Abstract
Well-defined, humane endpoints aid in monitoring animal health status during disease development. Body condition scoring (BCS) is a method for assessing health status in mouse studies where wasting and death are potential endpoints. Whether BCS is useful in monitoring animals with bleomycin-induced lung injury has not been reported. Body weight (BW) is a common humane endpoint for this model, but because the lungs increase in weight as BW decreases, the animal's true physical condition could be masked when using BW as the sole endpoint criterion. Therefore, our goal here was to assess the usefulness of BCS in monitoring health status in a mouse model of lung injury. Lung injury was caused by acute instillation of the fibrogenic antibiotic bleomycin into the lungs through the trachea. Male C57BL/6 mice received bleomycin (0.075 U) dissolved in saline or saline alone. Bleomycin instillation led to a doubling of lung weight and decreases in both BW and BCS, compared with saline instillation. The changes in BW and BCS were significantly correlated with lung weight. When the adjusted BW was used (corrected for the increase in lung weight), the correlation was unchanged, suggesting that the increase in lung weight did not significantly mask the decrease in BW. Bleomycin instillation caused decreases in both soleus and visceral epididymal fat masses. The change in BCS was significantly correlated with both soleus and VEF mass, suggesting that BCS is reflective of the systemic loss of muscle and fat mass. Our findings suggest that BW and BCS are significantly correlated to lung injury in the bleomycin model of lung fibrosis and that BCS is an appropriate alternative humane endpoint in this mouse model.
Abbreviations: BCS, body condition score; BW, body weight; VEF, visceral epididymal fat
In studies with potential for animal pain and distress, well-defined, humane endpoints are important for identifying animals that need to be removed from study and euthanized. Typical methods for identifying these animals include observation of behavior, assessment of physical appearance, and measurement of body weight (BW).3 Decrease in BW is a commonly used endpoint for animal studies but is not an ideal parameter for monitoring health status in studies involving weight gain (for example, organ enlargement or tumor development).3,10
Body condition scoring (BCS) is a method for assessing health status in mouse studies. BCS is performed by gently holding the mouse by the base of the tail and passing a finger over the sacroiliac bones to assess fat and skeletal muscle stores.22 In mice, BCS range from 1 (emaciated) to 5 (obese). Typically, a BCS of 2 or lower is considered an appropriate endpoint for removal from the study protocol and euthanasia. The effectiveness of BCS in assessing health status in the murine model of bleomycin-induced lung fibrosis has not been examined.
Human idiopathic pulmonary fibrosis is a progressive and irreversible disease that causes fibrosis of the lungs and leads to respiratory failure.8 The median survival time after diagnosis is less than 3 y.2 Therefore, there is a critical need to develop and test therapies in animal models of the disease. Several murine models of lung fibrosis have been developed (for example, bleomycin, irradiation, asbestos, genetic modification).17 Intratracheal instillation of bleomycin is the most common, clinically relevant, well-characterized model that closely resembles human idiopathic pulmonary fibrosis.12,17
The bleomycin murine model is relatively easy to develop and causes reliable lung injury. Bleomycin causes DNA strand breakage, as well as oxidative stress, leading to inflammation and fibrosis of the lungs.13,20 The initial inflammatory stage lasts as long as 7 to 8 d and is followed by the development of fibrosis thereafter.16 The inflammatory stage is characterized by activation of macrophages and neutrophils, with subsequent abnormal deposition of collagen ultimately leading to disruption of normal lung architecture during the fibrotic stage.16,17 In addition, changes in respiratory physiology occur, including increased breathing cycle time, decreased minute ventilation, and decreased forced vital capacity.7,15 Consistent with these effects, exercise capacity is dramatically reduced.19 Consequently, the model exhibits high mortality,4,9 and thus well-defined, humane endpoints are needed to minimize animal pain and distress.
Although BW loss is typically used as a humane endpoint in this model, it may not accurately reflect the animal's true physical condition because while BW loss is occurring, the weight of the lungs is increasing. Moreover, the BW of untreated control animals increases during the observation period in this model, which can be as long as 28 d. Therefore, the BW loss of bleomycin-treated animals may be underestimated when referenced to their initial weight. The use of BCS may better reflect an animal's true physical condition because the BCS is independent of BW.18 Therefore, our goal here was to evaluate the usefulness of BCS as a humane endpoint for the murine model of bleomycin-induced lung fibrosis. We also examined whether changes in BCS were reflective of changes in muscle and fat loss. In the current study, lung injury in mice was caused by acute instillation of the fibrogenic antibiotic bleomycin into the lungs through the trachea. Bleomycin instillation led to lung inflammation, fibrosis, and increased lung weight. Changes in BW and BCS were correlated with lung weight. Changes in BCS were correlated with soleus and visceral epididymal fat (VEF) mass. Our findings show that BW and BCS correlate strongly with lung injury in bleomycin-treated animals and decreases in BCS are reflective of systemic loss of fat and skeletal muscle mass.
