Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 6, 2015).
Failure to respond to antiepileptic drugs in patients with uncontrolled seizure activity such as refractory status epilepticus (RSE) has led to the use of anaesthetic drugs. Coma is induced with anaesthetic drugs to achieve complete control of seizure activity. Thiopental sodium and propofol are popularly used for this purpose. Both agents have been found to be effective. However, there is a substantial lack of evidence as to which of the two drugs is better in terms of clinical outcomes.
Objectives
To compare the efficacy, adverse effects, and short‐ and long‐term outcomes of refractory status epilepticus (RSE) treated with one of the two anaesthetic agents, thiopental sodium or propofol.
Search methods
We searched the Cochrane Epilepsy Group Specialized Register (16 August 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 16 August 2016), MEDLINE (Ovid, 1946 to 16 August 2016), ClinicalTrials.gov (16 August 2016), and the South Asian Database of Controlled Clinical Trials (16 August 2016). Previously we searched IndMED, but this was not accessible at the time of the latest update.
Selection criteria
All randomised controlled trials (RCTs) or quasi‐RCTs (regardless of blinding) assessing the control of RSE using either thiopental sodium or propofol in patients of any age and gender.
Data collection and analysis
Two review authors screened the search results and reviewed the abstracts of relevant and eligible trials before retrieving the full‐text publications.
Main results
One study with a total of 24 participants was available for review. This study was a small, single‐blind, multicentre trial studying adults with RSE receiving either propofol or thiopental sodium for the control of seizure activity. This study was terminated early due to recruitment problems. For our primary outcome of total control of seizures after the first course of study drug, there were 6/14 patients versus 2/7 patients in the propofol and thiopental sodium groups, respectively (risk ratio (RR) 1.50, 95% confidence interval (CI) 0.40 to 5.61, low quality evidence). Mortality was seen in 3/14 patients versus 1/7 patients in the propofol and thiopental sodium groups, respectively (RR 1.50, 95% CI 0.19 to 11.93, low quality evidence). Our third primary outcome of length of ICU stay was not reported. For our secondary outcomes of adverse events, infection was seen in 7/14 patients versus 5/7 patients in the propofol and thiopental sodium groups, respectively (RR 0.70; 95% CI 0.35 to 1.41). Hypotension during administration of study drugs and requiring use of vasopressors was seen in 7/14 patients versus 4/7 patients in the propofol and thiopental sodium groups, respectively (RR 0.87; 95% CI 0.38 to 2.00). The other severe complication noted was non‐fatal propofol infusion syndrome in one patient. Patients receiving thiopental sodium required more days of mechanical ventilation when compared with patients receiving propofol: (median (range) 17 days (5 to 70 days) with thiopental sodium versus four days (2 to 28 days) with propofol). At three months there was no evidence of a difference between the drugs with respect to outcome measures such as control of seizure activity and functional outcome.
Authors' conclusions
Since the last version of this review we have found no new studies.
There is a lack of robust, randomised, controlled evidence to clarify the efficacy of propofol and thiopental sodium compared to each other in the treatment of RSE. There is a need for large RCTs for this serious condition.
Keywords: Adult; Humans; Anesthetics, Intravenous; Anesthetics, Intravenous/therapeutic use; Anticonvulsants; Anticonvulsants/therapeutic use; Drug Resistant Epilepsy; Drug Resistant Epilepsy/drug therapy; Propofol; Propofol/therapeutic use; Respiration, Artificial; Status Epilepticus; Status Epilepticus/drug therapy; Thiopental; Thiopental/therapeutic use
Plain language summary
Propofol versus thiopental sodium for the treatment of refractory status epilepticus (RSE)
Review question: In this review we evaluated the evidence for the use of these anaesthetic drugs in controlling seizure activity in patients with RSE.
Background: Persistent convulsions (lasting 30 minutes or more) are a major medical emergency associated with significant morbidity and mortality. At times, these convulsions fail to respond to first‐ and second‐line drug therapy, and may occur in up to 31% of patients suffering from persistent seizure or convulsive activities. Persistent seizure activity may become unresponsive to antiepileptic drugs. Anaesthetics such as thiopental sodium and propofol are frequently given for control of seizures in such situations. Both agents have their own side effects and complications.
Study characteristics: The evidence is current to August 2016. We could only identify one trial, which was terminated early due to recruitment problems. This study enrolled only 24 participants of the required 150. This study was a small, single‐blind, multicentre trial studying adults with RSE receiving either propofol or thiopental sodium for the control of seizure activity.
Key results: There was no difference between the two drugs in their ability to control seizure activity. The only difference noted was the requirement for prolonged mechanical ventilation for patients in the thiopental sodium group. This could be due to the prolonged presence of the drug in the body due to its slow removal.
Quality of evidence: We judged the quality of the evidence for our primary outcomes of total control of seizures and in‐hospital mortality to be low. There is a clear need for a large randomised controlled trial to study the efficacy of anaesthetic agents in the treatment of RSE.
