Abstract
Background
Propranolol is one of the most commonly prescribed drugs for migraine prophylaxis.
Objectives
We aimed to determine whether there is evidence that propranolol is more effective than placebo and as effective as other drugs for the interval (prophylactic) treatment of patients with migraine.
Search methods
Potentially eligible studies were identified by searching MEDLINE/PubMed (1966 to May 2003) and the Cochrane Central Register of Controlled Trials (Issue 2, 2003), and by screening bibliographies of reviews and identified articles.
Selection criteria
We included randomised and quasi‐randomised clinical trials of at least 4 weeks duration comparing clinical effects of propranolol with placebo or another drug in adult migraine sufferers.
Data collection and analysis
Two reviewers extracted information on patients, methods, interventions, outcomes measured, and results using a pre‐tested form. Study quality was assessed using two checklists (Jadad scale and Delphi list). Due to the heterogeneity of outcome measures and insufficient reporting of the data, only selective quantitative meta‐analyses were performed. As far as possible, effect size estimates were calculated for single trials. In addition, results were summarised descriptively and by a vote count among the reviewers.
Main results
A total of 58 trials with 5072 participants met the inclusion criteria. The 58 selected trials included 26 comparisons with placebo and 47 comparisons with other drugs. The methodological quality of the majority of trials was unsatisfactory. The principal shortcomings were high dropout rates and insufficient reporting and handling of this problem in the analysis. Overall, the 26 placebo‐controlled trials showed clear short‐term effects of propranolol over placebo. Due to the lack of studies with long‐term follow up, it is unclear whether these effects are stable after stopping propranolol. The 47 comparisons with calcium antagonists, other beta‐blockers, and a variety of other drugs did not yield any clear‐cut differences. Sample size was, however, insufficient in most trials to establish equivalence.
Authors' conclusions
Although many trials have relevant methodological shortcomings, there is clear evidence that propranolol is more effective than placebo in the short‐term interval treatment of migraine. Evidence on long‐term effects is lacking. Propranolol seems to be as effective and safe as a variety of other drugs used for migraine prophylaxis.
Keywords: Adult, Humans, Adrenergic beta‐Antagonists, Adrenergic beta‐Antagonists/therapeutic use, Calcium Channel Blockers, Calcium Channel Blockers/therapeutic use, Migraine Disorders, Migraine Disorders/prevention & control, Propranolol, Propranolol/therapeutic use, Randomized Controlled Trials as Topic, Treatment Refusal
Propranolol for migraine prophylaxis
Propranolol, a beta‐blocker, is one of the most commonly prescribed drugs for the prevention of migraine. This systematic review identified 58 trials, and these provide evidence that propranolol reduces migraine frequency significantly more than placebo. We did not find any clear differences between propranolol and other migraine‐preventing drugs, but firm conclusions cannot be drawn about the relative efficacy of propranolol and other drugs due to the small sample size of most of the trials.
Background
Migraine is a common disabling condition. It typically manifests as attacks of severe, pulsating, one‐sided headache and is often accompanied by nausea, phonophobia, or photophobia. Population‐based studies from the US and elsewhere suggest that six to seven per cent of men and 15% to 18% of women experience migraine headaches (Lipton 2001; Stewart 1994). Preventive drugs are used by a small proportion of migraineurs. Available guidelines commonly recommend beta‐blockers as the first choice for migraine prophylaxis (e.g., Pryse‐Phillips 1997). It is not certain exactly how beta‐blockers decrease the frequency of migraine attacks, but they may affect the central catecholaminergic system and brain serotonin receptors.
Among the many different beta‐blockers, propranolol is one of the most commonly prescribed for migraine prophylaxis (Ramadan 1997). It has been subjected to a number of placebo‐controlled trials and is now sometimes used as a comparator drug when testing newer agents for migraine prophylaxis (Gray 1999; Holroyd 1991). While propranolol is well‐tolerated in general, it is associated with a variety of adverse effects (such as bradycardia, hypotension, bronchospasm, gastrointestinal complaints, vertigo, hypoglycaemia, etc).
Objectives
We aimed to systematically review the available randomised and quasi‐randomised controlled trials of propranolol for migraine prophylaxis. Specifically, we aimed to determine whether there is evidence that propranolol is:
more effective than placebo;
as effective as other pharmacological agents.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (using methods such as alternation) clinical trials were included. Trials that did not make an explicit statement about the allocation method, but were described as double‐blind (referring to blinding of patients and blinding of recruiting, treating, and evaluating staff), were included unless there were clear reasons to assume that allocation was not randomised.
Types of participants
Study participants were required to be adult migraine sufferers. Trials including individual participants under 18 years of age were included provided that the mean age of trial participants clearly indicated that the majority of patients were adults (e.g., if the age range was 16 to 61 years, with a mean age of 41 years). Trials conducted among patients who suffered from other types of headaches in addition to migraine were included. Trials studying patients with migraine and patients with other types of headache were included only if results for the subgroup of migraine sufferers were presented separately.
Types of interventions
Included studies were required to have at least one arm in which oral propranolol was used for migraine prophylaxis. Acceptable control groups included other migraine‐prophylactic drugs (e.g., flunarizine, metoprolol, cyclandelate) and placebo. Trials comparing only different doses of propranolol, without a non‐propranolol group, were excluded. Trials comparing propranolol solely with non‐drug interventions (e.g., biofeedback or relaxation) were also excluded.
Types of outcome measures
At least one of the following outcomes must have been measured (but not necessarily reported in sufficient detail to allow effect size calculation): number of migraine attacks, number of headache days, pain intensity, headache index, or global response. Trials reporting only physiological or laboratory parameters were excluded, as were trials focusing on the treatment of acute migraine attacks and trials with an observation period of less than 4 weeks after the start of treatment.
Search methods for identification of studies
We searched the following sources:
The basic search was performed in MEDLINE (1966 through September 2001) using the search terms 'propranolol or propanolol' and 'headache (exploded)' combined with the optimal search strategy for randomised controlled trials described in the Cochrane Reviewers' Handbook (Clarke 2003). To update this search, we regularly screened citations from the search 'migraine AND propranolol' in PubMed for eligible studies or reviews that might include eligible studies (last update May 2003; limited to publication date 2000 or later).
The Cochrane Central Register of Controlled Trials (CENTRAL) was searched using the terms 'propranolol or propanolol' and 'migraine or headache' (last update Issue 2, 2003).
In addition, we screened bibliographies of reviews and identified articles.
Data collection and analysis
Eligibility
One reviewer screened titles and abstracts of all references identified and excluded all citations that were clearly ineligible (e.g., trials with children or on non‐migraine headaches). Full copies of all remaining articles were obtained. Two independent reviewers then checked whether trials met inclusion criteria using a special form. Disagreements were resolved by discussion.
Data extraction
Two independent reviewers extracted the following information using a pre‐tested form: On the patient population:
number randomised and analysed;
diagnoses (and headache classification systems used);
age;
sex;
duration of disease;
setting.
On methods:
study design;
use of a headache diary;
duration of baseline, therapy, and follow‐up periods; for crossover studies, we also documented washout periods;
randomisation;
concealment;
handling of dropouts and withdrawals.
On interventions:
type of intervention;
dosage;
regimen;
duration.
Outcomes and results:
withdrawals and dropouts due to adverse events, lack of efficacy, or other reasons, with total number;
results for headache days, attack frequency, pain intensity, medication use, headache index, and other outcomes at baseline, after up to 4 weeks of treatment, after more than 4 weeks of treatment, and at follow‐up after completion of treatment;
number of responders, with definition of 'response';
number of patients reporting adverse events
Assessment of quality
Methodological quality was assessed using the scale by Jadad et al. (Jadad 1996) and the Delphi list (Verhagen 1998). The Jadad scale has three items and a maximum score of 5: randomisation (statement that the study was randomised = 1 point; if the method used to generate the random sequence was described, an additional point is given), double‐blinding (statement that the study was double‐blind = 1 point; additional point if a credible description of how blinding was achieved was given), and dropouts and withdrawals (a point was given if the number and reasons for dropouts and withdrawals were presented for each group separately). The Delphi list has nine questions, which concern randomisation; concealment of randomisation; baseline comparability; specification of eligibility criteria; blinding of the care provider, patients, and outcome evaluator; adequacy of reporting of main results; and analysis according to the intention‐to‐treat principle. Each question can be answered 'yes = 1', 'no = 0', or 'unclear = ?'.
In addition, the adequacy of observation and reporting of key clinical issues was assessed using a checklist developed by one of the reviewers. It included questions on: description of the sampling strategy (source of patients, recruitment); description of headache diagnoses (description of specific diagnoses, use of transparent diagnostic criteria); description of patient characteristics (age, sex, duration, baseline severity); inclusion of a baseline period (of at least 4 weeks); description of co‐interventions (amount of analgesic use); use of a headache diary; detailed presentation of data on headache frequency and intensity (central tendency, variability); completeness of follow up at 2 months (results for at least 90% of included patients 2 months after the start of treatment) and at 6 months (follow up of at least 6 months after start of the treatment, with results available for at least 80% of patients). Each item could be scored as met ('yes = 1') or not met or unclear ('no/unclear = 0').
Summarising the results
As anticipated, the reporting of the complex outcome data in the included trials was highly variable (various measurement methods, different time points) and often insufficient (lack of variance measures, unclear number of observations). The only outcomes reported in a substantial number of papers were:
various headache frequency measures (number of migraine attacks, number of migraine days, and number of headache days; mostly reported for the last 4 weeks of treatment, but sometimes for other time frames);
headache indices;
number of 'responders' (with 'response' most often defined as at least a 50% reduction in number of migraine attacks, but also sometimes as at least a 50% reduction in headache index or by global patient assessment); and
number of patients reporting adverse events.
Furthermore, a relevant proportion of trials had a crossover design and reported results only in a pooled manner for both treatment periods.
Using RevMan 4.2, we calculated standardized mean differences for headache frequency, and relative risks for number of responders, number of patients with adverse events, and number of dropouts due to adverse events for individual trials. As the measure for headache frequency we used, if available, the number of migraine attacks in the last 4 weeks or the last month of (full‐dose) treatment. However, a number of trials presented data either for different time frames (for example, 8 weeks) or for another frequency measure (migraine or headache days). For the calculation of the relative risk of response to treatment (here referred to as the 'responder ratio') we used, if available, the number of patients with a reduction of at least 50% in the number of migraine attacks. If this was not available, we used the number of patients with a reduction of at least 50% in the number of headache days, the number of patients with a reduction of at least 50% in headache index, or global response measures. In the 'Characteristics of included studies' table, we indicate which measures were actually used for each trial. As denominators we used the number of patients included in the analysis for responder ratios and the relative risk of adverse events, and the number of patients randomised for the relative risk of dropouts due to adverse events. In many instances there was uncertainty about precise numbers, for example, when only percentage values were reported for proportion of responders, with denominators not fully clear, or when the total number of patients randomised was presented but not the number randomised to each group. In other cases, standard deviations had to be calculated from standard errors or 95% confidence intervals. We tried to calculate effect sizes for single trials as often as possible despite these uncertainties. Therefore, the effect size estimates must be interpreted with caution. In the 'Characteristics of included studies' table, we generally describe the data as reported in the publications.
For effect size calculation we used, in the first instance, only data from trials with a parallel‐group design and first‐period data from those crossover studies that reported data for the first treatment period separately. As many crossover trials did not present separate data for the first treatment period, we calculated, in a second step, effect size estimates for all trials providing data, including crossover trials that reported only 'pooled' data (data from all treatment periods combined as if they were from a parallel‐group trial). If crossover trials reported both first‐period and pooled data, then we used the first‐period data for the first analyses and pooled data for the second; if crossover trials reported data only for separate phases, with no pooled data, then we used first‐period data for both analyses. Comparisons of propranolol versus placebo, versus calcium antagonists, versus other beta‐blockers, and versus other drugs were included.
In our protocol we prespecified that we would not perform quantitative meta‐analysis for a given comparison if fewer than half of the included trials provided usable data. For the comparison of propranolol versus placebo, fewer than half of the trials in fact provided sufficient data for effect size calculation. However, because the descriptive results, simple vote counts (see next paragraph), and Jadad scores were similar for trials providing data for effect size calculation and those not providing data, we decided post hoc to perform quantitative meta‐analyses for the propranolol versus placebo comparison to get at least a crude estimate of the overall effect sizes. Quantitative meta‐analyses were also performed for the comparisons with calcium antagonists and other beta‐blockers. We calculated both fixed‐effect and random‐effects estimates, but only fixed‐effect estimates (which give more weight to larger trials) are presented in the 'Results' section. Due to the heterogeneity of trials (regarding patients, dosages, observation periods, and methods for outcome measurements), the lack of detailed data for a relevant proportion of the trials, and the problem of pooled crossover data (described in the preceding paragraph), all overall effect size estimates presented below must be interpreted with great caution. Because of these problems, we did not calculate measures like numbers‐needed‐to‐treat, which suggest direct applicability of effect size estimates to routine use in practice.
Because of the difficulties with the quantitative analysis, we also provide a systematic descriptive summary of results for each study in the table on the 'Characteristics of included studies'. If available, results were summarized for the following outcomes: response, headache frequency, analgesic use, headache indices, adverse events, and dropouts due to adverse events. In addition, we performed a simple vote count to provide a crude estimate of the overall outcome of each study. For this vote count, each reviewer independently categorized the results of each study using the following scale: + = propranolol significantly better than control (primary or most clinically relevant outcome measures statistically significantly better with propranolol than with control); (+) = propranolol trend better than control (significant differences for only some clinically relevant outcomes, or no statistically significant differences, but potentially clinically relevant trends in favour of propranolol); 0 = no difference; (‐) = control trend better than propranolol; ‐ = control significantly better than propranolol. Disagreements were resolved by discussion and consensus was reached on each study.
Results
Description of studies
We identified 96 potentially relevant publications. Seventy‐two publications describing 58 separate trials met the inclusion criteria (see table on the 'Characteristics of included studies'). One publication presented separate data on patients suffering from migraine alone and patients suffering from migraine plus interval headaches, but no analysis of all patients together; it was, therefore, analysed as two separate trials (Mathew 1981‐Study 1; Mathew 1981‐Study 2).
Twenty‐four publications were excluded: six were review articles (Amery 1988; Anonymous 1979; Montastruc 1992; Raveau‐Landon 1988; Tfelt‐Hansen 1986; Turner 1984); nine described studies that were not randomised or quasi‐randomised (Cortelli 1985; de Bock 1997; Diamond 1987; Julien 1976; Rosen 1983; Schmidt 1991; Verspeelt 1996a; Verspeelt 1996b; Wober 1991); one unblinded trial did not describe the method of allocation to groups (Steardo 1982); two trials were on the treatment of acute attacks (Banerjee 1991; Fuller 1990); one had an observation period of less than 4 weeks (Winther 1990); and five were randomised trials, but did not include placebo or another drug as a control (Carroll 1990; Havanka‐Kann. 1988; Holroyd 1995; Penzien 1990; Sovak 1981) (for details see table on the 'Characteristics of excluded studies'). We have so far been unable to obtain a copy of two articles (Bernik 1978; Rao 2000); they are categorized here as studies 'awaiting assessment'.
The 58 trials included a total of 73 comparisons relevant to this review: 26 trials included a comparison with placebo, and 40 trials included a total of 47 comparisons of propranolol with another drug. Eight trials had both a placebo and an active comparator group. Three trials additionally included comparisons of propranolol alone versus propranolol in combination with another drug, one a comparison with another propranolol dose, and one a comparison with d‐propranolol. There were 15 comparisons with calcium antagonists (10 flunarizine, three nifedipine, one nimodipine, one verapamil), 12 with other beta‐blockers (five metoprolol, five nadolol, one atenolol, one timolol), and 20 with various other agents (three amitriptyline, two femoxetine, two methysergide, two cyclandelate, two divalproex sodium, two tolfenamic acid, one dihydroergotamine, one dihydroergocryptine, one mefenamic acid, one acetylsalicylic acid, one clonidine, one 5‐hydroxytryptophan, one naproxen). Thirty‐three trials had a crossover design. The included trials were published between 1972 and 2002; four were available only as abstracts. Ten studies reported the source of funding.
The median number of patients per trial was 49 (range 9 to 810), and the total number of included patients was 5072. The proportion of patients excluded from analysis varied from zero to 50%. Eight trials were restricted to patients with migraine without aura, and one to patients with migraine with aura. Twelve studies used the International Headache Society's criteria (IHS 1988) for confirming the diagnosis, 20 the Ad Hoc Committee's criteria (Ad Hoc 1962), and nine other criteria; in 17 trials, the diagnostic criteria used were not reported. Propranolol doses ranged from 60 to 320 mg per day. Baseline periods varied from 0 to 10 weeks (median 4 weeks), and treatment phases from 4 to 30 weeks (median 12 weeks). Only a few studies included a follow‐up period after completion of treatment, and dropout rates in these studies were high, making any interpretation difficult.
Risk of bias in included studies
The Jadad score for each study is given in the 'Characteristics of included studies' table, results of the assessment with the Delphi list are reported in Table 5, and results of the assessment of the adequacy of observation are described in Table 6 and Table 7.
Table 1.
Methodological quality ‐ Delphi list
| Study | Randomisation | Concealment | Baseline comparab. | Inclusion criteria | Blind evaluators | Blind care providers | Blind patients | Reporting of results | Intent‐to‐treat |
| Ahuja 1985 | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | ? |
| Al‐Qassab 1993 | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | ? |
| Albers 1989 | 1 | ? | ? | 1 | 0 | 0 | 0 | 1 | 0 |
| Andresson 1981 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Baldrati 1983 | 1 | ? | ? | ? | 1 | 1 | 1 | 0 | 0 |
| Behan 1980 | ? | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 |
| Bonuso 1998 | 1 | ? | ? | 0 | ? | ? | ? | 0 | 0 |
| Bordini 1997 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 |
| Borgesen 1974 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Dahlöf 1987 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 1 |
| Diamond 1976 | 1 | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 |
| Diamond 1982 | 1 | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 |
| Diener 1996 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diener 2002 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diener 1989 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 1 | ? |
| Formisano 1991 | 1 | ? | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Forssman 1976 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Gawel 1991 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Gerber 1995 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Grotemeyer 1987 | ? | ? | ? | ? | 1 | 1 | 1 | 1 | 0 |
| Hedman 1986 | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | 0 |
| Holdorff 1977 | 1 | ? | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Johnson 1986 | 1 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 |
| Kangasniemi 1983 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Kangasniemi 1984 | 1 | ? | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Kaniecki 1997 | 1 | ? | ? | 1 | ? | ? | ? | 0 | 0 |
| Kass 1980 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Kjaesgard‐Rasmussen 1994 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 1 | ? |
| Klapper 1994 | 1 | ? | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kuritzky 1987 | 1 | ? | ? | ? | ? | ? | ? | 0 | 0 |
| Lücking 1988a | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Lücking 1998b | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Ludin 1989 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Maissen 1985 | ? | ? | 0 | 1 | 1 | 1 | 1 | 1 | ? |
| Malvea 1973 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 |
| Mathew 1981‐Study 1 | 1 | ? | ? | 0 | 0 | 0 | 0 | 1 | 0 |
| Mathew 1981‐Study 2 | 1 | ? | ? | 0 | 0 | 0 | 0 | 1 | 0 |
| Micieli 2001 | 1 | ? | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Mikkelsen 1986 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Nadelmann 1986 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Nicolodi 1997 | 1 | ? | ? | ? | 1 | 1 | 1 | 1 | ? |
| Olerud 1986 | 1 | ? | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| Olsson 1984 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Palferman 1983 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 |
| Pita 1977 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Pradalier 1989 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 1 | ? |
| Ryan 1984 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 |
| Sargent 1985 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Scholz 1987 | 1 | ? | ? | ? | 0 | 1 | 1 | 1 | 0 |
| Shimell 1990 | 1 | ? | 1 | 1 | 1 | 1 | 1 | 0 | ? |
| Solomon 1986 | ? | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 |
| Stensrud 1976 | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | 0 |
| Stensrud 1980 | 1 | ? | ? | 0 | 1 | 1 | 1 | 0 | 0 |
| Sudilovski 1987 | 1 | ? | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Tfelt‐Hasen 1984 | 1 | ? | ? | 1 | 1 | 1 | 1 | 1 | 0 |
| Weber 1972 | 1 | ? | ? | ? | 1 | 1 | 1 | 1 | 0 |
| Wideroe 1976 | 1 | ? | ? | 0 | 1 | 1 | 1 | 1 | 0 |
| Ziegler 1987 | 1 | ? | ? | 1 | 1 | 1 | 1 | 0 | 0 |
Table 2.
