Abstract
Advances in proteomics methodology and instrumentation have allowed detailed characterization of the composition of lymph. Far from being a simple ultrafiltrate of blood plasma, lymph has been shown to carry a rich repertoire of proteins and peptides reflecting the tissue of origin and its physiological state. Peptides derived from lymph can be loaded on the MHCII proteins, particularly those present on immature and/or inactivated antigen presenting cells, and may play an important role in maintenance of peripheral tolerance.
Lymph Formation
The pre-nodal or afferent lymph originates from the interstitial fluid of all parenchymal organs through a process of ultrafiltration of the blood plasma circulating through the capillaries (Fig. 1).1–4 During the last decades, several principles thought to control lymph formation have been revisited. For example, the classical Starling principle that the fluid filtering from the arterial end of the capillary bed is absorbed at its venous end has been experimentally challenged.1 Indeed, it is now known that the majority of extravasated fluid will not re-enter the circulatory system by uptake into the microcirculation, but instead will be drained into the lymphatics. It also appears that a low level of fluid filtration takes place throughout the entire microcirculation of most parenchymal organs and not only at the arterial end of the capillary bed.1 Finally, the idea that the colloid osmotic pressure driving the fluid filtration rate is generated primarily from the protein concentration in the plasma has also been challenged. It is now thought that the filtration rate between plasma and interstitial fluid is limited by the glycocalyx present at the luminal surface of the endothelial cells.1 The glycocalyx acts as a sieve, which filtrates proteins according to their size. Water and small molecules pass easily through the glycocalyx and through intracellular clefts. Proteins and bigger molecular complexes move across the epithelial barrier via openings or pores in the glycocalix (Fig. 1).
FIG. 1.

Schematic of fluid and protein exchange across the microvessels with continuous endothelium. Water molecules pass through the glycocalyx and intracellularly. Proteins are sieved through large pores between endothelial cells and through opening of the glycocalyx. A color version of this figure is available www.liebertpub.com/lrb.
In addition to proteins, small molecules, and water originating from plasma ultrafiltration, several other products from tissue/organ catabolism/metabolism will further enrich the interstitial fluid. Upon collection into the open end capillaries, the interstitial fluid becomes lymph.5–10
Several parameters are known to affect lymph formation, including the blood capillary filtration rate, composition and permeability of the glycocalix, metabolic state of parenchymal cells, and draining ability of the lymphatic capillaries and collectors. All these factors are continually varying, depending on the location and physiological or pathological state of the tissue or organ from which the lymph is drained.11–17 Additionally, the contribution of tissue metabolism/catabolism to the lymph composition is likely to generate a unique molecular signature specific for the organ from where the lymph is collected.18–27
Lymph and Plasma Proteomes
Very comprehensive databases have been assembled for the protein composition of human and rodent plasma under physiological and pathological conditions.28–34 Up to a few years ago, much less information was available for the lymph, partially due to the difficulty in collecting lymph samples and partially due to the low sensitivity of mass spectrometric techniques in detecting low abundance proteins in scarce material. Even though cannulating a lymphatic collector is still a difficult technique to master and so lymph samples are still limited, newly-developed techniques for depletion of abundant proteins, and the availability of sensitive and accurate mass spectrometers and proteomics methodologies, have allowed for a far more detailed characterization of the lymph proteome.
Recently, several groups have published extensive proteomic profiles of the lymph collected both in human, rodents, bovine, and ovine.11–27 The first lymph proteomic profile, performed on ovine lymph by Leak et al.,8 challenged the notion that the lymph was a mere ultrafiltrate of the plasma. Indeed, although the majority of proteins were present in both lymph and plasma samples, two proteins were identified as uniquely expressed in lymph; glial fibrillary acidic protein and neutrophil cytosol factor-1.8 During the last decade, several more comprehensive proteomic analyses have appeared, reporting lymph proteins originating from plasma ultrafiltration, and others that are specifically generated in the tissue from where the lymph is collected.7–12,17,19–27 Our group recently reported the first human proteomic analysis of afferent lymph as compared to the plasma using a combination of immune-affinity depletion methods for albumin and IgG, coupled with 1D SDS-PAGE and 2D-DIGE together with nano-LC-LTQ-Orbitrap tandem MS/MS.10 The two biological fluids shared a common proteome, but we also observed a lymph-enriched proteome consisting of products derived from extracellular matrix processing, cellular apoptosis, and tissue metabolism/catabolism.
