Abstract
Background
The rising prevalence of autism spectrum disorder (ASD) has increased the need for evidence‐based treatments to lessen the impact of symptoms. Presently, no therapies are available to effectively treat individuals with all of the symptoms of this disorder. It has been suggested that hyperbaric oxygen therapy may alleviate the biochemical dysfunction and clinical symptoms of ASD.
Objectives
To determine whether treatment with hyperbaric oxygen:
1. improves core symptoms of ASD, including social communication problems and stereotypical and repetitive behaviors;
2. improves noncore symptoms of ASD, such as challenging behaviors;
3. improves comorbid states, such as depression and anxiety; and
4. causes adverse effects.
Search methods
On 10 December 2015, we searched CENTRAL, Ovid MEDLINE, Embase, and 15 other databases, four of which were Chinese language databases. We also searched multiple trial and research registers.
Selection criteria
We selected randomized controlled trials (RCTs) and quasi‐RCTs of any dose, duration, and frequency for hyperbaric oxygen therapy compared with no treatment or sham treatment for children and adults with ASD.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration, in that three review authors independently selected studies, assessed them for risk of bias, and extracted relevant data. We also assessed the quality of the evidence by using the GRADE approach.
Main results
We included one trial with a total of 60 children with a diagnosis of ASD who randomly received hyperbaric oxygen therapy or a sham treatment. Using GRADE criteria, we rated the quality of the evidence as low because of the small sample size and wide confidence intervals (CIs). Other problems included selection bias and short duration or follow‐up.
Overall, study authors reported no improvement in social interaction and communication, behavioral problems, communication and linguistic abilities, or cognitive function. With regard to the safety of hyperbaric oxygen therapy (adverse events), they reported minor‐grade ear barotrauma events. Investigators found significant differences between groups in total number of side effect events (Peto odds ratio (OR) 3.87, 95% CI 1.53 to 9.82) and in the number of children who experienced side effects (Peto OR 4.40, 95% CI 1.33 to 14.48).
Authors' conclusions
To date, there is no evidence that hyperbaric oxygen therapy improves core symptoms and associated symptoms of ASD. It is important to note that adverse effects (minor‐grade ear barotrauma events) can occur. Given the absence of evidence of effectiveness and the limited biological plausibility and possible adverse effects, the need for future RCTs of hyperbaric oxygen therapy must be carefully considered.
Keywords: Child; Child, Preschool; Humans; Hyperbaric Oxygenation; Hyperbaric Oxygenation/adverse effects; Autism Spectrum Disorder; Autism Spectrum Disorder/therapy; Cognition; Communication; Interpersonal Relations; Randomized Controlled Trials as Topic
Plain language summary
High‐pressure oxygen therapy for children and adults with autism spectrum disorder (ASD)
Background
Autism spectrum disorder (ASD) is associated with problems in social communication and restricted behaviors. High‐pressure (hyperbaric) oxygen therapy has been proposed as treatment for these ASD symptoms. We reviewed the evidence on effects of high‐pressure (hyperbaric) oxygen therapy among children and adults with ASD. We also assessed the evidence on the safety of high‐pressure oxygen therapy.
Review question
Does high‐pressure oxygen therapy improve social communication or other aspects of function in children and adults with ASD, and how safe is this therapy?
Study characteristics
We searched electronic databases and identified randomized controlled trials (in which participants are randomly allocated to one of two or more treatment groups) consisting of participants who received high‐pressure oxygen therapy or room air or no treatment as a control.
The evidence is current up to December 2015.
Key results
We found a single, small study of 60 children that evaluated high‐pressure oxygen therapy for ASD.
There was no evidence that high‐pressure oxygen therapy improved social interaction, behavioral problems, speech or language communication, or mental function in children with ASD. However, children who received high‐pressure (hyperbaric) oxygen therapy showed an increased occurrence of ear barotrauma events when compared with those in the control group.
Quality of the evidence
The quality of the evidence is low. Evidence is insufficient to confirm that high‐pressure oxygen is an effective treatment for individuals with ASD.
Summary of findings
Summary of findings for the main comparison. Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children.
| Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children | ||||||
|
Patient or population: children with ASD
Settings: trial conducted in Thailand
Intervention: hyperbaric oxygen therapy Comparison: control group (same chambers as those in hyperbaric oxygen therapy group with an oxygen concentration of 21%) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary outcome | |||||
| Social interaction and communication ‐ Parental ATEC score (scale from 0 to 40) Follow‐up: 20 sessions of interventions | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.21 higher (2.21 lower to 4.63 higher) | Not estimable | 60 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Social interaction and communication ‐ Clinician ATEC score (scale from 0 to 40) Follow‐up: 20 sessions of interventions | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.55 higher (1.35 lower to 4.45 higher) | Not estimable | 60 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Behavioral problems ‐ Parental ATEC score Follow‐up: mean 20 sessions | Mean score in the control groups was 20.41 | Mean score in the intervention groups was 0.24 lower (4.80 lower to 4.32 higher) | Not estimable | 60 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Behavioral problems ‐ Clinician ATEC score (scale from 0 to 40) Follow‐up: 20 sessions of interventions | Mean score in the control groups was 13.52 | Mean score in the intervention groups was 1.28 lower (4.47 lower to 1.91 higher) | Not estimable | 60 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATEC: Autism Treatment Evaluation Checklist; CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSmall sample size (60 participants). bWide CIs, which include no effect AND appreciable harm and benefit.
Background
Description of the condition
Autism spectrum disorder (ASD) consists of a group of neurodevelopmental disorders classically characterized by impaired social interaction and communication, repetitive behaviors (Ghanizadeh 2012), and selective (inward) attention (Hughes 2008). Three different types of ASD have been defined: autistic disorder, Asperger syndrome, and pervasive developmental disorder not otherwise specified (PDD‐NOS) (APA 2013). People with ASD often present with challenging behaviors, including aggression, tantrums, irritability, hyperactivity, inattention, impulsivity, self‐injury, and pica (Matson 2011).
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) states that ASD is characterized by persistent deficits in social communication and social interaction, as well as restricted, repetitive patterns of behavior, interests, or activities. Furthermore, these symptoms must be present in the early developmental period and must not be attributable to another medical or neurological condition. Severity levels for ASD include level one (requiring support), level two (requiring substantial support), and level three (requiring very substantial support). These levels are based on the severity of social communication impairments and restricted, repetitive patterns of behavior (APA 2013).
The number of people with a diagnosis of ASD has escalated over the past decade, and prevalence rates continue to rise. In the United States, 1% of individuals are reported to have ASD (Gal 2012). ASD has a strong genetic basis. High‐throughput, next‐generation sequencing of large cohorts has exposed a heterogeneous and complex genetic landscape and has revealed novel risk genes (De Rubeis 2015). Current estimates suggest that approximately 1000 genes are likely to be involved in ASD (De Rubeis 2014). Two recent population‐based studies have converged on a more refined estimate of greater than 50% heritability, with most cases due to common variations (Gaugler 2014; Sandin 2014). A person carrying one of the individual variants is, at most, 1.2 times more likely to develop ASD (Anitha 2012). Although the causes are unclear, studies on gray and white matter implicate brain development abnormalities in ASD pathology. These abnormalities predominantly affect the association areas and undermine functional integration (O'Hearn 2008). Changes to the structure and function of synapses and dendrites have been reported (Pardo 2007). Impaired microglial function may contribute to several major etiological factors in ASD (Maezawa 2011). Recent studies have discovered hundreds of ASD risk genes, which indicate a strong genetic basis for ASD susceptibility (Devlin 2012). Multiple genetic loci predispose a person to ASD (Yang 2007). A specific mutation or deletion of a gene may determine one's susceptibility to ASD, and interactions between multiple genes may cause 'idiopathic' ASD. Evidence suggests that impaired methylation and genetic polymorphisms of cytochrome enzymes are linked to ASD (Currenti 2010). Other studies show that environmental factors interact with underlying genetic profiles to produce the clinical heterogeneity observed in ASD (Dufour‐Rainfray 2011; Muhle 2004).
Evidence supports early intensive behavioral and developmental intervention to improve cognitive performance, language skills, and adaptive behavior in children with ASD (AHRQ 2011; Eldevik 2009; Howlin 2009; Maglione 2012; Reichow 2012;Warren 2011). Interventions that provide parent training and cognitive‐behavioral therapy (CBT) for bolstering social skills and managing challenging behaviors may be useful for improving social communication, language use, and symptom severity in children with ASD. The Treatment and Education of Autistic and Related Communication Handicapped Children (TEACCH) program produced some improvements in motor skills and cognitive measures (AHRQ 2011).
In the field of pharmacotherapy, no agents have been proven to change the core features of autism, but some compounds can be effective for treating individuals with specific behavioral or mental health problems. The antipsychotic drugs risperidone and aripiprazole improve challenging behaviors, such as emotional distress, aggression, hyperactivity, and self‐injury, but both drugs have a high incidence of harm (AHRQ 2011; Ching 2012; Jesner 2007; McPheeters 2011). Use of antipsychotic medications for managing behavior should be considered when psychosocial or other interventions are insufficient or cannot be delivered because of the severity of the behavior (NICE 2013). Little evidence is available on other behavioral interventions, allied health therapies, or complementary and alternative medicine (AHRQ 2011).
Description of the intervention
The Undersea and Hyperbaric Medical Society defines hyperbaric oxygen therapy as an intervention in which the patient breathes near 100% oxygen intermittently while inside a hyperbaric chamber pressurized to greater than sea level pressure (one atmosphere absolute, or ATA) (UHMS 2016). With this method, it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. As reported by the Undersea and Hyperbaric Medical Society, hyperbaric oxygen therapy has been shown to be effective in the treatment of patients with various conditions, including air or gas embolism, carbon monoxide poisoning, clostridial myositis and myonecrosis (gas gangrene), crush injuries, compartment syndrome and other acute traumatic peripheral ischemias, decompression sickness, arterial insufficiencies, severe anemia, intracranial abscess, necrotizing soft tissue infections, osteomyelitis (refractory), delayed radiation injury, compromised grafts and flaps, acute thermal burn injuries, and idiopathic sudden sensorineural hearing loss (UHMS 2016). Systematic reviews and randomized controlled trials (RCTs) support the clinical use of hyperbaric oxygen for refractory diabetic wound healing (Huang 2015; Stoekenbroek 2014), radiation injury (Hoggan 2014), and compromised flaps and grafts (Goldman 2009). Use of this treatment for ischemia‐reperfusion disorders is supported by animal studies (Wei 2015) and by a small number of clinical trials (Thom 2011; Zhou 2008). Several other hyperbaric oxygen trials on frost injury, hypoxic ischemic encephalopathy, osteoradionecrosis, and traumatic brain injury are ongoing. Hyperbaric oxygen is well tolerated, but middle ear barotrauma is a common adverse effect (Muller‐Bolla 2006).
Hyperbaric oxygen therapy may also help individuals with autism. One case series showed that seven Thai autistic children benefited from hyperbaric oxygen, as evidenced by improvement in social interaction and language and motor skills (Chungpaibulpatana 2008).
In addition to the 'classical' hyperbaric oxygen therapy defined by the Undersea and Hyperbaric Medical Society (UHMS 2016), some ASD trials have used nonclassical hyperbaric oxygen therapy (Granpeesheh 2010; Rossignol 2009). In these trials, nonclassical hyperbaric oxygen therapy consisted of a chamber pressurized to greater than one ATA with less than 100% oxygen concentration. In one study, both classical and nonclassical hyperbaric oxygen therapies produced significant improvement in children with autism, as evidenced by normal levels of oxidative stress and inflammation markers (Rossignol 2007a). As outlined in our protocol (Xiong 2014), we assessed only the use of classical hyperbaric oxygen therapy in this review.
How the intervention might work
The core theoretical basis for hyperbaric oxygen therapy is that it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. To date, the potential mechanisms of hyperbaric oxygen therapy for ASD have been proposed on the basis of animal experiments. In this section of the review, we summarize these possible mechanisms of hyperbaric oxygen therapy (more than one ATA with 100% oxygen).
Recently, studies in ASD have reported many possible specific dysfunctions such as cerebral hypoperfusion (Ito 2005), inflammation (Vargas 2005), immune dysregulation (Li 2009a), neurotransmitter abnormalities (Connors 2006), and mitochondrial dysfunction (Rossignol 2007b; Rossignol 2012b).
In autistic children, it has been demonstrated that hypoperfusion in ASD is positively correlated with severity of autistic behaviors (Gendry 2005). Lower cerebral perfusion is significantly correlated with increasing age in children with ASD (Rossignol 2012a). The outcome of hypoperfusion occurs as hypoxia in the brain, which can be improved with increased oxygen delivery via hyperbaric therapy. The application of hyperbaric oxygen therapy is based on the theory that inhalation of oxygen at increased atmospheric pressure produces a marked elevation in the partial pressure of oxygen in arterial blood, and thus improves hypoxia in the brain (Calvert 2007). In neonatal rats, hyperbaric oxygen therapy improved hypoxic‐ischemic brain injury in multiple cerebral regions, including the cortex (Wang 2009), white matter (Wang 2007b), and hippocampus (Bai 2008). In addition, hyperbaric oxygen therapy improved the oxygen delivery capacity of the brain by inducing angiogenesis in mature rats with traumatic brain injury (Lin 2012a). Direct angiogenesis may be attributed to the proliferation of neural stem cells induced by hyperbaric oxygen therapy (Ichim 2007).