Adequacy of observation and reporting of key clinical issues (questions 1‐5)
| Study | Sampling described | Clear diagnosis | Patient character. | > 4 weeks baseline | Co‐interventions |
| Ahuja 1985 | 0 | 1 | 0 | 0 | 0 |
| Al‐Qassab 1993 | 0 | 1 | 1 | 1 | 0 |
| Albers 1989 | 0 | 1 | 1 | 0 | 1 |
| Andersson 1981 | 0 | 0 | 0 | 0 | 0 |
| Baldrati 1983 | 0 | 1 | 1 | 1 | 0 |
| Behan 1980 | 0 | 0 | 0 | 0 | 0 |
| Bonuso 1998 | 0 | 1 | 0 | 0 | 0 |
| Bordini 1997 | 0 | 1 | 0 | 0 | 0 |
| Borgesen 1974 | 1 | 1 | 1 | 1 | 0 |
| Dahlöf 1987 | 0 | 1 | 0 | 1 | 0 |
| Diamond 1976 | 0 | 0 | 0 | 0 | 0 |
| Diamond 1982 | 0 | 1 | 0 | 1 | 0 |
| Diener 1996 | 0 | 1 | 1 | 1 | 1 |
| Diener 2002 | 1 | 1 | 1 | 1 | 1 |
| Diener 1989 | 0 | 1 | 1 | 1 | 0 |
| Formisano 1991 | 0 | 1 | 0 | 1 | 0 |
| Forssman 1976 | 0 | 0 | 1 | 1 | 1 |
| Gawel 1992 | 0 | 1 | 1 | 1 | 0 |
| Gerber 1995 | 0 | 1 | 1 | 1 | 0 |
| Grotemeyer 1987 | 0 | 1 | 0 | 0 | 0 |
| Hedman 1986 | 0 | 1 | 0 | 0 | 1 |
| Holdroff 1977 | 0 | 1 | 0 | 0 | 0 |
| Johnson 1986 | 0 | 0 | 0 | 1 | 0 |
| Kangasniemi 1983 | 0 | 0 | 0 | 1 | 0 |
| Kangasniemi 1984 | 0 | 1 | 1 | 1 | 0 |
| Kaniecki 1997 | 1 | 1 | 0 | 1 | 0 |
| Kass 1980 | 0 | 1 | 0 | 1 | 1 |
| Kjaesgard‐Rasmussen 1994 | 0 | 1 | 1 | 1 | 1 |
| Klapper 1994 | 0 | 0 | 0 | 0 | 0 |
| Kuritzky 1987 | 0 | 0 | 0 | 0 | 0 |
| Lücking 1988a | 0 | 0 | 0 | 0 | 0 |
| Lücking 1988b | 0 | 0 | 0 | 0 | 0 |
| Ludin 1989 | 0 | 1 | 0 | 1 | 0 |
| Maissen 1985 | 0 | 1 | 0 | 1 | 0 |
| Malvea 1973 | 1 | 0 | 0 | 1 | 0 |
| Mathew 1981‐study 1 | 0 | 0 | 0 | 1 | 0 |
| Mathew 1981‐study 2 | 0 | 0 | 0 | 1 | 0 |
| Micieli 2001 | 0 | 1 | 1 | 1 | 1 |
| Mikkelsen 1986 | 0 | 1 | 0 | 0 | 0 |
| Nadelmann 1986 | 0 | 1 | 1 | 1 | 0 |
| Nicolodi 1997 | 0 | 0 | 0 | 1 | 0 |
| Olerud 1986 | 0 | 1 | 0 | 1 | 0 |
| Olsson 1984 | 1 | 1 | 1 | 1 | 1 |
| Palferman 1983 | 0 | 0 | 0 | 0 | 0 |
| Pita 1977 | 1 | 1 | 1 | 0 | 0 |
| Pradlier 1989 | 0 | 1 | 1 | 1 | 0 |
| Ryan 1984 | 0 | 0 | 0 | 1 | 0 |
| Sargent 1985 | 0 | 0 | 0 | 0 | 0 |
| Scholz 1987 | 0 | 0 | 0 | 1 | 0 |
| Shimell 1990 | 1 | 1 | 1 | 0 | 0 |
| Solomon 1986 | 0 | 0 | 0 | 0 | 0 |
| Stensrud 1976 | 0 | 1 | 0 | 0 | 0 |
| Stensrud 1980 | 0 | 1 | 0 | 0 | 0 |
| Sudilovsky 1987 | 0 | 1 | 0 | 1 | 0 |
| Tfelt‐Hansen 1984 | 1 | 1 | 1 | 1 | 0 |
| Weber 1972 | 0 | 1 | 0 | 0 | 0 |
| Wideroe 1976 | 1 | 1 | 0 | 0 | 0 |
| Ziegler 1987 | 0 | 0 | 0 | 1 | 0 |
Table 3.
Adequacy of observation and reporting of key clinical issues (questions 6‐10)
| Study | Diary used | Frequency data | Intensity data | 2‐month follow up | 6‐month follow up |
| Ahuja 1985 | 0 | 1 | 0 | 0 | 0 |
| Al‐Qassab 1993 | 1 | 1 | 1 | 0 | 0 |
| Albers 1989 | 1 | 1 | 0 | 0 | 0 |
| Andersson 1981 | 1 | 1 | 1 | 0 | 0 |
| Baldrati 1983 | 1 | 0 | 0 | 0 | 0 |
| Behan 1980 | 1 | 0 | 0 | 0 | 0 |
| Bonuso 1998 | 0 | 0 | 0 | 0 | 1 |
| Bordini 1997 | 1 | 0 | 0 | 0 | 0 |
| Borgesen 1974 | 1 | 1 | 0 | 0 | 0 |
| Dahlöf 1974 | 1 | 0 | 0 | 1 | 1 |
| Diamond 1976 | 0 | 0 | 0 | 0 | 0 |
| Diamond 1982 | 1 | 0 | 0 | 0 | 0 |
| Diener 1996 | 1 | 0 | 0 | 1 | 0 |
| Diener 2002 | 1 | 1 | 1 | 1 | 0 |
| Diener 1989 | 1 | 0 | 0 | 0 | 0 |
| Formisano 1991 | 1 | 1 | 0 | 0 | 0 |
| Forssman 1976 | 1 | 0 | 0 | 0 | 0 |
| Gawel 1992 | 1 | 0 | 1 | 1 | 0 |
| Gerber 1995 | 1 | 0 | 0 | 0 | 0 |
| Grotemeyer 1987 | 0 | 1 | 0 | 0 | 0 |
| Hedman 1986 | 0 | 1 | 0 | 0 | 0 |
| Holdorff 1977 | 0 | 0 | 0 | 0 | 0 |
| Johnson 1986 | 1 | 1 | 0 | 0 | 0 |
| Kangasniemi 1983 | 1 | 1 | 0 | 0 | 0 |
| Kangasniemi 1984 | 1 | 1 | 0 | 1 | 0 |
| Kaniecki 1997 | 1 | 0 | 0 | 0 | 0 |
| Kass 1980 | 1 | 1 | 0 | 1 | 0 |
| Kjaesgard‐Rasmussen 1994 | 0 | 1 | 1 | 0 | 0 |
| Klapper 1994 | 1 | 0 | 0 | 0 | 0 |
| Kuritzky 1987 | 1 | 0 | 0 | 0 | 0 |
| Lücking 1988a | 1 | 1 | 1 | 0 | 0 |
| Lücking 1988b | 1 | 1 | 1 | 0 | 0 |
| Ludin 1989 | 1 | 1 | 1 | 0 | 0 |
| Maissen 1985 | 1 | 1 | 1 | 1 | 0 |
| Malvea 1973 | 1 | 0 | 0 | 0 | 0 |
| Mathew 1981‐study 1 | 1 | 0 | 0 | 0 | 0 |
| Mathew 1981‐study 2 | 1 | 0 | 0 | 0 | 0 |
| Micieli 2001 | 1 | 1 | 0 | 0 | 0 |
| Mikkelsen 1986 | 1 | 1 | 1 | 0 | 0 |
| Nadelmann 1986 | 1 | 0 | 0 | 0 | 0 |
| Olerud 1986 | 1 | 1 | 1 | 1 | 0 |
| Olsson 1984 | 1 | 1 | 0 | 1 | 0 |
| Palferman 1983 | 1 | 0 | 0 | 0 | 0 |
| Pita 1977 | 0 | 1 | 1 | 0 | 0 |
| Pradalier 1989 | 1 | 1 | 0 | 0 | 0 |
| Ryan 1984 | 1 | 0 | 0 | 1 | 0 |
| Sargent 1985 | 1 | 1 | 1 | 0 | 0 |
| Scholz 1987 | 1 | 0 | 0 | 0 | 0 |
| Shimell 1990 | 0 | 0 | 0 | 1 | 0 |
| Solomon 1986 | 1 | 0 | 0 | 0 | 0 |
| Stensrud 1976 | 0 | 1 | 0 | 0 | 0 |
| Stensrud 1980 | 1 | 0 | 0 | 0 | 0 |
| Sudilovsky 1987 | 1 | 1 | 1 | 0 | 0 |
| Tfelt‐Hansen 1984 | 1 | 1 | 1 | 0 | 0 |
| Weber 1972 | 0 | 0 | 0 | 0 | 0 |
| Wideroe 1976 | 1 | 1 | 0 | 0 | 0 |
| Ziegler 1987 | 1 | 0 | 0 | 0 | 0 |
| Nicolodi 1997 | 1 | 1 | 0 | 0 | 0 |
The methodological quality of studies and the observation and reporting of key clinical issues were unsatisfactory in the majority of trials. The main shortcoming was the reporting and handling of dropouts and withdrawals. Twenty‐five studies reported numbers of and reasons for dropouts and withdrawals, but only three included an intention‐to‐treat (ITT) analysis, even as a secondary analysis. In comparisons between active drugs, an ITT analysis would seem highly desirable as a sensitivity analysis given the high dropout rates in most studies. Only two studies adequately described allocation concealment, and none tested the success of blinding. Fifty‐one studies were described as double‐blind. The median Jadad score was 2 (range 1 to 5); the median number of criteria met on the Delphi list was 5 (range 1 to 9). A transparent description of patient recruitment was available for only nine studies. Forty‐seven trials used headache diaries, but only 29 presented detailed results (means and standard deviations, or median and ranges, etc.) for frequency measures, and 15 for intensity measures. Eleven trials had data for at least 90% of randomised patients for a period of at least 2 months, and three for 80% of patients for at least 6 months. Crossover studies rarely reported analyses of carryover or period effects.
Effects of interventions
Comparisons with placebo
Twenty‐six trials compared propranolol with placebo.
Four trials reported data on response (with variable definitions) that could be used to estimate effect sizes, nine if crossover trials with pooled data are included. In all nine trials, the proportion of responders was higher with propranolol than with placebo, and in five trials the difference between the two interventions was statistically significant. The overall relative risk of response to treatment (here called the 'responder ratio') was 1.94 (95% confidence interval [CI] 1.61 to 2.35) for all nine trials (see Comparison No. 01 02), and 1.73 (95% CI 1.23 to 2.42) for the four trials with parallel‐group or first‐period crossover data (Comparison No. 01 01), indicating a significant effect of propranolol over placebo. Results were statistically rather heterogeneous (I² = 50.1%) in the set of four trials, but not in the set of nine trials (I² = 13.8%). Responder ratios tended to be higher in trials with higher dosages of propranolol, but the trial with the lowest dosage (80 mg) had the most positive result (Weber 1972).
Four trials, or 10 if pooled crossover trials are included, reported sufficient data on headache frequency. Here, too, all 10 trials showed at least a trend in favour of propranolol. The overall standardized mean difference was ‐0.45 (95% CI ‐0.75 to ‐0.14) for the four trials with parallel‐group or first‐period crossover data (Comparison No. 01 03), and ‐0.40 (95% CI ‐0.56 to ‐0.24) for all 10 trials (Comparison No. 01 04). The results suggest that propranolol 160 mg may be slightly more effective than 120 mg, but the results from the four trials using 160 mg were highly heterogeneous (I² = 60.8%).
Data on headache intensity were reported inconsistently, but effects over placebo seemed minor at best. There was no consistent trend for larger effects with higher doses.
Only two trials, or six if pooled crossover trials are included, reported data on the number of patients with adverse events. Adverse events were more often reported by patients receiving propranolol (relative risk 1.33, 95% CI 0.97 to 1.82 for the two studies reporting parallel‐group or first‐period crossover data [Comparison No. 01 05]; and 1.43, 95% CI 1.12 to 1.81 for all six trials [Comparison No. 01 06]).
For three, respectively 13, trials the number of patients dropping out due to adverse events was reported. Patients receiving propranolol dropped out more often than patients receiving placebo (relative risk 1.90, 95% CI 0.36 to 10.14 in the three trials with parallel‐group or first‐period crossover data [Comparison No. 01 07]; and 2.11, 95% CI 1.09 to 4.08 in all 13 trials [Comparison No. 01 08]).
Both for the number of patients reporting adverse events and the number of patients dropping out due to adverse events, the results of trials with parallel‐group or first‐period crossover data were statistically highly heterogeneous (Comparisons No. 01 05 and No. 01 07), while this heterogeneity strongly decreased when pooled crossover data were considered (Comparisons No. 01 06 and No. 01 08).
The descriptive review of the placebo‐controlled trials confirms the impression that propranolol is significantly more effective than placebo, mainly by reducing headache frequency. Any effect on headache intensity seems at best minor. According to our vote count, 17 trials showed a significant superiority over placebo, seven a trend in favour of propranolol, and two no difference. All these results apply only to effects during the treatment phase (most often during the last month).
Comparisons with calcium antagonists
This category included 13 trials with 15 comparisons, plus one trial comparing propranolol alone with a combination of propranolol and flunarizine. All trials providing data for effect size calculations in this subset had a parallel‐group design.
Responder data were available for 11 comparisons between propranolol in variable doses and calcium antagonists (flunarizine in nine cases), and for one comparison between propranolol alone and a combination of propranolol and flunarizine. No trial found a significant difference; in most studies response rates were very similar in both groups. The overall responder ratio was 1.00 (95% CI 0.93 to 1.09; Comparison No. 02 01).
Attack frequency data were available for seven comparisons and indicated no statistically significant differences between groups. The pooled standardized mean difference was ‐0.02 (95% CI ‐0.12 to 0.08; Comparison No. 02 02).
Nine trials comparing propranolol with a calcium antagonist (flunarizine in seven cases), and the trial comparing propranolol alone with a combination of propranolol and flunarizine reported the number of patients experiencing adverse events. These were similar in propranolol and flunarizine groups, but tended to be higher with nifedipine and nimodipine. The overall relative risk for all trials was 1.02 (95% CI 0.91 to 1.15; Comparison No. 02 03); for comparisons of propranolol and flunarizine, the overall relative risk was 1.06 (95% CI 0.94 to 1.20; Comparison No. 02 04).
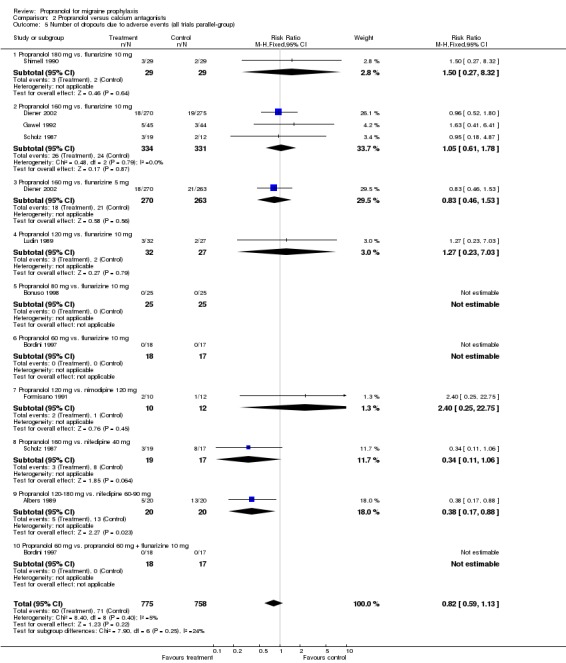
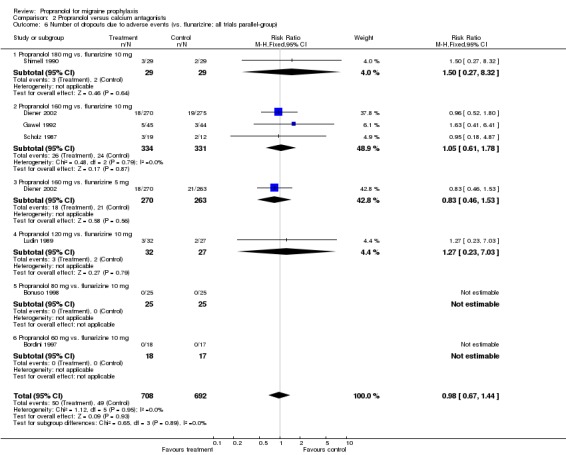
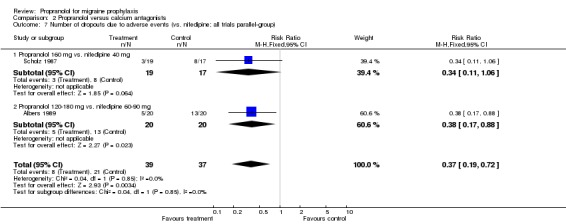
Results were similar for the number of patients dropping out due to adverse events in the 12 trials describing this outcome (Comparison No. 02 05). The overall relative risk for trials comparing propranolol and flunarizine was 0.98 (95% CI 0.67 to 1.44; Comparison No. 02 06). Patients receiving nifedipine dropped out more often due to side effects (relative risk 0.37, 95% CI 0.19 to 0.72; Comparison No. 02 07).
The vote count yielded the following result: two comparisons with a trend in favour of propranolol, 12 showing no difference, one with a trend in favour of flunarizine, and one with a trend in favour of the combination of propranolol and flunarizine.
Comparisons with other beta‐blockers
This category included 10 trials with 12 comparisons, plus one trial comparing propranolol with d‐propranolol.