Proteomic approaches have also been used to qualitatively and quantitatively characterize changes in lymph under pathological conditions. A study on ovine lymph, collected from control animal or animals infected with a nematode (Teladorsagia circumcincta), confirmed the presence of tissue-specific proteins in the lymph versus the plasma, and outlined for the first time a strong correlation between an inflammatory condition and the protein expression profile of the lymph.19 This study also demonstrated that an inflammatory tissue condition can be reflected in proteomic changes in lymph more so than in plasma.19 Following in the same line of work, several other groups characterized the lymph proteome during different pathological conditions with the ultimate goal of identifying tissue-specific proteins as markers of pathological conditions. Using this approach, several inflammatory factors and enzymes were mapped in the mesenteric lymph following induction of acute pancreatitis, including carboxypeptidases, lipases, amylases, and trypsinogen.20 Similarly, proteomic profiling of mesenteric lymph following hemorrhagic shock allowed the identification of several proteins released by tissue injury that were upregulated in post-shock versus pre-shock lymph.21–24 A concomitant depletion of coagulation factors and protease inhibitors and an increased level of hemolysis products also were observed.21–24 Markers of hemolysis, oxidative stress, matrix degradation, and general tissue damage could be mapped in the mesenteric lymph collected from patients with different injuries.26
Collectively all the experimental findings point to the lymph fluid as an important milieu for discovery of tissue and pathology-specific protein biomarkers.
Plasma and Lymph Peptidome and Degradome
The presence of low molecular weight serum and lymph proteomes composed of fragments derived from protein processing and cleavage has been recently recognized. Mapping of these “degradomes” was initially difficult due to the limitations in identification of species represented by a single peptide, as compared to conventional proteomic analysis of tryptic digests wherein each protein is represented by multiple peptide species. Improved strategies of peptide separation, database searching, and increases in mass resolution allowed the uncovering of the remarkable richness of protein fragments and naturally processed peptides observed in the low molecular weight proteome.5,6,16,30,32,35,36 One of the most comprehensive approaches towards the mapping of the low molecular weight cleaved proteome and peptidome of mouse serum has been reported by Brian L. Hood and colleagues.35 By using 18O labeling, they compared the low molecular weight serum proteome of tumor bearing mice with control mice. The low molecular weight degradome/peptidome identified in the tumor bearing mice encompassed over 6000 processed proteins and peptides. Analysis of the naturally processed proteome identified differential processing pathways between the controls and tumor bearing mice.35
Recently, several more peptidomes/degradomes have been mapped, including serum, plasma, lymph, synovial fluid, urine, and cerebrospinal fluid.30–38 In all the analyses, the peptides were identified with a near zero false discovery rate.30–38 The high confidence of peptide identification and amino acid assignment allowed prediction of the processing enzymes involved in the cleavage events that generated each peptide. Processing analysis identified over 500 proteases likely to be involved in cleaving the peptides found in the human plasma including caspases, cathepsins, MMPs, plasmin, calpains and granzyme A.35–39 Some of the sequenced peptides were extensively post-translationally modified, with acetylation, acetylhexosamine addition, amidation, cysteinylation, dehydration, and oxidation as the major post-translational modifications.