Hyperbaric oxygen therapy has shown anti‐inflammatory effects in animal studies. In a middle cerebral artery occlusion adult rat model, hyperbaric oxygen therapy inhibited neutrophil infiltration in the injured brain by reducing inflammation (Miljkovic‐Lolic 2003). In a mature rat model of inflammatory pain, hyperbaric oxygen therapy decreased inflammation and subsequent pain (Wilson 2006). In the immune system of an adult rat, hyperbaric oxygen therapy modulated immune‐mediated delayed neurological dysfunction following carbon monoxide poisoning (Thom 2006).
An insufficient supply of the neurotransmitter serotonin may be linked to autism. Mice genetically depleted of brain serotonin displayed symptoms of autism (Kane 2012). Hyperbaric oxygen therapy exerted antidepressant effects similar to those produced by some selective serotonin reuptake inhibitors in a rat model of forced swimming (Sumen‐Secgin 2005). Alternatively, impairments in neuroplasticity may contribute to the pathophysiology of ASD (Abdallah 2012). Increasing the level of dissolved oxygen with hyperbaric oxygen therapy appeared to activate neuroplasticity in patients with chronic neurologic deficiencies resulting from stroke (Efrati 2013). This therapy intensified neuroplastic responses by promoting axonal sprouting and synapse remodeling, which contributed to the recovery of locomotor performance in rats (Brkic 2012).
Mitochondrial dysfunction has been observed in approximately 5% of children with ASD (Anitha 2012). Mitochondrial dysfunction is associated with apoptosis, and thus, the causes of autism may include apoptotic mechanisms (El‐Ansary 2012). Members of the caspase family, the most important pre‐apoptosis molecules, are significantly increased in children with ASD (Siniscalco 2012). Hyperbaric oxygen therapy may ameliorate mitochondrial dysfunction and apoptosis in ASD. Hyperbaric oxygen therapy reduces apoptosis via the mitochondrial pathway after ischemia‐reperfusion brain injury (Li 2009; Yin 2013). The opening of mitochondrial, adenosine triphosphate (ATP)‐sensitive potassium channels plays a role in this effect (Lou 2006). Hyperbaric oxygen therapy significantly increased neuronal survival (Malek 2013). Another study showed that hyperbaric oxygen therapy increased cell proliferation and reduced infarct size in the hippocampus after stroke (Mu 2013).
Neural stem cells can differentiate into neurons, induce angiogenesis, and nonspecifically modulate the immune response (Ichim 2007). Hyperbaric oxygen therapy promotes the proliferation, migration, and differentiation of neural stem cells (Wang 2007a; Wang 2009), and this may contribute to neural recovery in children with ASD.
Hyperbaric oxygen therapy may play a neuroprotective role via modulation of gene regulation and protein expression in neurons in ASD. The genes and proteins regulated by hyperbaric oxygen therapy include factors associated with stress responses, transport, neurotransmission, signal transduction, and transcription factors (Chen 2009a). Hyperbaric therapy has been shown to activate ion channels (Mrsić‐Pelcić 2004), upregulate superoxide dismutase (SOD) (Freiberger 2006), decrease caspases (Chen 2009b), suppress p38 mitogen‐activated protein kinase (Yamashita 2009), induce heat shock protein (HSP) 70 overexpression (Lin 2012b), and activate Wnt signaling (Wang 2007a).
In children with ASD, hyperbaric oxygen therapy may improve behavioral symptoms, such as memory, social interaction, cognitive function, coloring skills, and speech and self‐help skills, and may alleviate other symptoms such as eczema, chronic diarrhea, and abdominal distention. Children completing 80 sessions of hyperbaric oxygen therapy showed improvement on the clinician‐rated Clinical Global Impression ‐ Improvement (CGI‐I) scale and on several parent‐based measures of behavior (Bent 2012).
Why it is important to do this review
Investigations into effective interventions for improving the core features of autism without adverse effects are ongoing. Hyperbaric oxygen as a potential treatment for ASD remains controversial. Although some evidence supports the benefits of hyperbaric oxygen (Chungpaibulpatana 2008; Rossignol 2009a; Rossignol 2012a), other findings do not (Ghanizadeh 2012; Granpeesheh 2010). In addition to other treatment options, the effectiveness of hyperbaric oxygen was assessed in a systematic review conducted by Rossignol 2009b. This review, now seven years old, included three observational studies and nonrandomized trials. Review authors did not assess the risk of bias in included studies and provided a purely narrative report of study findings. Since 2009, additional RCTs on the efficacy of hyperbaric oxygen have been completed. Therefore, a systematic review that synthesizes contemporary evidence of the potential benefits and harms of hyperbaric oxygen as reported in RCTs is timely.
Objectives
To determine whether treatment with hyperbaric oxygen:
improves core symptoms of ASD, including social communication problems and stereotypical and repetitive behaviors;
improves noncore symptoms, such as challenging behaviors;
improves comorbid states, such as depression and anxiety; and
causes adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐RCTs (i.e. when allocation is made by not truly random methods such as alternation, date of birth, or case record number), including cross‐over trials (Higgins 2011a).
Types of participants
Participants of any age with a diagnosis of autism spectrum disorder (ASD) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013); or individuals with a diagnosis of one of the four pervasive developmental disorders from the fourth edition text revision version of the DSM (DSM‐IV‐TR) (APA 2000), including autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD‐NOS); or from theInternational Classification of Mental and Behavioural Disorders, 10th Edition (ICD‐10) WHO 1993). We accepted diagnoses that were derived following use of assessment tools, such as the Autism Diagnostic Observation Scale (ADOS) (Lord 1997) and the Autism Diagnostic Interview ‐ Revised (ADI‐R) (Lord 1994).
Types of interventions
All forms of hyperbaric oxygen therapy (more than one atmosphere absolute (ATA) with 100% oxygen concentration), regardless of dose, duration, frequency, and pressure of treatment. Control interventions consisted of no treatment or a sham treatment. Sham treatment refers to treatment at one ATA or room air (21% oxygen), or both. We included studies that conducted four different comparisons.
Hyperbaric oxygen therapy only versus no treatment.
Hyperbaric oxygen therapy only versus sham treatment.
Hyperbaric oxygen therapy plus baseline treatment versus the same baseline treatment alone.
Hyperbaric oxygen therapy plus baseline treatment versus sham treatment plus the same baseline treatment.
We excluded trials that compared only different forms of hyperbaric oxygen therapy.
Types of outcome measures
Primary outcomes
The core features of ASD, which include impaired social interaction and communication and behavioral problems such as stereotypy or restricted repetitive patterns of behavior, interests, or activities, as measured by validated instruments and behavioral observation tools such as the Aberrant Behavior Checklist (ABC) (Aman 1987), the Ritvo‐Freeman Real Life Rating Scale (RFRLRS) (Freeman 1986), the Autism Treatment Evaluation Checklist (ATEC) (Rimland 1999), and the ADOS (Lord 1997).
Secondary outcomes
Communication and linguistic abilities, as measured by standardized instruments such as the Reynell Language Developmental Scale (RLDS) (Edwards 1997) and the Symbolic Play Test (SPT) (Lowe 1976)
Cognitive function, as measured by standardized instruments such as the Griffiths Mental Developmental Scale (GMDS) (Griffiths 1996), the Leiter International Performance Scale ‐ Revised (Leiter‐R) (Leiter 1980), and the Stanford‐Binet Intelligence Scale ‐ Fourth Edition (SB4) (Thorndike 1986)
Global function, as measured by standardized instruments such as the Pediatric Evaluation Disability Inventory (PEDI) (Haley 1992) and the Functional Independence Measure for Children (WeeFIM) (Msall 1994)
Safety of hyperbaric oxygen therapy, as measured by the incidence of adverse reactions such as barotrauma to the ear, round window blowout, "sinus squeeze," visual refractive changes, and numb fingers (Phillips 2005)
As stated in our protocol (Xiong 2014), we presented the primary outcomes of impaired social interaction and communication and behavioral problems in Table 1.
Search methods for identification of studies
Electronic searches
We searched the electronic databases and trial registers listed below on 25 November 2014, and updated the search in December 2015. We searched all available years and applied no language limits.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 11) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialized Register (searched 14 December 2015).
Ovid MEDLINE (1946 to November week 3 2015).
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations (10 December 2015).
Embase Ovid (1980 to 2015 week 50).
PsycINFO Ovid (1967 to December week 2 2015).
Science Citation Index Web of Science (SCI; 1970 to 14 December 2015).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 14 December 2015).
Conference Proceedings Citation Indexes ‐ Science Web of Science (CPCI‐S; 1990 to 14 December 2015).
Conference Proceedings Citation Indexes ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H; 1990 to 14 December 2015).
WorldCat (limited to theses; worldcat.org; searched 14 December 2015).
Cochrane Database of Systematic Reviews (CDSR; 2015, Issue 12) in the Cochrane Library (searched 14 December 2015).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) in the Cochrane Library (searched 14 December 2015).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to current; searched 14 December 2015).
ERIC EBSCOhost (Education Resources Information Center; 1966 to current; searched 14 December 2015).
HBO Evidence. The Database of Randomised Controlled Trials in Diving and Hyperbaric Medicine (hboevidence.unsw.wikispaces.net; searched 14 December 2015).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 15 December 2015).
ClinicalTrials.gov (clinicaltrials.gov; searched 15 December 2015).
metaRegister of Controlled Trials (mRCT; controlled‐trials.com/mrct; searched 21 November 2013. This service was subsequently unavailable).
Research Autism (researchautism.net/pages/welcome/home.ikml; searched 14 December 2015).
Autism Data (autism.org.uk/about‐autism/research/autism‐data.aspx; searched 14 December 2015).
Australian New Zealand Clinical Trials Registry (ANZCTR; anzctr.org.au; searched 26 November 2014).
China National Knowledge Infrastructure (CKNI; cnki.net; searched 10 December 2015).
WEIPU periodical database (cqvip.com; searched 10 December 2015).
Wan Fang Data (wanfangdata.com.cn; searched 10 December 2015).
Chinese Biologic Medical Database (CBM; sinomed.imicams.ac.cn; searched 10 December 2015).
The search strategies are outlined in Appendix 1.
Searching other resources
On 11 November 2014, we contacted the authors of RCTs identified by the electronic searches, to ask if they knew of any additional published or unpublished trials. We emailed the following experts using the addresses provided in published papers: Dr DA Rossignol, Dr C Schneider, Dr J Neubrander, Dr G Hintz, Dr LW Rossignol, Dr S Logerquist, Dr EM Madren, Dr B Grushkin, Dr S Smith, Dr A Usman, Dr EA Mumper, Dr D Granpeesheh, Dr CT Chaiyakul, and Dr J Tarbox. Three experts (Dr DA Rossignol, Dr G Hintz, and Dr J Tarbox) responded, and one expert (DA Rossignol) attached four citations (Xiong 2014 [pers comm]).
We manually searched the reference lists of relevant studies for additional published or unpublished trials. We checked the publication status of each trial identified by our search of trial registers, and we contacted study authors to request the results of completed but unpublished trials.
We searched the Chinese gray literature, which is available online, using the academic search engine "Baidu Scholar."
For non‐English language articles, we read the English title or abstract first, to determine relevance. If deemed relevant, we translated the main text into English. We completed this process for several Chinese studies, which we subsequently excluded because they did not meet the inclusion criteria (see Excluded studies).
Data collection and analysis
Selection of studies
We assessed all potentially relevant, published articles identified by our literature search. TX and HC independently read abstracts retrieved from the search in an effort to identify all trials that met the inclusion criteria. If needed, we retrieved full‐text articles. We involved a third review author (RL) to help resolve differences in opinion. We contacted trial authors to request clarification if details of the primary trials were not clear. We recorded our selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
We designed a form onto which we extracted the data described below.
-
Study methods.
Design.
Randomization method (including list generation).
Method of allocation concealment.
Blinding method.
Stratification factors.
-
Participants.
Inclusion/exclusion criteria.
Number (total/per group).
Age and sex distribution.
Specific diagnosis/diagnostic subtypes.
Comorbidities.
Duration of disorder.
Previous treatments.
-
Intervention and control.
Types of hyperbaric oxygen therapy.
Details of treatment, including duration of treatment.
Types of controls.
Details of control treatment, including drug dosage.
Details of cointerventions.
-
Follow‐up data.
Duration of follow‐up.
Dates of treatment withdrawal and reasons for treatment withdrawal.
Withdrawal rates.
Outcome data (as described in the subsection on Types of outcome measures).
-
Data analysis.
Methods of analysis (intention‐to‐treat/per‐protocol analysis).
Comparability of groups at baseline (yes/no).
Statistical techniques.
Two review authors (TX and HC) used the data extraction form to independently extract, assess, and code all data for each available study. We involved a third review author (RL) to negotiate differences in opinion. TX entered the data into Review Manager 5 (RevMan 5) (RevMan 5 2014), and DM checked the accuracy of the data input.
Assessment of risk of bias in included studies
Two review authors (TX and HC) independently assessed the risk of bias of the included study using the Cochrane 'Risk of bias' tool (Higgins 2011b). We resolved disagreements by consulting with the third review author (RL).
We evaluated risk of bias as low, high, or unclear for each of the domains listed below. We entered the results into the corresponding 'Risk of bias' table, beneath the Characteristics of included studies tables (Higgins 2011b).
Sequence generation (selection bias)
For each included study, we categorized the risk of selection bias regarding sequence generation as follows.
Low risk of bias: adequate (e.g. any truly random process such as a random number table or computerized random number generator).
