Abstract
Background
Guillain‐Barré syndrome (GBS) is an acute paralysing disease caused by inflammation of the peripheral nerves, which corticosteroids would be expected to benefit.
Objectives
To examine the ability of corticosteroids to hasten recovery and reduce the long‐term morbidity from GBS.
Search methods
On 12 January 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase. We also searched trials registries.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs of any form of corticosteroid or adrenocorticotrophic hormone versus placebo or supportive care alone in GBS. Our primary outcome was change in disability grade on a seven‐point scale after four weeks. Secondary outcomes included time from randomisation until recovery of unaided walking, time from randomisation until discontinuation of ventilation (for those ventilated), death, death or disability (inability to walk without aid) after 12 months, relapse, and adverse events.
Data collection and analysis
The review authors used standard methods expected by Cochrane.
Main results
The review authors discovered no new trials in the new searches in June 2009, November 2011, or January 2016. Six trials with 587 participants provided data for the primary outcome. According to moderate quality evidence, the disability grade change after four weeks in the corticosteroid groups was not significantly different from that in the control groups, mean difference (MD) 0.36 less improvement (95% confidence intervals (CI) 0.16 more to 0.88 less improvement). In four trials of oral corticosteroids with 120 participants in total, there was very low quality evidence of less improvement after four weeks with corticosteroids than without corticosteroids, MD 0.82 disability grades less improvement (95% CI 0.17 to 1.47 grades less). In two trials with a combined total of 467 participants, there was moderate quality evidence of no significant difference of a disability grade more improvement after four weeks with intravenous corticosteroids (MD 0.17, 95% CI ‐0.06 to 0.39). According to moderate quality evidence, there was also no significant difference between the corticosteroid treated and control groups for improvement by one or more grades after four weeks (risk ratio (RR) 1.08, 95% CI 0.93 to 1.24) or for death or disability after one year (RR 1.51, 95% CI 0.91 to 2.5). We found high quality evidence that the occurrence of diabetes was more common (RR 2.21, 95% CI 1.19 to 4.12) and hypertension less common (RR 0.15, 95% CI 0.05 to 0.41) in the corticosteroid‐treated participants.
Authors' conclusions
According to moderate quality evidence, corticosteroids given alone do not significantly hasten recovery from GBS or affect the long‐term outcome. According to very low quality evidence, oral corticosteroids delay recovery. Diabetes requiring insulin was more common and hypertension less common with corticosteroids based on high quality evidence.
Plain language summary
Corticosteroids for Guillain‐Barré syndrome
Review question
Do corticosteroids hasten recovery from disability in people with Guillain‐Barré syndrome compared with dummy (placebo) treatment or supportive care alone?
Background
Guillain‐Barré syndrome is an uncommon paralysing illness, usually caused when the person's immune system attacks their own nerves, which consequently become inflamed. In 25% of people affected, the disease leads to a need for artificial ventilation. About 5% of people with the disease die and about 10% are left disabled. Corticosteroids (such as prednisolone) reduce inflammation and so should reduce nerve damage.
Study characteristics
There were eight clinical trials with altogether 653 participants. Only six trials with altogether 587 participants gave information about the primary outcome measure for this review, which was change in a seven‐point disability scale. Financial support came from Baxter Bioscience for one trial, research councils for two trials, the National Institutes of Health for one trial, and unstated sources for the others.
Key results and quality of the evidence
According to moderate quality evidence, when we pooled the results of the six trials with the necessary information there was no significant difference in change in disability grade after four weeks. Also according to moderate quality evidence, there was no difference in the percentage of participants who died or were left disabled after one year. We considered the evidence about disability unreliable because of marked variations between the trials. In four small trials of oral corticosteroids, with 120 participants, there was significantly less improvement after four weeks with corticosteroids than without corticosteroids but we considered the evidence quality very low. By contrast, according to moderate quality evidence, in two large trials of intravenous (injected into a vein) corticosteroids with a combined total of 467 participants, there was a slight improvement in disability after four weeks, but the results allowed for the possibility of no effect. Corticosteroids were not associated with a significant increase in harm except that diabetes was significantly more common than with placebo or supportive treatment alone. Although high blood pressure is a known harmful effect of corticosteroids, high blood pressure was unexpectedly much less common in the corticosteroid‐treated participants. The lack of benefit from corticosteroids is not understood but might be because the drugs have a harmful effect on muscles which counteracts the benefit from reducing inflammation in nerves.
The review is assessed as up to date to January 2016.
Summary of findings
Summary of findings for the main comparison. Corticosteroid versus control for Guillain‐Barré syndrome.
| Corticosteroid versus control for Guillain‐Barré syndrome | ||||||
| Patient or population: people with Guillain‐Barré syndrome Settings: hospital Intervention: corticosteroid versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Corticosteroid | |||||
| Disability grade change after 4 weeks GBS disability grade. Scale from: 0 to 6 | The mean disability grade change after 4 weeks in the control groups was ‐0.89 GBS disability grade change1 | The mean disability grade change after 4 weeks in the intervention groups was 0.36 lower (0.88 lower to 0.16 higher) | ‐ | 587 (6 studies) | ⊕⊕⊕⊝ moderate2 | Lower change in disability grade means less improvement so the corticosteroid group did worse, but not significantly |
| Disability grade change after 4 weeks ‐ oral regimens GBS disability grade. Scale from: 0 to 6 | The mean disability grade change after 4 weeks ‐ oral regimens in the control groups was ‐1.33 GBS disability grade1 | The mean disability grade change after 4 weeks ‐ oral regimens in the intervention groups was 0.82 lower (1.47 to 0.17 lower) | ‐ | 120 (4 studies) | ⊕⊝⊝⊝ very low3,4,5 | Lower change in disability grade means less improvement so the corticosteroid group did worse, significantly in this analysis |
| Disability grade change after 4 weeks ‐ intravenous regimens GBS disability grade. Scale from: 0 to 6 | The mean disability grade change after 4 weeks ‐ intravenous regimens in the control groups was ‐0.78 GBS disability grade1 | The mean disability grade change after 4 weeks ‐ intravenous regimens in the intervention groups was 0.17 higher (0.39 higher to 0.06 lower) | ‐ | 467 (2 studies) | ⊕⊕⊕⊝ moderate6 | Higher change in disability grade means more improvement so the corticosteroid group did better, but not significantly |
| Improvement by ≥ 1 grades after 4 weeks GBS disability grade scale | 543 per 10007 | 586 per 1000 (505 to 673) | RR 1.08 (0.93 to 1.24) | 567 (5 studies) | ⊕⊕⊕⊝ moderate8 | Slightly more corticosteroid participants improved but the difference was not significant |
| Death or disability after 1 year | 92 per 10007 | 139 per 1000 (84 to 230) | RR 1.51 (0.91 to 2.5) | 491 (3 studies) | ⊕⊕⊕⊝ moderate9 | More corticosteroid participants had died or were disabled but the difference was not significant |
| Adverse events ‐ diabetes mellitus requiring insulin | 56 per 10007 | 124 per 1000 (67 to 231) | RR 2.21 (1.19 to 4.12) | 467 (2 studies) | ⊕⊕⊕⊕ high | Significantly more corticosteroid participants needed insulin |
| Adverse events ‐ hypertension | 117 per 10007 | 18 per 1000 (6 to 48) | RR 0.15 (0.05 to 0.41) | 467 (2 studies) | ⊕⊕⊕⊕ high | Significantly fewer corticosteroid participants developed hypertension, the opposite of what was expected. See Discussion |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GBS: Guillain‐Barré syndrome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Based on mean scores of control groups. 2 We downgraded once for imprecision. 95% CI consistent with either a clinically significant 0.88 grade less improvement with corticosteroids or slight benefit. High heterogeneity but not downgraded because explained by route of administration. 3 We downgraded because of limitations in study design. 3 of 4 trials had inadequate allocation concealment and 3 of 4 inadequate blinding. 4 We downgraded for heterogeneity; the I2 test for heterogeneity was 51%. 5 We downgraded for imprecision. Wide CIs consistent with no difference or clinically significant worse outcome with corticosteroids. 6 We downgraded for limitations in design and implementation. In 1 of the trials, plasma exchange was used more often in the placebo group, which might have biased against detecting the efficacy of corticosteroids. See Discussion. 7 Based on mean of control participants in all studies. 8 We downgraded once for limitations in design and implementation of the studies. 3 of 6 trials had inadequate allocation concealment, 3 inadequate blinding and in 1, more placebo than corticosteroid participants received plasma exchange which might have biased against detecting an effect from corticosteroids.
9 We downgraded for limitations in design and implementation of the studies and for imprecision.
Background
Guillain‐Barré syndrome (GBS) is an acute paralysing illness usually due to inflammation of the peripheral nerves and nerve roots. It causes tingling and numbness in the limbs and rapidly progressive weakness so that people lose the ability to walk. It may affect the face and swallowing muscles and 25% of people with GBS become so weak that they require artificial ventilation. About 5% of people with GBS die in the acute stages and 10% are left with permanent severe disability. It affects 1 to 2 per 100,000 of the population throughout the world and is more common in the elderly than the young. The cause of GBS is not definitely known. It is probably an autoimmune disease in which the autoimmune response, often triggered by an infection, is directed against antigens in the nerve. This leads to inflammation and nerve damage. Two principal subtypes of GBS are now recognised, acute inflammatory demyelinating polyradiculoneuropathy and acute motor axonal neuropathy. Acute inflammatory demyelinating polyradiculoneuropathy accounts for most of the disease in Europe and North America and acute motor axonal neuropathy is more common in Central America and South East China. In acute inflammatory demyelinating polyradiculoneuropathy the attack focusses on the myelin sheath but the precise target is unknown. In acute motor axonal neuropathy there is good evidence that the target is one or more ganglioside (a form of glycolipid) molecules on the outer membrane of the axon, the central conducting core of the nerve fibre (Willison 2016). The trials conducted so far have not distinguished the different subtypes of GBS. We do not know whether the subtype influences the prognosis from GBS but older age, preceding history of diarrhoea, and greater disease severity all adversely affect prognosis (Walgaard 2011).
Two treatments designed to reduce the presumptive autoimmune response do work in GBS. One Cochrane review concluded from randomised controlled trials (RCTs) that replacing the blood plasma by plasma exchange is beneficial (Raphaël 2012). Another review concluded that intravenous immunoglobulin (IVIg) is just as helpful (Hughes 2014). Theoretically, corticosteroids would be expected to reduce inflammation and so lessen nerve damage in inflammatory neuropathy. Corticosteroids have been shown to hasten recovery in a rat model of GBS, experimental autoimmune neuritis, but only when used in large doses (Hughes 1981; King 1985; Watts 1989). However, corticosteroids introduce risks, including that of increased susceptibility to infection (Bromberg 2004). Consequently the risk‐benefit ratio of corticosteroid administration in GBS requires careful study.
Corticosteroid treatment has been used in GBS in individual case reports from the early 1950s onwards. Some authors reported apparently favourable responses from small series or comparative but not controlled studies. No consensus about the efficacy of steroid treatment emerged from this work (see Hughes 1990 and Ropper 1991 for reviews). A retrospective cohort study compared 50 participants treated with prednisolone 1 mg/kg daily, or equivalent doses of dexamethasone, with 47 participants treated without corticosteroids (Peter 1996). The baseline characteristics of the two groups appeared similar. There was no significant difference between the groups in mortality, intensive care unit stay, or improvement in disability by the time of hospital discharge. Complications were more common in the corticosteroid group. However, a comparison of one series of corticosteroid‐treated participants with historical controls suggested a beneficial effect from corticosteroids when given in combination with IVIg (Dutch GBS Group 1994). In that study, 25 participants were treated with intravenous methylprednisolone 500 mg daily for five days in addition to IVIg 0.4 g/kg daily for five days. They were compared with a historical comparative group of 74 participants treated without steroids but also without IVIg (Dutch GBS Group 1994). After four weeks, 19 of the 25 methylprednisolone‐treated participants (76%) improved by one or more disability grades on a seven‐point scale, compared with only 39 of 74 (53%) participants treated in a previously controlled trial with IVIg but not corticosteroids (P = 0.04).
