Abstract
SiO 2 and carbon produced by kraft lignin pyrolyzed at 600°C can generate stable reactive oxygen species (ROS) by reaction with atmospheric oxygen. In this study, we systematically investigate the photochemistry of peroxyl radicals in carbon-supported silica (PCS) and assess its effects on the methylene blue (MB) photodegradation. Characterization revealed that the higher ROS generation ability of SiO 2/carbon under UV light irradiation was attributed to its abundant photoactive surface-oxygenated functional groups.
Keywords: ROS, photochemistry, methylene blue, degradation, UV
Introduction
Consistent access to clean water has come into focus this millennium due to high pollution; a reduced amount of drinkable water could be the next challenge for the future due to overpopulation 1– 3. The application of photocatalytic technology using semiconductors to solve the environmental problems, like the degradation of organic effluents have been received much attention 4– 8. Heterogeneous photocatalysis using semiconductors is an interesting method falling into advance oxidation processes (AOPs) 9– 11 that can produce highly reactive species containing oxygen (ROS). In fact, with this method is possible to produce oxidizing molecules like hydrogen peroxide and singlet oxygen ( 1O 2) together with radicals like hydroxyl radical (OH .) and superoxide radical anion ( O 2 .- ) 12– 13. These reactants can decompose organic pollutants in wastewater giving harmless compounds 14.
Recently, N. Chen et al. reported that reactive oxygen species generation in hydrochar and photochemistry of Sulfadimidine degradation in water 15. Y. Chen et al. reported the photo degradation of tetracycline in aqueous solution under simulated sunlight irradiation through the singlet oxygen 16. Li et al. reported that the degradation of ibuprofen by UV–visible light irradiation included direct photolysis and self-sensitization via ROS 17. Wang et al. reported that when a simpler molecule without visible-light absorption is degraded, the Fe-hydroxyl complexes still promote the generation of ROS and thus accelerate degradation, although the pathway of electron transfer, and the mechanism of photocatalysis was not completely understood 18.
In literature are present many methods for photoassisted AOPs like photo-electrochemical cells composed by an anode made with boron-doped diamond and cathode in carbon nanotubes; with this system, a model azo dye was depleted 19. Also exfoliated graphene, decorated with titanium dioxide and nanoparticles, is effective for photo-catalytic water treatment 20, 21.
In our current scenario, stable peroxyl radicals in carbon-supported silica (PCS) are prepared from cheap starting materials. The method used is the pyrolysis under vacuum of kraft lignin deposited onto silica. Vacuum pyrolysis produced defective carbon bearing carbon radicals. These radicals are quickly transformed into peroxyl radicals by reaction with oxygen molecules present in the atmosphere.
Methods
The materials and methods to produce PCS using high-vacuum pyrolysis are clearly explained and characterized previously 22. In brief, kraft lignin was absorbed onto silica and pyrolyzed under vacuum at 600 °C. For the kinetic data analysis, linear quadratic fitting and other kinetic fitting (reaction order checking) were performed by using Origin v6.0.
Degradation of MB dye procedures and analyses
100-ml of air-equilibrated 10 -6 M solutions of MB (Sigma Aldrich, India) in water containing 100 mg (1 mg/ml) of neat SiO 2 or PCS were poured in quartz cylindrical reactors (90 mm diameter x 25 mm height). Solutions were magnetically stirred in the dark for 10 min before irradiation and kept under stirring during the experiment. The light source consisted of two 15-W phosphor-coated lamps (center of emission, 366 nm). Aliquots (4 ml) were withdrawn at 5-min intervals (for a total of 10-12 samples) during the irradiation until the disappearance of the color. Solids were removed by syringe filtration with a 0.4-µm pore size, and the filtrates immediately examined by UV-visible absorption spectroscopy in 1-cm quartz cuvettes using a JASCO V-630 UV-visible spectrophotometer. The absorbance was normalized by dividing the absorbance at 668 nm of the sample (A) with the absorbance of the initial solution (A 0).
Results and discussion
Degradation of MB
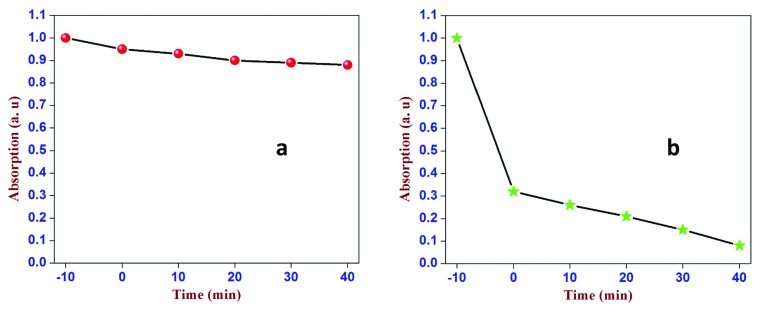
To assess the respective photocatalytic activity of PCS and of neat SiO 2, we carried out competitive experiments with MB ( Figure 1). PCS did not react with MB, in fact, solutions left for 24 hours in the dark does not show a decrease of MB concentration. Nonetheless, under dark conditions the dye was absorbed by PCS to a nearly tenfold greater extent than with pristine SiO 2 (dark region between −10 and 0 min, Figure 1b).
