Abstract
Background
Patients with brain tumour usually suffer from increased pressure in the skull due to swelling of brain tissue. A swollen brain renders surgical removal of the brain tumour difficult. To ease surgical tumour removal, measures are taken to reduce brain swelling, often referred to as brain relaxation. Brain relaxation can be achieved with intravenous fluids such as mannitol or hypertonic saline. This review was conducted to find out which of the two fluids may have a greater impact on brain relaxation.
Objectives
The objective of this review was to compare the effects of mannitol versus those of hypertonic saline on intraoperative brain relaxation in patients undergoing craniotomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 10), MEDLINE via Ovid SP (1966 to October 2013) and EMBASE via Ovid SP (1980 to October 2013). We also searched specific websites, such as www.indmed.nic.in, www.cochrane‐sadcct.org and www.Clinicaltrials.gov. We reran the search in January 2017 and found five potential studies of interest which have been added to a list of ‘Studies Awaiting Classification' and will be incorporated into the formal review findings during the review update.
Selection criteria
We included randomized controlled trials (RCTs) that compared the use of hypertonic saline versus mannitol for brain relaxation. We also included studies in which any other method used for intraoperative brain relaxation was compared with mannitol or hypertonic saline. Primary outcomes were longest follow‐up mortality, Glasgow Outcome Scale score at three months and any adverse events related to mannitol or hypertonic saline. Secondary outcomes were intraoperative brain relaxation, intensive care unit (ICU) stay, hospital stay and quality of life.
Data collection and analysis
We used standardized methods for conducting a systematic review, as described by the Cochrane Handbook for Systematic Reviews of Interventions. Two review authors independently extracted details of trial methodology and outcome data from reports of all trials considered eligible for inclusion. All analyses were made on an intention‐to‐treat basis. We used a fixed‐effect model when no evidence was found of significant heterogeneity between studies, and a random‐effects model when heterogeneity was likely.
Main results
We included six RCTs with 527 participants. Only one RCT was judged to be at low risk of bias. The remaining five RCTs were at unclear or high risk of bias. No trial mentioned the primary outcomes of longest follow‐up mortality, Glasgow Outcome Scale score at three months or any adverse events related to mannitol or hypertonic saline. Three trials mentioned the secondary outcomes of intraoperative brain relaxation, hospital stay and ICU stay; quality of life was not reported in any of the trials. Brain relaxation was inadequate in 42 of 197 participants in the hypertonic saline group and in 68 of 190 participants in the mannitol group. The risk ratio for brain bulge or tense brain in the hypertonic saline group was 0.60 (95% confidence interval (CI) 0.44 to 0.83, low‐quality evidence). One trial reported ICU and hospital stay. The mean (standard deviation (SD)) duration of ICU stay in the mannitol and hypertonic saline groups was 1.28 (0.5) and 1.25 (0.5) days (P value 0.64), respectively; the mean (SD) duration of hospital stay in the mannitol and hypertonic saline groups was 5.7 (0.7) and 5.7 (0.8) days (P value 1.00), respectively
Authors' conclusions
From the limited data available on the use of mannitol and hypertonic saline for brain relaxation during craniotomy, it is suggested that hypertonic saline significantly reduces the risk of tense brain during craniotomy. A single trial suggests that ICU stay and hospital stay are comparable with the use of mannitol or hypertonic saline. However, focus on other related important issues such as long‐term mortality, long‐term outcome, adverse events and quality of life is needed.
Plain language summary
Mannitol versus hypertonic saline for intraoperative brain relaxation in patients undergoing surgery for brain tumour
Review question: We reviewed evidence on the effectiveness of mannitol and hypertonic saline for brain relaxation in people having surgery (craniotomy) for brain tumour.
Background: People with brain tumour undergo a craniotomy, or opening of the skull bone, for its removal. A relaxed brain allows the surgeon to remove the skull bone easily and to remove the tumour without damaging other brain tissue. Brain relaxation is achieved often by using mannitol, which is a hypertonic fluid. Hypertonic solutions are those that have higher solute concentrations when compared with body fluids and tissue. Some surgeons use hypertonic saline instead of mannitol. We wanted to discover whether using hypertonic saline was better or worse than using mannitol.
Study characteristics: The evidence is current to October 2013. We included studies in children (age > 28 days and < 18 years) and adult patients (age > 18 years) of either gender who received mannitol or hypertonic saline during craniotomy for brain tumour. We reran the search in January 2017 and found five potential studies of interest which have been added to a list of ‘Studies Awaiting Classification' and will be incorporated into the formal review findings during the review update.
Key results: We found six studies with 527 participants.
Three studies reported the level of brain relaxation. Hypertonic saline may provide better brain relaxation than mannitol.
The length of intensive care unit stay and hospital stay was reported by one study.
No study reported on the effects of mannitol and hypertonic saline on mortality, the condition of the patient three months after the operation or patient quality of life. Based on our results, we would expect that of 100 patients who received hypertonic saline during surgery, around 22 patients would fail to have adequate brain relaxation compared with 36 patients given mannitol.
Quality of evidence
The quality of evidence for brain relaxation with use of hypertonic saline is low. Further research is needed to assess more important issues such as long‐term mortality, long‐term outcomes, adverse events and quality of life with use of the two fluids.
Summary of findings
Summary of findings for the main comparison. Mannitol versus hypertonic saline for brain relaxation in patients undergoing craniotomy.
| Mannitol versus hypertonic saline for brain relaxation in patients undergoing craniotomy | ||||||
| Patient or population: patients with brain relaxation undergoing craniotomy Settings: Intervention: mannitol versus hypertonic saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mannitol | Hypertonic saline | |||||
| Inadequate brain relaxation 3‐ or 4‐point scalesa | Study population | RR 0.6 (0.44 to 0.83) | 387 (3 studies) | ⊕⊕⊝⊝ lowb | ||
| 358 per 1000 | 215 per 1000 (157 to 297) | |||||
| Moderate | ||||||
| 302 per 1000 | 181 per 1000 (133 to 251) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a3‐ or 4‐point scales were used by study authors to assess brain relaxation. b Downgraded two levels owing to serious concerns about allocation, blinding and potential sources of other bias noted in the included studies
Background
One of the important goals of anaesthetic management for patients undergoing craniotomy is to provide a relaxed brain on which the surgeon can operate. This allows easy surgical manipulation and causes less damage to normal brain tissue. This, in turn, results in less secondary injury to the brain, which improves the patient's neurological outcome. Raised intracranial pressure results in a tense brain during the intraoperative period. Administration of mannitol is generally considered to be a "gold standard" for the treatment of raised intracranial pressure. Hypertonic saline is another intravenous fluid that has effects comparable with those of mannitol in terms of reduction in intracranial pressure (Battinson 2005; Harutjunyan 2005; Schwarz 2002; Vialet 2003). Earlier works on the use of hypertonic saline in neurosurgical patients have shown promising results (De Vivo 2001; Gemma 1997).
