Version Changes
Revised. Amendments from Version 1
Version 2 of our manuscript addresses the points from the peer-reviewers, whom we thank for their constructive feedback. We clarified the installation procedure for the package, which is currently in the development version of Bioconductor, due to be released in Spring 2019. Furthermore, we corrected typos, improved the documentation, and clarified potential differences in the output of the code examples that can arise due to updates of the OMA database.
Abstract
The Orthologous Matrix (OMA) is a well-established resource to identify orthologs among many genomes. Here, we present two recent additions to its programmatic interface, namely a REST API, and user-friendly R and Python packages called OmaDB. These should further facilitate the incorporation of OMA data into computational scripts and pipelines. The REST API can be freely accessed at https://omabrowser.org/api. The R OmaDB package is available as part of Bioconductor at http://bioconductor.org/packages/OmaDB/, and the omadb Python package is available from the Python Package Index (PyPI) at https://pypi.org/project/omadb/.
Keywords: orthologs, paralogs, hierarchical orthologous groups, comparative genomics, orthologous matrix, oma, API, R, python, REST, bioconductor
Introduction
Orthologs are pairs of protein coding genes that have common ancestry and have diverged due to speciation events 1. The detection of orthologs is of fundamental importance in many fields in biology, such as comparative genomics, as it allows us to propagate existing biological knowledge to ever growing newly sequenced data 2, 3.
The Orthologous Matrix (OMA) is a method and resource for the inference of orthologs among complete genomes 4. The OMA database ( https://omabrowser.org) features broad scope and size with currently over 2,100 species from all three domains of life.
The OMA browser has supported multiple ways of exporting the underlying data from its beginning. Users can download data either via bulk archives or interactively through the browser—using where possible standard file formats, such as FASTA, OrthoXML 5, or PhyloXML 6. For programmatic access, early OMA database releases offered an Application Programming Interface (API) in the form of the Simple Object Access Protocol (SOAP). However, the complexity and limited adoption of SOAP has prompted us to recently switch to the simpler, faster, and more widely used Representational State Transfer (REST) protocol for the OMA API 4. Here, we provide a description of this new OMA REST API.
Furthermore, the R environment is widely used in bioinformatics due to its flexibility as a high-level scripting language, statistical capabilities, and numerous bioinformatics libraries. In particular, the Bioconductor open source framework contains over 2,000 packages to facilitate either access to or manipulation of biological data 7. This motivated us to develop the OmaDB Bioconductor package which provides a more idiomatic and user-friendly access to OMA data in R implemented on top of the REST API.
Finally, to also enable Python users to easily interact with the database, we have developed a similar package in that language, compliant with the conventions and with support of typical complementary Python packages as outlined below.
Methods
We start by describing the OMA REST API, before moving on to detail the OmaDB Bioconductor package, and finally outline the omadb Python package.
OMA REST API
The REST framework is an API architectural style that is based on URLs and HTTP protocol methods. It was designed to be stateless and thus is context independent. That is, it does not save data internally between the HTTP requests which minimises server-side application state, thus easing parallelism. The combination of the HTTP and JSON data formats makes it particularly suitable for web applications and easily supported by most programming languages.
Since the backend of the OMA browser is almost fully based on Python and its frontend is supported by the Django web framework 8, we have opted to use the Django Rest Framework (DRF) to implement a REST API in our latest release 4. Most API calls require querying the OMA database, stored in HDF5 9, using a custom Python library (“pyoma”). The query results are serialised in the format requested by the user — typically JSON.
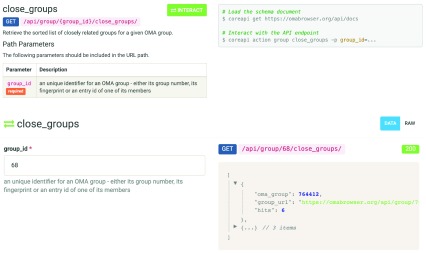
Most data available through the OMA browser is now also accessible via the API, with the exception of the local synteny data. This includes individual genes and their attributes such as protein or cDNA sequences, cross-references, pairwise orthologs, hierarchical orthologous groups 10, as well as species trees and the corresponding taxonomy. The API documentation as well as the interactive interface can be found at https://omabrowser.org/api/docs ( Figure 1).
Figure 1. Showcase of the OMA REST API documentation page, with an example of the interactive query and response.