Figure 1.
Normalized spectral intensity of the 668 nm band of methylene blue (MB) during ( a) the UV-irradiation of the MB/SiO 2 suspension at 366 nm at different time intervals, and ( b) the same process for the MB/peroxyl radicals in carbon-supported silica (PCS) suspensions under otherwise identical conditions. The region between −10 and 0 min refers to the extent of adsorption of the MB dye under dark conditions. It shows the first-order kinetics of the photodegradation of the MB dye by MB/PCS. 3 repeats performed.
Normally photocatalysts produce radicals able to degrade organics but in the case of PCS the catalyst already possesses reactive radicals.
Simple mechanism of established photocatalysts in MB
The net effect of PCS on the photodegradation of MB is a threefold increase in the kinetics of photodegradation ( Table 1). Without the assistance of an active photocatalyst, the only reaction mechanism that is applicable is the generation of singlet oxygen by sensitization ( Equation 2) via the excited state of the dye. The singlet oxygen can react with MB, giving rise to photobleaching ( Equation 3).
Table 1. Extent of adsorption and first-order kinetics of photodegradation of methylene blue (MB) (1.0 μM) on pristine SiO 2 and on SiO 2/graphene in aqueous media under ambient atmospheric conditions and under UV irradiation at 366 nm.
| Dye | k (min −1) | Adsorption, % | ||
|---|---|---|---|---|
| SiO 2 | PCS | SiO 2 | PCS | |
| MB | 0.027 ± 0.005 | 0.092 ± 0.006 | 24 | 91 |
Dye + photon = Dye* (1)
Dye* + O 2 T =Dye + O 2 s (2)
O 2 S + Dye = oxidation products (3)
With PCS, MB is strongly absorbed onto the pyrolytic carbon present on the catalyst surface. Moreover, pyrolytic carbon possesses a high concentration of peroxyl radicals. The enhancement on the reaction kinetic could be due to a local increase of concentration of dye and active oxygen. Since the oxygen is reversibly absorbed on the carbon giving peroxyl radicals 22, the surface of the catalyst is never depleted due to the presence of oxygen in solution.
In fact, in these conditions, we can have, together with Equation 1– Equation 3, a possible reaction of the excited state of the reactant with peroxyl radicals or adsorbed oxygen on PCS ( Equation 4).
Dye* + PCS-OO = PCS + dye oxidation (4)
The peroxyl radicals are reversibly formed by capture of atmospheric oxygen due to the presence of highly active pyrolytic carbon on PCS:
PCS + O 2 = PCS-OO (5)
Another possibility is the transfer of energy (or sensitization) of the excited state of the absorbed dye directly to the defective pyrolytic carbon, giving rise to formation of ROS. All these mechanism lead to an enhancement on the degradation of MB.
Copyright: © 2018 Vadivel D and Malaichamy I
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Conclusion
This study has shown that silica can be coated successfully with pyrolytic carbon obtained from inexpensive waste materials, such as kraft lignin and silica. The pyrolytic process performed at 600°C did not affect the crystalline state of silica when it was coated with carbon. The photocatalytic activity was measured against pristine SiO 2 through an examination of the kinetics of degradation of MB by UV-vis spectroscopy. Under UV light irradiation, the degradation was threefold greater for the MB-PCS compared with MB-silica.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Vadivel D and Malaichamy I
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1: Raw data for the article ‘Pyrolytic formation and photoactivity of reactive oxygen species in a SiO2/carbon nanocomposite from kraft lignin’ are presented, 10.5256/f1000research.16080.d218907 23
Acknowledgments
We wish to thank Prof. Nick Serpone of the PhotoGreen Laboratory of the Department of Chemistry at the University of Pavia for useful discussions.
