Abstract
Background
People with cystic fibrosis are at an increased risk of fat‐soluble vitamin deficiency including vitamin E. Vitamin E deficiency can cause a host of conditions such as haemolytic anaemia, cerebellar ataxia and cognitive difficulties. Vitamin E supplementation is widely recommended in cystic fibrosis and aims to ameliorate this deficiency. This is an updated version of the review.
Objectives
To determine the effects of any level of vitamin E supplementation on the frequency of vitamin E deficiency disorders in people with cystic fibrosis.
Search methods
We searched the Cochrane Group's Cystic Fibrosis Trials Register and also searched international trial registers for any ongoing clinical trials that were not identified during our register search.
Date of last search of the Register: 10 October 2016. Date of last search of international trial registers: 15 February 2017.
Selection criteria
Randomised controlled trials and quasi‐randomised controlled trials comparing any preparation of vitamin E supplementation to placebo or no supplement, regardless of dosage or duration.
Data collection and analysis
Two authors extracted outcome data from each study (published information) and assessed the risk of bias of each included study.
Main results
Four studies with a total of 141 participants were included in the review, two of these were in children (aged six months to 14.5 years), and the other two did not specify participants’ age. All studies used different formulations and doses of vitamin E for various durations of treatment (10 days to six months). Two studies compared the supplementation of fat‐soluble as well as water‐soluble formulations to no supplementation in different arms of the same study. A third study compared a water‐soluble formulation to a placebo; and in the fourth study a fat‐soluble formulation of vitamin E was assessed against placebo.
At one month, three months and six months, water‐soluble vitamin E significantly improved serum vitamin E levels compared with control: at one month, two studies, mean difference 17.66 (95% confidence interval 10.59 to 24.74); at three months, one study, mean difference 11.61 (95% confidence interval 4.77 to 18.45); and at six months, one study, mean difference 19.74 (95% confidence interval 13.48 to 26.00). At one month fat‐soluble vitamin E significantly improved serum vitamin E levels compared with control: one month, two studies, mean difference 13.59 (95% CI 9.52 to 17.66). The findings at three months were imprecise; one study; mean difference 6.40 (95% confidence interval ‐1.45 to 14.25).
None of the studies report the review's primary outcomes of vitamin E total lipid ratio or the incidence of vitamin E‐specific deficiency disorders, or the secondary outcomes lung function or quality of life. Only one study, comparing water‐soluble vitamin E with placebo, reported the secondary outcome of growth and nutritional status (weight), but the results are uncertain due to imprecision around the effect estimate.
There was limited detail about randomisation and blinding in the included studies which compromises the quality of the evidence base for the review. The heterogeneous mix of the formulations with differing biovailabilities among these studies also limits the generalisability of the data to the wider cystic fibrosis population.
Authors' conclusions
Vitamin E supplementation led to an improvement in vitamin E levels in people with cystic fibrosis, although the studies may have been at risk of bias. No data on other outcomes of interest were available to allow conclusions about any other benefits of this therapy.
In future, larger studies are needed, especially in people already being treated with enteric‐coated pancreatic enzymes and supplemented with vitamin E, to look at more specific outcome measures such as vitamin E status, lung function and nutritional status. Future studies could also look at the optimal dose of vitamin E required to achieve maximal clinical effectiveness.
Plain language summary
Vitamin E supplementation in people with cystic fibrosis
Review question
We wanted to know what effects, if any, vitamin E supplementation (at any dose) has on how often people with cystic fibrosis have health problems due to vitamin E deficiency.
Background
Approximately 85% to 90% of people with cystic fibrosis do not produce enough enzymes in their pancreas and are not able to absorb fat when digesting food. These individuals are also likely to have problems absorbing the fat‐soluble vitamins A, D, E and K. If levels of vitamin E are too low, this may cause problems with the nervous system, blood disorders and memory and thinking skills.
Search date
We last searched for evidence on 15 February 2017.
Study characteristics
The review identified four studies including 141 participants; two of these were in children (aged six months to 14.5 years) and the other two did not specify the age of the participants. The people taking part in the studies received different forms of vitamin E supplements (either water‐soluble or fat‐soluble), placebo (a substance containing no medication) or no supplements. Three studies stated that the treatment for each person was chosen at random, but one study only said the people were split into different groups.
Key results
Three of the studies showed an improvement in vitamin E levels after supplementation, but result should be interpreted with caution due to potential risks of bias. No studies reported any disorders related to vitamin E deficiency. As the studies used different forms of supplements and different doses, it was difficult to combine the results and apply them to the wider cystic fibrosis population, but the results did show that vitamin E supplementation can lead to an improvement in vitamin E levels in people with cystic fibrosis and may help avoid problems caused by vitamin E deficiency.
Future trials, especially in people already receiving treatment with pancreatic enzymes and vitamin E supplements, should look at more specific outcomes such as vitamin E status, lung function and nutritional status. They could also look at the best level of vitamin E supplements needed to be most clinically effective.
Quality of the evidence
We do not think that any of the people taking part in the studies could tell whether they received the supplements or the placebo, so that would not have affected the results; although they would have known if they were taking supplements or not taking anything. We could not tell from the information we have whether most of the studies were designed so all people had an equal chance of being in any of the groups. We also could not tell if anyone would have been able to guess in advance which group they would be in. It was also not clear if there were results reported for everyone taking part in the studies and the reasons why anyone might have dropped out of the studies. We do not know if these facts will affect our confidence in the results.
Background
Description of the condition
Cystic fibrosis (CF) is caused by a genetic defect in the CF transmembrane conductance regulator (CFTR) protein in exocrine glands (e.g. the pancreas, airways, lungs, liver, salivary glands, reproductive tract and sweat glands) (Goss 2004; Kerem 2005; Welsh 2001). The disease is clinically characterized by thick, sticky secretions which impair numerous body systems, primarily the digestive and respiratory tracts. Within the respiratory system, CFTR dysfunction leads to a breakdown in mucociliary clearance, mucous retention, infection and inflammation. These in turn result in respiratory signs and symptoms including cough, copious production of sputum, difficulty in breathing, decreased lung function and respiratory tract infections most importantly due to Pseudomonas aeruginosa (Aaron 2004; Kozlowska 2008; Linnane 2008). Within the digestive system, pancreatic secretions are reduced with low volumes of fluid and bicarbonate in people with CF. This causes duct obstruction and retention and activation of secreted proenzymes in pancreatic ducts leading to destruction and fibrosis of the pancreas itself. This process begins in utero and continues after birth, leading to exocrine pancreatic insufficiency. Pancreatic insufficiency (decreased pancreatic enzyme release), failure of CFTR‐mediated intestinal bicarbonate secretion, deranged bile acid function and inactivation of enzymes by hyperacidity in the upper intestine all contribute to malabsorption (Taylor 2010).
Approximately 85% to 90% of people with CF are pancreatic insufficient and have impaired fat absorption. These individuals are also prone to malabsorption of the fat soluble vitamins A, D, E and K and require pancreatic enzyme replacement therapy (PERT). Low vitamin E levels in people with CF put them at an increased risk of the detrimental effects of vitamin E deficiency. Vitamin E deficiency disorders are rare (Dodge 2006; Sinaasappel 2002; Welsh 2001). They include, but are not limited to, cerebellar ataxia, peripheral neuropathy, myopathy, pigmented retinopathy and visual field contrition with loss of vision (Ueda 2009). Sensory motor neuropathy, which manifests as loss of reflexes and generalized weakness, may occur late in the course of vitamin E deficiency (Suskind 2009). Vitamin E deficiency can also manifest as cognitive impairment (Koscik 2005) and haemolytic anemias (Swann 1998; Wilfond 1994).
Current guidelines on the treatment of CF support PERT in individuals with pancreatic enzyme insufficiency, including fat‐soluble vitamin supplementation, which includes vitamin E (Dodge 2006; Sinaasappel 2002). Recommendations include a dosage of 100 IU to 400 IU per day for all individuals with CF (Sinaasappel 2002).
Description of the intervention
Vitamin E is a generic term for a group of eight fat‐soluble compounds, the tocopherols and tocotrienols, of which α‐tocopherol has the highest biological activity (Suskind 2009). It functions as an antioxidant that protects cell membranes from oxidative damage (Peters 1996); its deficiency may worsen the burden of oxidative stress that results from constant inflammation, especially in respiratory and digestive systems which occurs in CF (Brigelius‐Flohé 2009). In addition to its antioxidant properties, vitamin E also helps improve nerve conduction (Cynamon 1988), maintain the structural integrity of the haemoglobin membrane (Swann 1998) and, along with vitamin A, plays a role in vision (Bines 2005). The specific mechanism of action for most of its effects is still relatively unknown (Brigelius‐Flohé 2009).
