Abstract
Background
Cancer‐related fatigue is reported as the most common and distressing symptom experienced by patients with cancer. It can exacerbate the experience of other symptoms, negatively affect mood, interfere with the ability to carry out everyday activities, and negatively impact on quality of life. Educational interventions may help people to manage this fatigue or to cope with this symptom, and reduce its overall burden. Despite the importance of education for managing cancer‐related fatigue there are currently no systematic reviews examining this approach.
Objectives
To determine the effectiveness of educational interventions for managing cancer‐related fatigue in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), and MEDLINE, EMBASE, CINAHL, PsycINFO, ERIC, OTseeker and PEDro up to 1st November 2016. We also searched trials registries.
Selection criteria
We included randomised controlled trials (RCTs) of educational interventions focused on cancer‐related fatigue where fatigue was a primary outcome. Studies must have aimed to evaluate the effect of educational interventions designed specifically to manage cancer‐related fatigue, or to evaluate educational interventions targeting a constellation of physical symptoms or quality of life where fatigue was the primary focus. The studies could have compared educational interventions with no intervention or wait list controls, usual care or attention controls, or an alternative intervention for cancer‐related fatigue in adults with any type of cancer.
Data collection and analysis
Two review authors independently screened studies for inclusion and extracted data. We resolved differences in opinion by discussion. Trial authors were contacted for additional information. A third independent person checked the data extraction. The main outcome considered in this review was cancer‐related fatigue. We assessed the evidence using GRADE and created a 'Summary of Findings' table.
Main results
We included 14 RCTs with 2213 participants across different cancer diagnoses. Four studies used only 'information‐giving' educational strategies, whereas the remainder used mainly information‐giving strategies coupled with some problem‐solving, reinforcement, or support techniques. Interventions differed in delivery including: mode of delivery (face to face, web‐based, audiotape, telephone); group or individual interventions; number of sessions provided (ranging from 2 to 12 sessions); and timing of intervention in relation to completion of cancer treatment (during or after completion). Most trials compared educational interventions to usual care and meta‐analyses compared educational interventions to usual care or attention controls. Methodological issues that increased the risk of bias were evident including lack of blinding of outcome assessors, unclear allocation concealment in over half of the studies, and generally small sample sizes. Using the GRADE approach, we rated the quality of evidence as very low to moderate, downgraded mainly due to high risk of bias, unexplained heterogeneity, and imprecision.
There was moderate quality evidence of a small reduction in fatigue intensity from a meta‐analyses of eight studies (1524 participants; standardised mean difference (SMD) ‐0.28, 95% confidence interval (CI) ‐0.52 to ‐0.04) comparing educational interventions with usual care or attention control. We found low quality evidence from twelve studies (1711 participants) that educational interventions had a small effect on general/overall fatigue (SMD ‐0.27, 95% CI ‐0.51 to ‐0.04) compared to usual care or attention control. There was low quality evidence from three studies (622 participants) of a moderate size effect of educational interventions for reducing fatigue distress (SMD ‐0.57, 95% CI ‐1.09 to ‐0.05) compared to usual care, and this could be considered clinically significant. Pooled data from four studies (439 participants) found a small reduction in fatigue interference with daily life (SMD ‐0.35, 95% CI ‐0.54 to ‐0.16; moderate quality evidence). No clear effects on fatigue were found related to type of cancer treatment or timing of intervention in relation to completion of cancer treatment, and there were insufficient data available to determine the effect of educational interventions on fatigue by stage of disease, tumour type or group versus individual intervention.
Three studies (571 participants) provided low quality evidence for a reduction in anxiety in favour of the intervention group (mean difference (MD) ‐1.47, 95% CI ‐2.76 to ‐0.18) which, for some, would be considered clinically significant. Two additional studies not included in the meta‐analysis also reported statistically significant improvements in anxiety in favour of the educational intervention, whereas a third study did not. Compared with usual care or attention control, educational interventions showed no significant reduction in depressive symptoms (four studies, 881 participants, SMD ‐0.12, 95% CI ‐0.47 to 0.23; very low quality evidence). Three additional trials not included in the meta‐analysis found no between‐group differences in the symptoms of depression. No between‐group difference was evident in the capacity for activities of daily living or physical function when comparing educational interventions with usual care (4 studies, 773 participants, SMD 0.33, 95% CI ‐0.10 to 0.75) and the quality of evidence was low. Pooled evidence of low quality from two of three studies examining the effect of educational interventions compared to usual care found an improvement in global quality of life on a 0‐100 scale (MD 11.47, 95% CI 1.29 to 21.65), which would be considered clinically significant for some.
No adverse events were reported in any of the studies.
Authors' conclusions
Educational interventions may have a small effect on reducing fatigue intensity, fatigue's interference with daily life, and general fatigue, and could have a moderate effect on reducing fatigue distress. Educational interventions focused on fatigue may also help reduce anxiety and improve global quality of life, but it is unclear what effect they might have on capacity for activities of daily living or depressive symptoms. Additional studies undertaken in the future are likely to impact on our confidence in the conclusions.
The incorporation of education for the management of fatigue as part of routine care appears reasonable. However, given the complex nature of this symptom, educational interventions on their own are unlikely to optimally reduce fatigue or help people manage its impact, and should be considered in conjunction with other interventions. Just how educational interventions are best delivered, and their content and timing to maximise outcomes, are issues that require further research.
Keywords: Adult; Female; Humans; Male; Middle Aged; Activities of Daily Living; Anxiety; Anxiety/therapy; Fatigue; Fatigue/etiology; Fatigue/therapy; Neoplasms; Neoplasms/complications; Neoplasms/therapy; Patient Education as Topic; Patient Education as Topic/methods; Problem Solving; Quality of Life; Randomized Controlled Trials as Topic; Reinforcement, Psychology
Plain language summary
Education for the management of cancer‐related fatigue
Objectives
This systematic review sought to find out how well educational interventions worked for managing cancer‐related fatigue.
Condition
Fatigue is a common and problematic symptom for people with cancer that is greater than the tiredness experienced in everyday life. It can make the experience of other symptoms worse, negatively affect mood, interfere with the ability to carry out everyday activities, and negatively impact on quality of life.
Interventions
Education can provide people with information about what fatigue is and how to manage it. For example, managing fatigue may involve conserving energy throughout the day, and learning about the benefits of exercise, diet, relaxation, and good sleep routines. These approaches may help people to manage their fatigue and help them cope with its effects. In November 2016 we found 14 trials using education for cancer‐related fatigue compared to the usual care people received or to an attention control such as providing general information about cancer. All of the included studies were randomised controlled trials. These trials were undertaken with adults with any type or stage of cancer.
Results
The review found that education may have a small effect on reducing the intensity of fatigue, its interference in daily activities or relationships, and general (overall) fatigue. It could have a moderate effect on reducing distress from fatigue amongst people with non‐advanced cancer. There may also be beneficial effects on anxiety and overall quality of life, although it is unclear whether it reduces depression. It is unknown if this result might differ between types of cancer treatment or if the education is provided during or after cancer treatment. Not enough is known about the type of education that is most effective, when it is best provided, or whether it is effective for people with advanced cancer.
Quality of evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low quality evidence means that we are very uncertain about the results. High quality evidence means that we are very confident in the results. There were problems with the design of some studies, and some were very small in size. The quality of the evidence therefore varied from very low to moderate overall and the results of this review need to be interpreted with caution.
Summary of findings
Summary of findings for the main comparison. Educational interventions versus control (usual care or attention control) for cancer‐related fatigue in adults.
| Educational interventions versus control (usual care or attention control) for cancer‐related fatigue in adults | ||||||
|
Patient or population: patients with cancer‐related fatigue (adults)
Settings: outpatients and community
Intervention: educational interventions Comparison: usual care or attention control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
|
General fatigue (at the end of the educational intervention) |
Not known | Not known | General fatigue in the educational intervention group was lower than in the control group (SMD ‐0.27, 95% CI ‐0.51 to ‐0.04) |
1711 (12 studies) | ⊕⊕⊝⊝ low1,2 | An SMD of ‐0.27 represents a small effect size with the upper end of the confidence interval suggesting this could be clinically significant for some people. |
|
Fatigue intensity (at the end of the educational intervention) |
Not known | Not known | Fatigue intensity in the educational intervention group was lower than in the control group (SMD ‐0.28, 95% CI ‐0.51 to ‐0.04) |
1524 (8 studies) | ⊕⊕⊕⊝ moderate1 | An SMD of ‐0.28 represents a small effect size with the upper end of the confidence interval suggesting this could be clinically significant for some people. |
|
Fatigue distress (at the end of the educational intervention) |
Not known | Not known | Fatigue distress in the educational intervention group was lower than in the control group (SMD ‐0.57, 95% CI 1.09 to 0.05) |
622 (3 studies) | ⊕⊕⊝⊝ low1,2 | An SMD of ‐0.57 represents a medium effect size that could be considered clinically significant. |
|
Fatigue interference (at the end of the educational intervention) |
Not known | Not known | Fatigue interference in the educational intervention group was lower than in the control group (SMD 0.35, 95% CI ‐0.54 to ‐0.16) |
439 (4 studies) | ⊕⊕⊕⊝ moderate1 | An SMD of ‐0.35 represents a small effect size with the upper end of the confidence interval suggesting this could be clinically significant for some people. |
|
Anxiety (at the end of the educational intervention) |
No assumed risk | Anxiety in the intervention group was lower than in the control group (MD ‐1.47, 95% CI ‐2.76 to ‐0.18) | 571 (3 studies) | ⊕⊕⊝⊝ low1,2 | An MD of ‐1.47 represents a small effect size in comparison to the scale range of 0 to 21, but the confidence interval suggests it may be clinically significant for some people. | |
|
Depression (at the end of the educational intervention) |
Not known | Not known | No significant difference in depression in the educational intervention group compared to the control group (SMD ‐0.12, 95% CI ‐0.47 to 0.23) |
881 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | Result is not statistically significant |
|
Global quality of life (at the end of the educational intervention) |
Not known | Not known | Global quality of life/health status in the educational intervention group was higher than in the control group (MD 11.47, 95% CI 1.29 to 21.65) |
477 (2 studies) | ⊕⊕⊝⊝ low1,3 | An MD of 11.47 (95% CI 1.29 to 21.65) on a 0‐100 scale represents a difference that would be clinically significant for some people. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Downgraded once: high risk of bias due to inadequate blinding of participants or assessors, and more than one other criterion had unknown risk of bias. 2 Downgraded once: high level of unexplained heterogeneity was evident. 3 Downgraded once: wide confidence interval for estimate.
Background
Description of the condition
Cancer‐related fatigue is reported by the National Comprehensive Cancer Network (NCCN; an alliance of many cancer centres based in the USA) as being the most common and, for some, the most distressing symptom experienced by people with cancer (NCCN 2016; Stark 2012). It can exacerbate the experience of other symptoms, negatively affect mood, interfere with the ability to carry out everyday activities, and negatively impact on quality of life (Mitchell 2006). Cancer‐related fatigue is different from normal fatigue in that it is not relieved by rest and can persist for months or even years after the completion of cancer treatment (Bower 2006). Although there is no agreed definition of cancer‐related fatigue, the most recent definition proposed by the NCCN defines cancer‐related fatigue as: "A distressing persistent, subjective sense of physical tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning" (NCCN 2016). Current understanding of the aetiology of cancer‐related fatigue is poor. It is likely that it is a result of a complex interaction of multiple factors related to both the disease process itself and side effects of treatment, but it is also likely to be influenced by a range of other factors such as medications, nutrition, sleep disturbance, pain, anxiety, and depression (Purcell 2009). The problem of cancer‐related fatigue is commonly reported in terms of its prevalence, with studies from Europe, the United States, and Japan reporting prevalence rates between 4% and 91% (Lawrence 2004), depending on factors such as tumour type, treatment type, time of measurement, and type of measurement tool used. Cancer‐related fatigue may be experienced at any stage of the disease trajectory or during cancer treatment. A number of studies have shown a pattern of increasing fatigue during treatment that often improves soon after the completion of treatment (Jacobsen 1999; Smets 1998a); but for some, fatigue may continue for long periods of time (Bower 2006; Smets 1998b). There is some research showing increases in fatigue amongst those receiving combination therapies (Woo 1998), and higher prevalence levels among those with advanced cancer (Stone 2000). However, the experience of cancer‐related fatigue may better be understood in terms of symptom characteristics such as its intensity, duration, and associated distress rather than prevalence statistics. These characteristics may be captured to varying degrees by components of symptom and quality of life measures such as the Functional Assessment of Cancer Therapy‐Fatigue (FACT‐F; Yellen 1997), European Organization for Research and Treatment of Cancer Core Questionnaire (EORTC QLQ‐C30; Aaronson 1993), and the Memorial Symptom Assessment Scale (MSAS; Portenoy 1994), and other measures of symptom distress such as the distress thermometer (Butt 2008). Fatigue has also been measured using multi‐dimensional fatigue measurement instruments that consider dimensions such as physical, cognitive, and emotional fatigue. Examples of these tools include the Multidimensional Fatigue Inventory (MFI‐20; Smets 1996), Fatigue Symptom Inventory (FSI; Hann 1998) and Revised Piper Fatigue Scale; Piper 1998).
Management of cancer‐related fatigue is hampered by lack of knowledge about its aetiology although attempts have been made to develop interventions taking into account the possible factors that may contribute to it. Guidelines developed by the NCCN recommend that treatable factors that may contribute to fatigue should be treated initially (NCCN 2016). These include pain, emotional distress, sleep disturbance, anaemia, nutrition, activity level, and co‐morbidities. Interventions recommended for those receiving active treatment, those receiving long term follow‐up, and for people at the end of life include education and counselling, general strategies for the management of fatigue such as energy conservation and distraction, and pharmacological and non‐pharmacological interventions (NCCN 2016).
Previous reviews of pharmacological interventions have included studies testing the effects of antidepressants, corticosteroids, medications to manage anaemia, and psychostimulants (Minton 2013 (a Cochrane systematic review); Morrow 2005), with some improvement in cancer‐related fatigue found with the psychostimulants (Minton 2013). Non‐pharmacological interventions designed to manage cancer‐related fatigue have the benefit of addressing multiple symptoms, have minimal, if any, side‐effects, and are acceptable to people with cancer. There has been increasing research on the effectiveness of these treatments for cancer‐related fatigue over the last decade with promising findings from randomised controlled trials (RCTs) for exercise (Mock 2005), psycho‐educational approaches (Yates 2005), and energy conservation (Barsevick 2004), amongst others. A review of 57 RCTs of non‐pharmacological interventions concluded that when considered as a whole, exercise and psychological interventions provided similar reductions in cancer‐related fatigue (Kangas 2008). Specifically, multimodal exercise and walking programs, restorative approaches, and supportive–expressive and cognitive–behavioral psychosocial interventions were identified in the review as having the potential for reducing cancer‐related fatigue. Systematic reviews have also been undertaken for specific interventions aimed at ameliorating fatigue, including exercise (Cramp 2012 (a Cochrane systematic review); Stricker 2004), complementary therapies (Sood 2007), and psychosocial interventions during cancer treatment (Goedendorp 2009 (a Cochrane systematic review)).
Description of the intervention
Patient education has been defined as "a systematic learning experience in which a combination of methods is generally used, such as the provision of information and advice and behaviour modification techniques, which influence the way the patient experiences his illness and/or his knowledge and health behaviour, aimed at improving or maintaining health or learning to cope with a condition, usually a chronic one...it may also involve influencing emotions and attitudes and is often aimed at altering behaviour" (van den Bourne 1998). In this systematic review, patient education or educational interventions are defined as any advice, information, or self‐management education, using any delivery format (verbal, written, or audiovisual, Internet), provided in order to help people understand and manage cancer‐related fatigue. This may incorporate information and advice about non‐pharmacological strategies (e.g. information about relaxation, information about cognitive behavioural therapy (CBT), or information about exercise), but would exclude trials that actually use these interventions. Educational interventions may use techniques such as providing advice and information, discussion, coaching, goal‐setting, feedback, and reinforcement that are also used in psychological therapies such as cognitive behavioural therapy (CBT), but the intervention would not be classified as CBT itself. Alternatively, educational interventions will be those which have been classified by the study's authors as such.
Education was often described as a component of the psychosocial interventions in the Cochrane review of psychosocial interventions to ameliorate cancer‐related fatigue during cancer treatment (Goedendorp 2009), and is recommended in the NCCN guidelines as the key management strategy. This review differs from the systematic review by Goedendorp 2009 in that it focuses on education as the sole intervention and does not include studies that may have also used psychological interventions such as relaxation training or CBT. A recent systematic review (Du 2015) purported to review education programs for cancer‐related fatigue, included 10 trials, and found limited evidence to support its use. However, three of the trials included other modalities (e.g. relaxation or exercise), and another did not have fatigue as the primary focus. Therefore the effectiveness of educational interventions has not been rigorously systematically reviewed until now, and is the focus of this review.
How the intervention might work
Education imparts information designed to improve knowledge and skills. Education is integral to the effective management of symptoms related to cancer and its treatment and in helping people with cancer manage side effects and make informed decisions (Chelf 2001). Having knowledge that fatigue is a common experience amongst those with cancer and that it increases during treatment, and knowledge of self‐management strategies to manage fatigue, may help people cope with this symptom and reduce its overall burden. Knowledge about cancer‐related fatigue and its management may relieve people's anxiety about the presence of this symptom, provide them with a sense of control, and help them develop necessary skills and motivation for behaviour changes that might assist in alleviating fatigue (Chelf 2001; Hinds 1995; Ream 1996). Such behaviours might include self care actions for promoting good sleep and rest, pacing and prioritising activities during the day, balancing exercise and rest, and using restorative activities (Yates 2005). Education about cancer‐related fatigue is particularly recommended for those commencing treatment (NCCN 2016), but may be useful at other time points, particularly for those who continue to find fatigue distressing or experience ongoing interference with everyday activities as a result of fatigue. In these instances, education about the management of cancer‐related fatigue may assist people to optimise their activity levels, participation, and quality of life within the confines of the fatigue levels they experience.
Why it is important to do this review
Despite the importance of education for managing cancer‐related fatigue, there are currently no systematic reviews examining this approach. In this systematic review we aim to clarify the effectiveness of educational interventions in the management of cancer‐related fatigue and this will, in turn, inform decision‐making and identify significant gaps in the research regarding this distressing symptom.
Objectives
To determine the effectiveness of educational interventions for managing cancer‐related fatigue in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were RCTs of interventions. Quasi‐randomised trials and crossover trials were excluded. No restrictions on language or publication status were applied.
Types of participants
Studies involving adults aged 18 years and older were included regardless of gender, stage of disease, tumour type, and type of treatment. Participants could have been receiving curative or palliative treatment or long‐term follow‐up, or could have had no evidence of active disease.