Materials and Methods
Mice, ethics approval, and husbandry procedures.
Adult male wild-type C57BL/6J (age, 12 wk; Jackson Laboratory Sacramento, CA) were maintained under SPF conditions at the San Francisco Veterans Affairs Medical Center. The mice were housed in sterile disposable IVC with a floor area of 523 cm2 and interior living space of 5653.5 cm3 (Innovive, San Diego, CA). Cages were bedded with irradiated corncob (depth, 0.25 in.; Envigo, Somerset, NJ) and changed every 7 to 10 d in a laminar-flow changing station. The mice received unrestricted access to chow (Teklad Global 16% protein, Envigo) and acidified water (pH 2.5 to 3.0, nonautoclaved) and were maintained on a 12:12-h light:dark cycle.
The health status of the colony was monitored by exposing sentinel cages to soiled bedding. Serology for ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, mouse hepatitis virus, murine norovirus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, reovirus type 3, Theiler murine encephalomyelitis virus, and Sendai virus was performed quarterly on sentinels. In addition, PCR testing for Aspiculuris tetraptera, Syphacia obvelata, Radfordia spp., and Myobia spp. was performed biannually on sentinels and colony animals (5 cages per rack).
This institution is AAALAC-accredited, and the study was approved by the Animal Care and Use Subcommittee of the San Francisco Veterans Affairs Medical Center and conformed to the Guide for the Care and Use of Laboratory Animals (revised 2011).11
Murine lung fibrosis model.
Lung injury was induced by using the fibrogenic antibiotic bleomycin which was instilled into the lungs through the trachea. Bleomycin (Sigma-Aldrich, St Louis, MO) was solubilized in sterile normal saline. Each mouse received a single instillation of 100 μL of saline with or without 0.075 U of bleomycin. The instillation was performed according to a modification of the technique for mouse lung intubation described in detail previously.14 At the beginning of the procedure, the mice were anesthetized with 4% isoflurane by inhalation; they were maintained with 1.5% isoflurane during the procedure using a nose cone. The anesthetized animal was placed on an angled board (approximately 60° from bench top) and suspended by its front teeth. The tongue was moved out of the way by using flat forceps, and a second pair of forceps was used to open the mouth. A 1-in., 22-gauge intravenous catheter (BD Biosciences, San Jose, CA) that incorporates a 0.5-mm fiberoptic cable (Edmunds Optics, Barrington, NJ) connected to a 150-W halogen light source was used to introduce the catheter into the trachea. The cable extended 2 to 3 mm beyond the tip of the catheter to illuminate the tracheal opening. Once the catheter was positioned in the trachea the cable was removed, plastic tubing that was attached to a syringe (containing the bleomycin or saline solution) was inserted through the catheter. The plastic tubing was cut to a length so that it would extend 2 to 3 mm beyond the catheter. The bleomycin or saline solution was administered; after the instillation procedure, the mice recovered on a warming pad. They were observed continuously for 15 min and then every 15 min until 1 h. All animals recovered within 5 min, and no complications occurred.
Assessment of BCS and BW.
BCS and BW were assessed daily, starting 1 d prior to tracheal instillation and ending on the day of euthanasia. BCS was performed before the mice were weighed, to avoid bias in the BCS, and without knowledge of the previous day's values. BCS was performed according to procedures outlined previously.22 Values can range from 1 to 5, with additional increments of plus or minus (for example, 2+) as necessary. All scores were given a numerical value (for example, BCS of 2+ = 2.33 or BCS of 3– = 2.66). A person with extensive experience in assessment of BCS in mice performed all BCS. For assessment of BW, mice were removed from their cages and placed in a small plastic dish on a scale; BW was recorded to the nearest 0.1 g.
Euthanasia and tissue harvest.
Mice were euthanized once they reached a humane endpoint or the study endpoint. The humane endpoints for this study were BW decrease of greater than 15%, inactivity, hunched posture, respiratory distress (labored or rapid, shallow breathing), and edema. The study endpoint occurred 10 to 12 d after bleomycin instillation. Mice were anesthetized by using 4% isoflurane; once a surgical plane of anesthesia was achieved (that is, no response to a toe pinch), thoracotomy was performed and the heart removed. In addition, the lungs were removed, trimmed of excess tissue, and weighed. The soleus muscle was dissected from the hindlimb musculature and weighed. The right and left VEF pads were dissected and weighed together.
Histology.
Lungs were fixed in 4% paraformaldehyde in phosphate buffer (Fisher Scientific, Fair Lawn, NY) at 4 °C for 24 h. The lungs were embedded in paraffin, and sections (thickness, 5 µm) were stained with hematoxylin, eosin, and picrosirius red.
Statistical analysis.
