Abstract
Background
Fluid restriction is often recommended as part of the management of infants with early or established bronchopulmonary dysplasia (BPD).
Objectives
To determine whether fluid restriction as part of the therapeutic intervention for early or established BPD improves clinical outcomes.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1) in the Cochrane Library (searched 16 February 2016), MEDLINE via PubMed (1966 to 16 February 2016), Embase (1980 to 16 February 2016), and CINAHL (1982 to 16 February 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Prospective randomised clinical trials comparing two distinct fluid administration volumes in preterm infants with early or established BPD.
Data collection and analysis
We used the standard methods of Cochrane Neonatal. For the included trial, we extracted data and assessed the risk of bias, and used GRADE methods to assess the quality of the evidence. The outcomes considered in this review are effects on mortality or requirement for oxygen at 36 weeks' postmenstrual age (primary outcome measure), the duration of supplemental oxygen therapy, proportion of infants discharged from hospital on oxygen, duration of assisted ventilation, duration of hospitalisation, weight gain, feeding tolerance, apnoea, necrotizing enterocolitis, renal dysfunction or nephrocalcinosis, lung mechanics, and use of diuretic therapy (secondary outcome measures).
Main results
One trial was found, including 60 preterm infants at 28 days of age with persistent oxygen requirements. Infants were randomised to either 180 mL/kg/day of standard formula or 145 mL/kg/day of concentrated formula. This single study did not provide data regarding our primary outcome. No effects of the intervention were found on any of our secondary outcomes. The quality of the evidence from this study was graded low.
Authors' conclusions
There is no evidence to support the practice of fluid restriction in infants with early or established BPD.
Plain language summary
Fluid restriction as a treatment for preterm infants developing chronic lung disease
Background Babies born prematurely are at risk of developing a form of chronic lung disease which is defined as persistent need for oxygen once the child has reached a corrected gestational age of 36 weeks. As fluid accumulation in the lung is one of the processes involved in the early phases of lung disease in premature babies, low fluid intake might halt the progression of the insult and result in lower rates of chronic lung disease of prematurity.
Study characteristics Our search identified only one study comparing two volumes of fluid intake in preterm infants with early signs of chronic lung disease. Unfortunately, this study did not report progression to established chronic lung disease. Hence, no infant could be included in this analysis.
Other outcomes, including days that the baby needed extra oxygen, proportion of infants discharged from hospital on oxygen, days of assisted ventilation, duration of hospital stay, weight gain and serious apnoeas were not affected by the volume of fluid received (from the evidence graded as low quality). The evidence is current to February 2016.
Key results There is no study comparing low to high fluid intake in a population of preterm babies with early signs of chronic respiratory disease to prevent progression to full blown chronic lung disease or death. Other outcomes were not improved by fluid restriction.
Quality of evidence Not applicable.
Summary of findings
Summary of findings for the main comparison. Fluid restricted compared to liberal fluids compared to placebo for treatment of preterm babies with chronic lung disease.
| Fluid restricted compared to liberal fluids compared to placebo for treatment of preterm babies with chronic lung disease | ||||||
| Participant or population: treatment of preterm babies with chronic lung disease Setting: Intervention: Fluid restricted compared to liberal fluids Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with fluid restricted compared to liberal fluids | |||||
| Duration of oxygen therapy | The mean duration of oxygen therapy was 27.5 days | median 0.5 days higher (0 to 0 ) | ‐ | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Duration of hospitalisation | The mean duration of hospitalisation was 85 days | MD 3 Days higher (7.64 lower to 13.64 higher) | ‐ | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Daily weight gain | The mean daily weight gain was 14.8 g/kg/d | MD 0.5 g/kg/d higher (1.22 lower to 2.22 higher) | ‐ | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Proportion with apnoea | Study population | RR 1.35 (0.98 to 1.86) | 60 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 630 per 1000 | 850 per 1000 (617 to 1000) | |||||
| Moderate | ||||||
| 630 per 1000 | 850 per 1000 (617 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Unmasked study
2 Wide confidence limits
Background
Description of the condition
Bronchopulmonary dysplasia (BPD), often referred to as chronic lung disease (CLD) of prematurity, is a frequent complication of neonatal intensive care. It occurs more frequently in the very immature infant and leads to persistent respiratory distress and a long‐term oxygen requirement. An inflammatory interstitial pulmonary oedema was described as part of the pathogenesis of early CLD in the preterm infant (Northway 1967; Brown 1978). It is not known if this is still the case in more recently treated infants in whom a reduction in alveolar development seems to be paramount in the development of "the new BPD" (Jobe 2011).
