Abstract
While investigating whether proteins retrieved by cervicovaginal lavages (CVL) from women with cervical intraepithelial neoplasia (CIN) might correlate with risk of progression to invasive cervical cancer, we unexpectedly identified HIV gag and env glycoprotein in CVL from women with HIV-negative serology. HIV antigens were consistently identified by mass spectrometry (MS) in CVL from 4 women but were absent in CVL from the remaining 16 women. HIV serologies of all 20 patients were negative for both HIV-1 and HIV-2 antibodies. To validate the unexpected MS findings we performed Western blot (WB) and immunoaffinity chromatography (IC) analysis of CVL for HIV proteins, viral load assays of paired CVL and blood samples, and immunohistochemical HIV p24 expression in cervical biopsy specimens. WB analysis of CVL for prostate-specific antigen (PSA) was performed to exclude semen contamination as the source of HIV proteins. WB and IC results demonstrated the presence of HIV-1 gp41 and p24 antigens in four CVL that were identified by MS to have the HIV proteins. Despite negative serology, HIV RNA in CVL and HIV p24 in cervix biopsies were detected in patients with HIV antigen-positive CVL. HIV p24-positive CVL were PSA negative. All 20 subjects remained HIV seronegative throughout the study. Women with HIV proteins and RNA were comparatively older. Our findings suggest that CVL HIV proteins in women with CIN could be markers for unrecognized HIV exposure or subclinical infection. Proteomic screening of cervical secretions may be useful in identifying seronegative women exposed to HIV and/or at risk for AIDS.
Introduction
Modern diagnostic criteria for many diseases now include DNA and/or RNA analyses.1 Protein markers may be more useful because disease-related alterations in cellular functions correlate more with post translational modifications of protein rather than with protein expression.2 For several years, we have been involved in studies investigating the risk factors associated with precursor cervix cancer lesions, referred to as cervical intraepithelial neoplasia (CIN).3–7 While investigating whether any of the proteins identified in cervicovaginal lavage (CVL) specimens from women with CIN might characterize the precursor cervix cancer state we unexpectedly found HIV proteins in CVL samples from four of our studied patients by peptide mass fingerprinting (PMF). Our study subjects were neither drug users nor at particularly high risk for HIV infection. They attended the gynecological clinics at a municipal hospital for routine Pap tests. The Pap smears were abnormal and subsequent cervical biopsies were diagnosed as CIN. Women with CIN 1 lesions were enrolled for the proteomic study to determine the correlation of cervix-specific protein expression with regression/progression patterns of cervical dysplasia. The unexpected findings of HIV proteins in CVL samples of our patients compelled us to undertake the present study.
An association of HIV and human papillomavirus (HPV)-related cervical disease in our patient population has been recognized since the late 1980s.8–10 An increased incidence of CIN is reported in HIV-infected women, most notably in patients with high risk HPV infection.11,12 Investigators have also shown that CIN is more common in immune-compromised women regardless of whether the immune suppression is congenital, iatrogenic, or acquired.13 In HIV-infected women CIN lesions are multifocal, progress rapidly, have high recurrence rates, and require more stringent monitoring and intervention.14 In Zambia, 30% of an unselected population of 150 HIV-positive women, many of whom were already on antiretroviral therapy, had advanced CIN and 20% had cervical cancer.15 In the United States, 1.3% of all women over the age of 13 years with AIDS were reported to have invasive cervical cancer, which prompted the Center for Disease Control and Prevention to include invasive cervical cancer on the list of AIDS-defining illnesses in 1993.16 HIV proteins have been detected in CVL from HIV-infected seropositive women.17,18 However, little is known about HIV proteins in cervicovaginal fluids in HIV-seronegative women. Our study confirms the presence of HIV viral proteins and RNA in CVL samples and in biopsied cervical tissues of HIV-negative patients, which is unrelated to semen contamination.
Materials and Methods
Subject recruitment
Twenty asymptomatic volunteer women aged 18–50 years, with abnormal Pap smears and histopathologically confirmed low grade dysplasia were recruited with informed consent from the gynecology clinics of Jacobi Medical Center (JMC), a municipal hospital in the Bronx, New York. Completed questionnaires provided routine demographic data, as well as information regarding reproductive and gynecological histories. The protocol was approved by the Institutional Review Board (IRB) of the Albert Einstein College of Medicine. HIV testing was offered as a part of the routine patient care protocol with the option to refuse testing. HIV serology was performed at the HIV testing facility of JMC.
The study subjects were followed for a period of 1 year at 3-month intervals. Eight of the patients were followed for a period of up to 2.5 years. As we became convinced that HIV proteins unrelated to semen contamination were consistently present in CVLs, the patients were referred for repeat HIV serologies to the JMC HIV clinic regardless of whether HIV proteins were detected in their CVLs or not.
Collection and processing of CVL samples
Approximately 10 ml of CVL was collected from each patient. Half of each CVL was collected in tubes containing a cocktail of protease inhibitors (Roche, Indianapolis, IN) and the other half was collected separately for the detection of HPV DNA as previously reported.19 CVL samples were placed on ice and processed within 2 h of collection. Soluble protein fractions of the CVL samples were prepared by centrifuging the samples at 229,000 × g for 20 min at 4°C. Supernatants were aliquoted and stored at −20°C until analysis.
Polyacrylamide gel electrophoresis and mass spectrometry of CVL samples
CVL protein concentrations were determined using a Nano Drop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Due to wide variations in protein concentrations among CVL samples, the samples were not normalized prior to electrophoresis, and 20-μl aliquots of CVL were analyzed by one-dimensional (1D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). CVL samples along with protein markers (Rainbow marker 800, GE Healthcare, Piscataway, NJ) were loaded onto precast 10% Tris–HCl polyacrylamide gels (Bio-Rad, Hercules, CA). The gels were either stained with Coomassie brilliant blue to examine the protein profiles or electroblotted onto nitrocellulose membranes (Schleicher Schuell Bioscience, Inc., Keene, NH) for Western blot (WB) analysis.