Spearman rank correlation coefficients were calculated for the change in BW compared with the change in BCS, change in BW or change in BCS compared with lung weight, and change in BCS compared with soleus or VEF mass. Independent-sample t tests were used to determine whether the dependent variables differed between saline- compared with bleomycin-treated animals. Paired t tests were used to determine whether the dependent variables differed before and after instillation for each group. Data are presented as mean ± SEM. The α level was set a priori at a P value less than 0.05. The statistical package Prism 7 (GraphPad Software, La Jolla, CA) was used for data analysis.
Results
Effects of bleomycin on the lungs, BW, and BCS of mice.
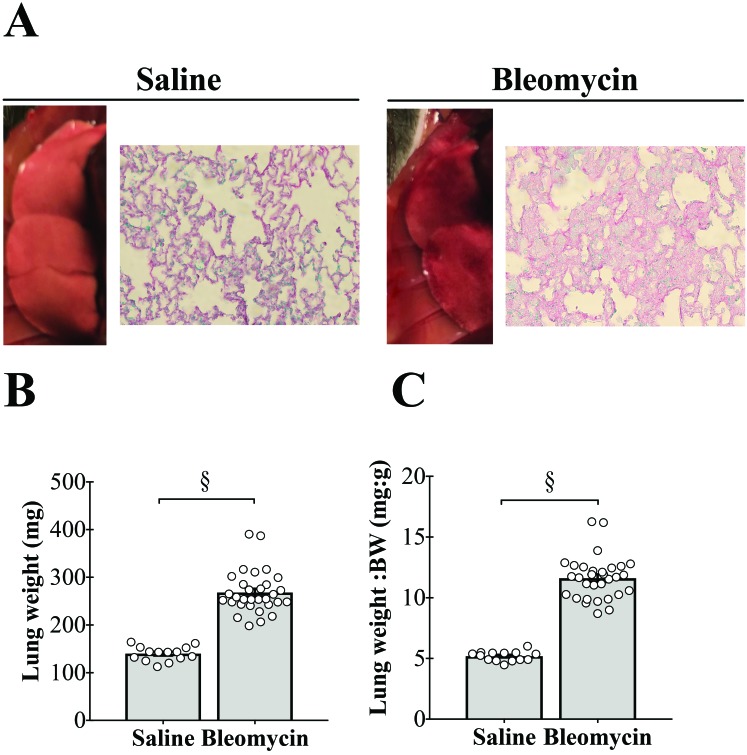
The morphology of lungs from animals receiving saline instillation was normal with a pink appearance and normal lung architecture (Figure 1 A, left). Gross examination of lungs from bleomycin-treated mice revealed multifocal lesions interspersed within what appeared to be normal areas of the lung. Some of the foci coalesced to form larger areas of lesions. This effect on the lungs involved multiple lobes and was bilateral. Histologic evaluation showed infiltration of inflammatory cells, disruption of alveoli, thickening of the alveoli walls, and increased deposition of collagen (Figure 1 A, right). These findings are consistent with diffuse interstitial lung disease.
Figure 1.
Tracheal instillation of bleomycin causes lung injury and increases lung weight. (A) Gross morphology and histology of right lung of a saline-treated mouse (left) and bleomycin-treated animal (right). Bleomycin instillation causes multifocal lesions on the lung, which are associated with infiltration of inflammatory cells, disruption of alveoli, thickening of the alveoli walls, and increased deposition of collagen. Note that gross lung images are not to scale. Hematoxylin, eosin, and picrosirius red staining; magnification, 400×. (B) Bleomycin instillation leads to doubling of lung wet weight, even when (C) expressed relative to body weight (BW). §, P < 0.0001.
Acute instillation of bleomycin led to a doubling of lung weight (P < 0.0001; Figure 1 B), which also was significantly increased when expressed as lung weight per body weight (P < 0.0001; Figure 1 C).
Both BW and BCS were significantly (P < 0.0001) decreased in bleomycin-treated animals (Table 1, Figure 2 A and B), but for saline-treated animals, BCS remained unchanged, and BW was significantly (P < 0.05) increased (Table 1).
Table 1.
Number of mice tested, body weight, and body condition score on day of bleomycin instillation and euthanasia
| Body weight (g) |
Body condition score |
||||
| Treatment group | No. of mice tested | Instillation | Euthanasia | Instillation | Euthanasia |
| Saline | 14 | 26.1 ± 0.5 | 27.0 ± 0.5a | 3.02 ± 0.02 | 3.02 ± 0.04 |
| Bleomycin | 31 | 26.7 ± 0.2 | 23.1 ± 0.3b | 3.04 ± 0.03 | 2.11 ± 0.05b |
Data shown are means ± SEM.
Values differ significantly (P < 0.05) between day of instillation and day of euthanasia.
Values differ significantly (P < 0.0001) between day of instillation and day of euthanasia.
