Abstract
Background
Potential benefits and harms of different lighting in neonatal units have not been quantified.
Objectives
• To determine effectiveness and safety of cycled light (CL) (approximately 12 hours of light on and 12 hours of light off) for growth in preterm infants at three and six months' corrected age (CA).
• In separate analyses, to compare effects of CL with those of irregularly dimmed light (DL) or near darkness (ND), and effects of CL with those of continuous bright light (CBL), on growth in preterm infants at three and six months' CA.
• To assess, in subgroup analyses, the effectiveness and safety of CL (vs control interventions (DL, ND and CBL)) introduced at different postmenstrual ages (PMAs) ‐ before 32 weeks', at 32 weeks' and from 36 weeks' PMA ‐ and to compare effectiveness and safety of CL for small for gestational age (GA) infants versus appropriately grown infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), MEDLINE via PubMed (1966 to January 2016), Embase (1980 to January 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 2016). We searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised trials of CL versus ND or CBL in preterm and low birth weight infants.
Data collection and analysis
We performed data collection and analyses according to the methods of the Cochrane Neonatal Review Group. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Main results
We identified one additional study enrolling 38 participants for inclusion in this update, for a total of nine studies reporting on 544 infants. In general, the quality of the studies was low, mainly owing to lack of blinding and small sample sizes.
Six studies enrolling 424 infants compared CL versus ND. No study reported on weight at three or six months. One study (n = 40) found no statistically significant difference in weight at four months between CL and ND groups. In another study (n = 62), the ratio of day‐night activity before discharge favoured the CL group (mean difference (MD) 0.18, 95% confidence interval (CI) 0.17 to 0.19), indicating 18% more activity during the day than during the night in the CL group compared with the ND group. Two studies (n = 189) reported on retinopathy of prematurity (stage ≥ 3) and reported no statistically significant differences between CL and ND groups (typical risk ratio (RR) 0.53, 95% CI 0.25 to 1.11, I2 = 0%; typical risk difference (RD) ‐0.09, 95% CI ‐0.19 to 0.01, I2 = 0%). Two studies (n = 77) reported length of hospital stay (days) and noted a significant reduction in length of stay between CL and ND groups favouring the CL group (weighted mean difference (WMD) ‐13 days, 95% CI ‐23 to ‐2, I2 = 0%; no heterogeneity). The quality of the evidence according to GRADE was low for this outcome. One study (n = 37) reported less crying at 11 weeks' corrected age (CA) in the CL group compared with the ND group (MD ‐0.57 hours/24 h, 95% CI ‐1.09 to ‐0.05). Tests for heterogeneity were not applicable.
Three studies enrolling 120 infants compared CL versus CBL. Two studies (n = 79) reported significantly shorter length of stay in the CL group compared with the CBL group (WMD ‐16.5 days, 95% CI ‐26.2 to ‐6.8, I2 = 0%; no heterogeneity). The quality of the evidence according to GRADE was low for this outcome. One study (n = 41) reported higher mean weight at three months' CA among infants cared for in the CL nursery (P value < 0.02) and a lower mean number of hours spent awake in 24 hours at three months of age (P value < 0.005). Data could not be entered into RevMan or GRADE. One study (n = 41) reported shorter time on the ventilator in the CL compared with the CBL group (MD ‐18.2 days, 95% CI ‐31.40 to ‐5.0). One study (n = 41) reported a shorter time to first oral feeding in the CL group (MD ‐6.8 days, 95% CI ‐13.29 to ‐0.31). We identified no safety issues.
Authors' conclusions
Trials assessing the effects of CL have enrolled 544 infants. No study reported on our primary outcome of weight at three or six months. Results from one additional study strengthen our findings that CL versus CBL shortens length of stay, as does CL versus ND. The quality of the evidence on both comparisons for this outcome according to GRADE was low. Future research should focus on comparing CL versus ND.
Plain language summary
Cycled light in the intensive care unit for preterm and low birth weight infants
Review question
Describe the effectiveness and safety of cycled light (approximately 12 hours of light on and 12 hours of light off) for growth in preterm infants at three and six months' corrected age. By exploring separate questions, we compared the effectiveness of cycled light with that of irregularly dimmed light or near darkness, and we compared cycled light with continuous bright light, for growth in preterm infants at three and six months' corrected age.
Background
Potential benefits and harms of different lighting in neonatal units have not been quantified. The pregnant woman is exposed to variable intensities of light and sound, and generally to lower levels at night. Some of the light and sound reaches the foetus within the womb and induces circadian rhythms. 'Circadian' is a term used to describe biological processes that recur naturally on a 24‐hour basis. After birth, preterm infants are cared for in an environment that has no planned light‐dark cycles and no other circadian entraining signals. Infants are exposed to continuous bright light, continuous near darkness or an unstructured combination of the two.
Study characteristics
We included a total of nine randomised and quasi‐randomised trials, which enrolled 544 infants.
Study funding resources
To our knowledge, no studies included in this review were funded by industry.
Key results
No study reported on weight at three or six months. One study reported improved growth at three months of age in infants exposed to cycled light compared with those exposed to continuous bright light. Another study found no difference in weight at four months of age. Length of hospital stay was shortened with cycled light in the nursery compared with near darkness or with continuous bright light. Only a few outcomes reached statistical significance, which is likely to be due to the small number of infants enrolled in these studies, but trends for most outcomes (weight gain, incidence of retinopathy of prematurity, time spent crying) favoured cycled light over near darkness, and cycled light over continuous bright light.
Quality of evidence
The quality of the evidence on outcomes assessed was low because the interventions could not be blinded to caregivers, and few infants were enrolled in these studies.
Summary of findings
Summary of findings for the main comparison. Cycled light (CL) compared with irregular dimmed light or near darkness (ND) for preterm or low birth weight infants.
| Cycled light (CL) compared with irregular dimmed light or near darkness (ND) for preterm or low birth weight infants | |||||
|
Patient or population: preterm or low birth weight infants undergoing hospital care Settings: hospital Intervention: cycled light (CL) Comparison: irregular dimmed light or near darkness (ND) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Dimmed light or near darkness (ND) | Cycled light | ||||
| Weight (g) at 4 months | Mean weight in control group (ND) was 6264 g | Mean weight in the CL group was non‐significantly higher than in the control group (MD 181.0 g, 95% CI ‐484.0 to 846) | 40 (1) |
⊕⊕⊝⊝ Low |
Bias: inevitable high risk of bias as the study could not be blinded Consistency: As only 1 study reported on this outcome, inconsistency was not a concern Precision: low precision as total sample size was small and CI was wide Indirectness: Study was conducted in the target population (no concerns about indirectness) |
| Length of stay (days) (CL from 32 weeks' PMA) | Mean length of stay ranged across control groups (ND) from 54 to 86 days | WM length of stay for CL groups was significantly shorter than for control groups (WMD ‐12.7 days, 95% CI ‐23.0 to ‐2.3) | 77 (2) | ⊕⊕⊝⊝ Low |
Bias: inevitable high risk of bias as studies could not be blinded Consistency: Findings of the 2 studies were consistent with I2 = 0% Precision: low precision as total sample size was small and CIs were wide Indirectness: Studies were conducted in the target population (no concerns about indirectness) |
| *The basis for the assumed risk was as follows: 'The mean [outcome] ranged across control groups from [value][measure].' Corresponding risk was as follows: 'The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] with 95% CI' CI: confidence interval; RR: risk ratio; WM: weighted mean; WMD: weighted mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
Summary of findings 2. Cycled light (CL) compared with continuous bright light (CBL) for preterm or low birth weight infants.
| Cycled light (CL) compared with continuous bright light (CBL) for preterm or low birth weight infants | |||||
|
Patient or population: preterm or low birth weight infants undergoing hospital care Settings: hospital Intervention: cycled light (CL) Comparison: continuous bright light (CBL) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Continuous bright light (CBL) | Cycled light (CL) | ||||
| Length of stay (days) (CL from birth) | Mean length of stay ranged across control groups (CBL) from 51 to 75 days | WM length of stay for intervention groups (CL) was significantly shorter than for control groups (WMD ‐16.5 days, 95% CI ‐26.2 to ‐6.8) | 79 (2) | ⊕⊕⊝⊝ low | Bias: inevitable high risk of bias as studies could not be blinded Consistency: Findings of the 2 studies were consistent, with I2 = 0% Precision: low precision as total sample size was small and CIs were wide Indirectness: Studies were conducted in the target population (no concerns about indirectness) |
| Days on ventilator (CL from birth) | Mean days on ventilator in the control group (CBL) was 29.3 | Mean number of days on ventilator for intervention group (CL) was significantly fewer than for control group (MD ‐18.2 days, 95% CI ‐31.4 to ‐5.00) | 41 (1) |
⊕⊕⊝⊝ low | Bias: inevitable high risk of bias as the study could not be blinded Consistency: As only 1 study reported on this outcome, inconsistency was not a concern Precision: low precision as total sample size was small and CI was wide Indirectness: Study was conducted in the target population (no concerns about indirectness) |
| *The basis for the assumed risk was as follows: 'The mean [outcome] ranged across control groups from [value][measure].' The corresponding risk was as follows: 'The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] with 95% CI' CI: confidence interval; RR: risk ratio; WM: weighted mean; WMD: weighted mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
Background
In the 1950s, Professor Franz Halberg introduced the term 'circadian' (Halberg 2003; Refinetti 2003), which was formed irregularly from the Latin words circa (about), dies (day) and anus (ring) (Halberg 1969). Circadian clocks are believed to have evolved in parallel with the geological history of the earth and have been fine‐tuned under selection pressures imposed by cyclical factors in the environment (Paranjpe 2005). All species on the planet are exposed to 24‐hour patterns of light and darkness as the earth rotates. In response to these regular daily oscillations in the natural light‐dark cycle, these species have evolved endogenous circadian rhythms that are repeated approximately every 24 hours. Circadian rhythms are observed in virtually all aspects of mammalian function, from expression of genes to complex physiological processes (Sukumaran 2010).
Physiology
The circadian rhythm influences the rhythmical production of several hormones (melatonin, cortisol, growth hormone), respiratory and cardiac function, the sleep‐wake state, level of alertness and body temperature (Seron‐Ferre 2001). The circadian clock in mammals is located in the suprachiasmatic nuclei in the anterior hypothalamus and is present by 18 weeks' gestation. This master circadian clock organises and orchestrates the timing of all biological functions ‐ from complicated physiological systems to single cells (Rea 2010). Maternal rest‐activity patterns may function as entraining signals for the foetus, as documented in animals, and include changes in heart rate, serum cortisol, melatonin levels and body temperature (Seron‐Ferre 2001). Rhythms of foetal movements, heart rate and breathing have been described in human foetuses and in preterm infants born at 28 to 34 weeks' gestation (Patrick 1982; Arduini 1987; Mirmiran 1990). More recently, Kintraia and co‐workers documented pronounced rhythms of activity and locomotion in healthy foetuses at a postmenstrual age (PMA) of 16 to 20 weeks (Kintraia 2005). The circadian clock in baboons is responsive to light very early in gestation; when the data are extrapolated to humans, this occurs at about 25 weeks' gestation (Stetson 1986; Hao 1999). These findings suggest maternal triggering of foetal rhythms long before birth at term. Potential advantages of circadian rhythms acquired during gestation are currently unknown but may include timing physiological functions of the foetus to match those of the mother and preparing the foetus for day and night changes and ambient temperature (Seron‐Ferre 2001). The circadian system matures progressively, and circadian rhythms of temperature have been documented by the end of the first week in a human infant exposed only to natural light and darkness. In the same infant, salivary melatonin circadian periodicity was noted by 45 days of age, and circadian rhythms of wake state by 45 days and of sleep state by 56 days of age (McGraw 1999). In adults, asynchrony between internal (clock) circadian rhythms and actual time is demonstrated in jet lag, which disrupts sleep and affects digestion and alertness (Rea 2008). Disruption of the natural 24‐hour pattern of light and dark from rapid flight across time zones or from rotating shift work can lead to a variety of conditions ranging from poor performance to sleep loss, weight gain, metabolic syndrome, inflammatory disease and cancer (Rea 2010; Sukumaran 2010). In rats, it has been documented that nociception exhibits robust daily rhythmicity. Sensitivity to pain is greatest late in the dark phase of the light‐dark cycle and least at the light‐dark transition (Christina 2004). This finding may have importance for the timing of painful procedures in neonates. Circadian rhythms in gene expression regulate both the action and the disposition of various drugs, and they affect therapeutic efficacy and toxicity in accordance with dosing time (Sukumaran 2010).
Description of the condition
For the purpose of this review, we named the condition under study 'Delays or disturbances in the development of circadian rhythms in preterm and low birth weight infants'. In an observational study that included 187 preterm and term infants cared for in a neonatal intensive care unit (NICU) with cycled lighting (CL) conditions (< 30 lux during the night, 300 to 580 lux during the day), circadian rhythms were documented as early as zero to three days' postnatal age (Begum 2006). Study authors concluded that the co‐existence of circadian cycles with low amplitude in preterm neonates may complementarily support immature homeostasis and function against unstable physiological conditions (Begum 2006). Entrainment and internal synchronisation are aspects of the circadian system that appear to be important for adaptation and optimal functioning of the organism (Mirmiran 1996). Circadian rhythms are normally entrained through natural exposure to light during the day and to darkness during the night over a 24‐hour period. In the preterm or low birth weight infant, it is plausible that interrupted maternal triggering of foetal rhythms due to preterm birth or exposure of the infant born preterm to near darkness (ND) or to continuous bright light (CBL) in the NICU could disturb or delay the development of circadian rhythms (Rivkees 2003; Rivkees 2004a). Such disturbances of circadian rhythms could result in adverse clinical outcomes such as poor growth, sleep disturbances, retinopathy of prematurity (ROP) and other adverse outcomes commonly seen in critically sick neonates. These outcomes, if present, are likely to affect length of stay in the hospital and long‐term neurodevelopmental outcomes.
Burden of illness
Potential benefits and harms of different types of light in the NICU and in stepdown units have not been quantified. CBL has been related to infant stress as manifested by increased levels of activity, decreased sleep and bradycardia (Gottfried 1985; Lotas 1992; Blackburn 1998; Rivkees 2000). Reducing exposure to light by covering the isolette, as recommended by the Neonatal Individualized Developmental Care and Assessment Program (NIDCAP), has not been shown to improve important short‐ or long‐term outcomes (Jacobs 2002; Symington 2006; Ohlsson 2007; Ohlsson 2009; Ohlsson 2013). Providing basic developmental care in the form of incubator covers and nesting in the NICU has had no effect on short‐term physical and neurological outcomes in infants born at less than 32 weeks' gestation (Maguire 2008). Follow‐up of the same study population at one and two years of age showed no positive effect on neurological and mental development or growth (Maguire 2009). Caring for preterm infants in the dark deprives them of the time‐of‐day information they would have received if they had been carried to term (40 weeks' gestation) (Rivkees 2004a). Phelps and Watts, in a Cochrane review, concluded that decreasing retinal ambient light exposure in preterm infants is very unlikely to reduce the incidence of ROP compared with no light reduction (Phelps 2001).
Description of the intervention
Clinical description of light exposure patterns associated with conventional neonatal care
In most nurseries, preterm infants are cared for in an environment that has no planned light‐dark cycles. Infants are exposed to CBL, continuous ND or an unstructured combination of the two. The Recommended Standards for Newborn ICU Design state the following for NICU Standard: 14 Ambient lighting in infant care area: "In very preterm infants, there has been no demonstrable benefit to exposure to light." After 28 weeks' gestation, some evidence suggests that diurnal CL has potential benefit for the infant. Caregivers benefit from moderate levels of ambient light in performing tasks and maintaining wakefulness (White 2007).
Near darkness is practiced in some nurseries because of its similarity to the relative darkness of the uterus. Infants receiving ND are exposed to minimal light throughout day and night except during times of shift change or handling. Some nurseries define ND as 5 to 10 lux. Light protective devices or light dimming may be used to achieve these settings. This approach overlooks the fact that the foetus develops in an environment that is relatively dark but is rich with auditory, tactile and kinaesthetic sensory stimuli. These maternal stimuli expose the foetus to circadian rhythms and help in synchronising the foetal clock with the external light‐dark cycle. Keeping preterm infants in the dark during their stay in the neonatal nursery deprives them of the time‐of‐day information they would have received throughout gestation (Rivkees 2003; Rivkees 2004a).
Clinical description of cycled light exposure pattern
No protocol or single definition is available for time cycles or for maximal and minimal lux lighting used for CL, which is usually provided in a 12‐hours‐on (11 to 13 hours)/12‐hours‐off (11 to 13 hours) pattern, similar to changes in natural light. A minimal time of transition between light and darkness occurs at the change of nursing shifts. A day versus night lighting difference is achieved by artificial lighting or by use of regular nursery lighting with uncovering of windows during the day. At that time, the incubator cover is folded on top of the incubator or is taken off, achieving 200 to 500 lux lighting or more (Brandon 2002; Mirmiran 2003; Begum 2006). At night‐time, windows are covered by dark, lined curtains, lights are dimmed or turned out and the only illumination consists of a low‐intensity night light (< 30 lux) (Begum 2006). Eye pads are used to protect the infant when light of greater intensity is needed for medical procedures. It still is not clear how early preterm infants in the nursery should be introduced to artificial circadian cycles to reach the same level of 'clock' maturity achieved by infants born at term. Studies show that a higher percentage of circadian rhythms with regard to body temperature and heart rate has been found to be appropriate for gestational age (GA) infants compared with small for GA, preterm infants (Glotzbach 1995). It is important to evaluate the influence of CL as an entraining signal in these groups of infants (preterm and small for GA infants).
How the intervention might work
Observational evidence concerning effects of light exposure on outcomes
The presence or absence of circadian rhythms in the newborn infant probably results from the combined influence of antenatal and postnatal environmental conditions (Mirmiran 2000). Postnatal development of human circadian rhythms may be hampered by maternal, foetal or perinatal disturbances. This is observed when the intimate mother‐foetus relationship is dramatically altered by preterm birth. Preterm infants are deprived of several important postnatal maternal entrainment factors, and they are exposed to CBL or irregular light for several weeks or months while in the NICU. This lack of maternal entrainment, exposure to irregular extrauterine lighting and care in the nursery may contribute to disturbances in body temperature, sleep and feeding patterns that are commonly experienced by preterm infants (Keener 1988; Thoman 1989a; Thoman 1989b). Using artificial entraining signals in the nursery, that is, CL, may prevent these disturbances and may help to promote growth while preventing other morbidities. CL has the potential to promote circadian rhythms that confer health benefits, including hormonal regulation, activity‐rest cycles and vital sign regulation, with the potential of promoting infant growth (Kennaway 1992; Rivkees 2003). CL could increase satisfaction with care experienced by parents and healthcare providers, leading to an indirect beneficial effect on the infant. In contrast, CL might decrease staff satisfaction and may adversely affect their ability to observe infants.