The first version of this review, published in 1999, included six RCTs and a total of 382 participants. We updated the review in 2007 to include a new trial with 225 participants (van Koningsveld 2004), and a newly discovered trial with 20 participants (Garcia 1985). We updated the review in June 2009, November 2011, and January 2016 but found no more new trials.
Objectives
To examine the ability of corticosteroids to hasten recovery and reduce the long‐term morbidity from Guillain‐Barré syndrome.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) or quasi‐RCTs (e.g. alternate allocation) of corticosteroid or adrenocorticotrophic hormone (ACTH) treatment for Guillain‐Barré syndrome (GBS). The type of additional therapy given, if any, did not affect inclusion in this review.
Types of participants
We included children and adults with GBS of all degrees of severity. We defined GBS according to internationally accepted diagnostic criteria as acute polyradiculoneuropathy causing progressive weakness of two or more limbs, an onset phase not more than four weeks, reduced or absent tendon reflexes, and lacking alternative causes (Asbury 1990). We included studies that did not conform exactly to these criteria provided that the authors regarded GBS or one of its synonyms, such as acute idiopathic neuropathy or acute inflammatory demyelinating polyradiculoneuropathy, as the preferred diagnosis. We noted any departure from the internationally accepted diagnostic criteria.
Types of interventions
We included treatment with any form of corticosteroid or adrenocorticotrophic hormone (ACTH).
Types of outcome measures
Primary outcomes
Improvement in disability grade four weeks after randomisation.
We accepted the disability scale used by the authors of each trial provided that it was closely similar to that described in one of the first trials (Hughes 1978), as follows.
0. Healthy. 1. Minor symptoms or signs of neuropathy but capable of manual work. 2. Able to walk without support of a stick but incapable of manual work. 3. Able to walk with a stick, appliance, or support. 4. Confined to bed or chair bound. 5. Requiring assisted ventilation. 6. Dead.
In the calculation of grade changes, we followed the convention that participants who died were assigned a disability score of six and retained in the analysis with this score at subsequent follow‐up intervals.
Secondary outcomes
Improvement by one or more disability grades on the scale described above, four weeks after randomisation. This measure had not been included in the first version of this review but was used in reviews of plasma exchange and intravenous immunoglobulin (IVIg) and has been added to this review for consistency and comparison.
Time from randomisation until recovery of unaided walking.
Time from randomisation until discontinuation of ventilation (for ventilated participants).
Death.
Death or disability (inability to walk without aid after 12 months).
Improvement in disability grade after six months.
Improvement in disability grade after 12 months.
Relapse (defined as a period of worsening lasting at least seven days that followed a period of improvement lasting at least seven days) during the first year after randomisation.
-
Occurrence of the following adverse events that are attributable to corticosteroids during or within one week after stopping treatment:
development of new infection treated with antibiotics;
gastrointestinal haemorrhage;
development of diabetes mellitus requiring insulin;
development of hypertension requiring drug treatment.
The most informative outcomes, selected for inclusion in the 'Summary of findings' table in this update, were the following.
Improvement by one or more disability grades four weeks after randomisation (primary outcome).
Death or disability (inability to walk without aid after 12 months).
Development of diabetes mellitus requiring insulin.
Development of hypertension requiring drug treatment.
Search methods for identification of studies
On 12 January 2016 we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Register of Studies Online), MEDLINE (January 1966 to December 2016), and Embase (January 1980 to January 2016). We also searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) international Clinical Trials Registry Platform (apps.who.int/trialsearch/) on 27 January 2016.
Electronic searches
The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register Appendix 1; CENTRAL Appendix 2; MEDLINE Appendix 3, Embase Appendix 4), and trials registries Appendix 5.
Searching other resources
We checked the bibliographies in reports of the RCTs and contacted the trial authors and other experts in the field to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
Two review authors independently checked titles and abstracts identified from the register. Two review authors obtained the full text of all potentially relevant studies for independent assessment. The review authors decided independently which trials fitted the inclusion criteria and graded their risk of bias. The review authors resolved disagreements about inclusion criteria by discussion.
Data extraction and management
Two review authors independently performed data extraction. We obtained some missing data from trial authors.
Assessment of risk of bias in included studies
The assessment of risk of bias in the trials included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias (e.g. differences in adherence to treatment or baseline differences not explained under other domains). We assessed blinding and incomplete outcome data separately for death and outcomes other than death.
We graded the 'Risk of bias' items as having high, low, or unclear risk. When agreement between review authors was poor, we reassessed the studies and reached agreement by consensus.
Measures of treatment effect
We calculated a treatment effect across trials using the Cochrane statistical package, Review Manager 5 (RevMan 2014). We expressed results as risk ratios with 95% confidence intervals (CIs) and risk differences with 95% CIs for dichotomous outcomes and mean differences and 95% CIs for continuous outcomes. We combined the effects of two trials using an inverse variance meta‐analysis generating log odds ratios (log OR), which could be converted to an adjusted OR in the results.
Data synthesis
We used a fixed‐effect model and tested for heterogeneity. Where we found genuine heterogeneity, not due to a few extreme studies, we substituted a random‐effects model. We analysed all the primary and secondary outcomes under consideration whenever the data allowed.
Subgroup analysis and investigation of heterogeneity
We planned to examine subgroups that had been defined in advance because of their prognostic importance as identified in previous prospective studies and trials. We defined the subgroups according to the status of the participants at randomisation as follows.
Younger and older (children and adults up to 49 years of age; adults aged 50 years or more).
More severely affected (requiring ventilation) or less severely affected (not requiring ventilation).
Having or not having documented relevant sensory deficit on routine neurological examination (symptoms alone were to be ignored).
Having or not having a history of diarrhoea (gastroenteritis) within the six weeks before the onset of neuropathic symptoms.
Time from onset of neuropathy to start of treatment (seven days or less after onset; more than seven, up to 14 days after onset; and more than 14 days after onset).
Before the outcome of the van Koningsveld 2004 trial was known, we decided to examine separately the effects of intravenous and oral regimens.
Sensitivity analysis
We undertook sensitivity analyses taking into account the risk of bias of the studies, specifically the effect of removing trials without adequate allocation concealment.
Results
Description of studies
Results of the search
The original search of the Cochrane Neuromuscular Specialised Register revealed eight references that might have been randomised controlled trials (RCTs) (Figure 1). We excluded three studies: Levchenko 1989 was not a randomised study, Mendell 1985 provided both plasma exchange and corticosteroids to the experimental group but neither to the control group, and Zagar 1995 was a review. Advertising for more trials among colleagues identified a further possible trial (Haass 1988), but this was an observational study with no control group (see Characteristics of excluded studies table). Our search of Embase revealed six references that might have represented RCTs, including three that had not been detected by the other searches. We included Bansal 1986, which was a quasi‐RCT, and excluded two other studies, which were observational (El Zunni 1997; Naylor 1986). We identified another RCT by personal contact with the author (Foyaca 2003). We excluded this trial because it did not meet our requirement for adequate diagnostic criteria as participants were included who had pure sensory deficit, facial diplegia with paraesthesiae, or hyper‐reflexia. In addition, the trial did not meet our criteria for adequate definition of a single primary outcome, and allocation concealment was unclear. When we repeated the searches in January 2016, the total number of papers retrieved from the Cochrane Neuromuscular Specialised Register was 60 (36 new), CENTRAL 76 (45 new), MEDLINE 606 (90 new), and Embase 349 (77 new). After deduplication, there were 212 new records but none were RCTs suitable for inclusion. There were no other or ongoing trials in the clinical trials registries.
1.
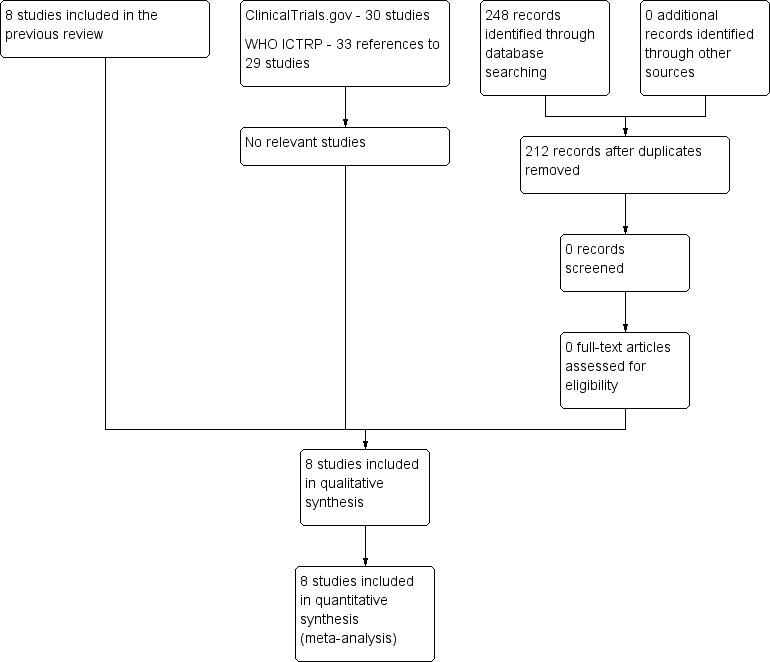
Study flow diagram.
Included studies
Eight trials, including 653 participants, fulfilled the selection criteria (see Characteristics of included studies). All the trials used internationally accepted diagnostic criteria (Asbury 1990) or closely similar explicit criteria.
Only six trials with 587 participants provided data for our primary outcome measure. The first trial compared intramuscular ACTH daily for 10 days with placebo (Swick 1976). Four trials with between 14 and 46 participants compared oral prednisolone with placebo (Shukla 1988; Singh 1996), or supportive treatment without steroids and no placebo (Bansal 1986; Hughes 1978). The oral regimens varied but all consisted of the equivalent of prednisolone 40 mg daily for at least two weeks. A trial with alternate allocation included 10 participants treated with methylprednisolone 1500 mg daily for five days and 10 participants who received supportive care (Garcia 1985). A trial with 242 participants compared intravenous methylprednisolone 500 mg daily for five days with an identical placebo saline solution (GBS Steroid 1993). This trial did not show a significant difference in any outcome between the corticosteroid‐ and placebo‐treated groups. One trial with 225 participants differed from the others in that all participants received IVIg 0.4 g/kg daily for five days in accordance with current practice and were randomly allocated to receive intravenous methylprednisolone 500 mg daily for five days, or an identical saline placebo (van Koningsveld 2004). In this trial, the authors reported a one disability grade improvement after four weeks in 63 of 113 (56%) of control‐ and 76 of 112 (68%) methylprednisolone‐treated participants (risk ratio (RR) 1.2, 95% confidence interval (CI) 1.0 to 1.5, P = 0.06), a difference which was not quite significant. After adjustment for age and severity of disability at randomisation, the treatment effect just achieved significance (RR 1.3, 95% CI 1.0 to 1.5, P = 0.03). When the authors also adjusted for other prognostic factors not defined in the protocol (number of days between onset of weakness and randomisation, preceding infection with cytomegalovirus, and the amplitude of compound muscle action potential (CMAP)) that were unbalanced between the treatment groups, the odds ratio (OR) favoured the primary outcome (OR 2.96, 95% CI 1.26 to 6.94, P = 0.01). Other outcomes, including the proportion of participants requiring ventilation, becoming able to walk unaided, and improving one or more disability grades during the first year, did not differ significantly between the groups.
Risk of bias in included studies
Figure 2 gives the risk of bias for each trial. Allocation concealment was adequate in four trials in which participants were randomly assigned to receive corticosteroids or ACTH or an identical‐appearing placebo (GBS Steroid 1993; Hughes 1978; Swick 1976; van Koningsveld 2004). One trial assigned participants to oral prednisolone or no corticosteroid treatment according to a central register of random numbers that was only revealed at the time of randomisation (Hughes 1978). In this trial, the treatment allocation concealment was also considered adequate. In Shukla 1988, participants were randomly allocated to corticosteroids or placebo but it was not clear whether allocation was concealed. In three trials, participants were alternately assigned to corticosteroid or supportive care and we considered the randomisation concealment inadequate (Bansal 1986; Garcia 1985; Singh 1996).