Types of interventions
To be included, studies must have stated that they aimed to evaluate the effect of educational interventions designed specifically to manage cancer‐related fatigue, or educational interventions targeting a constellation of physical symptoms or quality of life where fatigue was the primary focus. For the purpose of this review, educational interventions were defined as any advice, information, or self‐management education (verbal, written, or audiovisual) provided in order to help people understand and manage cancer‐related fatigue. These may have incorporated information and advice about non‐pharmacological strategies (e.g. information about relaxation, nutrition, CBT, or exercise), but cannot have actually used these interventions. Studies may have used techniques such as discussion, coaching, goal‐setting, feedback, and reinforcement that may also be used in psychological therapies such as CBT, but the intervention would not be classified as CBT itself. Interventions did not need to be delivered face to face but may have included interventions delivered via telephone, post, or the Internet. The intervention may have taken place in any setting, been delivered either to a group or an individual, involved a single education session or a series of sessions or delivered in either groups or with individuals. The studies could have compared educational interventions with no intervention or wait list controls, attention controls, or an alternative intervention for cancer‐related fatigue.
Types of outcome measures
Included studies must have considered fatigue or its management as the primary outcome of interest; this included fatigue if it was measured as a main outcome within a constellation of physical symptoms or quality of life, provided separate data for fatigue were available. Outcome measures of fatigue or its management were via self‐report, as fatigue is a subjectively experienced symptom. Self‐report of fatigue or its management was measured through questionnaires or diaries.
Primary outcomes
Fatigue was the primary outcome of interest for this review. It may have been assessed by validated fatigue scales or by any method of self‐evaluation. Fatigue may have been measured in terms of characteristics such as intensity, distress, interference, duration, or frequency, or as dimensions such as physical fatigue, mental fatigue, or general fatigue. In this review four separate measures of fatigue were considered; 1) fatigue intensity, 2) fatigue distress, 3) perceived fatigue interference, and 4) general fatigue. General fatigue was operationalised for this review as fatigue measures that combined different characteristics of fatigue (e.g. fatigue intensity, distress, and interference), that combined different dimensions of fatigue (e.g. cognitive and physical and emotional fatigue), or that stated that they were measures of general fatigue.
Secondary outcomes
Secondary outcomes included fatigue management concepts such as coping with fatigue, knowledge acquisition about fatigue, use of strategies for managing fatigue taught in the intervention, or fatigue self‐efficacy. Capacity to perform activities of daily living or physical functioning, anxiety, depression, and global quality of life were also considered. These were recorded regardless of the direction of effect. It should be noted that 'perceived fatigue interference' (a primary outcome) asks the individual directly about the degree to which fatigue affects their activities of daily living, whereas measures of the outcome 'activities of daily living' (secondary outcome) only ask about activities of daily living ‐ not the degree to which fatigue affects them. Adverse events were not recorded in studies within this review but educational interventions could conceivably increase anxiety, distress, and fatigue as a result of increasing the time and attention that individuals focus on these issues.
Search methods for identification of studies
Electronic searches
We searched:
The Cochrane Central Register of Controlled Trials (CENTRAL) via CRSO on 1/11/16;
MEDLINE (OVID; 1950 to October week 3 2016);
Embase (OVID; 1966 to October 2016 week 4);
CINAHL (EBSCO; 1982 to October 2016);
PsycINFO (OVID4; 1840 to October 2016);
ERIC (1966 to October 2016);
OTseeker (to October 2016);
PEDro (to October 2016).
We developed the search strategy for MEDLINE using the Cochrane filter for the identification of RCTs, as published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), and added relevant terms for this topic. The search strategies used can be found in Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5, Appendix 6, Appendix 7 and Appendix 8. Non‐English language studies were considered if they had an abstract published in English.
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials we:
checked reference lists of all relevant studies;
searched ongoing trials and research registers including ClinicalTrials.gov (www.clinicaltrials.gov/; accessed October 2016) and the Australian New Zealand Clinical Trials Registry (accessed October 2016);
contacted investigators known to be involved in research in this area;
handsearched nine relevant journals.
Data collection and analysis
Selection of studies
One review author (CD) initially screened titles and abstracts and eliminated those obviously not relevant to this review. Two review authors (SB and PM) independently screened the remaining titles and abstracts for their eligibility for inclusion in accordance with the above defined criteria. When the title and abstract did not provide all the information necessary to assess relevance, we retrieved full paper copies for screening. We retrieved full text copies of studies when either review author determined that the study possibly or definitely met the inclusion criteria (Figure 1).
1.

Study flow diagram.
For a trial to be included it must have contained fatigue as a primary outcome measure and one treatment arm must have been an educational intervention with the primary aim being management of fatigue.
We included a study if all of the following were met.
It was an RCT.
The primary aim of the study was to evaluate the effect of educational interventions to manage cancer‐related fatigue.
Participants were 18 years of age or older.
Participants were diagnosed with cancer.
At least one of the study arms received an educational intervention designed specifically to manage cancer‐related fatigue, or an educational intervention targeting a constellation of physical symptoms or quality of life where fatigue was the primary focus.
The primary outcome of interest included the measurement of fatigue or its management. Measurement could be as a separate measure of fatigue, or as part of a quality of life measure providing separate data for fatigue were available (e.g. as a sub scale).
We contacted study authors where information was unclear. We resolved disagreement about the selection of a study by consensus.
Data extraction and management
Two review authors (SB and TH) independently extracted data from the studies using a standard data extraction form. A third independent person not associated with any of the included trials checked the data extraction. We contacted authors in order to obtain any missing data. We collected the following data.
Study
Aim of study.
Participant characteristics
Demographic characteristics such as age and gender.
Disease characteristics such as tumour type and stage of disease.
Treatment characteristics such as type and duration of cancer treatment
Inclusion/exclusion criteria for participation in the study.
Intervention characteristics
We extracted the following information for each arm of the study where possible.
Aim, type of delivery/media used, and content of the intervention.
Time point of delivery of the intervention relative to completion of treatment/stage of disease.
Duration of the intervention, total number of sessions, and duration of each session.
Description of comparison intervention(s) (e.g. Usual care (which may or may not involve a degree of education), wait‐list control, or lower intensity educational intervention).
Setting of the intervention (where it was actually delivered; e.g. hospital, home, or community setting).
Group or individual intervention delivery
Outcomes
Timing, frequency, and duration of follow‐up for each outcome.
-
Key outcomes and measurement instruments used including:
fatigue or lack of energy (measured as fatigue intensity, fatigue distress, fatigue interference, general fatigue, or a combination of these);
knowledge acquisition about fatigue;
self‐reported use of strategies taught in the intervention;
perceived coping with fatigue;
self‐efficacy for the management of fatigue;
capacity to perform activities of daily living or physical functioning;
anxiety and depression;
global quality of life.
Other
Sample size and evidence of power calculation.
Follow‐up ‐ withdrawals/dropouts and intention to treat analysis.
Assessment of risk of bias in included studies
Two review authors (SB and CD) independently assessed the risk of bias of the selected studies (Figure 2, Figure 3), using the Cochrane 'Risk of bias' tool in accordance with methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Ascertaining risk of bias involved considering the following seven domains.
2.
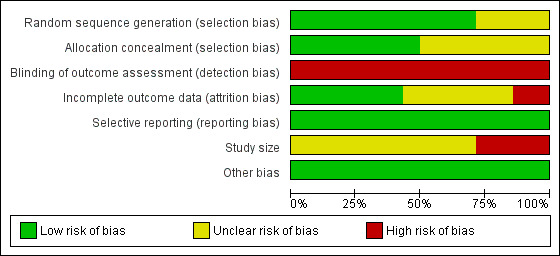
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
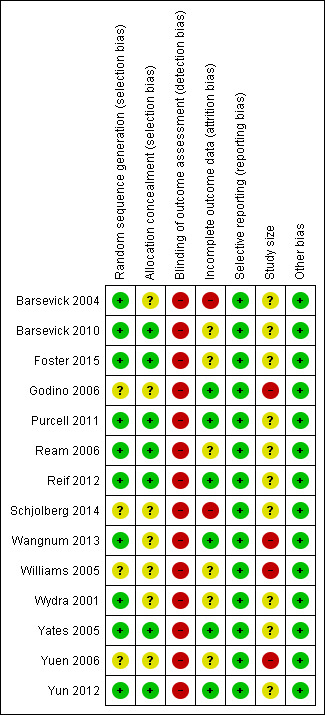
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation.
Allocation sequence concealment.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Study size.
Other bias.
Blinding of participants and personnel was not included as a domain in the 'Risk of bias' assessment because it is not achievable for any studies of educational interventions due to the nature of the intervention.
We assessed the size of the study in line with the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group's policy: low risk of bias for studies with 200 participants or more per treatment arm; unclear risk of bias for studies with 50 to 199 participants per treatment arm; or high risk of bias for studies with fewer than 50 participants per treatment arm.
We assessed each study as being at 'low risk of bias', 'high risk of bias', or 'unclear risk of bias' for each of the 'Risk of bias' items, based on the study reports and/or additional information provided by the study authors. We conducted these assessments in accordance with methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When we could not determine from the report if the criteria had or had not been met, we indicated an 'unclear risk of bias'.
Measures of treatment effect
We used Cochrane's Review Manager software, RevMan 5 (RevMan 2014), for all analyses. For continuous data, we used unadjusted post‐scores (outcomes that were recorded immediately after the end of the intervention period). We did not analyse data from follow‐up time points. We did not calculate change over time scores for the purposes of meta‐analyses in this review, but we reported change scores provided by study authors in the narrative description of results if post‐score data were not available. We calculated the mean difference (MD) and the 95% confidence interval (CI) for continuous data. For continuous outcomes where no standard deviations (SD) were reported, we planned to calculate the SD using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions. We calculated the standardised mean difference (SMD) and the 95% CI for continuous data that measured the same outcome using different measurement tools as per the plan for data synthesis described below. We planned to calculate the relative risks (RRs) and 95% CI and the number needed to treat (NNT) for dichotomous data.
Unit of analysis issues
Unit of analysis was the participant.
Dealing with missing data
We analysed data for all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised, we had planned to attempt to restore them to the correct group using data from the report or from the authors. However this was not possible as relevant data were not available.
Assessment of heterogeneity
We first assessed the studies for clinical homogeneity with respect to the population, intervention, and outcomes. We planned to describe studies that we judged to be too clinically heterogeneous separately and not combine them in a meta‐analysis. We tested studies without substantial clinical heterogeneity for statistical heterogeneity using the I2 statistic (I2 greater than 50% was considered substantial heterogeneity). If we suspected heterogeneity, we explored possible causes using subgroup analyses (where sufficient studies were available) and used the random‐effects model.
Assessment of reporting biases
Where we suspected reporting bias, we attempted to contact study authors to ask them to provide missing outcome data. Where this was not possible, and we suspected that the missing data introduced serious bias, we did not include the data for these outcomes in the meta‐analysis.
Data synthesis
We pooled clinically and statistically homogeneous studies using the fixed‐effect model, and clinically homogeneous and statistically heterogeneous studies using the random‐effects model. We combined continuous data only where (i) means and SDs were available or calculable and (ii) there was no clear evidence of skew in the distribution (using methods described in the Cochrane Handbook for Systematic Reviews of Interventions). When able to be combined, we used SMDs of scales measuring the same clinical outcomes in different ways in order to combine results across scales; otherwise we used MDs.
Quality of the evidence
One review author (SB) rated the quality of the outcomes: general fatigue, fatigue intensity, fatigue distress, fatigue interference, use of fatigue management strategies, activities of daily living or physical functioning, anxiety, depressive symptoms, and global quality of life. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (GRADEPro GDT 2015), and the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning a grade of evidence quality.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We decreased the grade if the following were present.
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of general fatigue, fatigue intensity, fatigue distress, fatigue interference, anxiety, depression, and global quality of life.
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, we undertook subgroup analysis based on type of cancer treatment, timing of intervention relative to completion of cancer treatment (e.g. intervention during cancer treatment or intervention delivered following completion of cancer treatment), tumour type, stage of disease, and group versus individual intervention. We completed subgroup analysis using the Deeks method (Deeks 2001). We restricted analyses to effects on the primary outcome (fatigue).
Sensitivity analysis
We planned to carry out sensitivity analysis, where appropriate, to explore the effects of risk of bias. We had planned to exclude studies at high risk of bias for concealment of allocation from the analysis in order to assess any substantive change in the overall result. We chose to focus on concealed allocation because, according to Pildal 2007 (p 854) "most conclusions favouring an intervention would lose support if trials with unclear or inadequate allocation concealment were excluded from the meta‐analysis." If no substantive difference existed, we left the studies in for the main analysis. We planned to conduct this sensitivity analysis for the primary outcome only.
Results
Description of studies
Results of the search
We retrieved a total of 2428 references from the searches and a further 61 through other sources such as hand searches. Following removal of duplicates there were 1884 references for consideration. After a screening of titles and abstracts by the authors, 1839 were discarded, leaving 45 reports to be considered for eligibility. Of these, we found 19 reports to be eligible; two reported on one study (Williams 2005), five reported on another (Foster 2015). Therefore, there were 14 studies that met the inclusion criteria and were included in the review, with a further four studies awaiting classification (Figure 1). Details of the 14 included studies are described in the Characteristics of included studies tables, and details of the studies awaiting classification are available in Characteristics of studies awaiting classification.
Included studies
Fourteen RCTs were included in this review and their details are provided in the Characteristics of included studies tables, including statements about their sources of funding.
Participants
In total, 2213 participants were included in these studies with a range of cancer diagnoses. Nine studies included participants with different cancer diagnoses (Barsevick 2004; Barsevick 2010; Foster 2015; Purcell 2011; Ream 2006; Reif 2012; Wydra 2001; Yuen 2006; Yun 2012), three specifically focused on women with breast cancer (Schjolberg 2014; Williams 2005; Yates 2005), one on people who had lung cancer (Wangnum 2013), and one on people with colon or gastric cancer (Godino 2006). Three studies included only females (Schjolberg 2014; Yates 2005; Williams 2005). The average ages for participants in each study were in the 50's. For detailed information on study participants see the Characteristics of included studies tables.
Interventions
The included studies used a range of educational interventions. Nine studies investigated the use of educational interventions commencing during cancer treatment (Barsevick 2004; Barsevick 2010; Godino 2006; Purcell 2011; Ream 2006; Wangnum 2013; Williams 2005; Wydra 2001;Yates 2005), with one of these studies using a factorial design with one study arm commencing post‐treatment (Purcell 2011). The remaining five studies commenced intervention with participants following the completion of their cancer treatment (Foster 2015; Schjolberg 2014; Reif 2012; Yuen 2006; Yun 2012). Four studies could be classified as purely having used 'information‐giving' educational strategies (Foster 2015; Williams 2005; Wydra 2001; Yun 2012), whereas the remainder used mainly information‐giving strategies coupled with some behavioural techniques such as problem‐solving and reinforcement, or support strategies. Of the 14 studies, eight included face to face delivery of the majority of the content (Godino 2006; Purcell 2011; Ream 2006; Reif 2012; Schjolberg 2014; Wangnum 2013; Yates 2005; Yuen 2006). These studies delivered the intervention for between two and nine sessions with the majority providing three sessions. The time frames for each session varied between 10 minutes and one day, with the median being 30 minutes. Two interventions that were delivered through the Internet allowed participants to determine the length of each session. The six remaining studies delivered the intervention verbally through three telephone sessions (Barsevick 2004; Barsevick 2010), a 20 minute audiotape format (Williams 2005), an interactive multi‐media module (Wydra 2001), or web‐based sessions (Foster 2015; Yun 2012). Four studies provided the intervention at the outpatient clinic where the patient was receiving treatment (Godino 2006; Purcell 2011; Reif 2012; Schjolberg 2014; Wydra 2001) with the remainder of studies providing the educational intervention to patients at home (face to face or by telephone). Three studies provided the intervention in a group format (Purcell 2011; Reif 2012; Schjolberg 2014). Interventions were mostly delivered by nurses, but two were delivered by occupational therapists or other allied health professionals (Purcell 2011; Yuen 2006). In the majority of studies (n = 9), the comparison arm was described as a 'standard care' or 'usual care' control group. In four of the 14 studies the participants received an attention control comprising phone calls about nutritional information (Barsevick 2004; Barsevick 2010), a leaflet about fatigue (Foster 2015), or general cancer education (Yates 2005). In the remaining study the comparison arm was a 'wait list' control (that is, the control group participants remained on a waiting list and were offered the intervention once the study was complete).
For detailed information on interventions see the Characteristics of included studies table.
Comparisons
This review compared educational interventions with no intervention or wait list controls (Reif 2012), usual care ( Godino 2006; Purcell 2011; Ream 2006; Schjolberg 2014; Wangnum 2013; Williams 2005; Wydra 2001; Yuen 2006; Yun 2012), attention controls providing information about nutrition (Barsevick 2004; Barsevick 2010) or general cancer education (Yates 2005), or with an alternative intervention (a leaflet on cancer‐related fatigue; Foster 2015).
Main Outcomes
Fatigue or its management was the primary outcome of interest. Seventeen different measures of fatigue were used in the studies contained in this review. In this review, we made a distinction between measurement of fatigue severity (measures that rated the intensity of fatigue), fatigue interference (measures that purely assessed interference with activities due to fatigue), and fatigue distress (a direct statement of how distressing the participant finds fatigue). We also considered measures of general (overall) fatigue. General fatigue measures included a combination of items covering characteristics of fatigue (fatigue intensity, fatigue interference, and fatigue distress), and/or multiple dimensions of fatigue (e.g. cognitive, physical, and emotional fatigue).
We were able to extract fatigue severity data from studies that used: single item visual analogue scales (VAS) such as the EORTC QLQ‐C30 fatigue scale that asks people to rate their fatigue (tiredness) on a 0‐100 scale (e.g. Reif 2012); the fatigue severity item of the Piper Fatigue Scale (e.g. Yates 2005); sub scales measuring fatigue intensity such as the Profile of Mood States ‐ Fatigue sub scale (e.g. Barsevick 2004); or the Lee Fatigue Scale ‐ Fatigue sub scale (Schjolberg 2014).
A direct, separate measurement of fatigue interference was available in four studies or from their authors. Fatigue interference was measured using a single VAS of subjective assessment of the effect of fatigue on chores/work or on pastimes/hobbies (Ream 2006), or by using the Inteference sub scale of the Brief Fatigue Inventory (Yun 2012). Although a number of other studies measured fatigue interference, the data for the specific items about interference were not available separately but were combined within scales that also measured other characteristics of fatigue such as fatigue intensity and fatigue distress. In this review we classified the latter measures as measures of general fatigue and reported on them below.