Description of the intervention
Because of the presence of excess lung water, diuretics have been investigated as a therapeutic option. They are now used frequently in the treatment of preterm infants with BPD. Physiological studies of diuretics have demonstrated short‐term effects on lung mechanics but no long‐term clinical benefits have been proven (Kao 1983; McCann 1985).
Possibly by extrapolation from the use of diuretics came the practice of restricting fluid intake as a treatment for preterm infants with CLD. Some neonatal textbooks recommend restricting fluid as a treatment for BPD (Niermeyer 1988; Bancalari 2002). By the time fluid restriction is contemplated, most infants are receiving enteral nutrition, either totally or at least partially.
Fluid restriction requires limiting total (enteral and parenteral) fluid intake to less than the amount usually recommended (i.e. to less than 150 mL/kg/day), with the goal of improving pulmonary function.
How the intervention might work
Fluid restriction is used in the hope that it will reduce pulmonary oedema and thus improve pulmonary function, and perhaps lessen lung injury.
Why it is important to do this review
There are concerns with the practice of fluid restriction as it may interfere with the delivery of adequate nutrition. It seems likely that restricting fluid intake will lead to a decrease in urine output; within clinically acceptable limits of fluid intake it is questionable whether fluid restriction will lead to changes in total body water, lung fluid balance or pulmonary function. In addition, the reduction of free water intake will probably lead to an increase in renal solute load and potentially to a greater incidence of renal dysfunction and nephrocalcinosis. On the other hand, larger fluid volumes could be associated with an increase in feeding intolerance or with a risk of necrotizing enterocolitis (Viswanathan 2015).
Randomised comparisons of differing levels of fluid intake in early life of the preterm infant as a means of preventing BPD have been performed and systematically reviewed (Bell 1998). These studies showed that reduced fluid intakes led to a non‐significant trend toward reduction in the risk of BPD and a significant reduction in death (Bell 1998).
Once BPD has developed or is developing, fluid restriction as part of treatment of the disease is sometimes considered by physicians, and continues to be recommended as a part of treatment. Review articles recommending fluid restriction usually provide no references, or refer solely to other review articles.
We wished to determine if there is adequate evidence to support this practice. Recommended fluid intake can be defined as 150 ml/kg/day or more (Gregory 2005); we therefore considered a fluid intake less than 150 ml/kg/day as 'restricted'. For the purpose of this review, we defined BPD as oxygen requirement at 28 postnatal days, and CLD as oxygen requirement at 36 weeks' postmenstrual age.
Objectives
To determine whether fluid restriction as part of the therapeutic intervention for early or established BPD improves clinical outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomised and quasi‐randomised trials. We excluded short‐term cross‐over studies as not being informative for our primary outcome measure.
Types of participants
Preterm infants (< 34 weeks) with early or established BPD. Established BPD was defined as oxygen need and evidence of respiratory insufficiency at 28 days of age. Early BPD was defined as a persistent oxygen need, respiratory insufficiency and high predicted likelihood of development of established BPD after a minimum of 21 days of age. Maximum age of enrolment into the study was 36 weeks' postmenstrual age.
Types of interventions
We searched for studies comparing two different levels of fluid intake. Because there is a wide range of possible fluid intakes in such infants, in order to group the studies we assumed that normal fluid intake is 140 to 150 ml/kg/day, and we intended to group the subjects into unrestricted (≥ 150 ml/kg/day) and fluid restricted (< 150 ml/kg/day).
Types of outcome measures
Primary outcomes
Mortality or requirement for oxygen at 36 weeks' postmenstrual age (CLD).
Secondary outcomes
Duration of supplemental oxygen in days from enrolment until discharge.
Proportion of infants discharged from hospital on oxygen.
Duration of assisted ventilation in days from enrolment until discharge.
Duration of hospitalisation in days from enrolment until discharge.
Daily weight gain from enrolment until the end of the intervention.
Apnoea: number (proportion) of infants with problematic apnoea.