Importantly, all the studies concur that in pathological conditions, the mapped peptidome/degradome of different biological fluids is highly enriched with new peptides as compared to the peptidome/degradome found in healthy physiological conditions.12–14 Indeed, in different diseases such as cancer, rheumatoid arthritis (RA), and osteoporosis, fragments derived from the most abundant proteins found in human plasma and serum have been shown to be processed differently by proteases upregulated during inflammation.30,31,36 As such, the degradome associated with different pathological states including tumor progression, invasion, and metastasis, has been proposed as a potential source of biomarkers for clinical diagnostic purposes.30–39
Our laboratories recently mapped the first peptidome transported by the human lymph.5 The hypothesis that motivated this work was that the lymph potentially could be a rich source of tissue-specific antigens, and additionally could carry an enriched processed proteome/peptidome since it directly collects from the extracellular milieu where products of tissue catabolism, tissue remodeling, cellular apoptosis, and extracellular matrix processing are collected before transport to the draining lymph nodes.5,6 More than 300 self-peptides were mapped that derived from the catabolic processing of both intracellular and extracellular proteins5 (Fig. 2 and Fig. 3). In this analysis, lymph was collected from the foot, and a large fraction of the peptidome was composed of processed proteins derived from extracellular matrix proteins, cell adhesion molecules, and plasma membrane/receptors.5 On the other hand, much of the intracellular-derived peptidome consisted of fragments of cytosolic, nuclear (transcription factors and regulators of gene expression), mitochondrial, golgi, and endoplasmic reticulum proteins. Most likely the intracellular peptidome originates from apoptotic cells, many of which have been found in the human lymph.40
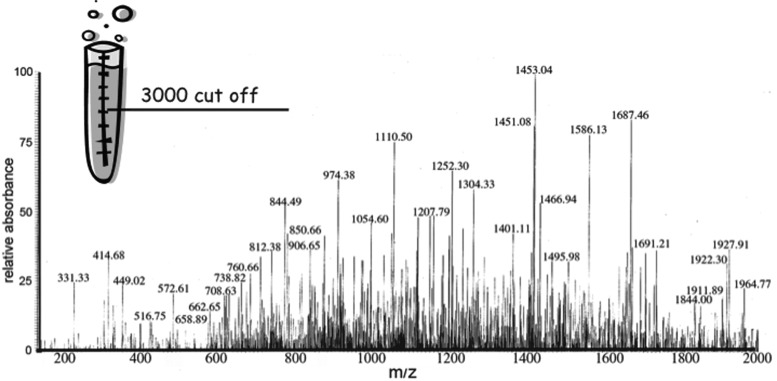
FIG. 2.
Comparative proteomic/peptidomic profile of human lymph and plasma samples. Comparative 2D-gel analysis of human lymph (red) and plasma (green), visualized by fluorescence staining (2CyeDye). Boxed area indicates the low molecular weight peptides. A Color version of this figure is available at www.liebertpub.com/lrb.
FIG. 3.
MS peptidomic profile of the human lymph. Mass spectroscopy scan (200–2000 m/z) of low MW (3000 Da) filtrate from lymph samples obtained after elution in 0.1% TFA.
Quantitative analysis of some of the most abundant peptides, performed using 14N/15N labeled peptide quantification and amino acid analysis from 2D-DIGE spots, indicated that many peptides were present in human lymph in at least nanomolar concentrations.5,6 Peptides present at these concentrations are immunologically relevant and can be loaded onto MHC class II molecules (MHCII) and displayed on antigen-presenting cells
Processing and MHCII Presentation of Lymph-Carried Antigens
There are several mechanisms by which tissue-derived self antigens are presented to the immune system. Tissue-resident dendritic cells (DCs) and macrophages (Mφ) are the primary antigen presenting cells that survey the self proteome of each parenchymal organ.41 Expression of tissue-specific self antigens by an AIRE-independent mechanism in lymphatic endothelial cells further expands the presentation of the self proteome.42 In addition, tissue-derived proteins carried by lymph can be phagocytosed by circulating DCs,43 lymphatic endothelial cells and nodal DCs, Mφ, and B cells.44,45 Transport of self-antigens directly to the node is a very efficient mechanism that expands the possibility of immunosurveillance.5,6
All the above described mechanisms rely on protein endocytosis and endosomal processing, thus creating an MHCII peptidome mostly restricted by endosomal cathepsins46 or by the proteasome for phagocytosed cross-presented antigens.47 MEROPS analysis, utilized to evaluate the processing enzymes involved in generating these peptides, indicates that several enzymes were responsible for cleaving of the lymph-carried peptidome, including cathepsins, granzymes, matrix metalloproteases (MMPs), and ADAMTs (A Disintegrin And Metalloproteinase with Thrombospondin motif).39 Apoptotic cells are known to be present in the lymph, and caspase-processed peptides likely derive from these cells.48 In addition, the high frequency of peptides apparently cleaved by MMPs and ADAMTs indicate that products from tissue remodeling and surface receptors editing are also present.48–56 The involvement of different processing pathways in the generation of the lymph-bound peptidome expands the peptidome available to MHCII molecules well beyond products generated by endosomal cathepsin processing.