2.
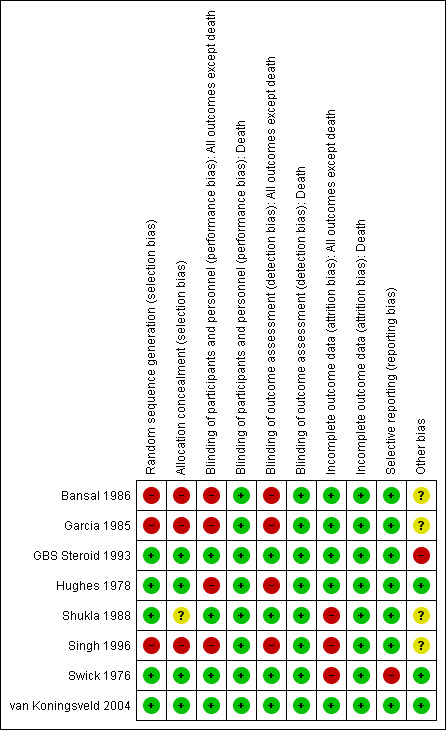
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Participant blinding was intended in five trials but not in three (Bansal 1986; Garcia 1985; Hughes 1978). None of the trials recorded effectiveness of blinding. One trial did not blind participants but did blind the observers (Hughes 1978). All trials considered baseline clinical features. In three trials the baseline clinical features were similar between intervention groups. In three trials, baseline differences were present: in the trial of Swick 1976, the time to nadir was shorter in the corticosteroid group, in Bansal 1986, the corticosteroid‐treated participants were 10 years older, and in Singh 1996, corticosteroid‐treated participants were older and more disabled than the control groups. Doubt also arose concerning this question in GBS Steroid 1993, in which there was an imbalance not at baseline but in the subsequent treatment of the two groups, since more of the participants in the placebo group received plasma exchange than participants in the corticosteroid group (see Discussion). In one trial, there was a slight imbalance at randomisation in the number of days between onset and randomisation (four days or less versus more than four days), amplitude of the CMAP, and presence or absence of preceding cytomegalovirus infection, all factors reported to affect prognosis (van Koningsveld 2004). Follow‐up continued for a year in three trials (GBS Steroid 1993; Hughes 1978; van Koningsveld 2004). The commonest problem was that the small trials did not state the primary outcome in the methods section of the paper. The two large trials did state the primary outcome (GBS Steroid 1993; van Koningsveld 2004). There was no evidence otherwise of selective reporting in any of the trials.
Effects of interventions
See: Table 1
Primary outcome measure
Change in disability grade four weeks after randomisation
Information for this outcome was available for six trials with 587 participants, of whom 297 received corticosteroids and 290 did not. In the analysis of all these trials there was no significant difference between the groups: the mean difference (MD) was 0.36 of a grade less improvement in the corticosteroid‐treated participants. The 95% CI ranged from 0.88 of a grade less improvement to 0.16 of a grade more improvement (see Figure 3 and Table 1). There was significant heterogeneity in this analysis so we used a random‐effects model for this computation. Inspection of the forest plot suggested more benefit from the intravenous regimens so that we undertook separate analyses of the trials that used oral and intravenous regimens. In the four small trials that used oral corticosteroids, there were 120 participants and there was significantly less improvement in corticosteroid‐treated participants than in controls (MD 0.82, 95% CI 0.17 to 1.47) (Bansal 1986; Hughes 1978; Shukla 1988; Singh 1996). In the two large trials with 467 participants that used intravenous methylprednisolone, the MD was 0.17 of a grade more improvement in participants treated with intravenous methylprednisolone (GBS Steroid 1993; van Koningsveld 2004). The 95% CIs ranged from 0.06 of a grade less improvement to 0.39 of a grade more improvement.
3.
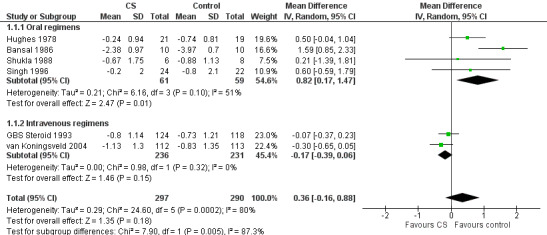
Forest plot of comparison: 1 Corticosteroid (CS) versus control, outcome: 1.1 Disability grade change after 4 weeks.
Secondary outcome measures
1. Improvement by one or more disability grades after four weeks
Information for this outcome was available for five trials with 567 participants. The information for this outcome was not available for Bansal 1986. The RR of improving by one or more grades from baseline was 1.08 (95% CI 0.93 to 1.24) more in the corticosteroid‐treated than the control participants (see Analysis 1.2). In other words, the absolute rate of participants improving one grade was 8% more with corticosteroids, with 95% CIs ranging from 7% less to 24% more.
1.2. Analysis.
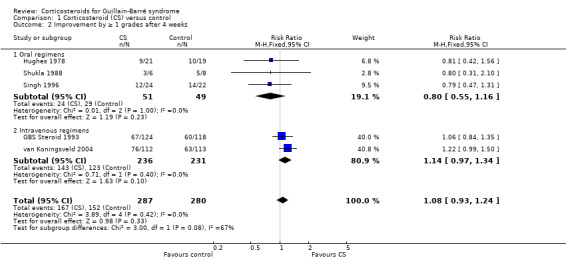
Comparison 1 Corticosteroid (CS) versus control, Outcome 2 Improvement by ≥ 1 grades after 4 weeks.
There was no significant heterogeneity in this analysis. In the three small oral corticosteroid trials there were 100 participants and the relative rate of improvement was less in the corticosteroid‐treated participants but the difference was not significant (MD 0.80, 95% CI 0.55 to 1.16). When we confined this analysis to the two large trials that used intravenous methylprednisolone, the RR of improvement did not achieve significance, being 1.14 (95% CI 0.97 to 1.34) greater in the intravenous methylprednisolone‐treated participants than the control group (see Figure 4 and Table 1). In other words, the absolute rate of participants improving one grade was 14% more with corticosteroids, with 95% CIs ranging from 3% less to 34% more.
4.
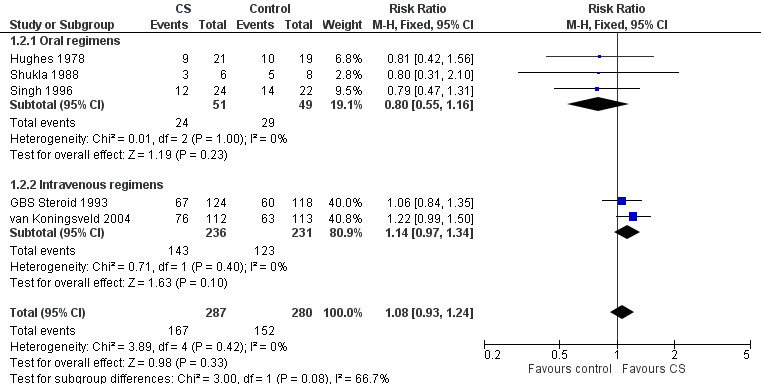
Forest plot of comparison: 1 Corticosteroid (CS) versus control, outcome: 1.2 Improvement by ≥ 1 grades after 4 weeks.
We checked that there were no interactions between treatment, age, or initial disability for this outcome in the two large trials that provided data. We tested the effect of age (less than 50 years and 50 years or more) and initial disability with an inverse variance meta‐analysis combining the results of the GBS Steroid 1993 and van Koningsveld 2004 trials. The result gave an adjusted log OR of 0.34 (95% CI ‐0.05 to 0.73) (see Analysis 1.3). This is equivalent to an adjusted OR of 1.41 (95% CI 0.95 to 2.07, P = 0.08), which was in favour of corticosteroids but not significant. Assuming that in the controls the probability of recovering one or more grades is the average of those observed in the two studies, that is 53.2%, then these adjusted log OR results are equivalent to an RR of improvement of 1.16 (95% CI 0.98 to 1.32, P = 0.08) more with corticosteroids than with placebo, again not significant at the 5% level. This is very little different to the unadjusted pooled RR of 1.14 (95% CI 0.97 to 1.34) (see Figure 4).
1.3. Analysis.
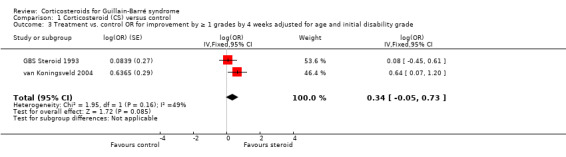
Comparison 1 Corticosteroid (CS) versus control, Outcome 3 Treatment vs. control OR for improvement by ≥ 1 grades by 4 weeks adjusted for age and initial disability grade.
2. Time from randomisation until recovery of unaided walking
We derived the outcome of time from randomisation until recovery of unaided walking for this review from the original data of Hughes 1978, in which the median time to walk unaided was 29 days (95% CI 16.4 to 73.8) in the group treated with oral prednisolone and 34 days (95% CI 18.8 to 181.4) in the control group. The difference between the groups, 5 days (95% CI ‐95 to 105) less in the corticosteroid group, was not significant (P = 0.37). The largest study also published data on this outcome (GBS Steroid 1993), in which the median time to walk unaided was 38 days in the group treated with steroids compared to 50 days in the placebo group. The difference between the groups, 12 days (95% CI ‐21.3 to 45.3) less in the corticosteroid group, was not significant. In the most recent trial (van Koningsveld 2004), the median time to walk unaided in the corticosteroid‐treated participants was 28 days (interquartile ratio (IQR) of the individual values 14 to 154 days), approximately equivalent to 95% CI of the median of 4 to 52 days) and 56 days (IQR 14 to 154 days, approximately equivalent to 95% CI of 32 to 80 days) in the placebo‐treated participants. The difference between the groups was 28 days less in the corticosteroid‐treated participants (95% CI ‐6.0 to 62.0), but this difference was not significant.
Only one other trial reported similar outcome measures. The study of ACTH included median time to complete recovery as an outcome measure, which was 4 months (95% CI 2 to 6) in the participants treated with ACTH and 10 months (95% CI 4.5 to 12) in the participants treated with placebo (Swick 1976). It is difficult to compute the significance of this observation since there were only nine participants in the corticosteroid group and seven in the placebo group; one participant in the treatment group died and was omitted from the data.
3. Time from randomisation until discontinuation of ventilation (for ventilated participants)
In GBS Steroid 1993, the median time from randomisation to discontinuation of ventilation was 18 days in the group treated with intravenous methylprednisolone and 27 days in the placebo group (difference 12 days shorter with corticosteroids, 95% CI 21.3 longer to 45.3 days shorter). In contradiction to this, in van Koningsveld 2004, the median time from randomisation to discontinuation of ventilation was longer at 30 days (95% CI 16.6 to 43.4) in the 24 participants treated with intravenous methylprednisolone and IVIg, compared with 26 days (95% CI 15.3 to 36.7) in the 26 control participants treated with placebo and IVIg.
4. Death
Mortality data were available from seven trials with 605 participants in total. Twenty of 308 (6.5%) participants treated with corticosteroids died during the trial follow‐up compared with 14 of 297 (4.7%) participants treated without corticosteroids, giving an insignificant difference with an RR of 1.28 (95% CI 0.67 to 2.47) more deaths in the corticosteroid‐treated participants (see Analysis 1.4). This analysis included all recorded deaths regardless of the length of follow‐up and even if the trial authors had excluded the case because they considered that the death was due to an unrelated cause. We included a participant who was randomised to ACTH in Swick 1976, left hospital against advice, and died at home. We also included a participant randomised to no steroid treatment in another trial, who died of suicide during convalescence (Hughes 1978).