High risk of bias: inadequate (e.g. any nonrandom process such as odd or even date of birth or hospital or clinic record number).
Unclear risk of bias: no or unclear information provided.
Allocation concealment (selection bias)
For each included study, we categorized the risk of selection bias regarding allocation concealment as follows.
Low risk of bias: adequate (e.g. telephone or central randomization or consecutively numbered sealed opaque envelopes).
High risk of bias: inadequate (e.g. open random allocation, unsealed or nonopaque envelopes, alternation, or date of birth).
Unclear risk of bias: no or unclear information provided.
Blinding of participants and personnel (performance bias)
For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received. We categorized the risk of performance bias regarding the methods used to blind participants and personnel as follows.
Low risk of bias: adequate for personnel (e.g. a normal pressure chamber, which could not be distinguished from a hyperbaric oxygen chamber, was used in the control group when no blinding was provided, but the outcome and outcome measurements were not likely to be influenced).
High risk of bias: inadequate (e.g. personnel aware of group assignment).
Unclear risk of bias: no or unclear information provided.
Blinding of outcome assessors (detection bias)
For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the risk of detection bias regarding methods used to blind outcome assessors as follows.
Low risk of bias: adequate (e.g. assessors blinded to the group at follow‐up).
High risk of bias: inadequate (e.g. assessors aware of group assignment at follow‐up).
Unclear risk of bias: no or unclear information provided.
Incomplete data addressed (attrition bias)
For each included study and each outcome, we described the completeness of data, including attrition and exclusions from analyses. We noted whether attrition and exclusions were reported, numbers included in the analyses at each stage (compared with the total number of randomly assigned participants), whether the reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or was supplied by the trial authors, we included missing data in the analyses. We categorized the risk of attrition bias regarding completeness of outcome data for each outcome as follows.
Low risk of bias: adequate (e.g. no missing outcome data, reasons for missing outcome data unlikely to be related to the true outcome, data missing for similar reasons across groups, or missing proportions were balanced).
High risk of bias: inadequate (e.g. reasons for missing outcome data likely to be related to true outcome or inappropriate imputation used for missing data).
Unclear risk of bias: no or unclear information provided.
Selective outcome reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and the results of this investigation. We assessed the risk of reporting bias as follows.
Low risk of bias: adequate (e.g. all of the study's pre‐specified outcomes and expected outcomes of interest to this review were clearly reported or the methods and results sections of the study agree with what was reported).
High risk of bias: inadequate (e.g. not all of the study's pre‐specified outcomes were reported, one or more reported primary outcomes were not pre‐specified, the outcomes of interest were incompletely reported and thus cannot be used, the study failed to include the results of a key outcome that was expected to be reported, or the methods and results sections of a study disagree with what was reported).
Unclear risk of bias: no or unclear information provided (e.g. the study protocol was not available).
Other bias
For each included study, we described any important concerns about other possible sources of bias not covered by the domains above (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early because of a data‐dependent process). We assessed the risk of other bias for each study as follows.
Low risk of bias: no concerns of other bias raised.
High risk of bias: potential source of bias related to the specific study design, fraudulent data or a similar problem, or a difference in the number of participants enrolled in the abstract and reported in the final publication.
Unclear risk of bias: concerns raised about potential sources of bias that could not be verified by contacting study authors.
Measures of treatment effect
We did not conduct a meta‐analysis, as we only included one study in this review. Please see Appendix 2 and our protocol (Xiong 2014) for measures of treatment effects that will be used in future updates of this review.
Unit of analysis issues
For most outcomes, the unit of analysis was the individual participant. We found no cluster‐randomized trials and no study with multiple intervention groups. Please see Appendix 2 and our protocol (Xiong 2014) for more information as to how we will resolve these issues in future updates of this review.
Dealing with missing data
We contacted the corresponding author of the one included study to request missing data but received no response.
We noted differential dropout in the intervention group, and in the published trials when reasons were provided. We reported reasons for missing data in the Characteristics of included studies tables. We explored the impact of missing data by examining the distribution of data and explanations for the missing information. In the Discussion section, we addressed the potential impact of missing data on the findings of our review (see Quality of the evidence).
Please see Appendix 2 and our protocol (Xiong 2014) for additional methods archived for use in future updates of this review.
Assessment of heterogeneity
We did not assess heterogeneity, as we were able to include only one study in this review. Please see Appendix 2 for methods to assess heterogeneity archived for future updates of this review.
Assessment of reporting biases
We were unable to obtain the protocol for the one included study, to compare outcomes reported in the protocol versus those reported in the published findings. Also, we tried to contact the study authors to request the missing outcome data, but no study authors replied.
We did not find a sufficient number of trials to test for publication bias by using funnel plots and the Egger test (Egger 1997); we included only one trial in this review, and a funnel plot requires 10 or more studies.
Please see Appendix 2 and our protocol (Xiong 2014) for methods archived for future updates of this review.
Data synthesis
We did not conduct a meta‐analysis, as we included only one study in this review. Instead, we presented a narrative description of the study's results (see Results).
For methods archived for future updates of this review, please see Appendix 2 and our protocol (Xiong 2014).
GRADE
We used the GRADE approach to describe the quality of the evidence and the strength of recommendations (GRADE 2013). We expressed the quality of the evidence on a four‐point adjectival scale ranging from 'high' to 'very low'. We initially gave evidence from RCTs a rating of high quality. However, we downgraded this rating on the basis of specific criteria: clinically important heterogeneity was unexplained; the study methods had risk of bias; the evidence was indirect; we noted important uncertainty about the estimates of effects; or we found evidence of publication bias. Thus, it was possible for data from RCTs to be given a very low quality rating if several of these concerns were present.
We used the GRADEpro: Guideline Development Tool (GRADEproGDT) (GRADEpro GDT 2014) to construct Table 1. This table presents information on the body of evidence (e.g. number of studies), review authors' judgements on the underlying quality of the evidence, key statistical results, and a grade for the quality of evidence for each outcome.
Subgroup analysis and investigation of heterogeneity
We did not perform a subgroup analysis because we identified only one small study consisting of 60 children. Please see Appendix 2 and our protocol (Xiong 2014) for subgroup analyses planned for future updates of this review.
Sensitivity analysis
We found only one study consisting of 60 children; thus, we performed no sensitivity analyses. Please see Appendix 2 and our protocol (Xiong 2014) for sensitivity analyses planned for future updates of this review.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search strategy yielded 813 citations. We deemed that 22 of these studies were potentially eligible on the basis of their title or abstract. We obtained and inspected the full‐text copies of these 22 studies. After reviewing all full‐text copies, we included one study (Sampanthavivat 2012) and excluded 20 studies (Bent 2012; Chungpaibulpatana 2008; Jepson 2011; Lerman 2008; Rossignol 2006; Rossignol 2007a; Rossignol 2009c; Rossignol 2012; VanEstenberg 2006; Yildiz 2008; Li 2012; Ou 2005; Ou 2010; Pan 2009; Wan 2013; Wu 2009; Yu 2010; Yu 2010a; Granpeesheh 2010; Rossignol 2009). See Figure 1 for more information.
1.

Flow diagram.
Footnotes
RCT: randomized controlled trial.
Included studies
We included one RCT (Sampanthavivat 2012), which is described below.
Participants
Sampanthavivat 2012 randomized 60 children with autism using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) (APA 2000). The study included 56 boys and four girls who had never received hyperbaric oxygen therapy. Children ranged from three to nine years of age, and their average age was 4.9 years. Children who had seizure disorders, uncontrolled asthma, a history of previous spontaneous pneumothorax, current ear or upper respiratory tract infection, emphysema, current or recent chemotherapy, severe claustrophobia, and ongoing chelating therapy were excluded.
Setting
The study was conducted in Thailand, at the Royal Thai Navy Medical Department (Sampanthavivat 2012).
Interventions and comparisons
Hyperbaric oxygen therapy was administered in a multiplace hyperbaric chamber for the Sampanthavivat 2012 study. The pressure of hyperbaric oxygen therapy was 1.5 ATA with 100% oxygen concentration. Hyperbaric oxygen therapy consisted of 20 one‐hour sessions held on weekdays over 10 weeks.
Children in the control group received identical sessions in the same chambers as those in the intervention group (hyperbaric oxygen therapy). The control group had an oxygen concentration of 21%. A slightly higher pressure of 1.15 ATA was used to mimic the experience of hyperbaric treatment, thus keeping the children blinded to their group assignment.
Outcomes
The study had multiple primary and secondary outcomes and used validated instruments to assess treatment effects on different clinical aspects of ASD. Sampanthavivat 2012 assessed autistic features by using the Autism Treatment Evaluation Checklist (ATEC) (Rimland 1999) and the Clinical Global Impression (CGI) scale of illness severity (CGIS) and change scores (CGIC) (Guy 1976). The ATEC consists of four subtests: speech or language communication; sociability; sensory or cognitive awareness; and health, physical or behavior (Rimland 1999). The CGIS provides a seven‐point scale to rate a range of responses from one (normal) and two (borderline mentally ill) to seven (among the most extremely ill patients) (Guy 1976). Investigators assessed CGIC scores after the intervention to score the improvement of each participant; CGIC scores ranged from one (very much improved) to seven (very much worse) (Guy 1976).
Social interaction and communication
In Sampanthavivat 2012, parents and the clinician assessed social interaction and communication by using the ATEC (Rimland 1999).
Behavioral problems
Sampanthavivat 2012 assessed behavioral problems by using the ATEC (Rimland 1999).
Communication and linguistic abilities
Sampanthavivat 2012 assessed communication and linguistic abilities by using the ATEC (Rimland 1999).
Cognitive function
Sampanthavivat 2012 assessed cognitive function by using the ATEC (Rimland 1999).
Safety of hyperbaric oxygen therapy
Sampanthavivat 2012 reported on possible adverse events.
Excluded studies
We excluded 20 studies: 14 because they were not a RCT, quasi‐RCT, or cluster‐RCT (Chungpaibulpatana 2008; Jepson 2011; Lerman 2008; Li 2012; Pan 2009; Rossignol 2006; Rossignol 2007a; Rossignol 2009c; Rossignol 2012; Wan 2013; Wu 2009; Yildiz 2008; Yu 2010; Yu 2010a); two because they did not include a control group (Bent 2012; Ou 2005); two because they used an inappropriate comparator (Ou 2010; VanEstenberg 2006); and two because the intervention was not hyperbaric oxygen therapy (24% oxygen, not 100% oxygen) (Granpeesheh 2010; Rossignol 2009). See the Characteristics of excluded studies tables for more information.
Risk of bias in included studies
Figure 2 provides an overview of the risk of bias in the Sampanthavivat 2012 study. See below for more detailed information.
2.
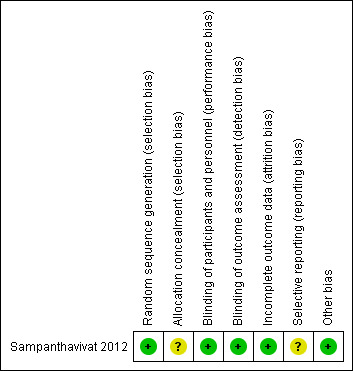
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators in Sampanthavivat 2012 randomized children by using a random number table; however, allocation concealment was unclear. We judged the sequence generation to be at low risk of bias and allocation concealment to be at unclear risk of bias in this study.
Blinding
The Sampanthavivat 2012 study used the term "double‐blind." Children and their parents, as well as all investigators and assessors involved in the study, were kept blinded to group assignments. Although the hyperbaric technician was not blinded, he or she was instructed not to discuss the intervention with anyone else. Therefore, we rated this study as having low risk of performance and detection bias.
Incomplete outcome data
Sampanthavivat 2012 reported loss of children to follow‐up. Three children withdrew: one child (gender not reported) because of parental refusal to enter the chamber because of a medical condition. This happened after consent, but before treatment allocation. After treatment allocation, one boy withdrew from the hyperbaric oxygen therapy group because of his uncooperative behavior, and another boy in the sham group dropped out following a febrile convulsion. Because of low withdrawal rates (2/60) overall, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. We judged this study to be at low risk of attrition bias.
Selective reporting
Reporting biases include outcome reporting bias, attrition bias, and duplicate (multiple) publication bias (Sterne 2011). We judged the Sampanthavivat 2012 study to have an unclear risk of reporting bias.
Outcome reporting bias
Selective reporting of certain outcomes depends on the nature and direction of results. Although the study authors reported both positive and negative results, we rated this study as having unclear risk of reporting bias. We did not find trial registry information for Sampanthavivat 2012, and no study authors replied to our email inquiry.
Attrition bias
This type of bias was low in the Sampanthavivat 2012 study because of low withdrawal rates (2/60). The proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. In addition, loss of participants was balanced between groups, and the reasons for missing data are unlikely to be related to the true outcome. One boy in the hyperbaric oxygen therapy group withdrew because of his uncooperative behavior, and another boy in the sham group dropped out following a febrile convulsion.
Duplicate (multiple) publication bias
We did not find duplicate publications of the one included study.
Other potential sources of bias
No other sources of bias appear to exist in the Sampanthavivat 2012 study, and hence, we judged the trial to be at low risk of other bias.
Effects of interventions
See: Table 1
For the current version of this review, the only included trial consisted of 60 children with autism who were treated for 20 sessions. As the review includes only one trial, we were unable to conduct a meta‐analysis for our two primary outcomes (social interaction and communication and behavioral problems) and three secondary outcomes (communication and linguistic abilities, cognitive function, and safety of hyperbaric oxygen therapy). We did not assess heterogeneity or reporting bias and we did not perform subgroup analyses or sensitivity analyses.
