Abstract
Background
Bowen's disease is the clinical term for in situ squamous cell carcinoma of the skin. Cutaneous lesions present as largely asymptomatic, well‐defined, scaly erythematous patches on sun‐exposed skin. In general, people with Bowen's disease have an excellent prognosis because the disease is typically slow‐growing and responds favourably to treatment. Lesions are persistent and can be progressive, with a small potential (estimated to be 3%) to develop into invasive squamous cell carcinoma. The relative effectiveness of the available treatments is not known for Bowen's disease, and this review attempts to address which is the most effective intervention, with the least side‐effects, for cutaneous Bowen's disease.
Objectives
To assess the effects of therapeutic interventions for cutaneous Bowen's disease.
Search methods
We searched the following databases up to September 2012: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2012, Issue 9), MEDLINE (from 1946), EMBASE (from 1974), PsycINFO (from 1806), and LILACS (from 1982). We also searched online trials registers. We checked the bibliographies of included and excluded studies and reviews, for further references to relevant randomised controlled trials (RCTs).
Selection criteria
We included all randomised controlled trials assessing interventions used in Bowen's disease, preferably histologically proven.
Data collection and analysis
Two authors independently carried out study selection and assessment of methodological quality.
Main results
The primary outcome measures were complete clearance of lesions after the first treatment cycle and recurrence rate at 12 months. Our secondary outcomes included the number of lesions that cleared after each treatment cycle, the number of treatment cycles needed to achieve clearance, the recurrence rates at > 12 months, cosmetic outcome, quality of life assessment, and adverse outcomes as reported by both participant and clinician.
We included 9 studies, with a total of 363 participants. One study demonstrated statistically significantly greater clearance of lesions of Bowen's disease with MAL‐PDT (methyl aminolevulinate with photodynamic therapy) when compared with placebo‐PDT (RR (risk ratio) 1.68, 95% CI (confidence interval) 1.12 to 2.52; n = 148) or cryotherapy (RR 1.17, 95% CI 1.01 to 1.37; n = 215), but there was no significant difference when MAL‐PDT was compared to 5‐FU (5‐fluorouracil). One study demonstrated statistically significantly greater clearance of lesions with ALA‐PDT (5‐aminolevulinic acid with photodynamic therapy) versus 5‐FU (RR 1.83, 95% CI 1.10 to 3.06; n = 66), but no statistically significant difference in recurrence rates at 12 months (RR 0.33, 95% CI 0.07 to 1.53).
Cryotherapy showed no statistically significant difference in clearance rates (RR 0.99, 95% CI 0.78 to 1.26) or recurrences at 1 year (RR 1.48, 95% CI 0.53 to 4.17) when compared to 5‐FU in 1 study of 127 participants.
One study compared imiquimod to placebo and demonstrated statistically significantly greater clearance rates in the imiquimod group (9/15 lesions) compared to placebo (0/16) (Fisher's Exact P value < 0.001). The imiquimod group did not report any recurrences at 12 months, but at 18 months, 2/16 participants in the placebo group had developed early invasive squamous cell carcinoma.
Authors' conclusions
Overall, there has been very little good‐quality research on treatments for Bowen's disease. There is limited evidence from single studies to suggest MAL‐PDT is an effective treatment. Although cosmetic outcomes appear favourable with PDT, five‐year follow‐up data are needed. Significantly more lesions cleared with MAL‐PDT compared to cryotherapy. No significant difference in clearance was seen when MAL‐PDT was compared with 5‐FU, but one study found a significant difference in clearance in favour of ALA‐PDT when compared to 5‐FU. There was no significant difference in clearance when cryotherapy was compared to 5‐FU.
The lack of quality data for surgery and topical cream therapies has limited the scope of this review to one largely about PDT studies. The age group, number, and size of lesions and site(s) affected may all influence therapeutic choice; however, there was not enough evidence available to provide guidance on this. More studies are required in the immunosuppressed populations as different therapeutic options may be preferable. Specific recommendations cannot be made from the data in this review, so we cannot give firm conclusions about the comparative effectiveness of treatments.
Keywords: Humans; Cryotherapy; Neoplasm Recurrence, Local; Aminolevulinic Acid; Aminolevulinic Acid/analogs & derivatives; Aminolevulinic Acid/therapeutic use; Aminoquinolines; Aminoquinolines/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Bowen's Disease; Bowen's Disease/therapy; Fluorouracil; Fluorouracil/therapeutic use; Imiquimod; Photochemotherapy; Photochemotherapy/methods; Photosensitizing Agents; Photosensitizing Agents/therapeutic use; Skin Neoplasms; Skin Neoplasms/therapy; Treatment Outcome
Plain language summary
Treatments for cutaneous Bowen's disease
Bowen's disease is the clinical term for a particular precancerous skin lesion. These lesions rarely cause patients any symptoms, but appear as well‐defined scaly patches on sun‐exposed skin, commonly in those over 60 years. They occur more in women and most frequently involve the lower legs of those affected in the UK. It is not known why, but the body sites most commonly affected vary across different countries. In general, people with Bowen's disease have an excellent prognosis because the disease is typically slow to develop and responds favourably to treatment. Lesions are usually slow‐growing, and although they are not life‐threatening, there is a small risk of progression to a skin cancer (estimated to be 3%) known as invasive squamous cell carcinoma.
This review attempted to find which is the most effective treatment for cutaneous Bowen's disease, with the least side‐effects.
There are a range of treatment options including the following: topical therapies, such as 5‐fluorouracil (5‐FU) and imiquimod creams; surgical interventions, such as excision and Mohs micrographic surgery; destructive therapies, such as cryotherapy (freezing); and light‐based therapies, such as photodynamic therapy (where a light‐sensitive cream is used in combination with visible light).
We included 9 randomised controlled trials, with a total of 363 participants. No studies examined surgical methods.
Photodynamic therapy appears to be an effective treatment and has the benefit of minimal scarring compared with cryotherapy or 5‐fluorouracil. Cryotherapy is convenient and less expensive, but does not appear to be as effective as photodynamic therapy and results in more scarring; 5‐aminolevulinic acid with photodynamic therapy (ALA‐PDT) appears to be more effective than 5‐fluorouracil, whereas methyl aminolevulinate with photodynamic therapy (MAL‐PDT) does not appear to be as good as 5‐fluorouracil. One study demonstrated benefit with imiquimod cream.
Specific recommendations cannot be made from these data, so this review cannot give firm conclusions about the comparative effectiveness of treatments. There is a clear need for future research to focus on a range of different studies comparing various therapies with each other, and in particular to surgical treatments to provide high‐quality evidence to guide clinical practice. The age group, number and size of lesions, sites affected, and immunological status may all influence therapeutic choices. Longer‐term follow up (up to 10 years) is needed to determine the effect of treatments on risk of progression of lesions of Bowen's disease to squamous cell carcinoma.
Background
Please note that we have explained unfamiliar terms in Table 1.
1. Glossary of terms.
| Medical term | Explanation |
| Metastasis | The spread of a malignant tumour from its original site to another part of the body, e.g. lungs, lymph nodes, liver, brain, bones, etc |
| Epidermis | The outermost layer of the skin |
| Transformation | A process by which cells acquire the properties of cancer |
| Subungual | Underneath the nail (finger or toe) |
| Periungual | Around the nail |
| Carcinogen | This is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer |
| HPV | Human papillomavirus |
| TH1 | T‐helper cells 1 |
| T cell | A type of white blood cell |
Description of the condition
John Templeton Bowen first described Bowen's disease in 1912 (Ali 2012). It is the clinical term for in situ squamous cell carcinoma, a type of non‐melanoma skin cancer (NMSC) that is confined to the epidermis (Arlette 2004). Typically, Bowen's disease lesions are slow‐growing, non‐pigmented reddish patches with irregular edges and a yellow or white crusting or scaling surface (Arlette 2004; Cox 1999; Ragi 1988). They are clearly demarcated from the surrounding normal skin. They are generally asymptomatic, although larger lesions may itch (Arlette 2004). Lesions are usually solitary, but multiple lesions occur in 10% to 20% of individuals (Eedy 1987; Kovacs 1996; Thestrup‐Pedersen 1988). Lesion size varies considerably, from a few millimetres to several centimetres in diameter, with the size of the lesion being directly related to its duration (Arlette 2004). The lesions are usually persistent and progressive and have a small potential for invasive malignancy.
Incidence and demographics
Bowen's disease can occur at any age in adults, although large‐population cohort studies suggest that it is commonly diagnosed in older people, between 60 and 90 years (Eedy 1987; Jaeger 1999; Kossard 1992; Kovacs 1996; Reizner 1994; Thestrup‐Pedersen 1988). These studies also reveal considerable worldwide variation in gender and body site distribution. Generally, Bowen's disease occurs more commonly in women, and varies in frequency between countries. In an Australian study, 57% of those with the disease were women (Kossard 1992); 56% to 61%, in 2 Danish studies (Jaeger 1999; Thestrup‐Pedersen 1988); 54%, in a Japanese study (Kovacs 1996); 74% to 80%, in 2 studies in the UK (Cox 1994; Eedy 1987); and 63%, in a study from the USA (Reizner 1994). The exception was in a study of a white population in Hawaii, where only 38% were women (Reizner 1994).
Although few studies have calculated incidence rates, there is considerable variation between the rates reported in America: 15 per 100,000 in Minneapolis (Chute 1991), 28 per 100,000 for men and 22 per 100,000 for women in Canada (Arlette 2004), and 174 per 100,000 for white men and 115 per 100,000 for white women living in Hawaii (Reizner 1994).
In Australia, the most common sites of Bowen's disease lesions are the head and neck (44%), followed by the lower limbs (30%), with 70% of lesions occurring below the knee. Australian men most commonly have lesions on the head and neck, while Australian women more commonly have Bowen's disease lesions on their lower limbs (Kossard 1992). The head and neck regions were also the most common site for lesions: in Denmark they made up 59% in 1 study (Thestrup‐Pedersen 1988) and 40% in another (Jaeger 1999), whereas in the USA they made up 66% (Reizner 1994). Reports from the UK suggest a different pattern of distribution of lesions: 13% of lesions on the head and neck (Eedy 1987) and 60% to 85% on the lower limbs (Cox 1994; Eedy 1987). Generally, few Bowen's disease lesions occur on the trunk, but a study in Japan (Kovacs 1996) and another on white people living in Hawaii (Reizner 1994) found a notably higher predominance of lesions on the trunk: 35% and 26%, respectively.
Other less common sites and variants include pigmented Bowen's disease, subungual/periungual, palmar, genital, and verrucous Bowen's disease.
Impact
In general, people with Bowen's disease have an excellent prognosis because the disease is typically slow‐growing and responds favourably to treatment, although a significant number of lesions of Bowen's disease are not treated due to its relatively benign nature and the demographics of the participants with the condition. The risk of progression of Bowen's disease to invasive squamous cell carcinoma (SCC) is generally considered to be about 3% (Kao 1986; Peterka 1961), of which approximately one third may metastasise (Arlette 2004; Cox 1999). These figures are high compared to what is actually seen in clinical practice and may reflect the inclusion of mucosal and anogenital Bowen's disease, where there is a higher risk of transformation and metastasis.
Bowen's disease may represent a risk marker for other non‐melanoma skin cancers. Studies that have investigated this association report that about one third of people have another NMSC (non‐melanoma skin cancer), most commonly basal cell carcinomas (BCC), at the time of diagnosis (Reizner 1994; Thestrup‐Pedersen 1988). There is also 4.3 times more risk of developing subsequent NMSC, which most likely reflects the shared ultraviolet light radiation aetiology (Jaeger 1999).
There has been much discussion about an association between Bowen's disease and internal malignancies, with several studies suggesting a significant relationship (summarised in Cox 1999). However, a 1989 meta‐analysis of 12 studies (10 cohort and 2 case‐control studies) found no significant relationship between Bowen's disease and internal malignancies (Lycka 1989). This result was subsequently confirmed by two large population‐based cohort studies in Denmark (Jaeger 1999) and in the USA. It is now generally accepted that there is no relationship between Bowen's disease and internal malignancies, and routine investigation for internal malignancies is not justified (Cox 2007).
Causes
Bowen's disease predominantly occurs in older age groups and on areas of the body subjected to chronic sun exposure (head and neck, and lower legs in women), suggesting a causal relationship between chronic exposure to ultraviolet light radiation and Bowen's disease (Cox 1994; Eedy 1987; Kossard 1992; Kovacs 1996; Reizner 1994; Thestrup‐Pedersen 1988). Exposure to carcinogens, e.g. arsenic through well water, older medications, and occupational chemicals, have been associated with the development of Bowen's disease. A time lag of more than 10 years between exposure and development of lesions is typical (Arlette 2004; Cox 1999). Viral aetiology has been postulated. The role of human herpes virus 8 (HHV8) is unclear.
In contrast, there is excellent evidence for a causative role for alpha‐papillomaviruses (mucosal HPV types) in periungual Bowen's and in mucosal and anogenital Bowen's disease, although the frequency with which these agents are detected in these lesions varies (Cox 2007; Grundmeier 2011; Riddel 2011).
Although not the subject of this review, mucosal HPV types are implicated in almost 100% of cases of mucosal and anogenital Bowen's disease (Iftner 2003).
Immunosuppression, either congenital, acquired, or iatrogenic, has also been associated with Bowen's disease (Bordea 2004; Cox 1999; Eedy 2005; Perrett 2007). One study demonstrated that 23% of skin cancers in renal transplant recipients were Bowen's disease (Bordea 2004).