Why it is important to do this review
The topic of 'Cycled light in the intensive care unit for preterm and low birth weight infants' has not been systematically reviewed, justifying this Cochrane review.
Objectives
Primary objectives
To determine effectiveness and safety of cycled light (CL) (approximately 12 hours of light on and 12 hours of light off) for growth in preterm infants at three and six months' corrected age (CA).
In separate analyses, to compare effects of CL with those of irregularly dimmed light (DL) or near darkness (ND), and effects of CL with those of continuous bright light (CBL), on growth in preterm infants at three and six months' CA.
Secondary objectives
To assess, in subgroup analyses, the effectiveness and safety of CL (vs control interventions (DL, ND and CBL)) introduced at different postmenstrual ages (PMAs) ‐ before 32 weeks', at 32 weeks' and from 36 weeks' PMA ‐ and to compare effectiveness and safety of CL for small for gestational age (GA) infants versus appropriately grown infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials. We included trials if randomisation was assigned by cluster (whole or part of an NICU) or at the individual participant level.
Types of participants
Preterm infants (< 37 weeks' PMA or low birth weight (< 2500 g)) admitted and cared for in an NICU or a stepdown unit.
Types of interventions
CL versus irregular DL or ND, or CBL initiated during hospitalisation in the NICU.
Types of outcome measures
Primary outcomes
Growth at three and six months' CA (grams/d, or actual weight).
Secondary outcomes
Time to full oral or nasogastric feeds (days).
Chronic lung disease (CLD) or bronchopulmonary dysplasia (BPD) (oxygen requirement > 0.21 at 28 days' and 36 weeks' PMA).
Days on assisted ventilation.
Days with oxygen above 0.21.
ROP; any stage and stage ≥ 3.
Days of initial hospitalisation.
Long‐term outcomes: growth and neurodevelopment, including visual and auditory outcomes at any age as reported by study authors using standardised and validated tests.
Any clinically important outcome not listed above but reported by study authors (not prespecified).
Caregivers' satisfaction or dissatisfaction with the intervention.
Parents' satisfaction or dissatisfaction with the intervention.
Potential adverse effects: any clinically important adverse outcome or side effect not listed above but reported by study authors (not prespecified).
Search methods for identification of studies
Both review authors identified studies using the search strategy recommended by the guidelines of the Cochrane Neonatal Review Group.
Electronic searches
For this update, we conducted a comprehensive search of the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) in The Cochrane Library; MEDLINE via PubMed (1966 to 12 January 2016); Embase (1980 to 12 January 2016); and Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 12 January 2016), using the following search terms: "cycling light" OR "cycled light" OR "periodicity", OR "circadian rhythm", OR "darkness", OR "light OR "lightening", plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions. On 16 March 2016, we searched Controlled‐trials.com and Clinicaltrials.gov for ongoing trials and performed electronic searches of the Abstracts2view website for abstracts from the Pediatric Academic Societies (PAS) Annual Meetings (2000 to 2015).
Searching other resources
Both review authors initiated the search by reviewing personal files and published reviews. We scanned the reference lists of identified studies for additional references and subsequently retrieved articles for review. We did not seek unpublished data, but we did contact authors of published trials to ask that they clarify or provide additional information.
Data collection and analysis
Both review authors performed data collection and analyses in accordance with the methods of the Cochrane Neonatal Review Group. We performed statistical analyses using Review Manager software (RevMan 2014). Estimates of treatment effects included risk ratio (RR), risk difference (RD) and, if the RD was statistically significant, the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. We reported all estimates of treatment effects with 95% confidence intervals (CIs). We used a fixed‐effect model for meta‐analyses and reported the results of one cluster trial separately.
Selection of studies
Both review authors assessed all abstracts and published full reports identified by the literature search as potentially relevant for inclusion in the review.
Data extraction and management
Each review author extracted data separately using a predesigned data abstraction form, then compared the results. One review author (AO) entered the data into Review Manager (RevMan 2014), and the other review author (IM) cross‐checked the printout against her own data abstraction forms. We corrected errors by consensus. For some studies identified as abstracts, we contacted primary authors to ascertain whether a full publication was available. We obtained information from the primary author if the published article provided inadequate information for inclusion in the review.
Assessment of risk of bias in included studies
Two review authors (IM and AO) independently assessed risk of bias (low, high or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Selection bias.
Performance bias.
Attrition bias.
Reporting bias.
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain. After conducting an independent evaluation, the two review authors discussed the results for each study and resolved discrepancies.
Dealing with missing data
We did not encounter a situation in which data were missing.
Assessment of heterogeneity
We performed heterogeneity tests including the I2 statistic to assess the appropriateness of pooling study data. We categorised the level of heterogeneity as < 25% (no heterogeneity), 25% to 49% (low heterogeneity), 50% to 74% (moderate heterogeneity) or ≥ 75% (high heterogeneity).
Assessment of reporting biases
We did not prepare funnel plots, as the maximum number of trials included in a meta‐analysis was two.
Data synthesis
Quality of evidence
We planned to use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: 'Growth at three and six months' CA (grams/d, or actual weight)' for the comparisons of interest. For 'Cycled light versus irregular dimmed light or near darkness (Comparison 1)', growth was not reported as an outcome at three and six months. For 'Cycled light versus continuous bright light (Comparison 2),' one study (Mann 1986) reported mean weight at 12 weeks' CA (three months) in graphic form only and could not be subjected to GRADE assessments or included in RevMan analyses. Therefore, the primary outcome for 'Growth at three and six months' CA (grams/d, or actual weight)' could not be subjected to GRADE assessments in either comparison.
As we had only continuous outcomes to report, we chose to use the 'Summary of findings' template, which is available under the heading 'Summary of findings tables' in RevMan's Table Editor, to report on selected outcomes for Comparison 1: weight at 4 months (grams), length of stay (CL from 32 weeks' PMA); and for Comparison 2: length of stay, days on ventilator.
Two review authors independently assessed the quality of the evidence. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based on the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2014 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence and assignment to one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
In planned subgroup analyses, we examined the effectiveness of CL (vs control interventions listed under secondary objectives) introduced at different PMAs: before 32 weeks', at 32 weeks' and from 36 weeks' PMA, and compared small for GA infants versus appropriately grown infants.
Sensitivity analysis
We planned no sensitivity analyses a priori, but we could have conducted these if warranted by the results. The only quasi‐randomised trials reported on the effects of CL versus CBL (Miller 1995; Vásquez‐Ruiz 2014), and the outcomes reported (length of stay) differed from those in the only other randomised trial that considered this comparison (Mann 1986). Therefore, we did not require a secondary analysis based on quality.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The study flow diagram presents results of searches conducted in January 2016 (Figure 1). This search identified one additional trial enrolling 38 infants (Vásquez‐Ruiz 2014), resulting in nine trials for inclusion. Six included studies (total n = 424) (Seiberth 1994; Boo N‐Y 2002; Brandon 2002; Mirmiran 2003; Rivkees 2004; Guyer 2012) compared CL versus ND, and three included studies (total n = 120) compared CL versus CBL (Mann 1986; Miller 1995; Vásquez‐Ruiz 2014). The included studies were conducted in England (n = 1), Germany (n = 1), the USA (n = 4), Malaysia (n = 1), Mexico (n = 1) and Switzerland (n = 1). We excluded four studies in the previous update: Blackburn 1991; Kennedy 2001; Hoogeveen 2004; Braz 2006. In this 2016 update, we excluded two studies (Aita 2012; Park 2015), and two studies are still awaiting classification (Kennaway 1996; Jung 2005; see Characteristics of studies awaiting classification table). The study by Jung and co‐workers was written in Korean, and we have been able to access only the abstract written in English, despite attempts to contact study authors (Jung 2005). We have not been able to ascertain clearly to which interventions infants in the study by Kennedy 2001 were randomised. We identified three ongoing trials (Sanadgol 2013; Aita 2014; NCT02146287).
1.
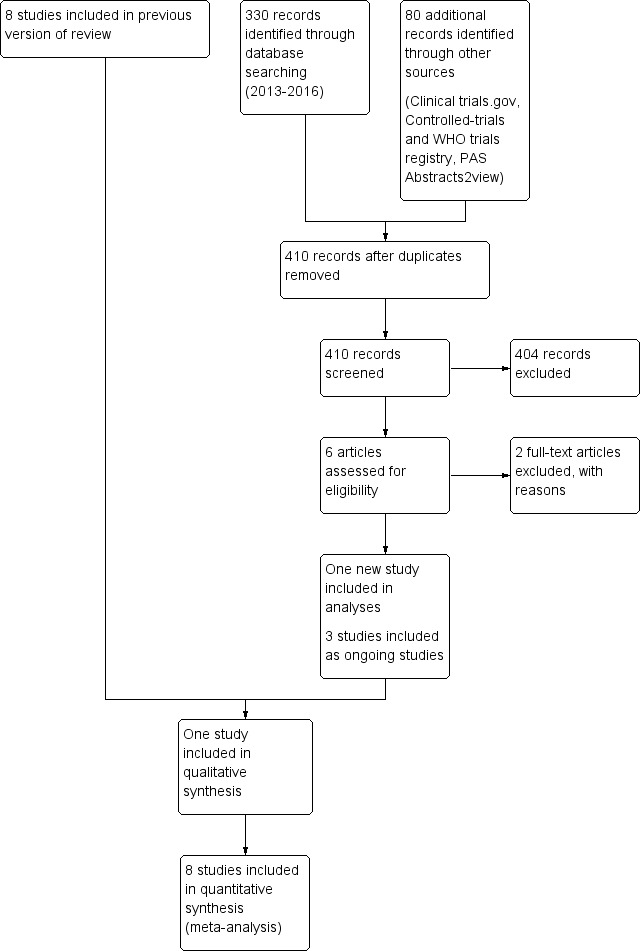
Study flow diagram: review update.
Included studies
We included nine studies. For details, see the Characteristics of included studies table.
Six studies compared cycled light versus near darkness
Table 3 describes lighting conditions for CL versus DL or ND. Light intensity applied to the infants was similar for studies using the term 'dimmed lighting' and those using the term 'near darkness.' In the only four studies for which results could be combined in meta‐analyses (Seiberth 1994; Boo N‐Y 2002; Brandon 2002; Guyer 2012), the contrast between daytime and night‐time light intensity was sufficiently similar to justify the combination of data from the two sets of two studies.
1. Lighting conditions for cycled light versus dimmed light or near darkness.
| Reference | Intervention | Daytime light intensity | Night‐time light intensity |
| Seiberth 1994 | Cycled light | Mean 342 lux (SD 55 lux) in the NICU Mean 415 lux (SD 42 lux) in the stepdown unit |
Mean 62 lux (SD 53 lux) in the NICU Mean 38 lux (SD 18 lux) in the stepdown unit |
| Near darkness | 99.9% light reduction | 99.9% light reduction | |
| Boo N‐Y 2002 | Cycled light | Mean 78.4 lux (SD 24.7 lux) | Mean 5.9 lux (SD 1.9 lux) |
| Dimmed light | Mean 5.9 lux (SD 1.9 lux) | Mean 5.9 lux (SD 1.9 lux) | |
| Brandon 2002 | Cycled light | Range 200‐225 lux | Range 5‐10 lux |
| Near darkness | Range 5‐10 lux | Range 5‐10 lux | |
| Mirmiran 2003 | Cycled light | 300 lux | < 20 lux |
| Dimmed light | < 20 lux | < 20 lux | |
| Rivkees 2004 | Cycled light | Mean 239 lux (SD 29 lux) | < 25 lux |
| Dimmed light | Mean 28.5 lux (SD 3 lux) | < 25 lux | |
| Guyer 2012 | Cycled light | 499.3 (SD 159.2) lux | 28.5 (SD 27.5) lux |
| Dimmed light | 97.6 (SD 45.3) lux | 20.8 (SD 20.7) lux |
NICU: neonatal intensive care unit; SD: standard deviation
Seiberth 1994: This single‐centre study was conducted by Seiberth and co‐workers in the NICU at Women's Hospital of the University of Heidelberg, Germany.
Objective: to investigate the influence of light on the incidence and severity of ROP.
Population: 169 infants (birth weight < 1500 g; PMA < 33 weeks) entered the study. Forty‐two infants were excluded.
Interventions.
65 infants (mean [standard deviation (SD)] birth weight 1091 g (233 g), mean (SD) PMA 29 weeks (1.7 weeks)) were assigned to CL. Reduced daylight was present during the day (mean (SD) illuminance, 342 lux (55 lux)). Light intensity was reduced during most of the night hours (mean (SD) intensity of light, 62 lux (53 lux)). When stable infants were transferred to the preterm unit (stepdown unit), the amount of daylight allowed was dampened during the day as well (mean (SD) intensity of light 415 lux (42 lux)), whereas at night, light was reduced to nearly complete darkness (mean (SD) intensity of light 26 (18) lux). Thus, CL conditions were present in both units.
62 infants (mean (SD) birth weight 1125 g (232 g), mean (SD) PMA 29.3 weeks (2.1 weeks)) were assigned to ND (patched eyes). Patches of black opaque plastic covered by cotton were placed over both eyes and were secured with adhesive tape to the temple on both sides. The light reduction achieved was greater than 99.9%. Eye patching was applied continuously from the first day after birth to 35 weeks' PMA. The intervention was started at birth.
Outcomes: ROP all stages and ROP stage > 2, duration of ventilation therapy (days), duration of hospital stay (days).
Boo N‐Y 2002: This single‐centre study was conducted by Boo and co‐workers at the Hospital Universiti Kebangsaan, Malaysia.
Objective: to compare weight gain between preterm infants exposed to 12 hours of CL and those exposed to a continuously dim environment.
Population: preterm infants (< 37 weeks' PMA; birth weight < 2001 g).
Interventions:
50 infants (mean (SD) birth weight 1482 g (236 g), mean (SD) PMA 31.6 weeks (2.2 weeks)) were assigned to the 'day‐and‐night' CL group. Intensity of light: mean (SD) 78.4 lux (24.7 lux). Lights in the cubicles were switched on between 7:00 and 19:00 hours, and were switched off between 19:00 and 7:00 hours.
46 infants (mean (SD) birth weight 1465 g (280 g), mean (SD) PMA 31.4 weeks (2.2 weeks)) were assigned to the 'continuously dimmed environment' group. Intensity of light: mean (SD) 5.9 lux (1.9 lux). Lights in the cubicles were switched off throughout day and night. Lights were switched on temporarily only during physical examination, treatment procedures and nursing care.
Outcomes: mean age of infants when they regained their birth weight, amount of weight gained by day 14 of life, duration of hospital stay, infant's weight on discharge, age when enteral feeds were introduced.
Brandon 2002: This single‐centre study was conducted at an intensive care and transitional care nursery of Duke University Medical Center, and at a Level II special care nursery of Durham Regional Hospital, Durham, North Carolina, USA.
Objective: to evaluate the benefits of CL versus ND for health of preterm infants born at less than 31 weeks' PMA.
Population: 62 infants born at less than 31 weeks' PMA.
Interventions:
Neonates (mean (SD) PMA 27.1 weeks (2.0 weeks), mean (SD) birth weight 1000 g (223 g), across the three intervention groups) were assigned randomly to one of three light intervention groups: group 1 ‐ CL from birth, group 2 ‐ CL at 32 weeks' PMA, group 3 ‐ CL at 36 weeks' PMA ‐ in transition for discharge home.
22 infants were assigned to CL from birth (group 1) and 19 infants were cared for in ND until 32 weeks' PMA, when they were cared for in CL (group 2). CL was provided in an 11‐hours‐on/11‐hours‐off pattern, with one transition hour at the change of shifts. The incubator cover was folded on top of the incubator, or the bassinet cover was off, to achieve daylight at 200 to 225 lux between 7:30 and 18:30 hours.
21 infants were assigned to ND (group 3) until 36 weeks. ND (5 to 10 lux) was provided by using protective devices during the daytime and dimming the room light or using protective devices at night‐time. Infants receiving ND were exposed to 5 to 10 lux of light throughout the day, except during 6:30 to 7:30 hours and 18:30 to 19:30 hours, when lighting levels varied on the basis of change of shift nursing care needs. These transition hours were applied to all groups.
Outcomes: mean weekly weight gain, total number of ventilation days, days in supplemental oxygen, hospital stay (days), calories per kilogram per day, days of life to start feeds, days of life to full feeds, brainstem auditory‐evoked response close to discharge from hospital (not included by us as an outcome), neurobehavioural organisation at 32 and 36 weeks' PMA, ROP.
Mirmiran 2003: This single‐centre study was conducted at Lucile Packard Children's Hospital in Stanford, Palo Alto, California, USA.
Objective: to address the hypothesis that CL before discharge may improve circadian organisation and sleep compared with continuous DL.
Population: preterm infants admitted to the NICU.
Interventions:
19 infants (mean (SD) PMA 30.7 weeks (1.3 weeks)) were allocated to the 'cycled' group and were cared for in a day‐night lighted room. Their incubator or bassinet was covered from 19:00 hours to 7:00 hours with a nearly opaque blanket, except during feeding or other interventions. The blanket was removed from 7:00 hours to 19:00 hours, at which time room lighting was turned up to standard lighting levels (approximately 300 lux) to produce a regular light‐dark cycled condition.
21 infants (mean (SD) PMA 29.8 weeks (1.7 weeks)) were allocated to the 'dimmed' group and were cared for in a dimly lit room (below 20 lux) around the clock. Their incubator/bassinet was covered with a nearly opaque blanket, except during feeding or other interventions by parents and caregivers.
Outcomes: weight at 35 weeks' and at four months' PMA. Body temperature was recorded continuously for up to three days at 36 weeks' PMA, as well as at home at one and three months' CA. Sleep was recorded at the same times using 24‐hour time‐lapse video recordings in conjunction with rectal temperature recordings.
Rivkees 2004: This single‐centre study was conducted in the Department of Pediatrics, at Yale School of Medicine, New Haven, Connecticut, USA.
Objective: to examine effects of nursery lighting conditions on the development of activity patterns in preterm infants.