Summary of findings
Summary of findings for the main comparison. Propofol compared to Thiopental sodium for the treatment of refractory status epilepticus.
| Propofol compared to Thiopental sodium for the treatment of refractory status epilepticus | ||||||
| Patient or population: patients with the treatment of refractory status epilepticus Settings: Hospital based Intervention: Propofol Comparison: Thiopental sodium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Thiopental sodium | Propofol | |||||
| Total control of seizures | Study population | RR 1.5 (0.4 to 5.61) | 21 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 286 per 1000 | 429 per 1000 (114 to 1000) | |||||
| In‐hospital mortality | Study population | RR 1.5 (0.19 to 11.93) | 21 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 143 per 1000 | 214 per 1000 (27 to 1000) | |||||
| Length of intensive care unit (ICU) stay | Not reported | Not reported | NA | NA | NA | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single blinded study: we downgraded one level for risk of bias 2 Wide confidence intervals crossing the line of "no effect" were noted; we downgraded one level for imprecision
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 6, 2015).
Although there is no universally accepted definition of status epilepticus (SE), it has been defined as a condition in which there is either continuous seizure activity for more than 30 minutes, or two or more seizure activities in a sequence without return of full consciousness between the episodes (Prasad 2005; Working Group 1993).
There is no consensus on the duration of seizure activity that may be required to define SE. SE may be broadly classified into two types, convulsive and non‐convulsive. The common aetiologies for SE are stroke, traumatic brain injury, brain tumours, central nervous system infection, metabolic or toxic encephalopathies, and electrolyte disorders. SE is a major medical emergency associated with significant morbidity and mortality (16% to 23%) (Rossetti 2007).
Refractory status epilepticus (RSE) is defined as SE that fails to respond to first‐ and second‐line therapy, and it is observed in 9% to 31% of patients with SE (Mayer 2002; Treiman 1998). To be categorised as RSE, some authors have suggested a time frame (Mayer 2002), whereas others have not (Holtkamp 2007; Rossetti 2005). The first line of treatment for SE includes benzodiazepines; the second line includes antiepileptic drugs such as phenytoin, phenobarbital, or valproic acid. The assistance of an anaesthetist is required for managing RSE, where coma may be induced with anaesthetic agents in order to achieve complete control of seizures. The use of anaesthetic agents such as thiopental sodium and propofol for managing RSE is common in many centres (Parviainen 2002; Van Gestel 2005). Thiopental sodium belongs to the barbiturates group of drugs unrelated to propofol, which is a phenolic compound. Both agents have been found to be effective in controlling seizures in RSE (Parviainen 2002; Van Gestel 2005). There is substantial lack of evidence as to which of the two drugs, thiopental sodium or propofol, is better in terms of clinical outcome of patients with RSE.
Description of the condition
RSE develops when patients become resistant to antiepileptic drugs with the passage of time. In hospital‐based treatment, it develops in 31% to 44% of patients with SE (Mayer 2002). Significant morbidity and mortality are associated with RSE. Failure to respond to antiepileptic drugs has led to the use of anaesthetic agents to control seizures. The popular anaesthetic agents are barbiturates, propofol, and isoflurane.
Description of the intervention
Anaesthetic agents have been used for the treatment of RSE. Barbiturates and propofol have been commonly used in this regard. However, most of the published literature is anecdotal. There is no consensus as to which of the two agents is better in terms of clinical outcome.
How the intervention might work
Thiopental sodium, a barbiturate, is a γ‐aminobutyric acid‐A (GABAA) agonist with possible actions on calcium channels (Rogowski 2004). Barbiturates have a prolonged duration of action, mainly due to their accumulation in the body. They are also known to produce hypotension during use. In contrast, propofol is gaining popularity because of its shorter duration of action and little tendency to accumulate in the body. Similar to barbiturates, propofol also produces hypotension, and reduces intracranial pressure and brain metabolic requirements (Marik 2004). Prolonged use of propofol as an infusion has been shown to result in potentially fatal cardiovascular collapse associated with lactic acidosis, hypertriglyceridaemia, and rhabdomyolysis, the so‐called "propofol infusion syndrome" (Zarovnaya 2007). Both agents are also N‐methyl‐D‐aspartate (NMDA) antagonists in vitro (Zhan 2001).
Why it is important to do this review
The current literature provides enough evidence to suggest that both thiopental sodium and propofol are effective in the treatment of RSE (Parviainen 2002; Van Gestel 2005). As both agents are associated with inherent side effects and complications, the choice of the agent is usually left at the discretion of the attending anaesthetist. There is a lack of evidence to suggest the superiority of one drug over the other. The aim of this Cochrane Review is to establish which of the two commonly used anaesthetic agents, thiopental sodium or propofol, is better suited for the treatment of RSE.
Objectives
To compare the efficacy, adverse effects, and short‐ and long‐term outcomes of refractory status epilepticus (RSE) treated with one of the two anaesthetic agents, thiopental sodium or propofol.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant RCTs or quasi‐RCTs, regardless of blinding. Diagnosis of RSE was based on any given standard definition specified in the articles and treatment consisting either of propofol or thiopental sodium. We excluded studies that did not define RSE, prior treatment with any other intravenous anaesthetic before treatment with thiopental sodium or propofol, and use of intermittent boluses of thiopental sodium or propofol for treating RSE.
Types of participants
We included individuals of any age and gender diagnosed with RSE of any aetiology.
Types of interventions
Patients receiving either thiopental sodium or propofol for the treatment of RSE, in addition to standard antiepileptic drugs used in status epilepticus (SE).
Types of outcome measures
Primary outcomes
Total control of seizures (after the first course of study drug).
In‐hospital mortality.
Length of intensive care unit (ICU) stay.
Secondary outcomes
Adverse events, such as infection, hypotension, and propofol infusion syndrome.
Duration of mechanical ventilatory support.
Duration of hospital stay.
Cognitive deficits.
