remains a viable alternative to enhance AAV gene transfer to target cells through alternative cellular receptors. However, approaches employing this strategy for CF gene therapy remain unexplored. To this end, we developed rAAV with genetic capsid modification to include a panel of putative airway epithelium-specific ligand sequences in the GH loop of AAV2 capsids and tested them in polarized human airway epithelial cells.
Materials and Methods
Cell culture, chemicals, and reagents
The human embryonic kidney cell line 293 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 units/ml) at 37°C and 5% CO2. The immortalized human airway epithelial cell line, Calu-3, derived from the serous glandular cells of airway submucosal glands, and the CFBE41o- (CFBE) cell line, derived from a CF patient homozygous for the ÄF508 mutation, were cultured in Minimum Essential Medium (MEM) supplemented with 10% FBS, penicillin (100 units/ml), 0.292 g/L l-glutamine, and streptomycin (100 units/ml) at 37°C and 5% CO2. To achieve polarization, 106 Calu-3 cells were seeded on 6.5-mm-diameter Transwell filters (Corning Life Sciences, Lowell, MA). After 2–3 days, the DMEM–10% FBS was replaced with DMEM containing 2% FBS, and the cells were cultured for an additional 7–9 days with medium in both the apical and basolateral compartments. Under these conditions, the cells differentiated at an air–liquid interface and formed monolayers with transepithelial resistances exceeding 1000 Ω · cm2 (Varga et al., 2004).
Restriction endonucleases and other DNA-modifying enzymes were purchased from Promega (Madison, WI) or New England BioLabs (Ipswich, MA). The AAV-2 capsid monoclonal antibody, B1, was obtained from American Research Products (Belmont, MA) and horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody was purchased from SouthernBiotech (Birmingham, AL).
Construction of AAV-2 capsid mutant plasmids and site-directed mutagenesis
The plasmid pSP62, containing the rep and cap genes of AAV2, was digested with the restriction enzyme ApaI to generate a 303-bp fragment between nucleotide junctions 3760 and 4042 in the AAV2 capsid region. This fragment was subcloned into pBluescript II KS+ plasmid (Stratagene, La Jolla, CA) for site-directed mutagenesis. Site-directed mutagenesis was performed with complementary oligonucleotide primers containing inserted ligand sequences, flanked by 15–20 bp on each side of amino acid positions 587 and 588 of the AAV-2 capsid.
Sequences of the oligonucleotides used for site-directed mutagenesis for tested ligands are as follows:
-
M1-HAIYPRH
FP: 5′-CCTCCAGAGAGGCAACCATGCGATCTATCCG-CGGCATAGACAAGCAGCTACCGC-3′
RP: 5′-GCGGTAGCTGCTTGTCTATGCCGCGGATAGA-TCGCATGGTTGCCTCTCTGGAGG-3′
-
M2-THALWHT
FP: 5′-ACTCACGCACTGTGGCACACA-3′
RP: 5′-TGTGTGCCACAGTGCGTGAGT-3′
-
M3-PLAEIDGIELTYC
FP: 5′-CCTCTCGCAGAGATCGACGGAATCGAGCTCA-CGTACTGC-3′
RP: 5′-GCAGTACGTGAGCTCGATTCCGTCGATCTCT-GCGAGAGG-3′
-
M4-THRPPMWSPVWP
FP: 5′-ACTCACCGACCTCCTATGTGGAGTCCTGTAT-GGCCT-3′
RP: 5′-AGGCCATACAGGACTCCACATAGGAGGTCG-GTGAGT-3′
-
M5-AWDWEPFGDPLR
FP: 5′-GCATGGGATTGGGAGCCATTTGGAGACCCAC-TACGA-3′
RP: 5′-TCGTAGTGGGTCTCCAAATGGCTCCCAATCC-CATGC-3′
-
M6-AWDMVQPAVRLS
FP: 5′-GCATGGGACATGGTCCAGCCAGCAGTTCGA-CTATCA-3′
RP: 5′-TGATAGTCGAACTGCTGGCTGGACCATGTCC-CATGC-3′
Sequences underlined in M1 correspond to regions in the AAV2 capsid flanking the ligand insert. This sequence is common to all six mutants. The mutagenesis reaction was performed in a volume of 50 μl for each of the six mutant constructs (pAW-2 to pAW-7): 100 ng of pAW-1, 200 ng each of forward primer (FP) and reverse primer (RP), 2 μl of 10 mM dNTP mix, 1 μl of Pfu polymerase (2.5 U/ml), and 5 μl of 10 × Pfu buffer. Polymerase chain reaction (PCR) cycling parameters of 95°C for 30 sec (denature), 65°C for 1 min (annealing), and 68°C for 6 min (extension) were repeated for 20 rounds, with a final extension of 7 min at 68°C. The template plasmid DNA was digested with DpnI for 1 hr at 37°C. The PCR products were transformed into XL-Blue competent cells and positive clones were identified by restriction digestion and automated sequencing, using the T7 primer. Mutated regions of the AAV2 capsid were isolated from the pBluescript vector and subcloned by partial digestion of plasmid pAAV/Ad (obtained from R.J. Samulski, University of North Carolina, Chapel Hill, NC) containing the AAV Rep- and Cap-coding regions, replacing the wild-type sequence. Positive clones were again confirmed by restriction digestion and automated sequencing.
