Abstract
Background
As part of liver transplantation, immunosuppression (suppressing the host immunity) is given to prevent graft rejections resulting from the immune response of the body against transplanted organ or tissues from a different person whose tissue antigens are not compatible with those of the recipient. The optimal maintenance immunosuppressive regimen after liver transplantation remains uncertain.
Objectives
To assess the comparative benefits and harms of different maintenance immunosuppressive regimens in adults undergoing liver transplantation through a network meta‐analysis and to generate rankings of the different immunosuppressive regimens according to their safety and efficacy.
Search methods
We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and trials registers until October 2016 to identify randomised clinical trials on immunosuppression for liver transplantation.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or publication status) in adult participants undergoing liver transplantation (or liver retransplantation) for any reason. We excluded trials in which participants had undergone multivisceral transplantation or participants with established graft rejections. We considered any of the various maintenance immunosuppressive regimens compared with each other.
Data collection and analysis
We performed a network meta‐analysis with OpenBUGS using Bayesian methods and calculated the odds ratio, rate ratio, and hazard ratio (HR) with 95% credible intervals (CrI) based on an available‐case analysis, according to National Institute of Health and Care Excellence Decision Support Unit guidance.
Main results
We included a total of 26 trials (3842 participants) in the review, and 23 trials (3693 participants) were included in one or more outcomes in the review. The vast majority of the participants underwent primary liver transplantation. All of the trials were at high risk of bias, and all of the evidence was of low or very low quality. In addition, because of sparse data involving trials at high risk of bias, it is not possible to entirely rely on the results of the network meta‐analysis. The trials included mainly participants undergoing primary liver transplantation of varied aetiologies. The follow‐up in the trials ranged from 3 to 144 months. The most common maintenance immunosuppression used as a control was tacrolimus. There was no evidence of difference in mortality (21 trials; 3492 participants) or graft loss (15 trials; 2961 participants) at maximal follow‐up between the different maintenance immunosuppressive regimens based on the network meta‐analysis. In the direct comparison, based on a single trial including 222 participants, tacrolimus plus sirolimus had increased mortality (HR 2.76, 95% CrI 1.30 to 6.69) and graft loss (HR 2.34, 95% CrI 1.28 to 4.61) at maximal follow‐up compared with tacrolimus. There was no evidence of differences in the proportion of people with serious adverse events (1 trial; 719 participants), proportion of people with any adverse events (2 trials; 940 participants), renal impairment (8 trials; 2233 participants), chronic kidney disease (1 trial; 100 participants), graft rejections (any) (16 trials; 2726 participants), and graft rejections requiring treatment (5 trials; 1025 participants) between the different immunosuppressive regimens. The network meta‐analysis showed that the number of adverse events was lower with cyclosporine A than with many other immunosuppressive regimens (12 trials; 1748 participants), and the risk of retransplantation (13 trials; 1994 participants) was higher with cyclosporine A than with tacrolimus (HR 3.08, 95% CrI 1.13 to 9.90). None of the trials reported number of serious adverse events, health‐related quality of life, or costs.
Funding: 14 trials were funded by pharmaceutical companies who would benefit from the results of the trial; two trials were funded by parties who had no vested interest in the results of the trial; and 10 trials did not report the source of funding.
Authors' conclusions
Based on low‐quality evidence from a single small trial from direct comparison, tacrolimus plus sirolimus increases mortality and graft loss at maximal follow‐up compared with tacrolimus. Based on very low‐quality evidence from network meta‐analysis, we found no evidence of difference between different immunosuppressive regimens. We found very low‐quality evidence from network meta‐analysis and low‐quality evidence from direct comparison that cyclosporine A causes more retransplantation compared with tacrolimus. Future randomised clinical trials should be adequately powered; performed in people who are generally seen in the clinic rather than in highly selected participants; employ blinding; avoid postrandomisation dropouts or planned cross‐overs; and use clinically important outcomes such as mortality, graft loss, renal impairment, chronic kidney disease, and retransplantation. Such trials should use tacrolimus as one of the control groups. Moreover, such trials ought to be designed in such a way as to ensure low risk of bias and low risks of random errors.
Plain language summary
Medical interventions to prevent graft rejection after liver transplantation
Background
Liver transplantation is the main treatment option for people with severe advanced liver disease. When organs or tissues are transplanted from one person (organ donor) to another (organ recipient), the body of the organ recipient identifies the donor organ (or graft) as a foreign body and mounts a response against it in a way similar to the natural body defence mechanism against infections (immune response). This can lead to graft rejection and graft loss resulting in death of the organ recipient. Various medical interventions are used either alone or in combination (immunosuppressive regimen) to prevent graft rejections. The combination of interventions used in the first few months after liver transplantation (induction immunosuppressive regimen) often differs from the combination used for the rest of the patient's life (maintenance immunosuppression). It is unclear which immunosuppressive regimen after liver transplantation is the best. We sought to identify the best maintenance immunosuppressive regimen by searching for existing studies on the topic. We included all randomised clinical trials reported until October 2016. We included only trials of participants who had previously undergone liver transplantation. We excluded trials of participants who had undergone multi‐organ transplantation (e.g. liver and kidney transplantations) or participants with established graft rejections. Apart from using standard Cochrane methods, which allow comparison of only two interventions at a time (direct comparison), we also employed advanced methods that allow comparison of the many different interventions individually compared in the trials (network meta‐analysis).
Study characteristics
We identified 26 randomised clinical trials with a total of 3842 participants. Of these, 23 randomised clinical trials (3693 participants) provided information for one or more outcomes. The trials mainly included participants undergoing liver transplantation for the first time, for various reasons.
Funding: 14 trials were funded by pharmaceutical companies who would benefit from the results of the trial; two trials were funded by parties who had no vested interest in the results of the trial; and 10 trials did not report the source of funding.
Quality of evidence
The overall quality of the evidence was low or very low, and all of the trials were at high risk of bias, which means it is possible that the conclusions made could overestimate the benefits or underestimate the harms of a given intervention because of the way the trials were conducted. In addition, because of insufficient information, the results of network meta‐analysis are not entirely reliable.
Key results
Several medical drugs were compared in the trials. We found no evidence of difference in the risk of death or graft loss between the different immunosuppressive regimens based on the network meta‐analysis. In the direct comparison, based on a single trial including 222 participants, the risk of death and graft loss was higher with tacrolimus plus sirolimus than with tacrolimus alone. There was no evidence of differences between the various immunosuppressive regimens in percentage of people who developed serious adverse events, percentage of people who developed any adverse events, risk of poor kidney function requiring dialysis or kidney transplantation (kidney dysfunction), prolonged kidney disease, graft rejections requiring treatment, and any graft rejections. The number of adverse events was lower with cyclosporine A than with many other immunosuppressive regimens. The risk of retransplantation was higher with cyclosporine A than with tacrolimus. None of the trials reported number of serious adverse events, health‐related quality of life, or costs.
There is significant uncertainty as to the optimal maintenance immunosuppressive regimen after liver transplantation; further well‐designed randomised clinical trials are required. Future trials should be performed in people who are generally seen in the clinic rather than in highly selected participants and report clinically important outcomes such as death, graft loss, kidney dysfunction, long‐term kidney disease, and retransplantation. Such trials should use tacrolimus as one of the control groups. Moreover, such trials ought to be designed in such a way as to ensure low risk of bias and low risks of random errors.
Summary of findings
Summary of findings for the main comparison. Maintenance immunosuppressive regimens for adults undergoing liver transplantation: a network meta‐analysis.
| Maintenance immunosuppressive regimens for adults undergoing liver transplantation: a network meta‐analysis | |||||||||
|
Patient or population: people undergoing liver transplantation Settings: tertiary care Intervention: various interventions Comparison: tacrolimus Follow‐up period: 6 months to 144 months | |||||||||
| Interventions | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No. of participants (trials) | Quality of the evidence of network meta‐analysis (GRADE) | |||||
| Assumed risk | Corresponding risk | ||||||||
| Tacrolimus | Various interventions (based on direct comparison) | Various interventions (based on indirect comparison) | Various interventions (based on network meta‐analysis) | Direct comparison | Indirect comparison | Network meta‐analysis | |||
| Mortality at maximal follow‐up | |||||||||
| Cyclosporine A | 154 per 1000 | 170 per 1000 (86 to 361) | 157 per 1000 (16 to 2125) | 173 per 1000 (112 to 278) |
HR 1.10 (0.56 to 2.34) Quality of evidence: very low1,2,4,5 |
HR 1.02 (0.11 to 13.80) Quality of evidence: very low1,2,3,5 |
HR 1.12 (0.73 to 1.81) |
1176 (8 trials) | ⊕⊝⊝⊝ very low6 |
| Cyclosporine A plus azathioprine | 154 per 1000 | 202 per 1000 (8 to 4917) | 172 per 1000 (105 to 290) | 206 per 1000 (85 to 459) |
HR 1.31 (0.05 to 31.94) Quality of evidence: very low1,4,5 |
HR 1.11 (0.68 to 1.89) Quality of evidence: very low1,2,3,5 |
HR 1.34 (0.55 to 2.98) |
202 (2 trials) | ⊕⊝⊝⊝ very low6 |
| Cyclosporine A plus azathioprine plus glucocorticosteroids | 154 per 1000 | ‐ | 1673 per 1000 (53 to 183391) | 1447 per 1000 (44 to 365629) | ‐ |
HR 10.87 (0.34 to 1191.54) Quality of evidence: very low1,2,3,5 |
HR 9.40 (0.28 to 2375.59) |
No direct comparison | ⊕⊝⊝⊝ very low6 |
| Cyclosporine A plus glucocorticosteroids | 154 per 1000 | ‐ | 106 per 1000 (37 to 281) | 107 per 1000 (37 to 292) | ‐ |
HR 0.69 (0.24 to 1.82) Quality of evidence: very low1,2,3,5 |
HR 0.70 (0.24 to 1.90) |
No direct comparison | ⊕⊝⊝⊝ very low6 |
| Cyclosporine A plus mycophenolate plus glucocorticosteroids | 154 per 1000 | ‐ | 2256 per 1000 (37 to 306010) | 1695 per 1000 (24 to 496517) | ‐ |
HR 14.66 (0.24 to 1988.23) Quality of evidence: very low1,2,3,5 |
HR 11.01 (0.16 to 3226.01) |
No direct comparison | ⊕⊝⊝⊝ very low6 |
| Everolimus | 154 per 1000 | 250 per 1000 (113 to 615) | 306 per 1000 (61 to 1447) | 251 per 1000 (101 to 709) |
HR 1.62 (0.73 to 3.99) Quality of evidence: very low1,4,5 |
HR 1.99 (0.40 to 9.40) Quality of evidence: very low1,2,3,5 |
HR 1.63 (0.66 to 4.60) |
474 (1 trial) | ⊕⊝⊝⊝ very low6 |
| Tacrolimus plus azathioprine | 154 per 1000 | 70 per 1000 (26 to 177) | 257 per 1000 (106 to 654) | 70 per 1000 (18 to 257) |
HR 0.46 (0.17 to 1.15) Quality of evidence: very low1,4,5 |
HR 1.67 (0.69 to 4.25) Quality of evidence: very low1,2,3,5 |
HR 0.46 (0.12 to 1.67) |
97 (1 trial) | ⊕⊝⊝⊝ very low6 |
| Tacrolimus plus everolimus | 154 per 1000 | 220 per 1000 (96 to 520) | 267 per 1000 (65 to 1571) | 218 per 1000 (67 to 727) |
HR 1.43 (0.63 to 3.38) Quality of evidence: very low1,4,5 |
HR 1.73 (0.43 to 10.21) Quality of evidence: very low1,2,3,5 |
HR 1.41 (0.44 to 4.73) |
488 (1 trial) | ⊕⊝⊝⊝ very low6 |
| Tacrolimus plus glucocorticosteroids | 154 per 1000 | ‐ | 83 per 1000 (25 to 304) | 93 per 1000 (22 to 355) | ‐ |
HR 0.54 (0.16 to 1.98) Quality of evidence: very low1,2,3,5 |
HR 0.60 (0.14 to 2.30) |
No direct comparison | ⊕⊝⊝⊝ very low6 |
| Tacrolimus plus mycophenolate plus glucocorticosteroids | 154 per 1000 | 89 per 1000 (22 to 294) | 59 per 1000 (8 to 451) | 81 per 1000 (20 to 287) |
HR 0.58 (0.14 to 1.91) Quality of evidence: very low1,4,5 |
HR 0.38 (0.05 to 2.93) Quality of evidence: very low1,2,3,5 |
HR 0.53 (0.13 to 1.87) |
195 (1 trial) | ⊕⊝⊝⊝ very low6 |
| Tacrolimus plus sirolimus | 154 per 1000 | 425 per 1000 (200 to 1029) | 75 per 1000 (22 to 246) | 435 per 1000 (123 to 1472) |
HR 2.76 (1.30 to 6.69) Quality of evidence: low1,4 |
HR 0.49 (0.14 to 1.60) Quality of evidence: very low1,2,3,5 |
HR 2.82 (0.80 to 9.56) |
222 (1 trial) | ⊕⊕⊝⊝ low6 |
| Health‐related quality of life | |||||||||
| None of the trials reported this outcome. | |||||||||
| *The basis for the assumed risk is the mean control group proportion (15.4%). The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI) for different types of estimates. CrI: credible intervals; HR: hazard ratio | |||||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||||
1Risk of bias: trial(s) were at high risk of bias (downgraded by one level). 2Heterogeneity: there were differences in the effect estimates obtained by fixed‐effect model and random‐effects model (downgraded by one level). 3Indirectness: sparse network made up of trials at high risk of bias (downgraded one level). 4Imprecision: small sample size (sample size required to measure 20% relative risk reduction from 15.4% = 3950) (downgraded by one level). 5Imprecision: credible intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (20% relative risk increase or reduction, i.e. 3.1% absolute increase or decrease from 15.4% was considered clinically significant) (downgraded by one level). 6Overall quality of evidence in network meta‐analysis: best of direct and indirect comparisons.
Background
Description of the condition
The liver is a complex organ with multiple functions including carbohydrate metabolism, fat metabolism, protein metabolism, drug metabolism, synthetic functions, storage functions, digestive functions, excretory functions, and immunological functions (Read 1972). The liver can be affected by acute or chronic diseases. The main causes of chronic liver disease are alcohol abuse and viral infections such as viral hepatitis B and C (Dam Fialla 2012; Ratib 2014). Other causes include autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, haemochromatosis, alpha‐1 antitrypsin deficiency, non‐alcoholic steatohepatitis, and cryptogenic cirrhosis (cirrhosis of unknown cause) (Dam Fialla 2012; Ratib 2014).
Chronic liver disease caused 10,000 deaths in 2009 in the UK and 36,000 deaths in 2013 in the USA (Davies 2012; CDC 2015). While the age‐standardised mortality due to cirrhosis (advanced liver fibrosis) has decreased from 18.6 per 100,000 per year to 15.6 per 100,000 per year overall, the proportion of all deaths caused by cirrhosis is increasing in some countries such as the UK (Lozano 2012; Murray 2013). Cirrhosis has two phases, an asymptomatic 'compensated cirrhosis' phase and a 'decompensated cirrhosis' phase characterised by clinical manifestations such as upper gastrointestinal bleeding from varices, ascites, encephalopathy, jaundice, or renal failure (D'Amico 2006). The median survival in people with compensated liver disease varies and can be more than 10 years, while for people with decompensated liver disease it is less than two years (D'Amico 2006). The only definitive treatment for decompensated liver cirrhosis is liver transplantation. Chronic liver failure is the most common indication for liver transplantation (Graziadei 2016). Other important indications are acute liver failure and hepatocellular carcinoma (Graziadei 2016). The median survival after liver transplantation is in excess of 10 years (Duffy 2010; SRTR 2012; Schoening 2013). There may also be an improvement in the quality of life of people with chronic liver disease after liver transplantation (Yang 2014).
Approximately 7000 liver transplantations are carried out in Europe and 6000 liver transplantations are carried out in the USA each year (SRTR 2012; ELTR 2017). The majority of the liver grafts are obtained from cadaveric donors (SRTR 2012; NHSBT 2014). Living‐donor liver transplantation is associated with increased complications and retransplantation and constitutes only a small proportion of liver transplantation (Wan 2014). Pretransplant deaths occur at a rate of 5.8 deaths per 100 waitlist years in the USA (SRTR 2012), and 12% of people on the UK waiting list died or became too unwell to be transplanted (NHSBT 2014), indicating organ shortage necessitating an organ allocation policy. The Model for End‐Stage Liver Disease (MELD) score, which is calculated based on serum bilirubin levels, creatinine levels, and International Normalised Ratio (INR) for prothrombin time and was first reported in 2001 (Kamath 2001), is the current method of selecting candidates and allocating organs in the USA. A similar scoring system with the additional parameter of sodium levels is used to calculate the United Kingdom Model for End‐Stage Liver Disease (UKELD), which is used by individual centres for prioritising people for transplantation in the UK (Barber 2011).
Description of the intervention
As part of liver transplantation, immunosuppression (suppressing the host immunity) is given to prevent graft rejections (Geissler 2009). Graft rejection can be described as an immune response (either cell‐mediated immunity (mediated by cytotoxic T cells) or humoral immunity (antibody‐mediated immunity mediated by B lymphocytes)) of the body against transplanted organ or tissues from a different person whose tissue antigens are not compatible with those of the recipient (NCBI 2014). Human leukocyte antigen (HLA) typing and matching is not used for organ allocation in liver transplantation, as there is no evidence of a difference in graft survival between HLA‐matched and HLA‐mismatched liver transplantation (Lan 2010). While transplanted liver grafts are less prone to graft rejection than other organ transplants, immunosuppression is routinely used for recipients of liver transplants (Geissler 2009). Various drugs have been used for immunosuppression, including calcineurin inhibitors (cyclosporine A and tacrolimus), antimetabolites (mycophenolate mofetil, mycophenolic acid, or azathioprine), mTOR (mammalian target of rapamycin) inhibitors (sirolimus, everolimus), corticosteroids (methylprednisolone), and antibody‐based therapies (thymoglobulin, antithymocyte globulin, alemtuzumab, basiliximab, daclizumab) (Haddad 2006; Geissler 2009; Fairfield 2015). These drugs may be used alone (usually calcineurin inhibitors or antimetabolites) or can be used in combination (usually a calcineurin inhibitor and a corticosteroid or a combination of calcineurin inhibitor, antimetabolite, and corticosteroid) (Lan 2014). Other combinations, such as calcineurin inhibitor and antimetabolite; antimetabolite and corticosteroids; antimetabolite and mTOR inhibitor; and mTOR inhibitor and corticosteroids may be used (Maheshwari 2006; Herlenius 2010). Antibodies may be used in addition to these interventions or as a replacement for corticosteroids (Penninga 2014a; Penninga 2014b). The main purpose of these combinations is to decrease the adverse effects of the individual drugs by reduction in dosage and to suppress immunity by multiple mechanisms (Geissler 2009). Initial immunosuppression (induction immunosuppression) often differs from long‐term immunosuppression (maintenance immunosuppression) because it is widely believed that graft rejections are more common during the first few months after liver transplantation.
Immunosuppression is associated with a variety of adverse effects. In general, immunosuppression is associated with increased risk of infections and malignancy (Geissler 2009; Rodriguez‐Peralvarez 2014). In addition, the adverse effects of different drugs include renal toxicity (calcineurin inhibitors), gastrointestinal adverse effects (antimetabolites), bone marrow suppression (antimetabolites), hepatic artery thrombosis (mTOR inhibitors), elevated cholesterol levels (mTOR inhibitors), diabetes (corticosteroids), hypertension (corticosteroids), osteoporosis (corticosteroids), and obesity (corticosteroids). Immunosuppression and related monitoring are the major costs associated with liver transplantation, costing approximately GBP 25,000 in 2003 (Longworth 2003).
How the intervention might work
Ciclosporin inhibits calcineurin, a calcium/calmodulin‐dependent phosphatase complex that inhibits the nuclear factor of activated T cells (NFAT) from entering the nucleus, an essential step in the activation of cytotoxic T cells (Geissler 2009). Mycophenolate mofetil and mycophenolic acid inhibit inosine‐5'‐monophosphate dehydrogenase (IMPDH), an important enzyme necessary for synthesis of guanosine nucleotides, which is in turn necessary for the growth of the B lymphocytes and T lymphocytes (Geissler 2009). Sirolimus and everolimus (mTOR inhibitors) inhibit mTORC1 (mammalian target of rapamycin complex 1) activity, which plays a key role in the proliferation of T cells in response to interleukin‐2 (Geissler 2009). Corticosteroids inhibit arachidonic acid metabolism, antigen presentation by dendritic cells, and interleukin‐1 dependent lymphocyte activation by decreasing interleukin‐1 transcription (Geissler 2009). Thymoglobulin, antithymocyte globulin, and alemtuzumab are antibodies against lymphocytes (Geissler 2009). Basiliximab and daclizumab are interleukin‐2 antibodies and so suppress T‐cell proliferation (Geissler 2009).
Why it is important to do this review
It is important to provide optimal maintenance immunosuppression so that the transplanted liver and the person can survive for the longest time possible. This is particularly important given the shortage of donor organs. Several maintenance immunosuppression regimens are available, and the optimal regimen in terms of clinical effectiveness or cost‐effectiveness is unknown. There have been several Cochrane systematic reviews on immunosuppression in liver transplantation (Haddad 2006; Penninga 2012; Fairfield 2015). There has been no previous network meta‐analysis on maintenance immunosuppressive regimens in people undergoing liver transplantation. Network meta‐analysis allows for a combination of direct evidence and indirect evidence and the ranking of different interventions in terms of the different outcomes (Salanti 2011; Salanti 2012). With this systematic review and network meta‐analysis we aimed to provide the best level of evidence for the role of different maintenance immunosuppressive regimens in people undergoing liver transplantation.
Objectives
To assess the comparative benefits and harms of different maintenance immunosuppressive regimens in adults undergoing liver transplantation through a network meta‐analysis and to generate rankings of the different immunosuppressive regimens according to their safety and efficacy.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this network meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other design because of the risk of bias in such studies. Inclusion of indirect observational evidence could weaken our network meta‐analysis, but it can also be viewed as a strength. It is well established that exclusion of non‐randomised studies increases the focus on potential benefits and reduces the focus on the risks of serious adverse events and those of adverse events.
Types of participants
We included randomised clinical trials with adult participants undergoing liver transplantation (or liver retransplantation) for any reason. We excluded randomised clinical trials in which participants had undergone multivisceral transplantation, since the immunosuppressive regimens may have to be tailored for the other organ. We also excluded randomised clinical trials that compared different regimens in treating established graft rejections, as the main purpose of routine maintenance immunosuppression is to prevent graft rejection.
Types of interventions
Any of the following possible maintenance immunosuppressive regimens after liver transplantation compared with each other. As we anticipated, none of the trials we identified had no immunosuppression in one of the intervention groups.
The following are some of the immunosuppressive regimens used alone or in combination that we considered:
calcineurin inhibitors (e.g. cyclosporine A and tacrolimus);
antimetabolites (e.g. mycophenolate mofetil, mycophenolate, or azathioprine);
mTOR inhibitors (e.g. sirolimus, everolimus);
glucocorticosteroids (e.g. methylprednisolone).
The above list is not exhaustive. If we identified immunosuppressive regimens of which we were unaware, we considered them to be eligible and included them in the network if they were used primarily for maintenance immunosuppression after liver transplantation. We reported the findings for these interventions in the Results and Discussion sections of the review. We considered only maintenance immunosuppressive for this review. We performed a subgroup analysis of trials in which the drug combination used for induction differed from that of maintenance therapy compared to trials in which the drug combination used for induction was the same as maintenance therapy (see Subgroup analysis and investigation of heterogeneity).
We evaluated the plausibility of transitivity assumption (the assumption that the participants included in the different trials with different immunosuppressive regimens can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the interventions) (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. This necessitates that information on potential effect‐modifiers such as primary transplantation versus retransplantation and the reasons for liver transplantation should be similar across trials. While we acknowledge that the relative effect of the different interventions may be different in people undergoing primary liver transplantation and those undergoing retransplantation and be based on different reasons for liver transplantation, we performed an analysis including all types of participants but planned to evaluate the treatment effect and ranking of different interventions in a subgroup analysis of people undergoing primary liver transplantation and people undergoing retransplantation (see Subgroup analysis and investigation of heterogeneity). If there was any concern that the clinical safety and effectiveness were dependent upon whether the participants had undergone primary liver transplantation or retransplantation or upon the different reasons for liver transplantation, we planned not to perform a network meta‐analysis on all participant subgroups.
Types of outcome measures
We assessed the comparative benefits and harms (and reported the relative ranking) of available maintenance immunosuppressive regimens in people with liver transplantation for the following outcomes.
Primary outcomes
Mortality at maximal follow‐up (time to death; maximal follow‐up).
Graft loss at maximal follow‐up (time to graft loss or death).
-
Adverse events (within three months after cessation of intervention). Depending on the availability of data, we attempted to classify adverse events as serious or non‐serious. We defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the intervention but resulting in a dose reduction or discontinuation of intervention (any time after commencement of intervention) (ICH‐GCP 1997). We defined a serious adverse event as any event that would increase mortality; is life‐threatening; requires hospitalisation; results in persistent or significant disability; is a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it. We used the definition used by study authors for non‐serious and serious adverse events:
serious adverse events;
any adverse events;
renal impairment (requiring renal support or renal transplantation);
chronic kidney disease (as defined by authors).
-
Health‐related quality of life as defined in the included trials using a validated scale such as the EQ‐5D or 36‐Item Short Form Health Survey (SF‐36) (EuroQol 2014; Ware 2014):
short term (up to one year);
medium term (one to five years);
long term (beyond five years).
We considered long‐term health‐related quality of life more important than short‐term or medium‐term health‐related quality of life, although short‐term and medium‐term health‐related quality of life were also important primary outcomes.
Secondary outcomes
Retransplantation (at maximal follow‐up).
-
Acute graft rejections (within one year) (Banff criteria if possible, otherwise as defined by authors) (Demetris 1997):
any acute graft rejections;
graft rejections requiring treatment (additional immunosuppression or increase in dosage of one or more components of the immunosuppression regimen).
Costs (maximal follow‐up). We planned to include costs related to the drugs and monitoring required as a result of the drugs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), and Science Citation Index Expanded (Web of Knowledge) from inception to 26th October 2016 for randomised clinical trials comparing two or more of the above interventions without applying any language restrictions (Royle 2003). We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/), which searches various trial registers, including ISRCTN and ClinicalTrials.gov. Appendix 1 shows the search strategies that we used and the time spans of the searches.
Searching other resources
We searched the references of the identified trials and the existing Cochrane reviews on immunosuppression to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (KG and MR or MG) independently identified the trials for inclusion by screening the titles and abstractsseeking full‐text articles for any references identified by at least one of the review authors for potential inclusion. We selected trials for inclusion based on the full‐text articles. The excluded full‐text references with reasons for their exclusion are provided in the Characteristics of excluded studies table. We also planned to list any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. Any discrepancies were resolved through discussion.
Data extraction and management
Two review authors (KG and MR or MG) independently extracted the following data.
-
Outcome data (for each outcome and for each intervention group whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events for count outcomes, and number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, comorbidities, proportion of participants undergoing liver transplantation for various reasons, and proportion of participants undergoing retransplantation;
details of the intervention and control (including dose, frequency, and duration) such as additional intervention for prevention of recurrence of disease that required transplantation, e.g. antiviral preparations for people who had undergone liver transplantation for chronic hepatitis C;
length of follow‐up;
risk of bias (Assessment of risk of bias in included studies).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria;
follow‐up time points of the outcome.
If available, we planned to obtain separate data for participants undergoing liver transplantation for different causes. We also planned to obtain separate data for participants undergoing primary liver transplantation (first liver transplantation) and those undergoing retransplantation if this information was available. We contacted the trial authors in the case of unclear or missing information. If there was any doubt as to whether trials shared the same participants, completely or partially (by identifying common authors and centres), we attempted to contact the trial authors to clarify whether the trial report was duplicated. Any differences in opinion were resolved through discussion.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions and described in the Cochrane Hepato‐Biliary Group Module to assess the risk of bias in included trials (Higgins 2011; Gluud 2016). Specifically, we assessed the risk of bias in included trials for the following domains using the methods below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the study.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We planned to only include such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so that the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We planned to only include such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: at least one of the outcomes related to the main reason for immunosuppression, namely, mortality or graft loss at maximal follow‐up along with intervention‐related adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial.
Uncertain risk of bias: the trial may or may not have been free of for‐profit bias, as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other components that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
Uncertain risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. baseline differences, early stopping).
We considered a trial to be at low risk of bias if we assessed the trial to be at low risk of bias across all domains. Otherwise, we considered trials to be at high risk of bias.
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we calculated the odds ratio (OR) with 95% credible interval (CrI) (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. health‐related quality of life reported on the same scale), we planned to calculate the mean difference (MD) with 95% Crl. We planned to use standardised mean difference (SMD) values with 95% Crl for health‐related quality of life if included trials use different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we calculated the rate ratio RR with 95% Crl. For time‐to‐event data (e.g. mortality at maximal follow‐up, graft loss at maximal follow‐up), we used hazard ratio (HR) with 95% Crl.
Relative ranking
We estimated the ranking probabilities for all interventions of being at each possible rank for each intervention. We then obtained the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
The unit of analysis was the participant undergoing liver transplantation according to the intervention group to which the participant was randomly assigned.
Cluster randomised clinical trials
As expected, we found no cluster randomised clinical trials. Had we found them, we would have included them provided that the effect estimate adjusted for cluster correlation was available.
Cross‐over randomised clinical trials
As expected, we found no cross‐over randomised clinical trials. Had we identified any, we planned to only include the outcomes after the period of first intervention since immunosuppressive regimens can potentially have a residual effect.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria. The codes for analysis we used account for the correlation between the effect sizes from studies with more than two groups.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992); otherwise, we used the data that were available to us (e.g. a trial may have reported only per‐protocol analysis results). As such 'per‐protocol' analyses may be biased, we planned to conduct best‐worst case scenario analysis (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analysis (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible.
For continuous outcomes, we planned to impute the standard deviation from P values according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation can decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We planned to assess the presence of clinical heterogeneity by comparing effect estimates in different reasons for liver transplantation, primary liver transplantation or retransplantation, different drugs from the class, and doses of the immunosuppressive regimen. Different study designs and risk of bias can contribute to methodological heterogeneity.
We assessed statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau2 and comparing this with values reported in study of the distribution of between‐study heterogeneity) (Turner 2012), and by calculating I2 (using Stata/SE 14.2). If we identified substantial heterogeneity, that is clinical, methodological, or statistical, we explored and addressed the heterogeneity in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity section).
Assessment of transitivity across treatment comparisons
We assessed the assumption of transitivity by comparing the distribution of the potential effect modifiers (clinical: primary transplantation or retransplantation, reasons for liver transplantation; methodological: risk of bias, year of randomisation, duration of follow‐up) across the different pairwise comparisons.
Assessment of reporting biases
For the network meta‐analysis, we judged the reporting bias by the completeness of the search (i.e. searching various databases and including conference abstracts), as we could find no meaningful order to perform a comparison‐adjusted funnel plot (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time) (Chaimani 2012).
Data synthesis
Methods for indirect and mixed comparisons
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We obtained a network plot to ensure that the trials were connected by interventions using Stata/SE 14.2 (Chaimani 2013). We excluded any trials that were not connected to the network. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2016). We modelled the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') using appropriate likelihood functions and links (Lu 2006b). We used binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and planned to use normal likelihood and identity link for continuous outcomes. We used tacrolimus as the reference group. We performed a fixed‐effect model and random‐effects model for the network meta‐analysis. We have reported both models for comparison with the reference group in a forest plot. For each pairwise comparison in a table, we have reported the fixed‐effect model if the two models reported similar results; otherwise, we reported the more conservative model.
We used a hierarchical Bayesian model using three different initial values, employing codes provided by NICE DSU (Dias 2016). We used a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we used a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assumed similar between‐trial standard deviation across treatment comparisons (Dias 2016). We used a 'burn‐in' of 5000 simulations, checked for convergence visually, and ran the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we planned to increase the number of simulations for 'burn‐in'. If we still did not obtain convergence, we planned to use alternate initial values and priors employing methods suggested by van Valkenhoef 2012. We also estimated the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2016).
Assessment of inconsistency
We assessed inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We used the inconsistency models employed in the NICE DSU manual, as we used common between‐study standard deviation (Dias 2014). In addition, we used design‐by‐treatment full interaction model and IF (inconsistency factor) plots to assess inconsistency (Higgins 2012; Chaimani 2013). In the presence of inconsistency, we planned to assess whether the inconsistency was due to clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the Subgroup analysis and investigation of heterogeneity section.
If there was evidence of inconsistency, we planned to identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We performed the direct comparisons using the same codes and the same technical details.
Calculation of required information size and Trial Sequential Analysis
For calculation of the required information size, see Appendix 2. We performed Trial Sequential Analysis to control the risk of random errors when at least two trials were included for the comparison of other interventions versus tacrolimus for the outcomes mortality at maximal follow‐up and health‐related quality of life, the two outcomes that determine whether the intervention should be given (Wetterslev 2008; Thorlund 2011; TSA 2011; Wetterslev 2017). We used an alpha error as per guidance of Jakobsen 2014, power of 90% (beta error of 10%), a relative risk reduction of 20%, a control group proportion observed in the trials, and the heterogeneity observed in the meta‐analysis. As the only outcome was mortality at maximal follow‐up, which is a time‐to‐event outcome, we performed the Trial Sequential Analysis using Stata/SE 14.2, employing methods suggested by Miladinovic 2013.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups using meta‐regression with the help of the codes provided in NICE DSU guidance if we included a sufficient number of trials (Dias 2012a). We planned to use the following trial‐level covariates for meta‐regression.
Trials at low risk of bias compared to trials at high risk of bias.
Different reasons for undergoing liver transplantation.
Primary liver transplantation compared to retransplantation.
Different drugs from the class (cyclosporine A compared to tacrolimus).
An additional drug used for induction compared to no additional drug used for induction (post hoc).
We calculated a single common interaction term when applicable (Dias 2012a). If the 95% credible intervals of the interaction term did not overlap zero, we considered this statistically significant.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to re‐analyse the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible.
Presentation of results
We presented the effect estimates with 95% CrI for each pairwise comparison calculated from the direct comparisons and network meta‐analysis. We also presented the cumulative probability of the treatment ranks (i.e. the probability that the intervention is within the top two, the probability that the intervention is within the top three, etc.) in graphs (SUCRA) (Salanti 2011). We also plotted the probability that each intervention was best, second best, third best, etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We presented 'Summary of findings' tables for mortality. In Table 1, we followed the approach suggested by Puhan and colleagues (Puhan 2014). First, we calculated the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), that is calculated the direct estimate for each comparison by including only trials in which there was direct comparison of interventions and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of interventions. Next we rated the quality of direct and indirect effect estimates using GRADE methodology, which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). We then presented the estimates of the network meta‐analysis and rated the quality of network meta‐analysis effect estimates as the best quality of evidence between the direct and indirect estimates (Puhan 2014). In addition, we have presented information on the number of trials and participants as per the standard 'Summary of findings' table.
