Abstract
Purpose:
To determine the efficacy of genetically distinct Bacillus Calmette-Guérin (BCG) strains in preventing disease recurrence for patients with non-muscle invasive bladder cancer.
Materials and Methods:
We conducted a systematic review and network meta-analysis of trials evaluating BCG strains against all possible comparators (different BCG strains, chemotherapy, and non-BCG biologic therapies) and intravesical chemotherapy as the common comparator. MEDLINE served as the primary data source, with search from inception to April 2014 for clinical trials involving patients with NMIBC receiving BCG. Primary outcome measure was bladder cancer recurrence defined as recurrent bladder tumor of any grade or stage. Random-effects network meta-analysis provided estimates for outcomes and is presented as odds ratios (ORs).
Results:
Across all possible comparators (n=65 trials, 12,246 patients, 9 strains) there were 2,177 (38.6%) recurrences among 5,642 treated patients compared with 2,316 (42.6%) recurrences among 5,441 comparators. With chemotherapy as the common comparator (n=28 trials, 5,757 patients, 5 strains), Tokyo (0.39 OR 95% CI 0.16–0.93), Pasteur (0.49 OR 95% CI 0.28–0.86), and TICE (0.61 OR 95% CI 0.40–0.93) strains were significantly better than chemotherapy at preventing recurrence. No BCG strain demonstrated significant superiority when compared against any other strain at preventing recurrence in our network meta-analysis.
Conclusions:
BCG strains exhibit significant differences in efficacy when compared against chemotherapy. However, no definitive conclusions are reached regarding strain superiority and head-to-head trials are greatly needed to further understand the importance of strain selection in determining BCG efficacy.
Keywords: bladder cancer, Bacillus Calmette-Guerin, Non-Muscle Invasive, Immunotherapy
Introduction
Urinary bladder cancer is the fourth most common malignancy in men 1. In 2016, there will be an estimated 76,960 new cases and bladder cancer will account for 16,390 deaths in the United States 1. The majority of patients diagnosed with bladder cancer present with non-muscle invasive bladder cancer (NMIBC) including carcinoma in-situ (CIS), Ta, and T1 stages. For these patients, the standard initial therapy is complete tumor removal via transurethral resection and bladder preservation is possible in most cases. Due to the high rates of disease recurrence, adjuvant therapy with intravesical instillation of Bacillus Calmette-Guérin (BCG) immunotherapy is indicated for all high-grade and some low-grade non-muscle invasive bladder tumors 2.
The term BCG comprises a family of related strains derived from a virulent strain of Mycobacterium bovis (M. bovis). Following 13 years of serial in-vitro passaging, Calmette and Guérin developed the original BCG strain, which was attenuated yet provided protection against virulent strains including Mycobacterium tuberculosis. Subsequent to its first use as a tuberculosis vaccine in 1921, BCG cultures were distributed globally for use in vaccine manufacturing. As a result of continuous passages under different conditions in various laboratories throughout the world, BCG strains began to diverge genetically, until the introduction of freeze-dried seed lots in the 1960’s. As a result, existing BCG vaccine strains have extensive genotypic diversity, including deletions and duplications, which can be linked to their dissemination patterns 3. In some studies, genetically distinct strains have been associated with differences in elicited immune responses including reactogenicity and immunogenicity 4; however, it is not known if such changes influence BCG’s efficacy in the treatment of bladder cancer.
Studies comparing BCG strains in head-to-head trials suggest that strains can influence clinical outcomes 5–9. Unfortunately, however, these trials have been relatively small, lacking statistical power to be able to reliably assess any effect related to strain differences. Therefore, we initiated a systematic review and network meta-analysis to determine if differences in BCG strains are associated with response to BCG in bladder cancer. A network meta-analysis integrates data from direct comparisons of treatments within trials with indirect comparisons of interventions assessed against a common comparator in different trials, to compare all investigated interventions.
