Abstract
Background
Intraoperative hypothermia during both open and laparoscopic abdominal surgery may be associated with adverse events. For laparoscopic abdominal surgery, the use of heated insufflation systems for establishing pneumoperitoneum has been described to prevent hypothermia. Humidification of the insufflated gas is also possible. Past studies on heated insufflation have shown inconclusive results with regards to maintenance of core temperature and reduction of postoperative pain and recovery times.
Objectives
To determine the effect of heated gas insufflation compared to cold gas insufflation on maintaining intraoperative normothermia as well as patient outcomes following laparoscopic abdominal surgery.
Search methods
We searched Cochrane Colorectal Cancer Specialised Register (September 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 8), Ovid MEDLINE (1950 to September 2016), Ovid Embase (1974 to September 2016), International Pharmaceutical Abstracts (IPA) (September 2016), Web of Science (1985 to September 2016), Scopus, www.clinicaltrials.gov and the National Research Register (1956 to September 2016). We also searched grey literature and cross references. Searches were limited to human studies without language restriction.
Selection criteria
Only randomised controlled trials comparing heated (with or without humidification) with cold gas insufflation in adult and paediatric populations undergoing laparoscopic abdominal procedures were included. We assessed study quality in regards to relevance, design, sequence generation, allocation concealment, blinding, possibility of incomplete data and selective reporting. Two review authors independently selected studies for the review, with any disagreement resolved in consensus with a third co‐author.
Data collection and analysis
Two review authors independently performed screening of eligible studies, data extraction and methodological quality assessment of the trials. We classified a study as low‐risk of bias if all of the first six main criteria indicated in the 'Risk of Bias Assessment' table were assessed as low risk. We used data sheets to collect data from eligible studies. We presented results using mean differences for continuous outcomes and relative risks for dichotomous outcomes, with 95% confidence intervals. We used Review Manager (RevMan) 5.3 software to calculate the estimated effects. We took publication bias into consideration and compiled funnel plots.
Main results
We included 22 studies in this updated analysis, including six new trials with 584 additional participants, resulting in a total of 1428 participants. The risk of bias was low in 11 studies, high in one study and unclear in the remaining studies, due primarily to failure to report methodology for randomisation, and allocation concealment or blinding, or both. Fourteen studies examined intraoperative core temperatures among heated and humidified insufflation cohorts and core temperatures were higher compared to cold gas insufflation (MD 0.31 °C, 95% CI, 0.09 to 0.53, I2 = 88%, P = 0.005) (low‐quality evidence). If the analysis was limited to the eight studies at low risk of bias, this result became non‐significant but remained heterogeneous (MD 0.18 °C, 95% CI, ‐0.04 to 0.39, I2= 81%, P = 0.10) (moderate‐quality evidence). In comparison to the cold CO2 group, the meta‐analysis of the heated, non‐humidified group also showed no statistically significant difference between groups. Core temperature was statistically, significantly higher in the heated, humidified CO2 with external warming groups (MD 0.29 °C, 95% CI, 0.05 to 0.52, I2 = 84%, P = 0.02) (moderate‐quality evidence). Despite the small difference in temperature of 0.31 °C with heated CO2, this is unlikely to be of clinical significance.
For postoperative pain scores, there were no statistically significant differences between heated and cold CO2, either overall, or for any of the subgroups assessed. Interestingly, morphine‐equivalent use was homogeneous and higher in heated, non‐humidified insufflation compared to cold insufflation for postoperative day one (MD 11.93 mg, 95% CI 0.92 to 22.94, I2 = 0%, P = 0.03) (low‐quality evidence) and day two (MD 9.79 mg, 95% CI 1.58 to 18.00, I2 = 0%, P = 0.02) (low‐quality evidence). However, morphine use was not significantly different six hours postoperatively or in any humidified insufflation groups.
There was no apparent effect on length of hospitalisation, lens fogging or length of operation with heated compared to cold gas insufflation, with or without humidification. Recovery room time was shorter in the heated cohort (MD ‐26.79 minutes, 95% CI ‐51.34 to ‐2.25, I2 = 95%, P = 0.03) (low‐quality evidence). When the one and only unclear‐risk study was removed from the analysis, the difference in recovery‐room time became non‐significant and the studies were statistically homogeneous (MD ‐1.22 minutes, 95% CI, ‐6.62 to 4.17, I2 = 12%, P = 0.66) (moderate‐quality evidence).
There were also no differences in the frequency of major adverse events that occurred in the cold or heated cohorts.
These results should be interpreted with caution due to some limitations. Heterogeneity of core temperature remained significant despite subgroup analysis, likely due to variations in the study design of the individual trials, as the trials had variations in insufflation gas temperatures (35 ºC to 37 ºC), humidity ranges (88% to 100%), gas volumes and location of the temperature probes. Additionally, some of the trials lacked specific study design information making evaluation difficult.
Authors' conclusions
While heated, humidified gas leads to mildly smaller decreases in core body temperatures, clinically this does not account for improved patient outcomes, therefore, there is no clear evidence for the use of heated gas insufflation, with or without humidification, compared to cold gas insufflation in laparoscopic abdominal surgery.
Keywords: Adult; Female; Humans; Male; Carbon Dioxide; Analgesics, Opioid; Analgesics, Opioid/administration & dosage; Body Temperature; Hot Temperature; Hot Temperature/therapeutic use; Humidity; Hypothermia; Hypothermia/prevention & control; Insufflation; Insufflation/methods; Intraoperative Complications; Intraoperative Complications/prevention & control; Laparoscopy; Laparoscopy/methods; Morphine; Morphine/administration & dosage; Pain, Postoperative; Pain, Postoperative/prevention & control; Pneumoperitoneum, Artificial; Pneumoperitoneum, Artificial/methods; Randomized Controlled Trials as Topic
Plain language summary
Heated CO2 for laparoscopic abdominal surgery
Background
In laparoscopic surgery, surgery is performed through small incisions using long instruments and video cameras. To create a working and viewing space in the abdomen, carbon dioxide (CO2) is insufflated to separate the abdominal wall from internal organs. Traditionally, unheated CO2 is used but there has been suggestions that heated CO2 may prevent hypothermia. Hypothermia has been associated with heart attacks, abnormal heart rhythms, increased infections, decreased clotting ability and increased blood loss. We aimed to investigate the role of heated compared with cold CO2 in laparoscopic abdominal surgery.
Study Characteristics
We searched the medical literature for randomised controlled trials (where people are allocated at random to one of two or more treatment groups) that compared heated and cold CO2. We analysed data from the trials for changes in core temperature. We also compared post‐operative pain scores and pain medication requirements, length of hospital stay, length of surgery and fogging of the surgical video camera lens. Evidence is current to September 2016.
Key results and quality of evidence
We identified and included 22 trials. There was an increase of 0.31 °C in the heated, humidified CO2 group compared to the cold CO2 group but the data were heterogeneous (highly variable). However, if the analysis was limited to the eight low‐risk‐of‐bias studies that reported core temperatures, no significant difference was found. Also, there was no temperature difference for heated and non‐humidified gas compared to cold gas.
There was no difference in postoperative pain with heated or cold insufflation. However, pain medication use was higher in only the heated, non‐humidified group on postoperative days one and two.
Heated gas apparently did not change length of hospitalisation, lens fogging or length of operation. Recovery room stay was shorter with heated gas but the data was heterogeneous (highly variable). When we only included studies at low risk of bias, the data became homogeneous (less variable) and the recovery room time was not significantly different between the heated and cold gas groups.
Authors' Conclusions
While heated, humidified gas leads to slightly smaller decreases in core body temperatures, this does not account for improvement in any patient outcomes. Therefore, there is no clear evidence for the use of heated gas insufflation, with or without humidification, in laparoscopic abdominal surgery.
Summary of findings
Summary of findings for the main comparison. Core temperature.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (core temperature) Setting: Operating room Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Change in core temperature (ºC) | The mean change in core temperature was ‐0.22 °C | The mean change in core temperature in the intervention group was 0.21 °C higher (0.06 to 0.36) | 1100 (19 RCTs) | ⨁⨁◯◯ LOW 1 2 | Negative temperature indicates core temperature dropped during surgery |
| Change in core temperature: heated, humidified vs cold | The mean change in core temperature: heated, humidified vs cold was ‐0.25 °C | The mean change in core temperature: heated, humidified vs cold in the intervention group was 0.31 °C higher (0.09 to 0.53) | 885 (14 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Change in core temperature: heated only vs cold | The mean change in core temperature: heated vs cold was ‐0.19 °C | The mean change in core temperature: heated vs cold in the intervention group was 0.02 °C higher (‐0.16 to 0.20) | 215 (7 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Change in core temperature for known low risk of bias studies | The mean change in core temperature for low risk of bias studies was ‐0.10 °C | The mean change in core temperature for low risk of bias studies in the intervention group was 0.16 °C higher (‐0.01 to 0.33) | 653 (10 RCTs) | ⨁⨁⨁◯ MODERATE4 | |
| Change in core temperature for known low risk of bias studies: heated, humidified vs cold | The mean change in core temperature for low risk of bias studies: heated, humidified vs cold was ‐0.09 °C | The mean change in core temperature for low risk of bias studies: heated, humidified vs cold in the intervention group was 0.18 °C higher (‐0.04 to 0.39) | 561 (8 RCTs) | ⨁⨁⨁◯ MODERATE4 | |
| Change in core temperature for low risk of bias studies: heated only vs cold | The mean change in core temperature for low risk of bias studies: heated vs cold was ‐0.10 °C | The mean change in core temperature for low risk of bias studies: heated vs cold in the intervention group was 0.12 °C higher (‐0.15 to 0.39) | 92 (3 RCTs) | ⨁⨁⨁◯ MODERATE 2 | |
| Change in core temperature with external warming | The mean change in core temperature with external warming was ‐0.14 °C | The mean change in core temperature with external warming in the intervention group was 0.29 °C higher (0.05 to 0.52) | 545 (8 RCTs) | ⨁⨁⨁◯ MODERATE1 | |
| Change in core temperature without external warming | The mean change in core temperature without external warming was ‐0.40 °C | The mean change in core temperature without external warming in the intervention group was 0.32 °C higher (‐0.11 to 0.75) | 340 (6 RCTs) | ⨁⨁⨁◯ MODERATE1 | |
| Change in core temperature for operations > 120 min | The mean change in core temperature for operations > 120 min was ‐0.74 °C | The mean change in core temperature for operations > 120 min in the intervention group was 0.70 °C higher (0.10 to 1.30) | 194 (4 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
- Risk of bias not clear
- Inconsistent effect
- Low‐risk studies only
- Wide confidence intervals
Summary of findings 2. Pain score.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (pain score) Setting: Hospital Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Day 1 pain score (0 to 10‐point VAS) | The mean day 1 pain score was 2.8 | The mean day 1 pain score in the intervention group was 0.04 fewer (‐0.42 to 0.34) | 991 (14 RCTs) | ⨁⨁◯◯ LOW 1 2 | Higher score indicates more pain for participants |
| Day 1 pain score: heated, humidified vs cold (abdominal) | The mean day 1 pain score: heated, humidified vs cold (abdominal) was 4 | The mean day 1 pain score: heated, humidified vs cold (abdominal) in the intervention group was 0.14 fewer (‐0.6 to 0.33) | 670 (10 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Day 1 pain score: heated, humidified vs cold (shoulder) | The mean day 1 pain score: heated, humidified vs cold (shoulder) was 2 | The mean day 1 pain score: heated, humidified vs cold (shoulder) in the intervention group was 0.35 fewer (‐1.75 to 1.05) | 171 (3 RCTs) | ⨁◯◯◯ VERY LOW 1 2 4 | |
| Day 1 pain score: heated only vs cold | The mean day 1 pain score: heated vs cold was 2.8 | The mean day 1 pain score: heated vs cold in the intervention group was 0.5 more (‐0.11 to 1.12) | 150 (3 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Day 2 pain score | The mean day 2 pain score was 2.2 | The mean day 2 pain score in the intervention group was 0.28 fewer (‐0.78 to 0.21) | 695 (10 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Day 2 pain score: heated, humidified vs cold (abdominal) | The mean day 2 pain score: heated, humidified vs cold (abdominal) was 3.2 | The mean day 2 pain score: heated, humidified vs cold (abdominal) in the intervention group was 0.4 fewer (‐1.07 to 0.28) | 442 (7 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Day 2 pain score: heated, humidified vs cold (shoulder) | The mean day 2 pain score: heated, humidified vs cold (shoulder) was 1.5 | The mean day 2 pain score: heated, humidified vs cold (shoulder) in the intervention group was 0.88 fewer (‐2.93 to 1.17) | 111 (2 RCTs) | ⨁◯◯◯ VERY LOW 1 2 4 | |
| Day 2 pain score: heated only vs cold | The mean day 2 pain score: heated vs cold was 1.9 | The mean day 2 pain score: heated vs cold in the intervention group was 0.41 more (‐0.44 to 1.27) | 142 (3 RCTs) | ⨁⨁◯◯ LOW 1 2 | |
| Day 1 pain score for low risk of bias studies | The mean day 1 pain score for low risk of bias studies was 2.7 | The mean day 1 pain score for low risk of bias studies in the intervention group was 0.17 more (‐0.21 to 0.55) | 570 (7 RCTs) | ⨁⨁⨁⨁ HIGH3 | |
| Day 1 pain score for low risk of bias studies: heated, humidified vs cold (abdominal) | The mean day 1 pain score for low risk of bias studies: heated, humidified vs cold (abdominal) was 4.3 | The mean day 1 pain score for low risk of bias studies: heated, humidified vs cold (abdominal) in the intervention group was 0.17 more (‐0.29 to 0.63) | 460 (7 RCTs) | ⨁⨁⨁⨁ HIGH3 | |
| Day 1 pain score for low risk of bias studies: heated, humidified vs cold (shoulder) | The mean day 1 pain score for low risk of bias studies: heated, humidified vs cold (shoulder) was 1.2 | The mean day 1 pain score for low risk of bias studies: heated, humidified vs cold (shoulder) in the intervention group was 0.25 more (‐0.81 to 1.31) | 110 (2 RCTs) | ⨁⨁⨁◯ MODERATE 4 | |
| Day 2 pain score for low risk of bias studies | The mean day 2 pain score for low risk of bias studies was 3.5 | The mean day 2 pain score for low risk of bias studies in the intervention group was 0.29 fewer (‐0.65 to 0.07) | 380 (5 RCTs) | ⨁⨁⨁⨁ HIGH3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
- Risk of bias not clear
- Inconsistent effect
- Low‐risk studies only
- Wide confidence intervals
Summary of findings 3. Morphine consumption.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (morphine consumption) Setting: Post‐operative Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Up to 6 h | The mean up to 6 h morphine consumption was 12.6 mg | The mean up to 6 h in the intervention group was 0.45 mg more (‐1.19 to 2.08) | 231 (4 RCTs) | ⨁⨁⨁◯ MODERATE 1 | Morphine consumption was presented as equivalent daily dose |
| Day 1 morphine | The mean day 1 morphine consumption was 32.4 mg | The mean day 1 morphine consumption in the intervention group was 0.64 mg less (‐4.48 to 3.20) | 573 (9 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Day 1 morphine: heated, humidified vs cold | The mean day 1 morphine consumption: heated, humidified vs cold was 31.2 mg | The mean day 1 morphine consumption: heated, humidified vs cold in the intervention group was 1.66 mg less (‐4.79 to 1.46) | 481 (7 RCTs) | ⨁⨁◯◯ LOW 14 | |
| Day 1 morphine: heated only vs cold | The mean day 1 morphine consumption: heated vs cold was 33.6 mg | The mean day 1 morphine consumption: heated vs cold in the intervention group was 11.93 mg more (0.92 to 22.94) | 92 (3 RCTs) | ⨁⨁◯◯ LOW 12 | |
| Day 2 morphine | The mean day 2 morphine consumption was 22.1 mg | The mean day 2 morphine consumption in the intervention group was 0.61 mg less (‐2.79 to 1.57) | 532 (7 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Day 2 morphine: heated, humidified vs cold | The mean day 2 morphine consumption ‐ Heated, humidified vs cold was 21.3 mg | The mean day 2 morphine consumption: heated, humidified vs cold in the intervention group was 0.94 mg less (‐1.9 to 0.01) | 410 (6 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Day 2 morphine: heated only vs cold | The mean day 2 morphine consumption: heated vs cold was 23 mg | The mean day 2 morphine consumption: heated vs cold in the intervention group was 9.79 mg more (1.58 to 18.00) | 122 (2 RCTs) | ⨁⨁◯◯ LOW 12 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1. Risk of bias not clear 2. Wide confidence intervals
