Abstract
Background
Subfertility due to anovulation is a common problem in women. First‐line oral treatment is with antioestrogens such as clomiphene citrate, but resistance may be apparent with clomiphene. Alternative and adjunctive treatments have been used including tamoxifen, dexamethasone, and bromocriptine. The effectiveness of these is to be determined.
Objectives
To determine the relative effectiveness of antioestrogen agents including clomiphene alone or in combination with other medical therapies in women with subfertility associated with anovulation, possibly caused by polycystic ovarian syndrome.
Search methods
We conducted a search of the Cochrane Gynaecology and Fertility Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, and CINAHL (all from inception to August 2016) to identify relevant randomised controlled trials (RCTs). We searched the United Kingdom National Institute for Clinical Excellence (NICE) guidelines and the references of relevant reviews and RCTs. We also searched the clinical trial registries for ongoing trials (inception until August 2016).
Selection criteria
We considered RCTs comparing oral antioestrogen agents for ovulation induction (alone or in conjunction with medical therapies) in anovulatory subfertility. We excluded insulin‐sensitising agents, aromatase inhibitors, and hyperprolactinaemic infertility.
Data collection and analysis
Two review authors independently performed data extraction and quality assessment. The primary outcome was live birth; secondary outcomes were pregnancy, ovulation, miscarriage, multiple pregnancy, ovarian hyperstimulation syndrome, and adverse effects.
Main results
This is a substantive update of a previous review. We identified an additional 13 studies in the 2016 update. The review now includes 28 RCTs (3377 women) and five RCTs awaiting classification. Five of the 28 included trials reported live birth/ongoing pregnancy. Secondary outcomes were poorly reported.
The quality of the evidence ranged from low to very low. The primary reasons for downgrading the evidence were imprecision and risk of bias associated with poor reporting.
Antioestrogen versus placebo
Live birth rate, miscarriage rate, multiple pregnancy rate, and ovarian hyperstimulation syndrome (OHSS)
No data were reported for these outcomes.
Clinical pregnancy rate
Clomiphene citrate was associated with an increased chance of a clinical pregnancy compared with placebo, though the size of the benefit was very uncertain (odds ratio (OR) 5.91, 95% confidence interval (CI) 1.77 to 19.68; 3 studies; 133 women; low‐quality evidence). If the chance of a clinical pregnancy was 5% in the placebo group, then between 8% and 50% of women would have a clinical pregnancy in the clomiphene group.
Clomiphene citrate versus tamoxifen
Live birth rate
There was no clear evidence of a difference in the chance of a live birth between the clomiphene citrate and tamoxifen groups (OR 1.24, 95% CI 0.59 to 2.62; 2 studies; 195 women; low‐quality evidence). If 20% of women in the tamoxifen group had a live birth, then between 13% and 40% of women in the clomiphene citrate group would have a live birth.
Miscarriage rate
There was no clear evidence of a difference in the chance of a miscarriage between the clomiphene citrate and tamoxifen groups (OR 1.81, 95% CI 0.80 to 4.12; 4 studies; 653 women; low‐quality evidence). If 3% of women in the tamoxifen group had a miscarriage, then between 2% and 10% in the clomiphene citrate group would have a miscarriage.
Clinical pregnancy rate
There was no clear evidence of a difference in the chance of a clinical pregnancy between the clomiphene citrate and tamoxifen groups (OR 1.30, 95% CI 0.92 to 1.85; 5 studies; 757 women; I2 = 69%; low‐quality evidence). If 22% of women in the tamoxifen group had a clinical pregnancy, then between 21% and 35% in the clomiphene citrate group would have a clinical pregnancy.
Multiple pregnancy rate
There was insufficient evidence of a difference in the chance of a multiple pregnancy between the clomiphene citrate group (OR 2.34, 95% CI 0.34 to 16.04; 3 studies; 567 women; very low‐quality evidence). If 0% of women in the tamoxifen group had a multiple pregnancy, then between 0% and 0.5% of women in the clomiphene group would have a multiple pregnancy.
OHSS
There were no instances of OHSS in either the clomiphene citrate or the tamoxifen group reported from three studies.
Clomiphene citrate with tamoxifen versus tamoxifen alone
Clinical pregnancy rate
There was insufficient evidence to determine whether there was a difference between groups (OR 3.32, 95% CI 0.12 to 91.60; 1 study; 20 women; very low‐quality evidence). No data were reported for the other outcomes.
Other comparisons of interest
Limited evidence suggested that compared with a gonadotropin, clomiphene citrate was associated with a reduced chance of a pregnancy, ongoing pregnancy, or live birth, with no clear evidence of a difference in multiple pregnancy rates.
The comparison of clomiphene citrate plus medical adjunct versus clomiphene alone was limited by the number of trials reporting the comparison and poor reporting of clinical outcomes relevant to this systematic review and by the number of adjuncts reported (ketoconazole, bromocriptine, dexamethasone, combined oral contraceptive, human chorionic gonadotropin, hormone supplementation). The addition of dexamethasone or combined oral contraceptive suggested a possible benefit in pregnancy outcomes, but findings were very uncertain and further research is required to confirm this.
There was limited evidence suggesting that a 10‐day regimen of clomiphene citrate improves pregnancy outcomes compared with a 5‐day regimen. Data for early versus late regimens of clomiphene citrate were insufficient to be able to make a judgement on differences for pregnancy outcomes.
Authors' conclusions
We found evidence suggesting that clomiphene citrate improves the chance of a clinical pregnancy compared with placebo, but may reduce the chance of live birth or ongoing pregnancy when compared with a gonadotropin. Due to low event rates, we advise caution interpreting these data.
The comparison of clomiphene citrate plus medical adjunctive versus clomiphene alone was limited by the number of trials reporting the comparison. The evidence was very low quality and no firm conclusions could be drawn, but very limited evidence suggested a benefit from adjunctive dexamethasone or combined oral contraceptives. Low‐quality evidence suggested that a 10‐day regimen of clomiphene citrate improves pregnancy rates compared with a 5‐day regimen, but further research is required.
Plain language summary
Clomiphene and other antioestrogens for subfertility associated with anovulation
Review question
Do antioestrogens including clomiphene improve fertility in women with anovulation associated with polycystic ovary syndrome?
Background
Subfertility due to the absence of ovulation is a common problem in women. Medical treatment may help these women ovulate. For example, oral antioestrogens such as clomiphene cause increased stimulation of the ovaries and aid ovulation.
Study characteristics
We added 13 new studies in the 2016 update, and the review now includes 28 trials (3377 women). Five of the 28 included trials reported live birth. Miscarriage, multiple pregnancy rates, and adverse events such as ovarian hyperstimulation syndrome were poorly reported. The evidence is current to August 2016.
Key results
We found evidence suggesting that clomiphene citrate improves the chance of a clinical pregnancy compared with placebo.
There was no evidence of a difference between clomiphene and tamoxifen, a similar antioestrogen drug. Women treated with clomiphene citrate were less likely to get pregnant or have a live baby compared with women who had received gonadotropins; there was no evidence for a difference in the chance of a multiple pregnancy. The numbers of women getting pregnant in these trials were very small, therefore we cannot be certain of the results.
Both dexamethasone (a steroid) and combined oral contraceptives are used to supplement clomiphene and show promise, but more studies are needed to confirm this. Few studies reported beyond the establishment of early pregnancy; given the reported risks of miscarriage with clomiphene treatment, no definitive conclusions can be drawn about effective treatment. We found evidence suggesting that a 10‐day regimen of clomiphene citrate improved pregnancy outcomes when compared with a 5‐day regimen, although the volume of data is limited and further research is required. There were insufficient data reported for early versus late regimens of clomiphene citrate to be able to make a judgement on differences for pregnancy outcomes.
Quality of the evidence
The quality of the evidence ranged from low to very low. The primary reasons for downgrading evidence were imprecision and risk of bias.
Summary of findings
Background
Description of the condition
Anovulation and oligo‐ovulation are estimated to be the cause 21% of female infertility. The World Health Organization (WHO) splits the causes into the following three categories (NICE 2013).
Group 1: hypothalamic pituitary failure or hypogonadotropic hypogonadism, accounting for around 10% of ovulatory disorders
Group 2: hypothalamic pituitary dysfunction or eugonadotropic, 85% of ovulatory disorders
Group 3: ovarian failure or hypergonadotropic hypogonadism, 4% to 5% of ovulatory disorders
Group 2 is the subject of this review. This group consists predominantly of women with polycystic ovary syndrome (PCOS) but may also include women with hyperprolactinaemia and those with unexplained anovulation. PCOS is a common condition of uncertain aetiology occurring in 4% to 7% of women of reproductive age (Lobo 2000). The syndrome was first described in 1935 and was first known as Stein‐Leventhal syndrome. In the past the diagnostic criteria for PCOS have varied. A recent consensus meeting between the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine decided on the criteria, based upon majority opinion and not clinical trial data (ESHRE/ASRM 2003). Two of the following three factors are required for diagnosis of PCOS, with exclusion of other aetiologies such as congenital adrenal hyperplasia, androgen‐secreting tumours, hyperprolactinaemia, and Cushing's syndrome:
oligo‐ovulation or anovulation;
clinical or biochemical signs of hyperandrogenism, or both;
polycystic ovaries as seen on ultrasound scanning.
Common symptoms and signs of PCOS include hirsutism, acne, irregular menstrual bleeding, and obesity. Investigations of women with PCOS may show raised luteinising hormone (LH) and free testosterone levels. Features on ultrasound scanning are enlarged ovaries (volume greater than 10 mL) or equal to or greater than 12 follicles 2 mm to 9 mm or greater in size diffusely distributed on one or both ovaries, or both (ESHRE/ASRM 2003). Women with PCOS may be at increased risk of pregnancy loss and complications and endometrial carcinoma. Their cardiovascular risk is also raised due to an increased risk of type 2 diabetes mellitus, hypertension, and altered serum lipid profiles (Fauser 2012; Hart 2015; Lobo 2000). Women with PCOS are more likely to be diagnosed with infertility and to undergo in vitro fertilisation (Hart 2015).
During normal menstruation, oestrogen levels are low, while follicle‐stimulating hormone (FSH) and LH levels begin to rise. This stimulates the development of an ovarian follicle which produces androgens (male sex hormones), some of which are bound to sex hormone binding globulin and some of which circulate freely in the bloodstream. Some androgens are converted to oestrogens. This causes a rise in the level of oestrogen, which in turn causes a fall in FSH and LH levels. The oestrogen levels continue to rise, eventually causing an LH surge, which triggers ovulation. Following ovulation a corpus luteum is formed which produces progesterone as well as oestrogen. The purpose of the corpus luteum is to prepare the endometrium for embryo implantation and for the maintenance of early pregnancy.
In PCOS there is a state of chronic anovulation characterised by small ovarian cysts, elevated ovarian production of androgens, and sometimes hypersecretion of LH. PCOS is the most common cause of anovulatory infertility. With the new criteria being wider than previously accepted definitions, its diagnosis is even more frequent (ESHRE/ASRM 2003).
Hyperprolactinaemia (which is included in the WHO group 2 category) is not included in this review.
Description of the intervention
A number of treatment options, used alone or in conjunction with other medical therapies, are available for the treatment of subfertility associated with anovulation.
Clomiphene citrate and tamoxifen
Medical ovulation induction with clomiphene citrate is currently the first‐line treatment for anovulatory women. Clomiphene citrate is an antioestrogen and competes for receptor‐binding sites with endogenous oestrogens. Recently published United Kingdom National Institute for Clinical Excellence (NICE) guidelines state that first‐line treatment for WHO group 2 anovulation should be clomiphene citrate (or tamoxifen) for up to 12 months (NICE 2013). The recommended daily dose of clomiphene citrate is 50 mg to 100 mg with a maximum of 250 mg. However, clomiphene resistance (failure to ovulate after taking clomiphene) is common, occurring in approximately 15% to 40% of women with PCOS (Kousta 1997; Pritts 2002; Wolf 2000). Definitions of clomiphene resistance vary, but the NICE definition is: "Anovulatory women who do not ovulate while receiving the 150 mg dose of clomiphene citrate" (NICE 2013). Resistance is associated with an increased body mass index, and weight loss programmes improve the success rates of clomiphene citrate therapy (Kousta 1997). Alternative and adjunctive treatments have been sought due to the high incidence of clomiphene resistance.
Dexamethasone as an adjunct
Addition of oral dexamethasone, a steroid hormone, to clomiphene citrate has been advocated in order to improve the chances of ovulation and subsequent pregnancy (Haas 2013).
Bromocriptine as an adjunct
Bromocriptine, a dopamine agonist used to treat hyperprolactinaemia, has been studied as an adjunctive treatment to clomiphene‐induced ovulation in anovulatory women with PCOS.
Aromatase inhibitors
The use of aromatase inhibitors (AIs) to treat anovulatory infertility is a new indication. Proponents of AIs believe that they are superior to, and safer than, clomiphene citrate. The latest form of these drugs ('third generation' anastrozole, letrozole, and exemestane) are currently being used as a treatment for breast cancer (Mitwally 2004). Aromatase inhibitors are not included in this review, as they are the subject of a separate review (Franik 2014).
CYP17a inhibitors
Ketoconazole is a CYP17a inhibitor. It inhibits a different part of the cytochrome P450 complex to AIs. Ketoconazole inhibits aromatase activity in the gonads (Hassan 2001; Parsanezhad 2003), and therefore may have similar effects to AIs with added antiandrogenic effects.
Metformin and other insulin‐sensitising agents alone or as an adjunct
A feature of PCOS is hyperinsulinaemia due to insulin resistance. This is thought to increase androgen production by the ovaries. Metformin and other insulin‐sensitising agents (e.g. troglitazone, rosiglitazone, pioglitazone, and D‐chiro‐inositol) are thought to help correct this and therefore increase ovulation and pregnancy rates in women with PCOS (Tang 2012). Use of insulin‐sensitising agents such as metformin are not included in this review, as they are the subject of a separate review (Tang 2012).
Gonadotropins
Gonadotropins are a long‐standing treatment for clomiphene‐resistant women. A variety of injectable drugs are available (human menopausal gonadotropins (hMG), urinary FSH, and recombinant FSH). These all have problems related to cost, risk of multiple pregnancy, and ovarian hyperstimulation syndrome (OHSS) (Weiss 2015).
Pulsatile gonadotropin‐releasing hormone (GnRH) is also sometimes used. This involves pulsatile GnRH infusion by intravenous or subcutaneous route using a portable pump. Cost and effect are likely similar to that of hMG treatment (Bayram 2004), but there may be a reduced risk of multiple pregnancy and OHSS (Tan 1996).
How the intervention might work
Clomiphene citrate and tamoxifen
By blocking receptors in the hypothalamus and pituitary, clomiphene citrate interferes with the feedback mechanism of endogenous oestrogen on the pituitary and hypothalamus. The result is an increase in FSH and LH secretion by the pituitary, which stimulates the production of ovarian follicles and ovulation. Estimates for numbers of women conceiving with clomiphene therapy vary from 30% to 50%, in Kousta 1997, to 15% (NICE 2013). Approximately 7% of pregnancies resulting from clomiphene‐induced ovulation are twin pregnancies, and 0.5% are triplet pregnancies (Wolf 2000). Miscarriage rates of 13% to 25% have been reported with clomiphene‐induced conceptions (Kousta 1997). This proportion may be higher than in women with normal fertility and unassisted conception, but this is uncertain (Haas 2013). A more advanced age may be responsible, and beyond that it is not possible to separate the adverse effects of treatment from the underlying process leading to subfertility. OHSS has been reported rarely following clomiphene citrate use. Tamoxifen has been used to induce ovulation but is used much less frequently than clomiphene citrate (Messinis 1982); its mode of action is similar to that of clomiphene citrate.
Dexamethasone as an adjunct
The proposed mechanism of action of dexamethasone in PCOS is suppression of the adrenal production of androgens, which should augment the action of clomiphene. It has also been suggested that dexamethasone may facilitate the growth of ovarian follicles by causing an increase in FSH levels. A third mechanism of action may be to reduce the high pulsatile levels of LH seen in PCOS and which contributes to anovulation (Brann 1991).
Bromocriptine as an adjunct
Dopamine can reduce elevated LH levels in PCOS and has also been reported to lead to a return in cyclical ovarian activity in normoprolactinaemic women with PCOS (Leblanc 1976; Siebel 1984).
Why it is important to do this review
We reviewed the available literature in an attempt to establish the effectiveness and complications of antioestrogen agents, alone or in combination with adjunctive treatments, in ovulation induction for women with anovulatory infertility.
This review has superseded the review on clomiphene citrate for ovulation induction (Hughes 1996), and covers WHO group 2 women (excluding hyperprolactinaemia).
Objectives
To determine the relative effectiveness of antioestrogen agents alone or in combination with other medical therapies in women with subfertility associated with anovulation, possibly caused by polycystic ovarian syndrome (PCOS).
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We did not include cross‐over trials unless phase‐one data were available.
Types of participants
Women of reproductive age with WHO group 2 anovulation. Anovulation was defined as a lack of evidence of serum progesterone in the luteal range for the reference laboratory or a failure of basal body temperature to rise by more than 0.4 ºC for 10 days or more. Age was as determined by trial authors.
Exclusion criteria
We excluded women with hyperprolactinaemia or Cushing's syndrome, or both, and trials which reported that women with these two conditions had been included. We excluded trials including women with WHO group 1 anovulation.
Types of interventions
The following interventions and comparisons were eligible for inclusion:
Antioestrogen versus no treatment or placebo
For example:
clomiphene citrate;
tamoxifen;
other.
Antioestrogen versus antioestrogen
For example:
clomiphene citrate versus tamoxifen;
clomiphene citrate versus other;
tamoxifen versus other;
other.
Antioestrogen versus gonadotropin
Follicle‐stimulating hormone (FSH)
Human menopausal gonadotropin (hMG)
Antioestrogen plus other medical therapy versus antioestrogen alone
For example:
dopamine agonist ‐ bromocriptine;
dopamine agonist ‐ cabergoline;
corticosteroid ‐ dexamethasone;
other.
Antioestrogen plus other medical therapy versus antioestrogen plus other medical therapy
We excluded trials utilising intrauterine insemination, as they are not relevant to the objective of this review. We included trials utilising natural intercourse or timed intercourse.
We did not include insulin‐sensitising agents such as metformin and aromatase inhibitors in this review, as they are the subject of separate reviews (El Daly 2006; Tang 2012).
Clomiphene citrate regimens
Regimen A versus Regimen B.
Types of outcome measures
Primary outcomes
Live birth/ongoing pregnancy rate (per woman).
Miscarriage rate (per woman), where miscarriage was defined as the involuntary loss of a pregnancy before 20 weeks gestation.
Secondary outcomes
Clinical pregnancy rate (per woman), where pregnancy was defined as evidence of intrauterine gestation on ultrasound; this includes pregnancies in the pre‐treatment phase.
Incidence of multiple pregnancy (per woman), where multiple pregnancy was defined as greater than one intrauterine pregnancy.
Incidence of ovarian hyperstimulation syndrome (OHSS) (per woman), defined according to the definition adopted by the reporting authors.
Incidence of women reported adverse effects (per woman), defined according to the definition of the reporting authors.
Search methods for identification of studies
This is a substantive update of the previous review, and we searched the following sources for relevant studies.
Electronic searches
We searched for all published and unpublished RCTs of clomiphene citrate and antioestrogens for ovulation induction in women with PCOS without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist (from database inception until 2 August 2016).
We searched the following electronic databases and trial registers on 2 August 2016.
(1) Cochrane Gynaecology and Fertility Group Specialised Register (Appendix 1), Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies Online) (Appendix 2), MEDLINE (Appendix 3), Embase (Appendix 4), PsycINFO (Appendix 5), and CINAHL (Appendix 6).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2, Chapter 6, 6.4.11)(Higgins 2011). The Embase, CINAHL, and PsycINFO searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/methodology/filters.html#random).
(2) We also searched the following trials registers to identify ongoing and registered clinical trials (17th August 2016).
ClinicalTrials.gov (a service of the US National Institutes of Health) (www.clinicaltrials.gov)
World Health Organization Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx).
We used the key words 'anovulation' and 'clomiphene citrate'.
Searching other resources
We handsearched the reference lists of included studies.
Data collection and analysis
Selection of studies
In the update of this review, the two review authors independently selected potentially eligible trials in accordance with the aforementioned criteria. We excluded trials from the systematic review if they made comparisons other than those prespecified above. Disagreements were resolved by discussion.
Data extraction and management
The two review authors independently extracted and verified study characteristics and outcome data from eligible studies using forms designed according to Cochrane guidelines. We sought additional information on trial methodology and actual trial data from the authors of six trial reports (Boonstanfar 2001; Branigan 2003; Hassan 2001; Parsanezhad 2002a; Parsanezhad 2002b; Vegetti 1999), but received no reply. We were unable to contact the authors of five trial reports (Cudmore 1966; Daly 1984; Garcia 1985; Johnson 1966; Suginami 1993). Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references.
Pregnancies that occurred in the pre‐treatment phase were included as a success in the analysis.
Assessment of risk of bias in included studies
The two review authors independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool, which addresses the following domains: selection bias (randomisation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias (Higgins 2011). Disagreements were resolved through discussion. We have fully described all judgements and summarised our conclusions in the 'Risk of bias' table in the Characteristics of included studies.
Measures of treatment effect
For dichotomous data (all of the outcome measures in this review), we used the numbers of events in the intervention and control groups of each study to calculate the Mantel‐Haenszel odds ratios. We presented 95% confidence intervals for all outcomes. Where data to calculate odds ratios were not available, we utilised the most detailed numeric data available that could facilitate similar analyses of included studies.
Unit of analysis issues
The primary analysis was per woman randomised. Per‐cycle data were not pooled, but if reported were included in an additional table. Where per‐cycle data were reported, we contacted the authors of the primary study and requested per‐woman randomised data. We counted multiple live birth such as twins and higher‐order births as a single live birth event. We included only the first arm of cross‐over trials in a pooled analysis.
Dealing with missing data
Where possible, we analysed the data on an intention‐to‐treat basis, and attempted to contact the original study authors for missing data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the I2 statistic, taking an I2 value above 50% to indicate substantial heterogeneity (Higgins 2002).
Assessment of reporting biases
In the view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and being alert for duplication of data. We had planned that if there were 10 or more trials in an analysis, we would produce a funnel plot to explore the possibility of small‐study effects. We were unable to make this assessment in this update of the review. In future updates we will seek to explore publication bias where sufficient trials are available.
Data synthesis
Where studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons.
-
Antioestrogen versus placebo
Clomiphene citrate versus placebo
-
Antioestrogen versus antioestrogen
Clomiphene citrate versus tamoxifen
Clomiphene citrate plus tamoxifen versus clomiphene
-
Antioestrogen versus gonadotropin
Clomiphene citrate versus FSH
Clomiphene citrate versus hMG
-
Antioestrogen plus medical adjunct versus antioestrogen alone
Clomiphene citrate plus ketaconazole versus clomiphene
Clomiphene citrate plus bromocriptine versus clomiphene
Clomiphene citrate plus dexamethasone versus clomiphene
Clomiphene citrate plus combined oral contraceptive versus clomiphene
Clomiphene citrate plus human chorionic gonadotropin versus clomiphene
Clomiphene citrate plus hormone supplement versus clomiphene
-
Clomiphene citrate regimens
Clomiphene citrate 5 days versus clomiphene citrate 10 days
Early clomiphene citrate versus late clomiphene citrate
Subgroup analysis and investigation of heterogeneity
If we detected substantial heterogeneity, we tried to explain it through subgroup analysis by comparing specific regimens (drug doses) where data were available.
Sensitivity analysis
We did not conduct any sensitivity analyses in this review update. In future updates we will conduct sensitivity analyses if there is evidence of substantial statistical heterogeneity. We will conduct sensitivity analysis on the primary outcome measure of live birth. We included studies with adequate evidence of allocation concealment. We undertook a random‐effects analysis to assess sensitivity to choice of model.
'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro GDT software for the main comparisons of the review (GRADEpro GDT 2014). The two review authors independently evaluated the overall quality of the evidence for the main outcomes of the review (live birth rate, miscarriage rate, clinical pregnancy rate, multiple pregnancy rate, and OHSS per woman randomised) using GRADE criteria (risk of bias, consistency, imprecision, indirectness, publication bias) (Atkins 2004).
We included 'Summary of findings' tables for the following comparisons.
Antioestrogen versus placebo
Antioestrogen versus antioestrogen
Antioestrogen plus medical adjunct versus antioestrogen alone
Antioestrogen regimens
The remaining comparisons of antioestrogen versus gonadotropin is discussed within the text of the review.
Timeline
The review authors intend that a new search for RCTs will be performed every two years and the review updated accordingly.
Results
Description of studies
Results of the search
See Characteristics of included studies and Characteristics of excluded studies.
The previous version of this review included 15 trials.