Production of tropism-modified rAAV-luciferase
rAAVs encoding luciferase in wild-type or mutant capsids were produced by triple calcium phosphate-mediated transfection of HEK-293 cells, using equimolar ratios of the packaging plasmid, plasmid containing AAV Rep and wild-type or modified capsids, and the plasmid pXX6, which supplied the adenoviral (Ad) helper genes E2a, VA, and E4. The cells were harvested after 65 hr and viral particles were purified by discontinuous iodixanol gradient and heparin-affinity chromatography. Particle titer of the purified virus was determined by quantitative slot-blot analysis and real-time PCR as previously described (Ren et al., 2007).
Characterization of mutant capsid expression by Western blot
For immunoblotting, 20 μg of protein from control and vector-transduced cells was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to Hybond-ECL nitrocellulose membrane (GE Healthcare Life Sciences, Piscataway, NJ) at 4°C. The membranes were blocked at 4°C for 2 hr with 5% nonfat milk in 1 × Tris-buffered saline (TBS; 20 mM Tris-HCl [pH 7.5], 150 mM NaCl). The membranes were probed with the AAV2 capsid monoclonal antibody B1, diluted 1:50, followed by the addition of horseradish peroxidase-conjugated goat antimouse IgG secondary antibody diluted to 1:1000. Membranes were washed three times with 1 × TBS containing 0.5% Tween 20 and capsid proteins were visualized by enhanced chemiluminescence detection (ECL; GE Healthcare Life Sciences).
Transduction of human airway and nonairway epithelial cells with rAAV encapsidated in wild-type and mutant capsids
Approximately 105 cells were seeded per well in 24-well tissue culture plates. The next day, medium was removed and cells were washed briefly with serum-free Opti-MEM (Invitrogen, Carlsbad, CA). One hundred multiplicities of infection (MOI; 1 MOI = 100 physical particles per cell) of rAAV-luc in wild-type or mutant capsids, resuspended in 100 μl of Opti-MEM, was dispensed onto the cells and incubated at 37°C for 2 hr, after which free virus was removed. The cells were washed twice with Opti-MEM, replenished with complete medium, and incubated at 37°C for an additional 48 hr. For the heparin blocking experiment, both cells and virus were preincubated with heparin (500 μg/ml) for 1 hr, after which the cells were infected with the virus for 2 hr in the presence of heparin.
rAAV infections of fully differentiated polarized human airway epithelia were performed by overlaying 100 MOI of rAAV-luc in 100 μl of Opti-MEM directly onto the apical compartment of Transwell inserts. Viral infections were performed for 2 hr, after which the medium and virus were removed from the apical compartment and cultures were returned to an air–liquid interface. At the time of removing the virus from the apical compartment, the lower and upper chambers were given fresh medium and the cells were incubated for 48 hr in 5% CO2 at 37°C.
Analysis of luciferase expression
Cells were harvested from 24-well plates 48 hr after the transduction experiments and resuspended in 100 μl of passive cell lysis buffer (Promega). Luciferase activity was determined by adding 20 μl of lysate from each well to 100 μl of luciferase substrate (Promega) and the luminescence index was recorded with a Sirius luminometer (Berthold Detection Systems, Pforzheim, Germany). Luciferase expression was expressed as relative light units (1 RLU = 10 photons) per second per well, normalized to the protein content in each lysate and determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA) with a CERES 900 spectrophotometer (BioTek Instruments, Winooski, VT) at 595 nm. All assays were performed in triplicate.
Statistics
Results are expressed as means ± SD. Statistical significance among means was determined by Student t test. Data were considered significant when p < 0.05.