Description of the condition
Raised intracranial pressure during the intraoperative period results in bulging of brain and poor surgical exposure. Various treatment methods have been used by anaesthetists to reduce this intraoperative brain bulge. These methods include hyperventilation (increasing respiratory rate); drainage of cerebrospinal fluid; use of intravenous anaesthetic agents such as propofol and thiopentone; facilitation of venous drainage by positioning of patients with head up; and use of osmotic agents, such as mannitol and hypertonic saline. These manoeuvres facilitate relaxation of the brain and surgery, as less retraction pressure is required to separate the lobes of the brain.
Description of the intervention
Mannitol is a six‐carbon sugar with a molecular weight of 182; it is available as 20% and 25% solution. Mannitol is rapidly infused intravenously in doses of 0.25 to 1 gm/kg. As it is hyperosmolar, that is, has greater osmolality than blood, mannitol facilitates the shift of water from the brain into the vasculature. Hypertonic saline is the hyperosmolar solution of normal saline, which is a sodium chloride solution. It is commonly available in concentrations of 3%, 5%, 7.5% and 23%. Hypertonic saline provides the advantage of not crossing the blood‐brain barrier; therefore, it remains in the intravascular compartment and does not enter brain tissue (White 2006). Hypertonic saline has less of a diuretic effect when compared with mannitol and thus maintains better cerebral perfusion pressure (White 2006).
How the intervention might work
Osmotic diuretics such as mannitol and hypertonic saline increase the osmolality of the blood, which shifts water from the brain to the intravascular compartment, that is, into the blood. Intravenous administration of hypertonic saline has been shown to improve cerebral perfusion. At the same time, brain oedema is reduced by the intervention, thus increasing compliance and decreasing intracerebral pressure.
Why it is important to do this review
Hyperosmolar solutions such as mannitol and hypertonic saline have been used routinely to achieve brain relaxation in neurosurgical patients undergoing craniotomy. Both agents offer advantages and disadvantages. Through this review, we sought to identify which of the two agents is better suited to intraoperative brain relaxation.
Objectives
The objective of this review was to compare the effects of mannitol versus those of hypertonic saline on intraoperative brain relaxation in patients undergoing craniotomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that compared use of hypertonic saline versus mannitol for brain relaxation. We also included studies in which any other method used for intraoperative brain relaxation was compared with mannitol or hypertonic saline.
We excluded studies in which other methods for producing brain relaxation such as hyperventilation and administration of drugs such as furosemide had not been uniformly used between the two study groups. Monitoring of intracranial pressure was not a prerequisite for inclusion of studies in our review.
Types of participants
We included paediatric and adult participants (> 18 years of age) of either gender who received mannitol or hypertonic saline during craniotomy for brain tumour.
We excluded neonates (younger than 28 days old) from this review.
Types of interventions
The experimental intervention was hypertonic saline, and the control treatment was mannitol.
Types of outcome measures
Primary outcomes
Longest follow‐up mortality.
Outcome at three months (Glasgow Outcome Scale score).
Adverse events such as electrolyte imbalance, haemodynamic disturbance, rebound oedema and kidney injury.
Secondary outcomes
Brain relaxation (as assessed on three‐, four‐ or five‐point scales and reported as dichotomized outcomes: good and poor).
Intensive care unit (ICU) stay.
Hospital stay.
Quality of life assessment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 10) (see Appendix 1), MEDLINE via Ovid SP (1966 to October 2013) (see Appendix 2) and EMBASE via Ovid SP (1980 to October 2013) (see Appendix 3) .
The MEDLINE search strategy was combined with the Cochrane highly sensitive search filter for identifying RCTs (Lefebvre 2011). The MEDLINE search strategy was adapted for searches of other databases.
We applied no language restrictions. We reran the search in January 2017 and found five potential studies of interest which have been added to a list of ‘Studies Awaiting Classification' and will be incorporated into the formal review findings during the review update.
Searching other resources
We searched for relevant ongoing trials on specific websites such as the following.
Data collection and analysis
Selection of studies
Using results of the above searches, we screened all titles and abstracts for eligibility. Two review authors (GPS and VA) independently performed this screening. We obtained and assessed for relevance the full articles for all potentially eligible RCTs relevance based on the preplanned checklist. Each review author documented the reason for exclusion of each excluded trial. We resolved disagreements between review authors through discussion with the third review author (HP), who decided on inclusion or exclusion of the study. We compiled a list of all eligible trials.
Data extraction and management
Two review authors (GPS and VA) independently extracted the data and assessed trial quality using a standardized data extraction form (see Appendix 4). We resolved disagreements through consultation with the third review author (HP). In cases in which additional information was required, GPS or HP contacted the first author of the relevant trial.
Assessment of risk of bias in included studies
Two review authors independently assessed the methodological quality of the included trials (VA and GPS). We resolved disagreements through discussion with the third review author (HP). We performed the assessment as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and judged the risk of bias of included studies on the basis of the following domains.
Random sequence generation.
Allocation concealment.
Blinding and outcome.
Incomplete outcome reporting.
Publication bias and any other bias.
Follow‐up of study participants.
We considered a trial as having low risk of bias if all domains were assessed as adequate. We considered a trial as having high risk of bias if one or more domains were assessed as inadequate or unclear. We included a 'Risk of bias' table as part of the Characteristics of included studies and a 'Risk of bias summary' figure, which detailed all judgements made for all studies included in the review.
Measures of treatment effect
We undertook statistical analysis using the statistical software, Review Manager 5.2, of The Cochrane Collaboration. We used risk ratios (RRs) to measure treatment effect for proportions (dichotomous outcomes) among primary outcomes and adverse effects. We converted continuous data to mean differences (MDs) using the inverse variance method, and we calculated an overall MD. We used a fixed‐effect model when no evidence of significant heterogeneity was found between studies, and a random‐effects or fixed‐effect model when heterogeneity was likely (DerSimonian 1986). As an estimate of the statistical significance of a difference between experimental and control interventions, we calculated RRs and MDs between groups, as well as 95% confidence intervals (CIs). A statistically significant difference between intervention and control groups was assumed if the 95% CI did not include the value of no differential effect.
Unit of analysis issues
We included in our review only RCTs with a parallel‐group design.
Dealing with missing data
We performed quantitative analysis on an intention‐to‐treat (ITT) basis and contacted study authors to obtain missing data. We analysed missing data, if any, by imputation using best case and worst case scenario methods. If we found insufficient data, the potential impact of the missing data was considered in the interpretation of results.
Assessment of heterogeneity
We did not perform meta‐analysis if we suspected important clinical heterogeneity on examination of the included trials. We used the Q statistic to test statistical heterogeneity between trials and considered a P value ≤ 0.05 as indicating significant heterogeneity; the I² statistic was used to assess the magnitude of heterogeneity (Higgins 2002). We considered I2 > 50% to indicate that a meta‐analysis was not appropriate and used a random‐effects model analysis if I2 was between 30% and 50%. However, the decision to use a random‐effects or fixed‐effect model did not rest solely on the value of I2 but rather was based on an overall assessment of the heterogeneity of included studies. When in doubt, we carried out both fixed‐effect and random‐effects models to examine potential differences.
Assessment of reporting biases
We assessed publication bias, funding bias and small‐study effect in a qualitative manner, using a funnel plot. We planned to test for funnel plot asymmetry if more than 10 studies were included in the meta‐analysis.
