Abstract
Gestational trophoblastic disease or neoplasia covers a spectrum of benign and malignant conditions arising from pregnancies with highly abnormal development of trophoblastic tissue. In this brief review, we discuss the different features of these different conditions and their origins and risk factors and introduce some of the more novel and controversial treatment options currently being explored.
Keywords: gestational trophoblast disease, hydatidiform mole, choriocarcinoma
Introduction
Gestational trophoblastic disease (GTD) or neoplasia (GTN) covers a spectrum of benign and malignant conditions arising from malformed pregnancies. In this brief review, we discuss the different features of these different conditions and their origins and risk factors and introduce some of the more novel and controversial treatment options currently being explored.
Normal trophoblast cell development
Fetal trophoblast cells are essential to facilitate embryo implantation into the uterus and are the major components of the placenta that ensure normal growth and development of the fetus 1. During placental development, the trophectoderm expands rapidly and starts to differentiate into both villous cytotrophoblasts (CTBs), which in turn differentiate into invasive extravillous trophoblast (EVT) and syncytiotrophoblast 2, 3. At the tips of anchoring villi, proliferating CTB generates columns to attach to the maternal decidua and differentiating EVT cells invade the maternal tissues, through the decidua and as far as the inner third of the myometrium 2. Within the trophoblast cell column, cells begin to express HLA-G as they differentiate from villous to extravillous trophoblast cells 4.
EVT cells invade the maternal tissues via two main routes, the interstitial and endovascular pathways, although there is also some evidence for endoglandular invasion 5, 6. Endovascular EVT cells invade the spiral arteries, retrograde to flow, plugging the artery openings so that for the first 10 weeks of gestation the developing embryo and placenta exist in a hypoxic environment 5. Interstitial EVT cells invade through the decidua and into the myometrium by breaking down the extracellular matrix and moving into the cleared spaces in a highly regulated manner. One of the main functions of invading interstitial EVT cells is in remodeling of the uterine spiral arteries, allowing adequate blood flow to the maternal–placental interface. Interstitial EVT cells also become terminally differentiated by forming non-invasive multinucleated giant cells, although the mechanism by which these cells are formed is not clear.
In addition to differentiating into EVT cells, CTB cells differentiate into the syncytiotrophoblast and continually fuse with it to replenish spent nuclei and organelles that are shed as apoptotic bodies known as syncytial knots, although the frequency with which this occurs is debated 1, 6. The syncytiotrophoblast is essential for gas and nutrient exchange across the placenta to and from the developing fetus.
Gestational trophoblastic disease
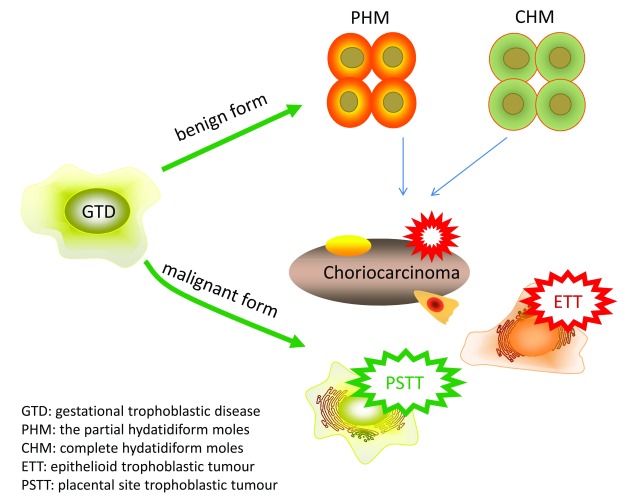
GTD is a heterogeneous group of pregnancy-associated growths, often termed tumors, including choriocarcinoma, invasive mole, hydatidiform mole (partial and complete), epithelioid trophoblastic tumor (ETT), and placental site trophoblastic tumor (PSTT) 7– 9, that arise from placental villous and extravillous trophoblast cells 10. GTD has benign and malignant forms; the benign forms include partial hydatidiform moles (PHMs) and complete hydatidiform moles, whereas the malignant forms are choriocarcinoma (which can arise from hydatidiform mole, normal term pregnancy, ectopic pregnancy, or miscarriage), ETT, and PSTT 11 ( Figure 1 and Table 1). The major forms of GTD are choriocarcinoma and hydatidiform mole, and ETT and PSTT are relatively rare 8. GTD can occur weeks or years after any pregnancy but they occur most commonly after a molar pregnancy, which adds about 1 to 2% risk of further complete and partial mole 8, 11, 12.