Feeding intolerance: number (proportion) of infants with feeding intolerance requiring alteration in feeds.
Necrotizing enterocolitis (NEC): number (proportion) of infants with NEC defined as Bell stage two or more.
Renal dysfunction: mean changes in serum creatinine between enrolment and the end of the intervention.
Nephrocalcinosis: proportion of infants with nephrocalcinosis on renal ultrasound or CT scan.
Lung mechanics: percentage change in dynamic compliance between enrolment and the end of the intervention.
Need for diuretics: number of doses of diuretic (loop diuretic or thiazide) needed per participant since the start of intervention.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1) in the Cochrane Library (searched 16 February 2016); MEDLINE via PubMed (1966 to 16 February 2016); Embase (1980 to 16 February 2016); and CINAHL (1982 to 16 February 2016) using the following search terms: (fluid OR food, formulated) AND (bronchopulmonary dysplasia OR BPD OR chronic lung disease OR CLD), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials' registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Clinical Trials Registry Platform www.who.int/ictrp/search/en/; and the ISRCTN Registry).
Searching other resources
We examined the references in all studies identified as potentially relevant.
Data collection and analysis
We used the standard methods of Cochrane and Cochrane Neonatal (Higgins 2011). Review authors independently assessed trials for inclusion and methodological quality. Differences were resolved by discussion. We planned to contact study authors if we needed additional information.
Selection of studies
All three review authors screened the title and abstract of all studies identified by the above search strategy. We assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until consensus was achieved.
Data extraction and management
All three review authors extracted the data separately. Any disagreements were discussed until consensus was achieved. We contacted trial authors for further information if data from the trial reports was insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of Cochrane Neonatal. The methodological quality of the studies was assessed using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement/assessment. For each criterion, assessment was 'yes', 'no' or 'unclear'. Additional information from the trial authors was requested to clarify methodology and results as necessary. This information was included in the table 'Characteristics of included studies'.
In addition, the review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The methodological quality of the studies was assessed using the following criteria.
Sequence generation (evaluating possible selection bias). For each included study, we described the method used to generate the allocation sequence as: adequate (any truly random process e.g. random number table; computer random number generator); inadequate (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or unclear.
Allocation concealment (evaluating possible selection bias). For each included study, we described the method used to conceal the allocation sequence as: adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or unclear.
Blinding (evaluating possible performance bias). For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We assessed the methods as: adequate, inadequate, or unclear for participants; adequate, inadequate, or unclear for study personnel; and adequate, inadequate, or unclear for outcome assessors.
Incomplete outcome data (evaluating possible attrition bias through withdrawals, dropouts, protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as: adequate (< 20% missing data); inadequate (> 20% missing data); or unclear;
Selective reporting bias. For each included study where the protocol is available (through trials' registers), we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported); inadequate (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or unclear.
Other sources of bias. We noted other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes; no; or unclear.
Funding sources: when reported we divided funding sources into funding from local funds (hospital, research centre, or university), charitable foundations, government agencies, or from industry. If funding was from industry, and industry also had substantial input into study design or conduct or analysis, then that was determined to be a high risk of bias. Funding from sources that had no potential financial benefit from the results was considered to be a low risk of bias. Unclear or partial funding was considered unclear risk of bias.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When it was deemed appropriate to combine two or more study arms, we obtained the treatment effects from the combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We determined the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for a statistically significant difference in the RD.
Unit of analysis issues
The only trial included was an individually randomised parallel group trial.
Dealing with missing data
We requested additional data from the trial investigators for data on important outcomes which were missing.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we planned to examine the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We planned to calculate the I² statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. The I² statistic was to be analysed as follows: less than 25% = no heterogeneity; 25% to 49% = low heterogeneity; 50% to 74% = moderate heterogeneity; and 75%+ = high heterogeneity. If moderate or high heterogeneity was detected (I² statistic > 50%), we would have explored the possible causes (for example, differences in study design, participants, interventions, or completeness of outcome assessments) in sensitivity analyses.
Assessment of reporting biases
We planned to investigate publication bias by using funnel plots if at least 10 clinical trials were included in the systematic review (Higgins 2011).
