Abstract
Background
To reduce the morbidity and mortality associated with preterm birth, home uterine activity monitoring aims for early detection of increased contraction frequency, and early intervention with tocolytic drugs to inhibit labour and prolong pregnancy. However, the effectiveness of such monitoring is disputed.
Objectives
To determine whether home uterine activity monitoring is effective in improving the outcomes for women and their infants considered to be at high risk of preterm birth, when compared with care that does not include home uterine activity monitoring.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2016), CENTRAL (Cochrane Library 2016, Issue 5), MEDLINE (1966 to 28 June 2016), Embase (1974 to 28 June 2016), CINAHL (1982 to 28 June 2016), and scanned reference lists of retrieved studies.
Selection criteria
Randomised control trials of home uterine activity monitoring, with or without patient education programmes, for women at risk of preterm birth, compared with care that does not include home uterine activity monitoring.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risks of bias, extracted data and checked them for accuracy. We did not attempt to contact authors to resolve queries. We assessed the evidence using the GRADE approach.
Main results
There were 15 included studies (6008 enrolled participants); 13 studies contributed data. Women using home uterine monitoring were less likely to experience preterm birth at less than 34 weeks (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.62 to 0.99; three studies, 1596 women; fixed‐effect analysis) (GRADE high). This difference was not evident when we carried out a sensitivity analysis, restricting the analysis to studies at low risk of bias based on study quality (RR 0.75, 95% CI 0.57 to 1.00; one study, 1292 women). There was no difference in the rate of perinatal mortality (RR 1.22, 95% CI 0.86 to 1.72; two studies, 2589 babies) (GRADE low).
There was no difference in the number of preterm births at less than 37 weeks (average RR 0.85, CI 0.72 to 1.01; eight studies, 4834 women; random‐effects, Tau2 = 0.03, I2 = 68%) (GRADE very low). Infants born to women using home uterine monitoring were less likely to be admitted to neonatal intensive care unit (average RR 0.77, 95% CI 0.62 to 0.96; five studies, 2367 babies; random‐effects, Tau2 = 0.02, I2 = 32%) (GRADE moderate). This difference was not maintained when we restricted the analysis to studies at low risk of bias (RR 0.86, 95% CI 0.74 to 1.01; one study, 1292 babies). Women using home uterine monitoring made more unscheduled antenatal visits (mean difference (MD) 0.48, 95% CI 0.31 to 0.64; two studies, 1994 women) (GRADE moderate). Women using home uterine monitoring were also more likely to have prophylactic tocolytic drug therapy (average RR 1.21, 95% CI 1.01 to 1.45; seven studies, 4316 women; random‐effects, Tau2 = 0.03, I2 = 62%), but this difference was no longer evident when we restricted the analysis to studies at low risk of bias (average RR 1.22, 95% CI 0.90 to 1.65; three studies, 3749 women; random‐effects, Tau2 = 0.05, I2 = 76%) (GRADE low). The number of antenatal hospital admissions did not differ between home groups (RR 0.91, 95% CI 0.74 to 1.11; three studies, 1494 women (GRADE low)). We found no data on maternal anxiety or acceptability.
Authors' conclusions
Home uterine monitoring may result in fewer admissions to a neonatal intensive care unit but in more unscheduled antenatal visits and tocolytic treatment; the level of evidence is generally low to moderate. Important group differences were not evident when we undertook sensitivity analysis using only trials at low risk of bias. There is no impact on maternal and perinatal outcomes such as perinatal mortality or incidence of preterm birth.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Early Diagnosis; Gestational Age; Intensive Care Units, Neonatal; Intensive Care Units, Neonatal/statistics & numerical data; Obstetric Labor, Premature; Obstetric Labor, Premature/diagnosis; Patient Admission; Patient Admission/statistics & numerical data; Premature Birth; Premature Birth/epidemiology; Premature Birth/prevention & control; Prenatal Care; Prenatal Care/statistics & numerical data; Randomized Controlled Trials as Topic; Uterine Monitoring; Uterine Monitoring/methods
Plain language summary
Monitoring pregnant women at home for detecting preterm labour
What is the issue?
Babies who are born too early are more likely to become ill or die. If preterm labour is detected, treatment can start to slow down or stop labour. This also gives time for treatment to improve the baby’s breathing at birth. Increased contractions can be a sign of labour starting early.
Why is this important?
Many women do not recognise these contractions in time for treatment. Pregnant women at risk of giving birth early could use a monitoring device at home. This would send data to the hospital, and help doctors and midwives to detect and treat preterm labour.
What evidence did we find?
We searched for evidence on 28 June 2016 and found 15 randomised studies, involving 6008 women. Thirteen of these studies provided data we could use. The quality of results ranged from very low to high (GRADE). Most studies had design limitations, which in some were serious. Most studies compared women taught how to check for signs of premature labour with women who were also given a home uterine activity monitor. In some studies both groups used a monitor but one group had a ‘sham’ monitor that did not actually send the data to the women’s healthcare providers.Using a monitor at home made very little difference to many of the outcomes for mother or baby, although not all studies measured all outcomes. Women using monitors were no less likely to experience preterm birth at less than 37 or 32 weeks of pregnancy (GRADE very low). Women using monitors were less likely to experience preterm birth at less than 34 weeks, but when we analysed only high‐quality studies, no clear difference remained (GRADE high). Babies born to women using the monitor were less likely to be admitted to neonatal intensive care (GRADE moderate) but there were no fewer deaths (GRADE low). Women using the monitor were more likely to make an unscheduled antenatal visit (GRADE moderate), but the number of antenatal hospital admissions did not differ (GRADE low). Women using monitors appeared to be more likely to receive tocolysis (treatment to stop labour) (GRADE low), but when we looked only at high‐quality studies there was no clear difference. We found no data to assess women's views, although one large trial reported low compliance with monitor use. In some studies, women with monitors had more contact with midwives or maternity nurses, but it is unclear what effect this had.
What does this mean?
Home uterine monitoring may result in fewer admissions to a neonatal intensive care unit, but more unscheduled antenatal visits and treatment for preterm labour. The level of evidence is generally low to moderate.
Summary of findings
Summary of findings for the main comparison. Home uterine monitoring for preventing preterm birth.
| Home uterine monitoring for preventing preterm birth | ||||||
| Patient or population: women undergoing home monitoring for preventing preterm birth versus women receiving standard care Settings: trials took place in the USA and France Intervention: home uterine monitoring | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Home uterine monitoring | |||||
| Perinatal mortality | Study population | RR 1.22 (0.86 to 1.72) | 2589 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 46 per 1000 | 56 per 1000 (39 to 79) | |||||
| Preterm birth less than 34 weeks' gestation | Study population | RR 0.78 (0.62 to 0.99) | 1596 (3 studies) | ⊕⊕⊕⊕ high | Sensitivity analysis included 1 study at low risk of bias (1292 women) and did not show any difference in results | |
| 166 per 1000 | 130 per 1000 (103 to 165) | |||||
| Antenatal hospital admissions | Study population | RR 0.91 (0.74 to 1.11) | 1494 (3 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 186 per 1000 | 169 per 1000 (137 to 206) | |||||
| Preterm birth less than 37 weeks' gestation | Study population | RR 0.85 (0.72 to 1.01) | 4834 (8 studies) | ⊕⊝⊝⊝ very low2,4,5 | ||
| 364 per 1000 | 310 per 1000 (262 to 368) | |||||
| Admission to NICU | Study population | RR 0.77 (0.62 to 0.96) | 2367 (5 studies) | ⊕⊕⊕⊝ moderate3 | Evidence not downgraded for moderate heterogeneity (I² = 32%) | |
| 290 per 1000 | 223 per 1000 (180 to 278) | |||||
| Number of unscheduled antenatal visits | The mean number of days ranged across control groups from approximately 1 to 2 days | The mean number of days in the monitored group was approximately half a day higher MD 0.48 (0.31 to 0.64) |
1994 (2 studies) | ⊕⊕⊕⊝ moderate1 | Variation in protocol and healthcare delivery structures make it difficult to generalise from 1 large study contributing 65% of the weight for this outcome | |
| Use of tocolysis | Study population | RR 1.21 (1.01 to 1.45) | 4316 (7 studies) | ⊕⊕⊝⊝ low4,6 | This outcome may no longer be useful, due to changes in clinical practice. Sensitivity analysis including only 3 studies at low risk of bias (3749 women) did not show any clear difference in results. | |
| 188 per 1000 | 228 per 1000 (190 to 273) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1All studies contributing data with design limitations (‐1). 2Wide confidence interval crossing the line of no effect (‐1). 3Most studies with design limitations (‐1). Outcome not blinded in 2 studies. 4Most studies with design limitations (‐1). 5Statistical heterogeneity (I2 = 68%) (‐1). 6Statistical heterogeneity (I2 = 62%) (‐1).
Background
Description of the condition
Preterm birth is a major cause of perinatal mortality and morbidity. Lockwood 2001 considers various methods used to predict women at high risk of preterm labour, noting problems with sensitivity and specificity of traditional tests, and problems with accuracy of some biochemical and biophysical tests. Home uterine activity monitoring is one of the methods that has been used to try to predict preterm birth in the belief that early detection of increased contraction frequency would allow early intervention with tocolytic drugs to inhibit labour and prolong pregnancy (Maxwell 2001). The rationale has been that many women do not recognise their contractions in time for tocolytic therapy to be applied to inhibit labour. No study so far has demonstrated that tocolysis has any role other than to allow time for the fetus to benefit from maternal steroid administration. One possible reason is that the tocolytic is being administered too late.
Description of the intervention
The various care packages developed to predict preterm birth include hospital admission to enhance clinical surveillance, and educational packages to help women identify the signs of early labour, with or without the use of electronic home uterine monitoring devices. Mothers are taught to use these devices at home for one‐ to two‐hour periods each day, and the data stored in the device are transmitted by modem to a base centre for interpretation by a midwife or doctor, with appropriate response if the level appeared abnormal. The ICSI 2002 committee report found that home uterine activity monitoring was a safe procedure, but noted that its effectiveness was not proven. One of the identified difficulties in assessing the effectiveness of home uterine activity monitoring is the different types of care packages used, and the difficulty of assessing whether it is the home uterine activity monitoring or the increased nursing support that is responsible for the changes in outcomes. Existing randomised controlled trials have often used different control groups because such intensive monitoring may only be aimed at women deemed at risk of preterm birth, and the risk profiles may differ.
How the intervention might work
The rationale for home uterine monitoring was that early detection of uterine activity is a sensitive and specific diagnostic test for the onset of preterm labour, but studies (for example, Iams 2002) suggest that the relationship between the maximum frequency of contractions and preterm delivery is weak. More recent research (De Lau 2013; Vinken 2009) on the electrohysterogram (EHG) indicates that it may be possible to distinguish physiological uterine activity from uterine contractions that will lead to preterm labour, but more computer modelling of uterine activation is likely to be necessary (Sharp 2013). Monitoring at home could allow mothers to avoid prolonged or additional hospital admissions, and to be cared for at home. On the other hand, some mothers might become more anxious during the monitoring, particularly if they were remote from hospital, and greater awareness might in itself lead to more frequent presentation at hospital. Reducing unnecessary hospital admissions may decrease antenatal healthcare costs but these savings could be offset by the increased costs associated with poor neonatal outcome.
The rationale for home uterine monitoring also requires that the tocolytic drugs that could be administered to prolong pregnancy are effective, but there is no clear evidence for the effectiveness of long‐term tocolysis (Duley 2011). Various types of tocolytic agents exist. Cochrane systematic reviews indicate that the possible adverse effects of the betamimetics should be weighed against the advantages of delayed delivery (Neilson 2014), and that calcium channel blockers may be as effective as betamimetics but with fewer adverse effects (Flenady 2014). Oral betamimetics for maintenance therapy after threatened preterm labour are not advised (Dodd 2012). The evidence on different dosing regimens for magnesium sulphate (McNamara 2015) as single‐agent tocolytic therapy is very limited. However, administration of magnesium sulphate to women considered at risk of preterm birth does reduce the risk of cerebral palsy (Doyle 2009). Nifedipine and atosiban (an oxytocin receptor agonist) have comparable effectiveness but the latter is expensive and is only available intravenously (Duley 2011). The evidence on different dosing regimens for magnesium sulphate (McNamara 2015) as single‐agent tocolytic therapy is very limited. A Health Technology Assessment review of screening to prevent spontaneous preterm birth (Honest 2009) concluded that non‐steroidal anti‐inflammatory agents were the most effective tocolytic agents in reducing spontaneous preterm birth and prolongation of pregnancy in symptomatic women, but there were doubts over their safety and they are not in routine use. Antenatal corticosteroids helped reduce the incidence of respiratory distress syndrome and the risk of intraventricular haemorrhage.
An overview (Piso 2014) of Cochrane systematic reviews on antenatal interventions to reduce preterm birth noted that a few interventions have been found effective (e.g. progesterone for some groups of women, to improve infant health (Dodd 2013)) and a small number appear harmful. For around half the interventions evaluated the evidence did not warrant recommendations for clinical practice (Piso 2014). The accuracy of tests to predict preterm birth has been judged generally poor (Honest 2009), although transvaginal cervical‐length screening has been advocated as a means of identifying women at risk of preterm birth (Berghella 2013; Conde‐Agudelo 2015). Fetal fibronectin testing and similar bed‐side tests on cervical secretions have also been used for screening (Berghella 2008; Sanchez‐Ramoz 2009; Vis 2009). Their benefit is that they have a greater than 95% negative predictive value for delivery within seven days, and studies have looked at combining transvaginal cervical‐length screening with fibronectin testing (e.g. Hadži‐Legal 2016) with variable results.
Why it is important to do this review
There is a lack of consensus on the effectiveness of home uterine monitoring (Reichmann 2008). There are a number of reasons for this, including the variability in study design and the uncertain benefits of early detection of uterine activity. There were two aspects to be explored in the review: (1) is home uterine monitoring effective at detecting uterine activity? and (2) is it worthwhile finding out if women have uterine activity?
Objectives
To determine whether home uterine activity monitoring is effective in improving the outcomes for women and their infants considered to be at high risk of preterm birth, when compared with care that does not include home uterine activity monitoring.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised and quasi‐randomised controlled trials in which the use of home uterine activity monitoring is compared with care that does not include home uterine activity monitoring.
Types of participants
Women considered by their obstetricians to be at risk for preterm birth. We included women with multiple pregnancies in the review, but treated them as a subgroup (where possible).
Types of interventions
The emphasis is on identifying the value of home uterine activity monitoring, which may be used as part of a care package to reduce the need for hospital admission or monitoring, or both, to reduce the need for additional educational support for the woman, or reduce the need for additional nursing contact. We also considered trials where home uterine monitoring is compared with a different form of extra surveillance for women defined as being at risk by their obstetricians.
Comparisons:
Care including home uterine monitoring versus routine care (without home uterine monitoring or with placebo or 'sham' home monitoring).
Care including home uterine monitoring versus care with an alternative form of additional surveillance.
Types of outcome measures
Primary outcomes
Infant outcomes
Perinatal mortality rate.
Preterm birth at less than 34 weeks' gestation.
Prenatal outcomes
Number of days in hospital antenatally.
Secondary outcomes
Infant outcomes
Preterm birth (less than 37 weeks).