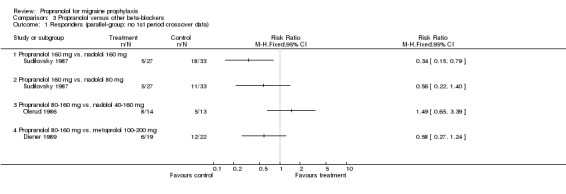
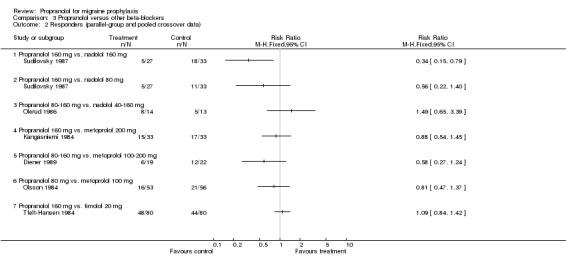
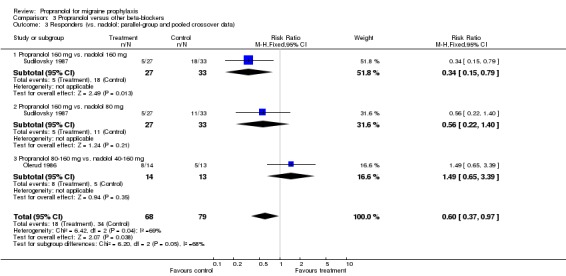
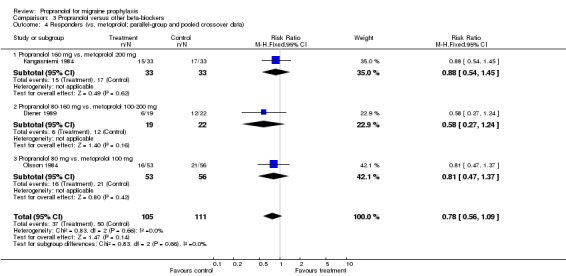
Responder data were reported for four comparisons (Comparison No. 03 01), or seven when including crossover trials with pooled data (Comparison No. 03 02). In one trial, nadolol 160 mg was significantly superior to propranolol 160 mg (Sudilovsky 1987); the other six trials did not yield significant differences. As trial results were statistically heterogeneous (I² = 53.5% for the four‐ and 48.0% for the seven‐trial set), and comparator drugs were nadolol in three trials and metoprolol in three trials, we did not combine results for all trials, but instead performed separate analyses for comparisons with nadolol (Comparison No. 03 03) and metoprolol (Comparison No. 03 04). In the three trials comparing propranolol and nadolol, the overall responder ratio favoured nadolol (0.60, 95% CI 0.37 to 0.97), but the results of the three trials were contradictory (I² = 68.8%). The three trials comparing propranolol and metoprolol had more consistent results (I² = 0%), but did not show significant differences (responder ratio 0.78, 95% CI 0.56 to 1.09).
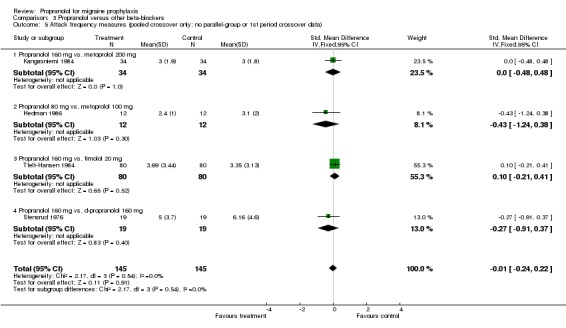
Only four trials, all crossover in design, reported attack frequency data, all pooled, and none reported significant differences; the overall standardized mean difference was ‐0.01 (95% CI ‐0.24 to 0.22; Comparison No. 03 05). There were also no clearcut differences in the number of patients with adverse events (one, respectively three, trials; Comparisons No. 03 06 and No. 03 07) or the number of patients dropping out due to adverse events (six, respectively 11, comparisons; Comparisons No. 03 08 and No. 03 09).
The vote count results were as follows: seven comparisons showing no difference, three with a trend in favour of the other beta‐blocker, one significantly in favour of the other beta‐blocker (vs. nadolol), and one not interpretable. Compared to d‐propranolol there was a trend in favour of propranolol.
Comparisons with other drugs
This category included 20 trials with 20 comparisons, plus two trials comparing propranolol alone with a combination of propranolol and amitriptyline. We did not perform quantitative meta‐analyses for the comparisons of propranolol and other drugs due to the great variety of comparator drugs used. Therefore, we provide only a descriptive summary of results here. Readers are referred to the 'Characteristics of included studies' table and the graphs in MetaView for further information.
Five trials, or 10 if pooled crossover trials are included, provided responder data that could be used for effect size calculation (Comparisons No. 04 01 and No. 04 02). None found a significant difference. Both trials comparing propranolol 160 mg and femoxetine 400 mg reported a possibly relevant trend in favour of propranolol (Andersson 1981; Kangasniemi 1983). Attack frequency data were reported in eight, respectively 10, trials (Comparisons No. 04 03 and No. 04 04). Our calculations yielded a significant superiority of propranolol 120 mg in one of two trials comparing it with tolfenamic acid 300 mg (Kjaersgard 1994) and to 5‐hydroxytryptophan 300 mg (Maissen 1991). Both comparisons with femoxetine again showed a trend in favour of propranolol. No differences were observed in other trials.
Four, respectively 10, trials described the number of patients reporting adverse events (Comparisons No. 04 05 and No. 04 06). The trial comparing propranolol 120 mg and naproxen 1100 mg reported significantly fewer adverse events with propranolol (Sargent 1985). Apart from the comparisons with cyclandelate (trend in favour of cyclandelate [Diener 1996]) and divalproex sodium (trend in favour of propranolol [Kaniecki 1997]), the numbers of patients reporting adverse events with propranolol and comparator drugs were very similar. The number of patients dropping out due to adverse events was reported for seven, respectively 18, comparisons (including the two comparisons of propranolol alone with a combination of propranolol and amitriptyline). There were no significant differences, but confidence intervals were wide due to the low number of events (Comparisons No. 04 07 and No. 04 08).
The vote count yielded the following result: one trial significantly in favour of propranolol (vs. amitriptyline), five with a trend in favour of propranolol, 11 showing no difference, two with a trend in favour of the comparator drug, and one not interpretable; one of the two comparisons of propranolol alone and propranolol in combination with amitriptyline was classified as no difference, and the other as showing a trend in favour of the combination.
Discussion
Despite the methodological limitations of the majority of the available trials, there is clear and consistent evidence that propranolol is superior to placebo in the interval treatment of patients suffering from migraine. Based on the available trials, it is not possible to draw reliable conclusions on whether different doses of propranolol have different effectiveness, or whether the prophylactic effects continue after propranolol is stopped. Propranolol seems to be as effective as other pharmacological agents used for migraine prophylaxis, and seems to have similar safety and tolerability, but definitive conclusions are not possible due to small sample sizes and the inconsistent reporting of results, which precluded a reliable meta‐analysis of the available studies.
The major problem encountered in this review was the highly variable and often insufficient reporting of the complex outcome data. Migraine prophylaxis trials typically use headache diaries to monitor the course of the disease. From these headache diaries a variety of outcomes can be extracted (headache days, migraine days, migraine attacks, days with a defined headache intensity, attack intensity, mean headache intensity, headache indices, headache hours, days with medication, use of analgesics, etc.). These outcomes can be assessed over different time frames (most often 4 weeks, but there were trials using 3 weeks, 8 weeks, total treatment periods, etc.) and presented in different manners (values at a certain time interval presented as means with standard deviations, standard errors, confidence intervals, or often no measure of variance; medians with range, quartiles, or nothing; as mean or median per cent change compared to baseline, etc.). The data reported in the included studies represent a highly heterogeneous mixture of these different options. This not only makes quantitative meta‐analysis technically difficult, but raises the question of why certain results have been presented and others not. Due to these problems, all overall effect size estimates from the quantitative meta‐analyses reported here must be interpreted with great caution.
Another problem was the high dropout rates reported. The majority of the trials were performed at a time when intention‐to‐treat analyses were not mandatory. Therefore, dropouts and withdrawals were typically excluded from analysis, which probably led to overly optimistic response rates (regardless of study group) and possibly to an over‐estimation of effects over placebo.
Due to the small sample size of most trials, the comparisons of propranolol with other drugs must be interpreted with great caution. Clinically relevant differences might exist but have not been detected. On the other hand, as there are very few trials or often only a single trial comparing a defined dose of propranolol with a comparator drug in a defined dose, any significant differences found in our effect size calculations also have to be interpreted with great care.
Taking all these problems into account, there is considerable uncertainty about the actual size of the effect of propranolol over placebo and effect sizes for propranolol in comparison with other pharmacological agents.
The main shortcoming of the available trials from a practical perspective is the lack of adequate follow up after stopping treatment. The few studies that had such a follow up reported very high withdrawal rates.
Our findings are very similar to those of a systematic review on drug treatments for the prevention of migraine headache performed for the US Agency of Health Care Policy and Research (now the US Agency for Healthcare Research and Quality) in 1999 (Gray 1999).
Authors' conclusions
Based on the available evidence, the use of propranolol for the prophylactic treatment of migraine is justified.
Since propranolol has been on the market for a long time, it seems unlikely that major studies will be performed in the future with propranolol as the primary experimental treatment. However, it will probably still be used as a comparator drug when new agents or uncommon dosing schemes are tested (as, e.g., in Diener 2002). We recommend that new trials follow the International Headache Society's guidelines for controlled trials of drugs for migraine (Tfelt‐Hansen 2000), so that future studies will be conducted according to a high methodological standard and will more readily permit quantitative meta‐analysis. However, as these guidelines recommend quite narrow inclusion criteria, it seems unclear whether the findings of such trials will be directly applicable to migraine treatment in primary care. Major topics for future research include the question of how stable the preventive effects of prophylactic drug treatment is once the treatment has been stopped and the extent to which migraine patients comply with prophylactic treatment in routine practice.
Acknowledgements
The authors would like to thank Dr HJ Jaster for extracting data from several studies, and Rebecca Gray and Douglas McCrory for their great help and input at various stages of the review.
Data and analyses
Comparison 1.
Propranolol versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (parallel‐group and 1st period crossover data) | 4 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.23, 2.40] |
| 1.1 Propranolol 160 mg | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.34, 5.14] |
| 1.2 Propranolol 120 mg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.85, 2.20] |
| 1.3 Propranolol 80‐120 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.42] |
| 1.4 Propranolol 80 mg | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.67 [0.93, 232.44] |
| 2 Responders (parallel‐group and pooled crossover data) | 9 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.61, 2.35] |
| 2.1 Propranolol 160 mg | 3 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.59, 2.87] |
| 2.2 Propranolol 80‐160 mg | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.26, 3.18] |
| 2.3 Propranolol 120 mg | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.97, 2.10] |
| 2.4 Propranolol 80‐120 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.42] |
| 2.5 Propranolol 80 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.5 [1.98, 28.40] |
| 2.6 Propranolol, dose unclear | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.02, 3.93] |
| 3 Attack frequency measures (parallel‐group and 1st period crossover data) | 4 | 172 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.75, ‐0.14] |
| 3.1 Propranolol 160 mg | 2 | 67 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.27, ‐0.27] |
| 3.2 Propranolol 120 mg | 2 | 105 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.64, 0.13] |
| 4 Attack frequency measures (parallel‐group and pooled crossover data) | 10 | 634 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.56, ‐0.24] |
| 4.1 Propranolol 240 mg | 1 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.08, 0.28] |
| 4.2 Propranolol 160 mg | 4 | 291 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| 4.3 Propranolol 120 mg | 5 | 309 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.55, ‐0.10] |
| 5 Number of patients with adverse events (parallel‐group; no 1st period crossover data) | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.97, 1.82] |
| 5.1 Propranolol 120 mg | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.97, 1.82] |
| 6 Number of patients with adverse events (parallel‐group and pooled crossover data) | 6 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.12, 1.81] |
| 6.1 Propranolol 240 mg | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.20, 21.48] |
| 6.2 Propranolol 160 mg | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.99, 2.34] |
| 6.3 Propranolol 80‐160 mg | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.79, 3.25] |
| 6.4 Propranolol 120 mg | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.95, 1.77] |
| 7 Number of dropouts due to adverse events (parallel‐group and 1st period crossover data) | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.36, 10.14] |
| 7.1 Propranolol 80‐320 mg | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Propranolol 160 mg | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.77] |
| 7.3 Propranolol 120 mg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.38 [0.35, 116.13] |
| 8 Number of dropouts due to adverse events (parallel‐group and pooled crossover data) | 13 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.09, 4.08] |
| 8.1 Propranolol 80‐320 mg | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Propranolol 240 mg | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.78] |
| 8.3 Propranolol 160 mg | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.77, 6.01] |
| 8.4 Propranolol 80‐160 mg | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.74, 48.75] |
| 8.5 Propranolol 120 mg | 3 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.91] |
| 8.6 Propranolol 80 mg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 1.1.
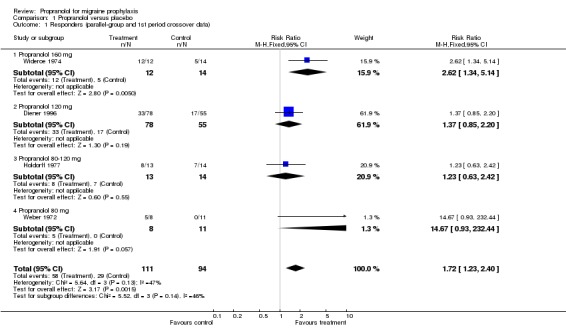
Comparison 1 Propranolol versus placebo, Outcome 1 Responders (parallel‐group and 1st period crossover data).
Analysis 1.2.
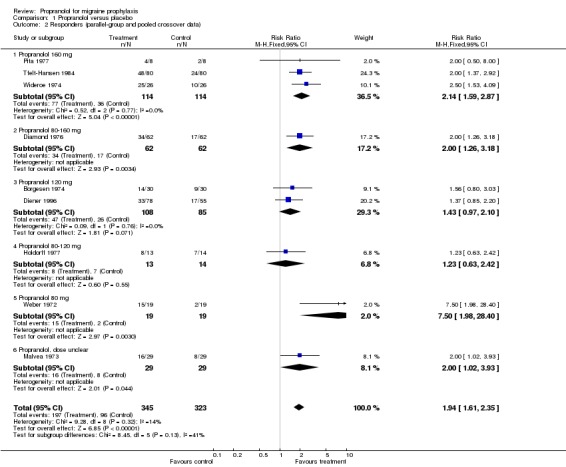
Comparison 1 Propranolol versus placebo, Outcome 2 Responders (parallel‐group and pooled crossover data).
Analysis 1.3.
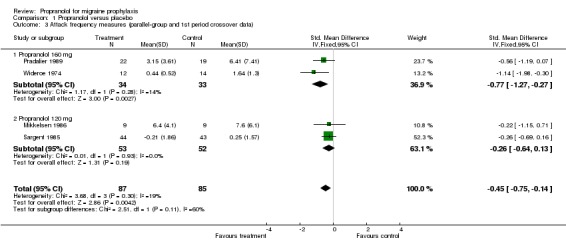
Comparison 1 Propranolol versus placebo, Outcome 3 Attack frequency measures (parallel‐group and 1st period crossover data).
Analysis 1.4.
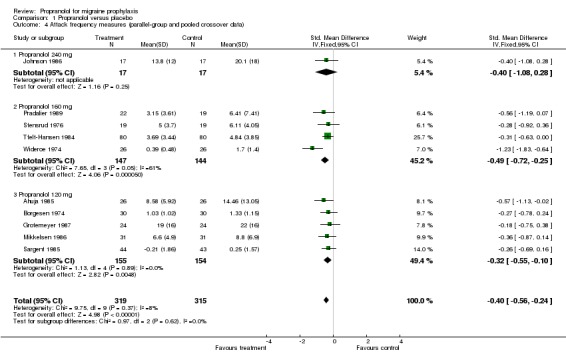
Comparison 1 Propranolol versus placebo, Outcome 4 Attack frequency measures (parallel‐group and pooled crossover data).
Analysis 1.5.
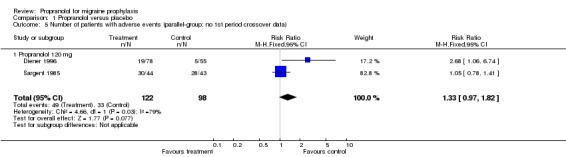
Comparison 1 Propranolol versus placebo, Outcome 5 Number of patients with adverse events (parallel‐group; no 1st period crossover data).
Analysis 1.6.
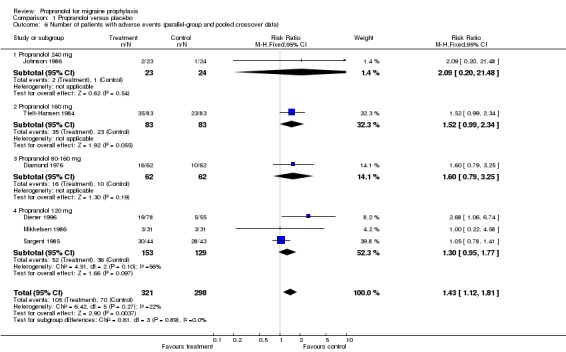
Comparison 1 Propranolol versus placebo, Outcome 6 Number of patients with adverse events (parallel‐group and pooled crossover data).
Analysis 1.7.
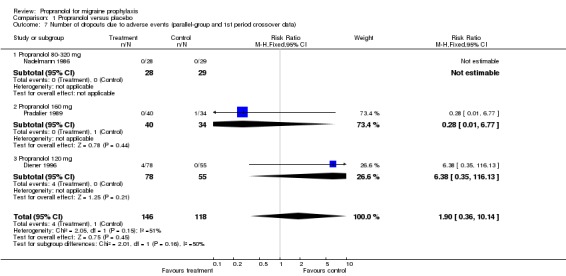
Comparison 1 Propranolol versus placebo, Outcome 7 Number of dropouts due to adverse events (parallel‐group and 1st period crossover data).
Analysis 1.8.
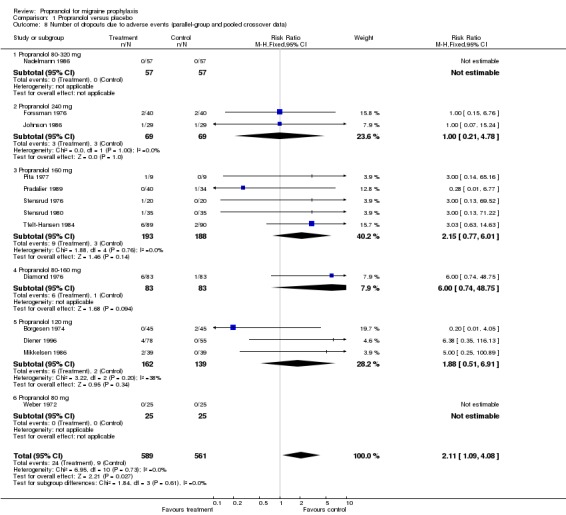
Comparison 1 Propranolol versus placebo, Outcome 8 Number of dropouts due to adverse events (parallel‐group and pooled crossover data).
Comparison 2.