Description of the intervention
There are a range of treatment options for Bowen's disease, including the following:
topical therapies, such as 5‐fluorouracil and imiquimod creams;
surgical interventions, such as excision and Mohs micrographic surgery;
destructive therapies, such as cryotherapy and curettage and cautery,
light‐based therapies, such as laser therapy and photodynamic therapy; and
radiotherapy.
How the intervention might work
The primary mechanism of action of 5‐fluorouracil is inhibition of DNA synthesis by competitive inhibition of thymidylate synthetase and incorporation into RNA and DNA.
Imiquimod is an immune‐response modifier that promotes a TH1‐driven cell‐mediated immune response.
Mohs micrographic surgery is a technique whereby 100% of the surgical margin is examined by mapping horizontal frozen sections from successive excision layers until complete clearance is achieved.
Cryotherapy uses liquid nitrogen to destroy tissue by freezing it to ‐196°C.
Electrodessication and cautery and curettage are generally known as 'scraping or burning‐off of skin growths'. Curettage is performed under local anaesthesia. The curette is either an oval, semisharp spoon‐shaped instrument or an open ring connected to a handle. The curette is designed to cut through abnormally soft or friable tissue with minimum force so that the diseased tissue can be selectively removed. Curettage should be combined with subsequent electrocautery that destroys additional tissue.
Laser surgery uses a highly focused beam of light that destroys only the cancer cells.
Photodynamic therapy is a visible light in the blue or red spectrum that is absorbed by a porphyrin or other light‐sensitive compound, in order to produce free radicals. These free radicals are what cause the cell damage and death.
Radiotherapy works by destroying the cancer cells in the treated area using high‐energy X‐rays and has included contact radiation, grenz ray therapy, strontium 90, proton radiotherapy, emitting radionuclides, orthovoltage therapy, and electrons.
Why it is important to do this review
The relative effectiveness of the available treatments for Bowen's disease is not known. The rationale for treatment is to prevent progression to a cancerous lesion and also to improve cosmetic appearance. Treatment of Bowen's disease needs to balance the burden of treatment against its benefit, particularly as the disease mainly affects the elderly and is predominantly slow‐growing in nature with a good prognosis. Some Bowen's disease lesions may deserve special consideration, for example, lesions of the lower limb and especially larger lesions, because of the potential for poor healing in the former and the high recurrence rates in the latter.
Given these issues, this review attempted to address the following:
(1) What are the most effective treatments for Bowen's disease, with the fewest side‐effects?
(2) How do the various therapies compare in the following participant subgroups:
participants with lower leg lesions (i.e. located below the knee)?
participants with lesions > 2 cm²?
participants with medical comorbidities leading to poor wounding healing, age greater than 70 years, or both?
Objectives
To assess the effects of therapeutic interventions for cutaneous Bowen's disease.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) of any design of interventions for cutaneous Bowen's disease.
Types of participants
All adults with histologically proven cutaneous Bowen's disease. We excluded people with genodermatoses (genetic disorders of the skin), mucosal, or anogenital Bowen's disease.
Types of interventions
Any interventions for the treatment of cutaneous Bowen's disease, including the following:
Surgical
Surgical excision
Mohs micrographic surgery
Destructive
Curettage, cautery, or electrodesiccation
Cryosurgery ‐ any number of cycles
Other techniques
Topical therapy, e.g. imiquimod, 5‐fluorouracil
Photodynamic therapy
Laser surgery
Radiotherapy
The comparators were any other type of accepted and commonly used treatment method, any interventions compared to control (placebo/no treatment), or different dosages/durations of the same interventions.
Types of outcome measures
Primary outcomes
Complete clearance of the lesion.
(a) Number of lesions or participants cleared after first treatment cycle. (b) Recurrence at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle.
Number of treatment cycles needed to achieve clearance.
Recurrence at > 12 months.
Cosmetic outcome using a recognised and validated instrument to measure cosmesis.
Consumer satisfaction with treatment modality, cosmesis, or pain at site, recorded on a Likert or Likert‐like scale.
Time to complete healing of lesion following treatment, by clinical examination or by participant assessment through a diary or similar mechanism.
Quality of life, by any validated quality‐of‐life instrument.
Adverse outcomes categorised using the following system: none; mild (transient, requires no treatment, non‐interference with social or occupational function); moderate (requires simple treatment, interferes with social or occupational function); or severe (requires vigorous treatment, hospitalisation, and interrupts social or occupational function).
Recurrence of Bowen's disease in same site determined by clinical examination.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, and in progress)
Electronic searches
We searched the following databases up to 7 September 2012:
the Cochrane Skin Group Specialised Register using the following terms: (bowen* and disease) or (bowenoid and papulosis) or (morbus and bowen) or (squamous and cell and carcinoma) or (in and situ and squamous and cell and carcinoma) or (intraepidermal and squamous and cell and carcinoma);
the Cochrane Central Register of Controlled Trials (CENTRAL), 2012, Issue 9, in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE via OVID (from 1946) using the strategy in Appendix 2;
EMBASE via OVID (from 1974) using the strategy in Appendix 3;
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 4; and
PsycINFO via OVID (from 1806) using the terms 'random.mp.' and 'squamous cell carcinoma.mp'.
Trials registers
We searched the following trials registers on 15 February 2012 using the following terms: (bowen* and disease) or (morbus and bowen) or (squamous and cell and carcinoma) or (in and situ and squamous and cell and carcinoma) or (intraepidermal and squamous and cell and carcinoma).
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The Ongoing Skin Trials Register (http://skin.cochrane.org/ongoing‐skin‐trials‐register).
Details of the trials found are in the 'Characteristics of ongoing studies' tables.
Searching other resources
Reference lists
We checked the bibliographies of included and excluded studies and published reviews for further references to relevant trials.
Correspondence
The authors did not contact any pharmaceutical companies.
Adverse events
We did not perform a separate search for adverse effects of interventions used for the treatment of Bowen's disease. We considered adverse and side‐effects described in included studies only.
Data collection and analysis
Selection of studies
Two authors (FB‐H and RM) reviewed the titles and abstracts identified from the searches. We did not seek the full text of studies that were clearly not randomised controlled trials of treatments for Bowen's disease. The same two authors independently assessed the full text version of the remaining studies against the predefined selection criteria. We resolved differences of opinion through discussion with a third author (JL‐B).
Data extraction and management
Two authors (JL‐B and RM) independently extracted the data using a specially designed data extraction form. The third author (FB‐H) resolved any differences of opinion. Two authors entered data into Review Manager (FB‐H and RM).
Cosmetic outcome, consumer satisfaction with cosmesis, consumer pain ratings, and severity of adverse effects are all ordinal data outcomes. Where possible, we translated these outcomes into dichotomous data and reported both the original and translated results.
All other outcomes were expressed as actual or percentage differences between treatment arms.
Assessment of risk of bias in included studies
The assessment of the methodological quality of included studies included an evaluation of the following components of internal and external validity for each included study, since there is some evidence that these are associated with biased estimates of treatment effect (Juni 2001): (a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ we considered it 'adequate' if the assignment could not be foreseen; (c) who was blinded and not blinded (participants, clinicians, outcome assessors), if appropriate; (d) the number of participants lost to follow up in each treatment arm, and if the reasons for losses were adequately reported; and (e) whether all participants were analysed according to the groups to which they were initially randomised (intention‐to‐treat principle).
In addition, we assessed baseline comparability between treatment arms ‐ this included consideration of age, gender of participants at baseline, and size and site of treated lesion(s) of Bowen's disease. Where these features were significantly different, we highlighted this as high risk of bias.
Measures of treatment effect
We expressed the results as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes.
Where we could not dichotomise ordinal data, we reported the results narratively.
We translated ordinal outcomes (cosmetic outcome, consumer satisfaction with cosmesis, consumer pain ratings, and severity of adverse effects) into dichotomous data using established cut‐off points, where possible.
For individual studies that had outcome data with zero event rates, we assessed whether there was a significant treatment effect by performing a Fisher's Exact test for parallel‐group studies and McNemar’s test for within‐participant studies (using an exact two‐sided P value).
Unit of analysis issues
The main unit of analysis was the lesion because the studies did not present their findings by individual participants. We accepted that this will yield 95% CIs that do not take the clustering at participant level into account; thus, the estimated standard errors were less conservative, resulting in narrower 95% CIs. Therefore, any significant findings are likely to be credible.
We analysed internally controlled trials using appropriate methods for paired designs, and we did not pool these studies with studies of other designs. Where a trial contained multiple intervention groups, we made pair‐wise comparisons of interventions versus placebo or other interventions.
Dealing with missing data
We dealt with missing data due to participant dropout through intention‐to‐treat analysis. We analysed all trial participants according to the group to which they were assigned, and we included, where possible, all participants in the analysis irrespective of whether their outcomes were actually collected. For dichotomous outcomes, we assumed that all the 'missings' had a poor outcome.
Assessment of heterogeneity
We assessed heterogeneity or variability between studies visually, and we quantified using the I² statistic. I² statistic describes the percentage of the variability in effect estimates that is due to variability among the studies rather than chance (Higgins 2011). Where I² statistic was > 85%, we did not perform meta‐analysis.
Assessment of reporting biases
We planned to use funnel plots to alert us to the potential of publication bias, although we are aware that factors other than publication bias can cause asymmetric funnel plots, and conversely, publication bias may be present with a symmetrical funnel plot.
Data synthesis
If the included studies had sufficient homogeneity, we performed a meta‐analysis to calculate a weighted treatment effect across trials. The degree of heterogeneity determined if we used a fixed‐effect or random‐effects model. Where data were not available to perform a meta‐analysis, we summarised the data for each trial narratively.
Subgroup analysis and investigation of heterogeneity
In our protocol, we planned that if substantial heterogeneity existed (I² statistic > 50%) between studies for the primary outcome, we would explore heterogeneity by examining the effects of excluding study subgroups, e.g. those studies with lower reported methodological quality (i.e. studies that did not clearly report randomisation or blinding, and which do not have an intention‐to‐treat analysis).
We also planned to investigate potential causes of the heterogeneity, including dosage and duration of treatment, lesion characteristics (size, body site), and age groups of participants.
We planned to conduct subgroup analyses where adequate information was available, with the groups including the following: body site of lesion (particularly lower leg lesions, i.e. located below the knee), size of lesion (particularly lesions > 2 cm), age of the participants (particularly those over 70 years of age), and participants with medical comorbidities (particularly those that could affect wound healing). However, in this review, we were unable to undertake such subgroup analyses, but may be able to in future updates.
Sensitivity analysis
In our protocol, we planned to conduct sensitivity analyses to assess the robustness of the results of the review, relative to the key assumptions; however, in this review, we did not undertake any sensitivity analyses. This may be possible in future updates.
Adverse outcomes
Where data were available, we gave a RR, and where this was not available, we summarised and described the information qualitatively.
Other
The consumer in our team (JD) ensured the final review was relevant, readable, and understandable.
Results
Description of studies
Results of the search
The electronic search identified 283 references to studies. We identified six additional ongoing studies. Of the 289 records screened, we excluded 273 references based on the titles and abstracts, and we sought the full text of 16 studies. After reading the full text, we included nine studies and excluded seven studies (see Figure 1).
1.
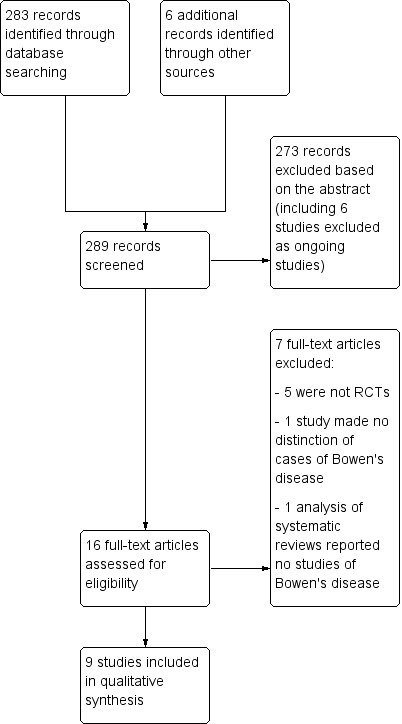
Study flow diagram
Included studies
We included a total of 9 studies, with 363 participants (132 men and 231 women), in the review, and we addressed the following comparisons:
Interventions
1. Photodynamic therapy
5‐aminolevulinic acid with photodynamic therapy (ALA‐PDT) single illumination versus ALA‐PDT two‐fold illumination (de Haas 2007; Puizina‐Ivic 2008)
5‐aminolevulinic acid with photodynamic therapy (ALA‐PDT) red light versus ALA‐PDT green light (Morton 2000)
Intravenous (IV) verteporfin with red light at 60 versus 120 versus 180 J/cm² (Lui 2004)
Methyl aminolevulinate with photodynamic therapy (MAL‐PDT) versus placebo versus cryotherapy versus 5‐FU (5‐fluorouracil) (Morton 2006)
2. Cryotherapy
Cryotherapy versus placebo versus MAL‐PDT versus 5‐FU (Morton 2006)
Cryotherapy versus ALA‐PDT (Morton 1996)
3. 5‐fluorouracil
5‐fluorouracil (5‐FU) versus placebo versus MAL‐PDT versus cryotherapy (Morton 2006)
5‐fluorouracil versus ALA‐PDT (Perrett 2007; Salim 2003)
4. Imiquimod
Imiquimod versus placebo (Patel 2006)
Design
Of the nine included RCTs, only one was a within‐participant study comparing PDT and 5‐FU in post‐transplant recipients (Perrett 2007). Two studies were placebo‐controlled; one compared imiquimod versus placebo (Patel 2006); and another compared MAL‐PDT to placebo‐PDT (Morton 2006).
