Abstract
Background
Surgical wounds (incisions) heal by primary intention when the wound edges are brought together and secured, often with sutures, staples, or clips. Wound dressings applied after wound closure may provide physical support, protection and absorb exudate. There are many different types of wound dressings available and wounds can also be left uncovered (exposed). Surgical site infection (SSI) is a common complication of wounds and this may be associated with using (or not using) dressings, or different types of dressing.
Objectives
To assess the effects of wound dressings compared with no wound dressings, and the effects of alternative wound dressings, in preventing SSIs in surgical wounds healing by primary intention.
Search methods
We searched the following databases: the Cochrane Wounds Specialised Register (searched 19 September 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2016, Issue 8); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and Epub Ahead of Print; 1946 to 19 September 2016); Ovid Embase (1974 to 19 September 2016); EBSCO CINAHL Plus (1937 to 19 September 2016).
There were no restrictions based on language, date of publication or study setting.
Selection criteria
Randomised controlled trials (RCTs) comparing wound dressings with wound exposure (no dressing) or alternative wound dressings for the postoperative management of surgical wounds healing by primary intention.
Data collection and analysis
Two review authors performed study selection, 'Risk of bias' assessment and data extraction independently.
Main results
We included 29 trials (5718 participants). All studies except one were at an unclear or high risk of bias. Studies were small, reported low numbers of SSI events and were often not clearly reported. There were 16 trials that included people with wounds resulting from surgical procedures with a 'clean' classification, five trials that included people undergoing what was considered 'clean/contaminated' surgery, with the remaining studies including people undergoing a variety of surgical procedures with different contamination classifications. Four trials compared wound dressings with no wound dressing (wound exposure); the remaining 25 studies compared alternative dressing types, with the majority comparing a basic wound contact dressing with film dressings, silver dressings or hydrocolloid dressings. The review contains 11 comparisons in total.
Primary outcome: SSI
It is uncertain whether wound exposure or any dressing reduces or increases the risk of SSI compared with alternative options investigated: we assessed the certainty of evidence as very low for most comparisons (and low for others), with downgrading (according to GRADE criteria) largely due to risk of bias and imprecision. We summarise the results of comparisons with meta‐analysed data below:
‐ film dressings compared with basic wound contact dressings following clean surgery (RR 1.34, 95% CI 0.70 to 2.55), very low certainty evidence downgraded once for risk of bias and twice for imprecision.
‐ hydrocolloid dressings compared with basic wound contact dressings following clean surgery (RR 0.91, 95% CI 0.30 to 2.78), very low certainty evidence downgraded once for risk of bias and twice for imprecision.
‐ hydrocolloid dressings compared with basic wound contact dressings following potentially contaminated surgery (RR 0.57, 95% CI 0.22 to 1.51), very low certainty evidence downgraded twice for risk of bias and twice for imprecision.
‐ silver‐containing dressings compared with basic wound contact dressings following clean surgery (RR 1.11, 95% CI 0.47 to 2.62), very low certainty evidence downgraded once for risk of bias and twice for imprecision.
‐ silver‐containing dressings compared with basic wound contact dressings following potentially contaminated surgery (RR 0.83, 95% CI 0.51 to 1.37), very low certainty evidence downgraded twice for risk of bias and twice for imprecision.
Secondary outcomes
There was limited and low or very low certainty evidence on secondary outcomes such as scarring, acceptability of dressing and ease of removal, and uncertainty whether wound dressings influenced these outcomes.
Authors' conclusions
It is uncertain whether covering surgical wounds healing by primary intention with wound dressings reduces the risk of SSI, or whether any particular wound dressing is more effective than others in reducing the risk of SSI, improving scarring, reducing pain, improving acceptability to patients, or is easier to remove. Most studies in this review were small and at a high or unclear risk of bias. Based on the current evidence, decision makers may wish to base decisions about how to dress a wound following surgery on dressing costs as well as patient preference.
Plain language summary
Dressings for the prevention of surgical site infection
Review question
This review aimed to assess whether use of different wound dressings (or leaving a wound exposed without a dressing) has an impact on the number of people who get wound infections following surgery where the wound is closed with stitches, staples, clips or glue. We also investigated whether different dressings resulted in less pain, less scarring or were more acceptable to patients and health professionals.
Background
Millions of surgical procedures are conducted globally each year. The majority of procedures result in wounds in which the edges are brought together to heal using stitches, staples, clips or glue; this is called 'healing by primary intention'. Afterwards, wounds are often covered with a dressing that acts as a barrier between it and the outside environment. One possible advantage of a dressing may be to protect the wound from infection (surgical site infection). Many different dressing types are available for use on surgical wounds. However, it is not clear whether one type of dressing is better than any other in preventing surgical site infection, or, indeed, whether it is better not to use a dressing at all.
Study characteristics
We conducted a review of all available, relevant evidence about the impact of dressings on the prevention of surgical site infections in surgical wounds healing by primary intention. This review examined data from 29 randomised controlled trials (which provide the most reliable evidence). These investigated the use of dressings in surgery that had a low risk of surgical site infection (clean surgery) and surgery with a higher risk (potentially contaminated surgery).
Key results
We found no clear evidence to suggest that one dressing type was better than any other at reducing the risk of surgical site infection, nor that covering wounds with any dressing at all reduced the risk of surgical site infection. Additionally, there was no clear evidence that any dressing type improves scarring, pain control, patient acceptability or ease of removal. Currently decision makers may opt to make decisions about whether and how to dress a wound based on patient and clinician preferences and dressing costs.
Certainty of the evidence
It is important to note that many trials in this review were small and the evidence was of low or very low certainty meaning that current information is uncertain.
Assessed as up to date September 2016.
Summary of findings
Background
Description of the condition
Millions of surgical procedures are conducted around the world each year. The majority of procedures result in surgical wounds that will heal by primary intention. This is where wound edges are re‐approximated using sutures, staples, clips or glue, either alone, or in combination. Following wound closure, surgical wounds commonly leak fluid or blood within the first 24 hours and they are frequently covered with different types of dressing ‐ including glue‐as‐a‐dressing (tissue glue applied over a wound that has already been closed) ‐ to manage the exudate, provide wound protection and prevent possible external contamination that might lead to surgical site infection (SSI) and delayed healing. A study in the USA found that in over 750,000 episodes of surgical hospitalisation, 1% resulted in an SSI (de Lissovoy 2009), and similar estimates have been found in France (Astagneau 2009). However, such values are known to underestimate the levels of SSI by not considering those that develop outside hospitals (Bruce 2001; Gibbons 2011). In the UK it has been estimated that 4% to 5% of patients undergoing a surgical procedure contract an SSI (Health Protection Agency 2002; Smyth 2008), but this percentage varies greatly depending on the circumstances. Whilst various patient factors can predict the likelihood of SSI, the type of surgical procedure performed exerts a major influence on risk. Surgical procedures involving 'clean' body cavities have much lower numbers of infection, around 3% to 5%, compared with procedures involving body cavities with infected, necrotic or dirty tissue, for example, colorectal surgery, which have surgical infection figures of around 10% to 30% (McLaws 2000). A widely used definition that describes the contamination classification of surgical procedures is given below:
Clean: non‐infective operative wounds in which no inflammation is encountered, and neither the respiratory, alimentary, genitourinary tract nor the oro‐pharyngeal cavity is entered. In addition these cases are elective, primarily closed, and drained with closed drainage system when required.
Clean/contaminated: operative wounds in which the respiratory, alimentary, genital or urinary tract is entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina and oropharynx are included in this category, provided no evidence of infection or a major break in sterile technique is encountered.
Contaminated: fresh, accidental wounds, operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract, and incisions in which acute, non‐purulent inflammation is encountered.
Dirty: old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera. This definition suggests that organisms causing postoperative infection were present in the operative field before the operation.
SSIs not only cause considerable patient morbidity, but also increase the consumption of healthcare resources. In the UK, the mean additional cost of treating an infected surgical wound (compared with a non‐infected wound) was estimated at GBP 1618 (Plowman 2001), with much of this extra cost attributable to an increased length of hospital stay (mean increase of 6.5 days) (Plowman 2001). In the USA, de Lissovoy 2009 estimated that the extended length of stay and increased treatment costs associated with SSIs over a one‐year period led to approximately 1 million additional inpatient‐days, costing an additional USD 1.6 billion.
Whilst SSIs can be difficult to define (one review identified 41 different definitions and 13 grading scales of SSI (Bruce 2001)), the Centers for Disease Control and Prevention (CDC) have published the following guidelines defining superficial and deep incisional SSIs (Horan 2008). A superficial SSI is defined as: an infection occurring within 30 days after the operation, that only involves the skin and subcutaneous tissue of the incision, and is associated with at least one of the following:
purulent drainage, with or without laboratory confirmation, from the surgical site;
organisms isolated from an aseptically‐obtained culture of fluid or tissue from the surgical site;
at least one of the following signs or symptoms of infection: pain or tenderness, localised swelling, redness or heat, and the superficial incision is deliberately opened by the surgeon and is culture‐positive or not cultured (a culture‐negative finding does not meet this criterion);
diagnosis of SSI by the surgeon or attending physician.
A deep incisional SSI is defined as: infection that occurs within 30 days after the operative procedure if no implant is left in place, or within one year if an implant is left in place, and the infection appears to be related to the operative procedure and involves deep soft tissues (e.g. fascial and muscle layers) of the incision associated with one of the following:
purulent drainage from the deep incision, but not from the organ/space component of the surgical site;
a deep incision spontaneously dehisces (opens up) or is deliberately opened by the surgeon and is culture‐positive or not cultured when the patient has at least one of the following symptoms: fever or localised pain or tenderness;
an abscess, or other evidence of infection involving the deep incision is found on direct examination, during re‐operation, or by histopathologic or radiologic examination;
diagnosis of a deep incisional SSI by a surgeon or attending physician.
Description of the intervention
Dressings are widely used in the care of wounds. Several attributes of an ideal wound dressing have been described (BNF 2016; Goldman 1992; NICE 2008); these include:
the ability of the dressing to absorb and contain exudate without leakage or strike‐through;
lack of particulate contaminants left in the wound by the dressing;
thermal insulation;
impermeability to water and bacteria;
suitability of the dressing for use with different skin closures (sutures, staples);
avoidance of wound trauma on dressing removal;
frequency with which the dressing needs to be changed;·
provision of pain relief;
cosmesis and comfort;
effect on formation of scar tissue;
transparency to aid visualisation of the wound.
Dressing products have evolved considerably in the last few decades, and now fall into broad, widely‐recognised categories, namely:
basic wound contact dressings such as gauze or cotton absorbents;
'advanced' dressings such as hydrogels, hydrocolloids and films;
antimicrobial and other specialist dressings; and, more recently
topical skin adhesives, which can be used to cover an already closed wound ‐ 'glue‐as‐a‐dressing'.
Within these groups there are many hundreds of dressing types available. For ease of comparison in this review, dressings have been classified into groups according to the British National Formulary (BNF) (BNF 2016). However, it is important to note that the distributors of dressings may vary from country to country, and that dressing names may also vary. Below we summarise key dressing groups as well as noting wound exposure where no dressing is used to cover a wound.
Wound exposure
In some cases wounds may be left uncovered following surgery. They may have no dressing at all applied or a simple pad placed on the closed wound to absorb leakage which is removed shortly after.
Basic wound contact dressings
Absorbent dressings and surgical absorbents
Absorbent dressings are applied directly to the wound. Surgical absorbents may be used as secondary absorbent layers in the management of heavily‐exuding wounds. Examples include Primapore® (Smith & Nephew), Mepore® (Mölnlycke), and absorbent cotton gauze, BP 1988.
Low‐adherence dressings and wound contact materials
Low adherence dressings and wound contact materials are usually cotton pads that are placed directly in contact with the wound. They are either non‐medicated (e.g. paraffin gauze dressing), or medicated (e.g. containing povidone iodine or chlorhexidine). Examples include paraffin gauze dressing, BP 1993, Xeroform Dressing® ‐ a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Advanced dressings
Vapour‐permeable films
Vapour‐permeable films are permeable to water vapour and oxygen, but not to water or micro‐organisms. They are normally transparent. Examples include OpSite® (Smith & Nephew) and Tegaderm® (3M).
Hydrocolloid dressings
Hydrocolloid dressings are occlusive dressings composed of a hydrocolloid matrix attached to a base (possibly film or foam). Fluid absorbed from the wound causes the hydrocolloid to liquefy. Examples include Comfeel® (Coloplast) and DuoDerm® (ConvaTec, UK).
Fibrous hydrocolloid dressing (hydrofibre, spun hydrocolloid dressings)
Fibrous hydrocolloid dressings are composed of sodium carboxymethylcellulose which forms a gel when it comes into contact with fluid. Examples include Aquacel® (ConvaTec, UK).
Polyurethane matrix hydrocolloid dressing
Polyurethane matrix hydrocolloid dressings consist of two layers ‐ a polyurethane gel matrix and a waterproof polyurethane top‐film designed to act as a bacterial barrier. There is only one dressing of this type listed in the BNF: Cutinova® Hydro (Smith & Nephew).
Antimicrobial dressings
Polyhexametylene biguanide (PHMB) dressing
PHMB dressings are impregnated with the antimicrobial agent polyhexanide.
Topical skin adhesives (glue‐as‐dressing)
Skin tissue adhesives are currently described in the BNF as being indicated for closure of minor skin wounds and for additional suture support. However, they can be used on an already closed wound as a dressing without an additional covering. They act as a barrier, are sterile before application and contain enbucrilate or octyl 2‐cyanoacrylate.
How the intervention might work
Current practice for some surgical wounds healing by primary intention involves placement of a dressing over the closed wound before the patient leaves the clean environment of the operating theatre. This practice assumes that the risk of SSIs may be reduced by providing a barrier to environmental contamination. Furthermore, dressings may have additional roles in managing wound exudate, protecting wounds and their staples or sutures, and meeting patients' expectations by 'hiding' the wound, or, alternatively, when transparent dressings are used, facilitating health professionals' observation of the wound. Conversely, in other practices (e.g. paediatric surgery) it is usual not to use a dressing. This practice assumes that the risk of SSIs may be reduced by allowing the wound to dry. When wounds are covered by 'glue‐as‐a‐dressing' it is also assumed that this acts as a barrier that may reduce external infection.
Why it is important to do this review
Surgical wounds healing by primary intention are commonplace within all elective and emergency surgical practice. It is important to assess whether wound dressings have a potential role in reducing the risk of SSI. Such information could inform allocation of resources to appropriate treatments. Currently these decisions are made with limited review data. In the UK, a government‐funded guideline reviewed the data from five trials that are relevant to this review, and concluded that existing studies did not show convincing differences in dressing effectiveness in terms of reducing SSI (NICE 2008). Whilst the review methods were robust, the search date was September 2007, and so studies published after this date were not assessed. Recent World Health Organisation guidelines have been published which assess one group of dressings, advanced dressings, compared with standard dressings (Allegranzi 2016).
Objectives
To assess the effects of wound dressings compared with no wound dressings, and the effects of alternative wound dressings, in preventing SSIs in surgical wounds healing by primary intention.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared the immediate postoperative application of wound dressings with no wound dressings, or compared alternative dressings, for surgical wounds expected to heal by primary intention.
Types of participants
Studies involving adults or children (aged two years and over) who had undergone surgical procedures where healing of the surgical wound was planned by primary intention. Wounds of any contamination level (clean, clean/contaminated, contaminated and dirty) were eligible for inclusion. We excluded procedures involving graft sites, and wounds of the mouth and eye. Participants were required to have dressings applied in the operating theatre, immediately after closure of the skin. We excluded studies where participants had infected wounds at the start of the study.
Types of interventions
The primary intervention was wound exposure or application of wound dressings that could be:
basic wound contact dressings: classed as surgical and non‐surgical absorbent dressings, low‐adherence dressings, impregnated/non‐impregnated gauze, and adhesive tape;
advanced wound dressings such as hydrogels, hydrocolloids and films;
antimicrobial and other specialist dressings;
tissue adhesive used as a dressing (glue‐as‐dressing) on an already closed wound.
We included comparisons of a dressing versus no dressing (exposed wound), and versus alternative dressings. We did not consider trials that compared different application durations of the same dressing (timing trials), as these will form a separate review. Nor did we include trials where the application of topical gels or ointments to wounds (in the absence of a dressing comparator) was evaluated, as we viewed these as different interventions. We did not include trials where the application of tissue adhesive was for the purpose of closing the wound only. The only difference between trial groups for included studies was the method of wound coverage used.
Types of outcome measures
Primary outcomes
Occurence of postoperative SSI as defined by the CDC criteria (Horan 2008), or the authors' definition of SSI. We did not differentiate between superficial and deep‐incisional infection.
Secondary outcomes
Scarring: as reported by the author.
Pain: reported using a validated scale or as reported by the author.
Acceptability (participant and clinician): as reported by the author.
Ease of removal (participant and clinician): as reported by the author.
Cost: any measure of cost of treatment, or other aspects of resource use i.e. other equipment.
Search methods for identification of studies
Electronic searches
In September 2016 for our second update of this review we searched the following electronic databases:
the Cochrane Wounds Specialised Register (searched 19 September 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2016, Issue 8);
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and Epub Ahead of Print; 1946 to 19 September 2016);
Ovid Embase (1974 to 19 September 2016);
EBSCO CINAHL Plus (1937 to 19 September 2016).
The search string for CENTRAL can be found in Appendix 1. The search methods used for the original version of this review can be found in Appendix 2.The search strategies for Ovid MEDLINE, Ovid Embase and EBSCO CINAHL can be found in: Appendix 3; Appendix 4; and Appendix 5. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions with respect to language, date of publication or study setting.
Searching other resources
We searched the bibliographies of all retrieved and relevant publications identified by these strategies for further studies. While handsearches were not performed for this review, they are conducted by Cochrane Wounds in order to inform the CENTRAL database, which we searched. We did not contact manufacturers of dressings regarding studies for inclusion.
We also searched the following clinical trials registries.
ClinicalTrials.gov (www.clinicaltrials.gov).
the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Data collection and analysis
Selection of studies
Two review authors independently assessed the studies' titles and abstracts against the review's inclusion criteria. After this initial assessment, we obtained all studies that might meet these criteria in full. Full papers were checked for eligibility by two review authors, with disagreements resolved by discussion and, where required, the input of a third review author. We extracted details of the eligible studies, and summarised them on a data extraction sheet. Two review authors extracted data independently. If data were missing from reports, we made attempts to contact the study authors to obtain the missing information. Studies that were published in duplicate we only included once, but extracted the maximum amount of data from the papers.
Data extraction and management
All data were extracted independently by two review authors. The following data were extracted:
country in which the trial was conducted;
type of surgery;
classification of surgical contamination (see Table 10 for classification guide);
eligibility criteria;
details of the dressing/treatment regimen received by each group, including the duration that the dressing was in situ;
primary and secondary outcome(s) (with definitions);
outcome data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group).
1. Classification of surgical contamination of included studies.
| Classification | Description | Study classification |
| Clean only | Non‐infective operative wounds in which no inflammation is encountered, and neither the respiratory, alimentary, genitourinary tract nor the oro‐pharyngeal cavity is entered. In addition these cases are elective, primarily closed, and drained with closed drainage system when required. | Burke 2012; Cosker 2005; De Win 1998; Dickinson Jennings 2015; Lawrentschuk 2002; Law 1987; Langlois 2015; Martin‐Trapero 2013; Michie 1994; Moshakis 1984; Politano 2011; Ravnskog 2011; Vogt 2007; Wikblad 1995; Wynne 2004 |
| Clean/contaminated only | Operative wounds in which the respiratory, alimentary, genital or urinary tract is entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina and oropharynx are included in this category, provided no evidence of infection or a major break in sterile technique is encountered. |
Persson 1995; Ruiz‐Tovar 2015 Not reported for Biffi 2012; Kriegar 2011; Siah 2011 but we put them in this class on the basis of details reported. |
| Contaminated only | Fresh, accidental wounds, operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract, and incisions in which acute, non‐purulent inflammation is encountered. | |
| Dirty only | Old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera. This definition suggests that organisms causing postoperative infection were present in the operative field before the operation. | |
| Mixed |
Bennett 2013 (clean and possibly clean/contaminated and contaminated) Gardezi 1983;(clean, clean/contaminated and possibly contaminated) Hewlett 1996; (predominately clean, some clean/contaminated and possibly contaminated) Holm 1998; (clean, clean/contaminated and contaminated) Ozaki 2015 (25/500 participants were clean‐contaminated and the remaining 475 were clean surgery) Phan 1993; (clean, clean/contaminated) Shinohara 2008; (clean, clean/contaminated and possibly contaminated) |
|
| No classification | Rohde 1979; Prather 2011 |
Assessment of risk of bias in included studies
Two review authors independently assessed each included study for risk of bias. Assessment was undertaken using the Cochrane `Risk of bias' tool (Higgins 2011). This tool considers six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, freedom from selective reporting, and other issues (i.e. serious baseline imbalance). A `Risk of bias' table was completed for each eligible study; these data were combined into a `Risk of bias' summary figure where we have tabulated judgements for each domain by study.
Measures of treatment effect
We presented results with 95% confidence intervals (CI). We reported estimates for dichotomous outcomes (e.g. infected: yes/no) as risk ratios (RR) (Deeks 2002). We reported continuous data (e.g. pain) as mean differences (MD), and we calculated overall effect sizes (with 95% CI).
Unit of analysis issues
When we located three‐armed trials where only two of the arms were relevant to the review, we did not extract data for the non‐relevant arm. When three‐armed studies had two arms randomised to receive different brands of the same dressing, we combined these into one group and treated the trial as a two‐armed trial. We did not combine arms in three‐armed trials when all the arms received different, relevant interventions, in those cases we included all relevant comparisons.
Dealing with missing data
We did not consider the issue of missing data in the protocol for this review. The problem of missing data is common in trials, especially those of poor quality. Excluding participants from the analysis after randomisation, or ignoring participants lost to follow‐up can, in effect, undo the process of randomisation, and thus, potentially, introduce bias into the trial. For our primary outcome, SSI, we assumed that where randomised participants were not included in an analysis, they did not have an SSI (that is they were considered in the denominator but not the numerator). Given the relatively small number of SSI events anticipated, this seemed the most appropriate assumption. When a trial did not specify participant group numbers prior to drop out, we presented only complete case data. We present data for all secondary outcomes as complete case analysis.
Assessment of heterogeneity
Our assessment of heterogeneity comprised an initial assessment of clinical and methodological heterogeneity and the appropriateness of combining study results: that is the degree to which the included studies varied in terms of participant, intervention, outcome and characteristics such as length of follow‐up. We supplemented this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity of the results ‐ assessed using the Chi² test (we considered that a significance level of P < 0.10 indicated statistically significant heterogeneity) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). In general I² values of 25%, or less, may mean a low level of heterogeneity (Higgins 2003), and values of 75%, or more, indicate very high heterogeneity (Deeks 2011). We also examined the variability of the point estimates and the overlap of the confidence intervals, when I² values were less than 50%. Where there was evidence of high heterogeneity we explored this further: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias, an across‐study reporting bias, is one of a number of possible causes of 'small study effects', that is, a tendency for estimates of the intervention effect to appear to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the effect estimates from individual RCTs against some measure of trial size or precision (Sterne 2011). If we had meta‐analyses that included 10 or more RCTs, we would have presented funnel plots using Cochrane Review Manager 5 software (RevMan 2014). However, we did not have sufficient studies for this.
Data synthesis
We combined details of included studies in a narrative review according to dressing type and stratified by surgical contamination level. We explored both clinical and statistical heterogeneity. Where appropriate, we pooled data using meta‐analysis (conducted using RevMan 5), that is, where studies were considered similar in terms of intervention type, duration, and outcomes. We assessed statistical heterogeneity using the Chi² test (we considered that a significance level of P value less than 0.1 indicated heterogeneity), and the I² test (Higgins 2003). In the absence of clinical heterogeneity, and in the presence of statistical heterogeneity (I² over 50%), we used a random‐effects model. Where there was no clinical or statistical heterogeneity, we applied a fixed‐effect model.
GRADE assessment and 'Summary of findings' tables
We presented the main results of the review in 'Summary of findings’ tables for the following comparisons:
basic wound contact dressings compared with exposed wounds;
specific advanced dressings compared with exposed wounds;
basic wound dressings compared with specific advanced dressings e.g. film, hydrocolloid;
basic wound contact dressings compared with antimicrobial dressings.
These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings’ tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach. This defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We present the following outcomes in the 'Summary of findings’ tables:
SSI;
scarring;
acceptability of dressing to patient;
ease of dressing removal.
For relevant outcomes reported for comparisons not listed above we have presented GRADE assessments without a 'Summary of findings' table.
In terms of the GRADE assessment, when making decisions for the risk of bias domain we downgraded only when we had classed studies as being at high risk of bias for one or more domains and/or they were classed as being at unclear risk of bias for both domains that contribute to selection bias. In assessing the precision of effect estimates for SSI we followed GRADE guidance (GRADE 2013), and calculated an optimal information size (OIS) using conventional sample size calculation methods. We used the OIS, along with the size of 95% CIs ‐ in terms of whether they spanned estimates of benefit and harm ‐ to assess for downgrading. We calculated the OIS based on GRADE guidance of using a relative risk reduction of between 20% and 30%. The OIS is summarised below but should not be treated as optimal sample sizes for any future research. Within a GRADE assessment the OIS is used to assess the stability of CIs rather than to assess the appropriateness of a sample size to detect a difference.
Our calculation was: reduction in SSI from 14% to 10% (80% power; alpha 5%) = 2070 participants.
We also followed GRADE guidance and downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciate harm.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies;Characteristics of studies awaiting classification; Characteristics of ongoing studies for full details of studies identified. We are contacting the authors of three studies to clarify their eligibility for the review (Goharshenasan 2016; Siddiqui 2016; Springer 2015). We identified four relevant on‐going studies (ISRCTN06792113; NCT02771015; NCT02904200; NCT02619773).
In searching trial registers we also located records for four studies marked as complete, which we could not link to published data on the basis of the information available (see Table 11). We have tried to contact representatives for these trials to locate possible unpublished data: this work is ongoing.
2. Information on studies listed as completed on trial register with unclear publication status.
| Studies listed as completed with no published record we are aware of | Relevant outcomes listed | Database | Listed contact |
| Efficacy of wound care and reduction of wound complications by use of AQUACEL® Ag surgical dressing | Yes | Clinical trials.gov clinicaltrials.gov/ct2/show/NCT02445300?term=dressing+AND+surgery&rank=8 |
Feng Chih Kuo Chang Gung Memorial Hospital |
| Prospective, randomised, controlled clinical investigation, comparing two postoperative wound dressings used after elective hip and knee replacement | Yes | Clinical trials.gov clinicaltrials.gov/ct2/show/NCT02653183?term=dressing+AND+surgery&rank=10 |
Being conducted in Belgium Molnlycke Health |
| Post‐op visible wound dressings in treatment of surgical incisions | Unclear | Clinical trials.gov clinicaltrials.gov/ct2/show/NCT01577225?term=dressing+AND+surgery&rank=11 |
Being conducted in China Smith & Nephew Medical (Shanghai) Ltd |
| Aquacel compared with traditional post surgical wound dressing in vascular surgery patients | Unclear | Clinical trials.gov clinicaltrials.gov/ct2/show/NCT00428623?term=dressing+AND+surgery&rank=42 |
Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 |
Included studies
A total of 29 RCTs met the inclusion criteria; nine being added in this 2016 update (Biffi 2012; Dickinson Jennings 2015; Kriegar 2011; Langlois 2015; Ozaki 2015; Politano 2011; Prather 2011; Ruiz‐Tovar 2015; Siah 2011). There are now 23 two‐arm trials and six three‐arm trials in the review. Ruiz‐Tovar 2015 was a three‐arm trial, but only two arms are relevant here and we did not extract data for the non‐relevant arm. In one three‐arm trial, two of the three arms were randomised to receive different brands of a film dressing (Cosker 2005). For this review, these two film‐dressing groups were combined into one group and the trial was treated as a two‐arm trial. Likewise, for Dickinson Jennings 2015 we combined two silver dressing arms. We did not combine arms for the remaining three‐arm trials, since all groups were deemed to have received different interventions, and so we included all relevant comparisons.
In all trials the surgical procedure took place in a hospital operating theatre.
In total 15 (52%) of the included trials have been published since 2007 (Bennett 2013; Biffi 2012; Burke 2012; Dickinson Jennings 2015; Kriegar 2011; Langlois 2015; Martin‐Trapero 2013; Ozaki 2015; Politano 2011; Prather 2011; Ravnskog 2011; Ruiz‐Tovar 2015; Shinohara 2008; Siah 2011; Vogt 2007).
The trials took place in several different countries: 17 were conducted in Europe, four in the UK (Cosker 2005; Hewlett 1996; Law 1987; Moshakis 1984), two in Belgium (De Win 1998; Phan 1993); two in Sweden (Persson 1995; Wikblad 1995), two in Denmark (Holm 1998; Vogt 2007), one in Germany (Rohde 1979), one in Ireland (Burke 2012), two in Spain (Martin‐Trapero 2013; Ruiz‐Tovar 2015), one in France (Langlois 2015), one in Italy (Biffi 2012), and one in Norway (Ravnskog 2011). Two of the remaining trials were conducted in Australia (Lawrentschuk 2002; Wynne 2004), one in Pakistan (Gardezi 1983), seven in the USA (Bennett 2013; Dickinson Jennings 2015; Kriegar 2011; Michie 1994; Ozaki 2015; Politano 2011; Prather 2011), one in Japan (Shinohara 2008), and one in Singapore (Siah 2011). One trial was published in German (Rohde 1979), and one in Spanish (Martin‐Trapero 2013), and we acquired translations of these.