OmaDB Bioconductor package
To facilitate simplified access to the API and downstream analyses in the R environment, we have also developed an API wrapper package in R, now available in Bioconductor 7 ( http://bioconductor.org/packages/OmaDB/). This allowed for abstraction of the server interface, eliminating the need to know structure of the database or the URL endpoints to access the required data.
The package consists of a collection of functions that import OMA data into R objects, the type of which depends on the query supplied. Due to the volume of the data available, some selected object attributes are at first given as URL endpoints. However, these are automatically loaded upon accession. OmaDB also facilitates further downstream analyses with other Bioconductor packages, such as GO enrichment analysis with topGO 11, sequence analysis with BioStrings 12, phylogenetic analyses using ggtree 13 or gene locus analyses with the help of GenomicRanges 14.
The open source code is hosted at https://github.com/DessimozLab/OmaDB/. In the results section we showcase usage of the latest version of the package (v2.0), which requires R version >= 3.6 and Bioconductor version >= 3.9. Note that as of the time of publication, this is in the Bioconductor development version. For details, see the Software Availability section.
Package Installation
if (!requireNamespace("BiocManager")) install.packages("BiocManager") BiocManager::install("OmaDB") # Load the package library(OmaDB)
omadb Python package
For Python users, we provide an analogous package named omadb. Results are supplied to users as a hybrid attribute-dictionary object. As such, both attribute and key-based access is possible. Where the URL of a further API call is listed in a response, this has been designed to be automatically requested for the user.
For data that can be represented as a table, the pandas package 15 is supported. HOGs can be analysed or displayed using the pyham library 16. Trees are retrievable as DendroPy 17 or ETE3 18 Tree objects. Gene Ontology enrichment analyses are possible through the use of the goatools package 19.
The open source code is hosted at https://github.com/DessimozLab/pyomadb/. The package requires Python >=3.6, as well as a stable internet connection. It is also available to download from PyPI, installable using pip.
Package Installation
# Install in shell, using pip $ pip install omadb # In Python, load the package >>>fromomadb import Client # Initialise the client >>> c = Client()
Results
We provide six illustrative examples in R. The first shows a direct call to the REST API, while the other five showcase the OmaDB R package (version 2.0). These examples are also available as a Jupyter notebook 20 as part of the OmaDB R code repository. We have also provided analogous examples in Python, also in the form of a Jupyter notebook, included in its code repository—with the exception of Example 6, which uses a package only available in R.
Note that the results of the queries using the API and the packages may change as we continue to update the OMA database. The OMA database release of June 2018 was used to generate the examples below.
Example 1 - Simply accessing the API, in R, via URLs
One way to access the API is to directly send a request using httr 21 in R. This approach requires the user to know the URL of the API endpoint, as well as the URL of the API function of interest. Some additional processing steps of the resultant response is usually needed. A simple example to retrieve information on the P53_RAT protein is provided below.
Here we first formulate our URL of interest and use it to send a GET request to the API. This gives us the response JSON object, which can then be parsed into an R list.
library(httr) url <-"https://omabrowser.org/api/protein/P53_RAT/" response <- GET(url) response_content_list <- httr::content(response, as ="parsed")
Example 2 - Using a sequence to find its gene family (Hierarchical Orthologous Group) and function via gene ontologies
Below is a simple workflow using the OmaDB package to annotate a given protein sequence, using the mapSequence() function.