The searches in the 2016 review update resulted in the retrieval of 41 full‐text papers (Figure 1). We included 13 new studies (Characteristics of included studies). We excluded 25 studies (Characteristics of excluded studies). Two studies are awaiting classification, as it is unclear if intrauterine insemination was used, which is an exclusion criterion (Craig 2015; Neuhausser 2011); we have contacted the authors and await a response. We moved one study from the excluded studies to the included studies, as it was eligible for the new comparison of antioestrogen versus gonadotropin (Badawy 2008).
1.
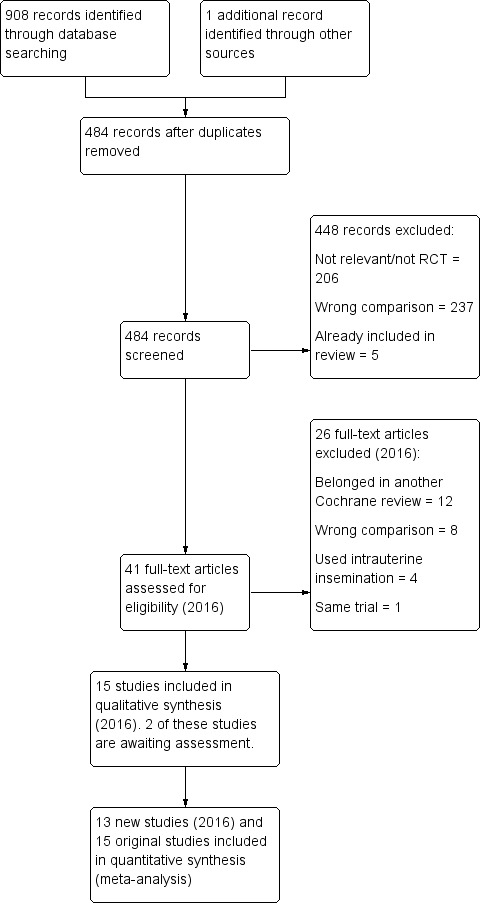
Study flow diagram for update 2016.
Included studies
We included a total of 28 studies in this 2016 update of the systematic review. Thirteen new studies were included (Badawy 2009; Badawy 2011; Dehbashi 2006; Elsedeek 2014; Esmaeilzadeh 2011; Ghafourzadeh 2004; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Omran 2011; Seyedoshohadaei 2012; Tripathy 2013). We moved one study from the excluded studies to the included studies, as it was eligible for the new comparison of antioestrogen versus gonadotropin (Badawy 2008).
Design
Twenty‐four studies were parallel‐design RCTs (Badawy 2008; Badawy 2009; Badawy 2011; Boonstanfar 2001; Branigan 2003; Branigan 2005; Daly 1984; Dehbashi 2006; Elkind‐Hirsch 2005; Elnashar 2006; Elsedeek 2014; Esmaeilzadeh 2011; Ghafourzadeh 2004; Hassan 2001; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Omran 2011; Parsanezhad 2002a; Parsanezhad 2002b; Seyedoshohadaei 2012; Tripathy 2013; Vegetti 1999; Yilmaz 2006), and four studies were cross‐over trials where phase‐one data were available (Cudmore 1966; Garcia 1985; Johnson 1966; Suginami 1993).
Setting
A variety of different settings were used to recruit women into the studies.
Not stated (Boonstanfar 2001; Cudmore 1966; Daly 1984; Hassan 2001; Homburg 2012; Johnson 1966; Omran 2011; Suginami 1993).
Infertility clinic (Branigan 2003; Branigan 2005; Elsedeek 2014; Ghafourzadeh 2004; Lopez 2004; Moslemizadeh 2008; Vegetti 1999; Yilmaz 2006).
Outpatient department (Badawy 2008; Badawy 2009; Badawy 2011; Elnashar 2006; Tripathy 2013).
Department of obstetrics and gynaecology (Garcia 1985).
Division of reproductive endocrinology (Parsanezhad 2002a; Parsanezhad 2002b).
Women's health institute (Elkind‐Hirsch 2005).
Infertility and reproductive health centre/infertility research centre (Dehbashi 2006; Esmaeilzadeh 2011).
Private clinic (Seyedoshohadaei 2012).
Country
The included studies were conducted in the following countries.
Turkey (Yilmaz 2006).
USA and Canada (Boonstanfar 2001; Branigan 2003; Branigan 2005; Cudmore 1966; Daly 1984; Elkind‐Hirsch 2005; Garcia 1985; Johnson 1966).
Japan (Suginami 1993).
Italy (Vegetti 1999).
Iran (Dehbashi 2006; Esmaeilzadeh 2011; Ghafourzadeh 2004; Moslemizadeh 2008; Parsanezhad 2002a; Parsanezhad 2002b; Seyedoshohadaei 2012).
Egypt (Badawy 2008; Badawy 2009; Badawy 2011; Elnashar 2006; Elsedeek 2014; Hassan 2001; Omran 2011).
India (Tripathy 2013).
Spain (Lopez 2004).
Multicentre (Homburg 2012).
Participants
The women ranged in age from 18 to 39 years. Daly 1984 and Omran 2011 did not state age.
Cycles of treatment
The number of treatment cycles ranged from one to six‐plus in the included trials, however in some trials this was not stated.
Not stated (Badawy 2008; Boonstanfar 2001; Daly 1984; Ghafourzadeh 2004; Omran 2011; Vegetti 1999).
One (Badawy 2011; Branigan 2005; Elkind‐Hirsch 2005; Elnashar 2006; Elsedeek 2014; Esmaeilzadeh 2011; Johnson 1966; Moslemizadeh 2008; Suginami 1993; Yilmaz 2006).
Up to two (Dehbashi 2006).
Up to three (Cudmore 1966; Homburg 2012; Lopez 2004; Tripathy 2013).
Up to four (Badawy 2009).
One to five (Garcia 1985).
Six or more, or to pregnancy (Branigan 2003; Parsanezhad 2002a).
Three to six (Hassan 2001).
Inclusion criteria
The main inclusion criteria reported in the trials are listed. Anovulatory PCOS was the principal inclusion criterion.
Anovulatory (Boonstanfar 2001; Branigan 2003; Cudmore 1966; Daly 1984; Garcia 1985; Homburg 2012; Johnson 1966; Lopez 2004; Seyedoshohadaei 2012; Suginami 1993; Vegetti 1999).
PCOS (Badawy 2008; Badawy 2011; Branigan 2005; Elnashar 2006; Hassan 2001; Lopez 2004; Moslemizadeh 2008; Tripathy 2013).
Insulin resistance (Hassan 2001).
Secondary amenorrhoea (longer than two years) or oligomenorrhoeic (Cudmore 1966; Daly 1984; Elkind‐Hirsch 2005; Yilmaz 2006).
No previous exposure to clomiphene or ovulation induction (Daly 1984; Yilmaz 2006).
No fertility treatment in previous three months (Cudmore 1966).
No other causes of infertility (Boonstanfar 2001; Branigan 2003; Cudmore 1966; Tripathy 2013; Yilmaz 2006).
Clomiphene‐resistant PCOS (Elsedeek 2014; Esmaeilzadeh 2011; Ghafourzadeh 2004; Parsanezhad 2002a).
Normoprolactinaemia (Suginami 1993; Tripathy 2013; Yilmaz 2006).
Tubal patency (Badawy 2008; Badawy 2011; Branigan 2003; Homburg 2012; Moslemizadeh 2008; Seyedoshohadaei 2012; Tripathy 2013).
Specified ages (Branigan 2003; Branigan 2005; Elkind‐Hirsch 2005; Elnashar 2006; Homburg 2012; Lopez 2004; Yilmaz 2006).
No medication for previous two months (Elnashar 2006).
Duration of primary infertility longer than two years (Elnashar 2006; Yilmaz 2006).
Normal semen analysis (Badawy 2008; Badawy 2011; Branigan 2005; Dehbashi 2006; Ghafourzadeh 2004; Homburg 2012; Lopez 2004; Seyedoshohadaei 2012; Yilmaz 2006).
Normal results on hysterosalpingogram (Branigan 2005; Dehbashi 2006; Ghafourzadeh 2004; Seyedoshohadaei 2012; Yilmaz 2006).
Normal endocrine function (Branigan 2005; Dehbashi 2006; Elnashar 2006; Lopez 2004; Seyedoshohadaei 2012; Yilmaz 2006).
Body mass index between 18 and 38 (Elkind‐Hirsch 2005), body mass index 20 to 30 kg/m2 (Tripathy 2013).
Comorbid disease (tuberculosis, abnormal glucose tolerance test) (Tripathy 2013).
No history of pelvic surgery or pelvic inflammatory disease (Lopez 2004).
No details (Badawy 2009; Omran 2011).
Interventions
Antioestrogen versus no treatment or placebo
Clomiphene citrate versus placebo
Three trials compared clomiphene citrate to placebo (Cudmore 1966; Garcia 1985; Johnson 1966), all of which were of cross‐over design (phase‐one data only). Doses varied from a 50 mg fixed dose to a variable dose of up to 250 mg (dependent on ovulatory response). Phase one of the trials lasted from one to five cycles. The total number of women was 133, 63 randomised to the control group and 70 to the treatment group.
Antioestrogen versus antioestrogen
Clomiphene citrate versus tamoxifen
Five trials compared clomiphene citrate to tamoxifen (Badawy 2011; Boonstanfar 2001; Moslemizadeh 2008; Seyedoshohadaei 2012; Vegetti 1999). In the Boonstanfar 2001 and Vegetti 1999 trials, the doses of clomiphene citrate ranged from 50 mg to 200 mg, as both trials varied dose dependent on ovulatory response. The Seyedoshohadaei 2012 trial used an initial dose of 50 mg, increasing by 50 mg per cycle to a maximum of 150 mg. The Badawy 2011 and Moslemizadeh 2008 trials used a dose of 100 mg daily. In the Boonstanfar 2001 and Vegetti 1999 trials, the doses of tamoxifen ranged from 20 mg to 60 mg, again as both trials varied the dose. The Badawy 2011 and Moslemizadeh 2008 trials used a dose of 20 mg of tamoxifen per day. The Seyedoshohadaei 2012 trial used an initial dose of 10 mg per day, increasing by 10 mg per cycle to a maximum of 30 mg per day. Boonstanfar 2001 and Vegetti 1999 did not state duration of treatment. Badawy 2011 and Moslemizadeh 2008 treated women for a single cycle. The total number of cycles of treatment was between 91 and 129 for women on clomiphene citrate and between 113 and 133 for women on tamoxifen. The Boonstanfar 2001 trial appears to have continued after publication in 2001; an abstract of a larger trial was published in 2002 that appears to include the women from Boonstanfar 2001. We have excluded this abstract from analysis while awaiting author clarification. A total of 657 women participated, of which 332 were randomised to clomiphene treatment and 325 to tamoxifen.
Clomiphene citrate plus tamoxifen versus clomiphene citrate
Suginami 1993 compared clomiphene citrate plus tamoxifen to clomiphene citrate alone. The trial was of cross‐over design with phase‐one data available. The dose of clomiphene citrate was 100 mg when used alone and 50 mg when used in combination with 20 mg tamoxifen. Up to three cycles of treatment were given in the first phase. Of the 20 participants, 10 were randomised to clomiphene citrate plus tamoxifen and 10 to clomiphene citrate alone.
Ghafourzadeh 2004 compared clomiphene citrate plus tamoxifen to clomiphene citrate alone in 100 women. The dose of clomiphene citrate was 100 mg when used alone and 50 mg when used in combination with 20 mg tamoxifen. The number of cycles of treatment was unclear.
Antioestrogen versus gonadotropin
Clomiphene citrate versus hMG
Badawy 2008 compared clomiphene citrate with hMG in 318 women. The dose of clomiphene citrate was 100 mg. The number of cycles of treatment was unclear.
Clomiphene citrate versus FSH
Homburg 2012 and Lopez 2004 compared clomiphene citrate with FSH in 378 women. In both trials the starting dose of clomiphene citrate was 50 mg, increasing to a maximum of 150 mg in subsequent cycles. Both trials used up to three cycles of treatment.
Antioestrogen plus other medical therapy versus antioestrogen alone
Clomiphene citrate plus bromocriptine versus clomiphene citrate
Parsanezhad 2002b and Tripathy 2013 compared clomiphene citrate plus bromocriptine to clomiphene citrate. In the Parsanezhad 2002b trial, the control group was given 200 mg clomiphene citrate and placebo continuously. The treatment group was given 200 mg clomiphene citrate plus 7.5 mg bromocriptine continuously. Both groups were administered human chorionic gonadotropin (hCG) (10,000 U) to trigger ovulation and were treated for up to six cycles. The dose of bromocriptine or placebo was gradually introduced before commencing clomiphene citrate. All 100 women had clomiphene‐resistant PCOS. In the Tripathy 2013 trial, the control group was given 50 mg of clomiphene citrate daily from Day 3 to Day 7. The treatment group was given clomiphene citrate 50 mg from Day 3 to Day 7 and bromocriptine 2.5 mg from Day 1 to Day 30. All of the women had a diagnosis of PCOS.
Clomiphene citrate plus dexamethasone versus clomiphene
Four trials compared clomiphene citrate plus dexamethasone to clomiphene citrate (Daly 1984; Elnashar 2006; Esmaeilzadeh 2011; Parsanezhad 2002a). The control groups were given 50 mg to 150 mg clomiphene citrate on Days 5 to 9 (Daly 1984); 200 mg clomiphene citrate on Days 5 to 9 and placebo from Day 5 to Day 14 (Parsanezhad 2002a); 100 mg clomiphene citrate on Days 3 to 7 and placebo from Days 3 to 12 (Elnashar 2006); or 100 mg clomiphene citrate on Days 3 to 7 and placebo from Days 5 to 14 (Esmaeilzadeh 2011). Treatment groups were given 50 mg to 150 mg clomiphene citrate plus 0.5 mg dexamethasone on Days 5 to 9 (Daly 1984); 200 mg clomiphene citrate on Days 5 to 9 plus 2 mg dexamethasone on Days 5 to 14 (Parsanezhad 2002a); 100 mg clomiphene citrate on Days 3 to 7 plus 2 mg dexamethasone on Days 3 to 12 (Elnashar 2006); or 100 mg of clomiphene citrate on Days 3 to 7 plus 2 mg dexamethasone on Days 5 to 14 (Esmaeilzadeh 2011). Parsanezhad 2002a and Elnashar 2006 administered hCG to both groups to trigger ovulation. Both groups were treated for up to six cycles in Parsanezhad 2002a and for only one cycle in Elnashar 2006 and Esmaeilzadeh 2011.
Clomiphene citrate plus ketoconazole versus clomiphene
Hassan 2001 compared clomiphene citrate plus ketoconazole versus clomiphene. The control group was given up to 150 mg clomiphene for three to six cycles. The treatment group was given 400 mg per day ketoconazole for 85 days and then 100 mg to 150 mg clomiphene for three to six cycles. In both groups "patients who persistently failed to respond to clomiphene 150 mg per day (clomiphene resistant) were shifted to hMG". The 97 women were all insulin resistant and had PCOS; 48 were randomised to the control group and 49 to the treatment group.
Clomiphene citrate plus combined oral contraceptive versus clomiphene citrate
Branigan 2003 compared clomiphene citrate plus combined oral contraceptive to clomiphene citrate. The control group had no treatment for 38 to 56 days (two cycles), in particular no progestin to induce menstruation, while the treatment group was given combined oral contraceptive (0.03 mg ethinyl estradiol and 0.15 mg desogestrel (Desogen)) continuously for 42 to 50 days. In the following cycle, each group received 100 mg clomiphene citrate on Days 5 to 9, with ovulation triggered by 10,000 U of hCG. Those women who ovulated but did not become pregnant in this cycle (from either group) repeated the clomiphene citrate dose for up to six cycles. It was unclear what treatment or follow‐up was provided to women who did not ovulate. The 51 participants were all clomiphene resistant; 25 were randomised to the control group and 26 to the treatment group.
Clomiphene citrate plus hCG versus clomiphene citrate alone
Two studies made this comparison (Branigan 2005; Yilmaz 2006). In the study by Branigan 2005, the experimental group received clomiphene citrate 100 mg daily on Days 5 to 9 with daily doses of 200 IU hCG intramuscularly; the control group received 150 mg clomiphene citrate daily on Days 5 to 9. Yilmaz 2006 administered 50 mg clomiphene citrate on Days 5 to 9 with 10,000 IU hCG administered when the follicle reached greater than 18 mm in diameter; the control group received clomiphene citrate only.
Clomiphene citrate plus hormone supplementation versus clomiphene citrate alone
Two trials made this comparison (Elkind‐Hirsch 2005; Moslemizadeh 2008). The control and experimental groups both received clomiphene citrate 100 mg daily on Days 3 to 7 in the Elkind‐Hirsch 2005 trial and on Days 3 to 9 in the Moslemizadeh 2008 trial. The experimental group received oral estradiol 1.5 mg twice daily commencing on Day 8 and discontinued when a LH surge was detected in the Elkind‐Hirsch 2005 trial. In the Moslemizadeh 2008 trial, 2 mg of estradiol was given daily from Day 8 to the hCG injection. A total of 167 women were randomised.
Clomiphene citrate regimen A versus clomiphene citrate regimen B
Two trials reported this comparison (Elsedeek 2014; Omran 2011). The trials compared clomiphene citrate 200 mg per day for 5 days with clomiphene citrate 100 mg per day for 10 days in women with clomiphene‐resistant PCOS.
Dehbashi 2006 compared clomiphene citrate 100 mg starting Day 1 of menstrual cycle for 5 days with clomiphene citrate 100 mg starting Day 5 of menstrual cycle for 5 days for a maximum of 3 cycles in 78 women with PCOS.
Badawy 2009 used an early (100 mg clomiphene citrate starting on the date after finishing medroxyprogesterone for five days) versus late (100 mg clomiphene citrate daily for five days starting on Day 3 of menses) regimen.
We found no RCTs for the following comparisons.
Tamoxifen versus placebo.
Any antioestrogen plus cabergoline versus antioestrogen.
Any antioestrogen plus medical adjunct versus antioestrogen plus medical adjunct.
Outcomes
Five trials reported live birth/ongoing pregnancy (Boonstanfar 2001; Elsedeek 2014; Homburg 2012; Lopez 2004; Seyedoshohadaei 2012).
Fifteen trials reported adverse events including miscarriage (Badawy 2008; Badawy 2009; Badawy 2011; Boonstanfar 2001; Branigan 2003; Cudmore 1966; Elkind‐Hirsch 2005; Elnashar 2006; Hassan 2001; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Seyedoshohadaei 2012; Vegetti 1999; Yilmaz 2006).
All of the trials reported pregnancy. Ghafourzadeh 2004 and Badawy 2008 reported a positive pregnancy test result and no data for any other pregnancy outcome measure (clinical pregnancy, ongoing pregnancy, live birth).
Thirteen trials reported incidence of multiple pregnancy (Badawy 2008; Badawy 2011; Boonstanfar 2001; Branigan 2003; Daly 1984; Elnashar 2006; Elsedeek 2014; Hassan 2001; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Seyedoshohadaei 2012; Yilmaz 2006).
Seven trials reported incidence of OHSS (Badawy 2008; Badawy 2011; Boonstanfar 2001; Lopez 2004; Moslemizadeh 2008; Seyedoshohadaei 2012; Suginami 1993).
Excluded studies
See Characteristics of excluded studies.
We excluded 37 initially identified trials from the review. Six of these were excluded in the 2016 update of the review (Dura 2015 (two publications); Kosar 2014; Moini 2015; Topcu 2010; Yari 2010). The primary reasons for exclusion of the studies were inclusion criteria and interventions.
Risk of bias in included studies
See Characteristics of included studies; Figure 2; Figure 3.
2.
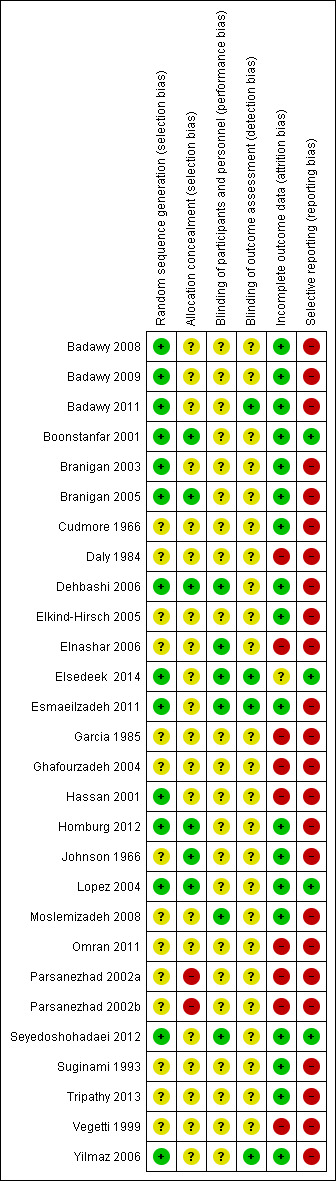
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
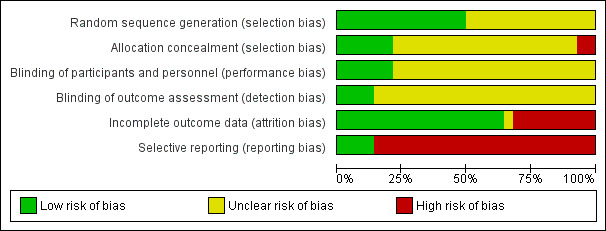
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
We judged 13 trials to be at low risk of bias for random sequence generation (Badawy 2008; Badawy 2009; Badawy 2011; Boonstanfar 2001; Branigan 2003; Branigan 2005; Dehbashi 2006; Elsedeek 2014; Esmaeilzadeh 2011; Hassan 2001; Homburg 2012; Lopez 2004; Seyedoshohadaei 2012; Yilmaz 2006). All 13 trials used random number tables. We judged random sequence generation to be unclear in the remaining studies due to inadequate details.
We judged only six trials to be at low risk of bias for allocation concealment (Boonstanfar 2001; Branigan 2005; Dehbashi 2006; Homburg 2012; Johnson 1966; Lopez 2004). We considered two trials to be at high risk of bias for allocation concealment, as allocation was conducted by a third party (pharmacist) using odd‐even numbers. The remaining studies were at unclear risk of bias for allocation concealment.
Blinding
Performance bias
We judged six studies that reported blinding of participants or personnel, or both to be at low risk of performance bias (Dehbashi 2006; Elnashar 2006; Elsedeek 2014; Esmaeilzadeh 2011; Moslemizadeh 2008; Seyedoshohadaei 2012).
The remaining studies provided insufficient detail to make a judgement and were considered to be at unclear risk of performance bias (Badawy 2008; Badawy 2009; Badawy 2011; Boonstanfar 2001; Branigan 2003; Branigan 2005; Cudmore 1966; Daly 1984; Elkind‐Hirsch 2005; Garcia 1985; Ghafourzadeh 2004; Hassan 2001; Homburg 2012; Johnson 1966; Lopez 2004; Omran 2011; Parsanezhad 2002a; Parsanezhad 2002b; Suginami 1993; Tripathy 2013; Vegetti 1999; Yilmaz 2006).
Detection bias
We judged four studies that reported blinding of outcome assessors to be at low risk of detection bias (Badawy 2011; Elsedeek 2014; Esmaeilzadeh 2011; Yilmaz 2006).
The remaining studies provided insufficient detail to make a judgement and were considered to be at unclear risk of detection bias (Badawy 2008; Badawy 2009; Boonstanfar 2001; Branigan 2003; Branigan 2005; Cudmore 1966; Daly 1984; Dehbashi 2006; Elkind‐Hirsch 2005; Elnashar 2006; Garcia 1985; Ghafourzadeh 2004; Hassan 2001; Homburg 2012; Johnson 1966; Lopez 2004; Moslemizadeh 2008; Omran 2011; Parsanezhad 2002a; Parsanezhad 2002b; Seyedoshohadaei 2012; Suginami 1993; Tripathy 2013; Vegetti 1999).
Incomplete outcome data
For the purposes of this review we defined a withdrawal as a woman who stopped taking the assigned trial drug but was followed up by the trial. We defined a loss to follow‐up as a woman who stopped participating in the trial and was not followed up. The number of dropouts was both these figures together. However, these terms are often used interchangeably by trial authors, without being defined.
Only Garcia 1985 and Esmaeilzadeh 2011 performed an intention‐to‐treat analysis; for Garcia 1985 the phase‐one data contained results for all but three women (who were lost to follow‐up). Thirteen studies reported no dropouts or all women randomised were analysed, or both (Badawy 2008; Badawy 2009; Badawy 2011; Cudmore 1966; Dehbashi 2006; Elnashar 2006; Esmaeilzadeh 2011; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Seyedoshohadaei 2012; Suginami 1993; Tripathy 2013); we considered these studies to be at low risk of attrition bias.