Results
Description of studies
Results of the search
We identified 5939 references through electronic searches of CENTRAL (n = 703), MEDLINE (n = 2985), Embase (n = 1357), Science Citation Index Expanded (n = 824), World Health Organization International Clinical Trials Registry Platform (n = 6), and ClinicalTrials.gov (n = 64). After removing 1603 duplicates, we obtained 4336 references. We then excluded 4075 clearly irrelevant references through screening titles and reading abstracts and retrieved 261 references for further assessment. We identified no references through scanning reference lists of the identified randomised trials. We excluded 171 references (110 studies) for the reasons stated in the Characteristics of excluded studies table. Two ongoing trials did not report any interim data (Simone 2014; Nashan 2015). A total of 88 references (describing 26 trials) met the inclusion criteria. The reference flow is summarised in the study flow diagram (Figure 1).
1.
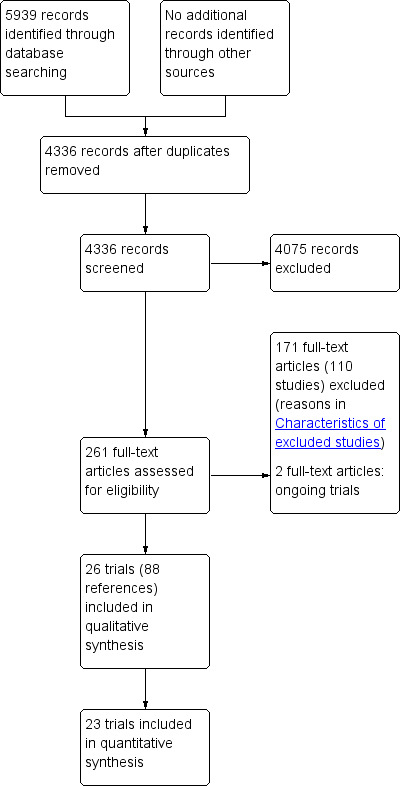
Study flow diagram.
Included studies
A total of 26 trials involving 3842 participants met the inclusion criteria for and were included in this review. Three trials did not contribute any information for this review (Fernandez‐Miranda 1998; Pham 1998; Baiocchi 2006), leaving a total of 3693 participants included in one or more outcomes in the review (after postrandomisation dropouts). The mean or median age of the participants ranged from 42 years to 55 years in the trials that reported this information. The proportion of females ranged from 28.1% to 58.7% in the trials that reported this information. Only one trial reported including participants undergoing retransplantation (Greig 2003). The proportion of participants who had undergone primary transplantation was more than 95% in all trials (Greig 2003). Three trials reported only participants who had undergone transplantation for chronic hepatitis C virus decompensated cirrhosis (Zervos 1998; Martin 2004; Manousou 2014). The remaining trials included participants with varied indications for liver transplantation. One trial was a three‐intervention group trial (De Simone 2012). The remaining trials had two intervention groups.
The interventions, controls, number of included participants, and reported follow‐up period for the different trials are provided in Table 2.
1. Characteristics of included studies (arranged by comparisons).
| Study name | Number of participants randomised | Postrandomisation dropouts | Number of participants for whom outcome was reported | Intervention 1 | Intervention 2 | Intervention 3 | Average follow‐up period (months) |
| Belli 1998 | 108 | ‐ | 108 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | ‐ | 41 |
| Pageaux 2004 | 174 | 0 | 174 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | ‐ | 6 |
| Masetti 2010 | 78 | 0 | 78 | Everolimus | Cyclosporine A | ‐ | 22 |
| Sterneck 2000 | 57 | ‐ | 57 | Cyclosporine A plus mycophenolate plus glucocorticosteroids | Cyclosporine A plus azathioprine plus glucocorticosteroids | ‐ | 6 |
| De Simone 2012 | 719 | 0 | 719 | Tacrolimus plus everolimus | Everolimus | Tacrolimus | 36 |
| Baiocchi 2006 | 20 | 0 | 20 | Cyclosporine A | Tacrolimus | ‐ | 3 |
| Cholongitas 2011 | 66 | 0 | 66 | Cyclosporine A | Tacrolimus | ‐ | 97 |
| Fernandez‐Miranda 1998 | 27 | ‐ | 27 | Cyclosporine A | Tacrolimus | ‐ | 22 |
| Fung 1991 | 81 | ‐ | 81 | Cyclosporine A | Tacrolimus | ‐ | 12 |
| Greig 2003 | 143 | 0 | 143 | Cyclosporine A | Tacrolimus | ‐ | 12 |
| Loinaz 2001 | 101 | 1 | 100 | Cyclosporine A | Tacrolimus | ‐ | 24 |
| O'Grady 2002 | 606 | 0 | 606 | Cyclosporine A | Tacrolimus | ‐ | 36 |
| Shenoy 2008 | 60 | 0 | 60 | Cyclosporine A | Tacrolimus | ‐ | 12 |
| Stegall 1997 | 71 | 0 | 71 | Cyclosporine A | Tacrolimus | ‐ | 6 |
| Zervos 1998 | 50 | 1 | 49 | Cyclosporine A | Tacrolimus | ‐ | 14 |
| Chen 2002 | 81 | 0 | 81 | Cyclosporine A plus azathioprine | Tacrolimus | ‐ | 124 |
| Jonas 2005 | 121 | 0 | 121 | Cyclosporine A plus azathioprine | Tacrolimus | ‐ | 144 |
| Manousou 2014 | 103 | 1 | 97 | Tacrolimus plus azathioprine | Tacrolimus | ‐ | 96 |
| Boudjema 2011 | 195 | 0 | 195 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus | ‐ | 11 |
| Asrani 2014 | 222 | 0 | 222 | Tacrolimus plus sirolimus | Tacrolimus | ‐ | 24 |
| Pham 1998 | 88 | 8 | 76 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | ‐ | 27 |
| Porayko 1994 | 37 | 0 | 37 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | ‐ | 12 |
| Martin 2004 | 85 | 6 | 79 | Cyclosporine A plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | ‐ | 12 |
| Jain 2001 | 350 | 0 | 350 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | ‐ | 34 |
| Fisher 1998 | 99 | 0 | 99 | Cyclosporine A plus mycophenolate | Tacrolimus plus mycophenolate | ‐ | 48 |
| Pelletier 2013 | 100 | 0 | 100 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus mycophenolate | ‐ | 69 |
Transitivity assumption
Table 3 contains a list potential modifiers in the trials arranged according to comparisons. As seen from the table, there was variability in the reasons for transplant, period of recruitment, and follow‐up in the trials, but these do not appear to vary by comparison, so the transitivity assumption appears reasonable. There were also no specific clinical reasons (based on inclusion and exclusion criteria listed in the Characteristics of included studies) to suggest that the type of participants under one comparison would be different from the type of participants in other comparisons.
2. Potential effect modifiers.
| Study name | Intervention 1 | Intervention 2 | Primary transplantation | Reason for transplantation: hepatitis C virus | Reason for transplantation: hepatitis B virus | Reason for transplantation: alcoholic cirrhosis | Reason for transplantation: other reasons | Years of randomisation | Additional drug used for induction | Average follow‐up in months | Risk of bias |
| Belli 1998 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | 104/104 (100.0%) | 42/104 (40.4%) | 24/104 (23.1%) | 9/104 (8.7%) | 21/104 (20.2%) | 1991 to 1995 | Yes | 41 | High |
| Pageaux 2004 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | 174/174 (100.0%) | 26/174 (14.9%) | 12/174 (6.9%) | 84/174 (48.3%) | 52/174 (29.9%) | 1999 to 2001 | Yes | 6 | High |
| Masetti 2010 | Everolimus | Cyclosporine A | 78/78 (100.0%) | Not stated | Not stated | Not stated | Not stated | 2006 to 2008 | Yes | 22 | High |
| Sterneck 2000 | Cyclosporine A plus mycophenolate plus glucocorticosteroids | Cyclosporine A plus azathioprine plus glucocorticosteroids | 57/57 (100.0%) | Not stated | Not stated | Not stated | Not stated | 1996 to 1998 | No | 6 | High |
| De Simone 2012 | Tacrolimus plus everolimus | Intervention 1: Everolimus Intervention 2: Tacrolimus |
719/719 (100.0%) | 175/719 (24.3%) | 49/719 (6.8%) | 171/719 (23.8%) | 258/719 (35.9%) | 2008 to 2011 | Yes | 36 | High |
| Baiocchi 2006 | Cyclosporine A | Tacrolimus | 20/20 (100.0%) | 8/20 (40.0%) | 4/20 (20.0%) | 3/20 (15.0%) | 1/20 (5.0%) | Not stated | No | 3 | High |
| Cholongitas 2011 | Cyclosporine A | Tacrolimus | 66/66 (100.0%) | Not stated | Not stated | Not stated | Not stated | 1996 to 1997 | No | 97 | High |
| Fernandez‐Miranda 1998 | Cyclosporine A | Tacrolimus | Not stated | Not stated | Not stated | Not stated | Not stated | 1993 to 1995 | Yes | 22 | High |
| Fung 1991 | Cyclosporine A | Tacrolimus | 81/81 (100.0%) | Not stated | 0/81 (0.0%) | Not stated | Not stated | 1990 | Yes | 12 | High |
| Greig 2003 | Cyclosporine A | Tacrolimus | 139/143 (97.2%) | 47/143 (32.9%) | 0/143 (0.0%) | 25/143 (17.5%) | 67/143 (46.9%) | 1996 | Yes | 12 | High |
| Loinaz 2001 | Cyclosporine A | Tacrolimus | 100/100 (100.0%) | Not stated | Not stated | Not stated | Not stated | Not stated | Yes | 24 | High |
| O'Grady 2002 | Cyclosporine A | Tacrolimus | 606/606 (100.0%) | 60/606 (9.9%) | 20/606 (3.3%) | 110/606 (18.2%) | 98/606 (16.2%) | 1997 to 1999 | Yes | 36 | High |
| Shenoy 2008 | Cyclosporine A | Tacrolimus | Not stated | 32/60 (53.3%) | 5/60 (8.3%) | 8/60 (13.3%) | 16/60 (26.7%) | 2002 to 2004 | Yes | 12 | High |
| Stegall 1997 | Cyclosporine A | Tacrolimus | Not stated | Not stated | 0/71 (0.0%) | Not stated | Not stated | Not stated | Yes | 6 | High |
| Zervos 1998 | Cyclosporine A | Tacrolimus | Not stated | 49/49 (100.0%) | 0/49 (0.0%) | 0/49 (0.0%) | 0/49 (0.0%) | 1995 to 1996 | Yes | 14 | High |
| Chen 2002 | Cyclosporine A plus azathioprine | Tacrolimus | 81/81 (100.0%) | 2/81 (2.5%) | 2/81 (2.5%) | 6/81 (7.4%) | 71/81 (87.7%) | 1990 to 1992 | No | 124 | High |
| Jonas 2005 | Cyclosporine A plus azathioprine | Tacrolimus | 121/121 (100.0%) | 35/121 (28.9%) | 30/121 (24.8%) | 20/121 (16.5%) | 37/121 (30.6%) | 1990 to 1992 | Yes | 144 | High |
| Manousou 2014 | Tacrolimus plus azathioprine | Tacrolimus | 97/97 (100.0%) | 97/97 (100.0%) | 0/97 (0.0%) | 0/97 (0.0%) | 0/97 (0.0%) | 2000 to 2007 | Yes | 96 | High |
| Boudjema 2011 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus | 195/195 (100.0%) | 16/195 (8.2%) | 4/195 (2.1%) | 83/195 (42.6%) | 91/195 (46.7%) | 2003 to 2007 | Yes | 11 | High |
| Asrani 2014 | Tacrolimus plus sirolimus | Tacrolimus | 222/222 (100.0%) | 72/222 (32.4%) | 30/222 (13.5%) | 79/222 (35.6%) | 40/222 (18.0%) | 2000 to 2003 | Yes | 24 | High |
| Pham 1998 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | 76/76 (100.0%) | Not stated | Not stated | Not stated | Not stated | 1990 to 1992 | No | 27 | High |
| Porayko 1994 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | 37/37 (100.0%) | Not stated | Not stated | 6/37 (16.2%) | 29/37 (78.4%) | 1990 to 1991 | No | 12 | High |
| Martin 2004 | Cyclosporine A plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | 79/79 (100.0%) | 79/79 (100.0%) | 0/79 (0.0%) | 0/79 (0.0%) | 0/79 (0.0%) | Not stated | Yes | 12 | High |
| Jain 2001 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | 350/350 (100.0%) | 95/350 (27.1%) | 15/350 (4.3%) | 70/350 (20.0%) | 160/350 (45.7%) | 1995 to 1998 | No | 34 | High |
| Fisher 1998 | Cyclosporine A plus mycophenolate | Tacrolimus plus mycophenolate | 99/99 (100.0%) | 37/99 (37.4%) | 7/99 (7.1%) | 11/99 (11.1%) | 44/99 (44.4%) | 1995 to 1997 | Yes | 48 | High |
| Pelletier 2013 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus mycophenolate | 100/100 (100.0%) | 54/100 (54.0%) | Not stated | 42/100 (42.0%) | 8/100 (8.0%) | 2002 to 2005 | No | 69 | High |
Source of funding
Fourteen trials were funded by pharmaceutical companies who would benefit from the results of the trial (Porayko 1994; Fisher 1998; Sterneck 2000; Chen 2002; O'Grady 2002; Greig 2003; Martin 2004; Pageaux 2004; Jonas 2005; Shenoy 2008; De Simone 2012; Pelletier 2013; Asrani 2014; Manousou 2014); two trials were funded by parties who had no vested interest in the results of the trial (Fung 1991; Boudjema 2011); and the remaining 10 trials did not report the source of funding.
Excluded studies
None of the excluded studies met the inclusion criteria. The reasons for exclusion are provided in the Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias is summarised in Figure 2, Figure 3, and Table 4. As none of the trials were at low risk of bias in all domains, we considered all trials to be at high risk of bias.
2.
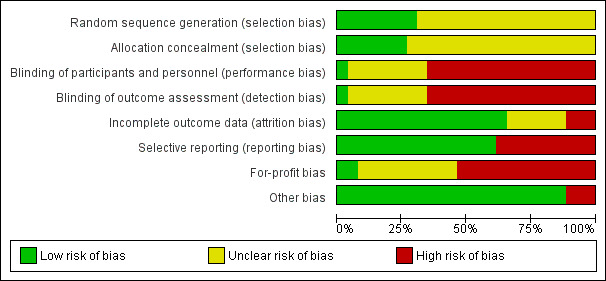
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
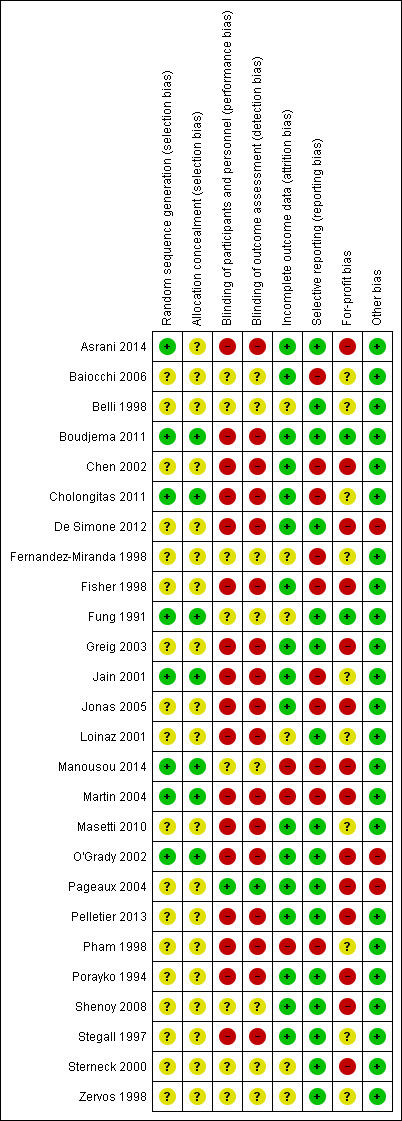
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3. Risk of bias (arranged by intervention).
| Name of studies | Intervention 1 | Intervention 2 | Random sequence generation | Allocation concealment | Blinding of participants and health professionals | Blinding of outcome assessors | Attrition bias | Selective outcome reporting | For‐profit bias | Overall risk of bias |
| Belli 1998 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Pageaux 2004 | Cyclosporine A plus glucocorticosteroids | Cyclosporine A | Unclear | Unclear | Low | Low | Low | Low | High | High |
| Masetti 2010 | Everolimus | Cyclosporine A | Unclear | Unclear | High | High | Low | Low | Unclear | High |
| Sterneck 2000 | Cyclosporine A plus mycophenolate plus glucocorticosteroids | Cyclosporine A plus azathioprine plus glucocorticosteroids | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High | High |
| De Simone 2012 | Tacrolimus plus everolimus | Intervention 1: Everolimus Intervention 2: Tacrolimus |
Unclear | Unclear | High | High | Low | Low | High | High |
| Baiocchi 2006 | Cyclosporine A | Tacrolimus | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | High |
| Cholongitas 2011 | Cyclosporine A | Tacrolimus | Low | Low | High | High | Low | Low | Unclear | High |
| Fernandez‐Miranda 1998 | Cyclosporine A | Tacrolimus | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | High |
| Fung 1991 | Cyclosporine A | Tacrolimus | Low | Low | Unclear | Unclear | Unclear | Low | Low | High |
| Greig 2003 | Cyclosporine A | Tacrolimus | Unclear | Unclear | High | High | Low | Low | High | High |
| Loinaz 2001 | Cyclosporine A | Tacrolimus | Unclear | Unclear | High | High | Unclear | Low | Unclear | High |
| O'Grady 2002 | Cyclosporine A | Tacrolimus | Low | Low | High | High | Low | Low | High | High |
| Shenoy 2008 | Cyclosporine A | Tacrolimus | Unclear | Unclear | Unclear | Unclear | Low | Low | High | High |
| Stegall 1997 | Cyclosporine A | Tacrolimus | Unclear | Unclear | High | High | Low | Low | Unclear | High |
| Zervos 1998 | Cyclosporine A | Tacrolimus | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Chen 2002 | Cyclosporine A plus azathioprine | Tacrolimus | Unclear | Unclear | High | High | Low | High | High | High |
| Jonas 2005 | Cyclosporine A plus azathioprine | Tacrolimus | Unclear | Unclear | High | High | Low | Low | High | High |
| Manousou 2014 | Tacrolimus plus azathioprine | Tacrolimus | Low | Low | Unclear | Unclear | High | Low | High | High |
| Boudjema 2011 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus | Low | Low | High | High | Low | Low | Low | High |
| Asrani 2014 | Tacrolimus plus sirolimus | Tacrolimus | Low | Unclear | High | High | Low | Low | High | High |
| Pham 1998 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | Unclear | Unclear | High | High | High | High | Unclear | High |
| Porayko 1994 | Cyclosporine A plus azathioprine plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | Unclear | Unclear | High | High | Low | Low | High | High |
| Martin 2004 | Cyclosporine A plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | Low | Low | High | High | High | Low | High | High |
| Jain 2001 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus glucocorticosteroids | Low | Low | High | High | Low | Low | Unclear | High |
| Fisher 1998 | Cyclosporine A plus mycophenolate | Tacrolimus plus mycophenolate | Unclear | Unclear | High | High | Low | Low | High | High |
| Pelletier 2013 | Tacrolimus plus mycophenolate plus glucocorticosteroids | Tacrolimus plus mycophenolate | Unclear | Unclear | High | High | Low | Low | High | High |
Allocation
Eight trials were at low risk of bias due to random sequence generation (Fung 1991; Jain 2001; O'Grady 2002; Martin 2004; Boudjema 2011; Cholongitas 2011; Asrani 2014; Manousou 2014); the remaining trials were at unclear risk of bias due to random sequence generation. Seven trials were at low risk of bias due to allocation concealment (Fung 1991; Jain 2001; O'Grady 2002; Martin 2004; Boudjema 2011; Cholongitas 2011; Manousou 2014); the remaining trials were at unclear risk of bias due to allocation concealment. Overall, seven trials were at low risk of selection bias (Fung 1991; Jain 2001; O'Grady 2002; Martin 2004; Boudjema 2011; Cholongitas 2011; Manousou 2014).
Blinding
One trial was at low risk of bias due to lack of blinding of participants and health professionals and bias due to lack of blinding of outcome assessors (Pageaux 2004); 17 trials were at high risk of bias due to lack of blinding of participants and health professionals and bias due to lack of blinding of outcome assessors (Porayko 1994; Stegall 1997; Fisher 1998; Pham 1998; Jain 2001; Loinaz 2001; Chen 2002; O'Grady 2002; Greig 2003; Martin 2004; Jonas 2005; Masetti 2010; Boudjema 2011; Cholongitas 2011; De Simone 2012; Pelletier 2013; Asrani 2014); the remaining trials were at unclear risk of bias due to lack of blinding of participants and health professionals and bias due to lack of blinding of outcome assessors.
Incomplete outcome data
Seventeen trials were at low risk of incomplete outcome data (attrition bias) (Porayko 1994; Stegall 1997; Fisher 1998; Jain 2001; Chen 2002; O'Grady 2002; Greig 2003; Pageaux 2004; Jonas 2005; Baiocchi 2006; Shenoy 2008; Masetti 2010; Boudjema 2011; Cholongitas 2011; De Simone 2012; Pelletier 2013; Asrani 2014); three trials were at high risk of incomplete outcome data (attrition bias) (Pham 1998; Martin 2004; Manousou 2014); the remaining trials were at unclear risk of incomplete outcome data (attrition bias).
Selective reporting
We did not find a published protocol for any of the trials. Sixteen trials were at low risk of selective reporting (reporting bias) (Fung 1991; Porayko 1994; Stegall 1997; Belli 1998; Zervos 1998; Sterneck 2000; Loinaz 2001; O'Grady 2002; Greig 2003; Pageaux 2004; Shenoy 2008; Masetti 2010; Boudjema 2011; De Simone 2012; Pelletier 2013; Asrani 2014); the remaining trials were at high risk of selecting outcome reporting bias.
Other potential sources of bias
For‐profit bias: 14 trials were at high risk of for‐profit bias (Porayko 1994; Fisher 1998; Sterneck 2000; Chen 2002; O'Grady 2002; Greig 2003; Martin 2004; Pageaux 2004; Jonas 2005; Shenoy 2008; De Simone 2012; Pelletier 2013; Asrani 2014; Manousou 2014); two trials were were at low risk of for‐profit bias (Fung 1991; Boudjema 2011); the remaining trials were at unclear risk of for‐profit bias.
Three trials were at high risk of other bias (O'Grady 2002; Pageaux 2004; De Simone 2012): O'Grady 2002 was stopped early for benefit; in De Simone 2012, recruitment to one of the intervention groups was stopped early; and in Pageaux 2004, despite following participants for 12 months, the authors have presented only the six‐month results, and have excluded two late deaths. The remaining trials were at low risk of other bias.
Effects of interventions
See: Table 1
The network plot for all outcomes with more than one trial is shown in Figure 4. As shown in Figure 4, only two outcomes (mortality at maximal follow‐up and graft rejections requiring treatment) have treatment comparisons in which direct and indirect estimates were available. Although 'closed loops' are present in some other outcomes (e.g. graft loss at maximal follow‐up, adverse events (proportion) and renal impairment, and graft rejections (any)), this was due to a three‐armed trial (De Simone 2012). The data used for the network meta‐analysis is available in Appendix 3. The ranking probabilities of different interventions for different outcomes in which network meta‐analysis was performed is shown in Table 5. These ranking probabilities are also presented as figures that show the cumulative probability of being best, second best, etc. (SUCRA) and rankogram, which shows the ranking probability of each intervention at each different rank (best intervention, second best, etc.). These ranking probabilities should be interpreted with extreme caution because the sparse networks were made up of trials at high risk of bias.
4.
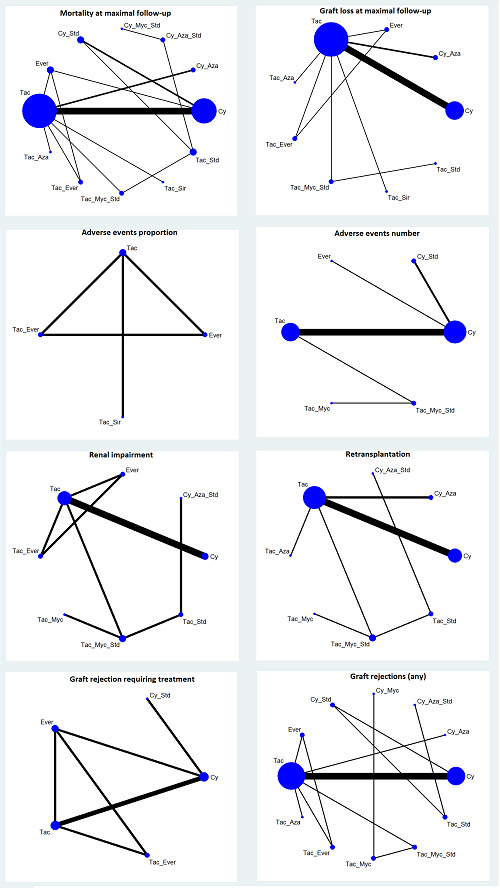
The network plots showing the comparisons in which there were at least two trials. The size of the node (circle) provides a measure of the number of trials in which the particular Intervention was included as one of the intervention groups. The thickness of the line provides a measure of the number of direct comparisons between two nodes (Interventions). Only two outcomes (mortality at maximal follow‐up and graft rejections requiring treatment) have treatment comparisons in which direct and indirect estimates were available. Although 'closed loops' are present in some other outcomes (e.g. graft loss at maximal follow‐up, adverse events (proportion), renal impairment, and graft rejections (any)), this was due to a trial with three intervention groups.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
4. Ranking probabilities of different interventions.
| Mortality at maximal follow‐up | ||||||||||||
| Rank | Tac | Cy | Cy_Aza | Cy_Aza_Std | Cy_Myc_Std | Cy_Std | Ever | Tac_Aza | Tac_Ever | Tac_Myc_Std | Tac_Sir | Tac_Std |
| 1 | 0.002 | 0.001 | 0.0081 | 0.0059 | 0.0429 | 0.08883 | 0.003833 | 0.4588 | 0.01477 | 0.2413 | 0.002833 | 0.1298 |
| 2 | 0.02697 | 0.0091 | 0.02223 | 0.0205 | 0.01723 | 0.1636 | 0.01063 | 0.1413 | 0.03433 | 0.2943 | 0.0058 | 0.254 |
| 3 | 0.06937 | 0.03153 | 0.0344 | 0.01453 | 0.02043 | 0.2026 | 0.0175 | 0.1347 | 0.04107 | 0.1896 | 0.007833 | 0.2364 |
| 4 | 0.1398 | 0.07723 | 0.05383 | 0.02217 | 0.02243 | 0.2261 | 0.02603 | 0.1165 | 0.06163 | 0.1054 | 0.0107 | 0.1381 |
| 5 | 0.2622 | 0.1618 | 0.0916 | 0.02023 | 0.0251 | 0.1223 | 0.0448 | 0.04737 | 0.089 | 0.05653 | 0.0152 | 0.0638 |
| 6 | 0.2462 | 0.2478 | 0.0984 | 0.01667 | 0.01947 | 0.0837 | 0.06113 | 0.03437 | 0.08167 | 0.0391 | 0.02023 | 0.05127 |
| 7 | 0.1561 | 0.2136 | 0.1886 | 0.01463 | 0.0179 | 0.04797 | 0.1067 | 0.022 | 0.1217 | 0.02753 | 0.03773 | 0.04547 |
| 8 | 0.0698 | 0.1476 | 0.1823 | 0.02453 | 0.02297 | 0.03197 | 0.1922 | 0.017 | 0.1797 | 0.02253 | 0.07307 | 0.03623 |
| 9 | 0.02213 | 0.0767 | 0.1805 | 0.0347 | 0.03257 | 0.0216 | 0.2746 | 0.01443 | 0.1841 | 0.0163 | 0.1118 | 0.03067 |
| 10 | 0.004433 | 0.02673 | 0.0916 | 0.073 | 0.05803 | 0.0086 | 0.1729 | 0.0083 | 0.1261 | 0.006067 | 0.4119 | 0.0123 |
| 11 | 0.000867 | 0.005967 | 0.038 | 0.3902 | 0.309 | 0.002 | 0.06763 | 0.0036 | 0.04383 | 0.001 | 0.1366 | 0.001367 |
| 12 | 6.67E‐05 | 0.000933 | 0.01047 | 0.363 | 0.412 | 0.0007 | 0.02197 | 0.001633 | 0.02207 | 0.0003 | 0.1663 | 0.0006 |
| Graft loss at maximal follow‐up | ||||||||||||
| Rank | Tac | Cy | Cy_Aza | Ever | Tac_Aza | Tac_Ever | Tac_Myc_Std | Tac_Sir | Tac_Std | |||
| 1 | 0.01193 | 0.007067 | 0.0211 | 0.05607 | 0.4405 | 0.05217 | 0.1118 | 0.017 | 0.2823 | |||
| 2 | 0.08647 | 0.02727 | 0.04603 | 0.097 | 0.1568 | 0.08173 | 0.2609 | 0.02827 | 0.2156 | |||
| 3 | 0.165 | 0.05783 | 0.06317 | 0.09837 | 0.1764 | 0.09153 | 0.1978 | 0.03217 | 0.1177 | |||
| 4 | 0.3136 | 0.1072 | 0.09 | 0.1128 | 0.06527 | 0.1136 | 0.0911 | 0.03983 | 0.06663 | |||
| 5 | 0.2383 | 0.1876 | 0.1108 | 0.1145 | 0.04677 | 0.1211 | 0.07753 | 0.04807 | 0.0552 | |||
| 6 | 0.1362 | 0.2272 | 0.1466 | 0.1295 | 0.03837 | 0.1243 | 0.07083 | 0.0675 | 0.05943 | |||
| 7 | 0.0397 | 0.2128 | 0.1777 | 0.1418 | 0.03157 | 0.149 | 0.07553 | 0.1117 | 0.06023 | |||
| 8 | 0.007867 | 0.1347 | 0.2162 | 0.1517 | 0.02537 | 0.1572 | 0.07533 | 0.1598 | 0.07187 | |||
| 9 | 0.0009 | 0.0383 | 0.1283 | 0.09827 | 0.01893 | 0.1094 | 0.03913 | 0.4957 | 0.07107 | |||
| Adverse events (proportion) | ||||||||||||
| Rank | Tac | Ever | Tac_Ever | Tac_Sir | ||||||||
| 1 | 0.269 | 0.4943 | 0.1828 | 0.05393 | ||||||||
| 2 | 0.3693 | 0.302 | 0.3059 | 0.02283 | ||||||||
| 3 | 0.3366 | 0.1819 | 0.4476 | 0.03387 | ||||||||
| 4 | 0.0251 | 0.02177 | 0.06377 | 0.8894 | ||||||||
| Adverse events (number) | ||||||||||||
| Rank | Tac | Cy | Cy_Std | Ever | Tac_Myc | Tac_Myc_Std | ||||||
| 1 | 0 | 0.3396 | 0.001967 | 0.6566 | 0.0008 | 0.001067 | ||||||
| 2 | 0.07187 | 0.6552 | 0.03233 | 0.2176 | 0.0061 | 0.01687 | ||||||
| 3 | 0.6274 | 0.0051 | 0.1901 | 0.05377 | 0.02423 | 0.09947 | ||||||
| 4 | 0.2581 | 6.67E‐05 | 0.3336 | 0.0368 | 0.03877 | 0.3327 | ||||||
| 5 | 0.03857 | 0 | 0.3133 | 0.0218 | 0.1179 | 0.5085 | ||||||
| 6 | 0.0041 | 0 | 0.1288 | 0.01347 | 0.8122 | 0.04143 | ||||||
| Renal impairment | ||||||||||||
| Rank | Tac | Cy | Cy_Aza_Std | Ever | Tac_Ever | Tac_Myc | Tac_Myc_Std | Tac_Std | ||||
| 1 | 0.0153 | 0.0404 | 0.504 | 0.1631 | 0.04607 | 0.08963 | 0.1122 | 0.0293 | ||||
| 2 | 0.0536 | 0.08217 | 0.08977 | 0.1705 | 0.0753 | 0.131 | 0.2908 | 0.1069 | ||||
| 3 | 0.1178 | 0.1174 | 0.05827 | 0.1112 | 0.0959 | 0.1464 | 0.2312 | 0.1219 | ||||
| 4 | 0.1731 | 0.1524 | 0.049 | 0.1023 | 0.0912 | 0.1429 | 0.1471 | 0.142 | ||||
| 5 | 0.2275 | 0.1838 | 0.0433 | 0.1099 | 0.1023 | 0.1152 | 0.09747 | 0.1204 | ||||
| 6 | 0.2333 | 0.1717 | 0.0468 | 0.0993 | 0.1192 | 0.1216 | 0.0716 | 0.1364 | ||||
| 7 | 0.1358 | 0.1452 | 0.064 | 0.1444 | 0.1694 | 0.115 | 0.03967 | 0.1866 | ||||
| 8 | 0.04363 | 0.1069 | 0.1449 | 0.09933 | 0.3006 | 0.1382 | 0.01 | 0.1565 | ||||
| Retransplantation | ||||||||||||
| Rank | Tac | Cy | Cy_Aza | Cy_Aza_Std | Tac_Aza | Tac_Myc | Tac_Myc_Std | Tac_Std | ||||
| 1 | 0.0925 | 0.002667 | 0.04833 | 0.1566 | 0.3022 | 0.0076 | 0.1543 | 0.2358 | ||||
| 2 | 0.2075 | 0.01133 | 0.06863 | 0.06533 | 0.146 | 0.01023 | 0.247 | 0.244 | ||||
| 3 | 0.2234 | 0.04117 | 0.1197 | 0.0679 | 0.1452 | 0.0175 | 0.2247 | 0.1604 | ||||
| 4 | 0.2633 | 0.0855 | 0.1304 | 0.04907 | 0.1466 | 0.02703 | 0.17 | 0.128 | ||||
| 5 | 0.1688 | 0.1677 | 0.1941 | 0.0683 | 0.1204 | 0.0381 | 0.1317 | 0.1109 | ||||
| 6 | 0.03903 | 0.3206 | 0.2627 | 0.07923 | 0.08443 | 0.06377 | 0.0573 | 0.09293 | ||||
| 7 | 0.005133 | 0.3006 | 0.1444 | 0.2519 | 0.0407 | 0.2169 | 0.01447 | 0.02593 | ||||
| 8 | 0.000267 | 0.07043 | 0.03177 | 0.2617 | 0.01447 | 0.6188 | 0.000467 | 0.0021 | ||||
| Graft rejections (any) | ||||||||||||
| Rank | Tac | Cy | Cy_Aza | Cy_Aza_Std | Cy_Myc | Cy_Std | Ever | Tac_Aza | Tac_Ever | Tac_Myc | Tac_Myc_Std | Tac_Std |
| 1 | 0.0015 | 0.0003 | 0.0619 | 0.0906 | 0.05987 | 0.05883 | 0.004033 | 0.01077 | 0.4311 | 0.09627 | 0.1642 | 0.02063 |
| 2 | 0.01683 | 0.002133 | 0.109 | 0.0657 | 0.0654 | 0.1025 | 0.01983 | 0.0222 | 0.1711 | 0.1295 | 0.2467 | 0.049 |
| 3 | 0.06013 | 0.0061 | 0.1157 | 0.0591 | 0.07787 | 0.132 | 0.02767 | 0.02877 | 0.1165 | 0.1298 | 0.1887 | 0.05763 |
| 4 | 0.1338 | 0.01607 | 0.1119 | 0.05787 | 0.0811 | 0.132 | 0.0359 | 0.03733 | 0.0984 | 0.1032 | 0.1329 | 0.0595 |
| 5 | 0.1982 | 0.04087 | 0.1146 | 0.05963 | 0.06117 | 0.1265 | 0.04947 | 0.04803 | 0.06617 | 0.0829 | 0.08977 | 0.0626 |
| 6 | 0.2194 | 0.08913 | 0.09947 | 0.05347 | 0.05667 | 0.1176 | 0.06387 | 0.0616 | 0.0371 | 0.0738 | 0.06087 | 0.06703 |
| 7 | 0.1777 | 0.1456 | 0.0916 | 0.05317 | 0.05853 | 0.1059 | 0.07933 | 0.08 | 0.02787 | 0.06903 | 0.03907 | 0.0722 |
| 8 | 0.1129 | 0.2133 | 0.0809 | 0.06043 | 0.06207 | 0.07973 | 0.09677 | 0.0964 | 0.01973 | 0.06597 | 0.03097 | 0.08087 |
| 9 | 0.05737 | 0.2036 | 0.0736 | 0.07683 | 0.06823 | 0.065 | 0.1299 | 0.1193 | 0.01433 | 0.07087 | 0.02283 | 0.09813 |
| 10 | 0.0187 | 0.1639 | 0.05877 | 0.08767 | 0.09003 | 0.05113 | 0.1546 | 0.1526 | 0.009267 | 0.07103 | 0.01567 | 0.1267 |
| 11 | 0.003133 | 0.09203 | 0.04827 | 0.1311 | 0.1047 | 0.02003 | 0.1594 | 0.1606 | 0.006167 | 0.08387 | 0.0063 | 0.1844 |
| 12 | 0.000267 | 0.02697 | 0.03423 | 0.2044 | 0.2143 | 0.008733 | 0.1793 | 0.1824 | 0.002267 | 0.0238 | 0.001933 | 0.1213 |
| Graft rejections requiring treatment | ||||||||||||
| Rank | Tac | Cy | Cy_Std | Ever | Tac_Ever | |||||||
| 1 | 0.07247 | 0.06747 | 0.1412 | 0.0279 | 0.691 | |||||||
| 2 | 0.4076 | 0.236 | 0.1216 | 0.08197 | 0.1527 | |||||||
| 3 | 0.3061 | 0.3952 | 0.1103 | 0.1127 | 0.07577 | |||||||
| 4 | 0.1736 | 0.2476 | 0.1811 | 0.3476 | 0.0502 | |||||||
| 5 | 0.04017 | 0.05377 | 0.4459 | 0.4299 | 0.0303 | |||||||
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
Mortality at maximal follow‐up
The network meta‐analysis of mortality at maximal follow‐up included a total of 21 trials (3492 participants) (Fung 1991; Porayko 1994; Stegall 1997; Belli 1998; Zervos 1998; Sterneck 2000; Jain 2001; Loinaz 2001; Chen 2002; O'Grady 2002; Greig 2003; Martin 2004; Pageaux 2004; Jonas 2005; Shenoy 2008; Masetti 2010; Boudjema 2011; Cholongitas 2011; De Simone 2012; Asrani 2014; Manousou 2014). In the network meta‐analysis, the between‐study standard deviation (τ) was 0.3949 (τ2 = 0.1559; lies within the 95% range for all‐cause mortality in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There was no evidence of inconsistency as evidenced by the model fit, treatment‐by‐design model, and inconsistency factor. The inconsistency plot is shown in Figure 5. As shown in Figure 5, there was only one comparison for which direct and indirect estimates were available. Forest plots of mortality (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 6. Both fixed‐effect model and random‐effects model for other interventions compared with tacrolimus are provided in Figure 6. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar except for tacrolimus plus sirolimus versus tacrolimus. Tacrolimus plus sirolimus causes more mortality at maximal follow‐up compared with tacrolimus in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis, but not in the random‐effects model of network meta‐analysis. Several other comparisons in which there was evidence of difference in fixed‐effect model showed no evidence of difference based on random‐effects model. We used the more conservative random‐effects model to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Figure 7. As shown in this figure, there was no evidence of difference in any of the pairwise comparisons in network meta‐analysis, although direct comparisons of single trials showed that tacrolimus plus sirolimus had higher mortality than tacrolimus (hazard ratio (HR) 2.76, 95% credible interval (CrI) 1.30 to 6.69) (1 trial; 222 participants), and tacrolimus plus glucocorticosteroids had lower mortality than cyclosporine A plus azathioprine plus glucocorticosteroids (HR 0.06, 95% CrI 0.00 to 0.91) (1 trial; 39 participants). The surface area under the curve for each intervention being best, second best, third best, and so on, and the ranking probabilities of each intervention being best, second best, third best, and so on, are shown in Figure 8. None of the interventions seems to be clearly better than any of the others.