library(OmaDB) sequence <-'MKLVFLVLLFLGALGLCLAGRRRSVQWCAVSQPEATKCFQWQRNMRKVRGPPVSCIKRD SPIQCIQAIAENRADAVTLDGGFIYEAGLAPYKLRPVAAEVYGTERQPRTHYYAVAVVKKGGSFQLNELQGL KSCHTGLRRTAGWNVPIGTLRPFLNWTGPPEPIEAAVARFFSASCVPGADKGQFPNLCRLCAGTGENKCAFS SQEPYFSYSGAFKCLRDGAGDVAFIRESTVFEDLSDEAERDEYELLCPDNTRKPVDKFKDCHLARVPSHAVV ARSVNGKEDAIWNLLRQAQEKFGKDKSPKFQLFGSPSGQKDLLFKDSAIGFSRVPPRIDSGLYLGSGYFTAI QNLRKSEEEVAARRARVVWCAVGEQELRKCNQWSGLSEGSVTCSSASTTEDCIALVLKGEADAMSLDGGYVY TAGKCGLVPVLAENYKSQQSSDPDPNCVDRPVEGYLAVAVVRRSDTSLTWNSVKGKKSCHTAVDRTAGWNIP MGLLFNQTGSCKFDEYFSQSCAPGSDPRSNLCALCIGDEQGENKCVPNSNERYYGYTGAFRCLAENAGDVAF VKDVTVLQNTDGNNNEAWAKDLKLADFALLCLDGKRKPVTEARSCHLAMAPNHAVVSRMDKVERLKQVLLHQ QAKFGRNGSDCPDKFCLFQSETKNLLFNDNTECLARLHGKTTYEKYLGPQYVAGITNLKKCSTSPLLEACEF LRK' seq_annotation <- mapSequence(sequence) length(seq_annotation$targets)# 1
The identified targets can be found in the seq_annotation$targets. As the length of this object attribute is 1, in this example the sequence mapping identified a single target sequence. From this object further information can be obtained as follows:
seq_annotation$targets[[1]]$canonicalid# 'TRFL_HUMAN'
Thus, our sequence is human lactotransferrin (also known as lactoferrin). Lactotransferrin is one of four subfamilies of transferrins in mammals 22.
To investigate the evolutionary history of genes more precisely, we turn to Hierarchical Orthologous Groups (HOGs)—sets of genes which have descended from a single common ancestral gene within a taxonomic range of interest 10. For an introduction to HOGs, we refer the interested reader to the following short video: https://youtu.be/5p5x5gxzhZA.
By knowing the ID of the HOG to which our sequence belongs, we can obtain a list of all the HOG members (i.e. all genes in the HOG), as follows:
hog_id <- seq_annotation$targets[[1]]$oma_hog_id# 'HOG:0413862.1a.1b' hog <- getHOG(id = hog_id, members = TRUE, level ='Mammalia') hog$members
Note that it is also possible to access information on a HOG using the getHOG() function. A HOG can be identified by its ID or the ID of one of its member proteins. Therefore the below will produce the same output.
hog <- getHOG(id ='TRFL_HUMAN', members = TRUE, level ='Mammalia')
We can easily retrieve the Gene Ontology (GO) terms 23 that are associated to each of the members using OmaDB.
go_annotations <- getProtein(hog$members$omaid,attribute ='gene_ontology')
The resultant list of GO terms per gene is in the “geneID2GO” format by default, which is used by the topGO 11 package.
To compare the function of lactotransferrins with their paralogous counterparts, we can retrieve a background set consisting of all members of the transferring HOG defined at the root of the eukaryotes
bgHOG <- getHOG(id ='TRFL_HUMAN', members = TRUE, level ='Eukaryota') bgAnnnot <- getProtein(bgHOG$members$omaid, attribute ='gene_ontology')
We can now construct a topGO object using the getTopGO function as seen below. Note that the background set of terms is set by getTopGO to all terms appearing in the list of annotations. This may not be appropriate in all cases—the choice of background set requires careful consideration 24.
bgAnnnotFormatted = formatTopGO(bgAnnnot, format ='geneID2GO') library(topGO) myGO <- getTopGO(annotations = bgAnnnotFormatted, format ='geneID2GO',foregroundGenes = hog$members$entry_nr, ontology ='BP') myRes <- runTest(myGO, algorithm ='classic', statistic ='fisher') print(GenTable(myGO, myRes))
As the output in Table 1 indicates, several enriched terms in the mammalian lactotransferrin are related to bone formation, consistent with previous reports in the literature (e.g. 25). So is the role of lactotransferrin in antimicrobial activity (e.g. 26).