Normal vitamin E blood levels generally range from about 23 μmol/L to 46 μmol/L. Plasma levels of 80 μmol/L and above are considered excessive (Biesalski 2009). However, vitamin E circulates in the blood bound to lipoproteins and, as a consequence, more accurate assessment of status should be assessed using the vitamin E to total lipid ratio, which has a sensitivity of 95% and a specificity of 99% for detecting vitamin E deficiency (Thurnham 1986). Normal ratios of α‐tocopherol to total lipid are greater than 0.6 mg and greater than 0.8 mg α‐tocopherol per gram total lipid in children and adults respectively. In a study in a non‐CF population, 47% of low vitamin E levels were normal and 58% with elevated plasma vitamin E were normal or low when re‐evaluated using vitamin E to total lipid ratio (Winbauer 1999).
Oral and parenteral multivitamin and vitamin E supplements have previously been used to ameliorate vitamin E deficiencies (Aparicio 2001; Peters 1996; Swann 1998; Ueda 2009; Winklhofer‐Roob 1996). In CF, this supplementation is usually oral and used in conjunction with PERT. Oral vitamin E may be in the form of chewable or non‐chewable tablets, liquids or powders (Mayo Clinic 2009). Treatment is usually begun as soon as the serum vitamin E levels are investigated and is often life‐long. Even in large doses, treatment has few adverse effects (Aparicio 2001), which were therefore not specifically measured in this review.
How the intervention might work
Vitamin E supplementation aims to correct the deficiency of this vitamin in the body.
Why it is important to do this review
Routine fat‐soluble multivitamin supplementation has become the accepted standard of care in management of people with CF (Bell 2002; Bines 2005; Sinaasappel 2002). The general recommendation of the UK Cystic Fibrosis Trust Nutrition Working Group is regular estimation of serum vitamin E (in addition to lipid ratio) levels to guide vitamin E supplementation of up to 100 mg to 200 mg daily depending on the age of the individual (UK Cystic Fibrosis Trust 2002). This is an updated version of a previous review (Okebukola 2011; Okebukola 2014).
Objectives
To determine the effects of supplementation of vitamin E (whether this was over and above the usual supplements or the only vitamin E supplement given) on vitamin E deficiency or frequency of vitamin E deficiency disorders in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised controlled trials (including cluster‐randomised trials).
Types of participants
All individuals with a diagnosis of CF who have not received a lung transplant. Diagnosis of CF should be based on a positive sweat test or genetic testing or both, plus one or more characteristic clinical features, a history of CF in a sibling, or a positive newborn screening test (Farrell 2008).
Vitamin E supplements are prescribed routinely only to people with CF who are pancreatic insufficient; people who are pancreatic sufficient are supplemented only when levels are found to be sub‐optimal from blood test results (usually in an antioxidant role). These are very different sets of individuals and vitamin E is supplemented for entirely different reasons. We therefore planned to analyse studies with people who were pancreatic insufficient separately from those studies with people who were pancreatic sufficient.
Types of interventions
Any preparation of vitamin E supplementation compared to placebo or no supplement, regardless of dosage or duration. This supplement could be over the usual amount of vitamin being given, or the only vitamin E supplement.
Types of outcome measures
Primary outcomes
Vitamin E total lipid ratio
Vitamin E levels in serum
-
Incidence of vitamin E‐specific deficiency disorders
peripheral neuropathy
retinopathy
myopathy and ataxia
cognitive impairment
haemolytic anemias
Secondary outcomes
-
Growth and nutritional status
weight
height
body mass index (BMI) percentile
-
Lung function tests
forced expiratory volume at one second (FEV₁) (% predicted or litres)
forced vital capacity (FVC) (% predicted or litres)
Quality of life (QoL) (using validated tools, e.g. the Cystic Fibrosis Questionnaire‐ Revised (CFQ‐R) (Quittner 2009) and Cystic Fibrosis Quality of Life Questionnaire (CFQoL) (Gee 2000))
Search methods for identification of studies
Electronic searches
We identified relevant studies from the Group's CF Trials Register using the term: vitamin E.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of theCochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of last search: 10 October 2016.
We searched the international trial registers the ISRCTN website and www.clinicaltrials.gov for any ongoing clinical trials. There were no language or date restrictions. The details of the search terms and resources are found in the appendices (Appendix 2).
Date of last search: 15 February 2017.
Searching other resources
We contacted manufacturers of vitamin E supplements for the results of studies that have been completed but not yet published, other ongoing or published studies. Where it was possible, we also contacted authors of included studies to obtain further information.
Data collection and analysis
Selection of studies
Two authors (PO and SK) independently reviewed titles and abstracts of all articles yielded from the literature search to identify potentially relevant studies. We assessed the full‐texts of these identified studies for inclusion using pre‐formulated screening criteria; and resolved disagreements by consensus. Due to a change in the author team between publication of the protocol and work commencing on the full review, only two authors selected studies instead of three as was indicated in the protocol.
Data extraction and management
Two authors (PO and SK) independently extracted pre‐determined variables into electronic data collection forms. As indicated above, due to a change in the author team, only two authors independently extracted data instead of three as was planned in the protocol. We resolved disagreements by discussion and reached a consensus. In addition to extracting data for all outcome variables, we also recorded the following data when available.
Study details
year of study
study setting
study design (cross‐over, cluster or parallel, single centre or multicentre)
source of funding
whether sample size was calculated
sample size (number enrolled or randomised versus the number analysed)
method of sequence generation
method of allocation concealment
who was blinded and for which outcomes
Participant details
age of participants
sex of participants
severity of disease
inclusion and exclusion criteria
pancreatic sufficient versus pancreatic insufficient
record of PERT
pre‐ or post‐lung transplant
presence or absence of diabetes mellitus
numbers of dropouts and reasons for withdrawal from study (clinical, side effects, refusal, other)
Intervention details
intervention formulation (i.e. capsule, solution, powder)
dose of intervention
frequency of administration
duration of therapy
concurrent medication(s)
Where available, we planned to extract data relevant for outcomes at one month, up to three months, up to six months, up to 12 months and annually thereafter. However, we were only able to extract data at one month, up to three months, up to six months. We also converted the units from md/dl to umol/L which is the SI unit.
We originally planned to present all formulations of vitamin E supplements combined, but have since decided it is more clinically appropriate to present the water‐soluble formulations separately from the fat‐soluble formulations.
As stated above, vitamin E supplements are prescribed routinely only to people who are pancreatic insufficient; those who are pancreatic sufficient are supplemented only when levels are found to be sub‐optimal. We therefore planned to analyse studies with people who are pancreatic insufficient separately from studies with people who are pancreatic sufficient. However, as the included studies did not present sufficient information to allow us to do this, we have presented all data combined.
Assessment of risk of bias in included studies
Two authors independently judged the risk of bias of each included study, following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the following domains as having a low, unclear or high risk of bias.
Randomisation (low risk ‐ random number table, computer‐generated lists or similar methods; unclear risk ‐ described as randomised, but no details given; high risk ‐ e.g. alternation, the use of case record numbers, and dates of birth or day of the week)
Concealment of allocation (low risk ‐ e.g. list from a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed opaque envelopes; unclear risk ‐ not described; high risk ‐ if allocation sequence was known to, or could be deciphered by the investigators who assigned participants or if the study was quasi‐randomised)
Blinding (of participants, personnel and outcome assessors)
Incomplete outcome data (whether investigators used an intention‐to‐treat analysis)
Selective outcome reporting
Other sources of bias
Measures of treatment effect
We reported the results from continuous outcomes as mean differences (MD) in change scores between groups and their 95% confidence intervals (CIs), as planned. Where only endpoint data were available, we planned to use the MD between endpoint scores, but no study reported endpoint data. All studies reported standard deviations (SD), which we used in the analysis. No studies reported standard errors (SEs).
We had planned to report the results from dichotomous outcomes using odds ratios (OR) and 95% CIs. However, all the data were reported as continuous outcomes. We did not include adverse effects as an outcome of interest and no studies reported data on relevant adverse event outcomes.