The types of surgical procedures undertaken were varied and included cardiac and/or vascular surgery (Shinohara 2008; Vogt 2007; Wikblad 1995; Wynne 2004); caesarean sections (Bennett 2013), abdominal surgery and/or gastrointestinal surgery (Biffi 2012; Holm 1998; Kriegar 2011; Persson 1995; Martin‐Trapero 2013; Rohde 1979), or a number of different surgical procedures within the same trial (Burke 2012; Gardezi 1983; Hewlett 1996; Siah 2011). The surgical procedures in each trial were classified as having been clean, clean/contaminated, contaminated or dirty, or a combination of these (see Table 10 for classification). We recorded when the type of surgery performed was unclear (Rohde 1979). Studies also compared a range of different dressing types and regimens as described below and in Table 12.
3. Trial data.
| Study ID | Groups | Primary outcome SSI | Cost | Scarring | Pain | Acceptability | Ease of removal |
| Bennett 2013 | Group A: standard soft cloth (n = 262) Group B: silver ion‐eluting dressings (n = 262) |
Infection Group A: 19/262 Group B: 25/262 |
Group A: USD 1.30 per dressing
(USD 306.80 group total), Group B: USD 46.36 per dressing (USD 11,080.04 group total) |
n/r | n/r | n/r | n/r |
| Biffi 2012 | Group A: standard absorbant dressing (n= 59) Group B: silver hydrofibre dressing (n = 62) |
Infection (clinical and microbiological assessment) Group A: 11/59 Group B: 9/62 |
n/r | n/r | n/r | n/r | n/r |
| Burke 2012 | Group A: absorbent dressing (n = 62: 35 THA and 27 TKA) Group B: hydrofiber inner layer and hydrocolloid outer layer (Jubilee dressing) (n = 62: 35 THA and 27 TKA). |
Infection Group A: 0/62 Group B: 0/62 Inflammation Group A: 3/62 Group B: 3/62 |
Mean no. of dressing change Group A: 1 = 8/62 2 = 35/62 3+=19/62 Group B: 1 = 38/62 2 = 19/62 3+= 5/62 |
n/r | n/r | n/r | n/r |
| Cosker 2005 | Group A: standard absorbent dressing (n = 100) Group B: transparent film dressing and pad (n = 100) Group C: film dressing (n = 100) |
Group A: 5/100 Group B: 5/100 Group C 4/100 | n/r | n/r | n/r | n/r | n/r |
| De Win 1998 | Group A: absorbent dressing (n = 6) Group B: transparent film dressing and pad (n = 8) |
Group A: 0/6 Group B: 0/8 | Mean total cost of dressings Group A = BEF 11.5 Group B = BEF 14.3 |
n/r | n/r | n/r | n/r |
| Dickinson Jennings 2015 | Group A: standard sterile dressing (n = 117) Group B: metallic silver dressing (n = 116) Group C: ionic silver dressing (n = 118) |
Group A: 3/117 Group B: 1/116 Group C: 2/118 |
n/r | n/r | Measured on a 10‐point scale with 0 = no pain and 10 = maximum pain): Group A: 0.98 Group B: 0.67 Group C: 0.75 No other data reported except a P value of 0.265 Pain at dressing removal: Group A: 2.37 Group B: 1.47 Group C: 2.38 No other data reported except a P value of 0.025 |
n/r | Measured on a 5‐point scale with 1 = very easy and 5 = very difficult. Authors presented data for % classed very easy. Not clear how this was calculated across removals. % classed very easy Group A: 0 (0%) Group B: 71 (70%) Group C: 50 (51%) |
| Gardezi 1983 | Group A: conventional gauze dressing (n = 50) Group B: film dressing (n = 50) |
Group A: 6/50 Group B: 3/50 | n/r | n/r | No data about how this was measured. Group A: 2/50 Group B: 1/50 |
n/r | n/r |
| Hewlett 1996 | Group A: absorbent dressing (n = 39) Group B: film dressing (n = 37) |
n/r | Dressing cost to complete healing (excluding procedure packs) Group A: GBP 1.60 Group B: GBP 1.46 Cost including procedure packs: Group A: GBP 4.36 Group B: GBP 2.84 |
n/r | n/r | n/r | n/r |
| Holm 1998 | Group A: absorbent dressing (n = 37) Group B: hydrocolloid dressing (n = 36) | Group A: 5/22 Group B: 1/28 |
Group A: 4 wounds required dressing change; Group B: 5 wounds required dressing change due to leakage or adherence issues. |
Mean width (mm) Group A: 1.78 (range 1–3) Group B: 2.26 (range 1–5) Total cosmetic and functional quality of scar (combined from 6 domain scores: elevation of scar, scar down‐binding, supposed inconveniences originating from scar, scar width, colour of scar, cosmetic result, not clear what scores refer to. Units unknown). Group A: 21.5 Group B: 22.6 |
n/r | n/r | n/r |
| Kriegar 2011 | Group A: gauze (n = 55) Group B: silver nylon dressing (n = 55) |
Group A: 18/55 (14 superficial and 4 deep) Group B: 7/55 (5 superficial, 2 deep) |
n/r | n/r | n/r | n/r | n/r |
| Law 1987 | Group A: gauze (n = 59) Group B: film dressing (n = 54) Group C: exposed wound (n = 53) | Group A: 3/59 Group B: 5/54 Group C: 1/53 |
Total dressing cost: Group A: GBP 6.60 Group B: GBP 42.00 Group C: GBP 0.80 |
n/r | n/r | n/r | n/r |
| Lawrentschuk 2002 | Group A: non‐adherent absorbable dressing (n = 25) Group B: paraffin tulle gras (n = 25) | Group A: 3/25 Group B: 0/25 |
n/r | n/r | n/r | n/r | n/r |
| Langlois 2015 | Group A: gauze (n = 40) Group B: hydrofibre (n = 40) |
Group A: 0/40 Group B: 0/40 |
n/r | Data on the appearance of scar was reported at 6 weeks ‐ blinded assessment Stoney Brook scale Medians with standard deviations Group A: 0 (SD 1.62) Group B: 1 (SD 1.71) The authors did not report what the scores on the Stoney Brook scale related too (what was low and what was high). Literature suggests that the total score is derived by adding the scores on the individual items of the scale and ranges from 0 (worst) to 5 (best). Categorical scale (poor, acceptable or excellent categories ‐ Medians with standard deviations Group A: 0 (SD 0.71) Group B: 0.5 (SD 0.63) Also present data using VAS but not clear whether high or low scores were better. |
All collected using a scale and analysed by study authors using means: 1 = not satisfied; 2 = fairly satisfied; 3 = satisfied; 4 = highly satisfied Medians with standard deviations Pain reported by participants Pain during dressing change Group A: 4 (SD 0.60) Group B: 4 (SD 0.48) Pain outside of dressing change Group A: 3 (SD 0.90) Group B: 3 (SD 0.97) Nurse views of participant pain (no further details) Group A: 4 (SD 0.69) Group B: 4 (SD 0.66) |
n/r | Collected using a scale and analysed by study authors using means. 1 = not satisfied; 2 = fairly satisfied; 3 = satisfied; 4 = highly satisfied Medians with standard deviations Nurse‐reported Group A: 3 (SD 0.59) Group B: 4 (SD 0.49) |
| Martin‐Trapero 2013 | Group A: non‐occlusive dressing (gauze) (n = 101) Group B: 0.2% (PHMB) dressing (n = 96) |
Group A: 5/101 Group B: 1/96 |
n/r | n/r | n/r | n/r | n/r |
| Michie 1994 | Group A: cotton gauze impregnated with bismuth tribromophenate (n = 28) Group B: hydrocolloid dressing (n = 28) | Group A: 0/28 Group B: 0/28 |
n/r | Participant ratings Evenness Group A: Excellent = 14 Good = 8 Fair = 0 Group B: Excellent = 22 Good = 0 Fair = 0 Colour Group A: Excellent = 13 Good = 9 Fair = 0 Group B: Excellent = 22 Good = 0 Fair = 0 Suppleness Group A: Excellent = 15 Good = 6 Fair = 0 Group B: Excellent = 21 Good = 0 Fair = 0 Investigator‐rated 4‐point rating scale scores for 3rd and 4th visits: Scar suppleness Group A: None = 1; Some = 2; Considerable = 13; Very much = 10; Group B: None = 1; Some = 1; Considerable = 4; Very much = 20 Scar raised Group A: No = 14; Some = 11; Considerable = 1 Group B: No = 21; Some = 5; Considerable = 0. Final visit scores (approximately 7 months) Scar suppleness Group A: No = 0; Some = 0; Considerable = 0; Very much = 19 Group B: No = 0; Some = 0; Considerable = 0; Very much = 19 Scar raised Group A: None = 16; Some = 2; Considerable = 0; Very much = 0Group B: None = 18; Some = 0; Considerable = 0; Very much = 0 Data on pigmentation pulling and itching also reported, but not extracted here. |
Past 48 h measured on a VAS where 0 = 'no pain' and 10 = 'most pain'): First visit: Group A = 0.89 (SD 1.35) Group B = 0.92 (SD 1.36) Second visit: Group A = 0.02 (SD 0.04) Group B = 0.008 (SD 0.03) |
n/r |
Participant's perception of pain on removal:1st visit (measured on a VAS, where 0 = 'no pain' and 10 = 'most pain'): Group A: 0.03 (SD 0.07) Group B: 0.24 (SD 0.79) 2nd visit Group A: 0.01 (SD 0.03) Group B: 0.42 (SD 0.68) Clinician's opinion dressing easy to remove? 1st visit: Yes: Group A: 18/25 Group B: 22/25 2nd visit Yes: Group A: 4/9 Group B: 9/9 (24 did not require dressing removal) |
| Moshakis 1984 | Group A: dry gauze dressing Group B: transparent film dressing | n/r | n/r | n/r | Assessed by participants on a linear scale 1 to 10 where 1 = no discomfort/pain and 10 = extremely uncomfortable/painful):
Group A: mean 5.1, SE (0.36), SD (2.76); Group B: mean 1.6, SE (0.19), SD (1.48) NOTE: SD calculated by review author as (SE* sqrtN) |
Assessed by participants on a linear scale 1–10 where 1 equated to no trouble at all, and 10 equated to very troublesome):
Group A: mean 4.2, SE (0.32) SD (2.46) Group B: mean 1.3, SE (0.15), SD (1.17) Acceptability: nurse assessed on a linear scale as for participants): Group A: mean 5.42, SE (0.44) Group B: mean 1.2, SE (0.08) NOTE: SD calculated by review author as (SE* sqrtN) |
n/r |
| Ozaki 2015 | Group A: standard gauze Group B: silver alginate dressing |
Group A: 0/250 Group B: 1/250 |
n/r | n/r | n/r | n/r | n/r |
| Persson 1995 | Group A: exposed wounds initially covered with an absorbent dressing removed morning after surgery (n = 30) Group B: occlusive hydrocolloid dressing (n = 31) | Group A: 2/30 Group B: 2/31 |
n/r | Estimated from graphical representation of VAS: 0‐100 mm, higher score indicating worse pain). Group A: 40 mm Group B: 32 mm |
From participants' perception, estimated from graphical representation of VAS: 0–100 mm for each domain listed, with a higher score indicating increased anxiety):
Thought about wound? Group A: 18 mm Group B: 32 mm Found it unpleasant to look at? Group A: 4 mm Group B: 4 mm Worried about infection? Group A: 7 mm Group B: 10 mm Worried about rupture? Group A: 5 mm Group B: 8 mm. Hesitated to shower? Group A: 5 Group B: 3 |
n/r | |
| Phan 1993 | Group A: standard gauze dressing (not named) (n = 86) Group B: surgical wound ointment with pure Vaseline (Qualifar) without gauze dressing (n = 93) | Group A: 21/86 Group B: 29/93 |
n/r | n/r | n/r | n/r | n/r |
| Politano 2011 | Group A: standard dressing Group B: silver‐impregnated dressing |
Group A: 25/70 Group B: 19/75 |
n/r | n/r | n/r | n/r | n/r |
| Prather 2011 | Group A: gauze Group B: silver nylon |
n/r | n/r | n/r | Reported pain data for 7 days post surgery ‐ using a scale measuring from 0‐10 ‐ with 0 being no pain and 10 being worst pain. At baseline the mean pain score in Groups A and B was 5. Paper presented subsequent data for each day until day 7, when the mean pain score was 4 in Group A and 2 in Group B. No standard deviation data were presented and no further analysis is presented here. |
n/r | n/r |
| Ravnskog 2011 | Group A: alginate dressing (n = 100) Group B: hydrofibre dressing (n = 100) |
n/r |
Length of hospital stay (mean days; SD) Group A: 8.05; (3.2) Group B: 8.71; (4.1) |
n/r |
Pain from the dressing during mobilisation (measured with 10‐point VAS where 0 = no
problems and 10 = unbearable problems) Mean (SD) Group A: 0.42 (1.2) Group B: 0.34 (1.0) |
All measured with 10‐point VAS where 0 = no
problems and 10 = unbearable problems. Mean (SD) Itching under the dressing Group A: 0.87 (1.6) Group B: 0.87 (1.6) Burning pain under the dressing Group A: 0.50 (1.3) Group B: 0.54 (1.2) Discomfort caused by use of the dressing Group A: 0.56 (1.2) Group B: 0.59 (1.1) |
Pain at removal of the dressing (yes) Group A: 2.1% Group B: 15% Pain score at removal (10‐point VAS where 0 = no problems and 10 = unbearable problems). Mean (SD) Group A: 0.21 (0.5) Group B: 0.57 (1.3) |
| Rohde 1979 | Group A: conventional dressing (n = 46) Group B: transparent drape (n = 44) |
Mild wound infection (reddening around stitches): Group A: 52% Group B: 32% (only % reported in paper, so n values calculated as: Group A: 24/46 Group B:14/44) Infection (not clear whether systemic infection or other type of wound infection): Group A: 7% Group B: 14% ( as above, n values calculated as: Group A: 3/46 Group B: 6/44) |
Cost (per participant): Group A: DEM 10.40 allowing for 3 changes after the operation Group B: DEM 3.60 . | n/r |
Comfortable Group A: 78% Group B: 80% |
n/r |
Easy to remove Group A: 89% Group B: 95% |
| Ruiz‐Tovar 2015 | Group A: gauze and plastic adhesive tape ‐ (n = 49) Group B: silver‐containing dressing (no further details) (n = 49) |
Group A: 10/49 Group B: 9/49 |
n/r | n/r | n/r | n/r | n/r |
| Shinohara 2008 | Group A: conventional gauze (n = 71) Group B: occlusive hydrocolloid dressing (n = 63) | Group A: 4/71 Group B: 3/63 (note in the paper there is a difference between table data and narrative results ‐ we have taken table data) |
Cost (of dressing per participant): Group A: JPY 779.9 Group B: JPY 714.9 | Mean width (standard deviation) Group A: 2.3 (2.4) mm Group B: 2.2 (2.4) mm. |
n/r | n/r | n/r |
| Siah 2011 | Group A: wound exposure (n = 83) Group B: ionic silver‐containing dressing (n = 83) |
Group A: 8/83 Group B: 1/83 |
n/r | n/r | n/r | n/r | n/r |
| Vogt 2007 | Group A: absorbent dressing (n = 80) Group B: hydrofibre/spun hydrocolloid dressing (n = 80) | 6 weeks: Group A: 7/66 (not full denominator of 80 as 14 of those randomised not included); Group B: 9/70 (not full denominator of 80 as 10 of those randomised not included) |
Cost/per participant: Group A: EUR 10‐11.8 Group B: EUR 20.3‐48.7 |
n/r | n/r | 1‐4 days after surgery (participant assessment: composite outcome from discomfort at mobilisation, pain at dressing change, and skin problems. All combined onto 3‐point scale where good = discomfort at all; moderate = minor problems and poor = severe problems):
Group A: good = 52(denominator unclear) Group B: good = 59(denominator unclear) |
n/r |
| Wikblad 1995 | Group A: absorbent dressing (n = 92) Group B: hydrocolloid dressing (n = 77) Group C: polyurethane matrix hydrocolloid dressing (n = 81) | 11 participants treated with antibiotics postoperatively; 8 of these had infections in the sternum (5 of these were in the absorbent dressing group). Not reported by group. | Days 1‐5 per participant: Group A: USD 0.73 Group B: USD 3.60 Group C: USD 3.34 |
n/r | At day 5 (rated on 3‐point scale, no pain to very painful, numerator/denominator data not provided):
No pain on removal Group A: 76% Group B: 61% Group C: 14% (Actual values calculated by review authors using the denominator from the ease of removal data, assuming both variables measured at the same time. Group A: 64/84; Group B: 37/61; Group C: 8/60) |
n/r |
Ease of removal (dressing assessed by clinician as difficult to remove: difficult to remove? Yes
Group A: 5/84 Group B: 13/61 Group C: 45/60 |
| Wynne 2004 | Group A: dry absorbent dressing (n = 243) Group B: hydrocolloid dressing (n = 267) Group C: film dressing (n = 227) | Group A: 6/243 Group B: 6/267 Group C: 9/227 |
Median cost per participant: Group A: AUD 0.52 Group B: AUD 3.93 Group C: AUD 1.59. |
n/r | n/r | Assessed by participants Dressing awareness Group A: 49/243 Group B: 77/267 Group C: 80/227 Movement limitation Group A: 30/243 Group B: 61/267 Group C: 60/227 Dissatisfied Group A: 46/243 Group B: 75/267 Group C: 80/227. |
n/r |
n/r = not reported
Excluded studies
In total, we excluded 99 studies after screening of the full text. There were a number of reasons for exclusions including 21 studies that were not RCTs and nine studies that included wounds healing by secondary intention, i.e. wounds that were left open, or had broken open, and were healing from deep to superficial layers. Full details are given in the Characteristics of excluded studies.
For a summary PRISMA flow chart see Figure 1.
1.
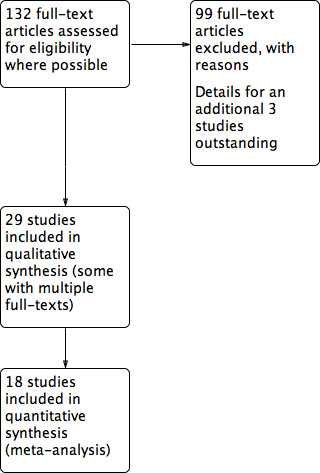
Study flow diagram.
Risk of bias in included studies
See Figure 2 for the `Risk of bias' summary: we judged 14 trials as being at high risk of bias for one or more domain (Bennett 2013; Cosker 2005; De Win 1998; Dickinson Jennings 2015; Holm 1998; Kriegar 2011; Moshakis 1984; Ozaki 2015; Persson 1995; Phan 1993; Ravnskog 2011; Vogt 2007; Wikblad 1995; Wynne 2004). We deemed one trial to be at low risk of bias (Ruiz‐Tovar 2015).
2.
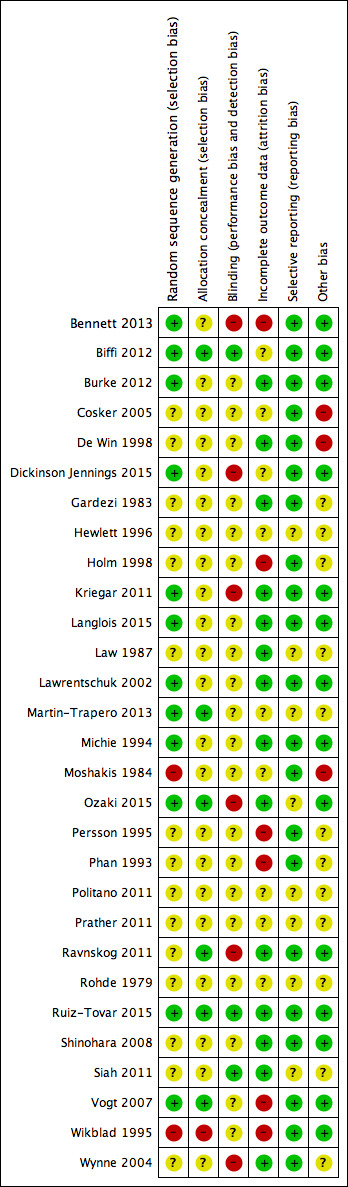
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. Basic wound contact dressing compared with exposed wound.
| Basic wound contact dressing compared with exposed wound | ||||||
| Patient or population: surgical wounds healing by primary intention Setting: postsurgical Intervention: exposed wounds Comparison: basic wound contact dressing | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact dressing | Risk with exposed wound | |||||
| SSI
Assessment method: clinical features of infection Follow‐up: 20 days (for other surgery, not reported for clean surgery) |
CLEAN SURGERY | |||||
| 51 per 1000 | 19 per 1000 (2 to 176) |
RR 0.37 (0.04 to 3.46) |
112 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1, 2 | It is uncertain whether leaving wounds exposed following clean surgery increases or reduces the risk of SSI compared with use of a basic wound contact dressing, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 32 fewer SSIs per 1000 with exposed wounds (49 fewer to 125 more) | ||||||
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| 206 per 1000 | 276 per 1000 (173 to 451) |
RR 1.34 (0.82 TO 2.19) |
207 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 3 4 | It is uncertain whether leaving wounds exposed reduces or increases the risk of SSI compared with use of a basic wound contact dressing following potentially contaminated surgery, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 70 more SSIs per 1000 with exposed wounds (33 fewer to 245 more) | ||||||
| Scarring (further information not reported) |
CLEAN SURGERY | |||||
| Not estimable | Not estimable | One study reported that there was no difference in quality of final scar between the exposed group and the basic wound contact‐dressed group, but no data were presented, nor was any information provided regarding who measured this outcome, how it was measured, or how long after surgery. | 112 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 5, 6 | It is uncertain whether there is any difference in scarring after leaving wounds exposed compared with use of basic wound contact dressings, as the certainty of the evidence has been assessed as very low. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Acceptability of dressing to participant Clean surgery Assessment method: VAS Follow‐up: not reported Unclear for other surgery |
CLEAN SURGERY | |||||
| Not estimable | Not estimable | One study reported no difference in dressing preference as measured on a linear analogue scale. No further information or data were presented. | 112 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 5 6 | It is uncertain whether leaving wounds exposed following clean surgery is more or less acceptable to patients compared with use of a basic wound contact dressings, as the certainty of the evidence has been assessed as very low. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Ease of dressing removal | CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (exposed wounds) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The study had a small sample size and low number of events: the OIS was not met. 95% CIs were wide ranging from a 96% reduced risk of SSI in the exposed group to a 246% increase risk. Downgraded twice for imprecision.
2 Risk of bias as unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
3 Study classed as being at high risk of bias for one domain. Downgraded once for study limitations.
4 The study had a small sample size and low number of events: OIS was not met. 95% CIs were wide ranging from a 18% reduced risk of SSI in the exposed group to a 119% increase risk. Downgraded twice for imprecision.
5 No data were available to asses this outcome ‐ downgraded twice for imprecision as un/certainty of estimates could not be assessed.
6 Risk of bias unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
Summary of findings 2. Film dressing compared with exposed wound.
| Film dressing compared with exposed wound | ||||||
|
Patient or population: surgical wounds resulting from clean surgery and healing by primary intention
Setting: postsurgical
Intervention: exposed wounds Comparison: film dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with film dressing | Risk with exposed wound | |||||
| SSI
Assessment method: undefined method Follow‐up: mean 20 days |
93 per 1000 | 19 per 1000 (2 to 156) |
RR 0.20 (0.02 to 1.69) |
107 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain whether leaving wounds exposed following clean surgery leads an increase or decrease in risk of SSI compared with use of a film dressing, as the certainty of the evidence has been assessed as very low. |
| Risk difference: 74 fewer SSIs per 1000 with exposed wounds (91 fewer to 64 more) | ||||||
| Scarring (further information not reported) |
Not estimable | Not estimable | One study reported that there was no difference in quality of final scar between the exposed group and the dressed group, but no data were presented, nor was any information provided regarding who measured this outcome, how it was measured, or how long after surgery. | 107 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 2 3 | It is uncertain whether there is any difference in scarring after wound exposure compared with use of film dressings following clean surgery, as the certainty of the evidence has been assessed as very low. |
| Acceptability of dressing to participant assessed with: VAS Follow‐up: not reported |
Not estimable | Not estimable | One study reported no difference in dressing preference as measured on a linear VAS. No further information or data were presented. | 107 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 2 3 | It is uncertain whether leaving wounds exposed is more or less acceptable to patients compared with use of a film dressing following clean surgery, as the certainty of the evidence has been assessed as very low |
| Ease of dressing removal | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (exposed wounds) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The study in this comparison was underpowered with a small sample size and a low number of events: the OIS was not met. 95% CIs were very wide ranging from a 98% reduction in SSI risk to a 69% increased risk for exposed wounds. Downgraded twice for imprecision.
2 Risk of bias as unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
3 No data were available to asses this outcome ‐ downgraded twice for imprecision as un/certainty of estimates could not be assessed.
Summary of findings 3. Silver dressing compared with exposed wound.
| Silver dressing compared with exposed wound | ||||||
|
Patient or population: surgical wounds resulting from surgery at risk of contamination and healing by primary intention
Setting: postsurgical
Intervention: exposed wounds Comparison: silver dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with silver dressing | Risk with exposed wound | |||||
| SSI
Assessment method: CDC definition of SSI Follow‐up: mean 30 days |
96 per 1000 | 771 per 1000 (98 to 1000) |
RR 8.00 (1.02 to 62.55) | 166 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain whether leaving wounds exposed following surgery at risk of contamination leads to an increase or decrease in risk of SSI compared with use of a silver dressing, as the certainty of the evidence has been assessed as very low. |
| Risk difference: 675 more SSIs per 1000 with exposed wounds (2 more to 1000 more) | ||||||
| Scarring | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Acceptability | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Ease of dressing removal | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (exposed wounds) and the relative effect of the intervention (and its 95% CI). CDC: Centers for Disease Control and Prevention; CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio SSI: surgical site infection; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The study in this comparison was underpowered with a small sample size and a low number of events: the OIS was not met. 95% CIs were very wide ranging from a 2% increase in SSI risk to a 525% increased risk for exposed wounds. Downgraded twice for imprecision.
2 Risk of bias as unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
Summary of findings 4. Basic wound contact dressing compared with film dressing.
| Basic wound contact dressing compared with film dressing | ||||||
|
Patient or population: surgical wounds healing by primary intention
Setting: postsurgical Intervention: film dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact dressing | Risk with film dressing | |||||
| SSI
Assessment method: various methods Follow‐up: unclear |
CLEAN SURGERY | |||||
| 34 per 1000 | 46 per 1000 (24 to 87) | RR 1.34 (0.70 to 2.55) | 897
(4 RCTs)* *One of the four included trials had no SSI outcome events |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain whether film dressings reduce or increase the risk of SSI compared with use of basic wound contact dressings following clean surgery, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 12 more SSIs per 1000 with film dressings (10 fewer to 53 more) | ||||||
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Two trials reported SSI data. Due to a lack of information about type of surgery, data were not pooled. One study reported 6/50 participants had an SSI in the basic wound contact group compared with 3/50 in the film‐dressed group. One study, where the level of surgical contamination was unclear, reported 26/46 participants with an SSI in the basic wound contact group compared with 14/44 participants in the film‐dressed group. These data were not pooled. **A third RCT did not collect SSI data. |
190 (2 RCTs)** |
⊕⊝⊝⊝ VERY LOW 3 4 | It is uncertain whether film dressings increase or reduce the risk of SSIs compared with basic wound contact dressings following surgery with potential for contamination, as the certainty of the evidence has been assessed as very low. | |
| Scarring | CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Acceptability of dressing to participant Clean surgery Assessment method:VAS, participants rated dressing acceptability (0 = no trouble and 10 = very troublesome) Follow‐up: (clean surgery) 6‐8 days Follow‐up: unclear for other surgery |
CLEAN SURGERY | |||||
| The mean acceptability score was 4.2 scale units | Mean difference: 2.9 scale units lower (3.59 lower to 2.21 lower) | n/a | 120 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 5 6 | It is uncertain whether film dressings are more or less acceptable to patients than basic wound contact dressings following clean surgery, as the certainty of the evidence has been assessed as very low. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Ease of dressing removal Assessment method: unclear Follow‐up: unclear |
CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| Not estimable | Not estimable | One study reported a proportion figure for ease of dressing removal, but provided no information about how these data were obtained or what the figures mean. The data cannot be interpreted and are not presented. | n/a | n/a | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (basic wound contact dressing) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study weighted at 38% in the meta‐analysis was classed as being at high risk of bias. Downgraded once for study limitations.
2 The total number of participants included in the analysis and the number of SSI events were low: the OIS was not met. The 95% CI intervals were wide ‐ ranging from a possible reduction in risk of SSI in the film group of 30% to an increase risk of SSI in the film group of 155%. Downgraded twice for imprecision.
