Abstract
Background
This is an updated review originally published in 2004 and first updated in 2007. This version includes substantial changes to bring it in line with current methodological requirements. Methadone is a synthetic opioid that presents some challenges in dose titration and is recognised to cause potentially fatal arrhythmias in some patients. It does have a place in therapy for people who cannot tolerate other opioids but should be initiated only by experienced practitioners. This review is one of a suite of reviews on opioids for cancer pain.
Objectives
To determine the effectiveness and tolerability of methadone as an analgesic in adults and children with cancer pain.
Search methods
For this update we searched CENTRAL, MEDLINE, Embase, CINAHL, and clinicaltrials.gov, to May 2016, without language restriction. We also checked reference lists in relevant articles.
Selection criteria
We sought randomised controlled trials comparing methadone (any formulation and by any route) with active or placebo comparators in people with cancer pain.
Data collection and analysis
All authors agreed on studies for inclusion. We retrieved full texts whenever there was any uncertainty about eligibility. One review author extracted data, which were checked by another review author. There were insufficient comparable data for meta‐analysis. We extracted information on the effect of methadone on pain intensity or pain relief, the number or proportion of participants with 'no worse than mild pain'. We looked for data on withdrawal and adverse events. We looked specifically for information about adverse events relating to appetite, thirst, and somnolence. We assessed the evidence using GRADE and created a 'Summary of findings' table.
Main results
We revisited decisions made in the earlier version of this review and excluded five studies that were previously included. We identified one new study for this update. This review includes six studies with 388 participants. We did not identify any studies in children.
The included studies differed so much in their methods and comparisons that no synthesis of results was feasible. Only one study (103 participants) specifically reported the number of participants with a given level of pain relief, in this case a reduction of at least 20% ‐ similar in both the methadone and morphine groups. Using an outcome of 'no worse than mild pain', methadone was similar to morphine in effectiveness, and most participants who could tolerate methadone achieved 'no worse than mild pain'. Adverse event withdrawals with methadone were uncommon (12/202) and similar in other groups. Deaths were uncommon except in one study where the majority of participants died, irrespective of treatment group. For specific adverse events, somnolence was more common with methadone than with morphine, while dry mouth was more common with morphine than with methadone. None of the studies reported effects on appetite.
We judged the quality of evidence to be low, downgraded due to risk of bias and sparse data. For specific adverse events, we considered the quality of evidence to be very low, downgraded due to risk of bias, sparse data, and indirectness, as surrogates for appetite, thirst and somnolence were used.
There were no data on the use of methadone in children.
Authors' conclusions
Based on low‐quality evidence, methadone is a drug that has similar analgesic benefits to morphine and has a role in the management of cancer pain in adults. Other opioids such as morphine and fentanyl are easier to manage but may be more expensive than methadone in many economies.
Keywords: Adult; Aged; Aged, 80 and over; Humans; Middle Aged; Analgesics, Opioid; Analgesics, Opioid/therapeutic use; Methadone; Methadone/therapeutic use; Morphine; Morphine/therapeutic use; Neoplasms; Neoplasms/complications; Pain; Pain/drug therapy; Pain/etiology; Pain Measurement; Randomized Controlled Trials as Topic
Plain language summary
Methadone (an opioid drug) for treating people with cancer pain
Bottom line
Methadone taken by mouth provided good pain relief for most adults with moderate or severe cancer pain.
Background
One person in two or three who gets cancer will suffer from pain that becomes moderate or severe in intensity. The pain tends to get worse as the cancer progresses. Methadone has been used for many years as one of a number of different pain killers for cancer pain.
Study characteristics
In this updated review we set out to estimate how well methadone worked, how many people had side effects, and how severe those side effects were – for example, whether they were so severe that participants stopped taking their methadone.
In May 2016, we found just six studies with 388 adult participants. The studies were often small, and compared different preparations.
Key findings
For pain relief there did not seem to be much difference between methadone and morphine. For most people pain was reduced from moderate or severe to mild or no pain with methadone. Methadone is associated with some unwanted effects, mainly sleepiness, constipation, and dry mouth. These can be severe enough to stop people taking methadone. No data were available about the use of methadone in children.
We would like to see more consistency in study design, and especially in study reporting, which should include information on unwanted effects and the outcome of pain reduced to tolerable levels, that is, no worse than mild pain, so that people with cancer are not bothered by pain.
Quality of the evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low quality evidence means that we are very uncertain about the results. High quality evidence means that we are very confident in the results. The quality of the evidence was low or very low.
Summary of findings
Summary of findings for the main comparison. Methadone compared to Morphine for cancer pain.
| Methadone compared to morphine for cancer pain | ||||||
| Patient or population: people with cancer pain Intervention: methadone Comparison: morphine | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Morphine | Methadone | |||||
| Participant‐reported pain intensity |
Pain intensity scores: One study (103 participants) reported > 20% improvement in pain scores for 76% of morphine and 75% methadone participants in those that completed No worse than mild pain (pain score of 3/10 or less after treatment): One study (54 participants) reported all achieved no worse than mild pain based on mean pain scores. Two studies (148 participants) reported mean pain scores very close to a score of 3 |
⊕⊕⊝⊝ low1,2 | Downgraded two points for reasons stated | |||
| Adverse events: appetite, thirst, somnolence | Not estimable | Not estimable | Not estimable | 342 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | Downgraded three points for reasons stated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Risk of bias: random allocation and allocation concealment unclear, all had sample size of less than 200 per treatment arm. 2 Imprecision: sample size is smaller than optimal information size; confidence interval around estimate of effect is wide and included no effect and appreciable benefit/harm. 3 Indirectness. Surrogates for appetite, thirst and somnolence such as sedation, drowsiness and dry mouth used.
Background
This is the second update of the review entitled 'Methadone for Cancer Pain' first published in 2004, and updated in 2007 (Nicholson 2007). This version includes substantial changes to bring it in line with current methodological requirements for Cochrane reviews. This review is one of a suite of reviews on opioids for cancer pain.
Description of the condition
Cancer‐related pain is a common problem. Worldwide prevalence data almost two decades ago suggested that there were 17 million people living with cancer (Payne 1998). Assessments of the prevalence of pain from nationwide studies in different countries have revealed consistent results, indicating that 30% to 40% of patients in active therapy experience pain, with this rate increasing to 70% to 90% in patients with advanced and progressive disease. Van den Beuken‐van Everdingen estimated a prevalence of pain in excess of 50% for patients at all stages and with all types of cancer (van den Beuken‐van Everdingen 2007).
Cancer‐related pain may be caused by the tumour pressing on adjacent organs/tissues, or by the tumour invading the tissue and damaging it.
Pain has a significant impact on function. Uncontrolled pain is incompatible with satisfactory quality of existence and it is well recognised that persistent pain impairs daily life and social interaction. Studies highlight the increased risk of anxiety, depression and even suicidal ideation in patients with uncontrolled pain (Graner 2016).
Description of the intervention
Methadone is a synthetic opioid in the structural class of diphenylpropylamines, which was developed in the 1930s. It is a potent agonist at the mu‐opioid and delta‐opioid receptors. The high affinity of methadone for mu‐receptors (key mediators in supra‐spinal analgesia) and delta‐receptors (probably important in spinal analgesia) has resulted in it being recommended for use as an analgesic in cancer pain.
Most clinical practice and research in most countries uses a racaemic mixture of two isomers, levorotatory (L) methadone and dextrorotatory (D) methadone, although in Germany L‐methadone alone is used (Bruera 2002). L‐methadone is 8 to 50 times more potent than D‐methadone in humans and is believed to be almost entirely responsible for its analgesic properties (Fainsinger 1993).
Methadone is available as a lipophilic hydrochloride salt and is available as formulations for oral, rectal and parenteral administration (Fainsinger 1993; Ripamonti 1997). It is well absorbed by all routes. Oral administration is followed by rapid gastrointestinal absorption with measurable plasma levels at 30 minutes. The peak plasma levels after an oral dose occur at four hours and begin to decline 24 hours after dosing. Oral bioavailability is high, generally over 85% (79% ± 21 in studies). The recommended dose to be given parenterally is between 50% and 80% of the oral dose (Gannon 1997; Davis 2001). Although local toxicity associated with subcutaneous administration has been reported (Bruera 1991), in many cases this is manageable (Mathew 1999).
