Unstructured summary
Children represent both a clinically important population susceptible to tuberculosis, but also a key group in whom to study intrinsic and vaccine-induced mechanisms of protection. Following exposure to Mycobacterium tuberculosis, young children are at high risk of progressing first to tuberculosis infection, then tuberculosis disease and possibly disseminated forms of tuberculosis, with accompanying high morbidity and mortality. Younger school-age children are relatively protected, before risk increases again in adolescence. Furthermore, children are the only group for whom there is proven benefit of Bacillus Calmette-Guérin (BCG) immunization. Case-control comparisons from key cohorts, which recruited more than 15,000 children and young people between them, have identified the monocyte:lymphocyte ratio, activated CD4 T cells, and an RNA signature as correlates of risk for developing tuberculosis. Further studies of protected and susceptible populations are necessary to guide development of novel tuberculosis vaccines that could facilitate the goal of zero childhood tuberculosis deaths.
Introduction
Traditionally, paediatric tuberculosis has been relatively neglected, although recent years have seen a welcome increase in policy focus including the goal of zero childhood tuberculosis deaths.1 To achieve this ambition, significant progress now needs to occur, given that more than a million children were estimated to develop tuberculosis in 2016, with 250,000 children dying of the disease.2 This represents 10% of the total burden of incident tuberculosis and 15% of associated total mortality.2 Children can equally be affected by resistant strains of Mycobacterium tuberculosis (M tuberculosis), with an estimated 25,000 children developing multidrug-resistant (MDR) and 1,200 extensively drug-resistant (XDR) tuberculosis in 2014 alone.3 There are numerous social, epidemiological, immunological, diagnostic, and therapeutic differences between childhood and adult tuberculosis,4 hence paediatric tuberculosis requires specific considerations in clinical, public health and research aspects.
Children and young people represent clinically important populations both with increased susceptibility to tuberculosis, but also key groups in whom to study mechanisms of protection. The precise definition of paediatric tuberculosis is debated: the World Health Organization (WHO) reports tuberculosis data for those less than 15 years old, the UN Convention on the Rights of the Child defines a child as someone less than 18 years old, and adolescence is increasingly considered to be until the age of 24.2,5,6 Here, we broadly refer to those under the age of 18 years old, whilst acknowledging that the burden of adolescent disease is therefore underestimated.
Young children have the highest risk of progressing to disease following infection, where infection is defined as mycobacterial sensitisation evidenced by a positive tuberculin skin test (TST) or interferon-γ release assay (IGRA). They are also at the highest risk of disseminated forms of tuberculosis such as miliary tuberculosis and tuberculous meningitis.7 Younger children are the most likely to die, with tuberculosis mortality rates, from the pre-treatment era, of nearly 50% in those less than five years of age, significantly higher than in older children.8
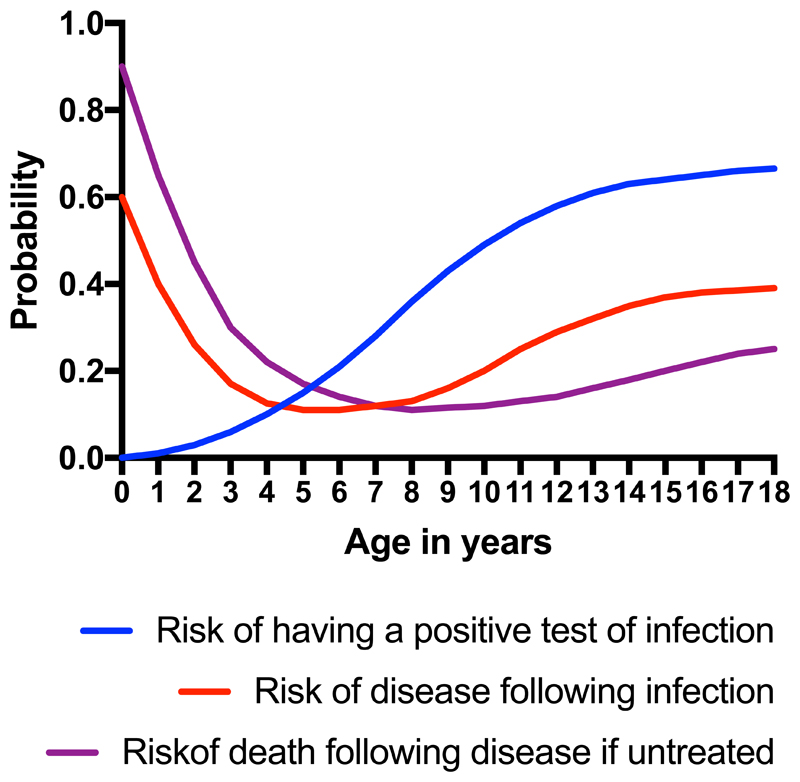
By contrast, younger school age children (five to ten years old) seem to be relatively protected against tuberculosis, prior to a second peak in incidence during adolescence and into adulthood (Figure 1).9 In fact, many children control M tuberculosis without intervention. Studies from the pre-chemotherapy era show that a) the majority of children survive tuberculosis disease without treatment,8 b) pathological appearances on pulmonary radiographs frequently clear spontaneously,10 and c) M tuberculosis can be cultured from recently infected asymptomatic children who do not proceed to become unwell.11 Contemporary data confirms that some children with culture-confirmed MDR-tuberculosis remain well and symptom free in the absence of treatment.12 Therefore the interactions between human host and the mycobacterial pathogen are increasingly recognised to develop along a spectrum rather than falling within clearly delineated categories.13–16
Figure 1.
Schematic representation of effect of age in high-burden communities upon a) risk of infection, b) risk of disease following infection, and c) risk of death following disease if untreated.
This diversity in human responses to M tuberculosis exposure raises the question of whether a protective immune response can be promoted by a new vaccine. The development of a protective vaccine by 2025 is a cornerstone of the WHO End TB Strategy.17 Children are the only group for whom there is strong evidence of inducible protection though vaccination.18–20 Bacillus Calmette-Guérin (BCG), a live, attenuated vaccine was first given to humans in 1921 and has been administered to more people than any other vaccine in history. Infants were the target population for the first phase II randomized placebo-controlled clinical trial of a new tuberculosis vaccine, the modified Vaccinia Ankara virus expressing antigen 85A (MVA85A). Unfortunately, the MVA85A vaccine showed no additional efficacy against tuberculosis disease or infection beyond that of BCG in South African infants.21 These results, not confirming prior animal models and human immunogenicity data, prompted much reflection in the field of tuberculosis vaccine development.22–24
The quest for human correlates of protection against M tuberculosis remains a major research priority.17,22 To date, our understanding of protection in children has been derived from three main research approaches. One such approach has been the use of case-control studies, nested within larger paediatric and adolescent interventional trials or cohorts. Commonly, children and adolescents who developed tuberculosis disease (cases), are compared to those that remained well (controls). Such studies have enrolled more than 15,000 children and young people between them, almost entirely from South Africa, and have utilised a range of laboratory approaches in the search for correlates of risk and protection (Table 1).14,21,25–37 A second approach has been active contact tracing, evaluation and follow-up of M tuberculosis exposed individuals, for example through household contact studies or outbreak investigations.38–48 A third approach has been identification of genetic defects in children suffering from severe forms of mycobacterial disease. By evaluating the immunological pathways involved, critical aspects of the human immune response necessary to contain M tuberculosis can be determined.49–51 In this review, we first summarise key components of the paediatric immune response to M tuberculosis (Figure 2) before focusing on the current understanding of risk and protective factors when children encounter M tuberculosis (Figure 3). For the purposes of clarity, whilst acknowledging that it is an oversimplification of the clinical spectrum, we have structured our discussion around the concepts of exposure, infection, pulmonary disease, severe disease and death.
Table 1.