All studies randomly assigned participants or comparable lesions of participants to one of the treatment groups.
Sample sizes
The number of participants evaluated in the studies varied from 8 to 225 participants. Each participant could have had up to three lesions treated.
Setting
All studies were undertaken in secondary and tertiary healthcare settings.
Five studies (Patel 2006; Perrett 2007; Morton 1996; Morton 2000; Salim 2003) were undertaken in the UK, and one (Salim 2003) was a multicentred study. One study (Lui 2004) was a multicentred phase II study based in four North American clinics; one was a single‐centred study from the Netherlands (de Haas 2007), and one was from Croatia (Puizina‐Ivic 2008). One RCT was carried out across 40 dermatology clinics in 11 European countries (Morton 2006).
Participants
Overall, there were 132 men and 231 women. The mean age of the participants was 71 years (range = 22 to 99 years). Three studies (Lui 2004; Morton 2000; Puizina‐Ivic 2008) did not provide gender distribution. One study (Puizina‐Ivic 2008) did not provide the age of the participants.
One study (Lui 2004) investigating a PDT dose escalation treatment included participants with both basal cell carcinoma and Bowen's disease. One study (Puizina‐Ivic 2008) analysed the effect of different treatment regimens with ALA‐PDT of both Bowen's disease and actinic keratoses.
One study (Perrett 2007) included only post‐transplant recipients.
Four studies (Lui 2004; Morton 2000; Puizina‐Ivic 2008; Salim 2003) did not provide lesion size at baseline. Mean lesion size at baseline was 13.6 mm² (range = 0.23 to 50 mm²).
We provide further details in the 'Characteristics of included studies' section.
Excluded studies
We excluded seven studies. On reading the full text, five (Ahmed 2000; Baron 2010; de Haas 2008; Kaminaka 2009; Mizutani 2012) were not randomised controlled trials. One study (Brown 2005) examined dysplastic skin lesions and not specifically areas of Bowen's disease. One study (Macbeth 2011) was an analysis of systematic reviews that did not report any RCTs of Bowen's disease.
Please refer to the 'Characteristics of excluded studies' tables for details.
Ongoing studies
We identified six studies as ongoing; please refer to the 'Characteristics of ongoing studies' tables for details.
Studies awaiting classification
We found no studies for this section.
Risk of bias in included studies
We used a subjective measure of quality, classifying trials as high‐, medium‐, or low‐quality based on the four main criteria (random sequence generation, concealment of allocation, blinding of outcome assessment, and handling of withdrawals and dropouts). There was no disagreement about trial quality. In general, the methodological quality of the trials was poor. We have summarised our judgements below, and these can be seen in Figure 2 and Figure 3.
2.
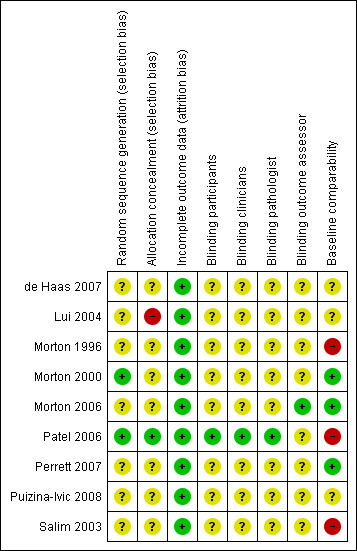
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.
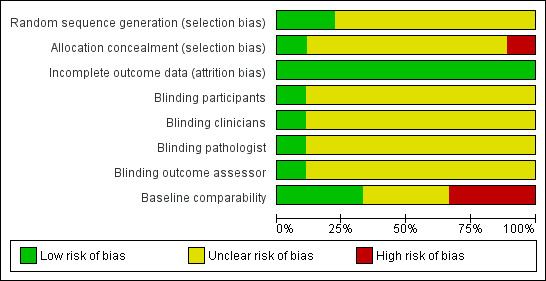
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
In seven studies, the method of generation of the randomisation sequence was either not described or unclear. In particular, one study (Puizina‐Ivic 2008) assigned participants to an intervention with no details of randomisation given, and another study (Perrett 2007) cited a textbook reference (Pocock 1983) but failed to provide a clear method for randomisation.
Two studies clearly described the randomisation: One study (Morton 2000) randomised lesions using a sealed envelope technique, and one study (Patel 2006) used an independent group prior to the start of the study to randomise participants to treatments; we judged these to be at low risk of bias.
Concealment of allocation was clear in only one study (Patel 2006), which we judged to be at low risk of bias. We judged one study (Lui 2004) to be at high risk of bias and the rest as 'unclear'.
Blinding
One study (Patel 2006) detailed blinding of participants, clinical investigators, and histopathologists, and we assessed it as at low risk of bias for these three domains. In the study by Morton 2006, there was evidence of partial blinding of clinicians and participants to MAL‐PDT and placebo‐PDT arms, but no blinding to the other arms of the study (cryotherapy and 5‐fluorouracil), and because of partial blinding, we classified this as unclear risk of bias for participants, clinicians, and pathologists.
Incomplete outcome data
Three of the studies (Morton 2006; Patel 2006; Salim 2003) provided clear flowcharts detailing completeness of data, but two studies (Lui 2004; Morton 2000) reported this in the text. There was no loss to follow up in the other four studies (de Haas 2007; Morton 1996; Perrett 2007; Puizina‐Ivic 2008). We assessed all the included studies as at low risk of bias for this domain.
Other potential sources of bias
Not all studies reported baseline comparability, and in three studies (Morton 1996; Patel 2006; Salim 2003) where baseline comparability was significantly different between treatment arms, the risk of bias was high.
Although we judged the study that only included organ transplant recipients on chronic immunosuppressive therapy (Perrett 2007) as at low risk of bias for baseline comparability, its results are of limited use to the immunocompetent population.
Effects of interventions
For our primary outcome 'Complete clearance of the lesion', we planned that ideally clearance of the lesion would be determined by histology, but clinical clearance at follow‐up was also accepted. Since a number of treatments require more than 1 cycle of therapy, we looked at 'Number of lesions or participants cleared after first treatment cycle' and 'Recurrence at 12 months'.
For our secondary outcomes, we had planned how we would assess some of these: We planned to assess cosmetic outcome using a recognised and validated instrument to measure cosmesis; we expected to assess consumer satisfaction that had been recorded on a Likert or Likert‐like scale; we planned to determine 'Time to complete healing of lesion following treatment' by clinical examination or by participant assessment through a diary or similar mechanism; we planned to determine 'quality of life' by any validated quality of life instrument; we planned to categorise adverse outcomes into four levels of severity and to determine the outcome of 'Recurrence of Bowen's disease in the same site' by clinical examination. However, we did not have the data with which to carry out these plans.
We planned to assess our prespecified outcomes in relation to any interventions for the treatment of Bowen's disease (see Types of interventions). However, we did not find RCTs that examined all of these interventions, so we have reported them below in the following order:
Photodynamic therapy
5‐aminolevulinic acid with photodynamic therapy (ALA‐PDT) single illumination versus ALA‐PDT 2‐fold illumination
5‐aminolevulinic acid with photodynamic therapy (ALA‐PDT) red light versus ALA‐PDT green light
Intravenous verteporfin red light
Methyl aminolevulinate cream with photodynamic therapy (MAL‐PDT) versus placebo cream‐PDT
Photodynamic therapy versus cryotherapy
Photodynamic therapy versus 5‐fluorouracil
Cryotherapy versus 5‐fluorouracil
Imiquimod
Photodynamic therapy (PDT)
We found seven studies. Two studies (de Haas 2007; Puizina‐Ivic 2008) reported single illumination versus two‐fold illumination. The third study (Morton 2000) reported red light versus green light, and the fourth study (Lui 2004) examined intravenous verteporfin with red light 60 J/cm² versus 120 J/cm² versus 180 J/cm². One study (Morton 2006) compared photodynamic therapy to placebo; two studies (Morton 1996; Morton 2006) compared photodynamic therapy to cryotherapy; and three studies (Morton 2006; Perrett 2007; Salim 2003) compared photodynamic therapy to 5‐FU.
ALA‐PDT single illumination versus ALA‐PDT two‐fold illumination
Two studies (de Haas 2007; Puizina‐Ivic 2008) compared ALA‐PDT single illumination to ALA‐PDT two‐fold illumination (separated by a two‐hour time interval). The methodology was different in the 2 studies, with 1 using ALA‐PDT single illumination at a dose of 75 J/cm² versus 2‐fold illumination at a dose of 20 + 80 J/cm² (de Haas 2007) and the other study (Puizina‐Ivic 2008) using ALA‐PDT single illumination at a higher dose of 100 J/cm² versus 2‐fold illumination at the equivalent dose of 50 + 50 J/cm². The primary outcome was measured by assessing residual tumour tissue using fluorescence intensity in one study, which is not an investigation that is routinely available in dermatology clinics. Histology was also used to confirm tumour failure (Puizina‐Ivic 2008).
Primary outcomes
Number of lesions that cleared after the first treatment cycle
There was no statistically significant difference in the number of lesions that achieved clearance after the first treatment cycle (RR 0.81, 95% CI 0.62 to 1.06; 2 studies: de Haas 2007; Puizina‐Ivic 2008) (Analysis 1.1).
1.1. Analysis.
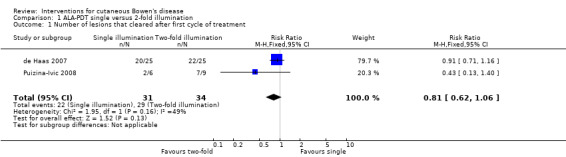
Comparison 1 ALA‐PDT single versus 2‐fold illumination, Outcome 1 Number of lesions that cleared after first cycle of treatment.
Recurrence at 12 months
There were no data for this outcome.
Secondary outcomes
Number of lesions that cleared after each treatment cycle/Number of treatment cycles needed to achieve clearance
Only one treatment cycle was given for each intervention, so we could not determine these outcomes.
Recurrence at > 12 months
There were no data for this outcome.
Cosmetic outcome
There was no statistically significant difference in good cosmetic outcome (RR 1.09, 95% CI 0.94 to 1.24; 1 study) (Analysis 1.2).
1.2. Analysis.
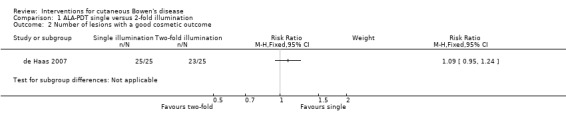
Comparison 1 ALA‐PDT single versus 2‐fold illumination, Outcome 2 Number of lesions with a good cosmetic outcome.
Consumer satisfaction with treatment modality, cosmesis, or pain at site
There was no statistically significant difference in reporting of pain during treatment (RR 0.11, 95% CI 0.01 to 1.96; 1 study) (Analysis 1.3).
1.3. Analysis.
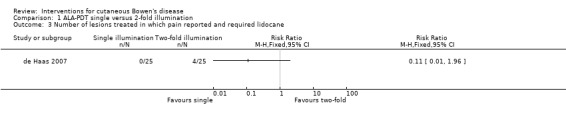
Comparison 1 ALA‐PDT single versus 2‐fold illumination, Outcome 3 Number of lesions treated in which pain reported and required lidocane.
Time to complete healing of lesion following treatment
One study (de Haas 2007) reported a three‐week maximum healing time, which was not different between intervention groups, although actual times were not provided.
Adverse outcomes
One study (de Haas 2007) provided data for adverse events and reported no serious adverse events, with the exception of a non‐statistically significant difference in reporting of pain (RR 0.11, 95% CI 0.01 to 1.96) (Analysis 1.3).
Quality of life
Recurrence of Bowen's disease in the same site
There were no data for these outcomes.
ALA‐PDT red light versus ALA‐PDT green light
One study (Morton 2000) compared ALA‐PDT red light versus ALA‐PDT green light.
Primary outcomes
Number of lesions that cleared after the first treatment cycle.
There was no significant difference in the number of lesions that cleared after the first cycle (RR 1.21, 95% CI 0.85 to 1.71; 1 study) (Analysis 2.1).
2.1. Analysis.
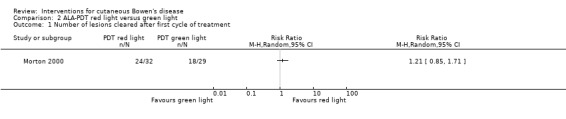
Comparison 2 ALA‐PDT red light versus green light, Outcome 1 Number of lesions cleared after first cycle of treatment.
Recurrence at 12 months
There were 74% fewer recurrences in the red light group (2/32) compared to the green light group (7/29) (RR 0.26, 95% CI 0.06 to 1.15; 1 study) (Analysis 2.2).
