Abstract
Background
This is an updated version of the original Cochrane review published in Issue 1, 2010, on 'Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults'. Breathlessness is one of the most common symptoms experienced in the advanced stages of malignant and non‐malignant disease. Benzodiazepines are widely used for the relief of breathlessness in advanced diseases and are regularly recommended in the literature. At the time of the previously published Cochrane review, there was no evidence for a beneficial effect of benzodiazepines for the relief of breathlessness in people with advanced cancer and chronic obstructive pulmonary disease (COPD).
Objectives
The primary objective of this review was to determine the efficacy of benzodiazepines for the relief of breathlessness in people with advanced disease. Secondary objectives were to determine the efficacy of different benzodiazepines, different doses of benzodiazepines, different routes of application, adverse effects of benzodiazepines, and the efficacy in different disease groups.
Search methods
This is an update of a review published in 2010. We searched 14 electronic databases up to September 2009 for the original review. We checked the reference lists of all relevant studies, key textbooks, reviews, and websites. For the update, we searched CENTRAL, MEDLINE, and EMBASE and registers of clinical trials for further ongoing or unpublished studies, up to August 2016. We contacted study investigators and experts in the field of palliative care asking for further studies, unpublished data, or study details when necessary.
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) assessing the effect of benzodiazepines compared with placebo or active control in relieving breathlessness in people with advanced stages of cancer, chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), motor neurone disease (MND), and idiopathic pulmonary fibrosis (IPF).
Data collection and analysis
Two review authors independently assessed identified titles and abstracts. Three review authors independently performed assessment of all potentially relevant studies (full text), data extraction, and assessment of methodological quality. We carried out meta‐analysis where appropriate.
Main results
Overall, we identified eight studies for inclusion: seven in the previous review and an additional study for this update. We also identified two studies awaiting classification in this update. The studies were small (a maximum number of 101 participants) and comprised data from a total of 214 participants with advanced cancer or COPD, which we analysed. There was only one study of low risk of bias. Most of the studies had an unclear risk of bias due to lack of information on random sequence generation, concealment, and attrition. Analysis of all studies did not show a beneficial effect of benzodiazepines for the relief of breathlessness (the primary outcome) in people with advanced cancer and COPD (8 studies, 214 participants) compared to placebo, midazolam, morphine, or promethazine. Furthermore, we observed no statistically significant effect in the prevention of episodic breathlessness (breakthrough dyspnoea) in people with cancer (after 48 hours: risk ratio of 0.76 (95% CI 0.53 to 1.09; 2 studies, 108 participants)) compared to morphine. Sensitivity analyses demonstrated no statistically significant differences regarding type of benzodiazepine, dose, route and frequency of delivery, duration of treatment, or type of control. Benzodiazepines caused statistically significantly more adverse events, particularly drowsiness and somnolence, when compared to placebo (risk difference 0.74 (95% CI 0.37, 1.11); 3 studies, 38 participants). In contrast, two studies reported that morphine caused more adverse events than midazolam (RD ‐0.18 (95% CI ‐0.31, ‐0.04); 194 participants).
Authors' conclusions
Since the last version of this review, we have identified one new study for inclusion, but the conclusions remain unchanged. There is no evidence for or against benzodiazepines for the relief of breathlessness in people with advanced cancer and COPD. Benzodiazepines caused more drowsiness as an adverse effect compared to placebo, but less compared to morphine. Benzodiazepines may be considered as a second‐ or third‐line treatment, when opioids and non‐pharmacological measures have failed to control breathlessness. There is a need for well‐conducted and adequately powered studies.
Keywords: Adult; Humans; Benzodiazepines; Benzodiazepines/adverse effects; Benzodiazepines/therapeutic use; Dyspnea; Dyspnea/drug therapy; Dyspnea/etiology; Lung Neoplasms; Lung Neoplasms/complications; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/complications; Randomized Controlled Trials as Topic
Plain language summary
Benzodiazepines for the relief of breathlessness in advanced diseases in adults
Background
Breathlessness is a common and distressing symptom in advanced cancer and other diseases at the end of life. Treating breathlessness sufficiently remains very difficult. Benzodiazepines are a group of sedating medicines (drugs), including lorazepam, clorazepate, diazepam, alprazolam, and temazepam, that are used mainly for sleep disturbance and anxiety, but are widely used for the relief of breathlessness.
Key results
In this updated systematic review we aimed to determine whether benzodiazepines relieved breathlessness in adults with advanced disease. In August 2016, we found eight studies.
Benzodiazepines caused more side effects such as drowsiness or somnolence when compared to placebo but caused less side effects when compared to morphine. Our review therefore supports the use of benzodiazepines only if other first‐line treatments, such as opioids and non‐drug treatments, have failed. However, there is still an urgent need for more studies in this field to find better ways to relieve this burdensome symptom in people with advanced diseases.
We concluded in summary that there is no evidence that benzodiazepines relieve breathlessness in adults with advanced disease.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews, Issue 1, 2010, on 'Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults'.
Description of the condition
The American Thoracic Society defines breathlessness as "a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioral responses" (Parshall 2012). This multidimensional concept of breathlessness as 'total breathlessness' is comparable with the concepts of 'total pain' or 'total suffering' (Booth 2006). The term 'breathlessness' is used interchangeably with dyspnoea, shortness of breath, breathing difficulty, and laboured breathing. Breathlessness is defined as refractory when it persists despite optimal treatment of the underlying condition (Dorman 2009), and can manifest as continuous breathlessness or episodic breathlessness (Simon 2012; Simon 2014).
Breathlessness is one of the most common symptoms in the last year of life (Higginson 2004). In advanced diseases, it is highly prevalent in chronic obstructive pulmonary disease (COPD) (56% to 98%), chronic heart failure (CHF) (18% to 88%), and cancer (16% to 77%) (Moens 2014). It is a distressing symptom for the patient, but also for the caregivers (Nordgren 2003). The frequency and severity of breathlessness increases during the course of the disease until death (Currow 2010; Seow 2011). Furthermore, breathlessness may be related to anxiety and depression (Neuman 2006), thus treatment of anxiety and depression may reduce this symptom. However, the contribution, the causal relationship, and the direction of influence are still unclear (Booth 2008).
Different diseases cause breathlessness, such as primary and secondary cancer, COPD, CHF, motor neurone disease (MND), and cryptogenic fibrosing alveolitis/idiopathic pulmonary fibrosis (IPF). The advanced stage of each disease must be defined separately because of the different disease trajectories. The pathophysiology of breathlessness depends mainly on the underlying cause. It includes, for example, airway obstruction, reduction of lung or gas exchange capacity, muscle weakness, degeneration of neurons, or reduction of blood diffusing capacity. The pathological pathway is complex and beyond a sole reduction of PO2 (partial pressure of oxygen) or increase of PCO2 (partial pressure of carbon dioxide) (Manning 1995). The medulla in the brain stem, the motor and sensory cortex, peripheral and central chemoreceptors, and mechanoreceptors in the airways and chest wall are the main sites of action responsible for the perception of breathlessness (Booth 2008). There are different explanations of how different parts interact and induce the sensation of breathlessness, such as corollary discharge, afferent‐reafferent dissociation, and receptor reaction. The corollary discharge describes the hypothesis that a sensory 'copy' of the motor output is sent from the motor cortex to the sensory cortex and imparts a conscious awareness of respiratory effort, and is the most widely accepted hypothesis (Beach 2006).
After treatment of the underlying cause, symptom management of breathlessness includes non‐pharmacological and pharmacological interventions. A recent Cochrane Review on non‐pharmacological interventions for the relief of breathlessness in advanced disease showed effectiveness of neuro‐electrical muscle stimulation, chest wall vibration, walking aids, and breathing training (Bausewein 2008). A recent review on the use of oxygen highlights that there is a statistically and clinically significant benefit for both ambulatory and long‐term oxygen in COPD, but no consistent evidence for their use in cancer (Cranston 2008).
Opioids are the first choice in the pharmacological management of refractory breathlessness. A Cochrane Review showed evidence for the use of oral and parenteral application of opioids, but there is currently no evidence for nebulised opioids (Jennings 2001; Parshall 2012). However, most of the studies were underpowered, and there is a need for further well‐designed studies to investigate the effectiveness in different diseases, applications, and doses. Besides opioids, there are other drugs for the palliation of breathlessness, such as steroids (for lymphangitis carcinomatosa), inhaled local anaesthetics, or more sedating drugs such as benzodiazepines, phenothiazines, buspirone, or chlorpromazine, with variable evidence in symptom control (Davis 2005; Parshall 2012).
Description of the intervention
Benzodiazepines are frequently used in the management of breathlessness in advanced diseases and are regularly recommended in textbooks for palliative medicine or clinical guidelines (Booth 2006; Bruera 2006). The most common drugs are diazepam, midazolam, alprazolam, and lorazepam. However, there are more than 40 different benzodiazepines (Hardman 2005).
How the intervention might work
It is increasingly evident that breathlessness interacts with mental health, for example anxiety and depression and anxiety or panic can trigger or worsen breathlessness (Booth 2008; Davis 1997). Benzodiazepines are anxiolytics, which have sedating effects and are intended to relieve anxiety and might therefore have palliating effects for breathlessness.
Benzodiazepines belong to the group of hypnotics and sedatives. Their core chemical structure is a fusion of the benzene and the diazepine ring with various modifications that are responsible for the different compounds of the drug. The interaction of benzodiazepines with specific subunits of GABA (gamma‐aminobutyric acid) receptors is responsible for their mechanism of action. The central and main effects of benzodiazepines are sedative‐hypnotic, muscle‐relaxant, anxiolytic, and anticonvulsant. Side effects include impairment of mental and motor function, light‐headedness, and nausea (Hardman 2005). Physical dependence is a huge problem in long‐term use of benzodiazepines. There is no effect on respiration (for example depression of respiration) in normal doses, and only a slight depression of ventilation in higher doses (Hardman 2005). The main therapeutic uses are insomnia, anxiety disorders, acute epilepsy, alcohol withdrawal, and anaesthetic premedication (Hardman 2005). The group of non‐benzodiazepines (for example zolpidem) act on the same receptors with similar effects, but have a different chemical structure. We have not included these in this review as they do not belong to the benzodiazepine group.
Why it is important to do this review
Despite the frequent use of benzodiazepines for the relief of breathlessness in palliative care, the evidence for their efficacy is still unclear.
Objectives
The primary objective of this review was to determine the efficacy of benzodiazepines for the relief of breathlessness in people with advanced disease.
Secondary objectives were to determine the efficacy of different benzodiazepines, different doses of benzodiazepines, different routes of application, adverse effects of benzodiazepines, and the efficacy in different disease groups.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We defined 'randomised' as studies described by the authors as 'randomised' anywhere in the manuscript.
Controlled clinical trials (CCTs).
While writing the protocol, we expected a limited number of studies and therefore decided also to include controlled trials, giving special consideration to the higher risk of bias in these trials in the analysis.
Types of participants
Adult participants described as suffering from either breathlessness, dyspnoea, shortness of breath, difficult breathing, or laboured breathing due to advanced malignant and non‐malignant diseases.
The advanced stages of diseases included the following.
Cancer: advanced local or metastatic disease.
COPD: stage III (severe) or IV (very severe) according to the Global Initiative for Obstructive Lung Disease (GOLD) classification. This includes people with airflow limitation of FEV1 < 50%, FEV1/FVC < 0.7 (FEV1: forced expiratory volume in one second; FVC: forced vital capacity) and symptoms such as more severe breathlessness, reduced exercise capacity, and repeated exacerbations (GOLD 2007).
CHF: stage III or IV of the New York Heart Association (NYHA) classification including symptoms such as breathlessness or palpitation and an increasing limitation of exercise capacity and discomfort at rest.
MND: all participants suffering from breathlessness.
IPF: all participants suffering from breathlessness as the most prominent and disabling symptom.
We excluded studies including participants with acute or chronic asthma, pneumonia, or other potentially curable diseases. Participants included in the studies could be in any care setting (for example hospital or home care).
We included studies evaluating participants on oxygen as long as oxygen was used in both the intervention and the control arm.
Types of interventions
The use of benzodiazepines (at any dose, any frequency (also single dose), any duration, and through any route) for the relief of breathlessness compared with placebo or active control. We included all drugs that belong to the pharmacological group of benzodiazepines (Hardman 2005).
Types of outcome measures
Primary outcomes
Primary outcomes included subjective measurements of breathlessness on validated and reliable scales such as:
uni‐dimensional scales (e.g. visual analogue scales (VAS), numeric rating scales (NRS), categorical scales, modified Borg scales); or
multidimensional scales (e.g. St. George's Respiratory Questionnaire (SGRQ), Chronic Respiratory Disease Questionnaire (CRQ)).
We included studies that measured breathlessness as a primary or secondary outcome, and also studies evaluating breathlessness at rest or on exercise.
Secondary outcomes
Secondary outcomes included:
measurement of anxiety;
measurement of depression;
adverse effects of benzodiazepines;
functional exercise capacity (e.g. walking tests);
measurement of quality of life; and
attrition.
Search methods for identification of studies
We ran the search for the original review on 12 September 2009 and ran a subsequent search on 23 August 2016.
Electronic searches
For the original review, we identified studies from a search of the following 14 databases:
the Cochrane Pain, Palliative and Supportive Care Trials Register (12 September 2009);
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2009, Issue 3) (12 September 2009);
the Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library (12 September 2009);
Database of Abstracts of Reviews of Effects (DARE) (12 September 2009);
MEDLINE (1950 to 12 September 2009);
EMBASE (1980 to 12 September 2009);
CINAHL (1980 to 12 September 2009);
PsycINFO (1806 to 12 September 2009);
American College of Physicians (ACP) Journal Club (12 September 2009);
Health Technology Assessment (HTA) Database (12 September 2009);
NHS Economic Evaluation Database (NHSEED) (12 September 2009);
Database of Halley Stewart Library (St. Christopher's Hospice) (12 September 2009);
International Pharmaceutical Abstracts (1970 to 12 September 2009); and
Iowa Drug Information System (IDIS) (1966 to 12 September 2009).
For the update, we searched:
CENTRAL Issue 7 2016 (the Cochrane Library) (searched 2009 to 2016);
MEDLINE & Medline in Process (OVID) (Sept 2009 to 23 August 2016);
EMBASE (OVID) (September 2009 to 23 August 2016).
We decided not to search the other databases searched for the original review as they did not yield any useful records.
We searched the following study registers or meta registers of clinical trials for ongoing or unpublished studies for the update:
ClinicalTrials.gov (08 July 2016);
metaRegister of Controlled trials (mRCT) ‐ active registers (08 July 2016);
WHO International Clinical Trials Registry Platform (08 July 2016).
We again contacted study investigators and experts in the field of palliative care to ask for further studies, unpublished data, or study details when necessary.
Please see Appendix 1 for the search strategies applied in the original review. Appendix 2 shows the search strategies applied in this update, with minor changes in lines 3 and 5 (in line 3 to ensure that both British and US spellings of labour/labor were picked up; in line 5 to ensure that both of the phrases 'shortness of breath' and 'short of breath' were included).
Searching other resources
Handsearching
We checked the reference lists of all relevant studies, key textbooks, and key websites for further relevant studies. We checked the reference lists of several reviews on the subject (Abernethy 2008; Allen 1984; Altose 1985; Bausewein 2008; Booth 2008; Davis 1997; De Conno 1991; Lanken 2008; Manning 2000; Ripamonti 1999; Rocker 2007; Runo 2001; Thomas 2002; Tobin 1990; Viola 2008; Williams 2006).
We handsearched the reference lists of the following 16 textbooks: Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Oxford Textbook of Palliative Medicine; Textbook of Palliative Medicine; Textbook of Palliative Nursing; Palliative Medicine; Management of Advanced Disease;Palliative Care Formulary 3; Oxford Handbook of Palliative Care; Palliative Medicine ‐ a Case‐Based Manual; Principles and Practice of Palliative Care and Supportive Oncology; Dyspnoea in Advanced Disease; Dyspnoea; Heart Failure and Palliative Care; Supportive Care in Respiratory Disease; Textbook of Respiratory Medicine; and Palliative Care in Neurology.
In addition, we searched seven websites to identify relevant data: www.benzo.org.uk; www.book.palliative.info; www.caresearch.com.au; www.cks.library.nhs.uk; www.controlled‐trials.com; www.palliativedrugs.com; and www.patient.co.uk.
We undertook no further handsearches for this review update.
Personal contact
We contacted the following authors of main studies and investigators who are known to be carrying out research in this area for further studies and unpublished data for the original review: Amy Abernethy, Sam Ahmedzai, Eduardo Bruera, Leandro Cerchietti, Jessica Corner, David Currow, Carol Davies, Deborah Dudgeon, Wesley Ely, Tim Harrison, Michio Hosaka, Miriam Johnson, Alfredo Navigante, Andrew Wilcock, and Ashley Woodcock. In addition, we asked all members of the Association of Palliative Medicine (UK) and all users of the bulletin board of www.palliativedrugs.com in a circular letter for additional studies or unpublished data. For the update, we contacted the above mentioned experts again and also asked the following newly identified study investigators for further information on their studies: Scott Bolesta, Eliza S. Daubert, Diana E. Hart, Neil K Hiliard, Fiona Horwood, Clare Randall, and Gerben Stege.
Language
There was no language restriction in the selection of studies.
Data collection and analysis
Selection of studies
Two review authors (STS, CB in the original review; VW, STS in the update) independently assessed the relevant titles and abstracts identified. Disagreement was resolved by consensus and with a third review author (IJH in the original review; CB in the update). Three review authors (STS, CB, SB in the original review; VW, STS, CB in the update) independently assessed the full text of all potentially relevant studies, and disagreement at this stage was again resolved by consensus and with a fourth review author (IJH).
Data extraction and management
Three review authors (STS, CB, SB) independently extracted data from each appropriate study in the original review; two review authors (STS, VW) did this for the update. We specifically designed an extraction form for collection of relevant data consisting of:
Study ID and publication details, including:
study aim.
Study design and methods, including:
randomisation procedure;
allocation concealment;
details of blinding;
number and time of follow‐ups;
handling of missing data; and
details of analysis.
Participant characteristics, including:
demographics;
diagnosis;
performance status;
number and description of participants in the intervention and control groups; and
setting.
Intervention, including:
the drug and its characteristics (e.g. half‐life);
route of administration;
dose;
frequency of application;
duration of therapy; and
description of placebo.
Primary outcomes, including:
measurement of breathlessness; and
change in level of breathlessness.
Secondary outcomes, including:
adverse effects of benzodiazepines;
functional exercise capacity;
dose modification;
number and reason of withdrawals/attrition;
measurement of anxiety;
measurement of depression;
measurement of quality of life; and
arterial blood gas measurements.
Additional information, including:
participant comments on intervention.
Methodological quality, including:
Risk of bias table (according to Cochrane standard);
Edwards Method Score (11 items as described below).
We contacted authors of studies to provide unpublished data for the meta‐analysis where required.
Assessment of risk of bias in included studies
Three review authors (STS, CB, SB in the original review; STS, VW in the update) independently assessed all selected studies for methodological quality. We used two measures of methodological quality. Firstly, we assessed the quality of studies using the Review Manager (RevMan) 'Risk of bias' table, categorising them as 'low risk', 'high risk', or 'unclear risk' according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; RevMan 2008; RevMan 2014). Secondly, we graded the quality of studies according to the Edwards Method Score (Edwards 2001; Edwards 2003). This checklist of methodological quality contains 11 items that assess the primary research quality of the studies and its published description. The following items were assessed and scored zero, one, or two for adequacy: definition of aims; sample formation; description of inclusion and exclusion criteria; description of participant characteristics; power calculation; objectivity of outcome measures used; adequacy of follow‐up; adequacy of analysis (intention‐to‐treat (ITT)); adjustment for baseline differences between groups; appropriate unit of allocation to groups; and randomisation method. We then constructed a total method score (max 22) and rated the overall quality of the studies as follows: low (12 and under), medium (13 to 14), high (15 and over) (Edwards, personal communication). We integrated the results of the quality assessment in data analysis, as well as in meta‐analysis (cumulative meta‐analysis only in high‐quality studies).