Summary of findings 4. Hospital stay.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (hospital stay) Setting: Hospital Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Hospital stay (days) | The mean hospital stay was 2.7 days | The mean hospital stay in the intervention group was 0.06 days less (‐0.31 to 0.19) | 685 (10 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Hospital stay: heated, humidified vs cold | The mean hospital stay: heated, humidified vs cold was 2.9 days | The mean hospital stay: heated, humidified vs cold in the intervention group was 0.13 days less (‐0.44 to 0.18) | 563 (9 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| Hospital stay: heated only vs cold | The mean hospital stay: heated vs cold was 2.6 days | The mean hospital stay: heated vs cold in the intervention group was 0.20 days more (‐0.23 to 0.62) | 122 (2 RCTs) | ⨁⨁⨁◯ MODERATE 1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1. Risk of bias not clear
Summary of findings 5. Recovery time.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (recovery time) Setting: Hospital Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Recovery time (minutes) | The mean recovery time was 106.8 min | The mean recovery time in the intervention group was 26.79 min less (‐51.34 to ‐2.25) | 327 (6 RCTs) | ⨁⨁◯◯ LOW 12 | |
| Recovery time for low risk of bias studies | The mean recovery time for low risk of bias studies was 90.1 min | The mean recovery time for low risk of bias studies in the intervention group was 1.22 min less (‐6.62 to 4.17) | 277 (5 RCTs) | ⨁⨁⨁◯ MODERATE2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1. Risk of bias not clear 2. Wide confidence intervals
Summary of findings 6. Lens fogging.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparscopic abdominal surgery (lens fogging) Setting: Operating room Intervention: Heated Gas Comparison: Cold Gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Times cleaned | The mean frequency of cleaning was 1.8 times | The mean times cleaned in the intervention group was 0.73 times more (‐0.32 to 1.77) | 341 (7 RCTs) | ⨁⨁◯◯ LOW 1 2 | The frequency of lens cleaning during surgery |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1. Risk of bias not clear 2. Inconsistent effect
Summary of findings 7. Operative time.
| Heated CO2 with or without humidification for laparoscopic abdominal surgery | |||||
| Patient or population: Laparoscopic abdominal surgery (operative time) Setting: Operating room Intervention: Heated gas Comparison: Cold gas | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cold gas | Risk with heated gas | ||||
| Operative time (minutes) | The mean operative time was 76.6 min | The mean operative time in the intervention group was 0.44 min less (‐3.91 to 3.04) | 1318 (20 RCTs) | ⨁◯◯◯ VERY LOW 1 2 3 | |
| Operative time: heated, humidified vs cold | The mean operative time: heated, humidified vs cold was 94.3 min | The mean operative time: heated, humidified vs cold in the intervention group was 2.01 min less (‐7.15 to 3.13) | 1033 (15 RCTs) | ⨁◯◯◯ VERY LOW 1 2 3 | |
| Operative time: heated only vs cold | The mean operative time: heated vs cold was 58.8 min | The mean operative time: heated vs cold in the intervention group was 0.91 min more (‐4.02 to 5.83) | 285 (7 RCTs) | ⨁⨁◯◯ LOW 13 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1. Risk of bias not clear 2. Inconsistent effect 3. Wide confidence intervals
Background
Description of the condition
Intraoperative hypothermia can occur with open or laparoscopic surgery. General anaesthesia is associated with impaired thermoregulation (Putzu 2007; Qadan 2009) and insufflation of gas at ambient temperature during laparoscopic abdominal surgery may contribute to worsened hypothermia due to prolonged procedure times. Perioperative hypothermia has been associated with myocardial ischaemia and stimulation of cardiac dysrhythmias, such as ventricular tachycardia (Frank 1993; Frank 1997; Putzu 2007). Generalised immunosuppression and increased surgical site infections have also been described in conjunction with hypothermia. Infections are thought to arise because of a reduction in oxygen delivery to healing tissue due to peripheral vasoconstriction, (Beilin 1998; Qadan 2009). Increased blood loss has been associated with intraoperative hypothermia, resulting in greater transfusion requirements (Putzu 2007; Rajagopalan 2008), which may in turn further worsen hypothermia. Certain patient populations, including the elderly, may be at a higher risk of hypothermia (Macario 2002).
Description of the intervention
A European survey of 8083 surgical cases determined that only 19.4% of patients received intraoperative temperature monitoring (TEMMP). Interventions to prevent hypothermia include passive techniques (such as blankets and covers), and active techniques (such as heated forced air systems, heated mattresses and blankets, warmed humidified ventilator circuits and warmed intravenous and irrigation fluids). These techniques have been suggested to limit perioperative complications due to hypothermia (Putzu 2007; Winkler 2000; Wong 2007). Warm and humidified gas insufflation during laparoscopic surgery has been suggested as another active method to prevent hypothermia. The gas is heated by using a tube with an inline heating coil and water reservoir. The gas may be heated and humidified using such systems. The insufflation gas of choice in laparoscopic surgery is CO2 but other possibilities include nitrous oxide, helium or argon.
How the intervention might work
Several studies have analysed the impact of using warmed CO2, with or without humidification, for abdominal insufflation in laparoscopic surgery on patient‐centred clinical outcomes. It has been suggested that warming up CO2 prior to insufflation may prevent hypothermia and peritoneal inflammation (Demco 2001). Other studies concluded that warmed insufflation decreases postoperative pain (Champion 2006; Farley 2004; Hamza 2005; Mouton 1999; Ott 1998) and improves recovery times. These studies typically involved small and specific patient populations. In contradiction, there are a number of studies that show no important clinical benefits of using heated insufflation (Davis 2006; Nguyen 2002) and one in particular showed increased postoperative pain in the heated group (Kissler 2004).
Why it is important to do this review
This systematic review is an update to our previous review (Birch 2011), to further clarify the role of heated CO2 on core temperature during laparoscopic abdominal surgery and its impact on relevant clinical outcomes.
We repeated our search for eligible trials with updated search strategies, identified additional studies and included them in the meta‐analyses.
Objectives
To determine the effect of heated gas insufflation compared to cold gas insufflation on maintaining intraoperative normothermia as well as patient outcomes following laparoscopic abdominal surgery.
Methods
Criteria for considering studies for this review
Types of studies
All types of randomised controlled trials (RCT) including parallel‐group, crossover, cluster and factorial trials.
Types of participants
Adults and children undergoing laparoscopic abdominal surgery.
Types of interventions
Heated, with or without humidification, gas insufflation versus cold gas insufflation.
Types of outcome measures
Primary outcomes
Change in intra‐operative core temperature preferably measured via the tympanic membrane, nasopharynx, oesophagus, bladder or rectum (Cork 1983).
Secondary outcomes
The following clinical outcomes:
postoperative pain score (10‐point visual analogue scale (VAS));
morphine consumption; preferably reported as morphine equivalent daily doses
hospital stay;
recovery room stay;
lens fogging;
operative time;
major adverse events defined as Clavien‐Dindo grade III or higher (Dindo 2004).
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restrictions in collaboration with the Cochrane Information Specialist (CIS) from Cochrane Colorectal Cancer. We searched the following electronic databases:
Cochrane Colorectal Cancer Group Specialised Register (September 2016);
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 8)) (September 2016) (Appendix 1);
Ovid MEDLINE (1950 to Septermber 2016) (Appendix 2);
Ovid Embase (1974 to September 2016) (Appendix 3);
SCOPUS (date to July 2016) (Appendix 4);
Web of Science (1985 to July 2016) (Appendix 5);
www.clinicaltrials.gov, International Pharmaceutical Abstracts, the National Research Register and Google Scholar for completed and ongoing trials (Appendix 6).
Searching other resources
We also searched Google Scholar, conference proceedings and reference lists of included studies for relevant studies.
Data collection and analysis
Selection of studies
Two review authors (JD, XS) performed study selection independently, with any subsequent disagreement resolved through discussion with a third co‐author (NS). Studies were included in the review irrespective of whether they reported measured outcome data.
Data extraction and management
Two review authors (XS, NS) independently collected data from the included studies into data sheets. We resolved disagreements through discussion with a third co‐author (JD). Two studies (Saad 2000; Wills 2001) that did not use standard visual analogue scales (VAS) had their 0 to 100 scores converted to a score from 0 to 10.
Assessment of risk of bias in included studies
We used the Cochrane ‘Risk of bias’ tool for assessing risk of bias of included trials (Higgins 2011). We assessed risk of bias of the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting bias;
other bias (conflicts of interest, reporting of data, reporting and balance of characteristics at baseline).
We judged each domain as low risk, high risk or unclear risk of bias according to criteria used in the Cochrane ‘Risk of bias’ tool (see Appendix 7) (Higgins 2011). We judged a study as low risk of bias if we assessed all of the first six domains as low risk. Two review authors (JD, XS) independently assessed the risk of bias and disagreements were resolved with a third author (NS).
Measures of treatment effect
We calculated the effect of the intervention for each trial, expressing categorical data as relative risks (RR) with 95% confidence intervals (CI) and continuous data as mean differences (MD) ± 95% CIs.
Unit of analysis issues
For individual trials, the unit of analysis we used was individual participants. There were no cluster‐randomised trials or cross‐over trials that would be at risk of unit of analysis issues eligible for inclusion in our review. If there are such studies in future updates, we will perform sensitivity analyses to determine the effect of these trials on outcome measures.
Dealing with missing data
If possible, we obtained missing data either from the original study authors or from similar reviews written by others (Lee 2011; Sajid 2008; Sammour 2008). We contacted nine authors, four responded with additional data, two had no further data, and three did not respond. When the original data only provided the mean, we used the largest standard deviation (SD) in the group of trials in the analysis.
Assessment of heterogeneity
We assessed clinical heterogeneity for differences in participant characteristics (paediatric vs adult), intervention characteristics (humidified vs non‐humidified, duration of surgery, external warming) and outcome measures (abdominal vs shoulder pain) with subgroup analysis where possible. Heterogeneity was tested using the Chi² test with significance set at P < 0.10 and the amount of heterogeneity quantified by the I² statistic. Heterogeneity was considered as low, moderate, and high based on I2 values of 25%, 50%, and 75%, respectively (Higgins 2003).
Assessment of reporting biases
We considered publication bias and compiled funnel plots for the studies to reveal this. We then applied Egger's linear regression analysis (Egger 1997) to each funnel plot to detect asymmetry.
Data synthesis
We used meta‐analysis to combine the outcomes and determine the estimated effect of intervention, which we calculated using Review Manager (RevMan) software version 5.3 (RevMan 2014). We applied the random‐effects method in our analysis, assuming that the true effect estimates varied among studies.
Subgroup analysis and investigation of heterogeneity
When significant heterogeneity was found among studies, we performed subgroup analysis to explore the source. We performed subgroup analysis for humidified vs non‐humidified subgroups for the following outcomes: core temperature, pain score, morphine consumption, hospital stay, and operative time. For the core temperature outcome, we also analysed subgroups with longer operative times (more than 120 minutes) and those with external warming. The 120‐minute threshold was decided after consulting with surgeons on the research team as there was no clear definition in the literature. Further, for pain scores, we performed subgroup analysis for shoulder and abdominal pain. Shoulder pain occurs in some patients after insufflation due to referred pain from irritation of the diaphragm. Additionally, we performed separate analysis with only low‐risk‐of‐bias studies for core temperature, pain score and recovery time.
Summary of Findings Table
We assessed the quality of evidence of core temperature, pain score, morphine consumption, hospital stay, recovery room stay, lens fogging and operative time for the heated gas group versus cold gas group using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2009) in the 'Summary of Findings' table(s).
The GRADE system classifies the quality of evidence in one of four grades:
| Grade | Definition |
| High | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
Factors that influence the quality of evidence:
| Downgrades the evidence | Upgrades the evidence |
| Study limitation | Large magnitude of effect |
| Inconsistency of results | All plausible confounding would reduce the demonstrated effect |
| Indirectness of evidence | Dose‐response gradient |
| Imprecision | |
| Publication bias |
Sensitivity analysis
Not all studies had adequately reported on sequence generation, allocation concealment, blinding, or number of and reasons for withdrawals, and were therefore at an unclear risk of bias. We therefore performed sensitivity analyses including only those trials with a known low risk of bias.
Results
Description of studies
Results of the search
The electronic searches identified a total of 2392 citations. After removing duplicate studies, we reviewed 1850 titles or abstracts, and excluded trials that involved non‐abdominal procedures, uncommon laparoscopic procedures, non‐human subjects and those not using cold gas as a control. We also excluded duplicated studies and non‐randomised controlled trials. Finally, the review authors DB, NS and XS agreed that 22 trials met the inclusion criteria and included them in this review. See PRISMA diagram (Moher 2009) (Figure 1).
1.
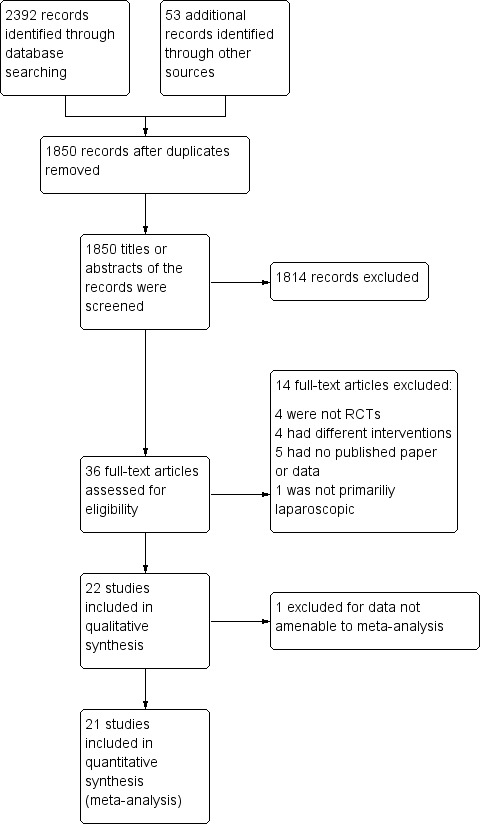
Study flow diagram
Included studies
All 22 included studies were RCTs comparing heated CO2 insufflation (with or without humidification) with standard cold CO2 insufflation. All the included studies used CO2 insufflation. We excluded from the review studies examining outcomes that were dissimilar to those relevant to this review and studies where we did not receive a response from the authors. Surgical procedures included in the studies were: gastric bypass (n = 168), gynaecologic surgery (n = 259), cholecystectomy (n = 500), Nissen fundoplication (n = 157), appendicectomy (n = 190), low anterior resection (n = 16), gastrectomy (n = 7), colonic surgery (n = 84), diagnostic laparoscopy (n = 40), hernioplasty (n = 4), myotomy (n = 2) and rectopexy (n = 1).
Primary outcome data were available for 1081 participants as three studies (Demco 2001; Klugsberger 2014; Slim 1999) did not report intraoperative changes in core temperature. Of these, 430 were in the heated, humidified gas group; 105 were in the heated‐only gas group; and 546 were in the cold gas group.
Five studies had relatively long operative times (more than 120 minutes) (Backlund 1998; Hamza 2005; Lee 2011; Ott 1991; Sammour 2010). Ten out of 22 studies used a warming blanket for simultaneous warming (Backlund 1998; Farley 2004; Hamza 2005; Lee 2011; Manwaring 2008; Nguyen 2002; Sammour 2010; Savel 2005; Wills 2001; Yu 2013). A heated insufflation company supported 11 of the 22 trials (Backlund 1998; Davis 2006; Farley 2004; Hamza 2005; Kissler 2004; Manwaring 2008; Mouton 1999; Nelskyla 1999; Ott 1998; Savel 2005; Wills 2001). Ten of the 22 studies demonstrated a benefit with the use of heated gas insufflation (Agaev 2013; Backlund 1998; Farley 2004; Hamza 2005; Klugsberger 2014; Lee 2011; Mouton 1999; Nelskyla 1999; Ott 1998; Puttick 1999). See Characteristics of included studies and Table 8; Table 9; Table 10 for full study details.
1. Demographics of included studies.
| Study | Number of participants | Mean age (years) | % Female | Mean BMI (kg/m2) or weight (kg) |
| Agaev 2013 | 150 | 52 | 72.7 | |
| Backlund 1998 | 26 | 49W/53C | 42.3 | 25W/25C (BMI) |
| Champion 2006 | 50 | 41.5WH/44C | 86 | 50W/52.9C (BMI) |
| Davis 2006 | 44 | 42.3WH/40.6W/44.8H/42.5C | 47.2WH/49.1W/48.5H/52.4C (BMI) | |
| Demco 2001 | 40 | 100 | ||
| Farley 2004 | 117 (16 excluded) | 52 | 68.3 | 29.5W/29.7C (BMI) |
| Hamza 2005 | 50 (6 excluded) | 44WH/45C | 89.1 | 125W/128C (weight) |
| Kissler 2004 | 90 (53 with data) | 37WH/33W/36C | 100 | 63WH/63W/65C (weight) |
| Klugsberger 2014 | 148 | 55.7 | 69.6 | 28.56 (BMI) |
| Lee 2011 | 30 | 60.1W/55.1C | 36.7 | |
| Manwaring 2008 | 60 | 30WH/30C | 100 | 25W/24C (BMI) |
| Mouton 1999 | 32 | 23‐89 (range) | ||
| Nelskyla 1999 | 37 | 46W/47C | 100 | 63W/66C (weight) |
| Nguyen 2002 | 20 | 43WH/45C | 45 | |
| Ott 1998 | 72 (50 with data) | 100 | ||
| Puttick 1999 | 30 | 46.2W/53.7C | ||
| Saad 2000 | 20 | 62W/51C | 60 | 75W/83C (weight) |
| Sammour 2010 | 82 (8 excluded) | 71WH/69C | 57.1W/59C | 26.5W/25.5C (BMI) |
| Savel 2005 | 30 | 41WH/39C | 80 | 50.6W/52.3C (BMI) |
| Slim 1999 | 108 (8 excluded) | 52W/53C | 58 | 26.9W/25.7C (BMI) |
| Wills 2001 | 41 (1 excluded) | 47.5W/52.2C | 45 | 27W/29.2C (BMI) |
| Yu 2013 | 195 (5 excluded) | 12 | 36.8 | 49.6W/50.3C (weight) |
W = warmed cohort, C = cold cohort, H = humidified cohort, WH = warmed and humidified cohort
2. Methodology of included studies.
| Study | Procedures | Method of temperature measurement | Insufflation gas | Gas temperature (°C) | Heating device | Humidification (%) | Duration of surgery (minutes) | External warming |
| Agaev 2013 | 110 laparoscopic cholecystectomy, 40 laparoscopic fundoplication | Carbon dioxide | WISAP Flow Thermo | Not specified | 42WH/56C | None | ||