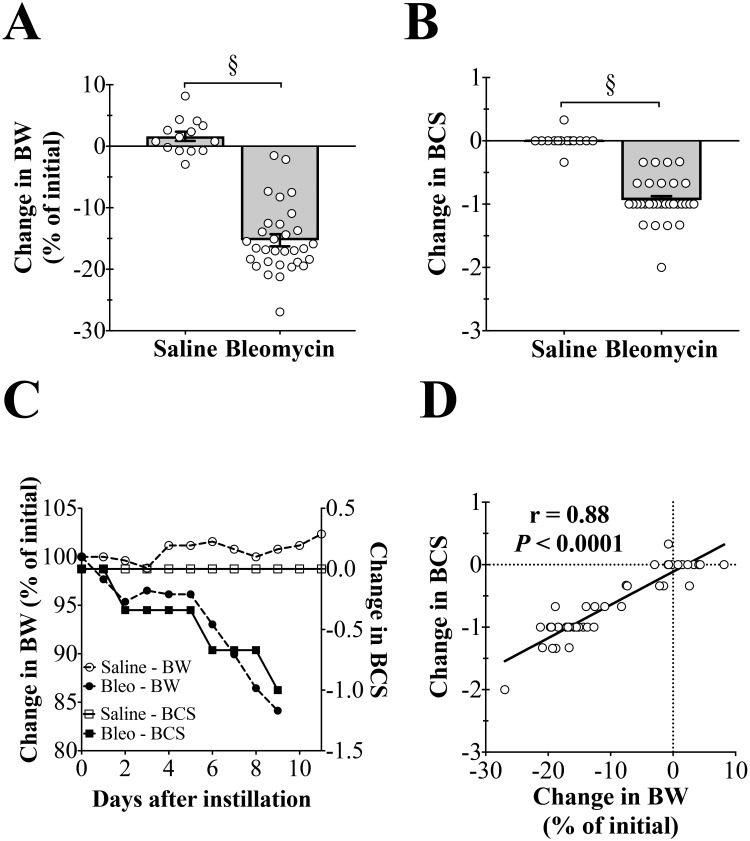
Figure 2.
Tracheal instillation of bleomycin causes decreases in body weight (BW) and body condition score (BCS). (A) Bleomycin instillation causes decreased BW. §, P < 0.0001. (B) Bleomycin instillation causes decreased BCS. §, P < 0.0001. (C) Representative changes in BW and BCS in a bleomycin-treated mouse and a saline control animal. Bleomycin causes an initial drop in BW that is stable until day 5, after which a precipitous drop in BW occurs. Decreases in BCS closely follow the decreases in BW. Saline instillation does not affect BCS, whereas BW slightly increases. (D) Decreases in BW and BCS are strongly correlated with each other.
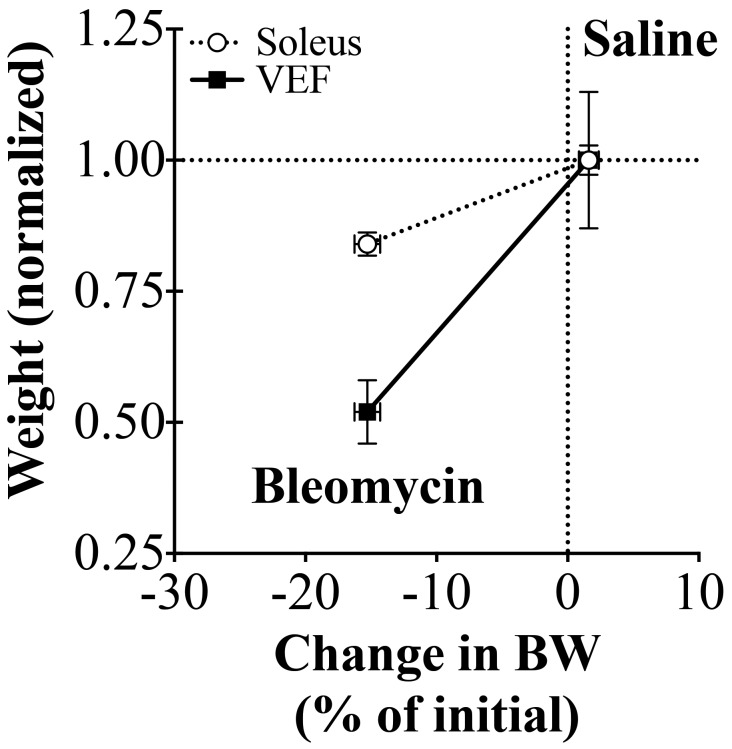
In a representative time course for the changes in BW and BCS for saline- and bleomycin-treated animals, BW decreased by approximately 15% in the bleomycin-treated animal, and BCS decreased by 1 unit (Figure 2 C). For the saline-treated mouse, BW increased by approximately 2.5%, whereas BCS remained unchanged. Lastly, BW and BCS were strongly correlated with one another (r = 0.88, P < 0.0001, Figure 2 D).