We considered a rate of less than 10% of women dropping out to be an acceptable attrition rate; six studies reported rates from 4.3% to 10% (Boonstanfar 2001; Branigan 2003; Branigan 2005; Elkind‐Hirsch 2005; Johnson 1966; Yilmaz 2006). We considered these studies to be at low risk of attrition bias.
A rate of more than 10% of women dropping out may be cause for concern. Three studies had high dropout rates: Daly 1984 (17%); Garcia 1985 (43%, though 94% of women were analysed in phase‐one data); and Hassan 2001 (21%). The reasons are detailed in the 'Risk of bias' tables. We considered these studies to be at high risk of attrition bias.
Five trials provided no details on attrition and were considered to be at high risk of bias (Ghafourzadeh 2004; Omran 2011; Parsanezhad 2002a; Parsanezhad 2002b; Vegetti 1999). Parsanezhad 2002b presented outcome rates as percentages; an attempt to calculate actual participant numbers from group sizes reported at randomisation indicated that women may have been lost to follow‐up. Elsedeek 2014 reported that 230 women were included in their study. Their power calculation required a minimum of 220 participants and they only report data for 220 participants. They do not explain how these 220 were selected from the 230 women included. We judged this study to be unclear risk of bias.
Selective reporting
Only five of 28 included trials reported on live birth (Boonstanfar 2001; Elsedeek 2014; Homburg 2012; Lopez 2004; Seyedoshohadaei 2012), and reporting of adverse effects was limited in all of the included trials.
There were differences in the number of cycles of treatment (one to six‐plus), and therefore the duration of follow‐up. This was detailed in a previous section of the review (see Characteristics of included studies for details).
The definitions used for some of the outcomes varied, which may have influenced reporting on PCOS, pregnancy, ovulation rate, and clomiphene resistance (see Characteristics of included studies for details).
We judged 21 trials in which no data were reported for live birth or outcomes were reported that were not prespecified, or both, to be at high risk of selective reporting bias (Badawy 2008; Badawy 2009; Badawy 2011; Branigan 2003; Branigan 2005; Cudmore 1966; Elkind‐Hirsch 2005; Elnashar 2006; Esmaeilzadeh 2011; Garcia 1985; Ghafourzadeh 2004; Hassan 2001; Homburg 2012; Johnson 1966; Moslemizadeh 2008; Omran 2011; Parsanezhad 2002a; Parsanezhad 2002b; Suginami 1993; Vegetti 1999; Yilmaz 2006).
One study did not prespecify or define outcomes (Tripathy 2013), and two studies did not prespecify any outcomes (Daly 1984; Dehbashi 2006); we judged these studies to be at high risk of selective reporting bias.
Other potential sources of bias
None of the trials performed compliance monitoring to assess adherence to the treatment regimen.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Antioestrogen versus placebo.
| Antioestrogen versus placebo | ||||||
|
Patient or population: ovulation induction in polycystic ovarian syndrome Setting: USA/Canada. 1 trial took place in a department of obstetrics and gynaecology; details of setting for 2 trials not provided. Intervention: antioestrogen Comparison: no treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo | Risk with antioestrogen | |||||
| Live birth rate ‐ not reported | See comment | See comment | Not estimable | ‐ | ‐ | No data for live birth reported for this comparison |
| Miscarriage rate ‐ not reported | See comment | See comment | Not estimable | ‐ | ‐ | No data for miscarriage reported for this comparison |
| Clinical pregnancy rate | 48 per 1000 | 228 per 1000 (81 to 496) | OR 5.91 (1.77 to 19.68) | 133 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Low event rates and small sample size observed in included trials |
| Multiple pregnancy rate ‐ not reported | See comment | See comment | Not estimable | ‐ | ‐ | No data for multiple pregnancy reported for this comparison |
| Ovarian hyperstimulation syndrome (OHSS) ‐ not reported | See comment | See comment | Not estimable | ‐ | ‐ | No data for OHSS reported for this comparison |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Wide confidence intervals with low event rates and small sample size suggest imprecision ‐ downgraded one level. 2There was insufficient detail for multiple aspects of risk of bias to be able to make a judgement in any of the included studies, none of the studies reported on live birth ‐ downgraded one level.
Summary of findings 2. Antioestrogen versus antioestrogen.
| Antioestrogen versus antioestrogen | ||||||
|
Patient or population: ovulation induction in polycystic ovarian syndrome Setting: Egypt, USA/Canada, Iran (2 trials), Italy. Trials conducted in outpatient department, infertility clinic (2 trials), private clinic, and 1 trial did not report setting. Intervention: antioestrogen Comparison: antioestrogen | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with antioestrogen | Risk with antioestrogen | |||||
| Clomiphene citrate versus tamoxifen ‐ Live birth rate | 204 per 1000 | 241 per 1000 (131 to 402) | OR 1.24 (0.59 to 2.62) | 195 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Low event rates and small sample size observed in the included studies |
| Clomiphene citrate versus tamoxifen ‐ Miscarriage rate | 27 per 1000 | 49 per 1000 (22 to 104) | OR 1.81 (0.80 to 4.12) | 653 (4 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Low event rates observed in included studies |
| Clomiphene citrate versus tamoxifen ‐ Clinical pregnancy rate | 221 per 1000 | 270 per 1000 (207 to 345) | OR 1.30 (0.92 to 1.85) | 757 (5 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | |
| Clomiphene citrate versus tamoxifen ‐ Multiple pregnancy | 4 per 1000 | 8 per 1000 (1 to 54) | OR 2.34 (0.34 to 16.04) | 567 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 6 7 | Low event rates observed in included studies |
| Clomiphene citrate versus tamoxifen ‐ ovarian hyperstimulation syndrome (OHSS) | Not pooled | Not pooled | Not estimable | 567 (3 studies) | ‐ | No events of OHSS reported in either intervention or control group in the included studies |
| Clomiphene citrate plus tamoxifen versus clomiphene citrate ‐ Clinical pregnancy rate | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.32 (0.12 to 91.60) | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 7 8 9 | Very low event rates and very small sample size (n = 20 women) observed in this study |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Low event rates and small sample size increase the chance of imprecision ‐ downgraded one level. 2Wide confidence intervals crossing the line of no effect and low event rates suggest imprecision ‐ downgraded one level. 3There was insufficient detail to be able to make a judgement regarding randomisation in three of four studies, blinding of researchers/women in two of four studies, and blinding of outcome assessors in three of four studies ‐ downgraded one level. 4I2 statistic was greater than 50% ‐ downgraded one level. 5Insufficient data to be able to make judgements on risk of bias for allocation concealment, random allocation, and blinding ‐ downgraded one level. 6Insufficient data to be able to make judgments on risk of bias for randomisation and blinding ‐ downgraded one level. 7Wide confidence intervals crossing the line of no effect suggesting substantive benefit and substantive harm. Event rates are low, suggesting high risk of imprecision ‐ downgraded one level. 8Evidence is based on data from a single small study (n = 20 women) ‐ downgraded one level. 9Insufficient detail for all aspects of risk of bias to be able to make a judgement. Live birth was not reported ‐ downgraded one level.
Summary of findings 3. Antioestrogen plus medical adjunct versus antioestrogen alone.
| Antioestrogen plus medical adjunct versus antioestrogen alone | ||||||
|
Patient or population: ovulation induction in polycystic ovarian syndrome Setting: Four studies from Iran, three from USA, two from Egypt, one from Turkey and one from India. Studies conducted in a University clinic, infertility outpatient clinic, Women's hospital clinic, Infertility and Reproductive Health Centre, private infertility clinic, Women's Health Research Institute, Infertiltiy Clinic and Research Clinic; three studies provided no information. Intervention: antioestrogen plus medical adjunct Comparison: antioestrogen alone | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with antioestrogen alone | Risk with antioestrogen plus medical adjunct | |||||
| Clomiphene citrate plus ketoconazole versus clomiphene citrate | Miscarriage rate | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size in this single study | |||
| 27 per 1000 | 8 per 1000 (0 to 164) | OR 0.28 (0.01 to 7.08) | 80 (1 RCT) | |||
| Clinical pregnancy rate | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size in this single study | ||||
| 216 per 1000 | 395 per 1000 (195 to 638) | OR 2.37 (0.88 to 6.40) | 80 (1 RCT) | |||
| Multiple pregnancy | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size in this single study | ||||
| 162 per 1000 | 186 per 1000 (67 to 423) | OR 1.18 (0.37 to 3.78) | 80 (1 RCT) | |||
| Clomiphene citrate plus bromocriptine versus clomiphene citrate ‐ Clinical pregnancy rate | 187 per 1000 | 191 per 1000 (99 to 337) | OR 1.03 (0.48 to 2.21) | 174 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Low event rates and small sample size observed in the included studies |
| Clomiphene citrate plus dexamethasone versus clomiphene citrate | Clinical pregnancy rate | ⊕⊝⊝⊝ VERY LOW 3 5 6 | ||||
| 81 per 1000 | 355 per 1000 (163 to 608) | OR 6.20 (2.20 to 17.48) | 434 (4 RCTs) | |||
| Multiple pregnancy rate | ⊕⊕⊝⊝ LOW 2 3 | Low event rates and small sample size observed in these studies. One study had no events in the intervention or the control group. | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | OR 7.71 (0.38 to 155.64) | 144 (2 RCTs) | |||
| Clomiphene citrate plus combined oral contraceptive versus clomiphene citrate ‐ Miscarriage rate | Miscarriage rate | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size observed in this single study | |||
| 42 per 1000 | 42 per 1000 (3 to 425) | OR 1.00 (0.06 to 16.97) | 48 (1 RCT) | |||
| Clinical pregnancy rate | ⊕⊝⊝⊝ VERY LOW 3 8 | Low event rates and small sample size observed in this single study | ||||
| 42 per 1000 | 542 per 1000 (120 to 911) | OR 27.18 (3.14 to 235.02) | 48 (1 RCT) | |||
| Multiple pregnancy | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size observed in this single study | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | OR 7.98 (0.39 to 163.33) | 48 (1 RCT) | |||
| Clomiphene citrate plus hCG versus clomiphene citrate alone | Ongoing pregnancy rate | ⊕⊝⊝⊝ VERY LOW 1 3 9 | Low event rates and small sample size observed in this single study | |||
| 277 per 1000 | 334 per 1000 (189 to 517) | OR 1.31 (0.61 to 2.80) | 125 (1 RCT) | |||
| Miscarriage rate | ⊕⊕⊕⊝ MODERATE 2 | Low event rates and small sample size observed in the included studies | ||||
| 61 per 1000 | 44 per 1000 (12 to 146) | OR 0.70 (0.19 to 2.62) | 192 (2 RCTs) | |||
| Clinical pregnancy rate | ⊕⊕⊕⊝ MODERATE 9 | Low event rates and small sample size observed in the included studies | ||||
| 235 per 1000 | 266 per 1000 (153 to 420) | OR 1.18 (0.59 to 2.36) | 192 (2 RCTs) | |||
| Multiple pregnancies | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size observed in this single study | ||||
| 15 per 1000 | 33 per 1000 (3 to 281) | OR 2.21 (0.19 to 24.98) | 125 (1 RCT) | |||
| Clomiphene citrate plus hormone supplementation versus clomiphene citrate alone | Miscarriage rate | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Low event rates and small sample size observed in this single study | |||
| 21 per 1000 | 21 per 1000 (1 to 259) | OR 1.00 (0.06 to 16.46) | 96 (1 RCT) | |||
| Clinical pregnancy rate | ⊕⊕⊝⊝ LOW 3 9 | Low event rates and small sample size observed in these studies | ||||
| 220 per 1000 | 186 per 1000 (94 to 331) | OR 0.81 (0.37 to 1.76) | 161 (2 RCTs) | |||
| Multiple pregnancy rate | ‐ | No events of multiple pregnancy were observed in either the intervention or control group in this single study. | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 96 (1 RCT) | |||
| OHSS | ‐ | No events of OHSS were observed in either the intervention or control group in this single study. | ||||
| 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 96 (1 RCT) | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; hCG: human chorionic gonadotropin; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Evidence is based on data from a single small study ‐ downgraded one level. 2Wide confidence intervals crossing the line of no effect, low event rates, and small sample size suggest imprecision ‐ downgraded one level. 3Insufficient detail to be able to make judgements on risk of bias. Live birth was not reported ‐ downgraded one level. 4Wide confidence interval and small sample size suggest imprecision ‐ downgraded one level. 5Wide confidence intervals observed ‐ downgraded one level. 6I2 statistic was greater than 50% ‐ downgraded one level. 7I2 statistic was greater than 80% ‐ downgraded two levels. 8Wide confidence intervals, low event rates, and small sample size observed. 9Low event rates and small sample size increase the likelihood of imprecision.
Summary of findings 4. Antioestrogen regimens.
| Antioestrogen regimens | ||||||
|
Patient or population: ovulation induction in polycystic ovarian syndrome Setting: Three studies took place in Egypt and one in Iran. One was conducted in a University Fertility Clinic, one in an Infertility Research Centre and one in a gynaecology outpatient department. The fourth research setting was not specified. Intervention: clomiphene citrate regimen A Comparison: clomiphene citrate regimen B | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with clomiphene citrate regimen B | Risk with clomiphene citrate regimen A | |||||
| Live birth ‐ Clomiphene citrate 5 days versus clomiphene citrate 10 days | 155 per 1000 | 18 per 1000 (4 to 76) | OR 0.10 (0.02 to 0.45) | 220 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Clinical pregnancy ‐ Clomiphene citrate 5 days versus clomiphene citrate 10 days | 173 per 1000 | 36 per 1000 (12 to 103) | OR 0.18 (0.06 to 0.55) | 220 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Multiple pregnancy ‐ Clomiphene citrate 5 days versus clomiphene citrate 10 days | 27 per 1000 | 9 per 1000 (1 to 82) | OR 0.33 (0.03 to 3.20) | 220 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | |
| Miscarriage rate ‐ Early versus late clomiphene citrate | 29 per 1000 | 36 per 1000 (8 to 147) | OR 1.25 (0.27 to 5.70) | 212 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | |
| Clinical pregnancy ‐ Early versus late clomiphene citrate | 195 per 1000 | 405 per 1000 (198 to 653) | OR 2.81 (1.02 to 7.75) | 78 (1 RCT) | ⊕⊕⊝⊝ LOW 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Evidence is based on a single study ‐ downgraded one level for serious imprecision. 2Event rates are low, which may increase the likelihood of imprecision ‐ downgraded one level. 3Insufficient data to allow judgement of risk of bias ‐ downgraded one level for serious risk of bias. 4Wide confidence intervals, low event rates, and small sample size observed in this single study ‐ downgraded one level for serious imprecision.
1 Antioestrogen versus no treatment or placebo
Clomiphene citrate (50 mg to 250 mg) versus placebo
There were three trials in this comparison (Cudmore 1966; Garcia 1985; Johnson 1966).
Primary outcomes
Live birth rate
No data were reported for this comparison.
Miscarriage rate
No data were reported for this comparison.
Secondary outcomes
Clinical pregnancy rate
Clomiphene citrate was associated with an increased chance of a clinical pregnancy compared with placebo (odds ratio (OR) 5.91, 95% confidence interval (CI) 1.77 to 19.68; 3 studies; 133 women; low‐quality evidence; Analysis 1.1; Figure 4). If the chance of a clinical pregnancy was 5% in the placebo group, then between 8% and 50% of women in the clomiphene group would have a clinical pregnancy. We downgraded the evidence for imprecision and insufficient methodological information to be able to judge risk of bias (Table 1).
1.1. Analysis.
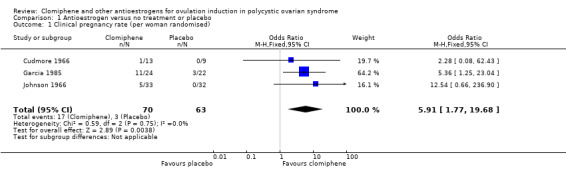
Comparison 1 Antioestrogen versus no treatment or placebo, Outcome 1 Clinical pregnancy rate (per woman randomised).
4.
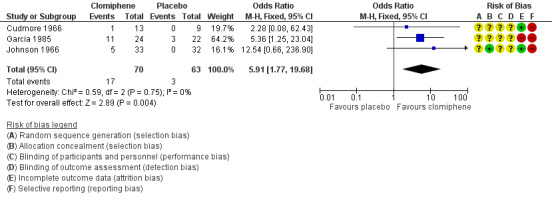
Forest plot of comparison: 1 Antioestrogen versus no treatment or placebo, outcome: 1.1 Clinical pregnancy rate (per woman randomised).
Multiple pregnancy
No data were reported for this comparison.
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
2 Antioestrogen versus antioestrogen
Clomiphene citrate (50 mg to 200 mg) versus tamoxifen (20 mg to 60 mg)
There were five studies in this comparison (Badawy 2011; Boonstanfar 2001; Moslemizadeh 2008; Seyedoshohadaei 2012; Vegetti 1999).
Primary outcomes
Live birth
Two studies reported on live birth (Boonstanfar 2001; Seyedoshohadaei 2012). There was no evidence of a difference in the chance of a live birth between the clomiphene citrate and tamoxifen groups (OR 1.24, 95% CI 0.59 to 2.62; 2 studies; 195 women; low‐quality evidence; Analysis 2.1; Figure 5). If 20% of women in the tamoxifen group had a live birth, then between 13% to 40% of women in the clomiphene citrate group would have a live birth. We downgraded the evidence for imprecision (Table 2).
2.1. Analysis.
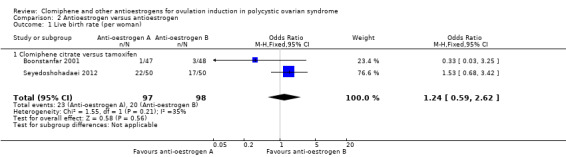
Comparison 2 Antioestrogen versus antioestrogen, Outcome 1 Live birth rate (per woman).
5.
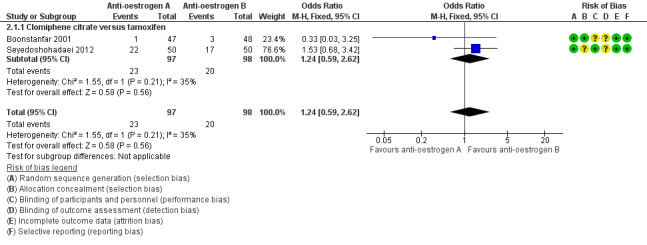
Forest plot of comparison: 2 Antioestrogen versus antioestrogen, outcome: 2.1 Live birth rate (per woman).
Miscarriage
Four studies reported on miscarriage (Badawy 2011; Boonstanfar 2001; Moslemizadeh 2008; Seyedoshohadaei 2012). There was no evidence of a difference in the chance of a miscarriage between the clomiphene citrate and tamoxifen groups (OR 1.81, 95% CI 0.80 to 4.12; 4 studies; 653 women; low‐quality evidence; Analysis 2.2; Figure 6). If 3% of women in the tamoxifen group had a miscarriage, then between 2% and 10% of women in the clomiphene citrate group would have a miscarriage (Table 2). We downgraded the evidence for imprecision and risk of bias.
2.2. Analysis.
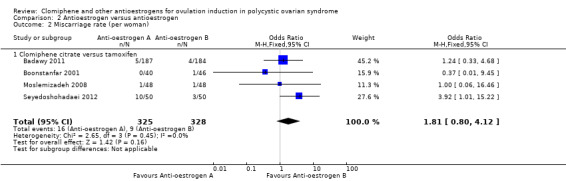
Comparison 2 Antioestrogen versus antioestrogen, Outcome 2 Miscarriage rate (per woman).
6.
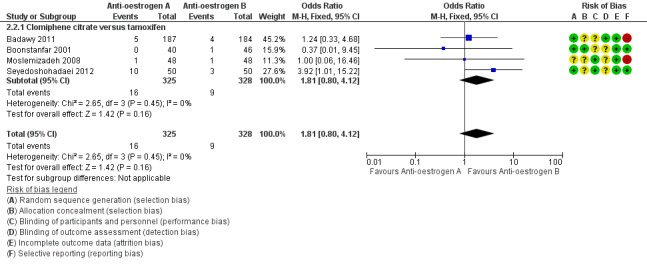
Forest plot of comparison: 2 Antioestrogen versus antioestrogen, outcome: 2.2 Miscarriage rate (per woman).
Secondary outcomes
Clinical pregnancy
All five studies reported data on clinical pregnancy (Badawy 2011; Boonstanfar 2001; Moslemizadeh 2008; Seyedoshohadaei 2012; Vegetti 1999). There was no evidence of a difference in the chance of a clinical pregnancy between the clomiphene citrate and tamoxifen groups (OR 1.30, 95% CI 0.92 to 1.85; 5 studies; 757 women; I2 = 69%; low‐quality evidence; Analysis 2.3). If 22% of women in the tamoxifen group had a clinical pregnancy, then between 21% and 35% of women in the clomiphene citrate group would have a clinical pregnancy (Table 2). We downgraded the evidence for inconsistency (heterogeneity) and risk of bias. The observed heterogeneity is most likely due to differences in study protocols; there were differences in number of cycles of treatment, dose of clomiphene citrate and tamoxifen, and start and end day of treatment in the menstrual cycles (refer to Characteristics of included studies).
2.3. Analysis.
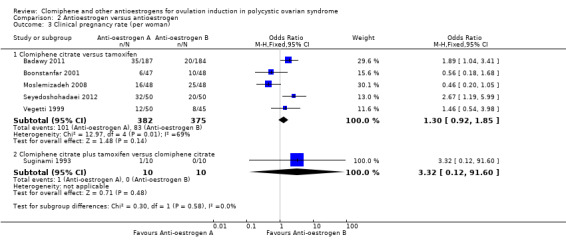
Comparison 2 Antioestrogen versus antioestrogen, Outcome 3 Clinical pregnancy rate (per woman).
Multiple pregnancy
Three studies reported on multiple pregnancy (Badawy 2011; Moslemizadeh 2008; Seyedoshohadaei 2012). There was insufficient evidence to determine whether there was a difference in the chance of a multiple pregnancy between the clomiphene citrate group (3 out of 285; 1%) and tamoxifen group (1 out of 282; < 1%) (OR 2.34, 95% CI 0.34 to 16.04; 3 studies; 567 women; very low‐quality evidence). The data suggests that if 0% of women in the tamoxifen group had a multiple pregnancy, then between 0% and 0.5% of women in the clomiphene group would have a multiple pregnancy (Table 2). We downgraded the evidence for risk of bias and imprecision.
OHSS
There were no instances of OHSS in either the clomiphene citrate or the tamoxifen group (Badawy 2011; Boonstanfar 2001; Moslemizadeh 2008).
Adverse effects
No data were reported for this comparison.
Clomiphene citrate (50 mg) plus tamoxifen (20 mg) versus clomiphene citrate (100 mg)
Two trials reported on this comparison (Ghafourzadeh 2004; Suginami 1993).
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
No data were reported for this comparison.
Secondary outcomes
Clinical pregnancy
One small study reported on clinical pregnancy (Suginami 1993). There was insufficient evidence to determine whether there was a difference between the clomiphene citrate plus tamoxifen group and the clomiphene‐alone group (OR 3.32, 95% CI 0.12 to 91.60; 1 study; 20 women). Caution is required in interpreting these data as they have high levels of imprecision with wide confidence intervals, small event rates, and small sample size (Analysis 2.3).
Multiple pregnancy
No data were reported for this comparison.
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
3 Antioestrogen versus gonadotropin
Three trials reported on this comparison. Two trials reported data for clomiphene citrate versus FSH (Homburg 2012; Lopez 2004), and one trial reported data for clomiphene citrate versus hMG (Badawy 2008).
Primary outcomes
Live birth/ongoing pregnancy
The evidence suggests that live birth/ongoing pregnancy is reduced with clomiphene citrate compared with gonadotropins (OR 0.64, 95% CI 0.41 to 0.98; 2 studies; 378 women; I2 = 0%; Analysis 3.1, Figure 7). Lopez 2004 reported on live birth, and Homburg 2012 reported on ongoing pregnancy. Both trials used FSH as the gonadotropin. The Badawy 2008 trial only reported data for biochemical pregnancy and was therefore not included in the meta‐analysis.
3.1. Analysis.
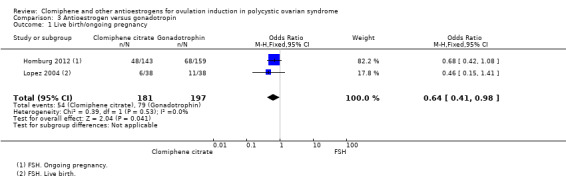
Comparison 3 Antioestrogen versus gonadotropin, Outcome 1 Live birth/ongoing pregnancy.
7.
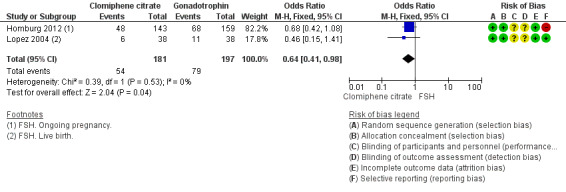
Forest plot of comparison: 3 Antioestrogen versus gonadotropin, outcome: 3.1 Live birth/ongoing pregnancy.