Data synthesis
We used the standard methods of the Cochrane Neonatal Review Group. For categorical outcomes, typical estimates for relative risk (RR) and risk difference (RD) were calculated along with their 95% confidence intervals. For significant results, we planned on reporting the number needed to treat for an additional beneficial outcome or number needed to treat for an additional harmful outcome (NNTB/NNTH). A fixed‐effect model was assumed. Continuous outcomes were analysed using weighted mean difference, assuming a fixed‐effect model.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: death, chronic lung disease at 36 weeks, duration of invasive and non‐invasive assisted ventilation and duration of oxygen therapy.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis for the severity of the disease, the different levels of fluid restriction and the duration of the intervention were planned but could not be performed as only one study was found and data for these subgroups was unavailable.
Sensitivity analysis
Sensitivity analysis was planned to eliminate trials considered of low quality, but only one trial was found.
Results
Description of studies
Results of the search
The initial search as described found 221 articles, Figure 1.
1.
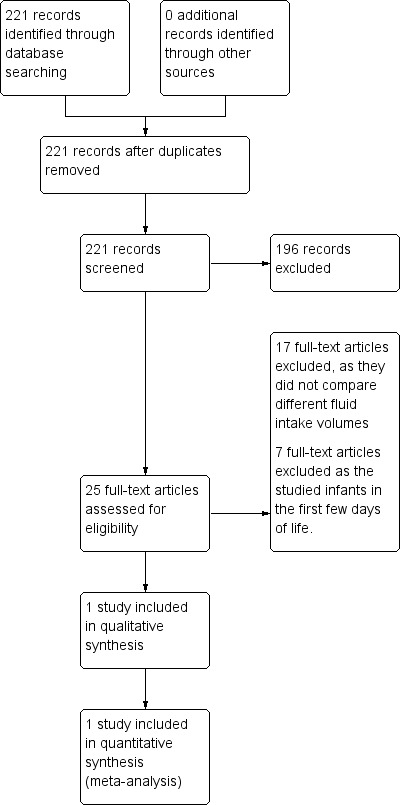
Study flow diagram.
Twenty‐five of the articles were reports of randomised controlled trials. Seventeen were randomised comparisons of maternal milk with artificial milk; maternal milk with or without fortification; or of different formulas or fortification of feeds given after discharge from the neonatal intensive care unit.
There were eight reports of trials comparing different fluid intakes in preterm infants in which one outcome reported was either BPD or CLD. Seven of those reports were of RCTs comparing fluid intakes in the first week of life.
There was only one relevant study in which two volumes of fluid intake were compared in infants with early or established BPD.
Included studies
The only relevant study was Fewtrell 1997, a randomised comparison of two different diet/fluid regimes in 60 preterm infants below 1500 grams' birth weight and less than 32 weeks' gestation, who were enrolled as they still required oxygen at 28 days of age. Infants received either standard formula (24 kcal/oz) at 180 mL/kg/day (n = 27) or a high nutrient‐density formula (30 kcal/oz) at 145 mL/kg/day (n = 33). The primary outcomes are listed as growth rate; duration and severity of respiratory disease; and energy and protein intake.
Excluded studies
The reasons for excluding studies are summarized in Figure 1.
Risk of bias in included studies
The 'Risk of bias' table revealed a moderate risk of bias in this trial. The intervention was not masked, as it was not feasible to do so; also the research staff performing some of the assessments were not masked. The trial was smaller than originally planned as there were more mothers providing breast milk than expected.
Allocation
Randomisation assignments held in sealed envelopes.
Blinding
Unblinded study.
Incomplete outcome data
All infants accounted for.
Selective reporting
No published or registered protocol was found.
Other potential sources of bias
Funded by industry (Ross Laboratories).
Effects of interventions
See: Table 1
Restricted compared with liberal fluid intake (comparison 1)
Primary outcomes
Mortality or CLD
This outcome was not reported.
Secondary outcomes
Duration of supplemental oxygen in days from enrolment until discharge
All but one infant in each group required oxygen during the study. The median duration of supplemental oxygen was 27.5 days (range 2 to 71) in the high‐fluid group, and 28 days (range 2 to 79) in the restricted group. The standard deviations are not reported.
Proportion of infants discharged from hospital on oxygen
Not reported.
Duration of assisted ventilation in days from enrolment until discharge
More of the fluid‐restricted infants were on assisted ventilation during the study period, and the median duration of assisted ventilation was longer in the fluid‐restricted group (27 days (range 5 to 48) compared to 1 day (range 1 to 16)), but was reported to be not statistically significant.
