Abstract
Background
Many antihypertensive agents exist today for the treatment of primary hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or both). Randomised controlled trials (RCTs) have been carried out to investigate the evidence for these agents. There is, for example, strong RCT evidence that thiazides reduce mortality and morbidity. Some of those trials used reserpine as a second‐line therapy. However, the dose‐related blood pressure reduction with this agent is not known.
Objectives
The primary objective of this review was to quantify the dose‐related efficacy of reserpine versus placebo or no treatment in reducing systolic blood pressure (SBP) or diastolic blood pressure (DBP), or both.
We also aimed to evaluate the dose‐related effects of reserpine on mean arterial blood pressure (MAP) and heart rate (HR), as well as the dose‐related effects on withdrawals due to adverse events.
Search methods
We searched the Cochrane Hypertension Group Specialised Register (January 1946 to October 2016), CENTRAL (2016, Issue 10), MEDLINE (January 1946 to October 2016), Embase (January 1974 to October 2016), and ClinicalTrials.gov (all dates to October 2016). We also traced citations in the reference sections of the retrieved studies.
Selection criteria
Included studies were truly randomised controlled trials (RCTs) comparing reserpine monotherapy to placebo or no treatment in participants with primary hypertension.
Data collection and analysis
We assessed methods of randomisation and concealment. We extracted and analysed data on blood pressure reduction, heart rate, and withdrawal due to adverse effects.
Main results
We found four RCTs (with a total of 237 participants) that met the inclusion criteria, none of which we found through the 2016 update search. The overall pooled effect demonstrates a statistically significant systolic blood pressure (SBP) reduction in participants taking reserpine compared with placebo (weighted mean difference (WMD) ‐7.92, 95% confidence interval (CI) ‐14.05 to ‐1.78). Because of significant heterogeneity across the trials, a significant effect in diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) could not be found. A dose of reserpine 0.5 mg/day or greater achieved the SBP effects. However, we could not determine the dose‐response pattern because of the small number of trials. We did not combine data from the trial that investigated Rauwiloid against placebo with reserpine data from the remaining three trials. This is because Rauwiloid is a different alkaloid extract of the plant Rauwolfia serpentina, and the dose used is not comparable to reserpine. None of the included trials reported withdrawals due to adverse effects.
Authors' conclusions
Reserpine is effective in reducing SBP roughly to the same degree as other first‐line antihypertensive drugs. However, we could not make definite conclusions regarding the dose‐response pattern because of the small number of included trials. More RCTs are needed to assess the effects of reserpine on blood pressure and to determine the dose‐related safety profile before the role of this drug in the treatment of primary hypertension can be established.
Plain language summary
Reserpine for lowering blood pressure
Reserpine, a root extract of the naturally occurring plant Rauwolfia serpentina, was used in the past as a first‐line therapy for reducing blood pressure. Nowadays, it is used less commonly as a second‐line treatment. This review aimed to assess reserpine's efficacy as a first‐line agent in reducing blood pressure in primary hypertension. The method involved finding and summarising the best existing evidence from randomised controlled trials. We considered the quality of the included studies to be reasonable, with acceptable randomisation and blinding methods overall, as shown in the 'Risk of bias' graphs. We noted a weakness when the studies did not provide a detailed description of the methods or results, thus, introducing potential reporting bias arising from selective reporting or other biases, such as lack of concealment of allocation. To ensure we include only good‐quality evidence, we only rated a study highly if there was clear evidence that all efforts had been made to ensure neither the participant nor the clinician or assessors were aware of what drug the participant was taking, through concealment of allocation and blinding throughout the study.
This systematic meta‐analysis concluded that reserpine is effective in reducing systolic blood pressure as a first‐line agent. The degree of this effect was mild to moderate. Because the four included studies did not investigate a wide range of doses, no data were available to infer a dose‐related response in blood pressure. Insufficient data were available to evaluate the adverse effects of reserpine therapy. This update did not reveal new studies; as such, the conclusions of this review remain unchanged and represent the most up‐to‐date evidence, current to October 2016.
Background
Description of the condition
Targeting blood pressure as a modifiable risk factor for all‐cause mortality and cardiovascular morbidity (such as myocardial infarctions and strokes) has been well established in the literature. Clinical trials have demonstrated the benefits of antihypertensive medications. Thiazides are the first‐line treatment due to their proven benefit in terms of morbidity and mortality (Wright 1999; ALLHAT 2002; Wright 2009). However, other drugs, such as angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta‐blockers, and calcium channel blockers, have been used as first‐line treatments as well. The evidence for these drugs is not as convincing as it is for thiazides; it is not known whether the differences in mortality and morbidity benefits are due to their different effects on blood pressure. Blood pressure‐lowering efficacy has been studied for most of the aforementioned classes of drugs (Law 2003; Heran 2008a; Heran 2008b).
Description of the intervention
Reserpine is an antihypertensive drug that has been used as a first‐line drug since the 1940s (Wilkins 1953), and more recently, it has been used as a second‐line therapy (SHEP 1991; ALLHAT 2002). The reasons for this change in use are not very clear, but it is generally believed that the development of newer antihypertensive medications with better side effect profiles rendered reserpine less favourable. However, the initial trials used high doses of reserpine (0.75 mg to 10 mg daily), which seemed to cause depression and various other gastrointestinal symptoms (Liebowitz 1957; Blackman 1959; Labarthe 1979).
How the intervention might work
Reserpine, marketed under the brand names Serpalan and Serpasil, is an alkaloid extract from the root of the plant Rauwolfia serpentina. This compound acts by depleting catecholamines, including norepinephrine, dopamine, and serotonin, from central and peripheral synapses (Chekman 1972). This mechanism of action has allowed reserpine to be used as an antihypertensive and antipsychotic medication.
Why it is important to do this review
The use of reserpine as an antihypertensive agent dates to the 1940s. Higher doses were initially reported to cause many side effects (Doyle 1954), but doses as low as 0.05 mg daily may be effective when combined with a diuretic (VACS 1982). No systematic evidence exists pertaining to the use of this agent as a first‐line therapy in primary hypertension. It is rational then to systematically evaluate the efficacy of this agent against placebo before undermining reserpine's potential benefit, especially when considering its convenient dosage schedule and low cost compared with the newer more expensive alternatives.
More data about the efficacy of various doses of reserpine in reducing blood pressure are therefore needed to guide future clinical practice and research. The goal of this Cochrane Review was to evaluate the dose‐related efficacy of reserpine, compared with placebo or no treatment, in reducing blood pressure.
Objectives
The primary objective of this review was to quantify the dose‐related efficacy of reserpine versus placebo or no treatment in reducing systolic blood pressure (SBP) or diastolic blood pressure (DBP), or both.
We also aimed to evaluate the dose‐related effects of reserpine on mean arterial blood pressure (MAP) and heart rate (HR), as well as the dose‐related effects on withdrawals due to adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with or without blinding, comparing reserpine monotherapy with placebo or no treatment in a parallel design. Participant follow up must have been at least three weeks in duration, and blood pressure must have been reported at baseline and at one or more points between three and 12 weeks post‐treatment.
Types of participants
We required the participants to have started the trial with primary hypertension defined as systolic blood pressure (SBP) > 140 mmHg or diastolic blood pressure (DBP) > 90 mmHg, or both, without the presence of a secondary cause of hypertension. There was no gender or age restriction.
Types of interventions
The intervention of interest was reserpine* as monotherapy at any dose** compared with placebo or no treatment***.
*We also considered the whole root extract and other alkaloid extracts of Rauwolfia serpentina.
**Trials in which titration to a higher dose was based on blood pressure response were not eligible if the titration occurred before three weeks of treatment because dose‐response relationships cannot be analysed if participants within each randomised group are taking different doses. However, trials in which a response‐dependent titration took place during or after the three‐ to 12‐week interval were eligible if pre‐titration data were given. For forced titration trials, we extracted data from the lowest dose, provided this dose was given for a three‐ to 12‐week period.
***The definition of placebo is an inert substance designed to resemble the drug being tested but which has no active ingredient and no treatment effect. In trials that use a placebo as a comparator, all participants in the placebo group usually receive the same medical treatment ‐ except for the drug being tested ‐ as the experimental group. This is due to the utilisation of a double‐blind study design in these trials. In trials that do not use treatment as a comparator, all participants in this group also usually receive the same medical treatment as the experimental group, except that no experimental drug is given. In these trials, the design is open‐label and not blinded.