Initially, we had planned that where both change scores and endpoint data were available, we would combine both using the MD method in RevMan, being careful to use the appropriate SD (of both endpoint data and or change from baseline change) for each study.The differences were reported as MD in RevMan (RevMan 2011). However, we did not combine change scores and endpoint data as standardised mean differences (SMDs).
We planned to use the SMD and 95% CIs and calculate pooled effects, if studies reported outcomes using different measurement scales, but this was unnecessary as all the studies reported using the same measurement system. We did, however, convert all measurements to the SI unit (umol/L).
Unit of analysis issues
If we had found any cross‐over studies, we intended to calculate the mean treatment differences where possible and enter these using the fixed‐effect generic inverse variance (GIV) analysis in RevMan, to provide summary weighted differences and 95% CIs (RevMan 2011). For any cross‐over studies we identify in future updates of this review, if we believe there is a carryover effect which will outlast any washout period included in the study or where second period data is unavailable, we will include only data from the first arm in the meta‐analysis (Elbourne 2002). Following peer review comments, we updated our planned methods and for multi‐arm studies, we now plan to include data from both arms, in line with the current guidance in the Cochrane Handbook of Systematic Reviews (Higgins 2011b).
Dealing with missing data
We planned to make up to three attempts to contact corresponding authors for studies in which there are missing data. If this was not productive, we planned to impute the missing data (SDs, standard errors (SEs) or other parameters) with replacement values based on statistical analysis as recommended in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We planned to treat these as if they were observed, under the assumption that these data are not missing at random. We contacted the authors of the Keljo study to obtain some of the missing data which were used in the abstract. The authors replied and sent us the complete unpublished study (Keljo 2000). We also planned to conduct a sensitivity analysis to test the effects of imputing missing data, but did not need to do this as we obtained the missing data directly from the authors.
Assessment of heterogeneity
If we had been able to combine sufficient data from multiple studies, we intended to describe any heterogeneity between the study results and test this to see if it reached statistical significance using the Chi² test. We considered heterogeneity to be significant when the P value is less than 0.10. We also planned to use the I² statistic (Higgins 2003), where heterogeneity is categorized such that a value around 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and over 70% to 100% is considerable heterogeneity as described in the Cochrane Handbook of Systematic Reviews (Higgins 2011c)
Assessment of reporting biases
We intended to analyse included studies for selective reporting by comparing study protocols with final reports to identify outcomes measured but not reported. Where protocols were not available, we cross‐referenced the 'Methods' section of the study report with the 'Results' section to assess whether study authors comment on all the outcomes they say they measured. We did not identify clinically important outcomes that were omitted in the included studies. We also attempted to reduce publication bias by searching unpublished sources and grey literature in addition to published sources.
Data synthesis
If possible, we planned to enter data into meta‐analyses, combining all dose regimens and include the 95% CI, estimated using a fixed‐effect model. We also planned to utilise the random‐effects model whenever we had identified moderate or substantial heterogeneity (I² greater than 50%).
Subgroup analysis and investigation of heterogeneity
We intended to study the following subgroups to investigate any heterogeneity that we might have identified, but were not able to do so due to a lack of data:
children (under 18 years) and adults (18 years of age and over);
method of diagnosis of CF (screened versus non screened);
presence versus absence of co‐morbid conditions like diabetes mellitus.
Sensitivity analysis
We also planned to use the following sensitivity analyses to assess the impact of the potentially important factors on the overall outcomes, but were not able to do so due to a lack of data:
analysis using a random‐effects model;
per protocol analysis;
high versus low risk of bias for each domain assessed;
analysis with or without imputed data.
Results
Description of studies
Results of the search
We identified 13 references to 12 studies from the search of our databases outlined above. We identified two additional studies from searching other databases (the ISRCTN website and www.clinicaltrials.gov). As illustrated in the PRISMA diagram, 14 studies were included for screening (Figure 1). Of the 14 studies, four were included and 10 were excluded; the reasons for their exclusion are discussed below.
1.
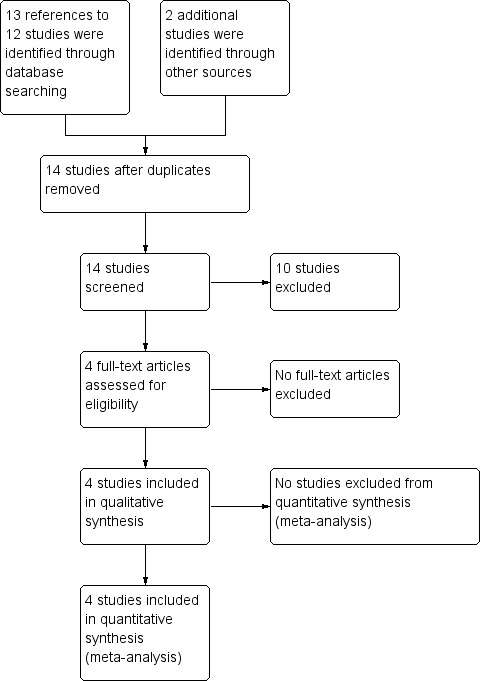
Study flow diagram
We contacted the manufacturers of vitamin E supplements; however, they responded that they did not have any information about any unpublished or ongoing studies.
Included studies
Four studies (n = 141) were included (Harries 1969; Levin 1961; Keljo 2000; Wong 1988). Two of these were only available as abstracts, which limited the amount of detail available for the review (Keljo 2000; Wong 1988); however, we were able to obtain additional information from the authors of the Keljo study. The fourth study is a full paper, but has only been published as part of conference proceedings and not in a journal (Harries 1969). Two of these studies are from the 1960s when PERT would not have been widely available and the management strategies for CF quite different from present times.
There were insufficient studies included to conduct subgroup analyses or sensitivity analyses.
Trial characteristics
Three of the four studies were described as randomised (Harries 1969; Levin 1961; Keljo 2000) and the fourth study was a controlled clinical study with no reference to randomisation during allocation of participants to study arms (Wong 1988). However, we assumed that the authors performed some form of randomisation. All studies were of parallel design; two consisted of two arms (Keljo 2000; Levin 1961) and two of three arms (Harries 1969; Wong 1988). All were single‐centre studies; two were conducted in the USA (Keljo 2000; Levin 1961), one was conducted in Canada (Wong 1988) and the fourth study was conducted in the UK (Harries 1969). Study duration ranged from between 10 and 14 days (Wong 1988) to six months (Levin 1961).
Participants
All studies were of similar size, the number of participants ranged from 22 (Wong 1988) to 49 (Levin 1961). Two studies recruited children only (Harries 1969; Levin 1961) and two studies did not specify if participants were children or adults or a mix of both (Keljo 2000; Wong 1988). Both paediatric studies gave details on the age of their participants: range six months to 14.5 years (Harries 1969); and mean (SD) age of participants in the treatment group 113.1 (65.12) months and in the placebo group 113.5 (63.99) months (Levin 1961). Only one study gave any details of the gender split of participants (26 males, 19 females) (Levin 1961). Two studies reported that participants had dropped out (Harries 1969; Levin 1961), but only one gave specific reasons for this (Levin 1961). In the remaining two studies there seemed be drop outs from the study, but these were not clearly discussed (Keljo 2000; Wong 1988). Disease status varied between studies; one study described participants as having been stabilized with a study‐specific disease severity score of 2.22 in the treatment group and 2.10 in the placebo group (where 1 is good condition and 5 is very severe illness) (Levin 1961). A second study described participants as having mild pulmonary disease (Keljo 2000). A third study recruited participants admitted to hospital for a pulmonary exacerbation (Wong 1988) and the remaining study did not comment on disease status (Harries 1969). None of the studies explicitly stated whether the participants were pancreatic sufficient or insufficient.
Interventions
Two studies compared fat‐soluble and water‐miscible supplements of vitamin E to no supplementation; both these studies used the dose of 10 mg/kg/day for the supplements (Harries 1969; Wong 1988). The water‐miscible formulation Harries used contained the surface active agent cremaphor El and glycerine (Harries 1969). Wong used an oral water‐soluble vitamin E preparation (Aquasol E) (Wong 1988). A further study compared the same dose of water‐miscible vitamin E supplement to placebo (Levin 1961). The remaining study compared a vegetable oil placebo to RRR alpha‐tocopherol which was given to participants weighing less than 20 kg at a dose of 600 IU/day and to participants weighing more than 20 kg at a dose of 1200 IU/day (Keljo 2000).