Multiple Coomassie-stained polypeptide bands were excised from each of the gel lanes and processed for in-gel trypsin digestion. Tryptic peptides were analyzed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry, peptide mass fingerprinting (MALDI-TOF MS, PMF). Portions of the gel from adjacent blank lane(s) corresponding to the regions of the Coomassie-stained polypeptide bands were cut and processed simultaneously to serve as controls. The tryptic peptides were purified using either C-18 ZipTips (Millipore Corporation, Bedford, MA) or PepClean C-18 Spin Columns (Pierce, Rockford, IL), as per the manufacturers’ instructions. Equal volumes (1.5 μl each) of the purified CVL peptide mixture and matrix solution (saturated α-cyano-4-hydroxycinnamic acid in 50% acetonitrile/water containing 0.1% TFA) were mixed, and 1.5 μl of the resultant mixture spotted onto a MALDI-TOF target plate. Mass spectrometry measurements were acquired on a Voyager-DE STR MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA) in positive ion reflector mode at a resolution of 4500. One hundred shots were summed to obtain the spectrum. Protonated molecular ions (M + H)+ of three trypsin autodigestion peptides (m/z 842.51, 1045.56, and 2211.10) and a matrix peak (m/z 568.14) were used as internal calibrants. Unique monoisotopic peaks (m/z values) with distinct peptide isotopic envelopes, not seen in the control spectra, were selected for protein identification (PMF) using ProFound and MASCOT (in-house) search engines against the NCBI nonredundant database. The taxonomic categories used were All Taxa, Homo sapiens, and Virus. The peptide mass tolerance ranged from 10 to 150 ppm. The proteins were considered as candidate proteins if (1) they were the top hits, (2) they had two to seven monoisotopic peaks matching with the identified proteins, (3) the sequence coverage was between 20% to 40%, and (4) the Profound and Mascot scores were significant (p < 0.05). Positive protein identification was based on the published guidelines of criteria for reporting identified proteins by mass spectrometry.20
Western blot assays of CVL for HIV and prostate-specific antigens
WB was performed on all CVL samples using HIV-specific p24 and gp41 mouse monoclonal antibodies (mAbs). Non-specific proteins were blocked with 3% milk in Tris-buffered saline (pH 7.4), containing 0.1% Tween-20 (TBST). Immunoreactive protein bands were visualized using an enhanced chemiluminescent reagent (GE Healthcare, Piscataway, NJ), as per the manufacturer's direction. The dilution of the primary antibodies used for WB analysis varied between 1:500 and 1:800. Secondary antibody was used at a dilution of 1:3000. HIV-1 p24 mouse mAbs were purchased from three different vendors (Abcam Inc., Cambridge, MA; ZeptoMetrix, Buffalo, NY, and Cliniqa, Fallbrook, CA) and another that was obtained from the New York Blood Bank (courtesy of Dr. Shibo Jian). Recombinant full-length HIV-1 p24 (BioWorld, Dublin, OH) was used as positive control. The other mAbs used for WB assays included HIV-1 gp41 (ZeptoMetrix, Buffalo, NY), human serum albumin (HSA, Abcam Inc. Cambridge, MA), phosphotyrosine 4G10 (Upstate Cell Signaling Inc., San Francisco, CA), and prostate specific antigen (PSA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), which corresponded to amino acid residues 1–261 of the full-length human PSA. The secondary antibody was a goat antimouse horseradish peroxidase-conjugated polyclonal antibody (BD Biosciences, Franklin Lakes, NJ). The presence of HIV p24 and gp41 antigens was examined in all CVL samples obtained at 3-month intervals for periods ranging from 1 to 2.5 years.
In addition to WB assays that were conducted to monitor for the presence of HIV antigens in the CVL samples at serial clinic visits, the following WB assays were performed to further confirm the presence of HIV p24 in the CVL: briefly, four equal aliquots of a CVL sample were loaded onto a gel. After transferring the proteins, the immunoblot was split into four sections, each section corresponding to one sample lane. Four different HIV-1 p24 mAbs were obtained from four different sources, and each immunoblot section was probed with a separate mAb. The remaining procedure of the WB assay was performed as described earlier. The specificity of the HIV antigen-positive bands on immunoblots was additionally verified by probing HIV antigen-positive and -negative immunoblots with a potent irrelevant mAb to HSA.
In another separate WB assay we examined whether the positive signals on immunoblots probed with HIV-1 p24 mAb were due to IgG present in the CVL sample. For this WB assay, one HIV antigen-negative and three HIV antigen-positive CVL were loaded in duplicate on a gel. After transferring the proteins, the immunoblot was divided into two. One-half of the immunoblot was probed with both primary and secondary antibodies, and the other half was probed with secondary antibody alone.
PSA in CVL was also analyzed by the WB method using mAb raised against human PSA, p30 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). PSA was analyzed from all women obtained at two different clinic visits and for women with HIV antigen-positive CVL (HAPC), for a minimum of three clinic visits.
Immunoaffinity chromatography of CVL
Immunoaffinity chromatography (IC) was performed using the Microlink Protein Coupling Kit (Pierce, Rockford, IL) as per the manufacturer's instructions. Compared to recombinant HIV p24 protein that was used as control, HIV p24 antigen in the CVL was found to migrate slowly in the immunoblots in all our WB assays. We therefore suspected that the viral protein in the CVL might be phosphorylated. We, hence used phosphotyrosine 4G10 mAb to prepare the affinity column. For each IC assay, 160 μl of CVL was loaded onto the columns and WB assays of the eluates were performed using HIV-1 p24 and phosphotyrosine 4G10 mAbs.
HIV viral load assays of paired blood and CVL samples
HIV viral load assays of paired CVL and blood samples were performed for two patients with HAPC employing the VERSANT HIV RNA 3.0 assay (bDNA) Test kit (Siemens, Tarrytown, NY) as per the manufacturer's instructions. The lower limit of sensitivity of the method used was 75 copies of HIV RNA/ml. The viral load data of the remaining two patients with HAPC were not determined because fresh CVL and blood specimens were not available for viral load studies and these two women were lost to follow-up.
Immunohistochemical localization of HIV p24 in biopsied cervix tissues
Immunohistochemical localization of HIV p24 antigen in cervix biopsy tissues was performed to clarify the source of p24 antigen in the CVL. Twenty cervix biopsies were analyzed. Tissue sections were cut from formalin-fixed paraffin-embedded tissue blocks that were available after diagnostic evaluations. The presence of large numbers of erythrocytes and blood vessels in cervix biopsies yielded high backgrounds when stained with DAB. Hence, the alkaline phosphatase staining method was employed. Tissue sections (4 μm) were subjected to heat-induced epitope retrieval in citrate buffer. HIV-1 p24 expression in the tissue was detected using HIV-1 p24 mAb (Dako, Carpinteria, CA). Each tissue served as its own control, and the immunohistochemistry (IHC) was carried out replacing the primary antibody with an equal amount of mouse IgG. An autopsied brain tissue that was known to express HIV-1 p24 was obtained from a patient with HIV encephalitis, and sections from this tissue were used as positive controls for the IHC assays. The antigen–antibody complex formed in the presence of alkaline phosphatase-conjugated secondary antibody (Southern Biotech, Birmingham, AL) was visualized using nitroblue tetrazolium/bromochloroindolyl phosphate (NBT/BCIP) as substrate. Endogenous phosphatase was blocked by levimasole. Tissue sections were counterstained with hematoxylin or Fast Red.