Below, we provide a narrative description of the results of the included study.
Primary outcomes
Social interaction and communication
Investigators in Sampanthavivat 2012 assessed social interaction by using the Autism Treatment Evaluation Checklist (ATEC). They reported no significant difference between intervention (hyperbaric oxygen therapy) and control groups (sham treatment, room air (21% oxygen under 1.15 ATA)) for parental score (mean difference (MD) 1.21, 95% confidence interval (CI) ‐2.21 to 4.63, n = 60; low‐quality evidence) or clinician score (MD 1.55, 95% CI ‐1.35 to 4.45, n = 60; low quality evidence). See Analysis 1.1.
1.1. Analysis.
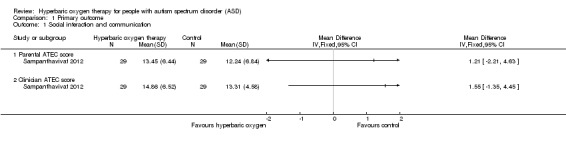
Comparison 1 Primary outcome, Outcome 1 Social interaction and communication.
Behavioral problems
Sampanthavivat 2012 assessed behavioral problems by using the ATEC. Results showed no significant differences between intervention (hyperbaric oxygen therapy) and control groups (sham treatment, room air (21% oxygen) under 1.15 ATA) for parental score (MD ‐0.24, 95% CI ‐4.80 to 4.32, n = 60; low‐quality evidence) or clinician score (MD ‐1.28, 95% CI ‐4.47 to 1.91, n = 60; low‐quality evidence). See Analysis 1.2.
1.2. Analysis.
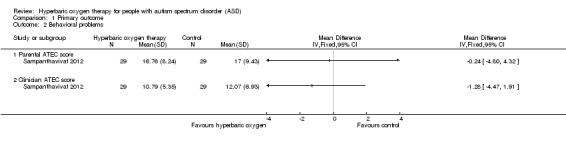
Comparison 1 Primary outcome, Outcome 2 Behavioral problems.
Secondary outcomes
Communication and linguistic abilities
Sampanthavivat 2012 assessed communication and linguistic abilities by using the ATEC and found no significant differences between intervention (hyperbaric oxygen therapy) and control groups (sham treatment, room air (21% oxygen) under 1.15 ATA) in parental score (MD 1.21, 95% CI ‐2.12 to 4.54, n = 60) or clinician score (MD ‐0.27, 95% CI ‐3.93 to 3.39, n = 60). See Analysis 2.1.
2.1. Analysis.
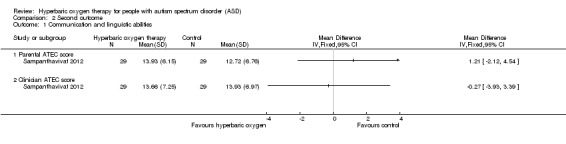
Comparison 2 Second outcome, Outcome 1 Communication and linguistic abilities.
Cognitive function
Sampanthavivat 2012 assessed cognitive function by using the ATEC and reported no significant differences between intervention (hyperbaric oxygen therapy) and control groups (sham treatment, room air (21% oxygen) under 1.15 ATA) in parental score (MD 0.93, 95% CI ‐2.71 to 4.57, n = 60) or clinician score (MD ‐0.38, 95% CI ‐3.06 to 2.30, n = 60). See Analysis 2.2.
2.2. Analysis.
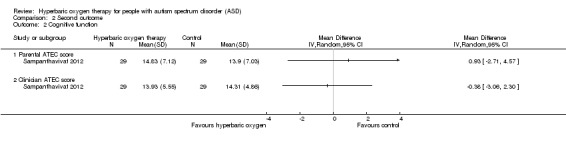
Comparison 2 Second outcome, Outcome 2 Cognitive function.
Safety of hyperbaric oxygen therapy
Sampanthavivat 2012 reported possible adverse events including minor‐grade ear barotrauma events. Investigators noted significant differences between intervention (hyperbaric oxygen therapy) and control groups (sham treatment, room air (21% oxygen) under 1.15 ATA) in total number of side effect events (Peto odds ratio (OR) 3.87, 95% CI 1.53 to 9.82, n = 60; risk ratio (RR) 5, 95% CI 1.46 to 17.18; risk difference (RD) 0.02, 95% CI 0.01 to 0.03) and in the number of children who had side effects (Peto OR 4.40, 95% CI 1.33 to 14.48; n = 60; RR 3.67, 95% CI 1.14 to 11.79; RD 0.28, 95% CI 0.07 to 0.48). The proportion of children with adverse events was significantly greater in the hyperbaric oxygen therapy group than in the control group. See Analysis 2.3.
2.3. Analysis.
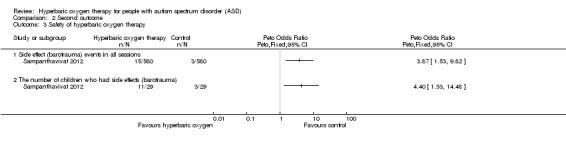
Comparison 2 Second outcome, Outcome 3 Safety of hyperbaric oxygen therapy.
Discussion
Summary of main results
To date, few rigorous studies have been conducted or reported on hyperbaric oxygen therapy for autism spectrum disorder (ASD). This review included only one trial involving 60 children (Sampanthavivat 2012) in which investigators used 100% oxygen at l.5 atmospheres absolute (ATA). Treatment consisted of 20 sessions of one hour duration. The included study reported data on two primary outcomes (social interaction and communication and behavioral problems) and three secondary outcomes (communication and linguistic abilities, cognitive function, and safety of hyperbaric oxygen therapy). We found no evidence of a treatment effect on the two primary outcomes or on two secondary outcomes (communication and linguistic abilities, cognitive function). Notably, the proportion of children with adverse events was significantly greater in the hyperbaric oxygen therapy group than in the control group.
Overall completeness and applicability of evidence
One trial consisting of 60 children is not sufficient to provide robust evidence for hyperbaric oxygen therapy in ASD. All of the included participants were children (participants' ages ranged from three to nine years), and they were predominantly male. Thus, these findings are applicable only to a subgroup of children with ASD and are not applicable to adults.
The study was performed before 2013 and used Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) constructs for diagnosis (APA 2000). Tools used in the study included the Autism Treatment Evaluation Checklist (ATEC) (Rimland 1999) and the Clinical Global Impression (CGI) scale (Leckman 1989). Although assessment tools did not change during the study period, interpretation of these results should consider recent papers on the use of the same diagnostic tools in evaluating the impact of Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria for diagnosis of the 'new' ASD (APA 2013). We were unable to conduct a meta‐analysis for our two primary outcomes (social interaction and communication and behavioral problems) and our three secondary outcomes (communication and linguistic abilities, cognitive function, and safety of hyperbaric oxygen therapy) because only one study was included. We could draw no robust conclusions.
Upon considering all available evidence, we found no evidence for the benefits of hyperbaric oxygen treatment for children with ASD. However, we did identify possible short‐term adverse events (minor‐grade ear barotrauma events) due to hyperbaric oxygen therapy. Thus, future clinical studies of hyperbaric oxygen treatment for children with ASD may not be appropriate, particularly given the absence of a persuasive theory of change from experimental and clinical studies, the unknown long‐term safety of the treatment, and the financial and opportunity costs of not participating in other proven therapies.
Quality of the evidence
Risk of bias was generally low or unclear in the included trial. Selection bias may exist in this study because details on allocation concealment were lacking. Attrition bias was low in this study because of low withdrawal rates (2/60), and the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. We considered performance and detection biases to be low because the study achieved and reported blinding of participants and outcome assessors to group assignments.
Using the GRADE approach, we rated the quality of this body of evidence as low (see Table 1) because of the small study sample size (only 60 children with ASD) and wide confidence intervals (CIs) for the estimates of effects.
Potential biases in the review process
Although we attempted to minimize publication bias by using a comprehensive search strategy and by searching numerous sources, we may have failed to identify potentially relevant trials or experiments. For example, although we applied no language limits in our searches, the search for this review was based on English and Chinese. Thus, we may have missed some trials in other non‐English databases. It is unclear how this may have affected our conclusions.
We planned to carry out a meta‐analysis and additional subgroup analyses to identify program components associated with more effective outcomes and factors that modified intervention effectiveness; however, we included only one study. Similarly, we identified too few studies to conduct sensitivity analyses to examine the impact of study design or quality.
Agreements and disagreements with other studies or reviews
A systematic review evaluating the effectiveness of hyperbaric oxygen therapy for ASD was published in 2012 (Ghanizadeh 2012). This review included two randomized controlled trials (RCTs) and other types of nonrandomized trials such as case series, case reports, pilot studies, and retrospective studies; did not perform data synthesis; and found that although some studies suggested that hyperbaric oxygen is effective for autism, these promising effects were not replicated by other studies. In addition, serious adverse effects were not reported in many of the studies, including two RCTs, three case series, one retrospective study, one open‐label pilot trial, and one case study report. The results of our review, consisting of only one small RCT of 60 children, do not support the recommendations made by Ghanizadeh 2012. Our review suggests that no persuasive evidence supports the effectiveness of hyperbaric oxygen for ASD. Furthermore, the proportion of children with adverse events was significantly higher in the hyperbaric oxygen therapy group than in the control group. The long‐term safety of hyperbaric oxygen therapy remains unknown.
Authors' conclusions
Implications for practice.
Evidence is insufficient to show that hyperbaric oxygen is an effective treatment for ASD. Adverse effects (minor‐grade ear barotrauma events) can occur.
Implications for research.
Given the absence of evidence on the effectiveness of hyperbaric oxygen therapy, and its limited biological plausibility, researchers must carefully consider the need for future RCTs. Researchers should first weigh the financial and opportunity costs (e.g. not participating in other proven therapies) of hyperbaric oxygen treatment before beginning any new RCTs. Given that the follow‐up period of the trial included in this review was short, long‐term effects and side effects of hyperbaric oxygen for ASD may not have been observed. Basic research results and clinical evidence for hyperbaric oxygen therapy are contradictory and weak. Thus, the focus of research should shift toward other, more important studies (such as behavioral and developmental interventions and pharmacotherapy) on ASD.
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2016 | Amended | Added second affiliations for all authors |
Acknowledgements
We thank the staff at the Cochrane Developmental, Psychosocial and Learning Problems Group for assistance in preparation of the review: Professor Geraldine Macdonald, Dr Joanne Wilson, Margaret Anderson, and Gemma O'Loughlin.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
CENTRAL 2015, Issue 11. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records]. CENTRAL 2014, Issue 10. Searched 25 November 2014, limited to publication years 2013‐2014 [1 record]. CENTRAL 2013, Issue 10. Searched 20 November 2013 [6 records].
#1 [mh ^"child development disorders, pervasive"] #2 [mh "Developmental Disabilities"] #3 pervasive development* disorder* #4 (pervasive near/3 child*) #5 (PDD or PDDs or PDD‐NOS or ASD or ASDs) #6 autis* #7 asperger* #8 kanner* #9 childhood next schizophrenia #10 {or #1‐#9} #11 [mh "Hyperbaric Oxygenation"] #12 (Hyperbaric near/3 oxygen*) #13 oxygen next therap* #14 (Hyperbaric near/3 therap*) #15 HBO #16 HBOP #17 {or #11‐#16} #18 #10 and #17
Ovid MEDLINE
Ovid MEDLINE 1946 to November week 3 2015. Searched 14 December 2015, limited to ed=20141101‐20151117 [2 records]. Ovid MEDLINE 1946 to November week 2 2014. Searched 25 November 2014, limited to ed=20131101 to 20141125 [4 records]. Ovid MEDLINE 1946 to November week 1 2013. Searched 19 November 2013 [44 records].
1 exp child development disorders, pervasive/ 2 Developmental Disabilities/ 3 pervasive development$ disorder$.tw. 4 (pervasive adj3 child$).tw. 5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 6 autis$.tw. 7 asperger$.tw. 8 kanner$.tw. 9 childhood schizophrenia.tw. 10 or/1‐9 11 Hyperbaric Oxygenation/ 12 (Hyperbaric adj3 oxygen$).tw. 13 oxygen therap$.tw. 14 (Hyperbaric adj3 therap$).tw. 15 HBO.tw. 16 HBOP.tw. 17 or/11‐16 18 10 and 17
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 10 December 2015. Searched 14 December 2015 [5 records]. Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 24 November 2014. Searched 25 November 2014 [2 records]. Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 18 November 2013. Searched 19 November 2013 [2 records].
1 pervasive development$ disorder$.tw. 2 (pervasive adj3 child$).tw. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 4 autis$.tw. 5 asperger$.tw. 6 kanner$.tw. 7 childhood schizophrenia.tw. 8 or/1‐7 9 (Hyperbaric adj3 oxygen$).tw. 10 oxygen therap$.tw. 11 (Hyperbaric adj3 therap$).tw. 12 HBO.tw. 13 HBOP.tw. 14 or/9‐13 15 8 and 14
Embase (Ovid)
Embase 1980 to 2015 Week 50. Searched 14 December 2015, limited to ed=201447‐201550 [12 records]. Embase 1980 to 2014 Week 47. Searched 25 November 2014, limited to ed=201346 to 201447 [10 records]. Embase 1980 to 2013 Week 46. Searched 19 November 2013 [82 records].