Materials and Methods
Evidence Acquisition
We conducted a systematic review of Medline (http://www.ncbi.nlm.nih.gov/pubmed), from 1966 to April 2014 in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 10, 11 using the following search criteria:
Medical Subject Headings (MeSH) terms: (“Urinary Bladder Neoplasms”[MeSH]) AND (“BCG Vaccine”[MeSH] OR “Mycobacterium bovis”[MeSH] OR “BCG Connaught” [Supplementary Concept])
Ovid text work terms: bladder cancer or BCG or TICE or Connaught
Linkage: 1 and 2, limited to human adults and publications in English
Selection Criteria
All publications involving patients with carcinoma in-situ (CIS), Ta or T1 stage bladder cancer that compared transurethral resection plus intravesical BCG (any strain) to either transurethral resection alone, transurethral resection plus intravesical chemotherapy, transurethral resection plus intravesical BCG (either different strain or different dose), transurethral resection plus a non-BCG biologic (defined as either: Interferon-α2b (INTRON® A), Bropirimine, autologous intravesical macrophage cell therapy (BEXIDEM®), or Maltose Tetrapalmitate), or transurethral resection plus a combination of intravesical treatments were reviewed.
Data collection
Data extracted from each publication was conducted independently by two investigators and included: sample size, number of patients per treatment group, exposure and outcome definition, study location, type of control, study design, strain type and dose, treatment schedule and summary of results. Any discrepancies were resolved by consensus in meeting with the presence of the moderator (JEC). Exclusion criteria were studies with fewer than 10 patients, case reports, non-randomized studies, or those studies not reporting any data on recurrence. Data from cohorts represented in more than one publication were only reported once, with the most up-to-date information represented. Maintenance treatment with intravesical BCG was defined as any additional treatment(s) following an initial induction treatment. Standard doses for all BCG strains are listed in Table 1. Any dose less than the standard dose was designated as “low-dose”.
Table 1.
Standard Dose of BCG Daughter Strains
| BCG Strain | Standard Dose |
|---|---|
| Armand Frappier | 120 mg (milligrams) |
| Connaught | 81 mg |
| Danish 1331 | 120 mg |
| Evans | 1–5 X 109 CFU (Colony Forming Units) |
| Glaxo | 1–3 X 109 CFU |
| Moreau | 80 mg |
| Pasteur | 150 mg |
| RIVM | 2 X 108 CFU |
| TICE | 50 mg |
| Tokyo | 80 mg |
Risk of Bias Assessment and Reporting
All of the extracted data was evaluated for internal consistency and contrasted with the trial’s protocol and all published reports. Data was extracted independently by two authors (BEB and JSO). Data was checked for validity, missing values, and completeness across all variables according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 12. The method of randomization and concealment was also assessed according to the Cochrane Handbook. All disagreements were resolved upon discussion with the moderator (RSS). The result of this meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 11.
Statistical Evaluation
The primary study endpoint was disease recurrence defined as pathologically confirmed low- and/or high-grade bladder cancer at any time following BCG treatment. Our initial approach to evaluate the comparative effectiveness among all unique BCG strains (n=10) was to perform a series of standard pairwise random-effect meta-analyses stratified by comparator and strain within comparator 13. The odds ratio (OR) was used to measure the relative effectiveness of the BCG strains in reducing recurrence. We grouped the meta-analyses by comparison type: BCG vs. chemotherapy, BCG + surgery vs. surgery only, BCG induction + maintenance vs. BCG induction only, BCG standard dose vs. BCG low dose. We used the standard 0.5 zero-cell continuity correction to account for zero-event arms. The standar test for heterogeneity and the index were used to assess statistical heterogeneity in treatment effects across studies. A significance level < 0.15 and/or> 50% were used to determine the presence of statistical heterogeneity 14.