Results
Pap smears obtained from women on the same day as their CVL samples ranged from “within normal limits” through high grade squamous intraepithelial lesions (HGSIL). HPV DNA was detected in 11 out of 20 (55%) women and categorized as follows: high-risk types 16, 18, 33, 35, 39, and 53; low-risk type 72; and unknown risk type 62 (Table 1). Four women were identified as having HIV p24 and gp41 antigens in their CVL samples. The mean ages of women with and without HIV protein-positive CVL samples were 43.2 years ±5.1 SD (n = 4) and 26.2 years ±6.9 SD (n = 16), respectively (p = 0.002 by Student's t test, assuming unequal variances). The PSA status of all 20 CVL samples is presented in Table 1. A positive PSA status in the table indicates that a signal on immunoblots was observed at least once when the CVL samples were examined at serial clinic visits. PSA-positive bands were noted in 7 out of 16 CVL samples analyzed. However, the possibility that the HIV proteins identified in the CVL samples were from semen contamination was excluded by the absence of PSA in samples that were positive for HIV p24 antigen (data not shown). Table 2 summarizes the available clinical information on our subjects, including sexual history, reproductive history, and the frequency of HIV testing.
Table 1.
Demographic Characteristics of Patients
| Patient | Age | Pap resulta | Smoking statusb | Contraception statusc | HPV DNA in CVL | PSA status of CVLd | HIV-1 p24 in CVL (MALDI TOF PMF) |
|---|---|---|---|---|---|---|---|
| 1 | 46 | LGSIL | Nonsmoker | Tubal ligation | Positive type 58 | Positive | Positive |
| 2 | 27 | ASCUS | Nonsmoker | Condom | Positive type 16 | Negative | Negative |
| 3 | 37 | ASCUS | Nonsmoker | Tubal ligation | Negative | Positive | Positive |
| 4 | 22 | Negative for CIN | Nonsmoker | Intrauterine device | Negative | Not analyzed | Negative |
| 5 | 25 | LGSIL | Nonsmoker | Condom | Positive type 72 | Negative | Negative |
| 6 | 50 | LGSIL | Nonsmoker | Condom | Positive type 39 | Negative | Positive |
| 7 | 34 | LGSIL | Nonsmoker | Condom | Negative | Positive | Negative |
| 8 | 46 | HGSIL | Smoker | Condom | Negative | Negative | Negative |
| 9 | 20 | LGSIL | Nonsmoker | Condom | Positive type 18 | Positive | Negative |
| 10 | 25 | ASCUS | Nonsmoker | Pills | Positive type 33 | Positive | Negative |
| 11 | 22 | ASCUS | Nonsmoker | Pills | Positive type 59 | Negative | Negative |
| 12 | 21 | ASCUS | Nonsmoker | Pills | Positive type 16 | Negative | Negative |
| 13 | 21 | ASCUS | Nonsmoker | Pills | Negative | Positive | Negative |
| 14 | 23 | Negative for CIN | Smoker | Pills | Negative | Not analyzed | Negative |
| 15 | 40 | LGSIL | Nonsmoker | Pills | Negative | Negative | Positive |
| 16 | 25 | HGSIL | Nonsmoker | Pills | Positive type 62 | Not analyzed | Negative |
| 17 | 31 | ASCUS | Nonsmoker | Condom | Negative | Negative | Negative |
| 18 | 18 | ASCUS | Nonsmoker | Condom | Positive type 16 | Not analyzed | Negative |
| 19 | 36 | Negative for CIN | Nonsmoker | Tubal ligation | Negative | Positive | Negative |
| 20 | 24 | Negative for CIN | Nonsmoker | Pills | Positive type 16 | Negative | Negative |
LGSIL/HGSIL, low/high grade squamous intraepithelial lesion; ASCUS, atypical cells of unknown significance.
Smokers smoked ½ to 2 packs a day for at least 1 year.
Women on contraceptive pills for at least a period of 6 months and on other various forms of contraception.
The PSA status of four CVL samples was not determined because fresh CVL was not available when PSA assays could be done.
Table 2.
HIV Testing, Sexual and Reproductive Histories of Study Subjects
| Patient | Frequency of HIV serologies | Number of lifetime male vaginal sex partners | Number of pregnancies | Number of live births |
|---|---|---|---|---|
| 1a | 3 | 2 | 5 | 5 |
| 2 | 4 | 4 | 2 | 2 |
| 3a | 1 | 1 | 1 | 1 |
| 4 | 4 | 3 | 1 | 0 |
| 5 | 1 | 3 | 2 | 1 |
| 6a | 3 | 4 | 2 | 2 |
| 7 | 4 | 1 | 5 | 5 |
| 8 | 1 | 4 | 3 | 2 |
| 9 | 8 | 2 | 3 | 1 |
| 10 | 4 | 3 | 1 | 1 |
| 11 | 1 | 6 | 3 | 2 |
| 12 | 8 | 21 | 2 | 0 |
| 13 | 1 | 2 | 1 | 0 |
| 14 | 6 | 2 | 2 | 1 |
| 15a | 1 | 1 | 4 | 2 |
| 16 | 4 | 8 | 2 | 0 |
| 17 | 2 | 8 | 1 | 1 |
| 18 | 5 | 1 | 2 | 1 |
| 19 | 1 | 2 | 5 | 3 |
| 20 | 2 | 10 | 1 | 1 |
Indicates subjects with HIV antigen-positive CVLs.
Protein expression profiling of CVL samples
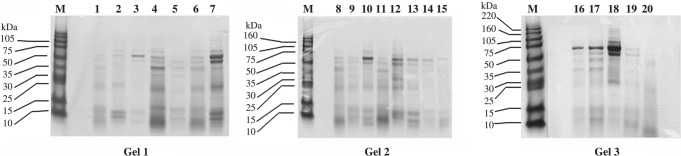
The protein concentrations of the 20 CVL samples ranged from 0.18 to 1.34 mg/ml. The gel patterns (Fig. 1) showed distinct polypeptide bands across a wide range of molecular weights in each of the lanes, with marked variations in the protein profiles among CVL samples.
FIG. 1.
Coomassie Brilliant blue-stained SDS gels showing protein profiles of cervicovaginal lavage samples. Protein concentrations of the CVL samples ranged from 0.18 to 1.34 mg/ml; 20-μl CVL sample was loaded per lane. The Coomassie-stained gel patterns of 20 individual CVL samples show distinct polypeptide bands across a wide range of molecular weights.