Data synthesis
We quantitatively reviewed the included data and combined them by intervention, outcome and population, using Review Manager 5.2. We synthesized data in the absence of important clinical or statistical heterogeneity and expressed risk ratios for proportions.
Subgroup analysis and investigation of heterogeneity
When appropriate, given obvious clinical or statistical (I2 > 40%) heterogeneity, we considered subgroup analysis based on age of participants (children vs adults) and on concentrations of hypertonic saline and mannitol. We considered doses of hypertonic saline and mannitol in subgroup analyses if the data indicated heterogeneity on that basis.
Sensitivity analysis
We performed sensitivity analysis to explore the consistency of effect size measures in trials with low risk of bias versus high risk of bias and to investigate the impact of missing data by using the imputation method described above.
Summary of findings
We planned to use the principles of the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) system (Guyatt 2008) in our review to assess the quality of the body of evidence associated with specific outcomes (mortality, outcome at three months, brain relaxation, ICU stay, hospital stay and adverse effects) and to construct a 'Summary of findings' (SoF) table using GRADEpro software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. We created the Table 1 for brain relaxation. We found low evidence recommending the use of hypertonic saline for intraoperative brain relaxation in patients undergoing surgery for brain tumour; therefore, use of hypertonic saline rather than mannitol is recommended.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1.
1.
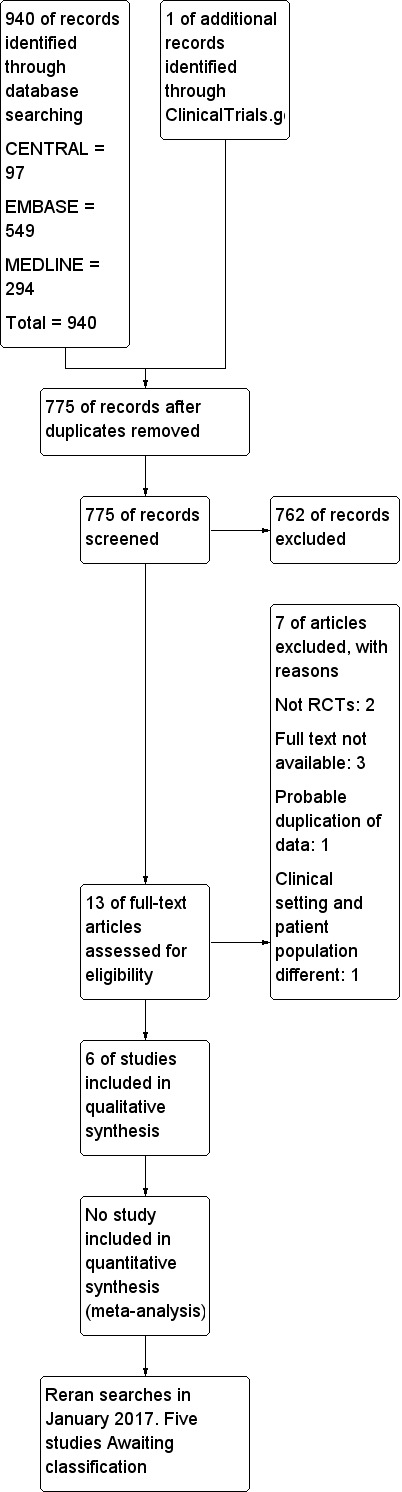
Study flow diagram.
Included studies
We included six studies in our review (Demneri 2011; De Vivo 2001; Gemma 1997; Rozet 2007; Vilas Boas 2011; Wu 2010). All included studies were of parallel design, and only three studies (Rozet 2007; Vilas Boas 2011; Wu 2010) used equiosmolar concentrations of fluids. None of the included studies reported our primary outcomes. Brain bulk was reported differently by all of the studies; however, appropriate data were not provided by authors of three studies (De Vivo 2001; Gemma 1997; Rozet 2007). A single study (Wu 2010) reported our secondary outcomes of ICU stay and hospital stay.
Excluded studies
We excluded seven studies for the reasons detailed in the Characteristics of excluded studies. Two studies were not RCTs (Levin 1979; Smedema 1993). We were unable to obtain the full text for three studies (Eldahab 2009; Erard 1999; Pausawasdi 1982); a probable duplication of data was noted in one study (Muangman 2005); and in another study (Harutjunyan 2005), the participant population and the clinical setting were different from those in our inclusion criteria.
Studies awaiting classification
We reran the search in January 2017 and found five potential studies of interest (Dostal 2015; Hernández‐Palazón 2016; Malik 2014; Raghava 2015; Souissi 2013). These studies will be incorporated into the formal review findings during the review update. For further details of the studies see the table Characteristics of studies awaiting classification
Risk of bias in included studies
We assessed the risk of bias of included studies by using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011). The risk of bias tool invites judgements on five items for each trial (selection bias, performance bias, detection bias, attrition bias and reporting bias). All review authors independently assessed risk of bias for each study and resolved disagreements by discussion. The characteristics of included studies used for our assessment of the risk of bias in included studies are shown in Figure 2 and Figure 3. Only one study (Gemma 1997) was found to be of high methodological quality.
2.
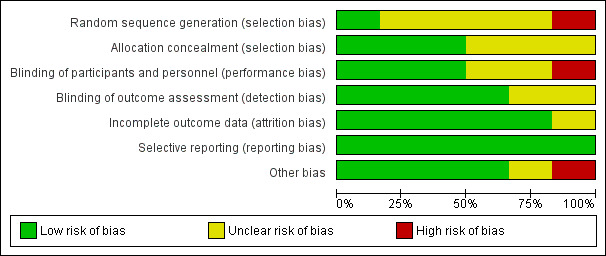
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
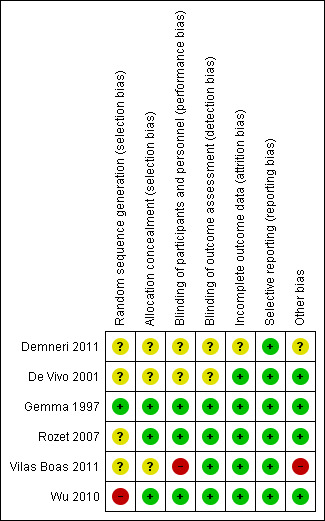
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the six included studies, only three (Gemma 1997; Rozet 2007; Wu 2010) reported allocation concealment. The remaining studies did not describe allocation concealment.
Blinding
Of the six included studies, only three (Gemma 1997; Rozet 2007; Wu 2010) reported blinding of participants and personnel; four studies reported blinding of the outcome assessor (Gemma 1997; Rozet 2007; Vilas Boas 2011; Wu 2010). The remaining studies did not describe blinding.
Incomplete outcome data
Five studies reported data on all participants (De Vivo 2001; Gemma 1997; Rozet 2007; Vilas Boas 2011; Wu 2010). However, this information remained unclear in one study (Demneri 2011), as it was presented as an abstract and study authors failed to include it.
Selective reporting
We found that all planned outcomes were reported in the studies. Study authors reported all outcomes mentioned in their methodology.