We performed a frequentist random-effects network meta-analysis on a subset of 28 trials with chemotherapy as the common comparator 15–17. It is the only subset of sufficient size that includes head-to-head direct comparisons, as well as indirect comparisons, to assess the comparative effectiveness among 5 BCG strains (Tokyo, Pasteur, TICE, Connaught, RIVM). Two BCG strains (Glaxo, Armand-Frappier), each represented by a single trial, were excluded from these analyses: (1) A fair-quality trial comparing the Glaxo BCG strain with the Pasteur BCG strain (no chemotherapy comparator) and (2) A very small fair-quality trial comparing Armand-Frappier BCG strain against chemotherapy. The 28 included trials are sufficiently similar with respect to patient populations and comparators to assure sufficient transitivity (similarity) across trials to meaningfully combine direct and indirect effect estimates of the relative effectiveness among the 5 BCG strains. Network meta-analysis also assumes consistency in effect estimates derived from direct head-to-head comparisons among the BCG strains and indirect comparisons derived based on the contrast between each BCG strain and chemotherapy. The method described by Lu and Andes (2006) 18 was used to assess inconsistency between direct and indirect comparisons.
Results from our network meta-analysis are summarized graphically and in league tables. Primary assessment of comparative effectiveness among the 5 BCG strains is expressed as odds ratios with 95% confidence intervals. Relative rankings among the 5 BCG strains were obtained by computing the Surface Under the Cumulative Ranking Curve (SUCRA) 19. SUCRA scores vary between 0 and 1, and they track with the mean rank for each strain based on the probability that a particular strain is ranked best among the comparator strains (rank = 1), second best (rank = 2), and so forth. The higher the SUCRA score the higher the overall mean rank for a particular BCG strain. There is a great deal of uncertainty associated with these relative rankings, and they must be interpreted within the context of the actual effect estimates computed form the network of trials 10, 20.
Results
Clinical Trials and Patient Characteristics
The PRISMA flowchart describing the search process is shown in Figure 1. Initially, our search yielded 258 potentially relevant publications. The titles of all 258 results were reviewed, and if the publication could not be eliminated based on title alone, the abstract was subsequently reviewed. The remaining 128 manuscripts were reviewed in full to determine eligibility. Finally, 65 studies contained data in accordance with the eligibility criteria. From these 65 studies, 12,246 participants were available for meta-analysis. For the 7,326 patients for which gender was identified, 6,076 (82.9%) and 1,250 (17.1%) were male and female, respectively. Regarding the 8,554 patients for which tumor stage was provided, 3,973 (46.4%), 3,479 (40.7%), and 1,102 (12.9%) were identified as Ta, T1, and CIS, respectively.
Figure 1.
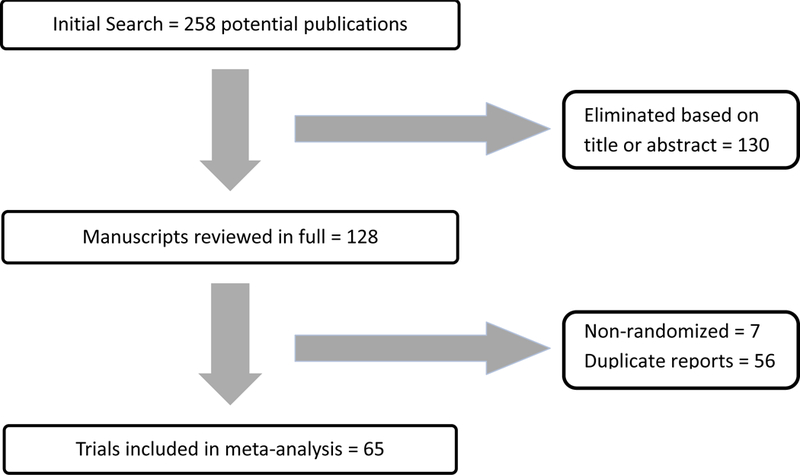
Flowchart Detailing Inclusion of Trials
Trial Characteristics
Characteristics of trials are detailed in Supplemental Table 1. The trial publication years ranged from 1978 to 2014. The trials did not consistently list follow-up as mean or median, and as such, an average was computed for the mean (24 months) and median (51.7 months). A wide range of chemotherapy and immunotherapy control groups were identified (the number in parentheses denotes number of studies in which each agent was utilized): Mitomycin C (17), Epirubicin (10), Interferon-α2b (3), Doxorubicin (3), Gemcitabine (3), Thiotepa (2), Maltose Tetrapalmitate (1), BEXIDEM® (1), Bropirimine (1), and Ofloxacin (1). In all, a total of 10 different strains of BCG were represented: Connaught (19), Pasteur (17), TICE (16), Tokyo (5), RIVM (4), Danish 1331 (3), Armand-Frappier (2), Moreau (1), Glaxo (1) and Evans (1). Lastly, a total of 21 studies utilized doses of BCG strains that differed from the standard dose ( Pasteur (11), Connaught (7), Danish 1331 (2), Tokyo (1)).