Summary of key paediatric case-control studies, their parent cohorts, the methodologies employed to explore correlates of protection, and major findings.
| Parent study | Cohort | Number of incident TB cases in analysis* | Methods used to identify correlates of risk/protection | Potential Correlates of Risk | Potential Correlates of Protection |
|---|---|---|---|---|---|
| Double-blind randomised placebo-controlled phase 2b trial of MVA85A vaccine boost of BCG.21 | 2,797 infants in South Africa. | 71 |
Ex vivo interferon-γ ELISpot assays: Ag85A IgG BCG Mycobacterial Growth Inhibition Assay Flow Cytometry31,37 |
% of HLADR+CD4+ T cells 31 | BCG ELISpot 31 % of D0 CD4+ T cells31 D28 post-boost vaccination Ag85A-IgG31 |
| 28 | Quantitative QuantiFERON-TB Gold In-Tube35 | QFT conversion at interferon-γ values higher than 4·00 IU/mL | None | ||
| Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children25 | 1,336 infants in South Africa & Botswana. | 187 | Full Blood Count Monocyte:lymphocyte ratio | Higher monocyte:lymphocyte ratio30 | None |
| Adolescent Cohort Study 14,27,32–34,36 | 6,363 adolescents aged 12–18 years in South Africa. | 30 | Flow Cytometry | % of HLADR+CD4+ T cells31 | None |
| 46 | RNA sequencing data of unstimulated whole blood→TB risk signature→qRT-PCR | 16 gene signature14 | None | ||
| 96 | Serial QuantiFERON-TB Gold In-Tube | Persistently positive QFT, converting from negative to positive QFT, converting from positive to negative QFT36 | None | ||
| Randomised trial to compare the incidence of tuberculosis over two years in infants vaccinated at birth with intradermal BCG or with percutaneous BCG26 |
5,724 infants in South Africa. | 29 | BCG stimulation: Flow cytometry,28 29-plex supernatant cytokine/chemokine,29 Lymphocyte proliferation assay,29 Cytotoxic marker assay29. Quantification of myeloid and lymphoid cell populations29 |
None | None |
| 46 | BCG stimulated PBMCs → Microarray RNA gene expression analysis29 | None. | None |
MVA85A = modified Vaccinia Ankara virus expressing antigen 85A; BCG= Bacille Calmette Guerin; PBMCs=Peripheral Blood Mononuclear Cells; ELISpot = Enzyme-Linked ImmunoSpot; PPD=Purified Protein Derivative; EBV= Epstein-Barr Virus; CMV=Cytomegalovirus; OR=Odds Ratio; FDR= False Discovery Rate; SFC=spot-forming cell; LTBI=Latent M. tuberculosis Infection; qRT-PCR=quantitative real-time Polymerase Chain Reaction, QFT= QuantiFERON-TB Gold In-Tube; OR=Odds Ratio; IRR=Incidence Rate Ratio. *Due to availability of samples etc, different numbers of cases were included in different analyses, despite being derived from the same cohort.
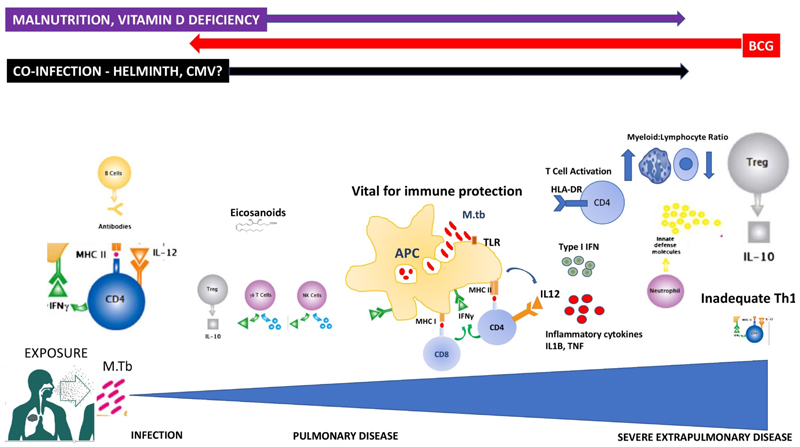
Figure 2.
Summary of key immunological players in paediatric response to Mycobacterium tuberculosis, with hypothesized associations with risk and protection.
(Adapted from illustration ©Hugh Gifford 2010, first published in first published in Jones C, Whittaker E, Bamford A, Kampmann B. Immunology and pathogenesis of childhood TB. Paediatr Respir Rev 2011; 12:3–8.)
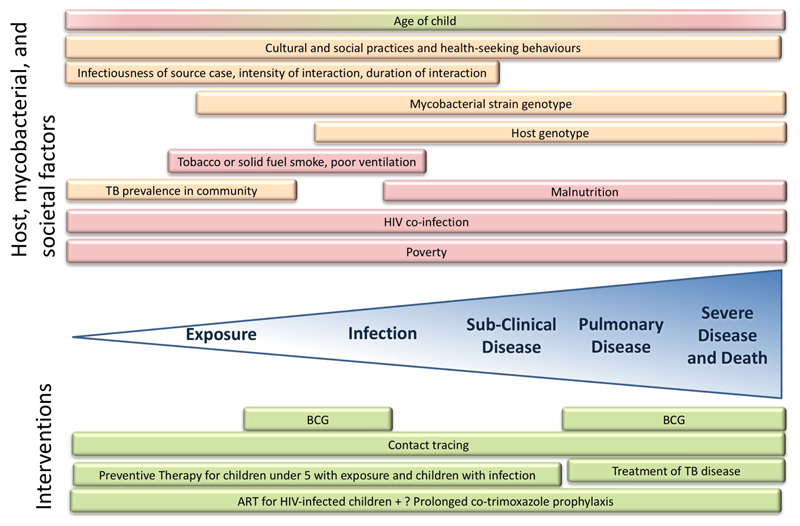
Figure 3.
Schematic representation of host, mycobacterial, and societal factors that influence risk along the spectrum of paediatric interactions with Mycobacterium tuberculosis from exposure to severe disease, together with protective interventions.
Red background denotes risk factors, green background denotes protective factors, and orange denotes factors that can be either protective or increase risk.
Key components of paediatric immunological response to M tuberculosis
There are several possible outcomes for M tuberculosis within the (paediatric) lung with dynamic shifts between status over time and within the same individual:16 elimination by the innate immune system; asymptomatic control accompanied by a cell-mediated immune memory response (known as tuberculosis infection) with or without persistence of viable bacteria; direct progression to pulmonary disease (previously known as primary tuberculosis) or delayed development of pulmonary disease (often described as reactivation); and extension beyond the lung and lymphatic system to cause severe disseminated disease. The immune mechanisms involved in regulating the fine balance between host control and disease remain poorly defined and a correlate of protective immunity remains elusive. It is often assumed that the differences in susceptibility to M tuberculosis between children and adults are attributable to age-related differences in the immune response, but only a limited number of studies have assessed this.
A range of innate and adaptive cell types and immune mediators are involved in the host response to M tuberculosis (Figure 2). Antigen presenting cells, including macrophages and dendritic cells are central to the initial response. Young children have fewer circulating dendritic cells than adults, with reduced functional capacity, macrophage phagocytosis and recruitment.52 Other components of the innate response to mycobacteria are also different in neonates and early infancy as compared to older children and adults. These include collectin levels and the complement pathway.53,54 Maturation of toll-like receptors over the first year of life may also be relevant.55–57 Neutrophils are the most commonly infected phagocyte in human tuberculosis,58 and drive a type I interferon-inducible transcript signature in adult whole blood that correlates with clinical severity, suggesting neutrophils may be involved in disease pathogenesis.13 The importance of CD4+ T-cells and interferon-γ (IFNγ) is evident through the significantly increased risk of tuberculosis in infants with HIV infection, and severe mycobacterial disease in those with defects in the IFNγ pathway.50,51,59 Whilst Th17 cells (producing IL17 and IL22) reportedly play a role in adult TB, paediatric studies have not demonstrated their contribution to protection or susceptibility.28,60–62 The potential immunoprotective role of non-conventional T cells which link the innate and adaptive immune responses, such as gammadelta (γδ) T cells and NK cells, has also been investigated.63,64 These cell types expand in response to mycobacterial infection and display effector T cell functions such as IFNγ production and granulysin release in children. Children with tuberculosis have higher levels of regulatory T cells than healthy controls, even after six months of TB treatment.60
Risk of exposure to M tuberculosis
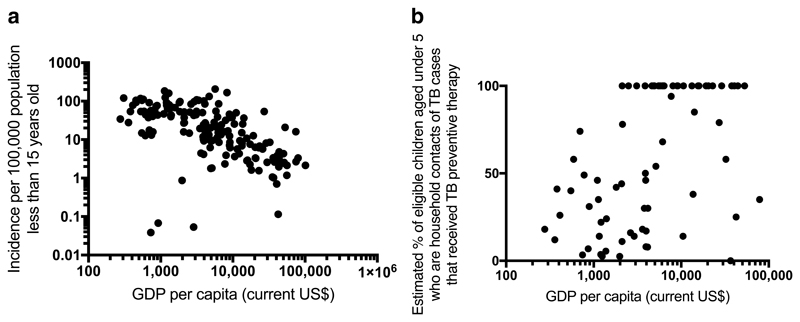
The relationship between paediatric tuberculosis and poverty is overwhelming (Figure 4) and confounds other risk factors.65,66 The influence of poverty extends across the whole spectrum of paediatric tuberculosis and is associated with increased risk of being exposed to tuberculosis, of becoming infected, of developing disease and of poor outcomes.67
Figure 4.