Measures of treatment effect
We used primary and secondary outcomes in the meta‐analysis when appropriate and possible. The primary outcome measures (breathlessness) were either in the form of continuous or ordinal data. We treated all ordinal data as continuous data because the scales used were long enough (following the recommendation of the Cochrane Handbook, Higgins 2011). In the meta‐analysis, we treated studies with cross‐over design in the same way as studies with a parallel design if there was no indication of a carry‐over effect (following the advice of the Cochrane Handbook and after discussion with the statistician of the Cochrane Review Group) (Higgins 2011). We judged the potential existence of a carry‐over effect on a theoretical basis after analysis of the study (for example drug persistency in the body into the next period). We estimated the effect by comparing the post‐treatment measurements of the intervention and the control groups. We calculated the standardised mean differences (SMD) for continuous data with a 95% confidence interval (CI) to show the size of the effect of interventions. Due to the diversity of measurement tools for breathlessness, we used SMD to measure the intervention effect in standardised units. A negative SMD was defined as a beneficial effect of the intervention. We calculated the risk ratio (RR) for dichotomous data to estimate the relative risk. We used risk difference when there was no event in one of the groups (for example for adverse effects or attrition), because the estimation of RR is not possible in this case. For all data we considered a P value of less than 0.05 as statistically significant.
Unit of analysis issues
We combined cross‐over trials and studies with a parallel design in the meta‐analysis and treated the cross‐over studies as parallel design. We did this after a critical analysis of all studies, review of the literature (Elbourne 2002; Higgins 2011), discussion with a statistician, and the following judgements:
the cross‐over design was suitable for the targeted research questions;
none of the cross‐over studies showed evidence of a carry‐over effect;
dropouts were excluded from analysis;
there was no evidence for a period effect. This approach can produce a unit‐of‐analysis error.
Dealing with missing data
When means and standard deviations (SD) were missing, we did not impute or estimate them for meta‐analysis, because none of the suggested imputations in the Cochrane Handbook for Systematic Reviews of Interventions were reliable (after consultation with the statistician of the Cochrane Review Group) (Higgins 2011). We therefore contacted the authors for additional data (means and SD). If they could not provide means or SD, we asked for the original data and calculated means and SD from these data. Data were only retrieved from graphs if exact numbers could be determined. With this procedure, we were able to retrieve all relevant data.
Assessment of heterogeneity
We anticipated clinical heterogeneity because of the differences in diagnostic groups, participants' disease, types of benzodiazepines, doses, duration of treatment, and route of delivery. We measured the impact of statistical heterogeneity (of effects) by quantifying inconsistency using the I2 statistic and based its interpretation on the recommendations given in the Cochrane Handbook (Higgins 2011).
Assessment of reporting biases
We assessed selective outcome reporting but did not assess further forms of reporting bias (no further assessment than those included in the 'Risk of bias' tool).
Data synthesis
We combined studies using RevMan (Version 5.0 for the original review, Version 5.3 for the update) (RevMan 2008; RevMan 2014). We attempted to obtain all relevant data from each paper.
We performed a meta‐analysis including all appropriate studies. We excluded studies from the meta‐analysis if the methodological quality of the study was low (Edwards Method Score 12 and lower). We used a random‐effects model because clinical heterogeneity was present. We used the fixed‐effect model only for the presentation of single studies or for studies with adequate homogeneity.
Subgroup analysis and investigation of heterogeneity
See section Assessment of heterogeneity.
Sensitivity analysis
We undertook sensitivity analysis to look for influences of different variables (for example participants, interventions, outcomes, and study design). We also performed sensitivity analysis taking into account methodological quality and the robustness of results.
Results
Description of studies
We have illustrated the study selection process for the update of this review in Figure 1.
1.
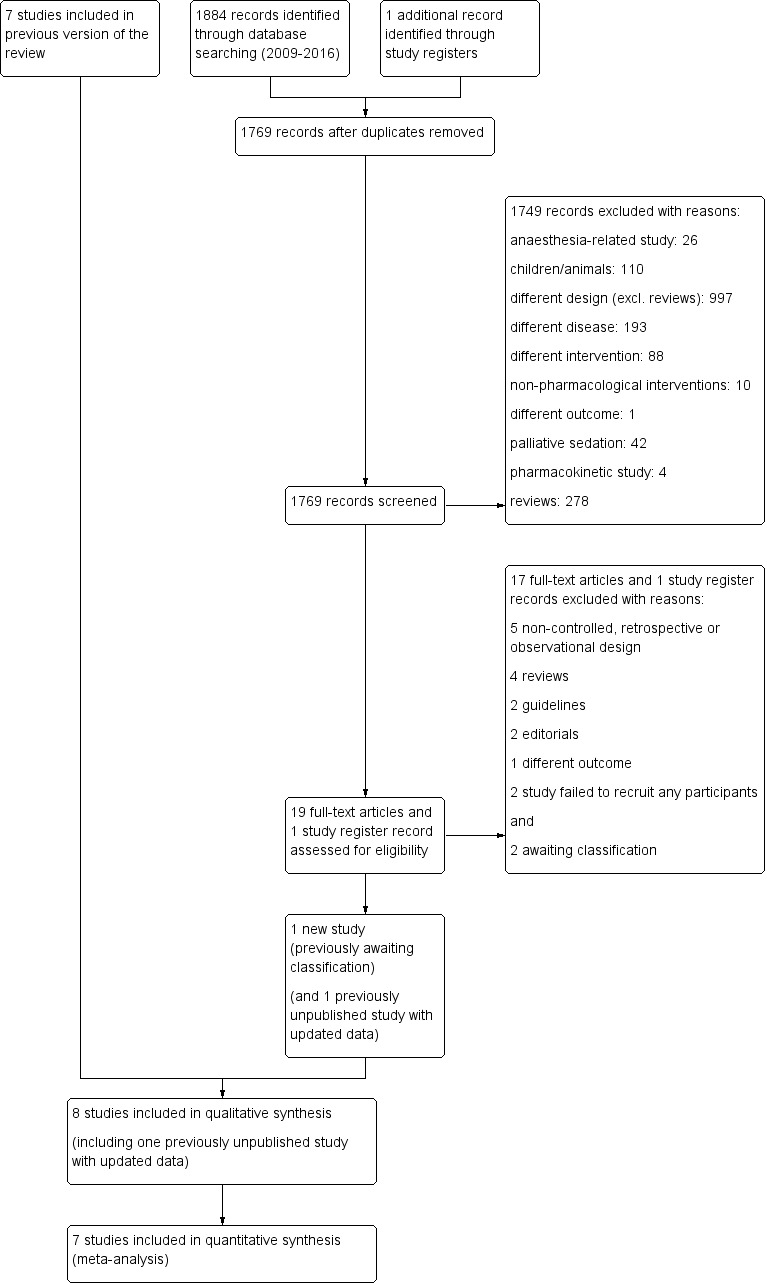
Study flow diagram.
Results of the search
For the update, we included eight studies in total (Eimer 1985, Harrison (unpublished), Man 1986, Navigante 2006, Navigante 2010 (updated data which was an unpublished study in the earlier review), Shivaram 1989, Stege 2010 (this study was awaiting assessment in the earlier review), Woodcock 1981). In addition in this update, we included two study for awaiting classification (Hart 2012, Hardy 2016).
For the earlier review, we identified a total of 1309 references through the search of 14 databases. We excluded 207 duplicates. We studied the titles and abstracts of each of the 1102 articles and selected relevant articles if they met the inclusion criteria. In the earlier review, we sorted the 1071 excluded articles into the following exclusion groups: different disease (241 articles), different drug (223), reviews (242), anaesthesia‐related study (111), psychology‐related study (61), pharmacokinetic study (45), different design (38), palliative/terminal sedation (37), non‐pharmacological interventions (14), and other (for example children) (59). We retrieved 31 articles for more detailed evaluation in the original review. In the earlier review, we identified 48 additional references from the reference lists of the original 31 articles by handsearching and the auxiliary function ‘Related Articles’ in Science Direct (www.sciencedirect.com). The search of 59 reviews, 16 textbooks, and seven websites did not yield any new articles. For the earlier review, we studied a total of 79 articles in more detail after obtaining the full text. Of these 79 articles, seven studies met our inclusion criteria and were included in the earlier review, and one study was awaiting classification (Stege 2010). We excluded a total of 74 articles for the earlier review. In addition, after sending a letter to all members of the Association of Palliative Medicine (UK) and after personal contact with several investigators (see above) at time of the original review, we were able to identify three new and unpublished studies (Harrison (unpublished), Navigante 2010, Stege 2010). We received data from two out of the three unpublished studies which we could include in the earlier review (Harrison (unpublished), Navigante 2010). The author of the third study could not send the data before submission and was set for "awaiting classification" of the earlier review (Stege 2010).
For the update of the review, we identified 1884 records: 32 articles in CENTRAL, 92 in MEDLINE, and 1760 in EMBASE. We also identified one potentially relevant clinical study report in trial registries. After de‐duplication, there were 1769 articles for assessment. After screening titles and abstracts and exclusion of 1749 studies (see Figure 1 for reasons of exclusion) we obtained full copies of 19 published studies and one study from trial registries. Of these 20 records, we excluded 17 studies and the record from trial registries (this was a pilot study which failed to recruit any participants and was therefore excluded (NCT01687751, personal communication with Neil Hilliard, June 2015)). One of the excluded records was a study protocol only, and the study was cancelled early before any participants were included (Daubert 2014; personal communication with Eliza Daubert and Scott Bolesta in June 2015). Two additional studies were considered for awaiting classification. One out of these two studies was published only as a conference abstract and we received no data until the time of submission of the review (see Characteristics of studies awaiting classification) (Hart 2012). The second study was published just before the submission of this review and the data will be considered at the next update (conclusion of this study supports the conclusion of the review) (see Characteristics of studies awaiting classification) (Hardy 2016). Finally for the update, we added one new study to the seven studies of the original review (Stege 2010 ‐ this study was unpublished and awaited assessment in the earlier review). In addition, we updated data of one previously unpublished but previously included study (Navigante 2010).
Included studies
See Characteristics of included studies.
Eimer 1985: a double‐blind, placebo‐controlled, cross‐over RCT tested clorazepate in five non‐anxious participants with severe COPD in a hospital setting to determine whether relieving breathlessness could be achieved. The study started with three arms (7.5 mg/day and 22.5 mg/day oral clorazepate compared to placebo), but the high‐dose arm (22.5 mg) was excluded from analysis after 3 out of 5 participants dropped out due to intolerable adverse effects. The duration of treatment was two weeks with a one‐week wash‐out period. Breathlessness was assessed weekly with a Breathlessness Grade from 1 (little breathlessness) to 6 (extreme breathlessness). Secondary outcomes were anxiety, depression, adverse effects, a 12‐minute walking test, and attrition.
Harrison (unpublished): the effectiveness of lorazepam in the relief of breathlessness was tested in a randomised, double‐blind, placebo‐controlled, cross‐over study of 26 participants with advanced cancer in an in‐ and outpatient setting (single‐centre). Seventeen participants completed the study and were included in the analysis. The study tested lorazepam 0.5 mg twice daily orally over five days with a two‐day wash‐out period. A visual analogue scale (VAS) (0 to 100) was used to measure breathlessness as primary outcome (responding to three questions: 1. breathlessness in general over the last 24 hours (summary); 2. breathlessness at its best over the last 24 hours; and 3. breathlessness at its worst over the last 24 hours). Secondary outcomes were anxiety and depression (measured on the Hospital Anxiety and Depression Scale (HADS)) and adverse effects after the treatment.
Man 1986: a double‐blind, placebo‐controlled, cross‐over RCT of 29 participants with advanced but clinically stable COPD in an outpatient setting assessed the efficacy and safety of alprazolam in relieving breathlessness. The analysis included 24 participants, and five participants dropped out. The study compared the effect of alprazolam 1.0 mg/day orally to placebo before and after one week of treatment (with a one‐week wash‐out period after cross‐over). Breathlessness was measured either by Grade of Dyspnoea with 5 (breathlessness at rest) to 2 (able to keep up with people of similar age on level, but not on hills and stairs) to 1 (other than 2 to 5), as well as with a Dyspnoea Scoring (VAS 0 to 10) at rest and during exercise (bicycle ergometer). The study measured adverse effects, attrition, and a 12‐minute walking test as additional outcomes.
Navigante 2006: a single‐blind RCT with a parallel design assessed the role of midazolam in the alleviation of severe breathlessness during the last week of life of 101 participants with advanced cancer. The investigators conducted a three‐arm trial in a hospital setting comparing morphine only (10 mg/day), midazolam only (20 mg/day), and the combination of morphine plus midazolam (10 plus 20 mg/day), with a treatment duration of 48 hours and subcutaneous administration. The dose was adjusted if the participant was not morphine naive (+25% on top of daily subcutaneous equivalent dose of morphine). Rescue medication was provided with 5 mg midazolam in the morphine group and 2.5 mg morphine in the midazolam and midazolam plus morphine group. Breathlessness was the primary outcome, assessed in four different ways:
Breathlessness intensity with the modified Borg scale (0 to 10) before the intervention and 24 and 48 hours after intervention;
Percentage of participants with breathlessness relief (yes/no) after 24 and 48 hours and no breathlessness relief after 48 hours;
Numbers of episodes of breathlessness ('breakthrough dyspnoea' = numbers of rescue medication) per participant after 24 and 48 hours; and
Percentage of participants with episodic breathlessness after 24 and 48 hours.
Other outcomes were adverse effects (total of clinical relevance and different adverse effects in grading 1 to 3), anxiety, and attrition.
Navigante 2010: a single‐blind RCT with a parallel design was undertaken with 63 participants with advanced cancer and breathlessness in a single‐centre outpatient clinic (two participants dropped out after randomisation). The aim was to assess the efficacy of oral midazolam for the relief of breathlessness in comparison to oral morphine. A fast titration phase (FTP) was used to determine the effective dose (effect of at least 50% reduction of breathlessness) for the follow‐up phase (FUP) starting with midazolam 2 mg every four hours (excluding sleeping time) and morphine 3 mg every four hours (excluding sleeping time) with incremental steps of 25% of the preceding dose every 30 minutes. The duration of treatment in the FUP was five days with daily assessment of the primary endpoint breathlessness intensity (numeric rating scale (NRS), 0 to 10) and the secondary outcomes number of episodes of breathlessness per day, descriptors the participant used for breathlessness, and the number of adverse effects. The study reported dose reduction, therapeutic failure, and additional procedures (for example antibiotics).
Shivaram 1989: a double‐blind, randomised, placebo‐controlled, cross‐over study of 12 participants with advanced COPD with anxiety (non‐psychiatric stage) in an unknown setting (probably hospital) assessed the effect of alprazolam to relieve breathlessness. Four participants dropped out and were excluded, leaving eight participants for analysis. The study compared the effect of oral alprazolam 0.75 mg/day to placebo at baseline and after two weeks of treatment (with two days wash‐out). Breathlessness was measured on a modified Borg scale (0 to 10). No other outcomes except adverse effects were assessed.
Stege 2010: a double‐blind, randomised, placebo‐controlled, cross‐over study of 17 participants with COPD (Global Initiative for Obstructive Lung Disease (GOLD) stages 3 or 4) with insomnia in an outpatient centre of a respiratory medicine hospital department. The aim of the study was to assess whether temazepam (10 mg daily over one week) influences indices of breathing and gas exchanges during sleep; the effect on dyspnoea sensation (assessed with 10‐point VAS) and other outcomes were secondary objectives. Three participants dropped out, leaving 14 participants for analysis.
Woodcock 1981: a double‐blind, placebo‐controlled, cross‐over RCT of 18 participants with severe COPD compared the effect of oral diazepam (25 mg/day) and promethazine (125 mg/day) on breathlessness. Three participants dropped out, leaving 15 participants for analysis. Breathlessness was the main outcome, assessed as 'daily dyspnoea' by VAS (0 to 10) and 'dyspnoea grade' 5 (breathlessness at rest) to 2 (able to keep up with people of similar age on level, but not on hills and stairs) to 1 (other than 2 to 5), after each intervention in an outpatient setting with a two‐week treatment duration (no wash‐out period). The study assessed adverse effects, dose modification, anxiety, depression, a 12‐minute walking test, treadmill, and ergometer measurement.
Study design
All studies were RCTs (Eimer 1985; Harrison (unpublished); Man 1986; Navigante 2006; Navigante 2010; Shivaram 1989; Woodcock 1981). Besides Navigante 2006 and Navigante 2010, who used a single‐blind, parallel, and morphine‐controlled design, all other studies were double‐blind, cross‐over, and placebo‐controlled (Eimer 1985; Harrison (unpublished); Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981).
Sample size
In general, the sample size was small (between five and 29 participants), except for two studies of Navigante and colleagues (Navigante 2006 with 101 participants and Navigante 2010 with 63 participants). One study finished data collection without dropouts (Eimer 1985). Five studies had between three and nine dropouts (dropout/N: 9/26, 5/29, 4/12, 3/17, 3/18) (Harrison (unpublished); Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981); one study lost two of 63 participants (Navigante 2010); and one study lost 31 participants due to death during the study (Navigante 2006), which were always excluded from the analysis. Four studies provided a power calculation (Harrison (unpublished); Navigante 2010; Shivaram 1989;Stege 2010), and three of them reached an appropriate number of participants (Navigante 2010; Shivaram 1989; Stege 2010). None of the studies presented an intention‐to‐treat analysis. A total of 214 participants were analysed, including 33 participants of the third intervention arm of the parallel‐designed study from Navigante 2006.
Participants
Three studies included participants with cancer (Harrison (unpublished); Navigante 2006; Navigante 2010), and five studies included participants with advanced COPD (Eimer 1985; Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981).
Outcomes
Breathlessness intensity was measured mainly on a VAS/NRS (Harrison (unpublished); Man 1986; Navigante 2010; Stege 2010; Woodcock 1981), a modified Borg scale (Navigante 2006; Shivaram 1989), and a Dyspnoea Grade 1 to 6 scale or 1 to 5 scale (Eimer 1985; Man 1986; Woodcock 1981). The majority of studies measured breathlessness at rest (Eimer 1985; Harrison (unpublished); Navigante 2006; Navigante 2010; Shivaram 1989); only three studies also assessed breathlessness on exercise (Eimer 1985; Man 1986; Woodcock 1981). Two studies assessed episodic breathlessness (Navigante 2006; Navigante 2010), and one study did not further specify breathlessness (Stege 2010). Other outcomes were anxiety (Harrison (unpublished); Navigante 2006; Woodcock 1981), depression (Harrison (unpublished); Woodcock 1981), adverse effects (all), walking tests (Eimer 1985; Man 1986; Woodcock 1981), and attrition (all).
Intervention
Two studies tested alprazolam, one with 1.0 mg/day (Man 1986), and one with 0.75 mg/day (Shivaram 1989). One study tested 25 mg/day diazepam within a three‐arm design with 125 mg/day promethazine compared to placebo (Woodcock 1981). Navigante 2006 applied midazolam 20 mg/day only and in combination with morphine 10 mg/day compared to morphine 10 mg/day only within a three‐arm design. Navigante 2010 studied oral midazolam 8 mg/day versus morphine 12 mg/day (both starting doses). Harrison (unpublished) examined lorazepam 1 mg/day. Eimer 1985 tested two different doses of clorazepate, 7.5 and 22.5 mg/day, compared to placebo; however, due to intolerable adverse effects, the 22.5 mg arm was excluded from analysis. Stege 2010 examined the effect of temazepam 10 mg/day orally. The treatment durations ranged between 48 hours, in Navigante 2006, and two weeks, in Eimer 1985, Shivaram 1989, and Woodcock 1981.