| Backlund 1998 | Laparoscopic fundoplication, hernioplasty, sigmoid colon resection, rectopexy | Pulmonary artery catheter | Carbon dioxide | 37 | WISAP Flow Thermo | None | 161W/163C | Warm blanket/ warm waterbath mattress |
| Champion 2006 | Laparoscopic Roux‐en‐Y gastric bypass | Rectal thermometer | Carbon dioxide | 35 | Lexion Insuflow | 95 | 61.7WH/61.7C | None |
| Davis 2006 | Laparoscopic Roux‐en‐Y gastric bypass | Foley catheter for bladder temperature | Carbon dioxide | 37 | Lexion Insuflow | 95 | 78‐84 (range) | None |
| Demco 2001 | Awake laparoscopy | Carbon dioxide | 35 | Lexion Insuflow | 95 | None | ||
| Farley 2004 | Laparoscopic cholecystectomy | Oesophageal probe | Carbon dioxide | 35 | Lexion Insuflow | 95 | 91.2 | Bair Hugger forced air warmer (32 °CW/34 °C C) |
| Hamza 2005 | Laparoscopic Roux‐en‐Y gastric bypass | Oesophageal/ tympanic membrane | Carbon dioxide | 37 | Lexion Insuflow | 95 | 120WH/132C | Warm cotton blankets |
| Kissler 2004 | Laparoscopic gynaecologic surgery | Intravesical temperature | Carbon dioxide | 38 | Laparo‐CO2‐Pneu2232 | 95‐100 | 62WH/51W/45C | None |
| Klugsberger 2014 | Laparoscopic cholecystectomy | Rectal probe | Carbon dioxide | 35 | Storz Optitherm | 95 | 63.88 | None |
| Lee 2011 | Laparoscopic low anterior resection, colectomy, gastrectomy | Oesophageal temperature probe | Carbon dioxide | 37 | WISAP Flow Thermo | None | 212W/230C | Bair Hugger forced air warmer/ warming mattress with circulating water at 38 °C |
| Manwaring 2008 | 49 laparoscopy for endometriosis, 16 laparoscopy for adhesions | Carbon dioxide | 37 | Fisher & Paykel | 100 | 49.6WH/46.8C | Upper body warming blanket | |
| Mouton 1999 | Laparoscopic cholecystectomy | Oesophageal thermoresistor | Carbon dioxide | 34‐37 | LINS‐1000 | 88‐90 | 40WH/48.3WH | None |
| Nelskyla 1999 | Laparoscopic hysterectomy | Tympanic and nasopharyngeal infrared technique | Carbon dioxide | 37 | None | 56W/51C | None | |
| Nguyen 2002 | Laparoscopic Nissen fundoplication | Oesophageal probe | Carbon dioxide | 37 | Georgia BioMedical Insuflow | 95 | 35.6WH/35.6C | Bair Hugger forced air warmer |
| Ott 1998 | Laparoscopic gynaecologic surgery | Endotracheal temperature probe | Carbon dioxide | 36.2 | Insuflow | 95 | 38‐262 (range) | None |
| Puttick 1999 | Laparoscopic cholecystectomy | Oesophageal probe | Carbon dioxide | 37 | WISAP Flow Thermo | None | 31.5W/32.1C | None |
| Saad 2000 | Laparoscopic cholecystectomy | Oesophageal probe | Carbon dioxide | 37 | WISAP Flow Thermo | None | 56W/61C | None |
| Sammour 2010 | Laparoscopic colon resection | Oesophageal probe | Carbon dioxide | 37 | Fisher & Paykel | 98 | 176.3WH/184.7C | Bair Hugger forced air warmer |
| Savel 2005 | Laparoscopic Roux‐en‐Y gastric bypass | Oesophageal probe | Carbon dioxide | 35 | Lexion Insuflow | 95 | 76WH/101C | Bair Hugger forced air warmer at discretion of blinded anaesthesiologist |
| Slim 1999 | Laparoscopic cholecystectomy, fundoplication, myotomy | Subdiaphragmatic thermometric probe | Carbon dioxide | 37 | ThermoFlator | None | 73W/67C | None |
| Wills 2001 | Laparoscopic fundoplication | Nasopharyngeal thermistor | Carbon dioxide | 37 | Cook LINS‐2000 | None | 69W/72C | Bair Hugger forced air warmer |
| Yu 2013 | Laparoscopic appendectomy | Naso‐oesophageal probe | Carbon dioxide | 37 | Fisher & Paykel | 98 | 69.8WH/71.6C | Forced‐air warming blanket |
W = warmed cohort, C = cold cohort, H = humidified cohort, WH = warmed and humidified cohort
3. Outcomes of included studies.
| Study | Mean change in core temperature (°C) | Adverse events (Clavien‐Dindo ≥ III) | ||||
| Heated and humidified | Heated only | Cold | Heated and humidified | Heated only | Cold | |
| Agaev 2013 | 0.49 | ‐0.06 | Not reported | Not reported | ||
| Backlund 1998 | 0.2 | ‐0.1 | Not reported | Not reported | ||
| Champion 2006 | ‐0.4 | ‐0.4 | Not reported | Not reported | ||
| Davis 2006 | 0.4 | 0.2 | 0.4 | Not reported | Not reported | Not reported |
| Demco 2001 | Not reported | Not reported | Not reported | Not reported | ||
| Farley 2004 | 0.29 | ‐0.03 | Not reported | Not reported | ||
| Hamza 2005 | ‐0.7 | ‐1.7 | Not reported | Not reported | ||
| Kissler 2004 | ‐0.5 | ‐0.6 | ‐0.4 | Not reported | Not reported | Not reported |
| Klugsberger 2014 | Not reported | Not reported | 0 | 0 | ||
| Lee 2011 | ‐0.4 | ‐0.7 | Not reported | Not reported | ||
| Manwaring 2008 | ‐0.2 | ‐0.13 | Not reported | Not reported | ||
| Mouton 1999 | ‐0.25 | ‐0.3 | 0 | 0 | ||
| Nelskyla 1999 | ‐0.2 | 0 | Not reported | Not reported | ||
| Nguyen 2002 | 0.4 | 0.3 | 0 | 0 | ||
| Ott 1998 | ‐0.3 | ‐1.64 | 0 | 0 | ||
| Puttick 1999 | ‐0.24 | ‐0.42 | Not reported | Not reported | ||
| Saad 2000 | 0 | ‐0.1 | Not reported | Not reported | ||
| Sammour 2010 | 0.64 | 0.48 | 3 (8.6%) | 5 (12.8%) | ||
| Savel 2005 | 0.4 | ‐0.3 | Not reported | Not reported | ||
| Slim 1999 | Not reported | Not reported | 0 | 0 | ||
| Wills 2001 | 0.2 | 0 | 0 | 1 (4.8%) | ||
| Yu 2013 | 0.1 | 0.1 | 3 (10.3%) | 0 | ||
Agaev 2013: originally published in Russian, this study examined 150 laparoscopic operations (110 cholecystectomies and 40 fundoplications), participants with standard CO2 vs. warmed, humidified CO2 during the operations. Their conclusion was warmed, humidified CO2 had advantages for maintaining a warmer intraoperative core temperature, having less postoperative pain and requiring fewer analgesic prescriptions.
Backlund 1998: examined the effect of 37 °C and room temperature‐insufflated CO2 during and after prolonged laparoscopic surgery (more than 120 minutes). Twenty six participants undergoing fundoplication, hernioplasty, resection of the sigmoid colon and rectopexy were randomly assigned to warm or cold gas groups. Core temperature, cardiac index, urine output and recovery room opioid usage and pain scores were recorded.
Champion 2006: was a trial of heated, humidified versus cold dry CO2 insufflation for laparoscopic gastric bypass, which examined 50 consecutive obese patients with homogeneous baseline characteristics (gender, age, preoperative weight, body mass index (BMI) and c‐reactive protein (CRP)) between groups. The ambient insufflation gas was at a temperature of 35 °C and 95% relative humidity. The sole difference identified in the heated group was a lower postoperative subjective shoulder pain score at 18 hours. There were no differences between groups in intraoperative core temperature, operating room temperature, litres of insufflation, operating time, number of lens cleanings, recovery room temperature, narcotic usage, length of hospitalisation, high‐sensitivity CRP at 24 hours or abdominal pain scores.
Davis 2006: with adequate allocation concealment, this study examined 44 laparoscopic Roux‐en‐Y gastric bypass patients in Ohio State University. There were four study groups with 11 participants in each and similar baseline characteristics across the groups. The groups included the following insufflation techniques: 1) cold dry, 2) cold humidified (97% relative humidity), 3) heated dry (37 °C) and 4) heated humidified (37 °C and 97% relative humidity) CO2. There were no differences in patient core temperature, intra‐abdominal humidity, postoperative narcotic usage, pain scale scores, recovery room time, length of hospitalisation, lens fogging or macrophage activity between groups, though participants in the heated, humidified insufflation group demonstrated increased macrophage activity in biopsies.
Demco 2001: 40 women undergoing diagnostic laparoscopy were randomised to heated, humidified insufflation or cold CO2. Outcomes were shoulder pain, fentanyl use, percent requiring general anaesthetic, percent requiring intravenous sedation, amount of gas instilled before experiencing pain, operating time, recovery room time and time to recovery of shoulder pain. Outcomes were presented as percentages of participants in various groups (e.g. operative time more than 10 minutes, 10 to 20 minutes, etc.), which could not be included in meta‐analysis.
Farley 2004: with adequate allocation concealment, randomised 101 people undergoing laparoscopic cholecystectomy to either cold or heated and humidified CO2 insufflation. The experimental group showed higher intraoperative core temperatures and decreased postoperative pain scores at day 14; the study authors questioned the clinical relevance of the latter outcome. They identified no differences in the rate of lens fogging, narcotic requirements, length of hospitalisation or time of return to baseline activity levels.
Hamza 2005: randomised 50 people undergoing laparoscopic Roux‐en‐Y gastric bypass surgery, with no information on allocation concealment, to cold or heated and humidified CO2 insufflation. Six were excluded. Mean operative times for each group were greater than 120 min. The heated group showed a higher intraoperative core temperature, a reduction in time in the recovery room and narcotic requirements, and a higher quality of recovery at 48 hours postoperatively. There were no differences in postoperative tympanic membrane temperatures, pain scores, shivering, overall morphine usage, nausea scores, Aldrete recovery assessment scores, length of hospital stay or lens fogging.
Kissler 2004: recruited 90 consecutive women scheduled for gynaecologic laparoscopic surgery into this study with randomisation to heated humidified, heated non‐humidified and cold gas insufflation groups, each with 30 participants. The trial was stopped following enrolment of 53 participants due to a tendency for less pain and higher postoperative satisfaction in the cold insufflation control group.
Klugsberger 2014: randomised 148 people undergoing laparoscopic cholecystectomy to standard gas or warmed, humidified gas groups. Intraoperative core temperature was significantly higher with less six‐hour postoperative pain in the warmed, humidified gas group. Pain was not significantly different on the first day after operation.
Lee 2011: randomised 30 people undergoing laparoscopic low anterior resection, colectomy or gastrectomy to heated CO2 or standard CO2 groups. Mean operative times were greater than 200 minutes for each cohort. They recorded acid‐base parameters and core temperature. Heated CO2 did not significantly change acid‐base parameters in participants but reduced the decrease in core body temperature 30 minutes after pneumoperitoneum.
Manwaring 2008: randomised 60 gynaecology patients to heated humidified or cold insufflation groups. Heated and humidified gas insufflation was not associated with any significant benefits as no difference was found in oesophageal temperature, pain scores or narcotic usage.
Mouton 1999): randomised 40 people undergoing cholecystectomy to heated, humidified insufflation or cold gas insufflation. Eight were excluded. Though they found no difference in core temperature during the relatively brief operations, there was significantly less pain compared to the experimental heated and humidified insufflation participants at six hours and on the first to third days postoperatively. Pain was also less on the 14th postoperative day.
Nelskyla 1999: randomised 40 women undergoing laparoscopic hysterectomy to heated or unheated gas insufflation groups. Three were excluded. Tympanic and nasopharyngeal intraoperative temperatures were not different between the groups.
Nguyen 2002: randomised 20 people undergoing laparoscopic Nissen fundoplication, without information on the allocation method, to heated and humidified or cold and dry gas insufflation groups. There were no differences in core temperature, pain scores, narcotic consumption, urine output or lens fogging.
Ott 1998: without stating the number of participants in each group, this study randomised 72 women undergoing laparoscopic gynaecologic surgery to heated and humidified or cold and dry gas insufflation. Most data was extracted from a systematic review (Sammour 2008) and was only available for 50 patients with no reason was given. The experimental heated group showed improved intraoperative normothermia and postoperative pain, and reduced recovery room stay.
Puttick 1999: randomised 30 people undergoing laparoscopic cholecystectomy to heated or cold gas insufflation. The study authors concluded that intraoperative cooling could be prevented by heating the insufflated gas.
Saad 2000: randomised 20 people undergoing laparoscopic cholecystectomy to heated or cold gas insufflation with no effects when comparing core temperature or postoperative pain. VAS pain scores were converted from a 0 to 100 scale to a standard 0 to 10 scale.
Sammour 2010: randomised 82 people undergoing laparoscopic colon surgery to heated, humidified or cold gas insufflation groups, each with 41 participants. Eight patients were excluded. They found no significant effects, including no effect on the early postoperative inflammatory cytokine response. Mean operative times were greater than 170 minutes for both cohorts.
Savel 2005: randomised 30 people undergoing laparoscopic Roux‐en‐Y gastric bypass to cold or heated and humidified gas insufflation groups. Length of hospitalisation and operative time were reduced in the experimental group but the study found no differences in pain sensation.
Slim 1999: enrolled 100 people undergoing laparoscopic cholecystectomy, fundoplication, or Heller's myotomy and randomised them to cold or heated insufflation. Shoulder and subcostal pain sensation was increased in the heated insufflation group and the study found no difference on core temperature or narcotic consumption.
Wills 2001: randomised 41 people to heated or cold gas insufflation during laparoscopic fundoplication. One was excluded. An increased core temperature was associated with the heated insufflation group, though the control group participants suffered less postoperative pain and required fewer narcotics. VAS pain scores were converted from a 0 to 100 scale to a standard 0 to 10 scale.
Yu 2013: randomised 195 children undergoing laparoscopic appendectomy to warm, humidified CO2 or standard CO2 groups. Five were excluded. The study assessed postoperative opioid usage, pain intensity, postoperative recovery and return to normal activities. Warm, humidified CO2 insufflation had no short‐term clinical benefits on postoperative outcomes in children.
Excluded studies
We excluded Beste 2006 and Benavides 2009 from this review because they compared heated, humidified CO2 with heated, non‐humidified CO2, a comparison not intended for this review. However, they were included in two previously published systematic reviews (Sajid 2008; Sammour 2008). Herrmann 2015 we excluded because it assessed laparoscopic‐assisted vaginal hysterectomies which is not primarily an abdominal, laparoscopic surgery. We excluded the remaining studies because they were not RCTs. Excluded studies were excluded from both quantitative and qualitative analyses. See section on Characteristics of excluded studies for details.
Risk of bias in included studies
We assessed risk of bias for all included studies (Figure 2; Figure 3). Eleven studies had an overall low risk of bias (low risk of bias for the six main criteria assessed) in the presentation of their results (Champion 2006; Davis 2006; Farley 2004; Hamza 2005; Lee 2011; Manwaring 2008; Nguyen 2002; Sammour 2010; Slim 1999; Wills 2001; Yu 2013).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
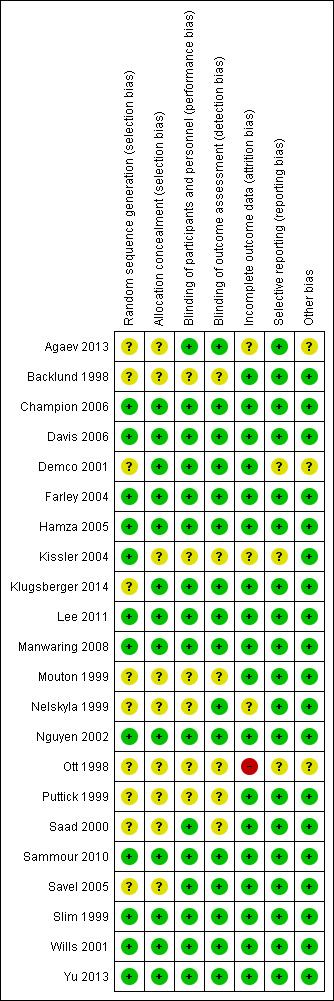
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We rated 11 studies at unclear risk of bias, with nine of these studies (Backlund 1998; Demco 2001; Kissler 2004; Mouton 1999; Nelskyla 1999; Ott 1998; Puttick 1999; Saad 2000; Savel 2005) failing to report on the methodology for randomisation or allocation concealment. Agaev 2013 stated that randomisation was done with a computer model post‐anaesthetic, but comparative groups were very uneven with 84 in the heated group and 66 in non‐heated. Klugsberger 2014 was unclear about randomisation and also had uneven groups (67 in heated and 81 in non‐heated).
Blinding
We judged five studies (Backlund 1998; Mouton 1999; Ott 1998; Puttick 1999; Saad 2000) at unclear risk of bias because they had no description of blinding. The remaining studies were adequately blinded with only one or two operating‐room personnel unblinded to initiate the intervention.
Incomplete outcome data
We deemed three studies an unclear risk of attrition bias. Agaev 2013 did not state the number of participants included in their analysis. Kissler 2004 was stopped early because the control group had less pain and improved satisfaction. Nelskyla 1999 excluded three participants without clear reasoning. Ott 1998 reported data on only 55 of 72 participants and did not state a reason for this missing data. This was assessed a high risk of bias.
Selective reporting
Demco 2001 did not report any core temperatures which would be expected from a study on heated insufflation. Klugsberger 2014 and Slim 1999 reported mean core temperatures but did not report on intraoperative changes in core temperature. However, Slim 1999 only measured subdiaphragmatic core temperatures once during the operation so this is not due to selective reporting.
Other potential sources of bias
Agaev 2013, originally published in Russian, was translated voluntarily by a research scientist employed by a surgical humidification device company. We deemed this an unclear risk of bias due to a possible conflict of interest. Many studies (Agaev 2013; Backlund 1998; Champion 2006; Davis 2006; Hamza 2005; Kissler 2004; Lee 2011; Mouton 1999; Nguyen 2002; Ott 1998; Saad 2000; Savel 2005; Wills 2001) were also missing standard deviations and this potentially distorted the true effects and potentially increased the error.
Demco 2001 did not report any baseline demographics, while Ott 1998 did not separate demographics between groups and potential imbalances in participant characteristics could have contributed to bias.
Industry supported eight trials by providing heated insufflation devices (Backlund 1998; Farley 2004; Kissler 2004; Manwaring 2008; Nelskyla 1999; Savel 2005; Wills 2001). Two trials received educational grants from industry (Davis 2006; Hamza 2005) and one trial reported industry assistance (Mouton 1999). We judged this a low risk of bias as there appeared to be no industry influence in the trials with industry support.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Primary outcome
Change in core temperature
(Analysis 1.1; Analysis 1.2; Analysis 1.3)
1.1. Analysis.