Results and Discussion
Inclusion of putative airway epithelium-specific targeting ligands does not affect rAAV packaging or heparin binding
During the initiation of this study, a few peptide ligands had been identified by in vitro phage display with high-affinity binding to human airway epithelium or to alternative receptors highly expressed on airway epithelium. The ligand sequences HAIYPRH and THRPPMWSPVWP were identified by screening a phage display peptide library based on a combinatorial library of 7 or 12 random amino acid peptides. These two peptide sequences were found to bind with high affinity to the human transferrin receptor (Jost et al., 2001). Ligands of apically located receptors on human airway epithelium, which bind the target cells more specifically and with higher affinity, are needed for most gene therapy applications. The peptide PLAEDIDGIELTY was isolated and shown to completely displace α9β1-integrin-expressing cells from binding to the third fibronectin type III repeat TNfn3 (Schneider et al., 1998). Furthermore, peptide–DNA complexes resulted in efficient gene transfer with targeting capability to cells overexpressing α9β1-integrins. Peptide sequences AWDWEPFGDPLR and AWDMVQPAVRLS were identified after four rounds of biopanning on polarized 16HBE14o- cells (Cozens et al., 1994). Either peptide sequence could mediate transduction from the apical or basolateral side of polarized 16HBE14o- cells. Isolation of the peptide THALWHT was accomplished through biopanning a 7-mer peptide library against the human bronchial epithelium cell line 16HBE14o-. Similar to Calu-3, this cell line forms polarized airway epithelium expressing the morphological characteristics of respiratory cells in vivo. The synthesized peptide motif THALWHT was coupled to a cationic DNA-binding moiety and was shown to mediate efficiently targeted gene delivery into 16HBE14o- cells, indicating the possibility of similar results when applied to other vector systems. THALWHT was not definitively associated with any known receptor when compared with proteins of known sequence (Jost et al., 2001).
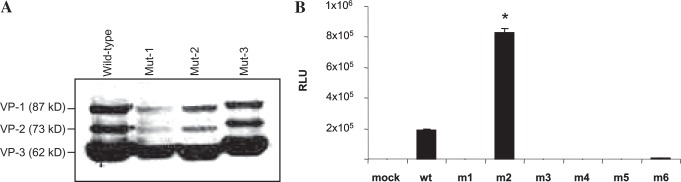
We created six different capsid mutants containing the previously described peptide sequences by site-directed mutagenesis. On positive sequence confirmation of the capsid open reading frame (ORF) of AAV-2 mutants, 293 cells were transfected and subjected to Western blot analysis. The results of Western blot analysis, shown in Fig. 1A, confirmed that genetic inclusion of targeting ligands does not affect capsid gene expression. Relative abundances of VP-1, VP-2, and VP-3 in wild-type AAV occur at an approximately 1:1:10 ratio. B1 antibody, which recognizes linear epitope IGTRYLTR in the C-terminal region of all three capsid proteins, was used to confirm that the plasmids containing genetically modified capsids expressed capsid proteins of the appropriate size and ratio (Wistuba et al., 1997; Wobus et al., 2000). A notable increase in the molecular masses of mutant capsid proteins further confirmed that heterologous peptide ligands were successfully incorporated into VP-1, VP-2, and VP-3.
FIG. 1.
Western blot analysis of 293 cell lysates containing wild-type (wt) and mutant rAAV capsids and transgene expression in 293 cells with rAAV-luc encapsidated in wild-type or mutant capsids. (A) Equal volumes of cell lysates containing wild-type and representative mutant capsids, after transfection of the respective plasmids along with the plasmid pXX6, containing adenovirus helper functions, were separated by 10% SDS–PAGE and analyzed by Western blotting with AAV capsid antibody B1 followed by horseradish peroxidase-conjugated anti-mouse secondary antibody. The molecular masses of VP-1, VP-2, and VP-3 are 87, 73, and 62 kDa, respectively. (B) HEK-293 cells were either mock-transduced or transduced with 100 MOI of rAAV-luciferase with wild-type or mutant capsid for 2 hr at 37°C. After infections, free viral particles were removed by washing with phosphate-buffered saline (PBS) and the cells were incubated for 48 hr. Luciferase activity was determine from cell lysates and expressed as relative light units (RLU), normalized to the protein content of each cell lysate. *p < 0.01 between wild type and m2.
After this, we packaged rAAV encoding luciferase in wild-type and the six mutant capsids. Purification of the recombinant vector encapsidated in wild-type and the six different mutant capsids was done by heparin column affinity chromatography. On the basis of the heparin-binding region in the AAV capsid, amino acids R585 and R588, it was thought that ligand insertion between amino acids 587 and 588 may abolish heparin binding due to the proximity of the insertion site to the heparan sulfate proteoglycan (HSPG)-binding region (Kern et al., 2003; Opie et al., 2003). However, analyses of heparin column flow-through and wash fractions by slot-blot analysis failed to detect AAV-2 particles, suggesting the heparin-binding region was not affected by ligand insertions in our studies. There was no significant difference in particle titer of mutant viruses compared with wild-type luciferase-encoding virus. The titers ranged from 5 × 1011 to 1 × 1012 particles/ml.
