Abstract
Background
Choroidal neovascularisation (CNV) is a common complication of pathological myopia. Once developed, most eyes with myopic CNV (mCNV) experience a progression to macular atrophy, which leads to irreversible vision loss. Anti‐vascular endothelial growth factor (anti‐VEGF) therapy is used to treat diseases characterised by neovascularisation and is increasingly used to treat mCNV.
Objectives
To assess the effects of anti‐vascular endothelial growth factor (anti‐VEGF) therapy for choroidal neovascularisation (CNV), compared with other treatments, sham treatment or no treatment, in people with pathological myopia.
Search methods
We searched a number of electronic databases including CENTRAL and Ovid MEDLINE, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform ICTRP). We did not use any date or language restrictions in the electronic searches for trials. Electronic databases were last searched on 16 June 2016.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing anti‐VEGF therapy with another treatment (e.g. photodynamic therapy (PDT) with verteporfin, laser photocoagulation, macular surgery, another anti‐VEGF), sham treatment or no treatment in participants with mCNV.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two authors independently screened records, extracted data, and assessed risk of bias. We contacted trial authors for additional data. We analysed outcomes as risk ratios (RRs) or mean differences (MDs). We graded the certainty of the evidence using GRADE.
Main results
The present review included six studies which provided data on the comparison between anti‐VEGF with PDT, laser, sham treatment and another anti‐VEGF treatment, with 594 participants with mCNV. Three trials compared bevacizumab or ranibizumab with PDT, one trial compared bevacizumab with laser, one trial compared aflibercept with sham treatment, and two trials compared bevacizumab with ranibizumab. Pharmaceutical companies conducted two trials. The trials were conducted at multiple clinical centres across three continents (Europe, Asia and North America). In all these six trials, one eye for each participant was included in the study.
When compared with PDT, people treated with anti‐VEGF agents (ranibizumab (one RCT), bevacizumab (two RCTs)), were more likely to regain vision. At one year of follow‐up, the mean visual acuity (VA) in participants treated with anti‐VEGFs was ‐0.14 logMAR better, equivalent of seven Early Treatment Diabetic Retinopathy Study (ETDRS) letters, compared with people treated with PDT (95% confidence interval (CI) ‐0.20 to ‐0.08, 3 RCTs, 263 people, low‐certainty evidence). The RR for proportion of participants gaining 3+ lines of VA was 1.86 (95% CI 1.27 to 2.73, 2 RCTs, 226 people, moderate‐certainty evidence). At two years, the mean VA in people treated with anti‐VEGFs was ‐0.26 logMAR better, equivalent of 13 ETDRS letters, compared with people treated with PDT (95% CI ‐0.38 to ‐0.14, 2 RCTs, 92 people, low‐certainty evidence). The RR for proportion of people gaining 3+ lines of VA at two years was 3.43 (95% CI 1.37 to 8.56, 2 RCTs, 92 people, low‐certainty evidence). People treated with anti‐VEGFs showed no obvious reduction (improvement) in central retinal thickness at one year compared with people treated with PDT (MD ‐17.84 μm, 95% CI ‐41.98 to 6.30, 2 RCTs, 226 people, moderate‐certainty evidence). There was low‐certainty evidence that people treated with anti‐VEGF were more likely to have CNV angiographic closure at 1 year (RR 1.24, 95% CI 0.99 to 1.54, 2 RCTs, 208 people). One study allowed ranibizumab treatment as of month 3 in participants randomised to PDT, which may have led to an underestimate of the benefits of anti‐VEGF treatment.
When compared with laser photocoagulation, there was more improvement in VA among bevacizumab‐treated people than among laser‐treated people after one year (MD ‐0.22 logMAR, equivalent of 11 ETDRS letters, 95% CI ‐0.43 to ‐0.01, 1 RCT, 36 people, low‐certainty evidence) and after two years (MD ‐0.29 logMAR, equivalent of 14 ETDRS letters, 95% CI ‐0.50 to ‐0.08, 1 RCT, 36 people, low‐certainty evidence).
When compared with sham treatment, people treated with aflibercept had better vision at one year (MD ‐0.19 logMAR, equivalent of 9 ETDRS letters, 95% CI ‐0.27 to ‐0.12, 1 RCT, 121 people, moderate‐certainty evidence). The fact that this study allowed for aflibercept treatment at 6 months in the control group might cause an underestimation of the benefit with anti‐VEGF.
People treated with ranibizumab had similar improvement in VA recovery compared with people treated with bevacizumab after one year (MD ‐0.02 logMAR, equivalent of 1 ETDRS letter, 95% CI ‐0.11 to 0.06, 2 RCTs, 80 people, moderate‐certainty evidence).
Of the included six studies, two studies reported no adverse events in either group and two industry‐sponsored studies reported both systemic and ocular adverse events. In the control group, there were no systemic or ocular adverse events reported in 149 participants. Fifteen people reported systemic serious adverse events among 359 people treated with anti‐VEGF agents (15/359, 4.2%). Five people reported ocular adverse events among 359 people treated with anti‐VEGF agents (5/359, 1.4%). The number of adverse events was low, and the estimate of RR was uncertain regarding systemic serious adverse events (4 RCTs, 15 events in 508 people, RR 4.50, 95% CI 0.60 to 33.99, very low‐certainty evidence) and serious ocular adverse events (4 RCTs, 5 events in 508 people, RR 1.82, 95% CI 0.23 to 14.71, very low‐certainty evidence). There were no reports of mortality or cases of endophthalmitis or retinal detachment.
There was sparse reporting of data for vision‐related quality of life (in favour of anti‐VEGF) in only one trial at one year of follow‐up. The studies did not report data for other outcomes, such as percentage of participants with newly developed chorioretinal atrophy.
Authors' conclusions
There is low to moderate‐certainty evidence from RCTs for the efficacy of anti‐VEGF agents to treat mCNV at one year and two years. Moderate‐certainty evidence suggests ranibizumab and bevacizumab are equivalent in terms of efficacy. Adverse effects occurred rarely and the trials included here were underpowered to assess these. Future research should be focused on the efficacy and safety of different drugs and treatment regimens, the efficacy on different location of mCNV, as well as the effects on practice in the real world.
Keywords: Humans; Laser Coagulation; Photochemotherapy; Angiogenesis Inhibitors; Angiogenesis Inhibitors/adverse effects; Angiogenesis Inhibitors/therapeutic use; Bevacizumab; Bevacizumab/therapeutic use; Choroidal Neovascularization; Choroidal Neovascularization/etiology; Choroidal Neovascularization/therapy; Macula Lutea; Macula Lutea/surgery; Myopia, Degenerative; Myopia, Degenerative/complications; Photosensitizing Agents; Photosensitizing Agents/therapeutic use; Porphyrins; Porphyrins/therapeutic use; Randomized Controlled Trials as Topic; Ranibizumab; Ranibizumab/therapeutic use; Receptors, Vascular Endothelial Growth Factor; Receptors, Vascular Endothelial Growth Factor/therapeutic use; Recombinant Fusion Proteins; Recombinant Fusion Proteins/therapeutic use; Vascular Endothelial Growth Factor A; Vascular Endothelial Growth Factor A/antagonists & inhibitors; Verteporfin
Plain language summary
Anti‐VEGF for treatment of choroidal neovascularisation (new blood vessels) in people with pathological (severe) myopia
What is the aim of this review? The aim of this Cochrane Review was to find out if anti‐vascular endothelial growth factor (called anti‐VEGF) treatment of new blood vessels in people with severe myopia (also known as nearsightedness or shortsightedness) prevents vision loss. Cochrane researchers collected and analysed all relevant studies to answer this question and found six studies.
Key messages People with severe myopia and growth of new blood vessels at the back of the eye may benefit from treatment with anti‐VEGF. It may prevent vision loss. SIde effects (harms) occur rarely.
What was studied in the review? Myopia occurs when the eyeball becomes too long. If the myopia is severe, sometimes the retina (light‐sensitive tissue at the back of the eye) becomes too thin and new blood vessels grow. These new blood vessels can leak and cause vision loss.
Anti‐vascular endothelial growth factor (anti‐VEGF) is a drug that may slow down the growth of these new vessels. Doctors can inject anti‐VEGF into the eye of people who have severe myopia and signs of new blood vessels growing at the back of the eye. This may prevent vision loss.
What are the main results of the review? The Cochrane researchers found six relevant studies. These studies took place in multiple clinical centres across three continents (Europe, Asia and North America), Three studies compared anti‐VEGF treatment with photodynamic therapy (PDT; a treatment with a light‐sensitive medicine and a light source that destroys abnormal cells); one study compared anti‐VEGF with laser treatment; one study compared anti‐VEGF with no treatment; and two studies compared different types of anti‐VEGF to each other. In some of the studies, the comparison group received anti‐VEGF after a short period which may mean that the results underestimate the beneficial effect of anti‐VEGF.
The results of the review show that:
• People with severe myopia who have anti‐VEGF treatment probably achieve better vision than people receiving PDT, laser or no treatment (moderate‐ and low‐certainty evidence). • Two different types of anti‐VEGF ‐ ranibizumab and bevacizumab ‐ probably have similar effects on vision (moderate‐certainty evidence). • Side effects (harms) occur rarely.
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to 16 June 2016.
Summary of findings
Summary of findings for the main comparison. Anti‐VEGF compared with control for choroidal neovascularisation in people with pathological myopia.
| Anti‐VEGF compared with control for choroidal neovascularisation in people with pathological myopia | ||||||
| Patient or population: CNV in people with pathologic myopia Setting: clinical centres Intervention: intravitreal injections of anti‐VEGF Comparison: PDT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PDT | Risk with anti‐VEGF | |||||
| Change in visual acuity at 1 year assessed with: logMAR | The mean change in visual acuity at 1 year ranged from ‐0.186 to 0.15 logMAR | The mean change in visual acuity at 1 year in the intervention group was 0.14 logMAR lower (better) (0.2 lower to 0.08 lower) | ‐ | 263 (3 RCTs) | ⊕⊕⊕⊝ Low1 2 | Overall heterogeneity: I2 = 68% |
| Gain 3+ lines of visual acuity at 1 year | 265 per 1000 | 493 per 1000 (337 to 724) | RR 1.86 (1.27 to 2.73) | 226 (2 RCTs) | ⊕⊕⊕⊝ Moderate 1 | Overall heterogeneity: I2 = 58% |
| Change in central macular thickness at 1 year | The mean change in central macular thickness at 1 year ranged from ‐14 μm to ‐60.8 μm | The mean change in central macular thickness at 1 year in the intervention group was 17.84 μm greater reduction (6.3 lower reduction to 41.98 greater reduction) | ‐ | 226 (2 RCTs) | ⊕⊕⊕⊝ Moderate 2 | Overall heterogeneity: I2 = 2% |
| CNV angiographic closure at 1 year | 562 per 1000 | 697 per 1000 (556 to 865) | RR 1.24 (0.99 to 1.54) | 208 (2 RCTs) | ⊕⊕⊝⊝ Low 1 2 | Overall heterogeneity: I2 = 83% |
| Systemic serious adverse events | 1 per 1000 | 5 per 1000 (1 to 34) | RR 4.50 (0.60 to 33.99) |
508 (4 RCTs) | ⊕⊝⊝⊝ Very low 3 | Estimate of effect taken from trials comparing anti‐VEGF to other, sham or no treatment. As no events reported in the control groups of these studies, we assumed a low absolute risk of 1 per 1000 in the comparator group for illustrative purposes only. |
| Ocular adverse events | 1 per 1000 | 2 per 1000 (0 to 15) | RR 1.82 (0.23 to 14.71) |
508 (4 RCTs) | ⊕⊝⊝⊝ Very low 4 | |
| Quality of life | Mean change in NEI‐VFQ (with sham) ‐2.58 | The mean change in score was 5.72 better (1.60 better to 9.84 better) | 121 (1 RCT) |
⊕⊕⊕⊝ Moderate 2 | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). One study allowed anti‐VEGF treatment (ranibizumab) as of month 3 in participants randomised to PDT. Anti‐VEGF: anti‐vascular endothelial growth factor; CI: confidence interval; CNV: choroidal neovascularisation; NEI‐VFQ: National Eye Institute Visual Function Questionnaire; PDT: photodynamic therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 1 level for inconsistency as I2 greater than 50%. Note: one study allowed anti‐VEGF (ranibizumab) treatment as of month 3 in participants randomised to PDT.
2 Downgraded 1 level for imprecision as confidence intervals include null value or clinically insignificant effect.
3 Downgraded 2 levels for imprecision as only 15 events, and downgraded 1 level for indirectness as people with previous cardiovascular events were excluded in these studies.
4 Downgraded 2 levels for imprecision as only 5 events, downgraded 1 level for indirectness as people with concomitant ocular disease such as glaucoma were excluded in these studies.
Background
Description of the condition
Myopia, also known as nearsightedness or shortsightedness, is a condition in which close objects are seen clearly, but objects further away appear blurred. Parallel light coming from the environment focuses in front of the retina, due to a higher refractive power of the cornea or the lens or a longer axial length of the eyeball. As a major cause of legal blindness in many countries, pathological myopia is characterised by a refractive error of ‐6.0 dioptres or more and an axial length of more than 26.5 mm. It affects almost 2% of the general population in the USA (Sperduto 1983), with a higher prevalence of approximately 9% in Asian countries (Wong 2000). Pathological myopia is the leading cause of blindness in Japan (Iwase 2006), and the second most frequent cause of low vision or blindness in people older than 40 years in China (Xu 2006).
Eyes with pathological myopia have progressive elongation of the eyeball and development of a posterior staphyloma which is a bulging of a weakened sclera at the posterior of the eyeball. This leads to thinning of the retinal pigment epithelium and choroid (Hsiang 2008). Myopic choroidal neovascularisation (mCNV) may develop in 5% to 10% of people with pathological myopia, and is mainly characterised by widespread chorioretinal degeneration in the posterior pole of the eye, growth of new blood vessels from the choroid capillary layer, breaks of Bruch's membrane, subsequent subretinal haemorrhage and fibrotic membrane formation under the foveola (Ikuno 2008; Ohno‐Matsui 2003). Once developed, 90.1% of eyes with mCNV experience a progression to macular atrophy (Hayashi 2010), which leads to irreversible vision loss. In one long‐term follow‐up study, visual acuity (VA) dropped to 20/100 in 96.3% of eyes after the onset of mCNV (Yoshida 2003).