Other potential sources of bias
We could find no other potential sources of bias in four of the included studies (De Vivo 2001; Gemma 1997; Rozet 2007; Wu 2010). In one study (Wu 2010), the intervention fluid was donated by a pharmaceutical company, and this could have introduced bias into the study. The source of the intervention fluid remained unclear in another study (Demneri 2011).
Effects of interventions
See: Table 1
Primary outcomes
1. Longest follow‐up mortality
No study reported this outcome.
2. Outcome at three months (Glasgow Outcome Scale score)
No study reported this outcome.
3. Adverse events such as electrolyte imbalance, haemodynamic disturbance, rebound oedema and kidney injury
No study reported these outcomes.
None of the studies reported our primary outcomes of longest follow‐up mortality, Glasgow Outcome Scale score at three months and adverse events such as electrolyte imbalance, haemodynamic disturbance, rebound oedema and kidney injury.
Secondary outcomes
1. Brain relaxation
Three studies enrolling 387 participants reported brain relaxation (73.4% of total participants in this review) (Demneri 2011 enrolled 140 participants; Vilas Boas 2011 enrolled 29 participants; and Wu 2010 enrolled 238 participants). These three trials suggest that the incidence of inadequate brain relaxation was reduced from 68 of 190 in the mannitol group to 42 of 197 in the hypertonic saline group (RR of brain bulge 0.60, 95% CI 0.44 to 0.83, P value 0.002). No heterogeneity was noted in these studies (see Analysis 1.1).
1.1. Analysis.
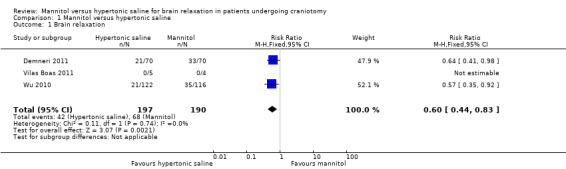
Comparison 1 Mannitol versus hypertonic saline, Outcome 1 Brain relaxation.
2. ICU and hospital stay
Only one study (Wu 2010) enrolling 238 participants reported ICU stay and hospital stay (45.2% of total participants in this review). This study suggested that the mean (standard deviation (SD)) duration of ICU stay in the mannitol and hypertonic saline groups was 1.28 (0.5) and 1.25 (0.5) days (P value 0.64), respectively; the mean (SD) duration of hospital stay in the mannitol and hypertonic saline groups was 5.7 (0.7) and 5.7 (0.8) days (P value 1.00), respectively.
3. Quality of life assessment
No study reported this outcome.
Discussion
This review concerns randomized evidence for the use of hypertonic saline and mannitol in patients undergoing surgery for brain tumour. We planned to collect data on clinically relevant outcomes such as mortality, outcome at three months and adverse events (primary), along with other parameters (secondary outcomes) such as intraoperative brain relaxation, length of ICU and hospital stay and quality of life. Data on the primary end points of our review are lacking. However, we were able to collect data for the incidence of intraoperative brain relaxation in study participants receiving the two fluids.
Summary of main results
None of the studies reported our primary outcomes. Only three studies reported our secondary outcomes. Our analysis suggests that hypertonic saline is beneficial in producing brain relaxation in patients undergoing surgery for brain tumour. Length of ICU stay and length of hospital stay were comparable after intraoperative use of hypertonic saline or mannitol.
Overall completeness and applicability of evidence
The overall methodological quality of these studies cannot be considered good, but no heterogeneity was noted. However, this evidence was obtained from a limited number of studies. We were unable to retrieve data on many clinically useful outcomes such as mortality, outcome at three months and quality of life. The evidence produced by this review, therefore, should be interpreted with caution, keeping in mind that it is only intraoperative brain relaxation that may be achieved more effectively with use of hypertonic saline.
Quality of the evidence
We selected randomized studies for our review, and most of these studies did not report details of randomization and allocation concealment. However, blinding was carried out in most. The overall methodological quality of these studies could not be considered good. The included studies had homogeneous populations, and no heterogeneity was noted. For brain relaxation, the quality of evidence was low, as suggested by the Table 1.
Potential biases in the review process
In an attempt to minimize bias, we followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions. Eligibility for inclusion and exclusion and assessment of risk of bias of different studies were carried out independently by two review authors.
Agreements and disagreements with other studies or reviews
We are unaware of any such review that compares hypertonic saline and mannitol in patients undergoing surgery for brain tumour.
Authors' conclusions
Implications for practice.
The finding of our review that hypertonic saline causes brain relaxation more effectively than mannitol was derived from a limited number of studies. Therefore, the authors of this review cannot draw firm conclusions on the benefits of any one fluid over another for use during the intraoperative period, as far as brain relaxation is concerned.
Implications for research.
The finding from this review is based on only two well‐reported studies; therefore, the results should be interpreted with caution. RCTs based on uniform and standard methodology are needed. Proper methods of randomization and blinding should be followed. Standard doses of mannitol and hypertonic saline, administered at a specified intraoperative time, should be important considerations in the RCT. It is imperative that patient‐related outcomes such as mortality, quality of life, outcome at three months or one year and ICU and hospital stay should be considered while the study is being designed. RCTs should be adequately powered. A multi‐centre trial involving centres in different parts of the world would probably be useful.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2017 | Amended | New search run to January 2017, five new studies not fully incorporated and awaiting classification |
Acknowledgements
We would like to thank Arash Afshari (content editor), Cathal Walsh (statistical editor) and Peter JD Andrews, Robert Wyllie, Federico Bilotta and Rainer Lenhardt (peer reviewers) for help and editorial advice provided during preparation of this systematic review. We would also like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)) for guiding us through this protocol, and Karen Hovhannisyan (Trials Search Co‐ordinator, CARG) for preparing our search strategy.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Mannitol explode all trees #2 mannitol* #3 (#1 OR #2) #4 MeSH descriptor Craniotomy explode all trees #5 MeSH descriptor Neurosurgical Procedures explode all trees #6 MeSH descriptor Neurosurgery explode all trees #7 MeSH descriptor Intracranial Pressure explode all trees #8 MeSH descriptor Intraoperative Period explode all trees #9 (brain near (surg* or manipulat* or procedur* or relax*)) or craniotom* or (neurosurg* near (patient* or procedur* or manipulat*)) or (intracranial near pressure) #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 (#3 AND #10)
Appendix 2. MEDLINE (Ovid SP) search strategy
exp Mannitol/ or mannitol*.af.
exp Craniotomy/ or Neurosurgical Procedures/ or Neurosurgery/ or Intracranial Pressure/ or Intraoperative Period/ or (brain adj3 (surg* or manipulat* or procedur* or relax*)).mp. or craniotom*.af. or (neurosurg* adj3 (patient* or procedur* or manipulat*)).mp. or (intracranial adj3 pressure).mp.