Types of outcome measures
Primary outcomes
The outcome of interest was changes in systolic and diastolic blood pressure from baseline at the three‐ to 12‐week interval in the treatment group compared with the control group.
Secondary outcomes
Outcomes of interest also included changes in mean arterial blood pressure, heart rate in the treatment group compared with the control group, and withdrawal from the study due to adverse events in the treatment group compared with the control group.
Search methods for identification of studies
Electronic searches
The Database of Abstracts of Reviews of Effectiveness (DARE) and the Cochrane Database of Systematic Reviews were searched for related reviews.
We searched the following databases up to 26 October 2016:
the Cochrane Hypertension Group Specialised Register (January 1946 to October 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10);
MEDLINE Ovid (January 1946 to October 2016);
Embase Ovid (January 1974 to October 2016); and
the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 26 October 2016).
The Cochrane Hypertension Group Specialised Register includes controlled trials from searches of AGRICOLA, the Allied and Complementary Medicine Database (AMED), BIOSIS, CAB Abstracts, CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature), Cochrane Central Register of Controlled Trials, Embase, Food Science and Technology Abstracts (FSTA), Global Health, International Pharmaceutical Abstracts (IPA), LILACS (Latin American and Caribbean Health Science Information database), MEDLINE, ProQuest Dissertations & Theses, PsycINFO, SCIRUS, Web of Science, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) with selected MeSH terms and free‐text terms relating to reserpine and hypertension. No language restrictions were used. The MEDLINE search strategy, Appendix 1, was translated into use in CENTRAL, Appendix 2; Embase, Appendix 3; the Cochrane Hypertension Group Specialised Register, Appendix 4; and ClinicalTrials.gov, Appendix 5, using the appropriate controlled vocabulary as applicable.
Searching other resources
We identified reference lists of all papers and relevant reviews and contacted authors of trials reporting incomplete information to provide missing information.
Data collection and analysis
Selection of studies
Two independent reviewers (SDS and MIP) blindly applied the search strategy and the inclusion criteria. We extracted titles and abstracts for relevance. We traced and further assessed relevant bibliographies. A third party (JW) helped with inclusion and exclusion decision‐making.
Data extraction and management
Two reviewers (SDS and MIP) independently reviewed the data from the included studies. We used a standardised form (Appendix 6) to extract the data. We used the Cochrane Review Manager software, RevMan 5, to synthesise the review (RevMan 2014).
Assessment of risk of bias in included studies
We assessed the risk of bias for each trial according to Cochrane guidelines, using the following five criteria.
Sequence generation
Allocation concealment
Blinding or objective assessment of primary outcomes
Incomplete outcome data
Selective outcome reporting
Measures of treatment effect
We used weighted mean systolic and diastolic blood pressure, mean arterial blood pressure (MAP), and heart rate change from baseline to report difference in blood pressure and heart rate versus placebo. We also quantified the total number of participants who withdrew.
Unit of analysis issues
For blood pressure measurements, we accepted mmHg. When reported, we preferred measurements in the sitting position followed by standing, then supine measurements.
Dealing with missing data
In the case of missing data in included trials, we contacted investigators by email, telephone, or fax. We imputed missing values for standard deviation of changes in blood pressure or heart rate from data provided in the trial using the following hierarchy of methods.
Pooled standard deviation calculated either from the t‐statistic corresponding to an exact P value reported or from the 95% confidence interval of the mean difference between treatment group and comparative group.
Standard deviation of blood pressure/heart rate at the end of treatment.
Standard deviation of blood pressure/heart rate at baseline (except if this measure was used for entry criteria).
Weighted mean standard deviation of change in blood pressure/heart rate calculated from at least three other trials using the same dose regimen.
Weighted mean standard deviation of change in blood pressure/heart rate calculated from other trials using any dose.
Assessment of heterogeneity
We applied random‐effects model analysis in case of heterogeneity.
Sensitivity analysis
Robustness of the results involved assessment of the following characteristics.
Quality of trials (adequate randomisation, concealment of allocation, blinding).
Industry‐sponsored trials versus non‐industry‐sponsored trials.
Results
Description of studies
Results of the search
Reported here are both the results of the original review and those of the 2016 update. The original search performed in 2009 yielded 242 studies (186 found in the Cochrane Central Register of Controlled Trials (CENTRAL), 11 in MEDLINE, 27 in Embase, and 18 from bibliographies), of which we excluded 183 (76%) based on reading the title and abstract. We retrieved the full text of the remaining 59 studies, which we reviewed in more detail. Of the retrieved studies, 49 did not meet our inclusion because of the following reasons.
Most of the retrieved potential trials (23) combined reserpine with other antihypertensive agents (Hughes 1955; Lee 1956; Krogsgaard 1958; Agnew 1963; Parkes 1969; Smith 1969; Bracharz 1971; Kennedy 1971; Gaskin 1972; Glazer 1972; Nicaise 1973; Ferguson 1975; Rösler 1975; Van Hoose 1976; Krämer 1977; Finnerty 1980; Salmela 1981; Seedat 1984; Leary 1989; Stein 1990; Schmidt 1991; Mattes 1977).
Furthermore, 13 trials added reserpine as a second‐line agent when the first‐line agent was insufficient (Wolff 1966; VA‐NHLBI 1978; Finnerty 1979; VACS 1982; SHEP 1989; VACS 1990; SHEP 2008; SHEP 1995; SHEP 1996; Manyemba 1997; SHEP 1998a; SHEP 1998b; SHEP 1998c; Wright 2008).
When reserpine was used as a first‐line agent in monotherapy, it was mostly compared to other agents and no placebo group was present. This included 14 trials (Finnerty 1954; Kuhns 1954; Torsti 1969; Safar 1975; Ferguson 1976; Josebury 1976; Kanda 1977; Smith 1977; INAGAKI 1978; De Divitiis 1981; Ogawa 1984; Griebenow 1997; Krönig 1997).
Certain trials had more than one reason for exclusion. For example, Griebenow 1997 used reserpine in a fixed‐dose combination with a thiazide and compared it with an angiotensin‐converting enzyme (ACE) inhibitor with no placebo control arm.
We further excluded six trials either because they did not report the outcomes of interest (two trials) or because they had a cross‐over design (four trials). As such, only four trials met our inclusion criteria and were include in the analysis. We provide a complete accounting of all the search results in the QUOROM flowchart of search results (Figure 1).
1.
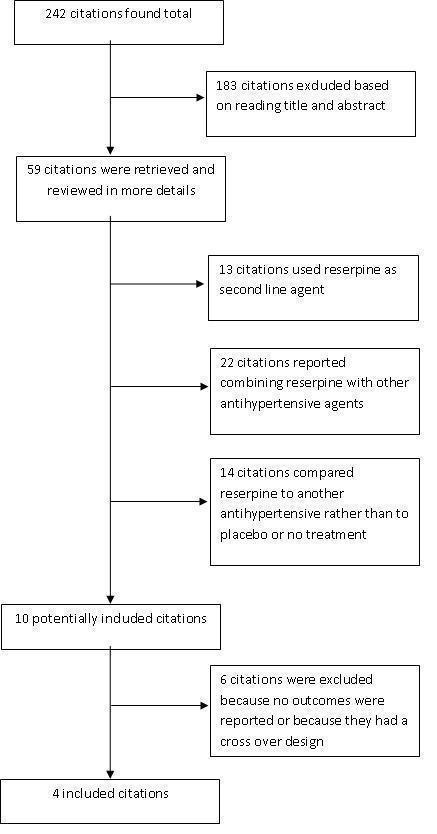
QUOROM flowchart of search results
The updated search was performed in October 2016 and yielded 1786 records. After removing duplicates (studies already reviewed), we screened 20 abstracts, of which we excluded 17 as irrelevant and retrieved three in full text and assessed for eligibility (Christman 1956; Suckle 1956; Johnson 1961). We excluded all three. Refer to Figure 2 for a flowchart of 2016 update search results.
2.
