Abstract
Background
Group therapy offers individuals the opportunity to learn behavioural techniques for smoking cessation, and to provide each other with mutual support.
Objectives
To determine the effect of group‐delivered behavioural interventions in achieving long‐term smoking cessation.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register, using the terms 'behavior therapy', 'cognitive therapy', 'psychotherapy' or 'group therapy', in May 2016.
Selection criteria
Randomized trials that compared group therapy with self‐help, individual counselling, another intervention or no intervention (including usual care or a waiting‐list control). We also considered trials that compared more than one group programme. We included those trials with a minimum of two group meetings, and follow‐up of smoking status at least six months after the start of the programme. We excluded trials in which group therapy was provided to both active therapy and placebo arms of trials of pharmacotherapies, unless they had a factorial design.
Data collection and analysis
Two review authors extracted data in duplicate on the participants, the interventions provided to the groups and the controls, including programme length, intensity and main components, the outcome measures, method of randomization, and completeness of follow‐up. The main outcome measure was abstinence from smoking after at least six months follow‐up in participants smoking at baseline. We used the most rigorous definition of abstinence in each trial, and biochemically‐validated rates where available. We analysed participants lost to follow‐up as continuing smokers. We expressed effects as a risk ratio for cessation. Where possible, we performed meta‐analysis using a fixed‐effect (Mantel‐Haenszel) model. We assessed the quality of evidence within each study and comparison, using the Cochrane 'Risk of bias' tool and GRADE criteria.
Main results
Sixty‐six trials met our inclusion criteria for one or more of the comparisons in the review. Thirteen trials compared a group programme with a self‐help programme; there was an increase in cessation with the use of a group programme (N = 4395, risk ratio (RR) 1.88, 95% confidence interval (CI) 1.52 to 2.33, I2 = 0%). We judged the GRADE quality of evidence to be moderate, downgraded due to there being few studies at low risk of bias. Fourteen trials compared a group programme with brief support from a health care provider. There was a small increase in cessation (N = 7286, RR 1.22, 95% CI 1.03 to 1.43, I2 = 59%). We judged the GRADE quality of evidence to be low, downgraded due to inconsistency in addition to risk of bias. There was also low quality evidence of benefit of a group programme compared to no‐intervention controls, (9 trials, N = 1098, RR 2.60, 95% CI 1.80 to 3.76 I2 = 55%). We did not detect evidence that group therapy was more effective than a similar intensity of individual counselling (6 trials, N = 980, RR 0.99, 95% CI 0.76 to 1.28, I2 = 9%). Programmes which included components for increasing cognitive and behavioural skills were not shown to be more effective than same‐length or shorter programmes without these components.
Authors' conclusions
Group therapy is better for helping people stop smoking than self‐help, and other less intensive interventions. There is not enough evidence to evaluate whether groups are more effective, or cost‐effective, than intensive individual counselling. There is not enough evidence to support the use of particular psychological components in a programme beyond the support and skills training normally included.
Plain language summary
Do group‐based smoking cessation programmes help people to stop smoking?
Background
One approach to help people who are trying to quit smoking is to offer them group‐based support. Participants meet regularly, with a facilitator who is typically trained in smoking cessation counselling. Programme components are varied. A perceived strength of this approach is that participants provide each other with support and encouragement. The outcome of interest was not smoking at least six months from the start of the group programme.
Study characteristics
We identified 66 trials comparing group‐based programmes to other types of support, or comparing different types of group programme. The most recent search was in May 2016.
Results & quality of evidence
In 13 trials (4395 participants) people in the control conditions were provided with a self‐help programme. There was a benefit for the group‐based approach, with the chance of quitting increased by 50% to 130%. This means that if five in 100 people were able to quit for at least six months using self‐help materials, eight to 12 in 100 might be successful if offered group support. We judged the quality of this evidence as moderate, because studies did not report methods in enough detail to exclude possible bias. There was also evidence of a benefit of group support compared to advice and brief support from a healthcare professional (14 trials, 7286 participants), although the difference was smaller and more variable. We rated this as low‐quality evidence, because of the variability as well as possible risk of bias. There was also low‐quality evidence of a benefit in studies that did not provide the control group with any help to quit (9 trials, 1098 participants). Six trials (980 participants) compared group format with individual face‐to‐face counselling; there was no sign that one approach was more helpful than the other. The remaining studies compared different types of group programmes; typically they did not show differences, so it is not possible to show which components of group‐based programmes are most helpful.
Summary of findings
Summary of findings for the main comparison. Group‐format behavioural programmes compared to alternative support for smoking cessation.
| Patient or population: People who smoke Setting: Smoking cessation clinics predominantly recruiting people interested in quitting smoking, from community and healthcare settings Intervention: Group‐format behavioural programmes Comparison: Various | ||||||
| Outcome: Smoking cessation assessed at least 6 months after start of treatment, based on self‐report, ± biochemical validation of abstinence | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Numbers quit in control conditions | Numbers quit after group programme | |||||
| Group programme compared to self‐help programme | Moderate | RR 1.88 (1.52 to 2.33) | 4395 (13 RCTs) 2 | ⊕⊕⊕⊝ MODERATE 3 | ||
| 5 per 100 1 | 9 per 100 (8 to 12) | |||||
| Group programme compared to brief support | Moderate | RR 1.25 (1.07 to 1.46) | 7601 (16 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 5 per 100 5 | 6 per 100 (5 to 7) | |||||
| Group programme compared to face‐to‐face individual intervention | Moderate | RR 0.99 (0.76 to 1.28) | 980 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 11 per 100 6 | 11 per 100 (8 to 14) | |||||
| Group programme plus pharmacotherapy versus pharmacotherapy and brief support alone | Moderate | RR 1.11 (0.93 to 1.33) | 1523 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 8 | ||
| 18 per 100 7 | 20 per 100 (17 to 24) | |||||
| Group programme versus 'no intervention' controls | Moderate | RR 2.60 (1.80 to 3.76) | 1098 (9 RCTs) | ⊕⊕⊝⊝ LOW 3 9 | ||
| 5 per 100 | 13 per 100 (9 to 19) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Moderate assumed control condition quit rate, mid way between higher crude average and lower weighted average. 2Including 4 cluster‐randomized studies. 3Most studies at unclear risk of bias for allocation and concealment, probably reflecting poor reporting in old studies. Downgraded, but potential for large over‐ or under‐estimation of effect size low. 4Heterogeneity in pooled estimate. 5Based on crude average in control conditions. The weighted average would be 4%; one large trial had very low quit rates from brief support. 6Based on average in studies of individual counselling without pharmacotherapy from review of individual counselling. 7Based on control condition rate from review of behavioural adjuncts to pharmacotherapy. 8Downgraded due to imprecision. A larger review of the effect of increasing behavioural support as an adjunct to pharmacotherapy detected a small benefit. 9Most studies did not biochemically validate abstinence.
Background
Group therapy is a common method of delivering smoking cessation interventions. Over 100 group therapies have been described (Hajek 1996). The purposes of group programmes have been summarized as: to analyse motives for group members' behaviour; to provide an opportunity for social learning; to generate emotional experiences; and to impart information and teach new skills (Hajek 1985; Hajek 1996). Group programmes may be led by professional facilitators such as clinical psychologists, health educators, nurses or physicians, or occasionally by successful users of the programme.
The implementation of smoking cessation programmes in groups has been a popular method of delivering behavioural interventions. Behavioural interventions typically include such methods as coping and social skills training, contingency management, self‐control, and cognitive‐behavioural interventions. The use of a group format for the delivery of a behavioural intervention appears to have two underlying rationales. Lying between self‐help methods with minimal therapist contact and intensive individual counselling/therapy, a group might offer better cessation rates than the former, with lower costs per smoker than the latter. There may be a specific therapeutic benefit of the group format in giving people who smoke the opportunity to share problems and experiences with others attempting to quit. This might lead to increased quit rates, even compared to individual face‐to‐face methods.
More recent research has focused on identifying the components that contribute most to the success of the intervention. In particular, there is interest in ways to enhance programmes with components which could be specifically helpful for those with poor success rates for quitting, such as people with histories of depressive disorder or substance abuse. In addition to evaluating the benefit of generic group behaviour therapy for smoking cessation, this review evaluates the evidence for including specific strategies or psychological techniques in group programmes.
Objectives
To determine the effect of group‐delivered behavioural interventions in achieving long‐term smoking cessation.
Methods
Criteria for considering studies for this review
Types of studies
Trials were eligible for inclusion if participants were randomly allocated to treatment conditions. We included trials of worksite smoking cessation programmes which randomized worksites to different programmes. We also included studies that randomized therapists, rather than smokers, to offer group therapy or control, provided that the specific aim of the study was to examine the effect of group therapy on smoking cessation.
Types of participants
Adult smokers of either gender, irrespective of their initial level of nicotine dependency, recruited from any setting, with the exception of trials recruiting pregnant women in antenatal care settings, since interventions for pregnant women are reviewed separately (Chamberlain 2013). Interventions recruiting only adolescent smokers are also reviewed separately (Grimshaw 2013).
Types of interventions
We considered studies in which smokers met for scheduled meetings and received some form of behavioural intervention, such as information, advice and encouragement or cognitive behavioural therapy (CBT) delivered over at least two sessions. We excluded studies of interventions where participants met once for an orientation or information session. We excluded studies which covered group meetings but which were primarily investigating the efficacy of aversive smoking, acupuncture, hypnotherapy, exercise or partner support, unless there were other relevant arms. Trials investigating these specific components have been separately reviewed by Hajek 2001, White 2014, Barnes 2010, Ussher 2014 and Park 2012 respectively. We exclude trials of components to prevent relapse, as they are covered by a separate review (Hajek 2013). Trials in which smokers received group therapy in addition to active or placebo pharmacotherapy were excluded unless there were other relevant arms. The effect of nicotine replacement therapy (NRT) is evaluated in a separate review (Stead 2012), but we include studies which tested group therapy as an adjunct to nicotine replacement.
Types of outcome measures
The main outcome was abstinence from cigarettes at follow‐up at least six months after the start of treatment. We excluded trials that reported only shorter follow‐up or had no measurement of smoking cessation. In each study we used the strictest available criteria to define abstinence. For example, in studies where biochemical validation of cessation was available, we counted as abstinent only those participants who met the criteria for biochemically‐confirmed abstinence. Wherever possible, we used a sustained cessation rate, rather than point prevalence. Where participants were lost to follow‐up, we regarded them as being continuing smokers.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Group Specialized Register for reports of studies with the keywords 'Behavio$r therapy', 'Group therapy' or 'Cognitive therapy' or free‐text terms 'behav*' and 'group*'. At the time of the search in May 2016 the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 4, 2016; MEDLINE (via OVID) to update 20160513; Embase (via OVID) to week 201621; PsycINFO (via OVID) to update 20160516. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and lists of other resources searched. The most recent search was conducted in May 2016. For earlier versions of the review we also checked the US Public Health Service Clinical Practice Guidelines on smoking cessation (Fiore 1996; Fiore 2008) reference lists for trials used in meta‐analyses assessing the efficacy of different treatment formats and the components of effective interventions.
Data collection and analysis
LS (Cochrane Information Specialist for the Tobacco Addiction Group) identified trials which met the screening criteria of having one group therapy arm and sufficient length of follow‐up. For the update in 2017, LS and AC independently checked and data‐extracted reports of potentially relevant interventions.
Assessment of risk of bias in included studies
We assessed risks of selection bias based on methodology in the Cochrane Handbook (section 8.5) (Higgins 2011). We assessed risk of detection bias based on whether self‐reported smoking cessation was biochemically validated. Methods for validating abstinence include measuring carbon monoxide in exhaled air, and measuring cotinine, a metabolite of nicotine, in saliva or urine. We judged studies using any method of validation to be at low risk of bias. We assessed risk of attrition bias as high if loss to follow‐up was both high and differed across study arms.
Measures of treatment effect & data synthesis
We summarized individual study results as a risk ratio, calculated as: (number of quitters in intervention group/ number randomized to intervention group) / (number of quitters in control group/ number randomized to control group). We assumed that participants lost to follow‐up were continuing smokers and included them in denominators. We excluded any deaths from denominators. Where appropriate, we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect method to estimate a pooled risk ratio with a 95% confidence interval (Greenland 1985). We estimated the amount of statistical heterogeneity between trials using the I2 statistic (Higgins 2003). Values over 50% can be regarded as moderate heterogeneity, and values over 75% as high.
If trial reports did not present results in a form which allowed extraction of the necessary key data, we attempted to contact investigators.
If a trial had both a comparable programme with non‐group delivery, and a waiting‐list or minimal‐intervention control, we included both in the appropriate comparisons. If two different group programmes were compared with another method or a control, we combined the group interventions in the comparison of group versus non‐group methods. We made the following comparisons, based on the characteristics of the comparator condition: 1.1 Groups versus self‐help programmes: 1.1.1 Group therapy plus self‐help manuals versus the same self‐help programme alone 1.1.2 Group therapy plus self‐help manuals versus a different self‐help programme 1.2 Group versus other less intensive interventions: 1.2.1 Group therapy versus physician, nurse, or pharmacist advice 1.2.2 Group therapy versus health education
1.3 Group therapy plus pharmacotherapy versus pharmacotherapy alone
1.4 Group therapy versus individual counselling sessions: 1.4.1 Group versus individual therapy, similar intensity, same programme content 1.4.2 Group versus individual therapy, similar intensity, different programme content 1.5 Group therapy versus no intervention (including usual care, minimal contact or a waiting‐list control) 2 Comparisons between group programmes (with and without matching for intensity and contact time) 2.1 Skills training 2.2 Mood management 2.3 Manipulation of group dynamics 2.4 Other miscellaneous comparisons
Results
Description of studies
We include 66 studies in this review. Forty studies compared a group programme with a non‐group‐based cessation intervention, or a no‐intervention control (Glasgow 1981; Pederson 1981; Cottraux 1983; Rabkin 1984; McDowell 1985; DePaul 1987; Curry 1988; Omenn 1988; DePaul 1989; Garcia 1989; Leung 1991; Ginsberg 1992; Gruder 1993; Hill 1993; Hilleman 1993; Hollis 1993; Sawicki 1993; Batra 1994; DePaul 1994; Rice 1994; Jorenby 1995; Nevid 1997; Bakkevig 2000; García 2000; Minthorn‐Biggs 2000; Camarelles 2002; Hall 2002; Grant 2003; Pisinger 2005; Romand 2005; Slovinec 2005; Otero 2006; Zheng 2007; Wilson 2008; Dent 2009; Rovina 2009; Pisinger 2010; Webb 2010; Gifford 2011; Schleicher 2012). Some of these compared group therapy with more than one alternative and were included in each relevant comparison group. Some compared more than one programme or used a factorial design, and in most cases we collapsed the factorial structure and combined different group programmes in the comparison with a non‐group control. The other 26 studies did not have a non‐group control and contribute only to comparisons between different group‐based programmes.
Most studies recruited community volunteers prepared to participate in group programmes. Three studies recruited in primary care settings (McDowell 1985; Hollis 1993; Pisinger 2010). Other studies recruited participants with a diagnosed cardiovascular health problem (Rice 1994), people with diabetes (Sawicki 1993), people with schizophrenia (George 2000), participants in an outpatient alcohol treatment programme (Grant 2003), and people in an inpatient alcohol treatment programme (Mueller 2012). Three studies conducted at DePaul University recruited employees in worksites which had been randomly assigned to provide different programme formats. One other study (Omenn 1988) also recruited at a worksite, but individual smokers were randomized to treatment.
Two studies recruited only women (Slovinec 2005; Schmitz 2007). One Chinese study recruited predominantly men (Zheng 2007). Two studies recruited only African‐American smokers (Matthews 2009; Webb 2010).
The group programmes varied in their length, format and content. The description in the table Characteristics of included studies gives the number and length of sessions and brief details of main components of the intervention. Most programmes used between six and eight sessions, with the first few sessions devoted to discussion of motivation for quitting, health benefits, and strategies for planning a quit attempt. Specific components at this stage may include signing a contract to quit, or making a public declaration, and nicotine fading (changing the type of cigarette smoked to a lower nicotine brand). Participants may also keep records of the number of cigarettes smoked and the triggers for smoking (self‐monitoring). Part of the group process also includes discussion and sharing of experiences and problems (intra‐treatment social support). Participants may also be instructed on ways to seek appropriate support from friends, colleagues and family (extra‐treatment social support). A range of other problem‐solving skills may also be introduced, including identifying high‐risk situations for relapse, generating solutions and discussing or rehearsing responses. Some programmes incorporate more specific components intended to help manage poor mood or depression associated with quitting and withdrawal.
Most studies followed participants for 12 months. Twenty‐two out of 66 (33%) had only six months follow‐up (Glasgow 1981; Pederson 1981; Rabkin 1984; Garcia 1989; Glasgow 1989; Goldstein 1989; Leung 1991; Sawicki 1993; Digiusto 1995; Jorenby 1995; Bushnell 1997; George 2000; Minthorn‐Biggs 2000; Camarelles 2002; Zheng 2007; Dent 2009; Matthews 2009; Macpherson 2010; Webb 2010; Aytemur 2012; Mueller 2012; Schleicher 2012). One study has reported five‐year follow‐up (Pisinger 2005). Of the studies with one‐year follow‐up, 23 reported an outcome requiring a sustained period of cessation; 13 with non‐group controls (DePaul 1987; Curry 1988; DePaul 1989; Gruder 1993; Hollis 1993; Batra 1994; DePaul 1994; Nevid 1997; Hall 2002; Romand 2005; Wilson 2008; Rovina 2009; Ramos 2010) and nine with only between‐group comparisons (Lando 1990; Lando 1991; Zelman 1992; Hall 1994; Hall 1996; Hall 1998; Brown 2001; Patten 2002; Batra 2010). Three of these did not require biochemical validation at longest follow‐up so there were 10 studies with one‐year sustained and validated quit rates contributing to the non‐group control comparisons (Curry 1988; DePaul 1989; Hollis 1993; DePaul 1994; Nevid 1997; Hall 2002; Romand 2005; Wilson 2008; Rovina 2009; Ramos 2010).
1 Comparisons between group therapy interventions and non‐group controls
1.1 Comparison of group versus self‐help programmes
Four studies compared a group programme with the same content provided by written materials alone. Curry 1988 tested two approaches, one emphasizing absolute abstinence and the other using a relapse prevention approach. Glasgow 1981 compared three different programmes suitable for self‐help use. Two were manuals using a structured behaviour therapy approach, the third was a multimedia quit kit with tips for quitting. All of these programmes lasted for eight weeks. García 2000 compared a 10‐session five‐week programme, a five‐session programme, and a five‐session programme plus self‐help manual, versus use of a self‐help manual alone. Rice 1994 used the shorter Smokeless programme. In this study the self‐help participants received five telephone calls during the two‐week programme to remind them to open the envelopes containing the appropriate booklet for the day. A further four trials included in this subgroup used a group programme as an adjunct to a televised cessation programme as well as self‐help materials. Three of these recruited smokers from worksites which had been randomly assigned to provide manuals or additional group meetings (DePaul 1987; DePaul 1989; DePaul 1994). In the fourth, smokers who had registered to receive a self‐help manual were randomized to receive the materials alone or additional group programmes (Gruder 1993). This study tested two different group programmes, both of three sessions. Their results are combined for comparison with self‐help.
Five studies did not use an identical programme manual for the group and self‐help conditions. In one the participants randomized to use self‐help were allowed a choice of manuals (Hollis 1993). In addition during a single meeting with the health counsellor they were encouraged to set a quit date, and one follow‐up telephone call was arranged. They were then mailed tip sheets and six bi‐monthly newsletters. Randomized participants who did not visit the health counsellor to receive their materials were mailed the appropriate programme, so a proportion of those assigned to group therapy effectively received a self‐help intervention. In a third treatment condition participants were randomized to make a choice between self‐help materials and attending a group programme, but we have not included this in a formal comparison. Hilleman 1993 gave no details of the programme used in the group format but the self‐help component consisted of a brief pamphlet. In this factorial trial of behavioural components and clonidine there was no evidence for an interaction with the pharmacotherapy, so the clonidine/placebo arms were collapsed. In Omenn 1988 participants with a stated preference for a group programme, and participants with no preference, were randomized to attend either a three‐ or an eight‐week group programme, or to use a self‐help guide alone. The two group programmes are combined in the analysis. Nevid 1997 compared a culturally‐tailored programme for Hispanic smokers with an enhanced self‐help programme which included one meeting and telephone contact. Batra 1994 compared a group and a self‐help approach.
1.2 Comparison of group therapy versus brief cessation support
Group therapy compared to physician or nurse advice
Of the 14 studies in this comparison, nine recruited from a healthcare setting. Two of the studies that compared different programme delivery formats also included an advice‐only control (Hollis 1993; Rice 1994). Hollis 1993 included a condition in which participants received the same 30‐second health provider advice as other arms, and in addition a brief pamphlet from the health counsellor. Rice 1994 included a no‐intervention control, but this included advice from a clinical nurse specialist to quit smoking because of the participants' cardiovascular health problems. In three other trials the physician advice was an alternative to a group programme. McDowell 1985 compared two different group programmes with an intervention in which participants were asked to attend a 15‐minute appointment with their physician for smoking cessation advice and a self‐help booklet. Sawicki 1993 compared referral to a group programme to referral for a 15‐minute physician advice session. Cottraux 1983 compared a three‐session group programme to two 10‐minute meetings with a doctor who prescribed a placebo. The authors describe this as a placebo control and the function of the doctor was to recommend the use of the tablets (which contained lactose) rather than to give other support. Bakkevig 2000 recruited community volunteers who were allocated to attend a group programme or to go and ask their physician for help. Only 36% consulted their general practitioner whilst 75% attended at least one programme session. In a factorial design with community volunteers, Hall 2002 randomized participants to pharmacotherapy with bupropion or nortriptyline or placebo, along with advice from a physician. Half of all these groups were randomized to an additional five‐session group‐based psychological intervention. Slovinec 2005 randomized women to either three physician visits alone or the addition of a group programme focused on stress management. Pisinger 2005 provided a single session of lifestyle counselling in a population‐based trial and offered the intervention group participation a six‐session group course over five months. Otero 2006 compared different schedules of group intervention to a single 20‐minute session. There was also randomization to nicotine patch or no‐patch conditions; the no‐patch conditions are used in this comparison. Wilson 2008 recruited people with chronic obstructive pulmonary disease attending outpatient appointments. All received standardized brief advice to stop smoking. Dent 2009 included Veterans in a VA hospital who were referred by their physician to the smoking cessation pharmacy clinic. Participants either attended the “Vets without cigarettes” group‐based programme, which is based on theories of behaviour change, or received a standard care 10‐ to 15‐minute counselling phone call from a pharmacist. All participants were offered free bupropion or NRT. Ramos 2010, in addition to the individual counselling control noted above, included a "minimal intervention" condition consisting of brief physician advice to quit. All participants were offered NRT or bupropion at the physician’s discretion. In a cluster‐randomized trial in Danish primary care centres, Pisinger 2010 compared group‐based treatment to usual physician‐delivered advice; NRT was recommended to all participants.
Group therapy compared to health education
Rabkin 1984 compared a group programme to an intervention described as health education, consisting of a single group meeting which included a lecture on the health consequences of smoking. Participants decided on a method and made a commitment to quit, then had a single individual counselling session one week later. Romand 2005 compared the 'Five Day Plan' programme to a single session of information on health consequences.
1.3 Comparison of group therapy plus pharmacotherapy versus pharmacotherapy with brief support
In the comparisons above, pharmacotherapy was not systematically provided to all participants. Five trials included comparisons in which both intervention and control conditions had access to cessation medication. Ginsberg 1992 compared a prescription of nicotine gum plus a four‐week behavioural programme to nicotine gum plus two group sessions at which participants were given educational materials. Jorenby 1995 compared an eight‐week group programme to a minimal‐contact control group in which participants just used 22 mg or 44 mg nicotine patches and attended weekly assessment sessions without counselling. Otero 2006, as noted above, compared multiple sessions to a single 20‐minute session, and this comparison included arms allocated to use nicotine patch for eight weeks. Rovina 2009 compared group counselling (either CBT or supportive counselling) to brief (< 15 minutes) physician advice; all participants received bupropion. Gifford 2011 compared bupropion plus 10 weeks of combined group and individual counselling using functional analytic psychotherapy and acceptance and commitment therapy to bupropion and medication instructions only.