Excluded studies
See Characteristics of excluded studies.
We excluded 90 out of 97 full‐text publications because they did not meet the inclusion criteria (30 'no subjective measurement of breathlessness'; 24 'reviews'; 15 'different drugs'; six 'different disease/healthy participant'; three 'combination of drugs'; six 'different study design', two 'guidelines', two 'editorials', and two studies 'failed in recruiting any participants'. We excluded a substantial number of studies because of a lack of subjective measurement of breathlessness, mainly older studies from the 1970s and 1980s that studied benzodiazepines in relation to spirometry, functional tests, or blood tests. Among them is the most cited paper in this area (Mitchell‐Heggs 1980a), which we had to exclude because of a lack of subjective measurement of breathlessness. Although the authors mentioned breathlessness as an outcome, we could determine no subjective measure, either in the text, tables, or graphs. Other reasons for excluding this study were the lack of systematic or standardised design and absence of control group. Four excluded studies used benzodiazepines only in combination with other drugs (Clemens 2011; Lichterfeld 1967; Navigante 1997; Navigante 2003), thus a separate assessment of the drug effect was not possible. Three excluded studies assessed breathlessness in healthy people (comparing diazepam, promethazine, and placebo) (Jones 1985; Stark 1981a; Stark 1981b). One study compared diazepam versus flupenthixol in people with psychosomatic disorders (breathlessness was only one of 12 associated symptoms and was not the primary outcome) (Jokinen 1984). Although we expected to find a substantial number of observational studies, there were only a few thematically relevant non‐controlled or retrospective studies, which we had to exclude. Among them was the case report from Greene 1989, a non‐controlled phase II study (Allcroft 2013), the above‐mentioned non‐controlled study on the combined use of opioids and benzodiazepines (Clemens 2011), a retrospective study on the management of dyspnoea in hospitalised palliative‐care patients (Gomutbutra 2013), and a prospective observational study on the patterns of benzodiazepine use among older adults with COPD in Canada (Vozoris 2013). We excluded the study Hosaka 1996 because four of the 22 participants did not meet our inclusion criteria regarding the underlying disease (asthma, tuberculosis), and the level of airways obstruction was above our inclusion criteria (mean FEV1 63% and FEV1/FVC 1.06), indicating that the disease stage was not as advanced as required for this review. This non‐randomised, placebo‐controlled, double‐blind, cross‐over trial studied the use of diazepam 10 to 12 mg/day over four weeks in 22 people with chronic respiratory insufficiency (mainly COPD and fibrosis) who received home oxygen therapy. The study, which was published in Japanese (abstract in English), showed a statistically significant improvement in breathlessness at rest in the diazepam group and a statistically non‐significant worsening in the placebo group, but the levels of breathlessness at baseline were different between the groups.
In the search for the update, we excluded the potentially relevant studies of Daubert 2014 and NCT01687751 (identified through register search), because no participants could be recruited in these trials and no future results are expected (personal communication with Neil Hilliard, Eliza Daubert and Scott Bolesta in June 2015).
Studies awaiting classification
In the update, we identified one potentially relevant study with the database search (Hart 2012), and one potentially relevant study in the clinical trial registries (Hardy 2016) (see Characteristics of studies awaiting classification).
Hardy 2016 is a randomised, placebo‐controlled, cross‐over trial testing the hypothesis that intranasal midazolam is superior to placebo for the palliation of dyspnoea in people with optimally treated life‐limiting disease. A sample size of 200 participants was planned but the study was terminated after interim analysis of 75 participants showing no difference between intervention arms. The primary outcome was breathlessness intensity at 15 min compared to baseline. There was no difference at any time points in breathlessness scores between arms. This study concludes that intranasal midazolam had no clinical benefit over intranasal placebo for the control of breathlessness.
The aim of the randomised, double‐blind, double‐dummy, placebo‐controlled pilot study of Hart 2012 was to compare the efficacy of intranasal midazolam with that of oral lorazepam tablets in relieving dyspnoea in people with severe respiratory disease. The study included 30 participants, and the findings showed a "worthwhile improvement in symptom control of dyspnoea and quality of life" (Hart 2012). Unfortunately, this study was only published as a conference abstract, and we did not receive data from the authors at time of the submission (personal contact with Dr. Hart in August 2016).
Risk of bias in included studies
See Characteristics of included studies, the corresponding 'Risk of bias' tables, Figure 2, and Figure 3.
2.
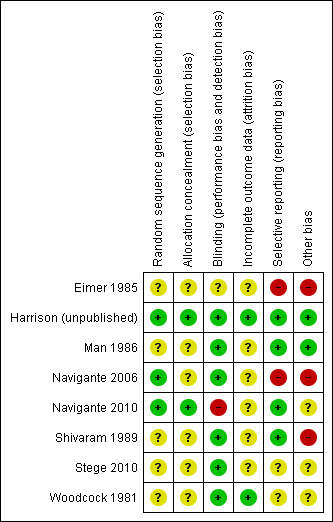
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
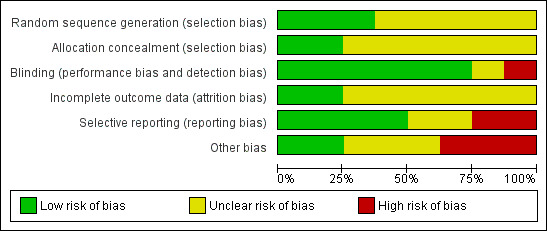
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
In the update, seven studies, Harrison (unpublished), Man 1986, Navigante 2006, Navigante 2010, Shivaram 1989, Stege 2010, and Woodcock 1981, showed high quality on the Edwards Method Score (Edwards 2001; Edwards 2003). Only one study had a high risk of bias due to lack of presented data and information and inappropriate presentation of data in figures (Eimer 1985). We therefore did not include this study in the meta‐analysis.
Allocation
All studies were RCTs. Four studies did not mention the denominator population that was screened for participation (Man 1986; Navigante 2010; Shivaram 1989; Woodcock 1981). There was a substantial gender imbalance due to more males than females participating in most studies (exact total numbers are not countable because of a different presentation of data for gender of randomised or analysed participants); only Navigante 2006 included more females than males. One study did not mention the gender distribution (Navigante 2010).
Blinding
Two studies were single‐blinded (Navigante 2006; Navigante 2010); one study did not define blinding appropriately, but was stated to be double‐blind (Eimer 1985); and the rest used a double‐blind design.
Incomplete outcome data
Seven studies with dropouts excluded them from the analysis (Harrison (unpublished); Man 1986; Navigante 2006; Navigante 2010; Shivaram 1989; Stege 2010; Woodcock 1981). None of the studies mentioned missing data or the handling of missing data.
Selective reporting
Woodcock 1981 stated that there was no effect of diazepam in the relief of breathlessness. However, a beneficial effect could be seen for diazepam, although it was not statistically significant (P = 0.06). Stege 2010 stated that no respiratory adverse events had occurred, however there was no information on other than respiratory adverse events.
Other potential sources of bias
Seven studies were published in indexed, peer‐reviewed journals (Eimer 1985; Man 1986; Navigante 2006; Navigante 2010Shivaram 1989; Stege 2010; Woodcock 1981), and one study was unpublished (Harrison (unpublished)). The author of the unpublished study sent us all the original data at time of the original review and was very helpful and supportive (Harrison (unpublished)). One study did not mention a wash‐out period between intervention and control phases (cross‐over design) (Woodcock 1981). However, sensitivity analysis showed no difference regarding the results compared to studies including a wash‐out period. Navigante 2006 used the comparative drug (midazolam plus morphine) for rescue medication. Since this combination of midazolam and morphine could have been used in all three treatment arms, a confident comparison or distinction between midazolam and morphine was not possible. Navigante 2010 allowed the use of treatments with potential impact on breathlessness besides the intervention and the control in the study (for example antibiotics, aspiration of pleural effusion, radiotherapy).
Effects of interventions
We included eight studies (eight RCTs; six cross‐over and two parallel designs; five COPD and three cancer studies) in the review with a total of 214 participants analysed (COPD, N = 66; cancer, N = 148). We have summarised the main findings of each of the studies below (see also the Characteristics of included studies tables). We carried out the meta‐analysis separately for placebo‐controlled studies, Harrison (unpublished), Man 1986, Shivaram 1989, Stege 2010, and Woodcock 1981, and morphine‐controlled studies (Navigante 2006; Navigante 2010).
Benzodiazepines for breathlessness in chronic obstructive pulmonary disease (COPD)
Five participants with advanced COPD were examined in a randomised, cross‐over trial. Breathlessness was measured at rest and after a 12‐minute walking test, after two weeks of treatment with clorazepate 7.5 mg/day compared to placebo. All participants completed the study. The change scores from baseline to postintervention showed no statistically significant difference between the intervention and the control group. The results were presented only in a figure without exact data, which was difficult to interpret.
Twenty‐nine participants with advanced COPD were randomised in a cross‐over design to alprazolam 1.0 mg/day or placebo over one week, but only 24 participants completed the study (five dropouts were excluded from analysis). There was no statistically significant effect of alprazolam versus placebo compared to baseline in the relief of breathlessness at rest and on exercise. Furthermore, no difference between the intervention and the control group was observed after treatment.
Twelve participants with advanced COPD were randomised (cross‐over), of which only eight participants completed the study (four dropouts were excluded from analysis). There was no improvement of breathlessness at rest with alprazolam 0.75 mg/day compared to baseline after two weeks, but there was an improvement with placebo (not statistically significant). Furthermore, no difference was observed after treatment between the intervention and the control group.
Seventeen participants with advanced COPD who experienced insomnia were randomised (cross‐over), of which 14 were analysed, excluding three dropouts. There was no difference of breathlessness intensity with temazepam 10 mg/day for one week compared to placebo.
Eighteen participants with advanced COPD were randomised in a cross‐over design to determine the effect of diazepam 25 mg/day in the relief of breathlessness compared to placebo and promethazine 125 mg/day (third arm). There were three dropouts, and 15 participants completed the study and were included in the analysis. Diazepam produced a statistically non‐significant effect in the relief of breathlessness at rest compared to placebo after two weeks. There was also no difference in breathlessness on exercise compared to placebo.
Meta‐analysis and summary
We included four out of five cross‐over studies (see Risk of bias in included studies) with a total of 61 participants with COPD (122 observations) in the meta‐analysis (Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981) (Analysis 2.1), comparing post‐treatment measures between intervention and control groups. We observed no statistically significant effect of alprazolam, diazepam, or temazepam with a standardised mean difference (SMD) estimated as ‐0.12 (95% confidence interval (CI) ‐0.52 to 0.29). The overall heterogeneity of effects was low (I2 = 18%).
2.1. Analysis.
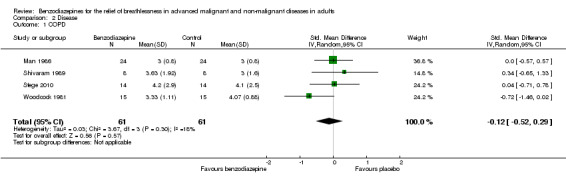
Comparison 2 Disease, Outcome 1 COPD.
Overall, the analysis (five studies) and meta‐analysis (four studies) with 66 and 61 participants, respectively, showed no statistically significant effect of four different benzodiazepines (clorazepate, diazepam, alprazolam, temazepam) in the relief of breathlessness in people with advanced COPD. One study showed a slight but statistically non‐significant advantage of diazepam compared to placebo (Woodcock 1981).
Benzodiazepines for breathlessness in cancer
Twenty‐six participants with advanced cancer were randomised (cross‐over), but only 17 participants completed the study and were included in the analysis. Lorazepam 1.0 mg/day had no statistically significant effect on breathlessness at rest compared to baseline and to placebo after five days of treatment. The result was similar for the overall level of breathlessness, breathlessness at its best, and breathlessness at its worst.
One hundred and one participants with terminal cancer were randomised in a three‐arm study with a parallel design that compared midazolam 20 mg/day, morphine 10 mg/day, and midazolam 20 mg/day plus morphine 10 mg/day after 24 and 48 hours of treatment (plus rescue doses). Thirty‐one participants died during the study after receiving the treatment (no difference between the study groups). Each treatment arm showed a statistically significant reduction of breathlessness compared to baseline, but without any difference when comparing the three arms after 48 hours. After 24 hours, morphine only and the combination of both drugs were slightly better than midazolam only. The highest percentage of participants who experienced a relief of breathlessness (92%) was in the midazolam plus morphine group after 24 hours; the lowest percentage was in the midazolam‐only group (46%). The midazolam group reported the highest percentage of participants with persistent and uncontrolled breathlessness after 48 hours (26%), the midazolam plus morphine group the lowest.
For meta‐analysis, we used the assessment after 48 hours unless stated otherwise.
Sixty‐three participants with advanced cancer were randomised in a parallel design to compare midazolam 8 mg/day and morphine 12 mg/day (control) over five days (starting doses with titration phase and rescue doses). Sixty‐one participants completed the study, with one drop‐out in each group (31 participants in the midazolam group and 30 participants in the morphine group). Both treatments showed a statistically significant reduction in breathlessness intensity after two, three, four, and five days compared to baseline. Midazolam reduced breathlessness significantly better than morphine when comparing the endpoints after all treatment days. Twenty‐one participants treated with midazolam reached a 50% reduction of breathlessness after the starting dose compared to only 11 participants in the morphine group (P = 0.023). Therapeutic failure was observed in 20% of participants in the morphine group compared to none in the midazolam group. A dose reduction was necessary in one participant in the midazolam group and in two participants in the morphine group due to excessive somnolence.
For meta‐analysis, we used the assessment after five days unless stated otherwise.
Meta‐analysis and summary
We could include all three studies of people with cancer in the meta‐analysis, but analysed the placebo‐controlled and morphine‐controlled studies separately. The placebo‐controlled study found no statistically significant effect with a SMD of ‐0.06 (95% CI ‐0.73 to 0.62) (Harrison (unpublished)) (Analysis 2.2). Pooling of the two morphine‐controlled studies also showed no statistically significant effect, with a SMD of ‐0.68 (95% CI ‐2.21 to 0.84) (Navigante 2006; Navigante 2010) (Analysis 2.3). One study demonstrated a statistically significant effect of midazolam compared to morphine (Navigante 2010), but this result was contrary to a similar study by the same research group where the morphine group showed a slightly better improvement of breathlessness than the midazolam group (Navigante 2006). We found no difference when comparing midazolam with midazolam plus morphine (third study arm in Navigante 2006).
2.2. Analysis.
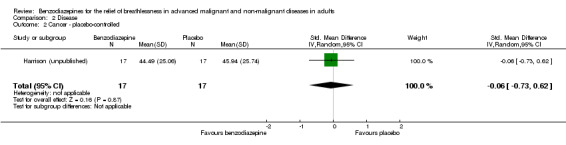
Comparison 2 Disease, Outcome 2 Cancer ‐ placebo‐controlled.
2.3. Analysis.
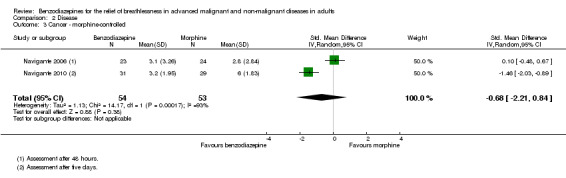
Comparison 2 Disease, Outcome 3 Cancer ‐ morphine‐controlled.
Overall, we found no statistically significant effect. Due to the high level of heterogeneity in the designs of the three studies (control group, study design, stage of disease, benzodiazepine, dose, and route of application), the meta‐analyses of all three studies should be interpreted with caution. There are conflicting results in the comparison of midazolam to morphine based on two studies from the same research group.
Benzodiazepines for the prevention of episodic breathlessness in cancer
The proportion of participants with episodic breathlessness was lower in the midazolam plus morphine group (21.2/24.0%) than in the morphine and midazolam groups after 24 and 48 hours, and highest in the midazolam group (36.4/38.5%). The median number of episodes of breathlessness per participant after 24 and 48 hours was higher in the morphine‐only group (two episodes) than in the midazolam‐only and midazolam plus morphine groups (one episode).
The proportion of participants with episodic breathlessness was lower in the midazolam group compared to the morphine group and reached a statistically significant level of P = 0.035 at three days, P = 0.034 at four days, and P < 0.001 at five days of treatment.
Meta‐analysis and summary
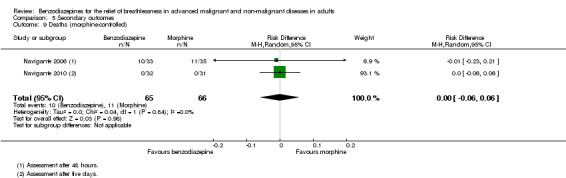
We could include both studies that examined episodic breathlessness in the meta‐analysis, comparing midazolam with morphine in 116 and 108 participants with cancer, respectively, after 24 and 48 hours (Navigante 2006; Navigante 2010). For the second study (Navigante 2010), we calculated the effect after 48 hours using the measurement at the third day when asked about episodic breathlessness on the day before (that is 48 hours). Overall, we found no statistically significant effect after 48 hours with a risk ratio of 0.76 (95% CI 0.53 to 1.09; 108 participants) (Analysis 4.3).
4.3. Analysis.
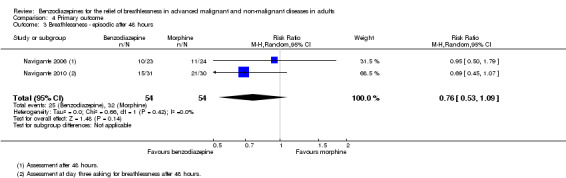
Comparison 4 Primary outcome, Outcome 3 Breathlessness ‐ episodic after 48 hours.
Although one study demonstrated a statistically significant positive effect with midazolam after three, four, and five days (Navigante 2010), the previous study from the same research group observed no difference between midazolam and morphine in preventing episodic breathlessness.
Overall ‐ benzodiazepines in breathlessness
Meta‐analysis and summary
We excluded one study from the meta‐analysis due to a lack of methodological quality and lack of data (see Risk of bias in included studies) (Eimer 1985). Therefore, we included seven studies in the overall meta‐analysis of the review update and analysed placebo‐controlled and morphine‐controlled studies separately. Pooling of the placebo‐controlled studies showed no significant effect with a SMD of ‐0.10 (95% CI ‐0.42 to 0.21) (Harrison (unpublished); Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981) (Analysis 1.1; Figure 4). The meta‐analysis of all placebo‐controlled studies included 156 observations equating to 78 participants. Pooling of the morphine‐controlled studies with 107 participants also showed no statistically significant effect with a SMD of ‐0.68 (95% CI ‐2.21 to 0.84) (Navigante 2006; Navigante 2010) (Analysis 1.2; Figure 5). Overall, there was no statistically significant beneficial effect of benzodiazepines in the relief of breathlessness at rest. These results must be interpreted with caution due to the presence of heterogeneity among the seven included studies regarding such components as disease group, control group, and benzodiazepine, among others.