2.2. Analysis.
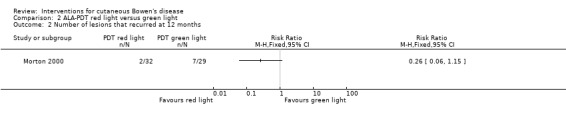
Comparison 2 ALA‐PDT red light versus green light, Outcome 2 Number of lesions that recurred at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
A significantly greater proportion of lesions cleared after the second cycle of treatment with red light compared with treatment with green light (RR 1.29, 95% CI 1.02 to 1.65; 1 study; n = 61) (Analysis 2.3).
2.3. Analysis.
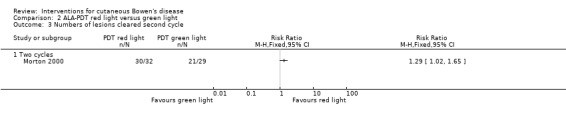
Comparison 2 ALA‐PDT red light versus green light, Outcome 3 Numbers of lesions cleared second cycle.
Number of treatment cycles needed to achieve clearance
Two treatment cycles were used in 9 participants who failed to respond to 1 treatment cycle.
Recurrence at > 12 months
There were no data available beyond 12 months.
Cosmetic outcome
No clinically‐obvious scars were present in either group at 12 months.
Adverse outcomes
There was no significant difference in the only adverse effect reported, which was perceived pain, between ALA‐PDT red light and ALA‐PDT green light treatment groups (RR 1.09, 95% CI 0.79 to 1.49; 1 study) (Analysis 2.4).
2.4. Analysis.
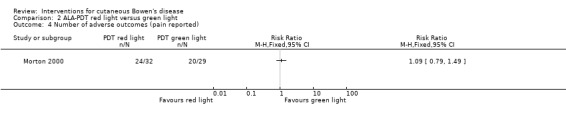
Comparison 2 ALA‐PDT red light versus green light, Outcome 4 Number of adverse outcomes (pain reported).
With regard to the other secondary outcomes, there were no data for these.
Intravenous (IV) verteporfin red light
One study (Lui 2004) compared IV verteporfin with red light at 3 different doses (60 J/cm² versus 120 J/cm² versus 180 J/cm²). In this study, only 34 of 421 participants had Bowen's disease, and these participants were included in the analysis.
Primary outcomes
Number of lesions cleared after the first treatment cycle
There was no statistically significant difference in the proportion of lesions that cleared after the first treatment cycle when IV verteporfin and red light at a dose of 60 J/cm² was compared to 120 J/cm² (RR 0.98, 95% CI 0.43 to 2.24; 1 study) (Analysis 3.1), or a dose of 60 J/cm² was compared to a dose of 180 J/cm² (RR 1.48, 95% CI 0.81 to 2.72; 1 study) (Analysis 3.2), or a dose of 120 J/cm² was compared to 180 J/cm² (RR 1.50, 95% CI 0.57 to 3.95) (Analysis 3.3).
3.1. Analysis.
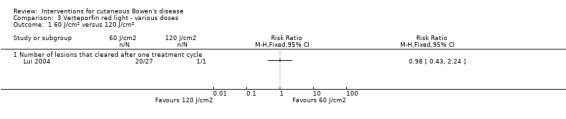
Comparison 3 Verteporfin red light ‐ various doses, Outcome 1 60 J/cm² versus 120 J/cm².
3.2. Analysis.
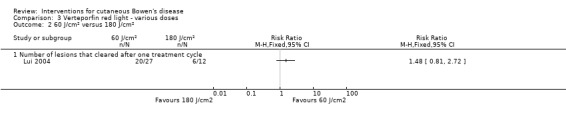
Comparison 3 Verteporfin red light ‐ various doses, Outcome 2 60 J/cm² versus 180 J/cm².
3.3. Analysis.
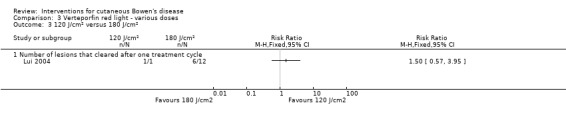
Comparison 3 Verteporfin red light ‐ various doses, Outcome 3 120 J/cm² versus 180 J/cm².
Recurrence at 12 months
Data stratified according to tumour type were not provided.
Secondary outcomes
Number of lesions that cleared after each treatment cycle/Number of treatment cycles needed to achieve clearance
A second cycle of treatment was given in some cases although these data were not given.
No data were given for the other secondary outcomes: recurrence at > 12 months; cosmetic outcome; consumer satisfaction with treatment modality, cosmesis, or pain; time to complete healing of lesion following treatment; quality of life, or recurrence of Bowen's disease in the same site. No separate data on adverse outcomes was provided for lesions of Bowen's disease.
MAL‐PDT versus placebo
One study (Morton 2006) compared MAL‐PDT (methyl aminolevulinate cream with photodynamic therapy) versus placebo cream‐PDT.
Primary outcomes
Number of lesions that cleared after the first treatment cycle
There were a statistically significantly greater proportion of lesions cleared with MAL‐PDT compared to placebo (RR 1.68, 95% CI 1.12 to 2.52; 1 study, n = 148) (Analysis 4.1).
4.1. Analysis.
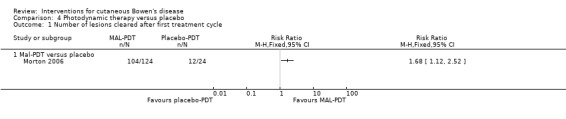
Comparison 4 Photodynamic therapy versus placebo, Outcome 1 Number of lesions cleared after first treatment cycle.
Recurrence at 12 months
There were statistically significantly fewer recurrences of lesions in the MAL‐PDT group compared with placebo (RR 0.29, 95% CI 0.10 to 0.86; 1 study, n = 107) (Analysis 4.2).
4.2. Analysis.
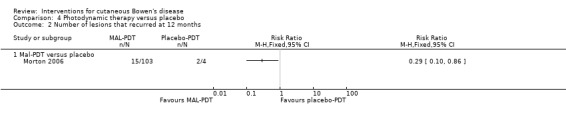
Comparison 4 Photodynamic therapy versus placebo, Outcome 2 Number of lesions that recurred at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
There was no statistically significant difference in the number of lesions cleared after 2 treatment cycles (RR 1.00, 95% CI 0.94 to 1.06) (Analysis 4.3).
4.3. Analysis.
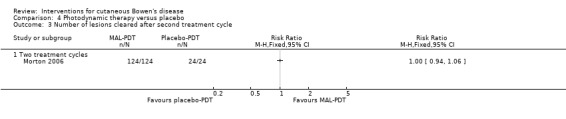
Comparison 4 Photodynamic therapy versus placebo, Outcome 3 Number of lesions cleared after second treatment cycle.
Number of treatment cycles needed to achieve clearance
In all cases, two treatment cycles were used to achieve clearance.
Consumer satisfaction with treatment modality, cosmesis, or pain at site
There was no significant difference in the number of participants reporting pain (RR 0.82, 95% CI 0.37 to 1.85; 1 study) (Analysis 4.4).
4.4. Analysis.
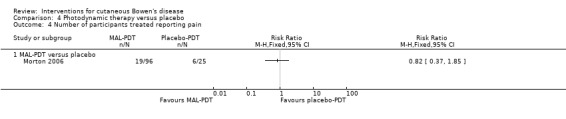
Comparison 4 Photodynamic therapy versus placebo, Outcome 4 Number of participants treated reporting pain.
Adverse events
There was no significant difference in the number of participants reporting more than 1 adverse event between the MAL‐PDT and placebo‐PDT groups (RR 1.06, 95% CI 0.69 to 1.63; 1 study) (Analysis 4.5).
4.5. Analysis.
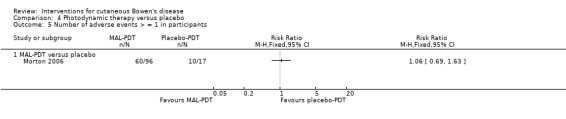
Comparison 4 Photodynamic therapy versus placebo, Outcome 5 Number of adverse events > = 1 in participants.
There were three serious adverse events in the MAL‐PDT group, but these were deemed unrelated to the intervention. Overall, 48/96 participants treated with MAL‐PDT reported greater than 1 of the following local adverse events: pain (19/96), erythema (8/96), burning sensation (16/96), crusting (8/96), stinging (9/96), irritation (3/96), itching (1/96), oedema (2/96), hyperpigmentation (3/96), or warmth (3/96) (Morton 2006).
With regard to the other secondary outcomes, no data were given for these.
Photodynamic therapy versus cryotherapy
Two studies compared PDT to cryotherapy: MAL‐PDT (Morton 2006) and ALA‐PDT (Morton 1996).
Primary outcomes
Number of lesions that cleared after the first treatment cycle
A significantly greater proportion of lesions cleared with MAL‐PDT compared to cryotherapy (RR 1.17, 95% CI 1.01 to 1.37; n = 215; Morton 2006) (Analysis 5.1). There was no significant difference in the number of lesions that cleared when ALA‐PDT was compared to cryotherapy (RR 1.50, 95% CI 0.90 to 2.49; Morton 1996) (Analysis 5.1).
5.1. Analysis.
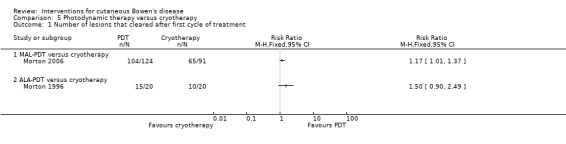
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 1 Number of lesions that cleared after first cycle of treatment.
Recurrence at 12 months
There was no statistically significant difference in recurrence at 12 months for either MAL‐PDT versus cryotherapy (RR 0.71, 95% CI 0.37 to 1.36) (Analysis 5.2) or ALA‐PDT versus cryotherapy (0/20 in the first group and 2/20 in the second group (Fisher's Exact P value = 0.49)).
5.2. Analysis.
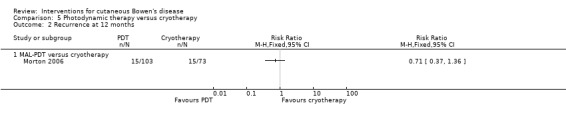
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 2 Recurrence at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
There was no statistically significant difference in the number of lesions that cleared in the ALA‐PDT group compared to cryotherapy after the second treatment cycle (RR 1.24, 95% CI 0.98 to 1.57; Morton 1996) (Analysis 5.3).
5.3. Analysis.
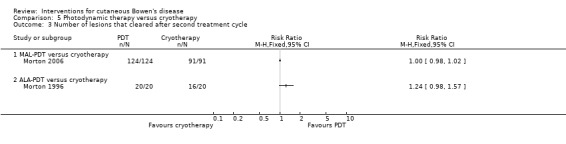
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 3 Number of lesions that cleared after second treatment cycle.
There was no statistically significant difference in the number of lesions that cleared in the MAL‐PDT group compared to cryotherapy (RR 1.00, 95% CI 0.98 to 1.02; Morton 2006) (Analysis 5.3).
Cosmetic outcome
At a final review at 12 months following clearance, a visible scar in the treatment field was observed in 4 lesions treated by cryotherapy, while visible scarring was absent in all lesions treated by ALA‐PDT (Morton 1996).
Cosmetic appearance at 12 months was statistically significantly better in the MAL‐PDT group compared to cryotherapy (RR 1.59, 95% CI 1.30 to 1.93; n = 147; Morton 2006) (Analysis 5.4).
5.4. Analysis.
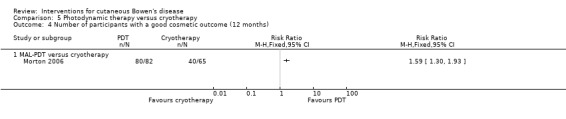
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 4 Number of participants with a good cosmetic outcome (12 months).
Adverse outcomes
Statistically significantly fewer participants reported pain in the ALA‐PDT group compared to cryotherapy (RR 0.58, 95% CI 0.38 to 0.87, n = 40; Morton 1996) (Analysis 5.5). There was no significant difference in reported pain when MAL‐PDT was compared to cryotherapy (RR 0.81, 95% CI 0.47 to 1.41; Morton 2006) (Analysis 5.5).
5.5. Analysis.
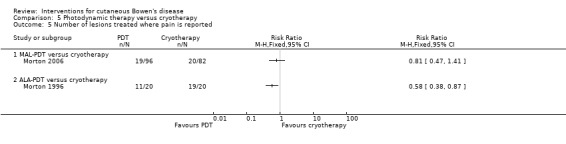
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 5 Number of lesions treated where pain is reported.
There was no statistically significant difference in the number of adverse events (> = 1) when MAL‐PDT was compared to cryotherapy (RR 1.07, 95% CI 0.84 to 1.36; Morton 2006) (Analysis 5.6).
5.6. Analysis.
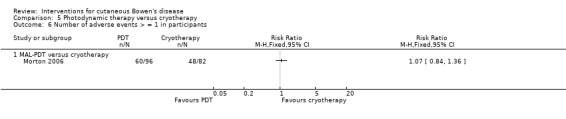
Comparison 5 Photodynamic therapy versus cryotherapy, Outcome 6 Number of adverse events > = 1 in participants.
Morton 1996 reported ulceration at the site of cryotherapy in 5/20 lesions, with 2/5 lesions subsequently requiring treatment with systemic antibiotics.
With regard to the other secondary outcomes, no data were given for these.