Table 1. Gene Ontology enrichment of Biological Process terms associated with mammalian lactotransferrins compared to all eukaryotic transferrins, as obtained from example 2.
| GO.ID | Term | P-value |
|---|---|---|
| GO:0001501 | skeletal system development | <1e-30 |
| GO:0001503 | ossification | <1e-30 |
| GO:0001649 | osteoblast differentiation | <1e-30 |
| GO:0001816 | cytokine production | <1e-30 |
| GO:0001817 | regulation of cytokine production | <1e-30 |
| GO:0001818 | negative regulation of cytokine production | <1e-30 |
| GO:0002237 | response to molecule of bacterial origin | <1e-30 |
| GO:0002682 | regulation of immune system process | <1e-30 |
| GO:0002683 | negative regulation of immune system process | <1e-30 |
| GO:0002761 | regulation of myeloid leukocyte differentiation | <1e-30 |
Example 3 - Taxonomic tree visualisation
The taxonomic data obtained using the OmaDB package can easily be plugged into ggtree 13 for phylogenetic tree visualisation. First, the tree is obtained using the getTaxonomy() function. In this example, the tree is rooted at the Hominoidea taxonomic level. The default format of the object returned is newick.
tax <- getTaxonomy(root ='Hominoidea')
The resultant object can directly be used to build a phylogenetic tree using the ggtree package as below:
library(ggtree) tree <- getTree(tax$newick) mytree <- ggtree(tree)
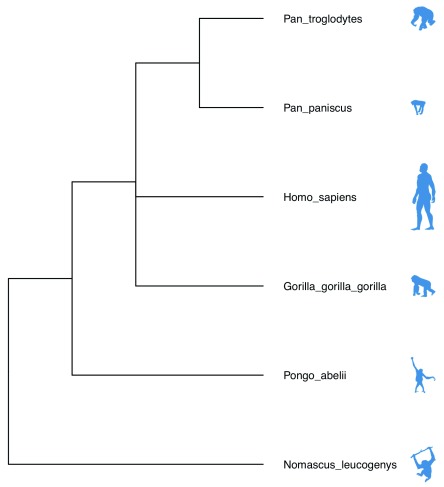
The tree can be further annotated using species silhouettes from PhyloPic ( http://phylopic.org/). This functionality is already enabled within the ggtree package and just requires obtaining the relevant image codes. The workflow to produce Figure 2 is below.
library(rphylopic) labels <- tree$tip.label labelsFormatted <- sapply(labels, FUN = function(x) gsub("_"," ", x, fixed = TRUE)) ids <- sapply(labelsFormatted, FUN = function(x) name_search(x)$canonicalName[1,1]) images <- sapply( as.character(ids), FUN = function(x) tryCatch(name_images(x)$same[[1]]$uid, error = function(w) name_images(x)$supertaxa[[1]]$uid) ) d <- data.frame(label = labels, images = as.character(images)) library(dplyr) library(ggimage)
mytree %<+% d + geom_tiplab(aes(image = images), geom ='phylopic', offset = 2.3, color ='steelblue') + geom_tiplab(offset = 0.3) + ggplot2::xlim(0, 7)
Figure 2. Species taxonomy tree obtained using example 3.
Example 4 - Visualising the distribution of PAM distances in the taxonomic space
To obtain all orthologous pairs between two genomes, we can use the getGenomePairs() function. To limit server load, the resultant response is paginated and by default only returns the first page, capped at 100 entries. This is easily adjustable by setting the ‘per_page’ parameter to either the number of orthologs required or simply to ‘all’.
In this example, we compare the distribution of PAM distances (Point accepted mutations; 27) between orthologs of two species-pairs, namely human-dog and human-mouse. First, we request the required data:
mouse_id = getGenome(id='Mus musculus')$taxon_id human_id = getGenome(id='Homo sapiens')$taxon_id dog_id = getGenome(id='Canis lupus familiaris')$taxon_id human_mouse <- getGenomePairs(genome_id1 = human_id, genome_id2 = mouse_id, rel_type ='1:1') human_dog <- getGenomePairs(genome_id1 = human_id, genome_id2 = dog_id, rel_type ='1:1')
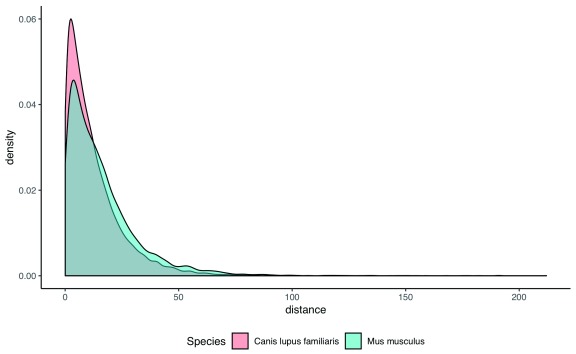
We can then bind the two resultant data frames and plot the results ( Figure 3), as so:
human_mouse$Species <-'Mus musculus' human_dog$Species <-'Canis lupus familiaris' all_pairs <- rbind(human_mouse, human_dog) all_pairs$Species <- as.factor(all_pairs$Species) library(ggplot2) g <- ggplot(all_pairs, aes(x =distance, fill = Species)) + geom_density(alpha = 0.5) + xlab('evolutionary distance [PAM]') + theme(legend.position ='bottom', panel.grid.major = element_blank(), panel.grid.minor = element_blank(), panel.background = element_blank(), axis.line = element_line(colour ='black')) print(g)
Figure 3. Distribution of evolutionary distances (in PAM units; 27) human-dog (red) and human-mouse (blue) pairs, obtained using example 4.