Mass spectrometry of CVL
PMF results using ProFound and MASCOT identified HIV-1 gag and HIV-1 env glycoprotein in CVL samples of 4 out of 20 women in the study. PMF results ranked HIV-1 env glycoprotein and HIV gag protein as the first significant candidate proteins with 16–46% coverage. The experimentally identified peptide sequences were found to be contiguous and overlapped with peptide sequences of identified HIV env glycoprotein and gag protein that exist in the Protein Data Bank. A representative mass spectrometry result of Well 26 Band 2 identifying HIV gag protein is described. Fourteen unique monoisotopic ions were obtained from the corresponding spectrum, compared to control. The PMF results, with mass tolerance set at 100 ppm, ranked HIV gag protein (accession no. gi 2959955) as the number one protein in the list, with an expectation value of 0.002 and with 30% sequence coverage. Out of a total 14 unique monoisotopic ions, 8 ions matched to the following 8 HIV gag peptides: FYKTLR (826.484 m/z), AEQASQEVK (988.418 m/z), DYVDRFYK (1104.499 m/z), EPFRDYVDR (1195.604 m/z), MYSPTSILDIR (1294.531 m/z), TLRAEQASQEVK (1358.717 m/z), MYSPTSILDIRQGPK (1704.904 m/z), and VHPVQAGPIPPGQLREPR (1947.016 m/z). Another representative mass spectrometry result of Well 8 Band 1 identifying HIV env glycoprotein is depicted as well. Twenty-seven unique monoisotopic ions were obtained from the corresponding spectrum, compared to control. The PMF results, with mass tolerance set at 150 ppm, ranked HIV env glycoprotein (accession no. gi 31075611) as the number one protein in the list with an expectation value of 0.001 and with 17% sequence coverage. Out of a total 27 unique monoisotopic ions, 9 ions matched to the following 9 HIV peptides: LTVWGIK (815.412 m/z), IEEGGGEQGSGR (1174.522 m/z), IKQIIINMWQR (1328.622 m/z), LLEDSQNQQEK (1330.572 m/z), VANQLGKHFPNK (1351.652 m/z), IVVTIISVVNRVR (1466.802 m/z), MIFWMLMISKATDK (1713.762 m/z), VSFEPIPIHYCTPAGFAILK (2258.962 m/z), and NIIVQFTESVPINCTRPNNNTR (2586.062 m/z).
PMF identified other significant candidate proteins from polypeptide bands of the same gels or other CVL sample-loaded gels. These included HPV-related early and late proteins, hepatitis C viral proteins, albumin, IgG heavy and light chains, T cell receptor chain, MHC Class II antigen, α2-macroglobulin precursor, β-globin chain, and sperm-associated antigen (data not shown).
Confirmation of HIV proteins in CVLs
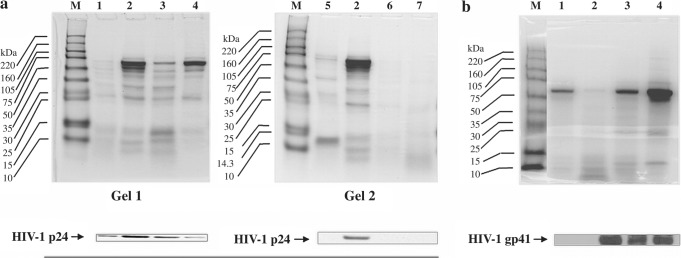
The WB and IC results are collectively depicted in Figs. 2–4. The immunoblot of Gel 1 in Fig. 2a depicts four HIV-1 p24 antigen-positive CVL samples. Gel 2 shows one HIV-1 p24 antigen-positive and three HIV-1 p24 antigen-negative CVL samples. The immunoblot of Fig. 2b shows one HIV-1 gp41 antigen-negative and three gp41 antigen-positive CVL samples. The Coomassie-stained protein profiles of lane 2 of both gels (Gel 2 Fig. 2a and Fig. 2b) show distinct polypeptide bands, yet the respective immunoblots show an absence of HIV-1 p24 and gp41 antigens, suggesting thereby that the presence of HIV proteins in the CVL samples was not dependent on the protein concentrations.
FIG. 2.
HIV-1 antigen-positive immunoblots of CVL samples and corresponding Coomassie-stained SDS gels. (a) Of the 20 CVL samples analyzed for the presence of HIV-1 p24 at serial clinic visits, the WB data of six representative CVL samples are shown. Lane 3 of both Gel 1 and Gel 2 represents the same CVL sample. Gel 1 of (a) shows the immunoblot of four HIV-1 p24-positive samples and their respective Coomassie-stained protein profiles. Gel 2 of (a) shows three representative HIV-1 p24-negative CVL in lanes 2, 4, and 5 of the immunoblot with their respective Coomassie-stained protein profiles shown above. (b) CVL samples of all 20 patients were also analyzed for the presence of HIV-1 gp41 antigen by WB and four representative WB data are shown: one HIV-1 gp41 antigen-negative CVL in lane 2 of the immunoblot, and three HIV-1 gp41 antigen-positive CVL samples in lanes 3, 4, and 5 of the immunoblot, respectively. The corresponding Coomassie-stained protein profiles of the samples are shown above.
FIG. 3.
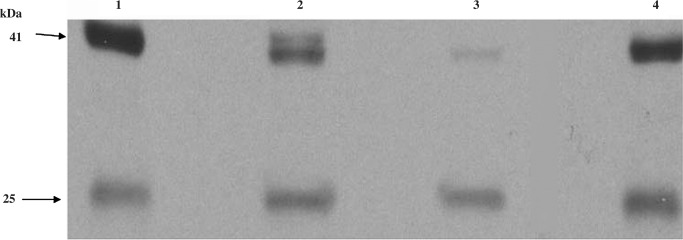
Immunoblots probed with four mouse HIV-1 p24 monoclonal antibodies raised from separate clones. Equal aliquots of a CVL sample were loaded four times on a gel. After transferring the proteins the immunoblot was split into four sections and probed with an HIV-1 p24 mAb obtained from the following sources: (1) Abcam Inc., Cambridge, MA; (2) Cliniqa, Fallbrook, CA; (3) ZeptoMetrix, Buffalo, NY; and (4) New York Blood Bank, New York, NY. Identical bands in all four immunoblots confirmed the presence of HIV-1 p24 in the CVL.
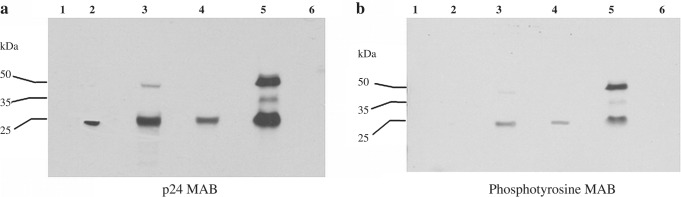
FIG. 4.