1.4. Analysis.
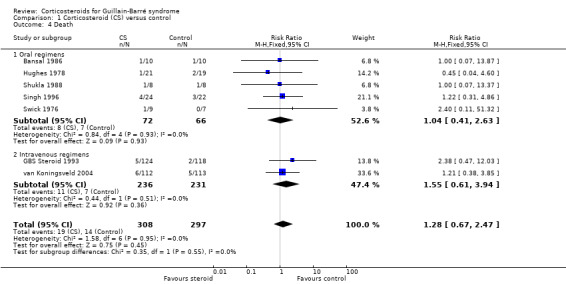
Comparison 1 Corticosteroid (CS) versus control, Outcome 4 Death.
5. Death or disability (inability to walk without aid) after 12 months
In the three trials in which this outcome was available (GBS Steroid 1993; Hughes 1978; van Koningsveld 2004), 35 of 252 (13.9%) participants treated with oral prednisolone or intravenous methylprednisolone were dead or disabled (needing aid to walk) after one year, compared with 22 of 239 (9.2%) control participants. The RR of this combined outcome was not significantly different, being 1.51 (95% CI 0.91 to 2.50) more in the corticosteroid than the placebo‐treated participants (see Table 1 and Analysis 1.5).
1.5. Analysis.
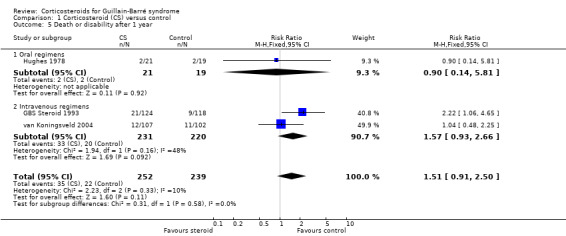
Comparison 1 Corticosteroid (CS) versus control, Outcome 5 Death or disability after 1 year.
6. Improvement in disability grade after six months
We obtained data for this outcome from the two largest trials, which had 455 participants (GBS Steroid 1993; van Koningsveld 2004). No significant difference was present between the corticosteroid‐treated and the placebo‐treated participants, MD 0.10 (95% CI ‐0.16 to 0.36) of a grade more improvement in the corticosteroid‐treated participants (see Analysis 1.6). The mean disability grades of the corticosteroid and control groups were not significantly different after six months in one other trial (Singh 1996), but the trial authors did not provide the standard deviations, so the meta‐analysis did not include this study.
1.6. Analysis.

Comparison 1 Corticosteroid (CS) versus control, Outcome 6 Disability grade change after 6 months.
7. Improvement in disability grade after 12 months
This outcome was available for three trials with 471 participants in total (GBS Steroid 1993; Hughes 1978; van Koningsveld 2004), showing almost no difference, MD of ‐0.03 (95% CI ‐0.29 to 0.23) less improvement in the corticosteroid group (see Analysis 1.7) (GBS Steroid 1993; Hughes 1978; van Koningsveld 2004).
1.7. Analysis.
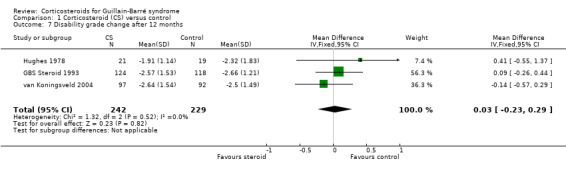
Comparison 1 Corticosteroid (CS) versus control, Outcome 7 Disability grade change after 12 months.
8. Relapse (defined as a period of worsening lasting at least seven days that followed a period of improvement lasting at least seven days) during the first year after randomisation
Three trials reported relapses during the first year after treatment (GBS Steroid 1993; Hughes 1978; van Koningsveld 2004). Eighteen of 251 participants treated with corticosteroids relapsed compared with 15 of 239 control participants, an insignificant difference, with an RR of 1.14 (95% CI 0.59 to 2.18) (see Analysis 1.8).
1.8. Analysis.
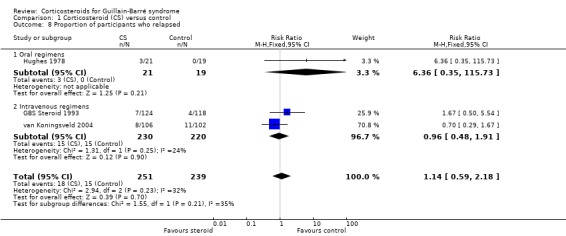
Comparison 1 Corticosteroid (CS) versus control, Outcome 8 Proportion of participants who relapsed.
9. Occurrence of specific adverse events that are attributable to corticosteroids during or within one week after stopping treatment
Shukla 1988 reported one participant out of eight treated with prednisolone who developed a perforated peptic ulcer and another who developed psychosis, but did not mention any such complications in eight placebo‐treated participants. Five trials did not report side effects or adverse events (Hughes 1978; Singh 1996; Swick 1976; van Koningsveld 2004). Two trials that used intravenous methylprednisolone provided information concerning the adverse events that had been preselected for this review and have been illustrated in the analyses. This is summarised as follows.
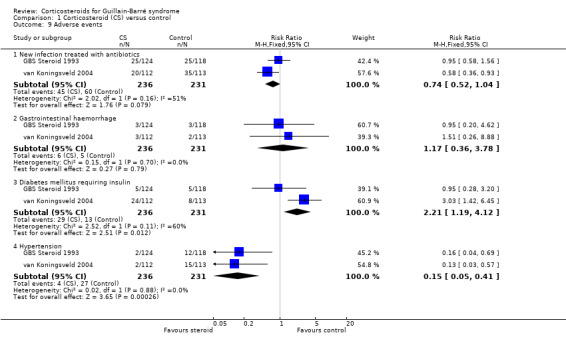
a. Development of new infection treated with antibiotics
In the first large trial, 25 of 124 (20.2%) participants treated with intravenous methylprednisolone had infection or septicaemia during the first four weeks of treatment; the proportion in the placebo group was similar, 25 of 118 (21.2%) (GBS Steroid 1993). In the second large trial, fewer participants treated with intravenous methylprednisolone (20/112, 18%) had urinary tract infections than in the placebo group (35/113, 31%, P = 0.01), the opposite of the result that might have been expected (van Koningsveld 2004). It is doubtful whether these two data sets should be analysed together but when this was done the RR of infection was not significantly different (RR 0.74, 95% CI 0.52 to 1.04) (see Analysis 1.9).
1.9. Analysis.
Comparison 1 Corticosteroid (CS) versus control, Outcome 9 Adverse events.
b. Gastrointestinal haemorrhage
The first large trial reported haemorrhage in 3 of 124 (2.4%) corticosteroid‐treated and 3 of 118 (2.5%) placebo‐treated participants but did not report the site of haemorrhage (GBS Steroid 1993). In the second large trial, gastrointestinal haemorrhage occurred in 3 of 112 (2.6%) corticosteroid‐treated and 2 of 113 (1.8%) placebo‐treated participants (van Koningsveld 2004). Again it is doubtful whether these two data sets should be analysed together but when this was done the RR of infection was not significant (RR 1.17, 95% CI 0.36 to 3.78) (see Analysis 1.9).
c. Development of diabetes mellitus requiring insulin
Our protocol included comparison of the proportions of participants who developed diabetes mellitus requiring the use of insulin. Neither trial stipulated this precise outcome, but measured related outcomes. In the first large trial, investigators reported diabetes mellitus in 5 of 124 (4.0%) corticosteroid‐treated and 5 of 118 (4.2%) placebo‐treated participants (GBS Steroid 1993). They did not state their definition of diabetes. In van Koningsveld 2004, a plasma glucose greater than 10 mmol/L occurred in 24 of 112 (21%) corticosteroid‐treated and 8 of 113 (7%) placebo‐treated participants (P = 0.01). When these results were combined, the RR of diabetes was significantly greater with corticosteroid treatment (RR 2.21, 1.19 to 4.12, P = 0.01) (see Table 1 and Analysis 1.9).
d. Development of hypertension requiring drug treatment
In both the large trials, hypertension was significantly less frequent in the steroid‐treated than the control group. When we combined the results, there were highly significantly fewer participants who developed hypertension in the intravenous methylprednisolone group, 4 of 236 (1.7%) than in the placebo group, 27 of 231 (7.4%) (RR 0.15, 95% CI 0.05 to 0.41, P = 0.0003) (see Table 1 and Analysis 1.9).
Subgroup analysis
In the absence of a significant main effect of corticosteroids on the primary outcome measure, it is questionable whether meta‐analysis of the effect on subgroups is appropriate. In one small trial of oral prednisolone, the improvement of six participants randomised to corticosteroids within the first week after onset was non‐significantly worse four weeks after randomisation and significantly worse three months after randomisation compared with 10 control participants randomised within the same period (Hughes 1978). Other information for the analyses of the subgroups we selected was not available in the published reports. However, published analyses in two of the large trials addressed this problem. In the published report of GBS Steroid 1993, a regression analysis found no interaction between age, severity at onset, or delay from onset until randomisation (within the maximum permitted period, which was 14 days) and the effect of treatment. Similarly, in van Koningsveld 2004, no treatment interactions with age (less than 50 years or 50 years and more), disability score, duration of weakness (four days or less), CMAP amplitude (4 mV or less or greater than 4 mV), or preceding cytomegalovirus infection. Consequently, neither trial identified subgroups in which intravenous methylprednisolone was more likely to be beneficial.
Sensitivity analysis
Because of the heterogeneity in the results from the primary outcome measure, mean improvement in disability grade after four weeks, we repeated the analysis following omission of the trials which did not have adequate allocation concealment (Bansal 1986; Garcia 1985; Shukla 1988; Singh 1996). This reduced but did not remove the heterogeneity and made little difference to the result, which showed almost no difference between the treatment groups, MD 0.01 (95% CI ‐0.37 to 0.39) more improvement in the corticosteroid‐treated participants. We have described results of the separate analyses of the intravenous and oral regimens above. There was no evidence of heterogeneity in the other analyses.
Discussion
We included eight studies examining the effects of corticosteroids in 653 participants. This is a relatively small number in relation to the known variability of severity and outcome of Guillain‐Barré syndrome (GBS). Our primary outcome measure, mean disability grade improvement after four weeks, was available for six trials with 587 participants and showed no significant difference. There was significant heterogeneity in the results, which could be explained by considering separately the results of the trials that tested oral and intravenous corticosteroids. In the four trials of oral corticosteroids, with 120 participants, there was less improvement in the corticosteroid groups than in the control groups, which was highly significant. In the meta‐analysis of the two trials of intravenous methylprednisolone, there was a non‐significant trend towards benefit from corticosteroids. No significant differences emerged from our analyses of the secondary outcome measures selected for this review. These were the number improving after four weeks, the time to walk unaided, duration of ventilation (for ventilated participants), number of deaths, and number left dead or disabled (unable to walk unaided) after one year).
There were differences between the two trials of intravenous methylprednisolone that provided the bulk of the evidence. Both tested intravenous methylprednisolone 500 mg daily for five days against placebo. One reported no difference in any outcome between the corticosteroid‐treated and placebo‐treated groups (GBS Steroid 1993). The other differed, in that both treatment groups also received intravenous immunoglobulin (IVIg), which has now become a standard treatment for GBS (van Koningsveld 2004). Their raw results were not significant for any of the outcome measures, but further analyses taking into account age and disability at randomisation showed a borderline significant result for their primary outcome measure (proportion of participants improving by one or more disability grade after four weeks). The review authors judged the methodological quality of both trials adequate, but there was one caveat about GBS Steroid 1993. Because of the variation in practice and ethical issues at the time of the trial, there was an imbalance in the numbers of participants who received plasma exchange in the two groups. Only 66 of 124 (53%) participants treated with intravenous methylprednisolone received plasma exchange compared with 77 of 118 (65%) placebo‐treated participants (P = 0.08). Furthermore, the physicians caring for the participants in that trial had been asked at the time of randomisation to declare whether each participant would definitely, definitely not, or possibly receive plasma exchange. For those participants for whom plasma exchange had been declared possible, only 8 of 45 (17.8%) randomised to intravenous methylprednisolone eventually received plasma exchange compared with 15 of 32 (46.9%) placebo‐treated participants (P = 0.0125). Since plasma exchange has been shown to be more effective than supportive treatment (GBS Group 1985; Raphaël 2012), this imbalance could have biased the results against detection of a beneficial effect from corticosteroids. However, an analysis after excluding the participants in whom the use of plasma exchange had been declared possible showed no beneficial effect from corticosteroids according to trial authors.