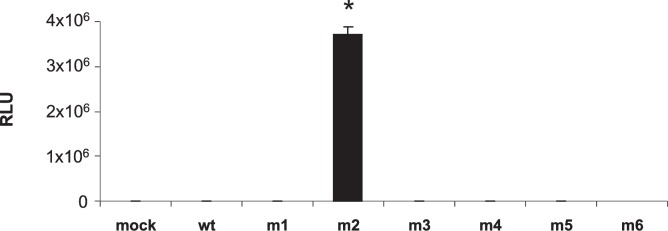
Initial studies to determine the transduction efficiency of the mutant capsids was performed in HEK-293 cells. These studies were intended to answer two primary questions: Because amino acid junction 587–588 is involved in heparin binding and internalization of the vector, disruption of the motif by inclusion of additional amino acids might affect either heparin binding or infectivity. Alternatively, the presence of a cellular receptor with binding affinity for genetically incorporated ligands on mutant capsids might augment vector transduction, synergistic to the heparan sulfate pathway.
Results, shown in Fig. 1B, indicate significant transduction of wild-type AAV-luc in HEK-293 cells, as expected. Interestingly, among the six different capsid mutants, only one (m2), containing the ligand sequence THALWHT, showed significant transduction whereas none of the other mutants (m1, m3, m4, m5, and m6) transduced HEK-293 cells. The normalized luciferase activity observed in m2 viral transduction was significantly higher than with unmodified wild-type capsid (p < 0.01).
Transduction of wild-type and genetic capsid-modified rAAV-luciferase in human airway epithelial cell line Calu-3
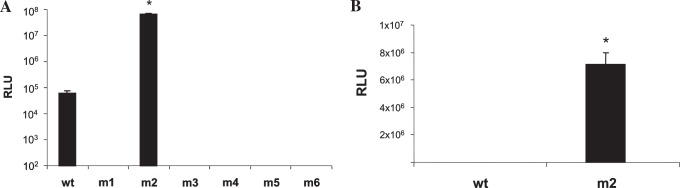
Having determined the relative transduction efficiency of rAAV-luc containing wild-type or mutant capsids in 293 cells, the next set of experiments was designed to analyze the transduction efficiency of these vectors in a human airway epithelial cell line. Initial studies were performed in Calu-3, a human cell line derived from the serous glandular cells of airway submucosal glands, and typically used to monitor interventions for CF (Finkbeiner et al., 1993; Shen et al., 1994). The cells form electrically high-resistance monolayers (500–1000 Ω · cm2). The choice of this cell line and culture conditions was determined on the basis of (1) the measurement of transepithelial resistance and (2) the demonstration of ZO-1 (tight junctional) staining and other location assays and (3) vectoral Cl− transport across monolayers (a sensitive measure that verifies tight junctional integrity). Many of these features have been reported previously by us and others for this cell type (Augeron et al., 1984; Morris et al., 1992, 1994; Finkbeiner et al., 1993; Shen et al., 1994; Weber et al., 1994; Auger et al., 1999). Similar to that of HEK-293 cells, transduction efficiency of both wild-type and mutant capsid vectors was determined in the human airway epithelial cell line Calu-3, grown as a monolayer in 24-well tissue culture plates. Results, shown in Fig. 2A, indicated an approximately 3-log increase in luciferase expression of the m2 virus over wild-type capsid. Although there was a significant increase in transduction efficiency of the m2 virus over the wild-type virus in 293 cells, the increase found in monolayer Calu-3 cells between the two vectors was highly dramatic. These data suggest that the m2 vector may also use an alternative receptor to internalize in Calu-3 cells, in addition to the HSPG receptor used by unmodified AAV2, and the fact that transduction enhancement with m2 was also observed in 293 cells, albeit to a lower extent compared with Calu-3 cells, suggests that the putative cellular receptor with binding affinity for the m2 ligand THALWHT might be expressed on 293 cells.
FIG. 2.
Luciferase activity in Calu-3 cells transduced with rAAV-luc containing wild-type and mutant capsids, and the effect of soluble heparin on transduction of wild-type and m2 rAAV-luc in Calu-3 cells. (A) Calu-3 cells, grown as a monolayer culture, were mock-transduced or transduced with 100 MOI of rAAV-luciferase with wild-type or the indicated mutant capsids for 2 hr at 37°C. After infection, free virus was removed by washing with PBS and the cells were incubated for 48 hr. Luciferase activity was determined from cell lysates and expressed as relative light units, normalized to the protein content of each cell lysate. *p < 0.01 between wild-type and m2. (B) Both virus and cells were preincubated with heparin (500 μg/ml) at 37°C for 1 hr, after which infection was performed for 2 hr, also in the presence of soluble heparin. After infection, cells were washed with PBS and grown in complete medium for 48 hr. The luciferase assay was done, using cell lysates from individual transductions, and values were normalized to the protein content of each lysate. *p < 0.0015.
