Abstract
Background
Hemoglobin A1c (HbA1c) is an important index of average glycemia in patients with diabetes mellitus that is widely used in clinical trials and large-scale epidemiological studies. Previous studies have shown that adverse sample storage conditions can cause erroneous HbA1c results. We examined the effect of storage at different temperatures with five current HbA1c methods: Tosoh G7 and G8 (Tosoh Bioscience, Inc., South San Francisco, CA) and Bio-Rad Variant™ II (Bio-Rad Laboratories, Hercules, CA) (all ion-exchange high-performance liquid chromatography); Siemens DCA 2000+ (Siemens Healthcare Diagnostics, Deerfield, IL) (immunoassay); and Trinity Biotech (Kansas City, MO) ultra2 (boronate-affinity high-performance liquid chromatography).
Methods
Five whole blood specimens with different HbA1c levels were analyzed by each assay method on Day 0 and then divided into aliquots that were stored at six different temperatures (−70°C, −20°C, 4°C, room temperature, 30°C, and 37°C) for analyses on subsequent days out to Day 84. Acceptance limits were defined as within ±3 SD of all −70°C results or ±0.2% HbA1c, whichever was wider, for each sample. Stability was considered acceptable for a given temperature only if results for all five specimens were acceptable on that day.
Results
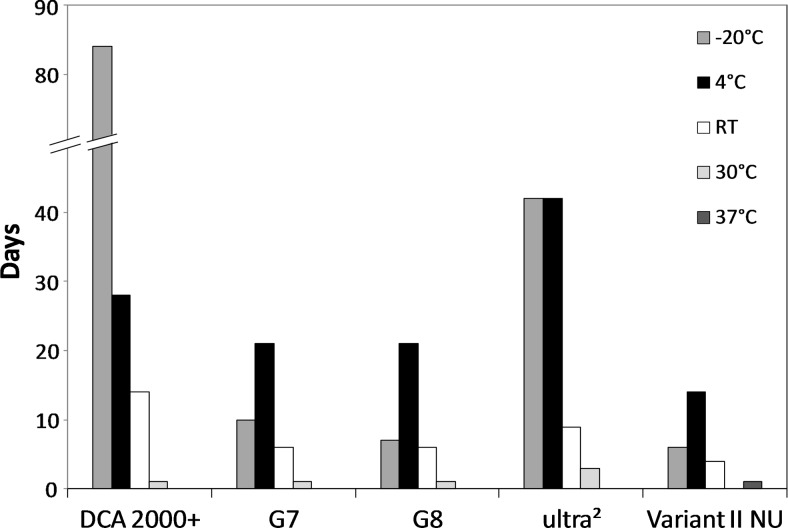
The DCA 2000+ demonstrated the best stability at −20°C and room temperature, whereas the ultra2 showed the best stability with specimens stored at 4°C. No methods demonstrated stability at 30°C or 37°C for more than 3 days.
Conclusions
Exposure of specimens to high temperatures should be avoided regardless of assay methodology. For the ion-exchange methods tested 4°C storage is preferable to −20°C (stability 14–21 days vs. 4–10 days). For studies where long-term stability is required, samples should be stored at −70°C or colder.
Introduction
Hemoglobin A1c (HbA1c) is an important index of average glycemia in patients with diabetes mellitus and is measured in many clinical trials and large-scale epidemiological studies. For these applications, specimens are often stored for a time and then shipped to a central laboratory. Previous studies have shown that exposure of whole blood (WB) samples to improper temperature conditions can cause erroneous HbA1c results and that, in general, ion-exchange assay methods tend to be more sensitive to storage issues than boronate affinity or immunoassay methods.1–5
For almost all current HbA1c assay methods, manufacturers claim stability at 4°C up to at least 1 week and for much longer if WB samples are stored at −70°C or colder (the exception to the latter being a few point-of-care methods that require fresh unfrozen blood). There are limited data in the literature regarding the stability of HbA1c measurements with currently available assay methods. Moreover, changes in the method over time may result in changes to stability characteristics for the same assay method. We previously reported on the stability of HbA1c results using five assay methods, specifically the Tosoh 2.2+ and G7 (Tosoh Bioscience, Inc., South San Francisco, CA), Bio-Rad Variant™ and Variant II (Bio-Rad Laboratories, Hercules, CA) (all ion-exchange high-performance liquid chromatography [HPLC]), and Trinity Biotech (Kansas City, MO) (Primus) CLC330/385 (which used boronate-affinity HPLC methodology).5 Here, we re-examine the effects of WB storage at different temperatures on HbA1c results with the G7 method and also report on four methods not previously tested: Tosoh G8 and Bio-Rad Variant II NU (ion-exchange HPLC), Trinity Biotech ultra2 (boronate-affinity HPLC), and Siemens DCA 2000+ (Siemens Healthcare Diagnostics, Deerfield, IL) (immunoassay).