1.4 Comparison of group versus individual format therapy
Six trials compared a group‐based intervention with a multisession individual counselling intervention. Three had comparable intensity in terms of number of visits; one trial (Rice 1994), already noted in previous comparisons, compared group treatment with an individual intervention using the same Smokeless programme. Participants met with a clinical nurse specialist therapist for the same schedule of meetings as in the group format. The second in this category (Garcia 1989) compared group therapy to individual sessions with a doctor; all participants also received nicotine gum. The third compared the same schedule of group or individual meetings with a nurse who offered nicotine patch to participants willing to make a quit attempt (Wilson 2008). The other three studies had less individual than group contact time: Jorenby 1995, in addition to the minimal contact control used, also tested an individual counselling condition consisting of three brief sessions from a nurse at one, two and four weeks. Participants in each format were also randomly assigned to receive one of two doses of nicotine patch. Camarelles 2002 compared a seven‐session group therapy programme to two individual sessions, with encouragement to use nicotine patch for addicted participants. Ramos 2010 compared a group versus individual intervention for smokers in the preparatory stage of smoking cessation. Care was delivered by a "microteam" comprising a physician and a nurse, who also offered NRT or bupropion, as medically appropriate. The authors reported that individuals in the group intervention received six times more contact time than participants in the individual intervention. One trial (Smith 2001) previously contributing to this category now contributes to the relapse prevention review (Hajek 2013), because the two interventions compared were not offered until after the quit date.
1.5 Comparison of group therapy versus 'no intervention' controls
Nine trials included control conditions which we considered to have little or no specific content to encourage cessation. Hill 1993 used an exercise programme as a placebo control condition. The exercise arm did however receive a self‐help stop‐smoking pamphlet and encouragement to quit. McDowell 1985 included a control of smokers who had volunteered for the study but were asked only to complete smoking diaries and questionnaires at follow‐up. In one study (Grant 2003), the controls had access to standard smoking cessation resources at the substance abuse treatment centre they were attending. Schleicher 2012 recruited college students with elevated depressive symptoms to participate in six sessions of a CBT mood management smoking cessation group or a nutrition group with equal contact time, but no smoking‐related content. The remaining five trials had waiting‐list controls (Pederson 1981; Cottraux 1983; Leung 1991; Minthorn‐Biggs 2000; Zheng 2007).
2. Comparisons between different group programmes
Trials in this comparison tested a range of different components for enhancing abstinence as part of group‐based programmes. We now exclude trials of relapse prevention components because they are covered by a separate review (Hajek 2013). We include other skills training or cognitive behavioural therapy (CBT) approaches that did not specifically address relapse prevention. We distinguish between trials that added a component and those that attempted to control for contact time by substituting an alternative component. We consider separately trials which specifically addressed mood management. We include as a separate subgroup in this comparison a trial comparing two public service programmes which differ in length.
2.1 Skills training
Nine trials contributed data to this category. Five trials substituted components in a programme, controlling for length. McDowell 1985 compared a nine‐session cognitive behaviour modification programme led by a psychologist to a programme led by a health educator. Goldstein 1989 compared two 11‐week courses; a behavioural programme which included skills training versus an educational programme which included non‐specific group support. Zelman 1992 compared two weeks of skills training or supportive counselling crossed with nicotine gum provision or a rapid smoking procedure. The nicotine exposure conditions are collapsed in this analysis. Ward 2001 added a cognitive counter‐conditioning (CCC) component to a four‐session programme which also included instruction in the use of NRT, and discussion of the concepts of self‐efficacy and the stages of change. In the CCC component participants jointly developed negative schema about smoking which they were to rehearse mentally whenever they had a cigarette. Rovina 2009, as noted above, compared CBT counselling to supportive group counselling composed of weekly sessions for one month followed by sessions every three weeks for the remaining 19 weeks. All participants in these groups were also given bupropion; the authors also included a condition in which participants attended CBT counselling but did not receive bupropion, but those data are not included in the meta‐analysis.
Four trials tested the effect of adding or extending sessions in a programme. Lando 1985 added six post‐quit sessions to a cessation programme using nicotine fading. Minthorn‐Biggs 2000 compared a 16‐session programme emphasizing social interaction and coping against a shorter American Lung Association programme. Huber 2003 compared a programme of five 90‐minute weekly meetings that included contracting, reinforcement, relaxation, skills training components to the same schedule of meetings lasting only 45 minutes where the focus was on sharing experiences. Nicotine gum was available to all participants. Otero 2006 compared programmes with three or four weekly hour‐long sessions to one or two sessions. Conditions with and without nicotine patch were collapsed in this analysis.
2.2 Mood Management
Seven studies investigated the use of a mood management intervention (either cognitive‐behavioural or behavioural activation) to manage the occurrence of negative mood. In five (Hall 1996; Brown 2001; Patten 2002; Brown 2007; Macpherson 2010) the contact time was matched. In two studies (Hall 1994; Hall 1998) the mood management intervention was compared with a shorter programme. Three of these studies had a factorial design with randomization to nicotine gum or placebo (Hall 1996), nortriptyline (Hall 1998) or bupropion (Brown 2007). These arms were collapsed in this meta‐analysis. Macpherson 2010 compared standard smoking cessation treatment to standard treatment plus behavioural activation, a treatment for depression that is one component of CBT, in which participants were taught to engage in non‐smoking reinforcing activities, activity monitoring and planning, and identification of values and goals. All participants also received eight weeks of nicotine patches.
2.3 Manipulation of group dynamics
Some of the studies already described had differences in group processes arising from the emphasis on skills or on discussion, but four studies specifically focused on manipulating the group dynamics. Digiusto 1995 compared a group programme which emphasized social support with one concentrating on self‐control. The organization of the groups differed, with the first emphasizing contact with other participants, the other using a didactic format and discouraging contact with other attenders. However other components were also varied, for example skills training instruction was given only in the self‐control group. The study hypothesis was that the treatments would show differential treatment effect with smokers of different personality types. Etringer 1984 and Lando 1991 manipulated the group environment in a less extreme way. Their programmes were intensive, lasting for 16 sessions over nine weeks. In an 'enriched cohesiveness' intervention, exercises focusing on the importance of self‐disclosure and feedback to other group members were introduced to facilitate positive group interaction. Etringer 1984 also compared a programme which included a satiation smoking procedure to one using nicotine fading. Their hypothesis was that group cohesiveness was already developed by the aversive smoking routine, so that the cohesiveness manipulation would be most effective in combination with nicotine fading. We collapse these two conditions. Schmitz 2007 compared a programme of CBT with a programme that focused on enhancing group support, both delivered over seven weekly meetings.
2.4 Other miscellaneous comparisons
Twelve studies do not fit within the broad categories above, either because they compared multiple different conditions, or because they did not use interventions comparable to other studies. They do not contribute substantially to the conclusions drawn in the review. All studies are described in the Results section, but not all are displayed in the summary meta‐analysis tables.
George 2000 used a programme developed to help smokers with schizophrenia and compared it to a standard programme. Two studies compared different procedures for altering smoking behaviour before the quit day. Glasgow 1989 compared two six‐week programmes, one emphasizing total abstinence, the other giving participants the option of cutting down their cigarette consumption if quitting was too difficult. Lando 1990 compared three programmes; the American Cancer Society Freshstart, the American Lung Association (ALA) Freedom from Smoking and a laboratory‐derived clinic approach. Bushnell 1997 compared Freshstart with a more intensive, small‐group approach. Glasgow 1981 compared three different group programmes, two based on social learning programmes developed by Pomerleau & Pomerleau, and Danaher & Lichtenstein, and the simpler I Quit Kit, intended to control for the non‐specific effects of a group programme. All groups had the same schedule of eight meetings. There were small numbers in each. García 2000 compared a 10‐session and a five‐session programme, each using the same components. Hertel 2008 manipulated participants expectations about smoking cessation during four sessions leading up to the target quit day, where participants in one group were encouraged to think about the positive consequences of smoking cessation (optimistic expectations) versus considering both the positive and negative consequences of smoking cessation (balanced expectations); the four sessions provided after the target quit day were identical between the conditions. In a sample of African‐American and mostly female smokers, Matthews 2009 compared a standard CBT‐plus‐NRT intervention to a culturally‐tailored CBT‐plus‐NRT intervention, in which approximately 40% of the session materials were modified to be culturally targeted. Only the results of one eight‐participant culturally‐tailored CBT group was reported. Batra 2010 compared standard treatment to targeted treatments for three high‐risk subgroups of smokers, including a highly dependent group, a depressive group, and a hyperactivity/novelty‐seeking group. All participants received the same amount of counselling plus NRT. In the meta‐analysis, the outcomes of the subgroups are collapsed to compare standard treatment compared to targeted treatment. We excluded from meta‐analysis participants in the standard treatment condition who were not classified as high‐risk smokers, because they were not randomized to treatment condition. Webb 2010 compared a six‐session CBT intervention culturally tailored for African‐American smokers to a time‐matched health education condition; all participants received eight weeks of nicotine patches. Aytemur 2012 evaluated the effect of adding a simultaneous eight‐week psychodrama training to a CBT group counselling plus smoking cessation pharmacotherapy intervention. Mueller 2012 compared a two‐week CBT programme to autogenic training for smokers on an inpatient alcohol detoxification unit.
Risk of bias in included studies
Selection bias
All the included trials were described as randomized, but most gave insufficient detail about the method of random sequence generation to be judged as low risk. Most also gave too little detail to judge whether the allocation sequence was concealed until a participant was enrolled. The risk of selection bias was therefore unclear for most studies and the GRADE quality of evidence for most comparisons was accordingly downgraded from high to moderate. In cases where more than one group method was compared, and recruitment was continuous, participants were generally allocated to treatment groups on the basis of their sequence of arrival. The group was then randomized to treatment. In studies in which randomization was individual, randomization schedules were in some cases reported to be interrupted in order to allocate families or friends to the same group. Both these features mean that people in a particular group may be more similar than would be expected by chance. This undermines the statistical assumption used to estimate the variance, which is that they are typical of the population as a whole. The same principle also applies when participants are treated in groups, because each person's chance of success may be influenced by the group in which they find themselves. The possibility that success rates varied beyond chance between the groups given the same treatment can be tested, but the power to detect these differences will generally be very low. All these features of group therapy trials are likely to lead to an underestimate of the true variance, and therefore to the estimation of confidence intervals which are too narrow. In those trials which randomized entire worksites to programme type this factor is even more relevant.
Detection bias
Most studies validated self‐reported cessation biochemically and were assessed as being at low risk of detection bias. Ten studies (Pederson 1981; Cottraux 1983; Etringer 1984; DePaul 1987; Leung 1991; Gruder 1993; Minthorn‐Biggs 2000; Camarelles 2002; Grant 2003;Otero 2006) did not report any use of biochemical validation of self‐reported smoking cessation. Some other studies used a mixture of biochemical measures and verification by family or colleagues, or only sought biochemical verification in a random sample of quitters, or used biochemical validation only during the treatment period and not at longer‐term follow‐up. Where only a sample of quitters was verified it was not always clear whether overall quit rates were corrected for the disconfirmation rate in the sample. One study (Glasgow 1981) gave self‐reported quit rates and quitting as measured by carbon monoxide (CO) separately. In most arms the self‐reported rate was lower, so we have used this measure. In the only arm where the CO‐validated rate was more conservative, self‐reported rates favour self‐help over group treatment, so is still conservative with respect to the hypothesis of the review.
Attrition bias
The majority of studies reported the number of participants who had dropped out; in most cases they were explicitly assumed to be smokers. In most studies dropout rates were low and did not differ substantially between conditions, so we rated the risk of bias as low. Early post‐randomization dropouts were not always identified by treatment group. Where the information was available we have generally included them to base the analysis on the numbers randomized. Since the assumption that dropouts are continuing smokers is the same whatever their treatment group, measures of relative effect will only be altered greatly if there is differential dropout. If dropout rates are higher in a minimal treatment control group, then the relative effectiveness of the intervention group may be inflated. We have noted in the 'Risk of bias' tables if there were substantial differences between the numbers randomized and those followed up. In Gruder 1993 the numbers followed up were so much lower than the numbers randomized that we have used the numbers followed up, but report also the effect of using numbers randomized.
1.
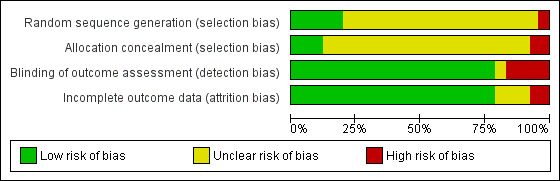
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
1 Comparisons between group therapy interventions and non‐group controls
1.1 Comparison of group versus self‐help programmes
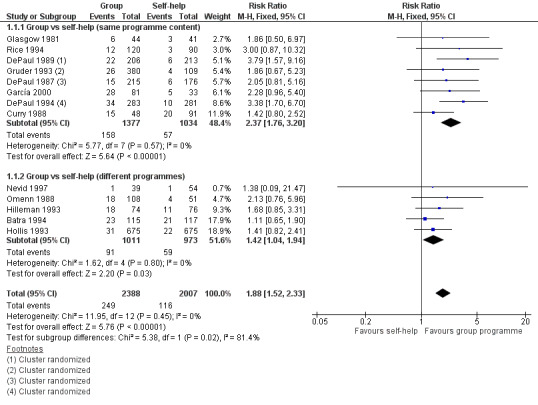
This comparison included 4395 participants from 13 studies. Results from all the studies had wide confidence intervals and only two detected a statistically significant effect. Quit rates in the self‐help control arms were typically 3% to 7% but were considerably higher in a few studies. In Table 1 we have assumed a control quit rate of 5% for estimating absolute effects. Pooling eight studies (N = 2411) that compared a group therapy programme with provision of the same content via a self‐help manual alone gave an estimated risk ratio (RR) of 2.37, 95% confidence interval (CI) 1.76 to 3.20); Analysis 1.1.1) for the effectiveness of the addition of group meetings. The estimate was smaller and of only borderline significance for the other five studies (N = 1984) that used different programmes for the group and self‐help formats, with a risk ratio of 1.42 (95% CI 1.04 to 1.94; Analysis 1.1.2), but since there was no evidence of heterogeneity (I2 = 0%) among the 13 studies we pooled the subgroups giving an estimated RR of 1.88 (95% CI 1.52 to 2.33; Analysis 1.1). Figure 2
1.1. Analysis.
Comparison 1 Group‐format behavioural programmes vs other format, Outcome 1 Smoking cessation. Group programme vs self‐help programme.
2.
Forest plot of comparison 1.1: Group programme vs self‐help programme.
Sensitivity analyses
Four studies (DePaul 1987; DePaul 1989; Gruder 1993; DePaul 1994) were carried out during a televised smoking cessation series which all participants were encouraged to watch. The three DePaul studies also took place in worksite settings, with the worksites rather than individuals randomized to condition. Statistically therefore their results may be less precise. When these studies were excluded, the RR for all other studies with the same or different programmes was 1.57 (95% CI 1.22 to 2.02). The result is therefore robust whether or not worksite trials using cluster randomization, or studies using group programmes as adjuncts to mass media interventions, are included. A sensitivity analysis using numbers randomized rather than numbers followed up in Gruder 1993 had no effect on the results. Restricting the analysis to the five studies (Curry 1988; DePaul 1989; Hollis 1993; DePaul 1994; Nevid 1997) reporting sustained and validated cessation at 12 months also left conclusions unchanged.
1.2. Comparison of group therapy versus brief support interventions
Physician, nurse or pharmacist advice for controls
Fourteen trials with 7286 participants contributed to this comparison. Quit rates in the brief support/advice control were typically 9% to 16%, although three trials reported quit rates under 3% in the advice control groups (Hollis 1993; Pisinger 2010; Ramos 2010). One trial had no quitters in either arm (Wilson 2008). There was evidence of statistical heterogeneity (I2 = 59%); we report a pooled estimate but with a GRADE evidence quality of low, due to inconsistency as well as risk of bias. The pooled estimate suggested a small benefit of group support over brief support with a confidence interval just excluding no effect (RR 1.22, 95% CI 1.03 to 1.43). Of the trials, only Hollis 1993 and Bakkevig 2000 found a statistically significant superiority of a group programme compared to advice from a healthcare provider and a pamphlet. Of the trials that did not detect significant effects, three (Cottraux 1983; Rice 1994; Sawicki 1993) had point estimates favouring the control condition.
Health Education for controls
There was heterogeneity (I2 = 84%) between the results of the two trials (315 participants) with this type of control. Rabkin 1984 found similar cessation rates for a full group programme compared to an intervention with a single session of health education and one individual counselling session. Romand 2005 detected a significant benefit of the 'Five Day Plan' programme over a single session on health consequences.
1.3 Comparison of group therapy plus pharmacotherapy with pharmacotherapy alone
Five trials with 1523 participants evaluated the effect of adding a group support programme to NRT or bupropion (Rovina 2009) and some individual behavioural support. Quit rates in the control conditions were 25% to 30%, except for Gifford 2011 which reported a quit rate of 8%. None of the trials (Ginsberg 1992; Jorenby 1995; Otero 2006; Rovina 2009; Gifford 2011) detected significant effects. There was no evidence of heterogeneity (I2 = 0%) and the pooled estimate was not significant, although not ruling out a clinical benefit (RR 1.11, 95% CI 0.93 to 1.33; Analysis 1.3). In a sensitivity analysis we included three trials from comparison 1.2 in which there was some offer of or access to pharmacotherapy although it was not provided systematically to all participants (Dent 2009; Pisinger 2010; Ramos 2010). This did not substantially change the estimate.
1.3. Analysis.
Comparison 1 Group‐format behavioural programmes vs other format, Outcome 3 Smoking cessation. Group plus pharmacotherapy vs pharmacotherapy alone.
1.4. Comparison of group and individual format therapy
The six trials in this comparison included 980 participants. The quit rate for the controls receiving individual counselling was typically between 10% and 26%, but one trial had no quitters in either arm, and does not contribute data (Wilson 2008). Although there was some clinical heterogeneity in the precise details of the intervention and control conditions, there was little evidence of statistical heterogeneity between the five trials contributing data (I2 = 9%), so we calculated a pooled estimate. This did not detect evidence of a significant difference (RR 0.99, 95% CI 0.76 to 1.28; Analysis 1.4). In two of the trials (Garcia 1989; Jorenby 1995), NRT was offered to all participants, in one trial (Ramos 2010), NRT or bupropion were offered to all participants, and in two others (Camarelles 2002; Wilson 2008) about half of the participants used NRT. It is possible that when pharmacotherapy is being used, small differences in type and amount of behavioural support may not affect long‐term success.
1.4. Analysis.
Comparison 1 Group‐format behavioural programmes vs other format, Outcome 4 Smoking cessation. Group programme vs individual therapy.
1.5 Group therapy compared to 'No Intervention' controls
Nine trials with 1098 participants contributed (Analysis 1.5). Heterogeneity was moderate (I2 = 55%). Because of this, the estimate size is unreliable (RR 2.60, 95% CI 1.80 to 3.76). Eight trials had higher quit rates with group programmes compared to a no‐intervention or a minimal‐contact control, but the two highly weighted studies had amongst the smallest effects.
1.5. Analysis.
Comparison 1 Group‐format behavioural programmes vs other format, Outcome 5 Smoking cessation. Group vs 'no intervention' controls.
2 Comparisons between different formats of group programme
2.1 Skills Training/Cognitive‐Behavioural components
Nine studies with 1599 participants compared group format programmes that differed in their use of specific components such as skills training or cognitive‐behavioural therapies. We distinguished between programmes that were matched for contact time (McDowell 1985; Goldstein 1989; Zelman 1992; Ward 2001; Rovina 2009) and those where the additional components increased the duration (Lando 1985; Minthorn‐Biggs 2000;Huber 2003; Otero 2006). Neither subgroup had evidence of much heterogeneity and the overall heterogeneity was also low (I2 = 10%), so we focus on the pooled estimate for all studies. Now that interventions addressing relapse prevention are not included the borderline significance disappears and there is no evidence for a benefit of more complex interventions (RR 1.16, 95% CI 0.98 to 1.37; Analysis 2.1). Only one trial (Goldstein 1989) showed a statistically significant benefit at long‐term follow‐up. The analysis includes almost 1600 participants but most of these were contributed by Otero 2006, with the other studies being small.
2.1. Analysis.
Comparison 2 Comparisons between different group programmes [Outcome Long term cessation for all comparisons], Outcome 1 "Skills training".
2.2 Mood Management components
Seven trials with 1367 participants tested specific interventions to help manage mood, using either CBT or behavioural activation. Five studies were matched for contact time (Hall 1996; Brown 2001; Patten 2002; Brown 2007; Macpherson 2010) and two had longer intervention than control programmes (Hall 1994; Hall 1998). There was little or no heterogeneity evident in the subgroups and none when pooling all studies (I2 = 0%). The pooled estimate did not detect evidence of an effect (RR 1.05, 95% CI 0.84 to 1.32; Analysis 2.2).
2.2. Analysis.
Comparison 2 Comparisons between different group programmes [Outcome Long term cessation for all comparisons], Outcome 2 Mood management.
2.3 Manipulation of Group Dynamics
There was no evidence from the four trials with 702 participants (Etringer 1984; Lando 1991; Digiusto 1995; Schmitz 2007) that there was an effect on cessation of attempts to change the interaction between participants in a group programme. There was little heterogeneity; none of the trials detected significant long‐term effects and the pooled estimate provided no evidence of a difference (RR 1.13, 95% CI 0.87 to 1.46; Analysis 2.3).
2.3. Analysis.
Comparison 2 Comparisons between different group programmes [Outcome Long term cessation for all comparisons], Outcome 3 Manipulation of group dynamics.
2.4 Other miscellaneous comparisons
The trials briefly noted here were mostly small and did not show significant long‐term effects on cessation, although with wide confidence intervals. One trial with 154 African‐American smokers (Webb 2010) did detect a benefit of a cognitive behavioural programme, compared to a contact matched group health education programme, with all participants given nicotine patches (RR 2.27 95% CI 1.20 to 4.29; Analysis 2.4.1). George 2000 (n=45) failed to show evidence that a programme designed for smokers with schizophrenia had a greater benefit than a standard intervention (RR 1.65, 95% CI 0.37 to 7.25; Analysis 2.4.2). Glasgow 1989 (n=66) did not detect a difference in six‐month quit rates using programmes differing in their emphasis on abstinence or controlled smoking (RR 0.94, 95% CI 0.32 to 2.78; Analysis 2.4.3). Pharmacotherapy use was similar between the psychodrama versus CBT‐only groups (80% versus 73%). Matthews 2009 did not find that culturally tailoring smoking cessation materials increased cessation rates (RR 1.04, 95% CI 0.28 to 3.81; Analysis 2.4.4), although they only reported the outcomes from one group of eight participants in the culturally‐tailored intervention. Batra 2010 (n=193) did not detect an overall effect of targeted versus standard treatment for three subgroups of smokers: highly dependent, depressive, and hyperactivity/novelty‐seeking (RR 1.05, 95% CI 0.65 to 1.69; Analysis 2.4.5), however, they reported higher rates of abstinence in the targeted versus standard treatment among the depressive subgroup (29% versus 12%). Moreover, more participants in the culturally‐tailored intervention used NRT (88% versus 51%). Mueller 2012 (n=103) did not detect a significant difference in cessation rates between a CBT smoking cessation group programme compared to a relaxation‐only group among smokers in a residential alcohol detoxification program, with the direction of effect favouring relaxation; none of the CBT group participants was abstinent at the six‐month follow‐up (RR 0.13, 95% CI 0.01 to 2.55; Analysis 2.4.5). Aytemur 2012 (n=127) did not find that adding psychodrama training to a CBT group programme improved smoking cessation outcomes (RR 1.39, 95% CI 0.89 to 2.16; Analysis 2.4.6).