Description of the intervention
Treatment strategies for mCNV mainly include: laser photocoagulation, macular surgeries, photodynamic therapy (PDT) with verteporfin and anti‐vascular endothelial growth factor (anti‐VEGF) therapy.
Prior to the 1990s, thermal laser photocoagulation was the only treatment for CNV (choroidal neovascularisation) in pathological myopia. It may still be an option for extrafoveal and juxtafoveal CNV today. However, its long‐term effect is guarded due to extension of atrophic laser scars (Brancato 1990) and recurrence of CNV (Johnson 1998).
Macular surgeries to tackle the problem involved primarily excision of CNV and macular translocation. Surgical CNV excision showed either no benefits (Hawkins 2004), or high rates of recurrence, ranging from 18% to 57% (Hamelin 2002; Ruiz‐Moreno 2001; Uemura 2000). Macular translocation might provide satisfactory results in some people (Kamei 2004; Takeuchi 2012), but it is rarely performed because there are other safer and more effective options.
PDT with verteporfin is so far the only approved treatment for mCNV by the US Food and Drug Administration (FDA). It could stabilise or improve vision in people with subfoveal CNV at one year of follow‐up (VIP Study Group 2001); however, the result at two years of follow‐up was not statistically significantly in favour of verteporfin therapy (Blinder 2003). Another long‐term follow‐up study showed rather disappointing VA results after PDT (Giansanti 2012).
Anti‐VEGF therapy has been widely used in treating diseases characterised by neovascularisation. Pegaptanib (Macugen; Eyetech Pharmaceuticals; Pfizer Inc, New York, NY, USA), a chemically synthesised ribonucleic acid (RNA) aptamer that targets only the VEGF 165 isoform, was approved by the FDA early in 2004 for the treatment of exudative age‐related macular degeneration (Gragoudas 2004). Two years later, pegatanib was granted marketing authorisation by the European Medicines Agency (EMA) to treat wet age‐related macular degeneration (Agency product number: EMEA/H/C/000620).
Ranibizumab (Lucentis; Genetech, San Francisco, CA, USA) was the second anti‐VEGF agent approved by the FDA specifically for treating neovascular age‐related macular degeneration in 2006 (Rosenfeld 2006). As a humanised, affinity‐maturated Fab fragment created from a full‐sized antibody (bevacizumab) and specifically designed for injections into the eye, ranibizumab was supposed to penetrate the inner retina and choroid more efficiently. In 2011, a broader application of ranibizumab to treat diabetic macular oedema was approved by the EMA, making the drug the first licensed therapy for diabetic macular oedema. In the USA, the FDA also approved ranibizumab to treat diabetic macular oedema in 2012 and to treat diabetic retinopathy in people with diabetic macular oedema in 2015. At present, ranibizumab has been approved to treat mCNV in Europe, Australia and Japan.
Bevacizumab (Avastin; Genetech, San Francisco, CA, USA), a full‐length monoclonal antibody that binds to all types of VEGF‐A, was approved by the FDA for its positive role in the treatment of metastatic colorectal cancer (Harris 2004). Then Michels 2005 tested its potential for the treatment of CNV via intravenous infusion, and intravitreal injections were further developed to avoid systemic adverse effects. It is now used off‐label for neovascular age‐related macular degeneration (Costa 2006). As a natural extension, scientists explored the use of anti‐VEGF therapy for CNV in pathological myopia. In 2005, Nguyen and coworkers reported that systemic bevacizumab was used in treating subfoveal CNV in pathological myopia. It was the first proof that VEGF‐A played an important role in the pathogenesis of CNV in pathological myopia (Nguyen 2005). Since then, a huge amount of research has been dedicated to exploring the efficacy and safety of different anti‐VEGF drugs in treating mCNV (Baba 2010; Bennett 2007; Hayashi 2012; Ruiz‐Moreno 2010; Voykov 2010; Yamamoto 2007).
Aflibercept (Eylea; Regeneron Pharmaceuticals, Tarrytown, New York, NY, USA) is a new molecule designed to couple with all members of the VEGF family, including VEGF‐A, ‐B, ‐C, ‐D, and even placental growth factor (PGF)‐1 and PGF‐2. It also has a higher VEGF‐A affinity (KD (the equilibrium dissociation constant between the antibody and its antigen) less than 1 pmol/L) than any other anti‐VEGF drug. Aflibercept has demonstrated a significant improvement in vision for people with neovascular age‐related macular degeneration (Brown 2011). In the USA, aflibercept was approved for treating neovascular age‐related macular degeneration in 2011 and for the treatment of macular oedema following central retinal vein occlusion in 2012. In 2014, aflibercept was approved for the treatment of diabetic macular oedema in the USA, Europe and Japan. At present, aflibercept has been approved for treating mCNV in Europe, Japan, Singapore, Korea and several other countries.
How the intervention might work
Pathological myopia is characterised by elongation of the axial length of the eyeball and subsequent progressive thinning of the choroid and sclera. Dysfunction of choroidal circulation causes atrophy of retinal pigment epithelium and release of VEGF‐A. Intraocular VEGF‐A levels correlate strongly with angiogenesis in people with age‐related macular degeneration, diabetic retinopathy, retinal vein occlusion and other retinal disorders (Adamis 1994; Aiello 1994; Boyd 2002; Kvanta 1996). Anti‐VEGF agents could counteract the angiogenic activity of VEGF by binding to a different VEGF protein subgroup, thus preventing receptor activation and later cascade reaction responsible for CNV.
Why it is important to do this review
Unlike age‐related macular degeneration, pathological myopia affects a younger middle‐aged population, which makes it a huge socioeconomic burden worldwide, especially for developing countries (Xu 2006). CNV in pathological myopia could result in a devastating threat to eyesight within a short period of time. PDT with verteporfin is the only approved approach by the US FDA to treat mCNV. However, it is not affordable or accessible to many people in low‐income countries, and its long‐term effects remain controversial (Blinder 2003). With a satisfactory short‐term therapeutic effect and few adverse effects, anti‐VEGF therapy has shown great potential to be the next generation treatment for mCNV. Long‐term results of several prospective, non‐randomised, consecutive interventional studies have been reported, though the results were inconsistent (Baba 2010; Bennett 2007; Hayashi 2010; Ikuno 2010; Ruiz‐Moreno 2010; Voykov 2010; Yamamoto 2007). As the VIP (Verteporfin In Photodynamic) trial has shown, promising positive results from one‐year follow‐up may not necessarily guarantee a good performance through the second year (Blinder 2003; VIP Study Group 2001). Similar to the complexity in treating age‐related macular degeneration (CATT 2011; CATT 2012; IVAN 2012), the choice of different anti‐VEGF agents and their different dosing regimens is also an inevitable problem in mCNV treatment. A systematic review to find an affordable and effective way to treat this blinding disease will be of great value.
Objectives
To assess the effects of anti‐vascular endothelial growth factor (anti‐VEGF) therapy for choroidal neovascularisation (CNV), compared with other treatments, sham treatment or no treatment, in people with pathological myopia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
We included participants who had CNV (commonly diagnosed with fluorescein angiography (FA) and optical coherence tomography (OCT)) secondary to pathological myopia (with a refractive error of ‐6.0 dioptres or more and an axial length greater than 26.5 mm). We did not impose any restrictions with regards to age, gender or ethnicity.
We excluded people with CNV associated with any condition other than pathological myopia; people with a previous history of treatment of mCNV; people who presented with macular hole, glaucoma, retinal degeneration, optic nerve neuropathy or systemic diseases affecting visual function; or people with uncompensated coronary artery disease, peripheral vascular disease, thromboembolism or stroke.
Types of interventions
Intervention: anti‐vascular endothelial growth factor (anti‐VEGF) therapy. Comparator: another treatment (e.g. photodynamic therapy (PDT) with verteporfin, laser photocoagulation, macular surgery, another anti‐VEGF), sham treatment or no treatment.
Types of outcome measures
Primary outcomes
Mean change from baseline in best‐corrected visual acuity (BCVA) at 1 year after treatment.
Proportion of participants with a gain of 3+ lines in BCVA at 1 year after treatment.
Secondary outcomes
Change in central macular thickness (CMT) assessed by OCT at 1 year after treatment.
Proportion of participants with CNV angiographic closure indicated by no evidence of dye leakage in FA at 1 year after treatment.
Percentage of participants with newly developed chorioretinal atrophy or progression of pre‐existing chorioretinal atrophy determined by fundus photography at 1 year after treatment.
Vision‐related quality of life (measured by questionnaires, e.g. Low Vision Quality of Life (LVQOL), Adaptation to Age‐related Vision Loss (AVL), Keele Participation Restriction Questionnaire (KAP)).
Adverse effects
Adverse events: transient visual disturbance (as defined by the investigator); infectious endophthalmitis; subconjunctival, vitreous or subretinal haemorrhage; retinal detachment; retinal pigment epithelium rip; sustained increase in intraocular pressure (as defined by the investigator); cataract and cardiovascular complications.
We also considered six months and 2 years as other time periods of outcome assessment.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 6), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2016), Embase (January 1980 to June 2016), the Chinese Biomedicine Database (CBM) (January 1980 to June 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 16 June 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), CBM (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of review articles and identified trial reports for details of further relevant publications. We also contacted experts in the field for details of upcoming trials, or completed trials awaiting publication.
Data collection and analysis
Selection of studies
Two authors (YZ and TZ) independently reviewed the abstracts of studies identified by the electronic and manual searches to decide on eligibility for inclusion in the review. For all the studies potentially eligible, we reassessed the study reports by reading the full text according to the inclusion criteria. We contacted the authors of these studies for further clarification when necessary. Any differences in study selection for inclusion were referred to a third author (GZX) and resolved by discussions, which were documented in the review.
Data extraction and management
Two authors (YZ and TZ) independently extracted trial data. One author (YZ) extracted data onto standard forms predesigned by Cochrane Eyes and Vision, and transcribed them into Review Manager 5 (RevMan 2014). A second author (TZ) verified them. We documented information related to VA measurement (including type of chart used, measurement protocol, test distance, etc.) in the Characteristics of included studies table. For further information, see Appendix 8.
We extracted data on how VA was measured and analysed for the analyses and whether standard deviations (SDs) were calculated on a logMAR scale. For our primary outcome, VA, we included studies using non‐logarithmic VA charts, but considered SDs calculated on a decimal scale as missing data, since the direct conversion from decimal to logMAR VA is straightforward for means, but not for SDs. Methods for dealing with missing data are described in the Dealing with missing data section.
Assessment of risk of bias in included studies
Two authors (YZ and TZ) independently assessed the risk of bias in each study by examining the methods of sequence generation used for randomisation, allocation concealment, study masking of participants and personnel, study masking of outcome assessors, incomplete outcome data and selective outcome reporting (Appendix 9), using the GRADE approach. When published data were insufficient to make the assessment, we contacted authors of these studies for further information by telephone, email or letter. With this information, we classified each study into one of three categories: 'low', 'high' or 'unclear' risk of bias according to the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
In order to appraise the effect of treatment, we calculated the mean difference (MD) for continuous variables and the risk ratio (RR) for dichotomous variables. We also reported 95% confidence intervals (CIs) for both individual results and pooled estimates.
Continuous outcome measures
-
Primary outcome
Mean change in BCVA at follow‐up compared to baseline.
-
Secondary outcomes
Change in CMT determined by OCT.
Vision‐related quality of life.
Dichotomous outcome measures
-
Primary outcome
Percentage of participants with a gain of 3+ lines in BCVA.
-
Secondary outcomes
Percentage of participants with CNV angiographic closure (no evidence of dye leakage in FA).
Percentage of participants with newly developed chorioretinal atrophy or progression of pre‐existing chorioretinal atrophy determined by fundus photography.
Unit of analysis issues
The unit of analysis was the participant. We anticipated in the protocol there would be only one eye included per participant for the following reasons: first, few people have mCNV in both eyes at the same time; second, intravitreous anti‐VEGF in one eye could be absorbed into systemic circulation and interfere with the evaluation of the other eye; and third, it would be more ethically acceptable to include only one eye due to safety concerns over an experimental intervention. All of the studies included in this review so far included only one eye per participant. If studies are found for future updates of the review adopt eyes, not participants, as the unit of analysis, we will still extract and use data from these studies but present them as a subgroup in the meta‐analysis; the other subgroup being studies using participants as unit of analysis. We did not accept paired studies (i.e. studies in which one eye of a participant was randomised to treatment and the fellow eye to control). We suggest this study design is unlikely to be used, but we will describe any such studies in the 'Discussion' if we find them in future updates.
Dealing with missing data
We first contacted the investigators to request missing data by email, post or any other method available. If we did not get a response in one month, we conducted a primary analysis based on participants with complete data assuming data missing at random. If data were unlikely to be missing at random (e.g. participants with poor prognosis are more likely to drop out), we would conduct analyses imputing the missing data with replacement values (e.g. values assuming all were poor outcomes, the mean value, or, if made available by study authors, values based on predicted values from a regression analysis) and state the assumptions we made. According to the risk of bias assessment, we felt it unnecessary to make any assumptions in this review. When SDs were missing or had been incorrectly calculated on a decimal scale, we contacted study authors for any missing data, and finally imputed SDs based on observed values from other studies if the authors did not reply or could not provide additional data.
Assessment of heterogeneity
We checked for statistical heterogeneity by examining the forest plot of the results, as well as using the Chi2 test and I2 statistic. Considering Chi2 had low power in analysing a meta‐analysis when studies were few in number or had a small sample size, we used a P value of 0.10 to indicate statistical significance. Results with an I2 statistic of less than 50% were considered as low heterogeneity. An I2 statistic between 50% and 75% was regarded as substantial heterogeneity and sources of heterogeneity were investigated. An I2 statistic between 75% and 100% indicated considerable statistical heterogeneity, and in this case we planned to present data in tables instead of performing a meta‐analysis. However, considering heterogeneity was poorly estimated if few studies were included, a CI for I2 was calculated. We also considered conducting a meta‐analysis if the few heterogeneous trials were mostly in the direction of benefit or harm.
Assessment of reporting biases
In future updates of the review, we plan to examine funnel plots of data if at least 10 trials are included to detect possible reporting biases, especially for those studies of small size. And in addition we will perform sensitivity analyses.