Propranolol versus calcium antagonists
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (all trials parallel‐group) | 10 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.09] |
| 1.1 Propranolol 180 mg vs. flunarizine 10 mg | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.01, 1.68] |
| 1.2 Propranolol 160 mg vs. flunarizine 10 mg | 2 | 598 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 1.3 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.28] |
| 1.4 Propranolol 120 mg vs. flunarizine 10 mg | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 1.5 Propranolol 80 mg vs. flunarizine 10 mg | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.38] |
| 1.6 Propranolol 60 mg vs. flunarizine 10 mg | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 1.7 Propranolol 120‐180 mg vs. nifedipine 60‐90 mg | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.51] |
| 1.8 Propranolol 80‐160 mg vs. nifedipine 20‐40 mg | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.72, 40.20] |
| 1.9 Propranolol 60 mg vs. propranolol 60 mg + flunarizine 10 mg | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 2 Attack frequency measures (all trials parallel‐group) | 6 | 1543 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 2.1 Propranolol 160 mg vs. flunarizine 10 mg | 1 | 524 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.11, 0.23] |
| 2.2 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 518 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.24, 0.10] |
| 2.3 Propranolol 120 mg vs. flunarizine 10 mg | 3 | 462 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.24, 0.13] |
| 2.4 Propranolol 120‐180 mg vs. nifedipine 60‐90 mg | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.73, 1.11] |
| 2.5 Propranolol 120 mg vs. nimodipine 120 mg | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.09, 0.74] |
| 3 Number of patients with adverse events (all trials parallel‐group) | 8 | 1753 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 3.1 Propranolol 160 mg vs. flunarizine 10 mg | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.24] |
| 3.2 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 3.3 Propranolol 120 mg vs. flunarizine 10 mg | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.97, 1.52] |
| 3.4 Propranolol 60 mg vs. flunarizine 10 mg | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 3.5 Propranolol 120‐180 mg vs. nifedipine 60‐90 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 0.98] |
| 3.6 Propranolol 120 mg vs. nimodipine 120 mg | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.12] |
| 3.7 Propranolol 60 mg vs. propanolol 60 mg + flunarizine 10 mg | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.33] |
| 4 Number of patients with adverse events (vs. flunarizine; all trials parallel‐group) | 6 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.20] |
| 4.1 Propranolol 160 mg vs. flunarizine 10 mg | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.24] |
| 4.2 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 4.3 Propranolol 120 mg vs. flunarizine 10 mg | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.97, 1.52] |
| 4.4 Propranolol 60 mg vs. flunarizine 10 mg | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 5 Number of dropouts due to adverse events (all trials parallel‐group) | 9 | 1533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 5.1 Propranolol 180 mg vs. flunarizine 10 mg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.32] |
| 5.2 Propranolol 160 mg vs. flunarizine 10 mg | 3 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.61, 1.78] |
| 5.3 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.53] |
| 5.4 Propranolol 120 mg vs. flunarizine 10 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.23, 7.03] |
| 5.5 Propranolol 80 mg vs. flunarizine 10 mg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Propranolol 60 mg vs. flunarizine 10 mg | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Propranolol 120 mg vs. nimodipine 120 mg | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [0.25, 22.75] |
| 5.8 Propranolol 160 mg vs. nifedipine 40 mg | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.06] |
| 5.9 Propranolol 120‐180 mg vs. nifedipine 60‐90 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.88] |
| 5.10 Propranolol 60 mg vs. propranolol 60 mg + flunarizine 10 mg | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number of dropouts due to adverse events (vs. flunarizine; all trials parallel‐group) | 7 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.44] |
| 6.1 Propranolol 180 mg vs. flunarizine 10 mg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.32] |
| 6.2 Propranolol 160 mg vs. flunarizine 10 mg | 3 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.61, 1.78] |
| 6.3 Propranolol 160 mg vs. flunarizine 5 mg | 1 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.53] |
| 6.4 Propranolol 120 mg vs. flunarizine 10 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.23, 7.03] |
| 6.5 Propranolol 80 mg vs. flunarizine 10 mg | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Propranolol 60 mg vs. flunarizine 10 mg | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Number of dropouts due to adverse events (vs. nifedipine; all trials parallel‐group) | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.19, 0.72] |
| 7.1 Propranolol 160 mg vs. nifedipine 40 mg | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.06] |
| 7.2 Propranolol 120‐180 mg vs. nifedipine 60‐90 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.88] |
Analysis 2.1.
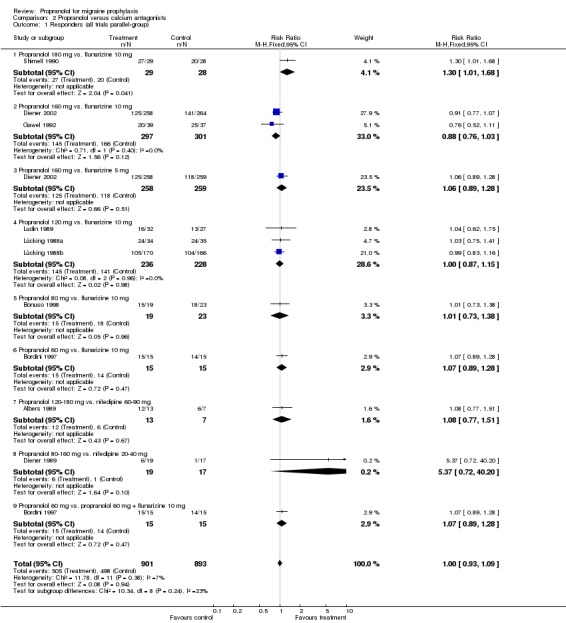
Comparison 2 Propranolol versus calcium antagonists, Outcome 1 Responders (all trials parallel‐group).
Analysis 2.2.
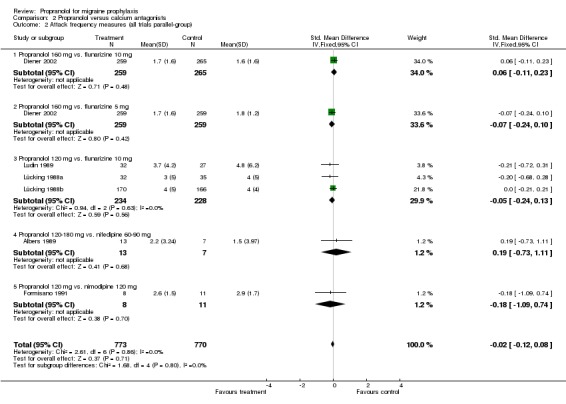
Comparison 2 Propranolol versus calcium antagonists, Outcome 2 Attack frequency measures (all trials parallel‐group).
Analysis 2.3.
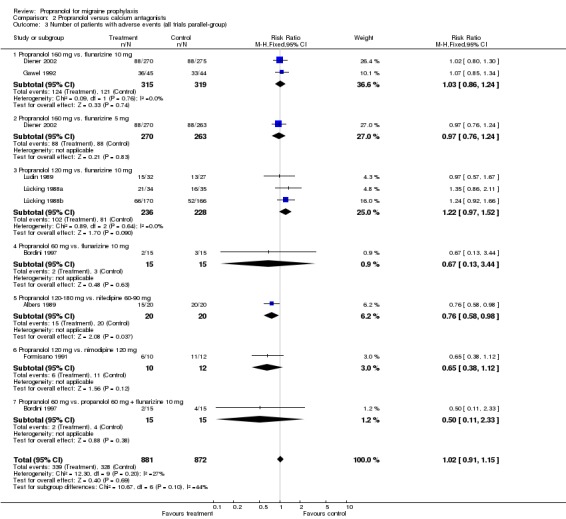
Comparison 2 Propranolol versus calcium antagonists, Outcome 3 Number of patients with adverse events (all trials parallel‐group).
Analysis 2.4.
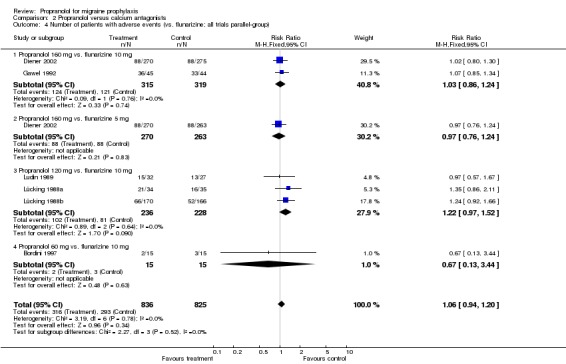
Comparison 2 Propranolol versus calcium antagonists, Outcome 4 Number of patients with adverse events (vs. flunarizine; all trials parallel‐group).
Analysis 2.5.
Comparison 2 Propranolol versus calcium antagonists, Outcome 5 Number of dropouts due to adverse events (all trials parallel‐group).
Analysis 2.6.
Comparison 2 Propranolol versus calcium antagonists, Outcome 6 Number of dropouts due to adverse events (vs. flunarizine; all trials parallel‐group).
Analysis 2.7.
Comparison 2 Propranolol versus calcium antagonists, Outcome 7 Number of dropouts due to adverse events (vs. nifedipine; all trials parallel‐group).
Comparison 3.
Propranolol versus other beta‐blockers
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (parallel‐group; no 1st period crossover data) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Propranolol 160 mg vs. nadolol 160 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Propranolol 160 mg vs. nadolol 80 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Propranolol 80‐160 mg vs. metoprolol 100‐200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Responders (parallel‐group and pooled crossover data) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Propranolol 160 mg vs. nadolol 160 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Propranolol 160 mg vs. nadolol 80 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Propranolol 160 mg vs. metoprolol 200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Propranolol 80‐160 mg vs. metoprolol 100‐200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Propranolol 80 mg vs. metoprolol 100 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Propranolol 160 mg vs. timolol 20 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Responders (vs. nadolol; parallel‐group and pooled crossover data) | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.97] |
| 3.1 Propranolol 160 mg vs. nadolol 160 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| 3.2 Propranolol 160 mg vs. nadolol 80 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.40] |
| 3.3 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.65, 3.39] |
| 4 Responders (vs. metoprolol; parallel‐group and pooled crossover data) | 3 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] |
| 4.1 Propranolol 160 mg vs. metoprolol 200 mg | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.45] |
| 4.2 Propranolol 80‐160 mg vs. metoprolol 100‐200 mg | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.27, 1.24] |
| 4.3 Propranolol 80 mg vs. metoprolol 100 mg | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.47, 1.37] |
| 5 Attack frequency measures (pooled crossover only; no parallel‐group or 1st period crossover data) | 4 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.24, 0.22] |
| 5.1 Propranolol 160 mg vs. metoprolol 200 mg | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.48, 0.48] |
| 5.2 Propranolol 80 mg vs. metoprolol 100 mg | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.24, 0.38] |
| 5.3 Propranolol 160 mg vs. timolol 20 mg | 1 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.21, 0.41] |
| 5.4 Propranolol 160 mg vs. d‐propranolol 160 mg | 1 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.91, 0.37] |
| 6 Number of patients with adverse events (parallel‐group; no 1st period crossover data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of patients with adverse events (parallel‐group and pooled crossover data) | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.24] |
| 7.1 Propranolol 160 mg vs. timolol 20 mg | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 7.2 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.93] |
| 7.3 Propranolol 80 mg vs. metoprolol 100 mg | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Number of dropouts due to adverse events (parallel‐group; no 1st period crossover data) | 4 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.10] |
| 8.1 Propranolol 160 mg vs. nadolol 160 mg | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.41, 11.09] |
| 8.2 Propranolol 160 mg vs. nadolol 80 mg | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.43, 11.57] |
| 8.3 Propranolol 120 mg vs. nadolol 160 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Propranolol 120 mg vs. nadolol 80 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.62] |
| 8.5 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 8.6 Propranolol 160 mg vs. metoprolol 200 mg | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 2.01] |
| 9 Number of dropouts due to adverse events (parallel‐group and pooled crossover data) | 9 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.61, 1.80] |
| 9.1 Propranolol 160 mg vs. nadolol 160 mg | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.41, 11.09] |
| 9.2 Propranolol 160 mg vs. nadolol 80 mg | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.43, 11.57] |
| 9.3 Propranolol 120 mg vs. nadolol 160 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Propranolol 120 mg vs. nadolol 80 mg | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.62] |
| 9.5 Propranolol 80‐160 mg vs. nadolol 40‐160 mg | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 9.6 Propranolol 160 mg vs. metoprolol 200 mg | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.33, 2.72] |
| 9.7 Propranolol 80 mg vs. metoprolol 100 mg | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Propranolol 160 mg vs. atenolol 100 mg | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.22] |
| 9.9 Propranolol 160 mg vs. timolol 20 mg | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.79] |
| 9.10 Propranolol 160 mg vs. d‐propranolol 160 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
Analysis 3.1.
Comparison 3 Propranolol versus other beta‐blockers, Outcome 1 Responders (parallel‐group; no 1st period crossover data).
Analysis 3.2.
Comparison 3 Propranolol versus other beta‐blockers, Outcome 2 Responders (parallel‐group and pooled crossover data).
Analysis 3.3.
Comparison 3 Propranolol versus other beta‐blockers, Outcome 3 Responders (vs. nadolol; parallel‐group and pooled crossover data).
Analysis 3.4.
Comparison 3 Propranolol versus other beta‐blockers, Outcome 4 Responders (vs. metoprolol; parallel‐group and pooled crossover data).
Analysis 3.5.
Comparison 3 Propranolol versus other beta‐blockers, Outcome 5 Attack frequency measures (pooled crossover only; no parallel‐group or 1st period crossover data).
Analysis 3.6.
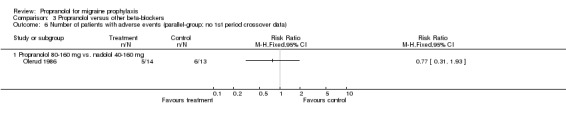
Comparison 3 Propranolol versus other beta‐blockers, Outcome 6 Number of patients with adverse events (parallel‐group; no 1st period crossover data).
Analysis 3.7.
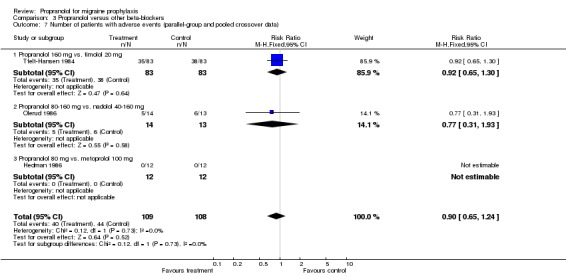
Comparison 3 Propranolol versus other beta‐blockers, Outcome 7 Number of patients with adverse events (parallel‐group and pooled crossover data).
Analysis 3.8.
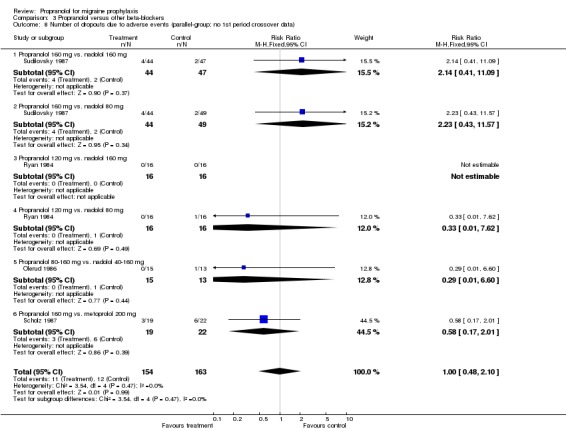
Comparison 3 Propranolol versus other beta‐blockers, Outcome 8 Number of dropouts due to adverse events (parallel‐group; no 1st period crossover data).
Analysis 3.9.
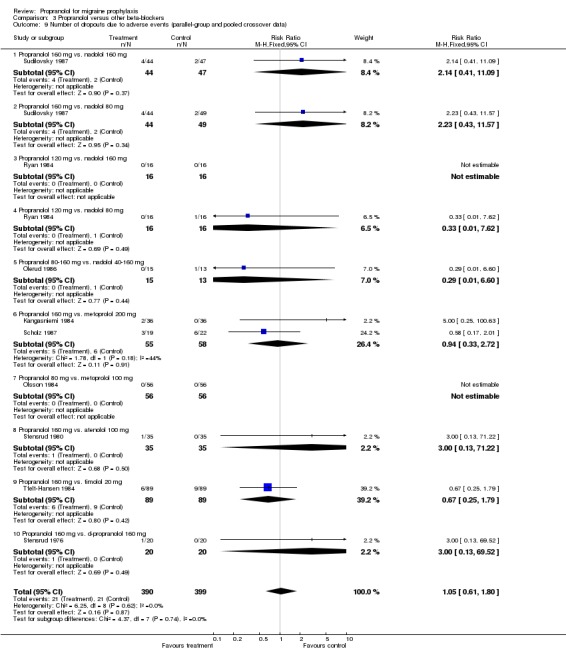
Comparison 3 Propranolol versus other beta‐blockers, Outcome 9 Number of dropouts due to adverse events (parallel‐group and pooled crossover data).
Comparison 4.
Propranolol versus other drugs
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (parallel‐group and 1st period crossover data) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Propranolol 160 mg vs. femoxetine 400 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Propranolol 120‐160 mg vs. cyclandelate 1200‐1600 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Propranolol 60‐240 mg vs. divalproex sodium 1000‐2000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Responders (parallel‐group and pooled crossover data [one 1st period]) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Propranolol 160 mg vs. femoxetine 400 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Propranolol 120‐160 mg vs. cyclandelate 1200‐1600 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Propranolol 60‐240 mg vs. divalproex sodium 1000‐2000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Propranolol 120 mg vs. methysergide 3 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Propranolol 160 mg vs. clonidine 100 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Propranolol 80‐240 mg vs. amitriptyline 50‐150 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Propranolol 1.8 mg/kg vs. ASA 13.5 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Attack frequency measures (parallel‐group and 1st period crossover data) | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Propranolol 160 mg vs. femoxetine 400 mg | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Propranolol 120 mg vs. tolfenamic acid 300 mg | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Propranolol 120 mg vs. naproxen 1100 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Propranolol 1230 mcg/kg vs. methysergide 30.8 mcg/kg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Propranolol 80 mg vs. alpha‐dihydroergocryptine 20 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Attack frequency measures (parallel‐group and pooled crossover data [two 1st period]) | 10 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Propranolol 160 mg vs. femoxetine 400 mg | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Propranolol 120 mg vs. tolfenamic acid 300 mg | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Propranolol 120 mg vs. naproxen 1100 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Propranolol 1230 mcg/kg vs. methysergide 30.8 mcg/kg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Propranolol 240 mg vs. mefenamic acid 1500 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Propranolol 160 mg vs. clonidine 100 mcg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Propranolol 80 mg vs. alpha‐dihydroergocryptine 20 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of patients with adverse events (parallel‐group; no 1st period crossover data) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Propranolol 120‐160 mg vs. cyclandelate 1200‐1600 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Propranolol 120 mg vs. naproxen 1100 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of patients with adverse events (parallel‐group and pooled crossover data) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Propranolol 120‐160 mg vs. cyclandelate 1200‐1600 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Propranolol 120 mg vs. naproxen 1100 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Propranolol 120 mg vs. tolfenamic acid 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Propranolol 120 mg vs. methysergide 3 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Propranolol 160 mg vs. clonidine 100 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Propranolol 60‐240 mg vs. divalproex sodium 1000‐2000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.9 Propranolol 1.8 mg/kg vs. ASA 13.5 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.10 Propranolol 240 mg vs. mefenamic acid 1500 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of dropouts due to adverse events (parallel‐group; no 1st period crossover data) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Propranolol 160 mg vs. dihydroergotamine 10 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Propranolol 120‐160 mg vs. amitriptyline 50‐75 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Propranolol 120‐160 mg vs. amitriptyline 50‐75 mg + propranolol 120‐160 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of dropouts due to adverse events (parallel‐group and pooled crossover data) | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Propranolol 160 mg vs. femoxetine 400 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Propranolol 120 mg vs. cyclandelate 1200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Propranolol 80‐240 mg vs. divalproex sodium 750‐1500 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Propranolol 60‐240 mg vs. divalproex sodium 1000‐2000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Propranolol 120 mg vs. 5‐hydroxytryptophan 300 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Propranolol 240 mg vs. mefenamic acid 1500 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Propranolol 120 mg vs. tolfenamic acid 300 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Propranolol 120 mg vs. methysergide 3 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Propranolol 160 mg vs. dihydroergotamine 10 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Propranolol 80 mg vs. alpha‐dihydroergocryptine 20 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.11 Propranolol 160 mg vs. clonidine 100 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.12 Propranolol 1.8 mg/kg vs. ASA 13.5 mg/kg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.13 Propranolol 120‐160 mg vs. amitriptyline 50‐75 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.14 Propranolol 120‐160 mg vs. amitriptyline 50‐75 mg + propranolol 120‐160 mg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 4.1.