Population: 62 infants less than 32 weeks' PMA, who were medically stable in NICU rooms, were randomly assigned between 32 and 34 weeks' PMA to CL or continuous DL.
Interventions:
29 infants (mean (SD) PMA 28.5 weeks (0.5 weeks), mean (SD) birth weight 1072 g (62 g)) were assigned to CL (mean (SD) 239 lux (29 lux), from 7:00 to 19:00 hours; < 25 lux, from 7:00 to 19.00 hours) for 25 days.
33 infants (mean (SD) PMA 28.5 weeks (0.4 weeks), mean (SD) birth weight 1110 g (64 g)) were assigned to DL (mean (SD) 28.5 lux (3 lux), from 7:00 to 19:00 hours; mean (SD) 15 lux (5 lux), from 19:00 to 7:00 hours) for 24 days. The intervention was started between 32 and 34 weeks' PMA.
Outcomes: total number of movements per day in 10‐day intervals (10 to 0 days before discharge; 1 to 10 days after discharge; 11 to 20 days after discharge; 21 to 30 days after discharge). Ratios of day‐night activity: 10 to 0 days before discharge; 1 to 10 days after discharge; 11 to 20 days after discharge; 21 to 30 days after discharge. Period analysis of circadian rhythms over the first 10 days at home.
Guyer 2012: This single‐centre study was conducted at the Clinic for Neonatology of the University Hospital Zurich, in Switzerland.
Objective: to examine whether CL conditions during neonatal care in very preterm infants (< 32 weeks' PMA) decreased crying and fussing behaviour, improved consolidation of sleep and influenced activity behaviour at 5 and 11 weeks' post term CA compared with preterm infants cared for in DL conditions.
Population: very preterm infants (≤ 32 weeks' and 0 days' PMA). Exclusion criteria: major cerebral injuries such as intraventricular haemorrhage grade III, periventricular leukomalacia or venous infarction, ROP grade 3 and 4, congenital malformations, small for GA (birth weight less than the third percentile), antenatal infection or intrauterine drug exposure. For 62 possibly eligible infants, 21 parents refused consent for study participation, mainly because the study was too time‐consuming. Forty‐one infants were enrolled (22 boys, 19 girls). Four additional infants had to be excluded from further analysis (dropped out after the first recording (n = 2), incomplete diary (n = 1) or unexplained high amount of parent‐reported 'un‐soothe‐able crying' as an outlier (n = 1)). A total of 37 preterm infants (DL: n = 20, CL: n = 17) were included in the final analysis. Enrolment took place after the transfer from intensive to intermediate care, at a mean age of 32.16 ± 1.35 weeks' PMA.
Intervention: 37 preterm infants were randomly assigned to CL (7:00 to 19:00 hours lights on, 19:00 to 7:00 hours lights off) (n = 17; mean PMA 30.6 ± 0.95 weeks; nine girls) or DL (lights off whenever the infant was asleep (n = 20; mean PMA 29.5 ± 2.1 weeks; eight girls) conditions.
Outcomes: daily weight gain (grams/d) during exposure. Sleeping, crying and activity behaviour were recorded in parental diaries and by Actogram at five and 11 weeks' CA.
Three studies compared cycled light versus continuous bright light
Table 4 describes lighting conditions for CL and CBL in the three studies. In the study by Mann and co‐workers, light intensity was not expressed in lux, but it is likely that conditions were similar (Mann 1986).
2. Lighting conditions for cycled light versus continuous bright light.
| Reference | Intervention | Daytime light intensity | Night‐time light intensity |
| Mann 1986 | Cycled light | Lit by bright fluorescent strip lights | Windows were covered with dark, lined curtains, lights were turned out and the only illumination was provided by a low‐intensity night light |
| Continuous bright light | Lit by bright fluorescent strip lights that remained permanently on | ||
| Miller 1995 | Cycled light | Range 156‐201 lux | Range 20‐32 lux |
| Continuous bright light | Range 172‐232 lux | Range 206‐274 lux | |
| Vásquez‐Ruiz 2014 | Cycled light | Range 249 ± 11 lux | Light/dark condition was achieved by placing from 19:00 to 07:00 an acrylic helmet, covered with blue surgical drapes; surgical cloths were placed on helmets, and the frontal part was open, allowing good airflow. This helmet was placed individually above the head and thorax of each baby, resulting in reduced illumination, with light intensity of 27 ± 0.8 lux at the level of the eyes |
| Continuous bright light | Range 249 ± 11 lux | ||
Mann 1986: This single‐centre study was conducted in Nottingham, England.
Objective: to see whether exposure to a cyclical day and night environment before discharge would influence subsequent behaviour of preterm infants.
Population: 41 preterm infants with PMA 27 to 35 weeks, postnatal age at study entry one to 63 days.
Interventions:
20 infants (mean (SD) PMA 32.0 weeks (2.0 weeks) (range 28 to 35 weeks), mean (SD) birth weight 1620 g (350 g) (range 1130 to 2370 g)) were cared for in a day and night nursery. During the daytime, the environment in the two nurseries was identical, but at 19:00 hours, windows in the night and day nursery were covered by dark, lined curtains, lights were turned out and the only illumination was provided by a low‐intensity night light. The radio was turned off, and staff and visitors were urged to make as little noise as possible. Low light and noise intensity were then maintained until 7:00 hours.
21 infants (mean (SD) PMA 31.6 weeks (1.9 weeks), mean birth weight (SD) 1640 g (390 g) (range 1020 to 2430 g)) were cared for in a control nursery. The control nursery included six cots, measured 23 × 13 feet (7 × 4 m), had a large exterior window and smaller internal windows without curtains and was lit by bright fluorescent strip lights that remained permanently on. No attempt was made at any time to reduce noise from the radio, staff, parents or other visitors.
Outcomes: number of hours spent awake in 24 hours; number of hours spent feeding in 24 hours; weight on discharge, at expected date of birth, at six weeks' PMA and at 12 weeks' (3 months') PMA. Mean time spent asleep, awake or feeding was calculated from 48 hours of recordings. All outcome results were presented in graphic form only and could not be entered into Review Manager (RevMan 2014).
Miller 1995: This single‐centre study was conducted at Notre Dame, Indiana, USA.
Objective: to assess how CL versus non‐CL affected growth and medical status of preterm infants in the NICU. To assess effects of lighting conditions on the amount and type of care delivered by staff, as well as whether lighting effects on infants were modified by staff behaviour.
Population: 41 infants at less than 37 weeks' PMA with birth weight less than 2500 g.
Interventions:
20 infants (mean (SD) birth weight 1151 g (360 g), mean (SD) PMA 28.0 weeks (2.2 weeks)) were assigned to CL.
Infants received a day‐night lighting pattern in both the NICU and the continuing care room. During daytime (7:00 to 18:00 hours), this group received 156 to 201 lux, and during night‐time (18:00 to 7:00 hours), they received 20 to 32 lux.
21 infants (mean (SD) birth weight 1049 g (330 g), mean (SD) PMA 28.0 weeks (2.1 weeks)) were assigned to CBL. Infants received 176 to 232 lux during daytime, and 206 to 274 lux during night‐time. The intervention was started at birth.
Outcomes: length of stay, days requiring supplemental oxygen, days on ventilator, days to first oral feeding, mean daily caloric intake, percent weight gain per week, nursing behaviour.
Vásquez‐Ruiz 2014: This single‐centre study was conducted at Hospital Juárez de Mexico.
Objective: to evaluate effects of an alternating light‐dark cycle (CL) in the NICU of a Mexican public hospital on weight gain and early discharge in preterm infants, and to evaluate the effect of reducing intensity of light at night in development of the preterm newborn.
Population: 38 preterm infants 28 to 36.3 weeks' PMA.
Interventions:
19 infants (mean (SEM) birth weight 1564 g (131 g), mean (SEM) PMA 31.7 weeks (0.55 weeks)) were assigned to CL. The light‐dark condition was achieved by placing from 19:00 to 07:00 an acrylic helmet, covered with blue surgical drapes; surgical cloths were placed on helmets, and the frontal part was open, allowing good airflow. This helmet was placed individually above the head and thorax of each baby, resulting in reduced illumination, with a light intensity of 27 lux ± 0.8 at the level of the eyes.
19 infants (mean (SEM) birth weight 1452 g (102 g), mean (SEM) PMA 31.7 weeks (0.31 weeks)) were assigned to CBL, with a range of 249 ± 11 lux during day and night.
The intervention was started at birth.
Outcomes: length of stay, melatonin levels in saliva (in a subsample of infants, n = 8), weight gain, milk intake.
Excluded studies
We excluded six studies (Blackburn 1991; Kennedy 2001; Hoogeveen 2004; Braz 2006; Aita 2012; Park 2015). For details, see the Characteristics of excluded studies table.
Risk of bias in included studies
For details, see the 'Risk of bias' table for each included study, the summary of findings tables (Table 1; Table 2) and the risk of bias summary (Figure 2) and graph (Figure 3).
2.
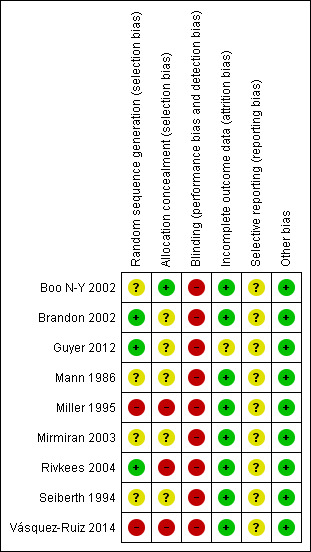
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
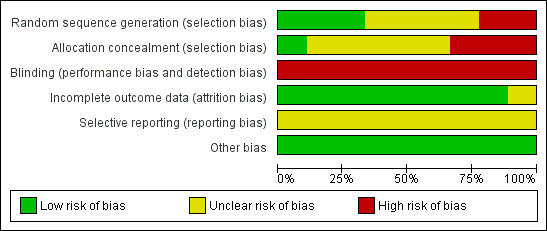
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
These studies could not be blinded. Outcomes were reported for all enrolled infants. To our knowledge, no trial had been entered into a trials registry at the protocol stage. Study protocols were not available to us.
One study randomised 20 infants to be cared for in a nursery with CL and reduced noise, and 21 to be cared for in a nursery with no reduction in intensity of light or noise (Mann 1986). Thus, this study had a co‐intervention of reduced noise, in addition to CL. All data for this study were reported in graphic form only and could not be entered into Review Manager (RevMan 2014).
Sequence generation
Three of the included studies had adequate sequence generation (low risk of bias) (Rivkees 2004 and Guyer 2012 used a table of random numbers; Brandon 2002 used a computer‐generated random list).
Two studies had no random sequence generation (high risk of bias) (Miller 1995; Vásquez‐Ruiz 2014), and the random sequence generation was unclear in the remaining four studies (unclear risk of bias) (Mann 1986; Seiberth 1994; Boo N‐Y 2002; Mirmiran 2003).
Allocation
One study had adequate allocation concealment (low risk of bias) (Boo N‐Y 2002: sequentially numbered sealed envelopes, which were opened in order). Information on adequate allocation concealment was lacking in five studies (unclear risk of bias) (Mann 1986; Seiberth 1994; Brandon 2002; Mirmiran 2003; Guyer 2012). Two studies had high risk of bias for allocation concealment (Miller 1995; Vásquez‐Ruiz 2014). One study (Miller 1995) was a quasi‐randomised trial in which infants were assigned to CL or CBL, subject to the availability of space and staff (quasi‐randomised trial). In Vásquez‐Ruiz 2014, infants were assigned by the sequential randomisation method, by which the first infant was assigned to the CBL group, the second to the CL group and so on. No single study had documented both adequate sequence generation and concealed allocation.
Blinding
The study intervention could not be blinded to caregivers nor to researchers in any of the included studies.
Incomplete outcome data
All studies, except Guyer 2012, provided complete outcome data. All 37 randomised infants were accounted for, but the mental development index (MDI) and the physical development index (PDI) were reported for only 15 infants in the CL group and for 16 in the DL group.
Selective reporting
Risk of bias for this item was unclear in all studies, as we did not have access to the study protocol for any trial; therefore we could not judge whether any deviations from the protocol had occurred.
Other potential sources of bias
We judged the risk for all studies as low, as we detected no other bias in any study.
Effects of interventions
Cycled light versus irregular dimmed light or near darkness (Comparison 1)
Tests for heterogeneity were not applicable in analyses that included only one trial.
Primary outcome
No study reported weight at three or six months of age.
Secondary outcomes
Weight at four months (Outcome 1.1)
One study (n = 40) reported weight at four months (Mirmiran 2003) and revealed no statistically significant differences between CL and ND groups (MD 181.0 g, 95% CI ‐484.0 to 846.0; Analysis 1.1).
1.1. Analysis.
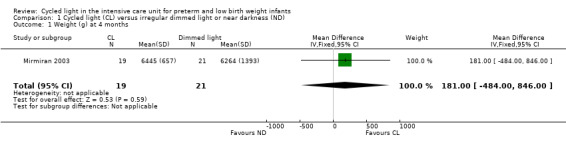
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 1 Weight (g) at 4 months.
Weight at 35 weeks' postmenstrual age (Outcome 1.2)
One study (n = 40) reported weight at 35 weeks' PMA (Mirmiran 2003) and noted no statistically significant differences between CL and ND groups (MD 106.0 g, 95% CI ‐41.66 to 253.66; Analysis 1.2).
1.2. Analysis.
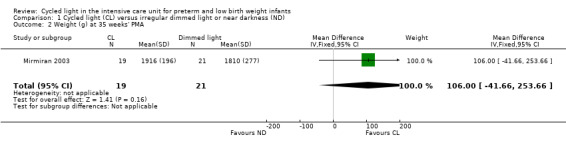
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 2 Weight (g) at 35 weeks' PMA.
Weight on day 14 (cycled light from 32 weeks' postmenstrual age) (Outcome 1.3)
One study (n = 96) reported weight on day 14 (Boo N‐Y 2002) and described no statistically significant differences between CL and ND groups (MD 0.00 g, 95% CI ‐103.88 to 103.88; Analysis 1.3).
1.3. Analysis.
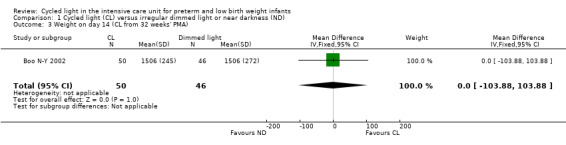
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 3 Weight on day 14 (CL from 32 weeks' PMA).
Weight gain by day 14 (cycled light from 32 weeks' postmenstrual age) (Outcome 1.4)
One study (n = 96) reported weight gain by day 14 (Boo N‐Y 2002) and revealed no statistically significant differences between CL and ND groups (MD ‐8.60 g, 95% CI ‐51.37 to 34.17; Analysis 1.4).
1.4. Analysis.
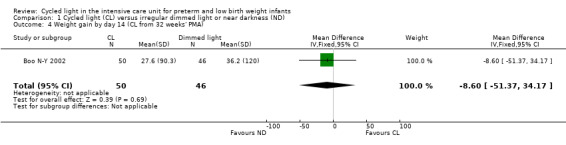
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 4 Weight gain by day 14 (CL from 32 weeks' PMA).
Mean age when birth weight was regained (cycled light from 32 weeks' postmenstrual age) (Outcome 1.5)
One study (n = 96) reported mean age when birth weight was regained (Boo N‐Y 2002) and noted no statistically significant differences between CL and ND groups (MD 0.10 days, 95% CI ‐2.57 to 2.77; Analysis 1.5).
1.5. Analysis.
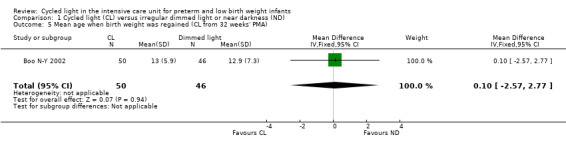
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 5 Mean age when birth weight was regained (CL from 32 weeks' PMA).
Cumulative mean weekly weight gain (grams/wk) (cycled light from birth) (Outcome 1.6)
One study (n = 43) reported cumulative mean weekly weight gain (Brandon 2002) and described no statistically significant differences between CL and ND groups (MD 24.00 grams/wk, 95% CI ‐50.97 to 98.97; Analysis 1.6).
1.6. Analysis.
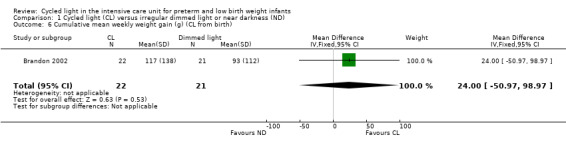
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 6 Cumulative mean weekly weight gain (g) (CL from birth).
Cumulative mean weekly weight gain (grams/wk) (cycled light from 32 weeks' postmenstrual age) (Outcome 1.7)
One study (n = 40) reported cumulative mean weekly weight gain (Brandon 2002) and described no statistically significant differences between CL and ND groups (MD 29.00 grams/wk, 95% CI ‐53.36 to 111.36; Analysis 1.7).
1.7. Analysis.
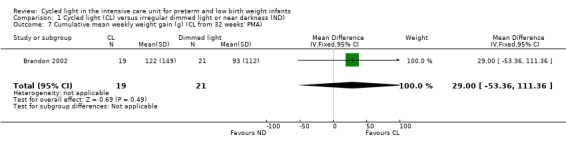
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 7 Cumulative mean weekly weight gain (g) (CL from 32 weeks' PMA).
Daily weight gain (grams/d) during neonatal care (cycled light from 32 weeks' postmenstrual age) (Outcome 1.8)
Two studies (n = 128) reported daily weight gain during neonatal care (Boo N‐Y 2002; Guyer 2012) and reported no statistically significant differences between CL and ND groups (typical MD 2.84 grams/d, 95% CI ‐0.67 to 6.36; Analysis 1.8). Results showed no heterogeneity for this outcome (I2 = 0%).
1.8. Analysis.
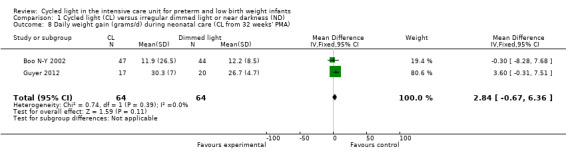
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 8 Daily weight gain (grams/d) during neonatal care (CL from 32 weeks' PMA).