The two-sample Kolmogorov-Smirnov test can be performed on the two distributions, using the command:
ks.test(human_dog$distance, human_mouse$distance)
This returns p-value < 2.2e-16. The median distance between dog and human is shorter than that of mouse and human (8.8 vs. 11.8). This is consistent with previous observations that the rodent has a longer branch than humans and carnivores, in part due to their shorter generation time 28.
Example 5 - Annotating protein sequences not present in OMA
Although the OMA database currently analyses over 2,100 genomes, many more have been sequenced, and the gap keeps on widening. It is nevertheless possible to use OMA to infer the function of custom protein sequences through a fast approximate search against all sequences in OMA 4.
# Our mystery sequence is cystic fibrosis transmembrane conductance # regulator in the Emperor penguin (UniProt ID: A0A087RGQ1_APTFO) mysterySeq <- 'FFFLLRWTKPILRKGYRRRLELSDIYQIPSADSADNLSEKLEREWDRELATSKKKPKLINALRRCFFWKFM FYGIILYLGEVTKSVQPLLLGRIIASYDPDNSDERSIAYYLAIGLCLLFLVRTLLIHPAIFGLHHIGMQMRI AMFSLIYKKILKLSSRVLDKISTGQLVSLLSNNLNKFDEGLALAHFVWIAPLQVALLMGLLWDMLEASAFSG LAFLIVLAFFQAWLGQRMMKYRNKRAGKINERLVITSEIIENIQSVKAYCWEDAMEKMIESIRETELKLTRK AAYVRYFNSSAFFFSGFFVVFLAVLPYAVIKGIILRKIFTTISFCIVLRMTVTRQFPGSVQTWYDSIGAINK IQDFLLKKEYKSLEYNLTTTGVELDKVTAFWDEGIGELFVKANQENNNSKAPSTDNNLFFSNFPLHASPVLQ DINFKIEKGQLLAVSGSTGAGKTSLLMLIMGELEPSQGRLKHSGRISFSPQVSWIMPGTIKENIIFGVSYDE YRYKSVIKACQLEEDISKFPDKDYTVLGDGGIILSGGQRARISLARAVYKDADLYLLDSPFGHLDIFTEKEI FESCVCKLMANKTRILVTSKLEHLKIADKILILHEGSCYFYGTFSELQGQRPDFSSELMGFDSFDQFSAERR NSILTETLRRFSIEGEGTGSRNEIKKQSFKQTSDFNDKRKNSIIINPLNASRKFSVVQRNGMQVNGIEDGHN DPPERRFSLVPDLEQGDVGLLRSSMLNTDHILQGRRRQSVLNLMTGTSVNYGPNFSKKGSTTFRKMSMVPQT NLSSEIDIYTRRLSRDSVLDITDEINEEDLKECFTDDAESMGTVTTWNTYFRYVTIHKNLIFVLILCVTVFL VEVAASLAGLWFLKQTALKANTTQSENSTSDKPPVIVTVTSSYYIIYIYVGVADTLLAMGIFRGLPLVHTLI TVSKTLHQKMVHAVLHAPMSTFNSWKAGGMLNRFSKDTAVLDDLLPLTVFDFIQLILIVIGAITVVSILQPY IFLASVPVIAAFILLRAYFLHTSQQLKQLESEARSPIFTHLVTSLKGLWTLRAFGRQPYFETLFHKALNLHT ANWFLYLSTLRWFQMRIEMIFVVFFVAVAFISIVTTGDGSGKVGIILTLAMNIMGTLQWAVNSSIDVDSLMR SVGRIFKFIDMPTEEMKNIKPHKNNQFSDALVIENRHAKEEKNWPSGGQMTVKDLTAKYSEGGAAVLENISF SISSGQRVGLLGRTGSGKSTLLFAFLRLLNTEGDIQIDGVSWSTVSVQQWRKAFGVIPQKVFIFSGTFRMNL DPYGQWNDEEIWKVAEEVGLKSVIEQFPGQLDFVLVDGGCVLSHGHKQLMCLARSVLSKAKILLLDEPSAHL DPVTSQVIRKTLKHAFANCTVILSEHRLEAMLECQRFLVIEDNKLRQYESIQKLLNEKSSFRQAISHADRLK LLPVHHRNSSKRKPRPKITALQEETEEEVQETRL' myAnnotations <- annotateSequence(mysterySeq)
This results in 54 GO annotations. By comparison, this sequence has merely 15 GO annotations in UniProt-GOA 29 — all of which are also predicted by this method in OMA.