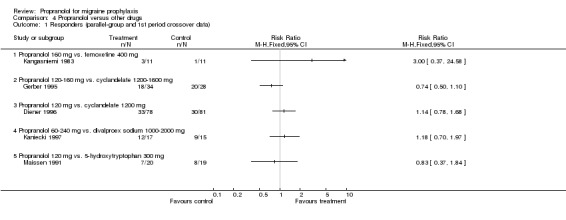
Comparison 4 Propranolol versus other drugs, Outcome 1 Responders (parallel‐group and 1st period crossover data).
Analysis 4.2.
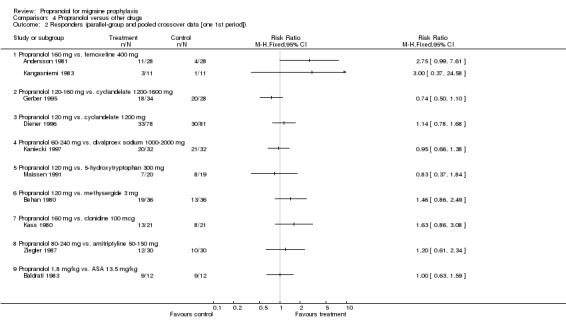
Comparison 4 Propranolol versus other drugs, Outcome 2 Responders (parallel‐group and pooled crossover data [one 1st period]).
Analysis 4.3.
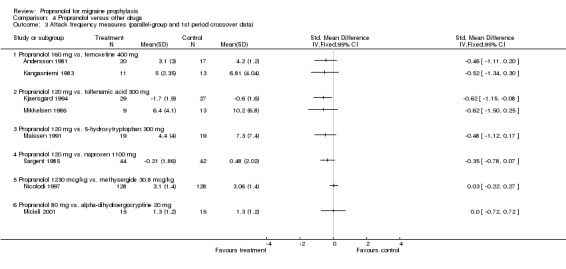
Comparison 4 Propranolol versus other drugs, Outcome 3 Attack frequency measures (parallel‐group and 1st period crossover data).
Analysis 4.4.
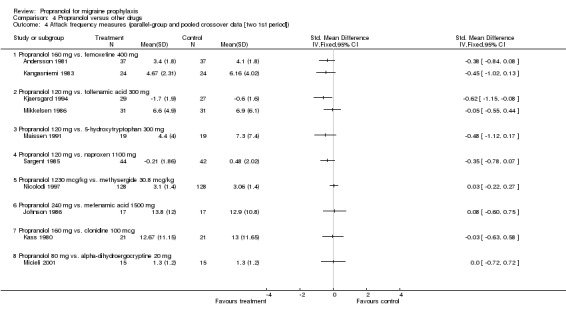
Comparison 4 Propranolol versus other drugs, Outcome 4 Attack frequency measures (parallel‐group and pooled crossover data [two 1st period]).
Analysis 4.5.
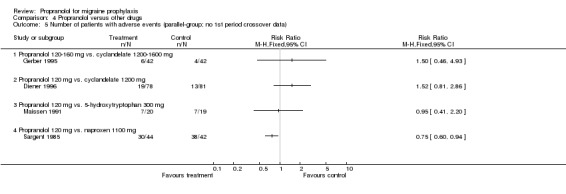
Comparison 4 Propranolol versus other drugs, Outcome 5 Number of patients with adverse events (parallel‐group; no 1st period crossover data).
Analysis 4.6.
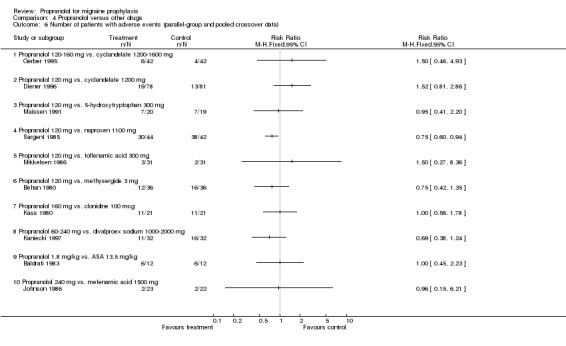
Comparison 4 Propranolol versus other drugs, Outcome 6 Number of patients with adverse events (parallel‐group and pooled crossover data).
Analysis 4.7.
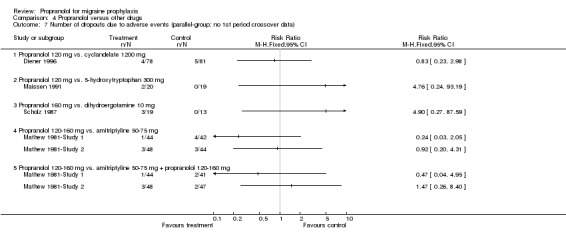
Comparison 4 Propranolol versus other drugs, Outcome 7 Number of dropouts due to adverse events (parallel‐group; no 1st period crossover data).
Analysis 4.8.
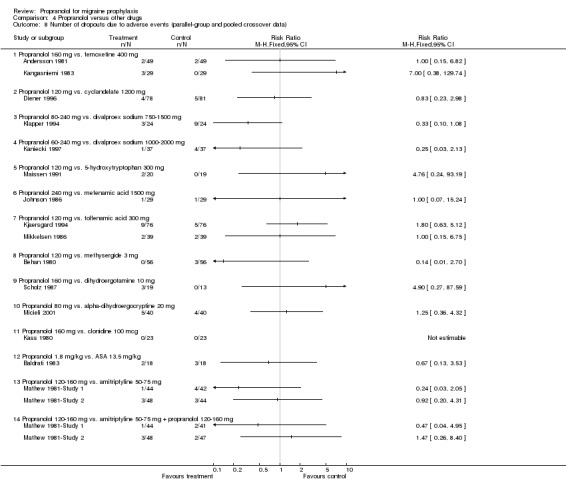
Comparison 4 Propranolol versus other drugs, Outcome 8 Number of dropouts due to adverse events (parallel‐group and pooled crossover data).
What's new
| Date | Event | Description |
|---|---|---|
| 10 June 2019 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 9 April 2015 | Amended | This review has been withdrawn. See Published notes. |
| 1 October 2012 | Amended | Contact details updated. |
| 12 March 2012 | Amended | Information about the updating of this review has been added to the Published notes section. |
| 10 August 2009 | Amended | Contact details updated. |
| 29 January 2009 | Amended | Contact details updated. |
| 26 August 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | D: crossover C: unclear B: double WD: unclear J: 1‐1‐0 DU: ‐/2x8w (no washout)/‐ | |
| Participants | N: 26/unclear D: migraine C: Ad Hoc F: 46% A: 17‐55 DU: unclear S: neurology clinic in India | |
| Interventions | P: 120 mg C: Placebo | |
| Outcomes | R: not reported F: 8.6 (sd 5.9) vs. 14.5 (13.1) attacks in 8 weeks AU: not reported HI: 20.7 (sd 16.8) vs. 38.0 (39.1) AEs: 'no significant side effects' Dropouts‐AEs: not reported V: + | |
| Notes | Dropouts/withdrawals unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 15/45 J: 1‐1‐1 DU: 4w/3x2mo (1w)/‐ | |
| Participants | N: 45/30 D: 27 migraine with, 17 without aura (1 unclear) C: unclear F: 80% A: 17‐55 years DU: 1‐49 years S: general neurology clinic in London | |
| Interventions | P1: 160 mg (long‐acting) P2: 80 mg (long‐acting) C: Placebo | |
| Outcomes | R: not reported F: median attack frequency 3.8 (P1) vs. 3.8 (P2) vs. 3.2 (C) AU: similar in all 3 groups HI: not reported AEs: not reported Dropouts‐AEs: not reported V: 0 | |
| Notes | High dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: unblinded WD: 20/40 J: 2‐0‐1 DU: ‐/6m/‐ | |
| Participants | N: 40/20 D: migraine C: Ad Hoc F: 89% A: 23‐47 years DU: not reported S: probably neurological university outpatient clinic in the US | |
| Interventions | P: 120‐180 mg C: Nifedipine 60‐90 mg | |
| Outcomes | R: 12/13 vs. 6/7 (subjective rating of at least 50% improvement) F: 2.2 (sd 3.2) vs. 1.5 (4.0) attacks/month during months 4‐6 AU: not reported HI: not reported AEs: 15/20 vs. 20/20 Dropouts‐AEs: 5/20 vs. 13/20 V: (+) | |
| Notes | Very high dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 12/49 J: 1‐1‐1 DU: 1m/2x3m (no washout)/‐ | |
| Participants | N: 49/37 D: 31 migraine with, 18 without aura C: unclear F: 69% A: 22‐68 years DU: 2‐40 years S: probably neurological practice in Denmark | |
| Interventions | P: 160 mg C: Femoxetine 400 mg | |
| Outcomes | R: 11/28 vs. 4/28 (reported only for 28 patients with baseline data) F: 1st period: 3.1 (sem 0.5) vs. 4.2 (0.3); pooled: 3.4 (sem 0.3) vs. 4.1 (0.3) attacks per 30 days in last 2 months AU: not reported HI: 15.4 vs. 18.0 AEs: more side effects with propranolol (30 vs. 14) Dropouts‐AEs: 2/49 vs. 2/49 V: (+) | |
| Notes | Baseline data for 28 patients only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 6/18 J: 1‐1‐1 DU: 1m/2x3m (2w)/‐ | |
| Participants | N: 18/12 D: all migraine without aura C: Ad Hoc F: 89% A: 18‐49 years DU: 3‐38 years S: probably neurological university outpatient clinic in Italy | |
| Interventions | P: 1.8 mg/kg C: Acetylsalicylic acid 13.5 mg/kg | |
| Outcomes | R: 9/12 vs. 9/12 (at least 50% index reduction) F: not reported AU: not reported HI: 65% reduction in both groups AEs: 6/12 vs. 6/12 Dropouts‐AEs: 2/18 vs. 3/18 V: 0 | |
| Notes | Small trial with relatively high dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 20/56 J: 0‐1‐0 DU: ‐/2x3m (1m)/‐ | |
| Participants | N: 56/36 D: 12 migraine with, 44 without aura C: unclear F: 66% A: 18‐56 years DU: 1‐33 years S: probably neurology clinic in Glasgow | |
| Interventions | P: 120 mg C: Methysergide 3 mg | |
| Outcomes | R: 19/36 vs. 13/36 F: frequency lower with propranolol AU: not reported HI: not reported AEs: 12/36 vs. 16/36 Dropouts‐AEs: 0/56 vs. 3/56 V: (+) | |
| Notes | High dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: unclear WD: 8/50 J: 1‐0‐0 DU: unclear/2m/4m | |
| Participants | N: 50/42 D: all migraine without aura C: IHS F: 68% A: 20‐45 years DU: unclear S: unclear, Italy | |
| Interventions | P: 80 mg C: Flunarizine 10 mg | |
| Outcomes | R: 15/19 vs. 18/23 F: not reported AU: not reported HI: not reported AEs: 4 AEs vs. 7 Dropouts‐AEs: 0 vs. 0 (number of patients randomised to groups not fully clear) V: 0 | |
| Notes | Study focusing on the fronto‐temporal nitrogylcerin test and reporting only responder data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 7/52 J: 1‐1‐0 DU: 3w/17w/6w | |
| Participants | N: 52/45 D: all migraine without aura C: IHS F: 91% A: 17‐48 years DU: not reported S: outpatient department of a university hospital in Brazil | |
| Interventions | P: 60 mg C1: Flunarizine 10 mg C2: Propranolol + flunarizine | |
| Outcomes | R: 15/15 vs. 14/15 vs. 14/15 (global assessment at least 'good') F: 1.3 vs. 1.2 vs. 1.1 attacks/20 days (no variance data) AU: not reported HI: 23.4 vs. 18.7 vs. 14.4 AEs: 2/15 vs. 3/15 vs. 4/15 Dropouts‐AEs: 0 vs. 0 vs. 0 (number of patients randomized to groups not fully clear) V: 0 vs. C1, (‐) vs. C2 | |
| Notes | Small groups, low propranolol dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 15/45 J: 1‐1‐0 DU: 4w/2x3m/‐ | |
| Participants | N: 45/30 D: 15 migraine with, 15 without aura C: Ad Hoc F: 83% A: 18‐59 years DU: 1‐50 years S: neurology department in Denmark | |
| Interventions | P: 120 mg C: Placebo | |
| Outcomes | R: 14/30 vs. 9/30 F: 1.03 (sd 1.02) vs. 1.33 (1.15) attacks per week AU: similar in both groups HI: not reported AEs: not reported Dropouts‐AEs: 0/45 vs. 2/45 V: + | |
| Notes | High dropout rate Responder rates and attack frequency calculated from single patient data presented in the publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: none J: 1‐1‐1 DU: 4w/2x1m/2x5m (5m washout) | |
| Participants | N: 28/28 D: 20 migraine with, 8 without aura C: World Federation of Neurology Research Group F: 83% A: 18‐60 years DU: > 2 years S: Sweden | |
| Interventions | P: 120 mg C: Placebo | |
| Outcomes | R: not reported F: significantly better than placebo AU: significantly better than placebo HI: significantly better than placebo AEs: more with propranolol Dropouts‐AEs: not reported V: + | |
| Notes | Available only as expanded abstract Long follow up suggesting no long‐lasting benefit of 1‐month treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 21/83 J: 1‐1‐0 DU‐/2x4‐8w (no washout)/‐ | |
| Participants | N: 83/62 D: Migraine with or without aura C: unclear F: 81% A: 21‐62 years DU: not reported S: neurology department in Chicago, USA | |
| Interventions | P: 80‐160 mg C: Placebo | |
| Outcomes | R: 34/62 preferred propranolol, 17/62 placebo F: not reported AU: not reported HI: not reported AEs: 16/62 vs. 10/62 Dropouts‐AEs: 6/83 vs. 1/83 V: + | |
| Notes | High dropout rate, unclear presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel (with optional crossover) C: unclear B: double WD: 48/148 J: 1‐1‐0 DU: 2m/4w/optional crossover for 6‐12 m | |
| Participants | N: 148/100 D: migraine C: Ad Hoc F: not reported A: not reported DU: not reported S: unclear, USA | |
| Interventions | P: 80‐160 mg C: Placebo | |
| Outcomes | R: not reported F: not reported AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: not reported V: not interpretable | |
| Notes | Trial meets the inclusion criteria, but primarily investigates long‐term treatment with propranolol and includes only a 4‐week RCT, whose results, however, are not presented in detail | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 8/58 J: 1‐1‐0 DU: 2m/7m/1‐2m | |
| Participants | N: 58/50 D: 4 migraine with, 54 without aura C: Ad Hoc F: 81 A: mean age 43 years DU: 1‐55 years S: unclear, Germany | |
| Interventions | P: 80‐160 mg C1: Metoprolol 100‐200 mg C2: Nifedipine 20‐40 mg | |
| Outcomes | R: 6/19 vs. 12/22 vs. 1/17 (according to time series analysis) F: greater reduction in metoprolol group, but relevant baseline differences AU: no significant differences HI: not measured AEs: more circulatory disturbances with propanolol, more fatigue with metoprolol Dropouts‐AEs: not reported V: (‐) vs. C1, (+) vs. C2 | |
| Notes | Rigorous study Dropout rates not fully clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 40/214 J: 1‐1‐1 DU: 4w/16w/‐ | |
| Participants | N: 214/214 (174 in per protocol analysis) D: 58 migaine with, 156 without aura C: IHS F: 78% A: 39 (SD 12) years DU: 19 (SD 12) years S: multiple centers in Germany and Italy | |
| Interventions | P: 120 mg C1: Placebo C2: Cyclandelate 1200 mg | |
| Outcomes | R: 33/78 vs. 17/55 vs. 30/81 F: not reported AU: not reported HI: not reported AEs: 19/78 vs. 5/55 vs. 13/81 Dropouts‐AEs: 4/78 vs. 0/55 vs. 5/81 V: (+) vs. C1, 0 vs. C2 | |
| Notes | Good quality trial Intent‐to‐treat analysis Presentation of results lacks detail | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: adequate B: double WD: 144/810 J: 2‐2‐1 DU: 4w/16w/‐ | |
| Participants | N: 810/783 (in intent‐to‐treat analysis, 808 in safety analysis) D: 72% migraine without aura C: IHS F: 81% A: 17‐66 years DU: 0.8 to 57 years S: 130 centres in 8 countries | |
| Interventions | P: 160 mg (slow‐release) C1: Flunarizine 10 mg C2: Flunarizine 5 mg | |
| Outcomes | R: 125/258 vs. 141/264 vs. 118/259 F: 1.7 (sd 1.6) vs. 1.6 (1.6) vs. 1.8 (1.2) AU: 0.9 vs. 1.1 vs. 1.1 migraine attacks treated symptomatically AEs: 88/270 vs. 88/275 vs. 88/263 Dropouts‐AEs: 18/270 vs. 19/275 vs. 21/263 V: 0 vs. C1, 0 vs. C2 | |
| Notes | Excellent, large, multicentre study Standard deviations for frequency calculated from reported 95% CIs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | D: parallel C: unclear B: none WD: 3/22 J: 1‐0‐1 DU: 4w/16w/4w | |
| Participants | N: 22/19 D: migraine with and without aura C: IHS F: 55% A: mean 39 years DU: mean 20 years S: unclear, Italy | |
| Interventions | P: 120 mg C: Nimodipine 120 mg | |
| Outcomes | R: not reported F: 2.6 (sd 1.5) vs. 2.9 (1.7) AU: not reported HI: not reported AEs: probably 6/10 vs. 11/12 (numbers not fully clear) Dropouts‐AEs: 2/10 vs. 1/12 V: 0 | |
| Notes | Small study focusing on endocrinological effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 8/40 J: 1‐2‐1 DU: 10w/2x12w (no washout)/‐ | |
| Participants | N: 40/32 D: 27 common, 5 classic migraine C: unclear F: 97% A: 17‐51 years DU: 2‐40 years S: probably university outpatient clinic in Sweden | |
| Interventions | P: 240 mg C: Placebo | |
| Outcomes | R: 11 responders in propranolol phase, no data for placebo F: not reported AU: significantly lower during propranolol phase HI: significantly better during propranolol phase AEs: more frequent in propranolol phase Dropouts‐AEs: 2/40 vs. 2/40 V: + | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 18/94 J: 1‐1‐0 DU: 1m/4m/‐ | |
| Participants | N: 94/76 (89 in safety analysis) D: 37 migraine with and 57 without aura C: World Federation of Neurology Research Group F: 90% A: mean 36 years DU: mean 17 years S: 4 Canadian centers | |
| Interventions | P: 160 mg C: Flunarizine 10 mg | |
| Outcomes | R: 20/39 vs. 25/37 (patient global assessment; threshold used to define positive response not specified; denominators not fully clear, only percentages given) F: slightly better reduction with flunarizine AU: similar reduction HI: not reported AEs: 36/45 vs. 33/44 Dropouts‐AEs: 5/45 vs. 3/44 V: (‐) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 22/84 J: 1‐1‐0 DU: 8w/16w/‐ | |
| Participants | N: 84/62 (84 in safety analysis) D: 19 migraine with and 43 without aura C: IHS F: 90% A: mean 41 years DU: mean 20 years S: unclear, Germany | |
| Interventions | P: 120‐160 mg C: Cyclandelate 1200‐1600 mg | |
| Outcomes | R: 18/34 vs. 20/28 F: Slightly more reduction with propranolol AU: similar in both groups AEs: 6/42 vs. 4/42 Dropouts‐AEs: not reported V: 0 | |
| Notes | High dropout rate A second publication reports on a follow‐up study, which is, however, uninterpretable due to a very high dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 6/30 J: 0‐1‐0 DU: ‐/2x12w (no washout)/‐ | |
| Participants | N: 30/24 D: migraine without aura C: Ad Hoc and other F: 73% A: 36 (sd 11) years DU: not reported S: probably neurological university outpatient department in Germany | |
| Interventions | P: 120 mg C: Placebo | |
| Outcomes | R: not reported F: 19 (sd 16) attacks during 8 weeks vs. 22 (16) AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: not reported V: (+) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: unclear J: 1‐1‐0 DU: 2w/2x1m (2w)/‐ | |
| Participants | N: 12/12 (numbers not fully clear) D: migraine with aura C: World Federation of Neurology Research Group F: 67% A: 30‐49 years DU: unclear S: unclear, Denmark | |
| Interventions | P: 80 mg C: Metoprolol 100 mg | |
| Outcomes | R: not reported F: 2.4 (sem 0.3) vs. 3.1 (0.6) AU: not reported HI: not reported AEs: 0 vs. 0 Dropouts‐AEs: not reported V: uninterpretable | |
| Notes | Small study focusing on laboratory parameters | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 26/53 J: 1‐1‐0 DU: ‐/3m/‐ | |
| Participants | N: 53/27 D: mostly migraine without aura C: Ad Hoc F: unclear A: unclear DU: unclear S: probably neurological university hospital outpatient department in Germany | |
| Interventions | P: 80‐120 mg C: Placebo | |
| Outcomes | R: 8/13 vs. 7/14 (at least 50% index reduction) F: not reported AU: not reported HI: no difference AEs: not reported Dropouts‐AEs: not reported V: 0 | |
| Notes | Very high dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: adequate B: double WD: 12/29 J: 1‐2‐1 DU: 1m/3x3m (no washout)/‐ | |
| Participants | N: 29/17 (22‐24 for adverse events) D: 10 migraine with, 19 without aura C: unclear F: 69% A: 22‐80 years DU: 4‐50 years S: probably university outpatient department in New Zealand | |
| Interventions | P: 240 mg C1: Placebo C2: Mefenamic acid 1500 mg | |
| Outcomes | R: not reported F: 13.8 (sd 12.0) vs. 20.1 (18.0) vs. 12.9 (10.8) attacks in 3 months AU: not reported HI: median migraine hours 75 vs. 138 vs. 66 AEs: 2/23 vs. 1/24 vs. 2/22 Dropouts‐AEs: 1/29 vs. 1/29 vs. 1/29 V: + vs. C1; 0 vs. C2 | |
| Notes | High dropout rate; otherwise rigorous crossover trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | D: crossover C: unclear B: double WD: 5/29 J: 1‐1‐0 DU: 4w/2x8w (4w)/‐ | |
| Participants | N: 29/24 D: 4 migraine with, 25 without aura C: unclear F: 86% A: 24‐47 years DU: 4‐40 years S: private practice, Finland | |
| Interventions | P: 160 mg C: Femoxetine 400 mg | |