Long‐term outcomes, such as dependence for daily activities (walking, eating, bathing, dressing, and toileting).
Search methods for identification of studies
Searches were run for the original review in May 2011. Subsequent searches were run in June 2011, March 2014, and March 2015. For the latest update we searched the following databases.
Cochrane Epilepsy Group Specialized Register (16 August 2016) using the search strategy outlined in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 16 August 2016) using the search strategy outlined in Appendix 2.
MEDLINE (Ovid, 1946 to 16 August 2016) using the search strategy outlined in Appendix 3.
ClinicalTrials.gov (16 August 2016) using the search strategy outlined in Appendix 4.
South Asian Database of Controlled Clinical Trials (16 August 2016) using the search strategy outlined in Appendix 5.
Previously we searched IndMED (26 March 2015) using the search terms: 'Propofol AND (thiopental OR thiopentone) AND (epilepsy OR epileptic)', but this was not accessible at the time of the latest update.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Using the results of the above searches, we screened all titles and abstracts for eligibility. Two review authors (HP and MK) independently performed this screening. We obtained and assessed the full articles of all eligible RCTs for relevance based on the pre‐planned checklist. Each author documented the reason for each trial that was excluded. We resolved any disagreement by discussion and decided on the inclusion or exclusion of the study. We compiled a list of all eligible trials. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram' (Moher 2009).
Data extraction and management
Two review authors (HP and MK) planned to independently extract the data and assess the trial quality. We resolved any disagreement through consultation and discussion. In case of additional information being required, HP was chosen to contact the first author of the relevant trial. Data extracted from the included trial, Rossetti 2011, were total control of seizures, mortality, adverse events, long‐term outcome, and the duration of mechanical ventilation.
Assessment of risk of bias in included studies
Two review authors assessed the methodological quality of the eligible trials independently (HP and MK). We resolved any disagreement by discussion. We performed the assessment as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the quality of the study on the basis of the following.
Random sequence generation.
Allocation concealment.
Blinding and outcome assessment.
Incomplete outcome data.
Selective reporting.
Any other bias.
We included a 'Risk of bias' table as part of the 'Characteristics of included studies' and a 'Risk of bias' summary figure (Figure 1), which details all of the judgements made for all included studies in the review.
1.
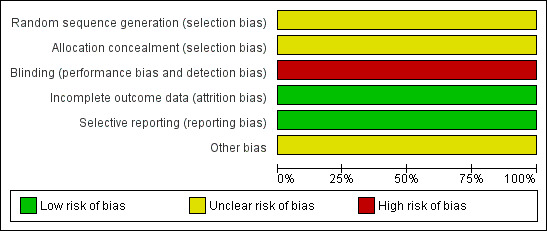
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Measures of treatment effect
We planned to record the number (percentage) of participants experiencing each categorical outcome, such as total control of seizures, mortality, adverse events, and long‐term outcomes. We planned to record the mean (standard deviation (SD))/median (range) for continuous outcomes, such as duration of mechanical ventilation, per randomised group. We carried out all primary analyses by 'intention‐to‐treat'. We used risk ratios (RRs) to measure the treatment effect for proportions (dichotomous outcomes) among the various outcomes. We planned to convert continuous data to mean differences (MDs) using the inverse variance method and calculate an overall MD. We planned to use a fixed‐effect model when we found no evidence of significant heterogeneity between studies, and a random‐effects or fixed‐effect model when heterogeneity was likely.
Unit of analysis issues
We planned to include only RCTs with parallel design in our review. The nature of the intervention here suggested that unit of analysis issues, such as those associated with cluster‐randomisation, were unlikely to arise. If we had included any cluster‐randomised studies we would have assessed the risk of bias following the suggestions in Section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the approach to analysis suggested in the subsequent sections. We would have taken a similar approach to any cross‐over trials included.
Dealing with missing data
We contacted the first author of the relevant trial and collected the required information.
Assessment of heterogeneity
We planned to assess the clinical heterogeneity of included studies, defined as methodological diversity, such as distribution of patient characteristics (age, seizure type, and number of drugs taken at the time of randomisation) and trial factors (randomisation concealment, blinding, and loss to follow‐up). We planned to use the Q statistic to test the statistical heterogeneity between trials and the I2 statistic to assess the magnitude of heterogeneity (Higgins 2002).
Assessment of reporting biases
We planned to assess publication bias/small‐study effects in a qualitative manner, using a funnel plot. Due to limited data, we did not assess this bias.
Data synthesis
We quantitatively reviewed the included data and combined data by intervention, outcome and population using the Cochrane Collaboration's statistical software, Review Manager (RevMan 2014). We planned to assess statistical heterogeneity using the Chi2 test and consider P values of 0.05 or less as statistically significant. We planned to assess the level of inconsistency across the studies using the I2 statistic, where an I2 value greater than 50% indicates substantial heterogeneity. Had we found statistically significant heterogeneity that we could not readily explain, we would have assessed it using a random‐effects model.
Due to only one study meeting our inclusion criteria, we did not perform a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for the following: age groups (children (< 14 years of age) and adults), gender, aetiology, and type of seizure (convulsive or non‐convulsive).
Due to limited data, we did not perform a subgroup analysis.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess the influence of including studies judged to have low methodological quality and characteristics of the interventions; that is, the doses of propofol and thiopental used.
Due to limited data, we did not perform a sensitivity analysis.