MHCII molecules are present under noninflammatory conditions on professional APC and certain endothelial cells including lymphatic endothelium. Lymph-carried peptides could be loaded onto MHCII on the surface of these cells, either by direct binding to peptide-free molecules or by peptide exchange.57–63 Lymph-carried peptides could also either be loaded on surface MHC class II molecules expressed on parenchymal tissue DCs and Mφ, migrating DCs, lymphatic endothelial cells, or nodal APC.41,43–45,58,60–63 Immature DCs and nonactivated APCs have been shown experimentally to be more receptive to surface MHC II loading as compared to activated DCs.60 This may be due to the differential DO/DM ratio between the two maturation states. The amount of DM is similar for immature and mature/activated APC, however the amount of the DM inhibitor DO is higher on immature and nonactivated cells.64,65 Thus, immature DCs and nonactivated Mφ or B cells display an MHC II peptidome which is less DM edited as compared to that of mature and activated APC. A MHCII peptidome less edited by DM would be broader, less stable, and more easily exchanged.60–65 Thus, lymph-carried peptides would be more easily loaded onto MHCII of immature DCs and nonactivated APC. The lymph-bound peptidome displayed by these cells might have particular relevance for the induction and maintenance of peripheral tolerance to non-endosomal processed peptides.66
In addition, MHC class I molecules (MHCI) are expressed on every cell type. Lymph-carried peptides could bind directly to MHC class I on endothelial cells, and nodal stromal, DC, Mφ, B, or T cells, by direct binding to empty MHCI molecules or through exchange with previously loaded peptides.57,59
Conclusions
Over the past 10 years, there have been huge advances in the analysis of the lymph. In particular, improvements in proteomic techniques have allowed a broader and more comprehensive analysis of the lymph “omics” (proteomic, peptidomic, degradomic, and metabolomic). The first glimpses of lymph collected from different tissues support the notion that lymph can carry an “omic” signature from the organ where it originates. More extensive analyses need to be done to provide a complete molecular characterization of the tissue-specific self antigens collected by the lymph draining different organs and tissues. Additionally, further mapping of the lymph peptidome/degradome is necessary to determine the many processing pathways producing the vast array of peptides available for T cell presentation. Finally, the immunological relevance of the lymph-carried self proteome and peptidome in maintenance of peripheral tolerance will also be an important question to address further.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Levick JR. Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 2.Aukland K. Kramer GC. Renkin EM. Protein concentration of lymph and interstitial fluid in the rat tail. Am J Physiol. 1984;247:4–9. doi: 10.1152/ajpheart.1984.247.1.H74. [DOI] [PubMed] [Google Scholar]
- 3.Rockson SG. The lymphatic continuum revisited. Ann NY Acad Sci. 2008:1131. doi: 10.1196/annals.1413.000. [DOI] [PubMed] [Google Scholar]
- 4.Rockson SG. Molecular insights into the microvascular regulation of lymph formation. Lymphat Res Biol. 2007;5:149–150. doi: 10.1089/lrb.2007.5301. [DOI] [PubMed] [Google Scholar]
- 5.Clement CC. Rotzschke O. Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol. 2011;2:6–11. doi: 10.1016/j.it.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement CC. Cannizzo ES. Nastke MD. Sahu R. Olszewski W. Miller NE. Stern LJ. Santambrogio L. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One. 2010;5:e9863. doi: 10.1371/journal.pone.0009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Interewicz B. Olszewski WL. Leak LV. Petricoin EF. Liotta LA. Profiling of normal human leg lymph proteins using the 2-D electrophoresis and SELDI-TOF mass spectrophotometry approach. Lymphology. 2004;37:65–72. [PubMed] [Google Scholar]