Determination of heparan sulfate proteoglycan-independent transduction of mutant capsid AAV
HSPG has been identified as the primary cellular receptor for AAV2 (Summerford and Samulski, 1998). Data from studies with m2 virus indicated significant transduction of this vector in HEK-293 and Calu-3 cells. Because the mutant virus was purified to homogeneity by passage through a heparin affinity column, it is likely that the transduction pathway used by the m2 virus could also involve the native HSPG entry mechanism identified for wild-type AAV2. It is also possible that addition of the ligand sequence THAL-WHT could synergize transduction enhancement rather than allowing entry through an entirely different receptor.
To determine whether the m2 virus increases transduction only in the presence of heparin-binding domains or transduces cells entirely through an alternative receptor, independent of the HSPG pathway, heparin-blocking experiments were performed. Calu-3 cells were transduced with rAAV-luc packaged in wild-type and m2 capsids after pretreatment with heparin (500 μg/ml), and luciferase activity was determined 48 hr after transduction. There was significantly higher luciferase activity from the m2 virus even after preincubation with soluble heparin, whereas heparin binding significantly abolished transduction of rAAV-luc packaged in wild-type capsid (p < 0.0015). These data are represented in Fig. 2B.
Transduction of wild-type and capsid-modified virus in polarized human airway epithelial cell line Calu-3
Although data from the previously described experiments strongly suggested that the m2 virus is capable of transducing Calu-3 cells in a heparan sulfate-independent manner, a major limitation in the transduction of human airway epithelium in vivo is the lack of virus accessibility to the basolateral sides of well-differentiated cells. Previous studies with recombinant Ad and AAV have in fact reported transduction through the basolateral surface, where sufficient AAV receptor is present, but not from the apical surface (Duan et al., 1998; Bals et al., 1999). Thus, it was important to determine the ability of m2 virus to transduce human airway epithelial cells on differentiation to polarized epithelium. Airway epithelial cells including Calu-3 form confluent polarized epithelia with transepithelial resistance when grown on a semipermeable support membrane. The cells also differentiate to form ciliated cells and constitutively express CFTR, features that mimic the actual in vivo physiology in the human airway (Foster et al., 2000). To test this, Calu-3 cells were differentiated on Transwell filters. After 2–3 days, the medium containing 10% FBS was exchanged to 2% FBS-containing medium for 1–2 days, and cells were cultured for an additional 7–9 days with medium containing 10% FBS at the apical and basolateral compartments. Under these conditions, the cells differentiated as monolayers with transepithelial resistance (Mathia et al., 2002; Varga et al., 2004). During this time, Calu-3 cells were transduced with rAAV-luc in wild-type or mutant capsids. Luciferase activity was determined 48 hr after transduction. Results are given in Fig. 3. Interestingly, the m2 mutant virus containing the ligand THALWHT displayed significant transduction enhancement (p < 0.0091), whereas all other mutants and rAAV-luc in wild-type capsid failed to show any transduction.
FIG. 3.
Transduction of rAAV-luc in wild-type or mutant capsids in polarized human airway epithelium. Calu-3 cells were grown in Transwell filters for 7 days to allow polarization. The cells were either mock-transduced or transduced with rAAV-luc encapsidated in wild-type or mutant capsids, as indicated previously, for 2 hr at 37°C. After infection, free viral particles were removed by washing with PBS and cells were grown for an additional 48 hr. Luciferase activity was measured from cell lysates and expressed as relative light units, normalized to the protein content of each lysate. *p < 0.0091 between m2 and the other vector types.
Transduction efficiency of wild-type and mutant vector in cystic fibrosis bronchial epithelial cells
To determine whether transduction of mutant AAV-luc can recapitulate human airway epithelial cells other than Calu-3 cells, CFBE41o- (CFBE) cells were chosen as an additional target. CFBE cells were derived from the airway epithelium of a CF patient homozygous for the ΔF508 mutation (Dragomir et al., 2004). Infection with mutant vectors was performed on differentiated CFBE cells as in Calu-3 cells. The cells were lysed after 48 hr and luciferase activity was determined as a measure of transduction efficiency. The results are given in Fig. 4.
FIG. 4.