1 and 2
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp mannitol/ or mannitol*.af. 2. exp craniotomy/ or neurosurgery/ or intracranial pressure/ or intraoperative period/ or (brain adj3 (surg* or manipulat* or procedur* or relax*)).mp. or craniotom*.af. or (neurosurg* adj3 (patient* or procedur* or manipulat*)).mp. or (intracranial adj3 pressure).mp. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. Data extraction form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Reference details |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (insert eligibility criteria for each characteristic as defined in the protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial (RCT) | |||||
| Controlled clinical trial (quasi‐randomized trial) | ||||||
|
Participants |
|
|||||
| Types of interventions | |
|||||
| Types of outcome measures | |
|||||
| INCLUDE | EXCLUDE | |||||
|
Reason for exclusion |
||||||
|
Notes: | ||||||
N.B. DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW.
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes No Unclear |
||
|
Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Aim of study |
|||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, clusters/groups or body parts) |
|||
|
Start date |
|
||
|
End date |
|
||
|
Total study duration |
|||
| Ethical approval needed/obtained for study | Yes No Unclear |
||
|
Notes: | |||
5. Risk of bias assessment
See Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear risk | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: all/ |
||||
| (if required) |
Outcome group: |
||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: all/ |
||||
| (if required) |
Outcome group: |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias |
|||||
|
Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total pop at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Severity of illness | ||
|
Co‐morbidities |
||
| Other treatment received(additional to study intervention) | ||
|
Other relevant sociodemographics |
||
|
Subgroups measured |
||
|
Subgroups reported |
||
|
Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group.
Intervention group 1
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
|
Theoretical basis(include key references) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity, etc., if relevant) |
||
|
Co‐interventions |
||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
|
Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1. Mortality
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 2. Outcome at 3 months
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 3. Brain relaxation
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 4. ICU stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 5. Hospital stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 6. Adverse events
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Outcome 7. Quality of life
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
Mortality
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, clusters/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Outcome at 3 months
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, clusters/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Brain relaxation
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, clusters/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Adverse events
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, clusters/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Continuous outcome
ICU stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Hospital stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Other outcome
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
|
Notes: | ||
11. Other information
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Key conclusions of study authors |
||
|
References to other relevant studies |
||
| Correspondence required for further study information(from whom, what and when) | ||
|
Notes: | ||
Data and analyses
Comparison 1. Mannitol versus hypertonic saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Brain relaxation | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Vivo 2001.
| Methods | RCT, parallel design, Department of Anesthesiology and Intensive Care, University of Naples ‘Federico’, Naples Sample size: details on sample size calculation not mentioned |
|
| Participants | Total: 30 participants (17 females; 13 males) Inclusion criteria: ASA I, II, 17 to 75 years of age, scheduled for intracranial supratentorial tumour surgery Exclusion criteria: not mentioned |
|
| Interventions | Control: mannitol (18%) Mannitol. Participants in this group had mannitol (0.5 gm/kg as bolus) at the start of the skin incision. During the postoperative period, they received mannitol (0.5 gm/kg daily) 3 times a day for 3 days (72 hours) Hypertonic saline/Mannitol. Participants in this group had mannitol (0.25 gm/kg as bolus) at the start of the skin incision plus 3% HTS, 20 mL/h, in the intraoperative period and mannitol (0.25 gm/kg daily) 3 times a day for 3 days plus HTS in the concentration of 3% on the first day, and 2% and 1% on the second and third days after surgery Hypertonic saline. Participants in this group had 3% HTS (3.5 ml/kg as bolus) at the start of the skin incision plus 3% HTS, 20 mL/h, in the intraoperative period and 3% HTS, 20 mL/h, on the first day and 2% and 1% on the second and third days after surgery |
|
| Outcomes |
Dural tension Mean arterial pressure, central venous pressure and heart rate Overall mortality Diuresis, serum osmolality, sodium, potassium, creatinine and urea blood values noted thrice a day |
|
| Notes | Hunter's scale (4‐point score) for dural tension (1 = excellent and 4 = impossible dural incision) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. Study authors contacted. No response |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Study authors contacted. No response |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. Study authors contacted. No response |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Study authors contacted. No response |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology are reported |
| Other bias | Low risk | Nothing suggestive |
Demneri 2011.
| Methods | RCT, parallel design, University Hospital Center, Department of Anaesthesiology and Intensive Care, Tirana, Albania Duration of study period: 2007 to 2009 Sample size: details on sample size calculation not mentioned |
|
| Participants | Total participants: 140 (females; males not provided) Adult patients undergoing craniotomy for excision of supratentorial brain tumour Exclusion criteria: not mentioned Inclusion criteria: not mentioned |
|
| Interventions | 2 mL/kg hypertonic saline 7.5% over 30 minutes Control: 4.75 mL/kg mannitol 20% over 30 minutes Total osmolar dose: 5.1 mOsmol/kg |
|
| Outcomes | Brain bulk Plasma and urine concentration of sodium |
|
| Notes | Abstract Limited data available. Study authors contacted for details Brain bulk measured on 4‐point scale 1 = excellent with no swelling 2 = minimal swelling, acceptable 3 = swollen but no treatment required 4 = swollen, needing treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. Study authors contacted. No response |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Study authors contacted. No response |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. Study authors contacted. No response |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Study authors contacted. No response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. Study authors contacted. No response |
| Selective reporting (reporting bias) | Low risk | Study authors have reported all outcomes mentioned in methodology |
| Other bias | Unclear risk | Nothing suggestive |
Gemma 1997.
| Methods | RCT, parallel design, Department of Anesthesiology, University of Milano, IRCCS H San Rafaele, Milano, Italy Sample size: details on sample size calculation not mentioned |
|
| Participants | Total: 50 participants Age mean (standard deviation) years: mannitol group: 51 (14); hypertonic saline group: 54 (13) Gender (male/female): mannitol group: 14/11; hypertonic saline group: 11/14 Inclusion: ASA I patients scheduled for supratentorial elective procedures (clipping of an aneurysm, repair of arteriovenous malformation or resection of tumour (n = 20 in mannitol group; n = 21 in HS group)) Exclusion: patients with ventricular shunt in place, obstructive hydrocephalus (which could obstruct the CSF pathway between the lateral ventricles and the lumbar space), fluid and electrolyte disturbances or preoperative treatment with diuretics and/or osmotic agents |
|
| Interventions | 7.5% hypertonic saline, 2.5 mL/kg, measured osmolality 2560 mOsm/kg, given over a 15‐minute period Control: mannitol 20%, 0.5 gm/kg, measured osmolality 1.401 mOsmol/kg |
|
| Outcomes | Brain bulk Lumbar cerebrospinal fluid pressure |
|
| Notes | Scale for assessment of brain bulk: satisfactory or unsatisfactory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On communication: Randomization list was generated before the beginning of the study with a computerized random number generator |
| Allocation concealment (selection bias) | Low risk | On communication: An anaesthesia fellow, not involved in participant care, provided M or HS to the anaesthesiologist in charge of the participant after wrapping it with an opaque band‐aid and according to a randomization list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | On communication: Both the anaesthesiologist and the neurosurgeon were blind to randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | On communication: Both the anaesthesiologist and the neurosurgeon were blind to randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data given on all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methodology reported |
| Other bias | Low risk | Nothing suggestive. |
Rozet 2007.