Figure 1. Schematic of the different benign and malignant forms of gestational trophoblastic diseases.
Schematic of the different benign and malignant forms of gestational trophoblastic diseases.
Table 1. Clinical features of gestational trophoblastic disease 13– 26.
| Gestational
trophoblastic Disease |
Clinical Manifestations | Incidence | Risk Factors | Cellular Origin |
|---|---|---|---|---|
| Choriocarcinoma | Irregular vaginal bleeding, cough, hemoptysis, headache,
vomiting, convulsions, purple blue vaginal nodules, enlarged uterus, ovarian flavin cyst, intraperitoneal hemorrhage, high hCG levels |
1 in 40,000
pregnancies |
Age, deep myometrial invasion,
tumor size, molar pregnancy, smoking, spontaneous miscarriage, ectopic pregnancy, site of metastases, disease duration, hCG level, stage |
Villous trophoblast |
| Hydatidiform mole
(complete) |
Karyotype of 46XX, 46XY, diffuse villous enlargement with
hydropic changes. varying atypia of trophoblast, absence of fetal tissue and villous capillaries, uterine enlarge , high hCG levels for gestational age, hypertension and hyperemesis gravidarum, abnormal bleeding, ovarian theca lutein cysts |
0.57–1.1 per 1000
pregnancies |
Age, ethnicity, and genetic basis,
spontaneous miscarriage, nutrient, family history |
Villous trophoblast |
| Hydatidiform mole
(partial) |
Karyotype of 69XXX, 69XXY or 69XYY,
placental villi are focal edema and denatured that vary in size and shape, trophoblastic cells proliferate, fetal tissue is present, few full-term babies born, uteri are small for gestational age, low hCG level for gestational age, few medical complications |
0.57–1.1 per 1000
pregnancies |
Age, ethnicity, and genetic basis,
spontaneous miscarriage, nutrient status, family history |
Villous trophoblast |
| Epithelioid
trophoblastic tumor |
Abnormal vaginal bleeding | 1 in 100,000
pregnancies |
Stage, family history, deep
invasion, tumor size |
Intermediate trophoblast |
| Placental site
trophoblastic tumor |
Abnormal vaginal bleeding, trophoblastic infiltration confined
to the endometrium and myometrium, hCG may be absent, resistant to chemotherapy |
1 in 100,000
pregnancies |
Age, deep invasion, tumor size,
mitotic rate, disease stage, hCG level |
Intermediate trophoblast |
Hydatidiform mole
Hydatidiform mole is a benign trophoblastic tumor 27 and accounts for the majority of GTD; about 80% of GTDs are hydatidiform moles 13. Hydatidiform mole is associated with abnormal gametogenesis and fertilization. The incidence varies around the world and is higher in Asia (~1 in 500) and the Middle East and Africa (~1 in 1000) than in Europe and North America (~1 in 1500) 14, 28. Risk factors include extremes of age, ethnicity, genetic basis, spontaneous miscarriage, and nutrient restriction 8. Women from 21 to 35 years of age have a lower risk of complete mole than women older than 35 years and younger than 21 years 15. Women with a history of prior spontaneous miscarriage have a two- to three-fold risk of molar pregnancy in comparison with the general population 29. Women with a history of molar pregnancy have a 10- to 20-fold risk of repeat molar pregnancy, and about 20% of patients will develop malignant transformation after evacuation of the mole.