- 8.Leak LV. Liotta LA. Krutzsch H, et al. Proteomic analysis of lymph. Proteomics. 2004;4:753–765. doi: 10.1002/pmic.200300573. [DOI] [PubMed] [Google Scholar]
- 9.Mittal A. Middleditch M. Ruggiero K, et al. The proteome of rodent mesenteric lymph. Am J Physiol Gastroint Liver Physiol. 2008;295:G895–G903. doi: 10.1152/ajpgi.90378.2008. [DOI] [PubMed] [Google Scholar]
- 10.Clement CC. Aphkhazava D. Nieves E. Callaway M, Olszewski W, Rotzschke O, Santambrogio L. Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J Proteomics. 2013;78:172–187. doi: 10.1016/j.jprot.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszewski WL. CRC Press; Boca Raton, Florida: 1991. LYMPH STASIS–Pathophysiology, Diagnosis and Treatment. chapters 10–11: 235280. [Google Scholar]
- 12.Meng Z. Veenstra TD. Proteomic analysis of serum, plasma, and lymph for the identification of biomarkers. Proteomics Clin Appl. 2007;1:747–757. doi: 10.1002/prca.200700243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Z. Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. J Proteomics. 2011;74:2650–2659. doi: 10.1016/j.jprot.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra TD. Conrads TP. Hood BL. Avellino AM. Ellenbogen RG. Morrison RS. Biomarkers: Mining the biofluid proteome. Mol Cell Proteomics. 2005;4:409–418. doi: 10.1074/mcp.M500006-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Ahn SM. Simpson RJ. Body fluid proteomics: Prospects for biomarker discovery. Proteomics Clin Appl. 2007;1:1004–1015. doi: 10.1002/prca.200700217. [DOI] [PubMed] [Google Scholar]
- 16.Geho DH. Liotta LA. Petricoin EF. Zhao W. Araujo RP. The amplified peptidome: The new treasure chest of candidate biomarkers. Curr Opin Chem Biol. 2006;1:50–55. doi: 10.1016/j.cbpa.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Nanjee MN. Cooke CJ. Olszewski WL. Miller NL. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41:1317–1327. [PubMed] [Google Scholar]
- 18.Armandola EA. Proteome profiling in body fluids and in cancer cell signaling. Med Gen Med. 2003;5:18–22. [PubMed] [Google Scholar]
- 19.Goldfinch GM. Smith WD. Imrie L. McLean K. Inglis NF. Pemberton AD. The proteome of gastric lymph in normal and nematode infected sheep. Proteomics. 2008;8:1909–1918. doi: 10.1002/pmic.200700531. [DOI] [PubMed] [Google Scholar]
- 20.Mittal A. Phillips AR. Middleditch M. Ruggiero K. Loveday B. Delahunt B. Cooper GJ. Windsor JA. The proteome of mesenteric lymph during acute pancreatitis and implications for treatment. JOP. 2009;10:130–142. [PubMed] [Google Scholar]
- 21.Masuno T. Moore EE. Cheng AM. Sarin EL. Banerjee A. Bioactivity of postshock mesenteric lymph depends on the depth and duration of hemorrhagic shock. Shock. 2006;26:285–289. doi: 10.1097/01.shk.0000223132.72135.52. [DOI] [PubMed] [Google Scholar]
- 22.Jordan JR. Moore EE. Damle SS, et al. Gelsolin is depleted in post-shock mesenteric lymph. J Surg Res. 2007;3:130–135. doi: 10.1016/j.jss.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltz ED. Moore EE. Zurawel AA, et al. Proteome and system ontology of hemorrhagic shock: Exploring early constitutive changes in postshock mesenteric lymph. Surgery. 2009;6:347–357. doi: 10.1016/j.surg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurawel A. Moore EE. Peltz ED, et al. Proteomic profiling of the mesenteric lymph after hemorrhagic shock: Differential gel electrophoresis and mass spectrometry analysis. Clin Proteomics. 2010;8:1–6. doi: 10.1186/1559-0275-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszewski WL. Pazdur J. Kubasiewicz E. Zaleska M. Cooke CJ. Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheumat. 2001;44:541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Dzieciatkowska M. Wohlauer MV. Moore EE, et al. Proteomic analysis of human mesenteric lymph. Shock. 2011;5:331–338. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang JF. Shih LY. Yuan KC. Fang KY. Hwang TL. Hsieh SY. Proteomic analysis of post-hemorrhagic shock mesenteric lymph. Shock. 2010;4:291–298. doi: 10.1097/SHK.0b013e3181ceef5e. [DOI] [PubMed] [Google Scholar]