Since analyses of van Koningsveld 2004 taking into account some prognostic factors showed significant differences in favour of corticosteroids for the primary outcome measure, the number of participants improving one disability grade after four weeks, we undertook a similar analysis of GBS Steroid 1993 taking into account age and disability, the factors for which we had information. We then used the inverse variance method to combine the results of these two trials taking into account these two factors; the log OR was in favour of corticosteroid group but the result was not significant.
The difference between the effects of oral and intravenous corticosteroids was unexpected. Multiple factors may play a role in this finding. The first question is whether the effect is real. The trials of oral corticosteroids that contributed the available evidence were of lower quality: in only two of the four trials was allocation concealment adequate. Second, the numbers in each trial were small; the total number of participants was only 120. Third, the unfavourable effect of oral corticosteroids was only detected for our primary outcome and not for improvement by one grade or for death, the only other outcomes for which data were available and where there was no difference. Fourth, corticosteroids are readily absorbed and the route of administration itself is unlikely to be responsible. Fifth, the oral corticosteroid courses lasted more than two weeks in all four trials since the treatment could be continued longer at the discretion of the treating physician. The intravenous courses only lasted five days. It is possible that early corticosteroid treatment helps by reducing inflammation and later treatment harms by inhibiting macrophage clearance of myelin debris or other repair mechanisms. Sixth, the intravenous trials tested higher initial doses, approximately eight‐fold higher for the five days that they were given, and rapid intravenous infusion might have a different effect on the immune system compared with oral absorption from tablets.
The question now arises as to the data that provide the best evidence on which to base practice. Our analysis of the oral corticosteroid trials indicates an important negative effect that physicians caring for people with GBS will want to take into account. Our combined analysis of the intravenous methylprednisolone trials showed no significant difference in any outcome but one of the included trials did not use IVIg, which is now part of standard care. The only trial that has tested the effect of adding corticosteroids to IVIg is van Koningsveld 2004; physicians may place more reliance on the data from this trial, which reflects current practice more accurately than the earlier studies. This argument assumes that corticosteroids given with IVIg are more efficacious than either treatment given on its own. However, there is a lack of other evidence to support this hypothesis. Whatever view is taken, the analyses presented in this review indicate that the true effect of intravenous corticosteroids is at best small and was absent for long‐term outcomes.
Serious adverse events were infrequent with the short courses of corticosteroids used in these trials. This was not surprising since observational studies and clinical experience suggest that short courses of corticosteroids rarely cause serious adverse effects. The only differences in adverse events between groups in the two intravenous methylprednisolone trials were in increased blood glucose concentrations, which were, as expected, significantly more common; and in hypertension, which was, surprisingly, significantly less common in the intravenous methylprednisolone group than in the placebo group. This latter result is difficult to explain. An association between glomerulonephritis and GBS has been reported (Bettinelli 1989; Olbricht 1993). It is possible that intravenous methylprednisolone had a favourable effect on renal function so as to prevent hypertension. Future trials of immunomodulatory treatment in GBS should monitor blood pressure and renal function. Few studies formally recorded the absence of the adverse events selected for consideration in this review. Particular adverse events that were recorded have been included but this list may be incomplete. Consequently, the absence of evidence cannot be construed as the absence of occurrence of these events. We encourage organisers of future trials to collect systematically information about adverse events and disease complications that are known to occur with GBS, such as infections requiring antibiotics, cardiac arrhythmia requiring insertion of a pacemaker or use of anti‐arrhythmic drugs, postural hypotension, systemic hypertension requiring treatment, deep vein thrombosis, and pulmonary embolism.
Our searches for this review were comprehensive and it is unlikely that there are any significant unpublished trials. There are also no ongoing trials.
This review prompts recommendations about the design of future GBS trials. There is no information about whether the response to corticosteroids, or any other treatment, differs between the principal subtypes of GBS, acute inflammatory demyelinating polyradiculoneuropathy and acute motor axonal neuropathy. Possible differences should be investigated. In the trials reviewed, the principal outcome measures have involved crude clinical end points, or a simple disability scale that may seem rather coarsely graded to consumers and may be insufficiently responsive to detect meaningful clinical effects. Furthermore, the principally used GBS disability score is an ordinal scale in which the distances between different score points are unlikely to be comparably important. Future trials should incorporate more responsive, validated linear disability scales, such as the Rasch ordered disability scale, which has been validated for use in GBS (van Nes 2011). The present GBS disability scale should still be retained for comparison but might be relegated to a secondary outcome. Linear measures of strength, in particular grip strength, should also be considered. Electrophysiological outcome measures have not been used and might provide objective surrogate end points, having continuous scales that would allow the use of parametric statistics. However, such measures may not reflect disability or handicap and are affected by difficulties in standardisation between laboratories, which would make it difficult to draw useful conclusions. Future trials should consider measuring fatigue, which is a persistent problem in many people with GBS. No trial has included health‐related quality of life measures or incorporated cost‐effectiveness calculations. Since corticosteroids are inexpensive, cost would not be a significant bar to their use in GBS.
The absence of an easily demonstrable beneficial effect of corticosteroids in GBS is difficult to explain. GBS resembles the animal model of experimental autoimmune neuritis in which early treatment with high‐dose corticosteroids does suppress the clinical deficit and hasten recovery (Hughes 1981; King 1985; Watts 1989). In addition, the related condition, chronic inflammatory demyelinating polyradiculoneuropathy, showed a significant response to oral prednisone in the only RCT ever performed (Dyck 1982; Mehndiratta 2015). It is possible that the anticipated beneficial effects of suppressing the inflammatory response that underlies the pathology of most cases of GBS in North America and Europe is counteracted by some other unexpected and unwanted effect of corticosteroids on the repair process. Following nerve transection in the rat, administration of corticosteroids causes prolonged loss of muscle electrical excitability due to loss of sodium channels or activation of calcium release channels (Rich 1998; Riggs 1998). The same phenomenon, if it occurs in humans, would explain why the use of corticosteroids in severe cases of GBS, in which denervation is anticipated, does not have the beneficial effect predicted from its known anti‐inflammatory properties. If this hypothesis is correct, then corticosteroids might be beneficial in a subgroup of people with conduction block but not denervation. In practice, it would be difficult to identify people in whom denervation is not already occurring or is about to occur. It would be helpful to develop indicators that would reliably identify people with GBS destined to have a poor prognosis.
Authors' conclusions
Implications for practice.
According to moderate quality evidence, corticosteroids do not significantly hasten recovery from GBS or affect the long‐term outcome. There is low quality evidence to suggest that oral corticosteroids delay recovery and moderate quality evidence that intravenous corticosteroids given in combination with intravenous immunoglobulin might hasten recovery. There was no evidence of harm from corticosteroids except that increased blood glucose concentrations requiring insulin were significantly more common. Unexpectedly, hypertension was significantly less common.
Implications for research.
More research into more effective treatments for Guillain‐Barré syndrome should be undertaken. More responsive outcome measures should be designed and validated. Future trials should report serious complications of GBS as well as possible side effects of drugs. They should also report separately results in different subtypes of GBS and subgroups of patients having clinical features at randomisation that are known to affect prognosis. This would require large groups of participants. An explanation should be sought for the absence of more benefit from corticosteroids in GBS.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2016 | New citation required but conclusions have not changed | No new RCTs identified. Ruth Brassington and Angela Gunn became authors. |
| 18 January 2016 | New search has been performed | New EMBASE filter used, CENTRAL and Cochrane Neuromuscular Specialised Register searches run via the CRSO & CRS |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 4 April 2012 | New citation required but conclusions have not changed | New search incorporated. Dr AV Swan retired from authorship. |
| 17 February 2012 | New search has been performed | No trials found, minor changes to text. |
| 20 January 2010 | New citation required but conclusions have not changed | Dr R van Koningsveld retired from authorship. |
| 10 June 2009 | New search has been performed | New search to June 2009: no new trials found. New MEDLINE filter used. |
| 22 July 2008 | Amended | Converted to new review format. |
| 28 February 2007 | New search has been performed | The searches were updated in February 2007, but no new studies were identified. |
| 31 January 2006 | New citation required and conclusions have changed | A large trial has been added almost doubling the amount of evidence. This trial showed a trend towards more improvement on short‐term benefit which became significant after taking into account prognostic factors that were imbalanced between the groups at randomisation. This did not alter the conclusion of the meta‐analysis from all trials that corticosteroids do not produce significant benefit. |
| 21 October 1999 | Amended | An error in entering the standard deviations for change in disability grade after one year for the trial of Hughes 1978, has been corrected in the table of comparisons. This has resulted in the difference between the groups becoming not significant for that outcome, in agreement with the other outcome measures. |
Acknowledgements
We thank Professor F van der Meché, co‐author of the first version of this review, Dr R van Koningsveld co‐author of the second version, and Dr A Swan co‐author of the first three updates. RACH and PvD have consultancies with firms that manufacture human immunoglobulin (see Declarations of interest), a drug used in the treatment of GBS. This review did not consider the comparison of corticosteroids and human immune globulin.
This project was supported by the United Kingdom National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases in the Institute of Neurology, University College London.