Materials and Methods
Five EDTA whole blood samples representing a range of HbA1c values between 5% and 10% HbA1c were collected; single-use 50-μL aliquots were prepared from each level and stored at −70°C, −20°C, 4°C, room temperature (17–23°C), 30°C, and 37°C. The specimens were initially analyzed by each method on Day 0, and then one aliquot from each temperature was analyzed by each method on days 1, 2, 3, 4, 7, 10, 14 (2 weeks), 21 (3 weeks), 28 (4 weeks), 42 (6 weeks), 56 (8 weeks), and 84 (12 weeks).
HbA1c methods evaluated were the Tosoh G7 and G8, Bio-Rad Variant II NU, Trinity Biotech ultra2, and Siemens DCA 2000+. The G7, G8, and Variant II NU are ion-exchange HPLC methods, the ultra2 is a boronate-affinity HPLC method, and the DCA 2000+ is a point-of-care immunoassay method.
Storage of whole blood at −70°C for at least 1 year has been shown to be acceptable for several different assay methodologies.5,6 In addition, we compared results from the specimens stored at −70°C with those from 4°C storage out to day 7; overall absolute mean differences were <0.03% HbA1c for all methods, indicating no significant freeze/thaw effect. Therefore the acceptance range for all methods was chosen to be the mean±3 SD of the 12 results (each time point from Day 1 through 84) from −70°C storage that were analyzed on the same days as the aliquots stored at the other temperature conditions. In cases where the ±3 SD range was less than ±0.2% HbA1c, the mean±0.2% HbA1c was used to define acceptability. A given storage temperature and time frame was only considered acceptable if results were acceptable at all five HbA1c levels evaluated. In one case the ultra2 results for two of the specimens for −20°C (Day 14) were just below (0.1% and 0.2% HbA1c) their acceptable limits, but −70°C results indicated this was a low analytical run, and subsequent results for all five specimens were acceptable through Day 42; these results were therefore considered acceptable.
There were cases where the Day 7 result was not acceptable but previous results were; also, samples stored at 30°C and 37°C storage went dry in some cases because of a low volume of blood and the storage vials used for these temperatures. Therefore, a follow-up study that used two samples at different HbA1c levels (6.1% and 8.1%) was performed to more precisely define the stability interval between Days 4 and 7 and also to repeat/verify the 30°C and 37°C results.
Results
Results for each method, storage temperature, and HbA1c level are summarized in Figure 1. The time point just prior to the first unacceptable result is indicated as the number of days stable. Manufacturer claims for stability are shown in Table 1; stability for all methods exceeded manufacturers' claims. Figures 2–5 show chromatograms with acceptable (a) and unacceptable (b and c) results for each method.
FIG. 1.
Stability of hemoglobin A1c. Each column indicates stability in days for each method, at each storage temperature. RT, room temperature.
Table 1.
Manufacturer Claims for Stability
| Method | Manufacturer claim |
|---|---|
| Siemens DCA 2000+ | 2 weeks at −70 to 5°C, 1 week at room temperature |
| Tosoh G7 | 1 week at 4–8°C, 8 h at room temperature |
| Tosoh G8 | 2 weeks at 4–8°C, 24 h at room temperature |
| Trinity Biotech ultra2 | At least 7 days at 4°C, longer at −70°C |
| Bio-Rad Variant II NU | 1 week at 2–8°C |
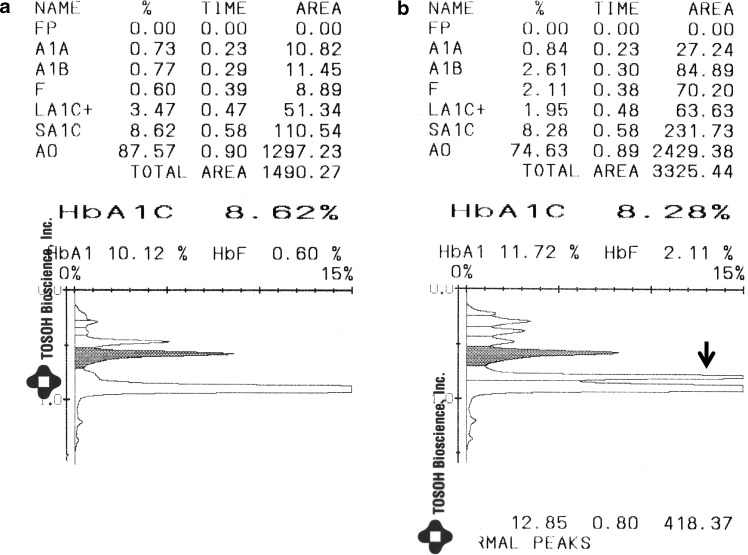
FIG. 2.