- 28.Anderson NL. Polanski M. Pieper R, et al. The human plasma proteome: A non redundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M. Lucas DA. Chan KC, et al. An investigation into the human serum “interactome”. Electrophoresis. 2004;25:1289–1298. doi: 10.1002/elps.200405866. [DOI] [PubMed] [Google Scholar]
- 30.Antwi K. Hostetter G. Demeure MJ, et al. Analysis of the plasma peptidome from pancreas cancer patients connects a peptide in plasma to overexpression of the parent protein in tumors. J Proteome Res. 2009;10:4722–4731. doi: 10.1021/pr900414f. [DOI] [PubMed] [Google Scholar]
- 31.Zheng X. Wu SL. Hincapie M. Hancock WS. Study of the human plasma proteome of rheumatoid arthritis. J Chromatogr A. 2009;1216:3538–3545. doi: 10.1016/j.chroma.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 32.Shen Y. Liu T. Tolić N, et al. Strategy for degradomic-peptidomic analysis of human blood plasma. J Proteome Res. 2010;9:2339–2346. doi: 10.1021/pr901083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koomen JM. Li D. Xiao LC. Liu TC. Coombes KR. Abbruzzese J. Kobayashi R. Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. J Proteome Res. 2005;3:972–981. doi: 10.1021/pr050046x. [DOI] [PubMed] [Google Scholar]
- 34.Shen Z. Want EJ. Chen W. Keating W. Nussbaumer W. Moore R. Gentle TM. Siuzdak G. Sepsis plasma protein profiling with immunodepletion, three-dimensional liquid chromatography tandem mass spectrometry, and spectrum counting. J Proteome Res. 2006;5:3154–3160. doi: 10.1021/pr060327k. [DOI] [PubMed] [Google Scholar]
- 35.Hood BL. Lucas DA. Kim G, et al. Quantitative analysis of the low molecular weight serum proteome using 18O stable isotope labeling in a lung tumor xenograft mouse model. J Am Soc Mass Spectrom. 2005;8:1221–1230. doi: 10.1016/j.jasms.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y. Tolić N. Liu T, et al. Blood peptidome-degradome profile of breast cancer. PLoS One. 2010;5:e13133. doi: 10.1371/journal.pone.0013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrah T. Deutsch EW. Omenn GS, et al. A high confidence human plasma proteome reference set with estimated concentrations in Peptide Atlas. Mol Cell Proteomics. 2011:9. doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omenn GS. States DJ. Adamski M, et al. Overview of the HUPO Plasma Proteome Project: Results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005;13:3226–3245. doi: 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- 39.Dittwald P. Ostrowski J. Karczmarski J. Gambin A. Inferring serum proteolytic activity from LC-MS/MS data. BMC Bioinformat. 2012;13:S7. doi: 10.1186/1471-2105-13-S5-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olszewski WL. Human afferent lymph contains apoptotic cells and “free” apoptotic DNA fragments—Can DNA be reutilized by the lymph node cells? Lymphology. 2001;34:179–183. [PubMed] [Google Scholar]
- 41.Savina A. Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JN. Guidi CJ. Tewalt EF, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–698. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavanagh LL. Von Andrian UH. Travellers in many guises: The origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez SF. Degn SE. Pitcher LA. Woodruff M. Heesters BA. Carroll MC. Trafficking of B cell antigen in lymph nodes. Annu Rev Immunol. 2011;29:215–233. doi: 10.1146/annurev-immunol-031210-101255. [DOI] [PubMed] [Google Scholar]