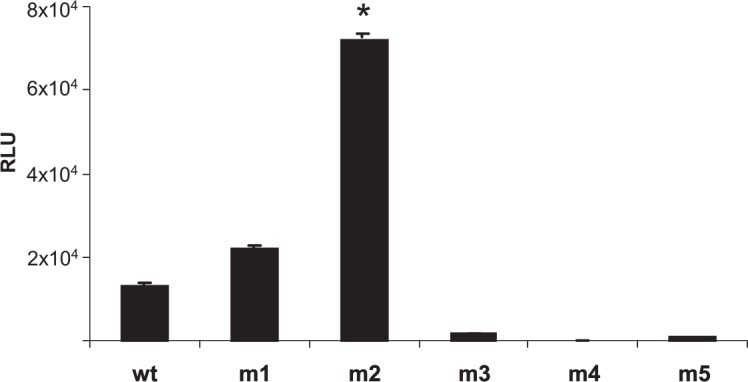
Transduction efficiency of rAAV with mutant capsids in CFBE cells. CFBE cells were mock-transduced or transduced with rAAV-luc encapsidated in wild-type or the indicated mutant (m) capsids for 2 hr at 37°C. Free viral particles were removed by washing with PBS. The cells were harvested 48 hr later and luciferase activity was determined from the cell lysates. Luciferase activity from individual samples was normalized to the protein content of each lysate and expressed as relative light units. *p < 0.03 between m2 and the other types.
It was interesting to note that transduction enhancement of the m2 virus was also evident in CBFE cells. Further, in addition to the m2 virus, significant transgene expression was observed from m1 virus, which showed no transduction in either 293 or Calu-3 cells. Because the ligand incorporated in the m1 mutant, HAIYPRH, has been reported to bind to the human transferrin receptor, it remains possible that these cells may be expressing higher levels of transferrin receptor compared with 293 and Calu-3 cells. However, other mutants (m3, m4, m5, and m6) did not show transduction of CFBE cells, as observed in Calu-3 cells.
A previous study, which identified the ligand THALWHT to be specific for the polarized airway epithelial cell line 16HBE14o-, did not identify any cellular protein-sharing consensus. However, a homology search using the BLAST database (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) in the present study revealed a consensus in the last five amino acids (ALWHT) with human thrombospondin precursor protein. Thrombospondin receptor (CD47) is a multifunctional glycoprotein secreted by endothelial cells and by α granules of platelets after activation by thrombin; it interacts with a wide variety of molecules to play a role in platelet aggregation, tumor metastasis, vascular smooth muscle growth, and microorganism adhesion (Rosseau et al., 2000). Monocyte migration across the alveolar epithelium also depends largely on CD47 (Rosseau et al., 2000) and in response to inflammatory challenge, the alveolar epithelium orchestrates enhanced monocyte traffic to the apical side by polarized chemokine secretion and upregulation of intercellular adhesion molecule-1 (ICAM-1) or vascular cell adhesion molecule-1 (VCAM-1). Thus, it is possible that the target cell receptor for the ligand THALWHT is CD47. Testing the vector containing the m2 ligand in mouse airway epithelium in vivo did not show a significant increase in gene transfer efficiency. It remains possible that species variation in the target molecule for the incorporated ligand may account for this limitation. Previous studies on phylogenetic differences in CD47 interactions have reported the existence of significant variation in the binding affinity of this protein for cognate ligands (Subramanian et al., 2006, 2007).
Studies have demonstrated that by combining permeability-enhancing compounds with the use of transduction-enhancing compounds, gene transfer through the apical surface of human airway epithelium could be augmented (Duan et al., 2000; Ding et al., 2003). Whereas a permeability-enhancing compound such as EGTA allows physical display of the vector to target cells, transduction-enhancing compounds act on endosomal processing of the vector. Elucidation of the crystal structure of AAV serotypes that have so far shown promise in transducing airway epithelium, and identification of amenable domains on their capsids for ligand incorporation, should in future allow us to design vectors with augmented transduction for gene therapy of CF.
Acknowledgments
Financial support from the Cystic Fibrosis Foundation (grant R464-CR02) is greatly acknowledged.
Author Disclosure Statement
No competing financial interests exist.