Two studies stated that participants continued to receive pancreatic enzyme supplementation during the study (Harries 1969; Wong 1988). One study stated participants continued to receive their standard vitamin supplements ADEKs® (Keljo 2000).
Outcomes
Only the measurement of serum vitamin E levels was common to all four included studies (Harries 1969; Keljo 2000; Levin 1961; Wong 1988). Other outcomes measured in the blood included peroxide red blood cell (RBC) haemolysis (Harries 1969), serum glutamic oxalacetic transaminase (Levin 1961), tumour necrosis factor (TNF) alpha and interleukin‐6 levels (Keljo 2000) and alpha tocopherol to cholesterol ratio (Wong 1988). In addition, Levin reported on weight gain, muscle strength and subjective improvement (Levin 1961).
Excluded studies
A total of 10 studies were excluded from this review. Seven studies were excluded as they compared various vitamin E formulations, but not to a placebo or no supplement arm, and therefore not meeting the selection criteria for the review (Munck 2010; Nasr 1993; Jacquemin 2009; Papas 2007; Winklhofer‐Roob 1992; Winklhofer‐Roob 1996; Wood 2003). A further study was excluded because it described the use of vitamin E with placebo compared to vitamin E with ursodeoxycholic acid in a single participant, therefore the only difference in treatment was ursodeoxycholic acid (Thomas 1995). One study was excluded because it was not randomised or quasi‐randomised (Sagel 2011). In the final study, which is still ongoing, tocotrienol is being used as a genetic modifier in people with CF (Kerem 2009). One of the arms of the study entails supplementation with tocotrienol followed by a washout period; however, this study was excluded because it is not a study of vitamin E supplementation compared to placebo or control.
Risk of bias in included studies
Two of the included studies were published as short abstracts, hence the information available about the design and robustness was limited (Keljo 2000; Wong 1988). However, one of the authors provided additional information by sending us the unpublished manuscript which contained further details (Keljo 2000). Clear information was available for the remaining two studies (Harries 1969; Levin 1961).
Allocation
In three of the included studies the risk of bias from sequence generation was unclear (Harries 1969; Keljo 2000; Wong 1988). Two of these were described as randomised, but gave no details of the randomisation process (Harries 1969; Keljo 2000); the third merely stated that participants were divided into one of three groups (Wong 1988). In the final study, there was a clear description of the randomisation process using labelled cards being placed in sealed envelopes and then each child accepted into the study group was assigned an envelope; we judged this to have a low risk of bias for sequence generation (Levin 1961).
Three of the studies did not discuss concealment of allocation and are judged to have an unclear risk of bias (Harries 1969; Keljo 2000; Wong 1988) the fourth study used sealed envelopes to conceal the allocation sequence and is judged to have a low risk of bias (Levin 1961).
Blinding
In two studies, vitamin E supplementation is compared to no supplementation, so these studies cannot be blinded (Harries 1969; Wong 1988). Since we are reporting on objective outcome measurements, the absence of blinding is unlikely to increase the risk of bias in these studies and we have judged them to have an low risk of bias. Another study was double‐blinded and used a vegetable oil placebo, we therefore judged it to have a low risk of performance and detection bias (Keljo 2000). In the study by Levin, the participants and the testers are described as being blinded; serum tocopherol levels were also not known to the examiners (Levin 1961). We felt overall that it had a low risk of bias.
Incomplete outcome data
Two studies reported that participants had dropped out (Harries 1969; Levin 1961). Only one of these gave specific reasons for these withdrawals and the attrition is balanced among the two groups; hence we judged this study to have a low risk of bias (Levin 1961). In the study by Harries, vitamin E levels at the end of one month of supplementation were not available for one participant in the fat‐soluble group and two in the water‐miscible supplementation group (Harries 1969). An intention‐to‐treat analysis was not used; however, these missing data are unlikely to have a clinically significant impact on the results and we therefore judged the study to have a low risk of bias (Harries 1969).
In the study by Wong, the initial number of participants allocated to each group is not clear and hence it is difficult to judge the number of dropouts across each group (Wong 1988). In view of this insufficient reporting we judged it have an unclear risk of bias due to incomplete outcome data (Wong 1988). In the remaining study, there appeared to be dropouts, but these were not clearly discussed and we judged this study to also have an unclear risk of bias (Keljo 2000).
Selective reporting
No study protocol was available to inform the risk of reporting bias in any of the four studies (Harries 1969; Keljo 2000; Levin 1961; Wong 1988). All studies report results for the outcomes stated in their 'Methods' sections. In the absence of a protocol and no apparent risk of bias, we judge the overall risk of bias to be unclear in three studies (Harries 1969; Levin 1961; Wong 1988). The authors of the Keljo study sent us the full study article on request, but the full article did not elucidate the methods of randomisation and did not fully report the results of the control group, which gives it a high risk for bias (Keljo 2000).
Other potential sources of bias
There is no information on how the diagnosis of CF was reached in any of the included studies or whether participants were pancreatic sufficient or insufficient, which may introduce a potential risk of bias. We therefore conclude a unclear risk of other potential bias for all studies. However, in the earlier studies the participants would not have had their pancreatic insufficiency treated with effective pancreatic enzymes as enteric‐coated preparations were not available. Instead, they would have been treated with a low‐fat diet to control symptoms of fat malabsorption. In addition, the dosages used in the Keljo study are higher than current international recommendations of: age up to 12 months, 40 IU to 80 IU; age one to three years, 50 IU to 150 IU; age four to seven years, 150 IU to 300 IU; and age eight years to adult, 150 IU to 500 IU (Sinaasappel 2002).
Effects of interventions
Water‐soluble vitamin E versus control
Three studies (n = 101) reported on this comparison (Levin 1961; Harries 1969; Wong 1988).
Primary outcomes
1. Vitamin E: total lipid ratio
None of the studies using this comparison reported on this outcome.
2. Vitamin E levels in serum
Three studies of water‐soluble vitamin E preparations reported on this outcome (Levin 1961; Harries 1969; Wong 1988). Data are available from two studies at the one‐month time point (Harries 1969; Wong 1988) and show a statistically significant result in favour of the supplemented group, MD 17.66 (95% CI 10.59 to 24.74). Levin reported statistically significant improvement in vitamin E levels at three months and six months, MD 11.61 (95% CI 4.77 to 18.45) and MD 19.74 (95% CI 13.48 to 26.00) respectively (Analysis 1.1).
1.1. Analysis.
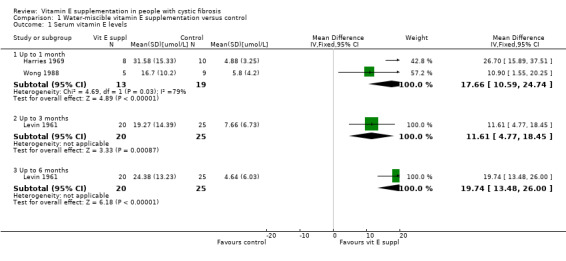
Comparison 1 Water‐miscible vitamin E supplementation versus control, Outcome 1 Serum vitamin E levels.
3. Incidence of vitamin E‐specific deficiency disorders
None of the studies include reported peripheral neuropathy, retinopathy, myopathy and ataxia, or cognitive impairment in either the supplemented or the placebo group. The studies did not report on clinical occurrence of haemolytic anaemia in either group.
Secondary outcomes
1. Growth and nutritional status
a. Weight
Levin reported an increase in weight in both the placebo and supplemented arms from the baseline to the one‐month and six‐month mark. However, the improvement in weight, which although was higher in the placebo group, was not statistically significant between groups at one month, MD ‐2.80 kg (95% CI ‐8.98 to 3.38) or after six months, MD ‐3.60 kg (95% CI ‐13.36 to 6.16) (Analysis 1.2).
1.2. Analysis.
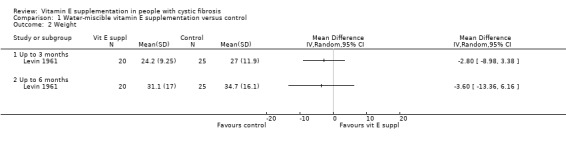
Comparison 1 Water‐miscible vitamin E supplementation versus control, Outcome 2 Weight.
b. Height
None of the included studies reported any changes in height as an outcome measure.
c. BMI percentile
None of the studies reported BMI percentile as an outcome measure.