Risk of Bias
Risk of bias in contributing to the primary outcome of recurrence following BCG therapy was generally moderate (Figure 2). A large portion of the studies demonstrate an unclear risk of selection, performance, and detection bias (Supplemental Table 2). The vast majority of publications do not explicitly state any information concerning blinding, allocation concealment, and randomization. Nevertheless, > 85% of the studies were considered “low risk” of bias when evaluating attrition and reporting bias. In addition, no major inclination was noted for small studies to over- or under-estimate treatment effects.
Figure 2.
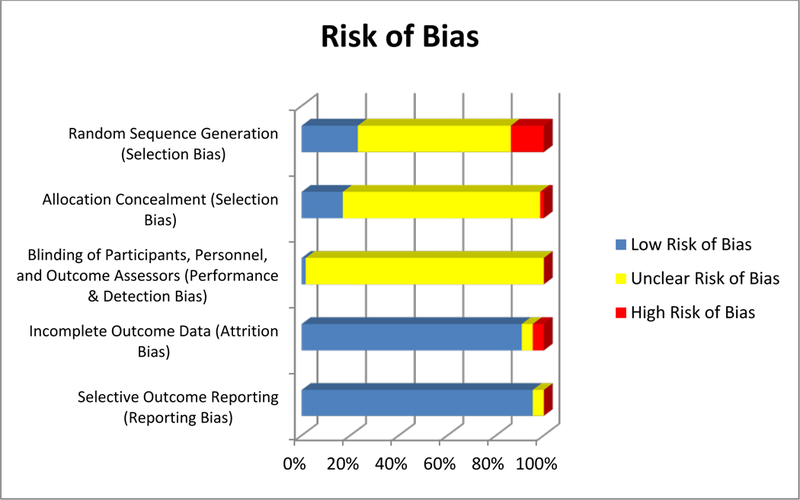
Risk of Bias Graph
Recurrence
Figure 3 shows the random-effects pairwise meta-analysis for recurrence stratified by comparator. BCG significantly reduced recurrence compared to chemotherapy (OR = 0.68 95% CI 0.54–0.85, ) and surgery (OR = 0.48 95% CI 0.28–0.8,). Pairwise meta-analysis of BCG versus chemotherapy broken down by BCG strain demonstrated Pasteur (OR = 0.51, 95% CI 0.32–0.80, ) and TICE (OR = 0.66, 95% CI 0.45–0.98,) strains as demonstrating significant decreases in recurrence (Supplemental Figure 1). TICE strain was associated with an increased risk of recurrence compared to Connaught strain (OR = 1.93, 95% CI 1.19–3.11) based on one randomized trial. RIVM strain was associated with an increased risk of recurrence compared to TICE strain (OR = 1.29, 95% CI 1.01–1.64) based upon one randomized trial.
Figure 3.