Associations between a) GDP per capita and paediatric TB incidence and b) GDP per capita and provision of TB preventive therapy to eligible children under 5 years old.
a) Spearman rank correlation coefficient= - 0.669, p<0.001, n=168 countries with available data and b) Spearman rank correlation coefficient= 0.533, p<0.001, n=75 countries with available data. Note the relatively few countries in b) reporting data on provision of TB preventive therapy to eligible children under 5 years old, and that 33/75 countries that do report perfect provision (100%). (Data sources: 2015 GDP data: World Bank; 2015 Population under 15: United Nations, Population Division, Department of Economic and Social Affairs; 2015: TB cases under 15 years old & estimated % of children received TB preventive therapy aged under 5 who are household contacts of TB cases and who are eligible for TB preventive therapy: World Health Organization)
The risk of exposure to M tuberculosis is a combination of epidemiological, environmental, sociocultural and behavioural factors that reflect how children, adolescents, and adults interact within societies. The probability of exposure relates to background prevalence of tuberculosis, although this can be heterogeneous within countries and cities.9 Age- and culture-related factors such as sleeping practices, care-giving, play, religious practices and school will influence how and where children interact with adults who might have tuberculosis and how much risk these interactions carry.68 Similarly, population density, household composition, crowding, transport systems, and ventilation, both at home and in healthcare facilities, all contribute to the risk of exposure.69,70 Adults living with HIV have a higher risk of tuberculosis,71 and therefore children in the same households are automatically at increased risk of exposure, in addition to the direct risks associated with vertical transmission of HIV and subsequent suppression of protective cellular immune responses. Social factors such as household alcohol consumption are known to enhance the risk of exposure, as adults who abuse alcohol are more likely to develop tuberculosis, and are also less likely to seek care or have successful treatment outcomes.72
Risk of infection with M tuberculosis
Following exposure, the risk that a child will develop M tuberculosis infection is influenced by the infectiousness of the source case, the duration and intensity of the interaction, the infectivity of the organism and the immune responses of the child.68 Source cases are more infectious if they have a high bacterial load, clinically reflected by sputum smear-positivity.40 Extensive pulmonary disease in the source case, defined as affecting multiple zones on a chest radiograph, is associated with increased risk of TST positivity in contacts, independent of mycobacterial load.40,41 The duration of cough increases the risk of transmission, with children more likely to be infected if the source case has been coughing for a longer period of time.41 If the source case is a first degree relative then child contacts are more likely to become infected than if the source case is a more distant relative,41 and the physical proximity of sleeping arrangements in households influences transmission.41,73 The influence of solid fuel smoke and cigarette smoke is complex but children are more likely to become infected if someone in the household smokes cigarettes or if solid fuels are used for cooking or heating.74
Whether certain mycobacterial strains are more infectious is unclear with conflicting findings regarding an increased infectiousness and virulence of the Beijing strain, as compared to other strains.75,42 A large Indian study showed that isoniazid-resistant strains were associated with higher rates of infection but similar rates of disease compared to isoniazid-susceptible strains,76 whilst a recent study from Peru suggested that contacts of MDR-tuberculosis cases were less likely to develop tuberculosis than contacts of drug-susceptible cases.48
Most contact studies demonstrate that children are more likely to have a positive TST with increasing age (Figure 1).41,77,78 Given that these tests reflect infection at any point in their lives, these results are not surprising as older children are more likely to have been exposed to infectious cases of tuberculosis in the community in addition to the identified household source case. BCG vaccination and exposure to non-tuberculous mycobacteria are also known to influence TST results. Whether uninfected older children are more likely than uninfected younger children to develop a positive TST following known exposure is unclear. Outbreak investigations in low prevalence settings suggest that perhaps younger children are more vulnerable, but this could be explained by increased intensity of exposure.44
Co-infections also play a role.79 Even when vertical transmission is interrupted, HIV-exposed but uninfected infants have transient altered responses to BCG vaccination in vitro early in life and are at increased risk of tuberculosis infection.80–84 The impact of parasite co-infection upon risk of tuberculosis infection in exposed children remains unclear.68,79,85 Helminth infections, in particular Ascaris lumbricoides and hookworm, induce a T helper type 2 associated immune responses, characterised by the presence of cytokines including IL4, IL5, IL9, IL10 and IL13.85 A recent randomized clinical trial showed no impact of deworming on either the TST or IGRAs in children86 and helminth infections have been shown to both increase87 or reduce88 the risk of TST positivity following exposure to an infectious source case.
Protection against infection by Mycobacterium tuberculosis
Understanding why some children who are exposed to M tuberculosis show no signs of infection is critical in understanding an effective early human immune response to M tuberculosis. This knowledge could definitively inform the design of vaccines able to induce protection against infection.89,90 In the absence of a gold standard, protection against infection is considered as absence of a positive TST or IGRA despite documented exposure or living in high prevalence regions. A Colombian household contact study demonstrated approximately 65% of the variability in TST results is attributable to genetic contributions.91 Specifically, a genome-wide linkage study of 128 families including 350 children in Cape Town, South Africa studied 6,000 single-nucleotide polymorphisms and identified a major locus for TST positivity (TST=0 vs >0mm) at chromosome region 11p14, and a locus for quantitative TST reaction at chromosome region 5p15.92 The 11p14 result was recently replicated in a prospective household contact study of 97 families in Paris, a low tuberculosis prevalence region,93 and the locus overlaps a region involved in Tumour Necrosis Factor alpha (TNFα) production.94 Investigation of the persistently TST negative phenotype in both children and adults in Uganda suggests that persistent TST negativity is not attributable to clinical or epidemiological characteristics alone, but also has genetic contributions and differential IFNγ responses.45,46,95
In addition to genetic contributions, modifiable factors such as vaccination or co-infection also play a role. The improved specificity of IGRAs to identify infection has enabled detection of a vaccine-inducible protective effect, with BCG having an estimated 19% efficacy (95% CI 8-29%) to protect against infection.19,90 To date, this has proven difficult to improve upon, with the MVA85A phase IIb vaccine trial showing no additional efficacy against M tuberculosis infection (–3·8%, 95% confidence interval: –28·1 to 15·9). However, the study was not powered for this endpoint.21 There are tentative associations that protozoal infections may protect against tuberculosis infection.87,96
Risk of pulmonary tuberculosis disease
Data from the pre-chemotherapy era established clearly that age is one of the most significant factors in determining which children will progress to disease. In the absence of any preventive therapy, infected infants have a 50% risk of progression to disease. However, this risk reduces between 5 and 10 years of age, before then rising again as children enter adolescence (Figure 1).10,97–99 The pattern of increased susceptibility with young age is confirmed by recent data, with progression rates for untreated patients with IGRA-confirmed infection of 43% for children under five years, significantly higher than the equivalent rate of 10.3% for adults.100 A recent Dutch study of contacts of patients with TB again showed a significant age-related effect with a 5-year risk of prevalent and incident TB of 33.3% in contacts less than 5 years compared to 6.7% in those older than 15 years.99
HIV co-infection is critical in this context - infants with HIV are twenty-four times more likely to develop tuberculosis than infants without HIV,59 with falling CD4 counts increasing risk.101,102 Antiretroviral therapy significantly reduces the risk, with most of the effect achieved within the first year of treatment.103,104 However, the risk of tuberculosis remains consistently elevated above that of the general population.103 Restoration of CD4+ numbers is not associated with significant increases in production of IFNγ in response to mycobacteria.105 Children who are malnourished or who have other forms of immune deficiency have been shown to be more vulnerable as well.106,107 However, it remains difficult to establish whether the tuberculosis leads to malnutrition or vice versa.