Kilocalories per kilogram per day (cycled light from birth) (Outcome 1.9)
One study (n = 43) reported kilocalories per kilogram per day (CL from birth) (Brandon 2002) and noted no statistically significant differences between CL and ND groups (MD 7.70 kcal/kg/d, 95% CI ‐18.96 to 34.36; Analysis 1.9).
1.9. Analysis.
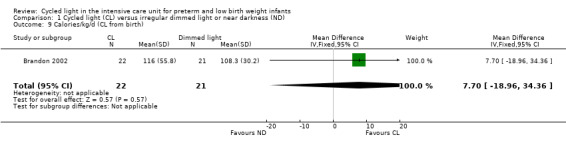
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 9 Calories/kg/d (CL from birth).
Kilocalories per kilogram per day (cycled light from 32 weeks' postmenstrual age) (Outcome 1.10)
One study (n = 40) reported kilocalories per kilogram per day (CL from 32 weeks' PMA) (Brandon 2002) and observed no statistically significant differences between CL and ND groups (MD 0.20 cal/kg/d, 95% CI ‐17.62 to 18.02; Analysis 1.10).
1.10. Analysis.
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 10 Calories/kg/d (CL from 32 weeks' PMA).
Days of life to start feeds (cycled light from birth) (Outcome 1.11)
One study (n = 43) reported days of life to start feeds (CL from birth) (Brandon 2002) and described no statistically significant differences between CL and ND groups (MD 2.00 days, 95% CI ‐6.64 to 10.64; Analysis 1.11).
1.11. Analysis.
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 11 Day of life to start feeds (CL from birth).
Days of life to start feeds (cycled light from 32 weeks' postmenstrual age) (Outcome 1.12)
One study (n = 40) reported days of life to start feeds (CL from 32 weeks' PMA) (Brandon 2002) and noted no statistically significant differences between CL and ND groups (MD ‐1.50 days, 95% CI ‐5.40 to 2.40; Analysis 1.12).
1.12. Analysis.
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 12 Day of life to start feeds (CL from 32 weeks' PMA).
Days of life to full feeds (cycled light from birth) (Outcome 1.13)
One study (n = 43) reported days of life to full feeds (CL from birth) (Brandon 2002) and reported no statistically significant differences between CL and ND groups (MD 0.80 days, 95% CI ‐20.83 to 22.43; Analysis 1.13).
1.13. Analysis.
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 13 Day of life to full feeds (CL from birth).
Days of life to full feeds (cycled light from 32 weeks' postmenstrual age) (Outcome 1.14)
One study (n = 40) reported days of life to full feeds (CL from 32 weeks' PMA) (Brandon 2002) and revealed no statistically significant differences between CL and ND groups (MD ‐6.70 days, 95% CI ‐25.15 to 11.75; Analysis 1.14).
1.14. Analysis.
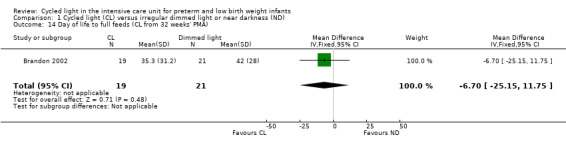
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 14 Day of life to full feeds (CL from 32 weeks' PMA).
One study published in abstract form (n = 39) reported statistically significantly shorter time to reach full oral feeds in the CL group (26 days with CL vs 38 days with ND; P value = 0.04) (Mirmiran 2003).
Ventilator days (cycled light from birth) (Outcome 1.15)
Two studies (n = 170) reported ventilator days (CL from birth) (Seiberth 1994; Brandon 2002) and described no statistically significant differences between CL and ND groups (MD 0.67 days, 95% CI ‐2.07 to 3.41; Analysis 1.15). Results showed low heterogeneity for this outcome (I2 = 39%).
1.15. Analysis.
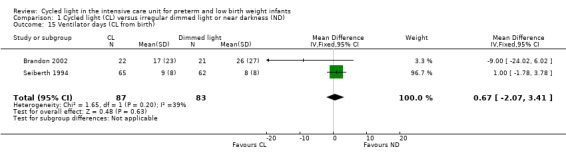
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 15 Ventilator days (CL from birth).
Ventilator days (cycled light from 32 weeks' postmenstrual age) (Outcome 1.16)
One study (n = 40) reported ventilator days (CL from 32 weeks' PMA) (Brandon 2002) and observed no statistically significant differences between CL and ND groups (MD ‐3.00 days, 95% CI ‐19.43 to 13.43; Analysis 1.16).
1.16. Analysis.
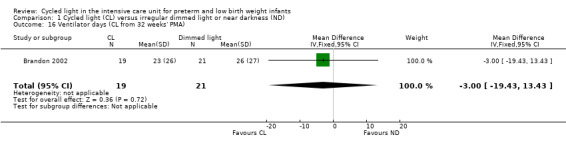
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 16 Ventilator days (CL from 32 weeks' PMA).
Days in supplemental oxygen (cycled light from birth) (Outcome 1.17)
One study (n = 43) reported supplemental oxygen (CL from birth) (Brandon 2002) and noted no statistically significant differences between CL and ND groups (MD ‐16.40 days, 95% CI ‐46.98 to 14; Analysis 1.17).
1.17. Analysis.
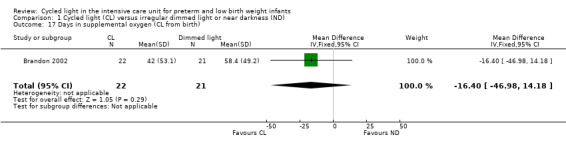
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 17 Days in supplemental oxygen (CL from birth).
Days in supplemental oxygen (cycled light from 32 weeks' postmenstrual age) (Outcome 1.18)
One study (n = 40) reported supplemental oxygen (CL from 32 weeks' PMA) (Brandon 2002) and described no statistically significant differences between CL and ND groups (MD ‐17.60 days, 95% CI ‐47.26 to 12.06; Analysis 1.18).
1.18. Analysis.
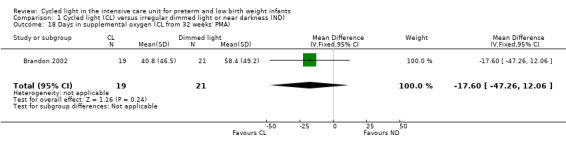
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 18 Days in supplemental oxygen (CL from 32 weeks' PMA).
One study published in abstract form (n = 39) reported no statistically significant differences in "days in supplemental oxygen" between CL and ND (11 days with CL vs 25 days with ND; P value = 0.09) (Mirmiran 2003).
Length of stay (cycled light from birth) (Outcome 1.19)
Two studies (n = 170) reported length of stay (days) (CL from birth) (Seiberth 1994; Brandon 2002) and reported no statistically significant differences between CL and ND groups (MD ‐ 4.67 days, 95% CI ‐14.81 to 5.47; Analysis 1.19). Results showed no heterogeneity for this outcome (I2 = 0%).
1.19. Analysis.
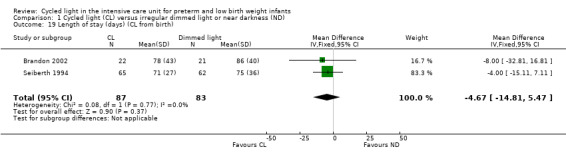
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 19 Length of stay (days) (CL from birth).
Mirmiran 2003 (n = 39) reported in abstract form results of shorter length of stay (six weeks with CL vs eight weeks with ND; P value = 0.05).
Length of stay (cycled light from 32 weeks' postmenstrual age) (Outcome 1.20)
Two studies (n = 77) reported length of stay (days) (CL from 32 weeks' PMA) (Brandon 2002; Guyer 2012) and described a statistically significant reduction in length of stay between CL and ND groups, favouring the CL group (MD ‐12.66 days, 95% CI ‐23 to ‐2.33; Analysis 1.20). Results showed no heterogeneity for this outcome (I2 = 0%) (Figure 4).
1.20. Analysis.
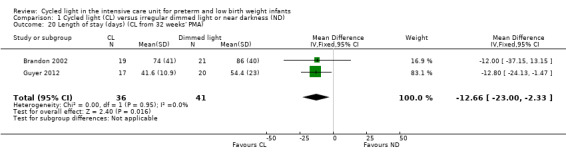
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 20 Length of stay (days) (CL from 32 weeks' PMA).
4.
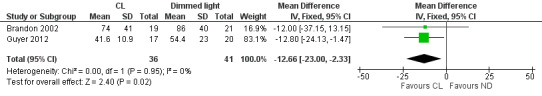
Forest plot of comparison: 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), outcome: 1.20 Length of stay (days) (CL from 32 weeks' PMA).
Retinopathy of prematurity (any stage) (cycled light from birth or from 32 weeks' postmenstrual age) (Outcome 1.21)
Two studies (n = 189) reported ROP (any stage) (Seiberth 1994; Brandon 2002) and noted no statistically significant differences between CL and ND groups (typical RR 0.83, 95% CI 0.60 to 1.13, I2 = 0%; typical RD ‐0.09, 95% CI ‐0.23 to 0.06, I2 = 15%; Analysis 1.21). Results showed no heterogeneity for this outcome.
1.21. Analysis.
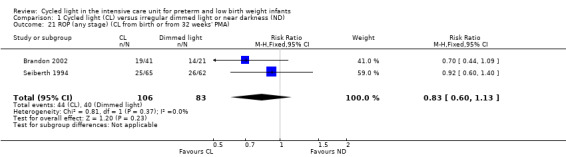
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 21 ROP (any stage) (CL from birth or from 32 weeks' PMA).
Retinopathy of prematurity (stage ≥ 3) (cycled light from birth or from 32 weeks' postmenstrual age) (Outcome 1.22)
Two studies (n = 189) reported ROP (stage ≥ 3) (Seiberth 1994; Brandon 2002) and revealed no statistically significant differences between CL and ND groups (typical RR 0.53, 95% CI 0.25 to 1.11, I2 = 0%; typical RD ‐0.09, 95% CI ‐0.19 to 0.01, I2 = 0%; Analysis 1.22). Results showed no heterogeneity for this outcome.
1.22. Analysis.
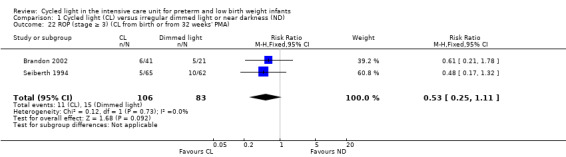
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 22 ROP (stage ≥ 3) (CL from birth or from 32 weeks' PMA).
Infants requiring laser surgery (cycled light from birth or from 32 weeks' postmenstrual age) (Outcome 1.23)
One study (n = 62) reported infants requiring laser surgery (Brandon 2002) and indicated no statistically significant differences between CL and ND groups (RR 0.51, 95% CI 0.11 to 2.32; RD ‐0.07, 95% CI ‐0.24 to 0.10; Analysis 1.23).
1.23. Analysis.
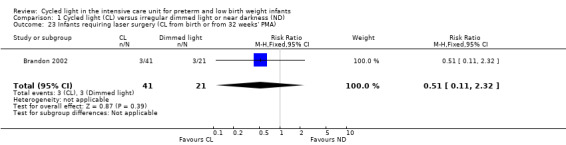
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 23 Infants requiring laser surgery (CL from birth or from 32 weeks' PMA).
Post hoc analyses
Ratio of day‐night activity over the 10 days preceding discharge from the hospital (cycled light from 32 weeks' postmenstrual age) (Outcome 1.24)
One study (n = 62) reported the ratio of day‐night activity over the 10 days preceding discharge from the hospital (Rivkees 2004), showing a statistically significant difference between CL and ND groups (MD 0.18, 95% CI 0.17 to 0.19; Analysis 1.24).
1.24. Analysis.
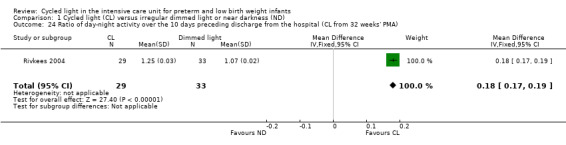
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 24 Ratio of day‐night activity over the 10 days preceding discharge from the hospital (CL from 32 weeks' PMA).
Period of entrained circadian rhythms over the first 10 days at home (cycled light from 32 weeks' postmenstrual age) (Outcome 1.25)
One study (n = 51) reported on the period of entrained circadian rhythms over the first 10 days at home (Rivkees 2004) and described a statistically significant difference between CL and ND groups (MD ‐0.78, 95% CI ‐0.89 to ‐0.67; Analysis 1.25).
1.25. Analysis.
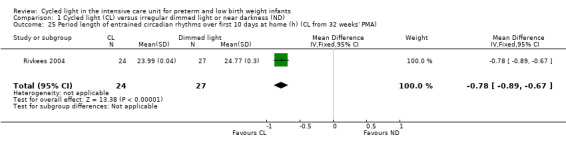
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 25 Period length of entrained circadian rhythms over first 10 days at home (h) (CL from 32 weeks' PMA).
Total number of movements per day (10 to 0 days before discharge) (cycled light from 32 weeks' postmenstrual age) (Outcome 1.26)
One study (n = 62) reported total number of movements per day (Rivkees 2004) and noted a statistically significant difference in total number of movements per day between CL and ND groups (MD 73.00 movements/d, 95% CI 43.62 to 102.38; Analysis 1.26).
1.26. Analysis.
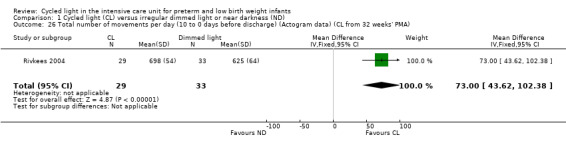
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 26 Total number of movements per day (10 to 0 days before discharge) (Actogram data) (CL from 32 weeks' PMA).
Total number of movements per day (1 to 10 days after discharge) (cycled light from 32 weeks' postmenstrual age) (Outcome 1.27)
One study (n = 51) reported total number of movements per day (Rivkees 2004) and noted a statistically significant difference in total number of movements per day between CL and ND groups (MD 82.00 movements/d, 95% CI 22.92 to 141.08; Analysis 1.27).
1.27. Analysis.
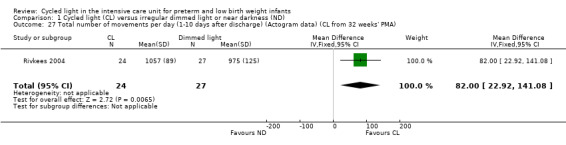
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 27 Total number of movements per day (1‐10 days after discharge) (Actogram data) (CL from 32 weeks' PMA).
Total number of movements per day (11 to 20 days after discharge) (cycled light from 32 weeks' postmenstrual age) (Outcome 1.28)
One study (n = 51) reported total number of movements per day (Rivkees 2004) and observed a statistically significant difference in total number of movements per day between CL and ND groups (MD 429.00 movements/d, 95% CI 341.94 to 516.06; Analysis 1.28).
1.28. Analysis.
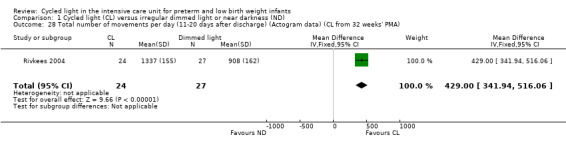
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 28 Total number of movements per day (11‐20 days after discharge) (Actogram data) (CL from 32 weeks' PMA).
Total number of movements per day (21 to 30 days after discharge) (cycled light from 32 weeks' postmenstrual age) (Outcome 1.29)
One study (n = 29) reported total number of movements per day (Rivkees 2004) and described a statistically significant difference in total number of movements per day between CL and ND groups (MD 536.00 movements/d, 95% CI 396.83 to 675.17; Analysis 1.29)
1.29. Analysis.
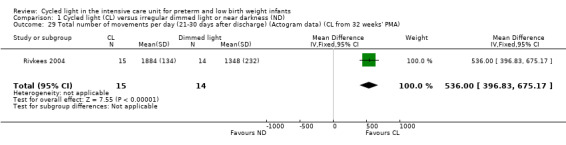
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 29 Total number of movements per day (21‐30 days after discharge) (Actogram data) (CL from 32 weeks' PMA).
Sleep (hours/24 h) at five weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.30)
One study (n = 37) reported the number of hours of sleep per 24 hours at five weeks' CA (Guyer 2012) and revealed no statistically significant differences in hours of sleep/24 h between CL and ND groups (MD 0.55, 95% CI ‐0.37 to 1.47; Analysis 1.30).
1.30. Analysis.
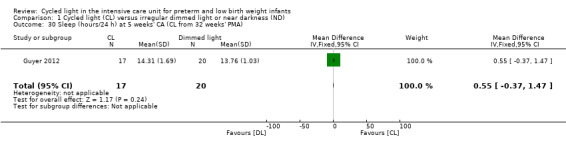
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 30 Sleep (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA).
Sleep (hours/24 h) at 11 weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.31)
One study (n = 37) reported the number of hours of sleep per 24 hours at 11 weeks' CA (Guyer 2012) and noted no statistically significant differences in hours of sleep/24 h between CL and ND groups (MD ‐0.09, 95% CI ‐0.88 to 0.70; Analysis 1.31).
1.31. Analysis.
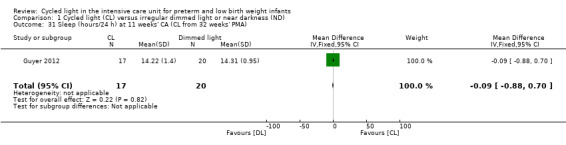
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 31 Sleep (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA).
Activity count per 24 hours at five weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.32)
One study (n = 37) reported activity count per 24 hours at five weeks' CA (Guyer 2012) and described no statistically significant differences in activity count between CL and ND groups (MD 27.45, 95% CI ‐2.07 to 56.97; Analysis 1.32).
1.32. Analysis.
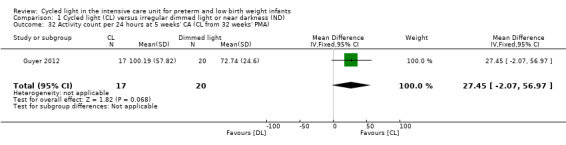
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 32 Activity count per 24 hours at 5 weeks' CA (CL from 32 weeks' PMA).
Activity count per 24 hours at 11 weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.33)
One study (n = 37) reported activity count per 24 hours at 11 weeks' CA (Guyer 2012) and revealed no statistically significant differences in activity count between CL and ND groups (MD 5.1, 95% CI ‐46.5 to 56.7; Analysis 1.33).