Data synthesis
Data analysis followed the guidelines set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If there were three or fewer trials included, we performed a fixed‐effect model analysis. If there was minimal statistical heterogeneity and minimal clinical heterogeneity between trials, we performed a meta‐analysis using a random‐effects model. If there was substantial statistical (i.e. I2 value more than 75%) or clinical heterogeneity, we reported results in tables instead of pooling data across trials. However, if the forest plot indicated all the estimates of treatment effect were in the same direction of benefit or harm, we combined study results even when there was substantial statistical heterogeneity.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses of the primary outcome based on the types of anti‐VEGF therapy: ranibizumab, bevacizumab or aflibercept.
In future updates of the review with more data available, we may perform other subgroup analysis based on: RCTs versus quasi‐RCTs, dosing regimens of anti‐VEGF medication (monthly, quarterly, 1+PRN, 3+PRN), location of CNV (subfoveal, parafoveal or juxtafoveal).
Sensitivity analysis
Decisions made through this systematic review might be biased by exclusion of studies with low methodological quality, exclusion of industry‐funded studies, exclusion of unpublished studies, inclusion of an 'outlier' study (a study with results very different from the rest of the studies) or inclusion of studies with missing data. If more studies are included in future updates of the review, we will perform a sensitivity analysis to evaluate the impact of the possible arbitrariness.
Summary of findings
We prepared a summary of findings table presenting relative and absolute risks. We graded the certainty of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/). We included the following outcomes in the summary of findings table.
Change in BCVA from baseline at 1 year
Gain 3+ lines of visual acuity at 1 year
CNV angiographic closure at 1 year
Change in central macular thickness at 1 year
Quality of life
Systemic serious adverse events
Ocular adverse events
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The electronic searches yielded 542 references (Figure 1). The Cochrane Information Specialist removed 153 duplicate records and we screened the remaining 389 reports. We rejected 376 records after reading the abstracts and obtained the full‐text reports of 13 references for further assessment. We included seven reports of six studies (Gharbiya 2010; Iacono 2012; MYRROR 2010; Parodi 2010; RADIANCE 2010; Ruiz‐Moreno 2013), and excluded three studies (Heier 2011; REPAIR 2010; Saviana 2014). We also identified three ongoing studies which meet the inclusion criteria (NCT01716026 (BENEMCOR); NCT01809223 (SHINY); NCT01922102 (Brilliance)). When the review is next updated, we will check to see if these studies have published data available.
1.
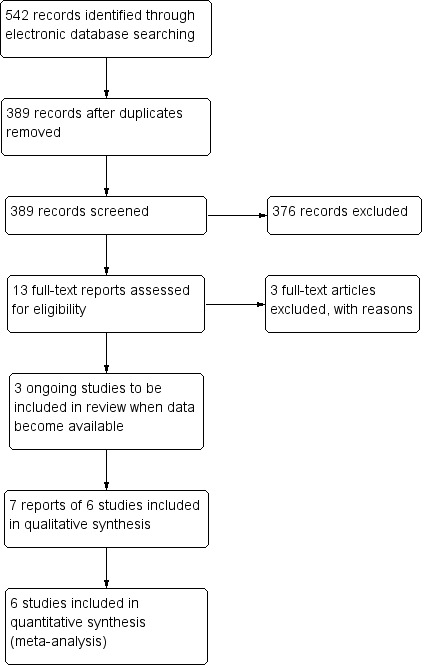
Study flow diagram.
Included studies
We included six studies in this review, all of which were RCTs. MYRROR 2010 and RADIANCE 2010 were industry‐sponsored, international, multicentre RCTs. Gharbiya 2010; Iacono 2012; and Parodi 2010 were all conducted in Italy and Ruiz‐Moreno 2013 was conducted in Spain. See Characteristics of included studies table for details.
Types of participants
Studies included participants with mCNV diagnosed clinically. Pathological myopia was typically defined as a refractive error of ‐6.0 dioptres or more and axial length greater than 26.5 mm. Active CNV was confirmed with leakage on FA. Only treatment‐naive participants were included and participants with CNV caused by reasons other than pathological myopia were excluded.
Types of interventions
Among the six included studies, one study compared ranibizumab with PDT (RADIANCE 2010), two studies compared bevacizumab with PDT (Parodi 2010; Ruiz‐Moreno 2013), one study compared bevacizumab with laser (Parodi 2010), one study compared aflibercept with sham treatment (MYRROR 2010), and two studies compared bevacizumab with ranibizumab (Gharbiya 2010; Iacono 2012).
The drug dosage was identical in all the included studies (ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2.0 mg). The treatment regimen for anti‐VEGF was 3+PRN in Ruiz‐Moreno 2013 and 1+PRN in all other included studies. Retreatment decision was primarily based on disease activity monitored by OCT or FA. RADIANCE 2010 included two arms of anti‐VEGF treatment (group I guided by VA stabilisation criteria and group II guided by disease activity criteria). Considering the consistency between studies, we chose group II (anti‐VEGF treatment guided by disease activity) as the experimental group of RADIANCE 2010 for major outcome analysis. It is worth mentioning that the control group in RADIANCE 2010 and MYRROR 2010 were allowed to receive anti‐VEGF treatment after three months (RADIANCE 2010) and six months (MYRROR 2010), which needs to be taken into consideration in analysis of efficacy.
Types of outcome measures
The primary outcome for all studies was change in VA although it was presented in different ways. For statistical reasons, VA was extracted as logMAR scale and no study used decimal scale. Two studies reported VA at baseline and different time points of the study instead of change in VA during follow‐up and further communication with authors retrieved no additional data (Iacono 2012; Parodi 2010). So the mean of change in VA was calculated and the SDs of change in VA had to be imputed from similar studies (see footnotes of forest plots). All studies reported the proportion of participants gaining 3+ lines of VA except the data of Ruiz‐Moreno 2013 where participants gained 2+ lines of VA.
For secondary outcomes, all studies mentioned CMT but not all the results were available for analysis. Parodi 2010 did not report CMT in separate groups, so we deleted CMT in the comparison of anti‐VEGF versus laser. Gharbiya 2010 did not report the SDs of change in CMT and Iacono 2012 described a trend in CMT reduction without detailed data, so the comparison of ranibizumab versus bevacizumab did not include CMT either. Ruiz‐Moreno 2013 did not report the SDs of change in CMT and the data were imputed as mean SD of change in CMT from RADIANCE 2010. Four studies reported the proportion of participants with CNV angiographic closure (Gharbiya 2010; Parodi 2010; RADIANCE 2010; Ruiz‐Moreno 2013), and three studies reported the change in mean CNV size (Iacono 2012; MYRROR 2010; RADIANCE 2010). So far, no study analysed the incidence of newly developed chorioretinal atrophy or progression of pre‐existing atrophy. Only one study reported quality of life outcomes (MYRROR 2010). All studies reported adverse events.
Excluded studies
We excluded three studies after full‐text assessment: Heier 2011 included participants with CNV not caused by pathological myopia and there was no subgroup analysis; REPAIR 2010 contained no comparator arm and Saviana 2014 included people previously treated with laser or PDT. See Characteristics of excluded studies table for details.
Risk of bias in included studies
See Figure 2 for a summary of risk of bias in included studies.
2.
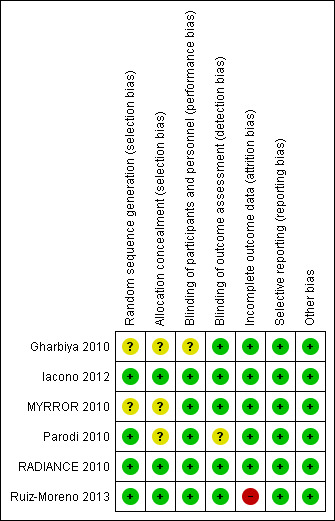
Risk of bias summary: authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, risk of bias was low in four studies (Iacono 2012; Parodi 2010; RADIANCE 2010; Ruiz‐Moreno 2013), and unclear in two studies (Gharbiya 2010; MYRROR 2010). Three studies did not report methods for allocation concealment (Gharbiya 2010; MYRROR 2010; Parodi 2010).
Blinding
Five studies fulfilled masking of participants and personnel and one study did not report it (Gharbiya 2010). Five studies obtained masking of outcome assessment and one study did not report it (Parodi 2010).
Incomplete outcome data
All studies but one were at low risk of attrition bias. Ruiz‐Moreno 2013 had a loss to follow‐up of over 20% at two years and no reason was reported, though the numbers were balanced across groups.
Selective reporting
In studies where the protocol was identified, there were no discrepancies between the protocol and published study. In studies without a protocol, outcome measures were listed in the methods section and no key outcome was missing (Iacono 2012; Parodi 2010).
Other potential sources of bias
No other potential sources of bias were identified in the included studies.
Effects of interventions
See: Table 1
See: Table 1.
Anti‐VEGF versus PDT
Outcomes at one year
Three studies compared anti‐VEGF therapy with PDT. One study used ranibizumab (RADIANCE 2010, 277 participants) and two studies used bevacizumab (Parodi 2010; Ruiz‐Moreno 2013, 99 participants).
Primary outcomes
At one‐year follow‐up, meta‐analysis showed an improvement in VA in participants treated with anti‐VEGF treatment compared with participants treated with PDT (MD ‐0.14 logMAR better, equivalent of 7 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, 95% CI ‐0.20 to ‐0.08, 3 trials, 263 participants) (Figure 3). Though heterogeneity was relatively high (I2 = 68%, P = 0.05), these trials were all in the same direction of benefit. Reasons contributing to the high heterogeneity may include: 1. the PDT group in RADIANCE 2010 was allowed to receive anti‐VEGF treatment after three months, which might narrow the difference between the two groups; and 2. the loss to follow‐up rate was over 20% in Ruiz‐Moreno 2013, which may lead to a larger SD of change in VA. Even though the I2 statistic for subgroup differences was 81.5% (P = 0.02), we cannot attribute the difference in VA change to different types of anti‐VEGF agent.
3.
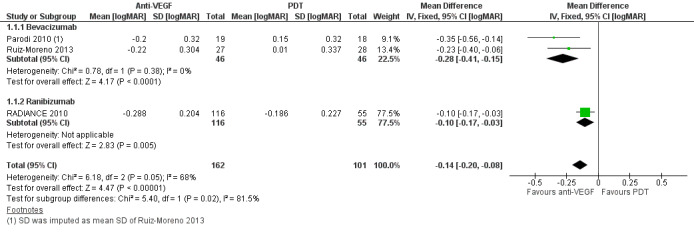
Forest plot of comparison: 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), outcome: 1.1 Change in visual acuity at 1 year [logMAR].
Two studies reported the proportion of participants gaining 3+ lines of VA at one year (RADIANCE 2010; Ruiz‐Moreno 2013) (Figure 4). The RR was 1.86 (95% CI 1.27 to 2.73, 2 trials, 226 participants, moderate‐certainty evidence) and about four people had to be treated with anti‐VEGF therapy, compared to PDT, to allow one person to markedly improve their vision.
4.
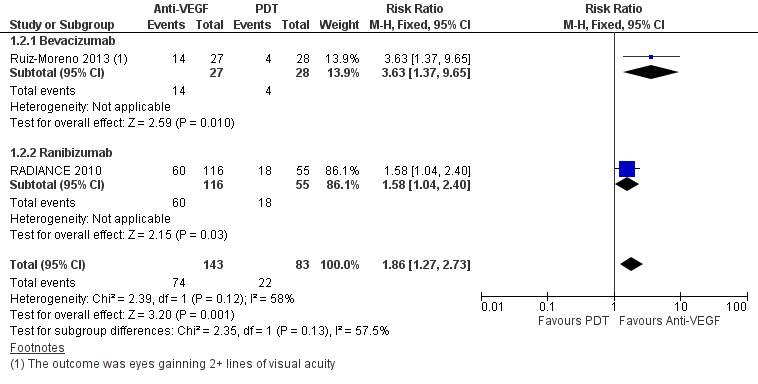
Forest plot of comparison: 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), outcome: 1.2 Gain 3+ lines of visual acuity at 1 year.
We assessed the certainty of the evidence as low for mean change of BCVA and moderate for proportion of gaining 3+ lines of BCVA, because there was high or unclear risk of bias for one or more domains in some of the included studies.
Secondary outcomes
Two studies reported change in CMT (RADIANCE 2010; Ruiz‐Moreno 2013). Meta‐analysis showed that reduction of CMT in participants treated with anti‐VEGF was 17.64 μm greater than in participants treated with PDT, though the estimate of effect was uncertain (95% CI ‐41.98 to 6.3, 2 trials, 226 participants) (Analysis 1.4). The rate of CNV angiographic closure favoured anti‐VEGF treatment, but the estimate was imprecise (RR 1.24, 95% CI 0.99 to 1.54, 2 trials, 208 participants, P = 0.06) (Analysis 1.3). However, it is worth noting that the control group in RADIANCE 2010 was allowed to receive anti‐VEGF treatment after three months, which might narrow the difference between anti‐VEGF and PDT in anatomic results. Parodi 2010 did not report CMT in separate groups, so it was excluded from the analysis of change in CMT.
1.4. Analysis.
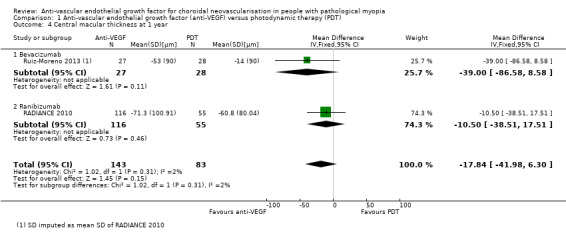
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 4 Central macular thickness at 1 year.
1.3. Analysis.
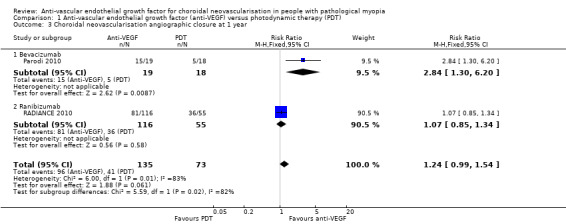
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 3 Choroidal neovascularisation angiographic closure at 1 year.
We assessed the certainty of the evidence as moderate for change in CMT and low for rate of CNV angiographic closure, because heterogeneity was high and the 95% CI value was null.
Outcomes at two years
Two studies reported the data at two years (Parodi 2010; Ruiz‐Moreno 2013).