The other trials are not shown graphically. Lando 1990 found that the American Lung Association (ALA) Freedom from Smoking programme was more successful than the American Cancer Society (ACS) Freshstart programme. Sustained one‐year quit rates were 12%, 19% and 22% for the ACS, ALA and clinic‐derived programme respectively. This was a large, multicentre study, and since treatment was allocated by group the authors estimated the design effect to allow for the correlation in outcome between people treated together. The corrected Chi2 for the three‐way comparison was significant (P < 0.014) for the one‐year sustained abstinence measure. The difference between the ALA and Lando programmes was not significant at one year. Bushnell 1997 compared Freshstart to a more intensive clinic‐based approach. This study did not show significant long‐term differences between the programmes, although early results favoured the intensive approach. Glasgow 1981 also compared three different programmes. They found no significant differences but numbers allocated to each programme were small. In García 2000 a five‐week 10‐session programme was associated with lower 12‐month quit rates than a five‐session programme (16% versus 39%). The rates for the more intensive programme were significantly lower when compared to a five‐session programme combined with a self‐help manual (16% vs 48%, P < 0.05). Hertel 2008 manipulated pre‐quit expectations (optimistic versus balanced) for four sessions, followed by four sessions of identical treatment. They found no overall difference in outcomes for people given optimistic versus balanced expectations before quitting (19% versus 21%), with some evidence of interaction with participants' prior experiences of quitting.
Take‐up rates for group programmes
The variation in take‐up rates for group therapy was partly determined by the method of recruitment and randomization. However, even in trials where eligible smokers agreed to attend group meetings prior to randomization, the non‐participation rate was often high. Curry 1988 enrolled participants who attended an information meeting. More group participants (88%) than self‐help participants (59%) began treatment (defined as completing the first week of self‐monitoring), and completed treatment. Because of the differential dropout the difference in quit rates is greater when we conducted an intention‐to‐treat analysis (including all randomized participants) than when only those who began treatment are included. Participation in the Glasgow 1981 trial was higher, with almost all those enrolled taking part and available for six‐month follow‐up.
Attrition following randomization was particularly high in Gruder 1993, which was carried out in conjunction with a television programme, because eligible smokers who had registered by mail for support materials were randomized before they were contacted. Only 70% could be reached and 62% scheduled for group meetings. Non‐participation at this stage was due to lack of interest or problems with timing or location of meetings. Of those who were scheduled, 50% then failed to attend any meetings.
Rice 1994 also had a high non‐attendance rate, even though participants were volunteers. Overall 34% dropped out of the trial on learning their treatment allocation. Thirty‐one per cent of those randomized to the group treatment refused to participate, whilst the dropout from the follow‐up‐only group was 48%. Hertel 2008 reported that only 79% of participants who completed the baseline session and were randomized to treatment attended at least two out of the four baseline sessions. On the other hand, Cottraux 1983 reported that just over half those enrolled attended all three behaviour therapy sessions, Batra 2010 reported that 74% of participants attended at least five out of six sessions, and Schleicher 2012 reported that 75% of participants attended at least four out of six sessions. Hilleman 1993 also did not report any dropout from group treatment, but this trial involved volunteers for a drug trial, and is probably not typical.
The lowest participation rates were seen in Hollis 1993 and Pisinger 2010. Hollis 1993 recruited smokers during visits to primary care offices. Of those randomized for referral to a group programme, 11% chose to attend, whilst of those given a choice of self‐help or groups just 8% attended a group programme. Pisinger 2010 cluster‐randomized GPs to recruit participants to a group counselling programme, an internet‐based programme, or usual care. Only 7% of participants attended a counselling group and 16% logged into the internet programme. A higher take‐up rate was seen in a Norwegian trial (Bakkevig 2000) which allocated community volunteers to either a smoking cessation group, which 75% attended, or to visit their physician for help (GP), which only 36% chose to do. In one study not included because the intervention offered NRT as well as referral to a behavioural programme as a covered benefit in a health plan, only 1.2% of the intervention group participated in a behavioural programme (Schauffler 2001). Pisinger 2005 had a 26.5% take‐up rate amongst people given brief counselling and offered group support.
Discussion
This review provides evidence that a behaviour therapy programme delivered in a group format aids smoking cessation. The effect is clearest when group support is compared to a self‐help programme providing information in written materials. We estimated that if five in 100 people could give up for at least six months assisted by written materials, eight to 12 in 100 could quit when given group support. We rate the GRADE evidence quality as moderate because these trials were done some years ago and most were at unclear risk of bias. Group support was also more effective than brief support such as advice from a physician or nurse, but we judged the GRADE quality to be low, because of the possibility of bias and variability in the effect size in different studies; see Table 1.
Combining the results of five trials that examined group therapy as an adjunct to pharmacotherapy did not detect a significantly increased quit rate for combined therapy over pharmacotherapy without group support. In all studies the comparison arm had at least brief behavioural support. This finding was not changed by the addition of three studies for this update (Otero 2006, Rovina 2009; Gifford 2011). We graded the evidence quality as moderate, downgraded due to imprecision. The overall increase in success rates attributable to pharmacotherapy might make small relative differences attributable to the type and amount of behavioural support more difficult to detect. The Cochrane Review of individual counselling (Lancaster 2017) has noted a similar failure to detect a significant additional benefit of individual counselling when added to the systematic use of NRT. In both cases evidence comes from a small number of trials (Jorenby 1995 contributes data to both reviews). A separate Cochrane Review (Stead 2015) has assessed the effect of increasing the amount of any type of behavioural support when used alongside pharmacotherapy. It analysed 47 studies including relevant studies from this review, and concluded that "increasing the amount of behavioural support is likely to increase the chance of success by about 10% to 25%". The confidence interval for the estimate based on five trials is consistent with a similar small benefit. In the absence of clear evidence to the contrary, it seems reasonable to assume that behavioural interventions and pharmacotherapies independently contribute to successful quitting.
The results from six studies provide no evidence that group therapy is more effective than individual counselling, whether or not the number of sessions was matched. There was therefore a lack of evidence that meeting with a group of other smokers was a critical element in an intensive smoking cessation programme. In two of the trials (Garcia 1989; Jorenby 1995), NRT was offered to all participants, in one trial (Ramos 2010) all participants were offered NRT or bupropion at the physician's discretion, and in two others (Camarelles 2002; Wilson 2008) about half of the participants used NRT. As suggested above, the use of pharmacotherapy may make it difficult to detect differences between the effects of behavioural components, if the relative increase in quit rates is small. Additionally, in Ramos 2010 the treatment team for each arm comprised a nurse and a physician, but the group intervention was more likely to be delivered by the nurse and the individual intervention was more likely to be delivered by the physician, which may have influenced the outcomes. Although we did not specifically seek cost data, none of these studies was designed to compare the costs of different formats. Using a group format ought to allow more people to be treated by a therapist, and therefore could be more cost‐effective if outcomes were similar, but there is not enough evidence about comparative efficacy.
Problems of conducting a systematic review of behavioural interventions should be noted. First, many trials of behavioural interventions use multiple treatment arms in an attempt to identify the precise therapeutic element leading to success. This makes the pre‐definition of explicit comparison groups difficult. Second, as with all behavioural as opposed to pharmacological therapies, the choice of an appropriate control condition presents problems when evaluating efficacy. There is no obvious equivalent for the drug placebo to control for the non‐specific effects of a treatment method. Evaluating group therapies against a waiting‐list control does not provide very good evidence for the specific effect of the group format. Whilst we took account of the broad nature of the support offered to the control group when pooling studies, variation in the components used as part of, for example, a usual care control, may still give rise to heterogeneity. Treatment effects could be underestimated if those studies using effective interventions tended to provide relatively helpful usual care or brief advice. An ongoing systematic review is conducting a detailed analysis of behavioural intervention and control elements, and is expected to provide more evidence about this (de Bruin 2016).
A limitation of research in which participants are treated in groups is that typically there may be only two or three groups in each treatment condition. Participants' chances of success are almost certainly not completely independent. There may be variation by the group in which they were treated, due to aspects of the group process. This aspect is generally ignored in trial analyses. We also cannot exclude the possibility of publication bias. Although group programmes have been widely offered for smoking cessation, often under the auspices of cancer prevention or lung health charities, we found relatively few studies meeting our criteria. It is possible that there are other published or unpublished studies we have not located.
This review has taken a broad approach to group programmes, without distinguishing between treatments on the basis of their theoretical approach, therapists or intensity. There is still limited evidence from which to identify those elements of group therapy which are most important for success. In the main analyses there are too few studies to compare subgroups of studies according to content, provider or length. The number of studies directly comparing different programmes is also small, although now that group therapy is well established as a treatment, more effort is being devoted to optimizing interventions. The largest number of newly included studies in this update were in this category. Some studies compare programmes using different theoretical approaches. Most commonly, they distinguish between approaches that stress the acquisition of specific skills, and those that aim to increase motivation and confidence in quitting without emphasis on cognitive and behavioural skills, (e.g. Hall 1998; Zelman 1992; Brown 2001 for comparisons between approaches). At present the evidence supporting the use of additional skills‐based components is weak, although it is consistent with the US guideline meta‐analyses (Fiore 2008), discussed further below. Although pooled point estimates suggest a small benefit, confidence intervals are sensitive to the studies included and the way interventions are categorized.
Others focus on additional components, such as adding psychodrama (as in Aytemur 2012), tailoring interventions for different cultural groups (e.g. Matthews 2009), or manipulating pre‐quit expectations (Hertel 2008). However, none of these studies detected differences between groups. Furthermore, at this time there are few similarities between studies to allow for pooled analyses.
A further focus of research is to identify whether specific subgroups of smokers benefit differentially (e.g. Batra 2010). This could allow tailoring of intensive interventions for specific target groups, for example people with histories of depression or other addictions, or with smoking‐related medical problems (Brandon 2001). Research addressing these questions is likely to contribute more to future updates of these reviews. At the moment there is not sufficient evidence to support using one programme type over another for smokers with any particular characteristics. A number of studies that were included in earlier versions of this review are now separately considered in a Cochrane Review of relapse prevention interventions (Hajek 2013). That review has detected no evidence of proven effective behavioural approaches for reducing relapse rates at long‐term follow‐up.
The US Public Health Service Guideline, Treating Tobacco Use & Dependence, updated in 2008 (Fiore 2008), is based on meta‐analyses using logistic regression. This approach allows the contribution of data from trials which did not directly compare different formats. The guideline includes estimates of the comparative cessation rates using different formats for delivering interventions. In the Guideline analysis, the estimated odds ratio (OR) for success using group counselling compared to no intervention was 1.3 (95% CI 1.1 to 1.6, Table 6.13). In an earlier version of the guideline the estimated benefit of group therapy was somewhat larger (Fiore 1996). Another guideline meta‐analysis considered the components provided within group and individual counselling programmes. This suggested that general problem‐solving elements (including skills training, relapse prevention and stress management) were likely to be beneficial (OR 1.5, 95% CI 1.3 to 1.8, Table 6.18). Intra‐treatment social support (OR 1.3, 95% CI 1.1 to 1.6) was also recommended. The analysis did not show relaxation exercises, contingency contracting, cigarette fading or negative affect components to be useful. The guideline authors stress that the strength of evidence underlying recommendations on use of these components is not of the highest level because of the correlation of the types of counselling and behavioural therapies with other treatment characteristics such as programme length or type of therapist. The conclusions of this Cochrane Review are consistent with the guideline finding in relation to the inclusion of general problem‐solving components, and are strengthened by being limited to unconfounded comparisons. They are still limited by the small number of studies and the heterogeneity of approaches.
There is further evidence from studies which did not meet our inclusion criteria that group programmes are effective. The Lung Health Study (Kanner 1996) was a trial of a smoking intervention and a bronchodilator in smokers with mild pulmonary obstructive disease. The programme consisted of 12 weeks of group therapy with a cognitive‐behavioural approach, and nicotine gum was available to all participants. In addition they all received a strong physician message about quitting followed by a meeting with a smoking intervention specialist. A maintenance programme was also provided. We excluded the study from our meta‐analysis because the effect of the group was confounded by the effects of nicotine replacement. However the quit rate achieved is greater than might be expected from the use of NRT alone, and it is reasonable to assume that the group programme contributed to the effect. Twenty‐two per cent of the intervention participants achieved smoking cessation for five years compared to 5% of the usual‐care control . Nine‐year follow‐up of a cohort of people treated in large group‐format community‐based interventions suggests a quit rate somewhere between 16% and 48%, depending on the extent to which the 34% of the cohort reached were representative of those treated (Carlson 2000). More recent results based on longer follow‐up report a difference in health outcomes between the intervention groups (Anthonisen 2005).
The drawback to group programmes as a public health strategy is their limited reach to the smoking population. Participation rates in a number of the studies considered here were low. Participating smokers need to be sufficiently motivated not only to attempt to stop, but also to commit themselves to the time and effort involved in attending meetings.
Authors' conclusions
Implications for practice.
There is reasonable evidence that groups are better than self‐help, and other less intensive interventions, in helping people stop smoking, although they may be no better than advice from a healthcare provider. There is not enough evidence to determine how effective they are in comparison to intensive individual counselling. From the point of view of the consumer who is motivated to make a quit attempt, it is probably worth joining a group if one is available; it will increase the likelihood of quitting. Group therapy may also be valuable as part of a comprehensive intervention which includes pharmacotherapy. From a public health perspective, the impact of groups on smoking prevalence will depend on their uptake. Providers need to make a judgement about the cost effectiveness of the gains achieved by group therapy compared to other interventions.
Implications for research.
The general efficacy of multicomponent programmes which include problem‐solving and social support elements has been established. Demonstrating the efficacy of specific components or procedures requires large sample sizes which can be difficult to achieve, given the difficulty of attracting smokers to intensive programmes. Identifying subgroups of smokers who are differentially helped by particular components may be possible, and this could lead to the development of targeted interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 15 December 2016 | New search has been performed | Searches updated, 13 new studies included. 'Summary of findings' table added |
| 15 December 2016 | New citation required but conclusions have not changed | No change to conclusions |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 17 February 2009 | Amended | Source of support amended |
| 8 October 2008 | New search has been performed | Updated for issue 1, 2009 with 9 new studies. Relapse prevention studies were removed, as now covered in another review. |
| 16 February 2005 | New citation required and minor changes | Updated for issue 2, 2005 with 4 new studies. No changes to the main conclusions. |
| 22 May 2002 | New citation required and minor changes | Updated for issue 3, 2002, expanding the inclusion criteria to include trials comparing more than one variant or type of group based programme. No changes to the main conclusions. |
Acknowledgements
Dr B Bonevski for assistance with data extraction. Prof V Rice for additional data. Peer referees for valuable suggestions.
Data and analyses
Comparison 1. Group‐format behavioural programmes vs other format.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation. Group programme vs self‐help programme | 13 | 4395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.52, 2.33] |
| 1.1 Group vs self‐help (same programme content) | 8 | 2411 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.76, 3.20] |
| 1.2 Group vs self‐help (different programmes) | 5 | 1984 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.04, 1.94] |
| 2 Smoking cessation. Group programme vs brief support | 16 | 7601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.46] |
| 2.1 Physician, nurse, or pharmacist advice | 14 | 7286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.03, 1.43] |
| 2.2 Health Education | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.94, 3.46] |
| 3 Smoking cessation. Group plus pharmacotherapy vs pharmacotherapy alone | 5 | 1523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.33] |
| 4 Smoking cessation. Group programme vs individual therapy | 6 | 980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.76, 1.28] |
| 4.1 Group vs individual (similar intensity & content) | 4 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.68, 1.81] |
| 4.2 Group vs individual (different intensity/content) | 2 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
| 5 Smoking cessation. Group vs 'no intervention' controls | 9 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.80, 3.76] |
1.2. Analysis.
Comparison 1 Group‐format behavioural programmes vs other format, Outcome 2 Smoking cessation. Group programme vs brief support.
Comparison 2. Comparisons between different group programmes [Outcome Long term cessation for all comparisons].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 "Skills training" | 9 | 1599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.98, 1.37] |
| 1.1 Substitution of components (controlling for programme length) | 5 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.67] |
| 1.2 Addition of components (not controlled for programme length) | 4 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.92, 1.37] |
| 2 Mood management | 7 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 2.1 Same contact time | 5 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.41] |
| 2.2 Longer contact time | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.73, 1.52] |
| 2.3 Mood Management versus motivational interviewing | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Manipulation of group dynamics | 4 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| 4 Other miscellaneous comparisons | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 CBT vs group health education | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Programme for people with schizophrenia vs standard programme | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Total abstinence vs controlled smoking programme emphasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Culturally‐targetted vs standard treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Programme for at‐risk subgroups vs standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 CBT vs relaxation training | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Additional psychodrama compared to group programme | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.4. Analysis.
Comparison 2 Comparisons between different group programmes [Outcome Long term cessation for all comparisons], Outcome 4 Other miscellaneous comparisons.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aytemur 2012.
| Methods | Study design: Randomized controlled trial Country: Turkey Recruitment: Smoking cessation clinics Group size: 10 participants/group |
|
| Participants | 127 smokers; 62% women, av. age 47, av. cpd 20 Therapists: 2 psychodramatists (1 pulmonary diseases specialist, 1 specialized psychiatry nurse) | |
| Interventions | 1. CBT + pharmacotherapy (NRT or bupropion), 30 minutes initial session and 6, 10‐minute booster sessions 2. As 1, plus psychodrama programme, with 8 weekly 120‐minute sessions |
|
| Outcomes | Continuous abstinence at 6 m Validation: none, self‐report |
|
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons ‐ Additional psychodrama compared to group programme'. Treatment allocation refusers (n = 14) included as smokers. 80% of psychodrama group and 73% of CBT only group used pharmacotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization and randomization code methods not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were prepared before enrolment by the head nurse; no other details |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Abstinence not validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91% of psychodrama and 87% of CBT‐only group completed follow‐up, all included in ITT analyses |
Bakkevig 2000.
| Methods | Study design: Randomized controlled trial Country: Norway Recruitment: Community volunteers Group size: not stated |
|
| Participants | 139 smokers; 67% women, av. age 44, av. cpd 19 Therapists: ex‐smokers who have previously used programme | |
| Interventions | 1. Physician (GP) advice; participants instructed to visit their GP for support. GP told to offer NRT as appropriate and provide 1 follow‐up visit 2. Group therapy; participants asked to attend 'Smokenders'. 7 weekly sessions + 1 follow‐up 4 wks later Quit day after 5 wks. Multifaceted including cognitive therapies | |
| Outcomes | Abstinence 1 yr post‐quit date Validation: < 83 mmol/L TCN and/or < 75 ng/mL cotinine. Only 10% of group 1 and 35% of 2 attended 1‐yr follow‐up | |
| Notes | Comparison 1.3.1. 'Real world' study. Treatment allocation refusers and other non‐compliers included as smokers. 36% consulted GP, 75% attended Smokenders | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and differential loss; 90% of controls & 65% of intervention lost/withdrew by end of study, all included in ITT analysis |
Batra 1994.
| Methods | Study design: Randomized controlled trial Country: Germany Recruitment: Community volunteers Group size: not stated |
|
| Participants | 232 smokers; 53% women, av. age 41, av cpd 25 | |
| Interventions | Both conditions received nicotine patch 1. Group therapy, 9 weekly 90‐min sessions 2. Self‐help materials | |
| Outcomes | Continuous abstinence at 12 m Validation: not described | |
| Notes | Comparison 1.1.2, different S‐H | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about validation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomized participants included in analysis, no further information. |
Batra 2010.
| Methods | Study design: Randomized controlled trial Country: Germany Recruitment: Smoking cessation treatment clinic volunteers Group size: 4 ‐ 12 participants |
|
| Participants | 193 smokers; Overall group characteristics not provided Therapists: 3 clinical psychologists |
|
| Interventions | At‐risk subgroups of smokers (highly dependent, depressive, or hyperactive/novelty‐seeking) were randomized to: 1. Standard pharmacobehavioural group treatment 2. Augmented psychotherapy tailored to subgroup |
|
| Outcomes | Continuous abstinence at 12 m Validation: CO < 10 ppm, only bioverified 1 participant per group who reported abstinence |
|
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons ‐ Programme for at‐risk subgroups vs standard' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the cluster randomization order was set in advance for each of the smoker profile groups using random numbers generated by a spreadsheet program" |
| Allocation concealment (selection bias) | Unclear risk | "The second author completed the profile assignment and managed the data randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% of intervention and 66% of standard participants completed follow‐up; all participants included in ITT analyses |
Brown 2001.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: not stated |
|
| Participants | 179 smokers with history of MDD; 60% women, av. age 45, av. cpd 27 Therapists: 2 for each group, clinical psychologists | |
| Interventions | 1. Standard group therapy. 8 x 2 hrs over 6 wks, TQD session 5. Including nicotine fading, RP, homework 2. As 1. + CBT for depression. Same schedule + coping skills to control depressive symptoms | |
| Outcomes | Sustained abstinence at 12 m (confirmed at post‐Rx, 1 m, 6 m). (PPA was main trial outcome) Validation: CO ≤ 10 ppm + saliva cotinine ≤ 46 ng/ml (abstinence was only verified by significant others in 6.5% of cases) | |
| Notes | No non‐group control. Comparison 2.2.1 ‐ testing effect of depression/mood management programme. Direction of effect opposite for sustained and PPA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, stratified on gender, current depressive symptoms, FTQ, using urn method |
| Allocation concealment (selection bias) | Low risk | No details given, but use of urn technique makes it likely that enrolment occurred before allocation known |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% lost to follow‐up at 12 m, included in ITT analysis |
Brown 2007.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: not specified |
|
| Participants | 524 smokers; 48% women, av. age 44, av. cpd 25 Therapists: 2 PhD psychologists for each group. All conducted both types of treatment | |
| Interventions | Factorial trial including bupropion versus placebo comparison 1. CBT for cessation; 12 x 90‐min over 12 wks, 2/week then 1/week then monthly. TQD session 7 2. As 1, plus CBT for depression, same contact time | |
| Outcomes | Abstinence at 12 m (sustained at 2 m & 6 m) Validation: CO ≤ 10 ppm & cotinine ≤ 15 ng/ml (8.2% verified by 'significant other') | |
| Notes | No non‐group control. Comparison 2.2.1 for effect of mood management. Pharmacotherapy conditions collapsed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned to one of two treatment sites, where they were to receive one of two manualized group treatments ... Participants were then randomly assigned to receive one of two medication conditions, bupropion or placebo, using the urn randomization technique" |
| Allocation concealment (selection bias) | Unclear risk | 'Whereas we were able to balance the drug and placebo conditions on an individual basis, behavioral treatments were randomized by group and thus were more susceptible to fluctuations in recruitment and to the availability at both sites of pairings of a senior and a junior therapist trained in CBTD". Knowledge of behavioural assignment was probably not concealed but seems unlikely to have led to individual selection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% provided complete outcome data at all follow‐ups, not related to treatment condition. All participants included in ITT analyses |
Bushnell 1997.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: max 50 ACS or 15 Vanderbilt University Medical Center (VUMC) |
|
| Participants | 314 military and civilian smokers, excludes 198 people, assignment NS, who did not attend any sessions after randomization. 44% women, age and smoking not described Therapists: ACS‐trained volunteers. VUMC‐healthcare professionals | |
| Interventions | All participants offered free NRT (in group 2 conditional on attending 75% classes) 1. ACS: 4 x 1‐hr large group sessions, no TQD 2. VUMC: 8 x 1‐hr sessions, RP model including stress management, diet, exercise | |
| Outcomes | Abstinence at 6m (PPA) Validation: CO < 8 ppm, salivary cotinine ≤ 10 mg/ml | |
| Notes | No non‐group control. Results not shown in graphs. No sig diff in 6 m quit, 12% (17/143) for ACS vs 13% (22/171) for VUMC Take‐up rate: 61% of screened population attended 1 or more classes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not stated, stratified by military or civilian |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | People who attended no classes were not included, other noncompleters included in ITT analysis |
Camarelles 2002.
| Methods | Study design: Randomized controlled trial Country: Spain Recruitment: Primary care Group size: 10 ‐ 14 |
|
| Participants | 106 smokers (any amount); 54% women, av. age 47, av. cpd 25 Therapists: 1 doctor, 3 nurses, trained and experienced | |
| Interventions | 72 participants eligible for nicotine patch, 53 used 1. Group therapy, 7 x 2 hrs over 3 wks, TQD after wk 3 2. Individual counselling, not matched for intensity, 2 sessions over 2 wks, with S‐H materials | |
| Outcomes | Sustained abstinence at 6 m Validation: none | |
| Notes | Comparison 1.2.2 between group and shorter individual therapy Slightly higher and longer use of NRT in group condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes, but chosen by participant. Since all received a cessation intervention, potential for selection bias probably low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation but all participants received a cessation intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on losses to follow‐up but all participants included in denominators |
Cottraux 1983.