Separate data for fatigue distress were available from three studies or their authors (Ream 2006; Reif 2012; Yuen 2006), and in each study fatigue distress was measured using a single VAS.
Measures of 'general fatigue' were operationalised for this review as measures that included multiple characteristics of fatigue (such as severity, distress, and fatigue interference) and/or multiple dimensions of fatigue (e.g. cognitive, physical, and emotional). Examples of these general fatigue measures used by studies in this review include the Functional Assessment of Cancer Therapy ‐ Fatigue sub scale (used by Yates 2005); the Fatigue Assessment Questionnaire (used by Reif 2012); the General Fatigue sub scale of the MFI‐20 (used by Purcell 2011); the General Fatigue Scale (used by Barsevick 2004 and Barsevick 2010); the Piper Fatigue Total Score (used by Yuen 2006); and the mean score of four VAS items measuring severity, distress, and interference (Ream 2006).
Where data were unavailable for a particular outcome, we were able to obtain data relevant to the main outcome directly from authors of four studies. Godino 2006 provided data for the FACT‐F and its sub scales; we obtained data on fatigue intensity from Purcell 2011; Reif 2012 provided data for fatigue distress; and Yuen 2006 provided data for fatigue intensity, distress, and interference as separate items.
Data for outcomes of interest to this review came from post intervention scores measured soon after the completion of the intervention. However, many of the trials also had further follow‐up time points. We did not use data from these additional time points in this review due to substantial differences in these measurement time points.
Studies awaiting classification
We identified four studies that are awaiting classification (Bigatao 2016; Littlechild 2016; Sandler 2015; Velji 2006).
Excluded studies
On close inspection of the 45 full text articles retrieved, we excluded 22 studies from this review because they did not meet the inclusion criteria (Characteristics of excluded studies). Of these, eight were not RCTs, a further nine did not use an intervention meeting the inclusion criteria, and five did not have fatigue as the primary outcome.
Risk of bias in included studies
Allocation
In ten studies, a variety of acceptable methods for sequence generation were described (Barsevick 2004; Barsevick 2010; Foster 2015; Purcell 2011; Ream 2006; Reif 2012; Wangnum 2013; Wydra 2001; Yates 2005; Yun 2012). However, the remaining studies (Godino 2006; Schjolberg 2014; Williams 2005; Yuen 2006) did not provide enough information to determine if acceptable methods were used for random sequence allocation or not, with most simply stating that participants were randomised to groups (Figure 2; Figure 3). Seven studies provided an adequate description of allocation concealment (Barsevick 2010; Foster 2015; Purcell 2011; Ream 2006; Reif 2012; Yates 2005; Yun 2012).
Blinding
Blinding was a limitation for all trials within this review. Due to the nature of the intervention (education) it was not possible to achieve adequate blinding of participants or study personnel and therefore we did not consider this for the risk of bias judgments. When blinding is not possible, other methods can be used to compensate for this and to understand the risk of bias, including measuring the between‐group equivalence of patients' expectations of benefit, therapists' allegiance to treatment, and degree of adherence to treatments (Yates 2005). No studies in this review measured expectation of benefit or allegiance to treatments and only two studies measured adherence of personnel to a manualised intervention and the control condition (Barsevick 2004; Barsevick 2010). The main outcome measure is subjective (fatigue) and is completed through self‐report measures, so it was also not possible to achieve assessor blinding because the participants provide their own data. It is quite possible that overestimation of results may therefore have occurred in these studies.
Incomplete outcome data
We judged the majority of studies to be at low risk of attrition bias. We judged the risk of bias due to incomplete outcome data to be unclear in six studies (Barsevick 2010; Foster 2015; Ream 2006; Williams 2005; Wydra 2001; Yuen 2006), and unclear in two.
Selective reporting
All studies reported results for the outcomes that they had described in their methods sections.
Other potential sources of bias
Size of study
All included studies were at unclear or high risk of bias for size. The majority of studies had fewer than 200 participants per treatment arm, and Godino 2006, Wangnum 2013, Williams 2005, and Yuen 2006 had fewer than 50 per treatment arm. It has been suggested that small studies may be more prone to bias and distort the effects of a meta‐analysis (Nuesche 2010).
Other
There were no other obvious sources of bias that we could determine from the reports of any of the studies.
Effects of interventions
See: Table 1
Primary outcome
Fatigue
General (overall) fatigue (post‐scores)
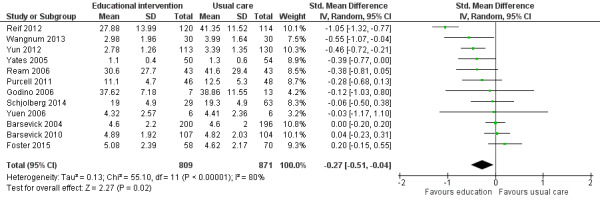
Twelve studies (1711 participants) compared educational interventions with usual care or attention controls and provided post‐intervention data for general fatigue (Barsevick 2004; Barsevick 2010; Foster 2015; Godino 2006; Purcell 2011; Ream 2006; Reif 2012; Schjolberg 2014; Wangnum 2013; Yates 2005; Yuen 2006; Yun 2012). Pooled analysis of these studies showed a statistically significant between‐group difference in favour of the educational intervention group (SMD ‐0.27, 95% CI ‐0.51 to ‐0.04) but there was substantial statistical heterogeneity (Analysis 1.1; Figure 4). We undertook a pre‐specified subgroup analysis to consider sources of heterogeneity but found no clear effects related to any of the pre‐specified variables. Using the guidelines by Cohen 1988, the SMD of ‐0.27 would be considered a small effect size. We back transformed the SMD to express it in the units of the Mean Fatigue item from Ream 2006 (which is an average of four VAS items covering fatigue intensity, distress, and interference with activities), to aide interpretation of the effect size. Using this approach, an effect size of ‐0.27 would translate into an estimated mean difference of ‐7.8 on the Mean Fatigue item (which has a possible scale range of 0‐100). Blinding of participants and assessors was not achieved in any of these studies, unclear risk of bias was evident for other risk of bias criteria, and unexplained statistical heterogeneity was evident. For these reasons, this outcome was downgraded from high to low quality as per the GRADE guidelines (see Table 1).
1.1. Analysis.
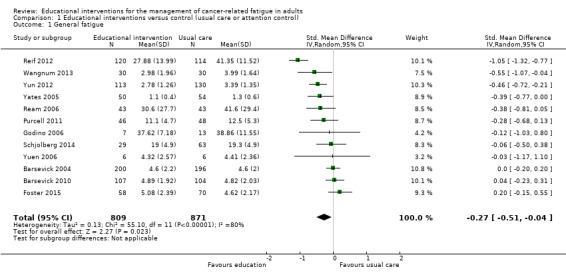
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 1 General fatigue.
4.
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.1 General fatigue.
General fatigue by type of cancer treatment, timing of intervention relative to completion of cancer treatment, tumour type, stage of disease, and group versus individual intervention
We undertook subgroup analysis to determine results for general fatigue in relation to the type of cancer treatment, timing of intervention relative to completion of cancer treatment, stage of disease, tumour type and group versus individual intervention. We found no clear effects related to type of cancer treatment or timing of the intervention relative to completion of cancer treatment (e.g. during or after cancer treatment), and it was not possible to extract data to examine differences by stage of disease or tumour type. Only three studies specifically focused on people with breast cancer (Schjolberg 2014; Williams 2005; Yates 2005), one specifically focused on people with lung cancer (Wangnum 2013), and one focused specifically on people with gastric or colon cancer (Godino 2006). The remaining studies included participants with various tumour types. There was insufficient information to determine the effects of group versus individual intervention. We did not undertake the pre‐specified sensitivity analysis to consider the risk of bias due to inadequately concealed allocation (determined by high risk of bias), as there were no studies with high risk of bias related to allocation concealment methods for those studies that reported general fatigue.
Fatigue intensity (post‐scores)
Eight studies comparing educational interventions with usual care or attention control measured intensity of fatigue and provided post‐score data that were suitable for meta‐analysis (Barsevick 2004; Barsevick 2010; Purcell 2011; Ream 2006; Reif 2012; Schjolberg 2014; Yates 2005; Yun 2012). Among these studies that provided data immediately following completion of intervention, there was a reasonably high level of statistical heterogeneity when we pooled all data, and the result was statistically significant in favour of the educational intervention group (SMD ‐0.28, 95% CI ‐0.52 to ‐0.04; Analysis 1.2; Figure 5). Using the guidelines by Cohen 1988 this would be considered a small effect size. The SMD of ‐0.28 can be re‐expressed in the units of the 'extent of fatigue' numeric rating scale (0 to 100) used by Ream 2006 as an example to assist interpretation. This would equate to an estimated mean difference of ‐8.05.
1.2. Analysis.
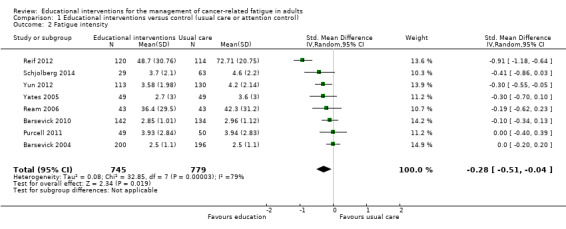
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 2 Fatigue intensity.
5.

Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.2 Fatigue intensity.
Participants in the trial by Reif 2012 had substantially higher baseline fatigue levels than most of the other studies. Therefore we conducted a sensitivity analysis excluding the Reif 2012 trial. After excluding these data there was still a small significant result and almost no statistical heterogeneity (SMD ‐0.14, 95% CI ‐0.25 to ‐0.03; P = 0.41; I² = 2%), indicating that this source contributed to the heterogeneity observed. We chose to leave the data from the Reif 2012 trial in Figure 5 to allow readers to compare the data from each study (Analysis 1.2; Figure 5). We were unable to extract suitable data for meta‐analysis on fatigue intensity from two trials. No statistically significant differences in fatigue intensity between the educational interventions and usual care were found in the trials by Williams 2005 and Wydra 2001. We downgraded this outcome (fatigue intensity) from high to moderate quality when using the GRADE guidelines due to the risk of bias introduced by lack of blinding and unclear risk of bias for other risk of bias criteria.
Fatigue intensity by type of cancer treatment, timing of intervention relative to completion of cancer treatment, tumour type, stage of disease, and group versus individual intervention
We found no clear effects related to type of cancer treatment or timing of the intervention relative to completion of cancer treatment (e.g. during or after cancer treatment) and it was not possible to extract data to examine differences by tumour type or stage of disease. There was insufficient information to determine the effects of group versus individual intervention. We did not undertake the pre‐specified sensitivity analysis to consider the risk of bias due to inadequately concealed allocation (determined by high risk of bias) as there were no studies with high risk of bias related to allocation concealment methods for the outcome of fatigue intensity.
Fatigue distress (post‐scores)
Three trials of educational interventions compared with usual care provided data from post‐intervention measurement of distress from fatigue (Ream 2006; Reif 2012; Yuen 2006). In these trials participants were asked to directly rate the amount of distress they had experienced because of fatigue using a VAS. Pooled data from these three studies (immediately following completion of the interventions) produced a statistically significant effect in favour of the educational intervention groups although statistical heterogeneity was evident (SMD ‐0.57, 95% CI ‐1.09 to ‐0.05). Using the guidelines by Cohen 1988 this would be considered a moderate effect size. Using the VAS for fatigue distress (Ream 2006) as an example, the SMD was back transformed to aide interpretation of the effect size. Using this approach an effect size of ‐0.57 would translate into an estimated mean difference of ‐18.1 on the fatigue distress VAS which had a possible scale range of 0 to 100. Regardless of the presence of heterogeneity, we have chosen to show the pooled data from all three studies in Figure 6 to allow readers to compare the studies (Analysis 1.3; Figure 6). Blinding of participants and assessors was not achieved in any of these studies and unexplained heterogeneity was evident, and for these reasons, we downgraded the level of quality of this outcome from high to low as per the GRADE guidelines.
6.
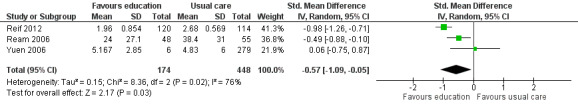
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.3 Fatigue distress.
1.3. Analysis.
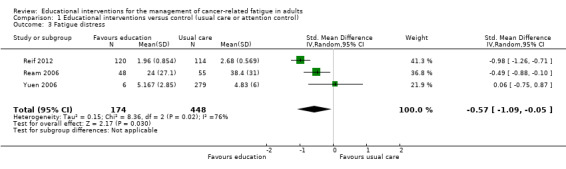
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 3 Fatigue distress.
Fatigue distress by type of cancer treatment, timing of intervention relative to completion of cancer treatment, tumour type, stage of disease, and group versus individual intervention
There were insufficient studies to determine any differences in fatigue distress related to type of cancer treatment, timing of the intervention relative to completion of cancer treatment (e.g. during or after cancer treatment), tumour type or stage of disease. There was insufficient information to determine the effects of group versus individual intervention. We did not undertake the pre‐specified sensitivity analysis to consider the risk of bias due to inadequately concealed allocation (determined by high risk of bias) as there were no studies with high risk of bias related to allocation concealment methods for the outcome of fatigue distress.
Fatigue interference (post‐scores)
Based on meta‐analysis of four trials (439 participants) that measured Interference in activities of daily living as a result of fatigue, we found a significant effect on fatigue interference in favour of educational interventions (SMD ‐0.35, 95% CI ‐0.54 to ‐0.16) compared with usual care or attention control (Analysis 1.4; Figure 7). To aid interpretation, the SMD of ‐0.35 can be re‐expressed in the units of the Piper fatigue interference sub scale used by Yates 2005 which has a numeric rating of 0 to 10. This would equate to an estimated mean difference of ‐0.93. No statistically significant between group difference was reported in a trial by Wydra 2001 but it was not possible to extract relevant data to report here. We judged the quality of the evidence to be moderate, downgrading the quality of evidence by one level due to the risk of bias in these studies.
1.4. Analysis.
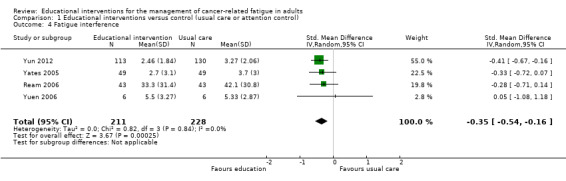
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 4 Fatigue interference.
7.
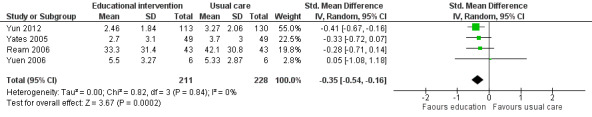
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.4 Fatigue interference.
Fatigue interference by type of cancer treatment, timing of intervention relative to completion of cancer treatment, tumour type, stage of disease, and group versus individual intervention
There were not enough studies to determine any differences in fatigue interference related to type of cancer treatment, timing of the intervention relative to completion of cancer treatment, tumour type or stage of disease. There was insufficient information to determine the effects of group versus individual intervention. We did not undertake the pre‐specified sensitivity analysis to consider the risk of bias due to inadequately concealed allocation (determined by high risk of bias) as there were no studies with high risk of bias related to allocation concealment methods for the outcome of fatigue interference.
Secondary outcomes
Fatigue management
Knowledge acquisition about fatigue
Only one study (Reif 2012) measured knowledge acquisition about fatigue using the Fatigue Knowledge Test (F‐WT) that has a scale range of 0‐34; the trial found a statistically significant difference in fatigue knowledge at the end of the intervention phase in favour of the intervention group (mean difference 6.38, 95% CI 4.99 to 7.77). We judged the quality of this evidence to be moderate, downgrading the quality of evidence by one level due to the risk of bias in this study.
Self‐efficacy for managing fatigue
One study measured self‐efficacy to manage fatigue (Foster 2015) using the Perceived Self‐Efficacy for Fatigue Management scale that had a possible score range of 0‐10, whereas Yates 2005 measured confidence with managing fatigue. There was no statistically significant difference in fatigue self‐efficacy at the end of the intervention phase for either study. It was not possible to blind participants or achieve blinding of outcome assessment in either of these studies and therefore we downgraded the level of quality of this outcome from high to moderate as per the GRADE guidelines.
Perceived coping with fatigue
No studies measured perceived coping with fatigue.
Use of fatigue management strategies
Five studies measured the use of fatigue management strategies that had been taught in the intervention and compared this to usual care or attention control (Barsevick 2004; Barsevick 2010; Williams 2005; Yates 2005; Yun 2012). When we pooled data from four of these studies (1019 participants) we found a statistically significant between group difference in favour of the educational intervention for number of strategies used (SMD 0.23, 95% CI 0.04 to 0.41; Analysis 1.5; Figure 8). One study that could not be included in the analysis due to the type of data reported found no between group differences for the number of self‐care strategies used for managing fatigue (Williams 2005). We judged this evidence to be of moderate quality, downgrading it by one level due to the risk of bias present in these studies.
1.5. Analysis.
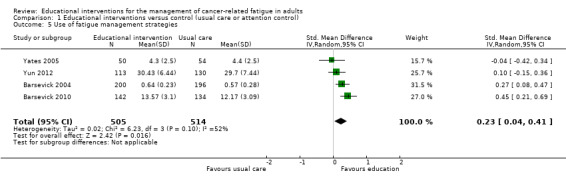
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 5 Use of fatigue management strategies.
8.
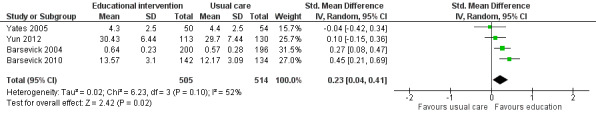
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.5 Use of fatigue management strategies.
Capacity to perform activities of daily living or physical functioning
Capacity to perform activities of daily living or physical functioning was measured in seven studies that compared educational interventions to usual care or attention controls. In these studies, capacity to perform activities of daily living or physical functioning was measured using sub scale scores of health related quality of life measures (e.g. the EORTC QLQ‐C30 or the 36‐Item Short Form Health Survey). We were able to pool data from four studies measuring physical functioning (773 participants) which showed no difference between groups (SMD 0.33 95% CI ‐0.10 to 0.76; Analysis 1.6; Figure 9). No significant difference was found for physical functioning immediately after cancer treatment in a further three trials (Ream 2006; Yates 2005; Yun 2012). Data from these three studies could not be combined because they were not suitable for meta‐analysis. In accordance with GRADE guidelines we downgraded the quality of this evidence from high to low because of risk of bias related to the inability to blind participants or achieve blinding of outcome assessment, and the presence of unexplained statistical heterogeneity.