- 45.Skokos D. Shakhar G. Varma R, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 46.Honey K. Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 47.Rock KL. Shen L. Cross-presentation: Underlying mechanisms and role in immunesurveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 48.Pang B. Neijssen J. Qiao X, et al. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J Immunol. 2009;183:1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 49.Badylak SF. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Dustin ML. deFougerolles AR. Reprogramming T cells: The role of extracellular matrix in coordination of T cell activation and migration. Curr Opin Immunol. 2009;3:286–290. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 51.Cauwe B. Van den Steen PE. Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–128. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 52.Reiss K. Saftig P. The“ADisintegrin And Metalloprotease” (ADAM family of sheddases: Physiological and cellular functions. Seminars Cell Devel Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci. 2009;56:148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Lai ZW. Steer DL. Smith AI. Membrane proteomics: The development of diagnostics based on protein shedding. Curr Opin Mol Ther. 2009;11:623–631. [PubMed] [Google Scholar]
- 55.Murphy G. Regulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’. Semin Cell Dev Biol. 2009;20:138–145. doi: 10.1016/j.semcdb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Heinrich J. Wiegert T. Regulated intramembrane proteolysis in the control of extracytoplasmic functions. Res Microbiol. 2009;160:696–703. doi: 10.1016/j.resmic.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 57.De Bruijn ML. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur J Immunol. 1991;12:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 58.Rabinowitz JD. Vrljic M. Kasson PM. Liang MN. Busch R. Boniface JJ. Davis MM. McConnell HM. Formation of a highly peptide-receptive state of class II MHC. Immunity. 1998;9:699–709. doi: 10.1016/s1074-7613(00)80667-6. [DOI] [PubMed] [Google Scholar]
- 59.Eisen HN. Hou XH. Shen C, et al. Promiscuous binding of extracellular peptides to cell surface class I MHC II protein. Proc Natl Acad Sci USA. 2012;109:4580–4585. doi: 10.1073/pnas.1201586109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatraman P. Nguyen TT. Sainlos M. Bilsel O. Chitta S. Imperiali B. Stern LJ. Fluorogenic probes for monitoring peptide binding to class II MHC proteins in living cells. Nat Chem Biol. 2007;3:222–228. doi: 10.1038/nchembio868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potolicchio I. Chitta S. Xu X, et al. Conformational variation of surface class II MHC proteins during myeloid dendritic cell differentiation accompanies structural changes in lysosomal MIIC. J Immunol. 2005;175:4935–4947. doi: 10.4049/jimmunol.175.8.4935. [DOI] [PubMed] [Google Scholar]
- 62.Santambrogio L. Sato AK. Carven GJ. Belyanskaya SL. Strominger JL. Stern LJ. Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci USA. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santambrogio L. Sato AK. Fischer FR. Dorf ME. Stern LJ. Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc Natl Acad Sci USA. 2009;96:15050–15055. doi: 10.1073/pnas.96.26.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denzin LK. Fallas JL. Prendes M. Yi W. Right place, right time, right peptide: DO keep DM focused. Immunol Rev. 2005;207:279–292. doi: 10.1111/j.0105-2896.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 65.Jensen PE. Antigen processing: HLA-DO a hitchhiking inhibitor of HLA-DM. Curr Biol. 1998;8:128–131. doi: 10.1016/s0960-9822(98)70988-1. [DOI] [PubMed] [Google Scholar]
- 66.Thomas SN. Rutkowski JM. Pasquier M. Kuan EL. Alitalo K. Randolph GJ. Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189:2181–2190. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]