Immunoblot of immunoaffinity column eluates probed with HIV-1 p24 and phosphotyrosine 4G10 mAbs. The samples loaded on the gel were as follows: lane 1 = molecular weight marker; lane 2 = HIV-1 p24 full length recombinant protein (positive control); lane 3 = untreated CVL sample; lane 4 = CVL sample treated with protein A + protein G treated to remove IgG; lane 5 = eluate from the immunoaffinity column in which the CVL sample was loaded; lane 6 = eluate from the control immunoaffinity column. The HIV-1 p24-positive band in lane 5 (chromatography column eluate) confirmed the presence of p24 antigen in the CVL. Two additional bands were noted in lane 5, one corresponding to 41 kDa and the other to 55 kDa. These are probably HIV 41 and 55 gag proteins that could be visualized only when 160 μl of CVL was loaded onto the column (a). (b) The WB results when the same immunoblot was reprobed with phosphotyrosine, recombinant 4G10 mAb.
Figure 3 depicts the results of the four immunoblots that were probed with four different HIV-1 p24 mAbs. The identical results of the four immunoblots, even though the four mAbs originated from different clones, confirmed the presence of HIV p24 in the CVL. The WB assay that was conducted to evaluate whether IgG in CVL was responsible for the immunopositive bands showed that the signals were absent when the primary antibody was omitted, thereby validating that the immunopositive bands were not due to immunoglobulins (data not shown). All HIV antigen-positive and-negative immunoblots were also probed with mAb to HSA to verify the specificity of the viral protein positive bands. Distinct immunopositive bands were seen in all immunoblots probed with HSA that corresponded to approximately 65 kDa molecular weight (data not shown), thereby validating that the positive bands seen when probed with HIV antibodies were HIV antigen specific. A representative IC result is shown in Fig. 4.
The presence of an HIV-1 p24-positive signal in affinity column eluate (lane 5) confirmed the presence of HIV p24 in the CVL sample. An increase in the intensity of the p24 signal was noted in lane 5 of the immunoblot compared to lanes 3 and 4 (Fig. 4a). Perhaps the 8-fold increase in sample volume loaded onto the immunoaffinity column (160 μl compared to 20 μl in each of lane 3 and 4) accounted for the difference in signal strengths. The difference in sample volume could also explain why HIV p55 was seen only in lane 5 of the immunoblot and not in the other two sample lanes. The WB results after reprobing the previous immunoblot with recombinant phosphotyrosine 4G10 mAb showed phosphotyrosine-positive signals in lanes 3, 4, and 5 of the immunoblot and not in lane 2 or 6 (Fig. 4b). When the HIV p24-positive and phosphotyrosine-positive immunoblots were overlaid on top of each other the p24 and phosphotyrosine-positive bands were found to coincide, suggesting thereby that HIV p24 protein in CVL was phosphorylated on tyrosine.
Viral load of paired CVL and blood samples
The viral loads of the two CVL samples positive for HIV proteins were 407 and 2780 copies/ml, even though the viral load of both paired plasma samples was below the detectable level of 75 copies/ml. When adjusted for the total CVL sample retrieved during the lavage process (∼10 ml per lavage), the calculated viral loads were 4070 copies and 27,800 copies per lavage, suggesting that these two women had in fact been exposed to HIV.
Immunohistochemistry of biopsied cervix tissues
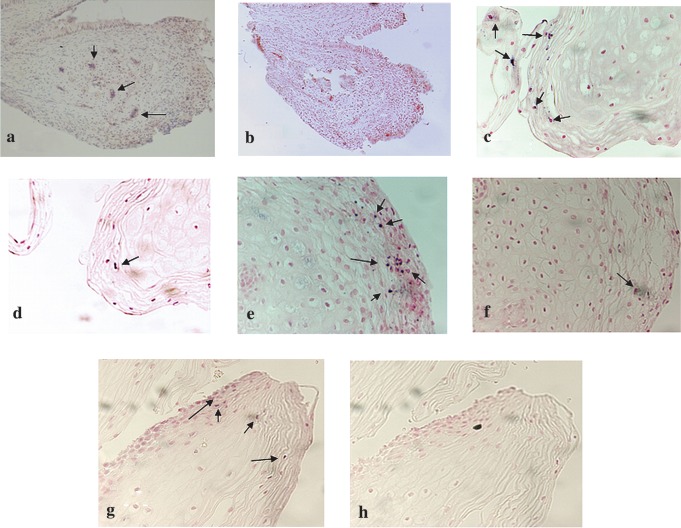
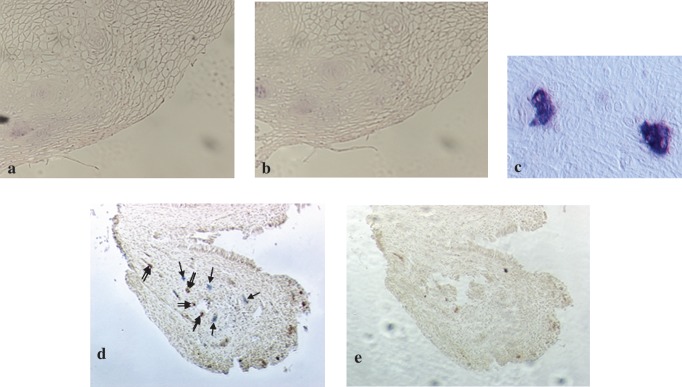
HIV p24 antigen-positive signals were noted in small foci in cervical biopsy tissues from four women with HIV antigen-positive CVL (HAPC) (Fig. 5a, c, e, and g). In tissues of the remaining 16 women with HIV antigen-negative CVL the HIV p24 antigen could not be detected. Two representative tissue sections, obtained from a woman with HIV antigen-negative CVL, in which HIV p24 antigen-positive signals were not detected, are shown in Fig. 6a and b: a tissue section that was probed with the primary antibody HIV-1 p24 mAb (Fig. 6a) and the other section from the same biopsy tissue was probed with mouse IgG to serve as its control (Fig. 6b). Figure 6c shows HIV p24-positive multinucleated giant cells in human brain tissue from an HIV-infected subject that was used as positive control for the IHC assays. One HIV p24 antigen-positive cervical biopsy section stained for the presence of HLA-DR antigen showed that the antigen was also present. However, the two antigens did not colocalize (Fig. 6d).
FIG. 5.
HIV p24 expression in cervical biopsy specimens from women with HAPC. The immunohistochemistry results when cervical biopsy specimens from women with HAPC were assayed by alkaline phosphatase staining method are depicted. (a, c, e, g) The presence of HIV p24 antigen in the tissues (as indicated with arrows, magnification 200 × ). (b, d, f, h) The results when the cervix tissues were processed without the primary antibody (HIV p24 mAb) to serve as negative controls (magnification 200 × ). Increased endogenous phosphatase activity, which could not be blocked with levimasole, was noted in all HIV p24-positive cervix tissues that were processed without the primary antibody to serve as controls (d, f).