3 Trial data were imprecise with small sample sizes and wide 95% CIs. Downgraded twice for imprecision.
4 Risk of bias as unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
5 Study was classed as being at high risk of bias for two domains. Downgraded twice for study limitations.
6 Study did not take into account potentially clustered nature of data which could lead to an underestimated standard error. Downgraded once for imprecision.
Summary of findings 5. Basic wound contact dressing compared with hydrocolloid dressing.
| Basic wound contact dressing compared with hydrocolloid dressing | ||||||
|
Patient or population: surgical wounds healing by primary intention Setting: postsurgical Intervention: hydrocolloid dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact | Risk with hydrocolloid dressing | |||||
| SSI
Clean surgery Assessment method: CDC definition of SSI Follow‐up: mean 28 days Other surgery Assessment method: various clinical measures Follow‐up: 83 days but unclear for one of the RCTs |
CLEAN SURGERY | |||||
| 25 per 1000 | 22 per 1000 (7 to 69)** |
RR 0.91 (0.30 to 2.78) |
510 (1 RCT)** **One further trial reported no SSI events and was not included in this presentation of data as it was a split‐site study. One further RCT did not report SSI data. |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain if hydrocolloid dressings increase or reduce the risk of SSI compared with use of basic wound contact dressings following clean surgery, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 3 fewer SSIs per 1000 with hydrocolloid dressings (17 fewer to 44 more) | ||||||
| OTHER SURGERY (WITH POTENTIAL FOR CONTAMINATION) | ||||||
| 80 per 1000 | 46 per 1000 (18 to 120) |
RR 0.57 (0.22 to 1.51) |
268 (3 RCTs) |
⊕⊝⊝⊝ VERY LOW 3, 4 | It is uncertain if hydrocolloid dressings increase or reduce the risk of SSI compared with basic wound contact dressings following potentially contaminated surgery, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 34 fewer SSIs per 1000 with hydrocolloid dressings (62 fewer to 41 more) | ||||||
| Scarring Clean surgery Assessment method: participants assessed different aspects of scarring as either: excellent, good or fair Follow‐up: 4 weeks potentially contaminated surgery Assessment method:measurement of scar width (mm) Follow‐up: 3 months |
CLEAN SURGERY | |||||
| Not estimable | Not estimable | 22/28 (79%) participants reporting on the hydrocolloid dressing rated their scar evenness as excellent compared with 14/28 (50%) reporting on the basic wound contact dressing. P value reported by study authors as 0.008. 22/28 (79%) participants reporting on the hydrocolloid dressing rated their scar colour as excellent compared with 13/28 (46%) reporting on the basic wound contact dressing. P value reported by study authors as 0.004. 21/28 (75%) participants reporting on the hydrocolloid dressing rated their scar suppleness as excellent compared with 15/28 (54%) reporting on the basic wound contact dressing. P value reported by study authors as 0.003. |
28 (1 RCT) |
⊕⊕⊝⊝ LOW 5 | Hydrocolloid dressings may lead to some improvement in cosmetic appearance of scars compared with basic wound contact dressings following clean surgery. | |
| Potentially contaminated surgery | ||||||
| The mean scar width was 2.3 mm | Mean difference 0.1 mm lower (0.91 lower to 0.7 higher) | n/a | 134 (1 RCT)** **One other study reported scar width, but reported no standard deviation or related measure. |
⊕⊕⊝⊝ LOW 6 |
Hydrocolloid dressings may lead to little or no improvement in cosmetic appearance of scars compared with basic wound contact dressings. | |
| Acceptability of dressing to participant Clean surgery Assessment method: participants rated whether they were dissatisfied with the dressing Follow‐up: 4 weeks Unclear for other surgery |
CLEAN SURGERY | |||||
| 189 per 1000 (dissatisfied) | 280 per 1000 (203 to 388) |
RR 1.48 (1.07 to 2.05) | 510 (1 RCT) |
⊕⊕⊝⊝ LOW 7 8 | Hydrocolloid dressings may lead to more dressing dissatisfaction compared with basic wound contact dressings following clean surgery. | |
| Risk difference: 91 more dissatisfied per 1000 with hydrocolloid dressings (13 more to 199 more) | ||||||
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Ease of dressing removal Clean surgery Assessment method: questions regarding dressing removal Follow‐up: mean 4 days No details for potentially contaminated surgery Unclear for potentially contaminated surgery |
CLEAN SURGERY | |||||
| Not estimable | Not estimable | Two studies reported ease of removal. One trial was a split‐site study. Data were not pooled because of this and other inconsistencies. One study reported 5/84 (6%) of respondents classified basic wound contact dressings as difficult to remove, compared with 13/61 (21%) in the hydrocolloid group. The second study reported at 3 days postoperatively that 22/28 (79%) participants reporting on the hydrocolloid dressing noted that the dressing was easy to remove compared with 18/28 (64%) reporting on the basic wound contact dressing. |
173 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW 9 10 11 | It is uncertain whether there are differences between hydrocolloid dressings and basic wound contact dressings in terms of ease of removal following clean surgery, as the certainty of the evidence has been assessed as very low. | |
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDC: Centers for Disease Control and Prevention; CI: Confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study at high risk of bias for outcome assessment. Downgraded once for risk of bias for study limitations.
2 Studies were small with low numbers of SSI events: the OIS was not met. The 95% CIs around the estimate are wide ranging from a 70% reduction in risk of SSI in the hydrocolloid group to a 178% increase. Downgraded twice for imprecision.
3Two studies (weighted 65% in the meta‐analysis) were at high risk of bias. Downgraded twice for risk of bias for study limitations.
4 Studies were small with low numbers of SSI events: the OIS was not met. 95% CIs that ranged from a 78% reduction in SSI risk in the hydrocolloid group to a 51% increase. Downgraded twice for imprecision.
5This was a split‐site study with half of a wound treated with one dressing and half with the other. The authors assessed scar colour, texture and colour on a 3‐point scale; the lack of independence seems to have been considered by authors, but they only present P values (favouring the hydrocolloid dressing). We have not reproduced the analysis, so given the lack of precision data and the small number of wounds in the study, have downgraded twice for imprecision.
6 Whilst the study resulted in precise estimates, data are available from just one study with a relatively small number of participants. Further data from more participants would add to certainty. Downgraded once for imprecision. Scarring can also be assessed in a number of ways with width being just one measure, use of a validated tool would be a more useful for decision making. Downgraded once for indirectness.
7The study had 95% CIs that ranged from a 7% increase risk of dissatisfaction in the hydrocolloid group to 105% increase risk, downgraded once for imprecision.
8 Risk of bias as unclear for sequence generation and allocation concealment. Downgraded once for study limitations.
9One study was classed at high risk of bias in two domains. Downgraded twice in limitations.
10 Studies had small sample sizes. Downgraded once for imprecision.
11Results were inconsistent ‐ the reason for inconsistency is not clear. Downgraded once for inconsistency.
Summary of findings 6. Basic wound contact dressing compared with fibrous‐hydrocolloid dressing.
| Basic wound contact dressing compared with fibrous‐hydrocolloid dressing | ||||||
| Patient or population: surgical wounds resulting from clean surgery and healing by primary intention Setting: postsurgical Intervention: fibrous‐hydrocolloid dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact dressing | Risk with fibrous‐hydrocolloid dressing | |||||
| SSI
Assessment method: signs of infection (redness, tenderness, swelling or exudate) Follow‐up: mean 6 weeks |
49 per 1000 | 63 per 1000 (25 to 162) | RR 1.29 (0.50 to 3.28) | 364
(3 RCTs)* * only 1 trial had SSI events |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain whether fibrous‐hydrocolloid dressings increase or reduce the risk of SSI compared with basic wound contact dressings following clean surgery, as the certainty of the evidence has been assessed as very low. |
| Risk difference: 14 more SSIs per 1000 with fibrous‐hydrocolloid dressings (25 fewer to 112 more) | ||||||
| Scarring | Not estimable | Not estimable | Available data could not be summarised | 80 (1 RCT) |
n/a | It is uncertain whether fibrous‐hydrocolloid dressings increase or reduce the quality of scarring compared with basic wound contact dressings following clean surgery, as the data available could not be analysed. |
| Acceptability of dressing to participant | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Ease of dressing removal | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study with outcome data at high risk of bias for outcome assessment was the only study providing data in this analysis. Downgraded once for risk of bias for study limitations
2 The studies were small and the number of SSI events low: the OIS was not met. The 95% CIs around the estimate are wide ranging from a 50% reduction in risk of SSI in the hydrocolloid group to a 228% increase. Downgraded twice for imprecision.
Summary of findings 7. Basic wound contact dressing compared with polyurethane matrix hydrocolloid dressings.
| Basic wound contact dressing compared with polyurethane matrix hydrocolloid dressing | ||||||
| Patient or population: surgical wounds resulting from clean surgery and healing by primary intention Setting: postsurgical Intervention: polyurethane matrix hydrocolloid dressings Comparison: basic wound contact dressing | ||||||
| Outcomes |
Anticipated absolute effects (95% CI)* |
Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact dressing | Risk with polyurethane matrix hydrocolloid dressing | |||||
| SSI | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. Secondary outcomes only were assessed. |
| Scarring | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Acceptability of dressing to participant | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Ease of dressing removal Assessment method: asked whether dressings were difficult to remove (yes) Follow‐up: 5 days |
60 per 1000 | 750 per 1000 (317 to 1000) |
RR 12.60 5.32 to 29.85 |
173 (only reported data for144 here) (1 RCT) |
⊕⊝⊝⊝ VERY LOW1, 2 | It is uncertain whether there are differences between matrix‐hydrocolloid dressings and basic wound contact dressings in terms of ease of removal, as the certainty of the evidence has been assessed as very low.. |
| Risk difference: 690 more difficult to remove per 1000 with matrix hydrocolloid dressings (261 more to 1000 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n/a: not applicable; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The study was classed being at high risk of bias in two domains. Downgraded twice for study limitations.
2 The study had a small sample size and very wide 95% CIs. Downgraded twice for imprecision.
Summary of findings 8. Basic wound contact dressing compared with silver dressing.
| Basic wound contact dressing compared with silver dressing | ||||||
|
Patient or population: surgical wounds resulting from a range of surgical procedures with some risk of contamination healing by primary intention Setting: postsurgical Intervention: silver dressing Comparison: basic wound contact dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact | Risk with silver dressing | |||||
| SSI potentially contaminated surgery SSI Assessment method: various clinical measures Mean follow‐up: 30 days Clean surgery Follow‐up unclear |
CLEAN SURGERY | |||||
| 253 per 1000 | 357 per 1000 (218 to 588) |
RR 1.11 (0.47 to 2.62) | 496 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW 1 2 | It is uncertain whether silver‐containing dressings increase or decrease the risk of SSI compared with basic wound contract dressings following clean surgery, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 104 more SSIs per 1000 with silver dressings (35 fewer to 334 more) | ||||||
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| 86 per 1000 | 71 per 1000 (44 to 118) | RR 0.83 (0.51 to 1.37) | 1353 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | It is uncertain if silver‐containing dressings increase or reduce the risk of SSI compared with basic wound contact dressings, as the certainty of the evidence has been assessed as very low. | |
| Risk difference: 15 fewer SSIs per 1000 with silver dressings (42 fewer to 32 more) | ||||||
| Scarring | CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Acceptability of dressing to participant | CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| Ease of dressing removal | CLEAN SURGERY | |||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| POTENTIALLY CONTAMINATED SURGERY | ||||||
| Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (basic wound contact dressing) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The studies were small and number of SSI events low: the OIS was not met. The 95% CIs ranged from a 53% reduction in risk of SSI in the silver‐treated group to an increased risk of 162%. Downgraded twice for imprecision.
2 Risk of bias as unclear for sequence generation and allocation concealment in one study and high risk of bias for blinded outcome assessment in second study. Downgraded once or study limitations.
3 Two studies which together contributed 53% of weight to the pooled analysis were classed as being at high risk of bias for two domains. Downgraded twice for risk of bias for study limitations.
4 The OIS was not met. The 95% CIs ranged from a 49% reduction in risk of SSI in the silver treated group to an increased risk of 37%. There number of SSI events was also low. Downgraded twice for imprecision.
Summary of findings 9. Basic wound contact dressing compared with non‐silver antimicrobial dressings.
| Basic wound contact dressing compared with non‐silver antimicrobial dressing | ||||||
| Patient or population: surgical wounds resulting from clean surgery and healing by primary intention Setting: postsurgical Intervention: polyhexametylene biguanide antimicrobial dressings Comparison: basic wound contact dressings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic wound contact dressing | Risk with polyhexametylene biguanide anti‐microbial dressing | |||||
| SSI Assessment method: CDC definition of SSI Mean follow‐up: 30 days |
CLEAN SURGERY | |||||
| 50 per 1000 | 11 per 1000 (1 to 88) | RR 0.21 (0.03 to 1.77) | 197 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | It is not clear whether polyhexametylene biguanide antimicrobial dressings reduce SSI risk in postsurgical wounds following clean surgery compared with basic wound contact dressings; the 95% CIs include clinical benefit and harms. | |
| Risk difference: 39 fewer SSIs per 1000 with antimicrobial dressings (49 fewer to 38 more) | ||||||
| Scarring | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Acceptability of dressing to participant | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| Ease of dressing removal | Not estimable | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDC: Centers for Disease Control and Prevention; CI: confidence interval; n/a: not applicable; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio; SSI: surgical site infection | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The study was small and number of SSI events low: the OIS was not met. The 95% CIs ranged from a 97% reduction in risk of SSI in the anti‐microbial treated group to an increased risk of 77%. Downgraded twice for imprecision.
A summary of all extracted trial data can be found in Table 12
Wound dressings compared with exposed wounds (no dressing)
Comparison 1: Basic wound contact dressings compared with exposed wound (no dressing) (2 trials; 319 participants)
Two trials, involving a total of 319 participants, compared wound exposure with basic wound contact dressings. Law 1987 conducted a three‐arm trial where the surgical procedure had a 'clean' contamination classification (112 participants). The trial compared a basic wound contact dressing (removed after five days and changed if wound was discharging), with exposed wounds. Phan 1993 undertook a surgical procedure with clean and 'clean/contaminated' contamination classification and compared a basic wound contact dressing (changed twice daily) with exposed wounds that were treated with petroleum jelly (Vaseline).
Primary outcome: SSI
Clean surgery
Law 1987: it is uncertain whether there is a difference in SSI risk between basic wound contact‐dressed wounds (3/59; 5%) and exposed wounds (1/53; 2%) (RR for developing SSI in the exposed group compared with the basic wound contact = 0.37, 95% CI 0.04 to 3.46; Analysis 1.1;very low certainty evidence downgraded once for risk of bias and twice for imprecision,Table 1).
1.1. Analysis.
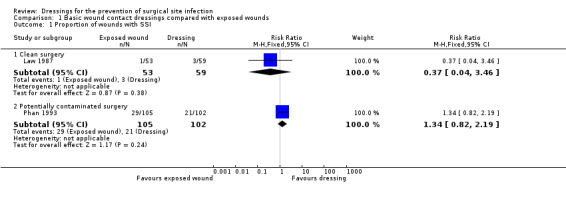
Comparison 1 Basic wound contact dressings compared with exposed wounds, Outcome 1 Proportion of wounds with SSI.
Potentially contaminated surgery Phan 1993: it is uncertain whether there is a difference in SSIs risk between basic wound contact‐dressed wounds (21/102; 21%) and exposed wounds (29/105; 28%) (RR for developing an SSI in the exposed group compared with the basic wound contact group = 1.34; 95% CI 0.82 to 2.19; Analysis 1.1;very low certainty evidence downgraded once for risk of bias and twice for imprecision,Table 1).
Secondary outcomes
Clean surgery: scarring
Law 1987 reported that there was no difference in quality of final scar between the exposed group and the basic wound contact‐dressed group, but did not present data or was any information regarding who measured this outcome, how it was measured, or how long after surgery. The effect of these interventions on scarring is uncertain (very low certainty evidence downgraded once for risk of bias and twice for imprecision, Table 1).
Clean surgery: acceptability
The trial reported no difference in dressing preference as measured on a linear analogue scale, and presented no further information or data. The effect of these interventions on acceptability is uncertain (very low certainty evidence downgraded once for risk of bias and twice for imprecision, Table 1).
Clean surgery: costs
Law 1987 reported that the mean total dressing costs per participant for the basic wound contact‐dressed group were GBP 6.60 compared with GBP 0.80 in the exposed group. No detailed information was presented i.e. the cost of complications, duration of stay in hospital and nurse time. The cost of dressing data alone is of limited value to decision makers.
Potentially contaminated surgery Phan 1993 did not present data on secondary outcomes.
Summary: Basic wound contact dressings compared with exposed wounds
It is uncertain whether leaving surgical wounds exposed (no dressing) when healing by primary intention increases or decreases SSI risk compared with use of a basic wound contact dressing following clean surgery or surgery with the potential for contamination; we assessed the certainty of the evidence as very low (downgraded once for risk of bias and twice for imprecision,Table 1).
The effect of these interventions on scarring and acceptability of dressings to patients is also uncertain as the certainty of evidence has been assessed as very low (downgraded once for risk of bias and twice for imprecision). The use of dressings incurs additional unit costs, but there are no cost‐effectiveness data available from these studies to facilitate informed decision making.
Comparison 2: Film dressings compared with exposed wounds (1 trial; 107 participants)
Law 1987 compared an exposed wound with a film‐dressed wound in 107 participants in clean surgery.
Primary outcome: SSI
Clean surgery
Law 1987: it is uncertain whether leaving surgical wounds exposed (1/53; 2%) leads to an increase or decrease in SSI risk compared with film‐dressed wounds (5/54; 9%) (RR 0.20, 95% CI 0.02 to 1.69; Analysis 2.1; very low certainty evidence, downgraded once for risk of bias and twice for imprecision,Table 2).
2.1. Analysis.
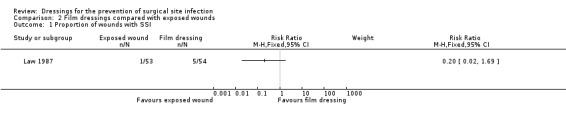
Comparison 2 Film dressings compared with exposed wounds, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
Clean surgery: scarring
Law 1987 reported that there was no difference in quality of final scar between the exposed group and the film group, but presented no data or any information regarding who measured this outcome, how it was measured, or how long after surgery. The effect of these interventions on scarring is uncertain (very low certainty evidence downgraded once for risk of bias and twice for imprecision, Table 2).
Clean surgery: acceptability
The trial reported no difference in dressing preference as measured on a linear analogue scale. No further information or data were presented. The effect of these interventions on acceptability is uncertain (very low certainty evidence downgraded once for risk of bias and twice for imprecision, Table 2).
Clean surgery: costs
Law 1987: total mean dressing costs per participant for the film group were GBP 42.00 compared with GBP 0.80 in the exposed group.
Summary: Film dressings compared with exposed wound
It is uncertain whether leaving surgical wounds to heal by primary intention exposed (no dressing) following clean surgery increases or reduces SSI risk compared with use of a film dressing; we assessed the certainty of the evidence as very low (downgraded once for risk of bias and twice for imprecision,Table 2). One trial reported that film dressings were more costly than leaving wounds exposed, but there are no cost‐effectiveness data available from the trial to facilitate informed decision making.
Comparison 3: Silver dressings compared with exposed wounds (1 trial; 166 participants)
Siah 2011 compared a silver dressing with wound exposure in 166 participants undergoing various types of elective colorectal surgery which were classed by review authors as clean/contaminated.
Primary outcome: SSI
Potentially contaminated surgery
Siah 2011: it is unclear whether leaving a surgical wound exposed (8/83; 10%) leads to an increase or a decrease in SSI risk compared with a silver‐dressed wound (1/83; 1%). RR: 8.00, 95% 1.02 to 62.55 (Analysis 3.1; very low certainty evidence downgraded once for risk of bias and twice for imprecision,Table 3).
3.1. Analysis.
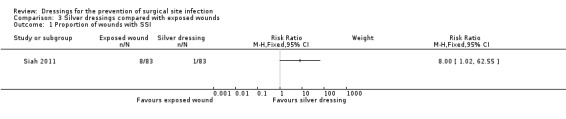
Comparison 3 Silver dressings compared with exposed wounds, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
Siah 2011 reported no relevant secondary outcomes.
Summary: Silver dressings compared with exposed wound
It is uncertain whether leaving surgical wounds that are healing by primary intention exposed (no dressing) following surgery at risk of contamination increases or reduces SSI risk compared with use of a silver dressing; we assessed the certainty of the evidence as very low (downgraded once for risk of bias and twice for imprecision,Table 3).
Dressings compared with other types of dressings
Comparison 4: Comparisons between different basic wound contact dressings (1 trial; 50 participants)
Lawrentschuk 2002 undertook a surgical procedure with a 'clean' contamination classification and compared a paraffin tulle dressing with a non‐adherent dressing in 50 participants (25 in each arm). Both dressing types were applied in the same way. In both groups a compressible, combined dressing was placed over the evaluated dressings with an adhesive elastic dressing then placed over these.
Primary outcome: SSI
Clean surgery
Lawrentschuk 2002: There was no clear difference in SSI risk between paraffin tulle‐dressing (0/25; 0%) compared with the non‐adherent dressings (3/25; 12%: RR 0.14, 95% CI 0.01 to 2.63; Analysis 4.1; low certainty evidence ‐ downgraded twice for imprecision); the 95% CI are wide and include both clinical benefit (in terms of reduced SSI risk) and harm (in terms of increased SSI risk).
4.1. Analysis.
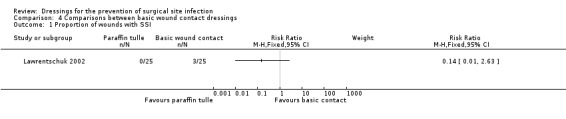
Comparison 4 Comparisons between basic wound contact dressings, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
Lawrentschuk 2002 reported no relevant secondary outcomes.
Summary: Basic wound contact dressings compared with different basic wound contact dressings
It is not clear whether paraffin tulle dressings reduce the risk of SSI events in surgical wounds healing by primary intention following clean surgery compared with use of a non‐adherent dressing; the 95% CI are wide and include both clinical benefit (in terms of reduced SSI risk) and harm (in terms of increased SSI risk) (low certainty evidence; downgraded twice for imprecision).
Comparison 5: Basic wound contact dressings compared with film dressings (8 trials; 1087 participants)
Eight trials compared a basic wound contact dressing with a film dressing. Five of these trials evaluated wounds resulting from 'clean' surgical procedures (Cosker 2005; De Win 1998; Law 1987; Moshakis 1984; Wynne 2004), and three evaluated wounds resulting from surgical procedures with mixed, or unclear, contamination classifications (Gardezi 1983; Hewlett 1996; Rohde 1979). The trials included a variety of basic wound contact dressings including gauze and surgical absorbents. Similarly, whilst the comparators were all film dressings, different brands were evaluated (five trials evaluated Opsite (Smith & Nephew), three Tegaderm (3M Healthcare), and one an unnamed brand (Cosker 2005 evaluated two film dressings).
Primary outcome: SSI
Clean surgery
Using a fixed‐effect model we pooled data from the four trials with participants who had 'clean' surgery and that reported SSI data (Cosker 2005; De Win 1998; Law 1987; Wynne 2004) (Analysis 5.1). One further trial, Moshakis 1984 did not report SSI data. Whilst Law 1987 and Wynne 2004 were three‐arm trials, this was the only meta‐analysis conducted with their data, so the complete groups relevant to this pooling were used. De Win 1998 reported zero SSI events. There is uncertain evidence on the risk of SSI between basic wound contact‐dressed wounds and film‐dressed wounds (RR 1.34; 95% CI 0.70 to 2.55; Analysis 5.1; very low certainty evidence downgraded once for risk of bias and twice for imprecision,Table 4).
5.1. Analysis.
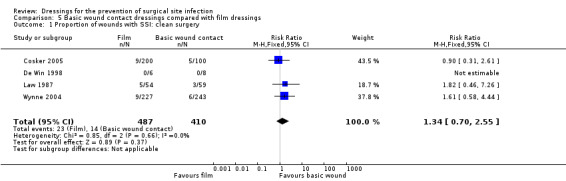
Comparison 5 Basic wound contact dressings compared with film dressings, Outcome 1 Proportion of wounds with SSI: clean surgery.
Potentially contaminated surgery
Gardezi 1983 conducted several surgical procedures that were classified as clean, clean/contaminated and possibly contaminated. There was no clear evidence of a difference in the risk of SSI in the basic wound contact‐dressed group (6/50; 12%) compared with the film‐dressed group (3/50; 6%) (RR 0.50 95% CI 0.13 to 1.89; Analysis 5.2; very low certainty evidence downgraded once for risk of bias and twice for imprecision;Table 4).
5.2. Analysis.
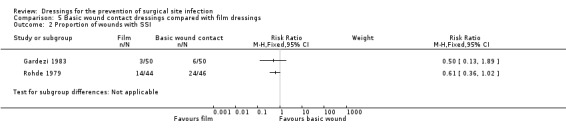
Comparison 5 Basic wound contact dressings compared with film dressings, Outcome 2 Proportion of wounds with SSI.
Hewlett 1996: did not report SSI data.
We could not be sure of the surgical classification of one further trial Rohde 1979. In total, 24/46 (52%) of participants in the basic wound contact dressing group had a mild wound infection compared with 14/44 (32%) in the film‐dressed group (RR 0.61; 95% CI 0.36 to 1.02 in favour of the film dressing. Given the difficulty in classifying the type of surgical procedure(s) undertaken in Rohde 1979, we did not pool this trial with Gardezi 1983. Overall it is unclear whether there is a difference in the risk of SSI in surgeries with different levels of potential contamination (very low certainty evidence downgraded once for risk of bias and twice for imprecision;Table 4).
Secondary outcomes
Clean surgery: pain
Moshakis 1984: reported the levels of pain in each group. This was measured using a patient‐assessed linear scale (1 to 10) where 1 corresponded to 'no discomfort' and 10 to 'extremely uncomfortable or painful'. Mean pain levels in the basic wound contact group were 5.1 (SD 2.78) compared with 1.6 (SD 1.48) in the film‐dressed group, favouring film dressings: mean difference (MD ‐3.50; 95% CI ‐4.29 to ‐2.71; Analysis 5.3). We deemed this trial to be at high risk of bias for two domains and it did not take into account the cluster nature of data. Evidence is of very low certainty and all analyses in this trial must be interpreted with caution.
5.3. Analysis.
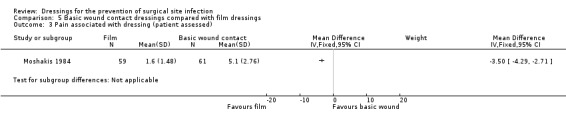
Comparison 5 Basic wound contact dressings compared with film dressings, Outcome 3 Pain associated with dressing (patient assessed).
Clean surgery: acceptability
Moshakis 1984: participants and treating nurses were asked to rate their acceptability of the dressings, which were measured using a linear scale where 1 corresponded to 'no trouble' and 10 to 'very troublesome'. The mean response of basic wound contact‐dressed participants was 4.2 (SD 2.46) and the mean response of the film‐dressed group was 1.3 (SD 1.17; (MD ‐2.90; 95% CI ‐3.59 to ‐2.21, favouring the film‐dressed group; Analysis 5.4;very low certainty evidence downgraded twice for risk of bias and once for imprecision;Table 4).
5.4. Analysis.
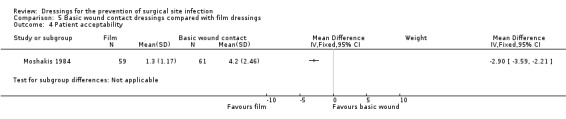
Comparison 5 Basic wound contact dressings compared with film dressings, Outcome 4 Patient acceptability.
The mean acceptability response of the treating nurse was 5.4 (SD unknown) in the basic wound contact group and 1.2 (SD unknown) in the film group.
Clean surgery: costs
De Win 1998: reported the total mean cost of dressings per participant in Belgian francs (now replaced by the Euro (EUR)): BEL 11.5 in the basic wound contact‐dressed group and BEL 14.3 in the film‐dressed group. No further economic data were presented in this trial. Law 1987 reported the mean per participant cost of the dressings in each group: GBP 6.60 in the basic wound contact group and GBP 42.00 in the film group.
Potentially contaminated surgery: ease of removal
Rohde 1979: also reported figures for ease of dressing removal, but as there was no information about how these data were obtained or what they meant, we cannot interpret them (Table 12).
Potentially contaminated surgery: pain
Gardezi 1983: reported a measure for pain in each group, no details were provided regarding collection of these data or how they could be interpreted (Table 12).
Potentially contaminated surgery: costs
Hewlett 1996: reported the mean per participant cost of dressings (including and excluding procedure pack) as GBP 1.60 for the basic wound contact‐dressed group compared with GBP 1.46 for the film‐dressed group or GBP 4.36 compared with GBP 2.84 when procedure packs were included. Rohde 1979 reported the cost per participant in Deutsch marks (now replaced by EUR) as DEM 10.40 in the basic wound contact‐dressed group and DEM 3.60 in the film group.