1.1. Analysis.
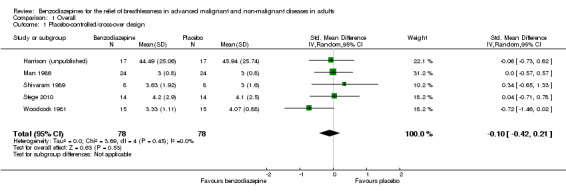
Comparison 1 Overall, Outcome 1 Placebo‐controlled/cross‐over design.
4.
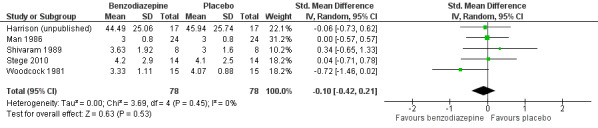
Forest plot of comparison: 1 Overall, outcome: 1.1 Placebo‐controlled/cross‐over design.
1.2. Analysis.
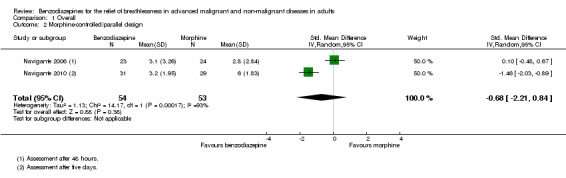
Comparison 1 Overall, Outcome 2 Morphine‐controlled/parallel design.
5.
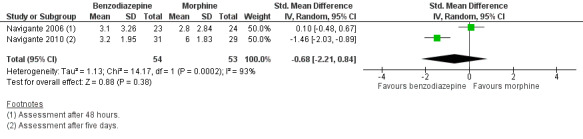
Forest plot of comparison: 1 Overall, outcome: 1.2 Morphine‐controlled/parallel design.
In the sensitivity analysis, a comparison to baseline of studies that presented baseline and after‐treatment measures demonstrated a positive effect for benzodiazepines, but this did not reach statistical significance (data not shown). However, changes from baseline have a higher risk of confounders (for example regression to the mean) compared to after‐treatment measures and should be avoided (Higgins 2011). Two studies looked at breathlessness on exercise (Man 1986; Woodcock 1981), but we could not include these in the meta‐analysis due to a lack of appropriate data (data presented only in graphs).
In summary, all but one study showed no beneficial effect of benzodiazepines (Eimer 1985; Harrison (unpublished); Man 1986; Navigante 2006; Shivaram 1989; Stege 2010). Only one study showed a statistically significant effect of midazolam compared to morphine (Navigante 2010), but as mentioned above this result was in contrast to a previous study by the same research group (Navigante 2006). One study demonstrated a beneficial effect of diazepam, but this was not statistically significant (Woodcock 1981).
Secondary outcomes
Anxiety
Four out of seven studies measured anxiety with different scales (Eimer 1985; Harrison (unpublished); Navigante 2006; Woodcock 1981). Benzodiazepines did not reduce anxiety, either as a change from baseline or compared to the control group after treatment.
Depression
Three studies examining depression found no statistically significant difference between the intervention and the placebo group (Eimer 1985; Harrison (unpublished); Woodcock 1981).
Adverse effects
All studies assessed adverse effects, but Stege 2010 only reported on respiratory adverse effects (none occurred). Two studies observed no adverse effects (Eimer 1985; Shivaram 1989). Harrison (unpublished) described three adverse effects in the intervention group that lead to withdrawal compared to one case in the placebo group. Man 1986 and Woodcock 1981 observed significantly more adverse effects (mainly drowsiness) in the benzodiazepine group compared to placebo. Navigante 2006 reported more adverse effects (mainly somnolence) in the morphine group (19/45) compared to midazolam (15/45). Surprisingly, the fewest adverse effects were reported for the combination group (11/45; third arm with midazolam plus morphine). The authors defined an adverse effect as clinically relevant with Grade 2 to 4 (Grade 1 mild, 2 moderate, 3 severe, 4 life‐threatening ‐ but observed no Grade 4) and found the highest number in the morphine group (10/16) compared to midazolam (3/16) and midazolam plus morphine (3/16). These results were confirmed in the following study (Navigante 2010), which found significantly more adverse effects (mainly somnolence) in the morphine group compared to the midazolam group.
Regarding adverse effects, we observed a beneficial but not statistically significant effect in the control group when studies used placebo as a control (Analysis 5.1, Figure 6). Studies comparing midazolam with morphine showed a statistically significant favourable effect for midazolam (Analysis 5.2). Drowsiness and somnolence were mainly reported with a statistically significant difference between intervention and control group when placebo was used as a control (benzodiazepines caused more drowsiness or somnolence) (Analysis 5.4).
5.1. Analysis.
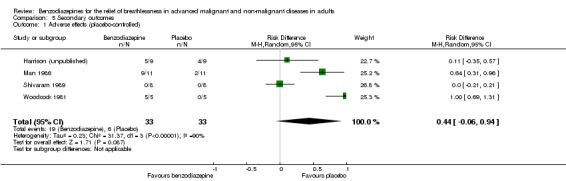
Comparison 5 Secondary outcomes, Outcome 1 Adverse effects (placebo‐controlled).
6.
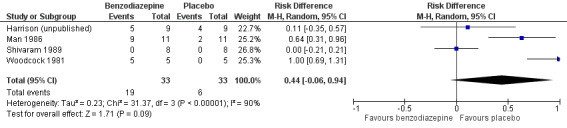
Forest plot of comparison: 5 Secondary outcomes, outcome: 5.1 Adverse effects (placebo‐controlled).
5.2. Analysis.
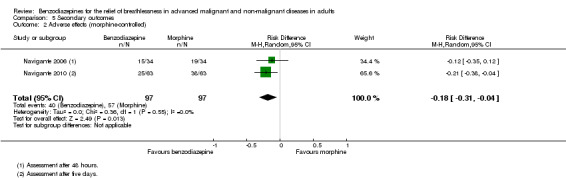
Comparison 5 Secondary outcomes, Outcome 2 Adverse effects (morphine‐controlled).
5.4. Analysis.
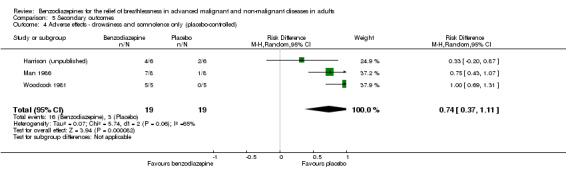
Comparison 5 Secondary outcomes, Outcome 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled).
Exercise tolerance
Only three out of seven studies looked at breathlessness on exercise. Eimer 1985 used a 12‐minute walking distance test and Man 1986 used a 12‐minute walking distance test and bicycle exercise. They did not find any difference between benzodiazepines and placebo regarding exercise tolerance. However, Woodcock 1981 demonstrated a significant impairment in walking distance after 12 minutes in the intervention group compared to placebo, and a non‐significant decline in time to exhaustion on treadmill and workload on bicycle ergometer.
Quality of life
None of the included studies studied quality of life.
Attrition
Regarding attrition, there was no difference between the intervention and control groups, either for the placebo‐controlled studies or the morphine‐controlled studies. Only one study reported results in favour of the intervention group, with four dropouts in the placebo group mainly due to increasing breathlessness and drowsiness (Shivaram 1989). One study had no attrition, either in the intervention or in the control group (Eimer 1985). One study reported three dropouts: one participant was excluded from the intervention group due to an exacerbation of COPD during the study, one participant was excluded due to obstructive sleep apnoea‐hypopnoea syndrome after baseline polysomnography, and one withdrew due to burden of the measurements in the control group (Stege 2010). One study reported five dropouts (Man 1986); one dropout was assigned to the placebo group (unknown adverse effect), and the other four dropouts were missing appointments without assignment to one group. The Harrison (unpublished) study reported twice as many dropouts in the placebo group (six) as in the intervention group (three). Alternatively, Woodcock 1981 counted twice as many dropouts in the intervention group (two) as in the placebo group (one). The reason for attrition was mainly drowsiness, which occurred in the intervention group in both studies. Both dropouts in Navigante 2010 were missing follow‐ups. Navigante 2006 had a very high attrition rate, and all dropouts were due to death (31 deaths in all three study arms with a total of 101 participants), but without a difference when comparing the three arms. We could find no difference between intervention and control group regarding deaths in all studies (Analysis 5.8; Analysis 5.9).
5.8. Analysis.
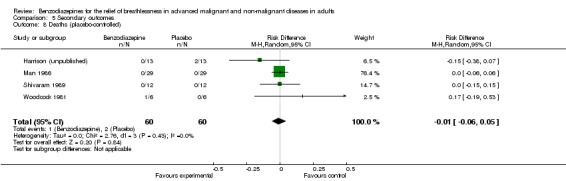
Comparison 5 Secondary outcomes, Outcome 8 Deaths (placebo‐controlled).
5.9. Analysis.
Comparison 5 Secondary outcomes, Outcome 9 Deaths (morphine‐controlled).
Others
Blood gases
Only one study reported a slightly but almost statistically significant difference (P = 0.05) in blood gases between alprazolam and placebo (PaO2 at rest higher and PaCO2 after exercise lower with placebo) (Man 1986). All other studies that measured oxygen saturation, PaO2, or PaCO2 found no significant change from baseline or between intervention and control group (Eimer 1985; Navigante 2006; Navigante 2010; Shivaram 1989; Stege 2010; Woodcock 1981).
Spirometric tests
Only one study found a slightly but almost statistically significant difference (P = 0.05) in spirometric tests with higher levels for FEV1 (forced expiratory volume in one second), TLC (total lung capacity), and FRC (functional residual capacity) in the placebo group (Man 1986). Three other studies measured the functional lung capacity, but did not find any significant change from baseline or between intervention and control group (Eimer 1985; Shivaram 1989; Woodcock 1981).
Discussion
Summary of main results
Benzodiazepines are widely used drugs in the treatment of breathlessness, but very few studies have evaluated their effectiveness. On the basis of eight RCTs including 214 participants, we conclude that there is no evidence for a beneficial effect of benzodiazepines for the relief of breathlessness at rest in people with advanced cancer or chronic obstructive pulmonary disease (COPD). However, this conclusion is based on a small number of studies with a limited number of participants, heterogeneity among included studies, and some inconsistency across the studies. Furthermore, we could observe no statistically significant effect in the prevention of episodic breathlessness in people with cancer. Sensitivity analysis demonstrated no statistically significant differences regarding the type of benzodiazepine, dose, route and frequency of delivery, duration of treatment, or type of control.
Overall completeness and applicability of evidence
The study from Navigante 2010 is the only RCT that showed a statistically significant beneficial effect, although it used morphine as a control. This result is surprising, as morphine has been shown to be effective in the relief of breathlessness (Jennings 2001). Furthermore, a study from the same research group of people with terminal cancer two years earlier demonstrated a contrary result, with an advantage of morphine over midazolam, and best results for the combination of the two drugs (Navigante 2006). However, the results of the earlier study must be interpreted with caution as there were some methodological difficulties: the authors studied people with terminal cancer and observed a very high attrition rate due to death (31/101); and they allowed rescue medication during the study, therefore all three treatment arms might have included both drugs and a valid differentiation of the effect was not possible without uncertainty (Navigante 2006). Further studies are needed to examine the comparison between morphine and midazolam and to verify the results of Navigante 2010.
Episodic breathlessness was only studied in people with cancer, and the focus was on preventing breakthrough dyspnoea (no evaluation of the effect on relief of episodic breathlessness) (Navigante 2006; Navigante 2010). The extent of the beneficial preventative effect of midazolam compared to morphine was larger after 48 hours than after 24 hours, but statistically non‐significant at both times. RCTs assessing the treatment (not prevention) of episodic breathlessness with benzodiazepines are still missing.
Most studies observed adverse effects. Drowsiness and somnolence were mainly reported with a significantly higher occurrence in the benzodiazepine group when a placebo was the control. In contrast to the other studies, Shivaram 1989 reported attrition only in the placebo group, due to increasing breathlessness and drowsiness. We excluded these three cases from analysis. It could be argued that the occurrence of increasing breathlessness only in the placebo group favours the intervention group in the relief of breathlessness, and this might have changed their conclusion. Shivaram 1989 argued that this could be a suggestive effect, as participants were told that the treatment might cause increasing breathlessness. However, as only 12 participants were included, it is not possible to judge if this is a random effect.
There was no difference between the intervention and control groups in respect to attrition and deaths, either for the placebo‐controlled studies or for the morphine‐controlled studies. However, the reporting of dropouts in cross‐over studies was not always sufficient to assess when the dropout occurred (first or second period of the study), in order to calculate the attrition (Eimer 1985; Harrison (unpublished); Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981). Given the small numbers of dropouts, the potential miscalculation is likely to be small for the present cross‐over studies. However, attrition must still be interpreted with caution. Navigante 2006 observed a high attrition rate due to deaths (31/101) without any difference between the three study arms. As mentioned before, all three treatment arms allowed a combination of midazolam and morphine, therefore the high death rate could not been attributed to a single drug. The authors argued that most of the deaths were caused by the underlying advanced disease. As they studied people with terminal cancer and a life expectancy of less than a week, this seems likely. However, a relation between treatment and death could not be excluded entirely because of the relatively high doses of midazolam. There is some evidence from a large cohort study that benzodiazepines alone might increase mortality, but concurrent benzodiazepines and opioids do not (Ekstrom 2014).
Different types of benzodiazepines were tested as well as different doses, long‐ and short‐acting drugs, and different durations of treatment and modes of administration. However, we could find no differences when conducting sensitivity analysis regarding all these criteria. Furthermore, different comparators were used: five studies used placebo (Harrison (unpublished); Man 1986; Shivaram 1989; Stege 2010; Woodcock 1981), and two studies used morphine as a control treatment (Navigante 2006; Navigante 2010). We therefore conducted separate meta‐analyses for each group with the same control treatment.
The measurement tools for examining breathlessness in all but one study were validated and frequently used (Eimer 1985).
Quality of the evidence
Overall, this review analysed 214 participants in eight studies, including 33 participants of the third intervention arm of the parallel‐designed study from Navigante 2006. However, the number of participants in each single study was small (between five and 35 in each comparison group). We intended to evaluate the effect of benzodiazepines in five different disease groups (cancer, COPD, chronic heart failure, motor neurone disease, and idiopathic pulmonary fibrosis), but could only identify studies of people with COPD and cancer. Studies of the other patient groups are also needed. One study identified in the review update included people with different life‐limiting diseases (see Hardy 2016 in Characteristics of studies awaiting classification section).
We judged seven out of eight studies to be of high quality on the Edwards Method Score (15 or more on the scale) (Edwards 2003). To minimise the risk of bias, we excluded one study from the meta‐analysis due to low methodological quality (high risk of bias) based on both the Edwards Method Score and the Cochrane 'Risk of bias' tool (Eimer 1985). However, the single‐blinding process in two studies (Navigante 2006; Navigante 2010), and the exclusion of dropouts from analysis, increased the risk of bias in all studies. The 'Risk of bias' tool also revealed that most studies were at unclear risk regarding allocation concealment and potential attrition bias (see "Risk of bias in included studies") (Figure 3). The Edwards Method Score has previously been used successfully for judging the quality of non‐pharmacological interventions (for example communication sessions and treatments of breathlessness) (Bausewein 2008; Edwards 2003). Our experience of using the Edwards Method Score to assess the quality of a pharmacological intervention in this review was good, however the tool seems to overestimate the quality of studies compared to the Cochrane 'Risk of bias' tool.
Potential biases in the review process
We combined cross‐over trials and studies with a parallel design in the meta‐analysis and treated the cross‐over studies as parallel design. We did this after a critical analysis of all studies, review of the literature (Elbourne 2002; Higgins 2011), discussion with a statistician, and the following judgements:
the cross‐over design was suitable for the targeted research questions;
none of the cross‐over studies showed evidence of a carry‐over effect;
dropouts were excluded from analysis;
there was no evidence for a period effect. This approach can produce a unit‐of‐analysis error.
However, this error leads to a conservative analysis and under‐weighs the cross‐over studies (Higgins 2011). Most cross‐over studies did not show an effect of benzodiazepines in the relief of breathlessness. This conservative analysis therefore supports our conclusion. In addition, sensitivity analysis showed no difference when analysing the cross‐over studies separately (Figure 4).
Two studies presented only median scores for post‐treatment measures because of skewed data (Navigante 2006; Navigante 2010). The mean and standard deviation (SD) was needed to include these studies in the meta‐analysis. Instead of calculating the mean and SD from the median and range, we followed the advice of the statistician of the Cochrane Pain, Palliative and Supportive Care Review Group and obtained the mean and SD from the raw data provided by the author. With this approach we were able to include these studies in the meta‐analysis.
Although we used a broad search strategy, we could have missed some unpublished data, such as PhD or Masters theses. However, we identified two unpublished studies through circular mails and personal contact with authors in the original review (Harrison (unpublished); Navigante 2010) (see Characteristics of ongoing studies).
Agreements and disagreements with other studies or reviews
Three excluded studies assessed breathlessness in healthy participants and could not find a beneficial effect of diazepam (Jones 1985; Stark 1981a; Stark 1981b). The trial from Hosaka 1996 was the only study excluded because the participants were at non‐advanced disease stage (chronic respiratory insufficiency but lung function tests above our inclusion criteria). This study was also the only non‐RCT study, but with a high risk of bias. A statistically significant improvement in the relief of breathlessness was seen in the intervention group in change from baseline. However, baseline data between intervention and control groups were different. Other methodological problems in this study (no wash‐out phase, breathlessness not primary outcome, sample of participants included a few people with potentially curable disease) necessitate careful interpretation of results. Apart from this trial, we identified no study that looked at people with a non‐advanced disease stage. Furthermore, we included only RCTs in order to reduce the risk of bias, but expected some uncontrolled trials in this area. Surprisingly, we could only find one uncontrolled study (Greene 1989). This case study reported a beneficial effect of alprazolam in one participant. One excluded study was a single‐site, open‐label pilot study of clonazepam together with sustained‐release morphine with 10 participants who completed the study showed promising results (safety, feasibility) in order to justify a definitive phase III study (Allcroft 2013).
After little research activity in this field, with a few studies in the 1980s and no studies during the 1990s, the set‐up of four trials during the last conduction of this review possibly offers hope for further studies, which are urgently needed.
Authors' conclusions
Implications for practice.
For the update of this review, we identified one additional study for inclusion, but the conclusions remain unchanged.
There is no evidence for a beneficial effect of benzodiazepines in the relief of breathlessness in people with advanced cancer and COPD. There is a non‐significant beneficial effect, but the overall effect size is small. Benzodiazepines caused more drowsiness as an adverse effect compared to placebo but less compared to morphine. These results justify considering benzodiazepines as second‐ or third‐line treatment, when opioids and non‐pharmacological measures have failed to control breathlessness. Although we included a few low‐ to high‐quality studies in this review, there is still a further need for well‐conducted and adequately powered studies in this field.
There is currently not enough evidence to support the use of benzodiazepines in the prevention of episodic breathlessness in people with cancer. There are no data from controlled trials for the treatment of episodic breathlessness with benzodiazepines.
There are no differences regarding the type of benzodiazepine, dose, mode and frequency of administration, and duration of treatment.
Implications for research.
Although we included a few high‐quality studies in this review, there is still a further need for more well‐conducted and larger studies in this field. Further research should pay attention to the following issues.
Larger studies with more participants to reach a statistically sound conclusion are required.
More attention should be paid to the reporting of methodological details of randomised controlled trials because this is essential for interpretation of the results.
Studies in chronic heart failure (CHF), motor neurone disease (MND), idiopathic pulmonary fibrosis (IPF), and other life‐threatening diseases with breathlessness (e.g. advanced renal failure) are needed.
Treatment of episodic breathlessness with benzodiazepines has not yet been studied in controlled trials.
Benzodiazepines in the relief of breathlessness with panic attacks might be worth studying.