1.6. Analysis.
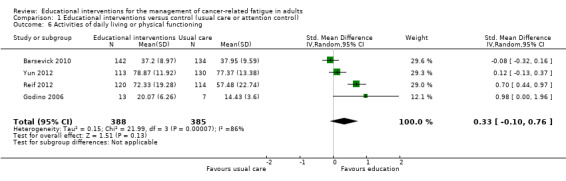
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 6 Activities of daily living or physical functioning.
9.
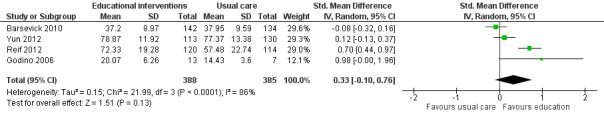
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.4 Activities of daily living or physical functioning.
Anxiety
We combined data from three studies that compared educational interventions with usual care (Reif 2012; Purcell 2011; Yun 2012; 571 participants), and provided the post‐intervention scores for the anxiety sub scale of the Hospital Depression and Anxiety scale which has a scale range of 0 to 21. The pooled result was statistically significant in favour of the educational intervention group (MD ‐1.47, 95% CI ‐2.76 to ‐0.18; Analysis 1.7; Figure 10). When considering the GRADE guidelines for quality, we downgraded this outcome from high to low quality due to the risk of bias introduced by lack of blinding of participants and assessors and presence of unexplained heterogeneity. Three other studies measured anxiety but it was not possible to extract appropriate data to allow them to be included in the meta‐analysis. Ream 2006 reported a statistically significant between‐group difference in favour of the intervention group as did Williams 2005. Yates 2005, however, reported no statistically significant difference in anxiety (although data were not provided).
1.7. Analysis.
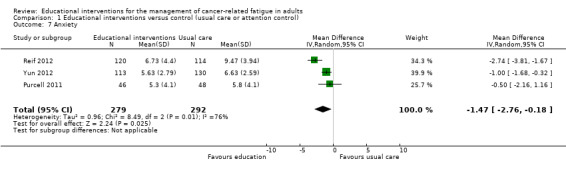
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 7 Anxiety.
10.
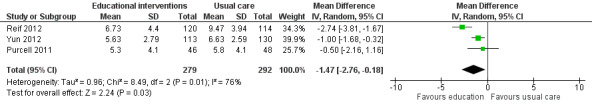
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.7 Anxiety.
Depression
When we pooled results from four studies comparing the effect of educational interventions to usual care or attention controls on depressive symptoms (881 participants), we found no between group differences (SMD ‐0.12, 95% CI ‐0.47 to 0.23; Analysis 1.8; Figure 11). We were not able to pool data from a further four studies due to insufficient data with three of these studies finding no significant between group differences in depressive symptoms (Purcell 2011; Wangnum 2013; Yates 2005). The study by Ream 2006, however, reported a statistically significant between group difference in mean ranks (P = 0.02). We judged the quality of this evidence to be very low. We downgraded the evidence three levels due to the risk of bias in the included studies, unexplained statistical heterogeneity, and imprecision of the data.
1.8. Analysis.
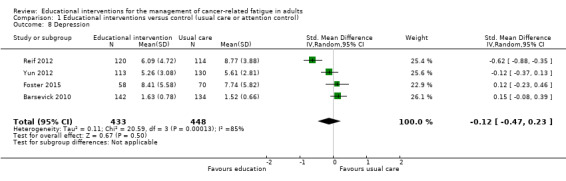
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 8 Depression.
11.
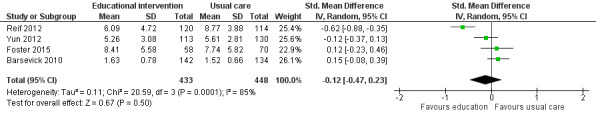
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.8 Depression.
Global Quality Of Life
When comparing educational interventions to usual care, only one study measured global quality of life as a separate construct to health status, finding no statistically significant between group differences (Wydra 2001). Three studies used the global score from the EORTC QLQ‐C30 that includes one item about overall health status and one item about global quality of life (scale range 0 ‐ 100); however, we could only pool data from two of these studies showing a small to moderate effect in favour of the educational intervention (MD 11.47, 95% CI 1.29 to 21.65; Analysis 1.9; Figure 12). In contrast, Yates 2005 reported no statistically significant difference, however no data were available to use in meta‐analysis. In accordance with the GRADE guidelines, we judged this evidence to be low quality due to the risk of bias in each of the studies and the presence of unexplained heterogeneity.
1.9. Analysis.
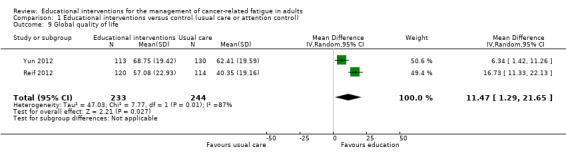
Comparison 1 Educational interventions versus control (usual care or attention control), Outcome 9 Global quality of life.
12.
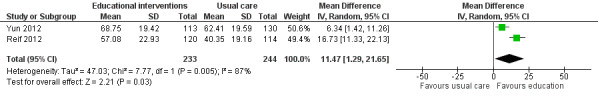
Forest plot of comparison: 1 Educational interventions versus control (usual care or attention control), outcome: 1.9 Global quality of life.
Discussion
The aims of this systematic review were to determine the effectiveness of educational interventions for managing cancer‐related fatigue in adults and to explore the effectiveness of educational interventions for managing cancer‐related fatigue in different types of adult cancer populations where data were available. Educational interventions may have a small effect on reducing fatigue intensity, fatigue's interference with daily life, and general fatigue, and could have a moderate effect on reducing fatigue distress. Educational interventions focused on fatigue may also help reduce anxiety and improve global quality of life, but at this point it is unclear what effect they might have on capacity for activities of daily living or depressive symptoms. Additional studies undertaken in the future are likely to impact on our confidence in the conclusions.
Summary of main results
Overall the results of this review provide preliminary evidence for the beneficial effect of educational interventions for reducing general cancer‐related fatigue (a composite measurement of multiple characteristics or dimensions of fatigue), fatigue intensity, fatigue distress, and fatigue interference compared with usual care or attention controls. Using the guidelines by Cohen 1988, we found a small effect size for reduction in general fatigue (12 studies, N = 1711), fatigue intensity (eight studies, N = 1524) and fatigue interference (four studies, N = 439), and a moderate effect size for fatigue distress (three studies, N = 622). A small increase in the use of fatigue management strategies for those receiving an educational intervention compared with usual care or attention control was also evident. Substantial heterogeneity was evident for analyses of fatigue distress and general fatigue, with one trial appearing to be an outlier (Reif 2012). The risk of bias present in these studies, and an inability to include data from all studies in the meta‐analyses, means the true effect sizes could be smaller.
There were no clear effects found related to type of cancer treatment or the timing of the intervention relative to completion of cancer treatment, and it was not possible to extract sufficient data to examine differences by stage of disease or tumour type. There was insufficient information to determine the effect of group versus individually delivered intervention. Further research is required to clarify the role of these different factors.
It is not known what aspects incorporated in 'general fatigue' are being impacted by educational interventions. Separate analysis of fatigue characteristics may help clarify this. In this review, we did not undertake analysis for different 'dimensions' of fatigue (e.g. cognitive, affective, or physical fatigue) used in a few of the studies; this review specifically sought to consider the different fatigue characteristics such as intensity, distress, and interference from fatigue.
Fatigue has been demonstrated by numerous studies to change across the course of cancer treatment and following its completion making it difficult for trialists to determine when to measure fatigue. This also needs to be considered when interpreting the findings of the pooled analysis of post‐intervention scores in this review. Post intervention measurements of fatigue intensity occurred soon after the completion of the interventions in most studies, but there was significant clinical heterogeneity regarding when interventions (and measurements) occurred in relation to cancer treatment.
It appears that educational interventions focused on fatigue may reduce anxiety, but their effect on depressive symptoms is less clear. Whether or not educational interventions focused on fatigue may impact depressive symptoms in studies with higher baseline fatigue and depression scores than studies within this review is unknown. Measuring depression in future studies is important due to the potential relationship between fatigue and depression.
There is insufficient evidence at this point regarding the effect of educational interventions focused on fatigue for improving capacity to perform activities of daily living or physical functioning, but preliminary evidence exists about their benefit on global quality of life. Further study is required to confirm these findings.
Adverse events were not recorded in studies within this review but educational interventions could conceivably increase anxiety, distress, and fatigue as a result of increasing the time and attention that individuals focus on these issues.
Overall completeness and applicability of evidence
We found fourteen studies that used RCT design testing educational interventions focused on cancer‐related fatigue management, with fatigue as the primary outcome. Pooled data for general fatigue were available from 1711 participants, 1524 participants for fatigue intensity, 622 participants for fatigue distress, and 439 participants for fatigue interference. The interventions were heterogeneous in terms of delivery format including: mode of delivery (face to face, web‐based, audiotape, telephone); delivery to groups or to individuals; number of sessions provided (ranging from 2 to 12 sessions); and timing of commencement of intervention in relation to cancer treatment (during or after cancer treatment). Further, 18 different measures of fatigue were used across the studies contained in this review. These outcome measures are summarised in Characteristics of included studies. This heterogeneity makes it difficult to interpret the findings from this review. Most data in this review came from post intervention scores measured soon after the completion of the intervention. However, many of the trials also had a further follow‐up time point, but we did not consider data from these additional time points in this review due to substantial differences in these measurement time points. The investigation of delivery of educational or supportive interventions by different modes of delivery and in relation to the timing of cancer treatment is important given the nature of the symptom in question. In particular, the ability of people to receive education and support through methods that take into consideration their lack of energy for attending appointments in person needs further consideration.
Although there is some evidence about the effects of educational interventions for reducing general fatigue, fatigue intensity, fatigue distress, and fatigue interference, measurement of these constructs in future trials could improve our understanding of this complex symptom. It is surprising that only eight studies provided data for depression and six for anxiety given the potential relationship between fatigue and depression in particular. Further well‐designed and reported trials are required to evaluate the effects of educational interventions on cancer‐related fatigue.
Quality of the evidence
We rated the quality of the evidence for outcomes included in the 'Summary of findings' tables using the GRADE system. Overall, the quality of the evidence in this review varied from very low to moderate quality due to the risk of bias in the included studies, the presence of unexplained heterogeneity in many of the analyses, and lack of precision for a number of outcomes. Additional studies undertaken in the future are likely to have an impact on our confidence in the conclusions of this review.
The included studies had methodological issues that increased their risk of bias. Random sequence generation was adequate in seven of the 14 trials but only six used adequately concealed allocation. The main risk of bias came from lack of any type of blinding in all studies for both the main outcome (fatigue) and secondary outcomes. Blinding of participants is not possible to achieve in studies of this nature and the ability to attain blinding of outcome assessment is particularly problematic given that the outcomes are subjective in nature and data were provided by self‐report. To date there are no accepted objective measures of cancer‐related fatigue, although objective measurement of its effects on cognition, motor function, and sleep quality may be achievable. The likelihood of overestimation of results must therefore be carefully considered. Only seven trials had adequate completeness of outcome data, and for three studies there was evidence of selective reporting. All studies had unclear or high risk of bias due to low numbers of participants in each arm of the study. It has been suggested that small studies may be more prone to bias and distort the effects of a meta‐analysis (Nuesche 2010). We attempted a pre‐specified subgroup analysis to explore statistical heterogeneity for the main outcome of fatigue but no clear explanation was evident. High statistical heterogeneity did not preclude pooling data, but instead resulted in downgrading of the quality of the evidence. Imprecision was apparent for two outcomes (depression and quality of life) and further research is needed to clarify or confirm the results.
Potential biases in the review process
Strengths of this review include the comprehensive literature searches, the potential for inclusion of non‐English publications, selection of studies and data extraction by two independent researchers, and contacting authors when relevant data were missing from reports. However, despite the comprehensive search undertaken, it is possible that we may have missed unpublished studies. It should also be noted that three of the review authors (SB, TH, AP) were involved in one of the studies in the review (Purcell 2011).
The studies in this review were heterogeneous in terms of outcome measures, interventions, and participants; however, we judged the overall aims and purposes of these studies to be clinically similar enough to warrant meta‐analyses. The statistical heterogeneity that resulted in some of these analyses could partly be explained by the inclusion of the trial by Reif 2012, which differed in nature from the majority of studies in two ways: participants started with a higher level of fatigue intensity than participants in all other trials, and the intervention was delivered in groups.
It was difficult to extract meaningful data allowing syntheses of results from two of the 14 studies (Williams 2005; Wydra 2001). Results reported by authors of these studies have been provided in Effects of interventions. In a number of instances, data from participants from other studies could not be included in the pooled analyses due to the nature of the data or lack of clarity of the data provided. Data for non‐significant findings were also not provided by two studies for outcomes of relevance to this review (depression, anxiety, and physical function). Ideally, authors should have provided summary statistics for all assessed outcomes, regardless of study results.
Agreements and disagreements with other studies or reviews
A recent systematic review purporting to review education programs for cancer‐related fatigue included 10 trials and found limited evidence to support its use (Du 2015). However, three of the trials included other modalities (e.g. relaxation or exercise) and another did not have fatigue as the primary focus. This current review identified eight additional trials not included in Du 2015 and included trials that focused on education, making it a more comprehensive and focused review. Another Cochrane systematic review by Goedendorp 2009 asked a broader question examining psychosocial interventions for reducing fatigue, included three of the same trials from this review. Similar to our review, Goedendorp 2009 concluded that the most promising psychosocial interventions educated people about fatigue, self‐care or coping techniques, and how to manage their activity. A few systematic reviews have considered the broad question of the effectiveness of non‐pharmacological interventions for cancer‐related fatigue (e.g. Jacobsen 2007; Kangas 2008; Wanchai 2011), finding support for psychological interventions and exercise, with the review by Wanchai 2011 identifying the potential benefit of educational interventions. Finally, The National Comprehensive Cancer Network guidelines for cancer‐related fatigue recommend the use of educational interventions for its management (NCCN 2016).
Authors' conclusions
Implications for practice.
This review found some evidence from RCTs that educational interventions may have a small effect in reducing general (overall) fatigue, fatigue intensity, and fatigue interference, and may reduce the distress from fatigue. However the research is hampered by methodological issues that may mean the results are overestimated.
For people with cancer
Knowing that fatigue is a common experience amongst those with cancer, and that it increases during treatment, may be reassuring for people with this symptom. Educational interventions can also help people develop knowledge and skills for managing fatigue such as pacing and prioritising daily activities.
For clinicians
Researchers suggest that cancer‐related fatigue is under‐diagnosed and under‐treated (Jameson 2016; Smith 2007), in part due to health professionals thinking little can be done to manage it (Borneman 2007). Providing education about fatigue and its management appears to have some effect on this symptom and incorporating education as part of routine care seems reasonable. The NCCN guidelines recommend that screening for fatigue should occur for all people receiving treatment for cancer at their initial clinical visit and that education about cancer‐related fatigue be offered as soon as possible regarding the potential for fatigue to develop and possible treatment options (Koornstra 2014; NCCN 2016).
For policy makers
Educational interventions may play a small role in reducing general (overall) fatigue, fatigue intensity, and fatigue interference, and may reduce the distress from fatigue. The incorporation of education for the management of fatigue as part of routine care appears reasonable, but given the complex nature of this symptom, educational interventions should be considered in conjunction with other interventions.
For funders of the intervention
Costs associated with providing educational interventions for the management of cancer‐related fatigue are largely personnel costs. These costs are likely to differ depending on the nature of the specific educational intervention used. No cost‐effectiveness studies have been undertaken.
Implications for research.
General implications
Further research to understand the best approach to providing educational interventions for the management of cancer‐related fatigue is recommended. A number of specific implications for research are evident.
There was insufficient research to allow conclusions to be drawn with respect to the effect of educational interventions by tumour type, timing of intervention relative to completion of cancer treatment, stage of disease, type of cancer treatment being received, or delivery of the intervention by group versus individual formats. For these factors to be better understood, trials that are designed specifically to address these questions are needed. No studies were identified that specifically considered educational interventions with people with advanced cancer and this could be a focus for future research. The majority of studies involved participants with a mild to moderate level of fatigue. Whether studies targeted to those with moderate to severe fatigue would have greater impact on fatigue could also be considered.
People experiencing fatigue may benefit from the reassurance from face to face contact, however they may also appreciate support without having to leave home. The comparative benefits of different delivery options have not been tested. Asking preferences of those receiving treatment may be informative. Much remains to be understood about timing and formats of educational interventions for the management of cancer‐related fatigue. There have been no studies that have specifically considered the dose of the educational intervention and only a few (e.g. Barsevick 2004; Barsevick 2010; Foster 2015; Yates 2005) attempted to control for the potential effect of social support, time, and attention ‐ further issues to be considered in research.
Measurement (endpoints)
A number of fatigue measures incorporate questions about fatigue distress and interference together with measurement of fatigue severity. There may also be an advantage to measuring these constructs separately. This can be understood if one considers that the intensity of fatigue a person might experience might not always be highly correlated with fatigue distress of interference from fatigue. For example, in a study with hospitalised medical‐surgical patients, Kris 2004 found that fatigue severity had only a moderate correlation (r = 0.52) with fatigue distress.
The relationship between depression and fatigue is unclear, but given the potential for people who have fatigue to develop depressive symptoms and that people who have depression are often more fatigued (Mitchell 2006), measurement of depression in future trials should be routine. No studies investigated the impact on work outcomes or cost‐effectiveness of the interventions and these could be considered in future research.
Design
Large, well‐designed RCTs are needed to further investigate the effectiveness of educational interventions for managing cancer‐related fatigue. It was difficult to ascertain the details of methods used in many of these studies. Authors are urged to use the CONSORT guidelines (Schulz 2010; including its various elaborations) when planning their research and writing up their reports.
What's new
| Date | Event | Description |
|---|---|---|
| 28 November 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2016
| Date | Event | Description |
|---|---|---|
| 9 November 2009 | Amended | Contact details updated. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in four years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We acknowledge the support of the PaPaS Review Group editorial team and the following reviewers: Teresa Corbett, Scott Strassels, Nancy Thornton, and Julie Gildie.
This review was funded by a Palliative Care Development Grant from the National Health and Medical Research Council of Australia, Reference Number 519793. This funding body played no role in this research.