Photodynamic therapy versus 5‐fluorouracil
Three studies (Morton 2006; Perrett 2007; Salim 2003) compared PDT versus 5‐FU (5‐fluorouracil). One study (Perrett 2007) was an intrapatient comparison undertaken in organ‐transplant recipients, which compared MAL‐PDT (75 J/cm²) to 5‐FU. One study (Morton 2006) compared MAL‐PDT (dose 75 J/cm² given x 2 treatment cycles) to 5‐FU. One study (Salim 2003) compared ALA‐PDT (100 J/cm²) to 5‐FU.
Primary outcomes
Number of lesions that cleared after the first treatment cycle
There was no statistically significant difference in number of lesions that cleared when MAL‐PDT was compared to 5‐FU (RR 1.16, 95% CI 0.93 to 1.44; Morton 2006) (Analysis 6.1).
6.1. Analysis.
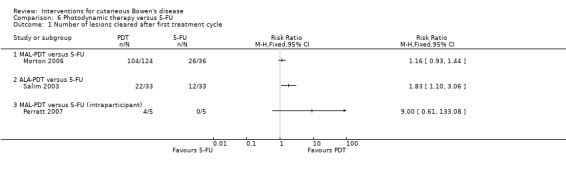
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 1 Number of lesions cleared after first treatment cycle.
When ALA‐PDT was compared to 5‐FU, a significantly greater proportion of lesions were cleared in the ALA‐PDT group compared to treatment with 5‐FU (RR 1.83, 95% CI 1.10 to 3.06; n = 66; Salim 2003) (Analysis 6.1).
The study that included an immunosuppressed population found no significant difference in the number of lesions that cleared when MAL‐PDT was compared with 5‐FU (McNemar's test ‐ P value = 0.125 (2‐sided exact P value)) (RR 9.00, 95% CI 0.61 to 133.08; Perrett 2007) (Analysis 6.1).
Recurrence at > 12 months
There were no statistically significant differences in recurrence at 12 months or greater when either MAL‐PDT (RR 1.09, 95% CI 0.39 to 3.08; Morton 2006) or ALA‐PDT (RR 0.33, 95% CI 0.07 to 1.53; Salim 2003) was compared to 5‐FU (Analysis 6.2).
6.2. Analysis.
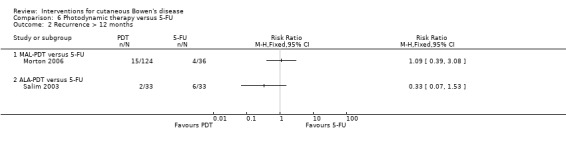
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 2 Recurrence > 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
All lesions were cleared after the second treatment cycle in both treatment groups when MAL‐PDT was compared to 5‐FU (RR 1.00, 95% CI 0.96 to 1.04; Morton 2006) (Analysis 6.3).
6.3. Analysis.
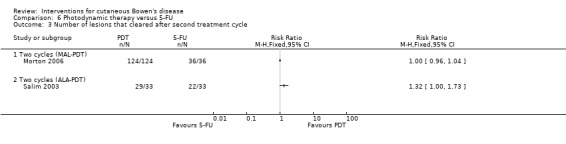
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 3 Number of lesions that cleared after second treatment cycle.
After the second treatment cycle when ALA‐PDT was compared to 5‐FU, statistically significantly more lesions cleared in the ALA‐PDT group compared to 5‐FU (RR 1.32, 95% CI 1.0 to 1.73; n = 66; Salim 2003) (Analysis 6.3).
Cosmetic outcome
Cosmetic outcome was statistically significantly better in the MAL‐PDT group compared to 5‐FU (RR 0.26, 95% CI 0.08 to 0.80; n = 103; Morton 2006) (Analysis 6.4). For the intrapatient study (Perrett 2007), there was no statistically significant difference in cosmetic outcome when MAL‐PDT was compared to 5‐FU (0/9 in the first group and 4/9 in the second group (Fisher's Exact P value = 0.08)).
6.4. Analysis.
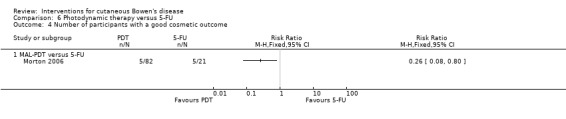
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 4 Number of participants with a good cosmetic outcome.
In one study (Salim 2003), 3/33 lesions treated with 5‐FU became ulcerated resulting in prominent scarring, with 0/33 lesions treated with ALA‐PDT demonstrating clinically obvious scarring at 12 months.
Consumer satisfaction with treatment modality, cosmesis, or pain at site
There was no statistically significant difference in pain reported by participants treated with either MAL‐PDT compared to 5‐FU (RR 0.59, 95% CI 0.31 to 1.13; Morton 2006) or ALA‐PDT compared to 5‐FU (RR 1.11, 95% CI 0.71 to 1.73) (Analysis 6.5).
6.5. Analysis.
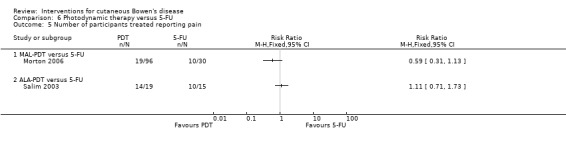
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 5 Number of participants treated reporting pain.
Adverse outcomes
There was no statistically significant difference in adverse events reported in a study comparing MAL‐PDT versus 5‐FU (RR 0.82, 95% CI 0.63 to 1.05; Morton 2006) (Analysis 6.6). The most frequently reported treatment‐related local adverse events for 5‐FU were pain (10/30), erythema (10/30), burning sensation (2/30), crusting (4/30), stinging (2/30), application site reaction (1/30), irritation (4/30), itching (5/30), and hyperpigmentation (1/30).
6.6. Analysis.
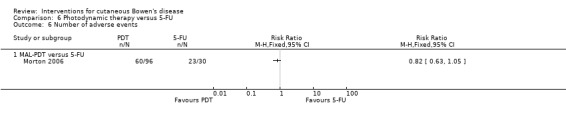
Comparison 6 Photodynamic therapy versus 5‐FU, Outcome 6 Number of adverse events.
There were statistically significantly fewer adverse events in the ALA‐PDT group as compared to 5‐FU (0/33 in the first group and 12/33 in the second group (Fisher's Exact P value < 0.001)) (Salim 2003).
Three participants (five lesions) who were treated with 5‐FU developed 'widespread dermatitic reactions' over the entire treated limbs and withdrew from the study (Salim 2003). One participant (two lesions) developed a similar reaction but completed therapy. Three lesions ulcerated; two lesions developed into painful erosions on completion of the treatment cycle. Ulcerated lesions healed with prominent scarring (Salim 2003).
In the intrapatient study (Perrett 2007), all participants experienced crusting of the treatment area following treatment with MAL‐PDT; 3/8 participants experienced pruritus; and 1/8 developed postinflammatory hyperpigmentation. Reported local reactions with 5‐FU included superficial erosions, crusting, and pruritus. This study did have a mixed population of people with actinic keratoses and Bowen's disease, and adverse events were not stratified according to skin lesion.
With regard to the other secondary outcomes, there were no data for these.
Cryotherapy versus 5‐fluorouracil
One study (Morton 2006) compared cryotherapy to 5‐FU.
Primary outcomes
Number of lesions that cleared after first treatment cycle
There was no statistical significant difference in the number of lesions that cleared after the first treatment cycle (RR 0.99, 95% CI 0.78 to 1.26) (Analysis 7.1).
7.1. Analysis.
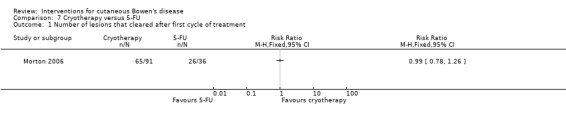
Comparison 7 Cryotherapy versus 5‐FU, Outcome 1 Number of lesions that cleared after first cycle of treatment.
Recurrence at 12 months
There was no statistical significant difference in recurrence at 12 months (RR 1.48, 95% CI 0.53 to 4.17) (Analysis 7.2).
7.2. Analysis.
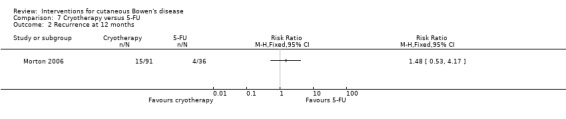
Comparison 7 Cryotherapy versus 5‐FU, Outcome 2 Recurrence at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
There was no statistically significant difference in the number of lesions that cleared after the second treatment cycle when cryotherapy was compared to 5‐FU (RR 1.00, 95% CI 0.96 to 1.04) (Analysis 7.3).
7.3. Analysis.
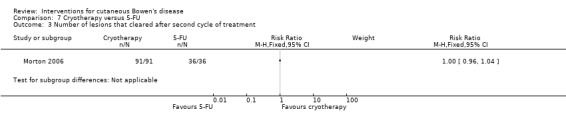
Comparison 7 Cryotherapy versus 5‐FU, Outcome 3 Number of lesions that cleared after second cycle of treatment.
Number of treatment cycles needed to achieve clearance
Thirty‐six participants needed 2 treatment cycles to achieve clearance.
Cosmetic outcome
There was no statistically significant difference in cosmetic outcome (RR 0.87, 95% CI 0.65 to 1.17) (Analysis 7.4).
7.4. Analysis.
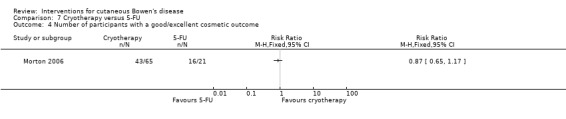
Comparison 7 Cryotherapy versus 5‐FU, Outcome 4 Number of participants with a good/excellent cosmetic outcome.
Consumer satisfaction with treatment modality, cosmesis, or pain at site
The cryotherapy group compared to 5‐FU experienced no statistically significant difference in pain (RR 0.73, 95% CI 0.39 to 1.38) (Analysis 7.5).
7.5. Analysis.
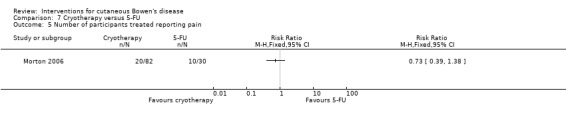
Comparison 7 Cryotherapy versus 5‐FU, Outcome 5 Number of participants treated reporting pain.
Adverse outcome
The cryotherapy group compared to 5‐FU experienced statistically significantly fewer adverse events (RR 0.64, 95% CI 0.47 to 0.86; n = 112) (Analysis 7.6).
7.6. Analysis.
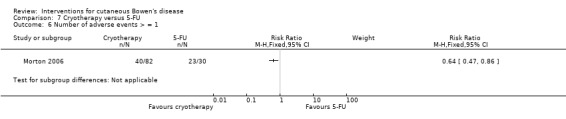
Comparison 7 Cryotherapy versus 5‐FU, Outcome 6 Number of adverse events > = 1.
With regard to the other secondary outcomes, there were no data given for these.
Imiquimod
One study (Patel 2006) compared imiquimod cream to placebo. This study included pre‐ and post‐treatment biopsies of lesions.
Primary outcomes
Number of lesions that cleared after the first treatment cycle
Statistically significantly more lesions cleared in the imiquimod group (9/15 lesions) compared to placebo (0/16) (Fisher's Exact P value < 0.001).
Recurrence at 12 months
The imiquimod group reported no recurrences at 12 months.
Secondary outcomes
Number of lesions that cleared after each treatment cycle
See the results for our primary outcome after one treatment cycle.
Number of treatment cycles needed to achieve clearance
Only one treatment cycle was given, so we could not determine this.
Recurrence at > 12 months
Follow‐up at 72 weeks revealed no recurrence in the imiquimod‐treated group. At 72 weeks, 2/16 participants in the placebo‐group had developed early invasive SCC. No statistical analysis was provided.
Cosmetic outcome
Clinical resolution was reported to be 'often' associated with faint residual blanching erythema or postinflammatory hyperpigmentation, although actual numbers were not provided.
Adverse outcomes
Almost all the participants treated with imiquimod developed a localised inflammatory reaction. Overall, 19 participants out of 31 experienced transient itching, oedema, or weeping. Four participants experienced adverse events related to the treatment, and 17 were deemed unrelated to the treatment.
Histological assessment at the end of the trial revealed progression to early invasive squamous cell carcinoma in 2/16 participants in the placebo group.
With regard to the other secondary outcomes, there were no data for these.
Discussion
Summary of main results
In summary, of our 9 included studies, which included 363 participants, the interventions included photodynamic therapy, cryotherapy, 5‐fluorouracil, and imiquimod.
Photodynamic therapy (PDT)
There was no statistically significant difference in clearance of lesions between ALA‐PDT (5‐aminolevulinic acid with photodynamic therapy) red light with ALA‐PDT green light, but there were significantly fewer recurrences at 12 months in those lesions treated with red light. There appears to be no superiority of ALA‐PDT two‐fold illumination versus single illumination (de Haas 2007; Puizina‐Ivic 2008), nor between increased doses of red light with intravenous verteporfin (Lui 2004). There were no RCTs directly comparing treatment with ALA‐PDT versus MAL‐PDT (methyl aminolevulinate with photodynamic therapy). MAL‐PDT was significantly better at clearing lesions (103/111 lesions) compared with placebo‐PDT (12/24 lesions). Although participant tolerability was greater and cosmetic outcomes were considered significantly better in both MAL‐PDT and ALA‐PDT‐treated groups compared with cryotherapy, only MAL‐PDT appears to be significantly more effective in clearing the lesions of Bowen's disease (103/111 versus 73/85 lesions treated with cryotherapy) (Morton 2006). In both cases, freeze‐thaw cycles of 20 seconds were used.