Appendices
Appendix 1. Cochrane Neuromuscular Register (CRS) search strategy
#1 "guillain barre syndrome" [REFERENCE] [STANDARD] #2 polyradiculoneuropath* or polyneuropath* or "acute polyradiculoneuritis" or "acute polyneuritis" [REFERENCE] [STANDARD] #3 inflammatory NEAR5 neuropath* or inflammatory NEAR5 polyneuropath* [REFERENCE] [STANDARD] #4 #1 or #2 or #3 [REFERENCE] [STANDARD] #5 MeSH DESCRIPTOR steroids Explode All [REFERENCE] [STANDARD] #6 glucocorticoid* or steroid* [REFERENCE] [STANDARD] #7 MeSH DESCRIPTOR Anti‐Inflammatory Agents Explode All [REFERENCE] [STANDARD] #8 anti‐inflammatory agent* or antiinflammatory agent* [REFERENCE] [STANDARD] #9 prednisone* or prednisolone* or cortisone* [REFERENCE] [STANDARD] #10 #5 or #6 or #7 or #8 or #9 [REFERENCE] [STANDARD] #11 #4 and #10 [REFERENCE] [STANDARD] #12 (#4 and #10) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (CRSO) search strategy
#1("guillain barre syndrome"):TI,AB,KY #2(polyradiculoneuropath* or polyneuropathy* or "acute polyneuritis" or "acute polyradiculoneuritis"):TI,AB,KY #3(inflammatory NEAR5 neuropath* or inflammatory NEAR5 polyneuropathy*):TI,AB,KY #4#1 OR #2 OR #3 #5MESH DESCRIPTOR Steroids EXPLODE ALL TREES #6MESH DESCRIPTOR Anti‐Inflammatory Agents EXPLODE ALL TREES #7(steroid* or glucocortico*):TI,AB,KY #8("anti‐inflammatory agent*" or "anti‐inflammatory agent*"):TI,AB,KY #9(prednisone* or prednisolone* or cortisone*):TI,AB,KY #10#5 OR #6 OR #7 OR #8 OR #9 #11#4 AND #10
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to December Week 5 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (402815) 2 controlled clinical trial.pt. (89885) 3 randomized.ab. (300110) 4 placebo.ab. (153924) 5 drug therapy.fs. (1807402) 6 randomly.ab. (212708) 7 trial.ab. (308971) 8 groups.ab. (1348986) 9 or/1‐8 (3423544) 10 exp animals/ not humans.sh. (4169575) 11 9 not 10 (2914775) 12 guillain barre syndrome.tw. or Guillain‐Barre Syndrome/ (7017) 13 POLYRADICULONEUROPATHY/ or POLYNEUROPATHIES/ (8124) 14 (acute polyradiculoneuritis or acute polyneuritis).mp. (176) 15 (inflammatory adj5 neuropath$3).tw. (1916) 16 (inflammatory adj5 polyneuropath$3).tw. (1515) 17 or/12‐16 (15109) 18 steroid$.tw. or exp steroids/ (851802) 19 exp Anti‐inflammatory Agents/ or anti‐inflammatory agent$.tw. (438813) 20 exp Glucocorticoids/ or glucocorticoid$.tw. (193542) 21 (prednisone$ or prednisolone$ or cortisone$).mp. (102536) 22 or/18‐21 (1077345) 23 11 and 17 and 22 (612) 24 remove duplicates from 23 (606)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 02> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (45414) 2 double‐blind procedure.sh. (124985) 3 single‐blind procedure.sh. (21252) 4 randomized controlled trial.sh. (388894) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1207245) 6 trial.ti. (190167) 7 controlled clinical trial/ (391213) 8 or/1‐7 (1458199) 9 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (21940450) 10 human/ or human cell/ or human tissue/ or normal human/ (16517365) 11 9 not 10 (5455817) 12 8 not 11 (1294364) 13 limit 12 to embase (1068750) 14 guillain barre syndrome.tw. or Guillain Barre Syndrome/ (12954) 15 acute polyradiculoneuritis.mp. or acute polyneuritis.tw. (184) 16 Polyneuropathies/ or Polyradiculoneuropathy/ (9896) 17 (inflammatory adj5 neuropath$3).tw. (2977) 18 (inflammatory adj5 polyneuropath$3).tw. (2375) 19 or/14‐18 (24802) 20 exp Steroid/ (1198754) 21 (steroid$ or glucocorticoid$).mp. (419452) 22 steroid therap$.tw. or Steroid Therapy/ (28896) 23 Anti‐inflammatory Agents.mp. or Antiinflammatory Agent/ (54431) 24 exp Immunosuppressive Agent/ (592965) 25 (prednisone$ or prednisolone$ or cortisone$).mp. (259880) 26 or/20‐25 (1567398) 27 13 and 19 and 26 (350) 28 remove duplicates from 66 (349)
Appendix 5. Trials registry search terms
Guillain Barre syndrome
ClinicalTrials.gov ‐ 30
WHO international Clinical trials Registry ‐ 33 references to 29 studies
Data and analyses
Comparison 1. Corticosteroid (CS) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disability grade change after 4 weeks | 6 | 587 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.16, 0.88] |
| 1.1 Oral regimens | 4 | 120 | Mean Difference (IV, Random, 95% CI) | 0.82 [0.17, 1.47] |
| 1.2 Intravenous regimens | 2 | 467 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.39, 0.06] |
| 2 Improvement by ≥ 1 grades after 4 weeks | 5 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.24] |
| 2.1 Oral regimens | 3 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.16] |
| 2.2 Intravenous regimens | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 3 Treatment vs. control OR for improvement by ≥ 1 grades by 4 weeks adjusted for age and initial disability grade | 2 | log(OR) (Fixed, 95% CI) | 0.34 [‐0.05, 0.73] | |
| 4 Death | 7 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.67, 2.47] |
| 4.1 Oral regimens | 5 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.41, 2.63] |
| 4.2 Intravenous regimens | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.61, 3.94] |
| 5 Death or disability after 1 year | 3 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.91, 2.50] |
| 5.1 Oral regimens | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.14, 5.81] |
| 5.2 Intravenous regimens | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.93, 2.66] |
| 6 Disability grade change after 6 months | 2 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 7 Disability grade change after 12 months | 3 | 471 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.23, 0.29] |
| 8 Proportion of participants who relapsed | 3 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.18] |
| 8.1 Oral regimens | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.36 [0.35, 115.73] |
| 8.2 Intravenous regimens | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.91] |
| 9 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 New infection treated with antibiotics | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.04] |
| 9.2 Gastrointestinal haemorrhage | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.36, 3.78] |
| 9.3 Diabetes mellitus requiring insulin | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.19, 4.12] |
| 9.4 Hypertension | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.41] |
1.1. Analysis.
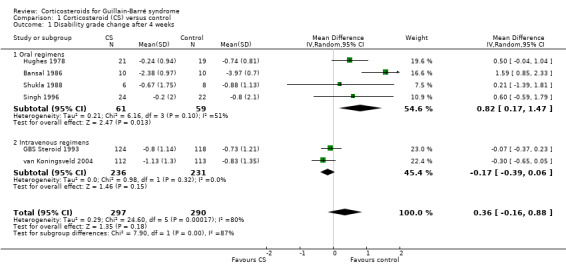
Comparison 1 Corticosteroid (CS) versus control, Outcome 1 Disability grade change after 4 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bansal 1986.
| Methods | Open parallel‐group controlled trial with alternate allocation | |
| Participants | 20 people with GBS defined according to criteria similar to those of Asbury 1990 | |
| Interventions | Prednisolone 15 mg 4 times daily for 4 days, 10 mg 4 times daily for 3 days, 10 mg 3 times daily for 10 days, and then tapered or no treatment | |
| Outcomes | Multiple outcomes including changes in the disability grade of Hughes 1978 after 1, 2, 3, and 4 weeks and 3 months; disease duration; residual disability; death; relapse | |
| Funding | Not stated | |
| Conflicts of interest among primary investigators | Not stated | |
| Notes | Single centre Conducted in India Mean (SD) age 54.5 (15.1) years in steroid group and 39.6 (14.4) years in control group. Unusually large improvements after 4 weeks in both groups, mean 2.4 grades in steroid group and 4.0 grades in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomised alternatively into two groups" |
| Allocation concealment (selection bias) | High risk | "Patients were randomised alternatively into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes except death | High risk | Control group received no treatment |
| Blinding of participants and personnel (performance bias) Death | Low risk | Blinding was unlikely to affect reporting of death |
| Blinding of outcome assessment (detection bias) All outcomes except death | High risk | Control group received no treatment |
| Blinding of outcome assessment (detection bias) Death | Low risk | Blinding was unlikely to affect reporting of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | Follow‐up for 12 weeks was complete |
| Incomplete outcome data (attrition bias) Death | Low risk | Follow‐up for 12 weeks was complete |
| Selective reporting (reporting bias) | Low risk | Only disability grade was specified in methods and was reported in full. Disease duration, residual disability, death, and relapse were also reported |
| Other bias | Unclear risk | Insufficient information to judge |
Garcia 1985.
| Methods | Open parallel‐group controlled trial with alternate allocation | |
| Participants | 20 mostly adults with GBS defined according to criteria of Asbury 1990 | |
| Interventions | Intravenous methylprednisolone 1500 mg daily for 5 days versus supportive care | |
| Outcomes | Time to recovery: not significantly different between the 2 groups. After 6 months, 7 of 10 corticosteroid‐treated participants had returned to work or resumed domestic duties at 95% of normal. No adverse effects attributable to corticosteroids were reported | |
| Funding | Not stated | |
| Conflicts of interest among primary investigators | Not stated | |
| Notes | Single centre Conducted in Mexico | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | High risk | Alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes except death | High risk | Control group received supportive care and no placebo |
| Blinding of participants and personnel (performance bias) Death | Low risk | Reporting of death is unlikely to have been affected by inadequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | High risk | Control group received supportive care and no placebo |
| Blinding of outcome assessment (detection bias) Death | Low risk | Reporting of death is unlikely to have been affected by inadequate blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | No drop‐outs were reported |
| Incomplete outcome data (attrition bias) Death | Low risk | No drop‐outs were reported |
| Selective reporting (reporting bias) | Low risk | The only outcome described in the methods, the recovery curve, was reported |
| Other bias | Unclear risk | Insufficient information to judge |
GBS Steroid 1993.
| Methods | Double‐blind parallel‐group randomised controlled trial | |
| Participants | 242 adults with GBS diagnosed according to the criteria of Asbury 1990. Unable to run. Disease onset < 15 days | |
| Interventions | Intravenous methylprednisolone 500 mg daily for 5 days or placebo infusions | |
| Outcomes | Primary: 0.5 disability grade (Hughes 1978) difference after 4 weeks. Secondary: 0.5 disability grade difference after 12 weeks, reduction of times to cease artificial ventilation, and to recover ability to walk unaided. After 4 weeks, mean (SD) disability grade improvement in corticosteroid group was 0.73 (1.21) grade compared with 0.8 (1.14) grade in the placebo group; difference 0.06 grade (95% CI ‐0.23 to 0.36) | |
| Funding | Funded by the British Medical Research Council | |
| Conflicts of interest among primary investigators | Not stated in the paper but none known | |
| Notes | International multicentre Plasma exchange permitted at the discretion of the participating neurologist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A telephone call to the trial office led to allocation of a random code number for a patient, stratified in blocks of 12 for each centre" |
| Allocation concealment (selection bias) | Low risk | "A telephone call to the trial office led to allocation of a random code number for a patient, stratified in blocks of 12 for each centre. The number led to the pharmacy preparing a coded 100 ml bag of either 5% dextrose with 500 mg methylprednisolone or normal saline alone (placebo). The seals on the bags that contained no methylprednisolone were punctured so that the bags could not be distinguished" |
| Blinding of participants and personnel (performance bias) All outcomes except death | Low risk | See above |
| Blinding of participants and personnel (performance bias) Death | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes except death | Low risk | See above |
| Blinding of outcome assessment (detection bias) Death | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 2 participants were withdrawn because of incorrect diagnosis (botulism). All other participants (of 240) remained in the trial for 48 weeks and all outcomes were reported |
| Incomplete outcome data (attrition bias) Death | Low risk | See above |
| Selective reporting (reporting bias) | Low risk | See above. All outcomes were reported |
| Other bias | High risk | "The proportion of patients for whom plasma exchange was stated as possible at randomisation and who then went on to have the procedure was significantly lower in the intravenous methylprednisolone group than in the placebo group (8/45 vs 15/32, p = 0.0125). Therefore it is possible that the patients on placebo were considered by their neurologists to be faring worse than those receiving IVMP and were therefore given plasma exchange; this would have had a beneficial effect and biased the analysis of the trial against detecting an effect of IVMP." |
Hughes 1978.