Correlation between BW or BCS and lung injury.
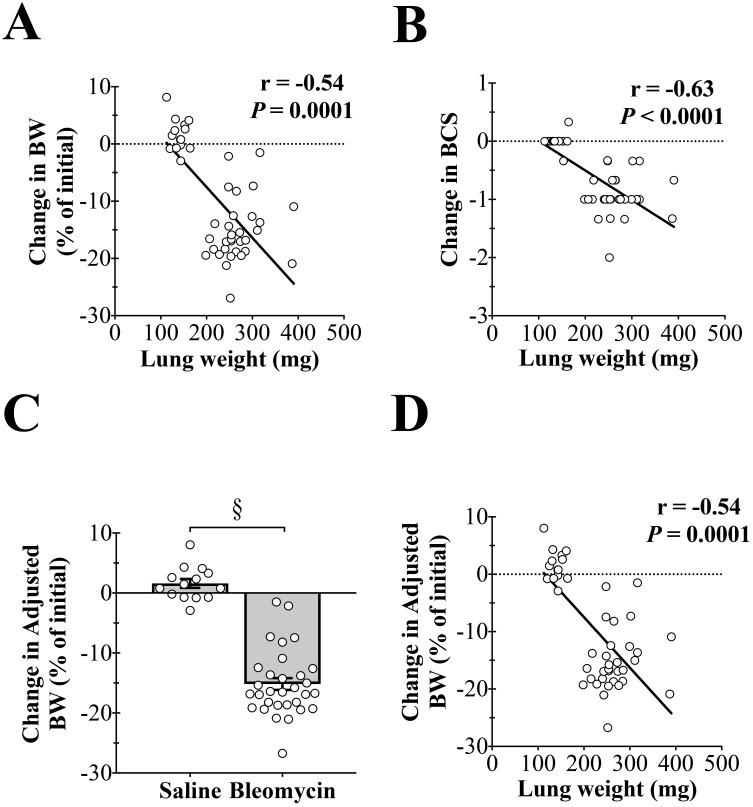
The increase in lung weight correlates well with histopathologic findings and Ashcroft scoring analysis, therefore lung weight is an appropriate indication of lung injury.7 BW (change in % initial) was significantly correlated (r = –0.54, P = 0.0001) with lung weight (Figure 3 A), as was the change in BCS (r = –0.63, P < 0.0001, Figure 3 B). For bleomycin-treated mice, the increase in lung weight could mask the animal's true BW loss. To account for this possibility, the lung weight was subtracted from the BW to derive the adjusted BW (change in % initial, Figure 3 C). Like BW, adjusted BW (change in % initial) was significantly correlated (r = –0.54, P = 0.0001) with lung weight (Figure 4 D). This finding suggests that the increase in lung weight does not significantly mask the animal's BW loss.
Figure 3.
Decreases in body weight (BW) and body condition score (BCS) are correlated with lung weight. (A) The change in BW is highly correlated with lung weight (r = –0.54, P = 0.0001). (B) The change in BCS is highly correlated with lung weight (r = –0.63, P < 0.0001). (C) Adjusted BW (corrected for the increase in lung weight) is significantly decreased by bleomycin instillation (§, P < 0.0001). (D) The change in adjusted BW is highly correlated with lung weight (r = –0.54, P = 0.0001).
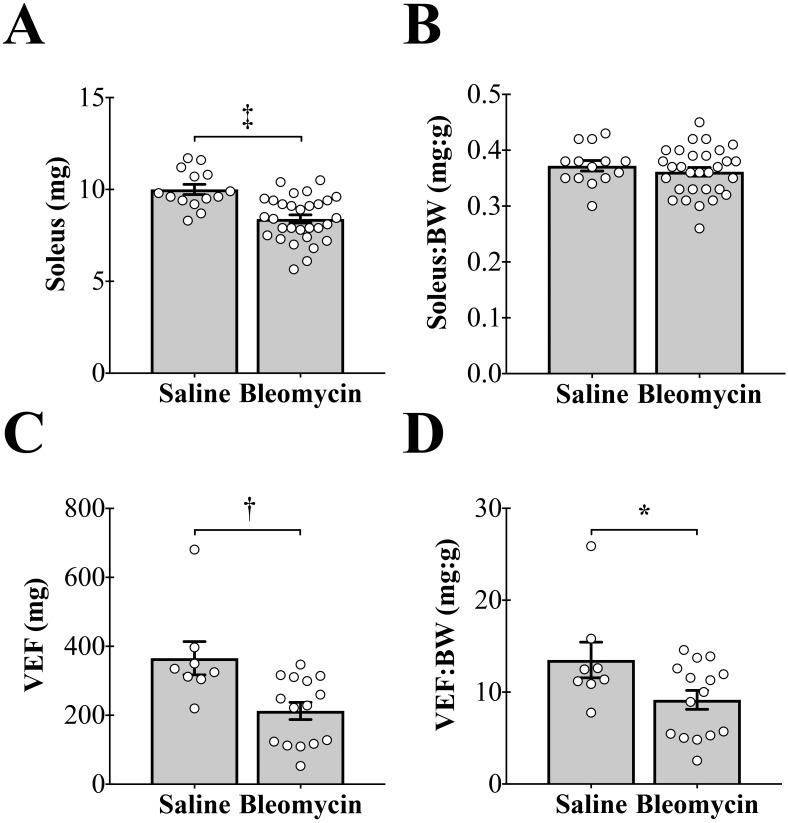
Figure 4.