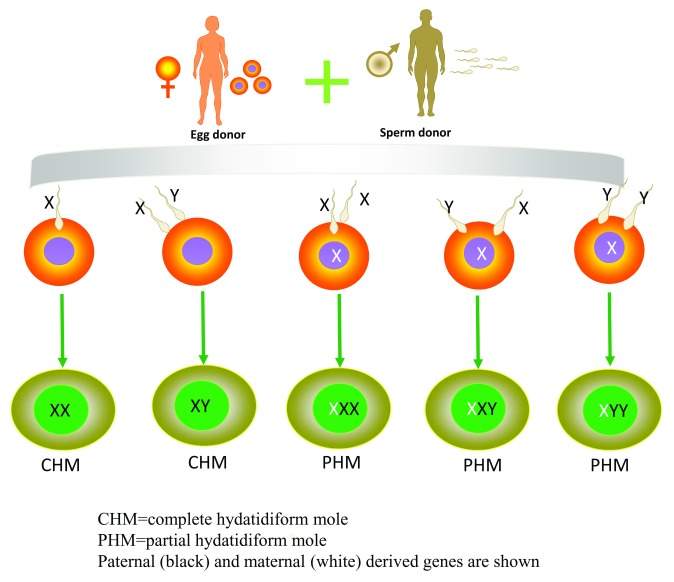
Hydatidiform moles are edematous immature placentas which are broken down into complete and partial moles. A complete mole occurs when an empty ovum is fertilized by a sperm, about 90% of complete hydatidiform moles are 46XX which originate from duplication of the chromosomes of a haploid sperm and the other 10% are 46XY ( Figure 2) 15, and the chromosomes are paternally derived. Complete hydatidiform moles take on the appearance of a “bunch of grapes” which undergo diffuse villous enlargement with hydropic changes. The trophoblast has varying degrees of atypia and villous capillaries are absent. Fetal tissue or the embryo is absent in complete moles. In complete hydatidiform moles, the uterus is typically significantly enlarged for gestational age, and patients always have an elevated human chorionic gonadotropin (hCG) level for gestational age. Often, there can be early onset of medical complications such as pregnancy-induced hypertension, hyperthyroid, and hyperemesis gravidarum 15, 30. The most common presentation of molar pregnancy is abnormal vaginal bleeding during the first trimester and ovarian theca lutein cysts greater than 6 cm in diameter 8, 31.
Figure 2. Schematic of the different karyotypes of complete and partial hydatidiform moles.
Schematic of the different karyotypes of complete and partial hydatidiform moles.
A partial mole occurs when an empty ovum is fertilized by two sperm, the normal karyotype being 69XXX, 69XXY, or 69XYY, although a diploid karyotype may also exist ( Figure 2) 15. In PHMs, placental villi have focal edema and denatured areas of varying size and shape and pathological trophoblast cell proliferation. Fetal tissue or a recognizable embryo will be present. Most often, the fetus is not living, although occasionally there will be a small living fetus. Rarely, a term infant will be born.
Choriocarcinoma
Choriocarcinomas are malignant trophoblastic tumors developing in the uterus from villous CTB cells 15, 32. About 50% of all choriocarcinomas arise from a complete molar gestation, 25% following a normal pregnancy, and 25% after a spontaneous miscarriage or ectopic pregnancy 33. Choriocarcinoma occurs in about 1 in 20,000 to 40,000 pregnancies in the United States and three to nine per 40,000 pregnancies in Southeast Asia and Japan 34. However, in all populations, the incidence rates of choriocarcinoma have declined over the past 30 years, although the precise incidence of choriocarcinoma may be under-reported because hemorrhage with biopsy precludes tissue diagnosis 15, 16.
Choriocarcinomas produce high levels of angiogenic growth factors and are able to remodel the uterine vasculature which can lead to hemorrhage 35. The clinical manifestation most often includes irregular vaginal bleeding, enlarged uterus, cough, hemoptysis, headache, and vomiting 17, 36– 40. Choriocarcinoma in the vagina can appear as purple/blue nodules and the uterus often becomes asymmetrically enlarged but not all women will present with all of these symptoms. In addition, intraperitoneal hemorrhage and high serum hCG levels exist 32, 41.
There are many potential predictive factors for choriocarcinoma. Rodabaugh et al. 18 found that a pretreatment hCG level of more than 100,000 mIU/mL, disease duration greater than 4 months from delivery, and the presence of liver or brain metastases were important predictors of outcome in patients with choriocarcinoma. The risk of choriocarcinoma is increased in women younger than 20 and in women older than 39 14. Deep myometrial invasion, tumor size, tumor stage, and site of metastases also influence the outcome of patients with choriocarcinoma 15, 32, 41. Usually, choriocarcinoma occurs secondary to molar pregnancy, spontaneous miscarriage, or ectopic pregnancy but can also occur after full-term normal pregnancy 8.