Beyond age-related differences, the ability to distinguish children with high risk of progressing from infection to disease on the basis of currently available clinical tests remains poor. In a multi-site study in Europe of 5,020 tuberculosis contacts, where 10% of contacts were <14 years, the positive predictive values for IGRAs were below 2%.47 A recent systematic review and meta-analysis, where 8/15 of the included trials involved paediatric participants, showed similar incidence rate ratios for tuberculosis disease progression for a TST>10mm (1·60 [0·94–2·72]) or a positive IGRA (2·11 [95% CI 1·29–3·46]).108 Prospective follow-up of a South African Adolescent Cohort study showed that IGRA conversion from a baseline negative test result to a positive result had a positive predictive value for the development of tuberculosis disease within 2 years of 2.6%, representing an 8-fold higher risk than those who did not convert.32 An increased relative risk remained even in cases where a positive IGRA reverted to negative.36
Whether the magnitude of TST reactions or IFNγ responses can further stratify risk is debatable.35,100,108,109 A study of nearly 20,000 children in Hong Kong, where newborn BCG vaccinations rates are high, who were administered a TST aged 6-10 years showed that those with a result >15mm had a 12-fold increased risk of developing TB during adolescence compared to those with a result between 10mm (the WHO threshold for a positive TST result) and 14mm.109 The odds of developing TB nearly doubled with every unit of IFNγ measured per millilitre in IGRAs in another study,100 but did not significantly affect risk of TB in other populations.47,108 A recently published analysis of the infant cohort from the MVA85A vaccine trial showed that infants with conversion to a positive IGRA with conventional thresholds showed no greater risk of developing TB than those with a persistent negative IGRA. However, those who had a quantitative result ten times greater than the conventional threshold for positivity had significantly higher risk of developing tuberculosis than both IGRA negative, and lower magnitude IGRA positive infants.35
Analyses of the MVA85A trial and South African Adolescent Cohort study have identified additional novel risk factors for developing disease (Table 1).14,32,35 A 16-gene, whole blood RNA signature was identified in adolescents that could predict progression from a positive IGRA to tuberculosis prior to developing symptoms or clinical diagnosis, with increasing sensitivity the closer to the time of diagnosis (Table 1).14 Whether this signature reflects early subclinical disease or could be used to stratify treatment and follow-up for children infected with M tuberculosis remains to be established. In both the MVA85A and Adolescent Cohort Study populations, activated HLA-DR expressing CD4+ T-cells were associated with increased risk of tuberculosis (Table 1).31 This observation, alongside the recently recognized importance of type I interferons,13 has prompted much discussion as to whether part of the susceptibility to tuberculosis is related to viral co-infections including CMV. In the MVA85A cohort, CMV was identified as a co-factor of interest, with a statistical association with HLA-DR+CD8+ cells, that in turn was associated with HLA-DR+CD4+ cells. CMV responses were however not directly associated with HLA-DR+CD4+ cells themselves, nor with risk of tuberculosis in this study.31 In an unrelated study, CD27 effector memory phenotype of CMV-specific IFNγ-producing CD4+ T cells differed in HIV-uninfected adults with TB compared to controls, although no effect was detectable in children.110 An elevated monocyte:lymphocyte ratio has been identified as a further risk factor for progression from case-control comparisons within a cohort of HIV-exposed infants (Table 1) and in a Madagascan household contact study.30,111
Genome-wide association studies to investigate genetic contributions to pulmonary tuberculosis susceptibility at a population level have primarily focused on adults. Several significant loci have been identified, but with relatively small effect sizes, challenges with replication in other populations, and uncertain functional significance.112–114 Involvement of HLA Class II 115,116 and SLC11A1 (formerly NRAMP1) 117 represent the most robust findings. Enzymes involved in inflammatory eicosanoid pathways may also play a role in paediatric susceptibility to TB.118 Through focusing on relatively early age of onset of pulmonary tuberculosis, several additional genetic loci have recently been identified (FOXP1 and AGMO,119 TOX,120 and STAT4 121 with tuberculosis onset <25 years). Vitamin D levels, in the context of genetic polymorphisms in receptors and the interaction with HIV, are additionally implicated in risk of disease.122,123 Seasonal peaks in childhood tuberculosis have been described in different communities and likely reflect a mixture of environment-related exposure factors combined with seasonal variations in vitamin D levels.124
Once again, the influence of helminth infection is unclear. In vitro studies show that pre-exposure or co-incident infection with filaria, hookworm, strongyloides and schistosoma is associated with down regulated or suppressed Th1 and Th17 responses to mycobacterial antigens.85 These immunological in vitro findings have not been replicated in patients with tuberculosis disease however.
Protection against pulmonary tuberculosis disease
Existing, safe, and effective interventions to prevent tuberculosis-exposed or tuberculosis-infected children from progressing to disease already exists.125,126 WHO makes a dual recommendation that household or close contacts of patients with tuberculosis are actively traced with particular focus on children,127 and that children <5 years of age, found not to have tuberculosis disease, should be given isoniazid daily for 6 months.128 The decades of experience with isoniazid preventive therapy in children show an estimated risk reduction for developing tuberculosis of 59%129 and a protective effect that can last at least 30 years.130 In one study the positive predictive value of a positive IGRA for progressing to disease was 17% in patients who did not receive preventive therapy, compared to 4% for the whole study population. A third of the patients were children.131 Negative predictive values for developing TB for both IGRAs and TST are consistently high at >98%, reinforcing that many tuberculosis-exposed children are able to effectively control the pathogen.47,100 The risk of developing isoniazid resistance through preventive therapy is extremely low,132 with a theoretical indirect benefit to drug-resistant strains; surveillance for drug resistance is key to identifying this.133 Shorter regimens, including 3 months of isoniazid and rifampicin, and 12 weekly doses of isoniazid and rifapentine are equally efficacious in children and young people, and may help improve treatment completion rates, known to be lower in adolescents.134,135 Appropriately chosen preventive therapy regimes in paediatric contacts of patients with MDR-TB appears to be effective.136 However, contact tracing and preventive therapy are not widely implemented in high tuberculosis-burden countries (Figure 4B) and this is an example of the need for health system strengthening alongside advocacy and human rights approaches to implement such strategies with already proven success.5,137
The evidence behind administering primary isoniazid prophylaxis without a known tuberculosis contact in HIV-infected children is less clearcut.25,128,129,138,139 In a study prior to the roll-out of antiretroviral therapy in sub-Saharan Africa, isoniazid decreased both all-cause mortality and confirmed or probable tuberculosis leading to the study being stopped early.138,139 However, more recently, 96 weeks of isoniazid primary prophylaxis showed no effect in HIV-exposed children, whether HIV-infected or not.25 Although acknowledging the low quality of the evidence, WHO recommends six months of isoniazid for children living with HIV over one year of age in high tuberculosis prevalence areas in the absence of symptoms of tuberculosis or a known tuberculosis contact.128 A trial comparing the continuation or cessation of co-trimoxazole in children living with HIV and receiving antiretroviral therapy beyond 96 weeks showed a three-fold reduction in tuberculosis incidence among those continuing, compared to those stopping.103 Whether this is a direct effect of co-trimoxazole on M tuberculosis or an effect on co-infections that may in turn affect the ability to contain M tuberculosis is unclear and merits further evaluation.
There is likely to be a vaccine-inducible protective effect against pulmonary tuberculosis disease in children.20,126,140,141 Neonatal BCG vaccination, as advised by WHO in high tuberculosis prevalence countries, appears to provide 60% protection against pulmonary tuberculosis in childhood (95% CI:42-71%), but offers much less protection against adult forms of pulmonary disease that contribute to transmission.20 A transient cessation in universal BCG vaccination between 1991 and 1996 in Greenland offered a well-controlled opportunity outside of a randomized trial to demonstrate a vaccine effectiveness of 50% against TB.140 A Peruvian contact tracing study showed a 65% decrease in prevalent and incident TB in BCG-vaccinated children under the age of ten years old, compared to their unvaccinated peers.126
The findings from case-control analyses which have investigated correlates of protection have been inconsistent to date. In the MVA85A cohort, where all infants were BCG-vaccinated, BCG IFNγ ELISpot responses correlated with reduced risk of tuberculosis.31 However, neither flow cytometric cellular analyses with intracellular cytokine profiling, nor gene expression profiing, yielded significant correlations between cases and controls from a cohort of infants enrolled in a trial of intradermal versus percutaneous BCG administration (Table 1).26,28,29 The many potential explanations for the different results between these studies include the number of cases identified, number of controls per case, type of matching between cases and controls, case definitions, difference in age of infants, choice of laboratory methodology, and statistical design. Data from the MVA85A cohort suggested that IgG against Ag85A 28 days after intervention might also correlate with protection.31 Overall, immunological correlates of protection remain distinctly lacking. Other potential correlates of protection include fetal growth, as higher birth weight is associated with lower risk of TB.107,142
Risk of severe tuberculosis disease and death