| Methods | Observer blinded parallel‐group randomised controlled trial | |
| Participants | 40 participants of any age with acute polyneuropathy of undetermined aetiology fulfilling criteria similar to those of Asbury 1990 | |
| Interventions | Prednisolone 15 mg 3 times daily for 1 week, 10 mg 3 times daily for 4 days, 5 mg 4 times daily for 3 days followed by continued treatment at discretion or no steroid treatment | |
| Outcomes | Changes in a 7‐point disability grade scale after 1, 3, and 12 months; time to onset of improvement; time to recover ability for manual work; and proportion with residual disability were all measured. A primary outcome measure was not predefined. There were no significant differences at any of these times except for the subgroup of participants who were randomised within 1 week from the onset. In this subgroup, the 6 control participants improved by a mean (SD) of 2.5 (0.43) grades whereas the 10 corticosteroid participants only improved by 0.9 (0.46) grade (P < 0.05) | |
| Funding | Funded by the British Medical Research Council | |
| Conflicts of interest among primary investigators | Not stated in the paper but none known | |
| Notes | Multicentre in south‐east England It was decided during the trial that participants who had begun to improve before trial entry should be excluded and so 1 participant was removed from each group. The 4‐week grade change SD is not in the paper and has been derived from the original data. 1 suicide in the control group was excluded by the authors from their analysis but has been included in the death table in this review. There were no significant differences at any of these times except for the subgroup of participants who were randomised within 1 week from the onset. In this subgroup, the 6 control participants improved by a mean (SD) of 2.5 (0.43) grades whereas the 10 corticosteroid participants only improved 0.9 (0.46) grade (P < 0.05) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At entry participants were randomised to prednisolone or control treatment according to a central register. The central register was prepared from a table of random numbers by the trial statistician |
| Allocation concealment (selection bias) | Low risk | The treatment allocation was obtained by telephoning a central reference point |
| Blinding of participants and personnel (performance bias) All outcomes except death | High risk | The comparison was of prednisolone versus no treatment. Assessments were made within 24 hours of entry by 1 of 2 assessors who had no knowledge of the treatment schedule |
| Blinding of participants and personnel (performance bias) Death | Low risk | Death would not be affected by any deficiency in blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | High risk | The comparison was of prednisolone versus no treatment. Assessments were made within 24 hours of entry by 1 of 2 assessors who had no knowledge of the treatment schedule |
| Blinding of outcome assessment (detection bias) Death | Low risk | Death would not be affected by any deficiency in blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 1 of 22 prednisolone‐treated participants and 3 of 22 controls were withdrawn. 1 from each group because they had been started on steroids before randomisation in contradiction of a protocol amendment, and 2 more participants were withdrawn from the control group: 1 because the diagnosis was later considered to be incorrect and 1 because prednisolone had been given before entry to the trial |
| Incomplete outcome data (attrition bias) Death | Low risk | Deaths reported in full |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | None detected |
Shukla 1988.
| Methods | Double‐blind randomised parallel‐group controlled trial | |
| Participants | 16 participants with GBS fulfilling diagnostic criteria of Asbury 1990 | |
| Interventions | Prednisolone 60 mg daily in divided doses for 1 week, 40 mg daily for 1 week, 30 mg daily for 2 weeks, and thereafter at the discretion of the physician or identical appearing placebo tablets | |
| Outcomes | Changes in the disability grade of Hughes 1978 after 1, 4, and 6 weeks. After 4 weeks, 5 of 8 corticosteroid and 3 of 6 placebo participants had improved ≥ 1 grades. 2 placebo participants dropped out | |
| Funding | Not stated | |
| Conflicts of interest among primary investigators | Not stated | |
| Notes | Single centre Conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised: packets of drugs had been prepared and numbered randomly well in advance" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes except death | Low risk | "Placebo tablets of the same size, shape and colour were given in the equivalent number and duration" |
| Blinding of participants and personnel (performance bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | Low risk | "Assessments were made...by a doctor who had no knowledge of the treatment schedule" |
| Blinding of outcome assessment (detection bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | High risk | 3 of 8 corticosteroid and 1 of 8 control participants were omitted at 4‐week follow‐up, including 1 from each group who died |
| Incomplete outcome data (attrition bias) Death | Low risk | Deaths were reported |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | Insufficient information to judge |
Singh 1996.
| Methods | Double‐blind controlled parallel‐group trial with participants allocated alternately to 1 blinded treatment or the other | |
| Participants | 52 participants with GBS fulfilling the diagnostic criteria of Asbury 1990 | |
| Interventions | Prednisolone 40 mg twice daily for 2 weeks and thereafter gradually tapered or identical appearing placebo tablets | |
| Outcomes | Disability grade of Hughes 1978 after 2, 4, and 24 weeks. After 4 weeks, 14 of 22 placebo and 12 of 24 corticosteroid participants had improved ≥ 1 grades. 6 dropped out | |
| Funding | Not stated | |
| Conflicts of interest among primary investigators | Not stated | |
| Notes | Single centre Conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Alternately randomized" |
| Allocation concealment (selection bias) | High risk | "Alternately randomized" |
| Blinding of participants and personnel (performance bias) All outcomes except death | High risk | "Alternately randomized", "identical placebo tablets" |
| Blinding of participants and personnel (performance bias) Death | Low risk | Reporting of death is unlikely to have been affected by inadequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | High risk | "Alternately randomized", "identical placebo tablets" |
| Blinding of outcome assessment (detection bias) Death | Low risk | Reporting of death is unlikely to have been affected by inadequate blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | High risk | 6 of 52 participants dropped out. The reasons and treatment assignment were not reported |
| Incomplete outcome data (attrition bias) Death | Low risk | Reporting of death is unlikely to have been affected by attrition bias |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in methods reported. Primary outcome not specified |
| Other bias | Unclear risk | Insufficient information to judge |
Swick 1976.
| Methods | Double‐blind parallel‐group randomised controlled trial | |
| Participants | 38 participants with idiopathic polyneuritis diagnosed according to criteria of Osler 1960. People requiring artificial ventilation or with contraindications to steroids were excluded | |
| Interventions | Active treatment for adults 100 units and children 2 units/kg aqueous ACTH intramuscularly daily for 10 days or equal volumes of diluent as placebo | |
| Outcomes | Duration of hospitalisation, time to complete recovery. Excluding 1 participant who died in the ACTH group, the median time to recovery was 9.0 days in the placebo and 4.4 days in the ACTH group (P = 0.05) | |
| Funding | "Supported in part by Special Traineeship Award" from National Institutes of Health, USA | |
| Conflicts of interest among primary investigators | Not stated | |
| Notes | Single centre Conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random groups were established using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Random groups were established using a table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes except death | Low risk | Placebo was an equal volume of the diluent for ACTH |
| Blinding of participants and personnel (performance bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | Low risk | Placebo was an equal volume of the diluent for ACTH |
| Blinding of outcome assessment (detection bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | High risk | Data for the outcomes required by this review not provided. However, 1 participant in the ACTH group who died was not included in the analysis of time to recovery, which was the main analysis considered by the authors |
| Incomplete outcome data (attrition bias) Death | Low risk | Deaths were reported |
| Selective reporting (reporting bias) | High risk | Selective reporting not applicable as data for the outcomes required by this review are not provided. However, 1 participant in the ACTH group who died was not included in the analysis of time to recovery, which was the main analysis considered by the authors |
| Other bias | Low risk | None detected |
van Koningsveld 2004.
| Methods | Double‐blind parallel‐group randomised controlled trial | |
| Participants | 225 people with GBS fulfilling the diagnostic criteria of Asbury 1990 | |
| Interventions | All participants received IVIg 0.4 g/kg daily for 5 days and also either intravenous methylprednisolone 500 mg daily for 5 days or placebo infusions | |
| Outcomes | Primary: improvement of 1 disability grade (modified after Hughes 1978) after 4 weeks. Secondary: ability to walk unaided after 8 weeks, number of days to walk independently. After 4 weeks, 63 of 113 (56%) placebo‐treated participants improved ≥ 1 grades compared with 76 of 112 (68%) corticosteroid‐treated participants (P = 0.06). See text for more details | |
| Funding | Baxter Bioscience, Brussels, Belgium, provided financial support | |
| Conflicts of interest among primary investigators | Paper states "None declared" | |
| Notes | Multicentre centre study in the Netherlands, Belgium, and Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We stratified randomisation according to age (<50 or ≥50 years) because of its effect on prognosis. We used block randomisation with random block sizes of 4, 6, or 8 generated by computer. When a neurologist identified a participant, they phoned a 24‐h hotline and were given a number, according to the randomisation list. The local hospital pharmacist subsequently prepared the trial medication (methylprednisolone or placebo), according to the randomisation number" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes except death | Low risk | See above |
| Blinding of participants and personnel (performance bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Blinding of outcome assessment (detection bias) All outcomes except death | Low risk | Report states, "In most cases neurologists responsible for patients' day‐to‐day care were not involved in assessment of treatment effect. We therefore assumed that unmasking was not an important issue in this trial" |
| Blinding of outcome assessment (detection bias) Death | Low risk | Reporting of death unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | Only 8 of 233 randomised participants were withdrawn, 4 from each group, and the withdrawals were for legitimate reasons that we judged unlikely to have biased the results |
| Incomplete outcome data (attrition bias) Death | Low risk | Only 8 of 233 randomised participants were withdrawn, 4 from each group, and the withdrawals were for legitimate reasons that we judged unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | None detected |
ACTH: adrenocorticotrophic hormone CI: confidence interval GBS: Guillain‐Barré syndrome IVIg: intravenous immunoglobulin SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| El Zunni 1997 | Not an RCT. Observational study in which 5 participants with disability grade 1 or 2 were given placebo, 5 with disability grade 2 who were already on steroids were continued on them and 6 with disability grade 3 to 5 were given IVIg. 1 of the last 6 died. There was no significant difference in the amount of improvement in disability grade between the groups |
| Foyaca 2003 | The participants were 29 HIV‐positive in a single South African centre randomly allocated to prednisone 1 mg/kg daily or placebo. After 36 days the mean (SD) improvement on the disability scale used in this review was significantly greater, 0.8 (0.676) in the 15 prednisone‐treated and 0.07 (0.27) in the 14 placebo‐treated participants, mean difference 0.73 (95% CI 0.33 to 1.13). 10 of 15 prednisone‐treated and 1 of 14 placebo‐treated participants had improved ≥ 1 grades and the relative rate of improving by this amount was significant, 9.3 (95% CI 1.4 to 63.8). Follow‐up data were not available for 1 placebo participant for unstated reasons Allocation concealment unclear, diagnostic criteria inadequate (criteria in methods contradicted by inclusion of non‐eligible participants described in discussion) |
| Haass 1988 | Non‐randomised open study |
| Levchenko 1989 | Not a trial of corticosteroid treatment but a personal series of people with severe GBS all treated with prednisolone or methylprednisolone, 8 with and 11 without plasma exchange as well |
| Mendell 1985 | Compared corticosteroids and plasma exchange against no treatment |
| Naylor 1986 | Not an RCT |
| Zagar 1995 | Review, not an RCT |
| Zhang 2000 | Controlled trial comparing 22 participants treated with the Chinese herbal medicine Tripterygium with 21 treated with dexamethasone 15 mg to 20 mg intravenously for 15 days and then 5 mg to 10 mg daily for 7 days followed by oral prednisone 30 mg to 60 mg daily reduced by 5 mg to 10 mg every 2 weeks. Method of randomisation unclear. None of the outcomes selected for this review available. After 8 weeks, 20 of 22 improved in the Tripterygium group and 13 of 21 in the dexamethasone group. Tripterygium lowered serum interleukin‐6 significantly |
CI: confidence interval
GBS: Guillain‐Barré syndrome
HIV: human immunodeficiency virus
IVIg: intravenous immunoglobulin
RCT: randomised controlled trial
SD: standard deviation
Differences between protocol and review
In the 2009 update, RH and PvD reassessed risk of bias. The review authors added a 'Risk of bias' figure and 'Summary of findings' table and removed the previous Table of methodological quality.
Angela Gunn and Ruth Brassington became authors. Tony Swan was an author of the protocol and the original version of this review but retired before the 2012 update.
Contributions of authors
RH prepared the first draft of the initial review and extracted the data from the studies.
PvD checked the data for the update of the review in January 2004.
RH and RvK undertook data extraction for the update in 2005.
AG undertook the searches in all versions of the review.
RH and PvD reviewed the results of the searches in 2009, 2011, and 2016.
RB edited the 2016 update.
All the current authors contributed to and agreed the 2016 update.
Sources of support
Internal sources
-
King's College, London, UK.
Infrastructure
-
University College London, UK.
Infrastructure
-
Erasmus Medical Centre Rotterdam, Netherlands.
Infrastructure
-
National Institute of Health Research (NIHR), UK.
Salary of Angela Gunn and Ruth Brassington at University College London NHS Foundation Trust is paid from NIHR Cochrane Infrastructure funding to Cochrane Neuromuscular
External sources
Guillain‐Barré Syndrome Foundation International, USA.
Declarations of interest
Richard Hughes holds consultancies with CSL Behring and LFB which make immunoglobulin which is used for treating GBS. He was chief investigator of one of the trials included in this review and an investigator in another.
RB is Managing Editor of Cochrane Neuromuscular. She has no known financial conflicts of interest. She did not take part in the approval processes for this review.