FIG. 6.
HIV p24 and HLA-DR expression in cervical biopsy specimens and HIV p24-positive control. (a) A representative IHC result showing HIV p24 antigen-negative cervix tissue after being probed with HIV p24 mAb (magnification 400 × ). (b) A section of the same tissue as in (a) processed without the primary antibody to serve as control (magnification 400 × ). (c) HIV p24-positive multinucleated giant cells in brain tissue that was used as positive control for the IHC assay (magnification 400 × ). An HIV p24-positive cervix tissue was examined for the presence of HLA-DR antigen. Blue stains (indicated with a single arrow) show the presence of HIV p24 and brown spots (double arrow) show the presence of HLA-DR antigens in the tissue (d, magnification 200 × ). (e) The same tissue processed without primary antibodies (magnification 200 × ).
Discussion
While monitoring the regression/progression patterns of cervix dysplasia in women with CIN we evaluated proteins in CVL for a possible association with cervical pathology. Bacteria, fungi, spermatozoa, leukocytes, and other contaminants of the cervical milieu are frequently retrieved by a lavage. We used the soluble protein fractions from CVL to circumvent these resident contaminants and to focus primarily on cervix-associated proteins. The identification of HIV gag and env glycoprotein in the CVL samples of four women by MALDITOF peptide mass fingerprinting was unexpected. The significant PMF results from two different search engines, ProFound and Mascot, provided confidence that HIV gag and env glycoprotein were likely to be present in these four CVL samples. CVL samples obtained at follow-up clinic visits showed the continued presence of HIV env and gag proteins by mass spectrometry PMF in the same four women and the absence of the HIV antigens in the remaining 16 women. These results were of particular interest because all 20 women were negative for HIV-1 and -2 antibodies.
These findings prompted us to reexamine the CVL samples and confirm the presence of these viral proteins using conventional and unconventional techniques: enzyme-linked immunosorbent assay (ELISA), WB, and IC. ELISA was performed only once (data not shown) while WB and IC assays were performed repeatedly, and all three techniques confirmed the presence of HIV-1 p24 in the four CVL samples and their absence in the remaining 16 CVL samples. WB studies additionally confirmed that the positive signals seen on the immunoblots probed with HIV p24 mAb were not due to IgG, they were HIV protein specific, and that the HIV proteins in CVL were not recent contributions from male partners’ semen. The patients were referred to the HIV testing facility of JMC for follow-up HIV serologies. Review of their electronic charts indicated that all patients were consistently seronegative for both HIV-1 and HIV-2 antibodies. Follow-up HIV testing was not initiated until we were confident that HIV proteins were present in CVL, and since patients entered the study at different times the number of HIV serologies was not the same for all subjects (Table 2). Noncompliance with referral to the HIV service may have also played a role in this variability. IC results of the present study suggested that HIV p24 protein in CVL was phosphorylated on tyrosine (Fig. 4). These results are similar to those reported by other investigators who have shown that cultured CD4+ lymphocytes infected with HIV released p24 protein phosphorylated at serine residues21,22 in the culture medium.
Although the detection of HIV antibodies in serum is currently the hallmark of HIV diagnosis, investigators have shown that appreciable amounts of HIV antigens and RNA can also be detected in cervical secretions from HIV-infected women.17,18 The viral loads of the two HAPCs in this study were 4070 and 27,800 copies even though HIV RNA was undetectable in their paired blood samples. Our findings are analogous to those reported by other investigators who have shown that in HIV-seropositive women, the viral loads and HIV genotypes of paired cervicovaginal secretions and blood are independently regulated and can differ. Active HIV viral replication in cervical secretions did not always correlate with a detectable plasma viral load.23
Numerous CD4+ cells reside in cervical mucosa, including T lymphocytes, macrophages, and dendritic cells.24 Cultured cervical epithelial cells isolated from hysterectomized uteri express CD4 and CCR5 coreceptors.25 Significantly elevated expression of CCR5 was also detected in cervical tissues from HIV-infected women compared to biopsies from healthy normal women.26 Leukocytes, endocervical, exocervical, and vaginal cells are common constituents of CVL. The HIV proteins identified in CVLs in our study may well have originated from any of these cells or from other CVL contaminants. Cervical biopsy specimens from the studied patients were therefore examined for HIV p24 antigen to determine the site(s) of protein expression. Immunopositive signals were noted in the four cervical biopsies from women with HAPC (Fig. 5a, c, e, and g) and were undetectable in the biopsies from women with HIV antigen-negative CVL (Fig. 6a). In two of these HIV-positive specimens, immunopositive spots were seen both in tissue sections probed with HIV p24 mAb and that which was probed with mouse IgG to serve as controls (Fig. 5 c, e, d, and f, respectively). The number of immunopositive spots in the tissue sections probed with the primary antibody was, however, greater. Levimasole was used to block the endogenous phosphatase levels. Our data suggest that in these two tissues, the endogenous phosphatase level was perhaps increased because the amount of levimasole used should have completely blocked the endogenous phosphatase level, but was found to be insufficient. Protein tyrosine phosphatases are a large group of enzymes that regulate many signal transduction processes that are critical for maintaining the homeostasis and efficient cellular activation.27 In disease states, specifically during HIV infection, this delicate balance between tyrosine kinase and tyrosine phosphatase is disturbed.
It is reported that following HIV infection, the host responds by increasing endogenous phosphatase levels to activate the resident T cells.27,28 Hence, increased endogenous phosphatase in the cervix tissue in our study could be a manifestation of HIV infection. HLA-DR is a transmembrane glycoprotein that is expressed on antigen-presenting cells such as B lymphocytes, monocytes, macrophages, thymic epithelial cells, and activated T cells. We investigated whether the HIV-positive cells in cervix biopsy tissue were also HLA-DR-positive. The tissue section was doubly probed with HIV p24 and HLA-DR mAbs. The data showed that both HIV p24 and HLA-DR antigens were present in the tissue but the two antigens did not colocalize (Fig. 6d). Confirmation of HIV p24 expression in cervical tissue by antigen blocking and phenotype characterization of the HIV p24-positive cells would have been useful, but the biopsy tissue samples were small and the number of tissue sections available for study was limited.