| Methods | RCT, parallel, Department of Anesthesiology, Harborview Medical cCenter, Seattle, Washington Sample size: For calculation of power analysis, the review authors considered a difference of 1 point in brain relaxation score between groups to be clinically significant. A power analysis based on 95% confidence interval and beta‐error of 20% revealed a sample size of 12 participants (6 in each treatment group) |
|
| Participants | Total: 40 adult participants Age,[mean (standard deviation)] years: 49 (13) in HS group and 48 (11) in mannitol group Gender: 12 female in HS group and 13 female in mannitol group Inclusion: patients scheduled to undergo craniotomy for various neurological pathologies, requiring intraoperative lumbar CSF drainage (tumours, 4 in HS group and 7 in mannitol group) Exclusion: age younger than 18 years, ASA V, preoperative hyponatraemia or hypernatraemia (serum Na < 130 or > 150 mEq/L), treatment with any hyperosmotic fluid (mannitol or HS) in the previous 24 hours or history of congestive cardiac failure or kidney disease |
|
| Interventions | 5 mL/kg of 3% hypertonic saline Control: 20% mannitol 5 mL/kg (osmolarity of 1 gm/kg is 1098 mOsmol/L) |
|
| Outcomes | Brain bulk | |
| Notes | 4‐point scale: 1 = perfectly relaxed; 2 = satisfactory; 3 = firm; 4 = bulging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Fluid blinded to both surgeon and anaesthesiologist" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Fluid blinded to both surgeon and anaesthesiologist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nothing suggestive |
Vilas Boas 2011.
| Methods | RCT, parallel design, Hospital Municipal Odilon Behrens, Brazil Sample size: details on sample size calculation not mentioned |
|
| Participants | Total: 29 adult patients, ASA I/II (female; male) Age [mean (standard deviation)]: mannitol group: 44 (3.34) years; HIS group: 49.5 (4.52) years Gender (male/female): mannitol group: 8/9; HIS group: 6/6 Inclusion: patients undergoing elective craniotomy and cerebral aneurysm clipping, arteriovenous malformations or cerebral tumours (4 in mannitol group and 5 in HIS group) Exclusion: age < 21 years, initial serum Na < 130 or > 150 mEq/L, metabolic disorders, treatment with hyperosmotic solution up to 24 hours before surgery or history of past heart or renal failure |
|
| Interventions | HIS 360 mL/h for 20 minutes Control: 20% mannitol at 750 mL/h for 20 minutes |
|
| Outcomes | Brain bulk | |
| Notes | 4‐point scale: 1 = perfect relaxation; 2 = satisfactory relaxation; 3 = firm brain; 4 = swollen brain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No appropriate information provided |
| Allocation concealment (selection bias) | Unclear risk | No appropriate information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The anaesthetist was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Cerebral relaxation was evaluated by the same surgeon who was blind to the hyperosmolar therapy used….." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methodology have been reported |
| Other bias | High risk | Communication: "Isoncotic Hypertonic Solution was a donation by the Fresenius Kabi AG" |
Wu 2010.
| Methods | RCT, parallel, Ching‐Tang Wu, Department of Anesthesiology, Tri‐service General Hospital, National Defence Medical Center, #325, Section 2, Chenggung Rd, Neihu 114, Taipei Sample size: 'A minimum of 106 patients was required in each group to detect a decrease in the incidence of tight‐brain condition from 36% to 18%, with a power of 80% and a confidence interval of 95%. To compensate for potential dropouts, we enrolled a minimum of 116 patients in each group' |
|
| Participants | Total: 238 participants (female; male) Age,[median (range)] years: mannitol: 54 (18‐80); HTS: 56 (18‐80) Gender (male/female): mannitol: 56/66; HTS: 56/60 Inclusion: patients who were enrolled to undergo elective craniotomy for supratentorial brain tumour Exclusion: age < 18 years, Glasgow Coma Scale score < 13, ASA IV/V, signs of raised ICP, perioperative hyponatraemia or hypernatraemia (serum Na < 135 or > 150 mEq/L, respectively), history of treatment with any hyperosmotic fluid (HTS or mannitol) within 24 hours preceding surgery and history of congestive heart failure or severe renal function impairment |
|
| Interventions | 160 mL of 3% HTS over 5 minutes Control: 150 mL of 20% mannitol |
|
| Outcomes | 1. Brain bulk 2. ICU stay 3. Hospital days |
|
| Notes | 3‐point scale for brain bulk: 1 = tight; 2 = adequate; 3 = soft | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not mentioned On communication: "we prepared 250 sealed envelopes. After a participant has been recruited, the next sealed envelope is opened and the treatment is indicated" Comment: The correct method of randomization was not used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The surgeons and the anaesthesiologists were blinded to the identity of the agents under study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Surgeon blinded to the anaesthetic techniques assessed the degree of brain relaxation..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes have been reported |
| Other bias | Low risk | Nothing suggestive |
ASA: American Society of Anesthesiologists physical status.
CSF: cerebrospinal fluid.
HS: hypertonic saline
HIS: hypertonic isoncotic saline.
HTS: hypertonic saline.
ICP: intracranial pressure.
ICU: intensive care unit.
M: mannitol.
M/F: male/female.
Na: sodium.
RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eldahab 2009 | Only abstract available, which does not give complete information. Contact details of study authors not available |
| Erard 1999 | Abstract. Study authors not responding to emails. Outcomes of interest not assessed |
| Harutjunyan 2005 | Participants are not patients with brain tumour undergoing surgery. The study is being conducted in the ICU, not in the operating theatre |
| Levin 1979 | Not an RCT. Participant population is different |
| Muangman 2005 | Probable duplication of data in the Rozet 2007 study |
| Pausawasdi 1982 | Study authors cannot be contacted. Full text could not be retrieved. Failed communication with the Editor of the journal |
| Smedema 1993 | Abstract. Unclear whether it is an RCT. Study authors cannot be contacted. Full text not available |
ICU: intensive care unit.
RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Dostal 2015.
| Methods | RCT, parallel design, Departments of *Anesthesia and Intensive Care;Neurosurgery, Faculty of Medicine Hradec Kralove, Charles University in Prague, University Hospital Hradec Kralove, Hradec Kralove; and Department of Anesthesia and Intensive Care, 1st Faculty of Medicine Prague, Charles University in Prague, Military University
Hospital, Prague, Czech Republic Sample size: 'A difference of 1 point in brain relaxation score between the groups was considered clinically significant for the power analysis. A power analysis based on an a error of 0.05 and a b error of 0.2 was performed using G*Power 3.0.9 (Franz Faul, University Kiel, Germany). The sample size needed for the Wilcoxon‐Mann‐Whitney (2 groups) test (expected mean difference of 1.0, SD in both groups of 1.2) with the minimal asymptotic relative efficiency setting was calculated. This calculation produced a sample size of 56 subjects (28 subjects in each treatment group). Sample size was increased to at least 35 patients per treatment group to compensate for potential dropouts and possible inaccuracy of predictions used for the power analysis. |
| Participants | Total: 74 adult patients (18 ‐ 70 years), ASA I/II/III (44 female; 30 male) Age [mean (standard deviation)]: mannitol group: 53.5 (13.0) years; HTS group: 52.1 (13.1) years Gender (male/female): mannitol group: 14/24; HIS group: 16/20 Inclusion: age 18 to 70 years, elective intracranial tumour surgery with indication for perioperative osmotherapy, American Society of Anesthesiologists physical status I to III, and preoperative natraemia of 135 to 145mmol/L Exclusion: history or presence of congestive heart failure (New York Heart Association class III to IV), history or presence of renal failure, presence of preoperative disturbance of water or sodium metabolism (diabetes insipidus, cerebral salt wasting syndrome, or syndrome of inappropriate antidiuretic hormone secretion), preoperative Glasgow Coma Scale score r13, preoperative need for haemodynamic support, preoperative presence of obstructive hydrocephalus, treatment with cyclosporine within the last month, or a neurosurgical procedure within the last 3 months |
| Interventions | HTS: 3.75 mL/kg body weight of 3.2% HTS Control: 20% mannitol at 0.75 g/kg body weight mannitol over 30 minutes |
| Outcomes | 1. Brain relaxation 2. ICU stay 3. Hospital stay |
| Notes | 4‐point scale: 1=perfectly relaxed, 2=satisfactorily relaxed, 3=firm brain, 4=bulging brain |
Hernández‐Palazón 2016.