1 exp autism/ 2 developmental disorder/ 3 autis*.tw. 4 pervasive development$ disorder$.tw. 5 (pervasive adj3 child$).tw. 6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 7 childhood schizophrenia.tw. 8 asperger$.tw. 9 kanner$.tw. 10 or/1‐9 11 hyperbaric oxygen/ 12 (Hyperbaric adj3 oxygen$).tw. 13 oxygen therap$.tw. 14 (Hyperbaric adj3 therap$).tw. 15 HBO.tw. 16 HBOP.tw. 17 or/11‐16 18 10 and 17 19 limit 18 to em=201346‐201447 20 from 19 keep 1‐10
PsycINFO (Ovid)
PsycINFO 1667 to December Week 2 2015. Searched 14 December 2015, limited to up=20141101‐20151207 [1 record]. PsycINFO 1967 to November Week 3 2014. Searched 25 November 2014, limited to up=20131118 to 20141125 [0 records]. PsycINFO 1967 to November Week 2 2013. Searched 19 November 2013 [18 records].
1 exp pervasive developmental disorders/ 2 developmental disabilities/ 3 pervasive development$ disorder$.tw. 4 (pervasive adj3 child$).tw. 5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 6 autis$.tw. 7 asperger$.tw. 8 kanner$.tw. 9 childhood schizophrenia.tw. 10 or/1‐9 11 oxygenation/ 12 (Hyperbaric adj3 oxygen$).tw. 13 oxygen therap$.tw. 14 (Hyperbaric adj3 therap$).tw. 15 HBO.tw. 16 HBOP.tw. 17 or/11‐16 18 10 and 17 19 limit 18 to up=20131118‐20141125
Web of Science databases
Science Citation Index (SCI) 1970 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [1 record]. SCI 1970 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013‐2014 [3 records]. SCI 1970 to 15 November 2013. Searched 20 November 2013 [21 records].
Social Sciences Citation Index (SSCI) 1970 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records]. SSCI 1970 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013 to 2014 [0 records]. SSCI 1970 to 15 November 2013. Searched 20 November 2013 [13 records].
Conference Proceedings Citation Index ‐ Science (CPCI‐S) and Conference Proceedings Citation Index ‐ Social Sciences & Humanities 1990 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records]. CPCI‐S & CPCI‐SS&H 1990 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013 to 2014 [0 records]. CPCI‐S & CPCI‐SS&H 1990 to 15 November 2013. Searched 20 November 2013 [2 records].
#12 #11 AND #6 DocType=All document types; Language=All languages; #11 #10 OR #9 OR #8 OR #7 DocType=All document types; Language=All languages; #10 TS= (HBO OR HBOP) DocType=All document types; Language=All languages; #9 TS=(Hyperbaric near/3 therap*) DocType=All document types; Language=All languages; #8 TS=("oxygen therap*") DocType=All document types; Language=All languages; #7 TS=(Hyperbaric near/3 oxygen*) DocType=All document types; Language=All languages; #6 #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; #5 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs) DocType=All document types; Language=All languages; #4 TS=("childhood schizophrenia") DocType=All document types; Language=All languages; #3 TS= (pervasive near/3 child*) DocType=All document types; Language=All languages; #2 TS= ("pervasive development* disorder*") DocType=All document types; Language=All languages; #1 TS=(autis* or asperger* or kanner*) DocType=All document types; Language=All languages;
WorldCat
(worldcat.org)
14 December 2015 [0 records]. 25 November 2014 [0 records]. 21 November 2013 [1 record].
Advanced Search; kw:autis* AND hyperbaric then limited to Theses
Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE), in the Cochrane Library
CDSR 2015, Issue 12. Searched 14 December 2015 [0 records]. CDSR 2014, Issue 11. Searched 25 November 2014 [0 records]. CDSR 2013, Issue 11. Searched 20 November 2013 [1 record].
DARE 2015, Issue 2. Searched 14 December 2015 [0 records]. DARE 2014, Issue 4. Searched 25 November 2014 [0 records]. DARE 2013, Issue 4. Searched 20 November 2013 [0 records].
#1[mh ^"child development disorders, pervasive"] #2[mh "Developmental Disabilities"] #3(pervasive development* disorder*):ti,ab #4(pervasive near/3 child*):ti,ab #5(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab #6(autis*):ti,ab #7(asperger*):ti,ab #8(kanner*):ti,ab #9(childhood next schizophrenia):ti,ab #10{or #1‐#9} #11[mh "Hyperbaric Oxygenation"] #12(Hyperbaric near/3 oxygen*):ti,ab #13(oxygen next therap*):ti,ab #14(Hyperbaric near/3 therap*):ti,ab #15"HBO":ti,ab #16HBOP:ti,ab #17{or #11‐#16} #18#10 and #17 in Cochrane Reviews (Reviews only) and Other Reviews
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature)
CINAHL 1937 to current. Searched 14 December 2015, limited to EM 20141101‐current [4 records]. CINAHL 1937 to current. Searched 26 November 2014, limited to EM=20131101‐current [1 record]. CINAHL 1937 to current. Searched 20 November 2013 [31 records].
S17 S10 AND S16 S16 S11 OR S12 OR S13 OR S14 OR S15 S15 HBO or HBOP S14 (Hyperbaric N3 therap*) S13 oxygen therap* S12 (Hyperbaric N3 oxygen*) S11 (MH "Hyperbaric Oxygenation") S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S8 childhood schizophren* S7 kanner* S6 asperger* S5 autis* S4 (pervasive N3 child*) S3 pervasive development* disorder* S2 (MH "Developmental Disabilities") S1 (MH "Child Development Disorders, Pervasive+")
ERIC EBSCOhost (Education Resources Information Center)
ERIC 1966 to current. Searched 14 December 2015 [0 records]. ERIC 1966 to current. Searched 25 November 2014, limited by publication year 2013 to current [0 records].
S17 S10 AND S16 S16 S11 OR S12 OR S13 OR S14 OR S15 S15 HBO or HBOP S14 (Hyperbaric N3 therap*) S13 oxygen therap* S12 (Hyperbaric N3 oxygen*) S11 (MH "Hyperbaric Oxygenation") S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 pervasive development* disorder* S8 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S7 childhood schizophren* S6 kanner* S5 asperger* S4 autis* S3 (pervasive N3 child*) S2 (MH "Developmental Disabilities") S1 (MH "Child Development Disorders, Pervasive+")
ERIC (ProQuest)
ERIC 1966 to current. Searched 21 November 2013 [15 records].
Set#: S1 Searched for: SU.EXACT.EXPLODE("Pervasive Developmental Disorders") or autis* or Asperger* or kanner* or "pervasive development* disorder*" or "childhood schizophrenia" or pervasive near/3 child* or pdd or pdds or asd or asds or pdd‐nos Databases: ERICSet#: S5 Searched for: Hyperbaric or oxygen* or HBO OR HBOP Databases: ERIC Set#: S6 Searched for: (SU.EXACT.EXPLODE("Pervasive Developmental Disorders") OR autis* OR Asperger* OR kanner* OR "pervasive development* disorder*" OR "childhood schizophrenia" OR pervasive NEAR/3 child* OR pdd OR pdds OR asd OR asds OR pdd‐nos) AND (Hyperbaric OR oxygen* OR HBO OR HBOP) Databases: ERIC
HBO Evidence. Database of Randomised Controlled Trials in Diving and Hyperbaric Medicine
(hboevidence.unsw.wikispaces.net)
HBO accessed 14 December 2015 [0 records]. HBO accessed 26 November 2014 [0 records]. HBO accessed 21 November 2013 [2 records].
Search term: autism
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
(apps.who.int/trialsearch)
15 December 2015 [10 records]. 25 November 2014 [0 records]. 21 November 2013 [10 records].
Basic search : autism AND oxygen OR autism AND hyperbaric
ClinicalTrials.gov
(clinicaltrials.gov)
15 December 2015 [8 records]. 25 November 2014 [0 records]. 21 November 2013 [8 records].
Basic search: hyperbaric AND autism
metaRegister of Controlled Trials (mRCT)
(isrctn.com/mrct)
14 December 2015. Not searched. Website message " service under review". 26 November 2014. Not searched. Website message "service under review". 21 November 2013 [4 records].
Selected: All registers Search terms: hyperbaric AND autism
Research Autism
(researchautism.net)
14 December 2015 [3 records]. 26 November 2014 [0 records). 21 November 2013 [9 records].
Browsed the alpabetical list from the Interventions tab for "hyperbaric" and downoaded the webpage.
Autism Data
(autism.org.uk)
14 December 2015 [0 records]. 25 November 2014 [1 record]. 21 November 2013 [15 records].
Australian New Zealand Clinical Trials Registry (ANZCTR)
(anzctr.org.au)
Searched 26 November 2014 [0 records]. This registry was not searched separately after this date as the content feeds into WHO ICTRP.
Chinese databases
China National Knowledge Infrastructure (CNKI) (cnki.net). Searched 18 December 2013, 5 December 2014 and 10 December 2015. WEIPU periodical database (cqvip.com). Searched 18 December 2013, 5 December 2014 and 10 December 2015. Wan Fang Data (wanfangdata.com.cn). Searched 18 December 2013, 5 December 2014 and 10 December 2015. Chinese Biologic Medical Database (CBM) (sinomed.imicams.ac.cn). Searched 18 December 2013, 5 December 2014 and 10 December 2015.
1 高压氧 2 孤独症 或 自闭症 3 随机 4 1 and 2 and 3
Appendix 2. Additional methods archived for future updates of this review
| Analysis | Methods |
| Measures of treatment effect |
Continuous data For continuous data, we will calculate the mean difference (MD) and 95% confidence intervals (CIs). Because different scales may be used to measure the same outcomes in trials on autism spectrum disorder (ASD), standardized mean differences (SMDs) may be used widely in our review. Final values and changes from baseline data should not be combined together as SMDs. When final values and changes from baseline data are available in included trials, we shall analyze them separately. Apart from analyzing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. Dichotomous data For dichotomous data, we will calculate the risk ratio (RR), odds ratio (OR), and risk difference (RD) with 95% CIs. |
| Unit of analysis issues | For most outcomes, the unit of analysis will be the individual participant. Cluster‐randomized trials We will include cluster‐randomized trials along with individually randomized trials in the analysis. We will analyze them, as detailed in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from another source. If we use ICCs from other sources, we will report this fact and we will conduct sensitivity analyses to investigate effects of variation in the ICC. If we identify both cluster‐randomized trials and individually randomized trials, we will synthesize relevant information. We will consider it reasonable to combine the results derived from both if little heterogeneity between the study designs is noted, and if interaction between the effect of the intervention and the choice of randomization unit is considered unlikely. We will also acknowledge heterogeneity in the randomization unit, and we will perform a separate meta‐analysis. Multiple intervention groups We were not faced with multiple intervention studies until now. In the future, for a study with multiple intervention groups, if appropriate, we will combine groups to create a single pairwise comparison. The recommended method in most situations is to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group (Higgins 2011a). Indirect comparisons are not randomized comparisons. They are observational findings across trials and may suffer the biases of observational studies (Higgins 2011a). Thus, we will exclude indirect comparisons. |
| Dealing with missing data | When published data are incomplete, we will try to obtain the missing data from the primary investigator, if possible. If this approach is unsuccessful, we will restrict the analyses to available data. We will use sensitivity analyses to examine whether overall findings are robust to the potential influence of missing data. We will assess how sensitive results are to reasonable changes in the assumptions made. We will critically appraise issues of the intention‐to‐treat (ITT) analysis and will compare them with specifications of primary outcome parameters and power calculations. |
| Assessment of heterogeneity | We will consider 3 types of heterogeneity: clinical, methodological, and statistical. We will assess clinical heterogeneity by comparing the distribution of important participant factors between trials such as age, gender, specific diagnoses or diagnostic subtypes (or both), duration of the disorder, and associated neuropsychiatric diseases. We will assess methodological heterogeneity by comparing trial characteristics such as randomization concealment, blinding, and losses to follow‐up (see Quality of the evidence). We will assess statistical heterogeneity by examining Chi² and I². We will use the Chi² test (P ≤ 0.10 shows substantial or considerable heterogeneity) to determine whether statistically significant heterogeneity is present. The Chi² test is not very reliable when a few studies or small sample sizes form the dataset. This means that a nonsignificant result cannot be taken as evidence of no heterogeneity. We will also assess the degree of statistical heterogeneity by examining I². We will grade the degree of heterogeneity as follows (Deeks 2011):
We will examine the trials to investigate possible explanations for heterogeneity. If heterogeneity is identified among a group of studies, we will check the data and establish potential reasons for the observed heterogeneity. For heterogeneity that cannot be readily explained, we intend to divide the data into subgroups if an appropriate basis is identified. Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has long been challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014), or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Assessment of reporting biases | We will try to obtain the study protocols of all included studies so that we can compare outcomes reported in the protocol versus those reported in the findings. When we suspect reporting bias, we will attempt to contact study authors to ask them to provide missing outcome data. When this is not possible, and the missing data are thought to introduce serious bias, we will conduct a sensitivity analysis to evaluate the impact of including such studies in the overall assessment of results. We will assess publication bias by using funnel plots or the Egger test (Egger 1997), depending on the number of clinical trials included in the systematic review. The funnel plot should be seen as a generic means of displaying small‐study effects. Asymmetry may arise as a result of publication bias or a relationship between trial size and effect size. True heterogeneity in intervention effects is only one cause of funnel plot asymmetry (Egger 1997; Sterne 2011). |
| Data synthesis | If more than one eligible trial is identified and sufficient homogeneity is observed among studies with respect to participants and reported outcomes, we will perform meta‐analyses using Review Manager 5 (RevMan 5) (RevMan 5 2014). We will use both the fixed‐effect model and random‐effects model in the meta‐analysis. Both models will yield similar results if no significant heterogeneity and no publication bias are noted among the trials. If no significant heterogeneity is present, we will report results of the fixed‐effect model only. However, the asymmetry of the funnel plot may be due to true heterogeneity. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effect model. For continuous data, we will use the inverse variance method, which is available in RevMan 5 2014. When data are sparse, in terms of low event rates or small study size, the Mantel‐Haenszel methods have better statistical properties than the inverse variance method for dichotomous data (Deeks 2011). In such cases, we will choose the Mantel‐Haenszel method for calculating the RR and RD for dichotomous data. Both Mantel‐Haenszel and inverse variance methods are poor when event rates are very low. In such cases, Peto's method works well; however, Peto's method can be used only to pool odds ratios (Deeks 2011). |
| Subgroup analysis and investigation of heterogeneity | We will perform subgroup analysis based on the factors below.