There was no difference in efficacy between MAL‐PDT and 5‐fluorouracil in the treatment of Bowen's disease in either immunocompetent (Morton 2006) or immunosuppressed (Perrett 2007) individuals. However, ALA‐PDT demonstrated significantly greater efficacy compared with 5‐fluorouracil (Salim 2003 ), but there was no difference in recurrence rates at 12 months with either MAL‐PDT or ALA‐PDT when compared with 5‐fluorouracil. Longer‐term follow‐up studies are required.
Cryotherapy
There was no difference in efficacy, recurrence rates, or cosmetic outcome between cryotherapy and 5‐fluorouracil (Morton 2006).
5‐fluorouracil
5‐fluorouracil was as effective as PDT, but had significantly more adverse reactions than ALA‐PDT (Salim 2003) and caused significantly more pain than cryotherapy (Morton 2006).
Imiquimod
Imiquimod was superior to placebo in clearing the lesions of Bowen's disease (Patel 2006). Histological examination post‐treatment demonstrated no recurrences at 72 weeks, but early invasive SCC was reported in the placebo‐treated group. Transient localised inflammatory reactions were commonly reported. There were no studies comparing imiquimod to other treatments.
Overall completeness and applicability of evidence
In this review, we attempted to address the following:
(1) What are the most effective treatments for Bowen's disease, with the fewest side‐effects?
Specifically, the lack of quality data limited this systematic review, such that we only included nine randomised controlled studies, and seven of those involved photodynamic therapy (PDT). Therefore, the majority of evidence available for the treatment of Bowen's disease is PDT. However, surgery is the most common treatment for Bowen's, and topical treatments such as 5‐fluorouracil and imiquimod creams are more widely available, more frequently used, and cheaper than PDT. Moreover, there is considerable variation in the efficacy of PDT, which is operator‐dependent. There is clearly better evidence in terms of RCTs for the use of PDT in Bowen's disease, but the lack of evidence for topical creams and surgery does not necessarily equate to a lack of efficacy. The bias towards PDT rather limits the usefulness of this review for a general practitioner or dermatologist working in a clinic without PDT resources. The efficacy of cryotherapy is also operator‐dependent, and in both studies using cryotherapy, a 20‐second freeze‐thaw cycle was used. Variation in clinical practice exists, and this was not studied.
(2) How do the various therapies compare in the following participant subgroups:
participants with lower leg lesions (i.e. located below the knee)?
participants with lesions > 2 cm²?
participants with medical comorbidities leading to poor wounding healing, age greater than 70 years, or both?
Data for specific participant subgroups were not available from these studies. Only one study (Morton 2000) reported lesions exclusively located on the lower leg comparing red and green light with ALA‐PDT, and one study (Salim 2003) treated lesions on the legs only with PDT, but all sites were included in the 5‐fluorouracil‐treated arm.
Retrospective analysis of lesions that were successfully treated with imiquimod found no difference in age, sex, lesion size, duration, lesion symptoms, lesion characteristics, or occurrence of adverse events between the nine participants who responded compared with those who did not, using Fishers exact test (Patel 2006). The small numbers in this study and the posthoc analysis is not able to provide any information for clinicians.
None of the other studies assessed the impact of the site of Bowen's disease on response to treatment.
None of the studies provided a subgroup analysis of lesions exceeding 2 cm², nor did any studies specifically report participants with medical comorbidities leading to poor wound healing, or those with an age greater than 70 years.
Nevertheless, the review provides reassurance to clinicians that the treatments used have an evidence base, and hence options can be selected in a clinically relevant manner. Guidance is now available on which studies should be undertaken to generate the evidence needed.
Quality of the evidence
The quality of the evidence is limited in terms of the number of trials, number of participants in trials, and the treatment comparisons available. Available treatments, including photodynamic therapy, cryotherapy, 5‐fluorouracil, and imiquimod, appear to be effective, but firm conclusions about comparative effectiveness cannot be drawn. Because of the presentation of the findings of the studies, with variable lesions treated per participant, we had to expand our primary outcome to include the number of lesions. Therefore, because of the methods available in Review Manager, we were unable to take into account any clustering effects by participants even if reported, and thus the precision of the measures of effect are likely to be less conservative.
Potential biases in the review process
We identified no potential biases in relation to the review process.
Agreements and disagreements with other studies or reviews
This is the only systematic review available on this topic.
Authors' conclusions
Implications for practice.
There is only limited quality data available to guide clinical practice. Clinicians need to carefully consider the needs of individuals, characteristics, sites of the lesions, and any comorbidities. Some people may not require any treatment at al; others will benefit from either surgical excision, other destructive treatments, cryotherapy, or topical treatments such as 5‐fluorouracil or photodynamic therapy. Photodynamic therapy appears to be an effective and safe non‐scarring treatment, which should be considered if available. The limited data suggest that 5‐fluorouracil is as effective as PDT, that cryotherapy is possibly less effective than PDT, and that imiquimod is also effective, but has not been compared with PDT. Cost, likely adverse events, and patient preference will all play a part in the choice of treatment.
Implications for research.
The lack of good‐quality research on common treatments for Bowen's disease has influenced this review towards PDT studies. There is a clear need for a range of different studies comparing various therapies with each other and potentially with placebo, in order to provide high‐quality evidence to guide clinical practice. In particular, studies comparing interventions to surgical treatments are lacking, for example, quality RCTs comparing surgery with topical treatments, topical treatments with each other, e.g. 5‐fluorouracil with imiquimod, and topical treatments versus PDT, e.g. imiquimod with PDT.
Although there is evidence that imiquimod is an effective treatment, studies comparing this intervention to other standard therapies are needed. With these data, we have been unable to stratify treatments according to lesion size, site, or number because of the small numbers of participants. Larger studies are therefore required to better provide guidance to clinicians. Finally, one study reported increased development of squamous cell carcinoma (SCC) in the placebo‐treated group suggesting that treatment may reduce the risk of progression from Bowen's disease. None of the other studies attempted to assess impact of the intervention on progression to SCC, and future studies should consider this as a clinically important outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2016 | Review declared as stable | A search of MEDLINE, PubMed, and Embase in October 2016 found no further studies other than the four studies identified last year, which our Co‐ordinating Editor and authors deemed small and not consequential. Thus, this review has been marked stable because an update has not been considered necessary for three successive years. Our Information Specialist will run a new search in October 2017 to re‐assess whether an update is needed. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 29 September 2015 | Review declared as stable | A search of MEDLINE, PubMed, and Embase in September 2015 found only four studies, which our Co‐ordinating Editor and authors deemed small and not consequential. Thus, this review has been marked stable because an update has not been considered necessary for two successive years. Our Trials Search Co‐ordinator will run a new search in September 2016 to re‐assess whether an update is needed. |
| 27 September 2014 | Amended | A search of MEDLINE, PubMed, and Embase in September 2014 found only 2 small studies, which provide incremental knowledge about variations in photodynamic therapy. Thus, an update has not been considered necessary at this time. Our Trials Search Co‐ordinator will run a new search in 2015 to re‐assess whether an update is needed. |
Notes
A search of MEDLINE, PubMed, and Embase in October 2016 found no further studies other than the four studies identified last year, which our Co‐ordinating Editor and authors deemed small and not consequential. Thus, this review has been marked stable because an update has not been considered necessary for three successive years. Our Information Specialist will run a new search in October 2017 to re‐assess whether an update is needed.
Acknowledgements
We would like to acknowledge our debt to Seaver Soon, Tracy Bialy, Calvin McCall, Robyn Whyte, Aditya Gupta, and Suephy Chen who started a protocol for a systematic review of interventions for Bowen's disease, but were unable to proceed with the review. Since publication of the protocol, Fiona Bath‐Hextall has remained as lead reviewer. Jo Leonardi‐Bee and David Wilkinson have remained as co‐reviewers, and Rubeta Matin has joined as a co‐reviewer. Jim Delitt agreed to be the consumer for the review under the new team.
The Cochrane Skin Group editorial base wishes to thank Dedee Murrell who was the Key Editor for this review; Matthew Grainge and Ching‐Chi Chi who were the Statistical and Methods Editors, respectively; the clinical referees, Charlotte Proby and Catherine Harwood; and the consumer referee, Colette O'Sullivan.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 (bowen* disease):ti,ab,kw #2 MeSH descriptor Bowen's Disease explode all trees #3 (bowenoid papulosis) #4 morbus bowen #5 (squamous cell carcinoma):ti,ab,kw #6 (in situ squamous cell carcinoma) #7 (intraepidermal squamous cell carcinoma) #8 MeSH descriptor Carcinoma, Squamous Cell explode all trees #9 (skin) #10 (#5 AND #9) #11 (#8 AND #9) #12 (#1 OR #2 OR #3 OR #4 OR #6 OR #7 OR #10 OR #11)
Appendix 2. MEDLINE (OVID) search strategy
1. Bowen$ disease.mp. or exp Bowen's Disease/ 2. bowenoid papulosis.mp. 3. morbus bowen.mp. 4. exp Carcinoma, Squamous Cell/ or in situ squamous cell carcinoma.mp. 5. intraepidermal squamous cell carcinoma.mp. 6. in situ squamous cell carcinoma.mp. 7. exp Skin/ 8. 4 and 7 9. 1 or 2 or 3 or 5 or 6 or 8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. clinical trials as topic.sh. 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp animals/ not humans.sh. 19. 17 not 18 20. 9 and 19
Appendix 3. EMBASE (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. crossover$.mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. assign$.mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. Bowen$ disease.mp. or exp Bowen Disease/ 15. bowenoid papulosis.mp. or exp Bowenoid Papulosis/ 16. morbus bowen.mp. 17. in situ squamous cell carcinoma.mp. 18. intraepidermal squamous cell carcinoma.mp. 19. squamous cell carcinoma.mp. or exp Squamous Cell Carcinoma/ 20. exp SKIN/ 21. 19 and 20 22. 14 or 15 or 16 or 17 or 18 or 21 23. 13 and 22
Appendix 4. LILACS (OVID) search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Palavras] and (bowen$ and disease) or (enfermedad and bowen) or (papulosis and bowenoide) or (morbus and bowen) or ((squamous and cell and carcinoma) and skin) or ((epitelioma and espinocelular) and piel) [Palavras]
Data and analyses
Comparison 1. ALA‐PDT single versus 2‐fold illumination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions that cleared after first cycle of treatment | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.06] |
| 2 Number of lesions with a good cosmetic outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Number of lesions treated in which pain reported and required lidocane | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. ALA‐PDT red light versus green light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions cleared after first cycle of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Number of lesions that recurred at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Numbers of lesions cleared second cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Two cycles | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of adverse outcomes (pain reported) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Verteporfin red light ‐ various doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 60 J/cm² versus 120 J/cm² | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Number of lesions that cleared after one treatment cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 60 J/cm² versus 180 J/cm² | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Number of lesions that cleared after one treatment cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 120 J/cm² versus 180 J/cm² | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number of lesions that cleared after one treatment cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Photodynamic therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions cleared after first treatment cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mal‐PDT versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of lesions that recurred at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mal‐PDT versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of lesions cleared after second treatment cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Two treatment cycles | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants treated reporting pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 MAL‐PDT versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of adverse events > = 1 in participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 MAL‐PDT versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Photodynamic therapy versus cryotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions that cleared after first cycle of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ALA‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recurrence at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of lesions that cleared after second treatment cycle | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 ALA‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with a good cosmetic outcome (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of lesions treated where pain is reported | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 ALA‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse events > = 1 in participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 MAL‐PDT versus cryotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Photodynamic therapy versus 5‐FU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions cleared after first treatment cycle | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 MAL‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ALA‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 MAL‐PDT versus 5‐FU (intraparticipant) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recurrence > 12 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 MAL‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ALA‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of lesions that cleared after second treatment cycle | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Two cycles (MAL‐PDT) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Two cycles (ALA‐PDT) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with a good cosmetic outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 MAL‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants treated reporting pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 MAL‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 ALA‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 MAL‐PDT versus 5‐FU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Cryotherapy versus 5‐FU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions that cleared after first cycle of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of lesions that cleared after second cycle of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Number of participants with a good/excellent cosmetic outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Number of participants treated reporting pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Number of adverse events > = 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Haas 2007.
| Methods | D: This was a single‐centre, randomised, comparative study. The study sequentially recruited participants attending the department RS: Patches of Bowen's disease were randomised, but the paper provided no method of randomisation AC: This was unclear B: This was unclear |
|
| Participants | Netherlands: 40 participants (17 men and 23 women with 50 biopsy‐proven lesions of Bowen's disease) Mean treated lesion diameter = 14.5 mm (range = 5 to 40 mm) Mean age = 74 years (49 to 91 years) |
|
| Interventions | For both groups, surface scales and rusts were removed before the application of topical aminolevulinic acid (ALA). Topical ALA was left in place for 4 hours, with a margin of 1 cm
|
|
| Outcomes | The lesion was the unit of analysis ‐ all sites included
|
|
| Notes | Each illumination was delivered at 50 mWcm‐². A diode laser and light‐emitting diode provided illumination at a wavelength of 630 nm Intervention product information/details
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not describe the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | The paper gave no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no mention of a pathologist |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | Unclear risk | The paper gave no details about baseline characteristics of lesions |
Lui 2004.