Summary: Basic wound contact dressings compared with film dressings
It is uncertain whether covering surgical wounds that are healing by primary intention with a film dressing increases or decreases the risk of SSI compared with use of a basic wound contact dressing following clean surgery or following surgery with other (or uncertain) contamination levels; we assessed the certainty of the evidence as very low (downgraded once for risk of bias and twice for imprecision,Table 4).
It is uncertain whether people with wounds treated with film dressings reported better or worse acceptability compared with basic wound contact dressings (very low certainty evidence downgraded twice for risk of bias and once for imprecision;Table 4). The cost data presented were too limited to allow us to draw any conclusions based on costs versus benefits.
Comparison 6: Basic contract wound dressings compared with hydrocolloid dressings (6 trials; 792 participants)
Six trials investigated the effect of a basic wound contact dressing compared with a hydrocolloid dressing (Holm 1998; Michie 1994; Persson 1995; Shinohara 2008; Wikblad 1995; Wynne 2004). The basic wound contact dressings were predominantly gauze.
Primary outcome: SSI
Clean surgery
Michie 1994: none of the 28 wound halves randomised to the basic wound contact dressing or the hydrocolloid dressing developed an SSI.
Wikblad 1995 presented no clear SSI data. The authors reported that 11 participants were treated with antibiotics postoperatively, and eight of these participants had infections in the sternum (five of these participants were in the basic wound contact dressing group). No further information was provided. We classed this trial as being at a high risk of bias due to a large amount of missing data.
Wynne 2004: it is uncertain whether there is a difference in SSI risk between hydrocolloid‐dressed (6/267; 2%) and basic wound contact‐dressed wounds (6/243; 3%) (RR 0.91; 95% CI 0.30 to 2.78; Analysis 6.1; very low certainty evidence downgraded once for risk of bias and twice for imprecision;Table 5).
6.1. Analysis.
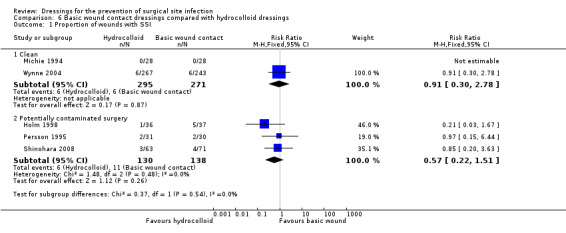
Comparison 6 Basic wound contact dressings compared with hydrocolloid dressings, Outcome 1 Proportion of wounds with SSI.
Potentially contaminated surgery
We pooled data from all trials in this comparison that presented SSI data (Holm 1998; Persson 1995; Shinohara 2008). It is uncertain whether there is a difference in SSI risk between hydrocolloid‐dressed and basic wound contact‐dressed wounds: (RR 0.57; 95% CI 0.22 to 1.51; I² = 0%; Analysis 6.1; very low certainty evidence downgraded twice for risk of bias and twice for imprecision;Table 5).
Secondary outcomes
Clean surgery: scarring
Michie 1994: participants were asked to assess different aspects of scarring as either: excellent, good or fair. This was a split‐site trial with halves of the same wound randomised to different dressings.
Four weeks postoperatively:
22/28 (79%) of participants reporting on the hydrocolloid dressing rated their scar evenness as excellent compared with 14/28 (50%) reporting on the basic wound contact dressing. P value reported by trial authors as 0.008.
22/28 (79%) of participants reporting on the hydrocolloid dressing rated their scar colour as excellent compared with 13/28 (46%) reporting on the basic wound contact dressing. P value reported by trial authors as 0.004.
21/28 (75%) of participants reporting on the hydrocolloid dressing rated their scar suppleness as excellent compared with 15/28 (54%) reporting on the basic wound contact dressing. P value reported by trial authors as 0.003.
Data for these outcomes were also extracted at a seven‐month visit but are not presented here, as there were more missing data at this later time point. Further scarring assessments by investigators are reported in Table 12
Hydrocolloid dressings may lead to some improvement in cosmetic appearance of scars compared with basic wound contact dressings, but these data were low certainty evidence downgraded twice for imprecision (Table 5).
Clean surgery: pain
Wikblad 1995 reported pain at dressing removal; 76% of participants (raw data calculated by review author as 64/84) in the basic wound contact group reported no pain on removal, compared with 61% (calculated as 37/61) in the hydrocolloid group: RR 0.80; 95% CI 0.63 to 1.01 (Analysis 6.2). However, a large number of participants were missing from this trial, which we classed as being at high risk of bias.
6.2. Analysis.
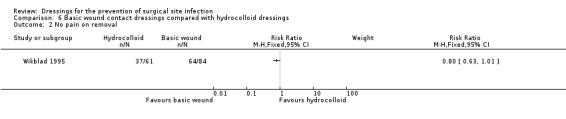
Comparison 6 Basic wound contact dressings compared with hydrocolloid dressings, Outcome 2 No pain on removal.
Clean surgery: acceptability
Wynne 2004: in the basic wound contact group 46/243 (19%) of participants reported that they were dissatisfied with the dressing compared with 75/267 (28%) in the hydrocolloid group (RR 1.48; 95% CI 1.07 to 2.05). It is not possible to tell how this dissatisfaction was influenced by the short time for which the basic wound contact dressing was in situ. It was unclear if participants were blinded to treatment. Hydrocolloid dressings may lead to more dressing dissatisfaction compared with basic wound contact dressings, but these data were low certainty evidence, downgraded once risk of bias and once for imprecision, Table 5).
Clean surgery: ease of removal
Wikblad 1995 reported at five days postoperatively that 5/84 (6%) of respondents (clinicians) classified basic wound contact dressings as difficult to remove, compared with 13/61 (21%) in the hydrocolloid group (RR 3.58, 95% CI 1.35 to 9.51).
Michie 1994 reported at three days postoperatively that 22/28 (79%) participants reported that the hydrocolloid dressing was easy to remove compared with 18/28 (64%) who reported that the basic wound contact dressing was easy to remove. This was a split‐site randomised trial.
It is uncertain whether there are differences between hydrocolloid dressings and basic wound contact dressings in terms of ease of removal as the certainty of the evidence has been assessed as very low (downgraded twice for risk of bias, once for imprecision and once for inconsistency;Table 5).
Clean surgery: costs
Wikblad 1995 reported the mean dressing cost per participant at USD 0.73 in the basic wound contact dressing group, and USD 3.60 in the hydrocolloid group.
Wynne 2004 reported the median cost per participant of the basic wound contact dressing group as AUD 0.52, compared with AUD 3.93 for the hydrocolloid group. Again this value included only the cost of the dressings themselves, and not other important measures of resource‐use that should be considered when using cost as a decision tool, i.e. amount of nurse time, and cost of complications.
Potentially contaminated surgery: scarring
Shinohara 2008: The mean scar width for both groups was very similar; 2.3 mm (standard deviation 2.4 mm) in the basic wound contact group compared with 2.2 mm (standard deviation 2.4 mm) in the hydrocolloid group (mean difference ‐0.10, 95% CI ‐0.91 to 0.70). We judged the data to provide low certainty evidence, downgraded once for imprecision and once for indirectness (Table 5).
Holm 1998 also reported the mean width of scars as 2.26 mm (range 1 mm to 5 mm) in the basic wound contact group and 1.78 mm (range 1 mm to 3 mm) in the hydrocolloid group (no standard deviation or related data presented) (Table 5).
Potentially contaminated surgery: pain
Persson 1995 reported participants' perceived pain associated with the wound, measured on a visual analogue scale (VAS) (0 to 100 mm) where a higher score indicted worse pain. The mean score for the basic wound contact group was 40 mm compared with 32 mm in the hydrocolloid group (no standard deviation data presented). We cannot interpret the data further.
Potentially contaminated surgery: costs
Shinohara 2008: reported the mean cost of dressings per patient in the basic wound contact group (in Japanese Yen) as JPY 780, compared with JPY 715 in the hydrocolloid group.
Summary: Basic wound contact dressings compared with hydrocolloid dressings
It is uncertain whether covering surgical wounds healing by primary intention with a hydrocolloid dressing increases or decreases the risk of SSI compared with a basic wound contact dressing following clean surgery (very low certainty evidence, downgraded for once risk of bias and twice for imprecision) or following surgery with other contamination levels (very low certainty evidence downgraded twice for risk of bias and twice for imprecision) (Table 5).
There was some low certainty evidence that hydrocolloid dressings may lead to some improvement in cosmetic appearance of scarring following clean surgery and other surgery types. Conversley there was low certainty evidence that hydrocolloid dressings may lead to more dissatisfaction with the dressing than basic wound contact dressings. It is uncertain whether there are differences between the dressings in terms of ease of dressing removal, as we assessed the certainty of the evidence as very low, (Table 5).
Wikblad 1995 report that a basic wound contact dressing was less painful at removal than a hydrocolloid dressing, but the analysis had a large amount of missing data and we judged it to be at high risk of bias, as well as being imprecise: we assessed the evidence from this trial as being of very low certainty.
Comparison 7: Basic wound contact dressings compared with fibrous‐hydrocolloid (hydrofibre) dressings (3 trials; 364 participants)
Two trials compared a basic wound contact dressing with a hydrofibre dressing (Burke 2012; Vogt 2007). Vogt 2007 randomised 160 participants undergoing elective vascular surgery to either an absorbant dressing or a hydrofibre dressing, while Burke 2012 randomised 124 participants undergoing hip or knee replacement to either an absorbent or a Jubilee dressing. The Jubilee dressing was described as having a hydrofibre inner layer and hydrocolloid outer layer. We considered the hydrofibre layer to be the contact dressing. Langlois 2015 randomised 80 participants undergoing hip or knee replacement to receive a gauze dressing or a hydrofibre dressing.
Primary outcome: SSI
Clean surgery
We included all three studies in this analysis (Burke 2012; Langlois 2015; Vogt 2007). For Vogt 2007 we have used the results of an intention‐to‐treat analysis including withdrawals in the denominator only (did not have an SSI) as our methodology stipulated. We have also presented the raw data reported for reference purposes (Analysis 7.2).
7.2. Analysis.
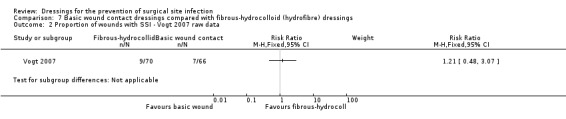
Comparison 7 Basic wound contact dressings compared with fibrous‐hydrocolloid (hydrofibre) dressings, Outcome 2 Proportion of wounds with SSI ‐ Vogt 2007 raw data.
We pooled data from the studies: there was a total of 364 participants, but only one trial had outcome events (Vogt 2007), so the results are driven by this. It is uncertain whether covering surgical wounds that are healing by primary intention with a fibrous hydrocolloid dressing increases or decreases risk of SSI compared with a basic wound dressing following clean surgery, (RR 1.29; 95% CI 0.50 to 3.28; Analysis 7.1; very low certainty evidence downgraded once due to risk of bias and twice due to imprecision;Table 6).
7.1. Analysis.
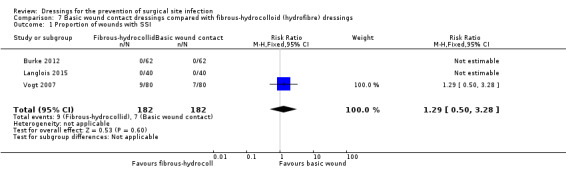
Comparison 7 Basic wound contact dressings compared with fibrous‐hydrocolloid (hydrofibre) dressings, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
Clean surgery: scarring
Langlois 2015 measured patient satisfaction with appearance of scar in three ways. One was using a VAS for which it was not clear whether a low or high score was better. We have not reported this here. The trial also reported data on a categorical scale (poor, acceptable or excellent) and results of the Stoney Brook scale (see Table 12 for these data). Data are reported as medians with standard deviations (usually used to summarise mean data). Since data have not been presented using the mean or categories we have not analysed them further.
Clean surgery: pain
Langlois 2015 assessed by patients and nurses using a four‐point scale where 1 was `not satisfied'; 2 was `fairly satisfied'; 3 was `satisfied'; and 4 was `highly satisfied' ‐ see Table 12. The data were presented using medians and standard deviations, which means that further analyses within the review are not possible.
Clean surgery: costs
Vogt 2007: while this trial reported the mean cost per participant (which included dressings, nurse time and other equipment, such as gloves), no further information was provided. The per participant cost of the basic wound contact group was reported in Euros as a range spanning EUR 10 to EUR 11.8 compared with EUR 20.3 to EUR 48.7 for the fibrous‐hydrocolloid group.
Burke 2012 reported the mean number of dressing changes for each group, with more participants in the hydrofibre group requiring only one dressing change 61% (38/62), and fewer requiring two dressing changes (31%; 19/62), or three or more dressing changes (8%; 5/62) when compared with the absorbent dressing arm where 56% (35/63) of participants required two dressing changes and 31% (19/62) required three or more dressing changes.
Summary: Basic wound contact dressings compared with fibrous‐hydrocolloid (hydrofibre) dressings
It is uncertain whether covering surgical wounds that are healing by primary intention with a fibrous hydrocolloid dressing increases or decreases risk of SSI compared with a basic wound dressing following clean surgery; we assessed the certainty of the evidence as very low (downgraded once due to risk of bias and twice due to imprecision; Table 6). The cost of fibrous‐hydrocolloid dressings was higher than the cost of basic wound contact dressings, but they may require changing less often.
Comparison 8: Basic wound contact dressings compared with polyurethane matrix hydrocolloid dressings (1 trial; 144 participants (estimated))
Wikblad 1995 was a three‐arm trial, presented in comparison 6. It investigated the effect of a basic wound contact dressing compared with a polyurethane matrix hydrocolloid dressing after heart surgery.
Primary outcome: SSI
Clean surgery
Wikblad 1995 reported no interpretable data for SSI. The authors reported that 11 participants were treated with antibiotics postoperatively, and eight of these had infections in the sternum (of which five were in the basic wound contact dressing group). No further information was provided and the outcome was considered to be unreported (Table 7).
Secondary outcomes
Clean surgery: ease of removal
Wikblad 1995: at five days postoperatively 5/84 (6%) of respondents (clinicians) reported that the basic wound contact dressings were difficult to remove, compared with 45/60 (75%) in the hydrocolloid group (RR 12.60, 95% CI 5.32 to 29.85 (no analysis presented); very low certainty evidence downgraded twice for risk of bias and once for imprecision; Table 7).
Clean surgery: pain
Wikblad 1995 reported pain at dressing removal and ease of removal; 76% of participants (calculated by review author as 64/84) in the basic wound contact dressing group reported no pain on removal, compared with only 14% (calculated by review authors as 8/60) in the hydrocolloid group. Fewer participants in the basic wound contact dressing group reported pain on dressing removal than in the matrix hydrocolloid group (RR 0.17; 95% CI 0.09 to 0.34; Analysis 8.1). A large number of participants were missing from this trial, which we classed as being at a high risk of bias.
8.1. Analysis.
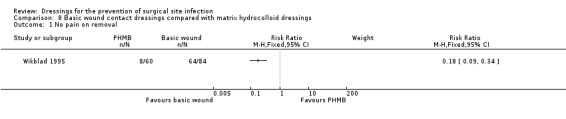
Comparison 8 Basic wound contact dressings compared with matrix hydrocolloid dressings, Outcome 1 No pain on removal.
Clean surgery: costs
Wikblad 1995 reported the mean dressing cost per patient at USD 0.73 for the basic wound contact dressing group and USD 3.34 for the matrix hydrocolloid group.
Summary: Basic wound contact dressings compared with polyurethane matrix hydrocolloid dressings
It is uncertain whether covering surgical wounds that are healing by primary intention with a polyurethane matrix hydrocolloid dressing increases or decreases the risk of SSI compared with a basic wound contact dressing following clean surgery; we assessed the certainty of the evidence as very low. The only trial to contribute data was poorly reported and at high risk of attrition bias with no SSI outcome data that could be used (Table 7). It was uncertain whether the basic wound contact dressing was easier to remove than the hydrocolloid dressing (very low certainty evidence;Table 7). The unit cost of the hydrocolloid dressing was higher than that of the basic wound contact dressing.
Comparison 9: Basic wound contact dressings compared with silver dressings (8 trials; 1959 participants)
Eight studies were considered in this comparison. Two studies included participants undergoing clean surgery (Dickinson Jennings 2015; Politano 2011). Politano 2011 randomised 145 participants to either a basic wound contact dressing or a silver‐containing dressing. Twenty‐five SSIs were reported in the silver dressing group compared with 19 in the standard dressing group. It was not clear from the report whether these events occurred in separate people, but we have assumed this in our treatment of the data here. Dickinson Jennings 2015 was a three‐arm trial that compared a basic wound dressing to two types of silver dressing. For the purpose of the review, we pooled the silver dressing arms.
Six studies compared the use of a basic wound dressing with a silver‐containing dressing in surgery at risk of contamination. Four studies involved colorectal surgery (Biffi 2012; Kriegar 2011; Prather 2011; Ruiz‐Tovar 2015). Bennett 2013 randomised 524 participants who had undergone a caesarean section; these can be clean, clean/contaminated, contaminated or dirty depending on timing of membrane rupture and other operative conditions ‐ data on the contamination level of the operations was not presented and so we classed it as mixed although it is likely that most operations were clean. Ozaki 2015 randomised 500 people undergoing a non‐emergency surgical procedure for peripheral vascular disease.
Primary outcome: SSI
Clean surgery
We pooled SSI data from the two clean surgery studies using a random‐effects model (I² = 34%; Chi² P value = 0.22). Based on the average effect, it is uncertain whether silver dressings increase or reduce the risk of SSI compared with a basic wound dressing (RR 1.11; 0.47 to 2.62; Analysis 9.1; very low certainty evidence downgraded once for risk of bias and twice for imprecision; Table 8).
9.1. Analysis.
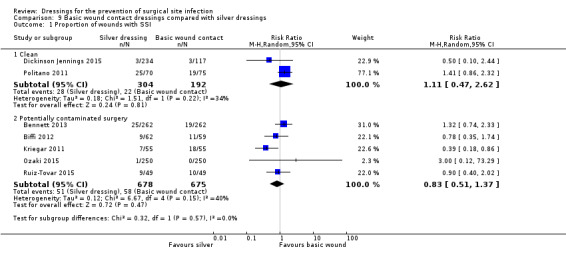
Comparison 9 Basic wound contact dressings compared with silver dressings, Outcome 1 Proportion of wounds with SSI.
Potentially contaminated surgery
We pooled SSI data from the studies where surgery was at risk of contamination using a random‐effects model ( I² = 40%; Chi² P value = 0.15) (Bennett 2013; Biffi 2012; Kriegar 2011; Ozaki 2015; Ruiz‐Tovar 2015). Based on the average effect of silver dressings it is uncertain whether use of silver dressings reduces the risk of SSI after potentially contaminated surgery compared with the basic wound contact dressings (RR 0.83; 95% CI 0.51 to 1.37; Analysis 9.1; very low certainty evidence downgraded twice for risk of bias and twice for imprecision;Table 8).
Secondary outcomes
Clean surgery: pain
Dickinson Jennings 2015 reported pain data for the three trial arms, but no variation data were reported and we have not considered these data further (see Table 12).
Clean surgery: ease of removal
Dickinson Jennings 2015 reported ease of removal data for the two types of silver dressings, but the data were not clear for the comparator group and so are not considered further (see Table 12).
Potentially contaminated surgery: pain
Prather 2011 measured pain using a 0 to 10 scale with 0 being 'no pain' and 10 being 'worst pain'. At baseline the mean pain score in the silver dressing group was 5 and in the gauze group was 5. Subsequent data were presented for each day until day seven when the mean pain score was 4 in the control group and 2 in the silver group. No standard deviation data were presented and no further analysis is presented here.
Potentially contaminated surgery: costs
Bennett 2013 presented the total dressing costs per group. The group total for the basic wound contact group was USD 307 and the total for the silver group was USD 11,080. No standard deviation data were presented and data are not analysed further.
Summary: Basic wound contact dressings compared with silver‐containing dressings
It is uncertain whether covering surgical wounds that are healing by primary intention with a silver‐containing dressing increases or decreases the risk of SSI compared with a basic wound contact dressing following clean surgery (very low certainty evidence downgraded once for risk of bias and twice for imprecision) or following potentially contaminated surgery (very low certainty evidence downgraded twice for risk of bias and twice for imprecision;Table 8). Data for secondary outcomes were very limited.
Comparison 10: Basic wound contact dressing compared with non‐silver antimicrobial dressings (1 trial; 197 participants)
Martin‐Trapero 2013 randomised participants undergoing elective laparoscopic cholecystectomy to a basic wound contact or PHMB antimicrobial dressing.
Primary outcome: SSI
Clean surgery
It is not clear whether there is a difference in SSI risk between the basic wound contact dressing group (5/101; 5%) and the PHMB dressing‐treated group (1/96; 1%), as the 95% CIs are wide and include benefits (in terms of reduced SSI risk) and harms (in terms of increased SSI risks) (RR 0.21, 95% CI 0.03 to 1.77; Analysis 10.1; low certainty evidence downgraded twice due to imprecision; Table 9).
10.1. Analysis.
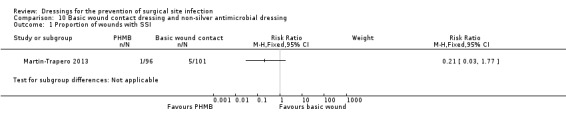
Comparison 10 Basic wound contact dressing and non‐silver antimicrobial dressing, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
This trial provided no data for our secondary outcomes..
Summary: Basic wound contact dressings compared with non‐silver antimicrobial dressings
It is not clear whether PHMB dressings reduce SSI risk in surgical wounds healing by primary intention compared with a basic wound contact dressing following clean surgery; the 95% CIs are wide and include benefits (in terms of reduced SSI risk) and harms (in terms of increased SSI risks) (low certainty evidence downgraded twice due to imprecision; Table 9).
Comparison 11: Comparisons between advanced dressings (3 trials; 694 participants)
We considered three studies in this comparison (Ravnskog 2011; Wikblad 1995; Wynne 2004). We included two arms of Wynne 2004 (a three‐arm trial): one arm received a film dressing (left in situ for five days) and another a hydrocolloid dressing (also left in situ for five days). Ravnskog 2011 compared an alginate dressing with a hydrofibre dressing in 200 participants undergoing hip replacement. The trial reported only pain data that could be included in this review.
Primary outcome: SSI
Clean surgery
Wikblad 1995 presented no clear data for SSIs.
In Wynne 2004 it was uncertain whether use of a film‐dressing reduces risk of SSI (9/227; 4%) compared with a hydrocolloid‐dressing (6/267; 2%) (RR 0.57; 95% CI 0.20 to 1.57; Analysis 11.1; very low certainty evidence downgraded once due to risk of bias and twice due to imprecision).
11.1. Analysis.
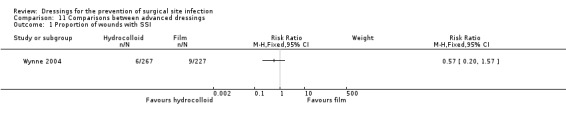
Comparison 11 Comparisons between advanced dressings, Outcome 1 Proportion of wounds with SSI.
Secondary outcomes
Clean surgery: acceptability
Wynne 2004: in the hydrocolloid group 75/267 (28%) of participants reported that they were dissatisfied with the hydrocolloid dressing compared with 80/227 (35%) in the film group (RR 0.80; 95% CI 0.61 to 1.03) (no analysis presented). It was unclear if participants were blinded to treatment.
Clean surgery: ease of removal
In Wikblad 1995 13/61 (21%) respondents (clinicians) in the hydrocolloid group reported that the dressing was not difficult to remove compared with 45/60 (75%) in the matrix hydrocolloid group (RR 3.52, 95% CI 2.13 to 5.82) (no analysis presented). However, a large number of participants were missing from this trial, which was classed as being at high risk of bias and data were imprecise and uncertain (very low certainty evidence).
Clean surgery: pain
Ravnskog 2011 used a VAS scale to measure: pain from the dressing during mobilisation; Itching under the dressing; burning pain under the dressing; discomfort caused by use of the dressing; and pain score at dressing removal. The data from the VAS scale were not clear, so these were not analysed further. Participants were also asked whether they had pain at removal of the dressing. In total 2.1% in the alginate dressing group had experienced pain compared with 15% in the hydrofibre dressing group (numerator and denominator data were not presented in the trial report).
In Wikblad 1995, 14% of participants (calculated by review authors as 8/60) in the hydrocolloid group reported no pain at dressing removal compared with 61% (calculated as 37/61) in the matrix‐hydrocolloid group. However, a large number of participants was missing from this trial, which we classed as being at high risk of bias, and the data are imprecise. The effect of these interventions on pain is uncertain (very low certainty evidence).
Clean surgery: costs
Wikblad 1995 reported the mean dressing cost per participant at USD 3.60 for the hydrocolloid dressing group and USD 3.34 for the matrix hydrocolloid group.
Wynne 2004 reported the median cost of the hydrocolloid dressing group in Australian dollars as AUD 3.93, compared with AUD 1.59 for a film dressing. Again this value included only the cost of the dressings themselves, and not other important measures of resource use that should be considered when using cost as a decision tool, i.e. amount of nurse time, and cost of complications.
Summary: Advanced dressings compared with another advanced dressing
It is uncertain whether covering surgical wounds that are healing by primary intention with a hydrocolloid dressing increases or decreases the risk of SSI compared with a film dressing following clean surgery, as we have assessed the certainty of evidence as very low (downgraded once due to risk of bias and twice due to imprecision). The limited data means that there is uncertainty about whether any one advanced dressing confers better acceptability or usage.
Discussion
Summary of main results
The primary aim of this systematic review was to present and appraise all existing evidence regarding the relative effectiveness of various surgical dressings, including not using a dressing, and using glue as a dressing, on the risk of developing surgical site infections (SSIs) in surgical wounds that are healing by primary intention. We found insufficient evidence that covering surgical wounds with any dressing compared with leaving them exposed influences the subsequent risk of SSI. Similarly there was insufficient evidence on which to base solid conclusions regarding whether any single type of dressing reduces risk of SSIs in wounds resulting from surgery. GRADE assessments of the evidence resulted predominantly in judgements of very low certainty. The studies included in the analyses were small and had low numbers of events. This means that the available evidence had low statistical power and that for most comparisons we could not exclude the possibility that there might be differences in effectiveness; currently, there is not enough information of a high enough certainty to be sure. Some studies were at a high risk of bias and were lacking important details in reports about trial populations or how outcomes were defined and when outcome data were collected.
We included a range of contamination levels for the many trials that investigated 'non clean' surgery, and it was not possible to draw conclusions for each.
For secondary outcomes, there is again uncertainty due to the certainty of the evidence being low or very low. The results of Moshakis 1984 suggested that film dressings might be less painful for patients than other basic wound contact dressings. However, we judged this trial to be at high risk of bias due to inadequate allocation concealment, and the absence of evidence that appropriate statistical procedures had been employed to accommodate the inclusion of some participants as their own controls (bilateral excisions). Wikblad 1995 reported that basic wound contact dressings were significantly less painful on removal than hydrocolloid dressings. However, a large amount of data were missing from this analysis, and we deemed it, too, to be at a high risk of bias. A number of trials suggested that advanced dressings were more expensive than basic wound contact dressings. However, all cost evaluations were very limited, and did not capture all relevant resource‐use data, or consider the costs versus the benefits of treatments ‐ which is best practice in economic evaluation. In short the economic data included in these studies did not lend themselves to decision making.
Overall completeness and applicability of evidence
There are many different dressing options to use postoperatively on surgical wounds. We identified 29 studies to include in this review, these assessed several dressing types, as well as leaving wounds exposed. There are no studies that evaluated glue‐as‐dressing. The included studies reported limited outcome data, even for the primary outcome of SSI. Where SSI was reported, the process used to define infection was often not reported, nor was it clear over what follow‐up period data collection took place. Outcomes for other important patient outcomes such as scarring were also poorly reported, with a range of measures used for assessment, which were sometimes unclear. Frequently, studies were very small in terms of number of participants and outcome events, which meant most included studies were very underpowered. Paradoxically the small, underpowered nature of studies means, that as well as being at risk of type 2 errors (that is missing important differences), they are at increased risk of type I errors as statistically significant findings are more likely to be spurious (Button 2010). Overall, the limited methodological reporting, the small sample sizes and the limited quality of outcome data collection, results in an insufficient volume of potentially biased evidence, which may be selectively reported.
Quality of the evidence
In general, the quality of the studies was very low and difficult to assess due to the lack of methodological detail reported. The majority of the included studies were more than 10 years old and did not follow current trial conduct and reporting guidelines, i.e. CONSORT (Schulz 2010). Key areas of good practice include the robust generation of a randomisation sequence (e.g. by a computer‐generated randomisation schedule); robust allocation concealment (e.g. through the use of a telephone randomisation service); and blinded outcome assessment where this is possible. Blinded outcome assessment is also crucial for assessment of outcomes such as SSI where there may be a subjective element in decision making, as non‐blinded assessment can introduce detection/observer bias (Hróbjartsson 2012), however, blinded assessment was not implemented in six studies, and not clearly reported in 20 more.
