Abstract
According to a single study in dogs that was conducted in 1949, the diabetic effects of the β-cell toxin alloxan are dependent on age. The current study examined whether this age-dependence of alloxan is present in the clinically relevant Ossabaw miniature swine (Sus scrofa domestica) model of metabolic syndrome. Juvenile swine (n = 8; age, 4.3 ± 0.2 mo) and adult swine (n = 8; age, 7.4 ± 0.2 mo) received alloxan (average dosage, 140 mg/kg IV) and were placed on a hypercaloric, atherogenic diet for 6 mo. The metabolic syndrome profile was confirmed by measuring body weight, cholesterol, and triglycerides. Intravenous glucose tolerance testing was used to assess glucose clearance and peripheral plasma insulin levels. The β-cell mass was calculated by immunohistochemical staining of pancreatic tissue. Although juvenile and adult swine exhibited comparable severity of metabolic syndrome, adult swine developed impaired glucose clearance and elevated fasting blood glucose levels at 6 mo after alloxan administration on the atherogenic diet. Peripheral plasma insulin levels in juvenile and adult swine were comparable at all time points and lower than in nonalloxan-treated age-matched controls, which is reflected in the lower pancreatic β-cell mass of the 2 treated groups. However, compared with adult pigs, juvenile swine exhibited greater insulin response recovery (complete or partial restoration of peripheral insulin levels to reference values) at 6 mo after alloxan administration. Overall, these results indicate that youth can confer some protection against the diabetogenic effects of alloxan in swine, potentially due in part to the greater insulin response recovery of young pigs. This study supports previous research that the effects of alloxan are dependent on the developmental maturity of the animal.
Abbreviations: HOMA-IR, homeostasis model assessment–insulin resistance; IVGTT, intravenous glucose tolerance test
Since the diabetic effect of alloxan was discovered in 1943, this drug has been used in biomedical research to recapitulate the physiology of diabetes in various animal models.45 Alloxan is a glucose analog that is specific to glucose transporter 2, which is expressed almost exclusively by hepatocytes and pancreatic β cells.35,45 When glucose transporter 2 conveys alloxan into the cytosol of pancreatic β cells, the toxin reacts with intracellular glutathione in a redox reaction, resulting in the formation of reactive oxygen species that cause β-cell death.22,34,35 After administration of alloxan, extensive β-cell necrosis is often observed.45 Some studies show long-term recovery after alloxan-induced diabetes, which is suspected to be due to either multiplication of surviving β cells or the formation of new β cells from the duct epithelium or the exocrine portion of the pancreas.10,32,41,45
The efficacy of alloxan can be unpredictable and is dependent on several factors, including species,34 route of administration,22,32 speed of administration,32 and diet.45 Although it is widely accepted that alloxan and streptozotocin, another diabetogenic agent, affect younger animals to a lesser extent than older animals, this belief is based only on a few studies in mice.28,48 The only study to cite the age-dependence of alloxan in a large animal model was published almost 70 y ago in dogs.15,22,32,45 With the variation between species and even between subjects of the same species, the possible age-dependent effects of alloxan in large animal models need to be reexamined.
In this study, we used the clinically relevant Ossabaw miniature swine (Sus scrofa domestica) model of metabolic syndrome—the clustering of risk factors including obesity, dyslipidemia, glucose intolerance, insulin resistance, and hypertension—to determine whether age-conferred protection against the diabetic effects of alloxan is present in swine. This study will either contradict the alloxan–age dogma or provide additional evidence of the age-dependent effects of alloxan in large animal models.
Materials and Methods
Animals, housing, and diet.
All experimental procedures involving animals were approved by the IACUC at Indiana University School of Medicine, according to recommendations outlined by the National Research Council and the American Veterinary Medical Association Panel on Euthanasia.3,31 Swine were obtained from a closed, SPF breeding colony at Purdue University (West Lafayette, IN). This herd has historically tested negative for Brucella spp., pseudorabies, vesicular stomatitis virus serovars Indiana and New Jersey, Mycoplasma hyopneumoniae, porcine reproductive and respiratory syndrome virus, porcine parvovirus, swine influenza virus serotypes H1N1 and H3N2, antibodies to transmissible gastroenteritis virus, and Leptospira interrogans serovars (canicola, grippotyphosa, hardjo, icterohaemorrhagiae, pomona, and bratislava). All pigs were housed in pairs in 24-ft2 pens under a 12:12-h light:dark cycle. Temperature was maintained at 20 to 22 °C throughout the study, and humidity was not controlled. After the induction of diabetes, all swine were immediately placed for 6 mo on a hypercaloric atherogenic diet (1000 to 1350 g daily for both groups; KT324, Purina Test Diet, Richmond, IN) consisting of 43% of total caloric intake from fat, 16% from protein, and 41% from carbohydrates, similar to previous studies.19 Swine were fed once daily to promote gorging behavior to ensure that the daily allotment of food was consumed completely. Feed intake and blood glucose were measured daily, and body weight was monitored weekly. Water was provided without restriction.
Central venous line placement.
Swine were restrained in a sling and anesthetized with 5% isoflurane in 100% O2 administered by mask. The unconscious pig was placed in the supine position, and a percutaneous needle with a syringe attached was inserted into the angle formed by the sternum and clavicle. Negative pressure was maintained in the syringe, and when it began to fill with dark-red venous blood, the needle was held in place and the syringe removed. A guidewire was fed through the percutaneous needle, at which point the needle was removed. A central venous catheter was fed over the guidewire, which was then removed. The catheter was capped and secured to the animal by using nonabsorbable suture. The pig was returned to the sternal position and isoflurane was discontinued, allowing the animal to regain consciousness under observation. The central line catheter was flushed twice each week, to ensure its patency.