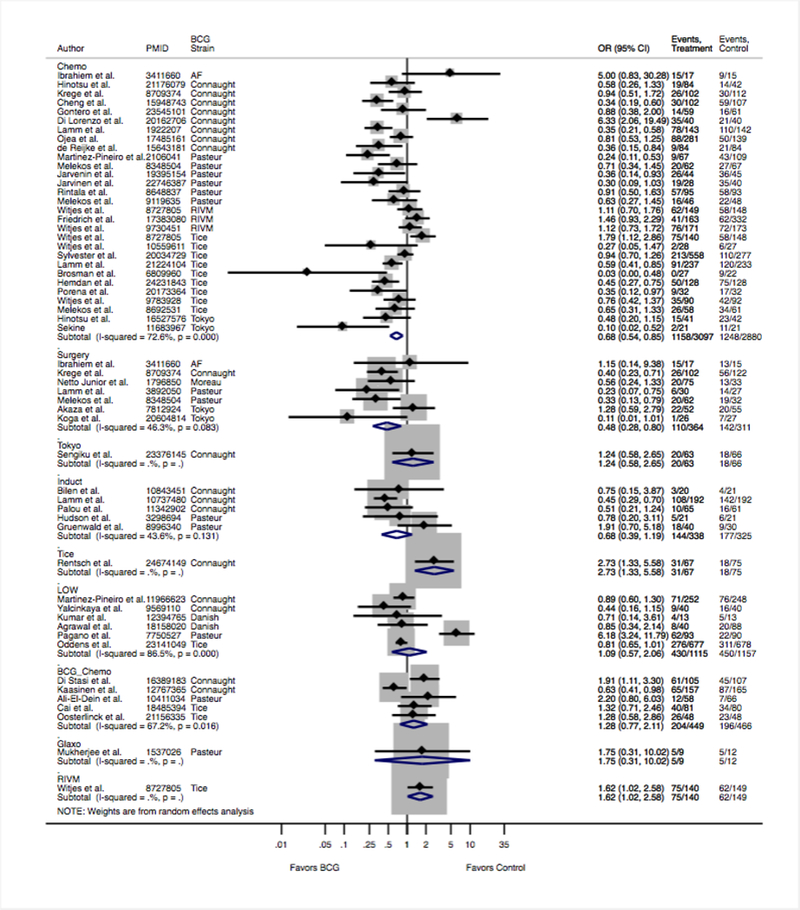
Impact of individual treatments on disease relapse of direct pairwise meta-analysis.
Networks considering the impact of BCG on disease recurrence with eligible comparisons considering all possible compators are shown in Figure 4a, demonstrating predominately indirect comparisons of BCG against chemotherapy and limited number of direct, head-to-head, comparisons among BCG strains. A network meta-analysis of trials using chemotherapy as the common comparator was conducted (Figure 4b and Supplmental Figure 2). Tokyo (OR = 0.39, 95% CI 0.16 – 0.93), Pasteur (OR = 0.50, 95% CI 0.28 – 0.86), and TICE (OR = 0.61, 95% CI 0.40 – 0.93) were associated with a decreased risk of recurrence compared to chemotherapy alone (Table 2). Tokyo strain was associated with a non-significant decrease in disease recurrence compared to all BCG strains while Connaught strain was associated with a non-significant increase in disease recurrence compared to Pasteur, TICE, and Tokyo BCG strains. SUCRA identified Tokyo strain as having the highest probability for superiority; followed by Pasteur, TICE, Connaught and RIVM (Supplemental Figures 3 and 4).
Figure 4. Network Maps.