Both mycobacterial and host factors influence the risk of disease progression to disseminated disease or death.143–145 Beijing strain has been shown in some studies to be associated with disseminated disease in adults.75 However, this pattern has not been demonstrated convincingly in children.145–147 Young age, MDR-TB, HIV infection, malnutrition, extrapulmonary TB and a TST result of <5mm, have all been found to be associated with death from TB in children.7,8,143,144,148 Immunological explanations for why some children succumb to severe TB however remain elusive.149 One of the major contributions to the field of tuberculosis biology has been the description of genetic susceptibilities underlying rare and severe infections by normally avirulent mycobacteria in children.49–51 The subsequent identification of inherited defects in the IFNγ receptors, IFNγ genes or associated signaling pathways provided key mechanistic insights into the important role of IFNγ in humans. A hallmark of the clinical presentation in patients with complete IFNγ receptor deficiency is impaired granuloma formation, in line with the importance of IFNγ for containment of mycobacteria. Mutations in STAT1, the IL-12 pathway, and NEMO are amongst those more recently identified that lead to the clinical constellation of Mendelian susceptibility to mycobacterial disease.51 Increased susceptibility to mycobacteria in more generalized primary immunodeficiencies such as Severe Combined Immunodeficiency, Chronic Granulomatous Disease and GATA2 deficiency confirm the crucial role that T cells, neutrophils, and antigen-presenting cells respectively play in the paediatric response to mycobacteria.51 Iatrogenic immunodeficiency, through treatment of chronic inflammatory disorders with anti-TNFα monoclonal antibody treatment, is known to cause severe mycobacterial disease in children. However, it occurs relatively rarely in the context of screening for and treatment of infection prior to commencing immunosuppressive therapy.150
Protection against severe tuberculosis disease
Evidence for the efficacy of BCG is strongest for prevention of severe disease. BCG protects young children from tuberculous meningitis and miliary tuberculosis with an efficacy of 75-85%.18,20 However, this varies in different geographical regions, with a reported efficacy in the United Kingdom of up to 80% versus 0-20% in low income countries nearer the equator, where there is greater prevalence of helminth infections and exposure to environmental mycobacteria.20,141 The immunogenicity of BCG vaccines is decreased by suppressed cellular immune responses to mycobacterial antigens and increased TGF-beta production in individuals with helminth infections.19,151
WHO states that tuberculosis contact investigations “contribute to early identification of active tuberculosis, thus decreasing its severity.”127 Ten percent of children under the age of five years, evaluated through contact tracing in low-middle income countries, are found to have prevalent tuberculosis,39 with small numbers of severe cases identified in clinical trials with active follow-up. Combined with the natural history of tuberculosis in children, it is firmly established that early case identification and treatment initiation through contact tracing prevents progression to severe disease.10
Future directions
As discussed in this review, despite many years of substantial research, through combinations of hypothesis-based and hypothesis-generating methodologies, with the evaluation of unstimulated and mycobacteria-stimulated samples, and with more than 15,000 infants and adolescents enrolled into trials that enabled case-control comparisons, true correlates of protective paediatric immunity remain elusive (Table 1). It remains essentially unknown why BCG protects some children but not others. Differential responses to BCG vaccination identified through gene expression may have provided clues, although these findings also do not correlate with protection.29 Although some correlates of risk have now been identified (e.g. HLA-DR+CD4+ T-cells,31 higher monocyte:lymphocyte ratios,30,111 and a 16-gene RNA signature14) the underlying biology still remains to be elucidated and these parameters are not easily tractable to inform vaccine design. There are at least 14 novel vaccines in clinical trials up to Phase IIb,152 but to date it has proven difficult to exceed the effectiveness of BCG,21 which is approaching 100 years since it was first used in humans. Furthermore, despite failing to prevent adolescent and adult tuberculosis that drive the ongoing epidemic, BCG induces strong IFNγ responses and not only protects children against infection, pulmonary disease, and disseminated tuberculosis, it also protects against leprosy with an estimated efficacy of 26%,153 and appears to have heterologous protective effects.154–156 Thus, even though BCG vaccination is clearly not halting the epidemic, BCG is proving to be a hard act to follow and it remains difficult to move beyond its use in infants when assessing novel vaccines. In areas where TB incidence is high enough to enable large studies with sufficient statistical power, HIV-exposed infants represent a specific subpopulation where such vaccines can be trialled as a true alternative to BCG, given the known potential of BCG to cause dissemination in HIV-positive infants.157 A range of approaches including combinations of novel vaccines with BCG in prime-boost strategies, post-exposure vaccination, and improved prevention of infection are all also under consideration.17,22,90 These endeavours are inevitably hamstrung by the limited knowledge of what immune response an ideal tuberculosis vaccine should induce.
To make progress in protecting children against tuberculosis, parallel and multidisciplinary approaches are needed. Poverty remains the most significant risk factor (Figure 4) and therefore universal health coverage, social protection and justice, and poverty alleviation are all part of the solution.5,65,67,158 Furthermore, there are already existing and efficacious interventions: contact tracing, preventive therapy for children under the age of 5; prevention, diagnosis and treatment of paediatric HIV; infection control precautions; and training of front line health workers to improve diagnosis.5 Alongside implementing what we already know works, the research agenda needs to focus on unravelling the biology behind the glimmers of insight into innate, adaptive and trained immunity, both in susceptible and protected populations, to enable more rational vaccine development and make progress towards zero childhood tuberculosis deaths (Box 1). 1,22
Box 1. Proposed research priorities to accelerate understanding of risk and protection of children affected by Mycobacterium tuberculosis.
Studying populations at extremes of susceptibility and protection:
- Highly vulnerable with exposure to TB:
-
○Under 3 years old
-
○Adolescents
-
○Immunocompromise
-
○TB meningitis/miliary TB/death
-
○
- Relatively protected with exposure to TB:
-
○Exposed uninfected children
-
○School-age/pre-adolescence
-
○
Sociocultural and epidemiological factors:
-
○
Pre-school children – interactions between the child’s health and that of the primary caregiver, role of nutrition and poverty.
-
○
Adolescence – pregnancy, sexual behaviours including risk of HIV infection, drugs, alcohol, tobacco, health-seeking behaviours, treatment concordance, poverty.
Pathogen determinants of risk and protection:
Impact of mycobacterial strain on host immune response
Impact of drug resistance on host immune response
Improved understanding of existing interventions
Heterogeneity of responses to BCG
Heterologous effects of BCG
Re-evaluation of preventive therapy in HIV-infected children
Optimal preventive therapy in those exposed to MDR-TB
Interpretation of host immune responses:
In vitro and in vivo experimental follow-up of correlates of risk and protection identified from clinical studies
Biological exploration of gene expression data
Ontogeny of the immune system through childhood into adulthood.
Maximising yield from prospective studies
Collaborative and co-ordinated approach
Multiple study sites
Combinations of hypothesis-testing and hypothesis-generating approaches
Methodological developments to minimize required sample volumes
Combinations of use of fresh and frozen samples
Study of non-stimulated and mycobacterial-stimulated samples.
Beyond blood
Where ethically and clinically appropriate, further exploration of advanced radiological imaging in children exposed to TB
Use of readily available non-sputum based samples e.g. urine, stool, saliva and nasopharyngeal aspirates
Where ethically and clinically appropriate, use of samples from site of disease e.g. cerebrospinal fluid, lymph nodes, alveolar macrophages
Co-infection
Exploration of immunological basis for increased risk of TB infection in HIV-exposed but uninfected children
Exploration of influence of non-tuberculous mycobacteria, helminth, protozoal, cytomegalovirus, influenza, and microbiota interactions on paediatric response to mycobacteria.
Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles, through combinations of the search terms “tuberculosis” “risk” “susceptibility” “immune correlate” “epidemiology” “infection” “disease” “correlate” “preventive” “prophylactic” “protect*” “genome-wide association study” “genetics” “immunology” restricted to humans and children (0-18 years) without language restrictions until 30th April 2017. Articles resulting from these searches and relevant references cited in those articles were reviewed with preference for inclusion in this review of the latest evidence for publications within the last ten years.
Acknowledgements
RB’s Clinical Research Training Fellowship is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union (MR/K023446/1). EW’s and JAS’ Academic Clinical Lectureships are funded by the National Institute of Health Research (NIHR). JAS is also supported by the Academy of Medical Sciences and a Fulbright Scholarship. BK is supported by MRC Program Grant MR/K011944/1, MRC-GCRF Foundation award MR/P024270/1 and NIHR Senior Fellowship SRF-2009-02-07.
Role of funding source:
Funding sources were not involved in scope of the review, its preparation, nor the decision to submit the paper for publication
Footnotes
Authors' contributions:
RB, JAS and BK initiated the review. RB, EW, JAS, and BK developed the scope of the manuscript. RB, EW, and JAS conducted the literature searches and prepared the first draft. BK critically reviewed the data and draft, and all authors subsequently modified the manuscript jointly. All authors approved the final submitted version of the manuscript.
Conflict of interest statements:
BK holds a patent for a paediatric diagnostic biosignature. RB was a consultant for FIND, Geneva. All other authors declare no competing interests.
Ethics committee approval: Not applicable.