Miscarriage
There was no evidence of a difference between clomiphene citrate and gonadotropin for chance of miscarriage (OR 0.84, 95% CI 0.39 to 1.78; 3 studies; 696 women; I2 = 0%; Analysis 3.2, Figure 8). Homburg 2012 and Lopez 2004 used FSH, and Badawy 2008 used hMG.
3.2. Analysis.
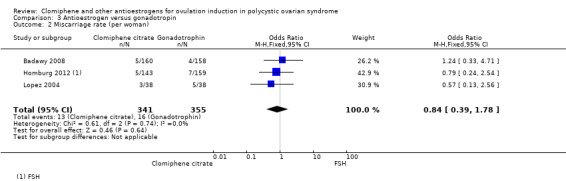
Comparison 3 Antioestrogen versus gonadotropin, Outcome 2 Miscarriage rate (per woman).
8.
Forest plot of comparison: 3 Antioestrogen versus gonadotropin, outcome: 3.2 Miscarriage rate (per woman).
Secondary outcomes
Clinical pregnancy
The evidence suggests that clinical pregnancy is reduced with clomiphene citrate compared with gonadotropins (OR 0.61, 95% CI 0.40 to 0.93; 2 studies; 378 women; I2 = 0%). Both trials used FSH as the gonadotropin.
Multiple pregnancy
There was no evidence of a difference for chance of a multiple pregnancy between clomiphene citrate (2 out of 341; < 1%) and gonadotropins (9 out of 355; 3%) (OR 0.26, 95% CI 0.06 to 1.06; 3 studies; 696 women; I2 = 0%). Homburg 2012 and Lopez 2004 both used FSH, and Badawy 2008 used hMG. Caution is advised in interpreting these data due to low event rates.
OHSS
There was no evidence of a difference between groups for chance of developing OHSS (OR 0.19, 95% CI 0.02 to 1.67; 2 studies; 394 women; I2 = 0%). Lopez 2004 used FSH, and Badawy 2008 used hMG.
Adverse effects
No data were reported for this comparison.
4 Antioestrogen plus other medical therapy versus antioestrogen alone
Clomiphene citrate (up to 150 mg) plus ketoconazole (400 mg) versus clomiphene citrate (up to 150 mg)
One study reported this comparison (Hassan 2001).
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
One instance of miscarriage was reported in the clomiphene citrate‐alone group in the Hassan 2001 study. There were no events in the clomiphene citrate plus ketoconazole group (OR 0.28, 95% CI 0.01 to 7.08; 1 study; 80 women; very low‐quality evidence; Table 3). The evidence suggests that if 2.7% of women in the clomiphene group had a miscarriage, then between 0% and 16% of women the in the clomiphene plus ketoconazole group would have a miscarriage. We downgraded the evidence for low event rates, small sample size, and the evidence being based on a single study (imprecision) (Table 3).
Secondary outcomes
Clinical pregnancy
There was no evidence of a difference between clomiphene citrate plus ketoconazole and clomiphene citrate alone for chance of a clinical pregnancy (OR 2.37, 95% CI 0.88 to 6.40; 1 study; 80 women; very low‐quality evidence; Table 3). The evidence suggests that if 22% of women in the clomiphene group had a clinical pregnancy, then between 20% and 64% of women in the clomiphene plus ketoconazole group would have a clinical pregnancy. We downgraded the evidence for low event rates, small sample size, and the evidence being based on a single study (imprecision) (Table 3).
Multiple pregnancy
There was no evidence of a difference for chance of a multiple pregnancy between clomiphene plus ketoconazole (8 out of 43; 19%) and clomiphene alone (6 out of 37; 16%) (OR 1.18, 95% CI 0.37 to 3.78; 1 study; 80 women; very low‐quality evidence; Table 3). The evidence suggests that if 16% of women in the clomiphene group had a multiple pregnancy, then between 7% and 42% of women in the clomiphene plus ketoconazole group would have a multiple pregnancy. We downgraded the evidence for low event rates, small sample size, and the evidence being based on a single study (imprecision) (Table 3).
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Clomiphene citrate (50 mg to 200 mg) plus bromocriptine (2.5 mg to 7.5 mg) versus clomiphene citrate (50 mg to 200 mg)
Two trials reported this comparison (Analysis 4.2) (Parsanezhad 2002b; Tripathy 2013).
4.2. Analysis.
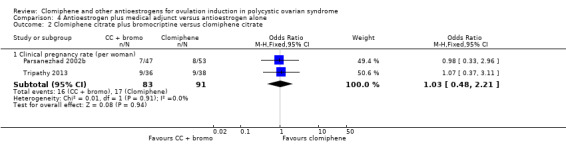
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 2 Clomiphene citrate plus bromocriptine versus clomiphene citrate.
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
No data were reported for this comparison.
Secondary outcomes
Clinical pregnancy
There was no evidence of a difference in chance of a clinical pregnancy between clomiphene citrate plus bromocriptine compared with clomiphene citrate alone (OR 1.03, 95% CI 0.48 to 2.21; 2 studies; 174 women; low‐quality evidence). The evidence suggests that if 19% of women in the clomiphene group had a clinical pregnancy, then between 10% and 34% of women in the clomiphene plus bromocriptine group would have a clinical pregnancy. We downgraded the evidence for low event rate and small sample size (imprecision) (Table 3).
Multiple pregnancy
No data were reported for this comparison.
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Clomiphene (50 mg to 200 mg) plus dexamethasone (0.5 mg to 2.0 mg) versus clomiphene citrate (50 mg to 200 mg)
Four trials reported on this comparison (Analysis 4.3) (Daly 1984; Elnashar 2006; Esmaeilzadeh 2011; Parsanezhad 2002a).
4.3. Analysis.
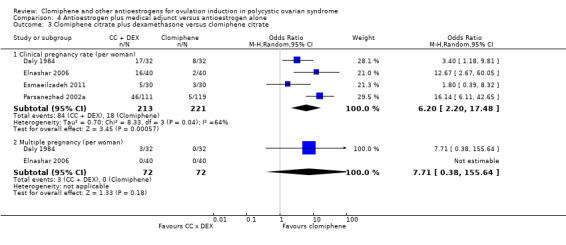
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 3 Clomiphene citrate plus dexamethasone versus clomiphene citrate.
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
No data were reported for this comparison.
Secondary outcomes
Clinical pregnancy
All four trials reported on clinical pregnancy. Clomiphene plus dexamethasone was associated with an increase in the chance of a clinical pregnancy (average OR 6.20, 95% CI 2.20 to 17.48; 4 studies; 434 women; random‐effects I2 = 64%; very low‐quality evidence). Three trials used a 2 mg dose of dexamethasone (Elnashar 2006; Esmaeilzadeh 2011; Parsanezhad 2002a), and one trial used a 0.5 mg dose (Daly 1984). The removal of the Daly 1984 trial did not affect the direction of the treatment effect or the statistical significance. The evidence suggests that if 8% of women in the clomiphene group had a clinical pregnancy, then between 16% and 61% of women in the clomiphene plus dexamethasone group would have a clinical pregnancy. We downgraded the evidence for risk of bias, imprecision, and inconsistency (Table 3).
Multiple pregnancy
There was no evidence of a difference in the incidence of multiple pregnancy per woman between clomiphene citrate plus dexamethasone compared with clomiphene citrate alone (OR 7.71, 95% CI 0.38 to 155.64; 2 studies; 144 women; low‐quality evidence) (Daly 1984; Elnashar 2006). We could not calculate absolute risk, as no events were reported in either group in one of the trials. Three multiple pregnancies were reported in the clomiphene plus dexamethasone group (Daly 1984); no other cases of multiple pregnancy were reported. The Daly 1984 trial used a 0.5 mg dose of dexamethasone, and the Elnashar 2006 trial used a 2 mg dose of dexamethasone. It is unclear if the dosage of dexamethasone influenced the outcome due to the small number of events, if any, that were reported.
OHSS
No data were reported for this comparison.
Adverse effects
No side effects were reported by Elnashar 2006 in either group.
Clomiphene citrate (100 mg) plus combined oral contraceptive versus clomiphene citrate (100 mg)
One study reported on this comparison (Branigan 2003).
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
There was no evidence of a difference in miscarriage rate between women treated with clomiphene citrate plus combined oral contraceptive and women treated with clomiphene citrate alone (OR 1.0, 95% CI 0.06 to 16.97; 1 study; 48 women; very low‐quality evidence). The evidence suggests that if 4% of women in the clomiphene citrate group had a miscarriage, then between 0% and 43% of women in the clomiphene citrate plus oral contraceptive group would have a miscarriage. We downgraded the evidence for low event rate and small sample size and the evidence being based on a single study (imprecision) (Table 3).
Secondary outcomes
Clinical pregnancy
Clomiphene citrate plus combined oral contraceptive was associated with an increased chance of a clinical pregnancy compared with clomiphene citrate alone (OR 27.18, 95% CI 3.14 to 235.02; 1 study; 48 women; very low‐quality evidence). The evidence suggests that if 4% of women in the clomiphene citrate group had a clinical pregnancy, then between 12% and 91% of women in the clomiphene citrate plus combined oral contraceptive group would have a clinical pregnancy. We downgraded the evidence for low event rate and small sample size and the evidence being based on a single study (imprecision) (Table 3).
Multiple pregnancy
There was no evidence of a difference in the chance of a multiple pregnancy between clomiphene citrate plus combined oral contraceptive and clomiphene citrate alone (OR 7.98, 95% CI 0.39 to 163.33; 1 study; 48 women; very low‐quality evidence). We could not calculate absolute risk estimates, as there were no events in the control group. We downgraded the evidence for low event rate and small sample size and the evidence being based on a single study (imprecision) (Table 3).
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Clomiphene citrate plus hCG versus clomiphene citrate
Two trials reported this comparison (Analysis 4.5) (Branigan 2005; Yilmaz 2006).
4.5. Analysis.
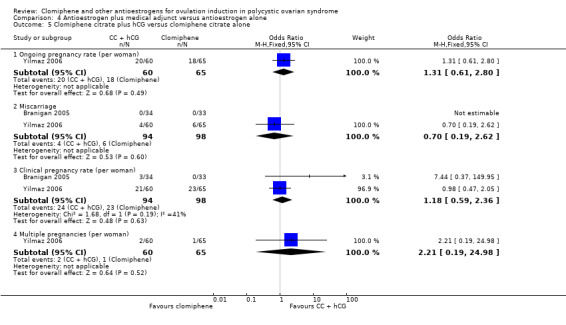
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 5 Clomiphene citrate plus hCG versus clomiphene citrate alone.
Primary outcomes
Live birth/ongoing pregnancy rate
There was no evidence of a difference between groups for ongoing pregnancy reported by Yilmaz 2006 (OR 1.31, 95% CI 0.61 to 2.80; 1 study; 125 women; very low‐quality evidence). The evidence suggests that if 28% of women had an ongoing pregnancy in the clomiphene citrate group, then between 19% and 52% of women in the clomiphene citrate plus hCG group would have an ongoing pregnancy. We downgraded the evidence for low event rate and small sample size and the evidence being based on a single study (imprecision) (Table 3).
Miscarriage
There was no evidence of a difference in the chance of miscarriage between clomiphene citrate plus hCG and clomiphene citrate alone (OR 0.70, 95% CI 0.19 to 2.62; 2 studies; 192 women; moderate‐quality evidence). The evidence suggests that if 6% of women in the clomiphene citrate group had a miscarriage, then between 1% and 15% of women in the clomiphene citrate plus hCG group would have a miscarriage. We downgraded the evidence for low event rate and small sample size (imprecision) (Table 3).
Secondary outcomes
Clinical pregnancy rate
There was no evidence of a difference in the chance of a clinical pregnancy between clomiphene citrate plus hCG and clomiphene citrate alone (OR 1.18, 95% CI 0.59 to 2.36; 2 studies; 192 women; moderate‐quality evidence). The evidence suggests that if 24% of women in the clomiphene group had a clinical pregnancy, then between 15% and 42% of women in the clomiphene plus hCG group would have a clinical pregnancy. We downgraded the evidence for low event rate and small sample size (imprecision) (Table 3).
Multiple pregnancy rate
Only Yilmaz 2006 reported on multiple pregnancies, and there was no evidence of a difference between clomiphene citrate plus hCG (2 out of 60; 3%) and clomiphene citrate alone (1 out of 65; 2%) (OR 2.21, 95% CI 0.19 to 24.98; 1 study; 125 women; very low‐quality evidence). The evidence suggests that if 2% of women in the clomiphene group had a multiple pregnancy, then between 0% and 28% of women in the clomiphene plus hCG group would have a multiple pregnancy. We downgraded the evidence for low event rate and small sample size and the evidence being based on a single study (imprecision) (Table 3).
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Clomiphene citrate plus hormone supplementation versus clomiphene citrate
Two trials reported this comparison (Analysis 4.6) (Elkind‐Hirsch 2005; Moslemizadeh 2008).
4.6. Analysis.
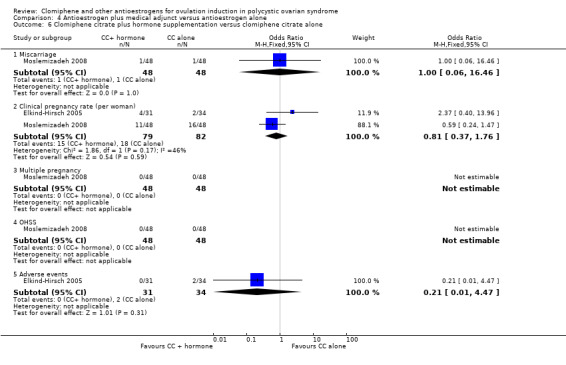
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 6 Clomiphene citrate plus hormone supplementation versus clomiphene citrate alone.
Primary outcomes
Live birth rate
No data were reported for this comparison.
Miscarriage rate
One event of miscarriage was reported for both the clomiphene citrate plus hormone supplementation and the clomiphene citrate‐alone groups. There was no statistical difference between the groups (OR 1.00, 95% CI 0.06 to 16.46; 1 study; 96 women; very low‐quality evidence). The evidence suggests that if 2% of women in the clomiphene citrate group had a miscarriage, then between 0% and 26% of women in the clomiphene citrate plus hormone supplementation group would have a miscarriage. We downgraded the evidence for low event rates and small sample size and the evidence being based on a single trial (imprecision) (Table 3).
Secondary outcomes
Clinical pregnancy rate
There was no evidence of a difference in the chance of a clinical pregnancy between the clomiphene citrate plus hormone supplementation and the clomiphene citrate‐alone groups (OR 0.81, 95% CI 0.37 to 1.76; 2 studies; 161 women; low‐quality evidence). The evidence suggests that if 22% of women in the clomiphene citrate group had a clinical pregnancy, then between 9% and 33% of women in the clomiphene citrate plus hormone supplementation group would have a clinical pregnancy. We downgraded the evidence for low event rates and small sample size (imprecision) (Table 3).
Multiple pregnancy rate
There were no events of multiple pregnancy in either the clomiphene citrate plus hormone supplementation or the clomiphene citrate‐alone group, reported in one trial of 96 women (Moslemizadeh 2008), therefore we could not calculate absolute risk.
OHSS
There were no events of OHSS in either the clomiphene citrate plus hormone supplementation or the clomiphene citrate‐alone group, reported in one trial of 96 women (Moslemizadeh 2008), therefore we could not calculate absolute risk.
Adverse effects
There were no reports of adverse effects in either the clomiphene citrate plus hormone supplementation or the clomiphene citrate‐alone group (OR 0.21, 95% CI 0.01 to 4.47; 1 study; 65 women). Caution is required in interpreting these data due to low event rates and small sample size, which increases the risk of imprecision. There were wide confidence intervals that cross the line of no effect.
5 Antioestrogen plus other medical therapy versus antioestrogen plus other medical therapy
No trials were found reporting data for this comparison.
6 Clomiphene citrate regimens
Clomiphene citrate for 5 days versus clomiphene citrate for 10 days
Two trials reported this comparison (Elsedeek 2014; Omran 2011). Both trials used a regimen of 200 mg clomiphene citrate per day for 5 days or 100 mg clomiphene citrate per day for 10 days.
Primary outcomes
Live birth rate
One trial reported that clomiphene citrate for 10 days was associated with an increased chance of a live birth compared with the 5‐day regimen (OR 0.10, 95% CI 0.02 to 0.45; 1 study; 220 women; low‐quality evidence) (Elsedeek 2014). The evidence suggests that if 16% of women in the 10‐day regimen group had a live birth, then between 0% and 8% of women in the 5‐day regimen group would have a live birth. We downgraded the evidence for low event rates and the evidence being based on a single trial (imprecision) (Table 4).
Miscarriage rate
No data were reported for this comparison.
Secondary outcomes
Clinical pregnancy rate
One trial reported that clomiphene citrate for 10 days was associated with an increased chance of a clinical pregnancy (OR 0.18, 95% CI 0.06 to 0.55; 1 study; 220 women; low‐quality evidence) (Elsedeek 2014). The evidence suggests that if 17% of women in the 10‐day regimen group had a clinical pregnancy, then between 1% and 10% in the 5‐day regimen group would have a clinical pregnancy.We suggest caution when interpreting these data due to low event rates and the evidence being based on a single trial (imprecision) (Table 4). Further research is needed to confirm this benefit for an extended regimen.
Multiple pregnancy rate
There was no evidence of a difference between the 5‐day regimen (1 out of 110; < 1%) and the 10‐day regimen (3 out of 110; 3%), reported by Elsedeek 2014 (OR 0.33, 95% CI 0.03 to 3.20; 1 study; 220 women; very low‐quality evidence). The evidence suggests that if 3% of women in the 10‐day regimen group had a multiple pregnancy, then between 0% and 8% in the 5‐day regimen group would have a multiple pregnancy. We downgraded the evidence for low event rates and small sample size and the evidence being based on a single trial (imprecision) (Table 4.
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Early versus late clomiphene citrate
Two trials reported this comparison (Badawy 2009; Dehbashi 2006). Badawy 2009 used a regimen of 5 days of 100 mg per day clomiphene citrate starting on the day after finishing medroxyprogesterone (early regimen) compared with 5 days of 100 mg per day clomiphene citrate starting on Day 3 of menses (late regimen). Dehbashi 2006 used a regimen of 100 mg clomiphene citrate per day on Days 1 to 5 of the menstrual cycle (early regimen) compared with 100 mg clomiphene citrate per day on Days 5 to 9 of the menstrual cycle (late regimen).
Primary outcomes
Live birth
No data were reported for this comparison.
Miscarriage
One trial reported no evidence of a difference in chance of a miscarriage between early and late clomiphene citrate regimens (OR 1.25, 95% CI 0.27 to 5.70; 1 study; 212 women; very low‐quality evidence) (Badawy 2009). The evidence suggests that if 3% of women in the late‐regimen group had a miscarriage, then between 1% and 15% of women in the early‐regimen group would have a miscarriage. We downgraded the evidence for being based on a single trial (imprecision) and risk of bias (Table 4).
Secondary outcomes
Clinical pregnancy
One trial reported that an early regimen of clomiphene citrate was associated with an increased chance of a clinical pregnancy compared with a late regimen of clomiphene citrate (OR 2.81, 95% CI 1.02 to 7.75; 1 study; 78 women; low‐quality evidence) (Dehbashi 2006). The evidence suggests that if 20% of women in the late‐regimen group had a clinical pregnancy, then between 20% and 65% of women in the early‐regimen group would have a clinical pregnancy. We downgraded the evidence for being based on a single trial (imprecision) (Table 4).
Multiple pregnancy
No data were reported for this comparison.
OHSS
No data were reported for this comparison.
Adverse effects
No data were reported for this comparison.
Discussion
Summary of main results
Clomiphene citrate versus placebo
Analysis of the three trials comparing clomiphene with placebo showed that clomiphene improves the chance of pregnancy (Table 1).
Clomiphene citrate versus tamoxifen
Five trials comparing clomiphene with tamoxifen showed no clear evidence of a difference in live birth, clinical pregnancy, miscarriage, or multiple pregnancy rate. No cases of OHSS were reported.
Clomiphene citrate plus tamoxifen versus clomiphene citrate
Two trials compared clomiphene citrate plus tamoxifen with clomiphene citrate alone. The evidence was insufficient to determine whether there was a difference in clinical pregnancy rates. No other outcomes were reported for this comparison (Table 2).
Clomiphene citrate versus gonadotropin
Three trials compared clomiphene citrate with gonadotropins. Clomiphene citrate was associated with a reduced chance of a clinical pregnancy, ongoing pregnancy, or live birth. There was no evidence of a difference between groups for chance of a multiple pregnancy, although event rates were very low and therefore data should be interpreted with caution.
Clomiphene citrate plus medical adjunct versus clomiphene citrate
Data were reported for six different medical adjuncts used with clomiphene citrate and compared with clomiphene citrate alone (Table 3).
One small study of 80 women reported limited evidence using ketoconazole as an adjunct. There was no evidence of a difference between clomiphene plus ketoconazole and clomiphene alone for miscarriage, clinical pregnancy, or multiple pregnancy. Event rates were low and therefore data should be interpreted with caution. The results are open to some misinterpretation, as the trial authors moved women who failed to respond to 150 mg clomiphene to hMG treatment. No data were provided on the numbers from each group, however it would be reasonable to assume that more women from the control group, with its higher rates of clomiphene resistance, required this.
Two trials reported the use of bromocriptine as an adjunct. There was no evidence of a difference for clinical pregnancy rates between groups. No other relevant outcomes were reported.
Four trials reported using dexamethasone as an adjunct. Clomiphene citrate plus dexamethasone was associated with an increased chance of a clinical pregnancy when compared with clomiphene citrate alone. Three of the four trials used a dose of 2 mg dexamethasone. There was no evidence of a difference between groups for multiple pregnancy. None of the trials used the same protocol for treatment, which could explain the observed heterogeneity. Despite this, dexamethasone shows potential as an inexpensive and non‐invasive treatment option for women with PCOS, perhaps especially those who have failed to respond to standard therapy.
One small trial reported on combined oral contraceptive (COC) pill used as an adjunct. Clomiphene citrate plus COC was associated with an increase in clinical pregnancy, with no evidence of a difference in miscarriage or multiple pregnancy rate when compared with clomiphene citrate alone. Event rates and sample size (n = 48 women) were small and therefore data should be interpreted with caution. Further trials are needed to establish if COC is indeed a safe and effective adjunct to clomiphene citrate.
The use of hCG as an adjunct showed no evidence of a difference in miscarriage, clinical pregnancy, or multiple pregnancy rate when compared with clomiphene citrate alone.
Two studies reported hormone supplementation as an adjunct, finding no evidence of a difference in miscarriage or clinical pregnancy. No events were reported for multiple pregnancy or OHSS.
Clomiphene citrate regimens
Clomiphene citrate given for 10 days was associated with an increased chance of live birth and clinical pregnancy compared with a 5‐day regimen. There was no evidence of a difference for multiple pregnancy (Table 4).
Data for early versus late regimens were insufficient to be able to draw any conclusions regarding the benefits of one over the other.
Overall completeness and applicability of evidence
We believe we have used rigorous methods to identify published and unpublished trials by searching multiple electronic databases with no restriction on language. Trials were reported from various countries. Many of the comparisons included in this review only had one or two relevant trials, and the sample sizes were small, which increases the risk of imprecision. In particular, data for multiple pregnancies were very limited. The trials that compared an antioestrogen with no treatment or placebo did not report multiple pregnancy as a clinical outcome. There were no data on self reported adverse effects.
Quality of the evidence
Limitations of the review
All of the trials included in this review have methodological flaws, including lack of clarity around randomisation and allocation concealment, lack of blinding, and attrition, which weaken the results.
Using GRADE methodology, we judged the evidence to be of low quality for the comparisons of antioestrogen versus no treatment or placebo (Table 1) and antioestrogen versus antioestrogen (Table 2). We downgraded much of the evidence for risk of bias and imprecision. More rigorous RCTs are required for all of the interventions.
Live birth rate is the gold‐standard primary outcome for RCTs of this nature (Vail 2003). Only five of the 28 included trials in this review reported this outcome (Boonstanfar 2001; Elsedeek 2014; Homburg 2012; Lopez 2004; Seyedoshohadaei 2012). Using pregnancy rate as a surrogate endpoint is of dubious accuracy. Other poorly reported outcomes included adverse effects and incidence of OHSS. OHSS is a rare but potentially life‐threatening complication of ovulation induction therapy; it is an important outcome, but if it did not occur in the trial populations it may not have been reported. Multiple pregnancy was poorly reported, and where data were available, event rates were low, making it uncertain if the interventions were influencing the outcome.
Potential biases in the review process
We believe we have conducted a thorough review of the literature searching for relevant published and unpublished trials, unrestricted by date or language. There were insufficient trials (fewer than 10) for each comparison to allow us to investigate publication bias via inspection of funnel plots.