What's new
| Date | Event | Description |
|---|---|---|
| 21 October 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 15 September 2016 | New citation required but conclusions have not changed | We included one new study in the update, but the conclusion did not change. We identified two studies awaiting assessment. |
| 29 August 2016 | New search has been performed | We updated this review to include the results of new search (August 2016). We have added new 'Risk of bias' summary tables and updated contact details. |
| 6 October 2010 | Amended | We have updated contact details. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We are grateful to John Plummer (statistician of the Cochrane Review Group) for his very helpful comments and discussions regarding statistical methods and analysis. We are also grateful to Dr Yoshie Shizusawa, who helped with the extraction of data from the Japanese paper. Many thanks to Dr Fliss Murtagh for comments on an earlier draft of this review. We thank Joanne Abbott from the Cochrane Pain, Palliative and Supportive Care Review Group for her great support with the literature searches for the update.
The following peer referees contributed feedback to initial drafts of the review: Fiona Cramp, Ollie Minton, and Paul Perkins.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies used for the original review
MEDLINE search strategy via OVID
1. exp dyspnea 2. dyspn$.mp. 3. breathing adj3 labour$ 4. breathless$.mp. 5. shortness of breath.mp. 6. breathing difficult$.mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp benzodiazepines 9. benzodiazepine$.mp. 10. adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam 11. 8 or 9 or 10 12. 7 AND 11
EMBASE search strategy via OVID
1. exp DYSPNEA 2. dyspn$.mp. 3. breathing adj3 labour$ 4. breathless$.mp. 5. shortness of breath.mp. 6. breathing difficult$.mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Benzodiazepine Derivative 9. benzodiazepine$.mp. 10. adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam 11. 8 or 9 or 10 12. 7 AND 11
CINAHL search strategy via OVID
1. MH “dyspnea+” 2. dyspn* 3. breathing N3 labour* 4. breathless* 5. shortness of breath 6. breathing difficult* 7. 1 or 2 or 3 or 4 or 5 or 6 8. MH “Anxiety Agents, Benzodiazepine+” 9. benzodiazepine 10. adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam 11. 8 or 9 or 10 12. 7 AND 11
PsycINFO search strategy via OVID
1. exp DYSPNEA 2. dyspn$.mp. 3. breathing adj3 labour$ 4. breathless$.mp. 5. shortness of breath.mp. 6. breathing difficult$.mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp BENZODIAZEPINES 9. benzodiazepine$.mp. 10. adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam 11. 8 or 9 or 10 12. 7 AND 11
CENTRAL search strategy
#1 MeSH descriptor Dyspnea explode all trees #2 dyspn* #3 breathing adj3 labour* #4 breathless* #5 shortness of breath #6 breathing difficult* #7 1 or 2 or 3 or 4 or 5 or 6 #8 exp benzodiazepines #9 benzodiazepine* #10 adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam #11 8 or 9 or 10 #12 7 AND 11
PaPaS Register search strategy
((dyspn* or (breathing AND (laboured or labored)) or breathless* or "shortness of breath" or "breathing difficult*") AND (benzodiazepines or adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam))
Search strategy for Cochrane DSR, ACP Journal Club, DARE, CCTR, CMR, HTA, and NHSEED via OVID
1. dyspn$.mp. 2. breathing adj3 labour$ 3. breathless$.mp. 4. shortness of breath.mp. 5. breathing difficult$.mp. 6. 1 or 2 or 3 or 4 or 5 7. benzodiazepine$.mp. 8. adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam 9. 7 or 8 10. 6 AND 9
Search strategy for Iowa Drug Information System (IDIS) and International Pharmaceutical Abstracts
((benzodiazepines or tetrazepam or diazepam or oxatepam or lorazepam or lormetazepam or clotiazepam or pinazepam or uldazepam or quazepam or temazepam or metaclazepam nordazepam or fludiazepam or flunitrazepam or halazepam or clonazepam or nitrazepam or zolazepam or flurazepam or flutoprazepam or prazepam or clazepam or meclonazepam or fosazepam or midazolam or medazepam or clotiazepam or doxefazepam or premazepam or camazepam or ritazepam or delorazepam or bentazepam or bromazepam) AND ((abnormality, resp & dyspnea) or (apnea, unspecified)))
Appendix 2. Search strategy for the review update 2016
MEDLINE search strategy via OVID (update)
1. exp dyspnea/
2. dyspn$.mp.
3. (breathing adj3 labo?r$).mp.
4. breathless$.mp.
5. (short* adj2 breath).mp.
6. breathing difficult$.mp.
7. or/1‐6
8. exp benzodiazepines/
9. benzodiazepine$.mp.
10. (adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam).mp.
11. or/8‐10
12. 7 and 11
13. (200909* or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016*).ed.
14. 12 and 13
EMBASE search via OVID (update)
1. exp dyspnea/
2. dyspn$.mp.
3. (breathing adj3 labo?r$).mp.
4. breathless$.mp.
5. (short* adj2 breath).mp.
6. breathing difficult$.mp.
7. or/1‐6
8. exp Benzodiazepine Derivative/
9. benzodiazepine$.mp.
10. (adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam).mp.
11. or/8‐10
12. 7 and 11
13. (200909* or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014 or 2015* or 2016*).dd
14. 12 and 13
CENTRAL search strategy via the Cochrane Library (update)
#1 MeSH descriptor: [Dyspnea] explode all trees
#2 dyspn*:ti,ab,kw (Word variations have been searched)
#3 (breathing near/3 labo?r*):ti,ab,kw (Word variations have been searched)
#4 breathless*:ti,ab,kw (Word variations have been searched)
#5 (short* near/2 breath):ti,ab,kw (Word variations have been searched)
#6 breathing difficult*:ti,ab,kw (Word variations have been searched)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Benzodiazepines] explode all trees
#9 benzodiazepine*:ti,ab,kw (Word variations have been searched)
#10 (adinazolam or alprazolam or bentazepam or bromazepam or brotizolam or chlordiazepoxide or cinolazepam or clobazam or clonazepam or clorazepate or clotiazepam or cloxazolam or delorazepam or demoxepam or desmethyldiazepam or diazepam or estazolam or etizolam or etozolam or fludiazepam or flunitrazepam or flurazepam or flutoprazepam or halazepam or haloxazolam or ketazolam or loprazolam or lorazepam or lormetazepam or medazepam or metaclazepam or mexazolam or midazolam or nimetazepam or nitrazepam or nordazepam or oxazepam or oxazolam or pinazepam or prazepam or quazepam or temazepam or tetrazepam or tofisopam or triazolam):ti,ab,kw (Word variations have been searched)
#11 #8 or #9 or #10
Data and analyses
Comparison 1. Overall.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Placebo‐controlled/cross‐over design | 5 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 2 Morphine‐controlled/parallel design | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
Comparison 2. Disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 COPD | 4 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.52, 0.29] |
| 2 Cancer ‐ placebo‐controlled | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.73, 0.62] |
| 3 Cancer ‐ morphine‐controlled | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
Comparison 3. Intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepines ‐ alprazolam | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.41, 0.57] |
| 2 Benzodiazepines ‐ diazepam | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 3 Benzodiazepines ‐ midazolam | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 4 Benzodiazepines ‐ temazepam | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.91, 2.11] |
| 5 Benzodiazepines ‐ ultra short‐acting | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 6 Benzodiazepines ‐ intermediate‐acting | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.38] |
| 7 Benzodiazepines ‐ long‐acting | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours) | 2 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.74, 0.01] |
| 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days) | 5 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 10 Benzodiazepines ‐ morphine + midazolam‐controlled | 1 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.54, 0.61] |
| 11 Benzodiazepines ‐ promethazine‐controlled | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
3.1. Analysis.
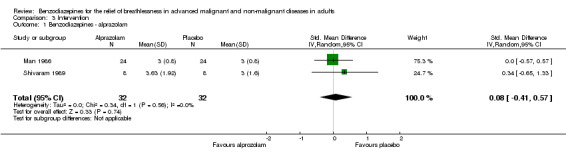
Comparison 3 Intervention, Outcome 1 Benzodiazepines ‐ alprazolam.
3.2. Analysis.
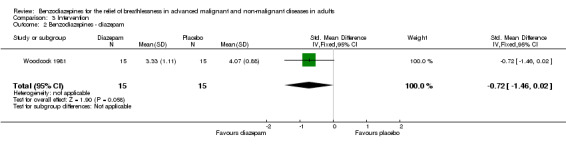
Comparison 3 Intervention, Outcome 2 Benzodiazepines ‐ diazepam.
3.3. Analysis.
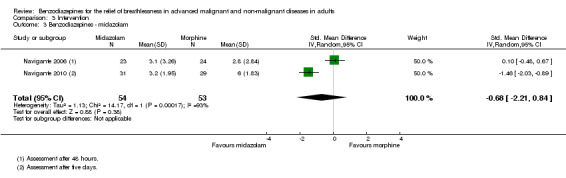
Comparison 3 Intervention, Outcome 3 Benzodiazepines ‐ midazolam.
3.4. Analysis.
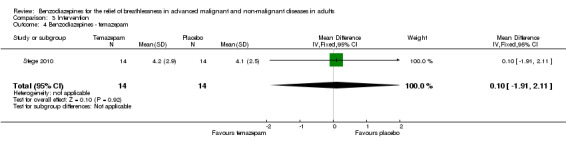
Comparison 3 Intervention, Outcome 4 Benzodiazepines ‐ temazepam.
3.5. Analysis.
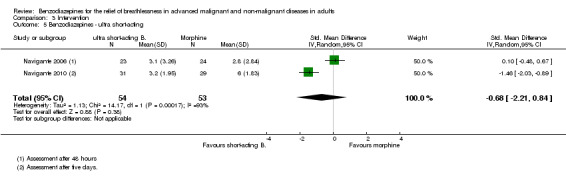
Comparison 3 Intervention, Outcome 5 Benzodiazepines ‐ ultra short‐acting.
3.6. Analysis.
Comparison 3 Intervention, Outcome 6 Benzodiazepines ‐ intermediate‐acting.
3.7. Analysis.
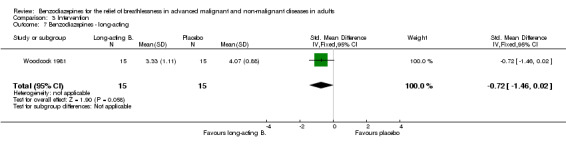
Comparison 3 Intervention, Outcome 7 Benzodiazepines ‐ long‐acting.
3.8. Analysis.
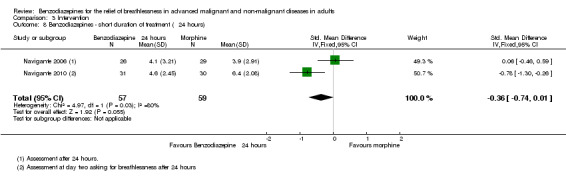
Comparison 3 Intervention, Outcome 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours).
3.9. Analysis.
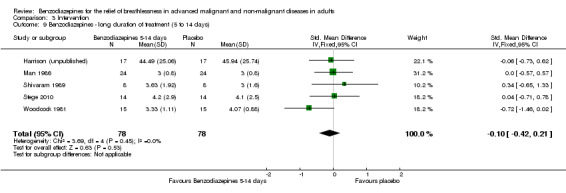
Comparison 3 Intervention, Outcome 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days).
3.10. Analysis.
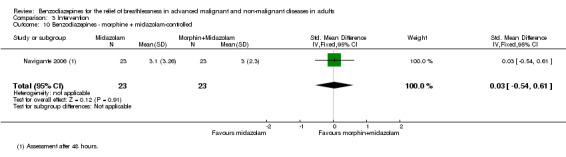
Comparison 3 Intervention, Outcome 10 Benzodiazepines ‐ morphine + midazolam‐controlled.
3.11. Analysis.
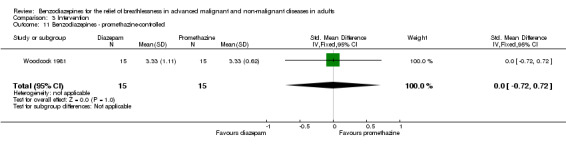
Comparison 3 Intervention, Outcome 11 Benzodiazepines ‐ promethazine‐controlled.
Comparison 4. Primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness ‐ no relief (placebo‐controlled) | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.39] |
| 2 Breathlessness ‐ no relief (morphine‐controlled) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.91, 3.32] |
| 3 Breathlessness ‐ episodic after 48 hours | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.09] |
| 4 Breathlessness ‐ episodic after 24 hours | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |
4.1. Analysis.
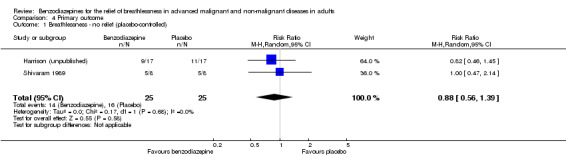
Comparison 4 Primary outcome, Outcome 1 Breathlessness ‐ no relief (placebo‐controlled).
4.2. Analysis.
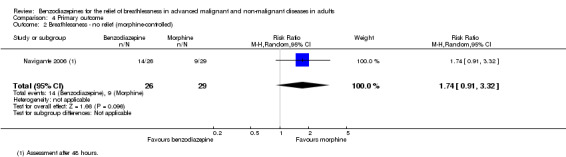
Comparison 4 Primary outcome, Outcome 2 Breathlessness ‐ no relief (morphine‐controlled).
4.4. Analysis.
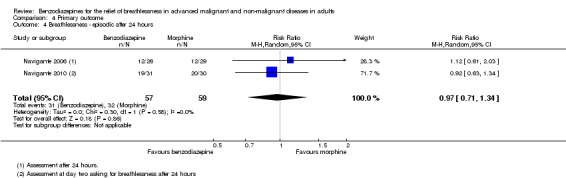
Comparison 4 Primary outcome, Outcome 4 Breathlessness ‐ episodic after 24 hours.
Comparison 5. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects (placebo‐controlled) | 4 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [‐0.06, 0.94] |
| 2 Adverse effects (morphine‐controlled) | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.31, ‐0.04] |
| 3 Adverse effects ‐ clinical relevance only (morphine‐controlled) | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled) | 3 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.74 [0.37, 1.11] |
| 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled) | 2 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| 6 Attrition (placebo‐controlled) | 4 | 146 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 7 Attrition (morphine‐controlled) | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| 8 Deaths (placebo‐controlled) | 4 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 9 Deaths (morphine‐controlled) | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.06] |
5.3. Analysis.
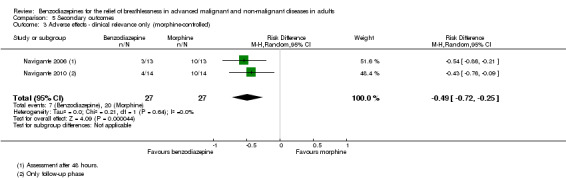
Comparison 5 Secondary outcomes, Outcome 3 Adverse effects ‐ clinical relevance only (morphine‐controlled).
5.5. Analysis.
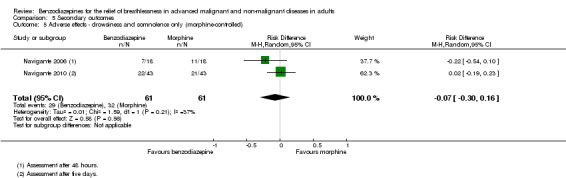
Comparison 5 Secondary outcomes, Outcome 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled).
5.6. Analysis.
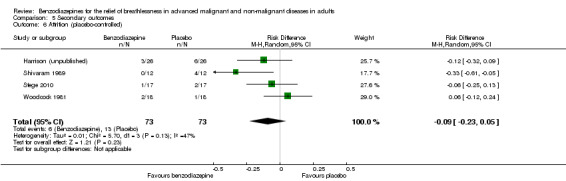
Comparison 5 Secondary outcomes, Outcome 6 Attrition (placebo‐controlled).
5.7. Analysis.
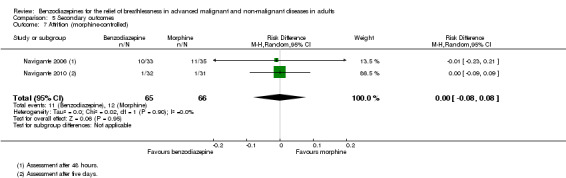
Comparison 5 Secondary outcomes, Outcome 7 Attrition (morphine‐controlled).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eimer 1985.
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 10/22 (Edwards Method Score) |
|
| Participants | Disease: COPD Number (randomised): N = 5 Setting: hospital Age (years, mean): not stated (only range: 51 to 68) Sex (male/female): 4/1 Participant pool: 56 Randomised: 5; study completed: 5 Withdrawals/dropouts: 0 (intervention 2 (clorazepate 22.5 mg) with 3 dropouts; whole intervention excluded from analysis) Reason for drop‐out (intervention 2): intolerable AEs (which AEs not mentioned) Baseline parameters: FEV1 less than 50% SpO2 (mmHg): 65.36; SpCO2 (mmHg): 41.58 |
|
| Interventions | Drug (dose): 1. clorazepate (7.5 mg) at bedtime; 2. clorazepate (22.5) mg at bedtime; 3. placebo Delivery: oral Duration of treatment: 2 weeks |
|
| Outcomes | Breathlessness grade (1 to 6) Results: no significant difference between clorazepate and placebo regarding dyspnoea and walking test (no numbers given; dyspnoea change only in graphs) Adverse effects: none within the 5 participants in intervention 1 and placebo SpO2 and SpCO2: no significant change |
|
| Notes | Author conclusion: this study failed to demonstrate that placebo or clorazepate consistently relieved breathlessness in non‐anxious people with severe COPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned (“Patients were assigned in a randomised double‐blind manner”) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinding stated in the abstract, but not mentioned further |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | High risk | Anxiety, depression, etc. were assessed but data not reported |
| Other bias | High risk |
|
Harrison (unpublished).
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double‐blind Methodological quality: 18/22 (Edwards Method Score) |
|
| Participants | Disease: advanced cancer (12/17 lung cancer) Number (randomised): N = 26 Setting: outpatient and inpatient Age (years, SD): 67.2 (8.3) Sex (male/female): 16/1 Participant pool: 54 Randomised: 26; study completed: 17 Withdrawals/dropouts: 9 (4 drowsiness, 1 deterioration, 1 dysphagia, 2 death, 1 unclear) (excluded from analysis) |
|
| Interventions | Drug (dose): lorazepam (0.5 mg twice daily = 1 mg per day) Delivery: oral Duration of treatment: 5 days (2 days wash‐out) |
|
| Outcomes | Dyspnoea VAS 0 to 100 ("How much trouble has your breathing caused you over the last 24 hours?") Results (mean): baseline to 5 days after intervention: 1. lorazepam 49.18 to 44.49; 2. placebo 48.06 to 45.94 Adverse effects (number of AEs/number with withdrawals): 1. lorazepam: 5/3; 2 placebo: 4/1 No change or differences in anxiety and depression (HADS) |
|
| Notes | Author conclusion: there were no differences between lorazepam and placebo in relieving breathlessness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...was randomly determined by computer prior to the study commencement" |
| Allocation concealment (selection bias) | Low risk | "A randomisation list was kept by the pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were presented The study author sent the raw data in addition |
| Selective reporting (reporting bias) | Low risk | Study protocol is available |
| Other bias | Low risk | Study appeared to be free of other bias |
Man 1986.