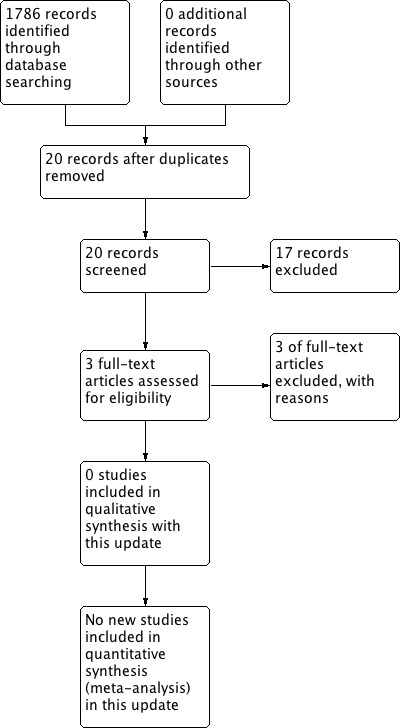
Search results (updated review 2016)
Included studies
We included four trials in the final analysis (Kfogsgaard 1957; Shapiro 1957; VACS 1960; Velasco 1975). Kfogsgaard 1957 is an outpatient, double‐blind RCT that aimed to compare the hypotensive effects of reserpine, phenobarbital, and placebo in a cross‐over design. As such, we included in the analysis only the first treatment interval, where 10 participants received reserpine and seven participants received placebo in parallel and before the cross‐over. This interval was eight to nine weeks long. The authors reported individual systolic blood pressure (SBP) and diastolic blood pressure (DBP) data, which allowed us to perform the desired calculations here. The dosage of reserpine during this first interval was 0.25 mg in this period. VACS 1960 is also a double‐blind controlled trial where participants at the Veterans Administrations Hospitals were randomised into one of three groups based on the severity of their hypertension (mild, moderately severe, and severe). Reserpine monotherapy was only investigated in the mild and moderately severe group. The duration of the study was 12 months long; however, it reported SBP and DBP data at baseline and at three, six, nine, and 12 months. We used the data at three months to perform our calculations. The study used a daily dose of 1 mg for the first two weeks followed by a maintenance dose of 0.5 mg. As this was not based on blood pressure response, we used the maintenance dose in our analysis. Velasco 1975 investigated the effect of a reserpine‐furosemide combination on blood pressure reduction compared with reserpine only, furosemide only, and placebo. Mean arterial pressure (MAP) was the main outcome. Nine participants had a dose of 0.3 mg reserpine daily for eight weeks, and there were seven participants in the placebo group. Shapiro 1957 used a different alkaloid extract of Rauwolfia serpentina, called Rauwiloid (also described elsewhere) (Finnerty 1954; Kfogsgaard 1957). Rauwiloid, phenobarbital, and placebo were studied for 30 weeks in an outpatient hypertension clinic. The trial used a daily dose of 6 mg of Rauwiloid. We included no new studies from the 2016 updated search results.
Excluded studies
We reviewed a total of nine trials in detail and excluded these because of the following reasons:
they did not report outcomes according to allocation (Palmer 1955; Sosa 1960):
they had a cross‐over design (Achor 1955; Bello 1956; Lee 1956; Sheldon 1957);
they did not report relevant outcomes (Christman 1956);
they lacked a parallel cross‐over design (Johnson 1961);
they lacked a double‐blind, placebo‐controlled design (Suckle 1956). (Please see the 'Characteristics of excluded studies' tables.)
Risk of bias in included studies
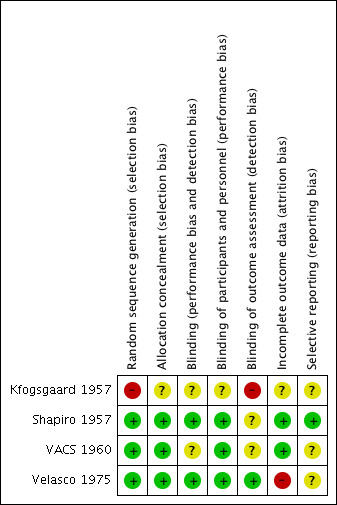
Although all included trials described a double‐blind technique, the description of the methodology was poor in all except Velasco 1975. For example, Kfogsgaard 1957 did not provide the method of randomisation and concealment of allocation. VACS 1960 stratified the randomisation according to the severity of the hypertension, but the paper did not describe the method of randomisation and concealment of allocation. Velasco 1975 described the use of a randomisation table, a code number, and tablets with the same appearance in colour, size, and shape, while Shapiro 1957 involved the pharmacist in randomising participants; thus, we assumed that this trial concealed allocation. For a complete description of risk of bias, refer to the 'Characteristics of included studies' tables and the 'Risk of bias' tables and graphs (Figure 3; Figure 4).
3.
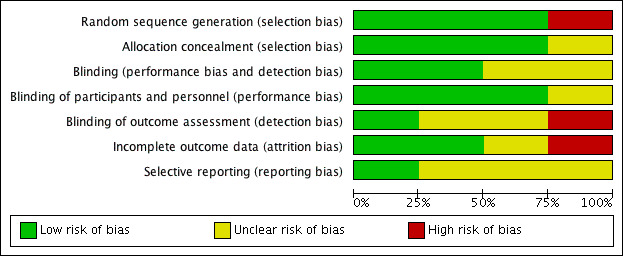
4.
Effects of interventions
Weighted mean change in systolic blood pressure
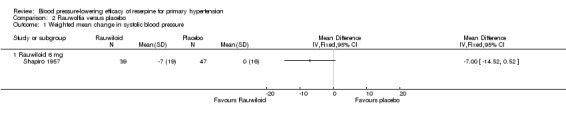
Of the four included trials, three reported systolic blood pressure (BP) data (Kfogsgaard 1957; Shapiro 1957; VACS 1960). Because Shapiro 1957 used Rauwiloid (6 mg/day), a different alkaloid extract of the plant Rauwolfia serpentina, we did not pool BP data from this trial with the remaining two. But the effect of Rauwiloid 6 mg/day from Shapiro 1957 (weighted mean difference (WMD) ‐7.00, 95% confidence interval (CI) ‐14.52 to 0.52) was similar to that achieved by 0.5 mg of reserpine in the VACS 1960 trial.
The pooled effect from the two trials Kfogsgaard 1957 and VACS 1960, which included 90 participants in the reserpine group and 45 in the placebo group, showed a statistically significant reduction in SBP in favour of reserpine (WMD ‐7.92, 95% CI ‐14.05 to ‐1.78). Furthermore, there was a dose‐response pattern when comparing 0.25 mg of reserpine in Kfogsgaard 1957 (WMD ‐1.00, 95% CI ‐17.67 to 15.67) with 0.5 mg in VACS 1960 (WMD ‐9.00, 95% CI ‐15.60 to ‐2.40) (see Figure 5).
5.
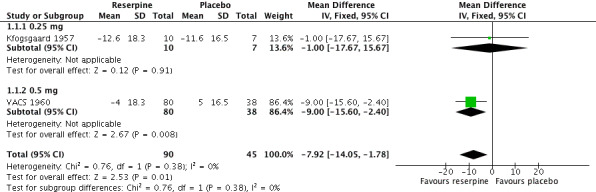
Forest plot of comparison: 1 Reserpine versus placebo, outcome: 1.1 Weighted mean change in systolic blood pressure.
Weighted mean change in diastolic blood pressure
The same trials also reported changes in diastolic blood pressure. The pooled effect showed a non‐statistically significant reduction in DBP in favour of reserpine (WMD ‐4.15, 95% CI ‐9.19 to 0.90). However, there was a trend of greater effect with reserpine 0.5 mg compared to 0.25 mg. Unlike the case with SBP, Rauwiloid seemed to induce a reduction in DBP greater than any of the other reserpine doses (WMD ‐10.00, 95% CI ‐14.44 to ‐5.56).
Weighted mean change in mean arterial pressure
Both Velasco 1975 and Kfogsgaard 1957 reported MAP, and it was possible to calculate it for VACS 1960 using the formula MAP = DBP + (1/3)(SBP‐DBP). The pooled effect from these three trials showed a statistically significant reduction in MAP (WMD ‐7.10, 95% CI ‐11.81 to ‐2.38). However, there was statistically significant heterogeneity across trials (I² = 55%), and this effect was no longer significant when we applied a random‐effects model (see Figure 6).
6.
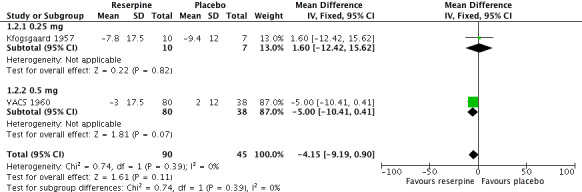
Forest plot of comparison: 1 Reserpine versus placebo, outcome: 1.2 Weighted mean change in diastolic blood pressure.
Shapiro 1957 did not report MAP results for Rauwiloid 6 mg/day, and it was not possible to calculate as the study only reported change in systolic and diastolic BP.