Agreements and disagreements with other studies or reviews
NICE 2013 recommends clomiphene as a first‐line treatment option for women with WHO group 2 anovulatory infertility, taking into account potential adverse effects, ease and mode of use, the woman's body mass index, and monitoring needed.
Authors' conclusions
Implications for practice.
We have not found strong evidence in favour of one antioestrogen or adjunctive agent. We found evidence supporting the effectiveness of the current first‐line treatment, clomiphene citrate, in terms of pregnancies, although there is a reduced chance of clinical pregnancy, ongoing pregnancy, or live birth when compared with gonadotropins. It is unclear whether there is a difference in effect between clomiphene plus tamoxifen and clomiphene alone, as the number of women studied was too small to be conclusive. We could find no trials comparing tamoxifen and placebo.
There were insufficient data to determine the place of ketoconazole, tamoxifen, bromocriptine, human chorionic gonadotropin, or hormone supplementation as an adjunct to clomiphene versus clomiphene alone in anovulatory, normoprolactinaemic women. Due to the limited reporting of multiple pregnancy as a clinical outcome, we are unable to judge the effect of antioestrogens on this outcome and therefore suggest that the monitoring of ovulation induction with serum hormones and preferably vaginal ultrasound should be considered in order to minimise the risk of multiple gestation.
Implications for research.
Clomiphene is currently widely accepted as an effective treatment, and it is unlikely that further trials against placebo will be conducted. Large, well‐designed randomised controlled trials are needed comparing the long‐standing interventions such as clomiphene with the medical adjunctive drugs (in particular dexamethasone), and the newer drugs such as aromatase inhibitors. In addition, studies on the duration of treatment with clomiphene should be planned. Differentiation between results by aetiology of anovulation is also needed in new trials.
We suggest further research is required to confirm the potential benefit in the improved clinical pregnancy rate observed in a single trial comparing a 10‐day with a 5‐day regimen.
This review reports that currently available trials are often of poor quality and have potentially serious methodological and selective‐reporting flaws, primarily due to lack of data on live birth. Randomised controlled trials should follow the CONSORT guidelines (Moher 2001). Trials should be of sufficient duration to have live birth as their primary outcome and should ideally report all secondary outcomes listed in this review, in particular incidence of multiple pregnancy and miscarriage. All rates should be reported per woman, not per cycle, and in actual numbers of participants, not percentages.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2017 | Review declared as stable | Further evidence is unlikely to change the conclusions of this review. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 13 October 2016 | New search has been performed | 13 new studies were included in the 2016 update (Badawy 2008; Badawy 2009; Badawy 2011; Dehbashi 2006; Elsedeek 2014; Esmaeilzadeh 2011; Ghafourzadeh 2004; Homburg 2012; Lopez 2004; Moslemizadeh 2008; Omran 2011; Seyedoshohadaei 2012; Tripathy 2013). 2 studies were added to awaiting classification (Craig 2015; Neuhausser 2011). |
| 13 October 2016 | New citation required but conclusions have not changed | The addition of 13 studies did not change the conclusions of this review. |
| 23 June 2009 | New search has been performed | 9 new studies identified, review updated. |
| 16 June 2009 | Amended | Aromatase inhibitors removed from text as part of separate review. |
| 16 June 2009 | New citation required but conclusions have not changed | In June 2009 the title was changed to 'Clomiphene and antioestrogens for ovulation induction in polycystic ovarian syndrome' and new search completed. 9 additional studies identified in update. |
| 19 February 2009 | Amended | Changes made to structure of text and presentation of findings. |
| 9 June 2008 | Amended | Converted to new review format |
| 7 November 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors of the 2016 update thank Drs James Beck, Clare Boothroyd, J Collins, P Vandekerckhove and Edward Hughes for their contributions to previous versions of the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group search strategy
Procite platform
Inception until 2 August 2016
Keywords CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or "PCOS" or "anovulation" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligo‐ovulatory" or "oligoamenorrhea" or "oligoanovulatory"or "hirsutism" or Title CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or "PCOS" or "anovulation" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligo‐ovulatory" or "oligoamenorrhea" or "oligoanovulatory"or "hirsutism"
AND
Keywords CONTAINS "anti‐estrogen" or "antiestrogens" or "antioestrogens"or "Clomiphene" or "clomiphene citrate" or "clomiphene citrate resistance" or "clomiphene citrate resistant PCOS" or "clomiphene resistance" or "clomiphene resistant" or "clomiphene resistant patients" or "tamoxifen" or "tamoxifen citrate" or "*tamoxifen" or Title CONTAINS "anti‐estrogen" or "antiestrogens" or "antioestrogens"or "Clomiphene" or "clomiphene citrate" or "clomiphene citrate resistance" or "clomiphene citrate resistant PCOS" or "clomiphene resistance" or "clomiphene resistant" or "clomiphene resistant patients" or "tamoxifen" or "tamoxifen citrate" or "*tamoxifen" (416 hits)
Appendix 2. CENTRAL search strategy
CRSO web platform
Inception until 2 August 2016
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES (857) #2 MESH DESCRIPTOR Anovulation EXPLODE ALL TREES (110) #3 (Polycystic Ovar*):TI,AB,KY (1630) #4 (PCOS or PCOD):TI,AB,KY (1251) #5 (stein‐leventhal or leventhal):TI,AB,KY (15) #6 Anovulation:TI,AB,KY (274) #7 (oligo ovulat* or oligoovulat*):TI,AB,KY (14) #8 (ovar* adj3 (scelerocystic or polycystic or degeneration)):TI,AB,KY (570) #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 (1969) #10 MESH DESCRIPTOR Estrogen Receptor Modulators EXPLODE ALL TREES (2386) #11 (Estrogen Receptor Modulator*):TI,AB,KY (695) #12 antiestrogen*:TI,AB,KY (311) #13 (anti estrogen*):TI,AB,KY (76) #14 (anti oestrogen*):TI,AB,KY (63) #15 antioestrogen*:TI,AB,KY (39) #16 MESH DESCRIPTOR Clomiphene EXPLODE ALL TREES (417) #17 Clomiphene:TI,AB,KY (955) #18 MESH DESCRIPTOR Tamoxifen EXPLODE ALL TREES (1816) #19 Tamoxifen:TI,AB,KY (3618) #20 Nolvadex:TI,AB,KY (74) #21 Clomifene:TI,AB,KY (345) #22 Androxal:TI,AB,KY (3) #23 Clomid:TI,AB,KY (27) #24 Serophene:TI,AB,KY (2) #25 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 (5422) #26 #9 AND #25 (477)
Appendix 3. MEDLINE search strategy
Ovid MEDLINE(R) Epub Ahead of Print, In Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE (R)
Ovid Platform
1946 to 2 August 2016
1 exp Polycystic Ovary Syndrome/ (11628) 2 Polycystic Ovary Syndrome.tw. (9143) 3 (PCOS or PCOD).tw. (8204) 4 (stein‐leventhal or leventhal).tw. (708) 5 exp Anovulation/ (2078) 6 Anovulation.tw. (2385) 7 oligo ovulat$.tw. (77) 8 oligoovulat$.tw. (49) 9 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (79) 10 or/1‐9 (16924) 11 Estrogen Receptor Modulators/ (1932) 12 Estrogen Receptor Modulator$.tw. (2701) 13 antiestrogen$.tw. (6142) 14 antioestrogen$.tw. (491) 15 anti estrogen$.tw. (2219) 16 anti oestrogen$.tw. (787) 17 exp Clomiphene/ (4998) 18 Clomiphene.tw. (4629) 19 exp Tamoxifen/ (19471) 20 Tamoxifen.tw. (20024) 21 Nolvadex.tw. (138) 22 Clomifene.tw. (117) 23 Androxal.tw. (2) 24 Clomid.tw. (172) 25 Serophene.tw. (4) 26 or/11‐25 (38622) 27 10 and 26 (1618) 28 randomized controlled trial.pt. (425556) 29 controlled clinical trial.pt. (91298) 30 randomized.ab. (364322) 31 randomised.ab. (74905) 32 placebo.tw. (181508) 33 clinical trials as topic.sh. (178394) 34 randomly.ab. (260232) 35 trial.ti. (159000) 36 (crossover or cross‐over or cross over).tw. (70377) 37 or/28‐36 (1106446) 38 exp animals/ not humans.sh. (4282678) 39 37 not 38 (1020128) 40 27 and 39 (426)
Appendix 4. Embase search strategy
Ovid platform
1974 to 2 August 2016
1 exp ovary polycystic disease/ (20337) 2 Polycystic ovar$ disease.tw. (883) 3 Polycystic Ovary Syndrome.tw. (12122) 4 (PCOS or PCOD).tw. (11848) 5 (stein‐leventhal or leventhal).tw. (697) 6 exp anovulation/ (4438) 7 Anovulation.tw. (2905) 8 oligo ovulat$.tw. (88) 9 oligoovulat$.tw. (69) 10 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (84) 11 or/1‐10 (26127) 12 exp selective estrogen receptor modulator/ (6746) 13 Estrogen Receptor Modulator$.tw. (3523) 14 antiestrogen$.tw. (6672) 15 antioestrogen$.tw. (542) 16 anti estrogen$.tw. (2878) 17 anti oestrogen$.tw. (840) 18 exp clomifene/ (5313) 19 Clomiphene.tw. (5300) 20 Clomifene.tw. (193) 21 Androxal.tw. (18) 22 Clomid.tw. (935) 23 Serophene.tw. (189) 24 exp tamoxifen/ (52033) 25 Tamoxifen.tw. (26838) 26 Nolvadex.tw. (1369) 27 or/12‐26 (72819) 28 11 and 27 (2531) 29 Clinical Trial/ (861243) 30 Randomized Controlled Trial/ (412045) 31 exp randomization/ (71505) 32 Single Blind Procedure/ (22598) 33 Double Blind Procedure/ (130362) 34 Crossover Procedure/ (48133) 35 Placebo/ (279213) 36 Randomi?ed controlled trial$.tw. (140934) 37 Rct.tw. (21143) 38 random allocation.tw. (1553) 39 randomly allocated.tw. (25331) 40 allocated randomly.tw. (2143) 41 (allocated adj2 random).tw. (761) 42 Single blind$.tw. (17780) 43 Double blind$.tw. (164421) 44 ((treble or triple) adj blind$).tw. (577) 45 placebo$.tw. (236902) 46 prospective study/ (345031) 47 or/29‐46 (1600545) 48 case study/ (39626) 49 case report.tw. (311445) 50 abstract report/ or letter/ (968973) 51 or/48‐50 (1312826) 52 47 not 51 (1559063) 53 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5576565) 54 52 not 53 (1499261) 55 28 and 54 (863)
Appendix 5. PsycINFO search strategy
Ovid Platform
1806 to 2 August 2016
1 exp Endocrine Sexual Disorders/ (1057) 2 Polycystic Ovary Syndrome.tw. (215) 3 Polycystic Ovary disease.tw. (8) 4 (PCOS or PCOD).tw. (206) 5 (stein‐leventhal or leventhal).tw. (268) 6 Anovulation.tw. (61) 7 or/1‐6 (1526) 8 Estrogen Receptor Modulator$.tw. (134) 9 antiestrogen$.tw. (125) 10 antioestrogen$.tw. (2) 11 anti estrogen$.tw. (51) 12 anti oestrogen$.tw. (6) 13 Clomiphene.tw. (46) 14 Tamoxifen.tw. (453) 15 Clomifene.tw. (0) 16 Clomid.tw. (1) 17 or/8‐16 (700) 18 random.tw. (47326) 19 control.tw. (366342) 20 double‐blind.tw. (19967) 21 clinical trials/ (9724) 22 placebo/ (4606) 23 exp Treatment/ (657106) 24 or/18‐23 (1012755) 25 7 and 17 and 24 (9)
Appendix 6. CINAHL search strategy
Ebsco platform
1982 to 2 August 2016
| S36 | S23 AND S35 | 90 |
| S35 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 | 1,064,216 |
| S34 | TX allocat* random* | 5,168 |
| S33 | (MH "Quantitative Studies") | 14,720 |
| S32 | (MH "Placebos") | 9,760 |
| S31 | TX placebo* | 39,044 |
| S30 | TX random* allocat* | 5,168 |
| S29 | (MH "Random Assignment") | 41,345 |
| S28 | TX randomi* control* trial* | 107,910 |
| S27 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 843,769 |
| S26 | TX clinic* n1 trial* | 187,859 |
| S25 | PT Clinical trial | 79,689 |
| S24 | (MH "Clinical Trials+") | 200,782 |
| S23 | S8 AND S22 | 174 |
| S22 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 | 5,474 |
| S21 | TX Nolvadex | 19 |
| S20 | TX Tamoxifen | 3,668 |
| S19 | (MM "Tamoxifen+") | 1,775 |
| S18 | TX Clomid | 9 |
| S17 | TX Clomifene | 18 |
| S16 | TX Clomiphene | 346 |
| S15 | (MM "Clomiphene") | 119 |
| S14 | TX anti oestrogen* | 44 |
| S13 | TX anti estrogen* | 139 |
| S12 | TX antiestrogen* | 255 |
| S11 | TX antioestrogen* | 18 |
| S10 | TX Estrogen Receptor Modulator* | 1,314 |
| S9 | (MM "Estrogen Receptor Modulators+") | 2,486 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 3,156 |
| S7 | TX oligoovulat* | 6 |
| S6 | TX oligo ovulat* | 3 |
| S5 | TX Anovulation | 310 |
| S4 | (MM "Anovulation") | 98 |
| S3 | TX (PCOS or PCOD) | 1,620 |
| S2 | TX Polycystic Ovary Syndrome | 2,145 |
| S1 | (MM "Polycystic Ovary Syndrome") | 1,399 |
Data and analyses
Comparison 1. Antioestrogen versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy rate (per woman randomised) | 3 | 133 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.91 [1.77, 19.68] |
Comparison 2. Antioestrogen versus antioestrogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate (per woman) | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.59, 2.62] |
| 1.1 Clomiphene citrate versus tamoxifen | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.59, 2.62] |
| 2 Miscarriage rate (per woman) | 4 | 653 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.80, 4.12] |
| 2.1 Clomiphene citrate versus tamoxifen | 4 | 653 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.80, 4.12] |
| 3 Clinical pregnancy rate (per woman) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clomiphene citrate versus tamoxifen | 5 | 757 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.92, 1.85] |
| 3.2 Clomiphene citrate plus tamoxifen versus clomiphene citrate | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.32 [0.12, 91.60] |
| 4 Multiple pregnancy | 3 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.34, 16.04] |
| 5 OHSS | 3 | 567 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.4. Analysis.
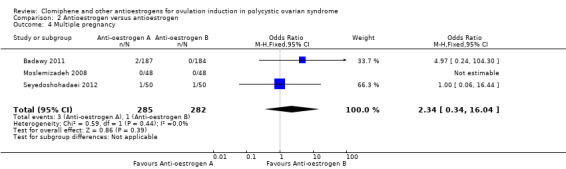
Comparison 2 Antioestrogen versus antioestrogen, Outcome 4 Multiple pregnancy.
2.5. Analysis.
Comparison 2 Antioestrogen versus antioestrogen, Outcome 5 OHSS.
Comparison 3. Antioestrogen versus gonadotropin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancy | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.98] |
| 2 Miscarriage rate (per woman) | 3 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.78] |
| 3 Clinical pregnancy rate (per woman) | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.93] |
| 4 Multiple pregnancy | 3 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.06, 1.06] |
| 5 OHSS | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.67] |
3.3. Analysis.
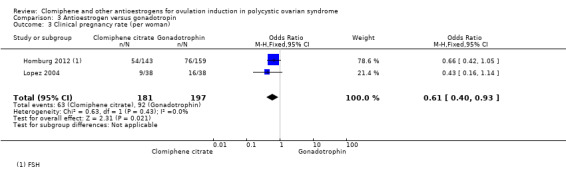
Comparison 3 Antioestrogen versus gonadotropin, Outcome 3 Clinical pregnancy rate (per woman).
3.4. Analysis.
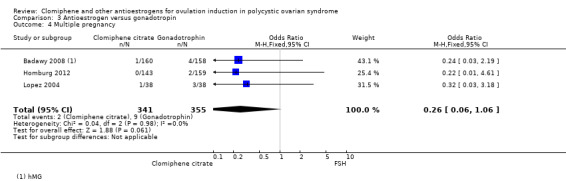
Comparison 3 Antioestrogen versus gonadotropin, Outcome 4 Multiple pregnancy.
3.5. Analysis.
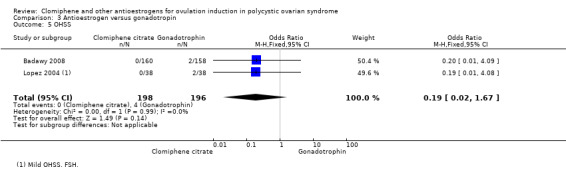
Comparison 3 Antioestrogen versus gonadotropin, Outcome 5 OHSS.
Comparison 4. Antioestrogen plus medical adjunct versus antioestrogen alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clomiphene citrate plus ketoconazole versus clomiphene citrate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Clinical pregnancy rate (per woman) | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.88, 6.40] |
| 1.2 Multiple pregnancy (per woman) | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.37, 3.78] |
| 1.3 Miscarriage rate | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.08] |
| 2 Clomiphene citrate plus bromocriptine versus clomiphene citrate | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Clinical pregnancy rate (per woman) | 2 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.48, 2.21] |
| 3 Clomiphene citrate plus dexamethasone versus clomiphene citrate | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Clinical pregnancy rate (per woman) | 4 | 434 | Odds Ratio (M‐H, Random, 95% CI) | 6.20 [2.20, 17.48] |
| 3.2 Multiple pregnancy (per woman) | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 7.71 [0.38, 155.64] |
| 4 Clomiphene citrate plus combined oral contraceptive versus clomiphene citrate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Miscarriage rate (per woman) | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.97] |
| 4.2 Clinical pregnancy rate (per woman) | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 27.18 [3.14, 235.02] |
| 4.3 Multiple pregnancy (per woman) | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.98 [0.39, 163.33] |
| 5 Clomiphene citrate plus hCG versus clomiphene citrate alone | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Ongoing pregnancy rate (per woman) | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.80] |
| 5.2 Miscarriage | 2 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.19, 2.62] |
| 5.3 Clinical pregnancy rate (per woman) | 2 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.59, 2.36] |
| 5.4 Multiple pregnancies (per woman) | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.19, 24.98] |
| 6 Clomiphene citrate plus hormone supplementation versus clomiphene citrate alone | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Miscarriage | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.46] |
| 6.2 Clinical pregnancy rate (per woman) | 2 | 161 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.37, 1.76] |
| 6.3 Multiple pregnancy | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 OHSS | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Adverse events | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.47] |
4.1. Analysis.
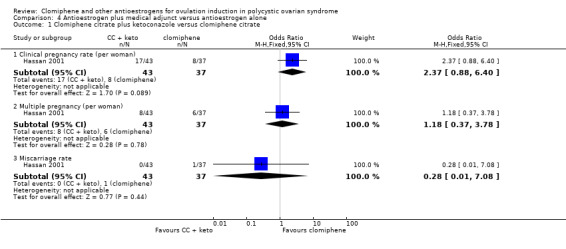
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 1 Clomiphene citrate plus ketoconazole versus clomiphene citrate.
4.4. Analysis.
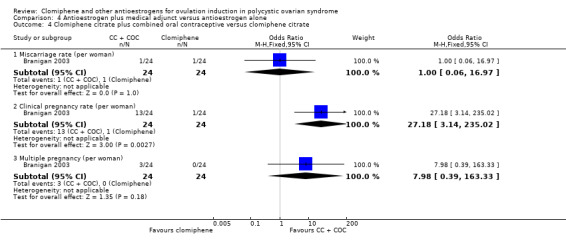
Comparison 4 Antioestrogen plus medical adjunct versus antioestrogen alone, Outcome 4 Clomiphene citrate plus combined oral contraceptive versus clomiphene citrate.
Comparison 5. Clomiphene citrate regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.45] |
| 1.1 Clomiphene citrate 5 days versus clomiphene citrate 10 days | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.45] |
| 2 Miscarriage rate | 1 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.27, 5.70] |
| 2.1 Early versus late clomiphene citrate | 1 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.27, 5.70] |
| 3 Clinical pregnancy | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.37, 1.33] |
| 3.1 Clomiphene 5 days v clomiphene 10 days | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.06, 0.55] |
| 3.2 Early v late clomiphene citrate | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.02, 7.75] |
| 4 Multiple pregnancy | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.20] |
| 4.1 Clomiphene 5 days v clomiphene 10 days | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.20] |
5.1. Analysis.
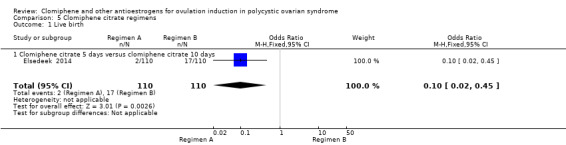
Comparison 5 Clomiphene citrate regimens, Outcome 1 Live birth.
5.2. Analysis.
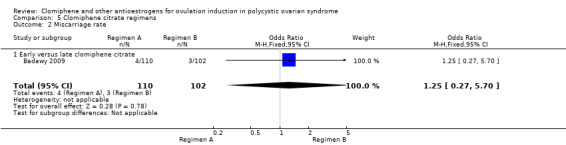
Comparison 5 Clomiphene citrate regimens, Outcome 2 Miscarriage rate.
5.3. Analysis.
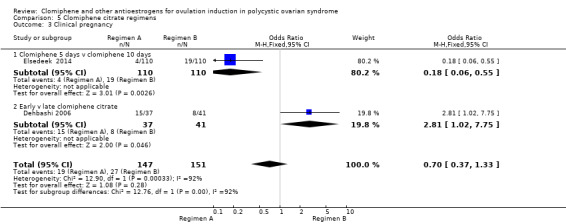
Comparison 5 Clomiphene citrate regimens, Outcome 3 Clinical pregnancy.