Individuals who remain seronegative despite repeated exposure to HIV have previously been reported by other investigators, and various mechanisms are postulated by the authors to explain these phenomena.29–38 The finding of mucosal HIV-1 proteins in four asymptomatic HIV-seronegative women in our study is unusual. The participants did not report intravenous drug use or sex work and were otherwise not at obvious high risk for HIV (Table 2). It is worth reemphasizing that patients recruited for this study voluntarily attended the gynecology clinics for routine Pap tests to screen for cervix cancer. Women with abnormal Pap smears were referred to the Colposcopy Clinic where they underwent colposcopically directed cervical biopsies. Those whose biopsies demonstrated grade 1 CIN were enrolled in a study that was initially designed to investigate whether proteins retrieved by cervical lavage correlated in any way with subsequent regression of CIN or progression to invasive cervix cancer. Our findings suggest the following possibilities: (1) HIV-1-specific proteins can be expressed in genital tract secretions and tissues due to mucosal infection that may never be associated with seropositivity; (2) early HIV infection limited to the genital tract will be followed by seroconversion after a latent period, the duration of which is currently unknown; (3) HIV infection might have been aborted with persistence of some residual protein; and/or (4) the women might have distinct genetic profiles that conferred resistance to HIV infection. Based on our findings we suggest that identification of HIV proteins in CVL from sexually active females might be an earlier predictor of HIV exposure and/or subclinical infection than a positive HIV serology.
Eighty percent of newly diagnosed HIV infections occur in women aged 20–49 years.39 The female genital tract may be more susceptible to HIV infection because of the repeated shedding and remodeling of the endometrium with menstruation. Leukocytes play active roles in this hormonally controlled rebuilding process. Many leukocytes have cell surface receptors for HIV, as do cervical epithelial cells.24–26 The availability of large numbers of HIV target cells may make the female genital tract vulnerable for HIV infection over the course of many menstrual cycles. Perhaps the older mean age of women with HAPC in the present study reflects a greater opportunity for HIV to access the host tissues during these periods of enhanced vulnerability.
The prevalence of HIV seropositivity among women in outpatient clinics in the Bronx has been reported to be less than 6%,40 yet 20% of our small sample of women with CIN had HIV proteins and RNA in their CVL. This discrepancy might be because we may have screened for HIV antigen in CVL at an early time before HIV antibodies appeared in the blood. We have thus identified a cohort of HIV-seronegative women who nevertheless appear to have been exposed to and infected with HIV. Future studies are needed to evaluate (1) the sensitivity and specificity of MALDI-TOF and WB for the detection of HIV protein in CVL, (2) the possible value of these techniques as early screens for HIV exposure prior to seroconversion, (3) whether HIV-seronegative patients with HAPC are capable of transmitting the virus to noninfected peripheral blood mononuclear cells, to their sexual partners, or to their own fetuses, and (4) what defines the immunological profile of women in this category.
We recognize that detection of proviral HIV DNA and determination of the HIV DNA sequence would have further substantiated HIV infection in these women. However, the study was not originally designed as an investigation of HIV and we did not have a provision for DNA testing in our informed consent form. Since New York State law prohibits HIV DNA testing without patient consent, proviral DNA assay or HIV DNA sequencing could not be performed retrospectively.
Detection of HIV antigens and/or RNA in CVL could be an adjunct to plasma testing for the diagnosis of HIV. Immunological responses to HIV develop over several weeks or months and a lag exists between entry of the virus and the appearance of HIV antibodies in the blood. This latent period is not usually recognized and HIV exposure without seropositivity is currently undiagnosed by routinely available methods. In the effort to develop an effective vaccine against HIV, early identification of women soon after HIV exposure can give us insights as to the earliest pathogenic processes that must be understood in stimulating effective immunological responses and protection. We believe that screening for HIV antigens in cervical secretions may offer such an investigative opportunity and may promote new insights regarding mucosal HIV infection in women.
Acknowledgments
This work is dedicated to the memory of the late Dr. George A. Orr for his invaluable contributions to this research. The study was supported by the Friends of Gynecologic Cancer Research of the Albert Einstein College of Medicine. The authors are thankful to the Center for AIDS Research of the Albert Einstein College of Medicine, particularly to Drs. Harris Goldstein for viral load studies and Sunhee Lee and Meng Liang Zhao for immunohistochemistry; Robert D. Burk for HPV typing; Gloria Ho for providing epidemiological data; Susan B. Horwitz, Charles S. Rubin, Irwin R. Merkatz, Louis M. Weiss, and Chandralekha Duttagupta for their continued support; Dr. Benjamin Romney at Columbia University School of Medicine for his editorial comments; Ms. Mary Sanvardeker for interviewing and recruiting patients; Ms. Berta Burd for her technical assistance, and to our volunteer participants.
Disclosure Statement
No competing financial interests exist.
References
- 1. Broeckel U, Maresso K, and Kugathasan S: Functional genomics and its implications for molecular medicine. Pediatr Clin North Am 2006;53:807–816 [DOI] [PubMed] [Google Scholar]
- 2. Nilsson CL. and Davidsson P: New separation tools for comprehensive studies of protein expression by mass spectrometry. Mass Spectrom Rev 2000;19:390–397 [DOI] [PubMed] [Google Scholar]
- 3. Burk RD, Kadish AS, Calderin S, and Romney SL: Human papillomavirus infection of the cervix detected by cervicovaginal lavage and molecular hybridization: Correlation with biopsy results and Papanicolaou smear. Am J Obstet Gynecol 1986;154:982–989 [DOI] [PubMed] [Google Scholar]
- 4. Basu J, Mikhail MS, Ahn CW, et al. : Plasma uric acid levels in women with cervical intraepithelial neoplasia. Nutr Cancer 2005;51:25–31 [DOI] [PubMed] [Google Scholar]
- 5. Kadish AS, Timmins P, Wang Y, et al. : Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev 2002;11:483–488 [PubMed] [Google Scholar]
- 6. Basu J, Romney SL, Angeletti RH, et al. : Proteomic analysis of cervicovaginal lavage samples: Identification of human immunodeficiency virus proteins. 9th International Meeting of Human Virology, Baltimore, MD, August28, 2005 [Google Scholar]