Induction of diabetes.
To induce diabetes, alloxan (Sigma Chemical, St Louis, MO), a pancreatic β-cell toxin, was administered rapidly intravenously to male and female Ossabaw miniature swine (age, 4 to 8 mo). At time of alloxan administration, swine were placed into 1 of 2 age groups: juvenile (age, younger than 6 mo; n = 8) and sexually mature adult52 (age, 6 mo or older; n = 8). Briefly, alloxan was dissolved in 14 mL of 1 M NaOH and 20 mL of 0.9% NaCl, for a final volume of 34 mL and a pH of 7.4. The alloxan solution was delivered through a 0.20-μm sterile filter into a central venous line in the jugular vein at an average dose of 140 mg/kg (range, 125 to 175 mg/kg). All 8 adult swine and 2 juvenile swine each received only one dose, whereas the remaining 6 juvenile swine were given 2 doses over the course of 1 wk, due to lack of hyperglycemic response to the first dose. The average first dose was 140 mg/kg (range, 75 to 175 mg/kg), and the average second dose was 120 mg/kg (range, 125 to 150 mg/kg). Different doses were used due to interindividual variability in the response to alloxan. To protect against possible renal toxicity, pigs were given 250 mL of 0.9% NaCl through intravenous drip prior to and after alloxan dosing. The pigs were fed free-choice and received 24 h of critical care after diabetes induction, to monitor for hypoglycemic shock. Swine received daily insulin glargine (Sanofi, Bridgewater, NJ) through subcutaneous injection to the flank, according to an algorithm previously published by our lab4,8 to maintain glycemic control below 300 mg/dL, a clinically relevant hyperglycemic level.40 The insulin dose for all pigs was between 0.1 and 0.6 U/kg and was individually adjusted according to daily blood glucose measurements, weekly weight measurements, and the presence or absence of lethargic behavior.4 The diabetic disease state was allowed to stabilize for 1 wk after alloxan administration, at which point all swine were placed on the hypercaloric atherogenic diet for 6 mo. Nonalloxanized age-matched controls on the same hypercaloric atherogenic diet were used in some analyses.
Metabolic phenotyping.
Blood for analysis (Antech Diagnostics, Fishers, IN) was collected 1 wk before alloxan dosing and 1 wk and 6 mo afterward. Homeostasis model assessment-insulin resistance (HOMA-IR) is a calculation that takes into account fasting glucose and insulin concentrations to assess insulin resistance.55 Increased HOMA-IR values indicate greater insulin resistance.26 HOMA-IR was calculated by using the following equation:
Blood pressure measurement.
From the beginning of the study, pigs were acclimated to a low-stress sling42,43 for conducting intravenous glucose tolerance testing (IVGTT) and blood pressure measurement. Blood pressures were measured by using a tail-cuff sphygmomanometer.42
IVGTT.
Insulin treatments were suspended 2 d prior to testing to determine endogenous glucose regulation. To assess pancreatic β-cell response to glucose, conscious swine that had been fasted overnight received 50% glucose solution intravenously (dose, 1 g glucose/kg body weight) through the central venous line. To obtain fasting glucose concentration, pigs were placed in a low-stress restraint sling, and blood samples (3 mL) were taken at 10, 5, and 0 min before glucose injection and then at 5, 10, 20, 30, 40, 50, and 60 min afterward. Blood glucose values were monitored (Advantage glucose monitor, Accu-Chek, Roche, Indianapolis, IN), and plasma insulin values were obtained through assays done at the Diabetes Research Core (School of Medicine, Indiana University).
Euthanasia.
After an overnight fast, swine were anesthetized through intramuscular injection of 2.2 mg/kg xylazine and 5.5 mg/kg tiletamine–zolazepam (Fort Dodge Animal Health, Fort Dodge, IA). Swine were intubated, and anesthesia was maintained with 2% to 4% isoflurane in 100% O2. The isoflurane level was adjusted to maintain anesthesia with stable hemodynamics. Pigs were euthanized through cardiectomy.
Immunohistochemistry.
Sections from the tail of the pancreas were placed in 10% phosphate-buffered formalin for 24 to 48 h and then embedded in paraffin. Tissue sections were stained (Department of Pathology, School of Medicine, Indiana University) by using guinea pig antiinsulin polyclonal antibody (Agilent, Santa Clara, CA) as a marker for β cells. Images were captured by using a photomicroscope (model DM 3000, Leica, Wetzlar, Germany) and analyzed with ImageJ software.50. Relative β-cell mass was quantified by calculating the percentage of 3,3′ diaminobenzidine–stained nuclear area relative to the total nuclear area by using the ImmunoRatio ImageJ plugin.
Statistics.
Statistical analysis was performed by using Prism 5.0 (GraphPad San Diego, CA). The Student t test, nonparametric Kruskal–Wallis one-way ANOVA with Dunn posthoc analysis, or 2-way ANOVA with Bonferroni posthoc analysis was performed. Data are represented as mean ± SEM, and nonparametric data are represented by dot plots expressing the median and interquartile range. A P value less than 0.05 was considered statistically significant.
Results
Insulin resistance in swine.