Very preterm birth delivery (less than 32 weeks).
Extremely preterm birth delivery (less than 28 weeks).
Air leak syndrome.
Necrotising enterocolitis.
Patent ductus arteriosus requiring treatment.
Chronic lung disease.
Retinopathy of prematurity.
Use of antenatal corticosteroids.
Respiratory distress syndrome.
Neuropathology on ultrasound (intraventricular haemorrhage all grades, severe grades three or four, periventricular leukomalacia).
Use of mechanical ventilation.
Admission to neonatal intensive care unit (NICU).
Mode of delivery.
Prenatal outcomes
Number of antenatal visits.
Number of antenatal hospital admissions.
Number of midwife/nurse home visits.
Use of tocolysis.
Maternal outcomes
Maternal anxiety.
Maternal acceptability of home uterine monitoring.
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results are screened and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched CENTRAL (Cochrane Library 2016, Issue 5), MEDLINE (1966 to 28 June 2016), Embase (1974 to 28 June 2016), CINAHL (1982 to 28 June 2016) (See:Appendix 1)
Searching other resources
We also scanned the reference lists of articles identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeUrquhart 2012.
For this update, the search identified one new report for our consideration (NCT02379351). We used the following methods, which are based on a standard template used by the Cochrane Pregnancy and Childbirth Group, to assess the 15 studies already included in the review.
Selection of studies
Two review authors independently assessed for inclusion all the studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager 5 software (RevMan 2014) and checked them for accuracy.
When information regarding any of the above was unclear, we had planned to contact authors of the original reports to provide further details, but we did not do this because all the studies are over 15 years old. We consulted some other reviews (ICSI 2002; Keirse 1993).
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcome.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcome.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcome, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data unbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that we would have expected to have been reported);
unclear risk of bias.
(6) Other potential bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook. We assessed evidence relating to the following outcomes for the comparison home uterine monitoring versus standard care.
Perinatal mortality
Preterm birth less than 34 weeks' gestation
Number of antenatal hospital admissions
Preterm birth less than 37 weeks' gestation
Admission to neonatal intensive care unit (NICU)
Number of unscheduled antenatal visits
Use of tocolysis
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014), in order to create a ’Summary of findings’ table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
We presented results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We have not included cluster‐randomised trials in this review. If relevant for the next update, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Section 16.3.4 or 16.3.6), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if we consider that the interaction between the effect of intervention and the choice of randomisation unit is unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We have not included cross‐over trials in this review and do not plan to do so in future updates. This design is not relevant to our review question.
Other unit of analysis issues
We have included trials involving women with multiple pregnancies in this review. Where possible, we have analysed multiple pregnancy in subgroups (see analyses for the outcomes 'Preterm birth less than 34 weeks'; 'Preterm birth less than 37 weeks'; and 'Number of antenatal visits'), but only one study for each of the outcomes provided subgroup data.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we conducted analyses as far as possible on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes we knew to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if an I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If we identified substantial heterogeneity (above 30%) for primary outcomes, we explored it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if we considered an average treatment effect across trials was clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we present the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses:
singleton gestation versus twin gestation.
We had also planned to carry out the following subgroup analyses:
gestational age at which home uterine activity monitoring (HUAM) began;
type of HUAM used;
reason HUAM was used.
The outcomes to be used in the subgroup analysis were:
preterm birth less than 34 weeks;
perinatal mortality.
We assessed subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We reported the results of subgroup analyses, quoting the Chi2 statistic and P value, and the interaction test I2 value.
We conducted additional subgroup analyses (singleton/twin) for the following outcomes:
preterm birth less than 37 weeks;
respiratory distress syndrome;
number of unscheduled antenatal visits.
Sensitivity analysis
We undertook sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, excluding poor‐quality studies from the analyses, to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
We identified 15 studies (6008 enrolled participants); 13 studies contributed data. All 15 included studies were randomised controlled trials, apart from one quasi‐randomised trial (Morrison 1987). The earliest trials, according to recruitment details in the studies, started in 1986 (Dyson 1991; Iams 1987), while the last ones (Brown 1999; Dyson 1998) completed recruiting in 1996. See also: Description of studies and Figure 1
1.

Study flow diagram.
Two included studies did not contribute to the data analysis: Porto 1987 (no relevant outcomes); Scioscia 1988 (problems with back‐calculation from percentages provided). For Hill 1990a, we have relied on the Keirse 1993 re‐analysis to identify the trial reports associated with this study, as sites appear to have reported separately.
From the updated search in May 2016, we retrieved one report from the Pregnancy and Childbirth Group Register (NCT02379351). We found no further trial reports in CENTRAL (the Cochrane Library), and one each in MEDLINE, Embase and CINAHL. We screened out these three at title and abstract as not being within in the scope of this review.
Included studies
Setting
All trials except one (Blondel 1992, conducted in France) were conducted in the USA.
Sample size
The smallest trials had fewer than 100 participants (e.g. Iams 1990; Lyons 1990; Morrison 1987; Nagey 1993); the largest trials had over 1000 participants (e.g. CHUMS 1995; Dyson 1998). In total, we collected data from 6008 enrolled and randomised participants.
Participants
Participants were women who had successfully been treated for preterm labour in the current pregnancy (Brown 1999; Hill 1990a; Iams 1990; Nagey 1993) or who were judged to be at risk (without prior treatment for preterm labour). One study (Blondel 1992) included both categories. The risk factors used in the studies varied. Some studies included twin and singleton gestations within the sample (e.g. Blondel 1992), others specified that only singleton gestations were included (e.g. Brown 1999), others included twin and singleton gestations but separated the groups for analysis (e.g. Dyson 1991; Dyson 1998), and one report (Hill 1990a) only studied twin gestations. Several trials focused on prenatal care for socially disadvantaged women, and two trials specified Medicaid coverage as one of the criteria for inclusion (Brown 1999; Morrison 1987). Other criteria for inclusion include possession of a telephone, and ability to use the monitoring device. There were differences among studies between the number of those judged eligible on medical and social demographic criteria and those randomised. For example, in CHUMS 1995, a large 18‐centre trial, 1355 women were enrolled and 1292 were randomised (95% of those enrolled). In a trial among 30 Kaiser Permanente clinics in northern California (Dyson 1998), 3455 women were identified as eligible, 2480 were enrolled, and 2422 eventually randomised (to one of three treatment groups) (97% of those enrolled). Corwin 1996 used the Creasy risk score; of those judged eligible (n = 509) on criteria including a Creasy risk score of greater than or equal to 10, 377 were enrolled and randomised (74% of those enrolled). In screening, many women did not meet the criterion of a Creasy risk score greater than or equal to 10, and of 509 who met initial screening criteria, 37 (7.3%) did not possess a telephone. One small study (Lyons 1990) examined military dependents.
Interventions
The type of interventions may be categorised into:
home uterine monitoring plus perinatal nursing contact versus standard care, with perinatal nursing contact varying from acknowledgement of receipt of transmissions (e.g. Corwin 1996; Wapner 1995) through to discussion on preterm labour management (Dyson 1998; Iams 1987);
home uterine monitoring (active versus sham device), with scripted protocol used for re‐monitoring (e.g. CHUMS 1995) or more general discussion between the monitoring centre perinatal nurses and participants about other records of signs of preterm labour (Dyson 1991).
In most studies, authors described how both experimental and control groups received education in self‐palpation, signs and symptoms of preterm labour, instructions on when to call for further professional advice as required (Brown 1999; Corwin 1996; Hill 1990a; Morrison 1987; Nagey 1993; Wapner 1995), and the following additionally asked both groups to make their own records (Dyson 1991; Dyson 1998; Iams 1987; Iams 1990).
All included studies were randomised controlled trials, apart from one quasi‐randomised trial (Morrison 1987). The earliest trials, according to recruitment details in the studies, started in 1986 (Dyson 1991; Iams 1987) and the last ones (Brown 1999; Dyson 1998) completed recruiting in 1996. See also: Description of studies.
Two included studies did not contribute to the data analysis: (Porto 1987) (no relevant outcomes) and Scioscia 1988 (problems with back‐calculation from percentages provided). For one included study (Hill 1990a), we have relied on the Keirse 1993 re‐analysis to identify the trial reports associated with this study, as sites appear to have reported separately.
We did not identify any trials comparing home monitoring with an alternative form of surveillance.
Excluded studies
The following excluded studies contained insufficient clinical data for analysis, or indeed for confirmation that the trials were truly in scope: Ogburn 1993; Tõrõk 1994. We did not identify any further reports of these studies. In Birnie 2000; Blondel 1988; Dawson 1999; Goulet 1999; Goulet 2001; Iedema 1994; Iedema‐Kuiper 1996; Monincx 1997; Monincx 2001; Reece 1992; Spira 1981; Spira 1986, Su 2002, the intervention was out of scope. Merkatz 1991 was a general review, as was Blondel 1990.
Risk of bias in included studies
Allocation
We rated four studies at low risk of bias for sequence generation. The CHUMS 1995 and Dyson 1998 trials used computer‐generated randomisation sequence allocation schemes and were assessed as being at low risk of bias for sequence generation. Corwin 1996 and Wapner 1995 used external randomisation services. Wapner 1995 used separate blocked randomisation at each site; Corwin 1996 used different random‐number sequences for each site; Dyson 1998 and Corwin 1996 stratified by gestation status (singleton or twin) and by site. We rated two studies at high risk of bias for sequence generation: Morrison 1987 used hospital numbers and Iams 1987 did not describe how the sequence was generated and reported an unbalanced sample. We judged the remaining studies to be at unclear risk, as no or unclear information was provided on sequence generation.
We assessed six studies at low risk of bias for allocation concealment (Brown 1999; CHUMS 1995; Corwin 1996; Hill 1990a; Nagey 1993; Wapner 1995); these studies used external randomisation services or sealed opaque envelopes for allocation concealment. In Morrison 1987 and Dyson 1991 staff carrying out randomisation may have been aware of allocation and we rated these studies at high risk of bias for these domains. In the remaining studies there was insufficient information or the method used to conceal allocation at the point of randomisation was not described at all. (see Table 2)
1. Methodological quality of trials.
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random‐number tables, lot drawing, coin‐tossing, shuffling cards, throwing dice | Case number, date of birth, date of admission, alternation |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially‐sealed opaque envelopes | Open allocation sequence, any procedure based on inadequate generation |
Blinding
Only in the two studies that used active versus sham devices (CHUMS 1995; Dyson 1991) were the participants, and monitoring centre staff unaware of the group allocation. Some studies mention specific instructions to participants not to inform caregivers of their group allocation on admission to hospital, or checks to ensure that caregivers were not informed of group allocation. There was an attempt at blinding staff caring for women and we rated these studies as unclear for performance bias (Corwin 1996; Dyson 1998; Morrison 1987; Wapner 1995). Many studies provided few details or indicated that caregivers were aware of at least some of the allocations; we rated these studies at high risk of bias for performance blinding (Figure 2).
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
We judged four studies to be at low risk of detection bias, as either a sham device was used or we considered that the outcomes reported were objective and unlikely to be affected by lack of blinding (CHUMS 1995; Corwin 1996; Nagey 1993; Porto 1987). In the remaining studies the risk of detection bias was either unclear, or at high risk of bias due to lack of blinding and the type of outcomes reported.
Incomplete outcome data
Four of the 13 studies had fewer than 5% withdrawals (Blondel 1992; Dyson 1991; Dyson 1998; Morrison 1987), while one study (Corwin 1996) had between 5% and 9.9% attrition (fetal deaths prior to observation, and noncompletion), and we rated these studies at low risk of attrition bias. The largest study in this group (Dyson 1998) notes that all those randomised completed the study, but 93 women (4%) did not receive the surveillance to which they were assigned. The statistical analysis presented is based on intention‐to‐treat, with the results on a completion‐of‐protocol basis stated to be similar. It seems that Lyons 1990 may have had no withdrawals, although this was not explicitly reported and we rated this study as being at unclear risk.
We judged two studies to be at high risk of bias due to high attrition or loss that may have related to outcomes (Hill 1990a; Scioscia 1988). Hill 1990a notes withdrawals and fetal/medical complications. Hill 1990a presents data for women who experienced preterm labour after entry to the trial, but there are discrepancies among the various reports of this study (Keirse 1993), and it is difficult to back‐calculate with certainty from the data provided. In the Scioscia 1988 trial five of the 72 women randomised were removed from the analysis post randomisation; it wasn't clear how many of these women were lost from the intervention and control groups, and so group denominators weren't clear, and we were unable to include data from this study.
We rated the remaining studies as unclear for attrition bias. Four of them (Brown 1999; Iams 1987; Iams 1990; Wapner 1995) had between 10% and 19.9% attrition. Brown 1999 states the main reason for attrition was a change in circumstances; Iams 1987 notes problems over noncompliance with the protocol, with women who did not monitor as requested for more than three days deemed to be noncompliant. The differences in noncompliance between years one and two of this study were statistically significant. A companion project (Iams 1990) also found noncompliance with the protocol to be a problem; Wapner 1995 notes a variety of reasons for withdrawals.
Nagey 1993 reports that only one woman in the control group was lost to follow‐up, but two women were excluded from the analysis on medical grounds, and four women in the experimental group never left hospital and thus did not receive the intervention.
Two studies (CHUMS 1995; and one contributory report to Hill 1990a (Knuppel 1990a)) had an attrition rate exceeding 20%, although CHUMS 1995 (one of the largest trials) followed up all women who started monitoring, including noncompliant ones, and withdrawals until delivery. Participants who did not transmit for over 48 hours were deemed noncompliant (unless the reason concerned hospital admission or delivery). The results are presented on an intention‐to‐treat basis (but only for those who started monitoring); the authors state that the per‐protocol results are similar. No details are provided for the 127 women who were randomised but did not start monitoring.
Selective reporting
In six studies we did not find evidence of outcome reporting bias and we rated them at low risk of bias (Blondel 1992; Brown 1999; CHUMS 1995; Dyson 1998; Iams 1987; Morrison 1987).
We assessed four studies at high risk of reporting bias. Iams 1990 states that all births before 37 completed weeks were reviewed in detail, but data are only provided for births before 36 weeks, and the end point is different from the companion trial (Iams 1987). Wapner 1995 does not provide the total number of preterm births (mean gestational age only), and outcomes are mostly reported for women diagnosed with preterm labour, a subset of the participants. The Watson 1990 trial report for Hill 1990a presents preterm birth data for the 34 of 86 participants with recurrent preterm labour, but there are data missing for the entire group of participants. It is unclear from the reports for Hill 1990a how the subgroups were formed, whether different sites followed the same or slightly different protocols, or how reporting was organised among the sites (Keirse 1993). Many of the end points in the included studies and outcome measurements (e.g. changes in cervical dilatation) do not map to those selected for the review. In Scioscia 1988 there were insufficient data to support results.
In the remaining six studies it was unclear whether there was outcome reporting bias; for example, Corwin 1996 does not report twin gestation outcome data, and Dyson 1991 includes the number of unscheduled visits for the twin data, but does not report the singleton data separately (reports "all women").
Other potential sources of bias
We assessed five studies to be at high risk of other bias, due to lack of lack of power calculations, being underpowered, protocol deviations or for poor reporting (Blondel 1992; Dyson 1991; Hill 1990a; Iams 1990; Nagey 1993). We rated three studies at low risk of other bias, and seven were at unclear risk.