| Methods | RCT, parallel design,Department of Anaesthesia, Hospital Universitario ‘‘Virgen de la Arrixaca’’, Murcia, Spain; Department of Neurosurgery, Hospital Universitario ‘‘Virgen de la Arrixaca’’, Murcia, Spain Sample size: 'An expected mean difference of 1.0, SD in both the groups of 1.2 in brain relaxation score, with error of 0.05 and error of 0.2 were considered as clinically significant for the power analysis.This calculation produced a sample size of 60 subjects (30 subjects per group) considering a loss ratio of 10%.' |
| Participants | Total: 60 adult patients (18 ‐ 70 years), ASA I/II/III ( 26 female; 34 male) Age [mean (standard deviation)]: mannitol group: 50 (16) years; HTS group: 49 (15) years Gender (male/female): mannitol group: 17/13; HTS group: 17/13 Inclusion:aged 18–70 years, ASA I/II/III Exclusion:perioperative hypo‐ or hyper‐natraemia (serum sodium <130 or >150 mEq/l), treatment with mannitol or HTS in previous 24 h, kidney disease, disturbance of water or sodium metabolism, preoperative Glasgow Coma Scale Score < or = 13, preoperative presence of obstructive hydrocephalus and congestive heart failure. |
| Interventions | HTS: 3 ml/kg of 3% HTS Control: 3 ml/kg of 20% mannitol at 0.6 g/kg body weight mannitol over 15 minutes |
| Outcomes | 1. Brain relaxation 2. ICU stay 3. Hospital stay 4. Mortality |
| Notes | 4‐point scale: 1=perfectly relaxed, 2=satisfactorily relaxed, 3=firm brain, 4=bulging brain |
Malik 2014.
| Methods | RCT, parallel design,Departments of Anaesthesiology and Critical Care and Neurosurgery, Sher‑I‑Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India Sample size: |
| Participants | Total: 114 adult patients (>18 years), ASA I/II/III ( 55 female; 59 male) Age [mean (standard deviation)]: mannitol group: 46.93 (12.1) years; HTS group: 43.39 (13.6) years Gender (male/female): mannitol group: 28/30 HTS group: 31/25 Inclusion:ASA II and III, age >18 years, of either sex Exclusion:history of unstable angina or myocardial infarction within past 6 months, congestive cardiac failure, Glasgow coma score <13, uncontrolled diabetes, severe renal impairment, preoperative hyponatraemia (serum sodium <130 meq/L) or hypernatraemia (serum sodium >150 meq/L), treatment with mannitol or hypertonic saline (HTS) during previous 24 h. |
| Interventions | HTS: 5 ml/kg of 3% HTS Control: 5 ml/kg of 20% mannitol over 15 minutes |
| Outcomes | 1. Brain relaxation 2. ICU stay 3. Hospital stay |
| Notes | 4‐point scale: 1=perfectly relaxed, 2=satisfactorily relaxed, 3=firm brain, 4=bulging brain |
Raghava 2015.
| Methods | RCT, parallel design,Department of Anesthesiology and Critical Care, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry,
India Sample size:'For power analysis calculation, we considered a difference of 1 point in brain relaxation score between the groups to be clinically significant. A power analysis based on 95% confidence interval with 90% power, the sample size of 25 in each group was sufficient.The total sample size required was 50 for 90% statistical power and 5% level of significance assuming 1 point difference of brain relaxation between two groups.' |
| Participants | Total: 50 adult patients (18 ‐ 65 years), ASA I/II/III ( 29 female; 21 male) Age [mean (standard deviation)]: mannitol group: 38.8 (11.9) years; HTS group: 41.6 (12.9) years Gender (male/female): mannitol group: 9/16 HTS group: 12/13 Inclusion: age group 18–65 years, with Glasgow coma scale (GCS) >13, and ASA physical status 1–3 Exclusion: presence of raised ICP, electrolyte imbalance, with severe cardiac, respiratory, or renal disease were excluded from the study. Patients who are already on mannitol or HS treatment were also excluded from the study |
| Interventions | HTS: 5 ml/kg of 3% HTS Control: 5 ml/kg of 20% mannitol over 15 minutes |
| Outcomes | 1. Brain relaxation |
| Notes | 4‐point scale: 1=perfectly relaxed, 2=satisfactorily relaxed, 3=firm brain, 4=bulging brain |
Souissi 2013.
| Methods | RCT, parallel design,National Institute of Neurology, La Rabta, Tunisia. Sample size: Not mentioned |
| Participants | Total: 30 adult patients (> 18 years), Age [mean (standard deviation)]: Not mentioned Gender (male/female): Not mentioned Inclusion:aged > 18 years Exclusion:ASA physical status IV or V, preoperative electrolyte disorder, pregnant woman, patient with history of congestive heart failure or kidney disease, and patient undergoing surgery for <1 hour |
| Interventions | HTS: 7.5% HTS Control: 20% mannitol |
| Outcomes | 1. Brain relaxation |
| Notes | 4‐point scale: No details available |
Contributions of authors
Hemanshu Prabhakar (HP), Gyaninder Pal Singh (GPS), Vidhu Anand (VA), Mani Kalaivani (MK)
Conceiving of the review: HP.
Co‐ordinating the review: HP.
Undertaking manual searches: GPS, VA.
Screening search results: HP, GPS.
Organizing retrieval of papers: GPS, VA.
Screening retrieved papers against inclusion criteria: GPS, VA.
Appraising quality of papers: GPS, VA.
Abstracting data from papers: GPS, VA.
Writing to authors of papers for additional information: HP, GPS.
Providing additional data about papers: HP, GPS.
Obtaining and screening data on unpublished studies: HP, GPS.
Managing data for the review: HP, GPS.
Entering data into Review Manager 5.2: HP, GPS.
Performing statistical analysis in Review Manager 5.2: HP, MK.
Performing other statistical analyses not using Review Manager 5.2: MK.
Interpreting data: HP, MK.
Making statistical inferences: HP, MK.
Writing the review: HP.
Serving as guarantor for the review: HP.
Reading and checking the review before submission: HP, GPS.