- 7. Ho GY, Palan PR, Basu J, et al. : Viral characteristics of human papillomavirus infection and antioxidant levels as risk factors for cervical dysplasia. Int J Cancer 1998;78:594–599 [DOI] [PubMed] [Google Scholar]
- 8. Feingold AR, Vermund SH, Burk RD, et al. : Cervical cytologic abnormalities and papillomavirus in women infected with human immunodeficiency virus. J Acquir Immune Defic Syndr 1990;3:896–903 [PubMed] [Google Scholar]
- 9. Vermund SH, Kelley KF, Klein RS, et al. : High risk of human papillomavirus infection and cervical squamous intraepithelial lesions among women with symptomatic human immunodeficiency virus infection. Am J Obstet Gynecol 1991; 165:392–400 [DOI] [PubMed] [Google Scholar]
- 10. Klein RS, Ho GYF, Vermund SH, Fleming I, and Burk RD: Risk factors for squamous epithelial lesions on Pap smear in women at risk for human immunodeficiency virus infection. J Infect Dis 1994;170:1404–1409 [DOI] [PubMed] [Google Scholar]
- 11. Ellerbrock TV, Chiasson MA, Bush TJ, et al. : Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000;283:1031–1037 [DOI] [PubMed] [Google Scholar]
- 12. Delmas MC, Larsen C, van Benthem B, et al. : Cervical squamous intraepithelial lesions in HIV-infected women: Prevalence, incidence and regression. European study group on natural history of HIV infection in women. AIDS 2000;14:1775–1784 [DOI] [PubMed] [Google Scholar]
- 13. Petry KU, Scheffel D, Bode U, et al. : Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer 1994;57:836–840 [DOI] [PubMed] [Google Scholar]
- 14. Fruchter RG, Maiman M, Sedlis A, Bartley L, Camilien L, and Arrastia CD: Multiple recurrences of cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Obstet Gynecol 1996;87:338–344 [DOI] [PubMed] [Google Scholar]
- 15. Castle PE, Schiffman M, Gravitt PE, et al. : Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol 2002;68:417–423 [DOI] [PubMed] [Google Scholar]
- 16. Klevens RM, Fleming PL, Mays MA, and Frey R: Characteristics of women with AIDS and invasive cervical cancer. Obstet Gynecol 1996;88:269–273 [DOI] [PubMed] [Google Scholar]
- 17. Iversen AKN, Larsen AR, Jensen T, et al. : Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis 1998;177:1214–1220 [DOI] [PubMed] [Google Scholar]
- 18. Hart CE, Lennox JL, Pratt-Palmore M, et al. : Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis 1999;179:871–882 [DOI] [PubMed] [Google Scholar]
- 19. Carr S, Aebersold R, Baldwin M, Burlingame A, Clauser K, and Nesvizhskii A: The need for guidelines in publication of peptide and protein identification data: Working group on publication guidelines for peptides and protein identification data. Mol Cell Proteomics 2004;3:531–533 [DOI] [PubMed] [Google Scholar]
- 20. Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, et al. : Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol 2006;103:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mervis RJ, Ahmad N, Lillehoj EP, et al. : The gag gene products of human immunodeficiency virus type 1: Alignment within the gag open reading frame, identification of posttranslation modifications, and evidence for alternative gag precursors. J Virol 1988;62:3993–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veronese FD, Copeland TD, Oroszlan S, Gallo RC, and Sarngadharan MG: Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol 1988;62:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rasheed S, Li Z, Xu D, and Kovacs A: Presence of cell-free human immunodeficiency virus in cervicovaginal secretions is independent of viral load in the blood of human immunodeficiency virus-infected women. Am J Obstet Gynecol 1996;175:122–130 [DOI] [PubMed] [Google Scholar]
- 24. Pudney J, Quayle AJ, and Anderson DJ: Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005;73:1253–1263 [DOI] [PubMed] [Google Scholar]
- 25. Yeaman GR, Asin S, Weldon S, et al. : Chemokine receptor expression in the human ectocervix: Implications for infection by the human immunodeficiency virus-type I. Immunology 2004;113:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prakash M, Kapembwa MS, Gotch F, and Patterson S: Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. J Reprod Immunol 2001;52:101–111 [DOI] [PubMed] [Google Scholar]
- 27. Ouellet M, Barbeau B, and Tremblay MJ: Protein tyrosyl phosphatases in T cell activation: Implication for human immunodeficiency virus transcriptional activity. Prog Nucleic Acid Res Mol Biol 2003;73:69–105 [DOI] [PubMed] [Google Scholar]
- 28. Nekhai S, Jerebtsova M, Jackson A, and Southerland W: Regulation of HIV-1 transcription by protein phosphatase 1. Curr HIV Res 2007;5:3–9 [DOI] [PubMed] [Google Scholar]
- 29. Patterson BK, Landay A, Andersson J, et al. : Repertoire of chemokine receptor expression in the female genital tract: Implications for human immunodeficiency virus transmission. Am J Pathol 1998;153:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samson M, Libert F, Doranz BJ, et al. : Resistance to HIV-1 infection in caucasian individuals bearing mutants alleles of the CCR-5 chemokine receptor gene. Nature 1996;382:722–725 [DOI] [PubMed] [Google Scholar]
- 31. Liu R, Paxton WA, Choe S, et al. : Homozygous defect in HIV-1 co-receptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996;86:367–377 [DOI] [PubMed] [Google Scholar]
- 32. Devito C, Hinkula J, Kaul R, et al. : Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 2000;14:1917–1920 [DOI] [PubMed] [Google Scholar]
- 33. Trabattoni D, Caputo SL, Maffeis G, et al. : Human [alpha] defensin in HIV-exposed but uninfected individuals. J Acquir Immune Defic Syndr 2004;35:455–463 [DOI] [PubMed] [Google Scholar]
- 34. Dorrell L, Hessell AJ, Wang M, et al. : Absence of specific mucosal antibody response in HIV-exposed uninfected sex workers from the Gambia. AIDS 2001;14:1117–1122 [DOI] [PubMed] [Google Scholar]
- 35. Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, and Lusso P: Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995;270:1811–1815 [DOI] [PubMed] [Google Scholar]
- 36. Becker Y: The molecular mechanism of human resistance to HIV-1 infection in persistently infected individuals—A review, hypothesis and implications. Virus Genes 2005;31:113–119 [DOI] [PubMed] [Google Scholar]
- 37. Kunanusont C, Foy HM, Kreiss JK, et al. : HIV-1 subtypes and male to female transmission in Thailand. Lancet 1995; 345:1078–1083 [DOI] [PubMed] [Google Scholar]
- 38. McNeeley TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, and Wahl SM: Inhibition of human immunodeficiency virus type I infectivity by secretory leukocyte protease inhibitor occurs prior to virus reverse transcription. Blood 1997;90:1141–1149 [PubMed] [Google Scholar]
- 39. Hall HI, Lee LM, Glynn MK, and Song DVM: Heterosexual transmission of HIV—29 states, 1999–2002. MMWR Morb Mortal Wkly Rep 2004;53:125–129 [PubMed] [Google Scholar]
- 40. Irwin KL, Pau CP, Lupo D, et al. : Presence of human immunodeficiency virus type I subtype A infection in a New York community with high prevalence. A sentinel site for monitoring HIV genetic diversity in North America. J Infect Dis 1997;176:1629–1633 [DOI] [PubMed] [Google Scholar]