Several studies report power calculations, but there is little consistency in the estimation assumptions. CHUMS 1995 (one of the largest trials) was designed to have 80% power, for a group difference of 1 cm (variance 1.4 cm) in change of cervical dilatation from the previous visit, when preterm labour was diagnosed, and assumed a 20% occurrence rate of preterm labour. Dyson 1998 used similar assumptions. Other studies (e.g. Brown 1999; Iams 1987; Nagey 1993) based their power calculations on a percentage reduction in preterm deliveries before 37 weeks. Other studies (e.g. Corwin 1996) did a power calculation based on the proportion of early detection of preterm labour possible, and assumed 30% would develop preterm labour.
Effects of interventions
See: Table 1
Primary outcomes
Infant primary outcomes
Perinatal mortality rate
Only two studies (Blondel 1992; Dyson 1998) with 2589 women, reported this outcome (Analysis 1.1) and although home uterine monitoring was associated with higher perinatal mortality (risk ratio (RR) 1.22, 95% confidence interval (CI) 0.86 to 1.72), the confidence interval was wide and crossed the line of no effect. No subgroup analysis was possible for singleton/twin pregnancy.
1.1. Analysis.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 1 Perinatal mortality.
Preterm birth at less than 34 weeks' gestation
There were fewer preterm births at less than 34 weeks' gestation in the home uterine monitoring group compared with controls (RR 0.78, 95% CI 0.62 to 0.99; three studies; n = 1596 (Analysis 1.2)). A fourth study (Scioscia 1988) provided no usable data. Of the three studies (CHUMS 1995; Dyson 1991; Nagey 1993) that measured this outcome, two of them (contributing over 93% by weight) compared active versus sham home uterine monitoring. The largest study (CHUMS 1995) provided data for those both randomised and monitored, and the authors state that the findings for the "subset who completed the protocol" were similar. If 36 women in a 1000 are likely to experience preterm birth at less than 34 weeks (CDC 2007), then home uterine monitoring might reduce the number at risk to between 21 and 36.
1.2. Analysis.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 2 Preterm birth < 34 weeks.
However, in a sensitivity analysis, the largest trial (CHUMS 1995: 72% contribution to this outcome) has low risk of bias for allocation, blinding, and selective reporting. The other two contributing trials (Dyson 1991; Nagey 1993) were at greater risk of bias (Figure 3). Excluding the data from the two trials at higher risk of bias, results show a slight difference in the upper confidence interval (changed from 0.99 to 1.00) but still favouring the home uterine monitoring group (RR 0.75, 95% CI 0.57 to 1.00; P = 0.05). Using the same scenario as above (36 women in a 1000 are likely to experience preterm birth at less than 34 weeks), then home uterine monitoring might change the number at risk to between 19 and 36. Only one study (Dyson 1991) at higher risk of bias provided singleton and twin data separately for preterm birth at less than 34 weeks' gestation. Home uterine monitoring was not associated with a decrease in the number of preterm twin births at less than 34 weeks' gestation (RR 0.55, 95% CI 0.26 to 1.17; one study, n = 109). Similarly, there was no statistically significant difference in the number of preterm singleton births at less than 34 weeks' gestation (RR 1.12, 95% CI 0.55 to 2.27; one study, n = 138); seeAnalysis 1.3.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
1.3. Analysis.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 3 Preterm birth < 34 weeks (Subgroup analysis).
Prenatal primary outcomes
Number of days in hospital antenatally
Lyons 1990 reports that women using home uterine monitoring spent 59 days in hospital antenatally (mean 1.9 days), compared with the control group that spent 169 days (mean 5.4 days). No other data are given.
Secondary outcomes
Infant secondary outcomes
Preterm birth (less than 37 weeks)
Nine studies (out of 15) assessed this outcome, but it was not possible to use the Scioscia 1988 data, and the data were analysed differently among the studies (see footnotes in Analysis 2.1). Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks (average RR 0.85, 95% CI 0.72 to 1.01; eight studies, n = 4834; random‐effects, Tau2 = 0.03, I2 = 68% (Analysis 2.1)). However, there was substantial heterogeneity. In Hill 1990a, data analysis focused on the women who experienced preterm labour, and the data presented in Analysis 2.1 are based on a back‐calculation of figures presented in the discussion. Given the difficulties of assessing how many women were actually included in this study (Keirse 1993), the figures presented are only a best estimate. Excluding the two studies at high risk of bias (Hill 1990a; Morrison 1987) does not change the findings (average RR 0.94, 95% CI 0.82 to 1.06; six studies, n = 4521; random‐effects, T2 = 0.01, I2 = 39%). Limiting the analysis to the studies at lower risk of bias (Blondel 1992; CHUMS 1995; Dyson 1998) suggests no clear difference (average RR 0.97, 95% CI 0.84 to 1.11; three studies, n = 3881; random‐effects, Tau2 = 0.031, I2 = 49%).
2.1. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 1 Preterm birth < 37 weeks.
A small group of studies included data for twin gestations. One report of Hill 1990a apparently presents some data for the twin gestations, focusing on the 30 women who experienced preterm labour. The authors indicate that for the group "in general", 50% of the monitored women delivered preterm compared with 81% of the controls, but it is unclear what denominator is being used. Dyson 1998 presented singleton and twin gestation data. The analysis indicates that women using home uterine monitoring with twin gestation were no less likely to experience preterm birth before 37 weeks (RR 0.96, 95% CI 0.71 to 1.30; n = 844). Dyson 1998 showed no difference in the number of women with singleton gestation using home uterine monitoring who experienced birth before 37 weeks (RR 0.95, 95% CI 0.62 to 1.45; one study, n = 2422). SeeAnalysis 2.2.
2.2. Analysis.
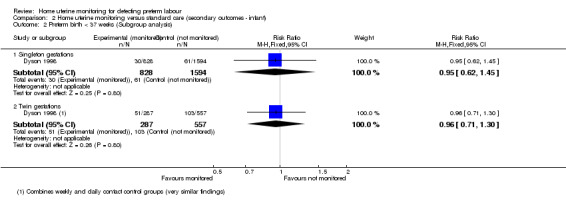
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 2 Preterm birth < 37 weeks (Subgroup analysis).
Very preterm birth delivery less than 32 weeks
Women using home uterine monitoring were no less likely to experience preterm birth at less than 32 weeks (average RR 0.76, 95% CI 0.31 to 1.85; three studies, n = 2550; random‐effects, Tau2 = 0.36, I2 = 56%), seeAnalysis 2.3. Only Dyson 1998, Morrison 1987 and Nagey 1993 assessed this outcome.
2.3. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 3 Preterm birth < 32 weeks.
Extremely preterm birth delivery less than 28 weeks
No data available.
Air leak syndrome
No data available.
Necrotising enterocolitis
No data available.
Patent ductus arteriosus requiring treatment
No data available.
Chronic lung disease
No data available.
Retinopathy of prematurity
No data available.
Use of antenatal corticosteroids
Brown 1999 reports that the use of antenatal corticosteroids was similar in both groups (56 women, 69.1% versus 54 women, 67.5%; RR 1.01, 95% CI 0.82 to 1.25; one study; n = 162 (Analysis 2.4)).
2.4. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 4 Use of antenatal corticosteroids.
Respiratory distress syndrome (RDS)
Dyson 1998 reports that for women with singleton gestations, and less than 34 weeks' gestation, five out of 19 infants from the home uterine monitoring group developed RDS, compared with four out of 19 in the control group (RR 1.25, 95% CI 0.40 to 3.95; one study, n = 38 (Analysis 2.5)), no difference.
2.5. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 5 Respiratory distress syndrome.
Similarly, we found no difference for twins at less than 34 weeks' gestation where four out of 44 infants from women using home uterine monitoring developed RDS, compared with 10 out of 42 in the control group (with sham device) (RR 0.38, 95% CI 0.13 to 1.12; n = 86 (Analysis 2.5)).
Neuropathology on ultrasound (intraventricular haemorrhage all grades, severe grades three or four, periventricular leukomalacia)
No data available.
Use of mechanical ventilation
Two relatively small studies assessed this outcome (Brown 1999; Corwin 1996), the latter providing singleton data only, for women with preterm labour. Infants from the monitored group were not significantly less likely to need mechanical ventilation (average RR 0.31, 95% CI 0.04 to 2.38; two studies, n = 539; random‐effects, T2 = 0.86, I2 = 37% (Analysis 2.6)).
2.6. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 6 Use of mechanical ventilation.
Admission to neonatal intensive care unit
Infants born to women in the home uterine monitoring group were less likely to be admitted to a neonatal intensive care unit than infants born to control group women (average RR 0.77, 95% CI 0.62 to 0.96; five studies, n = 2367; random‐effects, Tau2 = 0.02, I2 = 32% (Analysis 2.7)). Five studies measured this outcome (Brown 1999; CHUMS 1995; Corwin 1996; Dyson 1991; Wapner 1995), but the reporting is incomplete (Wapner 1995), limited to singleton gestations (Corwin 1996), or apparently flawed (Dyson 1991).
2.7. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 7 Admission to neonatal intensive care unit.
CHUMS 1995, the study with the lowest risk of bias in the group, states that 28.5% of all infants born to women in the monitored group were admitted to neonatal intensive care, compared with 32% of all infants born to control group women. However, a sensitivity analysis, excluding the studies at higher risk of bias, leaves CHUMS 1995, which did not find such a big difference (RR 0.86, 95% CI 0.74 to 1.01; n = 1292).
Mode of delivery
Brown 1999 found the same level of caesarean delivery (7/82 in monitored group, 7/80 in controls) (RR 0.98, 95% CI 0.36 to 2.66; one study, n = 162 (Analysis 2.8)).
2.8. Analysis.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 8 Mode of delivery.
(2) Secondary outcomes (prenatal)
Number of antenatal visits (unscheduled)
Blondel 1992 cites the average number of "visits to the outpatient clinic" as 3 ± 2.3 for the monitored group (n = 84), 3 ± 1.9 (n = 83) for the control group, in a care delivery system that relied on home visiting by midwives, and three scheduled visits to the outpatient clinic. CHUMS 1995 states that 3% of both the experimental and control groups made unscheduled emergency visits.
Five other studies (Dyson 1991; Dyson 1998; Hill 1990a; Morrison 1987; Wapner 1995) measured the mean number of unscheduled visits to an obstetrician but standard deviations were not available for three studies (Dyson 1991; Morrison 1987; Wapner 1995). Consequently data from only two studies (Dyson 1998; Hill 1990a) contributed to the analysis. The mean number of unscheduled visits was higher among the home uterine monitoring group than in the control group (mean difference (MD) 0.48, 95% CI 0.31 to 0.64; two studies, n = 1994 (Analysis 3.1)).
3.1. Analysis.

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 1 Number of antenatal visits (unscheduled).
A review (Reichmann 2009) examined whether home uterine monitoring might be more effective for multiple gestations. One study (Dyson 1998) provided data on twin pregnancies separately: for the twin pregnancies the mean number of unscheduled visits among the home uterine monitoring group is higher (MD 0.60, 95% CI 0.24 to 0.96; one study; n = 564), comparing the daily contact and home uterine monitoring groups. The data for singleton gestations were not reported separately, but have been estimated, with the mean number of unscheduled visits among the home uterine monitoring group higher (MD 0.40, 95% CI 0.15 to 0.65; one study; n = 1060) Analysis 3.3)).
3.3. Analysis.
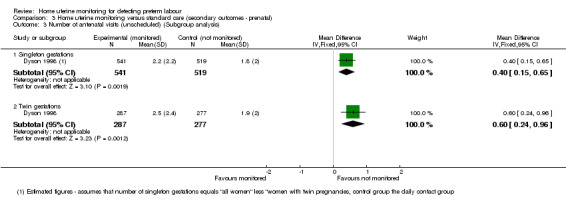
Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 3 Number of antenatal visits (unscheduled) (Subgroup analysis).
Number of antenatal hospital admissions
Three studies (Blondel 1992; Brown 1999; CHUMS 1995) assessed this outcome. There is no statistically significant difference between the number of antenatal hospital admissions in the monitoring and control groups (RR 0.91, 95% CI 0.74 to 1.11; three studies, n = 1494 (Analysis 3.2)).
3.2. Analysis.

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 2 Number of antenatal hospital admissions.
Number of midwife/nurse home visits
This was not a variable measured in most of the studies. In Blondel 1992, the control group received home visits by community midwives, whereas the experimental group received weekly visits from the monitoring centre midwife. In other studies, home uterine monitoring was an addition to standard high‐risk care (Brown 1999; Corwin 1996; Hill 1990a; Iams 1987; Iams 1990; Morrison 1987; Nagey 1993; Wapner 1995). The pattern of visits was determined by the protocol. In the studies that compared the active versus sham monitoring device (CHUMS 1995; Dyson 1991), protocols dictated the scope and frequency of interactions with monitoring centre perinatal nurses. Dyson 1998 used a three‐group design to compare: 1) monitoring with daily contact; 2) daily contact (control); 3) weekly contact (control) with the perinatal monitoring centre.
Use of tocolysis
Use of prophylactic tocolytic drug therapy was higher for the home uterine monitoring group compared with the control group (average RR 1.21, 95% CI 1.01 to 1.45; seven studies, n = 4316; random‐effects,Tau2 = 0.03, I2 = 62% (Analysis 3.4)). However, we observed substantial heterogeneity.
3.4. Analysis.

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 4 Use of tocolysis.
A sensitivity analysis, excluding studies at higher risk of bias from the analysis of the use of tocolysis, leaves three trials (Blondel 1992; CHUMS 1995; Dyson 1998). A random‐effects meta‐analysis showed no difference in the use of tocolysis among the women using home uterine monitoring (average RR 1.22, 95% CI 0.90 to 1.65, random‐effects, Tau2 = 0.05, I2 = 76%). CHUMS 1995 reports that tocolysis use was 31% in both monitored and control groups at any time during pregnancy, and 60% for participants diagnosed with preterm labour after enrolment.
(3) Secondary outcomes (maternal)
Maternal anxiety
No data provided.
Maternal acceptability of home uterine monitoring
No studies assessed maternal acceptability directly. One of the largest trials (Dyson 1998) notes that women in the home uterine monitoring group complied with the requirement of at least one daily session of monitoring 86% of the time. In another large trial (active versus sham device), CHUMS 1995 found that noncompliance was 12.5% in the experimental group and 12.7% in the control (noncompliance was assessed as failure to transmit for more than 48 hours).
Discussion
Summary of main results
Primary outcomes
Home uterine monitoring was not associated with a difference in perinatal mortality (on the basis of two studies). Home uterine monitoring was associated with fewer preterm births at 34 weeks (based on three studies). However, this difference was no longer apparent when we conducted a sensitivity analysis based on trial quality, restricting the analysis to a single study graded as being at low risk of bias. One study reported that women using home uterine monitoring spent fewer days in hospital than the control group.