Tracheal instillation of bleomycin causes decreases in muscle and fat mass. (A) Bleomycin instillation causes a significant decrease in soleus mass (‡, P < 0.001). (B) There is no effect of bleomycin instillation on soleus mass when expressed relative to body weight (BW). (C) Bleomycin instillation causes a significant decrease in visceral epididymal fat (VEF) mass (†, P < 0.01). (D) Bleomycin instillation causes a significant decrease in VEF mass when expressed relative to BW (*, P < 0.05).
Effects of bleomycin on soleus and VEF masses.
The soleus muscle was dissected from the hindlimb musculature and used an indicator of muscle mass, and the right and left VEF pads were harvested and weighed together as an indicator of fat mass. Tracheal instillation of bleomycin decreased both soleus and VEF masses (Figure 4 A and C). When expressed relative to BW, soleus mass did not differ between treatment groups (Figure 4 B). In contrast, VEF mass relative to BW was significantly decreased in the bleomycin-treated group compared with saline control mice (Figure 4 D).
When soleus and VEF masses were normalized to saline-treated controls and plotted against the percentage change in BW, the slope of the VEF mass compared with BW relationship was significantly steeper than the slope of the soleus mass compared with BW relationship (P = 0.001, Figure 5). This finding suggests that as BW decreases, a disproportionate loss of fat compared with muscle mass occurs.
Figure 5.
Tracheal instillation of bleomycin causes a disproportionate loss of visceral epididymal fat (VEF) compared with soleus mass. Soleus and VEF masses were normalized to the average weight of the saline-treated control group (Saline). When normalized soleus and VEF masses are correlated with BW (% of initial), the slope of the VEF mass compared with BW (% of initial) is significantly steeper (P = 0.001).
Correlation between soleus or VEF mass and BCS.
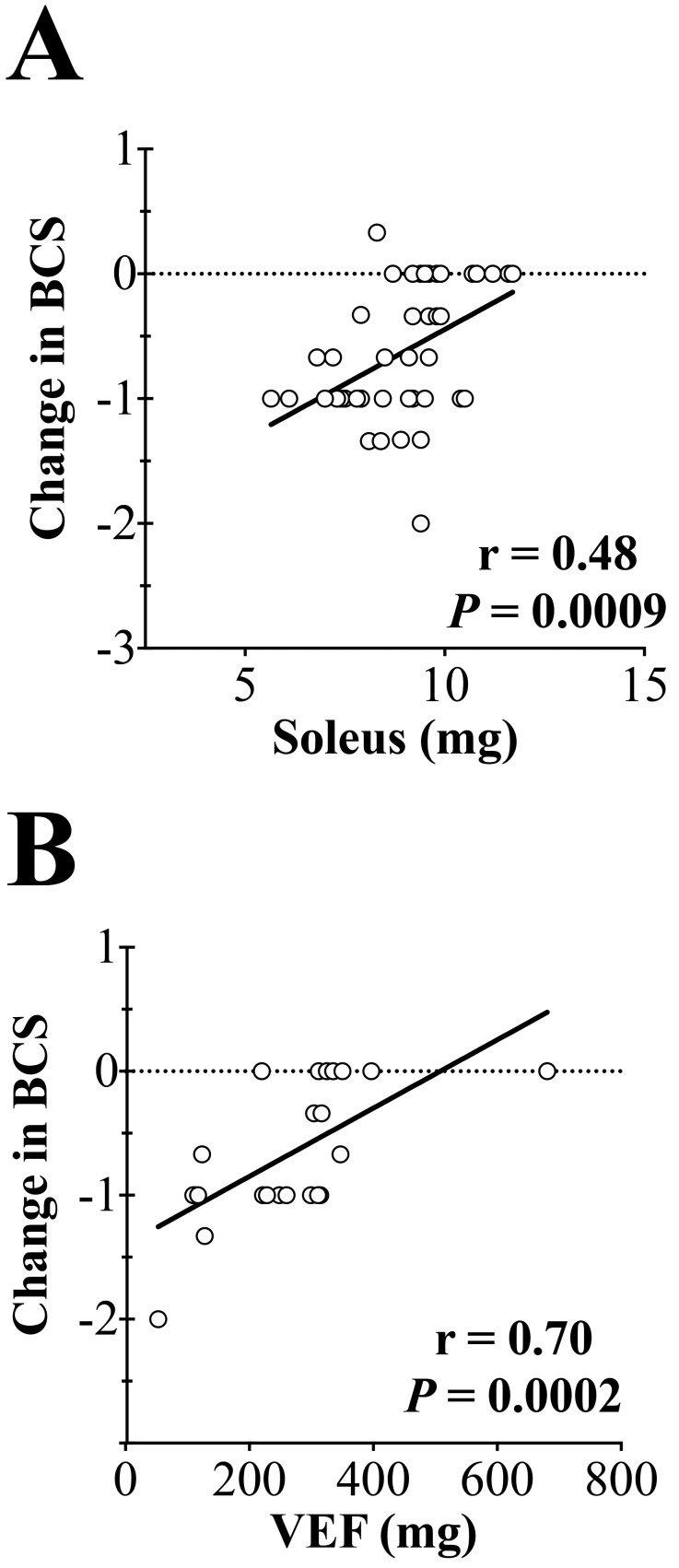
BCS correlates strongly with the amount of muscle and fat covering the sacroiliac bones.5 We asked whether BCS was reflective of systemic losses in muscle and fat mass. Soleus mass was strongly correlated with BCS (r = 0.48; P < 0.001, Figure 6 A), as was VEF (r = 0.70; P < 0.001, Figure 6 B). These findings suggest that decreases in BCS reflect systemic losses in fat and muscle mass.
Figure 6.
Decreases in body condition score (BCS) are correlated with soleus and visceral epididymal fat (VEF) masses. (A) The change in BCS is highly correlated with soleus mass (r = 0.48, P = 0.0009). (B) The change in BCS is highly correlated with VEF mass (r = 0.70, P = 0.0002).
Discussion
The major findings of this study are: 1) decreases in BW and BCS are highly related to the presence of lung injury in this mouse model; 2) the increase in lung weight from bleomycin instillation does not significantly mask an animal's loss in BW, and 3) decreases in BCS are related to systemic losses in muscle and fat mass. The significance of this study is that it shows BCS is a suitable alternative for assessing health status in the bleomycin mouse model of lung fibrosis.