2. Lung function tests
No change in FEV₁ or FVC, either as % predicted or litres, was reported in any of the included studies.
3. QoL
None of the included studies reported on QoL.
Fat‐soluble vitamin E versus control
Three studies (n = 101) reported on this comparison (Harries 1969; Keljo 2000; Wong 1988).
Primary outcomes
1. Vitamin E: total lipid ratio
A single study measured alpha tocopherol to cholesterol ratio at baseline, but did not report post‐intervention levels (Wong 1988)
2. Vitamin E levels in serum
Three studies of fat‐soluble vitamin E preparations reported on this outcome (Harries 1969; Keljo 2000; Wong 1988). At up to one month data were combined for two studies and showing a statistically significant result in favour of supplementation, MD 13.59 (95% CI 9.52 to 17.66). Data were also available at up to three months for a single study but were not statistically significant, MD 6.40 (95% CI ‐1.45 to 14.25) (Analysis 2.1).
2.1. Analysis.
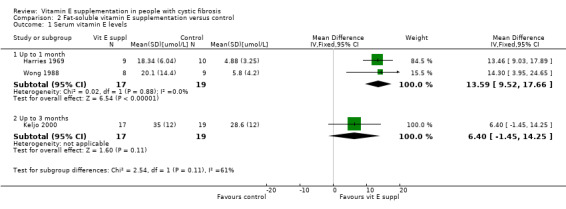
Comparison 2 Fat‐soluble vitamin E supplementation versus control, Outcome 1 Serum vitamin E levels.
3. Incidence of vitamin E‐specific deficiency disorders
None of the studies include reported peripheral neuropathy, retinopathy, myopathy and ataxia, or cognitive impairment in either the supplemented or the placebo group. The studies did not report on clinical occurrence of haemolytic anaemia in either group.
Secondary outcomes
1. Growth and nutritional status
None of the included studies reported any changes in weight, height or BMI percentile as an outcome measure.
2. Lung function tests
No change in FEV₁ or FVC, either as % predicted or litres, was reported in any of the included studies.
3. QoL
None of the included studies reported on QoL.
Discussion
People with cystic fibrosis (CF) and who are pancreatic insufficient are at an increased risk of malabsorption of fat‐soluble vitamins. Vitamin E supplementation, usually in combination with pancreatic enzyme replacement therapy (PERT), is universally recommended for people with CF who are pancreatic insufficient. We attempted to evaluate the effect of vitamin E supplementation compared with placebo in improving vitamin E levels and rectifying this deficiency in people with CF. We also wanted to assess the effect of vitamin E supplementation on the avoidance of vitamin E deficiency, growth, nutrition and respiratory status in people with CF.
A state of vitamin E deficiency, as characterised by low levels of the vitamin, has been shown to be associated with problems such as haemolysis and cognitive difficulties. Vitamin E supplementation may have additional beneficial effects as an antioxidant and possibly also an anti‐inflammatory role. It was not in the remit of this review to explore the latter role.
Summary of main results
Four studies with a total of 141 participants were included in the review (Harries 1969; Keljo 2000; Levin 1961; Wong 1988). All studies used different formulations and doses of vitamin E for various durations of treatment. There were three eligible trials comparing fat‐soluble vitamin supplementation to control (Harries 1969; Keljo 2000; Wong 1988), two of these studies also used water‐miscible formulations (Harries 1969; Wong 1988). One further study compared a water‐miscible formulation to control (Levin 1961). The duration of supplementation in the studies also differed from 10 days (Wong 1988) to six months (Levin 1961). Vitamin E supplementation is usually undertaken in conjunction with PERT, as was case in at least three of the four included studies (Harries 1969; Keljo 2000; Wong 1988).
Our review suggests that vitamin E supplementation is useful in improving the serum vitamin E levels in people with CF, despite the limitations of the included studies, as supplementation led to statistically significant improvements in vitamin E levels in three out of the four included studies (Harries 1969; Levin 1961; Wong 1988). Three studies reporting the use of water‐soluble supplements showed statistically significant improvements in serum vitamin E levels compared to placebo or no supplement (Harries 1969; Levin 1961; Wong 1988). Two of the three studies that reported fat‐soluble supplementation also indicated similar improvements in vitamin E levels in serum (Harries 1969; Wong 1988). While both the water‐soluble and fat‐soluble preparations led to an improvement in the levels of vitamin E, this improvement was not statistically significant if the participants were receiving the usual supplements of vitamin E (Keljo 2000). It is, however, difficult to definitively draw the conclusion that additional supplementation (over the usual amount) does not lead to a statistically significant improvement since the dosage of vitamin E supplementation may impact the results.
None of the studies report any symptoms typical of vitamin E deficiency, but baseline (untreated) vitamin E levels were low in all participants in three of the four included studies and these continued to be so in the placebo or no supplementation groups (Harries 1969; Levin 1961; Wong 1988). No study reported any other beneficial effects of interest (secondary outcomes), except weight in one study, which did not improve significantly more in the treatment group compared to the control group (Levin 1961). None of the included studies looked at improvements in lung function or cognition following supplementation; and hence it is difficult to draw any conclusions about the effect of vitamin E on these parameters. It is also instructive that none of the studies reported on many of the review outcomes and none of them had a common set of outcomes. It may thus be necessary to consider developing a core set of outcomes for research in this area.
Overall completeness and applicability of evidence
Vitamin E levels were the primary outcome measures in only two of the four included studies (Harries 1969; Wong 1988). One of these studies also reported the alpha tocopherol to cholesterol ratio at baseline, which is also a valid measure of vitamin E levels; however, this study did not report alpha tocopherol to cholesterol ratios post‐intervention (Wong 1988). None of the studies detail the basis for diagnosis of CF or pancreatic insufficiency. Varying formulations of vitamin E supplementation were used in the studies; three used fat‐soluble formulations (Harries 1969; Keljo 2000; Wong 1988) and three used water‐miscible formulations (Harries 1969; Levin 1961; Wong 1988). Bioavailability of vitamin E formulations can be variable. Comparison between various formulations was not in the remit of our review; however, this can potentially impact the serum vitamin levels thereby impacting the generalizability of the data. It is also possible that duration of supplementation may be a limitation to overall applicability as the longest study only lasted six months. Some of the included studies were conducted more than 50 years ago, when PERT was not available. Since PERT helps in fat (and fat‐soluble vitamin) absorption, the routine use of PERT will affect vitamin E levels in clinical practice.
Quality of the evidence
Two of these studies were published as short abstracts only (Keljo 2000; Wong 1988). Assessment of one of these was limited (Wong 1988), but the author of the second abstract provided us with additional data (Keljo 2000). One study was available as a full paper, but only presented at a conference and not published in a peer‐reviewed journal (Harries 1969). In the remaining three papers, the methods of randomisation and blinding are detailed only in one paper (Levin 1961). In one paper there is no presentation of standard deviation (SD) of the effect in the control group introducing a risk of bias (Keljo 2000).
The limitations of the available data due to imprecision and inconsistency compromise the quality of the evidence base for the review and restrict the applicability of the evidence.
Potential biases in the review process
The two authors individually appraised all the studies and the risk of any further biases, other than those detailed in risk of bias tables, is very unlikely. None of the authors have any conflicts of interest to declare.
Agreements and disagreements with other studies or reviews
Vitamin E deficiency in CF is reported to cause haemolytic anaemia (Wilfond 1994). None of the participants in the included studies were reported to have this complication. The exact relationship between vitamin E and lung health in CF is unclear. One study reported that vitamin E status is associated with an increased rate of pulmonary exacerbations in CF (Hakim 2007). However, a second study did not find any evidence to implicate vitamin E deficiency in the development of lung disease or airway inflammation (Bines 2005). None of the included studies reported lung function parameters for us to examine this relationship.
A Cochrane review examined the effect of antioxidant supplementation (including Vitamin E) on lung function in people with CF (Ciofu 2014). This review did not find any positive treatment effect of antioxidants on any clinical outcomes (lung function, quality of life, antibiotic days, adverse events).
As part of the Wisconsin CF Neonatal Screening Project, Koscik reported low cognitive scores in people with CF who had low serum vitamin E levels at diagnosis (Koscik 2005). None of the studies included in the review report cognitive scores as an outcome measure. We are thus unable to draw any firm conclusion about the effect of vitamin E on cognitive function in CF.