Change in heart rate
Kfogsgaard 1957 and Velasco 1975 reported heart rate data. The pooled effect showed a statistically significant reduction in heart rate (HR) by reserpine (WMD ‐8.82, 95% CI ‐14.20 to ‐3.43, P = 0.001). However, there was significant heterogeneity (I² = 86%), and when we applied the random‐effects model, this effect was no longer significant.
The results for Rauwiloid (6 mg/day) in Shapiro 1957 showed a significant reduction in HR (WMD ‐6.00, 95% CI ‐9.84 to ‐2.16, P = 0.002).
Withdrawal due to adverse effects
None of the included trials reported withdrawals due to adverse events. Yet, all trial reports generally discussed withdrawals, with the exception of Kfogsgaard 1957, which had no withdrawals as it reported data for the same number of included participants. All trials discussed reports of side effects from the intervention.
Discussion
This is the first systematic review investigating the efficacy of reserpine in participants with essential hypertension. Reserpine has been used for many years as an antihypertensive agent. Doses ranging from 0.05 mg to 2 mg daily have been tried (Kfogsgaard 1957; Ferguson 1976; SHEP 1991; ALLHAT 2002). Large trials, such as 'Systolic Hypertension in the Elderly Program' (SHEP) and the 'Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial' (ALLHAT), used reserpine as a second‐line agent. The SHEP trial used reserpine in a dose of 0.05 mg to 0.1 mg when atenolol was not tolerated (SHEP 1998a), while ALLHAT used it in a dose of 0.05 mg to 0.2 mg, as an alternative to either clonidine or atenolol at the physician's discretion (ALLHAT 2002). No rationale is provided for these regimens.
We searched the literature for evidence evaluating reserpine as a first‐line agent against placebo or no treatment in essential hypertension. The four trials that we included in this review used the following daily doses: 0.25 mg (Kfogsgaard 1957), 0.5 mg (VACS 1960), and 0.3 mg (Velasco 1975), while Shapiro 1957 used 6 mg of an alternative extract of Rauwolfia serpentina called Rauwiloid.
Appropriate pooling of the various reported results was possible in three of the four included trials (Kfogsgaard 1957; VACS 1960; Velasco 1975), which showed that reserpine causes a statistically significant reduction in systolic blood pressure (SBP) (weighted mean difference (WMD) ‐7.92, 95% CI ‐14.05 to ‐1.78). There was also a statistically significant reduction in mean arterial blood pressure (MAP) (WMD ‐7.10, 95% CI ‐11.81 to ‐2.38, P = 0.003) and heart rate (HR) (WMD ‐8.82, 95% CI ‐14.20 to ‐3.43, P = 0.001), but the heterogeneity test was significant (I² = 55%, I² = 86%, respectively), and the effect disappeared when we applied a random‐effects model. Although the pooled effect showed a reduction in diastolic blood pressure (DBP) in favour of reserpine, this result was not significant (WMD ‐4.15, 95% CI ‐9.19 to 0.90).
Because of the small number of included trials and because none of them investigated different doses, the dose‐related response was inconsistent amongst outcomes. However, 0.5 mg of reserpine seemed to induce a better reduction in SBP than 0.25 mg. Furthermore, Shapiro 1957 used Rauwiloid, a different alkaloid extract from the plant Rauwolfia serpentina, and it is unclear how a dose of 6 mg Rauwiloid compares with reserpine, although it is generally documented that the less pure the alkaloid is, the higher the dose needed to achieve a response and that reserpine is the most refined active ingredient to be isolated (Achor 1955). The doses in the included trials for reserpine range from 0.25 mg to 0.5 mg while doses have been reported that are as low as 0.05 mg daily, Palmer 1955, and as high as 2 mg daily (Doyle 1954; Stuppy 1955). This does not include investigations that report the use of the entire root extract, Rauwolfia serpentina (Chakravarty 1951; Wilkins 1953; Finnerty 1954; Sheldon 1957). Our search did not identify trials that investigated these doses and extract variations against placebo in a parallel randomised controlled trial (RCT) design. However, the dose‐related hypotensive effect of reserpine has been studied in other designs that did not meet the criteria of this systematic review (Doyle 1954; VACS 1982).
We therefore analysed Rauwiloid (6 mg/d) separately. Shapiro 1957 was the only trial in this comparison, and it reported SBP, DBP, and HR data in 39 participants taking Rauwiloid against 47 taking placebo. Reduction in SBP was not significant (WMD ‐7.00, 95% CI ‐14.52 to 0.52) while reduction in DBP (WMD ‐10.00, 95% CI ‐14.44 to ‐5.56) and HR (WMD ‐6.00, 95% CI ‐9.84 to ‐2.16) were both significant.
Summary of main results
The overall results of this Cochrane Review, which included four trials, indicate that reserpine has a statistically significant hypotensive effect on SBP as first‐line agent (Analysis 1.1). It also lowers DBP (Analysis 1.2), MAP (Analysis 1.3), and HR (Analysis 1.4), but these results were not statistically significant. Rauwiloid, an alternative Rauwolfia serpentina alkaloid to reserpine, seemed to reduce DBP (Analysis 2.2) and HR (Analysis 2.3) with statistical significance. The included trials did not report withdrawals due to adverse effects, and as such, we could not quantify them. While there was a general pattern towards a dose‐related response in SBP, some inconsistencies existed, mostly with MAP and HR. Therefore, the dose‐response data are weak, and trials with a more inclusive dosage range are lacking. As well, parallel placebo‐controlled data on the various Rauwolfia serpentina alkaloids and the whole root are also lacking to draw conclusive evidence. Since we found no new studies with the updated search in 2016, the results and outcomes of the analysis of this review remain unchanged.
1.1. Analysis.
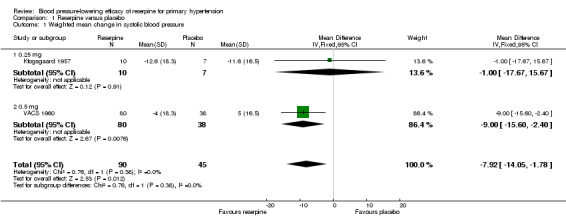
Comparison 1 Reserpine versus placebo, Outcome 1 Weighted mean change in systolic blood pressure.
1.2. Analysis.
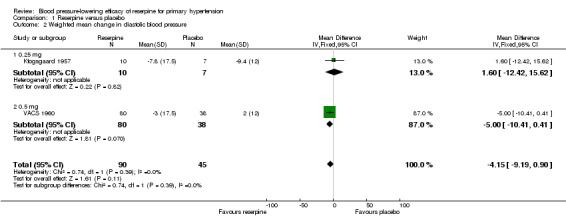
Comparison 1 Reserpine versus placebo, Outcome 2 Weighted mean change in diastolic blood pressure.
1.3. Analysis.
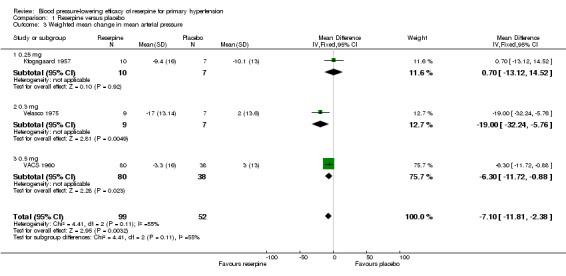
Comparison 1 Reserpine versus placebo, Outcome 3 Weighted mean change in mean arterial pressure.
1.4. Analysis.
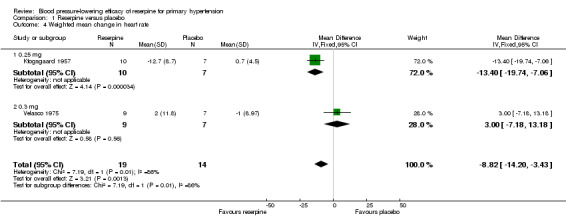
Comparison 1 Reserpine versus placebo, Outcome 4 Weighted mean change in heart rate.
2.2. Analysis.
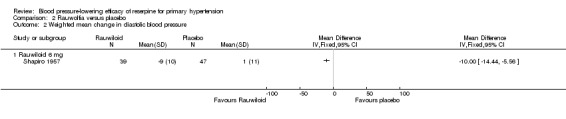
Comparison 2 Rauwolfia versus placebo, Outcome 2 Weighted mean change in diastolic blood pressure.
2.3. Analysis.
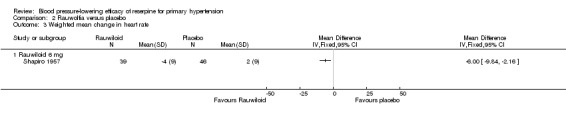
Comparison 2 Rauwolfia versus placebo, Outcome 3 Weighted mean change in heart rate.