Elimination of methadone is mediated by hepatic oxidative biotransformation, renal N‐demethylation, and urinary and faecal clearance. Chronic administration results in increased metabolite to methadone ratios, suggesting that autoinduction of hepatic microsomal enzymes occurs. Renal impairment is not thought to impair clearance; methadone may be useful in the management of pain in patients with renal failure. Some drug interactions are significant (Davis 2001).
Methadone has also been demonstrated in animal studies to have antagonist activity at the N‐methyl‐D‐aspartate (NMDA) receptor, resulting in interest in the clinical application of the drug in neuropathic pain syndromes. A combination of NMDA receptor antagonism and opioid agonism might provide valuable analgesic effects with fewer side effects than other analgesics (Gorman 1997).
How the intervention might work
Single dose studies have shown potency marginally greater than morphine; repeated administration results in greater potency. Methadone's properties of high oral bioavailability, rapid onset of analgesic effect, long half‐life (resulting in infrequent dosing schedules), lack of active metabolites, low rate of induction of tolerance, and low cost are characteristics that result in its use in the management of pain in cancer patients (Fainsinger 1993; Hanks 1998; Twycross 1998).
The perceived drawbacks of methadone include high potential for accumulation leading to delayed toxicity, highly variable pharmacokinetics between individuals, relative ease of use of modified‐release morphine preparations, possible drug interactions, and concerns over dose titration and conversion from other opioids (Fainsinger 1993). Potential side effects of methadone include constipation, drowsiness, confusion, nausea, hypotension, miosis (constriction of pupils), antidiuresis, exacerbation of asthma, and respiratory depression. Clinical situations where particular caution is advised include:
use in the elderly;
pain only partially responsive to methadone, where there is a risk of rapid dose escalation;
pain thought to have a predominantly psychological component; and
people in whom sensitivity to low doses of opioids has previously been demonstrated.
Particular concern has grown in recent years about two aspects of methadone pharmacology: interactions with other drugs and also the potential for prolongation of the QT interval (a measure of cardiac function) resulting in the potentially fatal arrhythmia called torsade de pointes (van den Beuken‐van Everdingen 2013). Weschules, Bain and Richeimer (Weschules 2008) conducted a systematic review of the literature to quantify the available evidence relating to methadone‐related drug interactions. They concluded that the evidence base was not well developed and much of the available literature consisted of case reports and case series considering inpatients being managed on methadone maintenance treatment programmes for opioid substance misuse. These patients had a high rate of HIV infection (and associated prescribing) and many of them were tobacco smokers with consequent potential induction of the cytochrome P1A2 enzyme, which may confound conclusions. Whilst the authors urge caution in extrapolating the findings of their review to the chronic and cancer pain patient populations, they draw attention to possible interactions of interest to pain management clinicians in a table summarising those attributed to interactions with anticonvulsants (phenytoin, phenobarbital and carbamazepine), selective serotonin reuptake inhibitor (SSRI) antidepressants (fluvoxamine, fluoxetine, paroxetine, sertraline and citalopram), tricyclic antidepressants (desipramine, amitriptyline, imipramine) and benzodiazepine anxiolytics (diazepam and alprazolam) (Weschules 2008). They also highlight concern that genetic polymorphism associated with the cytochrome‐P enzymes may have a bearing on both methadone metabolism per se, and the occurrence ‐ and potential severity ‐ of methadone‐related drug interactions.
Following reports of sudden death in people taking methadone in the early to mid 2000s, interest in prolongation of the QT interval by methadone has arisen. This risk has received specific attention in published guidance about use of methadone for pain in cancer and palliative care patients. For example, the editors of the Palliative Care Formulary (Twycross 2014) included a specific reference to methadone risks in the chapter on QT prolongation in the Fourth Edition which was not present in the Third edition. Cruciani reviewed the available literature in an attempt to answer the questions of whether, and when, ECG examination should be undertaken in patients prescribed methadone (Cruciani 2008). From an examination of available studies published between 1973 and 2007, and considering the evidence in favour of performing ECG testing set against those that raise questions about the significance of cardiac toxicity related to methadone, it was concluded that there are reasons to be cautious and a low threshold for recommending ECG testing is advised. Pending further evidence, Cruciani's recommendations are that ECG examination should be conducted at baseline (methadone initiation), on dose escalation and on addition of relevant medication in the following situations:
patients co‐administered methadone with drugs that are substrates of the cytochrome‐P3A4 or cytochrome‐P2D26 enzymes;
patients treated with drugs which may block the HERG (human ether‐a‐go‐go related gene) protein in the potassium channel of cardiac tissue, including some macrolide antibiotics (erythromycin, clarithromycin), antipsychotics (chlorpromazine, haloperidol, olanzapine, risperidone) and antidepressants (tricyclics and sertraline and venlafaxine);
patients who are medically frail or who have other risk factors for prolonged QT interval including cardiac disease, hypokalaemia, hypomagnesaemia and family history of sudden death.
The Palliative Care Formulary seeks to put this into a clinical context (Twycross 2014), also recommending baseline ECG and repeated ECG on dose titration or other relevant circumstances, with methadone discontinuation in favour of an alternative opioid being advocated if the QT interval is prolonged to greater than 500 ms. The authors do emphasise the need for clinical judgment over the balance of burden versus benefit when weighing the risk, and the importance of communication of this with the patient and those close to them.
Why it is important to do this review
Methadone is considered to be a useful analgesic for the management of moderate to severe cancer pain. The earlier review in 2007 indicated that it had similar efficacy to morphine, but adverse events may be problematic with repeated dosing, based on limited evidence. Standards for systematic reviews have strengthened since then and it is important to identify any new studies and reassess the evidence for methadone's place in managing cancer pain.
This review is one of a suite of reviews on cancer pain and will be incorporated into an overview of opioids for cancer pain.
Objectives
To determine the effectiveness and tolerability of methadone as an analgesic in adults and children with cancer pain.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs). We required full journal publication, with the exception of online clinical trial results summaries of otherwise unpublished clinical trials and abstracts with sufficient data for analysis. We did not include short abstracts (usually meeting reports). In this update we excluded studies with treatment groups of fewer than 10 participants.
Types of participants
Inclusion criteria
Male and female adults and children with cancer pain
Any pain with a malignant etiology
Etiology may be primary or secondary malignancy, solid or haematological tumours
Pain of at least moderate intensity
Participants being treated in any setting
Exclusion criteria
People taking methadone for suppression of cough
People taking, or who have previously taken, methadone for rehabilitation from opioid dependence
Types of interventions
Methadone given specifically for relief of cancer‐related pain, at any dose and by any route
Methadone compared with placebo or any other active comparison
Types of outcome measures
We sought to asses the outcome of 'no worse than mild pain' and the impact of methadone on consciousness, appetite and thirst. These are issues of concern to patients and their relatives and care providers, highlighted in the UK but of international relevance. (DH 2013;Ma 2016; Wiffen 2014).
Primary outcomes
Participant‐reported pain intensity or pain relief, or both, measured using a visual analogue scale, verbal rating scale or numerical rating scale
Participants with 'no worse than mild pain' on treatment
We did not use physician or carer assessments of pain.
Secondary outcomes
Participants with adverse events. We collected information on all reported adverse events, but were particularly interested in consciousness; drowsiness and confusion; loss of appetite; thirst; constipation; nausea and vomiting; and respiratory effects.
Withdrawals for any reason including non‐compliance, adverse events, and death
Search methods for identification of studies
We searched electronic databases for published studies and clinicaltrails.gov for unpublished studies.
Electronic searches
For this update we searched the four databases listed below, without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Register of Studies Online) on 4 May 2016
MEDLINE (via Ovid) January 2006 to 4 May 2016
Embase (via Ovid) January 2006 to 4 May 2016
CINAHL (via EBSCO) to 4 May 2016
For the original review in 2004 and the first update in 2007, we also searched CancerLit, which has been retired and the Cochrane Pain, Palliative and Supportive Care Trials Register, which is no longer updated.