| Outcomes | R: 3/11 vs. 1/11 (1st period; no pooled data) F: 1st period: 5.00 (sem 0.71) vs. 6.81 (1.12); pooled: 4.67 (sem 0.64) vs. 6.16 (0.82) AU: propranolol better HI: propranolol significantly better AEs: more side effects with propranolol Dropouts‐AEs: 3/29 vs. 0/29 V: (+) | |
| Notes | Responder data presented only for separate phases; frequency data reported for separate phases and for both phases pooled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 3/36 J: 1‐2‐1 DU: 4w/2x3m (no washout)/1m (placebo) | |
| Participants | N: 36/33 D: 6 migraine with, 30 without aura C: World Federation of Neurology Research Group F: 89% A: 18‐51 years DU: mean 16 years S: unclear | |
| Interventions | P: 160 mg C: Metoprolol 200 mg | |
| Outcomes | R: 15/33 vs. 17/33 (at least 50% severity score reduction) F: 3.0 (sd 1.9) vs. 3.0 (1.8) AU: 4.7 vs. 4.7 HI: 5.4 vs. 4.9 AEs: similar in both groups Dropouts‐AEs: 2/36 vs. 0/36 V: 0 | |
| Notes | Well‐reported crossover study with low dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: probably none WD: 5/37 J: 1‐0‐1 DU: 8w/2x12w (4w)/‐ | |
| Participants | N: 37/32 D: all migraine without aura C: IHS F: 81% A: not reported DU: not reported S: neurological practice, USA | |
| Interventions | P: 60‐240 mg C: Divalproex sodium 1000‐2000 mg | |
| Outcomes | R: 1st period: 12/17 vs. 9/15; pooled: 20/32 vs. 21/32 F: similar reduction AU: not reported HI: not reported AEs: 11/32 vs. 16/32 Dropouts‐AEs: 1/37 vs. 4/37 V: 0 | |
| Notes | Pragmatic crossover study with some reporting shortcomings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 2/23 J: 1‐1‐0 DU: 4w/2x16w (no washout)/‐ | |
| Participants | N: 23/21 D: 6 migraine with, 17 without aura C: World Federation of Neurology Research Group F: 70% A: 22‐62 years DU: not reported S: neurology department, Norway | |
| Interventions | P: 160 mg C: Clonidine 100 mcg | |
| Outcomes | R: 13/21 vs. 8/21 (at least 50% headache days reduction) F: 12.7 (sd 11.2) vs. 13.0 (11.7) headache days during last 12 treatment weeks AU: less with propranolol HI: not reported AEs: 11/21 vs. 11/21 Dropouts‐AEs: 0/23 vs. 0/23 V: (+) | |
| Notes | Small crossover trial with interesting outcome measures and data presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 20/76 J: 1‐2‐1 DU: 4w/2x12w (4w)/‐ | |
| Participants | N: 76/56 D: 14 migraine with, 62 without aura C: IHS F: 79% A: 19‐65 years DU: 1‐40 years S: 2 outpatient neurology departments in Denmark | |
| Interventions | P: 120 mg C: Tolfenamic acid 300 mg | |
| Outcomes | R: not reported F: reduction migraine days vs. baseline 1.7 (sd 1.9) vs. 0.6 (1.6) (1st period; no pooled data) AU: not reported HI: not reported AEs: 28 vs. 26 adverse effects Dropouts‐AEs: 9/76 vs. 5/76 V: 0 | |
| Notes | High dropout rate Unclear statistics No significant carryover effect detected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 12/24 J: 1‐0‐1 DU: ‐/2x2m (2w)/‐ | |
| Participants | N: 24/12 D: migraine C: IHS F: unclear A: unclear DU: unclear S: unclear, USA | |
| Interventions | P: 80‐240 mg C: Divalproex sodium 750‐1500 mg | |
| Outcomes | R: not reported F: divalproex significantly better AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: 3/24 vs. 9/24 V: uninterpretable | |
| Notes | Extremely high dropout rate, insufficient reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: unclear WD: 7/38 J: 1‐0‐0 DU: ‐/2x8w (unclear whether washout)/‐ | |
| Participants | N: 38/31 D: 7 migraine with, 24 without aura C: unclear F: unclear A: 17‐53 years DU: mean 14 years S: unclear, Israel | |
| Interventions | P: 160 mg (long‐acting) C: Placebo | |
| Outcomes | R: not reported F: 3.23 vs. 5.56 attacks (p = 0.014; no variance data presented) AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: not reported V: + | |
| Notes | Available only as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 12/59 J: 1‐2‐1 DU: 1m/4m/‐ | |
| Participants | N: 59/59 (intent‐to‐treat analysis) D: 15 migraine with, 44 without aura C: unclear F: 71% A: mean 34 years DU: not reported S: 15 neurological practices in Switzerland | |
| Interventions | P: 120 mg C: Flunarizine 10 mg | |
| Outcomes | R: 16/32 vs. 13/27 F: 3.7 (sd 4.2) vs. 4.8 (6.2) AU: 3.4 (sd 5.5) vs. 4.1 (6.9, number of analgesics) HI: 67 (sd 74) vs. 93 (154) AEs: 15/32 vs. 13/27 Dropouts‐AEs: 3/32 vs. 2/27 V: 0 | |
| Notes | Well‐reported study In flunarizine group, either good response or deterioration; with propranolol, fewer strong responders, but better on average | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 18/87 J: 1‐1‐0 DU: ‐/4m/‐ | |
| Participants | N: 87/69 D: mainly migraine with aura C: Ad Hoc F: 74% A: mean 42 years DU: not reported S: 12 hospital outpatient departments (probably in Germany) | |
| Interventions | P: 120 mg C: Flunarizine 10 mg | |
| Outcomes | R: 24/34 vs. 24/35 (investigator global assessment 'very good' or 'good') F: 3 (sd 5) vs. 4 (5) AU: decreased in 16/34 vs. 18/35 HI: not reported AEs: 21/34 vs. 16/35 Dropouts‐AEs: not reported V: 0 | |
| Notes | Relevant dropout; no intent‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 98/434 J: 1‐1‐0 DU: ‐/4m/‐ | |
| Participants | N: 434/336 D: mainly migraine with aura C: Ad Hoc F: 82% A: mean 42 years DU: not reported S: 99 medical practices (probably in Germany) | |
| Interventions | P: 120 mg C: Flunarizine 10 mg | |
| Outcomes | R: 105/170 vs. 104/166 (investigator global assessment 'very good' or 'good') F: 4 (sd 5) vs. 4 (4) AU: decreased in 83/170 vs. 95/166 HI: not reported AEs: 66/170 vs. 52/166 Dropouts‐AEs: not reported V: 0 | |
| Notes | Large study with high dropout rate; no intent‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 7/39 J: 0‐2‐1 DU: 1m/4m/3m | |
| Participants | N: 39/38 D: 8 migraine with, 31 without aura C: own F: 67% A: mean 39 years DU: not reported S: 7 neurologists in Switzerland | |
| Interventions | P: 120 mg C: 5‐hydroxytryptophan 300 mg | |
| Outcomes | R: 7/20 vs. 8/19 F: reduction from 7.1 (sd 5.8) vs. 9.4 (5.7) to 4.4 (4.0) vs. 7.3 (7.4) AU: reduction from 16.3 (sd 20.1) vs. 9.1 (7.4) to 4.3 (3.4) vs. 5.0 (5.5) HI: not reported AEs: 7/20 vs. 7/19 Dropouts‐AEs: 2/20 vs. 0/19 V: 0 | |
| Notes | Relevant baseline differences, high attack frequency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 2/31 J: 1‐1‐0 DU: 1m/2x6w (no washout)/‐ | |
| Participants | N: 31/29 D: all migraine without aura C: unclear F: 87% A: 25‐57 years DU: not reported S: headache clinic in Boston, USA | |
| Interventions | P: Dose unclear C: Placebo | |
| Outcomes | R: 16 preferred propranolol, 8 placebo, 5 none F: not reported AU: similar in both groups HI: 18.6 vs. 23.3 AEs: listed only for propranolol Dropouts‐AEs: not reported V: + | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: none WD: 67/340 J: 1‐0‐1 DU: 1m/6m/‐ | |
| Participants | N: 340/273 D: migraine (no interval headaches allowed) C: unclear F: 90% A: mean 35 years DU: not reported S: unclear, USA | |
| Interventions | P: 120‐160 mg C1: No prophylactic therapy C2: Amitriptyline 50‐75 mg C3: Biofeedback C4: Propranolol + biofeedback C5: Amitriptyline + biofeedback C6: Amitriptyline + propranolol C7: Propranolol + amitriptyline + biofeedback | |
| Outcomes | R: not reported F: not reported AU: not reported HI: index reduction 62% vs. 20% (C1), 42% (C2), 35% (C3), 64% (C4), 74% (C5), 48% (C6), 73% (C7) AEs: not reported Dropouts‐AEs: 1/44 (P) vs. 4/45 (C1) vs. 4/42 (C2) vs. 0/48 (C3) vs. 2/39 (C4) vs. 2/43 (C5) vs 2/41 (C6) vs. 3/38 (C7) V: + vs. C2 | |
| Notes | Uncommon 8‐armed trial Dropout rates highly variable between groups Only headache indices presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: none WD: 94/375 J: 1‐0‐1 DU: 1m/6m/‐ | |
| Participants | N: 375/281 D: migraine + interval headaches C: unclear F: 95% A: mean 40 years DU: not reported S: unclear, USA | |
| Interventions | P: 120‐160 mg C1: No prophylactic therapy C2: Amitriptyline 50‐75 mg C3: Biofeedback C4: Propranolol + biofeedback C5: Amitriptyline + biofeedback C6: Amitriptyline + propranolol C7: Propranolol + amitriptyline + biofeedback | |
| Outcomes | R: not reported F: not reported AU: not reported HI: index reduction 52% vs. 18% (C1), 60% (C2), 48% (C3), 69% (C4), 62% (C5), 66% (C6), 76% (C7) AEs: not reported Dropouts‐AEs: 3/48 (P) vs. 9/49 (C1) vs. 3/44 (C2) vs. 1/52 (C3) vs. 3/43 (C4) vs. 4/46 (C5) vs. 2/47 (C6) vs. 4/46 (C7) V: (‐) vs. C2 | |
| Notes | Uncommon 8‐armed trial Dropout rates highly variable between groups Only headache indices presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 10/40 J: 1‐1‐1 DU: 1m/2x3m (1m)/3m | |
| Participants | N: 40/30 D: migraine without aura C: IHS F: 75% A: 35 (sd 10) years DU: 18 years S: unclear, Italy | |
| Interventions | P: 80 mg C: Alpha‐dihydroergocryptine 20 mg | |
| Outcomes | R: not reported F: reduction from 5.2 (sd 1.4) to 1.3 (1.2) vs. 5.4 (1.3) to 1.3 (1.2) (1st period; no pooled data) AU: reduction from 8.5 (sd 4.2) to 2.2 (2.1) vs. 7.7 (3.0) to 2.0 (2.1; analgesic doses) HI: not reported AEs: not reported Dropouts‐AEs: 5/40 vs. 4/40 V: 0 | |
| Notes | High dropout rate Significant baseline differences in duration of attacks and psychological profile No significant carryover effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 8/39 J: 1‐2‐1 DU: ‐/3x12w (no washout)/‐ | |
| Participants | N: 39/31 D: 10 migraine with, 21 without aura C: Ad Hoc F: 84% A: 15‐65 years DU: not reported S: neurology outpatient department of a hospital in Denmark | |
| Interventions | P: 120 mg C1: Placebo C2: Tolfenamic acid 300 mg | |
| Outcomes | R: not reported F: 1st period: 6.4 (sd 4.1) vs. 7.6 (6.1) vs. 10.2 (6.8); pooled: 6.6 (sd 4.9) vs. 8.8 (6.9) vs. 6.9 (6.1) AU: trend in favor of tolfenamic acid HI: not reported AEs: 3/31 vs. 3/31 vs. 2/31 Dropouts‐AEs: 2/39 vs. 0/39 vs. 2/39 V: + vs. C1, (‐) vs. C2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 21/64 J: 1‐2‐1 DU: 4w/6+2x12w (no washout)/‐ | |
| Participants | N: 57/41 (67 entered baseline, 57 entered treatment phases) D: 35 migraine with, 27 migraine with and without aura C: Ad Hoc F: 83% A: 18‐60 years DU: not reported S: unclear, USA | |
| Interventions | P: 80‐320 mg C: Placebo | |
| Outcomes | R: not reported F: not reported AU: better with propranolol HI: better with propranolol AEs: more frequent with propranolol Dropouts‐AEs: 1st period: 0/28 vs. 0/29; pooled: 0/57 vs. 0/57 V: + | |
| Notes | Detailed subgroup analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: unclear J: 1‐1‐0 DU: 1m/3m/‐ | |
| Participants | N: 256/unclear D: migraine C: unclear F: 76% A: mean 35 years DU: mean 4 years S: unclear | |
| Interventions | P: 1230 mcg/kg C: Methysergide 30.8 mcg/kg | |
| Outcomes | R: not reported F: 3.1 (sd 1.4) vs. 3.1 (1.4) AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: not reported V: 0 | |
| Notes | Large trial, reported briefly with other studies in one publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 1/28 J: 1‐1‐1 DU: 4‐17w/24w/‐ | |
| Participants | N: 28/27 D: 2 migraine with, 26 without aura C: Ad Hoc F: 79% A: 17‐61 years DU: 2‐45 years S: unclear, Sweden | |
| Interventions | P: 80‐160 ng C: Nadolol 40‐160 mg | |
| Outcomes | R: 8/14 vs. 5/13 F: reduction from 3.6 to 1.9 vs. 5.6 to 2.7 (sd not reported) AU: reduction from 11.5 to 4.1 vs. 17.1 to 9.2 HI: not reported AEs: 5/14 vs. 6/13 Dropouts‐AEs: 0/15 vs. 1/13 V: 0 | |
| Notes | Small, rigorous trial with relevant baseline imbalances | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 3/56 J: 1‐2‐1 DU: 4w/2x8w (4w)/‐ | |
| Participants | N: 56/53 D: 22 migraine with, 34 without aura C: World Federation of Neurology Research Group F: 73% A: 19‐59 years DU: 5‐43 years S: 6 neurology clinics in Sweden | |
| Interventions | P: 80 mg C: Metoprolol 100 mg | |
| Outcomes | R: 16/53 vs. 21/56 (at least 50% index reduction) F: reduction from 5.4 to 4.2 in both groups (sd not reported) AU: reduction from 9.1 to 5.7 vs. 7.6 tablets/4 weeks HI: reduction from 12.4 to 8.7 vs. 9.7 AEs: similar in both groups Dropouts‐AEs: 0/56 vs. 0/56 V: 0 | |
| Notes | Well‐reported crossover study Strong deterioration during washout phase between the two treatment phases | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 6/16 J: 1‐1‐0 DU: ‐/2x8 (no washout)/‐ | |
| Participants | N: 16/10 D: migraine C: unclear F: 80% A: mean 41 years DU: mean 17 years S: outpatient department, UK | |
| Interventions | P: 120 mg C: Placebo | |
| Outcomes | R: not reported F: 21 vs. 24 headache days in 56 days (sd not reported) AU: not reported HI: 47 vs. 52 AEs: not reported Dropouts‐AEs: not reported V: (+) | |
| Notes | Small study with high dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 1/9 J: 1‐1‐1 DU: ‐/2x2m (no washout)/‐ | |
| Participants | N: 9/8 D: 4 migraine with, 5 without aura C: Ad Hoc F: 78% A: 23‐39 years DU: 1‐27 years S: clinical pharmacology department in Granada, Spain | |
| Interventions | P: 160 mg C: Placebo | |
| Outcomes | R: 4/8 vs. 2/8; 7 preferred propranolol, 1 no preference F: significantly fewer attacks with propranolol AU: not reported HI: not reported AEs: unclear Dropouts‐AEs: 1/9 vs. 0/9 V: + | |
| Notes | Extremely small trial with very positive results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 14/55, additional 19 patients dropped out in post‐randomisation baseline J: 1‐2‐1 DU: 4w/12w/‐ | |
| Participants | N: 74/41 D: 8 migraine with, 61 without, 5 both C: IHS F: 76% A: mean 37 years DU: mean 17 years S: muliple centers in France | |
| Interventions | P: 160 mg (long‐acting) C: Placebo | |
| Outcomes | R: not reported F: 3.15 (sem 0.77) vs. 6.4 (1.70) AU: not reported HI: not reported AEs: similar in both groups Dropouts‐AEs: 0/40 vs. 1/34 V: + | |
| Notes | Uncommon design with randomisation before baseline High dropout rate Intent‐to‐treat analysis unclear No placebo effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 3/48 J: 1‐1‐1 DU: 4w/12w/‐ | |
| Participants | N: 48/45 D: migraine C: unclear F: 73% A: 21‐60 years DU: not reported S: unclear, USA | |
| Interventions | P: 120 mg C1: Nadolol 80 mg C2: Nadolol 160 mg | |
| Outcomes | R: not reported F: reduction 2.88 vs. 3.39 vs. 2.63 (sd not reported) AU: not reported HI: reduction 6.70 vs. 7.89 vs. 4.60 AEs: not reported Dropouts‐AEs: 0/16 vs. 1/16 vs. 0/16 (denominators not fully clear) V: (‐) vs. C1, 0 vs. C2 | |
| Notes | Insufficient reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 20/149, additional 21 dropped before treatment started J: 1‐1‐0 DU: 2w/15w/‐ | |
| Participants | N: 149/129 D: migraine with and without aura C: unclear F: 79% A: 18‐62 years DU: mean 20 years S: unclear, USA | |
| Interventions | P: 120 mg C1: Placebo C2: Naproxen 1100 mg | |
| Outcomes | R: patient rating (1 = poor to 4 = excellent) 2.80 vs. 2.38 vs. 2.86 F: Decrease in headache days per week 0.21 (sd 1.86) vs. ‐0.25 (1.57) vs. ‐0.48 (2.02) AU: not reported HI: not reported AEs: 30/44 vs. 28/43 vs. 38/42 Dropouts‐AEs: not reported V: (+) vs. C1, 0 vs. C2 | |
| Notes | Insufficient reporting Results seem partly contradictory Small effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 45/109 J: 1‐1‐0 DU: 8w/24w/3m | |
| Participants | N: 83/83? (109 patients entered the study, 26 dropped out in the baseline period, 19 stopped treatment early ‐ not clear whether fully analyzed) D: 9 migraine with, 74 without aura C: unclear F: 77% A: mean 40 years DU: mean 19 years S: unclear, Germany | |
| Interventions | P: 160 mg C1: Metoprolol 200 mg C2: Flunarizine 10 mg C3: Nifedipine 40 mg C4: Dihydroergotamine 10 mg | |
| Outcomes | R: 33% vs. 60% vs. 17% vs. 31% vs. 8% (single case statistics) F: reduction of headache days by 30% vs. 54% vs. 11% vs. 5% vs. 37% AU: reduction by 40% vs. 40% vs. 41% vs. 45% vs. 33% HI: not reported AEs: not reported Dropouts‐AEs: 3/19 vs. 6/22 vs. 2/12 vs. 8/17 vs. 0/13 V: (‐) vs. C1, 0 vs. C2, 0 vs. C3, (+) vs. C4 | |