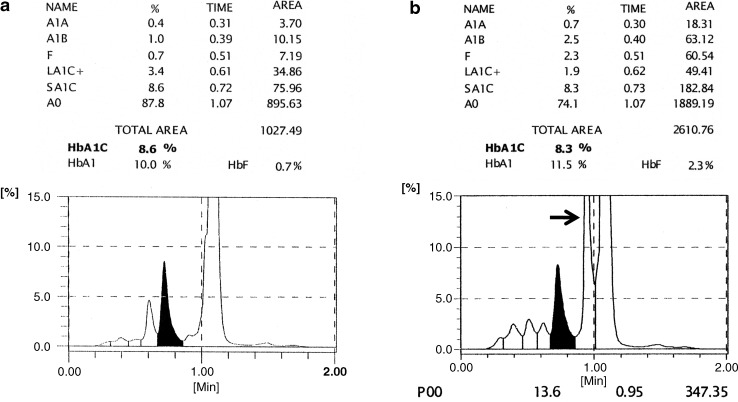
Tosoh G7 chromatograms from (a) a sample with an acceptable result and (b) a sample with an unacceptable result. The hemoglobin A1c (HbA1c) peak is the shaded peak. The chromatogram in (b) shows an extra peak next to the HbA0 peak; in this case the large peak at the end (arrow) is integrated as the “P00” peak.
FIG. 5.
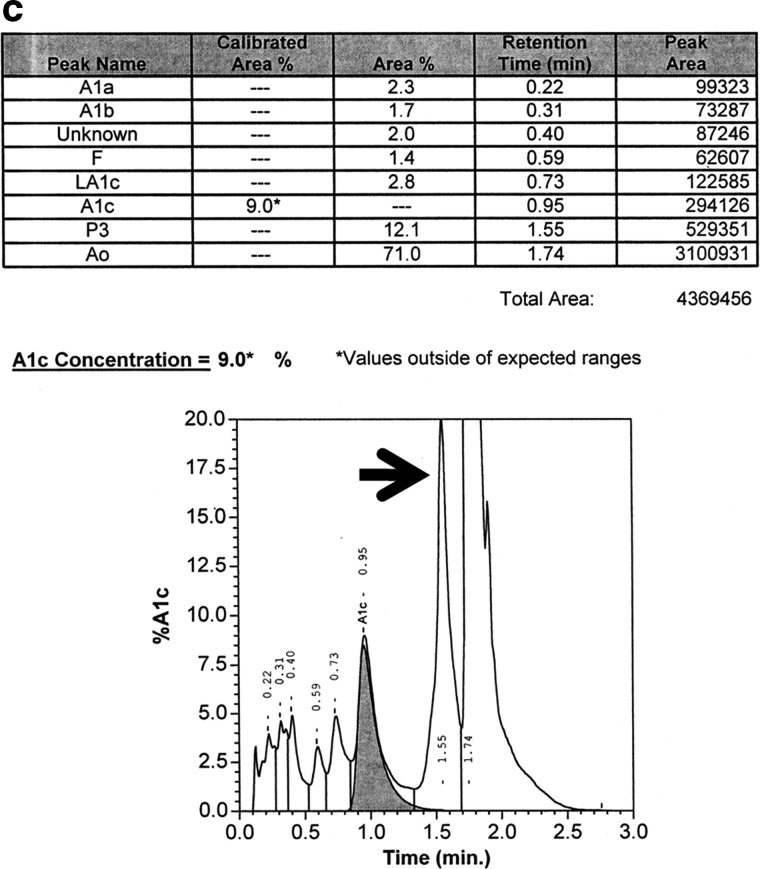
Variant II NU chromatograms from (a) a sample with an acceptable result and (b and c) two samples with unacceptable results. The hemoglobin A1c peak is the shaded peak. In (b) the P3 area is 7.0%, which is still within the manufacturer's acceptable limit of ≤10%. In (c) the P3 area is 12.1%, which exceeds the manufacturer's acceptable limit (arrow).