Comparison 1 Core temperature (ºC), Outcome 1 Change in core temperature.
1.2. Analysis.

Comparison 1 Core temperature (ºC), Outcome 2 Change in core temperature for low risk of bias studies.
1.3. Analysis.
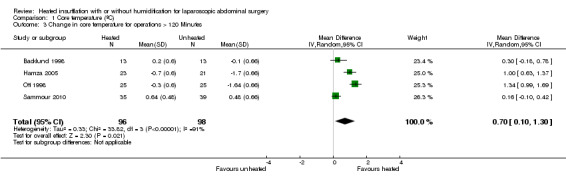
Comparison 1 Core temperature (ºC), Outcome 3 Change in core temperature for operations > 120 Minutes.
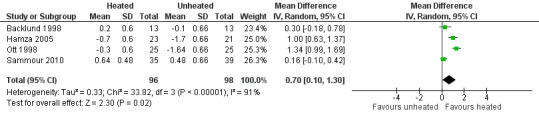
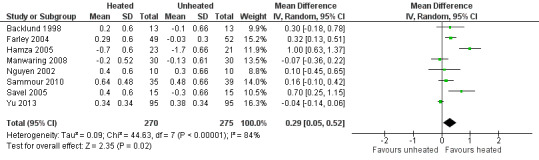
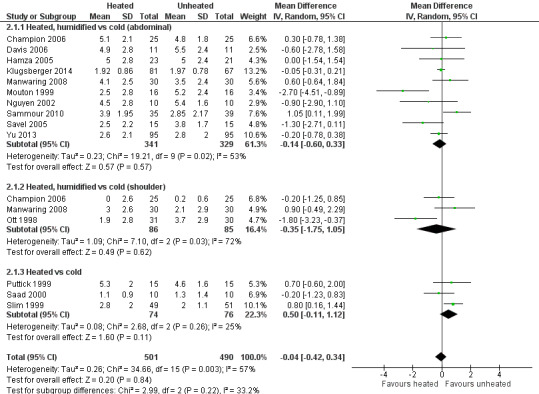
Nineteen studies reported change in intraoperative core temperatures. Overall, core temperature was slightly higher with heated CO2 (MD 0.21 °C, 95% CI 0.06 to 0.36, P = 0.007) (Figure 4). Heterogeneity was substantial (I2 = 86%), therefore subgroup analyses were performed for humidified and non‐humidified CO2. Heated gas with humidification had a small, but positive effect on core temperature intraoperatively compared to cold CO2 (MD 0.31 °C, 95% CI 0.09 to 0.53, P = 0.005) (Figure 4). When only studies with low risk of bias were assessed, this effect became statistically non‐significant (Figure 5). No apparent effect was found in the non‐humidified, heated‐gas group compared to cold gas, regardless of analysis based on all studies or only low‐risk studies. A subgroup analyses for operations lasting less and more than 120 minutes were also performed. There was no difference detected in temperature between heated and cold CO2 for operations lasting less than 120 minutes. However, for operations lasting over 120 minutes (Backlund 1998; Hamza 2005; Ott 1998; Sammour 2010), temperature was significantly higher with warming and humidification, but the studies exhibited significant statistical heterogeneity (I2 = 91%) (Figure 6). When subgroup analyses of studies using external warming were conducted, core temperatures were significantly higher in the heated, humidified group (MD 0.29 °C, 95% CI 0.05 to 0.52) (Figure 7); but the studies were once again statistically heterogenous (I2 = 84%). The only trial with a known low risk of bias (Savel 2005) showed no statistically significant difference between groups, however, with only 30 participants, such a small trial would unlikely be adequately powered to detect a difference between groups even if one was present (Figure 7). When only trials not using external warming were analysed, heated, humidified gas had no apparent effect on core temperature compared to cold gas. (Figure 8).
4.
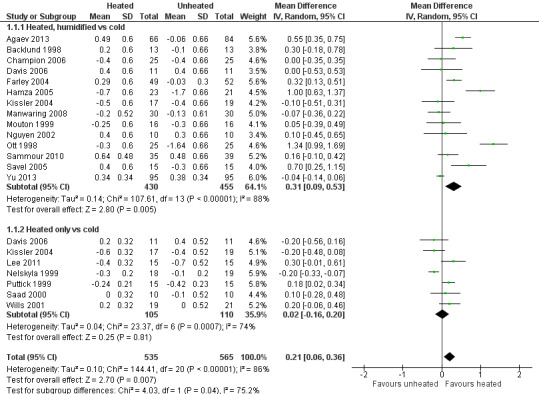
Forest plot of comparison: 2 Core temperature, outcome: 2.1 Change in core temperature
5.
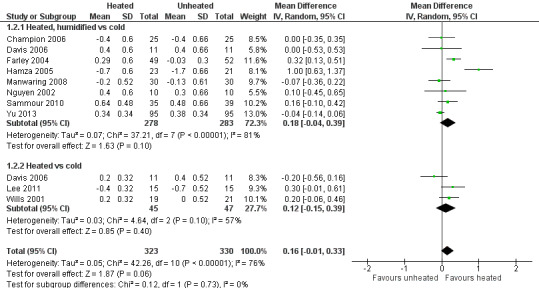
Forest plot of comparison: 2 Core temperature, outcome: 2.2 Change in core temperature for low risk of bias studies
6.
Forest plot of comparison: 1 Core temperature, outcome: 1.5 Change in core temperature in heated, humidified vs cold groups with OR > 120 Minutes
7.
Forest plot of comparison: 2 Core temperature, outcome: 2.3 Change in core temperature in heated, humidified vs cold groups with external warming
8.
Forest plot of comparison: 1 Core temperature, outcome: 1.4 Change in temperature in heated, humidified vs cold groups without external warming
Secondary outcomes
Pain scores
(Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4)
2.1. Analysis.
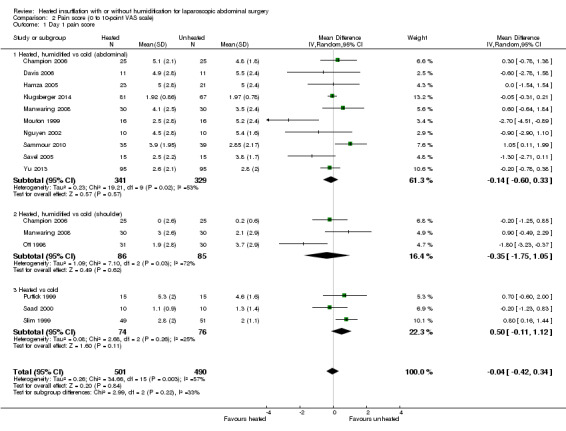
Comparison 2 Pain score (0 to 10‐point VAS scale), Outcome 1 Day 1 pain score.
2.2. Analysis.
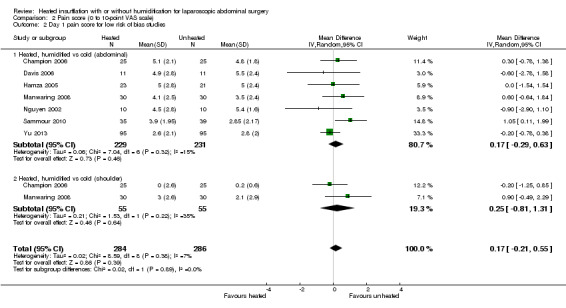
Comparison 2 Pain score (0 to 10‐point VAS scale), Outcome 2 Day 1 pain score for low risk of bias studies.
2.3. Analysis.