We acknowledge the contribution of Jenny Fleming to the protocol.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy (via OVID)
exp Neoplasms/
exp Bone Marrow Diseases/
exp Bone Marrow Transplantation/
exp Stem Cell Transplantation/
exp Radiotherapy/
exp Chemotherapy, Adjuvant/
exp Antineoplastic Combined Chemotherapy Protocols/
exp Salvage Therapy/
exp Palliative care
(neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or (bone adj marrow adj5 transplant*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
exp Fatigue/
(fatigue* or tired* or sleepy or sleepi* or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre or ((asthenia or asthenic) adj3 syndrome) or ((lack or loss or lost) adj3 (energy or vigour))).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
12 or 13
exp Consumer Health Information/
exp Patient Education as Topic/
(self‐manag* or "self manag*").mp. [mp=title, original title, abstract, name of substance word, subject heading word]
((Education* or teach* or train* or advice or information) adj3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or DVD or CD or Internet or Web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
15 or 16 or 17 or 18
19 and 14 and 11
Appendix 2. CINAHL search strategy (via EBSCO)
S34 S22 and S33
S33 S31 NOT S32
S32 (animals not (humans and animals))
S31 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30
S30 groups
S29 trial
S28 randomly
S27 drug therapy
S26 placebo
S25 randomi?ed
S24 controlled clinical trial
S23 randomized controlled trial
S22 S12 and S16 and S21
S21 S17 or S18 or S19 or S20
S20 ((Education* or teach* or train* or advice or information) and (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or DVD or CD or Internet or Web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*))
S19 (self‐manag* or "self manag*" or self care or self‐care)
S18 (MH "Patient Education+")
S17 (MH "Consumer Health Information")
S16 S13 or S14 or S15
S15 (lack N3 energy) or (loss N3 energy) or (lost N3 energy) or (lack N3 vigo#r) or (loss N3 vigo#r) or (lost N3 vigo#r)
S14 fatigue* or tired* or sleepy or sleepi* or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre or (asthenia N3 syndrome) or (asthenic N3 syndrome)
S13 (MH "Fatigue+")
S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S11 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or (bone N1 marrow N5 transplant*))
S10 (MH "Salvage Therapy")
S9 (MH "Antineoplastic Agents, Combined")
S8 (MH "Chemotherapy, Adjuvant")
S7 (MH "Chemotherapy, Cancer+")
S6 (MH "Radiotherapy+")
S5 (MH "Hematopoietic Stem Cell Transplantation")
S4 (MH "Bone Marrow Transplantation+")
S3 (MH "Bone Marrow Diseases+")
S2 (MH "Palliative Care")
S1 (MH "Neoplasms+")
Appendix 3. PsycINFO search strategy via OVID
1 exp Chemotherapy/ 2 exp Palliative Care/ 3 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or (bone adj marrow adj5 transplant*)).tw. 4 exp Neoplasms/ 5 bone marrow/ 6 exp Antineoplastic Drugs/ 7 (radiotherap* or salvage therap*).tw. 8 (stem cell adj5 transplantation).tw. 9 (bone marrow adj5 (disease* or transplant*)).tw. 10 or/1‐9 11 exp Fatigue/ 12 (fatigue* or tired* or sleepy or sleepi* or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre or ((asthenia or asthenic) adj3 syndrome) or ((lack or loss or lost) adj3 (energy or vigour))).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 13 11 or 12 14 health promotion/ or health education/ 15 exp Health Care Services/ 16 (self‐manag* or "self manag*").mp. 17 ((Education* or teach* or train* or advice or information) adj3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or DVD or CD or Internet or Web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)).mp. 18 14 or 15 or 16 or 17 19 10 and 13 and 18 20 clinical trials/ 21 (randomis* or randomiz*).tw. 22 (random$ adj3 (allocat$ or assign$)).tw. 23 ((clinic$ or control$) adj trial$).tw. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 25 (crossover$ or "cross over$").tw. 26 random sampling/ 27 Experiment Controls/ 28 Placebo/ 29 placebo$.tw. 30 exp program evaluation/ 31 treatment effectiveness evaluation/ 32 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 33 or/20‐32 34 19 and 33
Appendix 4. Embase (OVID) search strategy
1 exp Neoplasm/ 2 exp bone marrow disease/ 3 exp bone marrow transplantation/ 4 exp stem cell transplantation/ 5 exp radiotherapy/ 6 exp antineoplastic agent/ 7 exp salvage therapy/ 8 exp palliative therapy/ 9 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or (bone adj marrow adj5 transplant*)).tw. (4130659) 10 or/1‐9 11 exp fatigue/ 12 (fatigue* or tired* or sleepy or sleepi* or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre or ((asthenia or asthenic) adj3 syndrome) or ((lack or loss or lost) adj3 (energy or vigour))).tw. 13 11 or 12 14 exp consumer health information/ 15 exp patient education/ 16 exp self care/ 17 (self‐manag* or "self manag*").tw. 18 ((Education* or teach* or train* or advice or information) adj3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or DVD or CD or Internet or Web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)).tw. 19 14 or 15 or 16 or 17 or 18 20 10 and 13 and 19 21 random$.tw. 22 factorial$.tw. 23 crossover$.tw. 24 cross over$.tw. 25 cross‐over$.tw. 26 placebo$.tw. 27 (doubl$ adj blind$).tw. 28 (singl$ adj blind$).tw. 29 assign$.tw. 30 allocat$.tw. 31 volunteer$.tw. 32 Crossover Procedure/ 33 double‐blind procedure.tw. 34 Randomized Controlled Trial/ 35 Single Blind Procedure/ 36 or/21‐35 37 (animal/ or nonhuman/) not human/ 38 36 not 37 39 20 and 38
Appendix 5. Education Resources Information Center (ERIC) search strategy
Search History ERIC (CSA)
#1 Search Query #1 DE="cancer"
#2 Search Query #2 DE="drug therapy"
#3 Search Query #3 DE="radiology"
#4 Search Query #4 (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or drug therap* or palliative) or (bone marrow within 5 (transplant* or disease*))
#5 Search Query #5 #1 or #2 or #3 or #4 (DE="cancer") or (DE="drug therapy") or (DE="radiology") or (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or drug therap* or palliative) or (bone marrow within 5 (transplant* or disease*))
#6 Search Query #6 DE="fatigue biology"
#7 Search Query #7 (fatigue* or tired* or sleepy or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre) or ((asthenia or asthenic) within 3 syndrome) or ((lack or loss or lost) within 3 (energy or vigour))
#8 Search Query #8 #6 or #7 (DE="fatigue biology") or ((fatigue* or tired* or sleepy or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre) or ((asthenia or asthenic) within 3 syndrome) or ((lack or loss or lost) within 3 (energy or vigour)))
#9 Search Query #9 DE="health education"
#10 Search Query #10 DE="patient education"
#11 Search Query #11 self manag* or self‐manag*
#12 Search Query #12 (education* or teach* or train* or advice or advise or information) within 3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or videos or dvd or dvds or cd or cds or internet or web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)
#13 Search Query #13 #9 or #10 or #11 or #12 (DE="health education") or (DE="patient education") or (self manag* or self‐manag*) or ((education* or teach* or train* or advice or advise or information) within 3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or videos or dvd or dvds or cd or cds or internet or web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*))
#14 Search Query #14 #5 and #8 and #13 ((DE="cancer") or (DE="drug therapy") or (DE="radiology") or (neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or drug therap* or palliative) or (bone marrow within 5 (transplant* or disease*))) and ((DE="fatigue biology") or ((fatigue* or tired* or sleepy or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre) or ((asthenia or asthenic) within 3 syndrome) or ((lack or loss or lost) within 3 (energy or vigour)))) and ((DE="health education") or (DE="patient education") or (self manag* or self‐manag*) or ((education* or teach* or train* or advice or advise or information) within 3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or videos or dvd or dvds or cd or cds or internet or web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)))
#15 Search Query #15 AB=(randomized or randomised)
#16 Search Query #16 AB=randomly
#17 Search Query #17 AB=placebo
#18 Search Query #18 AB=trial
#19 Search Query #19 AB=groups
#20 Search Query #20 AB=controlled
#21 Search Query #21 #15 or #16 or #17 or #18 or #19 or #20 (AB=(randomized or randomised)) or(AB=randomly) or(AB=placebo) or(AB=trial) or(AB=groups) or(AB=controlled)
#22 Search Query #22 #14 and #21 (((DE="cancer") or(DE="drug therapy") or (DE="radiology") or((neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or drug therap* or palliative) or (bone marrow within 5 (transplant* or disease*)))) and ((DE="fatigue biology") or ((fatigue* or tired* or sleepy or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre) or ((asthenia or asthenic) within 3 syndrome) or ((lack or loss or lost) within 3 (energy or vigour)))) and ((DE="health education") or (DE="patient education") or(self manag* or self‐manag*) or ((education* or teach* or train* or advice or advise or information) within 3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or videos or dvd or dvds or cd or cds or internet or web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*)))) and ((AB=(randomized or randomised)) or (AB=randomly) or (AB=placebo) or (AB=trial) or (AB=groups) or (AB=controlled))
Appendix 6. CENTRAL search strategy
#1MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES #2MESH DESCRIPTOR Bone Marrow Diseases EXPLODE ALL TREES #3MESH DESCRIPTOR Bone Marrow Transplantation EXPLODE ALL TREES #4MESH DESCRIPTOR Stem Cell Transplantation EXPLODE ALL TREES #5MESH DESCRIPTOR Radiotherapy EXPLODE ALL TREES #6MESH DESCRIPTOR Chemotherapy, Adjuvant EXPLODE ALL TREES #7MESH DESCRIPTOR Antineoplastic Combined Chemotherapy Protocols EXPLODE ALL TREES #8MESH DESCRIPTOR Salvage Therapy EXPLODE ALL TREES #9MESH DESCRIPTOR Palliative care EXPLODE ALL TREES #10 ((neoplasm* or cancer* or leukaemi* or leukemi* or tumour* or tumor* or malignan* or carcino* or lymphoma* or adenocarcinoma* or radioth* or radiat* or irradiat* or radiochemo* or chemotherap* or (bone adj marrow near5 transplant*))):TI,AB,KY #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12MESH DESCRIPTOR Fatigue EXPLODE ALL TREES #13 ((fatigue* or tired* or sleepy or sleepi* or drows* or lassitude or letharg* or weary or weariness or exhaustion or exhausted or lacklustre or ((asthenia or asthenic) near3 syndrome) or ((lack or loss or lost) near3 (energy or vigour)))):TI,AB,KY #14 #12 OR #13 #15MESH DESCRIPTOR Consumer Health Information EXPLODE ALL TREES #16MESH DESCRIPTOR Patient Education as Topic EXPLODE ALL TREES #17 ((self‐manag* or "self manag*")):TI,AB,KY #18 (((Education* or teach* or train* or advice or information) adj3 (patient* or consumer* or client* or group* or individual* or program* or session* or intervention* or strateg* or visit* or video or DVD or CD or Internet or Web or telephon* or printed or written or material* or booklet* or pamphlet* or leaflet*))):TI,AB,KY #19 #15 OR #16 OR #17 OR #18 #20 #11 AND #14 AND #19
Appendix 7. OTseeker search strategy
The first search combined the terms 'cancer' AND 'fatigue' in the Keyword search field.
The second search combined the term 'fatigue' in the Keyword search field with the results from the oncology/palliative care category within the Diagnosis/Subdiscipline field (using the default operator "AND").
Appendix 8. PEDro search strategy
The search combined the terms 'cancer' AND 'fatigue' and education* in the Keyword search field.
Data and analyses
Comparison 1. Educational interventions versus control (usual care or attention control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General fatigue | 12 | 1680 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.51, ‐0.04] |
| 2 Fatigue intensity | 8 | 1524 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.51, ‐0.04] |
| 3 Fatigue distress | 3 | 622 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.09, ‐0.05] |
| 4 Fatigue interference | 4 | 439 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.54, ‐0.16] |
| 5 Use of fatigue management strategies | 4 | 1019 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.04, 0.41] |
| 6 Activities of daily living or physical functioning | 4 | 773 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.10, 0.76] |
| 7 Anxiety | 3 | 571 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.76, ‐0.18] |
| 8 Depression | 4 | 881 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.47, 0.23] |
| 9 Global quality of life | 2 | 477 | Mean Difference (IV, Random, 95% CI) | 11.47 [1.29, 21.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barsevick 2004.
| Methods | RCT; 2 groups; stratified by job status, type of treatment, and diagnosis | |
| Participants | 396 people with cancer Mean age: 56.3 years N = 337 females, N = 59 males Note: only 234 provided post intervention data but no information about the number of participants in each group at follow‐up were available. Diagnosed with breast, lung, colorectal, advanced prostate, gynaecologic, or testicular cancer, or lymphoma Receiving chemotherapy or RT with curative intent Excluded if: treatment plan included stem cell transplantation, interleukins, interferons, or tumour necrosis factor; patients with chronic fatigue syndrome; patients enrolled in other studies involving psycho‐educational interventions; patients with a psychiatric disorder; and patients receiving treatment for anaemia or depression. The study was conducted at a university health science centre and a comprehensive cancer centre in the USA. |
|
| Interventions | Experimental group: energy conservation and activity management condition received 3 telephone sessions from a trained oncology nurse. Participants were given information on cancer‐related fatigue and learned energy conservation skills. 2 sessions x 30 minutes and final session 15 minutes For participants receiving chemotherapy CTX or concurrent therapy the intervention was administered during the first 3 weeks of treatment For participants receiving RT the intervention occurred during week 3 to 5 of treatment Control group: received 3 telephone sessions with information on nutrition, informing and discussing maintenance of a healthy diet and use of vitamins and minerals 2 sessions x 30 minutes and final session 15 minutes |
|
| Outcomes | Measures taken at baseline, 48 hours after 2nd and 3rd chemotherapy cycle or during last week of RT, and 1 month following its completion. Outcomes Fatigue: measured with 3 scales: 1) Short Form of the Profile of Mood States (5 items measuring fatigue intensity); 2) Schwartz Cancer Fatigue Scale; and 3) General Fatigue Scale. Functional performance: Functional Performance Inventory |
|
| Notes | Aim: to evaluate the efficacy of energy conservation and activity management for fatigue reduction and maintenance of functional performance in adults with cancer who are undergoing treatment No sample size calculation evident Funding source: National Institute of Nursing Research (R01NR04573) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors indicate that participants were stratified and then randomised (p 1304) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however, it is possible to discern group allocation and therefore blinding was not achieved.The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 396 randomised; 234 provided follow‐up data (59%). It is unclear how many participants providing follow‐up data were in each group. However, authors state: "Failure to complete all fatigue measures was unrelated to intervention group assignment. Thus, complete & incomplete cases were distributed evenly across both study groups." (p 1305) |
| Selective reporting (reporting bias) | Low risk | Study outcomes have been reported as per listed in study methods |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Barsevick 2010.
| Methods | RCT; 2 groups, stratified by diagnosis (breast cancer vs. non‐breast cancer), random assignments were generated by the statistician and placed in sealed envelopes that were numbered and selected sequentially for each stratification group | |
| Participants | N = 292 Mean age: 53.95 years N = 228 females, N = 48 males Note: the number of participants used in the analysis of post scores were provided by the study author. Diagnoses: breast, lung, colorectal, prostate, gynaecologic, bladder, or testicular cancer, or lymphoma Receiving CTX with curative intent Exclusion criteria: treatment plan included marrow or stem cell transplantation, interleukins, interferons, or tumour necrosis factor; had a chronic fatigue disorder; were being treated for a diagnosed sleep disorder (such as narcolepsy or sleep apnoea); were enrolled in another study that involved a psychoeducational intervention; had a communication impairment; had overt evidence of psychiatric disorder; or initiated treatment for anaemia or depression during the previous three weeks Setting: the study was conducted at 4 clinical sites: 2 university health science centres, a community cancer centre, and a comprehensive cancer centre, USA |
|
| Interventions | Experimental group: received 3 telephone sessions with a specially trained oncology nurse. Written intervention materials included a handbook specific to Energy and Sleep Enhancement intervention. The intervention was delivered using an interactive approach that built on the individual's existing knowledge of energy conservation strategies, sleep management, and his or her unique response to symptoms. Between sessions 1 and 2, participants completed a daily diary (concerning symptoms and sleep patterns) and a priority list of usual activities. Intervention occurred in the 2nd, 3rd, and 4th week after the first CTX treatment Control group: received 3 telephone sessions with a specially trained oncology nurse, focused on information about nutrition and a healthy diet. The participant kept a 24‐hour dietary record as homework in preparation for the 2nd session. Written intervention materials included a handbook specific to the control group. Intervention occurred in the 2nd, 3rd, and 4th week after the first CTX treatment |
|
| Outcomes | Measures were taken at baseline and on day 4 after the first CTX. Follow‐up data points were days 43‐46 or 57‐60 depending on the length of the CTX cycle. At both measurement points, patients wore the Actigraph on the nondominant wrist on day 1 and removed it 72 hours later (which was day 4, the equivalent of 3 24‐hour periods). Outcomes Fatigue: 1) General Fatigue Scale (used for 'general fatigue' outcome in this review); 2) Fatigue sub scale of the Profile of Mood States (used for 'fatigue intensity' outcome in this review); Sleep: The Pittsburgh Sleep Quality Index; "Octagonal Basic Motionlogger Actigraph"; Adapted Morin Sleep Diary Activities of daily living or physical functioning: Interference items from the Brief Pain Inventory; 12‐Item Short Form Health Survey (a shorter version of the 36‐item Short Form Health Survey); Eastern Cooperative Oncology Group Performance Status Depression: Depressive symptom sub scale of the Profile of Mood States Pain: Brief Pain Inventory Side Effects: 5‐point Likert‐type scale, side‐effect checklist |
|
| Notes | Aim: to evaluate the efficacy of an "energy and sleep enhancement" intervention to relieve fatigue and sleep disturbance and improve health‐related functional status. No sample size calculation evident Funding source: National Institute of Nursing Research (R01NR04573) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p 203 column 1 states: "Random assignments were generated by the statistician and placed in sealed envelopes that were numbered and selected sequentially for each stratification group." |
| Allocation concealment (selection bias) | Low risk | p 203 column 1 states: "Random assignments were generated by the statistician and placed in sealed envelopes that were numbered and selected sequentially for each stratification group." ‐ although no detail regarding opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however it is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p 206 Results states: "Sixteen participants were excluded from the analysis, leaving 276 analysed cases. Reasons for exclusion included severity of illness (n = 4), loss to follow‐up (n = 10), and change of treatment (n = 2). However authors go on to state that "of these 276 participants, 60 had some missing data." It is not clear for which outcomes data were missing or for whom. |
| Selective reporting (reporting bias) | Low risk | p 202 Study Design ‐ data for outcome measures are provided as per study methods. Appears to have low risk of selective outcome reporting |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Foster 2015.