Example 6 - Combining OmaDB with BgeeDB for gene expression
We go back to the lactotransferrin gene family from Example 2. We can use OmaDB in conjunction with the BgeeDB Bioconductor package 30 to retrieve expression data from the Bgee database 31 as follows.
BiocManager::install("BgeeDB") library(BgeeDB) # Bgee uses Ensembl gene IDs, obtainable using OmaDB’s cross-references. trfl_xrefs <- getProtein(id='TRFL_HUMAN')$xref trfl_ens_id <- subset(trfl_xrefs, source =='Ensembl Gene')$xref # The Ensembl gene IDs need to be without version suffix trfl_ens_id <- strsplit(trfl_ens_id,'.',fixed=TRUE)[[1]][1] my_stage <-'UBERON:0034920'# Infant stage bgee.expr <- Bgee$new(species='Homo_sapiens') expr.data <- loadTopAnatData(bgee.expr, stage = my_stage) gene.expr.tissue.ids <- unlist(expr.data$gene2anatomy[trfl_ens_id], use.names = F) tissues <- expr.data$organ.names print(tissues[tissues$ID %in% gene.expr.tissue.ids, ])
Among the tissues in which lactotransferrin is expressed according to Bgee ( Table 2), we note the bone marrow and the palpebral conjunctiva (the eyelid inner surface). This is consistent with the aforementioned involvement of lactotransferrin in bone formation and anti-microbial activity.
Table 2. Human tissues in which lactotransferrin is expressed in infant stage, according to the Bgee database version 14 (output of Example 6).
| ID | Name |
|---|---|
| UBERON:0001812 | palpebral conjunctiva |
| UBERON:0000178 | blood |
| UBERON:0002371 | bone marrow |
| UBERON:0001154 | vermiform appendix |
| UBERON:0002084 | heart left ventricle |
Further tutorials on the OmaDB package can be found in the accompanying vignettes:
browseVignettes('OmaDB')
Discussion and outlook
Orthology is used for various purposes, such as species tree inference, gene evolution dynamic, or protein function prediction. The retrieval of orthologs is thus typically just the starting point of a larger analysis. Therefore, this overhaul and expansion of the OMA programmatic interface will facilitate the incorporation of OMA data in such larger analyses.
Our R package will continue to be maintained in line with the biannual Bioconductor releases. Further work to improve the package includes improvement in performance. For example, the responses are currently fully loaded into an R object of choice which, depending on the response size, may create some time lag in the response. We will also continue to update the package and the API in sync with the OMA browser to incorporate new functionalities of OMA.
Likewise, we will also maintain and further develop the Python package. In particular, we will explore the possibility of further integration with the BioPython library 32.
More generally, in OMA we will keep supporting the various ways of accessing the underlying data, including the interactive web browser and flat files in a variety of formats. The REST API is also complemented by a new SPARQL interface that enables highly specific queries, as well as federated queries over multiple resources 4. However, the query language is more complex.
We very much welcome feedback and questions from the community. We also highly appreciate contributions to the code in the form of pull requests. Our preferred channel for support is the BioStar website 33, where we monitor all posts with keyword “oma”.