| Notes | Complex five‐armed trial Dropout reporting difficult to follow | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 10 /58 J: 1‐2‐1 DU: ‐/4m/‐ | |
| Participants | N: 58/57 D: 27 migraine with, 30 without aura D: IHS F: 70% A: 16‐61 years DU: mean 27 years S: neurology outpatient clinic in Johannesburg, South Africa | |
| Interventions | P: 180 mg C: Flunarizine 10 mg | |
| Outcomes | R: 94% vs. 70% (patient global rating excellent/good) F: reduction from 5.7 to 1.2 vs. 4.6 to 1.4 (sd not presented) AU: not reported HI: not reported AEs: similar in both groups Dropouts‐AEs: 3/29 vs. 2/29 V: 0 | |
| Notes | No headache diary Possibly intent‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: unclear J: 0‐1‐0 DU: unclear/3x2m (unclear whether washout)/unclear | |
| Participants | N: unclear/15 D: migraine with or without aura C: unclear F: unclear A: unclear DU: unclear S: unclear | |
| Interventions | P: 120 mg C1: Placebo C2: Verapamil 240 mg | |
| Outcomes | R: not reported F: 4.5 vs. 5.0 vs. 4.5 (sd not presented) AU: not reported HI: not reported AEs: unclear Dropouts‐AEs: not reported V: + vs. C1, 0 vs. C2 | |
| Notes | Small crossover study, available only as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 1/20 J: 1‐1‐1 DU: ‐/3x4w (1w)/‐ | |
| Participants | N: 20/19 D: 3 migraine with, 17 without aura C: Ad Hoc F: 70% A: 15‐60 years DU: not reported S: unclear, Norway | |
| Interventions | P: 160 mg C1: Placebo C2: d‐propranolol 160 mg | |
| Outcomes | R: not reported F: 5.0 (sd 3.7) vs. 6.1 (4.1) vs. 6.2 (4.6) AU: not reported HI: 7.5 vs. 12.3 vs. 10.9 AEs: not reported Dropouts‐AEs: 1/20 vs. 0/20 vs. 0/20 V: + vs. C1, (+) vs. C2 | |
| Notes | No headache diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 7/35 J: 1‐2‐0 DU: ‐/2x6w (1w)/‐ | |
| Participants | N: 35/28 D: 6 migraine with, 29 without aura C: Ad Hoc F: 69% A: 25‐60 years DU: not reported S: unclear, Norway | |
| Interventions | P: 160 mg C1: Placebo C2: Atenolol 100 mg | |
| Outcomes | R: not reported F: 9.2 vs. 10.3 vs. 8.8 (headache days; sd not reported) AU: not reported HI: not reported AEs: more with propranolol Dropouts‐AEs: 1/35 vs. 0/35 vs. 0/35 V: (+) vs. C1, 0 vs. C2 | |
| Notes | Results reported insufficiently | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: parallel C: unclear B: double WD: 42/140 J: 1‐2‐0 DU: 4‐8w/12w/‐ | |
| Participants | N: 140/98 D: migraine with or without aura C: Ad Hoc F: 76% A: mean 39 years DU: mean 21 years S: 6 centers in the USA | |
| Interventions | P: 160 mg C1: Nadolol 80 mg C2: Nadolol 160 mg | |
| Outcomes | R: 5/27 vs. 11/33 vs. 18/33 F: not reported AU: at least 50% reduction in 11/26 vs. 10/33 vs. 16/32 HI: 1.31 vs. 1.24 vs. 2.22 (higher values indicate better response) AEs: not reported Dropouts‐AEs: 4/44 vs. 2/49 vs. 2/47 V: 0 vs. C1, ‐ vs. C2 | |
| Notes | High dropout rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 16/96 J: 1‐2‐0 DU: 4w/3x12w (no washout)/‐ | |
| Participants | N: 96/80 (83 for adverse events; 90 started placebo and 89 both active treatments) D: migraine C: Ad Hoc F: 74% A: mean 40 years DU: mean 21 years | |
| Interventions | P: 160 mg C1: Placebo C2: Timolol 20 mg | |
| Outcomes | R: 48/80 vs. 24/80 vs. 44/80 F: 3.69 (sd 3.44) vs. 4.84 (3.85) vs. 3.35 (3.13) AU: not reported HI: 6.66 (sd 5.87) vs. 9.03 (7.28) vs. 5.71 (5.14) AEs: 35/83 vs. 23/83 vs. 38/83 Dropouts‐AEs: 6/89 vs. 2/90 vs. 9/89 V: + vs. C1, 0 vs. C2 | |
| Notes | Rigorous, well‐reported crossover trial Probably subgroup analysis in publication by Standnes (see references) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 6/25 J: 1‐1‐0 DU: ‐/2x3m (no washout)/‐ | |
| Participants | N: 25/19 D: 6 migraine with, 13 without aura C: Ad Hoc F: 52% A: 19‐61 years DU: not reported S: unclear, USA | |
| Interventions | P: 80 mg C: Placebo | |
| Outcomes | R: 1st period: 5/8 vs. 0/11; pooled: 15/19 vs. 2/19 F: not reported AU: not reported HI: not reported AEs: unclear Dropouts‐AEs: 0/25 vs. 0/25 V: + | |
| Notes | Small crossover trial with extremely positive results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 4/30 J: 1‐1‐0 DU: ‐/2x3m (no washout)/‐ | |
| Participants | N: 30/26 D: 6 migraine with, 24 without aura C: Ad Hoc F: 87% A: 18‐55 years DU: not reported S: neurology outpatient department in Trondheim, Norway | |
| Interventions | P: 160 mg C: Placebo | |
| Outcomes | R: 1st period: 12/12 vs. 5/14; pooled: 25/26 vs. 10/26 F: mean monthly attack frequency, 1st period: 0.44 (sd 0.52) vs. 1.64 (1.30); pooled: 0.39 (sd 0.48) vs. 1.70 (1.40) AU: not reported HI: not reported AEs: not reported Dropouts‐AEs: not reported V: + | |
| Notes | Included only patients who had responded to propranolol before | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | D: crossover C: unclear B: double WD: 24/54 J: 1‐1‐0 DU: 4‐8w/3x8w (4w)/‐ | |
| Participants | N: 54/30 D: migraine C: Ad Hoc F: 73% A: 22‐57 years DU: not reported S: unclear, USA | |
| Interventions | P: 80‐240 mg C1: Placebo C2: Amitriptyline 50‐150 mg | |
| Outcomes | R: 12/30 vs. unclear vs. 10/30 (at least 50% index reduction) F: not reported AU: not reported HI: 405 vs. 511 vs. 429 AEs: not reported Dropouts‐AEs: not reported V: + vs. C1, 0 vs. C2 | |
| Notes | High dropout rate Insufficient presentation of results Complex design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Abbreviations: 'Methods' column: D = design; C = concealment of allocation; B = blinding; WD = dropouts and withdrawals; J = Jadad score; DU = duration of baseline/treament (in case of crossover studies, washout period in parentheses)/follow‐up period in m = months or w = weeks
'Participants' column: N = number of patients randomized/analyzed; D = diagnoses; C = classification of headaches (IHS = International Headache Society; ad hoc = Ad Hoc Committee); F = percentage of female participants; A = age (range or mean); DU = duration (migraine since); S = setting (if unclear, the country of the first author is provided)
'Interventions' column: P = propranolol dosage; C = control intervention
'Outcomes' column: R = responder (at least 50% reduction in number of migraine attacks, unless otherwise indicated); F = attack frequency (number of migraine attacks in the last 4 weeks/month of treatment, unless otherwise indicated); AU = analgesic use (typically tablets/time period); HI = headache index; AEs = adverse events (unless otherwise indicated, number of patients with at least one adverse event); Dropouts‐AEs = number of patients dropping out due to adverse events; V = vote count (+ = propranolol significantly better, (+) = propranolol trend better, 0 = no difference, (‐) = control trend better, ‐ = control significantly better). If numbers are presented, the first number always refers to the propranolol group, the second to the control group. If there was more than one control group, the order of results follows the numbering of control groups in the Interventions column.
'Allocation concealment' column: A = adequate, B = unclear, C = inadequate, D = not used
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amery 1988 | No original data ‐ review |
| Anonymous 1979 | No original data ‐ review |
| Banerjee 1991 | Study on treatment of acute migraine attacks |
| Carroll 1990 | Did not include a comparison with placebo or another drug |
| Cortelli 1985 | Not randomised or quasi‐randomised clinical study |
| de Bock 1997 | Not randomised or quasi‐randomised clinical study |
| Diamond 1987 | Not randomised or quasi‐randomised clinical study |
| Fuller 1990 | Study on treatment of acute migraine attacks |
| Havanka‐Kann. 1988 | Did not include a comparison with placebo or another drug |
| Holroyd 1995 | Did not include a comparison with placebo or another drug |
| Julien 1976 | Not randomised or quasi‐randomised clinical study |
| Montastruc 1992 | No original data ‐ review |
| Penzien 1990 | Did not include a comparison with placebo or another drug |
| Raveau‐Landon 1988 | No original data ‐ review |
| Rosen 1983 | Not randomised or quasi‐randomised clinical study |
| Schmidt 1991 | Not randomised or quasi‐randomised clinical study |
| Sovak 1981 | Did not include a comparison with placebo or another drug |
| Steardo 1982 | Open trial with unclear method of allocation |
| Tfelt‐Hansen 1986 | No original data ‐ review |
| Turner 1984 | No original data ‐ review |
| Verspeelt 1996a | Not randomised or quasi‐randomised clinical study |
| Verspeelt 1996b | Not randomised or quasi‐randomised clinical study |
| Winther 1990 | Observation period less than 4 weeks |
| Wober 1991 | Not randomised or quasi‐randomised clinical study |
Contributions of authors
Klaus Linde conceived the review, collected the literature, extracted data, performed analyses, and wrote the manuscript. Karin Rossnagel contributed to the protocol, extracted data, and contributed to the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Karl and Veronica Carstens Foundation, Germany.
International Headache Society (for administrative costs associated with editorial review and peer review), Other.
Declarations of interest
None known.
Notes
The original authors of this review are unable to update it and this review has now been withdrawn. The Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) is seeking new authors for the update. If you are interested, please contact the Managing Editor of PaPaS (contact details provided under 'Contact Person').
We are aware that this is a priority topic for the World Health Organisation (WHO) and encourage new author teams to get in touch. A future approach for updating this review may mean splitting the review per individual beta‐blocker.
Withdrawn from publication for reasons stated in the review
References
References to studies included in this review
- Ahuja GK, Verma AK. Propranolol in prophylaxis of migraine. Indian Journal of Medical Research 1985;82:263‐5. [PubMed] [Google Scholar]
- Albers GW, Simon LT, Hamik A, Peroutka SJ. Nifedipine versus propranolol for the initial prophylaxis of migraine. Headache 1989;29(4):215‐8. [DOI] [PubMed] [Google Scholar]
- Al‐Qassab HK, Findley LJ. Comparison of propranolol LA 80 mg and propranolol LA 160 mg in mirgraine prophylaxis: a placebo controlled study. Cephalalgia 1993;13(2):128‐31. [DOI] [PubMed] [Google Scholar]
- Andersson PG, Petersen EN. Propranolol and femoxetine, a 5HT‐uptake inhibitor, in migraine prophylaxis. A double‐blind crossover study. Acta Neurologica Scandinavica 1981;64(4):280‐8. [DOI] [PubMed] [Google Scholar]
- Baldrati A, Cortelli P, Procaccianti G, Gamberini G, D'Alessandro R, Baruzzi A, et al. Propranolol and acetylsalicylic acid in migraine prophylaxis. Double‐blind crossover study. Acta Neurologica Scandinavica 1983;67(3):181‐6. [DOI] [PubMed] [Google Scholar]
- Behan PO, Reid M. Propranolol in the treatment of migraine. Practitioner 1980;224(1340):201‐3. [PubMed] [Google Scholar]
- Bonuso S, Stasio E, Marano E, Angelis S, Amato D, Scellini T. Long‐term outcome of migraine therapy: predictive value of the frontotemporal nitroglycerin test. Neurology 1998;51(5):1475‐8. [DOI] [PubMed] [Google Scholar]
- Bordini CA, Arruda MA, Ciciarelli MC, Speciali JG. Propranolol vs flunarizine vs flunarizine plus propranolol in migraine without aura prophylaxis. A double‐blind trial. Arquivos de Neuro‐Psiquiatria 1997;55(3B):536‐41. [DOI] [PubMed] [Google Scholar]
- Borgesen SE. Treatment of migraine with propranolol. Postgraduate Medical Journal 1976;52 Suppl 4(0):163‐5. [PubMed] [Google Scholar]; Borgesen SE, Nielsen JL, Moller CE. Prophylactic treatment of migraine with propranolol. A clinical trial. Acta Neurologica Scandinavica 1974;50(5):651‐6. [DOI] [PubMed] [Google Scholar]; Borgesen SE, Nielsen JL, Moller CE. Propranolol in migraine. Lancet 1974;2(7871):58. [DOI] [PubMed] [Google Scholar]
- Dahlöf C. No clearcut longterm prophylactic effect of one month of treatment with propranolol in migraineurs. Cephalalgia 1987;7 Suppl 6:459‐60. [Google Scholar]
- Diamond S, Medina JL. Double blind study of propranolol for migraine prophylaxis. Headache 1976;16(1):24‐7. [DOI] [PubMed] [Google Scholar]
- Diamond S, Kudrow L, Stevens J, Shapiro DB. Long‐term study of propanolol in the treatment of migraine. Headache 1982;22(6):268‐71. [DOI] [PubMed] [Google Scholar]
- Diener HC, Scholz E, Dichgans J, Gerber WD, Jäck A, Bille A, et al. Central effects of drugs used in migraine prophylaxis evaluated by visual evoked potentials. Annals of Neurology 1989;25(2):125‐30. [DOI] [PubMed] [Google Scholar]; Gerber WD, Diener HC, Scholz E, Niederberger U. Responders and non‐responders to metoprolol, propanolol and nifidepine treatment in migraine prophylaxis: a dose‐range study based on time‐series analysis. Cephalalgia 1991;11(1):37‐45. [DOI] [PubMed] [Google Scholar]
- Diener HC, Foh M, Iaccarino C, Wessely P, Isler H, Strenge H, et al. Cyclandelate in the prophylaxis of migraine: a randomized, parallel, double‐blind study in comparison with placebo and propanolol. Cephalalgia 1996;16(6):441‐7. [DOI] [PubMed] [Google Scholar]
- Diener HC. Efficacy and tolerability of flunarizine and propranolol in the prophylactic treatment of migraine. Cephalalgia 1999;19(4):374. [Google Scholar]; Diener HC, Matias‐Guiu J, Hartung E, Pfaffenrath V, Ludin HP, Nappi G, et al. Efficacy and tolerability in migraine prophylaxis of flunarizine in reduced doses: a comparison with propranolol 160 mg daily [published erratum appears in Cephalalgia 2002;22(6):488]. Cephalalgia 2002;22(3):209‐21. [DOI] [PubMed] [Google Scholar]
- Formisano R, Falaschi P, Cerbo R, Proietti A, Catarci T, D'Urso R, et al. Nimodipine in migraine: clinical efficacy and endocrinological effects. European Journal of Clinical Pharmacology 1991;41(1):69‐71. [DOI] [PubMed] [Google Scholar]
- Forssman B, Henriksson KG, Johannsson V, Lindvall L, Lundin H. Propranolol for migraine prophylaxis. Headache 1976;16(5):238‐45. [DOI] [PubMed] [Google Scholar]
- Gawel M, Kreeft J, Nelson R, Simard D. Flunarizine is comparable to propranolol in the prophylaxis of migraine with and without aura. Cephalalgia 1991;11 Suppl 11:156. [Google Scholar]; Gawel MJ, Kreeft J, Nelson RF, Simard D, Arnott WS. Comparison of the efficacy and safety of flunarizine to propranolol in the prophylaxis of migraine. Canadian Journal of Neurological Sciences 1992;19(3):340‐5. [PubMed] [Google Scholar]
- Gerber WD, Schellenberg R, Thom M, Haufe C, Bölsche F, Wedekind W, et al. Cyclandelate versus propranolol in the prophylaxis of migraine ‐ a double‐blind placebo‐controlled study. Functional Neurology 1995;10(1):27‐35. [PubMed] [Google Scholar]; Schellenberg R, Schwarz A, Niederberger U, Bölsche F, Schindler M, Gerber WD, et al. On the long‐term effect of cyclandelate and propranolol in migraine after stopping a four‐month drug prophylaxis [Zur Langzeitwirkung von Cyclandelat und Propranolol bei Migräne nach Beendigung einer viermonatigen medikamentösen Prophylaxe]. Nervenheilkunde 1997;16:183‐7. [Google Scholar]
- Grotemeyer KH, Husstedt IW, Schlake HP. Betablocker vs placebo in vasomotor headache. A double‐blind crossover study [Betablocker vs Placebo bei vasomotorischem Kopfschmerz. Eine doppelblinde Cross‐over‐Studie]. Deutsche Medizinische Wochenschrift 1987;112(45):1740‐3. [DOI] [PubMed] [Google Scholar]
- Hedman C, Winther K, Knudsen JB. The difference between non‐selective and beta 1‐selective beta‐blockers in their effect on platelet function in migraine patients. Acta Neurologica Scandinavica 1986;74(6):475‐8. [DOI] [PubMed] [Google Scholar]
- Holdorff B, Sinn M, Roth G. Propranolol for migraine prophylaxis: a double‐blind study [Propranolol in der Migräneprophylaxe. Eine Doppelblindstudie]. Medizinische Klinik 1977;72(25):1115‐8. [PubMed] [Google Scholar]
- Johnson RH, Hornabrook RW, Lambie DG. Comparison of mefenamic acid and propranolol with placebo in migraine prophylaxis. Acta Neurologica Scandinavica 1986;73(5):490‐2. [DOI] [PubMed] [Google Scholar]