The search strategies for the four databases can be found in Appendix 1.
Searching other resources
For this update, we searched the clinical trials registry ClinicalTrials.gov up to May 2016.
Data collection and analysis
Selection of studies
PW and SD independently screened the title and abstract (where available) of all studies identified by the searches and selected studies that appeared to satisfy the inclusion criteria. Where there was any uncertainty, we requested the full text. We also reviewed all the included studies from the 2007 version of the review. All authors agreed the studies to be included. See Figure 1 for a flow chart of the screening process (Moher 2009).
1.
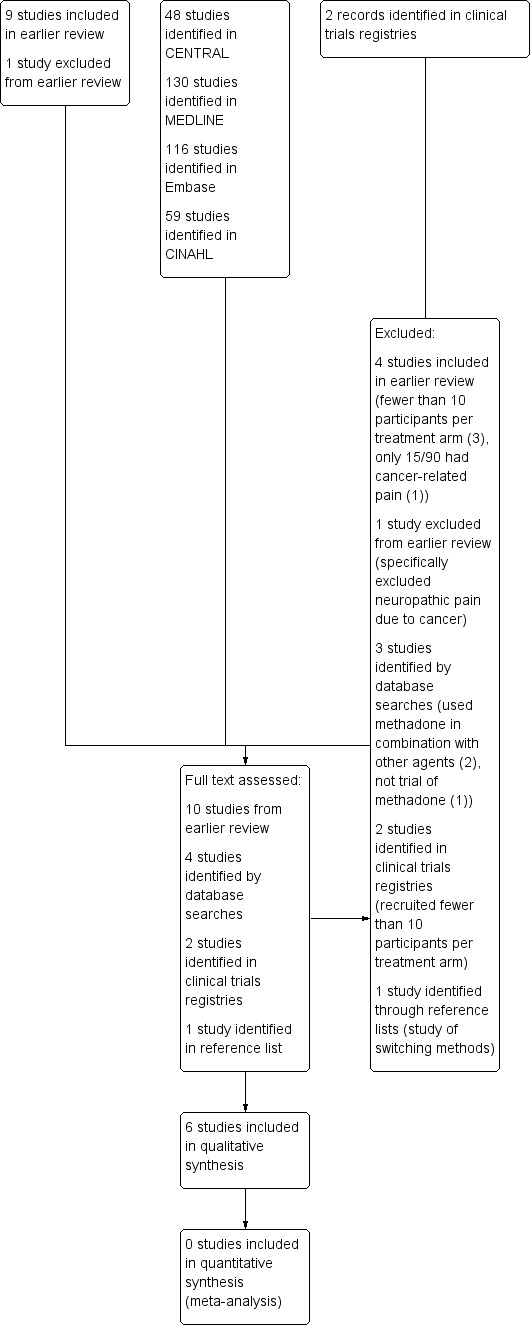
Study flow diagram
Data extraction and management
One author extracted data from the included studies into a standardised data collection sheet to include, where available:
publication details;
method, including trial design, allocation concealment, blinding and duration;
patient demographic details ‐ population, number of patients, age, sex, location of study (inpatient or outpatient);
details of malignant primary and secondary diagnoses, and previous and current drug history;
details of pain characteristics and types, range of intensity and how determined, assessed and documented;
description of the intervention (route, dose, etc) and control;
measures of effect (e.g. patient‐reported pain scores, patient satisfaction, quality‐of‐life scores);
adverse events, withdrawals, and dropouts.
A second author checked the data extraction before entry into Cochrane software Review Manager (RevMan 2014).
Assessment of risk of bias in included studies
All authors assessed all included studies for risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, and resolved any disagreements by discussion (Higgins 2011). The effect of risk of bias for included studies is shown in Figure 2 and Figure 3.
2.
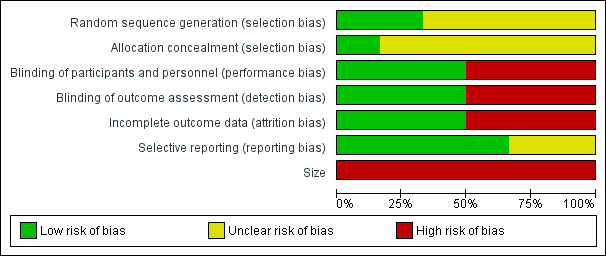
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
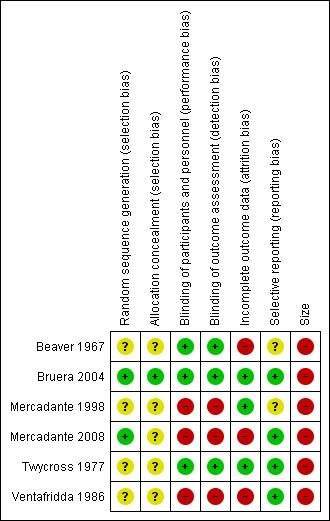
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process: random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a nonrandom process, which were therefore at high risk of bias (odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether the intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively‐numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation, which were therefore at high risk of bias (open list).
Blinding of participants, personnel, and outcome assessments (checking for possible performance and detection bias). We assessed the methods used to blind study participants, personnel, and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding: identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how blinding was achieved); high risk of bias (study not blinded).
Incomplete outcome data (attrition bias). We assessed the risk of bias from missing data as: low risk of bias (ITT analysis, or all participants accounted for with adequate reasons for loss and equally distributed between groups); low risk of bias (minimal loss of data); unclear risk of bias (other issues, such as cross‐over studies); high risk of bias (completer analysis, attrition 10% or more).
Selective reporting (reporting bias). We assessed the risk of reporting bias as: low risk of bias (all intended outcomes reported); unclear risk of bias (any anomaly in reporting, such as participants contributing more than one set of data, or some outcomes not participant‐reported); high risk of bias (prespecified outcome of interest not reported).
Size (checking for possible biases confounded by small size). Small studies may overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised. We considered studies to be at low risk of bias if they had 200 participants or more per treatment arm, at unclear risk if they had 50 to 200 participants per treatment arm, and at high risk if they had fewer than 50 participants per treatment arm.
Measures of treatment effect
It was not possible to combine studies for meta‐analysis to measure any treatment effect.
Unit of analysis issues
It was not possible to combine studies for meta‐analysis and therefore no unit of analysis issues were encountered.
Dealing with missing data
We took a decision not to impute missing data.
Assessment of heterogeneity
No statistical assessment of heterogeneity was made as no meta‐analysis was possible.
Assessment of reporting biases
We carried out extensive searches to identify relevant studies, but were unable to carry out any formal test for publication or other reporting biases.
Data synthesis
We did not pool data.
Grading of evidence
This section is taken from Cochrane Drugs and Alcohol recommended text. The overall quality of the evidence for each outcome was assessed using the GRADE system, and presented in Table 1, to present the main findings of a review in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grade of evidence:
High: we are very confident that the true effect lies close to that of the estimate of the effect;
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We downgraded the evidence for:
serious (‐1) or very serious (‐2) limitation to study quality;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1);
high probability of reporting bias (‐1).
In addition, where there were circumstances where the overall rating for a particular outcome needed to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used last observation carried forward (LOCF) imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by 3 levels, to very low quality. In circumstances where there were no data reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings in a transparent and simple tabular format. We have included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of participant‐reported pain intensity and adverse events ‐ appetite, thirst and somnolence.
Subgroup analysis and investigation of heterogeneity
We expected from previous versions of this review that the included studies would be heterogeneous and planned to undertake subgroup analysis based upon variations in methods such as titration schedule, route of administration, different comparators, and duration of studies. However no data were available for pooled analysis.
Sensitivity analysis
No sensitivity analysis was planned, or possible.
Results
Description of studies
Results of the search
We reviewed all the included and excluded studies in the earlier review, and carried out updated searches to May 2016. After deduplication and screening we assessed the full texts of 10 studies from the earlier review and four new studies identified by database searching. We subsequently identified one further potential paper from reference lists (Moksnes 2011) but this was excluded. Searches of ClinicalTrials.gov identified two studies, both of which had been terminated due to slow accrual, and were excluded from this review.
A flow chart of the process is shown in Figure 1 (Moher 2009).