Software availability
Please note that this manuscript uses version 2.0 of the OmaDB R package, which is in the development version of Bioconductor (v.3.9). Until the release of Bioconductor v.3.9 in Spring 2019, there are two possible ways of installing it:
-
1)
Install the development version of R (v.3.6) — required for Bioconductor v.3.9 — and install OmaDB using the command:
BiocManager::install('OmaDB', version ='devel') –or–
-
2)
Install OmaDB 2.0 directly from the github repo using the devtools R package:
install.packages('devtools') library(devtools) install_github('dessimozlab/omadb')
REST API available from: https://omabrowser.org/api
Documentation available from: https://omabrowser.org/api/docs
R OmaDB package available from: http://bioconductor.org/packages/OmaDB/
Source code available from: https://github.com/DessimozLab/OmaDB/
Archived source code as at time of publication: http://doi.org/10.5281/zenodo.2595086 34
License: GPL-2
omadb Python package available from: https://pypi.org/project/omadb/
Source code available from: https://github.com/DessimozLab/pyomadb/
Archived source code as at time of publication: http://doi.org/10.5281/zenodo.2530250 35
License: GPL-3
We also provide a binder to reproduce in Python the analyses done in R. This is available from: https://mybinder.org/v2/gh/DessimozLab/pyomadb/master?filepath=examples%2Fpyomadb-examples.ipynb
Acknowledgements
We thank Natasha Glover for helpful feedback on the manuscript, and Frédéric Bastian for help on the example involving BgeeDB.
Funding Statement
We acknowledge support by Swiss National Science Foundation grant 150654, UK BBSRC grant BB/M015009/1, the Swiss State Secretariat for Education, Research and Innovation (SERI), as well as a UCL Genetics, Evolution and Environment Departmental Summer Bursary (to KK).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Fitch WM: Distinguishing homologous from analogous proteins. Syst Zool. 1970;19(2):99–113. 10.2307/2412448 [DOI] [PubMed] [Google Scholar]
- 2. Sonnhammer EL, Gabaldón T, Sousa da Silva AW, et al. : Big data and other challenges in the quest for orthologs. Bioinformatics. 2014;30(21):2993–8. 10.1093/bioinformatics/btu492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forslund K, Pereira C, Capella-Gutierrez S, et al. : Gearing up to handle the mosaic nature of life in the quest for orthologs. Bioinformatics. 2018;34(2):323–329. 10.1093/bioinformatics/btx542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altenhoff AM, Glover NM, Train CM, et al. : The OMA orthology database in 2018: retrieving evolutionary relationships among all domains of life through richer web and programmatic interfaces. Nucleic Acids Res. 2018;46(D1):D477–85. 10.1093/nar/gkx1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmitt T, Messina DN, Schreiber F, et al. : Letter to the editor: SeqXML and OrthoXML: standards for sequence and orthology information. Brief Bioinform. 2011;12(5):485–8. 10.1093/bib/bbr025 [DOI] [PubMed] [Google Scholar]
- 6. Han MV, Zmasek CM: phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics. 2009;10:356. 10.1186/1471-2105-10-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huber W, Carey VJ, Gentleman R, et al. : Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12(2):115–21. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Django Software Foundation. Django. [cited 2018]. Reference Source [Google Scholar]
- 9. Folk M, Heber G, Koziol Q, et al. : An overview of the HDF5 technology suite and its applications. Proceedings of the EDBT. 2011. 10.1145/1966895.1966900 [DOI] [Google Scholar]
- 10. Altenhoff AM, Gil M, Gonnet GH, et al. : Inferring hierarchical orthologous groups from orthologous gene pairs. PLoS One. 2013;8(1):e53786. 10.1371/journal.pone.0053786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexa A, Rahnenfuhrer J: topGO: Enrichment analysis for Gene Ontology.R package version 2.28.0. Bioconductor2016. 10.18129/B9.bioc.topGO [DOI] [Google Scholar]
- 12. Pagès H, Aboyoun P, Gentleman R, et al. : Biostrings: Efficient manipulation of biological strings. R Package Version.2017;2(0). Reference Source [Google Scholar]
- 13. Yu G, Smith DK, Zhu H, et al. : ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data.McInerny G editor. Methods Ecol Evol. 2017;8(1):28–36. 10.1111/2041-210X.12628 [DOI] [Google Scholar]