Summary of findings
In this review update, we used the principles of the GRADE approach (Guyatt 2008) to assess the quality of the body of evidence associated with our primary outcomes (total control of seizures, in‐hospital mortality, length of ICU stay), and we constructed a Summary of Findings table (Table 1) using the GRADE software. When using the GRADE approach, one appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded evidence from 'high quality' by one level for serious (and by two levels for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect or potential publication bias.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
Our searches yielded 48 references (13 from MEDLINE, 7 from CENTRAL, 2 from the Cochrane Epilepsy Group Specialized Register, 22 from the South Asian Database of Controlled Clinical Trials, and 4 from ClinicalTrials.gov). The search of IndMED yielded no references. After de‐duplication, 38 references remained. After further scrutiny, we identified only one study for inclusion in this review.
Figure 2 shows the results of our searches.
2.
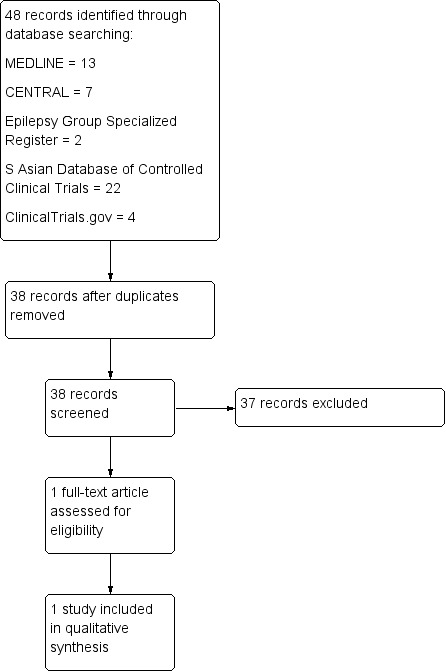
Study flow diagram.
Included studies
We included only one study in the review (Rossetti 2011). An overview of the study is given in Table 2. This study was supported in part by grants from the Swiss League against Epilepsy, AstraZeneca (Switzerland) and UCB (Switzerland).
1. Overview of study populations.
| Study ID | Interventions | Screened (n) | Randomised (n) | Safety analysis (n) | ITT (n) | Finishing study (n) | [%] of randomised participants finishing study |
| Rossetti 2011 | I1 Propofol I2 Barbiturate (thiopental (n = 7) and pentobarbital (n = 3)) |
I1 14 I2 10 |
I1 14 I2 10 |
I1 14 I2 10 |
I1 14 I2 10 |
I1 14 I2 9 |
I1 100 I2 90 |
I1: intervention 1; I2: intervention 2; ITT: intention‐to‐treat; n: number.
The study conducted by Rossetti et al was a randomised, single‐blind, multicentre trial studying adults with RSE (Rossetti 2011). Patients received either propofol or barbiturates for control of seizures. Fourteen patients received propofol. In the barbiturates group, seven patients received thiopental sodium, and three patients received pentobarbital. The primary endpoint was the proportion of patients with RSE controlled after the first course of the study drug, and secondary endpoints included drug tolerability. The trial was terminated after three years with only 24 patients recruited of the 150 required. The trial was terminated before completion due to inadequate recruitment and the prolonged mechanical ventilation requirement identified in the barbiturate arm. Treatment‐related complications were comparable for both propofol and barbiturates.
Excluded studies
None.
Risk of bias in included studies
The study conducted by Rossetti et al was single‐blind (Rossetti 2011). The small sample size and lack of double‐blinding could have influenced the results.
Allocation
It is difficult to understand how allocation was done, as the authors have not commented on this in their paper. Personal communication with the authors failed to clarify this issue.
Blinding
This was a single‐blind study where only the patient was blinded, so there is a chance of performance bias and detection bias.
Incomplete outcome data
Attrition bias is unlikely, as there were no incomplete outcome data.
Selective reporting
Reporting bias is unlikely as the authors reported all the outcomes that they described in the methodology.
Other potential sources of bias
This study was supported in part by grants from the Swiss League against Epilepsy, AstraZeneca (Switzerland) and UCB (Switzerland).
Effects of interventions
See: Table 1
Propofol versus thiopental sodium
Primary outcomes
Total control of seizures
There was no statistically significant difference between propofol and thiopental sodium in total control of seizures after the first course of the study drug; 6/14 patients versus 2/7 patients in the propofol and thiopental sodium groups, respectively (risk ratio (RR) 1.50, 95% confidence interval (CI) 0.40 to 5.61) (Analysis 1.1).
1.1. Analysis.
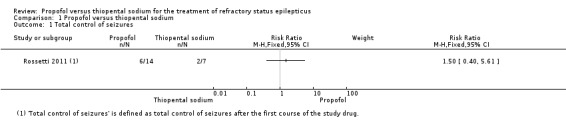
Comparison 1 Propofol versus thiopental sodium, Outcome 1 Total control of seizures.
In‐hospital mortality
There was no statistically significant difference in mortality between propofol and thiopental sodium; 3/14 patients versus 1/7 patients in the propofol and thiopental sodium groups, respectively (RR 1.50, 95% CI 0.19 to 11.93). In the propofol group, deaths presumed to be in‐hospital were secondary to Creutzfeldt‐Jakob disease (day five), cardiac asystole (day six) and progressive brain tumour (day 11). In the thiopental sodium group, one patient died on day five, secondary to colic ischaemia. Deaths on days 21, 29, and 42 were due to paraneoplastic encephalitis, pneumonia, and sepsis, respectively, which are presumed to be out‐of‐hospital (Analysis 1.2).