| Methods | RCT; 2 groups | |
| Participants | N = 163 randomised Mean age: 57.8 years N = 122 females, N = 35 males Diagnoses: breast, lung, prostate, gynaecologic, bladder, gastrointestinal, or head and neck cancer Completed treatment with surgery or chemotherapy with curative intent Eligibility: ≥ 18 years old; ≤ 5 years post treatment for non‐metastatic disease; moderate to severe fatigue; access to Internet Exclusion criteria: not able to give informed consent; mental health condition that could be exacerbated; too ill to participate Setting: participants were recruited from oncology clinics in the UK. Interventions were delivered online. |
|
| Interventions | Experimental group: 5 sessions, with access to the material over a 6 week period Internet based education program called RESTORE, based on Macmillan Cancer Backups leaflet ‐ Coping with Fatigue. Content included information about cancer‐related fatigue, coping, and optional sessions on diet, sleep, exercise, home work life, thoughts and feelings, and talking to others. Used activities designed to support self‐efficacy, e.g. goal setting, tailored feedback, and patient stories. Control group: received the leaflet about coping with fatigue |
|
| Outcomes | Perceived Self‐Efficacy for Fatigue Management (data from this scale were obtained from the author and used for the self‐efficacy outcome in this review) Brief Fatigue Inventory (data from this sub scale were obtained from the author and used for the general fatigue outcome in this review) Personal Wellbeing Index (data from this sub scale were obtained from the author and used for the quality of life outcome in this review) Patient Health Questionnaire (data from this sub scale were obtained from the author and used for the depression outcome in this review) |
|
| Notes | Aim: the aim of this exploratory RCT was to test the proof of concept of RESTORE; a web‐based resource designed to increase self‐efficacy to manage cancer‐related fatigue through structured activities including goal setting, tailored feedback, and patient stories. Sample size calculation was not undertaken as authors state it was a proof of concept study with sample size decided by pragmatic considerations Funding source: Macmillan Cancer Support as part of the Macmillan Survivorship Research Group programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors report that participants were randomised in blocks of four |
| Allocation concealment (selection bias) | Low risk | Authors report that "A statistician independently generated a random allocation sequence using 'R'." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however, it is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. Data analysts were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data were imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and outcome data were reported as per the aims and methods described in this paper. |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Godino 2006.
| Methods | RCT; 2 groups; stratified based on diagnosis and treatment with mono‐chemotherapy or multi‐chemotherapy schedule | |
| Participants | N = 40 Mean age: 58.5 years (SD 11.34, min. 32, max. 74) N = 19 females, N = 21 males Diagnosed with gastric or colon cancer Receiving CTX with curative intent Exclusion criteria were previous cancer treatment; presence of respiratory, cardiac, or hepatic dysfunctions; learning disability; central nervous system metastasis, and previous RT. The study was conducted at a chemotherapy unit in a comprehensive cancer centre in Barcelona (Spain) |
|
| Interventions | Experimental group: received an individualised intervention over 3 sessions ‐ a patient education programme delivered by nurses which was multifaceted; it included one‐to‐one education, training, and counselling, as well as audio‐visual and computerised educational materials. 3 sessions, duration of sessions not stated; session 1 during 1st cycle of CTX, session 2 during 2nd cycle of CTX, the 3rd session occurring 1 month after completion of CTX Control group: receiving the usual information provided to patients by cancer nurses, 2 sessions, duration of sessions not specified, session 1 during 1st cycle of CTX, the last session occurring 1 month after completion of treatment |
|
| Outcomes | Measures taken at baseline (session 1, 1st CTX cycle), session 2 (2nd CTX cycle), and session 3 (1 month after completion of CTX) Outcomes Fatigue: Functional Assessment of Cancer Therapy Fatigue scores. The sub scale called 'additional concerns' are items specially assessing fatigue (data from this sub scale were obtained from the author and used for the 'general fatigue' outcome in this review) Satisfaction: assessed using a previously piloted self‐completed questionnaire consisting of 10 items. Every item was measured on a scale from 0 to 10, with higher scores indicating more satisfaction. |
|
| Notes | Aim: to determine whether nursing education decreases the perception of fatigue in patients with a colon or gastric cancer diagnosis. The specific objectives were: 1) to evaluate the fatigue level and severity in patients diagnosed with colon or gastric cancer before, during, and after chemotherapy treatment; 2) to measure the degree of the patients' satisfaction with a nursing intervention aimed at decreasing fatigue. No sample size calculation evident Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however it is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "each group was reduced by 10, because some patients had fast disease progression, which prevented them from finishing the study" (p 154). Missing data balanced in numbers and cause across groups. |
| Selective reporting (reporting bias) | Low risk | Both primary and second outcomes have been reported as per study methods, however sub scale scores were not presented |
| Study size | High risk | Fewer than 50 participants per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Purcell 2011.
| Methods | RCT; 4 groups; computerised random number generated sequence into opaque, sequentially numbered envelopes stored securely in a research office | |
| Participants | N = 110 Mean age: 57.65 years N = 52 females, N = 58 males Patients undergoing outpatient RT treatment for cancer with curative intent Exclusion criteria: < 18 years of age, (1) low performance status (Karnofsky level of < 60/100), (2) undergoing treatment with palliative intent, (3) undergoing other concurrent cancer treatments (e.g. chemotherapy), (4) involvement in other programs or research specifically targeting fatigue, (5) inability to complete questionnaires due to cognitive or literacy levels This study was conducted at the radiation oncology department of a single major metropolitan hospital in Brisbane, Australia. |
|
| Interventions | This was a trial of the 'Cancer‐related fatigue intervention trial' (CAN‐FIT) Participants were randomly allocated into 4 groups receiving: 1) pre‐RFES and post‐RFES sessions; 2) pre‐RFES sessions only; 3) post‐RFES session only; 4) no RFES session (standard care). Session content was developed by a multidisciplinary team and provided information about RT and its processes; potential treatment side effects including fatigue; behavioural strategies to reduce fatigue including activity modification; information about the benefits of participation in exercise/activity and maintaining weight/nutrition; sleep hygiene tips; and relaxation strategies. Subjects received a goal setting sheet and progress diary. RFES sessions (60 minutes) were delivered using a Microsoft PowerPoint presentation 1 week prior to RT planning and/or 1‐2 weeks after the completion of RT. 2 follow‐up phone calls were made 2 and 4 weeks after each education session. Control group: no RFES session. Patients received standard care via one‐to‐one verbal nursing education about the RT process, patient‐specific diagnosis, and standardised written information about RT treatment. A 1‐page flyer was provided with generic information about fatigue. |
|
| Outcomes | Measures taken at baseline (assessment 1), pre‐RT (assessment 2) and post‐RT (assessment 3). Outcomes Fatigue: Multidimensional Fatigue Inventory (data from the general fatigue sub scale was used for the 'general fatigue' outcome in this review) Single item VAS of fatigue intensity (data were obtained from the primary author) Activities of daily living or physical functioning: Karnofsky performance status scale, Frenchay Activities Index, International Physical Activity Questionnaire short form Anxiety: Hospital Anxiety and Depression Scale Depression: Hospital Anxiety and Depression Scale Global quality of life: EuroQual‐5D Sleep disturbance: Medical Outcomes Study sleep measure Paid/domestic labour: Health and Labour Questionnaire |
|
| Notes | Aim: to evaluate the effectiveness of pre‐ and post‐ RT education in reducing cancer‐related fatigue Sample size calculation evident with sample size of 110 participants estimated to have 80% power to detect a previously identified minimally clinically significant difference of 2.1 units of the Multidimensional Fatigue Inventory general fatigue sub scale between intervention and control groups, for either the main effect of the pre‐ or post‐treatment interventions. Funding source: indirect funding through Queensland Health Cancer Control Team, Queensland Health Health Practitioners Scheme, Princess Alexandra Hospital Cancer Collaborative Group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Procedure states: "Participants were subsequently allocated into groups as determined by a simple randomisation sequence developed using a computerised random number generator." |
| Allocation concealment (selection bias) | Low risk | Procedure states: "A researcher independent of the investigative team supervised both the development of the random allocation sequence and placing of the sequence into opaque, sequentially numbered envelopes stored securely in a research office." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A research assistant administered assessments and was blinded to group allocation." However the outcomes of interest in this review (e.g. fatigue) are subjective and data is provided through self‐report. As participants weren't blinded and provided the data this introduces the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1 and tables 3 & 4 ‐ missing outcome data balanced across groups with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes reported on as per study methods |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Ream 2006.
| Methods | RCT; 2 groups; stratified according to the centre where they received treatment and the chemotherapy regimen they were given | |
| Participants | N = 103 Mean age: 56.5 N = 46 females, N = 57 males Diagnosed with non‐Hodgkin's lymphoma or gastrointestinal, non‐small cell lung, colorectal, breast, or unknown primary cancer Receiving CTX with curative intent Excluded if they were unable to understand, speak, read, and write English; or if they were being treated for psychiatric illness The study was conducted at 2 regional cancer centres in the United Kingdom. |
|
| Interventions | Experimental group: received an information pack presenting information on exercising, balancing activity with rest, prioritising and delegating activities, dietary supplements, relaxation, diversion, and sleep‐enhancement techniques, prior to chemotherapy Experienced cancer nurse with a counselling qualification and knowledge of cancer‐related fatigue visited patients at home once during each treatment cycle to review fatigue diary, and review use of strategies from the information pack The intervention was provided over the first three CTX treatment cycles. Control group: received standard care over the first 3 CTX treatment cycles delivered in each centre, with no written resources available to patients at either centre. |
|
| Outcomes | Measures taken at baseline and prior to cycle 4 CTX treatment Outcomes Fatigue: 1) Fatigue Diary: patients completed a diary for the first 7 days of each of the 3 treatment cycles over which the intervention ran; 2) 4 VASs: subjective quantification of fatigue, subjective distress because of fatigue, and subjective assessment of effects of fatigue on chores/work and on pastimes/hobbies; 3) mean score of the 4 VASs listed above (data used for the 'general fatigue' outcome in this review) Coping: measured by a single VAS of perceived general coping and the COPE inventory (shortened version) Emotional wellbeing: Hospital Anxiety and Depression scale General Health Status: 36‐Item Short Form Health Survey |
|
| Notes | Aim: to reduce the symptom of fatigue (primary outcome), improve individuals' emotional well being and general health status, and assist individuals' adoption of adaptive coping behaviours and enhance their perceived ability to cope A sample size calculation determined that 45 patients were needed in each study arm to yield 80% power to detect a significant reduction in fatigue of 10%. Funding source: Cancer Research Campaign Project Grant (ce1162/0101) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Individuals within these strata were then allocated at random between the intervention and control groups by a computer‐generated randomization table." (p 150) |
| Allocation concealment (selection bias) | Low risk | Authors state: "allocation concealment was possible" (p 150) and go on to describe the methods used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p 153 paragraph 2 & Fig 1; authors state "There were few missing data (<1%)," however Fig 2 shows that 17/103 or 16% were lost to follow‐up and data from 20/103 were not available for analysis with an imbalance of subjects lost across groups. The authors state that missing data were imputed; however, for the VAS scales, insufficient information is provided about what was actually done. |
| Selective reporting (reporting bias) | Low risk | It appears that all outcomes listed in the methods are reported on in the results |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Reif 2012.
| Methods | RCT; 2‐group multi centre wait‐list controlled trial | |
| Participants | N = 260 Mean age: 57.65 years N = 187 females, N = 74 males Disease‐free cancer survivors at any time point following active treatment and remission of acute toxic side effects; in stable condition (Eastern Cooperative Oncology Group Performance Status 0‐2). The patients' fatigue level had to be rated on a 0‐10 scale as moderate (4‐6) or severe (7‐10). Exclusion criteria: life expectancy less than 12 months, brain tumours or brain metastases, cognitive disorders or psychiatric conditions The study was conducted at ten German centres |
|
| Interventions | Experimental group: a structured patient education program about fatigue and fatigue management consisting of 6 weekly sessions (90 min each) designed for groups of 8 cancer survivors. Format: lectures, discussions, individual tasks, behavioural training, and home tasks. Between sessions, the patients were encouraged to keep a diary, perform home tasks, and implement lifestyle changes. 2 additional meetings after 3 and 6 months were offered to patients to share their experiences in daily life. Control group: wait list control All patients received standard information on fatigue as a lecture |
|
| Outcomes | Baseline measures were obtained prior to randomisation; post‐intervention measures after completion of intervention and then at 6 months following participation Fatigue Assessment Questionnaire (used for 'general fatigue' outcome in this review; separate data for fatigue interference supplied by author) Cancer‐related fatigue knowledge: the Fatigue Knowledge Test was developed for this study. The concepts were drawn from clinical recommendations with emphasis on self‐care. A fatigue education satisfaction scale to measure the patients' satisfaction was developed based on a scale for asthma education. Quality of life was measured with the European Organization for Research and Treatment of Cancer Core Questionnaire (single VAS item for fatigue from this questionnaire was used in this review for 'fatigue intensity') General self‐efficacy was assessed by using the General Self‐Efficacy Scale Exercise self‐efficacy was measured with the Physical Exercise Self‐Efficacy Scale Physical activity was measured by the Freiburg Questionnaire on Physical Activity Anxiety and depression were measured by the German version of the Hospital Anxiety and Depression Scale |
|
| Notes | Aim: to evaluate a patient education program that aims at reducing perceived fatigue in cancer survivors A sample size calculation determined that to detect a clinically relevant difference of 4 points in the mean with 80% power and a two‐sided 0.05 significance, 120 patients were needed in each group. Funding source: German Federal Ministry of Education and Research (FKZ 01GT0605) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation lists were used for concealed allocation by central telephone calls." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated randomisation lists were used for concealed allocation by central telephone calls." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 234/261 (89.66%) provided data at follow up; data from 27 patients couldn't be analysed as there were no data available (Fig 1); 9 in the experimental group didn't attend the program and data from 18 in control group not available |
| Selective reporting (reporting bias) | Low risk | Results for all outcomes listed in the methods were reported |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Schjolberg 2014.
| Methods | RCT; 2 groups | |
| Participants | N = 160 Women with early stage (Stage 1 or Stage 2) breast cancer Mean age: 55.3 years Receiving chemotherapy, RT, or hormone therapy with curative intent Particpants recruited from outpatient clinics of cancer centre in Norway |
|
| Interventions | Educational packages that contained information about fatigue, strategies to ease the experience of fatigue, and information about physical exercise activities were provided to groups of 10 patients. 3 2‐hour sessions were held once a week, tailored to the specific needs of patient groups. The information was provided using Microsoft PowerPoint presentation of the material, a written patient booklet, and face to face group discussion. Control group: received the standard education and care given to all patients (no group education) |
|
| Outcomes | Fatigue Questionnaire (total score used in the analysis in this review) Lee Fatigue Scale (fatigue sub scale used in the analysis in this review) Measures taken at baseline, immediately after completion of intervention, and three months after intervention |
|
| Notes | Aim: to evaluate the effects of a 3‐week educational intervention on patient levels of fatigue in women with breast cancer. Funding source: Oslo and Akershus University College, Norway and supported by the Norwegian Cancer Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; the outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding of participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were only available for 92 of 160 (57.5%) participants immediately following the intervention; no reasons for missing data provided |
| Selective reporting (reporting bias) | Low risk | Data in the results are consistent with the outcomes listed in the methods for this study |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Wangnum 2013.
| Methods | RCT; 2 groups | |
| Participants | N = 60 people with stage 3 or 4 lung cancer Mean age: 56.10, years range 45 to 65 years Male = 67.33% Eligibility criteria: participants received at least 1 treatment of platinum‐based chemotherapy within the 2nd and 4th round of therapy, Eastern Cooperative Oncology Group performance status = 0‐1, good physical fitness, self‐care, minor side effects, no history of tinnitus, able to read and write Thai, willing to participate, and had given written informed consent Recruited from outpatient cancer unit, Thailand |
|
| Interventions | Multidisciplinary education in self‐care 4 face to face individual sessions over a 9‐week period. Patients met with physical therapists, nutritionists, and a psychological nurse to receive information about exercise, breathing, nutrition, and prevention of depression. Patients were provided written information and home programs, and asked to keep a record of their diet and exercise. At each session the health professional reviewed how they had gone during the last week and provided recommendations for adjusting their diet or exercise. Note: in the second session, the capability of patients to exercise was assessed and guidelines about exercise were provided accordingly. Control: usual care. This involved the nurse giving patients training for 30 minutes on how to exercise during their course of chemotherapy sessions, and instructions were given to the patients to take home and review. |
|
| Outcomes | Piper Fatigue Scale Beck Depression Inventory Measured before and after the intervention |
|
| Notes | Aim: to examine fatigue scores in patients with lung cancer after chemotherapy treatment, and to compare the scores of the group receiving the multidisciplinary education program with those of the control group. No sample size calculation evident Funding source: the Institute of Medical Research and Technology Assessment, Rajavithi Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p 1062 states: "Patients were randomised using the "Block 4" pattern." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all participants |
| Selective reporting (reporting bias) | Low risk | Data in the results is consistent with the outcomes listed in the methods for this study |
| Study size | High risk | Fewer than 50 participants per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Williams 2005.
| Methods | RCT; 2 groups. This study appears to be the same as Williams 2004 although on p 140 of Williams 2005, it refers to "a preliminary study (Williams & Schreier, 2004)". Data for participants at baseline are almost exactly the same as are a number of outcome data. We assume they are the same study and report on them as such below. | |
| Participants | N = 71 Mean age: 50.42 years N = 71 females Newly diagnosed with breast cancer Receiving chemotherapy with curative intent Excluded if: undergoing any therapy other than chemotherapy The study was conducted at a tertiary medical centre in the Southeastern United States and a satellite cancer treatment clinic. |
|
| Interventions | Experimental group: received the standard education and care from staff nurses, plus a 20‐minute professionally recorded audiotape that consisted of education about exercise and relaxation to manage anxiety, fatigue, and sleep problems, and a printed self care diary of self care behaviours that mirrored the audiotape. Subjects were instructed to listen to the audiotape 12‐24 hours before the start of chemotherapy cycles and as often as desired during the course of their treatment. Control group: received the standard education and care given to all patients during chemotherapy, including verbal instructions on potential side effects from the staff nurses at the time of treatment, and American Cancer Society literature related to treatment (duration of session not stated). |
|
| Outcomes | All subjects were interviewed by the same interviewer 3 times by telephone: before the first CTX treatment, 1 month later, and 3 months later. The interviewers were graduate level nursing students. Measures taken at baseline, 1 month later, and 3 months later Outcomes Fatigue, anxiety, and sleep disturbance: data for the presence (yes/no) and severity (5 point scale) of side effects were obtained Anxiety: State‐Trait Anxiety Inventory |
|
| Notes | Aim: to examine the effect of informational audiotapes on patients' self care behaviours to manage chemotherapy side effects of fatigue, anxiety, and sleep disturbance. No sample size calculation evident Funding sources: Pitt County Chapter of the American Cancer Society, an Oncology Nurses Grant supported by GlaxoSmithKline, the Leo Jenkins Cancer Centre, and the American Cancer Society Institutional Research Grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p 139 Intervention: "Patients were randomly assigned to either the control group or the experimental group." ‐ insufficient randomisation information |
| Allocation concealment (selection bias) | Unclear risk | p 139 Intervention: "Patients were randomly assigned to either the control group or the experimental group." ‐ insufficient allocation information |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however, it is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was attributed to a hurricane and flooding affecting many participants in this study |
| Selective reporting (reporting bias) | Low risk | It appears that all outcomes listed in the methods are reported on in the results |
| Study size | High risk | Fewer than 50 participants per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Wydra 2001.