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 16/22 (Edwards Method Score) |
|
| Participants | Disease: COPD Number (randomised): N = 29 Setting: outpatient Age (years, mean): 65.4 Sex (male/female): 16/8 (complete) Participant pool: not stated Randomised: 29; study completed: 24 Withdrawals/dropouts: 5 (excluded from analysis) Reason for drop‐out: 1 AE (placebo), 4 missed appointments Baseline parameters: FEV1/FVC: 54% SpO2 (mmHg): 73.4; SpCO2 (mmHg): 32.8 |
|
| Interventions | Drug (dose): alprazolam 1.0 mg/day (0.5 mg twice daily) Control: placebo Delivery: oral Duration of treatment: 1 week |
|
| Outcomes | Dyspnoea grade at rest (1 to 5); dyspnoea scoring at rest and exercise (VAS 0 to 10) Results (mean, baseline to after intervention): alprazolam: 3.0 to 3.0; placebo: 3.2 to 3.0 No significant change in dyspnoea scoring during rest and exercise Adverse effects: 11 reported (7/11 drowsiness), 9/11 on alprazolam Functional test (12‐minute walking test in metres; baseline to after intervention): alprazolam: 896.5 to 880.88; placebo: 902.17 to 931.29 The resting SpO2 was significantly higher with placebo and exercising SpCO2 was significantly lower with placebo |
|
| Notes | Author conclusion: the subjective perception of dyspnoea was the same before and after alprazolam, at rest and during exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...designed as a randomized...” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “double‐blind...using alprazolam and matching placebo” Labelled bottles with tablets (alprazolam‐placebo‐wash‐out) described in detail Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total screened patients not mentioned No intention‐to‐treat analysis (5/29 lost were excluded from analysis), however only one with AE (placebo) |
| Selective reporting (reporting bias) | Low risk | Broad information available, good summaries, good presentation |
| Other bias | Low risk | Pharmaceutical funding (company with alprazolam), although negative results |
Navigante 2006.
| Methods | Design: RCT, parallel, multi‐arm (3), control: morphine and morphine + midazolam Blinding: single‐blind (only participant blinded) Methodological quality: 17/22 (Edwards Method Score) |
|
| Participants | Disease: terminal cancer (life expectancy less than a week) Number (randomised): N = 101 Setting: hospital inpatient Age (years, mean): 57.3 Sex (male/female): 47/54 Participant pool: n = 146 Randomised: 101; study completed: 70 Withdrawals/dropouts: 31 (all deaths) (excluded from analysis) |
|
| Interventions | Drug (dose): 1. morphine (Mo ‐ 10 mg/day); 2. midazolam (Mi ‐ 20 mg/day); 3. morphine + midazolam (MM ‐ Mo 10 mg/day + Mi 20 mg/day) Rescue dose: 1. Mi 5 mg; 2. Mo 2.5 mg; 3. Mo 2.5 mg (this means that all 3 treatment arms could include a combination of morphine and midazolam) Delivery: subcutaneous Duration of treatment: 48 hours |
|
| Outcomes | Dyspnoea intensity: modified Borg scale 0 to 10 Results presented in the paper: baseline (mean) to after intervention (24/48 hours = median and P‐values): 1. (Mo) 7.1 to 3/2 (P=0.002/P=0.0001); 2. (Mi) 6.9 to 4/2 (P=0.018/P=0.004); 3. (MM) 6.8 to 3/2 (P=0.003/P<0.0001) (Mean and CI (95%) for 24‐ and 48‐hour measures received from the authors (data skewed): 1. (Mo) 24 hours: 3.9 (2.8 to 5.0), 48 hours: 2.8 (1.6 to 4.0); 2. (Mi) 24 hours: 4.1 (2.8 to 5.4), 48 hours: 3.1 (1.7 to 4.5); 3. (MM) 24 hours: 3.4 (2.4 to 4.4), 48 hours: 3.0 (2.0 to 4.0)) Percentages of participants with breakthrough dyspnoea (24/48 hours): 1. (Mo) 34.3%/38%; 2. (Mi) 36.4%/38.5%; 3. (MM) 21.2%/24% Numbers of breakthrough episodes of dyspnoea per participant (24/48 hours): 1. (Mo) 2/2; 2. (Mi) 1/1; 3. (MM) 1/1 Percentages of participants with dyspnoea relief after 24 hours: 1. (Mo) 69% (P=0.03); 2. (Mi) 46% (P=0.004); 3. (MM) 92% (P‐values compare to MM) Percentages of participants with persistent, uncontrolled dyspnoea after 48 hours: 1. (Mo) 12.6%; 2. (Mi) 26% (P=0.04 compare to MM); 3. (MM) 4% Adverse effects: the most frequently recorded AE was somnolence (Mo > MM > Mi) Oxygen saturation (mean; baseline to after intervention; 24/48 hours): 1. (Mo) 72% to 72%/70%; 2. (Mi) 73% to 70%/70%; 3. (MM) 73% to 73%/71.5% Anxiety: significant correlation between dyspnoea and anxiety at all times |
|
| Notes | Author conclusion: the data demonstrate that the beneficial effects of morphine in controlling baseline levels of dyspnoea could be improved with the addition of midazolam to the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...using a random number generator in 1:1:1 ratio in blocks of nine” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how it was done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Drug administrations were performed in a single‐blind fashion.” “One potential limitation of our study is the single‐blinded nature of the design. The treating physicians’ knowledge of which schedule of drugs the patient received could influence their need for administering rescue medications. A double‐blind design can avoid this, but was considered not appropriate for our study population by the Ethics Committee at our institution. Nevertheless, the risk for underestimation of rescue needs was minimized by a double assessment of breakthrough episodes carried out by caregivers and research physicians.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45/146 excluded with statement of reasons Attrition (deaths) clearly mentioned Missing data not stated Unclear if participants who experienced relief of dyspnoea was assessed on the whole number of participants or only on participants alive at the end of the study (30% died) |
| Selective reporting (reporting bias) | High risk | Unclear which other symptoms were measured (only anxiety‐dyspnoea is reported). Results of ECOG and MMSE not reported. Dyspnoea relief (only after 24 hours) and uncontrolled dyspnoea (only after 48 hours) |
| Other bias | High risk | Using cross‐over rescue medication (midazolam for the morphine group and vice versa) could produce confusion for separate analysis |
Navigante 2010.
| Methods | Design: RCT, parallel, control: morphine Blinding: single‐blind (only participant and caregiver blinded) Methodological quality: 21/22 (Edwards Method Score) |
|
| Participants | Disease: advanced cancer Number (randomised): N = 63 Setting: outpatient clinic Age (years, median, intervention group 1/2): 59/55 Sex (male/female): not mentioned Participant pool: not mentioned Randomised: 63; study completed: 61* Withdrawals/dropouts: 2* (unable or unwilling to comply with the programmed follow‐up visits) (excluded from analysis) *Data at day 5 for the morphine group were only available for 29 participants, therefore all calculations at day 5 were done with 29 participants |
|
| Interventions | Drug (starting dose within fast titration phase): 2 mg for oral midazolam or 3 mg for oral morphine with incremental steps of 25% of the preceding dose every 30 min until dyspnoea was alleviated 50% or more ("effective dose" used in follow‐up assessment) Delivery: oral Duration of follow‐up treatment: 5 days using the "effective dose" every 4 hours (except the sleep hours) |
|
| Outcomes | Dyspnoea relief (fast titration phase): 5‐category scale (0% none, 25% slight, 50% moderate, 75% a lot, 100% complete) Dyspnoea intensity for the chronic component of dyspnoea (baseline and follow‐up assessment): NRS 0 to 10 Proportion of participants with BTD episodes Number of BTD episodes per day Results presented in the published paper: In the fast titration phase dyspnoea relief of at least 50% was achieved in all participants in both arms (after starting dose: midazolam 21/32 vs morphine 11/31 (P = 0.023), after dosing step 1: 9/32 vs 11/31 (P = 0.59), and after dosing step 2: 2/32 vs 9/31 (P = 0.022)); in the follow‐up phase, mean (95% CI) baseline dyspnoea intensity is presented in a table: midazolam 8.8 (±0.3) vs morphine 8.7 (±0.3) (P = 0.62), but follow‐up results on dyspnoea intensity are only presented in figure with box plots (participants receiving midazolam maintained a significantly lower dyspnoea intensity level in comparison with the morphine group, during the 4 days of follow‐up) and as median at the second day in text: midazolam 6 vs morphine 4.5 (P = 0.003); number of participants with 1 or more BTD episodes at baseline was 25 in both arms, and the proportion of participants with BTD episodes was significantly different at days 3 to 5, favouring the midazolam arm (data only presented by a figure); therapeutic failure (i.e. NRS 8 to 10 by day 5) midazolam 0/31 vs morphine 6/30; AE (n during fast titration phase): mild somnolence midazolam 18/32 vs morphine 15/31, mild agitation 2/32 vs 2/31, mild and moderate nausea only morphine 2/31 and 1/31; AE (n during follow‐up): somnolence (time spent sleeping during daytime) 3 h to 5 h midazolam 4/31 vs morphine 5/30, 6.11 h only morphine 1/30; agitation grade 1/2 only in morphine arm 2/1/30; nausea grade 1 only morphine 1/30; constipation grade 2 only morphine 2/30; others midazolam 1/31 (cognitive disturbance) vs morphine 2/31 (cough g1, pruritus g2, xerostomia g1, flushing g1); dose reduction (because of excessive somnolence): midazolam 1 vs morphine 2; Oxygen saturation: no change in either group Additional results received from the authors: Mean (95% CI) dyspnoea intensity for baseline and day 5 measures (data skewed): 1. (midazolam) baseline: 8.84 (8.50 to 9.19), day 5: 3.23 (2.51 to 3.94); 2. (morphine) baseline: 8.74 (8.44 to 9.04), day 5: 6.00 (5.31 to 6.69) |
|
| Notes | Author conclusion: the data demonstrate the beneficial effect of midazolam versus morphine in the relief of chronic dyspnoea intensity and the number of episodes of breathlessness (breakthrough dyspnoea), while adverse events occurred and were comparable between both arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number generator in 1:1 ratio in blocks of six" |
| Allocation concealment (selection bias) | Low risk | "Numbered envelopes that were used to implement the randomization were concealed until interventions were assigned. The researchers had final responsibility for patient enrollment" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dealing with missing data or presence of it |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
| Other bias | Unclear risk | None |
Shivaram 1989.
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 15/22 (Edwards Method Score) |
|
| Participants | Disease: COPD Number (randomised): N = 12 Setting: unclear Age (years, mean): 64.9 Sex (male/female): 8/0 Participant pool: not stated Randomised: 12; study completed: 8 Withdrawals/dropouts: 4 (excluded from analysis) Reason for drop‐out: all on placebo (3/4 increasing dyspnoea and drowsiness, 1/4 acute exacerbation) Baseline parameters: FEV1/FVC: all less than 65% SpO2 (mmHg): 76.0; SpCO2 (mmHg): 38.0 |
|
| Interventions | Drug (dose): alprazolam 0.75 mg/day (0.25 mg 3 times a day) Control: placebo Delivery: oral Duration of treatment: 2 weeks |
|
| Outcomes | Dyspnoea (modified Borg scale 0 to 10) Results* (baseline to after intervention): alprazolam: 3.6 to 3.6; placebo: 3.6 to 3.0 (*not explicitly stated if mean or median, but must be mean because of decimal numbers) Adverse effects: none within the 8 participants SpO2 and SpCO2: no significant change |
|
| Notes | Author conclusion: alprazolam did not alter the sensation of breathlessness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were started on a double‐blind, randomized crossover regimen” “The patients then received either placebo or alprazolam 0.25 mg in a double‐blind fashion” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how it was done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The medication code was known only to the hospital pharmacist.” “Patients were started on a double‐blind, randomized crossover regimen” “The patients then received either placebo or alprazolam 0.25 mg in a double‐blind fashion” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Described the attrition and reasons for it Excluded from analysis, but stated that they did not differ with regard to spirometric measures Demographics only from included participants (8/12) No predicted FEV1 and FVC mentioned |
| Selective reporting (reporting bias) | Low risk | No indication for selective reporting |
| Other bias | High risk | Only men (Veterans Affairs Medical Center) |
Stege 2010.
| Methods | Design: RCT, placebo‐controlled, cross‐over design Blinding: double Methodological quality: 16/22 (Edwards Method Score) |
|
| Participants | Disease: COPD (stages III to IV) Number (randomised/analysed): n = 17/14 Setting: outpatient center of a respiratory medicine department Age (years, mean, SD): 61.6 ∓ 8.0 Sex (male/female): 10/4 Participant pool: 199 Randomised: 17; study completed: 14 Withdrawals/dropouts: 3 (excluded from analysis) Reason for drop‐out: 1/3 on intervention: (exacerbation of COPD), 2/3 on placebo (1 obstructive sleep apnoea‐hypopnoea syndrome, 1 withdrew due to burden of the measurements) Baseline parameters: FEV1/FVC (mean, SD) 32.7 ∓ 13, PaCO2, kPa 5.4 ∓ 0.4, PaO2, kPa 9.6 ∓ 0.7 Baseline sleep‐related complaints: difficulty maintaining sleep (experienced by 8 participants), a prolonged sleep‐onset latency (experienced by 7 participants), extensive daytime sleepiness (experienced by 6 participants), and nocturnal dyspnoea (experienced by 2 participants) Baseline medication: inhaled corticosteroids 14/14, anticholinergics 13/14, β2‐antagonists 9/14, oral steroids 4/14, proton pump inhibitors 4/14, anticoagulants 4/14, other antihypertensives 4/14, theophylline 3/14, diuretics 3/14, acetylcysteine 1/14 |
|
| Interventions | Drug (dose): temazepam 10 mg/day (30 min before bedtime) Control: placebo Delivery: oral Duration of treatment: 1 week each, with 1‐week wash‐out time |
|
| Outcomes | Subjective dyspnoea (10‐point VAS) Results (mean (SD)): subjective dyspnoea: baseline 3.8 (2.6), temazepam 4.2 (2.9), placebo 4.1 (2.5), P = 0.90; transcutaneous carbon dioxide (PtcCO2) during sleep: baseline 6.2 (0.6), temazepam 5.9 (1.0), placebo 6.3 (1.4), P = 0.27; oxygen saturation (SpO2) during sleep: baseline 92 (2), temazepam 92 (3), placebo 92 (2), P = 0.31; total sleep time, h (mean (SD)): baseline 5.7 (1.2), temazepam 6.3 (1.0), placebo 5.4 (1.1), P = 0.03; sleep latency (10‐point VAS): baseline 4.4 (3.2), temazepam 3.3 (2.8), placebo 4.6 (3.2), P = 0.03; amount of stage 2 sleep, minutes (non‐rapid eye movement sleep): baseline 130.8 (54.5), temazepam 168.8 (34.4), placebo 140.0 (44.6), P = 0.03; no statistically significant changes for the other secondary outcomes; Adverse effects: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. (...) Randomization was done by the hospital pharmacy." Not mentioned of how this was done |
| Allocation concealment (selection bias) | Unclear risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. Randomization was done by the hospital pharmacy." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. Randomization was done by the hospital pharmacy. Subjects were instructed to take the study medication 30 min before they went to bed. (…) Sleep was manually staged according to standard methods by two qualified sleep technicians blinded to the subject’s treatment status." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Described the attrition (3/17) and the reasons for it, but did not describe participant characteristics of dropouts. No intention‐to‐treat analysis (14 of 17 enrolled participants analysed) |
| Selective reporting (reporting bias) | Unclear risk | The article addresses only respiratory adverse events; it is unclear if other than respiratory events had occurred |
| Other bias | Unclear risk |
|
Woodcock 1981.
| Methods | Design: RCT, cross‐over, placebo‐controlled, multi‐arm (3) Blinding: double Methodological quality: 15/22 (Edwards Method Score) |
|
| Participants | Disease: COPD Number (randomised): N = 18 Setting: outpatient Age (years, mean): 60.5 Sex (male/female): 15/3 Participant pool: not stated Randomised: 18; study completed: 15 Withdrawals/dropouts: 3 (excluded from analysis) Reason for drop‐out: 1 death (diazepam), 1 intolerable drowsiness (diazepam), 1 hypercapnia (placebo) Baseline parameters: FEV1: 25.3%; FEV1/FVC: 0.38 SpO2 (kPa): 9.5 (= 71.25 mmHg); SpCO2 (kPa): 4.6 (= 34.5 mmHg) |
|
| Interventions | Drug (dose): 1. diazepam 25 mg/day (5 mg 3 times a day plus 10 mg at bedtime); 2. promethazine 125 mg/day (25 mg 3 times a day plus 50 mg at bedtime); 3. placebo Delivery: oral Duration of treatment: 2 weeks |
|
| Outcomes | Dyspnoea grade (1 to 5) after each intervention and daily dyspnoea by VAS (0 to 10) at rest and after exercise (only by graph) Results (mean): dyspnoea grade: 1. 3.46 (diazepam); 2. 3.29 (P < 0.05) (promethazine); 3. 4.00 (placebo) Adverse effects (6 ‐ reduce dosage): all drowsiness: 5/6 diazepam; 1/6 promethazine; 5/5 drowsiness incidents (like falling down stairs) with diazepam Functional test (12‐minute walking test in metres): 1. 642 (P < 0.05) (diazepam); 2. 707 (P < 0.05) (promethazine); 3. 675 (placebo) SpO2 and SpCO2: no significant change No significant change in anxiety and depression |
|
| Notes | Author conclusion: diazepam had no significant effect on breathlessness and noticeably reduced exercise tolerance. Promethazine reduced breathlessness and improved exercise tolerance without altering lung function. Review author: however, there is a beneficial effect of diazepam, although not significant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The treatments were given in a randomized order.” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Double‐blind” procedure was described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 3/18 participants were lost and excluded from the analysis, they would underline the presented results rather than bias them |
| Selective reporting (reporting bias) | Unclear risk | All main outcomes are presented in detail The effect of diazepam in the relief of breathlessness is nearly statistically significant, but was discussed as "diazepam had no effect on breathlessness" |
| Other bias | Unclear risk | It is not explicitly stated if a wash‐out phase was used (on contacting the author, there was no wash‐out) Results of compliance test are not mentioned Screening method and numbers are not mentioned |
AEs = adverse effects BTD = breakthrough dyspnoea CI = confidence interval COPD = chronic obstructive pulmonary disease ECOG = Eastern Cooperative Oncology Group FEV1 = forced expiratory volume in one second FVC = forced vital capacity HADS = Hospital Anxiety and Depression Scale Mi = midazolam MM = midazolam + morphine MMSE = Mini‐Mental State Exam Mo = morphine NRS = numeric rating scale RCT = randomised controlled trial SD = standard deviation VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allcroft 2013 | Non‐controlled study (phase II) |
| Allen 1984 | Review |
| Anonymous 1980a | Review |
| Anonymous 1980b | Review |
| Appel 1989 | No subjective measurement of breathlessness; different drug (flumazenil) |
| Argyropoulou 1993 | Different drug (buspirone) |
| Bar‐Or 1982 | Review |
| Beaupre 1988 | No subjective measurement of breathlessness |
| Borson 1992 | Different drug (nortriptyline) |
| Bottomley 1990 | No subjective measurement of breathlessness; observational design |
| Boyden 2015 | Systematic review |
| Catchlove 1971a | No subjective measurement of breathlessness |
| Catchlove 1971b | No subjective measurement of breathlessness |
| Cherny 2014 | Guideline |
| Clark 1971 | No subjective measurement of breathlessness; case series |
| Clemens 2011 | Non‐controlled study (before‐after design) |
| Cohn 1992 | No subjective measurement of breathlessness |
| Daubert 2014 | Study protocol; study was cancelled before any participants were enrolled |
| De Sousa 1988 | No subjective measurement of breathlessness; letter/observational design |
| Denaut 1974 | No subjective measurement of breathlessness |
| Dolly 1982 | No subjective measurement of breathlessness; healthy participants |
| Dowson 2004 | Review |
| Ekstrom 2015 | Review |
| Fonsmark 2015 | Guideline |
| Forster 1983 | No subjective measurement of breathlessness; healthy participants |
| Gaddie 1972 | Review |
| Garrett 2015 | Review |
| Geddes 1976 | No subjective measurement of breathlessness |
| Gomutbutra 2013 | Non‐controlled, retrospective study |
| Greene 1989 | Non‐controlled experimental study (case report) |
| Guilleminault 1993 | No subjective measurement of breathlessness; observational design |
| Guz 1980 | Review |
| Heinonen 1972 | No subjective measurement of breathlessness; sedation for artificial ventilation |
| Hoeijer 1994 | No subjective measurement of breathlessness; different disease (sleep apnoea) |
| Horfarter 2006 | Review |
| Hosaka 1996 | Non‐advanced disease stage; a few participants with a different disease (asthma, tuberculosis) |
| Huttemann 1971 | Different drug (laevomepromazine) |
| Johanson 1993 | Review |
| Jokinen 1984 | Different disease (psychosomatic disorder) |
| Jolly 1996 | No subjective measurement of breathlessness; observational design |
| Jones 1985 | Different disease (healthy participants) |
| Kaltsas 2014 | Editorial |
| Kann 1968 | Review |
| Kronenberg 1975 | No subjective measurement of breathlessness; observational design |
| Lakshminarayan 1976 | No subjective measurement of breathlessness; healthy participants |
| Lareau 1999 | No drug intervention (secondary analysis) |
| Laros 1982 | No subjective measurement of breathlessness; case report; no benzodiazepine |
| Lichterfeld 1967 | Benzodiazepine only in combination (oxazepam + orciprenaline) |
| Light 1996 | Different drug (promethazine) |
| Marin 1987 | No drug intervention (retrospective study) |
| McIver 1994 | Different drug (chlorpromazine) |
| Mitchell‐Heggs 1980a | No subjective measurement of breathlessness; no control group; no standardised or systematic design |
| Mitchell‐Heggs 1980b | No drug intervention |
| Mouzi 2014 | No subjective measurement of breathlessness; case report |
| Murciano 1990 | No subjective measurement of breathlessness |
| Murciano 1993 | No subjective measurement of breathlessness |
| Navigante 1997 | Benzodiazepine only in combination (midazolam + morphine) |
| Navigante 2003 | Benzodiazepine only in combination (midazolam + morphine) |
| NCT01687751 | Study failed to recruit any participants |
| Nordt 1997 | Review |
| O'Donnell 1992 | No drug intervention (observational study) |
| O'Donnell 1994 | Review |
| O'Donnell 1998 | Review |
| O'Neill 1985 | Different drug (chlorpromazine) |
| Rao 1973 | No subjective measurement of breathlessness |
| Rapoport 1991 | No subjective measurement of breathlessness; healthy participants |
| Rice 1986 | Review |
| Rice 1987 | Different drug (promethazine) |
| Rodriguez‐Roisin 2014 | Editorial |
| Rose 2002 | Review |
| Rudolf 1978 | No subjective measurement of breathlessness |
| Runo 2001 | Review |
| Schultze‐Werninghaus 2007 | Review |
| Sen 1983 | No subjective measurement of breathlessness; no control group |
| Singh 1993 | Different drug (promethazine) |
| Smith 2015 | Review |
| Stark 1981a | Different disease (healthy participants) |
| Stark 1981b | Different disease (healthy participants) |
| Stark 1983 | Different drug (dihydrocodeine) |
| Stark 1988 | Review |
| Steens 1993 | No subjective measurement of breathlessness |
| Tenorio 2012 | Non‐controlled, retrospective study; congress abstract |
| Timms 1988 | No subjective measurement of breathlessness |
| Vozoris 2013 | Observational design |
| Walsh 1993 | Review |
| Wanrooij 2005 | Review |
| Wiedemann 1995 | Review |
| Wilson 1954 | No subjective measurement of breathlessness; different drug (oxygen, morphine, barbiturate) |
| Woodcock 1981a | Different drug (dihydrocodeine, alcohol, caffeine) |
| Woodcock 1981b | Different drug (oxygen) |
Characteristics of studies awaiting assessment [ordered by study ID]
Hardy 2016.