Secondary outcomes
Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks (on the basis of eight contributing studies). Infants born to women in the home uterine monitoring group were less likely to be admitted to a neonatal intensive care unit (NICU) than infants born to control group women, although this difference was no longer apparent when we included only those studies assessed as being at lower risk of bias. There was no difference between the number of antenatal hospital admissions for the monitoring and control groups. The number of unscheduled hospital visits appeared to be higher among the monitored women. Use of prophylactic tocolytic drug therapy was higher among the home uterine monitoring group than the control group. However, the difference was no longer apparent when we restricted our analysis to high‐quality studies.
Overall completeness and applicability of evidence
The trials were clinically heterogeneous. Risk factors for preterm labour were assessed differently, and the delivery of the home uterine monitoring intervention varied. Some trials (e.g. Corwin 1996) used non‐professional call centre staff to receive the transmissions sent by the pregnant women, while in others the contact was more active (e.g. Dyson 1991; Dyson 1998; Iams 1987; Iams 1990), and CHUMS 1995 used a scripted protocol. There appears to be no consensus from these trials on the extent of professional midwifery support deemed appropriate. We do not know what the women thought of their care regimen in the trials where the centre staff receiving the transmissions only acted as conduits, giving no advice directly. It is possible that the lower withdrawal rates in Dyson 1991 and Dyson 1998 could be attributed to the more intensive and personal nursing or midwifery contact, but the organisational setting (a Health Maintenance Organization) for those two trials was different from the settings in the other trials. Only one study (Morrison 1987) included costings, reporting the financial incentive in this case to reduce the number of hospital days associated with a preterm birth among pregnant women receiving Medicaid public assistance. Although a home uterine monitoring system might be appropriate for women in socio‐economically disadvantaged groups, only Brown 1999 targeted this group, and other studies excluded those who could not speak English, or who did not have a telephone.
Many telemedicine trials assess the acceptability of the system to participants and providers, but none of the trials presented data directly on this, and only one related trial (Blondel 1990) presents data on mothers' views of prenatal care with a home visiting system, with later overview of three trials involving home visiting. Arguably, the system architectures for home uterine monitoring involve choices between a) objective monitoring (checking up on education provided) or empowering women to monitor themselves, asking advice as necessary, or b) maintaining or enhancing participant contact (Urquhart 2010). Analysis of the included trials provides few clues on the best way to implement a care delivery system, including how monitoring should be organised, although the topic has been considered (e.g. Merkatz 1991).
All the included studies are based on the premise that increased uterine activity can be used as a predictor of possible preterm labour, and thus the effectiveness of home uterine monitoring as a screening tool is being assessed here. Pregnancy outcomes then depend on the effectiveness of the management of diagnosed preterm labour. Determining the contribution of home uterine monitoring to outcomes is therefore complex, especially in multicentre studies and studies in different countries and with different populations, where approaches to the management of preterm labour may differ. ‘Usual care’ for control groups was not the same in all studies, and some authors set out to investigate the effects of intensive nursing care compared with, or combined with, home uterine monitoring. In most of the studies the women in the home uterine monitoring group also had daily contact with the nurses monitoring their transmitted results, but in, for example, Wapner 1995, the daily nurse contact was withheld from the monitored group. The role of intensive nursing support was not the primary intervention being assessed in any of these studies, and although some authors have attempted to assess its role alongside, or instead of, home uterine monitoring, this is not possible from the data presented. The use of intensive antenatal care (from midwives and others) in the emotional and social support of pregnant women has been the subject of another Cochrane Review (Hodnett 2010) and demonstrates the difficulty of assessing the precise contribution of nursing care to pregnancy outcomes. The home uterine monitoring study authors who conclude that intensive nursing care may be as effective as home uterine monitoring and may provide other benefits for women at risk, are right to suggest that further targeted research in this area is required.
Quality of the evidence
The main reason for downgrading the quality of the evidence was design limitations in the studies contributing data. In the studies that were not testing sham versus real home uterine monitoring devices, it would have been impossible to blind the participants to their allocation, and, depending on the way care was organised, some of the caregivers might easily learn the allocation. This could affect, for example, the use of tocolytic drugs. For objective outcomes, the fact that many of the trials scored 'unclear' for blinding is not a major concern.
One of the main difficulties with the meta‐analyses conducted for the review was the low number of studies that contributed to any particular outcome measurement, and subgroup analysis for any outcome was only possible for one study within each outcome group. It was therefore impossible to clarify whether home uterine monitoring might be more effective for twin gestations, as considered in one review (Reichmann 2009). In addition, if we exclude studies at higher risk of bias from some of the meta‐analyses there is no clear evidence of any difference between the experimental and control groups (Figure 2).
For the primary outcome of perinatal mortality, the two contributing studies are of equal quality scores (Figure 3). The inadequate blinding should not affect this outcome, but the quality of evidence is low (GRADE).
For the primary outcome of the number of preterm births at 34 weeks or less, the largest trial (CHUMS 1995, 75% contribution to this outcome) is one of the higher‐quality studies for allocation, blinding, and selective reporting. Relying solely on the findings from this trial very marginally reduces the size of effect. The overall quality of evidence is high (GRADE).
For secondary outcomes, excluding the studies at high risk of bias from the meta‐analysis of the data for NICU admission reduced the strength of the evidence of the difference between monitoring and control groups. Evidence for admission to NICU was graded overall as of moderate quality (GRADE). The analysis of the two contributing studies (Dyson 1998; Hill 1990a) on unscheduled antenatal visits indicates that the monitored women made more visits, but another study (CHUMS 1995) which used the number of women as the unit of analysis indicates that there was no clear difference between the groups. We rated the evidence for the outcome of number of unscheduled antenatal visits as of moderate quality (GRADE). There were no group differences in the numbers of women admitted to hospital during the antenatal period, and we rated this evidence as low quality (GRADE).
In the analysis of the use of tocolysis, there was no strong evidence of effect when we excluded studies at high risk of bias from the analysis. Clinical protocols for use of tocolysis very likely varied from study to study. The overall quality of evidence is low (GRADE). Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks, and the quality of evidence was very low (GRADE). We cannot determine the role of tocolysis from these studies.
We selected outcomes that we considered best reflected the potential benefits and harms of home uterine monitoring. We included infant outcomes that were covered in a recently‐published core outcome set for studies on preterm birth prevention (Van't Hooft 2015). Our infant outcomes include those relating to gestational age at delivery (birth before 37, 34, 32 and 28 weeks' gestation), and we also included infant mortality and morbidity outcomes. Our key maternal outcomes relate specifically to home uterine monitoring: maternal anxiety, and acceptance of home monitoring rather than to more general maternal morbidity outcomes which form part of the core outcome set. We will consider including these outcomes in future updates.
Potential biases in the review process
We attempted to reduce bias in the review process by ensuring that at least two of the review authors assessed all study reports. Two review authors independently assessed risks of bias in the individual trial reports. Although we took steps to try to minimise bias, we are aware that evaluation of risk of bias and evidence quality is partly a matter of judgement, and accept that a different review team may have made different judgements.
Agreements and disagreements with other studies or reviews
The ICSI 2002 committee report found that home uterine activity monitoring was a safe procedure, but noted that its effectiveness was not proven. Reichmann 2008 reviewed home uterine monitoring studies that included women in current preterm labour at enrolment and concluded that home uterine activity monitoring was not proven to be effective. Reichmann 2009 also concluded that home uterine monitoring was not useful for women with multiple gestations, and cited a study that showed that uterine contractions did not indicate preterm birth in twins. An earlier review (Keirse 1993) re‐analysed data from Hill 1990a, and concluded that this set of studies on the "Term Guard" system appeared to be flawed in design and execution. In addition, the studies provided no data on infant morbidity and mortality. Grimes 1992 also concludes that there were serious methodologic deficiencies in the published trials. On the other hand, Newman 2005 reports in a review of trials that "home uterine contraction monitoring with or without frequent perinatal nursing contact can reduce the risk of preterm birth and improve perinatal outcomes and that both are independently superior to standard preterm birth prevention education and care". Newman 2005 mentions another meta‐analysis by Colton 1995 that included the same studies as in Grimes 1992, plus some other studies. One reason for the discrepancies in the conclusions of these various meta‐analyses is that, as Newman 2005 acknowledges, the benefits become insignificant when the denominator is changed from the women in preterm labour to the entire randomised cohort. The meta‐analysis by Colton 1995 (in favour of home uterine monitoring) was partially supported by one of the device manufacturers.
Authors' conclusions
Implications for practice.
Home uterine monitoring may result in fewer admissions to a neonatal intensive care unit but more unscheduled antenatal visits and tocolytic treatment; however, the level of evidence is generally low to moderate. Important group differences were not evident when we conducted sensitivity analysis using only trials at low risk of bias. There is no impact on maternal and perinatal outcomes such as perinatal mortality or incidence of preterm birth.
Implications for research.
The assumption that home uterine monitoring can help predict premature labour needs to be re‐assessed. More research is necessary to identify a clinically useful remote monitoring system that is both closely linked to effective treatment to prevent preterm birth, and makes cost‐effective use of professional nursing and midwifery staff. New studies and their outcomes and reporting should reflect current evidence‐based obstetric practice.
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2016 | New search has been performed | Search updated, one new report identified and excluded (NCT02379351). New background material found and incorporated into review. |
| 28 June 2016 | New citation required but conclusions have not changed | Review still includes 15 studies, conclusions remain unchanged. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. |
Acknowledgements
Therese Dowswell, for assistance with the editorial queries for the 2016 update.
Nancy Medley, for preparing the 'Summary of findings' table for the 2014 update. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The review authors thank the Cochrane Pregnancy and Childbirth Group (Liverpool, UK) for their support, and the Cochrane Effective Practice and Organisation of Care Group for their earlier support in the telemedicine systematic review work that was the start of this particular review.
As part of the prepublication editorial process, the previous version of this review (Urquhart 2012) was commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
Author searches
CENTRAL (The Cochrane Library 2016, Issue 5)
#1 OBSTETRIC LABOR, PREMATURE (MeSH) #2 PREMATURE BIRTH (MeSH) #3 preterm birth OR premature labor OR premature labour): ti, ab, kw #4 UTERINE MONITORING (MeSH) OR UTERINE CONTRACTION (MeSH) #5 (home OR domiciliary OR ambulatory): ti, ab, kw #6 #3 AND #4 #7 (#1 OR #2 OR #3) #8 (#6 AND #7) limit to Trials
MEDLINE (1966 to 28 June 2016) #1 OBSTETRIC LABOR, PREMATURE [MeSH terms] OR PREMATURE BIRTH [MeSH terms] #2 premature labor OR premature labour OR preterm labor OR preterm labour OR preterm birth #3 #1 or #2 # 4 home OR ambulatory OR domiciliary # 5 HOME CARE SERVICES [MeSH terms] #6 HUM OR HUAM OR HUCA #7 UTERINE MONITORING [MeSH terms] OR fetal monitoring OR uterine monitor$ OR uterine contraction OR uterine activity monitor$ #8 (#4 OR #5 OR #6) #9 (#7 AND #8) #10 randomized controlled trial[Publication Type] OR controlled clinical trial[Publication Type] #11 RCT OR randomised OR randomized OR clinical trial$ #12 (#10 or #11) #13 (#3 AND #9 AND #12)
EMBASE (1974 to 28 June 2016) #1 ("premature labour" OR "preterm labour" OR "pre term labour") #2 ("premature labor" OR "preterm labor" OR "pre term labor") #3 PREMATURE LABOR/. #4 #1 OR #2 OR #3 #5 (home OR ambulatory).ti,ab #6 UTERUS CONTRACTION/ #7 "uterine contraction*".ti,ab #8. "uterine activity".ti,ab #9 #6 OR #7 OR #8 #10 #5 AND #9. #11. ("home uterine monitor*" OR "home uterine activity monitor*").ti,ab #12 (HUM OR HUAM OR HUCA).ti,ab #13 AMBULATORY MONITORING/ OR SENSOR/ #14 HOME MONITORING/ #15 PATIENT MONITORING/ #16 #10 OR #11 OR #12 OR #13 OR #14 OR #15 #17 ("randomised controlled trial*" OR "randomized controlled trial*" OR RCT).ti,ab #18 RANDOMIZED CONTROLLED TRIAL/ #19 #17 OR #18 #20 #4 AND #16 AND #19
CINAHL (1982 to 28 June 2016). #1 premature labour OR preterm labour #2 pre term labour #3 premature labor OR preterm labor #4 pre term labor #5 MH "Labor, Premature" #6 #1 OR #2 OR #3 OR #4 OR #5 #7 home OR ambulatory #8 MH "Uterine Contraction" #9 MH "Uterine Monitoring" #10 uterine contraction* OR uterine activity #11 #8 OR #9 OR #10
#12 #7 AND #11
#13 home uterine monitor* OR home uterine activity monitor*
#14 HUM OR HUAM OR HUCA
#15 MH "Wearable Sensors"
#16 wearable AND (monitor* OR sensor*)
#17 MH "Home Health Care Information Systems"
#18 #12 OR #13 OR #14 OR #15 OR #16 OR #17
#19 randomised controlled trial* OR randomized controlled trial* OR RCT
#20 MH "Randomized Controlled Trials" OR MH "Clinical Trials"
#21 19 OR 20
#22 #6 AND #18 AND #21
Data and analyses
Comparison 1. Home uterine monitoring versus standard care ‐ primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 2 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 2 Preterm birth < 34 weeks | 3 | 1596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 3 Preterm birth < 34 weeks (Subgroup analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.55, 2.27] |
| 3.2 Twin gestations | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
Comparison 2. Home uterine monitoring versus standard care (secondary outcomes ‐ infant).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks | 8 | 4834 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 2 Preterm birth < 37 weeks (Subgroup analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Singleton gestations | 1 | 2422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 2.2 Twin gestations | 1 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 3 Preterm birth < 32 weeks | 3 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.31, 1.85] |
| 4 Use of antenatal corticosteroids | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| 5 Respiratory distress syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Singleton gestations | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.40, 3.95] |
| 5.2 Twin gestations | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.12] |
| 6 Use of mechanical ventilation | 2 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.38] |
| 7 Admission to neonatal intensive care unit | 5 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.96] |
| 8 Mode of delivery | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.36, 2.66] |
Comparison 3. Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of antenatal visits (unscheduled) | 2 | 1994 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.31, 0.64] |
| 2 Number of antenatal hospital admissions | 3 | 1494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.11] |
| 3 Number of antenatal visits (unscheduled) (Subgroup analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 1060 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.15, 0.65] |
| 3.2 Twin gestations | 1 | 564 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.24, 0.96] |
| 4 Use of tocolysis | 7 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.01, 1.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blondel 1992.