|
| Sensitivity analysis | We will perform sensitivity analyses for missing data and for study risk of bias. We will employ sensitivity analysis using different approaches to impute missing data. We will critically appraise last observation carried forward (LOCF), ITT, and per‐protocol (PP) analysis and will compare them with primary outcome parameters and power calculations. If appropriate, we will conduct sensitivity analyses by study risk of bias based on the presence or absence of a reliable random allocation method, concealment of allocation, and blinding of participants or outcome assessors. We will test robustness of the results by including or excluding studies of poor quality. |
Data and analyses
Comparison 1. Primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Social interaction and communication | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Behavioral problems | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Second outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Communication and linguistic abilities | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Cognitive function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Safety of hyperbaric oxygen therapy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.1 Side effect (barotrauma) events in all sessions | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 The number of children who had side effects (barotrauma) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sampanthavivat 2012.
| Methods |
Date: published 3 September 2012 Design: randomized controlled trial, parallel groups (1 hyperbaric oxygen therapy group, 1 control group) Stratification factors: no |
|
| Participants |
Inclusion/exclusion criteria:
Number (total/per group): N = 60; hyperbaric oxygen therapy 29; control 29 Age: 3 to 9 years Sex distribution: total 56 boys and 4 girls. Hyperbaric oxygen therapy 29 boys and 1 girl. Sham air 27 boys and 3 girls Specific diagnoses/diagnostic subtypes: no Comorbidities: unclear Duration of disorder: unclear Previous treatments: unclear |
|
| Interventions |
Type of hyperbaric oxygen therapy: multiplace hyperbaric chamber Details of treatment: at 153 kPa (1.5 ATA) with 100% oxygen for a total of 20 sessions, each lasting 1 hour on weekdays Types of controls: multiplace hyperbaric chamber Details of control treatment, including drug dosage: pressurized room air at 116 kPa (1.15 ATA) for a total of 20 sessions, each lasting 1 hour, on weekdays Details of cointerventions: risperidone, nutrition supplements, and other medications |
|
| Outcomes |
Primary outcome measures: parental and clinician total/subscale ATEC scores Secondary outcome measures: parental and clinician CGIS scores, adverse events, and tolerance |
|
| Notes |
Follow‐up data Duration of follow‐up: 20 sessions of interventions Dates of treatment withdrawal: 1 child was excluded after consent, before treatment allocation. 1 boy in the hyperbaric oxygen therapy group dropped out during the intervention procedure, and another boy in the sham group dropped out following a febrile convulsion. Reasons for treatment withdrawal: 1 child (gender not specified) withdrew owing to parental refusal to enter the chamber because of a medical condition; 1 boy in the hyperbaric oxygen therapy group withdrew because of his uncooperative behavior; and another boy in the sham group dropped out following a febrile convulsion. Withdrawal rates: 3/60 overall |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Investigators, parents, participants, nursing staff, and all other clinical staff were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, parents, nursing staff, and all other clinical staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low withdrawal rates: 2/60. One child withdrew because of parental refusal to enter the chamber as the result of a medical condition. This happened after consent, but before treatment allocation. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was unavailable. Unclear whether outcomes were reported as per trial registration |
| Other bias | Low risk | Appears to be free from other sources of bias |
ATA: atmosphere absolute. ATEC: Autism Treatment Evaluation Checklist. CGIS: Clinical Global Impression ‐ Severity scale. DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth Edition ‐ Text Revision.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bent 2012 | No control group |
| Chungpaibulpatana 2008 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Granpeesheh 2010 | Intervention was provided at 1.3 ATA and 24% oxygen, not 100% oxygen. It is not HBO. |
| Jepson 2011 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Lerman 2008 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Li 2012 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Ou 2005 | No control group |
| Ou 2010 | Inappropriate comparator. Study compared hyperbaric oxygen plus psychological nursing and conventional comprehensive therapy with conventional therapy. |
| Pan 2009 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Rossignol 2006 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Rossignol 2007a | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Rossignol 2009 | Intervention was given at 1.3 ATA and 24% oxygen, not 100% oxygen. It is not HBO. |
| Rossignol 2009c | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Rossignol 2012 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| VanEstenberg 2006 | Inappropriate comparator. Study compared hyperbaric oxygen at 1.75 ATA with 1.3 ATA. |
| Wan 2013 | Not an RCT, quasi‐RCT, or cluster‐RCT. This was a case‐controlled study. |
| Wu 2009 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Yildiz 2008 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Yu 2010 | Not an RCT, quasi‐RCT, or cluster‐RCT |
| Yu 2010a | Not an RCT, quasi‐RCT, or cluster‐RCT |
ATA: atmosphere absolute. HBO: hyperbaric oxygen. RCT: randomized controlled trial.
Differences between protocol and review
We created a table to detail the differences between the protocol and the review (see Table 4).
1. Differences between protocol and review.
| Review section | Change |
| Description of the intervention | We added the paragraph below on 'nonclassical' hyperbaric oxygen therapy: In addition to the 'classical' hyperbaric oxygen therapy defined by the Undersea and Hyperbaric Medical Society (UHMS 2016), some ASD trials have used nonclassical hyperbaric oxygen therapy (Granpeesheh 2010; Rossignol 2009). In these trials, nonclassical hyperbaric oxygen therapy consisted of a chamber pressurized to greater than one ATA with less than 100% oxygen concentration. In one study, both classical and nonclassical hyperbaric oxygen therapies produced significant improvement in children with autism, as evidenced by normal levels of oxidative stress and inflammation markers (Rossignol 2007a). As outlined in our protocol (Xiong 2014), we assessed only the use of classical hyperbaric oxygen therapy in this review. |
| Types of participants | We revised this section as follows: Participants of any age with a diagnosis of autism spectrum disorder (ASD) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013); or individuals with a diagnosis of one of the four pervasive developmental disorders from fourth edition text revision version of the DSM (DSM‐IV‐TR) (APA 2000), including autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD‐NOS); or from theInternational Classification of Mental and Behavioural Disorders, 10th Edition (ICD‐10; WHO 1993). We accepted diagnoses that were derived following use of assessment tools, such as the Autism Diagnostic Observation Scale (ADOS) (Lord 1997) and the Autism Diagnostic Interview ‐ Revised (ADI‐R) (Lord 1994). |
| Electronic searches | We have specified that, when searching online clinical trial registries such as ClinicalTrials.gov and WHO ICTRP, we checked the publication status of each trial identified and contacted study authors for results of finished unpublished trials. |
| Searching other resources | We have clarified that we handsearched reference lists of relevant studies. We also searched the gray literature from the Internet using the academic search engine "Baidu Scholar." |
| Measures of treatment effect | We have clarified how we will handle final values and changes from baseline data, as follows: When final values and changes from baseline data are available in included trials, we shall analyse them separately. Apart from analysing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. |
| Unit of analysis issues | We explained how we would handle multiple intervention groups. See Appendix 2. |
| Assessment of heterogeneity | We regraded the degree of heterogeneity, as follows (Deeks 2011).
We added the following information: Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has been long challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014) or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We also specified that: We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Data synthesis | We have clarified when we will report results of the fixed‐effect model and when we will report results of the random‐effects model, as follows: If no significant heterogeneity is present, we will report the results of the fixed‐effect model only. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effects model. |
ASD: autism spectrum disorder. ATA: atmosphere absolute. DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. MD: mean difference. WHO ICTRP: World Health Organisation International Clinical Trials Registry Platform.
Contributions of authors
Conceiving the review: Tao Xiong. Designing the review: Tao Xiong. Coordinating the review: Tao Xiong and Dezhi Mu. Designing search strategies: Tao Xiong, Hongju Chen, and Rong Luo. Extracting data: Tao Xiong, Hongju Chen, and Rong Luo. Writing the review: Tao Xiong and Hongju Chen. Providing general advice on the review: Dezhi Mu. Securing funding for the review: Tao Xiong and Dezhi Mu.
Sources of support
Internal sources
-
Department of Pediatrics, West China, Second University Hospital, Sichuan University, Chengdu, China.
Salary support for Tao Xiong, Hongju Chen, and Dezhi Mu
External sources
-
Chinese Cochrane Center, West China, Second University Hospital, Sichuan University, Chengdu, China.
Academic support to the team
-
The National Natural Science Foundation of China (No.81330016, 81630038 to Dezhi Mu; No. 81300525 to Tao Xiong); the Foundation of Health and Family Planning Commission of Sichuan Province (No.140044 to Tao Xiong), China.
Financial support to Dezhi Mu and Tao Xiong
-
Cochrane Developmental, Psychosocial and Learning Problems Group, Other.
Academic support to the team
Declarations of interest
Tao Xiong ‐ none known. Hongju Chen ‐ none known. Rong Luo ‐ none known. Dezhi Mu ‐ none known.