Epithelioid trophoblastic tumor and placental site trophoblastic tumor
ETT and PSTT are malignant trophoblastic tumors that arise from intermediate trophoblast cells of the placental bed after a full-term pregnancy or a non-molar miscarriage 14, 19. The incidence is about 1 in 100,000 pregnancies and ETT and PSTT represent just 0.2 to 2% of GTD cases but have the highest mortality rate 19, 20, 42. Unlike other forms of GTD, hCG may be absent in PSTT and it is fairly resistant to chemotherapy and the treatment is often complete hysterectomy, although more recently fertility-sparing surgery has been offered with limited success but it requires careful post-surgery monitoring to ensure that it has been curative 14, 43. Trophoblast cell infiltration is confined to the endometrium and myometrium in PSTT, and invasion is characterized by cells infiltrating the muscle fibers. These patients often present with lung metastases 14, 16. Advanced age (greater than 34 years), deep myometrial invasion, and tumor size have been associated with a worse outcome for patients 19, 21, 44.
Treatment options for gestational trophoblastic disease
Standard treatment options
Standard treatment options for GTD differ depending on the type and stage of disease and include chemotherapy, dilatation and curettage (D&C), or hysterectomy or a combination of these. In general, D&C is used for molar pregnancy and where a woman wishes to retain her fertility; however, careful post-treatment monitoring is required to ensure no recurrence of disease. For more severe and chemoresistant disease and when fertility preservation is not a concern, hysterectomy is the more common option, particularly if there are no distant metastases. Some types of GTD respond well to chemotherapy, either single or combined therapy; however, chemotherapy is not effective for all types of disease. Common chemotherapeutic agents include methotrexate, actinomycin D, etoposide, cyclophosphamide, vincristine, and cisplatin. There is a high risk of metastatic spread of some types of GTD and therefore a combination treatment modality of hysterectomy and chemotherapy is often employed. Careful β-hCG or human placental lactogen testing or both are required to ensure efficacy of any treatment modality. The majority of GTDs are treatable with one of the above common options; however, some more novel and controversial treatment modalities have recently been introduced and will be discussed further here.
Prophylactic chemotherapy
One aspect of the treatment of GTD that is still controversial is whether to initiate prophylactic chemotherapy in a subpopulation of women with hydatidiform mole who are at high risk of persistence rather than following their hCG levels until they achieve the criteria for declaring no evidence of disease or meet the definition of persistent GTD. The idea is to reduce the need for more intense chemotherapy regimens in a smaller group of women by administering a more modest regimen to increase the chance of complete resolution. Several non-randomized trials demonstrated impressive reductions in the risk of recurrent/persistent disease. For example, in Korea, Kim et al. reported on 262 patients who were identified as having high-risk hydatidiform mole 45. Fifty (19%) received prophylactic chemotherapy and the remaining 216 patients served as controls. There were no cases of persistent GTD in the 59 patients who received prophylactic chemotherapy, but 59% of the control group developed persistent GTD 45.
In a randomized trial of a single dose of actinomycin D, 18% of the high-risk patients who received prophylactic chemotherapy and 34% of the high-risk patients who did not developed recurrent GTD (risk ratio [RR] = 0.54, 95% confidence interval [CI] = 0.35–0.82, number needed to treat = 7). Adverse events were similar in the two groups, and progression was not associated with increased disease severity in the chemotherapy group. Also, costs were lower with the prophylactic chemotherapy strategy 46.
A recent Cochrane review identified only three randomized trials, including the study of actinomycin D summarized above. The combined studies demonstrated a reduced risk of GTD (RR 0.37, 95% CI 0.24 to 0.57) but the Cochrane authors judged two of the three studies to be of low quality. The Cochrane authors concluded that prophylactic chemotherapy might reduce the risk of progression to GTD in women at high risk, but the strength of the conclusion is limited by the poor quality of the studies. Concerns about increased drug resistance, delays in treatment of GTD, and toxic side effects remain, so they conclude: “it is not possible to strongly recommend the practice” 47.
Second dilatation and curettage
Another area where there is some divergence of typical practice in the treatment of GTD is whether to perform a second uterine curettage when a patient’s hCG trend is non-reassuring after initial diagnosis and evacuation of a molar pregnancy. The classic teaching was that owing to the risk of life-threatening hemorrhage or uterine perforation (or both), a second D&C should not be performed.
However, in some centers with substantial experience in treating GTD, second uterine evacuation appears to prevent the development of persistent disease and reduce the requirement for chemotherapy 48. In the GOG 242 trial, 60 women with a first diagnosis of low-risk GTD underwent a second uterine curettage and 24 (40%) subsequently experienced complete resolution of disease without the need for chemotherapy. The study noted that no patient with an hCG level of greater than 100,000 mIU/mL was cured and no patient with an International Federation of Gynecology and Obstetrics/World Health Organization (FIGO/WHO) score of more than 4 was cured. Success rate also appeared to be lower in women younger than 19 and older than 40. No surgical complications were reported 49.