References
- 1.The World Health Organization. The Roadmap for Childhood TB: Toward Zero Deaths (WHO/HTM/TB/2013.12) 2013. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2017. 2017. [Google Scholar]
- 3.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16:1193–201. doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348–61. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 5.Basu Roy R, Brandt N, Moodie N, et al. Why the Convention on the Rights of the Child must become a guiding framework for the realization of the rights of children affected by tuberculosis. BMC Int Health Hum Rights. 2016;16:32. doi: 10.1186/s12914-016-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387:2423–78. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:947–57. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:285–95. doi: 10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donald PR, Marais BJ, Barry CE. Age and the epidemiology and pathogenesis of tuberculosis. Lancet. 2010;375:1852–4. doi: 10.1016/S0140-6736(10)60580-6. [DOI] [PubMed] [Google Scholar]
- 10.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intrathoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 11.Wallgren A. Primary pulmonary tuberculosis in childhood. Am J Dis Child. 1935;49:1105–1136. [Google Scholar]
- 12.Loveday M, Sunkari B, Marais BJ, Master I, Brust JCM. Dilemma of managing asymptomatic children referred with ‘culture-confirmed’ drug-resistant tuberculosis. Arch Dis Child. 2016;101:608–13. doi: 10.1136/archdischild-2015-310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry MPR, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387:2312–22. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esmail H, Lai RP, Lesosky M, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[18F]fluoro-D-glucose positron emission and computed tomography. Nat Med. 2016:1–6. doi: 10.1038/nm.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenaerts A, Barry CE, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lienhardt C, Lönnroth K, Menzies D, et al. Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions. PLoS Med. 2016;13:e1001965. doi: 10.1371/journal.pmed.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 19.Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349 doi: 10.1136/bmj.g4643. g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin Infect Dis. 2014;58:470–80. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 21.Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp CL, Wilson CB, Stuart LM. Tuberculosis vaccines: Barriers and prospects on the quest for a transformative tool. Immunol Rev. 2015;264:363–81. doi: 10.1111/imr.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tameris M, McShane H, McClain JB, et al. Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG. Tuberculosis. 2013;93:143–9. doi: 10.1016/j.tube.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashangura R, Sena ES, Young T, Garner P. Effects of MVA85A vaccine on tuberculosis challenge in animals: systematic review. Int J Epidemiol. 2015;44:1970–81. doi: 10.1093/ije/dyv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhi S, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkridge A, Hatherill M, Little F, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahomed H, Ehrlich R, Hawkridge T, et al. TB incidence in an adolescent cohort in South Africa. PLoS One. 2013;8:e59652. doi: 10.1371/journal.pone.0059652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagina BMN, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher HA, Filali-Mouhim A, Nemes E, et al. Human newborn bacille Calmette-Guérin vaccination and risk of tuberculosis disease: a case-control study. BMC Med. 2016;14:76. doi: 10.1186/s12916-016-0617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naranbhai V, Kim S, Fletcher H, et al. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med. 2014;12:120. doi: 10.1186/s12916-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7 doi: 10.1038/ncomms11290. 11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machingaidze S, Verver S, Mulenga H, et al. Predictive Value of Recent QuantiFERON Conversion for Tuberculosis Disease in Adolescents. Am J Respir Crit Care Med. 2012;186:1051–6. doi: 10.1164/rccm.201206-1134OC. [DOI] [PubMed] [Google Scholar]
- 33.Mahomed H, Hawkridge T, Verver S, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15:331–6. [PubMed] [Google Scholar]
- 34.Mahomed H, Hawkridge T, Verver S, et al. The Tuberculin Skin Test versus QuantiFERON TB Gold® in Predicting Tuberculosis Disease in an Adolescent Cohort Study in South Africa. PLoS One. 2011;6:e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med. 2017;5:282–90. doi: 10.1016/S2213-2600(17)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews JR, Hatherill M, Mahomed H, et al. The Dynamics of QuantiFERON-TB Gold In-Tube Conversion and Reversion in a Cohort of South African Adolescents. Am J Respir Crit Care Med. 2015;191:584–91. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris SA, Satti I, Matsumiya M, et al. Process of assay selection and optimization for the study of case and control samples from a phase IIb efficacy trial of a candidate tuberculosis vaccine, MVA85A. Clin Vaccine Immunol. 2014;21:1005–11. doi: 10.1128/CVI.00128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill PC, Ota MOC. Tuberculosis case-contact research in endemic tropical settings: design, conduct, and relevance to other infectious diseases. Lancet Infect Dis. 2010;10:723–32. doi: 10.1016/S1473-3099(10)70164-X. [DOI] [PubMed] [Google Scholar]
- 39.Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gessner BD, Weiss NS, Nolan CM. Risk factors for pediatric tuberculosis infection and disease after household exposure to adult index cases in Alaska. J Pediatr. 1998;132:509–13. doi: 10.1016/s0022-3476(98)70029-0. [DOI] [PubMed] [Google Scholar]
- 41.Lienhardt C, Sillah J, Fielding K, et al. Risk factors for tuberculosis infection in children in contact with infectious tuberculosis cases in the Gambia, West Africa. Pediatrics. 2003;111:e608–14. doi: 10.1542/peds.111.5.e608. [DOI] [PubMed] [Google Scholar]
- 42.Marais BJ, Hesseling AC, Schaaf HS, Gie RP, van Helden PD, Warren RM. Mycobacterium tuberculosis transmission is not related to household genotype in a setting of high endemicity. J Clin Microbiol. 2009;47:1338–43. doi: 10.1128/JCM.02490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutherford ME, Hill PC, Maharani W, et al. Risk factors for Mycobacterium tuberculosis infection in Indonesian children living with a sputum smear-positive case. Int J Tuberc Lung Dis. 2012;16:1594–9. doi: 10.5588/ijtld.12.0389. [DOI] [PubMed] [Google Scholar]
- 44.Gillman A, Berggren I, Bergstrom SE, Wahlgren H, Bennet R. Primary tuberculosis infection in 35 children at a Swedish day care center. Pediatr Infect Dis J. 2008;27:1078–82. doi: 10.1097/INF.0b013e31817e83f4. [DOI] [PubMed] [Google Scholar]
- 45.Ma N, Zalwango S, Malone LL, et al. Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC Infect Dis. 2014;14:352. doi: 10.1186/1471-2334-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahan CS, Zalwango S, Thiel Ba, et al. Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. Am J Trop Med Hyg. 2012;86:690–7. doi: 10.4269/ajtmh.2012.11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zellweger J-P, Sotgiu G, Block M, et al. Risk Assessment of Tuberculosis in Contacts by IFN-γ Release Assays. A Tuberculosis Network European Trials Group Study. Am J Respir Crit Care Med. 2015;191:1176–84. doi: 10.1164/rccm.201502-0232OC. [DOI] [PubMed] [Google Scholar]
- 48.Grandjean L, Gilman RH, Martin L, et al. Transmission of Multidrug-Resistant and Drug-Susceptible Tuberculosis within Households: A Prospective Cohort Study. PLoS Med. 2015;12:e1001843. doi: 10.1371/journal.pmed.1001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcaïs A, Fieschi C, Abel L, Casanova J-L. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med. 2005;202:1617–21. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 51.Boisson-Dupuis S, Bustamante J, El-Baghdadi J, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev. 2015;264:103–20. doi: 10.1111/imr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith S, Jacobs RF, Wilson CB. Immunobiology of childhood tuberculosis: a window on the ontogeny of cellular immunity. J Pediatr. 1997;131:16–26. doi: 10.1016/s0022-3476(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 53.Davis CA, Vallota EH, Forristal J. Serum complement levels in infancy: age related changes. Pediatr Res. 1979;13:1043–6. doi: 10.1203/00006450-197909000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Cosar H, Ozkinay F, Onay H, et al. Low levels of mannose-binding lectin confers protection against tuberculosis in Turkish children. Eur J Clin Microbiol Infect Dis. 2008;27:1165–9. doi: 10.1007/s10096-008-0573-8. [DOI] [PubMed] [Google Scholar]