References
- Aitken M.L., Moss R.B., Waltz D.A., Dovey M.E., Tonelli M.R., McNamara S.C., Gibson R.L., Ramsey B.W., Carter B.J., and Reynolds T.C. (2001). A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12, 1907–1916 [DOI] [PubMed] [Google Scholar]
- Auger R., Robin P., Camier B., Vial G., Rossignol B., Tenu J.P., and Raymond M.N. (1999). Relationship between phosphatidic acid level and regulation of protein transit in colonic epithelial cell line HT29-cl19A. J. Biol. Chem. 274, 28652–28659 [DOI] [PubMed] [Google Scholar]
- Augeron C., and Laboisse C.L. (1984). Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 44, 3961–3969 [PubMed] [Google Scholar]
- Auricchio A., O'Connor E., Weiner D., Gao G.P., Hildinger M., Wang L., Calcedo R., and Wilson J.M. (2002). Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 110, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Xiao W., Sang N., Weiner D.J., Meegalla R.L., and Wilson J.M. (1999). Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J. Virol. 73, 6085–6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büning H., Ried M., Perabo L., Gerner F., Huttner N., Enssle J., and Hallek M. (2003). Receptor targeting of adeno-associated virus. Gene Ther. 10, 1142–1151 [DOI] [PubMed] [Google Scholar]
- Büning H., Braun-Falco M., and Hallek M. (2004). Progress in the use of adenoassociated viral vectors for gene therapy. Cells Tissues Organs 177, 139–150 [DOI] [PubMed] [Google Scholar]
- Carter B.J. (2005). Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 16, 541–550 [DOI] [PubMed] [Google Scholar]
- Cozens A.L., Yezzi M.J., Kunzelmann K., Ohrui T., Chin L., Eng K., Finkbeiner W.E., Widdicombe J.H., and Gruenert D.C. (1994). CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10, 38–47 [DOI] [PubMed] [Google Scholar]
- Ding W., Yan Z., Zak R., Saavedra M., Rodman D.M., and Engelhardt J.F. (2003). Second-strand genome conversion of adeno-associated virus type 2 (AAV-2) and AAV-5 is not rate limiting following apical infection of polarized human airway epithelia. J. Virol. 77, 7361–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragomir A., Bjorstad J., Hjelde L., and Roomans G.M. (2004). Curcumin does not stimulate cAMP-mediated chloride transport in cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun. 322, 447–451 [DOI] [PubMed] [Google Scholar]
- Duan D., Yue Y., Yan Z., McCray P.B., and Engelhardt J.F. (1998). Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum. Gene Ther. 9, 2761–2776 [DOI] [PubMed] [Google Scholar]
- Duan D., Yue Y., Yan Z., Yang J., and Engelhardt J.F. (2000). Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associted virus. J. Clin. Invest. 105, 1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner W.E., Carrier S.D., and Teresi C.E. (1993). Reverse transcription-polymerase chain reaction (RT-PCR) phenotypic analysis of cell cultures of human tracheal epithelium, tracheobronchial glands, and lung carcinomas. Am. J. Respir. Cell Mol. Biol. 9, 547–556 [DOI] [PubMed] [Google Scholar]
- Foster K.A., Avery M.L., Yazdanian M., and Audus K.L. (2000). Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int. J. Pharm. 208, 1–11 [DOI] [PubMed] [Google Scholar]
- Gao G., Alvira M.R., Somanathan S., Lu Y., Vandenberghe L.H., Rux J.J., Calcedo R., Sanmiguel J., Abbas Z., and Wilson J.M. (2003). Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. U.S.A. 100, 6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., and Wilson J.M. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L., Allen J.M., and Miller A.D. (2001). Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared with that of AAV2 vectors. J. Virol. 75, 6615–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L., Allen J.M., and Miller A.D. (2002). Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat. Biotechnol. 20, 697–701 [DOI] [PubMed] [Google Scholar]
- Jost P.J., Harbottle R.P., Knight A., Miller A.D., Coutelle C., and Schneider H. (2001). A novel peptide, THALWHT, for the targeting of human airway epithelia. FEBS Lett. 489, 263–269 [DOI] [PubMed] [Google Scholar]
- Kern A., Schmidt K., Leder C., Muller O.J., Wobus C.E., Bettinger K., von der Lieth C.W., King J.A., and Kleinschmidt J.A. (2003). Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J. Virol. 77, 11072–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis M.P., and Wilson J.M. (2006). Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U.S.A. 103, 12993–12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathia N.R., Timoszyk J., Stetsko P.I., Megill J.R., Smith R.L., and Wall D.A. (2002). Permeability characteristics of calu-3 human bronchial epithelial cells: In vitro–in vivo correlation to predict lung absorption in rats. J. Drug Target. 10, 31–40 [DOI] [PubMed] [Google Scholar]
- Morris A.P., Cunningham S.A., Benos D.J., and Frizzell R.A. (1992). Cellular differentiation is required for cAMP but not Ca2+ -dependent Cl− secretion in colonic epithelial cells expressing high levels of cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 267, 5575–5583 [PubMed] [Google Scholar]