Overall completeness and applicability of evidence
Although the evidence from four RCTs (with 237 participants) demonstrates a statistically significant hypotensive action of reserpine on SBP, this can be considered sparse evidence, and the great possibility of publication bias precludes the applicability of this evidence to a general population with mild to moderate hypertension. Without systematic data on withdrawals due to adverse effects and a better dose‐response pattern, it is challenging to compare reserpine to other antihypertensive agents in terms of its benefit as a first‐line agent.
Authors' conclusions
Implications for practice.
The randomised controlled trial (RCT) evidence shows that reserpine monotherapy is effective in reducing systolic blood pressure (SBP) roughly to the same degree as other first‐line antihypertensive drugs. However, we could not make definite conclusions as the sample size from these RCTs was small. For the same reason, we could not establish a dose‐response pattern.
Implications for research.
With its long‐standing history and usage, it is surprising that we could only find four trials evaluating the antihypertensive efficacy of reserpine in a double‐blind, parallel RCT against placebo or no treatment. More RCTs are needed to assess the effects of reserpine monotherapy on blood pressure and to determine the dose‐related safety profile before the role of this drug in the treatment of primary hypertension can be established.
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2016 | New search has been performed | Review updated based on 26 October 2016 search results |
| 7 November 2016 | New citation required but conclusions have not changed | Update |
Acknowledgements
The authors would like to acknowledge the assistance provided by Cochrane Hypertension.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1948 to Present with Daily Update Search Date: 26 October 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 reserpine.mp. 2 (raunervil or rausedil or rausedyl or serpalan or serpasil or serpivite).mp. 3 (rauvolfia? or rauwolfia?).mp. 4 or/1‐3 5 hypertension/ 6 hypertens$.tw. 7 exp blood pressure/ 8 blood pressure.mp. 9 or/5‐8 10 randomized controlled trial.pt. 11 controlled clinical trial.pt. 12 randomized.ab. 13 placebo.ab. 14 drug therapy.fs. 15 randomly.ab. 16 trial.ab. 17 groups.ab. 18 or/10‐17 19 animals/ not (humans/ and animals/) 20 18 not 19 21 4 and 9 and 20
Appendix 2. CENTRAL search strategy
Database: Wiley ‐ Cochrane Central Register of Controlled Trials <2016 Issue 4> Search Date: 26 October 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1reserpine:ti,ab,kw in Clinical Trials #2(raunervil or rausedil or rausedyl or serpalan or serpasil or serpivite):ti,ab,kw in Clinical Trials #3(rauvolfia* or rauwolfia*):ti,ab,kw in Clinical Trials #4(#1 OR #2 OR #3) #5MeSH descriptor Hypertension, this term only #6hypertens*:ti,ab in Clinical Trials #7MeSH descriptor Blood Pressure explode all trees #8blood pressure:ti,ab,kw in Clinical Trials #9(#5 OR #6 OR #7 OR #8) #10(#4 AND #9)
Appendix 3. Embase search strategy
Database: Embase <1980 to 2016 October 26> Search Date: 26 October 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 reserpine.mp. 2 (raunervil or rausedil or rausedyl or serpalan or serpasil or serpivite).mp. 3 (rauvolfia? or rauwolfia?).mp. 4 or/1‐3 5 exp hypertension/ 6 (anti‐hypertens$ or hypertens$).tw. 7 exp blood pressure/ 8 (blood pressure or bloodpressure).mp. 9 or/5‐8 10 randomized controlled trial/ 11 crossover procedure/ 12 double‐blind procedure/ 13 random$.tw. 14 (crossover$ or cross‐over$).tw. 15 placebo$.tw. 16 (doubl$ adj blind$).tw. 17 allocat$.tw. 18 comparison.ti. 19 trial.ti. 20 or/10‐19 21 (animal$ not (human$ and animal$)).mp. 22 20 not 21 23 4 and 9 and 22
Appendix 4. Cochrane Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search Date: 26 October 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 reserpin* #2 ((raunervil or rausedil or rausedyl or serpalan or serpasil or serpivite)) #3 ((rauvolfia* or rauwolfia*)) #4 #1 OR #2 OR #3 #5 #4 AND (RCT or Review or Meta‐Analysis):DE
Appendix 5. ClinicalTrials.gov search strategy
Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search Date: 26 October 2016 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Study type: Interventional Studies Conditions: hypertension Interventions: reserpine
Appendix 6. Data Extraction Form
Data Extraction Form (modified from (Jüni 2001))
| First author | Journal | Year |
Study eligibility
| RCT |
Relevant participants (essential hypertension) |
Relevant interventions (reserpine, placebo) |
Relevant outcomes (SBP/DBP reduction) |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
Do not proceed if any of the above answers are 'No'. If study to be included in 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies'.
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc.) | |
| Sex of participants (numbers/%, etc.) | |
| Disease status (secondary cause of HTN?) | |
| Other | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| Method used to generate allocation and reasons for grading | GRADE |
| |
Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| Method used to conceal allocation and reasons for grading | GRADE (circle) |
| Adequate | |
| Inadequate | |
| Unclear | |
| Blinding | |
| Person responsible for participants care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| % excluded | |
| Not analysed as 'intention‐to‐treat' | |
| Unclear | |
Were withdrawals described? Yes? No? Unclear?
Data extraction
| Outcomes relevant to your review | |
| Reported in paper (circle) | |
| Outcome 1: systolic/diastolic BP reduction | Yes/No |
| Outcome 2: effect on heart rate | Yes/No |
| Outcome 3: withdrawal due to adverse effects | Yes/No |
| For continuous data | |||||||
| Code of paper | Outcomes |
Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| N | Mean (SD) | N | Mean (SD) | ||||
| A, etc. | SBP/DBP reduction | ||||||
| Heart rate | |||||||
| Withdrawal due to adverse effects | |||||||
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| Trial characteristics | Further details |
| Single centre/multicentre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomised? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/frequency of administration | |
| Duration of treatment (State weeks/months, etc., if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months, or years or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in Meta‐View | |
| Trial design (e.g. parallel/cross‐over) | |
| Other |
Data and analyses
Comparison 1. Reserpine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weighted mean change in systolic blood pressure | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐14.05, ‐1.78] |
| 1.1 0.25 mg | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐17.67, 15.67] |
| 1.2 0.5 mg | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐15.60, ‐2.40] |
| 2 Weighted mean change in diastolic blood pressure | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐4.15 [‐9.19, 0.90] |
| 2.1 0.25 mg | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐12.42, 15.62] |
| 2.2 0.5 mg | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐10.41, 0.41] |
| 3 Weighted mean change in mean arterial pressure | 3 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐11.81, ‐2.38] |
| 3.1 0.25 mg | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐13.12, 14.52] |
| 3.2 0.3 mg | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐19.0 [‐32.24, ‐5.76] |
| 3.3 0.5 mg | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐11.72, ‐0.88] |
| 4 Weighted mean change in heart rate | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐14.20, ‐3.43] |
| 4.1 0.25 mg | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐13.40 [‐19.74, ‐7.06] |
| 4.2 0.3 mg | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.18, 13.18] |
Comparison 2. Rauwolfia versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weighted mean change in systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Rauwiloid 6 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐14.52, 0.52] | |
| 2 Weighted mean change in diastolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Rauwiloid 6 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐14.44, ‐5.56] | |
| 3 Weighted mean change in heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Rauwiloid 6 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐9.84, ‐2.16] |
2.1. Analysis.
Comparison 2 Rauwolfia versus placebo, Outcome 1 Weighted mean change in systolic blood pressure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kfogsgaard 1957.
| Methods | Double‐blind cross‐over study | |
| Participants | 26 participants with benign primary hypertension for 4.1 years | |
| Interventions |
The duration of the control period was 7 weeks followed by each 1 of the interventions for 9 weeks. |
|
| Outcomes |
|
|
| Notes | The study used data from the first treatment period; therefore, we included only parallel comparisons before cross‐over occurred. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was no description of methodology used: the paper only stated that "the order of administration varied from one patient to another" without further details. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of methodology to ensure allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Although the authors stated that they used a double‐blind technique and that "the examiner did not know which tablet a patient received at a given time," they provided no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the authors stated that they used a double‐blind technique and all tablets had the same appearance, there was no further details explaining other factors such as taste. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no explanation of methodology to assess the blinding applied to outcomes, such as blood pressure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not describe how many participants met the inclusion criteria, were randomised, and were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The authors did not describe how many participants met the inclusion criteria, were randomised, and were included in the BP analysis. |
Shapiro 1957.