Selective uterine surgery
Women with chemoresistant disease typically are counseled to consider hysterectomy; however, many women in this position wish to retain their fertility. A review of the literature reports on women with PSTT who received fertility-saving treatment. Of 11 women who had laparotomy with uterine preservation, six patients had what was considered a successful procedure and the remaining five required total hysterectomy 43. Therefore, although this therapy may be effective in about 50% of cases, careful monitoring of excision margins and further disease progression are required. However, in the case of PSTT, hCG monitoring is not useful and a better marker of disease presence is human placental lactogen. In addition, studies on the effectiveness of these treatments for saving fertility have yet to be conducted.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Shannon Salvador, Division of Gynecologic Oncology, Segal Cancer Center, Jewish General Hospital, McGill University, Montreal, Quebec, H3T 1E2, Canada
Ewa Nowak-Markwitz, Gynecologic Oncology Department, Poznan University of Medical Sciences, Poznan, Poland
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Hemberger M, Udayashankar R, Tesar P, et al. : ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet. 2010;19(12):2456–67. 10.1093/hmg/ddq128 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Pijnenborg R, Robertson WB, Brosens I, et al. : Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2(1):71–91. 10.1016/S0143-4004(81)80042-2 [DOI] [PubMed] [Google Scholar]
- 3. Early development of the human placenta.In: Benirschke K, Kaufmann P. Pathology of the human placenta New York: Springer-Verlag,1990;13–21. 10.1007/978-1-4757-4193-3_2 [DOI] [Google Scholar]
- 4. Gude NM, Roberts CT, Kalionis B, et al. : Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407. 10.1016/j.thromres.2004.06.038 [DOI] [PubMed] [Google Scholar]
- 5. Pijnenborg R, Vercruysse L, Hanssens M: The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–58. 10.1016/j.placenta.2005.12.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Moser G, Huppertz B: Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta. 2017;56:19–26. 10.1016/j.placenta.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Eysbouts YK, Ottevanger PB, Massuger LFAG, et al. : Can the FIGO 2000 scoring system for gestational trophoblastic neoplasia be simplified? A new retrospective analysis from a nationwide dataset. Ann Oncol. 2017;28(8):1856–61. 10.1093/annonc/mdx211 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Bruce S, Sorosky J: Gestational Trophoblastic Disease.Stat Pearls Publishing.2017. Reference Source [PubMed] [Google Scholar]
- 9. Shih IM: Gestational trophoblastic neoplasia--pathogenesis and potential therapeutic targets. Lancet Oncol. 2007;8(7):642–50. 10.1016/S1470-2045(07)70204-8 [DOI] [PubMed] [Google Scholar]
- 10. Vree M, van Trommel N, Kenter G, et al. : The influence of lung metastases on the clinical course of gestational trophoblastic neoplasia: a historical cohort study. BJOG. 2016;123(11):1839–45. 10.1111/1471-0528.13622 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Vargas R, Barroilhet LM, Esselen K, et al. : Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy, and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med. 2014;59(5–6):188–94. [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Garrett LA, Garner EI, Feltmate CM, et al. : Subsequent pregnancy outcomes in patients with molar pregnancy and persistent gestational trophoblastic neoplasia. Obstet Gynecol Surv. 2008;63(11):704–5. 10.1097/01.ogx.0000335639.50781.79 [DOI] [PubMed] [Google Scholar]
- 13. Nadhan R, Vaman JV, C N, et al. : Insights into dovetailing GTD and Cancers. Crit Rev Oncol Hematol. 2017;114:77–90. 10.1016/j.critrevonc.2017.04.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Altieri A, Franceschi S, Ferlay J, et al. : Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4(11):670–8. 10.1016/S1470-2045(03)01245-2 [DOI] [PubMed] [Google Scholar]