5.
IF (Inconsistency Factor) plots of outcomes in which there were comparisons for which direct and indirect estimates were available (i.e. mortality at maximal follow‐up and graft rejection requiring treatment). There was no evidence of inconsistency in these outcomes, as the confidence intervals of the inconsistency factor overlapped one.
6.
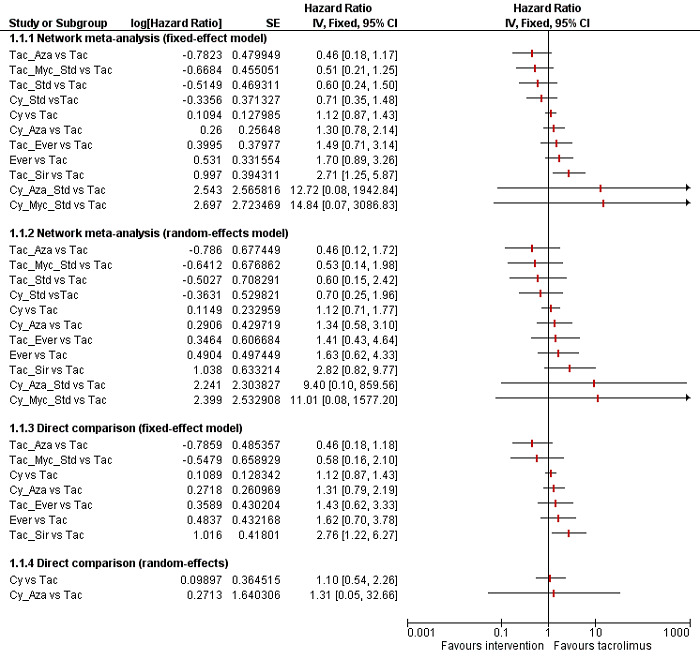
Forest plot of mortality at maximal follow‐up (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other Interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar except for tacrolimus plus sirolimus versus tacrolimus. Tacrolimus plus sirolimus causes more mortality at maximal follow‐up in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis but not in the random‐effects model of network meta‐analysis. We used the more conservative random‐effects model to arrive at conclusions.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
7.
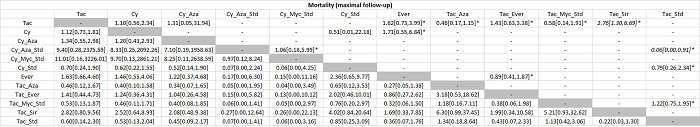
The table provides the effect estimate (hazard ratio) of each pairwise comparison for mortality at maximal follow‐up. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, there is no evidence of difference in any of the pairwise comparisons in the network meta‐analysis, although direct comparison showed that tacrolimus plus sirolimus had higher mortality than tacrolimus, and tacrolimus plus glucocorticosteroids had lower mortality than cyclosporine A plus azathioprine plus glucocorticosteroids in single trials.
* = single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
8.
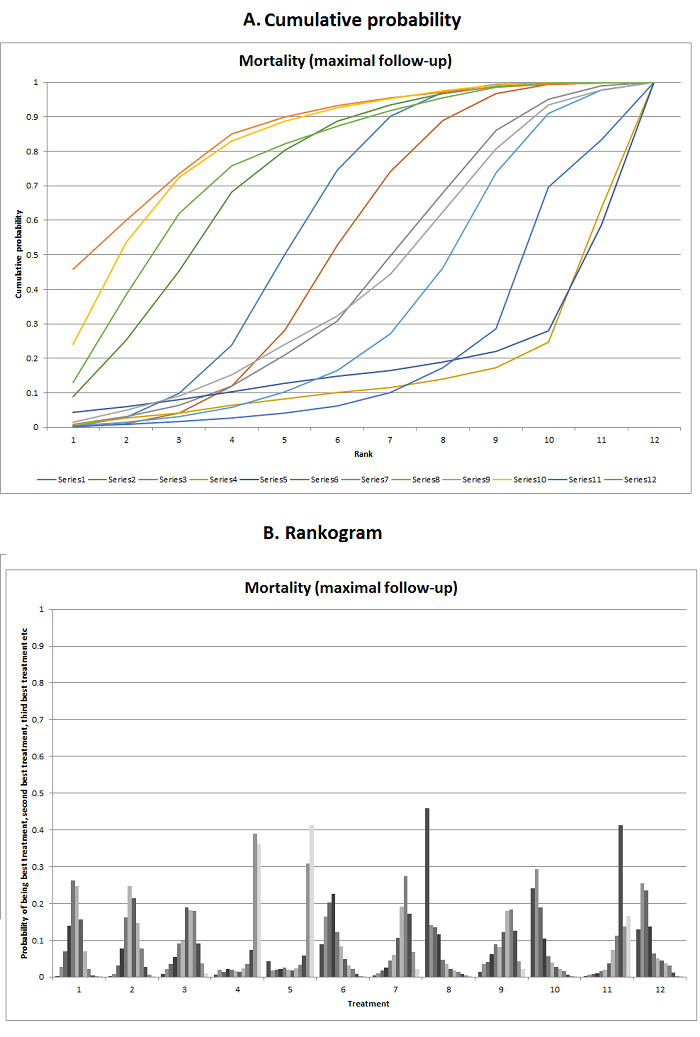
Mortality (maximal follow‐up)
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one Intervention is clearly better than any of the other Interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus azathioprine; 4: cyclosporine A plus azathioprine plus glucocorticosteroids; 5: cyclosporine A plus mycophenolate plus glucocorticosteroids; 6: cyclosporine A plus glucocorticosteroids; 7: everolimus; 8: tacrolimus plus azathioprine; 9: tacrolimus plus everolimus; 10: tacrolimus plus mycophenolate plus glucocorticosteroids; 11: tacrolimus plus sirolimus; 12: tacrolimus plus glucocorticosteroids.
Graft loss at maximal follow‐up
The network meta‐analysis of graft loss at maximal follow‐up included a total of 15 trials (2961 participants) (Fung 1991; Stegall 1997; Zervos 1998; Jain 2001; Loinaz 2001; Chen 2002; O'Grady 2002; Greig 2003; Jonas 2005; Shenoy 2008; Boudjema 2011; Cholongitas 2011; De Simone 2012; Asrani 2014; Manousou 2014). The between‐study standard deviation (τ) was 0.6253 (τ2 = 0.3910; lies within the 95% range for semi‐objective outcomes in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Forest plots of graft loss (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 9. Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided in Figure 9. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar except for tacrolimus plus sirolimus versus tacrolimus. Tacrolimus plus sirolimus causes more graft loss at maximal follow‐up than tacrolimus in the direct comparison (HR 2.34, 95% CrI 1.28 to 4.61) (1 trial; 222 participants) and fixed‐effect model of network meta‐analysis but not in the random‐effects model of network meta‐analysis. As in the case of mortality at maximal follow‐up, several other comparisons in which there was evidence of difference in fixed‐effect model did not show any evidence of difference based on random‐effects model. We used the more conservative random‐effects model to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Figure 10. As shown in Figure 10, there was no evidence of difference in any of the pairwise comparisons in the network meta‐analysis. The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best, and so on are shown in Figure 11. None of the interventions seems to be clearly better than any of the others.
9.
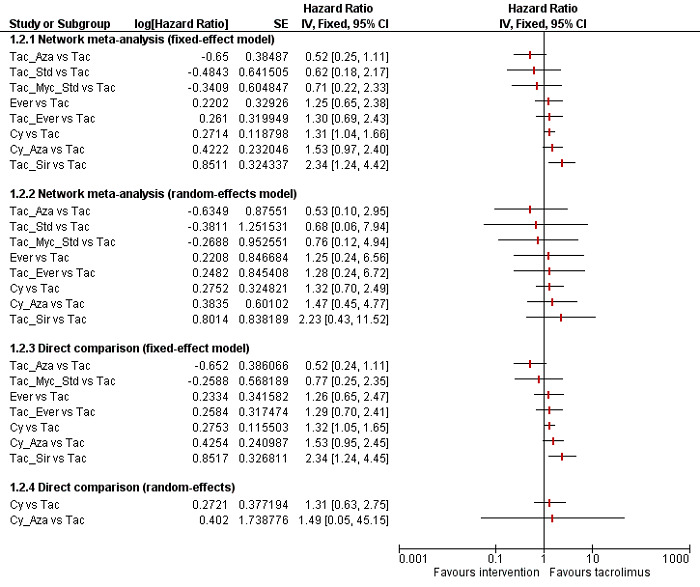
Forest plot of graft loss at maximal follow‐up (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other Interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar except for tacrolimus plus sirolimus versus tacrolimus. Tacrolimus plus sirolimus causes more graft loss at maximal follow‐up in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis but not in the random‐effects model of network meta‐analysis. We used the more conservative random‐effects model to arrive at conclusions.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
10.
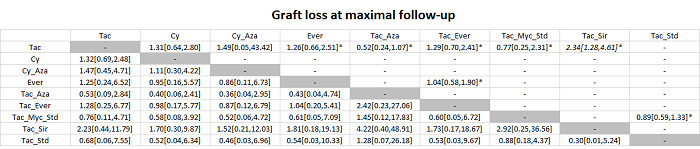
The table provides the effect estimate (hazard ratio) of each pairwise comparison for graft loss at maximal follow‐up. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, there was no evidence of difference in any of the pairwise comparisons in the network meta‐analysis, although tacrolimus plus sirolimus appears to increase graft loss at maximal follow‐up compared to tacrolimus.
* single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
11.
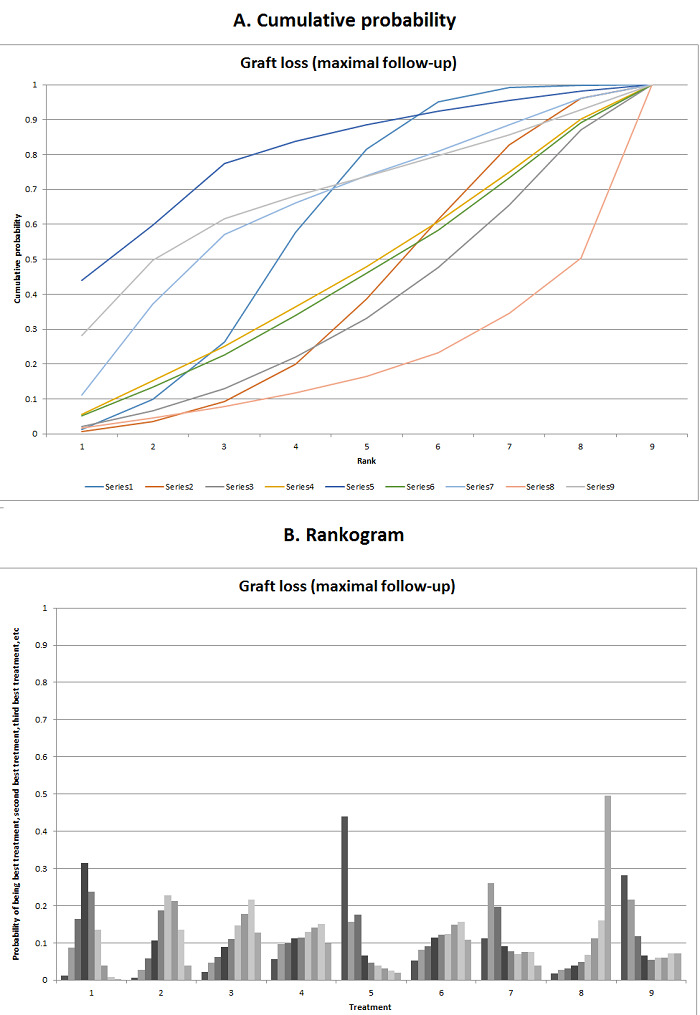
Graft loss (maximal follow‐up)
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one intervention is clearly better than any of the other interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus azathioprine; 4: everolimus; 5: tacrolimus plus azathioprine; 6: tacrolimus plus everolimus; 7: tacrolimus plus mycophenolate plus glucocorticosteroids; 8: tacrolimus plus sirolimus; 9: tacrolimus plus glucocorticosteroids.
Adverse events
Serious adverse events (proportion)
One trial (719 participants) reported on proportion of people with serious adverse events (De Simone 2012). There was no evidence that any of the pair‐wise comparisons affected the proportion of people with serious adverse events:
everolimus versus tacrolimus: odds ratio (OR) 1.40 (95% CrI 0.98 to 2.03);
everolimus plus tacrolimus versus tacrolimus: OR 1.21 (95% CrI 0.85 to 1.75);
everolimus plus tacrolimus versus everolimus: OR 0.86 (95% CrI 0.60 to 1.24).
Serious adverse events (number)
None of the trials reported the number of serious adverse events.
Any adverse events (proportion)
A total of two trials including 940 participants reported proportion of people with adverse events and were included in the network meta‐analysis (De Simone 2012; Asrani 2014). The between‐study standard deviation was so small that it was very close to the prior value (average standard deviation of the uniform distribution of 2.5). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Forest plots of adverse events (proportion) (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 12. Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided in Figure 12. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. There was no evidence of difference between any of the interventions (Figure 13), despite the surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best showing that tacrolimus plus sirolimus may be the worst intervention in terms of adverse events (proportion) (Figure 14).
12.
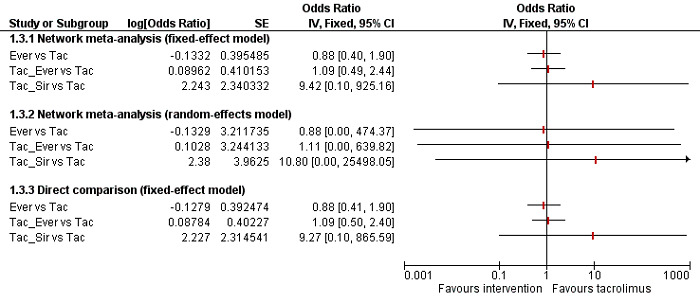
Forest plot of adverse events (proportion) (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared with tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar. As there was only trial for each comparison, the random‐effects model for the direct comparisons is not provided. There was no evidence of difference between any of the comparisons.
Abbreviations: Tac = tacrolimus; Ever = everolimus; Sir = sirolimus
13.
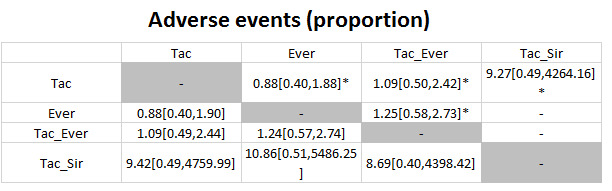
The table provides the effect estimate (odds ratio) of each pairwise comparison for adverse events (proportion) corresponding to intervention B. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison. Treatment effects with evidence of difference are shown in italics. As presented, there was no evidence of difference in any of the pairwise comparisons.
* single trial
Abbreviations: Tac = tacrolimus; Ever = everolimus; Sir = sirolimus
14.
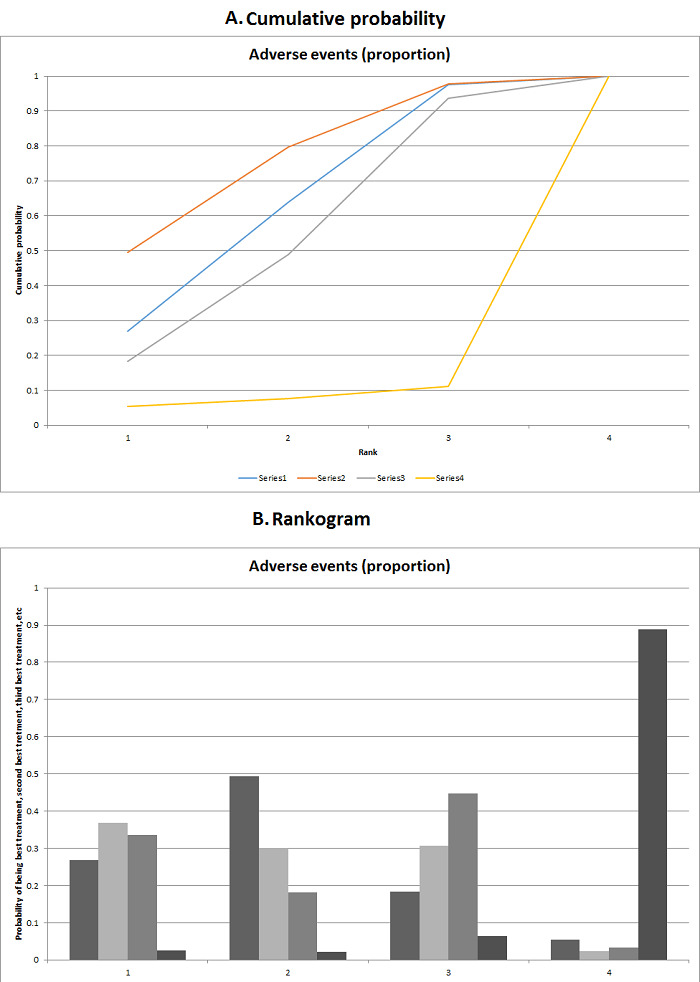
Adverse events (proportion)
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. Although the figure shows that tacrolimus plus sirolimus was the worst intervention in terms of adverse events (proportion), there was no evidence of differences in the odds ratios between the different interventions. Legend: 1: tacrolimus; 2: everolimus; 3: tacrolimus plus everolimus; 4: tacrolimus plus sirolimus.
Any adverse events (number)
The network meta‐analysis included a total of 12 trials (1748 participants) that reported the number of adverse events (Fung 1991; Stegall 1997; Belli 1998; Zervos 1998; Loinaz 2001; O'Grady 2002; Greig 2003; Pageaux 2004; Shenoy 2008; Masetti 2010; Boudjema 2011; Pelletier 2013). The between‐study standard deviation was so small that it was very close to the prior value (average standard deviation of the uniform distribution of 2.5). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Figure 15 shows forest plots of adverse events (numbers) (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided in Figure 15. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. We have reported the fixed‐effect model. The number of adverse events appears to be lower in the cyclosporine A group than in the tacrolimus group. Figure 16 shows the effect estimates of each pairwise comparison from network meta‐analysis and direct comparisons. As shown in Figure 16, cyclosporine A appears to be associated with fewer adverse events than tacrolimus and cyclosporine A plus glucocorticosteroids (based on both network meta‐analysis and direct comparisons), and fewer adverse events than tacrolimus plus mycophenolate and tacrolimus plus mycophenolate plus glucocorticosteroids groups based on network meta‐analysis. Tacrolimus plus mycophenolate also appears to be associated with more adverse events than everolimus based on network meta‐analysis. The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best show that tacrolimus plus mycophenolate may be the worst intervention in terms of number of adverse events (Figure 17). Cyclosporine A, which has a high probability of being in the top‐two ranks, was associated with fewer adverse events than most of the other interventions except everolimus in the pairwise comparisons, as mentioned above.
15.
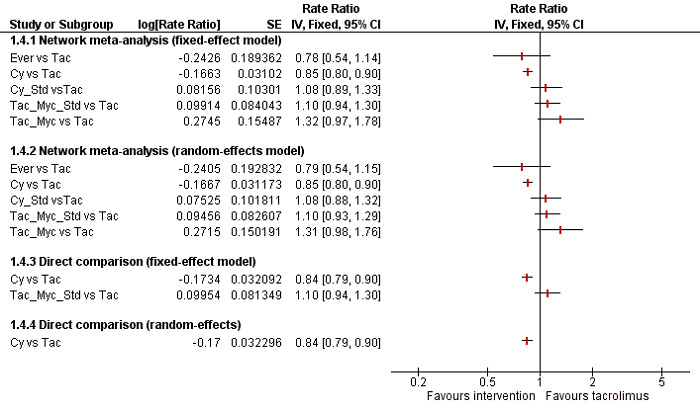
Forest plot of adverse events (number) (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar. The number of adverse events appears to be higher for cyclosporine A than for tacrolimus. There was no evidence of difference in the remaining comparisons with tacrolimus.
Abbreviations: Cy = cyclosporine A; Tac = tacrolimus; Ever = everolimus; Myc = mycophenolate; Sir = sirolimus; Std = glucocorticosteroids
16.
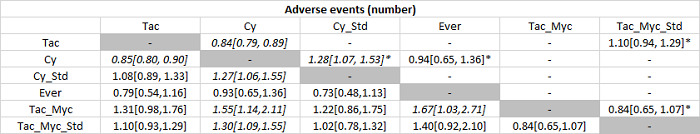
The table provides the effect estimate (rate ratio) of each pairwise comparison for adverse events (number) corresponding to intervention B. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison. Treatment effects with evidence of difference are shown in italics. As presented, cyclosporine A appears to be associated with fewer adverse events than tacrolimus and cyclosporine A plus glucocorticosteroids (based on both network meta‐analysis and direct comparisons), and fewer adverse events than tacrolimus plus mycophenolate and tacrolimus plus mycophenolate plus glucocorticosteroids based on network meta‐analysis. Tacrolimus plus mycophenolate also appears to be associated with more adverse events than everolimus based on network meta‐analysis.
* single trial
Abbreviations: Cy = cyclosporine A; Tac = tacrolimus; Ever = everolimus; Myc = mycophenolate; Sir = sirolimus; Std = glucocorticosteroids
17.
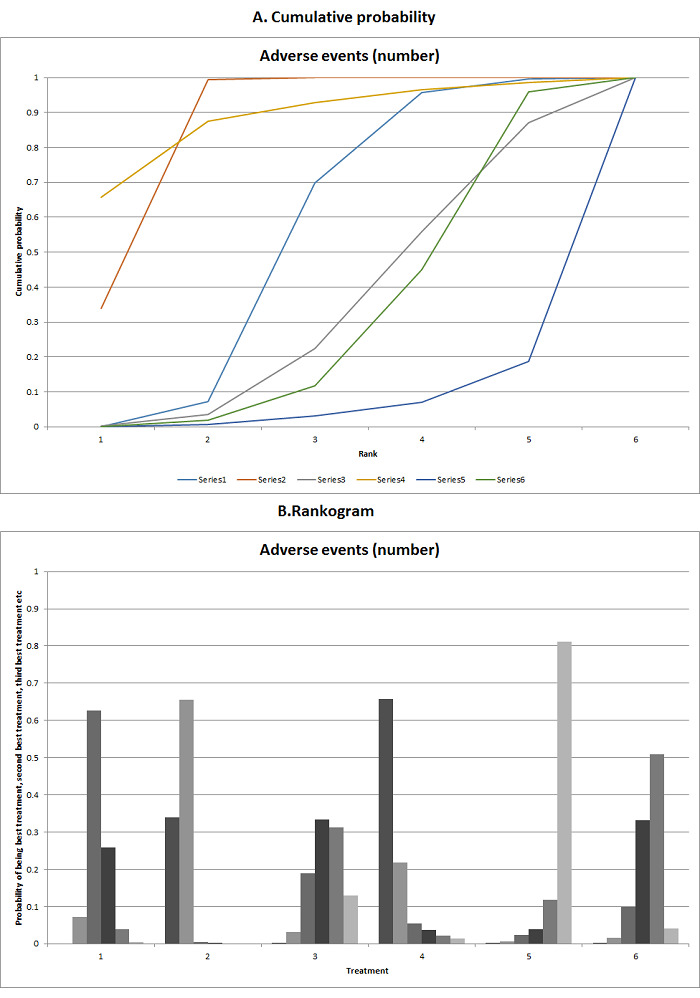
Adverse events (number)
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. Although the figure shows that tacrolimus plus mycophenolate appears to be the worst intervention in terms of number of adverse events, the pairwise comparisons show that there was no evidence of difference between tacrolimus plus mycophenolate and other comparisons except everolimus. Cylosporine A, which has a high probability of being in the top two ranks, was associated with fewer adverse events than other interventions with the exception of everolimus in the pairwise comparisons. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus glucocorticosteroids; 4: everolimus; 5: tacrolimus plus mycophenolate; 5: tacrolimus plus mycophenolate plus glucocorticosteroids.
Renal impairment
The network meta‐analysis included a total of eight trials (2233 participants) for renal impairment (Fung 1991; Porayko 1994; Jain 2001; O'Grady 2002; Greig 2003; Boudjema 2011; De Simone 2012; Pelletier 2013). The between‐study standard deviation (τ) was 1.273 (τ2 = 1.6205; lies outside the 95% range for semi‐objective outcomes in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Figure 18 shows forest plots of renal impairment (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided in Figure 18. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar except for tacrolimus plus mycophenolate plus glucocorticosteroids versus tacrolimus. Tacrolimus plus mycophenolate plus glucocorticosteroids causes less renal impairment compared with tacrolimus in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis, but not in the random‐effects model of network meta‐analysis. We used the more conservative random‐effects model to arrive at conclusions. Figure 19 shows the pairwise meta‐analysis estimates of the random‐effects model. As shown in Figure 19, there is no evidence of difference in any of the pairwise comparisons in network meta‐analysis, although direct comparison of a single trial showed tacrolimus plus mycophenolate plus glucocorticosteroids to have less renal impairment compared with tacrolimus. The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best, and so on are shown in Figure 20. None of the interventions seems to be clearly better than any of the others.
18.
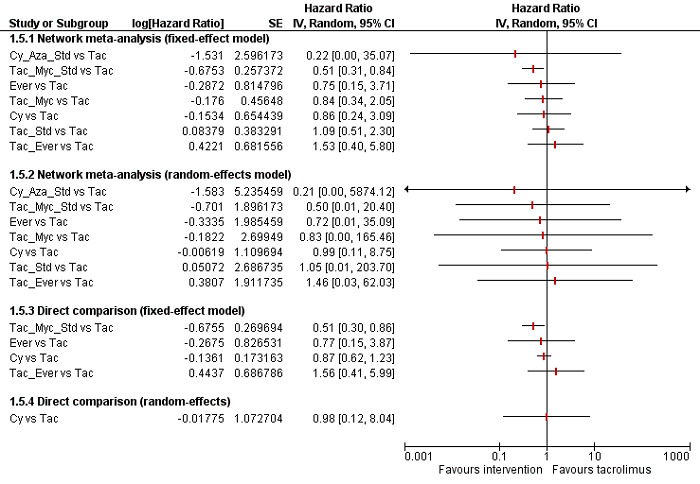
Forest plot of renal impairment (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar except for tacrolimus plus mycophenolate plus glucocorticosteroids versus tacrolimus. Tacrolimus plus mycophenolate plus glucocorticosteroids causes less renal impairment in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis but not in the random‐effects model of network meta‐analysis. We used the more conservative random‐effects model to arrive at conclusions.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Ever = everolimus; Std = glucocorticosteroids; _ = plus
19.
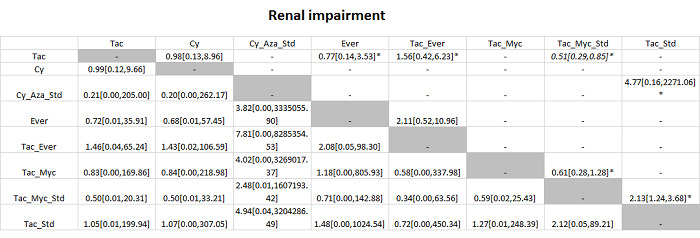
The table provides the effect estimate (hazard ratio) of each pairwise comparison for renal impairment. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, there was no evidence of difference in any of the pairwise comparisons in the network meta‐analysis, although tacrolimus plus mycophenolate plus glucocorticosteroids was associated with less renal impairment than tacrolimus in a single trial.
* single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Ever = everolimus; Std = glucocorticosteroids; _ = plus
20.
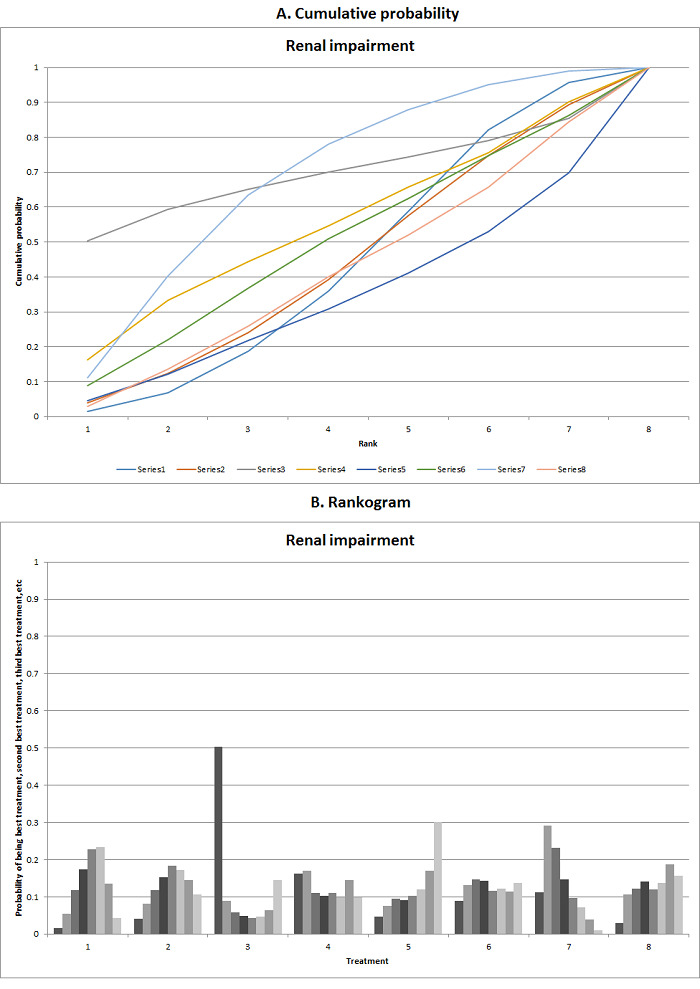
Renal impairment
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one intervention is clearly better than any of the other interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus azathioprine plus glucocorticosteroids; 4: everolimus; 5: tacrolimus plus everolimus; 6: tacrolimus plus mycophenolate; 7: tacrolimus plus mycophenolate plus glucocorticosteroids; 8: tacrolimus plus glucocorticosteroids.