1.33. Analysis.
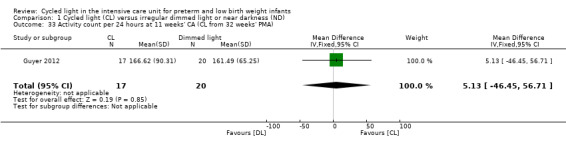
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 33 Activity count per 24 hours at 11 weeks' CA (CL from 32 weeks' PMA).
Wake and content (hours/24 h) at five weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.34)
One study (n = 37) reported on 'wake and content' (hours/24 h) at five weeks' CA (Guyer 2012) and reported no statistically significant differences in 'wake and content' between CL and ND groups (MD 0.89, 95% CI ‐0.59 to 2.37; Analysis 1.34).
1.34. Analysis.
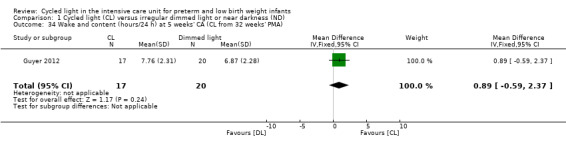
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 34 Wake and content (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA).
Wake and content (hours/24 h) at 11 weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.35)
One study (n = 37) reported on 'wake and content' (hours/24 h) at 11 weeks' CA (Guyer 2012) and described no statistically significant differences in 'wake and content' between CL and ND groups (MD 1.47, 95% CI ‐0.36 to 3.30; Analysis 1.35).
1.35. Analysis.
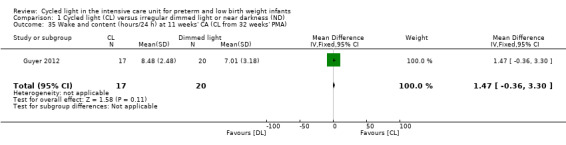
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 35 Wake and content (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA).
Crying (hours/24 h) at five weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.36)
One study (n = 37) reported on crying (hours/24 h) at five weeks' CA (Guyer 2012) and noted no statistically significant differences in crying at five weeks' CA between CL and ND groups (MD ‐0.47, 95% CI ‐0.95 to 0.01; Analysis 1.36).
1.36. Analysis.
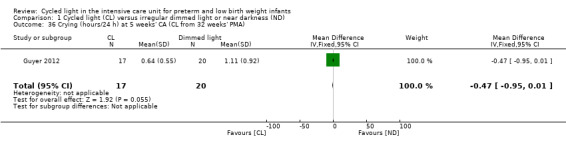
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 36 Crying (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA).
Crying (hours/24 h) at 11 weeks' corrected age (cycled light from 32 weeks' postmenstrual age) (Outcome 1.37)
One study (n = 37) reported on crying (hours/24 h) at 11 weeks' CA (Guyer 2012) and observed a statistically significant difference in crying at 11 weeks' CA between CL and ND groups favouring the CL group (MD ‐0.57, 95% CI ‐1.09 to 0.05; Analysis 1.37).
1.37. Analysis.
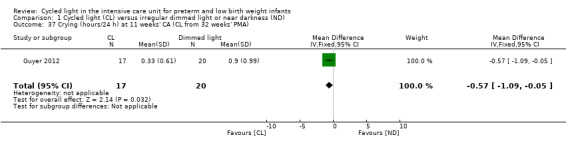
Comparison 1 Cycled light (CL) versus irregular dimmed light or near darkness (ND), Outcome 37 Crying (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA).
Neurobehavioural assessment of the preterm infant at 32 and 36 weeks' postmenstrual age (Outcome 1.38)
One study (56 of 62 randomised infants) reported on neurobehavioural assessment of the preterm infant at 32 and 36 weeks' PMA (Brandon 2002). Researchers noted no significant effect of lighting on short‐term developmental outcomes in preterm infants, except for popliteal angle, which was significantly less for infants receiving CL at 32 weeks' PMA. This was the only significant effect of the lighting environment among 18 variables examined and was probably a chance finding.
Cycled light versus continuous bright light (Comparison 2)
Three studies randomised infants to be cared for in a nursery with CL or a nursery with CBL (Mann 1986; Miller 1995; Vásquez‐Ruiz 2014).
One study randomised 20 infants to be cared for in a nursery with CL and 21 infants to be cared for in a nursery with no reduction in intensity of light and noise (Miller 1995). One study randomised 19 infants to be cared for in CL and 19 infants to be cared for in CBL (Vásquez‐Ruiz 2014). The following outcomes refer to these two studies. Results from the study by Vásquez‐Ruiz 2014 were included for the outcome of length of stay (cycled light from birth) (Outcome 2.3). For all other outcomes, results pertain to Miller 1995. In Mann 1986, results were reported in graphic form only and could not be entered into Review Manager (RevMan 2014). Results of that study are reported below.
Tests for heterogeneity are not applicable for analyses that include only one study.
Primary outcome
Weight at three or six months of age was not reported in Miller 1995 nor in Vásquez‐Ruiz 2014.
Secondary outcomes
Mean daily caloric intake (kcal/kg/d) (cycled light from birth) (Outcome 2.1)
Investigators reported no statistically significant differences in mean caloric intake between CL and CBL groups (MD 9.60 kcal/kg/d, 95% CI ‐0.50 to 19.70; Analysis 2.1) (Miller 1995).
2.1. Analysis.
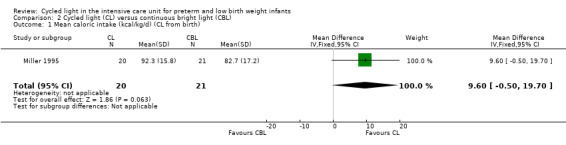
Comparison 2 Cycled light (CL) versus continuous bright light (CBL), Outcome 1 Mean caloric intake (kcal/kg/d) (CL from birth).
Days to first oral feeding (cycled light from birth) (Outcome 2.2)
Researchers reported a statistically significant difference in the number of days to first oral feeding between CL and CBL groups (MD ‐6.80 days, 95% CI ‐13.29 to ‐0.31; Analysis 2.2) (Miller 1995).
2.2. Analysis.
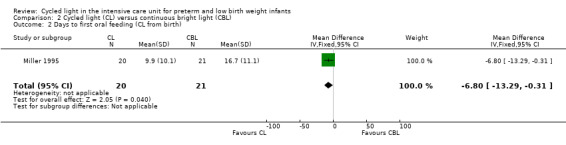
Comparison 2 Cycled light (CL) versus continuous bright light (CBL), Outcome 2 Days to first oral feeding (CL from birth).
Length of stay (cycled light from birth) (Outcome 2.3)
Two studies (Miller 1995; Vásquez‐Ruiz 2014) (n = 79) reported on length of stay and described a statistically significantly shorter length of stay in the CL group compared with the CBL group (MD ‐16.48 days, 95% CI ‐26.16 to ‐6.79; Analysis 2.3; Figure 5) (I2 = 0%) (no heterogeneity).
2.3. Analysis.
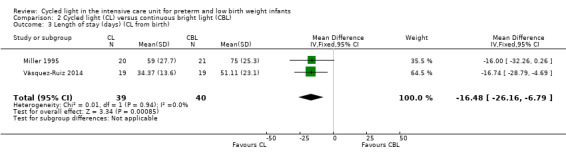
Comparison 2 Cycled light (CL) versus continuous bright light (CBL), Outcome 3 Length of stay (days) (CL from birth).
5.
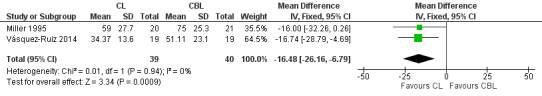
Forest plot of comparison: 2 Cycled light (CL) versus continuous bright light (CBL), outcome: 2.3 Length of stay (days) (CL from birth).
Days requiring supplemental oxygen (cycled light from birth) (Outcome 2.4)
Results showed no statistically significant differences in the number of days requiring supplemental oxygen between CL and CBL groups (MD ‐20.80 days, 95% CI ‐44.29 to 2.69; Analysis 2.4) (Miller 1995).
2.4. Analysis.
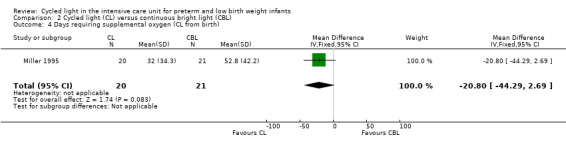
Comparison 2 Cycled light (CL) versus continuous bright light (CBL), Outcome 4 Days requiring supplemental oxygen (CL from birth).
Days on ventilator (cycled light from birth) (Outcome 2.5)
Study authors noted a statistically significant difference in the number of days on ventilator between CL and CBL groups (MD ‐18.20, 95% CI ‐31.40 to ‐5.00; Analysis 2.5) (Miller 1995).
2.5. Analysis.
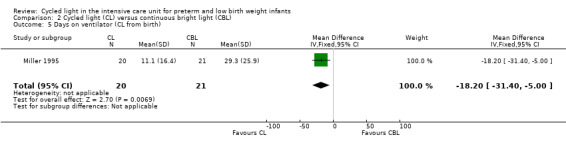
Comparison 2 Cycled light (CL) versus continuous bright light (CBL), Outcome 5 Days on ventilator (CL from birth).
Infant growth (cycled light from birth) (Outcome 2.6)
Researchers found a statistically significant difference in weight gain among infants in the CL group, who gained a mean of 9.4% of their weight over the course of one week, whereas infants in the CBL group gained a mean of 7.4% (P value < 0.05) (Miller 1995).
Nursing behaviour (cycled light from birth) (Outcome 2.7)
Results showed no significant relationships between lighting condition and nursing behaviour (Miller 1995) and no evidence that staff behaviours mediated the effects of lighting conditions.
One study (CL from 32 weeks' PMA) randomised 20 infants to be cared for in a nursery with CL and reduced noise, and 21 to be cared for in a nursery with no reduction in intensity of light or noise (Mann 1986).
All data were reported in graphic form only and could not be entered into Review Manager (RevMan 2014). The following outcomes refer to this study (Mann 1986).
Primary outcomes
Growth at three and six months
Growth at six months of age was not reported, but growth at three months (12 weeks) of age was noted.
Mean weight at 12 weeks' corrected age (Outcome 2.8)
Mean weight at 12 weeks' CA (three months) was statistically significantly higher among infants cared for in the CL nursery (P value < 0.02) (Mann 1986).
Mean weight at six weeks' corrected age (Outcome 2.9)
Mean weight at six weeks' CA was statistically significantly higher among infants cared for in the CL nursery (P value < 0.05) (Mann 1986).
Mean weight at expected date of birth (Outcome 2.10)
Mean weight at expected date of birth was not statistically significantly different between the two groups (Mann 1986).
Mean weight at discharge from hospital (Outcome 2.11)
Mean weight at discharge was not statistically significantly different between the two groups (Mann 1986).
Post hoc analyses
Mean number of hours spent awake in 24 hours at 12 weeks' corrected age (Outcome 2.12)
Mean number of hours spent awake at 12 weeks' CA (three months) was statistically significantly lower among infants cared for in the CL light nursery (P value < 0.005) (Mann 1986).
Mean number of hours spent awake in 24 hours at six weeks' corrected age (Outcome 2.13)
Mean number of hours spent awake at six weeks' CA was statistically significantly lower among infants cared for in the CL light nursery (P value < 0.01) (Mann 1986).
Mean number of hours spent awake in 24 hours at expected date of birth (Outcome 2.14)
Mean number of hours spent awake at the expected date of birth was statistically significantly lower among infants cared for in the CL light nursery (P value < 0.05) (Mann 1986).
Mean number of hours spent awake in 24 hours at discharge from hospital (Outcome 2.15)
Mean hours spent awake at discharge from hospital was not statistically significantly different between groups (Mann 1986).
Mean number of hours spent feeding in 24 hours at 12 weeks' corrected age (Outcome 2.16)
Mean number of hours spent feeding in 24 hours at 12 weeks' CA was statistically significantly lower among infants cared for in the CL nursery (P value < 0.02) (Mann 1986).
Mean number of hours spent feeding in 24 hours at six weeks' corrected age (Outcome 2.17)
Mean number of hours spent feeding in 24 hours at six weeks' CA was not statistically significantly different between the two groups (Mann 1986).
Mean number of hours spent feeding in 24 hours at expected date of birth (Outcome 2.18)
Mean number of hours spent feeding in 24 hours at expected date of birth was not statistically significantly different between the two groups (Mann 1986).
Mean number of hours spent feeding in 24 hours at discharge from hospital (Outcome 2.19)
Mean number of hours spent feeding in 24 hours at discharge from hospital was not statistically significantly different between the two groups (Mann 1986).
No study reported on any adverse effects of the interventions, nor did they report on caregivers' or parents' satisfaction/dissatisfaction with the interventions.
Trials identified no safety issues.
Discussion
Summary of main results
Cycled light versus near darkness
We identified one additional study for this comparison in 2013 (Guyer 2012). That study enrolled 37 infants, and the intervention was started at 32 weeks' postmenstrual age (PMA). We identified no new studies for the 2016 update. Thus, we identified six studies, including 424 infants. All studies had small sample sizes (range 37 to 127 infants). Investigators reported a wide variety of outcomes, none of which included our primary outcomes of growth at three and six months' PMA. However, one study reported on weight at four months' PMA (Mirmiran 2003). We reported on 39 different outcomes; of these only six outcomes included data from two trials with a maximum of 189 infants (Analysis 1.8; Analysis 1.15; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22); all other outcomes included data from only one study. Some studies used the term 'dim lighting' and others 'near darkness' for the reduced lighting condition (Table 3). We consider the light intensity levels similar enough to use the term 'dim lighting' and 'near darkness' interchangeably.
Studies reported no statistically significant differences in weight at four months of age, although results favoured the cycled light (CL) group (mean difference (MD) 181.00 g, 95% confidence interval (CI) ‐484.00 to 846.00). In one study, infants showed a significantly increased total number of movements from 10 days before discharge to 30 days after discharge (Actogram data), and the ratio of day‐night activity over the 10 days preceding discharge from hospital favoured the CL group by 18% (95% CI 17% to 19%) (Rivkees 2004). These data indicate that infants in the CL group showed a greater number of movements, but that most movements occurred during daytime. In this study, the mean period of entrained circadian rhythms over the first 10 days at home (hours) was 24 hours versus 24.77 hours for infants in the near darkness (ND) group, indicating synchronisation to the 24‐hour solar day (MD ‐0.78, 95% CI ‐0.89 to ‐0.67).
The 2013 update of the review noted a statistically significant reduction in length of stay of 12.66 days (95% CI ‐23.00 to ‐2.33; two studies, n = 77) and no heterogeneity for this outcome (I2 = 0%). The quality of the evidence was rated as low according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (Table 3).
One study found a significant reduction in time spent crying in 24 hours at 11 weeks' corrected age (CA), reporting less crying at 11 weeks' CA in the CL compared with the ND group.
In the study by Brandon and co‐workers, two groups were exposed to CL; one group started CL at birth and the other started CL at 32 weeks' PMA (Brandon 2002). None of the individual outcomes reached statistical significance compared with ND and no obvious trends favoured starting CL at birth versus starting at 32 weeks' PMA, which may have implications for the planning of future studies.
It is noteworthy that the incidence of retinopathy of prematurity (ROP), both any stage and stage ≥ 3 (n = 189), and of 'infants requiring laser surgery' (n = 62), showed important trends towards a reduction (risk difference (RD) ‐ 0.9, 95% CI ‐0.19 to 0.01 for ROP stage ≥ 3; RD ‐0.07, 95% CI ‐0.24 to 0.10 for laser surgery). Several other outcomes showed trends favouring the CL group. Phelps and Watts, in a Cochrane review, concluded that decreasing ambient light exposure in preterm infants is very unlikely to reduce the incidence of ROP (Phelps 2001). That review included one study (Seiberth 1994).
Although the only new study identified in 2013 included a small sample size, results added support for CL versus ND.
Six studies (n = 424) compared CL versus ND. Most outcomes were reported only in single studies. One study (n = 40) reported on weight at four months and noted no statistically significant differences between CL and ND groups (MD 181 g, 95% CI ‐484 to 846). One study (n = 39) reported statistically significantly shorter time to reach full oral feeds in the CL group (26 days with CL vs 38 days with ND; P value = 0.04). The ratio of day‐night activity before discharge favoured the CL group (MD 0.18, 95% CI 0.17 to 0.19). This update of the review describes a statistically significant reduction in length of stay of 12.66 days (95% CI ‐23.00 to ‐2.33; two studies, n = 77). One study found a significant reduction in time spent crying in 24 hours at 11 weeks' CA.
Two studies (n = 82) compared CL versus continuous bright light (CBL). Days on a ventilator were reduced in the CL group (MD ‐18 days, 95% CI ‐31 to ‐5), as were days to first oral feeding (MD seven days, 95% CI ‐13 to ‐0.3). One study (n = 41) reported statistically significantly higher mean weight at six weeks' (P value < 0.05) and at 12 weeks' (three months) (P value < 0.02) CA among infants cared for in the CL nursery. In the same study, the mean number of hours spent awake in 24 hours at 12 weeks' (three months') (P value < 0.005) and six weeks' (P value < 0.01) CA and at expected date of birth (P value < 0.05) was lower in the CL group.
Cycled light versus continuous bright light
We identified one additional study for this comparison in 2016 (Vásquez‐Ruiz 2014). This study enrolled 38 infants, and the intervention was started at birth. Thus, we identified three studies including 120 infants that have studied the effects of CL versus CBL (Mann 1986; Miller 1995; Vásquez‐Ruiz 2014). One study included our primary outcome of growth at three months (Mann 1986). We reported on many different outcomes, and of these, only one outcome included data from two trials with a maximum of 79 infants (Miller 1995; Vásquez‐Ruiz 2014); all other outcomes included data from only one study.
In Mann 1986, mean weight at six and 12 weeks' (three months') PMA was statistically significantly higher among infants cared for in the CL nursery, and at the same time points, the mean number of hours spent awake was statistically significantly lower among infants cared for in the CL nursery. The mean number of hours spent awake at the expected date of birth was statistically significantly lower among infants cared for in the CL nursery, and the mean number of hours spent feeding in 24 hours at 12 weeks' (three months') PMA was statistically significantly lower among infants cared for in the CL nursery. Results for these outcomes would indicate a positive effect of CL versus CBL.