Included studies
In this updated review, we included six studies, with 388 participants. Five of the nine included studies from the 2006 update are in this latest version (Beaver 1967; Bruera 2004; Mercadante 1998; Twycross 1977; Ventafridda 1986). One new study has been added (Mercadante 2008).
All studies were of adult participants with various types of cancer who required strong opioids to control their pain. Where reported the mean (or median) age was 48 to 65 years (range 18 to 87), and there were slightly more women than men in the studies (1.3:1). Most participants had previously been treated with World Health Organization (WHO) step 1 and 2 opioids (Ventafridda 1987), with inadequate response, but some had previously received step 3 opioids. Studies generally allowed titration to achieve the optimum balance of pain relief and adverse events. One study examined single doses of both intramuscular (IM) and oral morphine with some participants undergoing more than one round of treatments (Beaver 1967). The remaining studies used oral morphine.
Details of the individual studies are in the Characteristics of included studies table.
Excluded studies
We revised the excluded studies list for this update and this version of the review now excludes 11 studies. Four studies were included in the earlier review (Ferrer‐Brechner 1984; Gourlay 1986; Grochow 1989; Matts 1964), and one was excluded (Morley 2003). The new excluded studies were two studies identified in ClinicalTrials.gov (both excluded because they recruited only a single participant, NCT00573937; NCT00726830); Cubero 2010 and Lauretti 2013 (both used methadone in combination with other agents); Raptis 2013 (not a trial of methadone); and Moksnes 2011 (a study of switching methods).
See Characteristics of excluded studies for further details.
Risk of bias in included studies
Two authors independently assessed risk of bias for each study using the criteria outlined in Assessment of risk of bias in included studies. Summaries of the risk of bias assessment are provided in Figure 2 and Figure 3. The risks of bias for the included studies are described here.
Allocation
All studies were randomised, but only two adequately described the method used to generate the random sequence (Bruera 2004; Mercadante 2008). One study described the method used to conceal allocation of the random sequence and we judged this to be at low risk of bias (Bruera 2004). The remaining studies were judged at unclear risk of bias.
Blinding
Three studies adequately described the method used to maintain blinding (Beaver 1967; Bruera 2004; Twycross 1977). Two of the studies were open‐label and used commercial products so we judged them to be at high risk of bias (Mercadante 1998; Mercadante 2008). One study did not describe blinding and used different dosing regimens, and we judged it to be at high risk of bias (Ventafridda 1986).
Incomplete outcome data
We judged three studies to be at low risk of bias for this domain (Bruera 2004; Mercadante 1998; Twycross 1977), and two studies to be at high risk because they analysed only those who completed the study (Mercadante 2008; Ventafridda 1986). Beaver 1967 reported on participants who completed both cross‐over phases, and we judged this study to be at high risk of bias.
Selective reporting
We judged two studies to be at an unclear risk of bias (Beaver 1967, Mercadante 1998); all others were judged to be at low risk for this domain.
Other potential sources of bias
We judged all the studies to be at high risk due to small numbers of participants per treatment arm.
Effects of interventions
See: Table 1
A summary of results is presented in Appendix 2.
Efficacy
1. Methadone compared with oral morphine
a) Intramuscular (IM) methadone compared with IM morphine
A study by Beaver 1967 looked at 37 participants in a double‐blind double‐dummy cross‐over comparisons of IM methadone and IM morphine. Pain relief was good in both groups at six hours, with higher doses producing 'no worse than mild pain'. Methadone was found to be slightly more potent than morphine.
b) Oral methadone compared with immediate‐release oral morphine
Beaver 1967 also examined the efficacy of oral methadone and oral morphine in the IM study discussed above. However the study report does not provide separate data for the oral drugs. In a study of 66 participants by Ventafridda 1986, all who completed 14 days of treatment (n = 54) achieved 'no worse than mild pain'. We judged this to be very low‐quality evidence because of the small size of the study, the lack of blinding, and use of completer analysis.
c) Oral methadone compared with modified‐release oral morphine
Three studies (215 participants) compared oral methadone with oral morphine, modified‐release (MR) (Bruera 2004; Mercadante 1998; Mercadante 2008).
Bruera 2004 found that methadone at a daily dose of 15 mg was not superior to 60 mg/day of morphine MR. Pain reduction of at least 20% from baseline was the main pain outcome (methadone 24/49, morphine 30/54 at day 29). We judged this to be low‐quality evidence because of the small size of the treatment groups. We could not derive data for 'no worse than mild pain'.
Mercadante 1998 reported mean pain scores that were just above 'no worse than mild pain' (See Appendix 2). In the second study by Mercadante (Mercadante 2008) similar mean pain scores were reported for participants who did not drop out (completer analysis). We judged this to be low‐quality evidence because of the small size of the treatment groups, lack of blinding, and use of completer analysis in one study.
2. Oral methadone compared with transdermal (TD) fentanyl
In a three‐arm study by Mercadante 2008, there were no reported differences in pain intensity between the methadone and TD fentanyl groups for participants who did not drop out.
3. Oral methadone compared with diamorphine and cocaine combination
Twycross 1977 compared methadone with a diamorphine‐cocaine combination in 46 participants. The study examined the relative efficacy of the two interventions but no evaluable results were reported. Diamorphine‐cocaine combinations are no longer used so the study is of historical interest only.
Subgroup analysis
There were insufficient data to carry out any subgroup analysis.
Other efficacy measures
None of the studies reported quality‐of‐life or treatment‐satisfaction outcomes.
Adverse events
Specific adverse events
The most frequently observed adverse events were dry mouth, somnolence, and constipation. We have separately presented adverse events relating to appetite, consciousness and thirst, as well as more general adverse events in Appendix 3. No study reported the effects of the intervention on appetite. Sedation or somnolence was reported with both morphine and methadone; the results were inconsistent across studies, but the incidence was generally higher with methadone than with morphine. Dry mouth was reported in three studies (Beaver 1967; Mercadante 1998; Ventafridda 1986), but again results were not consistent. Both morphine and methadone can produce a dry mouth and, by association, thirst. The majority of studies reported nausea, vomiting, and constipation.
We judged the quality of the evidence relating to adverse events as very low because of the small size of the studies, lack of blinding and use of completer analysis in some studies, the small number of events, and indirectness, since surrogates for appetite, thirst and somnolence (such as sedation, drowsiness and dry mouth) were used.
Withdrawals due to adverse events, non compliance and death
Adverse event withdrawals were uncommon (12/202) and similar between groups.
There were no recorded withdrawals for noncompliance.
Deaths were uncommon except in one study where the majority of participants died irrespective of the treatment group (Twycross 1977).
Discussion
This is the second update of a review first published in 2004 and last updated in 2007. In this update, earlier decisions were revisited and current standards applied to the review.
Summary of main results
For studies in cancer pain, it is useful to know what proportion of people starting treatment are likely to be able to tolerate it, and what proportion of those who tolerate it are likely to obtain adequate pain relief. The studies did not report results in a way that unequivocally answered these questions.
Based on very limited amounts of data, there were no clear differences in participant‐reported pain intensity or pain relief between methadone and morphine or transdermal fentanyl: similar proportions of participants were able to tolerate each drug and achieve a level of pain that was probably similar to mild pain. All six included studies were small, and in addition, three used methods that put them at risk of bias. The results must therefore be interpreted with caution.
We were not able to obtain reliable data on the numbers of participants who achieved no worse than mild pain.
Adverse events were typical for opioids (e.g. sedation, somnolence, dry mouth, constipation) but inconsistently reported.
Overall completeness and applicability of evidence
Given the use of methadone in palliative care the amount of underpinning data is small. The most recent study is now eight years old and most studies were conducted before 2000. The more recent studies (251 participants randomised) compared methadone with either modified‐release morphine or transdermal fentanyl, which are commonly used in practice (Bruera 2004; Mercadante 1998; Mercadante 2008).
There were insufficient data to carry out any subgroup analysis. In particular, we were unable to investigate the influence of dose and titration regimen on either efficacy or tolerability. Included studies were underpowered to investigate serious adverse events, including arrhythmias.
We did not identify any studies in children.