Angela Gunn is the Information Specialist of Cochrane Neuromuscular. She did not take part in the approval processes for this review.
PvD and his institution have received consultancy fees from Talecris, Octapharma, and CSL Behring for membership of the Scientific Board of the ICE trial in CIDP, intravenous immunoglobulin (IVIg) in chronic polyneuropathy and a trial of IVIg in CIDP.
PvD's department has received research grants from Baxter, Sanquin, Talecris, and Baxalta, respectively, to conduct the following: an RCT comparing IVIg vs IVIg and steroids in GBS, an RCT investigating the effect of a second course of IVIg (SID‐trial) in GBS patients with a poor prognosis, a prospective international study on the effect of a second course of IVIg in GBS patients with a poor prognosis. and a dose‐finding study in CIDP.
PvD was an investigator in a trial of corticosteroids in GBS (van Koningsveld 2004).
The Co‐ordinating Editor of Cochrane Neuromuscular has assessed the authors' declarations of interest and considers that they do not represent a conflict in relation to this review. Additionally, the review does not consider comparisons of corticosteroids with intravenous immunoglobulin.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bansal 1986 {published data only}
- Bansal BC, Sood AK, Gupta AK, Yadav P. Role of steroids in the treatment of Guillain Barre syndrome ‐ a controlled trial. Neurology India 1986;34(5):329‐35. [EMBASE: 1987132329] [Google Scholar]
Garcia 1985 {published data only}
- Garcia AC, Vidal BE, Rebolledo FA, Texeira F, Ordaz FA, Futran YJ. Treatment of the acute phase of Guillain‐Barre‐Strohl syndrome with megadoses of methylprednisolone. Revista de Investigacion Clinica (Mexico City) 1985;37:119‐24. [PubMed] [Google Scholar]
GBS Steroid 1993 {published data only}
- Guillain‐Barré Syndrome Steroid Trial Group. Double‐blind trial of intravenous methylprednisolone in Guillain‐Barré syndrome. Lancet 1993;341(8845):586‐90. [PUBMED: 8094828] [PubMed] [Google Scholar]
- Guillain‐Barré Syndrome Steroid Trial Group. Double‐masked trial of intravenous methylprednisolone in Guillain‐Barré syndrome [Abstract]. Journal of Neurology, Neurosurgery and Psychiatry 1992;55:1214. [Google Scholar]
Hughes 1978 {published and unpublished data}
- Hughes RAC, Newsom‐Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet 1978;2(8093):750‐3. [PUBMED: 80682] [DOI] [PubMed] [Google Scholar]
Shukla 1988 {published data only}
- Shukla SK, Agarwal R, Gupta OP, Pande G, Singh M. Double blind controlled trial of prednisolone in Guillain‐Barré syndrome ‐ a clinical study. Clinician ‐ India 1988;52(5):128‐34. [EMBASE: 1988186155] [Google Scholar]
Singh 1996 {published data only}
- Singh NK, Gupta A. Do corticosteroids influence the disease course or mortality in Guillain‐Barré syndrome?. Journal of the Associations of Physicians of India 1996;44(1):22‐4. [PUBMED: 8773088] [PubMed] [Google Scholar]
Swick 1976 {published data only}
- Swick HM, McQuillen MP. The use of steroids in the treatment of idiopathic polyneuritis. Neurology 1976;26(3):205‐12. [PUBMED: 175314] [DOI] [PubMed] [Google Scholar]
van Koningsveld 2004 {published and unpublished data}
- Ruts L, Koningsveld R, Jacobs BC, Doorn PA. Determination of pain and response to methylprednisolone in Guillain‐Barre syndrome. Journal of Neurology 2007;254(10):1318‐22. [PUBMED: 17426908] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn PA, Koningsveld R, Schmitz PIM, Meché FGA, Visser LH, Meulstee J. Randomized trial on the effect of methyl prednisolone on standard treatment with intravenous immunoglobulin in Guillain‐Barré syndrome. Neuromuscular Disorders 2002;12:781. [Google Scholar]
- Koningsveld R, Schmitz PIM, Meché FGA, Visser VH, Meulstee J, Doorn PA. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain‐Barré syndrome: randomised trial. Lancet 2004;363(9404):192‐6. [PUBMED: 14738791] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
El Zunni 1997 {published data only}
- Zunni S, Prakash PS, Saiti KM, Busnaina IA. Guillain‐Barré syndrome (GBS): an appraisal. Central African Journal of Medicine 1997;43(4):99‐103. [Google Scholar]
Foyaca 2003 {published data only}
- Foyaca H, Ibanez‐Valdes L de F, Awotedu AA. Effect of prednisone on Guillain‐Barré syndrome in HIV positive patients. Internet Journal of Neurology 2003;2(1):1‐12. [Google Scholar]
Haass 1988 {published data only}
- Haass A, Trabert W, Gressnich N, Schimrigk K. High‐dose steroid therapy in Guillain‐Barré syndrome. Journal of Neuroimmunology 1988;20(2‐3):305‐8. [DOI] [PubMed] [Google Scholar]
Levchenko 1989 {published data only}
- Levchenko NI, Piradov MA, Pirogov VN. Plasmapheresis and hormone therapy in severe forms of Guillain‐Barré syndrome. Klinicheskaia Meditsina 1989;67(12):88‐91. [PubMed] [Google Scholar]
Mendell 1985 {published data only}
- Mendell JR, Kissel JT, Kennedy MS, Grinvalsky HT, Pittman GL, Kyler RS, et al. Plasma exchange in acute inflammatory polyradiculoneuropathy. A controlled randomized trial. [Abstract]. Annals of Neurology 1983;14:122. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Kennedy MS, Sahenk Z, Grinvalsky HT, Pittman GL, et al. Plasma exchange and prednisone in Guillain‐Barré syndrome. A controlled randomized trial. Neurology 1985;35(11):1551‐5. [DOI] [PubMed] [Google Scholar]
Naylor 1986 {published data only}
- Naylor C, Dos Santos Werneck AL, Lacativa M. Comparative study on steroids use and non‐use in the Guillain‐Barré syndrome. Arquivos de Neuro‐Psiquitria 1986;44(4):359‐63. [DOI] [PubMed] [Google Scholar]
Zagar 1995 {published data only}
- Zagar M. Treatment of Guillain‐Barré syndrome. Lijecnicki Vjesnik 1995;117(9‐10):246‐9. [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang X, Xia J, Ye H. Effect of tripterygium polyglycoside on interleukin‐6 in patients with Guillain‐Barré syndrome. Chung‐Kuo Chung Hsi Chieh Ho Tsa Chih 2000;20(5):332‐4. [PubMed] [Google Scholar]
Additional references
Asbury 1990
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Annals of Neurology 1990;27(Suppl):21‐4. [DOI] [PubMed] [Google Scholar]
Bettinelli 1989
- Bettinelli A, Giani M, Rossi L, Bardare M, Longetti A, Ghio L, et al. Ex novo episodes of acute glomerulonephritis and Guillain‐Barré syndrome: a case report. Clinical Nephrology 1989;31:269‐73. [PubMed] [Google Scholar]
Bromberg 2004
- Bromberg MB, Carter O. Corticosteroid use in the treatment of neuromuscular disorders: empirical and evidence‐based data. Muscle & Nerve 2004;30(1):20‐37. [DOI] [PubMed] [Google Scholar]
Dutch GBS Group 1994
- The Dutch Guillain‐Barré Study Group. Treatment of Guillain‐Barré syndrome with high‐dose immune globulins combined with methylprednisolone: a pilot study. Annals of Neurology 1994;35(6):749‐52. [DOI] [PubMed] [Google Scholar]
Dyck 1982
- Dyck PJ, O'Brien PC, Oviatt KF, Dinapoli RP, Daube JR, Bartleson JD, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Annals of Neurology 1982;11(2):136‐41. [DOI] [PubMed] [Google Scholar]
GBS Group 1985
- The Guillain‐Barré Syndrome Study Group. Plasmapheresis and acute Guillain‐Barré syndrome. Neurology 1985;35(8):1096‐104. [PubMed] [Google Scholar]
Hughes 1981
- Hughes RAC, Kadlubowski M, Hufschmidt A. Treatment of acute inflammatory polyneuropathy. Annals of Neurology 1981;9(Suppl):125‐33. [DOI] [PubMed] [Google Scholar]
Hughes 1990
- Hughes RAC. Guillain‐Barré Syndrome. Heidelberg: Springer‐Verlag, 1990. [Google Scholar]
Hughes 2014
- Hughes RAC, Swan AV, Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD002063.pub6] [DOI] [PubMed] [Google Scholar]
King 1985
- King RH, Craggs RI, Gross MLP, Thomas PK. Effects of glucocorticoids on experimental allergic neuritis. Experimental Neurology 1985;87(1):9‐19. [DOI] [PubMed] [Google Scholar]
Mehndiratta 2015
- Mehndiratta MM, Hughes RAC. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD002062.pub3] [DOI] [PubMed] [Google Scholar]
Olbricht 1993
- Olbricht CJ, Stark E, Helmchen U, Schulze M, Brunkhorst R, Koch KM. Glomerulonephritis associated with inflammatory demyelinating polyradiculoneuropathy: a case report and review of the literature. Nephron 1993;64(1):139‐41. [DOI] [PubMed] [Google Scholar]
Osler 1960
- Osler LD, Sidell AD. The Guillain‐Barre syndrome; the need for exact diagnostic criteria. New England Journal of Medicine 1960;12(262):964‐9. [DOI] [PubMed] [Google Scholar]
Peter 1996
- Peter JV, Gnanamuthu C, Cherian AM, Prabhakar S. Outcomes in the Guillain‐Barré syndrome: the role of steroids. Journal of the Association of Physicians of India 1996;44(3):172‐4. [PubMed] [Google Scholar]
Raphaël 2012
- Raphaël JC, Chevret S, Hughes RAC, Annane D. Plasma exchange for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2002, Issue 7. [DOI: 10.1002/14651858.CD001798.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rich 1998
- Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Annals of Neurology 1998;43(2):171‐9. [DOI] [PubMed] [Google Scholar]
Riggs 1998
- Riggs JE, Schochet SS Jr. Critical illness myopathy, steroids and cytochrome P450. Archives of Neurology 1998;55(12):1591. [DOI] [PubMed] [Google Scholar]
Ropper 1991
- Ropper AH, Wijdicks EFM, Truax BT. Guillain‐Barré Syndrome. Philadelphia: FA Davis Company, 1991. [Google Scholar]
van Nes 2011
- Nes SI, Vanhoutte EK, Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch‐built Overall Disability Scale (R‐ODS) for immune‐mediated peripheral neuropathies. Neurology 2011;76(4):337‐45. [DOI] [PubMed] [Google Scholar]
Walgaard 2011
- Walgaard C, Lingsma HF, Ruts L, Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain‐Barré syndrome. Nature Neurology 2011;76(11):968‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 1989
- Watts PM, Taylor WA, Hughes RAC. High‐dose methylprednisolone suppresses experimental allergic neuritis in the Lewis rat. Experimental Neurology 1989;103(1):101‐4. [DOI] [PubMed] [Google Scholar]
Willison 2016
- Willison HJ, Jacobs BC, Doorn PA. Guillain‐Barré syndrome. Lancet 2016;388(10045):717‐27. [DOI: 10.1016/S0140-6736(16)00339-1] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hughes 2000
- Hughes RAC, Meché FGA. Corticosteroids for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001446] [DOI] [Google Scholar]
Hughes 2006
- Hughes RAC, Swan AV, Koningsveld R, Doorn PA. Corticosteroids for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001446.pub2] [DOI] [PubMed] [Google Scholar]
Hughes 2010
- Hughes RAC, Swan AV, Doorn PA. Corticosteroids for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD001446.pub3] [DOI] [PubMed] [Google Scholar]
Hughes 2012
- Hughes RAC, Doorn PA. Corticosteroids for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001446.pub4] [DOI] [PubMed] [Google Scholar]