Duration of hospitalisation
There was no significant effect of restricted compared with liberal fluids on duration of hospitalisation; mean difference three days (95% CI −7.64 to 13.64). Tests for heterogeneity were not applicable (Analysis 1.1).
1.1. Analysis.
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 1 Duration of hospitalisation.
Daily weight gain from enrolment until the end of the intervention
There was no significant effect of restricted compared with liberal fluids; mean difference 0.50 g/day (95% CI −1.22 to 2.22). Tests for heterogeneity were not applicable (Analysis 1.2).
1.2. Analysis.
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 2 Daily weight gain.
Apnoea: number (proportion) of infants with problematic apnoea
There was no significant effect of restricted compared with liberal fluids; relative risk 1.35, 95% CI 0.98 to 1.86. Risk difference 0.22, 95% CI 0.00 to 0.44. Tests for heterogeneity were not applicable (Analysis 1.3).
1.3. Analysis.
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 3 Proportion with apnoea.
Feeding intolerance: number (proportion) of infants with feeding intolerance requiring alteration in feeds
The authors report that there was no significant effect of restricted compared with liberal fluids in the incidence of vomiting, abdominal distension or the volume of gastric aspirate but do not give the figures.
Necrotizing enterocolitis: number (proportion) of infants with NEC defined as Bell stage two or more
Not reported.
Renal dysfunction: mean changes in serum creatinine between enrolment and the end of the intervention
Not reported.
Nephrocalcinosis: proportion of infants with nephrocalcinosis on renal ultrasound or CT scan
Not reported.
Lung mechanics: percentage change in dynamic compliance between enrolment and the end of the intervention
Not reported.
Need for diuretics: number of doses of diuretic (loop diuretic or thiazide) needed per participant since the start of intervention
The median number of days of treatment with furosemide were 12 (interquartile range 2 to 41) days in the fluid restricted group versus 31 (interquartile range 6 to 60) days in the liberal fluid group, (P = 0.09).
There were no statistically significant differences between the restricted and liberal fluid regime in any of the primary or secondary outcomes.
The clinically important primary outcomes (death and CLD at 36 weeks) for the 'Summary of findings' tables were either not reported or were reported as median and range. Some data were presented about secondary outcomes that are indirectly related to the primary outcomes, and they are presented in the 'Summary of findings' table.
Discussion
Despite the frequent recommendation to restrict fluid intake in infants with evolving (early) or established BPD there is no evidence to support the practice. The one available trial did not provide data on the primary outcome and showed no statistically significant effect on the secondary outcomes which were reported. It seems unlikely that modest changes in fluid intake have any effect on pulmonary function. Only a drastic reduction in intake, with a reduction in total body water (i.e. dehydration) might be postulated to potentially have an effect. Adverse effects of fluid restriction may include nutritional inadequacy (avoided in Fewtrell 1997 by study design) and an increase in effective renal osmotic load, which was not investigated by Fewtrell and colleagues.
Summary of main results
The only applicable trial showed no benefit of restricting fluid intake to preterm infants who still required oxygen at, or after, 28 days of life. But our primary outcome was not reported.
Overall completeness and applicability of evidence
The power of this analysis is low because of the small number of infants studied.
Quality of the evidence
The quality of the evidence from the one trial was low, quality was downgraded for lack of blinding, imprecision of results, and indirectness for our primary outcomes.
Potential biases in the review process
We have no financial or other conflicts of interest.
Authors' conclusions
Implications for practice.
Fluid intake in BPD should be adequate to provide sufficient nutrients for growth and lung repair. Calorie requirements of infants with early or established BPD are increased compared to infants with normal lungs. There is no proven advantage of supplying highly concentrated, lower volume feeds. Available evidence is insufficient to either support or refute the use of fluid restriction for BPD.
Implications for research.
In order to provide a rationale for future RCTs of fluid restriction, physiological studies of short‐term fluid restriction (controlled and with adequate masking) are required. if such studies do demonstrate a physiological benefit on lung function or lung fluid content then further RCTs, adequately powered to detect clinically important differences in outcomes, may be justifiable.