Because juvenile and adult swine had comparable metabolic parameters before alloxan administration, they were pooled into the alloxan pretreatment group. Although the juvenile swine were significantly (P < 0.001) younger than the adult swine, both groups exhibited comparable weights and BMI measurements after 6 mo on an atherogenic diet. Compared with levels before alloxan administration, serum triglycerides in adult pigs were elevated both 1 wk and 6 mo after alloxan. Total cholesterol was elevated in all groups after 6 mo on an atherogenic diet. HOMA-IR calculations showed that the adult swine at 6 mo after alloxan treatment were more insulin resistant than adults before alloxan administration and compared with juveniles at 6 mo after alloxan dosing. To assess kidney function, creatinine and BUN levels were measured. Creatinine levels were comparable between groups, with untreated controls exhibiting slightly elevated (P < 0.05) levels compared with juvenile swine at 1 wk after alloxan, whereas adult swine at 1 wk after alloxan administration exhibited elevated BUN levels compared with before alloxan (P < 0.001) and juvenile levels (P < 0.01) that normalized by 6 mo after alloxan dosing. To measure liver function, serum levels of AST and ALT were measured and compared with reference ranges, and the AST:ALT ratio was calculated. At 6 mo after alloxanization, both juvenile and adult swine exhibited elevated AST and ALT levels, indicative of liver dysfunction.14,49 In addition, both groups had increased AST:ALT ratios at 6 mo after alloxan treatment, but these were equivalent to the AST:ALT ratio of nonalloxanized high-fat–fed control swine. Furthermore, systolic blood pressure and mean arterial pressure were greater in adult swine at 6 mo after alloxan administration compared with before treatment (Table 1).
Table 1.
Metabolic profiles show hyperlipidemia and insulin resistance in swine 6 mo after consumption of an atherogenic diet
| 1 wk after alloxan treatment | 6 mo after alloxan treatment | |||||
| Before alloxan | Juvenile | Adult | Juvenile | Adult | Nonalloxanized swine fed an atherogenic diet | |
| Age (mo) | — | 4.3 ± 0.2 | 7.4 ± 0.2b | 11.0 ± 0.2b,c | 14.0 ± 0.1b,c,d | 12.6 ± 1.1b,c |
| Sex (M/F) | 8/8 | 3/5 | 5/3 | 3/5 | 5/3 | 4/4 |
| Body weight (kg) | 24.9 ± 1.6 | 22.2 ± 1.0 | 29.7 ± 1.0 | 67.8 ± 2.6a,b,c | 73.5 ± 2.8a,b,c | 91.7 ± 2.6a,b,c,d |
| BMI (kg/m2) | — | — | — | 58.6 ± 2.4 | 59.4 ± 3.2 | 69.4 ± 1.3d |
| Serum TG (mg/dL) | 33 ± 16 | 66 ± 15 | 82 ± 16a | 41 ± 7 | 211 ± 87a,d | 40 ± 5e |
| Total cholesterol (mg/dL) | 91 ± 16 | 93 ± 5 | 110 ± 7 | 500 ± 114a,b,c | 988 ± 138a,b,c,d | 421 ± 59a,b,c,e |
| HOMA-IR | 2.9 ± 0.4 | 3.3 ± 0.3 | 4.6 ± 0.3 | 2.3 ± 0.3 | 5.4 ± 1.0a,d | 2.7 ± 0.2e |
| Creatinine (mg/dL) | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1b |
| BUN (mg/dL) | 14.7 ± 0.8 | 16.5 ± 3.1 | 27.4 ± 3.4a,b | 14.9 ± 0.9c | 16.9 ± 1.0c | 11.9 ± 1.5c |
| AST (IU/L) | 34 ± 2 | 35 ± 5 | 37 ± 3 | 98 ± 19a,b,c | 85 ± 11a,b,c | 47 ± 7d,e |
| ALT (IU/L) | 54 ± 5 | 35 ± 4 | 54 ± 8 | 62 ± 6b | 56 ± 6 | 34 ± 3a,d,e |
| AST:ALT | 0.66 ± 0.04 | 0.99 ± 0.06 | 0.74 ± 0.07 | 1.68 ± 0.43a | 1.73 ± 0.36a | 1.48 ± 0.20a |
| Systolic BP (mm Hg) | 131 ± 6 | — | — | 142 ± 10 | 158 ± 6 | 138 ± 9 |
| Diastolic BP (mm Hg) | 74 ± 5 | — | — | 83 ± 6 | 88 ± 3a | 90 ± 7 |
| MAP (mm Hg) | 93 ± 5 | — | — | 103 ± 6 | 112 ± 3a | 106 ± 7 |
BP, blood pressure; Chol, cholesterol; F, female; HOMA-IR, homeostasis model assessment-insulin resistance; M, male; MAP, mean arterial pressure; TG, triglycerides
Data are given as mean ± SEM. Values before alloxan represent pooled juvenile and adult swine.
P < 0.05 compared with prealloxan swine
P < 0.05 compared with juvenile swine 1 wk after alloxan
P < 0.05 compared with adult swine 1 wk after alloxan
P < 0.05 compared with juvenile swine 6 mo after alloxan
P < 0.05 compared with adult swine 6 mo after alloxan
Glucose clearance after alloxan treatment in adult compared with juvenile swine.