Key methodological information should be included in the trial report. In terms of analysis, data from all participants should be included in the analysis whenever possible, i.e. an intention‐to‐treat analysis should be conducted. Steps should be taken during a trial to minimise missing data as far as possible. Where missing data were an issue, imputation methods should be considered and clearly reported when implemented. When studies plan to evaluate more than one wound per person, or use participants as their own controls, or both, they should consult a statistician regarding both the trial design, sample size issues and the more advanced type of analysis that is required and, where possible, robust economic data should be collected. A number of trials included multiple surgical procedures with different levels of potential contamination; since they did not report data for each type separately, this limited the value of the data for analysis.
Potential biases in the review process
We conducted a comprehensive search that included trial registries, and obtained translations as required, so we do not believe that language bias is an issue. We were not able to explore publication bias using the studies we had, so the potential for bias for that is unknown. We did not deviate from the prepublished protocol, so do not believe bias has been introduced in terms of selective outcome reporting on our part.
It is noteworthy that we found four studies reported on a trial register (one of these trials was reported on two registers) which we are unable to link to published data. We contacted trial contacts to try to obtain any data we did not have. Where unpublished data exist and are not included in a review, there is an increase in the risk of publication bias.
Agreements and disagreements with other studies or reviews
We are not aware of any other published systematic reviews of dressings for surgical wounds that are healing by primary intention. Another review, however, regarding the use of wound dressings on surgical wounds was conducted as part of the development of a set of UK clinical guidelines addressing the prevention and treatment of SSIs (NICE 2008). This review generally reached similar conclusions to this systematic review. We would like to note, however, that the conclusions of this Cochrane Review are based on additional trials that were not included in the NICE review.
Our review does differ from the NICE review with regard to our use of healing data from Wikblad 1995. We did not utilise the limited infection data that were provided solely in the text of this three‐arm trial (which reported that 11 participants were treated with antibiotics postoperatively; eight had infections in the sternum: five were from the basic wound contact dressing group, but group(s) for the remaining three participants were not specified). The NICE review merged two of the three trial arms (a hydrocolloid‐dressed arm and a film‐dressed arm), and, as we cannot replicate their analysis, its authors may have obtained additional data (they reported six SSIs in the basic wound contact group and two in the merged hydrocolloid/film group). With regard to this trial, the NICE review concluded that: "there is limited evidence to suggest that there is a difference favouring the use of hydrocolloids or 'hydroactive' (film) dressings against the use of absorbent dressings in the prevention of SSI". Our review does not agree with this finding; however, the overall conclusions of the NICE review regarding wound dressings were limited to: "Cover surgical incisions with an appropriate interactive dressing at the end of the operation". The NICE report also conducted its own costing exercise, given that there were no data from the studies that could be used. They concluded that it is important to take into account the additional costs of changing dressings, as well as the initial price of each dressing type, when choosing dressings to use (NICE 2008).
The recent World Health Organisation guideline is supported by a systematic review containing 19 trials ‐ all included here. The review had a narrower remit comparing advanced dressings with standard dressings. The review reports advanced dressings as: Hydrocolloid; Silver‐impregnated Hydroactive and PHMB. The review also reports very low quality evidence for each of these comparisons (Allegranzi 2016). The guideline recommendation, suggests not using advanced dressings in preference to standard dressings on primarily closed surgical wounds for the purpose of preventing SSI and rates the evidence as low quality.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to determine whether covering surgical wounds that are healing by primary intention with wound dressings reduces the risk of surgical site infections (SSIs), or whether any particular type of wound dressing reduces the risk of infections more than another. Our review also failed to demonstrate any clear advantage of one dressing type over another (or wound exposure) for improved scarring, pain control, patient acceptability or ease of removal. It is important to note that many trials in this review were small and of poor quality, and at high or unclear risk of bias. Given the current evidence, decision makers may wish to make wound dressing choices on costs and clinician and patient preference. Additional steps to prevent SSIs can be based on other existing evidence and guidelines, for example the use of hand decontamination and antibiotic prophylaxis (NICE 2008).
Implications for research.
There is a lack of high quality research evidence regarding whether choice of wound dressing (or indeed use of wound dressings at all) affects the risk of SSIs in people whose surgical wounds are healing by primary intention. Whilst uncertainty remains regarding the best approach to dressing these surgical wounds, any investment in future research must maximise its value to decision‐makers. Given both the large number of dressing options and surgical procedures, the design of future trials should focus on those surgical procedures at highest risk of SSI, as well as evaluating the dressings or approaches that health professionals use most widely. In addition, as SSIs can be relatively rare events, very large trials are needed in terms of participant numbers. Such epidemiological information is vital to inform dressing trials and will become available through robust, routine data collection. Additionally, there may be value in asking decision‐makers (including patients) what they feel are the most pressing issues, e.g. type of dressing, or duration that a dressing remains in situ, as well as which outcomes are most important, including the ability of different dressings to manage specific symptoms such as absorption of exudate. Such planning means that research resources can be focused to address priorities. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting.
What's new
| Date | Event | Description |
|---|---|---|
| 15 December 2016 | New search has been performed | For this second update, glue‐as‐a‐dressing has been added as an intervention to the review. An update search has been run covering existing interventions and the new glue as dressing intervention. GRADE assessment has also been undertaken throughout the review and 'Summary of findings' tables added. Nine new studies have been included. |
| 15 December 2016 | New citation required but conclusions have not changed | No change to conclusions. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 7, 2011
| Date | Event | Description |
|---|---|---|
| 8 January 2015 | Amended | External sources of support updated. |
| 31 July 2014 | New citation required but conclusions have not changed | No change to conclusions |
| 31 July 2014 | New search has been performed | First update. Four new trials added (Bennett 2013; Burke 2012; Martin‐Trapero 2013; Ravnskog 2011) |
| 14 March 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to acknowledge the contribution of peer referees at both the protocol, review and update stage: Andrea Nelson, Joan Webster, Gill Worthy, Laura Bolton, John McCall, Sonya Osborne, Mark Rogers and Jane Nadel. We would also like to thank Elizabeth Royle for copy editing the review and the updated reviews.
Appendices
Appendix 1. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Bandages] explode all trees #2 MeSH descriptor: [Hydrogels] explode all trees #3 MeSH descriptor: [Alginates] explode all trees #4 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent):ti,ab,kw (Word variations have been searched) #5 MeSH descriptor: [Tissue Adhesives] explode all trees #6 MeSH descriptor: [Fibrin Tissue Adhesive] explode all trees #7 tissue next adhesive*:ti,ab,kw (Word variations have been searched) #8 MeSH descriptor: [Cyanoacrylates] explode all trees #9 octylcyanoacrylate*:ti,ab,kw (Word variations have been searched) #10 Dermabond:ti,ab,kw (Word variations have been searched) #11 MeSH descriptor: [Enbucrilate] explode all trees #12 Enbucrilate:ti,ab,kw (Word variations have been searched) #13 butylcyanoacrylate*:ti,ab,kw (Word variations have been searched) #14 MeSH descriptor: [Acrylates] explode all trees #15 acrylate*:ti,ab,kw (Word variations have been searched) #16 MeSH descriptor: [Bucrylate] explode all trees #17 bucrylate*:ti,ab,kw (Word variations have been searched) #18 {or #1‐#17} #19 MeSH descriptor: [Surgical Wound Infection] explode all trees #20 MeSH descriptor: [Surgical Wound Dehiscence] explode all trees #21 (surg* near/5 infect*):ti,ab,kw #22 (surg* near/5 wound*):ti,ab,kw #23 (wound* near/5 infection*):ti,ab,kw #24 (surg* near/5 incision*):ti,ab,kw #25 (surg* near/5 site*):ti,ab,kw #26 {or #19‐#24} #27 {and #18, #26} in Trials
Appendix 2. Search methods used in the original review
For the original review, we searched the following electronic databases:
Cochrane Wounds Specialised Register (searched 10 May 2011);
The Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2011, Issue 2);
Ovid MEDLINE (1950 to April Week 4 2011);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, 9 May, 2011);
Ovid Embase (1980 to 2011 Week 18);
EBSCO CINAHL (1982 to 6 May 2011)
The search used is listed below
#1 MeSH descriptor Bandages explode all trees#2 (dressing* or hydrocolloid* or gauze* or hydrogel* or alginate* or "bead" or "foam"):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Surgical Wound Infection explode all trees #5 MeSH descriptor Surgical Wound Dehiscence explode all trees #6 (surg* NEAR/5 infect*):ti,ab,kw #7 (surg* NEAR/5 wound*):ti,ab,kw #8 (wound* near/5 infection*):ti,ab,kw #9 (surg* NEAR/5 incision*):ti,ab,kw #10 (surg* NEAR/5 site*):ti,ab,kw #11 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #12 (#3 AND #11)
The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2009). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010). There were no restrictions on the basis of date or language of publication.
Appendix 3. Ovid MEDLINE search strategy
1. exp Bandages/
2. exp Hydrogels/
3. exp Alginates/
4. (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent).ti,ab.
5. exp Tissue Adhesives/
6. exp Fibrin Tissue Adhesive/
7. tissue adhesive$.mp.
8. exp Cyanoacrylates/
9. octylcyanoacrylate$.mp.
10. Dermabond.mp.
11. exp Enbucrilate/
12. Enbucrilate$.mp.
13. butylcyanoacrylate$.mp.
14. exp Acrylates/
15. acrylate$.mp.
16. exp Bucrylate/
17. bucrylate$.mp.
18. or/1‐17
19. exp Surgical Wound Infection/
20. exp Surgical Wound Dehiscence/
21. (surg* adj5 infection*).ti,ab.
22. (surg* adj5 wound*).ti,ab.
23. (wound* adj5 infection*).ti,ab.
24. surgical site*.mp.
25. or/19‐24
26. randomised controlled trial.pt.
27. controlled clinical trial.pt.
28. randomi?ed.ab.
29. placebo.ab.
30. clinical trials as topic.sh.
31. randomly.ab.
32. trial.ti.
33. or/26‐32
34. exp animals/ not humans.sh.
35. 33 not 34
36. and/18,25,35
Appendix 4. Ovid EMBASE search strategy
1. exp Wound Dressing/
2. exp Hydrogel/
3. exp Alginic Acid/
4. (dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent).ti,ab.
5. exp Tissue Adhesive/
6. exp Fibrin Glue/
7. (tissue adj adhesive$).mp.
8. exp Cyanoacrylate Derivative/
9. exp Cyanoacrylic Acid Octyl Ester/
10. octylcyanoacrylate$.mp.
11. Dermabond.mp.
12. exp ENBUCRILATE/
13. enbucrilate.mp.
14. butylcyanoacrylate$.mp.
15. exp Acrylic Acid/
16. acrylate$.mp.
17. exp Bucrilate/
18. bucrylate$.mp.
19. or/1‐18
20. exp Surgical Wound Infection/
21. exp Wound Dehiscence/
22. (surg* adj5 infection*).ti,ab.
23. (surg* adj5 wound*).ti,ab.
24. (wound* adj5 infection*).ti,ab.
25. surgical site*.ti,ab.
26. or/20‐25
27. Randomized controlled trials/
28. Single‐Blind Method/
29. Double‐Blind Method/
30. Crossover Procedure/
31. (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab.
32. (doubl$ adj blind$).ti,ab.
33. (singl$ adj blind$).ti,ab.
34. or/27‐33
35. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
36. human/ or human cell/
37. and/35‐36
38. 35 not 37
Appendix 5. EBSCO CINAHL search strategy
S34 S13 AND S21 AND S33
S33 S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32
S32 TX allocat* random*
S31 (MH "Quantitative Studies")
S30 (MH "Placebos")
S29 TX placebo*
S28 TX random* allocat*
S27 (MH "Random Assignment")
S26 TX randomi* control* trial*
S25 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S24 TX clinic* n1 trial*
S23 PT Clinical trial
S22 (MH "Clinical Trials+")
S21 S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 TI (postoperative* N5 infection* OR post‐operative* N5 infection*) or AB (postoperative* N5 infection* OR post‐operative* N5 infection*)
S19 TI wound* N5 infection* or AB wound* N5 infection*
S18 TI surg* N5 wound* or AB surg* N5 wound*
S17 TI surg* N5 infection* or AB surg* N5 infection*
S16 (MH "Surgical Wound")
S15 (MH "Surgical Wound Dehiscence")
S14 (MH "Surgical Wound Infection")
S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12
S12 TI Dermabond or AB Dermabond
S11 TI enbucrilate or AB enbucrilate
S10 TI bucrylate* or AB bucrylate*
S9 TI acrylate* or AB acrylate*
S8 TI butylcyanoacrylate* or AB butylcyanoacrylate*
S7 TI octylcyanoacrylate* or AB octylcyanoacrylate*
S6 TI cyanoacrylate* or AB cyanoacrylate*
S5 TI tissue adhesive* or AB tissue adhesive*
S4 (MH "Fibrin Tissue Adhesive")
S3 TI ( dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or non adherent or hydrocolloid* or alginat* or hydrogel* ) or AB ( dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or non adherent or hydrocolloid* or alginat* or hydrogel* )
S2 (MH "Alginates")
S1 (MH "Bandages and Dressings+")
Appendix 6. Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following:
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Basic wound contact dressings compared with exposed wounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Clean surgery | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.46] |
| 1.2 Potentially contaminated surgery | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.82, 2.19] |
Comparison 2. Film dressings compared with exposed wounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Silver dressings compared with exposed wounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Comparisons between basic wound contact dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 5. Basic wound contact dressings compared with film dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI: clean surgery | 4 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.70, 2.55] |
| 2 Proportion of wounds with SSI | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Pain associated with dressing (patient assessed) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Patient acceptability | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 6. Basic wound contact dressings compared with hydrocolloid dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Clean | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.30, 2.78] |
| 1.2 Potentially contaminated surgery | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.51] |
| 2 No pain on removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 7. Basic wound contact dressings compared with fibrous‐hydrocolloid (hydrofibre) dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.28] |
| 2 Proportion of wounds with SSI ‐ Vogt 2007 raw data | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 8. Basic wound contact dressings compared with matrix hydrocolloid dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No pain on removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 9. Basic wound contact dressings compared with silver dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Clean | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.47, 2.62] |
| 1.2 Potentially contaminated surgery | 5 | 1353 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.37] |
Comparison 10. Basic wound contact dressing and non‐silver antimicrobial dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 11. Comparisons between advanced dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds with SSI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennett 2013.
| Methods | 2‐arm RCT undertaken in the USA | |
| Participants | Women undergoing a caesarian section | |
| Interventions | Group A: standard soft cloth (MediporePad, 3M) (n = 262 total included in trial analysis = 236) Group B: silver ion‐eluting dressings (Silverlon, Argentum Medical) (n = 262 total included in trial analysis = 239). "The study population included a total of 524 women who consented to participate and met inclusion criteria; 475 cases were analyzed." Details from the trial author suggest that the trial had equal numbers in each arm. Thus for our analysis, in order to remain in line with our missing data methodology, we have assumed 262 in each arm. |
|
| Outcomes | Primary review outcome: SSI (signs of infection ‐ defined as an infection involving only the skin or subcutaneous tissue that occurred within the first 30 days after a surgical procedure ‐ from author) Secondary review outcomes: costs (of dressings USD ‐ no further details on data collection reported) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: participants were evaluated for signs of infection during their hospitalisation, and again at a visit made one‐week postpartum. Conference abstract ‐ author contacted for more information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomised block design using block sizes of 4 or 6 to ensure that an equal number of subjects were randomly assigned to each arm" ‐ we know that the sequence was computer generated from author correspondence. Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Details unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | None ‐ author correspondence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The study population included a total of 524 women who consented to participate and met inclusion criteria; 475 cases were analyzed." Further information from the author: 524 patients were consented, randomised meeting inclusion criteria [sic]; 475 cases were analysed with 49 participants excluded due to protocol deviations and missing data. Comment: text suggests that more participants were randomised than analysed. |
| Selective reporting (reporting bias) | Low risk | Key outcome reported. |
| Other bias | Low risk | None |
Biffi 2012.
| Methods | 2‐arm RCT conducted in Italy | |
| Participants | 121 participants undergoing elective surgery for colorectal cancer Inclusion criteria: undergoing elective surgery for colorectal cancer by laparotomic approach Exclusion criteria: history of allergy to dressing components; evidence of active infection at, or adjacent to, the operative site; coagulopathy (defined as platelet count < 50,000 cells/μL or a prothrombin time > 18 seconds); intestinal obstruction; active bowel bleeding; life expectancy < 6 months; inability to give written informed consent; or a programme of minimally invasive surgery planned (laparoscopy or robot‐assisted) |
|
| Interventions | Group A (n = 62): silver hydrofibre dressing (Aquacel Ag, ConvaTec) Group B (n = 59): standard absorbant dressing (Mepore, Molnlycke Health Care, Gothenburg, Sweden) All participants received a preoperative scrub and then painting with an aqueous solution of 10% povidone iodine, mechanical bowel preparation, and antibiotic prophylaxis in agreement with predefined protocols. |
|
| Outcomes | Primary review outcomes: SSI (clinical assessment) Secondary review outcomes: none |
|
| Notes | Follow‐up: 30 days We contacted the author about methods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To help match the two groups and address potential inter‐hospital differences, randomization was stratified by hospital with the use of computer‐generated randomization numbers without blocking." Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "In order to maintain the double‐blind characteristic of this trial, some actions were taken. First, the generator of the assignment was a data manager, who was separated from the executor." Comment: not clear if the executor of randomisation had access to the full randomisation schedule or was separate from it at point of randomisation. However, the staff were blinded to treatment and the separation was confirmed by the trial author. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The Aquacel Ag Hydrofiber dressing was covered by a common wound dressing in the experimental arm, whereas a double common dressing was applied to patients of the control group to blind the patient, the nursing and the medical staff and the independent data collector as to the nature of the dressing used. Whenever SSI was suspected or diagnosed, clinically relevant microbiologic samples were cultured. Investigators, who were unaware of the patients’ group assignments, assessed the seriousness of all adverse events and determined whether they were related to the trial." Comment: blinded outcome assessment conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow chart shows that 58/62 participants randomised to the intervention arm (4 lost to follow‐up) and 54/59 randomised to control arm were analysed (5 lost). Less than 10% lost in each arm, the impact of this loss unclear. |
| Selective reporting (reporting bias) | Low risk | None noted. Protocol not obtained. |
| Other bias | Low risk | None noted based on available information. |
Burke 2012.
| Methods | 2‐arm RCT, undertaken in single hospital centre in Ireland | |
| Participants | People undergoing elective total hip replacement (THR) and total knee replacement (TKR) (n = 124) Exclusion criteria:people undergoing revision surgery; taking immune‐suppressants (e.g. methotrexate); with chronic skin conditions (e.g. eczema, psoriases); with trophic skin changes (e.g. diabetes, peripheral vascular disease) |
|
| Interventions | Group A (n = 62: 35 THR and 27 TKR): absorbent dressing (Mepore, Mölnlycke Health Care) Group B (n = 62: 35 THR and 27 TKR): Jubilee dressing (hydrofiber inner layer (Aquacel, ConvaTec) with a viscoelastic hydrocolloid outer layer (DuoDerm, ConvaTec)) The TKR participants in both groups also had a layer of wool and crepe applied from the suprapatellar region of the knee to below the tibial tuberosity. This was removed on day 1 after surgery. Dressings were changed only when a > 50% strike through of the inner layer was visible. |
|
| Outcomes | Primary review outcome: SSI (not defined, although an erythematous, indurated wound with persistent copious discharge was taken to be suggestive of a deep SSI. Number of wounds with inflammation was also extracted, but inflamed wounds were not classed as infected in this trial) Secondary review outcomes: cost (number of dressing changes required and average hospital stay) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: not reported Given the use of a hydrofibre dressing as the contact layer here, we treated this intervention as a hydrofibre dressing. We contacted the author about methods. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were selected using the block randomisation method to have either the Jubilee dressing or a traditional adhesive dressing applied to their surgical wound." Author confirmed that a computer‐generated block randomisation was used, it was an Internet based program. Comment: details unclear |
| Allocation concealment (selection bias) | Unclear risk | Unable to make a decision based on available information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The outcomes were all assessed by the same tissue viability nurse and not by the medical/team involved in the surgery. She was not involved in the randomisation process." Comment: unclear if the nurse was blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All listed outcomes were reported ‐ there were no outcomes that were obviously missing. |
| Other bias | Low risk | Not noted |
Cosker 2005.
| Methods | 3‐arm RCT, undertaken in the UK | |
| Participants | People undergoing hip or knee surgery (trauma and elective cases). Those who failed to give consent, or who had dressing allergies were excluded. 100 participants were randomised to each dressing group (total n = 300). | |
| Interventions | Group A (n = 100): standard absorbent dressing (Primapore, Smith & Nephew)
Group B (n = 100): transparent film dressing and pad (Tegaderm and pad, 3M Healthcare)
Group C (n = 100): film dressing (Opsite Post‐Op, Smith & Nephew) Stated that all dressings were used according to manufacturers' instructions, but no further details provided. We merged Groups B and C and treated this as a 2‐arm trial in this review. |
|
| Outcomes | Primary review outcome: SSI (not defined); trial reported "numbers of patients in each group who progressed to overt infection" and required antimicrobial therapy. Secondary review outcomes: not reported | |
| Notes | Trial outcome data: see Table 12 Included some implants (i.e. screws) Follow‐up not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was effected by indicating the dressing in an envelope, which was opened by the theatre sister at the end of the operation." Comment: unclear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | See above. The paper notes that participants in the film dressing (Opsite) group were "significantly older" than in the other groups. No data presented. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 14 exclusions and it was unclear whether these were pre‐ or post‐randomisation. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes were reported. |
| Other bias | High risk | It seems that there was baseline imbalance in age, but no data were reported beyond the details in the text. |
De Win 1998.
| Methods | 2‐arm RCT undertaken in Belgium | |
| Participants | People > 18 years undergoing neuro‐ or cardiovascular surgery | |
| Interventions | Group A (n = 6): absorbent dressing (Mepore, Mölnlycke Health Care) Group B (n = 8): transparent film dressing and pad (Tegaderm and pad, 3M Healthcare) Dressing changes followed the in‐house wound care protocol (not described). | |
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: cost (mean total cost of dressings) |
|
| Notes | Trial outcome data: see Table 12 Report of interim analysis. Trial plans to recruit 60 people, paper reported the results of the 14 participants who had finished the trial at the time of writing. No further publications found. Follow‐up: participants enrolled for 7–10 days with dressing inspected every day. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided in paper. |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided in paper. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes were reported. |
| Other bias | High risk | Multiple wounds per participant not taken into account. |
Dickinson Jennings 2015.
| Methods | 3‐arm RCT undertaken in the USA | |
| Participants | 351 participants undergoing sternotomy Inclusion criteria: > 21 years of age; undergoing cardiac surgery requiring sternotomy incisions; hospitalised at the trial setting; English‐speaking; able to understand and give consent; have their surgeon approve participation; and not be sensitive to silver or alginates |
|
| Interventions | Group A (n = 117): standard sterile dressing (Primapore, Smith & Nephew). This dressing was left in place for either 24 or 48 hours. Group B (n = 116): metallic silver dressing (Acticoat Post‐Op, Smith & Nephew). This dressing was left in place over the incision for 5 days. Group C (n = 118): ionic silver dressing (Transeal, DeRoyal). This dressing was left in place over the incision for 5 days. All participants received the same skin preparation and antibiotic regime. Before placement of the silver dressings, a liquid barrier product (Skin Prep, ConvaTec) was applied to the area around the participant’s incision and permitted to dry to enhance adherence. All participants received intravenous antibiotics within the appropriate timeframe before surgery. |
|
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: ease of removal (5‐point scale: 1 = very easy, 2 = moderately easy, 3 = neither easy nor difficult, 4 = moderately difficult and 5 = very difficult); pain (comfort) (0‐10 scale with 0 signifying no pain and 10 signifying maximum pain). |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: 30 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The PI used a statistician‐generated, random numbers table to assign participants to each of the 3 dressing groups." Comment: considered adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Following randomization, the PI took the appropriate dressing to the operating room and communicated the dressing assignment directly to the nursing staff. Participants were not told of their group assignment until they awakened after surgery." Comment: not clear if PI was aware of sequence until point of randomisation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Due to the nature of the dressings, no aspect of this trial was blinded." Comment: no blinded outcome assessment was undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Thirty‐six participants were withdrawn from the trial because they did not wear their assigned dressing until the appropriate removal time due to additional surgeries or inadvertent removal." Comment: all participants should have been included in the trial regardless of adherence to protocol. The impact of this incomplete outcome data on findings is unclear: 11 participants were removed from Group A, 11 from Group B, and 14 from Group C. |
| Selective reporting (reporting bias) | Low risk | Given information presented in paper, all prespecified outcomes reported. |
| Other bias | Low risk | None noted |
Gardezi 1983.
| Methods | 2‐arm RCT undertaken in Pakistan | |
| Participants | People undergoing a general surgical operation ‐ 9 different types Exclusion criteria: children < 12 years of age; unconscious or unresponsive people |
|
| Interventions | Group A (n = 50): conventional gauze dressing changed after 48 hours Group B (n = 50): film dressing (polyurethane membrane) applied immediately postsurgery and left in situ until suture removal (fresh film applied on discharge and left until review at 1 week). No dressing details provided. | |
| Outcomes | Primary review outcome: SSI (not clearly defined); a number of relevant wound features i.e. redness were assessed, but it is not clear how this assessment informed diagnosis. Secondary review outcomes: pain (no details about how this was measured) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: no details provided Antibiotics were given when infection occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Pairs of participants were matched (age, sex and physical condition). One of each pair was assigned to each group. However, the authors stated that some pairing was done retrospectively. This makes the randomisation process difficult to understand. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation using pairs would be unconcealed, but it is not clear whether this process formed the basis of the allocation method. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no reported loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in paper, all prespecified outcomes reported. |
| Other bias | Unclear risk | Limited baseline information and unclear randomisation process |
Hewlett 1996.
| Methods | 2‐arm RCT undertaken in the UK | |
| Participants | People undergoing spinal, orthopaedic or abdominal surgery; in total 77 participants were randomised. Exclusion criteria: people admitted to hospital for minimally‐invasive surgical techniques |
|
| Interventions | Group A (n = 39): absorbent dressing (Mepore, Mölnlycke Health Care) Group B (n = 37): film dressing (Opsite, Smith & Nephew) Manufacturers' instructions were followed when applying and removing dressings (no further details provided). Treatment was for a maximum of 10 days. | |
| Outcomes | Primary outcome: not reported. Secondary outcomes: cost (dressing cost to complete healing) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: unclear. Trial information retrieved from a poster report only. Infection not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided in poster report. |
| Allocation concealment (selection bias) | Unclear risk | No information provided in poster report. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided in poster report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided in poster report. |
| Selective reporting (reporting bias) | Unclear risk | No information provided in poster report. |
| Other bias | Unclear risk | No information provided in poster report. |
Holm 1998.
| Methods | RCT undertaken in Denmark | |
| Participants | People undergoing abdominal surgical procedures with an incision > 5 cm Exclusion criteria: people suffering concomitant underlying disorders that influence the healing process (i.e. HIV, or receiving systemic corticosteroids or chemotherapy), potentially undergoing dirty procedures, or the creation of an enterstoma |
|
| Interventions | Group A (n = 37): absorbent dressing (Mepore, Mölnlycke Health Care) removed 2 days postoperatively (usual routine) Group B (n = 36): hydrocolloid dressing (Comfeel plus transparent dressing, Coloplast) left on until sutures removed at day 10 No difference in drain usage between groups. | |
| Outcomes | Primary review outcome: SSI (diagnosed in presence of pus, pyrexia and local tenderness) Secondary review outcomes: cost (number of dressing changes required); scarring (mean width in mm and total cosmetic quality of scar) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: average follow‐up time was 74.1 days in the absorbent dressing group and 80.2 days in the hydrocolloid group. Cosmetic outcome was assessed at final follow‐up 3 months after the operation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent patients were randomised..." Comment: not enough detail provided to enable us to judge the process. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinded for scarring, unclear regarding infection. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded post randomisation: 23 (6 deaths, 12 personal reasons (1 due to dressing), 3 re‐operation and 2 other). Infection (n = 6) was also classed as a reason for drop out. No ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes were reported. |
| Other bias | Unclear risk | Limited baseline data reported, more transverse wounds in the hydrocolloid group. |
Kriegar 2011.
| Methods | 2‐arm RCT conducted in the USA | |
| Participants | 110 participants undergoing anticipated colorectal surgery. Both open and laparoscopic operations were included. Inclusion criteria: anticipated colorectal surgery with an abdominal incision of at least 3 cm Exclusion criteria: known allergy to silver; signs of abdominal wall infection; condition that would prevent full closure of the skin at the primary operative site or prior abdominal mesh that was not planned to be fully removed at the time of operation; pregnant or breastfeeding women; and people who had received antibiotics within 1 week of surgery |
|
| Interventions | Group A (n = 55): sterile gauze held with tape; on discharge participants were instructed to change dressings as needed. Group B (n = 55): silver nylon dressing; the dressing was designed to stay in place for 7 days. The wounds were examined 48 hours after surgery. Silver‐dressed wounds that had dried before this point were hydrated, and if the gauze had become saturated it was changed. All participants received preoperative antibiotics 30 to 60 minutes before surgery (ertapenem or alternatives for participants with a penicillin allergy). All perioperative antibiotics were discontinued within 24 hours. |
|
| Outcomes | Primary review outcome: SSI (based on CDC classification) Secondary review outcome: none reported |
|
| Notes | Follow‐up for 30 days Author contacted for methods details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomistion was completed with nQuery software by a blinded statistician using sealed envelopes" Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomistion was completed with nQuery software by a blinded statistician using sealed envelopes" Comment: unclear if envelopes were numbered to ensure they were opened sequentially. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The surgical team was blinded to the surgical dressing until the time of skin closure at the end of the operation. .... determination of whether a wound was infected was made by an unblinded physician." Comment: not blinded, risk of bias of outcome assessment for SSI (only outcome). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart shows no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | None noted. Protocol not obtained. |
| Other bias | Low risk | None noted based on reporting. |
Langlois 2015.