| Methods | Design: multicentre, placebo‐controlled, cross‐over design, blinded (masking used) RCT |
| Participants | Inclusion criteria: adults with dyspnoea related to life‐limiting disease (malignant and non‐malignant) or its treatment, dyspnoea score > 3/10 on at least 3 occasions during the previous week, English speaking or have an interpreter available, AKPS scale > 30, able to operate a nasal spray device, able to understand all trial requirements and complete a dyspnoea diary, no changes in any medication likely to affect dyspnoea (e.g. steroids, opioids) within 48 hours of starting the study Target sample size: 200 > terminated after interim analysis including 75 participants |
| Interventions | Intranasal midazolam (3 inhalations (total dose of 1.5 mg active drug) vs placebo (citric acid 7.65 mg/ml in normal saline placebo nasal spray) |
| Outcomes | Primary outcome: dyspnoea intensity at 15 minutes compared to baseline Secondary outcomes: DID (dyspnoea intensity difference) at 5, 30, and 60 mins, sedation (NRS 0 = not at all drowsy to 10 = extremely drowsy), anxiety (NRS 0 = not at all anxious to 10 = extremely anxious) |
| Notes | The study has been published just before publication of this review update The study is registered at the Australian New Zealand Clinical Trials Registry (ACTRN), trial ID: ACTRN12609000506291, Title: Midazolam nasal spray for the treatment of breathlessness in patients with life‐limiting disease Contact information: Clare Randall, Arohanui Hospice 1 Heretaunga St Palmerston North 4414, New Zealand, clare.r@arohanuihospice.org.nz |
Hart 2012.
| Methods | Design: randomised, double‐blind, double‐dummy, placebo‐controlled pilot study |
| Participants | 30 people with severe respiratory disease (MRC dyspnoea score 4 or 5) |
| Interventions | Lorazepam tablets 0.5 mg twice daily with dummy nasal spray up to 4 times daily or intranasal midazolam (dose 400 mg) 2 sprays up to 4 times daily with placebo tablets |
| Outcomes | Primary outcome measures were designed to evaluate quality of life measures incorporating change in:
|
| Notes | The authors conclude in the abstract that intranasal midazolam is no less effective in this setting than oral lorazepam and suggest that intranasal midazolam is another useful tool for managing dyspnoea. However, only conference abstract is available; we contacted two of the authors asking for further details, but did not receive an answer until the review was published |
AKPS = Australia‐modified Karnofsky Performance Status DID = dyspnoea intensity difference MRC = Medical Research Council NRS = numeric rating scale RCT = randomised controlled trial
Differences between protocol and review
For the previous version of the review, we changed the title slightly by inserting 'advanced' in front of 'malignant'. This resulted in no changes to the included and excluded studies.
Contributions of authors
All review authors contributed to the development of the idea for this review, revised the manuscript, and approved the final version.
STS: developed and wrote the protocol, developed the search strategies and the data extraction form, searched for studies, obtained copies of the studies, extracted data from studies, entered data into RevMan, carried out analysis and meta‐analysis, drafted the review and finalised it after discussion with the other review authors. Responsible for further updates.
IJH: discussed and approved the protocol, the search strategy, and the data extraction form, discussed the outcomes and analysis with the other review authors, and provided epidemiological and wider systematic review expertise.
SB: discussed and approved the protocol, the search strategy, and the data extraction form, checked extracted information from studies, discussed the outcomes and analysis with the other review authors.
RH: discussed and approved the protocol, the search strategy, and the data extraction form, discussed the outcomes and analysis with the other review authors, and provided social science expertise.
VW: searched for studies for the update, extracted data from studies for the update, entered data into RevMan for the update, contributed to the meta‐analysis for the update, drafted the review update.
CB: supervised the protocol, contributed to the development of the search strategy and the data extraction form, searched the titles, extracted data from studies, supervised the analysis, discussed the outcomes and analysis with the other review authors, and provided wider systematic review expertise.
Sources of support
Internal sources
Department of Palliative Care, Policy and Rehabilitation, King's College London, UK.
Institute of Palliative Care, Germany.
Department of Palliative Medicine, University Hospital of Cologne, Germany.
External sources
The Werner Jackstaedt Foundation, Germany.
-
The Federal Ministry of Education and Research (BMBF; 01KG1509), Germany.
Research grant for the conduction of the review update (2015)
Declarations of interest
STS: none known. STS is a specialist in palliative care and works as a physician caring for patients with life‐limiting diseases.
IJH: none known. IJH is a specialist in palliative care and works as a researcher and physician caring for patients with life‐limiting diseases.
SB: none known. SB worked as a specialist in palliative care and and now supports patients with chronic illness.
RH: none known. RH is a reader in palliative care and works as a researcher in palliative care with focus on HIV/AIDS, Sub‐Saharan Africa and Global Health.
VW received reimbursement of travel costs from Teva Pharmaceutical Industries Ltd. for the 8th World Research Congress of the European Association of Palliative Care (Lleida, Spain, 2014) outside the submitted work. VW is a health economist with experience in research in palliative care (2011‐2015) and works as a research associate at an institute of quality and efficiency in health care (since 2015).
CB: none known. CB is a specialist in palliative care and works as a physician caring for patients with life‐limiting diseases.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Eimer 1985 {published data only}
- Eimer M, Cable T, Gal P, Rothenberg LA, McCue JD. Effects of clorazepate on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Journal of Family Practice 1985;21:359‐62. [PubMed] [Google Scholar]
Harrison (unpublished) {unpublished data only}
- Harrison TJR. A comparison of the effectiveness of oral lorazepam and placebo in relieving breathlessness associated with advanced cancer. (MSc thesis, 2004).
Man 1986 {published data only}
- Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832‐6. [DOI] [PubMed] [Google Scholar]
Navigante 2006 {published data only}
- Navigante AH, Cerchietti LC, Castro MA, Lutteral MA, Cabalar ME. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. Journal of Pain and Symptom Management 2006;31:38‐47. [DOI] [PubMed] [Google Scholar]
Navigante 2010 {published and unpublished data}
- Navigante AH, Castro MA, Cerchietti LC. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. Journal of Pain and Symptom Management 2010;39(5):820‐30. [DOI] [PubMed] [Google Scholar]
Shivaram 1989 {published data only}
- Shivaram U, Cash M, Finch P. Effects of alprazolam on gas exchange, breathing pattern, and lung function in COPD patients with anxiety. Respiratory Care 1989;34:196‐200. [Google Scholar]
Stege 2010 {published data only}
- Stege G, Heijdra YF, Elshout FJ, Ven MJ, Bruijn PJ, Sorge AA, et al. Temazepam 10 mg does not affect breathing and gas exchange in patients with severe normocapnic COPD. Respiratory Medicine 2010;104(4):518‐24. [DOI] [PubMed] [Google Scholar]
Woodcock 1981 {published data only}
- Woodcock AA, Gross ER, Geddes DM. Drug treatment of breathlessness: contrasting effects of diazepam and promethazine in pink puffers. British Medical Journal 1981;283:343‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Allcroft 2013 {published data only}
- Allcroft P, Margitanovic V, Greene A, Agar MR, Clark K, Abernethy AP, et al. The role of benzodiazepines in breathlessness: a single site, open label pilot of sustained release morphine together with clonazepam. Journal of Palliative Medicine 2013;16(7):741‐4. [PUBMED: 23597092] [DOI] [PubMed] [Google Scholar]
Allen 1984 {published data only}
- Allen SC. Treatment of dyspnoea: the role of drugs. Drugs of Today 1984;8:397‐403. [Google Scholar]
Anonymous 1980a {published data only}
- Anonymous (Lancet). Diazepam and breathlessness. Lancet 1980;2:242‐3. [PubMed] [Google Scholar]
Anonymous 1980b {published data only}
- Anonymous (British Medical Journal). Centrally acting drugs in chronic airways obstruction. British Medical Journal 1980;281:1232‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Appel 1989 {published data only}
- Appel M, Bron HN, Hooymans PM, Janknegt R. Efficacy of flumazenil in COPD patient with therapeutic diazepam levels. The Lancet 1989;1:392. [DOI] [PubMed] [Google Scholar]
Argyropoulou 1993 {published data only}
- Argyropoulou P, Patakas D, Koukou A, Vasiliadis P, Georgopoulos D. Buspirone effect on breathlessness and exercise performance in patients with chronic obstructive pulmonary disease. Respiration 1993;60:216‐20. [DOI] [PubMed] [Google Scholar]
Bar‐Or 1982 {published data only}
- Bar‐Or D, Marx JA, Good J. Breathlessness, alcohol, and opiates. The New England Journal of Medicine 1982;306(22):1363‐4. [DOI] [PubMed] [Google Scholar]
Beaupre 1988 {published data only}
- Beaupre A, Soucy R, Phillips R, Bourgouin J. Respiratory center output following zopiclone or diazepam administration in patients with pulmonary disease. Respiration 1988;54(4):235‐40. [DOI] [PubMed] [Google Scholar]
Borson 1992 {published data only}
- Borson S, McDonald GJ, Gayle T, Deffebach M, Lakshminarayan S, VanTuinen C. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics 1992;33(2):190‐201. [DOI] [PubMed] [Google Scholar]
Bottomley 1990 {published data only}
- Bottomley DM, Hanks GW. Subcutaneous midazolam infusion in palliative care. Journal of Pain and Symptom Management 1990;5:259‐61. [DOI] [PubMed] [Google Scholar]
Boyden 2015 {published data only}
Catchlove 1971a {published data only}
- Catchlove RF, Kafer ER. The effects of diazepam on the ventilatory response to carbon dioxide and on steady‐state gas exchange. Anesthesiology 1971;34(1):9‐13. [DOI] [PubMed] [Google Scholar]
Catchlove 1971b {published data only}
- Catchlove RF, Kafer ER. The effects of diazepam on respiration in patients with obstructive pulmonary disease. Anesthesiology 1971;34(1):14‐8. [DOI] [PubMed] [Google Scholar]
Cherny 2014 {published data only}
Clark 1971 {published data only}
- Clark TJH. Respiratory depression caused by nitrazepam in patients with respiratory failure. The Lancet 1971;2:737‐8. [DOI] [PubMed] [Google Scholar]
Clemens 2011 {published data only}
Cohn 1992 {published data only}
- Cohn MA, Morris DD, Juan D. Effects of estazolam and flurazepam on cardiopulmonary function in patients with chronic obstructive pulmonary disease. Drug Safety 1992;7(2):152‐8. [DOI] [PubMed] [Google Scholar]
Daubert 2014 {published data only}
- Daubert E, Bolesta S. Effect of lorazepam versus morphine on quality of life in hospice patients with dyspnea and anxiety. Journal of the American Pharmacists Association 2014, issue 2:e193.