Quality of the evidence
The evidence base identified by this review was small and limited in scope due to the small number of participants included and the diversity of the study methodologies and outcome reporting. Two of the more recent studies were at high risk of bias due to a lack of blinding (Mercadante 1998; Mercadante 2008).
Potential biases in the review process
We are unaware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
The search strategy for this updated review identified a systematic review conducted using Cochrane methods to examine the literature on methadone for cancer pain since the 2007 publication of this review (Good 2014). The review authors identified four randomised controlled trials. One is included in this update (Mercadante 2008). The other three were identified in our search strategy but have been allocated to the 'excluded studies' because they used methadone in combination with other agents (Lauretti 2013), or did not compare methadone with a different comparator. One examined outcomes for participants on a stable dose of morphine, who were switched to methadone with or without acetaminophen (paracetamol) (Cubero 2010). Good pain control and improved side‐effect burden and quality of life were reported, with a majority of participants expressing a preference for methadone treatment. However this was not a trial that examined methadone use in the way we defined for our review, which explains our decision to exclude it. The other study identified by Good 2014 compared two methods of switching to methadone (Moksnes 2011). Whilst interesting, the study addressed a different question from that considered by this review.
Authors' conclusions
Implications for practice.
For patients with cancer pain
There is limited evidence from RCTs that methadone is effective in managing severe pain due to cancer in adults. Given the issues of difficulties around titration to an effective dose and also the possibility of severe adverse effects (particularly cardiac arrhythmias), it is unlikely to have a role as the first line of treatment.
For clinicians managing cancer pain
Methadone is an opioid that has been used for many years to treat severe cancer pain although the evidence base from randomised controlled trials is sparse. If other opioids are not tolerated it may have a role, providing the issues of dose titration and possible severe adverse effects are considered. There is no information for use in paediatrics.
For policy makers
Methadone has a role if other opioids are not tolerated, providing the issues of dose titration and possible severe adverse effects are considered. There is no information for use in paediatrics.
For organisations or bodies making decisions about funding treatment options
Methadone has a place on formularies but needs to be managed by those clinicians specialising in pain management and palliative care.
Implications for research.
General implications
Research in this patient population is challenging, and no large, high‐quality trials with well‐managed bias have been conducted. A further consideration is the question of whether, with the increased concerns about the adverse effects of methadone on the prolongation of the QT interval, and greater recognition of significant methadone‐related drug interaction, methadone is likely to be used more widely than at present, even with further research. While it would be easy to suggest that further research is needed, in practice this is very unlikely to happen as this is an old drug and funding is not likely to be forthcoming.
Implications for study design
The major problem with research involving methadone relates to its unique pharmacological characteristics. Repeated dose studies using methadone at fixed dose intervals is potentially hazardous in the early phase of pain management in a switch from another opioid, or when treating opioid‐naive patients.
Implications for measurement and outcomes
As the distribution of response to analgesics is often bimodal, we strongly recommend the collection of dichotomous data in preference to mean pain scores. Data should be available to allow the estimation of the proportion of participants who achieve no worse than mild pain, defined as below 30 mm on a 100 mm VAS pain‐intensity scale. Adverse events should always be reported but we advocate specific reporting of events affecting appetite, thirst and consciousness in line with the Neuberger report (DH 2013).
Other considerations for future study design include:
use of standard and comparable pain intensity scores, which would allow closer comparison between different studies and potentially meta‐analysis;
inclusion of patient‐reported pain and other data only;
larger numbers of participants in studies where differentiation by pain syndrome has been attempted in order to answer the question as to whether methadone is particularly valuable in bone or neuropathic pain or other syndromes;
patient satisfaction and quality of life appraisal.
What's new
| Date | Event | Description |
|---|---|---|
| 13 February 2017 | Amended | Amended typo in appendix. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 8 February 2017 | Review declared as stable | See Published notes. |
| 4 May 2016 | New citation required but conclusions have not changed | Review substantially revised and now includes 6 studies (5 from earlier review, 1 new) with 388 participants. Conclusions not changed. |
| 4 May 2016 | New search has been performed | New searches in May 2016, and original included and excluded studies reassessed in line with current standards. |
| 7 August 2015 | New search has been performed | This review has been updated to include results of new searches. New risk of bias tables have been included and included studies tables updated. |
| 2 July 2008 | Amended | Converted to new review format. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Dr Gavin Stewart kindly reviewed the text at an early stage and made editorial adjustments. He also provided guidance on the feasibility or otherwise of meta‐analysis.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. 2016 Search strategies
CENTRAL
MESH DESCRIPTOR methadone EXPLODE ALL TREES (895)
(methadone or amidine or amidone or methadone or phenadone or heptadon or physeptone or eptadone or symoron or metasedin or sedo or ketalgine or "Martindale methadone mixture DTF" or methadose or methex or dolophine):TI,AB,KY (1857)
#1 or #2 (1867)
MESH DESCRIPTOR neoplasms EXPLODE ALL TREES (46032)
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*):TI,AB,KY (95750)
#4 or #5 (100496)
MESH DESCRIPTOR Pain EXPLODE ALL TREES (32221)
(pain* or nocicept* or neuropath*):TI,AB,KY (87637)
#7 or #8 (93445)
3 AND #6 AND #9 (48)
MEDLINE (via OVID)
exp Methadone/ (11005)
(methadone or amidine or amidone or methadone or phenadone or heptadon or physeptone or eptadone or symoron or metasedin or sedo or ketalgine or "Martindale methadone mixture DTF" or methadose or methex or dolophine).mp. (14357)
1 or 2 (14357)
exp Neoplasms/ (2821099)
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*).mp. (3083681)
4 or 5 (3400565)
exp Pain/ (328104)
(pain* or nocicept* or neuropath*).mp. (636872)
7 or 8 (708138)
randomized controlled trial.pt. (413578)
controlled clinical trial.pt. (90546)
randomized.ab. (310481)
placebo.ab. (157686)
drug therapy.fs. (1847843)
randomly.ab. (219181)
trial.ab. (320971)
10 or 11 or 12 or 13 or 14 or 15 or 16 (2482796)
3 and 6 and 9 and 17 (319)
limit 18 to yr="2006 ‐Current" (130)
Embase (via OVID)
exp Methadone/ (27180)
(methadone or amidine or amidone or methadone or phenadone or heptadon or physeptone or eptadone or symoron or metasedin or sedo or ketalgine or "Martindale methadone mixture DTF" or methadose or methex or dolophine).mp. (32962)
1 or 2 (32962)
exp Neoplasms/ (3660745)
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*).mp. (4191612)
4 or 5 (4648482)
exp Pain/ (974712)
(pain* or nocicept* neuropath*).mp. (1203279)
7 or 8 (1448357)
(random* or factorial* or crossover or "cross over" or cross‐over).tw. (1136282)
(placebo* or (doubl* adj blind*) or (singl* adj blind*)).tw. (307524)
(allocat* or allocat*).tw. (103527)
crossover Procedure/ (46919)
double‐blind procedure/ (130500)
single‐blind procedure/ (22010)
Randomized Controlled Trial/ (402622)
10 or 11 or 12 or 13 or 14 or 15 or 16 (1381772)
3 and 6 and 9 and 17 (195)
limit 18 to yr="2006 ‐Current" (116)
CINAHL (via EBSCO)
MH methadone OR TX methadone or amidine or amidone or methadone or phenadone or heptadon or physeptone or eptadone or symoron or metasedin or sedo or ketalgine or "Martindale methadone mixture DTF" or methadose or methex or dolophine (3328)
MH neoplasms OR TX ( neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma* ) (269,166)
MW pain OR TX ( pain* or nocicept* or neuropath* ) (173,682)
S1 AND S2 AND S3 (214)
TX ( (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) ) OR TX randomi#ed control$ trial$ OR MH random assignment OR TX random* allocat* OR TX placebo* OR MH Quantitative Studies OR TX allocat* random* (725,719)
S4 AND S5 (59)
Appendix 2. Results
| Study ID | Design | Total Participants | No worse than mild pain | Results Methadone | Results control |
| Beaver 1967 | Cross‐over | 37 | Not estimable | N/A | N/A |
| Bruera 2004 | Parallel | 103 | Not estimable | > 20% improvement 37/49 | > 20% improvement 41/54 |
| Mercadante 1998 | Parallel | 40 | Pain score < 4/10. Mean values morphine 3.3 +/‐ 0.2, methadone 3.4 +/‐ 0.1 | N/A | N/A |