- 55.Burl S, Townend J, Njie-Jobe J, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shey MS, Nemes E, Whatney W, et al. Maturation of Innate Responses to Mycobacteria over the First 9 Months of Life. J Immunol. 2014;192:4833–43. doi: 10.4049/jimmunol.1400062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi H, Sun L, Wu X, et al. Toll-like receptor 1(TLR1) Gene SNP rs5743618 is associated with increased risk for tuberculosis in Han Chinese children. Tuberc. 2015;95:197–203. doi: 10.1016/j.tube.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Eum S-Y, Kong J-H, Hong M-S, et al. Neutrophils Are the Predominant Infected Phagocytic Cells in the Airways of Patients With Active Pulmonary TB. Chest. 2010;137:122–8. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–14. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 60.Whittaker E, Nicol M, Zar HJ, Kampmann B. Regulatory T Cells and Pro-inflammatory Responses Predominate in Children with Tuberculosis. Front Immunol. 2017;8:448. doi: 10.3389/fimmu.2017.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scriba TJ, Kalsdorf B, Abrahams D-A, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 63.Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The Contribution of Non-Conventional T Cells and NK Cells in the Mycobacterial-Specific IFNγ Response in Bacille Calmette-Guérin (BCG)-Immunized Infants. PLoS One. 2013;8:e77334. doi: 10.1371/journal.pone.0077334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Liberto D, Buccheri S, Caccamo N, et al. Decreased serum granulysin levels in childhood tuberculosis which reverse after therapy. Tuberculosis. 2007;87:322–8. doi: 10.1016/j.tube.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janssens J-P, Rieder HL. An ecological analysis of incidence of tuberculosis and per capita gross domestic product. Eur Respir J. 2008;32 doi: 10.1183/09031936.00078708. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. Global Tuberculosis Report 2016. 2016. [Google Scholar]
- 67.World Health Organization. Addressing Poverty in TB Control. Options for National TB Control Programmes (WHO/HTM/TB/2005.352) 2005. [Google Scholar]
- 68.Lule SA, Mawa PA, Nkurunungi G, et al. Factors associated with tuberculosis infection, and with anti-mycobacterial immune responses, among five year olds BCG-immunised at birth in Entebbe, Uganda. Vaccine. 2015;33:796–804. doi: 10.1016/j.vaccine.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lygizos M, Shenoi SV, Brooks RP, et al. Natural ventilation reduces high TB transmission risk in traditional homes in rural KwaZulu-Natal, South Africa. BMC Infect Dis. 2013;13:300. doi: 10.1186/1471-2334-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guelar A, Gatell JM, Verdejo J, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;7:1345–9. doi: 10.1097/00002030-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Lonnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egere U, Togun T, Sillah A, et al. Identifying children with tuberculosis among household contacts in The Gambia. Int J Tuberc Lung Dis. 2017;21:46–52. doi: 10.5588/ijtld.16.0289. [DOI] [PubMed] [Google Scholar]
- 74.Jafta N, Jeena PM, Barregard L, Naidoo RN. Childhood tuberculosis and exposure to indoor air pollution: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:596–602. doi: 10.5588/ijtld.14.0686. [DOI] [PubMed] [Google Scholar]
- 75.Hanekom M, van der Spuy GD, Streicher E, et al. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J Clin Microbiol. 2007;45:1483–90. doi: 10.1128/JCM.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuberculosis Research Centre, Indian Council of Medical Research (ICMR), Chennai I. Risk of tuberculosis among contacts of isoniazid-resistant and isoniazid-susceptible cases. Int J Tuberc Lung Dis. 2011;15:782–8. doi: 10.5588/ijtld.09.0327. [DOI] [PubMed] [Google Scholar]
- 77.Zelner JL, Murray MB, Becerra MC, et al. Age-specific risks of tuberculosis infection from household and community exposures and opportunities for interventions in a high-burden setting. Am J Epidemiol. 2014;180:853–61. doi: 10.1093/aje/kwu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 79.Biraro IA, Egesa M, Toulza F, et al. Impact of Co-Infections and BCG Immunisation on Immune Responses among Household Contacts of Tuberculosis Patients in a Ugandan Cohort. PLoS One. 2014;9:e111517. doi: 10.1371/journal.pone.0111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones CE, Hesseling AC, Tena-Coki NG, et al. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination. AIDS. 2015;29:155–65. doi: 10.1097/QAD.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marquez C, Chamie G, Achan J, et al. Tuberculosis Infection in Early Childhood and the Association with HIV-exposure in HIV-uninfected Children in Rural Uganda. Pediatr Infect Dis J. 2016;35:524–9. doi: 10.1097/INF.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia-Knight MA, Nduati E, Hassan AS, et al. Altered Memory T-Cell Responses to Bacillus Calmette-Guerin and Tetanus Toxoid Vaccination and Altered Cytokine Responses to Polyclonal Stimulation in HIV-Exposed Uninfected Kenyan Infants. PLoS One. 2015;10:e0143043. doi: 10.1371/journal.pone.0143043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hesseling AC, Jaspan HB, Black GF, Nene N, Walzl G. Immunogenicity of BCG in HIV-exposed and non-exposed infants following routine birth or delayed vaccination. Int J Tuberc Lung Dis. 2015;19:454–62. doi: 10.5588/ijtld.14.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–84. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 85.Babu S, Nutman TB. Helminth-Tuberculosis Co-infection: An Immunologic Perspective. Trends Immunol. 2016;37:597–607. doi: 10.1016/j.it.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van der Zalm MM, van Soelen N, Mandalakas AM, et al. The Effect of Deworming on Tests of Tuberculosis Infection in Children With Recent Tuberculosis Exposure. Pediatr Infect Dis J. 2016;35:622–7. doi: 10.1097/INF.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verhagen LM, Hermans PW, Warris A, et al. Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis. 2012;92:505–12. doi: 10.1016/j.tube.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 88.van Soelen N, Mandalakas AM, Kirchner HL, et al. Effect of Ascaris Lumbricoides specific IgE on tuberculin skin test responses in children in a high-burden setting: a cross-sectional community-based study. BMC Infect Dis. 2012;12:211. doi: 10.1186/1471-2334-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: A new frontier in prevention. Immunology. 2014;141:506–13. doi: 10.1111/imm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hawn TR, Day TA, Scriba TJ, et al. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev. 2014;78:650–71. doi: 10.1128/MMBR.00021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cobat A, Barrera LF, Henao H, et al. Tuberculin skin test reactivity is dependent on host genetic background in Colombian tuberculosis household contacts. Clin Infect Dis. 2012;54:968–71. doi: 10.1093/cid/cir972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cobat A, Gallant CJ, Simkin L, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206:2583–91. doi: 10.1084/jem.20090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cobat A, Poirier C, Hoal E, et al. Tuberculin skin test negativity is under tight genetic control of chromosomal region 11p14-15 in settings with different tuberculosis endemicities. J Infect Dis. 2015;211:317–21. doi: 10.1093/infdis/jiu446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cobat A, Hoal EG, Gallant CJ, et al. Identification of a Major Locus, TNF1, That Controls BCG-Triggered Tumor Necrosis Factor Production by Leukocytes in an Area Hyperendemic for Tuberculosis. Clin Infect Dis. 2013;57:963–70. doi: 10.1093/cid/cit438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stein CM, Zalwango S, Malone LL, et al. Genome Scan of M. tuberculosis Infection and Disease in Ugandans. PLoS One. 2008;3:e4094. doi: 10.1371/journal.pone.0004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franke MF, Del Castillo H, Pereda Y, et al. Parasite infection and tuberculosis disease among children: a case-control study. Am J Trop Med Hyg. 2014;90:279–82. doi: 10.4269/ajtmh.13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 98.Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:278–85. [PubMed] [Google Scholar]
- 99.Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of Tuberculosis after Recent Exposure. A 10-Year Follow-up Study of Contacts in Amsterdam. Am J Respir Crit Care Med. 2014;190:1044–52. doi: 10.1164/rccm.201406-1159OC. [DOI] [PubMed] [Google Scholar]
- 100.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and Positive Predictive Value of a Whole-Blood Interferon-γ Release Assay for Developing Active Tuberculosis. Am J Respir Crit Care Med. 2011;183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 101.Dodd PJ, Seddon JA. Understanding the contribution of HIV to the risk of developing tuberculosis in children: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:S64. [Google Scholar]
- 102.Mansoor N, Scriba TJJ, de Kock M, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette-Guérin vaccine. J Infect Dis. 2009;199:982–90. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crook AM, Turkova A, Musiime V, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Med. 2016;14:50. doi: 10.1186/s12916-016-0593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edmonds A, Lusiama J, Napravnik S, Kitetele F, Van Rie A, Behets F. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38:1612–21. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kampmann B, Tena-Coki GN, Nicol MP, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS. 2006;20:1011–8. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 106.Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. J Infect Dis. 2012;206:1809–15. doi: 10.1093/infdis/jis608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olofin IO, Liu E, Manji KP, et al. Active Tuberculosis in HIV-Exposed Tanzanian Children up to 2 years of Age: Early-Life Nutrition, Multivitamin Supplementation and Other Potential Risk Factors. J Trop Pediatr. 2016;62:29–37. doi: 10.1093/tropej/fmv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis : a systematic review and meta-analysis. 2011;3099:1–11. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leung CC, Yew WW, Au KF, et al. A Strong Tuberculin Reaction in Primary School Children Predicts Tuberculosis in Adolescence. Pediatr Infect Dis J. 2012;31:150–3. doi: 10.1097/INF.0b013e318236ae2b. [DOI] [PubMed] [Google Scholar]