In Miller 1995, CL versus CBL reduced the number of days to first oral feeding by seven days (95% CI ‐13 to ‐0.3) and days on a ventilator by 18 days (95% CI ‐31 to ‐5) in favour of CL. Miller 1995 reported a statistically significant difference in weight gain, with infants in the CL group gaining a mean of 9.4% of their weight over the course of one week, whereas infants in the CL group gained a mean of 7.4% (P value < 0.05). Two studies (Miller 1995; Vásquez‐Ruiz 2014) reported on length of hospital stay, and the meta‐analysis showed a significant reduction in length of stay. The quality of the evidence for this outcome was rated as low according to GRADE. All other reported outcomes (days requiring supplemental oxygen, mean caloric intake) favoured the CL group, but results did not reach statistical significance. The Recommended Standards for Newborn ICU Design Standard 14 states: "Caregivers benefit from moderate levels of ambient light in order to perform tasks and maintain wakefulness" (White 2007). In the only study that addressed effects of aspects of lighting on healthcare providers, Miller 1995 found no significant relationships between lighting conditions and nursing behaviour and presented no evidence that staff behaviours mediated the effects of lighting conditions (Miller 1995).
These three small studies ‐ all with limitations ‐ suggest that CL offers an advantage over CBL, mainly resulting in improved growth and sleep, shortened hospital stay and fewer days on the ventilator. CBL has been related to infant stress as manifested by increased levels of activity, decreased sleep and bradycardia (Gottfried 1985; Lotas 1992; Blackburn 1998; Rivkees 2000). Statistically significant results and all trends for comparisons of CL versus CBL in the current review favoured CL. CBL does not occur in nature, and it is difficult to perceive how such a lighting condition could induce circadian rhythms.
Overall completeness and applicability of evidence
Small sample sizes of the included trials precluded any secondary analyses.
For this update, we identified a significantly shortened length of stay for both of the interventions studied: cycled light versus near darkness, and cycled light versus continuous bright light. We graded the evidence as low quality for this outcome. Results for length of stay should be interpreted with caution, as the outcome is highly dependent on PMA at birth of the neonate, availability of institutions providing Level II care to which the neonate can be transferred and the social situation of the family.
Included trials identified no safety issues.
Quality of the evidence
Quality varied, with few studies reporting sequence generation and concealed allocation. Few studies reported on sample size calculations.
In Table 1 and Table 2, we graded the quality of evidence for length of stay as low.
Mann and co‐workers provided no information on sequence generation nor on allocation concealment (Mann 1986). Miller 1995 and Vásquez‐Ruiz 2014 were quasi‐randomised trials. Mann 1986 reported data only in graphic form, but the data from two studies (Miller 1995; Vásquez‐Ruiz 2014) could be combined for length of stay. Mann 1986 provided a co‐intervention to the CL group, as noise as well as lighting was reduced at night.
For the few analyses in which more than one trial could be included, we noted no heterogeneity (I2 = 0). As no more than two studies were included in a single meta‐analysis, we could not assess publication bias (at least 10 trials were required).
Potential biases in the review process
We are not aware of any biases in the review process. Two review authors (IM and AO) selected the trials from literature searches and indicated complete agreement. Both review authors filled in predesigned forms for risk of bias assessments and data abstraction. These review authors checked assessments against each other's forms, discussed any discrepancies and reached agreement.
Agreements and disagreements with other studies or reviews
Engwall 2014, in a systematic review of intervention studies concerning cycled light compared with dim light/non‐cycled light, concluded that cycled light may be beneficial for preterm infant health. We are not aware of any other such reviews.
Authors' conclusions
Implications for practice.
Although results of this review favour the use of cycled light (CL) versus near darkness (ND), and CL versus continuous bright light (CBL), studies published to date preclude a clear conclusion. CL appears preferable to CBL.
Implications for research.
In view of positive trends for better outcomes with CL, we recommend that additional well‐designed studies of adequate sample size be conducted to confirm or refute the use of CL in neonatal intensive care units (NICUs) and stepdown units. Future research on preterm infants should focus on comparisons between CL and ND. Comparing CL versus ND is justifiable, as CL is of importance for induction of circadian rhythm, and ND is close (but not identical) to the lighting conditions the foetus is exposed to in utero until birth at term. In the CL group, studies should aim for a daytime (12 hours) light intensity greater than 200 lux and a night‐time (12 hours) light intensity less than 20 lux, which would also be the light intensity in the ND group for 24 hours. The sample size could be based on the increase in weight reported in this review.
Important questions remain to be investigated: weight gain, sleep patterns, effects on ROP and long‐term neurodevelopmental outcomes.
Future research should focus on comparisons of CL and ND, as some encouraging trends have been noted, especially with regards to reduction in ROP and increased weight gain. Sample size calculations could be based on the results of this review. For such studies, CL could be defined as less than 20 lux during the night‐time (12 hours) and greater than 200 lux during the daytime (12 hours), and ND as less than 20 lux for 24 hours.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Added external source of support |
| 27 July 2016 | New citation required but conclusions have not changed | Results from the additional included study strengthen our findings that CL shortens length of hospital stay compared with CBL. The quality of the evidence according to GRADE was low for this outcome |
| 16 March 2016 | New search has been performed | This updates the review "Cycled light in the intensive care unit for preterm and low birth weight infants" (Morag 2013). Through searches run on January 12, 2016, we identified for inclusion 1 new study (Vásquez‐Ruiz 2014), which enrolled 38 infants |
| 3 June 2013 | New citation required but conclusions have not changed | We identified 1 additional trial, which enrolled 37 infants. This study compared cycled light versus near darkness and reported a significant reduction in length of hospital stay. Results for length of stay should be interpreted with caution as the outcome is highly dependent on the PMA of the neonate at birth, the availability of institutions providing Level II care to which the neonate can be transferred and the social situation of the family. The same study found a significant reduction in time spent crying over 24 hours at 11 weeks' corrected age. The new findings support the use of CL, but the evidence is not strong enough for us to change our conclusions as provided in the previous update: "Although the results of this review favour the use of CL versus ND and CL versus CBL, the studies published to date preclude a clear recommendation because of a lack of power. Cycled light appears preferable to CBL" |
| 29 October 2008 | Amended | We prepared this review update according to the new review format |
Acknowledgements
We acknowledge Ms Elizabeth Uleryk, Chief Librarian, The Hospital for Sick Children, Toronto, for assistance in developing the search strategy for the first edition of this review.
Ms Yolanda Brosseau, Managing Editor, Cochrane Neonatal Review Group, conducted the literature searches in January 2013 and 2016.
We thank Dr DJ Kennaway for drawing our intention to his study: "Factors influencing the development of melatonin rhythmicity in humans" (Kennaway 1996). This study is currently listed under Studies awaiting classification.
We thank Dr Debra Brandon, Associate Professor, Director PhD and Postdoctoral Programs, Duke University School of Nursing, Durham, North Carolina, USA, for providing additional information on her ongoing studies.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) The Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
We evaluated the following issues and entered them into the 'Risk of bias' table.
-
Selection bias (random sequence generation and allocation concealment): For each included study, we categorised risk of selection bias as:
low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator);
high risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk ‐ no or unclear information provided.
-
Allocation concealment: For each included study, we categorised risk of bias regarding allocation concealment as:
low risk ‐ adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk ‐ no or unclear information provided.
-
Performance bias: For each included study, we categorised the methods used to blind study personnel from knowledge of which intervention a participant received. As our study population consisted of neonates, all would be blinded to the study intervention:
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
high risk ‐ inadequate, personnel aware of group assignment; or
unclear risk ‐ no or unclear information provided.
-
Detection bias: For each included study, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received. As our study population consisted of neonates, all would be blinded to the study intervention. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods used with regards to detection bias as:
low risk ‐ adequate, follow‐up performed with assessors blinded to group assignment;
high risk ‐ inadequate, assessors at follow‐up aware of group assignment; or
unclear risk ‐ no or unclear information provided.
-
Attrition bias: For each included study and for each outcome, we described completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised the methods with respect to risk of attrition bias as:
low risk ‐ adequate (< 10% missing data);
high risk ‐ inadequate (≥ 10% missing data); or
unclear risk ‐ no or unclear information provided.
-
Reporting bias: For each included study, we described how we investigated risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk ‐ inadequate (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk ‐ no or unclear information provided (study protocol was not available).
-
Other bias: For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ concerns raised about multiple looks at the data with the results made known to investigators, difference in number of participants enrolled in abstract and final publications of the paper; or
unclear risk ‐ concerns raised about potential sources of bias that could not be verified by contacting study authors.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Cycled light (CL) versus irregular dimmed light or near darkness (ND).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (g) at 4 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 181.0 [‐484.00, 846.00] |
| 2 Weight (g) at 35 weeks' PMA | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 106.0 [‐41.66, 253.66] |
| 3 Weight on day 14 (CL from 32 weeks' PMA) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐103.88, 103.88] |
| 4 Weight gain by day 14 (CL from 32 weeks' PMA) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐8.60 [‐51.37, 34.17] |
| 5 Mean age when birth weight was regained (CL from 32 weeks' PMA) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.57, 2.77] |
| 6 Cumulative mean weekly weight gain (g) (CL from birth) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 24.0 [‐50.97, 98.97] |
| 7 Cumulative mean weekly weight gain (g) (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 29.00 [‐53.36, 111.36] |
| 8 Daily weight gain (grams/d) during neonatal care (CL from 32 weeks' PMA) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 2.84 [‐0.67, 6.36] |
| 9 Calories/kg/d (CL from birth) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 7.70 [‐18.96, 34.36] |
| 10 Calories/kg/d (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐17.62, 18.02] |
| 11 Day of life to start feeds (CL from birth) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.64, 10.64] |
| 12 Day of life to start feeds (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐5.40, 2.40] |
| 13 Day of life to full feeds (CL from birth) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐20.83, 22.43] |
| 14 Day of life to full feeds (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐25.15, 11.75] |
| 15 Ventilator days (CL from birth) | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [‐2.07, 3.41] |
| 16 Ventilator days (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐19.43, 13.43] |
| 17 Days in supplemental oxygen (CL from birth) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐16.4 [‐46.98, 14.18] |
| 18 Days in supplemental oxygen (CL from 32 weeks' PMA) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐17.6 [‐47.26, 12.06] |
| 19 Length of stay (days) (CL from birth) | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐4.67 [‐14.81, 5.47] |
| 20 Length of stay (days) (CL from 32 weeks' PMA) | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐12.66 [‐21.00, ‐2.33] |
| 21 ROP (any stage) (CL from birth or from 32 weeks' PMA) | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.13] |
| 22 ROP (stage ≥ 3) (CL from birth or from 32 weeks' PMA) | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.11] |
| 23 Infants requiring laser surgery (CL from birth or from 32 weeks' PMA) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.11, 2.32] |
| 24 Ratio of day‐night activity over the 10 days preceding discharge from the hospital (CL from 32 weeks' PMA) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.17, 0.19] |
| 25 Period length of entrained circadian rhythms over first 10 days at home (h) (CL from 32 weeks' PMA) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐0.89, ‐0.67] |
| 26 Total number of movements per day (10 to 0 days before discharge) (Actogram data) (CL from 32 weeks' PMA) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 73.0 [43.62, 102.38] |
| 27 Total number of movements per day (1‐10 days after discharge) (Actogram data) (CL from 32 weeks' PMA) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [22.92, 141.08] |
| 28 Total number of movements per day (11‐20 days after discharge) (Actogram data) (CL from 32 weeks' PMA) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 429.0 [341.94, 516.06] |
| 29 Total number of movements per day (21‐30 days after discharge) (Actogram data) (CL from 32 weeks' PMA) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 536.0 [396.83, 675.17] |
| 30 Sleep (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.37, 1.47] |
| 31 Sleep (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.88, 0.70] |
| 32 Activity count per 24 hours at 5 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 27.45 [‐2.07, 56.97] |
| 33 Activity count per 24 hours at 11 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.13 [‐46.45, 56.71] |
| 34 Wake and content (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐0.59, 2.37] |
| 35 Wake and content (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [‐0.36, 3.30] |
| 36 Crying (hours/24 h) at 5 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.95, 0.01] |
| 37 Crying (hours/24 h) at 11 weeks' CA (CL from 32 weeks' PMA) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.09, ‐0.05] |
Comparison 2. Cycled light (CL) versus continuous bright light (CBL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean caloric intake (kcal/kg/d) (CL from birth) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 9.60 [‐0.50, 19.70] |
| 2 Days to first oral feeding (CL from birth) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐13.29, ‐0.31] |
| 3 Length of stay (days) (CL from birth) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐16.48 [‐26.16, ‐6.79] |
| 4 Days requiring supplemental oxygen (CL from birth) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐20.80 [‐44.29, 2.69] |
| 5 Days on ventilator (CL from birth) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐18.20 [‐31.40, ‐3.00] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Mann 1986.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no | |
| Participants | 41 preterm infants with PMA 27‐35 weeks, postnatal age on study entry 1‐63 days
Setting: single centre in Nottingham, England Study period: August‐December 1984 |
|
| Interventions | Day and night nursery group: 20 infants with mean PMA (SD) 32.0 weeks (2.0 weeks) (range 28‐35 weeks), mean birth weight (SD) 1620 g (350 g) (range 1130‐2370 g)
Control nursery group: 21 infants with mean PMA (SD) 31.6 weeks (1.9 weeks), mean birth weight (SD) 1640 g (390 g) (range 1020‐2430 g) Infants spent at least 10 days in 1 of the 2 nurseries Night and day nursery was identical in size and in number and distribution of windows as control nursery. During the daytime, the environment was identical in the 2 nurseries, but at 19:00 hours, windows in the night and day nursery were covered by dark, lined curtains, lights were turned off and the only illumination was provided by a low‐intensity night light. The radio was turned off, and staff and visitors were urged to make as little noise as possible. Low light and noise intensity were maintained until 7:00 hours. The control nursery contained 6 cots, measured 23 × 13 feet (7 × 4 m), had a large exterior window and smaller internal windows without curtains and was lit by bright fluorescent strip lights that remained permanently on. No attempt was made to reduce noise from the radio, staff, parents or other visitors at any time |
|
| Outcomes | Number of hours spent awake in 24 hours; number of hours spent feeding in 24 hours; and weight on discharge, at expected date of birth, at 6 weeks' CA and at 12 weeks' (3 months') CA. Mean time spent asleep, awake or feeding was calculated from 48 hours of recordings | |
| Notes | All outcome results were presented in graphic form only and could not be entered into Review Manager (RevMan 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "...randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants enrolled |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appeared free of other bias |
Seiberth 1994.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes for ROP but not for other outcomes | |
| Participants | 169 infants (birth weight < 1500 g; PMA < 33 weeks) entered the study. 42 infants were excluded (see Risk of bias table ‐ incomplete outcome data addressed?) Setting: single centre, NICU at Women's Hospital of the University of Heidelberg, Heidelberg, Germany Study period: 1 January 1987 to 31 July 1991 |
|
| Interventions | CL group: 65 infants, mean (SD) birth weight 1091 g (233 g) and mean (SD) PMA 29 weeks (1.7 weeks), were assigned to CL. In the NICU in the CL group, reduced daylight was present during the day (mean (SD) illuminance, 342 lux (55 lux)). Light intensity was reduced during most of the night hours (mean (SD) illuminance, 62 lux (53 lux)). When stable, infants were transferred to the preterm unit (stepdown unit), where the amount of daylight allowed was reduced during the day (mean (SD) illuminance 415 lux (42 lux)), whereas at night, light was reduced to nearly complete darkness (mean (SD) illuminance, 26 lux (18 lux)). Thus, CL conditions were present on both units ND group: 62 infants, mean (SD) birth weight 1125 g (232 g) and mean (SD) PMA 29.3 weeks (2.1 weeks), were assigned to ND (patched eyes). Patches of black opaque plastic covered by cotton were placed over both eyes and were secured with adhesive tape to the temple on both sides. Light reduction achieved was > 99.9%. Eye patching was applied continuously from the first day after birth to 35 weeks' PMA |
|
| Outcomes | ROP all stages and ROP stage > 2, duration of ventilation therapy (days), duration of hospital stay (days) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "... each infant was randomly assigned to the near darkness or cycled lighting group." Randomisation was done separately for 3 birth‐weight groups: < 1000 g, 1000‐1249 g and 1250‐1500 g |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded. "Examination for ROP was done by a single observer who was unaware of the infants' group assignment at least at five and eight weeks and at three and three and six months post partum" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 169 preterm infants who entered the study, 42 were excluded because of death (6 infants in the ND group, 8 in the CL group); parents' withdrawal of informed consent (3 infants in the ND group, 0 in the CL group) or transfer to another hospital (14 infants in the ND group, 11 in the CL group). 127 infants remained in the study |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appeared free of other bias |
Miller 1995.