Chronic kidney disease
Only one trial (100 participants) reported chronic kidney disease (Pelletier 2013). There was no evidence of difference between tacrolimus plus mycophenolate plus glucocorticosteroids and tacrolimus plus mycophenolate (OR 0.38, 95% CrI 0.11 to 1.17).
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Retransplantation
The network meta‐analysis included a total of 13 trials (1994 participants) for retransplantation (Fung 1991; Porayko 1994; Zervos 1998; Jain 2001; Chen 2002; O'Grady 2002; Greig 2003; Jonas 2005; Shenoy 2008; Boudjema 2011; Cholongitas 2011; Pelletier 2013; Manousou 2014). The between‐study standard deviation (τ) was 0.7429 (τ2 = 0.5519; lies within the 95% range for semi‐objective outcomes in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Forest plots of retransplantation (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 21. Both fixed‐effect model and random‐effects model for other interventions compared with tacrolimus are provided in Figure 21. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar. Cyclosporine A resulted in higher incidence of retransplantation compared with tacrolimus. As there were differences in the effect estimates in other comparisons, we used the more conservative random‐effects model to arrive at conclusions. The pair‐wise meta‐analysis estimates of the random‐effects model are shown in Figure 22. As shown in Figure 21, cyclosporine A had a higher incidence of retransplantation compared with tacrolimus (HR 3.08, 95% CrI 1.13 to 9.90), and tacrolimus plus mycophenolate plus glucocorticosteroids had a lower incidence of retransplantation compared with tacrolimus plus mycophenolate (HR 0.03, 95% CrI 0.00 to 0.90). The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best, and so on are shown in Figure 23. None of the interventions seems to be clearly better than any of the others.
21.
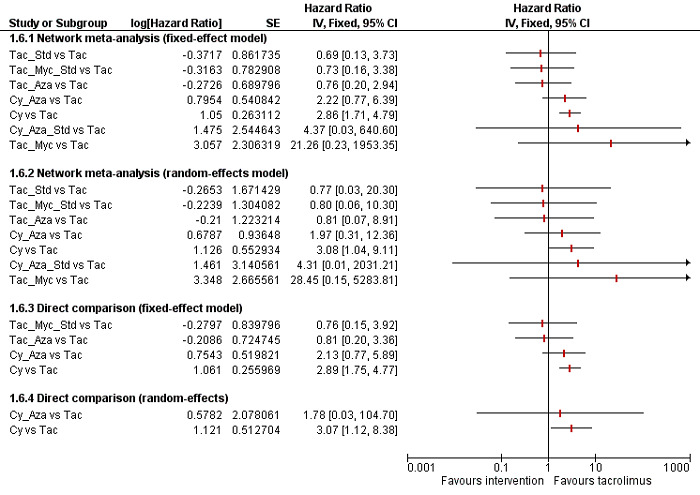
Forest plot of retransplantation (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar, although the random‐effects model was more conservative. Cyclosporine A resulted in higher incidence of retransplantation than tacrolimus.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Std = glucocorticosteroids; _ = plus
22.
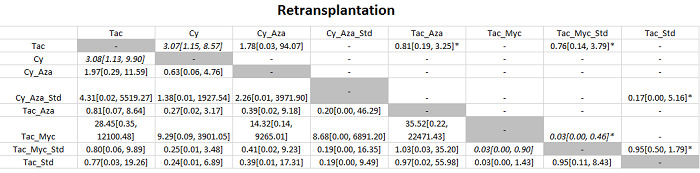
The table provides the effect estimate (hazard ratio) of each pairwise comparison for retransplantation. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, cyclosporine A is associated with a higher incidence of retransplantation than tacrolimus, and tacrolimus plus mycophenolate plus glucocorticosteroids showed a lower incidence of retransplantation than tacrolimus plus mycophenolate.
* single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Std = glucocorticosteroids; _ = plus
23.
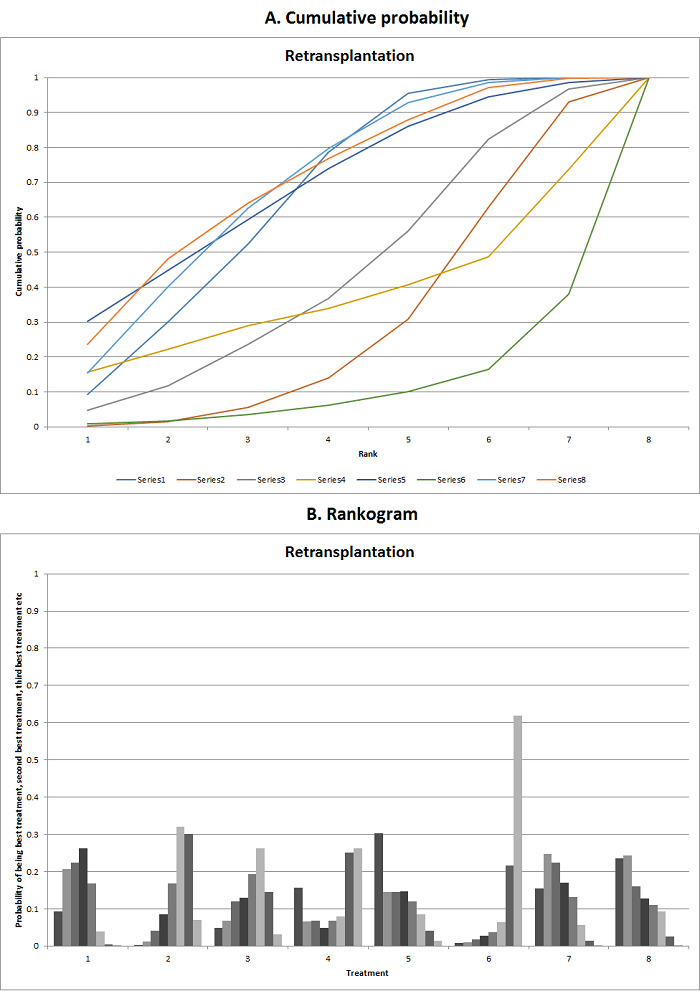
Retransplantation
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one intervention is clearly better than any of the other interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus azathioprine; 4: cyclosporine A plus azathioprine plus glucocorticosteroids; 5: tacrolimus plus azathioprine; 6: tacrolimus plus mycophenolate; 7: tacrolimus plus mycophenolate plus glucocorticosteroids; 8: tacrolimus plus glucocorticosteroids
Graft rejections
Graft rejections (any)
Network meta‐analysis of graft rejections (any) included a total of 16 trials (2726 participants) (Fung 1991; Porayko 1994; Fisher 1998; Zervos 1998; Loinaz 2001; O'Grady 2002; Greig 2003; Martin 2004; Pageaux 2004; Jonas 2005; Shenoy 2008; Boudjema 2011; Cholongitas 2011; De Simone 2012; Pelletier 2013; Manousou 2014). The between‐study standard deviation (τ) was 0.5755 (τ2 = 0.3312; lies within the 95% range for subjective outcomes in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There were no direct and indirect estimates for the same comparison, and so we did not assess inconsistency. Forest plots of graft rejections (any) (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 24. Both fixed‐effect model and random‐effects model for other interventions compared with tacrolimus are provided in Figure 24. As shown in the figure, the direct estimates (fixed‐effect model) and fixed‐effect network meta‐analysis estimates were similar and demonstrated fewer graft rejections (any) in tacrolimus plus everolimus and tacrolimus plus mycophenolate plus glucocorticosteroids than tacrolimus, while there were more graft rejections (any) with cyclosporine A and everolimus compared with tacrolimus. However, the random‐effects model did not demonstrate any evidence of difference in graft rejections (any) for any comparison. We used the more conservative random‐effects model to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Figure 25. As shown in Figure 25, there was no evidence of difference in any of the pairwise comparisons in network meta‐analysis, although direct comparisons involving single trials showed fewer graft rejections (any) in the tacrolimus plus everolimus and tacrolimus plus mycophenolate plus glucocorticosteroids groups compared with the tacrolimus group, while cyclosporine A and everolimus had more graft rejections (any) compared with tacrolimus in single trials. Tacrolimus plus everolimus also had fewer graft rejections (any) compared with everolimus based on evidence from a single trial. The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best, and so on are shown in Figure 26. None of the interventions seems to be clearly better than any of the others.
24.
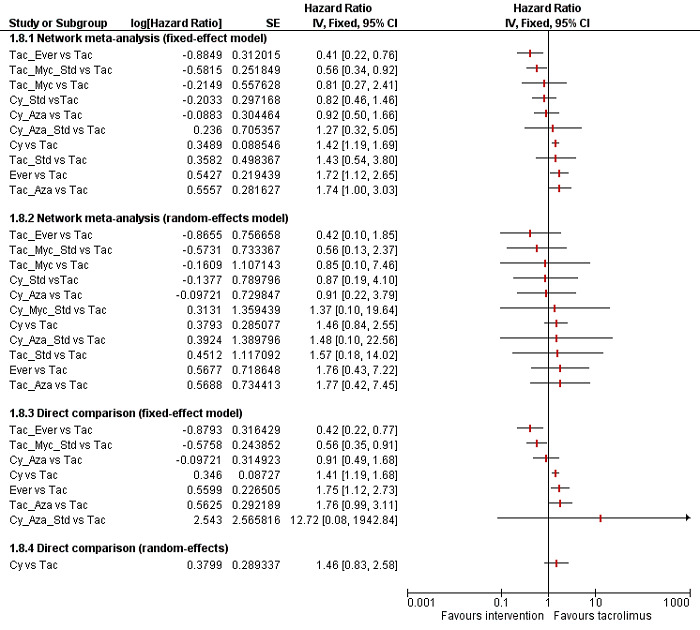
Forest plot of graft rejections (any) (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided. The direct estimates and fixed‐effect network meta‐analysis estimates are similar and demonstrate fewer graft rejections (any) for tacrolimus plus everolimus and tacrolimus plus mycophenolate plus glucocorticosteroids than for tacrolimus, while cyclosporine A and everolimus were associated with more graft rejections (any) than tacrolimus. However, the random‐effects model did not demonstrate any evidence of difference in graft rejections (any) for any comparison. We used the more conservative random‐effects model to arrive at conclusions.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Ever = everolimus; Std = glucocorticosteroids; _ = plus
25.
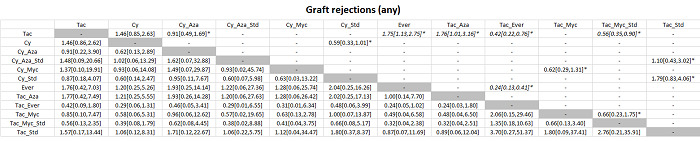
The table provides the effect estimate (hazard ratio) of each pairwise comparison for graft rejections (any). The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, there was no evidence of difference in any of the pairwise comparisons in the network meta‐analysis, although direct comparison showed fewer graft rejections (any) for tacrolimus plus everolimus and tacrolimus plus mycophenolate plus glucocorticosteroids than for tacrolimus, while cyclosporine A and everolimus were associated with more graft rejections (any) than tacrolimus in single trials. Tacrolimus plus everolimus also showed fewer graft rejections (any) than everolimus based on evidence from a single trial.
* = single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Ever = everolimus; Std = glucocorticosteroids; _ = plus
26.
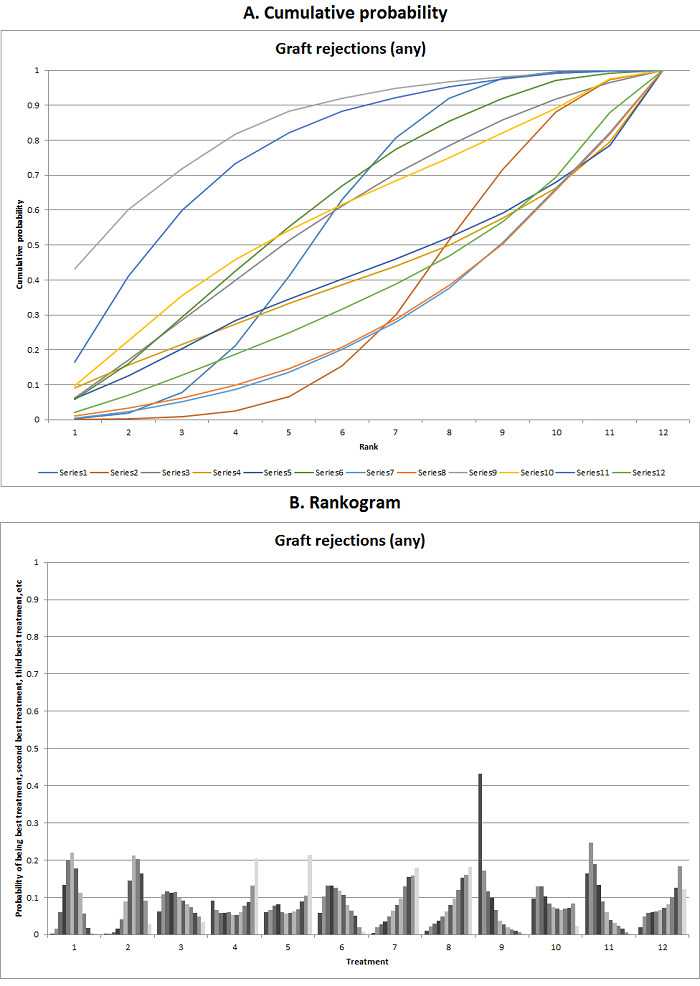
Graft rejections (any)
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one intervention is clearly better than any of the other interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus azathioprine; 4: cyclosporine A plus azathioprine plus corticosteroids; 5: cyclosporine A plus mycophenolate; 6: cyclosporine A plus glucocorticosteroids; 7: everolimus; 8: tacrolimus plus azathioprine; 9: tacrolimus plus everolimus; 10: tacrolimus plus mycophenolate; 11: tacrolimus plus mycophenolate plus glucocorticosteroids; 12: tacrolimus plus glucocorticosteroids.
Graft rejections requiring treatment
Network meta‐analysis included a total of five trials (1025 participants) for graft rejections requiring treatment (Stegall 1997; Belli 1998; Masetti 2010; Cholongitas 2011; De Simone 2012). In the network meta‐analysis, the between‐study standard deviation (τ) was 0.9127 (τ2 = 0.8330; lies within the 95% range for subjective outcomes in pharmacological comparisons) (Turner 2012). We could not estimate the I2. There was no evidence of inconsistency as evidenced by the model fit, treatment‐by‐design model, and inconsistency factor. The inconsistency plot is shown in Figure 5. However, as shown in Figure 5, there was only one comparison for which direct and indirect estimates were available. Forest plots of graft rejections requiring treatment (network meta‐analysis estimates and direct comparisons when available) are shown in Figure 27. Both fixed‐effect model and random‐effects model for other interventions compared with tacrolimus are provided in Figure 27. As shown in the figure, the direct estimates and network meta‐analysis estimates of different models were similar except for everolimus versus tacrolimus. Everolimus causes more graft rejections requiring treatment compared with tacrolimus in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis, but not in the random‐effects model of network meta‐analysis. One other comparison in which there was evidence of difference in fixed‐effect model did not show evidence of difference based on random‐effects model. We used the more conservative random‐effects model to arrive at conclusions. The pairwise meta‐analysis estimates of the random‐effects model are shown in Figure 28. As shown in Figure 28, there was no evidence of difference in any of the pairwise comparisons in network meta‐analysis, although direct comparisons involving single trials showed everolimus to have a higher incidence of graft rejections requiring treatment compared with tacrolimus and tacrolimus plus everolimus. The surface area under the curve for each intervention being best, second best, third best, and so on and the ranking probabilities of each intervention being best, second best, third best, and so on are shown in Figure 29. None of the interventions seems to be clearly better than any of the others.
27.
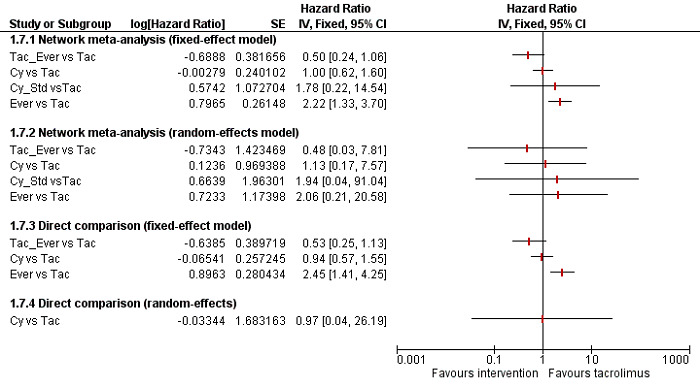
Forest plot of graft rejections requiring treatment (network meta‐analysis estimates and direct comparisons when available). Both fixed‐effect model and random‐effects model for other interventions compared to tacrolimus are provided. The direct estimates and network meta‐analysis estimates are similar except for everolimus versus tacrolimus. Everolimus causes more graft rejections than tacrolimus in the direct comparison involving one trial and fixed‐effect model of network meta‐analysis but not in the random‐effects model of network meta‐analysis. We used the more conservative random‐effects model to arrive at conclusions.
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
28.
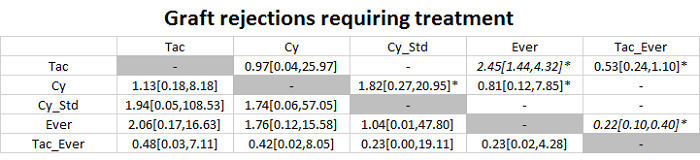
The table provides the effect estimate (hazard ratio) of each pairwise comparison for graft rejection requiring treatment. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Treatment effects with evidence of difference are shown in italics. As presented, there was no evidence of difference in any of the pairwise comparisons in the network meta‐analysis, although direct comparison showed that everolimus caused more graft rejections requiring treatment than tacrolimus and tacrolimus plus everolimus in a single trial.
* = single trial
Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus
29.
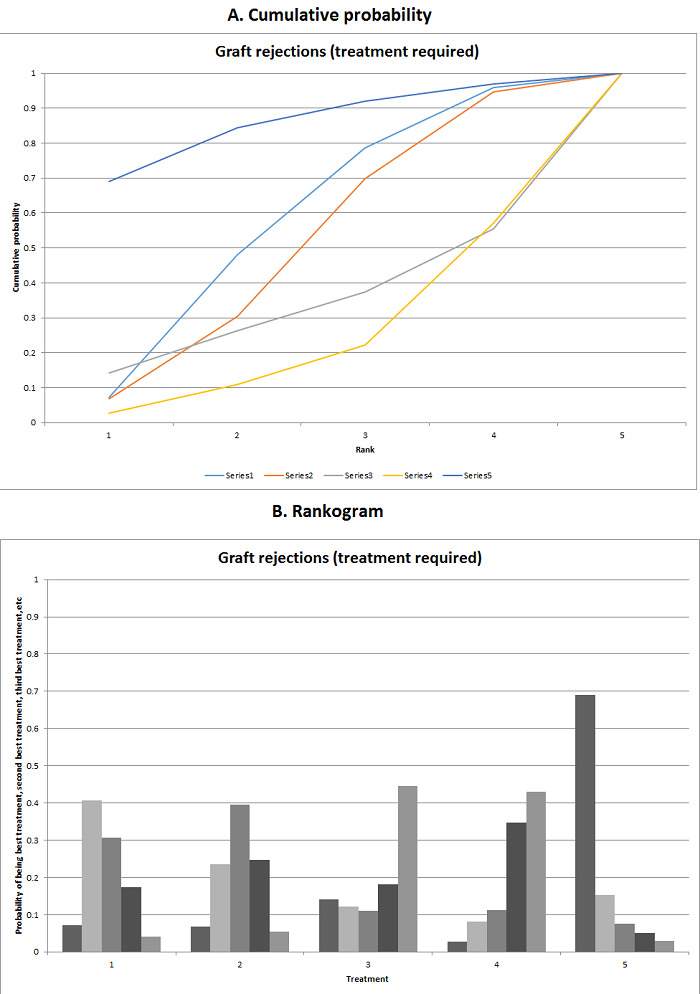
Graft rejections requiring treatment
A. The figure shows the surface area under the curve constructed on the basis of the ranking probabilities. B. The figure shows the probability of each Intervention being best, second best, third best, and so on. There was no evidence that one intervention is clearly better than any of the other interventions. Legend: 1: tacrolimus; 2: cyclosporine A; 3: cyclosporine A plus glucocorticosteroids; 4: everolimus; 5: tacrolimus plus everolimus.
Costs
None of the trials reported on costs.
Subgroup analyses
Because of the nature of the data (most trials included only participants undergoing primary transplantation; most trials included participants with varied aetiology without separate outcome data based on aetiology; and the absence of any one trial at low risk of bias), we did not perform these subgroup analyses. We considered tacrolimus and cyclosporine A as different interventions, therefore we did not perform a subgroup analysis of the same class of drugs. We performed a post hoc subgroup analysis of trials in which the drug combination of the induction immunosuppression differed from that of the maintenance immunosuppression (i.e. additional drug was used for induction) compared to trials in which the drug combination of the induction immunosuppression was the same as that of the maintenance immunosuppression (no additional drug was used for induction). The credible intervals of the interaction term were extremely wide and overlapped zero for all comparisons other than adverse events (number). The interaction term for the meta‐regression of adverse events (number) was 0.80 (95% CrI 0.37 to 1.70). However, it should be noted that in only one of the trials in this comparison did participants receive the same drug combination as the induction and maintenance immunosuppression (Pelletier 2013). A subgroup analysis did not alter the interpretation of the results.
Sensitivity analysis
We did not perform a sensitivity analysis of imputing information based on different scenarios because of the paucity of data to carry out these analyses (i.e. the postrandomisation dropouts when described were few, and the trial authors did not report the participant flow adequately to perform these sensitivity analyses). We did not impute standard deviation (as none of the trials reported health‐related quality of life or costs), therefore we did not perform a sensitivity analysis to assess the impact of imputing the standard deviation.
Trial Sequential Analysis
We performed a Trial Sequential Analysis for mortality at maximal follow‐up for various comparisons. As shown in Figure 30 and Figure 31, the cumulative Z‐curves (blue lines) did not cross any of the trial sequential monitoring boundaries (red lines) for any of the comparisons, and neither did they cross the conventional alpha boundary of 2.5% (green lines), suggesting a high risk of random error.
30.
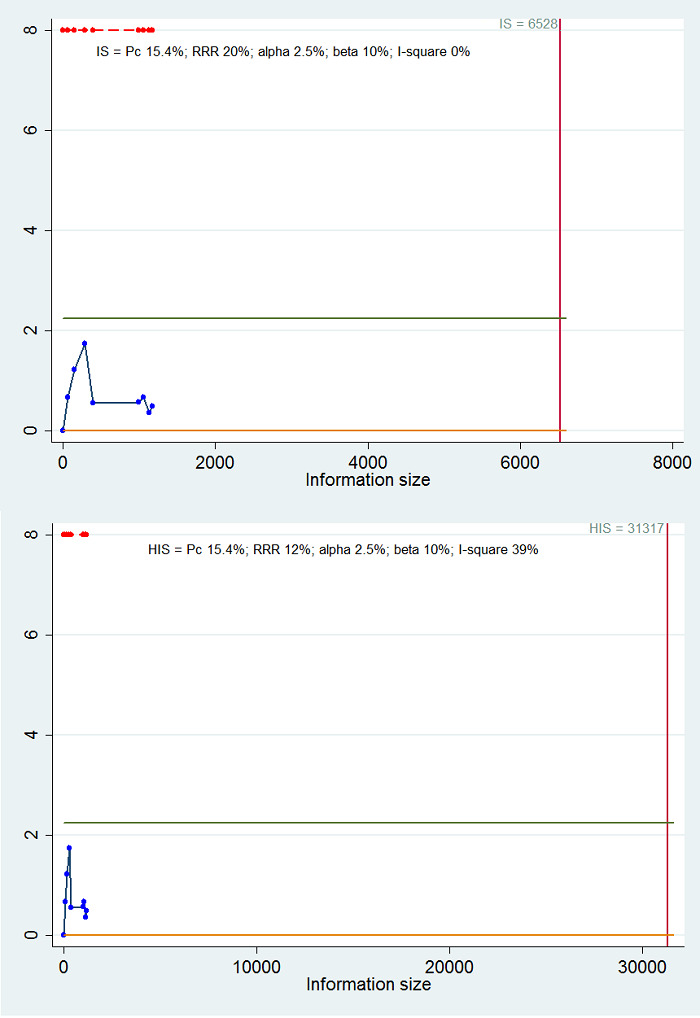
Trial Sequential Analysis of mortality at maximal follow‐up for cyclosporine A versus tacrolimus. We used an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20% (upper figure) and that observed in trials (12%) (lower figure), control group proportion (Pc) observed in the trials (15.4% mortality), and I2 of 0% (upper figure) and that observed in the trials (I2 = 39%) (lower figure). The accrued sample size (1176) is only a fraction of the information size (IS) (6528 trial participants) or heterogeneity‐adjusted information size (HIS) (31,317 trial participants). As shown in all of the comparisons, the cumulative Z‐curves (blue line) do not cross any of the trial sequential monitoring boundaries (red lines), and neither do they cross the conventional alpha boundary of 2.5% (green line).
31.
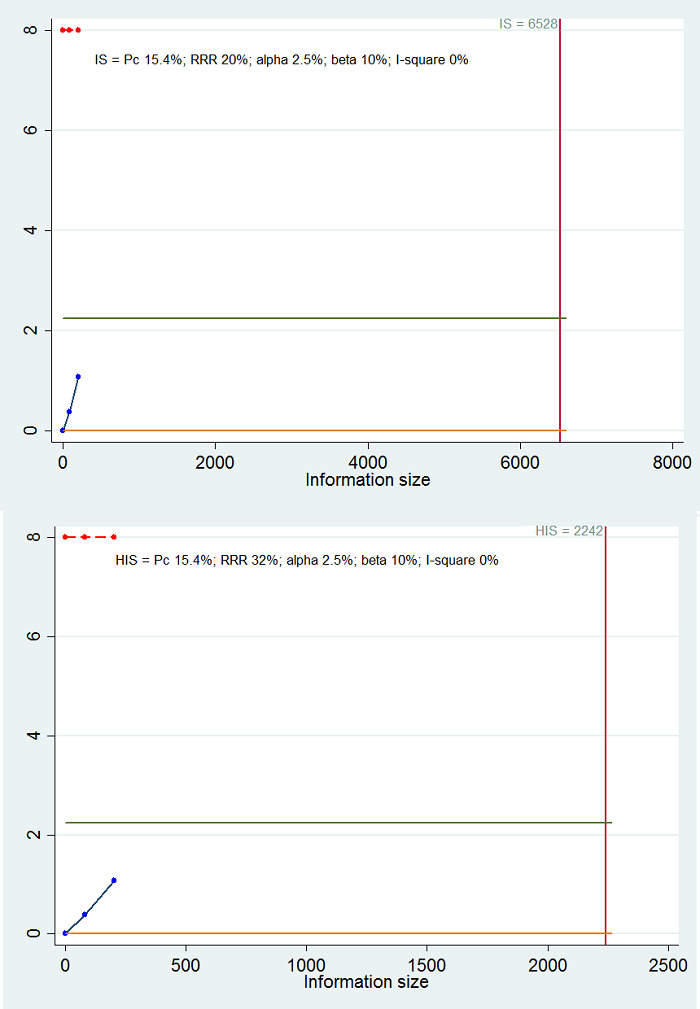
Trial Sequential Analysis of mortality at maximal follow‐up for cyclosporine A plus azathioprine versus tacrolimu. We used an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20% (upper figure) and that observed in trials (12%) (lower figure), control group proportion (Pc) observed in the trials (15.4% mortality), and I2 of 0% (heterogeneity observed in the trials). The accrued sample size (202) is only a fraction of the information size (IS) (6528 trial participants) or heterogeneity‐adjusted information size (HIS) (2242 trial participants). As shown in all of the comparisons, the cumulative Z‐curves (blue line) do not cross any of the trial sequential monitoring boundaries (red lines), and neither do they cross the conventional alpha boundary of 2.5% (green line).
Quality of evidence
The overall quality of the evidence was low or very low for all comparisons due to the high risk of bias in the trials (downgraded by one level); heterogeneity for indirect comparisons, as there were differences in the effect estimates obtained by fixed‐effect model and random‐effects model (downgraded by one level); indirectness in all indirect comparisons because of sparse network made up of trials of high risk of bias (downgraded one level); small sample size for direct comparisons (downgraded by one level); and imprecision for all comparisons in which the credible intervals overlapped a clinically significant increase or reduction and clinically insignificant increase or reduction (20% relative risk reduction was considered clinically significant) (downgraded by one level).
Discussion
Summary of main results
We included a total of 26 trials (3842 participants) in this review, and 3693 participants from 23 trials were included in one or more outcomes in this review assessing maintenance immunosuppression for adults undergoing liver transplantation. There was no evidence of inconsistency in the two networks (mortality at maximal follow‐up and graft rejections requiring treatment) in which we could assess this, and the effect estimates from direct comparisons and network meta‐analysis were reasonably similar.
The mortality (maximal follow‐up) and graft loss (maximal follow‐up) were higher for tacrolimus plus sirolimus compared with tacrolimus in a single trial including 222 participants based on direct comparisons, however there was no evidence of difference based on network meta‐analysis results. It appears that adding sirolimus to the standard immunosuppressive regimen worsens the outcomes. Most trials did not report serious adverse events, despite this being an important outcome for patients and healthcare funders. There were fewer adverse events with cyclosporine A compared with tacrolimus in our network meta‐analysis. As shown in Figure 16, cyclosporine A appears to be associated with fewer adverse events compared with most other interventions, but the implications of this are unclear, as the impact of these adverse events on the participant's health‐related quality of life was not reported. There was no evidence of differences in renal impairment based on network meta‐analysis. Only one trial reported the number of people with chronic kidney disease, despite this being one of the major aspects determining the immunosuppressive regimen. Most trials reported kidney function as the mean values in the groups, which is not helpful in identifying whether people receiving a particular immunosuppressive regimen developed chronic kidney disease more often. None of the trials reported health‐related quality of life. This is an important clinical outcome that should be reported in future trials.
Incidence of retransplantation was higher with cyclosporine A compared with tacrolimus. Again, this is an important clinical outcome, as it has huge implications for the patient and healthcare funders. A previous Cochrane systematic review that compared tacrolimus and cyclosporine A (and accepted other maintenance immunosuppressive agents as co‐interventions) concluded that tacrolimus was better than cyclosporine A in terms of patient survival (Haddad 2006). It should also be noted that most recent trials use tacrolimus monotherapy or tacrolimus‐based therapy as the control group, suggesting that tacrolimus is considered the standard against which other immunosuppressive agents are compared. We found no reliable evidence that any of the other interventions are better than tacrolimus in our review. Future trials on maintenance immunosuppression should therefore include tacrolimus as the control group.
Overall completeness and applicability of evidence
The trials included mainly people undergoing primary liver transplantation for various aetiologies. The findings of this review are therefore applicable to people undergoing primary liver transplantation for any aetiology. We have only considered maintenance immunosuppression in adults. As graft rejections are more frequent in the first few months after liver transplantation, higher doses of the immunosuppression may be needed. However, additional drugs (induction immunosuppression agents) are routinely used with a view to decrease the number of graft rejections without requiring a higher dose of maintenance immunosuppression. As we evaluated only maintenance immunosuppression in this review, our findings are applicable only to maintenance immunosuppression.
Quality of the evidence
The overall quality of the evidence was low or very low for all outcomes. The main reasons for this were the trials at high risk of bias, in particular the exclusion of participants from the analysis after randomisation in some trials; small sample size; and imprecision. Overall, there are serious concerns about whether the effect estimates observed are the true effect estimates.
Potential biases in the review process
We selected a range of databases to search without using any language restrictions and conducted the network meta‐analysis according to NICE DSU guidance. In addition, we have presented the results from fixed‐effect model and random‐effects model and used the more conservative model. These are the strengths of the review process.
We have excluded studies that compared variations in duration or dose in the different interventions. Hence this review does not provide information on whether one variation is better than another. Another major limitation of this review was the paucity of data. Few trials were included for each comparison; in many comparisons, only one trial was included. This makes it difficult to assess whether the effect estimates are reproducible. This paucity of data decreases the confidence in the results.
All of the network meta‐analyses included only sparse data from trials at high risk of bias. We were able to compare the direct and indirect estimates for very few comparisons. This means that the tests for inconsistency are underpowered. One of the underpinning assumptions of a network meta‐analysis is that the participants in the different comparisons are similar. As information on the potential effect modifiers such as the reason for liver transplantation was missing from some trials, we had to rely on our judgement to assess the transitivity assumption. While there is no reason to suggest that there is any difference in the type of people included under different comparisons (Table 3), making firm judgements based on such network meta‐analysis having missing information is inappropriate; for this reason we have also reported the results of direct comparison for major outcomes, that is mortality at maximal follow‐up and graft loss at maximal follow‐up, in the conclusion.
We only included randomised clinical trials, which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. According to our choice of studies (i.e. only randomised clinical trials), it is possible that we missed a large number of studies addressing reporting of harms. Accordingly, this review is biased towards benefits ignoring harms. We did not search for interventions and trials registered at regulatory authorities (e.g. US Food and Drug Administration, European Medicines Agency, etc.). We may have therefore overlooked trials, and as such trials are usually unpublished, this lack of inclusion could make our comparisons look more advantageous than they truly are. On the other hand, inclusion of non‐randomised studies in the network meta‐analysis can increase the differences in potential modifiers and decrease the reliability of the findings of the network meta‐analysis.
Agreements and disagreements with other studies or reviews
There has been no previous network meta‐analysis or systematic review on maintenance immunosuppression for adults undergoing liver transplantation. We agree with Haddad 2006 that tacrolimus appears to be superior to cyclosporine A. We also agree with Fairfield 2015 that there is uncertainty about the role of glucocorticosteroid therapy in immunosuppression. We were unable to compare our findings with those of Penninga 2012, since the trials included in Penninga 2012 did not report any of our outcomes of interest.
Authors' conclusions
Implications for practice.
Based on low‐quality evidence from a single small trial from direct comparison, tacrolimus plus sirolimus increases mortality and graft loss at maximal follow‐up compared with tacrolimus. Based on very low‐quality evidence from network meta‐analysis, we found no evidence of difference between immunosuppressive regimens. Based on very low‐quality evidence from network meta‐analysis and low‐quality evidence from direct comparison, cyclosporine A causes more retransplantation compared with tacrolimus.
Implications for research.
Trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement, Chan 2013, and CONSORT statement (Schulz 2010). Future randomised clinical trials ought to be adequately powered; performed in people who are generally seen in the clinic rather than in highly selected participants; employ blinding; avoid postrandomisation dropouts or planned cross‐overs; and use clinically important outcomes such as mortality, graft loss, renal impairment, chronic kidney disease, and retransplantation. Such trials should use tacrolimus as one of the control groups.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2017 | Amended | Some errors were noted in Figure 7 and Figure 19 where we have presented the fixed‐effect model rather than the random‐effects model as stated in the text. This was corrected. Some of the calculations for the Summary of findings table 1 were also based on the fixed‐effect model rather than the random‐effects model. This has now been corrected. None of the corrected errors require further changes within the review text. |
Notes
Some errors were noted in the Figure 7 and Figure 19, where we have presented the fixed‐effect model rather than the random‐effects model as stated in the text. This was corrected. Some of the calculations for the Table 1 were also based on the fixed‐effect model rather than the random‐effects model. This has now been corrected.
Considerable overlap is evident in the Methods section of this review and that of several other reviews written by the same group of authors.
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers of review: Georgina Salanti, Greece; Luit Penninga; Denmark; Khalid Mumtaz, USA Contact editor: Christian Gluud, Denmark Sign‐off editor: Christian Gluud, Denmark
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 10, 2016 | #1 (liver or hepatic) #2 (transplant* or graft*) #3 #1 and #2 #4 MeSH descriptor: [Liver Transplantation] explode all trees #5 #3 or #4 #6 immunosuppress* #7 MeSH descriptor: [Immunosuppression] explode all trees #8 MeSH descriptor: [Immunosuppressive Agents] explode all trees #9 #6 or #7 or #8 #10 MeSH descriptor: [Cyclosporine] explode all trees #11 MeSH descriptor: [Tacrolimus] explode all trees #12 MeSH descriptor: [Antimetabolites] explode all trees #13 MeSH descriptor: [Sirolimus] explode all trees #14 MeSH descriptor: [Glucocorticoids] explode all trees #15 MeSH descriptor: [Antilymphocyte Serum] explode all trees #16 (Calcineurin inhibitor* or cyclosporine* or ciclosporin* or tacrilimus or antimetabolite or mycophenolate or azathioprine or mTOR inhibitor* or sirolimus or rapamycin or everolimus or corticosteroids or glucocorticoids or prednisolone or prednisone or methylprednisolone or cortisol or cortisone or methylprednisolone or betamethasone or thymoglobulin or antithymocyte or antilymphocyte or anti‐thymocyte or thymocyte antibody or anti‐lymphocyte or alemtuzumab or basiliximab or daclizumab) #17 #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #5 and #9 and #17 |
| MEDLINE (OvidSP) | January 1947 to October 2016 | 1. (liver or hepatic).af. 2. (transplant* or graft*).af. 3. 1 and 2 4. exp Liver Transplantation/ 5. 3 or 4 6. exp Immunosuppression/ or exp Immunosuppressive Agents/ 7. immunosuppress*.ti,ab. 8. 6 or 7 9. exp Cyclosporine/ 10. exp Tacrolimus/ 11. exp Antimetabolites/ 12. exp Sirolimus/ 13. exp Glucocorticoids/ 14. exp Antilymphocyte Serum/ 15. (Calcineurin inhibitor* or cyclosporine* or ciclosporin* or tacrilimus or antimetabolite or mycophenolate or azathioprine or mTOR inhibitor* or sirolimus or rapamycin or everolimus or corticosteroids or glucocorticoids or prednisolone or prednisone or methylprednisolone or cortisol or cortisone or methylprednisolone or betamethasone or thymoglobulin or antithymocyte or antilymphocyte or anti‐thymocyte or thymocyte antibody or anti‐lymphocyte or alemtuzumab or basiliximab or daclizumab).ti,ab. 16. or/9‐15 17. 5 and 8 and 16 18. randomized controlled trial.pt. 19. controlled clinical trial.pt. 20. randomized.ab. 21. placebo.ab. 22. drug therapy.fs. 23. randomly.ab. 24. trial.ab. 25. groups.ab. 26. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. exp animals/ not humans.sh. 28. 26 not 27 29. 17 and 28 |
| Embase (OvidSP) | January 1974 to October 2016 | 1. (liver or hepatic).af. 2. (transplant* or graft*).af. 3. 1 and 2 4. exp liver transplantation/ 5. 3 or 4 6. exp immunosuppressive treatment/ or exp immunosuppressive agent/ 7. immunosuppress*.ti,ab. 8. 6 or 7 9. exp calcineurin inhibitor/ 10. exp antimetabolite/ 11. exp rapamycin/ or exp everolimus/ 12. exp glucocorticoid/ 13. exp thymocyte antibody/ 14. (Calcineurin inhibitor* or cyclosporine* or ciclosporin* or tacrilimus or antimetabolite or mycophenolate or azathioprine or mTOR inhibitor* or sirolimus or rapamycin or everolimus or corticosteroids or glucocorticoids or prednisolone or prednisone or methylprednisolone or cortisol or cortisone or methylprednisolone or betamethasone or thymoglobulin or antithymocyte or antilymphocyte or anti‐thymocyte or thymocyte antibody or anti‐lymphocyte or alemtuzumab or basiliximab or daclizumab).ti,ab. 15. or/9‐14 16. 5 and 8 and 15 17. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 18. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 19. 17 or 18 20. 16 and 19 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to October 2016 | #1 TS=((liver or hepatic) AND (transplant* or graft*)) #2 TS=(immunosuppress*) #3 TS=(Calcineurin inhibitor* or cyclosporine* or ciclosporin* or tacrilimus or antimetabolite or mycophenolate or azathioprine or mTOR inhibitor* or sirolimus or rapamycin or everolimus or corticosteroids or glucocorticoids or prednisolone or prednisone or methylprednisolone or cortisol or cortisone or methylprednisolone or betamethasone or thymoglobulin or antithymocyte or antilymphocyte or anti‐thymocyte or thymocyte antibody or anti‐lymphocyte or alemtuzumab or basiliximab or daclizumab) #4 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #5 #1 and #2 and #3 and #4 |
| World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) | October 2016 | liver transplant* AND immunosuppress* |
| ClinicalTrials.gov | October 2016 | Interventional Studies | liver transplant* | immunosuppression | Phase 2, 3, 4 |
Appendix 2. Sample size calculation
The 10‐year mortality in people undergoing liver transplantation is about 20% (SRTR 2012). The required information size based on a control group proportion of 20%, a relative risk reduction of 20% in the experimental group, type I error of 5%, and type II error of 20% is 2894 participants. Network analyses are more prone to risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than in direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors, such as the number of participants included for each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the required sample size is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups, and the sample size in the trials is more than the required information size, we will calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
(nAC x (1 ‐ IAC2)) x (nBC x (1 ‐ IBC2))/(nAC x (1 ‐ IAC2)) + (nBC x (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups.
Appendix 3. Data used for network meta‐analysis
| Mortality at maximal follow‐up | |||||||||||
| #Mort; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Aza; 4 = Cy_Aza_Std; 5 = Cy_Myc_Std; 6 = Cy_Std; 7 = Ever; 8 = Tac_Aza; 9 = Tac_Ever; 10 = Tac_Myc_Std; 11 = Tac_Sir; 12 = Tac_Std. | |||||||||||
| list(ns=21,nt=12) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 11 | 30 | 16 | 36 | NA | NA | 1 | 2 | NA | 2 | 97 | #Cholongitas 2011 |
| 3 | 41 | 7 | 40 | NA | NA | 1 | 2 | NA | 2 | 12 | #Fung 1991 |
| 2 | 71 | 8 | 72 | NA | NA | 1 | 2 | NA | 2 | 12 | #Greig 2003 |
| 14 | 51 | 5 | 49 | NA | NA | 1 | 2 | NA | 2 | 24 | #Loinaz 2001 |
| 71 | 301 | 80 | 305 | NA | NA | 1 | 2 | NA | 2 | 36 | #O'Grady 2002 |
| 2 | 30 | 3 | 30 | NA | NA | 1 | 2 | NA | 2 | 12 | #Shenoy 2008 |
| 5 | 35 | 2 | 36 | NA | NA | 1 | 2 | NA | 2 | 6 | #Stegall 1997 |
| 7 | 25 | 8 | 24 | NA | NA | 1 | 2 | NA | 2 | 14 | #Zervos 1998 |
| 12 | 40 | 14 | 41 | NA | NA | 1 | 3 | NA | 2 | 124 | #Chen 2002 |
| 16 | 61 | 21 | 60 | NA | NA | 1 | 3 | NA | 2 | 144 | #Jonas 2005 |
| 10 | 243 | 15 | 231 | 14 | 245 | 1 | 7 | 9 | 3 | 36 | #De Simone 2012 |
| 14 | 49 | 7 | 48 | NA | NA | 1 | 8 | NA | 2 | 96 | #Manousou 2014 |
| 7 | 100 | 4 | 95 | NA | NA | 1 | 10 | NA | 2 | 11 | #Boudjema 2011 |
| 9 | 112 | 22 | 110 | NA | NA | 1 | 11 | NA | 2 | 24 | #Asrani 2014 |
| 11 | 54 | 9 | 50 | NA | NA | 2 | 6 | NA | 2 | 41 | #Belli 1998 |
| 7 | 84 | 2 | 90 | NA | NA | 2 | 6 | NA | 2 | 6 | #Pageaux 2004 |
| 4 | 26 | 12 | 52 | NA | NA | 2 | 7 | NA | 2 | 22 | #Masetti 2010 |
| 3 | 29 | 3 | 28 | NA | NA | 4 | 5 | NA | 2 | 6 | #Sterneck 2000 |
| 3.5 | 18 | 0.5 | 21 | NA | NA | 4 | 12 | NA | 2 | 12 | #Porayko 1994 |
| 8 | 41 | 6 | 38 | NA | NA | 6 | 12 | NA | 2 | 12 | #Martin 2004 |
| 32 | 175 | 38 | 175 | NA | NA | 10 | 12 | NA | 2 | 34 | #Jain 2001 |
| END | |||||||||||
| Graft loss at maximal follow‐up | |||||||||||
| #GrLoss; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Aza; 4 = Ever; 5 = Tac_Aza; 6 = Tac_Ever; 7 = Tac_Myc_Std; 8 = Tac_Sir; 9 = Tac_Std. | |||||||||||
| list(ns=15,nt=9) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 13 | 30 | 21 | 36 | NA | NA | 1 | 2 | NA | 2 | 97 | #Cholongitas 2011 |
| 5 | 41 | 14 | 40 | NA | NA | 1 | 2 | NA | 2 | 12 | #Fung 1991 |
| 2 | 71 | 10 | 72 | NA | NA | 1 | 2 | NA | 2 | 12 | #Greig 2003 |
| 14 | 51 | 6 | 49 | NA | NA | 1 | 2 | NA | 2 | 24 | #Loinaz 2001 |
| 80 | 301 | 102 | 305 | NA | NA | 1 | 2 | NA | 2 | 36 | #O'Grady 2002 |
| 3 | 30 | 3 | 30 | NA | NA | 1 | 2 | NA | 2 | 12 | #Shenoy 2008 |
| 6 | 35 | 3 | 36 | NA | NA | 1 | 2 | NA | 2 | 6 | #Stegall 1997 |
| 8 | 25 | 9 | 24 | NA | NA | 1 | 2 | NA | 2 | 14 | #Zervos 1998 |
| 15 | 40 | 16 | 41 | NA | NA | 1 | 3 | NA | 2 | 124 | #Chen 2002 |
| 19 | 61 | 31 | 60 | NA | NA | 1 | 3 | NA | 2 | 144 | #Jonas 2005 |
| 18 | 243 | 21 | 231 | 23 | 245 | 1 | 4 | 6 | 3 | 36 | #De Simone 2012 |
| 19 | 49 | 11 | 48 | NA | NA | 1 | 5 | NA | 2 | 96 | #Manousou 2014 |
| 8 | 100 | 6 | 95 | NA | NA | 1 | 7 | NA | 2 | 11 | #Boudjema 2011 |
| 14 | 112 | 29 | 110 | NA | NA | 1 | 8 | NA | 2 | 24 | #Asrani 2014 |
| 53 | 175 | 48 | 175 | NA | NA | 7 | 9 | NA | 2 | 34 | #Jain 2001 |
| END | |||||||||||
| Serious adverse events (proportion) (only direct comparisons) | |||||||||||
| #SAE_Pro; intervention codes: 1 = Tac; 2 = Ever; 3 = Tac_Ever. | |||||||||||
| list(ns=1,nt=2) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | |
| 104 | 231 | 130 | 243 | 122 | 245 | 1 | 2 | NA | 3 | #De Simone 2012 | |
| END | |||||||||||
| Adverse events (proportion) | |||||||||||
| #AEPro; intervention codes: 1 = Tac; 2 = Ever; 3 = Tac_Ever; 4 = Tac_Sir. | |||||||||||
| list(ns=2,nt=4) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | |
| 229 | 243 | 216 | 231 | 232 | 245 | 1 | 2 | 3 | 3 | #De Simone 2012 | |
| 109.5 | 112 | 108.5 | 109 | NA | NA | 1 | 4 | NA | 2 | #Asrani 2014 | |
| END | |||||||||||
| Adverse events (number) | |||||||||||
| #AENum; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Std; 4 = Ever; 5 = Tac_Myc; 6 = Tac_Myc_Std. | |||||||||||
| list(ns=12,nt=6) | |||||||||||
| r[,1] | E[,1] | r[,2] | E[,2] | r[,3] | E[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | |
| 114 | 41.0 | 111 | 40.0 | NA | NA | 1 | 2 | NA | 2 | #Fung 1991 | |
| 367 | 71.0 | 331 | 72.0 | NA | NA | 1 | 2 | NA | 2 | #Greig 2003 | |
| 80 | 102.0 | 111 | 98.0 | NA | NA | 1 | 2 | NA | 2 | #Loinaz 2001 | |
| 1671 | 903.0 | 1350 | 915.0 | NA | NA | 1 | 2 | NA | 2 | #O'Grady 2002 | |
| 22 | 30.0 | 16 | 30.0 | NA | NA | 1 | 2 | NA | 2 | #Shenoy 2008 | |
| 6 | 12.5 | 12 | 16.5 | NA | NA | 1 | 2 | NA | 2 | #Stegall 1997 | |
| 41 | 29.2 | 33 | 28.0 | NA | NA | 1 | 2 | NA | 2 | #Zervos 1998 | |
| 283 | 91.7 | 296 | 87.1 | NA | NA | 1 | 6 | NA | 2 | #Boudjema 2011 | |
| 2 | 184.5 | 42 | 170.8 | NA | NA | 2 | 3 | NA | 2 | #Belli 1998 | |
| 188 | 42.0 | 214 | 45.0 | NA | NA | 2 | 3 | NA | 2 | #Pageaux 2004 | |
| 45 | 47.7 | 83 | 95.3 | NA | NA | 2 | 4 | NA | 2 | #Masetti 2010 | |
| 136 | 287.5 | 114 | 287.5 | NA | NA | 5 | 6 | NA | 2 | #Pelletier 2013 | |
| END | |||||||||||
| Renal impairment | |||||||||||
| #RenImp; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Aza_Std; 4 = Ever; 5 = Tac_Ever; 6 = Tac_Myc; 7 = Tac_Myc_Std; 8 = Tac_Std. | |||||||||||
| list(ns=8,nt=8) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 4 | 41 | 9 | 40 | NA | NA | 1 | 2 | NA | 2 | 12 | #Fung 1991 |
| 7 | 71 | 5 | 72 | NA | NA | 1 | 2 | NA | 2 | 12 | #Greig 2003 |
| 55 | 301 | 45 | 305 | NA | NA | 1 | 2 | NA | 2 | 36 | #O'Grady 2002 |
| 4 | 243 | 3 | 231 | 6 | 245 | 1 | 4 | 5 | 3 | 36 | #De Simone 2012 |
| 42 | 100 | 23 | 95 | NA | NA | 1 | 7 | NA | 2 | 11 | #Boudjema 2011 |
| 0.5 | 18 | 1.5 | 21 | NA | NA | 3 | 8 | NA | 2 | 12 | #Porayko 1994 |
| 18 | 50 | 12 | 50 | NA | NA | 6 | 7 | NA | 2 | 69 | #Pelletier 2013 |
| 20 | 175 | 39 | 175 | NA | NA | 7 | 8 | NA | 2 | 34 | #Jain 2001 |
| END | |||||||||||
| Chronic kidney disease (only direct comparison) | |||||||||||
| #CKD; intervention codes: 1 = Tac_Myc; 2 = Tac_Myc_Std. | |||||||||||
| list(ns=1,nt=2) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study | |
| 11 | 50 | 5 | 50 | NA | NA | 1 | 2 | NA | 2 | #Pelletier 2013 | |
| END | |||||||||||
| Retransplantation | |||||||||||
| #Retrans; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Aza; 4 = Cy_Aza_Std; 5 = Tac_Aza; 6 = Tac_Myc; 7 = Tac_Myc_Std; 8 = Tac_Std. | |||||||||||
| list(ns=13,nt=8) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 2 | 30 | 5 | 36 | NA | NA | 1 | 2 | NA | 2 | 97 | #Cholongitas 2011 |
| 2 | 41 | 7 | 40 | NA | NA | 1 | 2 | NA | 2 | 12 | #Fung 1991 |
| 0.5 | 72 | 5.5 | 73 | NA | NA | 1 | 2 | NA | 2 | 12 | #Greig 2003 |
| 14 | 301 | 37 | 305 | NA | NA | 1 | 2 | NA | 2 | 36 | #O'Grady 2002 |
| 1.5 | 31 | 0.5 | 31 | NA | NA | 1 | 2 | NA | 2 | 12 | #Shenoy 2008 |
| 1 | 25 | 4 | 24 | NA | NA | 1 | 2 | NA | 2 | 14 | #Zervos 1998 |
| 3 | 40 | 2 | 41 | NA | NA | 1 | 3 | NA | 2 | 124 | #Chen 2002 |
| 3 | 61 | 10 | 60 | NA | NA | 1 | 3 | NA | 2 | 144 | #Jonas 2005 |
| 5 | 49 | 4 | 48 | NA | NA | 1 | 5 | NA | 2 | 96 | #Manousou 2014 |
| 4 | 100 | 3 | 95 | NA | NA | 1 | 7 | NA | 2 | 11 | #Boudjema 2011 |
| 1.5 | 18 | 0.5 | 21 | NA | NA | 4 | 8 | NA | 2 | 12 | #Porayko 1994 |
| 6.5 | 51 | 0.5 | 51 | NA | NA | 6 | 7 | NA | 2 | 69 | #Pelletier 2013 |
| 21 | 175 | 20 | 175 | NA | NA | 7 | 8 | NA | 2 | 34 | #Jain 2001 |
| END | |||||||||||
| Graft rejections (treatment) | |||||||||||
| #GRT; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Std; 4 = Ever; 5 = Tac_Ever. | |||||||||||
| list(ns=5,nt=5) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 22 | 30 | 24 | 36 | NA | NA | 1 | 2 | NA | 2 | 97 | #Cholongitas 2011 |
| 11 | 26 | 15 | 32 | NA | NA | 1 | 2 | NA | 2 | 6 | #Stegall 1997 |
| 20 | 243 | 43 | 231 | 11 | 245 | 1 | 4 | 5 | 3 | 36 | #De Simone 2012 |
| 2 | 54 | 3 | 50 | NA | NA | 2 | 3 | NA | 2 | 41 | #Belli 1998 |
| 2 | 26 | 3 | 52 | NA | NA | 2 | 4 | NA | 2 | 22 | #Masetti 2010 |
| END | |||||||||||
| Graft rejections (any) | |||||||||||
| #GRAny; intervention codes: 1 = Tac; 2 = Cy; 3 = Cy_Aza; 4 = Cy_Aza_Std; 5 = Cy_Myc; 6 = Cy_Std; 7 = Ever; 8 = Tac_Aza; 9 = Tac_Ever; 10 = Tac_Myc; 11 = Tac_Myc_Std; 12 = Tac_Std. | |||||||||||
| list(ns=16,nt=12) | |||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | time[] | #study |
| 28 | 30 | 29 | 36 | NA | NA | 1 | 2 | NA | 2 | 97 | #Cholongitas 2011 |
| 16 | 41 | 33 | 40 | NA | NA | 1 | 2 | NA | 2 | 12 | #Fung 1991 |
| 25 | 71 | 31 | 72 | NA | NA | 1 | 2 | NA | 2 | 12 | #Greig 2003 |
| 21 | 51 | 25 | 49 | NA | NA | 1 | 2 | NA | 2 | 24 | #Loinaz 2001 |
| 143 | 301 | 179 | 305 | NA | NA | 1 | 2 | NA | 2 | 36 | #O'Grady 2002 |
| 8 | 30 | 10 | 30 | NA | NA | 1 | 2 | NA | 2 | 12 | #Shenoy 2008 |
| 6 | 25 | 12 | 24 | NA | NA | 1 | 2 | NA | 2 | 14 | #Zervos 1998 |
| 23 | 61 | 21 | 60 | NA | NA | 1 | 3 | NA | 2 | 144 | #Jonas 2005 |
| 34 | 243 | 53 | 231 | 15 | 245 | 1 | 7 | 9 | 3 | 36 | #De Simone 2012 |
| 22 | 49 | 31 | 48 | NA | NA | 1 | 8 | NA | 2 | 96 | #Manousou 2014 |
| 46 | 100 | 28 | 95 | NA | NA | 1 | 11 | NA | 2 | 11 | #Boudjema 2011 |
| 32 | 84 | 22 | 90 | NA | NA | 2 | 6 | NA | 2 | 6 | #Pageaux 2004 |
| 8 | 17 | 10 | 20 | NA | NA | 4 | 12 | NA | 2 | 12 | #Porayko 1994 |
| 18 | 50 | 12 | 49 | NA | NA | 5 | 10 | NA | 2 | 48 | #Fisher 1998 |
| 11 | 41 | 16 | 38 | NA | NA | 6 | 12 | NA | 2 | 12 | #Martin 2004 |
| 10 | 50 | 7 | 50 | NA | NA | 10 | 11 | NA | 2 | 69 | #Pelletier 2013 |
| END | |||||||||||
| Abbreviations: Tac = tacrolimus; Cy = cyclosporine A; Myc = mycophenolate; Aza = azathioprine; Sir = sirolimus; Ever = everolimus; Std = glucocorticosteroids; _ = plus; NA = not applicable | |||||||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asrani 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: International, multicentric Number randomised: 222 Postrandomisation dropouts: 0 (0%) Revised sample size: 222 Average age: 50 years Females: 65 (29.3%) Primary transplantation: 222 (100%) Retransplantation: 0 (0%) HCV: 72 (32.4%) HBV: 30 (13.5%) Alcoholic cirrhosis: 79 (35.6%) Other causes: 40 (18%) Average follow‐up period in months (for all groups): 24 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: sirolimus plus tacrolimus (n = 110). Further details: sirolimus: IV to attain a trough level 4 to 11 ng/mL. Tacrolimus: IV to attain 3 to 5 ng/mL. Group 2: tacrolimus (n = 112). Further details: tacrolimus: IV to attain 5 to 10 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized randomization" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "participants, care providers and those assessing outcomes were not blinded to randomized treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "participants, care providers and those assessing outcomes were not blinded to randomized treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "this study was conducted, monitored and paid for by Wyeth, which was acquired by Pfizer in October 2009." |
| Other bias | Low risk | Comment: no other bias noted. |
Baiocchi 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 20 Postrandomisation dropouts: 0 (0%) Revised sample size: 20 Average age: 49 years Females: 5 (25%) Primary transplantation: 20 (100%) Retransplantation: 0 (0%) HCV: 8 (40%) HBV: 4 (20%) Alcoholic cirrhosis: 3 (15%) Other causes: 1 (5%) Average follow‐up period in months (for all groups): 3 Additional treatment such as antiviral drugs: lamivudine in HBV patients Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Other important inclusion criteria:
Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 10). Further details: cyclosporine A: attain 250 ng/mL. Group 2: tacrolimus (n = 10). Further details: tacrolimus: attain 10 ng/mL. | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Belli 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 108 Postrandomisation dropouts: not stated Revised sample size: 108 Average age: not stated Females: not stated Primary transplantation: 104 (96.3%) Retransplantation: 0 (0%) HCV: 42 (38.9%) HBV: 24 (22.2%) Alcoholic cirrhosis: 9 (8.3%) Other causes: 21 (19.4%) Average follow‐up period in months (for all groups): 41 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus glucocorticosteroids (n = 50). Further details: cyclosporine A: attain 150 to 250 ng/mL; glucocorticosteroids: prednisolone 0.1 mg/kg/day. Group 2: cyclosporine A (n = 54). Further details: cyclosporine A: attain 150 to 250 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Boudjema 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 195 Postrandomisation dropouts: 0 (0%) Revised sample size: 195 Average age: 51 years Females: 52 (26.7%) Primary transplantation: 195 (100%) Retransplantation: 0 (0%) HCV: 16 (8.2%) HBV: 4 (2.1%) Alcoholic cirrhosis: 83 (42.6%) Other causes: 91 (46.7%) Average follow‐up period in months (for all groups): 11 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: mycophenolate plus tacrolimus (n = 95). Further details: mycophenolate: 1 g twice daily; tacrolimus: attain trough concentration of <= 6 ng/mL. Group 2: tacrolimus (n = 100). Further details: tacrolimus: attain trough concentration of >= 6 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the center‐stratified randomization was based on computer‐generated lists" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization lists were kept by the pharmacist of the coordinating center" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "since the trial was not blinded, the lists were balanced by blocks of 24 patients in order to ensure total unpredictability of the randomization sequence" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "since the trial was not blinded, the lists were balanced by blocks of 24 patients in order to ensure total unpredictability of the randomization sequence" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Low risk | Quote: "this study was conducted with financial support from the French Ministry of Health (2001 Clinical Research Hospital Program, PHRC 2001)" |
| Other bias | Low risk | Comment: no other bias noted. |
Chen 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 81 Postrandomisation dropouts: 0 (0%) Revised sample size: 81 Average age: 49 years Females: not stated Primary transplantation: 81 (100%) Retransplantation: 0 (0%) HCV: 2 (2.5%) HBV: 2 (2.5%) Alcoholic cirrhosis: 6 (7.4%) Other causes: 71 (87.7%) Average follow‐up period in months (for all groups): 124 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus azathioprine (n = 41). Further details: cyclosporine A: attain 100 to 200 ng/mL; azathioprine: 2 mg/kg/day. Group 2: tacrolimus (n = 40). Further details: tacrolimus: attain 0.5 to 1 ng/mL (plasma concentrations). | |
| Outcomes | The outcomes reported were:
|
|
| Notes | This was part of the European FK506 trial, which included multiple centres with different centres using their own immunosuppressive regimen. This report is in patients from Birmingham, UK. Some elements such as inclusion criteria and source of funding were obtained from the multicentric report, although the results of the multicentric trial were not included because of the different regimens used in different centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | High risk | Quote: "this study was sponsored by Fujisawa Pharmaceutical Co Ltd, Osaka, Japan" |
| Other bias | Low risk | Comment: no other bias noted. |
Cholongitas 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 66 Postrandomisation dropouts: 0 (0%) Revised sample size: 66 Average age: 48 years Females: 27 (40.9%) Primary transplantation: 66 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 97 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: not stated HBV only: not stated Alcoholic cirrhosis only: not stated Other causes: not stated | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 36). Further details: cyclosporine A: attain 100 to 300 ng/mL. Group 2: tacrolimus (n = 30). Further details: tacrolimus: attain 5 to 15 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was by using sealed opaque envelopes consecutively numbered and opened, containing the allocated treatment code, derived from a random numbers table". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was by using sealed opaque envelopes consecutively numbered and opened, containing the allocated treatment code, derived from a random numbers table". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol available; either mortality/graft loss or adverse events or both were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
De Simone 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: international, multicentric Number randomised: 719 Postrandomisation dropouts: 0 (0%) Revised sample size: 719 Average age: 54 years Females: 196 (27.3%) Primary transplantation: 719 (100%) Retransplantation: 0 (0%) HCV: 175 (24.3%) HBV: 49 (6.8%) Alcoholic cirrhosis: 171 (23.8%) Other causes: 258 (35.9%) Average follow‐up period in months (for all groups): 36 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Other important inclusion criteria:
Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: everolimus plus tacrolimus (n = 245). Further details: everolimus: attain a trough concentration of 3 to 8 ng/mL; tacrolimus: attain a trough concentration of 3 to 5 ng/mL. Group 2: everolimus (n = 231). Further details: everolimus: attain a trough concentration of 6 to 10 ng/mL. Group 3: tacrolimus (n = 243). Further details: tacrolimus: attain a trough concentration of 6 to 10 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "the study was funded by Novartis Pharma AG" |
| Other bias | High risk | Comment: recruitment to one of the intervention groups was stopped early. |
Fernandez‐Miranda 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 27 Postrandomisation dropouts: not stated Revised sample size: 27 Average age: not stated Females: not stated Primary transplantation: not stated Retransplantation: not stated HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 22 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: not stated Retransplantation only: not stated HCV only: not stated HBV only: not stated Alcoholic cirrhosis only: not stated Other causes: not stated | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 14). Further details: cyclosporine A: attain 100 to 250 ng/mL. Group 2: tacrolimus (n = 13). Further details: tacrolimus: attain 5 to 10 ng/mL. | |
| Outcomes | None of our outcomes of interest were reported. | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Fisher 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 99 Postrandomisation dropouts: 0 (0%) Revised sample size: 99 Average age: 48 years Females: 39 (39.4%) Primary transplantation: 99 (100%) Retransplantation: 0 (0%) HCV: 37 (37.4%) HBV: 7 (7.1%) Alcoholic cirrhosis: 11 (11.1%) Other causes: 44 (44.4%) Average follow‐up period in months (for all groups): 48 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus mycophenolate (n = 50). Further details: cyclosporine A: attain 200 to 300 ng/mL; mycophenolate mofetil: 1 g/day. Group 2: tacrolimus plus mycophenolate (n = 49). Further details: tacrolimus: attain 5 to 10 ng/mL; mycophenolate mofetil: 1 g/day. | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | High risk | Quote: "this study was supported in part by a grant from Hoffman, LaRoche Inc." |
| Other bias | Low risk | Comment: no other bias noted. |
Fung 1991.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 81 Postrandomisation dropouts: not stated Revised sample size: 81 Average age: 42 years Females: 33 (40.7%) Primary transplantation: 81 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: 0 (0%) Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 12 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: not stated HBV only: no Alcoholic cirrhosis only: not stated Other causes: not stated Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 40). Further details: cyclosporine A: attain 600 to 800 ng/mL. Group 2: tacrolimus (n = 41). Further details: tacrolimus: attain 1 to 5 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment assignment was determined by a computer program implementing the block randomization technique, to assure that the treatment groups remained reasonably balanced" |
| Allocation concealment (selection bias) | Low risk | Quote: "a sealed envelope method was implemented. Each envelope contained a single treatment assignment". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Low risk | Quote: "this work was supported by research grant OK 29961 from the National Institutes of Health, Bethesda, Maryland, and the Veterans Administration". |
| Other bias | Low risk | Comment: no other bias noted. |
Greig 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Canada Number randomised: 143 Postrandomisation dropouts: 0 (0%) Revised sample size: 143 Average age: 50 years Females: 56 (39.2%) Primary transplantation: 139 (97.2%) Retransplantation: 4 (2.8%) HCV: 47 (32.9%) HBV: 0 (0%) Alcoholic cirrhosis: 25 (17.5%) Other causes: 67 (46.9%) Average follow‐up period in months (for all groups): 12 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: no Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 72). Further details: cyclosporine A: attain 100 to 250 ng/mL. Group 2: tacrolimus (n = 71). Further details: tacrolimus: attain 5 to 15 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "Supported by Fujisawa Canada, Inc" |
| Other bias | Low risk | Comment: no other bias noted. |
Jain 2001.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 350 Postrandomisation dropouts: 0 (0%) Revised sample size: 350 Average age: 52 years Females: 148 (42.3%) Primary transplantation: 350 (100%) Retransplantation: 0 (0%) HCV: 95 (27.1%) HBV: 15 (4.3%) Alcoholic cirrhosis: 70 (20%) Other causes: 160 (45.7%) Average follow‐up period in months (for all groups): 34 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: tacrolimus plus mycophenolate plus glucocorticosteroids (n = 175). Further details: tacrolimus: attain 10 to 15 ng/mL; mycophenolate mofetil: 1 g twice daily; glucocorticosteroids: methyl prednisolone 20 mg/day. Group 2: tacrolimus plus glucocorticosteroids (n = 175). Further details: tacrolimus: attain 10 to 15 ng/mL; glucocorticosteroids: methyl prednisolone 20 mg/day. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was based on a sequential draw of assignments using a variable block randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "the statisticians gave sealed envelopes to clinicians." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | Unclear risk | Quote: "supported in part by research grants from the Veterans Administration and project grant no. DK‐29961 from The National Institutes of Health, Bethesda, MD" Comment: it is not clear if additional funding was received from drug manufacturers. |
| Other bias | Low risk | Comment: no other bias noted. |
Jonas 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany Number randomised: 121 Postrandomisation dropouts: 0 (0%) Revised sample size: 121 Average age: 48 years Females: 71 (58.7%) Primary transplantation: 121 (100%) Retransplantation: 0 (0%) HCV: 35 (28.9%) HBV: 30 (24.8%) Alcoholic cirrhosis: 20 (16.5%) Other causes: 37 (30.6%) Average follow‐up period in months (for all groups): 144 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus azathioprine (n = 60). Further details: cyclosporine A: attain 600 to 900 ng/mL; azathioprine: 1 to 2 mg/kg/day. Group 2: tacrolimus (n = 61). Further details: tacrolimus: 0.10 to 0.15 mg/kg/day. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | High risk | Quote: "this study was sponsored by Fujisawa Pharmaceutical Co Ltd, Osaka, Japan" |
| Other bias | Low risk | Comment: no other bias noted. |
Loinaz 2001.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 101 Postrandomisation dropouts: 1 (1%) Revised sample size: 100 Average age: 50 years Females: 31 (31%) Primary transplantation: 100 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 24 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: not stated Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 49). Further details: cyclosporine A: attain 100 to 150 ng/mL. Group 2: tacrolimus (n = 51). Further details: tacrolimus: attain 5 to 8 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were postrandomisation dropouts, but the reasons for them were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality/graft loss and adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Manousou 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 103 Postrandomisation dropouts: 6 (5.8%) Revised sample size: 97 Average age: 49 years Females: 29 (29.9%) Primary transplantation: 97 (100%) Retransplantation: 0 (0%) HCV: 97 (100%) HBV: 0 (0%) Alcoholic cirrhosis: 0 (0%) Other causes: 0 (0%) Average follow‐up period in months (for all groups): 96 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: yes HBV only: no Alcoholic cirrhosis only: no Other causes: no Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: tacrolimus plus azathioprine (n = 48). Further details: tacrolimus: attain 5 to 10 ng/mL; azathioprine: 1 mg/kg/day. Group 2: tacrolimus (n = 49). Further details: tacrolimus: attain 5 to 10 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: early complications, early retransplantation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "for randomization, sealed opaque envelopes were used; they were opened in a numbered sequence containing the allocated treatment in a 1:1 proportion derived from a random number table with a blocked code for each center" |
| Allocation concealment (selection bias) | Low risk | Quote: "for randomization, sealed opaque envelopes were used; they were opened in a numbered sequence containing the allocated treatment in a 1:1 proportion derived from a random number table with a blocked code for each center" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts, which were related to treatment complications. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | High risk | Quote: "AKB and APD have an unrestricted educational grant from Pfizer." |
| Other bias | Low risk | Comment: no other bias noted. |
Martin 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 85 Postrandomisation dropouts: 6 (7.1%) Revised sample size: 79 Average age: 50 years Females: 29 (36.7%) Primary transplantation: 79 (100%) Retransplantation: 0 (0%) HCV: 79 (100%) HBV: 0 (0%) Alcoholic cirrhosis: 0 (0%) Other causes: 0 (0%) Average follow‐up period in months (for all groups): 12 Additional treatment such as antiviral drugs: no antiviral therapy Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: yes HBV only: no Alcoholic cirrhosis only: no Other causes: no Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus glucocorticosteroids (n = 41). Further details: cyclosporine A: attain 100 to 250 ng/mL; glucocorticosteroids: prednisolone 5 mg/day. Group 2: tacrolimus plus glucocorticosteroids (n = 38). Further details: tacrolimus: attain 5 to 10 ng/mL; glucocorticosteroids: prednisolone 5 mg/day. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: did not receive transplant or did not meet inclusion/exclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prior to transplant, patients were assigned by a telephone randomization system to receive either tac‐rolimus or cyclosporine (Neoral) maintenance therapy beginning 12 hours after transplant". |
| Allocation concealment (selection bias) | Low risk | Quote: "prior to transplant, patients were assigned by a telephone randomization system to receive either tac‐rolimus or cyclosporine (Neoral) maintenance therapy beginning 12 hours after transplant". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation drop‐outs. Many were related to potential complications of treatment. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol available; either mortality/graft loss or adverse events or both were not reported. |
| For‐profit bias | High risk | Quote: "supported by an educational grant from Fujisawa Healthcare, Inc., Deerfield, IL". |
| Other bias | Low risk | Comment: no other bias noted. |
Masetti 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 78 Postrandomisation dropouts: 0 (0%) Revised sample size: 78 Average age: 54 years Females: 18 (23.1%) Primary transplantation: 78 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 22 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: not stated HBV only: not stated Alcoholic cirrhosis only: not stated Other causes: not stated Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: everolimus (n = 52). Further details: everolimus: attain 6 to 10 ng/mL. Group 2: cyclosporine A (n = 26). Further details: cyclosporine A: attain 125 to 175 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
O'Grady 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: international, multicentric Number randomised: 606 Postrandomisation dropouts: 0 (0%) Revised sample size: 606 Average age: 51 years Females: 256 (42.2%) Primary transplantation: 606 (100%) Retransplantation: 0 (0%) HCV: 60 (9.9%) HBV: 20 (3.3%) Alcoholic cirrhosis: 110 (18.2%) Other causes: 98 (16.2%) Average follow‐up period in months (for all groups): 36 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 305). Further details: cyclosporine A: attain 150 to 250 ng/mL. Group 2: tacrolimus (n = 301). Further details: tacrolimus: attain 5 to 15 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the data coordinating centre at the Medical Statistics Unit at the London School of Hygiene and Tropical Medicine generated stratified and blocked randomised sequences using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "cards, with details of treatment allocation on, were put in serially numbered, opaque envelopes and sent to each centre" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "Fujisawa and Novartis both approved the protocol, received interim reports simultaneously, and commented on the manuscript before submission for publication" |
| Other bias | High risk | Comment: trial was stopped early for benefit. |
Pageaux 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 174 Postrandomisation dropouts: 0 (0%) Revised sample size: 174 Average age: 52 years Females: 50 (28.7%) Primary transplantation: 174 (100%) Retransplantation: 0 (0%) HCV: 26 (14.9%) HBV: 12 (6.9%) Alcoholic cirrhosis: 84 (48.3%) Other causes: 52 (29.9%) Average follow‐up period in months (for all groups): 6 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus glucocorticosteroids (n = 90). Further details: cyclosporine A: attain 150 to 300 ng/mL; glucocorticosteroids: prednisolone 20 mg/day. Group 2: cyclosporine A (n = 84). Further details: cyclosporine A: attain 150 to 300 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "supported by a grant from Novartis Pharma" |
| Other bias | High risk | Comment: despite following participants for 12 months, the authors present only the 6‐month results and have excluded 2 late deaths. |
Pelletier 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 100 Postrandomisation dropouts: 0 (0%) Revised sample size: 100 Average age: 55 years Females: 24 (24%) Primary transplantation: 100 (100%) Retransplantation: 0 (0%) HCV: 54 (54%) HBV: not stated Alcoholic cirrhosis: 42 (42%) Other causes: 4 (4%) Average follow‐up period in months (for all groups): 69 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: no Alcoholic cirrhosis only: not stated Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: tacrolimus plus mycophenolate plus glucocorticosteroids (n = 50). Further details: tacrolimus: dosage not stated; mycophenolate mofetil: dosage not stated; glucocorticosteroids: tapering dose (dose not stated). Group 2: tacrolimus plus mycophenolate (n = 50). Further details: tacrolimus: dosage not stated; mycophenolate mofetil: dosage not stated. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "enrolled candidates were randomized to either the 'steroids' or 'no‐steroids' groups using a closed‐envelope system" Comment: further information about the closed‐envelope system was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "this study was supported by a grant from Astellas Pharma, Inc., Deerfield, IL, USA" |
| Other bias | Low risk | Comment: no other bias noted. |
Pham 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 88 Postrandomisation dropouts: 12 (13.6%) Revised sample size: 76 Average age: not stated Females: not stated Primary transplantation: 76 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 27 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: not stated HBV only: not stated Alcoholic cirrhosis only: not stated Other causes: not stated Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus azathioprine plus glucocorticosteroids (n = 38). Further details: cyclosporine: 1 to 6 mg/kg/day; azathioprine: 2 mg/kg/day; glucocorticosteroids: methyl prednisolone 0.3 mg/kg/day. Group 2: tacrolimus plus glucocorticosteroids (n = 38). Further details: tacrolimus: 0.3 mg/kg/day; glucocorticosteroids: methyl prednisolone 20 mg/day tapering dose. | |
| Outcomes | None of our outcomes of interest were reported. | |
| Notes | Reasons for postrandomisation dropouts: died postoperatively or cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: participants were excluded because of death or cross‐over. This will introduce bias. |
| Selective reporting (reporting bias) | High risk | Comment: no published protocol was available; either mortality/graft loss or adverse events, or both were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Porayko 1994.