| Methods | Study design: Randomized controlled trial Country: France Recruitment: Community volunteers Group size: 15 |
|
| Participants | 558 (418 in arms of interest) community volunteers; 24% women, av. cpd 31 Therapists; 2 per group, qualifications not described | |
| Interventions | 1. Behaviour therapy. Includes discussion, training in relaxation. 3 x 3‐hr sessions over 2 wks. Relaxation and stress‐desensitization audiotape for daily use 2. Acupuncture (not included in MA) 3. Placebo ‐ lactose capsules for 2 wks. Met 2 x 10‐min with a doctor 4. 1‐yr waiting‐list control. | |
| Outcomes | Abstinence at 12 m Validation: none. Assessor blind to treatment condition | |
| Notes | Although 3 described by authors as placebo the 2 meetings with a doctor make it more comparable with an advice intervention, so 1 vs 3 used in comparison 1.3.1 and 1 vs 4 in comparison 1.5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, stratified by presence of another smoker in household, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation and some participants were on a waiting‐list control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% lost to follow‐up, included in ITT analysis |
Curry 1988.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 12 |
|
| Participants | 139 smokers: 51% women, av. age 41, av. cpd 28 Therapists: 2 teams of 2 PhD psychologists. Each team led 1 group in each programme | |
| Interventions | Test of group vs S‐H format, and traditional vs RP programme. Groups met for 8 x 2‐hr weekly meetings which included relaxation training, enlisting social support and practising alternative behaviours 1. RP Group. Focused on smoking as learned behaviour. Quit day at 3rd session. Additional elements included identifying high‐risk situations, cognitive restructuring and role‐playing 2. RP Self‐help. 8 workbook units 3. 'Absolute Abstinence' (AA) Group. Focused on addictive component of smoking. Quit day at 5th session. Additional elements included focused smoking, health education and contingency contract. 4. Absolute Abstinence Self‐help. 8 workbook units | |
| Outcomes | Abstinence from months 9 to 12 of follow‐up Validation: saliva TCN and 2 collateral verifiers | |
| Notes | From 2009 RP & AA conditions collapsed so 1&3 vs 2&4 entered in comparison 1.1 instead of 2 substudies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Part by coin toss and part random‐number table. Friends co‐randomized to same programme but not necessarily same format. More assigned to S‐H than group by design |
| Allocation concealment (selection bias) | Unclear risk | No details given, but randomization procedure makes it likely that it was not concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 69% began treatment but all assigned to treatment included in ITT analysis |
Dent 2009.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Veterans by physician referral Group size: 2 ‐ 10 |
|
| Participants | 101 smokers: 7% women, av. age 55 ‐ 57, av. cpd 19 ‐ 20 Therapists: pharmacists and pharmacy students | |
| Interventions | 1. Individual, 1 x 10 ‐ 15‐minute phone session of 'standard care cessation' 2. Group therapy, 3 x face‐to‐face groups lasting 3 h, 2 h, and 1 h every 2 wks; TQD scheduled 2 ‐ 3 days prior to second session |
|
| Outcomes | Continuous abstinence at 6 m Validation: urinary cotinine |
|
| Notes | New for 2017 update Comparison 1.3.1 'Smoking cessation Group programme vs brief intervention ‐ Physician, nurse, or pharmacist advice' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization codes assigned to each participant were computer generated by the study statistician and stratified by sex in blocks of 6.” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98% of group and 94% of individual participants completed 6 m follow‐up; all included in ITT analyses |
DePaul 1987.
| Methods | Study design: Cluster‐randomized controlled trial Country: USA Recruitment: Employees at 43 worksites, recruited prior to a 3‐wk television smoking cessation programme |
|
| Participants | 233 smokers in group discussion worksites, 192 in non‐group worksites; 72% women, av. age 43, av. cpd 30 Groups led by employee with 3 hrs training | |
| Interventions | All participants were given S‐H manuals by company co‐ordinators and instructed to view the televised segments 1. Twice‐weekly 45‐min group meetings for 3 wks 2. S‐H alone | |
| Outcomes | Abstinence at 12 m (multiple PPA) No validation | |
| Notes | Percentage quit rates estimated from graphs and denominator assumed to be numbers followed‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomization by worksite, matched for size. 3 worksites did not enter allocated condition but excluding them did not alter findings |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8% lost to follow‐up in each group |
DePaul 1989.
| Methods | Study design: Cluster‐randomized controlled trial Country: USA Recruitment: Employees at 38 worksites, recruited prior to a 3‐wk television smoking cessation programme |
|
| Participants | 419 smokers who participated in the worksite programmes; 63% women in groups, 54% women in S‐H, av. age 38, av. cpd 21 | |
| Interventions | 1. 6 x twice‐weekly group meetings to coincide with the 3‐wk television series, then monthly meetings for 1 yr. Abstinent smokers and 5 of their family and 5 co‐workers entered for a lottery at the final group meeting and 12 m follow‐up 2. S‐H manuals only | |
| Outcomes | Abstinence from end of programme to 12 m Validation; saliva cotinine and co‐worker or relative confirmation | |
| Notes | Data based on participants in the programmes. Attrition was defined as not attending any group meetings, not reading the manual, not being located for post‐testing, refusing to be interviewed or changing jobs. The attrition rate was 17% for group worksites and 29% for non‐group worksite participants, so correcting the data for attrition would increase the apparent efficacy of the group condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomization by worksite, matched for size |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported, only those followed up used in MA, see Notes |
DePaul 1994.
| Methods | Study design: Cluster‐randomized controlled trial Country: USA Recruitment: Employees in 61 worksites who expressed interest |
|
| Participants | 564 smokers in relevant comparisons, 58% women, av. age 38, av. cpd 21 | |
| Interventions | The worksite interventions were timed to coincide with a mass media intervention consisting of a week‐long smoking cessation series on TV, and a complementary newspaper supplement 1. S‐H manual (ALA Freedom from Smoking in 20 days) 2. S‐H manual and incentive payment of USD 1 for each day abstinent up to USD 175 3. 6 group meetings over 3 wks followed by 14 booster meetings over 6 m. Incentive payments. Handouts from same S‐H manual. Maintenance manual (ALA A Lifetime of Freedom from Smoking) | |
| Outcomes | Sustained abstinence at 12 m Validation: CO < 9 ppm. Saliva cotinine at 6 m only | |
| Notes | 3 vs 2 in Group vs S‐H. Including 1 would increase effect. Treated as same S‐H programme, since same approach used, although group participants not given complete cessation manual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by company |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up high, but lower in group condition. All included in ITT analysis |
Digiusto 1995.
| Methods | Study design: Randomized controlled trial Country: Australia Recruitment: Community volunteers and physician referral |
|
| Participants | 137 smokers; 56% women, av. cpd 26, av. age 44 | |
| Interventions | 1. Social support. Emphasized interaction, social coping strategies. 5 treatment meetings of which 2 held after quit date 2. Self‐control. Interaction discouraged. Taught cognitive‐behavioral self‐control strategies. 4 meetings, 1 7 days after quit day | |
| Outcomes | Abstinence for 7 days at 6 m No validation at 6 m. (At 1 wk 5/82 claiming abstinence had cotinine > 250 nmol/L) | |
| Notes | No non‐group control. Study designed to test specific effects of social support aspect of group treatments. Included in comparison 2.3 ‐ effect of manipulating group dynamics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No validation but all participants had similar contact, likelihood of differential self‐report judged low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% of social support & 10% of self‐control lost to follow‐up, included in ITT analysis |
Etringer 1984.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group Size: 7 ‐ 13 |
|
| Participants | 72 smokers; 57% women, av. age 36, av. cpd 25 Therapists: doctoral candidates with 2 yrs in counselling psychology | |
| Interventions | Factorial design using 2 cessation programmes and an intervention on group cohesiveness. Not clear whether session patterns identical for each. 9‐wk course of 45 ‐ 60‐min sessions 1. Enriched cohesiveness using written commitments, exercises and video. Satiation smoking in preparation for cessation 2. Enriched cohesiveness. Nicotine fading in preparation phase 3. Standard group. Satiation smoking 4. Standard group. Nicotine fading | |
| Outcomes | Abstinence at 1 yr Validation by randomly contacting approx half of the 3 informants nominated | |
| Notes | No non‐group control. 1&2 vs 3&4 in comparison 2.3. Originally treated as 2 studies in MA but due to small size now collapsed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "For the most part, subjects were assigned to treatment on a random basis. However for logistical reason the requests of couples and friends who wished to be assigned to the same group were honoured" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No validation but all participants had similar contact, likelihood of differential self‐report judged low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of losses to follow‐up, all recruited participants included in analyses |
Garcia 1989.
| Methods | Study design: Randomized controlled trial Country: Spain Recruitment: Primary care clinic volunteers Group size: maximum 15 |
|
| Participants | 68 smokers (in relevant arms); 41% women, av. age 34, av. cpd 25 | |
| Interventions | 1. Group therapy, 7 sessions over 3 m, nicotine gum 2 mg 2. Individual counselling in clinic, same schedule as groups, nicotine gum as in 1 (A 3rd arm receiving group therapy and placebo gum is not included) | |
| Outcomes | Sustained abstinence (quit at previous follow‐ups) at 6 m Validation: CO < 7 ppm | |
| Notes | Contributes to comparison 1.2.1 vs individual counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81 (43%) did not begin treatment and are not included. No differences detected between dropouts, or between treatment groups |
García 2000.
| Methods | Study design: Randomized controlled trial Country: Spain Recruitment: Community volunteers Group size: 7 ‐ 16 |
|
| Participants | 162 volunteers for a multisession programme, smoking > 10 cpd; 52% women, av. age 32, av. cpd 26 Therapist: Psychologist | |
| Interventions | 1. Multicomponent programme, 10x 1‐hr sessions over 5 wks 2. Multicomponent, 5 x 1‐hr over 5 wks 3. As 2 plus S‐H manual 4. S‐H manual, 1 orientation session | |
| Outcomes | PPA (7‐day) at 12 m Validation: CO < 8 ppm + confirmation by informant | |
| Notes | 1+2+3 vs 4 in comparison 1.1.1 for effect of any group programme. 1 vs 2 described in text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts who did not attend any sessions after randomization were not included. Losses to follow‐up included in analyses |
George 2000.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: People with schizophrenic disorders Group size: 4 ‐ 6 |
|
| Participants | 45 smokers with schizophrenia or schizo‐affective disorder; 67% men, av. age 40, av. cpd 30. More people were prescribed atypical antipsychotics in ALA group | |
| Interventions | All used 21 mg nicotine patches from quit day in wk 3 1. ALA 7 x 60‐min sessions + 3 x supportive counselling 2. Special schizophrenia programme. 3 x 60‐min weekly sessions motivational enhancement + 7 x psycho‐education, social skills, RP | |
| Outcomes | PPA 6m from therapy completion Validation: CO < 10 ppm | |
| Notes | No non‐group control. 2 vs 1 in comparison 2.4 evaluating enhanced programme in specific population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned to groups by using a block randomization procedure such that when 4‐6 subjects were ... considered eligible ... they were assigned together [to one of the programmes]." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in analysis |
Gifford 2011.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: not stated |
|
| Participants | 303 smokers; 59% women, av. age 46, av. cpd 24 Therapists: 1 substance abuse therapist, 3 clinical psychology doctoral students; psychiatrist and psychiatry resident for medication management |
|
| Interventions | 1. 10 wks of bupropion and 1‐hr group of medication instructions 2. As 1, with 10 wks of 1 group and 1 individual counselling session per wk, focused on functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation |
|
| Outcomes | 7‐day PPA at 12 m Validation: CO ≤10 ppm |
|
| Notes | New for 2017 update 10 intervention and 2 control participants did not complete any intervention after randomization; Comparison 1.3 'Group plus pharmacotherapy vs pharmacotherapy alone' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “the research director used a random numbers generator (http://www.randomizer.org) to randomly assign participants to condition” |
| Allocation concealment (selection bias) | Low risk | Study co‐ordinator was notified about assignment after participants were accepted |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44% of intervention and 46% of controls completed follow‐up, no significant difference in attrition |
Ginsberg 1992.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 3 ‐ 6 |
|
| Participants | 99 smokers with an acquaintance willing to participate as a support partner; 54% women, av. age 38, av. cpd 26 Therapists: PhD psychologist or MSc health educator | |
| Interventions | 1. Nicotine gum (NG) and educational materials, 2 sessions over 2 wks 2. NG and behavioural programme including skill training, 5 sessions over 4 wks 3. NG and behavioural programme and partner support programme, 8 sessions over 5 wks | |
| Outcomes | Abstinence at 52 wks (not clear if abstinence required at prior assessment at wks 4,12, 26) Validation: CO < 10 ppm, urine cotinine < 50 ng/mL. Paper states that cotinine levels failed to confirm self‐report in 7 people, 3 of whom were still coded as abstinent on the balance of evidence | |
| Notes | Intervention 1 had only 2 brief sessions so not classified as group therapy, 2+3 vs 1 in comparison 1.4, effect of addition of group support to NG (excluding group 3 would increase effect size) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned to 3‐6 member groups in order of entrance into treatment within time constraints. Treatment for each group was randomly selected with the constraint that each cohort [of 9] have one group of each condition and an equal number of smoking partners across conditions". Potential for systematic bias probably low |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment dropouts and losses to follow‐up included in analyses, 1 death excluded |
Glasgow 1981.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 4 ‐ 6 |
|
| Participants | 88 smokers (85 included in analysis) Therapists: A clinical psychologist and 2 graduate students in behaviour therapy, crossed with treatment conditions | |
| Interventions | 3 x 2 factorial design for treatment programme and delivery format 1. Therapist administered programme based on either Danaher & Lichtenstein manual, Pomerleau & Pomerleau manual or I Quit Kit. 8 sessions over 8 wks 2. Self‐administered using same 3 manuals | |
| Outcomes | Abstinence at 6 m Validation: CO < 15 ppm. At follow‐up, self‐report gives lower success rates in 3/6 arms than using CO measure, so self‐report data used | |
| Notes | Early versions of review had a substudy for each programme; all 3 programmes now combined in comparison 1.1.1 vs self‐help format. There is a negligible change to MA The comparison between different programmes is discussed in text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% of participants completed treatment and available for follow‐up |
Glasgow 1989.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Media advertisements Group size: 4 ‐ 8 |
|
| Participants | 66 smokers; 56% women av. age 40, av. cpd 26 Therapists: 2 research assistants. Crossed with treatments | |
| Interventions | Both programmes had 6 weekly meetings 1. Abstinence‐based condition. TQD at 4th session. Post‐quit sessions emphasize RP 2. Cessation‐Controlled Smoking. Quitting recommended but alternative of controlled smoking offered Quit date between sessions 4 and 5 | |
| Outcomes | Abstinence for 7 days at 6 m follow‐up. Validation: CO ≤ 9 ppm. 11 people disconfirmed | |
| Notes | No non‐group control. Compares difference in emphasis on abstinence. Comparison 2.4 'Other miscellaneous comparisons ‐ Total abstinence vs controlled smoking programme emphasis' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by group, no other information |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% lost in 1 and 14% in 2, all included in ITT analysis |
Goldstein 1989.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 6 ‐ 13. |
|
| Participants | 107 smokers Therapists: all sessions co‐led by psychiatrist and clinical psychologist | |
| Interventions | All groups met for 10 x 1‐hr sessions over 11 wks 1. Behavioural treatment (including intensive skills training) + fixed schedule nicotine gum 2. Same as 1, but ad lib schedule of gum 3. Educational group, no specific skills training, didactic presentation, non‐specific group support + fixed schedule gum 4. Same as 3. + ad lib gum | |
| Outcomes | Abstinence at 6 m follow‐up Validation: saliva cotinine < 10 ng/ml, or expired CO < 8 ppm in people still using nicotine gum | |
| Notes | No non‐group control; Nicotine schedule arms collapsed 1+2 vs 3+4 in comparison 2.1.1 evaluating greater complexity of group programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized in 2 x 2 factorial design, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 early treatment dropouts re‐included in ITT analysis here |
Grant 2003.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Substance abuse treatment centre volunteers Group size: not stated |
|
| Participants | 20 alcoholic smokers; 93% men, av. age 44, 77% smoked 11 ‐ 30 cpd | |
| Interventions | All participants were attending an outpatient alcohol treatment programme 1. Education & group therapy, 5 weekly sessions, 8 wks trial of NRT offered unless contra‐indicated 2. No formal treatment, access to standard cessation resources including NRT | |
| Outcomes | Abstinence at 12 m follow‐up (7 day PPA) Validation: no biochemical, collateral informants at 6 m only | |
| Notes | Comparison 1.5. Use of NRT high in both conditions, 6/20 in treatment, 10/20 in control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals not included in denominators |
Gruder 1993.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Smokers registering to receive S‐H materials during advance promotion of a televised cessation programme, who indicated willingness to attend group sessions and had a non‐smoking 'buddy' Group size varied from 3 ‐ 22, mean approx 11 |
|
| Participants | 1440 smokers completing a registration form and assigned to this study Therapists: Mainly nurses and health educators randomly assigned and trained to lead either Social Support or Discussion meetings | |
| Interventions | All participants sent ALA Freedom from Smoking in 20 days manual and instructed to watch TV programme. 1. Social Support. 3 x 90‐min group meetings and copy of Quitters Guide for smokers, and 1 group meeting + Buddy Guide for buddies. Participants were instructed on how to get help from their buddies and others. Telephone calls to participants and buddies at 1 m and 2 m 2. Discussion. Same schedule of meetings and phone calls as 1, but general information and review of self‐help manual 3. No‐contact control | |
| Outcomes | Multiple PPA (post‐intervention, 6 m and 12 m). 24‐m rates also given but substantial loss to follow‐up by this time, so 12‐m rates used here. Validation attempted but abandoned due to participant refusal to provide samples | |
| Notes | 1&2 vs 3 in comparison 1.1. Social support manipulation reviewed in Park 2012. Although group participants also scheduled to receive phone calls, these occurred after the first follow‐up so will not have differentially affected the multiple PPA quit rates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization to group or no‐group at time of registration. No details on method. 1205 participants assigned to a group condition, and attempts made to contact them to schedule group meetings. Randomization between the 2 group conditions was by site. 26 sites offered social support condition, 24 discussion control |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation, difference in contact |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quit rates for group vs self‐help comparison based on numbers assigned to group treatments who were scheduled to a meeting, and includes 'no shows' who were still assessed |
Hall 1994.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers or referrals |
|
| Participants | 149 smokers ( > 10 cpd) 52% women, av. age 41, av. cpd 25, 31% had history of MDD Therapists: physician, psychologist. Both received training | |
| Interventions | 2 mg nicotine gum was prescribed for both groups 1. Standard group therapy. 5 sessions over 8 wks. Information and group support for planning and implementing individual strategies 2. Mood Management. 10 sessions over 8 wks. Similar to 1, plus specific cognitive‐behavioural components for developing skills for coping with situations leading to poor mood. Thought stopping, rational‐emotive techniques, relaxation, etc | |
| Outcomes | Continuous abstinence at 52 wks. (Confirmed quit at all prior assessments and no smoking in previous wk) Validation: CO ≤ 10 ppm and urine cotinine ≤ 60 ng/ml | |
| Notes | No non‐group control; 2 vs 1 in comparison 2.3.2 evaluating additional mood management component | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts included as smokers, numbers not specified |
Hall 1996.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 5 ‐ 12 |
|
| Participants | 201 smokers ( > 10 cpd) 48% men, av. age 40, av. cpd 24; 22% had history of MDD Therapists: not described | |
| Interventions | 2 x 2 factorial design. Nicotine gum/placebo arms collapsed All groups had 10 sessions over 8 wks. TQD at 3rd session 1. Standard group therapy including written exercises, handouts, homework. Group discussion 2. Cognitive behavioural Mood Management. Same programme as Hall 1994 arm 2 | |
| Outcomes | Continuous abstinence at 52 wks. (Confirmed quit at all prior assessments and no smoking in previous wk.) Validation: urine cotinine ≤ 60 ng/ml | |
| Notes | No non‐group control; 2 vs 1, in comparison 2.3.1 evaluating additional mood management component, controlling for contact time, nicotine/placebo arms collapsed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized after stratification by depression history and number of cigs smoked |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts included as smokers, numbers not specified |
Hall 1998.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers. Exclusion criteria included MDD within 3 m of baseline Group size: 5 ‐ 11 |
|
| Participants | 199 smokers of ≥ 10 cpd 55% women, av. age 40, av. cpd 21 ‐ 25; 33% had history of MDD Therapists: 3 doctoral level clinical psychologists | |
| Interventions | 2 x 2 factorial design. Alternative pharmacological interventions were nortriptyline titrated to therapeutic levels ‐ usually 75 ‐ 100 mg/day for 12 wks or placebo. Collapsed in this analysis 1. Health Education 2. Cognitive behavioural mood management (See Hall 1994 for description of each intervention) | |
| Outcomes | Abstinence at 64 wks (1 yr post‐treatment). Continuous abstinence rates not reported by psychological treatment group Validation: CO < 10 ppm and cotinine < 341 nmol/L | |
| Notes | No non‐group control; same behavioural interventions compared as Hall 1994, 2 vs 1 in comparison 2.3.2 evaluating additional mood management component. Nortriptyline/placebo arms collapsed, no drug x psychological treatment interaction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by computer, after stratification on history of MDD and number of cigs smoked |
| Allocation concealment (selection bias) | Low risk | Computer randomization after data collection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% lost to follow‐up at 1yr, no difference by group, included in ITT analysis |
Hall 2002.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers. Exclusion criteria included current MDD Group size: 3 ‐ 11 |
|
| Participants | 220 smokers of ≥ 10 cpd 40% ‐ 47% women, av. age 37 ‐ 43, av. cpd 20 ‐ 23; 33% had history of MDD Therapists: Masters‐level counsellors | |
| Interventions | 3 x 2 factorial design with pharmacotherapies: bupropion, nortriptyline, or placebo 1. Medical Management (MM) control: physician advice, S‐H, 10 ‐ 20 mins 1st visit, 5 mins at 2, 6, 11 wks) 2. Psychological Intervention (PI) as MM plus 5 x 90‐min group sessions at 4, 5, 5, 7, 11 wks) | |
| Outcomes | Prolonged abstinence at 1 yr (47 wks post‐quit date). PPA also reported Validation: CO ≤ 10 ppm, urine cotinine ≤ 60 ng/mL | |
| Notes | Comparison 1.3, group versus physician advice No significant interaction between pharmacotherapy and behaviour therapy, so pharmacotherapy arms collapsed in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not specified, "double blind" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost to follow‐up at 1 yr, no difference by group, included in ITT analysis |
Hertel 2008.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: not stated |
|
| Participants | 418 smokers; 52% ‐ 56% women, av. age 47, av. cpd 22 ‐ 23 | |
| Interventions | 8 weekly sessions, TQD scheduled between sessions 4 and 5. Matched contact time, and sessions 5 ‐ 8 were identical between groups 1. Optimistic expectations: sessions 1 ‐ 4 content focused on the benefits of quitting smoking 2. Balanced expectations: sessions 1 ‐ 4 content focused on the pros and cons of quitting smoking |
|
| Outcomes | 7‐day PPA at 16 m (self‐report) | |
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons' No differences in abstinence rates (˜20%) between participants in the optimistic vs balanced expectations groups, described in text, not displayed in analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No validation but matched contact time, so likelihood of differential misreport judged low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 529 smokers were randomized, but 111 participants were excluded from analyses because they did not attend 2 of first 4 sessions (21% of optimistic group and 21% of balanced group). Of N = 418, 78% of participants in the optimistic expectations group and 79% of participants in the balanced expectations group completed the 16‐m follow‐up |
Hill 1993.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers |
|
| Participants | 82 community volunteers aged 50+ who had smoked for over 30 yrs Therapists: Each group had 2 instructors from a pool of 6, all with experience in smoking cessation or exercise training, or both. | |
| Interventions | 1. Behavioural Training (BT) adapted from Lung Health Study programme. Included quit date setting, RP training with role play of coping responses. 12 x 90‐min sessions over 3 m 2. BT + nicotine gum 3. BT + additional physical exercise 4. Exercise and S‐H pamphlet. This was a placebo control matched for contact time to 3. Therapist, who was blind to study hypothesis, encouraged smokers to quit at the exercise meetings | |
| Outcomes | 5‐day abstinence at 12 m. (Abstinence at previous follow‐ups not required) Validation: CO < 10 ppm or informant confirmation | |
| Notes | 1 vs 4 in comparison 1.4 vs minimal intervention control. Exercise component considered in separate review (Ussher 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment in blocks of 8 ‐ 12 individuals |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 non‐participants & 4 dropouts not included in analysis |
Hilleman 1993.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers |
|
| Participants | 150 smokers; 67% women, av. age ˜50, av. cpd ˜32 Therapists; not described | |
| Interventions | 1. Behaviour modification training, 12 x 1‐hr classes over 3 m + transdermal clonidine 2. Same behaviour modification as 1, + placebo patches 3. S‐H printed material (I Quit Kit), transdermal clonidine 4. S‐H printed material, placebo patches | |
| Outcomes | Cessation at 1 yr Partial validation by random plasma TCN monitoring | |
| Notes | Drug arms collapsed as no evidence for a treatment group interaction reported. 1 + 2 vs 3 + 4 in comparison 1.1.2 vs S‐H control, although the I Quit Kit is only a brief pamphlet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Incomplete validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information on losses to follow‐up, all participants included in analyses |
Hollis 1993.