This study found that instillation of bleomycin significantly increased lung weight, which was caused by increased inflammation, fluid accumulation, and fibrosis in the lungs. Other signs of lung injury in bleomycin-treated mice included decreased activity and, at the time of euthanasia, periods of dyspnea (data not shown). In addition, bleomycin instillation caused significant decreases in both BW and BCS. These findings are consistent with previous studies showing that bleomycin instillation increases lung weight and decreases BW.4
The use of BW loss as a humane endpoint for this model could be confounded by the increase in lung weight. Thus, the animal's true physical condition could be masked by using BW loss as the sole endpoint criteria. In the current study, both BW and BCS were highly correlated to the presence of lung injury. In addition, when the adjusted BW was used, the correlation was unchanged, suggesting that the increase in lung weight does not significantly mask the decrease in BW. The increase in lung weight (0.13 g relative to the saline control) is small relative to the body weight (23 g). Therefore, in principle, the increase in lung weight could have a masking effect, but, in practice, the effect is so small that it is negligible in this particular model. These findings suggest that both BW and BCS are appropriate methods for monitoring animal health status during disease progression. As outlined previously,6 BCS has many advantages over the assessment of BW, including that 1) BCS is fast to perform, given that it can be completed within 30 to 60 s; 2) BW requires a scale, which might have to be transported into the animal facility, and 3) importantly, using BW as an endpoint requires a reference value (for example, an animal's initial weight or that of an untreated group).
Instillation of bleomycin caused decreases in both soleus and VEF masses, signifying the presence of significant wasting of skeletal muscle and fat, consistent with other rodent models of pulmonary artery hypertension.1,21 VEF mass dropped more precipitously than soleus mass (Figure 5), thus perhaps signifying greater reliance on fat for energy production. Indeed, bleomycin-treated mice ate less chow than saline-treated animals (data not shown); this behavior is consistent with increased reliance on fat stores for energy production.
BCS in mice has been validated by using microCT, in which measurements of skeletal muscle and fat from a transverse section taken midway between the second fused vertebra in the sacral region corresponded to BCS.5 The present study shows that in the bleomycin model, BCS is reflective of changes in fat and muscle mass that occur in the body but distant from the anatomic location where BCS is assessed. This finding suggests BCS reflects overall body condition in the bleomycin model of lung fibrosis.
BCS is a suitable humane endpoint for male C57BL/6J mice with bleomycin-induced lung injury. However, further study is needed to determine whether this technique is appropriate for use in female C57BL/6J, other mouse strains, or other diseases. In addition, whether other humane endpoints (that is, level of dyspnea or decreases in activity) aid in monitoring animal health status needs to be tested.