| Methods | RCT; 2 groups; random assignment list | |
| Participants | N = 174 Mean age: 55.7 years N = 86 females, N = 88 males Out‐patient of cancer centre Receiving chemotherapy, radiation therapy, biological agents, supportive therapy, or a combination of therapies with curative intent Exclusion criteria: < 18 years of age, < 5th‐grade English reading level, brain or visual dysfunction that could interfere with the study, and inability to provide written consent The study was conducted at four comprehensive cancer centres: the Norris Cotton Cancer Center at the Dartmouth‐Hitchcock Medical Center in Lebanon, NH; the University of Pennsylvania Radiation Oncology Clinic in Philadelphia; the Cancer Therapy and Research Center associated with the University of Texas Health Science Center in San Antonio; and the Kenneth Norris Jr. Cancer Research Center and Hospital at the University of Southern California in Los Angeles. |
|
| Interventions | Experimental group: self‐guided interactive videodisc module, focusing on 5 major areas: (a) using the computer, (b) fatigue, (c) saving, maintaining, and restoring energy, (d) managing stress, and (e) sleeping better. Equipment was set up by a research assistant. 1 session at treatment facility, fatigue instruction ranged from 22 minutes to 2 hours and 23 minutes; average of 1 hour and 3 minutes Control group: cancer treatment as usual receiving conventional fatigue instruction 1 session, 1‐20 minutes while at treatment facility |
|
| Outcomes | Measures taken at baseline (pre‐test), end of intervention/30 minutes prior to completion of clinic stay (post‐test), and follow‐up 1 to 3 months after participation. Outcomes Fatigue: 5‐point Likert scale measuring level of fatigue, impact of fatigue on daily work, physical activities, social activities, and quality of life, and the amount of change being made to compensate for fatigue Follow‐up questionnaire about self‐care tasks for saving energy and sleeping better Use of strategies taught in the intervention Perceived coping with fatigue: 5‐point Likert scale Activities of daily living or physical functioning: 5‐point Likert scale Global quality of life: 5‐point Likert scale |
|
| Notes | Aim: to develop and test an interactive multimedia fatigue management module prototype, designed to accommodate adults undergoing cancer treatment with limited literacy and without computer skills. Sample size calculation evident with minimum sample size of 68 subjects each in the treatment and control groups recommended for analysis of covariance, with 0.80 power, a 0.05 significance criterion, 2 levels, and an estimated medium size effect Funding sources: National Centre for Nursing Research, Phase 1, Small Business Innovation Research Grant (R43NR02207) and the National Cancer INstitute, Phase II, Small Business Innovation Research Grant (R44CA62562) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p 1402 column 2 ‐ states "statistician generated a random assignment list by computer based on random numbers that were used to determine placement in the treatment or control groups" |
| Allocation concealment (selection bias) | Unclear risk | p 1402 column 2 ‐ insufficient information and detail regarding method of concealment ‐ "statistician generated a random assignment list by computer based on random numbers that were used to determine placement in the treatment or control groups" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported; however it is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p 1402 Testing Procedure ‐ "no exclusions were necessary"; & Results ‐ "The data set contained 174 observations; as a result of missing values, the analysis included only 160 observations" ‐ insufficient reporting of attrition to permit judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in methods provide in the results |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Yates 2005.
| Methods | RCT; 2 groups; randomised through a central telephone system using computer‐generated random numbers | |
| Participants | N = 110 female only Mean age: 49.4 years All women > 18 years of age with stage I or II breast cancer Receiving CTX with curative intent Women were admitted to the study if they had an Eastern Cooperative Oncology Group performance rating of one or two and their haemoglobin level was at least 11.6 g/mL at recruitment. Day treatment units in 3 major metropolitan hospital settings in Australia |
|
| Interventions | Experimental group: received a patient booklet and 3 individualised sessions incorporating information giving, problem solving, rehearsal and reinforcement; to improve patients' knowledge and skills to enable them to perform self‐care behaviours designed to minimize fatigue. Intervention delivered by oncology nurse. The 1st session was ˜20 minutes in length and delivered face to face in the clinic at the patient's second course of CTX. The 2nd and 3rd sessions were conducted by phone 1 week apart and were ˜10 minutes in length. Control group: received general cancer education sessions (equivalent in number and timing of intervention) from on oncology nurse.The control sessions were delivered in 1 face to face session, followed by 2 phone sessions at 1‐week intervals. |
|
| Outcomes | Measures taken at baseline and at session 2 and 3 of CTX, or on first day of RT, and 2 weeks after completion of RT Outcomes Fatigue: 1) A list of 10 self‐care actions using an 11‐point numeric rating scale 2) Perceived confidence in managing fatigue 3) 4 11‐point numeric rating scales measured fatigue at worst, best, average, and currently 4) 11‐point numeric rating scales of severity, distress, and impact on 4 aspects of daily life from the Revised Piper Fatigue Scale 5) Functional Assessment of Cancer Therapy‐Fatigue (used for 'general fatigue' outcome in this review) measuring 20 fatigue‐related symptoms (range 0‐4) Depression: 14‐item Hospital Anxiety and Depression Scale Cancer self‐efficacy scale was assessed using a 24‐item instrument developed in earlier pilot studies: the 30‐item European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (version 3). |
|
| Notes | Aim: to evaluate the efficacy of a psychoeducational intervention in improving cancer‐related fatigue A sample size of 35 patients per group was estimated as necessary to detect a significant difference in treatment effects on fatigue measures with 80% power and type I error of 5% (2 sided). Funding source: Queensland Nursing Council, National Breast Cancer Foundation, Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p 6028 Study Design ‐ "The patient was then randomly assigned to intervention or control conditions through a central telephone system using computer‐generated random numbers" ‐ adequate randomisation |
| Allocation concealment (selection bias) | Low risk | p 6028 Study Design ‐ "The patient was then randomly assigned to intervention or control conditions through a central telephone system using computer‐generated random numbers. Group allocation was concealed from research assistants involved in recruitment and the baseline and follow‐up assessments" ‐ adequate concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although it states on p 6028 that "group allocation was concealed from research assistants involved in recruitment, baseline and follow‐up assessments" the outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding of participants providing the data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p 6030‐1 Results & Fig 2 ‐ missing outcome balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | It appears that all outcomes listed in the methods are reported on in the results |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Yuen 2006.
| Methods | RCT; 2 groups | |
| Participants | N = 12 Mean age: 55.4 years N = 5 females, N = 7 males Diagnosed with cancer Received RT with curative intent Exclusion criteria: (a) concurrent, systemic health problems such as anaemia, cardiopulmonary, endocrine, or neurologic diseases, which are known to contribute to increased fatigue levels; (b) documented past or current diagnosis of any major Axis I psychiatric disorder, such as melancholia, which may confound the evaluation of fatigue levels; (c) visual or hearing impairment that assistive devices cannot correct; (d) illiteracy, with no proxy to help with forms and materials used in the study; and (e) life expectancy of less than 6 months Participants recruited from one hospital in USA |
|
| Interventions | Experimental group: received 1 to 2 hours of individual, face to face energy conservation training from an occupational therapist followed by once a week telephone monitoring sessions in the subsequent 3 week Control group: received standard care from their oncologist |
|
| Outcomes | Measures taken at baseline/pre‐training (within first 2 weeks after subjects had completed radiation therapy) and post‐training (week following telephone monitoring sessions at the same time period for intervention and control groups) Outcomes Fatigue: Piper Fatigue Scale (data from the total score used for the 'general fatigue' outcome in this review) |
|
| Notes | Aim: to evaluate the effectiveness of energy conservation training to help post‐therapy cancer survivors manage their fatigue No sample size calculation evident Funding source: Association of Schools of Allied Health Professions New Investigator's Award and the South Carolina Occupational Therapy Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p e‐128 paragraph 2: "participants were randomly assigned to one of two groups" ‐ insufficient information |
| Allocation concealment (selection bias) | Unclear risk | p e‐128 paragraph 2: "participants were randomly assigned to one of two groups" ‐ insufficient information regarding allocation method |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Low risk | It appears that all outcomes listed in the methods are reported on in the results |
| Study size | High risk | Fewer than 50 participants per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
Yun 2012.
| Methods | RCT; 2 groups | |
| Participants | N = 273 Mean age not able to be calculated; approximately 53% were 45 to 65 years N = 199 females, N = 74 males Inclusion criteria: disease‐free cancer survivors with moderate to severe fatigue (worst fatigue in Brief Fatigue Inventory 4) for at least 1 week, cancer stages I to III, primary treatment completed within the past 24 months, and age 20 to 65 years Exclusion criteria: undergoing or planning surgery, RT, or chemotherapy; a major health problem that might cause fatigue; exercise or nutrition intervention was contraindicated; cardiovascular disease, pulmonary disease, uncontrolled hypertension, poorly controlled diabetes, or severe musculoskeletal disease; severe psychiatric disorders such as major depression or suicidal tendencies; dyspnoea; evidence of metastases or recurrence; Eastern Cooperation and Oncology Group Performance Status 3 to 4; or did not use the Internet or a mobile telephone (in addition to other blood counts, etc). Participants recruited from 4 Korean hospitals |
|
| Interventions | Experimental groups: 12‐week, Internet‐based, individually tailored cancer‐related fatigue education program Components of the 12‐week, individually tailored intervention program were based on 2008 National Comprehensive Cancer Network guidelines and covered six strategic areas: energy conservation, physical activity, nutrition, sleep hygiene, pain control, and distress management. A general introduction to cancer‐related fatigue was added. The user's Web page on the Health Navigation site covers 7 education areas offering different sessions (general introductory session, 2 sessions on energy conservation, 4 on nutrition, 10 on physical activity, 7 on sleep hygiene, 7 or 12 on pain control according to pain severity, and 8 on distress management). Control group: routine care |
|
| Outcomes | Measures taken at baseline and at 3 months (following completion of 12 week program) Brief Fatigue Inventory (separate sub scales for global fatigue, severity, and interference available; severity and interference sub scales used for relevant outcomes in this review) Fatigue Severity Scale Hospital Anxiety and Depression Scale European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Energy‐Conservation Strategies Inventory (contains 20 items that cover activities related to planning, distraction, labor saving, burden reduction, and comfort) MET (metabolic equivalent; a measure of physical activity) Medical Outcome Study–Sleep Scale Brief Pain Inventory |
|
| Notes | Aim: to determine whether an Internet‐based tailored education program is effective for disease‐free cancer survivors with cancer‐related fatigue Sample size calculation: "Anticipating a 15% dropout rate, we decided on 133 as the prospective number of participants in each group so that we could achieve a statistical power of 80% and an effect size of 0.375 by a two‐sided t‐test at the 0.05 level." Funding source: National Cancer Centre (0710420 and1010470‐1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician generated a randomizations table with NQuery Advisor 6.01 (Statistical Solutions, Saugus, MA) and used the table to assign each patient to either the intervention group or the usual care group." |
| Allocation concealment (selection bias) | Low risk | Quote:"Identification numbers, unrevealed to the recruiting physicians, were assigned to participants and entered into a computer for randomizations. An independent statistician generated a randomizations table with NQuery Advisor 6.01 (Statistical Solutions, Saugus, MA) and used the table to assign each patient to either the intervention group or the usual care group." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is possible to discern group allocation and therefore blinding was not achieved. The outcomes of interest are subjective with data provided through self‐report (e.g. fatigue) and are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 243/273 (89%) completed the course. Quote: "For intent‐to‐treat analysis, we used the approach of last observation carried forward to impute scores for missing values." |
| Selective reporting (reporting bias) | Low risk | It appears that all outcomes listed in the methods are reported on in the results |
| Study size | Unclear risk | 50‐199 per treatment arm |
| Other bias | Low risk | This study appears to be free of other sources of bias |
CTX: cyclophosphamide
Fig: figure
g/mL: grams per millilitre
Max.: maximum
Min.: minimum
N / n: number of participants
p: page
RCT: randomised controlled trial
RFES: Radiotherapy Fatigue Education and Support
RT: radiotherapy
SD: standard deviation
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allard 2006 | Primary focus not an educational intervention |
| Allison 2004 | Not a randomised controlled trial |
| Armes 2007 | Intervention included education but cognitive behavioural therapy was primary focus |
| Barsevick 2002 | Not a randomised controlled trial |
| Borthwick 2003 | Not a randomised controlled trial |
| Brown | Focus is on quality of life and intervention includes modalities other than education |
| Chan | Intervention included relaxation training as well as education |
| Corbett 2016 | Intervention was focused on cognitive behaviour therapy |
| Davidson 2001 | Main outcome not fatigue and included psychosocial interventions |
| Dirksen 2008 | Intervention focused on insomnia and used cognitive behavioural therapy |
| Evers | Not a randomised controlled trial |
| Fawzy 1995 | Fatigue is not a primary outcome of interest |
| Given 2002 | Multifaceted intervention; primary intervention not education |
| Holley 2001 | Not a randomised controlled trial |
| Kim 2002 | Fatigue was not the primary focus of the intervention |
| OBrien 2014 | Main outcome did not include measurement of fatigue or its management |
| Ream 2002 | Not a randomised controlled trial |
| Ream 2005 | Not a randomised controlled trial |
| Stanton 2005 | Although one arm of this trial is an educational intervention and one of the primary outcomes is fatigue, the focus of this intervention is not about managing fatigue. |
| Wengstrom 1999 | Primary outcome not fatigue |
| Willems 2016 | Focus of the intervention could be selected by individual participants. Not all participants sought help with fatigue |
| Yesilbalkan | Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Bigatao 2016.
| Methods | RCT |
| Participants | Adults with brain tumours |
| Interventions | Educational program |
| Outcomes | Fatigue, quality of life, mood |
| Notes |
Littlechild 2016.
| Methods | RCT |
| Participants | Hospice outpatients |
| Interventions | Group versus individual educational support |
| Outcomes | Fatigue, function, quality of life |
| Notes |
Sandler 2015.
| Methods | RCT |
| Participants | Adult cancer patients |
| Interventions | Participants were assigned to an education intervention, or a 12 week integrated cognitive behavioural therapy and graded exercise therapy intervention supervised by an exercise physiologist and clinical psychologist. |
| Outcomes | Self‐reported fatigue and functional status (role limitation due to physical health problems domain of the 36‐Item Short Form Health Survey) |
| Notes | Only conference abstract available |
Velji 2006.
| Methods | RCT |
| Participants | Women receiving radiation therapy for gynaecological cancers |
| Interventions | Individualized Symptom Education Program |
| Outcomes | Fatigue, pain, nausea, mood disturbance, and pelvic symptoms |
| Notes | PhD thesis (author has been contacted for more information) |
RCT: randomised controlled trial
Differences between protocol and review
The primary outcome (fatigue) was defined more clearly and specifically in the review compared with the protocol. Specifically, 'general fatigue' was operationalised for this review as fatigue measures that combine different characteristics of fatigue (e.g. fatigue intensity, distress, and interference), that combine different dimensions of fatigue (e.g. cognitive and physical and emotional fatigue), or that state that they are measures of general fatigue. In the protocol one of the inclusion criteria listed for studies was that they include a measurement of fatigue as a primary outcome. This was extended in this review to 'measurement of fatigue or its management' as having to be a primary outcome to be included. We added a comment about adverse events to the secondary outcomes section.
With respect to the type of intervention described in the methods section, the protocol stated: "Studies that use psycho‐behavioural methods such as meditation, relaxation or techniques to improve coping with fatigue will not be included, unless a comparative treatment arm of education only is used. Studies that combine psycho‐behavioural methods with education will be excluded to minimise confounding unless a comparative treatment arm of education only is used." In the full review we have stated this differently as follows: "For the purpose of this review educational interventions were defined as any advice, information, or self‐management education (verbal, written, or audiovisual), provided in order to help people understand and manage cancer‐related fatigue. This may incorporate information and advice about non‐pharmacological strategies (e.g. information about relaxation, nutrition, cognitive behavioural therapy (CBT), or information about exercise), but would exclude trials that actually use these interventions."
We also added an elaboration on the types of interventions used in this review in the methods section in response to feedback. The additional information is as follows: "Studies may have used techniques such as discussion, coaching, goal‐setting, feedback, and reinforcement that may also be used in psychological therapies such as CBT, but the intervention would not be classified as CBT itself."
We did not search LILACS, CancerLit, Dissertation Abstracts, or Science Citation Index using the cited reference search. We did not extract data about co‐interventions, participant adherence, or providers of the intervention.
Jenny Fleming contributed to the protocol but did not act as an author on the final review.
We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created a 'Summary of findings' table, plans for which were not included in the protocol.
Contributions of authors
Sally Bennett: content expert, conceived the project, developed the protocol, coordinated authors, appraised risk of bias, extracted data, graded the quality of the evidence, and was responsible for the full review. SB will be responsible for updates. Amanda Purcell: content expert and contributed to writing and review of the protocol and review. Pam Meredith: content expert, helped determine selection of studies, and contributed to protocol and review editing. Elaine Beller: methodological expert, helped extract data for the review, and contributed to review of the protocol and full review. Terry Haines: methodological expert and contributed to review of the protocol and full review. Christie Delaney: involved in study selection, assessment of risk of bias, and extracting information on characteristics of studies.
Sources of support
Internal sources
-
The University of Queensland, Australia.
Infrastructure support and time for authors to contribute to the protocol was provided by The University of Queensland
External sources
-
National Health & Medical Research Council Palliative Care Development Grant, Australia.
Provided funding for literature searching and for research assistant to assist with this review
Declarations of interest
SB: none known; SB was an investigator in one of the studies in this review (Purcell 2011).
AP: none known; AP was an investigator in one of the studies in this review (Purcell 2011).
EB: none known.
TH: none known; TH was an investigator in one of the studies in this review (Purcell 2011).
PM: none known.
CD: none known.