| Methods | Study design: Randomized controlled trial Country; USA Recruitment: People visiting outpatient internal medicine and family practice offices in a group practice Health Maintenance Organiation. |
|
| Participants | 2707 smokers who received provider (physician, physician assistant or nurse practitioner) advice to quit Therapists: Project nurse or health counsellor | |
| Interventions | If people refused to see a counsellor they were mailed information appropriate to their assignment 1. Advice ‐ In addition to provider advice, given brief pamphlet by health counsellor 2. Self‐quit ‐ Cessation advice, CO assessment, 10‐min video, stop‐smoking kit, and choice of S‐H manuals. Encouraged to set quit date. 1 follow‐up telephone call and series of mailings 3. Group referral. Cessation advice, CO assessment. Video encouraged use of intensive (9 meetings over 2 m) group programme, and waiver of fee. Effort made to schedule attendance 4. Combination. Participants shown video explaining both S‐H and group approaches, and encouraged to choose one | |
| Outcomes | 1 yr 2 x PPA (7 days at 3 m and 12 m) Validation: Saliva cotinine at 1 yr. Most conservative outcome is used in which self‐reported non‐smokers who did not provide saliva samples are recorded as smokers | |
| Notes | 3 vs 2 in comparison 1.1.2 vs S‐H programme. 3 vs 1 in 1.3.1 vs brief advice control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Two random digits contained in the patient's health record number were used to assign patients" |
| Allocation concealment (selection bias) | High risk | Use of health record number precludes concealment. All who received initial provider advice were considered participants, and providers who delivered initial message stated to be blind to assignment, so possibility of selection bias may be low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% lost to follow‐up at 12 m. Response rates not significantly different across conditions, all participants included in analysis |
Huber 2003.
| Methods | Study design: Randomized controlled trial Country: Germany Recruitment: Community volunteers |
|
| Participants | 174 smokers 55% women, av. age 38, av. cpd 28 Therapists: experienced counsellors, each took 2 groups in each condition | |
| Interventions | 1. 5 x 90‐min weekly meetings. Included contracting, reinforcement, relaxation, skills training, nicotine gum 2. Same schedule of meetings, 45 mins only, focus on sharing experiences. Nicotine gum 3. As 1, no nicotine gum. Not included in meta‐analysis | |
| Outcomes | PPA at 12 m Validation: CO ≤ 4 ppm | |
| Notes | Included in 2009 update. No non‐group control, in comparison 2.1.2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 people attending 2 or fewer meetings not included in analysis. Said to be evenly distributed. Later dropouts included as smokers |
Jorenby 1995.
| Methods | Study design: Randomized controlled trial Country: USA (2 sites) Recruitment: Community volunteers Group size: not specified |
|
| Participants | 504 smokers (≥ 15 cpd); ˜53% women, av. age 44, av. cpd 26 ‐ 29 Therapists: Trained smoking cessation counsellors | |
| Interventions | Compared 22 mg vs 44 mg nicotine patch and 3 types of adjuvant treatment. Patch groups collapsed. All participants had 8 weekly assessments by research staff 1. Minimal: Given S‐H pamphlet by physician during screening visit for trial entry, and instructed not to smoke whilst wearing patch. No further contact with counsellors. 2. Individual: Given S‐H pamphlet at screening visit along with motivational message. Also met nurse counsellor x 3 following quit date. Nurse helped generate problem‐solving strategies and provided praise and encouragement 3. Group: Given S‐H pamphlet at screening visit along with motivational message. Received 8 x 1‐hr weekly group sessions. Skills training, problem‐solving skills | |
| Outcomes | 7‐day PPA at 26 wks Validation: CO < 10 ppm | |
| Notes | No sig diff in dose‐related outcome and no dose‐counselling interaction at 26 wks reported, so patch arm collapsed in analysis. 3 vs 1 in comparison 1.4, group + NRT vs NRT with minimal support. 3 vs 2 in 1.2.1, group vs individual (different programme) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly ordered within blocks of 30 assignments" |
| Allocation concealment (selection bias) | Unclear risk | Allocation by research assistant, concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 78 (3.7%) excluded from ITT analysis due to death or too ill for follow‐up. 426 (20%) lost to follow‐up included in ITT analysis; higher loss in treatment than control |
Lando 1985.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Media advertising Group size: 8 ‐ 12 |
|
| Participants | 130 smokers (65 in relevant arms) 51% women, av. age 38, av. cpd 30 | |
| Interventions | All received orientation + 2 weekly + 6 consecutive sessions in wk 3, then quit day 1. Nicotine fading + 7 maintenance sessions over 6 wks 2. Nicotine fading. No post‐quit maintenance 3. Oversmoking + maintenance (not used in review) 4. Nicotine fading + oversmoking + maintenance (not used in review) | |
| Outcomes | Abstinence at 12 m (PPA) Validation: CO and informants | |
| Notes | No non‐group control. 2 vs 1 in comparison 2.1 for effect of extended contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about loss to follow‐up |
Lando 1990.
| Methods | Study design: Randomized controlled trial Country: USA, 3 sites Recruitment: Community volunteers Group size: av. 10 ‐ 11 |
|
| Participants | 1041 smokers; 57% women, av. age 43, av. cpd 29 Therapists: trained facilitators | |
| Interventions | 1. ACS FreshStart. Orientation + 4 x 1‐hr sessions over 2 wks. No TQD set 2. ALA Freedom from Smoking. Orientation + 7 x 90 ‐ 120‐min sessions over 7 wks. TQD at 3rd session 3. Laboratory‐derived programme. 16 x 45 ‐ 60‐min sessions over 9 wks. Nicotine fading procedure and smoke‐holding used during preparation phase | |
| Outcomes | Sustained abstinence (slips allowed) at 1 yr. (PPA and quit attempts also reported) Validation: attempted for 43% sample. serum TCN < 80 ‐ 100 ng/ml. Borderline cases required cotinine < 15 ng/ml | |
| Notes | No non‐group control. Results not displayed in graphs. Quit rates: 1. 12% (N = 331). 2. 19% (N = 363). 3. 22% (N= 347) P = 0.014 corrected for design effect. No facilitator effect found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned ... as a function of orientation session attended". 70 orientation sessions held and 97 treatment groups formed |
| Allocation concealment (selection bias) | Unclear risk | No details given, but participants only given general information about type of programme |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence for almost half, all participants had active intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% loss to follow‐up at 1 yr. All except 3 deaths included |
Lando 1991.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 4 ‐ 16, typically 6 ‐ 11 |
|
| Participants | 353 smokers; 52% women, av. age 42, av. cpd 30 Therapists: Trained facilitators, mainly graduates, including some who had quit through clinic programme | |
| Interventions | Both interventions included 16 x 45 ‐ 60‐min sessions over a 9 wk period. Nicotine fading schedule prior to quit date at 3 wks 1. Enriched cohesiveness intervention: included written commitments and exercises designed to facilitate positive group interaction 2. Standard group treatment | |
| Outcomes | 1‐yr sustained (relapse‐free) abstinence Validation: randomly selected subsample of those claiming abstinence tested for saliva TCN, but not clear whether reported data includes a correction for false reporting | |
| Notes | No non‐group control. In comparison 2.3.2 evaluating group cohesion. Originally a factorial design comparing satiation and nicotine fading in addition to cohesiveness manipulation, but satiation arm abandoned. Only data for nicotine fading procedure arms reported in paper. P values reported in the paper were corrected for the design effects of clustering | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned by information group attended. 32 information meetings and 41 treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Partial validation, all participants received active intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6.5% loss to follow‐up included in analyses |
Leung 1991.
| Methods | Study design: Randomized controlled trial Country: Hong Kong Recruitment: Community volunteers |
|
| Participants | 95 (63 in relevant arms); 26% women, av. age ˜37, av. cpd ˜26 | |
| Interventions | 1. Behavioural programme including self‐monitoring, management techniques, coping skills. 10 x 1½‐hr sessions over 2 wks 2. Auricular acupuncture. Same no. of sessions. Not used in review 3. Waiting‐list control | |
| Outcomes | Abstinence (not defined) at 6 m Validation by cohabitant and work colleague report | |
| Notes | 1 vs 3, comparison 1.5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation, waiting‐list control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 people lost to follow‐up re‐included in analyses for MA |
Macpherson 2010.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers with mild depressive symptoms Group size: 3 ‐ 8 participants |
|
| Participants | 68 smokers with mildly elevated depressive symptoms (BDI ≥ 10) but no current major depression; 49% women, av. age 44 Therapists: 2 clinical psychologists, 3 clinical psychology students; all therapists conducted at least 1 of each treatment groups |
|
| Interventions | 8 x 1‐hr weekly sessions, TQD scheduled for session 4, all participants received nicotine patch and conditions were matched for contact time 1. Standard smoking cessation treatment: Per 2008 PHS Guidelines (self‐monitoring, identifying previous effective cessation strategies, relaxation, coping with triggers, social support, lifestyle changes, relapse prevention, and homework) 2. Standard treatment + behavioral activation treatment for smoking: As 1, plus a focus on non‐smoking reinforcing activities, activity monitoring and planning, and identification of values and goals) |
|
| Outcomes | Continuous abstinence at 26 wks Validation: CO ≤ 10 ppm and cotinine ≤ 15 ng/ml |
|
| Notes | New for 2017 update Comparison 2.2.1 'Mood management ‐ Same contact time' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence. "Assessments conducted by research assistants blinded to treatment condition". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout in both groups, 36% of participants in standard treatment and 43% of participants in behavioural activation completed follow‐up |
Matthews 2009.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: ˜7 participants |
|
| Participants | 68 African‐American smokers (≥ 1 cpd) 90% women, av. age 45, av. cpd 13 Therapists: Intervention designed to be administered by Master's level clinicians; The culturally‐targeted group was led by white women, and the various standard treatment groups were led by white women, white men, Latino women, and bi‐racial women |
|
| Interventions | 6 weekly sessions, 75 ‐ 90 mins, TQD scheduled for session 3, treatments matched for contact time. All participants offered nicotine patches 1. Standard treatment: CBT, motivational interviewing, 12‐step skills 2. Culturally‐targeted: Standard treatment was adapted to focus on themes relevant to African‐American smokers via culturally‐targeted themes, images and messages. An estimated 40% of standard materials were modified to be culturally targeted |
|
| Outcomes | 7‐day PPA at 6 m Validation: CO ≤ 6 ppm |
|
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons ‐ Culturally‐targeted treatment vs standard treatment' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 1 culturally‐tailored group included; 100% of culturally‐tailored vs 58% of standard treatment completed follow‐up |
McDowell 1985.
| Methods | Study design: Randomized controlled trial Country: Canada Recruitment: Volunteers visiting family practices for scheduled appointments Groups size: 10 ‐ 15 |
|
| Participants | 366 smokers in 9 group family practices; 60% women; av. age 36, av. cpd 24 Therapists: depended on intervention | |
| Interventions | 1. Physician advice by 1 of 12 family physicians. 15‐min counselling session with US 'NCI Helping Smokers Quit Kit' and 1 postal follow‐up 2. Operation Kick‐It programme. 9 sessions. Therapists: public health nurse or health educator 3. Cognitive Behavior Modification programme. 9 sessions. Therapists: 1 of 2 M.Ed psychologists 4. Self‐monitoring control followed up at 2, 6 and 12 m | |
| Outcomes | Abstinence (over 1‐wk diary period) at 12 m Validation: participants warned that saliva TCN might be tested, but only a few sampled. No results reported | |
| Notes | 2 & 3 vs 1 in comparison 1.3.1 vs physician or nurse advice/counselling, and in 1.5 vs minimal intervention control. 3 vs 2 in 2.1.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details, allocation took place once potential participants returned questionnaires |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation, not all participants had active intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% lost to follow‐up, slightly higher for controls. All re‐included for this analysis |
Minthorn‐Biggs 2000.
| Methods | Study design: Randomized controlled trial Country: Canada Recruitment: Community volunteers Group size: not stated |
|
| Participants | 75 smokers; 68% women, av. age 41, av. cpd 25 Therapists: Study author or Lung Association facilitator | |
| Interventions | 1. Canadian Lung Association Countdown programme. 7 weekly sessions 2. Social interaction programme. 12 sessions over 6 wks + 4 weekly. Skills training 3. No‐treatment control | |
| Outcomes | Abstinence at 6 m (12 m‐rates only available for groups 1 and 2) Validation: none | |
| Notes | 1+2 vs 3 in comparison 1.5 vs no treatment. 3 vs 2 in 2.1.2, effect of additional skills training. No control for therapist effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation, no contact control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts included in analyses |
Mueller 2012.
| Methods | Study design: Randomized controlled trial Country: Switzerland Recruitment: People hospitalized for 21‐day alcohol detoxification Group size: not stated |
|
| Participants | 103 smokers, alcohol‐dependent (ICD‐10 criteria), no exclusion for other substance‐use disorders 29% women, av. age 44, av. cpd 25 ‐ 30 Therapists: 2 psychologists trained in CBT and addiction treatment |
|
| Interventions | 5 x 30‐min groups during last 2 weeks of inpatient hospitalization. Participants intending to quit smoking were offered nicotine patch during inpatient stay 1. CBT: CO‐level measurements, motivational processes, education about nicotine and its effects, psychological factors in addiction, setting TQD (if participants intended to quit), and RP. 2. Autogenic training: relaxation method, non‐evidence‐based method for smoking cessation |
|
| Outcomes | 7‐day PPA at 6 m Validation: CO < 10 ppm |
|
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons ‐ CBT vs relaxation training' Approximately 50% of participants had stopped using alcohol at follow‐up, no differences by group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47% of CBT and 66% of autogenic‐training participants attended follow‐up; authors reported no significant differences in attrition |
Nevid 1997.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers, via media and healthcare settings Group size: 3 ‐ 12, single‐sex groups, same‐sex therapists |
|
| Participants | 93 Hispanic smokers (excludes 56 people, 35 Gr, 21 S‐H who were randomized but did not attend any session and were not included in further analysis); 48% women, av. age 44, av. cpd 21 Therapists: bilingual Hispanic psychologists and social workers | |
| Interventions | 1. Group therapy. 8 x 2 hrs. Included videos using culturally‐specific components. Motivation, nicotine fading, quitting techniques, RP, 'buddy' support. TQD 5th week 2. S‐H with 1 group session for motivation and instructions and telephone contact. ALA Freedom from Smoking in 20 days in English & Spanish, also Guia para Dejar de Fumar Both conditions received same maintenance programme; ALA S‐H manual A Lifetime of Freedom from Smoking and 2 telephone calls a month for 6 m | |
| Outcomes | Abstinence at 12 m (sustained from post‐treatment). PPA rates also reported Validation. Saliva cotinine | |
| Notes | Comparison 1.1.2 vs different S‐H. Low take‐up rates. 33% of eligible attended orientation session, only 62% of enrollees attended any further session. Using 12‐m PPA rates would give 3/39 vs 4/54 quit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "pairwise random assignment ... a random numbers table was used to generate a sequence of odd and even numbers, which was then used as the basis for randomly assigning members of each pair of consecutively enrolled participants within each gender to either [the treatment or control]" |
| Allocation concealment (selection bias) | Unclear risk | Seems unlikely from description that schedule was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Post‐randomization dropouts are excluded. There was differential attendance |
Omenn 1988.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Single worksite (13,000 workers, 9 employers) Group size: typically 15 ‐ 20 |
|
| Participants | 159 smokers; 66% men, av. age 43, av. cpd 25, with preference for group programme or no preference (Smokers with preference for S‐H were not allocated to group programmes) Led by instructors trained in both programmes | |
| Interventions | 1. Multiple Component programme. 3 sessions over 3 wks. Didactic format 2. RP programme. 8 sessions over 8 wks. Interactive format, choice of immediate or phased quit 3. Minimal Treatment programme. S‐H materials only. ACS 22‐page Quitter's Guide 7‐day plan | |
| Outcomes | Abstinence at 12 m (single PPA) Validation: saliva cotinine ≤ 35 ng/ml | |
| Notes | 1+2 vs 3 in comparison 1.1.2 vs different S‐H. No difference in outcome at 12 m between 2 group programmes. Self‐reported quit rates similar across all 3 conditions but more missing saliva samples in S‐H so validated rates lower | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "nurses at aid stations using randomized assignment lists generated by research centre, within preference for format" |
| Allocation concealment (selection bias) | High risk | Mention of lists and not envelopes suggests that concealment unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At least 89% followed up in each arm |
Otero 2006.
| Methods | Study design: Randomized controlled trial Country: Brazil Recruitment: Community volunteers Group size: 12 |
|
| Participants | 1199 smokers (includes 254 non‐attenders); 63% women, av.age 42, 46% smoked > 20 cpd Therapists: trained doctors, nurses or psychologists | |
| Interventions | Factorial design with NRT 21 mg or 14 mg patch for 8 wks incl tapering and 5 levels of behavioural support collapsed into 3 for analysis 1. Single 20‐min session ‐ classified as 'brief intervention control' in meta‐analysis 2. Cognitive behavioural, 1 or 2 weekly x 1‐hr sessions 3. As 2, with 3 or 4 weekly sessions. Maintenance or recycling sessions provided to all groups at 3, 6, 12 m | |
| Outcomes | Abstinence at 12 m (7‐day PPA) Validation: none | |
| Notes | 2&3 vs 1 without patch in comparison 1.2.1. 2&3 vs 1 with patch in 1.3. 3 vs 2 (patch conditions collapsed) in 2.1.2. 29% of no‐patch group participants asked for nicotine patch after the 3‐m follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, stratified by age & sex, by independent specialist |
| Allocation concealment (selection bias) | Low risk | Trial administrators blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No validation but all participants received active treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about numbers lost; non‐participants and losses to follow‐up included in ITT analysis |
Patten 2002.
| Methods | Study design: Cluster‐randomized controlled trial Country: USA Recruitment: Volunteers attending Alcoholics Anonymous Group size: approx 8 |
|
| Participants | 48 smokers with history of alcohol dependence but 3 m of drug and alcohol abstinence; 47% women, av. age 42, av. cpd 28 Therapists: different clinical psychologist and doctoral student pair for each condition | |
| Interventions | 1. Behavioural counselling, 12 x 2‐hr weekly, TQD wk 8. Includes nicotine fading, skills training, homework, discussion 2. As 1 + Cognitive Behavioural Mood Management skills training. Same length | |
| Outcomes | Abstinence at 12 m, sustained at 1, 3 m. Validation: CO < 10 ppm (PPA rates and informant or CO‐validated rates also reported) | |
| Notes | No non‐group control. Comparison 2.3, effect of additional mood management component | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described; cluster‐randomized to small groups based on order of recruitment |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73% BC and 84% CBT completed 12‐m follow‐up. All participants included in ITT analysis |
Pederson 1981.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Volunteers for a S‐H smoking cessation programme |
|
| Participants | 40 smokers; 60% women, av. age 39, av. cpd 28 | |
| Interventions | 1. Pomerleau & Pomerleau manual, an introductory session, followed by 1‐hr group meetings at 2 and 6 wks 2. Danaher & Lichtenstein manual and same schedule of meetings as 1 3. Waiting‐list control | |
| Outcomes | Abstinence at 6 m for at least 3 m Validation: none | |
| Notes | 1&2 vs 3 in comparison 1.5. Described by the authors as a S‐H programme but the 3 meetings met criteria for a group programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described. Participants switched between the 2 manuals because of scheduling constraints |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No validation, waiting‐list control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in ITT analysis |
Pisinger 2005.
| Methods | Study design: Randomized controlled trial Country: Denmark Recruitment: proactive invitation to sample from a population register |
|
| Participants | 2408 daily smokers identified by questionnaire from the total sample; 40% women, av. age 46, 57% in precontemplation Therapists: Doctors or nurses trained in counselling | |
| Interventions | 1. 'Low intensity': single 15 ‐ 45‐min session of individual lifestyle counselling using motivational interviewing 2. 'High intensity': as 1 plus offer of participation in 6‐session group course over 5 m. Option to consider and be invited again in 3 m Untreated population control not included in this review | |
| Outcomes | PPA 5 yrs (follow‐up at 1 & 3 yrs also) Validation: serum cotinine | |
| Notes | Comparison 1.3.1. 5 yr outcomes reported in Pisinger 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random sample." More participants were randomized to the high‐intensity intervention |
| Allocation concealment (selection bias) | Low risk | "the sample was a priori randomized" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Deaths and emigrations excluded. 20% did not attend or return questionnaires at 5 yrs, included in ITT analysis |
Pisinger 2010.
| Methods | Study design: Cluster‐randomized controlled trial Country: Denmark Recruitment: People visiting general practice clinics Group size: not stated |
|
| Participants | 1518 smokers (from 24 general practitioners); 80% of those eligible were enrolled Therapists: not described |
|
| Interventions | General practitioners were randomized to 1 of 3 conditions, lasting 8 wks: 1. Usual care: general practitioners provide smoking cessation advice and assistance as usual 2. Internet‐based smoking cessation programme: General practitioners were encouraged to discuss smoking with all smokers, and provide smokers interested in quitting with information to enrol in an interactive website with 13 sessions delivered over 6 m. 16% of randomized participants enrolled in internet programme 3. Group counselling: General practitioners were encouraged to discuss smoking with all smokers, and provide smokers interested in quitting with contact information to enrol in group counselling consisting of 5 2‐hr sessions, and recommending NRT. 7% of randomized participants attended group counselling |
|
| Outcomes | PPA at 12 m Validation: urine cotinine < 200 ng/ml |
|
| Notes | New for 2017 update 1 vs 3 included in Comparison 1.3.1 'Physician, nurse, or pharmacist advice' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomized; "General practitioners were pre‐randomized at the Research Centre by a computer generated list to one of the three groups; Doctors sharing the same address were randomized by the investigators to the same group as the first drawn doctor." |
| Allocation concealment (selection bias) | High risk | General practitioner characteristics may not have been balanced across groups; they knew which group they were randomized to before accepting participation and characteristics of participating GPs were different to non‐participants. (All participants asked to complete baseline questionnaire to identify smokers, and were not aware of practice allocation, so risk of selection bias for participants low) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition by group not described. 50% of baseline smokers returned follow‐up questionnaires 70% of self‐reported abstinent smokers completed urine validation |
Rabkin 1984.