1.2. Analysis.
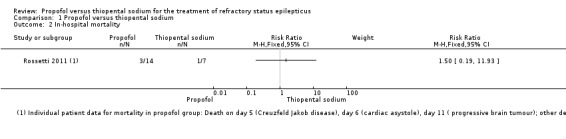
Comparison 1 Propofol versus thiopental sodium, Outcome 2 In‐hospital mortality.
Length of ICU stay
Data on this outcome were not reported.
Secondary outcomes
Adverse events
There was no statistically significant difference in adverse events between propofol and thiopental sodium (Table 3). Infection was seen in 7/14 patients versus 5/7 patients in the propofol and thiopental sodium groups, respectively (RR 0.70, 95% CI 0.35 to 1.41) (Analysis 1.3).
2. Adverse effects.
| Characteristic | Rossetti 2011 |
| I1 I2 |
Propofol Thiopental |
| Participants who died (n) Epilepsy‐related I1 Propofol I2 Thiopental |
0 0 |
| Participants who died (n) All causes I1 Propofol I2 Thiopental |
3 1 |
| Adverse events (n) I1 Propofol I2 Thiopental |
14 11 |
| Serious adverse events (n) I1 Propofol I2 Thiopental |
1 1 |
| Duration of ICU stay | Not reported |
| Duration of mechanical ventilation (median (range)) I1 Propofol I2 Thiopental |
17 days (5 to 70 days) 4 days (2 to 28 days) |
| Duration of hospitalisation | Not reported |
| Neurological deficits | Not reported |
| Cognitive deficits | Not reported |
| Haematological toxicity | Not reported |
| Liver toxicity | Not reported |
| Hypersensitivity or drug allergy | Not reported |
| Bronchopneumonia | Not reported |
| Other side effects | Not reported |
I1: intervention 1; I2: intervention 2; ICU: intensive care unit; n: number.
1.3. Analysis.
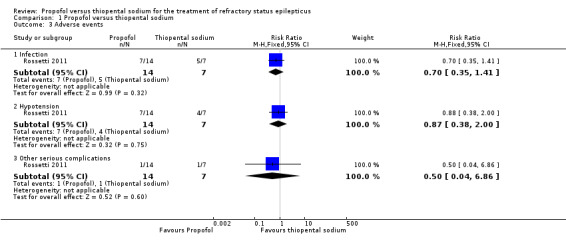
Comparison 1 Propofol versus thiopental sodium, Outcome 3 Adverse events.
Hypotension during administration of study drugs and requiring use of vasopressors was seen in 7/14 patients versus 4/7 patients in the propofol and thiopental sodium groups, respectively (RR 0.87, 95% CI 0.38 to 2.00) (Analysis 1.3).
The other severe complication noted was non‐fatal propofol infusion syndrome in one patient (RR 0.50, 95% CI 0.04 to 6.86) (Analysis 1.3).
Duration of mechanical ventilatory support
The number of days of mechanical ventilation was greater in the thiopental sodium group when compared with the propofol group (median (range): 17 days (5 to 70 days) with thiopental sodium versus four days (2 to 28 days) with propofol).
Duration of hospital stay
Data on this outcome were not reported.
Cognitive deficits
Data on this outcome were not reported.
Long‐term outcomes
There was no statistical difference in the functional outcome between propofol and thiopental sodium at three months; 5/14 patients versus 3/7 patients in the propofol and thiopental sodium groups, respectively (RR 0.83, 95% CI 0.28 to 2.52) (Analysis 1.5).
1.5. Analysis.
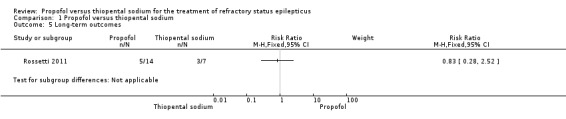
Comparison 1 Propofol versus thiopental sodium, Outcome 5 Long‐term outcomes.
Discussion
Our search identified only one study that addressed the issue of treatment of refractory status epilepticus (RSE) using thiopental sodium and propofol in a randomised, single‐blind, multicentric trial (Rossetti 2011). The trial was terminated before completion due to inadequate recruitment. Twenty‐four patients were recruited in five centres; 14 received propofol and seven received thiopental sodium. The primary endpoint, that is, control of seizure with first course of drug treatment, was achieved in 43% in the propofol group and 22% in the barbiturate group (seven patients received thiopental sodium and three patients received pentobarbital). The overall mortality was 43% and 34% in the propofol and barbiturate groups, respectively. However, the authors fail to report the in‐hospital mortality. Patients returning to baseline condition at the three‐month follow‐up were similar in the two groups. No information was provided on the length of intensive care unit (ICU) and hospital stay of the patients. However, days of mechanical ventilation were significantly more in the thiopental group. This could be due to the long elimination half‐life of the drug when compared with propofol. The fact that the trial was prematurely stopped could have introduced bias. At the same time, the under‐sampling resulted in loss of power to detect a difference between the two treatment arms.
This study confirms that RSE is a serious clinical condition carrying high morbidity and mortality. The authors of the study agree that a larger multicentric study is needed with a larger sample size and adequate funding to obtain conclusive results. The authors also suggest that a third treatment arm using midazolam as the treatment drug may be included in the study, which may address the issue of tolerability of the drugs, propofol and thiopental.