Blood glucose at 60 min after intravenous administration of a bolus of glucose was assessed at 1 wk (Figure 1 A through C) and 6 mo (Figure 1 D through F) after alloxan administration. At 1 wk after alloxan treatment, IVGTT showed that adult swine had significantly (P < 0.05) higher blood glucose measurements at all time points when compared with both juvenile swine and all swine before alloxan administration (Figure 1 A). This association is reflected in the higher baseline fasting glucose level (Figure 1 B) and greater glucose AUC (Figure 1 C). This hyperglycemia persisted at 6 mo after alloxan administration, with adult swine exhibiting significantly (P < 0.05) higher glucose concentrations at most time points during glucose challenge compared with juvenile pigs and adult swine before alloxan treatment (Figure 1 D), higher (P < 0.05) baseline fasting glucose level (Figure 1 E), and a greater (P < 0.05) glucose AUC (Figure 1 F). Juvenile swine had a significantly (P < 0.05) greater glucose AUC than nonalloxanized swine at 6 mo after alloxan administration (Figure 1 F), thus demonstrating a progression of glucose intolerance in juvenile pigs.
Figure 1.
Adult swine exhibited prolonged impaired glucose clearance after intravenous glucose tolerance testing. Blood glucose levels were tested for 60 min after a bolus of glucose (1 mg/kg body weight). (A) At 1 wk alloxan administration, adult swine (n = 8) showed a significant deficit in glucose clearance at all time points compared with pretreatment controls (n = 16) and juvenile swine (n = 8). (B) The fasting blood glucose level was elevated in juvenile animals at baseline and in the adult group at 1 wk after alloxan administration. (C) AUC analysis at 1 wk after alloxan administration shows impaired glucose clearance in adult alloxanized pigs compared with pretreatment controls and juvenile swine. (D) At 6 mo after alloxan administration, adult swine continued to exhibit impaired glucose clearance compared with nonalloxanized swine and juvenile swine at most time points. (E) The baseline fasting glucose concentration was elevated in adult swine at 6 mo after alloxan administration. (F) At 6 mo after alloxan administration, AUC analysis using the fasting blood glucose concentration as a baseline for each group shows impaired glucose clearance in juvenile alloxanized pigs compared with untreated controls; this impaired glucose clearance is exacerbated in adult alloxanized animals. *, P < 0.05.
Relationship between age and fasting blood glucose level in alloxanized swine.
The fasting blood glucose level of swine at 1 wk after the final alloxan dose was plotted against the age at which each pig was alloxanized (Figure 2). This analysis revealed a strong positive correlation between blood glucose level and age at the time of alloxan administration, even for comparable doses of alloxan.
Figure 2.
Correlation of fasting blood glucose level with age at alloxan administration. The fasting blood glucose concentration at 1 wk after alloxan administration is significantly positively correlated to the age of swine at time of alloxan administration (black). The dose of alloxan, however, is not correlated to age (red) and is not significantly different between groups. In animals given 2 doses of alloxan, the average of the doses was plotted.
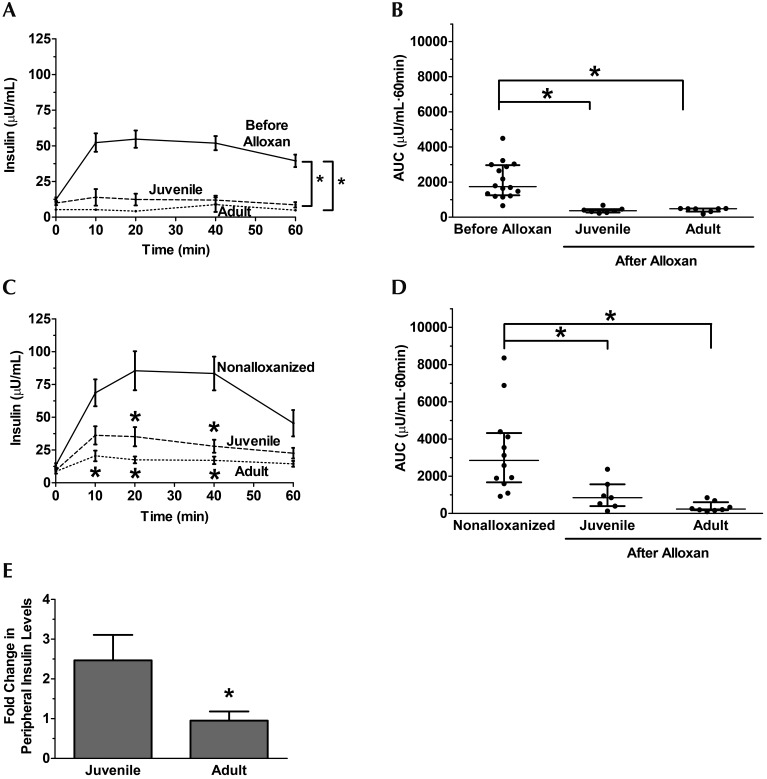
Hypoinsulinemia in swine.
Serum insulin levels were measured during IVGTT. At 1 wk after alloxan administration, both juvenile and adult swine exhibited lower glucose levels than before alloxan administration at all time points except t = 0 (Figure 3 A). This finding is reflected in the insulin AUC analysis (Figure 3 B). This hypoinsulinemia persists at 6 mo after alloxan administration, with juvenile and adult swine exhibiting lower insulin levels at several time points when compared with untreated, age-matched animals (Figure 3 C). Again, juvenile and adult alloxanized swine had smaller insulin AUC than nonalloxanized swine (Figure 3 D). An outlier in the juvenile group whose serum insulin concentration was 5fold greater than the standard deviation was removed from this analysis.
Figure 3.