| Methods | Randomised placebo‐controlled trial with double‐blind design | |
| Participants | 144 participants with mild to moderately severe hypertension in an outpatient clinic setting | |
| Interventions |
The duration of the trial was 30 weeks. |
|
| Outcomes |
|
|
| Notes | Rauwiloid = rauwolfia alkaloid extract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There was a clear description of how the study generated randomisation sequence, which was by the pharmacist randomly choosing treatment or placebo and the next participant automatically getting the next intervention. |
| Allocation concealment (selection bias) | Low risk | The authors knew the sequence, but the study blinded both the participants and the physicians recording the data. Participants were not informed that a placebo was also employed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study blinded the clinician and data assessors to what the participant was taking. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study blinded the participant, as the pharmacist randomly selected treatment after receiving the prescription. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors reported "alternation of drug was known only to us", indicating that the concealment was possibly revealed, but it is unclear whether the actual treatment was known as well. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported all measured outcomes; they had no exclusion criteria other than malignant hypertension, and they simply included all referred participants as the setting was a specialised "hypertension clinic". |
| Selective reporting (reporting bias) | Low risk | The study reported all outcomes and measures; it had no exclusion criteria other than malignant hypertension and simply included all referred participants as the setting was a specialised "hypertension clinic". The authors reported baseline participant characteristics. |
VACS 1960.
| Methods | A double‐blind controlled trial | |
| Participants | 425 participants admitted to the Veterans Administration Hospitals stratified based on severity of hypertension as assessed by 5 criteria: basal DBP; optic fundi; and cardiac, cerebral, and renal complications
|
|
| Interventions | Interventions differed based on severity. The trial time was 12 months. Mild
Severe
All ganglionic‐blocking agents in 1 to 3 were given in doses of up to 10 units depending on BP response, and reserpine 1 mg was given for 2 weeks then 0.5 mg thereafter Moderate
For moderate severity, reserpine tablets were 0.25 mg, hydralazine was 25 mg, and 1 unit of ganglionic‐blocking agents consisted of the following: mecamylamine 1 mg, chlorisondamine 8 mg, and pentolinium tartrate 10 mg. |
|
| Outcomes |
|
|
| Notes | Half of the participants were lost to follow‐up within the first 3 months; therefore, data were not complete. The BP measurements were reported at home and in clinic. Home measurements were in the sitting position, and we thus use them here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors reported randomising participants after stratification by severity using coded envelopes known only to the statistician. |
| Allocation concealment (selection bias) | Low risk | The authors described a complex code system for the different interventions used, to discourage the possibility of the physician remembering and thus associating a certain code with a certain BP response. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors reported that treatment and dose adjustments were done under certain circumstances for participant safety, suggesting possible unblinding risk. However, they stated that they used "code numbered" drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors stated that they used code‐numbered drugs at all times, so participants were not aware of their treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors reported that they used complex double codes in the treatment allocation, making it difficult for the physician and assessor to reveal the treatment. Yet the labelling of the groups with "antipressor" for reserpine and its placebo or "reduction" for hydralazine and its placebo might have narrowed down and thus revealed potential allocation to the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study well‐outlined the inclusion and exclusion criteria. The authors reported that they undertook treatment and dose adjustments under certain circumstances for participant safety. |
| Selective reporting (reporting bias) | Unclear risk | The study reported participants lost to follow‐up and cases of treatment discontinuation; however, there was no evidence that the study undertook intention‐to‐treat analysis unless the participant remained in the study for at least 3 months, in which case they carried foward the last blood pressure value. As such,the study made a partial attempt to account for dropout data. The authors reported that dropout rates were lower in those treated with placebo, suggesting the implication of side effects in the dropout rates, but there was no follow up to confirm this. |
Velasco 1975.
| Methods | Double‐blind RCT with parallel design | |
| Participants | 40 participants, aged 31 to 72 years with confirmed essential hypertension, were studied at an out‐patient hypertension service. By its end, the study excluded 8 participants because they discontinued the medications or used other treatments.
The study reported no other demographic details. |
|
| Interventions |
The placebo and drugs were administered 3 times per day. The duration of the trial was 8 weeks. |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors described using randomisation tables. |
| Allocation concealment (selection bias) | Low risk | The authors described the use of randomisation tables and similar tablets for placebo and treatment groups. They also mentioned the use of codes to blind physicians and participants. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study documented the blinding of both participants and participants using codes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study documented the blinding of both participants and participants using codes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study documented the blinding of both participants and participants using codes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study reported the exclusion of 8 participants due to discontinuation of the study or using other antihypertensive drugs; however, there was no mention of at what stage this exclusion happened and whether thery used their data in intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | The authors reported all outcomes and analysis for included participants but do not report further information about excluded participants, when they excluded them, and their outcomes |
MAP: mean arterial pressure. SBP: systolic blood pressure. DBP: diastolic blood pressure. BP: blood pressure. RCT: randomised controlled trial. Na: sodium K: potassium Cl: confidence interval
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achor 1955 | The study had a cross‐over design. |
| Bello 1956 | The study had a cross‐over design. |
| Christman 1956 | The study did not report the relevant outcomes. |
| Johnson 1961 | The study was lacking a parallel cross‐over design. |
| Lee 1956 | The study had a cross‐over design. |
| Palmer 1955 | The study did not report the outcomes of interest. |
| Sheldon 1957 | The study had a cross‐over design. |
| Sosa 1960 | The study did not report the outcomes of interest. |
| Suckle 1956 | The study was lacking a double‐blinded placebo‐controlled design. |
Differences between protocol and review
There were no differences between the protocol and this update.
Contributions of authors
Sandy Shamon took the lead role in searching for, identifying, and assessing studies; in data extraction and analyses; and in writing up the review. Marco Perez helped to develope optimal search strategies and identify trials, assess studies, and data extract, and he contributed to the writing of the final draft.
Sources of support
Internal sources
No sources of support supplied
External sources
University of British Columbia, Canada.