| Mercadante 2008 | Parallel | 108 | At Wweek 4 mean pain for morphine 2.5 (1.7 ‐ 3.3), fentanyl 2.4 (2 ‐ 2.8) and methadone 3.4 (2.6 ‐ 4.1) (completers only) | N/A | N/A |
| Twycross 1977 | Parallel | 46 | Not reported | Survival worse on methadone | N/A |
| Ventafridda 1986 | Parallel | 54 | All participants achieved no worse than mild pain based on mean pain scores (completers only) | N/A | N/A |
| Total | 388 |
Appendix 3. Adverse events including appetite, consciousness and thirst
| Summary of results: adverse events relating to appetite, consciousness and thirst | |||||||
| Study ID | No of Participants | Methadone | Control | Appetite | Consciousness | Thirst | Other adverse effects |
| Beaver 1967 | 37 | Oral and IM doses to 48 mg | Morphine oral and IM doses to 32 mg | Not reported | Sedation on morphine 16/92 doses; on methadone 25/92 doses. Hallucinations morphine 1/92 |
Dry mouth on methadone 4/92 doses | Vomiting on morphine 7/92 doses; on methadone 10/92 doses. Constipation not reported |
| Bruera 2004 | 103 | Oral 7.5 mg every 12 h + 5 mg 4 h as required | Morphine MR 15 mg oral every 12 h + 5 mg 4 h as required | Not reported | Sedation more severe in methadone group. Confusion similar between groups | Not reported | Nausea and constipation similar between groups |
| Mercadante 1998 | 40 | Oral liquid 2‐ to 3 times a day. Dose titrated according to need | Morphine MR tablets 2‐ to 3 times a day. Dose titrated according to need |
Not reported | Drowsiness on morphine 8/20; on methadone 3/20. Confusion 1/20 in both groups. | Dry mouth on morphine 8/20; on methadone 3/20 | Constipation on morphine 12/20; on methadone 6/20 |
| Mercadante 2008 | 108 | Oral 15 mg daily then titrated to need | Morphine MR 60 mg oral daily then titrated to need. TD fentanyl 0.6 mg daily then titrated to need | Not reported | Not reported | Not reported | Results not easy to interpret but appear low for all arms. ''No relevant differences among the groups" |
| Twycross 1977 | 46 | 5 to ‐20 mg daily | Diamorphine 5 to ‐40 mg + cocaine 10 mg daily | Not reported | Not reported | Not reported | Over 20 days, 17/20 died on methadone, and 21/27 died in diamorphine + cocaine group (from graph) |
| Ventafridda 1986 | 54 | 8 to ‐28 mg as oral liquid daily (as divided doses, 6‐hourly, then 8‐hourly) | Morphine average 4 to ‐24 mg oral (normal release) every 4 h | Not reported | Somnolence worse on morphine | Dry mouth worse on morphine | Constipation similar between groups |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beaver 1967.
| Methods | Randomised, double‐blind (double dummy), cross‐over study Single doses with assessment at 6 h |
|
| Participants | Chronic pain due to cancer N = 43 started, 37 completed at least 1 series (M: 11, F: 26) Mean age 48 years (range 34‐59 years) |
|
| Interventions | Morphine (IM or oral) or methadone (IM or oral) in a range of doses from 8 mg to 48 mg 4 doses for each series and 4 different series |
|
| Outcomes | PI: categorical scale (5 points), measured over 6 h 50% pain relief Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "in the oral‐parenteral study both capsules and an injection, one of which was a dummy, were administered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "in the oral‐parenteral study both capsules and an injection, one of which was a dummy, were administered" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only completed cross‐overs were analysed for efficacy |
| Selective reporting (reporting bias) | Unclear risk | Some participants contributed more than one set of data |
| Size | High risk | < 50 participants per treatment arm |
Bruera 2004.
| Methods | Randomised, double‐blind, parallel‐group; duration 28 days | |
| Participants | Outpatients with cancer pain determined by a validated clinical assessment. Differentiated between neuropathic and non‐neuropathic pain N = 103 patients randomised (methadone 49, morphine 54), 66 completed (methadone 29, morphine 37) M: 37, F: 66 Age: methadone median age 59 years (range 26‐84 years); morphine median age 60 years (range 31‐87 years) Excluded participants who had previously been treated with strong opioids All non‐opioids discontinued on recruitment; titration of antiemetics and laxatives allowed |
|
| Interventions | Oral methadone 7.5 mg twice daily with 5 mg 4‐hourly as needed. Oral morphine 15 mg twice daily with 5 mg 4‐hourly as needed Titration based on use of 'as needed' doses and physicians' assessment daily for days 1 to 8, then weekly | |
| Outcomes | Participant‐reported data, daily on days 1 to 8, then weekly thereafter: pain, sedation, confusion, nausea, constipation, recorded on 0‐10 NRS
Weekly assessment of cognitive function (tool not specified) and global assessment of overall benefit on VRS with 7 points and descriptors
End of study outcomes:
Pain response of 20% NRS reduction considered clinically relevant
Composite toxicity calculated as sum of NRS for side effects ‐ worsening by 20% or more considered clinically relevant
Pain response and stable composite toxicity deemed to be 'obvious benefit'
Participant‐reported global benefit ‐ VRS 4 or more on 7‐point scale Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "generated centrally by computer generated numbers stratified by center" |
| Allocation concealment (selection bias) | Low risk | Pharmacy at each centre advised of treatment assigned but the code was kept in a sealed envelope and not released to clinicians unless clinical emergency |
| Blinding of participants and personnel (performance bias) | Low risk | Stated to be double blind, "in identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated to be double blind, "in identical capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of withdrawals in both groups and for similar reasons. BOCF for dichotomised end points |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported upon |
| Size | High risk | 49 participants in one arm and 54 in the other |
Mercadante 1998.
| Methods | Prospective randomised parallel‐group study Participants treated until death |
|
| Participants | Advanced cancer requiring strong opioids for pain management N = 40 M: 19, F: 21 Age: morphine mean age 65 years ± 2.7; methadone mean age 61 years ± 2.9 |
|
| Interventions | Oral morphine modified‐release, titrated to need Oral methadone solution, titrated to need Other palliative care drugs allowed including non‐opioid analgesics. No anticancer therapy |
|
| Outcomes | Pain intensity self report (when possible, otherwise doctor‐rated) Symptoms related to cancer or adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be 'randomised' |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Commercially available products used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Commercially available products used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems identified |
| Selective reporting (reporting bias) | Unclear risk | Some pain measurements may have been physician‐ or carer‐assessed but not clearly specified |
| Size | High risk | 20 participants per treatment arm |
Mercadante 2008.
| Methods | Multicentre, randomised, open, parallel‐group study: duration 4 weeks Fixed starting dose of study medication, adjusted to balance analgesia and adverse effects Assessment at baseline and weekly intervals |
|
| Participants | Adults with advanced cancer requiring strong opioids who had received opioids for mild to moderate pain, including tramadol and codeine at doses of at least 300 mg and 180 mg respectively, without adequate analgesia. Expected survival > 3 months Breast cancer was the most frequent diagnosis (16 participants), and mixed nociceptive‐neuropathic syndromes (18 participants) the most dominant pain type N = 108 (70 completed) M: 36, F: 34 (completers) Mean age 59 years (range 18‐78) (completers) |
|
| Interventions | Oral methadone, 15 mg/d in 3 divided doses, n = 36 Modified‐release oral morphine, initially 60 mg/d, n = 36 Transdermal fentanyl patch, initially 0.6 mg/d (25 μg/h), n = 36 Rescue medication: oral morphine at 1/6 24‐h oral equivalent requirement |
|
| Outcomes | Symptoms associated with opioid therapy (e.g. nausea, drowsiness, confusion): 4‐point scale (not at all, slight, a lot, severe) Constipation: 4‐point scale (0 = 1 passage every 1‐2 days, 1 = one passage every 3‐4 days, 2 = one passage > 4 days, 3 = rectal measures) Distress score calculated from sum of symptom intensities PI: NRS (0‐10) Time to achieve dose stabilisation Number of daily dose changes Opioid escalation index QoL using Spitzer QoL index (activity, daily life, health perceptions, social support, Behaviour rated on Likert 3‐point scale (0‐2) Cost |
|
| Notes | Other medication for 'symptoms' were allowed during the study, which included anti‐inflammatories, antidepressants and anticonvulsants, which may have an impact on pain; these were either continued or introduced as needed during the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded; used "commercially available" products |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded; used "commercially available" products |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Efficacy data (group means) for participants remaining in study (completers). States numbers of dropouts equally distributed between groups |
| Selective reporting (reporting bias) | Low risk | No problems identified |
| Size | High risk | 36 per treatment arm |
Twycross 1977.