| Methods | Study design: Randomized controlled trial Country: Canada Recruitment: Media advertisements Group size: 10 |
|
| Participants | 168 community volunteers (67 in relevant arms) av. age 40, av. cpd 24 Therapist: "trained in group behaviour techniques" | |
| Interventions | 1. Behaviour modification. Multicomponent, 5 x 45 ‐ 90‐min meetings over 3 wks 2. Health Education. Single group meeting with didactic lectures by a health professional, film, discussion. Individual session with a therapist 1 wk later including a counselling element 3. Hypnosis 4. Waiting‐list control, with no long‐term follow‐up | |
| Outcomes | Self‐reported abstinence via questionnaire at 6 m follow‐up No validation at 6 m, Blood TSN at 3 wks | |
| Notes | 1 vs 2 in comparison 1.3.2 vs other method. 2 does not meet criterion of > 1 group session, and includes a session of individual counselling. 3&4 not used in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation but both arms contributing to review received active treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of dropouts similar, included in ITT analysis |
Ramos 2010.
| Methods | Study design: Randomized controlled trial Country: Spain Recruitment: People who attended health centres Group size: not stated |
|
| Participants | 287 smokers (from 10 health centres) in the preparatory phase of smoking cessation 54% women Therapists: 1 physician and nurse "microteam" per health centre |
|
| Interventions | All participants were offered NRT or bupropion 1. Minimal intervention: usual care provided by health worker with basic training 2. Individual intervention: 6 sessions, motivational interviewing to increase motivation to quit smoking and prevent relapses after smoking cessation 3. Group intervention: Same as 2 in group format, matched content and number of sessions |
|
| Outcomes | Continuous abstinence at 12 m Validation: CO, cutoff unclear |
|
| Notes | New for 2017 update 3 vs 2 included in Comparison 1.2.1 'Smoking cessation Group programme vs individual therapy ‐ Group vs individual (similar intensity & content)' although group participants were reported to have received more contact 3 vs 1 included in Comparison 1.3.1 'Smoking cessation Group programme vs brief intervention ‐ Physician, nurse, or pharmacist advice' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "An allocation concealment method based on the use of sequentially‐numbered, opaque, sealed envelopes was used…A block of 60 envelopes (20 for III, 20 for IGI and 20 for MI) was prepared in the central research unit for each participating health centre and subsequently sent out." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fewer minimal intervention participants completed CO validation than other groups; overall, very few participants completed 12 m CO follow‐up visit |
Rice 1994.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Health professional and self‐referral |
|
| Participants | 406 smokers with a cardiovascular health problem Therapists: Clinical nurse specialist who had undergone a 1‐wk teaching workshop for Smokeless (a multicomponent intervention in 6 booklets including elements of skills training, behavioural rehearsal, aversive puffing) | |
| Interventions | All except control received Smokeless 1. Individual Intervention: Met with nurse for 4 x 1‐hr sessions in wk 1 and single maintenance session in wk 2 2. Group Intervention: Met in groups of 5 ‐ 7 on same schedule 3. Written intervention: Given Smokeless materials in labelled envelopes to open on same schedule Prompted by call from project secretary 4. No Intervention: Advice from nurse to quit smoking | |
| Outcomes | PPA at 1 yr Saliva TCN tested but not used to correct self‐report | |
| Notes | The published data was based on 255 smokers willing to participate in the treatment allocated. Numbers randomized to treatment provided by author 2 vs 3 in comparison 1.1 vs self help; 2 vs 1 in 1.2.1 vs individual therapy, 2 vs 4 in 1.3.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described. Stratified by sex, smoking history and history of cardiovascular incident |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants provided samples for biochemical validation, likely to reduce misreporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34% chose not to participate after randomization, with differences between groups Reincluded in ITT analyses. 12 deaths not included |
Romand 2005.
| Methods | Study design: Randomized controlled trial Country: France, 6 towns Recruitment: Community volunteers, motivated to quit |
|
| Participants | 228 smokers 54% women, av. age 42, av. cpd 20 Therapists: 2 professionals per group, e.g. trained psychologist and qualified health adviser | |
| Interventions | 1. Five Day Plan (FDP); 5 sessions on consecutive nights, & supplementary sessions 1 ‐ 2 wks later 2. Control; 1 hr of general information on tobacco‐related health problems | |
| Outcomes | Abstinence at 12 m, lapse‐free (PPA also reported) Validation: CO < 10 ppm | |
| Notes | In comparison 1.3.2 Using the less stringent definition of abstinence would reduce the effect, 16% vs 11% quit. A small number of control group participants attended other FDP courses or used pharmacotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, stratified by town, "balanced every four individuals" |
| Allocation concealment (selection bias) | Unclear risk | No details given. The discrepancy in group sizes suggests the possibility of selection bias, but may be due to the stratification & chance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% & 15% lost at 12 m, included as smokers in ITT analysis |
Rovina 2009.
| Methods | Study design: Randomized controlled trial Country: Greece Recruitment: Smokers who attended smoking cessation clinic located in a hospital Group size: up to 10 participants |
|
| Participants | 205 smokers, motivated to quit; av. age 45 Therapists: "Specialized psychologist" conducted group counselling; brief advice provided by chest physicians |
|
| Interventions | Participants randomized to 1 of 4 groups. Group counselling lasted 19 wks, conducted in 60‐min sessions, weekly for first month and every 3 weeks for remainder 1. Bupropion only (< 15 minutes of brief advice to quit smoking) 2. Bupropion + Support group counselling: focus of counselling was on behavioural skills including learning and rehearsing new behaviours, response substitution, and monitoring and planning for high‐risk situations 3. Bupropion + CBT group counselling: focus of counselling was on changing thoughts, beliefs and attitudes to quitting and to alter negative mood in the formal way 4. CBT group counselling only: As 3, no bupropion. Not used in review |
|
| Outcomes | Continuous abstinence at 12 m Validation: CO ≤ 10 ppm |
|
| Notes | New for 2017 update 2&3 vs 1 included in Comparison 1.3 'Group plus pharmacotherapy vs pharmacotherapy alone' 3 vs 2 included in Comparison 2.1.1 'Skills training ‐ Substitution of components (controlling for programme length)' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% of all participants completed follow‐up; not reported by group |
Sawicki 1993.
| Methods | Study design: Randomized controlled trial Country: Germany Recruitment: From a university diabetic outpatients clinic |
|
| Participants | Diabetic smokers prepared to participate in a stop‐smoking programme; 40% women, av. age 37, av. cpd 21 | |
| Interventions | 1. Extensive behaviour therapy including self‐control. 10 x 90‐min weekly sessions. Led by a psychotherapist 2. Physician advice, 15‐min unstructured session. NRT offered in the case of severe addiction | |
| Outcomes | Abstinence at 6 m Validation: serum cotinine < 20 ng/ml | |
| Notes | Comparison 1.3.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25/44 participated in group programme and 31/45 received physician advice Non‐participants followed up and included in ITT analysis |
Schleicher 2012.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: College students Group size: not stated |
|
| Participants | 58 smokers, college students who reported smoking at least 25 out of the past 30 days and endorsed elevated depressive symptoms 51% women, av. age 21 Therapists: 2 ‐ 3 advanced graduate students in psychology or public health |
|
| Interventions | 6 group sessions, first 5 sessions took place weekly, last session occurred after 30 days. Equal contact time 1. Nutrition group: designed to increase the consumption of fruit and vegetables, including self‐monitoring of fruit and vegetable consumption, nutrition education, and motivational approaches to increase consumption 2. CBT mood management for smoking group: participants were taught that smoking is a learned behaviour and is related to mood, self‐monitoring to identify smoking triggers, thoughts, feelings, and behaviours, thought logs, relaxation training, pleasant event scheduling, motivational interviewing, and relapse prevention. TQD scheduled after session 4 |
|
| Outcomes | 30‐day PPA at 6 m Validation: none |
|
| Notes | New for 2017 update Comparison 1.5 'Smoking cessation. Group vs 'no intervention' controls' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table, blocking on smoking level and motivation to quit |
| Allocation concealment (selection bias) | High risk | Project personnel were not blinded to group assignment at time of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported, control group, although matched for contact, did not focus on smoking so potential for differential misreporting |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 69% of participants in the smoking group and 66% of participants in the nutrition group completed follow‐up |
Schmitz 2007.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers |
|
| Participants | 154 women smokers > 20 cpd, av.age 48, av. cpd 21 Therapists: Masters‐level therapists, 2 per group | |
| Interventions | Factorial trial of bupropion versus placebo (collapsed in analysis) and 2 group therapies 1. CBT based on relapse prevention model, 7 weekly 60‐min meetings, TQD morning of 1st session, 10 days after start of meds 2. Supportive therapy (ST), same schedule, emphasis on group support | |
| Outcomes | Abstinence at 12 m (7‐day PPA) Validation: CO ≤ 10 ppm, saliva cotinine < 15 ng/ml | |
| Notes | No non‐group control. There was no main effect of either type of treatment so pharmacotherapy arms collapsed. There was an interaction between behavioural support condition and pharmacotherapy; People receiving bupropion benefitted more from CBT whilst people on placebo had higher quit rates with ST. 2 vs 1 in comparison 2.3.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn procedure, balancing on a range of outcome‐related variables |
| Allocation concealment (selection bias) | Low risk | "Investigators and research staff blind to randomization codes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 "enrollment failures" who did not receive any treatment are excluded from analyses. Other non‐completers and losses to follow‐up included in ITT analysis |
Slovinec 2005.
| Methods | Study design: Randomized controlled trial Country: Canada Recruitment: Community volunteers |
|
| Participants | 332 women smokers of at least 10 cpd, av. age 40, av. cpd 20 | |
| Interventions | 1. 'Usual Care' 3 x 15‐min physician visits, 2 wks before & 4 & 8 wks after TQD. Nicotine patch, S‐H materials 2. As 1, plus Stress Management Training. 8 x 2‐hr, 2 & 1 wk before TQD, 1, 2, 3, 4, 5, 7 wks after. CBT targeted smoking‐specific and life stressors | |
| Outcomes | Abstinence at 12 m (7‐day PPA) Validation: CO ≤ 9 ppm for sample at 12 m, all quitters at 2 m. No disconfirmation out of 16 samples but 3 not reached (2UC, 1SM) | |
| Notes | Comparison 1.3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | "Treatment allocation was concealed until completion of baseline testing at which time participants were informed of their group assignment’; unclear that study staff blind until enrollment. 'Study physicians were blind to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27% UC and 21% SM lost at 12‐m follow‐up, included in ITT analysis |
Ward 2001.
| Methods | Study design: Randomized controlled trial Country: Jamaica Recruitment: Community volunteers |
|
| Participants | 75 smokers (+35 assigned to a waiting‐list control, not included in review); 57% women, av. age approx 39 Treated in 4 groups, Therapist: not described | |
| Interventions | 1. Group therapy with emphasis on self‐efficacy and stages of change, and use of NRT. 3 x 2‐hr weekly + follow‐up at 7 wks. Chose own quit date 2. as 1 plus cognitive counter‐conditioning component. Group developed negative images of smoking to be used when smoking. Same schedule | |
| Outcomes | Abstinence at 12 m (PPA) Validation: saliva cotinine. Cutoff not specified | |
| Notes | No non‐group control. In comparison 2.1.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 dropouts included in ITT analysis |
Webb 2010.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: ˜7 participants on average |
|
| Participants | 154 African‐American smokers; 56% women, av. age 44, av. cpd 13 Therapists: African‐American clinical psychologist and white Masters‐level counsellor |
|
| Interventions | All participants received 8 wks of NRT (patches). Participants were offered 6 x 60 ‐ 90‐min sessions over 2 wks 1. Health education group: educational series on medical conditions that are associated with or caused by smoking that are relevant to the African‐American community. During Session 1, participants received a handout containing smoking cessation strategies; however, specific topics concerning smoking cessation were not discussed in session, and coping skills training was not provided. 2. Culturally‐tailored CBT group: Participants were taught cognitive–behavioral cessation and relapse prevention strategies, and included discussion of barriers to cessation, previous quit attempts, risky situations, benefits observed after quitting, and homework. Participants were encouraged to reduce smoking and quit for session 1 |
|
| Outcomes | 28‐day continuous abstinence at 6 m Validation: none |
|
| Notes | New for 2017 update Comparison 2.4 'Other miscellaneous comparisons ‐ CBT vs group health education' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomization", "stratified random sampling to generate randomization codes in blocks of 15" |
| Allocation concealment (selection bias) | Unclear risk | "Eligible participants were assigned a tentative random assignment and scheduled for an orientation session" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation but all participants received active treatment and similar amount of contact, "6‐month assessment occurred via telephone by the RA, who was blinded to study condition." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 71% of CBT group and 69% of health education group completed follow‐up |
Wilson 2008.
| Methods | Study design: Randomized controlled trial Country: Northern Ireland, UK Recruitment: Respiratory outpatient dept |
|
| Participants | 91 smokers with COPD, 52% women, av. age 61, av. cpd 19 Therapists: trained respiratory nurses | |
| Interventions | 1. Usual care; brief advice from physician including assessment of stage of change and advice on NRT 2. As 1, plus 5 weekly 60‐min group sessions, offer of NRT in wk 2 3. Same schedule of individual sessions | |
| Outcomes | Sustained abstinence at 12 m ("intermittent cessation" also reported) Validation: CO ≤ 10 ppm & saliva cotinine ≤ 10 ng/ml | |
| Notes | No sustained abstainers in any group, 2 UC and 3 group participants achieved intermittent cessation 2 vs 3 in 1.2.1 vs individual counselling, 2 vs 1 in comparison 1.3.1 vs usual care. Only 24% attended 3 or more group meetings, 37% 3 or more individual sessions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Sequentially sealed envelope." "All study personnel blind to randomisation sequence." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Greater loss to follow‐up in individual and usual care but ≤ 30% in all. All included in ITT analysis |
Zelman 1992.
| Methods | Study design: Randomized controlled trial Country: USA Recruitment: Community volunteers Group size: 3 ‐ 6 |
|
| Participants | 116 smokers (excludes 10 early dropouts evenly spread across groups); 54% women, av. age approx 50 Therapists: clinical psychologists, 2 per group | |
| Interventions | Behavioural counselling with nicotine gum or rapid smoking conditions collapsed here 1. Coping Skills Training. 6 x 60+ min over 2 wks. TQD night before 1st session. Develop strategies, reframing, contracting, thought‐stopping 2. Informational and supportive counselling. Discussion, sharing of ideas and feelings. Same schedule of sessions and TQD as 1 | |
| Outcomes | Sustained abstinence at 12 m (no lapses > 3 days) Validation: Collateral report at 12 m (CO used up to 3 m follow‐up, blood cotinine at 6 m) | |
| Notes | No non‐group control. 1 vs 2 in comparison 2.1.1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence at some follow‐ups, both arms received active treatment and similar contact intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report that exclusion of early dropouts does not change results |
Zheng 2007.
| Methods | Study design: Randomized controlled trial Country: China Recruitment: Community volunteers Group size: 13 ‐ 15 |
|
| Participants | 232 smokers (no minimum daily amount specified); 94% men, av. age 56 in I, 53 in C (P < 0.05) Therapists: health education professionals | |
| Interventions | 1. Social cognitive group intervention, 5 x 2‐hr twice‐weekly sessions 2. Waiting‐list control | |
| Outcomes | Sustained abstinence at 6 m Validation urine cotinine < 25 ng/ml | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants took paper marked 1 or 2 from a box |
| Allocation concealment (selection bias) | High risk | Possibility that allocation could be changed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow‐up in I, 11 in C, included in ITT analysis |
ALA: American Lung Association ACS: American Cancer Society av: average (mean) BDI: Beck's Depression Index CBT: cognitive behavioural therapy CO: Carbon Monoxide cpd: cigarettes per day FTQ: Fagerstrom Tolerance Questionnaire hr: hour(s) m: month(s) MDD: Major Depressive Disorder min: minute. NCI: National Cancer Institute NRT: nicotine replacement therapy NS: statistically non‐significant PPA: Point prevalence abstinence ppm: parts per million RP: Relapse prevention Rx: treatment S‐H: self‐help. sig diff: statistically significant difference TCN: thiocyanate TQD: Target Quit Day vs: versus wk: week(s) yr: year(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asuzu 2013 | No long‐term follow up |
| Becona 1997 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Bernstein 1970 | No long‐term follow up |
| Bertera 1990 | Not randomized |
| Bowen 2009 | Only 1 wk follow‐up. Only 1 session intervention |
| Brewer 2011 | Only 17‐wk follow‐up (post‐treatment initiation) |
| Brown 1984 | Small study of nicotine fading and relapse prevention. No non‐group control |
| Campbell 1995 | Not randomized |
| Carlson 2003 | Not controlled |
| Carlson 2012 | Not randomized |
| Cinciripini 1994 | The minimal contact self‐help control condition included 8 weekly visits to the research centre to fill out questionnaires and review progress. Although participants did not receive a formal intervention they were encouraged to discuss their progress and were directed to the appropriate section of the self‐help materials (I Quit Kit) Allocation to treatment alternated for successive sequences of 5 participants |
| Cinciripini 1995 | All interventions received same basic group programme. 4 arms differed in pre‐cessation programme of scheduled smoking |
| Colletti 1979 | Primary outcome was reduction in smoking rate. Quit rates not given by treatment group. 42 participants randomized to 3 maintenance strategies following same cessation programme |
| Colletti 1980 | Primary outcome was reduction in smoking rate. Quit rates not given at maximum follow‐up, reported not to be significantly different. 29 participants randomized to 2 maintenance procedures, 1 involving 4‐wk additional therapis contact |
| Copeland 2015 | Only 4 m follow‐up |
| Costello 2011 | Only 5‐wk follow‐up |
| Cropsey 2008 | Female prisoners (N = 360) were randomized to 10‐wk group therapy + NRT or wait‐list control. However, wait‐list control participants then joined intervention group analyses |
| Culbertson 2012 | Compared 2 methods of cue exposure using virtual reality (smoking vs placebo cues); all participants received the same group programme |
| Davis 1986 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Decker 1989 | Not randomized ‐ run sequentially. Compared an identical programme delivered at group meetings or by weekly mailings |
| Dickson‐Spillmann 2013 | Compared hypnotherapy vs relaxation in a single‐session intervention |
| Dijkstra 2014 | Single‐session group training using Allen Carr's "Easyway to Stop Smoking." |
| Elliott 1978 | Primarily a study of aversive smoking |
| Erfurt 2015 | No difference in quit rates among participants who did or did not elect to use stop‐smoking medication; all participants received the same group programme |
| Frikart 2003 | Not controlled |
| Glasgow 1978 | No abstinence data reported at 3‐m or 6‐m follow‐up |
| Grassi 2011 | Compared group programmes with and without varenicline. No non‐group control |
| Green 2003 | Not controlled |
| Hall 1984 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Hall 1985 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Hall 1987 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Hall 2009 | All participants received the same 12‐wk group therapy + pharmacotherapy programme, and were then randomized to receive extended individual behaviour therapy, NRT, both, or neither. See also: Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug and Alcohol Dependence 2010 Jun 1;109(1‐3):114‐9. |
| Hall 2011 | All participants received the same 12‐wk group therapy + pharmacotherapy programme, and were then randomized to receive extended active vs placebo bupropion, ± individual therapy, or no intervention |
| Hamilton 1979 | No follow‐up of control group at 6 m. Treatment arms investigated addition of social support |
| Hamilton 1998 | Only 3‐m follow‐up. Randomization not reported in abstract |
| Hernández‐López 2009 | Not randomized |
| Hilleman 2004 | Not randomized; historical control |
| Hiscock 2013 | Not randomized |
| Kahler 2008 | Not group therapy, only individual counselling provided |
| Kapson 2010 | Only 3 m follow‐up See also: Kapson HS, Leddy MA, Haaga DAF. Specificity of effects of cognitive behavior therapy on coping, acceptance, and distress tolerance in a randomized controlled trial for smoking cessation. Journal of clinical psychology 2012;68(12):1231‐40. |
| Katz 1977 | Only 3 m follow‐up. Abstinence rates not reported by group. Compared 3 different group programmes |
| Killen 1984 | Evaluated effect of relapse prevention components. Now included in Hajek 2013b |
| Kisely 2003 | Not randomized |
| Klesges 1999 | Not group therapy: intervention was a single 50‐min group session using a computer‐interactive format |
| Kumar 2012 | Only 2‐m follow‐up |
| Lando 1982 | A small trial manipulating multiple factors |
| Larson 1999 | Only 35 participants split among 3 programme variants. Randomization and length of follow‐up not reported in abstract |
| Lowe 1980 | Evaluates the effect of adding covert sensitization training to a group programme. Covered by review of aversion therapy (Hajek 2001) |
| Martin 1997 | Compared group programmes with and without an exercise component. No non‐group control. Included in Cochrane Review of exercise for smoking cessation (Ussher 2014) |
| Mayer 2010 | Test of relapse prevention intervention; included in Hajek 2013 |
| McEwen 2006 | Not randomized and only 4‐wk follow‐up |
| McGovern 1991 | Compared 2 methods of nicotine fading; all participants received the same group programme (Early version of review included within miscellaneous comparison section) |
| McIntyre 1986 | Compared an additional spouse support element with a basic programme. No non‐group control |
| Moadel 2012 | Only 3‐m follow‐up |
| Mogielnicki 1986 | Assignment to a group programme or a mailed self‐help programme was sequential. There appeared to be limited follow‐up of participants receiving mailed programmes |
| Morris 2011 | Quitline ± group counselling for smokers with schizophrenia. Abstinence data not reported by group at 6‐ follow‐up |
| Moser 2011 | No group counseling, not randomized |
| NCT00960375 | Only 3 m follow‐up |
| NCT02072772 | Only 3‐m follow‐up. Individual, web‐based counselling |
| Nyborg 1986 | Couples were allocated to treatment and success rates were reported by couple |
| Park 2014 | Only 12‐wk follow‐up |
| Perkins 2001 | Primarily a study of CBT for weight control |
| Pirie 1992 | Compared additional weight control element with a standard programme, also effect of nicotine gum in a factorial design. No non‐group control |
| Powell 1981 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Razavi 1999 | Primarily a study of relapse prevention, see Cochrane Review of interventions for relapse prevention (Hajek 2013b) |
| Reid 2008 | Group counselling was counfounded with nicotine replacement therapy |
| Savant 2013 | Evaluated cessation of multiple tobacco products, and abstinence rates were not reported by tobacco product |
| Schauffler 2001 | Participants were randomized to be eligible for OTC NRT and a group behavioural cessation programme as part of their HMO benefit. NRT and group therapy were therefore confounded. Cessation rates were significantly higher in intervention group; 18% vs 13% at 12 m. However only 1.2% participated in a behavioural programme |
| Schoenberg 2016 | Only ˜4‐m follow‐up |
| Schwartz 1968 | Success was defined as a reduction in smoking of > 85%, not complete abstinence, and no period of continuous reduction was required at follow‐up. The study compared combinations of group vs individual vs no counselling and tranquillizer (equanil) vs placebo vs no prescription. It is included in the review of anxiolytics (Hughes 2000) |
| Simmons 2011 | Compared single‐session of either a web‐based smoking intervention, web‐based nutrition intervention, in‐person didactic smoking intervention, or in‐person group intervention |
| Smith 2001 | Compares 2 group interventions initiated after a cessation attempt as an adjunct to NRT and individual support. Now included in Hajek 2013b |
| Stevens 1989 | Compared a standard group programme to relapse prevention intervention. Now included in Hajek 2013b |
| Supnick 1984 | Compared 4 maintenance strategies after initial therapy. No. of abstainers not reported by group at 6‐m follow‐up. The differences in content and outcome between the 4 strategies were small |
| Thompson 1988 | A complete factorial design included combinations of physician advice, self‐help materials and referral to American Health Foundation Smoking cessation classes. Not primarily a trial of group therapy. Take‐up of group programme was very low |
| Thorndike 2006 | Short follow‐up (1 month). Compared CBT to time‐matched health education and scheduled reduced smoking |
| Tiffany 1986 | Primarily a trial of different forms of rapid smoking, included in aversion review (Hajek 2001). No non‐group control |
| Tonnesen 2008 | Compared group programmes with and without smokeless tobacco. No non‐group control |
| Vellisco 2001 | Not randomized. Participants were allocated to an information‐only or a psychological‐counselling group in order of attendance |
| Wagner 2012 | Only 12‐wk follow‐up |
| Wetter 2011 | Participants randomized after completing the same group counselling. Included in Hajek 2013 |
| Wittchen 2011 | Only individual counselling provided |
| Yu 2006 | Short follow‐up (3 m). (Assessed from abstract) |
| Yuhongxia 2012 | Only 3‐m follow‐up |
CBT: cognitive behavioural therapy HMO: Health Maintenance Organization m: month(s) min: minute(s) NRT: nicotine replacement therapy OTC: over‐the‐counter wk: week(s)
Differences between protocol and review
For the 2017 update, we include a 'Summary of findings' table for the main comparisons.