| Methods | Single‐centre, quasi‐randomised, controlled trial I. Blinding of randomisation ‐ no II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no, not for any of the outcomes of interest | |
| Participants | 41 infants < 37 weeks' PMA and birth weight < 2500 g Setting: single‐centre Notre Dame, IN, USA Study period: not stated |
|
| Interventions | CL group: 20 infants, mean (SD) birth weight 1151 g (360 g) and mean (SD) PMA 28.0 weeks (2.2) weeks Infants received a day‐night lighting pattern both in the NICU and in the continuing care room. During daytime (7:00‐18:00 hours), this group received 156‐201 lux, and during night‐time (18:00‐7:00 hours), they received 20‐32 lux CBL group: 21 infants, mean (SD) birth weight 1049 g (330 g) and mean (SD) PMA 28.0 weeks (2.1 weeks). Infants received 176‐232 lux during daytime and 206‐274 lux during night‐time |
|
| Outcomes | Length of stay, days requiring supplemental oxygen, days on ventilator, days to first oral feeding, mean daily caloric intake, % weight gain per week, nursing behaviour | |
| Notes | We did not include results of the Brazelton Neonatal Behavioral Assessment Scale, as to our knowledge, it has not been validated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No random sequence created |
| Allocation concealment (selection bias) | High risk | Infants were assigned to CL or CBL, subject to availability of space and staff (quasi‐randomised trial) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all infants assigned |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appears free of other bias |
Boo N‐Y 2002.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no | |
| Participants | Preterm infants < 37 weeks, weight < 2001 g, who were hospitalised in the NICU for at least 7 days and still were not ready for discharge at the time of recruitment Setting: single‐centre study at the Hospital Universiti Kebangsaan, Malaysia Study period: 1 August 1998 to 31 July 1999 |
|
| Interventions | "Day‐and‐night" CL group: 50 infants, mean (SD) birth weight of 1482 g (236 g) and mean (SD) PMA 31.6 weeks (2.2 weeks), were assigned to the "day‐and‐night" CL group. Intensity of light was mean (SD) 78.4 lux (24.7 lux). Duration of the intervention from day 7 of life to discharge. Lights in the cubicles were switched on between 7:00 and 19:00 hours and were switched off between 19:00 and 7:00 hours "Continuously dimmed environment" group: 46 infants, mean (SD) birth weight of 1465 g (280 g), were assigned to the "continuously dimmed environment" group. Intensity of light was mean (SD) 5.9 lux (1.9 lux). Lights in the cubicles were switched off throughout both day and night. Lights were switched on temporarily only during physical examination, treatment procedures and nursing care |
|
| Outcomes | Primary outcome measures were mean age of infants when they regained their birth weight and amount of weight gained by day 14 of life. Secondary outcomes included duration of hospital stay, infant's weight on discharge and age when enteral feeds were introduced | |
| Notes | Although light intensity during daytime in the CL group was lower than in other studies, we chose to include this study, as we were unable to clearly define the CL intervention a priori. Outcomes reported as median and range (interquartile range) were converted to mean and SD using the formulas proposed by Hozo and co‐workers (Hozo 2005) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes, which were opened in order |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported on all randomised infants. No study infant died after recruitment into the study |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appeared free of other bias |
Brandon 2002.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no | |
| Participants | 62 infants born at < 31 weeks' PMA Setting: intensive care and transitional care nursery of Duke University Medical Center, and Level II special care nursery of Durham Regional Hospital, Durham, NC, USA Study period: May 1998 to July 1999 |
|
| Interventions | Neonates, mean (SD) PMA 27.1 weeks (2.0 weeks), mean (SD) birth weight 1000 g (223 g) across the 3 intervention groups, were assigned randomly to 1 of 3 light intervention groups: (1) CL from birth, (2) CL at 32 weeks' PMA and (3) CL at 36 weeks' PMA in transition for discharge home (ND) CL from birth and CL at 32 weeks' PMA groups: 22 infants were assigned to CL from birth, and 19 infants were cared for in ND until 32 weeks' PMA, when they were cared for in CL. CL was provided in an 11‐hours‐on, 11‐hours‐off pattern, with 1 transition hour at change of shifts. The incubator cover was folded on top of the incubator, or the bassinet cover was off, to achieve daylight at 200‐225 lux between 7:30 and 18:30 hours. Light was provided with Philips Cool White fluorescent lamps (Philips, Somerset, NJ, USA), measured as illuminance Each lamp emitted 5.5% as ultraviolet A light and 94.5% as visible light. Filters over the lamps filtered out ultraviolet A light, so only visible light reached the infant ND group: 21 infants were assigned to the ND group. ND (5‐10 lux) was provided by using protective devices during daytime and by dimming the room light or using protective devices at night‐time. Infants receiving ND were exposed to 5‐10 lux light throughout the day, except during 6:30‐7:30 hours and 18:30‐19:30 hours, when lighting levels varied according to change of shift nursing care needs. These transition hours were applied to all groups |
|
| Outcomes | Mean weekly weight gain, total number of ventilation days, days in supplemental oxygen, hospital stay (days), calories/kg/d, days of life to start feeds, days of life to full feeds, brainstem auditory‐evoked response close to discharge from hospital (not included by us as an outcome), neurobehavioural organisation at 32 and 36 weeks' PMA, ROP | |
| Notes | For analyses, the group that received CL from birth and the group that received CL from 32 weeks' PMA were reported as exposed to CL (we report the data from these 2 CL groups separately). The ND group was exposed to CL at 36 weeks' PMA. We report data for this group as the ND group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | "Neonates were assigned randomly to one of 3 light intervention groups..." Each set of multiple births assigned to the same intervention group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appeared free of other bias |
Mirmiran 2003.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes. All data were scored by an experienced infant sleep researcher, who was masked to experimental condition of the infant | |
| Participants | Preterm infants admitted to the NICU Setting: single‐centre, Lucile Packard Children's Hospital at Stanford, Palo Alto, CA, USA Study period: not stated |
|
| Interventions | CL group: 19 infants, mean (SD) PMA 30.7 weeks (1.3 weeks), birth weight range 962‐1817 g, were allocated to the 'cycled' group and were cared for in a day‐night lighted room. Their incubator/bassinet was covered from 19:00 hours to 7:00 hours with a nearly opaque blanket, except during feeding or other interventions provided by parents and caregivers. The blanket was removed from 7:00 hours to 19:00 hours, at which time room lighting was turned up to standard lighting levels (approximately 300 lux) to produce a regular light‐dark cycled condition. Number of days of intervention was mean (SD) 35 days (25 days) DL group: 21 infants, mean (SD) PMA 29.8 weeks (1.7 weeks), birth weight range 751‐2280 g, were allocated to DL group and were cared for in a dimly lit room (< 20 lux) 24 hours a day. Their incubator/bassinet was covered with a nearly opaque blanket, except during feeding or other interventions provided by parents and caregivers. Number of days of intervention was mean (SD) 39 days (17 days) No attempt was made to modify the light‐dark cycle at home |
|
| Outcomes | Weight at 35 weeks' and 4 months' PMA. Body temperature was recorded continuously for up to 3 days at 36 weeks' PMA, as well as at home at 1 and 3 months' CA, using a digital ambulatory recorder. Sleep was recorded at the same times using 24‐hour time‐lapse video recordings in conjunction with rectal temperature recordings. This provided non‐invasive monitoring of sleep. Infrared and low light level cameras allowed for recording of the infant even during dark periods. Results for body temperature amplitude, active sleep time during the 12 hours of night‐time and quiet sleep during the 12 hours of night‐time were presented in graph form only, and data could not be entered into Review Manager (RevMan 2014) | |
| Notes | PAS abstract of the same study from 2001 indicates that 19 infants were assigned to the CL group and 20 to the DL group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "...randomly assigned to one of two groups" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors successfully recorded 19 infants in the CL group and 21 in the DL group. Study authors do not state how many infants were enrolled. See note above about different numbers of participants enrolled in the trial as reported in an abstract in 2001 |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appeared free of other bias |
Rivkees 2004.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ yes | |
| Participants | 62 infants < 32 weeks' PMA who were medically stable in NICU rooms were randomly assigned at 32‐34 weeks' PMA to CL or continuous DL Setting: single‐centre, Department of Pediatrics, Yale School of Medicine, New Haven, CT, USA Study period: not stated |
|
| Interventions | CL group: 29 infants, mean (SD) PMA 28.5 weeks (0.5 weeks) and mean (SD) birth weight 1072 g (62 g), were assigned to CL of mean (SD) 239 lux (29 lux), from 7:00 to 19:00 hours; < 25 lux from 19:00 to 7:00 hours for 25 days DL group: 33 infants, mean (SD) PMA 28.5 weeks (0.4 weeks) and mean (SD) birth weight 1110 g (64 g), were assigned to DL of mean (SD) 28.5 lux (3 lux) from 7:00 to 19:00 hours; 15 lux (5 lux) from 19:00 to 7:00 hours for 24 days Activity (using acti‐watches placed on 1 ankle) was continuously monitored from enrolment until approximately 1 month after discharge from the hospital Weight and head circumference were assessed up to 6 months after discharge from the hospital |
|
| Outcomes | Total number of movements per day in 10‐day intervals (10‐0 days before discharge; 1‐10 days after discharge; 11‐20 days after discharge; 21‐30 days after discharge). Ratios of day‐night activity (10‐0 days before discharge; 1‐10 days after discharge; 11‐20 days after discharge; 21‐30 days after discharge). Period analysis for circadian rhythms over the first 10 days at home Abstract stated that weight and head circumference were assessed up to 6 months after discharge from hospital. Those results are not presented in the main text |
|
| Notes | The number of infants enrolled in the 2 groups differs between the 'Table of patient characteristics' and the abstract. The numbers are switched. We used the numbers as presented in the main text. We have written to the first study author to clarify this difference, but we have not received an answer as of 10 March 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | High risk | One individual randomly assigned infants to control or experimental group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intervention could not be blinded. Investigators who analysed and interpreted data were blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 20 days after discharge activity, data were available for 24 experimental and 27 control infants. Total 51 infants At 30 days after discharge activity, data were available for 15 experimental and 14 control infants. Total 29 infants In 11 infants, activity data were not available through 20 days after discharge from the hospital as a result of premature removal of acti‐watches or mechanical failure |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered in a trials registry; therefore, we were unable to ascertain whether deviations from the protocol had occurred |
| Other bias | Low risk | Appears free of other bias |
Guyer 2012.
| Methods | Single‐centre, randomised, controlled trial I. Blinding of randomisation ‐ yes, table of random numbers II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no |
|
| Participants | Very preterm infants (≤ 32 0/7 weeks' PMA) Exclusion criteria: major cerebral injuries such as intraventricular haemorrhage grade III, periventricular leukomalacia or venous infarction ROP grade III and IV, congenital malformations, small for gestational age (birth weight less than third percentile), antenatal infection or intrauterine drug exposure Of 62 possibly eligible infants, 21 parents refused consent for study participation, mainly because the study was too time‐consuming. 41 infants were enrolled (22 boys, 19 girls). 4 additional infants had to be excluded from further analysis (dropped out after the first recording (n = 2), incomplete diary (n = 1) or unexplained high amount of parent‐reported "un‐soothe‐able crying" as an outlier (n = 1)). A total of 37 preterm infants (DL: n = 20; CL: n = 17) were included in the final analysis Enrolment took place after transfer from intensive to intermediate care at a mean age of 32.16 ± 1.35 weeks' PMA Setting: Clinic for Neonatology of the University Hospital, Zurich, Switzerland Study period: not stated |
|
| Interventions | 17 infants, mean (SD) PMA 30.6 weeks (0.95 weeks) and mean (SD) birth weight 1439 g (299 g), received CL mean (SD) 499.3 lux (159.2 lux), from 7:00 to 19:00 hours; and 28.5 lux (27.5 lux) from 19:00 to 7:00 hours for mean (SD) 30.8 days (11.2 days) 20 infants, mean (SD) PMA 29.5 weeks (2.1 weeks) and mean (SD) birth weight 1284 g (346 g), received DL mean (SD) 97.6 lux (45.3 lux) during the day and 20.8 lux (20.7 lux) at night for mean (SD) 34.6 days (16.3 days) |
|
| Outcomes | Of the vast number of outcomes reported by study authors, we chose to include daily weight gain (grams/d) during neonatal care, sleep (hours/24 h) at 5 and 11 weeks' CA, activity count per 24 hours at 5 and 11 weeks' CA, wake and content (hours/24 h) at 5 and 11 weeks' CA and crying (hours/24 h) at 5 and 11 weeks' CA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | 2 rooms in the intermediate care ward were equipped for each lighting condition, and randomisation was performed according to availability of free space at the time of transfer to intermediate care. In case of available space in both conditions, a table of random numbers was used. No information provided on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomised infants accounted for, but MDI and PDI reported only for 15 infants in the CL and 16 in the DL group |
| Selective reporting (reporting bias) | Unclear risk | This trial has been registered at www.clinicaltrials.gov (identifier NCT01513226). Registered on 11 January 2012, but only after enrolment had been completed September 2008 |
| Other bias | Low risk | Appears free of other bias |
Vásquez‐Ruiz 2014.
| Methods | Single‐centre, quasi‐randomised, controlled trial Setting: NICU of a Mexican public hospital (Hospital Juárez de Mexico). Study period not stated I. Blinding of randomisation ‐ no II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no |
|
| Participants | 38 preterm infants 28‐36.3 weeks PMA | |
| Interventions | Constant light vs light/dark cycle. 19 infants in each group | |
| Outcomes | Length of stay only outcome we could include. Discharge age, discharge weight not included as outcomes in our review | |
| Notes | Notes: 1. In the Methods section, study authors explain: "All infants were kept in individual incubators" in the photos, the infants are in warmers. Were the photos taken in warmers to more clearly show the acrylic helmets? Notes 2. We noted some discrepancies in the data: "In all cases, infants received oral feeding" – however, preterm infants born at 28 weeks' GA cannot be orally fed Notes 3. Study authors stated that nurses were blinded to treatment: "nursed every 3 h by 3 nurses blinded to the infants assigned group"; however, the intervention cannot be blinded, as infants in the study group were covered with acrylic helmets |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A random sequence was not generated |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. Infants were assigned by sequential randomisation method, by which the first infant was assigned to control constant light group (n = 19), the second to the experimental light‐dark cycle group (n = 19) and so on |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intervention could not be blinded Statements: "Two investigators, who were blinded to the patients’ conditions analyzed and interpreted the data" and "Infants were cleaned and nursed every 3 h by 3 nurses blinded to the infants assigned group" – cannot be true |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants, except for melatonin in the saliva, which was reported in 8 infants |
| Selective reporting (reporting bias) | Unclear risk | Study was not entered in an RCT registry, so we cannot judge whether deviations from the protocol occurred |
| Other bias | Low risk | Appears free of other bias |
CA: corrected age; CBL: continuous bright light; CL: cycled light; h: hour; NDI: mental development index; ND: near darkness; NICU: neonatal intensive care unit; PDI: physical development index; PMA: postmenstrual age; RCT: randomised controlled trial; ROP: retinopathy of prematurity; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aita 2012 | Intervention was applied for only 4 hours and included both light and noise reduction. Did not report on any of our outcomes of interest |
| Blackburn 1991 | Not a randomised controlled trial |
| Braz 2006 | Randomised controlled trial that compared ocular protection against ambient light in both eyes. Infants in the control group did not receive ocular protection and were kept under regular light conditions |
| Hoogeveen 2004 | Cohort study with 68 infants cared for in a cycled light room and 41 cared for in a dimmed light room |
| Kennedy 2001 | In this randomised controlled trial, goggles that reduced visible light by 97% were placed on the infant within 24 hours of birth and remained in use until 31 weeks' postmenstrual age, or for a minimum of 4 weeks. Control group was cared for in the standard lighting environment for each participating nursery. No group was exposed to cycled lighting |
| Park 2015 | Chart review was conducted for 94 extremely preterm infants who participated in a larger longitudinal randomised study (Brandon DH, et al. Timing for introduction of cycled light in NICU. Under review) |
Characteristics of studies awaiting assessment [ordered by study ID]
Jung 2005.
| Methods | Single centre, randomised, controlled trial I. Blinding of randomisation ‐ cannot determine II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement(s) ‐ no |
| Participants | 20 low birth weight infants Setting: 2 neonatal intensive care units in Seoul, South Korea Study period: not stated |
| Interventions | 10 infants received cycled light for 10 days 10 infants served as a control group |
| Outcomes | Weight, oxygen saturation, heart rate |
| Notes | Study was available in abstract form only. Full paper is written in Korean. We have written to the study author to ask for additional information and, if possible, translation of the full paper to English. As of 21 March 2016, we have not received an answer |
Kennaway 1996.
| Methods | See notes |
| Participants | See notes |
| Interventions | See notes |
| Outcomes | See notes |
| Notes | We have been in contact with Dr Kennaway to request to which interventions infants were randomised. We are awaiting further information so we can assess this study for inclusion |
Characteristics of ongoing studies [ordered by study ID]
Aita 2014.
| Trial name or title | Reducing NICU light and noise during kangaroo mother care: feasibility, acceptability and estimated effects on preterm infants' and mothers' outcomes |
| Methods | Randomised controlled trial |
| Participants | 30 NICU mother‐infant dyads born between 28 and 32 weeks' PMA |
| Interventions | Experimental group (KMC + NICU light and noise reduction) or control group (KMC only) |
| Outcomes | Infants' physiological stability was assessed by heart and respiratory rates and oxygen saturation, and quiet sleep was evaluated via videotaping. Maternal anxiety was assessed by the State‐Trait Anxiety Inventory Scale, and cortisol levels by saliva collection and analysis |
| Starting date | Study was ongoing in 2014 |
| Contact information | M. Aita, Faculty of Nursing, Universite de Montreal, Montreal, Canada |
| Notes |
NCT02146287.
| Trial name or title | Preterm infants: light effects on health and development |
| Methods | Randomised controlled trial |
| Participants | Infants < 7 days of age and born at 28 weeks or at < 28 weeks |
| Interventions | Cycled light was provided in an 11‐hours‐on, 11‐hours‐off pattern. Daylight (240‐700 lux) was provided with the incubator cover folded on top of the incubator, allowing light in from 4 sides, or with the bassinet cover off during day hours (07:30‐18:30). With the daylight range of 240‐700 lux and limited access to natural light, excessive daylight was prevented Continuous near darkness was provided as 5‐30 lux throughout the day, except from 06:30‐07:30 and 18:30‐19:30 hours, when lighting levels varied according to nursing care needs at change of shift. Near darkness (5‐30 lux) was provided by using incubator (totally covered or with the front flap back) and bassinet covers, and by dimming individual bedside light during day (07:30‐18:30) and night hours (19:30‐06:30)
|
| Outcomes |
Primary outcome measures: Infant weight gain trajectory [time frame: weekly inpatient up to 52 weeks' postmenstrual age (PMA) and outpatient visits to 18 months] [designated as safety issue: no]. This measure was single‐blinded
Change in sleep development during hospitalisations [time frame: every 3 weeks up to 52 weeks' PMA] [designated as safety issue: no] Change in developmental pattern of 4 sleep‐wake states (active, quiet, transition, awake) were evaluated during hospitalisation Change in sleep development after discharge home [time frame: every 5 months following hospital discharge up to 24 months' PMA] [designated as safety issue: no] Change in development of sleep and wake bouts evaluated following hospital discharge until infant reached 24 months' PMA Mental development [time frame: 9 months PMA] [designated as safety issue: no] Mental development measured using the Bayley Scales of Infant Development Psychomotor development [time frame: 9 months' PMA] [designated as safety issue: no] Psychomotor development measured using Bayley Scales of Infant Development Mental development [time frame: 18 months' PMA] [designated as safety issue: no] Mental development measured using Bayley Scales of Infant Development Psychomotor development [time frame: 18 months' PMA] [designated as safety issue: no] Psychomotor development measured using Bayley Scales of Infant Development Secondary outcome measures: length of hospitalisation in days [time frame: at hospital discharge from 0 to 222 days] [designated as safety issue: no] Length of hospitalisations from birth until discharge home Severity of retinopathy of prematurity (ROP) [time frame: up to 52 weeks] [designated as safety issue: no] ROP change over time and degree of severity assessed until 24 months' PMA Visual acuity [time frame: measured at 12 months' PMA] [designated as safety issue: no] Neurological development [time frame: 9 months' PMA] [designated as safety issue: no] Neurological development assessed by neurological examination as normal, suspect or abnormal, and presence or absence of cerebral palsy Brainstorm auditory evoked potentials [time frame: 6 months] [designated as safety issue: yes] Neurological development [time frame: 18 months' PMA] [designated as safety issue: no] Neurological development assessed by neurological examination as normal, suspect or abnormal, and presence or absence of cerebral palsy Change in retinopathy of prematurity (ROP) [time frame: every 2 weeks during hospitalisations after 30 weeks' PMA up to 52 weeks' PMA, and during outpatient visits up to 24 months' PMA] [designated as safety issue: no] ROP change over time and degree of severity assessed until 24 months' PMA |
| Starting date | June 2003 |
| Contact information | Debra H Brandon, PhD, Duke University School of Nursing; debra.brandon@duke.edu |
| Notes | ClinicalTrials.gov identifier: NCT02146287 Several abstracts based on this study have been published. Brandon DH, Ryan D, Barnes A. The effects of cycled light on short term health outcomes. Southern Nurses Research Society Annual Conference: Health Disparities‐Evidence into Action Conference Proceedings; February 2008; Birmingham, Alabama. Brandon D, Goldstein R, Malcolm W, Ryan D, Barnes A. Long‐term effects of day night cycled light on preterm infants' neurodevelopmental outcomes. International Conference of Infant Studies Conference Proceedings; 2010; Baltimore, Maryland. Brandon DH, Holditch‐Davis. Sleep development of ELBW infants following hospital discharge. 2012 State of the Science Congress in Nursing Research; September 2012; Washington, DC. None of the data presented in the abstracts could be incorporated into RevMan 2014. We are awaiting the full publication of this study |
Sanadgol 2013.