Comparison 2 Pain score (0 to 10‐point VAS scale), Outcome 3 Day 2 pain score.
2.4. Analysis.

Comparison 2 Pain score (0 to 10‐point VAS scale), Outcome 4 Day 2 pain score of low risk of bias studies.
For pain scores (measured using a 0 to 10 visual analogue scale), there was no statistically significant difference detected between groups overall on day 1 (MD ‐0.04, 95% CI ‐0.42 to 0.34) or day 2 (MD ‐0.28, 95% CI ‐0.78 to 0.21). Subgroup analyses were performed for the effect of humidified CO2 and non‐humidified CO2, on shoulder and abdominal pain separately, and for heated only versus cold CO2 (not by location of pain).
Day 1
The effect of heated and humidified gas on postoperative day one showed no statistically significant difference compared to cold gas (abdominal pain MD ‐0.14, 95% CI ‐0.60 to 0.33, P = 0.57; shoulder pain MD ‐0.35, 95% CI ‐1.75 to 1.05, P = 0.62) (Figure 9). Given the significant heterogeneity across studies (abdominal P = 0.02, I2 = 53%; shoulder P = 0.03, I2 = 72%), sensitivity analyses were performed and only studies with a known low risk of bias were included. The pain scores were still apparently not different with respect to either abdominal or shoulder pain and the test of heterogeneity was no longer statistically significant (abdominal P = 0.32, I2 = 15%; shoulder P = 0.22, I2 = 35%) (Figure 10). When heated only gas was compared to cold gas, the day‐one pain scores were not statistically significantly different (Figure 9).
9.
Forest plot of comparison: 1 Pain score, outcome: 1.1 Day 1 pain score
10.

Forest plot of comparison: 1 Pain score, outcome: 1.3 Day 1 pain score for low risk of bias study
Day 2
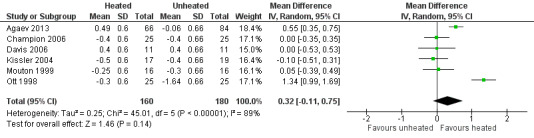
For pain on the second postoperative day, heated and humidified gas did not apparently improve abdominal or shoulder pain (abdominal MD ‐0.40, 95% CI ‐1.07 to 0.28, P = 0.25; shoulder MD ‐0.88, 95% CI ‐2.93 to 1.17, P = 0.40), but again, the studies were heterogenous (I2 62% and 92%, respectively) (Figure 11). When only low risk of bias studies were included, the conclusion remained unchanged (Figure 12) and I2 decreased to 0%. With heated only gas, the postoperative day‐two pain score was similar to the cold gas control (MD 0.41, 95% CI ‐0.44 to 1.27, P = 0.34) with no statistically significant heterogeneity across trials (P = 0.23, I2 = 33%).
11.

Forest plot of comparison: 1 Pain score, outcome: 1.2 Day 2 pain score
12.
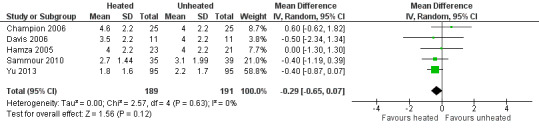
Forest plot of comparison: 1 Pain score, outcome: 1.4 Day 2 pain score of low risk of bias studies
Morphine consumption
(Analysis 3.1; Analysis 3.2; Analysis 3.3)
3.1. Analysis.
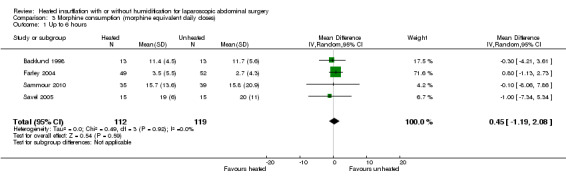
Comparison 3 Morphine consumption (morphine equivalent daily doses), Outcome 1 Up to 6 hours.
3.2. Analysis.
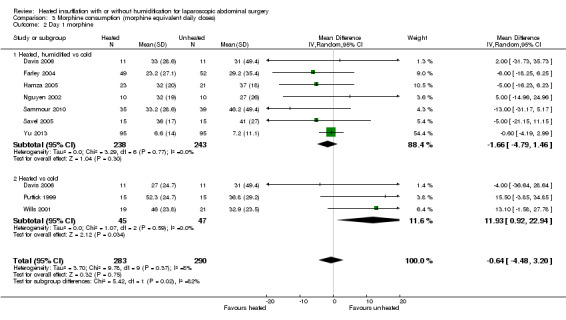
Comparison 3 Morphine consumption (morphine equivalent daily doses), Outcome 2 Day 1 morphine.
3.3. Analysis.

Comparison 3 Morphine consumption (morphine equivalent daily doses), Outcome 3 Day 2 morphine.
Four studies comparing heated and humidified CO2 with cold CO2 insufflation reported no statistically significant difference in morphine consumption up to six hours post‐operatively between groups (MD 0.45 mg, 95% CI ‐1.19 to 2.08, P = 0.59) (Figure 13). Heterogeneity was not statistically significant across studies (I2 = 0%). Morphine use on the first postoperative day was not significantly different either overall (MD ‐0.64 mg, 95% CI ‐4.48 to 3.20), or when CO2 was heated and humidified (MD ‐1.66 mg, 95% CI ‐4.79 to 1.46, P = 0.30), but was higher when CO2 was heated without humidification (MD 11.93 mg, 95% CI 0.92 to 22.94, P = 0.03) (Figure 14). A similar pattern was observed for the second postoperative day, where there was no difference overall or with humidification, but was higher with heated, non‐humidified CO2 (MD 9.79 mg, 95% CI 1.58 to 18.00, P = 0.02) (Figure 15).
13.

Forest plot of comparison: 3 Morphine consumption, outcome: 3.1 Up to 6 hours
14.

Forest plot of comparison: 3 Morphine consumption, outcome: 3.2 Day 1 morphine
15.
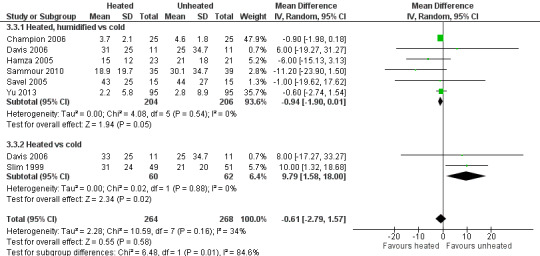
Forest plot of comparison: 3 Morphine consumption, outcome: 3.3 Day 2 morphine
Hospital stay
4.1. Analysis.
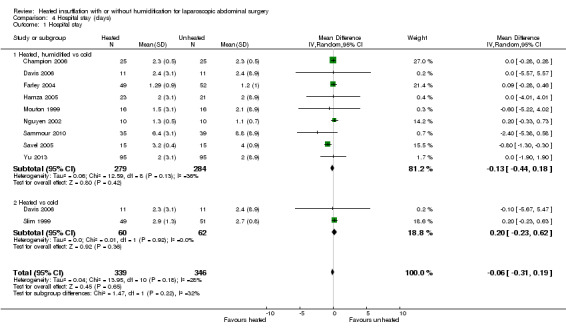
Comparison 4 Hospital stay (days), Outcome 1 Hospital stay.
Length of stay in hospital was not different between the heated (with or without humidification) and cold gas insufflation groups (MD ‐0.06 days, 95% CI ‐0.31 to 0.19, P = 0.65) (Figure 16). There was no statistically significant heterogeneity across studies (I2 = 28%).
16.

Forest plot of comparison: 4 Hospital stay, outcome: 4.1 Hospital stay
Recovery room stay
5.1. Analysis.

Comparison 5 Recovery room stay (minutes), Outcome 1 Recovery time.
5.2. Analysis.

Comparison 5 Recovery room stay (minutes), Outcome 2 Recovery time for low risk of bias studies.
Recovery room time was documented in six studies and there was substantial heterogeneity among them (I2 = 95%). Shorter recovery time (MD ‐26.79 minutes, 95% CI ‐51.34 to ‐2.25, P = 0.03) was found with heated insufflation (Figure 17). With exclusion of the only high risk study (Ott 1998), the studies were statistically homogenous (I2 =12%) but the difference in recovery room stay was statistically not significant (MD ‐1.22 minutes, 95% CI ‐6.62 to 4.17, P = 0.44) (Figure 18).
17.

Forest plot of comparison: 7 Recovery room stay, outcome: 7.1 Recovery time
18.

Forest plot of comparison: 7 Recovery room stay, outcome: 7.2 Recovery time for low risk of bias studies
Lens fogging
6.1. Analysis.

Comparison 6 Lens fogging, Outcome 1 Times cleaned.
Evidence of substantial heterogeneity was present (I2 = 78%) and no significant difference in the lens fogging scores was shown (MD 0.73, 95% CI ‐0.32 to 1.77, P = 0.17) (Figure 19).
19.
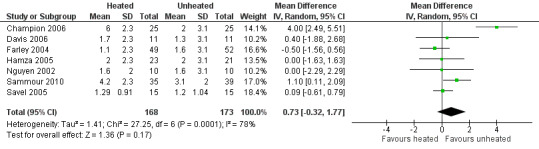
Forest plot of comparison: 5 Lens fogging, outcome: 5.1 Lens fogging
Operative time
7.1. Analysis.
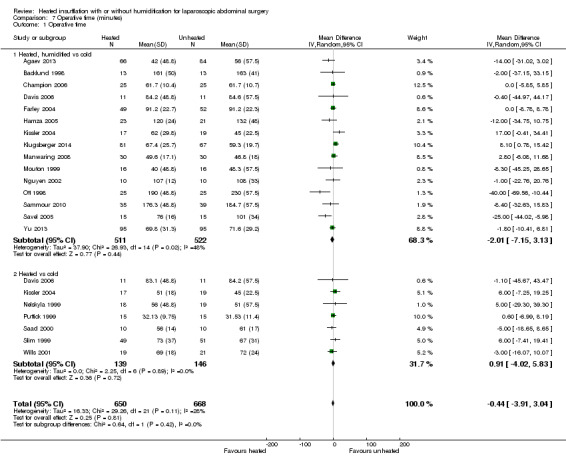
Comparison 7 Operative time (minutes), Outcome 1 Operative time.
Twenty studies reported their mean operative time; no evidence of statistically significant heterogeneity was found (I2 = 28%). The mean operative time was similar across groups (MD ‐0.44 minutes, 95% CI ‐3.91 to 3.04, P = 0.81) (Figure 20). Subgroup analyses on humidified and non‐humidified subgroups did not change the results.
20.

Forest plot of comparison: 6 Operative time, outcome: 6.1 Operative time
Adverse events
The majority of included studies did not report on adverse events (Table 10). There were a total of twelve major adverse events with six in the heated group and six in the cold group.
Discussion
Controversy exists on the use of heated CO2 insufflation during laparoscopic surgery. Laparoscopic procedures already demand higher operating expenses than conventional open techniques (Janson 2004) and the addition of further complex equipment only increases this limitation. In 2002, the European Association for Endoscopic Surgery published consensus guidelines for laparoscopic pneumoperitoneum and stated that, "the clinical benefits of warmed humidified insufflation gas are minor and contradictory" (Neudecker 2002).
Summary of main results
Evidence based on the 22 RCTs in this systematic review failed to demonstrate definitive evidence for the use of heated CO2 insufflation during laparoscopic abdominal surgery. While heated and humidified gas insufflation leads to slightly higher core body temperatures, these studies are quite heterogeneous and patient outcomes were not improved with respect to pain scores, morphine consumption and hospital length of stay. For longer operative cases (more than 120 minutes), heated gas is associated with improved core temperatures during surgery. However, these benefits disappeared when the analysis only included trials with a known low risk of bias.
Among the 11 trials at a known low risk of bias included in the review, only one study demonstrated both improved maintenance of normothermia, as well as a reduction in analgesic use in the early postoperative period (Hamza 2005). In this study, external warming blankets were used solely as a 'rescue' treatment, potentially confounding the effect of the experimental intervention. Another study reported higher intraoperative core temperatures (Farley 2004) and improved postoperative pain but no differences in other outcomes. One heated, non‐humidified gas insufflation study reported increased core temperatures but with higher operative pain scores and narcotics usage (Wills 2001). The remaining eight known low risk of bias studies did not find any beneficial effect for the intervention in terms of maintaining normothermia. The heterogeneity in core temperature outcomes across studies may be secondary to minor protocol differences between studies such as different insufflation gas temperatures (35 ºC to 37 ºC), humidity ranges (88% to 100%), gas volumes and location of the temperature probe.
Overall completeness and applicability of evidence
All 22 RCTs included in this review compared heated CO2 with cold CO2 insufflation. The majority (19 RCTs, n = 1100) reported the primary outcome, change in core temperature. Fifteen studies (n = 925) included humidified insufflation and ten studies (n = 617) used external warming. This allowed for various subgroup analyses on different modalities of heated insufflation and helps to determine whether changes such as humidification and external warming have any effect. The review also included a broad range of laparoscopic surgeries including cholecystectomy, gastric bypass, gynaecological, gastrectomy, colectomy, low anterior resection and fundoplication, proving its applicability to many different laparoscopic abdominal surgeries. However, this variability may have contributed to the heterogeneity of the results.
The majority of participants were 30 to 60 years old and were female, as some studies only included women. Few of the studies included participants more than 60 years old and results may not be generalisable to an older population, who may be at higher risk of hypothermia (Macario 2002). Additionally, only one study (Yu 2013) enrolled primarily adolescents, who are at higher risk of intraoperative hypothermia given their higher surface area to body mass. This risk is particularly high in neonatal populations (Macario 2002), who were not studied in any trial included in this review.
Quality of the evidence
The results of this review should be interpreted cautiously due to some limitations. Although the studies were all randomised controlled trials and applicable to the research question, some lacked design information making evaluation of study quality difficult. Many of the studies included small sample sizes, which made individual inferences difficult regarding the attribution of effects to random error or the heated insufflation intervention. This also affects precision of the results. The standard deviations used for meta‐analysis were missing from some studies and the largest standard deviation from that group was used instead. This potentially distorts the true effects and potentially increases error. Finally, some heterogeneity across studies could not be explained through subgroup analysis, and the results from studies were often inconsistent. Specifically, conclusions on the effectiveness of heated CO2 on core temperature is downgraded as heterogeneity remained significant despite subgroup analysis. See Table 1.
We also assessed publication bias for each outcome with funnel plots and Egger's linear regression test (Egger 1997) and we found no publication bias (Figure 21; Figure 22; Figure 23; Figure 24; Figure 25). We performed Egger's test on outcomes that included data from at least 10 trials: core temperature change (P = 0.697, 95% CI ‐4.26 to 2.94), day one pain score (P = 0.347, 95% CI ‐3.98 to 1.57), operating time (P = 0.662, 95% CI ‐0.41 to 0.63), day one morphine (P = 0.917, 95% CI ‐1.58 to 1.72) and length of stay (P = 0.477, 95% CI ‐3.38 to 1.75).
21.