Primary outcomes
Anti‐VEGF maintained its advantage over PDT in terms of VA change at two years (Analysis 1.5; Analysis 1.6). The mean VA in participants treated with anti‐VEGF was ‐0.26 logMAR better, equivalent of 13 ETDRS letters compared with participants treated with PDT (95% CI ‐0.38 to ‐0.14, 2 trials, 92 participants). The RR for proportion of participants gaining 3+ lines of VA at two years was 3.43 (95% CI 1.37 to 8.56, 2 trials, 92 participants).
1.5. Analysis.
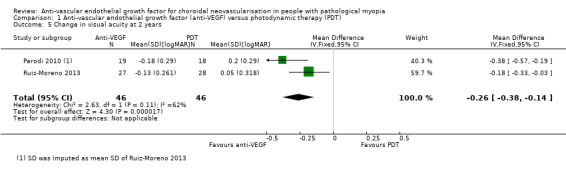
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 5 Change in visual acuity at 2 years.
1.6. Analysis.
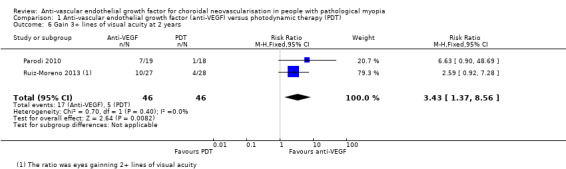
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 6 Gain 3+ lines of visual acuity at 2 years.
Secondary outcomes
Change in CMT and rate of CNV angiographic closure were similar in both groups at two years (Analysis 1.8; Analysis 1.7). Both treatments presented satisfactory anatomic results in the long term.
1.8. Analysis.
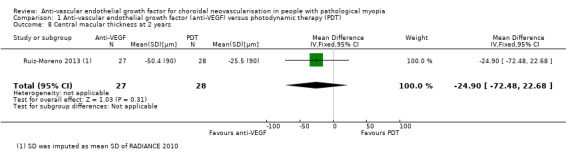
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 8 Central macular thickness at 2 years.
1.7. Analysis.
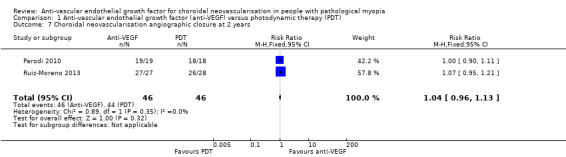
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 7 Choroidal neovascularisation angiographic closure at 2 years.
We judged the certainty of the evidence as low, because 'optimal information size' was not met and there were possible publication biases due to off‐label use of bevacizumab.
Anti‐VEGF versus laser
One study compared anti‐VEGF with laser treatment (36 participants) (Parodi 2010).
Outcomes at one year
Primary outcomes
Anti‐VEGF treatment had a better visual prognosis than laser treatment with an MD of ‐0.22 logMAR, equivalent of 11 ETDRS letters (95% CI ‐0.43 to ‐0.01, P = 0.04) (Analysis 2.1).
2.1. Analysis.
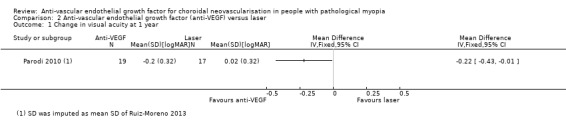
Comparison 2 Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser, Outcome 1 Change in visual acuity at 1 year.
Secondary outcomes
Parodi 2010 did not report change in CMT for different groups, so the comparison for change in CMT was not available. The rate of CNV angiographic closure favoured anti‐VEGF treatment, but estimates were imprecise and included little or no difference (RR 1.68, 95% CI 0.96 to 2.92, P = 0.07) (Analysis 2.2).
2.2. Analysis.
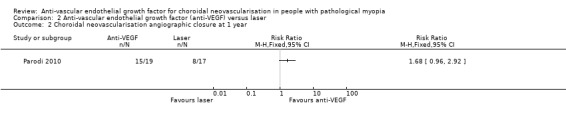
Comparison 2 Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser, Outcome 2 Choroidal neovascularisation angiographic closure at 1 year.
Outcomes at two years
Primary outcomes
At two years, the mean change in VA still favoured anti‐VEGF treatment (Analysis 2.3). The mean VA in participants treated with anti‐VEGFs was ‐0.29 logMAR better, equivalent of 14 ETDRS letters (95% CI ‐0.50 to ‐0.08, P = 0.007). There was no difference between anti‐VEGF and laser treatment in the proportion of participants gaining 3+ lines of VA (Analysis 2.4).
2.3. Analysis.
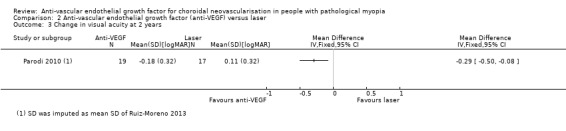
Comparison 2 Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser, Outcome 3 Change in visual acuity at 2 years.
2.4. Analysis.
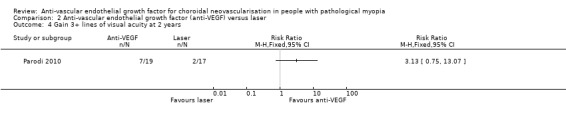
Comparison 2 Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser, Outcome 4 Gain 3+ lines of visual acuity at 2 years.
Secondary outcomes
No participants had subfoveal CNV recurrence in either group at two years (Analysis 2.5).
2.5. Analysis.
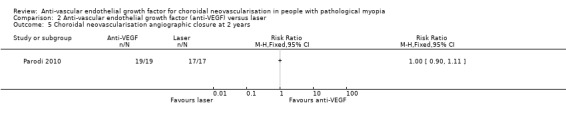
Comparison 2 Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser, Outcome 5 Choroidal neovascularisation angiographic closure at 2 years.
We downgraded the certainty of the evidence for the comparison of anti‐VEGF with laser from high to low, because 'optimal information size' was not met and possible negative results for bevacizumab might not be published.
Anti‐VEGF versus sham treatment
One study compared anti‐VEGF with sham treatment (121 participants) (MYRROR 2010). Since the primary efficacy end point was the mean change in BCVA from baseline to week 24 and the control group was allowed to have anti‐VEGF therapy after week 24, we analysed the data at six months to display the difference between anti‐VEGF and sham treatment better.
Outcomes at six months
Primary outcomes
At week 24, participants with anti‐VEGF treatment showed a greater improvement over sham treatment with an MD of ‐0.28 logMAR, equivalent of 14 ETDRS letters (95% CI ‐0.36 to ‐0.21, P < 0.00001) (Analysis 3.1). The anti‐VEGF group also had a higher rate of participants gaining 3+ lines of VA (RR 4.02, 95% CI 1.33 to 12.15) (Analysis 3.2).
3.1. Analysis.
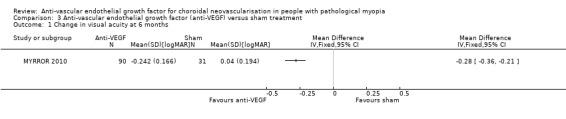
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 1 Change in visual acuity at 6 months.
3.2. Analysis.
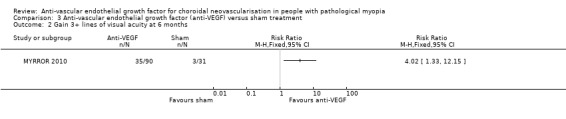
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 2 Gain 3+ lines of visual acuity at 6 months.
Secondary outcomes
Though the SDs of change in CMT were relatively high, there was a statistically significant difference that favoured the anti‐VEGF group (MD ‐66.80, 95% CI ‐114.87 to ‐18.73, P = 0.006) (Analysis 3.3). CNV angiographic closure ratio was not reported at week 24, but lesion area was compared between the two groups. Both CNV size and the area of fluorescein dye leakage presented a larger reduction in the anti‐VEGF group compared with sham treatment. Quality of life measured using the National Eye Institute Visual Function Questionnaire 25 (data available on the www.clinicaltrials.gov website) also favoured the anti‐VEGF group (MD 5.72, 95% CI 1.60 to 9.84, P = 0.007) (moderate‐certainty evidence) (Analysis 3.4).
3.3. Analysis.
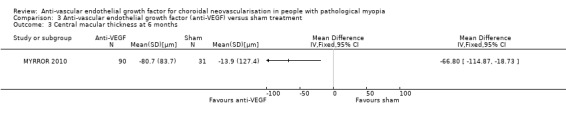
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 3 Central macular thickness at 6 months.
3.4. Analysis.
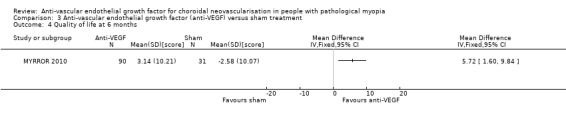
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 4 Quality of life at 6 months.
Outcomes at one year
Primary outcomes
The SDs of change in VA were imputed as mean SD of change in VA at six months since no data were reported in the manuscript or posted on the www.clinicaltrials.gov website. The advantage of anti‐VEGF over sham treatment in mean change in VA was less prominent but maintained at week 48 (MD ‐0.19, equivalent of 9 ETDRS letters, 95% CI ‐0.27 to ‐0.12) (Analysis 3.5). The proportion of people gaining 3+ lines of VA favoured anti‐VEGF versus sham treatment, but estimates were imprecise and included little or no difference (RR 1.72, 95% CI 0.96 to 3.10, P = 0.07) (Analysis 3.6).
3.5. Analysis.
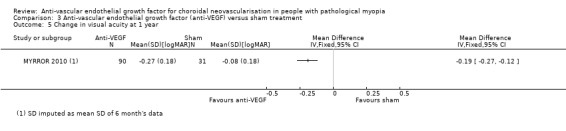
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 5 Change in visual acuity at 1 year.
3.6. Analysis.
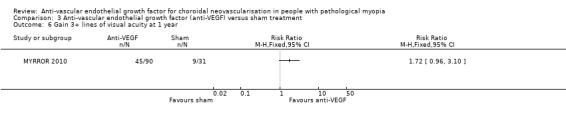
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 6 Gain 3+ lines of visual acuity at 1 year.
Secondary outcomes
The results were similar in the two groups in terms of change in CMT (Analysis 3.8). The rate of CNV angiographic closure at week 48 favoured anti‐VEGF versus sham treatment, but estimates were imprecise and included little or no difference (RR 1.31, 95% CI 0.99 to 1.72) (Analysis 3.7). Quality of life score at week 48 was not reported in the manuscript and no additional data were retrieved.
3.8. Analysis.
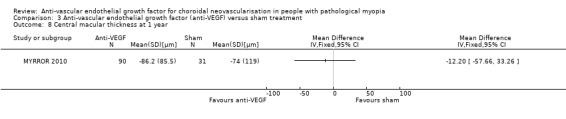
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 8 Central macular thickness at 1 year.
3.7. Analysis.
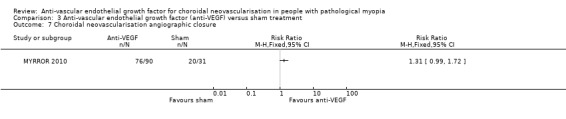
Comparison 3 Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment, Outcome 7 Choroidal neovascularisation angiographic closure.
We considered the certainty of the evidence on comparison of anti‐VEGF with sham treatment as moderate, because the 'optimal information size' was not met.
Adverse events: anti‐VEGF versus control
In the four studies comparing anti‐VEGF with other treatment or sham treatment, two studies reported no systemic or ocular serious adverse events in either group during follow‐up (Parodi 2010; Ruiz‐Moreno 2013). Two industry‐sponsored studies documented adverse events in the Medical Dictionary for Regulatory Activities (MedDRA) code (MYRROR 2010; RADIANCE 2010).
Serious systemic adverse events
There were 15 serious systemic adverse events in 359 participants (15/359, 4.2%). All the adverse events were in the anti‐VEGF group, but the estimate of RR was uncertain (RR 4.50, 95% CI 0.60 to 33.99; participants = 508). See Figure 5.
5.
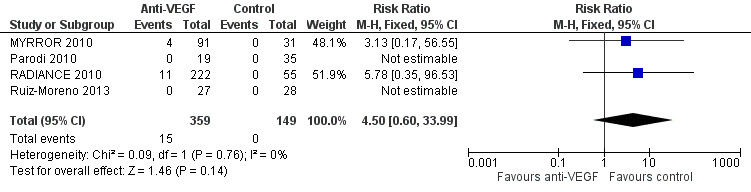
Forest plot of comparison: 4 Adverse events: anti‐vascular endothelial growth factor (anti‐VEGF) versus control, outcome: 4.1 Systemic serious adverse events.
The investigator considered that none of the serious systemic adverse events was related to study drug, injection or study procedures.
Serious ocular adverse events
There were five serious ocular adverse events in 359 participants (5/359, 1.4%). Though all the adverse events occurred in the anti‐VEGF group, the difference between two groups was uncertain due to low incidence of adverse events (RR 1.82, 95% CI 0.23 to 14.71; participants = 508; studies = 4, 508 participants). See Figure 6.
6.
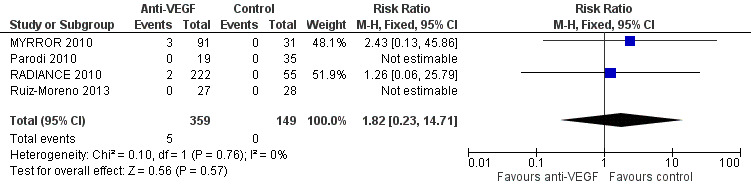
Forest plot of comparison: 4 Adverse events: anti‐vascular endothelial growth factor (anti‐VEGF) versus control, outcome: 4.2 Ocular serious adverse events.
One case of corneal erosion was suspected to be related to the ocular injection procedure of anti‐VEGF (RADIANCE 2010). Common ocular adverse events included conjunctival haemorrhage and punctate keratitis (with a similar incidence of about 10% for the former and 5% for the later in MYRROR 2010 and RADIANCE 2010). The incidence was similar in the anti‐VEGF and control groups.
The certainty of the evidence on serious adverse events was downgraded from high to very low, because of imprecision (two levels) and indirectness (one level) due to exclusion of participants with previous cardiovascular events in these studies.
Ranibizumab versus bevacizumab
Outcomes at one year
Two studies compared ranibizumab with bevacizumab (80 participants) (Gharbiya 2010; Iacono 2012). The follow‐up period for Gharbiya 2010 was six months.
Primary outcomes
Ranibizumab and bevacizumab showed similar results in change in VA (MD ‐0.02 logMAR, equivalent of 1 ETDRS letter, 95% CI ‐0.11 to 0.06, P = 0.59) (Analysis 5.1) and proportion of participants gaining 3+ lines of VA (RR 0.79, 95% CI 0.50 to 1.27, P = 0.33) (Analysis 5.2).