Authors' conclusions
Implications for practice.
People with CF with pancreatic insufficiency have impaired absorption of fats which forms the clinical basis to supplement fat‐soluble vitamins including vitamin E. Three of the four studies in our review demonstrate that supplementation of vitamin E in people with CF leads to an improvement in vitamin E levels (Harries 1969; Levin 1961; Wong 1988), but in none of these studies was the status of pancreatic sufficiency or insufficiency in participants clear. Although none of the studies reported any detrimental effects of low vitamin E, the consequences of low vitamin E are well‐known and a deficient state is best avoided. None of the included studies reported other outcomes of interest (except a non‐statistically significant change in weight in one study (Levin 1961), so it is difficult to draw any recommendations for treatment from vitamin E supplementation in people with CF. It may be prudent to extrapolate that clinical vitamin E deficiency would be detrimental to the health of people with CF and our review findings do not challenge the established practice of supplementation to avoid deficiency.
Implications for research.
It will be unethical to perform a study where vitamin E levels are not corrected (in the placebo group) to the point of clinical deficiency; however, studies looking at clinical or quality of life improvements with vitamin E supplementation are needed. Due to the heterogeneity of CF and the multiple concurrent therapies which people with CF are usually prescribed, it may be difficult to ascertain the extent of improvement related to vitamin E alone.
Despite these challenges, larger research studies are needed in people with CF who are pancreatic insufficient and who are being treated with enteric‐coated pancreatic enzymes and supplemented with vitamin E (usually fat‐soluble preparations, e.g. as in the UK). These studies should look at more specific outcome measures such as vitamin E status (as most participants will already be receiving supplements), lung function, nutritional status and perhaps the reduction in airway inflammation. It may be useful to consider developing a core set of outcomes for research in this area. It is, however, beyond the mandate of this review to recommend clinical doses of vitamin E for CF therapy. However, future studies may decide to test or confirm the optimal dose of vitamin E required to achieve maximal clinical effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 27 February 2017 | New citation required but conclusions have not changed | Since no additional references could be added to this review, our conclusions remain the same. |
| 10 October 2016 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register and searches of ongoing trials registries did not identify any new references potentially eligible for inclusion in this review. |
Acknowledgements
We would like to thank Larissa Shamseer for her input into an early draft of the protocol and also Helen McCabe who was an author on the published protocol. Thanks also to Nikki Jahnke for all her help with editing the review and other administrative matters. Finally we would like to thank the authors of the included Keljo study for providing additional data.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Glossary
| Term | Explanation |
| cerebellar ataxia | failure of muscular coordination and irregularity of muscular action characterized by defects in rate, range, force and direction of movement of limbs |
| myopathy | a disease of muscle or muscle tissue, especially skeletal muscle |
| peripheral neuropathy | any damage to the peripheral nerves (which are the nerves outside the brain and the spinal cord) |
| pigmented retinopathy | a disorder of the retina characterized by deposits of pigment and increasing loss of vision |
| visual field contrition | narrowing (or loss) of the visual fields ‐ which is the area simultaneously visible to one eye without movement |
Appendix 2. Search strategies
ISRCTN website and clinical trials.gov
The most recent searches were conducted on 15 February 2017. We used the following search terms:
cystic fibrosis
cystic fibrosis AND vitamin E
cystic fibrosis AND tocopherol
cystic fibrosis AND toco*
cystic fibrosis AND alpha tocopherol
Data and analyses
Comparison 1. Water‐miscible vitamin E supplementation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum vitamin E levels | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 1 month | 2 | 32 | Mean Difference (IV, Fixed, 95% CI) | 17.66 [10.59, 24.74] |
| 1.2 Up to 3 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 11.61 [4.77, 18.45] |
| 1.3 Up to 6 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 19.74 [13.48, 26.00] |
| 2 Weight | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Up to 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Up to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Fat‐soluble vitamin E supplementation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum vitamin E levels | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 1 month | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 13.59 [9.52, 17.66] |
| 1.2 Up to 3 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 6.40 [‐1.45, 14.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Harries 1969.
| Methods | Randomised controlled study of parallel design with 3 arms. Single‐centre study in UK. Duration 1 month. |
|
| Participants | 30 children with CF on pancreatic enzyme supplementation. Age range 6 months to 14.5 years. | |
| Interventions | Acute supplementation and long‐term supplementation. Children received either:
|
|
| Outcomes | Serum vitamin E levels and serum RBC haemolysis. | |
| Notes | Acute supplementation (called oral load test in the paper) was only done in 2 participants without controls. The study does not clarify whether the participants were pancreatic sufficient or insufficient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly specified in the the paper, states "chosen at random and arbitrarily assigned to one of three groups". |
| Allocation concealment (selection bias) | Unclear risk | Not clearly specified in the the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not clearly specified in the the paper. Very likely not blinded, as 1 of arms was no treatment. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clearly specified in the the paper. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 1 month reported data for 9 out of 10 participants in fat‐soluble group (i.e. 1 dropout) and for 8 out of 10 participants in water miscible group (i.e. 2 dropouts), no discussions of reasons for dropouts. However, these missing data are unlikely to have a significant impact on the results. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available, but outcomes stated in 'Methods' section reported in 'Results'. |
| Other bias | Unclear risk | Limited information the basis of diagnosis of CF, pancreatic sufficiency/insufficiency etc. |
Keljo 2000.
| Methods | Randomised double‐blind placebo‐controlled study of parallel design. Single centre in the USA. Participants stratified according to FEV₁ (70% ‐ 85% predicted and > 85% predicted) and use of DNAse. Duration 3 months. |
|
| Participants | 40 participants with CF and mild lung disease (FEV₁ % predicted > 70%). No details of age or gender split. Evenly distributed to treatment and placebo groups. | |
| Interventions | Participants randomised to take vegetable oil placebo or RRR alpha‐tocopherol (participants < 20 kg = 600 IU/Day < 20 kg; participants > 20 kg = 1200 IU/day). All participants also took ADEKs® for the duration of the study. |
|
| Outcomes | Beginnning and end of study: serum vitamin E levels; TNF‐alpha measurements; and IL‐6 measurements. End of study only: liver enzyme levels; PT measurements; and PTT measurements. Adverse effects reported. |
|
| Notes | Authors provided full manuscript later, but it did not have much more information. Vitamin E and placebo capsules donated by Henkel Corporation. ADEKs® vitamins and partial financial support provided by Axcan Scandipharm. The study does not clarify whether the participants were pancreatic sufficient or insufficient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind, but not stated who was blinded. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Implies some dropouts as at end of study 20 participants stated as taking DNAse and 15 as not taking DNAse, but not clearly stated. |
| Selective reporting (reporting bias) | High risk | Protocol later provided by authors but did not provide much more information, no presentation of SD of the effect in the control group. |
| Other bias | Unclear risk | Limited information on the basis for diagnosis of CF. Not clear if pancreatic sufficient or insufficient. |
Levin 1961.