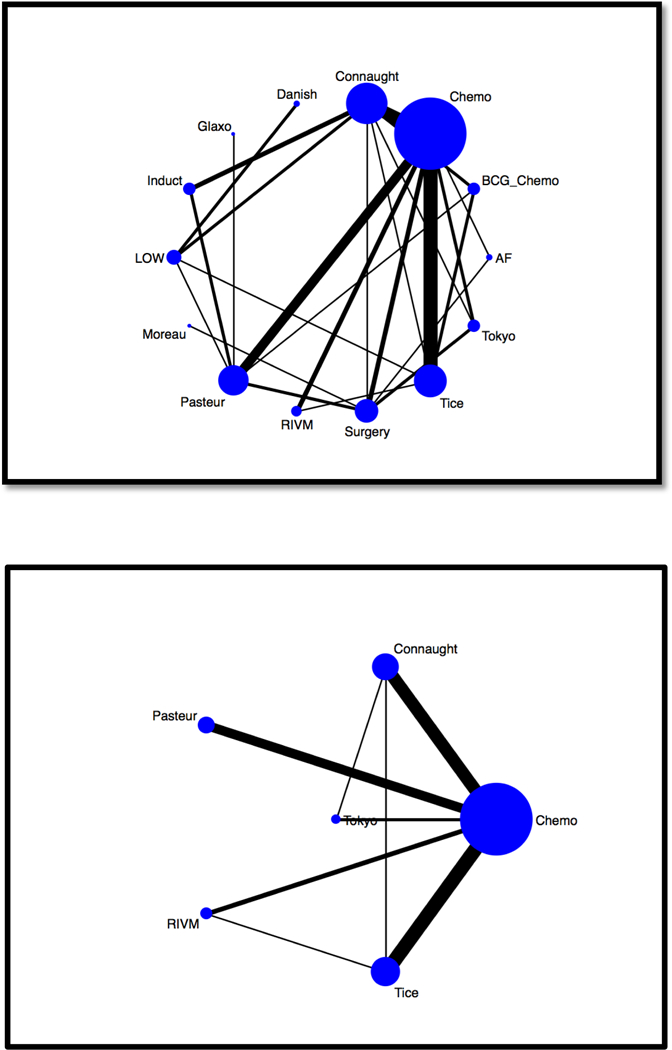
Network maps were created for bladder cancer recurrence based upon (a) all possible comparators and (b) chemotherapy as common comparator. The size of the nodes (blue circles) corresponds to the number of trials using each treatment. Each treatment is linked with a black line, the thickness of which corresponds to the number of trials that evaluated the treatments. Induct = BCG Induction only. LOW = low-dose BCG. Surgery = TURBT. AF = Armand-Frappier. BCG-Chemo = Combination BCG and chemotherapy. Chemo = chemotherapy.
Table 2.
League Table of Network Meta-Analysis Comparing BCG Strains: OR (95% CI)
| TREATMENT | CHEMOTHERAPY | TOKYO | TICE | RIVM | PASTEUR |
|---|---|---|---|---|---|
| TOKYO | 2.59 (1.07,6.22) | - | - | - | - |
| TICE | 1.64 (1.08,2.50) | 0.63 (0.24,1.66) | - | - | - |
| RIVM | 1.02 (0.54,1.94) | 0.40 (0.13,1.17) | 0.62 (0.31,1.28) | - | - |
| PASTEUR | 2.02 (1.16,3.51) | 0.78 (0.28,2.20) | 1.23 (0.62,2.46) | 1.97 (0.85,4.58) | - |
| CONNAUGHT | 1.38 (0.91,2.10) | 0.54 (0.22,1.31) | 0.84 (0.48,1.48) | 1.35 (0.63,2.88) | 0.68 (0.73, 1.37) |
Discussion
In the U.S., there are three BCG strains that are FDA approved for use in patients with NMIBC, including Armond-Frappier, Connaught, and TICE strains. However, TICE stain is the only BCG strain currently marketed in the U.S. Ongoing manufacturing problems are a result of challenges in sustaining lots of BCG 21. Resulting global shortages and BCG crisis highlight the importance of understanding strain differences in determining treatment response since alternative strains are available. While it has been demonstrated that these genetic differences between BCG vaccine strains can influence phenotypic properties such as immunogenicity and reactogenicity 3, it is unclear if such strain differences influence the clinical efficacy of BCG in treating bladder cancer.
This study provides evidence of marked efficacy in preventing disease recurrence across BCG strains using both direct and indirect comparisons from network metanalysis. These findings are important as regulatory agencies including the US Food and Drug Administration (FDA) grapple with handling of global BCG shortages due to manufacturing problems. In recent years, the FDA has not approved pleas for allowance of non-FDA approved BCG strains even in times of national BCG shortages due to lack of data regarding the oncologic efficacy of various daughter BCG strains. Importantly, we identified no evidence to support a statistically signficant difference in clinical efficacy across BCG strains.