| Methods | Randomised controlled trial | |
| Participants | 168 women who had been discharged and sent home after hospitalisation for threatened preterm labour (44%) and women at high risk for preterm labour (56%). Women enrolled between 24 and 34 weeks of pregnancy Setting: 4 public maternity units in Lyon, France, October 1988 to May 1989 | |
| Interventions | Intervention: home uterine monitoring (twice daily), daily telephone contact with midwife, and home visit once a week from midwife from the Tokos centre (supplier of device) Control group: standard care (home visits once or twice a week from a community midwife) Women with persistent symptoms or contractions outside baseline frequency were sent to outpatient clinic or the inpatient ward |
|
| Outcomes | Primary outcomes: perinatal mortality rate Secondary outcomes: 1) infant; preterm birth < 37 weeks, very preterm birth < 32 weeks, birthweight < 2500 g; 2) prenatal; number of antenatal visits, number of antenatal hospital admissions, use of tocolysis |
|
| Funding | Unclear: authors state that desired sample size not possible as the supplier of the home uterine monitoring device (Tokos Medical Corporation) was no longer funded in France. Tokos Medical Corporation provided the home uterine monitoring care system | |
| Notes | Provides other outcome measures not included in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. 2 groups of women (discharged after hospitalisation for threatened preterm labour, and women at high risk for preterm labour) included. "Allocated...to the monitored and control groups by randomization with sealed envelopes." Demographic characteristics of experimental and control groups comparable; authors note that the proportion of women with some risk factors smaller in the monitored (experimental) group |
| Allocation concealment (selection bias) | Unclear risk | "randomization with sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Midwives and participants knew allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Midwives referred women to hospital for treatment. Authors state doctors knew results of the monitoring, so not all prenatal outcomes were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals from intervention group, 1 from control group. Analysis by authors of 167 records (out of 168 recruited) |
| Selective reporting (reporting bias) | Low risk | No evidence that reporting incomplete. Author has published related papers |
| Other bias | High risk | Claims that 900 women required for suitable power (10% difference in groups), making the study underpowered. 13 in control group had no home visits |
Brown 1999.
| Methods | Randomised controlled trial | |
| Participants | Of 343 women treated with parenteral tocolytic therapy for preterm labour who met study criteria, 186 were enrolled initially, and 162 cases available for analysis (n = 82 experimental, n = 80 control). Study criteria included Medicaid coverage, recruitment between 24 and 34 weeks of pregnancy. \ Setting: Indiana, USA, between 1 July 1991 and 1 October 1996 |
|
| Interventions | Intervention: home uterine monitoring in addition to prenatal care of socio‐economically disadvantaged women who had received inpatient treatment for preterm labour. Experimental group transmitted monitor strip twice daily by telephone. Both experimental and control group had daily contact with perinatal nurse, and both groups on maintenance dose of oral terbutaline. Both groups received education in self‐palpation and were given instructions on how and when to call for further assistance if preterm labour was suspected | |
| Outcomes | Primary outcomes: see notes. Secondary outcomes: 1) infant, use of antenatal corticosteroids, use of mechanical ventilation, admission to neonatal ICU, mode of delivery, average birthweight; 2) prenatal; number of antenatal hospital admissions (unscheduled hospital observations), use of tocolysis (at least 1 readmission requiring tocolytic therapy | |
| Funding | Tokos Medical Corporation provided the monitor support. Indiana Office of Medicaid Policy and Planning supported the study | |
| Notes | Preterm birth < 35 weeks, and 35 to 37‐week births measured. Measured compliance with home uterine monitoring for < 35 and greater or equal to 35‐week deliveries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. "The random assignment process used sealed opaque envelopes to determine whether a patient would be in the monitored or control group." Maternal demographic and risk factors not statistically different between experimental and control groups |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and perinatal nurse in daily contact with both groups were aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Perinatal nurse in daily contact with both groups was aware of allocation; unclear whether hospital physicians aware, but perinatal nurse advised participant on management. Therefore some outcomes (unscheduled hospital observations, tocolysis) were not objective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 186 initially enrolled, for 24 of these circumstances changed |
| Selective reporting (reporting bias) | Low risk | No evidence that reporting incomplete |
| Other bias | Low risk | Power calculation based on reducing the risk of preterm delivery at < 37 weeks' gestation from 40% to 20%. Authors indicate that 82 women required for each group |
CHUMS 1995.
| Methods | Randomised controlled trial (double blinded) | |
| Participants | From 1355 recruited women between 24 and 36 weeks' gestation, and at high risk for preterm labour or birth, 1292 randomised, 1165 given device, active (n = 574) or sham (n = 591). Setting: Multicentre (18 sites), USA, 15 January 1992 to 27 May 1994 | |
| Interventions | Intervention group sent twice daily home uterine monitoring transmissions of 1 hour duration to base station and successful transmissions acknowledged by nurses. Control group also sent transmissions but the uterine activity data were not seen by nurses ‐ authors state they were "electronically buried". All participant interactions with base station nurses followed a scripted protocol, similar for both groups, whether for remonitoring, alerting of physicians or referral to hospitals | |
| Outcomes | Primary outcomes: preterm birth ≤ 34 weeks Secondary outcomes: 1) infant; admission to neonatal ICU, birthweight < 2500 g; 2) prenatal, unscheduled emergency visits, antepartum admissions, use of tocolysis. |
|
| Funding | The study was supported by Caremark Inc. (supplier of the monitoring device used) | |
| Notes | Provides other outcome measures not included in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation, with blocked random‐number sequences used. Both experimental and control groups similar in demographic and risk factors, and authors state that subgroups were also similar (and withdrawals) |
| Allocation concealment (selection bias) | Low risk | "Computer generated randomization scheme prepared for each investigational site was used to assign patients consecutively without regard to specific risk factor. The identity of group assignment was blinded to patients and their care givers through the completion of the entire study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state "identity of group assignment blinded to patients and their caregivers through the completion of the entire study". Those who were 'unblinded' initially were withdrawn from study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Group allocation unknown to caregivers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 1165 who were given devices, 842 completed (29.4%, n = 169 of experimental group, 25.7%, n = 152 of control group withdrew). All participants enrolled and monitored followed up until delivery, including women who were noncompliant or who withdrew voluntarily. Authors state analysis conducted on both per‐protocol (completed, n = 842) and on ITT basis (but this only includes those who started monitoring), and that both analyses showed comparable results. It is unclear what happened to the 63 experimental and 64 control women who were randomised but not subsequently monitored with a device |
| Selective reporting (reporting bias) | Low risk | Data summaries show "intent‐to‐treat" data. Authors state other analyses conducted (analysis of variance or logistic models) for all variables, and terms for site and group by site interaction effects included |
| Other bias | Low risk | Power calculation based on 80% power with 2‐tailed alpha of 0.05 for a group difference of 1 cm (variance 1.4 cm) in change of cervical dilatation from previous visit when preterm labour diagnosed. Authors state minimum enrolment of 310 participants for any individual risk factor subgroup to obtain the 62 patients for evaluation, required by power analysis. Trial failed to recruit sufficient multiple gestations for these subgroups. Authors note that preterm labour management varied among the sites, but claim that both arms of the study received the same or similar tocolytic treatment at any particular site |
Corwin 1996.
| Methods | Randomised controlled trial | |
| Participants | Women at risk of preterm labour in 3 hospital sites in USA, recruited between 26 and 32 weeks of gestation. From 2316 women screened, 432 were approached for informed consent, and 339 women with singleton gestations and 38 women with twin gestations agreed to participate (n = 198 experimental, n = 179 control) Setting: USA, from 01 September 1988 to 31 August 1989 | |
| Interventions | Intervention group received standard high‐risk obstetric care, and in addition used a home uterine monitoring device, twice daily for an hour, sending transmissions to the centre, where the receiver reported back the number of contractions to the participant. No nursing contact was provided. Both intervention and control group participants received education in self‐palpation, and were instructed to contact their physician if they suspected preterm labour. Control group received standard high‐risk obstetric care. Minimum care scheduled was a visit to obstetric facility once every 4 weeks until 30 weeks, at least every 2 weeks (30 to 36 weeks) and at least weekly thereafter | |
| Outcomes | Primary outcomes: see notes. Secondary outcomes: 1) infant; preterm birth < 37 weeks, admission to neonatal ICU | |
| Funding | Study designed for a Food and Drug Administration Premarket approval application, and supported by Matria Healthcare, supplier of the device | |
| Notes | Study analysed relative risk reduction for various adverse outcomes (early delivery, low birthweight categories). Data drawn from several publications, the Corwin paper essentially reworking the earlier papers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted separately for singleton and twin gestations, by "study personnel without direct patient care responsibilities". Different random‐number sequence used for each site. "Group assignment was made by means of opening consecutively numbered envelopes that randomized patients with a table of random numbers." Authors state that no statistically significant differences in demographic and risk factors between groups were detected (although some missing values noted) |
| Allocation concealment (selection bias) | Low risk | "Group assignment was made by means of opening consecutively numbered envelopes that randomized patients with a table of random numbers." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants aware, but were asked not to reveal their group allocation to caregivers. Caregivers were aware they were seeing a study participant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Caregivers not informed whether suspected uterine contractions were detected by the monitor or the participant. No nursing support provided to experimental group women as part of the monitoring package |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 339 recruited (174 experimental, 165 control), 14 (6 versus 8) did not complete, 7 fetal deaths prior to observation, available cases 164 versus 154 |
| Selective reporting (reporting bias) | Unclear risk | Twin gestation outcome data not reported (38 women); authors state group too small for analysis |
| Other bias | Unclear risk | Authors calculated that 320 women would need to be enrolled to have sufficient power (80%, with alpha = 0.05, beta = 0.20) to allow detection of improvement from 30% to 60% in the proportion of women with preterm labour with early diagnosis 8 < 2 cm cervical dilatation) |
Dyson 1991.
| Methods | Randomised controlled trial, with retrospective standard care group also included in study | |
| Participants | Women receiving care at Kaiser Permanente, before 28 weeks' gestation, and of these 251 gave consent. 138 (n = 68 experimental, n = 70 control) were singleton gestations, and 109 were twin (n = 57 experimental, n = 52 control), with 2 withdrawals from each arm of the study. Setting: California, USA, between 1 January 1986 and 1 January 1989 | |
| Interventions | Intervention and control groups received home uterine activity monitors, but only in the intervention group were the uterine activity data used in care. All participants were asked initially to monitor for an hour every day, and transmit daily ‐ this was later changed to twice daily monitoring and transmission. Both groups received education in self‐palpation and asked to record presence or absence of signs of preterm labour and number of contractions. Both groups were contacted at least 5 days a week by the study nurse, to discuss such signs and for the intervention group to review monitoring data. Tocolysis conducted according to protocol | |
| Outcomes | Primary outcomes: perinatal mortality rate (twin only), preterm birth < 34 weeks. Secondary outcomes: 1) infant; respiratory distress syndrome, admission to neonatal ICU; 2) prenatal; number of unscheduled visits |
|
| Funding | Supported in part by the Community Service Program of Kaiser Foundation Hospitals. Monitoring devices provided by Advanced Medical Systems | |
| Notes | Study reports findings for standard care group comparison (not included in review analyses) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. "patients were randomized into two groups, the home uterine monitoring group...and the education‐palpation group". Singleton and twin gestations not randomised separately. No comparisons of demographic data provided |
| Allocation concealment (selection bias) | High risk | No details provided. Nurses may have been aware of some group assignment because they were asked to "not analyze or respond to" errant transmissions made by women in the education‐palpation group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | For the control group "home uterine monitoring tracings were not analyzed or used in patient management". Participants not aware of group assignment. Nurses only aware which participants had transmitted and could not analyse uterine activity data for the control group (unless participants accidentally transmitted to a different monitor, in which case the nurse did not respond to the tracing) "The charts of all patients in the (control) group who experienced preterm labour were reviewed and in no case did it appear that a patient in the (control) group was referred by a nurse for increased uterine activity detected by one of these accidentally unblinded tracings" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Both groups discussed possible signs and symptoms of preterm labour with nurses and monitoring (experimental) group additionally discussed activity tracings with nurses. 1 outcome (number of unscheduled visits) therefore not objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes obtained for 247 of the 251 women who consented. Singleton and twin gestation data reported separately. |
| Selective reporting (reporting bias) | Unclear risk | Some data also provided about number of unscheduled visits, but for singletons only |
| Other bias | High risk | Figures for EP group, neonatal outcome unclear for singleton gestations. Authors state 16.4%, but this equates to 11.5 infants(?). Unclear whether other data flaws exist |
Dyson 1998.
| Methods | Randomised controlled trial with 3 arms | |
| Participants | Women receiving prenatal care at Kaiser Permanente clinics (n = 8), judged to be high risk. Enrolment between 24 and 30 weeks' gestation for pre‐existing risk factors, and before 33 weeks for risk factors developed during pregnancy. 2422 women enrolled, including 844 women with twins. Setting: Northern California, USA. Recruitment took place between July 1992 and August 1996. | |
| Interventions | Intervention group received daily contact with a nurse plus home uterine monitoring device for use twice daily for an hour each session. Data were reviewed immediately after transmission and the woman contacted if her threshold frequency was exceeded. All women in trial received education about symptoms and self‐palpation, and asked to record symptoms. Women in the weekly contact group were asked to assess themselves twice daily, and if persistent symptoms of preterm labour were detected, they were to call for professional advice. A nurse from the perinatal service centre called the women weekly to review their logs. For the daily contact group, the procedures were the same, but the nurse called daily | |
| Outcomes | Primary outcomes: perinatal mortality rate Secondary outcomes: 1) infant; preterm birth < 37 weeks, very preterm birth < 32 weeks; 2) prenatal; number of unscheduled visits, use of tocolysis. Singleton and twin gestation outcomes reported separately. Twin pregnancies analysed as if they had a single outcome | |
| Funding | Supported in part by a grant (01 41 9032) from the Sidney Garfield Memorial Fund | |
| Notes | Primary end point of the study was incidence of birth at less than 35 weeks' gestation, secondary end points (not included in the review) included cervical status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were assigned to one of three treatment groups in a ratio of 1:1:1 with the use of a computer‐generated randomization sequence." Randomisation stratified according to status (twin or at‐risk singleton) and according to treatment centre "to control for possible differences in treatment philosophy" (there were 8 tertiary centres for preterm labour care). Authors state no statistically significant differences in the demographic and risk factors for the 3 groups |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether a central randomisation office was used, no details of the implementation of randomisation at different centres |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women and perinatal nurses aware of allocation but "instructed not to inform the obstetricians of the group assignments. The women and the perinatal service nurses were also instructed not to divulge the method of detecting uterine activity when they reported increased uterine activity to the obstetricians". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Perinatal nurse contact could affect prenatal outcomes (unscheduled visits, and also, therefore, tocolysis). 3 outcomes objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 2422 women assigned completed the study but 93 (4% of those randomised) did not receive the surveillance to which they had been assigned |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Authors state the study has a power of more than 95% to detect a 1 cm difference between groups in cervical dilatation at the time of preterm labour diagnosis for all study participants Women in home uterine monitoring, and daily contact groups complied with at least 1 daily session 86% of the time, weekly contact compliance less at 79% of the time |
Hill 1990a.