5.4. Analysis.
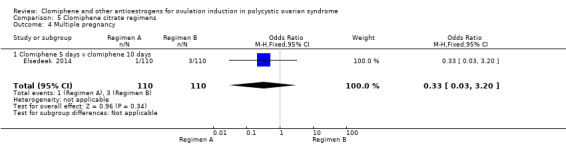
Comparison 5 Clomiphene citrate regimens, Outcome 4 Multiple pregnancy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badawy 2008.
| Methods | Parallel randomised controlled trial | |
| Participants | 318 women randomised Inclusion criteria: diagnosed with PCOS, patent fallopian tubes. Normal semen analysis in male partners. Exclusion criteria: Not stated Setting: outpatient clinic in Mansoura University Hospitals, Mansoura University, Egypt and a private practice setting Timing: May 2004 to May 2007 |
|
| Interventions | Clomiphene citrate ‐ 100 mg of clomiphene citrate daily starting on Day 2 of menses for 9 days (n = 160 women)
versus Gonadotropin ‐ human menopausal gonadotropin 75 IU intramuscularly daily for 5 days starting on Day 3 of menses (n = 158 women) |
|
| Outcomes | The primary outcome measures were the number of growing and mature follicles, serum oestradiol (pg/mL), serum progesterone (ng/mL), and endometrial thickness (mm). Secondary outcome measures were the occurrence of biochemical pregnancy and miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | Allocated by researcher |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the gonadotropin group was lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Only reported data for biochemical pregnancy |
Badawy 2009.
| Methods | Randomised controlled trial | |
| Participants | 212 women with PCOS Inclusion criteria: not clear, but included women with patent fallopian tubes and normal semen analysis in partners Exclusion criteria: not stated Setting: gynaecology outpatient clinic in Egypt Timing: November 2004 to March 2007 |
|
| Interventions | Withdrawal bleeding using 10 mg medroxyprogesterone acetate (MPA) for 10 days before stimulation Early CC ‐ 100 mg clomiphene citrate daily starting the next day after finishing MPA for 5 days (n = 110 women, 227 cycles) Late CC ‐ 100 mg clomiphene citrate daily for 5 days starting on Day 3 of menses (n = 102 women, 211 cycles) hCG 5000 to 10,000 IU given IM when at least 1 follicle was 18 mm or greater in diameter. Women advised to have intercourse 24 to 36 hours after hCG injection. |
|
| Outcomes | Primary outcomes: number of growing and mature follicles, serum E2, endometrial thickness Secondary outcomes: pregnancy (biochemical after 2 weeks), miscarriage |
|
| Notes | Sample size calculation: no ITT analysis: yes Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated using computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details, but blinding unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details, but blinding unlikely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all women randomised |
| Selective reporting (reporting bias) | High risk | Authors state that groups were balanced at baseline for demographics. However FSH and LH levels were significantly higher in the late‐CC group and the total number of follicles and the number of follicles ≥14 and 18 mm diameter were greater in the early‐CC group. Live birth data were not reported. |
Badawy 2011.
| Methods | Randomised controlled trial | |
| Participants | 371 women Age: clomiphene group 25.8 ± 2.1 years, tamoxifen group 26.2 ± 2.2 years Duration of infertility (months): clomiphene group 18.0 ± 7.2, tamoxifen group 16.8 ± 6.0 Inclusion criteria: women diagnosed with PCOS (ESHRE/ASRM 2003), patent fallopian tubes, normal semen analysis Setting: outpatient clinic, Egypt Timing: December 2005 to December 2009 |
|
| Interventions | Clomiphene citrate (n = 187) ‐ 1 cycle 100 mg/day from Day 3 for 5 days versus Tamoxifen (n = 184) ‐ 1 cycle 20 mg/day from Day 3 for 5 days |
|
| Outcomes | Primary: number of follicles, serum E2, endometrial thickness, ovulation rate Secondary: clinical pregnancy rate, miscarriage |
|
| Notes | Sample size calculation: yes, based on pregnancy rate ITT: yes Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random table" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation done by the investigators"; no other details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither women nor investigators were blinded, but unlikely to affect outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Multiple pregnancy and OHSS reported but not prespecified. Did not report on live birth. |
Boonstanfar 2001.
| Methods | Parallel randomised controlled trial | |
| Participants | 95 women (86 analysed) Inclusion criteria: anovulation with no other cause of infertility Age: CC 26.5 ± 4.3, TMX 26.6 ± 4.3 Duration of infertility: CC 3.7 ± 2.5 years, TMX 3.5 ± 2.9 years Exclusion criteria: uterine or adnexal pathology, abnormal HSG, abnormal semen analysis, age > 40 years, hyperprolactinaemia, hypo‐ or hyperthyroidism, FSH > 20 mIU/mL, progesterone > 3.0 ng/mL, previous exposure to ovulation induction agents, hepatic or renal disease, presence of a contraindication to trial drugs Location: Los Angeles, USA Setting: not stated |
|
| Interventions | Treatment(s): Both groups had a progesterone‐induced withdrawal bleed and then either 50 mg CC on Days 5 to 9, increased to 100 mg, and then 150 mg if woman remained anovulatory (n = 47); or 20 mg TMX on Days 5 to 9, increased to 40 mg, and then 60 mg if woman remained anovulatory (n = 48). Control or placebo: none Duration: not stated | |
| Outcomes | Relevant outcomes: live birth (incomplete follow‐up), pregnancy, ovulation, miscarriage (no definition), multiple birth, OHSS, and women‐reported adverse effects | |
| Notes | Contacted authors re: power calculation, blinding, funding, and ongoing pregnancy results; received no reply ITT: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for. Of 95 women randomised, 86 analysed, 9 did not return for follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported on. |
Branigan 2003.
| Methods | Parallel randomised controlled trial | |
| Participants | 48 women randomised (48 women analysed) Inclusion criteria: anovulation while receiving ≥ 150 mg clomiphene; under 36 years old; tubal patency (HSG/laparoscopy); normal fasting glucose and insulin; normal prolactin, thyroid‐stimulating hormone, and FSH levels; DHEAS ≤ 200 ug/mL; norm oestrogenic; no contraindication to COC use; and partner with normal semen analysis Age: 28.2 ± 3.4 Duration of infertility: 2.4 ± 0.8 Exclusion criteria: not stated Setting: Private tertiary infertility clinic; Bellingham, WA, USA |
|
| Interventions | Treatment(s): COC (0.03 mg ethinyl estradiol and 0.15 mg desogestrel (Desogen)) continuously for 42 to 50 days followed by 1 cycle of 100 mg CC (Days 5 to 9); 10,000 IU hCG ovulation trigger Control/placebo: 38 to 56 days no treatment followed by 1 cycle of 100 mg CC (Days 5 to 9); 10,000 IU hCG ovulation trigger Duration: up to 6 cycles of CC for those women who ovulated but did not become pregnant in the first cycle | |
| Outcomes | Relevant outcomes: ovulation, pregnancy, multiple pregnancy, miscarriage (no definition) | |
| Notes | Contacted authors re: randomisation and allocation concealment, treatment protocol, adverse effects, and definitions used; received no reply Power calculation: yes ITT: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised permuted blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Adequate ‐ opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details, but blinding unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised analysed |
| Selective reporting (reporting bias) | High risk | No report of live birth |
Branigan 2005.
| Methods | Randomised, parallel, 2‐arm study | |
| Participants | 70 women randomised (66 women analysed) Inclusion criteria: previously documented dominant follicle or follicles ≥12 mm mean diameter on transvaginal ultrasound follicular monitoring while receiving CC at the 100 mg dose but failed to ovulate; under the age of 40 years; documented normal uterine cavity and patent tubes by either HSG or laparoscopy and hysteroscopy; normal fasting glucose and insulin levels; normal prolactin, thyroid‐stimulating hormone, and FSH; DHEAS sulphate levels of 200 μg/mL or less; normal semen analysis according to WHO criteria in male partner Age: mean age of CC + hCG group 34.1 ± 1.1 years, mean age of CC‐only group 33.4 ± 1.3 years Duration of infertility: no details Exclusion criteria: not stated Setting: Private tertiary infertility clinic; USA |
|
| Interventions | Transvaginal ultrasound follicular monitoring started on Day 12 and was repeated every 1 to 2 days until mean diameter of lead follicle was greater than 20 mm. Treatment(s): CC + hCG: CC 100 mg on Days 5 to 9 plus daily IM injections of 200 IU hCG when the lead follicle was 12 mm or larger until 20 mm or larger was attained. (If the follicle diameter failed to increase by more than 1 mm per day after 14 mm or 14 mm was not achieved, monitoring was ceased and the cycle cancelled) (n =35) Control or placebo: CC‐only 150 mg Days 5 to 9 (n = 35) Both groups received 10,000 IU hCG IM injection when lead follicle diameter was 20 mm or greater. Timed intercourse advised on day of hCG injection and following day. Duration: 1 cycle |
|
| Outcomes | Relevant outcomes: ovulation rate, endometrial thickness, number of follicles, E2 levels, testosterone levels, P4 levels, pregnancy rate | |
| Notes | Pregnancy confirmed by serum hCG and 7‐week gestational ultrasound. BMI group 1: 21.3 ± 0.4, group 2: 21.2 ± 0.3 Power calculation: yes, based on expected ovulation rate ITT: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks with a block size of 4 used to generate the 2 groups. |
| Allocation concealment (selection bias) | Low risk | The group assignments were contained in consecutively numbered, opaque envelopes, which were opened after the women were enrolled in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of women randomised: 70 Number of women analysed: 66 Number of withdrawals and reasons: 1 woman in CC + hCG group and 3 women in CC‐only group did not begin the study. |
| Selective reporting (reporting bias) | High risk | Results section reported on additional relevant outcomes to those stated in the methods section. No details of adverse events, no report of live birth |
Cudmore 1966.
| Methods | Cross‐over trial | |
| Participants | 22 women randomised (22 women analysed) Inclusion criteria: All women stated as anovulatory. Secondary amenorrhoea (> 2 years) or oligomenorrhoea (no more than 4 periods a year and none in the 3 months prior to study) or anovulatory infertility (> 2 years); no infertility treatment in the 3 months prior to the study; no other cause of infertility found Exclusion criteria: not stated Age: treatment: 18 to 33, placebo: 20 to 29 Duration of infertility: not stated Setting: Halifax, Canada |
|
| Interventions | Treatment(s): 50 mg CC (Days 1 to 14) (n = 13) Control or placebo: placebo (Days 1 to 14) (n = 9) Duration: 3 cycles, then 3 cycles | |
| Outcomes | Relevant outcomes: ovulation and women‐reported adverse effects, hormonal responses | |
| Notes | Authors not contacted as trial published > 15 years ago. Power calculation: not stated ITT: yes Source of funding: Support and drug supplied by Wm S Merrell Company, Cincinnati, OH, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Entered treatment group by chance |
| Allocation concealment (selection bias) | Unclear risk | Coded, but unclear if this was centrally administered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but not stated who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, but not stated who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for. |
| Selective reporting (reporting bias) | High risk | Pregnancy not noted as an outcome in methods but described in results. No adverse events or miscarriage reported, no report of live birth. |
Daly 1984.
| Methods | Parallel randomised controlled trial | |
| Participants | 64 women randomised (45 women analysed) Inclusion criteria: either anovulatory as evidenced by basal body temperature charting or oligomenorrhoeic but responsive to progesterone. No previous exposure to clomiphene Age: not stated Duration of infertility: not stated Exclusion criteria: hyperprolactinaemia, hyper‐ or hypothyroidism, major male factor, tubal disease (HSG) Setting: USA |
|
| Interventions | Treatment(s): 50 mg CC (Days 5 to 9) plus 0.5 mg DEX. CC increased up to 150 mg if woman remained anovulatory. Women remaining anovulatory at 150 mg crossed to other arm of trial, as did women who ovulated but had an abnormal postcoital test or endometrial biopsy.
Control/placebo: 50 mg CC (Days 5 to 9). CC increased up to 150 mg if woman remained anovulatory. Women remaining anovulatory at 150 mg crossed to other arm of trial, as did women who ovulated but had an abnormal postcoital test or endometrial biopsy. Timed intercourse: no details Duration: not stated |
|
| Outcomes | Relevant outcomes: ovulation, pregnancy | |
| Notes | Authors not contacted as trial published > 15 years ago. Power calculation: not stated ITT: yes Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐randomised schedule |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details of blinding, but unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 women (4 CC, 5 CC + DEX) discontinued in the first cycle, and 10 women were found to have other infertility factors, leaving 22 women receiving CC alone and 23 receiving CC + DEX. |
| Selective reporting (reporting bias) | High risk | No outcome measures were reported in the methods section. No details of adverse events, no report of live birth |
Dehbashi 2006.
| Methods | Parallel randomised controlled trial | |
| Participants | 78 infertile women with PCOS. Inclusion criteria: not clearly stated, but included women who were anovulatory with laboratory or clinical evidence of hyperandrogenism with no apparent cause Setting: Infertility Research Centre, Iran Timing: June 2002 to May 2004 |
|
| Interventions | CC 100 mg starting day 1 of menstrual cycle for 5 days (n = 37 women, 71 cycles) for a maximum of 3 cycles CC 100 mg starting day 5 of menstrual cycle for 5 days (n = 41 women, 78 cycles) for a maximum of 3 cycles If women did not menstruate, then menstrual cycles were induced with a single 200 mg IM dose of progesterone. |
|
| Outcomes | Not stated a priori but reported on hormonal levels, follicle number and size, endometrial thickness, ovulation, pregnancy | |
| Notes | Sample size calculation: no ITT: yes Funding: Office for the Vice Chancellor for Research, Shiraz University of Medical Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised", standard random number table |
| Allocation concealment (selection bias) | Low risk | "Randomization was done by a nurse not aware of the objectives of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind"; nurses administering the regimen were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed. |
| Selective reporting (reporting bias) | High risk | No outcomes were prespecified. |
Elkind‐Hirsch 2005.
| Methods | Parallel randomised controlled trial | |
| Participants | 71 women randomised (65 analysed) Inclusion criteria: aged 21 to 35 years, oligo‐amenorrhoea, BMI > 18 and < 38 Exclusion criteria: pregnant, known endometrial or uterine anomaly, tubal occlusion, previously failed to ovulate in response to clomiphene, premature ovulation failure Age: median age 28 Duration of infertility: not stated Setting: Women's Health Research Institute (April 2003 to July 2004), USA |
|
| Interventions | Treatment(s): CC (100 mg orally for 5 days from Day 3 to 7 of cycle) Control or placebo: CC (100 mg orally for 5 days from Day 3 to 7 of cycle) + HS in the form of estradiol (E2) 1.5 mg (2 tablets) orally twice daily on cycle day 8. On cycle day 10, women commenced monitoring urine LH levels. E2 was discontinued with detection of LH surge. If woman was pregnant, vaginal progesterone was administered daily for an additional 10 weeks. Timed intercourse: encouraged from cycle day 10 Duration: 1 cycle |
|
| Outcomes | Relevant outcomes: pregnancy rate, ovulation rate | |
| Notes | Pregnancy assessed as serum hCG 2 weeks following LH surge. Power calculation indicated 458 women per group should have been randomised. Study stopped after 88 participants. ITT: yes for women with a P assay Source of funding: grant from Columbia Laboratories Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women had an abnormal scan and were discontinued without receiving treatment. |
| Selective reporting (reporting bias) | High risk | No details of adverse events, no report of live birth |
Elnashar 2006.
| Methods | Parallel randomised controlled study | |
| Participants | 80 women randomised (80 women analysed) All women had PCOS as defined by Rotterdam criteria, without hyperprolactinaemia, clinical evidence of hypercorticism, or thyroid dysfunction. Inclusion criteria: aged 18 to 39 years, period of infertility > 2 years, serum DHEAS within normal levels, no treatment during previous 2 months Age: CC + DEX 23.4 ± 3.6 years, CC + placebo 25.2 ± 2.4 years Duration of infertility: CC + DEX 2.1 ± 0.9 years, CC + placebo 3.2 ± 1.4 years Exclusion criteria: history of pelvic surgery or infertility factor other than anovulation Setting: Women's hospital clinic (March 2004 to December 2004); Egypt |
|
| Interventions | Induction of menses using progesterone‐in‐oil (100 mg); 10,000 IU IM hCG given when at least 1 follicle > 18 mm Treatment(s): CC 100 mg daily from Day 3 to 7 + DEX 2 mg daily orally in 2 divided doses from Day 3 to 12 Control or placebo: CC 100 mg daily from Day 3 to 7 + placebo (folic acid) from Day 3 to 12 Timed intercourse advised 24 to 36 hours after hCG. Duration: 1 cycle |
|
| Outcomes | Relevant outcomes: ovulation rate, number of follicles > 18 mm, endometrial thickness, and pregnancy rate | |
| Notes | All women had previously received clomiphene and were defined as clomiphene resistant. Clinical pregnancy defined as presence of gestational sac on ultrasound scanning 1 week after missed period. Power calculation: yes, based on results of study by Parsanezhad 2002a; Parsanezhad 2002b ITT: All women randomised were analysed. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned randomly, no further details |
| Allocation concealment (selection bias) | Unclear risk | Used closed, dark envelopes and allocated by a 3rd party (nurse) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and physician monitoring cycles were blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All women randomised were analysed, no dropouts. |
| Selective reporting (reporting bias) | High risk | No report of live birth and no details of adverse events |
Elsedeek 2014.
| Methods | Randomised controlled trial | |
| Participants | 230 women Age: CC 5‐days group 27.67 ± 3.65, CC 10‐days group 29.23 ± 4.43 Duration of infertility: CC 5‐days group 2.8 ± 1.3, CC 10‐days group 2.4 ± 0.9 Inclusion criteria: nulliparous; PCOS using NIH‐NICHD definition (presence of chronic anovulation, and clinical and/or biochemical evidence of hyperandrogenism excluding other related disorders); diagnosed as clomiphene resistant (lack of response to clomiphene at 100 mg/day for 5 days within 3 months of inclusion in the study) Exclusion criteria: baseline ovarian cysts or uterine pathology Setting: University infertility clinics, Egypt Timing: January 2009 to January 2012 |
|
| Interventions | Starting on Day 3 of progestin‐induced withdrawal bleeding Clomiphene citrate ‐ 1 cycle of 200 mg/day for 5 days Clomiphene citrate ‐ 1 cycle of 100 mg/day for 10 days Women also received 4 placebo tablets for 10 days. |
|
| Outcomes | Primary outcome: ovulation Secondary outcomes: number of dominant follicles, endometrial thickness, clinical pregnancy, live birth rate |
|
| Notes | Sample size calculation: yes, based on ovulation rate ITT: unclear Funding: no details in paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated tables" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation was placed in sealed envelopes opened on the first day of the treatment for each patient by infertility unit administrator". Not clear if envelopes were opaque or if they were handed out sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "placebo" controlled. Women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sonographers assessing follicle size were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors report including 230 women. The power calculation required 220 women, and that is what they report on. Unclear how these were selected from the 230 |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified were reported. The authors also reported multiple pregnancy and FSH levels. Adverse effects were not reported. |
Esmaeilzadeh 2011.
| Methods | Randomised controlled trial | |
| Participants | 60 women Mean age in CC + DEX group 24.8 ± 3.56 years, in CC + placebo group 23.1 ± 3.45 years Inclusion criteria: diagnosed with PCOS (Rotterdam criteria), age 18 to 35 years, infertility for 1 to 5 years, normal DHEAS level, diagnosed with clomiphene resistance (3 cycles of clomiphene 150 mg daily from Day 3 to 7) Exclusion criteria: hyperprolactinaemia, thyroidism, had a pelvic pathology or surgery, infertility factor other than anovulation Setting: Infertility and Reproductive Health Centre, Babal, Iran Timing: 2008 to 2010 |
|
| Interventions | No treatments for 3 months prior to trial entry 1 cycle of: CC 100 mg from Day 3 to 7 plus DEX 2 mg/day in divided doses Day 5 to 14 (n = 30) versus CC 100 mg from Day 3 to 7 plus placebo (folic acid 1 mg/day) Day 5 to 14 (n = 30) |
|
| Outcomes | Ovulation, clinical pregnancy rate, follicular development, hormonal status | |
| Notes | Power calculation: yes, but unclear on what it was based ITT: yes Funding: Babel University of Medical Science |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence |
| Allocation concealment (selection bias) | Unclear risk | "Concealed from study participants"; no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and doctor/nurse were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collector was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals after randomisation |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes were reported. Did not report on ongoing pregnancies, live births, or adverse effects |
Garcia 1985.
| Methods | Cross‐over randomised trial | |
| Participants | 49 women randomised (46 women analysed) Inclusion criteria: amenorrhoea (> 6 months), progesterone withdrawal bleeding, and no other known cause of infertility Age: mean 27.6 years Duration of infertility: not stated Exclusion criteria: not stated Setting: Department of Obstetrics and Gynecology; Philadelphia, USA |
|
| Interventions | Treatment(s): 50 mg clomiphene, increased by 50 mg if ovulation failed to occur, up to 250 mg (n = 24) Control or placebo: placebo, 1 tablet, increased up to 5 tablets similar to treatment (n = 22) Duration: 5 cycles, then 5 cycles | |
| Outcomes | Relevant outcomes: ovulation and pregnancy | |
| Notes | Authors not contacted as trial published > 15 years ago. Power calculation: not stated ITT: yes Source of funding: National Institute of Child Health and Human Development grant Notes: cross‐over trial, phase 1 data only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49 randomised and 46 analysed (21 withdrawals: 11 difficulty with protocol, 4 ambivalence towards pregnancy at time, 4 with medical difficulties, and 2 left the country) |
| Selective reporting (reporting bias) | High risk | No details of adverse events, no report of live birth |
Ghafourzadeh 2004.
| Methods | Randomised controlled trial | |
| Participants | 100 women Age range 18 to 39 years; mean age clomiphene + tamoxifen 25.53 ± 3.78 years, clomiphene alone 25.59 ± 4.65 years Duration of infertility: clomiphene alone 3.66 ± 1.83, clomiphene + tamoxifen 3.83 ± 2.0 Inclusion criteria: not clearly specified, but appears to be women with PCOS with normal hysterosalpingography, partners with normal semen analysis (WHO criteria), clomiphene resistant (failure with 3 previous cycles) Exclusion criteria: Cushing syndrome and adrenal hyperplasia Setting: Infertility clinic, Iran Timing: 2001 to 2003 |
|
| Interventions | Clomiphene 100 mg/day from Day 5 to 9 (n = 51) versus Clomiphene 50 mg/day + tamoxifen 20 mg/day from Day 5 to 9 (n = 49) |
|
| Outcomes | Development of at least 1 dominant follicle, positive pregnancy test | |
| Notes | Power calculation: no ITT: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised"; no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated, unclear if data are reported per women or per cycle |
| Selective reporting (reporting bias) | High risk | Ovulation rate is reported but was not prespecified. No data were reported for clinical pregnancy, live birth, or adverse effects. |
Hassan 2001.
| Methods | Parallel randomised controlled trial | |
| Participants | 97 women randomised (80 women analysed) Inclusion criteria: infertile women with PCOS and insulin resistance Exclusion criteria: male factor infertility Age: not stated Duration of infertility: not stated Setting: Alexandria, Egypt |
|
| Interventions | Treatment(s): ketoconazole 400 mg for 85 days pretreatment followed by CC 100 mg to 150 mg, women persistently failing to ovulate on 150 mg CC were shifted to hMG (n = 49) Control/placebo: CC 100 mg to 150 mg, women persistently failing to ovulate on 150 mg CC were shifted to hMG (n = 48) Duration: 3 to 6 cycles | |
| Outcomes | Relevant outcomes: pregnancy, multiple pregnancy, spontaneous abortion (after cord pulse) | |
| Notes | Authors contacted re: power calculation, allocation concealment, blinding, inclusion and exclusion criteria, exclusions and dropouts, age ranges, ITT analysis, hMG treatment, outcome definitions, and side effects; no reply received Incidence of clomiphene resistance: treatment 11.6% (5/43), control 32.4% (12/37) Power calculation: not stated ITT: not stated Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly divided using random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of women analysed: 80, treatment 43 and control 37 Number of withdrawals and reasons: control 11, treatment 6, reasons not stated |
| Selective reporting (reporting bias) | High risk | Pregnancy and multiple pregnancy are not specified as outcomes in the methods section, similar to antioestrogenic markers. No details of adverse events, no report of live birth |
Homburg 2012.
| Methods | Parallel randomised controlled trial (multicentre, n = 10) | |
| Participants | 302 women randomised Inclusion criteria: < 40 years old; normal uterine cavity and tubal patency; male partners had normal semen analysis; anovulatory or oligo‐anovulatory infertility associated with PCOS Exclusion criteria: no details Setting: multicentre across 10 European and South American sites Timing: August 2005 to March 2009 |
|
| Interventions | 3 cycles of: Clomiphene citrate (oral) ‐ starting dose 50 mg/day for 5 days from Day 4 of a progestin‐induced or spontaneous menstruation, rising by 50 mg/day up to 150 mg in subsequent cycles if ovulation not achieved (n = 143) versus FSH (s.c.) in a low‐dose protocol starting with 50 IU on cycle day 4, with weekly increments of 25 IU to induce a follicular response (n = 159) |
|
| Outcomes | Live birth, clinical pregnancy, ongoing pregnancy, miscarriage, multiple pregnancy | |
| Notes | ITT: yes Funding: unrestricted educational grant from Organon, Oss, Netherlands (MSD/Schering‐Plough) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation" |
| Allocation concealment (selection bias) | Low risk | "sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of blinding, and this would be unlikely given that one intervention was oral and one was subcutaneous. Blinding unlikely to have influenced fertility outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the clomiphene citrate group, 20 women did not complete the full study (5 intercycle, 1 chemical pregnancy, 14 personal reasons). In the FSH group, 27 women did not complete the full study (4 intercycle, 3 chemical pregnancy, 19 personal reasons, 1 following cycle cancellation for OHSS). |
| Selective reporting (reporting bias) | High risk | Although live birth was prespecified, no data were reported for this outcome. Ectopic pregnancy was reported as an outcome but was not prespecified in the methods. |
Johnson 1966.
| Methods | Cross‐over randomised trial (multicentre, n = 5) | |
| Participants | Inclusion criteria: anovulation for > 6 months, adequate endogenous oestrogen, no local or systematic defect that may interfere with CC action Age: not stated Duration of infertility: not stated Exclusion criteria: not stated Setting: USA | |
| Interventions | Treatment(s): 100 mg CC Days 6 to 10 Control or placebo: placebo Days 6 to 10 Duration: 1 cycle, then 1 cycle | |
| Outcomes | Relevant outcomes: pregnancy and ovulation | |
| Notes | Power calculation: not stated ITT: not stated Source of funding: supported by Wm S Merrell Company, Cincinnati, OH, USA Phase 1 data used only. Authors not contacted as trial published > 15 years ago. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Pharmacy coded drug boxes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind; no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of women randomised: 75 Number of women analysed: 65 (33 to CC and 32 to placebo) Number of withdrawals and reasons: 13, 8 failed to return or did not comply with protocol, and 5 became pregnant in the first phase |
| Selective reporting (reporting bias) | High risk | No details of adverse events, no report of live birth |
Lopez 2004.