| Methods | 2‐arm RCT undertaken in France | |
| Participants | 80 participants undergoing primary THA or TKA Inclusion criteria: aged 18‐95 years; able to understand information; undergoing primary THA or TKA Exclusion criteria: prior operative local procedure around the joint; past local infection; or advanced cancer |
|
| Interventions | Group A (n = 40): sterile gauze held in place with a crepe bandage Group B (n = 40): hydrofibre dressing (Aquacel, ConvaTec) In the sterile gauze group, the dressing change was scheduled between days 1and 3 postoperatively, with a second change on the day of discharge. Gauzes were then replaced by a conventional adhesive pad (Mepore, Mölnlycke Health Care, Göteborg, Sweden). In the hydrofibre dressing group, the only change was scheduled for the day of discharge. In both groups, an extra change of dressing was performed in case of saturation with leakage, major loss of adherence, bleeding, or suspected infection. The postoperative regimen included administration of systemic antibiotics for 48 hours, thromboprophylaxis with low molecular weight heparin for six weeks, and anti‐inflammatory medication (ketoprofen, 100 mg/day for 5 days). |
|
| Outcomes | Primary review outcome: SSI (not defined further) Secondary review outcome: scar cosmetic appearance; pain |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: 6 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised using a computer‐generated block randomization scheme to have either conventional or hydrofibre dressing." Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was based on the order of patient presentation, so each patient was randomised individually regardless of severity of osteoarthritis and co‐morbid situation." Comment: unclear if allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "scar cosmetic appearance evaluated six weeks after surgery by a plastic surgeon that was blinded to the dressing used and not involved in the surgical procedures." Comment: low risk for surgeon scar assessment unclear for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart suggests all participants analysed. |
| Selective reporting (reporting bias) | Low risk | None noted. Protocol not obtained. |
| Other bias | Low risk | None noted based on reported information. |
Law 1987.
| Methods | 3‐arm RCT undertaken in the UK | |
| Participants | People undergoing inguinal hernia repair or high saphenous ligation. 170 participants randomised. 4 participants lost to follow‐up, but unclear to which group(s) they belonged. No information provided regarding follow‐up. | |
| Interventions | Group A (n = 59): gauze, removed on day 5, or changed if wound was discharging Group B (n = 54): film dressing (Opsite; Smith & Nephew), removed on day 5. Discharge aspirated through dressing, and new dressing applied, if necessary. Group C (n = 53): exposed wound (if discharge, covered with gauze for as long as necessary) | |
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcome: cost (total dressing cost) | |
| Notes | Trial outcome data: see Table 12 Follow‐up: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly allocated to one of three surgical dressing options". Comment: not enough detail provided to understand process. |
| Allocation concealment (selection bias) | Unclear risk | No details provided in the report. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: trial notes that 4 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Lack of data for certain outcomes such as preference, scarring and comfort. |
| Other bias | Unclear risk | No baseline data presented. |
Lawrentschuk 2002.
| Methods | 2‐arm RCT undertaken in Australia | |
| Participants | People undergoing elective and emergency hip surgery. The trial stated that there were no exclusion criteria. | |
| Interventions | Group A (n = 25): non‐adherent absorbable dressing (Interpose) Group B (n = 25): paraffin tulle gras (Jelonet) In both groups: a compressible, combined dressing was placed immediately over the dressing being evaluated, and an adhesive elastic fabric dressing (Hyperfix) was placed over these 2 dressings. Dressings were placed with minimal force by the same resident in a standardised fashion, so as not to create tension in the skin. All dressings were sterile and non‐medicated. Wounds were checked at 48 hours ‐ all dressings were replaced after inspection and inspected again at 5 days. | |
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: not reported |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two different groups at the time of skin closure when a computer‐generated envelope was opened indicating which dressing to be [sic] used". Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | See above. Not clear if sequentially numbered. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Low risk | No other biases noted. |
Martin‐Trapero 2013.
| Methods | 2‐arm RCT undertaken in Spain | |
| Participants | People diagnosed with cholelithiasis undergoing elective laparoscopic cholecystectomy Exclusion criteria: > 70 years of age; diabetes; having a fever; and current treatment with immunosuppressants |
|
| Interventions | Group A (n = 101): non‐occlusive dressing (gauze) Group B (n = 96): 0.2% polyhexamethylene biguanide (PHMB) dressing The disinfection of the skin prior to surgery was performed 2 times with a solution of povidone iodine. A single dose of prophylaxis antibiotics was given at anaesthetic induction. Metal staples were used to close the surgical wound. Once incisions closed the wound was cleaned with povidone‐iodine 0.01% and the gauze or PHMB dressing was applied. |
|
| Outcomes | Primary review outcome: SSI (CDC definition) Secondary review outcomes: none (based on translation) |
|
| Notes | Trial outcome data: see Table 12 Information extracted from English abstract and limited translation of methods and results Follow‐up: 30 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on translation "....were assigned by an automatic method after the preparation of a spreadsheet (MS Excel) using the function ‐random tool where the researcher did not know the allocation of the next patient to be included in the study (concealment of random allocation)" |
| Allocation concealment (selection bias) | Low risk | Based on translation "....were assigned by an automatic method after the preparation of a spreadsheet (MS Excel) using the function ‐random tool where the researcher did not know the allocation of the next patient to be included in the study (concealment of random allocation)" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not able to assess |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not able to assess |
| Selective reporting (reporting bias) | Unclear risk | Not able to assess |
| Other bias | Unclear risk | Not able to assess |
Michie 1994.
| Methods | 2‐arm RCT undertaken in the USA | |
| Participants | People undergoing elective plastic and reconstructive surgery resulting in incision(s) not exceeding 200 mm Exclusion criteria: people with concomitant underlying disorders that might influence healing (e.g. use of corticosteroids, diabetes mellitus with a fasting blood sugar of > 250 mg/dL, or a compromised immunological status) 28 participants with 40 wounds took part in the trial. Participants served as their own controls, with half of each wound covered in a trial dressing. |
|
| Interventions | Group A (n = 28): cotton gauze impregnated with bismuth tribromophenate (Xeroform; Sherwood Medical Company) Group B (n = 28): hydrocolloid dressing (DuoDerm ExtraThin CGF; Convatec Bristol‐Myers Squibb) Dressings removed at 7‐10 days postoperatively (when sutures removed). | |
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: scarring (various outcomes); pain (past 48 hours); ease of removal (participant's perception of pain on removal and clinician's opinion as to whether the dressing was easy to remove) |
|
| Notes | Trial outcome data: see Table 12 Sponsored by Convatec. Data given for 28 participants rather than the 40 wounds. The statistical analysis allowed for this by using matched exact tests for proportions and matched asymptotic tests for trend for paired contingency data. Follow‐up: all wounds were evaluated at 2–3 days, 7–10 days, 4 weeks and 7 months postoperatively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated randomised table with blocks of 4 used to determine which dressing went to which end of the wound. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. Treating surgeon assessed cosmetic result. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Low risk | None noted |
Moshakis 1984.
| Methods | 2‐arm RCT undertaken in the UK | |
| Participants | People undergoing the excision of a breast lump | |
| Interventions | Group A (n = 59): dry gauze; 4–6 gauze dressings secured in place by 4 cm strips of tape (Transpore or Elastoplast, 3M Healthcare). Removed 1 day postoperatively and inspected; drain removed; a new dressing applied. Participants provided with dressings to take home, if further changes required. Group B (n = 61): transparent film (polyurethane membrane) dressing (Tegaderm, 3M Healthcare) left intact until day 6–8 for suture removal. If drain present, dressing was split along the length of the drain and it was removed. Any serious fluid collection was aspirated, and puncture covered with Transpore. | |
| Outcomes | Primary review outcome: not reported Secondary review outcomes: pain (assessed by participant on a linear scale); acceptability (assessed by participant on a linear scale also assessed by nurse on the same scale). Cost, scarring and ease of removal not reported. |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: nurse assessment of the wound took place before discharge, normally 1 day postoperatively. Participants gave their wound assessments at an outpatient visit (normally 6‐8 days postoperatively). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "..they were randomly allocated to receive.." However, some patients were undergoing excision of bilateral breasts lumps. In this case each wound was allocated to a different dressing. Therefore, some participants were their own control and some were not. Comment: unclear process |
| Allocation concealment (selection bias) | Unclear risk | "To diminish bias, allocation of the dressing to each patient was not known to the surgeon until the end of the operation" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 5 at outpatient follow‐up |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | High risk | Lack of baseline data. Analysis did not acknowledge the lack of independence in wounds on the same person. |
Ozaki 2015.
| Methods | 2‐arm RCT undertaken in the USA (2 medical centres) | |
| Participants | 500 adults were randomised. Eligible participants were undergoing an open (incision below the inguinal ligament) non‐emergency surgical procedure for peripheral vascular disease involving arteries or bypass grafts, with the anticipation that all incisions would be closed. | |
| Interventions | Group A (n = 250): standard gauze Group B (n = 250): silver alginate dressings No other dressing details provided. This original operating room dressing remained in situ until gross soiling, clinical need to remove, or postoperative day 3, whichever came first. Subsequent care was at the provider’s discretion. The wound‐closure technique was at the discretion of the surgeon. Cyanoacrylate tissue adhesives were considered as dressings and were not permitted. |
|
| Outcomes | Primary review outcome: assessment of wound complication which included SSI at 30 days (defined as no wound complication, superficial SSI or deep SSI ‐ noted that National Surgical Quality Improvement Program definitions were used). Secondary review outcomes: none reported |
|
| Notes | Trial outcome data: see Table 12 Noted that most cases were clean surgery (notes 25/500 participants had wounds classified as clean/contaminated) Follow‐up: 30 days We contacted the author to ask about methods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in the operating room by block design after wound closure was completed but before any dressing was applied." Quote from author: "Block 16 randomization per site. Generated via RAND in SAS" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote from author: "Patients and providers were blinded until time of reveal, which was at the end of the case (patient under anaesthesia) when the dressing is needed." Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | All outcomes Quote from paper: "In addition, although the patients and providers were not formally blinded to the type of original postoperative dressing, the study physicians generally reported an inability to recall which dressing the patient had received at the late follow‐up visits." Quote from author: "No one [sic] who assessed outcome was formally blinded, though in reality the evaluating clinicians noted that they frequently did not recall which early post‐operative dressing the patient had at 2 and 4 weeks." Comment: blinded outcome assessment was not conducted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In total 7/500 participants (3 in the silver group and 4 in the gauze group) were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Study reported and listed a number of other outcomes that were collected, including length of stay and EQ‐5D, but these data were not reported in the paper. |
| Other bias | Low risk | None noted |
Persson 1995.
| Methods | 2‐arm RCT undertaken in Sweden | |
| Participants | People having surgery for benign GI disease incurring a postoperative hospital stay of at least 5 days. 68 participants randomised. 7 participants excluded post‐randomisation (6 due to wrong dressing, 1 refused to be left without a dressing), but details of their allocation were not provided. | |
| Interventions | Group A (n = 30): exposed wounds initially covered with an absorbent dressing removed morning after surgery. Group B (n = 31): occlusive hydrocolloid dressing (DuoDerm E, Convatec/Bristol‐Myers Squibb) left in place until hospital discharge, or wound infection developed. | |
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: pain (estimated from graphical representation of linear scale); acceptability (from participants' perception) | |
| Notes | Trial outcome data: Table 12 Follow‐up: until discharge. No further information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On admission to the ward, they were randomised to have their wounds covered with a dressing or exposed..." Comment: limited detail provided to assess whether approach was adequate. |
| Allocation concealment (selection bias) | Unclear risk | No details mentioned in the report. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 randomised participants excluded (6 because wrong dressing applied and 1 who refused to have wound left uncovered). |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Unclear risk | Also varied timing as well as dressing type. No baseline table presented, but median age was 43 years in the dressing group and 36 years in the open group. |
Phan 1993.
| Methods | 2‐arm RCT undertaken in Belgium | |
| Participants | People with stage II, III and IV or recurrent head and neck cancer selected for extensive surgery (with or without radical neck dissection and flap reconstruction) Exclusion criteria: undergoing simple laryngectomy, partial glossectomy or pharyngoplasty. In total 207 participants randomised; 102 to receive the standard gauze (86 evaluated) and 105 to the ointment group (93 evaluated). |
|
| Interventions | Group A (n = 86): standard gauze dressing (not named). Changed twice daily with wound cleaning using alcoholic chlorhexidine solution
Group B (n = 93): surgical wound ointment with pure Vaseline (Qualifar) without gauze dressing. Vaseline was removed twice a day using sterile gauze, followed by cleaning of the wound with alcoholic chlorhexidine solution before application of a new cover with pure Vaseline. Duration for which the dressings remained in place was not recorded. |
|
| Outcomes | Primary review outcome: SSI (defined as a clinically documented infection localised at the surgical site and presenting with a purulent discharge with a severe inflammatory reaction > 5 cm of erythema and induration) Secondary review outcomes: not reported | |
| Notes | Trial outcome data: seeTable 12 32 participants in each group received antibiotic treatment. Follow‐up: 20 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was prospective and randomised". Comment: limited detail provided to assess |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed using sealed envelopes" Comment: not clear whether envelopes were numbered, or another method was employed to ensure concealment, so judged as unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26 participants excluded because lower GI surgery took place, surgery cancelled, protocol violation, participants could not be evaluated due to death or other circumstances. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Unclear risk | Differences at baseline: more stage IV cases in gauze group compared with Vaseline group (54% vs 39%) ‐ possibly due to exclusions? |
Politano 2011.
| Methods | 2‐arm RCT conducted in the USA | |
| Participants | 145 participants undergoing vascular reconstructions, documentation of other details limited. | |
| Interventions | Group A (n = 75): standard dressing (Primapore, Smith & Nephew) Group B (n = 70): silver impregnated dressing (Therabond 3D, Choice Therapeutics) |
|
| Outcomes | Primary review outcome: SSI (not defined) Secondary review outcomes: none reported |
|
| Notes | Trial outcome data: see Table 12 Abstract only ‐ only limited data available to extract. Unable to find author contact details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ no details reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ no details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear ‐ no details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ no details reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ no details reported |
| Other bias | Unclear risk | Unclear ‐ no details reported |
Prather 2011.
| Methods | 2‐arm RCT | |
| Participants | 110 participants undergoing colorectal surgery (no further details provided). | |
| Interventions | Group A (n = 54): gauze Group B (n = 56): silver nylon |
|
| Outcomes | Primary review outcome: not reported Secondary review outcomes: costs (costs of pain medication were calculated); pain (each day the level of pain was assessed in the morning and at night). Also reported that scores were collected at 30 days, but data not reported. A 0 to 10 pain scale was used where 0 = no pain and 10 = the worst pain imaginable. |
|
| Notes | Trial outcome data: see Table 12 Extraction based on abstract only ‐ limited information available Trial outcome data reported narratively in text Suggested that follow‐up was 30 days, but only reported data at 7 days Unable to find author contact details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ no details reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ no details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear ‐ no details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ no details reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ no details reported |
| Other bias | Unclear risk | Unclear ‐ no details reported |
Ravnskog 2011.
| Methods | 2‐arm RCT, undertaken in Norway | |
| Participants | People undergoing primary hip arthroplasty | |
| Interventions | Group A (n = 100): alginate dressing (Tegaderm Alginate, 3M) Group B (n = 100): hydrofibre dressing (Aquacel, ConvaTec) In theatre, participants received either a hydrofibre dressing (10 cm x 10 cm) or an alginate dressing (10 cm x 10 cm or 10 cm x 20 cm), both of which were folded to achieve a 3‐layer deep dressing. Both dressings were covered with the same adhesive polyurethane film (Mepore, Mölnlycke Healthcare). |
|
| Outcomes | Primary review outcome: not reported Secondary review outcomes: acceptability (measured as pain/discomfort during wear); ease of removal (pain at removal recorded using a VAS) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: not reported. Also reported on skin damage ‐ data not extracted for this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two members of hospital staff, who were in no other way connected to the trial, prepared the same number of cards with either ‘Aquacel’ or ‘Alginate’ written on them, and then put them into opaque sealed envelopes. Randomisation took place in the operating theatre, after incision, when the scrub nurse randomly chose and opened one of these sealed envelopes." Comment: difficult to be sure that the sequence was completely random. |
| Allocation concealment (selection bias) | Low risk | Quote: "Two members of hospital staff, who were in no other way connected to the trial, prepared the same number of cards with either ‘Aquacel’ or ‘Alginate’ written on them, and then put them into opaque sealed envelopes. Randomisation took place in the operating theatre, after incision, when the scrub nurse randomly chose and opened one of these sealed envelopes." Comment: method should preserve allocation concealment. Sequential numbering of the envelopes was not reported ‐ this would have been reassuring. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Patients were blinded to the dressing they received. Total blinding was not possible among staff as there is a slight visual difference between the two dressings." Comment: no blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Rohde 1979.
| Methods | 2‐arm RCT undertaken in Germany | |
| Participants | People undergoing elective abdominal procedure within a general surgery department | |
| Interventions | Group A (n = 46): conventional dressing (Fixomull‐stretch; Beiersdorf AG) Group B (n = 44): transparent drape (Opsite, Folie B. Braun Dexon GmbH, Spangenberg) | |
| Outcomes | Primary review outcome: SSI (unclearly defined) Secondary review outcomes: cost (per participant); pain (comfort); ease of removal (not defined) |
|
| Notes | Trial outcome data: see Table 12 Translated paper Follow‐up: not clear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Treatment' or 'control' cards were opened shortly after operation to determine which dressing should be applied. |
| Allocation concealment (selection bias) | Unclear risk | Not translated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not translated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not translated. |
| Selective reporting (reporting bias) | Unclear risk | Not translated. |
| Other bias | Unclear risk | Not translated. |
Ruiz‐Tovar 2015.
| Methods | 3‐arm RCT undertaken in Spain (only 2 arms relevant to this review and considered here) | |
| Participants | 98 people undergoing colorectal surgery (clean‐contaminated) Inclusion criteria: diagnosis of colorectal neoplasms and plans to undergo an elective operation with curative aims No exclusion criteria listed. |
|
| Interventions | Group A (n = 49): gauze and plastic adhesive tape, removed on day 5 as per protocol, or if SSI suspected Group B (n = 49): silver‐containing dressing (no further details), removed on day 5 as per protocol, or if SSI suspected All wounds: perioperative systemic antibiotics (cefuroxime 1500 mg and metronidazole 1500 mg; single dose preoperatively, within 30 minutes of incision, and redosed after 4 hours if the surgery exceeded 4 hours) were used in all groups. No mechanical bowel preparation took place in any participant. An aqueous solution of 10% povidone‐iodine was applied to the skin preoperatively. Skin closure was with staples after which povidone‐iodine solution was applied. All dressings were covered with a further standard dressing to blind participants, health professionals and data collectors. |
|
| Outcomes | Primary review outcome: SSI (SSI was suspected when the participant presented with fever, a red, painful, and tender region adjacent to the dressing, or the dressing was impregnated with a liquid that indicated purulent discharge ‐ any of these symptoms led to removal of the trial dressing. SSI was formally diagnosed using CDC criteria). Secondary review outcomes: none |
|
| Notes | Trial outcome data: see Table 12 Length of follow‐up was 30 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1:1 allocation scheme using a random‐number table into 3 groups" Comment: considered adequate |
| Allocation concealment (selection bias) | Low risk | Quoate: "In order to maintain the blind characteristic of this trial, some actions were taken. First, the generator of the assignment was a data manager, who was separated from those who applied dressings" Comment: considered adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "In order to maintain the blind characteristic of this trial, some actions were taken. First, the generator of the assignment was a data manager, who was separated from those who applied dressings (scrub nurses in the operating room at the end of each procedure). The groups received a common secondary dressing which blinded the medical staff, and the independent data collector ... Once the dressing was removed, the epidemiology nurse who diagnosed SSI on the basis of criteria developed by the Centers for Disease Control and Prevention (CDC) still remained unaware of the group assignments because she was not present at the time of dressing removal, and she evaluated the wound later" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised appear to have been included in the analysis. |
| Selective reporting (reporting bias) | Low risk | None noted, but trial protocol not obtained. |
| Other bias | Low risk | None noted |
Shinohara 2008.
| Methods | 2‐arm RCT undertaken in Japan | |
| Participants | People undergoing operations for GI surgery including gastric, duodenal, pancreatic and biliary surgery, and surgery on the colon and rectum Exclusion criteria: anal, perianal, peritonitis and emergency operations Follow‐up: dressings evaluated postoperatively by daily wound inspection until participant discharged. Cosmetic outcome assessed at 3 months after surgery. All participants were treated with cephamycin antibiotic postoperatively. |
|
| Interventions | Group A (n = 71): conventional gauze, removed postoperatively day 7 Group B (n = 63): occlusive hydrocolloid dressing (Karayahesive, Alcare) left in place until sutures removed 7 days postoperatively Dressings were changed if the dressing slipped or leaked. Dressings were discontinued if wound infection developed (defined as pus, pyrexia and local tenderness). | |
| Outcomes | Primary outcome: SSI (postoperative tissue and wound complications were defined as SSIs (superficial or deep wound infection, wound abscess) based on CDC guidelines for prevention of SSI). Secondary outcomes: cost (of dressing per participant); scarring: (mean scar width) |
|
| Notes | Trial outcome data: see Table 12 Mean follow‐up time noted as 90 days in both groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided in report on methods. |
| Allocation concealment (selection bias) | Unclear risk | No details provided in report on methods. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Low risk | No other biases noted. |
Siah 2011.
| Methods | 2‐arm RCT undertaken in Singapore | |
| Participants | 166 people undergoing various types of elective colorectal surgery Inclusion criteria: people undergoing abdominal surgery with incisions that penetrated the viscera Exclusion criteria: people who received non‐standard prophylaxis in the week prior to surgery or were listed as ‘dangerously ill’ and potentially at risk of dropping out of the trial before the end of its 4‐week duration; those on intensive immunosuppressant treatment, high‐dose steroids, radiation or chemotherapy or with a known allergy to silver; those who did not receive proper bowel preparation due to an emergency |
|
| Interventions | Group A (n = 83): wound exposure; a sterile, highly absorbent, low‐adherent pad was affixed immediately postoperatively by a low allergy, acrylic adhesive, spread onto the non‐woven backing surface, by the operating staff, immediately after wound closure. The dressing was then removed the next day (first postoperative day), in the surgical ward, and the wound was left exposed Group B (n = 83): ionic silver‐containing dressing (Aquacel Ag, ConvaTec, Wales, UK). Each dressing was covered with an adhesive skin contact layer. The dressing was left in place until discharge ‐ normally at 7 days Antibiotic prophylaxis was given, as per standard practice. |
|
| Outcomes | Primary review outcome: SSI (CDC criteria) Secondary review outcome: none reported. |
|
| Notes | Trial outcome data: see Table 12. Follow‐up: 30 days We contacted the author for information on methods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients included in this study were randomised into their respective group by means of drawing a sealed envelope stating either ‘control’ group or ‘study’ group. The randomisation was carried out by the researcher after patient consent was obtained." Comment: not enough information on which to judge risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients included in this study were randomised into their respective group by means of drawing a sealed envelope stating either ‘control’ group or ‘study’ group. The randomisation was carried out by the researcher after patient consent was obtained." Comment: not enough information on which to base a judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "As the intervention for the trial group was obvious to the researcher
and patients, compared with the no dressing control, blinding was impossible. All discharged patients were routinely given a 2‐week appointment to see their surgeon, who was blinded to the trial, for a wound assessment. On the 30th postoperative day, the ward staff nurses,who were also blinded to the trial, were given a CDC criteria checklist and phoned the patients to assess for SSI. Patients who were unable to describe their surgical site condition over the phone were asked to return to the clinic to have their surgical sites assessed by an advanced nurse practitioner, who was also blinded to the trial." Comment: considered blinded outcome assessment for SSI (only outcome) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From a figure in the paper it appears that 6 participants in total withdrew; 4 from Group A and 2 from Group B (for all dropouts reason given was medical complications unrelated to trial). We considered this to be a small number of withdrawals and of limited impact. |
| Selective reporting (reporting bias) | Unclear risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Unclear risk | No other biases noted. |
Vogt 2007.
| Methods | 2‐arm RCT undertaken in Denmark. | |
| Participants | People undergoing elective vascular surgery | |
| Interventions | Group A (n = 80): absorbent dressing (Mepore, Mölnlycke Health Care)
Group B (n = 80): hydrofibre/spun hydrocolloid dressing (Aquacel, ConvaTec) All dressings were applied at the end of surgery, and remained in situ for 4 days. After 4 days, no dressing was applied if the wound was dry. In the few cases where a dressing was still needed, standard treatment was used (not described). |
|
| Outcomes | Primary review outcome: SSI (defined as signs of infection ‐ redness, tenderness, swelling or exudate) Secondary review outcomes: cost (cost/per participant including dressing, nurse time and other equipment, e.g. gloves),acceptability (participant assessment: composite outcome from discomfort at mobilisation, pain at dressing change, and skin problems. All combined onto 3‐point scale where `good' = no discomfort at all; `moderate' = minor problems and `poor' = severe problems) | |
| Notes | Trial outcome data: see Table 12 Follow‐up: assessed daily for 4 days after surgery, at suture removal (typically at 14 days after surgery if in hospital), and at 6 weeks after surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before the study started a noninvolved person had mixed 160 notes, half of them marked Aquacel and half marked Mepore and put them in consecutive marked envelopes". |
| Allocation concealment (selection bias) | Low risk | "In the operating theatre the envelope was opened and the relevant dressing applied to the wound". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided in report on blinding for any outcome. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group A: 14 participants were not included (7 did not receive, or discontinued treatment, and 7 were lost to follow‐up). Group B: 10 participants were not included (5 did not receive, or discontinued treatment, and 5 were lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Low risk | None noted |
Wikblad 1995.
| Methods | 3‐arm RCT undertaken in Sweden | |
| Participants | People undergoing elective coronary bypass or valve‐replacement surgery | |
| Interventions | Group A (n = 92): absorbent dressing (no further details provided) Group B (n = 77): hydrocolloid dressing (DuoDerm, Convatec/Bristol‐Myers Squibb) Group C (n = 81): polyurethane matrix hydrocolloid dressing (Cutinova hydro, Beiersdorf AG) Dressings changed if signs of leakage or exudate. All dressing removed on day 5 postoperatively. | |
| Outcomes | Primary review outcome: SSI (definition of infection not given, although a culture was taken from the incision at day 5 postsurgery) Secondary review outcomes: cost (days 1‐5 per participant); pain (at day 5; rated on 3‐point scale); ease of removal (dressing assessed by clinician as difficult to remove) |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: outcome data collected from days 1 to 5 postoperatively. Participants self‐recorded information on wound appearance and feel 1 week after discharge, i.e. is wound red, does wound look swollen, is wound itchy? During fourth week after surgery 169 participants had the wound assessed by a nurse. Assessment included infection and treatment with antibiotics (yes/no). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: '... the secretary randomly selected a number from one to three (a number of each dressing type) and put the number on the anaesthesiologist's order sheet.' Comment: deemed to be at high risk of bias |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | On day 5, 2 independent reviewers assessed a photograph for redness, degree of wound healing and skin changes. Blinding not reported for infection. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: in the first week 34 dropped out (excessive bleeding = 15, reoperation = 7, postoperative complications = 1, died = 8, registration = 3). By the 4‐week assessment a further 47 had been lost. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Low risk | No other biases noted. |
Wynne 2004.