| Methods | Randomised controlled trial | |
| Participants | 299 women at risk for preterm labour enrolled from 4 tertiary care centres in the USA (n = 155 experimental, n = 144 control). Women were between 20 and 34 weeks' gestational age at entry. Participants in Watson 1990 (n = 86) had been successfully treated for preterm labour. Knuppel 1990a participants (n = 45) were twin gestations from 4 centres (presumably the same as the Hill 1990b report). Knuppel 1990b mentions enrolment of 385 women at risk for preterm labour Setting: USA, dates not generally provided, but late 1980s assumed, Knuppel 1990a report indicates 1987 ‐ 1988 | |
| Interventions | Intervention group used home uterine monitoring device, twice daily for an hour, and transmitted data to the centre. Perinatal nurses contacted the women daily, women also encouraged to call if an emergency problem was suspected. Both intervention and control groups received education in symptoms of preterm labour and self‐palpation. The control group were instructed to contact their physician if they became aware of any persistent sign of premature labour. The description of the Hill 1990a and Knuppel 1990b protocol appears similar In the Watson 1990 report, the control group received standard home management for the institution |
|
| Outcomes | Primary outcomes: see notes Secondary outcomes: 1) infant; preterm birth < 37 weeks (but analysis focuses on preterm labour group); 2) prenatal; number of unscheduled visits | |
| Funding | Supported in part by a medical service grant from Tokos Medical Corporation and Vicksburg Hospital Medical Foundation | |
| Notes | Study examined other outcomes not included in review, e.g. cervical status at first episode of preterm labour. Several reports associated with this study (e.g.Bentley 1990 and Martin 1990 discuss rationale for methods used), but it is unclear about extent of subgroup analysis. Keirse 1993 provides some additional details on procedures based on information obtained after publication of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided; "patients were assigned randomly". No comparison of demographic or risk factors in tables (apart from Watson 1990), authors state these were similar between the groups |
| Allocation concealment (selection bias) | Low risk | No details provided on implementation of randomisation procedures in the main study report, although Keirse 1993 mentions that one of the main investigators for Hill 1990a stated that sealed opaque envelopes were used and that randomisation was conducted separately for women, with and without an episode of preterm labour in the current pregnancy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women aware of their assignment. Nurses in the monitoring centre also aware. Unclear whether hospital staff were aware |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Perinatal nurses in the monitoring centre could influence prenatal outcomes (unscheduled visits) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis (Hill 1990a) excluded 25 (13 experimental, 12 control) withdrawals, and participants delivered for fetal or maternal medical complications (15 experimental, 14 control). Also excluded from some of the analyses were 13 participants in the experimental group who did not comply fully with the monitoring regimens. Women ceased to participate in the study when they reached 37 weeks' gestation Similarly Knuppel 1990a only provides data on the women who experienced preterm labour, and 13 of 58 enrolled were excluded from data analysis. Knuppel 1990b excluded 71 of 385 enrolled |
| Selective reporting (reporting bias) | High risk | Very unclear how the various reports for this study relate to one another, with discrepancies in the data |
| Other bias | High risk | No power calculation is reported. Authors (Hill 1990a) state the distribution of risk factors and demographic characteristics across both groups, but no tables are provided Participating physicians used the preterm labour protocol in place at the respective institution. This may account for some differences among the reports included within this multi‐site study, but it is unclear how many women in total were enrolled in the multi‐site study, and how the subgroup analysis was organised and reported |
Iams 1987.
| Methods | Randomised controlled trial, in 2 parts | |
| Participants | Women at risk of preterm labour (n = 157 year 1, n = 152 year 2) were recruited from area physicians in prenatal clinic (n = 205 experimental, n = 104 control). All women were between 20 and 34 weeks' gestational age at entry, none had experienced preterm labour prior to enrolment. Area physicians recruited 240, OSU 69 women Setting: Ohio, USA, and the Ohio State University (OSU), 1986 ‐ 1987 | |
| Interventions | Intervention group used home uterine monitoring device, and a perinatal nurse from the monitoring centre called daily to transmit and interpret uterine activity data. The control group received education in self‐palpation, and were asked to record contractions of 1 hour twice daily. They were contacted on weekdays by a perinatal nurse from the monitoring centre to discuss recorded contractions. Both groups were instructed to seek professional support if they experienced persistent symptoms above their baseline | |
| Outcomes | Primary outcomes: see notes. Secondary outcomes: 1) infant; preterm birth < 37 weeks; 2) prenatal; use of tocolysis (treated and prophylactic) | |
| Funding | Supported by a grant from the Tokos Medical Corporation (supplier of the monitoring device) and by March of Dimes Birth Defects Foundation Grant no 2‐1987/C‐185 | |
| Notes | Study measured other outcomes not included in protocol, e.g. preterm birth < 35 weeks. Study end point was number of women reaching 35 and 37 weeks at delivery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence not described. Authors comment " unfortunately did not stratify random assignment...within risk factors". A 2 to 1 (experimental:control) scheme applied. More women with multiple gestations allocated to the control group in both years of the study |
| Allocation concealment (selection bias) | Unclear risk | No details of the implementation of the randomisation procedures provided. Author comment "physicians who enrolled their patients in the study often forgot which study group the patient was in, suggesting they perceived similarly the care received by both groups" (Iams 1987) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women aware of allocation. Authors comment that "monitoring centre staff were aware of the crude preterm birth rates for both groups as the study progressed". Primary perinatal nurses aware of group allocation, and had participants in both groups. Authors comment that participating physicians were visited at least once to reinforce protocols. There was an apparent learning curve phenomenon among nursing staff over the course of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Preterm birth an objective outcome, but use of tocolysis not objective if perinatal nurses aware of group allocation, as they could influence visits to physicians |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors report significant differences in noncompliance between years 1 and 2 of the study. In year 1 there were 15 withdrawals (8.4% of experimental, 12% of controls) from a total of 157 enrolled, while in year 2 there were 28 withdrawals (12.2% of experimental, 29.6% of controls) from a total of 152 enrolled. Of the 309 women recruited, 266 completed the study (n = 184 experimental, n = 82 control) |
| Selective reporting (reporting bias) | Low risk | No gaps evident |
| Other bias | Unclear risk | Authors report a statistically significant decline in preterm births < 37 weeks overall from year 1 to year 2, which is not apparently correlated with changes in risk factors or demography or physician behaviour. Authors suggest that "something the nurses do in the course of their contact with the patient may actually inhibit the development of preterm labour" Authors report estimating that to detect a 30% reduction in deliveries < 37 weeks, 230 participants were required in each group to achieve power of 80%, 1‐tailed P of 0.05 |
Iams 1990.
| Methods | Randomised controlled trial | |
| Participants | Women with singleton gestations (n = 76) who had been successfully treated for preterm labour were recruited from private, transport and clinic populations served by the Centre. 2 to 1 allocation used with 46 in experimental group and 21 in control. Setting: Ohio State University tertiary perinatal centre, USA. Date not given in paper directly but authors state trial running concurrently with another trial (1986 ‐ 1987) | |
| Interventions | Intervention group used the home uterine activity monitoring device to record contractions twice daily for 1 hour. Staff at the monitoring centre contacted the women daily for transmission and reporting of activity data. Both groups received education in signs and symptoms of preterm labour from the centre staff. The control group performed self‐palpation for 1 hour twice daily. Centre staff phoned every weekday for reports and, if needed, at weekends. Both groups were instructed to seek professional support if they experienced persistent symptoms above their baseline. Nursing staff at the centre also contacted physicians sometimes | |
| Outcomes | Primary outcomes: see notes Secondary outcomes: 1) infant: N/A 2) prenatal; use of tocolysis | |
| Funding | Supported by the Tokos Medical Corporation and the March of Dimes | |
| Notes | Trial discontinued as the similarities between this and a companion trial (Iams 1987, above) were confusing participating physicians. Study end points in this trial were preterm births < 36 weeks, and parenteral tocolysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. "subjects were assigned randomly in a ratio of 1:2 " (control: experimental) No comparison of demographic and risk factors presented in tables |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation procedures provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors note multiple physicians and hospitals involved. Women and monitoring centre staff aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Tocolysis outcome could be affected by advice given by monitoring centre staff, who were aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Frequent contact permitted assessment of compliance. Withdrawal statistically significantly greater in the control group as 6 of 27 withdrew (22%) compared to 3 of 49 (6%) in experimental group. 67 out of 76 participants completed the study |
| Selective reporting (reporting bias) | High risk | Authors state all births before 37 completed weeks reviewed in detail, but data only provided for births before 36 weeks (defined as preterm) and end point different from companion trial |
| Other bias | High risk | Authors suggest the trial was underpowered |
Lyons 1990.
| Methods | Randomised controlled trial | |
| Participants | Women at risk of preterm birth in a dependent military population, allocated to experimental (n = 31) and control (n = 31) groups Setting: USA. Dates of recruitment not clear. | |
| Interventions | Women in the experimental group transmitted 1 hour of uterine activity data twice daily to the diagnostic centre, and women in the control were monitored weekly at the diagnostic centre. Subjective information obtained daily by nurses for the experimental group | |
| Outcomes | Primary outcomes: 1) prenatal, number of days in hospital antenatally | |
| Funding | No details provided in conference abstract | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomized prospective study was done....Sixty two patients at risk for preterm delivery were randomly assigned to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation in conference abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Subjective information was obtained by trained nursing personnel on a daily basis" in intervention group, "at each clinic visit" in control group, and "evaluated by the responsible physicians." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Women and nursing staff aware of allocation and prenatal outcome (number of days in hospital antenatally) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It appears that all women completed the study, but difficult to state for sure |
| Selective reporting (reporting bias) | Unclear risk | Few details provided in conference abstract |
| Other bias | Unclear risk | Few details provided in conference abstract |
Morrison 1987.
| Methods | Quasi‐randomised controlled trial | |
| Participants | Women (n = 75) supported through Medicaid, and judged at high risk for preterm labour, were identified as eligible from the clinic over a 9‐month period, between 14 and 24 weeks' gestational age. Of the 75 identified, 69 were randomised (n = 35 experimental, n = 34 control) Setting: University of Mississippi obstetric clinic, USA, 1987 or earlier | |
| Interventions | Intervention group used a home uterine activity monitoring device, twice daily for an hour, and data transmitted to a monitoring centre (with daily phone contact). If other symptoms developed the women were told to re‐monitor and to contact the study nurse. Both intervention and control groups received education in signs of preterm labour and were instructed to come to the hospital if symptoms developed. Both groups were examined once every 2 weeks. The control group were contacted by phone twice a week | |
| Outcomes | Primary outcomes: see notes. Secondary outcomes: 1) infant; preterm birth 37 weeks; 2) prenatal; number of unscheduled visits, use of tocolysis/admission for preterm labour | |
| Funding | Supported in part by the Vicksburg Hospital Medical Foundation | |
| Notes | Some cost analysis figures given. This study is related to a later and larger cost analysis study of 130 women, supported by Medicaid, who were recruited from clinics in Mississippi and Michigan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Tables show demographic and risk characteristics of the groups similar. Randomisation based on last digits of hospital number |
| Allocation concealment (selection bias) | High risk | Randomisation based on last digits of hospital number |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women aware and nursing staff aware of allocation. Authors state that women admitted for observation "were observed by staff unaware of the participants' involvement in the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors state that women admitted for observation "were observed by staff unaware of the participants' involvement in the study". Extent of nursing contact varied between the groups, and nursing advice could affect prenatal outcomes (number of unscheduled visits, use of tocolysis/admission for preterm labour) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women withdrew from 69 randomised, data obtained from 67 women |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting, several related publications on this trial |
| Other bias | Unclear risk | Authors state tocolysis protocol the same for both groups |
Nagey 1993.
| Methods | Randomised controlled trial | |
| Participants | Women who had been treated successfully for preterm labour, between 20 and 34 weeks' gestation, were recruited from University medical system, and randomised (n = 59) to experimental (n = 28) and control (n = 29) Setting: University of Maryland, USA, in the early 1990s | |
| Interventions | Intervention group used the home uterine monitoring device twice daily and transmitted data to the perinatal monitoring centre. Both intervention and control groups received education in signs of preterm labour and were instructed to call or return to hospital if signs were persistent. All women were seen once weekly in the office, and all women were given prescriptions for terbutaline | |
| Outcomes | Primary outcomes: preterm birth < 34 weeks Secondary outcomes: 1) infant; preterm birth < 37 weeks, very preterm birth < 32 weeks | |
| Funding | Supported in part by a grant from Tokos Medical Inc (supplier of the device) and by an Interagency project agreement project grant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Pseudo‐random number generator" used for randomisation Notes that 2 additional participants, initially randomised, were excluded from analysis as they were not found eligible medically |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors report that neither the women nor their caregivers were blinded to the allocation group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes preterm or term birth |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the experimental group, 4 women never left hospital and never received the intervention. 1 control participant lost to follow‐up. 2 participants initially randomised excluded from the analysis for medical reasons |
| Selective reporting (reporting bias) | Unclear risk | Authors comment that analysis on available cases did not change direction of significance (analysis presented as ITT) |
| Other bias | High risk | Power calculations (1‐sided alpha of 0.05. 80% power) based on incidences of preterm delivery of 0.45 in routine care and 0.15 in the monitoring group (based on previous randomised controlled trial, Morrison 1987). Estimated that 28 required in each arm, but authors also suggest that the trial may be underpowered |
Porto 1987.
| Methods | Randomised controlled trial with 3 arms | |
| Participants | Women "at high risk for preterm birth" allocated to 1) monitoring group with active analysis of uterine activity data (n = 44); 2) monitoring group, but with no active analysis of data (n = 46); 3) control group (standard high‐risk care) (n = 46). Porto 1990 appears to refer to same study but the total number stated is 148, not 136. Setting: USA, from May 1985 to September 1986, USA. | |
| Interventions | Women doing the monitoring transmitted 2 hours of data daily. For the active analysis group, contraction > 4 per hour referred for evaluation following a protocol. All women appear to have had daily phone contact with the study centre | |
| Outcomes | None of relevance to the review (only reports preterm birth < 36 weeks) | |
| Funding | Tradename of device mentioned | |
| Notes | This study is within scope, but not included in data tables or meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we undertook a randomized prospective study"; "patients were randomly assigned to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | No details of the method of implementation of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 1 of the 2 control groups received home uterine monitoring "but uterine activity data was blinded to patient management", other control group did not receive home uterine monitoring. Both control groups had daily participant telephone contact. Monitored participants deemed at risk "were evaluated at the hospital for possible preterm labour by strict protocol" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome of preterm birth objective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 1 outcome reported. Authors state that 7 noncompliant women were removed from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only 1 outcome reported in the abstract |
| Other bias | Unclear risk | Few details provided in conference abstract |
Scioscia 1988.
| Methods | Randomised controlled trial | |
| Participants | Women judged at risk for preterm labour were recruited from private and clinic populations, USA sites, and randomly allocated to experimental (home uterine monitoring) (n = 38) and control (self‐palpation) (n = 34) groups. Setting: USA. Dates of recruitment not reported. | |
| Interventions | All women monitored contractions for 1 hour daily, all women had daily contact with nurse or physician. Uterine monitoring data used to manage tocolytic dose | |
| Outcomes | Primary outcomes: preterm birth < 34 weeks Secondary outcomes: 1) infant; preterm birth < 37 weeks; 2) prenatal; number of unscheduled visits | |
| Funding | Manufacturer of device mentioned, no further details | |
| Notes | Not included in data tables and meta‐analysis as the results cannot be back‐calculated from the percentages provided (no sensible interpolations possible) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we performed a randomized clinical trial", authors state that groups comparable for risk factors, preterm delivery, referring physician and mean gestational age on entry |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation provided in conference abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All women aware of allocation, and all had daily contact with a perinatal nurse or physician, who would therefore be aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Prenatal outcome (number of unscheduled visits) would be affected by nature of advice from clinician who was aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors state that 5 participants were removed from analysis for calculation of mean gestational age; unclear whether this covers other outcome data. Raw frequency data impossible to back‐calculate from the percentages provided. |
| Selective reporting (reporting bias) | High risk | Few details provided in conference abstract; authors state that number of "emergency visits were similar" but no figures provided |
| Other bias | Unclear risk | Few details provided in conference abstract |
Wapner 1995.