- Kangasniemi PJ, Nyrke T, Lang AH, Petersen E. Femoxetine ‐ a new 5‐HT uptake inhibitor ‐ and propranolol in the prophylactic treatment of migraine. Acta Neurologica Scandinavica 1983;68(4):262‐7. [DOI] [PubMed] [Google Scholar]
- Kangasniemi P, Hedman C. Metoprolol and propranolol in the prophylactic treatment of classical and common migraine. A double‐blind study. Cephalalgia 1984;4(2):91‐6. [DOI] [PubMed] [Google Scholar]; Nyrke T, Kangasniemi P, Lang AH, Petersen E. Steady‐state visual evoked potentials during migraine prophylaxis by propranolol and femoxetine. Acta Neurologica Scandinavica 1984;69(1):9‐14. [DOI] [PubMed] [Google Scholar]
- Kaniecki RG. A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without aura. Archives of Neurology 1997;54(9):1141‐5. [DOI] [PubMed] [Google Scholar]
- Kass B, Nestvold K. Propranolol (Inderal) and clonidine (Catapressan) in the prophylactic treatment of migraine. A comparative trial. Acta Neurologica Scandinavica 1980;61(6):351‐6. [DOI] [PubMed] [Google Scholar]
- Kjaersgard Rasmussen MJ, Holt Larsen B, Borg L, Soelberg Sorensen P, Hansen PE. Tolfenamic acid versus propranolol in the prophylactic treatment of migraine. Acta Neurologica Scandinavica 1994;89(6):446‐50. [DOI] [PubMed] [Google Scholar]
- Klapper JA. An open label cross‐over comparison of divalproex sodium and propranolol HCl in the prevention of migraine headaches. Headache Quarterly 1994;5(1):50‐3. [Google Scholar]
- Kuritzky A, Hering R. Prophylactic treatment of migraine with long acting propranolol ‐ a comparison with placebo. Cephalalgia 1987;7 Suppl 6:457‐8. [Google Scholar]
- Lücking CH, Oestreich W, Schmidt R, Soyka D. Flunarizine versus propranolol in the prophylaxis of migraine: two double‐blind comparative studies in more than 400 patients. Cephalalgia 1988;8(Suppl 8):21‐6. [DOI] [PubMed] [Google Scholar]; Soyka D, Oestreich W. Flunarizine versus propranolol in migraine prophylaxis ‐ A multicenter double‐blind study in 12 hospitals [Flunarizin versus Propranolol in der Intervallprophylaxe der Migräne ‐ eine multizentrische Doppelblindstudie in 12 Kliniken]. Nervenheilkunde 1987;6:177‐83. [Google Scholar]
- Lücking CH, Oestreich W, Schmidt R, Soyka D. Flunarizine vs. propranolol in the prophylaxis of migraine: two double‐blind comparative studies in more than 400 patients. Cephalalgia 1988;8 Suppl 8:22‐6. [DOI] [PubMed] [Google Scholar]; Soyka D, Oestreich W. Flunarizine versus propranolol in the interval treatment of migraine [Flunarizin versus Propranolol in der Intervallbehandlung der Migräne ‐ multizentrische Doppelblindsudie bei niedergelassenen Allgemeinärzten und Internisten]. Nervenheilkunde 1990;9:45‐51. [Google Scholar]; Soyka D, Oestreich W. Therapeutic effectiveness of flunarizine and propranolol in the interval therapy of migraine. Cephalalgia 1987;7 Suppl 6:467‐8. [Google Scholar]
- Ludin HP. Flunarizine and propranolol in the treatment of migraine. Headache 1989;29(4):219‐24. [DOI] [PubMed] [Google Scholar]
- Maissen CP, Ludin HP. Comparative efficacy of 5‐hydroxytryptophan and propranolol in interval treatment of migraine [Vergleich der Wirksamkeit von 5‐Hydroxytrypthophan und von Propranolol in der Intervalltherapie der Migräne]. Schweizerische Medizinische Wochenschrift. Journal Suisse de Medecine 1991;121(43):1585‐90. [PubMed] [Google Scholar]
- Malvea BP, Gwon N, Graham JR. Propranolol prophylaxis of migraine. Headache 1973;12(4):163‐7. [DOI] [PubMed] [Google Scholar]
- Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21(3):105‐9. [Study 1 included patients with migraine only]. [DOI] [PubMed] [Google Scholar]
- Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21(3):105‐9 [Study 2 included patients with migraine + interval headaches]. [DOI] [PubMed] [Google Scholar]
- Micieli G, Cavallini A, Marcheselli S, Mailland F, Ambrosoli L, Nappi G. Alpha‐dihydroergocryptine and predictive factors in migraine prophylaxis. Int J Clin Pharmacol Ther 2001;39:155‐151. [DOI] [PubMed] [Google Scholar]
- Mikkelsen B, Pedersen KK, Christiansen LV. Prophylactic treatment of migraine with tolfenamic acid, propanolol and placebo. Acta Neurologica Scandinavica 1986;73(4):423‐7. [DOI] [PubMed] [Google Scholar]
- Nadelmann JW, Phil M, Stevens J, Saper JR. Propranolol in the prophylaxis of migraine. Headache 1986;26(4):175‐82. [DOI] [PubMed] [Google Scholar]
- Nicolodi M, Bianco PL, Sicuteri F. The way to serotonergic use and abuse in migraine. International Journal of Clinical Pharmacology Research 1997;17(2‐3):79‐84. [PubMed] [Google Scholar]
- Olerud B, Gustavsson CL, Furberg B. Nadolol and propranolol in migraine management. Headache 1986;26(10):490‐3. [DOI] [PubMed] [Google Scholar]
- Olsson JE, Behring HC, Forssman B, Hedman C, Hedman G, Johansson F, et al. Metoprolol and propranolol in migraine prophylaxis: a double‐blind multicentre study. Acta Neurologica Scandinavica 1984;70(3):160‐8. [DOI] [PubMed] [Google Scholar]
- Palferman TG, Gibberd FB, Simmonds JP. Prophylactic propranolol in the treatment of headache. British Journal of Clinical Practice 1983;37(1):28‐9. [PubMed] [Google Scholar]
- Pita E, Higueras A, Bolanos J, Perez N, Mundo A. Propranolol and migraine. A clinical trial. Archivos de Farmacologia y Toxicologia 1977;3(3):273‐8. [PubMed] [Google Scholar]
- Pradalier A, Serratrice G, Collard M, Hirsch E, Feve J, Masson M, et al. Beta‐blockers and migraine. Efficacy of time‐release propranolol versus placebo [Beta‐bloquants et migraine. Efficacité, contre placebo, du propranolol a libération prolongée]. Thérapie 1990;45(5):441‐5. [PubMed] [Google Scholar]; Pradalier A, Serratrice G, Collard M, Hirsch E, Feve J, Masson M, et al. Double‐blind placebo controlled study of the use of long‐acting propranolol in migraine prophylaxis. Cephalalgia 1989;9 Suppl 10:367‐8. [DOI] [PubMed] [Google Scholar]; Pradalier A, Serratrice G, Collard M, Hirsch E, Feve J, Masson M, et al. Long‐acting propranolol in migraine prophylaxis: results of a double‐blind, placebo‐controlled study. Cephalalgia 1989;9(4):247‐53. [DOI] [PubMed] [Google Scholar]
- Ryan RE Sr. Comparative study of nadolol and propranolol in prophylactic treatment of migraine. American Heart Journal 1984;108(4 Pt 2):1156‐9. [DOI] [PubMed] [Google Scholar]
- Sargent J, Solbach P, Damasio H, Baumel B, Corbett J, Eisner L, et al. A comparison of naproxen sodium to propranolol hydrochloride and a placebo control for the prophylaxis of migraine headache. Headache 1985;25(6):320‐4. [DOI] [PubMed] [Google Scholar]
- Scholz E, Gerber WD, Diener HC, Langohr HD, Reinecke M. Dihydroergotamine vs flunarizine vs nifidepine vs metoprolol vs propranolol in migraine prophylaxis: a comparative study based on time series analysis. In: Rose CF editor(s). Advances in headache research: proceedings of the 6th International Migraine Symposium. London: John Libbey, 1987:139‐45. [Google Scholar]
- Shimell CJ, Fritz VU, Levien SL. A comparative trial of flunarizine and propranolol in the prevention of migraine. South African Medical Journal 1990;77(2):75‐7. [PubMed] [Google Scholar]
- Solomon GD, Scott AFB. Verapamil and propranolol in migraine prophylaxis: a double‐blind, crossover study. Headache 1986;26(6):325. [Google Scholar]
- Stensrud P, Sjaastad O. Short‐term clinical trial of propranolol in racemic form (Inderal), D‐propranolol and placebo in migraine. Acta Neurologica Scandinavica 1976;53(3):229‐32. [DOI] [PubMed] [Google Scholar]
- Stensrud P, Sjaastad O. Comparative trial of Tenormin (atenol) and Inderal (propranolol) in migraine. Headache 1980;20(4):204‐7. [DOI] [PubMed] [Google Scholar]; Stensrud P, Sjaastad O. Comparative trial of Tenormin (atenolol) and Inderal (propranolol) in migraine. Upsala Journal of Medical Sciences ‐ Supplement 1980;31:37‐40. [PubMed] [Google Scholar]
- Sudilovsky A, Elkind AH, Ryan RE Sr, Saper JR, Stern MA, Meyer JH. Comparative efficacy of nadolol and propranolol in the management of migraine. Headache 1987;27(8):421‐6. [DOI] [PubMed] [Google Scholar]
- Standnes B. The prophylactic effect of timolol versus propranolol and placebo in common migraine: beta‐blockers in migraine. Cephalalgia 1982;2(3):165‐70. [DOI] [PubMed] [Google Scholar]; Tfelt‐Hansen P, Standnes B, Kangasneimi P, Hakkarainen H, Olesen J. Timolol vs propranolol vs placebo in common migraine prophylaxis: a double‐blind multicenter trial. Acta Neurologica Scandinavica 1984;69(1):1‐8. [DOI] [PubMed] [Google Scholar]
- Weber RB, Reinmuth OM. The treatment of migraine with propranolol. Neurology 1972;22(4):366‐9. [DOI] [PubMed] [Google Scholar]
- Wideroe TE, Vigander T. Propranolol in the treatment of migraine. BMJ 1974;2(921):699‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DK, Hurwitz A, Hassanein RS, Kodanaz HA, Preskorn SH, Manson J. Migraine prophylaxis. A comparison of propranolol and amitriptyline. Archives of Neurology 1987;44(5):486‐9. [DOI] [PubMed] [Google Scholar]; Ziegler DK, Hurwitz A, Preskorn S, Hassanein R, Seim J. Propranolol and amitriptyline in the prophylaxis of migraine. Pharmacokinetic and therapeutic effects. Archives of Neurology 1993;50(8):825‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Amery WK. Onset of action of various migraine prophylactics. Cephalalgia 1988;8 Suppl 8:11‐3. [DOI] [PubMed] [Google Scholar]
- Anonymous. Propranolol for prevention of migraine headaches. Medical Letter on Drugs & Therapeutics 1979;21(19):77‐8. [PubMed] [Google Scholar]
- Banerjee M, Findley L. Propranolol in the treatment of acute migraine attacks. Cephalalgia 1991;11(4):193‐6. [DOI] [PubMed] [Google Scholar]
- Carroll JD, Reidy M, Savundra PA, Cleave N, McAinsh J. Long‐acting propranolol in the prophylaxis of migraine: a comparative study of two doses. Cephalalgia 1990;10(2):101‐5. [DOI] [PubMed] [Google Scholar]
- Cortelli P, Sacquegna T, Albani F, Baldrati A, D'Alessandro R, Baruzzi A, et al. Propranolol plasma levels and relief of migraine. Relationship between plasma propranolol and 4‐hydroxypropranolol concentrations and clinical effects. Archives of Neurology 1985;42(1):46‐8. [DOI] [PubMed] [Google Scholar]
- Bock GH, Eelhart J, Marwijk HW, Tromp TP, Springer MP. A postmarketing study of flunarizine in migraine and vertigo. Pharmacy World & Science 1997;19(6):269‐74. [DOI] [PubMed] [Google Scholar]
- Diamond S, Solomon GD, Freitag FG, Mehta ND. Long‐acting propranolol in the prophylaxis of migraine. Headache 1987;27(2):70‐2. [DOI] [PubMed] [Google Scholar]
- Fuller GN, Guiloff RJ. Propranolol in acute migraine: a controlled study. Cephalalgia 1990;10(5):229‐33. [DOI] [PubMed] [Google Scholar]
- Havanka‐Kanniainen H, Hokkanen E, Myllylä VV. Long acting propranolol in the prophylaxis of migraine. Comparison of the daily doses of 80 mg and 160 mg. Headache 1988;28(9):607‐11. [DOI] [PubMed] [Google Scholar]
- Holroyd KA, France JL, Cordingley GE, Rokicki LA, Kvaal SA, Lipchik GL, et al. Enhancing the effectiveness of relaxation‐thermal biofeedback training with propranolol hydrochloride. Journal of Consulting & Clinical Psychology 1995;63(2):327‐30. [DOI] [PubMed] [Google Scholar]
- Julien J, Vallat JM, Lagueny A, Darriet M. Preventive treatment of migraine with propranolol (letter) [Le traitement prophylactique des migraines par le propranolol]. Nouvelle Presse Médicale 1976;5(10):653. [PubMed] [Google Scholar]
- Montastruc JL, Senard JM. Calcium channel blockers and prevention of migraine [Medicaments anticalciques et prophylaxie de la migraine]. Pathologie et Biologie 1992;40(4):381‐8. [PubMed] [Google Scholar]
- Penzien D, Johnson C, Carpenter D, Holroyd K. Drug vs. behavioral treatment of migraine: long‐acting propranolol vs. home‐based self‐management training. Headache 1990;30(5):300. [Google Scholar]
- Raveau‐Landon C, Bousser MG. Metoprolol, a new effective antimigraine agent [Le metoprolo, nouvel antimigraineux de fond]. Presse Medicale 1988;17(35):1805‐9. [PubMed] [Google Scholar]
- Rosen JA. Observations on the efficacy of propranolol for the prophylaxis of migraine. Annals of Neurology 1983;13(1):92‐3. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. Journal of Cardiovascular Pharmacology 1991;18 Suppl 8:S21‐6. [PubMed] [Google Scholar]
- Sovak M, Kunzel M, Sternbach RA, Dalessio DJ. Mechanism of the biofeedback therapy of migraine: volitional manipulation of the psychophysiological background. Headache 1981;21(3):89‐92. [DOI] [PubMed] [Google Scholar]
- Steardo L, Bonuso S, Stasio E, Marano E. Selective and non‐selective beta‐blockers: are both effective in prophylaxis of migraine? A clinical trial versus methysergide. Acta Neurologica 1982;4(3):196‐204. [PubMed] [Google Scholar]
- Tfelt‐Hansen P. Efficancy of beta‐blockers in migraine. A critical review. Cephalalgia 1986;6 Suppl 5:15‐24. [DOI] [PubMed] [Google Scholar]
- Turner P. Beta‐blocking drugs in migraine. Postgraduate Medical Journal 1984;60 Suppl 2:51‐5. [PubMed] [Google Scholar]
- Verspeelt J, Locht P, Amery WK. Post‐marketing cohort study comparing the safety and efficacy of flunarizine and propranolol in the prophylaxis of migraine. Cephalalgia 1996;16(5):328‐36. [DOI] [PubMed] [Google Scholar]
- Verspeelt J, Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. European Journal of Clinical Pharmacology 1996;51(1):15‐22. [DOI] [PubMed] [Google Scholar]
- Winther K, Hedman C, Flodgaard H. Platelet P1, P4‐Di (adenosine‐51) tetraphosphate (AP4A) in migraine patients before and during beta‐adrenoceptor blockade. European Journal of Clinical Investigation 1990;20(3):336‐8. [DOI] [PubMed] [Google Scholar]
- Wober C, Wober‐Bingel C, Koch G, Wessely P. Long‐term results of migraine prophylaxis with flunarizine and beta‐blockers. Cephalalgia 1991;11(6):251‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Bernik V, Maia E. The use of propranolol on migraine prophylaxis: a double‐blind clinical trials comparing propranolol with an analgesic drug (acetaminophen) and placebo [Uso do propranolol na profilaxia da enxacequa: Estudo duplo‐cego comparando propranolol a um analgesico (acetaminofen) e placebo]. Folha médica (Rio de Janeiro) 1978;77(4):501‐8. [Google Scholar]
- Rao BS, Das DG, Taraknath VR, Sarma Y. A double blind controlled study of propranolol and cyproheptadine in migraine prophylaxis. Neurology India 2000;48(3):223‐6. [PubMed] [Google Scholar]
Additional references
- Ad Hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179(9):717‐8. [Google Scholar]
- Clarke M, Oxman AD, editors. Optimal search strategy for RCTs. Cochrane Reviewers' Handbook 4.1.6 [updated January 2003]; Appendix 5c. In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software. [Google Scholar]
- Gray RN, Goslin RE, McCrory DC, Eberlein K, Tulsky J, Hasselblad V. Drug treatments for the prevention of migraine headache. Technical review 2.3. February 1999. Prepared for the Agency for Health Care Policy and Research under Contract No. 290‐94‐2025. Available at: http://www.clinpol.mc.duke.edu (accessed 20 February 2004). [PubMed]
- Holroyd KA, Penzien DB, Cordingley GE. Propranolol in the management of recurrent migraine: a meta‐analytic review. Headache 1991;31(5):333‐40. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646‐57. [DOI] [PubMed] [Google Scholar]
- Pryse‐Phillips WEM, Dodick DW, Edmeads JG, Gawel MJ, Nelson RF, Purdy RA, et al. Guidelines for the diagnosis and management of migraine in clinical practice [published erratum appears in CMAJ 1997;157(10):1354]. CMAJ 1997;156(9):1273‐87. [PMC free article] [PubMed] [Google Scholar]
- Ramadan NM, Schultz LL, Gilkey SJ. Migraine prophylactic drugs: proof of efficacy, utilization and cost. Cephalalgia 1997;17(2):73‐80. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population‐based studies. Neurology 1994;44(6 Suppl 4):S17‐S23. [PubMed] [Google Scholar]
- Tfelt‐Hansen P, Block G, Dahlöf C, Diener HC, Ferrari MD, Goadsby PJ, et al. Guidelines for controlled trials of drugs for migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
- Verhagen AP, Vet HCW, Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology 1998;51(12):1235‐41. [DOI] [PubMed] [Google Scholar]