Funnel plot of comparison: 2 Core temperature, outcome: 2.1 Change in core temperature
22.
Funnel plot of comparison: 1 Pain Score, outcome: 1.1 Day 1 pain score
23.

Funnel plot of comparison: 3 Morphine consumption, outcome: 3.2 Day 1 morphine
24.

Funnel plot of comparison: 4 Hospital stay, outcome: 4.1 Hospital stay
25.

Funnel plot of comparison: 6 Operative time, outcome: 6.1 Operative time
Potential biases in the review process
We could not identify any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Two previously published meta‐analyses revealed different conclusions from the current review (Sajid 2008; Sammour 2008). Both provided evidence for a reduction in postoperative pain and Sajid 2008 also demonstrated improved maintenance of core temperature and decreased narcotic requirements. The current review incorporates a greater number of studies in the analysis, including six recent trials showing equivocal results with heated insufflation compared to cold gas insufflation (Agaev 2013; Klugsberger 2014; Lee 2011; Manwaring 2008; Sammour 2010; Yu 2013). Finally, one study (Beste 2006) included in the previous reviews compared heated insufflation with humidification to heated insufflation without humidification, a comparison not in keeping with the aims of the current review and therefore excluded.
Authors' conclusions
Implications for practice.
Based on our review, heated CO2 insufflation with humidification leads to a small improvement in maintenance of core temperatures in people undergoing laparoscopic abdominal surgery. The clinical significance of a 0.31 °C difference in core temperature is unclear. One systematic review (Rajagopalan 2008) analysed the effect of mild hypothermia and found increased blood loss and transfusion requirements in hypothermia with a median temperature difference of 0.85 °C between hypothermic and normothermic groups. Whether this still applies for a smaller temperature difference has not been studied. However, heated insufflation did not reduce postoperative pain or analgesic requirements overall. There were also no differences in serious adverse events that occurred in the cold or heated cohorts to support the use of heated CO2 in preventing hypothermia‐associated complications. Additionally, heated insufflation did not seem to reduce hospital stay, recovery room stay, lens fogging, or operative time. If the maintenance of normothermia can be achieved through the use of warmed irrigation and external warming devices, perhaps less consideration can be given to the use of heated insufflation systems which adds expenses to procedures already more costly than open surgical approaches.
Implications for research.
Good quality studies of how heated and humidified CO2 affects patient outcomes have been completed. However, the studies have relatively small sample sizes making detection of differences between groups difficult due to low statistical power. In order to further clarify the effect of heated insufflation on patient outcomes, at least one large multi‐centre RCT with adequate power should be performed. Though some change in core temperature may be noted during intraoperative monitoring, one must question the clinical relevance of such findings and, therefore, other useful outcomes such as postoperative pain and adverse events may be more appropriate to use to calculate the size of an adequately powered study.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2016 | New citation required but conclusions have not changed | Updated to include six new trials |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 30 September 2015 | New search has been performed | Update and Amendment |
| 26 July 2010 | Amended | Final amendment |
| 12 July 2010 | Amended | Final draft |
Acknowledgements
Cochrane Colorectal Cancer editorial office for advice and careful copy editing.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 8))(September 2016)
#1 MeSH descriptor: [endoscopy] explode all trees
#2 MeSH descriptor: [minimal invasive surgical procedures] explode all trees
#3 MeSH descriptor: [pneumoperitoneum, artificial] explode all trees
#4 (endoscop* or laparoscop* or peritoneoscop* or laparotom*):ti,ab.kw
#5 (#1 or #2 or #3 or #4)
#6 MeSH descriptor: [carbon dioxide] explode all trees
#7 MeSH descriptor: [nitrous oxide] explode all trees
#8 MeSH descriptor: [argon] explode all trees
#9 MeSH descriptor: [helium] explode all trees
#10 (Gas* or carbon dioxide or CO2 or nitrous oxide or N2O or helium or argon or laughing gas):ti,ab,kw
#11 (#6 or #7 or #8 or #9 or #10)
#12 (Heat* or temperature* or warm* or isotherm*):ti,ab,kw
#13 (Humidification or humidif*):ti,ab,kw
#13 (#12 or #13)
#14 (#5 and #11 and #14)
Appendix 2. MEDLINE search strategy
MEDLINE (PubMed) (1950 to 23 September 2016)
1. Exp endoscopy/
2. Exp minimally invasive surgical procedures/
3. Exp pneumoperitoneum, artificial/
4. (endoscop* or laparoscop* or peritoneoscop* or laparotom*).mp.
5. 1 or 2 or 3 or 4
6. Exp carbon dioxide/
7. Exp Nitrous oxide/
8. Exp Argon/
9. Exp Helium/
10. (Gas* or carbon dioxide or CO2 or nitrous oxide or N2O or helium or argon or laughing gas).mp.
11. 6 or 7 or 8 or 9 or 10
12. (Heat* or temperature* or warm* or isotherm* or hypotherm* or thermoregulation).mp.
13. (Humidification or humidif*).mp.
14. 12 or 13
15. 5 and 11 and 14
16. Randomized controlled trial.pt.
17. Controlled clinical trial.pt.
18. Randomized.ab.
19. Placebo.ab.
20. Clinical trials as topic.sh.
21. Randomly.ab.
22. Trial.ti.
23. 16 or 17 or 18 or 19 or 20 or 21 or 22
24. Exp animals/ not humans.sh.
25. 23 not 24
26. 15 and 25
Appendix 3. Embase search strategy
Embase (1974 to 23 September 2016)
1. Exp abdominal‐surgery/
2. Exp minimally‐invasive‐surgery/
3. Exp endoscopic‐surgery/
4. Exp pneumoperitoneum/
5. (endoscop* or laparoscop* or laparotom* or peritoneoscop*).mp.
6. 1 or 2 or 3 or 4 or 5
7. (Gas* or carbon dioxide or CO2 or nitrous oxide or N2O or helium or argon or laughing gas).mp.
8. Exp carbon dioxide/
9. Exp nitrous oxide/
10. Exp Argon/
11. Exp Helium
12. 7 or 8 or 9 or 10 or 11
13. (heat* or temperature* or warm* or isotherm* or hypotherm* or thermoregulation).mp.
14. (Humidification or humidif*).mp.
15. 13 or 14
16. 6 and 12 and 15
17. crossover procedure.sh.
18. double‐blind procedure.sh.
19. single‐blind procedure.sh.
20. (crossover* or cross over*).ti,ab.
21. placebo*.ti,ab.
22. (doubl* adj blind*).ti,ab.
23. allocat*.ti,ab.
24. trial.ti.
25. randomized controlled trial.sh.
26. random*.ti,ab.
27. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
28. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
29. 27 not 28
30. 16 and 29
Appendix 4. Scopus, search strategy
1. TOPIC: (minimally invasive) OR TOPIC: (laparoscop*) OR TOPIC: (endoscop*) OR TOPIC: (artificial pneumoperitoneum) OR TOPIC: (peritoneoscop*)OR TOPIC: (laparotom*)
2. TOPIC: (carbon dioxide) OR TOPIC: (nitrous oxide) OR TOPIC: (argon) OR TOPIC: (helium) OR TOPIC: (Gas*) OR TOPIC: (CO2) OR TOPIC: (N2O)OR TOPIC: (laughing gas)
3. TOPIC: (Heat*) OR TOPIC: (temperature*) OR TOPIC: (warm*) OR TOPIC: (isotherm*) TOPIC: (thermoregulation) ORTOPIC: (hypotherm*)OR TOPIC: (humidif*)
4. #1 AND #2 AND #3
Appendix 5. Web of Science, search strategy
1. TITLE‐ABS‐KEY ( minimally invasive ) OR TITLE‐ABS‐KEY ( laparoscop* ) OR TITLE‐ABS‐KEY ( endoscop* ) OR TITLE‐ABS‐KEY ( artificial pneumoperitoneum ) OR TITLE‐ABS‐KEY ( peritoneoscop* ) OR TITLE‐ABS‐KEY ( laparotom* )
2. TITLE‐ABS‐KEY ( carbon dioxide ) OR TITLE‐ABS‐KEY ( co2 ) OR TITLE‐ABS‐KEY ( nitrous oxide ) OR TITLE‐ABS‐KEY ( n2o ) OR TITLE‐ABS‐KEY ( gas* ) OR TITLE‐ABS‐KEY ( laughing gas ) OR TITLE‐ABS‐KEY ( argon ) OR TITLE‐ABS‐KEY ( helium )
3. TITLE‐ABS‐KEY ( heat* ) OR TITLE‐ABS‐KEY ( temperature* ) OR TITLE‐ABS‐KEY ( warm* ) OR TITLE‐ABS‐KEY ( isotherm* ) OR TITLE‐ABS‐KEY ( humidif* ) OR TITLE‐ABS‐KEY ( hypotherm* ) OR TITLE‐ABS‐KEY ( thermoregulation )
4. #1 AND #2 AND #3
Appendix 6. Other searches
We performed keyword searches from the following websites:
‐ International Pharmaceutical Abstracts ‐ ClinicalTrials.gov ‐ National Research Register ‐ Google Scholar
Appendix 7. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
Random Sequence Generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of ‘Low risk’ of bias | The investigators describe a random component in the sequence generation process such as: · referring to a random number table; · using a computer random number generator; · coin tossing; · shuffling cards or envelopes; · throwing dice; · drawing of lots; · minimisation*. *Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘High risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · sequence generated by odd or even date of birth; · sequence generated by some rule based on date (or day) of admission; · sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: · allocation by judgement of the clinician; · allocation by preference of the participant; · allocation based on the results of a laboratory test or a series of tests; · allocation by availability of the intervention. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of ‘Low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); · sequentially numbered drug containers of identical appearance; · sequentially numbered, opaque, sealed envelopes. |
| Criteria for the judgement of ‘High risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · using an open random allocation schedule (e.g. a list of random numbers); · assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); · alternation or rotation; · date of birth; · case record number; · any other explicitly unconcealed procedure. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following: · no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; · blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following: · no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; · blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following: · insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · the study did not address this outcome. |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following: · no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; · blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following: · no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; · blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following: · insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · the study did not address this outcome. |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following: · no missing outcome data; · reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · missing data have been imputed using appropriate methods. |
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following: · reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; · potentially inappropriate application of simple imputation. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following: · insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided); · the study did not address this outcome. |
|
Selective reporting Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of ‘Low risk’ of bias | Any of the following: · the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; · the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following: · not all of the study’s pre‐specified primary outcomes have been reported; · one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; · one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; · the study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category. |
|
Other bias Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of ‘Low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘High risk’ of bias | There is at least one important risk of bias. For example, the study: · had a potential source of bias related to the specific study design used; or · has been claimed to have been fraudulent; or · had some other problem. |
| Criteria for the judgement of ‘Unclear risk’ of bias | There may be a risk of bias, but there is either: · insufficient information to assess whether an important risk of bias exists; or · insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Core temperature (ºC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in core temperature | 19 | 1100 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.06, 0.36] |
| 1.1 Heated, humidified vs cold | 14 | 885 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.09, 0.53] |
| 1.2 Heated only vs cold | 7 | 215 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.20] |
| 2 Change in core temperature for low risk of bias studies | 10 | 653 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.01, 0.33] |
| 2.1 Heated, humidified vs cold | 8 | 561 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.04, 0.39] |
| 2.2 Heated vs cold | 3 | 92 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.15, 0.39] |
| 3 Change in core temperature for operations > 120 Minutes | 4 | 194 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.10, 1.30] |
| 4 Change in core temperature with external warming | 8 | 545 | Mean Difference (IV, Random, 95% CI) | 0.29 [0.05, 0.52] |
| 5 Change in temperature without external warming | 6 | 340 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.11, 0.75] |
1.4. Analysis.
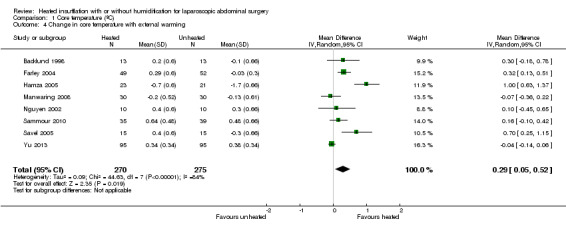
Comparison 1 Core temperature (ºC), Outcome 4 Change in core temperature with external warming.
1.5. Analysis.
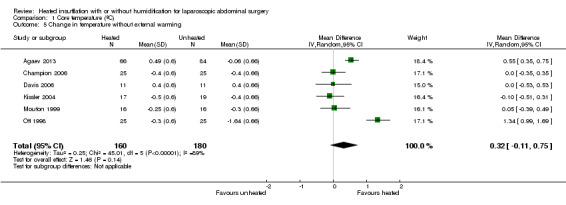
Comparison 1 Core temperature (ºC), Outcome 5 Change in temperature without external warming.
Comparison 2. Pain score (0 to 10‐point VAS scale).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Day 1 pain score | 14 | 991 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.42, 0.34] |
| 1.1 Heated, humidified vs cold (abdominal) | 10 | 670 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.60, 0.33] |
| 1.2 Heated, humidified vs cold (shoulder) | 3 | 171 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.75, 1.05] |
| 1.3 Heated vs cold | 3 | 150 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.11, 1.12] |
| 2 Day 1 pain score for low risk of bias studies | 7 | 570 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.21, 0.55] |
| 2.1 Heated, humidified vs cold (abdominal) | 7 | 460 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.29, 0.63] |
| 2.2 Heated, humidified vs cold (shoulder) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.81, 1.31] |
| 3 Day 2 pain score | 10 | 695 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.78, 0.21] |
| 3.1 Heated, humidified vs cold (abdominal) | 7 | 442 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.07, 0.28] |
| 3.2 Heated, humidified vs cold (shoulder) | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐2.93, 1.17] |
| 3.3 Heated vs cold | 3 | 142 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.44, 1.27] |
| 4 Day 2 pain score of low risk of bias studies | 5 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.65, 0.07] |
Comparison 3. Morphine consumption (morphine equivalent daily doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Up to 6 hours | 4 | 231 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.19, 2.08] |
| 2 Day 1 morphine | 9 | 573 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐4.48, 3.20] |
| 2.1 Heated, humidified vs cold | 7 | 481 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐4.79, 1.46] |
| 2.2 Heated vs cold | 3 | 92 | Mean Difference (IV, Random, 95% CI) | 11.93 [0.92, 22.94] |
| 3 Day 2 morphine | 7 | 532 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐2.79, 1.57] |
| 3.1 Heated, humidified vs cold | 6 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.90, 0.01] |
| 3.2 Heated vs cold | 2 | 122 | Mean Difference (IV, Random, 95% CI) | 9.79 [1.58, 18.00] |
Comparison 4. Hospital stay (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital stay | 10 | 685 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.31, 0.19] |
| 1.1 Heated, humidified vs cold | 9 | 563 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.44, 0.18] |
| 1.2 Heated vs cold | 2 | 122 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.23, 0.62] |
Comparison 5. Recovery room stay (minutes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery time | 6 | 327 | Mean Difference (IV, Random, 95% CI) | ‐26.79 [‐51.34, ‐2.25] |
| 2 Recovery time for low risk of bias studies | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐6.62, 4.17] |
Comparison 6. Lens fogging.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Times cleaned | 7 | 341 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.32, 1.77] |
Comparison 7. Operative time (minutes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative time | 20 | 1318 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐3.91, 3.04] |
| 1.1 Heated, humidified vs cold | 15 | 1033 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐7.15, 3.13] |
| 1.2 Heated vs cold | 7 | 285 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐4.02, 5.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agaev 2013.
| Methods | Double‐blinded RCT | |
| Participants | n = 110, laparoscopic cholecystectomy; n = 40, laparoscopic fundoplication | |
| Interventions | Warmed, humidified CO2 vs standard CO2 | |