| Methods | ||
| Participants | Country: USA Number randomised: 37 Postrandomisation dropouts: 0 (0%) Revised sample size: 37 Average age: 49 years Females: 14 (37.8%) Primary transplantation: 37 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: 6 (16.2%) Other causes: 29 (78.4%) Average follow‐up period in months (for all groups): 12 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: not stated HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus azathioprine plus glucocorticosteroids (n = 17). Further details: cyclosporine: attain 100 to 200 ng/mL; azathioprine: 2 mg/kg/day; glucocorticosteroids: prednisolone 10 mg/day. Group 2: tacrolimus plus glucocorticosteroids (n = 20). Further details: tacrolimus: attain 0.2 to 5 ng/mL; glucocorticosteroids: prednisolone 5 mg/day. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "this study was supported by a grant from Fujisawa Pharmaceutical Company, Deerfield, Illinois." |
| Other bias | Low risk | Comment: no other bias noted. |
Shenoy 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 60 Postrandomisation dropouts: 0 (0%) Revised sample size: 60 Average age: 53 years Females: 20 (33.3%) Primary transplantation: not stated Retransplantation: not stated HCV: 32 (53.3%) HBV: 5 (8.3%) Alcoholic cirrhosis: 8 (13.3%) Other causes: 16 (26.7%) Average follow‐up period in months (for all groups): 12 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: not stated Retransplantation only: not stated HCV only: no HBV only: no Alcoholic cirrhosis only: no Other causes: yes Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 30). Further details: cyclosporine A: attain 600 to 1000 ng/mL at 2 hours (C2). Group 2: tacrolimus (n = 30). Further details: tacrolimus: attain 5 to 10 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "supported by a research grant from Novartis" |
| Other bias | Low risk | Comment: no other bias noted. |
Stegall 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 71 Postrandomisation dropouts: 0 (0%) Revised sample size: 71 Average age: not stated Females: not stated Primary transplantation: not stated Retransplantation: not stated HCV: not stated HBV: 0 (0%) Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 6 Additional treatment such as antiviral drugs: no Important inclusion and exclusion criteria Primary transplantation only: not stated Retransplantation only: not stated HCV only: no HBV only: no Alcoholic cirrhosis only: not stated Other causes: not stated | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 36). Further details: cyclosporine A: attain 200 to 250 ng/mL. Group 2: tacrolimus (n = 35). Further details: tacrolimus: attain 8 to 10 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
Sterneck 2000.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany Number randomised: 57 Postrandomisation dropouts: not stated Revised sample size: 57 Average age: not stated Females: not stated Primary transplantation: 57 (100%) Retransplantation: 0 (0%) HCV: not stated HBV: not stated Alcoholic cirrhosis: not stated Other causes: not stated Average follow‐up period in months (for all groups): 6 Additional treatment such as antiviral drugs: none stated Important inclusion and exclusion criteria Primary transplantation only: yes Retransplantation only: no HCV only: no HBV only: not stated Alcoholic cirrhosis only: not stated Other causes: not stated Important exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A plus mycophenolate plus glucocorticosteroids (n = 28). Further details: cyclosporine A: attain 100 to 150 ng/mL; mycophenolate mofetil: 1 to 1.5 g twice daily; glucocorticosteroids: 6 mg/day. Group 2: cyclosporine A plus azathioprine plus glucocorticosteroids (n = 29). Further details: cyclosporine A: attain 100 to 150 ng/mL; azathioprine: 1 to 2 mg/kg/day; glucocorticosteroids: 6 mg/day. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | High risk | Quote: "the present work was partly supported by the company Hoffmann La Roche, Grenzach Whylen" |
| Other bias | Low risk | Comment: no other bias noted. |
Zervos 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 50 Postrandomisation dropouts: 1 (2%) Revised sample size: 49 Average age: 49 years Females: 23 (46.9%) Primary transplantation: not stated Retransplantation: not stated HCV: 49 (100%) HBV: 0 (0%) Alcoholic cirrhosis: 0 (0%) Other causes: 0 (0%) Average follow‐up period in months (for all groups): 14 Additional treatment such as antiviral drugs: yes (interferon therapy) Important inclusion and exclusion criteria Primary transplantation only: not stated Retransplantation only: not stated HCV only: yes HBV only: no Alcoholic cirrhosis only: no Other causes: no | |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cyclosporine A (n = 24). Further details: cyclosporine A: attain 300 to 400 ng/mL. Group 2: tacrolimus (n = 25). Further details: tacrolimus: attain 15 ng/mL. | |
| Outcomes | The outcomes reported were:
|
|
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were postrandomisation dropouts, but the reasons for them were not stated. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol was available; mortality/graft loss and adverse events were reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias noted. |
HBV: hepatitis B virus HCV: hepatitis C virus IV: intravenous TNM: Tumor, Node, Metastasis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelmalek 2012 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Barnes 1997 | Not on maintenance immunosuppression |
| Beckebaum 2004 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Becker 2008 | Not on maintenance immunosuppression |
| Benitez 2010 | Not on maintenance immunosuppression |
| Berenguer 2006 | Randomisation was performed at least 1 year after liver transplantation. |
| Biancofiore 2004 | Not on maintenance immunosuppression |
| Bilbao 2009 | Not on maintenance immunosuppression |
| Bogetti 2005 | Not on maintenance immunosuppression |
| Boillot 2000 | Not on maintenance immunosuppression |
| Boillot 2001 | Not on maintenance immunosuppression |
| Boillot 2005 | Not on maintenance immunosuppression |
| Boillot 2009 | Not on maintenance immunosuppression |
| Boleslawski 2004 | Details of maintenance immunosuppression not available. |
| Calmus 2010 | Not on maintenance immunosuppression |
| Chen 2005 | Comparison of different regimens of same drug |
| Cicinnati 2007 | Randomisation was performed at least 1 year after liver transplantation. |
| Cillo 2014 | Details of maintenance immunosuppression not available. |
| Cosimi 1987 | Not on maintenance immunosuppression |
| Cosimi 1990 | Not on maintenance immunosuppression |
| Cuervas‐Mons 2015 | Not on maintenance immunosuppression |
| Day 2004 | Details of maintenance immunosuppression not available. |
| De Simone 2009 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| De Simone 2015 | Comparison of different regimens of same drug |
| Duvoux 2015 | Randomisation was performed from 6 months to 10 years. |
| Eason 2003 | Not on maintenance immunosuppression |
| Ericzon 1997 | Not on maintenance immunosuppression |
| Farges 1994 | Not on maintenance immunosuppression |
| Filipponi 2004 | Not on maintenance immunosuppression |
| Firpi 2006 | Not a randomised clinical trial |
| Firpi 2010 | Randomisation was performed only after recurrence of hepatitis C infection. |
| Fischer 2012 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Fleckenstein 1996 | Not on maintenance immunosuppression |
| Garcia Gonzalez 2005 | Not on maintenance immunosuppression |
| Garcia‐Saenz‐de‐Sicilia 2014 | Not on maintenance immunosuppression |
| Geissler 2016 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Gerhardt 2009 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Gonzalez‐Pinto 2005 | Although this is a long‐term report of an included study (Loinaz 2001), after the randomisation period was complete, immunosuppression was left to local centre's protocol. |
| Grant 2012 | Not on maintenance immunosuppression |
| Hardinger 2004 | Details of maintenance immunosuppression not available. |
| Herlenius 2010 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Hodge 2002 | Randomisation was performed between 3 months and 27 months after liver transplantation. |
| Hytiroglou 1993 | Details of maintenance immunosuppression not available. |
| Junge 2005 | Randomisation was performed at least 1 year after liver transplantation. |
| Kato 2007 | Not on maintenance immunosuppression |
| Keiding 1997 | Not on maintenance immunosuppression |
| Klintmalm 1994 | No fixed immunosuppression regimen in either intervention group or control group. or both |
| Klintmalm 2007 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Klintmalm 2014 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Klupp 1998 | Not on maintenance immunosuppression |
| Langrehr 1997 | Not on maintenance immunosuppression |
| Langrehr 1998a | Not on maintenance immunosuppression |
| Langrehr 1998b | Not on maintenance immunosuppression |
| Langrehr 2001 | Not on maintenance immunosuppression |
| Langrehr 2002 | Not on maintenance immunosuppression |
| Lerut 2005 | Not on maintenance immunosuppression |
| Lerut 2008 | Not on maintenance immunosuppression |
| Levy 2004 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Levy 2006 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Levy 2014 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Llado 2006 | Details of maintenance immunosuppression not available. |
| Llado 2014 | Details of maintenance immunosuppression not available. |
| Lu 2006a | Details of maintenance immunosuppression not available. |
| Lupo 2008 | Not on maintenance immunosuppression |
| Margarit 2005 | Not on maintenance immunosuppression |
| McDiarmid 1991 | Not on maintenance immunosuppression |
| McDiarmid 1991a | Not on maintenance immunosuppression |
| McDiarmid 1993 | Includes paediatric population undergoing liver transplants |
| Moench 2007 | Not on maintenance immunosuppression |
| Mor 1994 | Includes paediatric population undergoing liver transplants |
| Nashan 1996 | Not on maintenance immunosuppression |
| Neuberger 2009 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Neuhaus 1993 | Not a randomised clinical trial |
| Neuhaus 1994 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Neuhaus 1997 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Neuhaus 2000 | Not on maintenance immunosuppression |
| Neuhaus 2002 | Not on maintenance immunosuppression |
| Neumann 2012 | Not on maintenance immunosuppression |
| Nevens 2007 | Randomisation was performed after development of renal impairment. |
| Northup 2006 | Details of maintenance immunosuppression not available. |
| Otero 2009 | Not on maintenance immunosuppression |
| Pageaux 1995 | Not on maintenance immunosuppression |
| Pageaux 2006 | Randomisation was performed after development of renal impairment. |
| Pascher 2015 | Details of maintenance immunosuppression not available. |
| Ramirez 2013 | Not on maintenance immunosuppression |
| Reding 1993 | Not on maintenance immunosuppression |
| Reggiani 2005 | Not on maintenance immunosuppression |
| Reich 2005 | Randomisation was performed after development of renal impairment. |
| Saliba 2016 | Details of maintenance immunosuppression not available. |
| Saliba 2016a | Comparison of different regimens of same drug |
| Salizzoni 2001 | Not clear whether the immunosuppressive regimens were continued beyond the induction phase |
| Schiano 2006 | Not on maintenance immunosuppression |
| Schmeding 2007 | Not on maintenance immunosuppression |
| Schmeding 2011 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Shaked 2016 | Randomisation was performed at an average of 17 months after liver transplantation. |
| Shenoy 2007 | Randomisation was performed at least 6 months after transplantation only in people with renal dysfunction. |
| Simone 2008 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Studenik 2005 | Not on maintenance immunosuppression |
| Takada 2013 | Not on maintenance immunosuppression |
| Teperman 2013 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Therapondos 2002 | Details of maintenance immunosuppression not available. |
| Timmermann 2002 | Details of maintenance immunosuppression not available. |
| Tisone 1998 | Not on maintenance immunosuppression |
| Trunečka 2015 | Not on maintenance immunosuppression |
| Villamil 2014 | Randomisation was performed at least 6 months after liver transplantation. |
| Washburn 2001 | Not on maintenance immunosuppression |
| Washburn 2008 | Not on maintenance immunosuppression |
| Watson 2007 | Randomisation was performed after development of renal impairment. |
| Wiesner 2001 | No fixed immunosuppression regimen in either intervention group or control group, or both |
| Yoshida 2005 | Not on maintenance immunosuppression |
Characteristics of ongoing studies [ordered by study ID]
Nashan 2015.
| Trial name or title | Hephaistos (NCT01551212) |
| Methods | Randomised clinical trial |
| Participants | People undergoing liver transplantation |
| Interventions | Everolimus plus tacrolimus versus tacrolimus |
| Outcomes | Graft loss, death, adverse events |
| Starting date | January 2012 |
| Contact information | nashan@uke.de |
| Notes | Trial registration: NCT01551212 |
Simone 2014.
| Trial name or title | REFLECT |
| Methods | Randomised clinical trial |
| Participants | People undergoing liver transplantation |
| Interventions | Everolimus plus tacrolimus versus tacrolimus |
| Outcomes | Graft loss, death |
| Starting date | March 2014 |
| Contact information | Novartis Pharmaceuticals (+41613241111) |
| Notes | Trial registration: NCT02115113 |
Differences between protocol and review
Although we implied in the methods of the protocol that we would compare maintenance immunosuppression, we have clarified this in the background and methods sections and included this in the title.
We have restricted the trials to only those including adults, as the treatment effects are likely to be different in adults and children.
We used tacrolimus as the reference intervention, as this was the most common intervention used as control.
We have used both the fixed‐effect model and random‐effects model and employed the more conservative model to arrive at conclusions, rather than using the model with the best fit as defined by deviance information criteria.
We have also revised the network meta‐analysis extensively to ensure that it reflects recent developments in the field.
We have analysed most outcomes as time‐to‐event outcomes, as the length of follow‐up between the trials was very variable. Ignoring this difference in length of follow‐up in a network meta‐analysis would have meant a major (and probably incorrect) assumption that the frequency of events was not dependent upon the length of follow‐up in the trials.
We planned to perform a sensitivity analysis excluding trials in which the drug combination used in induction immunosuppression differed from the drug combination used in maintenance immunosuppression. However, excluding such trials would have resulted in few trials being included in this review. We therefore performed a subgroup analysis of trials in which the drug combination used in induction immunosuppression differed from the drug combination used in maintenance immunosuppression compared to trials in which the drug combination used in induction immunosuppression was the same as the drug combination used in maintenance immunosuppression.
Contributions of authors
Kurinchi Gurusamy identified trials, extracted data, performed the analysis, and wrote the review. Manuel Rodríguez‐Perálvarez and Marta Guerrero‐Misas independently identified trials and extracted data. Douglas Thorburn, Brian Davidson, and Emmanuel Tsochatzis critically commented on the review. All authors approved of the published edition.
All review authors approved this version before publication.
Sources of support
Internal sources
University College London, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Kurinchi Gurusamy, Manuel Rodríguez‐Perálvarez, Marta Guerrero‐Misas, Emmanuel Tsochatzis, and Brian Davidson have no financial disclosures. Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. We have no other financial disclosures to report.
Edited (no change to conclusions)
References
References to studies included in this review
Asrani 2014 {published data only}
- Asrani SK, Wiesner RH, Trotter JF, Klintmalm G, Katz E, Maller E, et al. De novo sirolimus and reduced‐dose tacrolimus versus standard‐dose tacrolimus after liver transplantation: the 2000‐2003 phase II prospective randomized trial. American Journal of Transplantation 2014;14(2):356‐66. [DOI] [PubMed] [Google Scholar]
Baiocchi 2006 {published data only}
- Baiocchi L, Angelico M, Luca L, Ombres D, Anselmo A, Telesca C, et al. Cyclosporine a versus tacrolimus monotherapy. Comparison on bile lipids in the first 3 months after liver transplant in humans. Transplant International 2006;19(5):389‐95. [DOI] [PubMed] [Google Scholar]
Belli 1998 {published data only}
- Belli LS, Carlis L, Rondinara GF, Romani F, Alberti A, Pirotta V, et al. Prospective randomized trial of steroid withdrawal in liver transplant patients: Preliminary report. Transplant International 1994;7 Suppl 1:S88‐90. [DOI] [PubMed] [Google Scholar]
- Belli LS, Carlis L, Rondinara G, Alberti AB, Bellati G, Gasperi A, et al. Early cyclosporine monotherapy in liver transplantation: A 5‐year follow‐up of a prospective, randomized trial. Hepatology 1998;27(6):1524‐9. [DOI] [PubMed] [Google Scholar]
- Carlis L, Belli LS, Colella G, Rondinara GF, Slim AO, Alberti A, et al. Serum lipid changes in liver transplantation: Effect of steroids withdrawn in a prospective randomized trial under cyclosporine a therapy. Transplantation Proceedings 1999;31(1‐2):391‐3. [DOI] [PubMed] [Google Scholar]
- DeCarlis L, Belli LS, Rondinara GF, Alberti A, Sansalone CV, Colella G, et al. Early steroid withdrawal in liver transplant patients: Final report of a prospective randomized trial. Transplantation Proceedings 1997;29(1‐2):539‐42. [DOI] [PubMed] [Google Scholar]
Boudjema 2011 {published data only}
- Boudjema K, Camus C, Saliba F, Calmus Y, Salame E, Pageaux G, et al. Reduced‐dose tacrolimus with mycophenolate mofetil vs. standard‐dose tacrolimus in liver transplantation: a randomized study. American Journal of Transplantation 2011;11(5):965‐76. [DOI] [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen JW, Pehlivan M, Gunson BK, Buckels JA, McMaster P, Mayer D. Ten‐year results of a randomised prospective study of FK506 versus cyclosporine in management of primary orthotopic liver transplantation. Transplantation Proceedings 2002;34(5):1507‐10. [DOI] [PubMed] [Google Scholar]
- Dmitrewski J, Ayres S, Gunson BK, Buist LJ, Buckels JA, McMaster P, et al. Steroid withdrawal 3 months after liver transplantation ‐ does FK 506 confer any advantage over cyclosporin?. Transplant International 1994;7 Suppl 1:S85‐7. [DOI] [PubMed] [Google Scholar]
- Krentz AJ, Cramb R, Dousset B, Mayer D, McMaster P, Buckels J, et al. Serum lipids and apolipoproteins in liver transplant recipients: a comparative study of cyclosporin A and FK 506. Journal of Laboratory & Clinical Medicine 1994;124(3):381‐5. [PubMed] [Google Scholar]
- Krentz AJ, Dmitrewski J, Mayer D, McMaster P, Buckels J, Dousset B, et al. Postoperative glucose metabolism in liver transplant recipients. A two‐year prospective randomized study of cyclosporine versus FK506. Transplantation 1994;57(11):1666‐9. [PubMed] [Google Scholar]
- Krentz AJ, Dousset B, Mayer D, McMaster P, Buckels J, Cramb R, et al. Metabolic effects of cyclosporin a and FK 506 in liver transplant recipients. Diabetes 1993;42(12):1753‐9. [DOI] [PubMed] [Google Scholar]
Cholongitas 2011 {published data only}
- Chau TN, Quaglia A, Rolles K, Burroughs AK, Dhillon AP. Histological patterns of rejection using oral microemulsified cyclosporine and tacrolimus (FK506) as monotherapy induction after orthotopic liver transplantation. Liver 2001;21(5):329‐34. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Shusang V, Germani G, Tsochatzis E, Raimondo ML, Marelli L, et al. Long‐term follow‐up of immunosuppressive monotherapy in liver transplantation: Tacrolimus and microemulsified cyclosporin. Clinical Transplantation 2011;25(4):614‐24. [DOI] [PubMed] [Google Scholar]
- Rolles K, Davidson BR, Burroughs AK. A pilot study of immunosuppressive monotherapy in liver transplantation: tacrolimus versus microemulsified cyclosporin. Transplantation 1999;68(8):1195‐8. [DOI] [PubMed] [Google Scholar]
De Simone 2012 {published data only}
- Bernhardt P, Dong G, Lopez P, Hustache G, Bader G. Evaluation of the major adverse cardiac events risk with everolimus‐based calcineurin inhibitor reduction or withdrawal regimen in liver transplant recipients: 3‐year post‐hoc analysis of the randomized H2304 extension study. American Journal of Transplantation 2016;16:234. [Google Scholar]
- Charlton MR, Rinella ME, Heimbach J. Everolimus is associated with lower weight gain two years following liver transplantation ‐ results of a randomized multicenter study. Hepatology 2015;62:816A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone P, Nevens F, Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. American Journal of Transplantation 2012;12(11):3008‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone P, Nevens F, Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. Evolution of renal function with early everolimus‐facilitated reduction or elimination of tacrolimus in 719 de novo liver transplant recipients: 12 month data of the H2304 study. American Journal of Transplantation 2012;12:238. [Google Scholar]
- Simone P, Saliba F, Dong G, Escrig C, Fischer L. Do patient characteristics influence efficacy and renal outcomes in liver transplant patients receiving everolimus?. Clinical Transplantation 2016;30(3):279‐88. [DOI] [PubMed] [Google Scholar]
- Duvoux C, Durand F, Neau‐Cransac M, Hardwigsen J, Pageaux G, Giambattista F, et al. Impact of everolimus, an mtorc1 inhibitor, on hepatocellular carcinoma recurrence after liver transplantation: Results from the H2304 study. Transplant International 2016;29:5. [Google Scholar]
- Escrig C, Bader G, Dong G, Lopez P, Bernhardt P, Hustache G. Reduced renal function increases risk of cardiovascular events in liver transplant recipients at 2‐years post‐liver transplant. Transplant International 2015;28:90. [Google Scholar]
- Fischer L, Simone P, Dong G, Lopez P, Bernhardt P, Saliba F. Effect of donor‐recipient gender mismatch in liver transplant recipients: a post‐hoc analysis of the H2304 study. American Journal of Transplantation 2016;16:753. [Google Scholar]
- Fischer L, Saliba F, Kaiser GM, Carlis L, Metselaar HJ, Simone P, et al. Three‐year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: Follow‐up results from a randomized, multicenter study. Transplantation 2015;99(7):1455‐62. [DOI] [PubMed] [Google Scholar]
- Fischer L, Saliba F, Kaiser GM, Carlis L, Metselaar HJ, Nevens F, et al. Long‐term superior renal function with everolimus and reduced tacrolimus in liver transplant recipients: 3‐year results from the H2304 extension study. Liver Transplantation 2014;20:S171‐2. [Google Scholar]
- Fung J, Saliba F, Kaiser G, Carlis L, Metselaar H, Nevens F, et al. Everolimus with reduced tacrolimus preserves long‐term renal function in liver transplant recipients: 36 and 48 months results from the H2304e1 study. Transplantation 2014;98(Suppl 1):180. [Google Scholar]
- Junge G, Dumortier T, Schwende H, Fung J. Mtor inhibition in liver transplantation: How to dose for effective/safe CNI reduction?. Transplantation Proceedings 2013;45(5):1979‐80. [DOI] [PubMed] [Google Scholar]
- Junge G, Saliba F, Simone P, Fischer L, Dong G, Speziale A, et al. Impact of everolimus, an mTORC1 inhibitor, on hepatocellular carcinoma recurrence after liver transplantation: Results from the H2304 study. Transplantation 2015;99(7 Suppl 1):90‐1. [Google Scholar]
- Metselaar HJ, Saliba F, Schemmer P, Foltys D, Fabregat J, Ericzon BG, et al. Impact of early everolimus‐facilitated reduction or elimination of tacrolimus on the evolution of renal function in 719 de novo liver transplant recipients: 12 month data of the H2304 study. Transplantation 2012;94(10S):44. [Google Scholar]
- Nevens F, Carlis L, Metselaar HJ, Kaiser GM, Saliba F, Jonas S, et al. Everolimus‐based immunosuppression provides superior renal function and comparable efficacy versus standard tacrolimus in de novo liver transplant recipients: 24‐month results of a randomised controlled trial. Journal of Hepatology 2013;58:S12. [Google Scholar]
- Saliba F, Brown RS Jr, Metselaar H, Beckebaum S, Duvoux C, Navasa M, et al. Everolimus based immunosuppression in hepatitis C virus positive de novo liver transplant recipients: 24 month results from a randomized controlled trial. Liver Transplantation 2013;19(6 Suppl):S100‐1. [Google Scholar]
- Saliba F, Durand F, Neau‐Cransac M, Hardwigsen J, Pageaux G, Lopez P, et al. Everolimus with reduced tacrolimus preserves long‐term renal function in liver transplant recipients: 36 and 48 months results from the H2304e1 study. Transplant International 2016;29(S1):7. [Google Scholar]
- Saliba F, Fischer L, Simone P, Dong G, Escrig C, Lopez P. Everolimus with reduced tacrolimus provides improved renal function in liver transplant recipients: A MELD score subgroup analyses from the randomised controlled H2304 study at month 12 and 24. Transplant International 2015;28:199‐200. [Google Scholar]
- Saliba F, Fisher L, Simone P, Dong G, Escrig C, Lopez P. Clinical outcomes of transplantation by MELD score: Month 12 subgroup analysis of the H2304 study. Transplantation 2015;99(7 Suppl 1):151‐2. [Google Scholar]
- Saliba F, Metselaar HJ, Beckebaum S, Duvoux C, Navasa M, Dong G, et al. Everolimus‐based immunosuppression in HCV positive de novo liver transplant recipients: 24‐month results of a randomized controlled trial. Journal of Hepatology 2013;58:S79‐80. [Google Scholar]
- Saliba F, Simone P, Nevens F, Carlis L, Metselaar HJ, Beckebaum S, et al. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. American Journal of Transplantation 2013;13(7):1734‐45. [DOI] [PubMed] [Google Scholar]
- Song GW, Chen CL, Lee WC, Joh JW, Levy G, Lopez PM, et al. Efficacy and safety of everolimus with reduced tacrolimus versus standard exposure tacrolimus in living donor liver transplant recipients: H2307 study. Hepatology International 2013;7:S665. [Google Scholar]
Fernandez‐Miranda 1998 {published data only}
- Fernández‐Miranda C, Guijarro C, Calle A, Loinaz C, Gonzalez‐Pinto I, Gómez‐Izquierdo T, et al. Lipid abnormalities in stable liver transplant recipients ‐ effects of cyclosporin, tacrolimus, and steroids. Transplant International 1998;11(2):137‐42. [DOI] [PubMed] [Google Scholar]
Fisher 1998 {published data only}
- Fisher RA, Ham JM, Marcos A, Shiffman ML, Luketic VA, Kimball PM, et al. A prospective randomized trial of mycophenolate mofetil with neoral or tacrolimus after orthotopic liver transplantation. Transplantation 1998;66(12):1616‐21. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Shiffman ML, Naar JD, Ham JM, Seaman D, Luketic VA. A prospective randomized trial of mycophenolate mofetil (mmf) with neoral or tacrolimus induction following orthotopic liver transplantation (OLT). Hepatology 1996;24(4 (Pt 2)):511a. [Google Scholar]
- Fisher RA, Stone JJ, Wolfe LG, Rodgers CM, Anderson ML, Sterling RK, et al. Four‐year follow‐up of a prospective randomized trial of mycophenolate mofetil with cyclosporine microemulsion or tacrolimus following liver transplantation. Clinical Transplantation 2004;18(4):463‐72. [DOI] [PubMed] [Google Scholar]
Fung 1991 {published data only}
- DiMartini AF, Trzepacz PT, Pajer KA, Faett D, Fung J. Neuropsychiatric side effects of FK506 vs. cyclosporine a. First‐week postoperative findings. Psychosomatics 1997;38(6):565‐9. [DOI] [PubMed] [Google Scholar]
- Fung J, Abu‐Elmagd K, Jain A, Gordon R, Tzakis A, Todo S, et al. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplantation Proceedings 1991;23(6):2977‐83. [PMC free article] [PubMed] [Google Scholar]
- Fung J, Todo S, Abu‐Elmagd K, Jain A, Tzakis A, Martin M, et al. Randomized trial in primary liver transplantation under immunosuppression with FK 506 or cyclosporine. Transplantation Proceedings 1993;25(1 Pt 2):1130. [PMC free article] [PubMed] [Google Scholar]
- Kusne S, Fung J, Alessiani M, Martin M, Torre‐Cisneros J, Irish W, et al. Infections during a randomized trial comparing cyclosporine to FK 506 immunosuppression in liver transplantation. Transplantation Proceedings 1992;24(1):429‐30. [PMC free article] [PubMed] [Google Scholar]
Greig 2003 {published data only}
- Greig P, Lilly L, Scudamore C, Erb S, Yoshida E, Kneteman N, et al. Early steroid withdrawal after liver transplantation: the Canadian tacrolimus versus microemulsion cyclosporin a trial: 1‐year follow‐up. Liver Transplantation 2003;9(6):587‐95. [DOI] [PubMed] [Google Scholar]
Jain 2001 {published data only}
- Jain A. Prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil: Complete report on 350 primary adult liver transplantations. Transplantation Proceedings 2001;33(1‐2):1342‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Kashyap R, Demetris AJ, Eghstesad B, Pokharna R, Fung JJ. A prospective randomized trial of mycophenolate mofetil in liver transplant recipients with hepatitis C. Liver Transplantation 2002;8(1):40‐6. [DOI] [PubMed] [Google Scholar]
- Jain A, Kashyap R, Dodson F, Kramer D, Hamad I, Khan A, et al. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone and mycophenolate mofetil in primary adult liver transplantation: a single center report. Transplantation 2001;72(6):1091‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AB, Hamad I, Rakela J, Dodson F, Kramer D, Demetris J, et al. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil in primary adult liver transplant recipients: an interim report. Transplantation 1998;66(10):1395‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonas 2005 {published data only}
- Jonas S, Guckelberger O, Bechstein WO, Berg T, Muller AR, Platz KP, et al. Five‐year follow‐up of tacrolimus as primary immunosuppressant after liver transplantation. Transplantation Proceedings 1998;30(5):2179‐81. [DOI] [PubMed] [Google Scholar]
- Jonas S, Guckelberger O, Tullius SG, Steinmuller T, Muller AR, Grauhan O, et al. Corticosteroid‐free therapy after tacrolimus‐based dual immunosuppression versus cyclosporine‐based quadruple‐induction therapy. Transplantation Proceedings 2001;33(3):2232‐3. [DOI] [PubMed] [Google Scholar]
- Jonas S, Kling N, Bechstein WO, Blumhardt G, Lohmann R, Lobeck H, et al. Rejection episodes after liver transplantation during primary immunosuppression with FK506 or a cyclosporine‐based regimen: a controlled, prospective, randomized trial. Clinical Transplantation 1995;9(5):406‐14. [PubMed] [Google Scholar]
- Jonas S, Neuhaus R, Junge G, Klupp J, Theruvat T, Langrehr JM, et al. Primary immunosuppression with tacrolimus after liver transplantation: 12‐years follow‐up. International Immunopharmacology 2005;5(1):125‐8. [DOI] [PubMed] [Google Scholar]
- Mueller AR, Platz KP, Bechstein WO, Schattenfroh N, Stoltenburg‐Didinger G, Blumhardt G, et al. Neurotoxicity after orthotopic liver transplantation: a comparison between cyclosporine and FK506. Transplantation 1994;58(2):155‐69. [PubMed] [Google Scholar]
- Mueller AR, Platz KP, Blumhardt G, Bechstein WO, Steinmuller T, Christe W, et al. The optimal immunosuppressant after liver transplantation according to diagnosis: Cyclosporine a or FK506?. Clinical Transplantation 1995;9(3 Pt 1):176‐84. [PubMed] [Google Scholar]
- Mueller AR, Platz KP, Blumhardt G, Bechstein WO, Steinmuller T, Christe W, et al. The superior immunosuppressant according to diagnosis: Fk 506 or cyclosporine a. Transplantation Proceedings 1995;27(1):1117‐20. [PubMed] [Google Scholar]
- Mueller AR, Platz KP, Schattenfroh N, Bechstein WO, Christe W, Neuhaus P. Neurotoxicity after orthotopic liver transplantation in cyclosporin a‐ and FK 506‐treated patients. Transplant International 1994;7 Suppl 1:S37‐42. [DOI] [PubMed] [Google Scholar]
- Platz KP, Mueller AR, Blumhardt G, Bachmann S, Bechstein WO, Kahl A, et al. Nephrotoxicity after orthotopic liver transplantation in cyclosporin a and FK 506‐treated patients. Transplant International 1994;7 Suppl 1:S52‐7. [DOI] [PubMed] [Google Scholar]
- Platz KP, Mueller AR, Blumhardt G, Bachmann S, Bechstein WO, Kahl A, et al. Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation 1994;58(2):170‐8. [PubMed] [Google Scholar]