5.1. Analysis.
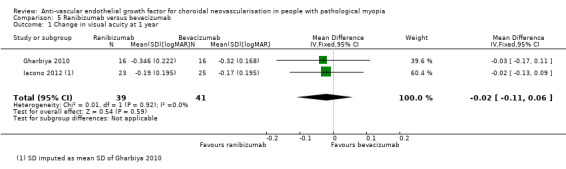
Comparison 5 Ranibizumab versus bevacizumab, Outcome 1 Change in visual acuity at 1 year.
5.2. Analysis.
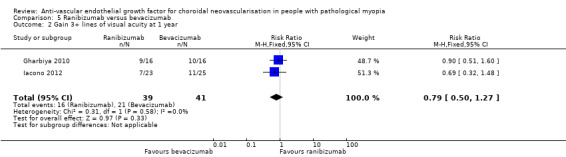
Comparison 5 Ranibizumab versus bevacizumab, Outcome 2 Gain 3+ lines of visual acuity at 1 year.
Secondary outcomes
Gharbiya 2010 did not report the SDs of change in CMT and Iacono 2012 described a trend in CMT reduction without detailed data, so the analysis of CMT was not available. For ratio of CNV angiographic closure, ranibizumab and bevacizumab had similar results (Analysis 5.3). In both studies, there were no systemic or serious ocular adverse events registered in either group during the follow‐up period.
5.3. Analysis.
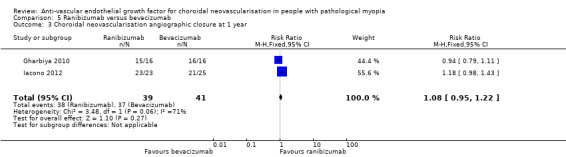
Comparison 5 Ranibizumab versus bevacizumab, Outcome 3 Choroidal neovascularisation angiographic closure at 1 year.
The certainty of the evidence on direct comparison of two anti‐VEGFs was downgraded from high to moderate, because the 'optimal information size' was not met.
Other outcomes
No studies included in the present review investigated the proportion of participants with newly developed chorioretinal atrophy or progression of pre‐existing chorioretinal atrophy determined by fundus photography. We plan to do this analysis when data become available.
We planned to do subgroup analyses based on dosing regimens of anti‐VEGF medication (monthly, quarterly, 1+PRN, 3+PRN) or location of CNV (subfoveal, parafoveal or juxtafoveal) in the protocol. However, the treatment regimen for anti‐VEGF was uniform in all studies except one trial (Ruiz‐Moreno 2013). Five included studies investigated subfoveal mCNV except one trial investigated juxtafoveal mCNV only (Parodi 2010). We did not conduct subgroup analyses due to insufficient data.
Discussion
Summary of main results
This systematic review provided moderate‐certainty evidence on the beneficial effect of anti‐VEGF over PDT on visual recovery in the treatment of pathological mCNV at one year. The MD of change in BCVA from baseline in participants treated with anti‐VEGFs was ‐0.14 logMAR unit, equivalent of seven ETDRS letters, and about four people needed to be treated to achieve a 3+ lines gain of vision in one person, compared with participants treated with PDT. At two years, anti‐VEGF maintained its advantage over PDT in terms of visual prognosis, but the certainty of the evidence was low. It is worth mentioning that the benefit of anti‐VEGF over PDT might be greater in the long term since one major study allowed rescue anti‐VEGF treatment after three months (RADIANCE 2010).
The comparison of anti‐VEGFs with laser photocoagulation showed that there was more improvement in VA among bevacizumab‐treated participants than among laser‐treated participants after one year (MD ‐0.22 logMAR, equivalent of 11 ETDRS letters, 95% CI ‐0.43 to ‐0.01, 1 trial, 36 participants) and after two years (MD ‐0.29 logMAR, equivalent of 14 ETDRS letters; 95% CI ‐0.50 to ‐0.08, 1 trial, 36 participants). However, the certainty of the evidence was low due to small sample size and possible publication bias.
When compared with sham treatment, participants treated with aflibercept had better vision at one year (MD ‐0.19 logMAR, equivalent of 9 ETDRS letters, 95% CI ‐0.27 to ‐0.12, 1 trial, 121 participants). The certainty of the evidence was downgraded to moderate due to small sample size.
Moderate‐certainty evidence on direct comparisons between different anti‐VEGF agents (ranibizumab versus bevacizumab) in two head‐to‐head trials did not demonstrate a difference in the effect on VA.
Evidence on safety assessment of anti‐VEGF agents was very low‐certainty in the included RCTs. Four studies reported no adverse events in either group and two industry‐sponsored studies reported both systemic and ocular adverse events. Only one case of corneal erosion was suspected to be related to the injection procedure of anti‐VEGF agent and all other adverse events were considered by the investigator to be unrelated to the study drug, injection or study procedures. However, since these trials excluded conditions such as uncontrolled hypertension or prior cerebrovascular accident, this could limit the applicability of evidence in the real‐world myopic population. Previous Cochrane Reviews showed good systemic safety of intravitreal anti‐VEGF therapy in age‐related macular degeneration (Solomon 2014) and diabetic macular oedema (Virgili 2014), as well as little difference between bevacizumab and ranibizumab (Moja 2014). The incidence of serious adverse events could be extremely rare in this myopic population, which is younger and at lower systemic risk than people with age‐related macular degeneration. Nonetheless, caution should be paid in applying these conclusions on safety to older and more frail people with myopia.
Overall completeness and applicability of evidence
The aim of this review was to assess effects of intravitreal injection of anti‐VEGF agents for the treatment of mCNV, compared with PDT, laser, sham treatment or another anti‐VEGF agent. The review included only RCTs. The primary outcomes were the mean change from baseline in BCVA and proportion of participants with a gain of 3+ lines in BCVA at one year of follow‐up. Secondary outcomes included other functional or morphological features, such as proportion of participants with CNV angiographic closure assessed by FA, central retinal thickness assessed by OCT, quality of life, and systemic and ocular adverse events. Relevant data were searched not only from journal publications, but also from clinical trial registries, conference abstracts, pharmaceutical company websites and FDA documents. When published data were insufficient, unclear or missing, we contacted study investigators for further information.
There are still insufficient data to analyse the change in CMT. Only three studies provided enough data for the analysis of CMT (MYRROR 2010; RADIANCE 2010; Ruiz‐Moreno 2013). The SDs of change in CMT in Ruiz‐Moreno 2013 had to be imputed from RADIANCE 2010 since we retrieved no additional data. Considering the SD of mean CMT at different time points, as well as the SD of mean change in CMT were both large, it is not surprising to have uncertain results in the comparison. However, CMT decreased significantly in participants treated with anti‐VEGF in one trial (aflibercept, MYRROR 2010) compared with sham at week 24 and in another trial (ranibizumab, RADIANCE 2010) compared with PDT at month three. In these two trials, participants from the control groups were allowed to receive anti‐VEGF treatment after week 24 and month three, respectively, which could partially explain why the change in CMT in the anti‐VEGF and control groups was similar at one year. Thus, there might still be a trend to suggest that CMT could be further decreased and maintained in people treated with anti‐VEGF agents compared with control group.
Data were insufficient for the analysis of efficacy difference based on CNV locations. Five included studies investigated subfoveal mCNV and one trial investigated juxtafoveal mCNV only (Parodi 2010). However, there was no subgroup analysis of this comparison.
This review included 594 participants from six trials conducted in multiple clinical centres from different countries, which could be representative of people with mCNV. However, this evidence was generated from clinical trials with adequate monitoring criteria. In the real‐world busy clinical practices, the change of vision might not be recognised in people with high myopia due to previous poor VA. Besides, economic burden is heavy in low‐income countries, thus prompt response and strict follow‐up is not always possible. A pragmatic RCT would be necessary to appraise the effect of anti‐VEGF on mCNV in real‐world situations. We would also like to add that pathological myopia is a complicated disease with mCNV commonly comorbid with other ocular diseases. For example, vitreous macular traction could develop as a result of posterior vitreous detachment. People might have received vitrectomy for these conditions before mCNV developed. Thus, the applicability of the available data to these people in clinical practice is still unknown. Pathological myopia is a condition evolving with time (e.g. progressive choroid atrophy develops with years), which makes it necessary to assess the long‐term (years of time) efficacy and safety of anti‐VEGF agents. Thus, information from the studies included is still limited because only two trials provided data at 24 months (Parodi 2010; Ruiz‐Moreno 2013).
Quality of the evidence
The certainty of the evidence is low regarding the comparison of anti‐VEGF with PDT because heterogeneity was high and the 'optimal information size' was not met for some outcomes. Certainty of evidence on the comparison of anti‐VEGF with laser is considered as low due to small sample size and possible publication biases. Certainty of evidence on the comparison of anti‐VEGF with sham treatment and the direct comparison of two anti‐VEGFs (bevacizumab versus ranibizumab) is considered as moderate due to small sample size.
Potential biases in the review process
We could have missed some small unpublished clinical trials on bevacizumab, because bevacizumab is an anti‐VEGF agent used off‐label to treat CNV in most countries. Some RCTs featuring bevacizumab may have been conducted but not published due to lack of efficacy, which could result in selective publication biases.
Agreements and disagreements with other studies or reviews
There are several reviews about anti‐VEGF treatment for mCNV.
Battaglia 2010; Cohen 2009; Gupta 2010; Lynch 2007; and Sun 2008 included studies conducted up to 2010, but their reviews on the pooled data of case series suggested possible promising efficacy of anti‐VEGFs for mCNV.
Ng 2012 summarised natural history and clinical features of mCNV and available therapies at that time. They included one RCT (Gharbiya 2010) and other case series, investigating bevacizumab and ranibizumab. They proposed that "intravitreal anti‐VEGF be the first‐line treatment of mCNV in patients of all ages".
Wang 2013 comprehensively reviewed evidence of anti‐VEGFs on mCNV and included four RCTs (Gharbiya 2010; Iacono 2012; Parodi 2010; Ruiz‐Moreno 2011), and other non‐randomised trials. They concluded that "first‐line therapy for mCNV eyes should be intravitreal anti‐VEGF injection, which could improve VA by two lines on average with considerable safety", and "there had been no difference observed regarding multiple anti‐VEGF agents". And as a natural history of mCNV, "chorioretinal atrophy (CRA) formation instead of CNV activity was thought to be related with long‐term poor vision prognosis. Future studies with long‐term observation are required to elucidate the ultimate prognosis of mCNV".
Authors' conclusions
Implications for practice.
There is low to moderate quality of evidence from randomised controlled trials (RCT) for the efficacy and safety of anti‐vascular endothelial growth factor (anti‐VEGF) agents to treat myopic choroidal neovascularisation over photodynamic therapy treatments at one year. Evidence from RCTs on comparisons between anti‐VEGF and other treatments (laser or sham treatment) is judged as low (laser) or moderate (sham treatment).
Direct comparison from two trials found no differences in the efficacy between ranibizumab and bevacizumab with moderate quality of evidence. It is not yet possible to determine the difference among other anti‐VEGF agents in this clinical context, but we will re‐evaluate this if data become available.
The investigation of safety of anti‐VEGF intravitreal injection suggests that incidence of severe systemic and ocular adverse outcomes is probably uncommon in one to two years of follow‐up but the studies were underpowered to assess relative effects. However, clinical practice should be adherent to treatment (as much as possible) and follow‐up standards used in RCTs since undertreatment in the real‐world practice could limit benefits.
Implications for research.
Future research should be focused on the efficacy of different drugs and treatment regimens, the efficacy on different location of myopic choroidal neovascularisation, as well as the effects in the real world. The economic burden on patients and healthcare systems of monthly reassessment and repeated injections is tremendous, especially in low‐income countries. Thus, studies focusing on medical economics will be appreciated. Possible prognostic factors should be further elucidated to allow better evaluation of appropriate candidates for anti‐VEGF intravitreal injection.
Acknowledgements
We are grateful to the editorial team of Cochrane Eyes and Vision (CEV) for advice and comments on the review. Information Specialist Iris Gordon created and executed the search strategies for the electronic databases. We thank Anupa Shah, Managing Editor of CEV, Jennifer Evans and Gianni Virgili, CEV Editors for their support throughout the whole process. We thank Catey Bunce, Ana Quartilho and Simone Donati for their comments on the protocol, review or both.
Dr Sebastian Wolf and Dr Margarita Gekkieva (Novartis Pharma AG) provided additional data for RADIANCE 2010. Dr Jose M Ruiz‐Moreno and Dr Maria I Lopez‐Galves provided additional data for Ruiz‐Moreno 2013. Dr Tummy Li (Bayer Pharma AG) provided additional data for MYRROR 2010. We appreciate their support for our additional data collection.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Myopia, Degenerative] explode all trees #2 myop* #3 #1 or #2 #4 MeSH descriptor: [Choroidal Neovascularization] this term only #5 choroidal neovascularization #6 CNV #7 #4 or #5 or #6 #8 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #9 MeSH descriptor: [Endothelial Growth Factors] this term only #10 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #11 MeSH descriptor: [Antibodies, Monoclonal] this term only #12 (macugen or pegaptanib or lucentis or rhufab or ranibizumab or bevacizumab or avastin or aflibercept or conbercept) #13 anti near/2 VEGF #14 endothelial near/2 growth near/2 factor #15 VEGF TRAP #16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #4 and #7 and #16
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Myopia, Degenerative/ 14. myop$.tw. 15. or/13‐14 16. Choroidal Neovascularization/ 17. choroidal neovascularization.tw. 18. CNV.tw. 19. or/16‐18 20. exp angiogenesis inhibitors/ 21. endothelial growth factors/ 22. exp vascular endothelial growth factors/ 23. Antibodies, Monoclonal/ 24. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$).tw. 25. (anti adj2 VEGF$).tw. 26. (endothelial adj2 growth adj2 factor$).tw. 27. VEGF TRAP$.tw. 28. or/20‐27 29. 15 and 19 and 28 30. 12 and 29
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. degenerative myopia/ 34. myop$.tw. 35. or/33‐34 36. subretinal neovascularization/ 37. choroidal neovascularization.tw. 38. CNV.tw. 39. or/36‐38 40. angiogenesis/ 41. angiogenesis inhibitor/ 42. angiogenesis factor/ 43. endothelial cell growth factor/ 44. vasculotropin/ 45. monoclonal antibody/ 46. pegaptanib/ 47. ranibizumab/ 48. bevacizumab/ 49. aflibercept/ 50. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$).tw. 51. (anti adj2 VEGF$).tw. 52. (endothelial adj2 growth adj2 factor$).tw. 53. VEGF TRAP$.tw. 54. or/40‐53 55. 35 and 39 and 54
Appendix 4. Chinese Medical Database (CBM)
Chinese terms for (("anti‐vascular endothelial growth factor" or "ranibizumab" or "bevacizumab" or "VEGF Trap" or "aflibercept" or "pegaptanib") and "choroidal neovascularization" and "pathological myopia" and "controlled clinical trial")
Appendix 5. ISRCTN search strategy
myopia and (pathologic or degenerative or malignant)
Appendix 6. ClinicalTrials.gov search strategy
Myopia AND (Pathologic OR Degenerative OR Malignant)
Appendix 7. WHO ICTRP search strategy
pathologic myopia OR degenerative myopia OR malignant myopia
Appendix 8. Data on study characteristics
| Heading in table in Review Manager 5 | Proposed subheadings | |
| Methods | Study design | •Parallel group RCT, i.e. people randomised to treatment •Paired eye or intra‐individual RCT, i.e. eyes randomised to treatment •Cluster RCT, i.e. communities randomised to treatment •Cross‐over RCT •Other |
| Eyes | •1 eye included in study
•2 eyes included in study, both eyes received same treatment
•2 eyes included in study, eyes received different treatments (pair matched)
|
|
| Participants | Country | |
| Setting | ||
| Number of participants | ||
| Number of men | ||
| Number of women | ||
| Mean age | ||
| Age range | ||
| Ethnic group | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | Intervention Comparator |
|
| Outcomes | List | |
| Notes | Date conducted | Indicating specific dates of recruitment of participants month/year to month/year |
| Sources of funding | ||
| Declaration of interest | ||
| Other |
RCT: randomised controlled trial.