SB, TH and AP did not extract data on trials they were involved in.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Barsevick 2004 {published data only}
- Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, Nail L. A randomized clinical trial of energy conservation for patients with cancer‐related fatigue. Cancer 2004;100(6):1302‐10. [DOI] [PubMed] [Google Scholar]
Barsevick 2010 {published data only}
- Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. Journal of Pain & Symptom Management 2010;40(2):200‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2015 {published and unpublished data}
- Foster C, Calman L, Grimmett C, Breckons M, Cotterell P, Yardley L, et al. Managing fatigue after cancer treatment: development of RESTORE, a web‐based resource to support self‐management. Psycho‐oncology 2015;24(8):940‐9. [DOI] [PubMed] [Google Scholar]
- Foster C, Grimmett C, May CM, Ewings S, Myall M, Hulme C, et al. A web‐based intervention (RESTORE) to support self‐management of cancer‐related fatigue following primary cancer treatment: a multi‐centre proof of concept randomised controlled trial. Support Care Cancer 2016;24(6):2445‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett C, Armes J, Breckons M, Calman L, Corner J, Fenlon D, et al. RESTORE: an exploratory trial of an online intervention to enhance self‐efficacy to manage problems associated with cancer‐related fatigue following primary cancer treatment: study protocol for a randomized controlled trial. Trials 2013;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett C, Breckons M, Calman L, Corner J, Fenlon D, Richardson A, et al. Restore: A web‐based intervention to support self‐management of cancer‐related fatigue following primary cancer treatment. Psycho‐oncology 2013;22:10. [Google Scholar]
- Grimmett C, Breckons M, Calman L, Corner J, Fenlon D, Richardson A, et al. Restore: An online intervention to enhance self‐efficacy to self manage cancer related fatigue; an exploratory trial protocol. Supportive Care in Cancer. 2013; Vol. 21:S100. [DOI] [PMC free article] [PubMed]
Godino 2006 {published data only}
- Godino C, Jodar L, Durán A, Martínez I, Schiaffino A. Nursing education as an intervention to decrease fatigue perception in oncology patients. European Journal of Oncology Nursing 2006;10(2):150‐5. [DOI] [PubMed] [Google Scholar]
Purcell 2011 {published data only}
- Purcell A, Fleming J, Burmeister B, Bennett S, Haines T. Is education an effective management strategy for reducing cancer‐related fatigue?. Support Care Cancer 2011;19(9):1429‐39. [DOI] [PubMed] [Google Scholar]
Ream 2006 {published data only}
- Ream E, Richardson A, Alexander‐Dann C. Supportive intervention for fatigue in patients undergoing chemotherapy: a randomized controlled trial. Journal of Pain & Symptom Management 2006;31(2):148‐61. [DOI] [PubMed] [Google Scholar]
Reif 2012 {published data only}
- Reif K, Vries U, Petermann F, Görres S. A patient education program is effective in reducing cancer‐related fatigue: a multi‐centre randomised two‐group waiting‐list controlled intervention trial. European Journal of Oncology Nursing 2013 Epub 2012;17(2):204‐13. [DOI] [PubMed] [Google Scholar]
Schjolberg 2014 {published data only}
- Schjolberg T, Dodd M, Henriksen N, Asplund K, Smastuen M, Rustoen K. Effects of an educational intervention for managing fatigue in women with early stage breast cancer. European Journal of Oncology Nursing 2014;18:286‐94. [DOI] [PubMed] [Google Scholar]
Wangnum 2013 {published data only}
- Wangnum K, Thanarojanawanich T, Chinwatanachai K, Jamprasert L, Maleehuan O, Janthakun V. Impact of the Multidisciplinary Education Program in Self‐Care on Fatigue in Lung Cancer Patients Receiving Chemotherapy. Journal of the Medical Association of Thailand 2013;96(12):1601‐8. [PubMed] [Google Scholar]
Williams 2005 {published data only}
- Williams SA, Schreier AM. The role of education in managing fatigue, anxiety, and sleep disorders in women undergoing chemotherapy for breast cancer. Applied Nursing Research 2005;18(3):138‐47. [DOI] [PubMed] [Google Scholar]
- Williams SA, Schreier AM, Hodgson H. The effect of education in managing side effects in women receiving chemotherapy for treatment of breast cancer. Oncology Nursing Forum 2004;31(1):E16‐23. [DOI] [PubMed] [Google Scholar]
Wydra 2001 {published data only}
- Wydra EW. The effectiveness of a self‐care management interactive multimedia module. Oncology Nursing Forum 2001;28(9):1399‐407. [PubMed] [Google Scholar]
Yates 2005 {published data only}
- Yates P, Aranda S, Hargraves M, Mirolo B, Clavarino A, McLachlan S, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early‐stage breast cancer. Journal of Clinical Oncology 2005;23(25):6027‐36. [DOI] [PubMed] [Google Scholar]
Yuen 2006 {published data only}
- Yuen HK, Mitcham M, Morgan L. Managing post‐therapy fatigue for cancer survivors using energy conservation training. Journal of Allied Health 2006;35(2):121E‐139E. [PubMed] [Google Scholar]
Yun 2012 {published data only}
- Yun YH, Lee KS, Kim YW, Park SY, Lee ES, Noh DY, et al. Web‐based tailored education program for disease‐free cancer survivors with cancer‐related fatigue: a randomized controlled trial. Journal of Clinical Oncology 2012;30(12):1296‐303. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allard 2006 {published data only}
- Allard N. Day surgery and recovery in women with a suspicious breast lesion: evaluation of a psychoeducational nursing intervention. Canadian Oncology Nursing Journal/Revue canadienne de soins infirmiers en oncologie 2006;16(3):137‐53. [DOI] [PubMed] [Google Scholar]
Allison 2004 {published data only}
- Allison PJ, Edgar L, Nicolau B, Archer J, Black M, Hier M. Results of a feasibility study for a psycho‐educational intervention in head and neck cancer. Psycho‐oncology 2004;13(7):482‐5. [DOI] [PubMed] [Google Scholar]
Armes 2007 {published data only}
- Armes J, Chalder T, Addington‐Hall J, Richardson A, Hotopf M. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer‐related fatigue. Cancer 2007;110(6):1385‐95. [DOI] [PubMed] [Google Scholar]
Barsevick 2002 {published data only}
- Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment‐related fatigue. Cancer Nursing 2002;25(5):333‐41. [DOI] [PubMed] [Google Scholar]
Borthwick 2003 {published data only}
- Borthwick D, Knowles G, McNamara S. Assessing fatigue and self‐care strategies in patients receiving radiotherapy for non‐small cell lung cancer. European Journal of Oncology Nursing 2003;7(4):231‐41. [DOI] [PubMed] [Google Scholar]
Brown {published data only}
- Brown P, Clark MM, Atherton P, Huschka M, Sloan J A, Gamble G, et al. Will improvement in quality of life (QOL) impact fatigue in patients receiving radiation therapy for advanced cancer?. American Journal of Clinical Oncology 2006;29(1):52‐8. [DOI] [PubMed] [Google Scholar]
Chan {published data only}
- Chan CW, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: results of a psychoeducational randomized controlled trial. J Pain Symptom Management 2011;41(2):347‐57. [DOI] [PubMed] [Google Scholar]
Corbett 2016 {published data only}
- Corbett T, Walsh JC, Groarke A, Moss‐Morris R, McGuire BE. Protocol for a pilot randomised controlled trial of an online intervention for post‐treatment cancer survivors with persistent fatigue. BMJ Open 2016;6(6):e011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2001 {published data only}
- Davidson JR, Waisberg JL, Brundage MD, MacLean AW. Nonpharmacologic group treatment of insomnia: a preliminary study with cancer survivors. Psycho‐oncology 2001;10(5):389‐97. [DOI] [PubMed] [Google Scholar]
Dirksen 2008 {published data only}
- Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. Journal of Advanced Nursing 2008;61(2):664‐75. [DOI] [PubMed] [Google Scholar]
Evers {published data only}
- Evers G, Giesberts K, Claeys P, Bogaert W, Paridaens R. Effect of self‐care instruction on self‐care knowledge and behaviour related to fatigue after radiotherapy and chemotherapy. Acta Hospitalia 1998;38(2):51‐61. [Google Scholar]
Fawzy 1995 {published data only}
- Fawzy NW. A psychoeducational nursing intervention to enhance coping and affective state in newly diagnosed malignant melanoma patients. Cancer Nursing 1995;18(6):427‐38. [PubMed] [Google Scholar]
Given 2002 {published data only}
- Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and Fatigue Management: Results of a Nursing Randomized Clinical Trial. Oncology Nursing Forum 2002;29(6):949‐56. [DOI] [PubMed] [Google Scholar]
Holley 2001 {published data only}
- Holley S, Borger D. Energy for living with cancer: preliminary findings of a cancer rehabilitation group intervention study. Oncology Nursing Forum 2001;28(9):1393‐6. [PubMed] [Google Scholar]
Kim 2002 {published data only}
- Kim Y, Roscoe JA, Morrow GR. The effects of information and negative affect on severity of side effects from radiation therapy for prostate cancer. Supportive Care in Cancer 2002;10(5):416‐21. [DOI] [PubMed] [Google Scholar]
OBrien 2014 {published data only}
- O'Brien L, Loughnan A, Purcell A, Haines T. Education for cancer‐related fatigue: Could talking about it make people more likely to report it?. Supportive Care in Cancer 2014;22:209‐15. [DOI] [PubMed] [Google Scholar]
Ream 2002 {published data only}
- Ream E, Richardson A, Alexander‐Dann C. Facilitating patients' coping with fatigue during chemotherapy ‐ Pilot outcomes. Cancer Nursing 2002;25(4):300‐8. [DOI] [PubMed] [Google Scholar]
Ream 2005 {published data only}
- Ream E, Richardson A, Evison M. A feasibility study to evaluate a group intervention for people with cancer experiencing fatigue following treatment. Clinical Effectiveness in Nursing 2005;9(3‐4):178‐87. [Google Scholar]
Stanton 2005 {published data only}
- Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, et al. Outcomes from the Moving Beyond Cancer psychoeducational, randomized, controlled trial with breast cancer patients. Journal of Clinical Oncology 2005;23(25):6009‐18. [DOI] [PubMed] [Google Scholar]
Wengstrom 1999 {published data only}
- Wengstrom Y, Haggmark C, Strander H, Forsberg C. Effects of a nursing intervention on subjective distress, side effects and quality of life of breast cancer patients receiving curative radiation therapy‐a randomized study. Acta Oncologica 1999;38(6):763‐70. [DOI] [PubMed] [Google Scholar]
Willems 2016 {published data only}
- Willems RA, Bolman CA, Mesters I, Kanera IM, Beaulen AA, Lechner L. Short‐term effectiveness of a web‐based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: Randomized controlled trial. Psycho‐oncology 2016;Online early. [DOI] [PubMed] [Google Scholar]
Yesilbalkan {published data only}
- Yesilbalkan OU, Karadakovan A, Göker E. The effectiveness of nursing education as an intervention to decrease fatigue in Turkish patients receiving chemotherapy. Oncology Nursing Forum 2009;36(4):E215‐22. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bigatao 2016 {published data only}
- Bigatao MR, Peria FM, Tirapelli DPC, Carlotti Junior CG. Educational program on fatigue for brain tumor patients: Possibility strategy?. Arquivos de neuro‐psiquiatria 2016;74(2):155‐60. [DOI] [PubMed] [Google Scholar]
Littlechild 2016 {published data only}
- Littlechild B. Managing fatigue: a trial of group and individual educational support for hospice outpatients. European Journal of Palliative Care 2016;23(4):166‐8. [Google Scholar]
Sandler 2015 {published data only}
- Sandler C, Goldstein D, Horsfield S, Bennett B, Friedlander M, Bastick P, et al. TOPS: A randomised controlled trial of a multidisciplinary intervention for post‐cancer fatigue. Journal of Clinical Oncology 2015;33(15):9571. [Google Scholar]
Velji 2006 {unpublished data only}
- Velji K. Effect of an individualized symptom education program on the symptom distress of women receiving radiation therapy for gynecological cancers. PhD thesis 2006.
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez N, et al. The European organization for research and treatment of cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. [DOI] [PubMed] [Google Scholar]
Borneman 2007
- Borneman T, Piper B, Chih‐Yi Sun V, Koczywas M, Uman G, Ferrell B. Implementing the Fatigue Guidelines at One NCCN Member Institution: Process and Outcomes. Journal of the National Comprehensive Cancer Network 2007;5(10):1092‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2006
- Bower JE. Management of cancer‐related fatigue. Clinical Advances in Hematology & Oncology 2006;4(11):828‐9. [PubMed] [Google Scholar]
Butt 2008
- Butt Z, Wagner LI, Beaumont JL, Paice J, Peterman A, Shevrin D, et al. Use of a single‐item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. Journal of Pain and Symptom Management 2008;35:20‐30. [DOI] [PubMed] [Google Scholar]
Chelf 2001
- Chelf JH, Agre P, Axelrod A, Cheney L, Cole DD, Conrad K, et al. Cancer‐related patient education: an overview of the last decade of evaluation and research. Oncology Nursing Forum 2001;28(7):1139‐47. [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. New Jersey: Lawrence Erlbaum, 1988. [Google Scholar]
Cramp 2012
- Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context (2nd edition). London (UK): BMJ Publication Group, 2001. [Google Scholar]
Du 2015
- Du S, Hu L, Dong J, Xu G, Jin S, Zhang H, et al. Patient education programs for cancer‐related fatigue: A systematic review. Patient Education and Counseling 2015;98:1308‐19. [DOI] [PubMed] [Google Scholar]
Goedendorp 2009
- Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006953.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.), 2015.
Hann 1998
- Hann DM, Jacobsen PB, Azzarello LM, Martin S, Curran S, Fields K, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of Life Research 1998;7(4):301‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Hinds 1995
- Hinds C, Streater A, Mood M. Functions and preferred methods of receiving information related to radiotherapy. Cancer Nursing 1995;18:374‐84. [PubMed] [Google Scholar]
Jacobsen 1999
- Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. Journal of Pain and Symptom Management 1999;18(4):233‐42. [DOI] [PubMed] [Google Scholar]
Jacobsen 2007
- Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta‐analysis of psychological and activity‐based interventions for cancer‐related fatigue. Health Psychology 2007;26(6):660‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jameson 2016
- Jameson G, VonHoff D. Fatigue. In: Alberts D, Lluria M, Kha S, Weihs K editor(s). Supportive Cancer Care. Springer International Publishing, 2016:163‐81. [Google Scholar]
Kangas 2008
- Kangas M, Bovbjerg D, Montgomery G. Cancer‐related fatigue: A systematic and meta‐analytic review of non‐pharmacological therapies for cancer patients. Psychological Bulletin 2008;134(5):700‐41. [DOI] [PubMed] [Google Scholar]
Koornstra 2014
- Koornstraa R, Peters M, Donofrio S, Borne B, Jong F. Management of fatigue in patients with cancer – A practical overview. Cancer Treatment Review 2014;40(6):791–9. [DOI] [PubMed] [Google Scholar]
Kris 2004
- Kris A, Dodd M. Symptom experience of adult hospitalized medical‐surgical patients. Journal of Pain and Symptom Management 2004;28(5):451–9. [DOI] [PubMed] [Google Scholar]
Lawrence 2004
- Lawrence D, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute Monographs 2004;32:40‐50. [DOI] [PubMed] [Google Scholar]
Minton 2013
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer‐related fatigue. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD006704.pub3] [DOI] [PubMed] [Google Scholar]
Mitchell 2006
- Mitchell SA, Berger AM. Cancer‐related fatigue: the evidence base for assessment and management. Cancer Journal 2006;12(5):374‐87. [DOI] [PubMed] [Google Scholar]
Mock 2005
- Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology 2005;14(6):464‐77. [DOI] [PubMed] [Google Scholar]
Morrow 2005
- Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer‐related fatigue. Cancer Investigation 2005;23(2):229‐39. [DOI] [PubMed] [Google Scholar]
NCCN 2016
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: cancer‐related fatigue. Available from: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (Accessed June 2016) 2016.
Nuesche 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Piper 1998
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncology Nursing Forum 1998;25(4):677‐84. [PubMed] [Google Scholar]
Portenoy 1994
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander‐Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer 1994;30A(9):1326‐36. [DOI] [PubMed] [Google Scholar]
Purcell 2009
- Purcell A, Fleming J, Haines T, Bennett S. Cancer‐related fatigue: A review and a conceptual framework to guide therapists' understanding. British Journal of Occupational Therapy 2009;72(2):79‐86. [Google Scholar]
Ream 1996
- Ream E, Richardson A. The role of information inpatients' adaptation to chemotherapy and radiotherapy: a review of the literature. European Journal of Cancer Care 1996;5:132‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smets 1996
- Smets EM, Garssen B, Cull A, Haes JC. Application of the multidimensional fatigue inventory (MFI‐20) in cancer patients receiving radiotherapy. British Journal of Cancer 1996;73(2):241‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smets 1998a
- Smets EM, Visser MR, Willems‐Groot AF, Garssen B, Oldenburger F, Tienhoven G, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. British Journal of Cancer 1998;78(7):899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smets 1998b
- Smets EM, Visser MR, Willems‐Groot AF, Garssen B, Schuster‐Uitterhoeve AL, Haes JC. Fatigue and radiotherapy: (B) experience in patients 9 months following treatment. British Journal of Cancer 1998;78(7):907‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2007
- Smith G, Toonen T. Primary Care of the Patient with Cancer. American Family Physician 2007;75(8):1207‐14. [PubMed] [Google Scholar]
Sood 2007
- Sood A, Barton DL, Bauer BA, Loprinzi CL. A critical review of complementary therapies for cancer‐related fatigue. Integrative Cancer Therapies 2007;6(1):8‐13. [DOI] [PubMed] [Google Scholar]
Stark 2012
- Stark L, Tofthagen C, Visovsky C, McMillan S. The Symptom Experience of Patients with Cancer. Journal of Hospice and Palliative Nursing 2012;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2000
- Stone P, Richards M, A'Hern R, Hardy J. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Annals of Oncology 2000;11:561‐7. [DOI] [PubMed] [Google Scholar]
Stricker 2004
- Stricker CT, Drake D, Hoyer KA, Mock V. Evidence‐based practice for fatigue management in adults with cancer: exercise as an intervention. Oncology Nursing Forum 2004;31(5):963‐76. [DOI] [PubMed] [Google Scholar]
van den Bourne 1998
- Bourne HW. The patient from receiver of information to informed decision‐maker. Patient Education and Counseling 1998;34(2):89‐102. [DOI] [PubMed] [Google Scholar]
Wanchai 2011
- Wanchai A, Armer JM, Stewart BR. Nonpharmacologic supportive strategies to promote quality of life in patients experiencing cancer‐related fatigue: a systematic review. Clinical Journal of Oncology Nursing 2011;15(2):203‐14. [DOI] [PubMed] [Google Scholar]
Woo 1998
- Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC. Differences in fatigue by treatment methods in women with breast cancer. Oncology Nursing Forum 1998;25(5):915‐20. [PubMed] [Google Scholar]
Yellen 1997
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management 1997;13(2):63‐74. [DOI] [PubMed] [Google Scholar]