Hypoinsulinemia in juvenile and adult swine at 6 mo after alloxanization. (A) Peripheral insulin during intravenous glucose tolerance testing was significantly lower in juvenile (n = 7) and adult (n = 8) swine compared with prealloxan controls (n = 16) at 1 wk after alloxan administration. (B) AUC analysis reveals comparable hypoinsulinemia in both the juvenile and adult alloxan-treated swine at 1 wk after alloxan administration. (C) At 6 mo after alloxan administration, juvenile and adult swine still exhibited hypoinsulinemia at several time points compared with nonalloxanized, age-matched controls (n = 8). (D) AUC analysis revealed comparable hypoinsulinemia in both juvenile and adult alloxanized swine 6 mo after alloxan administration. (E) The fold change in peripheral insulin from 1 wk to 6 mo after alloxan was greater in juvenile than adult swine. *, P < 0.05 compared with prealloxan or nonalloxanized controls.
Recovery of insulin response.
Insulin AUC (Figures 3 B and 3 D) were used to determine potential recovery from alloxan treatment. Juvenile swine exhibited an approximately 2.5fold increase in peripheral insulin levels from 1 wk to 6 mo after alloxan dosing (Figure 3 E). In contrast, peripheral insulin levels in adult swine remained constant between 1 wk and 6 mo after alloxan administration (Figure 3 E).
Pancreatic β-cell area.
Immunohistochemical analysis of pancreatic tissue from juvenile (Figure 4 A) and adult (Figure 4 B) swine showed that pancreatic β-cell area at 6 mo after alloxan administration is comparable between juvenile and adult swine (Figure 4 C). This value is lower than for nonalloxanized, high-fat–fed swine, whose pancreatic β-cell mass was calculated previously to be approximately 7.5%.4
Figure 4.

Change in β-cell mass in young and adult alloxanized swine. Immunohistochemical staining of insulin in pancreas from (A) juvenile and (B) adult swine revealed that, at 6 mo after alloxan administration, both juvenile and adult swine had a lower β-cell mass than nonalloxanized controls (in which the insulin-stained area is approximately 7.5%4). However, (C) β-cell mass did not differ significantly between alloxan-treated juvenile and adult swine.
Discussion
In this study, we show that alloxan-treated juvenile swine had a blunted response to the drug and did not have the severe diabetic effects that developed in adult swine given alloxan. Swine that received alloxan regardless of age exhibited decreased pancreatic β cell mass (Figure 4) and reduced peripheral plasma insulin after a bolus of glucose (Figure 3). However, only the juvenile swine exhibited recovery of the insulin response, which we defined as the recovery or partial recovery of peripheral insulin levels, at 6 mo after alloxan treatment (Figure 3 E). Juvenile and adult alloxanized swine developed comparable risk factors for metabolic syndrome, with adult swine exhibiting an elevated HOMA-IR (Table 1). Although alloxanized swine exhibited elevated ALT and AST levels, they did not exhibit critical levels of renal toxicity or hepatoxicity, as indicated by their BUN, creatinine, and AST and ALT levels (Table 1), similar to previous studies.19,42 In addition, fasting blood glucose was higher and significantly correlated with age of swine at 1 wk after alloxanization, even though the dosages of alloxan were comparable (Figure 2), and glucose clearance was more impaired in the adult swine as well (Figure 1). Taken together, these results show that fasting glucose and glucose clearance is more impaired in Ossabaw miniature swine that receive alloxan as adults than as juveniles and that juvenile swine can recover (at least partially) from alloxan treatment.
Pancreatic β cell mass and peripheral plasma insulin were not statistically different between the juvenile and adult alloxanized swine, even though adult swine exhibited greater hyperglycemia, both fasting and after a glucose challenge. Superficially, this result seems to be a paradox, but it can be explained partly by the increased peripheral resistance, as calculated by the HOMA-IR, in the adult swine.27,38 Under normal physiologic conditions, insulin resistance is counteracted by increased insulin secretion through β-cell compensation.1 However, the decreased β-cell mass due to alloxan treatment of adult swine was accompanied by a decreased functional compensation to increasing insulin secretion. Consequently, comparable levels of circulating insulin would have less of an effect on glucose clearance in the insulin-resistant adult swine as compared with juvenile swine.
Hyperglycemia-induced secondary insulin resistance is a well-documented phenomenon in type 1 diabetic humans36,58 and in animal models, including swine.18,30,37,42,44,46,47 In a previous study, our lab determined that the dramatic decrease in insulin sensitivity observed in alloxan-induced diabetic Yucatan swine is almost solely due to hyperglycemia.42 This hyperglycemia-induced insulin resistance is at least partially due to the downregulation of glucose transfer type 4 in skeletal muscle by high circulating glucose levels.37 The initial hyperglycemic event after alloxan administration causes the adult swine to become more insulin-resistant and thus partially explains why glucose clearance at 6 mo after alloxan treatment was more impaired in adult pigs than juvenile swine (Figure 1 D–F), even though peripheral insulin levels (Figure 3 C and D) and pancreatic β-cell mass (Figure 4) were comparable between these 2 groups. The initial differential effects of alloxan in juvenile compared with adult swine contribute to the greater hyperglycemia exhibited in adult swine.
The liver plays a vital role in glucose metabolism and may have contributed to the differential effects seen in the alloxanized juvenile pigs compared with treated adult swine. Insulin secreted from the pancreas travels through the portal vein to the liver, which binds 60% of the insulin delivered.57 The remaining insulin then goes into the peripheral circulation, where it binds to insulin-sensitive tissues, such as skeletal muscle and adipose. However, hepatic insulin binding doubles in streptozotocin-induced diabetes.16 The inverse relationship between circulating insulin levels and hepatic insulin binding16 may affect insulin circulation in the alloxanized pigs. Perhaps more insulin was secreted in juvenile pigs, but much of the insulin was bound by the liver, thereby resulting in greater insulin-mediated glucose uptake by the liver and lower fasting glucose compared with these parameters in adult pigs. Portal vein insulin levels or c-peptide levels are needed to provide direct evidence for this possibility.