- Steinmuller TM, Graf KJ, Schleicher J, Leder K, Bechstein WO, Mueller AR, et al. The effect of FK506 versus cyclosporine on glucose and lipid metabolism ‐ a randomized trial. Transplantation 1994;58(6):669‐74. [PubMed] [Google Scholar]
Loinaz 2001 {published data only}
- Loinaz C, Marin LM, Gonzalez‐Pinto I, Gomez R, Jimenez C, Moreno E. A single‐centre experience with cyclosporine microemulsion versus tacrolimus in 100 randomized liver transplant recipients: Midterm efficacy and safety. Transplantation Proceedings 2001;33(7‐8):3439‐41. [DOI] [PubMed] [Google Scholar]
Manousou 2014 {published data only}
- Manousou P, Cholongitas E, Samonakis D, Tsochatzis E, Corbani A, Dhillon AP, et al. Reduced fibrosis in recurrent HCV with tacrolimus, azathioprine and steroids versus tacrolimus: Randomised trial long term outcomes. Gut 2014;63(6):1005‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manousou P, Samonakis D, Cholongitas E, Patch D, O'Beirne J, Dhillon AP, et al. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transplantation 2009;15(12):1783‐91. [DOI] [PubMed] [Google Scholar]
- Manousou P, Samonakis D, Tsochatzis E, Cholongitas E, Davidson J, Patch D, et al. Long term ‐ 8 year follow up of a randomized trial of tacrolimus monotherapy versus triple therapy after liver transplantation for HCV cirrhosis. Journal of Hepatology 2013;58:S11. [Google Scholar]
- Samonakis DN, Cholongitas E, Triantos CK, Quaglia A, Senzolo M, Dhillon AP, et al. Randomised trial of tacrolimus monotherapy vs. tacrolimus/azathioprine/prednisolone after liver transplantation for HCV cirrhosis: Preliminary results. Journal of Hepatology 2005;42(Suppl 2):47. [Google Scholar]
- Samonakis DN, Mela M, Quaglia A, Triantos CK, Thalheimer U, Leandro G, et al. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transplant Infectious Disease 2006;8(1):3‐12. [DOI] [PubMed] [Google Scholar]
Martin 2004 {published data only}
- Martin P, Busuttil RW, Goldstein RM, Crippin JS, Klintmalm GB, Fitzsimmons WE, et al. Impact of tacrolimus versus cyclosporine in hepatitis C virus‐infected liver transplant recipients on recurrent hepatitis: a prospective, randomized trial. Liver Transplantation 2004;10(10):1258‐62. [DOI] [PubMed] [Google Scholar]
Masetti 2010 {published data only}
- Masetti M, Montalti R, Rompianesi G, Codeluppi M, Gerring R, Romano A, et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. American Journal of Transplantation 2010;10(10):2252‐62. [DOI] [PubMed] [Google Scholar]
- Montalti R, Masetti M, Rompianesi G, Romano A, Ballarin R, Denedetto F. Calcineurin inhibitor‐free immunosuppressive protocol with everolimus monotherapy in de novo liver transplantation. Liver Transplantation 2008;14(7 (Suppl 1)):S226. [Google Scholar]
O'Grady 2002 {published data only}
- Burroughs A, Rolles K, Gimson A, Jamieson N, Hudson M, Thick M, et al. Tacrolimus versus microemulsified cyclosporine in liver transplantation ‐ the TMC randomised trial final results at one year (abstract). Journal of Hepatology 2002;36(Suppl 1):26. [Google Scholar]
- O'Grady JG. TMC trial: 3 year follow‐up on randomised controlled trial of tacrolimus versus microemulsified cyclosporine in liver transplantation. Hepatology 2004;40(4 Suppl 1):551a. [Google Scholar]
- O'Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002;360(9340):1119‐25. [DOI] [PubMed] [Google Scholar]
- O'Grady JG, Hardy P, Burroughs AK, Elbourne D. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: Poststudy surveillance to 3 years. American Journal of Transplantation 2007;7(1):137‐41. [DOI] [PubMed] [Google Scholar]
Pageaux 2004 {published data only}
- Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. Steroid withdrawal at day 14 after liver transplantation: a double‐blind, placebo‐controlled study. Liver Transplantation 2004;10(12):1454‐60. [DOI] [PubMed] [Google Scholar]
Pelletier 2013 {published data only}
- Pelletier SJ, Ammori JB, Englesbe MJ, Sung RS, Magee JC, Fontana RJ. A prospective, randomized trial of complete steroid avoidance in liver transplantation. Liver Transplantation 2008;14(7 (Suppl 1)):S224. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Nadig SN, Lee DD, Ammori JB, Englesbe MJ, Sung RS, et al. A prospective, randomized trial of complete avoidance of steroids in liver transplantation with follow‐up of over 7 years. HPB 2013;15(4):286‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier SJ, Vanderwall K, Debroy MA, Englesbe MJ, Sung RS, Magee JC, et al. Preliminary analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplantation Proceedings 2005;37(2):1214‐6. [DOI] [PubMed] [Google Scholar]
Pham 1998 {published data only}
- Pham H, Lemoine A, Salvucci M, Azoulay D, Frenoy N, Samuel D, et al. Occurrence of gammopathies and lymphoproliferative disorders in liver transplant recipients randomized to tacrolimus (FK506)‐ or cyclosporine‐based immunosuppression. Liver Transplantation & Surgery 1998;4(2):146‐51. [DOI] [PubMed] [Google Scholar]
Porayko 1994 {published data only}
- Porayko MK, Textor SC, Krom RA, Hay JE, Gores GJ, Richards TM, et al. Nephrotoxic effects of primary immunosuppression with FK‐506 and cyclosporine regimens after liver transplantation. Mayo Clinic Proceedings 1994;69(2):105‐11. [DOI] [PubMed] [Google Scholar]
- Porayko MK, Textor SC, Krom RA, Hay JE, Gores GJ, Wahlstrom HE, et al. Nephrotoxicity of FK 506 and cyclosporine when used as primary immunosuppression in liver transplant recipients. Transplantation Proceedings 1993;25(1 Pt 1):665‐8. [PubMed] [Google Scholar]
Shenoy 2008 {published data only}
- Shenoy S, Hardinger K, Laflin MA, Kemp D, Desai N, Lowell J, et al. The economic impact of immunosuppression using cyclosporine (CsA) with 2 hour post dose monitoring vs tacrolimus (FK) following de novo liver transplantation ‐ results of a prospective, randomized trial (abstract no: 1377). American Journal of Transplantation 2005;5(Suppl 11):506. [Google Scholar]
- Shenoy S, Hardinger KL, Crippin J, Karenblat K, Lisker‐Melman M, Lowell JA, et al. A randomized, prospective, pharmacoeconomic trial of neoral 2‐hour postdose concentration monitoring versus tacrolimus trough concentration monitoring in de novo liver transplant recipients. Liver Transplantation 2008;14(2):173‐80. [DOI] [PubMed] [Google Scholar]
Stegall 1997 {published data only}
- Stegall MD, Everson GT, Wachs M, Schroter G, Karrer F, Bilir B, et al. Prednisone withdrawal 14 days after adult liver transplantation with mycophenolate mofetil (mm). Hepatology 1996;24(4 (Pt 2)):174a. [Google Scholar]
- Stegall MD, Wachs ME, Everson G, Steinberg T, Bilir B, Shrestha R, et al. Prednisone withdrawal 14 days after liver transplantation with mycophenolate ‐ a prospective trial of cyclosporine and tacrolimus. Transplantation 1997;64(12):1755‐60. [DOI] [PubMed] [Google Scholar]
Sterneck 2000 {published data only}
- Fischer L, Sterneck M, Gahlemann C, Gundlach M, Rogiers X, Broelsch CE. Safety and efficacy of mycophenolate mofetil versus azathioprine as primary immunosuppressive therapy in liver transplant recipients. Transplantationsmedizin 1999;11(4):274‐8. [Google Scholar]
- Sterneck M, Fischer L, Gahlemann C, Gundlach M, Rogiers X, Broelsch C. Mycophenolate mofetil for prevention of liver allograft rejection: Initial results of a controlled clinical trial. Annals of Transplantation 2000;5(1):43‐6. [PubMed] [Google Scholar]
Zervos 1998 {published data only}
- Zervos XA, Weppler D, Fragulidis GP, Torres MB, Nery JR, Khan MF, et al. Comparison of tacrolimus with microemulsion cyclosporine as primary immunosuppression in hepatitis C patients after liver transplantation. Transplantation 1998;65(8):1044‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelmalek 2012 {published data only}
- Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. American Journal of Transplantation 2012;12(3):694‐705. [DOI] [PubMed] [Google Scholar]
Barnes 1997 {published data only}
- Barnes D, Talenti D, Cammell G, Goormastic M, Farquhar L, Henderson M, et al. A randomized clinical trial of ursodeoxycholic acid as adjuvant treatment to prevent liver transplant rejection. Hepatology 1997;26(4):853‐7. [DOI] [PubMed] [Google Scholar]
Beckebaum 2004 {published data only}
- Beckebaum S, Cicinnati VR, Klein CG, Brokalaki E, Yu Z, Malago M, et al. Impact of combined mycophenolate mofetil and low‐dose calcineurin inhibitor therapy on renal function, cardiovascular risk factors, and graft function in liver transplant patients: Preliminary results of an open prospective study. Transplantation Proceedings 2004;36(9):2671‐4. [DOI] [PubMed] [Google Scholar]
- Beckebaum S, Klein CG, Sotiropoulos GC, Saner FH, Gerken G, Paul A, et al. Combined mycophenolate mofetil and minimal dose calcineurin inhibitor therapy in liver transplant patients: Clinical results of a prospective randomized study. Transplantation Proceedings 2009;41(6):2567‐9. [DOI] [PubMed] [Google Scholar]
Becker 2008 {published data only}
- Becker T, Foltys D, Bilbao I, D'Amico D, Colledan M, Bernardos A, et al. Patient outcomes in two steroid‐free regimens using tacrolimus monotherapy after daclizumab induction and tacrolimus with mycophenolate mofetil in liver transplantation. Transplantation 2008;86(12):1689‐94. [DOI] [PubMed] [Google Scholar]
Benitez 2010 {published data only}
- Benitez CE, Puig‐Pey I, Lopez M, Martinez‐Llordella M, Lozano JJ, Bohne F, et al. ATG‐fresenius treatment and low‐dose tacrolimus: Results of a randomized controlled trial in liver transplantation. American Journal of Transplantation 2010;10(10):2296‐304. [DOI] [PubMed] [Google Scholar]
Berenguer 2006 {published data only}
- Aguilera V, Prieto M, Rubin A, Risalde B, Ortiz C, San Juan F. Calcineurin inhibitors and outcome of liver transplantation in HCV‐positive recipients: Final results of a prospective randomized study. Hepatology 2009;50(4 (Suppl)):549a. [Google Scholar]
- Aguilera V, Prieto M, Rubín A, Risalde B, Ortiz Cantó C, San Juan F. Calcineurin inhibitors and outcome of liver transplantation in HCV‐positive recipients: Final results of a prospective randomized study. Journal of Hepatology 2010;52(Suppl 1):S43. [Google Scholar]
- Berenguer M, Aguilera V, Prieto M, San Juan F, Rayon JM, Benlloch S, et al. Effect of calcineurin inhibitors on survival and histologic disease severity in HCV‐infected liver transplant recipients. Liver Transplantation 2006;12(5):762‐7. [DOI] [PubMed] [Google Scholar]
Biancofiore 2004 {published data only}
- Biancofiore G, Della Rocca G, Bindi L, Romanelli A, Esposito M, Meacci L, et al. Use of fenoldopam to control renal dysfunction early after liver transplantation. Liver Transplantation 2004;10(8):986‐92. [DOI] [PubMed] [Google Scholar]
Bilbao 2009 {published data only}
- Bilbao I, Otto G, Becker T, D'Amico D, Colledan M, Bernardos A. Safety and efficacy of steroid‐free immunosuppression post‐liver transplantation: a randomised phase iii trial comparing tacrolimus monotherapy after daclizumab induction versus tacrolimus+mmf dual therapy. Journal of Hepatology 2009;50(Suppl 1):S58. [Google Scholar]
Bogetti 2005 {published data only}
- Bogetti D, Sankary HN, Jarzembowski TM, Manzelli A, Knight PS, Thielke J, et al. Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury. Clinical Transplantation 2005;19(4):507‐11. [DOI] [PubMed] [Google Scholar]
Boillot 2000 {published data only}
- Boillot O, Poncet G, Mechet I, Dumortier J, Delafosse B, Sagnard P, et al. Randomized trial of triple based immunosuppression using tacrolimus, mycophenolate mofetil and steroids versus quadruple regimen induction with thymoglobulin in liver transplantation. Hepatology 2000;32(4):599A. [Google Scholar]
Boillot 2001 {published data only}
- Boillot O, Baulieux J, Wolf P, Messner M, Cherqui D, Gugenheim J, et al. Low rejection rates with tacrolimus‐based dual and triple regimens following liver transplantation. Clinical Transplantation 2001;15(3):159‐66. [DOI] [PubMed] [Google Scholar]
Boillot 2005 {published data only}
- Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid‐free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transplantation 2005;11(1):61‐7. [DOI] [PubMed] [Google Scholar]
Boillot 2009 {published data only}
- Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, et al. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: a five‐year randomized prospective study. Liver Transplantation 2009;15(11):1426‐34. [DOI] [PubMed] [Google Scholar]
Boleslawski 2004 {published data only}
- Boleslawski E, BenOthman S, Grabar S, Correia L, Podevin P, Chouzenoux S, et al. Cd25, cd28 and cd38 expression in peripheral blood lymphocytes as a tool to predict acute rejection after liver transplantation. Clinical Transplantation 2008;22(4):494‐501. [DOI] [PubMed] [Google Scholar]
- Boleslawski E, Conti F, Sanquer S, Podevin P, Chouzenoux S, Batteux F, et al. Defective inhibition of peripheral cd8+ t cell il‐2 production by anti‐calcineurin drugs during acute liver allograft rejection. Transplantation 2004;77(12):1815‐20. [DOI] [PubMed] [Google Scholar]
Calmus 2010 {published data only}
- Calmus Y, Kamar N, Gugenheim J, Duvoux C, Ducerf C, Wolf P, et al. Assessing renal function with daclizumab induction and delayed tacrolimus introduction in liver transplant recipients. Transplantation 2010;89(12):1504‐10. [DOI] [PubMed] [Google Scholar]
- Calmus Y, Rostaing L, Gugenheim J, Duvoux C, Ducerf C, Wolf P. Evaluation of the benefit on renal function of a delayed introduction of tacrolimus in liver transplant recipients at 13 French centers. Hepatology 2007;46(4 (Suppl 1)):482a‐3a. [Google Scholar]
Chen 2005 {published data only}
- Chen G, He Y, Wang HZ, Lu Q, Yang SZ, Yang ZY, et al. Cyclosporine microemulsion C2 monitoring in Chinese adult liver transplant recipients: a preliminary randomized control trial. Chung‐Hua Wai Ko Tsa Chih 2005;43(19):1243‐7. [PubMed] [Google Scholar]
Cicinnati 2007 {published data only}
- Cicinnati VR, Yu Z, Klein CG, Sotiropoulos GC, Saner F, Malago M, et al. Clinical trial: Switch to combined mycophenolate mofetil and minimal dose calcineurin inhibitor in stable liver transplant patients ‐ assessment of renal and allograft function, cardiovascular risk factors and immune monitoring. Alimentary Pharmacology & Therapeutics 2007;26(9):1195‐208. [DOI] [PubMed] [Google Scholar]
Cillo 2014 {published data only}
- Cillo U, Vitale A, Salizzoni M, Coledan M, Rossi G, Baccarani U, et al. Early introduction of everolimus in de novo liver transplantation: 3‐months final results (primary endpoint) of a multicenter randomized clinical trial (EPOCAL). Liver Transplantation 2014;20:S105‐6. [Google Scholar]
- Cillo U, Vitale A, Salizzoni M, Colledan M, Rossi G, Baccarani U, et al. Early introduction of everolimus in de novo liver transplantation: Final results of a multicenter randomized clinical trial (EPOCAL). Transplant International 2015;28:74. [Google Scholar]
Cosimi 1987 {published data only}
- Cosimi AB, Cho SI, Delmonico FL. A randomized clinical trial comparing OKT3 and steroids for treatment of hepatic allograft rejection. Transplantation 1987;43(1):91‐5. [DOI] [PubMed] [Google Scholar]
Cosimi 1990 {published data only}
- Cosimi AB, Jenkins RL, Rohrer RJ, Delmonico FL, Hoffman M, Monaco AP. A randomized clinical trial of prophylactic OKT3 monoclonal antibody in liver allograft recipients. Archives of Surgery 1990;125(6):781‐4. [DOI] [PubMed] [Google Scholar]
Cuervas‐Mons 2015 {published data only}
- Cuervas‐Mons V, Herrero JI, Gomez MA, Gonzalez‐Pinto I, Serrano T, Mata M, et al. Impact of tacrolimus and mycophenolate mofetil regimen vs. a conventional therapy with steroids on cardiovascular risk in liver transplant patients. Clinical Transplantation 2015;29(8):667‐77. [DOI] [PubMed] [Google Scholar]
Day 2004 {published data only}
- Day CP, O'Grady J, Simpson K, Millson CE, Solomons N, Duncan J, et al. A randomised controlled trial of calcineurin inhibitor (CNI) replacement with mycophenolate mofetil and steroids in liver transplant patients with renal dysfunction. Hepatology 2004;40(4 Suppl 1):547a. [Google Scholar]
De Simone 2009 {published data only}
- Simone P, Metselaar HJ, Fischer L, Dumortier J, Boudjema K, Hardwigsen J, et al. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transplantation 2009;15(10):1262‐9. [DOI] [PubMed] [Google Scholar]
De Simone 2015 {published data only}
- Simone P, Carrai P, Coletti L, Precisi A, Campani D, Filipponi F. Efficacy and safety of once‐daily everolimus with minimized once‐daily tacrolimus after liver transplantation. American Journal of Transplantation 2015;15(S3):Abstract number: 1281. [Google Scholar]
- Simone P, Carrai P, Precisi A, Coletti L, Campani D, Filipponi F. Efficacy and safety of once‐daily everolimus with minimized once‐daily tacrolimus after liver transplantation. Transplantation 2015;99(7 Suppl 1):274‐5. [Google Scholar]
- Simone P, Carrai P, Precisi A, Coletti L, Ducci J, Campani D, et al. Efficacy and safety of a combination schedule with once‐daily everolimus and once‐daily tacrolimus in maintenance liver transplantation. Transplant International 2015;28:74. [Google Scholar]
Duvoux 2015 {published data only}
- Duvoux C, Villamil F, Renner EL, Grazi GL, Firpi RJ, Pageaux G, et al. Sustained virological response to antiviral therapy in a randomized trial of cyclosporine versus tacrolimus in liver transplant patients with recurrent hepatitis C infection. Annals of Transplantation 2015;20:25‐35. [DOI] [PubMed] [Google Scholar]
Eason 2003 {published data only}
- Eason JD, Blazek J, Mason A, Loss GE. Steroid‐free immunosuppression trough thymoglobulin induction in liver transplantation: Results of a prospective randomized trial (abstract). Hepatology 2000;32(4):208a. [Google Scholar]
- Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid‐free liver transplantation using rabbit antithymocyte globulin induction: Results of a prospective randomized trial. Liver Transplantation 2001;7(8):693‐7. [DOI] [PubMed] [Google Scholar]
- Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE Jr. Steroid‐free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation 2003;75(8):1396‐9. [DOI] [PubMed] [Google Scholar]
Ericzon 1997 {published data only}
- Ericzon BG, Eusufzai S, Soderdahl G, Duraj F, Einarsson K, Angelin B. Secretion and composition of bile after human liver transplantation: Studies on the effects of cyclosporine and tacrolimus. Transplantation 1997;63(1):74‐80. [DOI] [PubMed] [Google Scholar]
Farges 1994 {published data only}
- Farges O, Ericzon BG, Bressonhadni S, Lynch SV, Hockerstedt K, Houssin D, et al. A randomized trial of OKT3‐based versus cyclosporine‐based immunoprophylaxis after liver‐transplantation ‐ long‐term results of a European and Australian multicenter study. Transplantation 1994;58(8):891‐8. [DOI] [PubMed] [Google Scholar]
Filipponi 2004 {published data only}
- Filipponi F, Callea F, Salizzoni M, Grazi GL, Fassati LR, Rossi M, et al. Double‐blind comparison of hepatitis C histological recurrence rate in HCV+ liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation 2004;78(10):1488‐95. [DOI] [PubMed] [Google Scholar]
Firpi 2006 {published data only}
- Firpi RJ, Zhu H, Morelli G, Abdelmalek MF, Soldevila‐Pico C, Machicao VI, et al. Cyclosporine suppresses hepatitis C virus in vitro and increases the chance of a sustained virological response after liver transplantation. Liver Transplantation 2006;12(1):51‐7. [DOI] [PubMed] [Google Scholar]
Firpi 2010 {published data only}
- Firpi RJ, Soldevila‐Pico C, Morelli GG, Cabrera R, Levy C, Clark VC, et al. The use of cyclosporine for recurrent hepatitis C after liver transplant: a randomized pilot study. Digestive Diseases and Sciences 2010;55(1):196‐203. [DOI] [PubMed] [Google Scholar]
- Firpi RJ, Soldevila‐Pico C, Morelli GJ, Machicao VI, Levy C, Cabrera R. A randomized controlled trial of cyclosporine vs tacrolimus immunosuppression in patients receiving pegylated interferon and ribavirin for recurrence hepatitis C virus infection after liver transplantation. Hepatology 2006;44(4 (Suppl 1)):421a. [Google Scholar]
Fischer 2012 {published data only}
- Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin‐inhibitors to everolimus after liver transplantation ‐ PROTECT. American Journal of Transplantation 2012;12(7):1855‐65. [DOI] [PubMed] [Google Scholar]
- Fischer L, Simone P, Nevens F, Metselaar HJ, Dumortier J, Duvoux C. Calcineurin inhibitor withdrawal in the presence of everolimus is feasible in maintenance liver transplant recipients. Hepatology 2008;48(4 (Suppl)):566a. [Google Scholar]
- Sterneck M, Kaiser G, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. 5‐year follow‐up of the protect randomised liver transplantation study showed superior renal function with everolimus and early calcineurin inhibitor withdrawal. Transplant International 2015;28:90. [Google Scholar]
- Sterneck M, Kaiser G, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. Everolimus and early calcineurin inhibitor withdrawal is associated with superior renal function: 5‐year follow‐up of the randomized PROTECT liver transplantation study. American Journal of Transplantation 2015;15(Suppl 3):Abstract: 732. [Google Scholar]
- Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. 5‐year follow‐up results of the everolimus and calcineurin inhibitor withdrawal combination from the randomized PROTECT liver transplantation study. Transplantation 2015;99(7 Suppl 1):89‐90. [Google Scholar]
- Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. Everolimus and early calcineurin inhibitor withdrawal: 3‐year results from a randomized trial in liver transplantation. American Journal of Transplantation 2014;14(3):701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. Everolimus and early calcineurin inhibitor withdrawal: 3‐year results from a randomized trial in liver transplantation. American Journal of Transplantation 2014;14(3):701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, et al. Long‐term follow‐up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clinical Transplantation 2016;30(6):741‐8. [DOI] [PubMed] [Google Scholar]
Fleckenstein 1996 {published data only}
- Fleckenstein J, Parades M, Thuluvath PJ. A prospective randomized double‐blind trial evaluating the efficacy of ursodeoxycholic acid (UDCA) in preventing acute rejection. Hepatology 1996;24(4 (Pt 2)):177a. [DOI] [PubMed] [Google Scholar]
Garcia Gonzalez 2005 {published data only}
- Garcia Gonzalez M, Pera Madrazo C, Bernardos Rodriguez A, Gomez Gutierrez M, Ignacio Herrero J, Mir Pallardo J, et al. An open, randomized, multicenter clinical trial of oral tacrolimus in liver allograft transplantation: a comparison of dual vs. triple drug therapy. Liver Transplantation 2005;11(5):515‐24. [DOI] [PubMed] [Google Scholar]
- Gonzalez MG, Bernardos A, Gomez M, Ortiz de Urbina J. Both dual and triple regimens of tacrolimus are effective and safe in liver transplantation (abstract). Journal of Hepatology 2001;34(1):8. [Google Scholar]
Garcia‐Saenz‐de‐Sicilia 2014 {published data only}
- Garcia‐Saenz‐de‐Sicilia M, Olivera‐Martinez MA, Grant WJ, Mercer DF, Baojjang C, Langnas A, et al. Impact of anti‐thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Digestive Diseases and Sciences 2014;59(11):2804‐12. [DOI] [PubMed] [Google Scholar]
Geissler 2016 {published data only}
- Geissler EK, Schnitzbauer AA, Zolke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open‐label phase 3 trial. Transplantation 2016;100(1):116‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, et al. A prospective randomised, open‐labeled trial comparing sirolimus‐containing versus mTOR‐inhibitor‐free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer 2010;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerhardt 2009 {published data only}
- Gerhardt T, Terjung B, Knipper P, Palmedo H, Woitas RP, Kalff J, et al. Renal impairment after liver transplantation ‐ a pilot trial of calcineurin inhibitor‐free vs. calcineurin inhibitor sparing immunosuppression in patients with mildly impaired renal function after liver transplantation. European Journal of Medical Research 2009;14(5):210‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Pinto 2005 {published data only}
- Gonzalez‐Pinto IM, Rimola A, Margarit C, Cuervas‐Mons V, Abradelo M, Alvarez‐Laso C, et al. Five‐year follow‐up of a trial comparing tacrolimus and cyclosporine microemulsion in liver transplantation. Transplantation Proceedings 2005;37(4):1713‐5. [DOI] [PubMed] [Google Scholar]
Grant 2012 {published data only}
- Grant W, Botha J, Mercer D, Olivera‐Martinez M, McCashland T, Langnas A. A randomized, single‐center trial comparing thymoglobulin (TG) induction therapy and delayed initiation of tacrolimus to no induction with immediate initiation of tacrolimus in liver transplant (LT) recipients to assess the impact on renal function. American Journal of Transplantation 2012;12:539. [Google Scholar]
Hardinger 2004 {published data only}
- Hardinger KL, Chapman WC, Lowell J, Desai N, Crippin J, Lisker‐Melman M. Interim analysis of a single‐center, prospective, randomized trial compaing tacrolimus versus cyclosporine immunosuppression using 2 hour post dose (c2) monitoring in de novo liver transplant recipients. Transplantation 2004;78(2):377‐8. [Google Scholar]
Herlenius 2010 {published data only}
- Herlenius G, Felldin M, Norden G, Olausson M, Backman L, Gustafsson B, et al. Conversion from calcineurin inhibitor to either mycophenolate mofetil or sirolimus improves renal function in liver transplant recipients with chronic kidney disease: Results of a prospective randomized trial. Transplantation Proceedings 2010;42(10):4441‐8. [DOI] [PubMed] [Google Scholar]
Hodge 2002 {published data only}
- Hodge EE, Reich DJ, Clavien PA, Kim‐Schluger L. Use of mycophenolate mofetil in liver transplant recipients experiencing renal dysfunction on cyclosporine or tacrolimus ‐ randomized, prospective, multicenter study results. Transplantation Proceedings 2002;34(5):1546‐7. [DOI] [PubMed] [Google Scholar]
Hytiroglou 1993 {published data only}
- Hytiroglou P, Lee R, Sharma K, Theise ND, Schwartz M, Miller C, et al. Fk506 versus cyclosporine as primary immunosuppressive agent for orthotopic liver allograft recipients. Histologic and immunopathologic observations. Transplantation 1993;56(6):1389‐94. [DOI] [PubMed] [Google Scholar]
Junge 2005 {published data only}
- Junge G, Neuhaus R, Schewior L, Klupp J, Guckelberger O, Langrehr JM, et al. Withdrawal of steroids: A randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis. Transplantation Proceedings 2005;37(4):1695‐6. [DOI] [PubMed] [Google Scholar]
Kato 2007 {published data only}
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84(7):829‐35. [DOI] [PubMed] [Google Scholar]
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplantation Proceedings 2005;37(2):1217‐9. [DOI] [PubMed] [Google Scholar]
Keiding 1997 {published data only}
- Keiding S, Hockerstedt K, Bjoro K, Bondesen S, Hjortrup A, Isoniemi H, et al. The Nordic multicenter double‐blind randomized controlled trial of prophylactic ursodeoxycholic acid in liver transplant patients. Transplantation 1997;63(11):1591‐4. [DOI] [PubMed] [Google Scholar]
Klintmalm 1994 {published data only}
- Klintmalm GB. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. New England Journal of Medicine 1994;331(17):1110‐5. [DOI] [PubMed] [Google Scholar]
- Lake JR, Gorman KJ, Esquivel CO, Wiesner RH, Klintmalm GB, Miller CM, et al. The impact of immunosuppressive regimens on the cost of liver transplantation ‐ results from the U.S. Fk506 multicenter trial. Transplantation 1995;60(10):1089‐95. [DOI] [PubMed] [Google Scholar]
- Porayko MK, Wiesner RH. United States multicenter prospective randomized trial comparing primary immunosuppression with FK506 vs cyclosporine (CyA) following liver‐transplantation. Gastroenterology 1993;104(4):A974. [Google Scholar]
- Wiesner RH. A long‐term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation ‐ a report of the United States FK506 study group. Transplantation 1998;66(4):493‐9. [DOI] [PubMed] [Google Scholar]
Klintmalm 2007 {published data only}
- Fasola CG, Heffron TG, Sher L, Douglas DD, Brown R, Ham J, et al. Multicenter randomized hepatitis C (HCV) three trial post liver transplantation (OLT): a 90‐day report. Hepatology 2004;40(4 Suppl 1):163a. [Google Scholar]
- Klintmalm G, Fasola CG, Jennings LW. Hepatitis C (HCV)‐3 study: Day‐90 protocol biopsy (PB) grade is a surrogate marker for severe HCV recurrence (R), on PB, at years 1 and 2 post liver transplantation (OLT). Hepatology 2009;50(4 (Suppl)):1028a. [Google Scholar]
- Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, et al. A randomized, multicenter study comparing steroid‐free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transplantation 2011;17(12):1394‐403. [DOI] [PubMed] [Google Scholar]
- Klintmalm GB, Fasola CG, Jennings L, Heffron TG, Sher L, Mulligan D. Hepatitis C (HCV)‐3 study: Benefits of a steroid‐free immunosuppression (IS) regimen in HCV‐infected liver transplant recipients (OLT) may become evident only after long‐term follow up. Liver Transplantation 2008;14(7 (Suppl 1)):S105. [Google Scholar]
- Klintmalm GBG, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, et al. Corticosteroid‐free immunosuppression with daclizumab in HCV+ liver transplant recipients: 1‐year interim results of the HCV‐3 study. Liver Transplantation 2007;13(11):1521‐31. [DOI] [PubMed] [Google Scholar]
- Sher L, Jennings L, Rudich S, Alexopoulos SP, Netto G, Teperman L, et al. Results of live donor liver transplantation in patients with hepatitis C virus infection: the HCV 3 trial experience. Clinical Transplantation 2012;26(3):502‐9. [DOI] [PubMed] [Google Scholar]
Klintmalm 2014 {published data only}
- Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, et al. Belatacept‐based immunosuppression in de novo liver transplant recipients: 1‐year experience from a phase ii randomized study. American Journal of Transplantation 2014;14(8):1817‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klupp 1998 {published data only}
- Klupp J, Bechstein WO, Pratschke J, Tullius SG, Gebhard A, Lobeck H, et al. Risk and benefit of antibody induction therapy in combination with tacrolimus immunosuppression after liver transplantation. Transplantation Proceedings 1998;30(4):1443‐4. [DOI] [PubMed] [Google Scholar]
Langrehr 1997 {published data only}
- Langrehr JM, Nussler NC, Neumann U, Guckelberger O, Lohmann R, Radtke A, et al. A prospective randomized trial comparing interleukin‐2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation. Transplantation 1997;63(12):1772‐81. [DOI] [PubMed] [Google Scholar]
- Lemmens HP, Langrehr JM, Bechstein WO, Blumhardt G, Keck H, Lüsebrink R, et al. Interleukin‐2 receptor antibody vs ATG for induction immunosuppression after liver transplantation: Initial results of a prospective randomized trial. Transplantation Proceedings 1995;27(1):1140‐1. [PubMed] [Google Scholar]
Langrehr 1998a {published data only}
- Langrehr JM, Glanemann M, Guckelberger O, Klupp J, Neumann U, Machens C, et al. A randomized, placebo‐controlled trial with anti‐interleukin‐2 receptor antibody for immunosuppressive induction therapy after liver transplantation. Clinical Transplantation 1998;12(4):303‐12. [PubMed] [Google Scholar]
Langrehr 1998b {published data only}
- Langrehr JM, Glanemann M, Schneller A, Neumann U, Guckelberger O, Lohmann R, et al. A randomized trial comparing anti‐interleukin‐2 receptor antibody and placebo for immunosuppressive therapy after OLT. Transplantation Proceedings 1998;30(4):1445‐6. [DOI] [PubMed] [Google Scholar]
Langrehr 2001 {published data only}
- Langrehr JM, Klupp J, Junge G, Jonas S, Neuhaus R, Bechstein WO, et al. Quadruple versus dual tacrolimus‐based induction after liver transplantation: a prospective, randomized trial. Transplantation Proceedings 2001;33(3):2330‐1. [DOI] [PubMed] [Google Scholar]
- Langrehr JM, Klupp J, Pfitzmann R, Neumann U, Lohmann R, Jonas S, et al. A prospective, randomized trial with quadruple versus dual tacrolimus‐based induction after liver transplantation. Transplantation Proceedings 2001;33(1‐2):1520. [DOI] [PubMed] [Google Scholar]
Langrehr 2002 {published data only}
- Langrehr JM, Neumann UP, Lang M, Muller AR, Jonas S, Settmacher U, et al. First results from a prospective randomized trial comparing steroid‐free induction therapy with tacrolimus and mmf versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplantation Proceedings 2002;34(5):1565‐6. [DOI] [PubMed] [Google Scholar]
Lerut 2005 {published data only}