| Methods | Randomised, double‐blind, parallel‐group: duration 4 weeks approximately | |
| Participants | Cancer pain; participants with terminal cancer with severe pain
N = 46. Gender mix stated to be 'comparable' Median age 63 years |
|
| Interventions | Diamorphine elixir with cocaine 10 mg, n = 26 Oral methadone, n = 20 Dose titrated to pain relief |
|
| Outcomes | VAS for pain, nausea and mood, assessed twice daily | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated"; method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "drugs were dispensed in undistinguishable solutions" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "drugs were dispensed in undistinguishable solutions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | No problems identified |
| Size | High risk | < 50 participants per treatment arm (20 and 26) |
Ventafridda 1986.
| Methods | Randomised, parallel‐group: duration 14 days | |
| Participants | Advanced cancer requiring strong opioids for "excruciating pain" N = 66 (randomised), 54 (completed) M: 31, F: 23 |
|
| Interventions | Oral methadone 1 mg/ml, dose 8‐28 mg daily, given as divided dose every 6 h for 3 d, then every 8 h, n = 27 Oral morphine 4 mg/ml dose 4‐24 mg every 4 h, n = 27 All participants received diclofenac 150 mg daily and haloperidol 20 mg daily by injection |
|
| Outcomes | PI: 5‐point categorical scale (converted to integrated pain score, which took into account duration) Achieved no worse than mild pain (VAS ≤ 30/100) Adverse events Withdrawals |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be "randomised", method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Not described. Presumed open‐label, since different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. Presumed open‐label, since different dosing regimens |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis |
| Selective reporting (reporting bias) | Low risk | No problems identified |
| Size | High risk | < 50 participants per treatment arm |
BOCF: baseline observation carried forward; F: female; h: hours; IM: intramuscular; M: male; mg: milligrams; N: number of participants in study; n: number of participants in treatment arm; NRS: Numerical Rating Scale; PI: pain intensity; QoL: quality of life; VAS: Visual Analogue Scale; VRS: Verbal Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cubero 2010 | Study of methadone in combination with acetaminophen (paracetamol) |
| Ferrer‐Brechner 1984 | 28 participants across 4 groups. Likely to be fewer than 10 participants per treatment group |
| Gourlay 1986 | Fewer than 10 participants per treatment group |
| Grochow 1989 | Fewer than 10 participants per treatment group |
| Lauretti 2013 | Study of methadone in combination with other agents |
| Matts 1964 | Not specifically cancer pain ‐ only 15 out of 90 participants reported cancer‐related pain |
| Moksnes 2011 | Study of switching methods using methadone |
| Morley 2003 | Double‐blind randomised controlled cross‐over trial of methadone for neuropathic pain. Excluded cancer pain |
| NCT00573937 | Only 1 participant recruited |
| NCT00726830 | Only 1 participant recruited |
| Raptis 2013 | Not a study of methadone |
Differences between protocol and review
Philip Wiffen and Sheena Derry joined the author team in 2016.
For this 2017 update we:
included participants with baseline pain of at least moderate intensity;
excluded small studies (fewer than 10 participants per treatment arm);
looked for the outcome 'no worse than mild pain';
specifically looked for adverse events related to consciousness, appetite and thirst;
expanded the 'risk of bias' section and included an assessment of the quality of the evidence using GRADE, in line with current standards for Cochrane reviews.
Contributions of authors
GRW undertook the updated searches. GRW and ABN reviewed the search findings independently to determine studies which may meet the entry criteria and worked separately and then together to agree the final list. Subsequently SD and PW re‐ran searches and suggested changes to the included studies list.
All review authors were involved in data extraction and writing the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
South Tees Hospitals NHS Foundation Trust, UK.
Time
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
ABN: none known; ABN is a palliative care specialist who manages patients with cancer and prescribes methadone.
GRW: none known.
SD: none known.
PW: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Beaver 1967 {published data only}
- Beaver WT, Wallenstein SL, Houde RW, Rogers A. A clinical comparison of the analgesic effects of methadone and morphine administered intramuscularly, and of orally and parenterally administered methadone. Clinical Pharmacololgy and Therapeutics 1967;8(3):415‐26. [PUBMED: 5338385] [DOI] [PubMed] [Google Scholar]
Bruera 2004 {published data only}
- Bruera E, Palmer JL, Bosnjak S, Rico MA, Moyano J, Sweeney C, et al. Methadone versus morphine as a first‐line strong opioid for cancer pain: a randomized, double‐blind study. Journal of Clinical Oncology 2004;22(1):185‐92. [DOI: 10.1200/JCO.2004.03.172] [DOI] [PubMed] [Google Scholar]
Mercadante 1998 {published data only}
- Mercadante S, Casuccio A, Agnello A, Serretta R, Calderone L, Barresi L. Morphine versus methadone in the pain treatment of advanced cancer patients followed up at home. Journal of Clinical Oncology 1998;16(11):3656‐61. [PUBMED: 9817288] [DOI] [PubMed] [Google Scholar]
Mercadante 2008 {published data only}
- Mercadante S, Porzio G, Ferrera P, Fulfaro F, Aielli F, Verna L, et al. Sustained‐release oral morphine versus transdermal fentanyl and oral methadone in cancer pain management. European Journal of Pain 2008;12:1040‐6. [DOI: 10.1016/j.ejpain.2008.01.013] [DOI] [PubMed] [Google Scholar]
Twycross 1977 {published data only}
- Twycross R. A comparison of diamorphine with cocaine and methadone (letter). British Journal of Clinical Practice 1977;4:691‐93. [PUBMED: 69292]69292 [Google Scholar]
Ventafridda 1986 {published data only}
- Ventafridda V, Ripamonti C, Bianchi M, Sbanotto A, Conno F. A randomized study on oral administration of morphine and methadone in the treatment of cancer pain. Journal of Pain and Symptom Management 1986;1(4):203‐7. [DOI: 10.1016/S0885-3924(86)80042-2] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cubero 2010 {published data only}
- Cubero DIG, Giglio A. Early switching from morphine to methadone is not improved by acetaminophen in the analgesia of oncologic patients: a prospective, randomized, double‐blind, placebo‐controlled study. Supportive Care in Cancer 2010;18(2):235‐42. [DOI: 10.1007/s00520-009-0649-8] [DOI] [PubMed] [Google Scholar]
Ferrer‐Brechner 1984 {published data only}
- Ferrer‐Brechner T, Ganz P. Combination therapy with ibuprofen and methadone for chronic cancer pain. American Journal of Medicine 1984;77(1A):78‐83. [DOI: 10.1016/S0002-9343(84)80023-6] [DOI] [PubMed] [Google Scholar]
Gourlay 1986 {published data only}
- Gourlay GK, Cherry DA, Cousins MJ. A comparative study of the efficacy and pharmacokinetics of oral methadone and morphine in the treatment of severe pain in patients with cancer. Pain 1986;25(3):297‐312. [DOI: 10.1016/0304-3959(86)90234-4] [DOI] [PubMed] [Google Scholar]
Grochow 1989 {published data only}