- Morris A.P., Cunningham S.A., Tousson A., Benos D.J., and Frizzell R.A. (1994). Polarization-dependent apical membrane CFTR targeting underlies cAMP-stimulated Cl− secretion in epithelial cells. Am. J. Physiol. 266, C254–C268 [DOI] [PubMed] [Google Scholar]
- Nakai H., Storm T.A., and Kay M.A. (2000). Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 18, 527–532 [DOI] [PubMed] [Google Scholar]
- Opie S.R., Warrington K., Agbandje-McKenna M., Zolotukhin S., and Muzyczka N. (2003). Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J. Virol. 77, 6995–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard L.S., Rokhlina T., Karp P.H., Lashmit P., Afione S., Schmidt M., Zabner J., Stinski M.F., Chiorini J.A., and Welsh M.J. (2005). A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. U.S.A. 102, 2952–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi R.G., Ropper A.E., Dragos R., Reid A.C., Nakayama K., Tan Y., Ehteshami J.R., Coleman S.H., Silver R.B., Hackett N.R., Menez A., and Crystal R.G. (2003). In vivo trans-splicing of 5′ and 3′ segments of pre-mRNA directed by corresponding DNA sequences delivered by gene transfer. Mol. Ther. 8, 999–1008 [DOI] [PubMed] [Google Scholar]
- Ren C., White A.F., and Ponnazhagan S. (2007). Notch-1 augments intracellular trafficking of adeno-associated virus 2. J. Virol. 81, 2069–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenecker J., Huth S., and Rudolph C. (2006). Gene therapy for cystic fibrosis lung disease: Current status and future perspectives. Curr. Opin. Mol. Ther. 8, 439–445 [PubMed] [Google Scholar]
- Rosseau S., Selhorst J., Wiechmann K., Leissner K., Maus U.K., Mayer K., Grimminger F., Seeger W., and Lohmeyer J. (2000). Monocyte migration through the alveolar epithelial barrier: Adhesion molecule mechanisms and impact of chemokines. J. Immunol. 164, 427–435 [DOI] [PubMed] [Google Scholar]
- Schneider H., Harbottle R., Yokosaki Y., Kunde J., Sheppard D., and Coutelle C. (1998). A novel peptide, PLAEIDGIELTY, for the targeting of α9β1-integrins. FEBS Lett. 429, 269–273 [DOI] [PubMed] [Google Scholar]
- Shen B.Q., Finkbeiner W.E., Wine J.J., Mrsny R.J., and Widdicombe J.H. (1994). Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am. J. Physiol. 266, 493–501 [DOI] [PubMed] [Google Scholar]
- Subramanian S., Parthasarathy R., Sen S., Boder E.T., and Discher D.E. (2006). Species- and cell type-specific interactions between CD47 and human SIRPα. Blood 107, 2548–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S., Boder E.T., and Discher D.E. (2007). Phylogenetic divergence of CD47 interactions with human signal regulatory protein α reveals locus of species specificity: Implications for the binding site. J. Biol. Chem. 282, 1805–1818 [DOI] [PubMed] [Google Scholar]
- Summerford C., and Samulski R.J. (1998). Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72, 1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K., Jurkuvenaite A., Wakefield J., Hong J.S., Guimbellot J.S., Venglarik C.J., Niraj A., Mazur M., Sorscher E.J., Collawn J.F., and Bebök Z. (2004). Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J. Biol. Chem. 279, 22578–22584 [DOI] [PubMed] [Google Scholar]
- Wagner J.A., Moran M.L., Messner A.H., Daifuku R., Conrad C.K., Reynolds T., Guggino W.B., Moss R.B., Carter B.J., Wine J.J., Flotte T.R., and Gardner P. (1998). A phase I/II study of tgAAV-CF for the treatment of chronic sinusitis in patients with cystic fibrosis. Hum. Gene Ther. 9, 889–909 [DOI] [PubMed] [Google Scholar]
- Weber E., Berta G., Tousson A., St John P., Green M.W., Gopalokrishnan U., Jilling T., Sorscher E.J., Elton T.S., Abrahamson D.R., and Kirk K.L. (1994). Expression and polarized targeting of a rab3 isoform in epithelial cells. J. Cell. Biol. 125, 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba A., Kern A., Weger S., Grimm D., and Kleinschmidt J.A. (1997). Subcellular compartmentalization of adeno-associated virus type 2 assembly. J. Virol. 71, 1341–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus C.E., Hugle-Dorr B., Girod A., Petersen G., Hallek M., and Kleinschmidt J.A. (2000). Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: Epitope mapping and identification of capsid domains involved in AAV-2–cell interaction and neutralization of AAV-2 infection. J. Virol. 74, 9281–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhang Y., Duan D., and Engelhardt J.F. (2000). Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 6716–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zak R., Zhang Y., Ding W., Godwin S., Munson K., Peluso R., and Engelhardt J.F. (2004). Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J. Virol. 78, 2861–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Seiler M., Walters R., Kotin R.M., Fulgeras W., Davidson B.L., and Chiorini J.A. (2000). Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74, 3852–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]