Declarations of interest
Sandy Shamon: nothing to declare. Marco Perez: nothing to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Kfogsgaard 1957 {published data only}
- Kfogsgaard AR. Hypotensive effect of reserpine compared with phenobarbital and placebo. Acta Medica Scandinavica 1957;157(5):379‐85. [PubMed] [Google Scholar]
Shapiro 1957 {published data only}
- Shapiro AP, Teng HC. Technic of controlled drug assay illustrated by a comparative study of Rauwolfia serpentina, phenobarbital and placebo in the hypertensive patient. The New England Journal of Medicine 1957;256(21):970‐5. [DOI] [PubMed] [Google Scholar]
VACS 1960 {published data only}
- Veterans Administration Cooperative Group (VACS). A double blind control study of antihypertensive agents. I. Comparative effectiveness of reserpine, reserpine and hydralazine, and three ganglionic blocking agents, chlorisondamine, mecamyamine, and pentolinium tartrate. Archives of Internal Medicine 1960;106:81‐96. [PubMed] [Google Scholar]
Velasco 1975 {published data only}
- Velasco M, Arbona J, Guevara J, Torres J. A randomized double‐blind study of furosemide‐reserpine in essential hypertension. Current Therapeutic Research, Clinical and Experimental 1975;18(3):395‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Achor 1955 {published data only}
- Achor RW, Hanson NO, Gifford RW Jr. Hypertension treated with Rauwolfia serpentina and with reserpine; controlled study disclosing occasional severe depression. Journal of the American Medical Association 1955;159(9):841‐5. [DOI] [PubMed] [Google Scholar]
Bello 1956 {published data only}
- Bello CT, Turner LW. Reserpine as an antihypertensive in the outpatient clinic, a double blind clinical study. The American Journal of the Medical Sciences 1956;232(2):194‐7. [DOI] [PubMed] [Google Scholar]
Christman 1956 {published data only}
- Christman RS. Efficacy of Rauwolfia serpentina preparations in the treatment of hypertension in general medical practice: a clinical comparison of three regimens. American Practitioner and Digest of Treatment 1956;7(4):614‐7. [PubMed] [Google Scholar]
Johnson 1961 {published data only}
- Johnson RW, Sosa G, Morita Y, Mader IJ. Anti‐hypertensive effects of chlorothiazide, reserpne and placebo. A double‐blind evaluation. Michigan State Medical Society 1961;60:1420‐3. [PubMed] [Google Scholar]
Lee 1956 {published data only}
- Lee R, Seligmann AM, Goebel D, Fulton LA, Clark MA. Reserpine‐hydralazine combination therapy of hypertensive disease, with hydralazine in doses generally below the toxic range. Annals of Internal Medicine 1956;44(3):456‐65. [DOI] [PubMed] [Google Scholar]
Palmer 1955 {published data only}
- Palmer RS. The hypotensive action of Rauwolfia serpentina and reserpine: a double hidden placebo study of ambulatory patients with hypertension. American Practitioner and Digest of Treatment 1955;6(9):1323‐7. [PubMed] [Google Scholar]
Sheldon 1957 {published data only}
- Sheldon MB, Kotte JH. Effect of Rauwolfia serpentina and reserpine on the blood pressure in essential hypertension; a long‐term double‐blind study.. Circulation 1957;16(2):200‐6. [DOI] [PubMed] [Google Scholar]
Sosa 1960 {published data only}
- Sosa G, Morita Y, Mader IJ. A double blind evaluation of the antihypertensive effect of reserpine, phenobarbital, hydralazine, syrosingopine, and placebo in ambulatory subjects. Angiology 1960;11(5):381‐6. [Google Scholar]
Suckle 1956 {published data only}
- Suckle E. Comparison of Rauwolfia drugs in treatment of hypertension. Geriatrics 1956;11(11):509‐13. [PubMed] [Google Scholar]
Additional references
Agnew 1963
- Agnew TM, Irvine ROH, North JDK. Methyldopa and hydrochlorothiazide compared with reserpine and hydrochlorothiazide in hypertension. British Medical Journal 1963;2(5360):781‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
ALLHAT 2002
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981‐97. [DOI] [PubMed] [Google Scholar]
Blackman 1959
- Blackman JG, Campion DS, Fastier FN. Mechanism of action of reserpine in producing gastric haemorrhage and erosion in the mouse. British Journal of Pharmacology and Chemotherapy 1959;14(1):112‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bracharz 1971
- Bracharz H, Laas H. Clinical‐pharmacologic studies and therapy with anti‐hypertensive furosemide‐reserpine preparations. Medizinische Klinik 1971;66(23):1113‐7. [PubMed] [Google Scholar]
Chakravarty 1951
- Chakravarty NK, Rai Chaudhuri MN. Rauwolfia serpentina in essential hypertension. Indian Medical Gazette 1951;86(8):348‐54. [PMC free article] [PubMed] [Google Scholar]
Chekman 1972
- Chekman IS. Experimental investigation of the mechanism of action of reserpine. Bulletin of Experimental Biology and Medicine 1972;73(3):291‐3. [Google Scholar]
De Divitiis 1981
- Divitiis O, Petitto M, Somma S. Atenolol, chlorthalidone, and reserpine in mild‐moderate hypertension: double‐blind comparison. Drugs Under Experimental & Clinical Research 1981;7(6):773‐9. [Google Scholar]
Doyle 1954
- Doyle AE, Smirk FH. Hypotensive action of reserpine. Lancet 1954;266(6822):1096‐7. [DOI] [PubMed] [Google Scholar]
Ferguson 1975
- Ferguson RK, Rothenberg RJ. Comparative efficacy of reserpine and guanethidine in thiazide‐treated patients with mild to moderate essential hypertension. Clinical Pharmacology & Therapeutics 1975;17(2):233. [Google Scholar]
Ferguson 1976
- Ferguson RK, Rothenberg RJ, Nies AS. Patient acceptance of guanethidine as therapy for mild to moderate hypertension. A comparison with reserpine. Circulation 1976;54(1):32‐7. [DOI] [PubMed] [Google Scholar]
Finnerty 1954
- Finnerty FA. The value of rauwolfia serpentine in the hypertensive patient. American Journal of Medicine 1954;17(5):629‐40. [DOI] [PubMed] [Google Scholar]
Finnerty 1979
- Finnerty FA Jr, Gyftopoulos A, Berry C, McKenney A. Step 2 regimens in hypertension. An assessment. JAMA 1979;241(6):579‐81. [PubMed] [Google Scholar]
Finnerty 1980
- Finnerty FA Jr. Chlorthalidone plus reserpine versus hydrochlorothiazide plus reserpine in a stepped‐care approach to the treatment of essential hypertension. Journal of Clinical Pharmacology 1980;20(5‐6 Pt 1):357‐63. [DOI] [PubMed] [Google Scholar]
Gaskin 1972
- Gaskin R. The clinical effectiveness of dihydroergocristine ‐ reserpine clopamide combination in the treatment of hypertension. Standing Advisory Committee for Medical Research in the British Caribbean Bahamas 1972;17th Scientific Meeting:56p. [Google Scholar]
Glazer 1972
- Glazer N. Reserpine, hydralazine, hydrochlorothiazide combination(Ser‐AP‐ES) in essential hypertension. Current Therapeutic Research, Clinical and Experimental 1972;14(9):561‐72. [PubMed] [Google Scholar]
Griebenow 1997
- Griebenow R, Pittrow DB, Weidinger G, Mueller E, Mutschler E, Welzel D. Low‐dose reserpine/thiazide combination in first‐line treatment of hypertension: efficacy and safety compared to an ACE inhibitor. Blood Pressure 1997;6(5):299‐306. [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MM, Heran IK, James MW. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1955
- Hughes WH, Dennis E, Moyer JH. Treatment of hypertension with oral reserpine alone and in combination with hydralazine and hexamethonium. The American Journal of the Medical Sciences 1955;229(2):121‐34. [DOI] [PubMed] [Google Scholar]
INAGAKI 1978
- INAGAKI Yoshiaki, et al. Therapeutic efficacy of oxprenolol in essential hypertension: a double‐blind comparison with reserpine. Japanese Pharmacology and Therapeutics 1978;6(4):1097‐108. [Google Scholar]
Josebury 1976
- Joesbury HE, Phillips CA, Garrett RT, Wilkes E, Smith AJ. Mild hypertension: a clinical trial conducted in hospital general practice. British Medical Journal 1976;2(6050):1476‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanda 1977
- Kanda Y, et al. Clinical efficacy of apllobal for essential hypertension: a multicenter double‐blind controlled comparison with reserpine. The Japanese Journal of Clinical and Experimental Medicine 1977;54(11):3796‐804. [Google Scholar]
Kennedy 1971
- Kennedy CC, Spiekerman RE, Elveback L. Antihypertensive properties of cryptenamine used with reserpine and methyclothiazide. Journal of Pharmaceutical Sciences 1971;60(8):1139‐41. [DOI] [PubMed] [Google Scholar]
Krogsgaard 1958
- Krogsgaard AR. The role of reserpine in essential hypertension when used alone or combined with hydralazine. Acta Medica Scandinavica 1958;162(6):449‐63. [DOI] [PubMed] [Google Scholar]
Krämer 1977
- Krämer KD, Ghabussi P, Hochrein H. Antihypertensive combination‐therapy with inositol nicotinate in essential hypertension. Die Medizinische Welt 1977;28(27):1198‐201. [PubMed] [Google Scholar]
Krönig 1997