| Methods | D: This was an open‐label, randomised phase II multicentred study RD: The study randomised participants, although it did not provide details of the randomisation process. Randomisation was stratified by number of tumours and by centre AC: This was unclear B: There was no evidence of blinding in the study |
|
| Participants | North America: 54 participants (421 multiple non‐melanoma skin cancers, including superficial and nodular basal cell carcinoma, and 34 Bowen's disease); 4 North American university‐based clinics. The study recruited participants with at least 2 non‐pigmented biopsy‐proven non‐melanoma skin cancers Mean age = 55 years (22 to 79 years) Most had Fitzpatrick skin type II or III Inclusion criteria of the trial
|
|
| Interventions | Single intravenous infusion of 14 mg m‐² verteporfin followed 1 to 3 hours later by exposure to 1 of 3 different light doses from a non‐thermal LED panel:
The tumours were re‐treated 3 months after initial treatment if complete response was not achieved ‐ the re‐treatment dose increased to 18 mg m‐², but the dose remained the same |
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes |
Intervention product information/details
The exposed area included a 3 to 4 mm peritumoural margin Adverse events and cosmetic outcome was reported for all tumours with no stratification according to tumour type Follow‐up visits after month 6 were optional, and 2‐year follow‐up data were only available for 66% (276/421) of tumours (breakdown according to tumour subtype was not provided). 7 participants (51 lesions) withdrew from the study prior to 6 months (2 were lost to follow up; 4 withdrew for unspecified reasons; and 1 requested withdrawal owing to inconvenience of travel and treatment site pain requiring the use of codeine) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not detail the randomisation process |
| Allocation concealment (selection bias) | High risk | The paper gave no details, but it was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper provided details of loss to follow up: 7 participants (51 tumours ‐ number of Bowen's disease not specified) withdrew from the study prior to month 6; 2 were lost to follow up, 4 withdrew for unspecified reasons, and 1 requested withdrawal for inconvenience |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | Unclear risk | There were more participants with SCC in situ in the lower light‐dose treatment arm |
Morton 1996.
| Methods | D: This was a single‐centre, randomised, comparative study RS: This was unclear AC: This was unclear B: This was unclear |
|
| Participants | UK: 19 participants (40 lesions of Bowen's disease in 3 men and 16 women) Lesions were randomised to receive T1 (n = 20) or T2 (n = 20) Baseline characteristics: The lesions treated by PDT were overall larger (median size = 150 mm², range = 25 to 441 mm²) compared with those treated with cryotherapy (median size = 82 mm², range = 30 to 360 mm²) Mean age = 76 years (62 to 88 years) Inclusion criteria of the trial
|
|
| Interventions |
Participants were reviewed at 2‐monthly intervals. Treatment was repeated if required |
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes |
Intervention product information/details
Lesions treated in T1 were overall larger than in T2 group. The larger lesions also required more than 1 treatment cycle There was a 3 mm punch biopsy in lesions where doubt existed over clinical clearance or recurrence Analysis = intention‐to‐treat |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper gave no detail |
| Allocation concealment (selection bias) | Unclear risk | The paper did not provide details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | High risk | There were larger lesions in the PDT‐treated arm |
Morton 2000.
| Methods | D: This was a single‐centre, randomised comparison study RS: The study randomised individual lesions via the sealed envelope technique AC: This was unclear B: This was unclear |
|
| Participants | UK: 16 participants (61 lesions of Bowen's disease) The study randomised 70 lesions; all of the included lesions were on the lower limbs 1 participant (3 lesions) was lost to follow up, and 2 participants (with 6 lesions) died from chronic unrelated disease during the review period All lesions were biopsy‐proven < 21 mm diameter, previously untreated, and the number of lesions per participant was between 1 to 6 |
|
| Interventions |
Surface crusts were removed and the surface abraded before the application of ALA‐PDT (5‐aminolevulinic acid with photodynamic therapy). Topical ALA‐PDT was applied to lesions 4 hours before illumination. The cream was kept in place under occlusive dressing. Approximately 50 mg/cm² was applied to cover the entire field of illumination, including a clinically disease‐free margin of at least 4 mm |
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes |
Intervention product information/details
The analysis was not intention‐to‐treat |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They randomised lesions using a sealed envelope technique |
| Allocation concealment (selection bias) | Unclear risk | The paper did not specify the use of an opaque envelope |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper provided details of loss to follow up: 3 individuals (detailed above) were lost to follow up (9 lesions) |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | Low risk | Lesions were comparable at baseline; all were located on the legs |
Morton 2006.
| Methods | D: This was a randomised controlled study RS: This was unclear; the study randomised participants to interventions, although the paper did not provide the method of randomisation AC: This was unclear B: This was unclear |
|
| Participants | Europe: Histologically confirmed diagnosis of SCC in situ (biopsy taken within the preceding 5 months) in participants across 40 hospital outpatient dermatology clinics in 11 European countries T1: N = 96 (124 lesions), T2: N = 17 (24 lesions), T3: N = 82 (91 lesions), T4: N = 30 (36 lesions) Participants and lesion characteristics of the 4 intervention groups were similar at baseline Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | The study randomised participants to methyl aminolevulinate cream (160 mg/g), matching placebo cream, or standard therapy as chosen by the treating investigator (cryotherapy or fluorouracil)
Prior to application of MAL or placebo, the lesion was prepared by gentle surface debridement with a curette. Cream was applied for 3 hours then washed off with 0.9% saline before illumination with non‐coherent red light
Lesions with a partial response at 12 weeks were re‐treated Note: For PDT, treatment was repeated after 1 week for a complete treatment cycle. For cryotherapy, a hand‐held liquid nitrogen spray was used; initial ice field formation with a 2 mm rim of clinically healthy tissue, maintained for a minimum of 20 seconds |
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes |
Intervention product information/details
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not provide the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | The paper did not provide evidence of allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper provided details of participant disposition in the flowchart in the paper |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | The primary outcome was not blinded |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Low risk | An independent blinded observer assessed the cosmetic outcome from photographs |
| Baseline comparability | Low risk | The groups were similar at baseline |
Patel 2006.
| Methods | D: This was a single‐centre, randomised, controlled study RS: Prior to the start of the study, an independent group off‐campus carried out randomisation AC: An independent group provided investigators with a sequence of envelopes containing the allocation code. Each code defined identical boxes containing treatment sachets B: Clinicians and participants were blinded to treatment allocation |
|
| Participants | UK: The study included 31 participants (31 lesions in 11 men and 20 women) from a dermatology outpatient department, randomised to T1 or T2 Baseline characteristics: The 2 groups were similar at baseline, but mean duration and size of lesion was greater in the imiquimod group (23 mm² to 1176 mm² compared with 84 mm² to 555 mm² in the placebo group) Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
There was daily application at night for 16 weeks. No other treatment for cutaneous SCC in situ were allowed for the duration of the study. Treatment could be stopped for 5 days on 2 separate occasions in the event of a severe inflammatory reaction or 1 that was uncomfortable for the participant |
|
| Outcomes | The unit of analysis was the participant, but this was equivalent to lesion
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Double‐blind randomization was done by an independent group off campus at the beginning of the study" (page 1026) |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators were provided with a sequence of envelopes containing the allocation code" (page 1026) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper had a clear flowchart providing details regarding loss to follow‐up: 3 participants withdrew from the study (all from the imiquimod‐treated arm); 1 participant withdrew due to localised infection; 1 participant developed widespread reaction after incorrect application of cream; and 1 participant was lost to follow up after study staff were unable to contact them |
| Blinding participants | Low risk | The study blinded participants |
| Blinding clinicians | Low risk | The study blinded clinicians |
| Blinding pathologist | Low risk | The study blinded the pathologist to the treatment group |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | High risk | There were similar characteristics at baseline, but overall mean duration and size of lesion was greater in the imiquimod group |
Perrett 2007.
| Methods | D: This was an open‐label, single‐centre (dedicated dermatology clinic for organ transplant recipients), randomised intrapatient comparative study RS: Participants randomly assigned T1 to 1 lesional area or T2 to a parallel lesional area (Pocock 1983) AC: The study did not state a method of allocation concealment (intrapatient study) B: There was no evidence of blinding |
|
| Participants | UK: 8 post‐transplant participants (6 men, 2 women) with history of epidermal dysplasia (8 actinic keratoses, 10 lesions of Bowen's disease) The lesional size treated ranged from 39 mm² to 5010 mm², although specific sizes were not provided Inclusion criteria of the trial
|
|
| Interventions |
Prior to treatment, all lesions were gently abraded with curette to remove excess thick surface scale |
|
| Outcomes |
|
|
| Notes |
Intervention product information/details
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The only detail the paper gave was a textbook reference: Pocock 1983 |
| Allocation concealment (selection bias) | Unclear risk | The paper gave no detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 8 participants completed treatment and 6‐months of follow‐up. 1 participant assessed at 6 months died shortly afterwards of unrelated causes |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | The study did not blind assessments |
| Baseline comparability | Low risk | This was an intrapatient comparative study; therefore, there was low risk of bias as lesions treated were in the same participant and chosen to be comparable |
Puizina‐Ivic 2008.
| Methods | Croatia. D: This was a single‐centre comparative study of PDT and 5‐ALA using different treatment regimens RS: Participants were assigned to T1 and T2 AC: This was unclear B: This was unclear |
|
| Participants | Croatia: 51 participants (36 actinic keratoses, 15 lesions of Bowen's disease ‐ histologically proven) T1: 26 participants (20 actinic keratoses, 6 Bowen's disease) T2: 25 participants (16 actinic keratoses, 9 Bowen's disease) |
|
| Interventions |
|
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes | The paper did not provide the size and site of lesions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper reported randomly 'arranged' |
| Allocation concealment (selection bias) | Unclear risk | The paper provided no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There was no evidence of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | Unclear risk | The paper did not provide a table giving information about the baseline characteristics of each arm |
Salim 2003.
| Methods | D: This was a randomised comparative study (2 centres) RS: Participants were randomised to intervention, but no method of randomisation was provided AC: This was unclear B: There was no evidence of blinding |
|
| Participants | UK: 40 participants (8 men, 32 women) with 1 to 3 lesions Mean age = 76 years (65 to 88 years) T1: 20 participants (33 lesions exclusively located on legs) T2: 20 participants (33 lesions located on legs, arms, or face) No data were provided on the size of the lesions treated Inclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes | The lesion was the unit of analysis
|
|
| Notes | A repeat treatment cycle was performed after 6 weeks if required Intervention product information/details
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not detail the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | The paper did not provide details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper included a flowchart: There was no loss to follow up in the PDT arm; 3 participants discontinued 5‐FU treatment (representing 5 lesions) due to adverse reactions |
| Blinding participants | Unclear risk | There was no evidence of blinding |
| Blinding clinicians | Unclear risk | There were no details of blinding |
| Blinding pathologist | Unclear risk | There was no evidence of blinding |
| Blinding outcome assessor | Unclear risk | There was no evidence of blinding |
| Baseline comparability | High risk | Lesions in the PDT‐treated arm were located exclusively on legs |
METHODS D: design AC: method of allocation concealment RS: method of generating randomisation sequence B: blinding (participant, clinician, outcome assessment) T1: Treatment 1, etc
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2000 | There was no mention of randomisation. Participants were allocated treatment according to the established practice of the dermatologist in charge |
| Baron 2010 | This was not a RCT |
| Brown 2005 | This study looked at atypical skin on dorsal hands or forearms; it did not look specifically at Bowen's disease. Although some participants did have some Bowen's, it was not clear which cases these were |
| de Haas 2008 | This was not a RCT |
| Kaminaka 2009 | This was not a RCT |
| Macbeth 2011 | This was a systematic review of non‐melanoma skin cancers and did not include any new RCTs on Bowen's disease |
| Mizutani 2012 | This was not a RCT |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN30540872.
| Trial name or title | An open phase II study to assess the efficacy and safety of topical SR‐T100® gel in the treatment of human cutaneous squamous cell carcinoma in situ (actinic keratosis and Bowen's disease) |
| Methods | This is a single‐centre, phase II, open‐label, randomised study |
| Participants |
Inclusion criteria of the trial Participants must meet all of the inclusion criteria for entry into this study
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 December 2007 |
| Contact information | Dr Hamm‐Ming Sheu, National Cheng Kung University Hospital, Taiwan |
| Notes | ‐ |
NCT00384124.
| Trial name or title | Phase III trial of 6 weeks of imiquimod for the treatment of Bowens disease of the head and neck. Outcome is histologic clearance at 14 Weeks |
| Methods | This is a randomised, double‐blind, placebo‐controlled trial |
| Participants |
Inclusion criteria of the trial
|
| Interventions |
|
| Outcomes |
|
| Starting date | November 2006 |
| Contact information | Nicole M Owens, MD, Brooke Army Medical Centre Department of Dermatology |
| Notes | Final data collection date for primary outcome: November 2008 |
NCT00472459.
| Trial name or title | A multicentre, randomised study of photodynamic therapy (PDT) with Metvix® 160 mg/g cream in immuno‐compromised patients with non‐melanoma skin cancer |
| Methods | This is a randomised, open‐label, active‐controlled, parallel‐assignment safety/efficacy/prevention study |
| Participants |
Inclusion criteria of the trial
|
| Interventions | 2 contralateral areas with skin lesions within the participant will be compared
|
| Outcomes |
|
| Starting date | July 2003 |
| Contact information | PI: Ann‐Marie Wennberg, Sahlgrenska University Hospital, Gothenburg, Sweden |
| Notes | ‐ |
NCT00605709.
| Trial name or title | Dose‐ranging safety and efficacy study of topical creams containing API 31510 for the treatment of in situ cutaneous squamous cell carcinoma |
| Methods | This is a randomised, double‐blind, controlled study |
| Participants |
Inclusion criteria of the trial
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2008 |
| Contact information | Cytotech Labs |
| Notes | ‐ |
NCT00868088.
| Trial name or title | A clinical trial of ALA photodynamic therapy for treatment of actinic cheilitis in patients with squamous cell carcinoma of the lip |
| Methods | This is a randomised, single‐blind, parallel‐assignment safety/efficacy study Aim: to determine whether ALA‐PDT applied to the lips can effectively clear actinic cheilitis (AC) and squamous cell carcinoma in situ of the lip |
| Participants |
Inclusion criteria of the trial
|
| Interventions |
|
| Outcomes |
|
| Starting date | April 2009 |
| Contact information | Tuffs Medical Centre Department of Dermatology (Gary Rogers, MD) |
| Notes | ‐ |
NCT01245972.
| Trial name or title | A pilot study to examine the effectiveness of 595nM pulsed dye lasers in the treatment of basal cell carcinoma and squamous cell carcinoma in situ |
| Methods | This is a randomised controlled study |
| Participants |
Inclusion criteria of the trial
|
| Interventions |
|
| Outcomes |
|
| Starting date | August 2010 |
| Contact information | Shang I. Brian Jiang, MD, UCSD Medical Center, Division of Dermatology |
| Notes | ‐ |
Differences between protocol and review
The final version of this review differs from the original protocol for the following reasons:
Following comments from the clinical referees, we amended the title to 'cutaneous Bowen's disease' to make it explicit that this review does not apply to the mucosal or anogenital forms.