- Grochow L, Sheidler V, Grossman S, Green L, Enterline J. Does intravenous methadone provide longer lasting analgesia than intravenous morphine? A randomised, double blind study. Pain 1989;38(2):151‐7. [DOI: 10.1016/0304-3959(89)90233-9] [DOI] [PubMed] [Google Scholar]
Lauretti 2013 {published data only}
- Lauretti GR, Rizzo CC, Mattos AL, Rodrigues SW. Epidural methadone results in dose‐dependent analgesia in cancer pain, further enhanced by epidural dexamethasone. British Journal of Cancer 2013;108(2):259‐64. [DOI: 10.1038/bjc.2012.593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matts 1964 {published data only}
- Matts SGF, Swan CHF, Wharton BA. Double blind trial of dextromoramide, methadone and pethidine in the treatment of severe pain. Postgraduate Medical Journal 1964;40:103‐5. [PUBMED: 14122903] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moksnes 2011 {published data only}
- Moksnes K, Dale O, Rosland JH, Paulsen O, Klepstad P, Kaasa S. How to switch from morphine or oxycodone to methadone in cancer patients? A randomised clinical phase II trial. European Journal of Cancer 2011;47(16):2463‐70. [DOI: 10.1016/j.ejca.2011.06.047] [DOI] [PubMed] [Google Scholar]
Morley 2003 {unpublished data only}
NCT00573937 {unpublished data only}
- Prommer E (lead investigator). Methadone versus morphine for cancer related pain. clinicaltrials.gov/ct2/show/NCT00573937 (accessed 21 April 2016) 2016. [CTG: NCT00573937]
NCT00726830 {unpublished data only}
- Fisch MJ (lead investigator). Methadone, morphine, or oxycodone in treating pain in patients with cancer. clinicaltrials.gov/ct2/show/NCT00726830 (accessed 21 April 2016) 2012. [CTG: NCT00726830]
Raptis 2013 {published data only}
- Raptis E, Vadalouca A, Stavropoulou E, Argyra E, Melemeni A, Siafaka I. Pregabalin vs. opioids for the treatment of neuropathic cancer pain: a prospective, head‐to‐head, randomized, open‐label study. Pain Practice 2014;14(1):32‐42. [DOI: 10.1111/papr.12045] [DOI] [PubMed] [Google Scholar]
Additional references
Bruera 1991
- Bruera E, Fainsinger R, Moore M, Thibault R, Spoldi E, Ventafridda V. Local toxicity with subcutaneous methadone. Experience of two centers. Pain 1991;45(2):141‐3. [DOI: 10.1016/0304-3959(91)90179-2] [DOI] [PubMed] [Google Scholar]
Bruera 2002
- Bruera E, Sweeney C. Methadone use in cancer patients with pain: a review. Journal of Palliative Medicine 2002;5(1):127‐38. [DOI: 10.1089/10966210252785097] [DOI] [PubMed] [Google Scholar]
Cruciani 2008
- Cruciani RA. Methadone: to ECG or not to ECG.... That is still the question. Journal of Pain and Symptom Management 2008;36(5):545‐52. [DOI: 10.1016/j.jpainsymman.2007.11.003] [DOI] [PubMed] [Google Scholar]
Davis 2001
- Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Supportive Care in Cancer 2001;9(2):73‐83. [DOI: 10.1007/s005200000180] [DOI] [PubMed] [Google Scholar]
DH 2013
- Anon. More care, less pathway. A review of the Liverpool Care Pathway. Report by Baroness Julia Neuberger (Chair), available at www.gov.uk/government/uploads/system/uploads/attachment_data/file/212450/Liverpool_Care_Pathway.pdf (accessed 21 April 2016).
Fainsinger 1993
- Fainsinger R, Schoeller T, Bruera E. Methadone in the management of cancer pain: a review. Pain 1993;51(2):137‐47. [DOI: 10.1016/0304-3959(93)90125-9] [DOI] [PubMed] [Google Scholar]
Gannon 1997
- Gannon C. The use of methadone in the care of the dying. European Journal of Palliative Care 1997;4(5):152‐8. [Google Scholar]
Good 2014
- Good P, Afsharimani B, Movva R, Haywood A, Khan S Hardy J. Therapeutic challenges in cancer pain management: a systematic review of methadone. Journal of Pain and Palliative Care Pharmacotherapy 2014;28(3):197‐205. [DOI: 10.3109/15360288.2014.938883] [DOI] [PubMed] [Google Scholar]
Gorman 1997
- Gorman AL, Elliott KJ, Inturrisi CE. The d‐ and l‐isomers of methadone bind to the non‐competitive site on the N‐methyl‐D‐aspartate (NMDA) receptor in rat forebrain and spinal cord. Neuroscience Letters 1997;223(1):5‐8. [DOI] [PubMed] [Google Scholar]
Graner 2016
- Graner KM, Rolim GS, Moraes AB, Padovani CR, Lopes MA, Santos‐Silva AR, et al. Feelings, perceptions, and expectations of patients during the process of oral cancer diagnosis. Support Care Cancer 2016;24:2323‐32. [DOI: 10.1007/s00520-015-3030-0] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Hanks 1998
- Hanks GWC, Cherny N. Opioid analgesic therapy. In: Doyle D, Hanks GWC, MacDonald N editor(s). Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 1998:331‐51. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ma 2016
- Ma H, Liu Y, Huang L, Zeng XT, Jin SH, Yue GJ, et al. The adverse events of oxycodone in cancer‐related pain: a systematic review and meta‐analysis of randomized controlled trials. Medicine (Baltimore) 2016;95(15):e3341. [DOI: 10.1097/MD.0000000000003341] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 1999
- Mathew P, Storey P. Subcutaneous methadone in terminally ill patients: manageable local toxicity. Journal of Pain and Symptom Management 1999;18(1):49‐52. [DOI: 10.1016/S0885-3924(99)00020-2] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 1998
- Payne R, Gonzales GR. Pathophysiology of pain in cancer and other terminal diseases. In: Doyle D, Hanks GWC, MacDonald N editor(s). Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 1998:299‐310. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 1997
- Ripamonti C, Zecca E, Bruera E. An update on the clinical use of methadone for cancer pain. Pain 1997;70(2‐3):109‐15. [DOI: 10.1016/S0304-3959(96)03286-1] [DOI] [PubMed] [Google Scholar]
Twycross 1998
- Twycross R, Wilcock A, Thorpe S. Palliative Care Formulary. Oxford: Radcliffe Medical Press, 1998. [Google Scholar]
Twycross 2014
- Twycross R, Wilcock A (Eds). PCF5 Palliative Care Formulary. Fifth. Nottingham: palliativedrugs.com Ltd, 2014. [ISBN: 978‐0‐9552547‐9‐6] [Google Scholar]
van den Beuken‐van Everdingen 2007
- Beuken‐van Everdingen MH, Rijke JM, Kessels AG, Schouten HC, Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population‐based study in The Netherlands. Pain 2007;132:312‐20. [DOI] [PubMed] [Google Scholar]
van den Beuken‐van Everdingen 2013
- Beuken‐van Everdingen MH, Geurts JW, Patijn J. Prolonged QT interval by methadone: relevance for daily practice? A prospective study in patients with cancer and noncancer pain. Journal of Opioid Management 2013;9(4):263‐7. [DOI: 10.5055/jom.2013.0167] [DOI] [PubMed] [Google Scholar]
Ventafridda 1987
- Ventafridda V, Tamburini M, Caraceni A, Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer 1987;59(4):850‐6. [PUBMED: 3802043] [DOI] [PubMed] [Google Scholar]
Weschules 2008
- Weschules DJ, Bain KT, Richeimer S. Actual and potential drug interactions associated with methadone. Pain Medicine 2008;9(3):315‐44. [DOI: 10.1111/j.1526-4637.2006.00289.x] [DOI] [PubMed] [Google Scholar]
Wiffen 2014
- Wiffen PJ, Derry S, Moore RA. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nicholson 2002
- Nicholson A, Davies A, Reid C. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2002, Issue 11. [DOI: 10.1002/14651858.CD003971] [DOI] [Google Scholar]
Nicholson 2004
- Nicholson AB. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003971.pub2] [DOI] [PubMed] [Google Scholar]
Nicholson 2007
- Nicholson AB. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2007, Issue 10. [DOI: 10.1002/14651858.CD003971.pub3] [DOI] [PubMed] [Google Scholar]