Contributions of authors
LS & TL jointly conceived the review. LS & AC shared data extraction and all authors were involved in drafting the review.
Sources of support
Internal sources
Department of Primary Health Care, Oxford University, UK.
National Institute for Health Research (NIHR) School for Primary Care Research, UK.
External sources
NHS Research and Development National Cancer Programme, England, UK.
-
Predoctoral Individual National Research Service Award (F31 HL129494), USA.
Support for AC's effort and training
Declarations of interest
LS; None known. AC; None known. TL; None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aytemur 2012 {published data only}
- Aytemur ZA, Pismisoglu B, Kilinc O, Pismisoglu E, Hacievliyagil SS, Karaman C. Intensive clinic intervention plus psychodrama in smoking cessation and effects on cessation outcome. Turkiye Klinikleri Journal of Medical Sciences 2012;32(3):630‐7. [Google Scholar]
Bakkevig 2000 {published data only}
- Bakkevig O, Steine S, Hafenbradl K, Laerum E. Smoking cessation ‐ A comparative, randomised study between management in general practice and the behavioural programme SmokEnders. Scandinavian Journal of Primary Health Care 2000;18(4):247‐51. [DOI] [PubMed] [Google Scholar]
Batra 1994 {published data only}
- Batra A, Brömer A, Grüninger K, Schupp P, Buchkremer G. Behaviour therapy for smoking cessation in medical practices [Verhaltenstherapie Raucherentwoehnung in Arztpraxen]. Verhaltensmodifikation und Verhaltensmedizin 1994;15(4):364‐76. [Google Scholar]
- Batra A, Buchkremer G. Does length of nicotine replacement therapy predict long term abstinence in smokers?. Sucht 2000;46(6):414‐23. [Google Scholar]
- Oxley S, Batra A. Nicotine dependence and responsiveness to different standardised behavioural interventions combined with nicotine patch. Nicotine & Tobacco Research 1999;1(2):197. [Google Scholar]
Batra 2010 {published data only}
- Batra A, Collins SE, Schröter M, Eck S, Torchalla I, Buchkremer G, et al. A cluster‐randomized effectiveness trial of smoking cessation modified for at‐risk smoker subgroups.[Erratum appears in Journal of Substance Abuse Treatment. 2011 Mar;40(2):214]. Journal of Substance Abuse Treatment 2010;38(2):128‐40. [DOI] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, et al. Cognitive‐behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology 2001;69(3):471‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, et al. Patterns of change in depressive symptoms during smoking cessation: who's at risk for relapse?. Journal of Consulting and Clinical Psychology 2002;70(2):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology 2002;111(4):670‐5. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown RA, Niaura R, Lloyd‐Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive‐behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research 2007;9(7):721‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bushnell 1997 {published data only}
- Bushnell FK, Forbes B, Goffaux J, Dietrich M, Wells N. Smoking cessation in military personnel. Military Medicine 1997;162(11):715‐9. [PubMed] [Google Scholar]
Camarelles 2002 {published data only}
- Camarelles F, Asensio A, Jimenez‐Ruiz C, Becerril B, Rodero D, Vidaller O. Effectiveness of a group therapy intervention to quit smoking. Randomized clinical trial [Efectividad de la intervencion grupal para la deshabituacion tabaquica. Ensayo clinico aleatorizado]. Medicina Clinica (Barc) 2002;119(2):53‐7. [DOI] [PubMed] [Google Scholar]
Cottraux 1983 {published data only}
- Cottraux JA, Harf R, Boissel JP, Schbath J, Bouvard M, Gillet J. Smoking cessation with behaviour therapy or acupuncture ‐ a controlled study. Behaviour Research and Therapy 1983;21(4):417‐24. [DOI] [PubMed] [Google Scholar]
Curry 1988 {published data only}
- Curry SJ, Marlatt GA, Gordon J, Baer JS. A comparison of alternative theoretical approaches to smoking cessation and relapse. Health Psychology 1988;7(6):545‐56. [DOI] [PubMed] [Google Scholar]
Dent 2009 {published data only}
- Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist‐delivered program for smoking cessation. Annals of Pharmacotherapy 2009;43(2):194‐201. [DOI] [PubMed] [Google Scholar]
DePaul 1987 {published data only}
- Flay BR, Gruder CL, Warnecke RB, Jason LA, Peterson P. One year follow‐up of the Chicago televised smoking cessation program. American Journal of Public Health 1989;79(10):1377‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Gruder CL, Buckenberger L, Lesowitz T, Belgredan J, Flay BR, et al. A 12‐month follow‐up of a worksite smoking cessation intervention. Health Education Research 1987;2:185‐94. [Google Scholar]
- Jason LA, Gruder CL, Martino S, Flay BR, Warnecke R, Thomas N. Work site group meetings and the effectiveness of a televised smoking cessation intervention. American Journal of Community Psychology 1987;15(1):57‐72. [DOI] [PubMed] [Google Scholar]
DePaul 1989 {published data only}
- Jason LA, Lesowitz T, Michaels M, Blitz C, Victors L, Dean L, et al. A worksite smoking cessation intervention involving the media and incentives. American Journal of Community Psychology 1989;17(6):785‐99. [DOI] [PubMed] [Google Scholar]
- Salina D, Jason LA, Hedeker D, Kaufman J, Lesondak L, McMahon SD, et al. A follow‐up of a media‐based, worksite smoking cessation program. American Journal of Community Psychology 1994;22(2):257‐71. [DOI] [PubMed] [Google Scholar]
DePaul 1994 {published data only}
- Jason LA, McMahon SD, Salina D, Hedeker D, Stockton M, Dunson K, et al. Assessing a smoking cessation intervention involving groups, incentives, and self‐help manuals. Behavior Therapy 1995;26:393‐408. [Google Scholar]
- Jason LA, Salina D, McMahon SD, Hedeker D, Stockton M. A worksite smoking intervention: A 2 year assessment of groups, incentives and self‐help. Health Education Research 1997;12(1):129‐38. [DOI] [PubMed] [Google Scholar]
- McMahon SD, Jason LA. Stress and coping in smoking cessation: a longitudinal examination. Anxiety, Stress and Coping 1998;11:327‐43. [Google Scholar]
- McMahon SD, Jason LA, Salina D. Stress, coping, and appraisal in a smoking cessation intervention. Anxiety, Stress and Coping 1994;7:161‐71. [Google Scholar]
Digiusto 1995 {published data only}
- DiGiusto E, Bird KD. Matching smokers to treatment: Self‐control versus social support. Journal of Consulting and Clinical Psychology 1995;63(2):290‐5. [DOI] [PubMed] [Google Scholar]
Etringer 1984 {published data only}
- Etringer BD, Gregory VR, Lando HA. Influence of group cohesion on the behavioral treatment of smoking. Journal of Consulting and Clinical Psychology 1984;52(6):1080‐6. [DOI] [PubMed] [Google Scholar]
Garcia 1989 {published data only}
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Estruch Riba J, Fornes Ramis MV. Smoking addiction treatment, with nicotine chewing gum, in primary care. Double‐blind study [Tratamiento del tabaquismo, con chicle de nicotina, en atencion primaria. Estudio a doble ciego]. Revista Clinica Española 1993;192:157‐61. [PubMed] [Google Scholar]
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Granero Fernández EJ, Vila Coll MA, Estruch Riba J. Double‐blind study of the efficacy of nicotine chewing gum for smoking cessation in the primary care setting [Estudio doble ciego de la eficacia del chicle de nicotina en la deshabituacion tabaquica dentro del ambito de la atencion primaria]. Atencion Primaria 1989;6(10):719‐26. [PubMed] [Google Scholar]
García 2000 {published data only}
- Garcia MP, Becona E. Evaluation of the amount of therapist contact in a smoking cessation program. Spanish Journal of Psychology 2000;3(1):28‐36. [DOI] [PubMed] [Google Scholar]
George 2000 {published data only}
- George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry 2000;157(11):1835‐42. [DOI] [PubMed] [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700‐15. [DOI] [PubMed] [Google Scholar]
Ginsberg 1992 {published data only}
- Ginsberg D, Hall SM, Rosinski M. Partner support, psychological treatment, and nicotine gum in smoking treatment: an incremental study. International Journal of the Addictions 1992;27(5):503‐14. [DOI] [PubMed] [Google Scholar]
Glasgow 1981 {published data only}
- Glasgow RE, Schafer L, O'Neill HK. Self‐help books and amount of therapist contact in smoking cessation programs. Journal of Consulting and Clinical Psychology 1981;49(5):659‐67. [DOI] [PubMed] [Google Scholar]
Glasgow 1989 {published data only}
- Glasgow RE, Morray K, Lichtenstein E. Controlled smoking versus abstinence as a treatment goal: The hopes and fears may be unfounded. Behavior Therapy 1989;20:77‐91. [Google Scholar]
Goldstein 1989 {published data only}
- Goldstein MG, Niaura R, Follick MJ, Abrams DB. Effects of behavioral skills training and schedule of nicotine gum administration on smoking cessation. American Journal of Psychiatry 1989;146(1):56‐60. [DOI] [PubMed] [Google Scholar]
Grant 2003 {published data only}
- Grant KM, Northrup JH, Agrawal S, Olsen DM, McIvor C, Romberger DJ. Smoking cessation in outpatient alcohol treatment. Addictive Disorders and Their Treatment 2003;2:41‐6. [Google Scholar]
Gruder 1993 {published data only}
- Gruder CL, Mermelstein RJ, Kirkendol S, Hedeker D, Wong SC, Schreckengost J, et al. Effects of social support and relapse prevention training as adjuncts to a televised smoking‐cessation intervention. Journal of Consulting and Clinical Psychology 1993;61(1):113‐20. [DOI] [PubMed] [Google Scholar]
- Warnecke RB, Flay BR, Kviz FJ, Gruder CL, Langenberg P, Crittenden KS, et al. Characteristics of participants in a televised smoking cessation intervention. Preventive Medicine 1991;20:389‐403. [DOI] [PubMed] [Google Scholar]
Hall 1994 {published data only}
- Hall SM, Muñoz RF, Reus VI. Cognitive‐behavioral intervention increases abstinence rates for depressive‐history smokers. Journal of Consulting and Clinical Psychology 1994;62(1):141‐6. [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment ‐ a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64(5):1003‐9. [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Hall SM, Reus VI, Muñoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive‐behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55(8):683‐90. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Muñoz RF, Sees KL, Humfleet GL, Frederick S. Nortriptyline and cognitive‐behavioral treatment of cigarette smoking. CPDD Annual Meeting. San Juan, PR, 1996:52. [DOI] [PubMed]
Hall 2002 {published data only}
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Hartz DT, Maude‐Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59(10):930‐6. [DOI] [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Muñoz R. Cost‐effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32(4):381‐92. [DOI] [PubMed] [Google Scholar]
Hertel 2008 {published data only}
- Hertel AW, Finch EA, Kelly KM, King C, Lando H, Linde JA, et al. The impact of expectations and satisfaction on the initiation and maintenance of smoking cessation: An experimental test. Health Psychology 2008;27(3 Suppl):S197‐206. [DOI] [PubMed] [Google Scholar]
Hill 1993 {published data only}
- Hill R, Rigdon M, Johnson S. Behavioral smoking cessation treatment for older chronic smokers. Behavior Therapy 1993;24:321‐9. [Google Scholar]
Hilleman 1993 {published data only}
- Hilleman DE, Mohiuddin SM, Delcore MG, Lucas BD Jr. Randomized, controlled trial of transdermal clonidine for smoking cessation. Annals of Pharmacotherapy 1993;27(9):1025‐8. [DOI] [PubMed] [Google Scholar]
Hollis 1993 {published data only}
- Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse‐assisted counseling for smokers in primary care. Annals of Internal Medicine 1993;118(7):521‐5. [DOI] [PubMed] [Google Scholar]
Huber 2003 {published data only}
- Huber D, Gastner J. Smoking cessation: A comparison of behavior therapy, nicotine replacement therapy and their combination. Verhaltenstherapie und Verhaltenshmedizin 2003;24:167‐85. [Google Scholar]
Jorenby 1995 {published data only}
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274(17):1347‐52. [PubMed] [Google Scholar]
Lando 1985 {published data only}
- Lando HA, McGovern PG. Nicotine fading as a nonaversive alternative in a broad‐spectrum treatment for eliminating smoking. Addictive Behaviors 1985;10(2):153‐61. [DOI] [PubMed] [Google Scholar]
Lando 1990 {published data only}
- Lando HA, McGovern PG, Barrios FX, Etringer BD. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health 1990;80(5):554‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lando 1991 {published data only}
- Lando HA, McGovern PG. The influence of group cohesion on the behavioral treatment of smoking: A failure to replicate. Addictive Behaviors 1991;16(3‐4):111‐21. [DOI] [PubMed] [Google Scholar]
Leung 1991 {published data only}
- Leung J. Smoking cessation by auricular acupuncture and behavioural therapy. Psychologia 1991;34:177‐87. [Google Scholar]
Macpherson 2010 {published data only}
- Macpherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of Consulting and Clinical Psychology 2010;78(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2009 {published data only}
- Matthews AK, Sánchez‐Johnsen L, King A. Development of a culturally targeted smoking cessation intervention for African American smokers. Journal of Community Health 2009;34(6):480‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDowell 1985 {published data only}
- McDowell I, Mothersill KJ, Rosser WW, Hartman R. A randomized trial of three approaches to smoking cessation. Canadian Family Physician 1985;31:351‐5. [PMC free article] [PubMed] [Google Scholar]
- Mothersill KJ, McDowell I, Rosser WW. Subject characteristics and long term post‐program smoking cessation. Addictive Behaviors 1988;13(1):29‐36. [DOI] [PubMed] [Google Scholar]
- Rosser WW. The role of the family physician in smoking cessation. Canadian Family Physician 1984;30:160‐7. [PMC free article] [PubMed] [Google Scholar]
Minthorn‐Biggs 2000 {published data only}
- Minthorn‐Biggs MB. Smoking cessation using an interpersonal coping skills program. Dissertation Abstracts International Section A: Humanities and Social Sciences 2000;60(8‐A):2814. [Google Scholar]
Mueller 2012 {published data only}
- Mueller SE, Petitjean SA, Wiesbeck GA. Cognitive behavioral smoking cessation during alcohol detoxification treatment: a randomized, controlled trial. Drug and Alcohol Dependence 2012;126(3):279‐85. [DOI] [PubMed] [Google Scholar]
- Muller S, Petitjean S, Degen B, Wiesbeck GA. Smoking cessation in alcohol‐dependent patients: Results from a randomized, controlled trial. Alcohol and Alcoholism (Oxford, Oxfordshire) 2011;46:i7. [Google Scholar]
Nevid 1997 {published data only}
- Nevid JS, Javier RA. Preliminary investigation of a culturally‐specific smoking cessation intervention for Hispanic smokers. American Journal of Health Promotion 1997;11(3):198‐207. [DOI] [PubMed] [Google Scholar]
Omenn 1988 {published data only}
- Omenn GS, Thompson B, Sexton M, Hessol N, Breitenstein B, Curry S, et al. A randomized comparison of worksite‐sponsored smoking cessation programs. American Journal of Preventive Medicine 1988;4(5):261‐7. [PubMed] [Google Scholar]
- Thompson B, Omenn G, Sexton M, Breitenstein B, Hessol N, Curry S, et al. Worksite smoking cessation: a test of two programs. Progress in Clinical Biological Research 1987;248:93‐100. [PubMed] [Google Scholar]
Otero 2006 {published data only}
- Otero UB, Perez CA, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive‐behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Portuguese] [Ensaio clinico randomizado: efetividade da abordagem cognitivo‐comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439‐49. [DOI] [PubMed] [Google Scholar]
Patten 2002 {published and unpublished data}
- Patten CA, Drews AA, Myers MG, Martin JE, Wolter TD. Effect of depressive symptoms on smoking abstinence and treatment adherence among smokers with a history of alcohol dependence. Psychology of Addictive Behaviors 2002;16(2):135‐42. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Myers MG, Calfas KJ, Williams CD. Effectiveness of cognitive‐behavioral therapy for smokers with histories of alcohol dependence and depression. Journal of Studies on Alcohol 1998;59(3):327‐35. [DOI] [PubMed] [Google Scholar]
Pederson 1981 {published data only}
- Pederson LL, Baldwin N, Lefcoe NM. Utility of behavioral self‐help manuals in a minimal‐contact smoking cessation program. International Journal of the Addictions 1981;16(7):1233‐9. [DOI] [PubMed] [Google Scholar]
Pisinger 2005 {published data only}
- Jorgensen T, Borch‐Johnsen K, Thomsen TF, Ibsen H, Glumer C, Pisinger C. A randomized non‐pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. European Journal of Cardiovascular Prevention & Rehabilitation 2003;10(5):377‐86. [DOI] [PubMed] [Google Scholar]
- Pisinger C. Smoking cessation and smoking reduction in a general population. The Inter99 study. A randomised population‐based intervention study. Nordic Journal of Psychiatry 2006;60(1):67. [Google Scholar]
- Pisinger C, Glumer C, Toft U, Huth SL, Aadahl M, Borch‐Johnsen K, et al. High risk strategy in smoking cessation is feasible on a population‐based level. The Inter99 study. Preventive Medicine 2008;46(6):579‐84. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Vestbo J, Borch‐Johnsen K, Jorgensen T. It is possible to help smokers in early motivational stages to quit. The Inter99 study. Preventive Medicine 2005;40(3):278‐84. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Vestbo J, Borch‐Johnsen K, Jorgensen T. Smoking cessation intervention in a large randomised population‐based study. The Inter99 study. Preventive Medicine 2005;40(3):285‐92. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Vestbo J, Borch‐Johnsen K, Thomsen T, Jorgensen T. Acceptance of the smoking cessation intervention in a large population‐based study: the Inter99 study. Scandinavian Journal of Public Health 2005;33(2):138‐45. [DOI] [PubMed] [Google Scholar]
Pisinger 2010 {published data only}
- Pisinger C, Jorgensen MM, Moller NE, Dossing M, Jorgensen T. A cluster randomized trial in general practice with referral to a group‐based or an Internet‐based smoking cessation programme. Journal of Public Health (Oxford, England) 2010;32(1):62‐70. [DOI] [PubMed] [Google Scholar]
Rabkin 1984 {published data only}
- Rabkin SW, Boyko E, Shane F, Kaufert J. A randomized trial comparing smoking cessation programs utilizing behaviour modification, health education or hypnosis. Addictive Behaviors 1984;9(2):157‐73. [DOI] [PubMed] [Google Scholar]
Ramos 2010 {published data only}
- Ramos M, Ripoll J, Estrades T, Socias I, Fe A, Duro R, et al. Effectiveness of intensive group and individual interventions for smoking cessation in primary health care settings: a randomized trial. BMC Public Health 2010;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rice 1994 {published and unpublished data}
- Rice VH, Fox DH, Lepczyk M, Sieggreen M, Mullin M, Jarosz P, et al. A comparison of nursing interventions for smoking cessation in adults with cardiovascular health problems. Heart & Lung 1994;23(6):473‐86. [PubMed] [Google Scholar]
Romand 2005 {published data only}
- Romand R, Gourgou S, Sancho‐Garnier H. A randomized trial assessing the Five‐Day Plan for smoking cessation. Addiction 2005;100(10):1546‐54. [DOI] [PubMed] [Google Scholar]
Rovina 2009 {published data only}
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, et al. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Therapeutic Advances in Respiratory Disease 2009;3(6):279‐87. [DOI] [PubMed] [Google Scholar]
Sawicki 1993 {published data only}
- Sawicki PT, Didjurgeit U, Muhlhauser I, Berger M. Behaviour therapy versus doctor's anti‐smoking advice in diabetic patients. Journal of Internal Medicine 1993;234(4):407‐9. [DOI] [PubMed] [Google Scholar]
Schleicher 2012 {published data only}
- Schleicher HE, Harris KJ, Campbell DG, Harrar SW. Mood management intervention for college smokers with elevated depressive symptoms: a pilot study. Journal of American College Health 2012;60(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2007 {published data only (unpublished sought but not used)}
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive‐behavioral therapy for smoking cessation in women.[erratum appears in Nicotine & Tobacco Research 2007 Jul;9(7):785]. Nicotine & Tobacco Research 2007;9(6):699‐709. [DOI] [PubMed] [Google Scholar]
Slovinec 2005 {published data only}
- Slovinec D'Angelo ME, Reid RD, Hotz S, Irvine J, Segal RJ, Blanchard CM, et al. Is stress management training a useful addition to physician advice and nicotine replacement therapy during smoking cessation in women? Results of a randomized trial. American Journal of Health Promotion 2005;20(2):127‐34. [DOI] [PubMed] [Google Scholar]
Ward 2001 {published data only}
- Ward T. Using psychological insights to help people quit smoking. Journal of Advanced Nursing 2001;34(6):754‐9. [DOI] [PubMed] [Google Scholar]
Webb 2010 {published data only}
- Webb MS, Ybarra DR, Baker EA, Reis IM, Carey MP. Cognitive‐behavioral therapy to promote smoking cessation among African American smokers: a randomized clinical trial. Journal of Consulting and Clinical Psychology 2010;78(1):24‐33. [DOI] [PubMed] [Google Scholar]
Wilson 2008 {published data only (unpublished sought but not used)}
- Wilson JS, Fitzsimons D, Bradbury I, Stuart EJ. Does additional support by nurses enhance the effect of a brief smoking cessation intervention in people with moderate to severe chronic obstructive pulmonary disease? A randomised controlled trial. International Journal of Nursing Studies 2008;45:508‐17. [DOI] [PubMed] [Google Scholar]
Zelman 1992 {published data only}
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. Journal of Consulting and Clinical Psychology 1992;60(6):943‐52. [DOI] [PubMed] [Google Scholar]
Zheng 2007 {published data only}
- Zheng P, Guo F, Chen Y, Fu Y, Ye T, Fu H. A randomized controlled trial of group intervention based on social cognitive theory for smoking cessation in China. Journal of Epidemiology 2007;17:147‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asuzu 2013 {published data only}
- Asuzu C, Tondo J. Effects of covert sensitization and group therapy intervention in fostering tobacco smoking cessation among Nigerian commercial motorcyclists in Ibadan. Psycho‐oncology 2013;22(Suppl. 3):59‐60. [Google Scholar]
Becona 1997 {published data only}
- Becona E, Vazquez FL. Does using relapse prevention increase the efficacy of a program for smoking cessation? An empirical study. Psychological Reports 1997;81(1):291‐6. [DOI] [PubMed] [Google Scholar]
Bernstein 1970 {published data only}
- Bernstein DA. The modification of smoking behavior: a search for effective variables. Behaviour Research and Therapy 1970;8(2):133‐46. [DOI] [PubMed] [Google Scholar]
Bertera 1990 {published data only}
- Bertera RL, Oehl LK, Telephak JM. Self‐help versus group approaches to smoking cessation in the workplace: Eighteen‐month follow‐up and cost analysis. American Journal of Health Promotion 1990;4(3):187‐92. [DOI] [PubMed] [Google Scholar]
Bowen 2009 {published data only}
- Bowen S, Marlatt A. Surfing the urge: brief mindfulness‐based intervention for college student smokers. Psychology of Addictive Behaviors 2009;23(4):666‐71. [DOI] [PubMed] [Google Scholar]
Brewer 2011 {published data only}
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence 2011;119(1‐2):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1984 {published data only}
- Brown RA, Lichtenstein E, McIntyre KO, Harrington‐Kostur J. Effects of nicotine fading and relapse prevention on smoking cessation. Journal of Consulting and Clinical Psychology 1984;52(2):307‐8. [DOI] [PubMed] [Google Scholar]
Campbell 1995 {published data only}
- Campbell BK, Wander N, Stark MJ, Holbert T. Treating cigarette‐smoking in drug‐abusing clients. Journal of Substance Abuse Treatment 1995;12(2):89‐94. [DOI] [PubMed] [Google Scholar]
Carlson 2003 {published data only}
- Carlson LE, Taenzer P, Koopmans J, Casebeer A. Predictive value of aspects of the Transtheoretical Model on smoking cessation in a community‐based, large‐group cognitive behavioral program. Addictive Behaviors 2003;28(4):725‐40. [DOI] [PubMed] [Google Scholar]
Carlson 2012 {published data only}