Appendix 9. Parameters assessed for risk of bias
Random sequence generation
Low risk of bias: the sequence was generated using a computer random number generator, referring to a random number table, tossing a coin, shuffling cards, drawing of lots or throwing dice.
High risk of bias: there were some non‐random elements in the process of generation (e.g. quasi‐randomised studies: using dates, case record numbers as part of the rule to allocate participants).
Unclear risk of bias: the trial was described as randomised, but the method of sequence generation was not specified.
Allocation concealment
Low risk of bias: allocation could not be foreseen before or during enrolment by the application of central allocation, opaque and sealed envelopes or identical drug containers.
High risk of bias: allocation could possibly be foreseen because there was no appropriate safeguard of the allocation (e.g. unsealed or non‐opaque envelopes) or the study was quasi‐randomised.
Unclear risk of bias: the study was described as randomised but the method used to conceal the allocation was not described or not described in sufficient detail.
Masking of participants and personnel
Low risk of bias: masking of participants and key study personnel ensured, and it was unlikely that the masking could have been broken (e.g. centralised assessment of adverse effects, centralised preparation of treatment, injections of placebo or active treatment administered by an unblinded operator not involved in any other study procedure).
High risk of bias: no masking, incomplete masking or the masking could have been broken and the outcome was likely to be influenced by lack of masking.
Unclear risk of bias: the study did not address masking or not described in sufficient detail.
Masking of outcome assessment
Low risk of bias: masking of outcome assessment ensured, and unlikely that the masking could have been broken (e.g. outcome assessor not involved in treatment or centralised assessment of clinical examinations).
High risk of bias: no masking, incomplete masking or the masking could have been broken and the outcome was likely to be influenced by lack of masking.
Unclear risk of bias: the study did not address masking or did not described in sufficient detail.
Incomplete outcome data
Low risk of bias: no missing data or missing data were balanced across groups; the extent of data missing was not enough to have a clinically relevant impact on the estimate; missing data were imputed using appropriate methods.
High risk of bias: numbers or reasons for missing data were unbalanced across groups; the extent of data missing was enough to induce a clinically relevant bias on the results; inappropriate application of imputation.
Unclear risk of bias: insufficient information to classify the study as 'low risk' or 'high risk'.
Selective reporting bias
Low risk of bias: the study protocol was available and all the outcomes were reported as prespecified; the study protocol was not available, but clinically relevant and reasonably expected outcomes were all reported.
High risk of bias: not all prespecified or reasonably expected outcomes were reported; one or more reported primary outcomes was not prespecified unless well justified (e.g. an unexpected adverse effect).
Unclear risk of bias: insufficient information to classify the study as 'low risk' or 'high risk'.
Other biases
Low risk of bias: the study seemed to be free of other biases (e.g. no commercial support).
High risk of bias: the study had some commercial support and an important risk of bias was potentially introduced; the study had a marked baseline imbalance; the study had a potential source of bias related to the specific study design used.
Unclear risk of bias: the study had some commercial support, and we are unsure whether an important risk of bias existed.
Data and analyses
Comparison 1. Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in visual acuity at 1 year | 3 | 263 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.20, ‐0.08] |
| 1.1 Bevacizumab | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.41, ‐0.15] |
| 1.2 Ranibizumab | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.17, ‐0.03] |
| 2 Gain 3+ lines of visual acuity at 1 year | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.27, 2.73] |
| 2.1 Bevacizumab | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.37, 9.65] |
| 2.2 Ranibizumab | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.04, 2.40] |
| 3 Choroidal neovascularisation angiographic closure at 1 year | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.99, 1.54] |
| 3.1 Bevacizumab | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.30, 6.20] |
| 3.2 Ranibizumab | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.85, 1.34] |
| 4 Central macular thickness at 1 year | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | ‐17.84 [‐41.98, 6.30] |
| 4.1 Bevacizumab | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐39.0 [‐86.58, 8.58] |
| 4.2 Ranibizumab | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐10.5 [‐38.51, 17.51] |
| 5 Change in visual acuity at 2 years | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.38, ‐0.14] |
| 6 Gain 3+ lines of visual acuity at 2 years | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.37, 8.56] |
| 7 Choroidal neovascularisation angiographic closure at 2 years | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.96, 1.13] |
| 8 Central macular thickness at 2 years | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐24.9 [‐72.48, 22.68] |
1.1. Analysis.
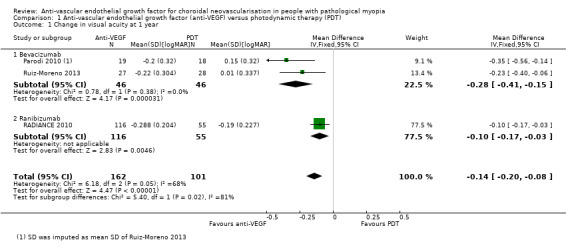
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 1 Change in visual acuity at 1 year.
1.2. Analysis.
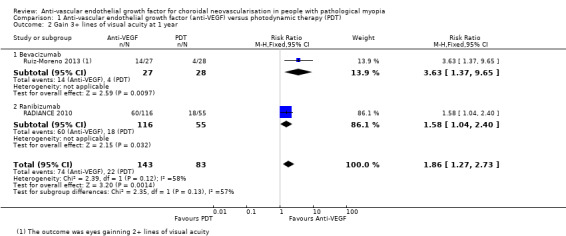
Comparison 1 Anti‐vascular endothelial growth factor (anti‐VEGF) versus photodynamic therapy (PDT), Outcome 2 Gain 3+ lines of visual acuity at 1 year.
Comparison 2. Anti‐vascular endothelial growth factor (anti‐VEGF) versus laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in visual acuity at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Choroidal neovascularisation angiographic closure at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Change in visual acuity at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Gain 3+ lines of visual acuity at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Choroidal neovascularisation angiographic closure at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Anti‐vascular endothelial growth factor (anti‐VEGF) versus sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in visual acuity at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Gain 3+ lines of visual acuity at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Central macular thickness at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of life at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in visual acuity at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Gain 3+ lines of visual acuity at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Choroidal neovascularisation angiographic closure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Central macular thickness at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Adverse events: anti‐vascular endothelial growth factor (anti‐VEGF) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic serious adverse events | 4 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.50 [0.60, 33.99] |
| 2 Ocular serious adverse events | 4 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.23, 14.71] |
4.1. Analysis.
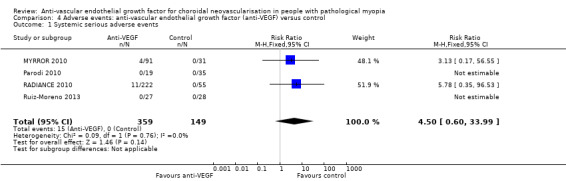
Comparison 4 Adverse events: anti‐vascular endothelial growth factor (anti‐VEGF) versus control, Outcome 1 Systemic serious adverse events.
4.2. Analysis.
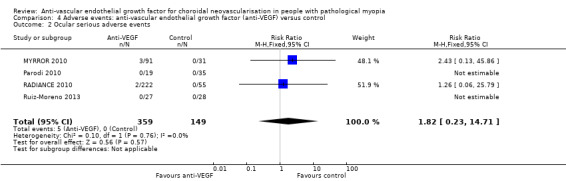
Comparison 4 Adverse events: anti‐vascular endothelial growth factor (anti‐VEGF) versus control, Outcome 2 Ocular serious adverse events.
Comparison 5. Ranibizumab versus bevacizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in visual acuity at 1 year | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.06] |
| 2 Gain 3+ lines of visual acuity at 1 year | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.27] |
| 3 Choroidal neovascularisation angiographic closure at 1 year | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gharbiya 2010.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (1 study eye per participant). If both eyes were eligible, the eye with worse VA was the study eye unless the other eye was deemed more suitable for medical reasons. |
|
| Participants |
Country: Italy Setting: Department of Ophthalmology, University of Rome, Rome, Italy Number of participants: 32 Number of men: 10 Number of women: 22 Age (mean ± SD): 60.63 ± 10.48 years in the ranibizumab group and 59.06 ± 11.42 years in the bevacizumab group Ethnic group: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: intravitreal ranibizumab 0.50 mg, retreatment afterwards based on FA or OCT changes every month (16 participants) Comparator: intravitreal bevacizumab 1.25 mg, retreatment afterwards based on FA or OCT changes every month (16 participants) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Follow‐up: 6 months VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: February 2008 to December 2008 Sources of funding: not reported Declaration of interest: Dr Fantozzi received a fellowship from Novartis Farma S.p.A ‐ Origgio, Varese, Italy, from 1 January 2008 to 31 December 2008. No other financial involvement with companies that directly compete with products in this manuscript had to be disclosed. Trial registration: ISRCTN49803272 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Best‐corrected visual acuity (BCVA) was measured according to a standardized refraction protocol, using the ETDRS chart at 4 meters distance by a single well‐trained and experienced orthoptist, who was masked to the study." "The leakage from the CNV was evaluated on fluorescein angiography (ImageNet; Topcon, Tokyo, Japan), performed by a trained photographer masked to the study, in the late phase (6‐8 min) compared with the early phase (first 1‐2 min)." "All FA and OCT evaluations were interpreted by 2 retinal specialists (M.G. and G.R.) in an unmasked fashion. If there were questions regarding interpretation of the study data, other retinal specialists (F.A. and N.F.) were approached in consultation." Comment: BCVA and leakage from the CNV on FA was evaluated using a masked method. Though FA and OCT evaluations were interpreted using an unmasked method, 2 independent specialists were involved and questions regarding interpretation were solved by future consultation with other retinal specialists. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The outcome measures were prespecified on ISRCTN registration. |
| Other bias | Low risk | No other bias identified. |
Iacono 2012.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (1 study eye per participant). It was not reported how the eye was chosen. |
|
| Participants |
Country: Italy Setting: Department of Ophthalmology of the Vita‐Salute University of Milan, Italy Number of participants: 55 Number of men: 13 Number of women: 42 Age (mean ± SD): 65 ± 12 years in the ranibizumab group and 61 ± 11 years in the bevacizumab group. Ethnic group: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: intravitreal ranibizumab 0.50 mg, retreatment afterwards based on FA or OCT changes every month (27 participants) Comparator: intravitreal bevacizumab 1.25 mg, retreatment afterwards based on FA or OCT changes every month (28 participants) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Follow‐up: 18 months VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: participants enrolled from April 2006 to July 2007, and followed for 18 months. Sources of funding: not reported Declaration of interest: F Bandello is an advisory board member for Novartis Pharma. The other authors had no proprietary or financial interest in any of the products mentioned in the study. Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patient randomisation to either IVR [intravitreal ranibizumab] or IVB [intravitreal bevacizumab] was performed by means of sequentially numbered envelopes according to a computer‐generated code list. A permuted block randomisation was performed with a final allocation ratio of 1:1." |
| Allocation concealment (selection bias) | Low risk | Quote: "by means of sequentially numbered envelopes according to a computer‐generated code list and stored by an investigator, unaware of the purpose of the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "by an experienced retinologist masked to the type of injection." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Best‐corrected visual acuity measurements were made at each visit by an expert examiner, unaware of the purpose of the study, whereas FA and OCT were interpreted separately by two experienced ophthalmologists masked to each other." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "after randomisation and before the administration of the first injection, four patients in the IVR group and three patients in the IVB group refused to participate in the study because they were unable to follow the strict study protocol." Comment: the reason for withdrawal was reported and balanced across arms. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol could not be found but outcome measures were specified in the methods section. |
| Other bias | Low risk | Comment: no other bias identified. |
MYRROR 2010.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (1 study eye per participant). The criteria for eye selection were not reported. |
|
| Participants |
Country: Asia (Hong Kong, Japan, Republic of Korea, Singapore and Taiwan) Setting: international, multicentre (see appendix of MYRROR 2010) Number of participants: 122 (only 121 participants were included in the full analysis set) Number of men: 29 Number of women: 92 Age (mean ± SD): 58.2 ± 13.3 years Ethnic group: Asian Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: intravitreal aflibercept 2.0 mg, retreatment afterwards in case CNV persisted or recurred (based on predefined criteria) at monthly follow‐up through week 44. If the assessment for retreatment was negative, participants were given sham injections for masking purpose (90 participants). Comparator: sham injections every month through to week 20. Intravitreal aflibercept 2.0 mg at week 24 and retreatment afterwards in case CNV persisted or recurred at monthly follow‐up from week 28 to week 44 (same treatment regimen as the intervention group) (31 participants). |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Follow‐up: 48 weeks VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: November 2010 to August 2013 Sources of funding: Bayer HealthCare Pharmaceuticals, Leverkusen, Germany Declaration of interest: "The authors take full responsibility for the scope, direction, and content of the manuscript, and have approved the submitted manuscript." Financial interests were not reported. Trial registration: NCT01249664 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "MYRROR was an international, phase III, multicenter, randomised, double‐masked, sham‐controlled study." "patients received sham injections only for masking purposes." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "MYRROR was an international, phase III, multicenter, randomised, double‐masked, sham‐controlled study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In total, 122 patients were randomised, of whom 91 received intravitreal aflibercept 2.0 mg and 31 received sham; 122 patients were included in the safety set. In the full analysis set, 121 patients were included (90 patients received intravitreal aflibercept 2.0 mg and 31 received sham)." Comment: according to participant flow data on ClinicalTrials.gov, 5 participants were withdrawn from the study and 1 participant did not complete visits to week 48 due to adverse events, both in the aflibercept group. However, only 1 participant failed to fulfil requirements of full analysis set after randomisation. |