Edited (no change to conclusions)
References
References to studies included in this review
Sampanthavivat 2012 {published data only}
- Sampanthavivat M, Singkhwa W, Chaiyakul T, Karoonyawanich S, Ajpru H. Hyperbaric oxygen in the treatment of childhood autism: a randomised controlled trial. Diving and Hyperbaric Medicine 2012;42(3):128‐33. [PUBMED: 22987458 ] [PubMed] [Google Scholar]
References to studies excluded from this review
Bent 2012 {published data only}
- Bent S, Bertoglio K, Ashwood P, Nemeth E, Hendren RL. Brief report: hyperbaric oxygen therapy (HBOT) in children with autism spectrum disorder: a clinical trial. Journal of Autism and Developmental Disorders 2012;42(6):1127‐32. [PUBMED: 21818676] [DOI] [PubMed] [Google Scholar]
Chungpaibulpatana 2008 {published data only}
- Chungpaibulpatana J, Sumpatanarax T, Thadakul N, Chantharatreerat C, Konkaew M, Aroonlimsawas M. Hyperbaric oxygen therapy in Thai autistic children. Journal of the Medical Association of Thailand 2008;91(8):1232‐8. [PUBMED: 18788696] [PubMed] [Google Scholar]
Granpeesheh 2010 {published data only}
- Granpeesheh D, Tarbox J, Dixon DR, Wilke AE, Allen MS, Bradstreet JJ. Randomized trial of hyperbaric oxygen therapy for children with autism. Research in Autism Spectrum Disorders 2010;4(2):268‐75. [DOI: 10.1016/j.rasd.2009.09.014] [DOI] [Google Scholar]
Jepson 2011 {published data only}
- Jepson B, Granpeesheh D, Tarbox J, Olive M, Stott C, Braud S, et al. Controlled evaluation of the effects of hyperbaric oxygen therapy on the behavior of 16 children with autism spectrum disorders. Journal of Autism and Developmental Disorders 2011;41(5):575‐88. [PUBMED: 20680427] [DOI] [PubMed] [Google Scholar]
Lerman 2008 {published data only}
- Lerman DC, Sansbury T, Hovanetz A, Wolever E, Garcia A, O'Brien E, et al. Using behavior analysis to examine the outcomes of unproven therapies: an evaluation of hyperbaric oxygen therapy for children with autism. Behavior Analysis in Practice 2008;1(2):50‐8. [PUBMED: 22477688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li Y. The Effect and Mechanism of Hyperbaric Oxygen on Autism [dissertation]. Wangfang Data: QingDao, 2012. [Google Scholar]
Ou 2005 {published data only}
- Ou HJ, Ji MY, Su XL, Feng XM. Effect of hyperbaric oxygen therapy as an assistant to treat children with autism. Chinese Nursing Research 2005;19(2):229‐30. [http://bit.ly/23aY73h] [Google Scholar]
Ou 2010 {published data only}
- Ou HJ, Ma LL, Wu R, Su XL, Lin H. Evaluation of hyperbaric oxygen therapy combined with psychological care for children with autism. Chinese General Nursing 2010;8(7):1698‐9. [http://bit.ly/25GxMMt] [Google Scholar]
Pan 2009 {published data only}
- Pan XS, Song XL, Wu Y, Fan QP. Combined therapy of hyperbaric oxygen for language and behavior disorders in children with autism. Clinical Focus 2009;24(12):1067‐8. [http://bit.ly/1Tw6ILb] [Google Scholar]
Rossignol 2006 {published data only}
- Rossignol D, Mumper E, James J, Melnyk S, Rossignol L. Hyperbaric oxygen therapy in autistic children improves symptomology, decreases markers of inflammation, and has neutral effects on oxidative stress. Neurotoxicology 2006;27(6):1155‐6. [DOI: 10.1016/S0161-813X(06)00273-7] [DOI] [Google Scholar]
Rossignol 2007a {published data only}
- Rossignol DA, Rossignol LW, James SJ, Melnyk S, Mumper E. The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: an open‐label pilot study. BMC Pediatrics 2007;7:36. [DOI: 10.1186/1471-2431-7-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 2009 {published data only}
- Rossignol DA, Rossignol LW, Smith S, Schneider C, Logerquist S, Usman A, et al. Hyperbaric treatment for children with autism: a multicenter, randomized, double‐blind, controlled trial. BMC Pediatrics 2009;9:21. [DOI: 10.1186/1471-2431-9-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 2009c {published data only}
- Rossignol DA, Rossignol LE, Smith S. Hyperbaric treatment for autistic children. The Brown University Child & Adolescent Psychopharmacology Update 2009;11(7):5‐6. [DOI: 10.1002/cpu.20095] [DOI] [Google Scholar]
Rossignol 2012 {published data only}
- Rossignol DA, Bradstreet JJ, Dyke K, Schneider C, Freedenfeld SH, O'Hara N, et al. Hyperbaric oxygen treatment in autism spectrum disorders. Medical Gas Research 2012;2:16. [DOI: 10.1186/2045-9912-2-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
VanEstenberg 2006 {published data only}
- VanEstenberg AM. The Reduction of Autistic Symptoms Through the Utilization of Hyperbaric Oxygen Therapy [PhD thesis]. Cincinnati (Ohio): Union Institute & University, 2006. [Google Scholar]
Wan 2013 {published data only}
- Wan JE, Li Y, Ma Y, Yi MJ, Gu J, Wang SZ, et al. Therapeutic effect of hyperbaric oxygen intervention on autism in children. Chinese Journal of Nautical Medicine and Hyperbaric Medicine 2013;20(1):25‐8. [DOI: 10.3760/cma.j.issn.1009-6906.2013.01.009] [DOI] [Google Scholar]
Wu 2009 {published data only}
- Wu R, Zeng XY, Su XL, Ou HJ. Effect of hyperbaric oxygen therapy for nuclear factor Κb and Child Autism Rating Scale in autistic children. Chinese Journal of Nautical Medicine and Hyperbaric Medicine 2009;16(2):125‐6. [DOI: 10.3760/cma.j.issn.1009-6906.2009.02.029] [DOI] [Google Scholar]
Yildiz 2008 {published data only}
- Yildiz S, Aktas S, Uzun G. Hyperbaric oxygen therapy in autism: is there evidence?. Undersea & Hyperbaric Medicine 2008;35(6):453‐5. [PUBMED: 19175200] [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu N, Pen JJ. Effect of hyperbaric oxygen therapy on rehabilitation training of children with autism. Journal of Clinical Pediatrics 2010;28(7):685‐7. [http://bit.ly/1RUjj8q] [Google Scholar]
Yu 2010a {published data only}
- Yu N, Pen JJ. Hyperbaric oxygen therapy associated with rehabilitation training for autistic children. Chinese Journal of Physical Medicine and Rehabilitation 2010;32(6):458‐60. [Google Scholar]
Additional references
Abdallah 2012
- Abdallah MW, Pearce BD, Larsen N, Greaves‐Lord K, Nørgaard‐Pedersen B, Hougaard DM, et al. Amniotic fluid MMP‐9 and neurotrophins in autism spectrum disorders: an exploratory study. Autism Research 2012;5(6):428‐33. [PUBMED: 23008271] [DOI] [PubMed] [Google Scholar]
AHRQ 2011
- Warren Z, Veenstra‐VanderWeele J, Stone W, Bruzek JL, Nahmias AS, Foss‐Feig JH, et al. Therapies for children with autism spectrum disorders. http://bit.ly/2cvtBRR (accessed 10 November 2012). [PubMed]
Aman 1987
- Aman MG, Richmond G, Stewart AW, Bell JC, Kissel RC. The Aberrant Behavior Checklist: factor structure and the effect of subject variables in American and New Zealand facilities. American Journal of Mental Deficiency 1987;91(6):570‐8. [PUBMED: 3591845] [PubMed] [Google Scholar]
Anitha 2012
- Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, et al. Brain region‐specific altered expression and association of mitochondria‐related genes in autism. Molecular Autism 2012;3(1):12. [PUBMED: 23116158] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders ‐ Text Revision. 4th Edition. (DSM‐IV‐TR). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. (DSM‐5). Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Bai 2008
- Bai J, Luan Z, Zhou CL, Qu SQ, Jiang Y, Wang ZY. Effect of hyperbaric oxygenation on the differentiation of implanted human neural stem cells into neurons in vivo. Chinese Journal of Contemporary Pediatrics 2008;10(2):195‐8. [PUBMED: 18433546] [PubMed] [Google Scholar]
Brkic 2012
- Brkic P, Stojiljkovic M, Jovanovic T, Dacic S, Lavrnja I, Savic D, et al. Hyperbaric oxygenation improves locomotor ability by enhancing neuroplastic responses after cortical ablation in rats. Brain Injury 2012;26(10):1273‐84. [PUBMED: 22571185] [DOI] [PubMed] [Google Scholar]
Calvert 2007
- Calvert JW, Cahill J, Zhang JH. Hyperbaric oxygen and cerebral physiology. Neurological Research 2007;29(2):132‐41. [PUBMED: 17439697] [DOI] [PubMed] [Google Scholar]
Chen 2009a
- Chen Y, Nadi NS, Chavko M, Auker CR, McCarron RM. Microarray analysis of gene expression in rat cortical neurons exposed to hyperbaric air and oxygen. Neurochemical Research 2009;34(6):1047‐56. [PUBMED: 19015983] [DOI] [PubMed] [Google Scholar]
Chen 2009b
- Chen S, Xiao N, Zhang X. Effect of combined therapy with ephedrine and hyperbaric oxygen on neonatal hypoxic‐ischemic brain injury. Neuroscience Letters 2009;465(2):171‐6. [PUBMED: 19765636] [DOI] [PubMed] [Google Scholar]
Ching 2012
- Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009043.pub2; Art. No.: CD009043] [DOI] [PubMed] [Google Scholar]
Connors 2006
- Connors SL, Matteson KJ, Sega GA, Lozzio CB, Carroll RC, Zimmerman AW. Plasma serotonin in autism. Pediatric Neurology 2006;35(3):182‐6. [PUBMED: 16939857] [DOI] [PubMed] [Google Scholar]
Cornell 2014
- Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random‐effects meta‐analysis of inconsistent effects: a time for change. Annals of Internal Medicine 2014;160(4):267‐70. [PUBMED: 24727843] [DOI] [PubMed] [Google Scholar]
Currenti 2010
- Currenti SA. Understanding and determining the etiology of autism. Cellular and Molecular Neurobiology 2010;30(2):161‐71. [PUBMED: 19774457] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Rubeis 2014
- Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014;515(7526):209‐15. [PUBMED: 25363760] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Rubeis 2015
- Rubeis S, Buxbaum JD. Recent advances in the genetics of autism spectrum disorder. Current Neurology and Neuroscience Reports 2015;15:553. [DOI: 10.1007/s11910-015-0553-1] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochanre Statistical Methods Group. Chaper 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org.
Devlin 2012
- Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Current Opinion in Genetics & Development 2012;22(3):229‐37. [PUBMED: 22463983] [DOI] [PubMed] [Google Scholar]
Dufour‐Rainfray 2011
- Dufour‐Rainfray D, Vourc'h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neuroscience and Biobehavioral Reviews 2011;35(5):1254‐65. [PUBMED: 21195109] [DOI] [PubMed] [Google Scholar]
Edwards 1997
- Edwards S, Fletcher P, Garman M, Hughes A, Letts C, SinkaI I. The Reynell Developmental Language Scales III. Windsor: NFER‐Nelson, 1997. [Google Scholar]
Efrati 2013
- Efrati S, Fishlev G, Bechor Y, Volkov O, Bergan J, Kliakhandler K, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients ‐ randomized, prospective trial. PLoS One 2013;8(1):e53716. [DOI: 10.1371/journal.pone.0053716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphic test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Ansary 2012
- El‐Ansary A, Al‐Ayadhi L. Neuroinflammation in autism spectrum disorders. Journal of Neuroinflammation 2012;9:265. [PUBMED: 23231720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldevik 2009
- Eldevik S, Hastings RP, Hughes JC, Jahr E, Eikeseth S, Cross S. Meta‐analysis of early intensive behavioral intervention for children with autism. Journal of Clinical Child and Adolescent Psychology 2009;38(3):439‐50. [DOI: 10.1080/15374410902851739] [DOI] [PubMed] [Google Scholar]
Freeman 1986
- Freeman BJ, Ritvo ER, Yokota A, Ritvo A. A scale for rating symptoms of patients with the syndrome of autism in real life settings. Journal of the American Academy of Child Psychiatry 1986;25(1):130‐6. [DOI: 10.1016/S0002-7138(09)60610-5] [DOI] [PubMed] [Google Scholar]
Freiberger 2006
- Freiberger JJ, Suliman HB, Sheng H, McAdoo J, Piantadosi CA, Warner DS. A comparison of hyperbaric oxygen versus hypoxic cerebral preconditioning in neonatal rats. Brain Research 2006;1075(1):213‐22. [PUBMED: 16458861] [DOI] [PubMed] [Google Scholar]
Gal 2012
- Gal G, Abiri L, Reichenberg A, Gabis L, Gross R. Time trends in reported autism spectrum disorders in Israel, 1986‐2005. Journal of Autism and Developmental Disorders 2012;42(3):428‐31. [PUBMED: 21567257] [DOI] [PubMed] [Google Scholar]
Gaugler 2014
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nature Genetics 2014;46(8):881‐5. [PUBMED: 25038753] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gendry 2005
- Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, et al. Autism severity and temporal lobe functional abnormalities. Annals of Neurology 2005;58(3):466‐9. [PUBMED: 16130096] [DOI] [PubMed] [Google Scholar]
Ghanizadeh 2012
- Ghanizadeh A. Hyperbaric oxygen therapy for treatment of children with autism: a systematic review of randomized trials. Medical Gas Research 2012;2:13. [PUBMED: 22577817] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 2009
- Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM&R 2009;1(5):471‐89. [PUBMED: 19627935] [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook. Introduction to GRADE handbook. Handbook for grading quality of evidence and strength of recommendations using the GRADE approach. Updated October 2013. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (10 December 2013).