| Outcomes | Core temperature, postoperative pain, analgesic requirements, lens fogging, postoperative pain and the need for anaesthesia. In addition , OR time, hospitalisation, complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to 2 groups using a computer model post‐anaesthesia but the groups were 84 in standard CO2 and 66 in heated, humidified CO2 Comment: with computer‐generated randomisation, it would be unlikely for the groups to be this uneven |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the surgical nurse knew the temperature of the CO2 feed." Comment: adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the surgical nurse knew the temperature of the CO2 feed." Comment: adequate blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data was unclear, number of participants included in analysis was not reported |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Unclear risk | Originally published in Russian, the study authors had a certified translator translate it into English. However, the translation and the qualification certificate of the translator were provided voluntarily by a research scientist from a surgical humidification device company |
Backlund 1998.
| Methods | RCT | |
| Participants | n = 26, prolonged (> 120 min) fundoplication, hernioplasty, resection of the sigmoid colon and rectopexy | |
| Interventions | Warmed, humidified CO2 vs standard CO2 | |
| Outcomes | Core temperature, cardiac index, urine output, recovery room opioid usage and pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only stated that the pain score was recorded by a trained nurse unaware of the temperature of the pneumoperitoneum |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device |
Champion 2006.
| Methods | RCT | |
| Participants | n = 50, consecutive, morbidly obese, laparoscopic antecolic proximal Roux‐en‐Y gastric bypass surgery | |
| Interventions | Heated and humidified CO2 vs cold and dry CO2 | |
| Outcomes | Intraoperative core temperature, room temperature, litres of CO2 insufflation, operating time, number of lens cleanings, recovery room temperature, narcotics usage, length of hospitalisation, high‐sensitivity CRP at 24 h, abdominal and shoulder pain scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blind draw by an impartial third party |
| Allocation concealment (selection bias) | Low risk | A draw was held to determine which type of insufflation was to be used on the first case, after which the insufflation method was alternated for the next 49 cases consecutively, with no interruption or exclusions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind study where participants were blinded as they were anaesthetized but personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The nursing personnel, who were unaware of the study, recorded the subjective pain score." Comment: adequate blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Davis 2006.
| Methods | Blinded RCT | |
| Participants | n = 44, laparoscopic gastric bypass | |
| Interventions | Cold CO2 vs cold humidified CO2 vs heated CO2 vs heated humidified CO2 | |
| Outcomes | Core temperature, humidity, intraoperative urine output, lens fogging, recovery room time, length of hospital stay, postoperative pain, total morphine sulphate equivalent | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block fashion randomisation |
| Allocation concealment (selection bias) | Low risk | Results in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind study where participants were blinded as they were anaesthetised but study personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Intraoperative outcomes were not blinded but they are objective measurements. Participants recorded postoperative pain and they remained blinded to their intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry funded research grant. |
Demco 2001.
| Methods | Double‐blinded RCT | |
| Participants | n = 40 women, diagnostic laparoscopy | |
| Interventions | Heated, humidified vs cold CO2 | |
| Outcomes | Shoulder pain, fentanyl use, percent requiring general anaesthetic, percent requiring intravenous sedation, amount of gas instilled before experiencing pain, operating time, recovery room time, time to recovery of shoulder pain | |
| Notes | This study presented outcomes as percentages of participants in each group (e.g. for operative time, percentage of participants in groups 0‐10 min, 10‐20 min, 20‐30 min, and 30‐40 min) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Sealed envelope: "The circulating nurse opened a sealed envelope directing her to have the unit turned on or off during the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the circulating nurse was not blinded: "To blind the surgeon further, the light on the unit could not be seen, and the plastic tubing was taped so the surgeon could not see condensation there." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the circulating nurse was not blinded: "To blind the surgeon further, the light on the unit could not be seen, and the plastic tubing was taped so the surgeon could not see condensation there." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Unclear risk | This study did not report any temperatures. |
| Other bias | Unclear risk | This study did not report any baseline demographics. |
Farley 2004.
| Methods | Double‐blinded RCT | |
| Participants | n = 117, laparoscopic cholecystectomy (16 excluded) | |
| Interventions | Heated, humidified CO2 vs cold CO2 | |
| Outcomes | Core temperature, lens fogging, postoperative pain, total morphine equivalents, hospital stay, return to baseline activity level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer model randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by surgical scrub nurse at the time of anaesthetic induction |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, surgeons, operative and floor nurses, study co‐ordinators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded when measuring intraoperative outcomes. Participants remained blinded when completing their pain scores. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 participants excluded from analysis due to 11 conversions to open, 3 requiring additional operations and 2 had the insufflation removed for technical reasons Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device. |
Hamza 2005.
| Methods | Double‐blinded RCT | |
| Participants | n = 50, laparoscopic gastric bypass (6 excluded) | |
| Interventions | Heated and humidified CO2 vs cold CO2 | |
| Outcomes | Core temperature, postoperatively tympanic temperature, pain score, shivering, morphine, nausea score, Aldrete recovery assessment score, hospital stay, lens fogging | |
| Notes | Warm blankets were used to cover the upper chest and arms in all control group participants for ethical considerations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | An OR nurse was responsible for connecting the device |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, surgeons, anaesthesiologist, data‐collecting personnel, recovery nurses were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel collecting data were blinded and participants remained blinded when completing their verbal rating scales |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants excluded from analysis (4 converted to open, 2 required rescuing with active warming for temperature < 34 °C) Comment: all excluded participants properly reported and not included in analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry funded research grant. |
Kissler 2004.
| Methods | Double‐blinded RCT | |
| Participants | n = 90 women, gynaecologic laparoscopic surgery (53 with data) | |
| Interventions | Humidified heated CO2 vs heated dry CO2 vs cold dry CO2 | |
| Outcomes | Analgesic requirements and postoperative pain | |
| Notes | The trial was stopped following enrolment of 53 participants because of a tendency toward less pain and higher postoperative satisfaction in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants, data analyst and interviewer were blinded to randomisation. However, no description of blinding of other participants (surgeon and nurses) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding of outcomes assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial was stopped early for there was a tendency toward less pain and higher postoperative satisfaction in participants in the control group |
| Selective reporting (reporting bias) | Unclear risk | Out of 90 participants, data only available on 53 participants |
| Other bias | Low risk | Industry provided heating device. |
Klugsberger 2014.
| Methods | Double‐blinded RCT | |
| Participants | n = 148, laparoscopic cholecystectomy | |
| Interventions | Warmed, humidified CO2 vs standard CO2 | |
| Outcomes | Core temperature, postoperative pain, time of first bowel movement after surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was unclear and treatment groups were uneven (67 received heated, humidified CO2 and 81 received standard CO2) Comment: randomisation likely not properly done |
| Allocation concealment (selection bias) | Low risk | Quote: "The secretary was privy to which method of gas was being used. The secretary opened a sealed opaque envelope to randomly allocate the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants, surgeons, nurses, and study co‐ordinator were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurses recording intraoperative outcomes were blinded. Participants remained blinded when recording their visual analogue pain scales. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Lee 2011.
| Methods | RCT | |
| Participants | n = 30, gastrectomy, colectomy or low‐anterior resection | |
| Interventions | Heated CO2 vs room temperature CO2 | |
| Outcomes | Acid‐base parameters, ETCO2, and core temperature | |
| Notes | An upper body blanket was applied to all participants and if their temperature fell below 35 °C, a Bair Hugger forced air warmer and a warming mattress with circulating water at 38 °C were applied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description in the article. Contacted study authors and they indicated that a random number table was used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No description but contacted study authors and they indicated that this was a blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No description but contacted study authors and they indicated that this was a blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Manwaring 2008.
| Methods | RCT | |
| Participants | n = 60 women, gynaecologic laparoscopic surgery | |
| Interventions | Heated humidified CO2 vs cold dry CO2 | |
| Outcomes | Core temperature, analgesic usage, postoperative pain, postoperative nausea and recovery room time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed in sequential opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All nursing staff were blinded and patient was blinded as they were anaesthetised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses recording outcome data were blinded. Participants remained blinded when nurses administered visual analogue scales. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device. |
Mouton 1999.
| Methods | RCT | |
| Participants | n = 40, laparoscopic cholecystectomy (8 excluded) | |
| Interventions | Heated, humidified CO2 vs cold CO2 | |
| Outcomes | Core temperature change, postoperative pain score, morphine usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants excluded due to conversion to open, pancreatitis or postoperative haematoma Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Data were available on 32 out of 40 participants and the reason was given by the study author. |
| Other bias | Low risk | Industry offered assistance for research. |
Nelskyla 1999.
| Methods | Double‐blinded RCT | |
| Participants | n = 40 women, laparoscopic hysterectomy (3 excluded) | |
| Interventions | Heated CO2 vs cold CO2 | |
| Outcomes | Tympanic temperature, heart rate variability | |
| Notes | Data on 37 women were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description on which personnel were blinded during operation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and staff in the postoperation care unit and ward were blinded. Intraoperative outcomes are objective so non‐blinding likely has less effect |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 excluded participants, 2 "did not fulfil the study protocol" and 1 "because of surgical problems." Comment: unclear reasons for exclusion |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device. |
Nguyen 2002.
| Methods | RCT | |
| Participants | n = 20, laparoscopic Nissen fundoplication | |
| Interventions | Heated and humidified CO2 vs cold CO2 | |
| Outcomes | Core temperature, pain score, morphine consumption, urine output, lens fogging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Intraoperative randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinded study where the participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Intraoperative outcomes were not blinded but they are objective measurements. Participants recorded postoperative pain and they remained blinded to their intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Ott 1998.
| Methods | Multi‐centre RCT | |
| Participants | n = 72 women, laparoscopic gynaecologic surgery (50 with data) | |
| Interventions | Heated and humidified CO2 vs cold CO2 | |
| Outcomes | Postoperative pain and recovery room length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were only available on 50 out of 72 participants and no reason was given. Some data was extracted from a different systematic review (Sammour 2008) as the original trial did not present all data. |
| Selective reporting (reporting bias) | Unclear risk | Data were only available on 50 out of 72 participants and no reason was given. |
| Other bias | Unclear risk | This study did not separate baseline demographics between groups. Industry provided heating device. |
Puttick 1999.
| Methods | RCT | |
| Participants | n = 30, laparoscopic cholecystectomy | |
| Interventions | Warmed CO2 vs cold CO2 | |
| Outcomes | Core temperature, intraperitoneal cytokines, pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Saad 2000.
| Methods | RCT | |
| Participants | n = 20, laparoscopic cholecystectomy | |
| Interventions | Heated CO2 vs cold CO2 | |
| Outcomes | Core temperature, intra‐abdominal temperature, postoperative pain, analgesics consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and ward nurses were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants remained blinded when assessing postoperative pain. Unclear if operating room nurses were blinded during measurement of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device. |
Sammour 2010.
| Methods | Multi‐centre RCT | |
| Participants | n = 82, laparoscopic colonic surgery (8 excluded) | |
| Interventions | Heated humidified CO2 vs cold CO2 | |
| Outcomes | Postoperative pain, intraoperative core temperature, camera fogging, morphine‐equivalent usage, postoperative parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed in opaque numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, surgeon and medical care staff were all blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, surgeon and medical care staff were all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight excluded after randomisation with clearly stated rationale Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Savel 2005.
| Methods | Blinded RCT | |
| Participants | n = 30, laparoscopic gastric bypass | |
| Interventions | Heated humidified CO2 vs cold CO2 | |
| Outcomes | Postoperative pain score, morphine consumption, OR time, core temperature, hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Participants randomised at the time of enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All clinicians except 1 author were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinicians except 1 author were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no treatment withdrawals, no trial group changes and no major adverse events. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Slim 1999.
| Methods | Double‐blinded RCT | |
| Participants | n = 108, laparoscopic cholecystectomy, fundoplication or Heller's myotomy (8 excluded) | |
| Interventions | Heated CO2 vs unheated CO2 | |
| Outcomes | Postoperative pain, core temperature, morphine consumption, nausea and vomiting, hospital stay, length of postoperative Ileus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table in sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened in the operating room |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and nurses were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses were blinded when collecting outcome data. Participants remained blinded when assessing postoperative pain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants excluded (4 conversion to open, 2 postoperative biliary collections, 1 technical problems with insufflator, 1 refused) Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
Wills 2001.
| Methods | Blinded RCT | |
| Participants | n = 41, laparoscopic fundoplication (1 excluded) | |
| Interventions | Heated CO2 vs cold CO2 | |