The aforementioned genetic differences among the various strains can be traced back to the early 20th century, 1921, when Albert Calmette (physician) and Camille Guérin (veterinarian) from the Pasteur Institute in Paris, France, cultivated an oral vaccine against tuberculosis through numerous serial passages of Mycobacterium bovis on potatoes enriched with glycerol 3. M. bovis was chosen as it is considered attenuated with respect to Mycobacterium tuberculosis due to loss of RD4 – RD11, the region of difference gene groups. This deletion leads to M. bovis, and all subsequently derived BCG strains, demonstrating resistance to pyrazinamide antibiotics. One further deletion, RD1, remains the essential difference between BCG and its parent, M. bovis, responsible for encoding the protein secretion system ESX-1. This original vaccine was distributed worldwide later that year, 1921, and continued to undergo serial passages using the method perfected by Calmette and Guérin, leading to several additional mutations that created the vast assortment of BCG strains. This process continued until the early 1960’s with the introduction of freeze-dryed seeds lots.
Despite these demonstrated genetic differences among BCG strains, very few trials have been conducted to compare the efficacy of different BCG strains. We identified 5 randomized head-to-head trials comparing different BCG strains 5–9. Four of these trials did not involve maintenance BCG therapy 5–9. Because maintenance BCG provides superior recurrence-free survival benefit compared to BCG therapy without maintenance 22, results from these four trials may not be applicable to practices where maintenance BCG is standard of care. As an example, a provocative study found 5-year recurrence-free survival of patients treated with BCG Connaught (74.0%) significantly improved compared to those treated with BCG TICE (48.0%) 7. However, patients in this study were not routinely treated with maintenance BCG as is standard of care in the U.S. Interestingly, the effect of specific BCG strains could be modified by the use of maintenance therapy. Outcomes across strains were recently reported in a large observational cohort of 2099 patients with primary T1G3 bladder cancer treated with BCG TICE (N=599) and Connaught (n=1546) 23. When no maintenance was given, Connaught was more effective than TICE for time to first recurrence (HR=1.34, P=0.004). However, when maintenance was given, TICE was more effective than Connaught for time to first recurrence (HR=0.64, P=0.01). In accordance with these findings, our analysis suggests that Connaught strain is not superior to any other BCG strain. In fact, based upon SUCRA, Tokyo was more than twice times as likely to be the best treatment for NMIBC compared to other BCG strains while Connaught ranked lower than Pasteur and TICE. Thus, studies evaluating differences in BCG strains should consider standard of care treatment patterns including the use of maintenance which has consistently demonstated superiority over induction alone 22, 24.
Results from other head-to-head trials could be limited in ability to detect differences in efficacy due to insufficient sample size. Mukherjee and colleagues 6 reported similar outcomes between Glaxo and Pasteur BCG strains (complete response rates of 64% to 56%, respectively at 12 months) but the trial was inadequatley powered with a total of 21 patients 6. Another randomized controlled trial was initiated to compare Tokyo versus Connaught, but was closed before complete accrual 8. Outcomes at a median follow-up of 2.4 years were reported on 129 patients including 66 treated with Tokyo and 63 treated with Connaught BCG strains. The study was powered to detect a recurrence free survival difference of 0.2 (alpha 0.05, Beta = 0.2) with a sample size of 96 patients per arm. The complete response rate was 90.3% and 85.0% in patients given the Tokyo and Connaught strains, respectively, which did not differ significantly (p = 0.896). The 2-year recurrence-free survival rate was 73.2% and 68.8%, respectively. To gain perspective on the size of an adequately powered superiority trial using this data, a study aimed to detect a 4.4% absolute difference in survival would require approximately 530 patients (251 per arm).
Our analysis is subject to potential limitations. First, because of non-standardized and scant reporting effects of BCG strains on adverse events we were unable to evaluate the tolerability of BCG strains and the extent to which this could influence ability to give complete doses. Second, many trials were included despite not utilizing routine maintenance BCG and as discussed this could influence the overall interpretations of oncologic efficacy between strains. Third, the risk of bias was unclear in many of the studies concerning selection bias due to under-reporting of blinding and concealment procedures. Finally, because many published reports provided only the number of patients experiencing disease recurrence, the odds of recurrence was determined without considering censoring or the time to event.