| Selective reporting (reporting bias) | Low risk | Comment: the outcome measures were prespecified on ClinicalTrials.gov registration. Though some outcomes (e.g. the proportion of participants losing ≥ 5, 10 or 15 letters) were not reported in the manuscript, data were shown in the "study results" section on ClinicalTrials.gov website. |
| Other bias | Low risk | Comment: no other bias identified. |
Parodi 2010.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (one study eye per participant). The criteria for eye selection were not reported. |
|
| Participants |
Country: Italy Setting: Department of Ophthalmology at the University of Udine and University of Trieste Number of participants: 54 Number of men: 17 Number of women: 37 Age (mean): 50.8 years in the bevacizumab group, 48.1 years in the PDT group and 44.5 years in the laser treatment group Ethnic group: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: intravitreal bevacizumab 1.25 mg, retreatment afterwards based on OCT or FA changes (19 participants) Comparator 1: PDT with verteporfin (following the Verteporfin in Photodynamic Therapy Study Group guidelines) (18 participants) Comparator 2: krypton laser photocoagulation, eyes developed recurrent CNV with subfoveal location during follow‐up could be retreated using PDT (17 participants) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Follow‐up: 24 months VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: participants enrolled from January 2006 to July 2006, and followed for 2 years Sources of funding: not reported Declaration of interest: not reported Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was randomly allocated to 1 of the 3 treatment groups through a computer‐generated number." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "At each scheduled examination, a complete ophthalmological assessment was carried out by an investigator who had had no previous contact with the subject and was unaware of the treatment previously administered." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol could not be found but outcome measures were specified in the methods section. |
| Other bias | Low risk | Comment: no other bias identified. |
RADIANCE 2010.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (1 study eye per participant). If both eyes were eligible, the eye with worse VA (assessed at visit 1) was selected for the study treatment. However, if medical reasons and local ethical requirements dictated, the investigator could select the eye with the better VA as the study eye. If needed, the fellow eye was treated as per the investigator's discretion. |
|
| Participants |
Country: Australia, Canada, France, Germany, Hong Kong, Hungary, India, Italy, Japan, Korea, Lithuania, Poland, Portugal, Singapore, Slovak Republic, Spain, Switzerland, Turkey, UK (76 study centres) Setting: international, multicentre (see appendix of RADIANCE 2010) Number of participants: 277 Number of men: 68 Number of women: 209 Age (mean ± SD): 54.0 ± 14.0 years in Group 1, 56.1 ± 14.4 years in Group 2 and 57.4 ± 12.8 years in Group 3 Ethnic group: white (56.6% in Group 1, 60.3% in Group 2 and 58.2% in Group 3), Asian (42.5% in Group 1, 39.7% in Group 2 and 41.8% in Group 3) and Other (0.9% in Group 1). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (Group 1): ranibizumab treatment guided by VA stabilisation criteria (intravitreal ranibizumab 0.50 mg on day 1 and month 1, with further treatment determined by the VA stabilisation criterion, defined as no change in BCVA as compared with 2 preceding monthly visits) (106 participants). Comparator 1 (Group 2): ranibizumab treatment guided by disease activity criteria (intravitreal ranibizumab 0.50 mg on day 1, with further treatment based on disease activity determined by OCT and FA) (116 participants). Comparator 2 (Group 3): verteporfin PDT, with ranibizumab allowed as of month 3 (verteporfin PDT on day 1, with further treatment determined by treating investigator using ranibizumab 0.50 mg guided by disease activity criteria, verteporfin PDT, or both through month 3 to 11) (55 participants). |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Follow‐up: 12 months VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: October 2010 to August 2012 Sources of funding: Novartis Pharma AG, Basel, Switzerland Declaration of interest: SW is on advisory boards of and has served as a consultant and a speaker for Allergan, Bayer, Heidelberg Engineering, Molecular Partners, Novartis, Roche and Optos. VJB reports grants and personal fees from Novartis during the conduct of the study and personal fees and non‐financial support from Bayer, Novartis, Allergan outside the submitted work. GL, UM, KO‐M and TS have nothing to disclose. TYW reports grants, personal fees, travel support and writing/reviewing fees from Novartis and Bayer and has served as a consultant for Abbott, Allergan, Bayer, Genentech, Novartis, Roche and Pfizer. RS reports grants from Novartis during the conduct of the study and has received grants from Bayer, Allergan, Thea and Alimera outside of the submitted work. SP and MG are employees of Novartis Pharma AG, Basel, Switzerland. Novartis Pharma AG, Switzerland, sponsored the study and was involved in the study conception and design, protocol writing, study drug provision, study co‐ordination, data collection, data analysis and data interpretation. Trial registration: NCT01217944 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list was produced by Novartis Drug Supply Management using a validated system that automates the random assignment of treatment groups to randomisation numbers in the specified ratio. At enrolment, patients received the lowest available randomisation number that then assigned them in a 2:2:1 ratio to 1 of the 3 treatment groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation numbers were generated using the following procedure to ensure that treatment assignment was unbiased and concealed from patients and investigator staff. A randomisation list was produced by or under the responsibility of Novartis DSM using a validated system that automates the random assignment of treatment arms to randomisation numbers in the specified ratio. The randomisation scheme for patients was reviewed and approved by a member of the Biostatistics Quality Assurance Group." (Email communication with Novartis Pharma AG, dated 10 June 2015) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "Due to the different appearances and routes of administration between the 2 treatments, all patients received either sham injection or PDT sham in conjunction with the study treatment. The PDT sham consisted of intravenous injection of 5% dextrose solution followed by light application of PDT." "The treating investigator was unmasked and administered the randomised study medication per the protocol; however, they were not involved in any other aspects of the study and could not communicate details of the treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "To ensure masking, 2 investigators were involved at each study center. All study assessments were made by the evaluating investigator, VA assessor, or other site personnel who were masked to the treatment assignment." "The images (OCT) were reviewed by a central reading center to ensure standardized evaluation." "The fundus photography and FA images were independently reviewed by the central reading center (CRC) to ensure standardized evaluation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10 participants (5.7% in Group 1 and 3.4% in Group 2) discontinued from the study and the reason was recorded (see Figure 2). |
| Selective reporting (reporting bias) | Low risk | Comment: the outcome measures were prespecified on ClinicalTrials.gov registration. |
| Other bias | Low risk | Comment: no other bias identified. |
Ruiz‐Moreno 2013.
| Methods |
Study design: parallel group randomised controlled trial. Unit of randomisation and analysis: the participant (1 study eye per participant). The criteria for eye selection was not reported. |
|
| Participants |
Country: Spain Setting: multicentre (Department of Ophthalmology, Castila La Mancha University, Albacete, Spain; IOBA, University of Valladolid, Valladolid, Spain; Pio del Rio Hortega University Hospital, Valladolid, Spain) Number of participants: 55 Number of men: not reported Number of women: not reported Age: not reported (similar in both groups) Ethnic group: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: PDT with Visudyne was performed at baseline and every 3 months if CNV activity was detected (28 participants). Comparator: 3 monthly intravitreal bevacizumab 1.25 mg at baseline, retreatment afterwards in cases with suspected CNV activity (27 participants). |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Follow‐up: 2 years VA measurement: ETDRS chart at a distance of 4 m. |
|
| Notes |
Date conducted: April 2008 to June 2011 Sources of funding: Instituto Universitario de Oftalmobiología Aplicada (IOBA) Declaration of interest: not reported Trial registration: NCT00967850 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was done by the promotor and was provided by the IOBA." (Email communication with Dr Lopez‐Galvez, dated 4 August 2015) |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was done by the promotor and was provided by the IOBA." (Email communication with Dr Lopez‐Galvez, dated 4 August 2015) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was doubled masked: (the follow‐up physician and the optometrist) and the patient were masked." (Email communication with Dr Lopez‐Galvez, dated 4 August 2015) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was doubled masked: (the follow‐up physician and the optometrist) and the patient were masked." (Email communication with Dr Lopez‐Galvez, dated 4 August 2015) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty‐four eyes in group 1 (86%) and 25 eyes in group 2 (92.6%) completed 1 year of follow‐up and 20 eyes in group 1 (71.4%) and 22 eyes in group 2 (78.6%) completed 2 years of follow‐up." Comment: loss to follow‐up was > 20% at 2 years and no reason was reported, though the numbers are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: the outcome measures were prespecified on ClinicalTrials.gov registration. |
| Other bias | Low risk | Comment: no other bias identified. |
BCVA: best‐corrected visual acuity; CNV: choroidal neovascularisation; D: dioptre; ETDRS: Early Treatment Diabetic Retinopathy Study; FA: fluorescein angiography; IU: international unit; mCNV: myopic choroidal neovascularisation; min: minute; OCT: optical coherence tomography; PDT: photodynamic therapy; SD: standard deviation; VA: visual acuity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Heier 2011 | Participants included people with CNV due to causes other than pathological myopia and there was no subgroup analysis for pathological myopia. |
| REPAIR 2010 | No comparator was included. All participants received the same intervention. |
| Saviana 2014 | The study included both naive and participants previously treated with anti‐VEGF drugs or laser and PDT treatment in the extrafoveal area. 11/17 participants in Group A and 5/17 participants in Group B received previous treatments. |
CNV: choroidal neovascularisation; PDT: photodynamic therapy; anti‐VEGF: anti‐vascular endothelial growth factor.
Characteristics of ongoing studies [ordered by study ID]
NCT01716026 (BENEMCOR).
| Trial name or title | Initial Treatment With Bevacizumab in Choroidal Neovascularization Associated to High Myopia (BENEMCOR) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double masked (participant, investigator) Primary purpose: treatment |
| Participants | 110 participants Country: Spain |
| Interventions | Experimental: single‐dose load phase Single‐dose treatment with bevacizumab, followed by 2 sham injections if conditions are met in month 2 and month 3, and followed with bevacizumab monthly as needed as per protocol. Active comparator: 3 month load with 9 month as needed. 3 monthly bevacizumab injections with 9 monthly doses as needed if participant meets the treatment criteria for each monthly visit. |
| Outcomes | Primary outcome measures:
|
| Starting date | October 2012 |
| Contact information | Jose Maria Ruiz‐Moreno, MD, PhD jm.ruiz@umh.es |
| Notes |
NCT01809223 (SHINY).
| Trial name or title | A Randomized, Double‐blind, Multicenter, Sham‐controlled, Safety and Efficacy Study of Conbercept in Patients With mCNV (SHINY) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double masked (participant, investigator) Primary purpose: treatment |
| Participants | 176 participants Country: China |
| Interventions | Experimental: conbercept treatment group Participants will receive conbercept injections 0.5 mg/eye, once a month for first 3 months. In the next 6 months, the investigator will decide whether repeat injections are needed base on the monthly assessment results. Sham comparator: sham injection group Participants will receive sham injections monthly for 3 months and will receive conbercept 0.5 mg/eye at month 4. The investigator will decide whether repeat injections are needed base on the monthly assessment results from month 5 to month 9. |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | August 2012 |
| Contact information | Chengdu Kanghong Biotech Co, Ltd. |
| Notes |
NCT01922102 (Brilliance).
| Trial name or title | Efficacy and Safety of Ranibizumab 0.5 vs Veteporfin PDT in Patients With Visual Impairment Due to Choroidal Neovascularization Secondary to Pathologic Myopia (Brilliance) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double masked (participant, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | 475 participants Countries: China, Hong Kong, India, Korea, Philippines and Thailand |
| Interventions | Experimental: Group 1: ranibizumab 0.5 mg driven by visual acuity stability criteria Experimental: Group 2: ranibizumab 0.5 mg driven by disease activity criteria Active comparator: Group 3: verteporfin PDT |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | September 2013 |
| Contact information | Novartis Pharmaceuticals +4163241111 |
| Notes |
BCVA: best‐corrected visual acuity; CNV: choroidal neovascularisation; ETDRS: Early Treatment Diabetic Retinopathy Study; OCT: optical coherence tomography; PDT: photodynamic therapy.
Differences between protocol and review
We made the following amendments to the protocol.
We added "proportion of participants with a gain of 3+ lines in BCVA at 12 months after treatment" as a primary outcome measure. It is a more straightforward index indicating visual prognosis than mean change in BCVA in logMAR scale, especially for non‐professionals.
Because it is interchangeable for statistics, the protocol prespecified logMAR scale as an estimate of primary outcome of mean change in BCVA. However, for clinical practice, we added "equivalent of ETDRS letters" to report the mean effect as an approximate, along with logMAR scale.
We added in time point of six months, which was not specified in the protocol, since it would be unethical to allow participants in the sham treatment group to have anti‐VEGF therapy after certain period of time, for instance after 24 weeks. It is not accurate to analyse the effect on intervention at one year after participants in the sham treatment group have been treated for six months. Thus, we analysed the data of six months to display the difference between anti‐VEGF and sham treatment better.
We decided to calculate 95% CIs for pooled estimates instead of calculating 99% CIs planned in the protocol. It is not easy to calculate different CIs for individual study results and pooled estimates and is unnecessary.
Contributions of authors
Conceiving the review: YZ, TZ.
Designing the review: YZ, TZ.
Co‐ordinating the review: GX, LP.
Data collection for the review
Designing search strategies: Cochrane Eyes and Vision Group editorial base.
Undertaking manual searches: YZ, TZ.
Screening search results: YZ, TZ.
Organising retrieval of papers: YZ.
Screening retrieved papers against inclusion criteria: YZ, TZ, GX.