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2017
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format. |
Appendices
Appendix 1. Standard search methodology
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Data and analyses
Comparison 1. Fluid restricted compared to liberal fluids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospitalisation | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.64, 13.64] |
| 2 Daily weight gain | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.22, 2.22] |
| 3 Proportion with apnoea | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.22 [‐0.00, 0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fewtrell 1997.
| Methods | Randomised controlled trial | |
| Participants | 60 preterm infants < 1500 grams and < 32 weeks, dependent on oxygen at more than 28 days of age | |
| Interventions | Standard formula (24 kcal/oz) at 180 mL/kg/day compared to high nutrient‐density formula (30 kcal/oz) at 145 mL/kg/day | |
| Outcomes | Growth variables, respiratory outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐determined randomisation sequence in block of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Randomisation sequences maintained in opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to mask the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measures done by a research nurse not blind to the dietary randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects accounted for in the results. |
| Selective reporting (reporting bias) | Unclear risk | No published or registered protocol was found. |
| Other bias | Unclear risk | The lack on concealment may have had an impact on the intervention itself: the medical staff were apparently reluctant to increase daily fluids, so the high‐volume group did not receive as much fluid as planned. |
| Funding Source | Unclear risk | Study was funded by industry (Ross Laboratories) who also created the formulae. No indication is given about other roles of the funding source. |
Differences between protocol and review
The search strategy was updated to include a search of CINAHL and clinical trial registries.
The methods for the assessment of risk of bias were altered to match current Cochrane standards.
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol.
Contributions of authors
KB designed the protocol and wrote the first draft.
All three authors reviewed the articles found by the literature search.
All three authors edited the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
None.
New
References
References to studies included in this review
Fewtrell 1997 {published data only}
- Fewtrell MS, Adams C, Wilson DC, Cairns P, Mcclure G, Lucas A. Randomized trial of high nutrient density formula versus standard formula in chronic lung disease. Acta Paediatrica. 1997;86(6):577‐82. [PUBMED: 9202790] [DOI] [PubMed] [Google Scholar]
Additional references
Bancalari 2002
- Bancalari E. Neonatal chronic lung disease. In: Fanaroff A, Martin R editor(s). Neonatal‐Perinatal Medicine. 7th Edition. Vol. 2, St. Louis, Missouri: Mosby Inc, 2002:1057‐70. [Google Scholar]
Bell 1998
- Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000503] [DOI] [Google Scholar]
Brown 1978
- Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. Journal of Pediatrics 1978;92(6):982‐4. [PUBMED: 660373] [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro [available from www.gradepro.org]. McMaster University, 2014.
Gregory 2005
- Gregory K. Update on nutrition for preterm and full‐term infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2005;34(1):98‐108. [PUBMED: 15673653] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jobe 2011
- Jobe AH. The New Bronchopulmonary Dysplasia. Current Opinion in Pediatrics 2011;23(2):167‐72. [PUBMED: 21169836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 1983
- Kao LC, Warburton D, Sargent CW, Platzker AC, Keens TG. Furosemide acutely decreases airway resistance in chronic bronchopulmonary dysplasia. Journal of Pediatrics 1983;103(4):624‐9. [PUBMED: 6620024] [DOI] [PubMed] [Google Scholar]
McCann 1985
- McCann EM, Lewis K, Deming DD, Donovan MJ, Brady JP. Controlled trial of furosemide therapy in infants with chronic lung disease. Journal of Pediatrics 1985;106(6):957‐62. [PUBMED: 3889258] [DOI] [PubMed] [Google Scholar]
Niermeyer 1988
- Niermeyer S. Nutritional and metabolic problems in infants with bronchopulmonary dysplasia. In: Bancalari E, Stocker JT editor(s). Bronchopulmonary dysplasia. Washington: Hemisphere Publishing Corporation, 1988. [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline‐membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357‐68. [PUBMED: 5334613] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook updated October 2013.
Viswanathan 2015
- Viswanathan S, McNelis K, Super D, Einstadter D, Groh‐Wargo S, Collin M. Standardized slow enteral feeding protocol and the incidence of necrotizing enterocolitis in extremely low birth weight infants. JPEN. Journal of Parenteral and Enteral Nutrition 2015;39(6):644‐54. [PUBMED: 25316681] [DOI] [PubMed] [Google Scholar]