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 30 November 2015. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Griffiths 1996
- Griffiths R, Huntley M. The Griffiths Mental Development Scales: From Birth to 2 Years. Henley‐on‐Thames, UK: Test Agency Ltd, 1996. [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Health, Education and Welfare, 1976. [http://www.psywellness.com.sg/docs/CGI.pdf] [Google Scholar]
Haley 1992
- Haley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Andrellos PJ. Pediatric Evaluation of Disability Inventory (PEDI). San Antonio (TX): Pearson, 1992. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochanre Statisticial Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoggan 2014
- Hoggan BL, Cameron AL. Systematic review of hyperbaric oxygen therapy for the treatment of non‐neurological soft tissue radiation‐related injuries. Supportive Care in Cancer 2014;22(6):1715‐26. [PUBMED: 24794980] [DOI] [PubMed] [Google Scholar]
Howlin 2009
- Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. American Journal on Intellectual Developmental Disabilities 2009;114(1):23‐41. [PUBMED: 19143460] [DOI] [PubMed] [Google Scholar]
Huang 2015
- Huang ET, Mansouri J, Murad MH, Joseph WS, Strauss MB, Tettelbach W, et al. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers. Undersea & Hyperbaric Medicine 2015;42(3):205‐47. [PUBMED: 26152105] [PubMed] [Google Scholar]
Hughes 2008
- Hughes JR. A review of recent reports on autism: 1000 studies published in 2007. Epilepsy & Behavior 2008;13(3):425‐37. [PUBMED: 18627794] [DOI] [PubMed] [Google Scholar]
Ichim 2007
- Ichim TE, Solano F, Glenn E, Morales F, Smith L, Zabrecky G, et al. Stem cell therapy for autism. Journal of Translational Medicine 2007;5:30. [DOI: 10.1186/1479-5876-5-30] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 2005
- Ito H, Mori K, Hashimoto T, Miyazaki M, Hori A, Kagami S, et al. Findings of brain 99mTc‐ECD SPECT in high‐functioning autism—3‐dimensional stereotactic ROI template analysis of brain SPECT. Journal of Medical Investigation 2005;52(1‐2):49‐56. [DOI] [PubMed] [Google Scholar]
Jesner 2007
- Jesner OS, Aref‐Adib M, Coren E. Risperidone for autism spectrum disorder. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005040.pub2; Art. No.: CD005040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kane 2012
- Kane MJ, Angoa‐Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, et al. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One 2012;7(11):e48975. [PUBMED: 23139830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leckman 1989
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician‐rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(4):566‐73. [PUBMED: 2768151] [DOI] [PubMed] [Google Scholar]
Leiter 1980
- Leiter RG. Leiter International Performance Scale Instruction Manual. Revised. Wood Dale: Stoelting, 1980. [Google Scholar]
Li 2009
- Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, et al. Hyperbaric oxygen preconditioning reduces ischemia‐reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience 2009;159(4):1309‐15. [DOI: 10.1016/j.neuroscience.2009.01.011] [DOI] [PubMed] [Google Scholar]
Li 2009a
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. Journal of Neuroimmunology 2009;207(1‐2):111‐6. [PUBMED: 19157572] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2012a
- Lin KC, Niu KC, Tsai KJ, Kuo JR, Wang LC, Chio CC, et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. Journal of Trauma and Acute Care Surgery 2012;72(3):650‐9. [PUBMED: 22491549] [DOI] [PubMed] [Google Scholar]
Lin 2012b
- Lin H, Chang CP, Lin HJ, Lin MT, Tsai CC. Attenuating brain edema, hippocampal oxidative stress, and cognitive dysfunction in rats using hyperbaric oxygen preconditioning during simulated high‐altitude exposure. Journal of Trauma and Acute Care Surgery 2012;72(5):1220‐7. [PUBMED: 22673248] [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview‐Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659‐85. [PUBMED: 7814313] [DOI] [PubMed] [Google Scholar]
Lord 1997
- Lord C, Rutter M, DiLavore PC. Autism Diagnostic Observation Scale ‐ Generic. Chicago, IL: University of Chicago Press, 1997. [Google Scholar]
Lou 2006
- Lou M, Chen Y, Ding M, Eschenfelder CC, Deuschl G. Involvement of the mitochondrial ATP‐sensitive potassium channel in the neuroprotective effect of hyperbaric oxygenation after cerebral ischemia. Brain Research Bulletin 2006;69(2):109‐16. [DOI: 10.1016/j.brainresbull.2005.11.009] [DOI] [PubMed] [Google Scholar]
Lowe 1976
- Lowe M, Costello AJ. Manual for the Symbolic Play Test. Windsor: National Foundation for Educational Research, 1976. [Google Scholar]
Maezawa 2011
- Maezawa I, Calafiore M, Wulff H, Jin LW. Does microglial dysfunction play a role in autism and Rett syndrome?. Neuron Glia Biology 2011;7(1):85‐97. [PUBMED: 22717189] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maglione 2012
- Maglione MA, Gans D, Das L, Timbie J, Kasari C. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics 2012;130 Suppl 2:S169‐78. [PUBMED: 23118248] [DOI] [PubMed] [Google Scholar]
Malek 2013
- Malek M, Duszczyk M, Zyszkowski M, Ziembowicz A, Salinska E. Hyperbaric oxygen and hyperbaric air treatment result in comparable neuronal death reduction and improved behavioral outcome after transient forebrain ischemia in the gerbil. Experimental Brain Research 2013;224(1):1‐14. [PUBMED: 23283415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matson 2011
- Matson JL, Sipes M, Fodstad JC, Fitzgerald ME. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs 2011;25(7):597‐606. [PUBMED: 21699271] [DOI] [PubMed] [Google Scholar]
McPheeters 2011
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics 2011;127(5):e1312‐21. [PUBMED: 21464191] [DOI] [PubMed] [Google Scholar]
Miljkovic‐Lolic 2003
- Miljkovic‐Lolic M, Silbergleit R, Fiskum G, Rosenthal RE. Neuroprotective effects of hyperbaric oxygen treatment in experimental focal cerebral ischemia are associated with reduced brain leukocyte myeloperoxidase activity. Brain Research 2003;971(1):90‐4. [PUBMED: 12691841] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mrsić‐Pelcić 2004
- Mrsić‐Pelcić J, Pelcić G, Vitezić D, Antoncić I, Filipović T, Simonić A, et al. Hyperbaric oxygen treatment: the influence on the hippocampal superoxide dismutase and Na+,K+‐ATPase activities in global cerebral ischemia‐exposed rats. Neurochemistry International 2004;44(8):585‐94. [PUBMED: 15016473] [DOI] [PubMed] [Google Scholar]
Msall 1994
- Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clinical Pediatrics 1994;33(7):421‐30. [PUBMED: 7525140] [DOI] [PubMed] [Google Scholar]
Mu 2013
- Mu J, Ostrowski RP, Soejima Y, Rolland WB, Krafft PR, Tang J, et al. Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of cAMP responsive element binding protein in the rat model of MCAo‐induced ischemic brain injury. Neurobiology of Disease 2013;51:133‐43. [PUBMED: 23146993] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Muhle 2004
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics 2004;113(5):e472‐86. [PUBMED: 15121991] [DOI] [PubMed] [Google Scholar]
Muller‐Bolla 2006
- Muller‐Bolla M, Collet JP, Ducruet T, Robinson A. Side effects of hyperbaric oxygen therapy in children with cerebral palsy. Undersea & Hyperbaric Medicine 2006;33(4):237‐44. [PUBMED: 17004410] [PubMed] [Google Scholar]
NICE 2013
- The Guideline Development Group, National Collaborating Centre, NICE project team. Autism: the management and support of children and young people on the autism spectrum. NICE guideline. Draft for Consultation, March 2013. http://bit.ly/2bHZr7K (accessed 10 February 2016).
Novianti 2014
- Novianti PW, Roes KC, Tweel I. Estimation of between‐trial variance in sequential meta‐analyses: a simulation study. Contemporary Clinical Trials 2014;37(1):129‐38. [PUBMED: 24321246] [DOI] [PubMed] [Google Scholar]
O'Hearn 2008
- O'Hearn K, Asato M, Ordaz S, Luna B. Neurodevelopment and executive function in autism. Developmental and Psychopathology 2008;20(4):1103‐32. [DOI: 10.1017/S09545794080005271103] [DOI] [PubMed] [Google Scholar]
Pardo 2007
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathology 2007;17(4):434‐47. [PUBMED: 17919129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2005
- Phillips JS, Jones SE. Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004617.pub2; Art. No.: CD004617] [DOI] [PubMed] [Google Scholar]
Reichow 2012
- Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD009260.pub2; Art. No.: CD009660] [DOI] [PubMed] [Google Scholar]
RevMan 5 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52‐60. [PUBMED: 25304503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rimland 1999
- Rimland B, Edelson SM. Autism Treatment Evaluation Checklist (ATEC). www.autism.com/index.php/ind_atec (accessed 15 December 2014).
Rossignol 2007b
- Rossignol DA. Hyperbaric oxygen therapy might improve certain pathophysiological findings in autism. Medical Hypotheses 2007;68(6):1208‐27. [PUBMED: 17141962] [DOI] [PubMed] [Google Scholar]
Rossignol 2009a
- Rossignol DA, Rossignol LW, Smith S, Schneider C, Logerquist S, Usman A, et al. Hyperbaric treatment for children with autism: a multicenter, randomized, double‐blind, controlled trial. BMC Pediatrics 2009;9:21. [DOI: 10.1186/1471-2431-9-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 2009b
- Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Annals of Clinical Psychiatry 2009;21(4):213‐36. [PUBMED: 19917212] [PubMed] [Google Scholar]
Rossignol 2012a
- Rossignol DA, Bradstreet JJ, Van Dyke K, Schneider C, Freedenfeld SH, O'Hara N, et al. Hyperbaric oxygen treatment in autism spectrum disorders. Medical Gas Research 2012;2:16. [DOI: 10.1186/2045-9912-2-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 2012b
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta‐analysis. Molecular Psychiatry 2012;17:290‐314. [DOI: 10.1038/mp.2010.136] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandin 2014
- Sandin S, Lichtenstein P, Kuja‐Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311(17):1770‐7. [DOI: 10.1001/jama.2014.4144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siniscalco 2012
- Siniscalco D, Sapone A, Giordano C, Cirillo A, Novellis V, Magistris L, et al. The expression of caspases is enhanced in peripheral blood mononuclear cells of autism spectrum disorder patients. Journal of Autism and Developmental Disorders 2012;42(7):1403‐10. [PUBMED: 21969075] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org.
Stoekenbroek 2014
- Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. European Journal of Vascular and Endovascular Surgery 2014;47(6):647‐55. [PUBMED: 24726143] [DOI] [PubMed] [Google Scholar]
Sumen‐Secgin 2005
- Sumen‐Secgin G, Cimsit M, Ozek M, Eroglu L. Antidepressant‐like effect of hyperbaric oxygen treatment in forced‐swimming test in rats. Methods and Findings in Experimental and Clinical Pharmacology 2005;27(7):471‐4. [PUBMED: 16258591] [DOI] [PubMed] [Google Scholar]
Thom 2006
- Thom SR, Bhopale VM, Fisher D. Hyperbaric oxygen reduces delayed immune‐mediated neuropathology in experimental carbon monoxide toxicity. Toxicology and Applied Pharmacology 2006;213(2):152‐9. [PUBMED: 16325878] [DOI] [PubMed] [Google Scholar]
Thom 2011
- Thom SR. Hyperbaric oxygen ‐ its mechanisms and efficacy. Plastic and Reconstructive Surgery 2011;127(Suppl 1):131‐41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorndike 1986
- Thorndike R, Hagen E, Sattler J. The Stanford‐Binet Intelligence Scale. 4th Edition. Itasca: The Riverside Publishing Company, 1986. [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [PUBMED: 22461129] [DOI] [PMC free article] [PubMed] [Google Scholar]
UHMS 2016
- Undersea & Hyperbaric Medical Society. Indications for hyperbaric oxygen therapy: definition of hyperbaric oxygen therapy. https://www.uhms.org/resources/hbo‐indications.html (accessed 10 February 2016).
Vargas 2005
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology 2005;57(1):67‐81. [DOI: 10.1002/ana.20315] [DOI] [PubMed] [Google Scholar]
Veroniki 2016
- Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Research Synthesis Methods 2016;7(1):55‐79. [PUBMED: 26332144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007a
- Wang XL, Yang YJ, Wang QH, Xie M, Yu XH, Liu CT, et al. Changes of Wnt‐3 protein during the proliferation of endogenous neural stem cells in neonatal rats with hypoxic‐ischemic brain damage after hyperbaric oxygen therapy. Chinese Journal of Contemporary Pediatrics 2007;9(3):241‐6. [PUBMED: 17582265] [PubMed] [Google Scholar]
Wang 2007b
- Wang XL, Yang YJ, Wang QH, Yu XH, Xie M, Liu CT, et al. Effect of hyperbaric oxygen therapy administered at different time on white matter damage following hypoxic‐ischemic brain damage in neonatal rats. Chinese Journal of Contemporary Pediatrics 2007;9(4):308‐12. [PUBMED: 17706027] [PubMed] [Google Scholar]
Wang 2009
- Wang XL, Yang YJ, Xie M, Yu XH, Wang QH. Hyperbaric oxygen promotes the migration and differentiation of endogenous neural stem cells in neonatal rats with hypoxic ischemic brain damage. Chinese Journal of Contemporary Pediatrics 2009;11(9):749‐52. [PUBMED: 19755026] [PubMed] [Google Scholar]
Warren 2011
- Warren Z, McPheeters ML, Sathe N, Foss‐Feig JH, Glasser A, Veenstra‐Vanderweele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics 2011;127(5):e1303‐11. [DOI: 10.1542/peds.2011-0426; PUBMED: 21464190] [DOI] [PubMed] [Google Scholar]
Wei 2015
- Wei L, Wang J, Cao Y, Ren Q, Zhao L, Li X, et al. Hyperbaric oxygenation promotes neural stem cell proliferation and protects the learning and memory ability in neonatal hypoxic‐ischemic brain damage. International Journal of Clinical and Experimental Pathology 2015;8(2):1752‐9. [PUBMED: 25973064] [PMC free article] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: WHO, 1993. [Google Scholar]
Wilson 2006
- Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Research 2006;1098(1):126‐8. [PUBMED: 16750177] [DOI] [PubMed] [Google Scholar]
Xiong 2014 [pers comm]
- Xiong T. Cochrane Review Group: call for new RCT information regarding hyperbaric oxygen therapy for autism spectrum disorder [personal communication]. Email to: DA Rossignol, C Schneider, J Neubrander, G Hintz, LW Rossignol, S Logerquist, EM Madren, B Grushkin, S Smith, A Usman, EA Mumper, D Granpeesheh, CT Chaiyakul, J Tarbox; 11 November 2014.
Yamashita 2009
- Yamashita S, Hirata T, Mizukami Y, Cui YJ, Fukuda S, Ishida K, et al. Repeated preconditioning with hyperbaric oxygen induces neuroprotection against forebrain ischemia via suppression of p38 mitogen activated protein kinase. Brain Research 2009;1301:171‐9. [PUBMED: 19747454] [DOI] [PubMed] [Google Scholar]
Yang 2007
- Yang MS, Gill M. A review of gene linkage, association and expression studies in autism and an assessment of convergent evidence. International Journal of Developmental Neuroscience 2007;25(2):69‐85. [DOI: 10.1016/j.ijdevneu.2006.12.002] [DOI] [PubMed] [Google Scholar]
Yin 2013
- Yin X, Meng F, Wang Y, Wei W, Li A, Chai Y, et al. Effect of hyperbaric oxygen on neurological recovery of neonatal rats following hypoxic‐ischemic brain damage and its underlying mechanism. International Journal of Clinical and Experimental Pathology 2013;6(1):66‐75. [PUBMED: 23236544] [PMC free article] [PubMed] [Google Scholar]
Zhou 2008
- Zhou BY, Lu GJ, Huang YQ, Ye ZZ, Han YK. Efficacy of hyperbaric oxygen therapy under different pressures on neonatal hypoxic‐ischemic encephalopathy. Chinese Journal of Contemporary Pediatrics 2008;10(2):133‐5. [PUBMED: 18433528] [PubMed] [Google Scholar]
References to other published versions of this review
Xiong 2014
- Xiong T, Chen H, Luo R, Mu D. Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children and adults. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010922; Art. No.: CD010922] [DOI] [PMC free article] [PubMed] [Google Scholar]