| Methods | 3‐arm RCT undertaken in Australia | |
| Participants | People having cardiac surgery that required a median sternotomy incision Exclusion criteria: immunosuppressed and non‐consenting people, and those under the care of surgeons who were not participating in the study |
|
| Interventions | Group A (n = 243): dry absorbent dressing (Primapore, Smith & Nephew) removed on day 2 postoperatively Group B (n = 267): hydrocolloid dressing (DuoDerm Thin, ConvaTec) in situ for 5 days Group C (n = 227): film dressing (Opsite, Smith & Nephew) in situ for 5 days | |
| Outcomes | Primary review outcome: SSI (definition of infection based on CDC guidelines for prevention of surgical site infection. Infection defined as superficial (involving skin and subcutaneous tissues), or deep (involving muscle, bone and mediastinum), in conjunction with one of the following: excision of wound tissue, a positive wound culture or treatment with antibiotics) Secondary review outcomes: cost (median per participant); acceptability (assessed by participants); ease of removal: (discomfort with removal ‐ assessed by participants). Scarring and pain not reported. |
|
| Notes | Trial outcome data: see Table 12 Follow‐up: outcome data collected daily on days 1‐5 postoperatively. Subsequent follow‐up via outpatient clinic, or phone call 4 weeks after discharge. At 4 weeks participants were questioned about their experiences with regard to pain, tenderness, redness, swelling, discharge or oozing from the chest wound; and whether they had sought medical attention or had antibiotic therapy initiated by doctor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the three treatments by the circulating nurse on the commencement of sternal skin closure". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was stratified equally across two operating theaters and was achieved using opaque envelopes". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not stated Personnel: not stated Outcome assessors: quote: "Blinding of data collectors to treatment was not feasible ...". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | SSI: denominator values suggested complete follow‐up for short‐term period. |
| Selective reporting (reporting bias) | Low risk | Given the information presented in the paper, all prespecified outcomes reported. |
| Other bias | Unclear risk | Varied timings as well as dressing types. |
Abbreviations
< = less than > = more than CDC = Centers for Disease Control and Prevention EQ‐5D = EuroQol five dimensions questionnaire (a standardised instrument for measuring generic health status) GI = gastrointestinal ITT = intention‐to‐treat (analysis) n = number in group PHMB = PI = principal investigator RAND = RCT = randomised controlled trial SAS = statistical analysis system SSI = surgical site infection THR = total hip replacement TKR = total knee replacement VAS = visual analogue scale vs = versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abejon 2012 | Not enough detail to confirm surgery type |
| Abejon 2013 | Not an RCT ‐ quasi‐randomised. |
| Ajao 1977 | Compared same dressing left in situ for different durations on surgical wounds healing by primary intention (timing trial). |
| Al‐Belasy 2003 | Oral surgery |
| Allan 1996 | Not surgical wounds |
| Alsbjorn 1990 | Dressings applied to drain sites 1‐2 days postoperatively |
| Anonymous 2013 | Unable to obtain abstract |
| Baker 1977 | Compared a plaster dressing that was placed over soft dressing material. No relevant outcomes reported. |
| Blondeel 2004 | Tissue‐adhesive used as a wound closure method |
| Borgognoni 2000 | Data were only available from an abstract, and no further information could be obtained from author. Outcome was the recurrence of keloids and associated immunohistochemical investigations. |
| Borkar 2011 | Included children < 2 years of age |
| Boyce 1995 | Wounds healing by secondary intention |
| Brehant 2009 | Open wounds without planned healing (stoma) |
| Cabrales 2014 | Open not closed wounds |
| Choi 2005 | Unable to obtain study report |
| Chou 2010 | Not relating to skin closure (dura) |
| Chrintz 1989 | Not an RCT |
| Colom Majan 2002 | Study included scars and not open wounds. |
| Decaillet 1998 | All patients received 2 hours of pressure dressings postoperatively. Not clear whether evaluated dressings were applied before or after this. |
| Dell 2001 | Covered by a different Cochrane review group (Eyes and Vision) |
| Di Maggio 1994 | Not thought to have measured relevant outcomes |
| Dillon 2008 | Do not believe to be an RCT; no contact from author. |
| Dixon 2006 | The trial compared ointments applied to the wounds, and not dressings. |
| Dobbelaere 2015 | Not an RCT |
| Dosseh Ekoue 2008 | Compared same dressing left in situ for different durations on surgical wounds healing by primary intention (timing trial). |
| Edwards 1967 | Not an RCT. Groups were formed arbitrarily and not randomised. |
| Eymann 2010 | Participants < 2 years old |
| Fries 2014 | Open not closed wounds ‐ confirmed after contact with author. |
| Furrer 1993 | Tissue‐adhesive applied prior to wound closure thus not glue as dressing |
| Garne 1989 | Compared same dressing left in situ for different durations on surgical wounds healing by primary intention (timing trial). |
| Gbolahan 2015 | Surgery of the mouth and in children < 2 years old |
| Giri 2004 | Included some wounds that were infected at baseline |
| Gonzalez 2002 | Not an RCT |
| Grauhan 2010 | Quasi‐randomised trial. Allocation of participants to the 2 study groups alternated according to the time of operation |
| Grover 2015 | Dressing/wound exposure was not the only systematic difference between the 2 arms. The exposure arm had daily applications of 5% povidone iodine solution that the dressing arm did not. |
| Guilbaud 1993 | Not surgical wounds |
| Guillotreau 1996 | Not surgical wounds |
| Gupta 1991 | Wounds healing by secondary intention |
| Heal 2009 | Compared same dressing left in situ for different durations on surgical wounds healing by primary intention (timing trial). |
| Hermans 2000 | Wounds healing by secondary intention |
| Hirose 2002 | Wounds healing by secondary intention |
| Hutchinson 1997 | Not surgical wounds |
| Igarza 1997 | Unable to obtain complete paper. |
| Johannesson 2008 | Vacuum dressing and no relevant outcome |
| Juergens 2011 | Wrong intervention |
| Kadar 2015 | Not an RCT |
| Kiefer 2016 | Not a wound dressing |
| Lambiris 1979 | Wrong intervention |
| Mandy 1985 | Not an RCT. 10 additional participants added to control group after initial randomisation. |
| Marinovic 2010 | Not sure if RCT or CCT ‐ unable to confirm design. |
| Martin‐Garcia 2005 | Not an RCT |
| Maw 1997 | Not an RCT |
| McVeigh 2011 | Not an RCT |
| Merei 2004 | Not an RCT, as participants were randomised by date of birth. |
| Meylan 2001 | Not an RCT. |
| Milne 1999 | Identification of blister formation |
| Moore 1997 | Wounds healing by secondary intention |
| Morales 2006 | Tissue‐adhesive as a wound closure method |
| Müller 1993 | No relevant outcomes included |
| Nearuy 2000 | Wounds healing by secondary intention |
| Palao i Domenech 2008 | Mixture of wound types, mainly chronic (most common were leg ulcers) |
| Palao i Domenech 2009 | Unclear whether dressings applied to wounds in theatre. |
| Parvizi 2013 | Method of wound closure varied between groups, as glue was used in 1 arm |
| Pastorfide 1989 | Sprays and ointments used as comparisons, rather than dressings. |
| Piromchai 2008 | Compared a pressure dressing with a non‐pressure dressing after thyroidectomy. Reported outcome was volume of fluid collected. Viewed as trial of applying pressure to wound rather than a dressing trial per se. |
| Pizarro Sule 2001 | Dressing was not only difference between trial arms |
| Ponnighaus 1999 | Study included wounds healing by secondary intention |
| Ravenscroft 2006 | No relevant outcomes measured. Whilst pain was assessed at dressing removal, there was no indication of how this was measured, or what the numbers 'meant'. |
| Reinicke 1990 | All participants operated on one day received the treatment and the following day received the control. Classed as quasi‐randomised. Additionally, the study included data from contaminated wounds that were not randomised at all, but dressed according to surgeon's preference. |
| Ridley 2016 | Not an RCT |
| Robson 2012 | Topical treatment rather than dressing |
| Romero 2011 | Method of wound closure also varied between groups, as glue was used in 1 arm |
| Rosenfeldt 2003 | Unclear comparison group |
| Rushbrook 2014 | Not an RCT |
| Schwartz 2014 | Not thought to be an RCT ‐ seems to use alternation. Not able to contact authors to confirm |
| Segers 2007 | The study included a range of types of wound to the hand including trauma and nail‐bed injuries. Not all wounds were planned to heal by primary intention. |
| Shamiyeh 2001 | Tissue‐adhesive used as a wound closure method |
| Sheppard 2014 | Conference abstract with limited data |
| Shima 1998 | Not an RCT |
| Signorini 2007 | The study treated keloid scars. |
| Singer 2002 | Tissue‐adhesive used as a wound closure method |
| Sinha 2001 | Tissue‐adhesive used as a wound closure method |
| Slawson 2002 | Tissue‐adhesive used as a wound closure method |
| Sondergaard 1982 | The trial included participants with wounds that had already become infected postoperatively. |
| Stanirowski 2016a | Following contact with the author, we did not consider this to be an RCT, due to use of alternation. |
| Stanirowski 2016b | Following contact with the author, we did not consider this to be an RCT, due to use of alternation. |
| Staveski 2013 | Trial appears to include children < 2 years old (information from conference poster). Author contacted for confirmation. No reply received to date. |
| Staveski 2016 | Not correct study population. |
| Terrill 2000 | Not an RCT, since participants were allocated by year of birth. |
| Teshima 2009 | Not an RCT. |
| Tofuku 2012 | Not an RCT. |
| Torra i Bou 2013 | Reported clinical comparative evaluation. No mention of randomisation. Contacted author to confirm whether an RCT. No reply received to date. |
| Ubbink 2008 | Open wounds (not planned primary closure) |
| Valente 2008 | We do not believe this to be an RCT. Unable to contact author |
| Widgerow 2009 | Not an RCT. |
| Wipke‐Tevis 1993 | Randomised to dressing 1 day postoperatively. |
| Wipke‐Tevis 1998 | Both groups had the same dressing applied for 24 hours and were then randomised. |
| Yamanaka 2012 | Unable to obtain paper after several attempts |
| Yang 2013 | Wounds healing by secondary intention |
Abbreviations
RCT = randomised controlled trial TKA = total knee arthroplasty
Characteristics of studies awaiting assessment [ordered by study ID]
Goharshenasan 2016.
| Methods | RCT |
| Participants | 52 participants having bilateral symmetric incisions in randomly selected plastic surgical patients (split wound randomisation) |
| Interventions | Honey and standard dressing |
| Outcomes | Infection |
| Notes | We think the honey was a topical treatment rather than dressing but require clarification from author |
Siddiqui 2016.
| Methods | RCT |
| Participants | 144 participants (3‐arm trial, 2 potentially relevant arms) |
| Interventions | Advanced dressing compared with a different advanced dressing |
| Outcomes | Infection |
| Notes | Unclear what the type of dressings are used. We think it may be film versus another type of film, but require confirmation from authors. |
Springer 2015.
| Methods | RCT |
| Participants | 143 participants having TKA |
| Interventions | Occlusive, antimicrobial surgical dressing or a standard surgical dressing |
| Outcomes | Unclear |
| Notes | No relevant outcomes reported ‐ contacted authors for more information on data collected |
Abbreviations
RCT = randomised controlled trial TKA = total knee arthroplasty
Characteristics of ongoing studies [ordered by study ID]
ISRCTN06792113.
| Trial name or title | HTA ‐ 12/200/04: The Bluebelle study: FeasiBiLity stUdy of complEx, simple and aBsEnt wound dressings in eLective surgery |
| Methods | Feasibilty work includes small RCT |
| Participants | People with surgical wounds healing by primary intention |
| Interventions | Simple dressings, glue as dressing and no dressing |
| Outcomes | SSI |
| Starting date | June 2014 |
| Contact information | Professor Jane Blazeby |
| Notes | www.nets.nihr.ac.uk/projects/hta/1220004 |
NCT02619773.
| Trial name or title | The use of mupirocin dressings and its effect on surgical site infections in elective colorectal surgery: a prospective, randomised controlled trial |
| Methods | RCT |
| Participants | Surgical patients |
| Interventions | Mupirocin dressing compared with island dressing |
| Outcomes | SSI |
| Starting date | November 2015 |
| Contact information | Stephen B Shapiro, MD |
| Notes |
NCT02771015.
| Trial name or title | Clinical trial to evaluate the performance of a flexible self‐adherent absorbent dressing coated with a soft silicone layer compared with a standard wound dressing after orthopedic or spinal surgery: study protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | 200 participants undergoing orthopedic or spinal surgery |
| Interventions | Mepilex Border Post‐Op versus a standard dressing (Cosmopor E adhesive) |
| Outcomes | Blistering incidence; pain |
| Starting date | September 2015 |
| Contact information | Department of Orthopedics and Trauma Surgery, University Hospital of Cologne, Kerpener Str. 62, Cologne D ‐ 50924, Germany |
| Notes | Ongoing |
NCT02904200.
| Trial name or title | A prospective, randomised, controlled clinical investigation, comparing trauma to peri‐wound skin and pain when using two different wound dressings |
| Methods | RCT |
| Participants | Vascular surgery patient ‐ not clear from database |
| Interventions | Silicon dressing compared with acrylic dressing |
| Outcomes | Skin condition. Unclear if SSI will be assessed |
| Starting date | September 2016 |
| Contact information | tina.kjellen@molnlycke.com |
| Notes | Contacted company for more information |
Abbreviations
RCT = randomised controlled trial SSI = surgical site infection
Differences between protocol and review
As a result of feedback from the peer referees the title of this review has been changed from: Wound dressings for surgical sites; to: Dressings for the prevention of surgical site infection.
Contributions of authors
Jo Dumville co‐ordinated the review, extracted data and checked the quality of data extraction, undertook and checked quality assessment, analysed and interpreted data, performed and checked the quality of the statistical analysis, completed the first draft of the review, performed part of the writing or editing, made an intellectual contribution to the review, approved the final version prior to submission, wrote to trial authors/experts/companies and is guarantor for the review and the review update.
Trish Gray contributed to the previous update of this review, checked the quality of data extraction, undertook and checked quality assessment, and checked quality of statistical analysis, performed part of the writing or editing, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Catherine Walter performed previous work that was the foundation of the current review.
Catherine Sharp performed previous work that was the foundation of the current review.
Tamara Page performed previous work that was the foundation of the current review.
Rhiannon Macefield contributed to the update of this review, checked the quality of data extraction, undertook and checked quality assessment, and checked quality of statistical analysis, performed part of the writing or editing, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Natalie Blencowe contributed to the update of this review, checked the quality of data extraction, undertook and checked quality assessment, performed part of the writing or editing, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Thomas KG Milne contributed to the update of this review, checked the quality of data extraction, undertook and checked quality assessment, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Barnaby Reeves contributed to the update of this review, checked the eligibility of additional studies, performed part of the writing or editing, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Jane Blazeby contributed to the update of this review, checked the quality of data extraction, performed part of the writing or editing, made an intellectual contribution to the review update, and approved the final version of the review update prior to submission.
Contributions of editorial base
Nicky Cullum (Editor): edited the review and the updated reviews, advised on methodology, interpretation and review content. Approved the final review and the updated reviews prior to submission. Gill Rizzello and Sally Bell‐Syer (Managing Editors) : co‐ordinated the editorial process. Edited the review and updated reviews. Ruth Foxlee designed the search strategy and Reetu Child ran the searches for this updated review.
Sources of support
Internal sources
Royal Adelaide Hospital, Adelaide, South Australia, Australia.
Division of Nursing, Midwifery & Social Work, School of Health Sciences, Faculty of Biology, Medicine & Health, University of Manchester, Manchester, UK.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 ‐ High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
The National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester, UK.
Declarations of interest
Jo Dumville: I receive research funding from the NIHR for the production of systematic reviews focusing on high priority Cochrane reviews in the prevention and treatment of wounds.
Trish Gray: none known
Catherine J Walter: none known.
Catherine Sharp: none known.
Tamara Page: none known.
Rhiannon Macefield: none known.
Natalie Blencowe: none known.
Thomas KG Milne: none known.
Barnaby Reeves is funded (both part salary and research consumables) in part by the NIHR Bristol Cardiovascular Biomedical Research Unit.
Jane Blazby: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bennett 2013 {published data only}
- Bennett K, Kellett W, Braun S, Spetalnick B, Huff B, Slaughter J, et al. Silver ion‐eluting dressings for prevention of post cesarean wound infection: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2013;208(Suppl 1):337. [Google Scholar]
Biffi 2012 {published data only}
- Biffi R, Fattori L, Bertani E, Radice D, Rotmensz N, Misitano P, et al. Surgical site infections following colorectal cancer surgery: a randomized prospective trial comparing common and advanced antimicrobial dressing containing ionic silver. World Journal of Surgical Oncology 2012;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 2012 {published data only}
- Burke NG, Green C, McHugh G, McGolderick N, Kilcoyne C, Kenny P. A prospective randomised study comparing the jubilee dressing method to a standard adhesive dressing for total hip and knee replacements. Journal of Tissue Viability 2012;21:84‐7. [DOI] [PubMed] [Google Scholar]
Cosker 2005 {published data only}
- Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. Journal of Wound Care 2005;14(1):27‐9. [DOI] [PubMed] [Google Scholar]
De Win 1998 {published data only}
- Win M, Blaere X, Scheers R. The effect of choice of surgical wound dressing on the direct cost of healing. Eighth European Conference on Advances in Wound Management, 1998 April 26‐28; Madrid, Spain. Madrid, 1998:54–7.
Dickinson Jennings 2015 {published data only}
- Dickinson Jennings C, Culver Clark R, Baker JW. A prospective, randomized controlled trial comparing 3 dressing types following sternotomy. Ostomy Wound Management 2015;61:42‐9. [PubMed] [Google Scholar]
Gardezi 1983 {published data only}
- Gardezi SA, Chaudhary AM, Sial AK, Ahmad I, Rashid M. Role of 'polyurethane membrane' in post operative wound management. The Journal of the Pakistan Medical Association 1983;33:219‐22. [PubMed] [Google Scholar]
Hewlett 1996 {published data only}
- Hewlett L. The evaluation of two post‐operative dressings in the management of surgical wounds. Fifth European Conference on Advances in Wound Management, 1995 November 21‐24; Harrogate, UK. 1996.
Holm 1998 {published data only}
- Holm C, Petersen JS, Gronboek F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal wounds. European Journal of Surgery 1998;164:179‐83. [DOI] [PubMed] [Google Scholar]
Kriegar 2011 {published data only}
- Krieger BR, Davis DM, Sanchez JE, Mateka JJ, Nfonsam VN, Frattini JC, et al. The use of silver nylon in preventing surgical site infections following colon and rectal surgery. Diseases of the Colon and Rectum 2011;54:1014‐9. [DOI] [PubMed] [Google Scholar]
Langlois 2015 {published data only}
- Langlois J, Zaoui A, Ozil C, Courpied J‐P, Anract P, Hamadouche M. Randomized controlled trial of conventional versus modern surgical dressings following primary total hip and knee replacement. International Orthopaedics 2015;39:1315‐9. [DOI] [PubMed] [Google Scholar]
Law 1987 {published data only}
- Law NH, Ellis H. Exposure of the wound ‐ a safe economy in the NHS. Postgraduate Medical Journal 1987;63:27‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrentschuk 2002 {published data only}
- Lawrentschuk N, Falkenburg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. Australian and New Zealand Journal of Surgery 2002;72:716‐9. [DOI] [PubMed] [Google Scholar]
Martin‐Trapero 2013 {published data only}
- Martin‐Trapero C, Martin‐Torrijos M, Fernandez‐Conde L, Torrijos‐Torrijos M, Manzano‐Martin E, Pacheco‐del Cerro JL, et al. Surgical site infections. Effectiveness of polyhexamethylene biguanide wound dressings. Enfermeria Clinica 2013;23:56‐61. [DOI] [PubMed] [Google Scholar]
Michie 1994 {published data only}
- Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective randomized, controlled study. Annals of Plastic Surgery 1994;32:57‐64. [DOI] [PubMed] [Google Scholar]
Moshakis 1984 {published data only}
- Moshakis V, Fordyce MJ, Griffiths JD, McKinna JA. Tegadern versus gauze dressing in breast surgery. British Journal of Clinical Practice 1984;38:149‐52. [PubMed] [Google Scholar]
Ozaki 2015 {published data only}
- Ozaki CK, Hamdan AD, Barshes NR, Wyers M, Hevelone ND, Belkin M, et al. Prospective, randomized, multi‐institutional clinical trial of a silver alginate dressing to reduce lower extremity vascular surgery wound complications. Journal of Vascular Surgery 2015;61:419‐27. [DOI] [PubMed] [Google Scholar]
Persson 1995 {published data only}
- Persson M, Svenberg T, Poppen B. To dress or not to dress surgical wounds? Patients' attitudes to wound care after major abdominal operations. European Journal of Surgery 1995;161:791‐3. [PubMed] [Google Scholar]
Phan 1993 {published data only}
- Phan M, Auwera P, Andry G, Aoun M, Chantrain G, Deramaecker R, et al. Wound dressing in major head and neck cancer surgery: a prospective randomised study of gauze dressing vs sterile vaseline ointment. European Journal of Surgical Oncology 1993;19:10–6. [PubMed] [Google Scholar]
Politano 2011 {published data only}
- Politano A, Tracci M, Strider D, Sawyer R, Kern J, Upchurch G, et al. A randomized, prospective study of surgical site infections following vascular reconstructive surgery: untreated vs. silver‐impregnated dressings. 34th Annual Meeting of the Surgical Infection Society, 2014 May 1‐3; Baltimore (MD). 2014.
Prather 2011 {published data only}
- Prather AD, Mateka JJ, Marcet JE. Silver nylon wound dressings are associated with decreased post‐operative pain. Diseases of the Colon and Rectum 2011;45:5. [Google Scholar]
Ravnskog 2011 {published data only}
- Ravnskog FA, Espehaug B, Indrekvam K. Randomised clinical trial comparing Hydrofiber and alginate dressings post‐hip replacement. Journal of Wound Care 2011;20:136‐42. [DOI] [PubMed] [Google Scholar]
Rohde 1979 {published data only}
- Rohde H, Thon K, Stoltzing H, Schirren J. The transparent adhesive drape as post‐operative dressing ‐ a randomised clinical study for comparison with a conventional dressing [Die Klarsicht‐Klebefolie als postoperativ Verband ‐ eine randomisierte, klinische Studie zum Vergleich mit einer konventionellen Verbandstechnik]. Der Chirurg 1981;52:46‐50. [PubMed] [Google Scholar]
Ruiz‐Tovar 2015 {published data only}
- Ruiz‐Tovar J, Llavero C, Morales V, Gamallo C. Total occlusive ionic silver‐containing dressing vs mupirocin ointment application vs conventional dressing in elective colorectal surgery: effect on incisional surgical site infection. Journal of the American College of Surgeons 2015;221:424‐9. [DOI] [PubMed] [Google Scholar]
Shinohara 2008 {published data only}
- Shinohara T, Yamashita Y, Satoh K, Mikami K, Yamauchi Y, Hoshino S, et al. Prospective evaluation of occlusive hydrocolloid dressing regarding the healing effect after abdominal operations: randomised controlled trial. Asian Journal of Surgery 2008;31(1):1–5. [DOI] [PubMed] [Google Scholar]
Siah 2011 {published data only}
- Siah CJ, Bacani CP, Yatim J. Effectivess of total occlusive ionic silver contained dressing in reducing bacterial growth and incidences of surgical site infections following open abdominal surgery‐a randomised controlled trial. Proceedings of Singapore Healthcare. 2011:193.
- Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver‐containing dressing combination in decreasing risk of surgical site infection: an RCT. Journal of Wound Care 2011;20:561‐8. [DOI] [PubMed] [Google Scholar]
Vogt 2007 {published data only}
- Vogt KC, Uhlyarik M, Schroeder TV. Moist wound healing compared with standard care of treatment of primary closed vascular surgical wounds: a prospective randomized controlled trial. Wound Repair and Regeneration 2007;15:624‐7. [DOI] [PubMed] [Google Scholar]
Wikblad 1995 {published data only}
- Wikblad K, Anderson B. A comparison of three wound dressings in patients undergoing heart surgery. Nursing Research 1995;44(5):312‐6. [DOI] [PubMed] [Google Scholar]
Wynne 2004 {published data only}
- Wynne R, Botti M, Stedman H, Holsworth L, Harinos M, Flavell O, et al. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds: a randomised trial. Chest 2004;125(1):43‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abejon 2012 {published data only}
- Abejon A. The management of surgical wound: importance of the dressing. European Wound Management Association Journal 2012;12:129. [Google Scholar]
Abejon 2013 {published data only}
- Abejón A, Casanova PL, Verdú SJ, Torra I, Bou J‐E. Open‐label clinical trial comparing the clinical and economic effectiveness of using a polyurethane film surgical dressing with gauze surgical dressings in the care of post‐operative surgical wounds. International Wound Journal 2013;12:285‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajao 1977 {published data only}
- Ajao OG. Surgical wound infection: a comparison between dressed and undressed wounds. Journal of Tropical Medicine and Hygiene 1977;80:192‐6. [PubMed] [Google Scholar]
Al‐Belasy 2003 {published data only}
- Al‐Belasy FA, Amer MZ. Hemostatic effect of n‐butyl‐2‐cyanoacrylate (histoacryl) glue in warfarin‐treated patients undergoing oral surgery. Journal of Oral and Maxillofacial Surgery 2003;61(12):1405‐9. [DOI] [PubMed] [Google Scholar]
Allan 1996 {published data only}
- Allen RB, Kerstein M, Klassen H, Friedmann PS, Pryce DW, Lawrence JC, et al. Prospective study of clinical infections in wounds dressed with occlusive versus conventional dressings. Ninth Annual Symposium on Advanced Wound Care and Sixth Annual Medical Research Forum on Wound Repair, 1996 April 20‐24; Atlanta (GA). 1996:116.
Alsbjorn 1990 {published data only}
- Alsbjorn BF, Ovesen H, Walther‐Larsen S. Occlusive dressing versus petroleum gauze on drainage wounds. Acta Chirurgica Scandinavica 1990;156(3):211‐3. [PubMed] [Google Scholar]
Anonymous 2013 {published data only}
- Anonymous. SiiverSeal® dressing improves surgical wound outcomes. Wounds 2013;25:A18. [Google Scholar]
Baker 1977 {published data only}
- Baker H, Barnes RW, Shurr DG. The healing of below‐knee amputations: a comparison of soft and plaster dressing. American Journal of Surgery 1977;133(6):716‐8. [DOI] [PubMed] [Google Scholar]
Blondeel 2004 {published data only}
- Blondeel PN, Murphy JW, Debrosse D, Nix II, Puls LE, Theodore N, Coulthard P. Closure of long surgical incisions with a new formulation of 2‐octylcyanoacrylate tissue adhesive versus commercially available methods. American Journal of Surgery 2004;188:307‐13. [DOI] [PubMed] [Google Scholar]
Borgognoni 2000 {unpublished data only}
- Borgognoni L, Martini L, Brandani P, Pelliccia L, Reali UM. The use of silicone gel sheeting in the prevention of recurrence after keloid excision: a clinical and immunohistochemical investigation. Wound Repair and Regeneration 2000;8(5):A408‐A409. [Google Scholar]
Borkar 2011 {published data only}
- Borkar NB, Khubalkar MV. Are postoperative dressings necessary?. Journal of Wound Care 2011;20:301‐3. [DOI] [PubMed] [Google Scholar]
Boyce 1995 {published data only}
- Boyce DE, Miller L, Moore K, Harding KG. An open comparative randomized parallel‐group clinical trial to evaluate the performance of Hyaff[TM] wound dressing in the management of pilonidal sinus excision wounds: an interim analysis. Sixth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1997:55‐6.
Brehant 2009 {published data only}
- Brehant O, Pessaux P, Regenet N, Tuech JJ, Panaro F, Mantion G, et al. Healing of stoma orifices: multicenter, prospective, randomized study comparing calcium alginate mesh and polyvidone iodine mesh. World Journal of Surgery 2009;33:1795‐801. [DOI] [PubMed] [Google Scholar]
Cabrales 2014 {published data only}
- Cabrales RA, Cobo RB, Patino YD, Quintero MF, Martinez JW, Upegui ML. Effectiveness of silver dressings in preventing surgical site infection in contaminated wounds. Latreia 2014;27:247‐54. [Google Scholar]
Choi 2005 {published data only}
- Choi JW, Hyun KB, Kim YO, Park BY. Comparing conventional suture method versus wound closure using tissue glue (Histoacryl Blue®): a prospective randomised clinical trial. Journal of the Korean Society for Plastic and Reconstructive Surgery 2005;32(1):19‐23. [Google Scholar]
Chou 2010 {published data only}
- Chou D, Cheng J, Chesnut R, Choudhri H, Gopinath S, Scott Graham R, et al. A prospective, multi‐center, randomized controlled study to compare a low swell formulation of a PEG hydrogel spinal sealant as an adjunct to sutured dural repair with common dural sealing methods. Fifth Annual Meeting of the North American Spine Society. 2010.
Chrintz 1989 {unpublished data only}
- Chrintz H, Vibits H, Cordtz TO, Harreby JS, Waaddegaard P, Larsen SO. Discontinuing postoperative wound dressings [Seponering af postoperativ sarforbinding]. Ugeskrift for Laeger 1989;151(41):2667‐8. [PubMed] [Google Scholar]
- Chrintz H, Vibits H, Cordtz TO, Harreby JS, Waaddegaard P, Larsen SO. Need for surgical wound dressing. British Journal of Surgery 1989;76:204‐5. [DOI] [PubMed] [Google Scholar]
Colom Majan 2002 {published data only}
- Colom Majan JI. Treatment with a self‐adherent soft silicone gel dressing versus no treatment on postoperative scars. Twelfth Conference of the European Wound Management Association, 2002 May 23‐25; Granada, Spain. 2002.
Decaillet 1998 {published data only}
- Decaillet JM. Use of a new hydrocolloid dressing on sutured wounds after hand surgery. Eighth Conference of the European Wound Management Association, 1998 April, Madrid, Spain. 1998.