- 110.Portevin D, Moukambi F, Mpina M, et al. Maturation and Mip-1β Production of Cytomegalovirus-Specific T Cell Responses in Tanzanian Children, Adolescents and Adults: Impact by HIV and Mycobacterium tuberculosis Co-Infections. PLoS One. 2015;10:e0126716. doi: 10.1371/journal.pone.0126716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rakotosamimanana N, Richard V, Raharimanga V, et al. Biomarkers for risk of developing active tuberculosis in contacts of TB patients: a prospective cohort study. Eur Respir J. 2015;46 doi: 10.1183/13993003.00263-2015. [DOI] [PubMed] [Google Scholar]
- 112.Thye T, Owusu-Dabo E, Vannberg FO, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet. 2012;44:257–9. doi: 10.1038/ng.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thye T, Vannberg FO, Wong SH, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet. 2010;42:739–41. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bellamy R, Beyers N, McAdam KP, et al. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc Natl Acad Sci. 2000;97:8005–9. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldfeld AE, Delgado JC, Thim S, et al. Association of an HLA-DQ Allele With Clinical Tuberculosis. JAMA. 1998;279:226. doi: 10.1001/jama.279.3.226. [DOI] [PubMed] [Google Scholar]
- 116.Sveinbjornsson G, Gudbjartsson DF, Halldorsson BV, et al. HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nat Genet. 2016;48:318–22. doi: 10.1038/ng.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bellamy R, Ruwende C, Corrah T, McAdam KPWJ, Whittle HC, Hill AVS. Variations in the NRAMP1 Gene and Susceptibility to Tuberculosis in West Africans. N Engl J Med. 1998;338:640–4. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 118.Shen C, Wu X-R, Wang B-B, et al. ALOX5 is associated with tuberculosis in a subset of the pediatric population of North China. Genet Test Mol Biomarkers. 2013;17:284–8. doi: 10.1089/gtmb.2012.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grant AV, Sabri A, Abid A, et al. A genome-wide association study of pulmonary tuberculosis in Morocco. Hum Genet. 2016;135:299–307. doi: 10.1007/s00439-016-1633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grant AV, El Baghdadi J, Sabri A, et al. Age-Dependent Association between Pulmonary Tuberculosis and Common TOX Variants in the 8q12–13 Linkage Region. Am J Hum Genet. 2013;92:407–14. doi: 10.1016/j.ajhg.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sabri A, Grant AV, Cosker K, et al. Association study of genes controlling IL-12-dependent IFN-γ immunity: STAT4 alleles increase risk of pulmonary tuberculosis in Morocco. J Infect Dis. 2014;210:611–8. doi: 10.1093/infdis/jiu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 123.Gupta A, Montepiedra G, Gupte A, et al. Low Vitamin-D Levels Combined with PKP3-SIGIRR-TMEM16J Host Variants Is Associated with Tuberculosis and Death in HIV-Infected and -Exposed Infants. PLoS One. 2016;11:e0148649. doi: 10.1371/journal.pone.0148649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaaf HS, Nel ED, Beyers N, Gie RP, Scott F, Donald PR. A decade of experience with Mycobacterium tuberculosis culture from children: a seasonal influence on incidence of childhood tuberculosis. Tuber Lung Dis. 1996;77:43–6. doi: 10.1016/s0962-8479(96)90074-x. [DOI] [PubMed] [Google Scholar]
- 125.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000:CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zelner JL, Murray MB, Becerra MC, et al. Bacillus Calmette-Guérin and Isoniazid Preventive Therapy Protect Contacts of Patients with Tuberculosis. Am J Respir Crit Care Med. 2014;189:853–9. doi: 10.1164/rccm.201310-1896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. WHO/HTM/TB/2012.9. 2012 [PubMed] [Google Scholar]
- 128.The World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. 2014. [PubMed] [Google Scholar]
- 129.Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta–analysis. BMC Infect Dis. 2014;14:91. doi: 10.1186/1471-2334-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hsu KHK. Thirty Years After Isoniazid: Its Impact on Tuberculosis in Children and Adolescents. JAMA. 1984;251:1283. doi: 10.1001/jama.251.10.1283. [DOI] [PubMed] [Google Scholar]
- 131.Altet N, Dominguez J, de Souza-Galvão M-L, et al. Predicting the Development of Tuberculosis with the Tuberculin Skin Test and QuantiFERON Testing. Ann Am Thorac Soc. 2015;12:680–8. doi: 10.1513/AnnalsATS.201408-394OC. [DOI] [PubMed] [Google Scholar]
- 132.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid Preventive Therapy and Risk for Resistant Tuberculosis. Emerg Infect Dis. 2006;12:744–51. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mills HL, Cohen T, Colijn C. Community-Wide Isoniazid Preventive Therapy Drives Drug-Resistant Tuberculosis: A Model-Based Analysis. Sci Transl Med. 2013;5:180ra49. doi: 10.1126/scitranslmed.3005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang S-H, Eitzman SR, Nahid P, Finelli MLU. Factors associated with failure to complete isoniazid therapy for latent tuberculosis infection in children and adolescents. J Infect Public Health. 2014;7:145–52. doi: 10.1016/j.jiph.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Cruz AT, Starke JR. Managing tuberculosis infection in children in the USA: an update. Future Microbiol. 2016;11:669–84. doi: 10.2217/fmb-2016-0027. [DOI] [PubMed] [Google Scholar]
- 136.Seddon JA, Hesseling AC, Finlayson H, et al. Preventive Therapy for Child Contacts of Multidrug-Resistant Tuberculosis: A Prospective Cohort Study. Clin Infect Dis. 2013;57:1676–84. doi: 10.1093/cid/cit655. [DOI] [PubMed] [Google Scholar]
- 137.Egere U, Sillah A, Togun T, et al. Isoniazid preventive treatment among child contacts of adults with smear-positive tuberculosis in The Gambia. Public Heal Action. 2016;6:226–31. doi: 10.5588/pha.16.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frigati LJ, Kranzer K, Cotton MF, Schaaf HS, Lombard CJ, Zar HJ. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax. 2011;66:496–501. doi: 10.1136/thx.2010.156752. [DOI] [PubMed] [Google Scholar]
- 139.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334 doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Michelsen SW, Soborg B, Koch A, et al. The effectiveness of BCG vaccination in preventing Mycobacterium tuberculosis infection and disease in Greenland. Thorax. 2014;69:851–6. doi: 10.1136/thoraxjnl-2014-205688. [DOI] [PubMed] [Google Scholar]
- 141.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 142.Villamor E, Iliadou A, Cnattingius S. Evidence for an effect of fetal growth on the risk of tuberculosis. J Infect Dis. 2010;201:409–13. doi: 10.1086/650313. [DOI] [PubMed] [Google Scholar]
- 143.Seddon JA, Visser DH, Bartens M, et al. Impact of Drug Resistance on Clinical Outcome in Children With Tuberculous Meningitis. Pediatr Infect Dis J. 2012;31:711–6. doi: 10.1097/INF.0b013e318253acf8. [DOI] [PubMed] [Google Scholar]
- 144.Hicks RM, Padayatchi N, Shah NS, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis. 2014;18:1074–83. doi: 10.5588/ijtld.14.0231. [DOI] [PubMed] [Google Scholar]
- 145.Pan Y, Yang Z, Liu R, Xing L, Peng Z, Zhu C. Host and Microbial Predictors of Childhood Extrathoracic Tuberculosis and Tuberculosis Meningitis. Pediatr Infect Dis J. 2015;34:1289–95. doi: 10.1097/INF.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 146.Hesseling AC, Marais BJ, Kirchner HL, et al. Mycobacterial genotype is associated with disease phenotype in children. Int J Tuberc Lung Dis. 2010;14:1252–8. [PubMed] [Google Scholar]
- 147.Maree F, Hesseling AC, Schaaf HS, et al. Absence of an association between Mycobacterium tuberculosis genotype and clinical features in children with tuberculous meningitis. Pediatr Infect Dis J. 2007;26:13–8. doi: 10.1097/01.inf.0000247044.05140.c7. [DOI] [PubMed] [Google Scholar]
- 148.Drobac PC, Shin SS, Huamani P, et al. Risk factors for in-hospital mortality among children with tuberculosis: the 25-year experience in Peru. Pediatrics. 2012;130:e373–9. doi: 10.1542/peds.2011-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sterling TR, Martire T, de Almeida AS, et al. Immune Function in Young Children With Previous Pulmonary or Miliary/Meningeal Tuberculosis and Impact of BCG Vaccination. Pediatrics. 2007;120:e912–21. doi: 10.1542/peds.2006-3150. [DOI] [PubMed] [Google Scholar]
- 150.Toussi SS, Pan N, Walters HM, Walsh TJ. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: systematic review of the literature. Clin Infect Dis. 2013;57:1318–30. doi: 10.1093/cid/cit489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 152.TB Vaccine Initiative. Pipeline of vaccines. [accessed March 12, 2017]; http://www.tbvi.eu/what-we-do/pipeline-of-vaccines/
- 153.Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6:162–70. doi: 10.1016/S1473-3099(06)70412-1. [DOI] [PubMed] [Google Scholar]
- 154.WHO Strategic Advisory Group of Experts. Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 — conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:221–36. [PubMed] [Google Scholar]
- 155.Higgins JPT, Soares-Weiser K, López-López JA, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355 doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Castro MJ, Pardo-Seco J, Martinon-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis. 2015;60:1611–9. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 157.Nemes E, Hesseling AC, Tameris M, et al. Safety and Immunogenicity of Newborn MVA85A Vaccination and Selective, Delayed Bacille Calmette-Guerin (BCG) for Infants of HIV Infected Mothers: A Phase 2 Randomized Controlled Trial. Clin Infect Dis. 2017 doi: 10.1093/cid/cix834. published online Sept 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stop TB Partnership. The Global Plan to End TB 2016 - 2020: The paradigm shift. 2015 [Google Scholar]
- 159.Jones C, Whittaker E, Bamford A, Kampmann B. Immunology and pathogenesis of childhood TB. Paediatr Respir Rev. 2011;12:3–8. doi: 10.1016/j.prrv.2010.09.006. [DOI] [PubMed] [Google Scholar]