| Methods | Parallel randomised controlled trial | |
| Participants | 76 women randomised Inclusion criteria: women aged < 40 years with anovulatory infertility due to PCOS of at least 1 year's duration and attending the infertility clinic at the Hospital Virgen de la Arrixaca in Murcia (Spain). Also ultrasonographic appearance of polycystic ovaries, a positive response to the progestin challenge test, normal serum prolactin, DHEAS sulphate, and fasting glucose concentrations, a normal HSG (and laparoscopy when appropriate), and no history of pelvic surgery or pelvic inflammatory disease. Normal semen analysis in male partner Exclusion criteria: previous pregnancy or previous treatment with ovarian stimulation drugs Setting: infertility clinic at the Hospital Virgen de la Arrixaca in Murcia (Spain) Timing: April 2000 to December 2001 |
|
| Interventions | Up to 3 consecutive cycles Clomiphene citrate (50 to 150 mg/day for 5 days): a daily dose of 50 mg for 5 days, starting on Day 5 following spontaneous or induced menstruation. If ovulation was documented but there was no pregnancy, the same dose was used in the next cycle. If there was no ovulatory response, the daily dose was increased by 50 mg for the subsequent cycle, up to a maximum daily dose of 150 mg in the 3rd treatment cycle (n = 38). versus Recombinant human FSH in a chronic, low‐dose, step‐up protocol (daily starting dose 75 IU) commenced on Day 3 following spontaneous or induced menstruation. The chronic, low‐dose, step‐up regimen consisted of a starting dose of 75 IU daily s.c., with dose increments of 37.5 IU daily every 7 days if there was no evidence of ovarian response by ultrasonography (i.e. no follicle > 10 mm in diameter). This stepwise increase was continued until ovarian activity was seen, at which time the dose was maintained. The starting dose of FSH could be modified in successive treatment cycles based on ovarian response in the previous cycle (n = 38). |
|
| Outcomes | Cumulative pregnancy, cycle cancellation, ovulation rate per cycle, cumulative ovulation rate, pregnancy rate per cycle, incidence of OHSS, cumulative live birth rate, and multiple birth rate | |
| Notes | Data are reported per cycle rather than per woman randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization table" |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes each containing a unique study number and prepared independently by a secretary." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but unlikely to affect pregnancy outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the FSH group withdrew after 1 unsuccessful treatment cycle. |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported. |
Moslemizadeh 2008.
| Methods | Randomised controlled trial | |
| Participants | 157 women enrolled Age of women in clomiphene‐alone group 27.6 ± 4.5 years, in tamoxifen group 27.1 ± 4.1 years, in clomiphene + estradiol group 27.3 ± 5.1 years Duration of infertility in clomiphene‐alone group 4.2 ± 2.7 years, in tamoxifen group 4.2 ± 2.3 years, in clomiphene + estradiol group 3.5 ± 2.7 years Inclusion criteria: PCOS, tubal patency Exclusion criteria: uterine, kidney, liver, or thyroid disease; uterine anomalies; uterine leiomyoma; male factor infertility; > 35 years old; secondary infertility; duration of infertility > 10 years; hyperprolactinaemia; hyper‐/hypothyroidism; FSH > 12 mIU ml‐1 in the 3rd day of the cycle; BMI > 30 kg/m2; clomiphene resistance; previous exposure to other ovulation induction agents; interval of earlier treatment with ovulatory agents less than 6 months; contraindication to one of the medications Setting: infertility clinic and research clinic, Sari, Iran Timing: August 2006 to August 2007 |
|
| Interventions | Clomiphene citrate 2 x 50 mg daily from Day 3 to 9 plus estradiol 2 mg daily from Day 8 to hCG injection day (n = 48) versus Clomiphene citrate 2 x 50 mg daily from Day 3 to 9 (n = 48) versus Tamoxifen 20 mg daily from Day 3 to 9 (n = 48) All groups received placebo. |
|
| Outcomes | Ovulation, clinical pregnancy rate | |
| Notes | Sample size calculation: yes, based on ovulation and pregnancy ITT: yes Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided"; no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women received placebo and were blinded to allocation. Paper states that the trial was double‐blind, but it was unclear who aside from the women was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 157 women enrolled, 13 were excluded due to ovulation failure, and 144 who were randomised completed the trial. |
| Selective reporting (reporting bias) | High risk | Additional outcomes were reported that were not prespecified. No report on live birth |
Omran 2011.
| Methods | Randomised controlled trial | |
| Participants | 220 cycles (number of women not reported) Inclusion criteria: not clearly stated, but included women with clomiphene‐resistant PCOS Exclusion criteria: not stated Setting: not stated. Egypt Timing: not stated |
|
| Interventions | 200 mg clomiphene citrate for 5 days 100 mg clomiphene citrate for 10 days |
|
| Outcomes | Ovulation rate, time to ovulation, number of follicles, endometrial thickness when largest follicle had a diameter of 18 mm, midluteal progesterone, pregnancy | |
| Notes | Sample size calculation: no ITT: unclear Funding: not reported Conference abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised"; no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double‐blind, but unclear who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Conference abstract only, no details on randomisation and attrition rates |
| Selective reporting (reporting bias) | High risk | Conference abstract only, no data reported |
Parsanezhad 2002a.
| Methods | Parallel randomised controlled trial | |
| Participants | 230 women randomised Inclusion criteria: PCOS as defined by a history of oligo‐ or amenorrhoea, increased basal LH and androgen levels, polycystic ovaries found on ultrasound. Plus clomiphene citrate resistance, defined as failure to ovulate and achieve normal luteal phase with 250 mg dose of CC for 5 days and at least 5 cycles Age: mean age treatment group 23.56 years, control group 23.36 years. Range 19 to 35 for both groups Duration of infertility: treatment mean 4 years, range 2 to 14; control mean 4.25 years, range 3 to 14.5 Exclusion criteria: not stated Setting: Reproductive and endocrinology division, university; Shiraz, Iran |
|
| Interventions | Treatment(s): 200 mg CC (Days 5 to 9), 2 mg DEX (Days 5 to 14), hCG (10,000 IU) as an ovulation trigger (n = 111) Control or placebo: 200 mg CC (Days 5 to 9), placebo 4 times a day (Days 5 to 14), hCG (10,000 IU) as an ovulation trigger (n = 119) Duration: up to 6 cycles | |
| Outcomes | Relevant outcomes: ovulation rate, pregnancy | |
| Notes | Authors contacted re: power calculation, randomisation, blinding, exclusion criteria, exclusions and dropouts, and ITT analysis; no reply received. Power calculation: not stated ITT: not stated Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | High risk | Unclear, 3rd party (pharmacist), odd‐even numbers given to treatment or control (no further explanation provided by authors) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up not clear |
| Selective reporting (reporting bias) | High risk | No details of adverse events, no report of live birth |
Parsanezhad 2002b.
| Methods | Parallel randomised controlled trial | |
| Participants | 100 women randomised Inclusion criteria: PCOS as defined by women with 3 of the following: infertility, oligo‐ or amenorrhoea, acne or hirsutism, obesity, increased testosterone, increased DHEAS, LH/FSH ratio > 2, polycystic ovaries on ultrasound. Plus clomiphene citrate resistance, defined as failure to ovulate and achieve normal luteal phase with the highest dose of CC for 5 days and at least 5 cycles. Plus normal prolactin (80 to 500 mIU/mL) Age: mean age treatment group 25.02 ± 2.7 years, control group 24.87 ± 2.9 years Duration of infertility: treatment mean 4.53 ± 3.1 years, range 2 to 22; control 4.02 ± 1.9 years, range 2 to 10 Exclusion criteria: not stated Setting: Shiraz, Iran |
|
| Interventions | Treatment(s): 200 mg CC on Days 5 to 9, bromocriptine gradual dose increase up to 2.5 mg 3 times a day continuously, hCG (10,000 IU) as an ovulation trigger on Day 16 or 17 (n = 47) Control or placebo: 200 mg CC on Days 5 to 9, placebo 3 times a day continuously, hCG (10,000 IU) as an ovulation trigger on Day 16 or 17 (n = 53) Duration: up to 6 cycles | |
| Outcomes | Relevant outcomes: ovulation rate, pregnancy, women‐reported adverse effects | |
| Notes | Authors contacted re: power calculation, randomisation, blinding, exclusion criteria, exclusions and dropouts, and ITT analysis; no reply received. ITT: not stated Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, no details |
| Allocation concealment (selection bias) | High risk | Unclear, 3rd party (pharmacist), odd‐even numbers given to treatment or control (no further explanation provided by authors) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but no details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up data not clear |
| Selective reporting (reporting bias) | High risk | No details of adverse events such as miscarriage, no report of live birth |
Seyedoshohadaei 2012.
| Methods | Randomised controlled trial | |
| Participants | 150 women randomised Mean age of clomiphene group 24.7 ± 4.7 years, tamoxifen group 25.4 ± 4.2 years; mean duration of infertility for the clomiphene group 2.95 ± 2.1 years, tamoxifen group 2.99 ± 2.0 years Inclusion criteria: anovulatory women, infertile at least 1 year, menstrual cycle 35 days to 6 months, normal serum prolactin, TSH, FSH, LH. Normal uterus and ovary, no evidence of polycystic ovary or dominant follicle at midcycle in ultrasonography, normal uterus and patent tubes on HSG. Normal semen analysis for partner Exclusion criteria: no details Setting: private clinics in Iran Timing: November 2007 to September 2009 |
|
| Interventions | Spontaneous or progesterone‐induced menses Clomiphene citrate (n = 50 women, 199 cycles) 50 mg daily from Day 3 to 9 (increased by 50 mg per failed cycle to a maximum of 150 mg daily) Tamoxifen (n = 50 women, 174 cycles) 10 mg daily from Day 3 to 9 (increased by 10 mg per cycle to a maximum of 30 mg daily) Letrozole (n = 50) 2.5 mg daily from Day 3 to 9 (increased by 2.5 mg per failed cycle to a maximum of 7.5 mg daily) If failed to ovulate after 5 days, then treatment continued up to 7 days. Treatment stopped if pregnant or failure to ovulate after 7 days of treatment or failed to conceive after 6 months despite ovulation, or severe adverse reaction |
|
| Outcomes | Primary outcomes: number of follicles 18 mm or more in diameter, endometrial thickness, ovulation rate Secondary outcomes: clinical pregnancy rate, spontaneous miscarriage rate, multiple pregnancy rate, OHSS |
|
| Notes | Sample size calculation: no ITT: yes Funding: Kurdistan University of Medical Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women not blinded, but unlikely to affect the outcome. Researchers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified were reported. |
Suginami 1993.
| Methods | Randomised cross‐over trial | |
| Participants | 20 women randomised (20 women analysed) Inclusion criteria: anovulation, normoprolactinaemic Age: Group A: 29.3 ± 3.1 years, Group B: 28.6 ± 3.0 years Duration of infertility: not stated Exclusion criteria: none stated Setting: Ehime, Japan |
|
| Interventions | Treatment(s): Both groups received combination pill (0.05 mg ethinyl E2 and 0.5 mg norgestrel) to induce withdrawal bleed, then Gp A ‐ 100 mg CC on Days 5 to 9 for 3 cycles and then 50 mg CC plus 20 mg TMX on Days 5 to 9 for 3 cycles. Gp B ‐ reverse sequence, otherwise identical (n = 10)
Control or placebo: None (n = 10) Timed intercourse: normal intercourse encouraged, no details Duration: 3 cycles then 3 cycles |
|
| Outcomes | Relevant outcomes: ovulation, pregnancy, and women‐reported adverse effects | |
| Notes | Unable to contact authors. Power calculation: not stated ITT: no Source of funding: not stated Notes: cross‐over trial, phase 1 data only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts; all women randomised were analysed |
| Selective reporting (reporting bias) | High risk | No details of adverse events such as miscarriage, no report of live birth |
Tripathy 2013.
| Methods | Parallel randomised trial | |
| Participants | 74 women randomised (74 women analysed) Inclusion criteria: diagnosed with PCOS (2 out of 3 criteria); serum prolactin ≤ 20 ng/mL, age less than 35 years, BMI 20 to 30 kg/m2 Exclusion criteria: hyperprolactinaemia (> 20 ng/mL), other causes of infertility (tubal, uterine), comorbid disease (tuberculosis, abnormal glucose tolerance test) Age: clomiphene citrate 25 ± 4.2 years, clomiphene + bromocriptine 25.13 ± 3.5 years Duration of infertility: not reported Setting: infertility outpatient department, Tamil Nadu, India Timing: not reported |
|
| Interventions | 3 cycles of treatment with: Clomiphene citrate 50 mg Day 3 to 7 (n = 38) versus Clomiphene citrate 50 mg Day 3 to 7 + bromocriptine 2.5 mg Day 1 to 30 (n = 36) |
|
| Outcomes | Relevant outcomes: ovulation rate, pregnancy rate | |
| Notes | Power calculation: not stated ITT: yes Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition, all women randomised were analysed |
| Selective reporting (reporting bias) | High risk | Outcomes were not specified or defined. Major adverse events such as OHSS were not reported. |
Vegetti 1999.
| Methods | Parallel randomised controlled trial | |
| Participants | 95 women randomised (95 women analysed) Inclusion criteria: normogonadotrophic anovulation, infertility for > 1 year, tubal patency shown by HSG or laparoscopy, normal semen analysis Age: not stated Duration of infertility: not stated Source of women: tertiary infertility centre Exclusion criteria: not stated Location: Milan, Italy |
|
| Interventions | Treatment(s): 100 mg CC on Days 3 to 7, if woman remained anovulatory for 2 cycles then dose doubled or 20 mg TMX on Days 3 to 7, if woman remained anovulatory for 2 cycles then dose doubled (n = 50) Control or placebo: none (n = 45) Duration: not stated | |
| Outcomes | Relevant outcomes: ovulation (per cycle, CC 108/129, TMX 92/133), pregnancy, and women‐reported adverse effects | |
| Notes | Authors contacted re: power calculation, random allocation, blinding, reasons for dropouts, external funding, anovulation definition, exclusion criteria, treatment time limit, ovulation rate per women; no reply received. ITT: not stated Source of funding: not stated Notes: abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reason given for dropouts |
| Selective reporting (reporting bias) | High risk | No details of adverse events such as miscarriage, no report of live birth |
Yilmaz 2006.
| Methods | Parallel randomised controlled trial | |
| Participants | 133 women randomised (125 women analysed) Inclusion criteria: normoprolactinaemic, normogonadotropic, primary infertility with oligomenorrhoea or amenorrhoea, age 20 to 40 years, duration of primary infertility > 2 years, no history of ovulation induction treatment and thyroid disease, normal results on HSG, husband with normal semen analysis according to WHO criteria Age: CC + hCG group 26.2 ± 3.4 years, CC‐alone group 26.7 ± 3.2 years Duration of infertility: CC + hCG group 2.91 ± 2.0 years, CC‐alone group 2.88 ± 2.0 years Exclusion criteria: no details Setting: Infertility units; Turkey Timing: May 2002 to April 2004 |
|
| Interventions | Day 1 was start of menses, clomiphene administered on Days 5 to 9 Treatment(s): CC 50 mg + hCG (Pregnyl 10,000 IU IM) when follicles reached 18 mm in diameter as determined by ultrasound (n = 60) Control or placebo: CC 50 mg (n = 65) Timed intercourse was advised 5 days after the last dose of clomiphene citrate for alternate days in both groups. Duration: 1 cycle |
|
| Outcomes | Relevant outcomes: ovulation and pregnancy rates, clinical pregnancy rate, fertilisation rate, implantation rate, twin rate, abortion rate (detected chemically but not by ultrasound scan at 7 weeks), corpus luteum function, mid‐luteal serum progesterone, and luteal phase length | |
| Notes | Pregnancy test (at 16th day after ovulation by serum ß‐hCG), positive foetal heart rate at 7 weeks Power calculation: yes, based on a previous trial ITT: no Source of funding: no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sonographers evaluating follicle size were blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 133 randomised, 125 completed the trial and were analysed. 8 women were lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | No details of adverse events, no report of live birth |
BMI: body mass index CC: clomiphene citrate COC: combined oral contraceptive DEX: dexamethasone DHEAS: dehydroepiandrosterone E2: estradiol FSH: follicle‐stimulating hormone hCG: human chorionic gonadotropin hMG: human menopausal gonadotropins HS: hormone supplementation HSG: hysterosalpingogram IM: intramuscular ITT: intention‐to‐treat analysis LH: luteinising hormone NIH‐NICHD: National Institutes of Health ‐ National Institute of Child Health and Human Development OHSS: ovarian hyperstimulation syndrome P4: progesterone PCOS: polycystic ovarian syndrome s.c.: subcutaneous TMX: tamoxifen TSH: thyroid‐stimulating hormone WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Omari 2002 | This was a conference abstract superseded by full paper (see Al‐Omari 2004). |
| Al‐Omari 2004 | Study using letrozole, excluded in 2009 update |
| Archer 1989 | Women not anovulatory |
| Armeanu 1992 | Not an RCT |
| Atay 2006 | Study using letrozole, excluded in 2009 update |
| Aygen 2007 | Study using letrozole, excluded in 2009 update |
| Bayer 2006 | Study using letrozole, excluded in 2009 update |
| Connaughton 1974 | Cross‐over trial, not all women anovulatory, was included in Hughes 1996 |
| Dura 2015 | Trial used intrauterine insemination. |
| Echt 1969 | Population selection based on diagnosis of luteal phase defect |
| el Tabbakh 1988 | Did not involve antioestrogen therapy |
| Gerhard 1979 | Not an RCT |
| Glasier 1989 | Women not anovulatory |
| Greenblatt 1961 | Not an RCT |
| Guedes Neto 2011 | Wrong comparison, no adjunct |
| Ito 1990 | Not an RCT |
| Johnson 1990 | Not oral agents |
| Koloszar 1996 | Does not appear to be an RCT |
| Kosar 2014 | Trial used intrauterine insemination. |
| Kubota 1992 | Not an RCT |
| Lisse 1980 | Not an RCT |
| Lobo 1982 | Not an RCT |
| Mendes 1999 | WHO group 1 women only |
| Mitwally 2001a | Not an RCT |
| Mitwally 2001b | Not an RCT |
| Moini 2015 | Trial used intrauterine insemination. |
| Presl 1984 | Does not appear to be an RCT |
| Roozenburg 1997 | Does not compare included interventions |
| Ruiz‐Velasco 1978 | Not an RCT |
| Senior 1978a | 6/9 women not anovulatory |
| Singh 1992 | Not an RCT |
| Topcu 2010 | Trial used intrauterine insemination. |
| Trott 1996 | Not an RCT |
| Tsuiki 1984 | Not an RCT |
| Williamson 1973 | Not an RCT |
| Yari 2010 | Wrong comparison |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Buvat 1987.
| Methods | Randomised trial |
| Participants | 66 infertile women, infertile for at least 1 year; n = 26 eugonadal anovulation, n = 40 luteal phase inadequacy; no other severe infertility factor |
| Interventions | Clomiphene citrate 25 to 50 mg/day versus tamoxifen 20 mg/day |
| Outcomes | Pregnancy, multiple pregnancy, adverse events |
| Notes | Unable to separate anovulatory data from luteal phase deficiency data. Unable to contact author |
Cabau 1990.
| Methods | Double‐blind randomised trial, randomisation using permuted blocks of 10. Numbered boxes from laboratory with no distinguishing marks |
| Participants | 300 women who had to be childless and referred for anovulatory cycles, irregular cycles with or without ovulation, or dysovulatory cycles. Also included women with slight insufficiency of mucus and those with idiopathic sterility. Trying to get pregnant for at least 1 year, or had already received treatment for sterility, or had suffered a miscarriage and tried for at least 6 months to get pregnant again Excluded all women to whom physician did not want to prescribe placebo, > 38 years old, amenorrhoea > 6 months' duration, known tubal sterility, distinctly insufficient or infected mucus, partners presenting with deficiency in semen, women undergoing artificial insemination |
| Interventions | Cyclofenil 400 mg taken on Days 4 to 8 of menstrual cycle or Days 5 to 8 (n = 114) versus placebo (n = 99) |
| Outcomes | Live birth, miscarriage, foetal death |
| Notes | Unable to separate anovulatory data. Unable to contact authors |
Craig 2015.
| Methods | Parallel randomised controlled trial |
| Participants | 160 women Inclusion criteria: 18 to 45 years old, anovulatory infertility Exclusion criteria: none specified Setting: USA Timing: not stated |
| Interventions | Traditional protocol: clomiphene followed by progestin withdrawal if anovulatory before increasing clomiphene dose (n = 60) versus Stair‐step protocol: clomiphene dose increased cycle Days 11 to 14 without progestin withdrawal if no follicle > 12 mm. Clomiphene dose started at 50 mg and increased up to 150 mg (n = 60). |
| Outcomes | Primary outcome: time to ovulation Secondary outcome: time to pregnancy |
| Notes | Conference abstract only. Unclear if this is in vitro fertilisation/intracytoplasmic sperm injection or intrauterine insemination |
Neuhausser 2011.
| Methods | Randomised trial |
| Participants | 50 anovulatory women Excluded: women with glucose intolerance on metformin |
| Interventions | Stair‐step protocol: if no dominant follicle clomiphene citrate increased from 50 mg/day to 100 mg for 5 days and then to 150 mg for 5 days versus Standard care: clomiphene citrate increased in 50 mg increments up to a maximum of 150 mg in subsequent menstrual cycles |
| Outcomes | Unclear from conference abstract, but does include pregnancy outcomes |
| Notes | It is unclear from the abstract whether or not intrauterine insemination was used. |
Senior 1978b.
| Methods | Randomised cross‐over trial |
| Participants | 9 infertile women (3 with anovulation and 6 with suspected luteal phase deficiency) |
| Interventions | Clomiphene for 2 months, tamoxifen for 2 months, and placebo for 1 month before and 1 month between interventions |
| Outcomes | Ovulation and pregnancy, hormonal assays |
| Notes | Unable to extract anovulatory women from luteal deficiency data; unable to contact authors |
Differences between protocol and review
In the 2009 review we widened the inclusion criteria of this review from that of the original protocol (women with anovulation attributed to polycystic ovarian syndrome (PCOS)) to include all World Health Organization (WHO) group 2 causes of anovulation, but excluding hyperprolactinaemia. We included trials that were non‐specific but appeared to describe PCOS‐like anovulation (e.g. women with progestin‐induced withdrawal bleeding). Due to the age of many of the trials, particularly for the comparison of clomiphene versus placebo, the most likely cause of anovulation was not fully described. In particular, the currently utilised diagnostic criteria for PCOS were not able to be met. These trials would have been excluded under the criteria of the protocol. We felt that their results were valid and important, and so widened the background and inclusion criteria sections of this review.
In the 2009 review we removed aromatase inhibitor comparisons from this review, as they have been addressed within a separate review (Franik 2014)
In the 2009 review we changed the title from 'Oral anti‐oestrogens and medical adjuncts for subfertility associated with anovulation' to 'Clomiphene and antioestrogens for ovulation induction in polycystic ovarian syndrome'.
In the 2016 update we removed as an outcome 'ovulation rate (per woman), where ovulation was defined as evidence of serum progesterone in the luteal range for the reference laboratory or a basal body temperature rise by > 0.4 ºC for 10 days or more as measured by a basal body temperature chart'.
Contributions of authors
Julie Brown: wrote the updated version of the review, including identification of new trials, data extraction, and analysis.
Cindy Farquhar: initiated and conceptualised the protocol, commented on drafts of the original and updated review, and assisted in the identification of new trials and data extraction for the review update.
Sources of support
Internal sources
-
University of Auckland, New Zealand.
Provided salary support for Julie Brown to update this review
External sources
None, Other.
Declarations of interest
Julie Brown: None known.