| Trial name or title | Creating an artificial night on physiological changes in preterm infants |
| Methods | Randomised controlled trial |
| Participants | 38 preterm infants (PMA 30‐34 weeks) hospitalised at Ghaem NICU, Iran, were evaluated within 10 days |
| Interventions | Infants were divided into 2 groups of 1200‐1700 and 1701‐2200 g on the basis of weight, and the weight of each group was randomised into artificial night (dark period from 19:00 to 7:00, during which the incubator was covered with linen cloth and light period was from 7:00 to 19:00 with the cover removed) and a control group (continuous lighting) |
| Outcomes | Physiologica; changes twice a day, weight & feeding tolerance collected daily |
| Starting date | April 2012 |
| Contact information | Vajihe Sanadgol, Principal Investigator, Mashhad University of Medical Sciences, Iran |
| Notes | NCT01833091 ‐ The study has been completed (completion date October 2012) |
KMC: kangaroo mother care; NICU: neonatal intensive care unit; PMA: postmenstrual age; ROP: retinopathy of prematurity
Differences between protocol and review
We reported several outcomes that were not predefined, including some physiological outcomes.
For this update in 2016, we added the methods and plan for Summary of findings tables and GRADE recommendations, which were not included in the original protocol.
Contributions of authors
Iris Morag and Arne Ohlsson contributed to all sections of the protocol, the full review and subsequent updates.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Canada.
Sheba Medical Center, Israel.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Iris Morage ‐ none.
Arne Ohlsson ‐ none.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Boo N‐Y 2002 {published data only}
- Boo NY, Chee SC, Rohana J. Randomized controlled study of the effects of different durations of light exposure on weight gain by preterm infants in a neonatal intensive care unit. Acta Pædiatrica 2002;91(6):674‐9. [DOI] [PubMed] [Google Scholar]
Brandon 2002 {published data only}
- Brandon DH, Holditch‐Davis D, Belyea M. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. Journal of Pediatrics 2002;140(2):192‐9. [DOI] [PubMed] [Google Scholar]
- Brandon DH, Holditch‐Davis D, Belyea M, Tivnan DM. Factors affecting early neurobehavioral and sleep outcomes in preterm infants. E‐PAS: http://www.abstracts2view.com/pasall/. Proceedings of the Pediatric Academic Societies Meeting; 2003 May 3 ‐ 6; Seatle (WA). 2003:2004.
- Brandon DH, Holditch‐Davis D, Winchester DM. Factors affecting early neurobehavioral and sleep outcomes in preterm infants. Infant Behavior and Development 2005;28(2):206‐19. [Google Scholar]
Guyer 2012 {published data only}
- Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, et al. Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics 2012;130(1):e145‐51. [DOI] [PubMed] [Google Scholar]
Mann 1986 {published data only}
- Mann NP, Haddow R, Stokes L, Goodley S, Rutter N. Effect of night and day on preterm infants in a newborn nursery: randomised trial. British Medical Journal (Clinical Research Ed.) 1986;293(6557):1265‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1995 {published data only}
- Miller CL, White R, Whitman TL, O'Callaghan MF, Maxwell SE. The effects of cycled versus noncycled lighting on growth and development in preterm infants. Infant Behavior and Development 1995;18(1):87‐95. [Google Scholar]
Mirmiran 2003 {published data only}
- Mirmiran M, Baldwin RB, Ariagno RL. Circadian and sleep development in preterm infants occurs independently from the influences of environmental lighting. Pediatric Research 2003;53(6):933‐8. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Pedu VD, Baldwin RB, Saporito AG, Ariagno RL. Cycled light in the intermediate nursery: effects on development of preterm infants. E‐PAS: http://www.abstracts2view.com/pasall/. Proceedings of the Pediatric Academic Societies Meeting; 2001 April 28 ‐ May 1; Baltimore (MD). 2001:2045.
- Peddu V, Mirmiran M, Boeddiker M, Baldwin RB, Ariagno RL. Effect of light‐dark (L/D) cycle on development of circadian rhythms in preterm infants. E‐PAS: http://www.abstracts2view.com/pasall/. Proceedings of the Pediatric Academic Societies Meeting; 2000 May 12 ‐ 16; Boston, MA. 2000:2507.
Rivkees 2004 {published data only}
- Rivkees SA, Mayes L, Jacobs H, Gross I. Rest‐activity patterns of premature infants are regulated by cycled lighting. Pediatrics 2004;113(4):833‐9. [DOI] [PubMed] [Google Scholar]
Seiberth 1994 {published data only}
- Seiberth V, Linderkamp O, Knorz MC, Liesenhof H. A controlled clinical trial of light and retinopathy of prematurity. American Journal of Ophthalmology 1994;118(4):492‐5. [DOI] [PubMed] [Google Scholar]
Vásquez‐Ruiz 2014 {published data only}
- Vásquez‐Ruiz S, Maya‐Barrios JA, Torres‐Narváez P, Vega‐Martínez BR, Rojas‐Granados A, Escobar C, et al. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Human Development 2014;90(9):535‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aita 2012 {published data only}
- Aita M, Johnston C, Goulet C, Oberlander TF, Snider L. Intervention minimizing preterm infants’ exposure to NICU light and noise. Clinical Nursing Research 2012;22(3):337–58. [DOI] [PubMed] [Google Scholar]
Blackburn 1991 {published data only}
- Blackburn S, Patteson D. Effects of cycled light on activity state and cardiorespiratory function in preterm infants. Journal of Perinatal & Neonatal Nursing 1991;4(4):47‐54. [DOI] [PubMed] [Google Scholar]
Braz 2006 {published data only}
- Braz RRT, Moreira MEL, Carvalho M, Lopes JM, Rodrigues MA, Cabral JA, et al. Effect of light reduction on the incidence of retinopathy of prematurity. Archives of Disease in Childhood Fetal and Neonatal Edition 2006;91(6):F443‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoogeveen 2004 {published data only}
- Hoogeveen E, Mirmiran M, Ariagno RL. Improved growth at four months in preterm infants receiving cycled light during newborn intermediate intensive care. E‐PAS: http://www.abstracts2view.com/pasall/. Proceedings of the Pediatric Academic Societies Meeting; 2004 May 1 ‐ 4; San Francisco, CA. 2004:2503.
Kennedy 2001 {published data only}
- Kennedy KA, Fielder AR, Hardy RJ, Tung B, Gordon DC, Reynolds JD, et al. Reduced lighting does not improve medical outcomes in very low birth weight infants. Journal of Pediatrics 2001;139(4):527‐31. [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park J, Knafl G, Thoyre S, Brandon D. Factors associated with feeding progression in extremely preterm infants. Nursing Research 2015;64(3):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jung 2005 {published data only}
- Jung IS. Effects of cycled lighting on body weight, physiological variables and behavioral states in low birth weight infants. Taehan Kanho Hakhoe Chi 2005;35(1):143‐53. [DOI] [PubMed] [Google Scholar]
Kennaway 1996 {published data only}
- Kennaway DJ, Goble FC, Stamp GE. Factors influencing the development of melatonin rhythmicity in humans. Journal of Clinical Endocrinology and Metabolism 1996;81(4):1525‐32. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Aita 2014 {published data only}
- Aita M, Stremler R, Feeley N, Barrington K, Nuyt AM. Reducing NICU light and noise during kangaroo mother care: feasibility, acceptability and estimated effects on preterm infants' and mothers' outcomes. Pediatric Critical Care Medicine. Istanbul, Turkey: Conference: 7th World Congress on Pediatric Intensive and Critical Care, PICC, 2014.
NCT02146287 {published data only}
- Brandon DH. Preterm infants: light effects on health and development. ClinicalTrials.gov 2014.
Sanadgol 2013 {published data only}
- Sanadgol V. Creating an artificial night on physiological changes in preterm infants. Version ClinicalTrials.gov Identifier: NCT01833091. ClinicalTrials.gov, 2013.
Additional references
Arduini 1987
- Arduini D, Rizzo G, Parlati E, Dell'Acqua S, Romanini C, Mancuso S. Loss of circadian rhythms of fetal behaviour in a totally adrenalectomized pregnant woman. Gynecologic and Obstetric Investigation 1987;23(4):226‐9. [DOI] [PubMed] [Google Scholar]
Begum 2006
- Begum EA, Bonno M, Obata M, Yamamoto H, Kawai M, Komada Y. Emergence of physiological rhythmicity in term and preterm neonates in a neonatal intensive care unit. Journal of Circadian Rhythms 2006;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackburn 1998
- Blackburn S. Environmental impact of the NICU on developmental outcomes. Journal of Pediatric Nursing 1998;13(5):279‐89. [DOI] [PubMed] [Google Scholar]
Christina 2004
- Christina A, Merlin N, Vijaya C, Jayaprakash S, Murugesh N. Daily rhythm of nociception in rats. Journal of Circadian Rhythms 2004;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engwall 2014
- Engwall M, Fridh I, Bergbom I, Lindahl B. Let there be light and darkness: findings from a prestudy concerning cycled light in the intensive care unit environment. Critical Care Nursing Quarterly 2014;37(3):273‐98. [DOI] [PubMed] [Google Scholar]
Glotzbach 1995
- Glotzbach SF, Edgar DM, Ariagno RL. Biological rhythmicity in preterm infants prior to discharge from neonatal intensive care. Pediatrics 1995;95(2):231‐7. [PubMed] [Google Scholar]
Gottfried 1985
- Gottfried A. Environmental neonatology. Infant Stress Under Intensive Care. Baltimore: University Park Press, 1985:252‐7. [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University, The Cochrane Collaboration. GRADEpro. Version 20150131. Hamilton: McMaster University, The Cochrane Collaboration, 2014.
Halberg 1969
- Halberg F. Chronobiology. Annual Review of Physiology 1969;31:675‐725. [DOI] [PubMed] [Google Scholar]
Halberg 2003
- Halberg F, Cornélissen G, Katinas G, Syutkina EV, Sothern RB, Zaslavskaya R, et al. Transdisciplinary unifying implications of circadian findings in the 1950's. Journal of Circadian Rhythms 2003;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hao 1999
- Hao HP, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proceedings of the National Academy of Sciences of the United States of America 1999;96(5):2426‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BioMed Central Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2002
- Jacobs SE, Sokol J, Ohlsson A. The Newborn Individualized Developmental Care and Assessment Program is not supported by meta‐analyses of the data. Journal of Pediatrics 2002;140(6):699‐706. [DOI] [PubMed] [Google Scholar]
Keener 1988
- Keener MA, Zeanah CH, Andreas TF. Infant temperament, sleep organization, and night time parental interventions. Pediatrics 1988;81(6):762‐71. [PubMed] [Google Scholar]
Kennaway 1992
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. Journal of Clinical Endocrinology and Metabolism 1992;75(2):367‐9. [DOI] [PubMed] [Google Scholar]
Kintraia 2005
- Kintraia PI, Zarnadze MG, Kintraia NP, Kashakashvili IG. Development of daily rhythmicity in heart rate and locomotor activity in the human fetus. Journal of Circadian Rhythms 2005;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lotas 1992
- Lotas MJ. Effects of light and sound in the neonatal intensive care unit environment on the low‐birth‐weight infant. NAACOGs' Clinical Issues in Perinatal and Women's Health Nursing 1992;3(1):34‐44. [PubMed] [Google Scholar]
Maguire 2008
- Maguire CM, Veen S, Sprij AJ, Cessie S, Wit JM, Walther FJ, Leiden Developmental Care Project. Effects of basic developmental care on neonatal morbidity, neuromotor development, and growth at term age of infants who were born at <32 weeks. Pediatrics 2008;121(2):e239‐45. [DOI] [PubMed] [Google Scholar]
Maguire 2009
- Maguire CM, Walther FJ, Zwieten PH, Cessie S, Wit JM, Veen S, Leiden Developmental Care Project. No change in developmental outcome with incubator covers and nesting for very preterm infants in a randomised controlled trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2009;94:F92‐7. [DOI] [PubMed] [Google Scholar]
McGraw 1999
- McGraw K, Hoffmann R, Harker C, Herman JH. The development of circadian rhythms in a human infant. Sleep 1999;22(3):303‐10. [DOI] [PubMed] [Google Scholar]
Mirmiran 1990
- Mirmiran M, Kok JH, Kleine MJ, Koppe JG, Overdijk J, Witting W. Circadian rhythms in preterm infants: a preliminary study. Early Human Development 1990;23(2):139‐46. [DOI] [PubMed] [Google Scholar]
Mirmiran 1996
- Mirmiran M, Lunshof S. Perinatal development of human circadian rhythms. Progress in Brain Research 1996;111:217‐26. [DOI] [PubMed] [Google Scholar]
Mirmiran 2000
- Mirmiran M, Ariagno RL. Influence of light in the NICU on the development of circadian rhythms in preterm infants. Seminars in Perinatology 2000;24(4):247‐57. [DOI] [PubMed] [Google Scholar]
Ohlsson 2007
- Ohlsson A, Jacob S. Meta‐regression can indicate if further NIDCAP studies are justified [Metaregression kan visa om fler NIDCAP‐studier är motiverade]. Läkartidningen 2007;104(3):134‐7. [PubMed] [Google Scholar]
Ohlsson 2009
- Ohlsson A. NIDCAP: new controversial evidence for its effectiveness. Pediatrics 2009;124(4):1213‐5. [DOI] [PubMed] [Google Scholar]
Ohlsson 2013
- Ohlsson A, Jacobs SE. NIDCAP: a systematic review and meta‐analyses of randomized controlled trials. Pediatrics 2013;131(3):e881‐93. [DOI] [PubMed] [Google Scholar]
Paranjpe 2005
- Paranjpe DA, Sharma VK. Evolution of temporal order in living organisms. Journal of Circadian Rhythms 2005;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patrick 1982
- Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross fetal body movements over 24‐hour observation intervals during the last 10 weeks of pregnancy. American Journal of Obstetetrics and Gynecology 1982;142(4):363‐71. [DOI] [PubMed] [Google Scholar]
Phelps 2001
- Phelps D, Watts J. Early light reduction for preventing retinopathy of prematurity in very low birth weight infants. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000122] [DOI] [PubMed] [Google Scholar]
Rea 2008
- Rea MS, Bierman A, Figueiro MG, Bullough JD. A new approach to understanding the impact of circadian disruption on human health. Journal of Circadian Rhythms 2008;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rea 2010
- Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. Journal of Circadian Rhythms 2010;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Refinetti 2003
- Refinetti R. Journal of Circadian Rhythms: 21st‐century publishing for 21st century science. Journal of Circadian Rhythms 2003;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivkees 2000
- Rivkees SA, Halo H. Developing circadian rhythmicity. Seminars in Perinatology 2000;24:232‐42. [DOI] [PubMed] [Google Scholar]
Rivkees 2003
- Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics 2003;112(2):373‐81. [DOI] [PubMed] [Google Scholar]
Rivkees 2004a
- Rivkees SA. Emergence and influences of circadian rhythmicity in infants. Clinics in Perinatology 2004;31(2):217‐28. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook 2013.
Seron‐Ferre 2001
- Seron‐Ferre M, Torres‐Farfan C, Forcelledo ML, Valenzuela GJ. The development of circadian rhythms in the fetus and neonate. Seminars in Perinatology 2001;25(6):363‐70. [DOI] [PubMed] [Google Scholar]
Stetson 1986
- Stetson MH, Elliott JA, Goldman BD. Maternal transfer of photoperiodic information influences the photoperiodic responses of prepubertal Djungarian hamsters (Phodopus sungorus sungorus). Biology of Reproduction 1986;34(4):664‐9. [DOI] [PubMed] [Google Scholar]
Sukumaran 2010
- Sukumaran S, Almon RR, DuBois DC, Jusko WJ. Circadian rhythms in gene expression: relationship to physiology, disease, drug disposition and drug action. Advances Drug Delivery Reviews 2010;62(9‐10):904‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Symington 2006
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001814.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoman 1989a
- Thoman EB, Ingersoll EW. The human nature of the youngest human: prematurely born babies. Seminars in Perinatology 1989;13(6):482‐94. [PubMed] [Google Scholar]
Thoman 1989b
- Thoman EB, Whitney MP. Sleep states of infants monitored in the home: individual differences, developmental trends, and origin of diurnal cyclicity. Infant Behavior and Development 1989;12(1):59‐75. [Google Scholar]
White 2007
- White RD. Recommended Standards for Newborn ICU Design: Report of the Seventh Census Conference on Newborn ICU Design Committee to Establish Recommended Standards for Newborn ICU Design, 2007. www.nd.edu/˜nicudes/index.html (accessed 29 July 2013).
References to other published versions of this review
Morag 2011
- Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006982] [DOI] [PubMed] [Google Scholar]
Morag 2013
- Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006982.pub2] [DOI] [PubMed] [Google Scholar]