Several studies have shown that creatinine increases progressively with time in alloxanized animals, signaling kidney dysfunction.2,54,56 However, at 6 mo after alloxan treatment, creatinine and BUN levels in our treated adult and juvenile pigs were comparable to those before treatment and in the nonalloxanized controls, and the AST:ALT ratio was comparable to that of nonalloxanized controls. These results indicate little kidney damage and no additional liver damage due to the drug.
Spontaneous recovery from streptozotocin- and alloxan-induced diabetes has been well-documented11,32,41,45 and occurs through several potential mechanisms. A recent study in mice showed that islet δ cells can be converted into β cells, an ability that is lost in adulthood.11 Other hypotheses involve the proliferation of surviving β cells,10,11,32,45 reprogramming of islet α cells into β cells,11,13 and conversion of duct epithelial cells to β cells.10,32,45 Our study is the first to show recovery from alloxan-induced diabetes in a large animal model. However, although the present study revealed that peripheral insulin levels can recover in juvenile swine, we did not delve into the mechanisms that drive this recovery. Future studies should focus on nonβ islet cell reprogramming in swine of different ages.
Swine do not reach developmental maturity until 6 mo of age.7,25,39,52 In the current study, although the 2 experimental groups were only about 3 mo different in age, developmentally this span was the difference between juvenile and adulthood.25,39,52 In addition to the blunted acute (1 wk) β-cell destruction by alloxan in juvenile pigs, they showed greater relative recovery of glucose tolerance and peripheral insulin responses at 6 mo after alloxan than did the adult pigs. This outcome speaks to the immense sensitivity of alloxan to animal age and the more robust resistance of juvenile pig β cells to alloxan.
The juvenile group contained 3 castrated males and 5 females, whereas the adult group contained 5 castrated males and 3 females (Table 1). The nonalloxanized control group contained 4 of each sex (Table 1). These differences in the numbers of animals of each sex comprising each group could potentially affect metabolic parameters, but the differences were not statistically significant. The effect of sex on weight gain varies among studies, depending on the diet and breed of pig.12,17 In Large White × Landrace pigs, intact boars were larger than females, whereas the weights of castrated males were comparable to those of females.17 Female swine reliably exhibit elevated serum cholesterol,5,6,12,33 but the effects of sex on triglyceride levels and insulin resistance are less predictable.6,12,33 Of note, the BUN, creatinine, ALT, and AST are comparable between male and female swine.6,33 In our study, even though the adult swine group contained more males than females, all of these swine exhibited greatly elevated serum triglycerides, cholesterol, and HOMA-IR (Table 1). If sex did affect the metabolic profile, then correcting for this effect would only make our results more robust.
Furthermore, the vasoprotective effects of estrogen in the circulation have been well documented,21,29,53 as have the harmful effects of testosterone.23,53 However, the sex-associated differences in our study might not be that simplistic, given that female Yucatan swine have similar serum testosterone levels as castrated males9 and that intact female dogs have comparable estradiol concentrations as neutered males.24 Although we did not measure serum estrogen levels in the current study, the literature supports the hypothesis that females and castrated males from various species have a similar sex hormone profiles.
The current study is the first since 1949 to investigate whether the effects of alloxan are dependent on age in large animals. Because the effects of alloxan can vary widely, different species at different stages of maturity need to be tested. Alloxan is widely used to elicit diabetes in animal models and characterizing responses to alloxan will enable researchers to predict whether the drug will have the desired effects in their particular model. Studies examining how the diabetic effects of alloxan are dependent on age in different species are of the utmost importance, given that different animal species and even different breeds within a species react differently. For example, pigs require a higher dose of alloxan to develop a similar diabetic state as in rats, dogs, and rabbits.34,51 Clearly, alloxan has very different effects based on the physiology of the species and breed. Therefore, a single study in one species is not adequate to make a sweeping generalization regarding the age of an animal on a drug's effectiveness. The field of diabetic animal models needs additional studies both to verify Creutzfeldt's 1949 findings and to determine whether this effect is typical among commonly used animal models of diabetes.
In the current study, we show that alloxan more efficaciously elicited acute and sustained hyperglycemia when administered to adult swine compared with juvenile swine. This result supports the 70-y-old dogma15 that the diabetic effects of alloxan are dependent on age, at least in dogs and minipigs. Future studies need to examine other laboratory animal species to determine the ubiquity of this age dependence. These studies should focus on several ages to create a dose–response curve for frequently used animal models. Our current study provides an important step in clarifying the effects of a widely used yet relatively unpredictable drug, thereby enabling researchers to better choose the animal species and stage of maturation that are best for their study design. This information can save researchers time and money and may reduce the number of research animals used.
Acknowledgments
We thank James P Byrd, Jane Hooker, and Benjamin Stivers for their technical assistance; the Indiana University School of Medicine Histology Core (Dr Keith Condon) for processing the histology and the use of their equipment; and Dr Robert Considine's laboratory for performing the insulin assays (P30 DK097512). The authors have no competing interests.