| Methods | Randomised double‐blind placebo‐controlled study of parallel design. Single‐centre trial in the USA. Duration 6 months, planned to see participants every 2 months when possible. No efforts made to counterbalance groups from which individuals were lost during study. |
|
| Participants | 49 participants accepted with proven diagnosis of CF and stabilised; 45 children followed for at least 2 months; 37 completed 6 months (18 in treatment group; 19 in placebo group). Each participant rated independently by 2 observers as to condition at the beginning of study on an arbitrary scale (1 = good condition up to 5 = very severe illness). Tocopherol Group 20 participants, mean (SD) age 113.1 (65.12) months, 9 males and 11 females, mean (SD) illness severity score 2.22 (1.09), mean (SD) weight 24.3 (9.75) kg. Placebo Group 25 participants, mean (SD) age 113.5 (63.99) months, 17 males and 8 females, mean (SD) illness severity score 2.10 (0.913), mean (SD) weight 26.8 (12.0) kg. |
|
| Interventions | 10 mg/kg/day of vitamin E tocopherol (d‐l alpha‐tocopheryl acetate) in water‐miscible solution in 2 or 3 divided doses (n = 20) versus placebo (n = 25). | |
| Outcomes | Weight, muscle power, serum tocopherol, activity of S‐GOT in serum and subjective improvement. | |
| Notes | Reported data at baseline, 2 and 6 months Tocopherol and placebo provided by U.S. Vitamin Corporation. Supported in part by grants from the National Institute of Arthritis and Metabolic Diseases, National Institutes of Public Health, Public Health Service and the Muscular Dystrophy Associations of America. Limited information on the basis for diagnosis of CF. The study does not clarify whether the participants were pancreatic sufficient or insufficient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed as follows: cards labelled 1 or 2 were individually placed in sealed envelopes in groups of 4 (2 for each mixture number). Envelopes were divided into 3 groups, according to age of participants: < 5 years, 5 ‐ 10 years, and ≥ 10 years and over. Each child accepted into the study group was assigned an envelope from the appropriate age group, and the enclosed card indicated the mixture to be given. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with designated randomisation, each child accepted into study was assigned an envelope from the appropriate age group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | From the paper in plan of study: "Patients and testers did not know which preparation was taken" (double‐blind method) also stated "Physicians blind to vitamin E results". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of the initial 49 participants accepted, 37 completed the study but intention‐to‐treat analysis was not carried out. 3 died within 6 months (all in placebo group), 2 declined to continue medication after 2 months, 1 participant removed due to diabetes mellitus and 7 participants studied for less than 6 months. However, we feel the attrition is balanced among the two groups; hence we judged this study to have a low risk of bias. Planned to measure data at baseline, end of study (6 months) and every 2 months where possible, reported data at baseline, 2 and 6 months. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but the paper reports data for each outcome measurement listed in the 'Methods' section of the paper. |
| Other bias | Unclear risk | None identified. Limited information on the basis for diagnosis of CF and whether pancreatic sufficient or insufficient. |
Wong 1988.
| Methods | Controlled clinical study. Single centre in Canada. Duration 10 ‐ 14 days. |
|
| Participants | 22 CF participants admitted for pulmonary infection. | |
| Interventions | Group A: oral fat‐soluble vitamin E 10 mg/kg/day (n = 8). Group B: oral water‐miscible vitamin E (Aquasol E) 10 mg/kg/day (n = 5). Group C: no supplementation (n = 9). |
|
| Outcomes | Serum vitamin E levels, alpha‐tocopherol/cholesterol ratio, 3‐day faecal fat excretion. | |
| Notes | All participants also received appropriate intravenous antibiotics, 10% Nutralipid 15 ml/kg/day and usual dosage of enteric‐coated pancreatic enzymes. The study does not clarify whether the participants were pancreatic sufficient or insufficient. Abstract only available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not stated, abstract states participants divided into 3 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, blinding of no treatment compared to either of treatment arms not possible, but not clear if blinded between treatment arms. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of no treatment compared to either of treatment arms not possible, but not clear if blinded between treatment arms. Objective outcome is measured so absence of blinding unlikely to increase risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants recruited and randomized not stated, only numbers of participants for whom results available given. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, abstract only so no 'Methods' section available to compare to 'Results' section. |
| Other bias | Unclear risk | None identified. Limited information on the basis for diagnosis of CF and if pancreatic sufficient or insufficient. |
CF: cystic fibrosis DNAse: dornase alfa IU: international units PI: pancreatic insufficient PS: pancreatic sufficient PT: prothrombin PTT: partial thromboplastin time RBC: red blood cell SD: standard deviation S‐GOT: glutamic oxalacetic transaminase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jacquemin 2009 | Compares bioavailability of preparations, no placebo or control arm. |
| Kerem 2009 | It is not a study of vitamin E supplementation compared to placebo or control. |
| Munck 2010 | Lack of placebo or control group. |
| Nasr 1993 | Compares two preparations of vitamin E, no control or placebo group. |
| Papas 2007 | Compares bioavailability of preparations, no control group. |
| Sagel 2011 | Not a randomised or quasi‐randomised study. |
| Thomas 1995 | Comparison of vitamin E given with placebo to vitamin E given with ursodeoxycholic acid in a single participant. |
| Winklhofer‐Roob 1992 | No placebo or control group. Compared 3 preparations of vitamin E to baseline status. |
| Winklhofer‐Roob 1996 | Compared levels of vitamin E after supplementation with 3 different preparations of vitamin E and compared to matched controls. |
| Wood 2003 | Compared low‐dose to high‐dose supplements. No placebo or control group. |
Differences between protocol and review
We originally planned to present all formulations of vitamin E supplements as a single intervention, but in the full review we have presented the comparisons of water‐soluble vitamin E supplements versus control and fat‐soluble vitamin E supplements versus control separately.
Contributions of authors
Peter Okebukola conceived of and wrote the text of the protocol and review alongside Sonal Kansra with comments from Joanne Barratt.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Harries 1969 {published data only}
- Harries JT, Muller DPR. Absorption of water miscible and fat soluble preparations of vitamin E in cystic fibrosis. 5th International Cystic Fibrosis Conference; 1969 Sept. 22‐26; Cambridge, England. 1969:298‐307. [CFGD Register: GN49]
Keljo 2000 {published and unpublished data}
- Keljo DJ, Giroir B, Jialal I. Circulating tumor necrosis factor alpha and interleukin‐6 levels in cystic fibrosis, effect of vitamin E therapy [abstract]. Pediatric Pulmonology 2000;30 Suppl 20:326. [CFGD Register: GN87] [Google Scholar]
Levin 1961 {published data only}
- Levin S. Muscular performance and vitamin E in cystic fibrosis [letter]. Archives of Disease in Childhood 1974;49(3):247. [CFGD Register: GN73a; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S, Gordon MH, Nitowsky HM, Goldman C, Sant'Agnese P, Gordon HH. Studies of tocopherol deficiency in infants and children: VI. Evaluation of muscle strength and effect of tocopherol administration in children with cystic fibrosis. Pediatrics 1961;27:578‐88. [CFGD Register: GN73b; DOI: 10.1136/adc.49.3.247-a; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 1988 {published data only}
- Wong LTK, Halstead C, Davidson AGF, Fang PM. Comparison of the efficacy of water‐miscible and fat soluble vitamin E in the therapy of vitamin E deficiency in cystic fibrosis patients [abstract]. Pediatric Pulmonology 1988;5 Suppl 2:144. [CFGD Register: GN52] [Google Scholar]
References to studies excluded from this review
Jacquemin 2009 {published data only}
- Jacquemin E, Hermeziu B, Kibleur Y, Friteau I, Mathieu D, Coz F, et al. Bioavailability of oral vitamin E formulations in adult volunteers and children with chronic cholestasis or cystic fibrosis. Journal of Clinical Pharmacy and Therapeutics 2009;34(5):515‐22. [CFGD Register: GN216; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kerem 2009 {published data only}
- NCT00889434. Efficacy and safety study of EGCG/Tocotrienol in 18 patients with splicing‐mutation‐mediated cystic fibrosis (CF). http://clinicaltrials.gov/show/NCT00889434 (accessed 10 March 2012). [Clinicaltrials.gov: NCT00889434]