Dell 2001 {published data only}
- Dell SJ, Hovanesian JA, Raizman MB, Crandall AS, Doane J, Snyder M. Randomized comparison of postoperative use of hydrogel ocular bandage and collagen corneal shield for wound protection and patient tolerability after cataract surgery. Journal of Cataract and Refractive Surgery 2011;37:1113‐21. [DOI] [PubMed] [Google Scholar]
Dillon 2008 {published data only}
- Dillon J, Sayer R, Clarke J, McLean I. Evaluation of a hydrofibre/hydrocolloid dressing in a district general hospital. European Wound Management Association Journal 2008;2(Supp):263, Abstract P320. [Google Scholar]
Di Maggio 1994 {published data only}
- Maggio MR, Sakamoto N, Curutchet HP. Comparative study of the evolution of surgical wounds treated with conventional healing topical agents or silicone oil [Estudio comparativo de la evolucion de las heridas quirurgicas cubiertas con curacion convencional o con aceite de siliconas]. La Prensa Médica Argentina 1994;81:194‐6. [Google Scholar]
Dixon 2006 {published data only}
- Dixon AJ, Dixon MP, Dixon JB. Randomized clinical trial of the effect of applying ointment to surgical wounds before occlusive dressing. British Journal of Surgery 2006;93:937‐43. [DOI] [PubMed] [Google Scholar]
Dobbelaere 2015 {published data only}
- Dobbelaere A, Schuermans N, Smet S, Straeten C, Victor J. Comparative study of innovative postoperative wound dressings after total knee arthroplasty. Acta Orthopedia Belgium 2015;81:454‐61. [PubMed] [Google Scholar]
Dosseh Ekoue 2008 {published data only}
- Dosseh Ekoue D, Doleaglenou A, Fortey YK, Ayite AE. Randomized trial comparing dressing to no dressing of surgical wounds in a tropical setting [Pansement versus absence de pansement au‐delà de 48 heures en milieu tropical: essai randomisé]. Journal de Chirurgie 2008;145(2):143–6. [PubMed] [Google Scholar]
Edwards 1967 {published data only}
- Edwards RH, Killen DA. Comparison of two methods of management of clean surgical wounds. JAMA 1967;201(1):137‐8. [DOI] [PubMed] [Google Scholar]
Eymann 2010 {published data only}
- Eymann R, Kiefer M. Glue instead of stitches: a minor change of the operative technique with a serious impact on the shunt infection rate. Acta Neurochirurgica. Supplement 2010;106:87‐9. [DOI] [PubMed] [Google Scholar]
Fries 2014 {published data only}
- Fries CA, Ayalew Y, Penn‐Barwell JG, Porter K, Jeffery SL, Midwinter MJ. Prospective randomised controlled trial of nanocrystalline silver dressing versus plain gauze as the initial post‐debridement management of military wounds on wound microbiology and healing. Injury 2014;45:1111‐6. [DOI] [PubMed] [Google Scholar]
Furrer 1993 {published data only}
- Furrer M, Inderbitzi R, Nachbur B. Does administration of fibrin glue prevent development of lymphoceles after radical lymphadenectomy?. Der Chirurg 1993;64(12):1044‐9. [PubMed] [Google Scholar]
Garne 1989 {published data only}
- Garne E, Merrild DB. Wound Infection following heart surgery. Ugeskrift for Laeger 1989;151:2192–3. [PubMed] [Google Scholar]
Gbolahan 2015 {published data only}
- Gbolahan OO, Ogunmuyiwa SA, Osinaike BB. Randomized controlled trial comparing dressing and no dressing of surgical wound after cleft lip repair. American Journal of Critical Care 2015;16:554‐8. [DOI] [PubMed] [Google Scholar]
Giri 2004 {published data only}
- Giri P, Kanti Das M, Majumdar A. Management of different types of wound by cyanoacrylate glue fixation: a random study of 213 patients. Journal of the Indian Medical Association 2004;102(11):625‐6. [PubMed] [Google Scholar]
Gonzalez 2002 {published data only}
- Gonzalez Llinares RM, Antolin Mugarza A, Salgado Larrea MV, Barandiaran Mugica MJ, Basurco Celaya R, Larranaga Garaikaoetxea N. Effectiveness of dressings in clean and clean‐contaminated surgical wounds 24‐48 hours after surgery. Enfermeria Clinica 2002;2:117‐21. [Google Scholar]
Grauhan 2010 {published data only}
- Grauhan O, Navasardyan A, Hofmann M, Muller P, Hummel M, Hetzer R. Cyanoacrylate‐sealed Donati suture for wound closure after cardiac surgery in obese patients. Interactive Cardiovascular and Thoracic Surgery 2010;11(6):763‐7. [DOI] [PubMed] [Google Scholar]
Grover 2015 {published data only}
- Grover A, Singh A, Sidhu DS. A prospective randomised trial of open wound treatment vs occlusive dressings in elective surgical cases with respect to surgical site infections. Journal of Clinical and Diagnostic Research 2015;9:PC26‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guilbaud 1993 {published data only}
- Guilbaud J, Honde C. Multicentre comparative clinical study of a new wound dressing: PA286 (Inerpan). European Journal of Plastic Surgery. 1993;16(2):73‐6. [Google Scholar]
Guillotreau 1996 {published data only}
- Guillotreau J, Flandrin Andre P, Moncade F. Calcium alginate and povidone iodine packs in the management of infected postoperative wounds: results of a randomized study [abstract]. British Journal of Surgery 1996;83:86. [Google Scholar]
Gupta 1991 {published data only}
- Gupta R, Foster ME, Miller E. Calcium alginate in the management of acute surgical wounds and abscesses. Journal of Tissue Viability 1991;1(4):115‐6. [Google Scholar]
Heal 2009 {published data only}
- Heal CF, Buettner PG, Cruickshank R, Graham D, Browning S, Pendergast J, et al. Does single application of topical chloramphenicol to high risk sutured wounds reduce incidence of wound infection after minor surgery? Prospective randomised placebo controlled double blind trial. BMJ 2009;338:a2812. [DOI: 10.1136/bmj.a2812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermans 2000 {published data only}
- Hermans M. A prospective clinical trial of wound dressings to investigate the rate of infection under occlusion. First World Wound Healing Congress, 2000 September 10‐13; Melbourne, Australia. 2000:92.
Hirose 2002 {published data only}
- Hirose T, Takahashi S, Shimizu T, Nishiyama N, Furuya R, Takeyama K, et al. Clinical and bacteriological outcomes of post‐operative surgical sites with and without antisepsis in the field of urology. Dermatology 2002;204(Suppl 1):122. [Google Scholar]
Hutchinson 1997 {published data only}
- Hutchinson J. Prospective study of clinical infections in wounds dressed with hydrocolloid versus conventional dressings. Proceedings of the sixth European Conference on Advances in Wound Management, 1996 October 1‐4; Amsterdam, the Netherlands. Amsterdam, 1997:263.
Igarza 1997 {unpublished data only}
- Igarza JL, Conejo JS. A clinical and microbiological evaluation of dressings in dermatological surgery: adhesive strips compared with a thin hydrocolloid. Sixth European Conference on Advances in Wound Management, 1995 November 21‐24; Harrogate, UK. 1995.
Johannesson 2008 {published data only}
- Johannesson A, Larsson GU, Oberg T, Atroshi I. Comparison of vacuum‐formed removable rigid dressing with conventional rigid dressing after transtibial amputation: similar outcome in a randomized controlled trial involving 27 patients. Acta Orthopaedica 2008;79 (3):361‐9. [DOI] [PubMed] [Google Scholar]
Juergens 2011 {published data only}
- Juergens S, Maune C, Kezze F, Mohr T, Scheuer K, Mallman P. A randomized, controlled study comparing the cosmetic outcome of a new wound closure device with prolene suture closing caesarean wounds. Internation Wound Journal 2011;8:329‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadar 2015 {published data only}
- Kadar A, Eisenberg G, Yahav E, Drexler M, Salai M, Steinberg EL. Surgical site infection in elderly patients with hip fractures, silver‐coated versus regular dressings: a randomised prospective trial. Journal of Wound Care 2015;24:441‐2,444‐5. [DOI] [PubMed] [Google Scholar]
Kiefer 2016 {published data only}
- Kiefer DG, Muscat JC, Santorelli J, Chavez MR, Ananth CV, Smulian JC, et al. Effectiveness and short‐term safety of modified sodium hyaluronic acid‐carboxymethylcellulose at cesarean delivery: a randomized trial. American Journal of Obstetrics and Gynecology 2016;214:e1‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambiris 1979 {published data only}
- Lambiris E, Friedebold G, Zilch H. Local treatment of surgical infections using PMMA‐spheres and chains ‐ results from Berlin. Aktuelle Probleme in Chirurgie und Orthopadie 1979;12:161‐9. [PubMed] [Google Scholar]
Mandy 1985 {published data only}
- Mandy SH. Evaluation of a new povidine‐iodine impregnated polyethylene oxide gel occlusive dressing. Journal of the American Academy of Dermatology 1985;13(4):655‐9. [DOI] [PubMed] [Google Scholar]
Marinovic 2010 {published data only}
- Marinović M, Cicvarić T, Juretić I, Grzalja N, Medved I, Ahel J. Application of wound closure Molndal technique after laparoscopic cholecystectomy‐initial comparative study. Collegium Antropologicum 2010;34(Suppl 2):243‐5. [PubMed] [Google Scholar]
Martin‐Garcia 2005 {published data only}
- Martin‐Garcia RF, Janer AL, Rullan FV. Octyl‐2‐cyanoacrylate liquid bandage as a wound dressing in facial excisional surgery: results of an uncontrolled pilot study. Dermatologic Surgery 2005;31:670‐3. [DOI] [PubMed] [Google Scholar]
Maw 1997 {published data only}
- Maw I, Quinn J, Wells G, Ducic Y, Odell P, Lamothe A, et al. A prospective comparison of octylcyanoacrylate tissue adhesive and suture for the closure of head and neck incisions. The Journal of Otolaryngology 1997;26:26‐30. [PubMed] [Google Scholar]
McVeigh 2011 {published data only}
- McVeigh T, Kovacic D, Tawfick W, Sultan S. Assessing the impact of techniques of wound closure on vascular surgical site infection rates. Irish Journal of Medical Science: Sylvester O'Halloran Meeting. 2011:S114.
Merei 2004 {published data only}
- Merei JM. Pediatric clean surgical wounds: is dressing necessary?. Journal of Pediatric Surgery 2004;39(12):1871‐3. [DOI] [PubMed] [Google Scholar]
Meylan 2001 {published data only}
- Meylan G, Tschantz P. Surgical wounds with or without dressing. Prospective comparative study [Pansement ou absence de pansement sur les plaies operatoires. Etude prospective comparative]. Annales de Chirurgie 2001;126(5):459‐62. [DOI] [PubMed] [Google Scholar]
Milne 1999 {published data only}
- Milne CT, Pierpont Barrere CC, McLaughlin T, Moore A. Surgical hip dressings: a comparison of taping methods. Orthopedic Nursing 1999;18(3):37‐42. [PubMed] [Google Scholar]
Moore 1997 {unpublished data only}
- Moore P, Foster L. The use of a hydrofibre dressings in surgical wounds. European Wound Management Association Conference, 1997 April 27‐29; Milan, Italy. 1997:42‐4.
Morales 2006 {published data only}
- Morales Moreira E, Jesus Hernandez M, Granados Hernandez D, Sardinas Lopez G. Use of tisuacryl as tissue adhesive in the healing of cutaneous facial wounds and those of the buccal mucosa [Empleo del Tisuacryl como adhesivo tisular en el cierre de heridas faciales cutáneas y de la mucosa bucal]. Mediciego 2006;12:Available from bvs.sld.cu/revistas/mciego/vol12_supl1_06/articulos/a5_v12_supl106.html. [Google Scholar]
Müller 1993 {unpublished data only}
- Müller K, Matzen E, Gottrup F. Treatment of incisional wound defects following laparotomy, in relation to treatment effect, time consumption and economy. A methodological description. Third European Conference on Advances in Wound Management, 1993 October 19‐22; Harrogate, UK. 1993.
Nearuy 2000 {unpublished data only}
- Nearuy PC, Watson G, Andriessen A. A randomised comparative evaluation of a hydrogel vs paraffin gauze in the management of surgical wounds. First World Wound Healing Congress, 2000 September 10‐13; Melbourne, Australia. 2000.
Palao i Domenech 2008 {published data only}
- Palao i Domenech R, Romanelli M, Tsiftsis DD, Slonkova V, Jortikka A, Johannesen N, et al. Effect of an ibuprofen‐releasing foam dressing on wound pain: a real‐life RCT. Journal of Wound Care 2008;17(8):342,344‐8. [DOI] [PubMed] [Google Scholar]
Palao i Domenech 2009 {published data only}
- Palao i Domenech R, Prantl L, Larsen AM, Jortikka A. Effects of a foam dressing with ibuprofen on wound pain in acute wounds. European Wound Manangement Association Journal 2009;9(2):102, Abstract P6. [Google Scholar]
Parvizi 2013 {published data only}
- Parvizi D, Friedl H, Schintler MV, Rappl T, Laback C, Wiedner M, et al. Use of 2‐octyl cyanoacrylate together with a self‐adhering mesh (DermabondTM PrineoTM) for skin closure following abdominoplasty: an open, prospective, controlled, randomized, clinical study. Aesthetic Plastic Surgery 2013;37:529‐37. [DOI] [PubMed] [Google Scholar]
Pastorfide 1989 {published data only}
- Pastorfide GB, Gorgonio NM, Ganzon AR, Alberto RM. Zinc chloride spray‐magnesium hydroxide ointment dual topical regimen in the treatment of obstetric and gynecologic incisional wounds. Clinical Therapeutics 1989;11(2):258‐63. [PubMed] [Google Scholar]
Piromchai 2008 {published data only}
- Piromchai P, Vatanasapt P, Reechaipichitkul W, Phuttharak W, Thanaviratananich S. Is the routine pressure dressing after thyroidectomy necessary? A prospective randomized controlled study. BMC Ear, Nose and Throat Disorders 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pizarro Sule 2001 {published data only}
- Pizarro Sule C, Silva Orrego V, Ordenes VM, Bozinovic AF, Cabezas MJ. Management of surgical wounds with occlusive dressings [Manejo de herida operatoria con aposito oclusivo]. Revista Chilena de Cirugía 2001;53:386‐9. [Google Scholar]
Ponnighaus 1999 {published data only}
- Ponnighaus JM, Kowalzick L. Polyurethane or calcium alginate dressings for temporary defects in tumour surgery?. Aktuelle Dermatologie 1999;25(5):133‐5. [Google Scholar]
Ravenscroft 2006 {published data only}
- Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Annals of the Royal Collage of Surgeons of England 2006;88(1):18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinicke 1990 {published data only}
- Reinicke G, Nowak W, Adler KP. Does the elastic wound‐dressing Ankerplast spray influence the healing process of wounds? [Beeinflusst der elastische Wundschnellverband Ankerplast‐Spray die Wundheilung?]. Experimentelle Chirurgie 1990;115:111‐6. [PubMed] [Google Scholar]
Ridley 2016 {published data only}
- Ridley N. An audit and trial aiming to reduce the rate of surgical site infections for women having a caesarean section. British Journal of Midwifery 2016;24:170‐1. [Google Scholar]
Robson 2012 {published data only}
- Robson V, Yorke J, Sen RA, Lowe D, Rogers SN. Randomised controlled feasibility trial on the use of medical grade honey following microvascular free tissue transfer to reduce the incidence of wound infection. British Journal of Oral and Maxillofacial Surgery 2012;50:321‐7. [DOI] [PubMed] [Google Scholar]
Romero 2011 {published data only}
- Romero P, Frongia G, Wingerter S, Holland‐Cunz S. Prospective, randomized, controlled trial comparing a tissue adhesive (Dermabond) with adhesive strips (Steri‐Strips) for the closure of laparoscopic trocar wounds in children. European Journal of Pediatric Surgery 2011;21(3):159‐62. [DOI] [PubMed] [Google Scholar]
Rosenfeldt 2003 {published data only}
- Rosenfeldt FL, Negri J, Holdaway D, Davis BB, Mack J, Grigg MJ, et al. Occlusive wrap dressing reduces infection rate in saphenous vein harvest site. Annals of Thoracic Surgery 2003;75(1):101‐5. [DOI] [PubMed] [Google Scholar]
Rushbrook 2014 {published data only}
- Rushbrook JL, White G, Kidger L, Marsh P, Taggart TF. The antibacterial effect of 2‐octyl cyanoacrylate (Dermabond) skin adhesive. Journal of Infection Prevention 2014;15:236‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2014 {published data only}
- Schwartz J, Goss S, Facchin F, Manizate F, Gendics C, Braitman E, et al. A prospective two‐armed trial assessing the efficacy and performance of a silver dressing used postoperatively on high‐risk, clean surgical wounds. Ostomy Wound Management 2014;60:30‐40. [PubMed] [Google Scholar]
Segers 2007 {published data only}
- Segers P, Jong AP, Spanjaard L, Ubbink DT, Mol BA. Randomized clinical trial comparing two options for postoperative incisional care to prevent post‐sternotomy surgical site infections. Wound Repair and Regeneration 2007;15(2):192–6. [DOI] [PubMed] [Google Scholar]
Shamiyeh 2001 {published data only}
- Shamiyeh A, Schrenk P, Stelzer T, Wayand WU. Prospective randomised blind controlled trial comparing sutures, tape, and octylcyanoacrylate tissue adhesive for skin closure after phlebectomy. Dermatologic Surgery 2001;27:877‐80. [DOI] [PubMed] [Google Scholar]
Sheppard 2014 {published data only}
- Sheppard C, Kent WD, Fedak P. Negative pressure dressing to decrease the incidence of leg wound complications after CABG: nursing perspectives for patient care. Canadian Journal of Cardiology 2014;30(10):S354. [Google Scholar]
Shima 1998 {published data only}
- Shima Y. Clinical efficacy of thin hydrocolloid dressing (KYD) for healing of surgical wound. Rinsho to Kenkyu (The Japanese Journal of Clinical and Experimental Medicine) 1998;75:669‐79. [Google Scholar]
Signorini 2007 {published data only}
- Signorini M, Clementoni MT. Clinical evaluation of a new self‐drying silicone gel in the treatment of scars: a preliminary report. Aesthetic Plastic Surgery 2007;31(2):183‐7. [DOI] [PubMed] [Google Scholar]
Singer 2002 {published data only}
- Singer AJ, Quinn JV, Hollander JE. Comparison of octylcyanoacrylate and standard wound closure methods for lacerations and Incisions: a multi‐center trial. Academic Emergency Medicine 2002;131:270‐6. [Google Scholar]
Sinha 2001 {published data only}
- Sinha S, Naik M, Wright V, Timmons J, Campbell AC. A single blind, prospective, randomised trial comparing n‐butyl 2‐cyanoacrylate tissue adhesive (Indermil) and sutures for skin closure in hand surgery. Journal of Hand Surgery 2001;26B:264‐5. [DOI] [PubMed] [Google Scholar]
Slawson 2002 {published data only}
- Slawson D. How does laceration and incision repair with the tissue adhesive octylcyanoacrylate (Dermabond) compare with standard wound closure methods?. Surgery 2002;5:2. [Google Scholar]
Sondergaard 1982 {published data only}
- Sondergaard JO, Galatius H. Debrisan compared with chloramine packs in the treatment of postoperative wounds. Ugeskrift for Laeger 1982;144(21):1523‐5. [PubMed] [Google Scholar]
Stanirowski 2016a {published data only}
- Stanirowski PJ, Kociszewska A, Cendrowski K, Sawicki W. Dialkylcarbamoyl chloride‐impregnated dressing for the prevention of surgical site infection in women undergoing cesarean section: a pilot study. Archives of Medical Science 2016;12:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanirowski 2016b {published data only}
- Stanirowski PJ, Bizon M, Cendrowski K, Sawicki W. Randomized controlled trial evaluating dialkylcarbamoyl chloride impregnated dressings for the prevention of surgical site infections in adult women undergoing cesarean section. Surgical Infections 2016;7:427‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staveski 2013 {published data only}
- Staveski S, Abrajano C, Casazza M, Dong E, Petty A, Felix K, et al. Silver dressings for sternotomy incision care in pediatric cardiac patients to decrease surgical site infections. Pediatric Critical Care Medicine 2013;14(Suppl 1):S97. [Google Scholar]
Staveski 2016 {published data only}
- Staveski S, Abrajano C, Casazza M, Bair E, Quan H, Dong E, et al. Silver‐Impregnated dressings for sternotomy incisions to prevent surgical site infections in children. American Journal of Critical Care 2016;25:402‐8. [DOI] [PubMed] [Google Scholar]
Terrill 2000 {published data only}
- Terrill PJ, Varughese G. A comparison of three primary non‐adherent dressings applied to hand surgery wounds. Journal of Wound Care 2000;9(8):359‐63. [DOI] [PubMed] [Google Scholar]
Teshima 2009 {published data only}
- Teshima H, Kawano H, Kashikie H, Nakamura K, Imada T, Oda T, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surgery Today 2009;39(1):848‐54. [DOI] [PubMed] [Google Scholar]
Tofuku 2012 {published data only}
- Tofuku K, Koga H, Yanase M, Komiya S. The use of antibiotic‐impregnated fibrin sealant for the prevention of surgical site infection associated with spinal instrumentation. European Spine Journal 2012;21:2027‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torra i Bou 2013 {published data only}
- Torra i Bou JE, Abejon Arroyo A, Garcia Veira M, Cabero Garcia B, Gonzalez Carbjose MJ, Garcis Caridad L, et al. Clinical comparison of a film surgical dressing versus gauze tape dressing in the management of post‐operative surgical wounds in orthopedic surgery patients. European Wound Management Association Journal 2013;13(Suppl 167):Abstract 277. [Google Scholar]
Ubbink 2008 {published data only}
- Ubbink DT, Vermeulen H, Goossens A, Kelner RB, Schreuder SM, Lubbers MJ. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Archives of Surgery 2008;143(10):950‐5. [DOI] [PubMed] [Google Scholar]
- Ubbink DT, Vermeulen H, Goossens A . Modern versus gauze dressings to treat open wounds in surgical patients: a randomised clinical trial. Australian Wound Management Association Seventh National Conference: Dreams, Diversity, Disasters, 2008 May 7‐10; Darwin, Australia. 2008.
Valente 2008 {published data only}
- Valente M, Patricio A, Sousa A, Brilhante F. The Molndal technique: a comparative assessment. European Wound Management Association Journal 2008;2(Suppl 2):Abstract P194. [Google Scholar]
Widgerow 2009 {published data only}
- Widgerow AD, Chait LA, Stals PJ, Stals R, Candy G. Multimodality scar management program. Aesthetic Plastic Surgery 2009;33(4):533‐43. [DOI] [PubMed] [Google Scholar]
Wipke‐Tevis 1993 {unpublished data only}
- Wipke‐Tevis D, Stotts NA. Dressings, cosmetic result, pain, distress and wound healing of surgical incisions. First Joint Meeting of the Wound Healing Society and the European Tissue Repair Society, 1993 August 22‐25; Amsterdam, the Netherlands. 1993.
Wipke‐Tevis 1998 {published data only}
- Wipke‐Tevis DD, Stotts NA. Effect of dressings on saphenous vein harvest incision pain, distress and cosmetic result. Progress in Cardiovascular Nursing 1998;13(3):3‐13. [PubMed] [Google Scholar]
Yamanaka 2012 {published data only}
- Yamanaka N, Tanaka M. Examination of film material suitable for wound management after abdominal surgery. Fourth Congress of the World Union of Wound Healing Societies, 2012 September 2‐6; Yokohama, Japan. 2012.
Yang 2013 {published data only}
- Yang X, Zhou H‐F, Zhang S‐M, Wang Y‐Z, Ye N, Wang Y, et al. Hydrofiber dressing with silver in wound healing after surgery for anal fistula. Chinese Journal of Tissue Engineering Research 2013;16:8835‐41. [Google Scholar]
References to studies awaiting assessment
Goharshenasan 2016 {published data only}
- Goharshenasan P, Amini S, Atria A, Abtahi H, Khorasani G. Topical application of honey on surgical wounds: a randomized clinical trial. Forschende Komplementarmedizin 2016;23:12‐5. [DOI] [PubMed] [Google Scholar]
Siddiqui 2016 {published data only}
- Siddiqui M, Bidaye A, Baird E, Abu‐Rajab R, Stark A, Jones B, et al. Wound dressing following primary total hip arthroplasty: a prospective randomised controlled trial. Journal of Wound Care 2016;40:42‐5. [DOI] [PubMed] [Google Scholar]
- Siddiqui M, Bidaye A, Baird E, Jones B, Stark A, Abu‐Rajab R, et al. Wound closure following primary total hip arthroplasty. Tenth Congress of the European Hip Society, 2012 September 20‐22; Milan, Italy. Milan, Italy: Wichtig Publishing Srl, 2012:425.
Springer 2015 {published data only}
- Springer BD, Beaver WB, Griffin WL. The role of surgical dressings in total knee arthroplasty: a randomized clinical trial. Current Orthopaedic Practice 2013;24(4):452‐3. [Google Scholar]
- Springer BD, Beaver WB, Griffin WL, Mason JB, Odum SM. Role of surgical dressings in total joint arthroplasty: a randomized controlled trial. American Journal of Orthopedics 2015;44:415‐20. [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN06792113 {published data only}
- ISRCTN06792113. The Bluebelle Study: complex, simple and absent wound dressings in elective surgery: Phase A. www.isrctn.com/ISRCTN06792113 (first received 18 March 2014).
NCT02619773 {published data only}
- NCT02619773. Effect of mupirocin dressings versus island dressings on surgical site infections in elective colorectal surgery. clinicaltrials.gov/ct2/show/NCT02619773 (first received 30 November 2015).
NCT02771015 {published data only}
- NCT02771015. Performance of flexible self‐adherent absorbent dressing coated with a soft silicone layer after hip‐, knee‐arthroplasty, primary spine surgery in comparison to a standard wound dressing (wound dressing). clinicaltrials.gov/ct2/show/NCT02771015 (first received 8 September 2015).
NCT02904200 {published data only}
- NCT02904200. Clinical investigation of two different wound dressings. clinicaltrials.gov/show/NCT02904200 (first received 13 September 2016).
Additional references
Allegranzi 2016
- Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, Jonge S, Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence‐based global perspective. Lancet Infectious Diseases 2016;S1473‐3099:30402‐9 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Astagneau 2009
- Astagneau P, L'Hériteau F, Daniel F, Parneix P, Venier AG, Malavaud S, et al. Reducing surgical site infection incidence through a network: results from the French ISO‐RAISIN surveillance system. Journal of Hospital Infection 2009;72(2):127‐34. [DOI] [PubMed] [Google Scholar]
BNF 2016
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary (BNF): Appendix 5: wound management products and elasticated garments. www.evidence.nhs.uk/formulary/bnf/current/a5‐wound‐management‐products‐and‐elasticated‐garments (accessed 4 November 2016).
Bruce 2001
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technology Assessment Monograph 2001;5(22):1‐194. [DOI] [PubMed] [Google Scholar]
Button 2010
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience 2010;14:365‐76. [DOI] [PubMed] [Google Scholar]
de Lissovoy 2009
- Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. American Journal of Infection Control 2009;37(5):387‐97. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21:1575‐600. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Gibbons 2011
- Gibbons C, Bruce J, Carpenter J, Wilson AP, Wilson J, Pearson A, et al. Identification of risk factors by systematic review and development of risk‐adjusted models for surgical site infection. Health Technology Assessment 2011;15(30):1‐156. [DOI] [PubMed] [Google Scholar]
Goldman 1992
- Goldman MP, Fronek A. Consensus paper on venous leg ulcer. Journal of Dermatology Surgery and Oncology 1992;18:592‐602. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE working group. GRADE Handbook. gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 5 February 2016).
Health Protection Agency 2002
- Health Protection Agency. Surveillance of surgical site infection in English hospitals 1997‐2002. A national surveillance and quality improvement programme. London (UK): Health Protection Agency, 2002. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horan 2008
- Horan TC, Andrus M, Dudeck MA. DC/NHSN surveillance definition of healthcare‐associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McLaws 2000
- McLaws ML, Murphy C, Whitby M. Standardising surveillance of nosocomial infections: the HISS program. Hospital Infection Standardised Surveillance. Journal of Quality in Clinical Practice 2000;20(1):6–11. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute of Health and Care Excellence (NICE). Surgical site infections: prevention and treatment. Clinical guideline [CG74]. October 2008. www.nice.org.uk/guidance/cg74 (accessed 4 November 2016).
Plowman 2001
- Plowman R, Graves N, Griffin MA, Roberts JA, Swan AV, Cookson B, et al. The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. Journal of Hospital Infection 2001;47(3):198‐209. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLOS Med 2010;7:e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 4 November 2016).
Smyth 2008
- Smyth ET, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four country healthcare associated infection prevalence survey 2006: overview of the results. Journal of Hospital Infection 2008;69(3):230‐48. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
References to other published versions of this review
Walter 2012
- Walter CJ, Dumville JC, Sharp CA, Page T. Systematic review and meta‐analysis of wound dressings in the prevention of surgical‐site infections in surgical wounds healing by primary intention. British Journal of Surgery 2012;9(9):1185‐1194. [DOI] [PubMed] [Google Scholar]