- 14. Lawrence M, Huber W, Pagès H, et al. : Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013;9(8):e1003118. 10.1371/journal.pcbi.1003118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKinney W: pandas: a foundational Python library for data analysis and statistics. Python for High Performance and Scientific Computing. 2011;1–9. Reference Source [Google Scholar]
- 16. Train CM, Pignatelli M, Altenhoff A, et al. : iHam & pyHam: visualizing and processing hierarchical orthologous groups. Bioinformatics. 2018. 10.1093/bioinformatics/bty994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sukumaran J, Holder MT: DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26(12):1569–71. 10.1093/bioinformatics/btq228 [DOI] [PubMed] [Google Scholar]
- 18. Huerta-Cepas J, Serra F, Bork P: ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol Biol Evol. 2016;33(6):1635–8. 10.1093/molbev/msw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klopfenstein DV, Zhang L, Pedersen BS, et al. : GOATOOLS: A Python library for Gene Ontology analyses. Sci Rep. 2018;8(1):10872. 10.1038/s41598-018-28948-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kluyver T, Ragan-Kelley B, Pérez F, et al. : Jupyter Notebooks-a publishing format for reproducible computational workflows.In: ELPUB2016;87–90. 10.3233/978-1-61499-649-1-87 [DOI] [Google Scholar]
- 21. Wickham H: httr: Tools for Working with URLs and HTTP.2018. Reference Source [Google Scholar]
- 22. Lambert LA, Perri H, Meehan TJ: Evolution of duplications in the transferrin family of proteins. Comp Biochem Physiol B Biochem Mol Biol. 2005;140(1):11–25. 10.1016/j.cbpc.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 23. Ashburner M, Ball CA, Blake JA, et al. : Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaudet P, Dessimoz C: Gene Ontology: Pitfalls, Biases, and Remedies. Methods Mol Biol.In: Dessimoz C, Škunca N, editors. The Gene Ontology Handbook. New York, NY: Springer New York;2017;1446:189–205. 10.1007/978-1-4939-3743-1_14 [DOI] [PubMed] [Google Scholar]
- 25. Naot D, Grey A, Reid IR, et al. : Lactoferrin--a novel bone growth factor. Clin Med Res. 2005;3(2):93–101. 10.3121/cmr.3.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orsi N: The antimicrobial activity of lactoferrin: current status and perspectives. Biometals. 2004;17(3):189–96. 10.1023/B:BIOM.0000027691.86757.e2 [DOI] [PubMed] [Google Scholar]
- 27. Dayhoff MO, Schwartz RM, Orcutt BC: A model of evolutionary change in proteins.In: Atlas of Protein Sequence and Structure1978;345–52. Reference Source [Google Scholar]
- 28. Easteal S: Generation time and the rate of molecular evolution. Mol Biol Evol. 1985;2(5):450–3. 10.1093/oxfordjournals.molbev.a040361 [DOI] [PubMed] [Google Scholar]
- 29. Huntley RP, Sawford T, Mutowo-Meullenet P, et al. : The GOA database: gene Ontology annotation updates for 2015. Nucleic Acids Res. 2015;43(Database issue):D1057–63. 10.1093/nar/gku1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Komljenovic A, Roux J, Wollbrett J, et al. : BgeeDB, an R package for retrieval of curated expression datasets and for gene list expression localization enrichment tests [version 2; referees: 2 approved, 1 approved with reservations]. F1000Res. 2016;5:2748. 10.12688/f1000research.9973.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bastian F, Parmentier G, Roux J, et al. : Bgee: Integrating and Comparing Heterogeneous Transcriptome Data Among Species.In: Data Integration in the Life Sciences (Lecture Notes in Computer Science). Springer Berlin Heidelberg.2008;124–31. 10.1007/978-3-540-69828-9_12 [DOI] [Google Scholar]
- 32. Cock PJ, Antao T, Chang JT, et al. : Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25(11):1422–3. 10.1093/bioinformatics/btp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parnell LD, Lindenbaum P, Shameer K, et al. : BioStar: an online question & answer resource for the bioinformatics community. PLoS Comput Biol. 2011;7(10):e1002216. 10.1371/journal.pcbi.1002216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. klarakaleb, Altenhoff A, bioc-gitadmin, et al. : DessimozLab/OmaDB: v1.99.1 (Version 1.99.2). Zenodo. 2019. 10.5281/zenodo.2595086 [DOI] [Google Scholar]
- 35. Alex WV, Altenhoff A: DessimozLab/pyomadb: v2.0.0 (Version 2.0.0). Zenodo. 2019. 10.5281/zenodo.2530250 [DOI] [Google Scholar]