Summary of main results
Both propofol and thiopental sodium are broadly comparable in terms of seizure control, mortality, rate of complications, adverse events and long‐term outcomes in patients with refractory status epilepticus (RSE). The 95% confidence interval was wide and allowed for up to a more than two‐fold difference between the two drugs. Patients receiving thiopental sodium required more days of mechanical ventilation when compared with patients receiving propofol.
Overall completeness and applicability of evidence
There is a lack of evidence as to the efficacy of propofol and thiopental sodium when compared with each other in the treatment of RSE. We are unable to detect a difference between the two drugs due to methodological issues. There is a need for a large randomised controlled trial (RCT) for this serious condition.
Quality of the evidence
This review included a single trial, which was terminated prior to completion due to inadequate recruitment of patients (Rossetti 2011). This trial was not double‐blinded, introducing a high risk of bias. We judged the quality of the limited evidence available for this review to be low (Table 1).
Potential biases in the review process
We followed the strict criteria for study selection and used a data extraction form for included studies as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We do not feel that any form of bias could have been introduced by the review authors during the preparation of this Cochrane Review.
Agreements and disagreements with other studies or reviews
To date this is the only eligible study available which has been included in our review. In a systematic review published in 2002 (Claassen 2002), the authors compared the efficacy of midazolam, propofol, and pentobarbital for terminating seizures in RSE patients. Considering all possible limitations, the authors concluded that pentobarbital was more effective than any other strategy suggested for treatment of RSE.
Authors' conclusions
Implications for practice.
There are insufficient data from randomised controlled trials (RCTs) to assess the efficacy of thiopental sodium and propofol in the treatment of refractory status epilepticus (RSE). Clinicians managing such cases should be aware of the adverse effects of these two anaesthetics drugs.
Implications for research.
RSE is a serious clinical condition and conducting clinical trials in this area may be difficult. Various ethical and methodological issues may arise. However, the problem itself is important and needs to be resolved. Use of thiopental sodium and propofol in RSE patients should be assessed in good quality, multicentric, randomised controlled trials for their effect and efficacy in terms of total control of seizures, mortality, length of intensive care unit (ICU) and hospital stay, adverse effects, and long‐term outcomes, such as dependence for daily activities. Many centres may have to be involved to enrol a suitable number of patients so that adequate study power can be achieved. A standard method may have to be followed with uniform outcome measures.
Since the last version of this review we have found no new studies.
What's new
| Date | Event | Description |
|---|---|---|
| 16 August 2016 | New search has been performed | Searches updated 16 August 2016. |
| 16 August 2016 | New citation required but conclusions have not changed | No new, relevant studies found. Conclusions are unchanged. |
History
Protocol first published: Issue 7, 2011 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 26 March 2015 | New citation required but conclusions have not changed | No new, relevant studies found. Conclusions are unchanged. |
| 26 March 2015 | New search has been performed | Searches updated 26 March 2015. |
Acknowledgements
We wish to thank the Cochrane Epilepsy Group for their continuous support in helping us prepare the review. We would like to thank Professor A Rossetti for providing the additional data and information on the included study. We wish to thank the South Asian Cochrane Network and Centre, CMC, Vellore, India, who conducted the workshop at the Prof. BV Moses and ICMR Center for Advanced Research and Training in Evidence‐Informed Healthcare, where this review was completed. We wish to acknowledge Gyaninder Pal Singh and Ashish Bindra who contributed to the original version of this review.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 status epilepticus
#2 MeSH DESCRIPTOR Status Epilepticus Explode All
#3 #1 OR #2
#4 thiopental OR thiopentone
#5 MeSH DESCRIPTOR Barbiturates Explode All
#6 MeSH DESCRIPTOR Thiopental Explode All
#7 #4 OR #5 OR #6
#8 MeSH DESCRIPTOR Propofol Explode All
#9 propofol
#10 #8 OR #9
#11 #3 AND #7 AND #10
#12 #11 AND > 26/03/2015:CRSCREATED AND INREGISTER
Appendix 2. CENTRAL via Cochrane Register of Studies Online (CRSO) search strategy
#1 MESH DESCRIPTOR Propofol EXPLODE ALL TREES
#2 anepol OR diprivan OR disoprivan OR disoprofol OR fresofol OR hypro OR lipuro OR plofed OR profol OR propofil OR propofol OR propofol2 OR propofol9 OR propofola OR propofolalfentanil OR propofolanasthesie OR propofoland OR propofolanestesi OR propofolapplikationssysteme OR propofolbased OR propofold OR propofoldosierungen OR propofole OR propofoleinsatz OR propofolem OR propofolfentanyl OR propofolformulierungen OR propofolgpi OR propofolis OR propofolketamine OR propofolom OR propofolon OR propofolondansetron OR propofolparavertebral OR propofolremifentanil OR propofols OR propofolsedierung OR propofolsufentanil OR propofolthiopentone OR propofolum OR propofolun OR propofolverbrauch OR propofolvs OR propofolzielkonzentration OR propovan OR propoven OR provive OR recofol
#3 #1 OR #2
#4 MESH DESCRIPTOR Thiopental EXPLODE ALL TREES
#5 penthiobarbital OR pentothal OR thiopental OR thiopentalem OR thiopentalor OR thiopentalum OR thiopentone OR tiopental OR tiopentale OR trapanal
#6 MESH DESCRIPTOR Barbiturates EXPLODE ALL TREES
#7 #4 OR #5 OR #6
#8 MESH DESCRIPTOR Status Epilepticus EXPLODE ALL TREES
#9 (Status Epilepticus):TI,AB,KY
#10 #8 OR #9
#11 #3 AND #7 AND #10
#12 26/03/2015 TO 16/08/2016:CD
#13 #11 AND #12
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2011.
1. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Status Epilepticus/
8. status epilepticus.tw.
9. 7 or 8
10. exp Thiopental/
11. exp Barbiturates/
12. (thiopental or thiopentone).tw.
13. 10 or 11 or 12
14. exp Propofol/
15. propofol.tw.
16. 14 or 15
17. 13 and 16
18. 6 and 9 and 17
19. limit 18 to ed=20150326‐20160816
20. remove duplicates from 19
Appendix 4. ClinicalTrials.gov search strategy
propofol | epilepsy | received on or after 03/26/2015
Appendix 5. South Asian Database of Controlled Clinical Trials search strategy
Keywords: propofol And thiopent Or Title: propofol And thiopent Or Abstract: propofol And thiopent
Data and analyses
Comparison 1. Propofol versus thiopental sodium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total control of seizures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 In‐hospital mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infection | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.41] |
| 3.2 Hypotension | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.38, 2.00] |
| 3.3 Other serious complications | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 6.86] |
| 4 Duration of mechanical ventilation | Other data | No numeric data | ||
| 5 Long‐term outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.4. Analysis.
Comparison 1 Propofol versus thiopental sodium, Outcome 4 Duration of mechanical ventilation.
| Duration of mechanical ventilation | ||
|---|---|---|
| Study | Propofol group | Thiopentone sodium group |
| Rossetti 2011 | Median: 4 days | Median: 17 days |
| Rossetti 2011 | Range: 2 to 28 days | Range: 5 to 70 days |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rossetti 2011.
| Methods | Pragmatic randomised controlled trial, single‐blind, multicentric | |
| Participants | Participants: adults (> 16 years) with RSE not due to cerebral anoxia, who clinically required coma Sex (female/male): propofol group: 50%/50%; barbiturate group: 66%/34% Age (median (range)): propofol group: 57 years (26 to 87 years); barbiturate group: 64 years (16 to 78 years) Ethnic groups: not reported Duration of epilepsy: not reported Inclusion criteria: patients > 16 years of age suffering from RSE receiving at least 1 first‐line and 1 second‐line drug in adequate doses Exclusion criteria: patients with known pregnancy, known intolerance to the study drugs, mitochondrial disorders, egg allergy, hypertriglyceridaemia (> 5 mmol/L) or significant rhabdomyolysis (creatinine kinase > 1500 U/L) on admission Diagnostic criteria: RSE not due to cerebral anoxia, defined as ongoing clinical or electrographic seizures, or repetitive seizures without return to baseline for at least 30 minutes despite administration of 1 first‐line (benzodiazepine) and 1 second‐line antiepileptic drug (phenytoin, valproate, phenobarbital and levetiracetam) in adequate doses Comorbidities: none Co‐medications: none Total randomised: 24 patients; 14 allocated to propofol group and 10 allocated to barbiturate group (thiopental and pentobarbital); (1 patient in thiopental sodium group did not require treatment and so was excluded, remaining 9 analysed) |
|
| Interventions | Number of control centres: 2 Country/location: Switzerland and the US Setting: CHUV et Université de Lausanne, Lausanne and Brigham and Women's Hospital, Harvard School of Medicaine, Boston Intervention I: 2 mg/kg titrated to burst suppression or 4 mg/kg until EEG was available Intervention II: 2 mg/kg iv titrated to burst suppression or 5 mg/kg if no EEG available Treatment before study: first‐ and second‐line antiepileptic drugs Time to treatment since onset of status: not reported Duration of follow‐up: 3 months 2 treatment arms: propofol and barbiturates (thiopental sodium or pentobarbital) |
|
| Outcomes | Primary outcomes (as stated in the publication): to assess the effectiveness (RSE control, adverse events) of a first course of propofol versus barbiturates Secondary outcomes (as stated in the publication): none Additional outcomes Outcomes used in our review:
|
|
| Notes | Stated aim of study: "This prospective study was undertaken to assess the effectiveness (SE control, adverse events) of a first course of PRO versus barbiturates, the two most commonly used agents according to the aforementioned surveys" Language of publication: English Commercial funding: yes Non‐commercial funding: no Publication status (peer review journal): yes Publication status (journal supplement): no Publication status (abstract): no Funded by AstraZeneca (Switzerland) and UCB (Switzerland) No conflict of interest Clinical Trial.gov ID: NCT00265616 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After written consent was obtained by proxy, randomisation was stratified by institution using sealed envelopes." Comment: The authors do not explain how the randomization was done. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Comment: authors contacted. No information provided. |
| Blinding (performance bias and detection bias) Subjective Outcomes | High risk | Being a single‐blind study, only the patient was blinded. Assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. All participants randomised completed the study and were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were mentioned in the methodology have been reported. |
| Other bias | Unclear risk | The trial was terminated before completion due to inadequate recruitment. The calculated sample size for this study was 150 patients, 75 in each arm. Funding by pharmaceutical companies. |
EEG: electroencephalography; iv: intravenous; RSE: refractory status epilepticus; SE: status epilepticus.
Differences between protocol and review
The primary outcome in our protocol 'total control of seizures' is now defined as total control of seizures after the first course of the study drug.