Sources of support
Internal sources
All India Institute of Medical Sciences, New Delhi, India.
External sources
No sources of support supplied
Declarations of interest
Hemanshu Prabhakar: none known.
Gyaninder Pal Singh: none known.
Vidhu Anand: none known.
Mani Kalaivani: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Demneri 2011 {published data only}
- Demneri M, Hoxha A, Pilika K, Saraci M. Comparison of 20% mannitol and 7.5% hypertonic saline for supratentorial craniotomy. European Journal of Anaesthesiology 2011;28:106‐7. [Google Scholar]
De Vivo 2001 {published data only}
- Vivo P, Gaudio A, Ciritella P, Puopolo M, Chiarotti F, Mastronardi E. Hypertonic saline solution: a safe alternative to mannitol 18% in neurosurgery. Minerva Anestesiologica 2001;67:603‐11. [PUBMED: 11731749] [PubMed] [Google Scholar]
Gemma 1997 {published data only}
- Gemma M, Cozzi S, Tommasino C, Mungo M, Calvi MR, Cipriani A, et al. 7.5% hypertonic saline versus 20% mannitol during elective neurosurgical supratentorial procedures. Journal of Neurosurgical Anesthesiology 1997;9:329‐34. [PUBMED: 9339405] [DOI] [PubMed] [Google Scholar]
Rozet 2007 {published data only}
- Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology 2007;107:697‐704. [PUBMED: 18073543] [DOI] [PubMed] [Google Scholar]
Vilas Boas 2011 {published data only}
- Vilas Boas WW, Marques MB, Alves A. Hydroelectrolytic balance and cerebral relaxation with hypertonic isoncotic saline versus mannitol (20%) during elective neuroanesthesia. Revista Brasileira De Anestesiologia 2011;61:456‐68. [PUBMED: 21724008] [DOI] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu CT, Chen LC, Kuo CP, Ju DT, Borel CO, Cherng CH, et al. A comparison of 3% hypertonic saline and mannitol for brain relaxation during elective supratentorial brain tumor surgery. Anesthesia and Analgesia 2010;110:903‐7. [PUBMED: 20185666] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Eldahab 2009 {published data only}
- Eldahab HA, Awad W, Wagh O. Should hypertonic saline 3% replace mannitol 20% for reduction of intracranial pressure in craniotomy for supratentorial tumors? A comparative study. Egyptian Journal of Anaesthesia 2009;25:413‐28. [Google Scholar]
Erard 1999 {published data only}
- Erard A, Bracco D, Walder B, Ravussin P. Effect of mannitol, NaCl 7.5% and NaCl 0.9% on osmolality and osmolarity: a randomized controlled study in neurosurgical patients [abstract]. British Journal of Anaesthesia 1999;82:Suppl 187. [Google Scholar]
Harutjunyan 2005 {published data only}
- Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients ‐ a randomized clinical trial. Critical Care Medicine 2005;9:R530‐40. [PUBMED: 16277715 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 1979 {published data only}
- Levin AB, Duff TA, Javid MJ. Treatment of increased intracranial pressure: a comparison of different hyperosmotic agents and the use of thiopental. Neurosurgery 1979;5:570‐5. [DOI] [PubMed] [Google Scholar]
Muangman 2005 {published data only}
- Muangman S, Rozet I, Vavilala MS, Lee LA, Lam AM. Iso‐osmolar solutions of mannitol vs hypertonic saline for intraoperative brain relaxation. Anesthesiology 2005;103:A76. [DOI] [PubMed] [Google Scholar]
Pausawasdi 1982 {published data only}
- Pausawasdi S, Bunyaratavej S. Furosemide and mannitol‐induced changes in intracranial pressure during the removal of intracranial tumours. Journal of Medical Association of Thailand 1982;65:413‐9. [PubMed] [Google Scholar]
Smedema 1993 {published data only}
- Smedema RJ, Gaab MR, Heissler HE. A comparison study between mannitol and glycerol therapy in reducing intracranial pressure. Intracranial Pressure 1993;VIII:605‐8. [Google Scholar]
References to studies awaiting assessment
Dostal 2015 {published data only}
- Dostal P, Dostalova V, Schreiberova J, Tyll T, Habalova J, Cerny V, Rehak S, Cesak T. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation in patients undergoing elective intracranial tumor surgery: a randomized clinical trial. Journal of Neurosurgical Anesthesiology 2015;27:51‐6. [PUBMED: 25036870 ] [DOI] [PubMed] [Google Scholar]
Hernández‐Palazón 2016 {published data only}
- Hernández‐Palazón J, Fuentes‐García D, Doménech‐Asensi P, Piqueras‐Pérez C, Falcón‐Araña L, Burguillos‐López S. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation during elective supratentorial craniotomy. British Journal of Neurosurgery 2016;30:70‐5. [PUBMED: 26571037 ] [DOI] [PubMed] [Google Scholar]
Malik 2014 {published data only}
- Malik ZA, Mir SA, Naqash IA, Sofi KP, Wani AA. A prospective, randomized, double blind study to compare the effects of equiosmolar solutions of 3% hypertonic saline and 20% mannitol on reduction of brain‐bulk during elective craniotomy for supratentorial brain tumor resection. Anesthesia: Essays and Researches 2017;8:388‐92. [PUBMED: 25886341 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raghava 2015 {published data only}
- Raghava A, Bidkar PU, Prakash MV, Hemavathy B. Comparison of equiosmolar concentrations of hypertonic saline and mannitol for intraoperative lax brain in patients undergoing craniotomy. Surgical Neurology International 2015;6:73. [PUBMED: 25984387 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Souissi 2013 {published data only}
- Souissi R, Tounsi M, Baccar K, Ben Hamza M, Baffoun N, Maatar N, Jemel H, Kaddour C. A Comparison of Equiosmolar Solutions ofMannitol and Hypertonic Saline for Brain Relaxation DuringCraniotomy (Preliminary Results). Journal of Neurosurgical Anesthesiology 2013;25:491. [DOI: 10.1097/ANA.0b013e3182a4d5ff] [DOI] [Google Scholar]
Additional references
Battinson 2005
- Battinson C, Andrews PJ, Graham C, Petty T. Randomized controlled trial on the effect of a 20% mannitol solution and a 7.5% saline / 6% dextran solution on increased intracranial pressure after brain injury. Critical Care Medicine 2005;33:196‐202. [PUBMED: 15644669] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;11(21):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Review Manager 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.2. Copenhagen. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Schwarz 2002
- Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke 2002;33:136‐40. [PUBMED: 11779902] [DOI] [PubMed] [Google Scholar]
Vialet 2003
- Vialet R, Albanese J, Thomachot L, Antonini F, Bourgouin A, Alliez B, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 ml/kg 7.5% saline is more effective than 2 ml/kg 20% mannitol. Critical Care Medicine 2003;31:1683‐7. [PUBMED: 12794404 ] [DOI] [PubMed] [Google Scholar]
White 2006
- White H, Cook D, Venkatesh B. The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesthesia and Analgesia 2006;102:1836‐46. [PUBMED: 16717334] [DOI] [PubMed] [Google Scholar]