| Methods | Randomised controlled trial | |
| Participants | Women (24 to 36 weeks' gestation) with a history of preterm delivery were recruited and randomised into monitored group (experimental) (n = 107) and control group (n = 111) Setting: 4 sites in the USA. Recruitment between February 1991 and February 1993 | |
| Interventions | Intervention group received routine high‐risk obstetric care and transmitted monitoring data twice daily to the receiving centre. The control group received routine high‐risk obstetric care. Both groups received education in self‐palpation and indications of preterm labour, and instructions on dealing with such indications. All participants were scheduled for routine office visits for evaluation at least once every 4 weeks until 30 weeks' gestation, once every 2 weeks (30 to 35 weeks' gestation) and weekly thereafter | |
| Outcomes | Secondary outcomes: 1) infant: admission to neonatal ICU 2) prenatal: Number of antenatal hospital visits | |
| Funding | Authors state "supported in part by a grant from Healthdyne Perinatal Services", manufacturers of the tocodynamometer uterine monitoring device | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate blocked random number sequences used at each study site. Randomisation carried out by study personnel not responsible for participant care |
| Allocation concealment (selection bias) | Low risk | Group assignments "carried out by study personnel not responsible for patient care, by opening consecutive numbered envelopes" at each site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women aware of allocation, and authors state that women were instructed "not to inform caretakers of their use or non‐use of the monitor" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Known allocation may have affected 1 outcome (number of antenatal hospital visits) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of withdrawals = 37 (17.8%). Unclear whether monitored group subject to more withdrawals; authors state that 9 women enrolled into monitoring group, but never received monitoring. Authors state that there were no significant differences in the "enrolled population" between monitored and control groups for mean scheduled and unscheduled office visits |
| Selective reporting (reporting bias) | High risk | Neonatal and pregnancy outcomes only reported for women who experienced preterm labour (n = 43, of which there were 21 monitored, and 22 control) |
| Other bias | Unclear risk | Authors state "neonatal and pregnancy outcomes not chosen as study end points in the design and sample size calculation". Sample size calculated for cervical dilatation at the time of diagnosis of preterm labour, the study endpoint |
ICU: intensive care unit ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bell 1992 | Not in scope, does not mention home uterine monitoring |
| Birnie 2000 | This paper and the others relating to this study (Monincx 1997; Monincx 2001) are all out of scope, and are therefore excluded. Midwives did the home monitoring and it was fetal heart rate that was transmitted. Uterine activity monitoring is not mentioned |
| Blondel 1988 | Not in scope, home visiting only |
| Blondel 1990 | Not in scope, deals with home visiting only, mentioned in discussion |
| Dawson 1999 | Not in scope, fetal monitoring only |
| Gookin 1994 | This is a letter, no data provided of relevance |
| Goulet 1999 | Not in scope, home visiting only |
| Goulet 2001 | Not in scope, home visiting only |
| Iedema 1994 | Not in scope, domiciliary care only |
| Iedema‐Kuiper 1996 | Not in scope, domiciliary care only |
| Merkatz 1991 | Not in scope, review discussing contribution of nursing care to monitoring |
| Monincx 1997 | Not in scope, see Birnie 2000 (above) |
| Monincx 2001 | Not in scope, see Birnie 2000 (above) |
| NCT02379351 | Not in scope, remote fetal monitoring only |
| O'Neil 1987 | Not in scope (personal communication comment) |
| Ogburn 1993 | Notice of trial registration data, and not clear whether trial was ever completed. No evidence found |
| Reece 1992 | Not in scope, fetal monitoring |
| Spira 1981 | Not in scope, domiciliary care only |
| Spira 1986 | Not in scope, domiciliary care only |
| Su 2002 | Not in scope, fetal monitoring |
| Tõrõk 1994 | The trial appears as if it should be in scope, but there is no report of any clinical data. The studies only describe the technology |
Differences between protocol and review
We have updated the methods to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Pregnancy and Childbirth Group's methodological guidelines.
In the protocol we stated that we intended to carry out the following subgroup analyses: singleton pregnancy; multiple pregnancy; gestational age at which home uterine activity monitoring (HUAM) began; type of HUAM used; reason HUAM was used. We planned to use the following outcomes: perinatal mortality and preterm birth less than 34 weeks. The studies provided only data on singleton pregnancy and multiple pregnancy, and only one study was involved. For this update, we added the following outcomes to the methods for subgroup analysis:
preterm birth less than 37 weeks;
respiratory distress syndrome;
number of unscheduled antenatal visits.
Contributions of authors
Rosemary Currell and Christine Urquhart jointly worked on the protocol, with assistance from Liz Callow for literature searching and development of the search strategy. Francoise Harlow advised on the protocol and commented on the draft review. The idea for the review emerged from a systematic review of telemedicine (for the Cochrane Effective Practice and Organisation of Care Group) which identified a discrete set of studies on home uterine monitoring that were more suitable for consideration as a separate review for the Cochrane Pregnancy and Childbirth Group. Both Rosemary Currell and Christine Urquhart were review authors on the telemedicine review.
Rosemary Currell and Christine Urquhart jointly worked on the 2014 and 2016 updates with contributions from Liz Callow (search strategy development) and Francoise Harlow (contribution to background).
Christine Urquhart is the contact author and guarantor for this review.
Sources of support
Internal sources
Aberystwyth University, UK.
External sources
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
2014 update
National Institute for Health Research (NIHR), UKNIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Christine Urquhart was a co‐author with Rosemary Currell on a Cochrane Review of telemedicine for the EPOC group.
Rosemary Currell: University of Wales Swansea received a grant from the Welsh Office of Research and Development for work on the Cochrane Review of telemedicine (published 2000), from which the current review originated.
Francoise Harlow: none known.
Liz Callow: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Blondel 1992 {published data only}
- Blondel B, Bréart G. Home uterine activity monitoring and prevention of preterm delivery. Journal of Perinatal Medicine 1992;20(Suppl 1):93. [Google Scholar]
- Blondel B, Bréart G, Berthoux Y, Berland N, Mellier G, Rudigoz RC, et al. Home uterine activity monitoring in France: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 1992;167(2):424‐9. [DOI] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown HL, Britton KA, Brizendine EJ, Hiett AK, Ingram D, Turnquest MA, et al. A randomized comparison of home uterine activity monitoring in the outpatient management of women treated for preterm labor. American Journal of Obstetrics and Gynecology 1999;180(4):798‐805. [DOI] [PubMed] [Google Scholar]
CHUMS 1995 {published data only}
- Collaborative. A multicenter randomized controlled trial of home uterine monitoring (HUAM): Active vs SHAM device. American Journal of Obstetrics and Gynecology 1995;172:253. [DOI] [PubMed] [Google Scholar]
- The Collaborative Home Uterine Monitoring Study (CHUMS) Group. A multicenter randomized controlled trial of home uterine monitoring: active versus sham device. American Journal of Obstetrics and Gynecology 1995;173(4):1120‐7. [DOI] [PubMed] [Google Scholar]
Corwin 1996 {published data only}
- Corwin MJ, Mou SM, Sunderji SG, Gall S, How H, Patel V, et al. Multicenter randomized clinical trial of home uterine activity monitoring: pregnancy outcomes for all women randomized. American Journal of Obstetrics and Gynecology 1996;175(5):1281‐5. [DOI] [PubMed] [Google Scholar]
- Mou S, Sunderji S, How H, Cummisky K, Kayne H, Gall S, et al. Use of home uterine activity monitoring in high risk women significantly improves gestational age at delivery. Pediatric Research 1990;27:241A. [Google Scholar]
- Mou SM, Sunderji SG, Gall S, How H, Patel V, Gray M, et al. Multicenter randomized clinical trial of home uterine activity monitoring for detection of preterm labor. American Journal of Obstetrics and Gynecology 1991;165(4 Pt 1):858‐66. [DOI] [PubMed] [Google Scholar]
- Sunderji S, Gall S, Mou S, How H, Corwin M. Earlier detection of preterm labor: multicenter prospective randomized clinical trial of home uterine activity monitoring. American Journal of Obstetrics and Gynecology 1991;164:259. [DOI] [PubMed] [Google Scholar]
Dyson 1991 {published data only}
- Dyson DC, Crites Y, Ray D, Armstrong MA, Wellman E. Preterm birth prevention ‐ the role of education ‐ palpation vs home uterine monitoring in non‐multiple gestations. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:38.
- Dyson DC, Crites Y, Ray D, Armstrong MA, Wellman E, Dyson J. Preterm birth prevention in twin gestations ‐ the role of education ‐ palpation vs home uterine monitoring. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:167.
- Dyson DC, Crites YM, Ray DA, Armstrong MA. Prevention of preterm birth in high‐risk patients: the role of education and provider contact versus home uterine monitoring. American Journal of Obstetrics and Gynecology 1991;164(3):756‐62. [DOI] [PubMed] [Google Scholar]
- Dyson DC, Wellman E, Dyson J, Armstrong MA. The role of home uterine monitoring in the prevention of preterm birth in high risk patients. Proceedings of 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3‐6; Las Vegas, Nevada, USA. 1988:4.
Dyson 1998 {published data only}
- Dyson D, Danbe K, Bamber J, Crites Y, Field R, Maier J, et al. A multicenter randomized trial of three levels of surveillance in patients at risk of preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S30. [Google Scholar]
- Dyson DC, Danbe KH, Bamber JA, Crites YM, Field DR, Maier JA, et al. Monitoring women at risk for preterm labor. New England Journal of Medicine 1998;338(1):15‐9. [DOI] [PubMed] [Google Scholar]
- Regenstein AC, Dyson DC, Danbe K, Bamber J, Field DR. Parity and the outcome of twin gestations. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S55. [Google Scholar]
Hill 1990a {published data only}
- Bentley DL, Bentley JL, Watson DL, Welch RA, Martin RW, Gookin KS, et al. Relationship of uterine contractility to preterm labor. Obstetrics and Gynecology 1990;76(1 Suppl):36S‐38S. [PubMed] [Google Scholar]
- Hill WC, Fleming AD. Martin RW, Hamer C, Knuppel RA, Lake MF, et al. Home uterine activity monitoring is associated with a reduction in preterm birth. Obstetrics and Gynecology 1990;76(1 Suppl):13S‐17S. [PubMed] [Google Scholar]
- Keirse MJ, Hoven M. Reanalysis of a multireported trial on home uterine activity monitoring. Birth 1993;20(3):117‐22. [DOI] [PubMed] [Google Scholar]
- Knuppel RA, Lake MF, Watson DK, Welch RA, Hill WC, Fleming AD, et al. Preventing preterm birth in twin gestation: home uterine activity monitoring and perinatal nursing support. Obstetrics and Gynecology 1990;76(1 Suppl):24S‐27S. [PubMed] [Google Scholar]
- Knuppel RA, Lake MF, Watson DL, Welch RA, Hill WC, Fleming ADF, et al. The contribution of symptomatology and/or uterine activity to the incidence of unscheduled visits. Obstetrics and Gynecology 1990;76(1 Suppl):28S‐31S. [PubMed] [Google Scholar]
- Martin RW, Gookin KS, Hill WC, Fleming AD, Knuppel RA, Lake MF, et al. Uterine activity compared with symptomatology in the detection of preterm labor. Obstetrics and Gynecology 1990;76(1 Suppl):19S‐23S. [PubMed] [Google Scholar]
- Watson DL, Welch RA, Mariona FG, Lake MF, Knuppel RA. Marting RW, et al. Management of preterm labor patients at home: does daily uterine activity monitoring and nursing support make a difference?. Obstetrics and Gynecology 1990;76(1 Suppl):32S‐35S. [PubMed] [Google Scholar]
Iams 1987 {published data only}
- Iams JD, Johnson FF, O'Shaughnessy RW. A prospective random trial of home uterine activity monitoring in pregnancies at increased risk of preterm labor. Proceedings of 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3‐6; Las Vegas, Nevada, USA. 1988:8.
- Iams JD, Johnson FF, O'Shaughnessy RW. A prospective random trial of home uterine activity monitoring in pregnancies at increased risk of preterm labor Part II. American Journal of Obstetrics and Gynecology 1988;159(3):595‐603. [DOI] [PubMed] [Google Scholar]
- Iams JD, Johnson FF, O'Shaughnessy RW, West LC. A prospective random trial of home uterine activity monitoring in pregnancies at increased risk of preterm labor. American Journal of Obstetrics and Gynecology 1987;157(3):638‐43. [DOI] [PubMed] [Google Scholar]
Iams 1990 {published data only}
- Iams JD, Johnson FF, Hamer C. Uterine activity and symptoms as predictors of preterm labor. Obstetrics & Gynecology 1990;76(1 Suppl):42S‐46S. [PubMed] [Google Scholar]
- Iams JD, Johnson FF, O'Shaughnessy RW. Ambulatory uterine activity monitoring in the post‐hospital care of patients with preterm labor. American Journal of Perinatology 1990;7(2):170‐3. [DOI] [PubMed] [Google Scholar]
Lyons 1990 {published data only}
- Lyons JD, Robertson AW. Uterine activity monitoring in the diagnosis and treatment of preterm labor: a randomized prospective comparison of clinic and home tocodynamometry services. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:182.
Morrison 1987 {published data only}
- Morrison JC, Martin JD Jr, Martin RW, Gookin KS, Wiser WL. Prevention of preterm birth by ambulatory assessment of uterine activity: a randomized study. American Journal of Obstetrics and Gynecology 1987;156(3):536‐43. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Martin JN Jr, Martin RW, Hess LW, Gookin KS, Wiser WL. Cost effectiveness of ambulatory uterine activity monitoring. International Journal of Gynecology & Obstetrics 1989;28(2):127‐32. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Martin JN, Martin RW, Hess LW, Gookin K, Wiser WL. A program of uterine activity monitoring and its effect on neonatal morbidity. Journal of Perinatology 1988;8(3):228‐31. [PubMed] [Google Scholar]
- Morrison JC, Martin JN, Martin RW, Hess LW, Gookin KS, Wiser WL. Cost effectiveness of ambulatory uterine monitoring to prevent preterm birth. Proceedings of 15th Annual Meeting of Southern Perinatal Association; 1988; New Orleans, USA. 1988.
Nagey 1993 {published data only}
- Nagey DA, Bailey‐Jones C, Herman AA. Randomized comparison of home uterine activity monitoring and routine care in patients discharged after treatment for preterm labor. Obstetrics and Gynecology 1993;82(3):319‐23. [PubMed] [Google Scholar]
- Nagey DA, Bailey‐Jones C, Herman AA. Randomized trial of home uterine activity monitoring (HUAM) vs routine care in patients discharged after treatment for preterm labor. American Journal of Obstetrics and Gynecology 1993;168:374. [PubMed] [Google Scholar]
- Olson G, Nagey D. Cost effectiveness of home uterine activity monitoring. American Journal of Obstetrics and Gynecology 1993;168:374. [Google Scholar]
Porto 1987 {published data only}
- Porto M. Home uterine activity monitoring: essential tool or expensive accessory?. Contemporary Ob/Gyn 1990;35:114‐9. [Google Scholar]
- Porto M, Nageotte P, Hill O, Keegan KA, Freeman RK. The role of home uterine activity monitoring in the prevention of preterm birth. Proceedings of 7th Annual Meeting of the Society of Perinatal Obstetricians; 1987 Feb 5‐7; Lake Buena Vista, Florida, USA. 1987:96.