Appraising quality of papers: YZ, TZ, GX.
Extracting data from papers: YZ, TZ.
Writing to authors of papers for additional information: YZ, TZ.
Providing additional data about papers: YZ.
Obtaining and screening data on unpublished studies: YZ, TZ.
Data management for the review
Entering data into Review Manager 5: YZ, TZ.
Analysis of data: YZ, TZ, GX, LP.
Interpretation of data
Providing a methodological perspective: YZ, TZ, LP.
Providing a clinical perspective: YZ, TZ, GX.
Providing a policy perspective: YZ, TZ, GX.
Writing the review: YZ, TZ. Providing general advice on the review: GX, LP. Securing funding for the review: GX. Guarantor for the review: GX.
Sources of support
Internal sources
Eye and Ear Nose Throat Hospital, Shanghai Medical School, Fudan University, China.
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
YZ: none. TZ: none. GX: none. LP: none.
New
References
References to studies included in this review
Gharbiya 2010 {published data only (unpublished sought but not used)}
- Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab ‐ a randomized controlled trial. American Journal of Ophthalmology 2010;149(3):458‐64. [DOI] [PubMed] [Google Scholar]
Iacono 2012 {published data only (unpublished sought but not used)}
- Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Cascavilla ML, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina 2012;32(8):1539‐46. [DOI] [PubMed] [Google Scholar]
MYRROR 2010 {published and unpublished data}
- Ikuno Y, Ohno‐Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR Study. Ophthalmology 2015;122(6):1220‐7. [DOI] [PubMed] [Google Scholar]
Parodi 2010 {published data only (unpublished sought but not used)}
- Parodi MB, Iacono P, Papayannis A, Sheth S, Bandello F. Laser photocoagulation, photodynamic therapy, and intravitreal bevacizumab for the treatment of juxtafoveal choroidal neovascularization secondary to pathologic myopia. Archives of Ophthalmology 2010;128(4):437‐42. [DOI] [PubMed] [Google Scholar]
RADIANCE 2010 {published and unpublished data}
- Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno‐Matsui K, Sharma T, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121(3):682‐92. [DOI] [PubMed] [Google Scholar]
Ruiz‐Moreno 2013 {published and unpublished data}
- Ruiz‐Moreno JM, Lopez‐Galvez MI, Donate J, Gomez‐Ulla F, Garcia‐Arumi J, Garcia‐Layana A, et al. Myopic choroidal neovascularization. Ophthalmology 2011;118(12):2521‐3. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Moreno JM, Lopez‐Galvez MI, Montero Moreno JA, Pastor Jimeno JC. Intravitreal bevacizumab in myopic neovascular membranes: 24‐month results. Ophthalmology 2013;120(7):1510‐1. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Heier 2011 {published data only}
- Heier JS, Brown D, Ciulla T, Abraham P, Bankert JM, Chong S, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age‐related macular degeneration: a phase I clinical trial. Ophthalmology 2011;118(1):111‐8. [DOI] [PubMed] [Google Scholar]
REPAIR 2010 {published data only}
- Tufail A, Narendran N, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, et al. Ranibizumab in myopic choroidal neovascularization: the 12‐month results from the REPAIR study. Ophthalmology 2013;120(9):1944‐5. [DOI] [PubMed] [Google Scholar]
Saviana 2014 {published data only}
- Saviano S, Piermarocchi R, Leon PE, Mangogna A, Zanei A, Cavarzeran Sc F, et al. Combined therapy with bevacizumab and photodynamic therapy for myopic choroidal neovascularization: a one‐year follow‐up controlled study. International Journal of Ophthalmology 2014;7(2):335‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01716026 (BENEMCOR) {published data only}
- NCT01716026. Establishment of the initial protocol with intravitreal bevacizumab for the treatment of choroidal neovascularization associated with high myopia:3 vs 1. clinicaltrials.gov/ct2/show/NCT01716026 Date first received: 25 October 2012.
NCT01809223 (SHINY) {published data only}
- NCT01809223. The safety and efficacy of conbercept in the treatment of choroidal neovascularization (CNV) secondary to high myopia. clinicaltrials.gov/ct2/show/NCT01809223 Date first received: 11 March 2013.
NCT01922102 (Brilliance) {published data only}
- NCT01922102. A 12‐month, phase III, randomized, double‐masked, multicenter, active‐controlled study to evaluate the efficacy and safety of two individualized regimens of 0.5mg ranibizumab vs. verteporfin PDT in patients with visual impairment due to choroidal neovascularization secondary to pathologic myopia. clinicaltrials.gov/ct2/show/NCT01922102 Date first received: 12 August 2013.
Additional references
Adamis 1994
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. American Journal of Ophthalmology 1994;118(4):445‐50. [DOI] [PubMed] [Google Scholar]
Aiello 1994
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine 1994;331(22):1480‐7. [DOI] [PubMed] [Google Scholar]
Baba 2010
- Baba T, Kubota‐Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S. Two‐year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. British Journal of Ophthalmology 2010;94(7):864‐70. [DOI] [PubMed] [Google Scholar]
Battaglia 2010
- Battaglia Parodi M, Iacono P, Bandello F. Antivascular endothelial growth factor for choroidal neovascularization in pathologic myopia. Developments in Ophthalmology 2010;46:73‐83. [DOI] [PubMed] [Google Scholar]
Bennett 2007
- Bennett MD, Yee W. Pegaptanib for myopic choroidal neovascularization in a young patient. Graefe's Archive for Clinical and Experimental Ophthalmology 2007;245(6):903‐5. [DOI] [PubMed] [Google Scholar]
Blinder 2003
- Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2‐year results of a randomized clinical trial ‐ VIP report No. 3. Ophthalmology 2003;110(4):667‐73. [DOI] [PubMed] [Google Scholar]
Boyd 2002
- Boyd SR, Zachary I, Chakravarthy U, Allen GJ, Wisdom GB, Cree IA, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Archives of Ophthalmology 2002;120(12):1644‐50. [DOI] [PubMed] [Google Scholar]
Brancato 1990
- Brancato R, Pece A, Avanza P, Radrizzani E. Photocoagulation scar expansion after laser therapy for choroidal neovascularization in degenerative myopia. Retina 1990;10(4):239‐43. [DOI] [PubMed] [Google Scholar]
Brown 2011
- Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap‐eye in wet age‐related macular degeneration. Ophthalmology 2011;118(6):1089‐97. [DOI] [PubMed] [Google Scholar]
CATT 2011
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2011;364(20):1897‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
CATT 2012
- Comparison of Age‐related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: two‐year results. Ophthalmology 2012;119(7):1388‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2009
- Cohen SY. Anti‐VEGF drugs as the 2009 first‐line therapy for choroidal neovascularization in pathologic myopia. Retina 2009;29(8):1062‐6. [DOI] [PubMed] [Google Scholar]
Costa 2006
- Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LA Jr, Scott IU. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose‐escalation study. Investigative Ophthalmology and Visual Science 2006;47(10):4569‐78. [DOI] [PubMed] [Google Scholar]
Giansanti 2012
- Giansanti F, Virgili G, Donati MC, Giuntoli M, Pieretti G, Abbruzzese G, et al. Long‐term results of photodynamic therapy for subfoveal choroidal neovascularisation with pathologic myopia. Retina 2012;32(8):1547‐52. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gragoudas 2004
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age‐related macular degeneration. New England Journal of Medicine 2004;351(27):2805‐16. [DOI] [PubMed] [Google Scholar]
Gupta 2010
- Gupta B, Elagouz M, Sivaprasad S. Intravitreal bevacizumab for choroidal neovascularisation secondary to causes other than age‐related macular degeneration. Eye 2010;24(2):203‐13. [DOI] [PubMed] [Google Scholar]
Hamelin 2002
- Hamelin N, Glacet‐Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G. Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. American Journal of Ophthalmology 2002;133(4):530‐6. [DOI] [PubMed] [Google Scholar]
Harris 2004
- Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncology 2004;5(5):292‐302. [DOI] [PubMed] [Google Scholar]
Hawkins 2004
- Hawkins BS, Miskala PH, Bass EB, Bressler NM, Childs AL, Mangione CM, et al. Surgical removal vs observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic: II. Quality‐of‐life findings from a randomized clinical trial: SST Group H Trial: SST Report No. 10. Archives of Ophthalmology 2004;122(11):1616‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayashi 2010
- Hayashi K, Ohno‐Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, et al. Long‐term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010;117(8):1595‐611. [DOI] [PubMed] [Google Scholar]
Hayashi 2012
- Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno‐Matsui K. Two‐year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina 2012;32(4):687‐95. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hsiang 2008
- Hsiang HW, Ohno‐Matsui K, Shimada N, Hayashi K, Moriyama M, Yoshida T, et al. Clinical characteristics of posterior staphyloma in eyes with pathologic myopia. American Journal of Ophthalmology 2008;146(1):102‐10. [DOI] [PubMed] [Google Scholar]
Ikuno 2008
- Ikuno Y, Sayanagi K, Soga K, Sawa M, Gomi F, Tsujikawa M, et al. Lacquer crack formation and choroidal neovascularization in pathologic myopia. Retina 2008;28(8):1124‐31. [DOI] [PubMed] [Google Scholar]
Ikuno 2010
- Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, et al. Two‐year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. American Journal of Ophthalmology 2010;149(1):140‐6. [DOI] [PubMed] [Google Scholar]
IVAN 2012
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age‐related macular degeneration: one‐year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399‐411. [DOI] [PubMed] [Google Scholar]
Iwase 2006
- Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 2006;113(8):1354‐62. [DOI] [PubMed] [Google Scholar]
Johnson 1998
- Johnson DA, Yannuzzi LA, Shakin JL, Lightman DA. Lacquer cracks following laser treatment of choroidal neovascularization in pathologic myopia. Retina 1998;18(2):118‐24. [DOI] [PubMed] [Google Scholar]
Kamei 2004
- Kamei M, Tano Y, Yasuhara T, Ohji M, Lewis H. Macular translocation with chorioscleral outfolding: 2‐year results. American Journal of Ophthalmology 2004;138(4):574‐81. [DOI] [PubMed] [Google Scholar]
Kvanta 1996
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age‐related macular degeneration express vascular endothelial growth factor. Investigative Ophthalmology and Visual Science 1996;37(9):1929‐34. [PubMed] [Google Scholar]
Lynch 2007
- Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Annals of Pharmacotherapy 2007;41(4):614‐25. [DOI] [PubMed] [Google Scholar]
Michels 2005
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 2005;112(6):1035‐47. [DOI] [PubMed] [Google Scholar]
Moja 2014
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2012
- Ng DS, Kwok AK, Chan CW. Anti‐vascular endothelial growth factor for myopic choroidal neovascularization. Clinical and Experimental Ophthalmology 2012;40(1):e98‐e110. [DOI] [PubMed] [Google Scholar]
Nguyen 2005
- Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA. Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. British Journal of Ophthalmology 2005;89(10):1368‐70. [PMC free article] [PubMed] [Google Scholar]
Ohno‐Matsui 2003
- Ohno‐Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. British Journal of Ophthalmology 2003;87(5):570‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenfeld 2006
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1419‐31. [DOI] [PubMed] [Google Scholar]
Ruiz‐Moreno 2001
- Ruiz‐Moreno JM, Vega C. Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. British Journal of Ophthalmology 2001;85(9):1041‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz‐Moreno 2010
- Ruiz‐Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2‐year outcome. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(7):937‐41. [DOI] [PubMed] [Google Scholar]
Ruiz‐Moreno 2011
- Ruiz‐Moreno JM, Lopez‐Galvez MI, Donate J, Gomez‐Ulla F, Garcia‐Arumi J, Garcia‐Layana A, et al. Myopic choroidal neovascularization. Ophthalmology 2011;118(12):2521‐3. [DOI] [PubMed] [Google Scholar]
Solomon 2014
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti‐vascular endothelial growth factor for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD005139.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperduto 1983
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Archives of Ophthalmology 1983;101(3):405‐7. [DOI] [PubMed] [Google Scholar]
Sun 2008
- Sun XD, Xu WQ, Rosenfeld PJ. Therapeutic efficacy and safety of off‐label use of bevacizumab for the treatment of neovascular diseases of the eye. Chinese Journal of Ophthalmology 2008;44(3):281‐4. [PubMed] [Google Scholar]
Takeuchi 2012
- Takeuchi K, Kachi S, Iwata E, Ishikawa K, Terasaki H. Visual function 5 years or more after macular translocation surgery for myopic choroidal neovascularisation and age‐related macular degeneration. Eye 2012;26(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uemura 2000
- Uemura A, Thomas MA. Subretinal surgery for choroidal neovascularization in patients with high myopia. Archives of Ophthalmology 2000;118(3):344‐50. [DOI] [PubMed] [Google Scholar]
VIP Study Group 2001
- Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1‐year results of a randomized clinical trial ‐ VIP report No. 1. Ophthalmology 2001;108(5):841‐52. [DOI] [PubMed] [Google Scholar]
Virgili 2014
- Virgili G, Parravano M, Menchini F, Evans JR. Anti‐vascular endothelial growth factor for diabetic macular oedema. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD007419.pub4] [DOI] [PubMed] [Google Scholar]
Voykov 2010
- Voykov B, Gelisken F, Inhoffen W, Voelker M, Bartz‐Schmidt KU, Ziemssen F. Bevacizumab for choroidal neovascularization secondary to pathologic myopia: is there a decline of the treatment efficacy after 2 years?. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(4):543‐50. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang E, Chen Y. Intravitreal anti‐vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta‐analysis. Retina 2013;33(7):1375‐92. [DOI] [PubMed] [Google Scholar]
Wong 2000
- Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Investigative Ophthalmology and Visual Science 2000;41(9):2486‐94. [PubMed] [Google Scholar]
Xu 2006
- Xu L, Wang Y, Li Y, Wang Y, Cui T, Li J, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 2006;113(7):1134.e1‐11. [DOI] [PubMed] [Google Scholar]
Yamamoto 2007
- Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. British Journal of Ophthalmology 2007;91(2):157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshida 2003
- Yoshida T, Ohno‐Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, et al. Myopic choroidal neovascularization: a 10‐year follow‐up. Ophthalmology 2003;110(7):1297‐305. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zhu 2014
- Zhu Y, Zhang T, Xu G, Peng L. Anti‐vascular endothelial growth factor for choroidal neovascularisation in people with pathologic myopia. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011160] [DOI] [PMC free article] [PubMed] [Google Scholar]