- Carlson LE, Lounsberry JJ, Maciejewski O, Wright K, Collacutt V, Taenzer P. Telehealth‐delivered group smoking cessation for rural and urban participants: feasibility and cessation rates. Addictive behaviors 2012;37(1):108‐14. [DOI] [PubMed] [Google Scholar]
Cinciripini 1994 {published data only}
- Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E, Vunakis H. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: initial findings. Addictive Behaviors 1994;19(1):13‐22. [DOI] [PubMed] [Google Scholar]
Cinciripini 1995 {published data only}
- Cinciripini PM, Lapitsky LG, Seay S, Wallfisch A, Kitchens K, Vunakis H. The effects of smoking schedules on cessation outcome ‐ can we improve on common methods of gradual and abrupt nicotine withdrawal?. Journal of Consulting and Clinical Psychology 1995;63(3):388‐99. [DOI] [PubMed] [Google Scholar]
Colletti 1979 {published data only}
- Colletti G, Kopel S. Maintaining behavior change: An investigation of three maintenance strategies and the relationship of self‐attribution to the long‐term reduction of cigarette smoking. Journal of Consulting and Clinical Psychology 1979;47(3):614‐7. [DOI] [PubMed] [Google Scholar]
- Colletti G, Stern L. Two‐year follow‐up of a nonaversive treatment for cigarette smoking. Journal of Consulting and Clinical Psychology 1980;48(2):292‐3. [DOI] [PubMed] [Google Scholar]
Colletti 1980 {published data only}
- Colletti G, Supnick JA. Continued therapist contact as a maintenance strategy for smoking reduction. Journal of Consulting and Clinical Psychology 1980;48(5):665‐7. [DOI] [PubMed] [Google Scholar]
Copeland 2015 {published data only}
- Copeland AL, McVay MA, Martin PD, Rash CJ, Kendzor DE, Baillie LE, et al. Smoking relapse and weight gain prevention program for postmenopausal weight‐concerned women: A pilot study. Eating Behaviors 2015;18:107‐14. [DOI: 10.1016/j.eatbeh.2015.05.0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costello 2011 {published data only}
- Costello MJ, Sproule B, Victor JC, Leatherdale ST, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomized trial with 6,987 smokers. Cancer Causes & Control 2011 Feb;22(2):167‐80. [DOI] [PubMed] [Google Scholar]
Cropsey 2008 {published data only}
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A. Smoking cessation intervention for female prisoners: addressing an urgent public health need. American Journal of Public Health 2008;98(10):1894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Culbertson 2012 {published data only}
- Culbertson CS, Shulenberger S, Garza R, Newton TF, Brody AL. Virtual reality cue exposure therapy for the treatment of tobacco dependence. Journal of Cyber therapy and Rehabilitation 2012;5(1):57‐64. [PMC free article] [PubMed] [Google Scholar]
Davis 1986 {published data only}
- Davis JR, Glaros AG. Relapse prevention and smoking cessation. Addictive Behaviors 1986;11(2):105‐14. [DOI] [PubMed] [Google Scholar]
Decker 1989 {published data only}
- Decker BD, Evans RG. Efficacy of a minimal contact version of a multimodal smoking cessation program. Addictive Behaviors 1989;14(5):487‐91. [DOI] [PubMed] [Google Scholar]
Dickson‐Spillmann 2013 {published data only}
- Dickson‐Spillmann M, Haug S, Schaub MP. Group hypnosis vs. relaxation for smoking cessation in adults: a cluster‐randomised controlled trial. BMC Public Health 2013;13:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson‐Spillmann M, Kraemer T, Rust K, Schaub M. Group hypnotherapy versus group relaxation for smoking cessation: an RCT study protocol. BMC Public Health 2012;12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dijkstra 2014 {published data only}
- Dijkstra A, Zuidema R, Vos D, Kalken M. The effectiveness of the Allen Carr smoking cessation training in companies tested in a quasi‐experimental design. BMC Public Health 2014;14:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 1978 {published data only}
- Elliott CH, Denney DR. A multiple‐component treatment approach to smoking reduction. Journal of Consulting and Clinical Psychology 1978;46(6):1330‐9. [DOI] [PubMed] [Google Scholar]
Erfurt 2015 {published data only}
- Erfurt L, Kroger CB. Use of medication in combination with a modern group programme for smoking cessation. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 2015;77(2):74‐80. [DOI] [PubMed] [Google Scholar]
Frikart 2003 {published data only}
- Frikart M, Etienne S, Cornuz J, Zellweger JP. Five‐day plan for smoking cessation using group behaviour therapy. Swiss Medical Weekly 2003;133(3‐4):39‐43. [DOI] [PubMed] [Google Scholar]
Glasgow 1978 {published data only}
- Glasgow RE. Effects of a self‐control manual, rapid smoking, and amount of therapist contact on smoking reduction. Journal of Consulting and Clinical Psychology 1978;46(6):1439‐47. [DOI] [PubMed] [Google Scholar]
Grassi 2011 {published data only}
- Grassi MC, Enea D, Ferketich AK, Lu B, Pasquariello S, Nencini P. Effectiveness of varenicline for smoking cessation: a 1‐year follow‐up study. Journal of Substance Abuse Treatment 2011;41(1):64‐70. [DOI] [PubMed] [Google Scholar]
Green 2003 {published data only}
- Green A, Yancy WS, Braxton L, Westman EC. Residential smoking therapy. Journal of General Internal Medicine 2003;18:275‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1984 {published data only}
- Hall SM, Rugg D, Tunstall C, Jones RT. Preventing relapse to cigarette smoking by behavioral skill training. Journal of Consulting and Clinical Psychology 1984;52(3):372‐82. [DOI] [PubMed] [Google Scholar]
Hall 1985 {published data only}
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53(2):256‐8. [DOI] [PubMed] [Google Scholar]
Hall 1987 {published data only}
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55(4):603‐5. [DOI] [PubMed] [Google Scholar]
Hall 2009 {published data only}
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009;104(6):1043‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011 {published data only}
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health 2011;101(12):2349‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1979 {published data only}
- Hamilton SB, Bornstein PH. Broad‐spectrum behavioral approach to smoking cessation: Effects of social support and paraprofessional training on the maintenance of treatment effects. Journal of Consulting and Clinical Psychology 1979;47(3):598‐600. [DOI] [PubMed] [Google Scholar]
Hamilton 1998 {published data only}
- Hamilton BD. Nicotine fading versus abrupt quitting: Testing educational and behavioral smoking cessation treatment strategies. Dissertation Abstracts International 1998;58(10‐B):5644. [Google Scholar]
Hernández‐López 2009 {published data only}
- Hernández‐López M, Luciano MC, Bricker JB, Roales‐Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors 2009;23(4):723‐30. [DOI] [PubMed] [Google Scholar]
Hilleman 2004 {published data only}
- Hilleman DE, Mohiuddin SM, Packard KA. Comparison of conservative and aggressive smoking cessation treatment strategies following coronary artery bypass graft surgery. Chest 2004;125(2):435‐8. [DOI] [PubMed] [Google Scholar]
Hiscock 2013 {published data only}
- Hiscock R, Murray S, Brose LS, McEwen A, Bee JL, Dobbie F, et al. Behavioural therapy for smoking cessation: the effectiveness of different intervention types for disadvantaged and affluent smokers. Addictive Behaviors 2013;38(11):2787‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahler 2008 {published data only}
- Kahler CW, Metrik J, Lachance HR, Ramsey SE, Abrams DB, Monti PM, et al. Addressing heavy drinking in smoking cessation treatment: A randomized controlled trial. Journal of Consulting and Clinical Psychology 2008;76(5):852‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapson 2010 {published data only}
- Kapson HS, Haaga DA. Depression vulnerability moderates the effects of cognitive behavior therapy in a randomized controlled trial for smoking cessation. Behavior Therapy 2010;41(4):447‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1977 {published data only}
- Katz RC, Heiman M, Gordon S. Effects of two self‐management approaches on cigarette smoking. Addictive Behaviors 1977;2(2‐3):113‐9. [DOI] [PubMed] [Google Scholar]
Killen 1984 {published data only}
- Killen JD, Maccoby N, Taylor CB. Nicotine gum and self‐regulation training in smoking relapse prevention. Behavior Therapy 1984;15:234‐48. [Google Scholar]
Kisely 2003 {published data only}
- Kisely SR, Wise M, Preston N, Malmgren S, Shannon P. A group intervention to reduce smoking in individuals with psychiatric disorder: brief report of a pilot study. Australian and New Zealand Journal of Public Health 2003;27(1):61‐3. [DOI] [PubMed] [Google Scholar]
Klesges 1999 {published data only}
- Klesges RC, Haddock CK, Lando H, Talcott GW. Efficacy of forced smoking cessation and an adjunctive behavioral treatment on long‐term smoking rates. Journal of Consulting and Clinical Psychology 1999;67(6):952‐8. [DOI] [PubMed] [Google Scholar]
Kumar 2012 {published data only}
- Kumar MS, Sarma PS, Thankappan KR. Community‐based group intervention for tobacco cessation in rural Tamil Nadu, India: a cluster randomized trial. Journal of Substance Abuse Treatment 2012;43(1):53‐60. [DOI] [PubMed] [Google Scholar]
Lando 1982 {published data only}
- Lando HA. A factorial analysis of preparation, aversion, and maintenance in the elimination of smoking. Addictive Behaviors 1982;7(2):143‐54. [DOI] [PubMed] [Google Scholar]
Larson 1999 {published data only}
- Larson ME. Behavioral substitutes and smoking cessation: Do they make quitting easier?. Dissertation Abstracts International: Section B: The Sciences and Engineering 1999;59(10‐B):5579. [Google Scholar]
Lowe 1980 {published data only}
- Lowe MR, Green L, Kurtz SMS, Ashenberg ZS, Fisher EB. Self‐initiated, cue extinction, and covert sensitization procedures in smoking cessation. Journal of Behavioral Medicine 1980;3(4):357‐72. [DOI] [PubMed] [Google Scholar]
Martin 1997 {published data only}
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: One‐year results of project SCRAP‐tobacco. Journal of Consulting and Clinical Psychology 1997;65(1):190‐4. [DOI] [PubMed] [Google Scholar]
Mayer 2010 {published data only}
- Mayer C, Vandecasteele H, Bodo M, Primo C, Slachmuylder J‐L, Kaufman L, et al. Smoking relapse prevention programs and factors which predict abstinence: A controlled study comparing the efficacy of workplace group counselling and proactive phone counselling. Journal of Smoking Cessation 2010;5(1):83‐94. [Google Scholar]
McEwen 2006 {published data only}
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one‐to‐one treatment in primary care. Addictive Behaviors 2006;31(9):1650‐60. [DOI] [PubMed] [Google Scholar]
McGovern 1991 {published data only}
- McGovern PG, Lando HA. Reduced nicotine exposure and abstinence outcome in two nicotine fading methods. Addictive Behaviors 1991;16(1‐2):11‐20. [DOI] [PubMed] [Google Scholar]
McIntyre 1986 {published data only}
- McIntyre KO, Lichtenstein E, Mermelstein RJ. Self‐efficacy and relapse in smoking cessation: a replication and extension. Journal of Consulting and Clinical Psychology 1983;51(4):632‐3. [DOI] [PubMed] [Google Scholar]
Moadel 2012 {published data only}
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV‐infected smokers. Journal of Acquired Immune Deficiency Syndromes 2012 Oct;61(2):208‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mogielnicki 1986 {published data only}
- Mogielnicki RP, Neslin S, Dulac J, Balestra D, Gillie E, Corson J. Tailored media can enhance the success of smoking cessation clinics. Journal of Behavioral Medicine 1986;9(2):141‐61. [DOI] [PubMed] [Google Scholar]
Morris 2011 {published data only}
- Morris CD, Waxmonsky JA, May MG, Tinkelman DG, Dickinson M, Giese AA. Smoking reduction for persons with mental illnesses: 6‐month results from community‐based interventions. Community Mental Health Journal 2011;47(6):694‐702. [DOI] [PubMed] [Google Scholar]
Moser 2011 {published data only}
- Moser C, Miedinger D, Frey F, Karli C, Leuppi JD. [A smoking cessation intervention in the Swiss Armed Forces]. [German]. Praxis 2011;100(22):1343‐50. [DOI] [PubMed] [Google Scholar]
NCT00960375 {published data only}
- NCT00960375. Smoking cessation for veterans with severe and persistent mental illness [Randomized trial of a smoking cessation program for persons with SMI]. www.clinicaltrials.gov/ct2/show/NCT00960375 First received August 14 2009.
NCT02072772 {published data only}
- NCT02072772. A trial of positively smoke free group therapy for HIV‐infected smokers. clinicaltrials.gov/show/NCT02072772 First received February 25 2014.
Nyborg 1986 {published data only}
- Nyborg KF, Nevid JS. Couples who smoke: A comparison of couples training versus individual training for smoking cessation. Behavior Therapy 1986;17:620‐5. [Google Scholar]
Park 2014 {published data only}
- Park CB, Choi JS, Park SM, Lee JY, Jung HY, Seol JM, et al. Comparison of the effectiveness of virtual cue exposure therapy and cognitive behavioral therapy for nicotine dependence. Cyberpsychology, Behavior and Social Networking 2014;17(4):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins 2001 {published data only}
- Perkins KA, Marcus MD, Levine MD, D'Amico D, Miller A, Broge M, et al. Cognitive‐behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight‐concerned women. Journal of Consulting and Clinical Psychology 2001;69(4):604‐13. [PubMed] [Google Scholar]
Pirie 1992 {published data only}
- Pirie PL, McBride CM, Hellerstedt WL, Jeffery RW, Hatsukami DK, Allen S, et al. Smoking cessation in women concerned about weight. American Journal of Public Health 1992;82(9):1238‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 1981 {published data only}
- Powell DR, McCann BS. The effects of a multiple treatment program and maintenance procedures on smoking cessation. Preventive Medicine 1981;10(1):94‐104. [DOI] [PubMed] [Google Scholar]
Razavi 1999 {published data only}
- Razavi D, Vandecasteele H, Primo C, Bodo M, Debrier F, Verbist H, et al. Maintaining abstinence from cigarette smoking: Effectiveness of group counselling and factors predicting outcome. European Journal of Cancer 1999;35(8):1238‐47. [DOI] [PubMed] [Google Scholar]
Reid 2008 {published data only}
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community‐based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment 2008;35(1):68‐77. [DOI] [PubMed] [Google Scholar]
- Reid MS, Jiang H, Fallon B, Sonne S, Rinaldi P, Turrigiano E, et al. Smoking cessation treatment among patients in community‐based substance abuse rehabilitation programs: exploring predictors of outcome as clues toward treatment improvement. American Journal of Drug and Alcohol Abuse 2011 Sep;37(5):472‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savant 2013 {published data only}
- Savant SC, Hegde‐Shetiya S, Agarwal D, Shirhatti R, Shetty D. Effectiveness of individual and group counseling for cessation of tobacco habit amongst industrial workers in Pimpri, Pune ‐ An interventional study. Asian Pacific Journal of Cancer Prevention 2013;14(2):1133‐9. [DOI] [PubMed] [Google Scholar]
Schauffler 2001 {published data only}
- Schauffler HH, McMenamin S, Olson K, Boyce‐Smith G, Rideout JA, Kamil J. Variations in treatment benefits influence smoking cessation: results of a randomised controlled trial. Tobacco Control 2001;10(2):175‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoenberg 2016 {published data only}
- Schoenberg NE, Studts CR, Shelton BJ, Liu M, Clayton R, Bispo JB, et al. A randomized controlled trial of a faith‐placed, lay health advisor delivered smoking cessation intervention for rural residents. Preventive Medicine Reports 2016;3:317‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 1968 {published data only}
- Schwartz JL, Dubitzky M. One‐year follow‐up results of a smoking cessation program. Canadian Journal of Public Health 1968;59(4):161‐5. [PubMed] [Google Scholar]
Simmons 2011 {published data only}
- Simmons VN, Heckman B, Fink AC, Patel R, Bello L, Brandon TH. Efficacy of an experiential, web‐based smoking intervention for college smokers [PA2‐2]. Toronto, Ontario, 2011:17.
Smith 2001 {published data only}
- Japuntich SJ, Smith SS, Jorenby DE, Piper ME, Fiore MC, Baker TB. Depression predicts smoking early but not late in a quit attempt. Nicotine & Tobacco Research 2007;9(6):677‐86. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped‐care treatments resurrect relapsing smokers?. Journal of Consulting and Clinical Psychology 2001;69(3):429‐39. [DOI] [PubMed] [Google Scholar]
Stevens 1989 {published data only}
- Stevens VJ, Hollis JF. Preventing smoking relapse, using an individually tailored skills‐training technique. Journal of Consulting and Clinical Psychology 1989;57(3):420‐4. [DOI] [PubMed] [Google Scholar]
Supnick 1984 {published data only}
- Supnick J, Colletti G. Relapse coping and problem solving training following treatment for smoking. Addictive Behaviors 1984;9(4):401‐4. [DOI] [PubMed] [Google Scholar]
Thompson 1988 {published data only}
- Thompson RS, Michnich ME, Friedlander L, Gilson B, Grothaus LC, Storer B. Effectiveness of smoking cessation interventions integrated into primary care practice. Medical Care 1988;26(1):62‐76. [DOI] [PubMed] [Google Scholar]
Thorndike 2006 {published data only}
- Thorndike FP, Friedman‐Wheeler DG, Haaga DA. Effect of cognitive behavior therapy on smokers' compensatory coping skills. Addictive Behaviors 2006;31(9):1705‐10. [DOI] [PubMed] [Google Scholar]
Tiffany 1986 {published data only}
- Tiffany ST, Martin EM, Baker TB. Treatments for cigarette smoking: An evaluation of the contributions of aversion and counseling procedures. Behaviour Research and Therapy 1986;24(4):437‐52. [DOI] [PubMed] [Google Scholar]
Tonnesen 2008 {published data only}
Vellisco 2001 {published data only}
- Vellisco Garcia A, Alvarez Gutiérrez FJ, Elías Hernández T, Romero J, Toral Marín J, Bordoy Sánchez C, et al. Results of a psychotherapeutic program for smoking after 12 months of follow‐up [Resultados de un programa psicoterapeutico de deshabituacion tabaquica tras 12 meses de seguimiento]. Archivos de Bronconeumologia 2001;37(1):14‐8. [DOI] [PubMed] [Google Scholar]
Wagner 2012 {published data only}
- Wagner FA, Sheikhattari P. The effectiveness of two smoking cessation interventions targeted for residents of disadvantaged neighborhoods and smokers with other addictions and co‐morbidities. European Psychiatry 2012;27:108. [Google Scholar]
Wetter 2011 {published data only}
- Wetter DW, McClure JB, Cofta‐Woerpel L, Costello TJ, Reitzel LR, Businelle MS, et al. A randomized clinical trial of a palmtop computer‐delivered treatment for smoking relapse prevention among women. Psychology of Addictive Behaviors 2011 Jun;25(2):365‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wittchen 2011 {published data only}
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care ‐ a randomized controlled trial of bupropione, nicotine replacements, CBT and a minimal intervention. International Journal of Methods in Psychiatric Research 2011;20(1):28‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2006 {published data only}
- Yu H, Zang Y, Lin J. The effect of abstinence from smoking with nicotine replacement therapy combining with psychological and behavior intervention. Zhongguo Kangfu Yixue Zazhi 2006;21:1104‐6. [Google Scholar]
Yuhongxia 2012 {published data only}
- Yuhongxia Y. The group behaviour intervention; smoking cessation; the abstinence rate. Heart (British Cardiac Society) 2012;98:E150‐1. [Google Scholar]
Additional references
Anthonisen 2005
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5‐year mortality: a randomized clinical trial. Annals of Internal Medicine 2005;142(4):233‐9. [DOI] [PubMed] [Google Scholar]
Barnes 2010
- Barnes J, Dong CY, McRobbie H, Walker N, Mehta M, Stead. Hypnotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD001008.pub2] [DOI] [PubMed] [Google Scholar]
Brandon 2001
- Brandon TH. Behavioral tobacco cessation treatments: yesterday's news or tomorrow's headlines?. Journal of Clinical Oncology 2001;19(18 Suppl):64S‐68S. [PubMed] [Google Scholar]
Carlson 2000
- Carlson LE, Taenzer P, Koopmans J, Bultz BD. Eight‐year follow‐up of a community‐based large group behavioral smoking cessation intervention. Addictive Behaviors 2000;25(5):725‐41. [DOI] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, O'Mara‐Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001055.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Bruin 2016
- Bruin M, Viechtbauer W, Eisma MC, Hartmann‐Boyce J, West R, Bull E, et al. Identifying effective behavioural components of Intervention and Comparison group support provided in SMOKing cEssation (IC‐SMOKE) interventions: a systematic review protocol. Systematic Reviews 2016;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 1996
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gtitz ER, et al. Clinical Practice Guideline No 18. AHCPR Publication No.96‐0692. books.google.co.uk/books?hl=en&lr=&id=nE8hb2HFO10C&oi=fnd&pg=PP15&dq=Fiore+MC,+Bailey+WC,+Cohen+SJ+et+al.+Smoking+Cessation.+Clinical+Practice+Guideline+No+18.+1996&ots=iTIUfUi94e&sig=RDNH3dtfV_2veoECZGNX8xvhuvY#v=onepage&q=Fiore%20MC%2C%20Bailey%20WC%2C%20Cohen%20SJ%20et%20al.%20Smoking%20Cessation.%20Clinical%20Practice%20Guideline%20No%2018.%201996&f=falseSmoking Cessation.. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, 1996.
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. AHRQ publication No. 00‐0032. bphc.hrsa.gov/buckets/treatingtobacco.pdf. Rockville, MD: US Dept of Health and Human Services. Public Health Services, 2008 (accessed 14th March 2017).
Greenland 1985
- Greenland S, Robbins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. [PubMed] [Google Scholar]
Grimshaw 2013
- Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003289.pub5] [DOI] [PubMed] [Google Scholar]
Hajek 1985
- Hajek P, Belcher M, Stapleton J. Enhancing the impact of groups: An evaluation of two group formats for smokers. British Journal of Clinical Psychology 1985;24(Pt 4):289‐94. [DOI] [PubMed] [Google Scholar]
Hajek 1996
- Hajek P. Current issues in behavioral and pharmacological approaches to smoking cessation. Addictive Behaviors 1996;21(6):699‐707. [DOI] [PubMed] [Google Scholar]
Hajek 2001
- Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000546.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2013
- Hajek P, Stead LF, West R, Jarvis M, Hartmann‐Boyce J, Lancaster, T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003999.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hughes 2000
- Hughes JR, Stead LF, Lancaster T. Anxiolytics for smoking cessation. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanner 1996
- Kanner RE. Early intervention in chronic obstructive pulmonary disease. A review of the Lung Health Study results. Medical Clinics of North America 1996;80(3):523‐47. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancester TR, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2012
- Park E‐W, Schultz JK, Tudiver F, Campbell T. Enhancing partner support to improve smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002928.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2015
- Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009670.pub3] [DOI] [PubMed] [Google Scholar]
Ussher 2014
- Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD002295.pub4] [DOI] [PubMed] [Google Scholar]
White 2014
- White AR, Rampes H, Jian PL, Stead LF, Campbell J. Acupuncture and related interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000009.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Stead 1998
- Stead LF, Bonevski B, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 1998, Issue 1. [Google Scholar]
Stead 1998a
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI] [PubMed] [Google Scholar]
Stead 2002
- Stead LF, Lancaster TR. Group therapy behaviour programmes for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI] [PubMed] [Google Scholar]
Stead 2005
- Stead LF, Lancaster TR. Group therapy behaviour programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2009
- Stead LF, Lancaster TR. Group therapy behaviour programmes for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [Google Scholar]