Scioscia 1988 {published data only}
- Scioscia A, Nickless N, Hodgson P, Belanger K, Hobbins JC. A randomized clinical trial of outpatient monitoring of uterine contractions in women at risk for preterm delivery: self‐palpation vs tocodynamometer. Proceedings of 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3‐6; Las Vegas, Nevada, USA. 1988:3.
Wapner 1995 {published data only}
- Wapner RJ, Cotton DB, Artal R, Librizzi RJ, Ross MG. A randomized multicenter trial assessing a home uterine activity monitoring device used in the absence of daily nursing contact. American Journal of Obstetrics and Gynecology 1995;172(3):1026‐34. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bell 1992 {published data only}
- Bell RJ, Lester AR, Lumley J. Antenatal uterine activity monitoring of women at increased risk of preterm labour. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1992;46(2‐3):65‐72. [DOI] [PubMed] [Google Scholar]
Birnie 2000 {published data only}
- Birnie E, Monincx WM, Zondervan HA, Bossuyt PM, Bonsel GJ. Comparing treatment valuations between and within subjects in clinical trials: does it make a difference?. Journal of Clinical Epidemiology 2000;53(1):39‐45. [DOI] [PubMed] [Google Scholar]
- Birnie E, Monincx WM, Zondervan HA, Bossuyt PM, Bonsel GJ. Cost‐minimization analysis of domiciliary antenatal fetal monitoring in high‐risk pregnancies. Obstetrics and Gynecology 1997;89(6):925‐9. [DOI] [PubMed] [Google Scholar]
Blondel 1988 {published data only}
- Blondel B, Llado J, Breart G. Prevention of preterm deliveries by home visiting system: results of a French randomized controlled trial. Advances in the Prevention of Low Birthweight: an International Symposium; 1988 May 8‐11; Cape Cod, Massachusetts, USA. 1988:141‐50.
Blondel 1990 {published data only}
- Blondel B, Bréart G, Llado J, Chartier M. Evaluation of the home‐visiting system for women with threatened preterm labor: results of a randomized controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1990;34(1‐2):47‐58. [DOI] [PubMed] [Google Scholar]
Dawson 1999 {published data only}
- Dawson A, Cohen D, Candelier C, Jones G, Sanders J, Thompson A, et al. Domiciliary midwifery support in high‐risk pregnancy incorporating telephonic fetal heart rate monitoring: a health technology randomized assessment. Journal of Telemedicine and Telecare 1999;5(4):220‐30. [DOI] [PubMed] [Google Scholar]
Gookin 1994 {published data only}
- Gookin KS. Randomized comparison of home uterine activity monitoring and routine care in patients discharged after treatment for preterm labor [letter; comment]. Obstetrics and Gynecology 1994;83(1):159‐60. [PubMed] [Google Scholar]
Goulet 1999 {published data only}
- Goulet C, Gevry H, Gauthier R, Aita M, Lepage L, Polomeno V. Randomized trial of two health care deliveries during premature labor. [French] [Essai randomise de deux modes de prestation des soins lors de travail premature.]. Recherche en Soins Infirmiers 1999;December(59):45‐56. [PubMed] [Google Scholar]
Goulet 2001 {published data only}
- Goulet C, Gevry H, Lemay M, Gauthier RJ, Lepage L, Fraser W, et al. A randomized clinical trial of care for women with preterm labour: home management versus hospital management. Canadian Medical Association Journal 2001;164(7):985‐91. [PMC free article] [PubMed] [Google Scholar]
Iedema 1994 {published data only}
- Iedema HR, Bruinse HW, Pal RS, Reuwer PJHM, Merkus JMWM, Alsbach GPJ. A randomised study of domiciliary antenatal care in high risk pregnancies: the effect of hospital admissions and fetal outcome. International Journal of Gynaecology and Obstetrics 1994;46:99. [Google Scholar]
Iedema‐Kuiper 1996 {published data only}
- Iedema‐Kuiper HR, Bruinse HW, Peddemors HE, Reuwer PJHM, Merkus JMWN, vdSalm PCM, et al. The Utrecht domicilliary care in high risk pregnancies study: the effect of hospital admission days, fetal and maternal outcome, maternal well being and cost effectiveness: a randomized study. Prenatal and Neonatal Medicine 1996;1(1 Suppl):10. [Google Scholar]
Merkatz 1991 {published data only}
- Merkatz RB, Merkatz IR. The contributions of the nurse and the machine in home uterine activity monitoring systems. American Journal of Obstetrics and Gynecology 1991;164(5 Pt 1):1159‐62. [DOI] [PubMed] [Google Scholar]
Monincx 1997 {published data only}
- Monincx WM, Zondervan HA, Birnie E, Ris M, Bossuyt PMM. High risk pregnancy monitored antenatally at home. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1997;75(2):147‐53. [DOI] [PubMed] [Google Scholar]
Monincx 2001 {published data only}
- Monincx WM, Birnie E, Zondervan HA, Bleker OP, Bonsel GJ. Maternal health, antenatal and at 8 weeks after delivery, in home versus in‐hospital fetal monitoring in high‐risk pregnancies. European Journal Obstetrics, Gynecology and Reproductive Biology 2001;184(1):197‐204. [DOI] [PubMed] [Google Scholar]
NCT02379351 {published data only}
- NCT02379351. Use of an in‐home non‐stress test device for remote fetal monitoring in a local high‐risk obstetric population. clinicaltrials.gov/ct2/show/NCT02379351 Date first received: 20 February 2015.
O'Neil 1987 {published data only}
- O'Neil G. Assessment of the value of abdominal pressure monitoring for one‐hour intervals between 20 and 32 weeks gestation. Personal communication 1987.
Ogburn 1993 {published data only}
- Ogburn PL. Trial to determine the best form of monitoring for preterm labor and preterm delivery prevention: home monitoring with Tokodynomometer vs daily home phone calls vs weekly cervical checks in patients at risk for preterm delivery. Personal communication 1993.
Reece 1992 {published data only}
- Reece EA, Hagay Z, Garofalo J, Hobbins JC. A controlled trial of self‐nonstress test vs assisted nonstress test in the evaluation of fetal well‐being. American Journal of Obstetrics and Gynecology 1992;166(2):489‐92. [DOI] [PubMed] [Google Scholar]
Spira 1981 {published data only}
- Spira N, Audras F, Chapel A, Debuisson J, Jacquelin C, Kirchhoffer C, et al. Domiciliary care of pathological pregnancies by midwives. Comparative controlled study on 996 women. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1981;10(6):543‐8. [PubMed] [Google Scholar]
Spira 1986 {published data only}
- Spira N. Evaluation of domiciliary care of pathological pregnancies by midwives. In: Papiernik E, Breart G, Spira N editor(s). Prevention of Preterm Birth. Vol. 138, Paris: INSERM, 1986:291‐308. [Google Scholar]
Su 2002 {published data only}
- Su F, Guo X. Clinical application of the expert type terminal of remote electronic fetal heart rate home monitoring system. Zhonghua Fu Chan Ke Za Zhi 2002;37(8):459‐61. [PubMed] [Google Scholar]
Tõrõk 1994 {published data only}
- Torok M. Hungarian tele‐medical preterm birth prevention project: a randomized multicenter clinical trial study. International Journal of Gynecology & Obstetrics 1994;46:58. [Google Scholar]
- Tõrõk M, Turi Z, Kovács F. Ten years' clinical experience with telemedicine in prenatal care in Hungary. Journal of Telemedicine and Telecare 1999;5 Suppl 1:S14‐S17. [DOI] [PubMed] [Google Scholar]
Additional references
Bentley 1990
- Bentley DL, Bentley JL, Watson DL, Welch RA, Martin RW, Gookin KS, et al. Relationship of uterine contractility to preterm labor. Obstetrics and Gynecology 1990;76(1 Suppl):36S‐38S. [PubMed] [Google Scholar]
Berghella 2008
- Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006843.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berghella 2013
- Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD007235.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2007
- CDC, Centers for Disease Control and Prevention. Preterm births ‐ United States, 2007. Morbidity and Mortality Weekly Report 2007;60(1):78‐9. [Google Scholar]
Colton 1995
- Colton T, Kayne HL, Zhang Y, Heeren T. A metaanalysis of home uterine activity monitoring. American Journal of Obstetrics and Gynecology 1995;173(5):1499‐505. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2015
- Conde‐Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2015;213(6):789‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Lau 2013
- Lau H, Rabotti C, Haazen N, Oei SG, Mischi M. Towards improving uterine electrical activity modeling and electrohysterography: ultrasonic quantification of uterine movements during labor. Acta Obstetricia et Gynecologica Scandinavica 2013;92(11):1323‐6. [DOI] [PubMed] [Google Scholar]
Dodd 2012
- Dodd JM, Crowther CA, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003927.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2013
- Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004947.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duley 2011
- Duley L, Bennett PR. Tocolysis for Women in Preterm Labour. RCOG Green‐top guidelines no. 1b. London: Royal College of Obstetricians and Gynaecologists, 2011. [Google Scholar]
Flenady 2014
- Flenady V, Wojcieszek AJ, Papatsonis DN, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 1992
- Grimes DA, Schulz KF. Randomized controlled trials of home uterine activity monitoring: a review and critique. Obstetrics and Gynecology 1992;79(1):137‐42. [PubMed] [Google Scholar]
Hadži‐Legal 2016
- Hadži‐Legal M, Markova AD, Stefanovic M, Tanturovski M. Combination of selected biochemical markers and cervical length in the prediction of impending preterm delivery in symptomatic patients. Clinical and Experimental Obstetrics & Gynecology 2016;43(1):154‐60. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 1990b
- Hill WC, Fleming AD. Martin RW, Hamer C, Knuppel RA, Lake MF, et al. Home uterine activity monitoring is associated with a reduction in preterm birth. Obstetrics and Gynecology 1990;76(1 Suppl):13S‐17S. [PubMed] [Google Scholar]
Hodnett 2010
- Hodnett ED, Fredericks S, Weston J. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD000198.pub2] [DOI] [PubMed] [Google Scholar]
Honest 2009
- Honest H, Forbes CA, Duree KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technology Assessment 2009;13(43):1‐334. [DOI] [PubMed] [Google Scholar]
Iams 2002
- Iams JD, Newman RB, Thom EA, Goldenberg RL, Mueller‐Heubach E, Moawa A, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. New England Journal of Medicine 2002;346(4):250‐5. [DOI] [PubMed] [Google Scholar]
ICSI 2002
- Institute for Clinical Systems Improvement. Home Uterine Activity Monitoring for Detection of Preterm Labor. Bloomington, MN: ICSI, 2002. [Google Scholar]
Keirse 1993
- Keirse MJ, Hoven M. Reanalysis of a multireported trial on home uterine activity monitoring. Birth 1993;20(3):117‐22. [DOI] [PubMed] [Google Scholar]
Knuppel 1990a
- Knuppel RA, Lake MF, Watson DK, Welch RA, Hill WC, Fleming AD, et al. Preventing preterm birth in twin gestation: home uterine activity monitoring and perinatal nursing support. Obstetrics and Gynecology 1990a;76(1 Suppl):24S‐27S. [PubMed] [Google Scholar]
Knuppel 1990b
- Knuppel RA, Lake MF, Watson DL, Welch RA, Hill WC, Fleming AD, et al. The contribution of symptomatology and/or uterine activity to the incidence of unscheduled visits. Obstetrics and Gynecology 1990b;76(1 Suppl):28S‐31S. [PubMed] [Google Scholar]
Lockwood 2001
Martin 1990
- Martin RW, Gookin KS, Hill WC, Fleming AD, Knuppel RA, Lake MF, et al. Uterine activity compared with symptomatology in the detection of preterm labor. Obstetrics and Gynecology 1990;76(1 Suppl):19S‐23S. [PubMed] [Google Scholar]
Maxwell 2001
- Maxwell CV, Amankwah KS. Alternative approaches to preterm labor. Seminars in Perinatology 2001;25(5):310‐5. [DOI] [PubMed] [Google Scholar]
McNamara 2015
- McNamara HC, Crowther CA, Brown J. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011200.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2014
- Neilson JP, West HM, Dowswell T. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD004352.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2005
- Newman RB. Uterine contraction assessment. Obstetrics & Gynecology Clinics of North America 2005;32(3):341‐67. [DOI] [PubMed] [Google Scholar]
Piso 2014
- Piso B, Zechmeister‐Koss I, Winkler R. Antenatal interventions to reduce preterm birth: an overview of Cochrane systematic reviews. BMC Research Notes 2014;7:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichmann 2008
- Reichmann JP. Home uterine activity monitoring: the role of medical evidence. Obstetrics and Gynecology 2008;112(1):325‐7. [DOI] [PubMed] [Google Scholar]
Reichmann 2009
- Reichmann JP. Home uterine activity monitoring: an evidence review of its utility in multiple gestations. Journal of Reproductive Medicine 2009;54(9):559‐62. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanchez‐Ramoz 2009
- Sanchez‐Ramos L, Delke I, Zamora J, Kaunitz AM. Fetal fibronectin as a short‐term predictor of preterm birth in symptomatic patients: a meta‐analysis. Obstetrics & Gynecology 2009;114(3):631‐40. [DOI] [PubMed] [Google Scholar]
Sharp 2013
- Sharp GC, Saunders PT, Norman JE. Computer models to study uterine activation at labor. Molecular Human Reproduction 2013;19(11):711‐7. [DOI] [PubMed] [Google Scholar]
Urquhart 2010
Van't Hooft 2015
- Van't Hooft J, Duffy JMN, Saade GR, Alfirevic Z, Meher S, Mol BWJ, et al. Core outcomes set for studies on primary prevention of preterm birth. Trials 2015;16(Suppl 1):11. [Google Scholar]
Vinken 2009
- Vinken MP, Rabotti C, Mischi M, Oei SG. Accuracy of the frequency‐related parameters of the electrohysterogram for predicting preterm delivery: a review of the literature. Obstetrical & Gynecological Survey 2009;64(8):529‐41. [DOI] [PubMed] [Google Scholar]
Vis 2009
- Vis JY, Wilms FF, Oudijk MA, Porath MM, Scheepers HC, Bloemenkamp KW, et al. Cost‐effectiveness of fibronectin testing in a triage in women with threatened preterm labor: alleviation of pregnancy outcome by suspending tocolysis in early labor (APOSTEL‐I trial). BMC Pregnancy Childbirth 2009;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1990
- Watson DL, Welch RA, Mariona FG, Lake MF, Knuppel RA. Marting RW, et al. Management of preterm labor patients at home: does daily uterine activity monitoring and nursing support make a difference?. Obstetrics and Gynecology 1990;76(1 Suppl):32S‐35S. [PubMed] [Google Scholar]
References to other published versions of this review
Urquhart 2012
- Urquhart C, Currell R, Harlow F, Callow L. Home uterine monitoring for detecting preterm labour. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006172.pub2] [DOI] [PubMed] [Google Scholar]