Cindy Farquhar is a director/shareholder of a gynaecology clinic and undertakes private practice within those premises.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Badawy 2008 {published data only}
- Badawy A, Allam A, Abulatta M. Extending clomiphene treatment in clomiphene‐resistant women with PCOS: a randomized controlled trial. Reproductive Biomedicine Online 2008;16(6):825‐9. [DOI] [PubMed] [Google Scholar]
Badawy 2009 {published data only}
- Badawy A, Inany H, Mosbah A, Abulatta M. Luteal phase clomiphene citrate for ovulation induction in women with polycystic ovary syndrome: a novel protocol. Fertility and Sterility 2009;91(3):838‐41. [DOI] [PubMed] [Google Scholar]
Badawy 2011 {published data only}
- Badawy A, Gibreal A. Clomiphene citrate versus tamoxifen for ovulation induction in women with PCOS: a prospective randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;159(1):151‐4. [DOI] [PubMed] [Google Scholar]
Boonstanfar 2001 {published data only}
- Boonstanfar R, Abbassi D, Tourgeman D, Saadat P, Jain JK, Mishell DR, et al. The comparative efficacy of tamoxifen and clomiphene citrate in obese and non‐obese anovulatory women: a prospective randomised trial. Fertility and Sterility 2002;77 Suppl 3(4):20‐1. [Google Scholar]
- Boonstanfar R, Jain JK, Mishell DR, Paulson RJ. A prospective randomized trial comparing clomiphene citrate with tamoxifen citrate for ovulation induction. Fertility and Sterility 2001;75(5):1024‐6. [DOI] [PubMed] [Google Scholar]
Branigan 2003 {published data only}
- Branigan EF, Estes MA. A randomized clinical trial of treatment of clomiphene citrate‐resistant anovulation with the use of oral contraceptive pill suppression and repeat clomiphene citrate treatment. American Journal of Obstetrics and Gynecology 2003;188(6):1424‐30. [DOI] [PubMed] [Google Scholar]
Branigan 2005 {published data only}
- Branigan E, Estes A. Use of micro‐dose human chorionic gonadotropin (hCG) after clomiphene citrate to complete folliculogenesis in previous CC‐resistant anovulation. American Journal of Obstetrics and Gynecology 2005;192:1890‐6. [DOI] [PubMed] [Google Scholar]
Cudmore 1966 {published data only}
- Cudmore DW, Tupper WRC. Induction of ovulation with clomiphene citrate. Fertility and Sterility 1966;17:363‐73. [DOI] [PubMed] [Google Scholar]
Daly 1984 {published data only}
- Daly DWC, Soto‐Albors C, Tohan N, Riddick D. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertility and Sterility 1984;41(6):844‐8. [DOI] [PubMed] [Google Scholar]
Dehbashi 2006 {published data only}
- Dehbashi S, Vafaei H, Parsanezhad MD, Alborzi S. Time of initiation of clomiphene citrate and pregnancy rate in polycystic ovarian syndrome. International Journal of Gynecology and Obstetrics 2006;93(1):44‐8. [DOI] [PubMed] [Google Scholar]
Elkind‐Hirsch 2005 {published data only}
- Elkind‐Hirsch K, Darensbourg C, Creasy G, Gipe D. Conception rates in clomiphene citrate cycles with and without hormone supplementation: a pilot study. Current Medical Research and Opinion 2005;21(7):1035‐40. [DOI] [PubMed] [Google Scholar]
Elnashar 2006 {published data only}
- Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethazone in treatment of clomiphene resistant polycystic ovary syndrome: a prospective placebo controlled study. Human Reproduction 2006;21(7):1805‐8. [DOI] [PubMed] [Google Scholar]
- Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethazone in treatment of clomiphene resistant polycystic ovary syndrome: a prospective placebo controlled study. Human Reproduction 2006;21(suppl 1):i69. [DOI] [PubMed] [Google Scholar]
Elsedeek 2014 {published data only}
- Elsedeek M, Elgindy E. Comparison between two clomiphene citrate protocols for induction of ovulation in clomiphene resistant polycystic ovary syndrome. Middle East Fertility Society Journal 2014;19(4):243‐7. [Google Scholar]
Esmaeilzadeh 2011 {published data only}
- Esmaeilzadeh S, Amiri MG, Basirat Z, Shirazi M. Does adding dexamethasone to clomiphene citrate improve ovulation in PCOS patients? A triple‐blind randomized clinical trial study. International Journal of Fertility & Sterility 2011;5(1):9‐12. [PMC free article] [PubMed] [Google Scholar]
Garcia 1985 {published data only}
- Garcia C‐R, Freeman ER, Rickels K, Wu C, Scholl G, Galle PC, et al. Behavioural and emotional factors and treatment responses in a study of anovulatory infertile women. Fertility and Sterility 1985;44(4):478‐83. [DOI] [PubMed] [Google Scholar]
Ghafourzadeh 2004 {published data only}
- Ghafourzadeh M, Karimi M, Karimazadeh MA, Bokai M. Comparison between two methods of ovulation induction: clomiphene alone and clomiphene + tamoxifen in PCOS patients. Iranian Journal of Reproductive Medicine 2004;2(3):74‐7. [Google Scholar]
Hassan 2001 {published data only}
- Hassan HA, El‐Gezeiry D, Nafaa TM, Baghdady I. Improved responsiveness of PCOS patients to clomiphene after CYP17a inhibitor. Journal of Assisted Reproduction and Genetics 2001;18(11):608‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Homburg 2012 {published data only}
- Homburg R, Hendriks ML, König TE, Anderson RA, Balen AH, Brincat M, et al. Clomifene citrate or low‐dose FSH for the first line treatment of infertile women with anovulation associated with polycystic ovary syndrome: a prospective randomized multinational study. Human Reproduction 2012;27(2):468‐73. [DOI] [PubMed] [Google Scholar]
Johnson 1966 {published data only}
- Johnson JE, Cohen MR, Goldfarb AF, Rakoff AE, Kistner RW, Plotz EJ, et al. The efficacy of clomiphene citrate for induction of ovulation. International Journal of Fertility 1966;11(4):265‐70. [PubMed] [Google Scholar]
Lopez 2004 {published data only}
- López E, Gunby J, Daya S, Parrilla JJ, Abad L, Balasch J. Ovulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low‐dose recombinant FSH as first line therapy. Reproductive Biomedicine Online 2004;9(4):382‐90. [DOI] [PubMed] [Google Scholar]
Moslemizadeh 2008 {published data only}
- Moslemizadeh N, Moghadam TG, Ehteshami S. Comparison of clomiphene citrate plus estradiol, with tamoxifen citrate effects in induction of ovulation and pregnancy in poly cystic ovarian syndrome patients. Journal of Medical Sciences 2008;8(8):734‐8. [Google Scholar]
Omran 2011 {published data only}
- Omran MS. Comparison between two clomiphene citrate protocols for induction of ovulation in clomiphene resistant PCOS. Fertility and Sterility 2011;96(3, Supplement):S90‐1. [Google Scholar]
Parsanezhad 2002a {published data only}
- Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Effect of high dose, short course dexamethasone in clomiphene citrate resistant women with polycystic ovary syndrome. Middle East Fertility Society Journal 2002;7(2):93‐7. [Google Scholar]
- Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate‐resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulphate levels: a prospective, double‐blind, placebo‐controlled trial. Fertility and Sterility 2002;78(5):1001‐4. [DOI] [PubMed] [Google Scholar]
Parsanezhad 2002b {published data only}
- Parsanezhad ME, Alborzi SA, Jahromi BN. A prospective, double‐blind, randomized, placebo‐controlled clinical trial of bromocriptine in clomiphene‐resistant patients with polycystic ovary syndrome and normal prolactin level. Archives of Gynecology and Obstetrics 2004;269:125‐9. [DOI] [PubMed] [Google Scholar]
- Parsanezhad ME, Alborzi SA, Jahromi BN. A prospective, double‐blind, randomized, placebo‐controlled clinical trial of bromocriptine in clomiphene‐resistant patients with polycystic ovary syndrome and normal prolactin level. International Journal of Fertility and Women's Medicine 2002;47(6):272‐7. [PubMed] [Google Scholar]
Seyedoshohadaei 2012 {published data only}
- Seyedoshohadaei F, Zandvakily F, Shahgeibi S. Comparison of the effectiveness of clomiphene citrate, tamoxifen and letrozole in ovulation induction in infertility due to isolated unovulation. Iranian Journal of Reproductive Medicine 2012;10(6):531‐6. [PMC free article] [PubMed] [Google Scholar]
Suginami 1993 {published data only}
- Suginami H, Kitagawa H, Nakahashi N, Yano K, Matsubara K. A clomiphene citrate and tamoxifen citrate combination therapy: a novel therapy for ovulation induction. Fertility and Sterility 1993;59:976‐9. [DOI] [PubMed] [Google Scholar]
Tripathy 2013 {published data only}
- Tripathy SS, Mohapatra MM, Chandrasekhar A. Induction of ovulation with clomiphene citrate versus clomiphene with bromocriptine in PCOS patients with normal prolactin: a comparative study. Journal of Clinical and Diagnostic Research 2013;7(11):2541‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vegetti 1999 {published data only}
- Vegetti W, Riccaboni A, Colombo M, Baroni E, Diaferia D, Ragni G, et al. Randomized study of induction of ovulation by two different molecules with antiestrogenic effects, in patients with chronic anovulation disorders. Fertility and Sterility 1999;72 Suppl 1(3):234‐5. [Google Scholar]
Yilmaz 2006 {published data only}
- Yilmaz B, Kelekci S, Savan K, Oral H, Mollamahmutoglu L. Addition of human chorionic gonadotropin to clomiphene citrate ovulation induction therapy does not improve pregnancy outcomes and luteal function. Fertility and Sterility 2006;85(3):783‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Omari 2002 {published data only}
- Al‐Omari WR, Al‐Hadithi N, Sulaiman WR, Izat B. 0‐243. Comparison of two aromatase inhibitors in clomiphene‐resistant PCOS. Human Reproductive Abstracts 2002;17:AB1. [Google Scholar]
Al‐Omari 2004 {published data only}
- Al‐Omari W, Sulaiman W, Al‐Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene resistant polycystic ovary syndrome. International Journal of Gynecology and Obstetrics 2004;85:289‐91. [DOI] [PubMed] [Google Scholar]
Archer 1989 {published data only}
- Archer DF, Hofmann G, Brzyski R, Ross B, Scott RT, Philput CB, et al. Effects of clomiphene citrate on episodic luteinizing hormone secretion throughout the menstrual cycle. American Journal of Obstetrics and Gynecology 1989;161(3):581‐92. [DOI] [PubMed] [Google Scholar]
Armeanu 1992 {published data only}
- Armeanu MC, Moss RJ, Schoemaker J. Ovulation induction with a single‐blind treatment regimen comparing naltrexone, placebo and clomiphene citrate in women with secondary amenorrhoea. Acta Endocrinologica 1992;126:410‐5. [DOI] [PubMed] [Google Scholar]
Atay 2006 {published data only}
- Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. Journal of International Medical Research 2006;34:73‐6. [DOI] [PubMed] [Google Scholar]
Aygen 2007 {published data only}
- Aygen E, Güzel Z, Özgun T, Atakul T, Şahin Y. The use of letrozole for ovulation induction in infertile women with polycystic ovarian syndrome [Polikistik over sendromlu infertil kadınlarda ovulasyon indüksiyonunda letrozolün kullanımı]. Erciyes Medical Journal 2007;29(3):195‐200. [Google Scholar]
Bayer 2006 {published data only}
- Bayer Ű, Basaran M, Kiran S, Coskun A, Gezer S. Use of an aromatase inhibitor in patients with polycystic ovary syndrome: a prospective randomised trial. Fertility and Sterility 2006;86(5):1447‐51. [DOI] [PubMed] [Google Scholar]
Connaughton 1974 {published data only}
- Connaughton J, Garcia C‐R, Wallach E. Cisclomiphene vs placebo for anovulation. Obstetrics and Gynecology 1974;43(5):697‐701. [PubMed] [Google Scholar]
Dura 2015 {published data only}
- Deveci CD, Demir B, Sengul O, Dilbaz B, Goktolga U. Clomiphene citrate 'stair‐step' protocol vs traditional protocol in patients with polycystic ovary syndrome: a randomized controlled trial. Archives of Gynecology and Obstetrics 2015;291:179‐84. [DOI] [PubMed] [Google Scholar]
- Dura C, Demir B, Dilbaz B, Goktolga U. Clomiphene 'stair step' protocol versus traditional protocol in patients with polycystic ovary syndrome (PCOS): a prospective trial. Ferility and Sterility 2011;96 (Volume 3 S1):S90. [Google Scholar]
Echt 1969 {published data only}
- Echt CR, Romberger FT, Goodman JA. Clomiphene citrate in the treatment of luteal phase defects. Fertility and Sterility 1969;20(4):564‐71. [DOI] [PubMed] [Google Scholar]
el Tabbakh 1988 {published data only}
- Tabbakh GH, Loutfi IA, Azab I, Rahman HA, Southren AL, Aleem FA. Bromocriptine in polycystic ovarian disease: a controlled clinical trial. Obstetrics and Gynecology 1988;71(3 part 1):301‐6. [PubMed] [Google Scholar]
Gerhard 1979 {published data only}
- Gerhard I, Runnebaum B. Comparison between tamoxifen and clomiphene therapy in women with anovulation. Archives of Gynecology and Obstetrics 1979;227:279‐88. [DOI] [PubMed] [Google Scholar]
Glasier 1989 {published data only}
- Glasier A, Irvine D, Wickings E, Hillier S, Baird D. A comparison of the effects on follicular development between clomiphene citrate, its two separate isomers and spontaneous cycles. Human Reproduction 1989;4(3):252‐6. [DOI] [PubMed] [Google Scholar]
Greenblatt 1961 {published data only}
- Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. JAMA 1961;178:101‐6. [DOI] [PubMed] [Google Scholar]
Guedes Neto 2011 {published data only}
- Guedes Neto EP, Moraes GS, Cristovam RA, Corleta HE, Lessey BA, Savaris RF. Prospective, randomized double blind comparison between raloxifene and clomiphene citrate for ovulation induction in polycystic ovary syndrome. Human Reproduction 2010;25:i45. [DOI] [PubMed] [Google Scholar]
- Paula Guedes Neto E, Savaris RF, Eye Corleta H, Silva de Moraes G, do Amaral Cristovam R, Lessey BA. Prospective, randomized comparison between raloxifene and clomiphene citrate for ovulation induction in polycystic ovary syndrome. Fertility and Sterility 2011;96(3):769‐73. [DOI] [PubMed] [Google Scholar]
Ito 1990 {published data only}
- Ito T, Michioka T, Kobayashi M, Narimatsu A, Yamashita S, Matsuzaki H, et al. A study on follicle stimulation and ovulation induction in polycystic ovary syndrome (PCOS). Hormone Research 1990;33 Suppl 2:32‐4. [DOI] [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson P, Pearce J. Recurrent spontaneous abortion and polycystic ovarian disease: a comparison of two regimens to induce ovulation. BMJ 1990;300:154‐6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Koloszar 1996 {published data only}
- Koloszar S, Szollosi J, Bartfai G. Ovulation induction with adjuvant antiandrogen treatment in Stein‐Leventhal syndrome [Az ovulatio inductio kiegeszitese adjuvans antiandrogen kezelessel Stein‐Leventhal‐syndromaban]. Orvosi Hetilap 1996;137(46):2569‐71. [PubMed] [Google Scholar]
Kosar 2014 {published data only}
- Kosar O, Ozaksit G, Taskin MI. Luteal phase clomiphene citrate for ovulation induction in women with polycystic ovary syndrome. Archives of Gynecology and Obstetrics 2014;290(4):771‐5. [DOI] [PubMed] [Google Scholar]
Kubota 1992 {published data only}
- Kubota T, Kamada S, Aso T. Combined therapy with bromocriptine and clomiphene citrate for patients with normoprolactinemic amenorrhea. International Journal of Fertility and Women's Medicine 1992;37:277‐82. [PubMed] [Google Scholar]
Lisse 1980 {published data only}
- Lisse K. Combined clomiphene‐dexamethasone therapy in cases with resistance to clomiphene [Kombinierte Clomiphen‐Dexamethason‐Therapie bei Clomiphenresistenz]. Zentralblatt fur Gynakologie 1980;102:645‐50. [PubMed] [Google Scholar]
Lobo 1982 {published data only}
- Lobo RA, Granger LR, Davajan V, Mishell DR. An extended regimen of clomiphene citrate in women unresponsive to standard therapy. Fertility and Sterility 1982;37(6):762‐6. [DOI] [PubMed] [Google Scholar]
Mendes 1999 {published data only}
- Mendes MC, Ferriani RA, Sala MM, Moura MD, Silva de Sa MF. Induction of ovulation with clomiphene citrate in combination with metoclopramide in patients with amenorrhea of hypothalamic origin. Gynecological Endocrinology 1999;13:149‐54. [DOI] [PubMed] [Google Scholar]
Mitwally 2001a {published data only}
- Mitwally MFM, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertility and Sterility 2001;75(2):305‐9. [DOI] [PubMed] [Google Scholar]
Mitwally 2001b {published data only}
- Mitwally MF, Casper RF. Aromatase inhibition: A novel method of ovulation induction in women with polycystic ovary syndrome. Reproductive Technologies 2001;10(5):244‐7. [Google Scholar]
Moini 2015 {published data only}
- Moini A, Ahmadi F, Jahangiri N, Ahmadi J, Reza Akhoond M. A randomized controlled trial evaluating the effect of ethinyl estradiol during clomiphene citrate cycles among women with polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics 2015;131:129‐32. [DOI] [PubMed] [Google Scholar]
Presl 1984 {published data only}
- Presl J. Tamoxifen for ovulation induction [Tamoxifen a induckce ovulace]. Ceskoslovenska Gynekologie 1984;49:740‐2. [PubMed] [Google Scholar]
Roozenburg 1997 {published data only}
- Roozenburg BJ, Dessel HJHM, Evers JLH, Bots RSGM. Successful induction of ovulation in normogonadotrophic clomiphene resistant anovulatory women by combined naltrexone and clomiphene citrate treatment. Human Reproduction 1997;12(8):1720‐2. [DOI] [PubMed] [Google Scholar]
Ruiz‐Velasco 1978 {published data only}
- Ruiz‐Velasco V, Arceo J. Induction of ovulation with tamoxifen [Induccion de la ovulacion con tamoxifen]. Ginecologia y obstetricia de Mexica 1978;43(258):251‐8. [PubMed] [Google Scholar]
Senior 1978a {published data only}
- Senior BE, Cawood ML, Oakey RE, McKiddie JM, Siddle DR. A comparison of the effects of clomiphene and tamoxifen treatment on the concentrations of oestradiol and progesterone in the peripheral plasma of infertile women. Clinical Endocrinology 1978;8:381‐9. [DOI] [PubMed] [Google Scholar]
Singh 1992 {published data only}
- Singh KB, Dunnihoo DR, Mahajan DK, Bairnsfather LE. Clomiphene‐dexamethasone treatment of clomiphene‐resistant women with and without the polycystic ovary syndrome. Journal of Reproductive Medicine 1992;37(3):215‐8. [PubMed] [Google Scholar]
Topcu 2010 {published data only}
- Topcu HO, Yuksel B, Islimye M, Karakaya J, Ozat M, Batioglu S. Tamoxifen as a first line treatment in ovulation induction for the infertile women with thin endometrium and high follicular response with clomiphene. Human Reproduction 2010;25 (Suppl 1):i312. [Google Scholar]
Trott 1996 {published data only}
- Trott EA, Plouffe L, Hansen K, Hines R, Brann DW, Mahesh VB. Ovulation induction in clomiphene‐resistant anovulatory women with normal dehydroepiandrosterone sulfate levels: beneficial effects of the addition of dexamethasone during the follicular phase. Fertility and Sterility 1996;66(3):484‐6. [DOI] [PubMed] [Google Scholar]
Tsuiki 1984 {published data only}
- Tsuiki A, Uehara S, Kyono K, Saito A, Hoshi K, Hoshiai H, et al. Induction of ovulation with an estrogen antagonist, tamoxifen. Tohoku Journal of Experimental Medicine 1984;144(1):21‐31. [DOI] [PubMed] [Google Scholar]
Williamson 1973 {published data only}
- Williamson JG, Ellis JD. The induction of ovulation by tamoxifen. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:844‐7. [DOI] [PubMed] [Google Scholar]
Yari 2010 {published data only}
- Yari F, Ghafarzadeh M, Khadish A, Vahabi S, Yari A. Clomiphene citrate and dexamethazone in treatment of polycystic ovary syndrome and infertility. International Journal of Gynecology & Obstetrics 2009;107:S384. [Google Scholar]
- Yari F, Ghafarzadeh M, Vahabi S, Khadish A, Yari A. Clomiphene citrate and dexamethasone in treatment of polycystic ovary syndrome and infertility in Khoramabad. Journal fur Reproduktionsmedizin und Endokrinologie 2010;7(4):369. [Google Scholar]
References to studies awaiting assessment
Buvat 1987 {published data only}
- Buvat JB‐HG, Marcolin G, Ardaens‐Boulier K. Antioestrogens as treatment of female and male infertilities. Hormone Research 1987;28:219‐29. [DOI] [PubMed] [Google Scholar]
Cabau 1990 {published data only}
- Cabau A, Krulik D, Reboul J. Hormonal and idiopathic sterility. Treatment with cyclofenil. A double‐blind controlled study [Sterilities de cause hormonale et sterilities inexpliquees. Traitement par le cyclofenil. Etude controlee a double insu]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 1990;19(1):96‐101. [PubMed] [Google Scholar]
Craig 2015 {published data only}
- Craig LB, Peck JD, Zhao D, Hansen K. Clomid stair‐step protocol may shorten the time to ovulation but not to pregnancy: a randomized clinical trial. Fertility and Sterility 2015;104(Suppl 3):e97. [Google Scholar]
Neuhausser 2011 {published data only}
- Neuhausser WM, Klein JU, Sauer MV, Lobo RA, Zimmermann RC. A prospective randomised controlled trial to evaluate a clomiphene stair step protocol vs standard clomiphene (SSTEPS) for ovulation induction in anovulatory women. Fertility and Sterility 2011;96 (Suppl 3):S29 O‐97. [Google Scholar]
Senior 1978b {published data only}
- Senior BE, Cawood ML, Oakey RE, McKiddie JM, Siddle DR. A comparison of the effects of clomiphene and tamoxifen treatment on the concentrations of oestradiol and progesterone in the peripheral plasma of infertile women. Clinical Endocrinology 1978;8:381‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayram 2004
- Bayram N, Wely M, Veen F. Pulsatile gonadotrophin releasing hormone for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000412.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brann 1991
- Brann D, Mahesh V. Role of corticosteroids in female reproduction. Federation of American Societies for Experimental Biology 1991;5:2691‐8. [DOI] [PubMed] [Google Scholar]
El Daly 2006
- Daly AA, Al‐Fozan HM, Al‐Inany HG, Bedawy MA, Saleh WF. Aromatase inhibitors for ovulation induction. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005635] [DOI] [Google Scholar]
ESHRE/ASRM 2003
- The Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2003;81(1):19‐25. [DOI] [PubMed] [Google Scholar]
Fauser 2012
- Fauser B, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM‐sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility 2012;97(1):38.e24. [DOI] [PubMed] [Google Scholar]
Franik 2014
- Franik S, Kremer JAM, Nelen WLDM, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010287.pub2] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- Hamilton (ON): GRADE Working Group. GRADEpro GDT. McMaster University, 2014: Hamilton (ON): GRADE Working Group, Version accessed prior to 12 November 2016.
Haas 2013
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003511.pub3] [DOI] [PubMed] [Google Scholar]
Hart 2015
- Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's long‐term health using data linkage. Journal of Clinical Endocrinology and Metabolism 2015;100(3):911‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hughes 1996
- Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for ovulation induction in women with oligo‐amenorrhoea. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000056] [DOI] [PubMed] [Google Scholar]
Kousta 1997
- Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Human Reproduction Update 1997;3(4):359‐65. [DOI] [PubMed] [Google Scholar]
Leblanc 1976
- Leblanc H, Lachelin G, Abu‐Fadil S, Yen S. Effects of dopamine infusion on pituitary hormone secretion in humans. Journal of Clinical Endocrinology and Metabolism 1976;43:668. [DOI] [PubMed] [Google Scholar]
Lobo 2000
- Lobo RA, Carmina E. The importance of diagnosing the polycystic ovary syndrome. Annals of Internal Medicine 2000;132(12):989‐93. [DOI] [PubMed] [Google Scholar]
Messinis 1982
- Messinis IE, Nillius SJ. Comparison between tamoxifen and clomiphene for induction of ovulation. Acta Obstetricia et Gynecologica Scandinavica 1982;61(4):377‐9. [PubMed] [Google Scholar]
Mitwally 2004
- Mitwally MFM, Casper RF. Aromatase inhibitors in ovulation induction. Seminars in Reproductive Medicine 2004;22(1):61‐78. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
NICE 2013
- National Collaborating Centre for Women's and Children's Health/National Institute for Clinical Excellence. Fertility problems: assessment and treatment. National Collaborating Centre for Women's and Children's Health 2013.
Parsanezhad 2003
- Parsanezhad ME, Alborzi S, Pakniat M, Schmidt EHS. A double‐blind, randomized, placebo‐controlled study to assess the efficacy of ketoconazole for reducing the risk of ovarian hyperstimulation syndrome. Fertility and Sterility 2003;805(5):1151‐5. [DOI] [PubMed] [Google Scholar]
Pritts 2002
- Pritts EA. Treatment of the infertile patient with polycystic ovarian syndrome. Obstetrical and Gynecological Survey 2002;57(9):587‐97. [DOI] [PubMed] [Google Scholar]
Siebel 1984
- Siebel M, Liskowitz, Kamnava M, Taylor M. Bromocriptine response in normoprolactinaemic patients with polycystic ovary disease. A preliminary report. Obstetrics and Gynecology 1984;64:213‐9. [PubMed] [Google Scholar]
Tan 1996
- Tan SL, Farhi J, Homburg R, Jacobs HS. Induction of ovulation in clomiphene‐resistant polycystic ovary syndrome with pulsatile GnRH. Obstetrics and Gynecology 1996;88:221‐6. [DOI] [PubMed] [Google Scholar]
Tang 2012
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003053.pub5] [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
Weiss 2015
- Weiss NS, Nahuis M, Bayram N, Mol BWJ, Veen F, Wely M. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD010290.pub2] [DOI] [PubMed] [Google Scholar]
Wolf 2000
- Wolf LJ. Ovulation induction. Clinical Obstetrics and Gynecology 2000;43(4):902‐15. [DOI] [PubMed] [Google Scholar]