References
- 1.Ahrén B, Pacini G. 2005. Islet adaptation to insulin resistance: mechanisms and implications for intervention. Diabetes Obes Metab 7:2–8. 10.1111/j.1463-1326.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 2.Altura BM, Lum G, Turlapaty PD, Altura BT. 1981. Sequential changes in serum glucose, triglycerides, and cholesterol in aging of normal and alloxan-diabetic rats. Experientia 37:224–226. 10.1007/BF01991622. [DOI] [PubMed] [Google Scholar]
- 3.AVMA Panel on Euthanasia. American Veterinary Medical Association. 2001. 2000 Report of the AVMA panel on euthanasia. J Am Vet Med Assoc 218:669–696. Erratum in: J Am Vet Med Assoc 2001 218:1884. [DOI] [PubMed] [Google Scholar]
- 4.Badin JK, Kole A, Stivers B, Progar V, Pareddy A, Alloosh M, Sturek M. 2018. Diabetes exacerbates coronary atherosclerosis and calcification in Ossabaw miniature swine with metabolic syndrome. J Transl Med 16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin E, Khan MA, Henderson GR, Kliman PG. 1985. Influence of age and sex on composition and lipid fluidity in miniature swine plasma lipoproteins. Atherosclerosis 54:187–203. 10.1016/0021-9150(85)90178-9. [DOI] [PubMed] [Google Scholar]
- 6.Bollen PJ, Madsen LW, Meyer O, Ritskes-Hoitinga J. 2005. Growth differences of male and female Gottingen minipigs during ad libitum feeding: a pilot study. Lab Anim 39:80–93. 10.1258/0023677052886565. [DOI] [PubMed] [Google Scholar]
- 7.Bottino R, Balamurugan AN, Smetanka C, Bertera S, He J, Rood PP, Cooper DK, Trucco M. 2007. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation 14:74–82. 10.1111/j.1399-3089.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 8.Boullion RD, Mokelke EA, Wamhoff BR, Otis CR, Wenzel J, Dixon JL, Sturek M. 2003. Porcine model of diabetic dyslipidemia: omnsulin and feed algorithms for mimicking diabetes in humans. Comp Med 53:42–52. [PubMed] [Google Scholar]
- 9.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. 2004. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol (1985) 96:2240–2248. 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bunnag SC, Warner NE, Bunnag S. 1967. Effect of alloxan on the mouse pancreas during and after recovery from diabetes. Diabetes 16:83–89. 10.2337/diab.16.2.83. [DOI] [PubMed] [Google Scholar]
- 11.Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. 2014. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514:503–507. 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christoffersen BO, Grand N, Golozoubova V, Svendsen O, Raun K. 2007. Gender-associated differences in metabolic syndrome-related parameters in gottingen minipigs. Comp Med 57:493–504. [PubMed] [Google Scholar]
- 13.Chung CH, Levine F. 2010. Adult pancreatic α-cells: a new source of cells for β-cell regeneration. Rev Diabet Stud 7:124–131. 10.1900/RDS.2010.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JM. 2006. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40 Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- 15.Creutzfeldt W. 1949. [Zur Histophysiologie des Inselapparates.] Z Zellforsch Mikrosk Anat 34:280–336. 10.1007/BF00388385. [Article in German]. [DOI] [Google Scholar]
- 16.Davidson MB, Kaplan SA. 1977. Increased insulin binding by hepatic plasma membranes from diabetic rats: normalization by insulin therapy. J Clin Invest 59:22–30. 10.1172/JCI108618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies AS, Pearson G, Carr JR. 1980. The carcass composition of male, castrated male and female pigs resulting from 2 levels of feeding J Agric Sci 95: 251–259. 10.1017/S0021859600039277 [DOI] [Google Scholar]
- 18.Dimitrakoudis D, Ramlal T, Rastogi S, Vranic M, Klip A. 1992. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J 284:341–348. 10.1042/bj2840341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dineen SL, McKenney ML, Bell LN, Fullenkamp AM, Schultz KA, Alloosh M, Chalasani N, Sturek M. 2015. Metabolic syndrome abolishes glucagon-like peptide 1 receptor agonist stimulation of SERCA in coronary smooth muscle. Diabetes 64:3321–3327. 10.2337/db14-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. 1999. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19:2981–2992. 10.1161/01.ATV.19.12.2981. [DOI] [PubMed] [Google Scholar]
- 21.Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. 2001. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension 37:640–644. 10.1161/01.HYP.37.2.640. [DOI] [PubMed] [Google Scholar]
- 22.Etuk E. 2010. Animals models for studying diabetes mellitus. Agr Biol J N Am 1:130–134. [Google Scholar]
- 23.Farhat MY, Wolfe R, Vargas R, Foegh ML, Ramwell PW. 1995. Effect of testosterone treatment on vasoconstrictor response of left anterior descending coronary artery in male and female pigs. J Cardiovasc Pharmacol 25:495–500. 10.1097/00005344-199503000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Frank LA, Rohrbach BW, Bailey EM, West JR, Oliver JW. 2003. Steroid hormone concentration profiles in healthy intact and neutered dogs before and after cosyntropin administration. Domest Anim Endocrinol 24:43–57. 10.1016/S0739-7240(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 25.Gad SC, Dincer Z, Svendsen O, Skaanild MT. 2015. The minipig, p 731–772. In: Gad SC, editor. Animal models in toxicology. Boca Raton (FL): CRC Press. [Google Scholar]
- 26.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, Quintela AG. 2013. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 13:1–10. 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. 2015. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 19:160–164. 10.4103/2230-8210.146874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammarström L, Hellman B, Ullberg S. 1967. On the accumulation of alloxan in the pancreatic beta-cells. Diabetologia 3:340–344. 10.1007/BF00429866. [DOI] [PubMed] [Google Scholar]