Denaut 1974 {published data only}
- Denaut M, Yernault JC, De CA. Double‐blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Current Medical Research and Opinion 1974;2:611‐5. [DOI] [PubMed] [Google Scholar]
De Sousa 1988 {published data only}
- Sousa E, Jepson BA. Midazolam in terminal care. The Lancet 1988;331:67‐8. [DOI] [PubMed] [Google Scholar]
Dolly 1982 {published data only}
- Dolly FR, Block AJ. Effect of flurazepam on sleep‐disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. American Journal of Medicine 1982;73(2):239‐43. [DOI] [PubMed] [Google Scholar]
Dowson 2004 {published data only}
- Dowson CA, Kuijer RG, Mulder RT. Anxiety and self‐management behaviour in chronic obstructive pulmonary disease: what has been learned?. Chronic Respiratory Disease 2004;1(4):213‐20. [DOI] [PubMed] [Google Scholar]
Ekstrom 2015 {published data only}
Fonsmark 2015 {published data only}
- Fonsmark L, Hein L, Nibroe H, Bundgaard H, Haas I, Iversen S, et al. Danish national sedation strategy. Targeted therapy of discomfort associated with critical illness. Danish Society of Intensive Care Medicine (DSIT) and the Danish Society of Anesthesiology and Intensive Care Medicine (DASAIM). Danish Medical Journal 2015; Vol. 62, issue 4:1‐5. [PubMed]
Forster 1983 {published data only}
- Forster A, Morel D, Bachmann M, Gemperle M. Respiratory depressant effects of different doses of midazolam and lack of reversal with naloxone: a double‐blind randomized study. Anesthesia Analgesia 1983;62:920‐4. [PubMed] [Google Scholar]
Gaddie 1972 {published data only}
- Gaddie J, Legge JS, Palmer KNV, Petrie JC, Wood RA. Effect of nitrazepam in chronic obstructive bronchitis. British Medical Journal 1972;2:688‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrett 2015 {published data only}
- Garrett Key R. Psychiatric care of lung cancer patients. Oncology 2015; Vol. 29, issue 3. [0890‐9091] [PubMed]
Geddes 1976 {published data only}
- Geddes DM, Rudolf M, Saunders KB. Effect of nitrazepam and flurazepam on the ventilatory response to carbon dioxide. Thorax 1976;31:548‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomutbutra 2013 {published data only}
Greene 1989 {published data only}
- Greene JG, Pucino F, Carlson JD, Storsved M. Effects of alprazolam on respiratory drive, anxiety, and dyspnea in chronic airflow obstruction: a case study. Pharmacotherapy 1989;9:34‐8. [DOI] [PubMed] [Google Scholar]
Guilleminault 1993 {published data only}
- Guilleminault C, Clerk A, Labanowski M, Simmons J, Stoohs R. Cardiac failure and benzodiazepines. Sleep 1993;16(6):524‐8. [PubMed] [Google Scholar]
Guz 1980 {published data only}
- Guz A, Minty K, Adams L, Rosser R. Quantifying dyspnoea and the use of drugs to alleviate the symptom. Clinical Respiratory Physiology 1980;16:209. [Google Scholar]
Heinonen 1972 {published data only}
- Heinonen J, Muittari A. The effect of diazepam on airway resistance in asthmatics. Anaesthesia 1972;27:37‐40. [DOI] [PubMed] [Google Scholar]
Hoeijer 1994 {published data only}
- Hoeijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double‐blind placebo‐controlled study. European Respiratory Journal 1994;7(11):2011‐5. [PubMed] [Google Scholar]
Horfarter 2006 {published data only}
- Horfarter B, Weixler D. Symptom control and ethics in final stages of COPD. Wiener Medizinische Wochenschrift 2006;156:275‐82. [DOI] [PubMed] [Google Scholar]
Hosaka 1996 {published data only}
- Hosaka M, Hoshimyama Y. Effects of diazepam on quality of life in patients receiving home oxygen therapy: a double‐blind, cross‐over, placebo‐controlled clinical trial [Japanese]. Journal of the Showa Medical Association 1996;5:522‐9. [Google Scholar]
Huttemann 1971 {published data only}
- Huttemann U, Kunkel G, Lode H, Hass E. The influence of laevomepromazine and prometazine on respiration in healthy subjects and patients with pulmonary emphysema. Arzneimittelforschung 1971;21(10):1594‐8. [PubMed] [Google Scholar]
Johanson 1993 {published data only}
- Johanson GA. Midazolam in terminal care. American Journal of Hospice and Palliative Care 1993;10:13‐4. [DOI] [PubMed] [Google Scholar]
Jokinen 1984 {published data only}
- Jokinen K. Flupenthixol versus diazepam in the treatment of psychosomatic disorders: a double‐blind, multi‐centre trial in general practice. Pharmatherapeutica 1984;3(9):573‐81. [PubMed] [Google Scholar]
Jolly 1996 {published data only}
- Jolly E, Aguirre L, Jorge E, Luna C. Acute effect of lorazepam on respiratory muscles in stable patients with chronic obstructive pulmonary disease. Medicina (B Aires) 1996;56:472‐8. [PubMed] [Google Scholar]
Jones 1985 {published data only}
- Jones AL, Cameron IR. The effect of promethazine and diazepam on respiratory control in breathless patients (abstract). Clinical Science 1985;69:6. [Google Scholar]
Kaltsas 2014 {published data only}
- Kaltsas K, Anevlavis S, Bouros D. Safety of opioids and benzodiazepines in patients with breathlessness and respiratory failure associated with chronic obstructive pulmonary disease. Pneumon 2014; Vol. 27, issue 3:197‐9. [1105‐848X]
Kann 1968 {published data only}
- Kann J, Jokl H. On the therapy of asthmatic dyspnea. Medizinische Klinik 1968;63(45):1814‐8. [PubMed] [Google Scholar]
Kronenberg 1975 {published data only}
- Kronenberg RS, Cosio MG, Stevenson JE, Drage CW. The use of oral diazepam in patients with obstructive lung disease and hypercapnia. Annals of Internal Medicine 1975;83(1):83‐4. [DOI] [PubMed] [Google Scholar]
Lakshminarayan 1976 {published data only}
- Lakshminarayan S, Sahn SA, Hudson LD, Weil JV. Effect of diazepam on ventilatory responses. Clinical Pharmacology and Therapeutics 1976;20(2):178‐83. [DOI] [PubMed] [Google Scholar]
Lareau 1999 {published data only}
- Lareau SC, Meek PM, Press D, Anholm JD, Roos PJ. Dyspnea in patients with chronic obstructive pulmonary disease: does dyspnea worsen longitudinally in the presence of declining lung function?. Heart & Lung 1999;28(1):65‐73. [DOI] [PubMed] [Google Scholar]
Laros 1982 {published data only}
- Laros CD, Bergstein PG. Relief of breathlessness in a case of progressive pulmonary fibrosis. Respiration 1982;43(6):452‐7. [DOI] [PubMed] [Google Scholar]
Lichterfeld 1967 {published data only}
- Lichterfeld A. Controlled therapeutic comparison of antiasthmatics in out‐patients in a double‐blind experiment. Arzneimittel‐Forschung 1967;17:1318‐21. [PubMed] [Google Scholar]
Light 1996 {published data only}
- Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest 1996;109(4):975‐81. [DOI] [PubMed] [Google Scholar]
Marin 1987 {published data only}
- Marin I, Andrieu J‐M, Chretien J. Bronchopulmonary carcinoma: a medical approach to the last weeks of life; a study of 191 cases. Annales de Medecine Interne 1987;138(2):90‐5. [PubMed] [Google Scholar]
McIver 1994 {published data only}
- McIver B, Walsh D, Nelson K. The use of chlorpromazine for symptom control in dying cancer patients. Journal of Pain and Symptom Management 1994;9(5):341‐5. [DOI] [PubMed] [Google Scholar]
Mitchell‐Heggs 1980a {published data only}
- Mitchell‐Heggs P, Murphy K, Minty K, Guz A, Patterson SC, Minty PS, et al. Diazepam in the treatment of dyspnoea in the 'Pink Puffer' syndrome. Quarterly Journal of Medicine 1980;49:9‐20. [PubMed] [Google Scholar]
Mitchell‐Heggs 1980b {published data only}
- Mitchell‐Heggs P, Guz A. Dyspnoea in 'pink puffers': the place of diazepam. British Journal of Diseases of the Chest 1980;74(4):418. [Google Scholar]
Mouzi 2014 {published data only}
Murciano 1990 {published data only}
- Murciano D, Aubier M, Palacios S, Pariente R. Comparison of zolpidem (Z), triazolam (T), and flunitrazepam (F) effects on arterial blood gases and control of breathing in patients with severe chronic obstructive pulmonary disease (COPD) (abstract). Chest 1990;97:51S‐2S. [DOI] [PubMed] [Google Scholar]
Murciano 1993 {published data only}
- Murciano D, Armengaud MH, Cramer PH, Neveux E, L'Heritier C, Pariente R, et al. Acute effects of zolpidem, triazolam and flunitrazepam on arterial blood gases and control of breathing in severe COPD. European Respiratory Journal 1993;6:625‐9. [PubMed] [Google Scholar]
Navigante 1997 {published data only}
- Navigante AH, Sauri A, Palazzo F, Coppola M, De CO, Kirchuk R, et al. Compared and randomized prospective trial between oxygenotherapy vs. morphine chlorhydrate + midazolam by subcutaneous route in patients with advanced cancer and dyspnea. Prensa Medica Argentina 1997;84:474‐6. [Google Scholar]
Navigante 2003 {published data only}
- Navigante AH, Cerchietti LCA, Cabalar ME. Morphine plus midazolam versus oxygen therapy on severe dyspnea management in the last week of life in hipoxemic advanced cancer patients. Medicina Paliativa 2003;10:14‐9. [Google Scholar]
NCT01687751 {unpublished data only}
- NCT01687751. Pilot study comparing treatment with dexmedetomidine to midazolam for symptom control in advanced cancer patients. clinicaltrials.gov/show/NCT01687751.
Nordt 1997 {published data only}
- Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. Journal of Emergency Medicine 1997;15(3):357‐65. [DOI] [PubMed] [Google Scholar]
O'Donnell 1992 {published data only}
- O'Donnell DE, Webb KA. Breathlessness in patients with severe chronic airflow limitation. Physiologic correlations. Chest 1992;102(3):824‐31. [DOI] [PubMed] [Google Scholar]
O'Donnell 1994 {published data only}
- O'Donnell DE. Breathlessness in patients with chronic airflow limitation. Mechanisms and management. Chest 1994;106(3):904‐12. [DOI] [PubMed] [Google Scholar]
O'Donnell 1998 {published data only}
- O'Donnell DE. Dyspnea in advanced chronic obstructive pulmonary disease. Journal of Heart & Lung Transplantation 1998;17(6):544‐54. [PubMed] [Google Scholar]
O'Neill 1985 {published data only}
- O'Neill PA, Morton PB, Stark RD. Chlorpromazine ‐ a specific effect on breathlessness?. British Journal of Clinical Pharmacology 1985;19:793‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1973 {published data only}
- Rao S, Sherbaniuk RW, Prasad K, Lee SJ, Sproule BJ. Cardiopulmonary effects of diazepam. Clinical Pharmacology & Therapeutics 1973;14(2):182‐9. [DOI] [PubMed] [Google Scholar]
Rapoport 1991 {published data only}
- Rapoport DM, Greenberg HE, Goldring RM. Differing effects of the anxiolytic agents buspirone and diazepam on control of breathing. Clinical Pharmacology and Therapeutics 1991;49:394‐401. [DOI] [PubMed] [Google Scholar]
Rice 1986 {published data only}
- Rice KL. Treatment of dyspnea with psychotropic agents. Chest 1986;90:789‐90. [DOI] [PubMed] [Google Scholar]
Rice 1987 {published data only}
- Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. British Journal of Disease of the Chest 1987;81(3):287‐92. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Roisin 2014 {published data only}
Rose 2002 {published data only}
- Rose C, Wallace L, Dickson R, Ayres J, Lehman R, Searle Y. The most effective psychologically‐based treatments to reduce anxiety and panic in patients with chronic obstructive pulmonary disease (COPD): a systematic review. Patient Education Counseling 2002;47(4):311‐8. [DOI] [PubMed] [Google Scholar]
Rudolf 1978 {published data only}
- Rudolf M. Depression of central respiratory drive by nitrazepam. Thorax 1978;33:97‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Runo 2001 {published data only}
- Runo JR, Ely EW. Treating dyspnea in a patient with advanced chronic obstructive pulmonary disease. Western Journal of Medicine 2001;175(3):197‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultze‐Werninghaus 2007 {published data only}
- Schultze‐Werninghaus G, Steinkamp G, Gillissen A, Pfeifer M, Lorenz J, Worth H, et al. The critically ill patient with COPD. Pneumologie 2007;61(6):410‐9. [DOI] [PubMed] [Google Scholar]
Sen 1983 {published data only}
- Sen D, Jones G, Leggat PO. The response of the breathless patient treated with diazepam. British Journal of Clinical Practice 1983;37:232‐3. [PubMed] [Google Scholar]
Singh 1993 {published data only}
- Singh NP, Despars JA, Stansbury DW, Avalos K, Light RW. Effects of buspirone on anxiety levels and exercise tolerance in patients with chronic airflow obstruction and mild anxiety. Chest 1993;103(3):800‐4. [DOI] [PubMed] [Google Scholar]
Smith 2015 {published data only}
- Smith TJ. Symptom management in the older adult. 2015 Update. Clinics in Geriatric Medicine 2015; Vol. 31, issue 2:155‐75. [0749‐0690] [DOI] [PMC free article] [PubMed]
Stark 1981a {published data only}
- Stark RD, Gambles SA. Does diazepam reduce breathlessness in healthy subjects? (abstract). British Journal of Clinical Pharmacology 1982;13:600. [Google Scholar]
Stark 1981b {published data only}
- Stark RD, Gambles SA, Lewis JA. Methods to assess breathlessness in healthy subjects: a critical evaluation and application to analyse the acute effects of diazepam and promethazine on breathlessness induced by exercise or by exposure to raised levels of carbon dioxide. Clinical Science 1981;61:429‐39. [DOI] [PubMed] [Google Scholar]
Stark 1983 {published data only}
- Stark RD, O'Neill PA. Dihydrocodeine for breathlessness in pink puffers. British Journal of Medicine 1983;286:1280‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stark 1988 {published data only}
- Stark RD. Dyspnoea: Assessment and pharmacological manipulation. European Respiratory Journal 1988;1(3):280‐7. [PubMed] [Google Scholar]
Steens 1993 {published data only}
- Steens RD, Pouliot Z, Millar TW, Kryger MH, George CF. Effects of zolpidem and triazolam on sleep and respiration in mild to moderate chronic obstructive pulmonary disease. Sleep 1993;16:318‐26. [DOI] [PubMed] [Google Scholar]
Tenorio 2012 {published data only}
- Tenorio Carmona B, Ramirez Rodriguez G, Rangel Selvera OA, Ales Siles I, Sanchez Del Corral Usaola F, Ruiperez Cantera I. Associated symptoms to terminality in elderly patient and the necessity of subcutaneous application at the home care assistance. European Geriatric Medicine 2012; Vol. 3:S47. [1878‐7649]
Timms 1988 {published data only}
- Timms RM, Dawson A, Hajdukovic RM, Mitler MM. Effect of triazolam on sleep and arterial oxygen saturation in patients with chronic obstructive pulmonary disease. Archives of Internal Medicine 1988;148:2159‐63. [PubMed] [Google Scholar]
Vozoris 2013 {published data only}
Walsh 1993 {published data only}
- Walsh D. Dyspnoea in advanced cancer. The Lancet 1993;342:450‐1. [DOI] [PubMed] [Google Scholar]
Wanrooij 2005 {published data only}
- Wanrooij B, Koelewijn M. Dyspnea relief in the palliative phase. Huisarts en Wetenschap 2005;48(5):239‐45. [Google Scholar]
Wiedemann 1995 {published data only}
- Wiedemann K, Diestelhorst C. The effect of sedation on pulmonary function. Anaesthetist 1995;44:588‐93. [PubMed] [Google Scholar]
Wilson 1954 {published data only}
- Wilson RH, Hoseth W, Dempsey ME. Respiratory acidosis. I. Effects of decreasing respiratory minute volume in patients with severe chronic pulmonary emphysema, with specific reference to oxygen, morphine and barbiturates. American Journal of Medicine 1954;17(4):464‐70. [DOI] [PubMed] [Google Scholar]
Woodcock 1981a {published data only}
- Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. The New England Journal of Medicine 1981;305(27):1611‐6. [DOI] [PubMed] [Google Scholar]
Woodcock 1981b {published data only}
- Woodcock AA, Gross ER, Geddes DM. Oxygen relieves breathlessness in "pink puffers". The Lancet 1981;317(8226):907‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hardy 2016 {published data only}
- Hardy J, Randall C, Pinkerton E, Flatley C, Gibbons K, Allan S. A randomised, double‐blind controlled trial of intranasal midazolam for the palliation of dyspnoea in patients with life‐limiting disease. Support Care Cancer 2016;24:3069. [DOI: 10.1007/s00520-016-3125-2] [DOI] [PubMed] [Google Scholar]
Hart 2012 {published data only (unpublished sought but not used)}
- Hart DE, Corna NE, Horwood F, Maingay G. Randomised control trial of intranasal midazolam or oral lorazepam for the relief of dyspnoea in severe respiratory disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185:A2953. [Google Scholar]
Additional references
Abernethy 2008
- Abernethy AP, Uronis HE, Wheeler JL, Currow DC. Pharmacological management of breathlessness in advanced disease. Progress in Palliative Care 2008;16(1):15‐20. [Google Scholar]
Altose 1985
- Altose MD. Assessment and management of breathlessness. Chest 1985;88:77S‐83S. [DOI] [PubMed] [Google Scholar]
Bausewein 2008
- Bausewein C, Booth S, Gysels M, Higginson IJ. Non‐pharmacological interventions for breathlessness in advanced stages of malignant and non‐malignant diseases. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005623.pub2] [DOI] [PubMed] [Google Scholar]
Beach 2006
- Beach D, Schwartzstein RM. The genesis of breathlessness ‐ what do we understand?. In: Booth S, Dudgeon D editor(s). Dyspnoea in Advanced Disease ‐ a Guide to Clinical Management. 1st Edition. Oxford: Oxford University Press, 2006:1‐18. [Google Scholar]
Booth 2006
- Booth S, Dudgeon D, editors. Dyspnoea in Advanced Disease ‐ a Guide to Clinical Management. 1st Edition. Oxford: Oxford University Press, 2006. [Google Scholar]
Booth 2008
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nature Clinical Practice Oncology 2008;5:90‐100. [DOI] [PubMed] [Google Scholar]
Bruera 2006
- Bruera E, Higginson IJ, Robb SD, Gunten CF. Textbook of Palliative Medicine. 1st Edition. London: Hodder Arnold, 2006. [Google Scholar]
Cranston 2008
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004769.pub2] [DOI] [PubMed] [Google Scholar]
Currow 2010
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. Journal of Pain and Symptom Management 2010;39(4):680‐90. [DOI] [PubMed] [Google Scholar]
Davis 1997
- Davis CL. ABC of palliative care. BMJ 1997;315(7113):931‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2005
- Davis C. Drug therapies. In: Ahmedzai SH, Muers MF editor(s). Supportive Care in Respiratory Disease. 1st Edition. Oxford: Oxford University Press, 2005:147‐63. [Google Scholar]
De Conno 1991
- Conno F, Spoldi E, Caraceni A, Ventafridda V. Does pharmacological treatment affect the sensation of breathlessness in terminal cancer patients?. Palliative Medicine 1991;5:237‐43. [Google Scholar]
Dorman 2009
- Dorman S, Jolley C, Abernethy A, Currow D, Johnson M, Farquhar M, et al. Researching breathlessness in palliative care: consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliative Medicine 2009;23(3):213‐27. [DOI] [PubMed] [Google Scholar]
Edwards 2001
- Edwards A, Elwyn G, Covey J, Matthews E, Pill R. Presenting risk information ‐ a review of the effects of "framing" and other manipulations on patient outcomes. Journal of Health Communication 2001;6(1):61‐82. [PUBMED: 11317424] [DOI] [PubMed] [Google Scholar]
Edwards 2003
- Edwards A, Unigwe S, Elwyn G, Hood K. Personalised risk communication for informed decision making about entering screening programs. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001865.pub2] [DOI] [PubMed] [Google Scholar]
Ekstrom 2014
- Ekstrom MP, Bornefalk‐Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 2014;348:g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
GOLD 2007
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD (2007). http://www.goldcopd.org (accessed 31 January 2008).
Hardman 2005
- Hardman JG, Limbird LE. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th Edition. New York: McGraw‐Hill, 2005. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, The Cochrane Collaboration. Available from www.cochrane‐handbook.org Version 5.1.0 [updated March 2011].
Higginson 2004
- Higginson IJ, Addington‐Hall J. The epidemiology of death and symptoms. In: Doyle D, Hanks G, Cherny N, Calman K editor(s). Oxford Textbook of Palliative Medicine. 3rd Edition. Oxford: Oxford University Press, 2004:14‐24. [Google Scholar]
Jennings 2001
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD002066] [DOI] [PubMed] [Google Scholar]
Lanken 2008
- Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen‐Flaschen J, Heffner JE, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Journal of Respiratory and Critical Care Medicine 2008;177(8):912‐27. [DOI] [PubMed] [Google Scholar]
Manning 1995
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. The New England Journal of Medicine 1995;333:1547‐53. [DOI] [PubMed] [Google Scholar]
Manning 2000
- Manning HL. Dyspnea treatment. Respiratory Care 2000;45(11):1342‐50. [PubMed] [Google Scholar]
Moens 2014
- Moens K, Higginson IJ, Harding R. Are there differences in the prevalence of palliative care‐related problems in people living with advanced cancer and eight non‐cancer conditions? A systematic review. Journal of Pain and Symptom Management 2014; Vol. 48, issue 4:660‐77. [PUBMED: 24801658] [DOI] [PubMed]
Neuman 2006
- Neuman A, Gunnbjornsdottir M, Tunsater A, Nystrom L, Franklin KA, Norrman E, et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respiratory Medicine 2006;100:1843‐9. [DOI] [PubMed] [Google Scholar]
Nordgren 2003
- Nordgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end‐stage heart failure. European Journal of Cardiovascular Nursing 2003;2:213‐7. [DOI] [PubMed] [Google Scholar]
Parshall 2012
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. American Journal of Respiratory and Critical Care Medicine 2012;185(4):435‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 1999
- Ripamonti C. Management of dyspnea in advanced cancer patients. Supportive Care in Cancer 1999;7(4):233‐43. [DOI] [PubMed] [Google Scholar]
Rocker 2007
- Rocker GM, Sinuff T, Horton R, Hernandez P. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. Journal of Palliative Medicine 2007;10(3):783‐97. [DOI] [PubMed] [Google Scholar]
Seow 2011
- Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2011;29(9):1151‐8. [DOI] [PubMed] [Google Scholar]
Simon 2012
- Simon ST, Higginson IJ, Benalia H, Gysels M, Murtagh FE, Spicer J, et al. Episodic and continuous breathlessness: a new categorization of breathlessness. Journal of Pain and Symptom Management 2012;45(6):1019‐29. [DOI] [PubMed] [Google Scholar]
Simon 2014
- Simon ST, Weingärtner V, Higginson IJ, Voltz R, Bausewein C. Definition, categorization, and terminology of episodic breathlessness: consensus by an International Delphi Survey. Journal of Pain and Symptom Management 2014;47(5):828‐38. [DOI] [PubMed] [Google Scholar]
Thomas 2002
- Thomas JR, Gunten CF. Clinical management of dyspnoea. The Lancet Oncology 2002;3(4):223‐8. [DOI] [PubMed] [Google Scholar]
Tobin 1990
- Tobin MJ. Dyspnoea. Archives of Internal Medicine 1990;150:1604‐13. [DOI] [PubMed] [Google Scholar]
Viola 2008
- Viola R, Kiteley C, Lloyd NS, Mackay JA, Wilson J, Wong RK, the Guideline Group. The management of dyspnea in cancer patients: a systematic review. Supportive Care Cancer 2008;16(4):329‐37. [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams CM. Dyspnea. Cancer Journal 2006;12(5):365‐73. [DOI] [PubMed] [Google Scholar]