In the background section, critical revision by a new author (RM), who was not involved at the stage of writing the protocol, has made minor changes to the text to make it clearer and easier to read. We also added new references to provide readers with the best and updated information on this subject.
With regard to outcomes, for the primary outcome, the unit of analysis has changed from number of participants to number of participants or lesions because the unit of analysis for most studies was number of lesions. In a number of studies, there were multiple lesions treated per participant. We provide more detail in the 'Characteristics of included studies' tables. For secondary outcomes, the unit of analysis has been changed to number of lesions. The reason for this change was that all studies included participants with variable numbers of lesions from one to six, and all studies reported outcomes as number of lesions that responded.
Contributions of authors
Draft the protocol: FB‐H, DA, and DW Search for trials: FB‐H and RM Obtain copies of trials: FB‐H Select which trials to include: FB‐H and RM Extract data from trials: FB‐H and RM Enter data into RevMan: FB‐H and RM Conduct analysis: FB‐H and JL‐B Interpret analysis: FB‐H, RM, JL‐B, and DW Draft final review: FB‐H, RM, and DW Update the review: FB‐H and RM
Disclaimer The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
The University of Nottingham, UK.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
de Haas 2007 {published data only}
- Haas ER, Sterenborg HJ, Neumann HA, Robinson DJ. Response of Bowen disease to ALA‐PDT using a single and a 2‐fold illumination scheme. Archives of Dermatology 2007;143(2):264‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lui 2004 {published data only}
- Lui H, Bobbs L, Tope WD, Lee PK, Elmets C, Provost N, et al. Photodynamic therapy of multiple nonmelanoma skin cancers with verteporfin and red light‐emitting diodes: two‐year results evaluating tumor response and cosmetic outcomes. Archives of Dermatology 2004;140(1):26‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morton 1996 {published data only}
- Morton CA, Whitehurst C, Moseley H, McColl JH, Moore JV, Mackie RM. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen's disease. British Journal of Dermatology 1996;135(5):766‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Morton 2000 {published data only}
- Morton CA, Whitehurst C, Moore JV, MacKie RM. Comparison of red and green light in the treatment of Bowen's disease by photodynamic therapy. British Journal of Dermatology 2000;143(4):767‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morton 2006 {published data only}
- Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or Fluorouracil for treatment of squamous cell carcinoma in situ: Results of a multicenter randomized trial. Archives of Dermatology 2006;142(6):729‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 2006 {published data only}
- Patel GK, Goodwin R, Chawla M, Laidler P, Price PE, Finlay AY, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen's disease): A randomised, double‐blind placebo‐controlled trial. Journal of the American Academy of Dermatology 2006;54(6):1025‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perrett 2007 {published data only}
- Perrett CM, McGregor JM, Warwick J, Karran P, Leigh IM, Proby CM, et al. Treatment of post‐transplant premalignant skin disease: a randomised intrapatient comparative study of 5‐fluorouracil cream and topical photodynamic therapy. British Journal of Dermatology 2007;156(2):320‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puizina‐Ivic 2008 {published data only}
- Puizina‐Ivic N, Zorc H, Vanjaka‐Rogosic L, Miric L, Persin A. Fractionated illumination improves the outcome in the treatment of precanerous lesions with photodynamic therapy. Collegium Antropologicum 2008;32(Suppl 2):67‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Salim 2003 {published data only}
- Salim S, Leman JA, McColl JH, Chapman R, Morton CA. Randomised comparison of photodynamic therapy with topical 5‐fluorouracil in Bowen's disease. British Journal of Dermatology 2003;148(3):539‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2000 {published data only}
- Ahmed I, Berth‐Jones J, Charles‐Holmes S, Callaghan CJ, Ilchyshyn A. Comparison of cryotherapy with curettage in the treatment of Bowen's disease: a prospective study. British Journal of Dermatology 2000;143(4):759‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baron 2010 {published data only}
- Baron ED, Malbasa CL, Santo‐Domingo D, Fu P, Miller JD, Hanneman KK, et al. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Lasers in Surgery & Medicine 2010;42(10):728‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2005 {published data only}
- Brown VL, Atkins CL, Ghali L, Cerio R, Harwood CA, Proby CM. Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high‐risk renal transplant recipients: randomized, double‐blind, placebo‐controlled trial. Archives of Dermatology 2005;141(8):985‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Haas 2008 {published data only}
- Haas ER, Vijlder HC, Sterenborg HJ, Neumann HA, Robinson DJ. Fractionated aminolevulinic acid‐photodynamic theapy provides additional evidence for the use of PDT for non‐melanoma skin cancer. Jouranal of the European Academy of Dermatology & Venereology 2008;22(4):426‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaminaka 2009 {published data only}
- Kaminaka C, Yamamoto Y, Yonei N, Kishioka A, Kondo T, Furukawa F. Phenol peels as a novel therapeutic approach for actinic keratosis and Bowen disease: a prospective pilot trial with assessment of clinical, histologic, and immunohistochemical correlations. Journal American Academy of Dermatology 2009;60(4):615‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macbeth 2011 {published data only}
- Macbeth AE, Grindlay DJC, Williams HC. What's new in skin cancer? An analysis of guidelines and systematic reviews published in 2008‐2009. Clinical & Experimental Dermatology 2011;36(5):453‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mizutani 2012 {published data only}
- Mizutani K, Akita Y, Yanagishita T, Kimura M, Tanaka T, Kinoshita Y, et al. Comparison of the efficacy of ALA‐PDT using an excimer‐dye laser (630nm) and a metal‐halide lamp (600‐740nm) for treatment of Bowen's disease. Photodermatology Photoimmunology & Photomedicine 2012;28(3):142‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN30540872 {published data only}
- ISRCTN30540872. Efficacy and safety of topical SR‐T100® gel in the treatment of human cutaneous squamous cell carcinoma in situ (actinic keratosis and Bowen's disease). www.controlled‐trials.com/ISRCTN30540872/ (accessed 15 February 2012).
NCT00384124 {published data only}
- NCT00384124. Topical Imiquimod for Bowen's Disease of the Head and Neck. clinicaltrials.gov/ct2/show/NCT00384124 (accessed 15 February 2012).
NCT00472459 {published data only}
- NCT00472459. PDT With Metvix® 160 mg/g Cream in Organ Transplant Recipients With Non‐melanoma Skin Cancer. clinicaltrials.gov/ct2/show/NCT00472459 (accessed 15 February 2012).
NCT00605709 {published data only}
- NCT00605709. Dose‐Ranging Safety and Efficacy Study of Topical Creams Containing API 31510 for the Treatment of in Situ Cutaneous Squamous Cell Carcinoma. clinicaltrials.gov/ct2/show/NCT00605709 (accessed 15 February 2012).
NCT00868088 {published data only}
- NCT00868088. Photodynamic Therapy to Treat Actinic Damage in Patients With Squamous Cell Carcinoma (SCC) of the Lip. clinicaltrials.gov/ct2/show/NCT00868088 (accessed 15 February 2012).
NCT01245972 {published data only}
- NCT01245972. Pilot Study of PDL to Treat BCC and SCCIS (PDLNMSC). clinicaltrials.gov/ct2/show/NCT01245972 (accessed 15 February 2012).
Additional references
Ali 2012
- Ali H, Shipman AR, Orpin SD. John Templeton Bowen, MD, 1857–1940: the centenary of his most famous publication. Clinical and Experimental Dermatology October 2012;37(7):825‐828. [PUBMED: 22998547] [DOI] [PubMed] [Google Scholar]
Arlette 2004
- Arlette JP, Trotter MJ. Squamous cell carcinoma in situ of the skin: history, presentation, biology and treatment. Australasian Journal of Dermatology 2004;45(1):1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bordea 2004
- Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal‐transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation 2004;77(4):574‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chute 1991
- Chute CG, Chuang TY, Bergstralh EJ, Su WP. The subsequent risk of internal cancer with Bowen's disease. A population‐based study. JAMA 1991;266(6):816‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Cox 1994
- Cox NH. Body site distribution of Bowen's disease. British Journal of Dermatology 1994;130(6):714‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cox 1999
- Cox NH, Eedy DJ, Morton CA. Guidelines for management of Bowen's disease. British Association of Dermatologists. British Journal of Dermatology 1999;141(4):633‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cox 2007
- Cox NH, Eedy DJ, Morton CA, Therapy Guidelines and Audit Subcommittee, British Association of Dermatologists. Guidelines for management of Bowen's disease: 2006 update. British Journal of Dermatology 2007;156(1):11‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eedy 1987
- Eedy DJ, Gavin AT. Thirteen‐year retrospective study of Bowen's disease in Northern Ireland. British Journal of Dermatology 1987;117(6):715‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eedy 2005
- Eedy DJ. Summary of inaugural meeting of the Skin Care in Organ Recipients Group, UK, held at the Royal Society of Medicine, 7 October 2004. British Journal of Dermatology 2005;153(1):6‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grundmeier 2011
- Grundmeier N, Hamm H, Weissbrich B, Lang SC, Bröcker EB, Kerstan A. High‐risk human papillomavirus infection in Bowen's disease of the nail unit: report of three cases and review of the literature. Dermatology 2011;223(4):293‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Iftner 2003
- Iftner A, Klug SJ, Garbe C, Blum A, Stancu A, Wilczynski SP, et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high‐risk genital types as possible risk factors. Cancer Research 2003;63(21):7515‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Jaeger 1999
- Jaeger AB, Gramkow A, Hjalgrim H, Melbye M, Frisch M. Bowen disease and risk of subsequent malignant neoplasms: a population‐based cohort study of 1147 patients. Archives of Dermatology 1999;135(7):790‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 1986
- Kao GF. Carcinoma arising in Bowen's disease. Archives of Dermatology 1986;122(10):1124‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Kossard 1992
- Kossard S, Rosen R. Cutaneous Bowen's disease. An analysis of 1001 cases according to age, sex, and site. Journal of the American Academy of Dermatology 1992;27(3):406‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kovacs 1996
- Kovacs A, Yonemoto K, Katsuoka K, Nishiyama S, Harhai I. Bowen's disease: statistical study of a 10 year period. Journal of Dermatology 1996;23(4):267‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lycka 1989
- Lycka BA. Bowen's disease and internal malignancy. A meta‐analysis. International Journal of Dermatology 1989;28(8):531‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peterka 1961
- Peterka ES, Lynch FW, Goltz RW. An association between Bowen's disease and internal cancer. Archives of Dermatology 1961;84:623‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pocock 1983
- Pocock, SJ. Clinical Trials: A Practical Approach. 1st Edition. Wiley‐Blackwell, 1983. [Google Scholar]
Ragi 1988
- Ragi G, Turner MS, Klein LE, Stoll HL. Pigmented Bowen's disease and review of 420 Bowen's disease lesions. Journal of Dermatologic Surgery & Oncology 1988;14(7):765‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reizner 1994
- Reizner GT, Chuang TY, Elpern DJ, Stone JL, Farmer ER. Bowen's disease (squamous cell carcinoma in situ) in Kauai, Hawaii. A population‐based incidence report. Journal of the American Academy of Dermatology 1994;31(4):596‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riddel 2011
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus‐associated squamous cell carcinoma: a review. Journal of the American Academy of Dermatology 2011;64(6):1147‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thestrup‐Pedersen 1988
- Thestrup‐Pedersen K, Ravnborg L, Reymann F. Morbus Bowen. A description of the disease in 617 patients. Acta Dermato‐Venereologica 1988;68(3):236‐9. [MEDLINE: ] [PubMed] [Google Scholar]