- Krönig B, Pittrow DB, Kirch W, Welzel D, Weidinger G. Different concepts in first‐line treatment of essential hypertension. Comparison of a low‐dose reserpine‐thiazide combination with nitrendipine monotherapy. German Reserpine in Hypertension Study Group. Hypertension 1997;29(2):651‐8. [DOI] [PubMed] [Google Scholar]
Kuhns 1954
- Kuhns K, Djuranovic R, Gehrs C, Koppen K. Comparative clinical studies on the effect of hydrazine phthalazine and the rauwolfia alkaloid reserpine on blood pressure. Klinische Wochenschrift 1954;32(37‐8):930‐5. [DOI] [PubMed] [Google Scholar]
Labarthe 1979
- Labarthe DR. Methodological variation in case‐control studies of reserpine and breast cancer. Journal of Chronic Diseases 1979;32(1‐2):95‐104. [DOI] [PubMed] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326(7404):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leary 1989
- Leary WP, Ryes AJ, Van DBK, Maharaj B. Antihypertensive effects of clopamide administered alone in combination with pindolol or with reserpine and dihydroergocristine. Current therapeutic Research, Clinical and Experimental 1989;46(6):1224‐35. [Google Scholar]
Liebowitz 1957
- Liebowitz D, Carbone JV. Effect of varying doses of reserpine on gastric secretion. The New England Journal of Medicine 1957;257(5):227‐8. [DOI] [PubMed] [Google Scholar]
Manyemba 1997
- Manyemba J. A randomised crossover comparison of reserpine and sustained‐release nifedipine in hypertension. The Central African Journal of Medicine 1997;43(12):344‐9. [PubMed] [Google Scholar]
Mattes 1977
- Mattes JA, Martin D. Propranolol in the treatment of essential hypertension. Journal of the American Medical Association 1977;237(21):2303‐10. [PubMed] [Google Scholar]
Nicaise 1973
- Nicaise J, Ghirardi P. Double‐blind cross‐over study of the hypotensive action of reserpine alone and associated with a diuretic (mebutizide). Bruxelles Medical 1973;53(11):683‐8. [PubMed] [Google Scholar]
Ogawa 1984
- Ogawa K, Ban M, Ito T, Watanabe T, Kobayashi T, Yamazaki N, et al. Diltiazem for treatment of essential hypertension: a double‐blind controlled study with reserpine. Clinical Therapeutics 1984;6(6):844‐53. [PubMed] [Google Scholar]
Parkes 1969
- Parkes WE, Agarwal AP, Hunt LB. Treatment of hypertension with quinethazone alone or in combination with reserpine. The Practitioner 1969;203(214):194‐8. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rösler 1975
- Rösler F, Wegener H, Eulenberger D, Dahme B. The effect of an antihypertensive drug during psychological stress. A double‐blind study. Arzneimittel‐Forschung 1975;25(6):965‐72. [PubMed] [Google Scholar]
Safar 1975
- Safar ME, Weiss YA, Corvol PL, Menard JE, London GM, Milliez PL. Anti‐hypertensive adrenergic‐blocking agents: effects on sodium balance, the renin‐angiotensin system and haemodynamics. Clinical Science and Molecular Medicine 1975;2:93s‐95s. [DOI] [PubMed] [Google Scholar]
Salmela 1981
- Salmela PI, Jounela AJ, Karppanen H. Double‐blind comparison of dihydralazine and prazosin in hypertensive patients on the diuretic‐reserpine regimen. Annals of Clinical Research 1981;13(6):433‐8. [PubMed] [Google Scholar]
Schmidt 1991
- Schmidt A, Vetter W, Dennler HJ, Groll S, Orengo P. Combined uni‐ and multicenter double‐blind studies in hypertensive patients. Comparison of blood pressure measurements. Schweizerische Rundschau fur Medizin Praxis 1991;80(34):849‐55. [PubMed] [Google Scholar]
Seedat 1984
- Seedat YK, Hoosen S, Bhigjee AI. Reserpine plus hydrochlorothiazide and sotalol plus hydrochlorothiazide in Black and Indian hypertensive patients. South African Medical Journal 1984;65(23):915‐7. [PubMed] [Google Scholar]
SHEP 1989
- Probstfield JL, Applegate WB, Borhani NO, Curb JD, Cutler JA, Davis BR, et al. The Systolic Hypertension in the Elderly Program (SHEP): an intervention trial on isolated systolic hypertension. SHEP Cooperative Research Group. Clinical and Experimental Hypertension. Part A, Theory and Practice 1989;11(5‐6):973‐89. [DOI] [PubMed] [Google Scholar]
SHEP 1991
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991;265(24):3255‐64. [PubMed] [Google Scholar]
SHEP 1995
- Kostis JB, Berge KG, Davis BR, Hawkins CM, Probstfield J. Effect of atenolol and reserpine on selected events in the systolic hypertension in the elderly program (SHEP). American Journal of Hypertension 1995;8(12 Pt 1):1147‐53. [DOI] [PubMed] [Google Scholar]
SHEP 1996
- Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996;276(23):1886‐92. [PubMed] [Google Scholar]
SHEP 1998a
- Davis BR, Vogt T, Frost PH, Burlando A, Cohen J, Wilson A, et al. Risk factors for stroke and type of stroke in persons with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. Stroke 1998;29(7):1333‐40. [DOI] [PubMed] [Google Scholar]
SHEP 1998b
- Pahor M, Shorr RI, Somes GW, Cushman WC, Ferrucci L, Bailey JE, et al. Diuretic‐based treatment and cardiovascular events in patients with mild renal dysfunction enrolled in the systolic hypertension in the elderly program. Archives of Internal Medicine 1998;158(12):1340‐5. [DOI] [PubMed] [Google Scholar]
SHEP 1998c
- Savage PJ, Pressel SL, Curb JD, Schron EB, Applegate WB, Black HR, et al. Influence of long‐term, low‐dose, diuretic‐based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: The Systolic Hypertension in the Elderly Program. Archives of Internal Medicine 1998;158(7):741‐51. [DOI] [PubMed] [Google Scholar]
SHEP 2008
- Patel AB, Kostis JB, Wilson AC, Shea ML, Pressel SL, Davis BR. Long‐term fatal outcomes in subjects with stroke or transient ischemic attack: fourteen‐year follow‐up of the systolic hypertension in the elderly program. Stroke 2008;39(4):1084‐9. [DOI] [PubMed] [Google Scholar]
Smith 1969
- Smith WM, Thurm RH, Bromer L. Comparative evaluation of Rauwolfia whole root and reserpine. Clinical Pharmacology and Therapeutics 1969;10(3):338‐43. [DOI] [PubMed] [Google Scholar]
Smith 1977
- Smith WM. Treatment of mild hypertension: results of a ten‐year intervention trial. Circulation Research 1977;40(5 Suppl 1):I98‐205. [PubMed] [Google Scholar]
Stein 1990
- Stein CM, Neill P, Mwaluko GM, Kusema T. Combination of a thiazide, a vasodilator and reserpine compared with methyldopa plus hydrochlorothiazide in the treatment of hypertension in Zimbabwe. South African Medical Journal 1990;77(5):243‐5. [PubMed] [Google Scholar]
Stuppy 1955
- Stuppy LJ, Tober JN. Treatment of hypertension with reserpine (serpasil) alone and in combination with hydralazine (apresoline). Angiology 1955;6(3):253‐9. [DOI] [PubMed] [Google Scholar]
Torsti 1969
- Torsti P, Neuvonen PJ, Vapaatalo HI, Idänpään‐Heikkilä JE. Circulatory and diuretic effects of hydrochlorothiazide and reserpine in man. Annals of Clinical Research 1969;1(2):126‐30. [PubMed] [Google Scholar]
VA‐NHLBI 1978
- Veterans Administration‐National Heart, Lung, and Blood Institute (VA‐NHLBI) Study Group for Evaluating Treatment in Mild Hypertension. Evaluation of drug treatment in mild hypertension: VA‐NHLBI feasibility trial. Plan and preliminary results of a two‐year feasibility trial for a multicenter intervention study to evaluate the benefits versus the disadvantages of treating mild hypertension. Prepared for the Veterans Administration‐National Heart, Lung, and Blood Institute Study Group for Evaluating Treatment in Mild Hypertension. Annals of the New York Academy of Sciences 1978;304:267‐92. [DOI] [PubMed] [Google Scholar]
VACS 1982
- Veterans Administration Cooperative Study (VACS) Participants. Low doses v standard dose of reserpine. A randomized, double‐blind, multiclinic trial in patients taking chlorthalidone. JAMA 1982;248(19):2471‐7. [PubMed] [Google Scholar]
VACS 1990
- Materson BJ, Cushman WC, Goldstein G, Reda DJ, Freis ED, Ramirez EA, et al. Treatment of hypertension in the elderly: I. Blood pressure and clinical changes. Results of a Department of Veterans Affairs Cooperative Study. Hypertension 1990;15(4):348‐60. [DOI] [PubMed] [Google Scholar]
Van Hoose 1976
- Hoose MC, Cutler RE. Antihypertensive efficacy of metolazone (Zaroxolyn) alone and combined with reserpine in treatment of essential hypertension. Current Therapeutic Research, Clinical and Experimental 1976;20(3):266‐76. [PubMed] [Google Scholar]
Wilkins 1953
- Wilkins RW, Judson WE. The use of Rauwolfia serpentina in hypertensive patients. The New England Journal of Medicine 1953;248(2):48‐53. [DOI] [PubMed] [Google Scholar]
Wolff 1966
- Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. Journal of Chronic Diseases 1966;19(3):227‐40. [DOI] [PubMed] [Google Scholar]
Wright 1999
- Wright JM, Lee CH, Chambers GK. Systematic review of antihypertensive therapies: does the evidence assist in choosing a first‐line drug?. CMAJ 1999;161(1):25‐32. [PMC free article] [PubMed] [Google Scholar]
Wright 2008
- Wright JT Jr, Harris‐Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2008;168(2):207‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]