- 29.Han SZ, Karaki H, Ouchi Y, Akishita M, Orimo H. 1995. 17β-estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation 91:2619–2626. 10.1161/01.CIR.91.10.2619. [DOI] [PubMed] [Google Scholar]
- 30.Hansen BF, Hansen SA, Ploug T, Bak JF, Richter EA. 1992. Effects of glucose and insulin on development of impaired insulin action in muscle. Am J Physiol 262:E440–E446. [DOI] [PubMed] [Google Scholar]
- 31.Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 32.Jain DK, Arya RK. 2011. Anomalies in alloxan-induced diabetic model: it is better to standardize it first. Indian J Pharmacol 43:91–91. 10.4103/0253-7613.75684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi H, Yamada T, Miura N, Noguchi M, Izumi H, Miyoshi N, Tanimoto A. 2013. Sex differences of serum lipid profile in novel microminipigs. In Vivo 27:617–621. [PubMed] [Google Scholar]
- 34.King AJ. 2012. The use of animal models in diabetes research. Br J Pharmacol 166:877–894. 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenzen S. 2008. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 51:216–226. 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 36.Maggs DG, Jacob R, Rife F, Caprio S, Tamborlane WV, Sherwin RS. 1997. Counterregulation in peripheral tissues. Effect of systemic hypoglycemia on levels of substrates and catecholamines in human skeletal muscle and adipose tissue. Diabetes 46:70–76. 10.2337/diab.46.1.70. [DOI] [PubMed] [Google Scholar]
- 37.Mathoo JMR, Shi ZQ, Klip A, Vranic M. 1999. Opposite effects of acute hypoglycemia and acute hyperglycemia on glucose transport and glucose transporters in perfused rat skeletal muscle. Diabetes 48:1281–1288. 10.2337/diabetes.48.6.1281. [DOI] [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.McAnulty PA, Barrow P, Marsden E. 2012. Reproductive system including studies in juvenile minipigs, p 263–276. In: McAnulty PA, Dayan AD, Ganderup N-C, Hastings KL, editors. The minipig in biomedical research. Boca Raton (FL): CRC Press. [Google Scholar]
- 40.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group. 2008. Translating the A1C assay into estimated average glucose values. Diabetes Care 31:1473–1478. 10.2337/dc08-0545. Erratum: Diabetes Care 32: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'hea EK, Allee GL, Leveille GA, Baker DH. 1971. Observations on the alloxan-diabetic pig. Int J Biochem 2:177–181. 10.1016/0020-711X(71)90209-6. [DOI] [Google Scholar]
- 42.Otis CR, Wamhoff BR, Sturek M. 2003. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53:53–64. [PubMed] [Google Scholar]
- 43.Panepinto LM, Phillips RW, Westmoreland NW, Cleek JL. 1982. Influence of genetics and diet on the development of diabetes in Yucatan miniature swine. J Nutr 112:2307–2313. 10.1093/jn/112.12.2307. [DOI] [PubMed] [Google Scholar]
- 44.Reaven GM, Sageman WS, Swenson RS. 1977. Development of insulin resistance in normal dogs following alloxan-induced insulin deficiency. Diabetologia 13:459–462. 10.1007/BF01234496. [DOI] [PubMed] [Google Scholar]
- 45.Rerup CC. 1970. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22:485–518. [PubMed] [Google Scholar]
- 46.Richardson JM, Balon TW, Treadway JL, Pessin JE. 1991. Differential regulation of glucose transporter activity and expression in red and white skeletal muscle. J Biol Chem 266:12690–12694. [PubMed] [Google Scholar]
- 47.Richter EA, Hansen BF, Hansen SA. 1988. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J 252:733–737. 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riley WJ, McConnell TJ, Maclaren NK, McLaughlin JV, Taylor G. 1981. The diabetogenic effects of streptozotocin in mice are prolonged and inversely related to age. Diabetes 30:718–723. 10.2337/diab.30.9.718. [DOI] [PubMed] [Google Scholar]
- 49.Rinella ME. 2015. Nonalcoholic fatty liver disease: a systematic review. JAMA 313:2263–2273. 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 50.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan K, Ramarao P. 2007. Animal models in type 2 diabetes research: an overview. Indian J Med Res 125:451–472. [PubMed] [Google Scholar]
- 52.Sturek M, Tune JD, Alloosh M. 2015. Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment, p 451–465. In: Swindle MM. editor. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. Boca Raton (FL): CRC Press. [Google Scholar]
- 53.Teoh H, Quan A, Leung SWS, Man RYK. 2000. Differential effects of 17β-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol 129:1301–1308. 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turlapaty PDMV, Lum G, Altura BM. 1980. Vascular responsiveness and serum biochemical parameters in alloxan diabetes mellitus. Am J Physiol 239:E412–E421. 10.1152/ajpendo.1980.239.6.E412. [DOI] [PubMed] [Google Scholar]
- 55.Wallace TM, Levy JC, Matthews DR. 2004. Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495. 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Wan R, Mo Y, Zhang Q, Sherwood LC, Chien S. 2010. Creating a long-term diabetic rabbit model. Exp Diabetes Res 2010:1–10. 10.1155/2010/289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilcox G. 2005. Insulin and insulin resistance. Clin Biochem Rev 26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 58.Yki-Järvinen H, Koivisto VA. 1986. Natural course of insulin resistance in type 1 diabetes. N Engl J Med 315:224–230. 10.1056/NEJM198607243150404. [DOI] [PubMed] [Google Scholar]