- 15. Seckl MJ, Sebire NJ, Berkowitz RS: Gestational trophoblastic disease. Lancet. 2010;376(9742):717–29. 10.1016/S0140-6736(10)60280-2 [DOI] [PubMed] [Google Scholar]
- 16. Brown J, Naumann RW, Seckl MJ, et al. : 15years of progress in gestational trophoblastic disease: Scoring, standardization, and salvage. Gynecol Oncol. 2017;144(1):200–7. 10.1016/j.ygyno.2016.08.330 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Mello JB, Ramos Cirilo PD, Michelin OC, et al. : Genomic profile in gestational and non-gestational choriocarcinomas. Placenta. 2017;50:8–15. 10.1016/j.placenta.2016.12.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Rodabaugh KJ, Bernstein MR, Goldstein DP, et al. : Natural history of postterm choriocarcinoma. J Reprod Med. 1998;43(1):75–80. [PubMed] [Google Scholar]
- 19. Horowitz NS, Goldstein DP, Berkowitz RS: Placental site trophoblastic tumors and epithelioid trophoblastic tumors: Biology, natural history, and treatment modalities. Gynecol Oncol. 2017;144(1):208–14. 10.1016/j.ygyno.2016.10.024 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Schmid P, Nagai Y, Agarwal R, et al. : Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. 2009;374(9683):48–55. 10.1016/S0140-6736(09)60618-8 [DOI] [PubMed] [Google Scholar]
- 21. Zhao J, Lv WG, Feng FZ, et al. : Placental site trophoblastic tumor: A review of 108 cases and their implications for prognosis and treatment. Gynecol Oncol. 2016;142(1):102–8. 10.1016/j.ygyno.2016.05.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Pradjatmo H, Dasuki D, Dwianingsih EK, et al. : Malignancy risk scoring of hydatidiform moles. Asian Pac J Cancer Prev. 2015;16(6):2441–5. 10.7314/APJCP.2015.16.6.2441 [DOI] [PubMed] [Google Scholar]
- 23. Lurain JR: Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204(1):11–8. 10.1016/j.ajog.2010.06.072 [DOI] [PubMed] [Google Scholar]
- 24. Sebire NJ: Histopathological diagnosis of hydatidiform mole: contemporary features and clinical implications. Fetal Pediatr Pathol. 2010;29(1):1–16. 10.3109/15513810903266138 [DOI] [PubMed] [Google Scholar]
- 25. Karimi-Zarchi M, Mortazavizadeh MR, Soltani-Gerdefaramrzi M, et al. : Investigation of Risk Factors, Stage and Outcome in Patients with Gestational Trophoblastic Disease since 2001 to 2011 in Iran-Yazd. Int J Biomed Sci. 2015;11(4):166–72. [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Baltazar JC: Epidemiological features of choriocarcinoma. Bull World Health Organ. 1976;54(5):523–32. [PMC free article] [PubMed] [Google Scholar]
- 27. Leenharattanarak P, Lertkhachonsuk R: Quality of life in gestational trophoblastic neoplasia patients after treatment in Thailand. Asian Pac J Cancer Prev. 2015;15(24):10871–4. 10.7314/APJCP.2014.15.24.10871 [DOI] [PubMed] [Google Scholar]
- 28. Newlands ES, Paradinas FJ, Fisher RA: Recent advances in gestational trophoblastic disease. Hematol Oncol Clin North Am. 1999;13(1):225–44, x. 10.1016/S0889-8588(05)70162-3 [DOI] [PubMed] [Google Scholar]
- 29. Parazzini F, Mangili G, La Vecchia C, et al. : Risk factors for gestational trophoblastic disease: a separate analysis of complete and partial hydatidiform moles. Obstet Gynecol. 1991;78(6):1039–45. [PubMed] [Google Scholar]
- 30. Higgins HP, Hershman JM, Kenimer JG, et al. : The thyrotoxicosis of hydatidiform mole. Ann Intern Med. 1975;83(3):307–11. 10.7326/0003-4819-83-3-307 [DOI] [PubMed] [Google Scholar]
- 31. Monchek R, Wiedaseck S: Gestational trophoblastic disease: an overview. J Midwifery Womens Health. 2012;57(3):255–9. 10.1111/j.1542-2011.2012.00177.x [DOI] [PubMed] [Google Scholar]
- 32. Seckl MJ, Fisher RA, Salerno G, et al. : Choriocarcinoma and partial hydatidiform moles. Lancet. 2000;356(9223):36–9. 10.1016/S0140-6736(00)02432-6 [DOI] [PubMed] [Google Scholar]