FIG. 3.
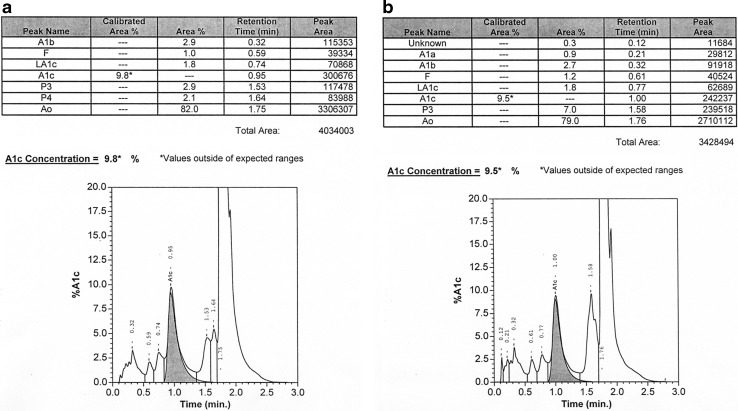
Tosoh G8 chromatograms from (a) a sample with an acceptable result and (b) a sample with an unacceptable result. The hemoglobin A1c (HbA1c) peak is the shaded peak. The chromatogram in (b) is unacceptable because of the extra peak between the HbA1c and HbA0 peaks (arrow).
WB samples were stable on the Siemens DCA 2000+ for at least 84 days at −20°C, 28 days at 4°C, 14 days at room temperature, 1 day at 30°C, and less than 24 h at 37°C. As with other immunoassays the only way to determine if a result is affected by improper storage, other than a patient result that does not match clinical impression, is if an error message is reported. In most cases the DCA 2000+ reported an error in lieu of an unacceptable result (24 errors were reported), but in 14 cases a result that was outside of acceptable limits was reported.
The Tosoh G7 showed stability of WB at −20°C for 10 days, 4°C for 21 days, room temperature for 6 days, 30°C for 1 day, and less than 24 h at 37°C. Stability for the G8 was identical to that of the G7 except for storage at −20°C (7 days). As recommended by the manufacturer, unacceptable chromatograms were identified by the appearance of an unidentified extra peak before the HbA0 peak (generally noted as “P00” or “P01”). For both methods this extra peak generally began to appear when HbA1c results were still within acceptable limits, but more important is that there were no cases where an unacceptable result was accompanied by an acceptable chromatogram. Stability for the G7 was comparable to that observed in the previous study with the exception of 4°C where results were stable for 21 days versus at least 57 days in the prior study.5
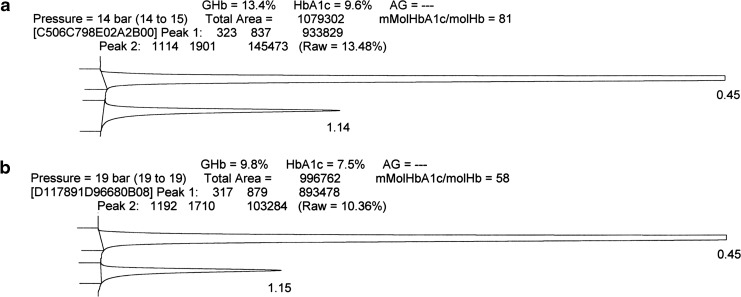
The Trinity Biotech ultra2 showed stability of WB at −20°C and 4°C for 42 days, room temperature for 9 days, 30°C for 3 days, and less than 24 h at 37°C. As previously seen with the prior generation CLC 330/385,5 ultra2 chromatograms generally did not clearly indicate when results were unacceptable (Fig. 4b), thus making likely that unacceptable HbA1c results could be reported. Of note is that the stability for the ultra2 was not as good as observed with the CLC 330/385 (e.g., 42 vs. 57 days at −20°C and 4°C and 9 vs. 14 days at room temperature).5
FIG. 4.
Trinity Biotech ultra2 chromatograms from (a) a sample with an acceptable result and (b) a sample with an unacceptable result. The first large peak includes the non-glycated hemoglobin (Hb); the second smaller peak includes the glycated Hb (GHb). There is no obvious difference between (a) and (b).