Funding Statement
We are grateful to the PANACEA - ERASMUS MUNDUS of the European Commission within the project Agreement Number 2012-2647/001-001 - EMA2 for an Action 2 scholarship in support of D.V.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Vinu R, Madras G: Environmental remediation by photocatalys. J Indian Inst Sci. 2010;90(2):189–230. Reference Source [Google Scholar]
- 2. Lachheb H, Puzenat E, Houas A, et al. : Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl Catal B. 2002;39(1):75–90. 10.1016/S0926-3373(02)00078-4 [DOI] [Google Scholar]
- 3. Qu X, Alvarez PJ, Li Q: Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47(12):3931–3946. 10.1016/j.watres.2012.09.058 [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues S, Ranjit KT, Uma S, et al. : Single-step synthesis of a highly active visible-light photocatalyst for oxidation of a common indoor air pollutant: acetaldehyde. Adv Mater. 2005;17(20):2467–2471. 10.1002/adma.200402064 [DOI] [Google Scholar]
- 5. Kisch H, Macyk W: Visible-light photocatalysis by modified titania. Chem Phys Chem. 2002;3(5):399–400. [DOI] [PubMed] [Google Scholar]
- 6. Davis AP, Green DL: Photocatalytic oxidation of cadmium-EDTA with titanium dioxide. Environ Sci Technol. 1999;33(4):609–617. 10.1021/es9710619 [DOI] [Google Scholar]
- 7. Choi H, Sofranko AC, Dionysiou DD: Nanocrystalline TiO 2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: synthesis, characterization, and multifunction. Adv Funct Mater. 2006;16(8):1067–1074. 10.1002/adfm.200500658 [DOI] [Google Scholar]
- 8. El-Bahy ZM, Ismail AA, Mohamed RM: Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J Hazard Mater. 2009;166(1):138–143. 10.1016/j.jhazmat.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 9. Saquib M, Muneer M: TiO 2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes Pigments. 2003;56(1):37–49. 10.1016/S0143-7208(02)00101-8 [DOI] [Google Scholar]
- 10. Muruganandham M, Swaminathan M: Solar photocatalytic degradation of a reactive azo dye in TiO 2-suspension. Sol Energy Mater Sol Cells. 2004;81(4):439–457. 10.1016/j.solmat.2003.11.022 [DOI] [Google Scholar]
- 11. Kaur S, Singh V: Visible light induced sonophotocatalytic degradation of Reactive Red dye 198 using dye sensitized TiO 2. Ultrason Sonochem. 2007;14(5):531–537. 10.1016/j.ultsonch.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 12. Ince NH, Tezcanli G, Belen RK, et al. : Ultrasound as a catalyzer of aqueous reaction systems: the state of the art and environmental applications. Appl Catal B. 2001;29(3):167–176. 10.1016/S0926-3373(00)00224-1 [DOI] [Google Scholar]
- 13. Ince NH, Tezcanli G: Reactive dyestuff degradation by combined sonolysis and ozonation. Dyes Pigments. 2001;49(3):145–153. 10.1016/S0143-7208(01)00019-5 [DOI] [Google Scholar]
- 14. Wang J, Zhang YY, Guo Y, et al. : Interaction of bovine serum albumin with Acridine Orange (C.I. Basic Orange 14) and its sonodynamic damage under ultrasonic irradiation. Dyes Pigments. 2009;80(3):271–278. 10.1016/j.dyepig.2008.07.013 [DOI] [Google Scholar]
- 15. Chen N, Huang Y, Hou X, et al. : Photochemistry of Hydrochar: Reactive Oxygen Species Generation and Sulfadimidine Degradation. Environ Sci Technol. 2017;51(19):11278–11287. 10.1021/acs.est.7b02740 [DOI] [PubMed] [Google Scholar]
- 16. Yong C, Hu C, Qu J, et al. : Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J Photochem Photobiol A Chem. 2008;197(1):81–87. 10.1016/j.jphotochem.2007.12.007 [DOI] [Google Scholar]
- 17. Li FH, Yao K, Lv WY, et al. : Photodegradation of ibuprofen under UV-Vis irradiation: mechanism and toxicity of photolysis products. Bull Environ Contam Toxicol. 2015;94(4):479–483. 10.1007/s00128-015-1494-8 [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Liu Z, Cai R: A new role for Fe 3+ in TiO 2 hydrosol: accelerated photodegradation of dyes under visible light. Environ Sci Technol. 2008;42(15):5759–5764. 10.1021/es800616b [DOI] [PubMed] [Google Scholar]
- 19. Vahid B, Khataee A: Photoassisted electrochemical recirculation system with boron-doped diamond anode and carbon nanotubes containing cathode for degradation of a model azo dye. Electrochimica Acta. 2013;88:614–620. 10.1016/j.electacta.2012.10.069 [DOI] [Google Scholar]
- 20. Zhang H, Lv X, Li Y, et al. : P25-graphene composite as a high performance photocatalyst. ACS Nano. 2010;4(1):380–386. 10.1021/nn901221k [DOI] [PubMed] [Google Scholar]
- 21. Lightcap IV, Kosel TH, Kamat PV: Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. Storing and shuttling electrons with reduced graphene oxide. Nano Lett. 2010;10(2):577–583. 10.1021/nl9035109 [DOI] [PubMed] [Google Scholar]
- 22. Vadivel D, Speltini A, Zeffiro A, et al. : Reactive carbons from Kraft lignin pyrolysis: Stabilization of peroxyl radicals at carbon/silica interface. J Anal Appl Pyrol. 2017;128:346–352. 10.1016/j.jaap.2017.09.016 [DOI] [Google Scholar]
- 23. Vadivel D, Malaichamy I: Dataset 1 in: Pyrolytic formation and photoactivity of reactive oxygen species in a SiO2/carbon nanocomposite from kraft lignin. F1000Research. 2018. 10.5256/f1000research.16080.d218907 [DOI] [PMC free article] [PubMed] [Google Scholar]