- Lerut J, Thuyne V, Mathijs J, Lemaire J, Talpe S, Roggen F, et al. Anti‐cd2 monoclonal antibody and tacrolimus in adult liver transplantation. Transplantation 2005;80(9):1186‐93. [DOI] [PubMed] [Google Scholar]
Lerut 2008 {published data only}
- Bonaccorsi‐Riani E, Sempoux C, Piette N, Julliard O, Kabamba B, Ciccarelli O, et al. Impact of steroid‐avoidance immunosuppression on long‐term outcome after liver transplantation for HCV cirrhosis: the need for well documented long‐term follow‐up. Acta Gastroenterologica Belgica 2012;75(4):411‐8. [PubMed] [Google Scholar]
- Lerut J, Mathys J, Verbaandert C, Talpe S, Ciccarelli O, Lemaire J, et al. Tacrolimus monotherapy in liver transplantation: One‐year results of a prospective, randomized, double‐blind, placebo‐controlled study. Annals of Surgery 2008;248(6):956‐67. [DOI] [PubMed] [Google Scholar]
- Lerut JP, Pinheiro RS, Lai Q, Stouffs V, Orlando G, Juri JM, et al. Is minimal, (almost) steroid‐free immunosuppression a safe approach in adult liver transplantation? Long‐term outcome of a prospective, double blind, placebo‐controlled, randomized, investigator‐driven study. Annals of Surgery 2014;260(5):886‐91; discussion 91‐2. [DOI] [PubMed] [Google Scholar]
Levy 2004 {published data only}
- Levy G, Grazi GL, Muhlbacher F, Samuel D, Friman S, Jones R, et al. 12‐month follow‐up analysis of a multicenter, randomized, prospective trial in de novo liver transplantation recipients (LIS2T) comparing cyclosporine microemulsion (c2 monitoring) and tacrolimus. Liver Transplantation 2006;12(10):1464‐72. [DOI] [PubMed] [Google Scholar]
- Levy G, Villamil F, Samuel D, Sanjuan F, Grazi GL, Wu Y, et al. Results of LIS2T, a multicenter, randomized study comparing cyclosporine microemulsion with c2 monitoring and tacrolimus with c0 monitoring in de novo liver transplantation. Transplantation 2004;77(11):1632‐8. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Lake J, Villamil F, Levy G, Marotta P, Mies S, et al. Comparison of cyclosporine microemulsion and tacrolimus in 39 recipients of living donor liver transplantation. Liver Transplantation 2005;11(11):1395‐402. [DOI] [PubMed] [Google Scholar]
Levy 2006 {published data only}
- Levy G, Schmidli H, Punch J, Tuttle‐Newhall E, Mayer D, Neuhaus P, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12‐ and 36‐month results. Liver Transplantation 2006;12(11):1640‐8. [DOI] [PubMed] [Google Scholar]
Levy 2014 {published data only}
- Levy G. REFINE: A multicenter, randomized open‐label study to compare liver fibrosis at 12 months after transplantation for hepatitis C cirrhosis using cyclosporine microemulsion (csa‐me) or tacrolimus (tac). Liver Transplantation 2013;1:S88. [Google Scholar]
- Levy G, Villamil FG, Nevens F, Metselaar HJ, Clavien PA, Klintmalm G, et al. REFINE: A randomized trial comparing cyclosporine a and tacrolimus on fibrosis after liver transplantation for hepatitis C. American Journal of Transplantation 2014;14(3):635‐46. [DOI] [PubMed] [Google Scholar]
- Lilly L, Tzakis A, Klintmalm G, Villamil FG, Jones RM. Comparison between steroid‐treated and steroid‐free cohorts in CNI‐treated patients: 6‐month interim analysis of the REFINE trial, a randomized study to compare the development of liver fibrosis at 12 months after transplantation for hepatitis C cirrhosis. Hepatology 2008;48(4 (Suppl)):548a‐9a. [Google Scholar]
Llado 2006 {published data only}
- Llado L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: Results from a prospective multicenter randomized study. Journal of Hepatology 2006;44(4):710‐6. [DOI] [PubMed] [Google Scholar]
- Lladó L, Fabregat J, Castellote J, Ramos E, Xiol X, Torras J, et al. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: Results of a prospective randomized study. Liver Transplantation 2008;14(12):1752‐60. [DOI] [PubMed] [Google Scholar]
Llado 2014 {published data only}
- Llado L, Fabregat J, Baliellas C, Ramos E, Torras J, Secanella L, et al. Influence of the type of calcineurin inhibitor on HCV recurrence after liver transplantation. Results of a prospective randomized study. Liver Transplantation 2014;20:S332. [Google Scholar]
Lu 2006a {published data only}
- Lu AW, Zheng SS, Wu J, Liang TB, Wang WL, Shen Y, et al. Dual, triple, and quadruple oral tacrolimus‐based immunosuppression regimens after orthotopic liver transplantation: a randomised comparative study of regimens. Chung‐Hua i Hsueh Tsa Chih 2006;86(48):3389‐92. [PubMed] [Google Scholar]
Lupo 2008 {published data only}
- Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation 2008;86(7):925‐31. [DOI] [PubMed] [Google Scholar]
- Lupo L, Ricci P, Caputi L, Tandoi F, Aquilino F, Palma G, et al. Basiliximab vs steroids in liver transplantation immunosuppression. A prospective randomized clinical trial. Liver Transplantation 2005;11(7):C75. [Google Scholar]
Margarit 2005 {published data only}
- Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transplant International 2005;18(12):1336‐45. [DOI] [PubMed] [Google Scholar]
McDiarmid 1991 {published data only}
- McDiarmid SV, Busuttil RW, Levy P, Millis MJ, Terasaki PI, Ament ME. The long‐term outcome of OKT3 compared with cyclosporine prophylaxis after liver transplantation. Transplantation 1991;52(1):91‐7. [DOI] [PubMed] [Google Scholar]
McDiarmid 1991a {published data only}
- McDiarmid SV, Millis MJ, Terasaki PI, Ament ME, Busuttil RW. OKT3 prophylaxis in liver transplantation. Digestive Diseases and Sciences 1991;36(10):1418‐26. [DOI] [PubMed] [Google Scholar]
- Millis JM, McDiarmid SV, Hiatt JR, Brems JJ, Colonna JO, Klein AS, et al. Randomized prospective trial of OKT3 for early prophylaxis of rejection after liver transplantation. Transplantation 1989;47(1):82‐8. [DOI] [PubMed] [Google Scholar]
McDiarmid 1993 {published data only}
- McDiarmid SV, Colonna JO 2nd, Shaked A, Ament ME, Busuttil RW. A comparison of renal function in cyclosporine‐ and FK‐506‐treated patients after primary orthotopic liver transplantation. Transplantation 1993;56(4):847‐53. [DOI] [PubMed] [Google Scholar]
Moench 2007 {published data only}
- Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, et al. Tacrolimus monotherapy without steroids after liver transplantation ‐ a prospective randomized double‐blinded placebo‐controlled trial. American Journal of Transplantation 2007;7(6):1616‐23. [DOI] [PubMed] [Google Scholar]
- Weiler N, Thrun I, Hoppe‐Lotichius M, Zimmermann T, Kraemer I, Otto G. Early steroid‐free immunosuppression with FK506 after liver transplantation: Long‐term results of a prospectively randomized double‐blinded trial. Transplantation 2010;90(12):1562‐6. [DOI] [PubMed] [Google Scholar]
Mor 1994 {published data only}
- Mor E, Patel T, Glabman S, Sheiner P, Emre S, Guy S, et al. Comparison of short and long‐term renal function in liver transplant patients receiving cyclosporin or FK 506. Transplant International 1994;7 Suppl 1:S77‐80. [DOI] [PubMed] [Google Scholar]
Nashan 1996 {published data only}
- Nashan B, Schlitt HJ, Schwinzer R, Ringe B, Kuse E, Tusch G, et al. Immunoprophylaxis with a monoclonal anti‐il‐2 receptor antibody in liver transplant patients. Transplantation 1996;61(4):546‐54. [DOI] [PubMed] [Google Scholar]
Neuberger 2009 {published data only}
- Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced‐dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. American Journal of Transplantation 2009;9(2):327‐36. [DOI] [PubMed] [Google Scholar]
Neuhaus 1993 {published data only}
- Neuhaus P, Bechstein WO, Blumhardt G, Wiens M, Lemmens P, Langrehr JM, et al. Comparison of quadruple immunosuppression after liver transplantation with ATG or IL‐2 receptor antibody. Transplantation 1993;55(6):1320‐7. [DOI] [PubMed] [Google Scholar]
Neuhaus 1994 {published data only}
- Devlin J, Williams R, Neuhaus P, McMaster P, Calne R, Pichlmayr R, et al. Renal complications and development of hypertension in the European study of FK 506 and cyclosporin in primary liver transplant recipients. Transplant International 1994;7 Suppl 1:S22‐6. [DOI] [PubMed] [Google Scholar]
- Ericzon B, Groth C, Bismuth H, Calne R, McMaster P, Neuhaus P, et al. Glucose metabolism in liver transplant recipients treated with FK 506 or cyclosporin in the European multicentre study. Transplant International 1994;7 Suppl 1:S11‐4. [DOI] [PubMed] [Google Scholar]
- McMaster P. Patient and graft survival in the European multicentre liver study ‐ FK 506 vs cyclosporin a. Transplant International 1994;7 Suppl 1:S32‐6. [DOI] [PubMed] [Google Scholar]
- Muhlbacher F. Tacrolimus versus cyclosporin microemulsion in liver transplantation: Results of a 3‐month study. Transplantation Proceedings 2001;33(1‐2):1339‐40. [DOI] [PubMed] [Google Scholar]
- Neuhaus P, McMaster P, Calne R, Pichlmayr R, Otto G, Williams R, et al. Neurological complications in the European multicentre study of FK 506 and cyclosporin in primary liver transplantation. Transplant International 1994;7 Suppl 1:S27‐31. [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Pichlmayr R, Williams R, Bechstein WO, Blumhardt G, McMaster P, et al. Randomized trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft‐rejection. Lancet 1994;344(8920):423‐8. [PubMed] [Google Scholar]
- Otto G, Bleyl J, Neuhaus P, McMaster P, Calne R, Pichlmayr R, et al. Corticosteroids and concomitant medication in the European multicentre study of FK 506 and cyclosporin in primary liver transplantation. Transplant International 1994;7 Suppl 1:S7‐10. [DOI] [PubMed] [Google Scholar]
- Samuel D. Primary treatment with tacrolimus after liver transplantation: Three‐year results of the European multicentre liver study. Tacrolimus in Organ Transplantation: Prevention and Treatment of Allograft Rejections 1998:58‐67. [Google Scholar]
- Undre N, Moller A. Pharmacokinetic interpretation of FK 506 levels in blood and in plasma during a European randomised study in primary liver transplant patients. The FK 506 European study group. Transplant International 1994;7 Suppl 1:S15‐21. [DOI] [PubMed] [Google Scholar]
- Williams R, Neuhaus P, Bismuth H, McMaster P, Pichlmayr R, Calne R, et al. Two‐year data from the European multicentre tacrolimus (FK506) liver study. Transplant International 1996;9 Suppl 1:S144‐50. [DOI] [PubMed] [Google Scholar]
Neuhaus 1997 {published data only}
- Neuhaus P, Langrehr JM, Williams R, Calne RY, Pichlmayr R, McMaster P. Tacrolimus‐based immunosuppression after liver transplantation: a randomised study comparing dual versus triple low‐dose oral regimens. Transplant International 1997;10(4):253‐61. [DOI] [PubMed] [Google Scholar]
Neuhaus 2000 {published data only}
- Neuhaus P, Klupp J, Langrehr JM, Neumann U, Gebhardt A, Pratschke J, et al. Quadruple tacrolimus‐based induction therapy including azathioprine and ALG does not significantly improve outcome after liver transplantation when compared with standard induction with tacrolimus and steroids: Results of a prospective, randomized trial. Transplantation 2000;69(11):2343‐53. [DOI] [PubMed] [Google Scholar]
Neuhaus 2002 {published data only}
- Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: Results from a double‐blind randomized placebo‐controlled trial. Liver Transplantation 2002;8(2):132‐42. [DOI] [PubMed] [Google Scholar]
Neumann 2012 {published data only}
- Neumann U, Samuel D, Trunecka P, Gugenheim J, Gerunda GE, Friman S. A randomized multicenter study comparing a tacrolimus‐based protocol with and without steroids in HCV‐positive liver allograft recipients. Journal of Transplantation 2012;2012:894215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nevens 2007 {published data only}
- Nevens F, Sterneck M, Metselaar H, Dumortier J, Giostra E, Boudjema K. Multicenter, randomized trial of conversion to everolimus with calcineurin inhibitor minimization or discontinuation in liver transplant patients with renal impairment. Hepatology 2007;46(4 (Suppl 1)):486a‐7a. [Google Scholar]
Northup 2006 {published data only}
- Northup PG, Chong TW, Iezzoni JC, Kerr SE, Burns AS, Pruett TL. Tacrolimus versus cyclosporine based immunosuppression in hepatitis C patients after liver transplantation: Long‐term follow‐up of a randomized controlled trial. Hepatology 2006;44(4 (Suppl 1)):412a‐3a. [Google Scholar]
Otero 2009 {published data only}
- Otero A, Varo E, Urbina JO, Martin‐Vivaldi R, Cuervas‐Mons V, Gonzalez‐Pinto I, et al. A prospective randomized open study in liver transplant recipients: Daclizumab, mycophenolate mofetil, and tacrolimus versus tacrolimus and steroids. Liver Transplantation 2009;15(11):1542‐52. [DOI] [PubMed] [Google Scholar]
Pageaux 1995 {published data only}
- Pageaux GP, Blanc P, Perrigault PF, Navarro F, Fabre JM, Souche B, et al. Failure of ursodeoxycholic acid to prevent acute cellular rejection after liver transplantation. Journal of Hepatology 1995;23(2):119‐22. [DOI] [PubMed] [Google Scholar]
Pageaux 2006 {published data only}
- Pageaux GP, Rostaing L, Calmus Y, Duvoux C, Vanlemmens C, Hardgwissen J, et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transplantation 2006;12(12):1755‐60. [DOI] [PubMed] [Google Scholar]
Pascher 2015 {published data only}
- Pascher A, De PS, Pratschke J, Salame E, Pirenne J, Isoneimi H, et al. Protein kinase C inhibitor sotrastaurin in de novo liver transplant recipients: a randomized phase II trial. American Journal of Transplantation 2015;15(5):1283‐92. [DOI] [PubMed] [Google Scholar]
Ramirez 2013 {published data only}
- Ramirez CB, Doria C, Frank AM, Armenti ST, Marino IR. Completely steroid‐free immunosuppression in liver transplantation: a randomized study. Clinical Transplantation 2013;27(3):463‐71. [DOI] [PubMed] [Google Scholar]
- Ramirez CB, Doria C, Frank AM, Navarro V, Herrine S, Rossi S. Enteric coated mycophenolate sodium with basiliximab induction, tacrolimus with or without steroids is safe and effective in adult liver transplantation. Liver Transplantation 2008;14(7 (Suppl 1)):S109. [Google Scholar]
- Ramirez CB, Doria C, Frank AM, Navarro V, Herrine S, Rossi S. Safety and efficacy of steroid‐free immunosuppression using basiliximab induction, tacrolimus and enteric coated mycophenolate sodium in adult liver transplantation. Liver Transplantation 2008;14(7 (Suppl 1)):S109. [Google Scholar]
Reding 1993 {published data only}
- Reding R, Feyaerts A, Vraux H, Latinne D, Parra B, Cornet A, et al. Prophylactic immunosuppression with anti‐interleukin‐2 receptor monoclonal antibody lo‐tact‐1 versus OKT3 in liver allografting. A two‐year follow‐up study. Transplantation 1996;61(9):1406‐9. [DOI] [PubMed] [Google Scholar]
- Reding R, Vraux H, Ville de Goyet J, Sokal E, Hemptinne B, Latinne D, et al. Monoclonal antibodies in prophylactic immunosuppression after liver transplantation. A randomized controlled trial comparing OKT3 and anti‐il‐2 receptor monoclonal antibody LO‐tact‐1. Transplantation 1993;55(3):534‐41. [DOI] [PubMed] [Google Scholar]
Reggiani 2005 {published data only}
- Reggiani P, Arru M, Regazzi M, Gatti S, Molinaro MD, Caccamo L, et al. A "steroid‐free" tacrolimus and low‐dose mycophenolate mofetil primary immunosuppression does not prevent early acute rejection after liver transplantation. Transplantation Proceedings 2005;37(4):1697‐9. [DOI] [PubMed] [Google Scholar]
Reich 2005 {published data only}
- Reich DJ, Clavien PA, Hodge EE. Mycophenolate mofetil for renal dysfunction in liver transplant recipients on cyclosporine or tacrolimus: Randomized, prospective, multicenter pilot study results. Transplantation 2005;80(1):18‐25. [DOI] [PubMed] [Google Scholar]
Saliba 2016 {published data only}
- Saliba F, Duvoux C, Dharancy S, Dumortier J, Calmus Y, Giambattista F, et al. Impact on renal function of stepwise withdrawal of tacrolimus combined with everolimus and EC‐MPS vs standard treatment combining tacrolimus and EC‐MPS in de novo liver transplant recipients: Results of the SIMCER study. American Journal of Transplantation 2016;16:263. [Google Scholar]
Saliba 2016a {published data only}
- Saliba F, Rostaing L, Gugenheim J, Durand F, Radenne S, Leroy V, et al. Corticosteroid‐sparing and optimization of mycophenolic acid exposure in liver transplant recipients receiving mycophenolate mofetil and tacrolimus: a randomized, multicenter study. Transplantation 2016;100(8):1705‐13. [DOI] [PubMed] [Google Scholar]
Salizzoni 2001 {published data only}
- Salizzoni M, Cavallari A, Risaliti A, Filipponi F, Gerunda GE, Forti D, et al. Tacrolimus‐based dual therapy is as efficacious and safe as the conventional tacrolimus‐based triple therapy in liver transplantation. Transplantation Proceedings 2001;33(3):2258‐62. [DOI] [PubMed] [Google Scholar]
Schiano 2006 {published data only}
- Schiano TD, Charlton M, Younossi Z, Galun E, Pruett T, Tur‐Kaspa R, et al. Monoclonal antibody HCV‐abxtl68 in patients undergoing liver transplantation for HCV: Results of a phase 2 randomized study. Liver Transplantation 2006;12(9):1381‐9. [DOI] [PubMed] [Google Scholar]
Schmeding 2007 {published data only}
- Schmeding M, Sauer IM, Kiessling A, Pratschke J, Neuhaus R, Neuhaus P, et al. Influence of basiliximab induction therapy on long term outcome after liver transplantation, a prospectively randomised trial. Annals of Transplantation 2007;12(3):15‐21. [PubMed] [Google Scholar]
Schmeding 2011 {published data only}
- Schmeding M, Kiessling A, Neuhaus R, Heidenhain C, Bahra M, Neuhaus P, et al. Mycophenolate mofetil monotherapy in liver transplantation: 5‐year follow‐up of a prospective randomized trial. Transplantation 2011;92(8):923‐9. [DOI] [PubMed] [Google Scholar]
Shaked 2016 {published data only}
- Shaked A, Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, et al. Early post‐transplant immunosuppression (IS) withdrawal ‐ final outcomes of the ITN030ST AWISH study. American Journal of Transplantation 2016;16:269. [Google Scholar]
Shenoy 2007 {published data only}
- Shenoy S, Hardinger KL, Crippin J, Desai N, Korenblat K, Lisker‐Melman M, et al. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single‐center trial. Transplantation 2007;83(10):1389‐92. [DOI] [PubMed] [Google Scholar]
- Shenoy S, Hardinger KL, Lowell J, Desai N, Mohanakumar T, Crippin J. Interim analysis of a prospective randomized trial to assess reversal of early stage calcineurin inhibitor induced nephrotoxicity using sirolimus based immunosuppression in stable long term liver recipients. American Journal of Transplantation 2004;4(Suppl 8):596. [Google Scholar]
Simone 2008 {published data only}
- Simone P, Nevens F, Metselaar HJ, Sterneck M, Dumortier J, Duvoux C. Everolimus facilitates calcineurin inhibitor withdrawal in maintenance liver transplant patients with renal impairment. Liver Transplantation 2008;14(7 (Suppl 1)):S225. [Google Scholar]
Studenik 2005 {published data only}
- Studenik P, Mejzlik V, Stouracova M, Ondrasek J, Cerny J. Steroid free tacrolimus and mycophenolate mofetil based immunosuppression in liver transplant recipients. Open label, randomised, prospective study. Liver Transplantation 2005;11(7):C42. [Google Scholar]
Takada 2013 {published data only}
- Takada Y, Kaido T, Asonuma K, Sakurai H, Kubo S, Kiuchi T, et al. Randomized, multicenter trial comparing tacrolimus plus mycophenolate mofetil to tacrolimus plus steroids in hepatitis C virus‐positive recipients of living donor liver transplantation. Liver Transplantation 2013;19(8):896‐906. [DOI] [PubMed] [Google Scholar]
Teperman 2013 {published data only}
- Marotta P, Sher L, Teperman LW, Sebastian A, Moonka D, Patel D. Interim 1‐year results of the spare‐the‐nephron (STN) trial in liver transplant recipients: Mycophenolate mofetil (MMF)/sirolimus (SRL) maintenance therapy after CNI withdrawal. Hepatology 2008;48(4 (Suppl)):547a. [Google Scholar]
- Teperman L, Marotta P, Sher L, Sebastian A, Moonka D, Patel D. Tolerability and renal sparing of mycophenolate mofetil (mmf)/sirolimus (SRL) maintenance therapy in liver transplant recipients: 12‐month results of the spare‐the‐nephron (STN) trial. Liver Transplantation 2008;14(7 (Suppl 1)):S224. [Google Scholar]
- Teperman L, Moonka D, Sebastian A, Sher L, Marotta P, Marsh C, et al. Calcineurin inhibitor‐free mycophenolate mofetil/sirolimus maintenance in liver transplantation: the randomized spare‐the‐nephron trial. Liver Transplantation 2013;19(7):675‐89. [DOI] [PubMed] [Google Scholar]
Therapondos 2002 {published data only}
- Therapondos G, Flapan AD, Dollinger MM, Garden OJ, Plevris JN, Hayes PC. Cardiac function after orthotopic liver transplantation and the effects of immunosuppression: a prospective randomized trial comparing cyclosporin (neoral) and tacrolimus. Liver Transplantation 2002;8(8):690‐700. [DOI] [PubMed] [Google Scholar]
Timmermann 2002 {published data only}
- Timmermann W, Erhard J, Lange R, Reck T, Kockerling F, Muller A, et al. A randomised trial comparing the efficacy and safety of tacrolimus with microemulsified cyclosporine after liver transplantation. Transplantation Proceedings 2002;34(5):1516‐8. [DOI] [PubMed] [Google Scholar]
Tisone 1998 {published data only}
- Tisone G, Angelico M, Palmieri G, Pisani F, Baiocchi L, Vennarecci G, et al. Immunosuppression without prednisone after liver transplantion is safe and associated with normal early graft function: Preliminary results of a randomized study. Transplant International 1998;11 Suppl 1:S267‐9. [DOI] [PubMed] [Google Scholar]
- Tisone G, Angelico M, Vennarecci G, Palmieri G, Buonomo O, Negrini S, et al. Metabolic findings after liver transplantation within a randomised trial with or without steroids. Transplantation Proceedings 1998;30(4):1447‐8. [DOI] [PubMed] [Google Scholar]
Trunečka 2015 {published data only}
- Bechstein W, Trunečka P, Klempnauer J, Pirenne J, Bennet W, Zhao A, et al. Renal function outcomes with tacrolimus qd after de novo liver transplantation for different primary diseases: Ad hoc analysis from the DIAMOND randomized, controlled trial. American Journal of Transplantation 2014;14(Suppl 3):160‐1. [Google Scholar]
- Tisone G, Klempnauer J, Bechstein W, Pirenne J, Friman S, Zhao A, et al. Renal function outcomes with tacrolimus qd after de novo liver transplantation for different baseline EGFR: Ad hoc analysis from the DIAMOND randomized controlled trial. Transplantation 2014;98:710‐1. [Google Scholar]
- Trunečka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, et al. Renal function in de novo liver transplant recipients receiving different prolonged‐release tacrolimus regimens ‐ the DIAMOND study. American Journal of Transplantation 2015;15(7):1843‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunečka P, Lehner F, Bechstein WO, Pirenne J, Bennet W, Zhao A, et al. Renal function outcomes with prolonged release tacrolimus according to donor age after de novo liver transplantation: a post hoc analysis from the DIAMOND randomized, controlled trial. Transplant International 2015;28:55. [Google Scholar]
Villamil 2014 {published data only}
- Villamil FG, Gadano AC, Zingale F, Perez R, Gil O, Yantorno S, et al. Fibrosis progression in maintenance liver transplant patients with hepatitis C recurrence: a randomised study of everolimus vs. calcineurin inhibitors. Liver International 2014;34(10):1513‐21. [DOI] [PubMed] [Google Scholar]
Washburn 2001 {published data only}
- Washburn K, Speeg KV, Esterl R, Cigarroa F, Pollack M, Tourtellot C, et al. Steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus, and mycophenolate mofetil. Transplantation 2001;72(10):1675‐9. [DOI] [PubMed] [Google Scholar]
Washburn 2008 {published data only}
- Washburn K, Ghobrial RM, Langnas AN, Freise C, Eckhoff D, Klintmalm G. Safety of thymoglobulin in minimizing maintenance immunosuppression in liver transplant recipients with renal dysfunction. Liver Transplantation 2008;14(7 (Suppl 1)):S105. [Google Scholar]
Watson 2007 {published data only}
- Watson CJ, Gimson AE, Alexander GJ, Allison ME, Gibbs P, Smith JC, et al. A randomized controlled trial of late conversion from calcineurin inhibitor (cni)‐based to sirolimus‐based immunosuppression in liver transplant recipients with impaired renal function. Liver Transplantation 2007;13(12):1694‐702. [DOI] [PubMed] [Google Scholar]
Wiesner 2001 {published data only}
- Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, et al. A randomized double‐blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transplantation 2001;7(5):442‐50. [DOI] [PubMed] [Google Scholar]
Yoshida 2005 {published data only}
- Cantarovich M, Yoshida EM, Peltekian KM, Marotta PJ, Greig PD, Kneteman NM, et al. Poor prediction of the glomerular filtration rate using current formulas in de novo liver transplant patients. Transplantation 2006;82(3):433‐6. [DOI] [PubMed] [Google Scholar]
- Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low‐dose tacrolimus regimen vs. a standard‐dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transplantation 2005;11(9):1064‐72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Nashan 2015 {published data only}
- Nashan B, Schemmer P, Braun F, Dworak M, Wimmer P, Schlitt H. Evaluating the efficacy, safety and evolution of renal function with early initiation of everolimus‐facilitated tacrolimus reduction in de novo liver transplant recipients: Study protocol for a randomized controlled trial. Trials 2015;16:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simone 2014 {published data only}
- Simone P, Cescon M, Cillo U, Tisone G, Guarisco R, Fagiuoli S. Exploring the impact of everolimus‐based immunosuppression on renal function: the REFLECT study. Liver Transplantation 2014;20:S319. [Google Scholar]
Additional references
Barber 2011
- Barber K, Madden S, Allen J, Collett D, Neuberger J, Gimson A, et al. Elective liver transplant list mortality: development of a United Kingdom end‐stage liver disease score. Transplantation 2011;92(4):469‐76. [DOI] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. Chronic liver disease and cirrhosis, 2015. www.cdc.gov/nchs/fastats/liver‐disease.htm (accessed 31 March 2015).
Chaimani 2012
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS ONE 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [DOI] [PubMed] [Google Scholar]
Dam Fialla 2012
- Dam Fialla A, Schaffalitzky de Muckadell OB, Touborg Lassen A. Incidence, etiology and mortality of cirrhosis: a population‐based cohort study. Scandinavian Journal of Gastroenterology 2012;47(6):702‐9. [DOI] [PubMed] [Google Scholar]
Davies 2012
- Davies S. Chief Medical Officer annual report 2011: volume 1. www.gov.uk/government/publications/cmo‐annual‐report‐2011‐volume‐one‐on‐the‐state‐of‐the‐public‐s‐health (accessed 10 October 2014).
Del Re 2013
- Re AC, Spielmans GI, Flückiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS ONE 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demetris 1997
- Demetris AJ, Batts KP, Dhillon AP, Ferrell L, Fung J, Geller SA, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology (Baltimore, Md.) 1997;25(3):658‐63. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). [PubMed]
Dias 2016
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pair wise and network meta‐analysis of randomised controlled trials, August 2011 (last updated September 2016). http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%202Sep2016v2.pdf (accessed 30 March 2017).
Duffy 2010
- Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, et al. Long‐term patient outcome and quality of life after liver transplantation: analysis of 20‐year survivors. Annals of Surgery 2010;252(4):652‐61. [DOI] [PubMed] [Google Scholar]
ELTR 2017
- European Liver Transplant Registry. Evolution of LTs in Europe. www.eltr.org/spip.php?article152 (accessed 17 March 2017).
EuroQol 2014
- EuroQol. About EQ‐5D, 2014. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Fairfield 2015
- Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. Glucocorticosteroid‐free versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD007606.pub3] [DOI] [PubMed] [Google Scholar]
Geissler 2009
- Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut 2009;58(3):452‐63. [DOI] [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 10. Art. No.: LIVER.
Graziadei 2016
- Graziadei I, Zoller H, Fickert P, Schneeberger S, Finkenstedt A, Peck‐Radosavljevic M, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (OGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wiener Klinische Wochenschrift 2016;128(19‐20):679‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Haddad 2006
- Haddad E, McAlister V, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005161.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: Concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamath 2001
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology (Baltimore, Md.) 2001;33(2):464‐70. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lan 2010
- Lan X, Zhang MM, Pu CL, Guo CB, Kang Q, Li YC, et al. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta‐analysis. World Journal of Gastroenterology 2010;16(27):3457‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 2014
- Lan X, Liu MG, Chen HX, Liu HM, Zeng W, Wei D, et al. Efficacy of immunosuppression monotherapy after liver transplantation: a meta‐analysis. World Journal of Gastroenterology 2014;20(34):12330‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longworth 2003
- Longworth L, Young T, Buxton MJ, Ratcliffe J, Neuberger J, Burroughs A, et al. Midterm cost‐effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transplantation 2003;9(12):1295‐307. [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2006b
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maheshwari 2006
- Maheshwari A, Torbenson MS, Thuluvath PJ. Sirolimus monotherapy versus sirolimus in combination with steroids and/or MMF for immunosuppression after liver transplantation. Digestive Diseases and Sciences 2006;51(10):1677‐84. [DOI] [PubMed] [Google Scholar]
Miladinovic 2013
- Miladinovic J, Hozo I, Djulbegovic B. Trial sequential boundaries for cumulative meta‐analyses. Stata Journal 2013;13(1):77‐91. [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Murray 2013
- Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381(9871):997‐1020. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- NCBI. Graft rejection, 2014. www.ncbi.nlm.nih.gov/mesh/68006084 (accessed 10 October 2014).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NHSBT 2014
- NHS Blood and Transplant. Organ donation. Activity report 2013‐2014. www.organdonation.nhs.uk/statistics/transplant_activity_report/ (accessed 8 October 2014).
OpenBUGS 3.2.3 [Computer program]
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
Penninga 2012
- Penninga L, Wettergren A, Chan AW, Steinbrüchel DA, Gluud C. Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008852.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2014a
- Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010252.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2014b
- Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010253.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Ratib 2014
- Ratib S, West J, Crooks CJ, Fleming KM. Diagnosis of liver cirrhosis in England, a cohort study, 1998‐2009: a comparison with cancer. American Journal of Gastroenterology 2014;109(2):190‐8. [DOI] [PubMed] [Google Scholar]
Read 1972
- Read AE. Clinical physiology of the liver. British Journal of Anaesthesia 1972;44(9):910‐7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Peralvarez 2014
- Rodriguez‐Peralvarez M, Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Current Opinion in Organ Transplantation 2014;19(3):253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schoening 2013
- Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, et al. Twenty‐year longitudinal follow‐up after orthotopic liver transplantation: a single‐center experience of 313 consecutive cases. American Journal of Transplantation 2013;13(9):2384‐94. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research Ed.) 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
SRTR 2012
- Scientific Registry of Transplant Recipients. OPTN/SRTR 2012 annual data report: liver. srtr.transplant.hrsa.gov/annual_reports/2012/pdf/03_liver_13.pdf (accessed 8 October 2014).
Stata/SE 14.2 [Computer program]
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station (TX): StataCorp LP, 2017.
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 30 November 2016).
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta‐analysis. Liver Transplantation 2014;20(4):425‐36. [DOI] [PubMed] [Google Scholar]
Ware 2014
- Ware JE. SF‐36® health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed on 8 October 2014).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014
- Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long‐term quality of life. Liver International 2014;34(9):1298‐313. [DOI] [PubMed] [Google Scholar]