Conclusion
The specific strain of BCG could influence bladder cancer recurrence rates. Tokyo, Pasteur, and TICE BCG strains all demonstrated a significant decrease in recurrence when compared with chemotherapy. However, no strain was significantly superior to another BCG strain during network analysis that incorporates direct and indirect comparisons. Tokyo strain was identified as having a high probability for superiority and should be compared to more commonly used TICE and Connaught BCG strains.
Supplementary Material
Acknowledgments
Grant support:
1. Max and Minnie Tomerlin Voelcker Fund
2. NIH 5K23CA178204–03
3. The Roger L. and Laura D. Zeller Charitable Foundation Chair in Urologic Cancer
Footnotes
The authors declare no potential conflicts of interest
References:
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin, 66: 7, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Association., A. U.: Guidelines for the Management of Non-Muscle Invasive Bladder Cancer (Stages Ta, T1, and TIS): 2007 Update. Linthicum, MD, Updated 2007
- 3.Gan C, Mostafid H, Khan MS et al. : BCG immunotherapy for bladder cancer--the effects of substrain differences. Nat Rev Urol, 10: 580, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Ritz N, Hanekom WA, Robins-Browne R et al. : Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev, 32: 821, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Fellows GJ, Parmar MK, Grigor KM et al. : Marker tumour response to Evans and Pasteur bacille Calmette-Guerin in multiple recurrent pTa/pT1 bladder tumours: report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). Br J Urol, 73: 639, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee A, Persad R, Smith PJ: Intravesical BCG treatment for superficial bladder cancer: long-term results using two different strains of BCG. Br J Urol, 69: 147, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Rentsch CA, Birkhauser FD, Biot C et al. : Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol, 66: 677, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Sengiku A, Ito M, Miyazaki Y et al. : A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol, 190: 50, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Witjes WP, Witjes JA, Oosterhof GO et al. : Update on the Dutch Cooperative Trial: mitomycin versus bacillus Calmette-Guerin-Tice versus bacillus Calmette-Guerin RIVM in the treatment of patients with pTA-pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Semin Urol Oncol, 14: 10, 1996 [PubMed] [Google Scholar]
- 10.Hutton B, Salanti G, Caldwell DM et al. : The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med, 162: 777, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Savitski MM, Mathieson T, Becher I et al. : H-score, a mass accuracy driven rescoring approach for improved peptide identification in modification rich samples. Journal of proteome research, 9: 5511, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT GS: Cochrane handbook for systematic reviews of interventions, version 5.1.0. http://www.cochrane-handbook.org (accessed February 10, 2015). . March 2011
- 13.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials, 7: 177, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med, 21: 1539, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Salanti G: Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods, 3: 80, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Chaimani A, Higgins JP, Mavridis D et al. : Graphical tools for network meta-analysis in STATA. PLoS One, 8: e76654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias S WN, Sutton AJ, Ades AE NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. . NICE DSU website Available: http://www.nicedsu.org.uk. Accessed 2013 Sep 4, 2011 [PubMed] [Google Scholar]
- 18.Lu G AA: Assessing evidence inconsistency in mixed treatment comparisons. J Amer Stat Assoc 101: 447, 2006 [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JP: Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol, 64: 163, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Trinquart L, Attiche N, Bafeta A et al. : Uncertainty in Treatment Rankings: Reanalysis of Network Meta-analyses of Randomized Trials. Ann Intern Med, 164: 666, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Mostafid AH, Palou Redorta J, Sylvester R et al. : Therapeutic options in high-risk non-muscle-invasive bladder cancer during the current worldwide shortage of bacille Calmette-Guerin. Eur Urol, 67: 359, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Lamm DL, Blumenstein BA, Crissman JD et al. : Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol, 163: 1124, 2000 [PubMed] [Google Scholar]
- 23.Shousha S: Oestrogen receptor status of breast carcinoma: Allred/H score conversion table. Histopathology, 53: 346, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Oddens J, Brausi M, Sylvester R et al. : Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol, 63: 462, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