Munck 2010 {published data only}
- Munck A, Ginies JL, Huet F, Wizla N, Gerardin M, Darviot E, et al. A new water‐soluble oral vitamin E formulation in cystic fibrosis (CF) children [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S91, Abstract no: 352. [CFGD Register: GN219; MEDLINE: ] [Google Scholar]
Nasr 1993 {published data only}
- Nasr SZ, O'Leary MH, Hillermeier C. Correction of vitamin E deficiency with fat‐soluble versus water‐miscible preparations of vitamin E in patients with cystic fibrosis. Journal of Pediatrics 1993;122(5 Pt 1):810‐2. [CFGD Register: GN42] [DOI] [PubMed] [Google Scholar]
Papas 2007 {published data only}
- Papas K, Kalbfleisch J, Mohon R. Bioavailability of a novel, water‐soluble vitamin E formulation in malabsorbing patients. Digestive Diseases and Sciences 2007;52(2):347‐52. [CFGD Register: GN114; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sagel 2011 {published data only}
- Sagel SD, Sontag MK, Anthony MM, Emmett P, Papas KA. Safety and efficacy of an antioxidant‐rich multivitamin supplement in cystic fibrosis. Journal of Cystic Fibrosis 2011;10(1):31‐6. [Clincaltrials.gov: NCT01018303] [DOI] [PubMed] [Google Scholar]
Thomas 1995 {published data only}
- Thomas PS, Bellamy M, Geddes D. Malabsorption of vitamin E in cystic fibrosis improved after ursodeoxycholic acid [letter]. Lancet 1995;346(8984):1230‐1. [CFGD Register: GN77; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winklhofer‐Roob 1992 {published data only}
- Winklhofer‐Roob BM, Shmerling DH, Schimek MG. Response of vitamin E deficient patients with cystic fibrosis (CF) to oral RRR‐alpha‐tocopherol or all‐rac‐alpha‐tocopheryl acetate [abstract]. Clinical Nutrition 1992;11 Suppl:67. [CFGD Register: GN48b] [Google Scholar]
Winklhofer‐Roob 1996 {published data only}
- Winklhofer Roob BM, van't Hof MA, Shmerling DH. Long‐term oral vitamin E supplementation in cystic fibrosis patients: RRR‐alpha‐tocopherol compared with all‐rac‐alpha‐tocopheryl acetate preparations. American Journal of Clinical Nutrition 1996;63(5):722‐8. [CFGD Register: GN48a; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wood 2003 {published data only}
- Wood LG, Fitzgerald DA, Lee AK, Garg ML. Improved antioxidant and fatty acid status of patients with cystic fibrosis after antioxidant supplementation is linked to improved lung function. American Journal of Clinical Nutrition 2003;77(1):150‐9. [CFGD Register: GN98] [DOI] [PubMed] [Google Scholar]
Additional references
Aaron 2004
- Aaron SD, Ramotar K, Ferris W, Vandemheen K, Saginur R, Tullis E, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2004;169:811‐5. [DOI] [PubMed] [Google Scholar]
Aparicio 2001
- Aparicio JM, Belanger‐Quintana A, Suarez L. Ataxia with isolated vitamin E deficiency: case report and review of the literature. Journal of Pediatric Gastroenterology and Nutrition 2001;33(2):206‐10. [DOI] [PubMed] [Google Scholar]
Bell 2002
- Bell SC, Shepherd RW. Optimising nutrition in cystic fibrosis. Journal of Cystic Fibrosis 2002;1(2):47‐50. [DOI: 10.1016/S1569-1993(02)00031-0] [DOI] [PubMed] [Google Scholar]
Biesalski 2009
- Biesalski HK. Vitamin E requirements in parenteral nutrition. Gastroenterology 2009;137(5):S92‐S104. [DOI: 10.1053/j.gastro.2009.07.073] [DOI] [PubMed] [Google Scholar]
Bines 2005
- Bines JE, Truby HD, Armstrong DS, Carzino R, Grimwood R. Vitamin A and E deficiency and lung disease in infants with cystic fibrosis. Journal of Paediatrics and Child Health 2005;41:663‐8. [DOI] [PubMed] [Google Scholar]
Brigelius‐Flohé 2009
- Brigelius‐Flohé R. Vitamin E: The Shrew Waiting to be Tamed. Free Radical Biology & Medicine 2009;46:543–54. [DOI] [PubMed] [Google Scholar]
Ciofu 2014
- Ciofu O, Lykkesfeldt J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD007020.pub3] [DOI] [PubMed] [Google Scholar]
Cynamon 1988
- Cynamon HA, Milov DE, Valenstein E, Wagner M. Effects of vitamin E deficiency on neurologic function in patients with cystic fibrosis. Pediatrics 1988;113:637‐9. [DOI] [PubMed] [Google Scholar]
Dodge 2006
- Dodge JA, Turck D. Cystic fibrosis: nutritional consequences and management. Best Practice & Research. Clinical Gastroenterology 2006;20(3):531‐46. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Farrell 2008
- Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. Journal of Pediatrics 2008;153(2):S4‐S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway S, Etherington C, Webb A. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goss 2004
- Goss CH, Rosenfeld M. Update on cystic fibrosis epidemiology. Current Opinion in Pulmonary Medicine 2004;10(6):510‐4. [DOI] [PubMed] [Google Scholar]
Hakim 2007
- Hakim F, Kerem E, Rivlin J, Bentur L, Stankiewicz H, Bdolach‐Abram T, et al. Vitamins A and E and pulmonary exacerbations in patients with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2007;45(3):347‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Higgins 2011c
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Kerem 2005
- Kerem E, Conway S, Elborn S, Heijerman H. Standards of care for patients with cystic fibrosis: a European consensus. Journal of Cystic Fibrosis 2005;4:7‐26. [DOI] [PubMed] [Google Scholar]
Koscik 2005
- Koscik RL, Lai HJ, Laxova A, Zaremba KM, Kosorok MR, Douglas JA, et al. Preventing early, prolonged vitamin E deficiency: an opportunity for better cognitive outcomes via early diagnosis through neonatal screening. Journal of Pediatrics 2005;147(3 Suppl):S51‐S56. [DOI] [PubMed] [Google Scholar]
Kozlowska 2008
- Kozlowska WJ, Bush A, Wade A, Aurora P, Carr SB, Castle RA, et al. Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2008;178(1):42‐9. [DOI] [PubMed] [Google Scholar]
Linnane 2008
- Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, et al. Lung function in infants with cystic fibrosis diagnosed by newborn screening. American Journal of Respiratory and Critical Care Medicine 2008;178(12):1238‐44. [DOI] [PubMed] [Google Scholar]
Mayo Clinic 2009
- Mayo Clinic. Vitamin E (oral route). www.mayoclinic.com/health/drug‐information/DR601967/FLUSHCACHE=0&UPDATEAPP=false (accessed 01 March 2011).
Peters 1996
- Peters SA, Kelly FJ. Vitamin E supplementation in cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 1996;22:341‐5. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sinaasappel 2002
- Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkampd G, Heijerman HG, et al. Nutrition in patients with cystic fibrosis: a European Consensus. Journal of Cystic Fibrosis 2002;1(2):51‐75. [PUBMED: 15463811] [DOI] [PubMed] [Google Scholar]
Suskind 2009
- Suskind DL. Nutritional Deficiencies During Normal Growth. Pediatric Clinics of North America 2009;56(5):1035‐53. [DOI] [PubMed] [Google Scholar]
Swann 1998
- Swann IL, Kendra JR. Anaemia, vitamin E deficiency and failure to thrive in an infant. Clinical and Laboratory Haematology 1998;20(1):61‐3. [DOI] [PubMed] [Google Scholar]
Taylor 2010
- Taylor C, Connolly S. Gastrointestinal disease and nutrition. In: Horsley A, Cunningham S, Innes JA editor(s). Cystic Fibrosis. Oxford: Oxford University Press, 2010:81‐93. [Google Scholar]
Thurnham 1986
- Thurnham DI, Davies JA, Crump BJ, Situnayake RD, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Annals of Clinical Biochemistry 1986;23(Pt 5):514‐20. [DOI] [PubMed] [Google Scholar]
Ueda 2009
- Ueda N, Suzuki Y, Rino Y, Takahashi T, Imada T, Takanashi Y, et al. Correlation between neurological dysfunction with vitamin E deficiency and gastrectomy. Journal of the Neurological Sciences 2009;287:216‐20. [DOI: 10.1016/j.jns.2009.07.020] [DOI] [PubMed] [Google Scholar]
UK Cystic Fibrosis Trust 2002
- UK Cystic Fibrosis Trust Nutrition Working Group. Nutritional Management of Cystic Fibrosis. UK Cystic Fibrosis Trust, April 2002. [ISBN 0‐9540536‐5‐6] [Google Scholar]
Welsh 2001
- Welsh M, Ramsey B, Accurso F, Cutting G. Cystic Fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D editor(s). The Metabolic and Molecular Basis of Inherited Disease. 8th Edition. New York: McGraw‐Hill, 2001:5121‐88. [Google Scholar]
Wilfond 1994
- Wilfond BS, Farrell PM, Laxova A, Mischler E. Severe hemolytic anemia associated with vitamin E deficiency in infants with cystic fibrosis. Implications for neonatal screening. Clinica Pediatrica 1994;33(1):2‐7. [DOI] [PubMed] [Google Scholar]
Winbauer 1999
- Winbauer AN, Pingree SS, Nuttall KL. Evaluating serum alpha‐tocopherol (vitamin E) in terms of a lipid ratio. Annals Clinical and Laboratory Science 1999;29(3):185‐91. [PubMed] [Google Scholar]
References to other published versions of this review
Okebukola 2011
- Okebukola PO, Kansra S, McCabe H. Vitamin E supplementation in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009422] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okebukola 2014
- Okebukola PO, Kansra S, Barrett J. Vitamin E supplementation in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD009422.pub2] [DOI] [PubMed] [Google Scholar]