| Outcomes | Core temperature, postoperative pain, analgesic requirement, postoperative recovery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Surgeons, anaesthetist, data analyst, participants and ward nurses were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Surgeons, anaesthetist, data analyst, participants and ward nurses were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant excluded for missing postoperative pain scores and one underwent repeat laparotomy. Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | Industry provided heating device. |
Yu 2013.
| Methods | Double‐blinded RCT | |
| Participants | n = 195 adolescents, laparoscopic appendectomy (5 excluded) | |
| Interventions | Warm, humidified CO2 vs standard CO2 | |
| Outcomes | Opioid usage, pain score, core temperature, postoperative recovery and return to normal activities | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online random number programme |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, numbered envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only one rotating scrub nurse assisted with randomisation. All other participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only one rotating scrub nurse assisted with randomisation. All other participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants excluded after randomisation for major protocol violation Comment: all excluded participants properly reported and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
CO2: carbon dioxide ETCO2: end tidal carbon dioxide VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barragan 2005 | Not a RCT |
| Benavides 2009 | Intervention was heated dry CO2 vs heated humidified CO2 |
| Beste 2006 | Intervention was heated dry CO2 vs heated humidified CO2 |
| Herrmann 2015 | Not primarily a laparoscopic abdominal surgery (laparoscopic‐assisted vaginal hysterectomy) |
| Monagle 1993 | Not a RCT |
| Mouton 2001 | Not a laparoscopic abdominal procedure (thoracoscopic) |
| Ott 1991 | Not a RCT |
| Pu 2014 | Different intervention: underbody warming system |
| Siebzehnrubl 2001 | This study was only presented as a poster and no published paper was found |
| Tohme 2010 | Published as an abstract only, study authors contacted for data. No response |
| Trevelyan 2011 | Published as an abstract only, authors contacted for data. No response |
| Yeh 2007 | Not a RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Sutton 2016.
| Methods | RCT |
| Participants | n = 101, minimally‐invasive colon resection |
| Interventions | Warmed, humidified CO2 vs standard CO2 |
| Outcomes | Core temperature, postoperative pain, analgesic requirements, length of stay, time to first flatus, and tolerance of solids |
| Notes | Recently published abstract awaiting classification |
Differences between protocol and review
There were no deviations from protocol.
Contributions of authors
DWB: protocol development, screening retrieved papers for eligibility criteria, analysing and editing review, providing guidance on methodology and quality control
JD: analysis and review editing
NS: analysis and review editing
NM: analysis and review editing
XS: literature search, screening search results, retrieving and analysing data, draft preparation
GH: protocol development, literature search, screening search results, draft preparation
SK: analysis and review editing, quality control
Sources of support
Internal sources
University of Alberta Library, Canada.
External sources
Cochrane Colorectal Cancer Group, Denmark.
Declarations of interest
DWB: no conflict of interest
JD: no conflict of interest
NS: no conflict of interest
NM: no conflict of interest
XS: no conflict of interest
GH: no conflict of interest
SK: no conflict of interest
Edited (no change to conclusions)
References
References to studies included in this review
Agaev 2013 {published data only}
- Agaev BA, Muslimov GF, Ibragimov TR, Alieva GR. The efficacy of the moisture and warmed CO(2) for laparoscopic surgery. Khirurgiia (Mosk) 2013;11:35‐9. [PubMed] [Google Scholar]
Backlund 1998 {published data only}
- Backlund M, Kellokumpu I, Scheinin T, Smitten K, Tikkanen I, Lindgren L. Effect of temperature of insufflated CO2 during and after prolonged laparoscopic surgery. Surgical Endoscopy 1998;12(9):1126‐30. [DOI] [PubMed] [Google Scholar]
Champion 2006 {published data only}
- Champion JK, Williams M. Prospective randomized trial of heated humidified versus cold dry carbon dioxide insufflation during laparoscopic gastric bypass. Surgery for Obesity and Related Diseases 2006;2(4):445‐9; discussion 449‐50. [DOI] [PubMed] [Google Scholar]
Davis 2006 {published data only}
- Davis SS, Mikami DJ, Newlin M, Needleman BJ, Barett MS, Fries R, et al. Heating and humidifying of carbon dioxide during pneumoperitoneum is not indicated: a prospective randomized trial. Surgical Endoscopy 2006;20(1):153‐8. [DOI] [PubMed] [Google Scholar]
Demco 2001 {published data only}
- Demco L. Effect of heating and humidifying gas on patients undergoing awake laparoscopy. The Journal of the American Association of Gynecologic Laparoscopists 2001;8(2):247‐51. [DOI] [PubMed] [Google Scholar]
Farley 2004 {published data only}
- Farley DR, Greenlee SM, Larson DR, Harrington JR. Double‐blind, prospective, randomized study of warmed, humidified carbon dioxide insufflation vs standard carbon dioxide for patients undergoing laparoscopic cholecystectomy. Archives of Surgery (Chicago, Ill: 1960) 2004;139(7):739‐4. [DOI] [PubMed] [Google Scholar]
Hamza 2005 {published data only}
- Hamza MA, Schneider BE, White PF, Recart A, Villegas L, Ogunnaike B, et al. Heated and humidified insufflation during laparoscopic gastric bypass surgery: effect on temperature, postoperative pain, and recovery outcomes. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2005;15(1):6‐12. [DOI] [PubMed] [Google Scholar]
Kissler 2004 {published data only}
- Kissler S, Haas M, Strohmeier R, Schmitt H, Rody A, Kaufmann M, et al. Effect of humidified and heated CO2 during gynecologic laparoscopic surgery on analgesic requirements and postoperative pain. The Journal of the American Association of Gynecologic Laparoscopists 2004;11(4):473‐7. [DOI] [PubMed] [Google Scholar]
Klugsberger 2014 {published data only}
- Klugsberger B, Schreiner M, Rothe A, Haas D, Oppelt P, Shamiyeh A. Warmed, humidified carbon dioxide insufflation versus standard carbon dioxide in laparoscopic cholecystectomy: a double‐blinded randomized controlled trial. Surgical Endoscopy 2014;28.9:2656‐60. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee KC, Kim JY, Kwak HJ, Lee HD, Kwon IW. The effect of heating insufflation gas on acid‐base alterations and core temperature during laparoscopic major abdominal surgery. Korean Journal of Anesthesiology 2011;61(4):275‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manwaring 2008 {published data only}
- Manwaring JM, Readman E, Maher PJ. The effect of heated humidified carbon dioxide on postoperative pain, core temperature, and recovery times in patients having laparoscopic surgery: A randomized controlled trial. Journal of Minimally Invasive Gynecology 2008;15(2):161‐5. [DOI: 10.1016/j.jmig.2007.09.007] [DOI] [PubMed] [Google Scholar]
Mouton 1999 {published data only}
- Mouton WG, Bessell JR, Millard SH, Baxter PS, Maddern GJ. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Surgical Endoscopy 1999;13(2):106‐8. [DOI] [PubMed] [Google Scholar]
Nelskyla 1999 {published data only}
- Nelskyla K, Yli‐Hankala A, Sjoberg J, Korhonen I, Korttila K. Warming of insufflation gas during laparoscopic hysterectomy: effect on body temperature and the autonomic nervous system. Acta Anaesthesiological Scandinavica 1999;43(10):974‐8. [DOI] [PubMed] [Google Scholar]
Nguyen 2002 {published data only}
- Nguyen NT, Furdui G, Fleming NW, Lee SJ, Goldman CD, Singh A, et al. Effect of heated and humidified carbon dioxide gas on core temperature and postoperative pain: a randomized trial. Surgical Endoscopy 2002;16(7):1050‐4. [DOI] [PubMed] [Google Scholar]
Ott 1998 {published data only}
- Ott DE, Reich H, Love B, McCorvey R, Toledo A, Liu CY, et al. Reduction of laparoscopic‐induced hypothermia, postoperative pain and recovery room length of stay by pre‐conditioning gas with the Insuflow device: a prospective randomized controlled multi‐center study. Journal of the Society of Laparoendoscopic Surgeons 1998;2(4):321‐9. [PMC free article] [PubMed] [Google Scholar]
Puttick 1999 {published data only}
- Puttick MI, Scott‐Coombes DM, Dye J, Nduka CC, Menzies‐Gow NM, Mansfield AO, et al. Comparison of immunologic and physiologic effects of CO2 pneumoperitoneum at room and body temperatures. Surgical Endoscopy 1999;13(6):572‐5. [DOI] [PubMed] [Google Scholar]
Saad 2000 {published data only}
- Saad S, Minor I, Mohri T, Nagelschmidt M. The clinical impact of warmed insufflation carbon dioxide gas for laparoscopic cholecystectomy. Surgical Endoscopy 2000;14(9):787‐90. [DOI] [PubMed] [Google Scholar]
Sammour 2010 {published data only}
- Sammour T, Kahokehr A, Hayes J, Hulme‐Moir M, Hill AG. Warming and humidification of insufflation carbon dioxide in laparoscopic colonic surgery. Annals of Surgery 2010;251(6):1024‐33. [DOI: 10.1097/SLA.0b013e3181d77a25] [DOI] [PubMed] [Google Scholar]
Savel 2005 {published data only}
- Savel RH, Balasubramanya S, Lasheen S, Gaprindashvili T, Arabov E, Fazylov RM, et al. Beneficial effects of humidified, warmed carbon dioxide insufflation during laparoscopic bariatric surgery: a randomized clinical trial. Obesity Surgery 2005;15(1):64‐9. [DOI] [PubMed] [Google Scholar]
Slim 1999 {published data only}
- Slim K, Bousquet J, Kwiatkowski F, Lescure G, Pezet D, Chipponi J. Effect of CO(2) gas warming on pain after laparoscopic surgery: a randomized double‐blind controlled trial. Surgical Endoscopy 1999;13(11):1110‐4. [DOI] [PubMed] [Google Scholar]
Wills 2001 {published data only}
- Wills VL, Hunt DR, Armstrong A. A randomized controlled trial assessing the effect of heated carbon dioxide for insufflation on pain and recovery after laparoscopic fundoplication. Surgical Endoscopy 2001;15(2):166‐70. [DOI] [PubMed] [Google Scholar]
Yu 2013 {published data only}
- Yu TC, Hamill JK, Liley A, Hill AG. Warm, humidified carbon dioxide gas insufflation for laparoscopic appendicectomy in children: a double‐blinded randomized controlled trial. Annals of Surgery 2013;257(1):44‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barragan 2005 {published data only}
- Barragan AB, Frezza EE. Impact of a warm gas insufflation on operating‐room ergonometrics during laparoscopic gastric bypass: a pilot study. Obesity Surgery 2005;15(1):70‐2. [DOI] [PubMed] [Google Scholar]
Benavides 2009 {published data only}
- Benavides R, Wong A, Nguyen H. Improved outcomes for lap‐banding using the Insuflow device compared with heated‐only gas.. Journal of the Society of Laparoendoscopic Surgeons 2009;13(3):302‐5. [PMC free article] [PubMed] [Google Scholar]
Beste 2006 {published data only}
- Beste TM, Daucher JA, Holbert D. Humidified compared with dry, heated carbon dioxide at laparoscopy to reduce pain. Obstetrics and Gynecology 2006;107(2 pt 1):263‐8. [DOI] [PubMed] [Google Scholar]
Herrmann 2015 {published data only}
- Herrmann A, Wilde RL. Insufflation with humidified and heated carbon dioxide in short‐term laparoscopy: a double‐blinded randomized controlled trial. BioMed Research International 2015;Epub 2015:Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monagle 1993 {published data only}
- Monagle J, Bradfield S, Nottle P. Carbon dioxide, temperature and laparoscopic cholecystectomy. The Australian and New Zealand Journal of Surgery 1993;63(3):186‐9. [DOI] [PubMed] [Google Scholar]
Mouton 2001 {published data only}
- Mouton WG, Naef M, Bessell JR, Otten KT, Wagner HE, Maddern GJ. A randomized controlled trial to determine the effect of humidified carbon dioxide (CO2) insufflation on postoperative pain following thoracoscopic procedures. Surgical Endoscopy 2001;15(6):579‐81. [DOI] [PubMed] [Google Scholar]
Ott 1991 {published data only}
- Ott DE. Correction of laparoscopic insufflation hypothermia. Journal of Laparoendoscopic Surgery 1991;1(4):183‐6. [DOI] [PubMed] [Google Scholar]
Pu 2014 {published data only}
- Pu Y, Cen G, Sun J, Gong J, Zhang Y, Zhang M, et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: a randomized controlled study. International Journal of Nursing Studies 2014;51(2):181‐9. [DOI] [PubMed] [Google Scholar]
Siebzehnrubl 2001 {published data only}
- Siebzehnruebl E, Haas M, Schmidt H, Lang N. Warm and humidified CO2 does not improve the postoperative pain score after laparoscopy‐a prospective, randomized and double blinded study. Human Reproduction June 2001;Abstracts of the 17th Annual Meeting of the ESHRE:216‐7. [Google Scholar]
Tohme 2010 {published data only}
- Tohme S, Shantha Kumara M, Yan CX, Nasar A, Cekic V, Whelan R. Effect of warmed, humidified CO2 gas on cytokine response to minimally invasive colorectal surgery: a randomized trial. Diseases of the Colon & Rectum 2010;53:4. [Google Scholar]
Trevelyan 2011 {published data only}
- Trevelyan S, Mason C, Chan A, Baird D, Flook D. Does humidified warmed CO2 insufflation gas improve post‐operative pain control in laparoscopic cholecystectomy? A randomized controlled trial. British Journal of Surgery 2011;98(s3):1‐79. [Google Scholar]
Yeh 2007 {published data only}
- Yeh CH, Kwok SY, Chan MK, Tjandra JJ. Prospective, case‐matched study of heated and humidified carbon dioxide insufflation in laparoscopic colorectal surgery. Colorectal Disease 2007;9(8):695‐700. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Sutton 2016 {published data only}
- Sutton E, Bellini G, Kumara HS, Yan X, Njoh L, Cekic V, et al. Warm and humidified vs cold and dry CO2 pneumoperitoneum in minimally invasive colon resection: a randomized controlled trial. Surgical Endoscopy 2016;30(S1):317. [DOI] [PubMed] [Google Scholar]
Additional references
Beilin 1998
- Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 1998;89(5):1133‐40. [9822001] [DOI] [PubMed] [Google Scholar]
Cork 1983
- Cork RC, Vaughan RW, Humphrey LS. Precision and accuracy of intraoperative temperature monitoring . Anesthesia & Analgesia 1983;62:211‐4. [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 1993
- Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;78(3):468‐76. [8457047] [DOI] [PubMed] [Google Scholar]
Frank 1997
- Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277(14):1127‐34. [PUBMED: 9087467] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Janson 2004
- Janson M, Bjorholt I, Carlsson P, Haglind E, Henriksson M, Lindholm E, et al. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. British Journal of Surgery 2004;91(4):409‐17. [DOI: 10.1002/bjs.4469] [DOI] [PubMed] [Google Scholar]
Macario 2002
- Macario A. What are the most important risk factors for a patient's developing intraoperative hypothermia?. Anesthesia and Analgesia 2002;94(1):215‐20. [11772832] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine 2009;6(7):e 1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neudecker 2002
- Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surgical Endoscopy 2002;16(7):1121‐43. [PUBMED: 12015619] [DOI] [PubMed] [Google Scholar]
Putzu 2007
- Putzu M, Casati A, Berti M, Pagliarini G, Fanelli G. Clinical complications, monitoring and management of perioperative mild hypothermia: anesthesiological features. Acta Bio‐Medica de l Ateneo Parmense 2007;78(3):163‐9. [PUBMED: 18330074] [PubMed] [Google Scholar]
Qadan 2009
- Qadan M, Gardner SA, Vitale DS, Lominadze D, Joshua IG, Polk HC Jr. Hypothermia and surgery: immunologic mechanisms for current practice. Annals of Surgery 2009;250(1):134‐40. [PUBMED: 19561472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajagopalan 2008
- Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108(1):71‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sajid 2008
- Sajid MS, Mallick AS, Rimpel J, Bokari SA, Cheek E, Baig MK. Effect of heated and humidified carbon dioxide on patients after laparoscopic procedures: a meta‐analysis. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2008;18(6):539‐46. [DOI: 10.1097/SLE.0b013e3181886ff4; 00129689‐200812000‐00001; Sajid:2008p530; PUBMED: 19098656] [DOI] [PubMed] [Google Scholar]
Sammour 2008
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. The British Journal of Surgery 2008;95(8):950‐6. [DOI: 10.1002/bjs.6304; Sammour:2008p528; PUBMED: 18618870] [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brozek J, Oxman A, editors. The GRADE Working Group 2009. GRADE handbook for grading quality of evidence and strength of recommendation. Available from http://www.cc‐ims/gradepro [updated March 2009]; Vol. version 3.2.
Winkler 2000
- Winkler M, Akca O, Birkenberg B, Hetz H, Scheck T, Arkiliç CF, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesthesia and Analgesia 2000;91:978‐84. [DOI] [PubMed] [Google Scholar]
Wong 2007
- Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. The British Journal of Surgery 2007;94(4):421‐6. [MEDLINE: 10.1002/bjs.5631; 17380549] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Birch 2011
- Birch DW, Manouchehri N, Shi X, Hadi G, Karmali S. Heated CO2 with or without humidification for minimally invasive abdominal surgery. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007821.pub2] [DOI] [PubMed] [Google Scholar]