The Bio-Rad Variant II NU showed stability of WB at −20°C for 6 days, 4°C for 14 days, room temperature for 4 days, less than 24 h at 30°C, and 1 day at 37°C. According to the manufacturer, a result should not be reported if the area of the peak between the HbA1c and HbA0 peaks (specifically “P3” or “P4”) exceeds 10%. In most cases unacceptable results (n=107) were accompanied by unacceptable chromatograms, but there were 14 instances of unacceptable results with acceptable chromatograms. Stability for the Variant II NU was not as good as for the Variant II in the previous study at −20°C (6 vs. 28 days), 4°C (14 vs. at least 57 days), or room temperature (4 vs. 7 days).5
Discussion
Boronate-affinity methods for measuring HbA1c have historically shown better stability compared with ion-exchange HPLC methods where hemoglobin adducts can influence the charge of the hemoglobin molecule and adversely affect peak separation.5 We found this to be the case with the ultra2 versus the G7 and G8, especially at −20°C and 4°C. However, G7 and G8 chromatograms clearly indicate degradation that may be affecting the HbA1c result, whereas this is not always the case with the ultra2.
Most routine clinical samples are stored properly (usually at 4°C) and delivered to the laboratory within a short period of time; therefore specimen storage is not usually an issue regardless of the assay method used to measure HbA1c. However, for clinical trials and research studies samples may be collected and shipped off-site, and the conditions specimens are exposed to prior to arrival in the laboratory cannot be determined with confidence. Specimens shipped promptly overnight at 4°C on cold packs or frozen at −70°C or colder and shipped on dry ice should be acceptable as long as they are packed with sufficient coolant and are stored properly by the receiving laboratory prior to analysis. When shipping at ambient temperature, there is always the risk that samples have been exposed to very high temperatures, and stability cannot be assumed based on room temperature stability data.
The DCA 2000+ demonstrated the best stability of the methods tested at −20°C. At 4°C the DCA 2000+ demonstrated better stability than the G7 and G8, although stability was not as good as that observed with the ultra2. It is important to note that with the DCA 2000+ there is a chance of reporting an inaccurate result for a specimen that has been exposed to adverse storage conditions. However, given that this method is mainly used for point-of-care, specimen storage is generally not a concern. Chromatograms for the G7 and G8 methods clearly showed evidence of possible degradation that could affect the HbA1c result. For the ultra2 and Variant II NU methods, unacceptable results could not always be reliably detected.
The only method for which HbA1c results showed greater stability at −20°C compared with 4°C was the DCA 2000+. Stability at these two temperatures was equivalent for the ultra2, and 4°C storage was preferable to −20°C for the ion-exchange methods tested; these findings were in accordance with those of our previous study.5 Exposure of samples to elevated temperatures (above room temperature) should be avoided regardless of the assay method used to measure HbA1c, and specimens should be kept frozen at −70°C or colder whenever long-term storage is required.
Author Disclosure Statement
No competing financial interests exist for any authors of this article.
References
- 1.Little RR. England JD. Wiedmeyer HM. Goldstein DE. Effects of whole blood storage on results for glycosylated hemoglobin as measured by ion-exchange chromatography, affinity chromatography and colorimetry. Clin Chem. 1983;29:1113–1115. [PubMed] [Google Scholar]
- 2.Little RR. England JD. Wiedmeyer HM. Goldstein DE. Glycosylated hemoglobin measured by affinity chromatography: micro-sample collection and room-temperature storage. Clin Chem. 1983;29:1080–1082. [PubMed] [Google Scholar]
- 3.Papadea C. Austin GE. Mullins RE. The effect of storage conditions on ion exchange and affinity chromatographic assays for glycated hemoglobin. Clin Biochem. 1984;17:296–301. doi: 10.1016/s0009-9120(84)90589-7. [DOI] [PubMed] [Google Scholar]
- 4.Youngman LD. Clark S. Manley S. Peto R. Collins R. Reliable measurement of glycated hemoglobin in frozen blood samples: implications for epidemiologic studies. Clin Chem. 2002;48:1627–1629. [PubMed] [Google Scholar]
- 5.Little RR. Rohlfing CL. Tennill AL. Connolly S. Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther. 2007;9:36–42. doi: 10.1089/dia.2006.0055. [DOI] [PubMed] [Google Scholar]
- 6.Jones W. Scott J. Leary S. Stratton F. Smith S. Jones R. Day A. Ness A. ALSPAC Study Team: Stability of whole blood at −70°C for measurement of hemoglobin A1c in healthy individuals. Clin Chem. 2004;50:2460–2461. doi: 10.1373/clinchem.2004.038521. [DOI] [PubMed] [Google Scholar]