By 'mortality' we now mean only in‐hospital mortality of the patients receiving study drugs. It does not include deaths after patients have been discharged from hospital.
A Google Scholar database search has not been conducted and so it has been removed from the list.
Contributions of authors
Conceiving the review: Hemanshu Prabhakar (HP).
Co‐ordinating the review: HP.
Undertaking manual searches: HP.
Screening search results: HP.
Organising retrieval of papers: HP.
Screening retrieved papers against inclusion criteria: HP.
Appraising quality of papers: HP.
Extracting data from papers: HP.
Writing to authors of papers for additional information: HP.
Providing additional data about papers: HP.
Obtaining and screening data on unpublished studies: HP.
Data management for the review: HP, Mani Kalaivani (MK).
Entering data into Review Manager (RevMan 2014): HP.
RevMan statistical data: HP, MK.
Other statistical analysis not using RevMan: HP, MK.
Double entry of data: (data entered by person one: HP; data entered by person two: MK).
Interpretation of data: HP, MK.
Statistical inferences: HP, MK.
Writing the review: HP.
Guarantor for the review (one author): HP.
Person responsible for reading and checking review before submission: HP, MK.
Sources of support
Internal sources
All India Institute of Medical Sciences, New Delhi, India.
External sources
-
National Institute of Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Declarations of interest
Hemanshu Prabhakar: None known.
Mani Kalaivani: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Rossetti 2011 {published data only}
- Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocritical Care 2011;14(1):4‐10. [DOI: 10.1007/s12028-010-9445-z] [DOI] [PubMed] [Google Scholar]
Additional references
Claassen 2002
- Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43(2):146‐53. [PUBMED: 11903460 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y. Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. British Medical Journal 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holtkamp 2007
- Holtkamp M. The anaesthetic and intensive care of status epilepticus. Current Opinion in Neurology 2007;20(2):188‐93. [PUBMED: 17351490 ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Marik 2004
- Marik PE. Propofol: therapeutic indications and side effects. Current Pharmaceutical Design 2004;10(29):3639‐49. [PUBMED: 15579060 ] [DOI] [PubMed] [Google Scholar]
Mayer 2002
- Mayer SA, Claasen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Archives of Neurology 2002;59(2):205‐10. [PUBMED: 11843690 ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. British Medical Journal 2009;339:2535.. [PMC free article] [PubMed] [Google Scholar]
Parviainen 2002
- Parviainen I, Uusaro A, Kalviainen R, Kaukanen E, Mervaala E, Ruokonen E. High‐dose thiopental in the treatment of refractory status epilepticus in intensive care unit. Neurology 2002;59(8):1249‐51. [PUBMED: 12391357 ] [DOI] [PubMed] [Google Scholar]
Prasad 2005
- Prasad K, Al‐Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003723.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogowski 2004
- Rogowski MA, Loscher W. The neurobiology of antiepileptic drugs. Nature Reviews Neuroscience 2004;5(7):553‐64. [PUBMED: 15208697 ] [DOI] [PubMed] [Google Scholar]
Rossetti 2005
- Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus. Effects of treatment aggressiveness on prognosis. Archives of Neurology 2005;62(11):1698‐702. [PUBMED: 16286542 ] [DOI] [PubMed] [Google Scholar]
Rossetti 2007
- Rossetti AO. Which anesthetic should be used in the treatment of refractory status epilepticus?. Epilepsia 2007;48(Suppl 8):52‐5. [PUBMED: 18330000 ] [DOI] [PubMed] [Google Scholar]
Treiman 1998
- Treiman DM, Meyers PD, Walton NY, Colling C, Rowan AJ, Handforth A, et al. A comparison of four treatments for generalized convulsive status epilepticus: Veterans Affairs Status Epilepticus Cooperative Study Group. New England Journal of Medicine 1998;339(12):792‐8. [PUBMED: 9738086] [DOI] [PubMed] [Google Scholar]
Van Gestel 2005
- Gestel JP, Blussé van Oud‐Alblas HJ, Malingré M, Ververs FF, Braun KP, Nieuwenhuizen O. Propofol and thiopental for refractory status epilepticus in children. Neurology 2005;65(4):591‐2. [PUBMED: 16116121 ] [DOI] [PubMed] [Google Scholar]
Working Group 1993
- Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA 1993;270(7):854‐9. [PUBMED: 8340986 ] [PubMed] [Google Scholar]
Zarovnaya 2007
- Zarovnaya EL, Jobst BC, Harris BT. Propofol‐associated fatal myocardial failure and rhabdomyolysis in an adult with status epilepticus. Epilepsia 2007;48(5):1002‐6. [PUBMED: 17381434 ] [DOI] [PubMed] [Google Scholar]
Zhan 2001
- Zhan RZ, Qi S, Wu C, Fujihara H, Taga K, Shimoji K. Intravenous anaesthetics differentially reduce neurotransmission damage caused by oxygen‐glucose deprivation in rat hippocampal slices in correlation with N‐methyl‐D‐aspartate receptor inhibition. Critical Care Medicine 2001;29(4):808‐13. [PUBMED: 11373474 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Prabhakar 2011
- Prabhakar H, Bindra A, Singh GP, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009202.pub2] [DOI] [PubMed] [Google Scholar]
Prabhakar 2012
- Prabhakar H, Bindra A, Singh GP, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009202.pub2] [DOI] [PubMed] [Google Scholar]
Prabhakar 2015
- Prabhakar H, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD009202.pub3] [DOI] [PubMed] [Google Scholar]