In conclusion, both BW and BCS correlate with the presence of lung injury in the murine model of bleomycin-induced lung fibrosis. BCS is an appropriate alternative to BW as a humane endpoint in this model. The benefits of BCS over BW include the rapidity with which BCS can be performed (that is, 30 to 60 s), without the need for any equipment or reference values.
Acknowledgments
This work was supported by American Heart Association Postdoctoral Fellowship 16POST30970031 (PMC) and Grant in Aid 15GRNT25550041 (AJB) and by the Department of Veterans Affairs Merit Review Award I01BX000740 (AJB).
References
- 1.Ahn B, Empinado HM, Al-Rajhi M, Judge AR, Ferreira LF. 2013. Diaphragm atrophy and contractile dysfunction in a murine model of pulmonary hypertension. PLoS One 8:1–7. 10.1371/journal.pone.0062702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. 1998. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 157:199–203. 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.Burkholder T, Foltz C, Karlsson E, Linton CG, Smith JM. 2012. Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol 2:145–165. 10.1002/9780470942390.mo110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowley PM, Wang G, Chang AN, Makwana O, Swigart PM, Lovett DH, Stull JT, Simpson PC, Baker AJ. 2015. The α1A-adrenergic receptor subtype mediates increased contraction of failing right ventricular myocardium. Am J Physiol Heart Circ Physiol 309:H888–H896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easterly ME, Foltz CJ, Paulus MJ. 2001. Body condition scoring: comparing newly trained scorers and microcomputed tomography imaging. Lab Anim (NY) 30:46–49. [PubMed] [Google Scholar]
- 6.Foltz CJ, Ullman-Cullere MH. 1999. Guidelines for assessing the health and condition of mice. Lab Anim (NY) 28:28–32. [PubMed] [Google Scholar]
- 7.Gilhodes JC, Jule Y, Kreuz S, Stierstorfer B, Stiller D, Wollin L. 2017. Quantification of pulmonary fibrosis in a bleomycin mouse model using automated histological image analysis. PLoS One 12:1–14. 10.1371/journal.pone.0170561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross TJ, Hunninghake GW. 2001. Idiopathic pulmonary fibrosis. N Engl J Med 345:517–525. 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 9.Hemnes AR, Zaiman A, Champion HC. 2008. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA–Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294:L24–L33. 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 10.Hickman DL, Swan M. 2010. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci 49:155–159. [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 12.Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Königshoff M, Kolb M, Laurent GJ, Nanthakumar CB, Olman MA, Pardo A, Selman M, Sheppard D, Sime PJ, Tager AM, Tatler AL, Thannickal VJ, White ESATS Assembly on Respiratory Cell and Molecular Biology 2017. An official American Thoracic Society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol 56:667–679. 10.1165/rcmb.2017-0096ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lown JW, Sim SK. 1977. The mechanism of the bleomycin-induced cleavage of DNA. Biochem Biophys Res Commun 77:1150–1157. 10.1016/S0006-291X(77)80099-5. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald KD, Chang H-YS, Mitzner W. 2009. An improved simple method of mouse lung intubation. J Appl Physiol (1985) 106:984–987. 10.1152/japplphysiol.91376.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milton PL, Dickinson H, Jenkin G, Lim R. 2012. Assessment of respiratory physiology of C57BL/6 mice following bleomycin administration using barometric plethysmography. Respiration 83:253–266. 10.1159/000330586. [DOI] [PubMed] [Google Scholar]
- 16.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. 2008. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40:362–382. 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore BB, Hogaboam CM. 2008. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L152–L160. 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 18.Paster EV, Villines KA, Hickman DL. 2009. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 59:234–241. [PMC free article] [PubMed] [Google Scholar]
- 19.Peng R, Sridhar S, Tyagi G, Phillips JE, Garrido R, Harris P, Burns L, Renteria L, Woods J, Chen L, Allard J, Ravindran P, Bitter H, Liang Z, Hogaboam CM, Kitson C, Budd DC, Fine JS, Bauer CMT, Stevenson CS. 2013. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for ‘active’ disease. PLoS One 8:1–15. 10.1371/journal.pone.0059348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sausville EA, Peisach J, Horwitz SB. 1976. A role for ferrous ion and oxygen in the degradation of DNA by bleomycin. Biochem Biophys Res Commun 73:814–822. 10.1016/0006-291X(76)90882-2. [DOI] [PubMed] [Google Scholar]
- 21.Steffen BT, Lees SJ, Booth FW. 2008. AntiTNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J Appl Physiol (1985) 105:1950–1958. 10.1152/japplphysiol.90884.2008. [DOI] [PubMed] [Google Scholar]
- 22.Ullman-Culleré MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]