- 33. Taylor S, Eisenstein K, Gildenstern V, et al. : Metastatic Choriocarcinoma Masquerading as a Congenital Glabellar Hemangioma. Pediatr Dev Pathol. 2019;22(1):59–64. 10.1177/1093526618765039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Smith HO, Qualls CR, Prairie BA, et al. : Trends in gestational choriocarcinoma: a 27-year perspective. Obstet Gynecol. 2003;102(5 Pt 1):978–87. 10.1016/S0029-7844(03)00669-0 [DOI] [PubMed] [Google Scholar]
- 35. Bagley R, Ren Y, Kurtzberg L, et al. : Human choriocarcinomas: placental growth factor-dependent preclinical tumor models. Int J Oncol. 2012;40(2):479–86. 10.3892/ijo.2011.1257 [DOI] [PubMed] [Google Scholar]
- 36. Candelier JJ, Frappart L, Diatta AL, et al. : Differential expression of E-cadherin, β-catenin, and Lewis x between invasive hydatidiform moles and post-molar choriocarcinomas. Virchows Arch. 2013;462(2):653–63. 10.1007/s00428-013-1427-z [DOI] [PubMed] [Google Scholar]
- 37. Slim R, Coullin P, Diatta AL, et al. : NLRP7 and the genetics of post-molar choriocarcinomas in Senegal. Mol Hum Reprod. 2012;18(1):52–6. 10.1093/molehr/gar060 [DOI] [PubMed] [Google Scholar]
- 38. Hirokawa K, Tomoda Y, Kaseki S, et al. : Recurrence of invasive moles and choriocarcinomas. Asia Oceania J Obstet Gynaecol. 1986;12(1):11–20. 10.1111/j.1447-0756.1986.tb00154.x [DOI] [PubMed] [Google Scholar]
- 39. McCormick JB: Gonadotrophin in urine and spinal fluid; quantitative studies for chorionic moles and choriocarcinomas. Obstet Gynecol. 1954;3(1):58–66. [PubMed] [Google Scholar]
- 40. Patten DK, Lindsay I, Fisher R, et al. : Gestational choriocarcinoma mimicking a uterine adenocarcinoma. J Clin Oncol. 2008;26(31):5126–7. 10.1200/JCO.2008.16.4129 [DOI] [PubMed] [Google Scholar]
- 41. Hammond CB, Weed JC, Barnard DE, et al. : Gestational trophoblastic neoplasia. CA Cancer J Clin. 1981;31(6):322–32. 10.3322/canjclin.31.6.322 [DOI] [PubMed] [Google Scholar]
- 42. Palmieri C, Fisher RA, Sebire NJ, et al. : Placental site trophoblastic tumour arising from a partial hydatidiform mole. Lancet. 2005;366(9486):688. 10.1016/S0140-6736(05)67143-7 [DOI] [PubMed] [Google Scholar]
- 43. Chiofalo B, Palmara V, Laganà AS, et al. : Fertility Sparing Strategies in Patients Affected by Placental Site Trophoblastic Tumor. Curr Treat Options Oncol. 2017;18(10):58. 10.1007/s11864-017-0502-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Bonazzi C, Urso M, Dell'Anna T, et al. : Placental site trophoblastic tumor: an overview. J Reprod Med. 2004;49(8):585–8. [PubMed] [Google Scholar]
- 45. Kim SJ, Na YJ, Jung SG, et al. : Management of high-risk hydatidiform mole and persistent gestational trophoblastic neoplasia: the Korean experience. J Reprod Med. 2007;52(9):819–30. [PubMed] [Google Scholar]
- 46. Uberti EM, Fajardo MdC, da Cunha AGV, et al. : Prevention of postmolar gestational trophoblastic neoplasia using prophylactic single bolus dose of actinomycin D in high-risk hydatidiform mole: a simple, effective, secure and low-cost approach without adverse effects on compliance to general follow-up or subsequent treatment. Gynecol Oncol. 2009;114(2):299–305. 10.1016/j.ygyno.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 47. Wang Q, Fu J, Hu L, et al. : Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2017;9:CD007289. 10.1002/14651858.CD007289.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Parker VL, Tidy JA: Current management of gestational trophoblastic disease. Obstet Gynaecol Reprod Med. 2017;27(11):338–45. 10.1016/j.ogrm.2017.08.004 [DOI] [Google Scholar]; F1000 Recommendation
- 49. Osborne RJ, Filiaci VL, Schink JC, et al. : Second Curettage for Low-Risk Nonmetastatic Gestational Trophoblastic Neoplasia. Obstet Gynecol. 2016;128(3):535–42. 10.1097/AOG.0000000000001554 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation